
® Third Quarter 2025 Update November 4, 2025

® Forward-Looking Statements This presentation contains forward-looking statements within the meaning of applicable U.S. securities law and forward-looking information within the meaning of applicable Canadian securities law. We caution investors that forward-looking statements are based on management’s expectations and assumptions as of the date of this presentation and involve substantial risks and uncertainties that could cause the actual outcomes to differ materially from what we currently expect. These risks and uncertainties include, but are not limited to, those associated with: total revenue; net product sales; the timing, design and results of clinical studies; and other risks and uncertainties identified in our filings with the U.S. Securities and Exchange Commission. Forward-looking statements in this presentation apply only as of the date made, and we undertake no obligation to update or revise any forward-looking statements to reflect subsequent events or circumstances. Additional information related to Aurinia, including a detailed list of the risks and uncertainties affecting Aurinia and its business, can be found in Aurinia’s most recent Annual Report on Form 10-K and its other public available filings available by accessing the Canadian Securities Administrators’ System for Electronic Document Analysis and Retrieval (SEDAR) website at www.sedarplus.ca or the U.S. Securities and Exchange Commission’s Electronic Document Gathering and Retrieval System (EDGAR) website at www.sec.gov/edgar, and on Aurinia’s website at www.auriniapharma.com. 2

® Third Quarter 2025 Financial Highlights and Recent Business Progress 3 • Third quarter 2025 LUPKYNIS sales experienced continued momentum following last year’s inclusion in American College of Rheumatology Guidelines, growing 27% • LUPKYNIS sales guidance for 2025 raised for second time this year, to $265 million to $270 million • New LUPKYNIS data analyses reinforce LUPKYNIS’ robust clinical benefit in the treatment of patients with lupus nephritis • Following positive Phase 1 results, aritinercept advances toward clinical studies in two autoimmune diseases

® Third Quarter 2025 Financial Update
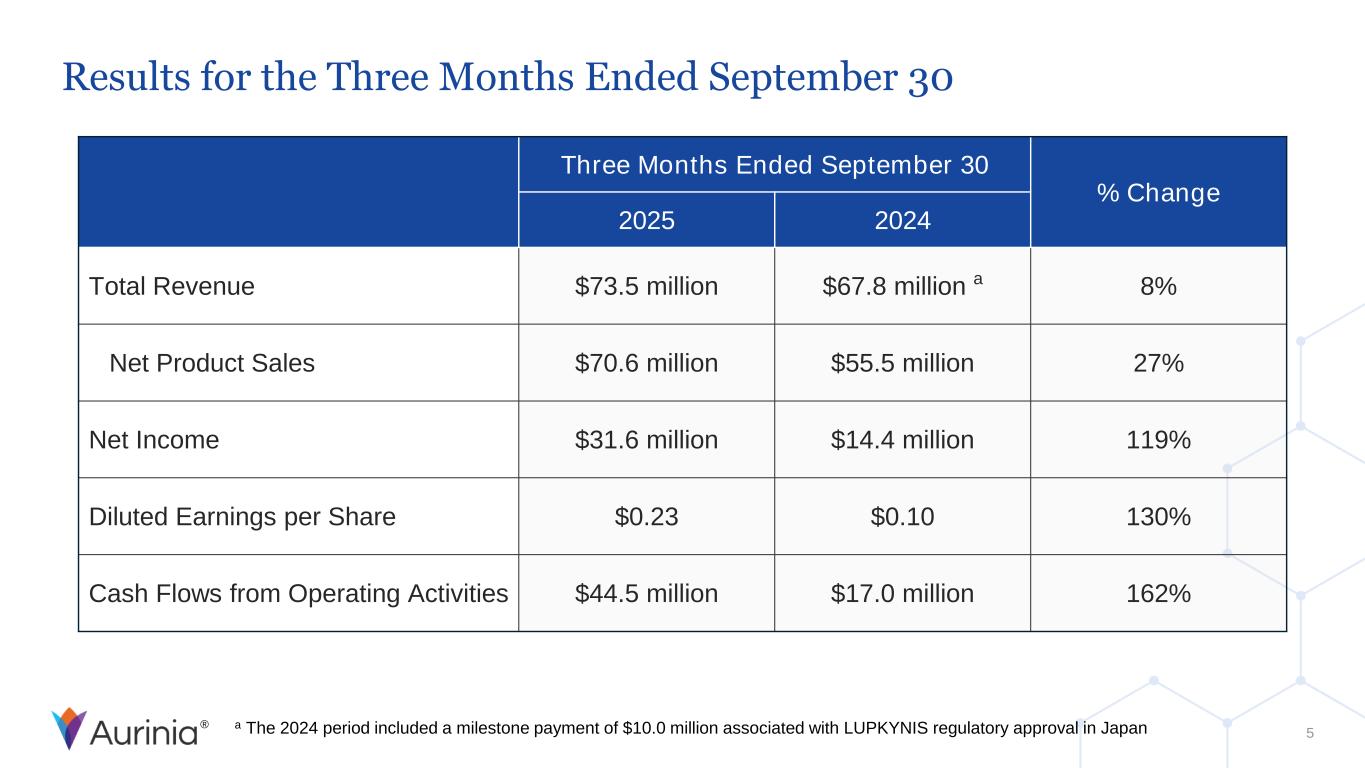
® Results for the Three Months Ended September 30 5 Three Months Ended September 30 % Change 2025 2024 Total Revenue $73.5 million $67.8 million a 8% Net Product Sales $70.6 million $55.5 million 27% Net Income $31.6 million $14.4 million 119% Diluted Earnings per Share $0.23 $0.10 130% Cash Flows from Operating Activities $44.5 million $17.0 million 162% a The 2024 period included a milestone payment of $10.0 million associated with LUPKYNIS regulatory approval in Japan
