
September 2025 AMPLIFY-7P Phase 2 Immunogenicity Data Nasdaq: ELTX .2

Disclaimers No Representation or Warranty We do not make and hereby expressly disclaim any representation or warranty, express or implied, as to the reasonableness of the assumptions made in the Presentation or the accuracy or completeness or the information contained in or incorporated by reference into the Presentation. We will not have any liability for any representations or warranties, express or implied, contained in, or omissions from, the Presentation. The data contained herein is derived from various internal and external sources. We do not assume any obligation to provide the recipient with access to any additional information or to update the information in the Presentation. Industry and Market Data The Presentation contains certain market data and other statistical information such as the size, growth and share of the industries and the market segments we operate in, which are based on information from independent industry organizations and other third-party sources, industry publications, surveys and forecasts. Such data may include projections based upon a number of assumptions. Neither we nor any third parties that provide information to us guarantee the accuracy, completeness, timeliness or availability of any information. We are not responsible for any errors or omissions (negligent or otherwise), regardless of the cause, or the results obtained from the use of such content. We do not give any express or implied warranties, including, but not limited to, any warranties of merchantability or fitness for a particular purpose or use, and we expressly disclaim any responsibility or liability for direct, indirect, incidental, exemplary, compensatory, punitive, special or consequential damages, costs, expenses, legal fees or losses (including lost income or profits and opportunity costs) in connection with the use of the information herein. The industry may not grow at the rate projected by market data, or at all. Failure of our industries to grow at the projected rate may have a material adverse effect on our business and the market price of our securities. In addition, if any one or more of the assumptions underlying the market data are later found to be incorrect, actual results may differ from the projections based upon these assumptions. You should not place undue reliance on these forward-looking statements.

Disclaimers Forward-Looking Statements This presentation contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, known as the PSLRA. Statements in this presentation that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding our planned clinical programs, including planned clinical trials and the potential of our product candidates, including the potential clinical benefits, the potential clinical utility, the potential benefits and market acceptance of our product candidates, the potential advantages of our product candidates over those of existing therapeutics and/or those of our competitors, the expected receipt of clinical data, the timing of initiation of our planned clinical trials, our plans to develop and commercialize our product candidates, including ELI-002, the timing of the availability of data from our clinical trials, including the disease-free survival final analysis from the ELI-002 7P Phase 2 trial, the potential for any correlation between the immunogenicity data from the Phase 2 AMPLIFY-7P trial and clinical efficacy outcomes, the timing of any planned investigational new drug application or new drug application, our plans to research, develop and commercialize our current and future product candidates, our estimates regarding future revenue, expenses, capital requirements and need for additional financing, and the advancement of and funding for our developmental programs generally. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors. New factors emerge from time to time, and it is not possible for us to predict all such factors, nor can we assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. These risks are more fully discussed in our Annual Report on Form 10-K filed with the SEC on March 31, 2025, and our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2025 filed with the SEC on May 13, 2025 and for the quarter ended June 30, 2025 filed with the SEC on August 7, 2025, under the heading “Risk Factors”, and any subsequent reports and other documents filed from time to time with the SEC. Forward-looking statements included in this release are based on information available to us as of the date of this release. We do not undertake any obligation to update such forward-looking statements to reflect events or circumstances after the date of this release, except to the extent required by law.
