
44th Annual J.P. Morgan Healthcare Conference January 12, 2026 BOB DUGGAN Chairman & Co-Chief Executive Officer DR. MAKY ZANGANEH President & Co-Chief Executive Officer

Forward Looking Statement Any statements in this presentation about the Company’s future expectations, plans and prospects, including but not limited to, statements about the clinical and preclinical development of the Company’s product candidates, entry into and actions related to the Company’s partnership with Akeso Inc., the Company's anticipated spending and cash runway, the therapeutic potential of the Company’s product candidates, the potential commercialization of the Company’s product candidates, the timing of initiation, completion and availability of data from clinical trials, the potential submission of applications for marketing approvals, the expected timing of BLA submissions or FDA decisions, potential acquisitions, statements about the previously disclosed At-The-Market equity offering program (“ATM Program”), the expected proceeds and uses thereof, the Company’s estimates regarding stock-based compensation, and other statements containing the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "would," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the Company’s ability to sell shares of our common stock under the ATM Program, the conditions affecting the capital markets, general economic, industry, or political conditions, including the effects of geopolitical developments, domestic and foreign trade policies, and monetary policies, the results of our evaluation of the underlying data in connection with the development and commercialization activities for ivonescimab, the outcome of discussions with regulatory authorities, including the Food and Drug Administration, the uncertainties inherent in the initiation of future clinical trials, availability and timing of data from ongoing and future clinical trials, the results of such trials, and their success, global public health crises, that may affect timing and status of our clinical trials and operations, whether preliminary results from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials or preclinical studies will be indicative of the results of later clinical trials, whether business development opportunities to expand the Company’s pipeline of drug candidates, including without limitation, through potential acquisitions of, and/or collaborations with, other entities occur, expectations for regulatory approvals, laws and regulations affecting government contracts and funding awards, availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements and other factors discussed in the "Risk Factors" and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of filings that the Company makes with the Securities and Exchange Commission. Summit defines a “positive study” as a clinical study that with one or more prespecified primary endpoints in which one of those endpoints achieves a statistically significant benefit according to the protocol or statistical analysis plan. Any change to our ongoing trials could cause delays, affect our future expenses, and add uncertainty to our commercialization efforts, as well as to affect the likelihood of the successful completion of clinical development of ivonescimab. Accordingly, readers should not place undue reliance on forward- looking statements or information. In addition, any forward-looking statements included in this presentation represent the Company’s views only as of the date of this release and should not be relied upon as representing the Company’s views as of any subsequent date. The Company specifically disclaims any obligation to update any forward-looking statements included in this presentation. Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Therapeutics and the Summit Therapeutics logo are trademarks of Summit Therapeutics Inc. Copyright 2026, Summit Therapeutics Inc. All Rights Reserved.2 Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026

Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). 3 Anti-VEGF Anti-PD-1 US Biologics License Application (BLA) Submitted to the FDA in the Fourth Quarter 2025 Ivonescimab + Chemo vs. Chemo in 2L+ EGFRm NSCLC SUBMITTED! Abbreviations: 2L+=second-line or later line; Chemo=Chemotherapy; EGFRm=epidermal growth factor receptor mutation-positive; FDA=US Food and Drug Administration; NSCLC=non-small cell lung cancer; PD-1=programmed cell death protein 1; VEGF=vascular endothelial growth factor; vs.=versus Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026
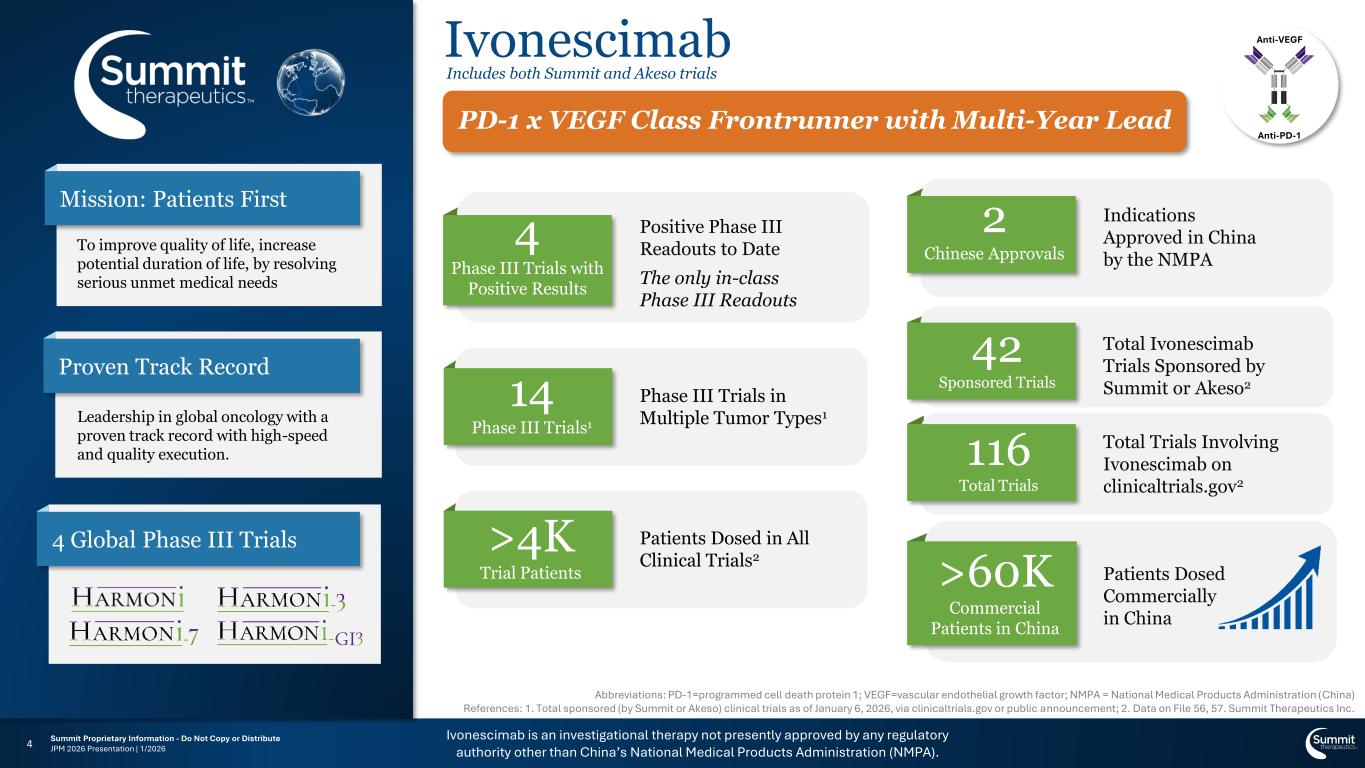
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/20264 Ivonescimab Anti-VEGF Anti-PD-1 Includes both Summit and Akeso trials Total Ivonescimab Trials Sponsored by Summit or Akeso2 Patients Dosed in All Clinical Trials2 Patients Dosed Commercially in China Phase III Trials in Multiple Tumor Types1 Positive Phase III Readouts to Date The only in-class Phase III Readouts Leadership in global oncology with a proven track record with high-speed and quality execution. Proven Track Record 4 Global Phase III Trials Mission: Patients First To improve quality of life, increase potential duration of life, by resolving serious unmet medical needs Indications Approved in China by the NMPA 4 Phase III Trials with Positive Results PD-1 x VEGF Class Frontrunner with Multi-Year Lead 14 Phase III Trials1 >4K Trial Patients 2 Chinese Approvals >60K Commercial Patients in China 42 Sponsored Trials Abbreviations: PD-1=programmed cell death protein 1; VEGF=vascular endothelial growth factor; NMPA = National Medical Products Administration (China) References: 1. Total sponsored (by Summit or Akeso) clinical trials as of January 6, 2026, via clinicaltrials.gov or public announcement; 2. Data on File 56, 57. Summit Therapeutics Inc. Total Trials Involving Ivonescimab on clinicaltrials.gov2 116 Total Trials
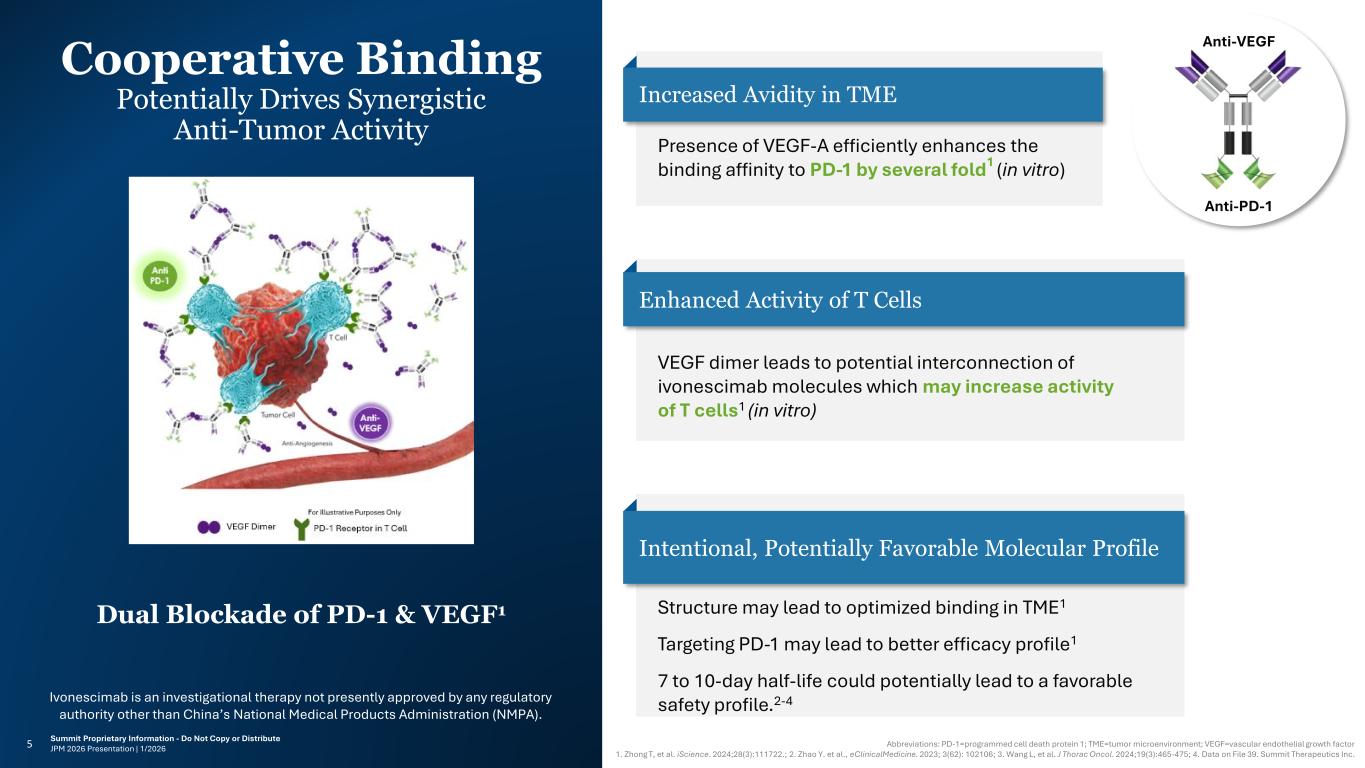
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | MAT-SMTxxx-xxxx | 1/20265 Cooperative Binding Potentially Drives Synergistic Anti-Tumor Activity Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Anti-VEGF Anti-PD-1 Intentional, Potentially Favorable Molecular Profile Structure may lead to optimized binding in TME1 Targeting PD-1 may lead to better efficacy profile1 7 to 10-day half-life could potentially lead to a favorable safety profile.2-4 Dual Blockade of PD-1 & VEGF1 Increased Avidity in TME Presence of VEGF-A efficiently enhances the binding affinity to PD-1 by several fold1 (in vitro) Enhanced Activity of T Cells VEGF dimer leads to potential interconnection of ivonescimab molecules which may increase activity of T cells1 (in vitro) Abbreviations: PD-1=programmed cell death protein 1; TME=tumor microenvironment; VEGF=vascular endothelial growth factor 1. Zhong T, et al. iScience. 2024;28(3):111722.; 2. Zhao Y. et al., eClinicalMedicine. 2023; 3(62): 102106; 3. Wang L, et al. J Thorac Oncol. 2024;19(3):465-475; 4. Data on File 39. Summit Therapeutics Inc. 1/2026