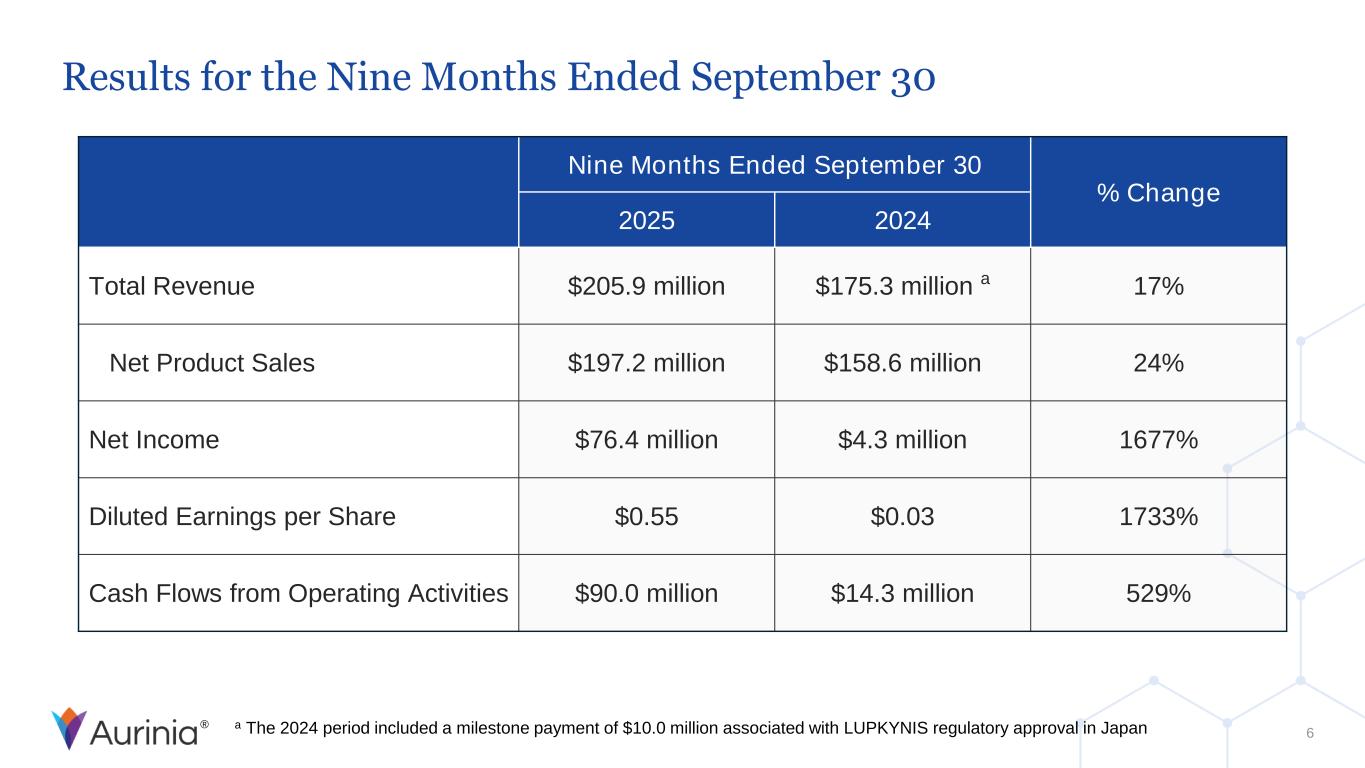
® Results for the Nine Months Ended September 30 6 Nine Months Ended September 30 % Change 2025 2024 Total Revenue $205.9 million $175.3 million a 17% Net Product Sales $197.2 million $158.6 million 24% Net Income $76.4 million $4.3 million 1677% Diluted Earnings per Share $0.55 $0.03 1733% Cash Flows from Operating Activities $90.0 million $14.3 million 529% a The 2024 period included a milestone payment of $10.0 million associated with LUPKYNIS regulatory approval in Japan
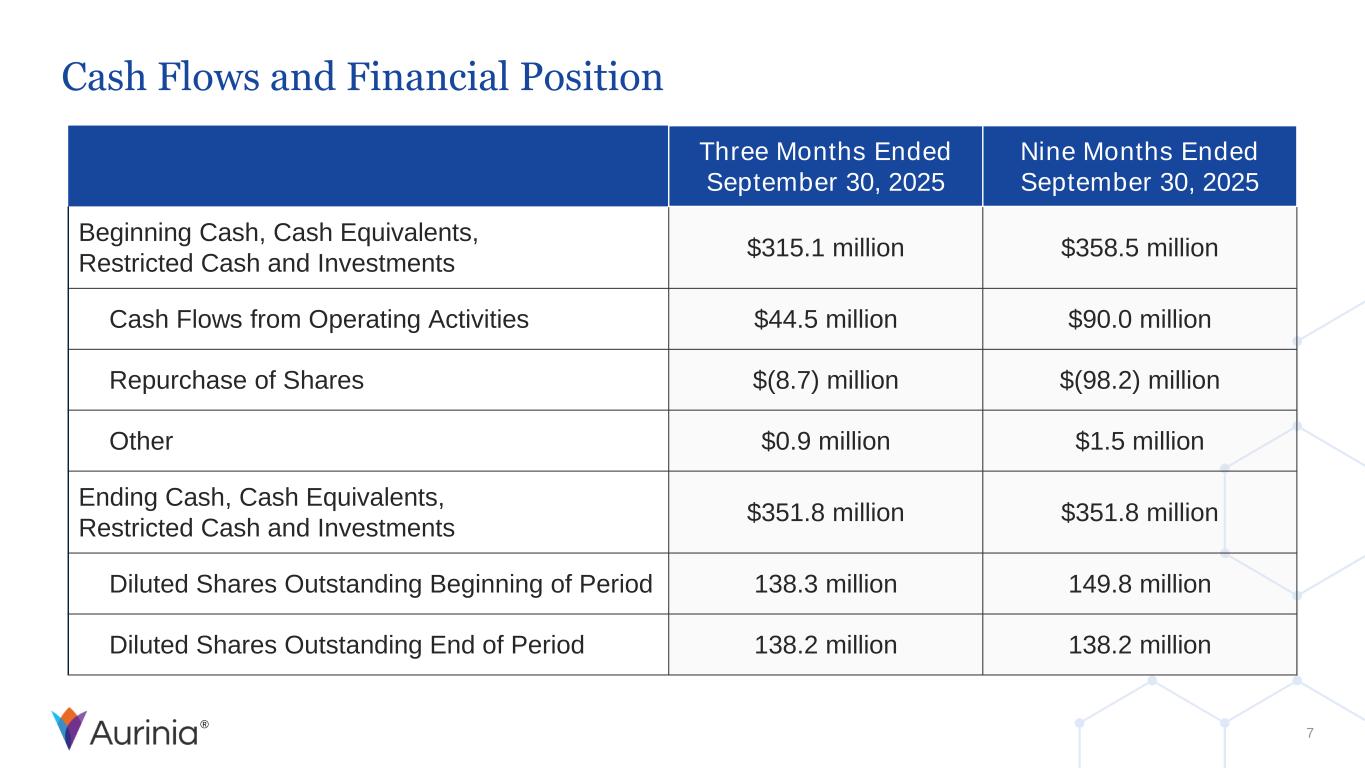
® Cash Flows and Financial Position 7 Three Months Ended September 30, 2025 Nine Months Ended September 30, 2025 Beginning Cash, Cash Equivalents, Restricted Cash and Investments $315.1 million $358.5 million Cash Flows from Operating Activities $44.5 million $90.0 million Repurchase of Shares $(8.7) million $(98.2) million Other $0.9 million $1.5 million Ending Cash, Cash Equivalents, Restricted Cash and Investments $351.8 million $351.8 million Diluted Shares Outstanding Beginning of Period 138.3 million 149.8 million Diluted Shares Outstanding End of Period 138.2 million 138.2 million
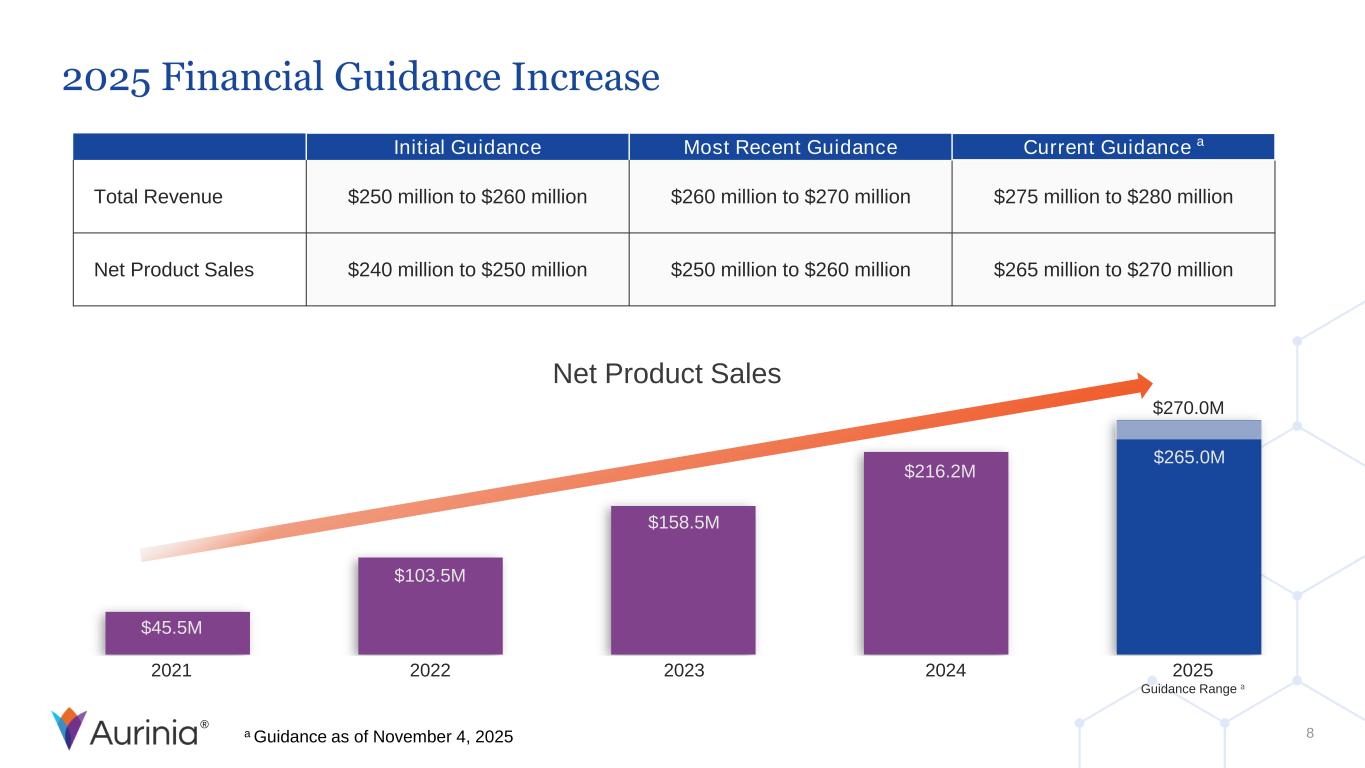
® Net Product Sales 2021 2022 2023 2024 2025 Guidance Range a $45.5M $103.5M $158.5M $216.2M $270.0M $265.0M 2025 Financial Guidance Increase 8 Initial Guidance Most Recent Guidance Current Guidance a Total Revenue $250 million to $260 million $260 million to $270 million $275 million to $280 million Net Product Sales $240 million to $250 million $250 million to $260 million $265 million to $270 million a Guidance as of November 4, 2025

® LUPKYNIS Update
® New Analyses Support LUPKYNIS’ Robust Clinical Benefit in the Treatment of Patients with Lupus Nephritis • Aurinia is sharing some new analyses of the results of the Phase 3 (AURORA 1 and AURORA 2) and Phase 2 (AURA-LV) studies that formed the basis of the 2021 FDA approval of LUPKYNIS and the 2024 FDA approval of the supplemental NDA for LUPKYNIS • These analyses were recently shared with the FDA in response to an information request • LUPKYNIS was granted full (not accelerated) FDA approval based on a statistically significant and clinically meaningful improvement in Complete Renal Response at Week 52 • The new analyses, which show that LUPKYNIS also was associated with a statistically significant and clinically meaningful reduction in the risk of Renal-Related Events or Death, reinforce the robust efficacy and favorable safety profile of LUPKYNIS 10
