
Inspire Medical Systems, Inc. JP Morgan Healthcare Conference January 12, 2026 NYSE: INSP

© Inspire Medical Systems, Inc. All Rights Reserved. Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as ‘‘may,’’ ‘‘will,’’ ‘‘should,’’ ‘‘expect,’’ ‘‘plan,’’ ‘‘anticipate,’’ ‘‘could,’’ “future,” “outlook,” ‘‘intend,’’ ‘‘target,’’ ‘‘project,’’ ‘‘contemplate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘continue,’’ or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. The forward-looking statements in this presentation relate to, among other things, statements regarding our preliminary, unaudited fourth quarter and full year results for fiscal 2025, our expectations regarding full year 2026 financial outlook, coding and reimbursement rates for our therapy, the planned investments in our business, our growth strategies, the expected timing of regulatory approval and market introduction for new products, the potential impact that our growth strategies and initiatives may have on our business, the ability of our SleepSync digital health platform to drive growth, and improvements in patient flow, care pathway capacity, market access, clinical data growth, product development, indication expansion, market development, and prior authorization approvals. These forward-looking statements are based on management’s current expectations and involve known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, our financial results may fluctuate significantly and may not fully reflect the underlying performance of our business; our history of operating losses and dependency on our Inspire therapy for revenues; commercial success and market acceptance of our Inspire therapy; our ability to achieve and maintain adequate and clear levels of coverage or reimbursement for our Inspire therapy or any future products we may seek to commercialize; competitive companies, technologies and pharmaceuticals in our industry; our involvement in current or future legal disputes or regulatory proceedings; our ability to expand our indications and develop and commercialize additional products and enhancements to our Inspire therapy; future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; our ability to accurately forecast customer demand for our Inspire therapy and manage our inventory; our dependence on third-party suppliers, vendors, and contract manufacturers; consolidation in the healthcare industry; our ability to expand, manage and maintain our direct sales and marketing organization, and to market and sell our Inspire therapy in markets outside of the U.S.; our ability to manage our growth; our ability to hire and retain our senior management and other highly qualified personnel; risk related to product liability claims and warranty claims; our ability to address quality issues that may arise with our Inspire therapy; our ability to successfully integrate any acquired business, products, or technologies; changes in global macroeconomic trends; our business model and strategic plans for products, technologies and business, including our implementation thereof; the impact of glucagon-like peptide 1 class of drugs on demand for our Inspire therapy; risks related to information technology and cybersecurity; our ability to commercialize or obtain regulatory approvals for our Inspire therapy, or the effect of delays in commercializing or obtaining regulatory approvals; and FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally. Other important factors that could cause actual results, performance or achievements to differ materially from those contemplated in this press release can be found under the captions “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations“ in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, as updated in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, and as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors page of our website at www.inspiresleep.com. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. We do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties. 2

