

Peak Trial: Bezuclastinib + Sunitinib in Gastrointestinal Stromal Tumors (GIST) Top-Line Results Investor Webcast November 10, 2025

Forward-Looking Statements and Risk Factors This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties and assumptions. If the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited to, any statements of the plans, strategies, and objectives of management for future operations, including our clinical development and commercialization plans; any projections of financial information; any statement about historical results that may suggest trends for our business; any statement of expectation or belief regarding future events; potential markets or market size, technology developments, our clinical product pipeline, clinical and pre-clinical data or the implications thereof, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any statements regarding the anticipated benefits of our collaborations or other strategic transactions; and any statements of assumptions underlying any of the items mentioned. These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited to, risks associated with: the potential impacts of raising additional capital, including dilution to our existing stockholders, restrictions on our operations or requirements that we relinquish rights to our technologies or product candidates; business interruptions resulting from the coronavirus disease outbreak or similar public health crises, which could cause a disruption of the development of our product candidates and adversely impact our business; the success, cost, and timing of our product development activities and clinical trials; the timing of our planned regulatory submissions to the FDA for our product candidate bezuclastinib and feedback from the FDA as to our plans; our ability to obtain and maintain regulatory approval for our bezuclastinib product candidate and any other product candidates we may develop, and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; the potential for our identified research priorities to advance our bezuclastinib product candidate; the ability to license additional intellectual property relating to our product candidates from third parties and to comply with our existing license agreements and collaboration agreements; the ability and willingness of our third-party research institution collaborators to continue research and development activities relating to our product candidates; our ability to commercialize our products in light of the intellectual property rights of others; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; the scalability and commercial viability of our manufacturing methods and processes; the commercialization of our product candidates, if approved; our plans to research, develop, and