
[Confidential] © 2025 Novocure GmbH© 2025 Novocure GmbH 1 PANOVA-3 Investor Event May 31, 2025 – Chicago .3
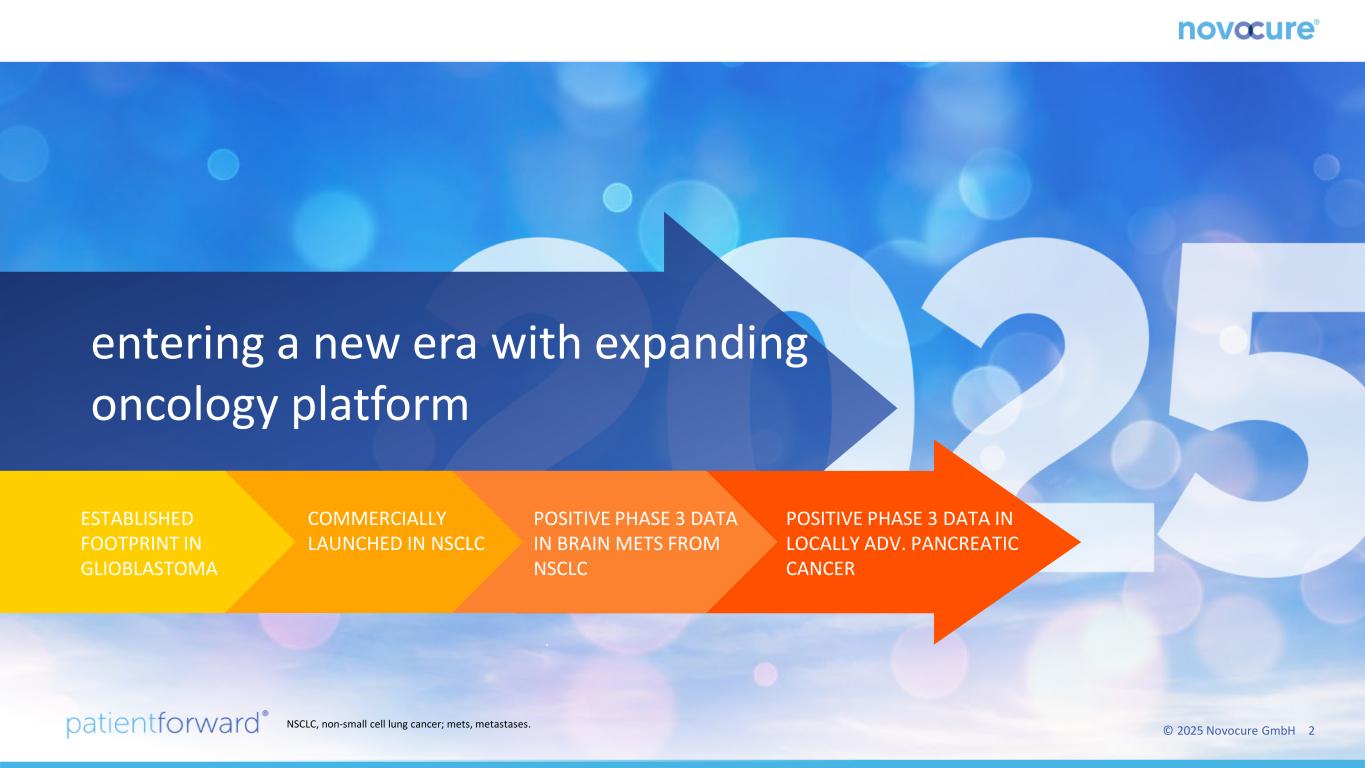
© 2025 Novocure GmbH 2 ` entering a new era with expanding oncology platform POSITIVE PHASE 3 DATA IN LOCALLY ADV. PANCREATIC CANCER POSITIVE PHASE 3 DATA IN BRAIN METS FROM NSCLC COMMERCIALLY LAUNCHED IN NSCLC ESTABLISHED FOOTPRINT IN GLIOBLASTOMA NSCLC, non-small cell lung cancer; mets, metastases.
© 2025 Novocure GmbH 3 agenda TTFields: A Multi-Indication Platform | Ashley Cordova Pancreatic Cancer Overview | Nicolas Leupin, M.D., Ph.D. PANOVA-3 Clinical Trial Results | Vincent Picozzi, M.D. Novocure Corporate Update | Ashley Cordova Q&A | Vincent Picozzi, M.D., Novocure Executive Team

© 2025 Novocure GmbH 4 together with our patients, we strive to extend survival in some of the most aggressive forms of cancer
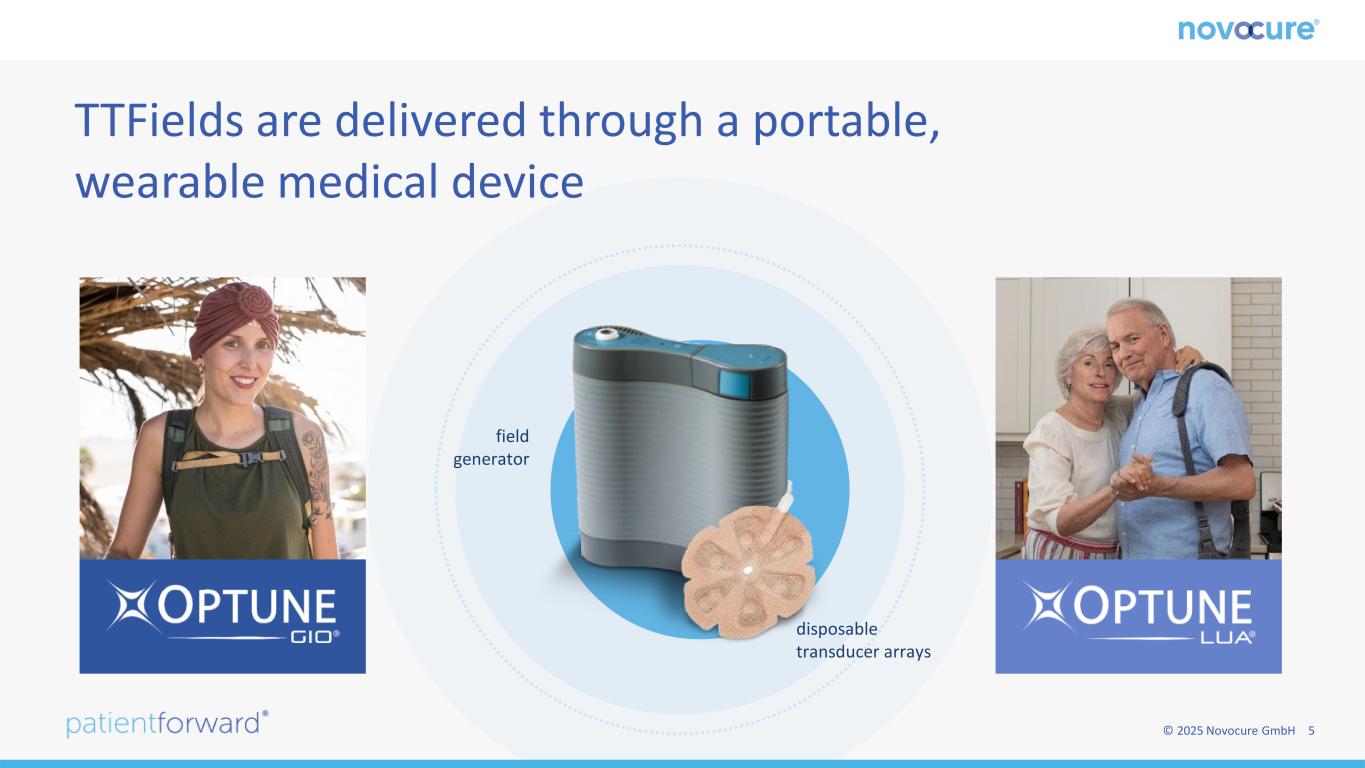
© 2025 Novocure GmbH 5 TTFields are delivered through a portable, wearable medical device disposable transducer arrays field generator
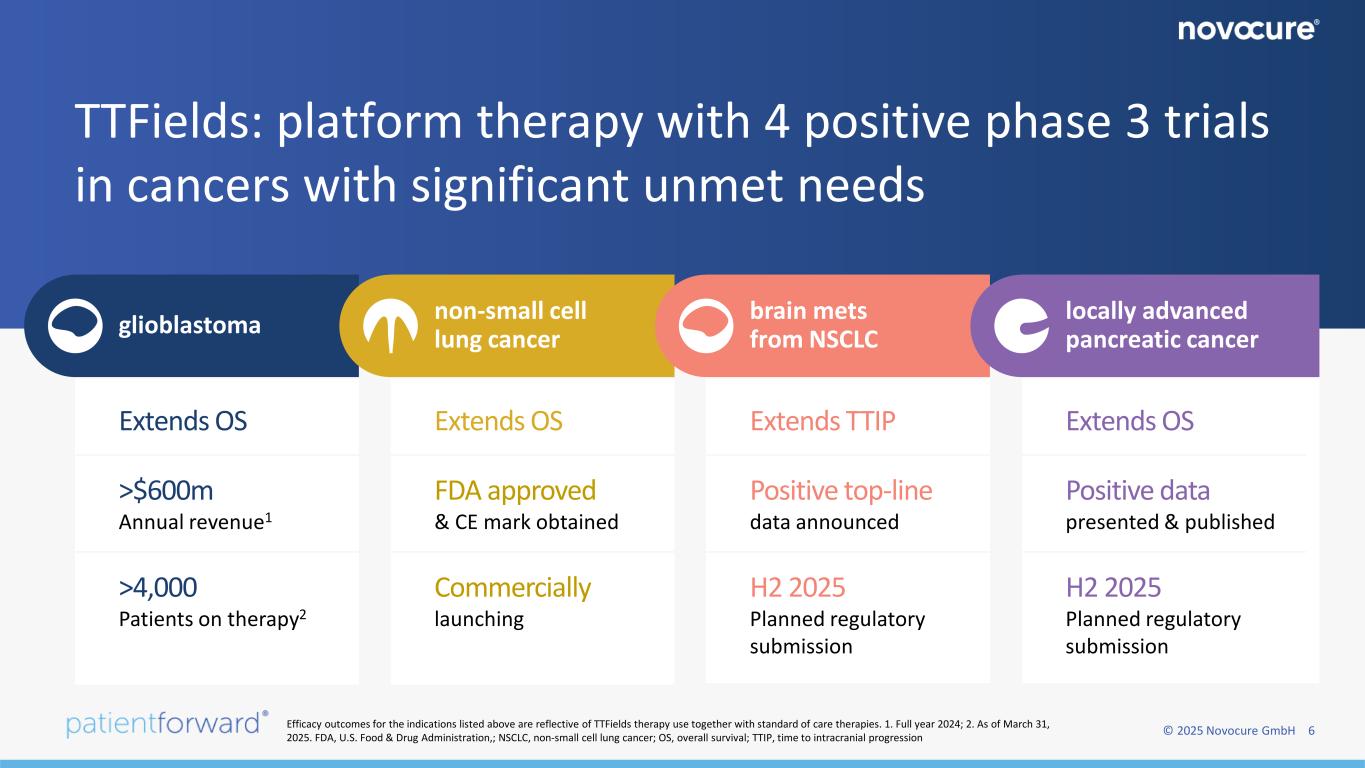
© 2025 Novocure GmbH 6 TTFields: platform therapy with 4 positive phase 3 trials in cancers with significant unmet needs glioblastoma non-small cell lung cancer brain mets from NSCLC locally advanced pancreatic cancer >$600m Annual revenue1 >4,000 Patients on therapy2 H2 2025 Planned regulatory submission Commercially launching Positive top-line data announced Extends TTIP FDA approved & CE mark obtained Extends OSExtends OS H2 2025 Planned regulatory submission Positive data presented & published Extends OS Efficacy outcomes for the indications listed above are reflective of TTFields therapy use together with standard of care therapies. 1. Full year 2024; 2. As of March 31, 2025. FDA, U.S. Food & Drug Administration,; NSCLC, non-small cell lung cancer; OS, overall survival; TTIP, time to intracranial progression

[Confidential] © 2025 Novocure GmbH © 2025 Novocure GmbH 7
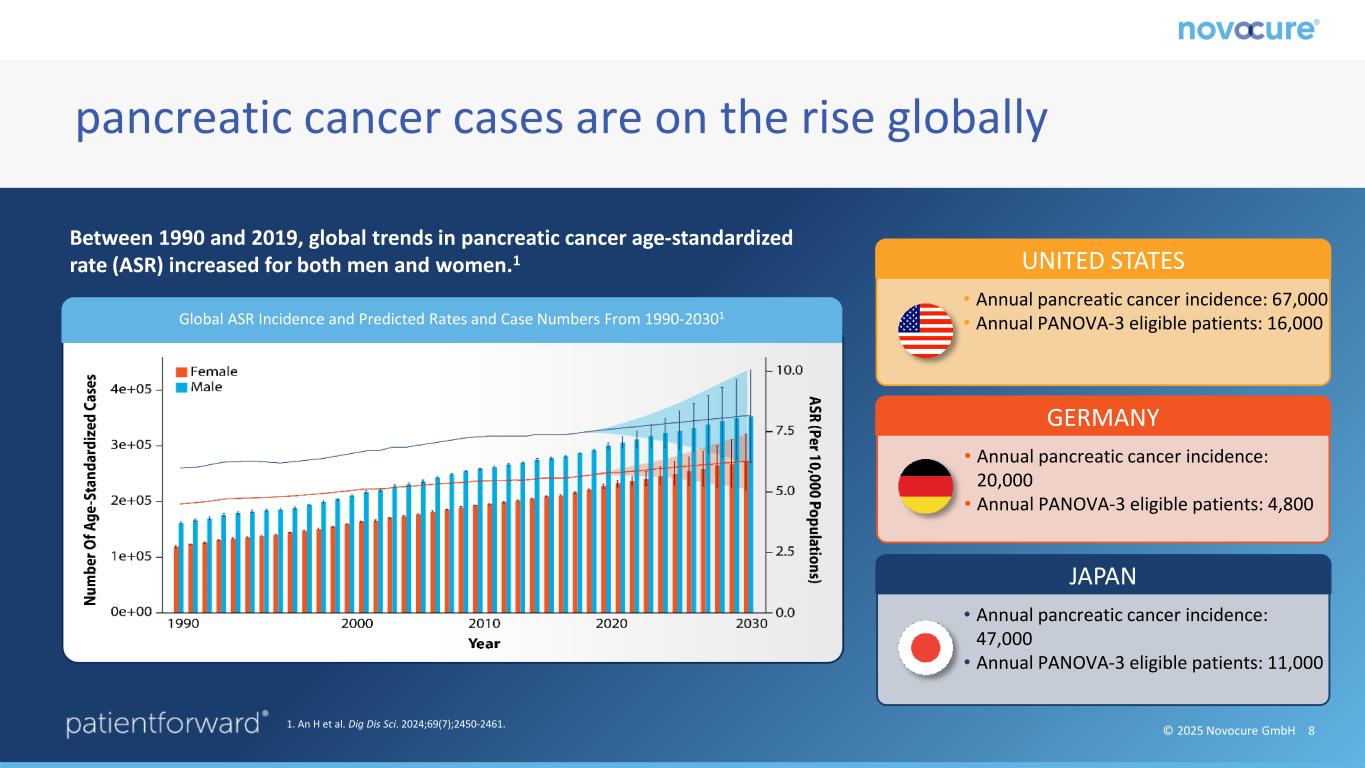
© 2025 Novocure GmbH 8 pancreatic cancer cases are on the rise globally Global ASR Incidence and Predicted Rates and Case Numbers From 1990-20301 UNITED STATES • Annual pancreatic cancer incidence: 67,000 • Annual PANOVA-3 eligible patients: 16,000 Between 1990 and 2019, global trends in pancreatic cancer age-standardized rate (ASR) increased for both men and women.1 1. An H et al. Dig Dis Sci. 2024;69(7);2450-2461. GERMANY • Annual pancreatic cancer incidence: 20,000 • Annual PANOVA-3 eligible patients: 4,800 JAPAN • Annual pancreatic cancer incidence: 47,000 • Annual PANOVA-3 eligible patients: 11,000
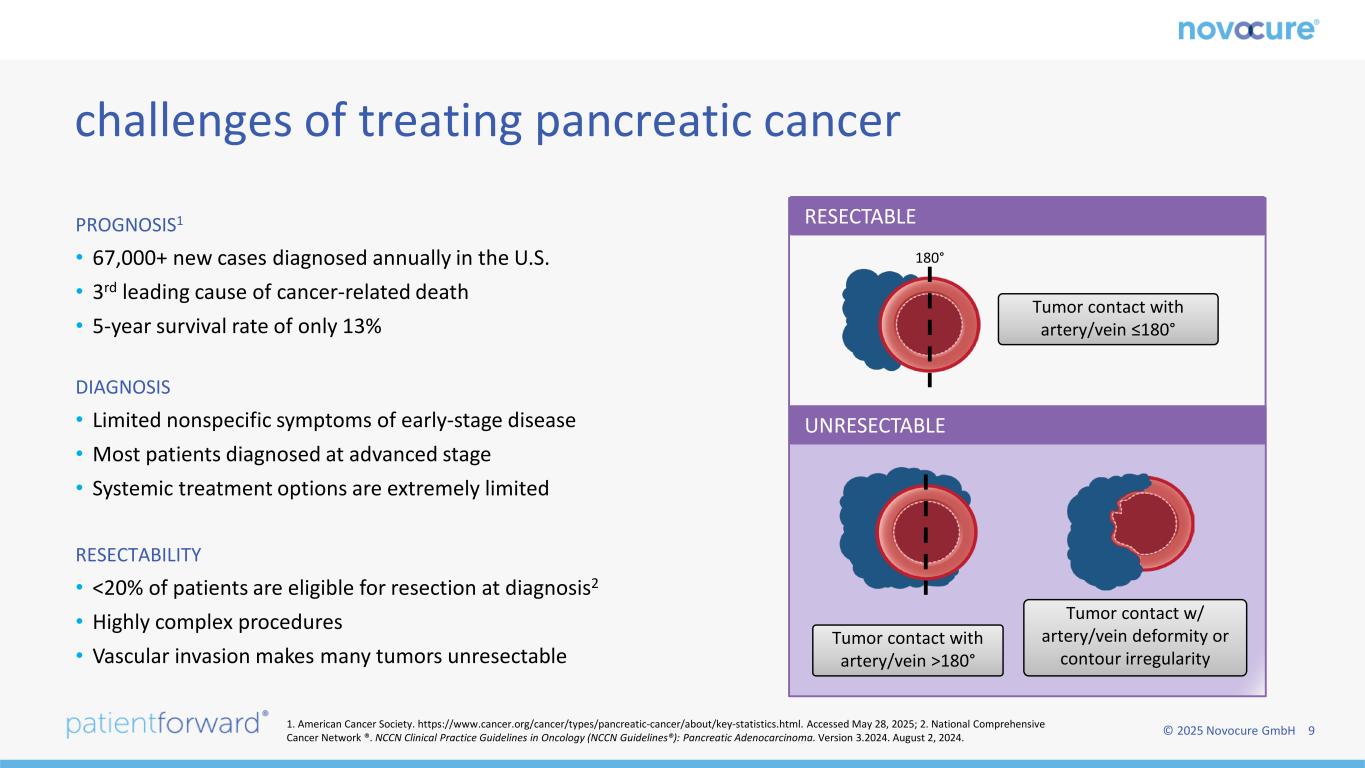
© 2025 Novocure GmbH 9 PROGNOSIS1 • 67,000+ new cases diagnosed annually in the U.S. • 3rd leading cause of cancer-related death • 5-year survival rate of only 13% DIAGNOSIS • Limited nonspecific symptoms of early-stage disease • Most patients diagnosed at advanced stage • Systemic treatment options are extremely limited RESECTABILITY • <20% of patients are eligible for resection at diagnosis2 • Highly complex procedures • Vascular invasion makes many tumors unresectable challenges of treating pancreatic cancer 180° Tumor contact with artery/vein ≤180° Tumor contact with artery/vein >180° Tumor contact w/ artery/vein deformity or contour irregularity RESECTABLE UNRESECTABLE 1. American Cancer Society. https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html. Accessed May 28, 2025; 2. National Comprehensive Cancer Network ®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma. Version 3.2024. August 2, 2024.
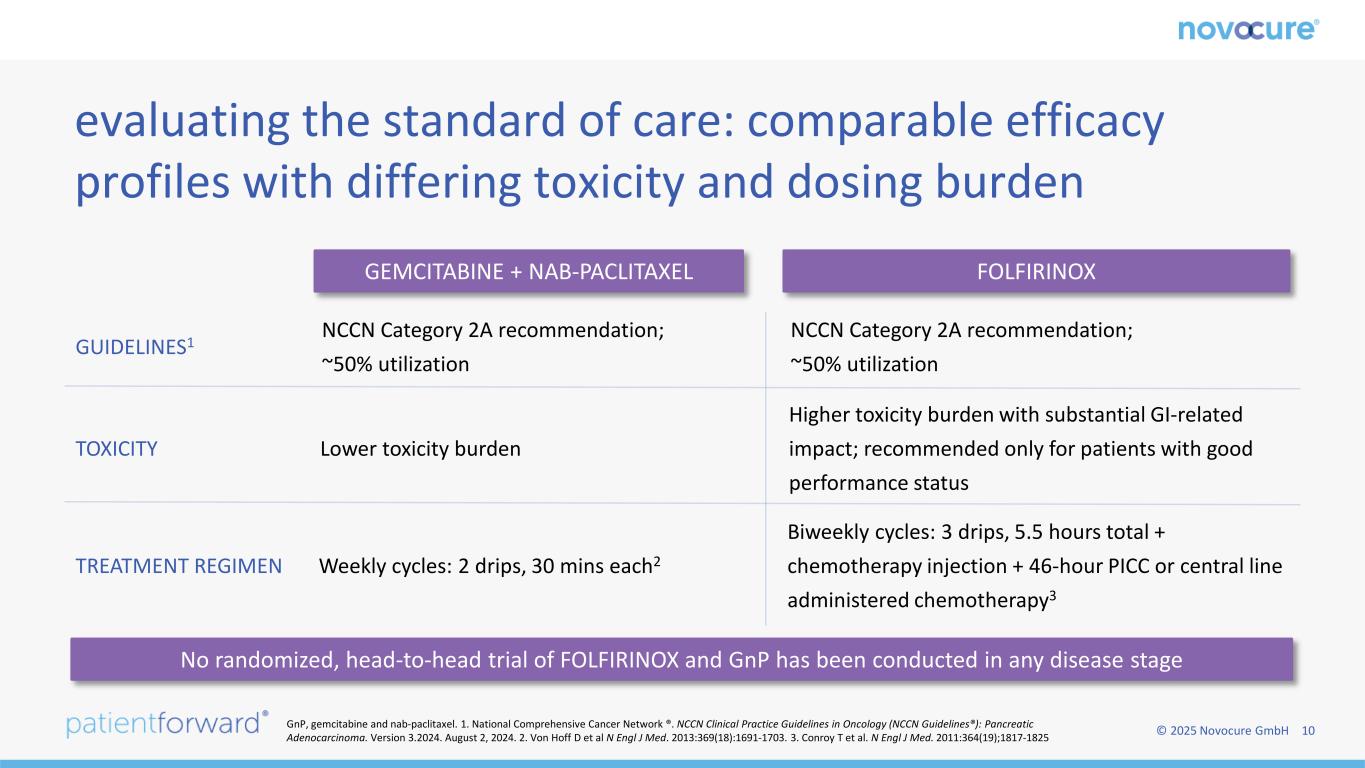
© 2025 Novocure GmbH 10 evaluating the standard of care: comparable efficacy profiles with differing toxicity and dosing burden GEMCITABINE + NAB-PACLITAXEL FOLFIRINOX GUIDELINES1 TOXICITY TREATMENT REGIMEN NCCN Category 2A recommendation; ~50% utilization NCCN Category 2A recommendation; ~50% utilization Lower toxicity burden Higher toxicity burden with substantial GI-related impact; recommended only for patients with good performance status Weekly cycles: 2 drips, 30 mins each2 Biweekly cycles: 3 drips, 5.5 hours total + chemotherapy injection + 46-hour PICC or central line administered chemotherapy3 No randomized, head-to-head trial of FOLFIRINOX and GnP has been conducted in any disease stage GnP, gemcitabine and nab-paclitaxel. 1. National Comprehensive Cancer Network ®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma. Version 3.2024. August 2, 2024. 2. Von Hoff D et al N Engl J Med. 2013:369(18):1691-1703. 3. Conroy T et al. N Engl J Med. 2011:364(19);1817-1825

© 2025 Novocure GmbH 11 late breaking oral abstract published in journal of clinical oncology featured in ‘best of ASCO’ positive phase 3 PANOVA-3 trial recognized at ASCO `

