
Summit Therapeutics ESMO Update & Q3 2025 Earnings Call October 20, 2025 8:00am ET

Forward Looking Statement Any statements in this press release about the Company’s future expectations, plans and prospects, including but not limited to, statements about the clinical and preclinical development of the Company’s product candidates, entry into and actions related to the Company’s partnership with Akeso Inc., the Company's anticipated spending and cash runway, the therapeutic potential of the Company’s product candidates, the potential commercialization of the Company’s product candidates, the timing of initiation, completion and availability of data from clinical trials, the potential submission of applications for marketing approvals, the expected timing of BLA submissions, potential acquisitions, statements about the previously disclosed At-The-Market equity offering program (“ATM Program”), the expected proceeds and uses thereof, the Company’s estimates regarding stock-based compensation, and other statements containing the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "would," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the Company’s ability to sell shares of our common stock under the ATM Program, the conditions affecting the capital markets, general economic, industry, or political conditions, including the effects of geopolitical developments, domestic and foreign trade policies, and monetary policies, the results of our evaluation of the underlying data in connection with the development and commercialization activities for ivonescimab, the outcome of discussions with regulatory authorities, including the Food and Drug Administration, the uncertainties inherent in the initiation of future clinical trials, availability and timing of data from ongoing and future clinical trials, the results of such trials, and their success, global public health crises, that may affect timing and status of our clinical trials and operations, whether preliminary results from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials or preclinical studies will be indicative of the results of later clinical trials, whether business development opportunities to expand the Company’s pipeline of drug candidates, including without limitation, through potential acquisitions of, and/or collaborations with, other entities occur, expectations for regulatory approvals, laws and regulations affecting government contracts and funding awards, availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements and other factors discussed in the "Risk Factors" and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of filings that the Company makes with the Securities and Exchange Commission. Any change to our ongoing trials could cause delays, affect our future expenses, and add uncertainty to our commercialization efforts, as well as to affect the likelihood of the successful completion of clinical development of ivonescimab. Accordingly, readers should not place undue reliance on forward-looking statements or information. In addition, any forward-looking statements included in this press release represent the Company’s views only as of the date of this release and should not be relied upon as representing the Company’s views as of any subsequent date. The Company specifically disclaims any obligation to update any forward-looking statements included in this press release. Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2025, Summit Therapeutics Inc. All Rights Reserved. 2