commercialize our product candidates; our ability to attract collaborators with development, regulatory, and commercialization expertise; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; and the fact that interim clinical data may not be indicative of future results, among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our periodic filings filed from time to time with the Securities and Exchange Commission. Unless as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to changes in our expectations. All of Cogent Biosciences, Inc. (“Cogent”) product candidates are investigational product candidates and their safety and efficacy have not yet been established. Cogent has not obtained marketing approval for any product, and there is no certainty that any marketing approvals will be obtained or as to the timelines on which they will be obtained. 2

Agenda and Speakers Andrew Robbins Neeta Somaiah, M.D. Jessica Sachs, M.D. President and Chief Executive Officer Professor and Department Chair, Chief Medical Officer Department of Sarcoma Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center • Introduction Andrew Robbins • GIST Disease Overview • Peak Top-Line Results Dr. Neeta Somaiah • Patient Cases • Summary Andrew Robbins • Q&A All 3
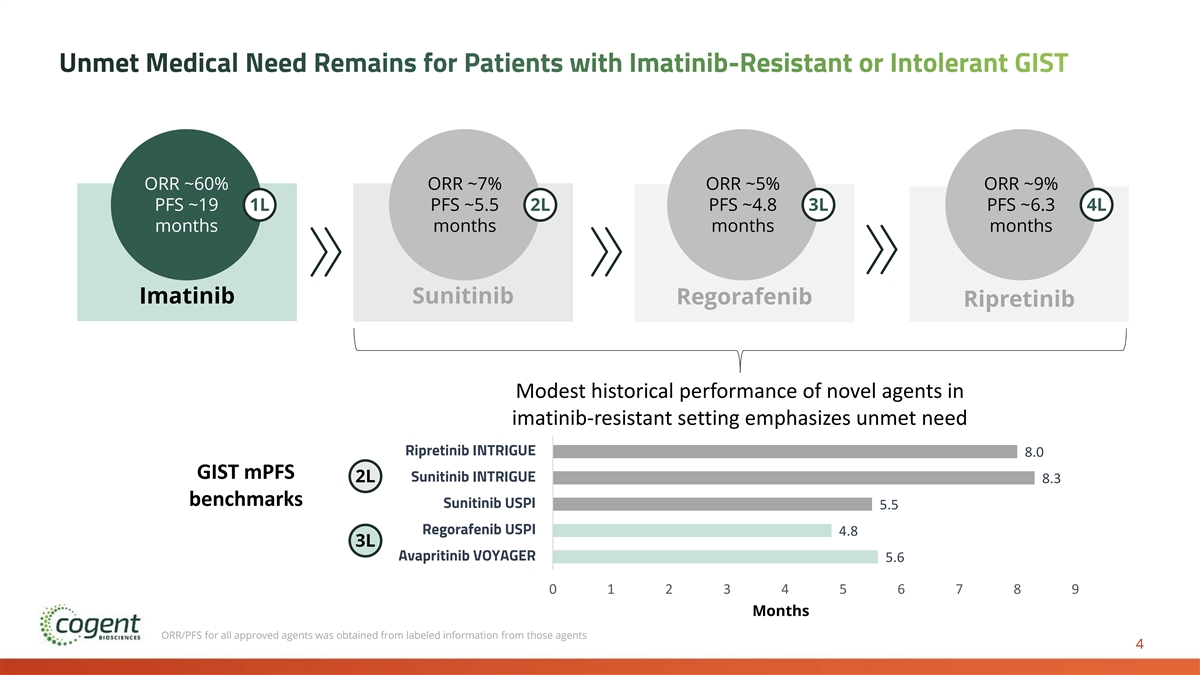
Unmet Medical Need Remains for Patients with Imatinib-Resistant or Intolerant GIST ORR ~60% ORR ~7% ORR ~5% ORR ~9% PFS ~19 1L PFS ~5.5 2L PFS ~4.8 3L PFS ~6.3 4L months months months months Imatinib Sunitinib Regorafenib Ripretinib Modest historical performance of novel agents in imatinib-resistant setting emphasizes unmet need Bezuclastinib + Sunitinib PEAK 1 Ripretinib INTRIGUE 8.0 GIST mPFS Sunitinib INTRIGUE 2L 8.3 benchmarks Sunitinib USPI 5.5 Regorafenib USPI 4.8 3L Avapritinib VOYAGER 5.6 0 1 2 3 4 5 6 7 8 9 Months ORR/PFS for all approved agents was obtained from labeled information from those agents 4

Peak Phase 3 Top-Line Results Full Results Expected to be Presented at an Upcoming Medical Meeting
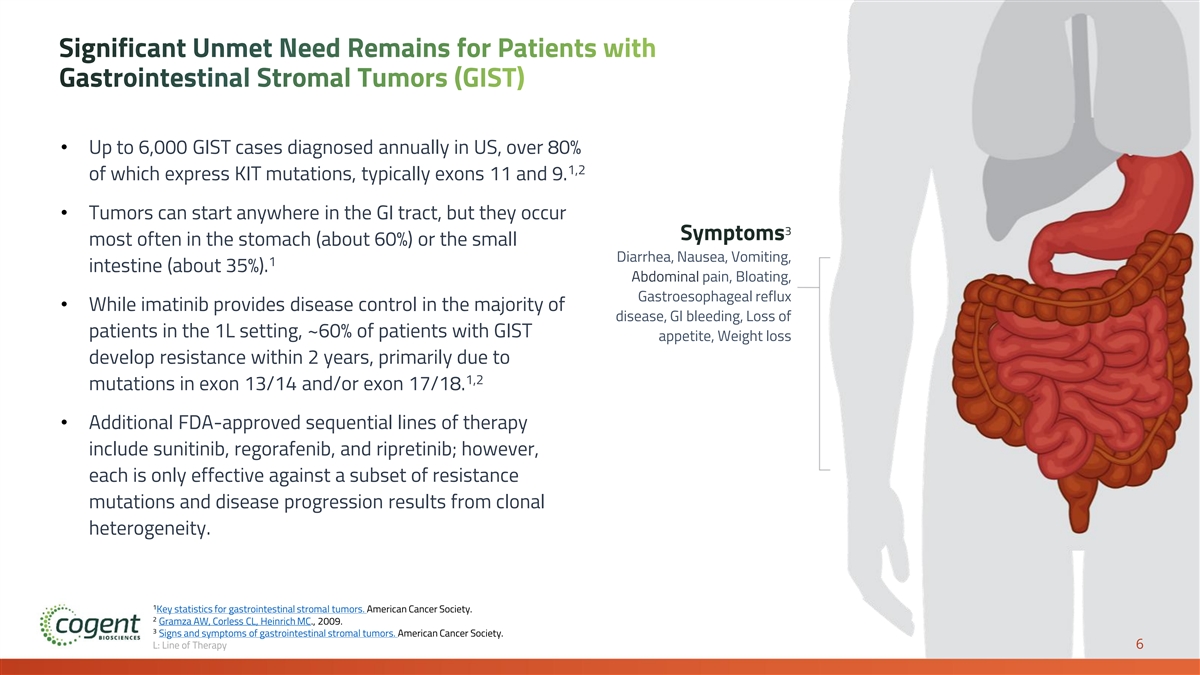
Significant Unmet Need Remains for Patients with Gastrointestinal Stromal Tumors (GIST) • Up to 6,000 GIST cases diagnosed annually in US, over 80% 1,2 of which express KIT mutations, typically exons 11 and 9. • Tumors can start anywhere in the GI tract, but they occur 3 Symptoms most often in the stomach (about 60%) or the small Diarrhea, Nausea, Vomiting, 1 intestine (about 35%). Abdominal pain, Bloating, Gastroesophageal reflux • While imatinib provides disease control in the majority of disease, GI bleeding, Loss of patients in the 1L setting, ~60% of patients with GIST appetite, Weight loss develop resistance within 2 years, primarily due to 1,2 mutations in exon 13/14 and/or exon 17/18. • Additional FDA-approved sequential lines of therapy include sunitinib, regorafenib, and ripretinib; however, each is only effective against a subset of resistance mutations and disease progression results from clonal heterogeneity. 