

Stoke Therapeutics Second Quarter 2025 Business Update Webcast for Investors & Analysts August 12, 2025
Long-Term 36-Month OLE Data Kimberly Parkerson, M.D., Ph.D., SVP, Head of Neurology Clinical Development Q&A Agenda CEO Opening Remarks Ian F. Smith, Interim Chief Executive Officer & Director Pipeline Update: ADOA Preclinical Data and Development Plan Barry Ticho, M.D., Ph.D., Chief Medical Officer Financial Update Thomas Leggett, Chief Financial Officer Phase 3 Design and Progress Barry Ticho, M.D., Ph.D., Chief Medical Officer CEO Closing Remarks Ian F. Smith, Interim Chief Executive Officer & Director

Forward-Looking Statements and Other Legal Notices This presentation has been prepared by Stoke Therapeutics, Inc. ("Stoke" or "us") for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter(s) or Stoke or any officer, director, employee, agent or advisor of Stoke. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Information provided in this presentation speaks only as of the date hereof. Stoke assumes no obligation to publicly update any information or forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments, subsequent events, or circumstances after the date hereof, or to reflect the occurrence of unanticipated events. This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: the ability of zorevunersen to treat the underlying causes of Dravet syndrome and reduce seizures or show improvements in behavior or cognition at the indicated dosing levels or at all; the design, timing and results of clinical studies, data readouts, regulatory decisions and other presentations for zorevunersen and STK-002; the potential for zorevunersen to be a first-in-class, disease-modifying therapy for Dravet syndrome; the timing of regulatory interactions or the outcomes thereof; the ability of STK-002 to treat the underlying causes of Autosomal Dominant Optic Atrophy (ADOA) and maintain or improve vision; our expectations, plans, aspirations and goals, including those related to the potential of zorevunersen and our collaborations with Biogen and Acadia; the anticipated market for zorevunersen; our future operating results, financial position and cash runway and ability to fund operations beyond Phase 3 and into launch readiness to mid-2028. Statements including words such as "anticipate," "believe," "hope," "plan," "will," "continue," expect," "ongoing," or "potential" and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and uncertainties, as well as assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks and uncertainties related to: our ability to advance, obtain regulatory approval of, and ultimately commercialize our product candidates, including zorevunersen and STK-002; the timing of data readouts and interim and final results of nonclinical and clinical studies; nonclinical and clinical data are voluminous and detailed, and regulatory authorities may interpret or weigh the importance of data differently and reach different conclusions than us