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). 19 October 2025 Phase III study of ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy as first-line treatment for advanced squamous non-small cell lung cancer (HARMONi-6) Shun Lu1, Fang Yang2, Zhou Jiang3, Longhua Sun4, Lin Wu3, Zhengxiang Han5, Yun Fan6, Yanqiu Zhao7, Xingya Li8, Haipeng Xu9, Xiangjiao Meng10, Ying Cheng11, Zhiye Zhang12, Zhiwei Chen1, Hui Luo13, Qin Shi14, Xuelei Ma15, Xuezhen Ma16, Zhongmin Zhang17, Michelle Xia18 1Shanghai Chest Hospital, Shanghai, China; 2Harbin Medical University Cancer Hospital, Harbin, China; 3Hunan Cancer Hospital, Changsha, China; 4The First Affiliated Hospital of Nanchang University, Nanchang, China; 5The Affiliated Hospital of Xuzhou Medical University, Xuzhou, China; 6Zhejiang Cancer Hospital, Hangzhou, China; 7Henan Cancer Hospital, Zhengzhou, China; 8The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; 9Fujian Provincial Tumor Hospital, Fuzhou, China; 10Shandong Cancer Hospital and Institute, Jinan, China; 11Jilin Cancer Hospital, Changchun, China; 12The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China; 13Jiangxi Cancer Hospital, Nanchang, China; 14Fuzhou pulmonary hospital of fujian, Fuzhou, China; 15West China Hospital of Sichuan University, Chengdu, China; 16Qingdao Central Hospital, Qingdao, China; 17Linyi People’s Hospital, Linyi, China; 18Akeso Biopharma, Inc., Zhongshan, China. Akeso Sponsored Trial HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 3
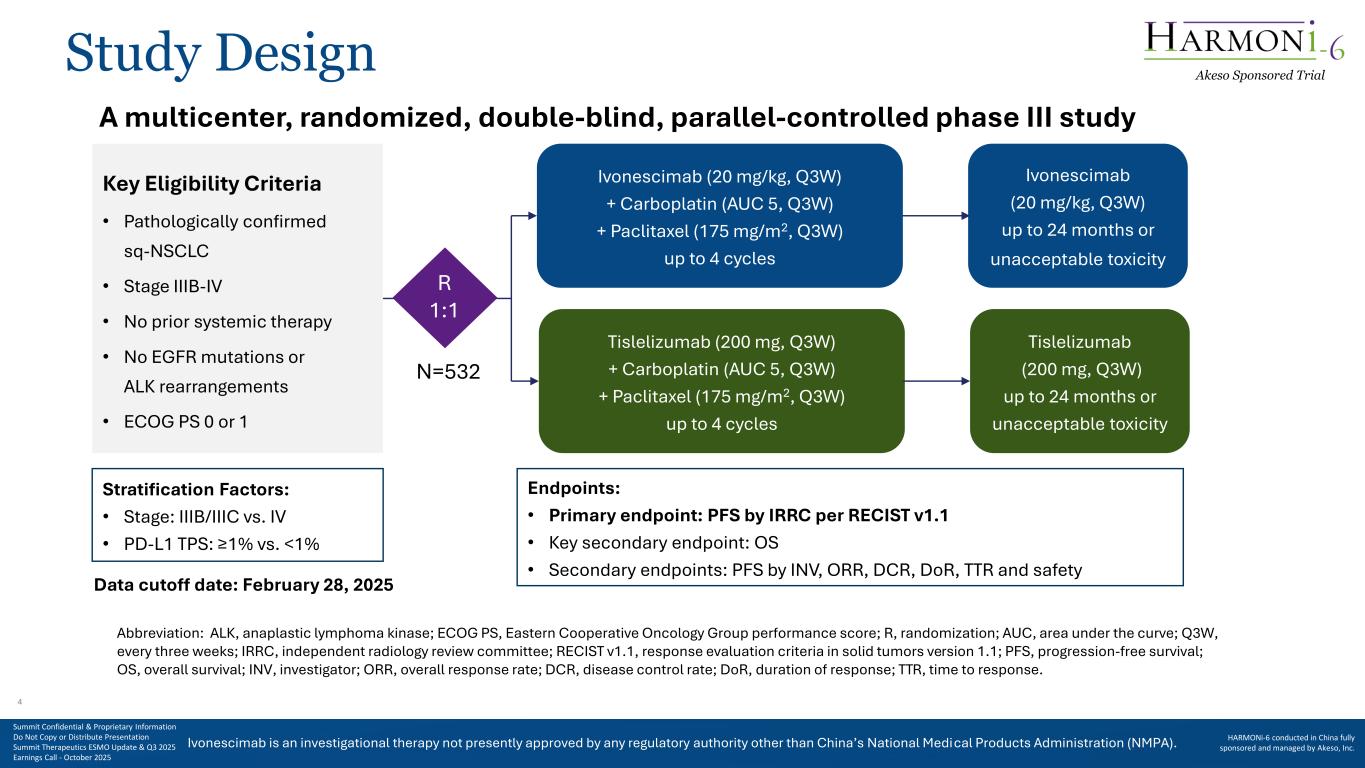
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Study Design A multicenter, randomized, double-blind, parallel-controlled phase III study Key Eligibility Criteria • Pathologically confirmed sq-NSCLC • Stage IIIB-IV • No prior systemic therapy • No EGFR mutations or ALK rearrangements • ECOG PS 0 or 1 Tislelizumab (200 mg, Q3W) + Carboplatin (AUC 5, Q3W) + Paclitaxel (175 mg/m2, Q3W) up to 4 cycles Ivonescimab (20 mg/kg, Q3W) up to 24 months or unacceptable toxicity Tislelizumab (200 mg, Q3W) up to 24 months or unacceptable toxicity N=532 Stratification Factors: • Stage: IIIB/IIIC vs. IV • PD-L1 TPS: ≥1% vs. <1% Endpoints: • Primary endpoint: PFS by IRRC per RECIST v1.1 • Key secondary endpoint: OS • Secondary endpoints: PFS by INV, ORR, DCR, DoR, TTR and safety Abbreviation: ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance score; R, randomization; AUC, area under the curve; Q3W, every three weeks; IRRC, independent radiology review committee; RECIST v1.1, response evaluation criteria in solid tumors version 1.1; PFS, progression-free survival; OS, overall survival; INV, investigator; ORR, overall response rate; DCR, disease control rate; DoR, duration of response; TTR, time to response. Data cutoff date: February 28, 2025 Ivonescimab (20 mg/kg, Q3W) + Carboplatin (AUC 5, Q3W) + Paclitaxel (175 mg/m2, Q3W) up to 4 cycles R 1:1 Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 4