© 2025 Novocure GmbH 12 ASCO phase 3 PANOVA-3 trial results VINCENT PICOZZI, M.D. Director of Pancreatic & Biliary Duct Cancer Virginia Mason Franciscan Health Leads the largest single practice in pancreatic cancer in the nation and has an active clinical research program, both at Virginia Mason and through the American College of Surgeons Oncology Group Member and former chair of Pancreatic Cancer Action Network (PanCAN) Medical Advisory Board

PRESENTED BY: PANOVA-3: Phase 3 study of Tumor Treating Fields (TTFields) with gemcitabine and nab-paclitaxel (GnP) for locally advanced pancreatic adenocarcinoma (LA-PAC) Vincent Picozzi, Hani Babiker, Sreenivasa Chandana, Bohuslav Melichar, Anup Kasi, Jin Gang, Javier Gallego, Andrea Bullock, Hao Chunyi, Lucjan Wyrwicz, Arsen Osipov, Christelle de la Fouchardiere, Tomislav Dragovich, Woojin Lee, Kynan Feeney, Philip A. Philip, Makoto Ueno, Eric Van Cutsem, Thomas Seufferlein, Teresa Macarulla on behalf of the PANOVA-3 study investigators Dr Vincent Picozzi 1Virginia Mason Medical Center, Seattle, Washington, USA; 2Mayo Clinic, Jacksonville, Florida, USA; 3The Cancer & Hematology Centers, Michigan, USA; 4Palacky University and University Hospital Olomouc, Olomouc, Czech Republic; 5University of Kansas Medical Center, Kansas City, Kansas, USA; 6Changhai Hospital, Shanghai, China; 7General University Hospital Elche, Elche, Spain; 8Harvard Medical School, Harvard University, Boston, Massachusetts, USA; Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; 9Beijing Cancer Hospital, Beijing, China; 10National Institute of Oncology; Maria Sklodowska Curie National Cancer Research Institute, Warsaw, Poland; 11Cedars-Sinai Medical Center, Los Angeles, CA; 12Centre Léon Bérard, Lyon, France; 13Baptist MD Anderson Cancer Center, Jacksonville, Florida, USA; 14National Cancer Center, Goyang, Republic of Korea; 15St John of God Murdoch Hospital, Murdoch, Western Australia, Australia; 16Wayne State University/Henry Ford Hospital, Jackson, Michigan, USA; 17Kanagawa Cancer Center, Yokohama, Japan; 18University Hospitals Gasthuisberg and University of Leuven (KUL), Leuven, Belgium; 19University Hospital, Ulm, Germany; 20Vall d’Hebron University Hospital, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain
PRESENTED BY: Key Takeaway Points/Conclusions 14 Survival benefit for patients is supported by significantly improved QoL and pain-free survival* compared with GnP alone Dr Vincent Picozzi The only frequently reported TTFields toxicity was localized skin reactions *as the time to a ≥ 20-point increase from baseline on a patient-reported visual analogue scale for pain or death. GnP, gemcitabine/nab-paclitaxel; LA-PAC, locally advanced pancreatic adenocarcinoma; OS, overall survival; TTFields, Tumor Treating Fields. PANOVA-3 is the first phase 3 trial in patients with unresectable LA-PAC to show an OS benefit over gemcitabine/nab-paclitaxel PANOVA-3 establishes TTFields with GnP as a potential new standard paradigm for unresectable LA-PAC
PRESENTED BY: LA- PAC, a high unmet need 15 • Pancreatic adenocarcinoma remains a high unmet need with a modest 5-year survival rate of 8%1,2 About 30–35% of patients present with LA-PAC* and only 10–15% of them will be eligible for potentially curative surgery3 The remaining patients are incurable and will experience debilitating symptoms, especially pain • The current SOC for unresectable LA-PAC is chemotherapy (GnP, FOLFIRINOX, NALIRIFOX) ± radiation4-6 • While most trials of novel agents have focused on patients with metastatic disease,7-9 recent studies in patients with LA-PAC have failed to demonstrate overall survival benefit over the current SOC10,11 Dr Vincent Picozzi *LA-PAC was defined as histological/cytological diagnosis of de novo adenocarcinoma of the pancreas, which was deemed unresectable, locally advanced by investigator. GnP, gemcitabine/nab-paclitaxel; FOLFIRINOX, 5-FU/leucovorin, irinotecan, and oxaliplatin; LA-PAC, locally advanced pancreatic adenocarcinoma; NALFIRINOX, liposomal irinotecan, oxaliplatin, 5-FU/leucovorin, SOC, standard of care; References: 1. National Cancer Institute 2025; 2. American Cancer Society. Cancer Facts & Figures 2025. 3. Park W, et al. JAMA. 2021;326(9):851-862; 4. Conroy T, et al. Ann Oncol 2023;34(11):987-1002; 5. National Comprehensive Cancer Network (NCCN). NCC Guidelines in Oncology. Pancreatic Adenocarcinoma. 2024; 6. Wainberg ZA, et al. Lancet 2023 Oct 7;402(10409):1272-1281; 7. Picozzi VJ, et al. J Clin Oncol 43, 2025 (suppl 4; abstr 673); 8. Hu ZI, et al. Nat Rev Gastroenterol Hepatol 2024; 21 (1):7-24; 1961-703; 9. Anderson EM, et al. Cancers (Basel)2021; 13(21):5510; 10. De La Fouchardiere C, et al. J Clin Oncol 2024;42(9):105-66; 7); 11. Hammel P et al. JAMA 2016;315(17):1844-53.