1 Key statistics for gastrointestinal stromal tumors. American Cancer Society. 2 Gramza AW, Corless CL, Heinrich MC., 2009. 3 Signs and symptoms of gastrointestinal stromal tumors. American Cancer Society. L: Line of Therapy 6
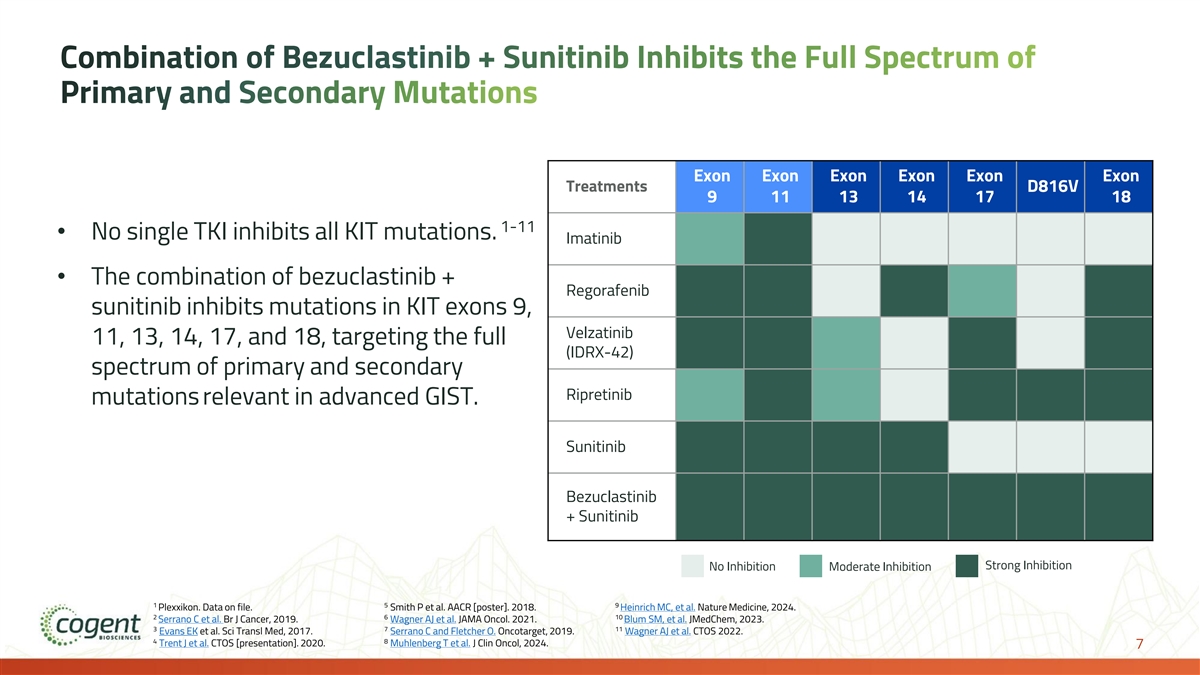
Combination of Bezuclastinib + Sunitinib Inhibits the Full Spectrum of Primary and Secondary Mutations Exon Exon Exon Exon Exon Exon Treatments D816V 9 11 13 14 17 18 1-11 • No single TKI inhibits all KIT mutations. Imatinib • The combination of bezuclastinib + Regorafenib sunitinib inhibits mutations in KIT exons 9, Velzatinib 11, 13, 14, 17, and 18, targeting the full (IDRX-42) spectrum of primary and secondary Ripretinib mutations relevant in advanced GIST. Sunitinib Bezuclastinib + Sunitinib Strong Inhibition No Inhibition Moderate Inhibition 1 5 9 Plexxikon. Data on file. Smith P et al. AACR [poster]. 2018. Heinrich MC, et al. Nature Medicine, 2024. 2 6 10 Serrano C et al. Br J Cancer, 2019. Wagner AJ et al. JAMA Oncol. 2021. Blum SM, et al. JMedChem, 2023. 3 7 11 Evans EK et al. Sci Transl Med, 2017. Serrano C and Fletcher O. Oncotarget, 2019. Wagner AJ et al. CTOS 2022. 4 8 Trent J et al. CTOS [presentation]. 2020. Muhlenberg T et al. J Clin Oncol, 2024. 7
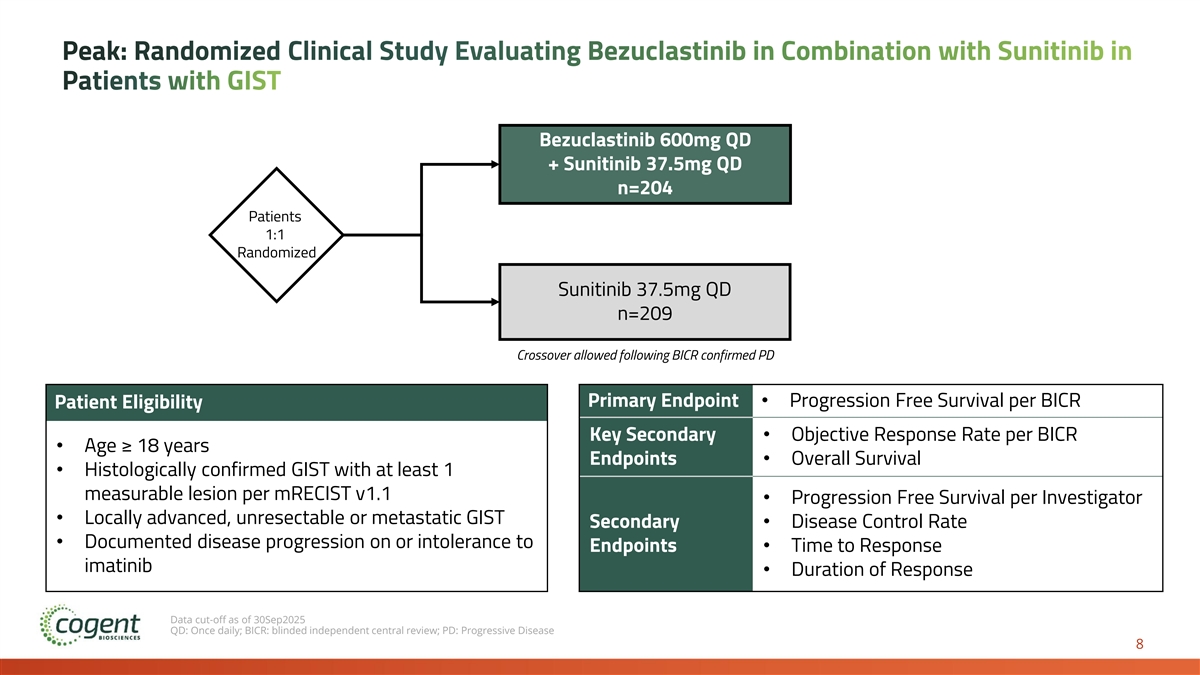
Peak: Randomized Clinical Study Evaluating Bezuclastinib in Combination with Sunitinib in Patients with GIST Bezuclastinib 600mg QD + Sunitinib 37.5mg QD n=204 Patients 1:1 Randomized C Sunitinib 37.5mg QD n=209 Crossover allowed following BICR confirmed PD Primary Endpoint• Progression Free Survival per BICR Patient Eligibility Key Secondary • Objective Response Rate per BICR • Age ≥ 18 years Endpoints• Overall Survival • Histologically confirmed GIST with at least 1 measurable lesion per mRECIST v1.1 • Progression Free Survival per Investigator • Locally advanced, unresectable or metastatic GIST Secondary • Disease Control Rate • Documented disease progression on or intolerance to Endpoints• Time to Response imatinib • Duration of Response Data cut-off as of 30Sep2025 QD: Once daily; BICR: blinded independent central review; PD: Progressive Disease 8
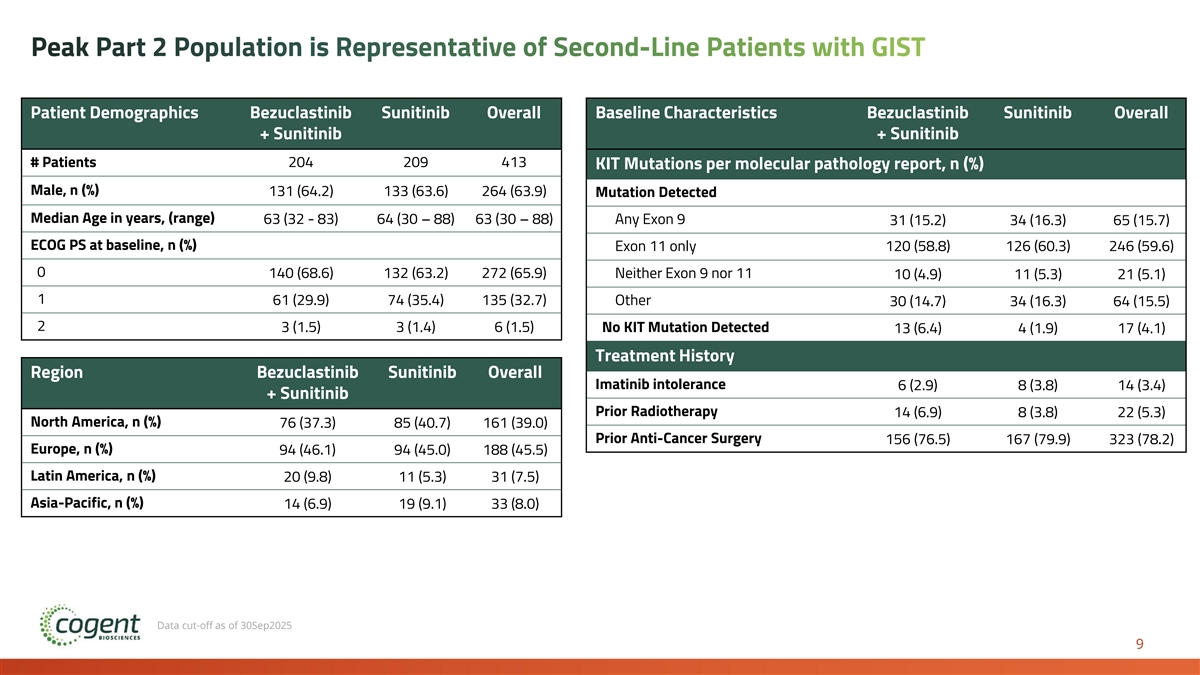