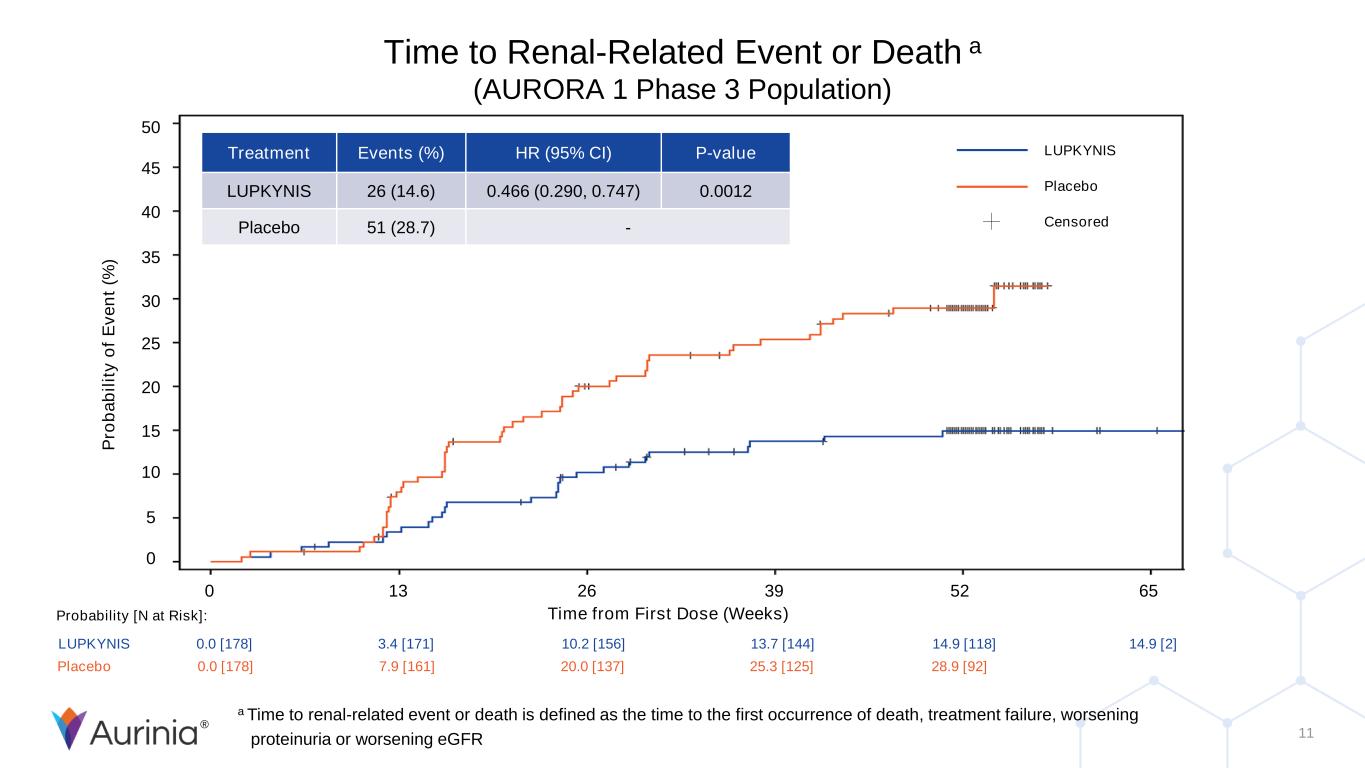
® 11 Time to Renal-Related Event or Death a (AURORA 1 Phase 3 Population) P ro b a b il it y o f E v e n t (% ) 50 45 40 35 30 25 20 15 10 5 0 0 13 26 39 52 65 Time from First Dose (Weeks) Treatment Events (%) HR (95% CI) P-value LUPKYNIS 26 (14.6) 0.466 (0.290, 0.747) 0.0012 Placebo 51 (28.7) - LUPKYNIS 0.0 [178] 3.4 [171] 10.2 [156] 13.7 [144] 14.9 [118] 14.9 [2] Placebo 0.0 [178] 7.9 [161] 20.0 [137] 25.3 [125] 28.9 [92] Probability [N at Risk]: LUPKYNIS Placebo Censored a Time to renal-related event or death is defined as the time to the first occurrence of death, treatment failure, worsening proteinuria or worsening eGFR

® Aritinercept Update
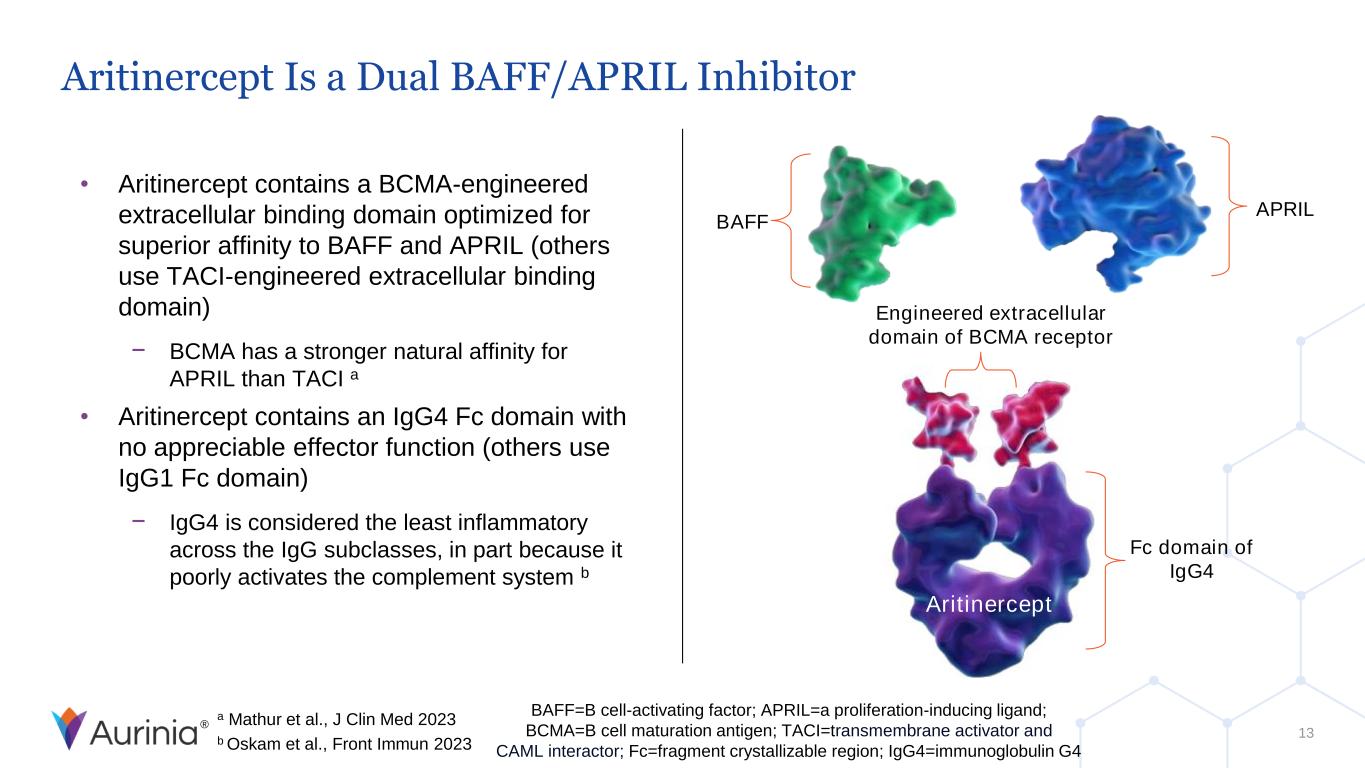
® Aritinercept Is a Dual BAFF/APRIL Inhibitor • Aritinercept contains a BCMA-engineered extracellular binding domain optimized for superior affinity to BAFF and APRIL (others use TACI-engineered extracellular binding domain) − BCMA has a stronger natural affinity for APRIL than TACI a • Aritinercept contains an IgG4 Fc domain with no appreciable effector function (others use IgG1 Fc domain) − IgG4 is considered the least inflammatory across the IgG subclasses, in part because it poorly activates the complement system b Engineered extracellular domain of BCMA receptor AUR200 BAFF APRIL Fc domain of IgG4 Aritinercept BAFF=B cell-activating factor; APRIL=a proliferation-inducing ligand; BCMA=B cell maturation antigen; TACI=transmembrane activator and CAML interactor; Fc=fragment crystallizable region; IgG4=immunoglobulin G4 a Mathur et al., J Clin Med 2023 b Oskam et al., Front Immun 2023 13
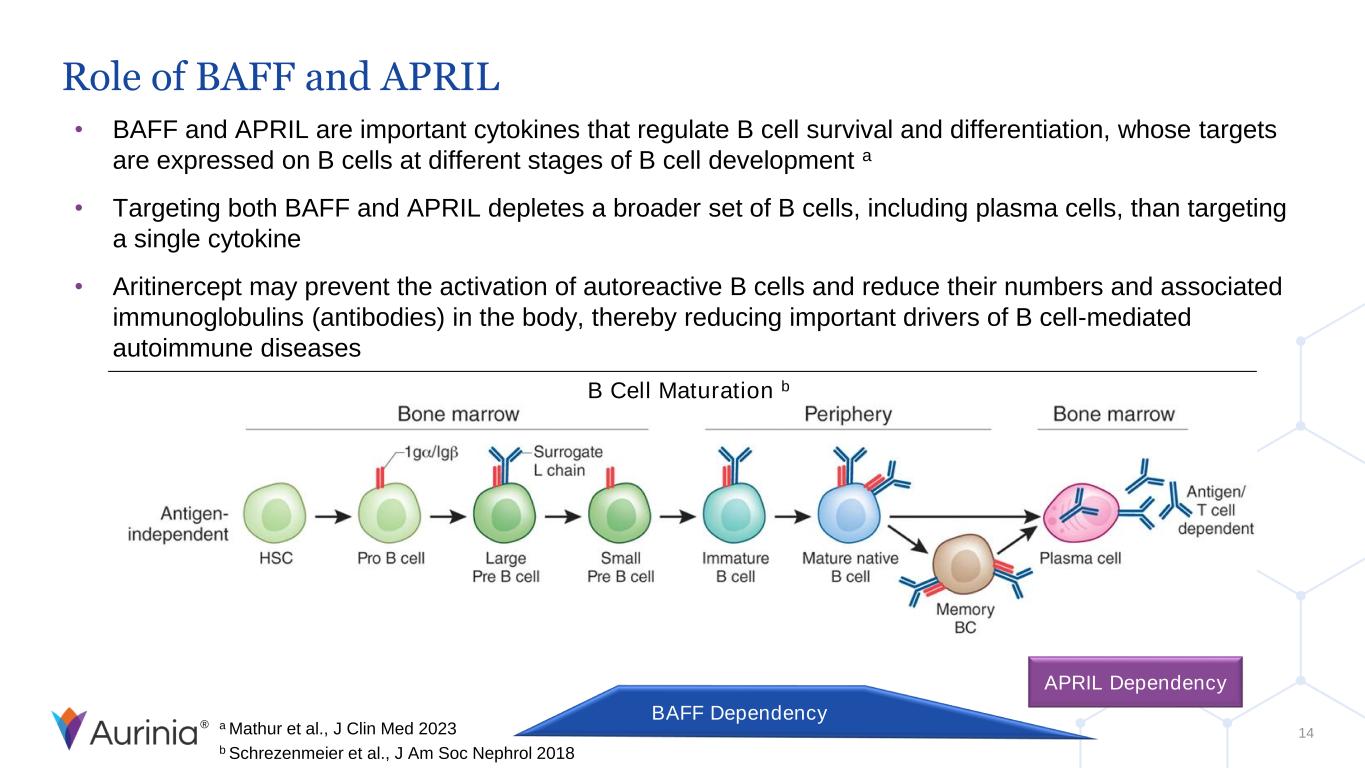
® Role of BAFF and APRIL • BAFF and APRIL are important cytokines that regulate B cell survival and differentiation, whose targets are expressed on B cells at different stages of B cell development a • Targeting both BAFF and APRIL depletes a broader set of B cells, including plasma cells, than targeting a single cytokine • Aritinercept may prevent the activation of autoreactive B cells and reduce their numbers and associated immunoglobulins (antibodies) in the body, thereby reducing important drivers of B cell-mediated autoimmune diseases B Cell Maturation b APRIL Dependency BAFF Dependency a Mathur et al., J Clin Med 2023 b Schrezenmeier et al., J Am Soc Nephrol 2018 14