PRESENTED BY: TTFields therapy 16 • TTFields are electric fields that disrupt processes critical for cancer cell division1-3 and may trigger an enhanced antitumor immune response • TTFields therapy is delivered noninvasively to the tumor site via a portable device that consists of a field generator and arrays placed on the skin4,5 • TTFields concomitant with systemic therapy is approved in the US and Europe for GBM, MPM, and metastatic NSCLC,6,7 and in Japan for GBM • TTFields with gemcitabine ± nab-paclitaxel was feasible and well tolerated in patients with advanced pancreatic adenocarcinoma in the phase 2 PANOVA pilot trial8 Dr Vincent Picozzi GBM, glioblastoma; MPM, malignant pleural mesothelioma; NSCLC, non-small cell lung cancer; TTFields, Tumor Treating Fields. 1. Kirson ED et al. Cancer Res. 2004;64(9):3288-3295. 2. Mun EJ et al. Clin Cancer Res. 2018;24(2):266-275. 3. Voloshin T et al. Cancers (Basel). 2020;12(10):3016; 4. Optune. Instructions for Use. Novocure; January 2019. 5. NovoTTF-100L. Instructions for Use for Unresectable Malignant Pleural Mesothelioma. Novocure; 2021; 6. Optune Lua® for Non-Small Cell Lung Cancer (NSCLC) – Patient Information and Operation Manual. Available at: https://assets.novocure.biz/optunelua/2024- 10/Optune%20Lua%20NSCLC%20ITE%20PIOM_v%2008.pdf; 7. Optune Gio®– Instructions for Use. Available at: https://www.optunegio.com/instructions-for-use; 8. Rivera F et al. Pancreatology 2017; 19(1):64-72. Actor portrayal
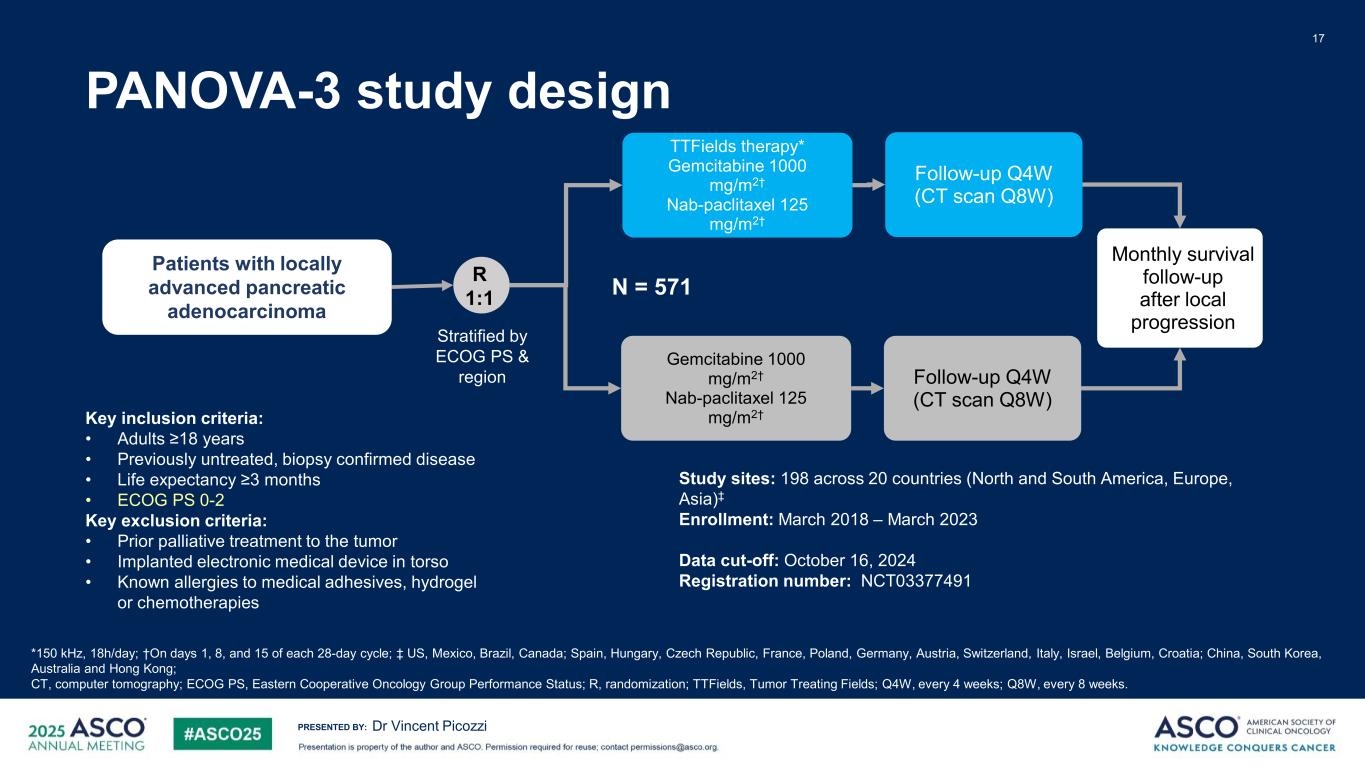
PRESENTED BY: PANOVA-3 study design 17 Dr Vincent Picozzi R 1:1 Gemcitabine 1000 mg/m2† Nab-paclitaxel 125 mg/m2† TTFields therapy* Gemcitabine 1000 mg/m2† Nab-paclitaxel 125 mg/m2† Follow-up Q4W (CT scan Q8W) Follow-up Q4W (CT scan Q8W) Monthly survival follow-up after local progression N = 571 Patients with locally advanced pancreatic adenocarcinoma Stratified by ECOG PS & region *150 kHz, 18h/day; †On days 1, 8, and 15 of each 28-day cycle; ‡ US, Mexico, Brazil, Canada; Spain, Hungary, Czech Republic, France, Poland, Germany, Austria, Switzerland, Italy, Israel, Belgium, Croatia; China, South Korea, Australia and Hong Kong; CT, computer tomography; ECOG PS, Eastern Cooperative Oncology Group Performance Status; R, randomization; TTFields, Tumor Treating Fields; Q4W, every 4 weeks; Q8W, every 8 weeks. Key inclusion criteria: • Adults ≥18 years • Previously untreated, biopsy confirmed disease • Life expectancy ≥3 months • ECOG PS 0-2 Key exclusion criteria: • Prior palliative treatment to the tumor • Implanted electronic medical device in torso • Known allergies to medical adhesives, hydrogel or chemotherapies Study sites: 198 across 20 countries (North and South America, Europe, Asia)‡ Enrollment: March 2018 – March 2023 Data cut-off: October 16, 2024 Registration number: NCT03377491
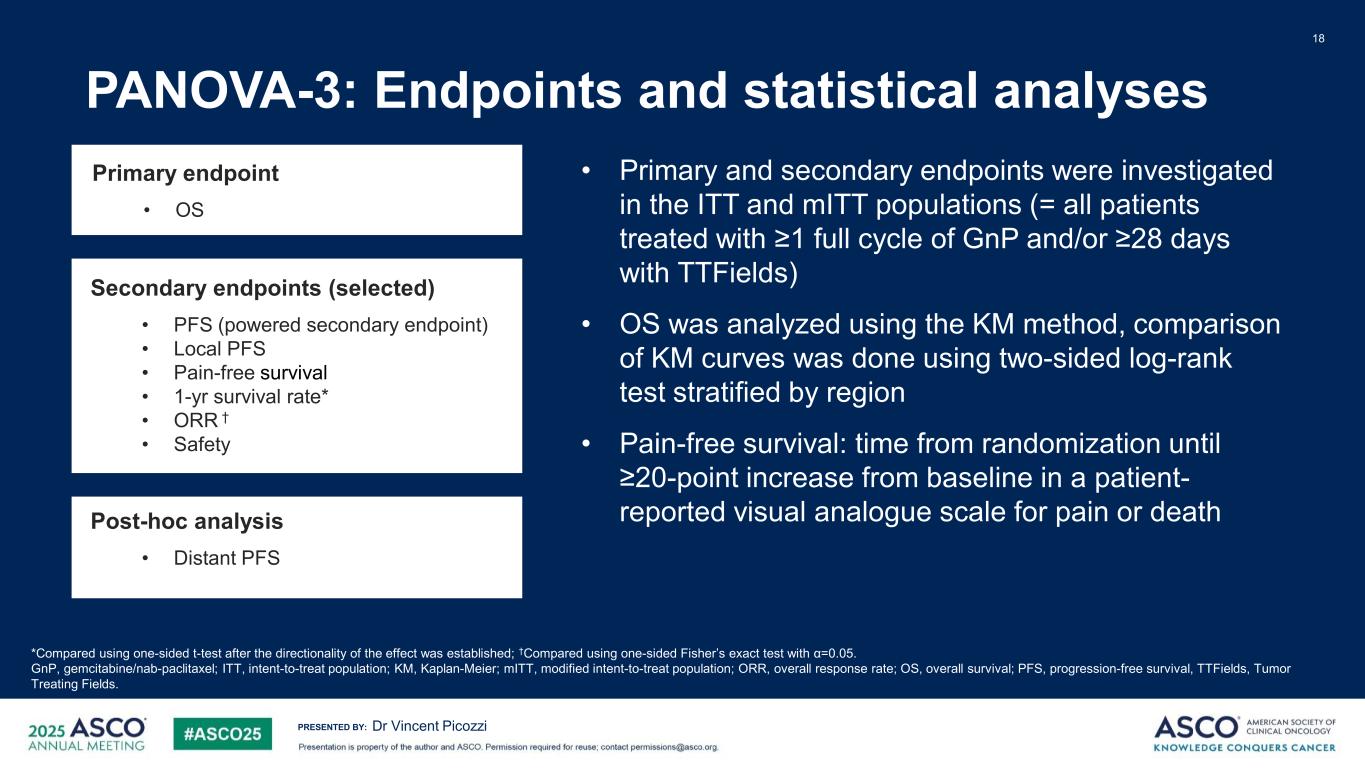
PRESENTED BY: PANOVA-3: Endpoints and statistical analyses 18 Dr Vincent Picozzi *Compared using one-sided t-test after the directionality of the effect was established; †Compared using one-sided Fisher’s exact test with α=0.05. GnP, gemcitabine/nab-paclitaxel; ITT, intent-to-treat population; KM, Kaplan-Meier; mITT, modified intent-to-treat population; ORR, overall response rate; OS, overall survival; PFS, progression-free survival, TTFields, Tumor Treating Fields. • Primary and secondary endpoints were investigated in the ITT and mITT populations (= all patients treated with ≥1 full cycle of GnP and/or ≥28 days with TTFields) • OS was analyzed using the KM method, comparison of KM curves was done using two-sided log-rank test stratified by region • Pain-free survival: time from randomization until ≥20-point increase from baseline in a patient- reported visual analogue scale for pain or death Secondary endpoints (selected) • PFS (powered secondary endpoint) • Local PFS • Pain-free survival • 1-yr survival rate* • ORR † • Safety Post-hoc analysis • Distant PFS Primary endpoint • OS
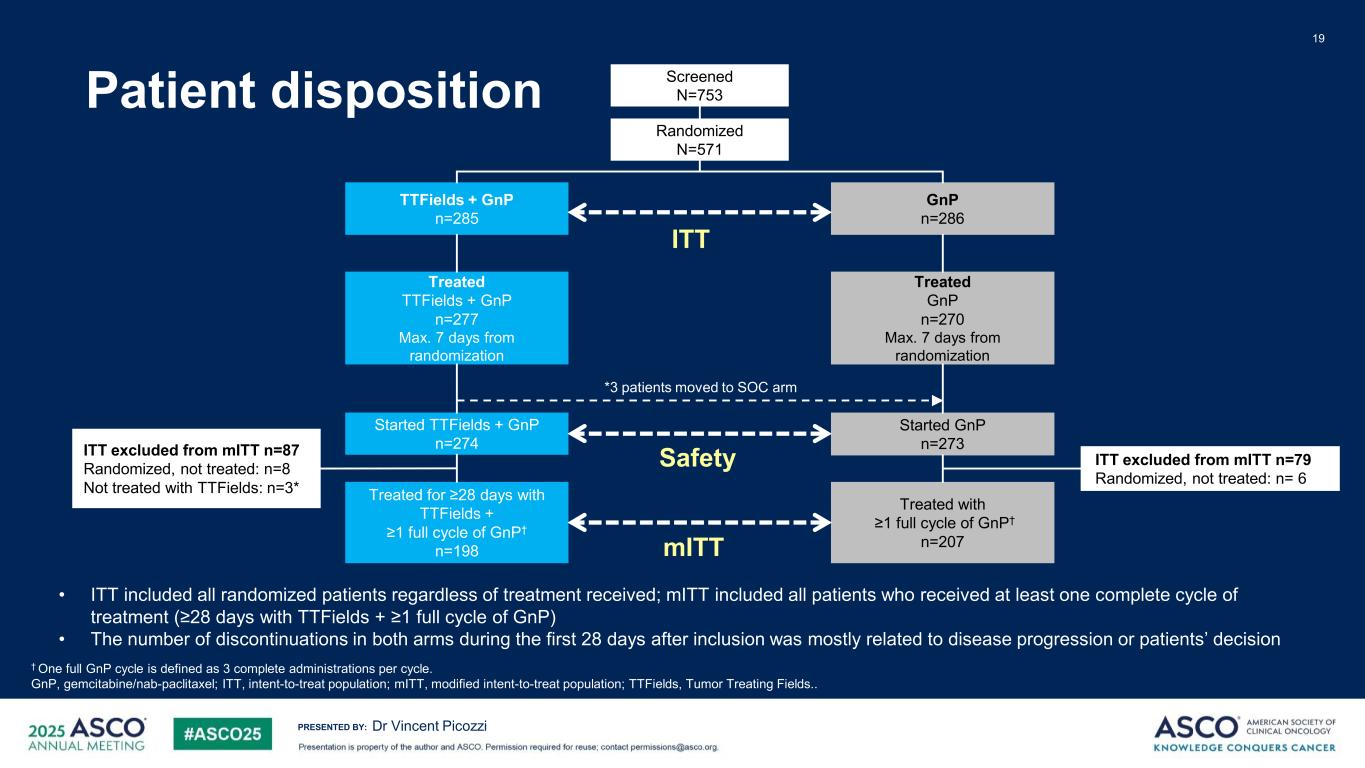
PRESENTED BY: Patient disposition 19 Dr Vincent Picozzi † One full GnP cycle is defined as 3 complete administrations per cycle. GnP, gemcitabine/nab-paclitaxel; ITT, intent-to-treat population; mITT, modified intent-to-treat population; TTFields, Tumor Treating Fields.. Screened N=753 Randomized N=571 TTFields + GnP n=285 GnP n=286 Treated TTFields + GnP n=277 Max. 7 days from randomization Treated GnP n=270 Max. 7 days from randomization Started TTFields + GnP n=274 Started GnP n=273 Treated for ≥28 days with TTFields + ≥1 full cycle of GnP† n=198 Treated with ≥1 full cycle of GnP† n=207 ITT excluded from mITT n=87 Randomized, not treated: n=8 Not treated with TTFields: n=3* ITT excluded from mITT n=79 Randomized, not treated: n= 6 ITT mITT Safety • ITT included all randomized patients regardless of treatment received; mITT included all patients who received at least one complete cycle of treatment (≥28 days with TTFields + ≥1 full cycle of GnP) • The number of discontinuations in both arms during the first 28 days after inclusion was mostly related to disease progression or patients’ decision *3 patients moved to SOC arm