or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; receiving Breakthrough Therapy Designation may not lead to a faster development or regulatory review or approval and does not mean zorevunersen will receive marketing approval; our ability to fund development activities and achieve development goals; our ability to protect our intellectual property; global business, political and macroeconomic conditions, including inflation, interest rate volatility, cybersecurity events, uncertainty with respect to the federal budget, instability in the global banking system and volatile market conditions, and global events, including public health crises and ongoing geopolitical conflicts, such as the conflicts in Ukraine and the Middle East; and other risks and uncertainties described under the heading "Risk Factors" in our Annual Report on Form 10-K for the year ended December 31, 2024, our quarterly reports on Form 10-Q and the other documentation we file from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. By attending or receiving this presentation you acknowledge that you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made, you will be solely responsible for your own assessment of the market and our market position, and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of Stoke. Certain information contained in or that may orally accompany this presentation relate to or are based on studies, research, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, research, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. This presentation discusses product candidates, including zorevunersen and STK-002, that have not yet been approved for marketing by the U.S. Food and Drug Administration or any other regulatory agency.

Opening Remarks Ian F. Smith Interim Chief Executive Officer & Director
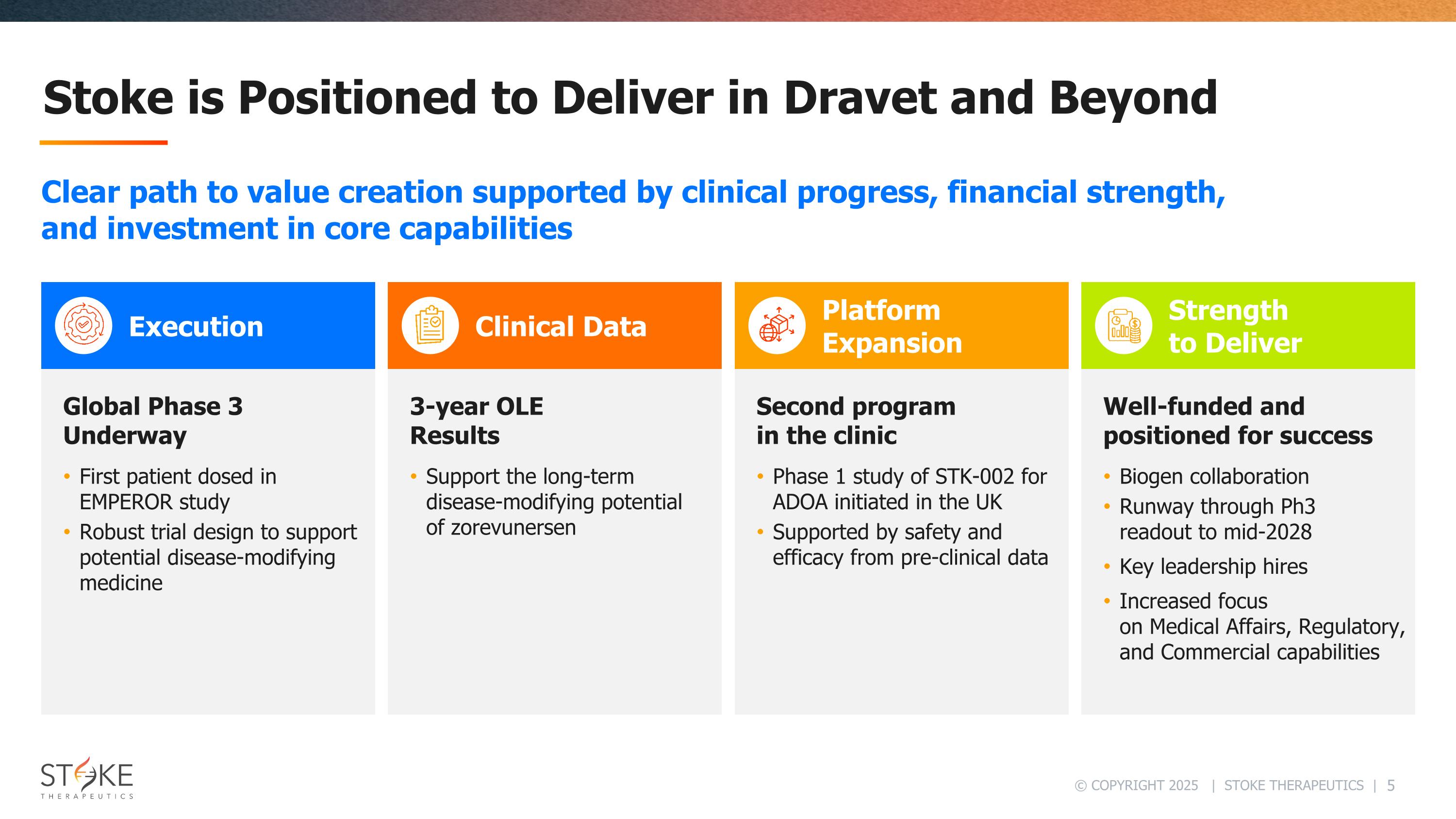
Stoke is Positioned to Deliver in Dravet and Beyond Execution Clinical Data Platform Expansion Strength to Deliver Clear path to value creation supported by clinical progress, financial strength, and investment in core capabilities Global Phase 3 Underway First patient dosed in EMPEROR study Robust trial design to support potential disease-modifying medicine 3-year OLE Results Support the long-term disease-modifying potential of zorevunersen Second program in the clinic Phase 1 study of STK-002 for ADOA initiated in the UK Supported by safety and efficacy from pre-clinical data Well-funded and positioned for success Biogen collaboration Runway through Ph3 readout to mid-2028 Key leadership hires Increased focus on Medical Affairs, Regulatory, and Commercial capabilities

Phase 3 Design and Progress Barry Ticho, M.D., Ph.D. Chief Medical Officer
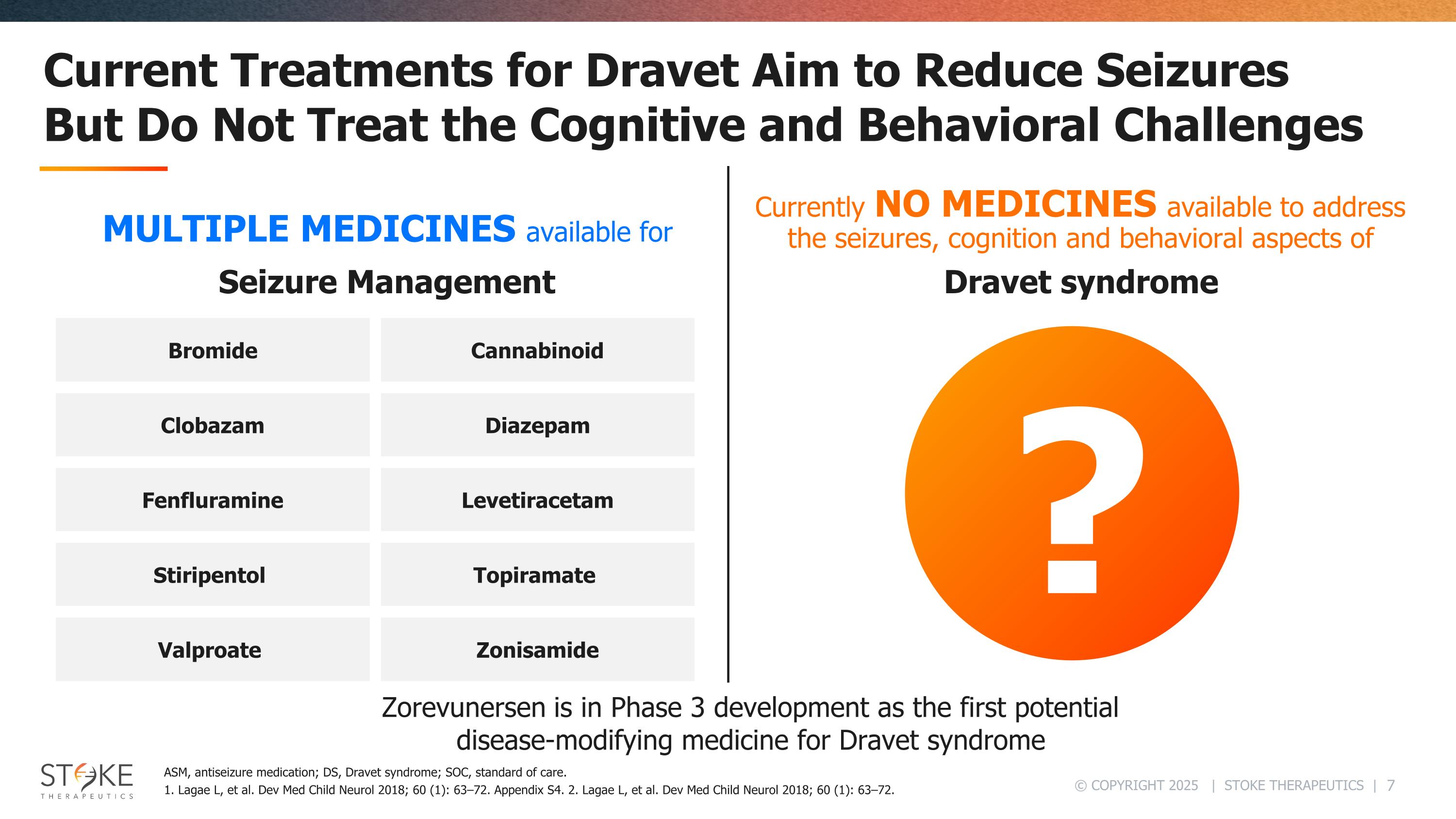