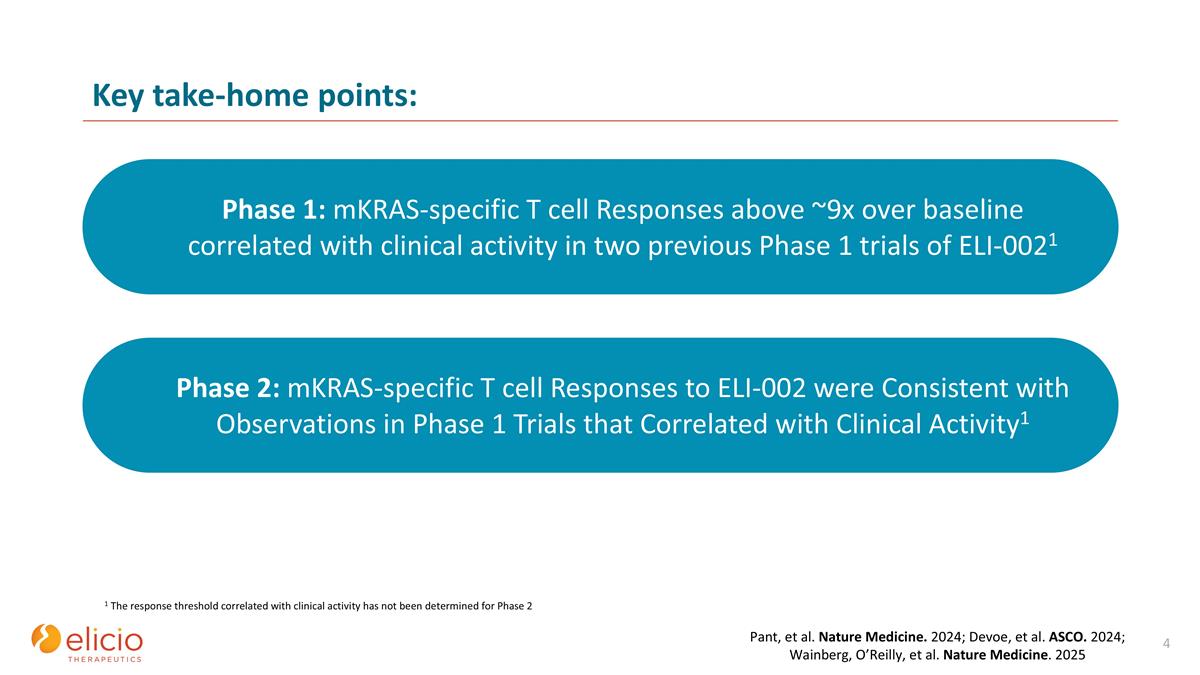
Key take-home points: Phase 2: mKRAS-specific T cell Responses to ELI-002 were Consistent with Observations in Phase 1 Trials that Correlated with Clinical Activity1 Phase 1: mKRAS-specific T cell Responses above ~9x over baseline correlated with clinical activity in two previous Phase 1 trials of ELI-0021 1 The response threshold correlated with clinical activity has not been determined for Phase 2 Pant, et al. Nature Medicine. 2024; Devoe, et al. ASCO. 2024; Wainberg, O’Reilly, et al. Nature Medicine. 2025
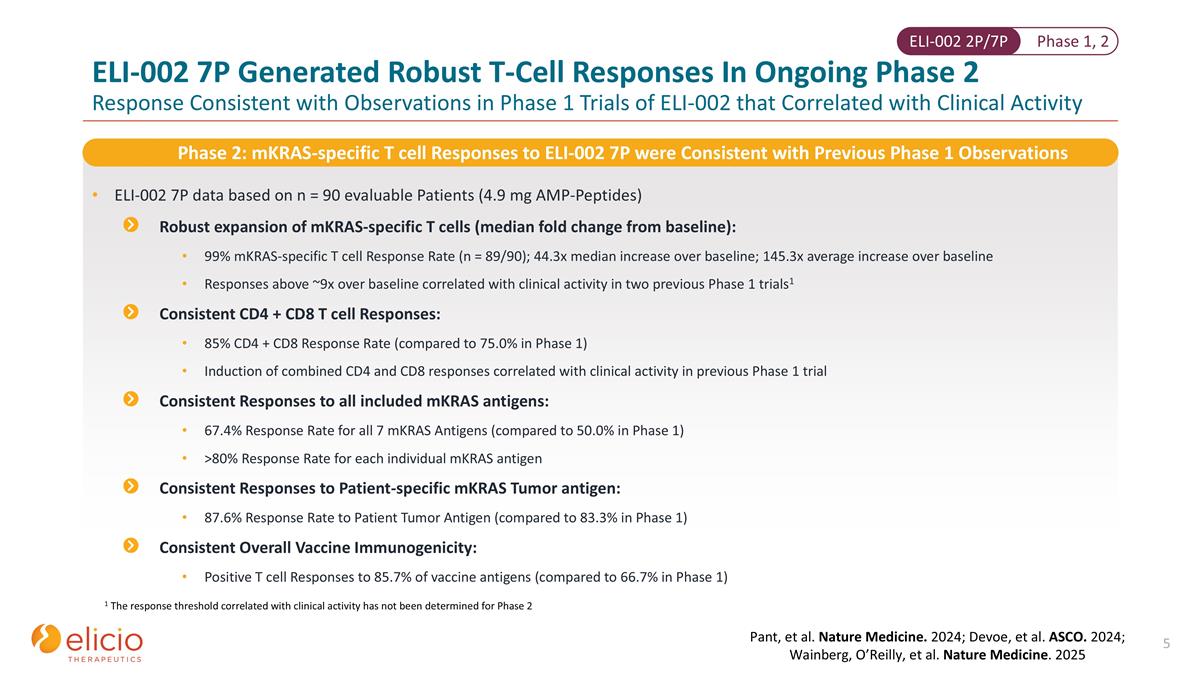
Phase 1, 2 ELI-002 2P/7P ELI-002 7P Generated Robust T-Cell Responses In Ongoing Phase 2 Response Consistent with Observations in Phase 1 Trials of ELI-002 that Correlated with Clinical Activity ELI-002 7P data based on n = 90 evaluable Patients (4.9 mg AMP-Peptides) Robust expansion of mKRAS-specific T cells (median fold change from baseline): 99% mKRAS-specific T cell Response Rate (n = 89/90); 44.3x median increase over baseline; 145.3x average increase over baseline Responses above ~9x over baseline correlated with clinical activity in two previous Phase 1 trials1 Consistent CD4 + CD8 T cell Responses: 85% CD4 + CD8 Response Rate (compared to 75.0% in Phase 1) Induction of combined CD4 and CD8 responses correlated with clinical activity in previous Phase 1 trial Consistent Responses to all included mKRAS antigens: 67.4% Response Rate for all 7 mKRAS Antigens (compared to 50.0% in