

December 2025 Corporate Presentation © Athira Pharma, Inc.

Disclaimer This presentation and any accompanying oral commentary contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “on track,” “ongoing” or the negative of these terms or other comparable terminology. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance and financial condition, business plans and objectives, our ability to obtain funding for our operations, including funding necessary to develop and commercialize our drug candidates and funding necessary for the payment of future milestone and other payments that may be earned by our collaboration partners, timing and success of our planned or future development activities, including our ability to consummate definitive agreements for our future development of lasofoxifene, our ability to obtain regulatory approval, the potential therapeutic benefits and economic value of (1) ATH-1105 as a potential treatment for Alzheimer’s disease, Parkinson’s disease, Parkinson’s disease dementia, amyotrophic lateral sclerosis, neuropathic pain and other neurodegenerative diseases and disorders, and (2) lasofoxifene as a potential treatment for breast cancer, the anticipated reporting of data, the potential learnings from preclinical studies and other nonclinical data and clinical studies and their ability to inform and improve future clinical development plans and to demonstrate the safety and efficacy of our drug candidates, our ability to initiate and successfully advance clinical trials for the development of our product candidates, the rate and degree of market acceptance of our drug candidates, anticipated milestone timelines, such as the timing of data releases, and our ability to meet such timelines, our plans related to commercializing our drug candidates, if approved, the success of competing therapies that are or may become available, potential growth opportunities, our plans for any potential financing, strategic transaction, or partnership, including for ATH-1105 and lasofoxifene, competitive position, and industry environment and potential market opportunities. We do not undertake any duty to update these forward-looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including, but not limited to, the risk that the conditions to the closing of any or all of the proposed transactions related to the definitive agreements for our future development of lasofoxifene are not satisfied (the “Transactions”); uncertainties as to the timing of the consummation of the Transactions and the ability of each of the parties thereto to consummate them; unexpected costs, charges or expenses resulting from the Transactions; potential adverse reactions or changes to business relationships resulting from the announcement or completion of the Transactions; risks associated with the possible failure to realize certain anticipated benefits of the Transactions, including with respect to future financial and operating results; the data from preclinical and clinical trials may not support the safety, efficacy and tolerability of our drug candidates; development of drug candidates may cease or be delayed; regulatory authorities could object to protocols, amendments and other submissions; future potential regulatory milestones for drug candidates, including those related to current and planned clinical studies, may be insufficient to support regulatory submissions or approval; whether our trials are sufficiently powered to meet the planned endpoints; we may not be able to recruit sufficient patients for our clinical trials; the outcome of legal proceedings that may in the future be instituted against us, our directors and officers; possible negative interactions of our drug candidates with other treatments; U.S. Food and Drug Administration (“FDA”) regulatory delays and uncertainty and new policies, including executive orders, changes in the leadership of federal agencies such as the FDA and U.S. Securities and Exchange Commission (“SEC”), staff layoffs, budget cuts to agency programs and research, and changes in drug pricing controls; our assumptions regarding our financial condition and the sufficiency of our cash, cash equivalents and investments to fund our planned operations may be incorrect; adverse conditions in the general domestic and global economic markets, including as a result of tariffs; the impact of competition; the impact of drug candidate development and clinical activities on operating expenses; the impact of new or changing laws and regulations; as well as the other risks detailed in our filings with the SEC from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that are described in greater detail in our filings with the SEC may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and readers are cautioned not to unduly rely upon these statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business. This presentation contains estimates, projections and other information concerning market, industry and other data. We obtained this data from our own internal estimates and research and from academic and industry research, publications, surveys, and studies conducted by third parties, including governmental agencies. These data involve a number of assumptions and limitations, are subject to risks and uncertainties, and are subject to change based on various factors, including those discussed in our filings with the SEC. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. While we believe such information is generally reliable, we have not independently verified any third-party information. This presentation concerns drug candidates that are under clinical investigation and which have not yet been approved for marketing by the FDA. The drug candidates are currently limited by federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. We announce material information to the public through a variety of means, including filings with the SEC, press releases, public conference calls, our website (www.athira.com/), our investor relations website (investors.athira.com), and our news site (investors.athira.com/news-and-events/press-releases). We use these channels, as well as social media, including our X account (@athirapharma) and Facebook page (https://www.facebook.com/athirapharmainc), to communicate with investors and the public about Athira, our products, and other matters. Therefore, we encourage investors, the media, and others interested in Athira to review the information we make public in these locations, as such information could be deemed to be material information. © Athira Pharma, Inc.

Athira Pharma Building a pipeline with the potential to change lives and create enduring value Rebranding coming soon! © Athira Pharma, Inc.

Management team with significant drug development and approval experience LEADERSHIP Mark Litton, PhD President and CEO Mark Worthington, JD General Counsel Kevin Church, PhD CSO Javier San Martin, MD CMO Robert Renninger CFO Mark Kubik Head of BD (consultant) Collectively involved in multiple developed products and exits Select prior companies Approved therapies © Athira Pharma, Inc. David Portman, MD CEO, Sermonix* *Expected to provide consulting services to the Company on a post-transaction basis.
Lasofoxifene Phase 3 registrational study ongoing with >50% enrolled ATH-1105 Phase 2 POC study in ALS planned to start in 1H2026 Expected key inflection points within ~2 years Topline results expected in mid-2027 Topline results expected 2027 © Athira Pharma, Inc.
Late-stage program diversifies pipeline Exciting opportunity in metastatic breast cancer Large Market Opportunity Differentiated Mechanism of Action Positive Phase 2 data Strategic Fit ER+/HER2- disease remains most common breast cancer subtype1 Represents approximately 70% of all breast cancers1 Global metastatic ER+/HER2- market expected to grow from ~$10.9B in 2025 to ~$15.9B by 20292 Designed to overcome resistance that limits current endocrine agents3,4,5 Tissue-selective pharmacology6 Strong combinability profile7 Demonstrated compelling signals of clinical activity7,8 Achieved 13 months of progression free survival (PFS)7 Favorable safety profile and well tolerated with potential QoL benefits6,7,8 Right company and team to bring this program forward Builds on Athira’s late-stage development infrastructure Leverages deep regulatory and development expertise of Athira leadership Sources: 1. NCI. Retrieved from https://seer.cancer.gov/statfacts/html/breast-subtypes.html; 2. Research and Markets. Retrieved from: https://www.researchandmarkets.com/reports/6076080/metastatic-hrher2-breast-cancer-global-market; 3. Venetis et al. Cancer Treat Rev 2023; 4. Laine et al. Breast Cancer Res 2021; 5. Adreano et al, Mol Cancer Ther 2020; 6. Goldfarb et al, Clinical Breast Cancer 2024; 7. Damodaran et. al, Annals of Oncology 2023; 8. Goetz et. al, Annals of Oncology 2023 © Athira Pharma, Inc.

7 Small molecule selective estrogen receptor modulator (SERM) Lasofoxifene for the potential treatment of metastatic breast cancer © Athira Pharma, Inc.
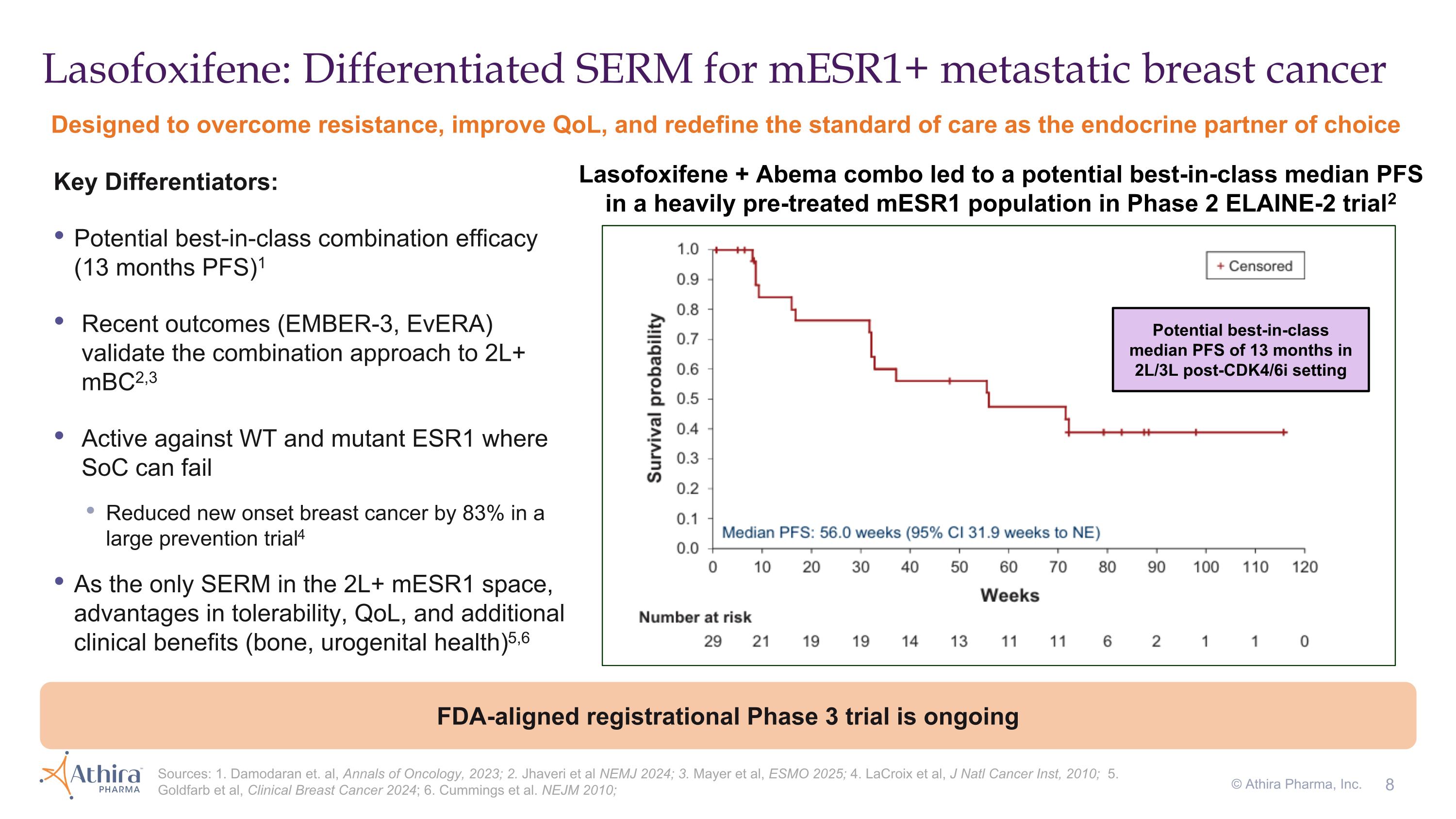
Lasofoxifene: Differentiated SERM for mESR1+ metastatic breast cancer Lasofoxifene + Abema combo led to a potential best-in-class median PFS in a heavily pre-treated mESR1 population in Phase 2 ELAINE-2 trial2 Potential best-in-class median PFS of 13 months in 2L/3L post-CDK4/6i setting Designed to overcome resistance, improve QoL, and redefine the standard of care as the endocrine partner of choice Key Differentiators: Potential best-in-class combination efficacy (13 months PFS)1 Recent outcomes (EMBER-3, EvERA) validate the combination approach to 2L+ mBC2,3 Active against WT and mutant ESR1 where SoC can fail Reduced new onset breast cancer by 83% in a large prevention trial4 As the only SERM in the 2L+ mESR1 space, advantages in tolerability, QoL, and additional clinical benefits (bone, urogenital health)5,6 FDA-aligned registrational Phase 3 trial is ongoing Sources: 1. Damodaran et. al, Annals of Oncology, 2023; 2. Jhaveri et al NEMJ 2024; 3. Mayer et al, ESMO 2025; 4. LaCroix et al, J Natl Cancer Inst, 2010; 5. Goldfarb et al, Clinical Breast Cancer 2024; 6. Cummings et al. NEJM 2010; © Athira Pharma, Inc.
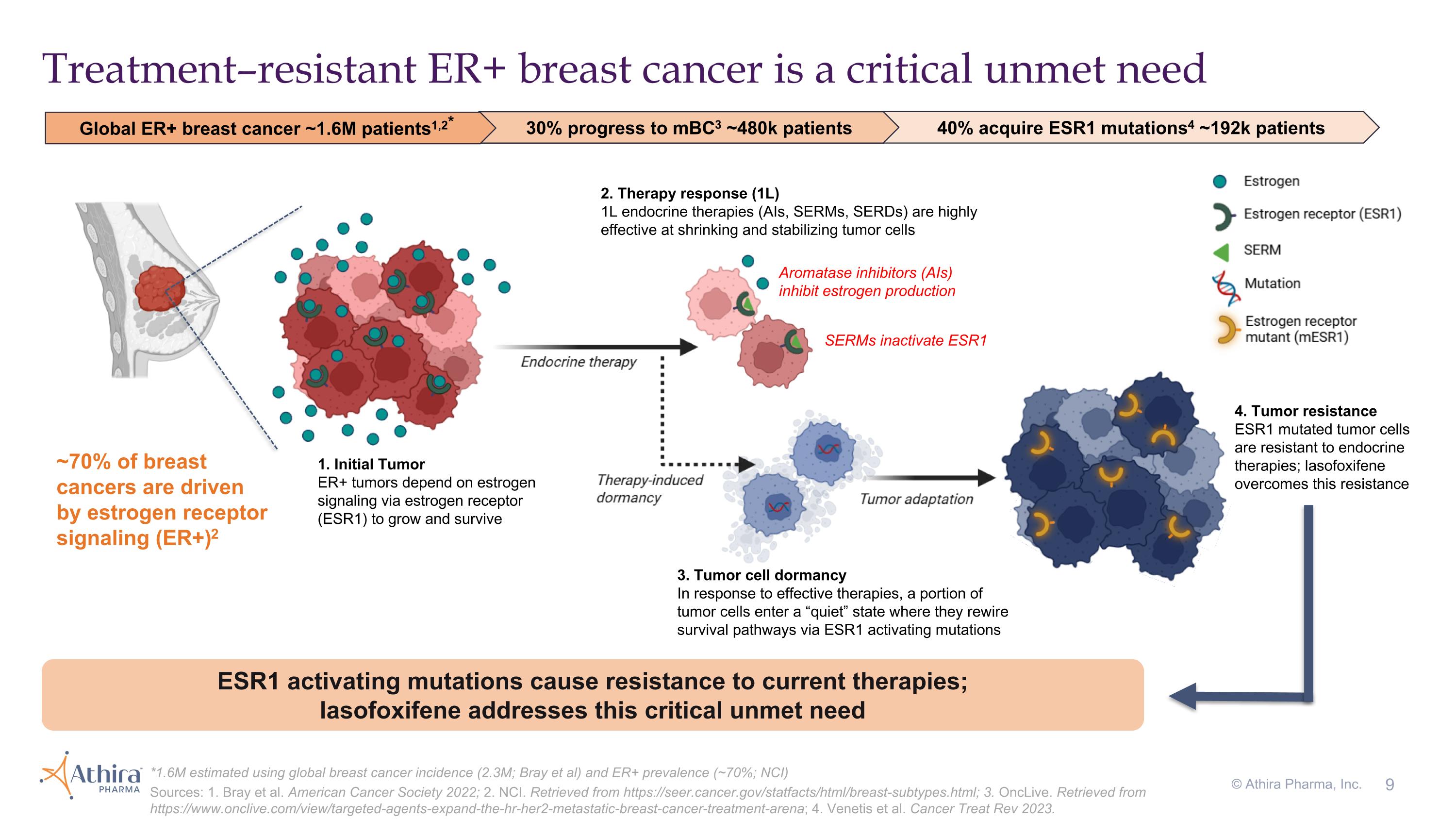
1. Initial Tumor ER+ tumors depend on estrogen signaling via estrogen receptor (ESR1) to grow and survive 2. Therapy response (1L) 1L endocrine therapies (AIs, SERMs, SERDs) are highly effective at shrinking and stabilizing tumor cells 3. Tumor cell dormancy In response to effective therapies, a portion of tumor cells enter a “quiet” state where they rewire survival pathways via ESR1 activating mutations 4. Tumor resistance ESR1 mutated tumor cells are resistant to endocrine therapies; lasofoxifene overcomes this resistance Aromatase inhibitors (AIs) inhibit estrogen production SERMs inactivate ESR1 ESR1 activating mutations cause resistance to current therapies; lasofoxifene addresses this critical unmet need Treatment–resistant ER+ breast cancer is a critical unmet need ~70% of breast cancers are driven by estrogen receptor signaling (ER+)2 30% progress to mBC3 ~480k patients Global ER+ breast cancer ~1.6M patients1,2* 40% acquire ESR1 mutations4 ~192k patients *1.6M estimated using global breast cancer incidence (2.3M; Bray et al) and ER+ prevalence (~70%; NCI) Sources: 1. Bray et al. American Cancer Society 2022; 2. NCI. Retrieved from https://seer.cancer.gov/statfacts/html/breast-subtypes.html; 3. OncLive. Retrieved from https://www.onclive.com/view/targeted-agents-expand-the-hr-her2-metastatic-breast-cancer-treatment-arena; 4. Venetis et al. Cancer Treat Rev 2023. © Athira Pharma, Inc.