enhancing patient lives through sleep innovation I t a l l s tarts and ends wi th our mission We are a medical technology company committed to
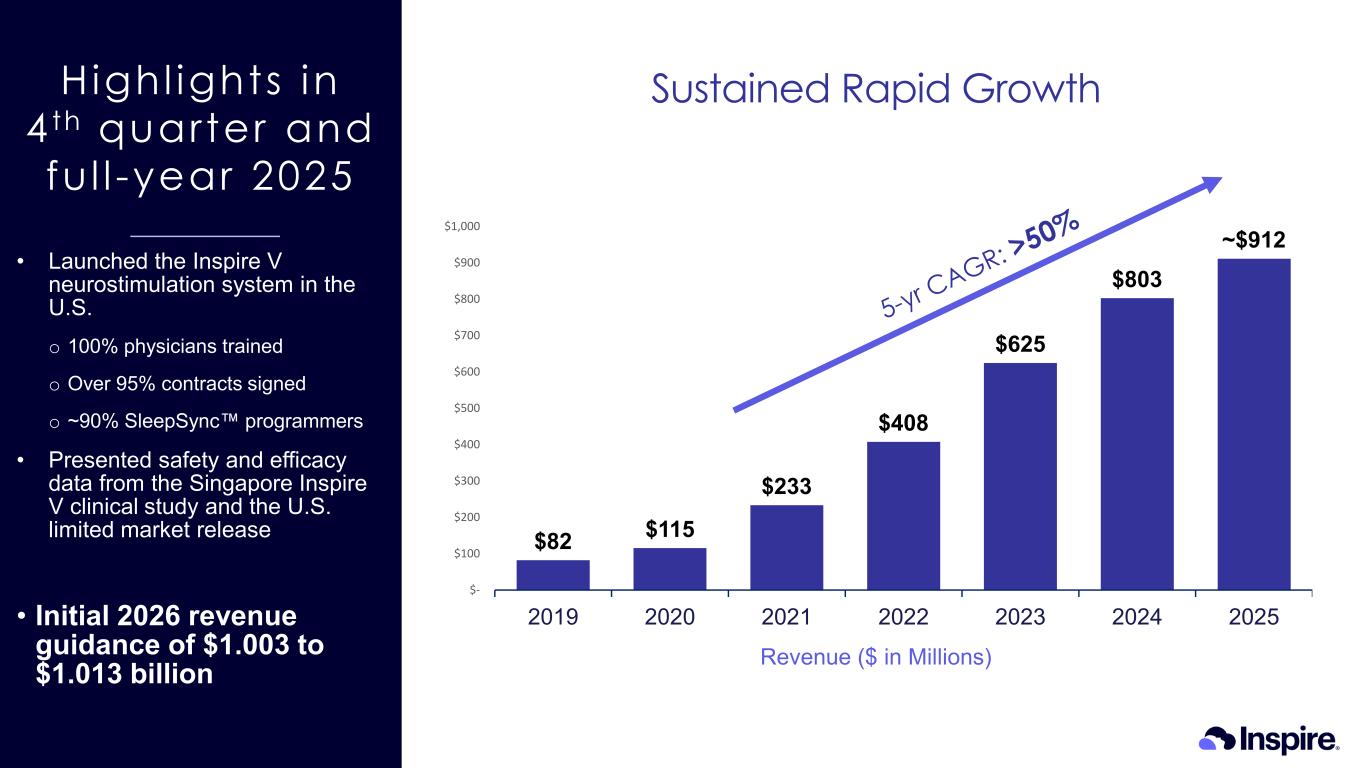
Highl ights in 4 th quarter and fu l l -year 2025 Sustained Rapid Growth $82 $115 $233 $408 $625 $803 ~$912 $- $100 $200 $300 $400 $500 $600 $700 $800 $900 $1,000 2019 2020 2021 2022 2023 2024 2025 • Launched the Inspire V neurostimulation system in the U.S. o 100% physicians trained o Over 95% contracts signed o ~90% SleepSync programmers • Presented safety and efficacy data from the Singapore Inspire V clinical study and the U.S. limited market release • Initial 2026 revenue guidance of $1.003 to $1.013 billion Revenue ($ in Millions)
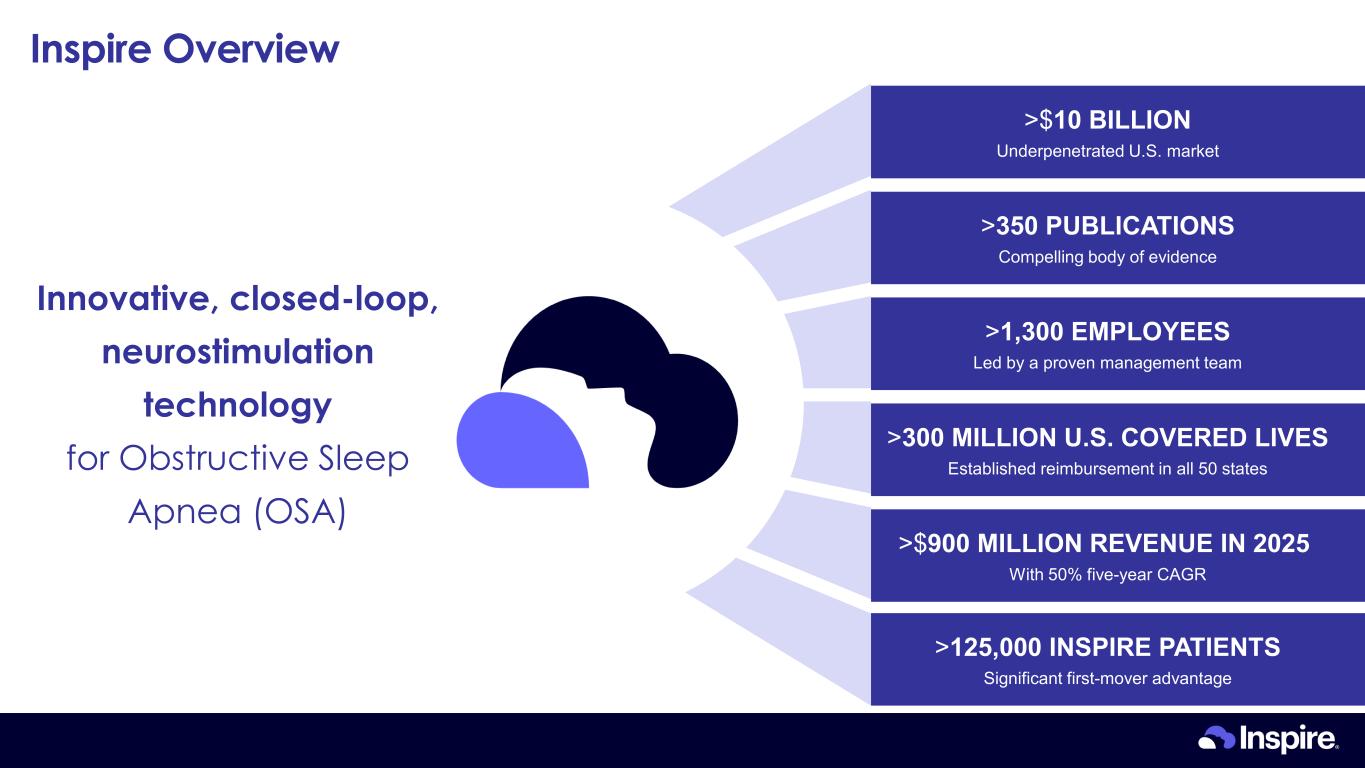
Inspire Overview >350 PUBLICATIONS Compelling body of evidence >300 MILLION U.S. COVERED LIVES Established reimbursement in all 50 states >1,300 EMPLOYEES Led by a proven management team >$900 MILLION REVENUE IN 2025 With 50% five-year CAGR >125,000 INSPIRE PATIENTS Significant first-mover advantage >$10 BILLION Underpenetrated U.S. market Innovative, closed-loop, neurostimulation technology for Obstructive Sleep Apnea (OSA)
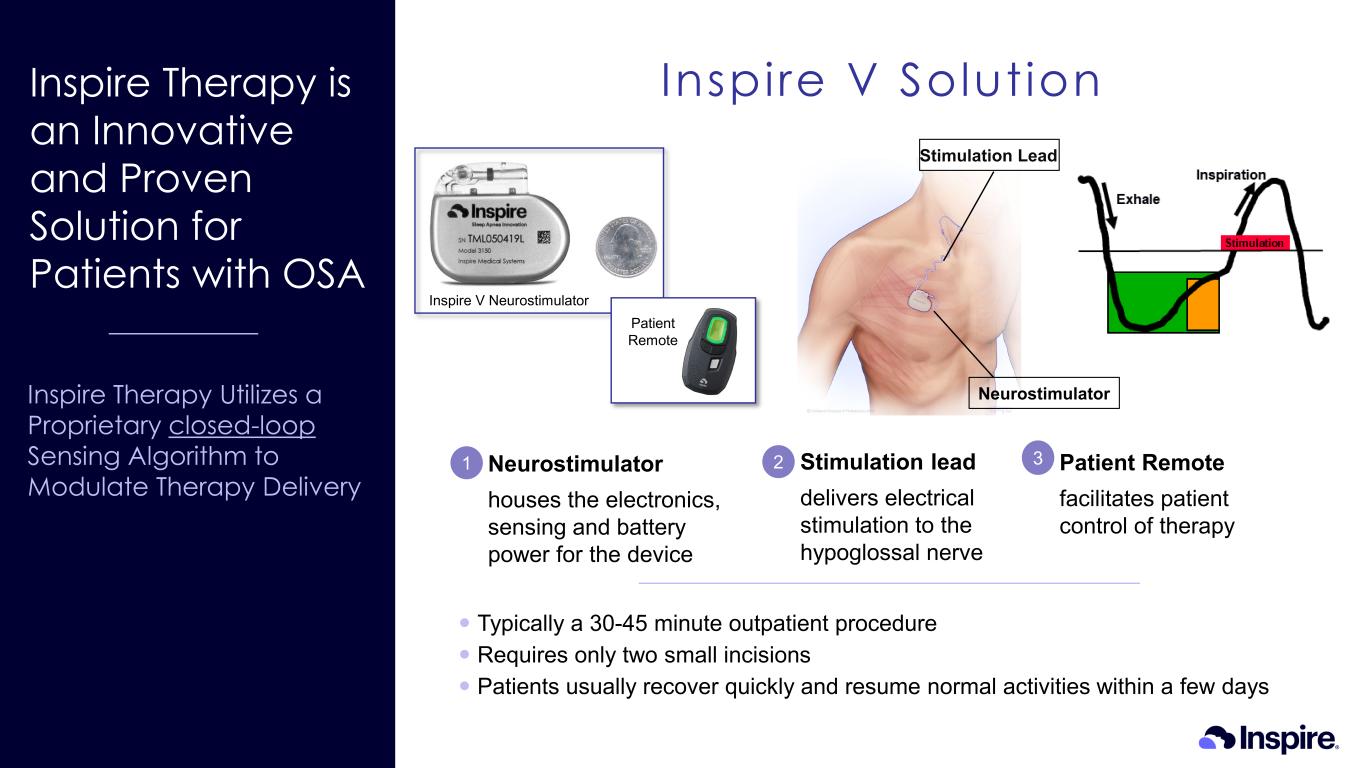
Inspire Therapy is an Innovative and Proven Solution for Patients with OSA Inspire Therapy Utilizes a Proprietary closed-loop Sensing Algorithm to Modulate Therapy Delivery Inspire V Solution 2 Typically a 30-45 minute outpatient procedure Requires only two small incisions Patients usually recover quickly and resume normal activities within a few days 31 Neurostimulator houses the electronics, sensing and battery power for the device Patient Remote facilitates patient control of therapy Stimulation lead delivers electrical stimulation to the hypoglossal nerve Stimulation Lead Neurostimulator Inspire V Neurostimulator Patient Remote
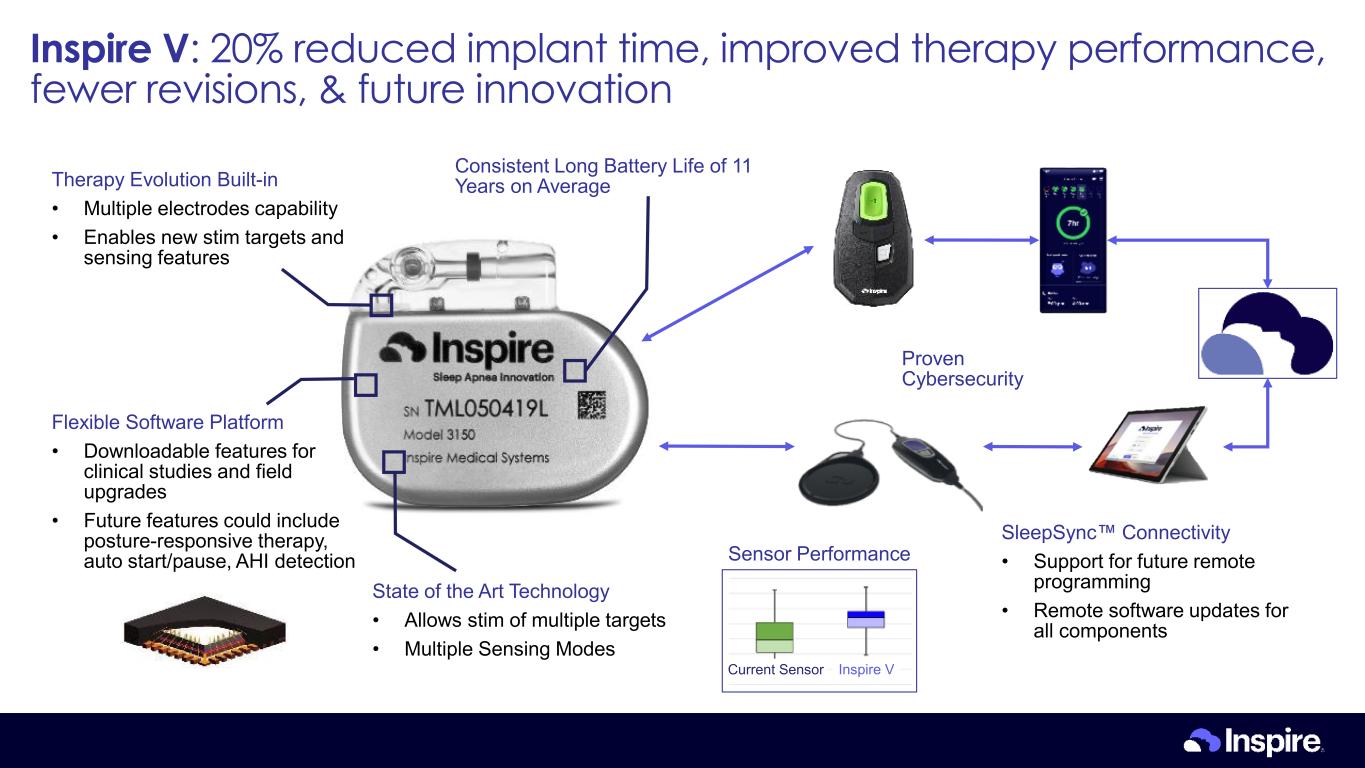
Current Sensor Inspire V Sensor Performance Therapy Evolution Built-in • Multiple electrodes capability • Enables new stim targets and sensing features Flexible Software Platform • Downloadable features for clinical studies and field upgrades • Future features could include posture-responsive therapy, auto start/pause, AHI detection State of the Art Technology • Allows stim of multiple targets • Multiple Sensing Modes Proven Cybersecurity Consistent Long Battery Life of 11 Years on Average SleepSync Connectivity • Support for future remote programming • Remote software updates for all components Inspire V: 20% reduced implant time, improved therapy performance, fewer revisions, & future innovation
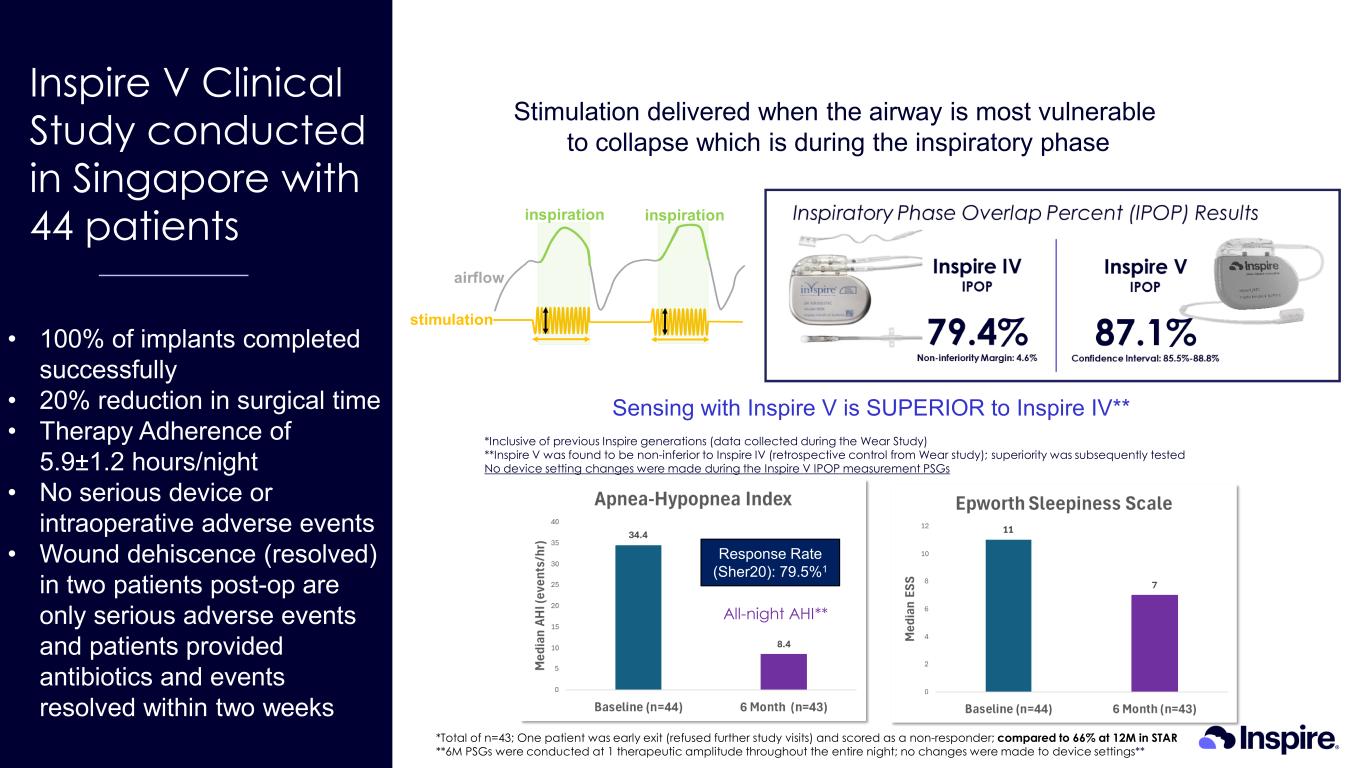
• 100% of implants completed successfully • 20% reduction in surgical time • Therapy Adherence of 5.9±1.2 hours/night • No serious device or intraoperative adverse events • Wound dehiscence (resolved) in two patients post-op are only serious adverse events and patients provided antibiotics and events resolved within two weeks Inspire V Clinical Study conducted in Singapore with 44 patients *Inclusive of previous Inspire generations (data collected during the Wear Study) **Inspire V was found to be non-inferior to Inspire IV (retrospective control from Wear study); superiority was subsequently tested No device setting changes were made during the Inspire V IPOP measurement PSGs *Total of n=43; One patient was early exit (refused further study visits) and scored as a non-responder; compared to 66% at 12M in STAR **6M PSGs were conducted at 1 therapeutic amplitude throughout the entire night; no changes were made to device settings** All-night AHI** Stimulation delivered when the airway is most vulnerable to collapse which is during the inspiratory phase Response Rate (Sher20): 79.5%1 Sensing with Inspire V is SUPERIOR to Inspire IV**
