

.2 Wave Life Sciences Corporate Presentation January 12, 2026

Forward-looking statements This document contains forward-looking statements. All statements other than statements of historical facts contained in this document, including statements regarding possible or assumed future results of operations, preclinical and clinical studies, business strategies, research and development plans, collaborations and partnerships, regulatory activities and timing thereof, competitive position, potential growth opportunities, use of proceeds and the effects of competition are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause the actual results, performance or achievements of Wave Life Sciences Ltd. (the “Company”) to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward- looking statements in this presentation are only predictions. The Company has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect the Company’s business, financial condition and results of operations. These forward-looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, including those listed under Risk Factors in the Company’s Form 10-K and other filings with the SEC, some of which cannot be predicted or quantified and some of which are beyond the Company’s control. The events and circumstances reflected in the Company’s forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, the Company operates in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that the Company may face. Except as required by applicable law, the Company does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. 2

Our Mission To unlock the broad potential of RNA medicines to transform human health 3
Building a leading RNA medicines company Differentiated RNA medicines Translating genetic insights into Unlocking platform and chemistry potentially best-in-class medicines emerging pipeline RNAi WVE-007 (obesity)• Extra-hepatic capabilities: with RNAi and RNA editing • Differentiated mechanism focused on fat loss and • Proprietary chemistry muscle preservation • Leveraging deep insights in human RNA editing WVE-006 (AATD) • Novel bifunctional modality: genetics WVE-008 (liver disease) simultaneously edit and silence with single • Restoration of functional • Strong and broad IP oligonucleotide construct protein production • In-house GMP manufacturing Other modalities: DMD and HD clinical programs 1 Well capitalized with ~$602 million and expected cash runway into 3Q 2028 4 1 Preliminary, unaudited cash and cash equivalents of ~$602 million are as of December 31, 2025. These preliminary, unaudited results are subject to adjustment. Wave expects to report its final and complete fourth-quarter and full-year 2025 financial results in late February 2026, and the actual results could be different from these preliminary, unaudited financial results
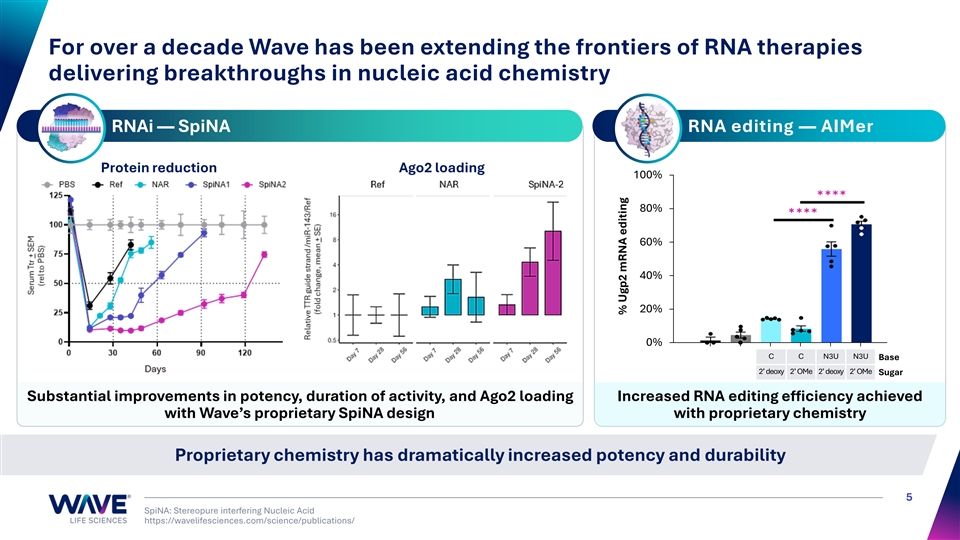
For over a decade Wave has been extending the frontiers of RNA therapies delivering breakthroughs in nucleic acid chemistry RNAi — SpiNA RNA editing — AIMer Protein reduction Ago2 loading 100% 80% 60% 40% 20% 0% C C N3U N3U Base 2’ deoxy 2’ OMe 2’ deoxy 2’ OMe Sugar Substantial improvements in potency, duration of activity, and Ago2 loading Increased RNA editing efficiency achieved with Wave’s proprietary SpiNA design with proprietary chemistry Proprietary chemistry has dramatically increased potency and durability 5 SpiNA: Stereopure interfering Nucleic Acid https://wavelifesciences.com/science/publications/ % Ugp2 mRNA editing
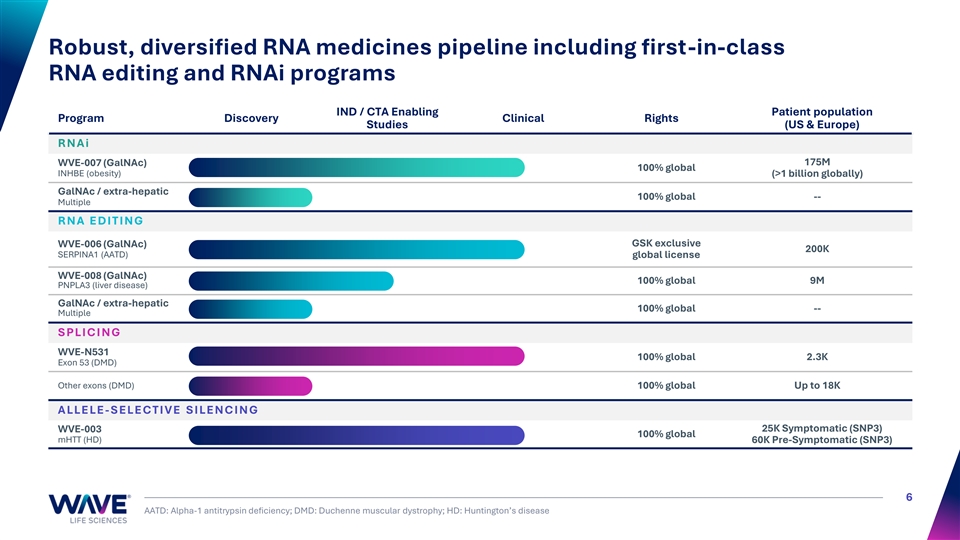
Robust, diversified RNA medicines pipeline including first-in-class RNA editing and RNAi programs IND / CTA Enabling Patient population Program Discovery Clinical Rights Studies (US & Europe) R N A i 175M WVE-007 (GalNAc) 100% global INHBE (obesity) (>1 billion globally) GalNAc / extra-hepatic 100% global -- Multiple R N A E D I T I N G GSK exclusive WVE-006 (GalNAc) 200K SERPINA1 (AATD) global license WVE-008 (GalNAc) 100% global 9M PNPLA3 (liver disease) GalNAc / extra-hepatic 100% global -- Multiple S PLI C I N G WVE-N531 100% global 2.3K Exon 53 (DMD) Other exons (DMD) 100% global Up to 18K A LLE LE - S E LE C T I V E S I LE N C I N G WVE-003 25K Symptomatic (SNP3) 100% global mHTT (HD) 60K Pre-Symptomatic (SNP3) 6 AATD: Alpha-1 antitrypsin deficiency; DMD: Duchenne muscular dystrophy; HD: Huntington’s disease

WVE-007 GalNAc-siRNA silencing Obesity 7
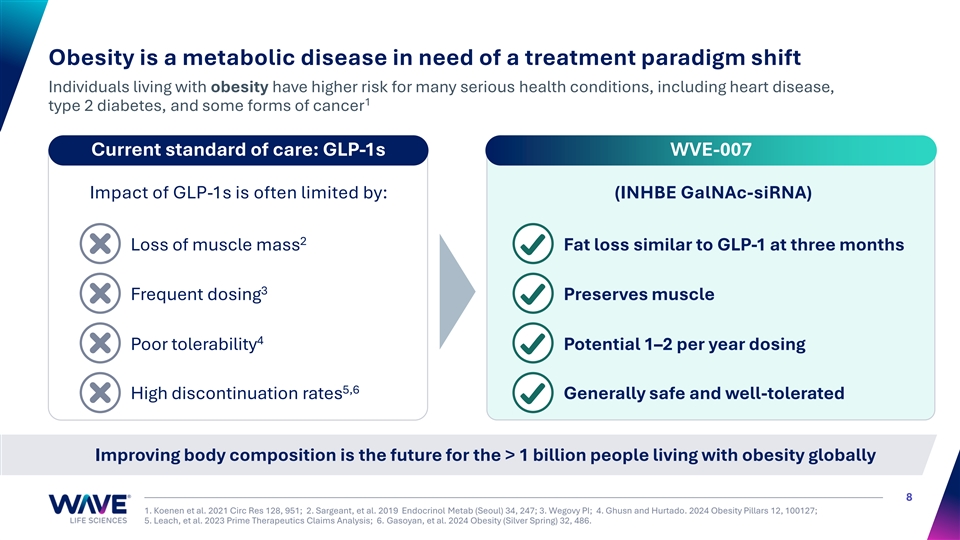
Obesity is a metabolic disease in need of a treatment paradigm shift Individuals living with obesity have higher risk for many serious health conditions, including heart disease, 1 type 2 diabetes, and some forms of cancer Current standard of care: GLP-1s WVE-007 Impact of GLP-1s is often limited by: (INHBE GalNAc-siRNA) 2 Loss of muscle mass Fat loss similar to GLP-1 at three months 3 Frequent dosing Preserves muscle 4 Poor tolerability Potential 1–2 per year dosing 5,6 High discontinuation rates Generally safe and well-tolerated Improving body composition is the future for the > 1 billion people living with obesity globally 8 1. Koenen et al. 2021 Circ Res 128, 951; 2. Sargeant, et al. 2019 Endocrinol Metab (Seoul) 34, 247; 3. Wegovy PI; 4. Ghusn and Hurtado. 2024 Obesity Pillars 12, 100127; 5. Leach, et al. 2023 Prime Therapeutics Claims Analysis; 6. Gasoyan, et al. 2024 Obesity (Silver Spring) 32, 486.
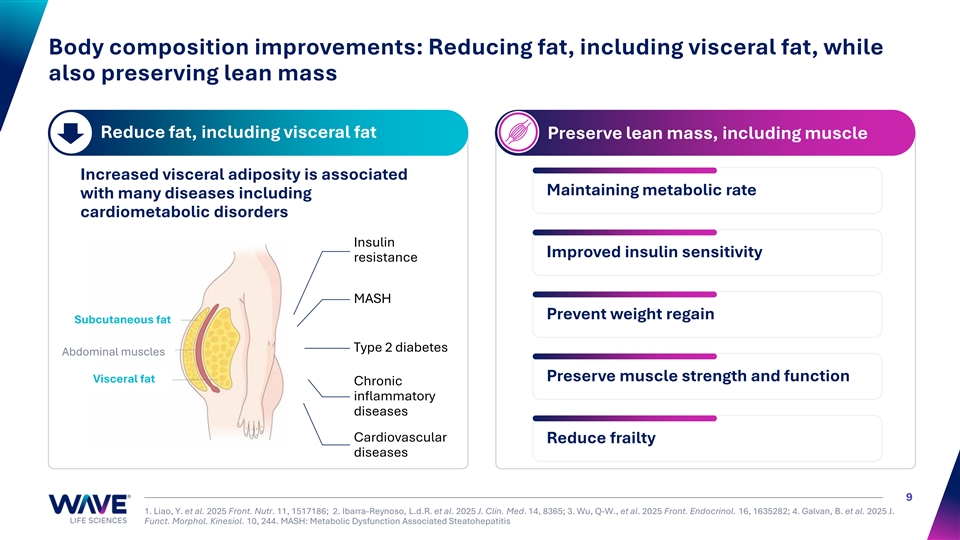
Body composition improvements: Reducing fat, including visceral fat, while also preserving lean mass Reduce fat, including visceral fat Preserve lean mass, including muscle Increased visceral adiposity is associated Maintaining metabolic rate with many diseases including cardiometabolic disorders Insulin Improved insulin sensitivity resistance MASH Prevent weight regain Subcutaneous fat Type 2 diabetes Abdominal muscles Preserve muscle strength and function Visceral fat Chronic inflammatory diseases Cardiovascular Reduce frailty diseases 9 1. Liao, Y. et al. 2025 Front. Nutr. 11, 1517186; 2. Ibarra-Reynoso, L.d.R. et al. 2025 J. Clin. Med. 14, 8365; 3. Wu, Q-W., et al. 2025 Front. Endocrinol. 16, 1635282; 4. Galvan, B. et al. 2025 J. Funct. Morphol. Kinesiol. 10, 244. MASH: Metabolic Dysfunction Associated Steatohepatitis
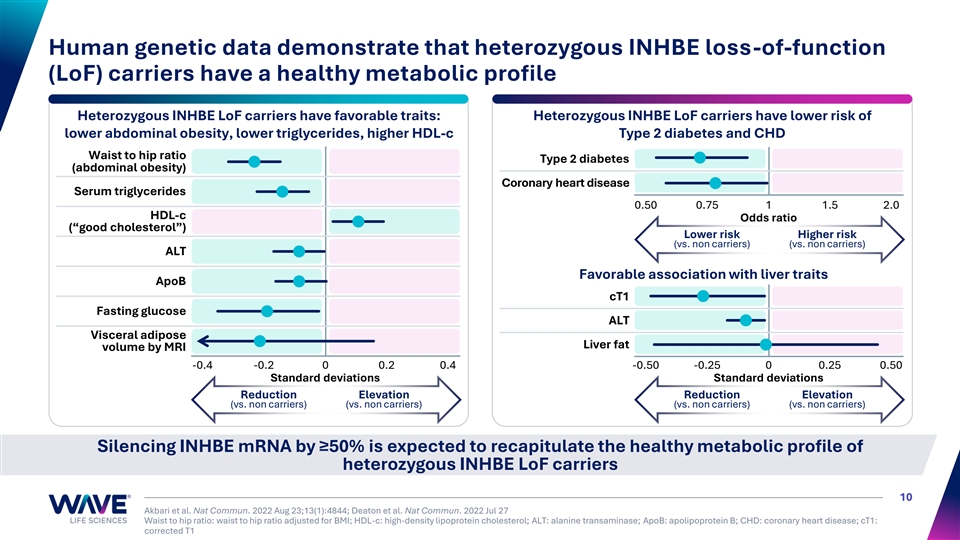
Human genetic data demonstrate that heterozygous INHBE loss-of-function (LoF) carriers have a healthy metabolic profile Heterozygous INHBE LoF carriers have favorable traits: Heterozygous INHBE LoF carriers have lower risk of lower abdominal obesity, lower triglycerides, higher HDL-c Type 2 diabetes and CHD Waist to hip ratio Type 2 diabetes (abdominal obesity) Coronary heart disease Serum triglycerides 0.50 0.75 1 1.5 2.0 HDL-c Odds ratio (“good cholesterol”) Lower risk Higher risk (vs. non carriers) (vs. non carriers) ALT Favorable association with liver traits ApoB cT1 Fasting glucose ALT Visceral adipose Liver fat volume by MRI -0.4 -0.2 0 0.2 0.4 -0.50 -0.25 0 0.25 0.50 Standard deviations Standard deviations Reduction Elevation Reduction Elevation (vs. non carriers) (vs. non carriers) (vs. non carriers) (vs. non carriers) Silencing INHBE mRNA by ≥50% is expected to recapitulate the healthy metabolic profile of heterozygous INHBE LoF carriers 10 Akbari et al. Nat Commun. 2022 Aug 23;13(1):4844; Deaton et al. Nat Commun. 2022 Jul 27 Waist to hip ratio: waist to hip ratio adjusted for BMI; HDL-c: high-density lipoprotein cholesterol; ALT: alanine transaminase; ApoB: apolipoprotein B; CHD: coronary heart disease; cT1: corrected T1
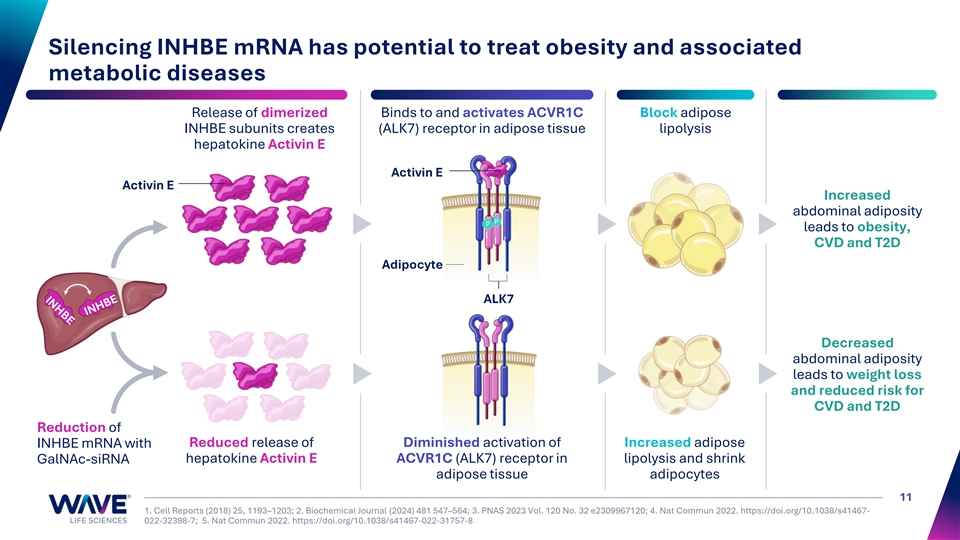
Silencing INHBE mRNA has potential to treat obesity and associated metabolic diseases Release of dimerized Binds to and activates ACVR1C Block adipose INHBE subunits creates (ALK7) receptor in adipose tissue lipolysis hepatokine Activin E Activin E Activin E Increased abdominal adiposity leads to obesity, I II I I CVD and T2D Adipocyte ALK7 Decreased abdominal adiposity leads to weight loss and reduced risk for CVD and T2D Reduction of Reduced release of Diminished activation of Increased adipose INHBE mRNA with hepatokine Activin E ACVR1C (ALK7) receptor in lipolysis and shrink GalNAc-siRNA adipose tissue adipocytes 11 1. Cell Reports (2018) 25, 1193–1203; 2. Biochemical Journal (2024) 481 547–564; 3. PNAS 2023 Vol. 120 No. 32 e2309967120; 4. Nat Commun 2022. https://doi.org/10.1038/s41467- 022-32398-7; 5. Nat Commun 2022. https://doi.org/10.1038/s41467-022-31757-8
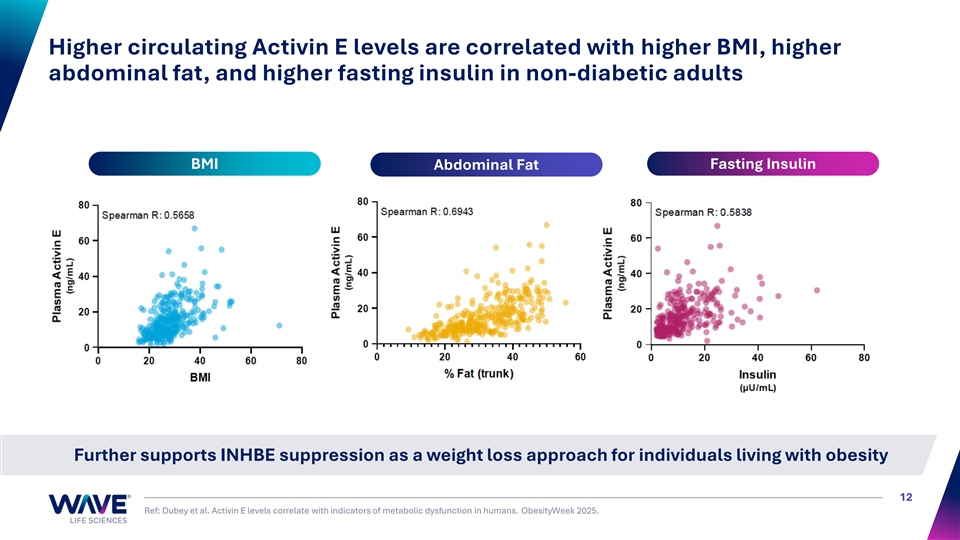
Higher circulating Activin E levels are correlated with higher BMI, higher abdominal fat, and higher fasting insulin in non-diabetic adults BMI Fasting Insulin Abdominal Fat Further supports INHBE suppression as a weight loss approach for individuals living with obesity 12 Ref: Dubey et al. Activin E levels correlate with indicators of metabolic dysfunction in humans. ObesityWeek 2025.