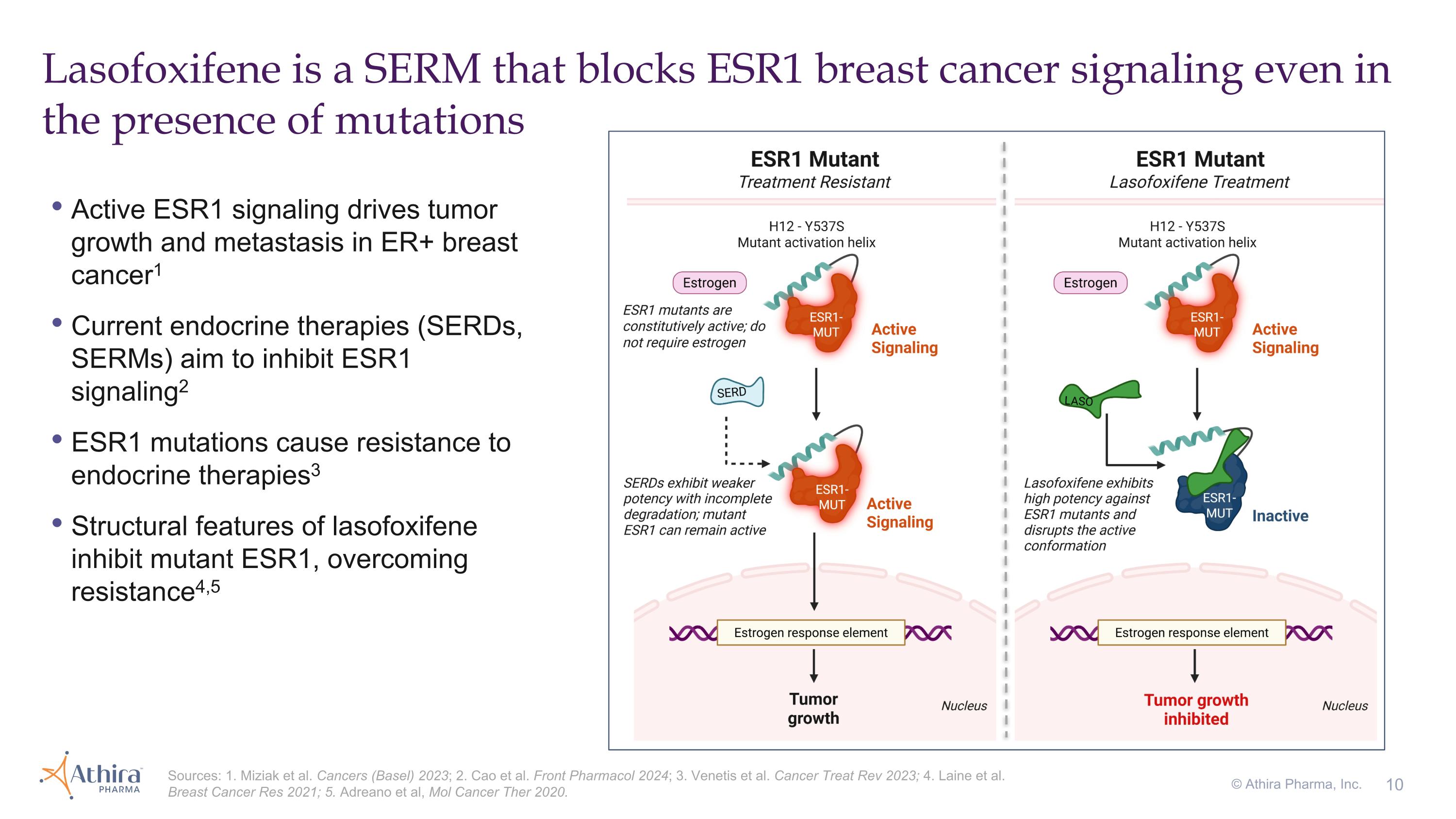
Active ESR1 signaling drives tumor growth and metastasis in ER+ breast cancer1 Current endocrine therapies (SERDs, SERMs) aim to inhibit ESR1 signaling2 ESR1 mutations cause resistance to endocrine therapies3 Structural features of lasofoxifene inhibit mutant ESR1, overcoming resistance4,5 Lasofoxifene is a SERM that blocks ESR1 breast cancer signaling even in the presence of mutations Sources: 1. Miziak et al. Cancers (Basel) 2023; 2. Cao et al. Front Pharmacol 2024; 3. Venetis et al. Cancer Treat Rev 2023; 4. Laine et al. Breast Cancer Res 2021; 5. Adreano et al, Mol Cancer Ther 2020. © Athira Pharma, Inc.
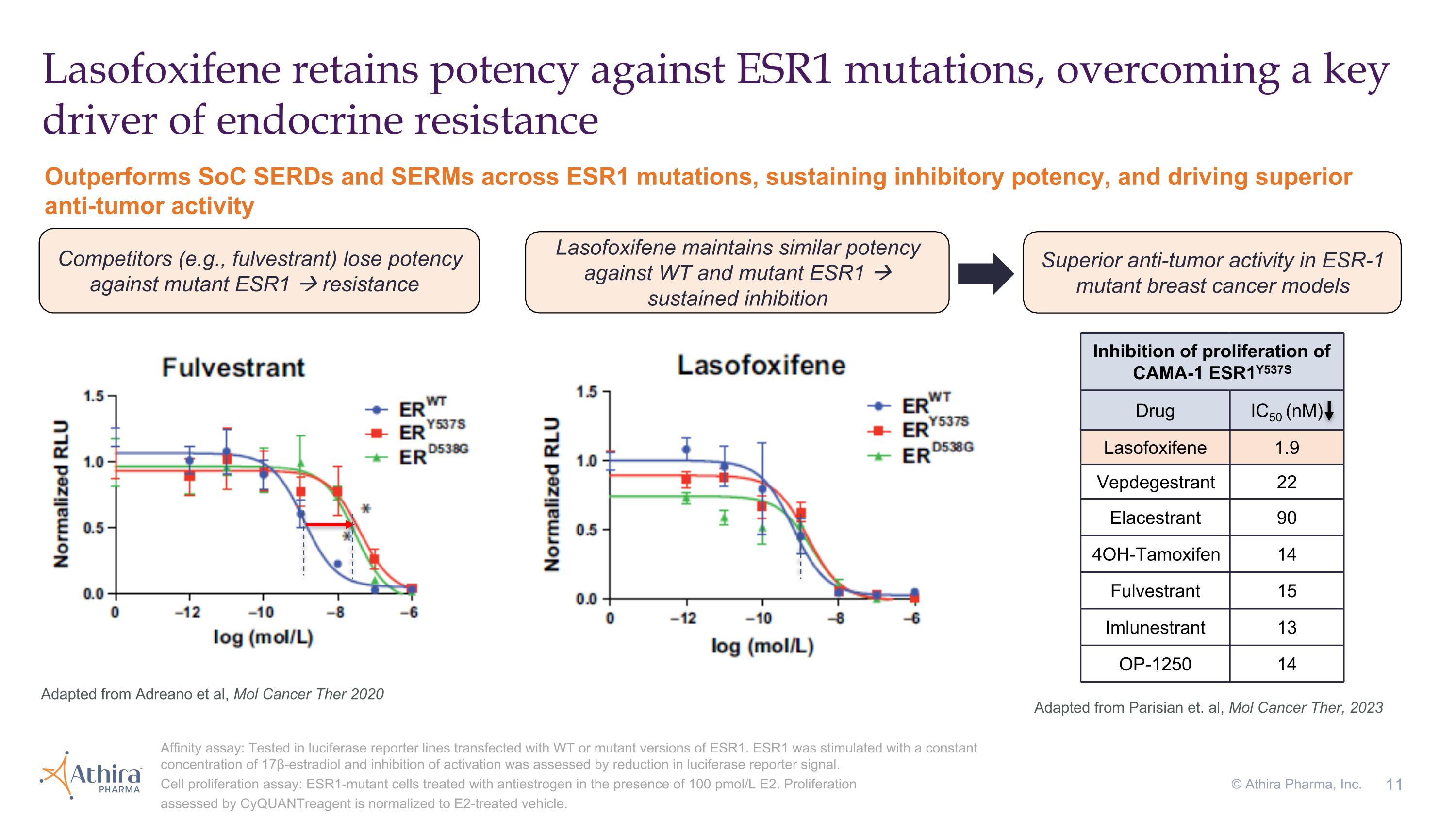
Lasofoxifene retains potency against ESR1 mutations, overcoming a key driver of endocrine resistance Affinity assay: Tested in luciferase reporter lines transfected with WT or mutant versions of ESR1. ESR1 was stimulated with a constant concentration of 17β-estradiol and inhibition of activation was assessed by reduction in luciferase reporter signal. Cell proliferation assay: ESR1-mutant cells treated with antiestrogen in the presence of 100 pmol/L E2. Proliferation assessed by CyQUANTreagent is normalized to E2-treated vehicle. Adapted from Adreano et al, Mol Cancer Ther 2020 Outperforms SoC SERDs and SERMs across ESR1 mutations, sustaining inhibitory potency, and driving superior anti-tumor activity Competitors (e.g., fulvestrant) lose potency against mutant ESR1 resistance Superior anti-tumor activity in ESR-1 mutant breast cancer models Inhibition of proliferation of CAMA-1 ESR1Y537S Drug IC50 (nM) Lasofoxifene 1.9 Vepdegestrant 22 Elacestrant 90 4OH-Tamoxifen 14 Fulvestrant 15 Imlunestrant 13 OP-1250 14 Adapted from Parisian et. al, Mol Cancer Ther, 2023 Lasofoxifene maintains similar potency against WT and mutant ESR1 sustained inhibition © Athira Pharma, Inc.
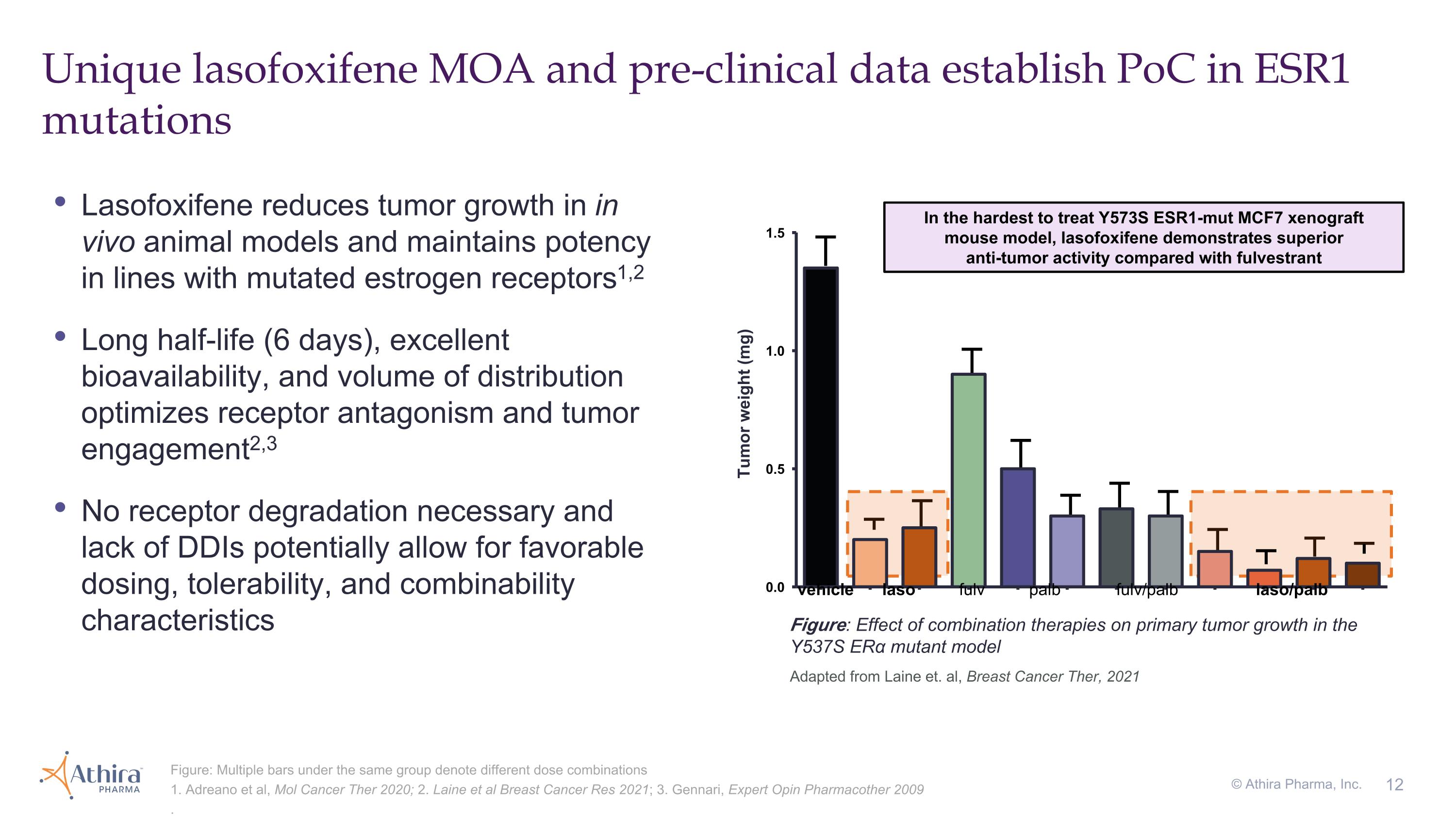
Lasofoxifene reduces tumor growth in in vivo animal models and maintains potency in lines with mutated estrogen receptors1,2 Long half-life (6 days), excellent bioavailability, and volume of distribution optimizes receptor antagonism and tumor engagement2,3 No receptor degradation necessary and lack of DDIs potentially allow for favorable dosing, tolerability, and combinability characteristics Unique lasofoxifene MOA and pre-clinical data establish PoC in ESR1 mutations Figure: Multiple bars under the same group denote different dose combinations 1. Adreano et al, Mol Cancer Ther 2020; 2. Laine et al Breast Cancer Res 2021; 3. Gennari, Expert Opin Pharmacother 2009 . Adapted from Laine et. al, Breast Cancer Ther, 2021 In the hardest to treat Y573S ESR1-mut MCF7 xenograft mouse model, lasofoxifene demonstrates superior anti-tumor activity compared with fulvestrant Tumor weight (mg) vehicle laso fulv palb fulv/palb laso/palb Figure: Effect of combination therapies on primary tumor growth in the Y537S ERα mutant model © Athira Pharma, Inc.
