
© Inspire Medical Systems, Inc. All Rights Reserved © 2025 Inspire Medical Systems, Inc. All Rights Reserved Inspire Product Theater Inspire V Real-World Experience One Less Step. Same Proven Results Presents

Inspire Product Theater – Faculty Ryan Soose, MD University of Pittsburgh School of Medicine Pittsburgh, PA Tom Kaffenberger, MD University of Pittsburgh School of Medicine Pittsburgh, PA C-00156.1 Maria Suurna, MD University of Miami Miami, FL Colorado ENT & Allergy Colorado Springs, CO Mass Eye & Ear Boston, MA Nic Beckmann, DO Phil Huyett, MD

Inspire Product Theater Agenda C-00156.1 • Early-User Implant Experience • Preliminary Patient Outcomes Data • Q&A Discussion with Expert Panel

Next Generation Hypoglossal Nerve Stimulation for the Treatment of Obstructive Sleep Apnea Results of the Inspire V Study Singapore General Hospital National University Hospital
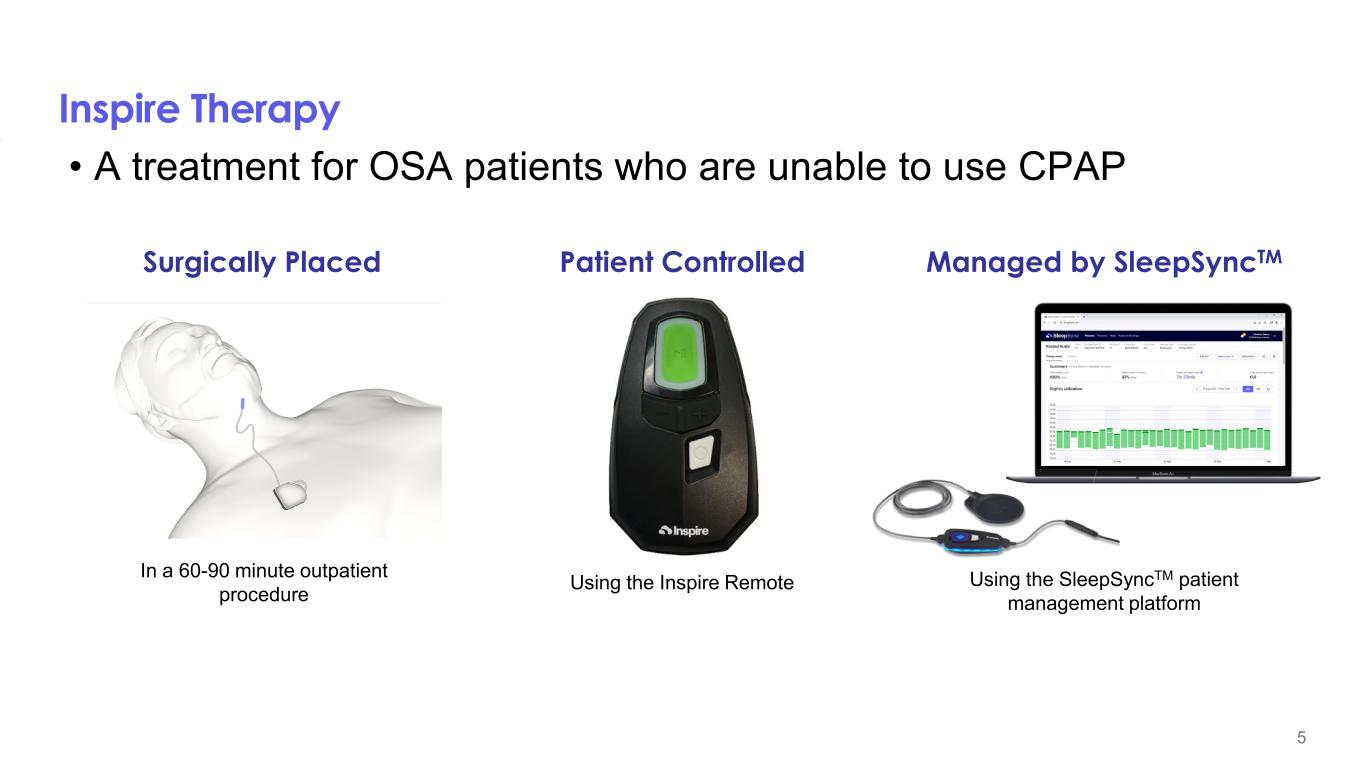
Inspire Therapy • A treatment for OSA patients who are unable to use CPAP In a 60-90 minute outpatient procedure Using the Inspire Remote Using the SleepSyncTM patient management platform 5 Surgically Placed Patient Controlled Managed by SleepSyncTM
Inspire V System 6 The Fifth Generation of a proven therapy for OSA is designed for a(n): • Simplified procedure • Enhanced patient comfort • More efficient patient management
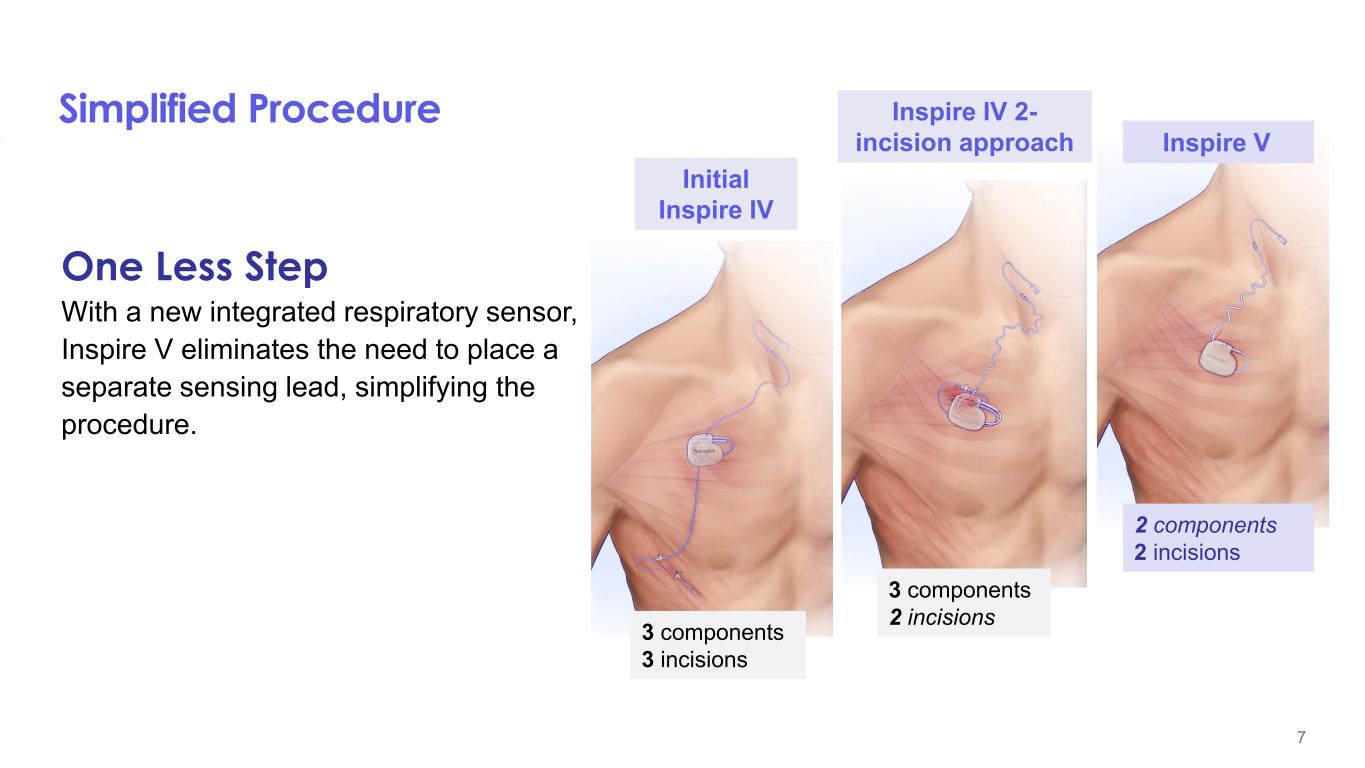
7 Simplified Procedure One Less Step With a new integrated respiratory sensor, Inspire V eliminates the need to place a separate sensing lead, simplifying the procedure. 2 components 2 incisions 3 components 2 incisions3 components 3 incisions Inspire V Inspire IV 2- incision approach Initial Inspire IV
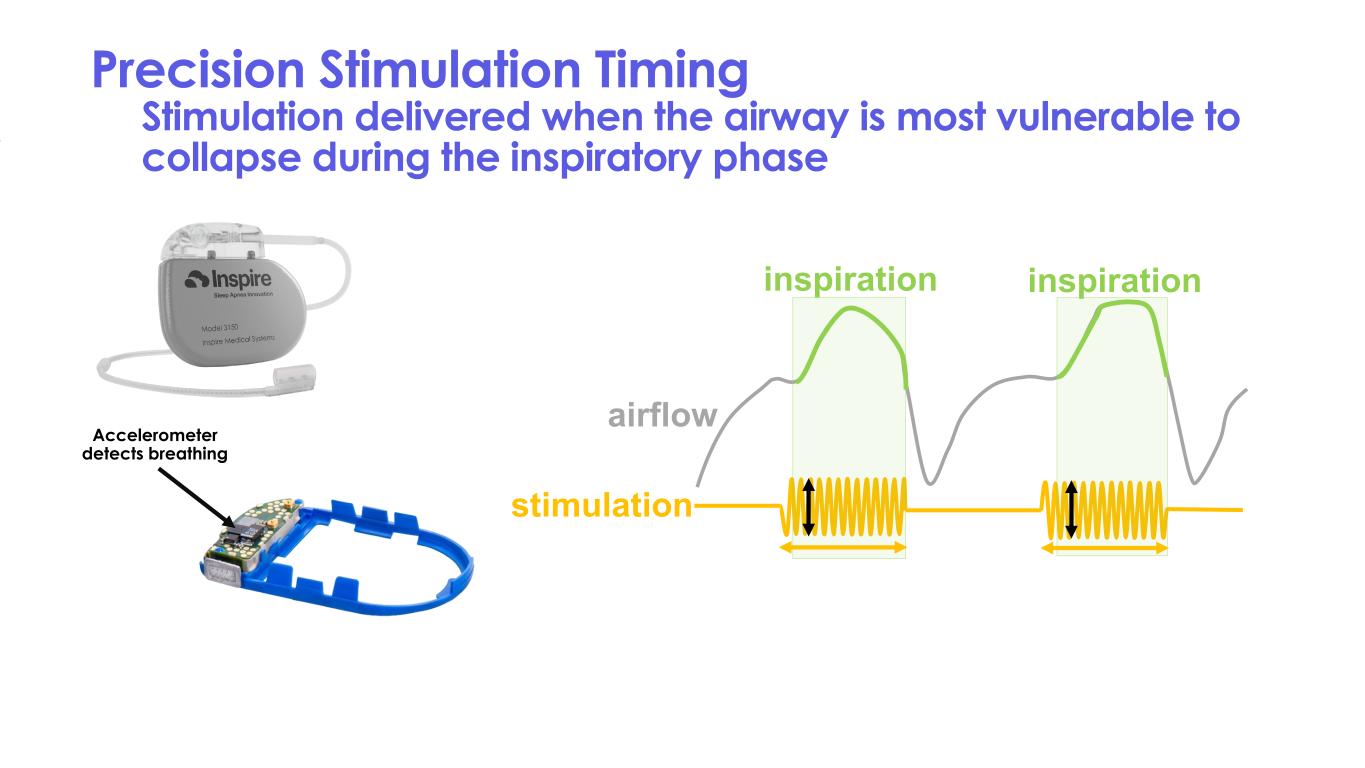