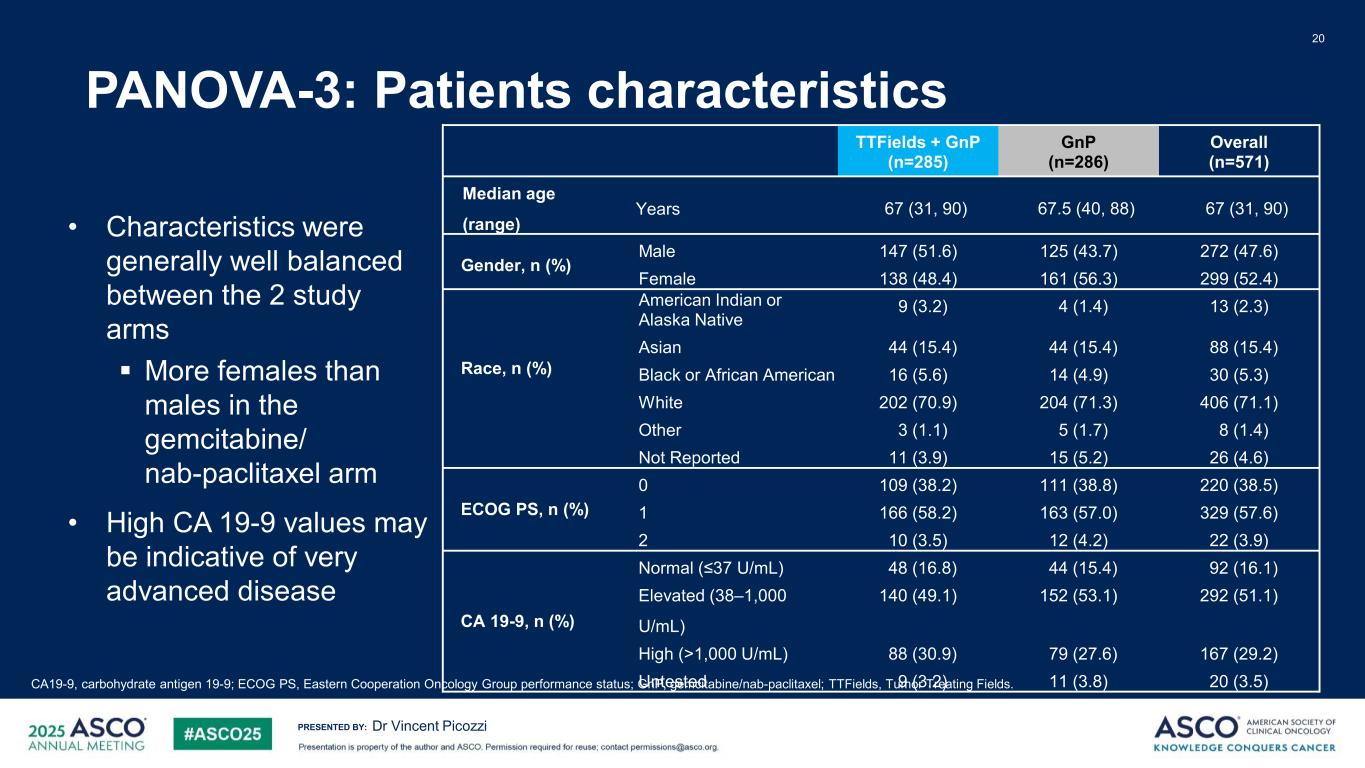
PRESENTED BY: PANOVA-3: Patients characteristics 20 Dr Vincent Picozzi CA19-9, carbohydrate antigen 19-9; ECOG PS, Eastern Cooperation Oncology Group performance status; GnP, gemcitabine/nab-paclitaxel; TTFields, Tumor Treating Fields. TTFields + GnP (n=285) GnP (n=286) Overall (n=571) Median age (range) Years 67 (31, 90) 67.5 (40, 88) 67 (31, 90) Gender, n (%) Male 147 (51.6) 125 (43.7) 272 (47.6) Female 138 (48.4) 161 (56.3) 299 (52.4) Race, n (%) American Indian or Alaska Native 9 (3.2) 4 (1.4) 13 (2.3) Asian 44 (15.4) 44 (15.4) 88 (15.4) Black or African American 16 (5.6) 14 (4.9) 30 (5.3) White 202 (70.9) 204 (71.3) 406 (71.1) Other 3 (1.1) 5 (1.7) 8 (1.4) Not Reported 11 (3.9) 15 (5.2) 26 (4.6) ECOG PS, n (%) 0 109 (38.2) 111 (38.8) 220 (38.5) 1 166 (58.2) 163 (57.0) 329 (57.6) 2 10 (3.5) 12 (4.2) 22 (3.9) CA 19-9, n (%) Normal (≤37 U/mL) 48 (16.8) 44 (15.4) 92 (16.1) Elevated (38–1,000 U/mL) 140 (49.1) 152 (53.1) 292 (51.1) High (>1,000 U/mL) 88 (30.9) 79 (27.6) 167 (29.2) U tested 9 (3.2) 11 (3.8) 20 (3.5) • Characteristics were generally well balanced between the 2 study arms More females than males in the gemcitabine/ nab-paclitaxel arm • High CA 19-9 values may be indicative of very advanced disease
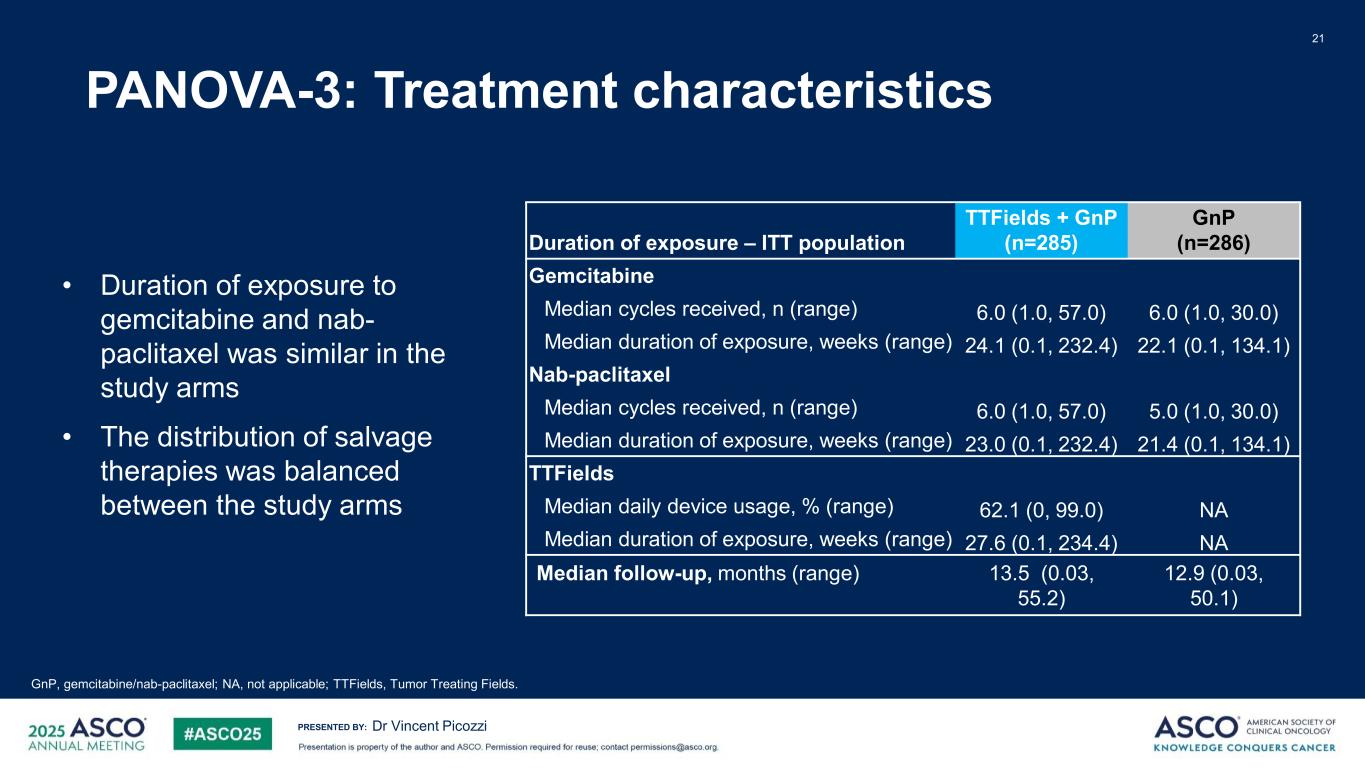
PRESENTED BY: PANOVA-3: Treatment characteristics 21 Dr Vincent Picozzi GnP, gemcitabine/nab-paclitaxel; NA, not applicable; TTFields, Tumor Treating Fields. • Duration of exposure to gemcitabine and nab- paclitaxel was similar in the study arms • The distribution of salvage therapies was balanced between the study arms Duration of exposure – ITT population TTFields + GnP (n=285) GnP (n=286) Gemcitabine Median cycles received, n (range) 6.0 (1.0, 57.0) 6.0 (1.0, 30.0) Median duration of exposure, weeks (range) 24.1 (0.1, 232.4) 22.1 (0.1, 134.1) Nab-paclitaxel Median cycles received, n (range) 6.0 (1.0, 57.0) 5.0 (1.0, 30.0) Median duration of exposure, weeks (range) 23.0 (0.1, 232.4) 21.4 (0.1, 134.1) TTFields Median daily device usage, % (range) 62.1 (0, 99.0) NA Median duration of exposure, weeks (range) 27.6 (0.1, 234.4) NA Median follow-up, months (range) 13.5 (0.03, 55.2) 12.9 (0.03, 50.1)
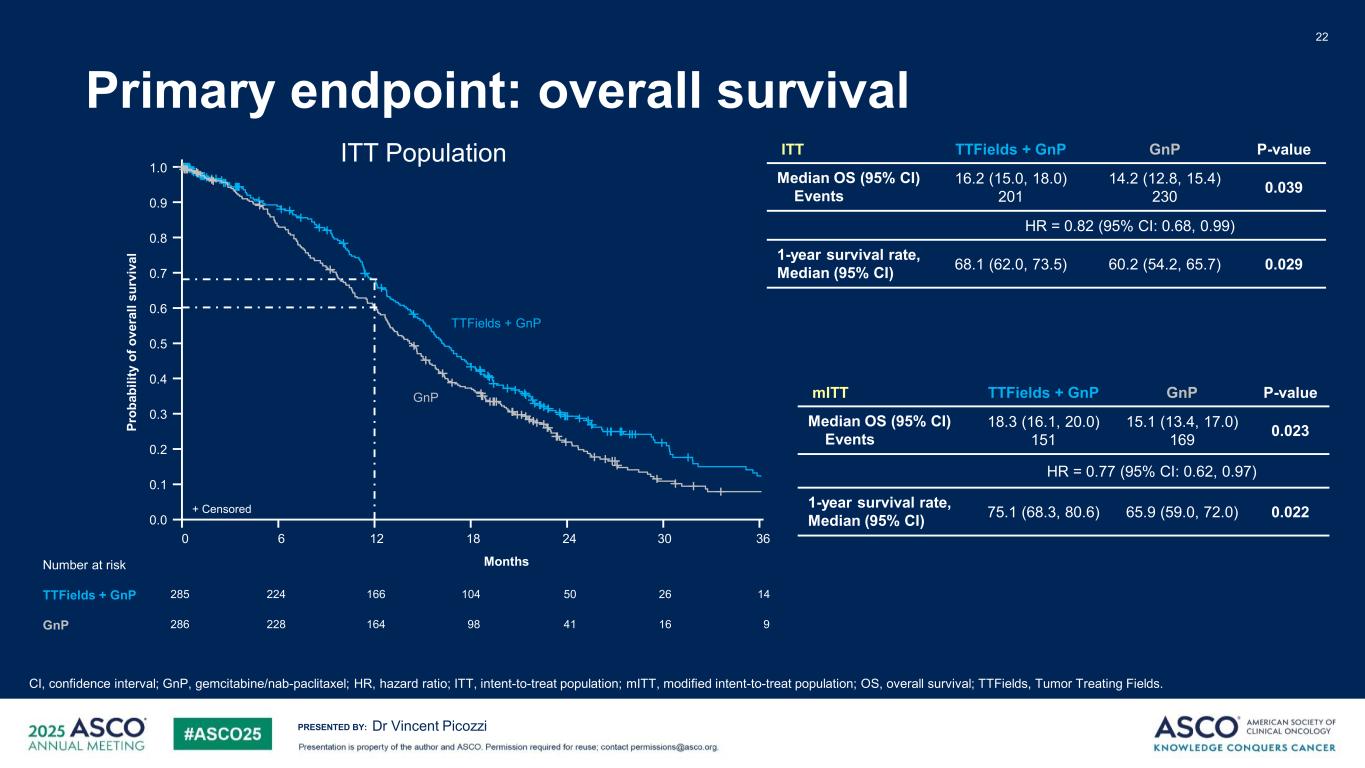
PRESENTED BY: Primary endpoint: overall survival Dr Vincent Picozzi ITT TTFields + GnP GnP P-value Median OS (95% CI) Events 16.2 (15.0, 18.0) 201 14.2 (12.8, 15.4) 230 0.039 HR = 0.82 (95% CI: 0.68, 0.99) 1-year survival rate, Median (95% CI) 68.1 (62.0, 73.5) 60.2 (54.2, 65.7) 0.029 22 ITT Population CI, confidence interval; GnP, gemcitabine/nab-paclitaxel; HR, hazard ratio; ITT, intent-to-treat population; mITT, modified intent-to-treat population; OS, overall survival; TTFields, Tumor Treating Fields. 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 2412 30186 14285 50166224 26104 9286 41164 1698228 Pr ob ab ili ty o f o ve ra ll su rv iv al MonthsNumber at risk TTFields + GnP GnP GnP TTFields + GnP + Censored 36 mITT TTFields + GnP GnP P-value Median OS (95% CI) Events 18.3 (16.1, 20.0) 151 15.1 (13.4, 17.0) 169 0.023 HR = 0.77 (95% CI: 0.62, 0.97) 1-year survival rate, Median (95% CI) 75.1 (68.3, 80.6) 65.9 (59.0, 72.0) 0.022
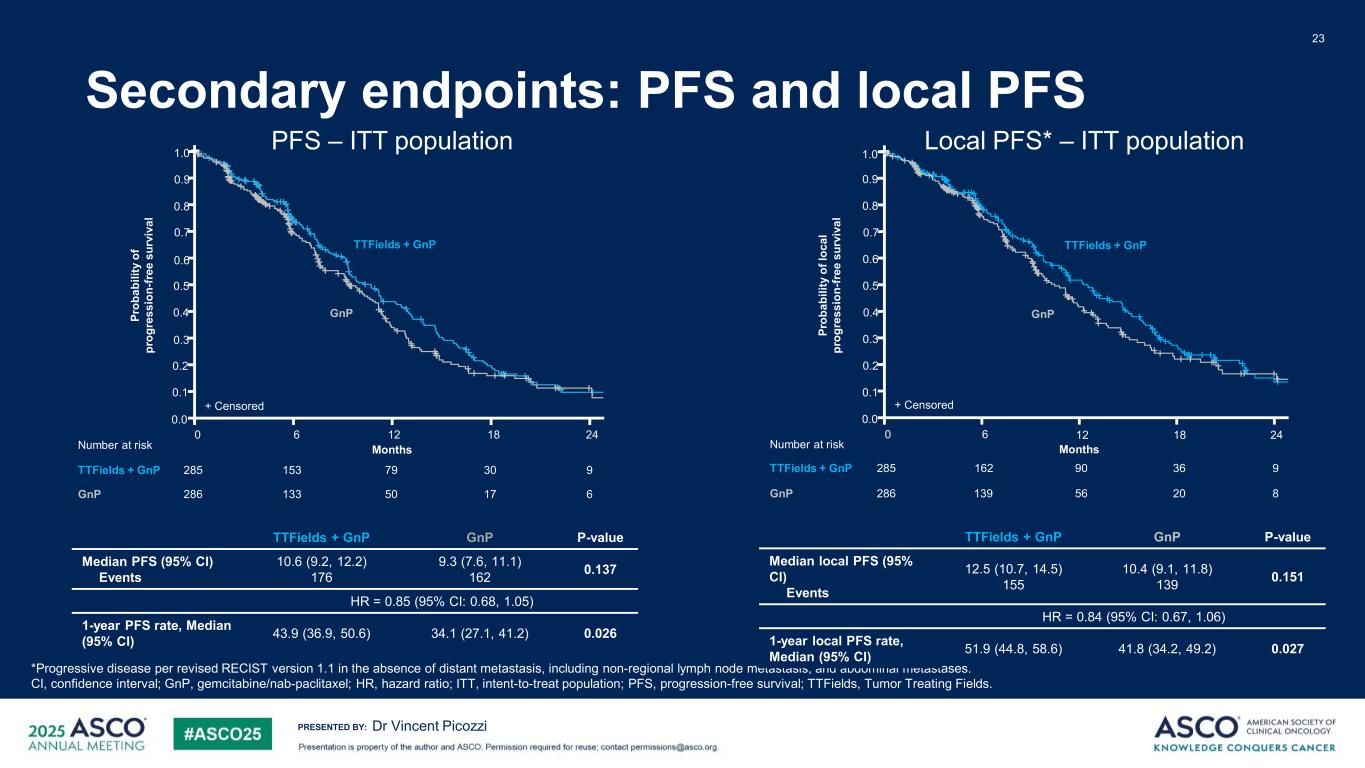
PRESENTED BY: Secondary endpoints: PFS and local PFS 23 Dr Vincent Picozzi PFS – ITT population Local PFS* – ITT population *Progressive disease per revised RECIST version 1.1 in the absence of distant metastasis, including non-regional lymph node metastasis, and abdominal metastases. CI, confidence interval; GnP, gemcitabine/nab-paclitaxel; HR, hazard ratio; ITT, intent-to-treat population; PFS, progression-free survival; TTFields, Tumor Treating Fields. 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 2418126 9 6 285 3079153 286 1750133 Number at risk TTFields + GnP GnP Pr ob ab ili ty o f pr og re ss io n- fr ee s ur vi va l Months + Censored GnP TTFields + GnP 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 2418126 9285 3690162 8286 2056139 Number at risk TTFields + GnP GnP Pr ob ab ili ty o f l oc al pr og re ss io n- fr ee s ur vi va l Months GnP + Censored TTFields + GnP TTFields + GnP GnP P-value Median PFS (95% CI) Events 10.6 (9.2, 12.2) 176 9.3 (7.6, 11.1) 162 0.137 HR = 0.85 (95% CI: 0.68, 1.05) 1-year PFS rate, Median (95% CI) 43.9 (36.9, 50.6) 34.1 (27.1, 41.2) 0.026 TTFields + GnP GnP P-value Median local PFS (95% CI) Events 12.5 (10.7, 14.5) 155 10.4 (9.1, 11.8) 139 0.151 HR = 0.84 (95% CI: 0.67, 1.06) 1-year local PFS rate, Median (95% CI) 51.9 (44.8, 58.6) 41.8 (34.2, 49.2) 0.027
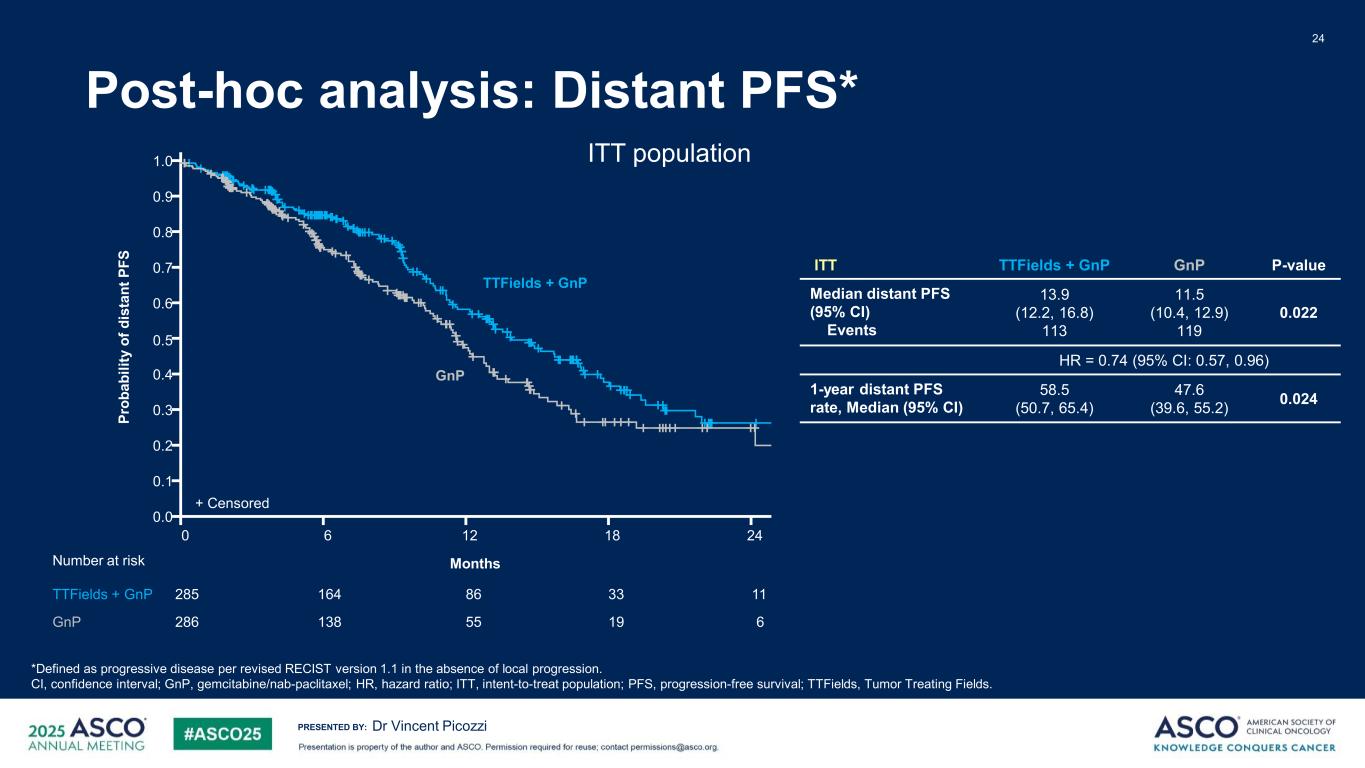
PRESENTED BY: Post-hoc analysis: Distant PFS* 24 Dr Vincent Picozzi *Defined as progressive disease per revised RECIST version 1.1 in the absence of local progression. CI, confidence interval; GnP, gemcitabine/nab-paclitaxel; HR, hazard ratio; ITT, intent-to-treat population; PFS, progression-free survival; TTFields, Tumor Treating Fields. ITT TTFields + GnP GnP P-value Median distant PFS (95% CI) Events 13.9 (12.2, 16.8) 113 11.5 (10.4, 12.9) 119 0.022 HR = 0.74 (95% CI: 0.57, 0.96) 1-year distant PFS rate, Median (95% CI) 58.5 (50.7, 65.4) 47.6 (39.6, 55.2) 0.024 ITT population1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 2418126 11285 3386164 6286 1955138 Number at risk TTFields + GnP GnP Months + Censored GnP TTFields + GnP Pr ob ab ili ty o f d is ta nt P FS