Phase 1) >80% Response Rate for each individual mKRAS antigen Consistent Responses to Patient-specific mKRAS Tumor antigen: 87.6% Response Rate to Patient Tumor Antigen (compared to 83.3% in Phase 1) Consistent Overall Vaccine Immunogenicity: Positive T cell Responses to 85.7% of vaccine antigens (compared to 66.7% in Phase 1) Phase 2: mKRAS-specific T cell Responses to ELI-002 7P were Consistent with Previous Phase 1 Observations 1 The response threshold correlated with clinical activity has not been determined for Phase 2 Pant, et al. Nature Medicine. 2024; Devoe, et al. ASCO. 2024; Wainberg, O’Reilly, et al. Nature Medicine. 2025
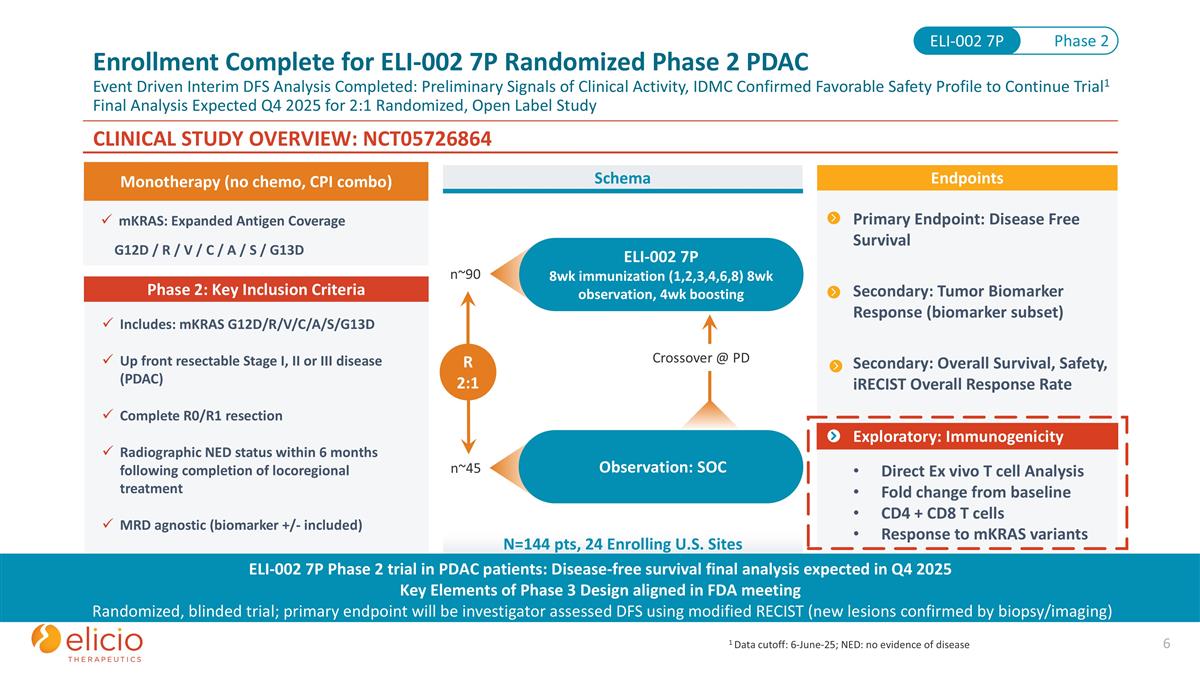
mKRAS: Expanded Antigen Coverage G12D / R / V / C / A / S / G13D CLINICAL STUDY OVERVIEW: NCT05726864 Includes: mKRAS G12D/R/V/C/A/S/G13D Up front resectable Stage I, II or III disease (PDAC) Complete R0/R1 resection Radiographic NED status within 6 months following completion of locoregional treatment MRD agnostic (biomarker +/- included) Phase 2: Key Inclusion Criteria Primary Endpoint: Disease Free Survival Secondary: Tumor Biomarker Response (biomarker subset) Secondary: Overall Survival, Safety, iRECIST Overall Response Rate Exploratory: Immunogenicity Direct Ex vivo T cell Analysis Fold change from baseline CD4 + CD8 T cells Response to mKRAS variants Endpoints ELI-002 7P 8wk immunization (1,2,3,4,6,8) 8wk observation, 4wk boosting Observation: SOC n~90 n~45 R 2:1 Crossover @ PD Schema N=144 pts, 24 