Peak Part 2 Population is Representative of Second-Line Patients with GIST Patient Demographics Bezuclastinib Sunitinib Overall Baseline Characteristics Bezuclastinib Sunitinib Overall + Sunitinib + Sunitinib # Patients 204 209 413 KIT Mutations per molecular pathology report, n (%) Male, n (%) 131 (64.2) 133 (63.6) 264 (63.9) Mutation Detected Median Age in years, (range) 63 (32 - 83) 64 (30 – 88) 63 (30 – 88) Any Exon 9 31 (15.2) 34 (16.3) 65 (15.7) ECOG PS at baseline, n (%) Exon 11 only 120 (58.8) 126 (60.3) 246 (59.6) 0 140 (68.6) 132 (63.2) 272 (65.9) Neither Exon 9 nor 11 10 (4.9) 11 (5.3) 21 (5.1) 1 61 (29.9) 74 (35.4) 135 (32.7) Other 30 (14.7) 34 (16.3) 64 (15.5) 2 3 (1.5) 3 (1.4) 6 (1.5) No KIT Mutation Detected 13 (6.4) 4 (1.9) 17 (4.1) Treatment History Region Bezuclastinib Sunitinib Overall Imatinib intolerance 6 (2.9) 8 (3.8) 14 (3.4) + Sunitinib Prior Radiotherapy 14 (6.9) 8 (3.8) 22 (5.3) North America, n (%) 76 (37.3) 85 (40.7) 161 (39.0) Prior Anti-Cancer Surgery 156 (76.5) 167 (79.9) 323 (78.2) Europe, n (%) 94 (46.1) 94 (45.0) 188 (45.5) Latin America, n (%) 20 (9.8) 11 (5.3) 31 (7.5) Asia-Pacific, n (%) 14 (6.9) 19 (9.1) 33 (8.0) Data cut-off as of 30Sep2025 9
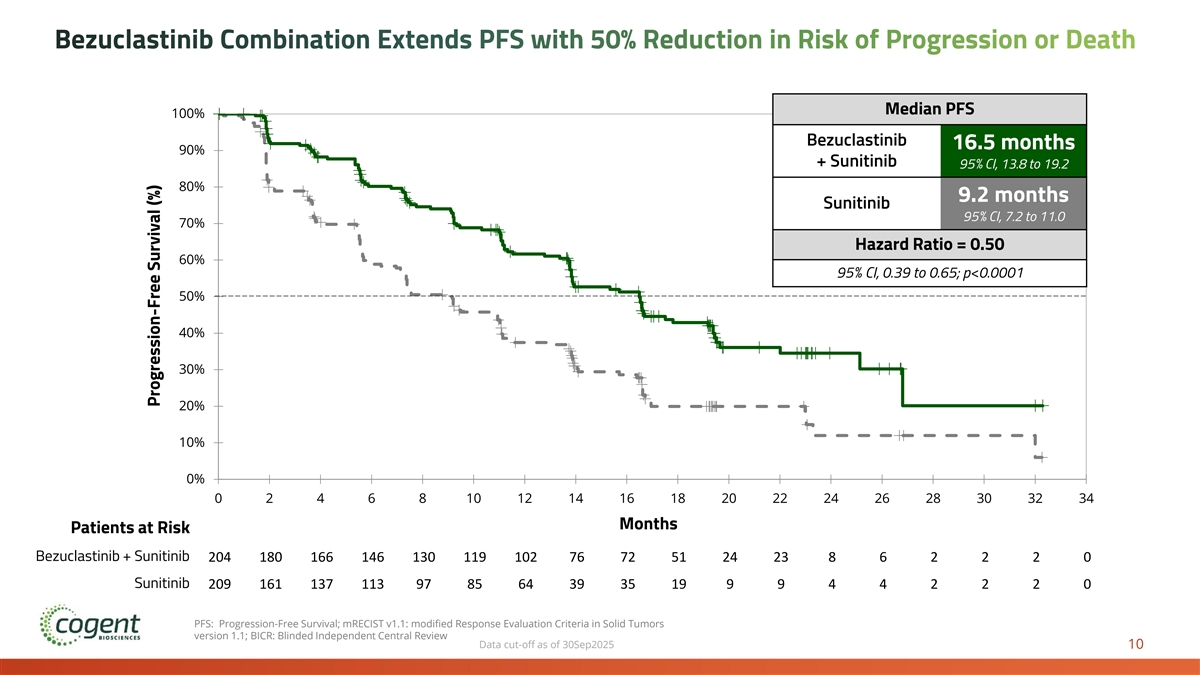
Bezuclastinib Combination Extends PFS with 50% Reduction in Risk of Progression or Death Median PFS 100% Bezuclastinib 16.5 months 90% + Sunitinib 95% CI, 13.8 to 19.2 80% 9.2 months Sunitinib 95% CI, 7.2 to 11.0 70% Hazard Ratio = 0.50 60% 95% CI, 0.39 to 0.65; p<0.0001 50% 40% 30% 20% 10% 0% 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 Time Months Patients at Risk Sunitinib Sunitinib + Bezuclastinib Bezuclastinib + Sunitinib 204 180 166 146 130 119 102 76 72 51 24 23 8 6 2 2 2 0 Sunitinib 209 161 137 113 97 85 64 39 35 19 9 9 4 4 2 2 2 0 PFS: Progression-Free Survival; mRECIST v1.1: modified Response Evaluation Criteria in Solid Tumors version 1.1; BICR: Blinded Independent Central Review Data cut-off as of 30Sep2025 10 Progression-Free Survival (%)
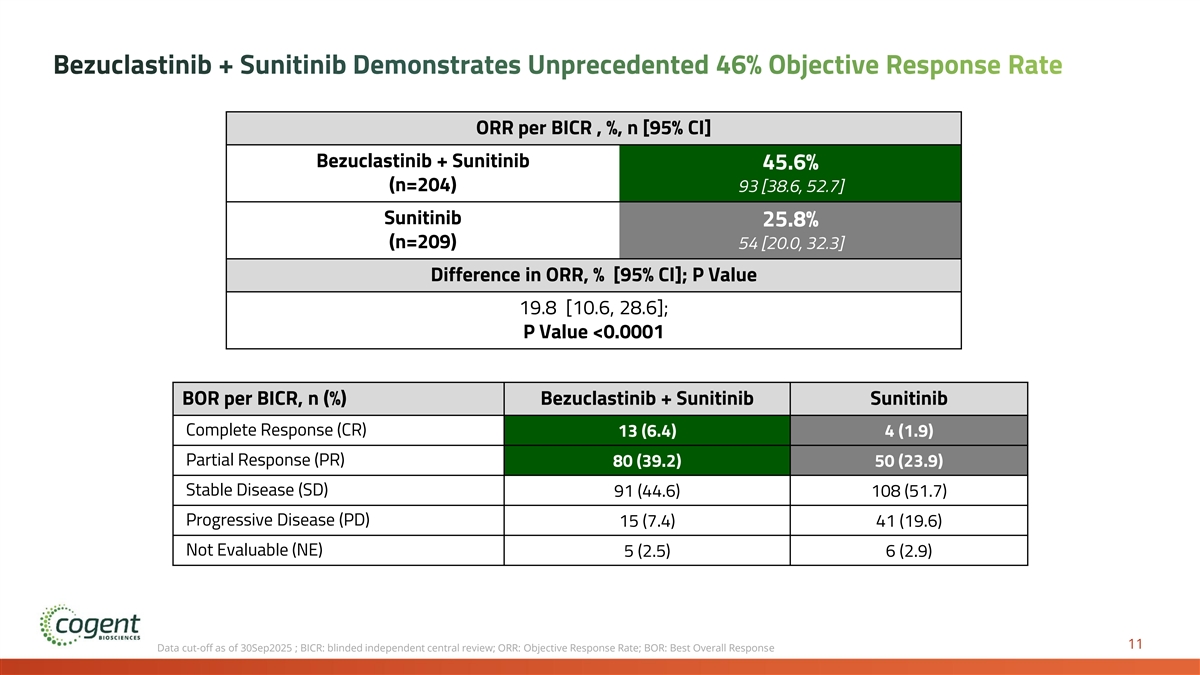
Bezuclastinib + Sunitinib Demonstrates Unprecedented 46% Objective Response Rate ORR per BICR , %, n [95% CI] Bezuclastinib + Sunitinib 45.6% (n=204) 93 [38.6, 52.7] Sunitinib 25.8% (n=209) 54 [20.0, 32.3] Difference in ORR, % [95% CI]; P Value 19.8 [10.6, 28.6]; P Value <0.0001 BOR per BICR, n (%) Bezuclastinib + Sunitinib Sunitinib Complete Response (CR) 13 (6.4) 4 (1.9) Partial Response (PR) 80 (39.2) 50 (23.9) Stable Disease (SD) 91 (44.6) 108 (51.7) Progressive Disease (PD) 15 (7.4) 41 (19.6) Not Evaluable (NE) 5 (2.5) 6 (2.9) 11 Data cut-off as of 30Sep2025 ; BICR: blinded independent central review; ORR: Objective Response Rate; BOR: Best Overall Response
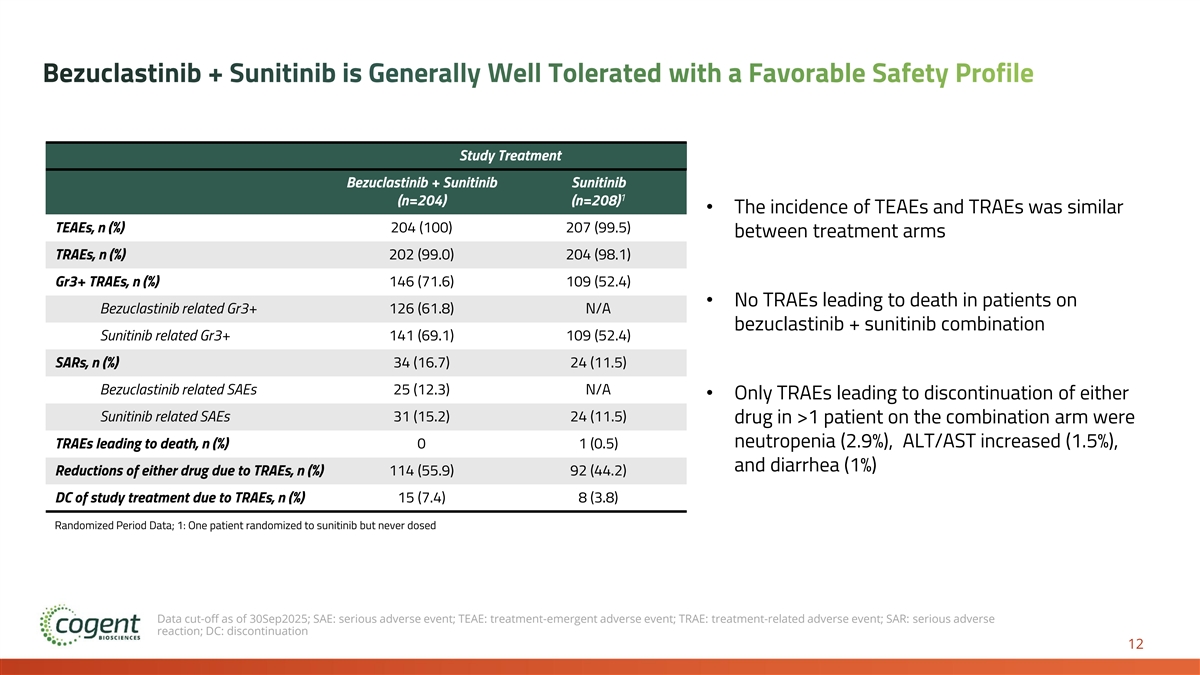
Bezuclastinib + Sunitinib is Generally Well Tolerated with a Favorable Safety Profile Study Treatment Bezuclastinib + Sunitinib Sunitinib 1 (n=204) (n=208) • The incidence of TEAEs and TRAEs was similar TEAEs, n (%) 204 (100) 207 (99.5) between treatment arms TRAEs, n (%) 202 (99.0) 204 (98.1) Gr3+ TRAEs, n (%) 146 (71.6) 109 (52.4) • No TRAEs leading to death in patients on Bezuclastinib related Gr3+ 126 (61.8) N/A