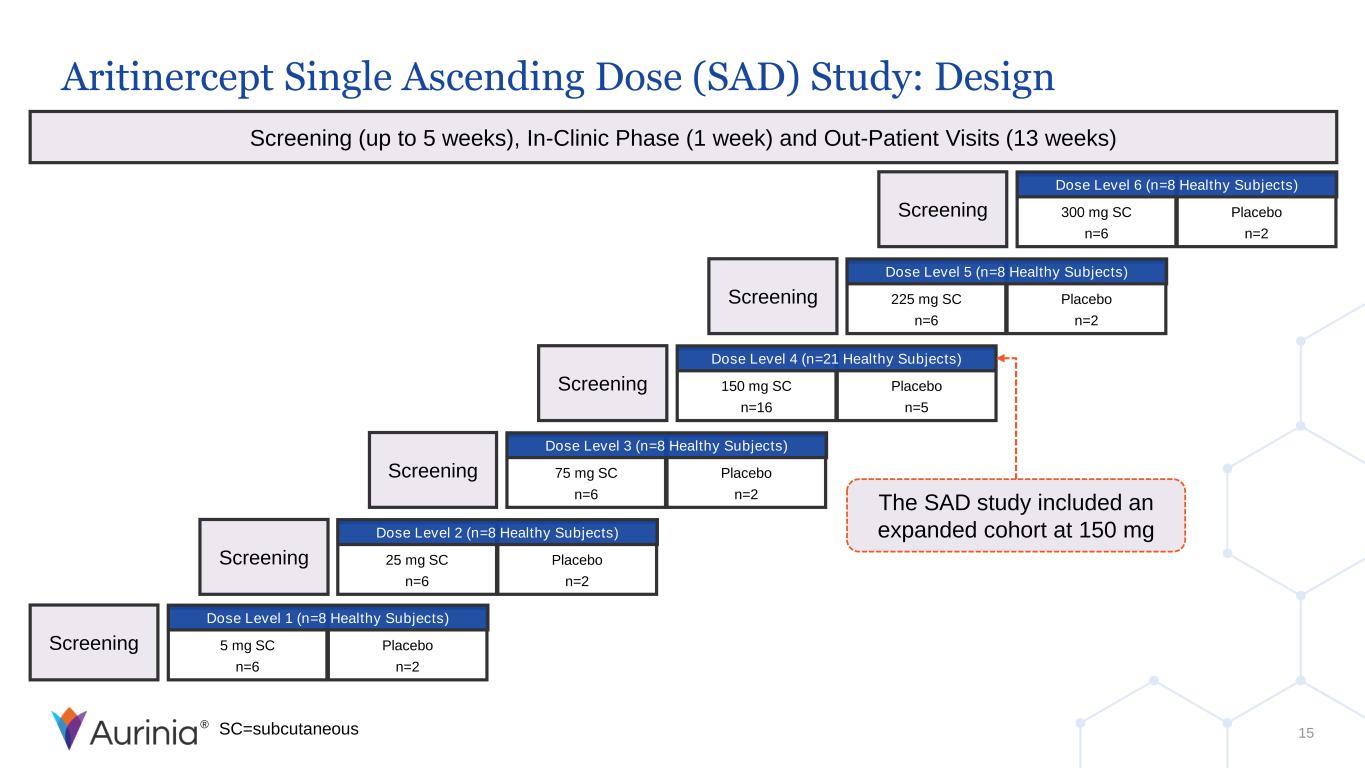
® Placebo n=2 5 mg SC n=6 Screening Dose Level 1 (n=8 Healthy Subjects) Screening (up to 5 weeks), In-Clinic Phase (1 week) and Out-Patient Visits (13 weeks) 15 Placebo n=2 25 mg SC n=6 Screening Dose Level 2 (n=8 Healthy Subjects) Placebo n=2 75 mg SC n=6 Screening Dose Level 3 (n=8 Healthy Subjects) Placebo n=5 150 mg SC n=16 Screening Dose Level 4 (n=21 Healthy Subjects) Placebo n=2 225 mg SC n=6 Screening Dose Level 5 (n=8 Healthy Subjects) Placebo n=2 300 mg SC n=6 Screening Dose Level 6 (n=8 Healthy Subjects) SC=subcutaneous The SAD study included an expanded cohort at 150 mg Aritinercept Single Ascending Dose (SAD) Study: Design
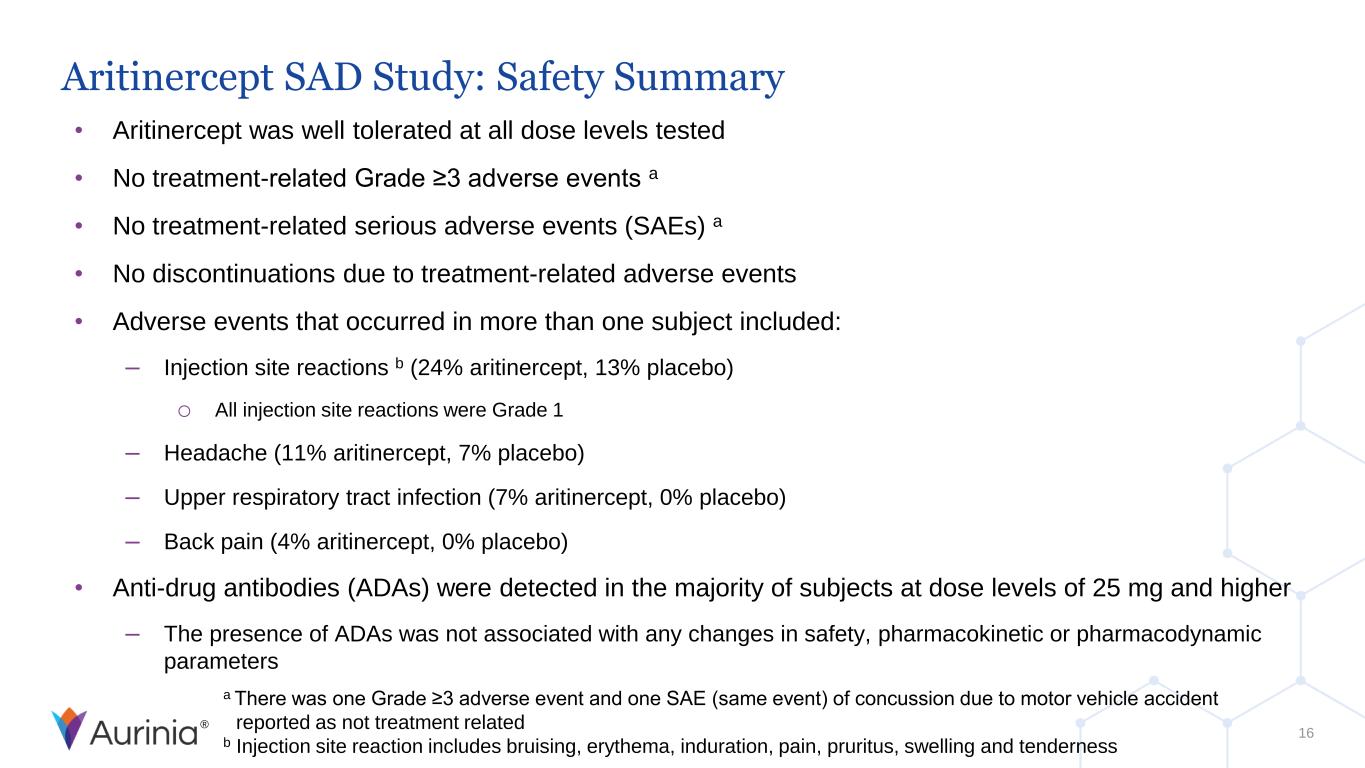
® Aritinercept SAD Study: Safety Summary • Aritinercept was well tolerated at all dose levels tested • No treatment-related Grade ≥3 adverse events a • No treatment-related serious adverse events (SAEs) a • No discontinuations due to treatment-related adverse events • Adverse events that occurred in more than one subject included: – Injection site reactions b (24% aritinercept, 13% placebo) o All injection site reactions were Grade 1 – Headache (11% aritinercept, 7% placebo) – Upper respiratory tract infection (7% aritinercept, 0% placebo) – Back pain (4% aritinercept, 0% placebo) • Anti-drug antibodies (ADAs) were detected in the majority of subjects at dose levels of 25 mg and higher – The presence of ADAs was not associated with any changes in safety, pharmacokinetic or pharmacodynamic parameters a There was one Grade ≥3 adverse event and one SAE (same event) of concussion due to motor vehicle accident reported as not treatment related b Injection site reaction includes bruising, erythema, induration, pain, pruritus, swelling and tenderness 16
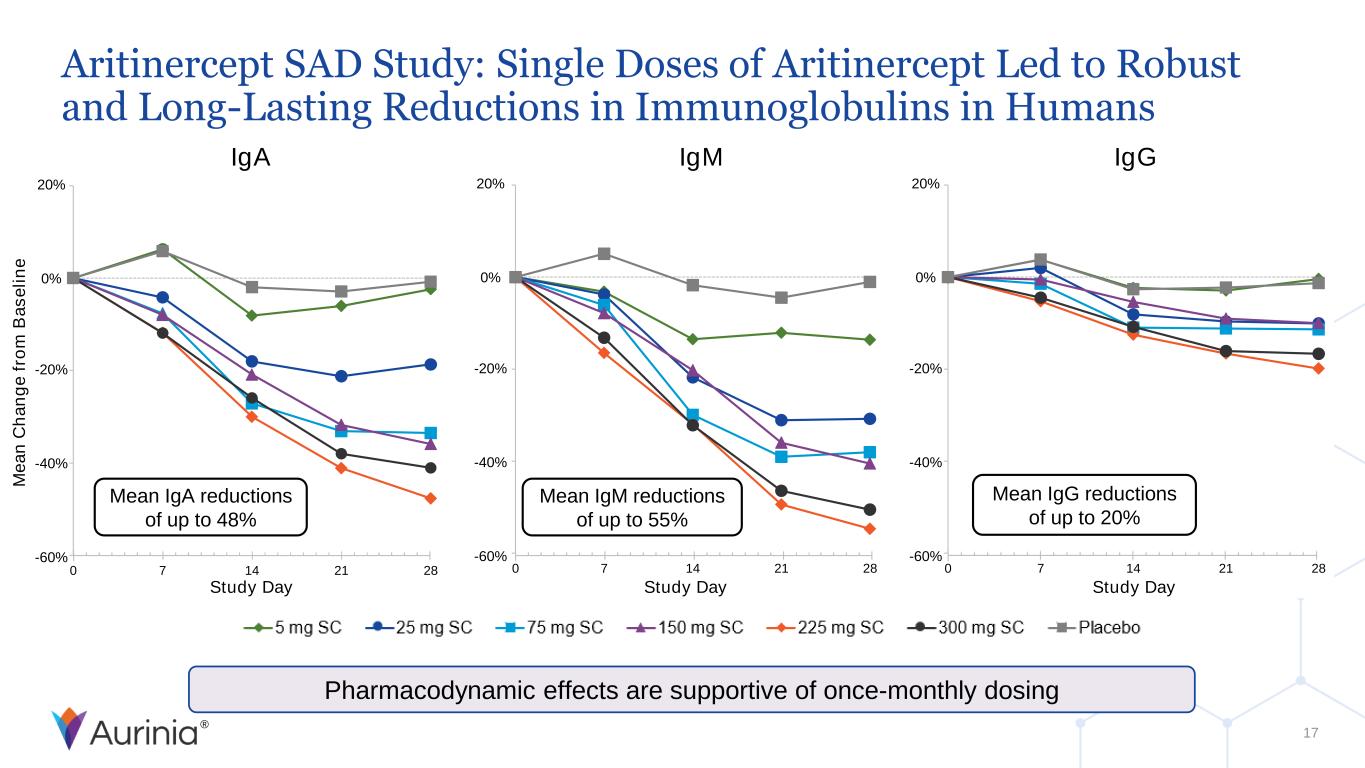
® 0 7 14 21 28 Study Day 20% 0% -20% -40% -60% 20% 0% -20% -40% -60% 0 7 14 21 28 M e a n C h a n g e f ro m B a s e li n e Study Day 20% 0% -20% -40% -60% 0 7 14 21 28 Study Day Aritinercept SAD Study: Single Doses of Aritinercept Led to Robust and Long-Lasting Reductions in Immunoglobulins in Humans 17 Pharmacodynamic effects are supportive of once-monthly dosing IgA IgM IgG Mean IgA reductions of up to 48% Mean IgM reductions of up to 55% Mean IgG reductions of up to 20%

