| © Rhythm® Pharmaceuticals, Inc. All rights reserved. ® November 7, 2025 Rhythm Pharmaceuticals |
| ® 2 On Today’s Call • David Connolly, VP, Investor Relations and Corporate Communications • David Meeker, MD, Chair, President and Chief Executive Officer • Alicia Fiscus, SVP, Head of Regulatory • Hunter Smith, Chief Financial Officer |
| ® 3 This presentation and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the safety, efficacy, potential benefits of, and clinical design or progress of any of our products or product candidates at any dosage or in any indication, including, setmelanotide, bivamelagon, and RM-718; the potential use of setmelanotide in patients with acquired hypothalamic obesity; our expectations surrounding potential regulatory submissions, progress, or approvals and timing thereof for any of our product candidates including in relation to the PDUFA goal date for the sNDA for setmelanotide in acquired hypothalamic obesity; our business strategy and plans, including regarding commercialization of setmelanotide and our other product candidates and the commercial growth of IMCIVREE; the estimated market size and addressable population for our drug products; the announcement of data from our clinical trials, including our Phase 3 trial evaluating setmelanotide for patients with acquired hypothalamic obesity, the substudy evaluating setmelanotide for patients with congenital hypothalamic obesity, the Phase 3 EMANATE trial evaluating setmelanotide in genetically caused MC4R pathway diseases, our Phase 2 and the anticipated Phase 3 trial evaluating the oral MC4R agonist bivamelagon in acquired hypothalamic obesity, Part C of the Phase 1 trial evaluating RM-718, and the open-label Phase 2 trial evaluating setmelanotide in patients with Prader-Willi syndrome; the ongoing enrollment in our clinical trials; existing or future collaboration agreements; our anticipated financial performance and financial position for any period of time, including estimated Non-GAAP Operating Expenses for 2025; and the sufficiency of our cash, cash equivalents and short-term investments to fund our operations for at least 24 months; and the timing of any of the foregoing. Statements using words such as “expect”, “anticipate”, “believe”, “may”, “will”, “aim” and similar terms are also forward-looking statements. Such statements are subject to numerous risks and uncertainties, including, but not limited to, our ability to enroll patients in