
FIRST AMENDMENT TO LICENSE AGREEMENT THIS FIRST AMENDMENT TO LICENSE AGREEMENT (this “Amendment”) is entered into this 11th day of November 2020 (the “Amendment Effective Date”), by and between NEWSOARA BIOPHARMA CO., LTD., a company organized under the laws of China, having a business address at Room 302-22, Building No. 1, 800 Na Xian Road, Shanghai Free Trade Zone, China (“Newsoara”), and VTV THERAPEUTICS LLC, a limited liability company organized under the laws of Delaware, having a business address at 3980 Premier Drive, Suite 310, High Point, NC 27265 (“vTv”). WHEREAS, Newsoara and vTv previously entered into the License Agreement on 31st day of May 2018 (the “Agreement”) whereby Newsoara obtained a license to the vTv Know- How, vTv Patent Rights, and vTv Compounds, which are PDE4 Inhibitors, including HPP737; WHEREAS, the Parties desire to amend the Agreement to address how certain vTv Patent Rights will be treated under the Agreement; NOW, THEREFORE, based on the foregoing premises and the mutual covenants and obligations set forth below, the parties agree as follows: 1. Amendments a. Article I (Definitions). i. The definitions of the below terms are hereby deleted from Article I of the Agreement and replaced as follows: “vTv Compound”. vTv Compound means any compound that (a) is Controlled by vTv as of the Effective Date and (b) is a PDE4 Inhibitor, including HPP737 and any backup compound thereto. For the avoidance of doubt, HPP737 as described on Schedule 1.28, as amended, is a vTv Compound. “vTv Know-How”. vTv Know-How means all Know-How that is Controlled by vTv or any of its Subsidiaries as of the Effective Date or thereafter during the Term (other than any Know-How included in Joint Intellectual Property) relating to, or that is necessary or useful for, the Development, Manufacture or Commercialization of vTv Compounds or Products, including HPP737. “vTv Patent Rights”. vTv Patent Rights means all Patent Rights that are Controlled by vTv or any of its Subsidiaries as of the Effective Date or thereafter during the Term (other than Joint Patent Rights) relating to, or that

is necessary or useful for, the Development, Manufacture or Commercialization of vTv Compounds or Products. The vTv Patent Rights in the Territory existing as of the Amendment Effective Date are set forth on Schedule 1.68, as amended. Notwithstanding the assignment, in whole or in part, of an interest in any vTv Patent Right by vTv to Newsoara pursuant to Section 9.3(c), such Patent Right shall continue to be treated as a vTv Patent Right for all purposes under the Agreement. ii. The following term is hereby added to Article I of the Agreement and is defined as follows: “Notice of Allowance” shall mean a notification from the Chinese National Intellectual Property Office of its decision to grant a patent right under Rule 39 of Chinese Patent Law, or its equivalent. b. Section 5.1. Section 5.1 shall be amended and restated as