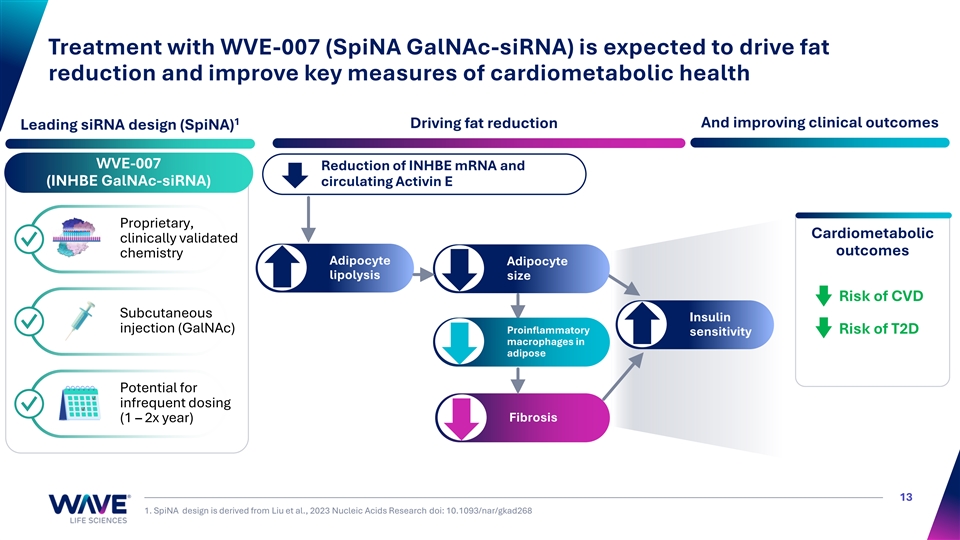
Treatment with WVE-007 (SpiNA GalNAc-siRNA) is expected to drive fat reduction and improve key measures of cardiometabolic health 1 Driving fat reduction And improving clinical outcomes Leading siRNA design (SpiNA) WVE-007 Reduction of INHBE mRNA and (INHBE GalNAc-siRNA) circulating Activin E Proprietary, Cardiometabolic clinically validated ✓ outcomes chemistry Adipocyte Adipocyte lipolysis size Risk of CVD Subcutaneous Insulin ✓ injection (GalNAc) Proinflammatory Risk of T2D sensitivity macrophages in adipose Potential for infrequent dosing ✓ Fibrosis (1 – 2x year) 13 1. SpiNA design is derived from Liu et al., 2023 Nucleic Acids Research doi: 10.1093/nar/gkad268
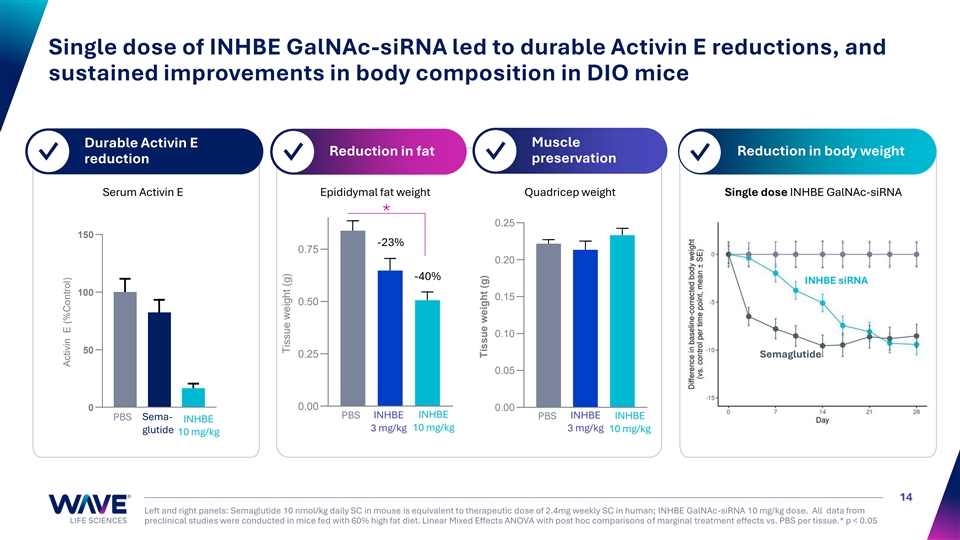
Single dose of INHBE GalNAc-siRNA led to durable Activin E reductions, and sustained improvements in body composition in DIO mice Muscle Durable Activin E Reduction in fat Reduction in body weight ✓ ✓ ✓ preservation ✓ reduction Serum Activin E Epididymal fat weight Quadricep weight Single dose INHBE GalNAc-siRNA * 0.25 150 -23% 0.75 0.20 -40% INHBE siRNA 100 0.15 0.50 0.10 50 0.25 Semaglutide 0.05 0 0.00 0.00 PBS INHBE INHBE INHBE PBS Sema- PBS INHBE INHBE 10 mg/kg 3 mg/kg 3 mg/kg 10 mg/kg glutide 10 mg/kg 14 Left and right panels: Semaglutide 10 nmol/kg daily SC in mouse is equivalent to therapeutic dose of 2.4mg weekly SC in human; INHBE GalNAc-siRNA 10 mg/kg dose. All data from preclinical studies were conducted in mice fed with 60% high fat diet. Linear Mixed Effects ANOVA with post hoc comparisons of marginal treatment effects vs. PBS per tissue.* p < 0.05 Activin E (%Control) Tissue weight (g) Tissue weight (g)
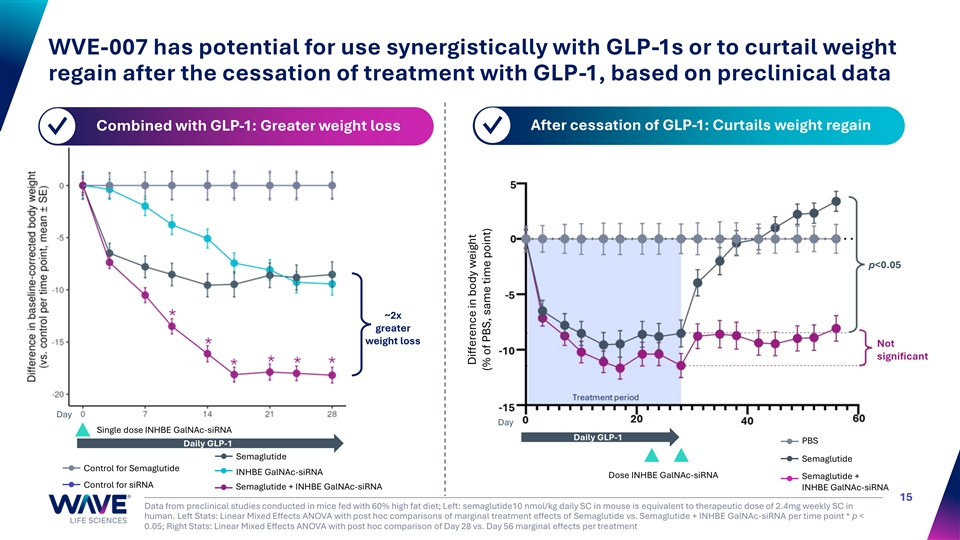
WVE-007 has potential for use synergistically with GLP-1s or to curtail weight regain after the cessation of treatment with GLP-1, based on preclinical data Combined with GLP-1: Greater weight loss After cessation of GLP-1: Curtails weight regain ✓ ✓ p<0.05 ~2x greater weight loss Not significant Day Day Single dose INHBE GalNAc-siRNA Daily GLP-1 PBS Daily GLP-1 Semaglutide Semaglutide Control for Semaglutide INHBE GalNAc-siRNA Dose INHBE GalNAc-siRNA Semaglutide + Control for siRNA Semaglutide + INHBE GalNAc-siRNA INHBE GalNAc-siRNA 15 Data from preclinical studies conducted in mice fed with 60% high fat diet; Left: semaglutide10 nmol/kg daily SC in mouse is equivalent to therapeutic dose of 2.4mg weekly SC in human. Left Stats: Linear Mixed Effects ANOVA with post hoc comparisons of marginal treatment effects of Semaglutide vs. Semaglutide + INHBE GalNAc-siRNA per time point * p < 0.05; Right Stats: Linear Mixed Effects ANOVA with post hoc comparison of Day 28 vs. Day 56 marginal effects per treatment Difference in body weight (% of PBS, same time point)
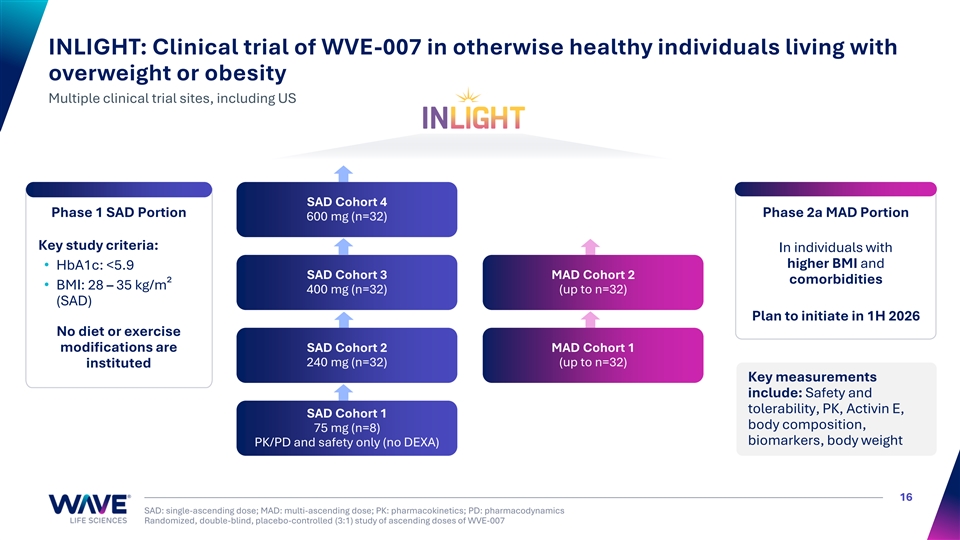
INLIGHT: Clinical trial of WVE-007 in otherwise healthy individuals living with overweight or obesity Multiple clinical trial sites, including US SAD Cohort 4 Phase 1 SAD Portion Phase 2a MAD Portion 600 mg (n=32) Key study criteria: In individuals with higher BMI and • HbA1c: <5.9 SAD Cohort 3 MAD Cohort 2 comorbidities • BMI: 28 – 35 kg/m² 400 mg (n=32) (up to n=32) (SAD) Plan to initiate in 1H 2026 No diet or exercise modifications are SAD Cohort 2 MAD Cohort 1 240 mg (n=32) (up to n=32) instituted Key measurements include: Safety and tolerability, PK, Activin E, SAD Cohort 1 body composition, 75 mg (n=8) biomarkers, body weight PK/PD and safety only (no DEXA) 16 SAD: single-ascending dose; MAD: multi-ascending dose; PK: pharmacokinetics; PD: pharmacodynamics Randomized, double-blind, placebo-controlled (3:1) study of ascending doses of WVE-007
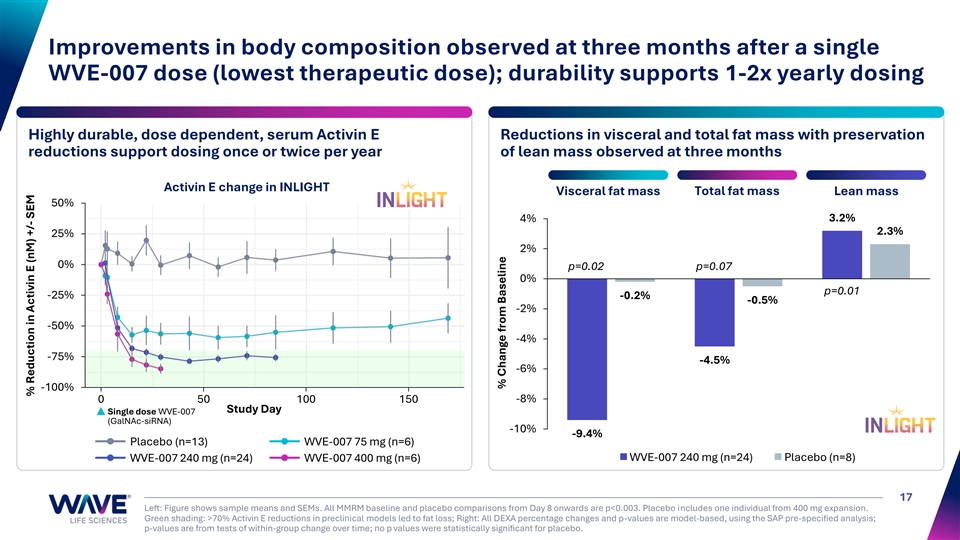
Improvements in body composition observed at three months after a single WVE-007 dose (lowest therapeutic dose); durability supports 1-2x yearly dosing Highly durable, dose dependent, serum Activin E Reductions in visceral and total fat mass with preservation reductions support dosing once or twice per year of lean mass observed at three months Activin E change in INLIGHT Total fat mass Visceral fat mass Lean mass 50% 3.2% 4% 2.3% 25% 2% 0% p=0.02 p=0.07 0% p=0.01 -25% -0.2% -0.5% -2% -50% -4% -75% -4.5% -6% -100% 0 50 100 150 -8% Study Day Single dose WVE-007 (GalNAc-siRNA) -10% -9.4% Placebo (n=13) WVE-007 75 mg (n=6) WVE-007 240 mg (n=24) Placebo (n=8) WVE-007 240 mg (n=24) WVE-007 400 mg (n=6) 17 Left: Figure shows sample means and SEMs. All MMRM baseline and placebo comparisons from Day 8 onwards are p<0.003. Placebo includes one individual from 400 mg expansion. Green shading: >70% Activin E reductions in preclinical models led to fat loss; Right: All DEXA percentage changes and p-values are model-based, using the SAP pre-specified analysis; p-values are from tests of within-group change over time; no p values were statistically significant for placebo. % Reduction in Activin E (nM) +/- SEM % Change from Baseline
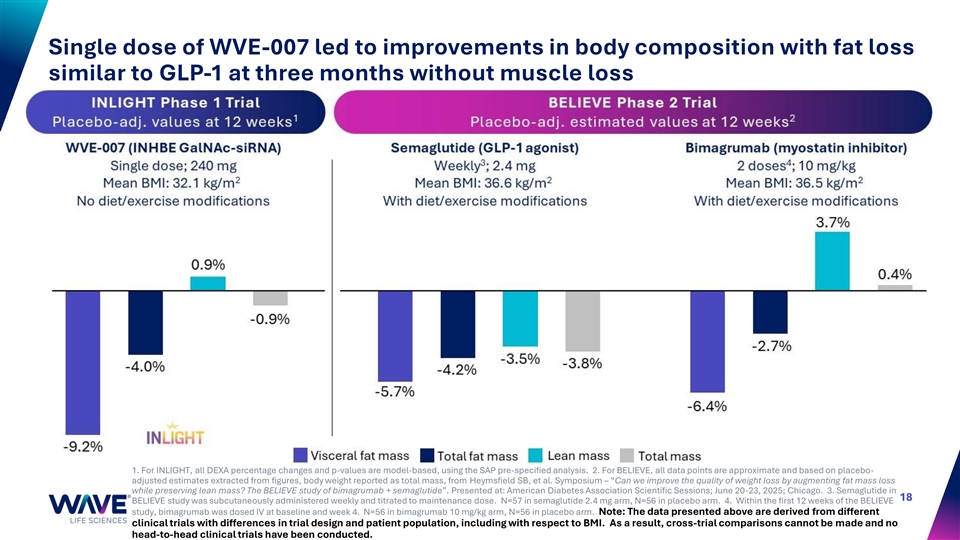