Enrolling U.S. Sites Monotherapy (no chemo, CPI combo) Phase 2 ELI-002 7P ELI-002 7P Phase 2 trial in PDAC patients: Disease-free survival final analysis expected in Q4 2025 Key Elements of Phase 3 Design aligned in FDA meeting Randomized, blinded trial; primary endpoint will be investigator assessed DFS using modified RECIST (new lesions confirmed by biopsy/imaging) Enrollment Complete for ELI-002 7P Randomized Phase 2 PDAC Event Driven Interim DFS Analysis Completed: Preliminary Signals of Clinical Activity, IDMC Confirmed Favorable Safety Profile to Continue Trial1 Final Analysis Expected Q4 2025 for 2:1 Randomized, Open Label Study Exploratory: Immunogenicity 1 Data cutoff: 6-June-25; NED: no evidence of disease
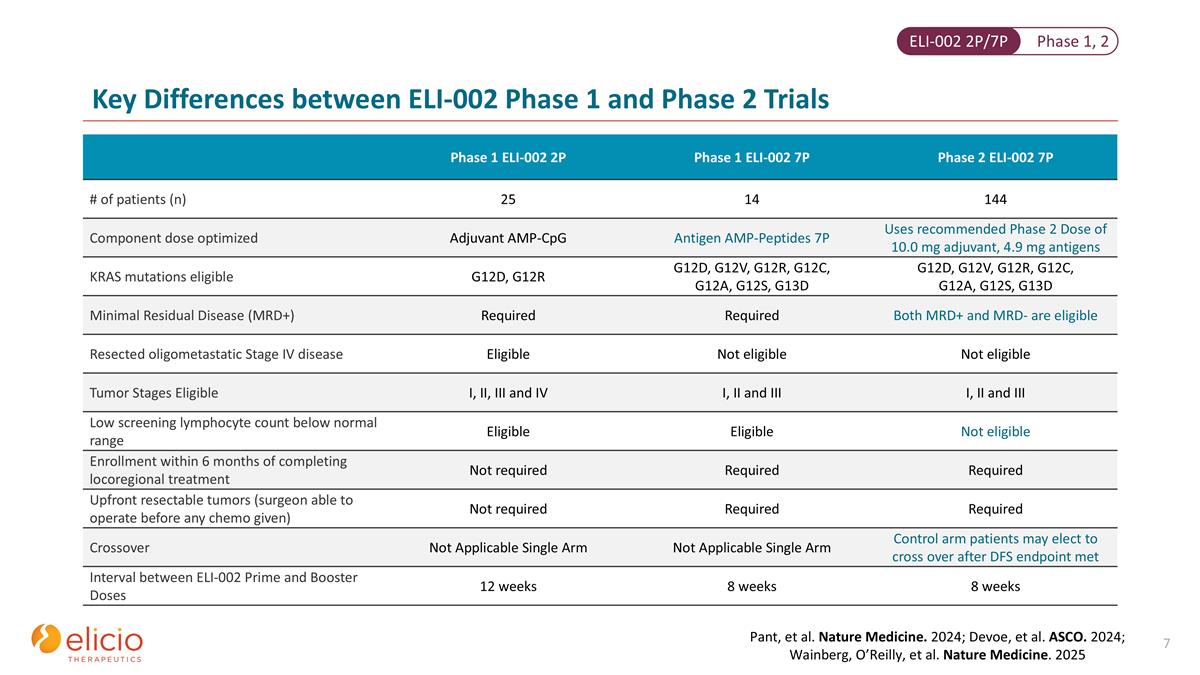
Phase 1 ELI-002 2P Phase 1 ELI-002 7P Phase 2 ELI-002 7P # of patients (n) 25 14 144 Component dose optimized Adjuvant AMP-CpG Antigen AMP-Peptides 7P Uses recommended Phase 2 Dose of 10.0 mg adjuvant, 4.9 mg antigens KRAS mutations eligible G12D, G12R G12D, G12V, G12R, G12C, G12A, G12S, G13D G12D, G12V, G12R, G12C, G12A, G12S, G13D Minimal Residual Disease (MRD+) Required Required Both MRD+ and MRD- are eligible Resected oligometastatic Stage IV disease Eligible Not eligible Not eligible Tumor Stages Eligible I, II, III and IV I, II and III I, II and III Low screening lymphocyte count below normal range Eligible Eligible Not eligible Enrollment within 6 months of completing locoregional treatment Not required Required