follows: “5.1 Diligence. During the Term, Newsoara shall, directly or through its Affiliates or Sublicensees, use Commercially Reasonable Efforts to (a) Develop at least one Compound and at least one Product in at least two different disease indications in the Field in Mainland China and (b) Commercialize at least one Product in at least two different disease indications in the Field in Mainland China. In addition, and without limiting the foregoing, Newsoara shall perform the obligation set forth in Section 5.3.” c. Section 5.3. The following new Section 5.3 shall be added to Article V immediately following Section 5.2 therein: “5.3. Specific Obligations. Newsoara shall dose the first patient in a Phase II Clinical Trial of a Product containing HPP737 on or before May 14, 2021.” d. Section 6.3(a). Section 6.3(a) shall be amended and restated in its entirety to read as follows: “6.3 Event Milestone Payments. (a) Newsoara shall pay to vTv the non-refundable, non-creditable, one-time payments set forth below after the earliest date on which the corresponding milestone event set forth below is achieved by Newsoara or any of its Affiliates or Sublicensees with respect to a Compound or Product, as the case may be: Milestone Event Payment
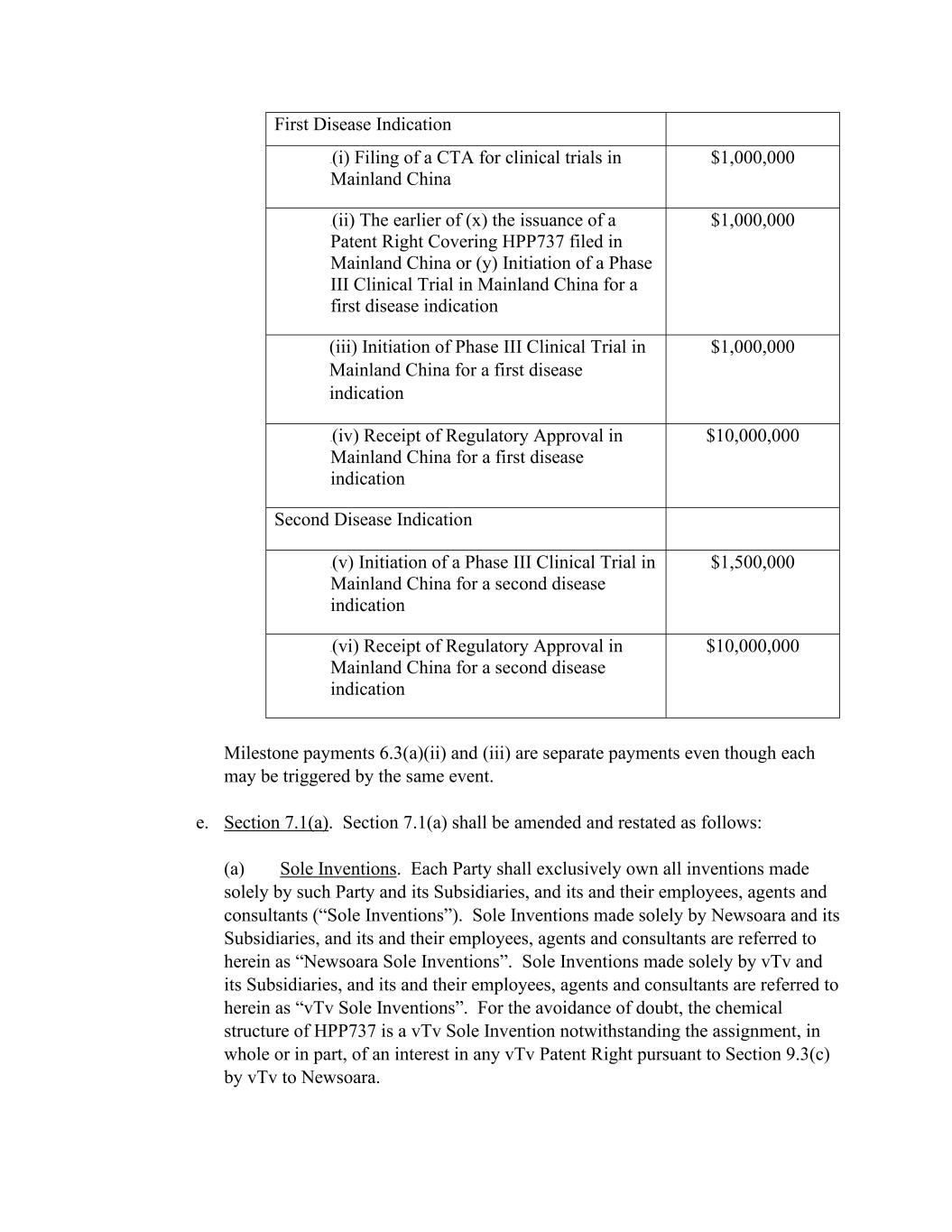
First Disease Indication 0B(i) Filing of a CTA for clinical trials in Mainland China $1,000,000 1B(ii) The earlier of (x) the issuance of a Patent Right Covering HPP737 filed in Mainland China or (y) Initiation of a Phase III Clinical Trial in Mainland China for a first disease indication $1,000,000 (iii) Initiation of Phase III Clinical Trial in Mainland China for a first disease indication $1,000,000 2B(iv) Receipt of Regulatory Approval in Mainland China for a first disease indication $10,000,000 Second Disease Indication 3B(v) Initiation of a Phase III Clinical Trial in Mainland China for a second disease indication $1,500,000 4B(vi) Receipt of Regulatory Approval in Mainland China for a second disease indication $10,000,000 Milestone payments 6.3(a)(ii) and (iii) are separate payments even though each may be triggered by the same event. e. Section 7.1(a). Section 7.1(a) shall be amended and restated as follows: (a) Sole Inventions. Each Party shall exclusively own all inventions made solely by such Party and its Subsidiaries, and its and their employees, agents and consultants (“Sole Inventions”). Sole Inventions made solely by Newsoara and its Subsidiaries, and its and their employees, agents and consultants are referred to herein as “Newsoara Sole Inventions”. Sole Inventions made solely by vTv and its Subsidiaries, and its and their employees, agents and consultants are referred to herein as “vTv Sole Inventions”. For the avoidance of doubt, the chemical structure of HPP737 is a vTv Sole Invention notwithstanding the assignment, in whole or in part, of an interest in any vTv Patent Right pursuant to Section 9.3(c) by vTv to Newsoara.