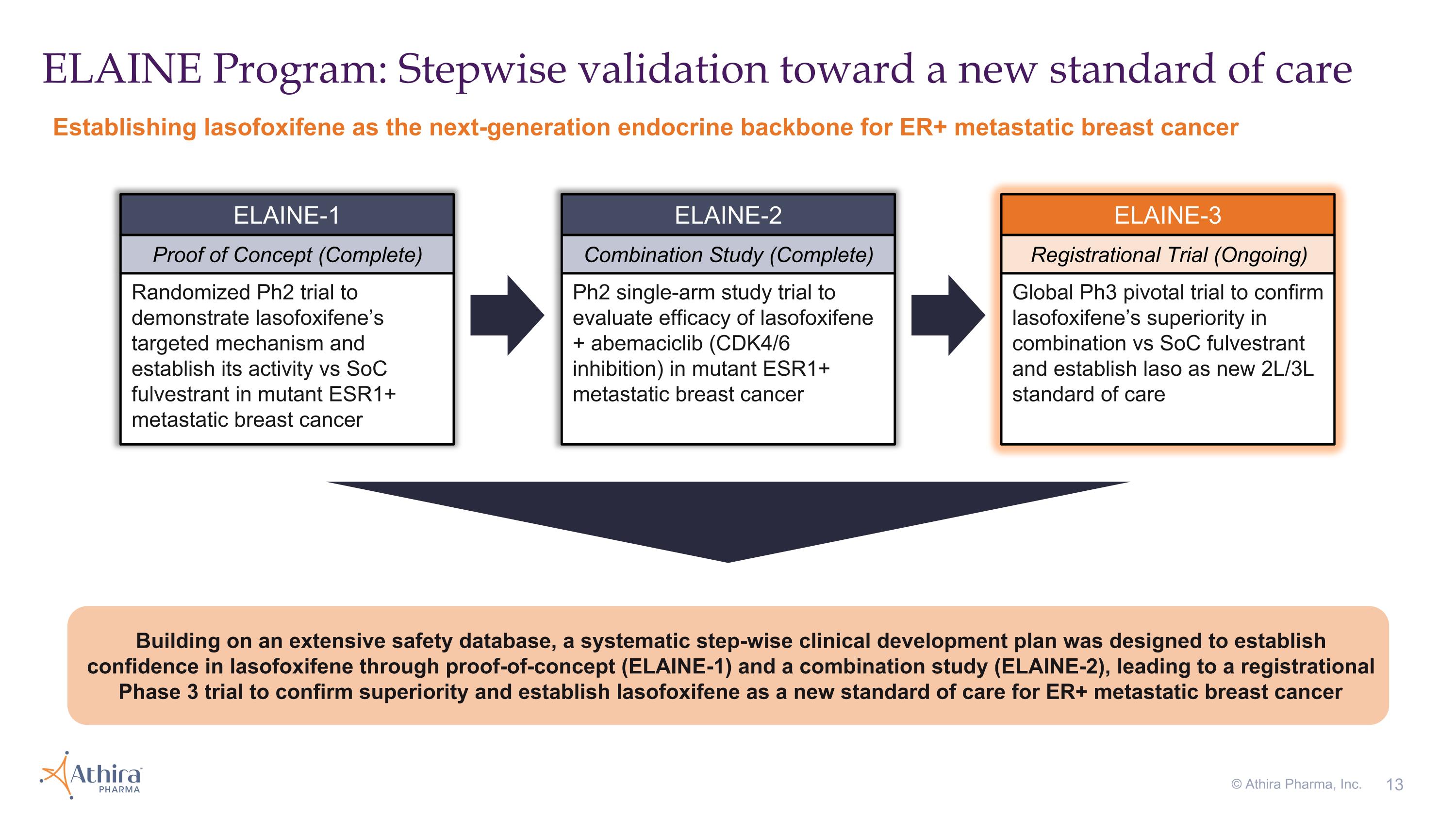
ELAINE Program: Stepwise validation toward a new standard of care Establishing lasofoxifene as the next-generation endocrine backbone for ER+ metastatic breast cancer © Athira Pharma, Inc. Randomized Ph2 trial to demonstrate lasofoxifene’s targeted mechanism and establish its activity vs SoC fulvestrant in mutant ESR1+ metastatic breast cancer ELAINE-1 Proof of Concept (Complete) Ph2 single-arm study trial to evaluate efficacy of lasofoxifene + abemaciclib (CDK4/6 inhibition) in mutant ESR1+ metastatic breast cancer ELAINE-2 Combination Study (Complete) Global Ph3 pivotal trial to confirm lasofoxifene’s superiority in combination vs SoC fulvestrant and establish laso as new 2L/3L standard of care ELAINE-3 Registrational Trial (Ongoing) Building on an extensive safety database, a systematic step-wise clinical development plan was designed to establish confidence in lasofoxifene through proof-of-concept (ELAINE-1) and a combination study (ELAINE-2), leading to a registrational Phase 3 trial to confirm superiority and establish lasofoxifene as a new standard of care for ER+ metastatic breast cancer
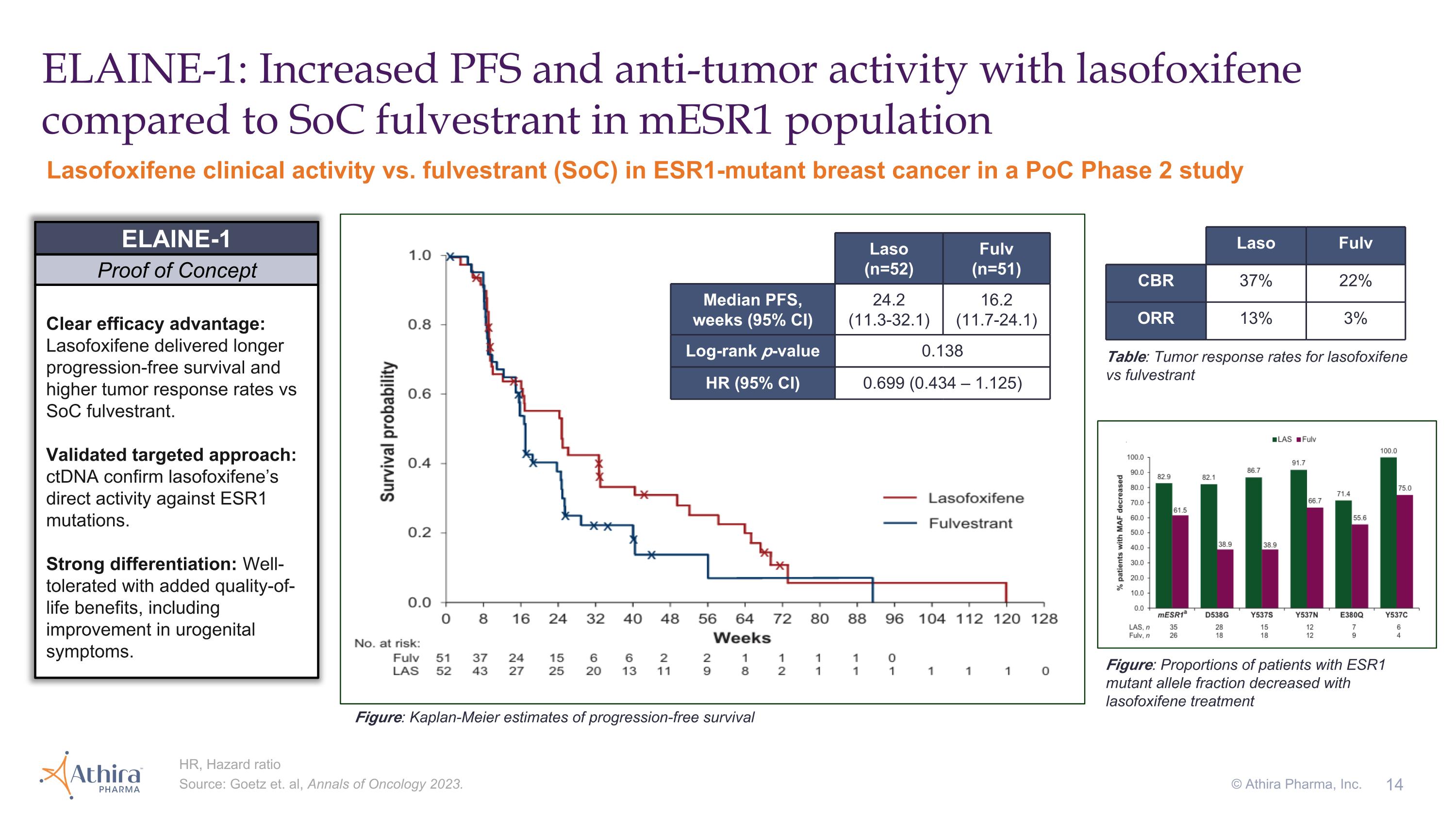
ELAINE-1: Increased PFS and anti-tumor activity with lasofoxifene compared to SoC fulvestrant in mESR1 population Clear efficacy advantage: Lasofoxifene delivered longer progression-free survival and higher tumor response rates vs SoC fulvestrant. Validated targeted approach: ctDNA confirm lasofoxifene’s direct activity against ESR1 mutations. Strong differentiation: Well-tolerated with added quality-of-life benefits, including improvement in urogenital symptoms. ELAINE-1 Proof of Concept Lasofoxifene clinical activity vs. fulvestrant (SoC) in ESR1-mutant breast cancer in a PoC Phase 2 study Table: Tumor response rates for lasofoxifene vs fulvestrant Laso (n=52) Fulv (n=51) Median PFS, weeks (95% CI) 24.2 (11.3-32.1) 16.2 (11.7-24.1) Log-rank p-value 0.138 HR (95% CI) 0.699 (0.434 – 1.125) Figure: Kaplan-Meier estimates of progression-free survival Laso Fulv CBR 37% 22% ORR 13% 3% Figure: Proportions of patients with ESR1 mutant allele fraction decreased with lasofoxifene treatment HR, Hazard ratio Source: Goetz et. al, Annals of Oncology 2023. © Athira Pharma, Inc.
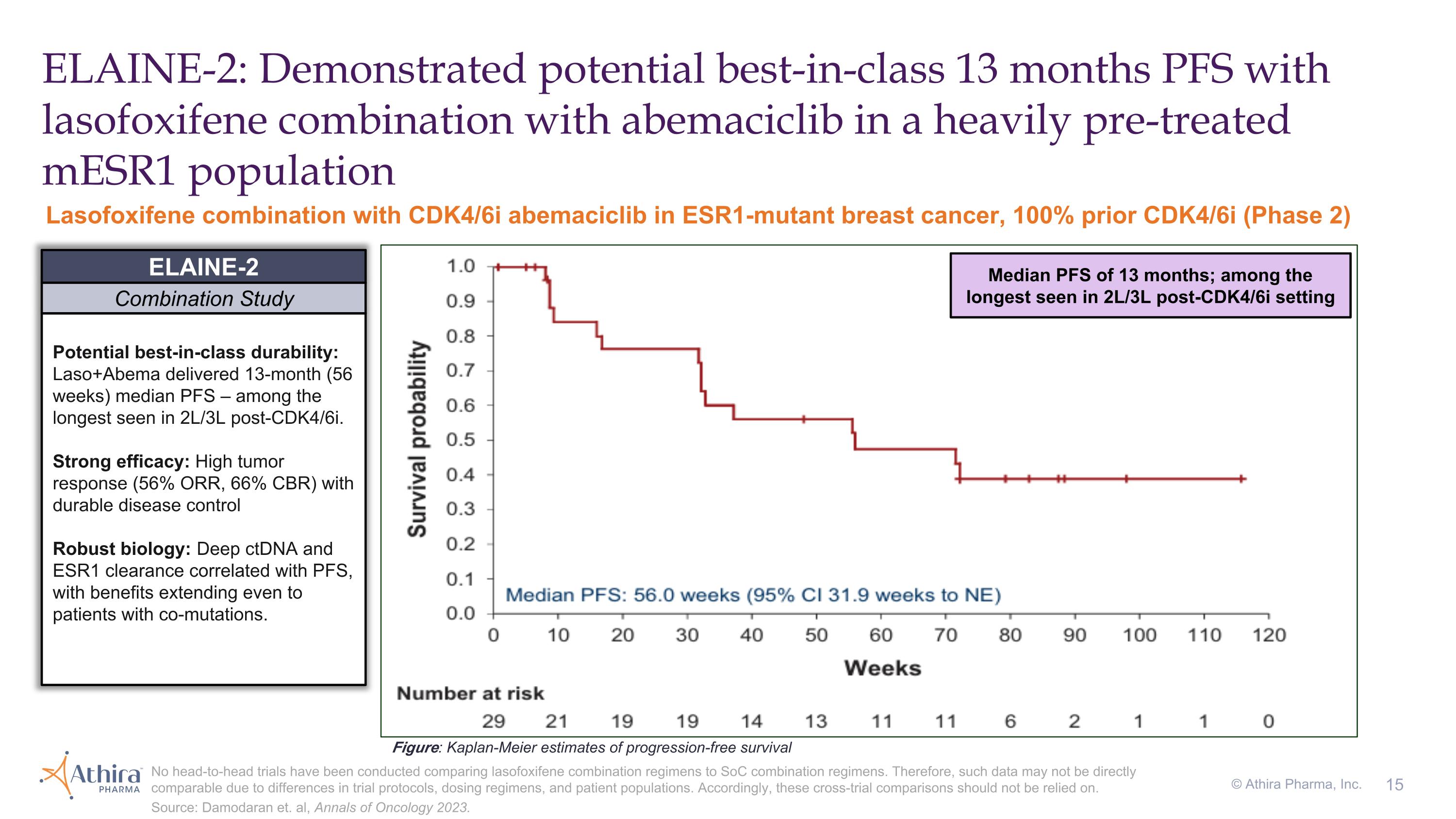
ELAINE-2: Demonstrated potential best-in-class 13 months PFS with lasofoxifene combination with abemaciclib in a heavily pre-treated mESR1 population Potential best-in-class durability: Laso+Abema delivered 13-month (56 weeks) median PFS – among the longest seen in 2L/3L post-CDK4/6i. Strong efficacy: High tumor response (56% ORR, 66% CBR) with durable disease control Robust biology: Deep ctDNA and ESR1 clearance correlated with PFS, with benefits extending even to patients with co-mutations. ELAINE-2 Combination Study Figure: Kaplan-Meier estimates of progression-free survival Lasofoxifene combination with CDK4/6i abemaciclib in ESR1-mutant breast cancer, 100% prior CDK4/6i (Phase 2) No head-to-head trials have been conducted comparing lasofoxifene combination regimens to SoC combination regimens. Therefore, such data may not be directly comparable due to differences in trial protocols, dosing regimens, and patient populations. Accordingly, these cross-trial comparisons should not be relied on. Source: Damodaran et. al, Annals of Oncology 2023. © Athira Pharma, Inc. Median PFS of 13 months; among the longest seen in 2L/3L post-CDK4/6i setting
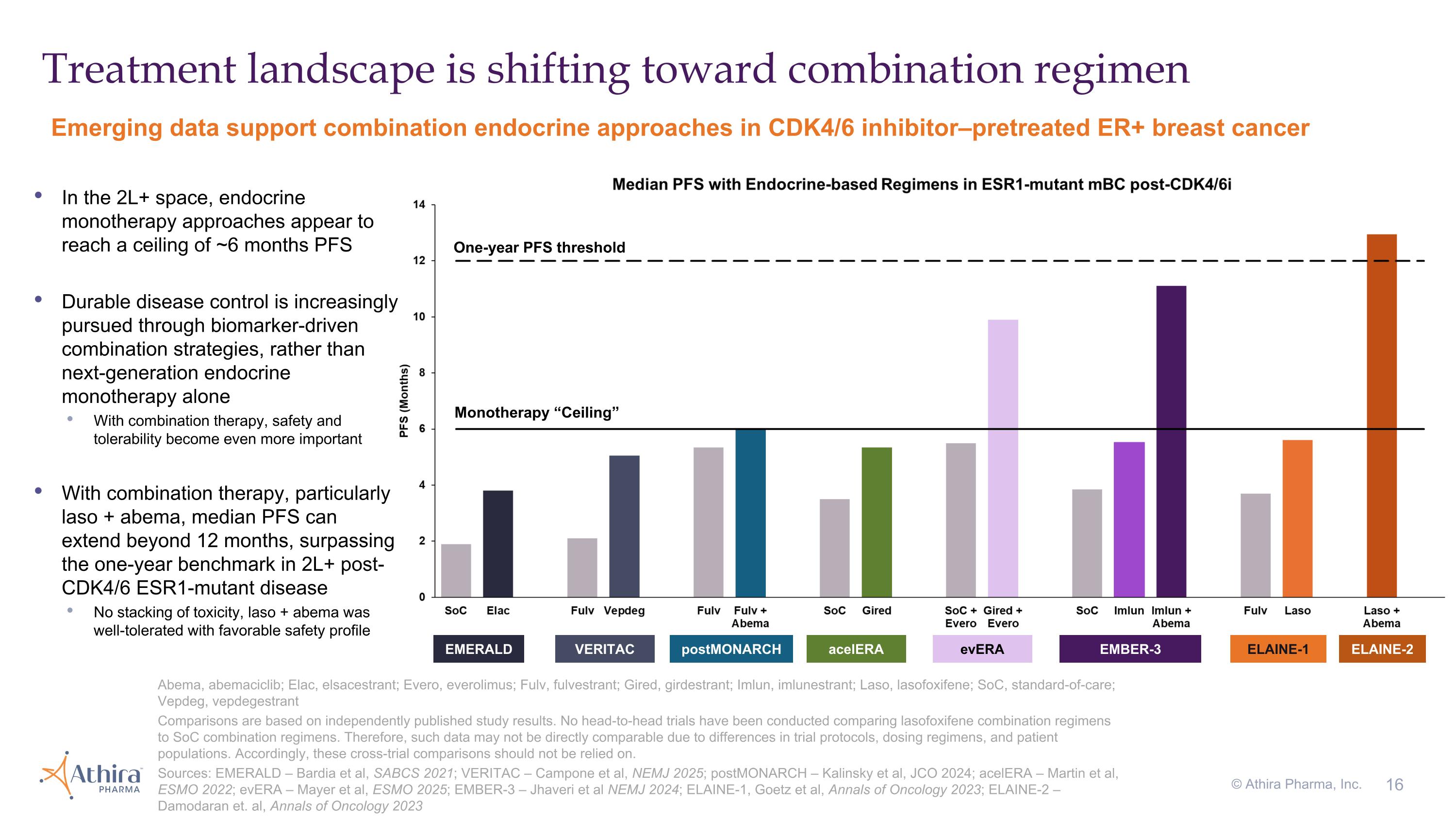
Treatment landscape is shifting toward combination regimen © Athira Pharma, Inc. In the 2L+ space, endocrine monotherapy approaches appear to reach a ceiling of ~6 months PFS Durable disease control is increasingly pursued through biomarker-driven combination strategies, rather than next-generation endocrine monotherapy alone With combination therapy, safety and tolerability become even more important With combination therapy, particularly laso + abema, median PFS can extend beyond 12 months, surpassing the one-year benchmark in 2L+ post-CDK4/6 ESR1-mutant disease No stacking of toxicity, laso + abema was well-tolerated with favorable safety profile Emerging