Single dose of WVE-007 led to improvements in body composition with fat loss similar to GLP-1 at three months without muscle loss 1. For INLIGHT, all DEXA percentage changes and p-values are model-based, using the SAP pre-specified analysis. 2. For BELIEVE, all data points are approximate and based on placebo- adjusted estimates extracted from figures, body weight reported as total mass, from Heymsfield SB, et al. Symposium – “Can we improve the quality of weight loss by augmenting fat mass loss while preserving lean mass? The BELIEVE study of bimagrumab + semaglutide”. Presented at: American Diabetes Association Scientific Sessions; June 20-23, 2025; Chicago. 3. Semaglutide in 18 BELIEVE study was subcutaneously administered weekly and titrated to maintenance dose. N=57 in semaglutide 2.4 mg arm, N=56 in placebo arm. 4. Within the first 12 weeks of the BELIEVE study, bimagrumab was dosed IV at baseline and week 4. N=56 in bimagrumab 10 mg/kg arm, N=56 in placebo arm. Note: The data presented above are derived from different clinical trials with differences in trial design and patient population, including with respect to BMI. As a result, cross-trial comparisons cannot be made and no head-to-head clinical trials have been conducted.
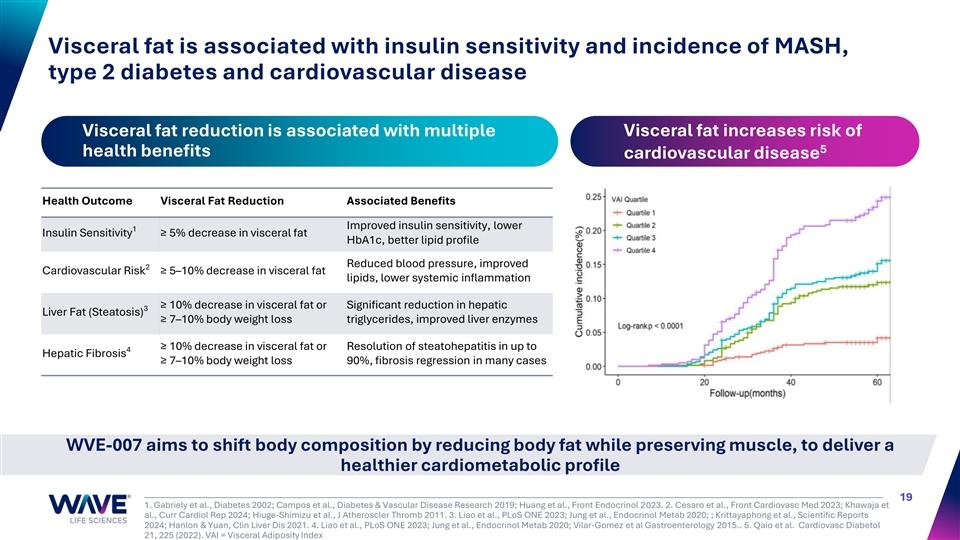
Visceral fat is associated with insulin sensitivity and incidence of MASH, type 2 diabetes and cardiovascular disease Visceral fat reduction is associated with multiple Visceral fat increases risk of 5 health benefits cardiovascular disease Health Outcome Visceral Fat Reduction Associated Benefits Improved insulin sensitivity, lower 1 Insulin Sensitivity ≥ 5% decrease in visceral fat HbA1c, better lipid profile Reduced blood pressure, improved 2 Cardiovascular Risk ≥ 5–10% decrease in visceral fat lipids, lower systemic inflammation ≥ 10% decrease in visceral fat or Significant reduction in hepatic 3 Liver Fat (Steatosis) ≥ 7–10% body weight loss triglycerides, improved liver enzymes ≥ 10% decrease in visceral fat or Resolution of steatohepatitis in up to 4 Hepatic Fibrosis ≥ 7–10% body weight loss 90%, fibrosis regression in many cases WVE-007 aims to shift body composition by reducing body fat while preserving muscle, to deliver a healthier cardiometabolic profile 19 1. Gabriely et al., Diabetes 2002; Campos et al., Diabetes & Vascular Disease Research 2019; Huang et al., Front Endocrinol 2023. 2. Cesaro et al., Front Cardiovasc Med 2023; Khawaja et al., Curr Cardiol Rep 2024; Hiuge-Shimizu et al., J Atheroscler Thromb 2011. 3. Liao et al., PLoS ONE 2023; Jung et al., Endocrinol Metab 2020; ; Krittayaphong et al., Scientific Reports 2024; Hanlon & Yuan, Clin Liver Dis 2021. 4. Liao et al., PLoS ONE 2023; Jung et al., Endocrinol Metab 2020; Vilar-Gomez et al Gastroenterology 2015.. 5. Qaio et al. Cardiovasc Diabetol 21, 225 (2022). VAI = Visceral Adiposity Index
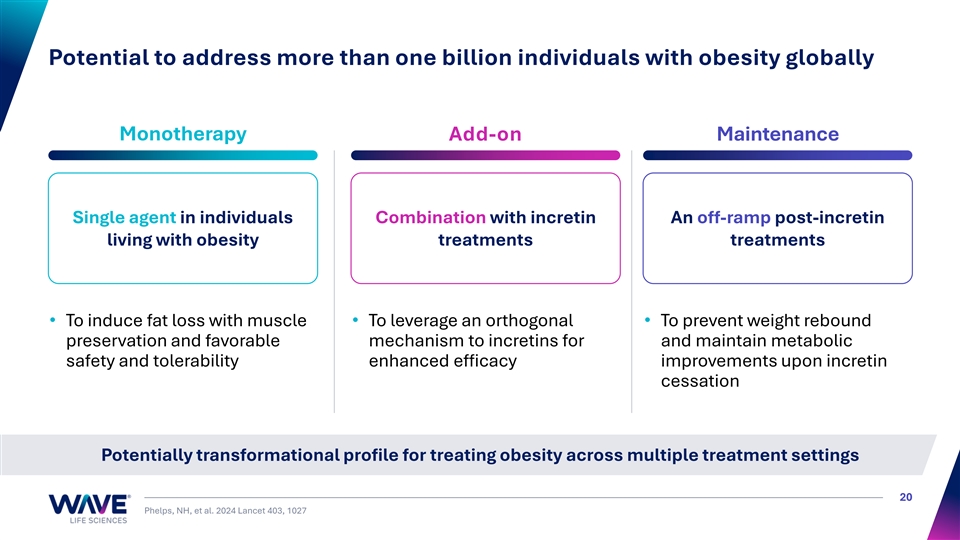
Potential to address more than one billion individuals with obesity globally Monotherapy Add-on Maintenance Single agent in individuals Combination with incretin An off-ramp post-incretin living with obesity treatments treatments • To induce fat loss with muscle • To leverage an orthogonal • To prevent weight rebound preservation and favorable mechanism to incretins for and maintain metabolic safety and tolerability enhanced efficacy improvements upon incretin cessation Potentially transformational profile for treating obesity across multiple treatment settings 20 Phelps, NH, et al. 2024 Lancet 403, 1027
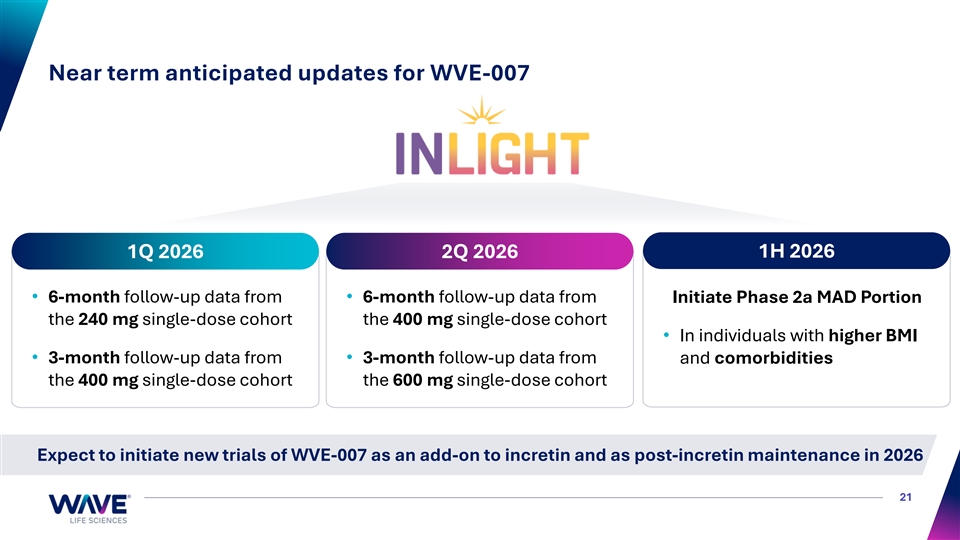
Near term anticipated updates for WVE-007 1H 2026 1Q 2026 2Q 2026 • 6-month follow-up data from • 6-month follow-up data from Initiate Phase 2a MAD Portion the 240 mg single-dose cohort the 400 mg single-dose cohort • In individuals with higher BMI • 3-month follow-up data from • 3-month follow-up data from and comorbidities the 400 mg single-dose cohort the 600 mg single-dose cohort Expect to initiate new trials of WVE-007 as an add-on to incretin and as post-incretin maintenance in 2026 21

WVE-006 RNA editing (AIMer) Alpha-1 antitrypsin deficiency (AATD) 22
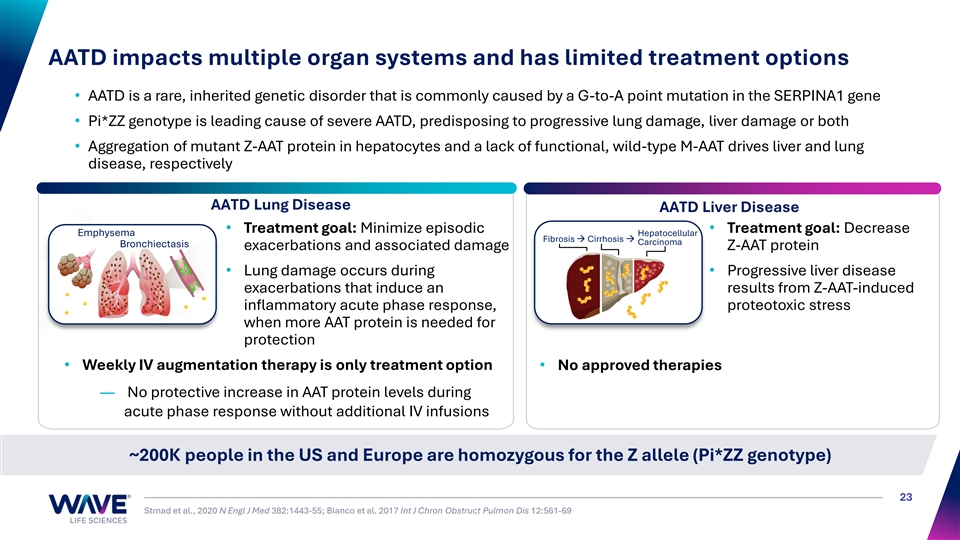
AATD impacts multiple organ systems and has limited treatment options • AATD is a rare, inherited genetic disorder that is commonly caused by a G-to-A point mutation in the SERPINA1 gene • Pi*ZZ genotype is leading cause of severe AATD, predisposing to progressive lung damage, liver damage or both • Aggregation of mutant Z-AAT protein in hepatocytes and a lack of functional, wild-type M-AAT drives liver and lung disease, respectively AATD Lung Disease AATD Liver Disease • Treatment goal: Minimize episodic • Treatment goal: Decrease Emphysema Hepatocellular Fibrosis → Cirrhosis → Carcinoma Bronchiectasis exacerbations and associated damage Z-AAT protein • Lung damage occurs during • Progressive liver disease exacerbations that induce an results from Z-AAT-induced inflammatory acute phase response, proteotoxic stress when more AAT protein is needed for protection • Weekly IV augmentation therapy is only treatment option• No approved therapies — No protective increase in AAT protein levels during acute phase response without additional IV infusions ~200K people in the US and Europe are homozygous for the Z allele (Pi*ZZ genotype) 23 Strnad et al., 2020 N Engl J Med 382:1443-55; Blanco et al. 2017 Int J Chron Obstruct Pulmon Dis 12:561-69