Required Upfront resectable tumors (surgeon able to operate before any chemo given) Not required Required Required Crossover Not Applicable Single Arm Not Applicable Single Arm Control arm patients may elect to cross over after DFS endpoint met Interval between ELI-002 Prime and Booster Doses 12 weeks 8 weeks 8 weeks Key Differences between ELI-002 Phase 1 and Phase 2 Trials Pant, et al. Nature Medicine. 2024; Devoe, et al. ASCO. 2024; Wainberg, O’Reilly, et al. Nature Medicine. 2025 Phase 1, 2 ELI-002 2P/7P
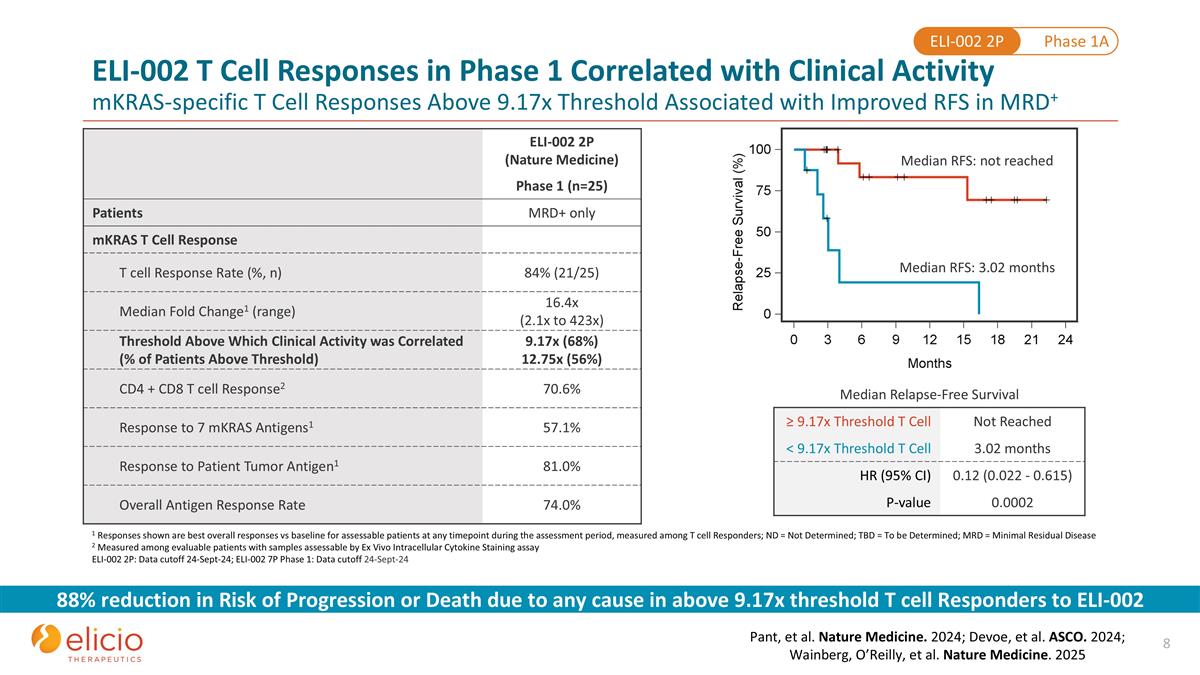
ELI-002 2P (Nature Medicine) Phase 1 (n=25) Patients MRD+ only mKRAS T Cell Response T cell Response Rate (%, n) 84% (21/25) Median Fold Change1 (range) 16.4x (2.1x to 423x) Threshold Above Which Clinical Activity was Correlated (% of Patients Above Threshold) 9.17x (68%) 12.75x (56%) CD4 + CD8 T cell Response2 70.6% Response to 7 mKRAS Antigens1 57.1% Response to Patient Tumor Antigen1 81.0% Overall Antigen Response Rate 74.0% 1 Responses shown are best overall responses vs baseline for assessable patients at any timepoint during the assessment period, measured among T cell Responders; ND = Not Determined; TBD = To be Determined; MRD = Minimal Residual Disease 2 Measured among evaluable patients with samples assessable by Ex Vivo Intracellular Cytokine Staining assay ELI-002 2P: Data cutoff 24-Sept-24; ELI-002 7P Phase 1: Data cutoff 24-Sept-24 88% reduction in Risk of Progression or Death due to any cause in above 9.17x threshold T cell Responders to ELI-002 Pant, et al. Nature Medicine. 