Current Treatments for Dravet Aim to Reduce Seizures But Do Not Treat the Cognitive and Behavioral Challenges Bromide Clobazam Fenfluramine Stiripentol Valproate Cannabinoid Diazepam Levetiracetam Topiramate Zonisamide ? ASM, antiseizure medication; DS, Dravet syndrome; SOC, standard of care. 1. Lagae L, et al. Dev Med Child Neurol 2018; 60 (1): 63–72. Appendix S4. 2. Lagae L, et al. Dev Med Child Neurol 2018; 60 (1): 63–72. Seizure Management MULTIPLE MEDICINES available for Dravet syndrome Currently NO MEDICINES available to address the seizures, cognition and behavioral aspects of Zorevunersen is in Phase 3 development as the first potential disease-modifying medicine for Dravet syndrome
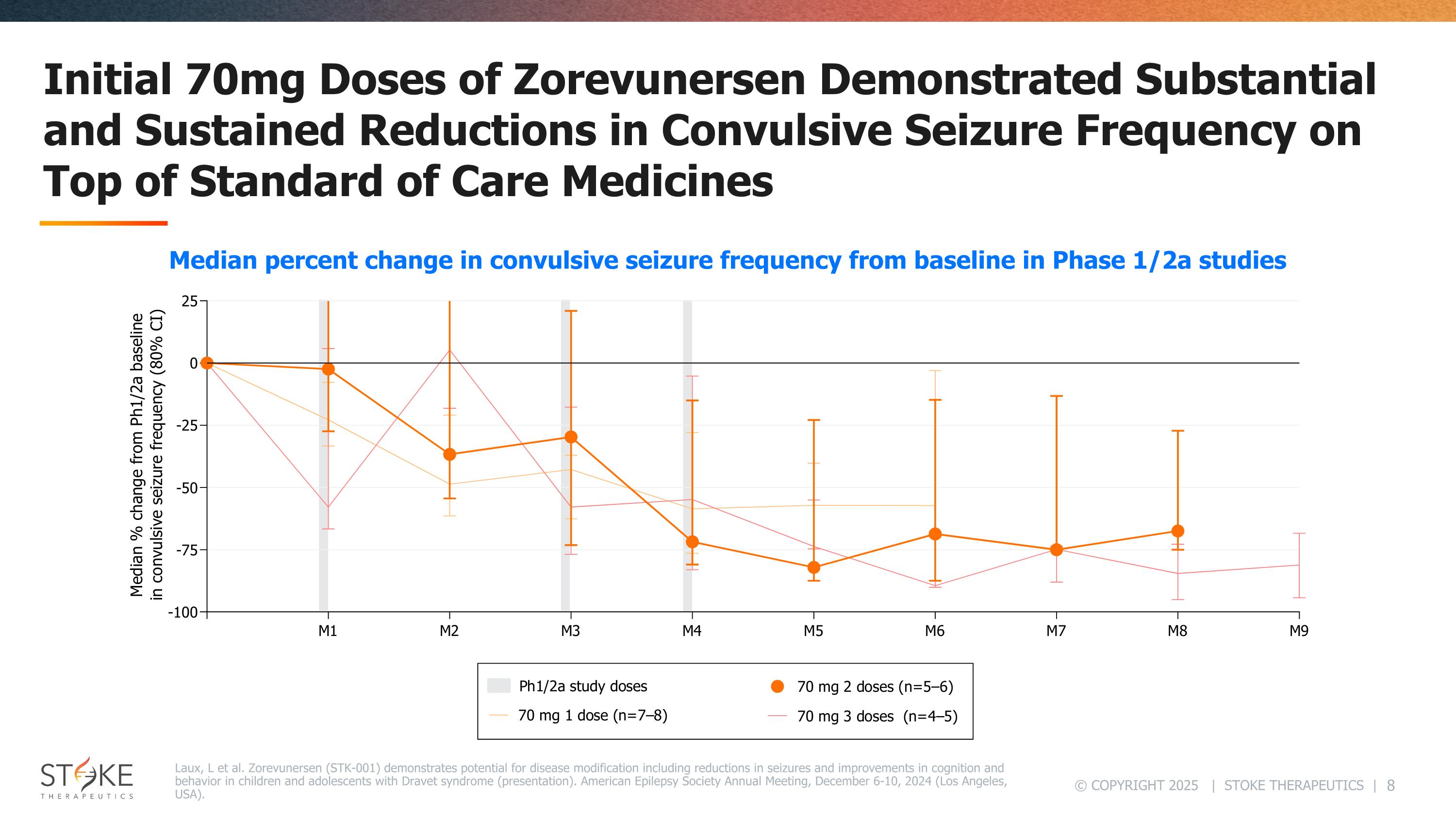
Initial 70mg Doses of Zorevunersen Demonstrated Substantial and Sustained Reductions in Convulsive Seizure Frequency on Top of Standard of Care Medicines Laux, L et al. Zorevunersen (STK-001) demonstrates potential for disease modification including reductions in seizures and improvements in cognition and behavior in children and adolescents with Dravet syndrome (presentation). American Epilepsy Society Annual Meeting, December 6-10, 2024 (Los Angeles, USA). Median percent change in convulsive seizure frequency from baseline in Phase 1/2a studies
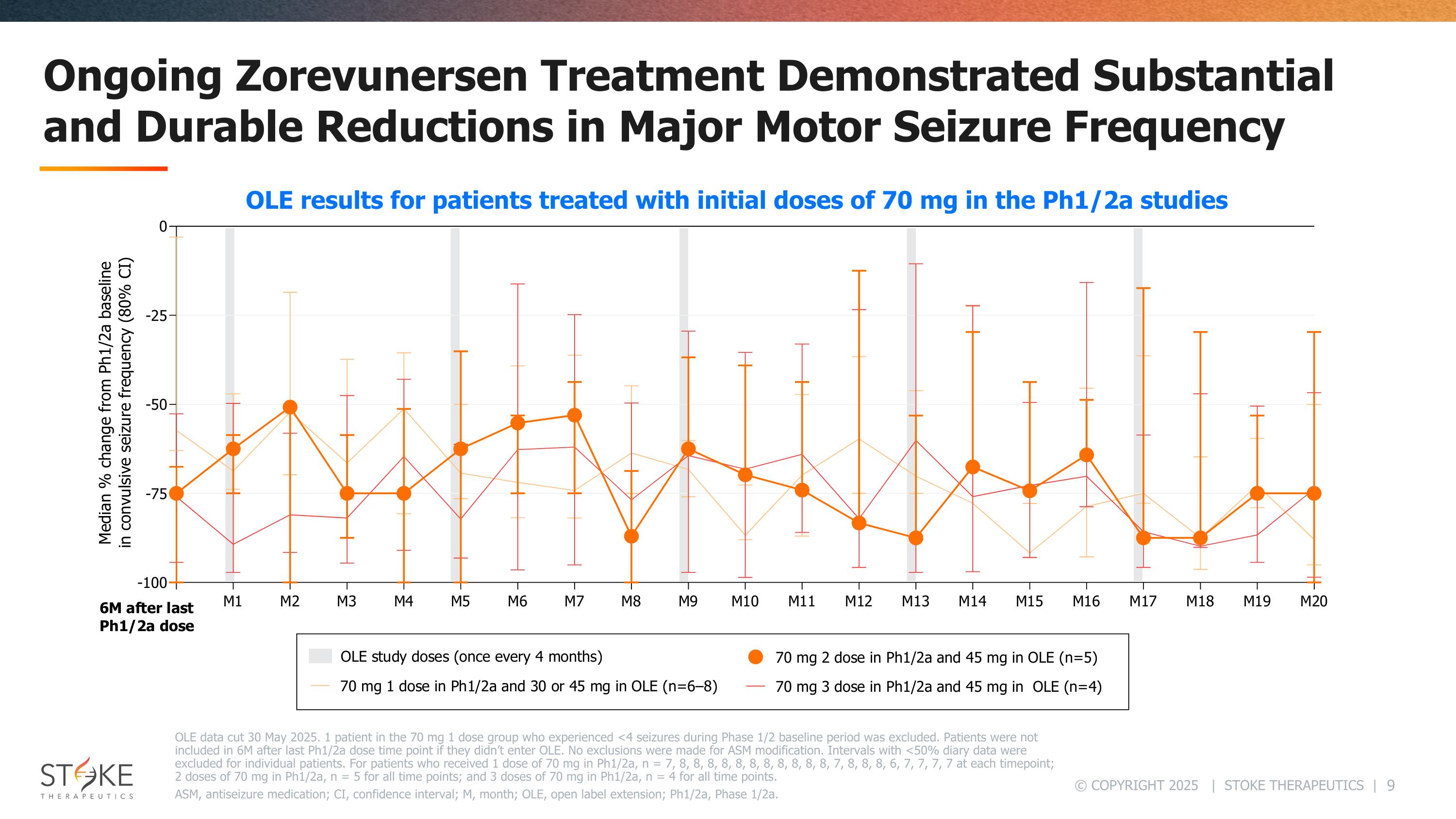
Ongoing Zorevunersen Treatment Demonstrated Substantial and Durable Reductions in Major Motor Seizure Frequency OLE data cut 30 May 2025. 1 patient in the 70 mg 1 dose group who experienced <4 seizures during Phase 1/2 baseline period was excluded. Patients were not included in 6M after last Ph1/2a dose time point if they didn’t enter OLE. No exclusions were made for ASM modification. Intervals with <50% diary data were excluded for individual patients. For patients who received 1 dose of 70 mg in Ph1/2a, n = 7, 8, 8, 8, 8, 8, 8, 8, 8, 8, 8, 8, 7, 8, 8, 8, 6, 7, 7, 7, 7 at each timepoint; 2 doses of 70 mg in Ph1/2a, n = 5 for all time points; and 3 doses of 70 mg in Ph1/2a, n = 4 for all time points. ASM, antiseizure medication; CI, confidence interval; M, month; OLE, open label extension; Ph1/2a, Phase 1/2a. OLE results for patients treated with initial doses of 70 mg in the Ph1/2a studies
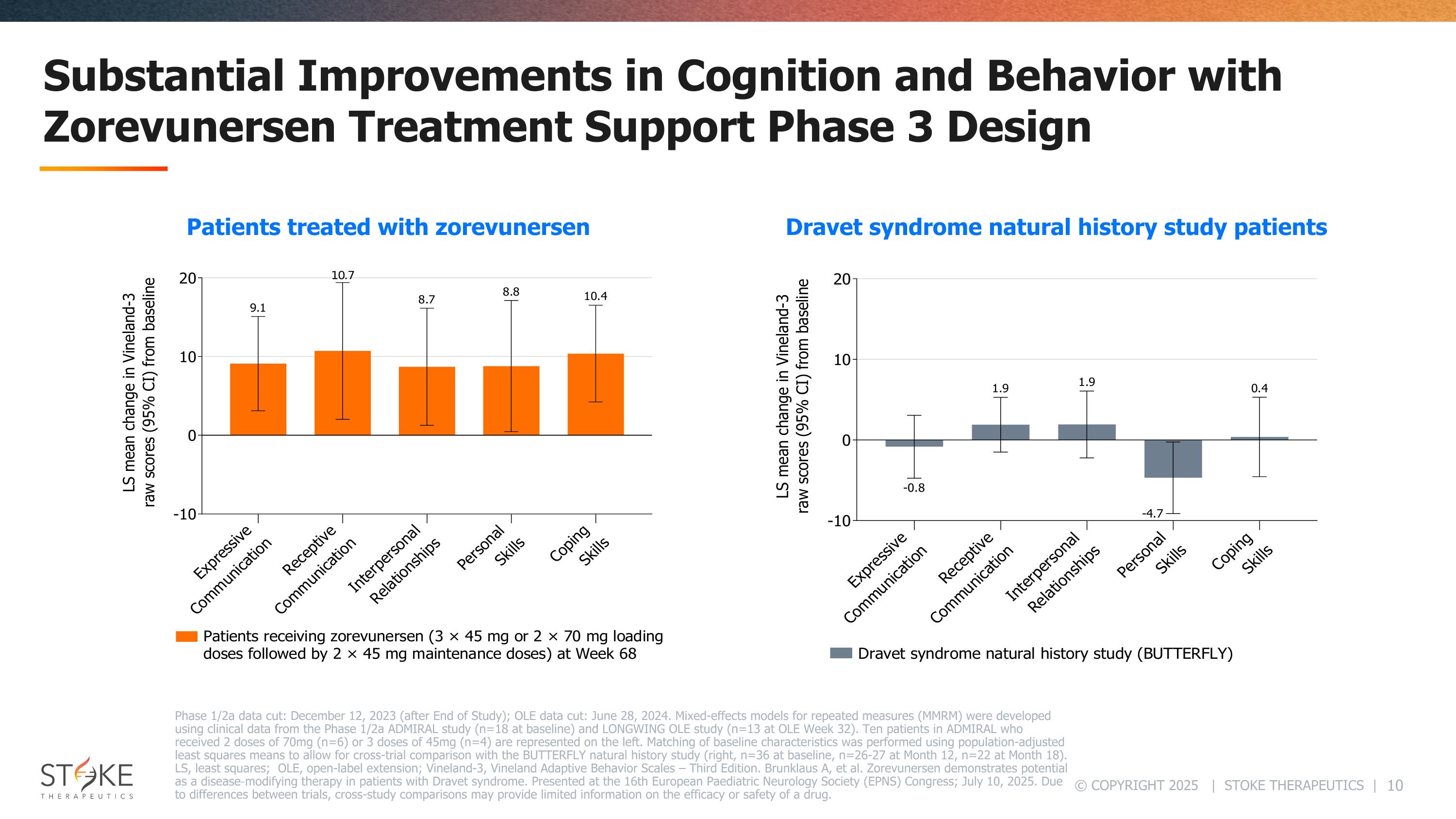
Substantial Improvements in Cognition and Behavior with Zorevunersen Treatment Support Phase 3 Design Dravet syndrome natural history study patients Patients treated with zorevunersen Phase 1/2a data cut: December 12, 2023 (after End of Study); OLE data cut: June 28, 2024. Mixed-effects models for repeated measures (MMRM) were developed using clinical data from the Phase 1/2a ADMIRAL study (n=18 at baseline) and LONGWING OLE study (n=13 at OLE Week 32). Ten patients in ADMIRAL who received 2 doses of 70mg (n=6) or 3 doses of 45mg (n=4) are represented on the left. Matching of baseline characteristics was performed using population-adjusted least squares means to allow for cross-trial comparison with the BUTTERFLY natural history study (right, n=36 at baseline, n=26-27 at Month 12, n=22 at Month 18). LS, least squares; OLE, open-label extension; Vineland-3, Vineland Adaptive Behavior Scales – Third Edition. Brunklaus A, et al. Zorevunersen demonstrates potential as a disease‑modifying therapy in patients with Dravet syndrome. Presented at the 16th European Paediatric Neurology Society (EPNS) Congress; July 10, 2025. Due to differences between trials, cross-study comparisons may provide limited information on the efficacy or safety of a drug.