f. Section 8.2. Section 8.2 shall be amended and restated as follows: “8.2 Confidential Information. “Confidential Information” means all trade secrets or other proprietary information, including any proprietary data and materials (whether or not patentable or protectable as a trade secret), regarding a Party’s or its Affiliate’s or licensor’s technology, products, business, financial status or prospects or objectives regarding the Products that is disclosed by a Party to the other Party. All information disclosed prior to the Effective Date by vTv to Newsoara pursuant to the Mutual Non-Disclosure Agreement by and between the Parties, dated as of December 19, 2016, as amended through the Effective Date (the “Confidentiality Agreement”), and the structure of HPP737 as set forth on Schedule 1.28, as amended, shall be deemed “Confidential Information” of vTv. For clarity, all data and information regarding Products and Compounds generated after the Effective Date by or on behalf of Newsoara, its Affiliates or their Sublicensees, shall be deemed “Confidential Information” of Newsoara. Notwithstanding the foregoing, there shall be excluded from the foregoing definition of Confidential Information any of the foregoing that: (a) either before or after the date of the disclosure to the receiving Party is lawfully disclosed to the receiving Party by a Third Party without any violation of any obligation to the other Party; or (b) either before or after the date of the disclosure to the receiving Party, becomes published or generally known to the public through no fault or omission on the part of the receiving Party or its Agents; or (c) is independently developed by or for the receiving Party without