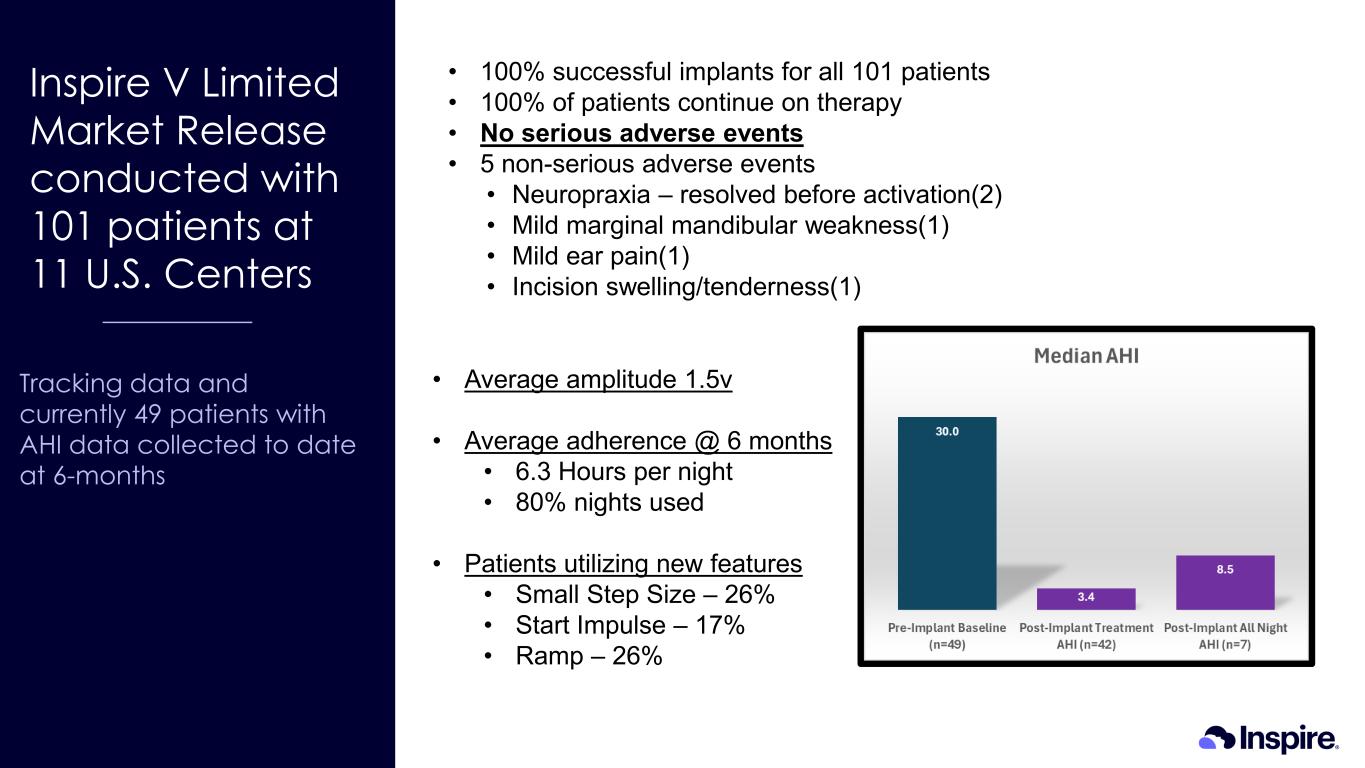
Inspire V Limited Market Release conducted with 101 patients at 11 U.S. Centers Tracking data and currently 49 patients with AHI data collected to date at 6-months • 100% successful implants for all 101 patients • 100% of patients continue on therapy • No serious adverse events • 5 non-serious adverse events • Neuropraxia – resolved before activation(2) • Mild marginal mandibular weakness(1) • Mild ear pain(1) • Incision swelling/tenderness(1) • Average amplitude 1.5v • Average adherence @ 6 months • 6.3 Hours per night • 80% nights used • Patients utilizing new features • Small Step Size – 26% • Start Impulse – 17% • Ramp – 26%
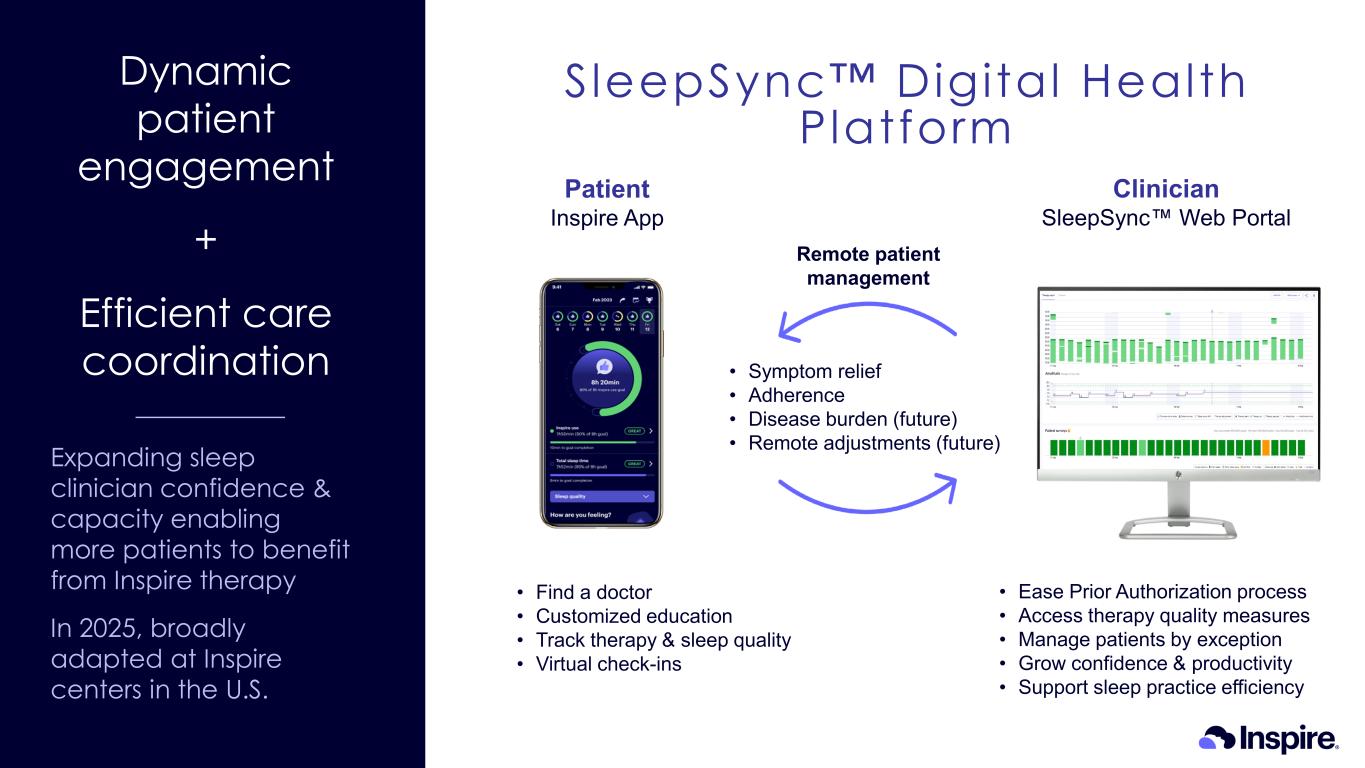
Dynamic patient engagement + Efficient care coordination Expanding sleep clinician confidence & capacity enabling more patients to benefit from Inspire therapy In 2025, broadly adapted at Inspire centers in the U.S. Remote patient management Patient Inspire App Clinician SleepSync Web Portal • Find a doctor • Customized education • Track therapy & sleep quality • Virtual check-ins • Ease Prior Authorization process • Access therapy quality measures • Manage patients by exception • Grow confidence & productivity • Support sleep practice efficiency • Symptom relief • Adherence • Disease burden (future) • Remote adjustments (future) SleepSync Digital Health Platform
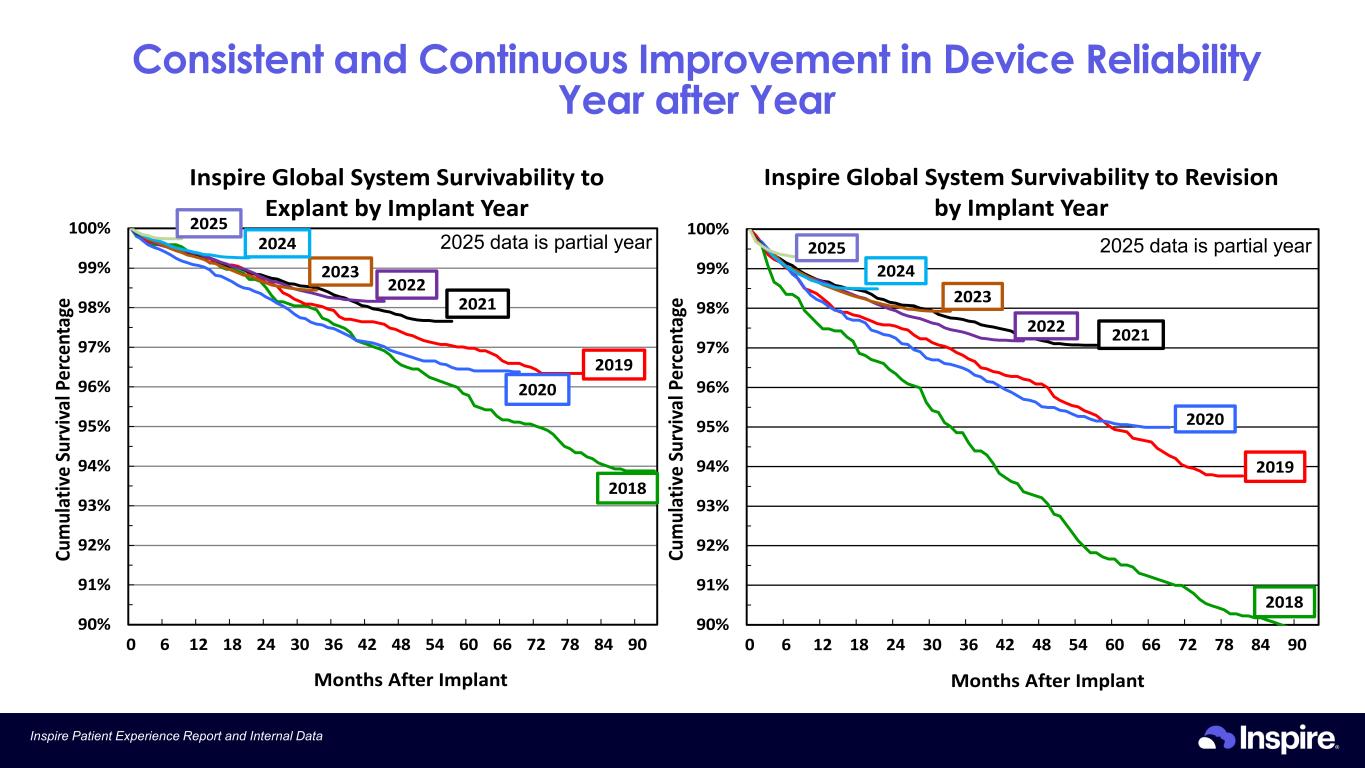
Consistent and Continuous Improvement in Device Reliability Year after Year 11Inspire Patient Experience Report and Internal Data 90% 91% 92% 93% 94% 95% 96% 97% 98% 99% 100% 0 6 12 18 24 30 36 42 48 54 60 66 72 78 84 90 Cu m ul at iv e Su rv iv al P er ce nt ag e Months After Implant Inspire Global System Survivability to Revision by Implant Year 2018 2020 2019 20212022 2023 2024 2025 90% 91% 92% 93% 94% 95% 96% 97% 98% 99% 100% 0 6 12 18 24 30 36 42 48 54 60 66 72 78 84 90 Cu m ul at iv e Su rv iv al P er ce nt ag e Months After Implant Inspire Global System Survivability to Explant by Implant Year 2018 2020 2019 2021 2022 2023 2024 2025 2025 data is partial year 2025 data is partial year
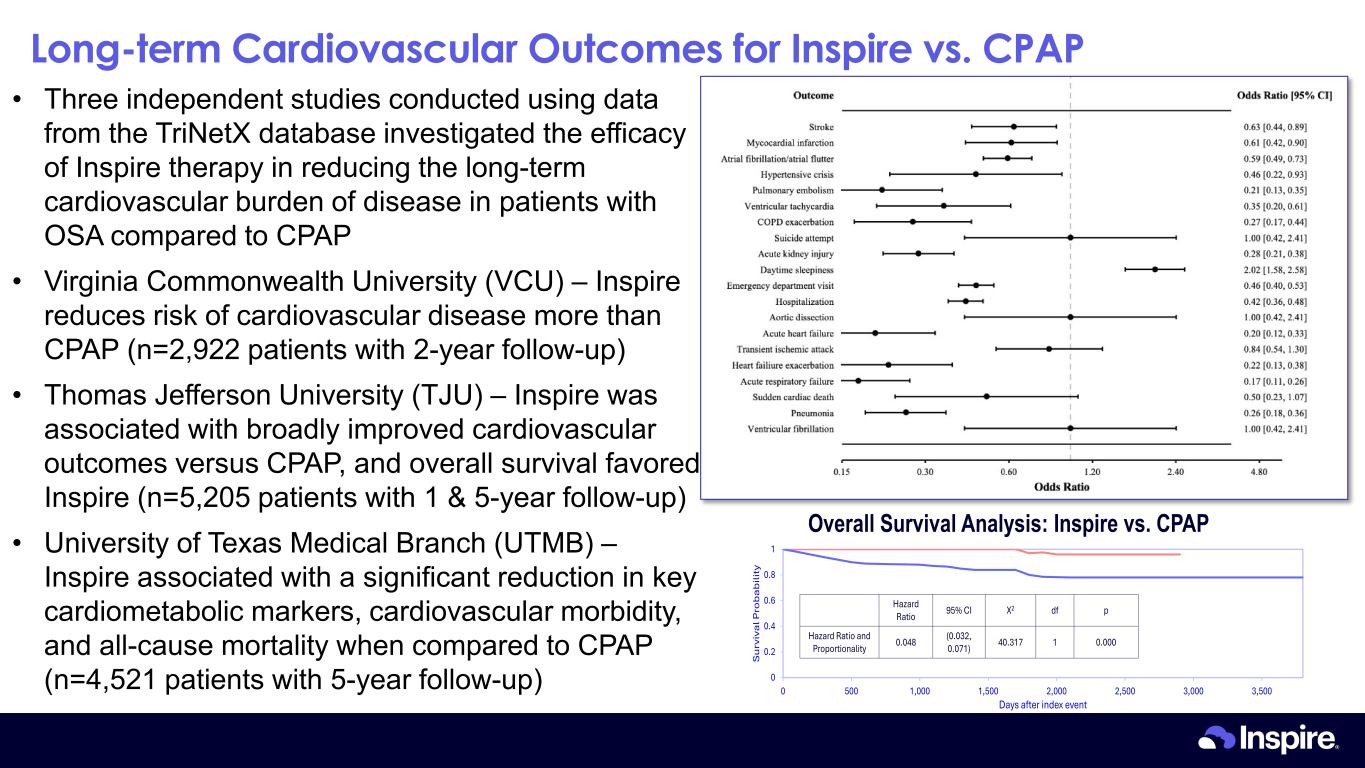
Long-term Cardiovascular Outcomes for Inspire vs. CPAP 12 • Three independent studies conducted using data from the TriNetX database investigated the efficacy of Inspire therapy in reducing the long-term cardiovascular burden of disease in patients with OSA compared to CPAP • Virginia Commonwealth University (VCU) – Inspire reduces risk of cardiovascular disease more than CPAP (n=2,922 patients with 2-year follow-up) • Thomas Jefferson University (TJU) – Inspire was associated with broadly improved cardiovascular outcomes versus CPAP, and overall survival favored Inspire (n=5,205 patients with 1 & 5-year follow-up) • University of Texas Medical Branch (UTMB) – Inspire associated with a significant reduction in key cardiometabolic markers, cardiovascular morbidity, and all-cause mortality when compared to CPAP (n=4,521 patients with 5-year follow-up) 0 0.2 0.4 0.6 0.8 1 0 500 1,000 1,500 2,000 2,500 3,000 3,500 S u rv iv a l P ro b a b ili ty Days after index event Overall Survival Analysis: Inspire vs. CPAP pdfX295% CIHazard Ratio 0.000140.317(0.032, 0.071)0.048Hazard Ratio and Proportionality

From our entrepreneurial beginnings, and with a focus on delivering life-changing outcomes, we’ve been enhancing the lives of patients for over 18 years… >125,000 Patients receiving Inspire >$900M Revenue >1,500 Implanters Founded 2007 IPO 2018 Today 4,000 Patients receiving Inspire $50M Revenue 200 Implanters With new innovations on the horizon and a big blue ocean of opportunity in front of us! … and we are still just getting started