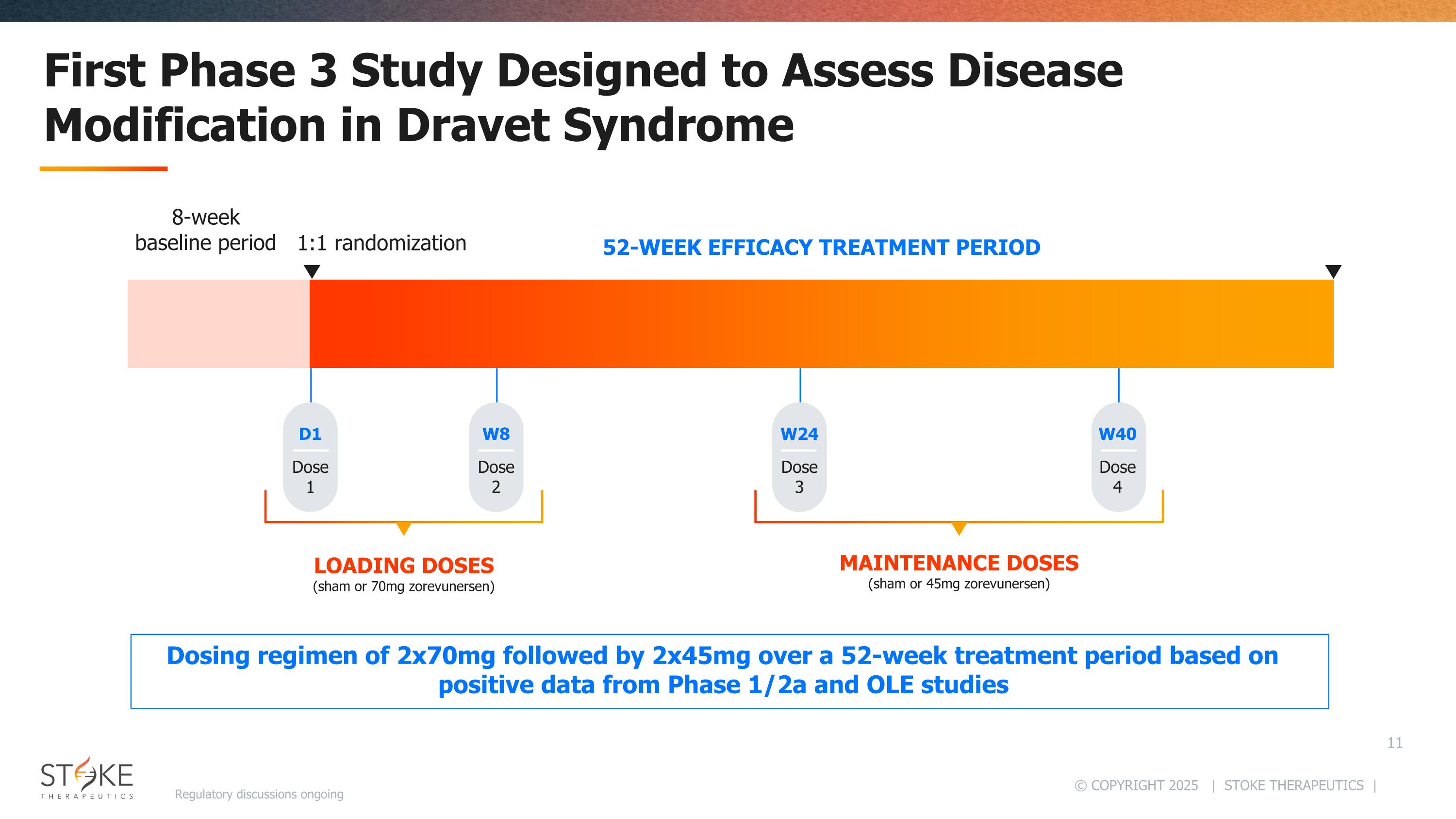
First Phase 3 Study Designed to Assess Disease Modification in Dravet Syndrome 1:1 randomization 52-WEEK EFFICACY TREATMENT PERIOD LOADING DOSES (sham or 70mg zorevunersen) MAINTENANCE DOSES (sham or 45mg zorevunersen) 8-week baseline period Dose 1 D1 Dose 4 W40 Dose 2 W8 Dose 3 W24 Dosing regimen of 2x70mg followed by 2x45mg over a 52-week treatment period based on positive data from Phase 1/2a and OLE studies Regulatory discussions ongoing

Primary endpoint Seizures Percent change from baseline in major motor seizure frequency in patients receiving zorevunersen as compared with sham at Week 28 Key secondaries Durability of effect on major motor seizure frequency measured at Week 52 Improvements in behavior & cognition measured by Vineland-3 subdomains Other Endpoints Safety, CGI-C, CaGI-C, BSID-IV, and others Study Design: Sham-controlled, 1:1 randomization Dosing Regimen: 2x70mg + 2x45mg Study Start: First patient dosed August 2025 Population: 2 to <18 years with a confirmed variant in the SCN1A gene not associated with gain of function Number of Patients Randomized: ~170 Sites: ~70 globally Duration: 8-week baseline, 52-week efficacy treatment period Data Anticipated: 2H 2027 EMPEROR Phase 3 Study Overview Regulatory discussions ongoing Phase 3 design and dose regimen aligned with Ph1/2a and OLE studies
EMPEROR Study Supported by Data; First Patient Dosed and Significant Demand to Enter Trial Large Data Set Informed Phase 3 Study Design Significant & Growing Demand Natural history reflects patient need Ph1/2 data established loading doses OLE data support maintenance regimen Improvements in cognition and behavior using Vineland-3 support key secondary endpoint selection and powering Pre-screening has identified ~130 patients >10 clinical trial sites now initiated in the U.S., UK, and Japan First patient dosed in August 2025 Increasing awareness of Dravet syndrome and the potential of zorevunersen

Potential for Disease Modification Kimberly Parkerson, M.D., Ph.D. Head of Neurology Clinical Development
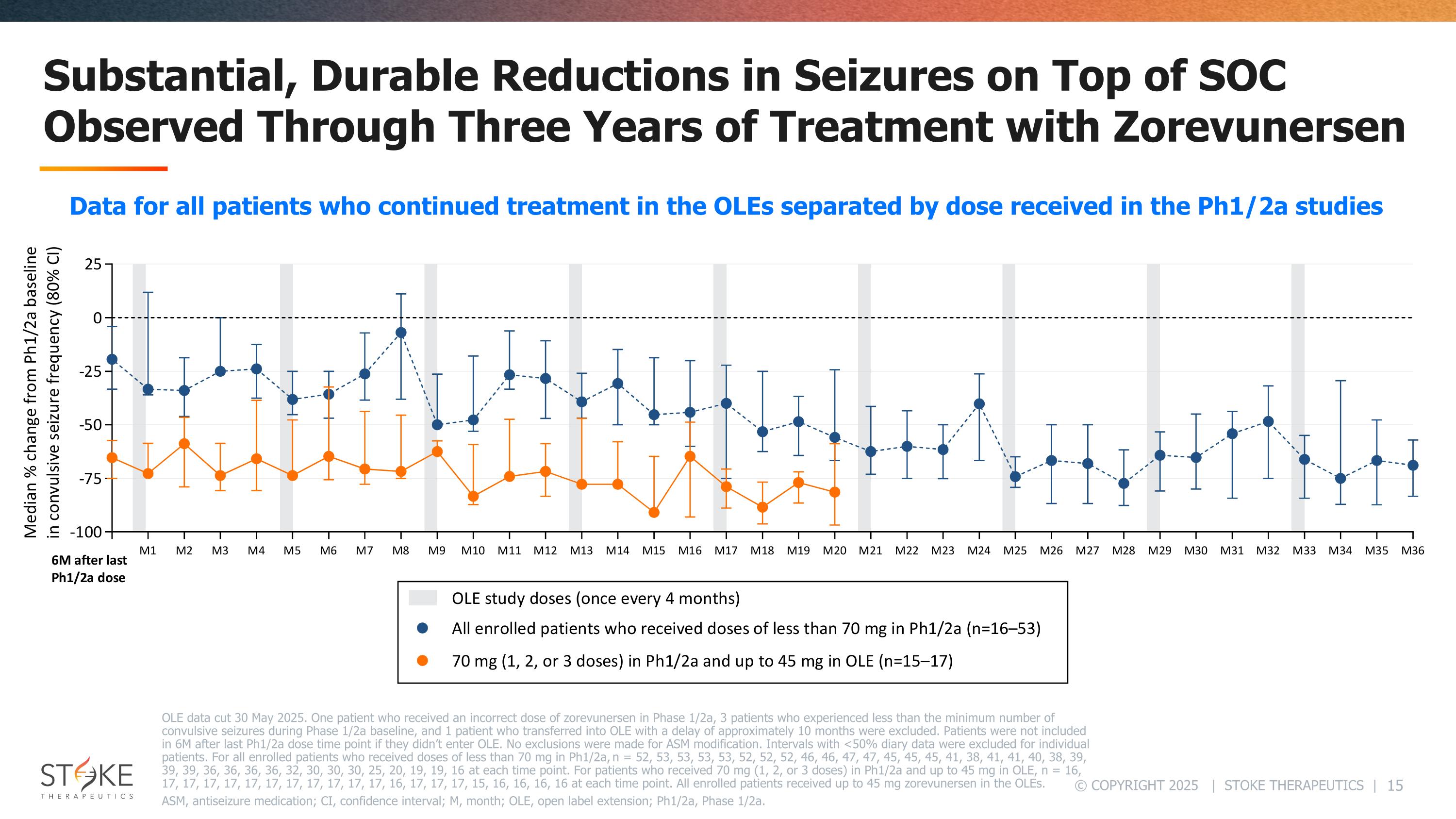
Substantial, Durable Reductions in Seizures on Top of SOC Observed Through Three Years of Treatment with Zorevunersen OLE data cut 30 May 2025. One patient who received an incorrect dose of zorevunersen in Phase 1/2a, 3 patients who experienced less than the minimum number of convulsive seizures during Phase 1/2a baseline, and 1 patient who transferred into OLE with a delay of approximately 10 months were excluded. Patients were not included in 6M after last Ph1/2a dose time point if they didn’t enter OLE. No exclusions were made for ASM modification. Intervals with <50% diary data were excluded for