Precision Stimulation Timing Stimulation delivered when the airway is most vulnerable to collapse during the inspiratory phase inspiration inspiration airflow stimulation Accelerometer detects breathing
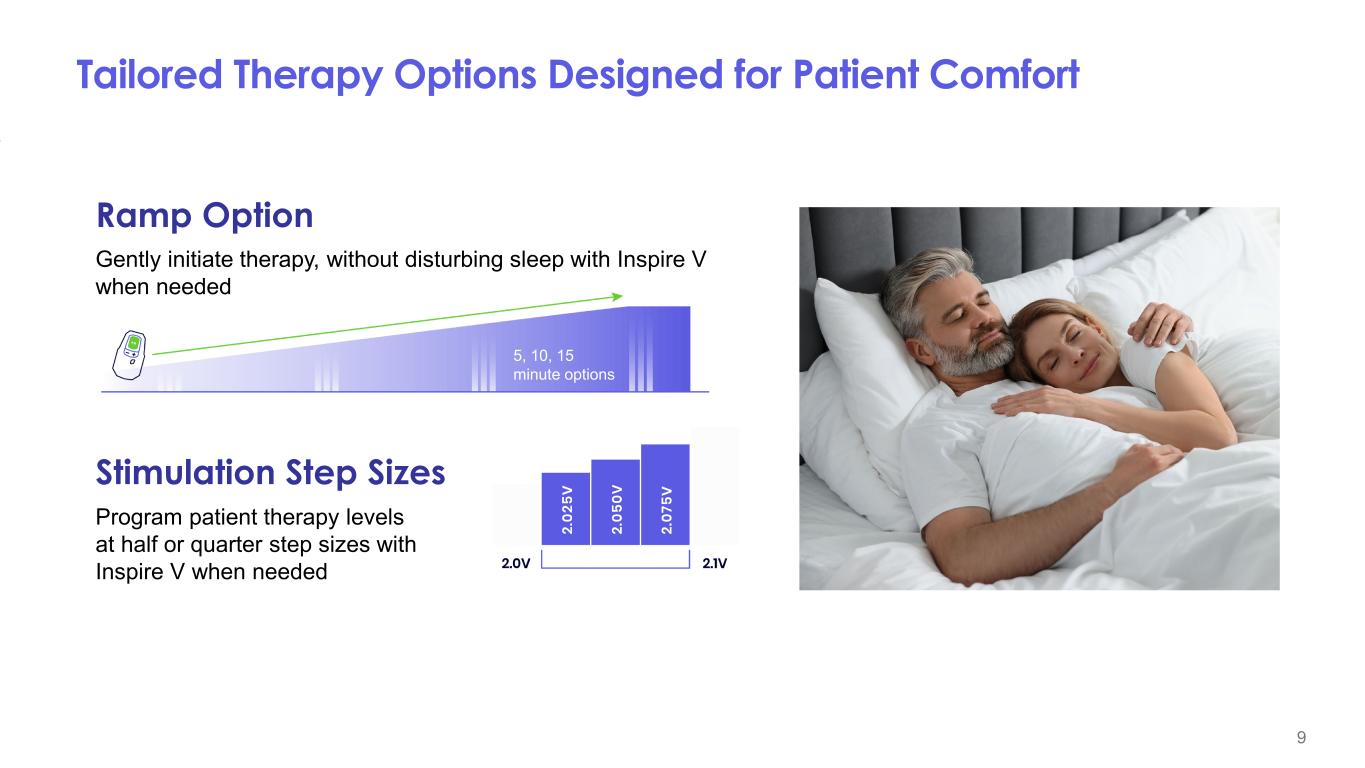
9 Tailored Therapy Options Designed for Patient Comfort Program patient therapy levels at half or quarter step sizes with Inspire V when needed Stimulation Step Sizes Gently initiate therapy, without disturbing sleep with Inspire V when needed Ramp Option 5, 10, 15 minute options

Inspire V Study Design • Prospective, multicenter cohort study • Designed to confirm effectiveness of Inspire V System • Two investigational centers (Singapore) • Singapore General Hospital (Prof. Toh Song Tar; Dr. Shaun Loh; Dr. Adele Ng) • National University Hospital (Dr. Crystal Cheong) • 44 Subjects • Patient Population • Subjects 21 years or age or older with moderate to severe OSA who meet the Inspire V UAS system indications for use and are willing to participate in the clinical trial

Study Objectives Primary Endpoint: Non-inferiority of Inspire V vs Inspire IV technology as measured by IPOP (Inspiratory Phase Overlap Percent) during in-lab polysomnography. Secondary Endpoints: • Change in Apnea – Hypopnea Index (AHI) at 6 Months post-activation • Mean Epworth Sleepiness Scale (ESS) is less than 10 at 6 Months post- activation Ancillary Information: • Therapy Adherence (hours/day) at 6 months post-activation • Procedure and Device/therapy Adverse Events • IPOP at 6 months post-activation • Mean Function Outcomes of Sleep Questionnaire (FOSQ) at 6 months post- activation
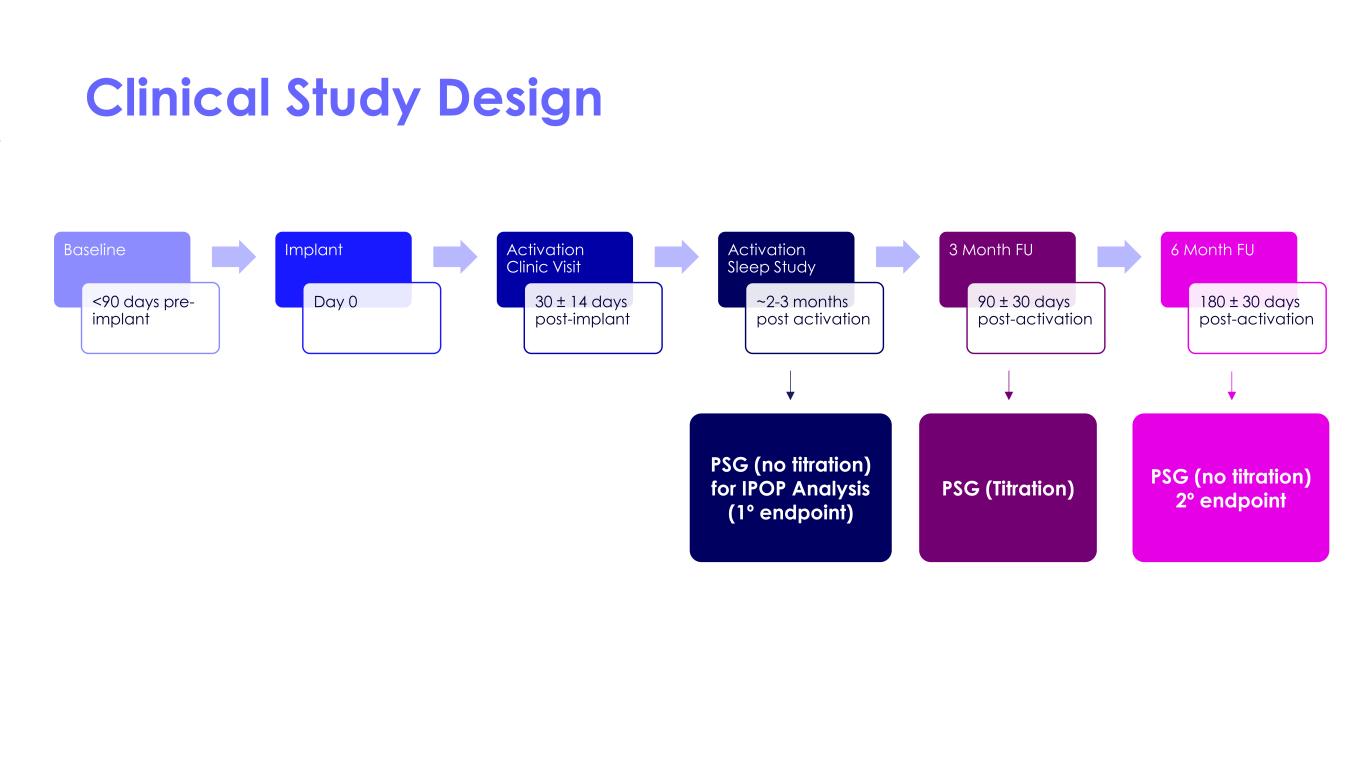