data support combination endocrine approaches in CDK4/6 inhibitor–pretreated ER+ breast cancer Abema, abemaciclib; Elac, elsacestrant; Evero, everolimus; Fulv, fulvestrant; Gired, girdestrant; Imlun, imlunestrant; Laso, lasofoxifene; SoC, standard-of-care; Vepdeg, vepdegestrant Comparisons are based on independently published study results. No head-to-head trials have been conducted comparing lasofoxifene combination regimens to SoC combination regimens. Therefore, such data may not be directly comparable due to differences in trial protocols, dosing regimens, and patient populations. Accordingly, these cross-trial comparisons should not be relied on. Sources: EMERALD – Bardia et al, SABCS 2021; VERITAC – Campone et al, NEMJ 2025; postMONARCH – Kalinsky et al, JCO 2024; acelERA – Martin et al, ESMO 2022; evERA – Mayer et al, ESMO 2025; EMBER-3 – Jhaveri et al NEMJ 2024; ELAINE-1, Goetz et al, Annals of Oncology 2023; ELAINE-2 – Damodaran et. al, Annals of Oncology 2023 EMERALD VERITAC acelERA evERA EMBER-3 ELAINE-1 ELAINE-2 Monotherapy “Ceiling” One-year PFS threshold postMONARCH
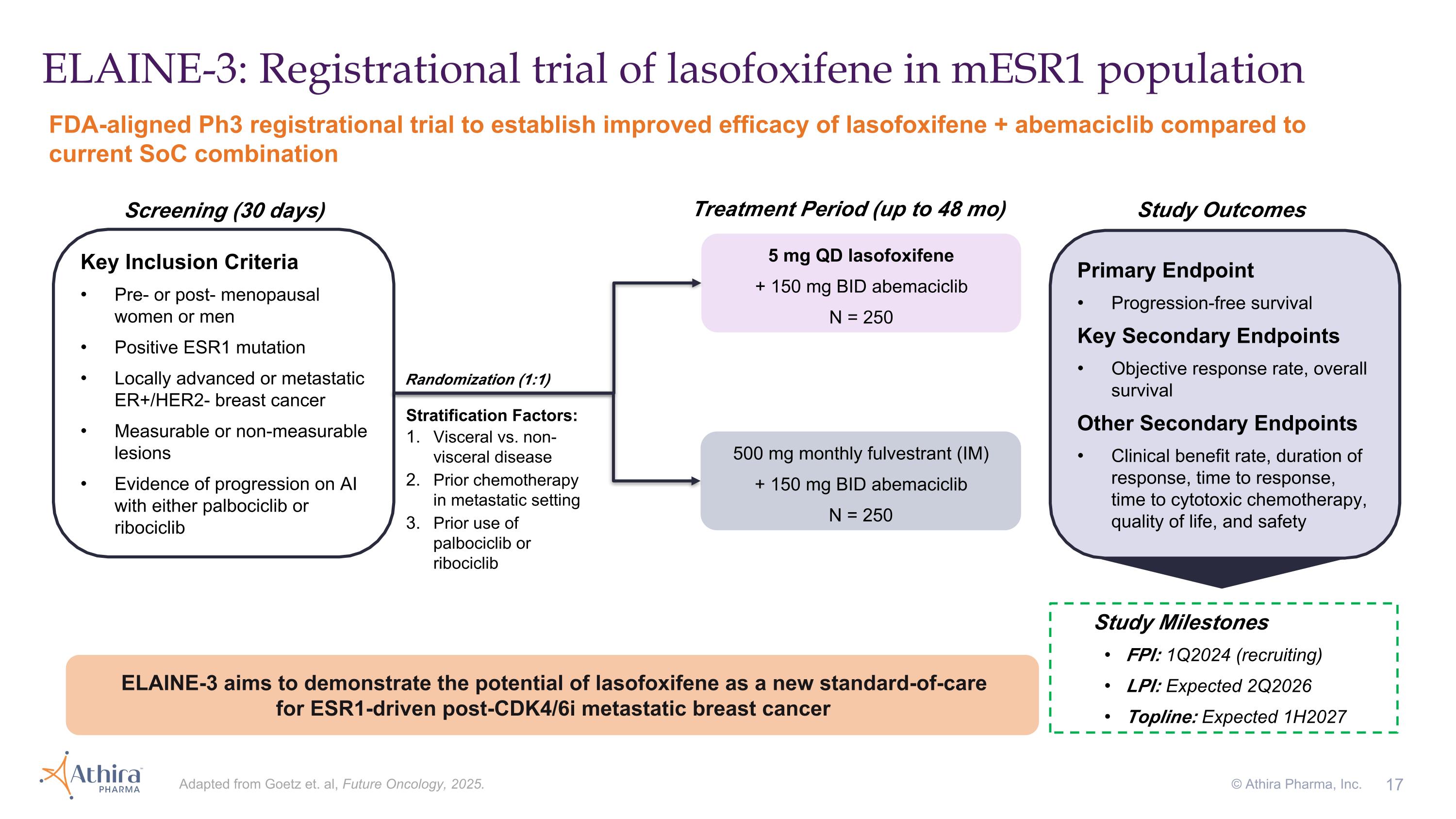
ELAINE-3: Registrational trial of lasofoxifene in mESR1 population FDA-aligned Ph3 registrational trial to establish improved efficacy of lasofoxifene + abemaciclib compared to current SoC combination Adapted from Goetz et. al, Future Oncology, 2025. © Athira Pharma, Inc. Stratification Factors: Visceral vs. non-visceral disease Prior chemotherapy in metastatic setting Prior use of palbociclib or ribociclib Key Inclusion Criteria Pre- or post- menopausal women or men Positive ESR1 mutation Locally advanced or metastatic ER+/HER2- breast cancer Measurable or non-measurable lesions Evidence of progression on AI with either palbociclib or ribociclib 500 mg monthly fulvestrant (IM) + 150 mg BID abemaciclib N = 250 5 mg QD lasofoxifene + 150 mg BID abemaciclib N = 250 Primary Endpoint Progression-free survival Key Secondary Endpoints Objective response rate, overall survival Other Secondary Endpoints Clinical benefit rate, duration of response, time to response, time to cytotoxic chemotherapy, quality of life, and safety Screening (30 days) Treatment Period (up to 48 mo) Study Milestones FPI: 1Q2024 (recruiting) LPI: Expected 2Q2026 Topline: Expected 1H2027 Study Outcomes ELAINE-3 aims to demonstrate the potential of lasofoxifene as a new standard-of-care for ESR1-driven post-CDK4/6i metastatic breast cancer Randomization (1:1)

Over 50% enrolled - expected to readout in mid-2027 Global study with >200 sites Europe, North America, and APAC (including China) Study is being executed by Medpace, a full-service global CRO with extensive experience running large, successful breast cancer trials Primary endpoint is determined by an independent review of centralized scans using industry standard RECIST criteria Primary readout conducted when 285 patients have been determined as meeting the RECIST criteria for progression ELAINE-3 is on track to complete enrollment by mid-2026 © Athira Pharma, Inc.
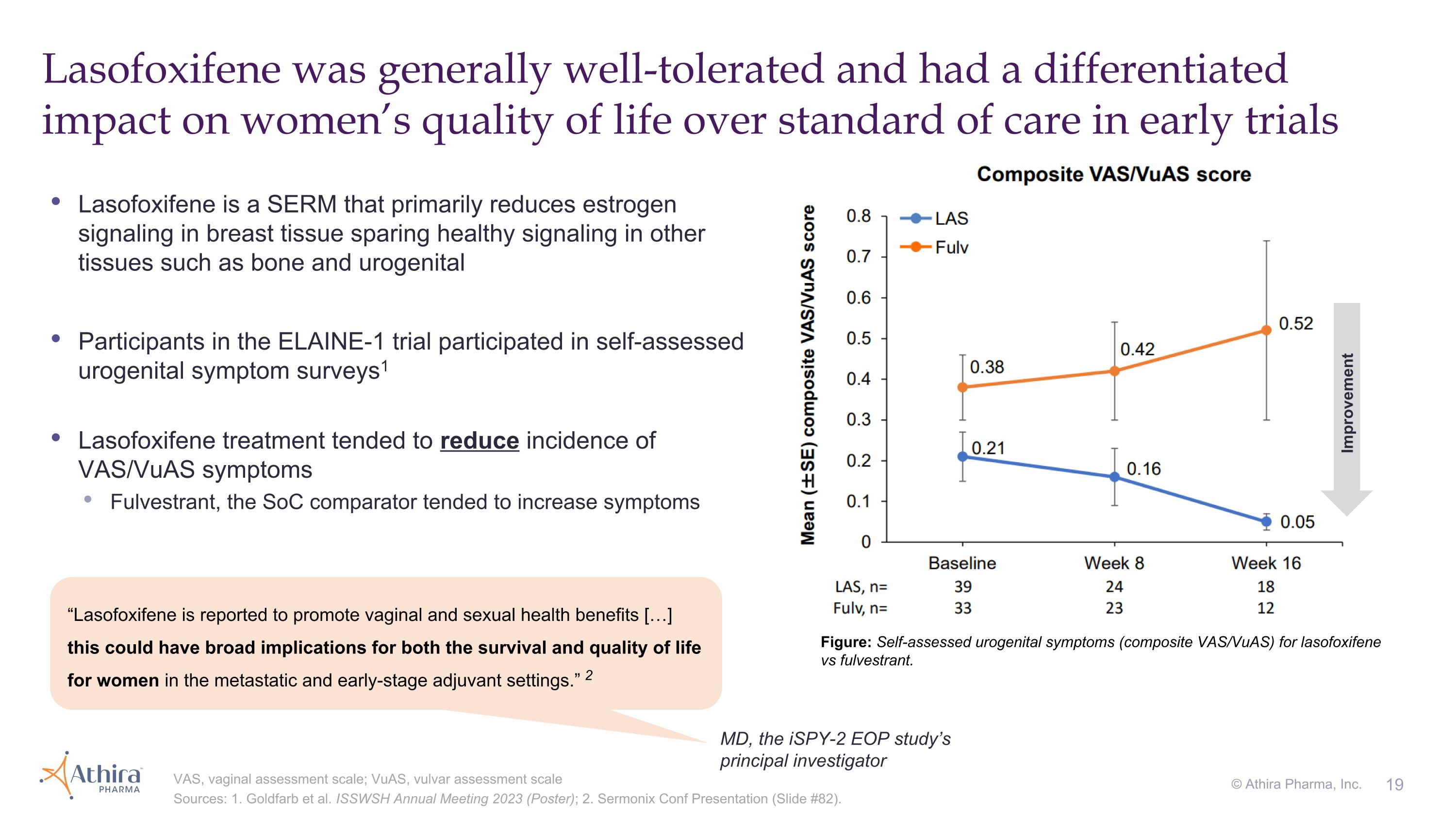
Lasofoxifene was generally well-tolerated and had a differentiated impact on women’s quality of life over standard of care in early trials VAS, vaginal assessment scale; VuAS, vulvar assessment scale Sources: 1. Goldfarb et al. ISSWSH Annual Meeting 2023 (Poster); 2. Sermonix Conf Presentation (Slide #82). Figure: Self-assessed urogenital symptoms (composite VAS/VuAS) for lasofoxifene vs fulvestrant. Improvement Lasofoxifene is a SERM that primarily reduces estrogen signaling in breast tissue sparing healthy signaling in other tissues such as bone and urogenital Participants in the ELAINE-1 trial participated in self-assessed urogenital symptom surveys1 Lasofoxifene treatment tended to reduce incidence of VAS/VuAS symptoms Fulvestrant, the SoC comparator tended to increase symptoms MD, the iSPY-2 EOP study’s principal investigator “Lasofoxifene is reported to promote vaginal and sexual health benefits […] this could have broad implications for both the survival and quality of life for women in the metastatic and early-stage adjuvant settings.” 2 © Athira Pharma, Inc.