clinical trials, the design and outcome of clinical trials, the ability to achieve necessary regulatory approvals, risks associated with data analysis and reporting, failure to identify and develop additional product candidates, unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, risks associated with the laws and regulations governing our international operations and the costs of any related compliance programs, the impact of competition, risks relating to product liability lawsuits, inability to maintain collaborations, or the failure of these collaborations, our reliance on third parties, risks relating to intellectual property, our ability to hire and retain necessary personnel, general economic conditions, risks related to internal control over financial reporting, and the other important factors discussed under the caption “Risk Factors” in our Form 10-Q for the quarter ended September 30, 2025 and our other filings with the Securities and Exchange Commission. Except as required by law, we undertake no obligations to make any revisions to the forward-looking statements contained in this press release or to update them to reflect events or circumstances occurring after the date of this press release, whether as a result of new information, future developments or otherwise. Forward-looking Statements |
| ® 4 Timeline of Information Requests and Responses Oct. 20 • FDA requests additional sensitivity analyses Oct. 27 • Rhythm submits response Nov. 6 • FDA notifies Rhythm that October 27th response deemed a major amendment • PDUFA goal date extended from December 20, 2025 to March 20, 2026 |
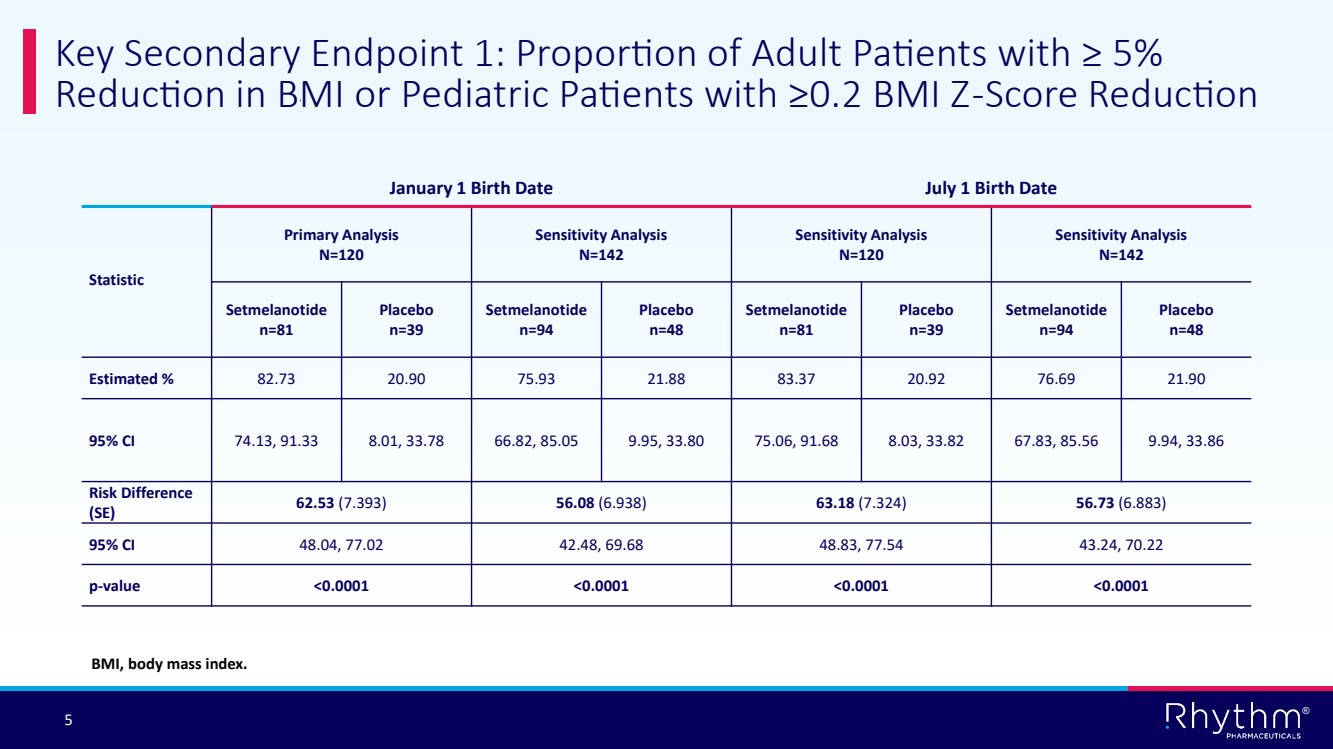
| ® 5 Key Secondary Endpoint 1: Proportion of Adult Patients with ≥ 5% Reduction in BMI or Pediatric Patients with ≥0.2 BMI Z-Score Reduction January 1 Birth Date July 1 Birth Date Statistic Primary Analysis N=120 Sensitivity Analysis N=142 Sensitivity Analysis N=120 Sensitivity Analysis N=142 Setmelanotide n=81 Placebo n=39 Setmelanotide n=94 Placebo n=48 Setmelanotide n=81 Placebo n=39 Setmelanotide n=94 Placebo n=48 Estimated % 82.73 20.90 75.93 21.88 83.37 20.92 76.69 21.90 95% CI 74.13, 91.33 8.01, 33.78 66.82, 85.05 9.95, 33.80 75.06, 91.68 8.03, 33.82 67.83, 85.56 9.94, 33.86 Risk Difference (SE) 62.53 (7.393) 56.08 (6.938) 63.18 (7.324) 56.73 (6.883) 95% CI 48.04, 77.02 42.48, 69.68 48.83, 77.54 43.24, 70.22 p-value <0.0001 <0.0001 <0.0001 <0.0001 BMI, body mass index. |
| ® 6 Questions |