bezuclastinib + sunitinib combination Sunitinib related Gr3+ 141 (69.1) 109 (52.4) SARs, n (%) 34 (16.7) 24 (11.5) Bezuclastinib related SAEs 25 (12.3) N/A • Only TRAEs leading to discontinuation of either Sunitinib related SAEs 31 (15.2) 24 (11.5) drug in >1 patient on the combination arm were neutropenia (2.9%), ALT/AST increased (1.5%), TRAEs leading to death, n (%) 0 1 (0.5) and diarrhea (1%) Reductions of either drug due to TRAEs, n (%) 114 (55.9) 92 (44.2) DC of study treatment due to TRAEs, n (%) 15 (7.4) 8 (3.8) Randomized Period Data; 1: One patient randomized to sunitinib but never dosed Data cut-off as of 30Sep2025; SAE: serious adverse event; TEAE: treatment-emergent adverse event; TRAE: treatment-related adverse event; SAR: serious adverse reaction; DC: discontinuation 12
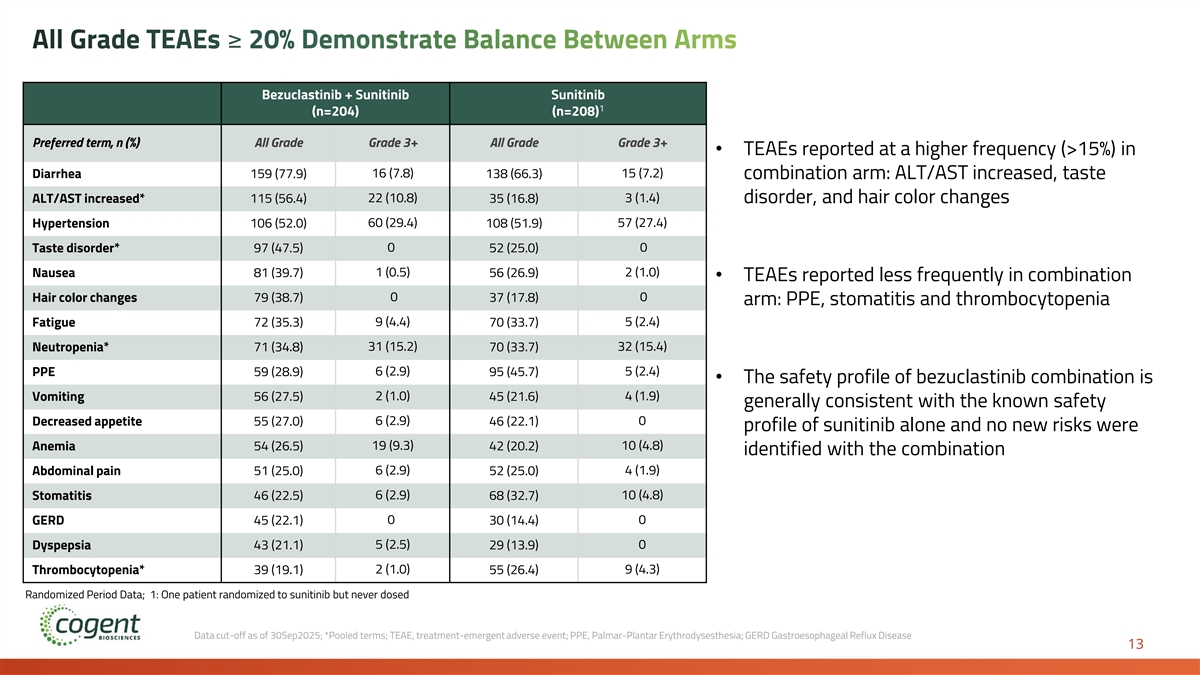
All Grade TEAEs ≥ 20% Demonstrate Balance Between Arms Bezuclastinib + Sunitinib Sunitinib 1 (n=204) (n=208) Preferred term, n (%) All Grade Grade 3+ All Grade Grade 3+ • TEAEs reported at a higher frequency (>15%) in 16 (7.8) 15 (7.2) Diarrhea 159 (77.9) 138 (66.3) combination arm: ALT/AST increased, taste ALT/AST increased* 115 (56.4) 22 (10.8) 35 (16.8) 3 (1.4) disorder, and hair color changes Hypertension 106 (52.0) 60 (29.4) 108 (51.9) 57 (27.4) Taste disorder* 97 (47.5) 0 52 (25.0) 0 1 (0.5) 2 (1.0) Nausea 81 (39.7) 56 (26.9) • TEAEs reported less frequently in combination 0 0 Hair color changes 79 (38.7) 37 (17.8) arm: PPE, stomatitis and thrombocytopenia Fatigue 72 (35.3) 9 (4.4) 70 (33.7) 5 (2.4) Neutropenia* 71 (34.8) 31 (15.2) 70 (33.7) 32 (15.4) PPE 59 (28.9) 6 (2.9) 95 (45.7) 5 (2.4) • The safety profile of bezuclastinib combination is 2 (1.0) 4 (1.9) Vomiting 56 (27.5) 45 (21.6) generally consistent with the known safety 6 (2.9) 0 Decreased appetite 55 (27.0) 46 (22.1) profile of sunitinib alone and no new risks were Anemia 54 (26.5) 19 (9.3) 42 (20.2) 10 (4.8) identified with the combination Abdominal pain 51 (25.0) 6 (2.9) 52 (25.0) 4 (1.9) Stomatitis 46 (22.5) 6 (2.9) 68 (32.7) 10 (4.8) 0 0 GERD 45 (22.1) 30 (14.4) 5 (2.5) 0 Dyspepsia 43 (21.1) 29 (13.9) Thrombocytopenia* 39 (19.1) 2 (1.0) 55 (26.4) 9 (4.3) Randomized Period Data; 1: One patient randomized to sunitinib but never dosed Data cut-off as of 30Sep2025; *Pooled terms; TEAE, treatment-emergent adverse event; PPE, Palmar-Plantar Erythrodysesthesia; GERD Gastroesophageal Reflux Disease 13
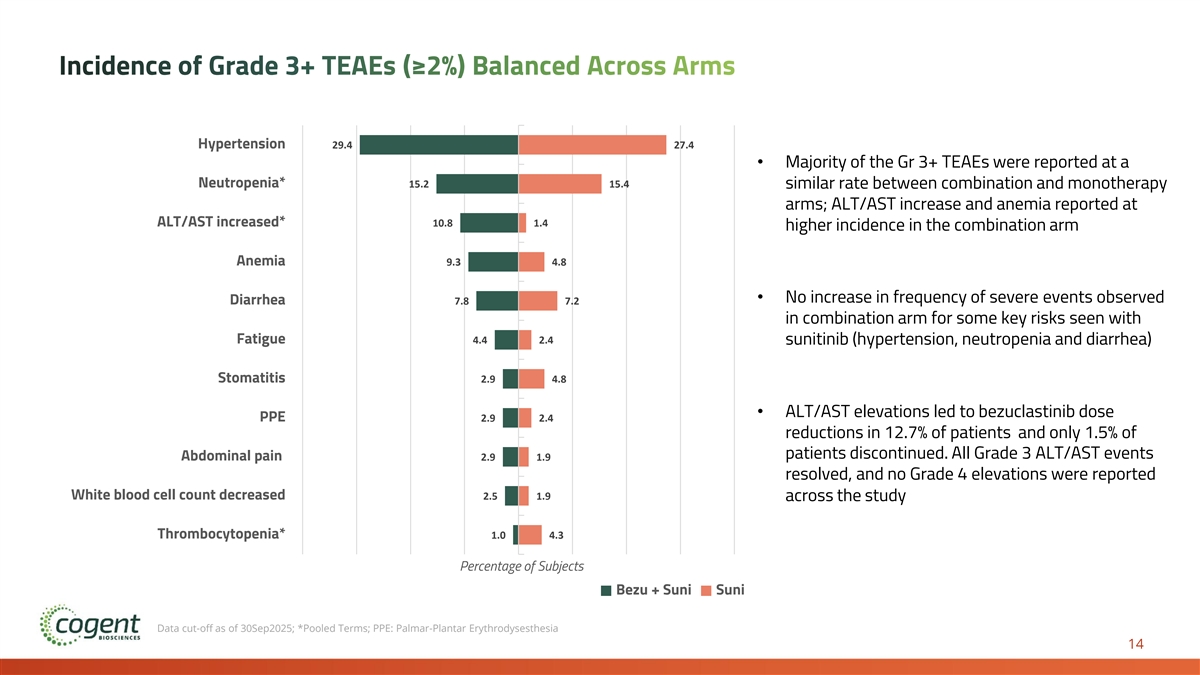