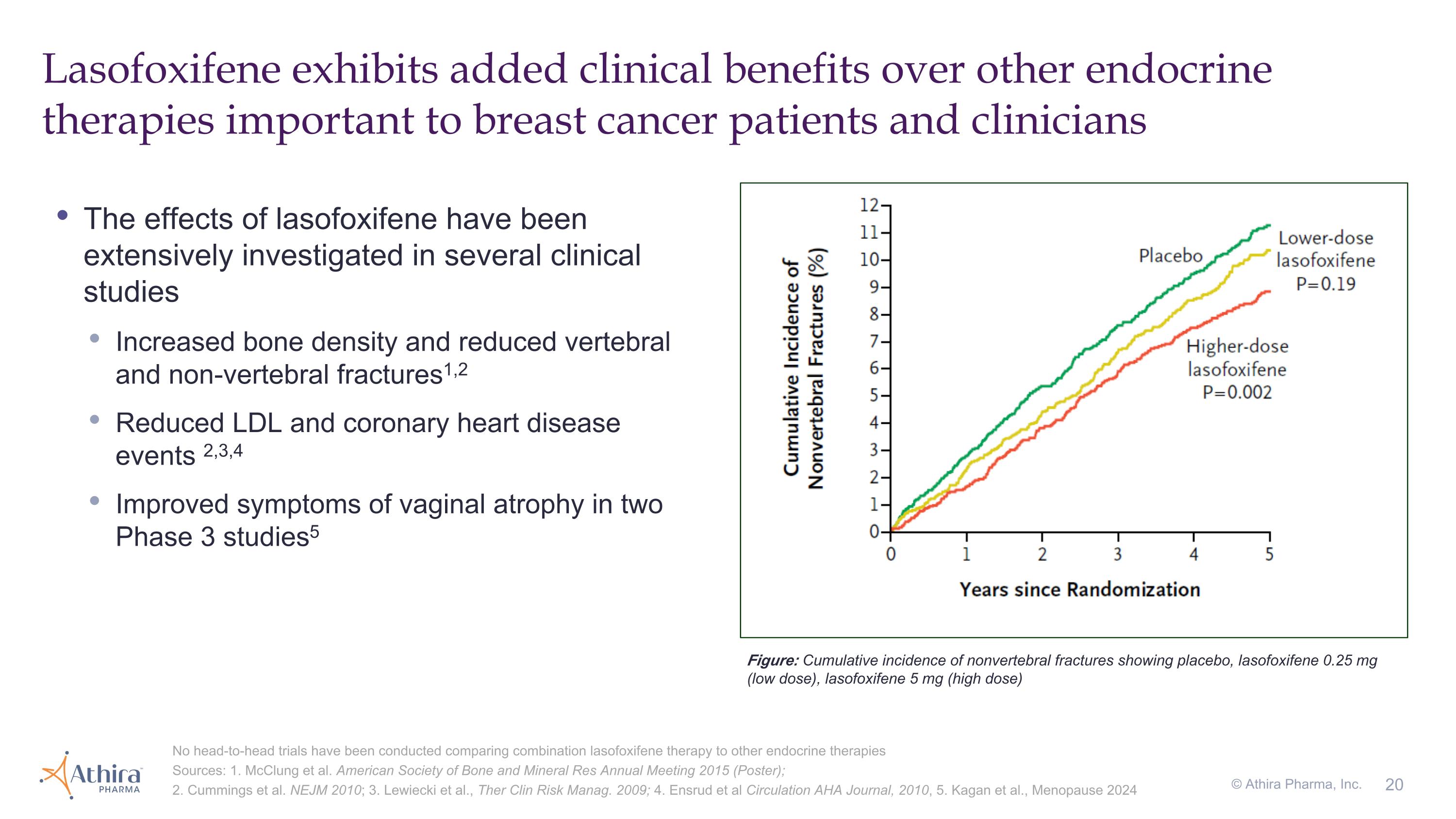
Lasofoxifene exhibits added clinical benefits over other endocrine therapies important to breast cancer patients and clinicians No head-to-head trials have been conducted comparing combination lasofoxifene therapy to other endocrine therapies Sources: 1. McClung et al. American Society of Bone and Mineral Res Annual Meeting 2015 (Poster); 2. Cummings et al. NEJM 2010; 3. Lewiecki et al., Ther Clin Risk Manag. 2009; 4. Ensrud et al Circulation AHA Journal, 2010, 5. Kagan et al., Menopause 2024 The effects of lasofoxifene have been extensively investigated in several clinical studies Increased bone density and reduced vertebral and non-vertebral fractures1,2 Reduced LDL and coronary heart disease events 2,3,4 Improved symptoms of vaginal atrophy in two Phase 3 studies5 © Athira Pharma, Inc. Figure: Cumulative incidence of nonvertebral fractures showing placebo, lasofoxifene 0.25 mg (low dose), lasofoxifene 5 mg (high dose)
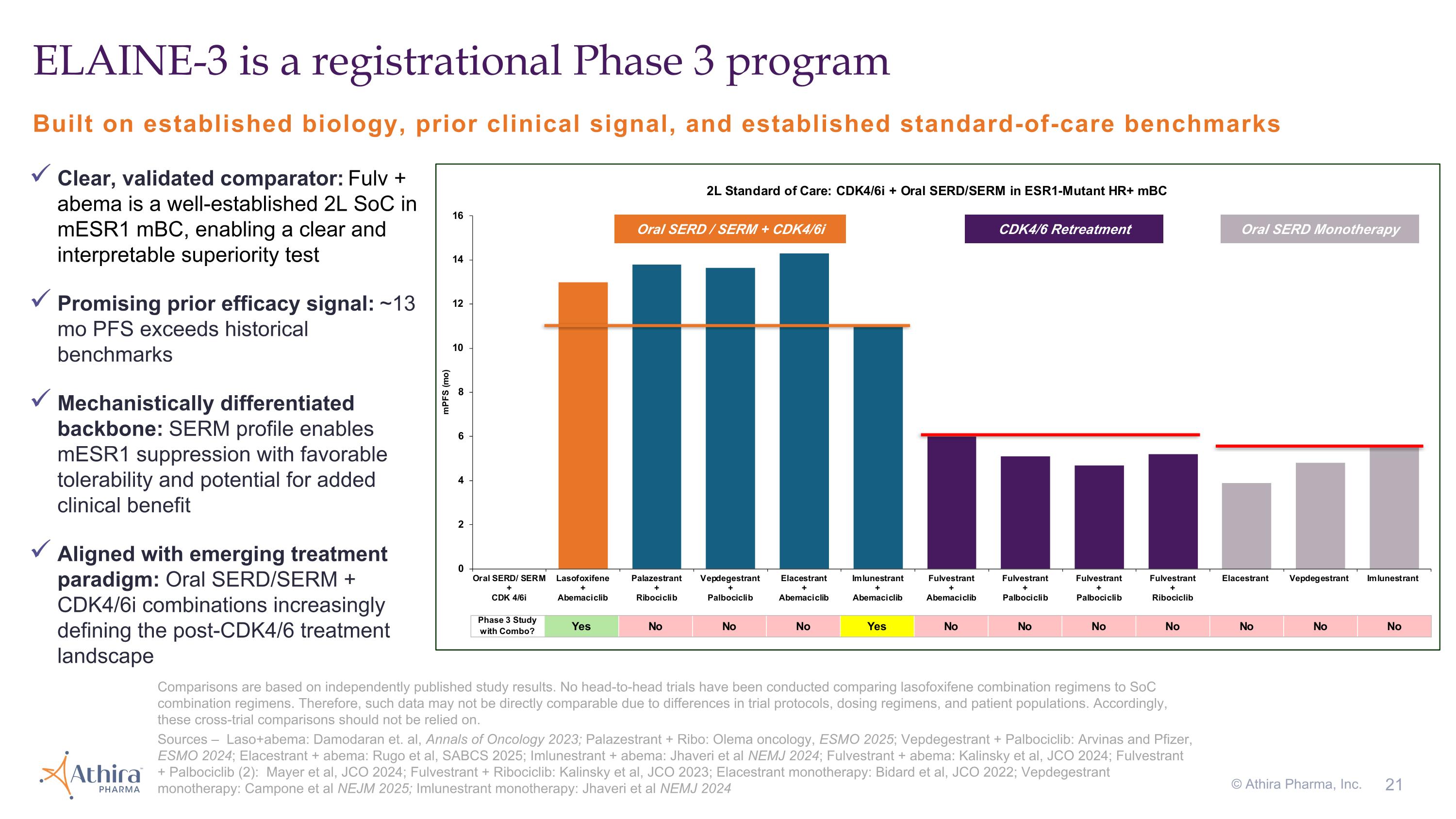
Built on established biology, prior clinical signal, and established standard-of-care benchmarks ELAINE-3 is a registrational Phase 3 program Comparisons are based on independently published study results. No head-to-head trials have been conducted comparing lasofoxifene combination regimens to SoC combination regimens. Therefore, such data may not be directly comparable due to differences in trial protocols, dosing regimens, and patient populations. Accordingly, these cross-trial comparisons should not be relied on. Sources – Laso+abema: Damodaran et. al, Annals of Oncology 2023; Palazestrant + Ribo: Olema oncology, ESMO 2025; Vepdegestrant + Palbociclib: Arvinas and Pfizer, ESMO 2024; Elacestrant + abema: Rugo et al, SABCS 2025; Imlunestrant + abema: Jhaveri et al NEMJ 2024; Fulvestrant + abema: Kalinsky et al, JCO 2024; Fulvestrant + Palbociclib (2): Mayer et al, JCO 2024; Fulvestrant + Ribociclib: Kalinsky et al, JCO 2023; Elacestrant monotherapy: Bidard et al, JCO 2022; Vepdegestrant monotherapy: Campone et al NEJM 2025; Imlunestrant monotherapy: Jhaveri et al NEMJ 2024 © Athira Pharma, Inc. Oral SERD / SERM + CDK4/6i CDK4/6 Retreatment Oral SERD Monotherapy Clear, validated comparator: Fulv + abema is a well-established 2L SoC in mESR1 mBC, enabling a clear and interpretable superiority test Promising prior efficacy signal: ~13 mo PFS exceeds historical benchmarks Mechanistically differentiated backbone: SERM profile enables mESR1 suppression with favorable tolerability and potential for added clinical benefit Aligned with emerging treatment paradigm: Oral SERD/SERM + CDK4/6i combinations increasingly defining the post-CDK4/6 treatment landscape
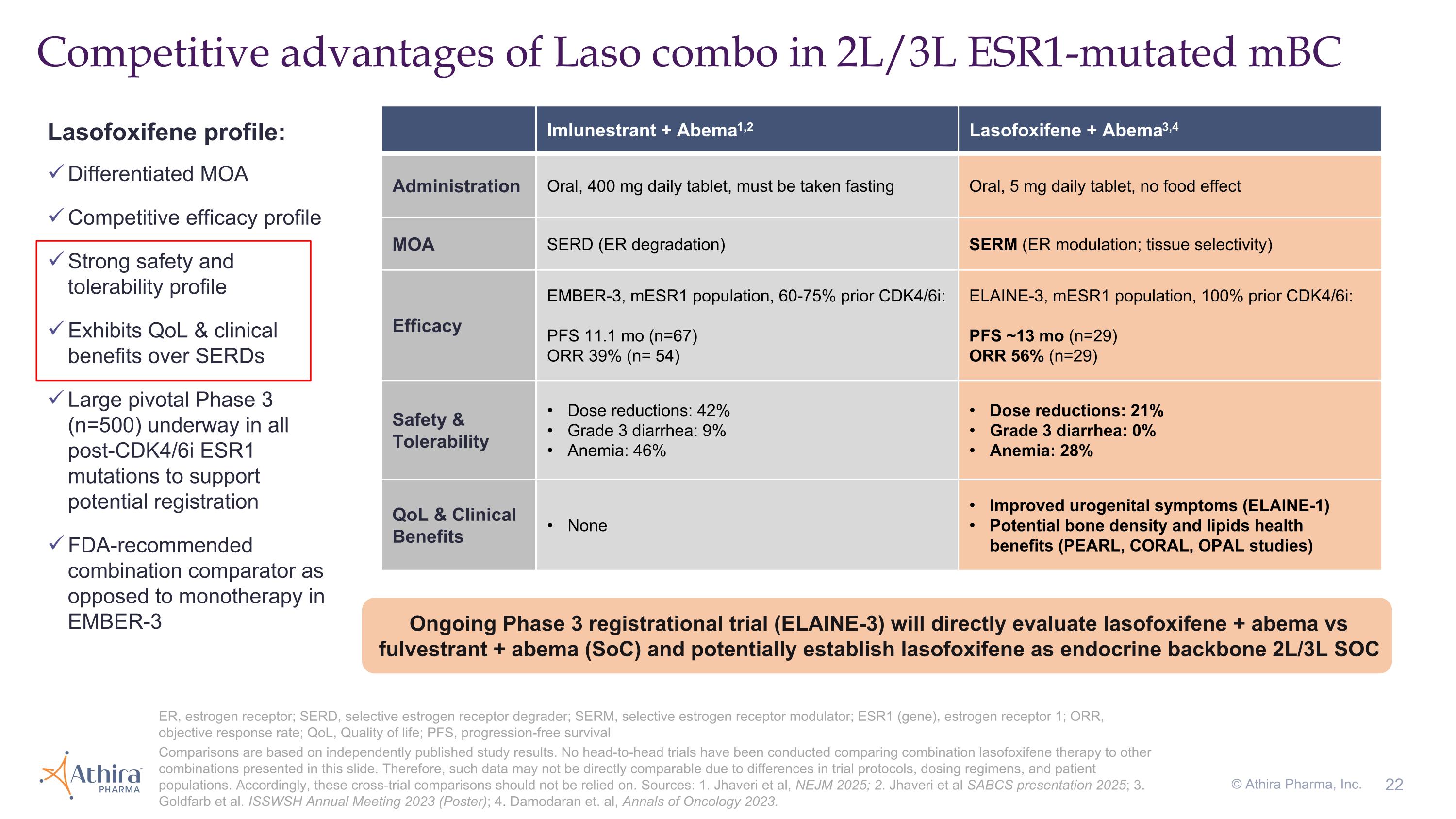
Competitive advantages of Laso combo in 2L/3L ESR1-mutated mBC Imlunestrant + Abema1,2 Lasofoxifene + Abema3,4 Administration Oral, 400 mg daily tablet, must be taken fasting Oral, 5 mg daily tablet, no food effect MOA SERD (ER degradation) SERM (ER modulation; tissue selectivity) Efficacy EMBER-3, mESR1 population, 60-75% prior CDK4/6i: PFS 11.1 mo (n=67) ORR 39% (n= 54) ELAINE-3, mESR1 population, 100% prior CDK4/6i: PFS ~13 mo (n=29) ORR 56% (n=29) Safety & Tolerability Dose reductions: 42% Grade 3 diarrhea: 9% Anemia: 46% Dose reductions: 21% Grade 3 diarrhea: 0% Anemia: 28% QoL & Clinical Benefits None Improved urogenital symptoms (ELAINE-1) Potential bone density and lipids health benefits (PEARL, CORAL, OPAL studies) ER, estrogen receptor; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulator; ESR1 (gene), estrogen receptor 1; ORR, objective response rate; QoL, Quality of life; PFS, progression-free survival Comparisons are based on independently published study results. No head-to-head trials have been conducted comparing combination lasofoxifene therapy to other combinations presented in this slide. Therefore, such data may not be directly comparable due to differences in trial protocols, dosing regimens, and patient populations. Accordingly, these cross-trial comparisons should not be relied on. Sources: 1. Jhaveri et al, NEJM 2025; 2. Jhaveri et al SABCS presentation 2025; 3. Goldfarb et al. ISSWSH Annual Meeting 2023 (Poster); 4. Damodaran et. al, Annals of Oncology 2023. © Athira Pharma, Inc. Lasofoxifene profile: Differentiated MOA Competitive efficacy profile Strong safety and tolerability profile Exhibits QoL & clinical benefits over SERDs Large pivotal Phase 3 (n=500) underway in all post-CDK4/6i ESR1 mutations to support potential registration FDA-recommended combination comparator as opposed to monotherapy in EMBER-3 Ongoing Phase 3 registrational trial (ELAINE-3) will directly evaluate lasofoxifene + abema vs fulvestrant + abema (SoC) and potentially establish lasofoxifene as endocrine backbone 2L/3L SOC

Endocrine backbone choice drives long-term risk and differentiation Comparisons are based on independently published study results. No head-to-head trials have been conducted comparing combination lasofoxifene therapy to other combinations presented in this slide. Therefore, such data may not be directly comparable due to differences in trial protocols, dosing regimens, and patient populations. Accordingly, these cross-trial comparisons should not be relied on. © Athira Pharma, Inc. Safety and tolerability While cross trial comparisons should be interpreted with caution, lasofoxifene exhibits a favorable safety and tolerability profile, specifically with regards to dose reduction and serious AEs, and additional QoL benefits No stacking with abemaciclib for diarrhea Combination treatment strategy drives long-term risk Regimens rely on chronic CDK4/6 inhibition, which defines the dominant toxicity burden Endocrine backbone choice therefore determines safety risk, tolerability, combinability and long-term adherence Chronic exposure considerations Oral SERDs are entering longer-duration use across lines and combinations While short-term safety is reassuring, SERD chronic toxicity profiles are still maturing, and longer-term use may impact ER in healthy tissues (e.g. bone, vagina) SERMs bring decades of human exposure and predictable long-term safety characteristics Lasofoxifene + abemaciclib vs. SERD + CDK4/6i regimens Lasofoxifene has the potential to deliver durable efficacy, minimize long-term safety risks, and provide QoL and clinical benefits that may position it as the next backbone endocrine therapy