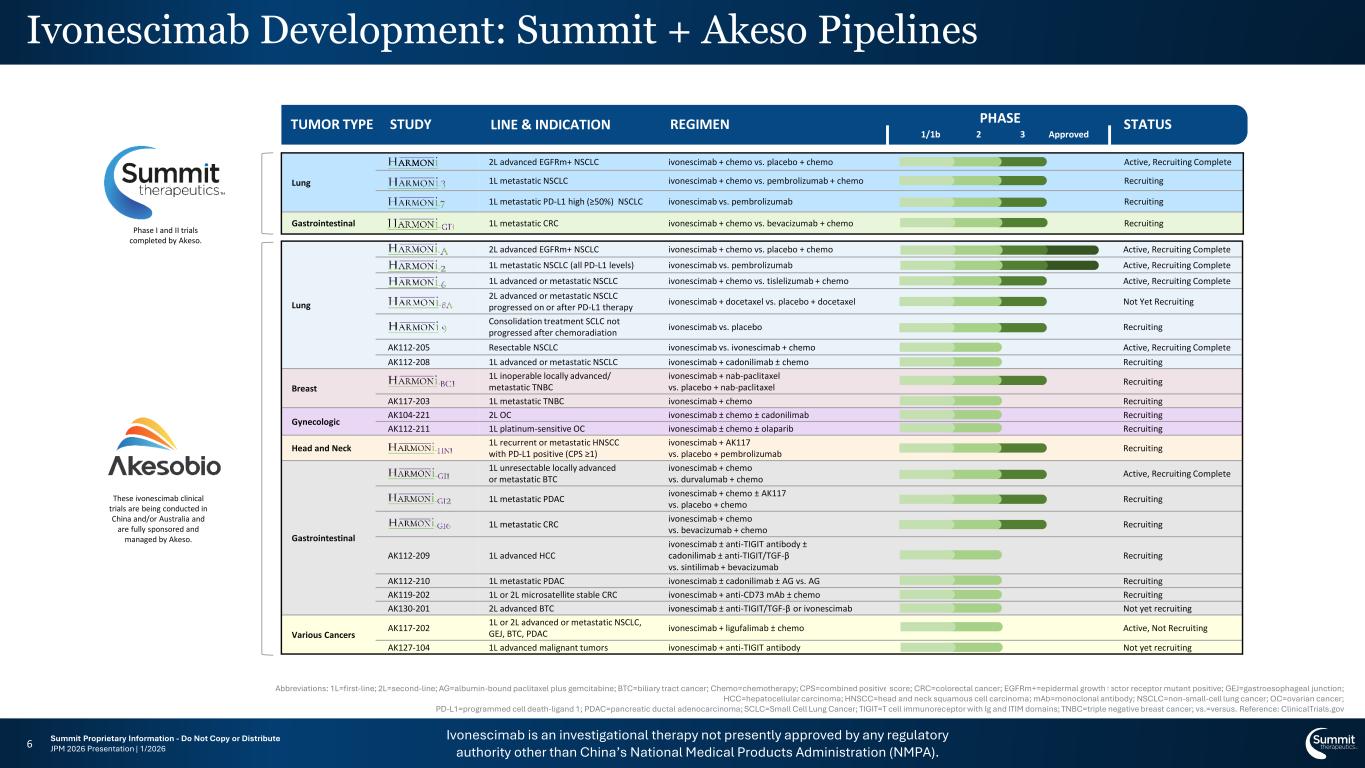
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/20266 Abbreviations: 1L=first-line; 2L=second-line; AG=albumin-bound paclitaxel plus gemcitabine; BTC=biliary tract cancer; Chemo=chemotherapy; CPS=combined positive score; CRC=colorectal cancer; EGFRm+=epidermal growth factor receptor mutant positive; GEJ=gastroesophageal junction; HCC=hepatocellular carcinoma; HNSCC=head and neck squamous cell carcinoma; mAb=monoclonal antibody; NSCLC=non-small-cell lung cancer; OC=ovarian cancer; PD-L1=programmed cell death-ligand 1; PDAC=pancreatic ductal adenocarcinoma; SCLC=Small Cell Lung Cancer; TIGIT=T cell immunoreceptor with Ig and ITIM domains; TNBC=triple negative breast cancer; vs.=versus. Reference: ClinicalTrials.gov These ivonescimab clinical trials are being conducted in China and/or Australia and are fully sponsored and managed by Akeso. TUMOR TYPE STUDY LINE & INDICATION REGIMEN STATUS Approved PHASE 1/1b 2 3 Lung 2L advanced EGFRm+ NSCLC ivonescimab + chemo vs. placebo + chemo Active, Recruiting Complete 1L metastatic NSCLC (all PD-L1 levels) ivonescimab vs. pembrolizumab Active, Recruiting Complete 1L advanced or metastatic NSCLC ivonescimab + chemo vs. tislelizumab + chemo Active, Recruiting Complete 2L advanced or metastatic NSCLC progressed on or after PD-L1 therapy ivonescimab + docetaxel vs. placebo + docetaxel Not Yet Recruiting Consolidation treatment SCLC not progressed after chemoradiation ivonescimab vs. placebo Recruiting AK112-205 Resectable NSCLC ivonescimab vs. ivonescimab + chemo Active, Recruiting Complete AK112-208 1L advanced or metastatic NSCLC ivonescimab + cadonilimab ± chemo Recruiting Breast 1L inoperable locally advanced/ metastatic TNBC ivonescimab + nab-paclitaxel vs. placebo + nab-paclitaxel Recruiting AK117-203 1L metastatic TNBC ivonescimab + chemo Recruiting Gynecologic AK104-221 2L OC ivonescimab ± chemo ± cadonilimab Recruiting AK112-211 1L platinum-sensitive OC ivonescimab ± chemo ± olaparib Recruiting Head and Neck 1L recurrent or metastatic HNSCC with PD-L1 positive (CPS ≥1) ivonescimab + AK117 vs. placebo + pembrolizumab Recruiting Gastrointestinal 1L unresectable locally advanced or metastatic BTC ivonescimab + chemo vs. durvalumab + chemo Active, Recruiting Complete 1L metastatic PDAC ivonescimab + chemo ± AK117 vs. placebo + chemo Recruiting 1L metastatic CRC ivonescimab + chemo vs. bevacizumab + chemo Recruiting AK112-209 1L advanced HCC ivonescimab ± anti-TIGIT antibody ± cadonilimab ± anti-TIGIT/TGF-β vs. sintilimab + bevacizumab Recruiting AK112-210 1L metastatic PDAC ivonescimab ± cadonilimab ± AG vs. AG Recruiting AK119-202 1L or 2L microsatellite stable CRC ivonescimab + anti-CD73 mAb ± chemo Recruiting AK130-201 2L advanced BTC ivonescimab ± anti-TIGIT/TGF-β or ivonescimab Not yet recruiting Various Cancers AK117-202 1L or 2L advanced or metastatic NSCLC, GEJ, BTC, PDAC ivonescimab + ligufalimab ± chemo Active, Not Recruiting AK127-104 1L advanced malignant tumors ivonescimab + anti-TIGIT antibody Not yet recruiting Lung 2L advanced EGFRm+ NSCLC ivonescimab + chemo vs. placebo + chemo Active, Recruiting Complete 1L metastatic NSCLC ivonescimab + chemo vs. pembrolizumab + chemo Recruiting 1L metastatic PD-L1 high (≥50%) NSCLC ivonescimab vs. pembrolizumab Recruiting Gastrointestinal 1L metastatic CRC ivonescimab + chemo vs. bevacizumab + chemo Recruiting Ivonescimab Development: Summit + Akeso Pipelines Phase I and II trials completed by Akeso.