® Summary and Next Steps • Aritinercept was well tolerated at all dose levels tested • Single doses of aritinercept led to robust and long-lasting reductions in immunoglobulins supportive of once-monthly dosing • Aurinia plans to initiate clinical studies of aritinercept in two autoimmune diseases by the end of 2025 18

® Q & A

® Appendix 1: Additional Analyses from LUPKYNIS Phase 3 and Phase 2 Clinical Studies
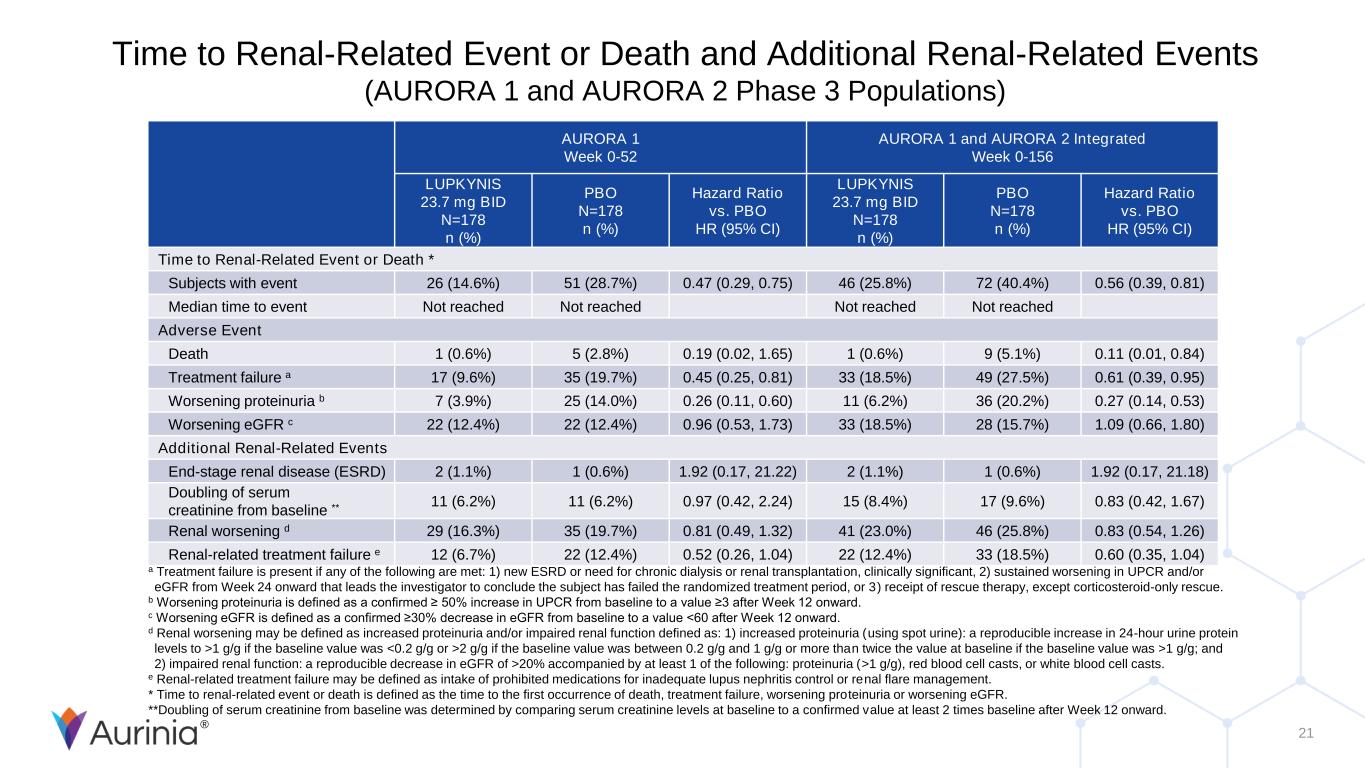
® 21 Time to Renal-Related Event or Death and Additional Renal-Related Events (AURORA 1 and AURORA 2 Phase 3 Populations) AURORA 1 Week 0-52 AURORA 1 and AURORA 2 Integrated Week 0-156 LUPKYNIS 23.7 mg BID N=178 n (%) PBO N=178 n (%) Hazard Ratio vs. PBO HR (95% CI) LUPKYNIS 23.7 mg BID N=178 n (%) PBO N=178 n (%) Hazard Ratio vs. PBO HR (95% CI) Time to Renal-Related Event or Death * Subjects with event 26 (14.6%) 51 (28.7%) 0.47 (0.29, 0.75) 46 (25.8%) 72 (40.4%) 0.56 (0.39, 0.81) Median time to event Not reached Not reached Not reached Not reached Adverse Event Death 1 (0.6%) 5 (2.8%) 0.19 (0.02, 1.65) 1 (0.6%) 9 (5.1%) 0.11 (0.01, 0.84) Treatment failure a 17 (9.6%) 35 (19.7%) 0.45 (0.25, 0.81) 33 (18.5%) 49 (27.5%) 0.61 (0.39, 0.95) Worsening proteinuria b 7 (3.9%) 25 (14.0%) 0.26 (0.11, 0.60) 11 (6.2%) 36 (20.2%) 0.27 (0.14, 0.53) Worsening eGFR c 22 (12.4%) 22 (12.4%) 0.96 (0.53, 1.73) 33 (18.5%) 28 (15.7%) 1.09 (0.66, 1.80) Additional Renal-Related Events End-stage renal disease (ESRD) 2 (1.1%) 1 (0.6%) 1.92 (0.17, 21.22) 2 (1.1%) 1 (0.6%) 1.92 (0.17, 21.18) Doubling of serum creatinine from baseline ** 11 (6.2%) 11 (6.2%) 0.97 (0.42, 2.24) 15 (8.4%) 17 (9.6%) 0.83 (0.42, 1.67) Renal worsening d 29 (16.3%) 35 (19.7%) 0.81 (0.49, 1.32) 41 (23.0%) 46 (25.8%) 0.83 (0.54, 1.26) Renal-related treatment failure e 12 (6.7%) 22 (12.4%) 0.52 (0.26, 1.04) 22 (12.4%) 33 (18.5%) 0.60 (0.35, 1.04) a Treatment failure is present if any of the following are met: 1) new ESRD or need for chronic dialysis or renal transplantation, clinically significant, 2) sustained worsening in UPCR and/or eGFR from Week 24 onward that leads the investigator to conclude the subject has failed the randomized treatment period, or 3) receipt of rescue therapy, except corticosteroid-only rescue. b Worsening proteinuria is defined as a confirmed ≥ 50% increase in UPCR from baseline to a value ≥3 after Week 12 onward. c Worsening eGFR is defined as a confirmed ≥30% decrease in eGFR from baseline to a value <60 after Week 12 onward. d Renal worsening may be defined as increased proteinuria and/or impaired renal function defined as: 1) increased proteinuria (using spot