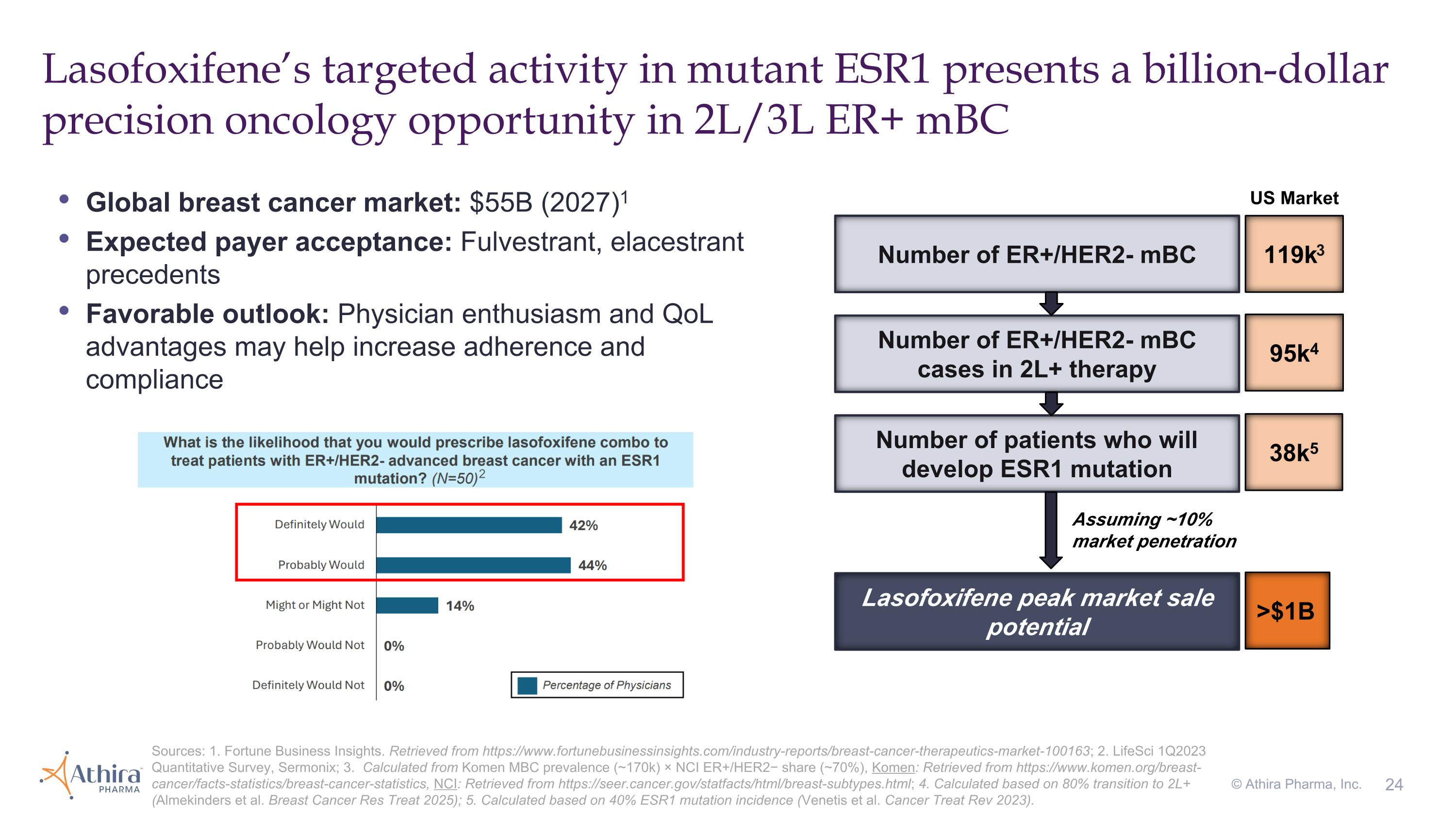
Lasofoxifene’s targeted activity in mutant ESR1 presents a billion-dollar precision oncology opportunity in 2L/3L ER+ mBC Number of ER+/HER2- mBC 119k3 Number of ER+/HER2- mBC cases in 2L+ therapy 95k4 Number of patients who will develop ESR1 mutation 38k5 Global breast cancer market: $55B (2027)1 Expected payer acceptance: Fulvestrant, elacestrant precedents Favorable outlook: Physician enthusiasm and QoL advantages may help increase adherence and compliance Lasofoxifene peak market sale potential >$1B Sources: 1. Fortune Business Insights. Retrieved from https://www.fortunebusinessinsights.com/industry-reports/breast-cancer-therapeutics-market-100163; 2. LifeSci 1Q2023 Quantitative Survey, Sermonix; 3. Calculated from Komen MBC prevalence (~170k) × NCI ER+/HER2− share (~70%), Komen: Retrieved from https://www.komen.org/breast-cancer/facts-statistics/breast-cancer-statistics, NCI: Retrieved from https://seer.cancer.gov/statfacts/html/breast-subtypes.html; 4. Calculated based on 80% transition to 2L+ (Almekinders et al. Breast Cancer Res Treat 2025); 5. Calculated based on 40% ESR1 mutation incidence (Venetis et al. Cancer Treat Rev 2023). Assuming ~10% market penetration 2 © Athira Pharma, Inc. US Market
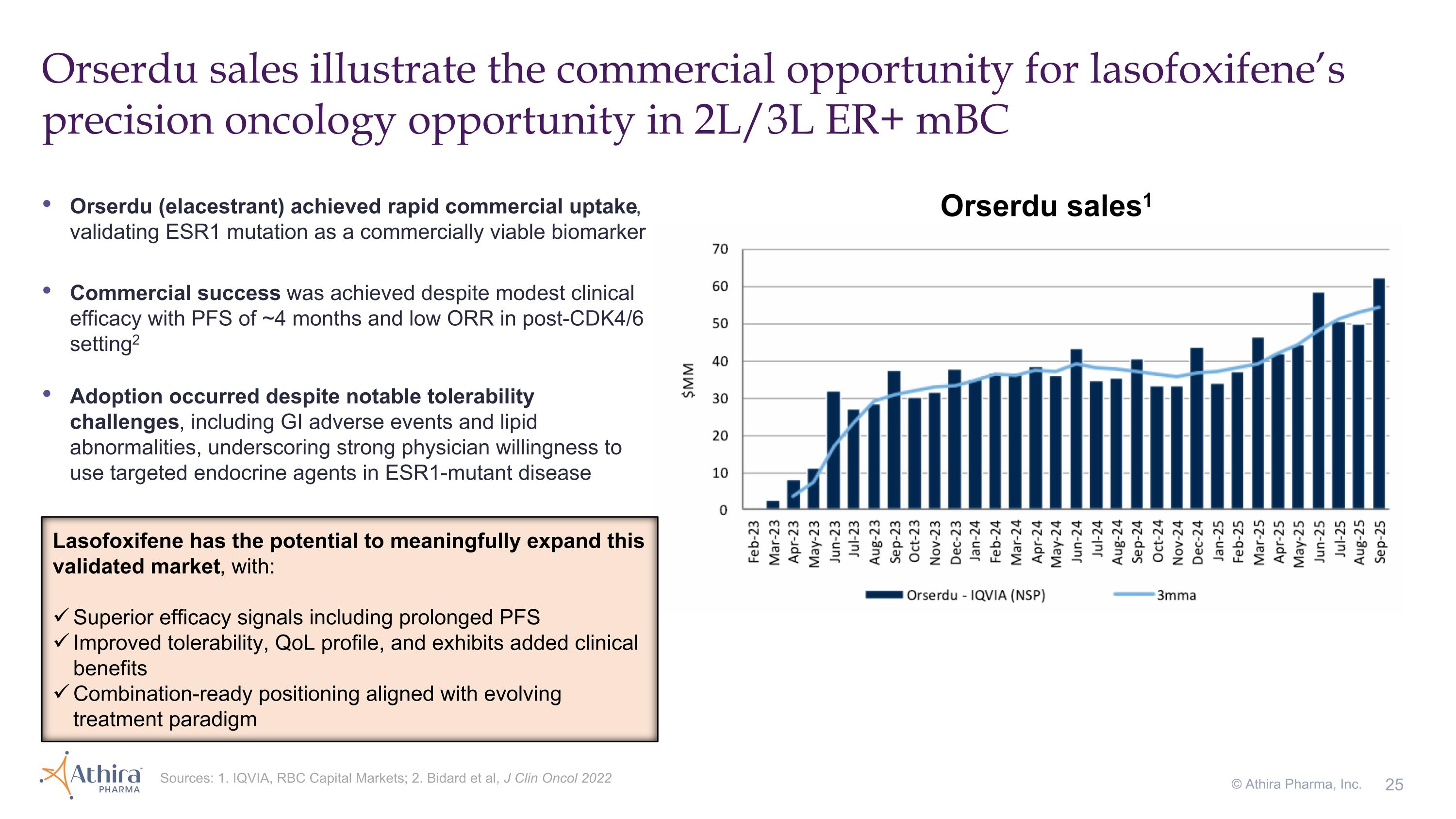
Orserdu sales illustrate the commercial opportunity for lasofoxifene’s precision oncology opportunity in 2L/3L ER+ mBC Sources: 1. IQVIA, RBC Capital Markets; 2. Bidard et al, J Clin Oncol 2022 © Athira Pharma, Inc. Orserdu (elacestrant) achieved rapid commercial uptake, validating ESR1 mutation as a commercially viable biomarker Commercial success was achieved despite modest clinical efficacy with PFS of ~4 months and low ORR in post-CDK4/6 setting2 Adoption occurred despite notable tolerability challenges, including GI adverse events and lipid abnormalities, underscoring strong physician willingness to use targeted endocrine agents in ESR1-mutant disease Lasofoxifene has the potential to meaningfully expand this validated market, with: Superior efficacy signals including prolonged PFS Improved tolerability, QoL profile, and exhibits added clinical benefits Combination-ready positioning aligned with evolving treatment paradigm Orserdu sales1
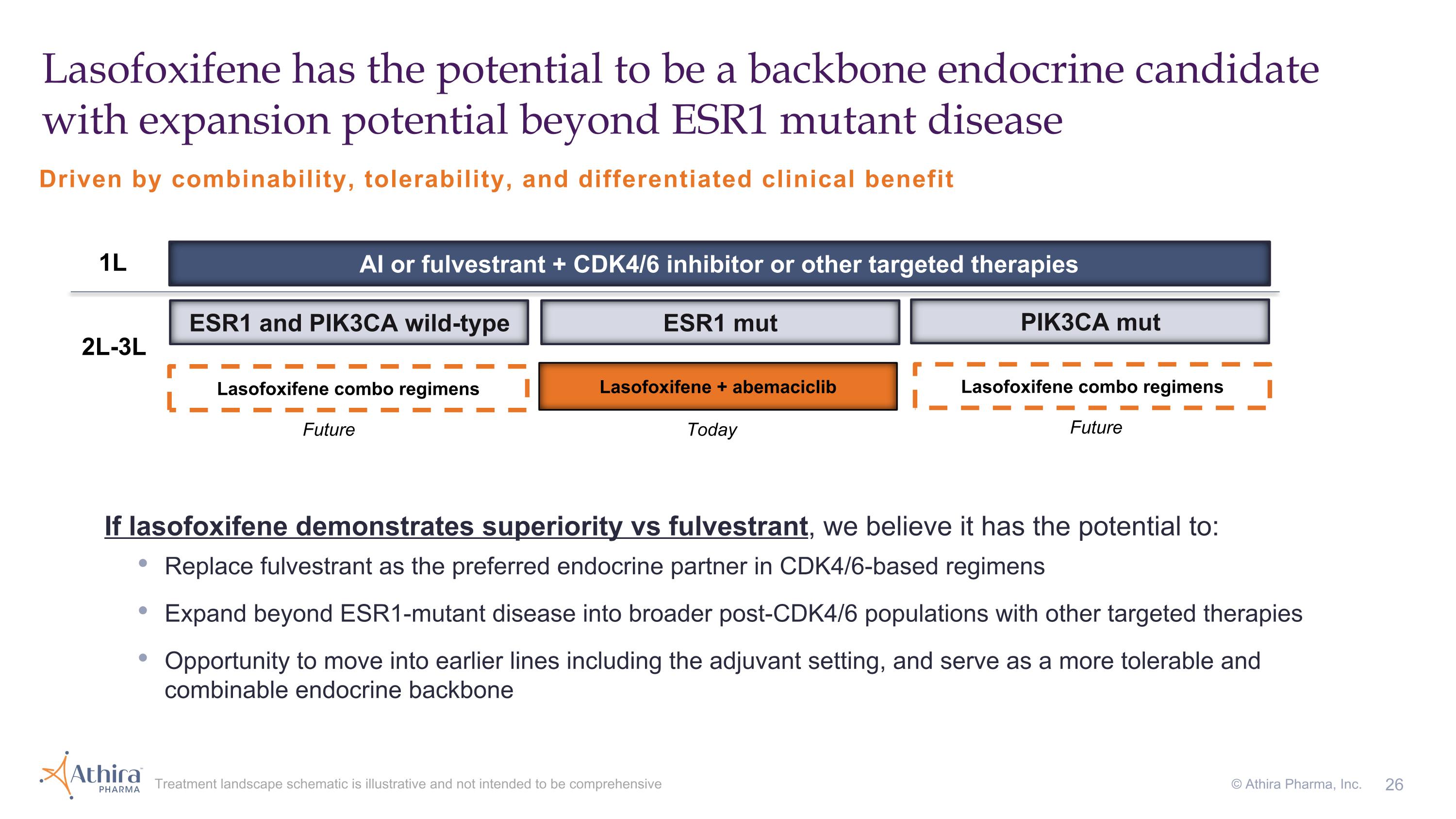
Lasofoxifene has the potential to be a backbone endocrine candidate with expansion potential beyond ESR1 mutant disease Treatment landscape schematic is illustrative and not intended to be comprehensive © Athira Pharma, Inc. ESR1 and PIK3CA wild-type ESR1 mut PIK3CA mut AI or fulvestrant + CDK4/6 inhibitor or other targeted therapies 1L 2L-3L Lasofoxifene + abemaciclib Lasofoxifene combo regimens Lasofoxifene combo regimens Driven by combinability, tolerability, and differentiated clinical benefit Today Future Future If lasofoxifene demonstrates superiority vs fulvestrant, we believe it has the potential to: Replace fulvestrant as the preferred endocrine partner in CDK4/6-based regimens Expand beyond ESR1-mutant disease into broader post-CDK4/6 populations with other targeted therapies Opportunity to move into earlier lines including the adjuvant setting, and serve as a more tolerable and combinable endocrine backbone
Expansion potential as endocrine partner of choice Oral administration Large unmet need Favorable safety profile and generally well-tolerated Superior PFS from Ph2 Exhibits improved QoL and clinical benefits Lasofoxifene: Ongoing registrational Phase 3 trial with readout in 2027 A differentiated therapy designed to overcome resistance, improve QoL, and establish a new standard of care in ER+ ESR1-mutant breast cancer © Athira Pharma, Inc.

28 ATH-1105 for the potential treatment of ALS Small molecule positive modulator of HGF © Athira Pharma, Inc.
Slowing neurodegeneration in ALS is a critical unmet need Sources: 1. Packard Center. Retrieved from https://packardcenter.org/answer-als-partners-to-launch-the-end-als-challenge-digital-competition; 2. CDC. Retrieved from https://www.cdc.gov/als/php/abstracts-publications-reports/prevalence-2022-2030.html; 3. ALS United. Retrieved from https://alsunitedchicago.org/als-life-expectancy-by-age/; 4. ALS Association. Retrieved from https://www.als.org/understanding-als; 5. ALS Association. Retrieved from https://www.als.org/advocacy/federal-public-policy-priorities; 6. ALS Association. https://www.als.org/navigating-als/living-with-als/therapies-care. ALS is a devasting neurodegenerative disease in which people progressively lose muscle control 225,000 people globally affected by ALS1 with ~33,000 of those cases in the US2 Average life expectancy is 2-5 years post-diagnosis3 Every 90 minutes someone is diagnosed with ALS4 Estimated annual out-of-pocket cost for care is $250,0005 Burden of ALS There is no cure for ALS and few effective treatment options Approved therapies only modestly slow progression and improve survival No current ALS drugs significantly protect motor neurons from the complex pathology and progressive degeneration Treatment Limitations6 There is an urgent need for therapies that meaningfully slow neurodegeneration, preserve motor function, improve quality of life, and prolong survival Significant Unmet Need © Athira Pharma, Inc.