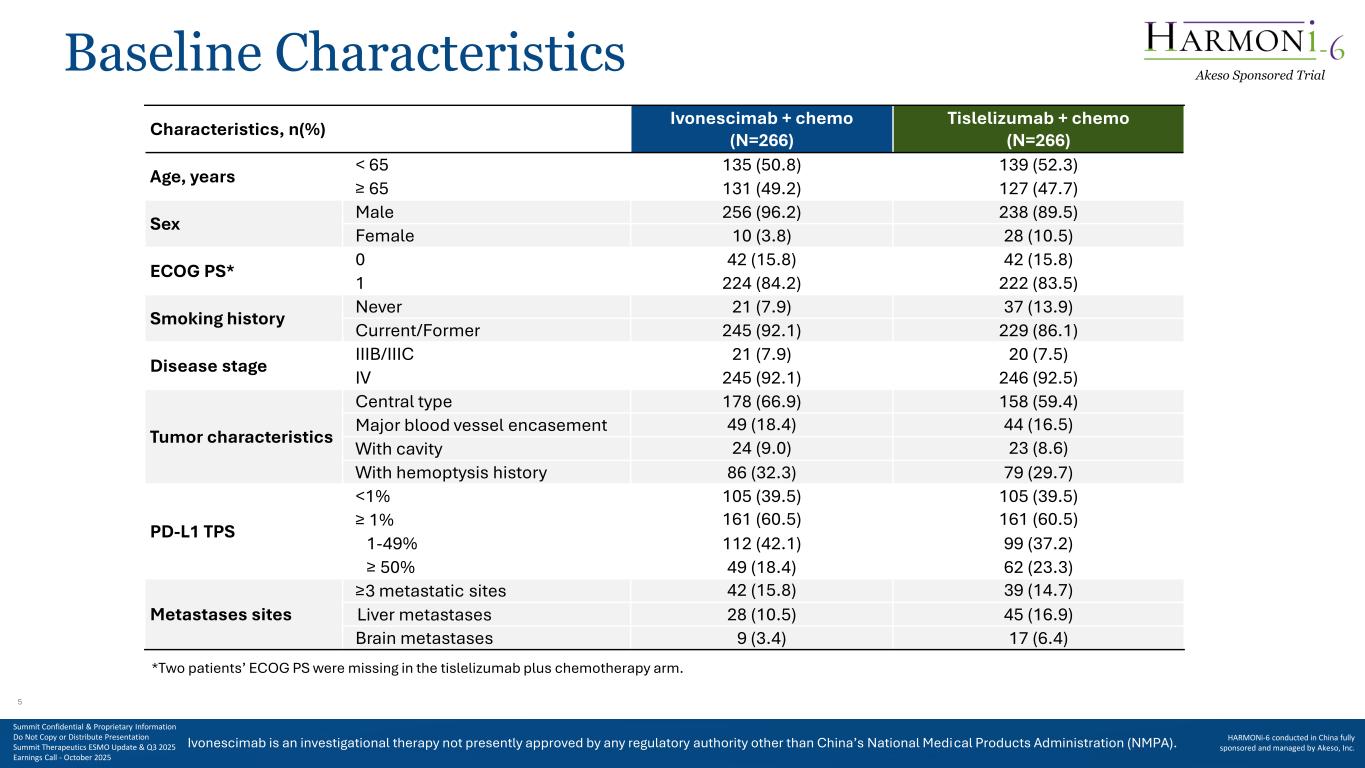
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Baseline Characteristics Characteristics, n(%) Ivonescimab + chemo (N=266) Tislelizumab + chemo (N=266) Age, years < 65 135 (50.8) 139 (52.3) ≥ 65 131 (49.2) 127 (47.7) Sex Male 256 (96.2) 238 (89.5) Female 10 (3.8) 28 (10.5) ECOG PS* 0 42 (15.8) 42 (15.8) 1 224 (84.2) 222 (83.5) Smoking history Never 21 (7.9) 37 (13.9) Current/Former 245 (92.1) 229 (86.1) Disease stage IIIB/IIIC 21 (7.9) 20 (7.5) IV 245 (92.1) 246 (92.5) Tumor characteristics Central type 178 (66.9) 158 (59.4) Major blood vessel encasement 49 (18.4) 44 (16.5) With cavity 24 (9.0) 23 (8.6) With hemoptysis history 86 (32.3) 79 (29.7) PD-L1 TPS <1% 105 (39.5) 105 (39.5) ≥ 1% 161 (60.5) 161 (60.5) 1-49% 112 (42.1) 99 (37.2) ≥ 50% 49 (18.4) 62 (23.3) Metastases sites ≥3 metastatic sites 42 (15.8) 39 (14.7) Liver metastases 28 (10.5) 45 (16.9) Brain metastases 9 (3.4) 17 (6.4) *Two patients’ ECOG PS were missing in the tislelizumab plus chemotherapy arm. Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 5
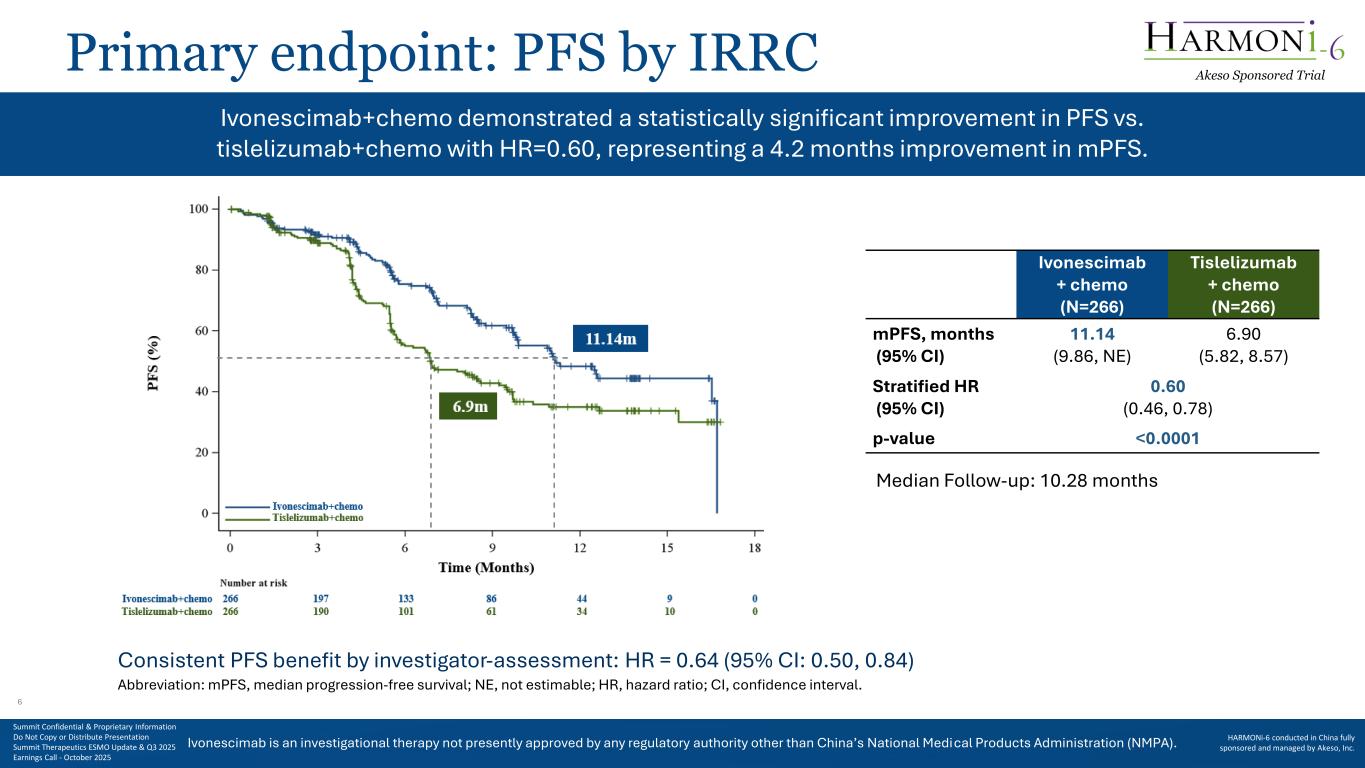
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Primary endpoint: PFS by IRRC Ivonescimab + chemo (N=266) Tislelizumab + chemo (N=266) mPFS, months (95% CI) 11.14 (9.86, NE) 6.90 (5.82, 8.57) Stratified HR (95% CI) 0.60 (0.46, 0.78) p-value <0.0001 Abbreviation: mPFS, median progression-free survival; NE, not estimable; HR, hazard ratio; CI, confidence interval. Median Follow-up: 10.28 months Ivonescimab+chemo demonstrated a statistically significant improvement in PFS vs. tislelizumab+chemo with HR=0.60, representing a 4.2 months improvement in mPFS. Consistent PFS benefit by investigator-assessment: HR = 0.64 (95% CI: 0.50, 0.84) Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 6
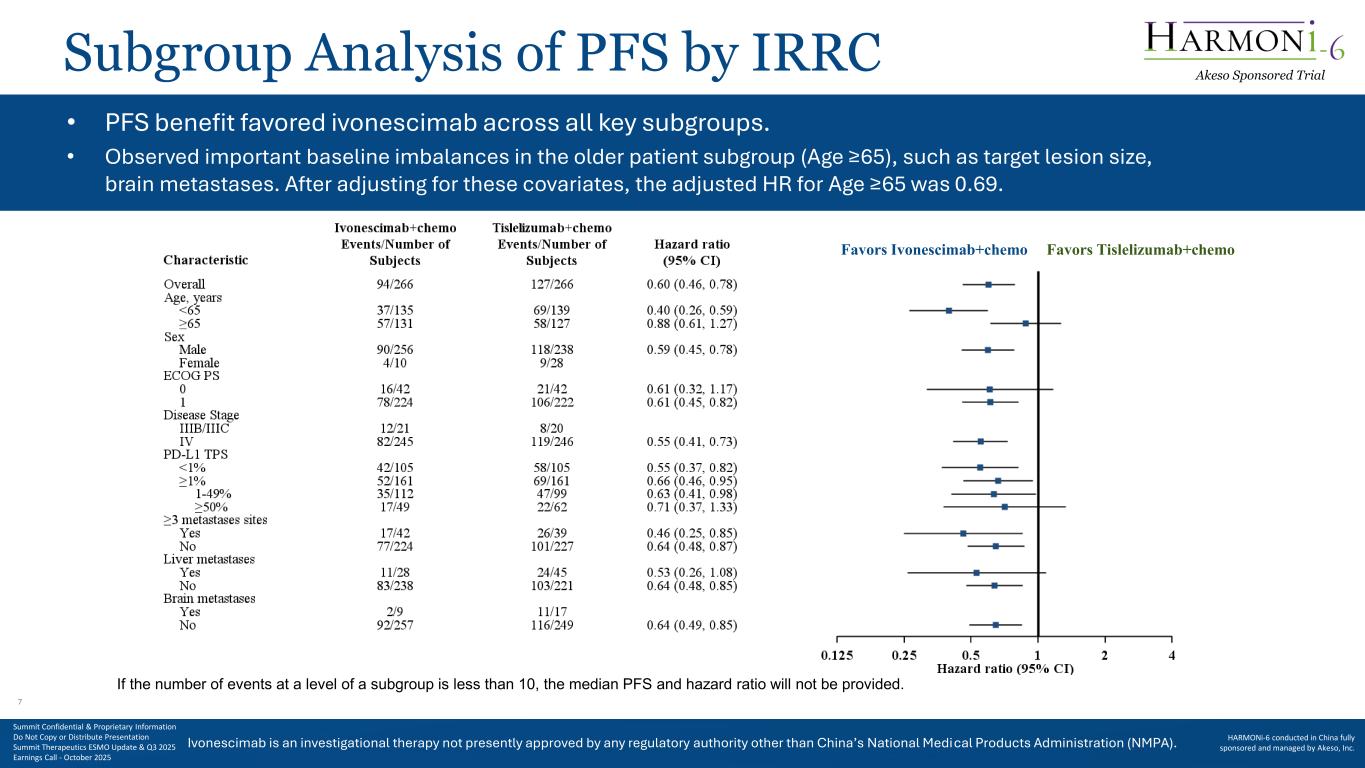
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Favors Ivonescimab+chemo Favors Tislelizumab+chemo Subgroup Analysis of PFS by IRRC • PFS benefit favored ivonescimab across all key subgroups. • Observed important baseline imbalances in the older patient subgroup (Age ≥65), such as target lesion size, brain metastases. After adjusting for these covariates, the adjusted HR for Age ≥65 was 0.69. If the number of events at a level of a subgroup is less than 10, the median PFS and hazard ratio will not be provided. Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 7
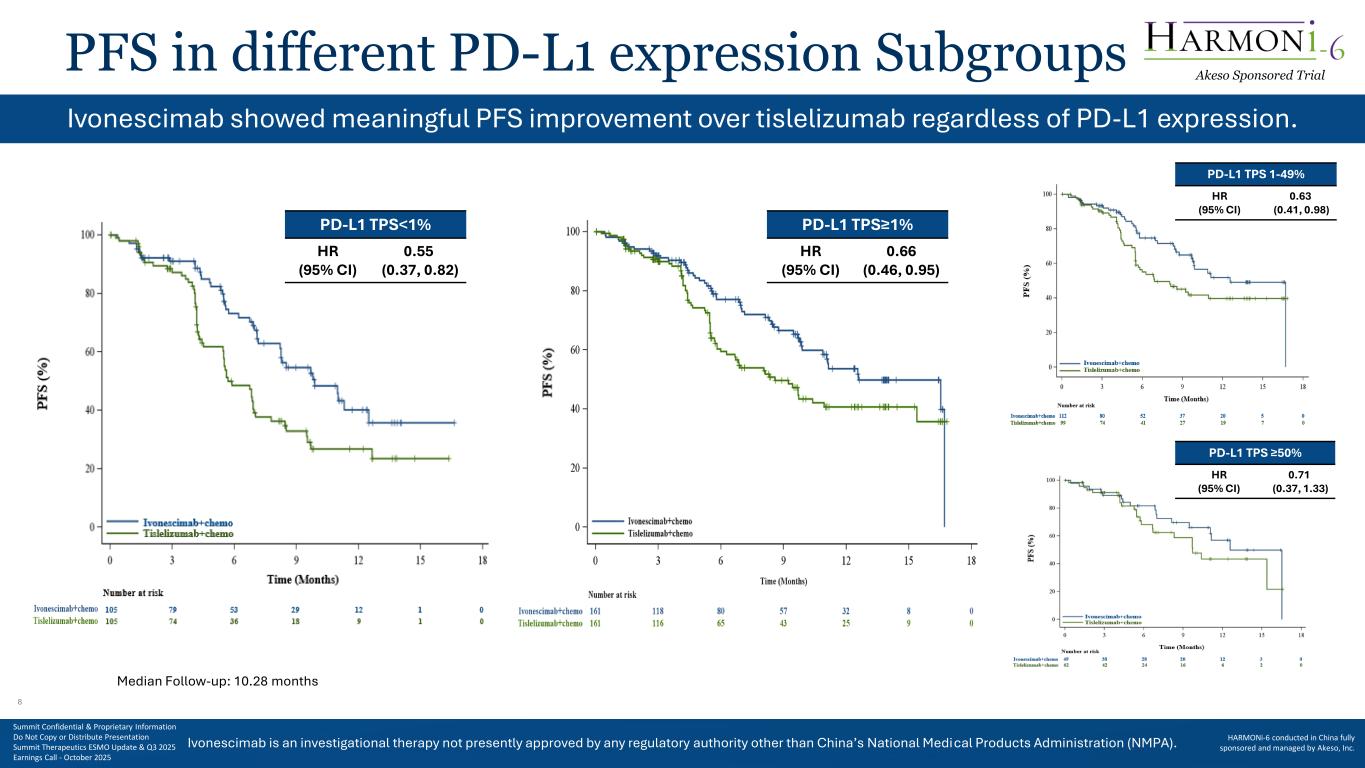
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). PFS in different PD-L1 expression Subgroups Ivonescimab showed meaningful PFS improvement over tislelizumab regardless of PD-L1 expression. PD-L1 TPS<1% HR (95% CI) 0.55 (0.37, 0.82) PD-L1 TPS 1-49% HR (95% CI) 0.63 (0.41, 0.98) PD-L1 TPS ≥50% HR (95% CI) 0.71 (0.37, 1.33) Median Follow-up: 10.28 months PD-L1 TPS≥1% HR (95% CI) 0.66 (0.46, 0.95) Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 8
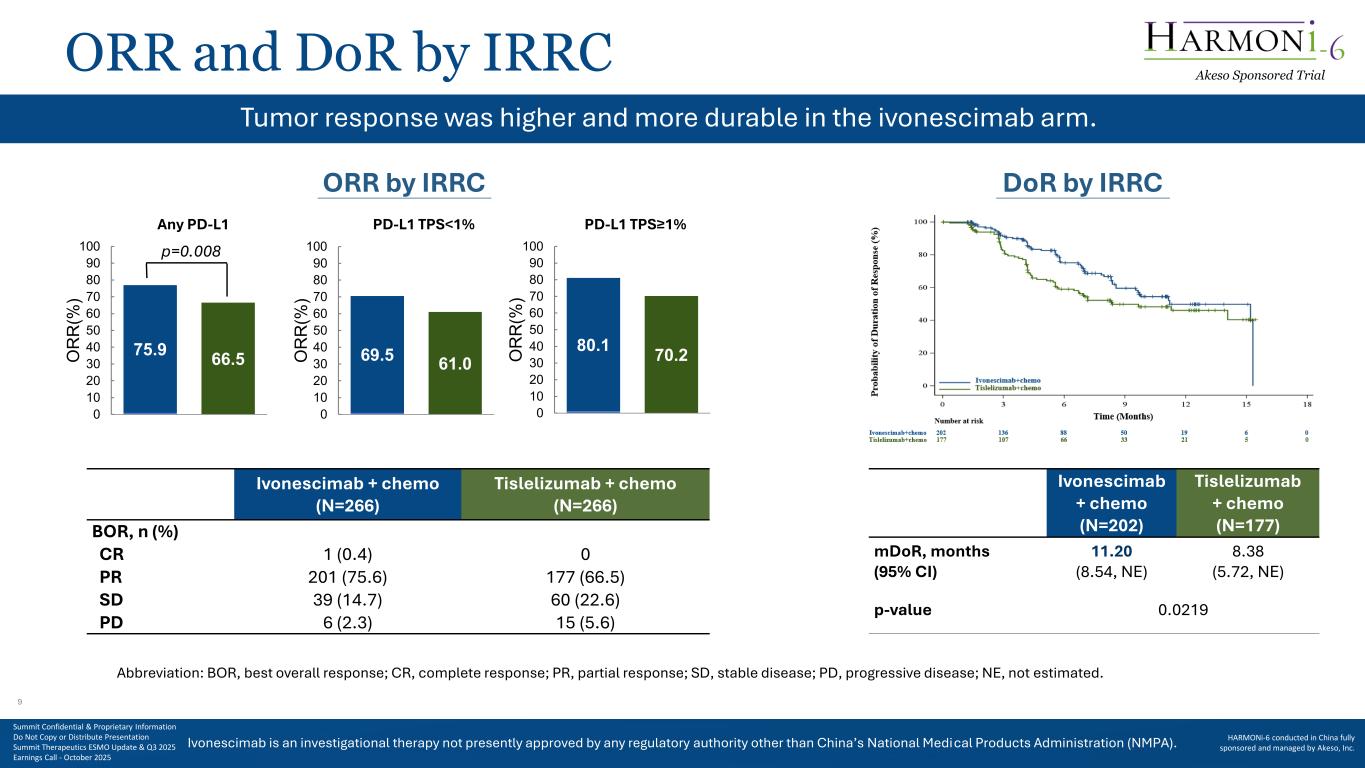