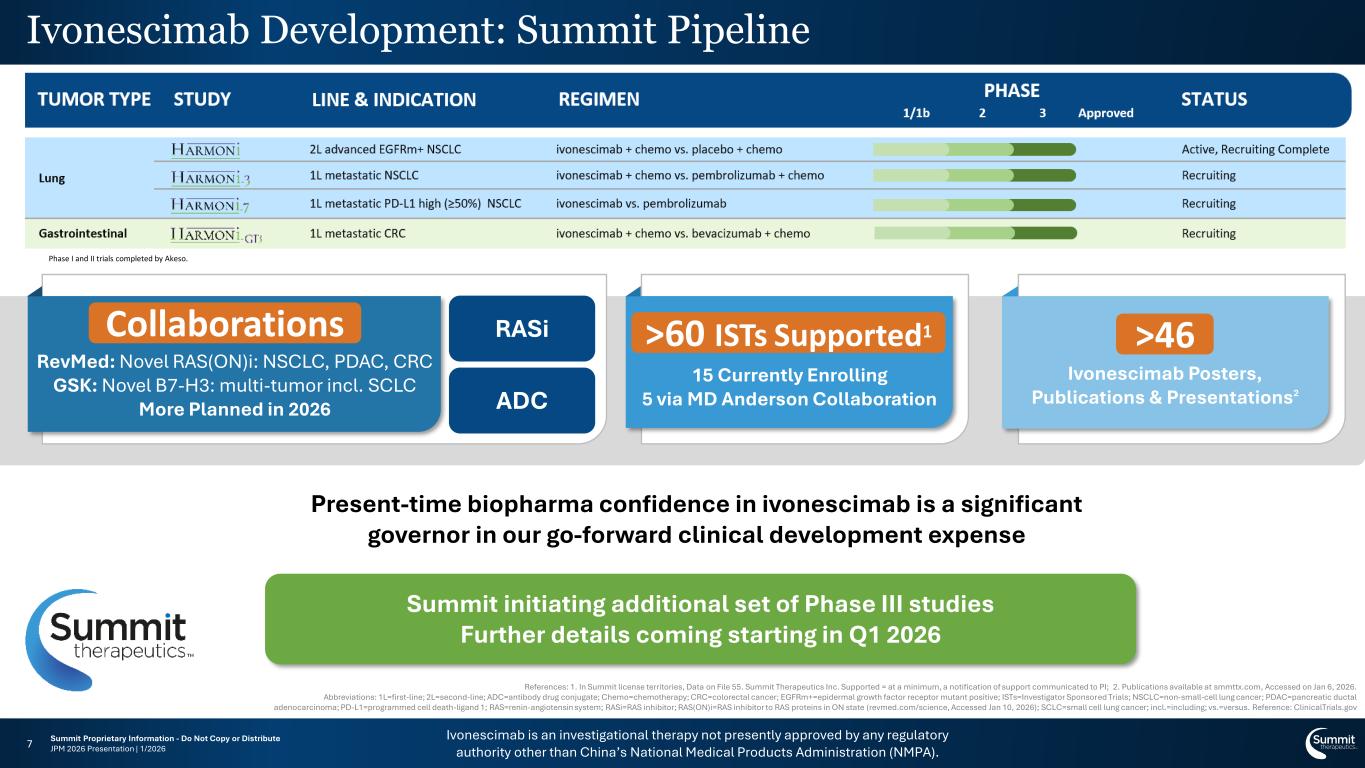
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/20267 Ivonescimab Development Plan Ivo esci ab evelop e t: Summit Pipeline Summit initiating additional set of Phase III studies Further details coming starting in Q1 2026 References: 1. In Summit license territories, Data on File 55. Summit Therapeutics Inc. Supported = at a minimum, a notification of support communicated to PI; 2. Publications available at smmttx.com, Accessed on Jan 6, 2026. Abbreviations: 1L=first-line; 2L=second-line; ADC=antibody drug conjugate; Chemo=chemotherapy; CRC=colorectal cancer; EGFRm+=epidermal growth factor receptor mutant positive; ISTs=Investigator Sponsored Trials; NSCLC=non-small-cell lung cancer; PDAC=pancreatic ductal adenocarcinoma; PD-L1=programmed cell death-ligand 1; RAS=renin-angiotensin system; RASi=RAS inhibitor; RAS(ON)i=RAS inhibitor to RAS proteins in ON state (revmed.com/science, Accessed Jan 10, 2026); SCLC=small cell lung cancer; incl.=including; vs.=versus. Reference: ClinicalTrials.gov Present-time biopharma confidence in ivonescimab is a significant governor in our go-forward clinical development expense Collaborations RevMed: Novel RAS(ON)i: NSCLC, PDAC, CRC GSK: Novel B7-H3: multi-tumor incl. SCLC More Planned in 2026 >60 ISTs1 15 Currently Enrolling 5 via MD Anderson Collaboration 46 Ivonescimab Posters, Publications & Presentations2 RASi ADC llaborations >60 ISTs Supported1 >46 Phase I and II trials completed by Akeso.