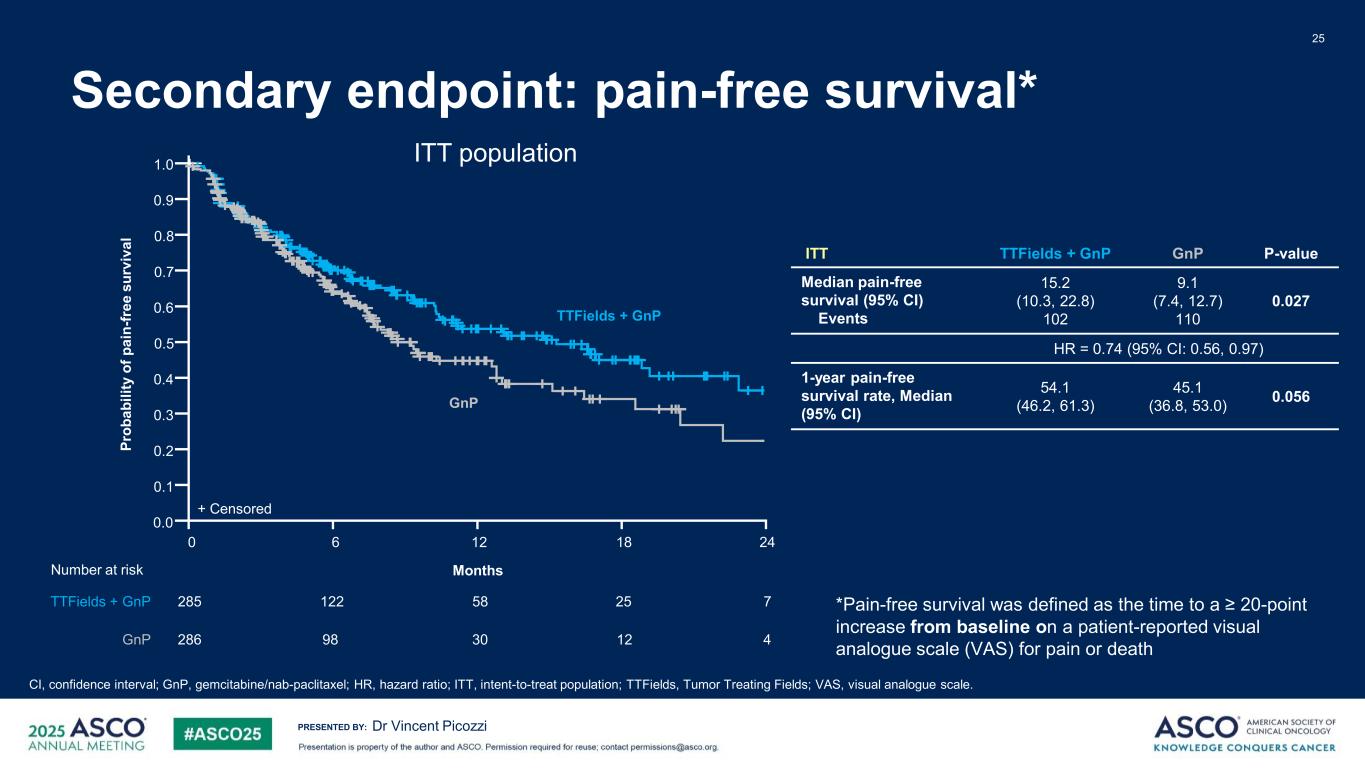
PRESENTED BY: Secondary endpoint: pain-free survival* 25 Dr Vincent Picozzi CI, confidence interval; GnP, gemcitabine/nab-paclitaxel; HR, hazard ratio; ITT, intent-to-treat population; TTFields, Tumor Treating Fields; VAS, visual analogue scale. *Pain-free survival was defined as the time to a ≥ 20-point increase from baseline on a patient-reported visual analogue scale (VAS) for pain or death ITT population1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 12 186 285 758122 25 286 430 1298 TTFields + GnP GnP + Censored Number at risk TTFields + GnP GnP Pr ob ab ili ty o f p ai n- fr ee s ur vi va l Months 24 ITT TTFields + GnP GnP P-value Median pain-free survival (95% CI) Events 15.2 (10.3, 22.8) 102 9.1 (7.4, 12.7) 110 0.027 HR = 0.74 (95% CI: 0.56, 0.97) 1-year pain-free survival rate, Median (95% CI) 54.1 (46.2, 61.3) 45.1 (36.8, 53.0) 0.056
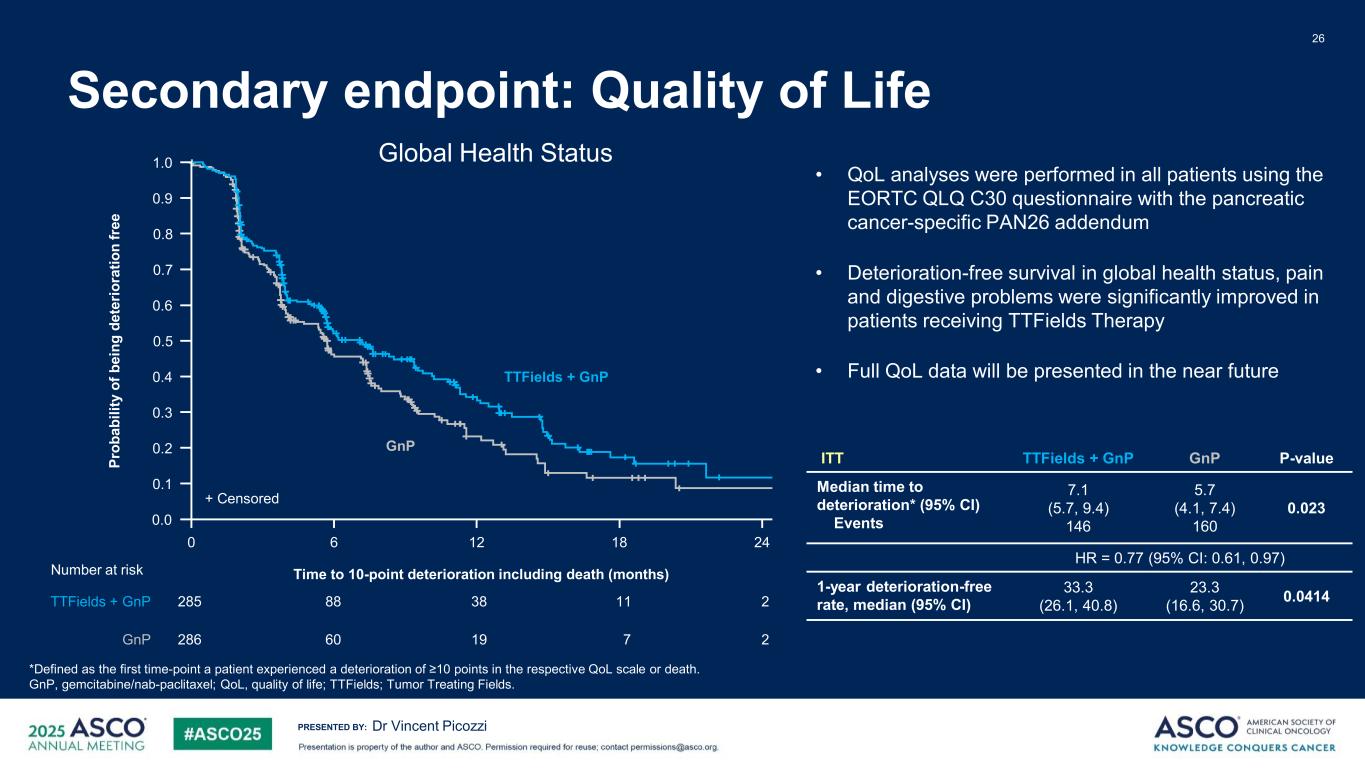
PRESENTED BY: 26 Dr Vincent Picozzi *Defined as the first time-point a patient experienced a deterioration of ≥10 points in the respective QoL scale or death. GnP, gemcitabine/nab-paclitaxel; QoL, quality of life; TTFields; Tumor Treating Fields. ITT TTFields + GnP GnP P-value Median time to deterioration* (95% CI) Events 7.1 (5.7, 9.4) 146 5.7 (4.1, 7.4) 160 0.023 HR = 0.77 (95% CI: 0.61, 0.97) 1-year deterioration-free rate, median (95% CI) 33.3 (26.1, 40.8) 23.3 (16.6, 30.7) 0.0414 • QoL analyses were performed in all patients using the EORTC QLQ C30 questionnaire with the pancreatic cancer-specific PAN26 addendum • Deterioration-free survival in global health status, pain and digestive problems were significantly improved in patients receiving TTFields Therapy • Full QoL data will be presented in the near future + Censored 0 12 186 Time to 10-point deterioration including death (months) 24 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Pr ob ab ili ty o f b ei ng d et er io ra tio n fr ee TTFields + GnP GnP 285 23888 11 286 219 760 Number at risk TTFields + GnP GnP Secondary endpoint: Quality of Life Global Health Status
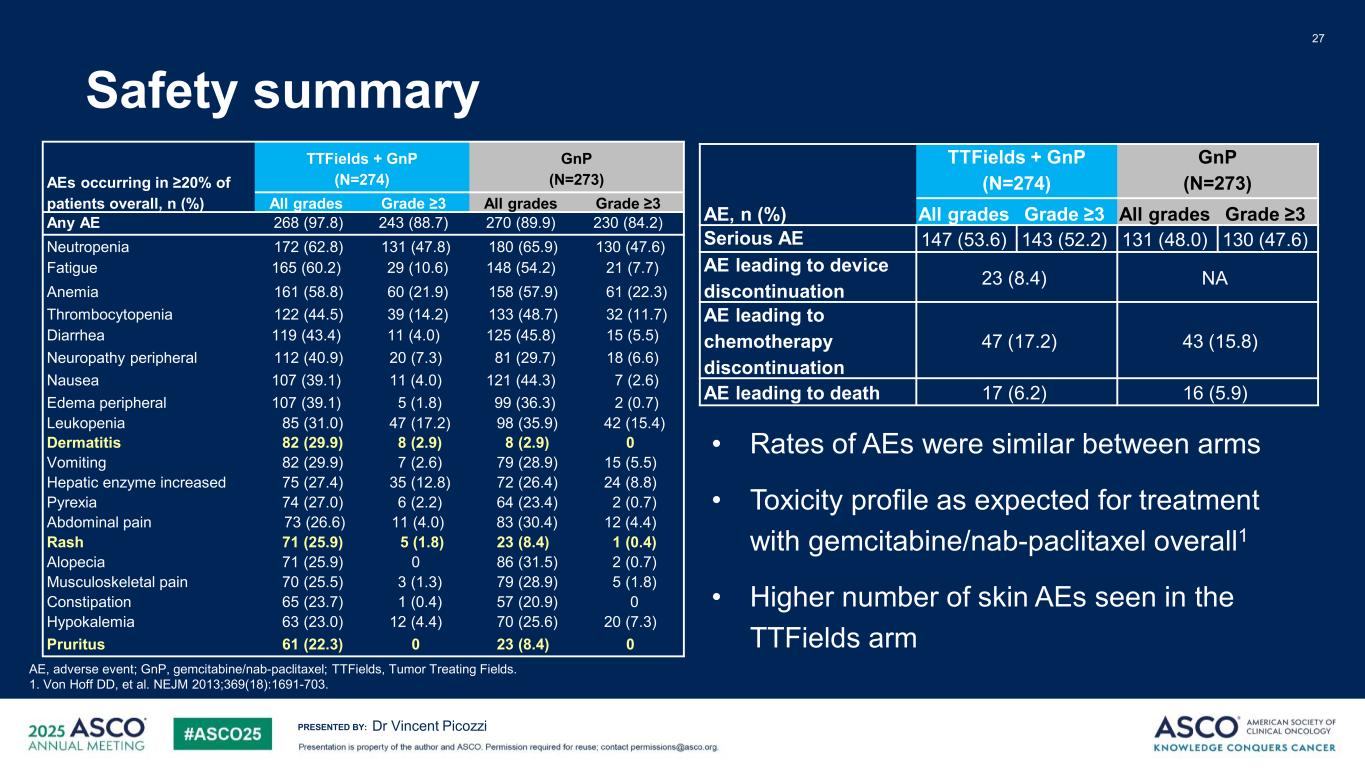
PRESENTED BY: Safety summary 27 Dr Vincent Picozzi AE, adverse event; GnP, gemcitabine/nab-paclitaxel; TTFields, Tumor Treating Fields. 1. Von Hoff DD, et al. NEJM 2013;369(18):1691-703. • Rates of AEs were similar between arms • Toxicity profile as expected for treatment with gemcitabine/nab-paclitaxel overall1 • Higher number of skin AEs seen in the TTFields arm AE, n (%) TTFields + GnP (N=274) GnP (N=273) All grades Grade ≥3 All grades Grade ≥3 Serious AE 147 (53.6) 143 (52.2) 131 (48.0) 130 (47.6) AE leading to device discontinuation 23 (8.4) NA AE leading to chemotherapy discontinuation 47 (17.2) 43 (15.8) AE leading to death 17 (6.2) 16 (5.9) AEs occurring in ≥20% of patients overall, n (%) TTFields + GnP (N=274) GnP (N=273) All grades Grade ≥3 All grades Grade ≥3 Any AE 268 (97.8) 243 (88.7) 270 (89.9) 230 (84.2) Neutropenia 172 (62.8) 131 (47.8) 180 (65.9) 130 (47.6) Fatigue 165 (60.2) 29 (10.6) 148 (54.2) 21 (7.7) Anemia 161 (58.8) 60 (21.9) 158 (57.9) 61 (22.3) Thrombocytopenia 122 (44.5) 39 (14.2) 133 (48.7) 32 (11.7) Diarrhea 119 (43.4) 11 (4.0) 125 (45.8) 15 (5.5) Neuropathy peripheral 112 (40.9) 20 (7.3) 81 (29.7) 18 (6.6) Nausea 107 (39.1) 11 (4.0) 121 (44.3) 7 (2.6) Edema peripheral 107 (39.1) 5 (1.8) 99 (36.3) 2 (0.7) Leukopenia 85 (31.0) 47 (17.2) 98 (35.9) 42 (15.4) Dermatitis 82 (29.9) 8 (2.9) 8 (2.9) 0 Vomiting 82 (29.9) 7 (2.6) 79 (28.9) 15 (5.5) Hepatic enzyme increased 75 (27.4) 35 (12.8) 72 (26.4) 24 (8.8) Pyrexia 74 (27.0) 6 (2.2) 64 (23.4) 2 (0.7) Abdominal pain 73 (26.6) 11 (4.0) 83 (30.4) 12 (4.4) Rash 71 (25.9) 5 (1.8) 23 (8.4) 1 (0.4) Alopecia 71 (25.9) 0 86 (31.5) 2 (0.7) Musculoskeletal pain 70 (25.5) 3 (1.3) 79 (28.9) 5 (1.8) Constipation 65 (23.7) 1 (0.4) 57 (20.9) 0 Hypokalemia 63 (23.0) 12 (4.4) 70 (25.6) 20 (7.3) Pruritus 61 (22.3) 0 23 (8.4) 0
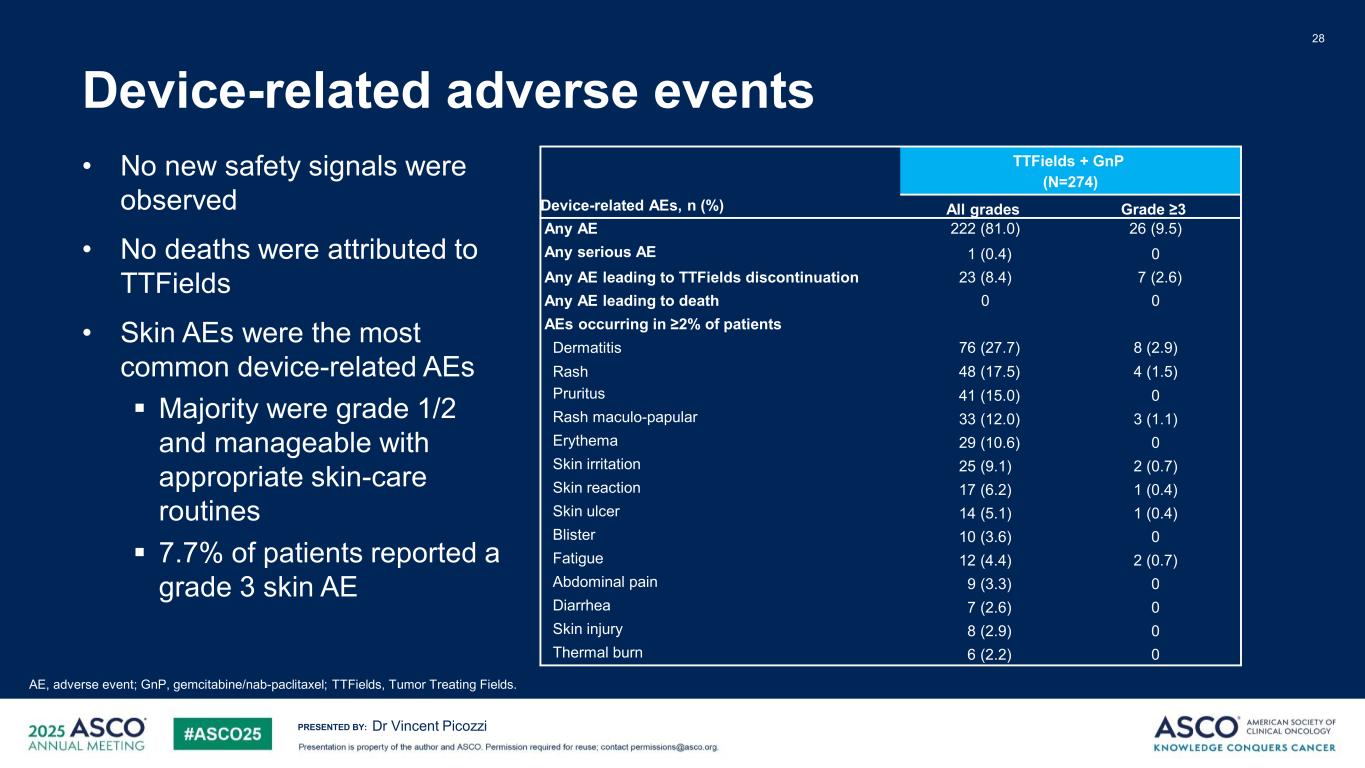
PRESENTED BY: 28 Dr Vincent Picozzi Device-related AEs, n (%) TTFields + GnP (N=274) All grades Grade ≥3 Any AE 222 (81.0) 26 (9.5) Any serious AE 1 (0.4) 0 Any AE leading to TTFields discontinuation 23 (8.4) 7 (2.6) Any AE leading to death 0 0 AEs occurring in ≥2% of patients Dermatitis 76 (27.7) 8 (2.9) Rash 48 (17.5) 4 (1.5) Pruritus 41 (15.0) 0 Rash maculo-papular 33 (12.0) 3 (1.1) Erythema 29 (10.6) 0 Skin irritation 25 (9.1) 2 (0.7) Skin reaction 17 (6.2) 1 (0.4) Skin ulcer 14 (5.1) 1 (0.4) Blister 10 (3.6) 0 Fatigue 12 (4.4) 2 (0.7) Abdominal pain 9 (3.3) 0 Diarrhea 7 (2.6) 0 Skin injury 8 (2.9) 0 Thermal burn 6 (2.2) 0 AE, adverse event; GnP, gemcitabine/nab-paclitaxel; TTFields, Tumor Treating Fields. Device-related adverse events • No new safety signals were observed • No deaths were attributed to TTFields • Skin AEs were the most common device-related AEs Majority were grade 1/2 and manageable with appropriate skin-care routines 7.7% of patients reported a grade 3 skin AE