Clinical Study Design Baseline <90 days pre- implant Implant Day 0 Activation Clinic Visit 30 ± 14 days post-implant Activation Sleep Study ~2-3 months post activation 3 Month FU 90 ± 30 days post-activation 6 Month FU 180 ± 30 days post-activation PSG (no titration) for IPOP Analysis (1º endpoint) PSG (Titration) PSG (no titration) 2º endpoint
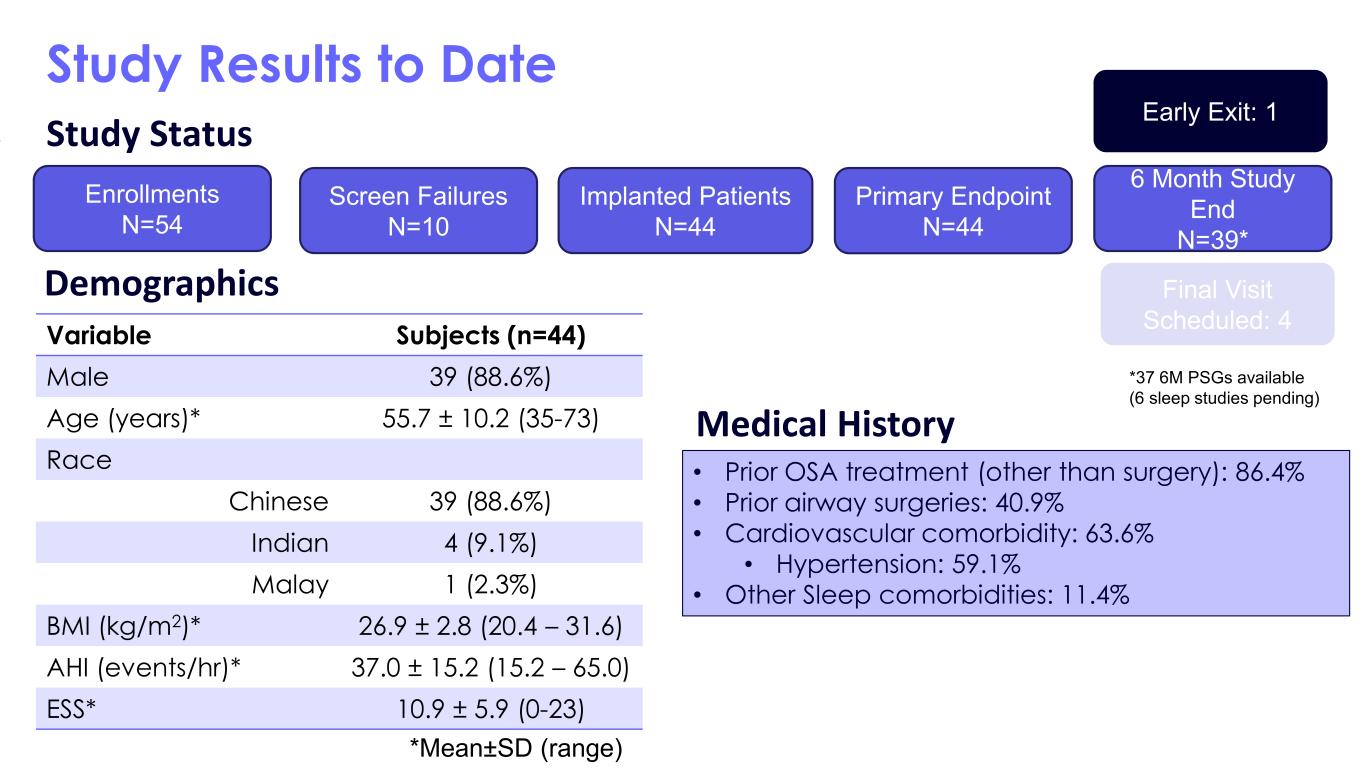
Study Results to Date Variable Subjects (n=44) Male 39 (88.6%) Age (years)* 55.7 ± 10.2 (35-73) Race Chinese 39 (88.6%) Indian 4 (9.1%) Malay 1 (2.3%) BMI (kg/m2)* 26.9 ± 2.8 (20.4 – 31.6) AHI (events/hr)* 37.0 ± 15.2 (15.2 – 65.0) ESS* 10.9 ± 5.9 (0-23) • Prior OSA treatment (other than surgery): 86.4% • Prior airway surgeries: 40.9% • Cardiovascular comorbidity: 63.6% • Hypertension: 59.1% • Other Sleep comorbidities: 11.4% Demographics Medical History Study Status Enrollments N=54 Screen Failures N=10 Implanted Patients N=44 Primary Endpoint N=44 6 Month Study End N=39* Early Exit: 1 Final Visit Scheduled: 4 *Mean±SD (range) *37 6M PSGs available (6 sleep studies pending)

Study Results to Date Procedure Details • 100% successful Implants (n=44) • 20.4% reduction in implant time (vs Inspire IV) • No serious intraoperative adverse events
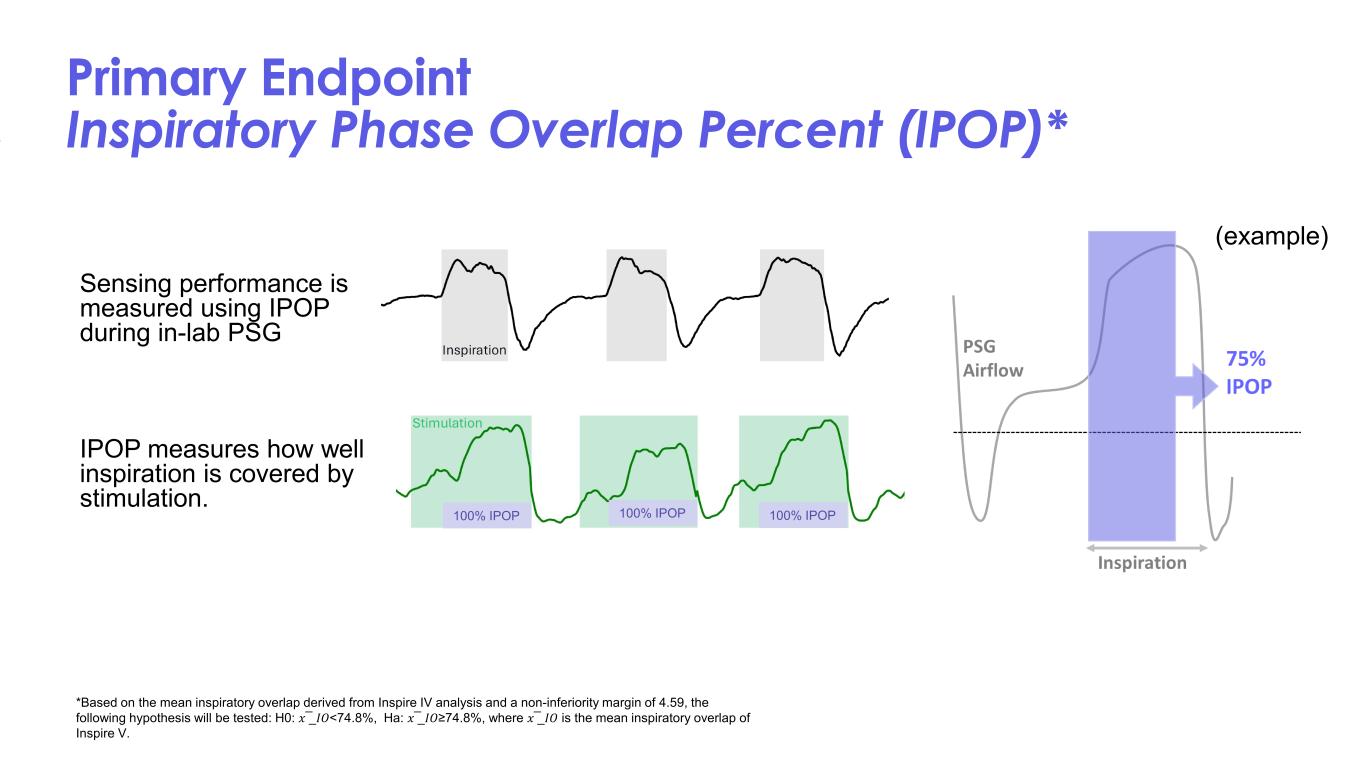
Primary Endpoint Inspiratory Phase Overlap Percent (IPOP)* Sensing performance is measured using IPOP during in-lab PSG IPOP measures how well inspiration is covered by stimulation. *Based on the mean inspiratory overlap derived from Inspire IV analysis and a non-inferiority margin of 4.59, the following hypothesis will be tested: H0: 𝑥𝑥 ̅_𝐼𝐼𝑂𝑂<74.8%, Ha: 𝑥𝑥 ̅_𝐼𝐼𝑂𝑂≥74.8%, where 𝑥𝑥 ̅_𝐼𝐼𝑂𝑂 is the mean inspiratory overlap of Inspire V. (example) 100% IPOP100% IPOP 100% IPOP
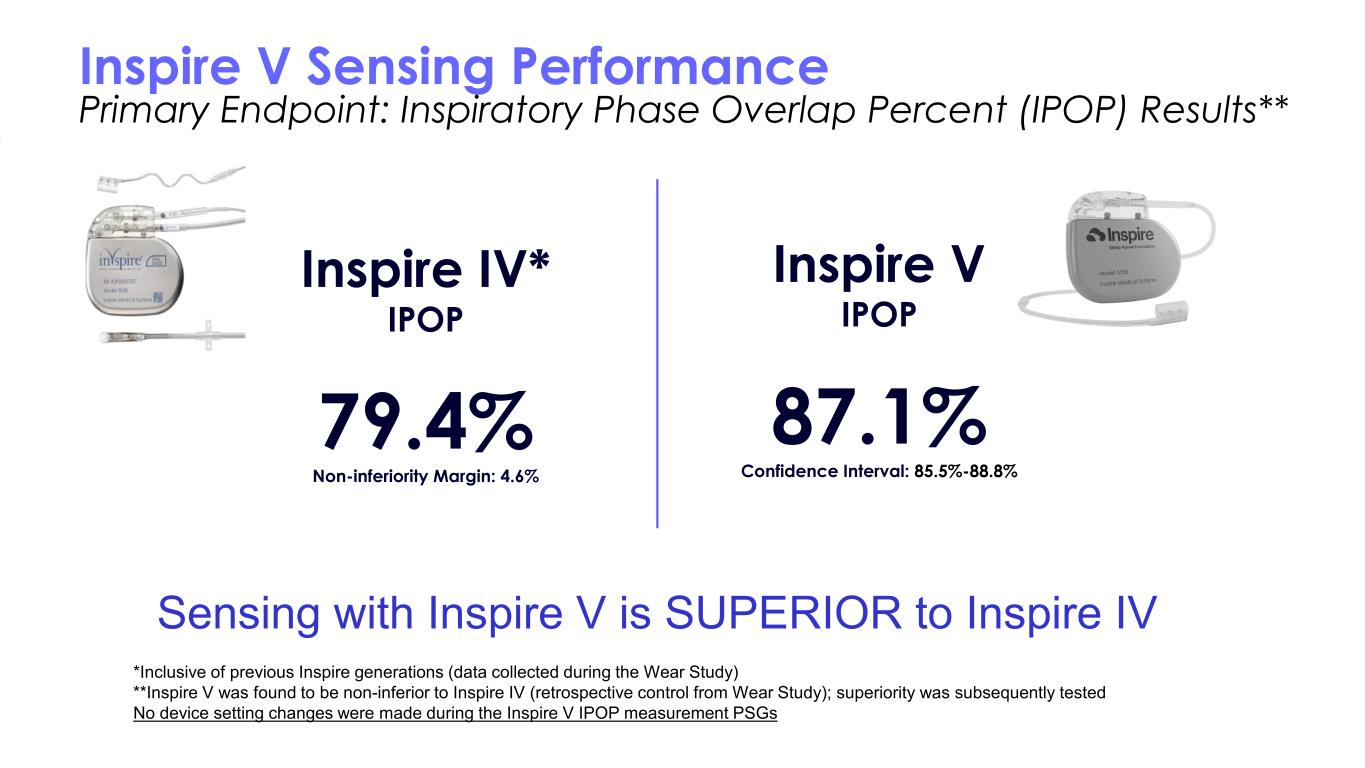