CPAP prescriptions annually ~2,000,000 CPAP non-compliant ~700,000 Inspire eligible ~500,000 Adults with moderate to severe OSA ~23,000,000 >$10B opportuni ty The domestic OSA market is huge… Internal estimates

© Inspire Medical Systems, Inc. All Rights Reserved.15 All Rights Reserved. INSPIRE CONFIDENTIAL Living Room Rescue • This slide contains an embedded video

© Inspire Medical Systems, Inc. All Rights Reserved.16 All Rights Reserved. INSPIRE CONFIDENTIAL Scrooge • This slide contains an embedded video

© Inspire Medical Systems, Inc. All Rights Reserved.17 All Rights Reserved. INSPIRE CONFIDENTIAL Chock’s Secret • This slide contains an embedded video

Inspire in a strong position to start 2026 • Matt Osberg announced as new CFO coming to Inspire from Apogee, Helen of Troy and Best Buy – will start at Inspire in late January • Inspire V fully launched in the U.S. with very good adoption and significant improvements in surgical time and patient outcomes – very good Q4 momentum • SleepSync broadly adopted and provides an effective tool in data tracking and longitudinal patient management – additional utility tools in development • Overall health outcomes continue to emerge demonstrating Inspire as delivering improvements in both sleep and overall cardiac health • Providing initial revenue guidance of $1.003-$1.013 billion, and our outlook does not include any contribution from increased reimbursement at this time • We view the recent reimbursement developments as a positive step and are continuing to work with the relevant agencies to gain clarification on coding

No mask. No hose. Just sleep. ®

© Inspire Medical Systems, Inc. All Rights Reserved. Appendix 20

Sustainability at Inspire Committed to improving the economic, social, and environmental impacts that our business has on the communities in which we operate, as well as our customers, business partners, suppliers, employees, and stockholders. E N V I R O N M E N TA L We work to operate our business responsibly and reduce our impact on the environment wherever feasible. • Our Board and executive officers are responsible for oversight, identification, and communication of climate-related risks and opportunities. • We are focused on building out foundational programmatic elements and oversight that enable meaningful future reductions in our environmental impact. S O C I A L Product safety and quality are of the utmost importance at Inspire. We also pride ourselves on our innovative and collaborative work environment, which we believe has driven our success and which we seek to uphold through an inclusive workforce, generous compensation and benefits, open communication, a focus on employee health, well- being and engagement, and robust training and development programs. • Our company’s success is built on our enduring commitment to product quality and patient outcomes. • InspireGives is our community outreach program and the foundation of our charitable giving and volunteer efforts. • We aim to foster a culture of continuous learning with significant investments in our people through programs focused on leadership and professional development. G O V E R N A N C E We strive to maintain strong governance practices and high standards of ethics, compliance, and accountability designed to provide long-term value creation opportunities. • Our governance practices include regular consideration and assessment of our governance structure, board and committee function, and board and management succession. • Our strong and diverse Board collectively possesses a range of qualifications, skills, and experiences that align with our long-term strategy and business needs. • Sustainability matters are overseen by our Board, executive leadership, and cross- functional team.