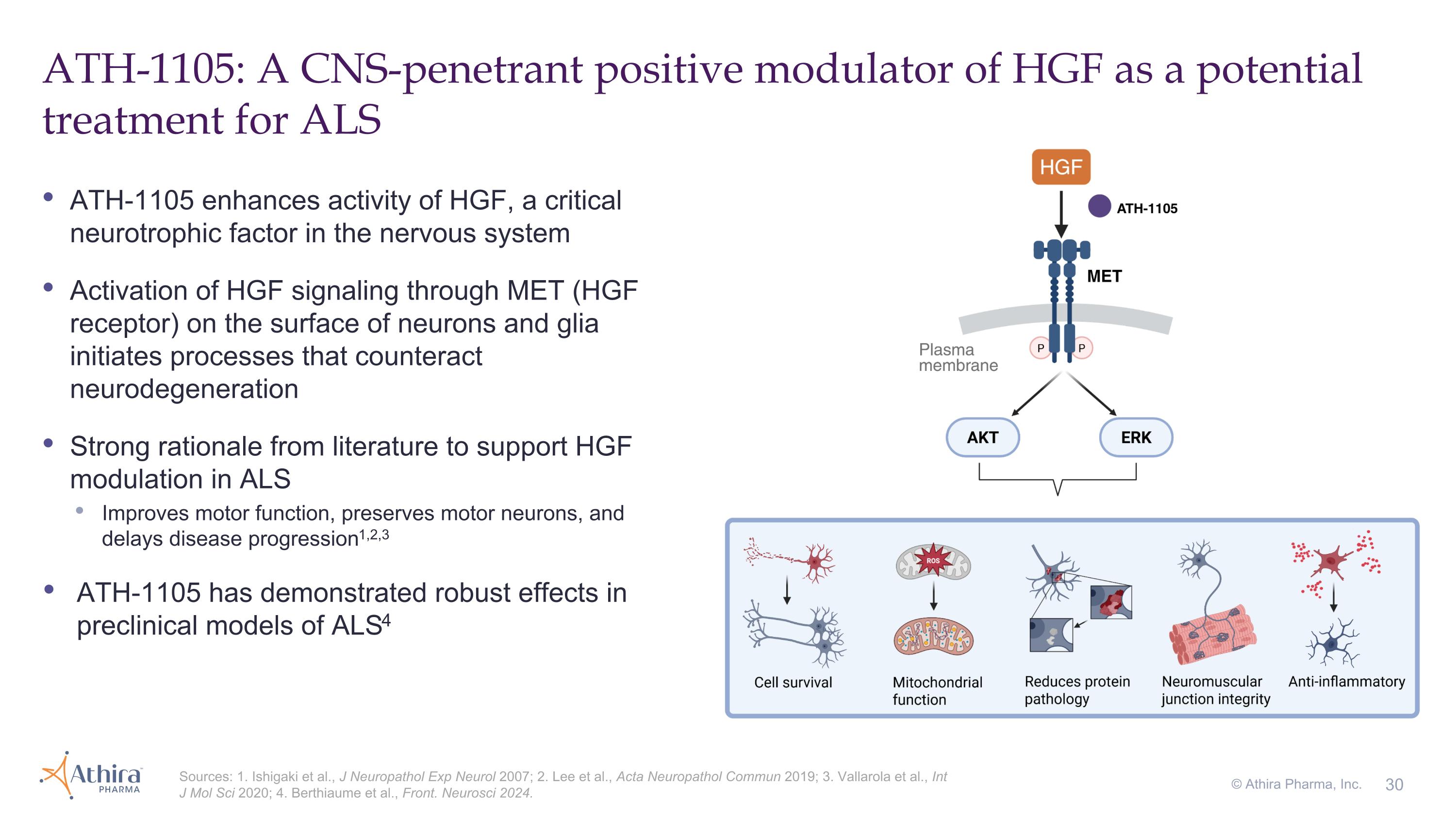
ATH-1105 enhances activity of HGF, a critical neurotrophic factor in the nervous system Activation of HGF signaling through MET (HGF receptor) on the surface of neurons and glia initiates processes that counteract neurodegeneration Strong rationale from literature to support HGF modulation in ALS Improves motor function, preserves motor neurons, and delays disease progression1,2,3 ATH-1105 has demonstrated robust effects in preclinical models of ALS4 ATH-1105: A CNS-penetrant positive modulator of HGF as a potential treatment for ALS Sources: 1. Ishigaki et al., J Neuropathol Exp Neurol 2007; 2. Lee et al., Acta Neuropathol Commun 2019; 3. Vallarola et al., Int J Mol Sci 2020; 4. Berthiaume et al., Front. Neurosci 2024. © Athira Pharma, Inc.
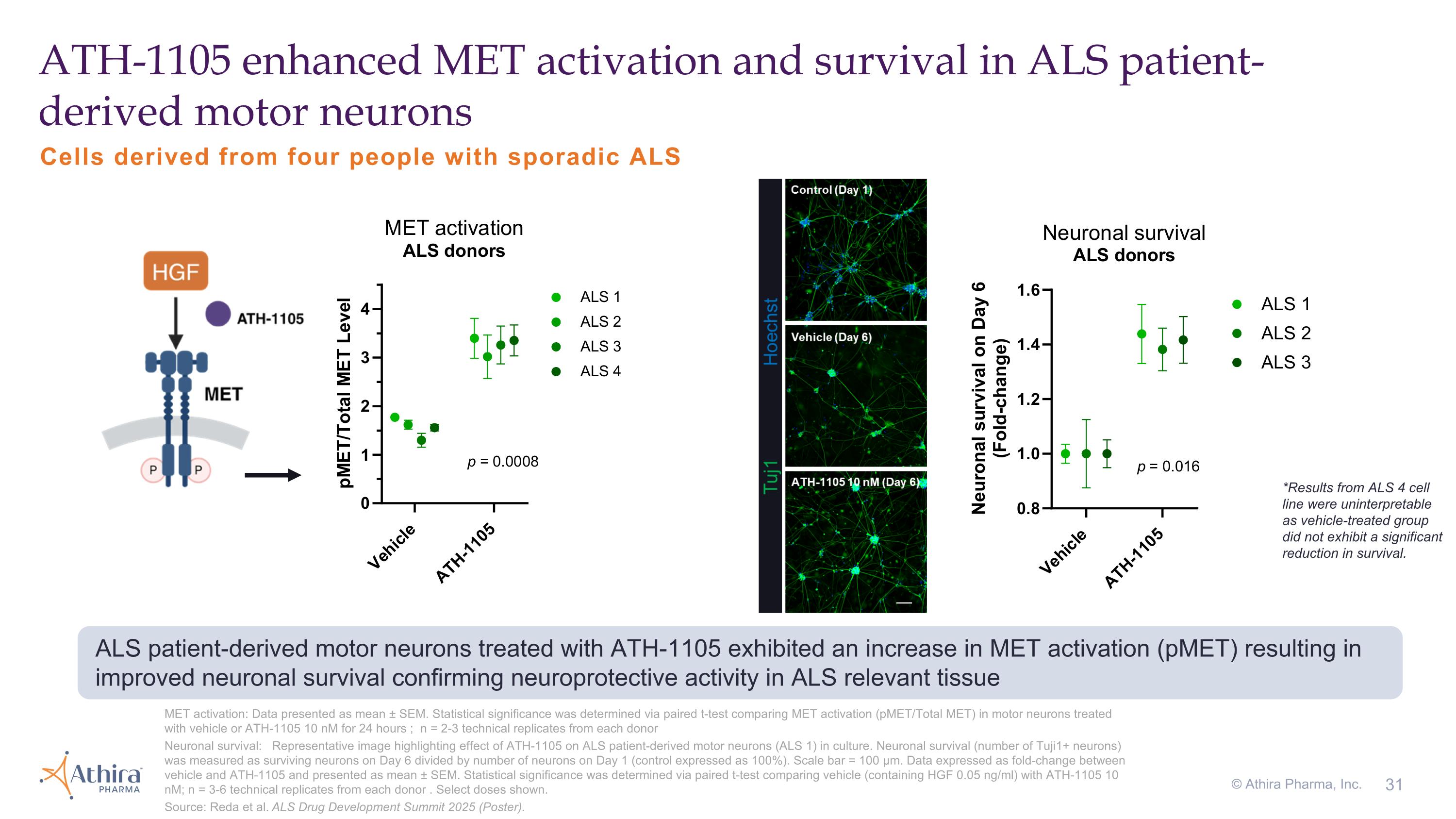
MET activation: Data presented as mean ± SEM. Statistical significance was determined via paired t-test comparing MET activation (pMET/Total MET) in motor neurons treated with vehicle or ATH-1105 10 nM for 24 hours ; n = 2-3 technical replicates from each donor Neuronal survival: Representative image highlighting effect of ATH-1105 on ALS patient-derived motor neurons (ALS 1) in culture. Neuronal survival (number of Tuji1+ neurons) was measured as surviving neurons on Day 6 divided by number of neurons on Day 1 (control expressed as 100%). Scale bar = 100 µm. Data expressed as fold-change between vehicle and ATH-1105 and presented as mean ± SEM. Statistical significance was determined via paired t-test comparing vehicle (containing HGF 0.05 ng/ml) with ATH-1105 10 nM; n = 3-6 technical replicates from each donor . Select doses shown. Source: Reda et al. ALS Drug Development Summit 2025 (Poster). ATH-1105 enhanced MET activation and survival in ALS patient-derived motor neurons ALS patient-derived motor neurons treated with ATH-1105 exhibited an increase in MET activation (pMET) resulting in improved neuronal survival confirming neuroprotective activity in ALS relevant tissue *Results from ALS 4 cell line were uninterpretable as vehicle-treated group did not exhibit a significant reduction in survival. Cells derived from four people with sporadic ALS © Athira Pharma, Inc.
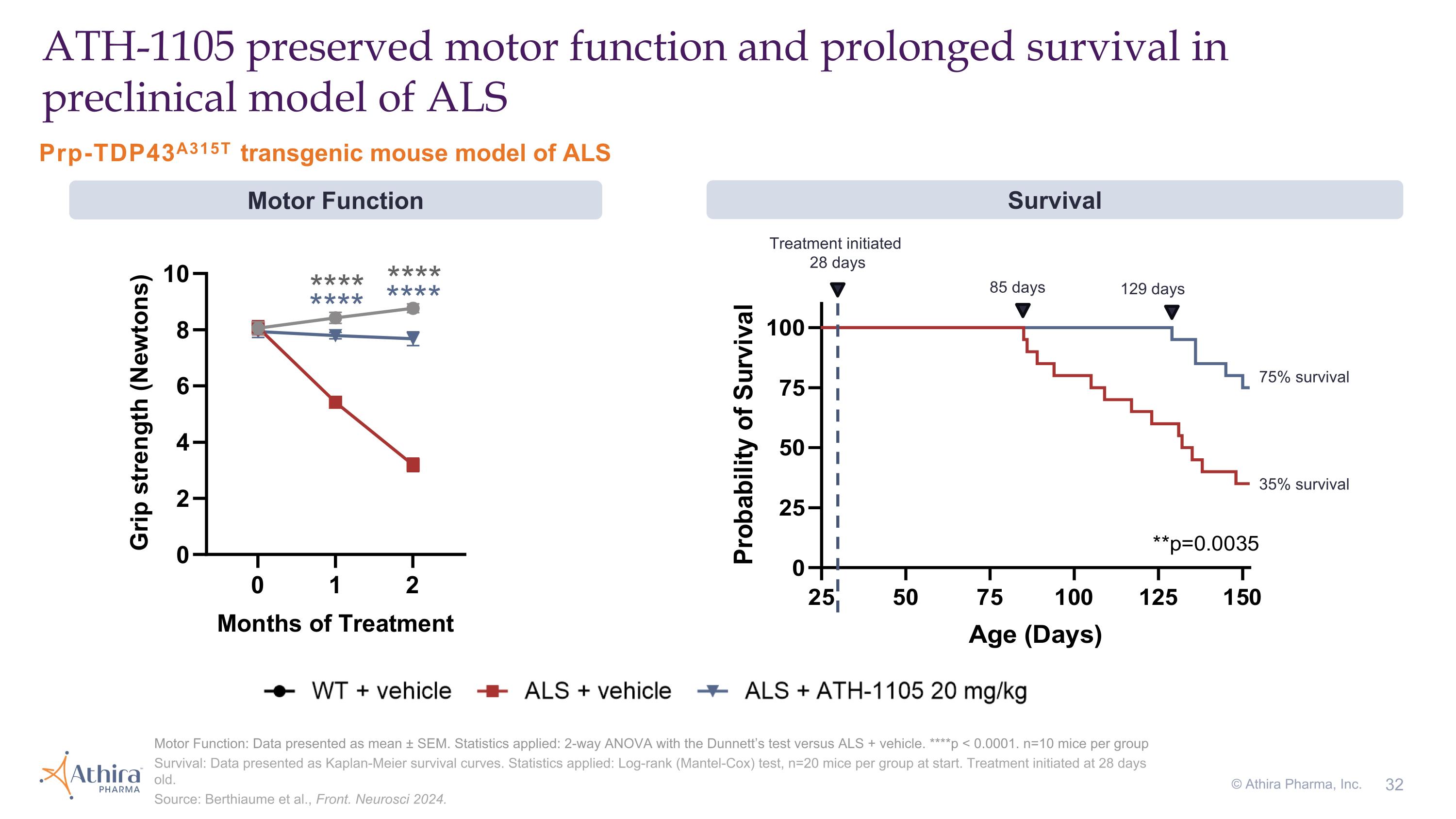
Prp-TDP43A315T transgenic mouse model of ALS ATH-1105 preserved motor function and prolonged survival in preclinical model of ALS Motor Function: Data presented as mean ± SEM. Statistics applied: 2-way ANOVA with the Dunnett’s test versus ALS + vehicle. ****p < 0.0001. n=10 mice per group Survival: Data presented as Kaplan-Meier survival curves. Statistics applied: Log-rank (Mantel-Cox) test, n=20 mice per group at start. Treatment initiated at 28 days old. Source: Berthiaume et al., Front. Neurosci 2024. 85 days 75% survival 35% survival 129 days Treatment initiated 28 days Survival Motor Function © Athira Pharma, Inc.
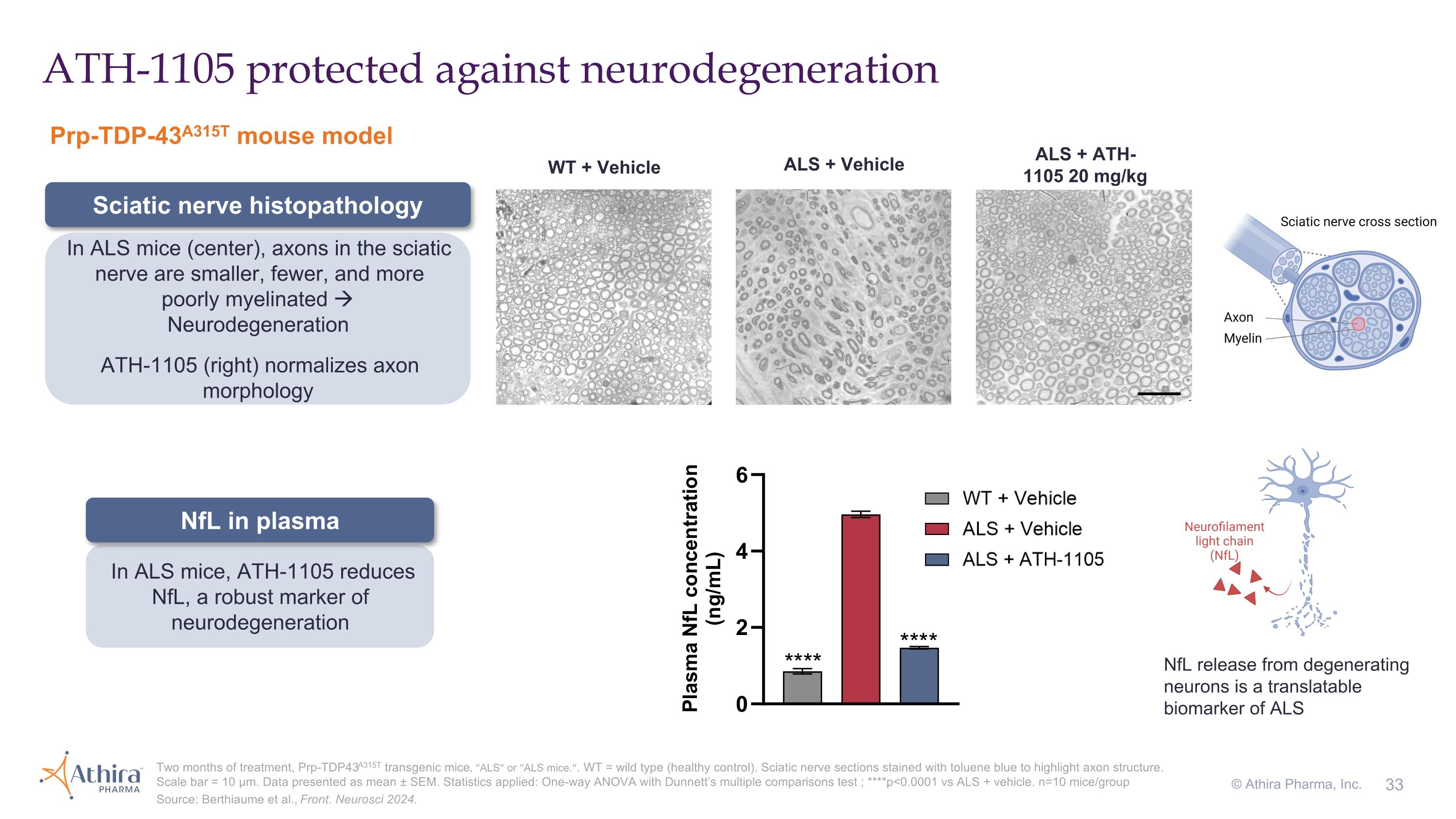
ATH-1105 protected against neurodegeneration Two months of treatment, Prp-TDP43A315T transgenic mice, ²ALS² or ²ALS mice.². WT = wild type (healthy control). Sciatic nerve sections stained with toluene blue to highlight axon structure. Scale bar = 10 µm. Data presented as mean ± SEM. Statistics applied: One-way ANOVA with Dunnett’s multiple comparisons test ; ****p<0.0001 vs ALS + vehicle. n=10 mice/group Source: Berthiaume et al., Front. Neurosci 2024. WT + Vehicle ALS + Vehicle ALS + ATH-1105 20 mg/kg In ALS mice, ATH-1105 reduces NfL, a robust marker of neurodegeneration In ALS mice (center), axons in the sciatic nerve are smaller, fewer, and more poorly myelinated Neurodegeneration ATH-1105 (right) normalizes axon morphology NfL release from degenerating neurons is a translatable biomarker of ALS Prp-TDP-43A315T mouse model Sciatic nerve histopathology NfL in plasma © Athira Pharma, Inc.