Inspire V Sensing Performance Primary Endpoint: Inspiratory Phase Overlap Percent (IPOP) Results** Inspire V IPOP 87.1% Confidence Interval: 85.5%-88.8% Inspire IV* IPOP 79.4% Non-inferiority Margin: 4.6% Sensing with Inspire V is SUPERIOR to Inspire IV *Inclusive of previous Inspire generations (data collected during the Wear Study) **Inspire V was found to be non-inferior to Inspire IV (retrospective control from Wear Study); superiority was subsequently tested No device setting changes were made during the Inspire V IPOP measurement PSGs
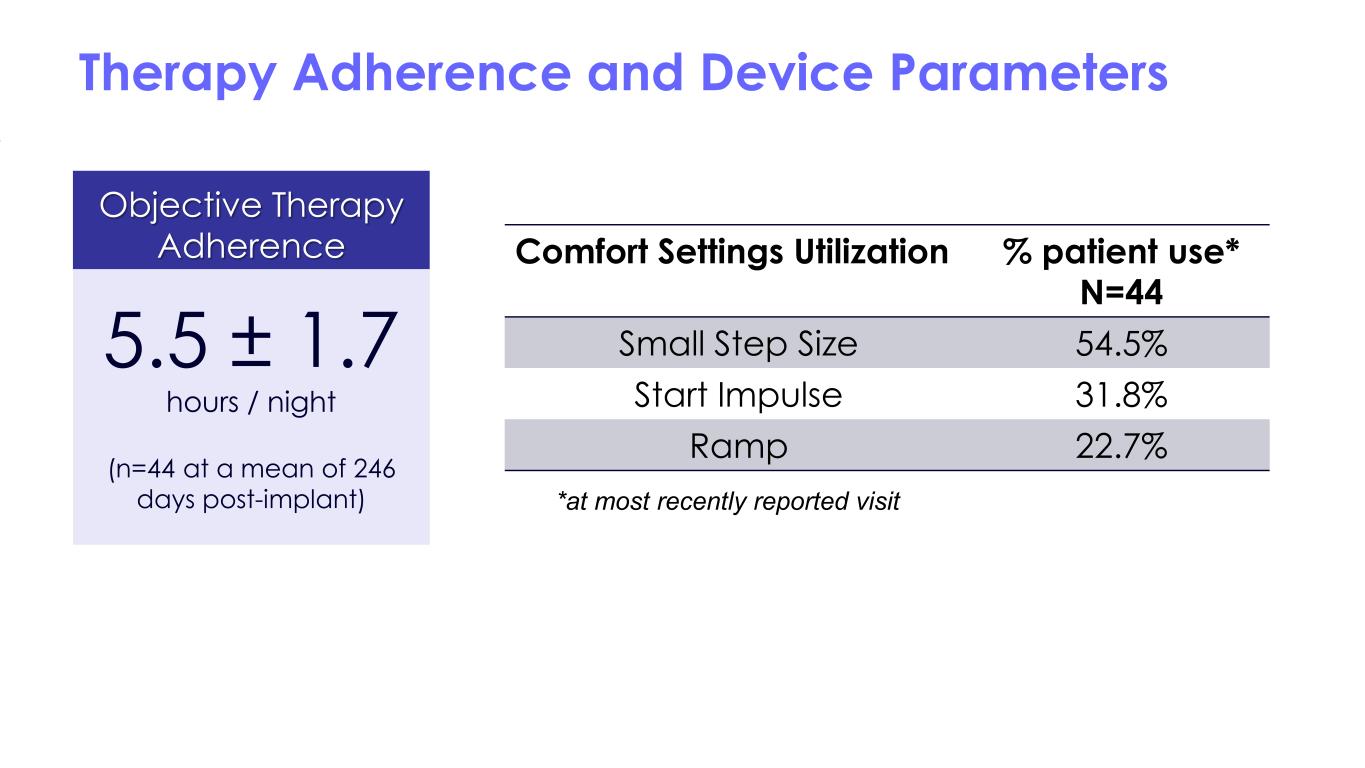
Therapy Adherence and Device Parameters Comfort Settings Utilization % patient use* N=44 Small Step Size 54.5% Start Impulse 31.8% Ramp 22.7% Objective Therapy Adherence 5.5 ± 1.7 hours / night (n=44 at a mean of 246 days post-implant) *at most recently reported visit
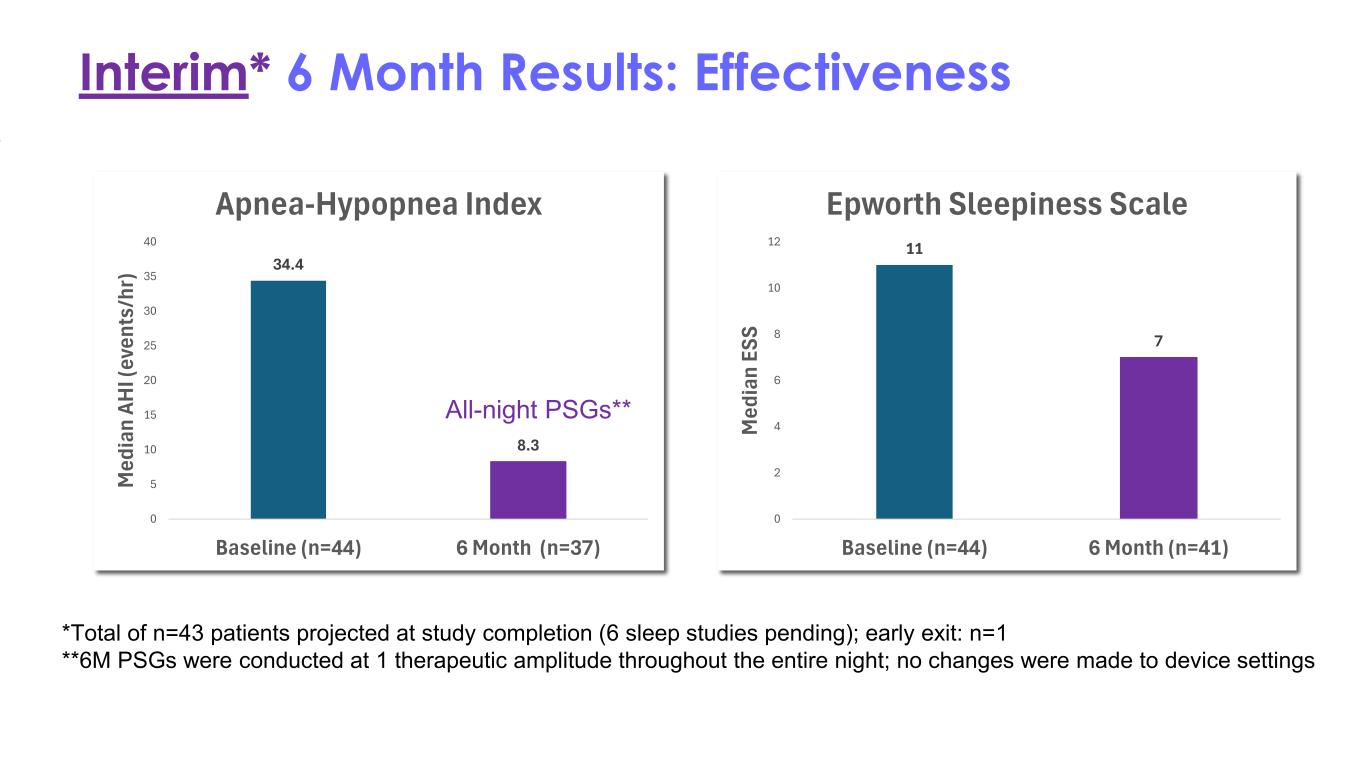
Interim* 6 Month Results: Effectiveness *Total of n=43 patients projected at study completion (6 sleep studies pending); early exit: n=1 **6M PSGs were conducted at 1 therapeutic amplitude throughout the entire night; no changes were made to device settings 34.4 8.3 0 5 10 15 20 25 30 35 40 Baseline (n=44) 6 Month (n=37) M ed ia n AH I ( ev en ts /h r) Apnea-Hypopnea Index 11 7 0 2 4 6 8 10 12 Baseline (n=44) 6 Month (n=41) M ed ia n ES S Epworth Sleepiness Scale All-night PSGs**

Interim 6 Month Results: Safety • No revisions/explants • Serious Adverse Events • Wound dehiscence (resolved): 2 • Patients provided antibiotics and event resolved within two weeks post-op • Non-serious Adverse Events • Neuropraxia (resolved): 1 • Right marginal mandibular weakness (resolved): 1 • Difficulty speaking (resolved): 1 • Hypotension during implant (resolved): 1 • Hypertrophic scar: 3