individual patients. For all enrolled patients who received doses of less than 70 mg in Ph1/2a, n = 52, 53, 53, 53, 53, 52, 52, 52, 46, 46, 47, 47, 45, 45, 45, 41, 38, 41, 41, 40, 38, 39, 39, 39, 36, 36, 36, 36, 32, 30, 30, 30, 25, 20, 19, 19, 16 at each time point. For patients who received 70 mg (1, 2, or 3 doses) in Ph1/2a and up to 45 mg in OLE, n = 16, 17, 17, 17, 17, 17, 17, 17, 17, 17, 17, 17, 16, 17, 17, 17, 15, 16, 16, 16, 16 at each time point. All enrolled patients received up to 45 mg zorevunersen in the OLEs. ASM, antiseizure medication; CI, confidence interval; M, month; OLE, open label extension; Ph1/2a, Phase 1/2a. Data for all patients who continued treatment in the OLEs separated by dose received in the Ph1/2a studies
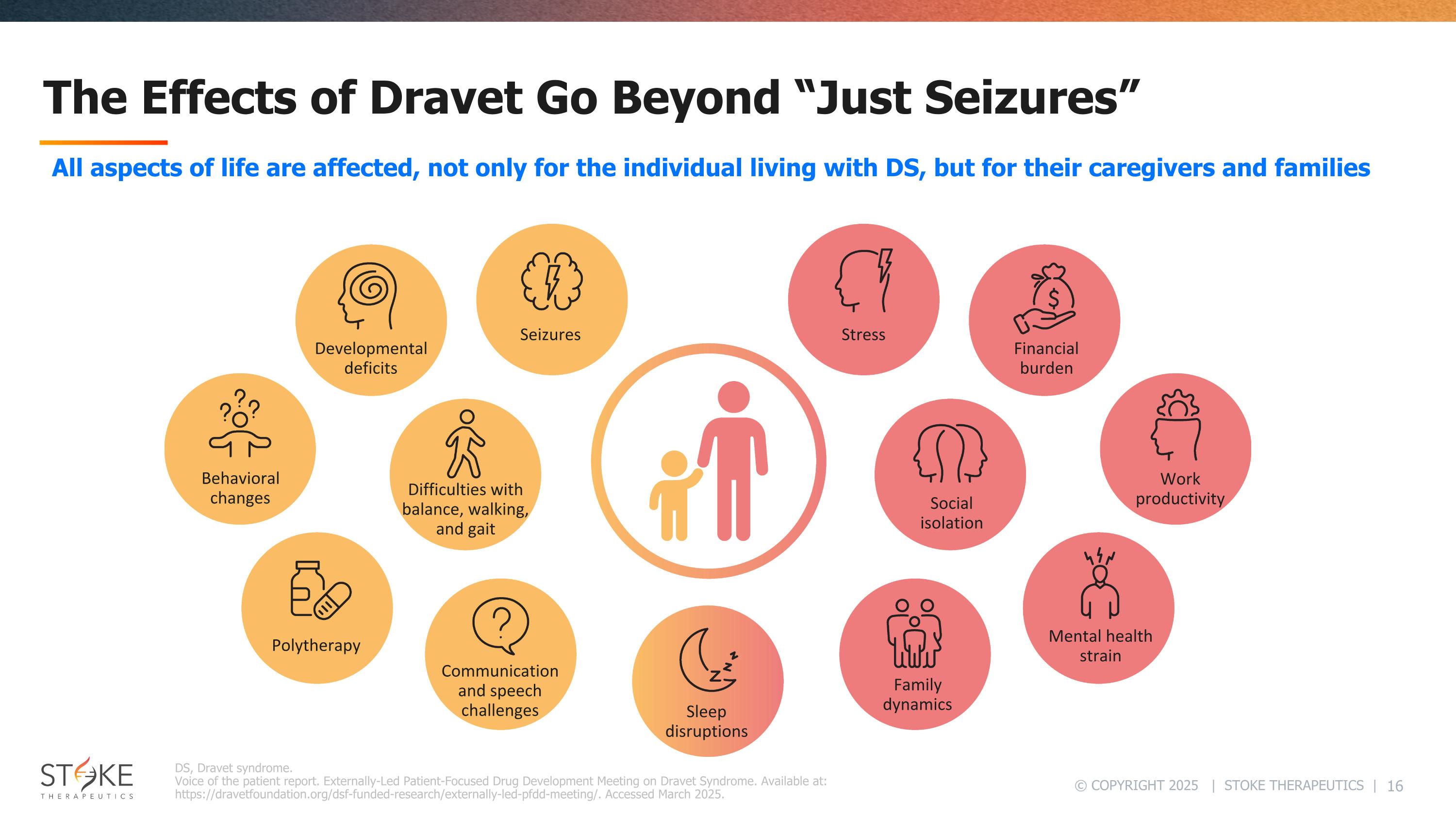
The Effects of Dravet Go Beyond “Just Seizures” DS, Dravet syndrome. Voice of the patient report. Externally-Led Patient-Focused Drug Development Meeting on Dravet Syndrome. Available at: https://dravetfoundation.org/dsf-funded-research/externally-led-pfdd-meeting/. Accessed March 2025. Difficulties with balance, walking, and gait Seizures Developmental deficits Behavioral changes Sleep disruptions Communication and speech challenges Polytherapy Stress Financial burden Work productivity Social isolation Mental health strain Family dynamics All aspects of life are affected, not only for the individual living with DS, but for their caregivers and families
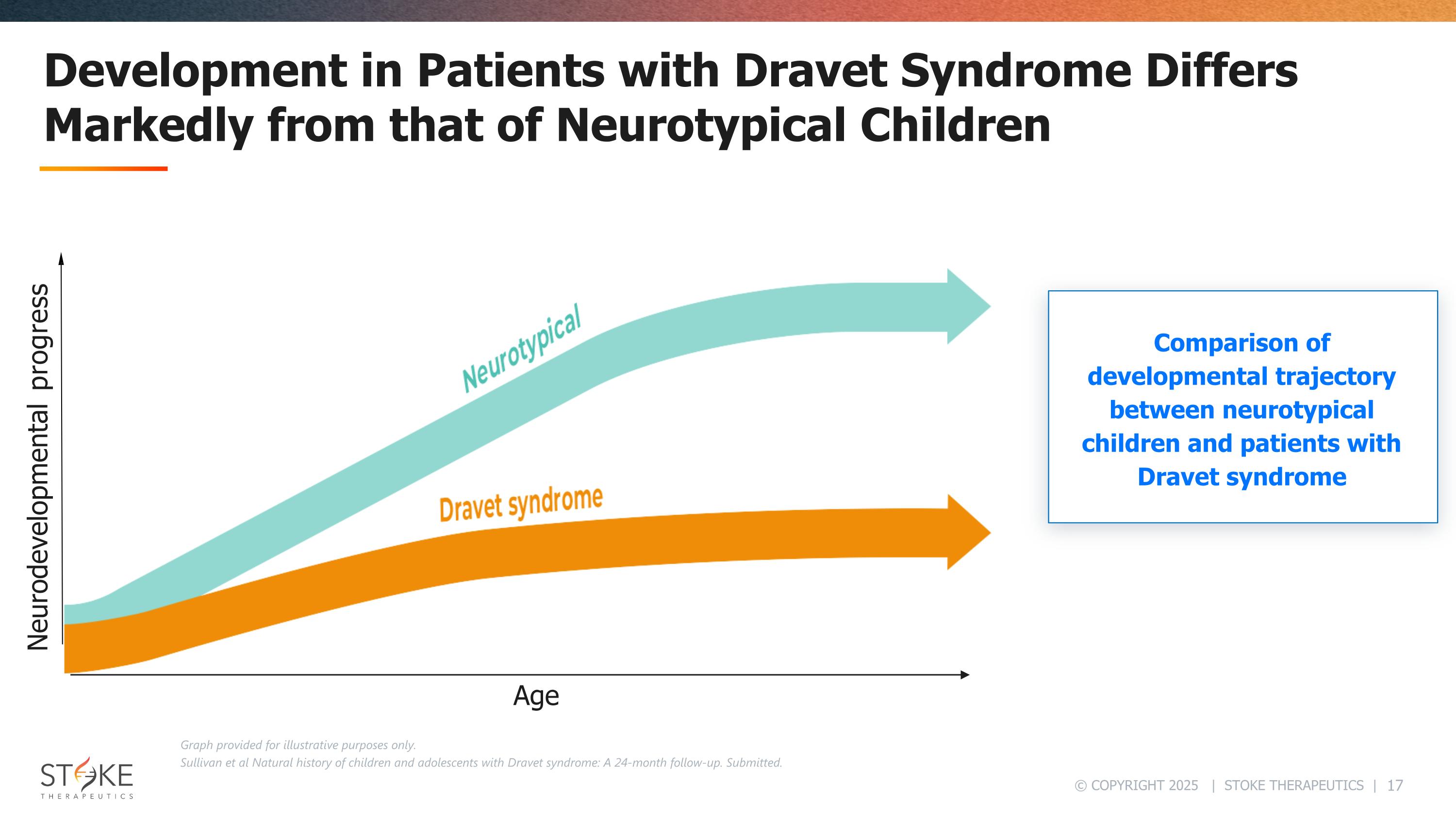
Development in Patients with Dravet Syndrome Differs Markedly from that of Neurotypical Children Comparison of developmental trajectory between neurotypical children and patients with Dravet syndrome Graph provided for illustrative purposes only. Sullivan et al Natural history of children and adolescents with Dravet syndrome: A 24-month follow-up. Submitted. Neurodevelopmental progress Age
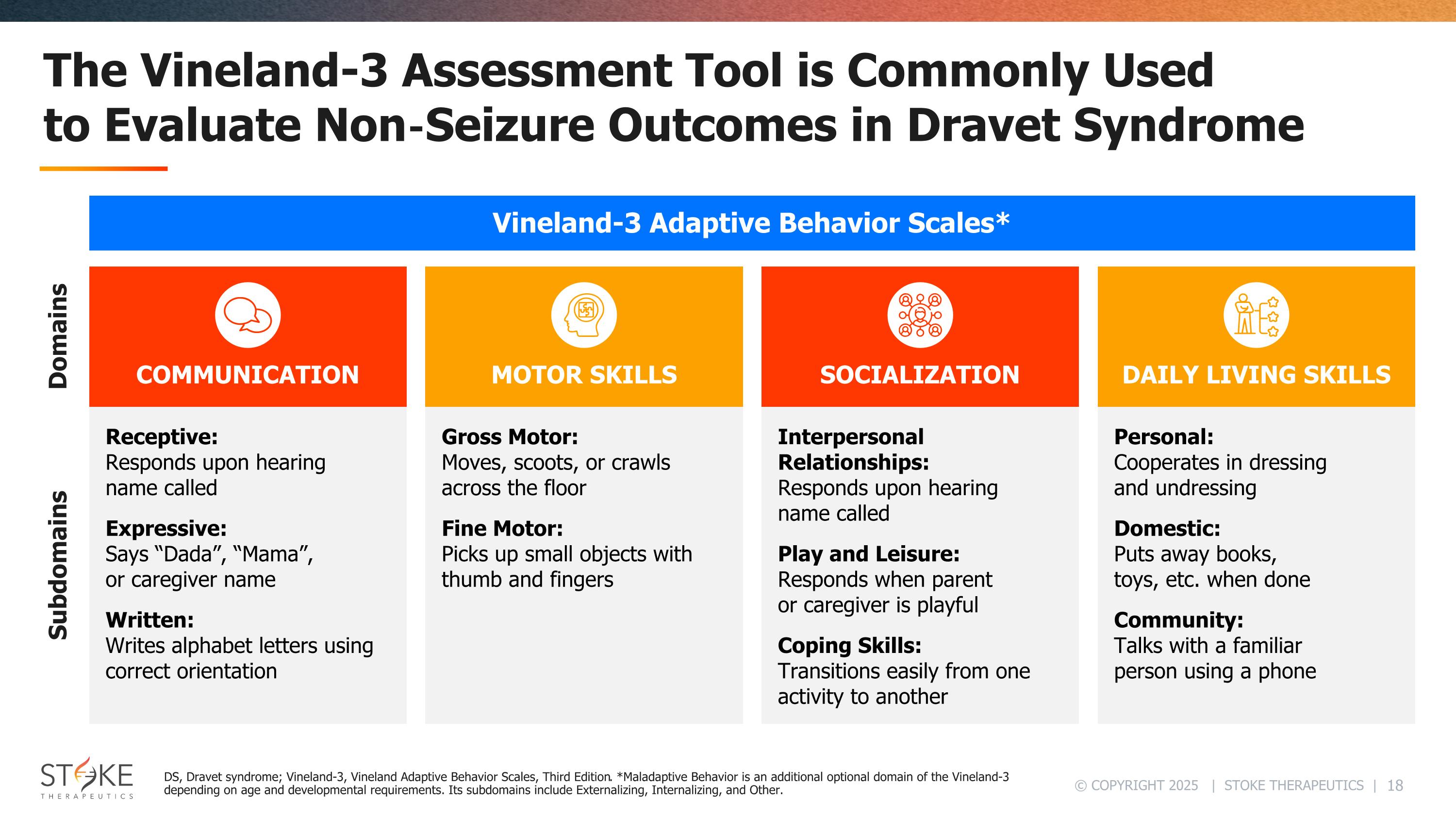
The Vineland-3 Assessment Tool is Commonly Used to Evaluate Non‑Seizure Outcomes in Dravet Syndrome COMMUNICATION Receptive: Responds upon hearing name called Expressive: Says “Dada”, “Mama”, or caregiver name Written: Writes alphabet letters using correct orientation MOTOR SKILLS Gross Motor: Moves, scoots, or crawls across the floor Fine Motor: Picks up small objects with thumb and fingers SOCIALIZATION Interpersonal Relationships: Responds upon hearing name called Play and Leisure: Responds when parent or caregiver is playful Coping Skills: Transitions easily from one activity to another DAILY LIVING SKILLS Personal: Cooperates in dressing and undressing Domestic: Puts away books, toys, etc. when done Community: Talks with a familiar person using a phone Domains Subdomains Vineland-3 Adaptive Behavior Scales* DS, Dravet syndrome; Vineland-3, Vineland Adaptive Behavior Scales, Third Edition. *Maladaptive Behavior is an additional optional domain of the Vineland-3 depending on age and developmental requirements. Its subdomains include Externalizing, Internalizing, and Other.
