| ® © Rhythm® Pharmaceuticals, Inc. All rights reserved. December 11, 2025 Rhythm Pharmaceuticals Positive Preliminary Results from Phase 2 Trial Evaluating Setmelanotide in Patients with Prader-Willi Syndrome |
| 2 ® This presentation and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the potential, safety, efficacy of setmelanotide, RM-718 and other product candidates; clinical design, enrollment, or progress, and preliminary, interim and final data readouts; potential regulatory submissions, approvals and timing thereof of setmelanotide, RM-718 and other product candidates; the potential benefits of any of the Company’s products or product candidates for any specific disease indication or at any dosage, including the potential benefits of setmelanotide or RM-718 for patients with PWS, BBS or POMC, PCSK1, or LEPR deficiency; expectations surrounding pending and potential regulatory submissions and approvals, including within the United States, the EU and other regions; business strategy and plans, including regarding commercialization of setmelanotide in the United States, the EU and other regions; our participation in upcoming events and presentations; and the timing of any of the foregoing. Statements using words such as “expect”, “anticipate”, “believe”, “may”, “will” and similar terms are also forward-looking statements. Such statements are subject to numerous risks, uncertainties, including, but not limited to, our ability to enroll patients in clinical trials, the design and outcome of clinical trials, the impact of competition, the ability to achieve or obtain necessary regulatory approvals, risks associated with data analysis and reporting, our ability to successfully commercialize setmelanotide, our liquidity and expenses, our ability to retain our key employees and consultants, and to attract, retain and motivate qualified personnel, and general economic conditions, and the other important factors, including those discussed under the caption “Risk Factors” in Rhythm’s Quarterly Report on Form 10-Q for the three months ended September 30, 2025 and our other filings with the Securities and Exchange Commission. Except as required by law, we undertake no obligations to make any revisions to the forward-looking statements contained in this release or to update them to reflect