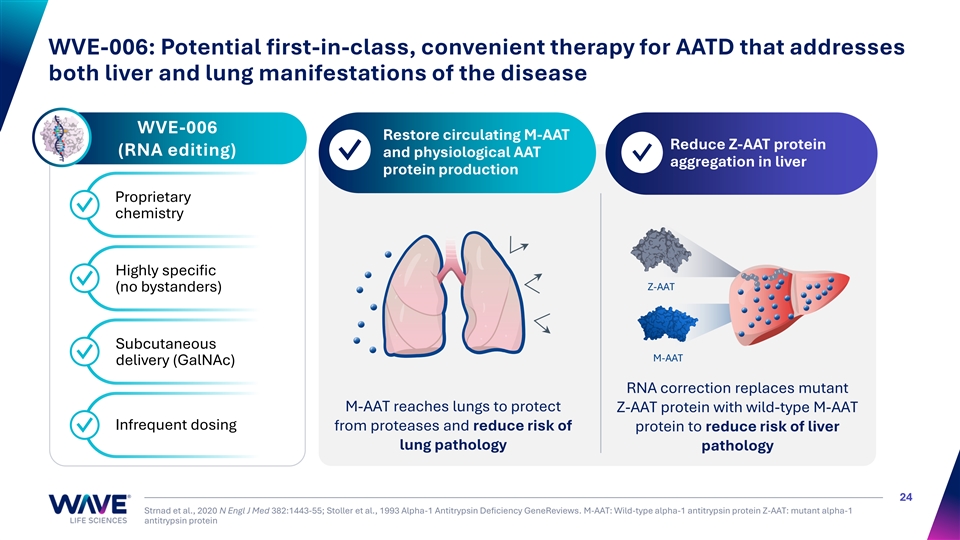
WVE-006: Potential first-in-class, convenient therapy for AATD that addresses both liver and lung manifestations of the disease WVE-006 Restore circulating M-AAT 1 2 Reduce Z-AAT protein (RNA editing) and physiological AAT ✓ ✓ aggregation in liver protein production Proprietary ✓ chemistry Highly specific ✓ Z-AAT (no bystanders) Subcutaneous ✓ M-AAT delivery (GalNAc) RNA correction replaces mutant M-AAT reaches lungs to protect Z-AAT protein with wild-type M-AAT Infrequent dosing from proteases and reduce risk of protein to reduce risk of liver ✓ lung pathology pathology 24 Strnad et al., 2020 N Engl J Med 382:1443-55; Stoller et al., 1993 Alpha-1 Antitrypsin Deficiency GeneReviews. M-AAT: Wild-type alpha-1 antitrypsin protein Z-AAT: mutant alpha-1 antitrypsin protein
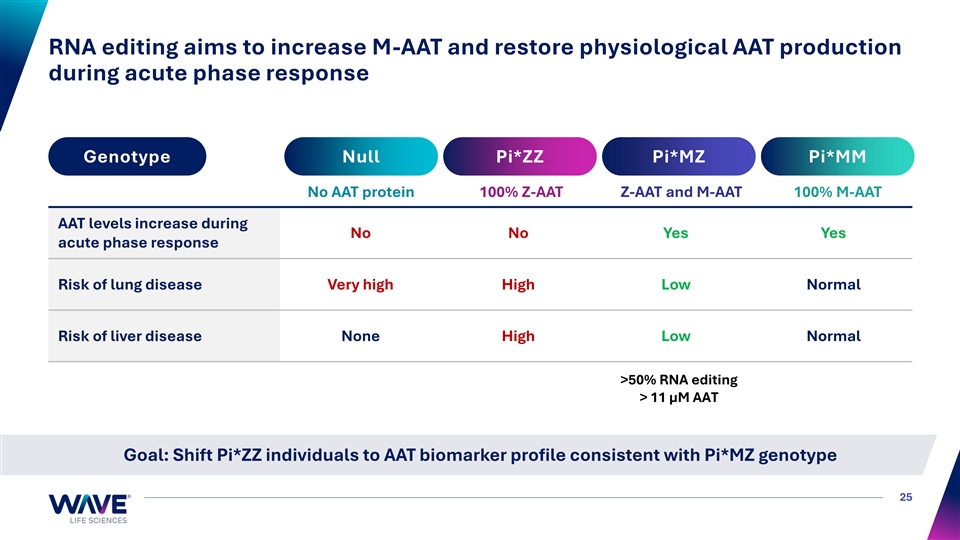
RNA editing aims to increase M-AAT and restore physiological AAT production during acute phase response Genotype Null Pi*ZZ Pi*MZ Pi*MM No AAT protein 100% Z-AAT Z-AAT and M-AAT 100% M-AAT AAT levels increase during No No Yes Yes acute phase response Risk of lung disease Very high High Low Normal Risk of liver disease None High Low Normal >50% RNA editing > 11 µM AAT Goal: Shift Pi*ZZ individuals to AAT biomarker profile consistent with Pi*MZ genotype 25
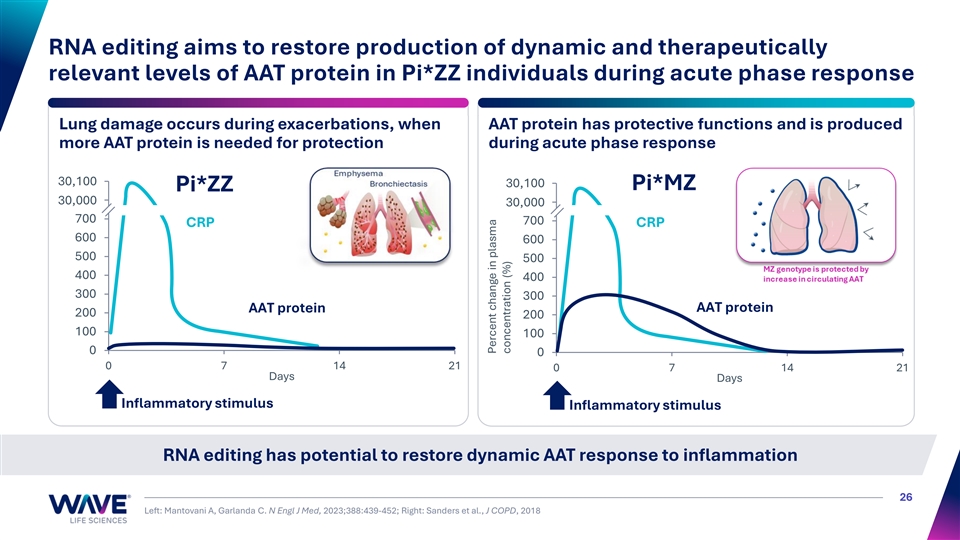
RNA editing aims to restore production of dynamic and therapeutically relevant levels of AAT protein in Pi*ZZ individuals during acute phase response Lung damage occurs during exacerbations, when AAT protein has protective functions and is produced more AAT protein is needed for protection during acute phase response 30,9 10 00 0 900 30,100 Pi*MZ Pi*ZZ 30,8 00 00 0 30,8 00 00 0 700 700 CRP CRP 600 600 500 500 400 400 300 300 AAT protein AAT protein 200 200 100 100 0 0 0 7 14 21 0 7 14 21 Days Days Inflammatory stimulus Inflammatory stimulus RNA editing has potential to restore dynamic AAT response to inflammation 26 Left: Mantovani A, Garlanda C. N Engl J Med, 2023;388:439-452; Right: Sanders et al., J COPD, 2018 Percent change in plasma concentration (%)
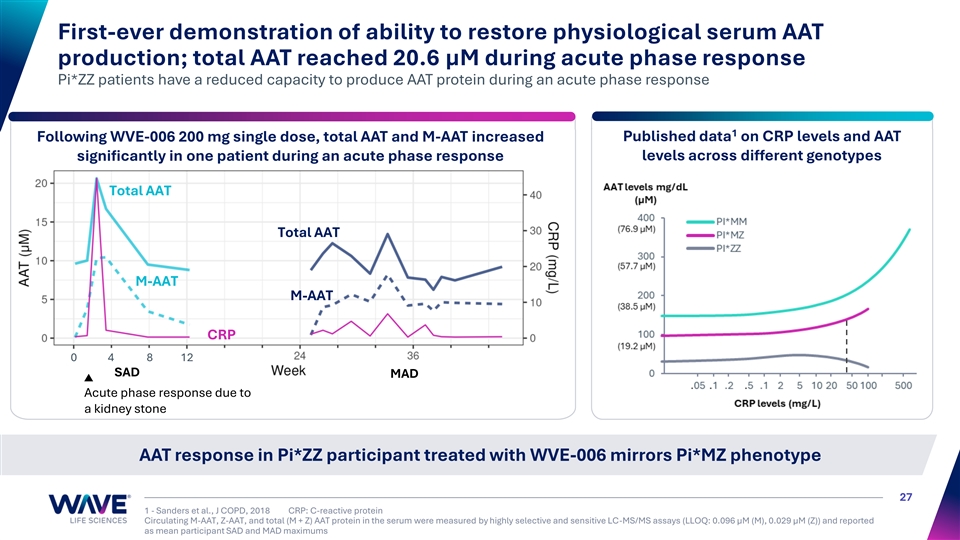
First-ever demonstration of ability to restore physiological serum AAT production; total AAT reached 20.6 µM during acute phase response Pi*ZZ patients have a reduced capacity to produce AAT protein during an acute phase response 1 Following WVE-006 200 mg single dose, total AAT and M-AAT increased Published data on CRP levels and AAT levels across different genotypes significantly in one patient during an acute phase response Total AAT Total AAT M-AAT M-AAT CRP 0 4 8 12 SAD MAD Acute phase response due to a kidney stone AAT response in Pi*ZZ participant treated with WVE-006 mirrors Pi*MZ phenotype 27 1 - Sanders et al., J COPD, 2018 CRP: C-reactive protein Circulating M-AAT, Z-AAT, and total (M + Z) AAT protein in the serum were measured by highly selective and sensitive LC-MS/MS assays (LLOQ: 0.096 µM (M), 0.029 µM (Z)) and reported as mean participant SAD and MAD maximums M-AAT + Z-AAT (µM) CRP levels (mg/L)
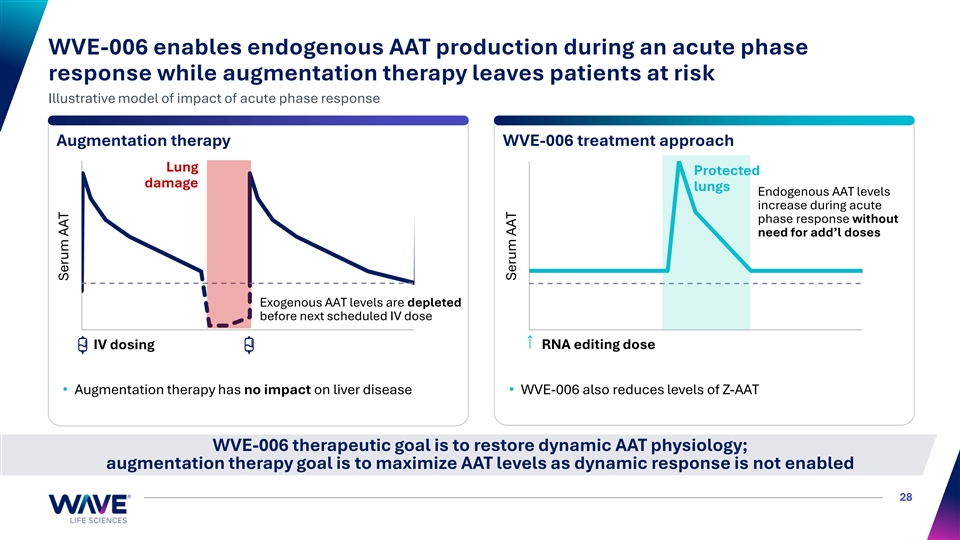
WVE-006 enables endogenous AAT production during an acute phase response while augmentation therapy leaves patients at risk Illustrative model of impact of acute phase response Augmentation therapy WVE-006 treatment approach Lung Protected damage lungs Endogenous AAT levels increase during acute phase response without need for add’l doses Exogenous AAT levels are depleted before next scheduled IV dose IV dosing RNA editing dose • Augmentation therapy has no impact on liver disease• WVE-006 also reduces levels of Z-AAT WVE-006 therapeutic goal is to restore dynamic AAT physiology; augmentation therapy goal is to maximize AAT levels as dynamic response is not enabled 28 Serum AAT Serum AAT
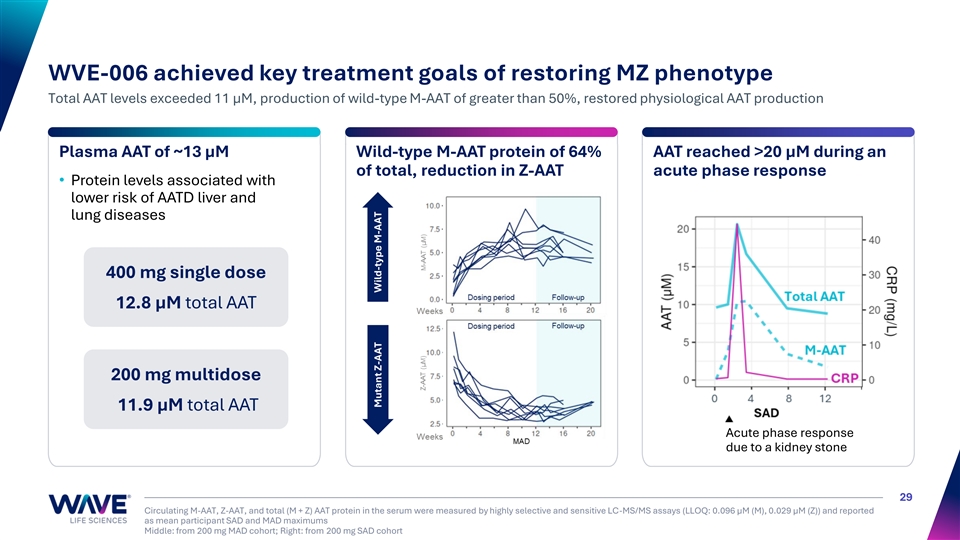
CRP (mg/L) WVE-006 achieved key treatment goals of restoring MZ phenotype Total AAT levels exceeded 11 µM, production of wild-type M-AAT of greater than 50%, restored physiological AAT production Plasma AAT of ~13 µM Wild-type M-AAT protein of 64% AAT reached >20 μM during an of total, reduction in Z-AAT acute phase response • Protein levels associated with lower risk of AATD liver and lung diseases 400 mg single dose 12.8 µM total AAT 200 mg multidose 11.9 µM total AAT Acute phase response due to a kidney stone 29 Circulating M-AAT, Z-AAT, and total (M + Z) AAT protein in the serum were measured by highly selective and sensitive LC-MS/MS assays (LLOQ: 0.096 µM (M), 0.029 µM (Z)) and reported as mean participant SAD and MAD maximums Middle: from 200 mg MAD cohort; Right: from 200 mg SAD cohort Mutant Z-AAT Wild-type M-AAT
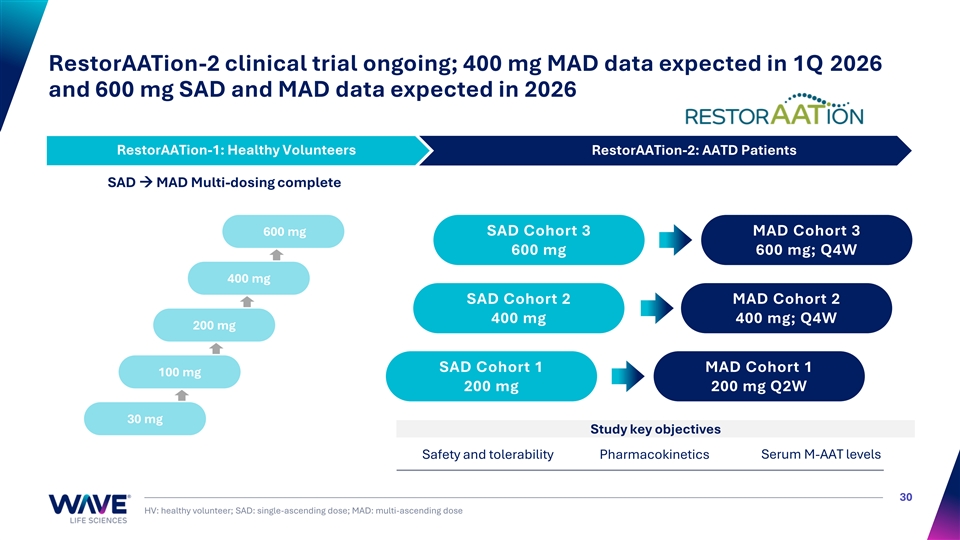