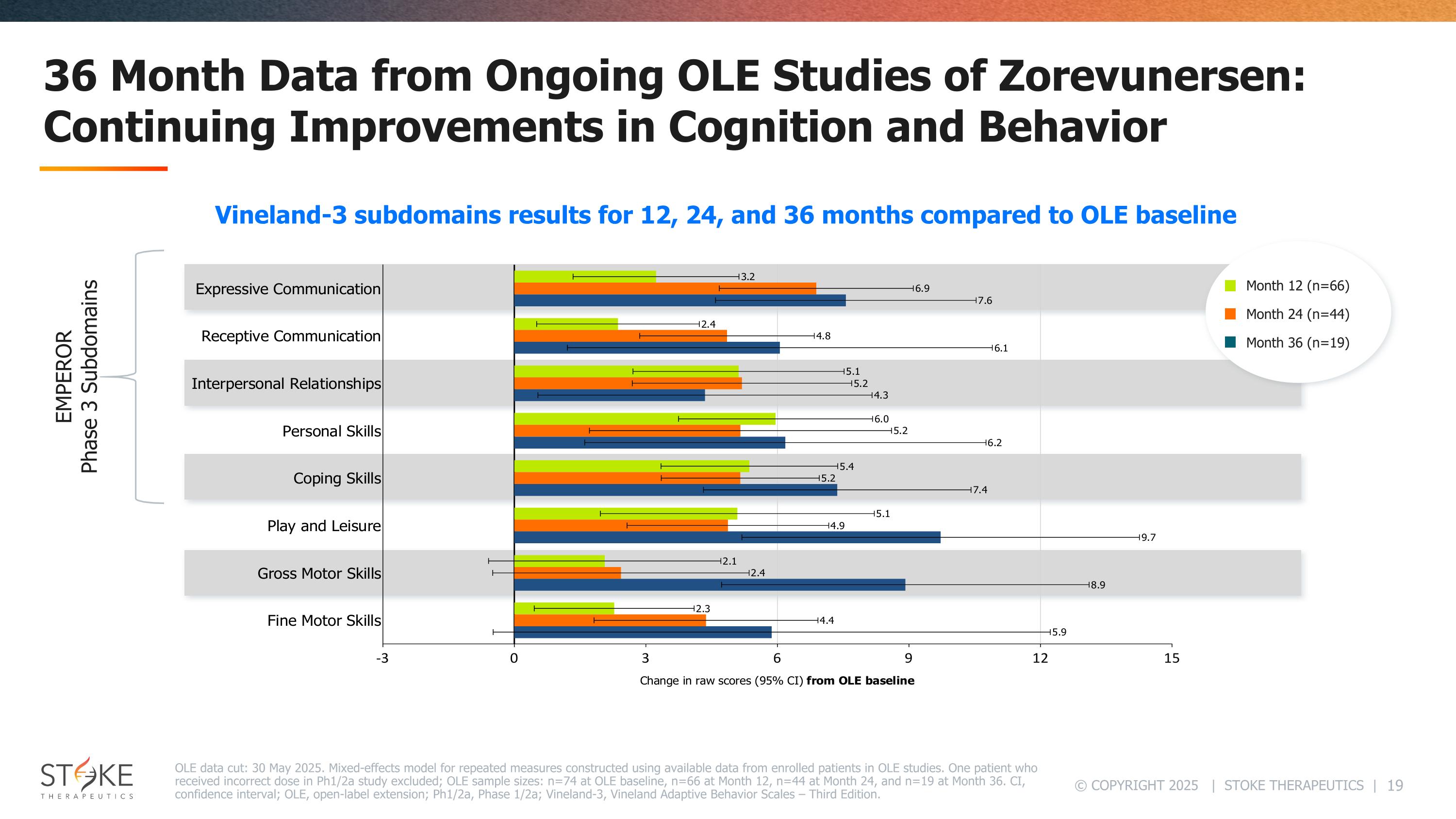
36 Month Data from Ongoing OLE Studies of Zorevunersen: Continuing Improvements in Cognition and Behavior OLE data cut: 30 May 2025. Mixed-effects model for repeated measures constructed using available data from enrolled patients in OLE studies. One patient who received incorrect dose in Ph1/2a study excluded; OLE sample sizes: n=74 at OLE baseline, n=66 at Month 12, n=44 at Month 24, and n=19 at Month 36. CI, confidence interval; OLE, open-label extension; Ph1/2a, Phase 1/2a; Vineland-3, Vineland Adaptive Behavior Scales – Third Edition. Month 12 (n=66) Month 24 (n=44) Month 36 (n=19) EMPEROR Phase 3 Subdomains Vineland-3 subdomains results for 12, 24, and 36 months compared to OLE baseline
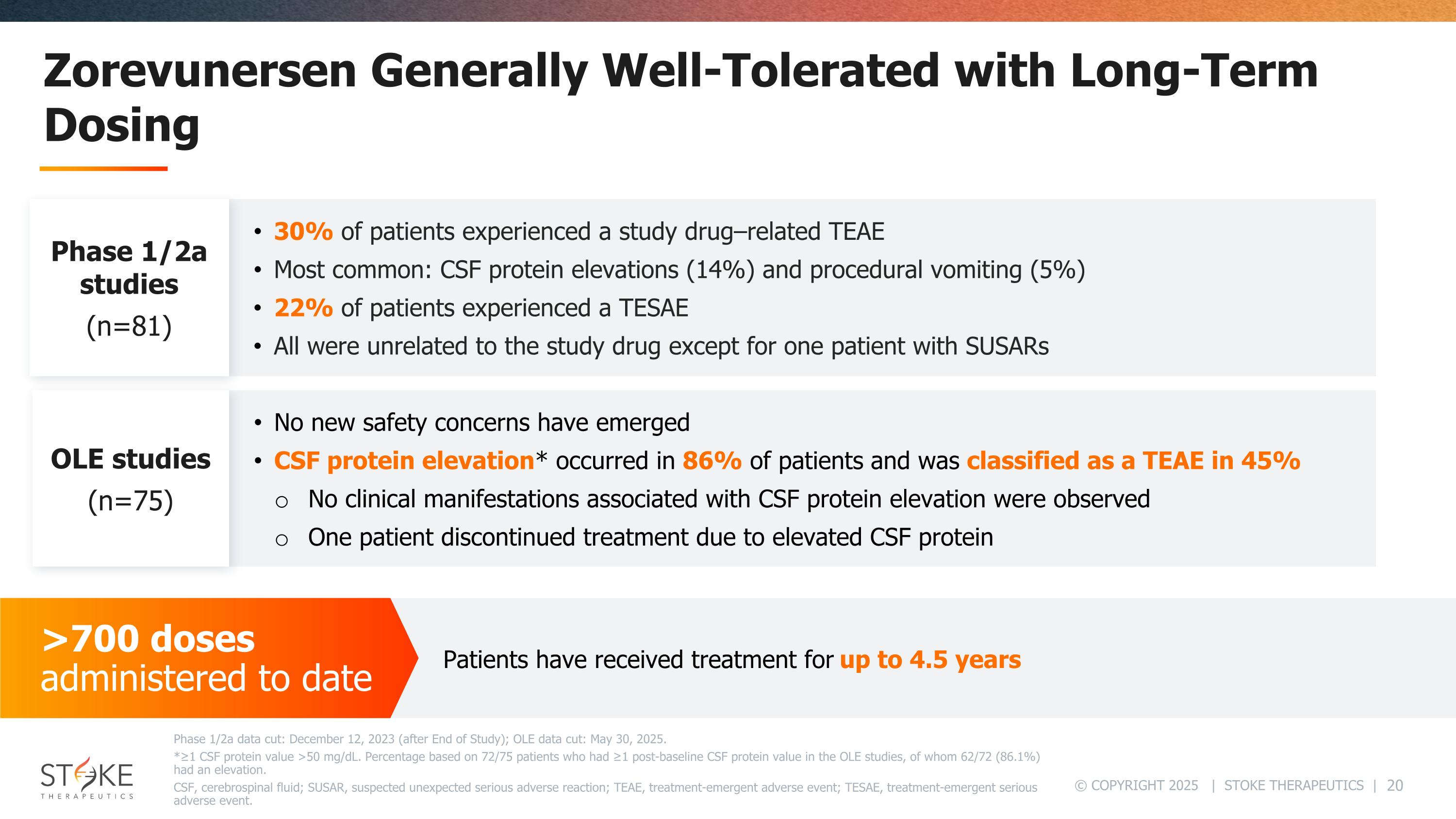
Zorevunersen Generally Well-Tolerated with Long-Term Dosing Phase 1/2a data cut: December 12, 2023 (after End of Study); OLE data cut: May 30, 2025. *≥1 CSF protein value >50 mg/dL. Percentage based on 72/75 patients who had ≥1 post-baseline CSF protein value in the OLE studies, of whom 62/72 (86.1%) had an elevation. CSF, cerebrospinal fluid; SUSAR, suspected unexpected serious adverse reaction; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event. >700 doses administered to date Patients have received treatment for up to 4.5 years No new safety concerns have emerged CSF protein elevation* occurred in 86% of patients and was classified as a TEAE in 45% No clinical manifestations associated with CSF protein elevation were observed One patient discontinued treatment due to elevated CSF protein 30% of patients experienced a study drug–related TEAE Most common: CSF protein elevations (14%) and procedural vomiting (5%) 22% of patients experienced a TESAE All were unrelated to the study drug except for one patient with SUSARs Phase 1/2a studies (n=81) OLE studies (n=75)

Pipeline Update Autosomal Dominant Optic Atrophy (ADOA) Preclinical Data and Development Plan
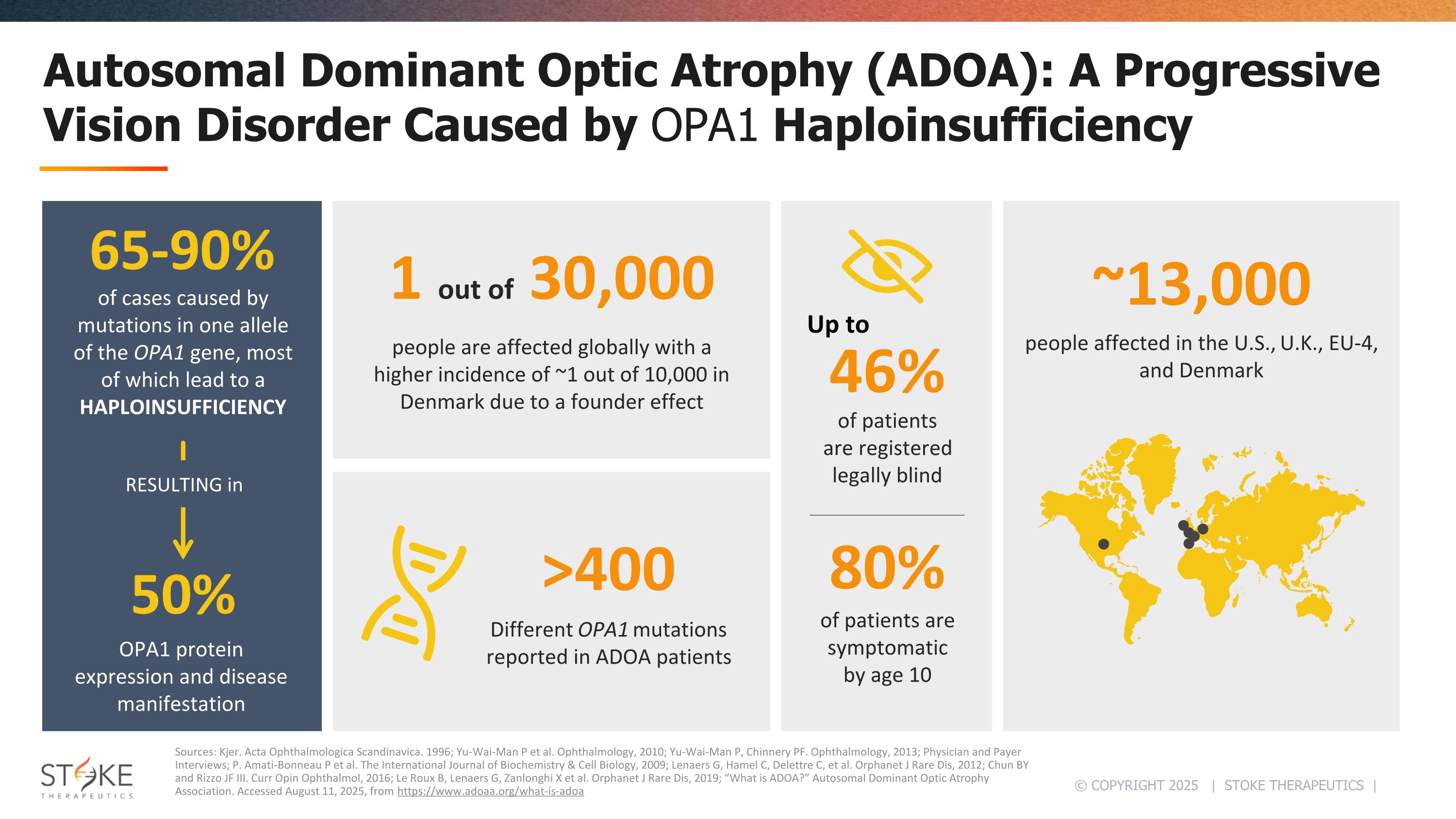