• Simplified implant procedure • 20.4% reduction in implant time • Elimination of respiratory sense lead • Accelerometer technology • Inspire V sensing is superior to previous Inspire generations • Strong objective therapy adherence at 5.5 hrs/night • Clinically significant reductions in OSA severity • Clinically significant improvements in key quality of life measures Inspire V Clinical Study Conclusions

© 2024 Inspire Medical Systems, Inc. All Rights Reserved Inspire V Limited Market Release Data as of October 3, 2025
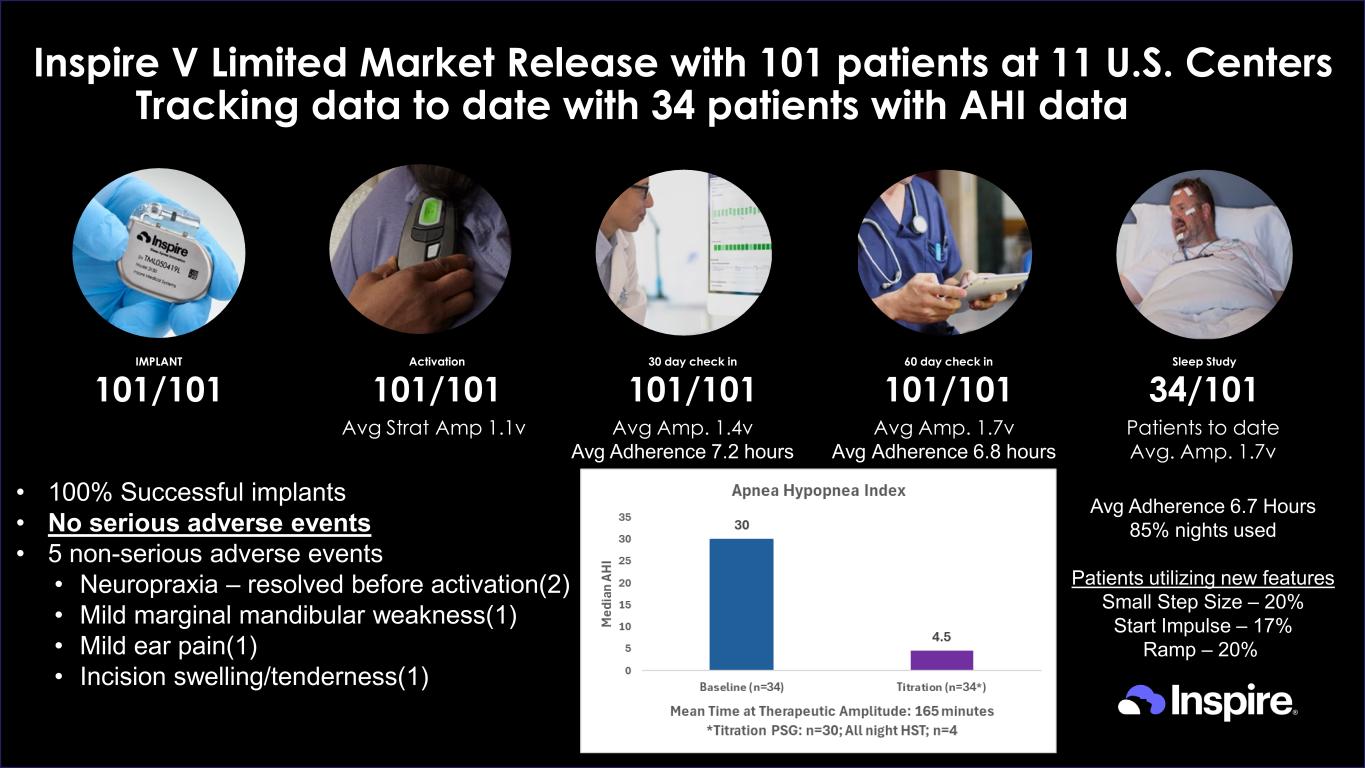
© Inspire Medical Systems, Inc. All Rights Reserved Inspire V Limited Market Release with 101 patients at 11 U.S. Centers Tracking data to date with 34 patients with AHI data 60 day check in 101/101 Activation 101/101 30 day check in 101/101 IMPLANT 101/101 Sleep Study 34/101 Avg Strat Amp 1.1v Patients to date Avg. Amp. 1.7v Avg Adherence 6.7 Hours 85% nights used Patients utilizing new features Small Step Size – 20% Start Impulse – 17% Ramp – 20% Avg Amp. 1.4v Avg Adherence 7.2 hours Avg Amp. 1.7v Avg Adherence 6.8 hours • 100% Successful implants • No serious adverse events • 5 non-serious adverse events • Neuropraxia – resolved before activation(2) • Mild marginal mandibular weakness(1) • Mild ear pain(1) • Incision swelling/tenderness(1)

Thank You. © 2024 Inspire Medical Systems, Inc. All Rights Reserved

Inspire V Experience at MEE Phil Huyett, MD Director of Sleep Surgery Massachusetts Eye and Ear Assistant Professor Harvard Medical School
Disclosures • Inspire medical systems – Research support – Educational consultant • Nyxoah – Research support • Part of limited release in February • Still having discussions with insurance!