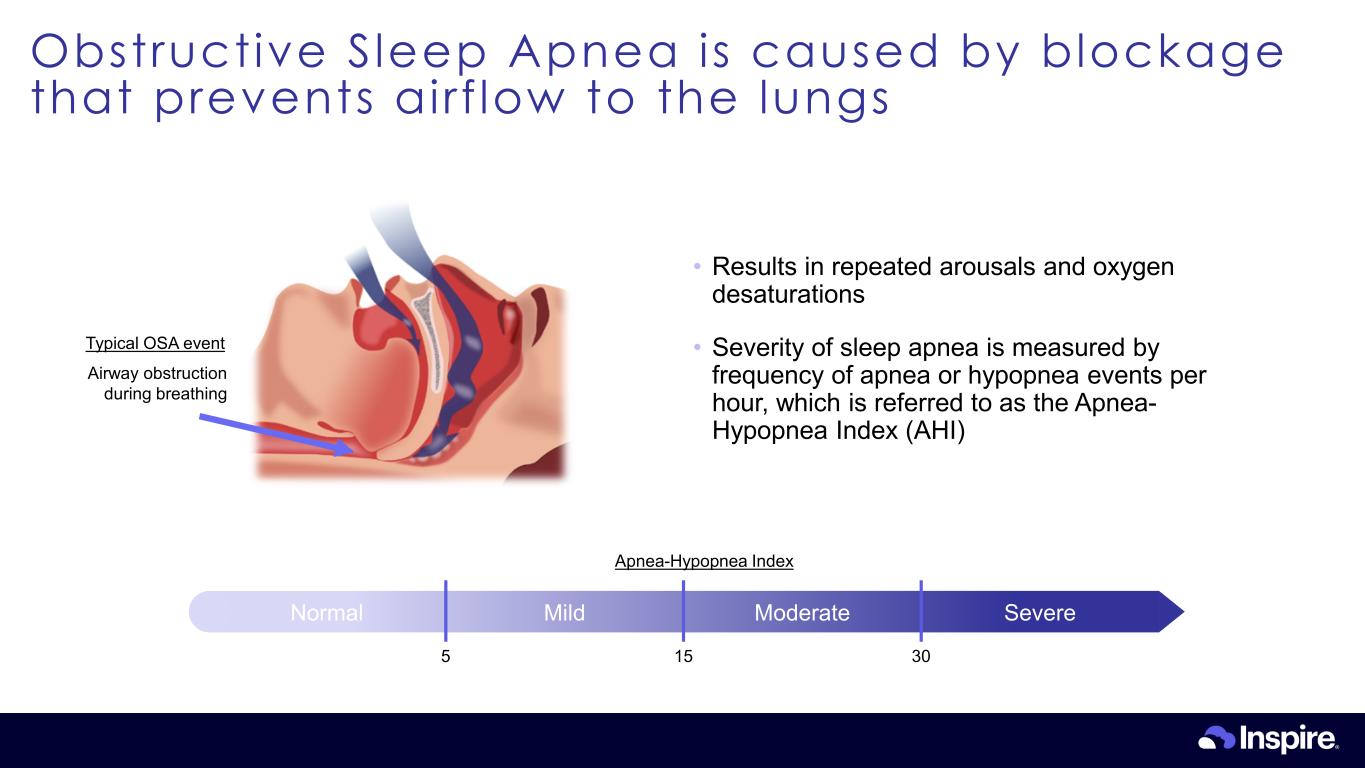
Obstructive Sleep Apnea is caused by blockage that prevents airf low to the lungs 22 Airway obstruction during breathing Typical OSA event • Results in repeated arousals and oxygen desaturations • Severity of sleep apnea is measured by frequency of apnea or hypopnea events per hour, which is referred to as the Apnea- Hypopnea Index (AHI) Normal Mild Moderate Severe 5 15 30 Apnea-Hypopnea Index
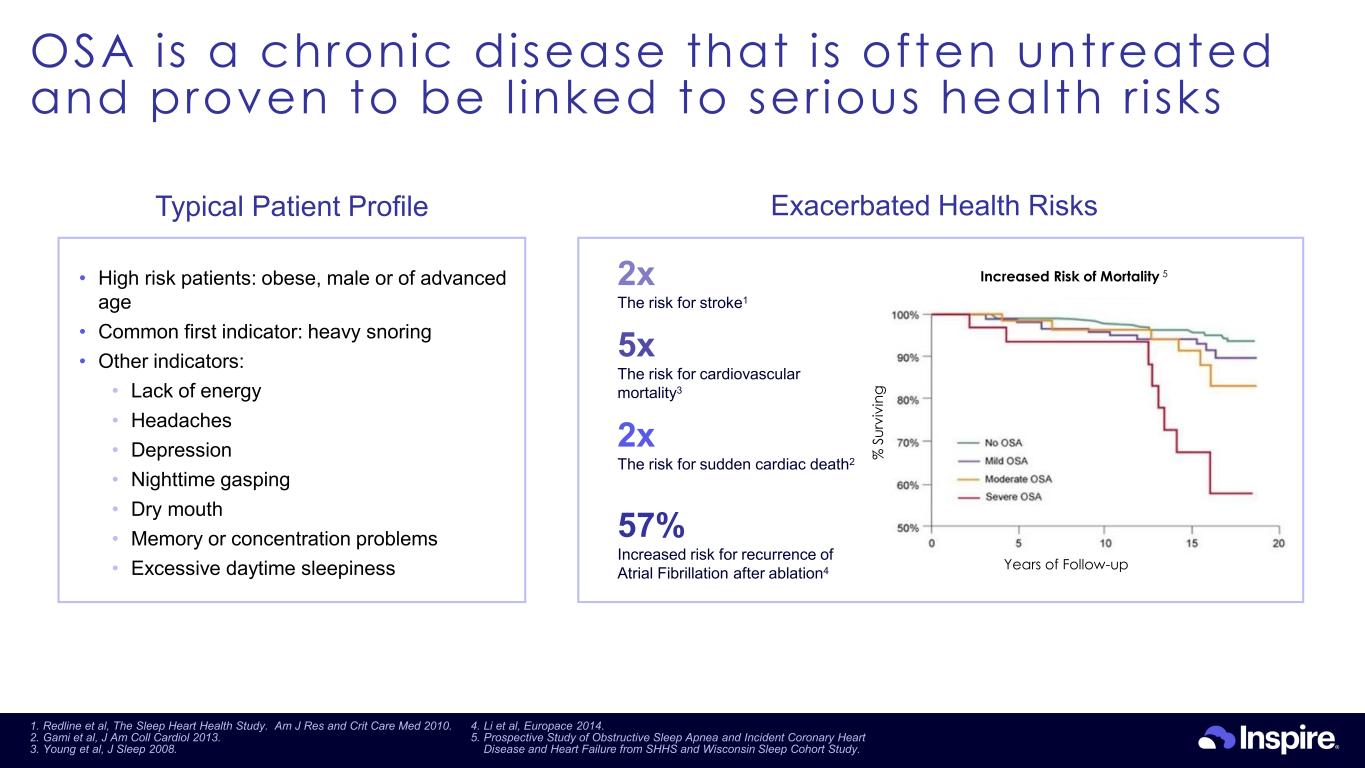
OSA is a chronic disease that is often untreated and proven to be l inked to ser ious health r isks 23 Exacerbated Health Risks • High risk patients: obese, male or of advanced age • Common first indicator: heavy snoring • Other indicators: • Lack of energy • Headaches • Depression • Nighttime gasping • Dry mouth • Memory or concentration problems • Excessive daytime sleepiness 2x The risk for stroke1 2x The risk for sudden cardiac death2 57% Increased risk for recurrence of Atrial Fibrillation after ablation4 5x The risk for cardiovascular mortality3 Years of Follow-up % S ur vi vi ng Increased Risk of Mortality 5 Typical Patient Profile 1. Redline et al, The Sleep Heart Health Study. Am J Res and Crit Care Med 2010. 2. Gami et al, J Am Coll Cardiol 2013. 3. Young et al, J Sleep 2008. 4. Li et al, Europace 2014. 5. Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure from SHHS and Wisconsin Sleep Cohort Study.