urine): a reproducible increase in 24-hour urine protein levels to >1 g/g if the baseline value was <0.2 g/g or >2 g/g if the baseline value was between 0.2 g/g and 1 g/g or more than twice the value at baseline if the baseline value was >1 g/g; and 2) impaired renal function: a reproducible decrease in eGFR of >20% accompanied by at least 1 of the following: proteinuria (>1 g/g), red blood cell casts, or white blood cell casts. e Renal-related treatment failure may be defined as intake of prohibited medications for inadequate lupus nephritis control or renal flare management. * Time to renal-related event or death is defined as the time to the first occurrence of death, treatment failure, worsening proteinuria or worsening eGFR. **Doubling of serum creatinine from baseline was determined by comparing serum creatinine levels at baseline to a confirmed value at least 2 times baseline after Week 12 onward.
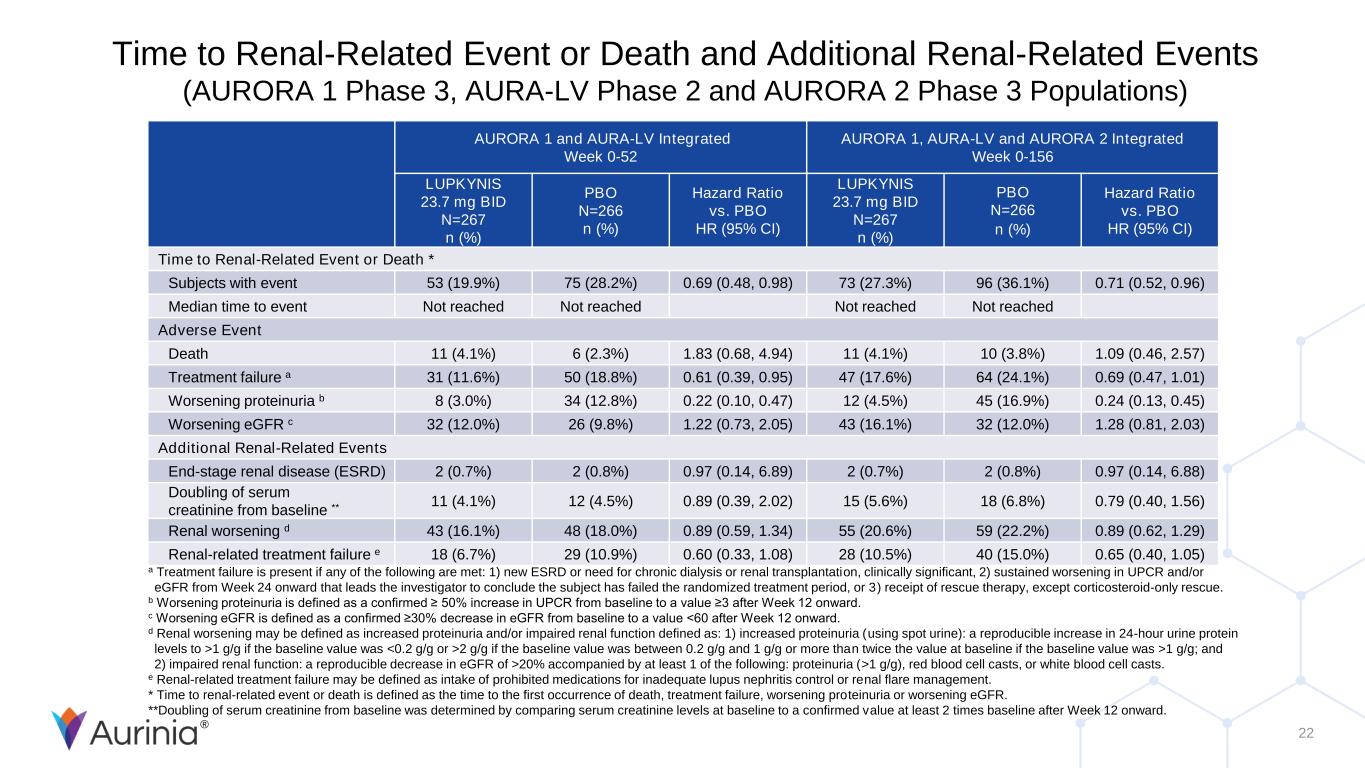
® 22 Time to Renal-Related Event or Death and Additional Renal-Related Events (AURORA 1 Phase 3, AURA-LV Phase 2 and AURORA 2 Phase 3 Populations) AURORA 1 and AURA-LV Integrated Week 0-52 AURORA 1, AURA-LV and AURORA 2 Integrated Week 0-156 LUPKYNIS 23.7 mg BID N=267 n (%) PBO N=266 n (%) Hazard Ratio vs. PBO HR (95% CI) LUPKYNIS 23.7 mg BID N=267 n (%) PBO N=266 n (%) Hazard Ratio vs. PBO HR (95% CI) Time to Renal-Related Event or Death * Subjects with event 53 (19.9%) 75 (28.2%) 0.69 (0.48, 0.98) 73 (27.3%) 96 (36.1%) 0.71 (0.52, 0.96) Median time to event Not reached Not reached Not reached Not reached Adverse Event Death 11 (4.1%) 6 (2.3%) 1.83 (0.68, 4.94) 11 (4.1%) 10 (3.8%) 1.09 (0.46, 2.57) Treatment failure a 31 (11.6%) 50 (18.8%) 0.61 (0.39, 0.95) 47 (17.6%) 64 (24.1%) 0.69 (0.47, 1.01) Worsening proteinuria b 8 (3.0%) 34 (12.8%) 0.22 (0.10, 0.47) 12 (4.5%) 45 (16.9%) 0.24 (0.13, 0.45) Worsening eGFR c 32 (12.0%) 26 (9.8%) 1.22 (0.73, 2.05) 43 (16.1%) 32 (12.0%) 1.28 (0.81, 2.03) Additional Renal-Related Events End-stage renal disease (ESRD) 2 (0.7%) 2 (0.8%) 0.97 (0.14, 6.89) 2 (0.7%) 2 (0.8%) 0.97 (0.14, 6.88) Doubling of serum creatinine from baseline ** 11 (4.1%) 12 (4.5%) 0.89 (0.39, 2.02) 15 (5.6%) 18 (6.8%) 0.79 (0.40, 1.56) Renal worsening d 43 (16.1%) 48 (18.0%) 0.89 (0.59, 1.34) 55 (20.6%) 59 (22.2%) 0.89 (0.62, 1.29) Renal-related treatment failure e 18 (6.7%) 29 (10.9%) 0.60 (0.33, 1.08) 28 (10.5%) 40 (15.0%) 0.65 (0.40, 1.05) a Treatment failure is present if any of the following are met: 1) new ESRD or need