events or circumstances occurring after the date of this release, whether as a result of new information, future developments or otherwise. Industry and Other Data Unless otherwise indicated, information contained in this presentation concerning our industry and the markets in which Rhythm operates, including its general expectations, market position and market opportunity, is based on its management’s estimates and research, as well as industry and general publications and research, surveys and studies conducted by third parties. While we believe the information from these third-party publications, research, surveys and studies is reliable, it does not guarantee the accuracy or completeness of such information, and Rhythm has not independently verified this information. Management’s estimates are derived from publicly available information, their knowledge of the company's industry and their assumptions based on such information and knowledge, which they believe to be reasonable. This data involves a number of assumptions and limitations which are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in our periodic reports filed with the Securities and Exchange Commission under the captions “Cautionary Note Regarding Forward Looking Statements,” “Summary Risk Factors” and “Risk Factors.” These and other factors could cause Rhythm’s future performance and market expectations to differ materially from its assumptions and estimates. Forward-looking Statements |
| 3 ® On Today’s Call David Meeker, MD Chairman, President & Chief Executive Officer, Rhythm Pharmaceuticals Jennifer Miller, MD Professor of Pediatric Endocrinology, University of Florida |
| 4 ® David Meeker, MD Chairman, President & Chief Executive Officer |
| 5 ® Potential therapeutic benefit with BMI, hyperphagia reductions observed in patients with Prader-Willi syndrome (PWS) treated with setmelanotide at Month 3 (n=8) and Month 6 (n=5) Promising preliminary results supportive of Phase 3, registrational trial of setmelanotide in PWS Initiated Part D in Phase 1 study to evaluate RM-718 in patients with Prader-Willi syndrome Promising Phase 2 Preliminary Results in Patients with Prader-Willi Syndrome |
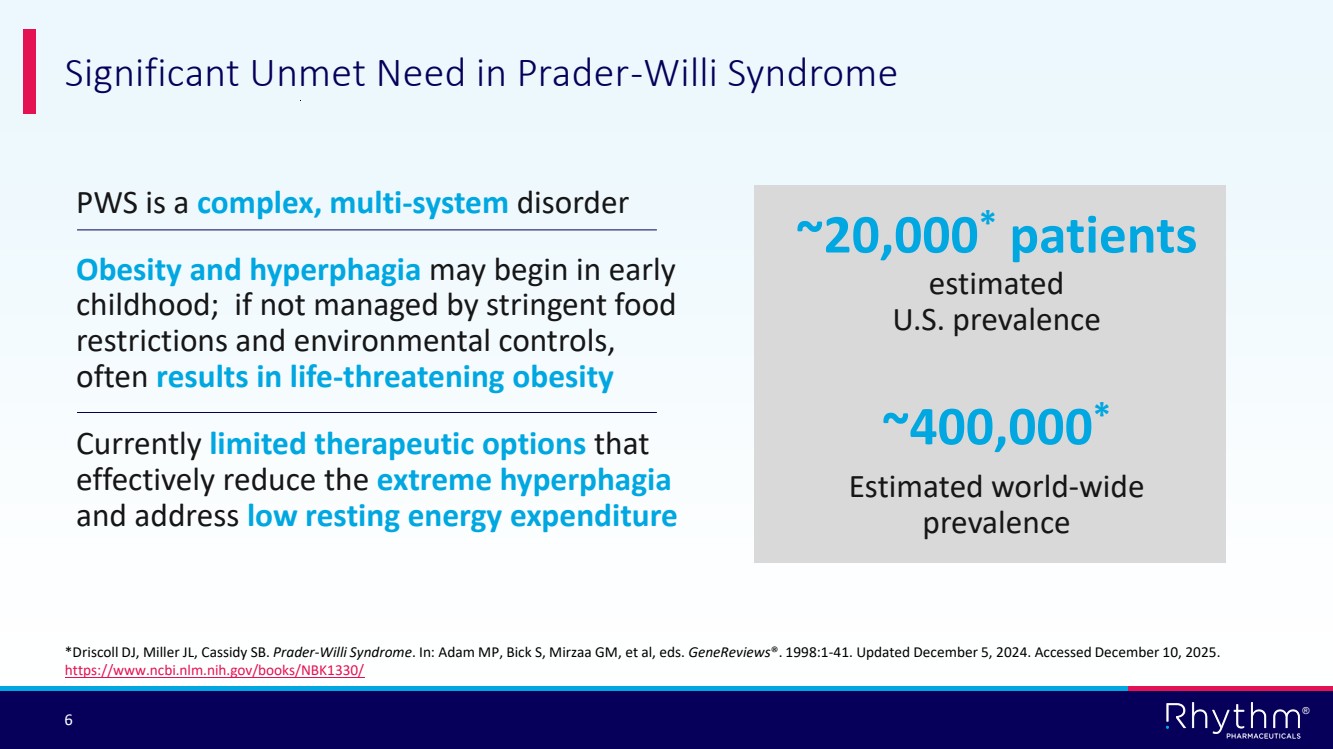