RestorAATion-2 clinical trial ongoing; 400 mg MAD data expected in 1Q 2026 and 600 mg SAD and MAD data expected in 2026 RestorAATion-1: Healthy Volunteers RestorAATion-2: AATD Patients RestorAATion-1: Healthy Volunteers SAD → MAD Multi-dosing complete 600 mg SAD Cohort 3 MAD Cohort 3 600 mg 600 mg; Q4W 400 mg SAD Cohort 2 MAD Cohort 2 400 mg 400 mg; Q4W 200 mg SAD Cohort 1 MAD Cohort 1 100 mg 200 mg 200 mg Q2W 30 mg Study key objectives Safety and tolerability Pharmacokinetics Serum M-AAT levels 30 HV: healthy volunteer; SAD: single-ascending dose; MAD: multi-ascending dose

WVE-008 RNA editing (AIMer) PNPLA3 I148M liver disease 31
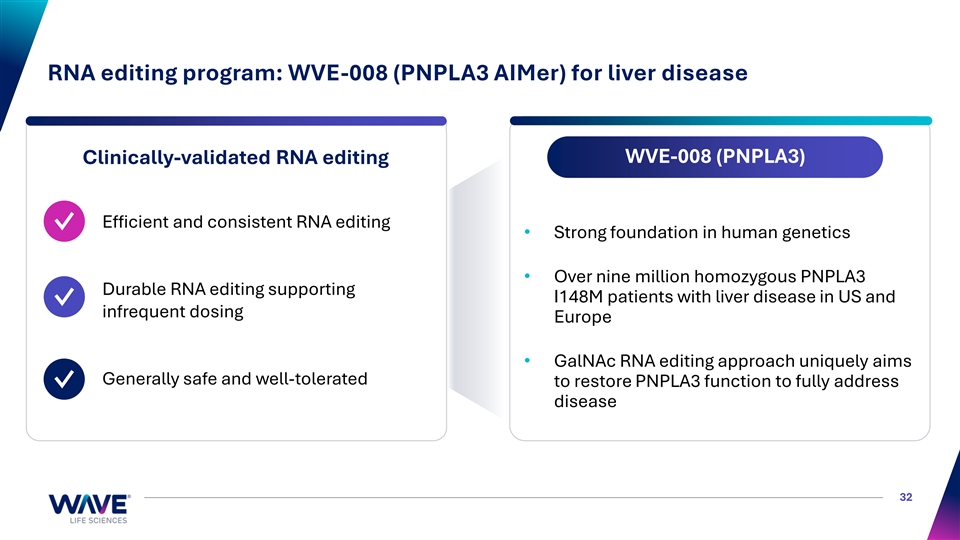
RNA editing program: WVE-008 (PNPLA3 AIMer) for liver disease WVE-008 (PNPLA3) Clinically-validated RNA editing Efficient and consistent RNA editing ✓ • Strong foundation in human genetics • Over nine million homozygous PNPLA3 Durable RNA editing supporting I148M patients with liver disease in US and ✓ infrequent dosing Europe • GalNAc RNA editing approach uniquely aims Generally safe and well-tolerated to restore PNPLA3 function to fully address ✓ disease 32
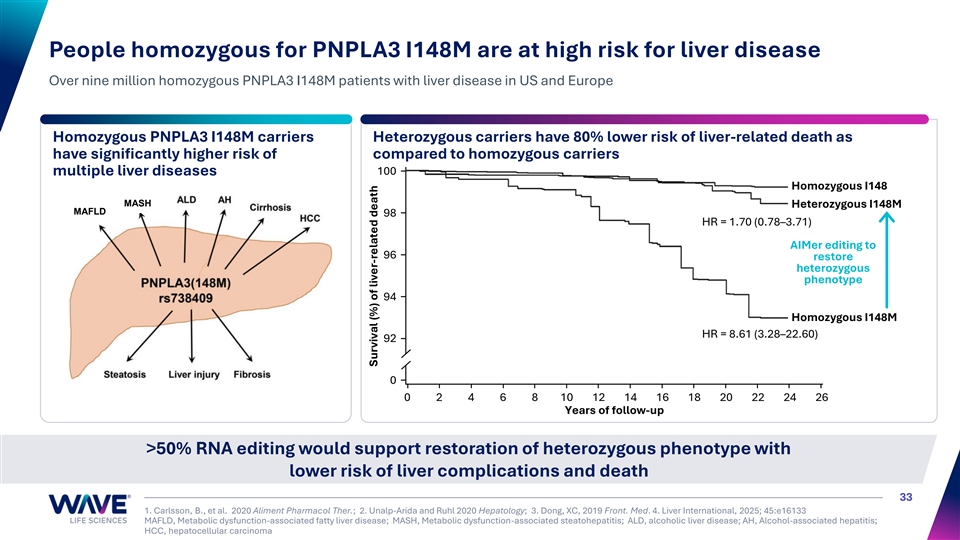
People homozygous for PNPLA3 I148M are at high risk for liver disease Over nine million homozygous PNPLA3 I148M patients with liver disease in US and Europe Homozygous PNPLA3 I148M carriers Heterozygous carriers have 80% lower risk of liver-related death as have significantly higher risk of compared to homozygous carriers 100 multiple liver diseases Homozygous I148 MASH Heterozygous I148M MAFLD 98 HR = 1.70 (0.78–3.71) AIMer editing to 96 restore heterozygous phenotype 94 Homozygous I148M HR = 8.61 (3.28–22.60) 92 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Years of follow-up >50% RNA editing would support restoration of heterozygous phenotype with lower risk of liver complications and death 33 1. Carlsson, B., et al. 2020 Aliment Pharmacol Ther.; 2. Unalp-Arida and Ruhl 2020 Hepatology; 3. Dong, XC, 2019 Front. Med. 4. Liver International, 2025; 45:e16133 MAFLD, Metabolic dysfunction-associated fatty liver disease; MASH, Metabolic dysfunction-associated steatohepatitis; ALD, alcoholic liver disease; AH, Alcohol-associated hepatitis; HCC, hepatocellular carcinoma Survival (%) of liver-related death
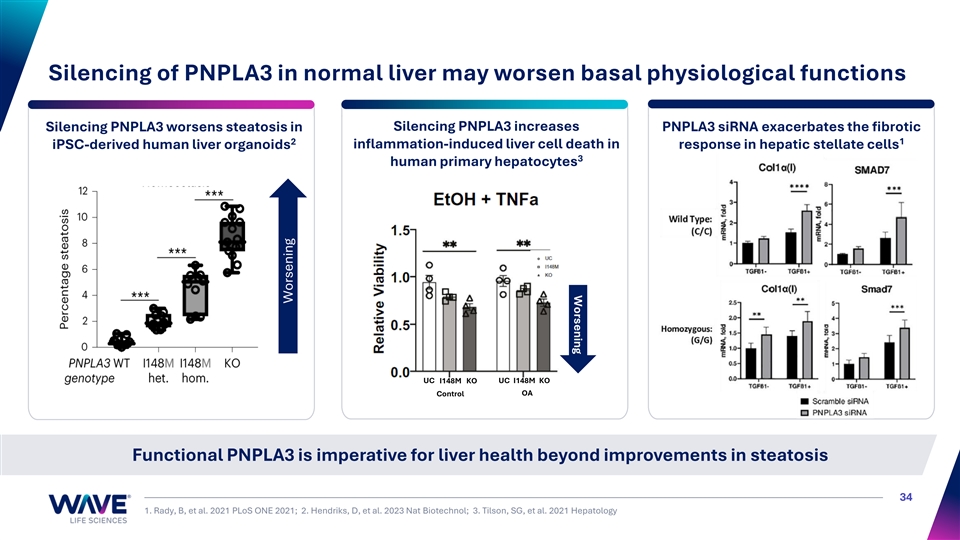
Worsening Silencing of PNPLA3 in normal liver may worsen basal physiological functions Silencing PNPLA3 increases Silencing PNPLA3 worsens steatosis in PNPLA3 siRNA exacerbates the fibrotic 1 2 iPSC-derived human liver organoids inflammation-induced liver cell death in response in hepatic stellate cells 3 human primary hepatocytes UC I148M KO UC I148M KO Control OA Functional PNPLA3 is imperative for liver health beyond improvements in steatosis 34 1. Rady, B, et al. 2021 PLoS ONE 2021; 2. Hendriks, D, et al. 2023 Nat Biotechnol; 3. Tilson, SG, et al. 2021 Hepatology Worsening
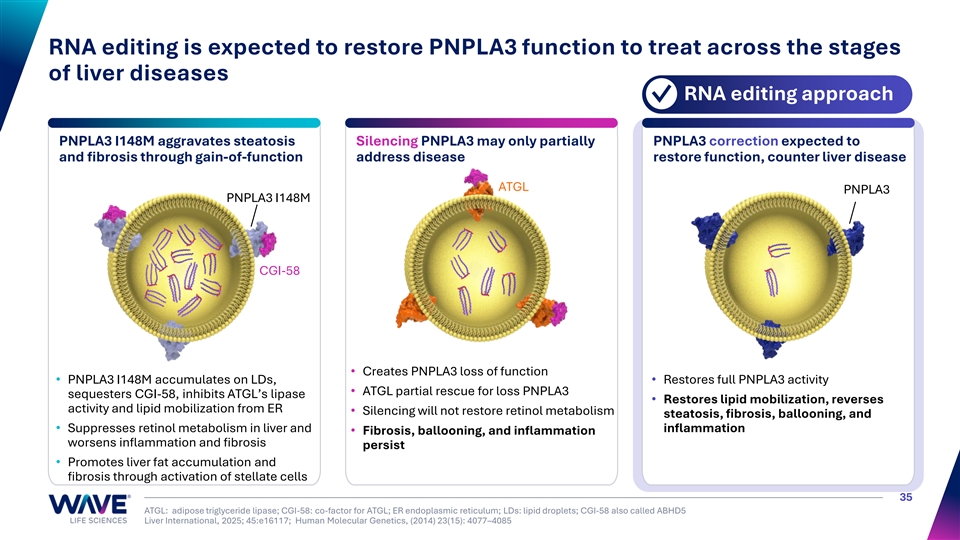
RNA editing is expected to restore PNPLA3 function to treat across the stages of liver diseases RNA editing approach ✓ PNPLA3 I148M aggravates steatosis Silencing PNPLA3 may only partially PNPLA3 correction expected to and fibrosis through gain-of-function address disease restore function, counter liver disease ATGL PNPLA3 PNPLA3 I148M CGI-58 • Creates PNPLA3 loss of function • PNPLA3 I148M accumulates on LDs, • Restores full PNPLA3 activity • ATGL partial rescue for loss PNPLA3 sequesters CGI-58, inhibits ATGL’s lipase • Restores lipid mobilization, reverses activity and lipid mobilization from ER • Silencing will not restore retinol metabolism steatosis, fibrosis, ballooning, and • Suppresses retinol metabolism in liver and inflammation • Fibrosis, ballooning, and inflammation worsens inflammation and fibrosis persist • Promotes liver fat accumulation and fibrosis through activation of stellate cells 35 ATGL: adipose triglyceride lipase; CGI-58: co-factor for ATGL; ER endoplasmic reticulum; LDs: lipid droplets; CGI-58 also called ABHD5 Liver International, 2025; 45:e16117; Human Molecular Genetics, (2014) 23(15): 4077–4085
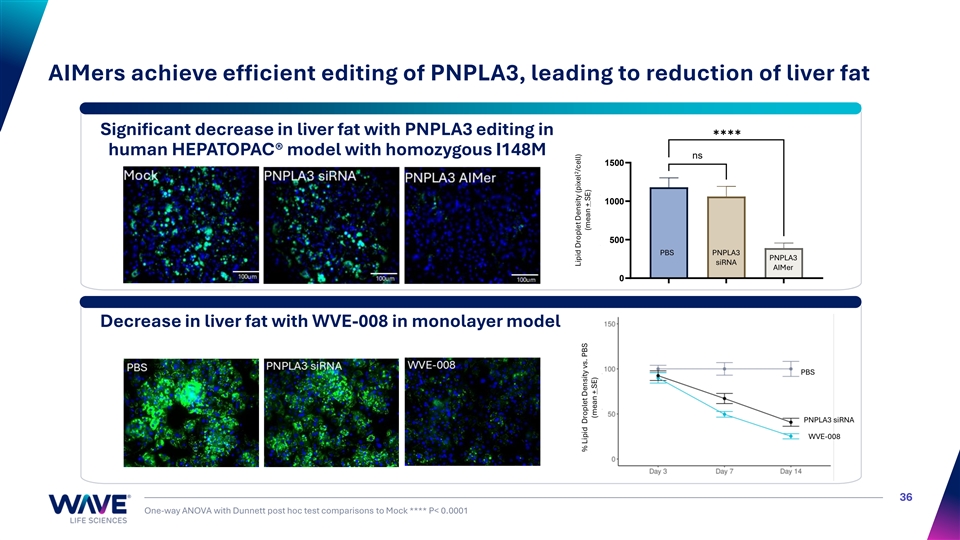
AIMers achieve efficient editing of PNPLA3, leading to reduction of liver fat ✱✱✱✱ Significant decrease in liver fat with PNPLA3 editing in human HEPATOPAC® model with homozygous I148M ns 1500 1000 500 PBS PNPLA3 PNPLA3 siRNA AIMer 0 Decrease in liver fat with WVE-008 in monolayer model PNPLA3 siRNA PBS PNPLA3 siRNA WVE-008 36 One-way ANOVA with Dunnett post hoc test comparisons to Mock **** P< 0.0001 Mock PNPLA3 siRNA PNPLA3 AIMer 2 Lipid Droplet Density (pixel /cell) 2 Lipid Droplet Density (pixel /cell) % Lipid Droplet Density vs. PBS (mean + SE) (mean + SE) (mean ± SE)
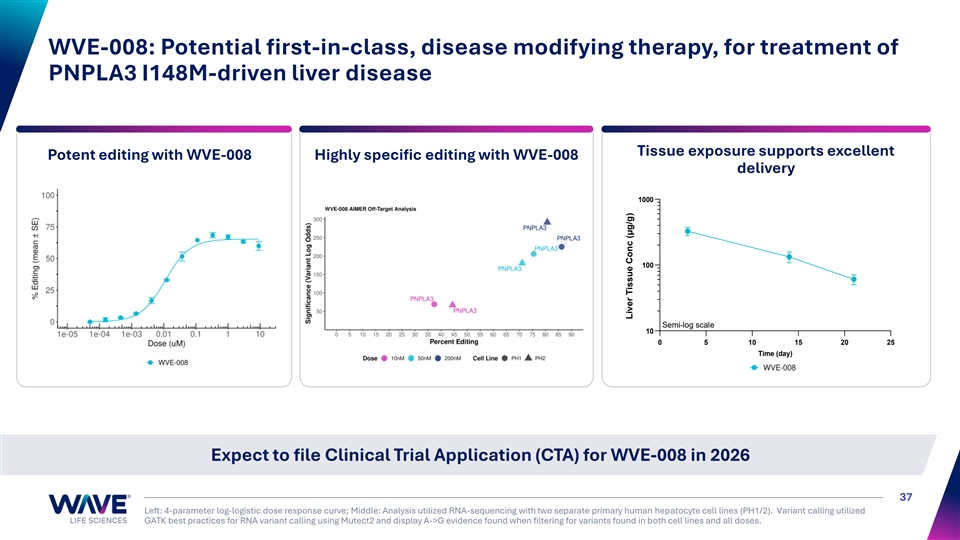
WVE-008: Potential first-in-class, disease modifying therapy, for treatment of PNPLA3 I148M-driven liver disease Tissue exposure supports excellent Potent editing with WVE-008 Highly specific editing with WVE-008 delivery 1000 100 Semi-log scale 10 0 5 10 15 20 25 Time (day) Expect to file Clinical Trial Application (CTA) for WVE-008 in 2026 37 Left: 4-parameter log-logistic dose response curve; Middle: Analysis utilized RNA-sequencing with two separate primary human hepatocyte cell lines (PH1/2). Variant calling utilized GATK best practices for RNA variant calling using Mutect2 and display A->G evidence found when filtering for variants found in both cell lines and all doses. Liver Tissue Conc (μg/g)