PRESENTED BY: Key Takeaway Points/Conclusions 29 Survival benefit for patients is supported by significantly improved QoL and pain-free survival* compared with GnP alone Dr Vincent Picozzi The only frequently reported TTFields toxicity was localized skin reactions *as the time to a ≥ 20-point increase from baseline on a patient-reported visual analogue scale for pain or death. GnP, gemcitabine/nab-paclitaxel; LA-PAC, locally advanced pancreatic adenocarcinoma; OS, overall survival; TTFields, Tumor Treating Fields. PANOVA-3 is the first phase 3 trial in patients with unresectable LA-PAC to show an OS benefit over gemcitabine/nab-paclitaxel PANOVA-3 establishes TTFields with GnP as a potential new standard paradigm for unresectable LA-PAC

PRESENTED BY: Tumor Treating Fields With Gemcitabine and Nab-Paclitaxel for Locally Advanced Pancreatic Adenocarcinoma: Randomized, Open-Label, Pivotal Phase III PANOVA-3 Study ascopubs.org QR code here Check out the accompanying podcast, “TTFields in Locally Advanced Pancreatic Adenocarcinoma,” with Drs. Eileen M. O’Reilly and Peter Li, located on the online publication’s main page or at asco.org/podcasts. Dr Vincent Picozzi

PRESENTED BY: 31 Special thank you to all participating patients, their families, and clinical research teams for your commitment and contributions. Dr Vincent Picozzi Acknowledgements Medical writing support for this presentation was provided by Chelsea Higgins, Janin Knop and Christine Mormont of Novocure and Christine el Jundi of Bioscript Group. We thank Prof. Daniel Von Hoff for his contributions to design, development of, and guidance on the trial. We thank Dr. Hani Babiker, chair of the P3 steering committee & co-first author for his unwavering support to this trial and to TTFields. This study was sponsored by Novocure. Editorial support funded by Novocure Thank you to all participating sites around the world Austria: Salzburg Uniklinik fur Innere Medizin, Landes-Krankenhaus Steyr, Medical University of Graz, Klinikum Klagenfurt am Wörthersee; Australia: Greenslopes Oncology, Westmead Hospital, Sydney Adventist Hospital, St. John of God Murdoch Hospital, Monash Health; Belgium: University of Leuven, Research UZ/KU Leuven, Clinique Universutaire Saint Luc - Institut Roi Albert; Brazil: Instituto Ribeiraopretano de Combate ao Cancer, Instituto Brasileiro de Controle do Cancer (IBCC), Hospital de Clínicas de Porto Alegre, COI, Ynova Pesquisa Clinica, Hospital de Caridade de Ijui (HCI), Centro de Pesquisa Clínica Multidisciplinar da Santa Casa de Porto Alegre, Hospital São Lucas da PUCRS, Oncoclinicas Rio de Janeiro S.A.’ Instituto D’Or de Pesquisa e Ensino – Matriz Rio de Janeiro, Instituto D’Or de Pesquisa e Ensino – Filial Salvador (Hospital São Rafael), Instituto D’Or de Pesquisa e Ensino; Canada: Centre Hospitalier Universitaire de Sherbrooke CIUSSS de l’Estrie – CHUS, London Health Science Center London Regional Cancer Program, Centre Hospitalier de l'Universite de Montreal- CHUM CIUSSS de l’Estrie – CHUS; People’s Republic of China: Beijing Cancer Hospital, Jilin Guowen Hospital, Henan Provincial People’s Hospital, Shanghai Changhai Hospital, Xingtai People’s Hospital, The First Affiliated Hospital of Zhengzhou University, First Affiliated Hospital of Xi'an Jiaotong University, Beijing University People's Hospital, Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology, Shanxi Province Cancer Hospital, Tongji Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology, Sun Yat- Sen Memorial Hospital of Sun Yat-Sen University, Guangdong Provincial People's Hospital, Bethune First Hospital of Jilin University, Sir Run Run Shaw Hospital affiliated to Zhejiang University School of Medicine, Linyi Cancer Hospital, Harbin Medical University Cancer Hospital, Beijing Union Medical College Hospital; Croatia: UHC Zagreb; Czech Republic: Olomouc Fakultni Nemocnice, Nemocnice Na Bulovce, General University Hospital in Prague, Nemocnice Novy Jicin, Masaryk Institute of Oncology; France: Institut de Cancérologie de l’Ouest (ICO), Hopital Saint-Antoine, Centre Léon Bérard, Hopital haut-Léveque CHU Bordeaux - Service d'Hépato- Gastroentérologie et d'Oncologie digestive, Strasbourg Oncologie Liberale, Hospital Group Bretagne Sud, Centre Armoricain d'Oncologie – CARIO, Centre de Lutte Contre le Cancer (CLCC) - Centre Paul Strauss; Germany: Klinik München Bogenhausen, Universitätsklinikum Ulm, Carl-von-Basedow-Klinikum Saalekreis, Medizinische Hochschule Hannover, Klinik fur Gastroenterologie, Hepatologie und Endokrinologie, Bonifatius Hospital Hematology and Oncology Lingen, Klinikum Chemnitz gGmbH; Hong Kong: Queen Mary Hospital; Hungary: Bacs-Kiskun County Teaching Hospital, Jasz-Nagykun-Szolnok Megyei Hetenyi Geza, National Institute of Oncology, Tolna County Balassa Janos Hospital, Békés Megyei Pándy Kálmán Kórháza Megyei Onkológiai