Incidence of Grade 3+ TEAEs (≥2%) Balanced Across Arms Hypertension 29.4 27.4 • Majority of the Gr 3+ TEAEs were reported at a Neutropenia* 15.2 15.4 similar rate between combination and monotherapy arms; ALT/AST increase and anemia reported at ALT/AST increased* 10.8 1.4 higher incidence in the combination arm Anemia 9.3 4.8 • No increase in frequency of severe events observed Diarrhea 7.8 7.2 in combination arm for some key risks seen with Fatigue 4.4 2.4 sunitinib (hypertension, neutropenia and diarrhea) Stomatitis 2.9 4.8 • ALT/AST elevations led to bezuclastinib dose PPE 2.9 2.4 reductions in 12.7% of patients and only 1.5% of patients discontinued. All Grade 3 ALT/AST events Abdominal pain 2.9 1.9 resolved, and no Grade 4 elevations were reported White blood cell count decreased 2.5 1.9 across the study Thrombocytopenia* 1.0 4.3 Percentage of Subjects Bezu + Suni Suni Data cut-off as of 30Sep2025; *Pooled Terms; PPE: Palmar-Plantar Erythrodysesthesia 14
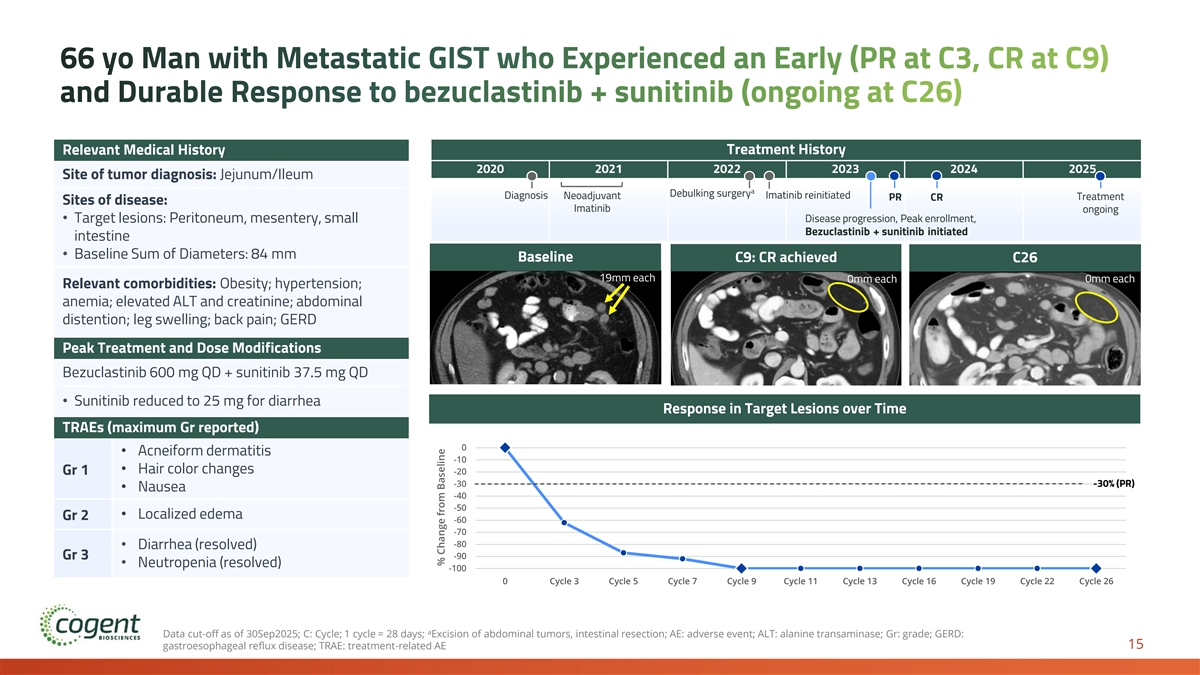
66 yo Man with Metastatic GIST who Experienced an Early (PR at C3, CR at C9) and Durable Response to bezuclastinib + sunitinib (ongoing at C26) Relevant Medical History Treatment History 2020 2021 2022 2023 2024 2025 Site of tumor diagnosis: Jejunum/Ileum a Debulking surgery Diagnosis Neoadjuvant Imatinib reinitiated Treatment PR CR Sites of disease: Imatinib ongoing • Target lesions: Peritoneum, mesentery, small Disease progression, Peak enrollment, Bezuclastinib + sunitinib initiated intestine • Baseline Sum of Diameters: 84 mm Baseline C9: CR achieved C26 19mm each 0mm each 0mm each Relevant comorbidities: Obesity; hypertension; anemia; elevated ALT and creatinine; abdominal distention; leg swelling; back pain; GERD Peak Treatment and Dose Modifications Bezuclastinib 600 mg QD + sunitinib 37.5 mg QD • Sunitinib reduced to 25 mg for diarrhea Response in Target Lesions over Time TRAEs (maximum Gr reported) 0 • Acneiform dermatitis -10 • Hair color changes Gr 1 -20 -30 -30% (PR) • Nausea -40 -50 • Localized edema Gr 2 -60 -70 -80 • Diarrhea (resolved) -90 Gr 3 • Neutropenia (resolved) -100 0 Cycle 3 Cycle 5 Cycle 7 Cycle 9 Cycle 11 Cycle 13 Cycle 16 Cycle 19 Cycle 22 Cycle 26 a Data cut-off as of 30Sep2025; C: Cycle; 1 cycle = 28 days; Excision of abdominal tumors, intestinal resection; AE: adverse event; ALT: alanine transaminase; Gr: grade; GERD: 15 gastroesophageal reflux disease; TRAE: treatment-related AE % Change from Baseline
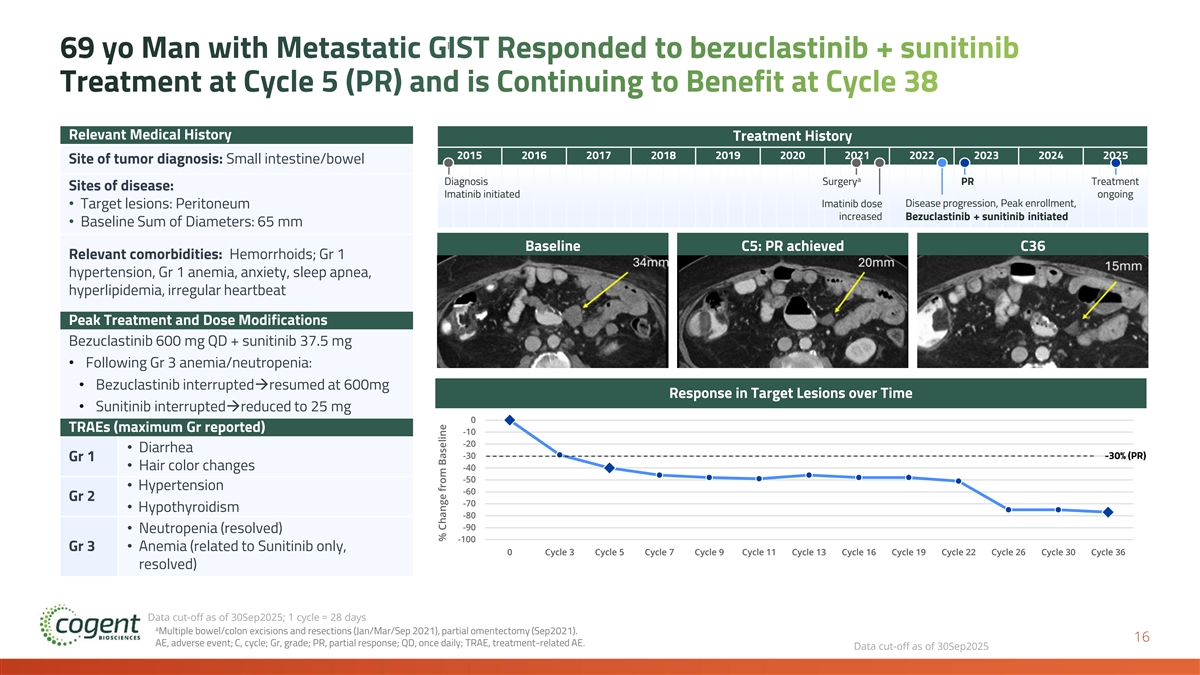
69 yo Man with Metastatic GIST Responded to bezuclastinib + sunitinib Treatment at Cycle 5 (PR) and is Continuing to Benefit at Cycle 38 Relevant Medical History Treatment History 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 Site of tumor diagnosis: Small intestine/bowel a Diagnosis Surgery PR Treatment Sites of disease: Imatinib initiated ongoing Imatinib dose Disease progression, Peak enrollment, • Target lesions: Peritoneum increased Bezuclastinib + sunitinib initiated • Baseline Sum of Diameters: 65 mm Baseline C5: PR achieved C36 Relevant comorbidities: Hemorrhoids; Gr 1 hypertension, Gr 1 