2024; Devoe, et al. ASCO. 2024; Wainberg, O’Reilly, et al. Nature Medicine. 2025 ELI-002 T Cell Responses in Phase 1 Correlated with Clinical Activity mKRAS-specific T Cell Responses Above 9.17x Threshold Associated with Improved RFS in MRD+ Median RFS: not reached Median RFS: 3.02 months Median Relapse-Free Survival ≥ 9.17x Threshold T Cell Not Reached < 9.17x Threshold T Cell 3.02 months HR (95% CI) 0.12 (0.022 - 0.615) P-value 0.0002 Phase 1A ELI-002 2P
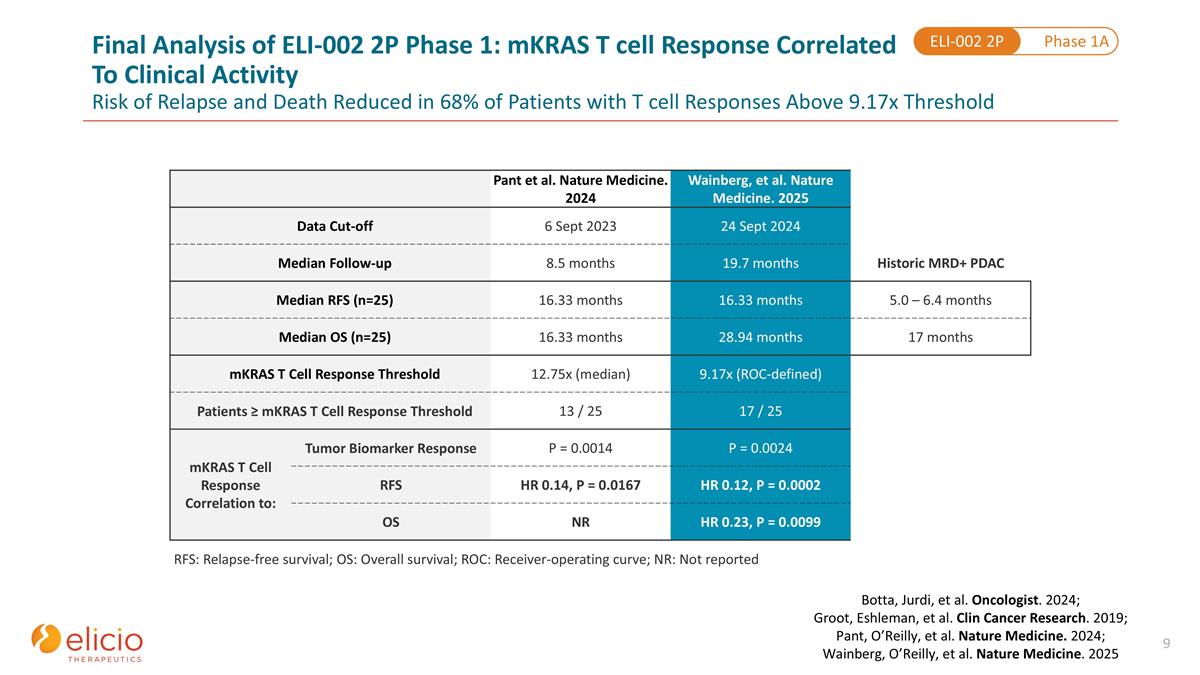
Pant et al. Nature Medicine. 2024 Wainberg, et al. Nature Medicine. 2025 Data Cut-off 6 Sept 2023 24 Sept 2024 Median Follow-up 8.5 months 19.7 months Historic MRD+ PDAC Median RFS (n=25) 16.33 months 16.33 months 5.0 – 6.4 months Median OS (n=25) 16.33 months 28.94 months 17 months mKRAS T Cell Response Threshold 12.75x (median) 9.17x (ROC-defined) Patients ≥ mKRAS T Cell Response Threshold 13 / 25 17 / 25 mKRAS T Cell Response Correlation to: Tumor Biomarker Response Tumor Biomarker Response P = 0.0014 P = 0.0024 RFS RFS HR 0.14, P = 0.0167 HR 0.12, P = 0.0002 OS OS NR HR 0.23, P = 0.0099 RFS: Relapse-free survival; OS: Overall survival; ROC: Receiver-operating curve; NR: Not reported Phase 1A ELI-002 2P Final Analysis of ELI-002 2P Phase 1: mKRAS T cell Response Correlated To Clinical Activity Risk of Relapse and Death Reduced in 68% of Patients with T cell Responses Above 9.17x Threshold Botta, Jurdi, et al. Oncologist. 2024; Groot, Eshleman, et al. Clin Cancer Research. 2019; Pant, O’Reilly, et al. Nature Medicine. 2024; Wainberg, O’Reilly, et al. Nature Medicine. 2025