Inspire V • Faster/less general anesthesia time • No more respiratory sensor revisions • ?reduced PTX % • ?faster recovery • ?less post op pain
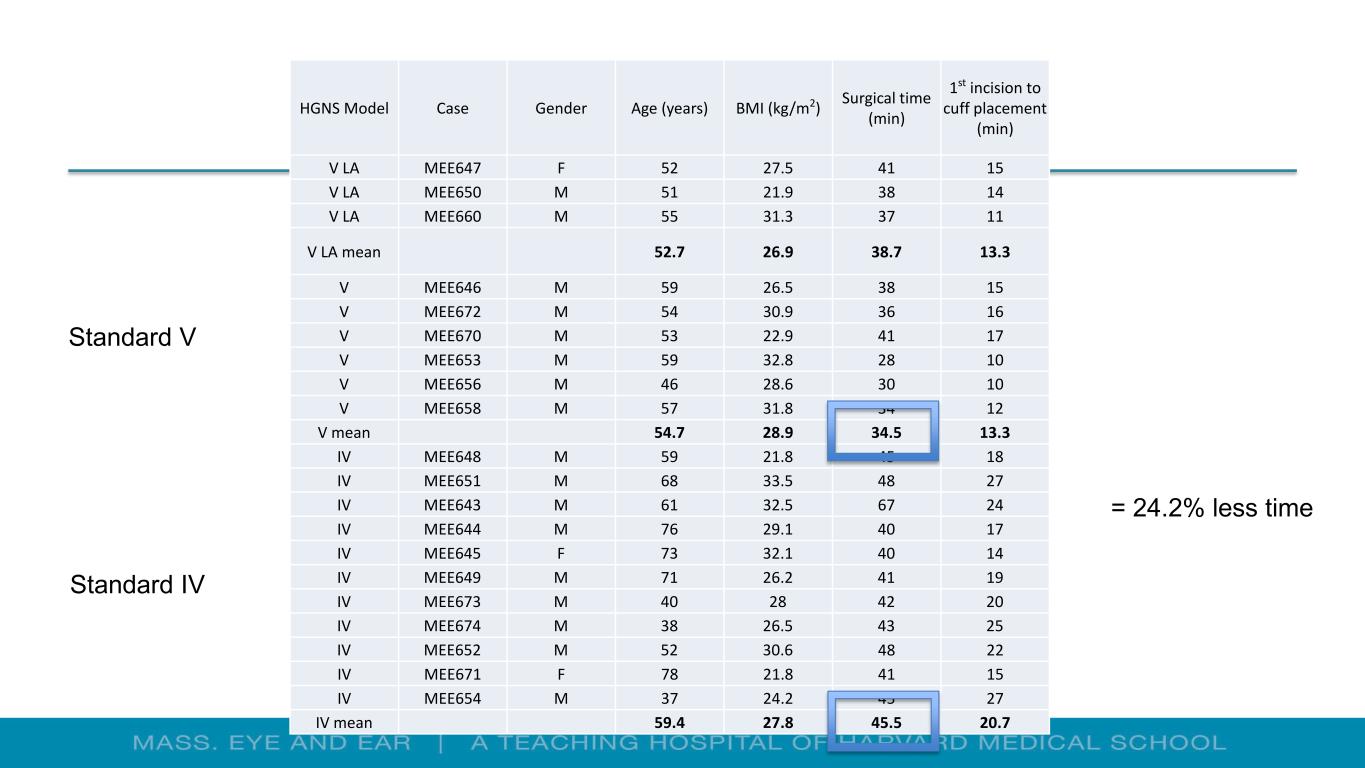
HGNS Model Case Gender Age (years) BMI (kg/m2) Surgical time (min) 1st incision to cuff placement (min) V LA MEE647 F 52 27.5 41 15 V LA MEE650 M 51 21.9 38 14 V LA MEE660 M 55 31.3 37 11 V LA mean 52.7 26.9 38.7 13.3 V MEE646 M 59 26.5 38 15 V MEE672 M 54 30.9 36 16 V MEE670 M 53 22.9 41 17 V MEE653 M 59 32.8 28 10 V MEE656 M 46 28.6 30 10 V MEE658 M 57 31.8 34 12 V mean 54.7 28.9 34.5 13.3 IV MEE648 M 59 21.8 45 18 IV MEE651 M 68 33.5 48 27 IV MEE643 M 61 32.5 67 24 IV MEE644 M 76 29.1 40 17 IV MEE645 F 73 32.1 40 14 IV MEE649 M 71 26.2 41 19 IV MEE673 M 40 28 42 20 IV MEE674 M 38 26.5 43 25 IV MEE652 M 52 30.6 48 22 IV MEE671 F 78 21.8 41 15 IV MEE654 M 37 24.2 45 27 IV mean 59.4 27.8 45.5 20.7 = 24.2% less time Standard IV Standard V
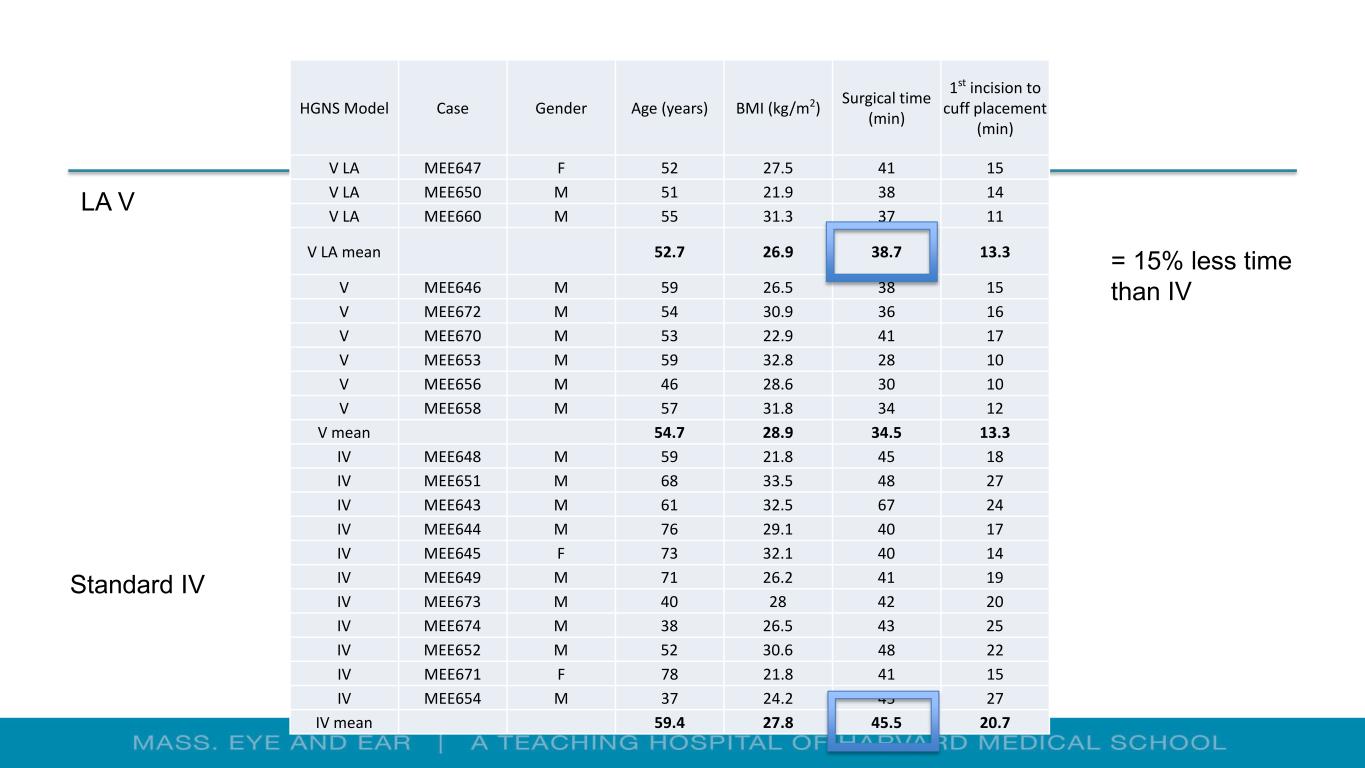
HGNS Model Case Gender Age (years) BMI (kg/m2) Surgical time (min) 1st incision to cuff placement (min) V LA MEE647 F 52 27.5 41 15 V LA MEE650 M 51 21.9 38 14 V LA MEE660 M 55 31.3 37 11 V LA mean 52.7 26.9 38.7 13.3 V MEE646 M 59 26.5 38 15 V MEE672 M 54 30.9 36 16 V MEE670 M 53 22.9 41 17 V MEE653 M 59 32.8 28 10 V MEE656 M 46 28.6 30 10 V MEE658 M 57 31.8 34 12 V mean 54.7 28.9 34.5 13.3 IV MEE648 M 59 21.8 45 18 IV