| 6 ® PWS is a complex, multi-system disorder Obesity and hyperphagia may begin in early childhood; if not managed by stringent food restrictions and environmental controls, often results in life-threatening obesity Currently limited therapeutic options that effectively reduce the extreme hyperphagia and address low resting energy expenditure Significant Unmet Need in Prader-Willi Syndrome ~20,000* patients estimated U.S. prevalence ~400,000* Estimated world-wide prevalence *Driscoll DJ, Miller JL, Cassidy SB. Prader-Willi Syndrome. In: Adam MP, Bick S, Mirzaa GM, et al, eds. GeneReviews®. 1998:1-41. Updated December 5, 2024. Accessed December 10, 2025. https://www.ncbi.nlm.nih.gov/books/NBK1330/ |
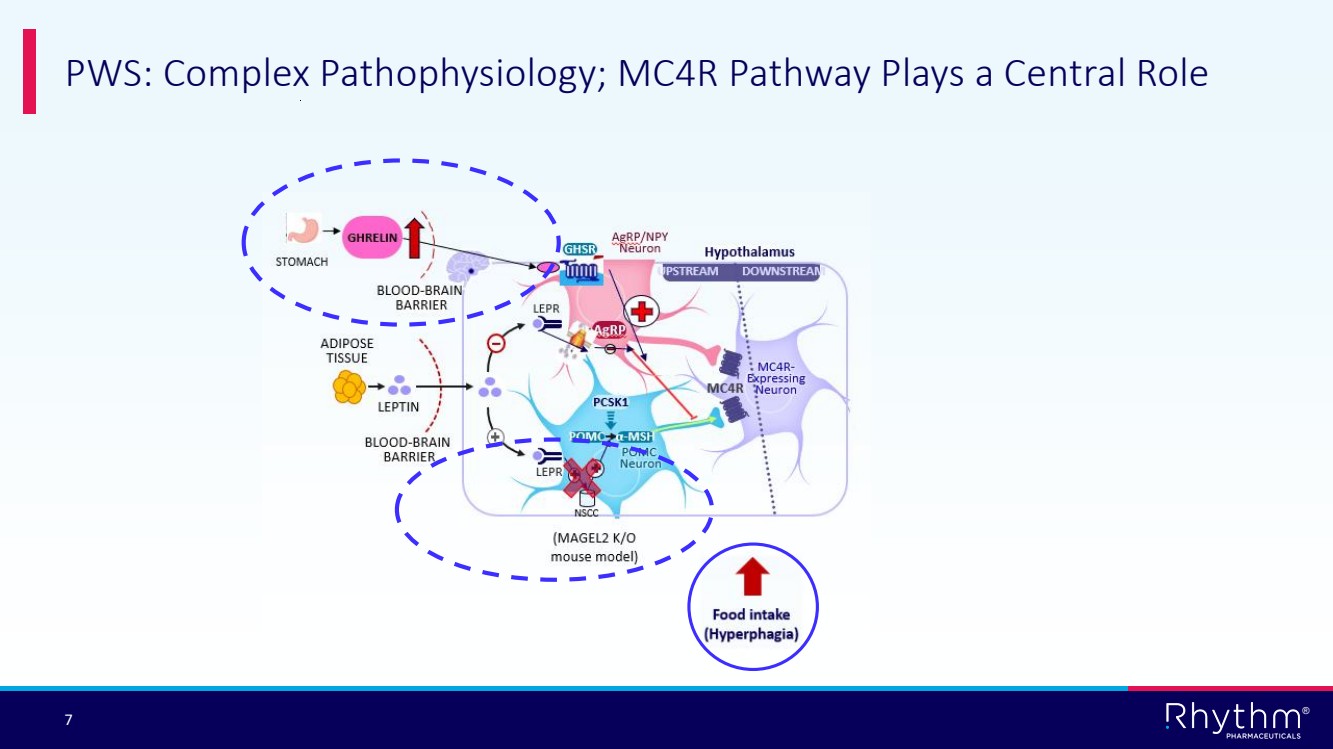
| 7 ® PWS: Complex Pathophysiology; MC4R Pathway Plays a Central Role |
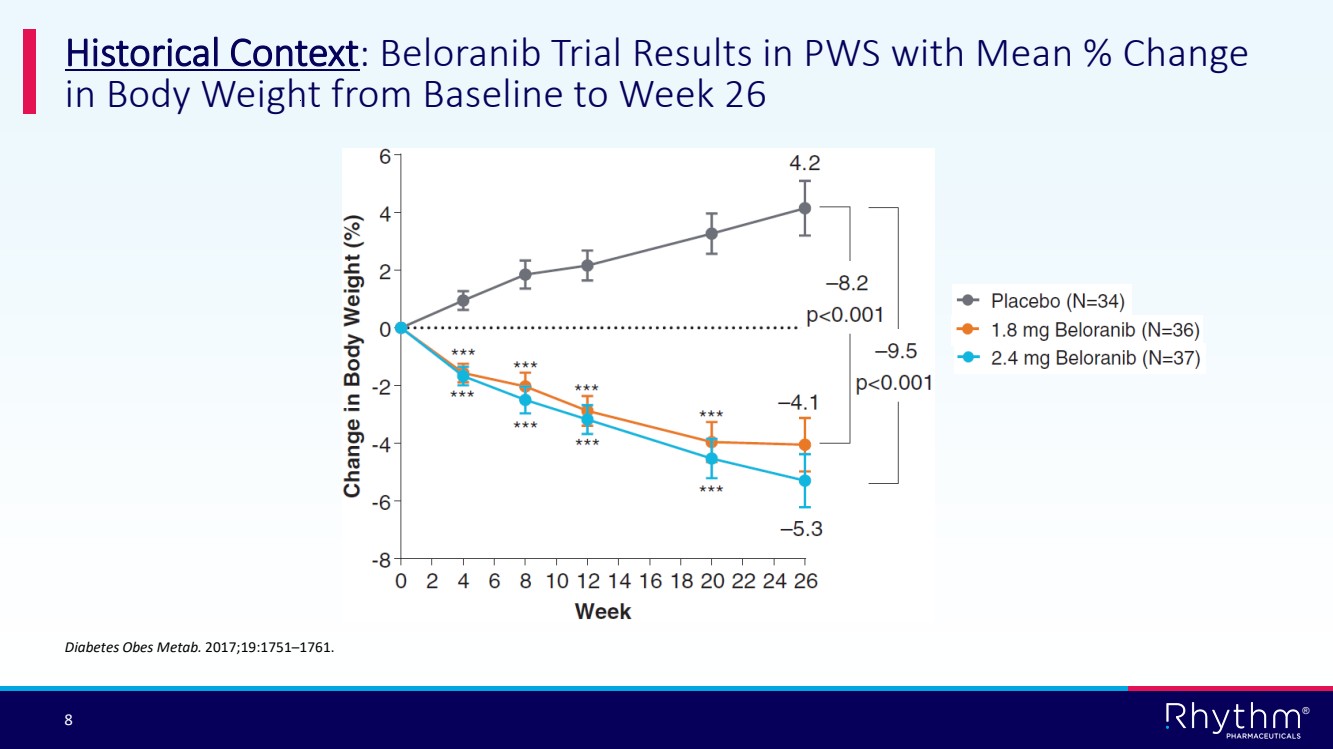
| 8 ® Historical Context: Beloranib Trial Results in PWS with Mean % Change in Body Weight from Baseline to Week 26 Diabetes Obes Metab. 2017;19:1751–1761. |
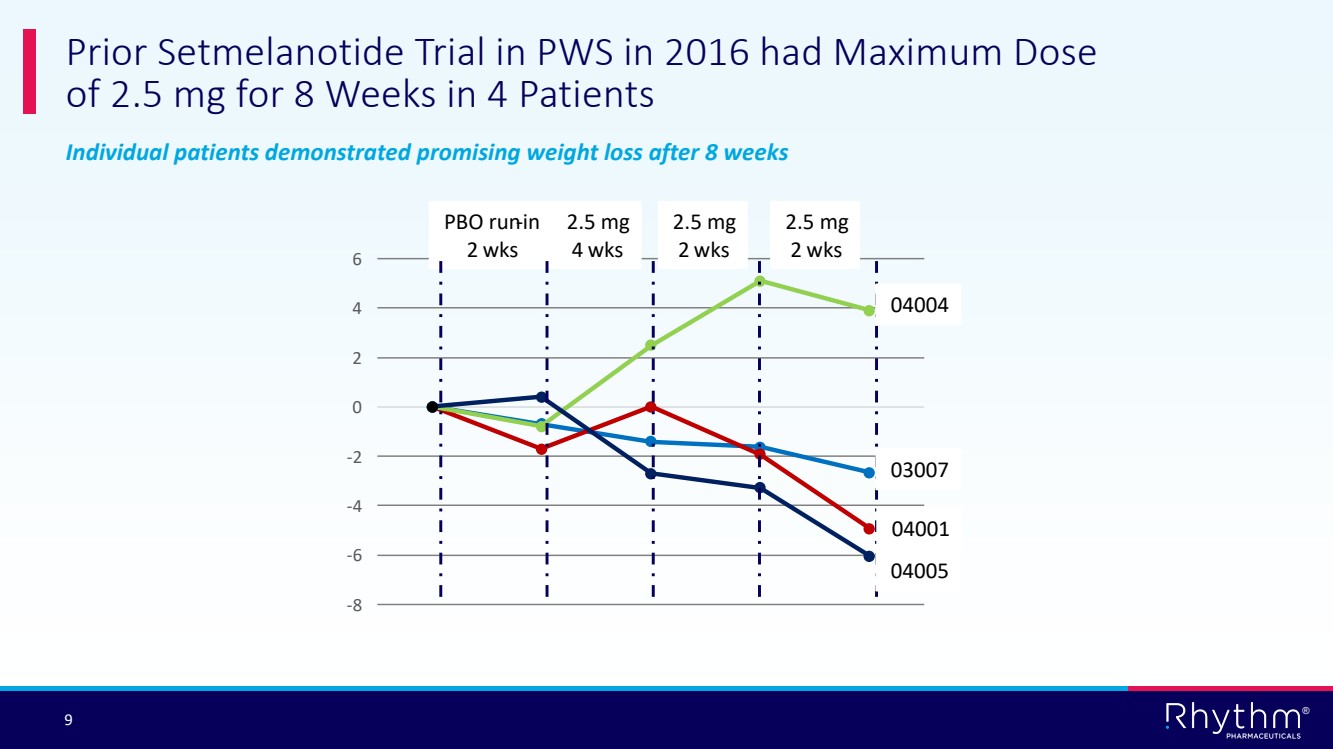
| 9 ® Individual patients demonstrated promising weight loss after 8 weeks Prior Setmelanotide Trial in PWS in 2016 had Maximum Dose of 2.5 mg for 8 Weeks in 4 Patients -8 -6 -4 -2 0 2 4 6 PBO run-in 2 wks 2.5 mg 4 wks 2.5 mg 2 wks 2.5 mg 2 wks 04005 03007 04004 04001 |