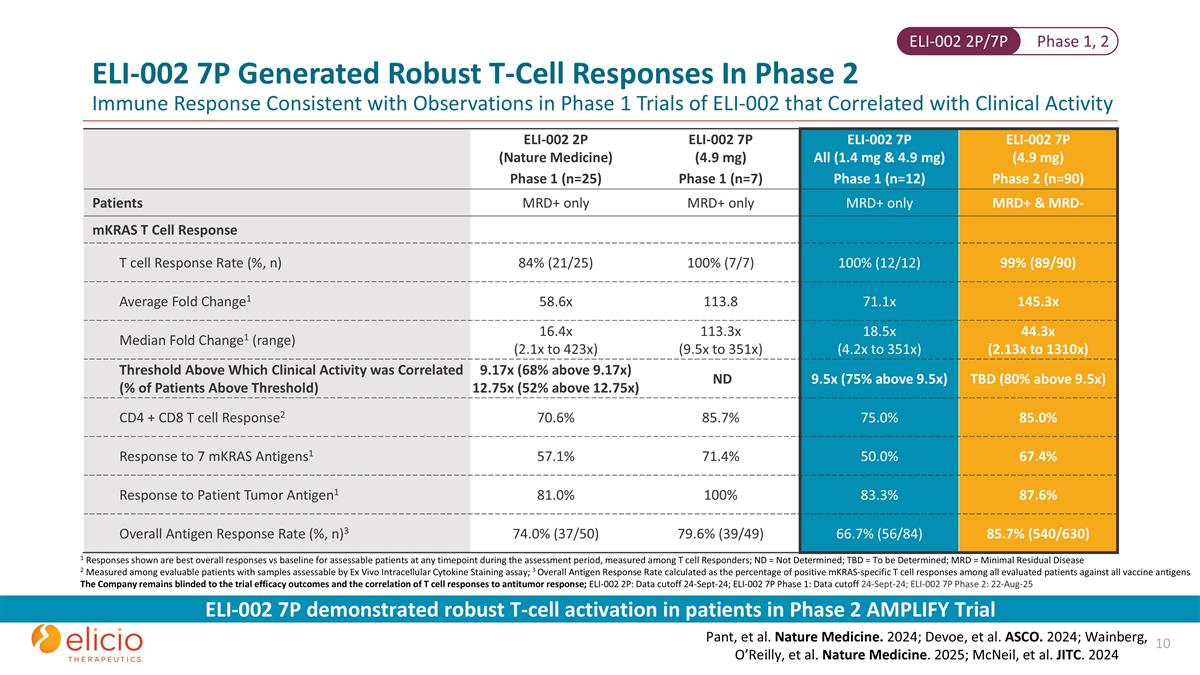
ELI-002 2P (Nature Medicine) ELI-002 7P (4.9 mg) ELI-002 7P All (1.4 mg & 4.9 mg) ELI-002 7P (4.9 mg) Phase 1 (n=25) Phase 1 (n=7) Phase 1 (n=12) Phase 2 (n=90) Patients MRD+ only MRD+ only MRD+ only MRD+ & MRD- mKRAS T Cell Response T cell Response Rate (%, n) 84% (21/25) 100% (7/7) 100% (12/12) 99% (89/90) Average Fold Change1 58.6x 113.8 71.1x 145.3x Median Fold Change1 (range) 16.4x (2.1x to 423x) 113.3x (9.5x to 351x) 18.5x (4.2x to 351x) 44.3x (2.13x to 1310x) Threshold Above Which Clinical Activity was Correlated (% of Patients Above Threshold) 9.17x (68% above 9.17x) 12.75x (52% above 12.75x) ND 9.5x (75% above 9.5x) TBD (80% above 9.5x) CD4 + CD8 T cell Response2 70.6% 85.7% 75.0% 85.0% Response to 7 mKRAS Antigens1 57.1% 71.4% 50.0% 67.4% Response to Patient Tumor Antigen1 81.0% 100% 83.3% 87.6% Overall Antigen Response Rate (%, n)3 74.0% (37/50) 79.6% (39/49) 66.7% (56/84) 85.7% (540/630) 1 Responses shown are best overall responses vs baseline for assessable patients at any timepoint during the assessment period, measured among T cell Responders; ND = Not Determined; TBD = To be Determined; MRD = Minimal Residual Disease 2 Measured among evaluable patients with samples assessable by Ex Vivo Intracellular Cytokine Staining assay; 3 Overall Antigen Response