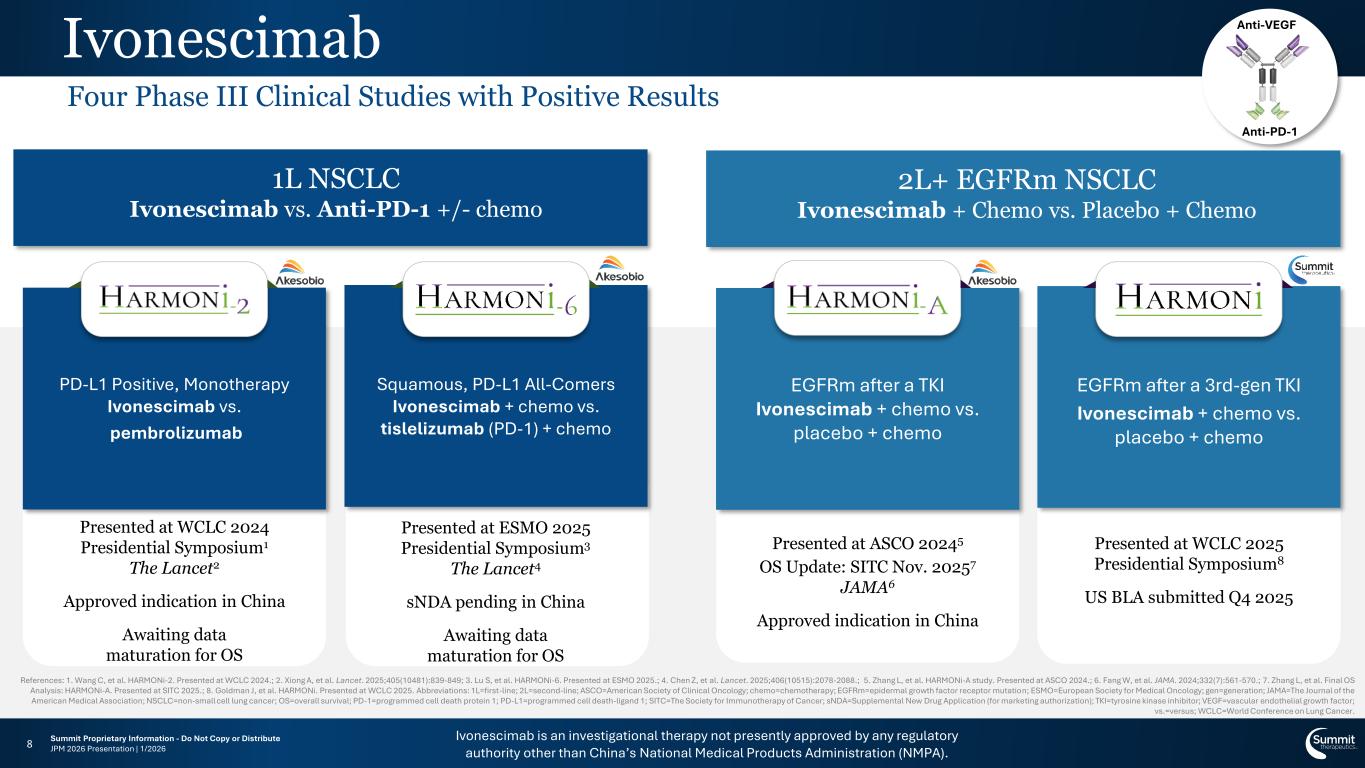
Anti-VEGF Anti-PD-1 Ivonescimab 1L NSCLC Ivonescimab vs. Anti-PD-1 +/- chemo Presented at WCLC 2024 Presidential Symposium1 The Lancet2 Approved indication in China Awaiting data maturation for OS Squamous, PD-L1 All-Comers Ivonescimab + chemo vs. tislelizumab (PD-1) + chemo Presented at ESMO 2025 Presidential Symposium3 The Lancet4 sNDA pending in China Awaiting data maturation for OS 2L+ EGFRm NSCLC Ivonescimab + Chemo vs. Placebo + Chemo EGFRm after a TKI Ivonescimab + chemo vs. placebo + chemo Presented at ASCO 20245 OS Update: SITC Nov. 20257 JAMA6 Approved indication in China EGFRm after a 3rd-gen TKI Ivonescimab + chemo vs. placebo + chemo Presented at WCLC 2025 Presidential Symposium8 US BLA submitted Q4 2025 Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). 8 PD-L1 Positive, Monotherapy Ivonescimab vs. pembrolizumab References: 1. Wang C, et al. HARMONi-2. Presented at WCLC 2024.; 2. Xiong A, et al. Lancet. 2025;405(10481):839-849; 3. Lu S, et al. HARMONi-6. Presented at ESMO 2025.; 4. Chen Z, et al. Lancet. 2025;406(10515):2078-2088.; 5. Zhang L, et al. HARMONi-A study. Presented at ASCO 2024.; 6. Fang W, et al. JAMA. 2024;332(7):561-570.; 7. Zhang L, et al. Final OS Analysis: HARMONi-A. Presented at SITC 2025.; 8. Goldman J, et al. HARMONi. Presented at WCLC 2025. Abbreviations: 1L=first-line; 2L=second-line; ASCO=American Society of Clinical Oncology; chemo=chemotherapy; EGFRm=epidermal growth factor receptor mutation; ESMO=European Society for Medical Oncology; gen=generation; JAMA=The Journal of the American Medical Association; NSCLC=non-small cell lung cancer; OS=overall survival; PD-1=programmed cell death protein 1; PD-L1=programmed cell death-ligand 1; SITC=The Society for Immunotherapy of Cancer; sNDA=Supplemental New Drug Application (for marketing authorization); TKI=tyrosine kinase inhibitor; VEGF=vascular endothelial growth factor; vs.=versus; WCLC=World Conference on Lung Cancer. Four Phase III Clinical Studies with Positive Results Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026
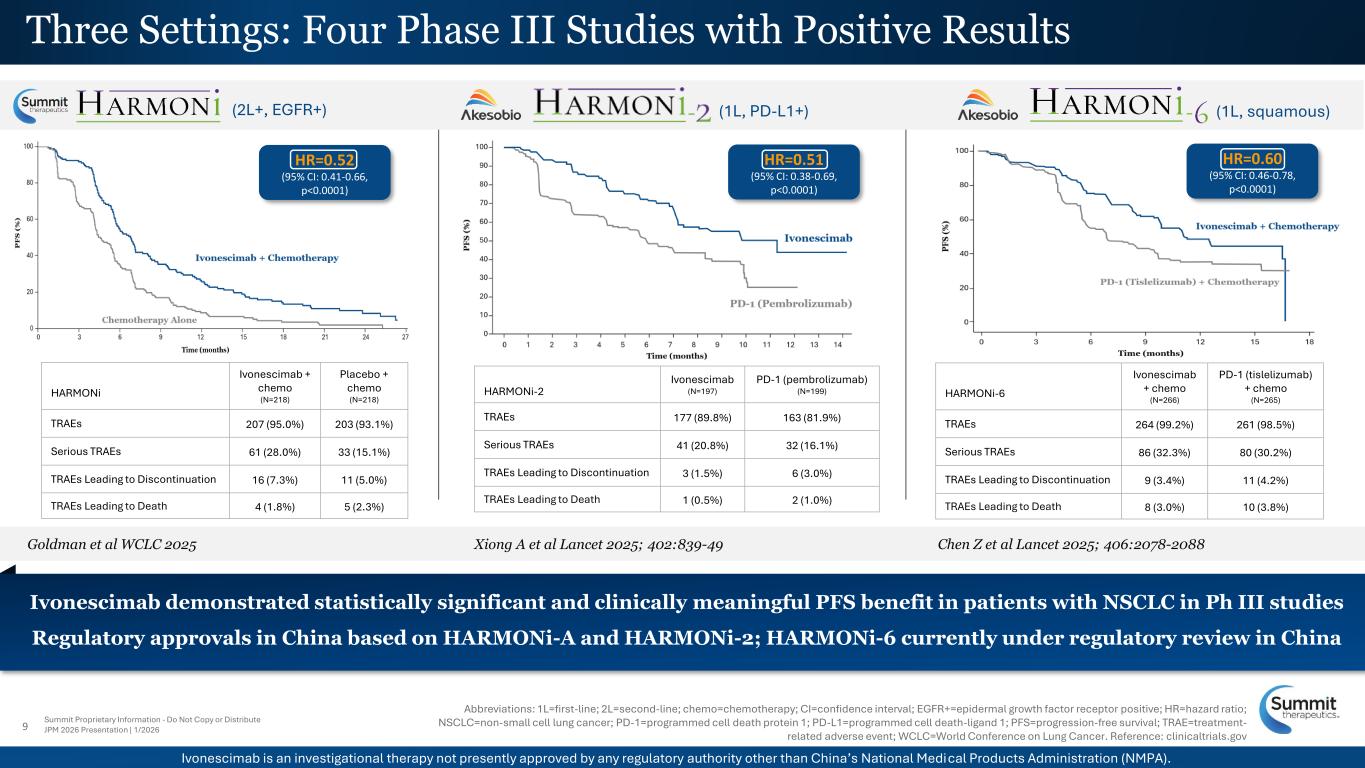