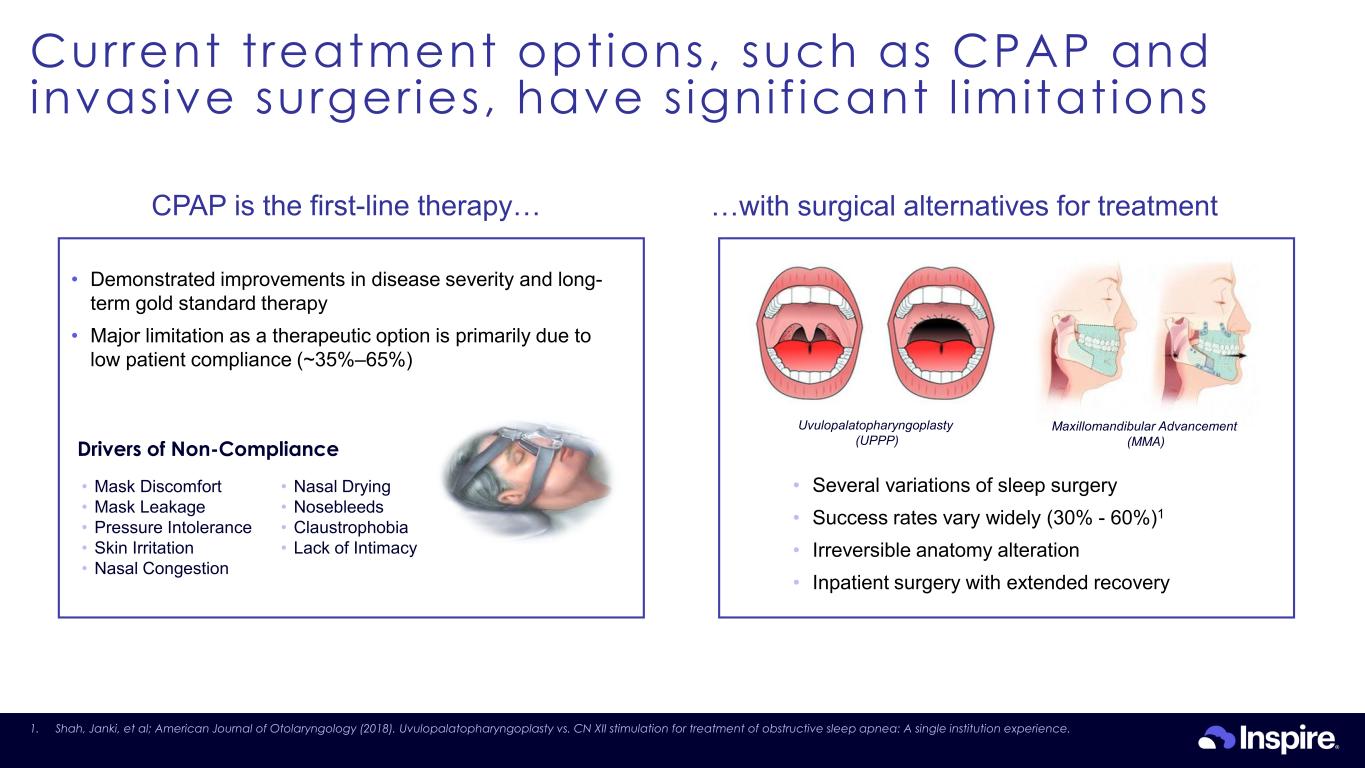
Current treatment options, such as CPAP and invasive surgeries, have s ignif icant l imitations 24 InaUvulopalatopharyngoplasty (UPPP) Maxillomandibular Advancement (MMA) • Several variations of sleep surgery • Success rates vary widely (30% - 60%)1 • Irreversible anatomy alteration • Inpatient surgery with extended recovery …with surgical alternatives for treatmentCPAP is the first-line therapy… Drivers of Non-Compliance • Demonstrated improvements in disease severity and long- term gold standard therapy • Major limitation as a therapeutic option is primarily due to low patient compliance (~35%–65%) • Mask Discomfort • Mask Leakage • Pressure Intolerance • Skin Irritation • Nasal Congestion • Nasal Drying • Nosebleeds • Claustrophobia • Lack of Intimacy 1. Shah, Janki, et al; American Journal of Otolaryngology (2018). Uvulopalatopharyngoplasty vs. CN XII stimulation for treatment of obstructive sleep apnea: A single institution experience.
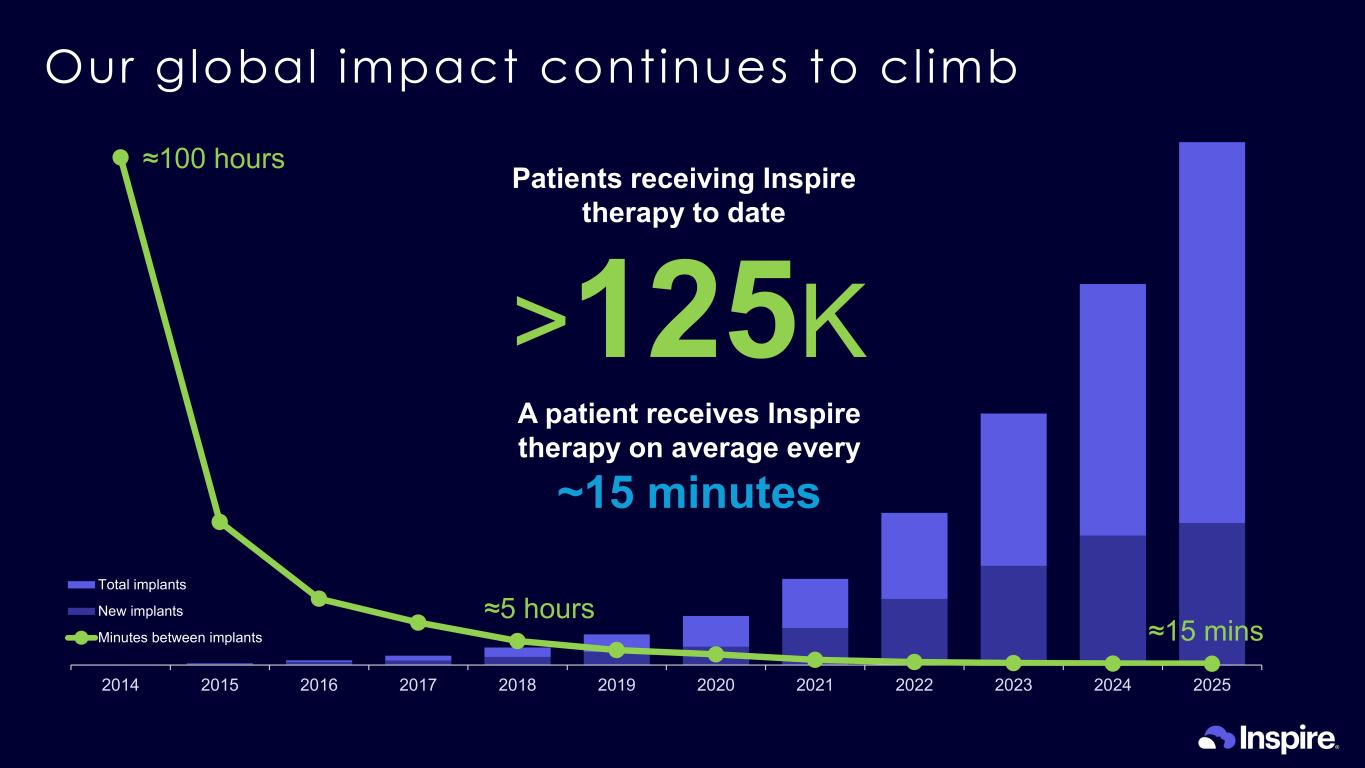
- 20,000 40,000 60,000 80,000 100,000 120,000 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 Total implants New implants Minutes between implants Our global impact continues to cl imb >125K Patients receiving Inspire therapy to date A patient receives Inspire therapy on average every ~15 minutes ≈100 hours ≈5 hours ≈15 mins
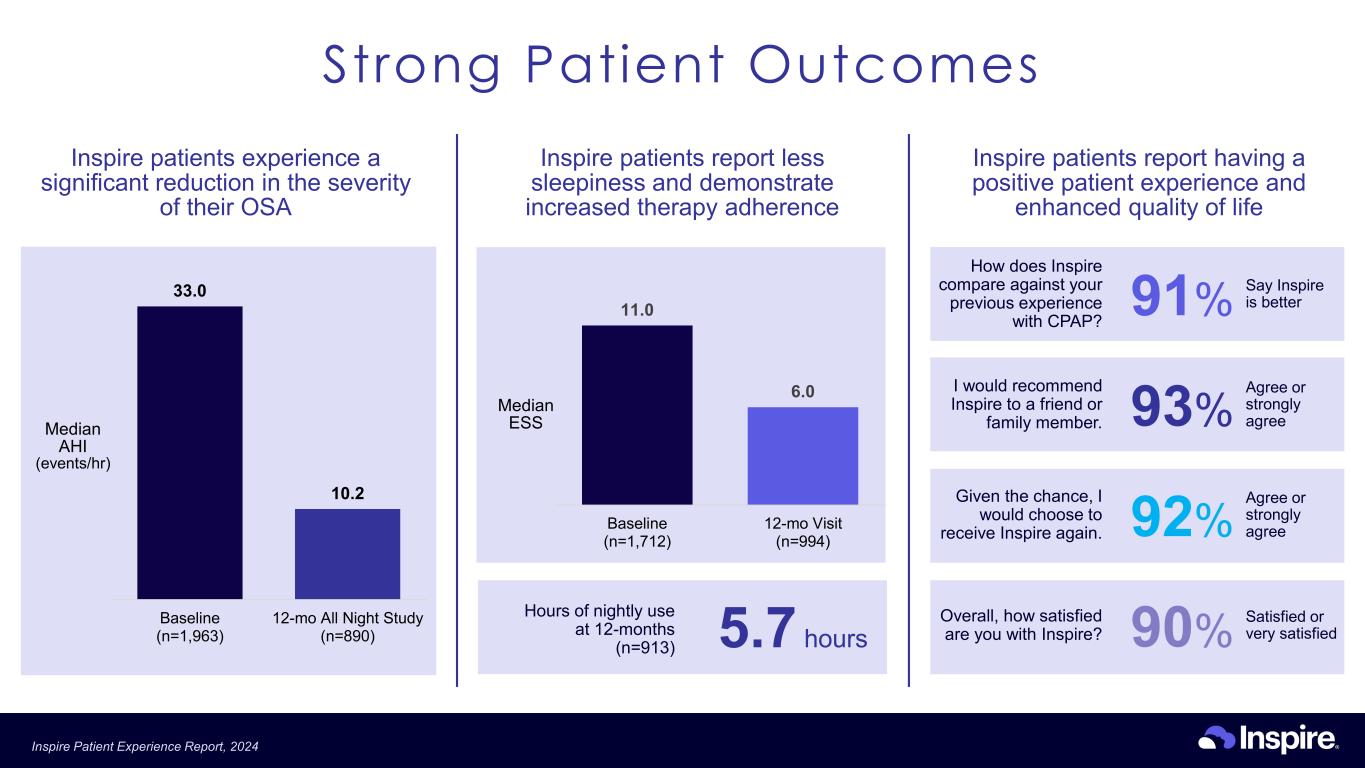
How does Inspire compare against your previous experience with CPAP? 91% Say Inspire is better I would recommend Inspire to a friend or family member. 93% Agree or strongly agree Overall, how satisfied are you with Inspire? 90% Satisfied or very satisfied Given the chance, I would choose to receive Inspire again. 92% Agree or strongly agree Inspire patients experience a significant reduction in the severity of their OSA 33.0 10.2 Baseline (n=1,963) 12-mo All Night Study (n=890) Median AHI (events/hr) Inspire patients report less sleepiness and demonstrate increased therapy adherence 11.0 6.0 Baseline (n=1,712) 12-mo Visit (n=994) Median ESS Hours of nightly use at 12-months (n=913) 5.7 hours Inspire patients report having a positive patient experience and enhanced quality of life Strong Patient Outcomes Inspire Patient Experience Report, 2024