for chronic dialysis or renal transplantation, clinically significant, 2) sustained worsening in UPCR and/or eGFR from Week 24 onward that leads the investigator to conclude the subject has failed the randomized treatment period, or 3) receipt of rescue therapy, except corticosteroid-only rescue. b Worsening proteinuria is defined as a confirmed ≥ 50% increase in UPCR from baseline to a value ≥3 after Week 12 onward. c Worsening eGFR is defined as a confirmed ≥30% decrease in eGFR from baseline to a value <60 after Week 12 onward. d Renal worsening may be defined as increased proteinuria and/or impaired renal function defined as: 1) increased proteinuria (using spot urine): a reproducible increase in 24-hour urine protein levels to >1 g/g if the baseline value was <0.2 g/g or >2 g/g if the baseline value was between 0.2 g/g and 1 g/g or more than twice the value at baseline if the baseline value was >1 g/g; and 2) impaired renal function: a reproducible decrease in eGFR of >20% accompanied by at least 1 of the following: proteinuria (>1 g/g), red blood cell casts, or white blood cell casts. e Renal-related treatment failure may be defined as intake of prohibited medications for inadequate lupus nephritis control or renal flare management. * Time to renal-related event or death is defined as the time to the first occurrence of death, treatment failure, worsening proteinuria or worsening eGFR. **Doubling of serum creatinine from baseline was determined by comparing serum creatinine levels at baseline to a confirmed value at least 2 times baseline after Week 12 onward.

® Appendix 2: Immunoglobulin Reductions from Aritinercept and Other BAFF/APRIL Inhibitors
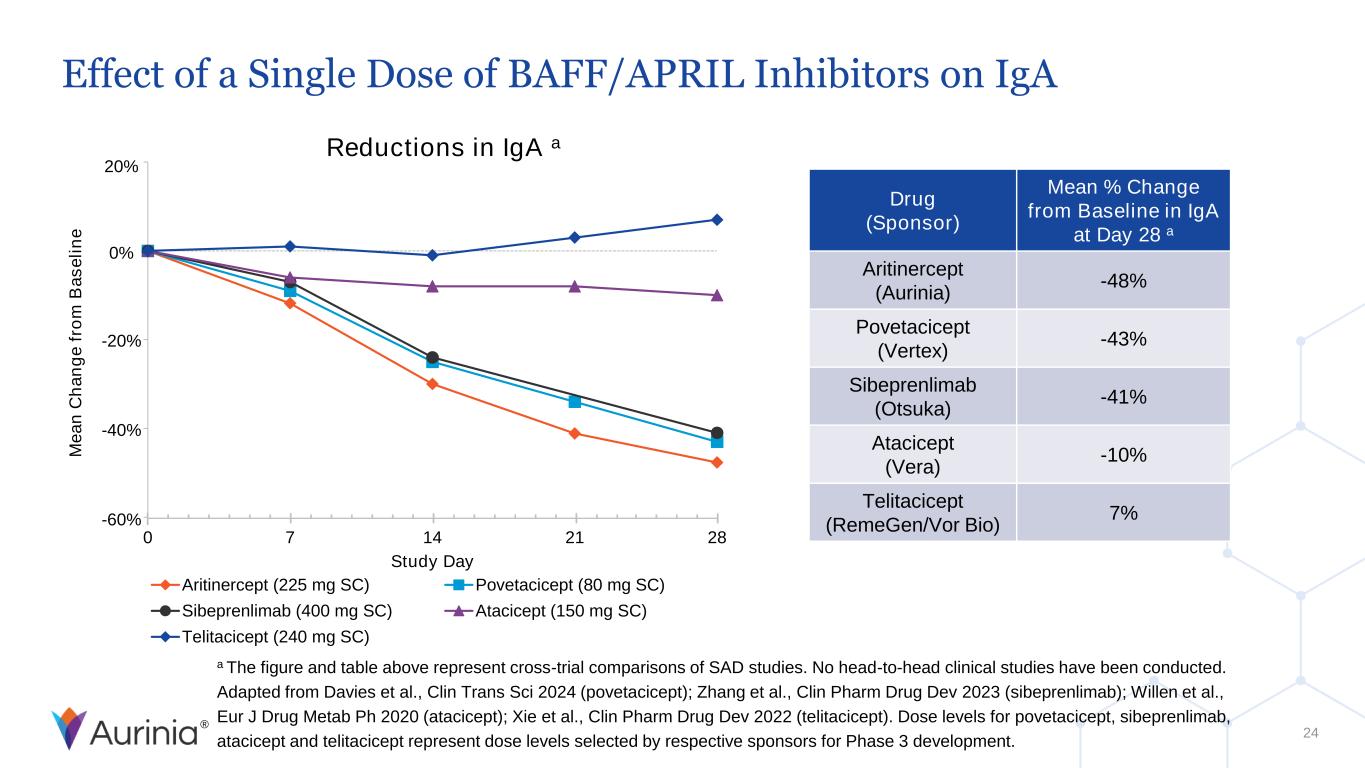
® 20% 0% -20% -40% -60% Effect of a Single Dose of BAFF/APRIL Inhibitors on IgA 24 a The figure and table above represent cross-trial comparisons of SAD studies. No head-to-head clinical studies have been conducted. Adapted from Davies et al., Clin Trans Sci 2024 (povetacicept); Zhang et al., Clin Pharm Drug Dev 2023 (sibeprenlimab); Willen et al., Eur J Drug Metab Ph 2020 (atacicept); Xie et al., Clin Pharm Drug Dev 2022 (telitacicept). Dose levels for povetacicept, sibeprenlimab, atacicept and telitacicept represent dose levels selected by respective sponsors for Phase 3 development. Reductions in IgA a Drug (Sponsor) Mean % Change from Baseline in IgA at Day 28 a Aritinercept (Aurinia) -48% Povetacicept (Vertex) -43% Sibeprenlimab (Otsuka) -41% Atacicept (Vera) -10% Telitacicept (RemeGen/Vor Bio) 7% 0 7 14 21 28 Study Day Aritinercept (225 mg SC) Povetacicept (80 mg SC) Sibeprenlimab (400 mg SC) Atacicept (150 mg SC) Telitacicept (240 mg SC) M e a n C h a n g e f ro m B a s e li n e