anemia, anxiety, sleep apnea, hyperlipidemia, irregular heartbeat Peak Treatment and Dose Modifications Bezuclastinib 600 mg QD + sunitinib 37.5 mg • Following Gr 3 anemia/neutropenia: • Bezuclastinib interrupted→resumed at 600mg Response in Target Lesions over Time • Sunitinib interrupted→reduced to 25 mg 0 TRAEs (maximum Gr reported) -10 -20 • Diarrhea -30 -30% (PR) Gr 1 • Hair color changes -40 -50 • Hypertension -60 Gr 2 -70 • Hypothyroidism -80 -90 • Neutropenia (resolved) -100 Gr 3• Anemia (related to Sunitinib only, 0 Cycle 3 Cycle 5 Cycle 7 Cycle 9 Cycle 11 Cycle 13 Cycle 16 Cycle 19 Cycle 22 Cycle 26 Cycle 30 Cycle 36 resolved) Data cut-off as of 30Sep2025; 1 cycle = 28 days a Multiple bowel/colon excisions and resections (Jan/Mar/Sep 2021), partial omentectomy (Sep2021). 16 AE, adverse event; C, cycle; Gr, grade; PR, partial response; QD, once daily; TRAE, treatment-related AE. Data cut-off as of 30Sep2025 % Change from Baseline

We Believe Peak Results are Transformative and Practice Changing • Bezuclastinib combination establishes first new benchmark for 2L GIST in 20 years • 50% reduced risk of progression or death compared to current standard of care • 16.5 months mPFS compared to 9.2 months for sunitinib alone (p<0.0001) • 46% ORR compared to 26% for sunitinib alone (p<0.0001) • OS immature with event rate of less than 20% at time of PFS analysis • Generally well tolerated with no unique risks observed when compared to the known safety profile of sunitinib • Estimated 19 months+ mean treatment duration for bezuclastinib combination patients based on projection for patients remaining on combination therapy • Active Expanded Access Program allowing immediate availability of the bezuclastinib combination for 2L patients with GIST • NDA submission for bezuclastinib in imatinib-resistant or intolerant GIST planned 1H 2026 based on results of the Peak trial 17
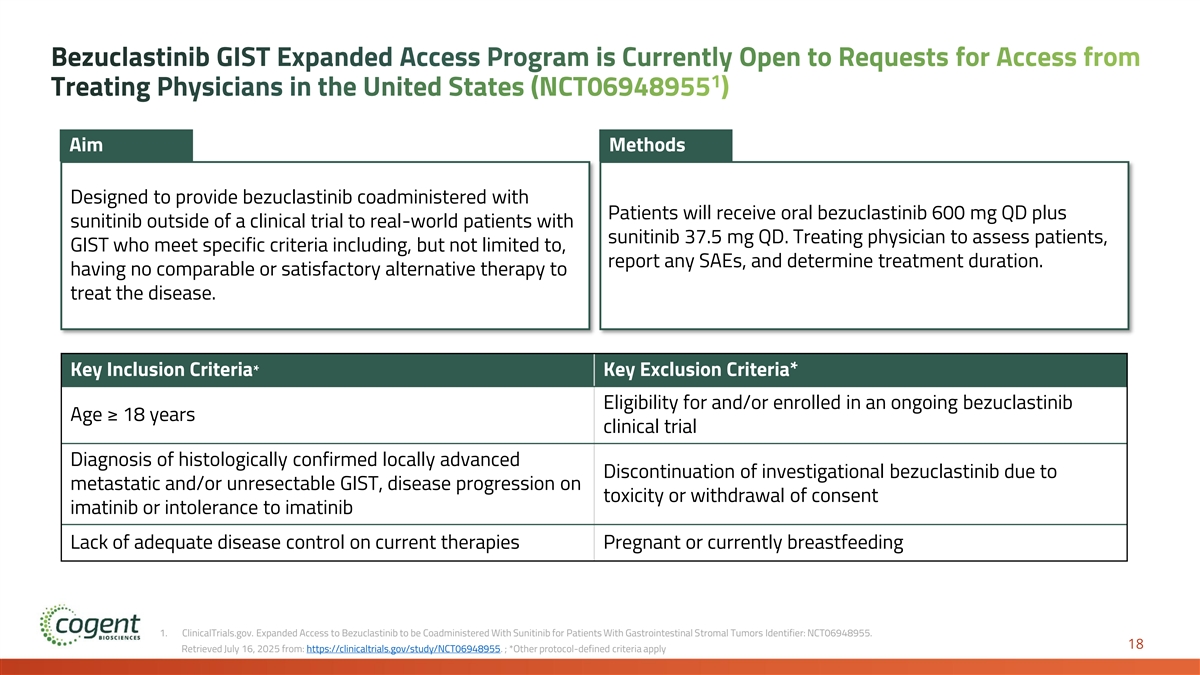
Bezuclastinib GIST Expanded Access Program is Currently Open to Requests for Access from 1 Treating Physicians in the United States (NCT06948955 ) Aim Methods Designed to provide bezuclastinib coadministered with Patients will receive oral bezuclastinib 600 mg QD plus sunitinib outside of a clinical trial to real-world patients with sunitinib 37.5 mg QD. Treating physician to assess patients, GIST who meet specific criteria including, but not limited to, report any SAEs, and determine treatment duration. having no comparable or satisfactory alternative therapy to treat the disease. Key Inclusion Criteria* Key Exclusion Criteria* Eligibility for and/or enrolled in an ongoing bezuclastinib Age ≥ 18 years clinical trial Diagnosis of histologically confirmed locally advanced Discontinuation of investigational bezuclastinib due to metastatic and/or unresectable GIST, disease progression on toxicity or withdrawal of consent imatinib or intolerance to imatinib Lack of adequate disease control on current therapies Pregnant or currently breastfeeding 1. ClinicalTrials.gov. Expanded Access to Bezuclastinib to be Coadministered With Sunitinib for Patients With Gastrointestinal Stromal Tumors Identifier: NCT06948955. 