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). ORR and DoR by IRRC Ivonescimab + chemo (N=202) Tislelizumab + chemo (N=177) mDoR, months (95% CI) 11.20 (8.54, NE) 8.38 (5.72, NE) p-value 0.0219 Abbreviation: BOR, best overall response; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not estimated. Tumor response was higher and more durable in the ivonescimab arm. Ivonescimab + chemo (N=266) Tislelizumab + chemo (N=266) BOR, n (%) CR 1 (0.4) 0 PR 201 (75.6) 177 (66.5) SD 39 (14.7) 60 (22.6) PD 6 (2.3) 15 (5.6) 75.9 66.5 0 10 20 30 40 50 60 70 80 90 100 O R R (% ) 69.5 61.0 0 10 20 30 40 50 60 70 80 90 100 O R R (% ) 80.1 70.2 0 10 20 30 40 50 60 70 80 90 100 O R R (% ) ORR by IRRC Any PD-L1 PD-L1 TPS<1% PD-L1 TPS≥1% DoR by IRRC p=0.008 Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 9
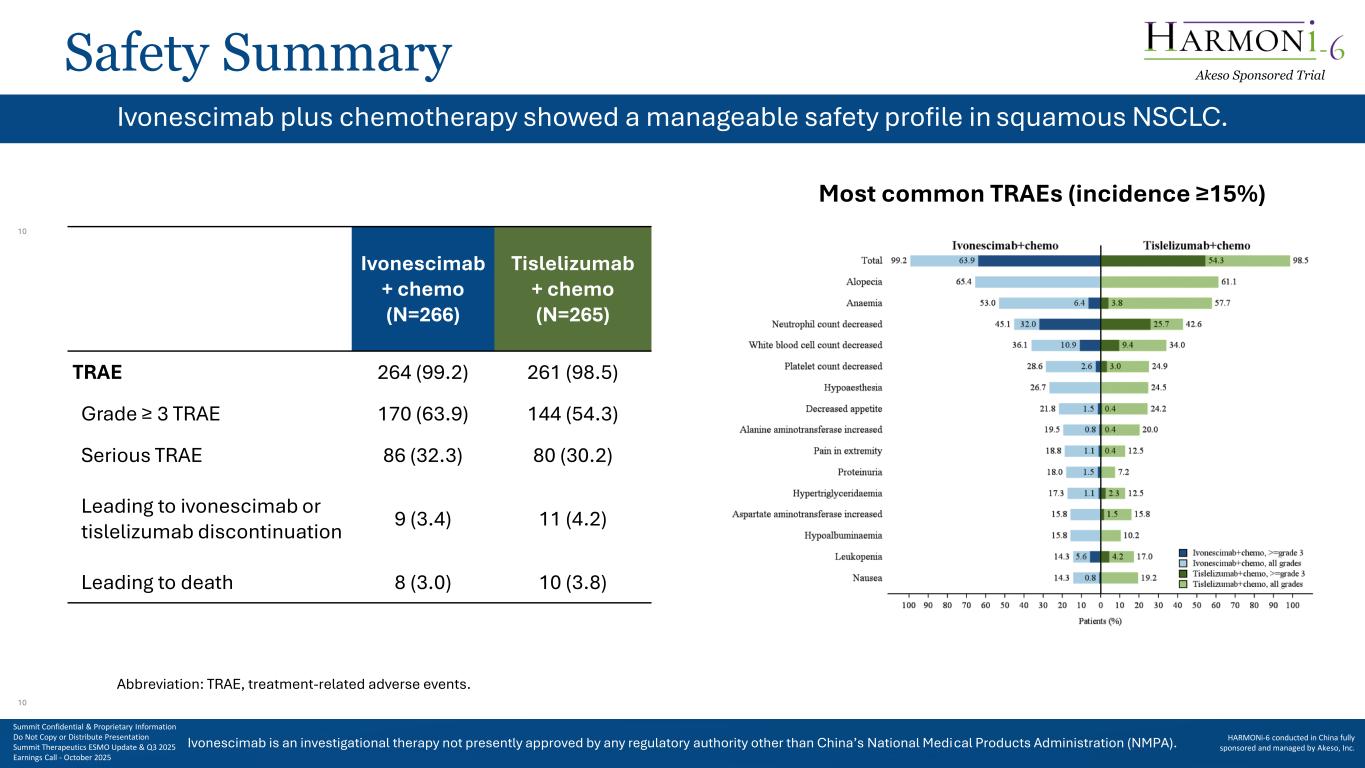
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Safety Summary b Most common TRAEs (incidence ≥15%) Ivonescimab + chemo (N=266) Tislelizumab + chemo (N=265) TRAE 264 (99.2) 261 (98.5) Grade ≥ 3 TRAE 170 (63.9) 144 (54.3) Serious TRAE 86 (32.3) 80 (30.2) Leading to ivonescimab or tislelizumab discontinuation 9 (3.4) 11 (4.2) Leading to death 8 (3.0) 10 (3.8) Abbreviation: TRAE, treatment-related adverse events. Ivonescimab plus chemotherapy showed a manageable safety profile in squamous NSCLC. Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 10 10
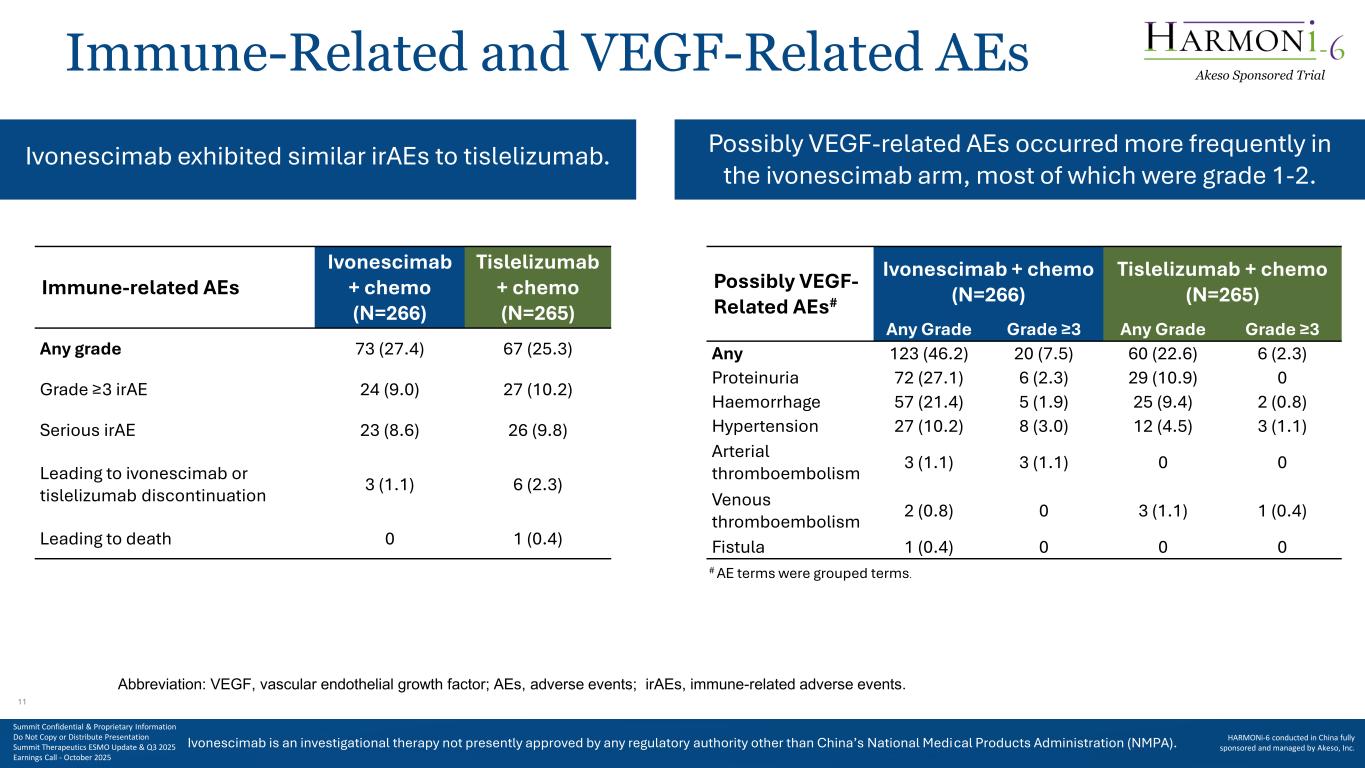
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Immune-Related and VEGF-Related AEs Ivonescimab exhibited similar irAEs to tislelizumab. Immune-related AEs Ivonescimab + chemo (N=266) Tislelizumab + chemo (N=265) Any grade 73 (27.4) 67 (25.3) Grade ≥3 irAE 24 (9.0) 27 (10.2) Serious irAE 23 (8.6) 26 (9.8) Leading to ivonescimab or tislelizumab discontinuation 3 (1.1) 6 (2.3) Leading to death 0 1 (0.4) Possibly VEGF- Related AEs# Ivonescimab + chemo (N=266) Tislelizumab + chemo (N=265) Any Grade Grade ≥3 Any Grade Grade ≥3 Any 123 (46.2) 20 (7.5) 60 (22.6) 6 (2.3) Proteinuria 72 (27.1) 6 (2.3) 29 (10.9) 0 Haemorrhage 57 (21.4) 5 (1.9) 25 (9.4) 2 (0.8) Hypertension 27 (10.2) 8 (3.0) 12 (4.5) 3 (1.1) Arterial thromboembolism 3 (1.1) 3 (1.1) 0 0 Venous thromboembolism 2 (0.8) 0 3 (1.1) 1 (0.4) Fistula 1 (0.4) 0 0 0 Abbreviation: VEGF, vascular endothelial growth factor; AEs, adverse events; irAEs, immune-related adverse events. # AE terms were grouped terms. Possibly VEGF-related AEs occurred more frequently in the ivonescimab arm, most of which were grade 1-2. Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 11
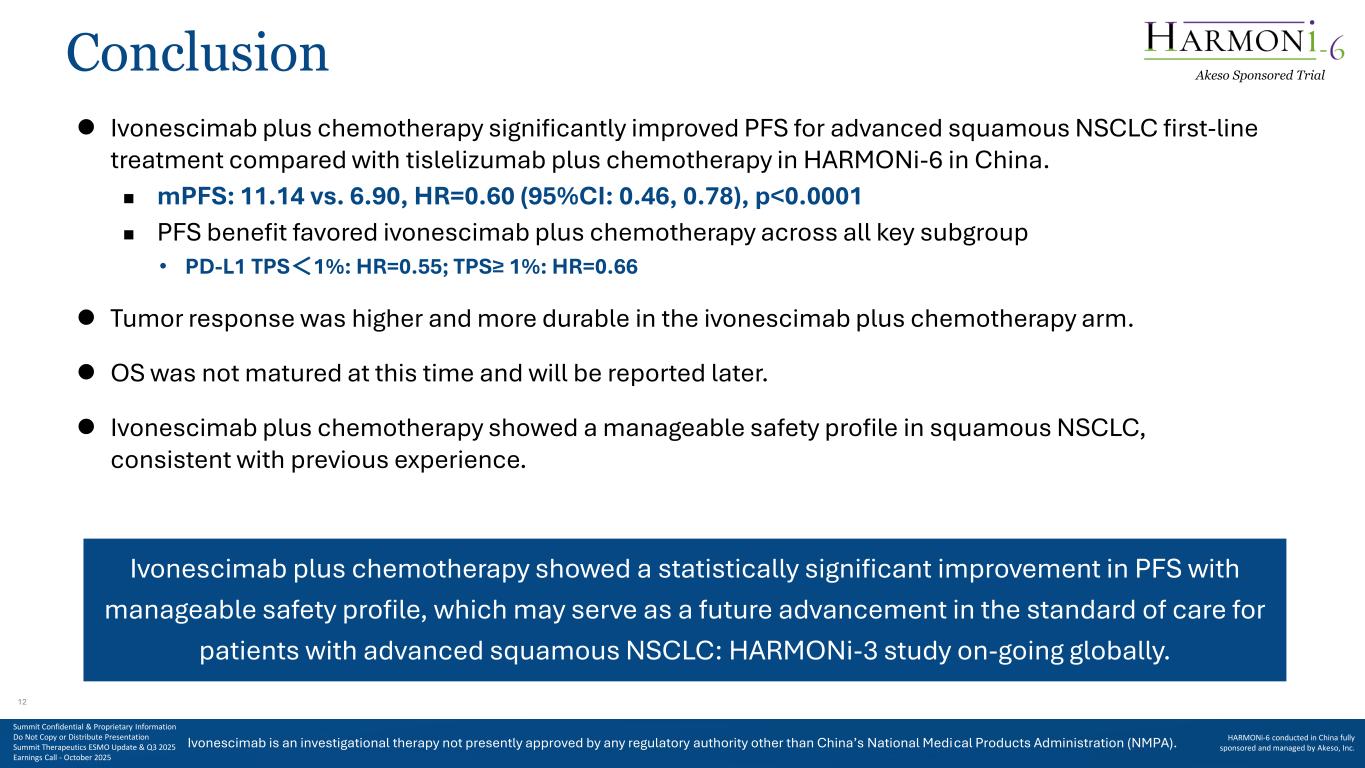
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Conclusion ⚫ Ivonescimab plus chemotherapy significantly improved PFS for advanced squamous NSCLC first-line treatment compared with tislelizumab plus chemotherapy in HARMONi-6 in China. ◼ mPFS: 11.14 vs. 6.90, HR=0.60 (95%CI: 0.46, 0.78), p<0.0001 ◼ PFS benefit favored ivonescimab plus chemotherapy across all key subgroup • PD-L1 TPS<1%: HR=0.55; TPS≥ 1%: HR=0.66 ⚫ Tumor response was higher and more durable in the ivonescimab plus chemotherapy arm. ⚫ OS was not matured at this time and will be reported later. ⚫ Ivonescimab plus chemotherapy showed a manageable safety profile in squamous NSCLC, consistent with previous experience. Ivonescimab plus chemotherapy showed a statistically significant improvement in PFS with manageable safety profile, which may serve as a future advancement in the standard of care for patients with advanced squamous NSCLC: HARMONi-3 study on-going globally. Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 12

Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Acknowledgement ⚫ All patients participating in the study as well as their families. ⚫ All investigators and team members involved in this trial. ⚫ Akeso Biopharma Inc. who sponsored this study. https://doi.org/10.1016/S0140-6736(25)01848-3 Published Online First at https://www.thelancet.com/journals/lancet/onlinefirst Akeso Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 HARMONi-6 conducted in China fully sponsored and managed by Akeso, Inc. 13

Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Phase 1-2 Phase 3 1L Biliary Tract: 1L Pancreatic: 1L Colorectal: 1L NSCLC: 2L+ NSCLC EGFR+: 1L TNBC: 2L+ NSCLC: 1L NSCLC: SCLC: 1L HNSCC: Conducted in China Fully Sponsored and Managed by Akeso Breast Head & Neck Gastric / GEJ Hepatocellular Gynecologic Ovarian Phase 3 30+ Approved Trials Being Initiated Investigator Sponsored Trials* Planned and Ongoing Studies Sponsored by Summit Therapeutics M.D. Anderson Collaboration Initiated* 5+ Pre-clinical and Clinical Ongoing Trials RAS(ON) + ivonescimab in multiple solid tumors Revolution Medicines Collaboration Enrolling 1L NSCLC: 1L NSCLC: Enrolling 2L+ NSCLC EGFR+: Enrollment Complete 1L CRC: Not Yet Enrolling *ISTs, M.D. Anderson collaboration trials not sponsored by Summit. Akeso Phase III clinical trials from Akeso’s 2025 First Half Interim Results (prnewswire.com; akesobio.com) and/or clinicaltrials.gov; Summit Therapeutics Press Release Revolution Medicines. Jun 30, 2025 Abbreviations: 1L=first-line; 2L=second-line; CDP=clinical development plan; CRC=colorectal cancer; EGFR=epidermal growth factor receptor; GEJ=gastroesophageal junction; HNSCC=head and neck squamous cell carcinoma; NSCLC=non small cell lung cancer; SCLC=small cell lung cancer; TNBC=triple negative breast cancer. Ivonescimab Development Plan Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 14
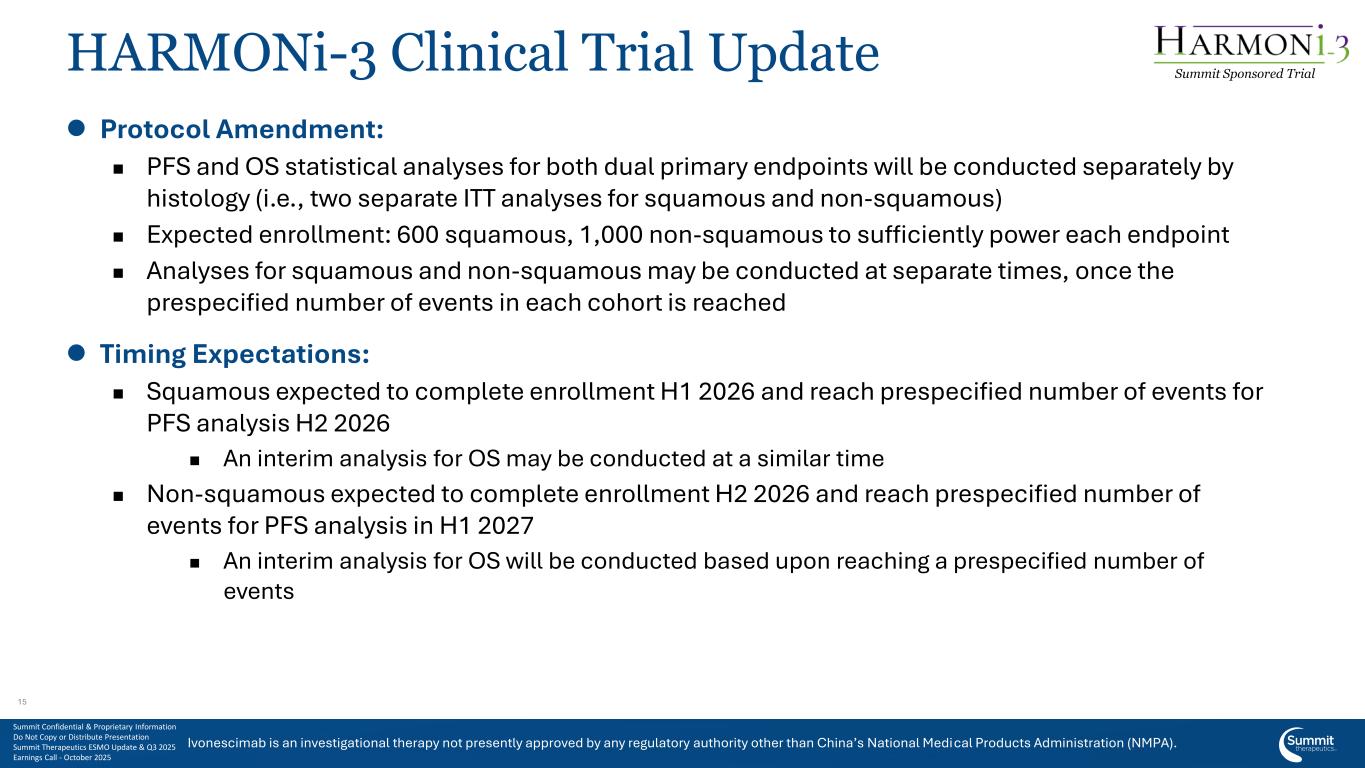
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). HARMONi-3 Clinical Trial Update ⚫ Protocol Amendment: ◼ PFS and OS statistical analyses for both dual primary endpoints will be conducted separately by histology (i.e., two separate ITT analyses for squamous and non-squamous) ◼ Expected enrollment: 600 squamous, 1,000 non-squamous to sufficiently power each endpoint ◼ Analyses for squamous and non-squamous may be conducted at separate times, once the prespecified number of events in each cohort is reached ⚫ Timing Expectations: ◼ Squamous expected to complete enrollment H1 2026 and reach prespecified number of events for PFS analysis H2 2026 ◼ An interim analysis for OS may be conducted at a similar time ◼ Non-squamous expected to complete enrollment H2 2026 and reach prespecified number of events for PFS analysis in H1 2027 ◼ An interim analysis for OS will be conducted based upon reaching a prespecified number of events Summit Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 15
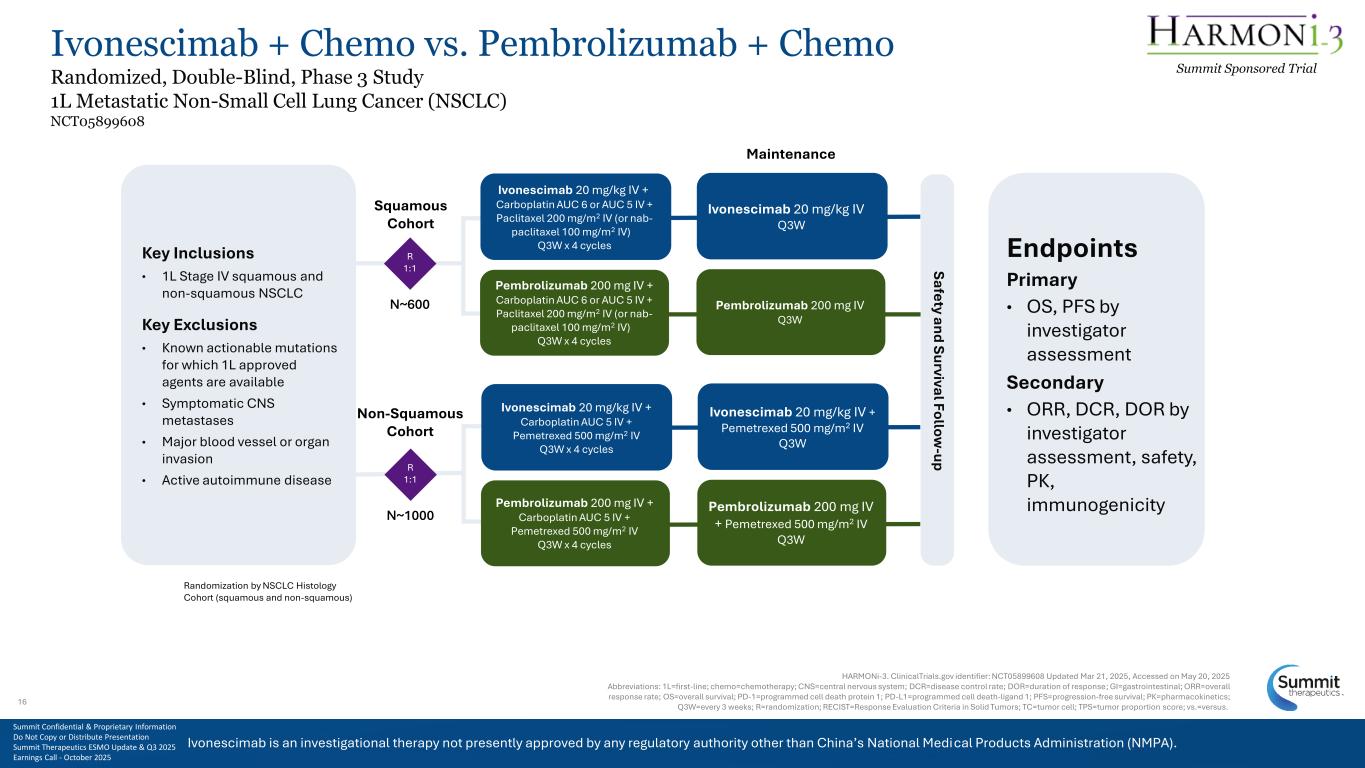