reference to or reliance upon the disclosing Party’s Confidential Information as demonstrated by contemporaneous written records of the receiving Party. Notwithstanding the foregoing, the receiving Party may disclose the disclosing Party’s Confidential Information if it is required to be disclosed to comply with applicable Laws, to defend or prosecute litigation or to comply with governmental regulations or the regulations or requirements of any stock exchange, provided that the receiving Party promptly provides prior notice of such disclosure to the other Party and uses Commercially Reasonable Efforts to avoid or minimize the degree of such disclosure. g. Section 9.3. The following vTv Covenant shall be added to Section 9.3 as follows: (c) As soon as practical following the Amendment Effective Date, vTv will execute an assignment to Newsoara of a one-half interest in (i) the vTv Patent numbered ZL200980102961.0 as filed in Mainland China and (ii) the vTv Patent application numbered 202010702357.X as filed in Mainland China.

h. Section 11.5. The following new provision shall be added to Section 11.5 as follows: (f) Assignment of Patents. Assignment by Newsoara to vTv of all of Newsoara’s right, title, and interest in the vTv Patents assigned to Newsoara by vTv pursuant to Section 9.3(c). i. Section 12.4. The notice address for vTv shall be amended and restated as follows: vTv Therapeutics LLC 3980 Premier Drive, Suite 310 High Point, NC 27265 ATTN: Law Department j. Schedule 1.28. Schedule 1.28 to the Agreement is hereby replaced in its entirety with Schedule 1.28 attached to this Amendment. k. Schedule 1.68. Schedule 1.68 to the Agreement is hereby replaced in its entirety with Schedule 1.68 attached to this Amendment. 