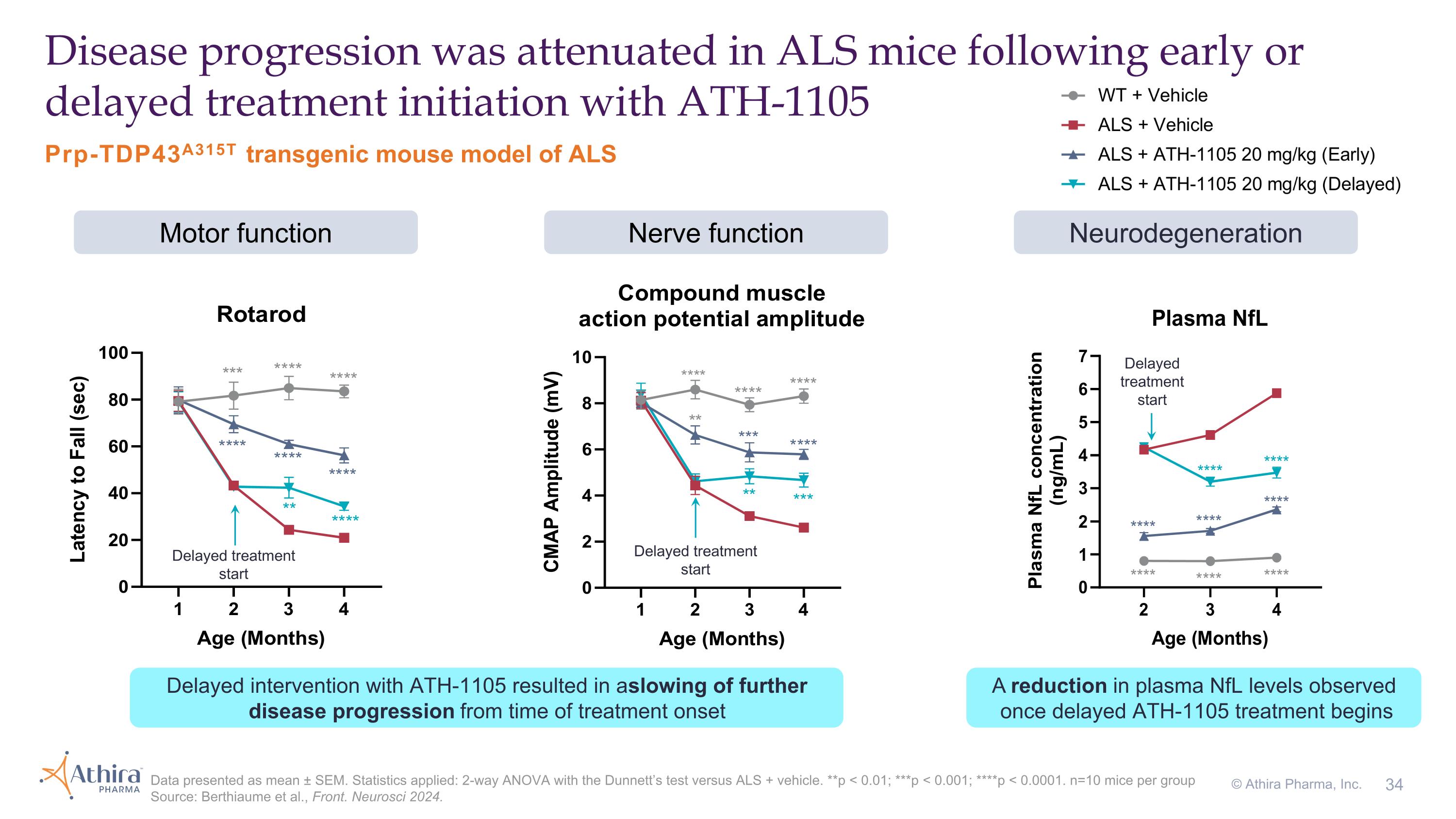
Delayed treatment start Delayed treatment start Prp-TDP43A315T transgenic mouse model of ALS Disease progression was attenuated in ALS mice following early or delayed treatment initiation with ATH-1105 Data presented as mean ± SEM. Statistics applied: 2-way ANOVA with the Dunnett’s test versus ALS + vehicle. **p < 0.01; ***p < 0.001; ****p < 0.0001. n=10 mice per group Source: Berthiaume et al., Front. Neurosci 2024. Motor function Nerve function Neurodegeneration Delayed treatment start Delayed intervention with ATH-1105 resulted in a slowing of further disease progression from time of treatment onset A reduction in plasma NfL levels observed once delayed ATH-1105 treatment begins © Athira Pharma, Inc.
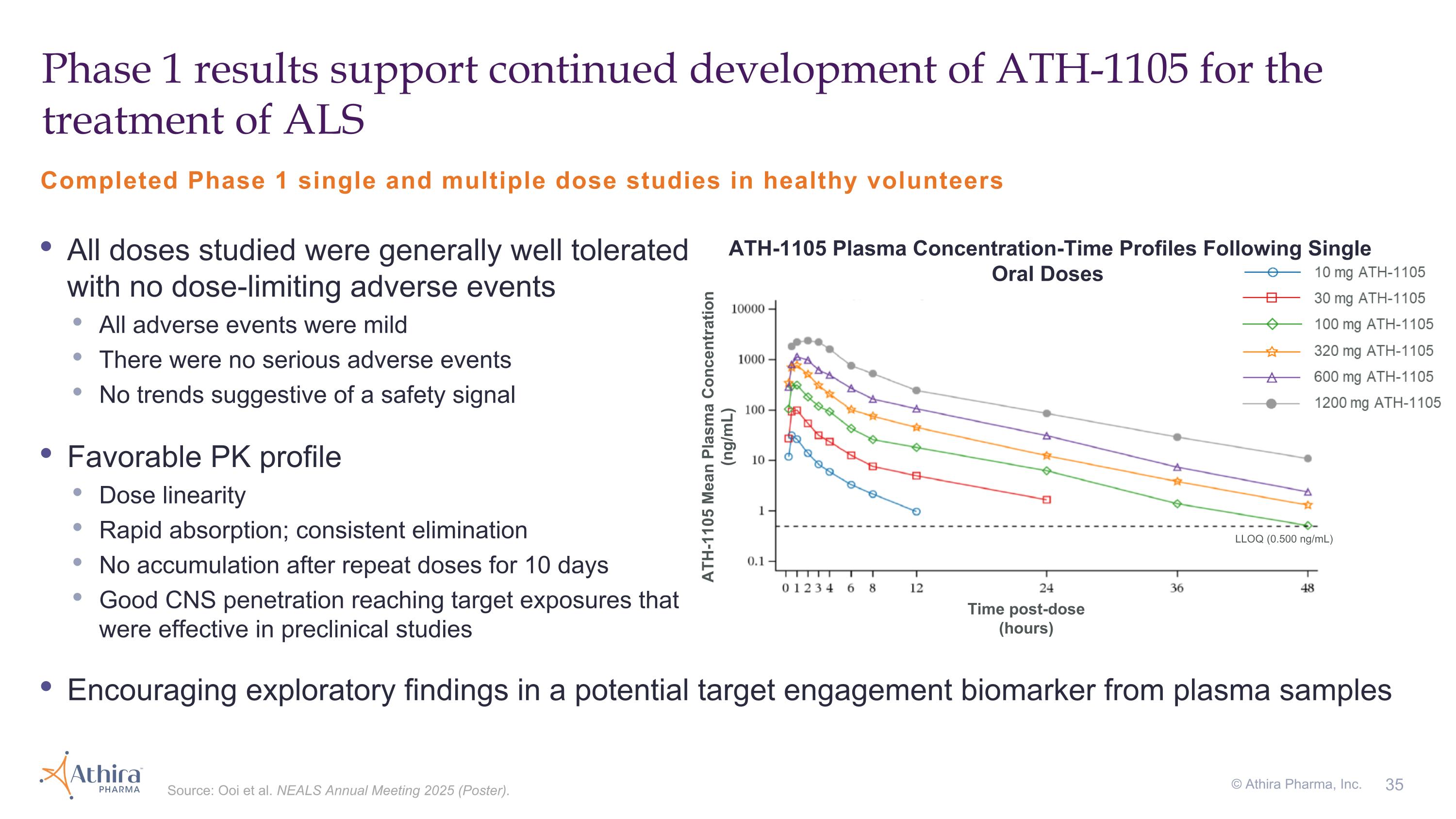
Phase 1 results support continued development of ATH-1105 for the treatment of ALS Completed Phase 1 single and multiple dose studies in healthy volunteers Source: Ooi et al. NEALS Annual Meeting 2025 (Poster). All doses studied were generally well tolerated with no dose-limiting adverse events All adverse events were mild There were no serious adverse events No trends suggestive of a safety signal Favorable PK profile Dose linearity Rapid absorption; consistent elimination No accumulation after repeat doses for 10 days Good CNS penetration reaching target exposures that were effective in preclinical studies Time post-dose (hours) ATH-1105 Mean Plasma Concentration (ng/mL) ATH-1105 Plasma Concentration-Time Profiles Following Single Oral Doses LLOQ (0.500 ng/mL) Encouraging exploratory findings in a potential target engagement biomarker from plasma samples © Athira Pharma, Inc.
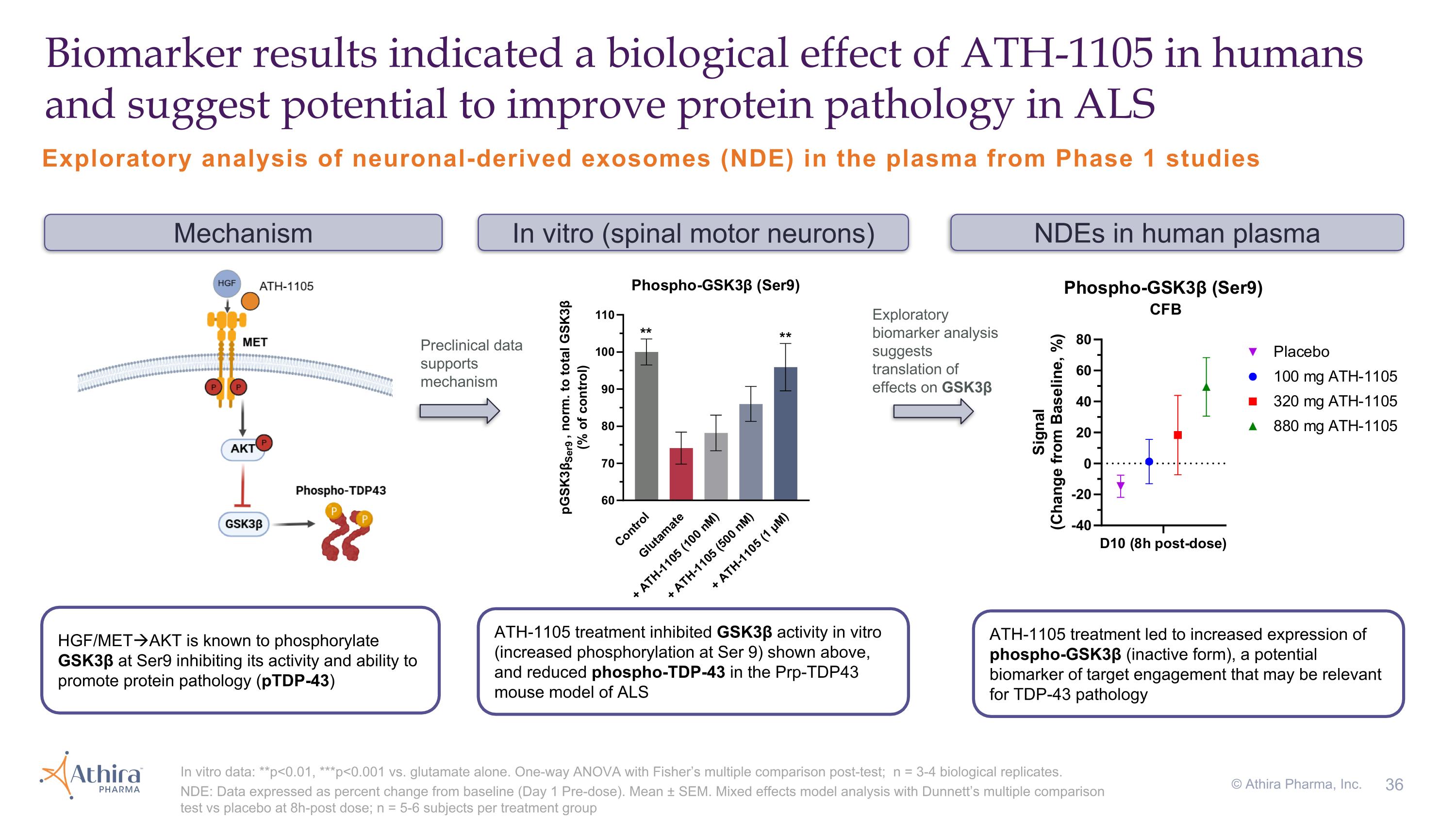
Exploratory analysis of neuronal-derived exosomes (NDE) in the plasma from Phase 1 studies Biomarker results indicated a biological effect of ATH-1105 in humans and suggest potential to improve protein pathology in ALS In vitro data: **p<0.01, ***p<0.001 vs. glutamate alone. One-way ANOVA with Fisher’s multiple comparison post-test; n = 3-4 biological replicates. NDE: Data expressed as percent change from baseline (Day 1 Pre-dose). Mean ± SEM. Mixed effects model analysis with Dunnett’s multiple comparison test vs placebo at 8h-post dose; n = 5-6 subjects per treatment group Mechanism NDEs in human plasma ATH-1105 treatment led to increased expression of phospho-GSK3β (inactive form), a potential biomarker of target engagement that may be relevant for TDP-43 pathology In vitro (spinal motor neurons) HGF/METAKT is known to phosphorylate GSK3β at Ser9 inhibiting its activity and ability to promote protein pathology (pTDP-43) ATH-1105 treatment inhibited GSK3β activity in vitro (increased phosphorylation at Ser 9) shown above, and reduced phospho-TDP-43 in the Prp-TDP43 mouse model of ALS © Athira Pharma, Inc. Preclinical data supports mechanism Exploratory biomarker analysis suggests translation of effects on GSK3β

Planning underway for a Phase 2 proof-of concept study to explore functional measures, biomarkers, and safety in people living with ALS Design considerations: ≥ 6 months treatment duration Biomarkers, including plasma NfL and NDEs Functional measures ALSFRS-R Vital capacity Grip strength Aim is to explore safety and preliminary efficacy to inform a potentially pivotal clinical trial ATH-1105 is ready for a Phase 2 study in people living with ALS © Athira Pharma, Inc.
Expansion potential ATH-1105 is a Phase 2 ready asset for the treatment of ALS A novel therapy designed to address the complex pathology and mitigate neurodegeneration in ALS Oral administration Neuroprotective* Favorable safety profile and generally well-tolerated Reduces plasma NfL* Biomarker signals in Phase 1 *in preclinical models © Athira Pharma, Inc.
Advancing 2 programs that have the potential to deliver life-changing therapies in breast cancer and ALS Transforming our future Compelling clinical development programs in areas of profound need Multibillion-dollar market opportunities Key clinical readouts within 2 years © Athira Pharma, Inc.

$90M upfront financing provides cash runway through data readout and into 2028 Up front financing supports lasofoxifene development program through topline data with sufficient capital runway into 2028 $90M upfront priced at a greater than 50% premium to our December 17, 2025 closing price [$6.35/share] If exercised, warrants provide up to additional $146M for further support PIPE financing up to $236 million © Athira Pharma, Inc.
Lasofoxifene Phase 3 registrational study ongoing with >50% enrolled ATH-1105 Phase 2 POC study in ALS planned to start in 1H2026 Expected key inflection points within ~2 years Topline results expected in mid-2027 Topline results expected 2027 © Athira Pharma, Inc.

Thank You © Athira Pharma, Inc.