9 Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026 Xiong A et al Lancet 2025; 402:839-49 Chen Z et al Lancet 2025; 406:2078-2088Goldman et al WCLC 2025 (2L+, EGFR+) (1L, squamous)(1L, PD-L1+) Abbreviations: 1L=first-line; 2L=second-line; chemo=chemotherapy; CI=confidence interval; EGFR+=epidermal growth factor receptor positive; HR=hazard ratio; NSCLC=non-small cell lung cancer; PD-1=programmed cell death protein 1; PD-L1=programmed cell death-ligand 1; PFS=progression-free survival; TRAE=treatment- related adverse event; WCLC=World Conference on Lung Cancer. Reference: clinicaltrials.gov Three Settings: Four Phase III Studies with Positive Results Ivonescimab demonstrated statistically significant and clinically meaningful PFS benefit in patients with NSCLC in Ph III studies Regulatory approvals in China based on HARMONi-A and HARMONi-2; HARMONi-6 currently under regulatory review in China V HARMONi-6 Ivonescimab + chemo (N=266) PD-1 (tislelizumab) + chemo (N=265) TRAEs 264 (99.2%) 261 (98.5%) Serious TRAEs 86 (32.3%) 80 (30.2%) TRAEs Leading to Discontinuation 9 (3.4%) 11 (4.2%) TRAEs Leading to Death 8 (3.0%) 10 (3.8%) HARMONi Ivonescimab + chemo (N=218) Placebo + chemo (N=218) TRAEs 207 (95.0%) 203 (93.1%) Serious TRAEs 61 (28.0%) 33 (15.1%) TRAEs Leading to Discontinuation 16 (7.3%) 11 (5.0%) TRAEs Leading to Death 4 (1.8%) 5 (2.3%) HARMONi-2 Ivonescimab (N=197) PD-1 (pembrolizumab) (N=199) TRAEs 177 (89.8%) 163 (81.9%) Serious TRAEs 41 (20.8%) 32 (16.1%) TRAEs Leading to Discontinuation 3 (1.5%) 6 (3.0%) TRAEs Leading to Death 1 (0.5%) 2 (1.0%) HR=0.52 (95% CI: 0.41-0.66, p<0.0001) HR=0.60 (95% CI: 0.46-0.78, p<0.0001) HR=0.51 (95% CI: 0.38-0.69, p<0.0001) Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA).
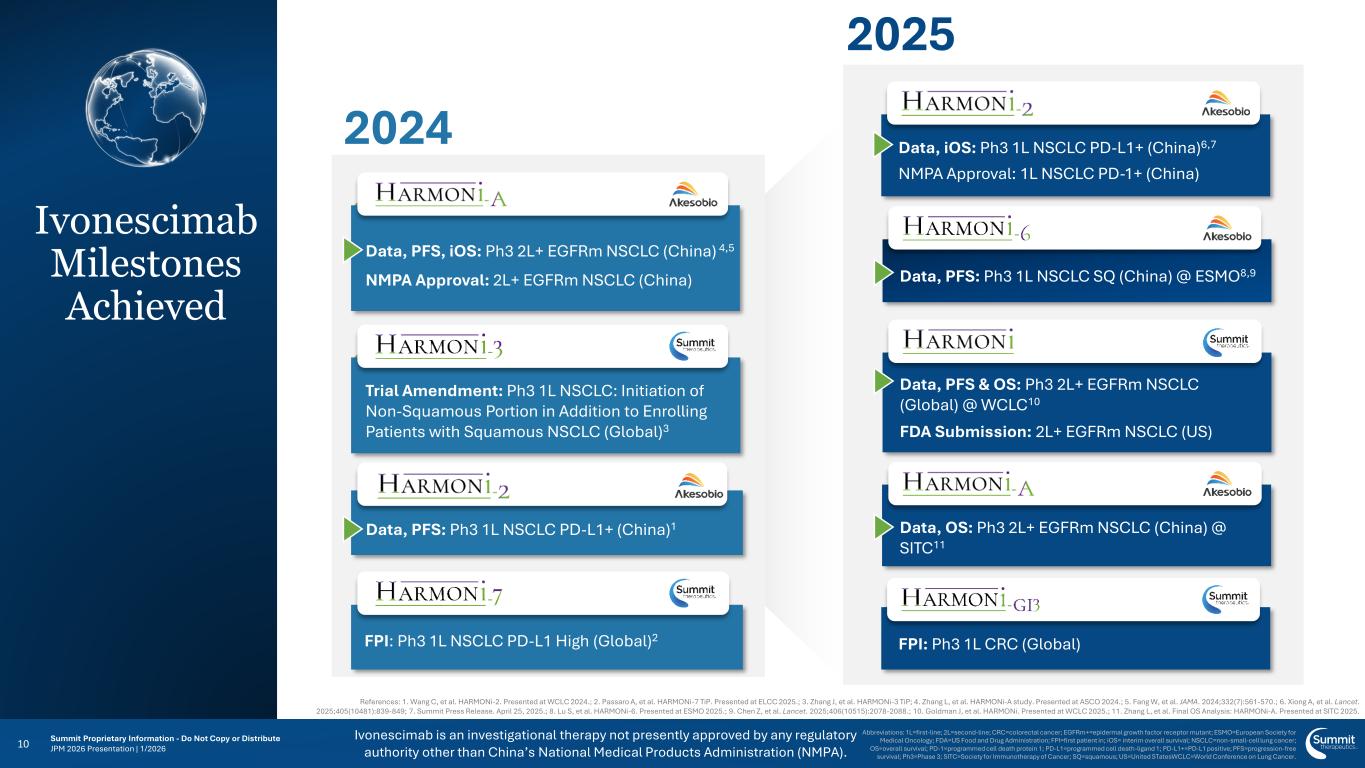
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). 10 Abbreviations: 1L=first-line; 2L=second-line; CRC=colorectal cancer; EGFRm+=epidermal growth factor receptor mutant; ESMO=European Society for Medical Oncology; FDA=US Food and Drug Administration; FPI=first patient in; iOS= interim overall survival; NSCLC=non-small-cell lung cancer; OS=overall survival; PD-1=programmed cell death protein 1; PD-L1=programmed cell death-ligand 1; PD-L1+=PD-L1 positive; PFS=progression-free survival; Ph3=Phase 3; SITC=Society for Immunotherapy of Cancer; SQ=squamous; US=United STatesWCLC=World Conference on Lung Cancer. 2024 2025 Data, iOS: Ph3 1L NSCLC PD-L1+ (China)6,7 NMPA Approval: 1L NSCLC PD-1+ (China) FPI: Ph3 1L CRC (Global) Data, PFS: Ph3 1L NSCLC SQ (China) @ ESMO8,9 Data, PFS & OS: Ph3 2L+ EGFRm NSCLC (Global) @ WCLC10 FDA Submission: 2L+ EGFRm NSCLC (US) Data, OS: Ph3 2L+ EGFRm NSCLC (China) @ SITC11 Trial Amendment: Ph3 1L NSCLC: Initiation of Non-Squamous Portion in Addition to Enrolling Patients with Squamous NSCLC (Global)3 Data, PFS, iOS: Ph3 2L+ EGFRm NSCLC (China) 4,5 NMPA Approval: 2L+ EGFRm NSCLC (China) References: 1. Wang C, et al. HARMONi-2. Presented at WCLC 2024.; 2. Passaro A, et al. HARMONi-7 TiP. Presented at ELCC 2025.; 3. Zhang J, et al. HARMONi-3 TiP; 4. Zhang L, et al. HARMONi-A study. Presented at ASCO 2024.; 5. Fang W, et al. JAMA. 2024;332(7):561-570.; 6. Xiong A, et al. Lancet. 2025;405(10481):839-849; 7. Summit Press Release. April 25, 2025.; 8. Lu S, et al. HARMONi-6. Presented at ESMO 2025.; 9. Chen Z, et al. Lancet. 2025;406(10515):2078-2088.; 10. Goldman J, et al. HARMONi. Presented at WCLC 2025.; 11. Zhang L, et al. Final OS Analysis: HARMONi-A. Presented at SITC 2025. FPI: Ph3 1L NSCLC PD-L1 High (Global)2 Data, PFS: Ph3 1L NSCLC PD-L1+ (China)1 Ivonescimab Milestones Achieved Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026

China Ivonescimab Clinical Questions Answered in 2025 Ivonescimab + Chemo vs. Tislelizumab + Chemo in 1L NSCLC 4,5 Ivo monotherapy: clinically meaningful early OS trend vs. pembro monotherapy (OS HR = 0.777 at 39% maturity based on health authority- requested interim analysis)2 Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Global Potential for positive results from China studies to translate to the west? Ivonescimab + Chemo vs. Chemo in 2L+ EGFRm NSCLC 1 Potential for positive PFS data to translate to positive OS data? Potential for positive monotherapy results to be sustained with addition of chemotherapy? Ivonescimab + Chemo vs. Chemo in 2L+ EGFRm NSCLC 2 References: 1. Goldman J, et al. HARMONi. Presented at WCLC 2025.; 2. Zhang L, et al. Final OS Analysis: HARMONi-A. Presented at SITC 2025.; 3. Summit Press Release. April 25, 2025.; 4. Lu S, et al. HARMONi-6. Presented at ESMO 2025.; 5. Chen Z, et al. Lancet. 2025;406(10515):2078-2088. Abbreviations: 1L=first-line; 2L=second-line; Chemo=chemotherapy; EGFRm+=epidermal growth factor receptor mutant positive; NSCLC=non-small- cell lung cancer; HR=hazard ratio; OS=overall survival; PFS=progression-free survival; vs.=versus. Reference: ClinicalTrials.gov Strong OS Trends, HR <0.80 1,3 Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/202611

Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/202612 Shaping the Path to Become a Commercial Entity 1Q26 Further details beginning this quarter on new global Phase IIIs 1H26 HARMONi-3 SQ: Completion of enrollment expected 2H26 HARMONi-3 SQ: PFS, interim OS data readout expected HARMONi-3 nSQ: Completion of enrollment expected HARMONi: BLA Decision in 2L+ EGFRm NSCLC expected 1H27 HARMONi-3 nSQ: PFS data readout expected Anti-VEGF Anti-PD-1 Abbreviations: 2L=second-line; BLA=Biologics License Application; EGFRm+=epidermal growth factor receptor mutant positive; NSCLC=non-small-cell lung cancer; nSQ=non-squamous; OS=overall survival; PD-1=programmed cell death protein 1; PD-L1=programmed cell death-ligand 1; PFS=progression-free survival; SQ=squamous; VEGF=vascular endothelial growth factor.
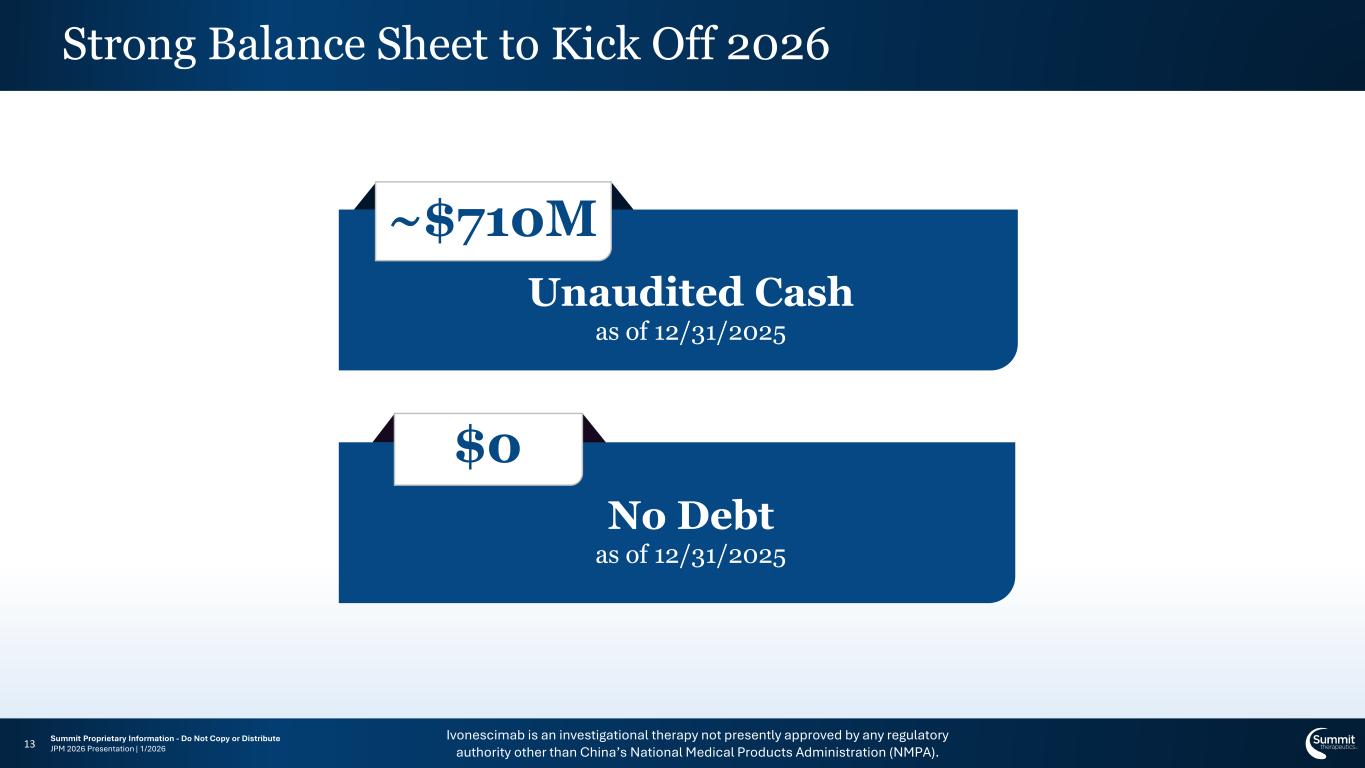
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026 Strong Balance Sheet to Kick Off 2026 13 ~$710M Unaudited Cash as of 12/31/2025 $0 No Debt as of 12/31/2025
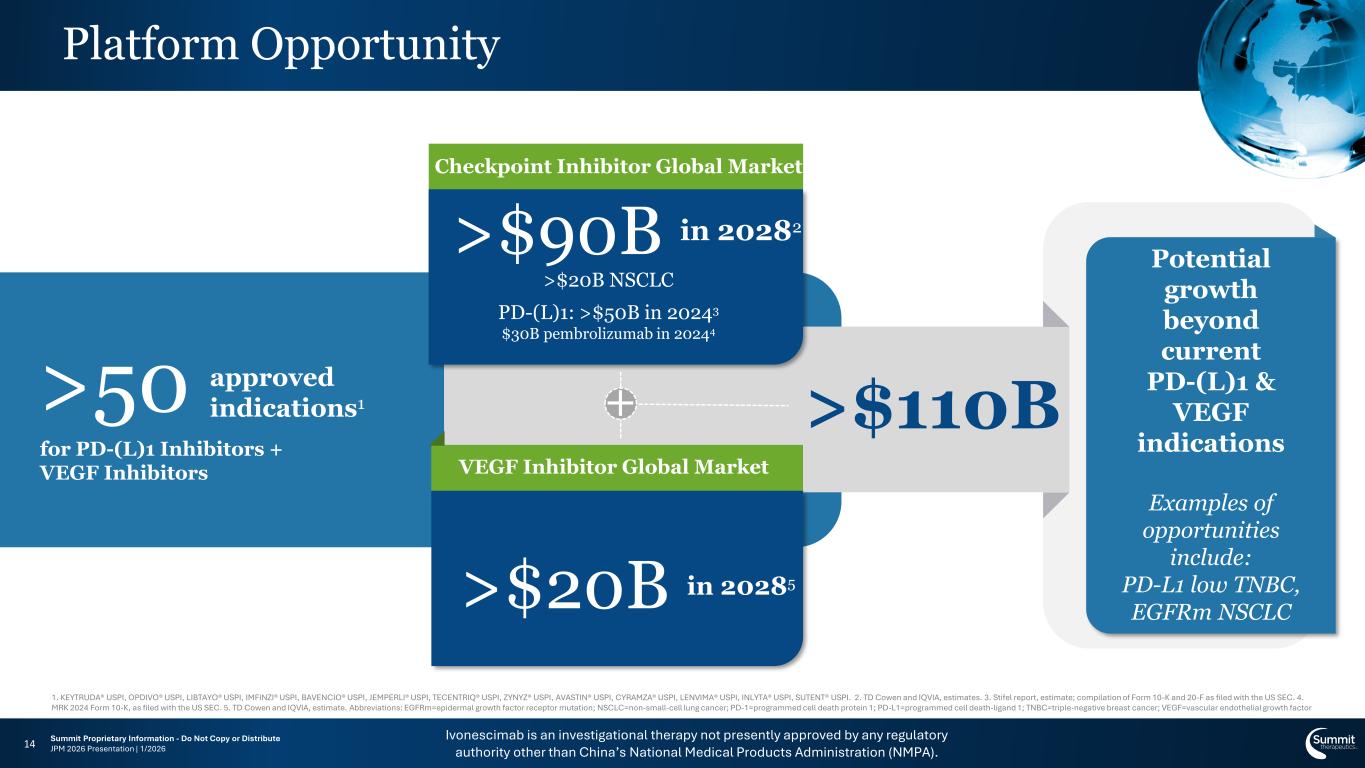
Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/2026 Potential growth beyond current PD-(L)1 & VEGF indications Examples of opportunities include: PD-L1 low TNBC, EGFRm NSCLC Platform Opportunity 14 for PD-(L)1 Inhibitors + VEGF Inhibitors >50 approved indications1 + Checkpoint Inhibitor Global Market >$90B in 20282 >$20B NSCLC PD-(L)1: >$50B in 20243 $30B pembrolizumab in 20244 >$20B in 20285 >$110B VEGF Inhibitor Global Market 1. KEYTRUDA® USPI, OPDIVO® USPI, LIBTAYO® USPI, IMFINZI® USPI, BAVENCIO® USPI, JEMPERLI® USPI, TECENTRIQ® USPI, ZYNYZ® USPI, AVASTIN® USPI, CYRAMZA® USPI, LENVIMA® USPI, INLYTA® USPI, SUTENT® USPI. 2. TD Cowen and IQVIA, estimates. 3. Stifel report, estimate; compilation of Form 10-K and 20-F as filed with the US SEC. 4. MRK 2024 Form 10-K, as filed with the US SEC. 5. TD Cowen and IQVIA, estimate. Abbreviations: EGFRm=epidermal growth factor receptor mutation; NSCLC=non-small-cell lung cancer; PD-1=programmed cell death protein 1; PD-L1=programmed cell death-ligand 1; TNBC=triple-negative breast cancer; VEGF=vascular endothelial growth factor

Ivonescimab is an investigational therapy not presently approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). Summit Proprietary Information - Do Not Copy or Distribute JPM 2026 Presentation | 1/202615 Bob Duggan Chairman & Co-Chief Executive Officer Dave Gancarz Chief Business & Strategy Officer Manmeet Soni Chief Operating Officer & Chief Financial Officer Dr. Maky Zanganeh President & Co-Chief Executive Officer Dr. Allen Yang Chief R&D Officer Dr. Fong Chow Chief Biometrics Officer Dr. Urte Gayko Chief Regulatory, Pharmacovigilance & Quality Officer Dr. Jack West VP, Medical Affairs and Thoracic Oncology Expert