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Ivonescimab + Chemo vs. Pembrolizumab + Chemo Randomized, Double-Blind, Phase 3 Study 1L Metastatic Non-Small Cell Lung Cancer (NSCLC) NCT05899608 Summit Sponsored Trial HARMONi-3. ClinicalTrials.gov identifier: NCT05899608 Updated Mar 21, 2025, Accessed on May 20, 2025 Abbreviations: 1L=first-line; chemo=chemotherapy; CNS=central nervous system; DCR=disease control rate; DOR=duration of response; GI=gastrointestinal; ORR=overall response rate; OS=overall survival; PD-1=programmed cell death protein 1; PD-L1=programmed cell death-ligand 1; PFS=progression-free survival; PK=pharmacokinetics; Q3W=every 3 weeks; R=randomization; RECIST=Response Evaluation Criteria in Solid Tumors; TC=tumor cell; TPS=tumor proportion score; vs.=versus. Ivonescimab 20 mg/kg IV + Carboplatin AUC 5 IV + Pemetrexed 500 mg/m2 IV Q3W x 4 cycles Key Inclusions • 1L Stage IV squamous and non-squamous NSCLC Key Exclusions • Known actionable mutations for which 1L approved agents are available • Symptomatic CNS metastases • Major blood vessel or organ invasion • Active autoimmune disease Safety and Survival Follow -up Ivonescimab 20 mg/kg IV + Carboplatin AUC 6 or AUC 5 IV + Paclitaxel 200 mg/m2 IV (or nab- paclitaxel 100 mg/m2 IV) Q3W x 4 cycles Pembrolizumab 200 mg IV + Carboplatin AUC 6 or AUC 5 IV + Paclitaxel 200 mg/m2 IV (or nab- paclitaxel 100 mg/m2 IV) Q3W x 4 cycles Endpoints Primary • OS, PFS by investigator assessment Secondary • ORR, DCR, DOR by investigator assessment, safety, PK, immunogenicity Squamous Cohort Non-Squamous Cohort Maintenance R 1:1 N~600 R 1:1 N~1000 Randomization by NSCLC Histology Cohort (squamous and non-squamous) Pembrolizumab 200 mg IV + Carboplatin AUC 5 IV + Pemetrexed 500 mg/m2 IV Q3W x 4 cycles Ivonescimab 20 mg/kg IV + Pemetrexed 500 mg/m2 IV Q3W Ivonescimab 20 mg/kg IV Q3W Pembrolizumab 200 mg IV Q3W Pembrolizumab 200 mg IV + Pemetrexed 500 mg/m2 IV Q3W Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 16
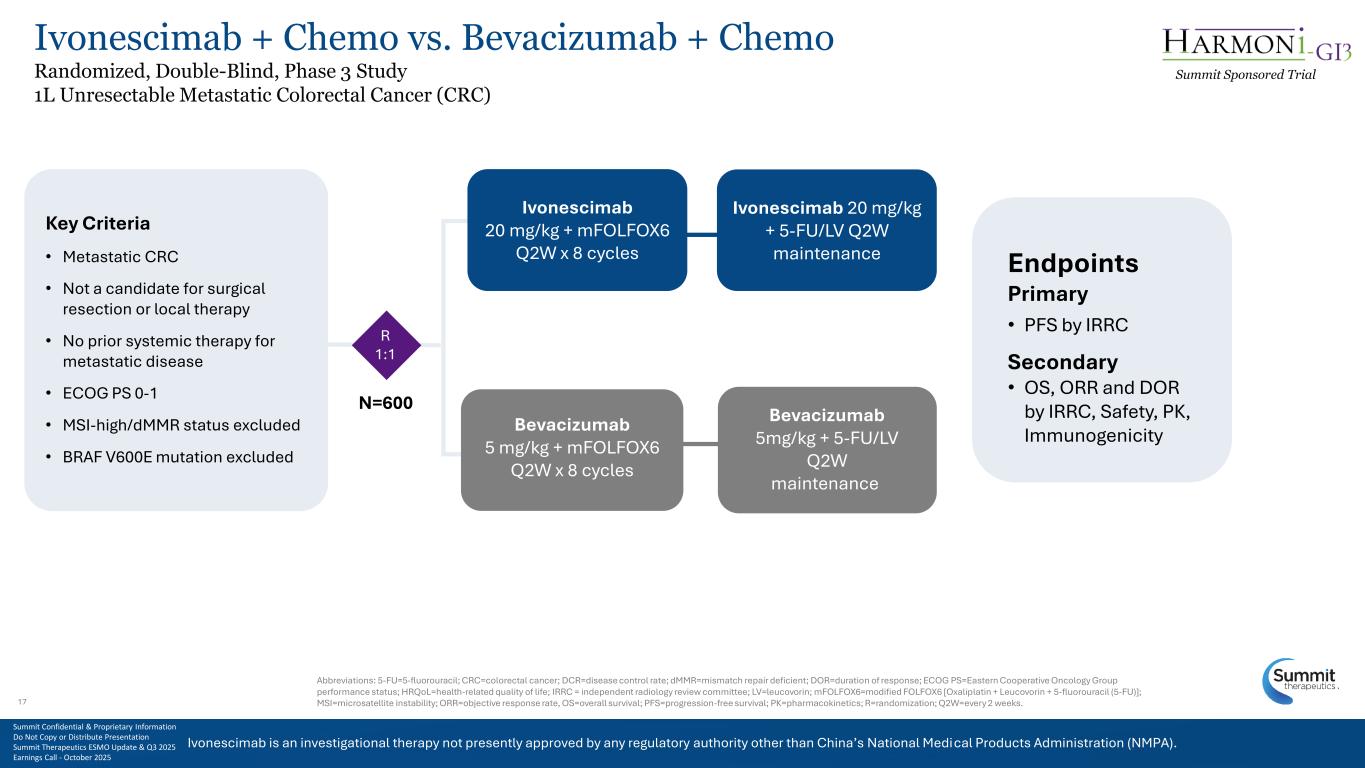
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). 17 Key Criteria • Metastatic CRC • Not a candidate for surgical resection or local therapy • No prior systemic therapy for metastatic disease • ECOG PS 0-1 • MSI-high/dMMR status excluded • BRAF V600E mutation excluded Bevacizumab 5 mg/kg + mFOLFOX6 Q2W x 8 cycles Ivonescimab 20 mg/kg + mFOLFOX6 Q2W x 8 cycles Bevacizumab 5mg/kg + 5-FU/LV Q2W maintenance Ivonescimab 20 mg/kg + 5-FU/LV Q2W maintenance R 1:1 N=600 Endpoints Primary • PFS by IRRC Secondary • OS, ORR and DOR by IRRC, Safety, PK, Immunogenicity Abbreviations: 5-FU=5-fluorouracil; CRC=colorectal cancer; DCR=disease control rate; dMMR=mismatch repair deficient; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group performance status; HRQoL=health-related quality of life; IRRC = independent radiology review committee; LV=leucovorin; mFOLFOX6=modified FOLFOX6 [Oxaliplatin + Leucovorin + 5-fluorouracil (5-FU)]; MSI=microsatellite instability; ORR=objective response rate, OS=overall survival; PFS=progression-free survival; PK=pharmacokinetics; R=randomization; Q2W=every 2 weeks. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Sponsored Trial Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 Ivonescimab + Chemo vs. Bevacizumab + Chemo Randomized, Double-Blind, Phase 3 Study 1L Unresectable Metastatic Colorectal Cancer (CRC) 17