Single oligonucleotide construct Bifunctional modality Simultaneously knockdown and edit RNA 38
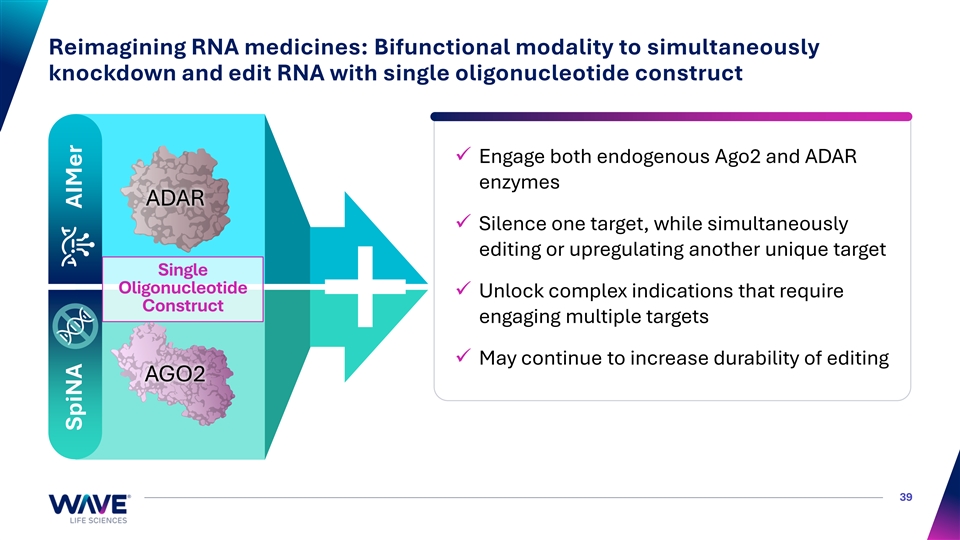
Reimagining RNA medicines: Bifunctional modality to simultaneously knockdown and edit RNA with single oligonucleotide construct ✓ Engage both endogenous Ago2 and ADAR enzymes ADAR ✓ Silence one target, while simultaneously editing or upregulating another unique target Single Oligonucleotide ✓ Unlock complex indications that require Construct engaging multiple targets ✓ May continue to increase durability of editing AGO2 39 SpiNA AIMer
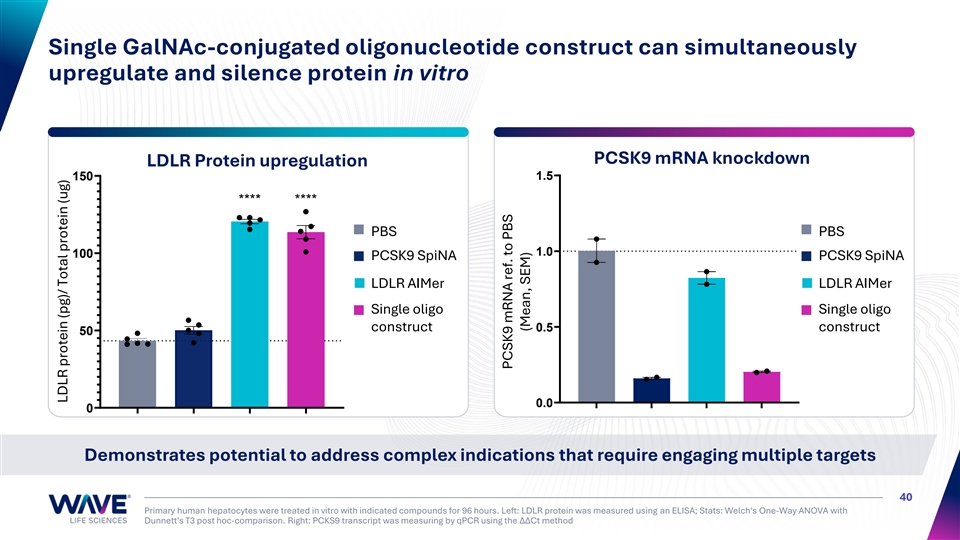
Single GalNAc-conjugated oligonucleotide construct can simultaneously upregulate and silence protein in vitro PCSK9 mRNA knockdown LDLR Protein upregulation 1.5 150 **** **** PBS PBS 1.0 100 PCSK9 SpiNA PCSK9 SpiNA LDLR AIMer LDLR AIMer Single oligo Single oligo construct 0.5 construct 50 0.0 0 Demonstrates potential to address complex indications that require engaging multiple targets 40 Primary human hepatocytes were treated in vitro with indicated compounds for 96 hours. Left: LDLR protein was measured using an ELISA; Stats: Welch's One-Way ANOVA with Dunnett's T3 post hoc-comparison. Right: PCKS9 transcript was measuring by qPCR using the ΔΔCt method LDLR protein (pg)/ Total protein (ug) PCSK9 mRNA ref. to PBS (Mean, SEM)

WVE-N531 Splicing Duchenne muscular dystrophy 41
Advancing WVE-N531 in exon 53 amenable DMD WVE-N531: exon skipping oligonucleotide designed to induce production of endogenous, functional dystrophin protein • High unmet need for therapies delivering more consistent dystrophin expression, as few patients today achieve dystrophin >5% of normal • Opportunity to extend dosing intervals beyond weekly standard of care to alleviate burden for patients and caregivers • Need to reach stem cells and distribute broadly to muscle tissues to potentially enable muscle regeneration and impact respiratory and cardiac function • WVE-N531 has Rare Pediatric Disease Designation and Orphan Drug Designation from FDA DMD impacts ~1 / 5,000 newborn boys annually; ~20,000 new cases annually worldwide 42 Duan, D. et al. 2021 Nat Rev Dis Primers 7, 13; Muscular Dystrophy Association; Aartsma-Rus, et al. 2009 Hum Mutat 30, 293.
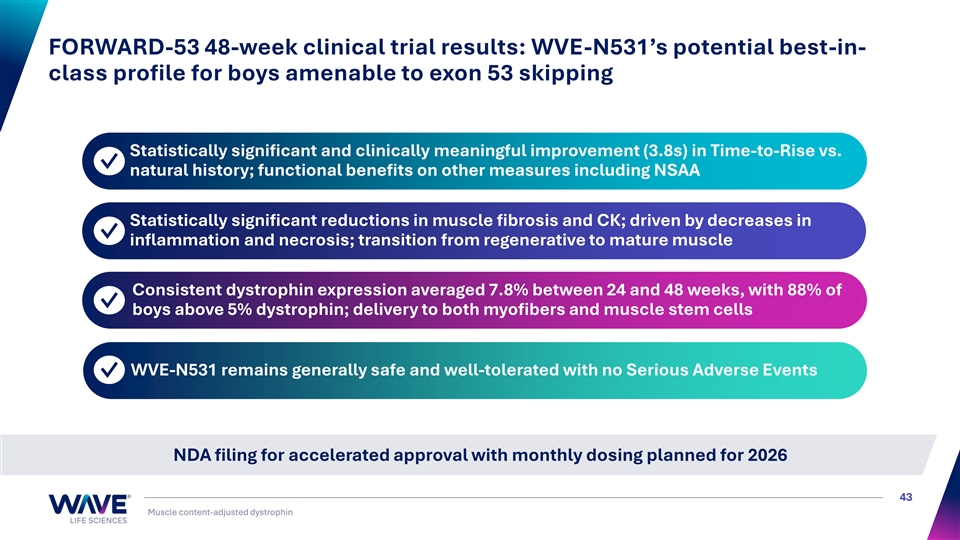
FORWARD-53 48-week clinical trial results: WVE-N531’s potential best-in- class profile for boys amenable to exon 53 skipping Statistically significant and clinically meaningful improvement (3.8s) in Time-to-Rise vs. ✓ natural history; functional benefits on other measures including NSAA Statistically significant reductions in muscle fibrosis and CK; driven by decreases in ✓ inflammation and necrosis; transition from regenerative to mature muscle Consistent dystrophin expression averaged 7.8% between 24 and 48 weeks, with 88% of ✓ boys above 5% dystrophin; delivery to both myofibers and muscle stem cells WVE-N531 remains generally safe and well-tolerated with no Serious Adverse Events ✓ NDA filing for accelerated approval with monthly dosing planned for 2026 43 Muscle content-adjusted dystrophin
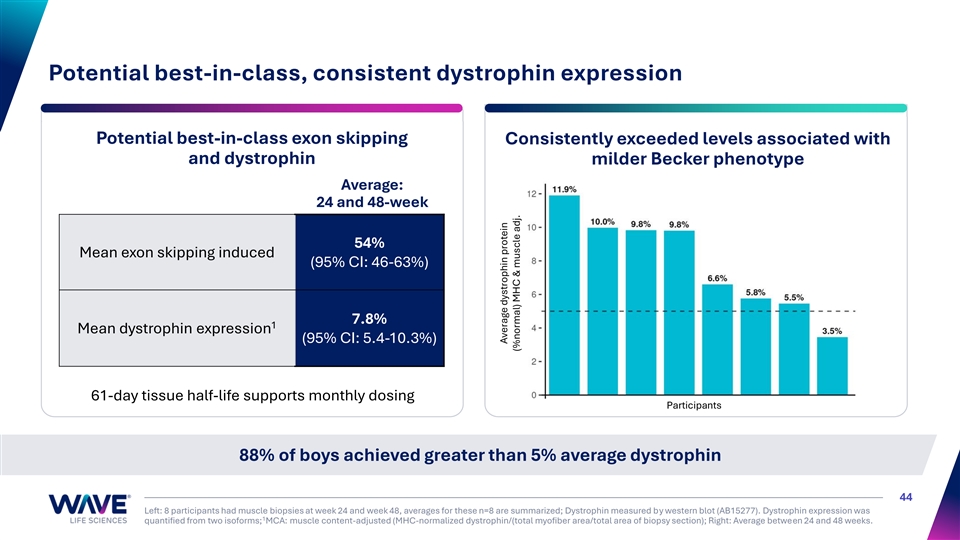
Potential best-in-class, consistent dystrophin expression Potential best-in-class exon skipping Consistently exceeded levels associated with and dystrophin milder Becker phenotype Average: 24 and 48-week 54% Mean exon skipping induced (95% CI: 46-63%) 7.8% 1 Mean dystrophin expression (95% CI: 5.4-10.3%) 61-day tissue half-life supports monthly dosing Participants 88% of boys achieved greater than 5% average dystrophin 44 Left: 8 participants had muscle biopsies at week 24 and week 48, averages for these n=8 are summarized; Dystrophin measured by western blot (AB15277). Dystrophin expression was 1 quantified from two isoforms; MCA: muscle content-adjusted (MHC-normalized dystrophin/(total myofiber area/total area of biopsy section); Right: Average between 24 and 48 weeks. Average dystrophin protein (%normal) MHC & muscle adj.
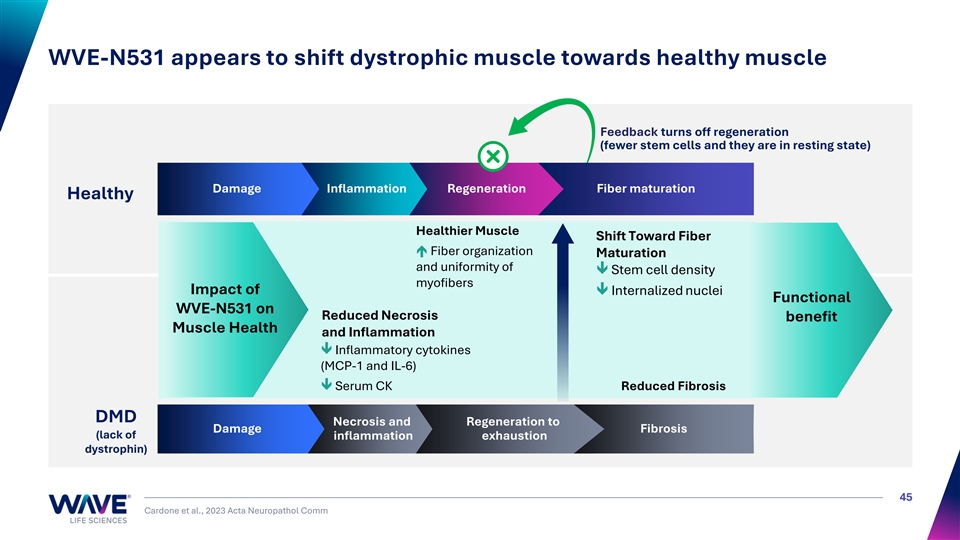
WVE-N531 appears to shift dystrophic muscle towards healthy muscle Feedback turns off regeneration (fewer stem cells and they are in resting state) Damage Inflammation Regeneration Fiber maturation Healthy Healthier Muscle Shift Toward Fiber é Fiber organization Maturation and uniformity of ê Stem cell density myofibers Impact of ê Internalized nuclei Functional WVE-N531 on Reduced Necrosis benefit Muscle Health and Inflammation ê Inflammatory cytokines (MCP-1 and IL-6) ê Serum CK Reduced Fibrosis DMD Necrosis and Regeneration to Damage Fibrosis (lack of inflammation exhaustion dystrophin) 45 Cardone et al., 2023 Acta Neuropathol Comm
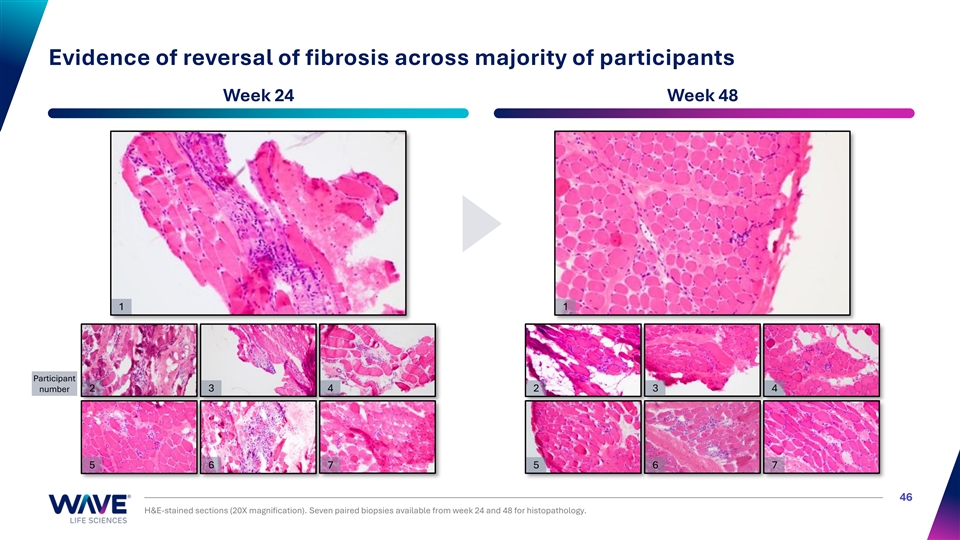
Evidence of reversal of fibrosis across majority of participants Week 24 Week 48 1 1 Participant number 2 3 4 2 3 4 5 6 7 5 6 7 46 H&E-stained sections (20X magnification). Seven paired biopsies available from week 24 and 48 for histopathology.