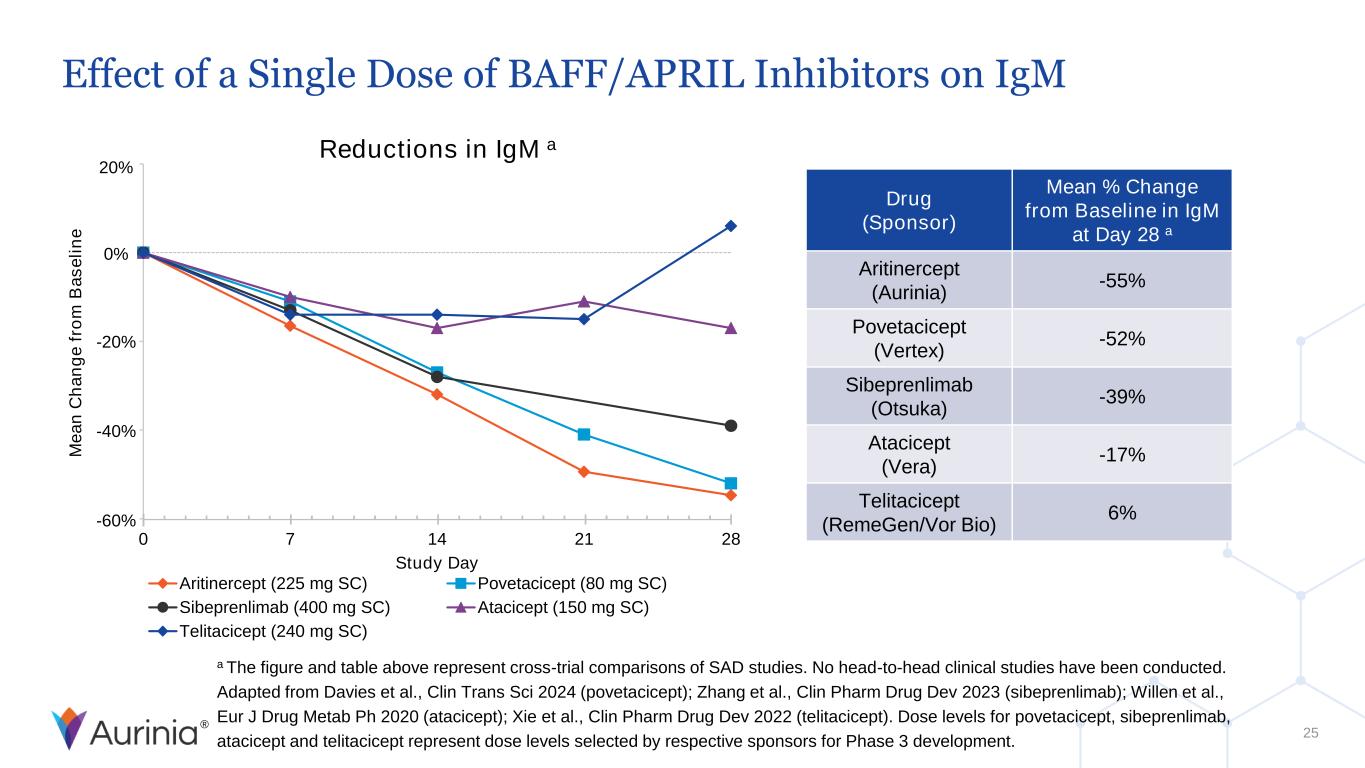
® Effect of a Single Dose of BAFF/APRIL Inhibitors on IgM 25 Reductions in IgM a 0 7 14 21 28 Study Day Aritinercept (225 mg SC) Povetacicept (80 mg SC) Sibeprenlimab (400 mg SC) Atacicept (150 mg SC) Telitacicept (240 mg SC) 20% 0% -20% -40% -60% Drug (Sponsor) Mean % Change from Baseline in IgM at Day 28 a Aritinercept (Aurinia) -55% Povetacicept (Vertex) -52% Sibeprenlimab (Otsuka) -39% Atacicept (Vera) -17% Telitacicept (RemeGen/Vor Bio) 6% M e a n C h a n g e f ro m B a s e li n e a The figure and table above represent cross-trial comparisons of SAD studies. No head-to-head clinical studies have been conducted. Adapted from Davies et al., Clin Trans Sci 2024 (povetacicept); Zhang et al., Clin Pharm Drug Dev 2023 (sibeprenlimab); Willen et al., Eur J Drug Metab Ph 2020 (atacicept); Xie et al., Clin Pharm Drug Dev 2022 (telitacicept). Dose levels for povetacicept, sibeprenlimab, atacicept and telitacicept represent dose levels selected by respective sponsors for Phase 3 development.
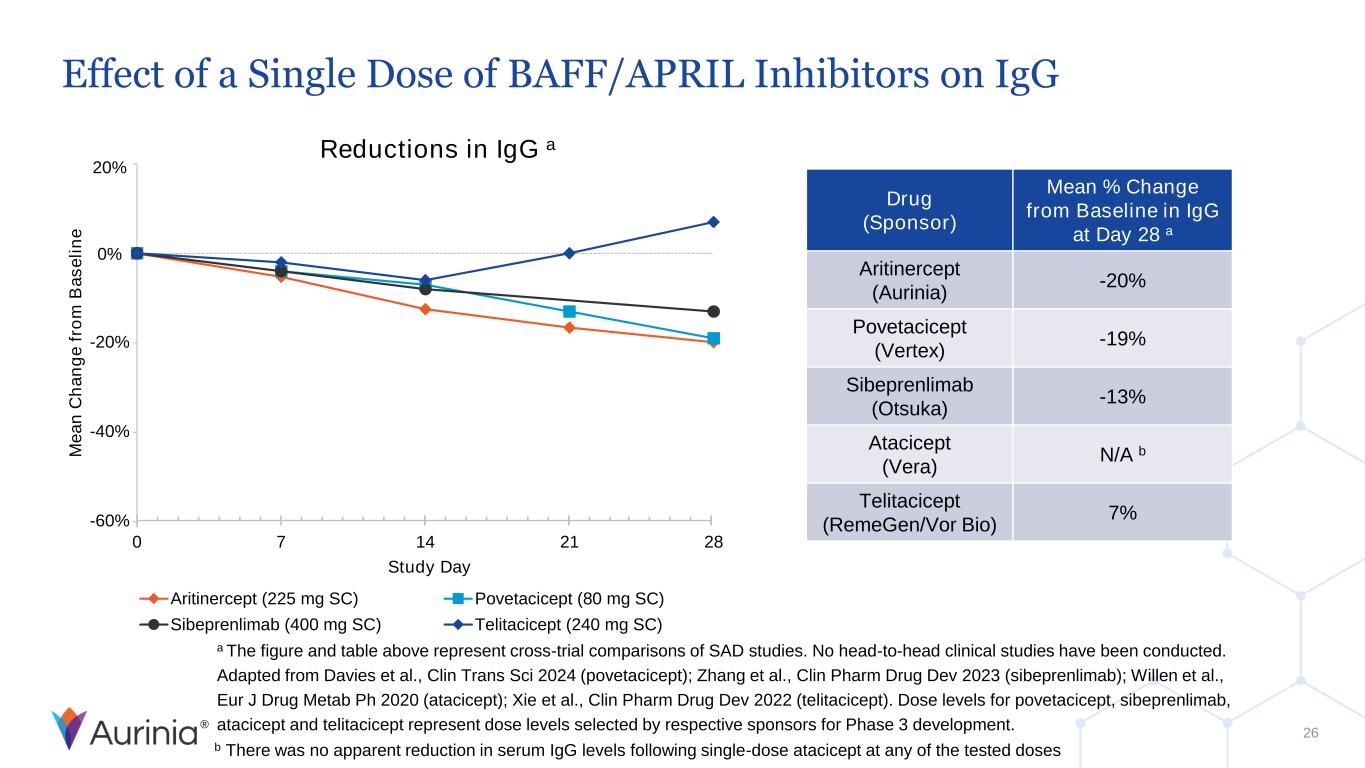
® Effect of a Single Dose of BAFF/APRIL Inhibitors on IgG 26 b There was no apparent reduction in serum IgG levels following single-dose atacicept at any of the tested doses Reductions in IgG a 0 7 14 21 28 Study Day Aritinercept (225 mg SC) Povetacicept (80 mg SC) Sibeprenlimab (400 mg SC) Telitacicept (240 mg SC) 20% 0% -20% -40% -60% Drug (Sponsor) Mean % Change from Baseline in IgG at Day 28 a Aritinercept (Aurinia) -20% Povetacicept (Vertex) -19% Sibeprenlimab (Otsuka) -13% Atacicept (Vera) N/A b Telitacicept (RemeGen/Vor Bio) 7% M e a n C h a n g e f ro m B a s e li n e a The figure and table above represent cross-trial comparisons of SAD studies. No head-to-head clinical studies have been conducted. Adapted from Davies et al., Clin Trans Sci 2024 (povetacicept); Zhang et al., Clin Pharm Drug Dev 2023 (sibeprenlimab); Willen et al., Eur J Drug Metab Ph 2020 (atacicept); Xie et al., Clin Pharm Drug Dev 2022 (telitacicept). Dose levels for povetacicept, sibeprenlimab, atacicept and telitacicept represent dose levels selected by respective sponsors for Phase 3 development.

® © Aurinia Pharmaceuticals Inc., 2025. All rights reserved. Trademarks and logos are the property of Aurinia Pharmaceuticals Inc.