MEE651 M 68 33.5 48 27 IV MEE643 M 61 32.5 67 24 IV MEE644 M 76 29.1 40 17 IV MEE645 F 73 32.1 40 14 IV MEE649 M 71 26.2 41 19 IV MEE673 M 40 28 42 20 IV MEE674 M 38 26.5 43 25 IV MEE652 M 52 30.6 48 22 IV MEE671 F 78 21.8 41 15 IV MEE654 M 37 24.2 45 27 IV mean 59.4 27.8 45.5 20.7 = 15% less time than IV Standard IV LA V
Inspire V LA surgical pearls • IPG replacement probably more challenging • Doesn’t add significant time • Be sure to parachute IPG medially • Alternative to other HGNS systems for patients who don’t want anterior chest incision

Initial Outcomes Inspire V Nic Beckmann, DO Colorado ENT and Allergy Colorado Springs, CO
Inspire V SYSTEM The Fifth Generation of a proven therapy for OSA is designed for a: • Simplified procedure • Enhanced patient comfort • More efficient patient management
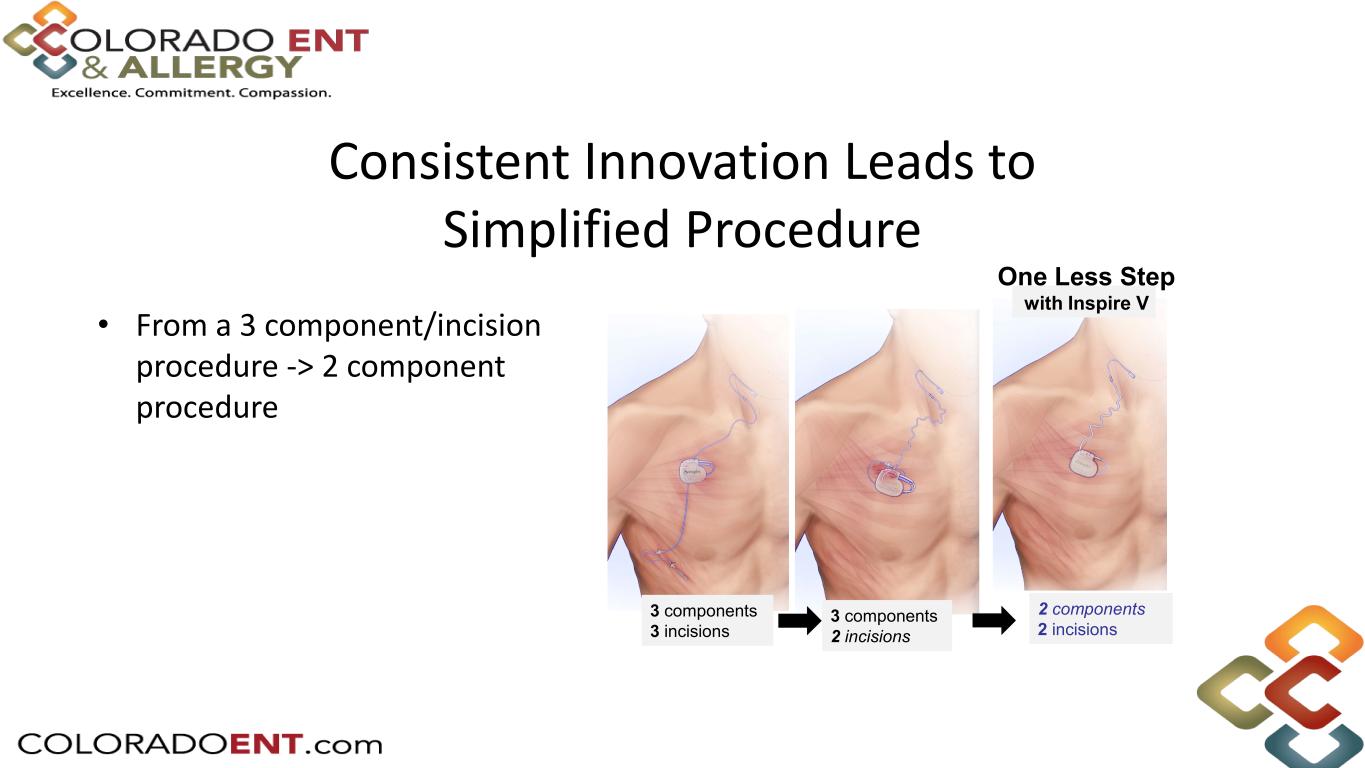
• From a 3 component/incision procedure -> 2 component procedure Consistent Innovation Leads to Simplified Procedure 2 components 2 incisions 3 components 2 incisions 3 components 3 incisions One Less Step with Inspire V
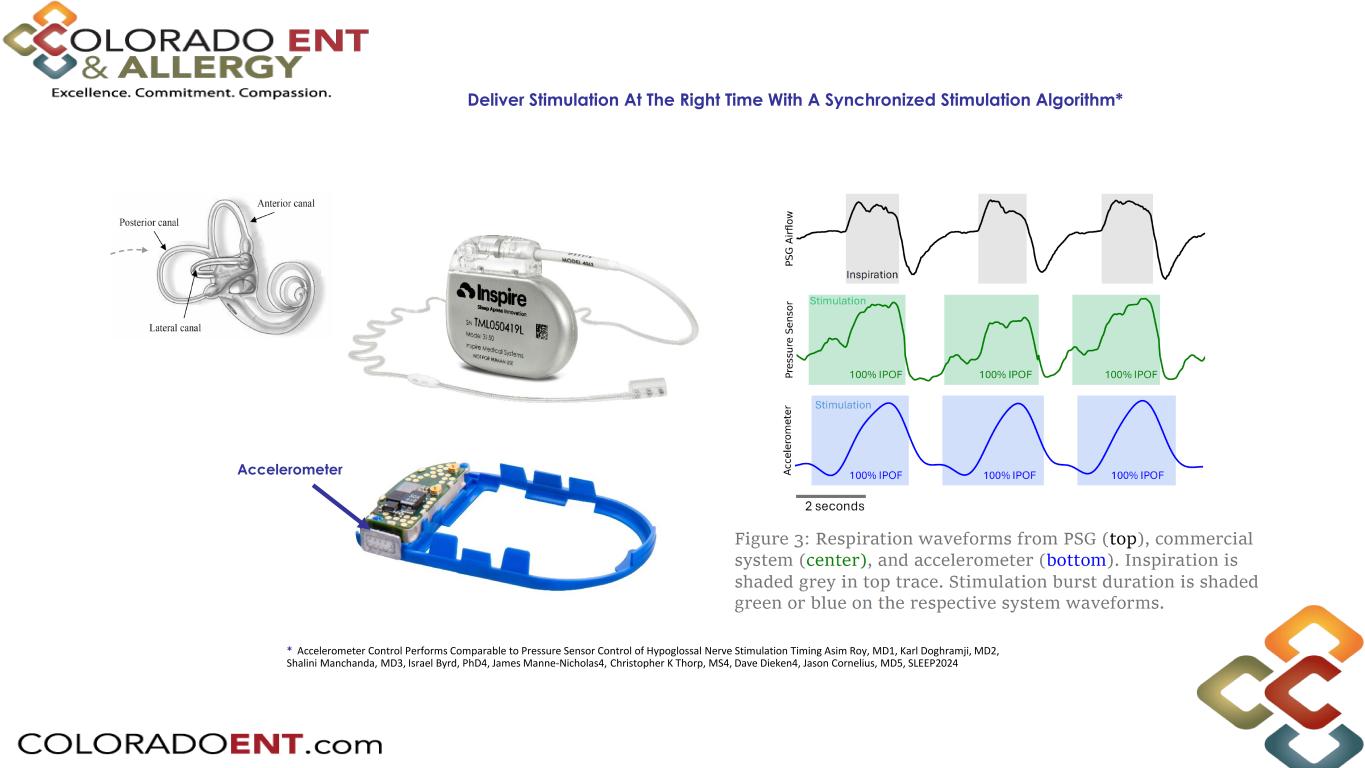
Deliver Stimulation At The Right Time With A Synchronized Stimulation Algorithm* * Accelerometer Control Performs Comparable to Pressure Sensor Control of Hypoglossal Nerve Stimulation Timing Asim Roy, MD1, Karl Doghramji, MD2, Shalini Manchanda, MD3, Israel Byrd, PhD4, James Manne-Nicholas4, Christopher K Thorp, MS4, Dave Dieken4, Jason Cornelius, MD5, SLEEP2024 Accelerometer