Autosomal Dominant Optic Atrophy (ADOA): A Progressive Vision Disorder Caused by OPA1 Haploinsufficiency Sources: Kjer. Acta Ophthalmologica Scandinavica. 1996; Yu-Wai-Man P et al. Ophthalmology, 2010; Yu-Wai-Man P, Chinnery PF. Ophthalmology, 2013; Physician and Payer Interviews; P. Amati-Bonneau P et al. The International Journal of Biochemistry & Cell Biology, 2009; Lenaers G, Hamel C, Delettre C, et al. Orphanet J Rare Dis, 2012; Chun BY and Rizzo JF III. Curr Opin Ophthalmol, 2016; Le Roux B, Lenaers G, Zanlonghi X et al. Orphanet J Rare Dis, 2019; “What is ADOA?” Autosomal Dominant Optic Atrophy Association. Accessed August 11, 2025, from https://www.adoaa.org/what-is-adoa NaV1.1 protein expression people are affected globally with a higher incidence of ~1 out of 10,000 in Denmark due to a founder effect ~13,000 people affected in the U.S., U.K., EU-4, and Denmark 1 out of 30,000 >400 Different OPA1 mutations reported in ADOA patients of patients are registered legally blind of patients are symptomatic by age 10 46% 80% 65-90% of cases caused by mutations in one allele of the OPA1 gene, most of which lead to a HAPLOINSUFFICIENCY 50% OPA1 protein expression and disease manifestation Up to RESULTING in
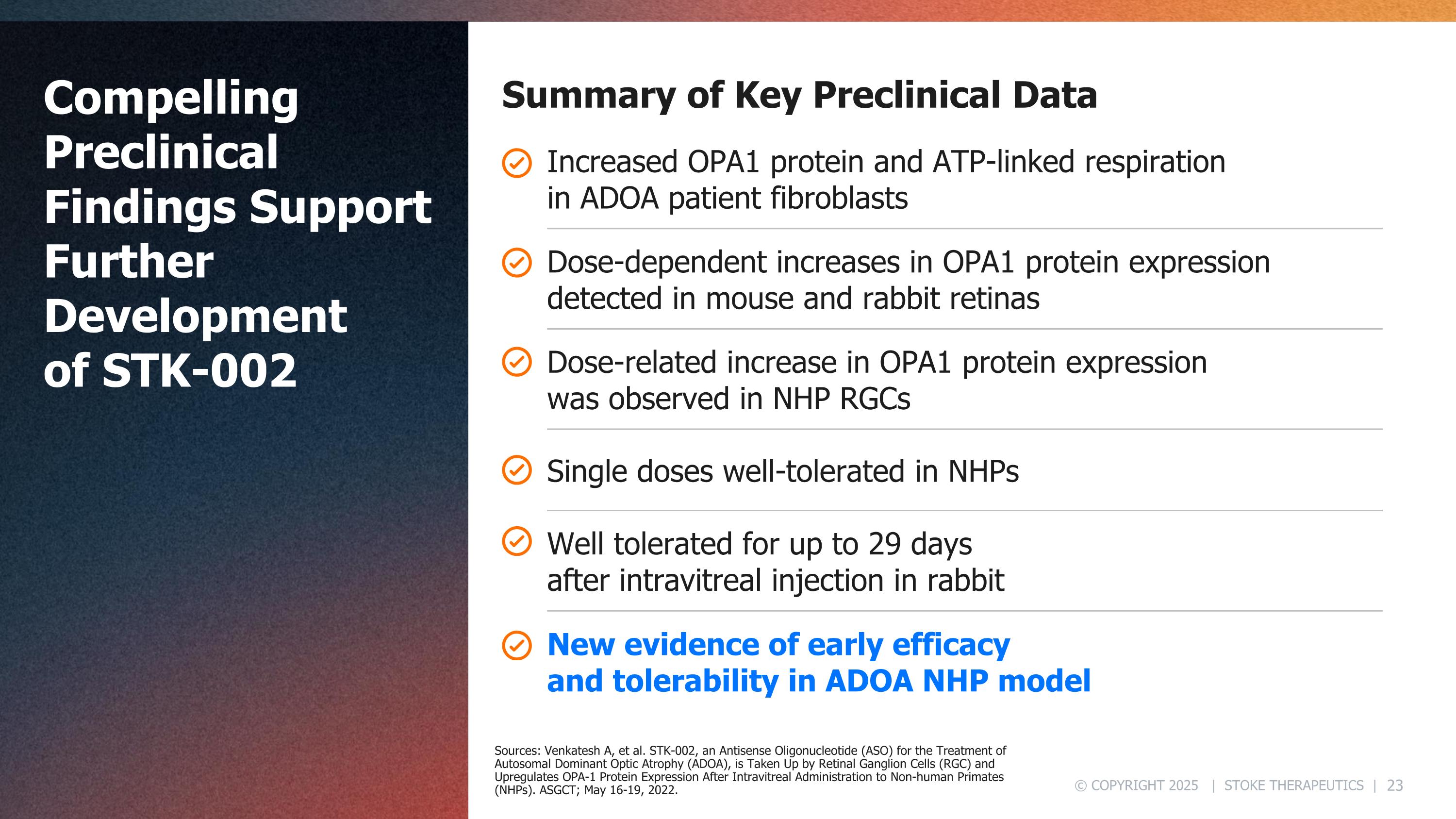
Compelling Preclinical Findings Support Further Development of STK-002 Summary of Key Preclinical Data Increased OPA1 protein and ATP-linked respiration in ADOA patient fibroblasts Dose-dependent increases in OPA1 protein expression detected in mouse and rabbit retinas Well tolerated for up to 29 days after intravitreal injection in rabbit Dose-related increase in OPA1 protein expression was observed in NHP RGCs Single doses well-tolerated in NHPs New evidence of early efficacy and tolerability in ADOA NHP model Sources: Venkatesh A, et al. STK-002, an Antisense Oligonucleotide (ASO) for the Treatment of Autosomal Dominant Optic Atrophy (ADOA), is Taken Up by Retinal Ganglion Cells (RGC) and Upregulates OPA-1 Protein Expression After Intravitreal Administration to Non-human Primates (NHPs). ASGCT; May 16-19, 2022.