Rate calculated as the percentage of positive mKRAS-specific T cell responses among all evaluated patients against all vaccine antigens The Company remains blinded to the trial efficacy outcomes and the correlation of T cell responses to antitumor response; ELI-002 2P: Data cutoff 24-Sept-24; ELI-002 7P Phase 1: Data cutoff 24-Sept-24; ELI-002 7P Phase 2: 22-Aug-25 ELI-002 7P demonstrated robust T-cell activation in patients in Phase 2 AMPLIFY Trial Pant, et al. Nature Medicine. 2024; Devoe, et al. ASCO. 2024; Wainberg, O’Reilly, et al. Nature Medicine. 2025; McNeil, et al. JITC. 2024 Phase 1, 2 ELI-002 2P/7P ELI-002 7P Generated Robust T-Cell Responses In Phase 2 Immune Response Consistent with Observations in Phase 1 Trials of ELI-002 that Correlated with Clinical Activity
Executive Summary: Key Takeaways from Phase 2 Immunogenicity Data T cell Responses from Phase 2 were consistent across numerous metrics of magnitude and functional quality with responses observed in previous Phase 1 studies of ELI-002 2P and ELI-002 7P where T cell Response was correlated to clinical activity Robust expansion of mKRAS-specific T cells Consistent CD4 + CD8 T cell responses Consistent responses to all included mKRAS antigens Consistent responses to patient-specific mKRAS tumor antigen Consistent overall vaccine immunogenicity Phase 2 ELI-002 7P-Induced mKRAS T cell Responses were Consistent with Prior Phase 1 Observations
September 2025 AMPLIFY-7P Phase 2 Immunogenicity Data Nasdaq: ELTX