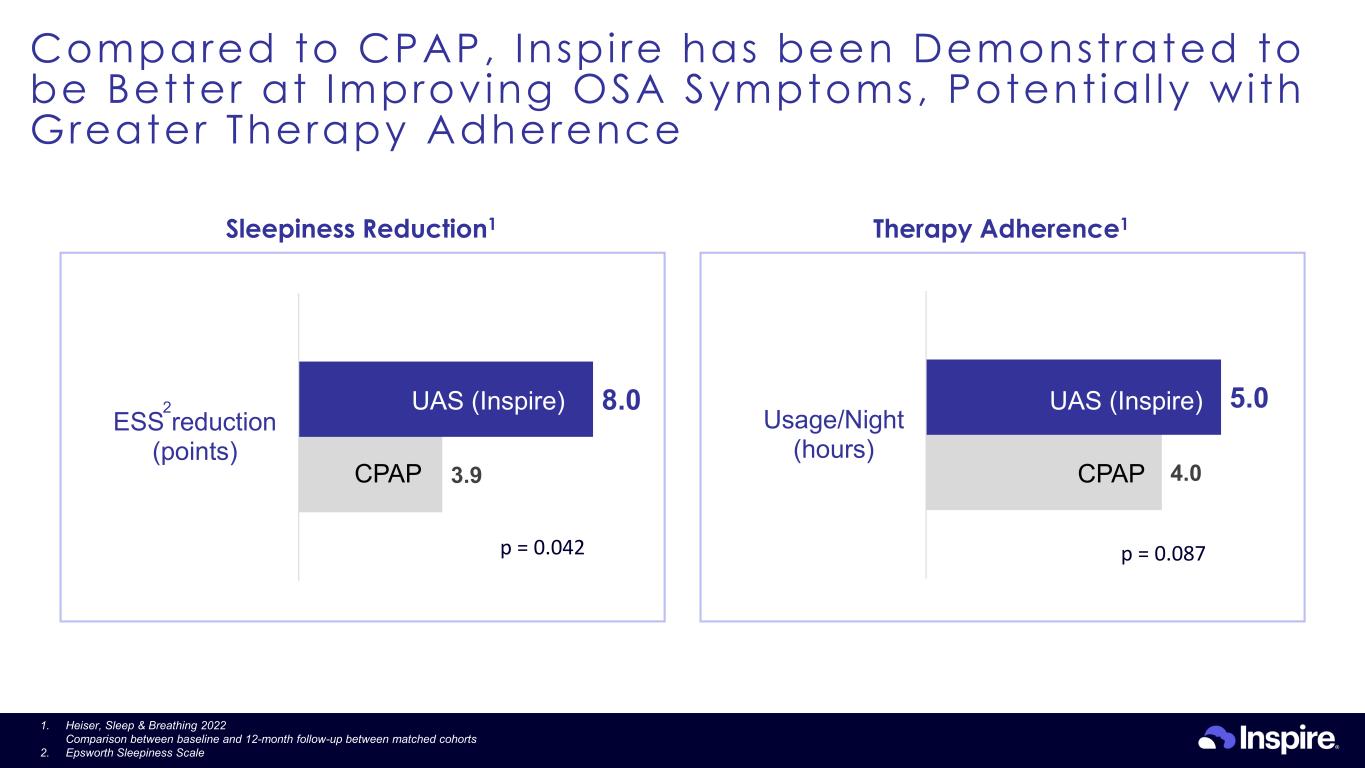
Compared to CPAP, Inspi re has been Demonstrated to be Better at Improving OSA Symptoms, Potent ia l ly wi th Greater Therapy Adherence 27 Therapy Adherence1 4.0 5.0 Usage/Night (hours) Sleepiness Reduction1 3.9 8.0 ESS reduction (points) 2 p = 0.042 p = 0.087 CPAP CPAP UAS (Inspire) UAS (Inspire) 1. Heiser, Sleep & Breathing 2022 Comparison between baseline and 12-month follow-up between matched cohorts 2. Epsworth Sleepiness Scale
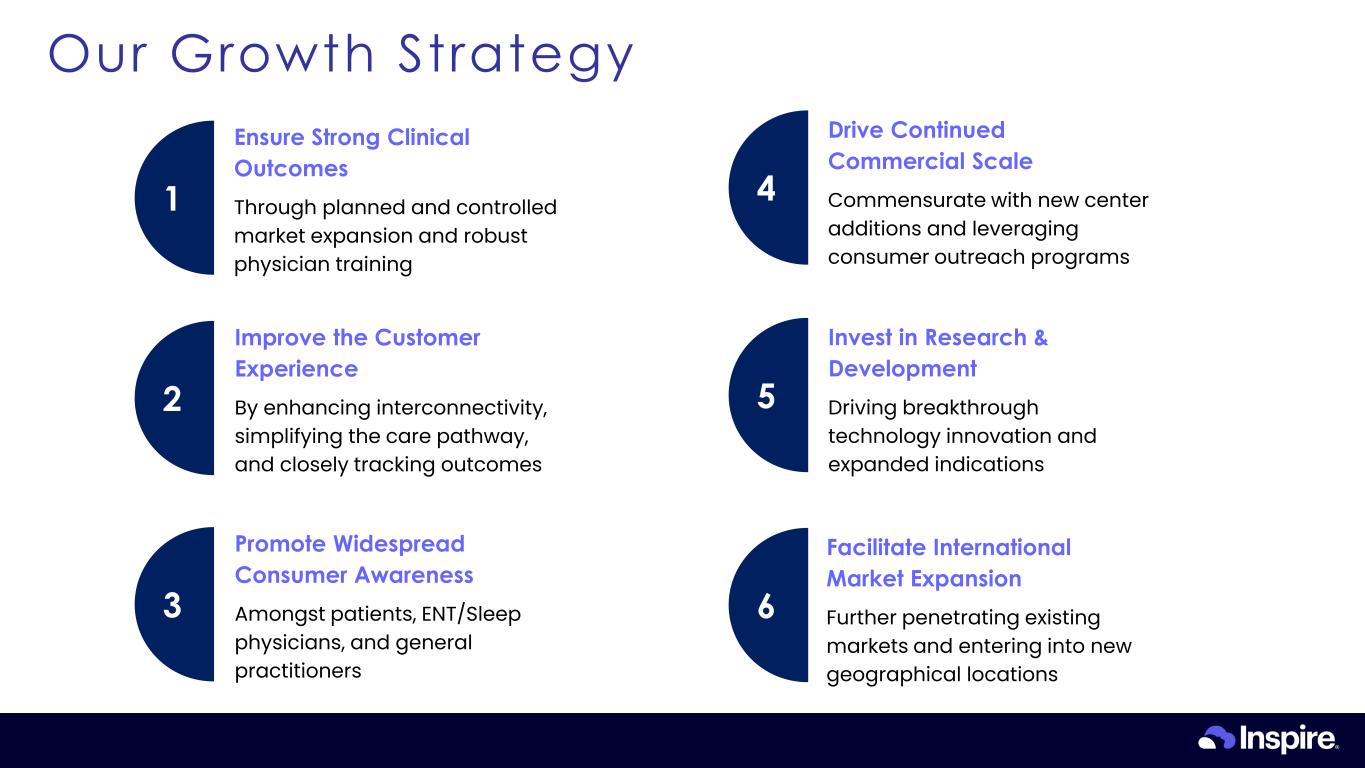
© Inspire Medical Systems, Inc. All Rights Reserved. Our Growth Strategy 28 1 Through planned and controlled market expansion and robust physician training Ensure Strong Clinical Outcomes 2 By enhancing interconnectivity, simplifying the care pathway, and closely tracking outcomes Improve the Customer Experience 3 Amongst patients, ENT/Sleep physicians, and general practitioners Promote Widespread Consumer Awareness 4 Commensurate with new center additions and leveraging consumer outreach programs Drive Continued Commercial Scale 5 Driving breakthrough technology innovation and expanded indications Invest in Research & Development 6 Further penetrating existing markets and entering into new geographical locations Facilitate International Market Expansion

Inspire Way We are a medical technology company committed to enhancing patient lives through sleep innovation “Put the patient first and you will never lose your way.” Demonstrate Operational Excellence Drive Therapy Adoption Strengthen Organizational Culture Focused on Outcomes. Fueled by Innovation. Grounded in Integrity. Committed to Compliance. Leading with Respect. Positively Persistent.