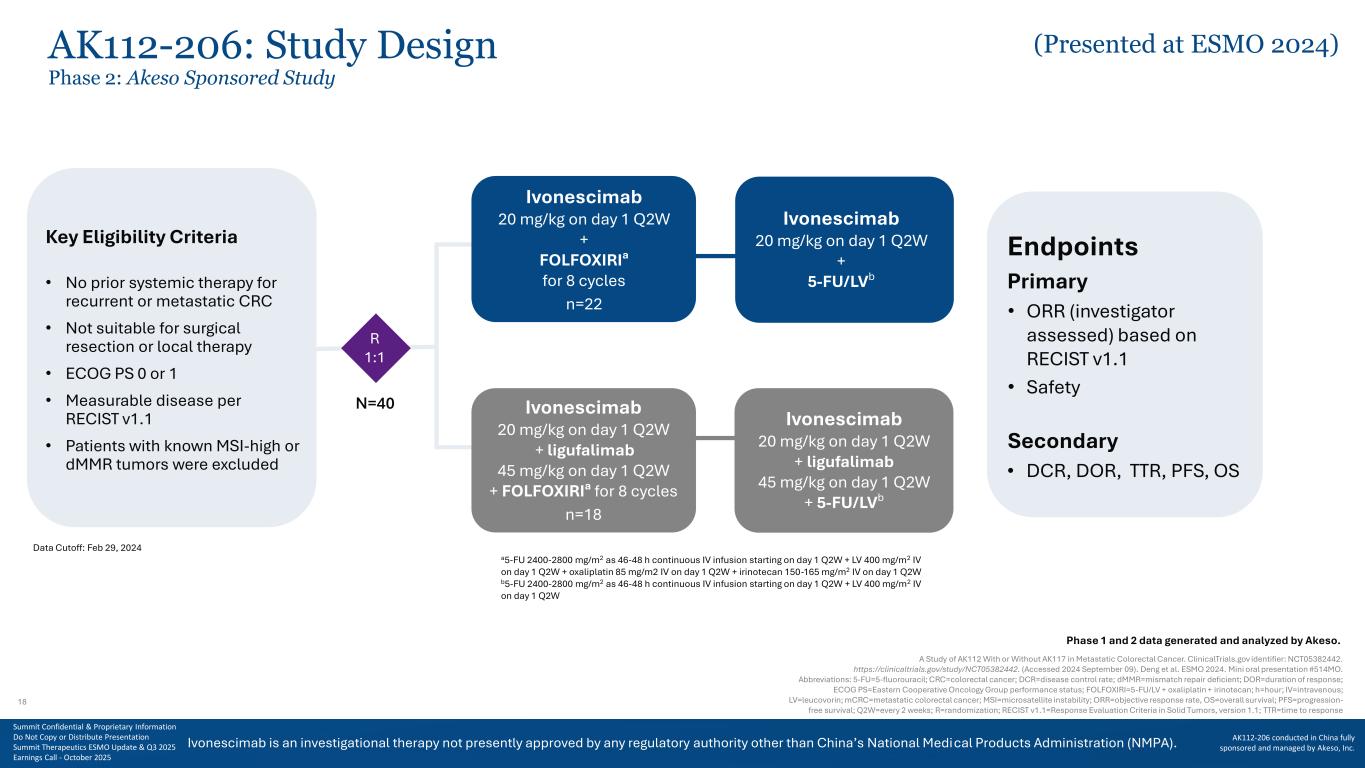
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). AK112-206: Study Design Phase 2: Akeso Sponsored Study A Study of AK112 With or Without AK117 in Metastatic Colorectal Cancer. ClinicalTrials.gov identifier: NCT05382442. https://clinicaltrials.gov/study/NCT05382442. (Accessed 2024 September 09). Deng et al. ESMO 2024. Mini oral presentation #514MO. Abbreviations: 5-FU=5-fluorouracil; CRC=colorectal cancer; DCR=disease control rate; dMMR=mismatch repair deficient; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group performance status; FOLFOXIRI=5-FU/LV + oxaliplatin + irinotecan; h=hour; IV=intravenous; LV=leucovorin; mCRC=metastatic colorectal cancer; MSI=microsatellite instability; ORR=objective response rate, OS=overall survival; PFS=progression- free survival; Q2W=every 2 weeks; R=randomization; RECIST v1.1=Response Evaluation Criteria in Solid Tumors, version 1.1; TTR=time to response a5-FU 2400-2800 mg/m2 as 46-48 h continuous IV infusion starting on day 1 Q2W + LV 400 mg/m2 IV on day 1 Q2W + oxaliplatin 85 mg/m2 IV on day 1 Q2W + irinotecan 150-165 mg/m2 IV on day 1 Q2W b5-FU 2400-2800 mg/m2 as 46-48 h continuous IV infusion starting on day 1 Q2W + LV 400 mg/m2 IV on day 1 Q2W Phase 1 and 2 data generated and analyzed by Akeso. Data Cutoff: Feb 29, 2024 Endpoints Primary • ORR (investigator assessed) based on RECIST v1.1 • Safety Secondary • DCR, DOR, TTR, PFS, OS R 1:1 Key Eligibility Criteria • No prior systemic therapy for recurrent or metastatic CRC • Not suitable for surgical resection or local therapy • ECOG PS 0 or 1 • Measurable disease per RECIST v1.1 • Patients with known MSI-high or dMMR tumors were excluded Ivonescimab 20 mg/kg on day 1 Q2W + FOLFOXIRIa for 8 cycles n=22 Ivonescimab 20 mg/kg on day 1 Q2W + ligufalimab 45 mg/kg on day 1 Q2W + FOLFOXIRIa for 8 cycles n=18 Ivonescimab 20 mg/kg on day 1 Q2W + 5-FU/LVb Ivonescimab 20 mg/kg on day 1 Q2W + ligufalimab 45 mg/kg on day 1 Q2W + 5-FU/LVb N=40 18 Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 AK112-206 conducted in China fully sponsored and managed by Akeso, Inc. (Presented at ESMO 2024)
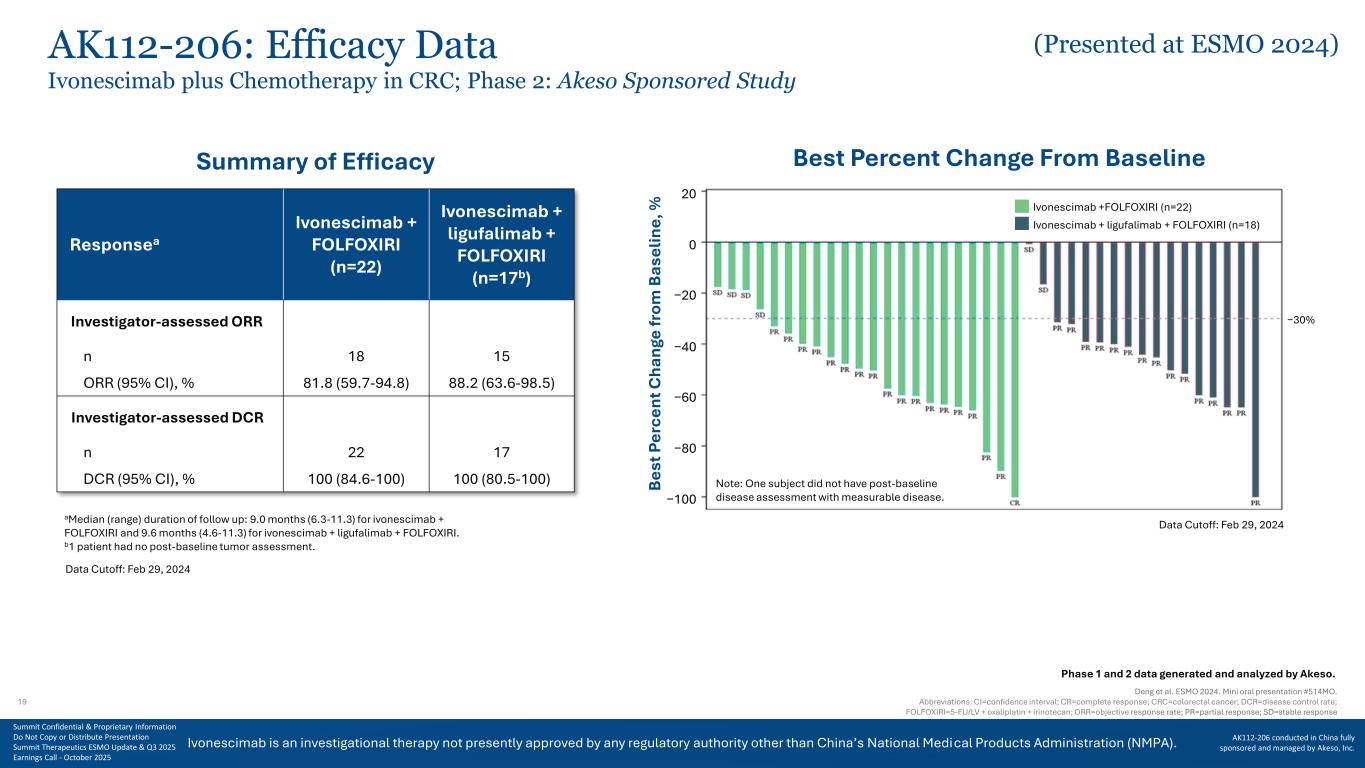
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). AK112-206: Efficacy Data Deng et al. ESMO 2024. Mini oral presentation #514MO. Abbreviations: CI=confidence interval; CR=complete response; CRC=colorectal cancer; DCR=disease control rate; FOLFOXIRI=5-FU/LV + oxaliplatin + irinotecan; ORR=objective response rate; PR=partial response; SD=stable response Data Cutoff: Feb 29, 2024 Note: One subject did not have post-baseline disease assessment with measurable disease. B es t P er ce nt C ha ng e fr om B as el in e, % Ivonescimab +FOLFOXIRI (n=22) Ivonescimab + ligufalimab + FOLFOXIRI (n=18) −30% 20 0 −20 −40 −60 −80 −100 Best Percent Change From Baseline Responsea Ivonescimab + FOLFOXIRI (n=22) Ivonescimab + ligufalimab + FOLFOXIRI (n=17b) Investigator-assessed ORR n 18 15 ORR (95% CI), % 81.8 (59.7-94.8) 88.2 (63.6-98.5) Investigator-assessed DCR n 22 17 DCR (95% CI), % 100 (84.6-100) 100 (80.5-100) aMedian (range) duration of follow up: 9.0 months (6.3-11.3) for ivonescimab + FOLFOXIRI and 9.6 months (4.6-11.3) for ivonescimab + ligufalimab + FOLFOXIRI. b1 patient had no post-baseline tumor assessment. Summary of Efficacy Ivonescimab plus Chemotherapy in CRC; Phase 2: Akeso Sponsored Study Data Cutoff: Feb 29, 2024 Phase 1 and 2 data generated and analyzed by Akeso. 19 Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 AK112-206 conducted in China fully sponsored and managed by Akeso, Inc. (Presented at ESMO 2024)