18 Retrieved July 16, 2025 from: https://clinicaltrials.gov/study/NCT06948955. ; *Other protocol-defined criteria apply
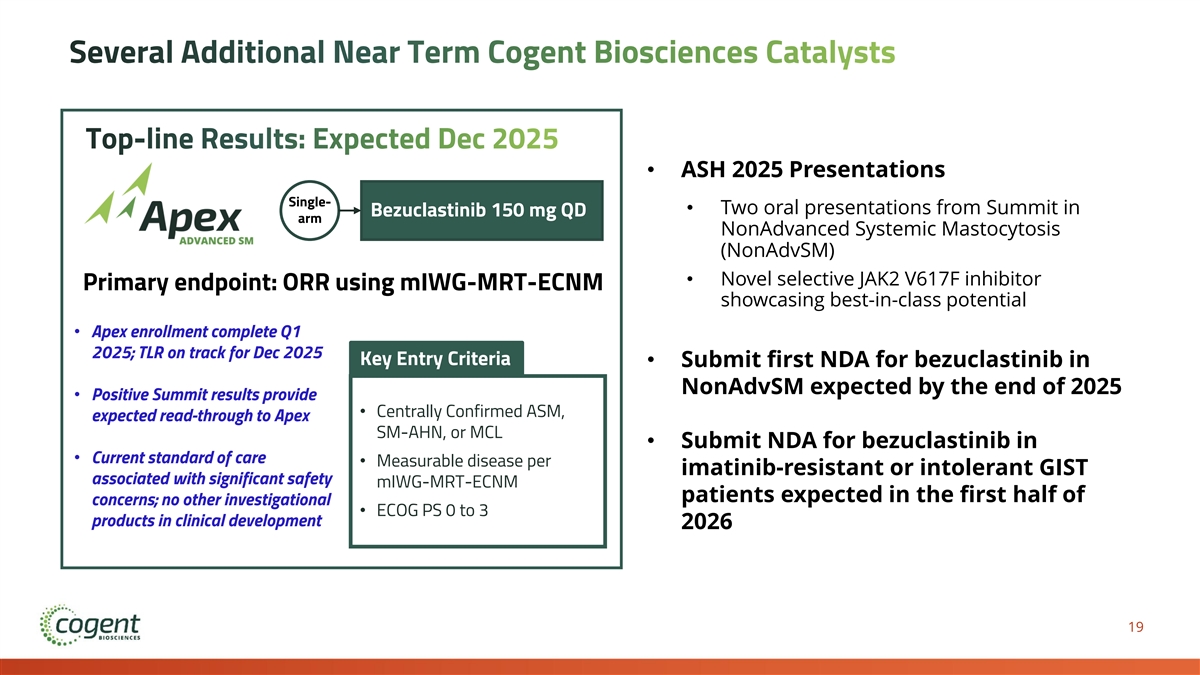
Several Additional Near Term Cogent Biosciences Catalysts Top-line Results: Expected Dec 2025 • ASH 2025 Presentations Single- • Two oral presentations from Summit in Bezuclastinib 150 mg QD arm NonAdvanced Systemic Mastocytosis (NonAdvSM) • Novel selective JAK2 V617F inhibitor Primary endpoint: ORR using mIWG-MRT-ECNM showcasing best-in-class potential • Apex enrollment complete Q1 2025; TLR on track for Dec 2025 Key Entry Criteria • Submit first NDA for bezuclastinib in NonAdvSM expected by the end of 2025 • Positive Summit results provide • Centrally Confirmed ASM, expected read-through to Apex SM-AHN, or MCL • Submit NDA for bezuclastinib in • Current standard of care • Measurable disease per imatinib-resistant or intolerant GIST associated with significant safety mIWG-MRT-ECNM patients expected in the first half of concerns; no other investigational • ECOG PS 0 to 3 products in clinical development 2026 19
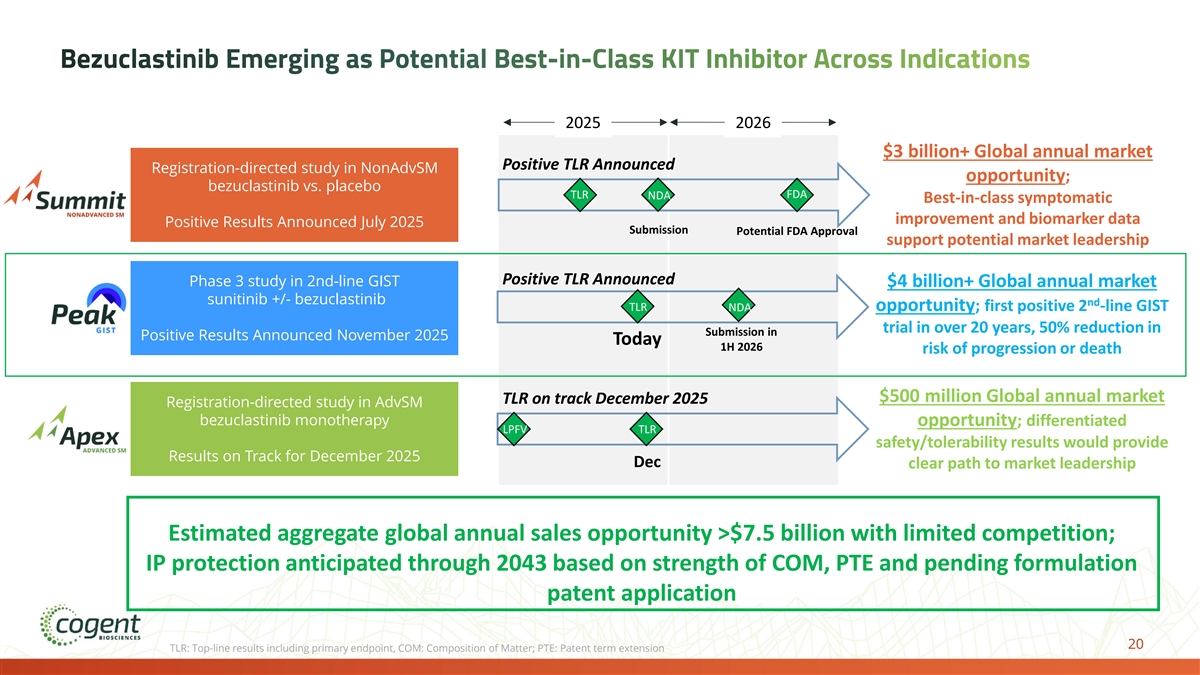
Bezuclastinib Emerging as Potential Best-in-Class KIT Inhibitor Across Indications 2025 2026 $3 billion+ Global annual market Positive TLR Announced Registration-directed study in NonAdvSM opportunity; bezuclastinib vs. placebo TLR FDA NDA Best-in-class symptomatic improvement and biomarker data Positive Results Announced July 2025 Submission Potential FDA Approval support potential market leadership Positive TLR Announced Phase 3 study in 2nd-line GIST $4 billion+ Global annual market sunitinib +/- bezuclastinib nd TLR NDA opportunity; first positive 2 -line GIST trial in over 20 years, 50% reduction in Submission in Positive Results Announced November 2025 Today 1H 2026 risk of progression or death $500 million Global annual market TLR on track December 2025 Registration-directed study in AdvSM bezuclastinib monotherapy opportunity; differentiated LPFV TLR safety/tolerability results would provide Results on Track for December 2025 Dec clear path to market leadership Estimated aggregate global annual sales opportunity >$7.5 billion with limited competition; IP protection anticipated through 2043 based on strength of COM, PTE and pending formulation patent application 20 TLR: Top-line results including primary endpoint, COM: Composition of Matter; PTE: Patent term extension

Q&A