2. Representations. Newsoara hereby represents and warrants as of the Amendment Effective Date that it has not filed a patent application in any country covering the chemical composition of HPP737. 3. Defined Terms. Capitalized terms used in this Amendment and not defined herein shall have the respective meanings ascribed to them in the Agreement. 4. Counterparts. This Amendment may be executed in one or more counterparts by original or facsimile signature, each of which shall be deemed to be an original, and all of which shall constitute one and the same instrument. 5. Effect. As amended by this Amendment, the Agreement remains in full force and effect. 6. Governing Law. This Amendment shall be governed by and interpreted in accordance with the internal laws of the State of New York, USA, without regard to its conflicts of laws rules. [Signature page follows]

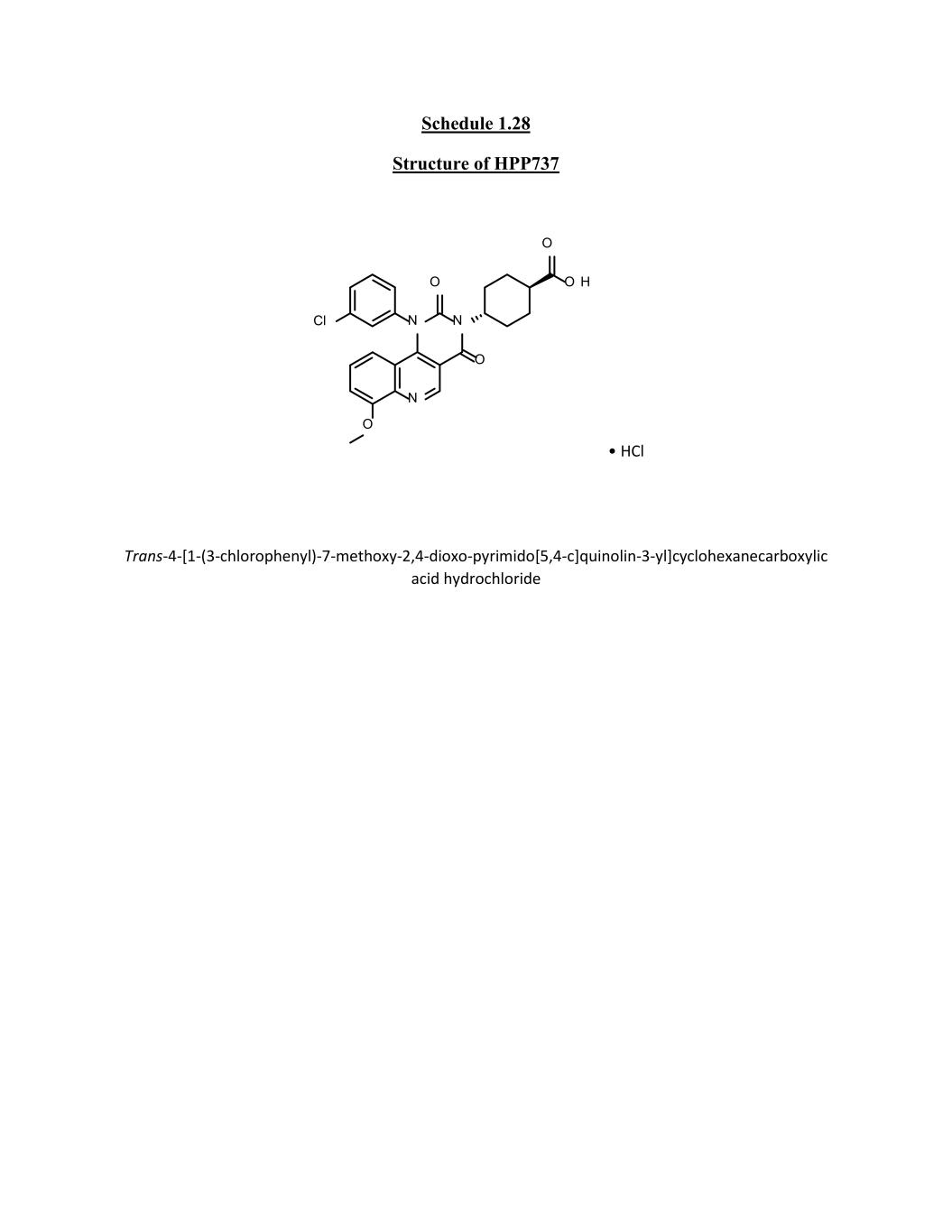
Schedule 1.28 Structure of HPP737 • HCl Trans-4-[1-(3-chlorophenyl)-7-methoxy-2,4-dioxo-pyrimido[5,4-c]quinolin-3-yl]cyclohexanecarboxylic acid hydrochloride O H O O N Cl N O N O
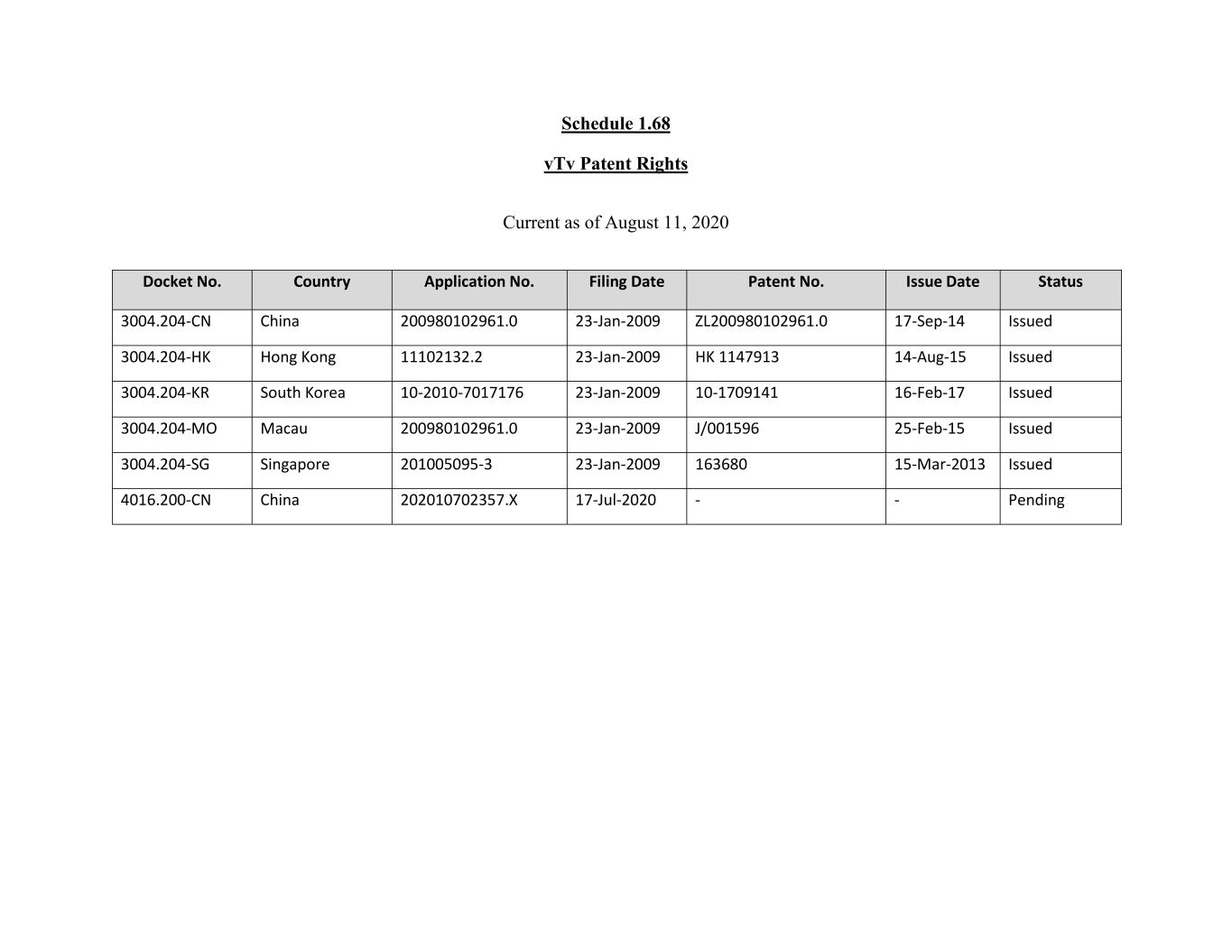
Schedule 1.68 vTv Patent Rights Current as of August 11, 2020 Docket No. Country Application No. Filing Date Patent No. Issue Date Status 3004.204-CN China 200980102961.0 23-Jan-2009 ZL200980102961.0 17-Sep-14 Issued 3004.204-HK Hong Kong 11102132.2 23-Jan-2009 HK 1147913 14-Aug-15 Issued 3004.204-KR South Korea 10-2010-7017176 23-Jan-2009 10-1709141 16-Feb-17 Issued 3004.204-MO Macau 200980102961.0 23-Jan-2009 J/001596 25-Feb-15 Issued 3004.204-SG Singapore 201005095-3 23-Jan-2009 163680 15-Mar-2013 Issued 4016.200-CN China 202010702357.X 17-Jul-2020 - - Pending