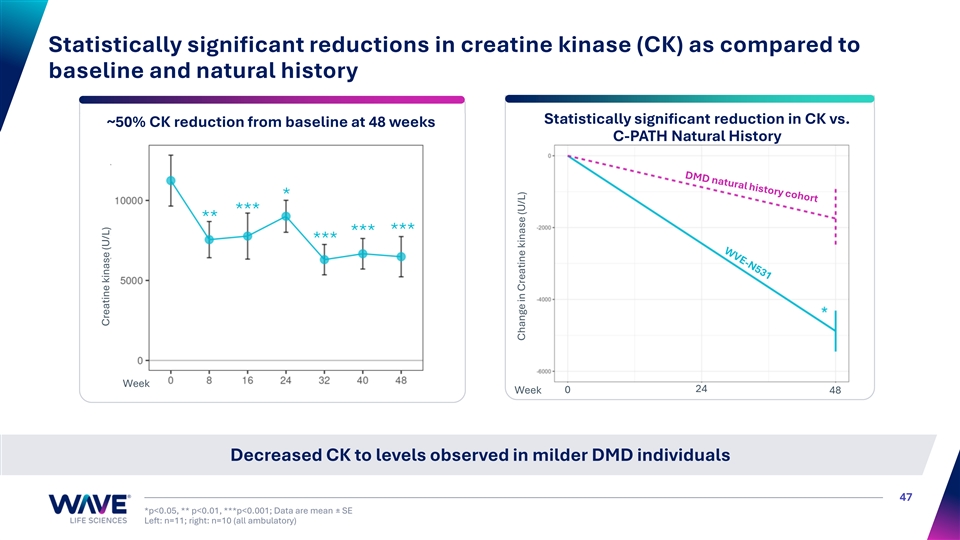
Statistically significant reductions in creatine kinase (CK) as compared to baseline and natural history Statistically significant reduction in CK vs. ~50% CK reduction from baseline at 48 weeks C-PATH Natural History * *** ** *** *** *** Week 24 Week 0 48 Decreased CK to levels observed in milder DMD individuals 47 *p<0.05, ** p<0.01, ***p<0.001; Data are mean ± SE Left: n=11; right: n=10 (all ambulatory) Creatine kinase (U/L) Change in Creatine kinase (U/L)
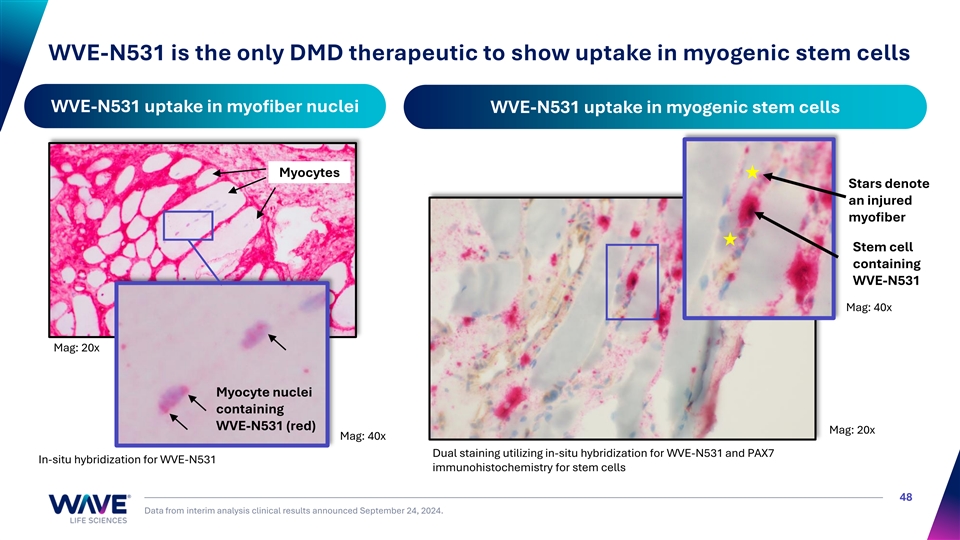
WVE-N531 is the only DMD therapeutic to show uptake in myogenic stem cells WVE-N531 uptake in myofiber nuclei WVE-N531 uptake in myogenic stem cells Myocytes Stars denote an injured myofiber Stem cell containing WVE-N531 Mag: 40x Mag: 20x Myocyte nuclei containing WVE-N531 (red) Mag: 20x Mag: 40x Dual staining utilizing in-situ hybridization for WVE-N531 and PAX7 In-situ hybridization for WVE-N531 immunohistochemistry for stem cells 48 Data from interim analysis clinical results announced September 24, 2024.
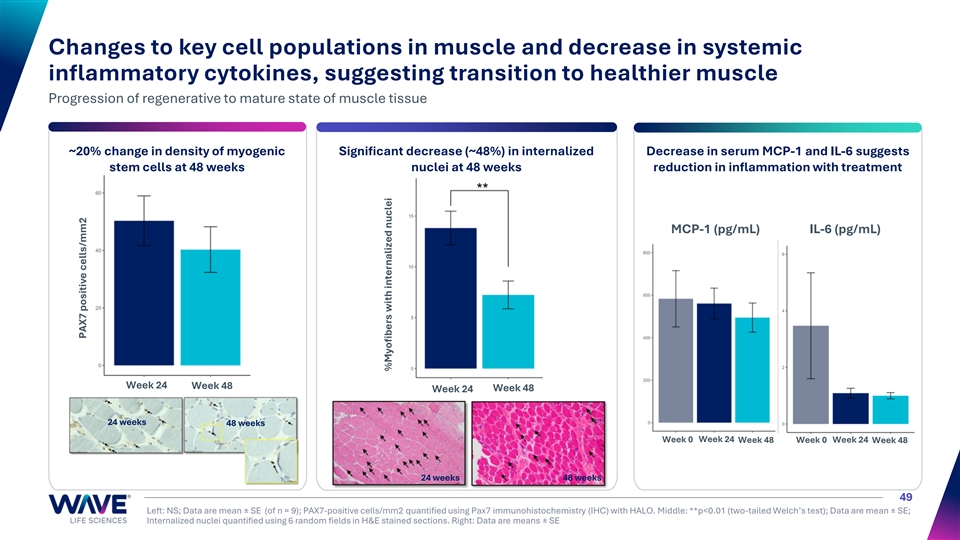
Changes to key cell populations in muscle and decrease in systemic inflammatory cytokines, suggesting transition to healthier muscle Progression of regenerative to mature state of muscle tissue ~20% change in density of myogenic Significant decrease (~48%) in internalized Decrease in serum MCP-1 and IL-6 suggests stem cells at 48 weeks nuclei at 48 weeks reduction in inflammation with treatment MCP-1 (pg/mL) IL-6 (pg/mL) Week 24 Week 48 Week 24 Week 48 24 weeks 48 weeks Week 0 Week 24 Week 48 Week 0 Week 24 Week 48 24 weeks 48 weeks 49 Left: NS; Data are mean ± SE (of n = 9); PAX7-positive cells/mm2 quantified using Pax7 immunohistochemistry (IHC) with HALO. Middle: **p<0.01 (two-tailed Welch’s test); Data are mean ± SE; Internalized nuclei quantified using 6 random fields in H&E stained sections. Right: Data are means ± SE PAX7 positive cells/mm2 %Myofibers with internalized nuclei
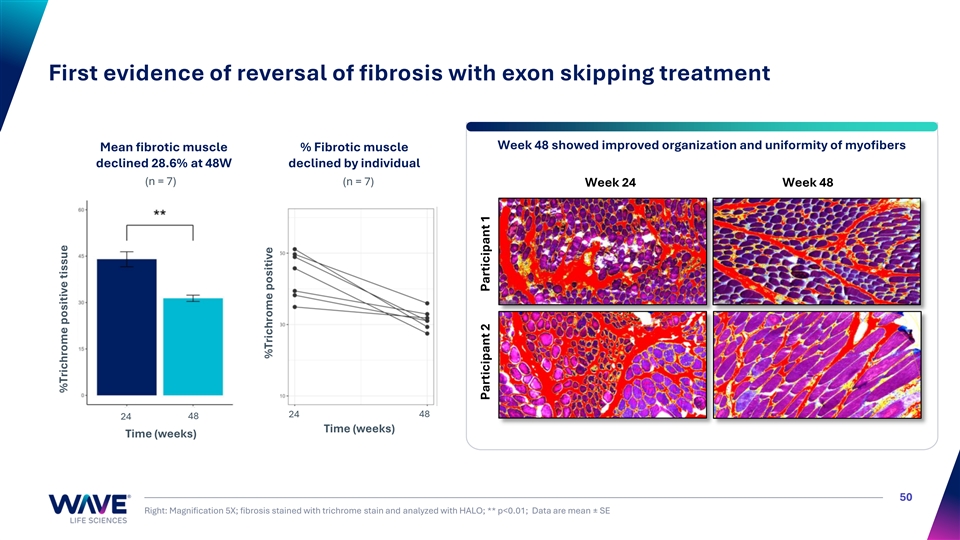
First evidence of reversal of fibrosis with exon skipping treatment Week 48 showed improved organization and uniformity of myofibers Mean fibrotic muscle % Fibrotic muscle declined 28.6% at 48W declined by individual (n = 7) (n = 7) Week 24 Week 48 24 48 24 48 Time (weeks) Time (weeks) 50 Right: Magnification 5X; fibrosis stained with trichrome stain and analyzed with HALO; ** p<0.01; Data are mean ± SE %Trichrome positive tissue %Trichrome positive Participant 2 Participant 1
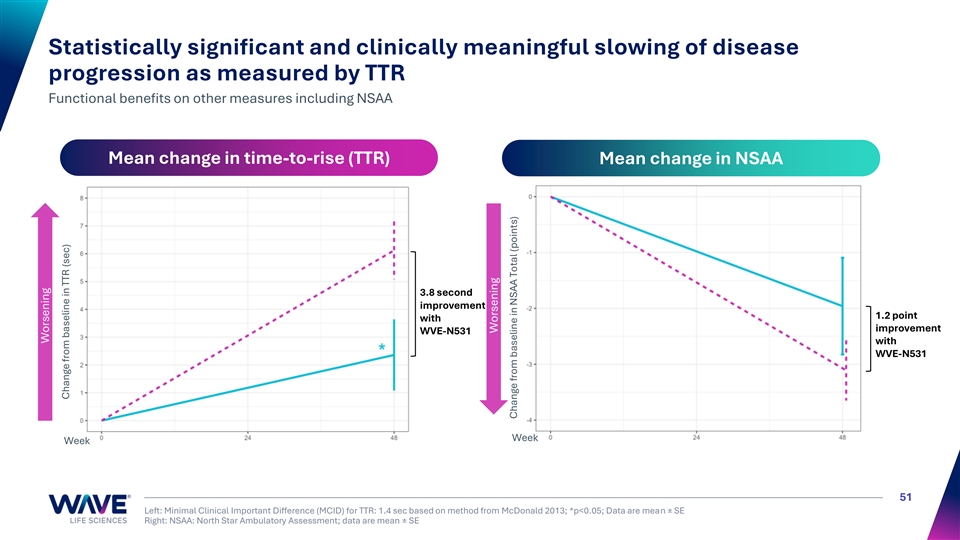
Statistically significant and clinically meaningful slowing of disease progression as measured by TTR Functional benefits on other measures including NSAA Mean change in time-to-rise (TTR) Mean change in NSAA 3.8 second improvement 1.2 point with improvement WVE-N531 with WVE-N531 Week Week 51 Left: Minimal Clinical Important Difference (MCID) for TTR: 1.4 sec based on method from McDonald 2013; *p<0.05; Data are mean ± SE Right: NSAA: North Star Ambulatory Assessment; data are mean ± SE Worsening Change from baseline in TTR (sec) Worsening Change from baseline in NSAA Total (points)
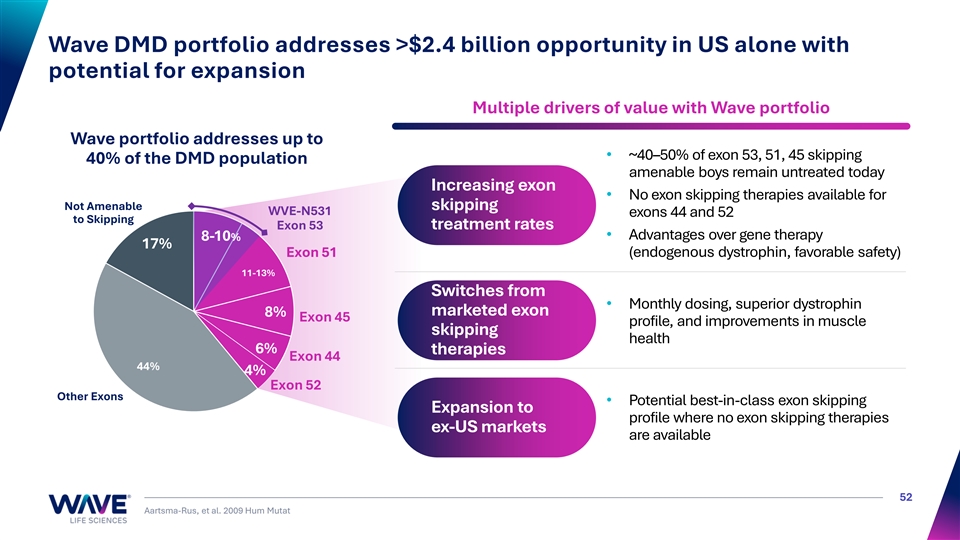
Wave DMD portfolio addresses >$2.4 billion opportunity in US alone with potential for expansion Multiple drivers of value with Wave portfolio Wave portfolio addresses up to • ~40–50% of exon 53, 51, 45 skipping 40% of the DMD population amenable boys remain untreated today Increasing exon • No exon skipping therapies available for Not Amenable skipping WVE-N531 exons 44 and 52 to Skipping Exon 53 treatment rates • Advantages over gene therapy 8-10% 17% E Ex xo on n 51 51 (endogenous dystrophin, favorable safety) 11-13% Switches from • Monthly dosing, superior dystrophin marketed exon 8% Exon 45 profile, and improvements in muscle skipping health 6% therapies Exon 44 44% 4% Exon 52 Other Exons • Potential best-in-class exon skipping Expansion to profile where no exon skipping therapies ex-US markets are available 52 Aartsma-Rus, et al. 2009 Hum Mutat

WVE-003 Allele-selective silencing Huntington’s Disease 53

Advancing WVE-003 to address HD across all stages of disease WVE-003 is a first-in-class, allele-selective oligonucleotide for the treatment of HD • HD is a monogenic autosomal dominant genetic disease; fully penetrant and affects entire brain • No current disease modifying therapies for HD • Characterized by cognitive decline, psychiatric illness, and chorea; ultimately fatal • Expanded CAG triplet repeat in HTT gene results in production of mutant huntingtin protein (mHTT) and loss of function of wild-type huntingtin protein (wtHTT) >200,000 patients with HD across all disease states Pre-Symptomatic HD Symptomatic HD (~160K in US and Europe) (~65K in US and Europe) 54 Sources on wtHTT: 1. Leavitt 2006 2. Cattaneo 2005 3. Kumar 2016 4. Franco-Iborra 2020 5. Hamilton 2015 6. Ochaba 2014 7. Wong 2014 8. Rui 2015 9. Caviston 2007 10. Twelvetrees 2010 11. Strehlow 2007 12. Milnerwood 2010 13. Smith-Dijak 2019 14. Tousley 2019 15. Zhang 2018 16. McAdam 2020 17. Altar 1997 18. Zuccato 2001 19. Gauthier 2004 20. Ferrer 2000 21. Baquet 2004 22. Liu 2011 23. Karam 2015