34 Tailored Therapy Options Designed for Patient Comfort Program patient therapy levels at half or quarter step sizes with Inspire V when needed Stimulation Step Sizes Gently initiate therapy, without disturbing sleep with Inspire V when needed Ramp Option 5, 10, 15 minute options
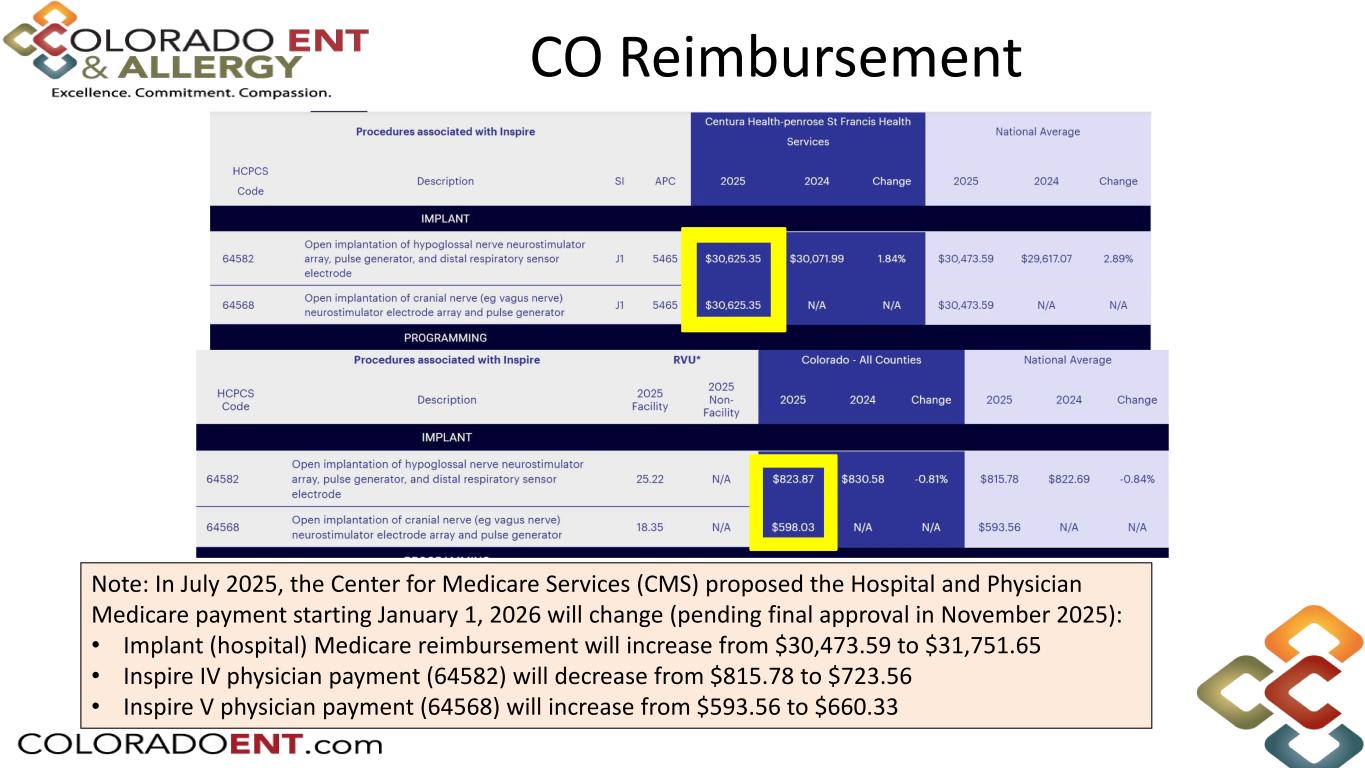
CO Reimbursement Note: In July 2025, the Center for Medicare Services (CMS) proposed the Hospital and Physician Medicare payment starting January 1, 2026 will change (pending final approval in November 2025): • Implant (hospital) Medicare reimbursement will increase from $30,473.59 to $31,751.65 • Inspire IV physician payment (64582) will decrease from $815.78 to $723.56 • Inspire V physician payment (64568) will increase from $593.56 to $660.33
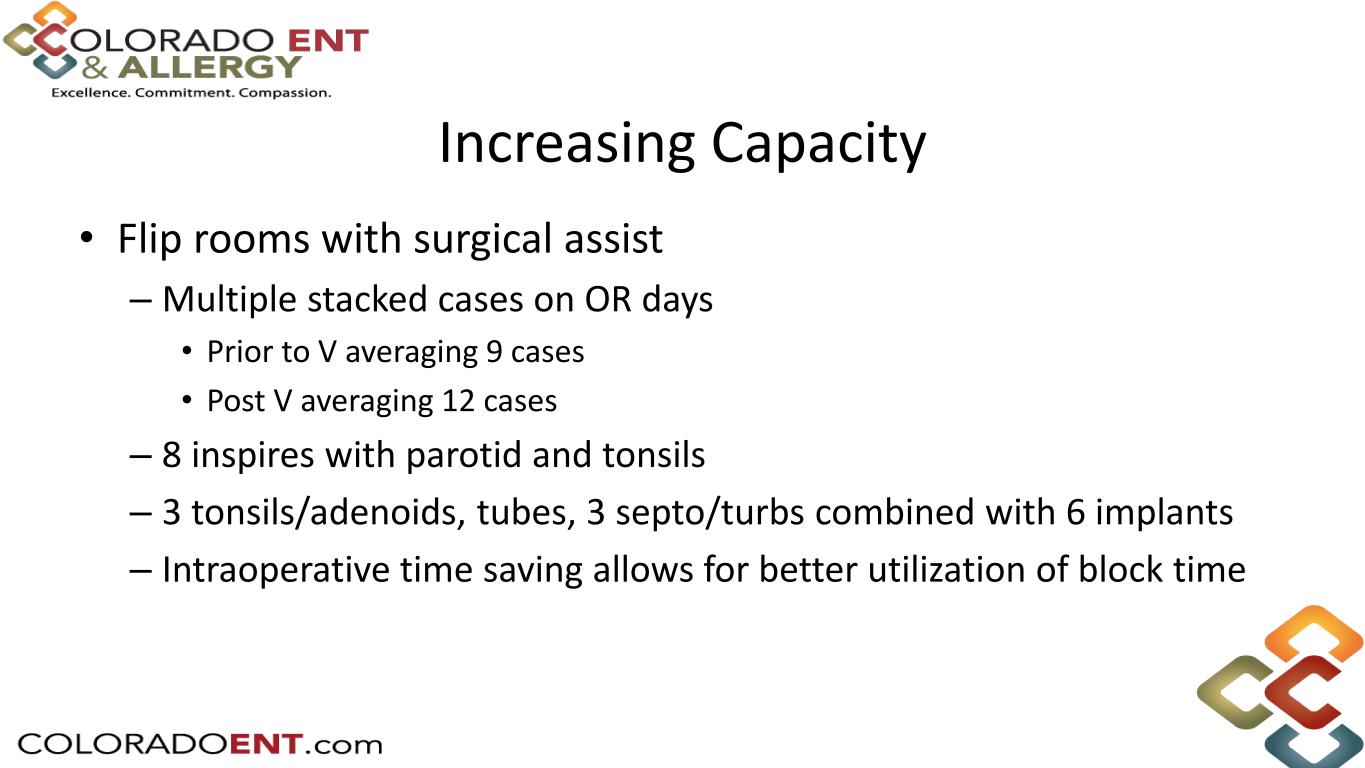
• Flip rooms with surgical assist – Multiple stacked cases on OR days • Prior to V averaging 9 cases • Post V averaging 12 cases – 8 inspires with parotid and tonsils – 3 tonsils/adenoids, tubes, 3 septo/turbs combined with 6 implants – Intraoperative time saving allows for better utilization of block time Increasing Capacity
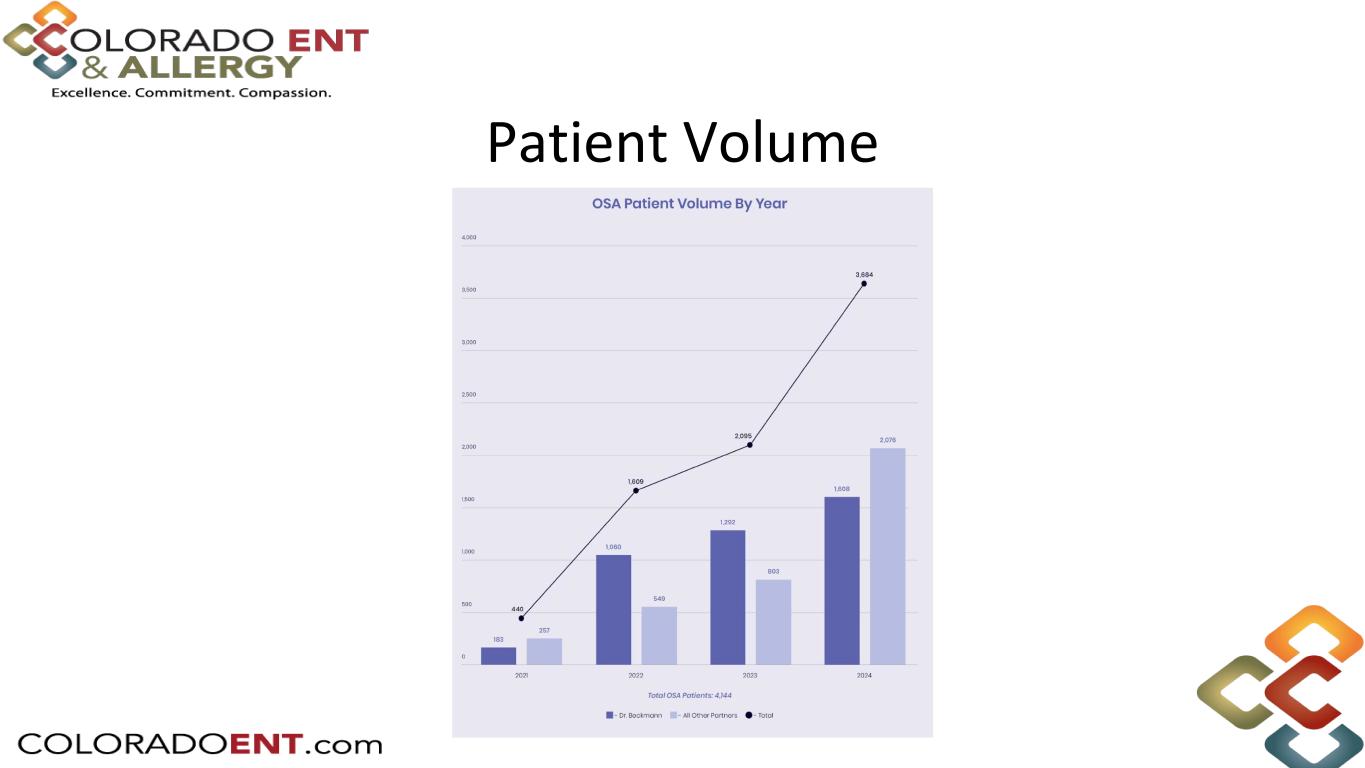
Patient Volume
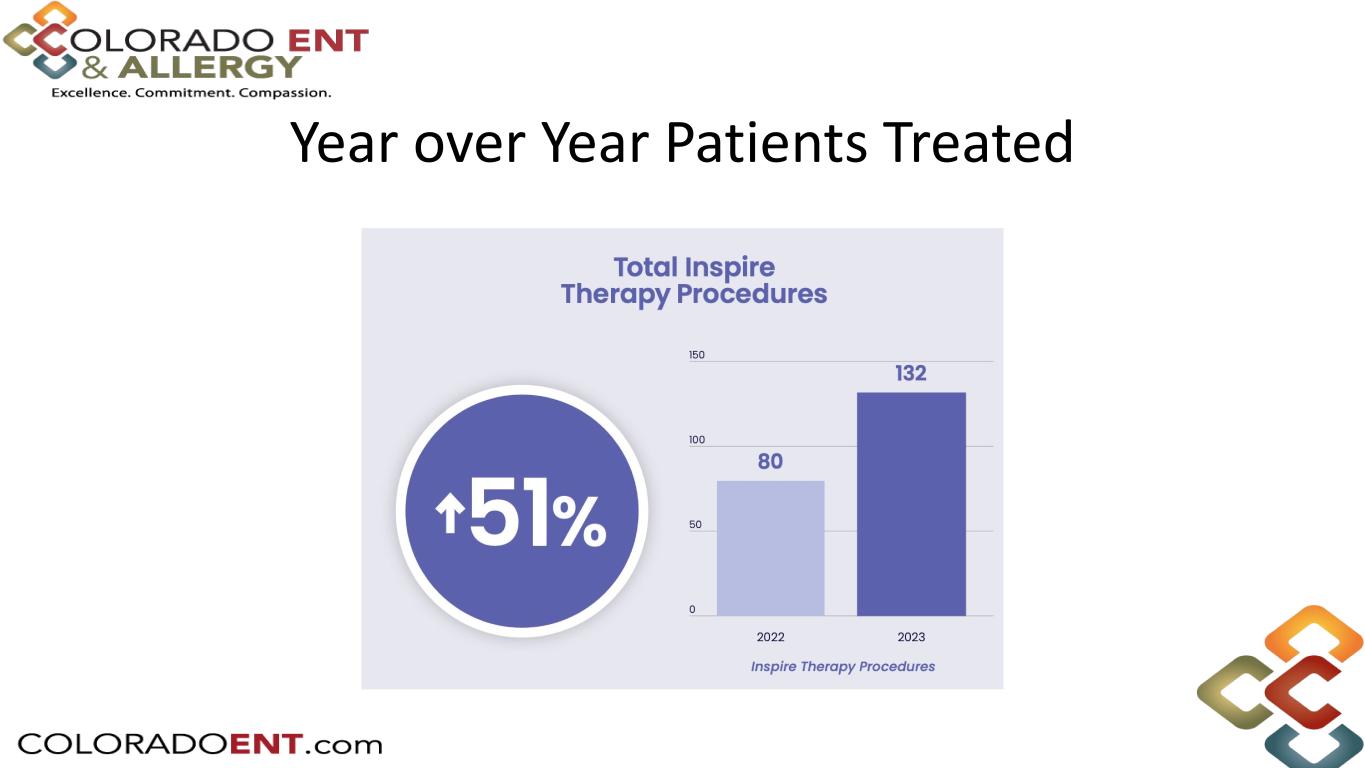
Year over Year Patients Treated

• Questions Thank you!