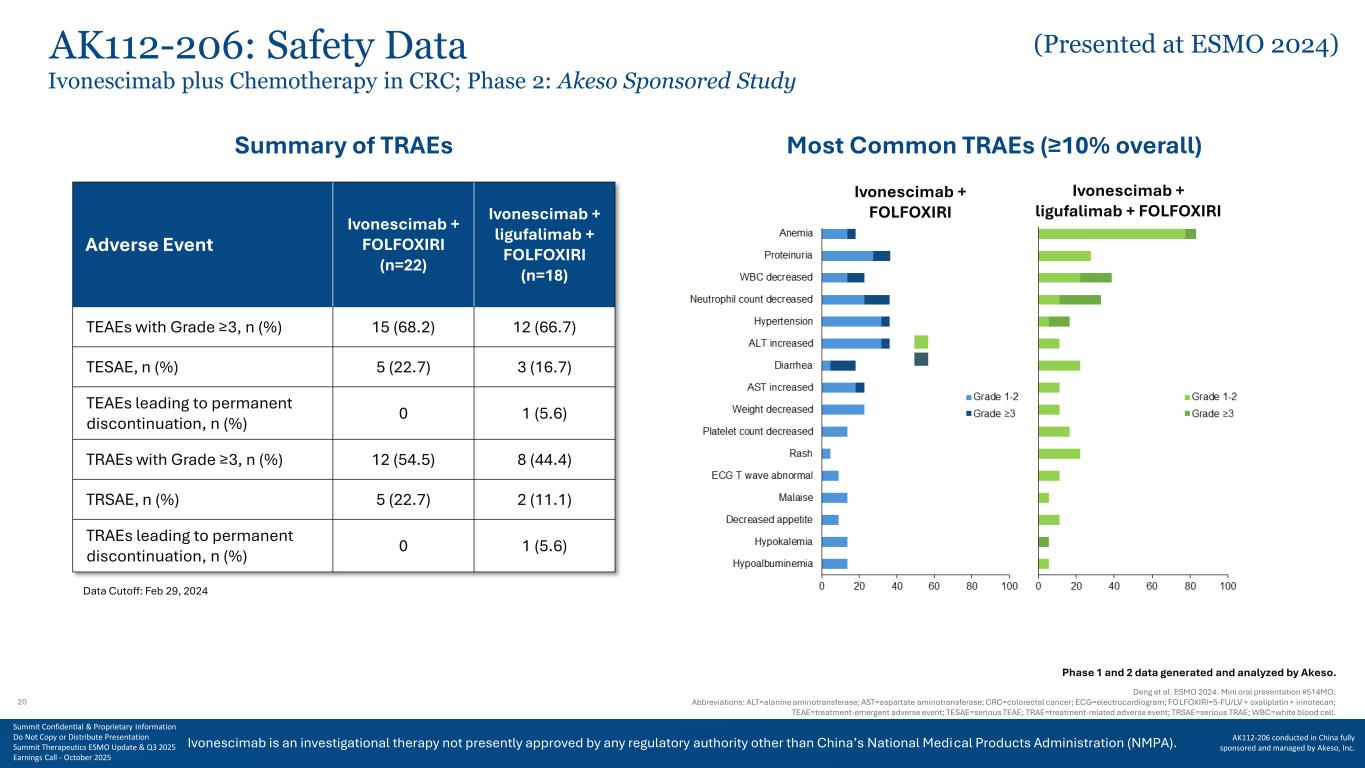
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Adverse Event Ivonescimab + FOLFOXIRI (n=22) Ivonescimab + ligufalimab + FOLFOXIRI (n=18) TEAEs with Grade ≥3, n (%) 15 (68.2) 12 (66.7) TESAE, n (%) 5 (22.7) 3 (16.7) TEAEs leading to permanent discontinuation, n (%) 0 1 (5.6) TRAEs with Grade ≥3, n (%) 12 (54.5) 8 (44.4) TRSAE, n (%) 5 (22.7) 2 (11.1) TRAEs leading to permanent discontinuation, n (%) 0 1 (5.6) Deng et al. ESMO 2024. Mini oral presentation #514MO. Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; CRC=colorectal cancer; ECG=electrocardiogram; FOLFOXIRI=5-FU/LV + oxaliplatin + irinotecan; TEAE=treatment-emergent adverse event; TESAE=serious TEAE; TRAE=treatment-related adverse event; TRSAE=serious TRAE; WBC=white blood cell. Data Cutoff: Feb 29, 2024 Summary of TRAEs Most Common TRAEs (≥10% overall) Ivonescimab + FOLFOXIRI Ivonescimab + ligufalimab + FOLFOXIRI AK112-206: Safety Data Ivonescimab plus Chemotherapy in CRC; Phase 2: Akeso Sponsored Study Phase 1 and 2 data generated and analyzed by Akeso. 20 (Presented at ESMO 2024) Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025 AK112-206 conducted in China fully sponsored and managed by Akeso, Inc.

Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Phase 1-2 Phase 3 1L Biliary Tract: 1L Pancreatic: 1L Colorectal: 1L NSCLC: 2L+ NSCLC EGFR+: 1L TNBC: 2L+ NSCLC: 1L NSCLC: SCLC: 1L HNSCC: Conducted in China Fully Sponsored and Managed by Akeso Breast Head & Neck Gastric / GEJ Hepatocellular Gynecologic Ovarian Phase 3 30+ Approved Trials Being Initiated Investigator Sponsored Trials* Planned and Ongoing Studies Sponsored by Summit Therapeutics M.D. Anderson Collaboration Initiated* 5+ Pre-clinical and Clinical Ongoing Trials RAS(ON) + ivonescimab in multiple solid tumors Revolution Medicines Collaboration Enrolling 1L NSCLC: 1L NSCLC: Enrolling 2L+ NSCLC EGFR+: Enrollment Complete 1L CRC: Not Yet Enrolling *ISTs, M.D. Anderson collaboration trials not sponsored by Summit. Akeso Phase III clinical trials from Akeso’s 2025 First Half Interim Results (prnewswire.com; akesobio.com) and/or clinicaltrials.gov; Summit Therapeutics Press Release Revolution Medicines. Jun 30, 2025 Abbreviations: 1L=first-line; 2L=second-line; CDP=clinical development plan; CRC=colorectal cancer; EGFR=epidermal growth factor receptor; GEJ=gastroesophageal junction; HNSCC=head and neck squamous cell carcinoma; NSCLC=non small cell lung cancer; SCLC=small cell lung cancer; TNBC=triple negative breast cancer. 21 Ivonescimab Development Plan Summit Confidential & Proprietary Information Do Not Copy or Distribute Presentation Summit Therapeutics ESMO Update & Q3 2025 Earnings Call - October 2025

Additional Comments, Questions & Answers