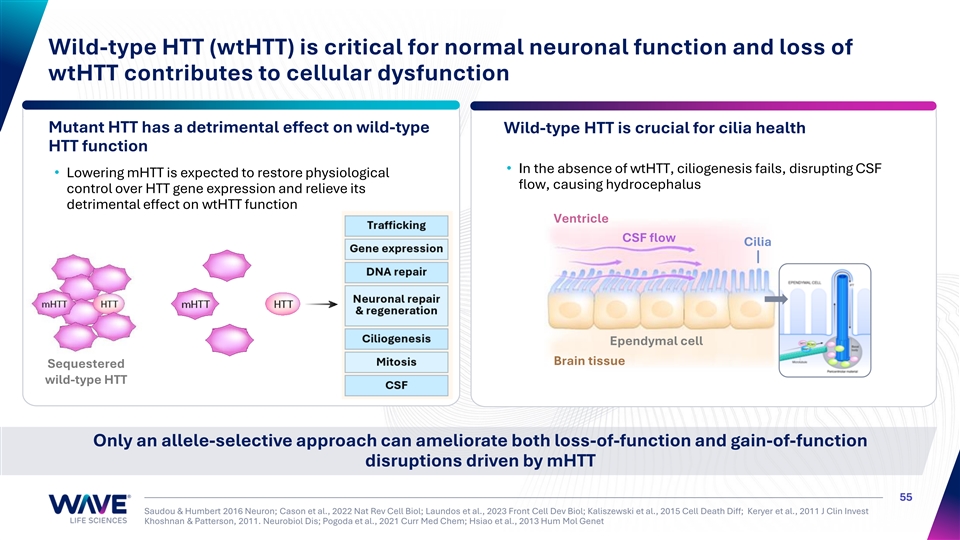
Wild-type HTT (wtHTT) is critical for normal neuronal function and loss of wtHTT contributes to cellular dysfunction Mutant HTT has a detrimental effect on wild-type Wild-type HTT is crucial for cilia health HTT function • In the absence of wtHTT, ciliogenesis fails, disrupting CSF • Lowering mHTT is expected to restore physiological flow, causing hydrocephalus control over HTT gene expression and relieve its detrimental effect on wtHTT function Ventricle CSF flow Cilia Ependymal cell Brain tissue Sequestered wild-type HTT Only an allele-selective approach can ameliorate both loss-of-function and gain-of-function disruptions driven by mHTT 55 Saudou & Humbert 2016 Neuron; Cason et al., 2022 Nat Rev Cell Biol; Laundos et al., 2023 Front Cell Dev Biol; Kaliszewski et al., 2015 Cell Death Diff; Keryer et al., 2011 J Clin Invest Khoshnan & Patterson, 2011. Neurobiol Dis; Pogoda et al., 2021 Curr Med Chem; Hsiao et al., 2013 Hum Mol Genet
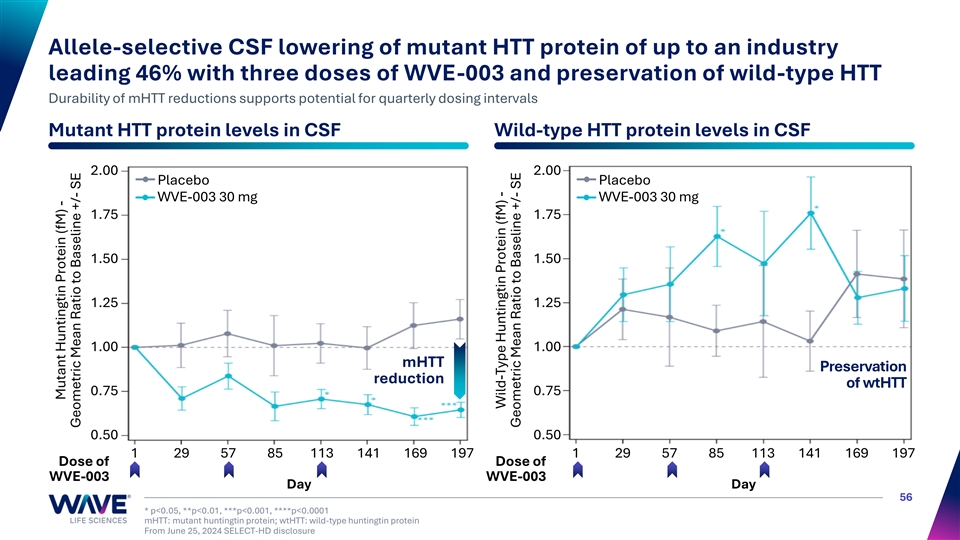
Allele-selective CSF lowering of mutant HTT protein of up to an industry leading 46% with three doses of WVE-003 and preservation of wild-type HTT Durability of mHTT reductions supports potential for quarterly dosing intervals Mutant HTT protein levels in CSF Wild-type HTT protein levels in CSF 2.00 2.00 Placebo Placebo WVE-003 30 mg WVE-003 30 mg 1.75 1.75 1.50 1.50 1.25 1.25 1.00 1.00 mHTT Preservation reduction of wtHTT 0.75 0.75 0.50 0.50 1 29 57 85 113 141 169 197 1 29 57 85 113 141 169 197 Dose of Dose of WVE-003 WVE-003 Day Day 56 * p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 mHTT: mutant huntingtin protein; wtHTT: wild-type huntingtin protein From June 25, 2024 SELECT-HD disclosure Mutant Huntingtin Protein (fM) - Geometric Mean Ratio to Baseline +/- SE Wild-Type Huntingtin Protein (fM) - Geometric Mean Ratio to Baseline +/- SE
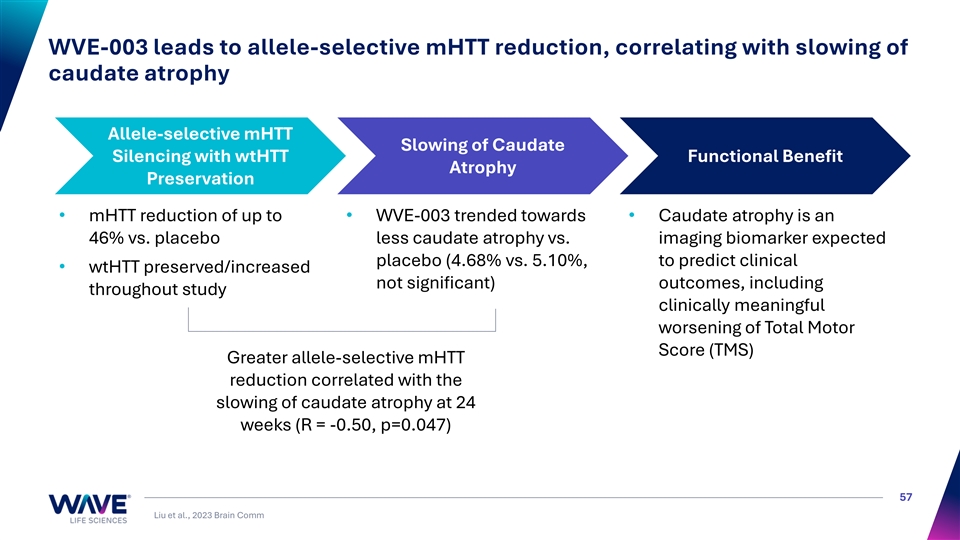
WVE-003 leads to allele-selective mHTT reduction, correlating with slowing of caudate atrophy Allele-selective mHTT Slowing of Caudate Silencing with wtHTT Functional Benefit Atrophy Preservation • mHTT reduction of up to • WVE-003 trended towards • Caudate atrophy is an 46% vs. placebo less caudate atrophy vs. imaging biomarker expected placebo (4.68% vs. 5.10%, to predict clinical • wtHTT preserved/increased not significant) outcomes, including throughout study clinically meaningful worsening of Total Motor Score (TMS) Greater allele-selective mHTT reduction correlated with the slowing of caudate atrophy at 24 weeks (R = -0.50, p=0.047) 57 Liu et al., 2023 Brain Comm
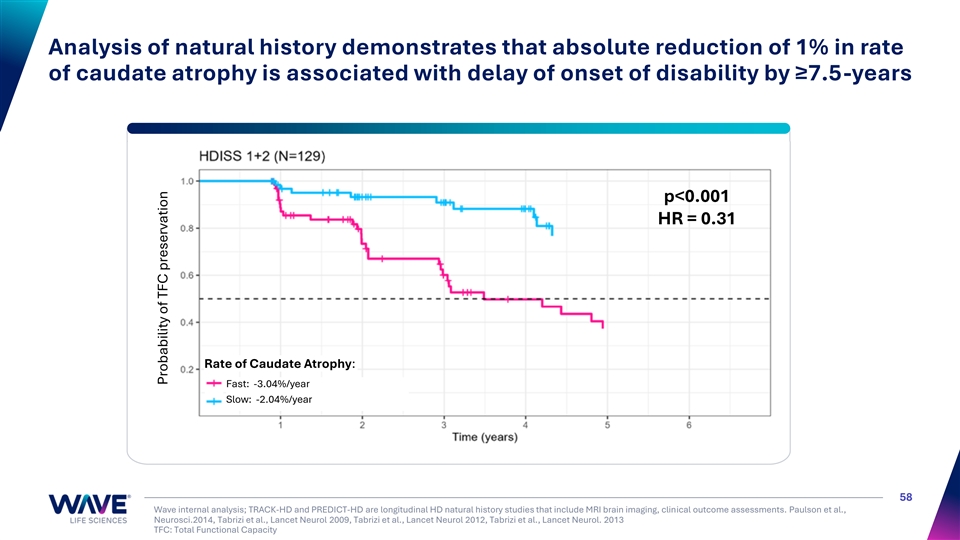
Analysis of natural history demonstrates that absolute reduction of 1% in rate of caudate atrophy is associated with delay of onset of disability by ≥7.5-years p<0.001 HR = 0.31 Rate of Caudate Atrophy: Fast: -3.04%/year Slow: -2.04%/year 58 Wave internal analysis; TRACK-HD and PREDICT-HD are longitudinal HD natural history studies that include MRI brain imaging, clinical outcome assessments. Paulson et al., Neurosci.2014, Tabrizi et al., Lancet Neurol 2009, Tabrizi et al., Lancet Neurol 2012, Tabrizi et al., Lancet Neurol. 2013 TFC: Total Functional Capacity Probability of TFC preservation

Reimagining RNA medicines 59
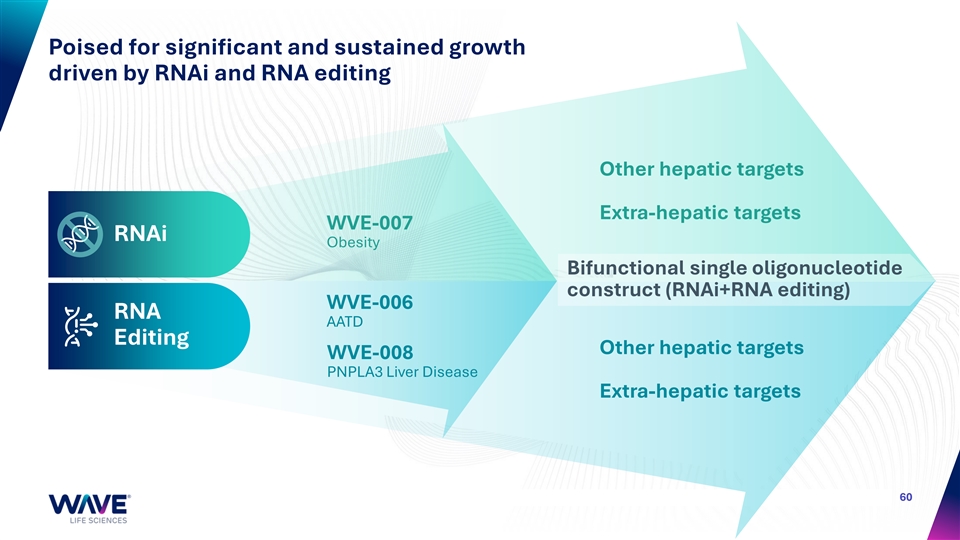
Poised for significant and sustained growth driven by RNAi and RNA editing Other hepatic targets Extra-hepatic targets WVE-007 RNAi Obesity Bifunctional single oligonucleotide construct (RNAi+RNA editing) WVE-006 RNA AATD Editing Other hepatic targets WVE-008 PNPLA3 Liver Disease Extra-hepatic targets 60
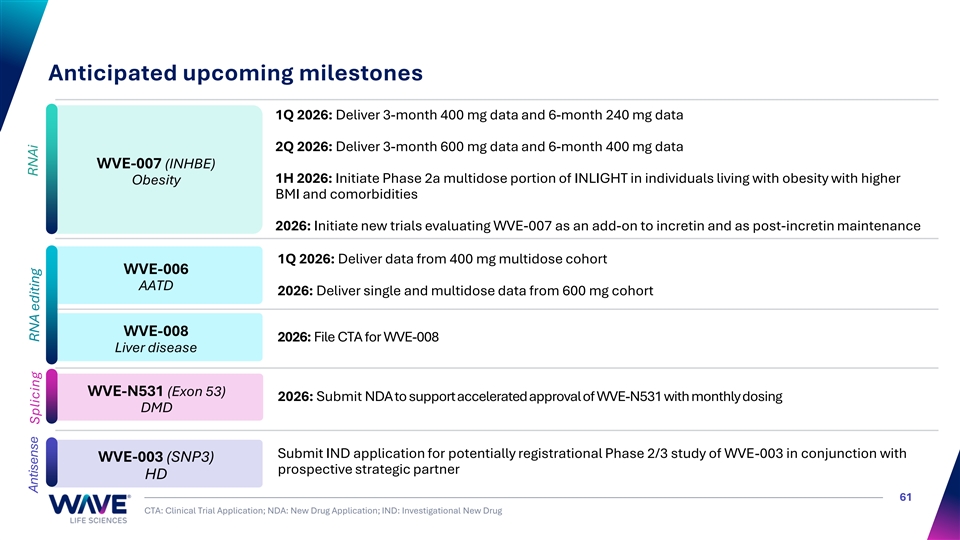
Anticipated upcoming milestones 1Q 2026: Deliver 3-month 400 mg data and 6-month 240 mg data 2Q 2026: Deliver 3-month 600 mg data and 6-month 400 mg data WVE-007 (INHBE) 1H 2026: Initiate Phase 2a multidose portion of INLIGHT in individuals living with obesity with higher Obesity BMI and comorbidities 2026: Initiate new trials evaluating WVE-007 as an add-on to incretin and as post-incretin maintenance 1Q 2026: Deliver data from 400 mg multidose cohort WVE-006 AATD 2026: Deliver single and multidose data from 600 mg cohort WVE-008 2026: File CTA for WVE-008 Liver disease WVE-N531 (Exon 53) 2026: Submit NDA to support accelerated approval of WVE-N531 with monthly dosing DMD Submit IND application for potentially registrational Phase 2/3 study of WVE-003 in conjunction with WVE-003 (SNP3) prospective strategic partner HD 61 CTA: Clinical Trial Application; NDA: New Drug Application; IND: Investigational New Drug RNAi Antisense Splicing RNA editing

For questions contact: investorrelations@wavelifesci.com