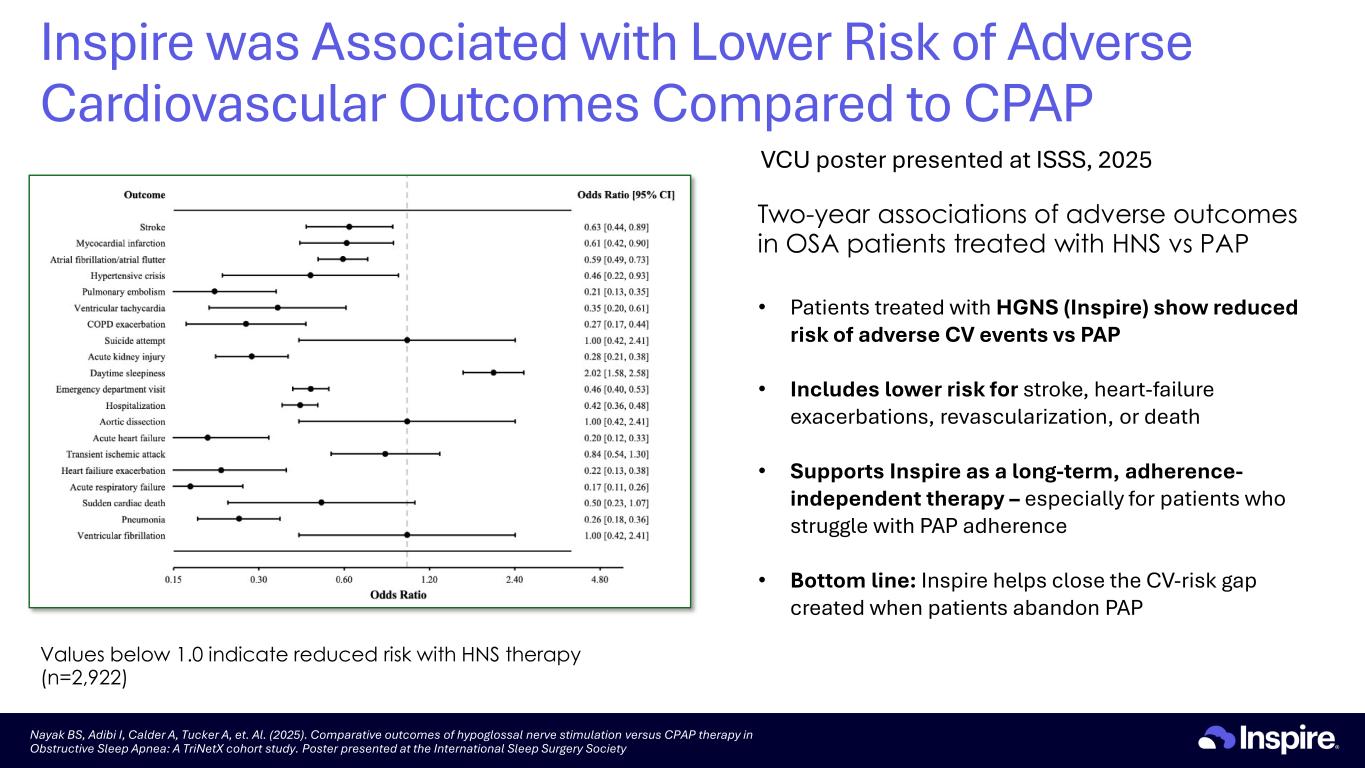
Two-year associations of adverse outcomes in OSA patients treated with HNS vs PAP Values below 1.0 indicate reduced risk with HNS therapy (n=2,922) Nayak BS, Adibi I, Calder A, Tucker A, et. Al. (2025). Comparative outcomes of hypoglossal nerve stimulation versus CPAP therapy in Obstructive Sleep Apnea: A TriNetX cohort study. Poster presented at the International Sleep Surgery Society • Patients treated with HGNS (Inspire) show reduced risk of adverse CV events vs PAP • Includes lower risk for stroke, heart-failure exacerbations, revascularization, or death • Supports Inspire as a long-term, adherence- independent therapy – especially for patients who struggle with PAP adherence • Bottom line: Inspire helps close the CV-risk gap created when patients abandon PAP Inspire was Associated with Lower Risk of Adverse Cardiovascular Outcomes Compared to CPAP VCU poster presented at ISSS, 2025
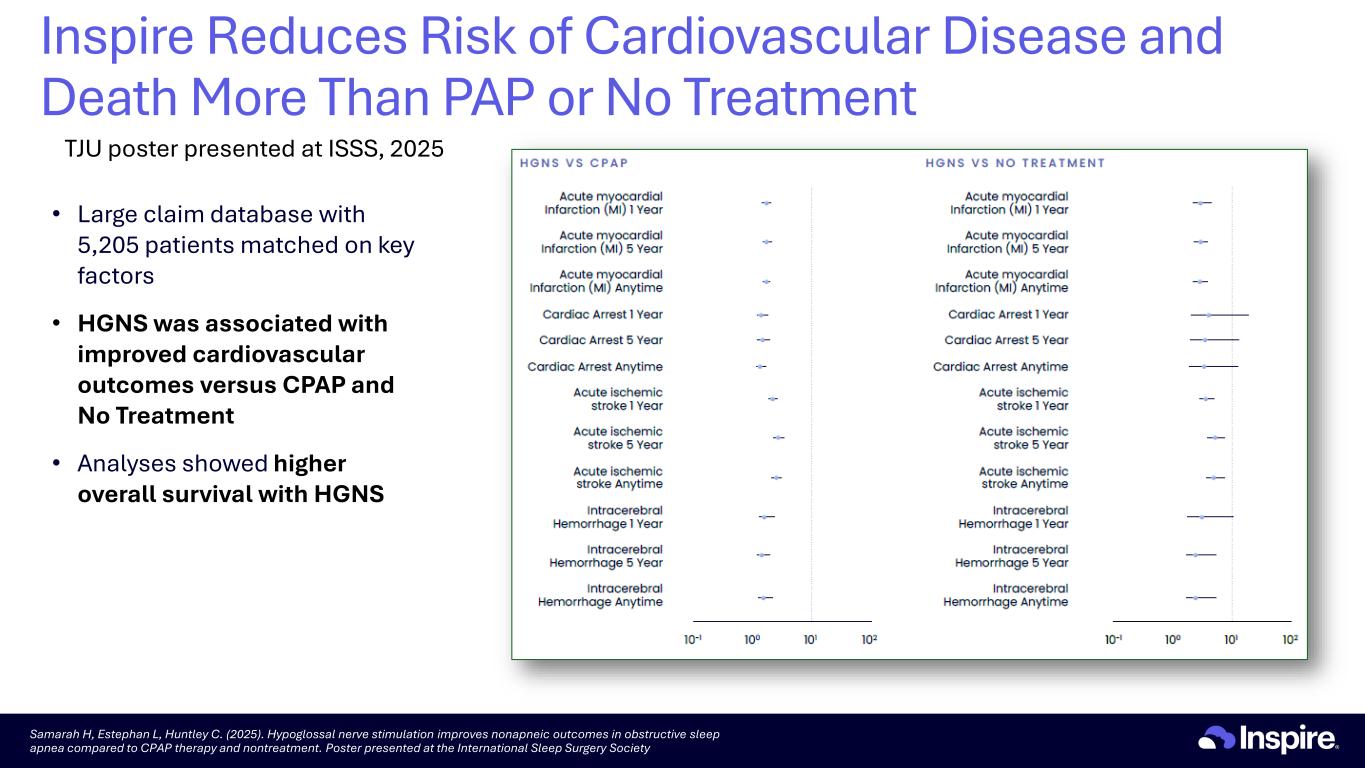
• Large claim database with 5,205 patients matched on key factors • HGNS was associated with improved cardiovascular outcomes versus CPAP and No Treatment • Analyses showed higher overall survival with HGNS Samarah H, Estephan L, Huntley C. (2025). Hypoglossal nerve stimulation improves nonapneic outcomes in obstructive sleep apnea compared to CPAP therapy and nontreatment. Poster presented at the International Sleep Surgery Society Inspire Reduces Risk of Cardiovascular Disease and Death More Than PAP or No Treatment TJU poster presented at ISSS, 2025
Inspire Reduces Risk of Cardiovascular Disease and Death more than PAP UTMB poster presented at ISSS, 2025 Burgess Z, Walawalkar R, Africa R, Pistone E, et. al. (2025). Long-Term Cardiovascular Outcomes for CPAP vs HGNS in Patients with OSA: A TriNetX Analysis. Poster presented at the International Sleep Surgery Society • Large claim database with 4,521 patients matched on key risk factors for cardiovascular disease • Compared Inspire therapy to those on PAP therapy • HGNS therapy is associated with a significant reduction in multiple cardiometabolic markers and cardiovascular morbidity over a 5-year period when compared to PAP therapy • Also profound benefits seen on all cause-mortality when compared to PAP