Központja; Israel: Haifa Rambam Medical Center, Tel Aviv Sourasky Medical Center, Hadassah Medical Center, Rabin Medical Center, Sheba Pancreatic Cancer Center; Italy:Università Campus Bio-Medico, Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Ospedale Civile Ss. Antonio e Biagio e Cesare Arrigo, Ospedale San Giovanni, Azienda Ospedaliero-Universitaria Careggi; Mexico: Accelerium Clinical Research, Centro Potosino de Investigación Médica, Mediadvance Clinical, Centro de Investigación Médica Aguascalientes (CIMA), FAICIC Clinical Research, Clinstile S.A de C.V., Centro de Estudios de Alta Especialidad de Sinaloa, Phylasis Clinicas Research, Hospitales Star Medica, PCR Toluca Corporativo Hospital Satelite, Clinica Integral Internacional de Oncologia, Hospital Angeles - Centro Medico del Potosi, Practice of Centro Hemato Oncologico Privado, Hospital Universitario "Dr. Jose Eleuterio Gonzalez“; Poland: M.Sklodowska-Curie Inst.of Oncol., Klinika Onkologii Uniwersytetu Medyczneg, Mrukmed Medical Center, Uniwersytecki Szpital Kliniczny we Wrocławiu, Oncology and Radiotherapy Clinic University Clinical Center Non-Invasive Medicine Center; South Korea: Chonnam National University Hwasun Hospital, Dong-A University Hospital, Gachon University Gil Hospital, Inha University Hospital, Keimyung University, Dongsan hospital, Korea University Guro Hospital, National Cancer Center, Samsung Medical Center, Seoul National University Bundang Hospital, The Catholic University of Korea, Seoul St. Mary's Hospital, Severance Hospital, Ajou University Hospital, CHA Bundang Medical Center; Spain: Vall d'Hebron Barcelona, Madrid, HM Hospitales CIOCC, Instituto Oncologico Dr. Rosell, Hospital Universitario Ramon Y Cajal Carretera de Colmenar Viejo, Hospital Regional Universitario de Málaga, Marqués de Valdecilla University Hospital, Elche Hospital General Universitario, Inst. Valenciano de Oncologia, Pamplona Clinica Universidad de Navarra,; Switzerland: Fribourg Hôpital, Winterthur Kantonsspital; USA: Tennessee Oncology, Cedars Sinai, Ochsner Clinic Foundation, Associated Neurologists of Southern Connecticut, University of Minnesota, The University of Arizona Cancer Center, Texas Oncology, Comprehensive Cancer Center of Nevada, Karmanos Cancer Center, Norton Cancer Institute, Beth Israel Deaconess Medical Center (BIDMC), Erlanger Health System, West Virginia University, Banner MD Anderson Cancer Center, Seattle Cancer Care Alliance, Pacific Cancer Medical Center, Virginia Mason Medical Center, Novant Health Oncology Specialists, HCA Midwest Division of Sarah Cannon Research Institute, Laura and Issac Perimutter Cancer Center at NYU Langone, Mount Sinai Comprehensive Cancer Center (MSCCC), Vita Medical Associates, Loyola University Chicago, University of Kansas Cancer Center (KUCC), Boca Raton Clinical Research Medical Center, Florida Hospital Tampa, Dignity Health Cancer Institute, Florida Cancer Specialists, Florida Cancer Specialists, Renown Regional Medical Center, Piedmont Cancer Institute (PCI), Nebraska Methodist Hospital, Geisinger Medical Center, North Shore University Health, University of Maryland Comprehensive Cancer Center, University of Oklahoma Health Sciences Center, Arizona Oncology Associates, Oncology and Hematology Associates of Southwest Virginia, Texas Oncology – Bedford, Texas Oncology – Tyler, Texas Oncology - El Paso Cancer, Baylor, Scott and White Medical Center, Cancer & Hematology Centers of Western Michigan, Florida Hospital Cancer Institute, Houston Methodist Cancer Center and Institute of Academic Medicine, Cotton O’Neil Cancer Center Stormont Vail Health Care, Illinois Cancer Specialist, Texas Oncology - Beaumont Mamie, Maryland Oncology Hematology, Willamette Valley Cancer Institution, NY Presbyterian Queens, Infirmary Health, White Plains Hospital, Vista Oncology Group, Sutter Institute for Medical Research, Rush University Medical Center, Methodist Richardson Cancer Center, UMass Memorial Hospital, Lynn Cancer Institute, Boca Raton Regional Hospital, Grandview Health, General Physician Cancer Care – Williamsville, Hematology Oncology Central Maine Medical Center (CMMC), Cancer Specialist of North Florida, Providence Medical Foundation, Gabrail Cancer Center Research, Toledo Clinic Cancer Center, OptumCare, Tennessee Cancer Specialists, Princeton Radiation Oncology (Regional Cancer Care Associates LLC), Bassett Cancer Institute, Ridley Tree Cancer Center, Mayo Clinic Jacksonville, St. Elizabeth Healthcare, St. Helena Healthcare - Martin O'Neil Cancer Center Scan to access JCO publication

[Confidential] © 2025 Novocure GmbH © 2025 Novocure GmbH 32

© 2025 Novocure GmbH 33 path to regulatory approval Topline analysis Data presentation at ASCO Data publication in JCO FDA submission 2025 2026 TUV and PMDA submissions Commercial launch Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4Q4 REGULATORY REVIEW PERIODSUBMISSION ✓ ✓ ✓ REGULATORY REVIEW PERIODSUBMISSION