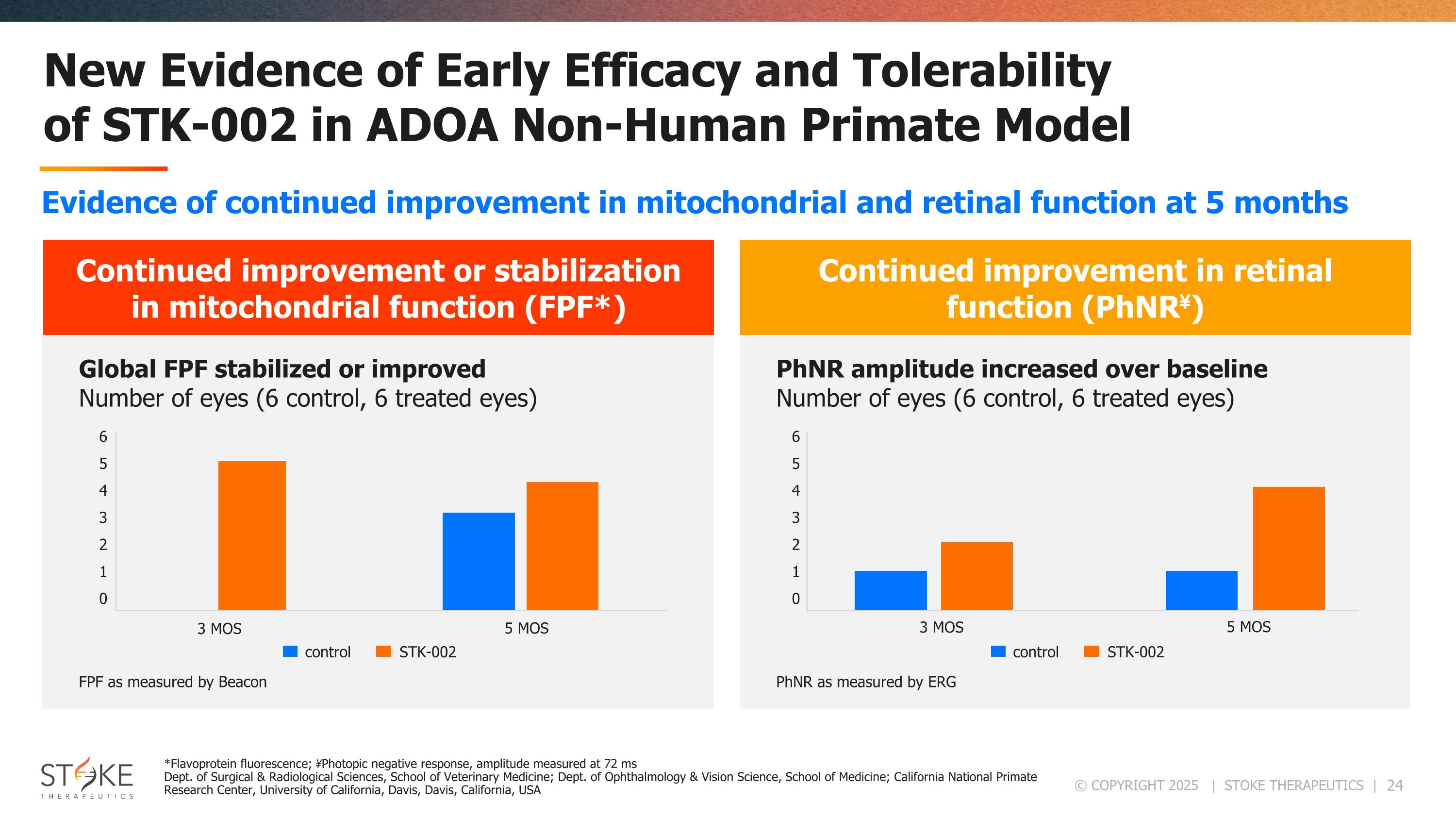
New Evidence of Early Efficacy and Tolerability of STK-002 in ADOA Non-Human Primate Model Continued improvement in retinal function (PhNR¥) PhNR as measured by ERG PhNR amplitude increased over baseline Number of eyes (6 control, 6 treated eyes) Continued improvement or stabilization in mitochondrial function (FPF*) FPF as measured by Beacon Global FPF stabilized or improved Number of eyes (6 control, 6 treated eyes) 6 5 4 3 2 1 0 3 MOS 5 MOS control STK-002 3 MOS 5 MOS 6 5 4 3 2 1 0 control STK-002 *Flavoprotein fluorescence; ¥Photopic negative response, amplitude measured at 72 ms Dept. of Surgical & Radiological Sciences, School of Veterinary Medicine; Dept. of Ophthalmology & Vision Science, School of Medicine; California National Primate Research Center, University of California, Davis, Davis, California, USA Evidence of continued improvement in mitochondrial and retinal function at 5 months
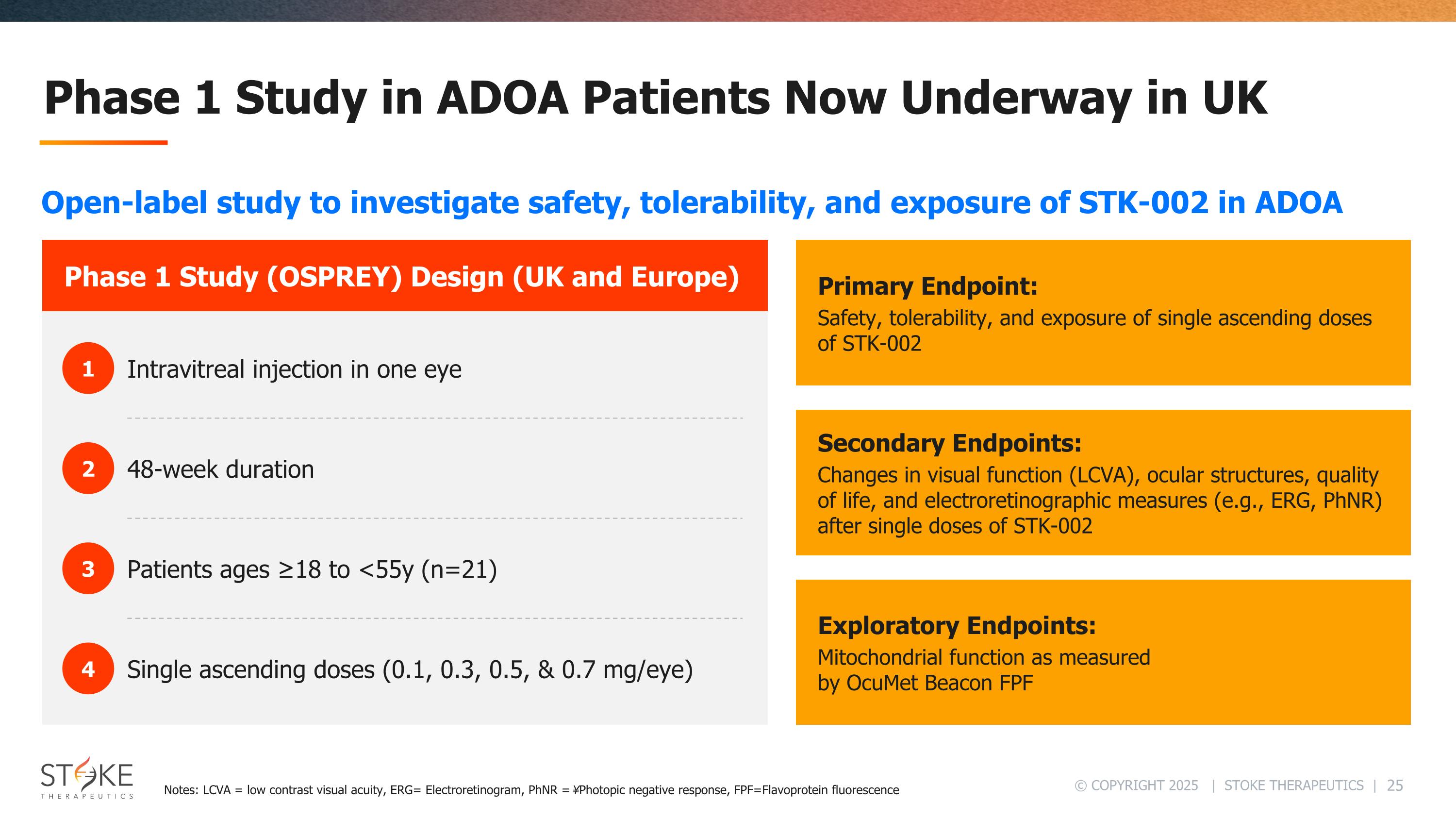
Phase 1 Study in ADOA Patients Now Underway in UK Phase 1 Study (OSPREY) Design (UK and Europe) 1 2 3 4 Intravitreal injection in one eye 48-week duration Patients ages ≥18 to <55y (n=21) Single ascending doses (0.1, 0.3, 0.5, & 0.7 mg/eye) Primary Endpoint: Safety, tolerability, and exposure of single ascending doses of STK-002 Secondary Endpoints: Changes in visual function (LCVA), ocular structures, quality of life, and electroretinographic measures (e.g., ERG, PhNR) after single doses of STK-002 Exploratory Endpoints: Mitochondrial function as measured by OcuMet Beacon FPF Open-label study to investigate safety, tolerability, and exposure of STK-002 in ADOA Notes: LCVA = low contrast visual acuity, ERG= Electroretinogram, PhNR = ¥Photopic negative response, FPF=Flavoprotein fluorescence

Financial Summary Tommy Leggett Chief Financial Officer

Closing Remarks Ian F. Smith Interim Chief Executive Officer & Director
Stoke is Positioned to Deliver in Dravet and Beyond Execution Clinical Data Platform Expansion Strength to Deliver Clear path to value creation supported by clinical progress, financial strength, and investment in core capabilities Global Phase 3 Underway First patient dosed in EMPEROR study Robust trial design to support potential disease-modifying medicine 3-year OLE Results Support the long-term disease-modifying potential of zorevunersen Second program in the clinic Phase 1 study of STK-002 for ADOA initiated in the UK Supported by safety and efficacy from pre-clinical data Well-funded and positioned for success Biogen collaboration Runway through Ph3 readout to mid-2028 Key leadership hires Increased focus on Medical Affairs, Regulatory, and Commercial capabilities

Q&A