© 2025 Novocure GmbH 34 PANOVA-4 builds on promising clinical data for TTFields therapy with PD-(L)1 inhibitors PANOVA-4 phase 2 study delves deeper into TTFields therapy’s capabilities in pancreatic cancer • Studying TTFields therapy with atezolizumab + nab-paclitaxel / gemcitabine in metastatic pancreatic cancer • Primary endpoint: disease control rate • Fully enrolled, 12-month patient follow-up ongoing • Top-line data anticipated H1 2026

© 2025 Novocure GmbH 35 2025 key objectives, unlocking TTFields potential DRIVING COMMERCIAL ADOPTION ADVANCING CLINICAL PIPELINE DELIVERING PRODUCT INNOVATION NSCLC: drive global active patient growth and pursue reimbursement NSCLC: CE Mark achieved, launch underway in Germany • NSCLC: PMDA approval and launch in Japan PANOVA-3: present and publish data • PANOVA-3: submit to FDA, CE Mark and PMDA • METIS: submit to FDA, publish clinical data • TRIDENT and PANOVA-4: patient follow up; prepare for H1 2026 top-line data Launch MyNovocure patient app • New array utilized by every Optune Gio patient • Advance next gen torso array

[Confidential] © 2025 Novocure GmbH © 2025 Novocure GmbH 36 Q&A Investor Event May 31, 2025 – Chicago

PRESENTED BY: 37 Appendix Dr Vincent Picozzi
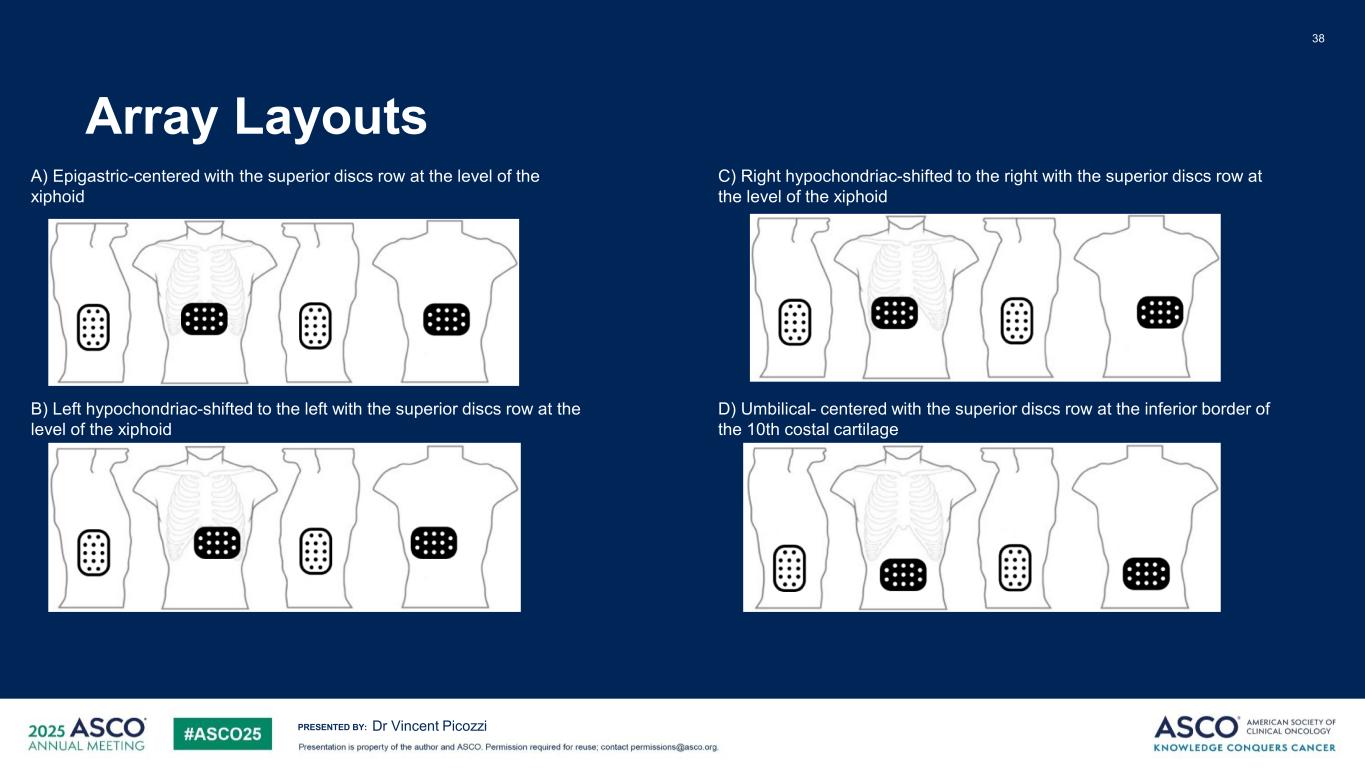
PRESENTED BY: Array Layouts 38 Dr Vincent Picozzi A) Epigastric-centered with the superior discs row at the level of the xiphoid B) Left hypochondriac-shifted to the left with the superior discs row at the level of the xiphoid C) Right hypochondriac-shifted to the right with the superior discs row at the level of the xiphoid D) Umbilical- centered with the superior discs row at the inferior border of the 10th costal cartilage
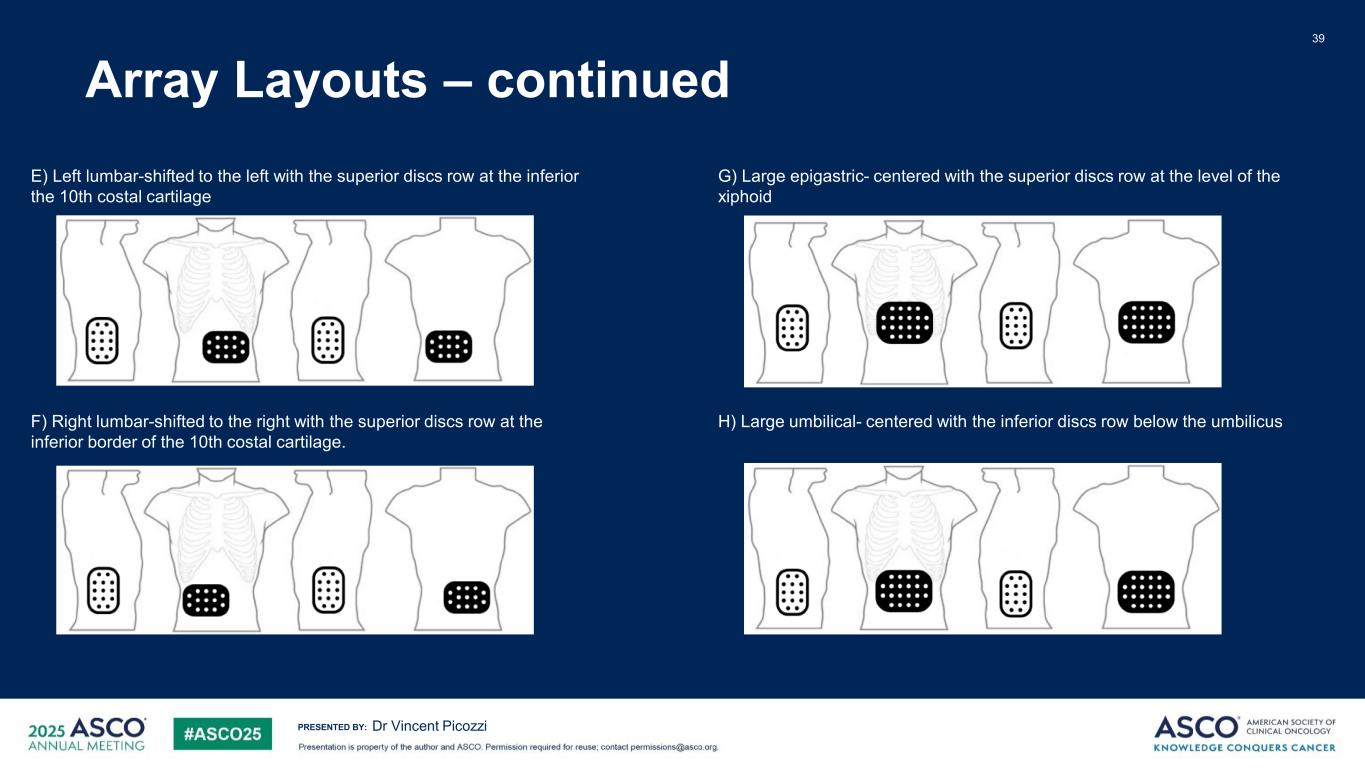
PRESENTED BY: Array Layouts – continued 39 Dr Vincent Picozzi E) Left lumbar-shifted to the left with the superior discs row at the inferior the 10th costal cartilage F) Right lumbar-shifted to the right with the superior discs row at the inferior border of the 10th costal cartilage. G) Large epigastric- centered with the superior discs row at the level of the xiphoid H) Large umbilical- centered with the inferior discs row below the umbilicus
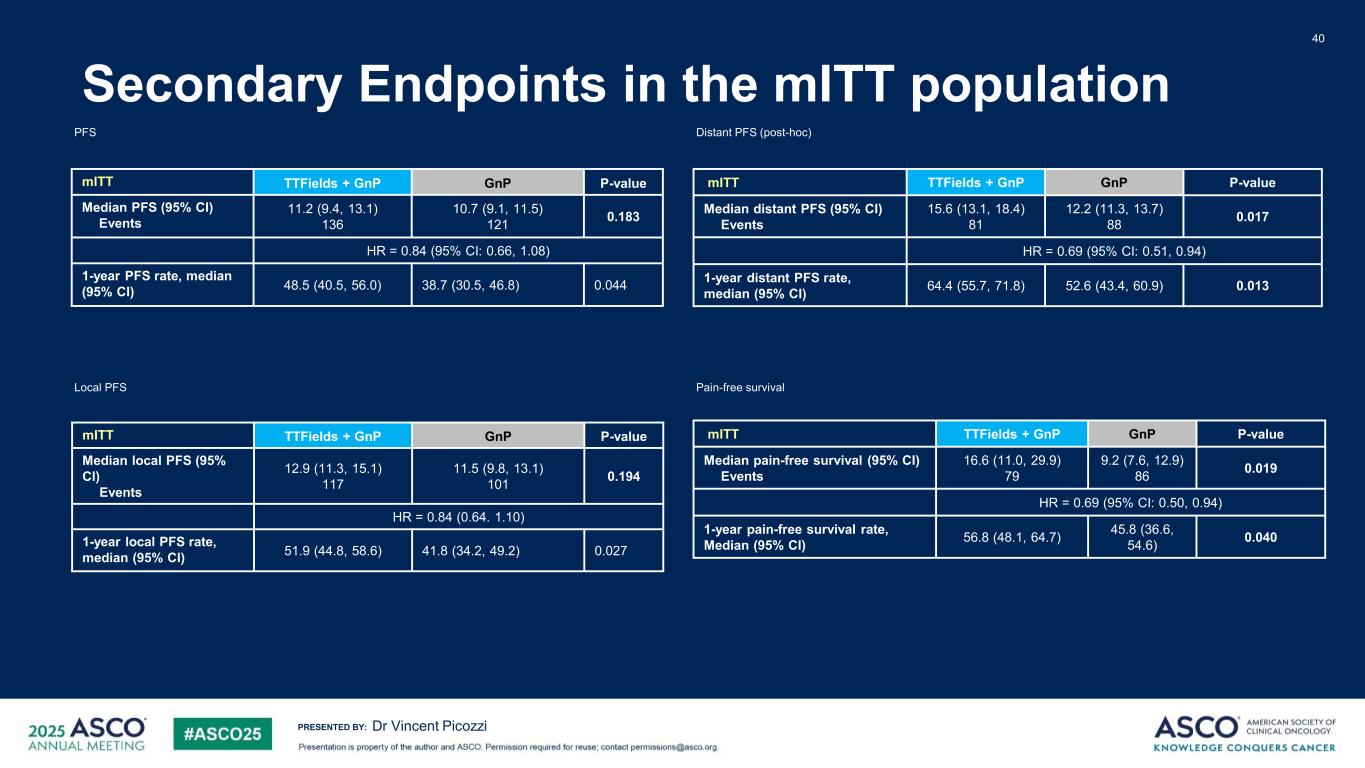
PRESENTED BY: Secondary Endpoints in the mITT population 40 Dr Vincent Picozzi mITT TTFields + GnP GnP P-value Median PFS (95% CI) Events 11.2 (9.4, 13.1) 136 10.7 (9.1, 11.5) 121 0.183 HR = 0.84 (95% CI: 0.66, 1.08) 1-year PFS rate, median (95% CI) 48.5 (40.5, 56.0) 38.7 (30.5, 46.8) 0.044 PFS Local PFS mITT TTFields + GnP GnP P-value Median local PFS (95% CI) Events 12.9 (11.3, 15.1) 117 11.5 (9.8, 13.1) 101 0.194 HR = 0.84 (0.64. 1.10) 1-year local PFS rate, median (95% CI) 51.9 (44.8, 58.6) 41.8 (34.2, 49.2) 0.027 mITT TTFields + GnP GnP P-value Median distant PFS (95% CI) Events 15.6 (13.1, 18.4) 81 12.2 (11.3, 13.7) 88 0.017 HR = 0.69 (95% CI: 0.51, 0.94) 1-year distant PFS rate, median (95% CI) 64.4 (55.7, 71.8) 52.6 (43.4, 60.9) 0.013 Distant PFS (post-hoc) mITT TTFields + GnP GnP P-value Median pain-free survival (95% CI) Events 16.6 (11.0, 29.9) 79 9.2 (7.6, 12.9) 86 0.019 HR = 0.69 (95% CI: 0.50, 0.94) 1-year pain-free survival rate, Median (95% CI) 56.8 (48.1, 64.7) 45.8 (36.6, 54.6) 0.040 Pain-free survival
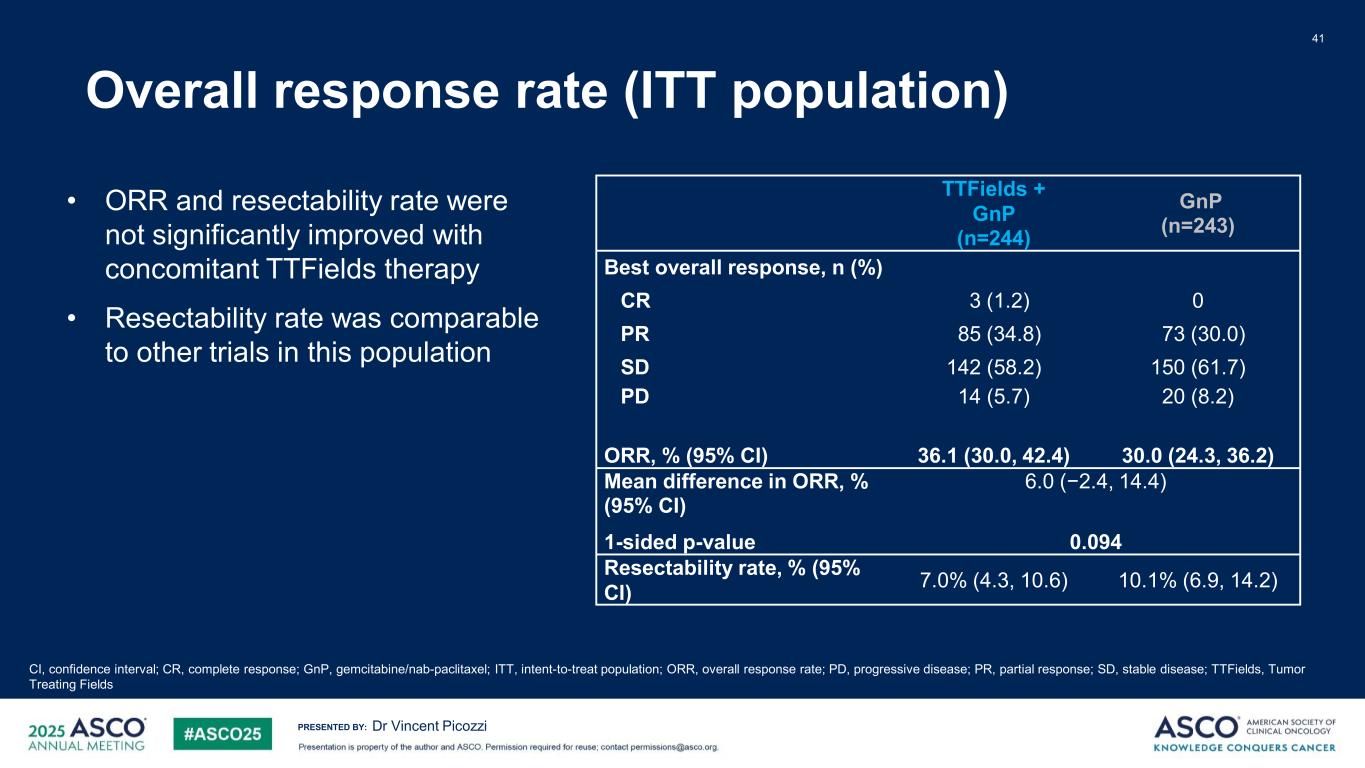
PRESENTED BY: Overall response rate (ITT population) 41 Dr Vincent Picozzi TTFields + GnP (n=244) GnP (n=243) Best overall response, n (%) CR 3 (1.2) 0 PR 85 (34.8) 73 (30.0) SD 142 (58.2) 150 (61.7) PD ORR, % (95% CI) 14 (5.7) 36.1 (30.0, 42.4) 20 (8.2) 30.0 (24.3, 36.2) Mean difference in ORR, % (95% CI) 1-sided p-value 6.0 (−2.4, 14.4) 0.094 Resectability rate, % (95% CI) 7.0% (4.3, 10.6) 10.1% (6.9, 14.2) CI, confidence interval; CR, complete response; GnP, gemcitabine/nab-paclitaxel; ITT, intent-to-treat population; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; TTFields, Tumor Treating Fields • ORR and resectability rate were not significantly improved with concomitant TTFields therapy • Resectability rate was comparable to other trials in this population
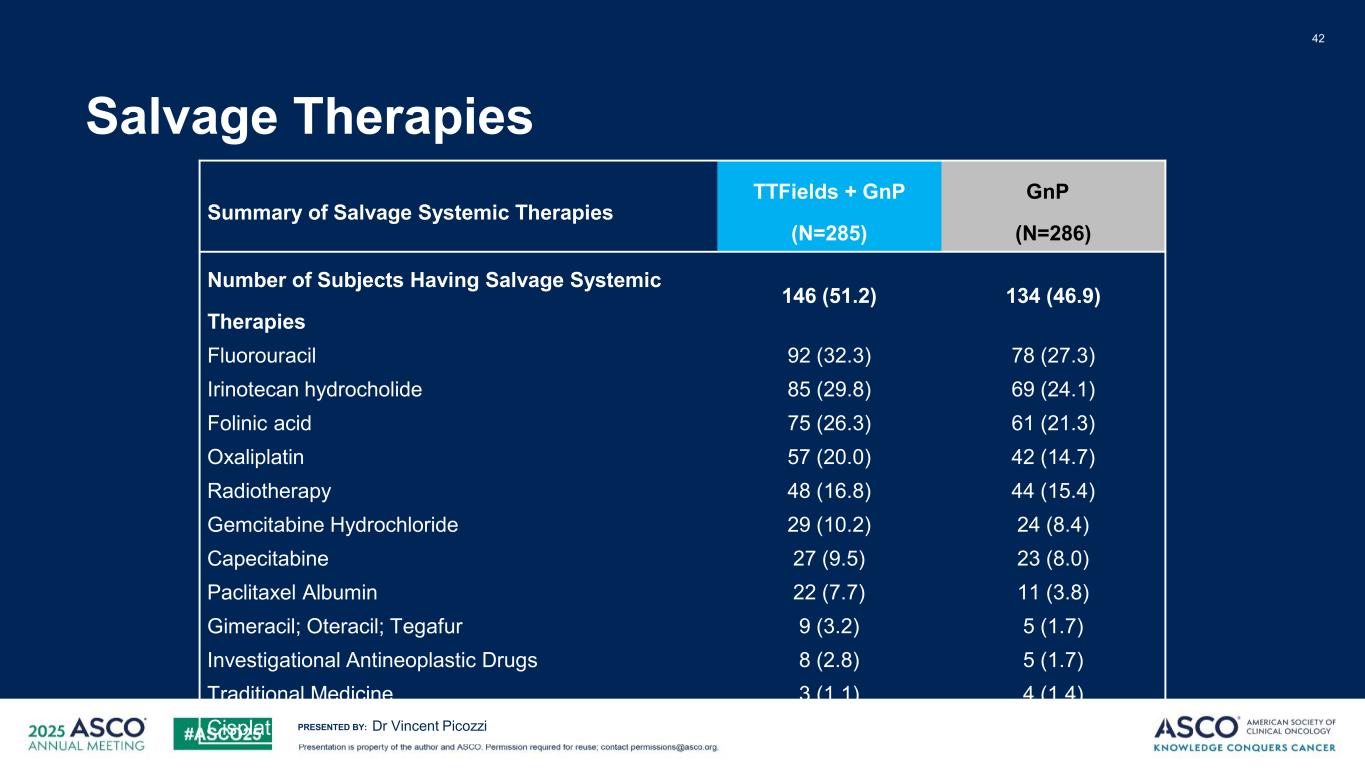
PRESENTED BY: Salvage Therapies 42 Dr Vincent Picozzi Summary of Salvage Systemic Therapies TTFields + GnP (N=285) GnP (N=286) Number of Subjects Having Salvage Systemic Therapies 146 (51.2) 134 (46.9) Fluorouracil 92 (32.3) 78 (27.3) Irinotecan hydrocholide 85 (29.8) 69 (24.1) Folinic acid 75 (26.3) 61 (21.3) Oxaliplatin 57 (20.0) 42 (14.7) Radiotherapy 48 (16.8) 44 (15.4) Gemcitabine Hydrochloride 29 (10.2) 24 (8.4) Capecitabine 27 (9.5) 23 (8.0) Paclitaxel Albumin 22 (7.7) 11 (3.8) Gimeracil; Oteracil; Tegafur 9 (3.2) 5 (1.7) Investigational Antineoplastic Drugs 8 (2.8) 5 (1.7) Traditional Medicine 3 (1.1) 4 (1.4) Cisplatin 3 (1.1) 2 (0.7)
PRESENTED BY: Limitations 43 • Investigator's assesment of CT scans for response determination • While protocol included a clear definition of resectability at baseline, there was no such guidance for follow up visits • Gender imbalance between both arms • High discontinuation rate within the first month in both arms • The median OS in the control arm is lower than in other phase 2 and 3 trials, albeit in line with RWE data • Open label trial Dr Vincent Picozzi