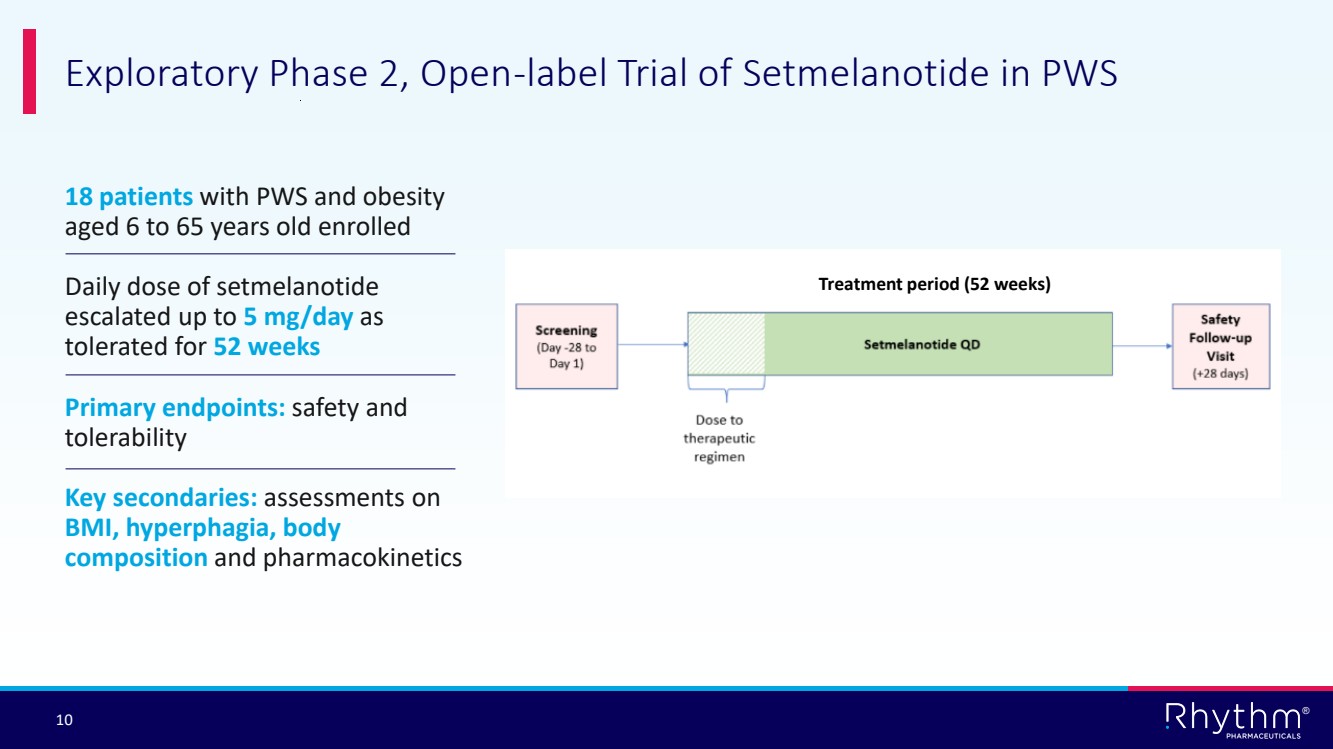
| 10 ® 18 patients with PWS and obesity aged 6 to 65 years old enrolled Daily dose of setmelanotide escalated up to 5 mg/day as tolerated for 52 weeks Primary endpoints: safety and tolerability Key secondaries: assessments on BMI, hyperphagia, body composition and pharmacokinetics Exploratory Phase 2, Open-label Trial of Setmelanotide in PWS Treatment period (52 weeks) |
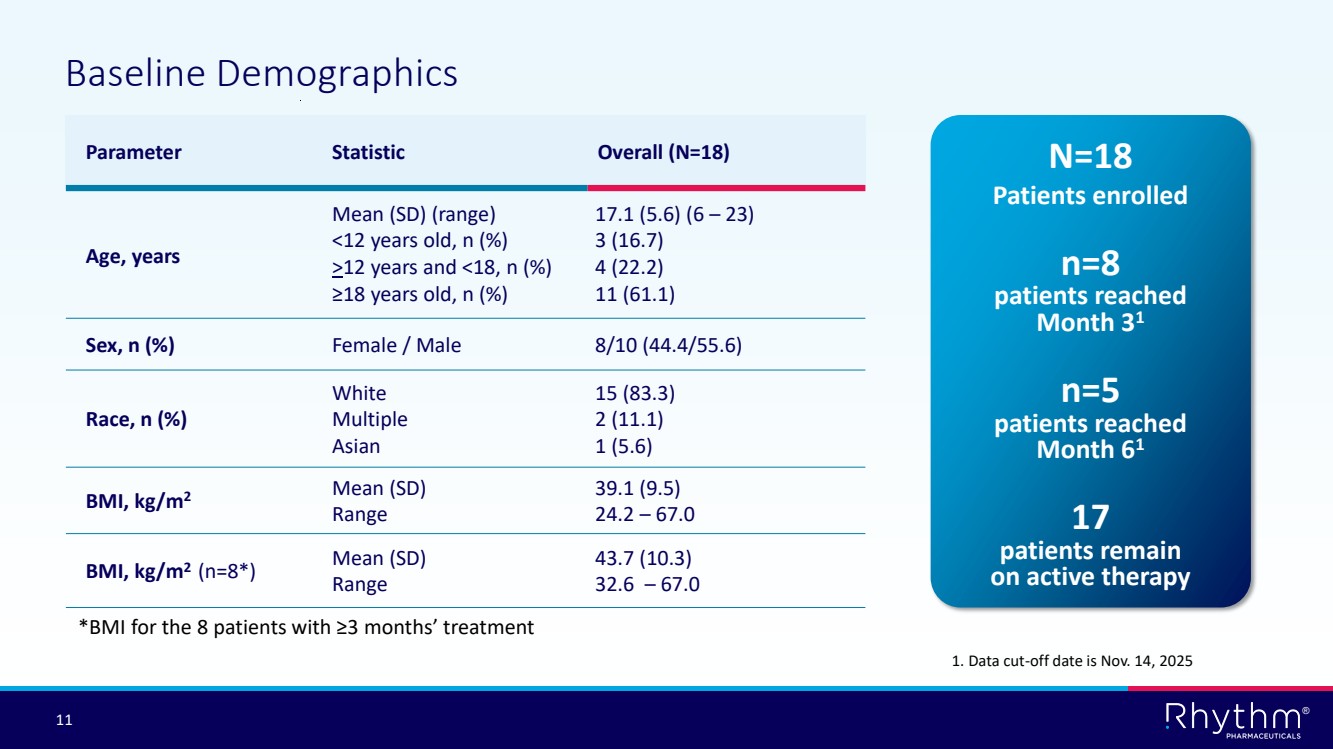
| 11 ® Baseline Demographics N=18 Patients enrolled Parameter Statistic Overall (N=18) Age, years Mean (SD) (range) <12 years old, n (%) >12 years and <18, n (%) ≥18 years old, n (%) 17.1 (5.6) (6 – 23) 3 (16.7) 4 (22.2) 11 (61.1) Sex, n (%) Female / Male 8/10 (44.4/55.6) Race, n (%) White Multiple Asian 15 (83.3) 2 (11.1) 1 (5.6) BMI, kg/m2 Mean (SD) Range 39.1 (9.5) 24.2 – 67.0 BMI, kg/m2 (n=8*) Mean (SD) Range 43.7 (10.3) 32.6 – 67.0 *BMI for the 8 patients with ≥3 months’ treatment n=8 patients reached Month 31 n=5 patients reached Month 61 17 patients remain on active therapy 1. Data cut-off date is Nov. 14, 2025 |
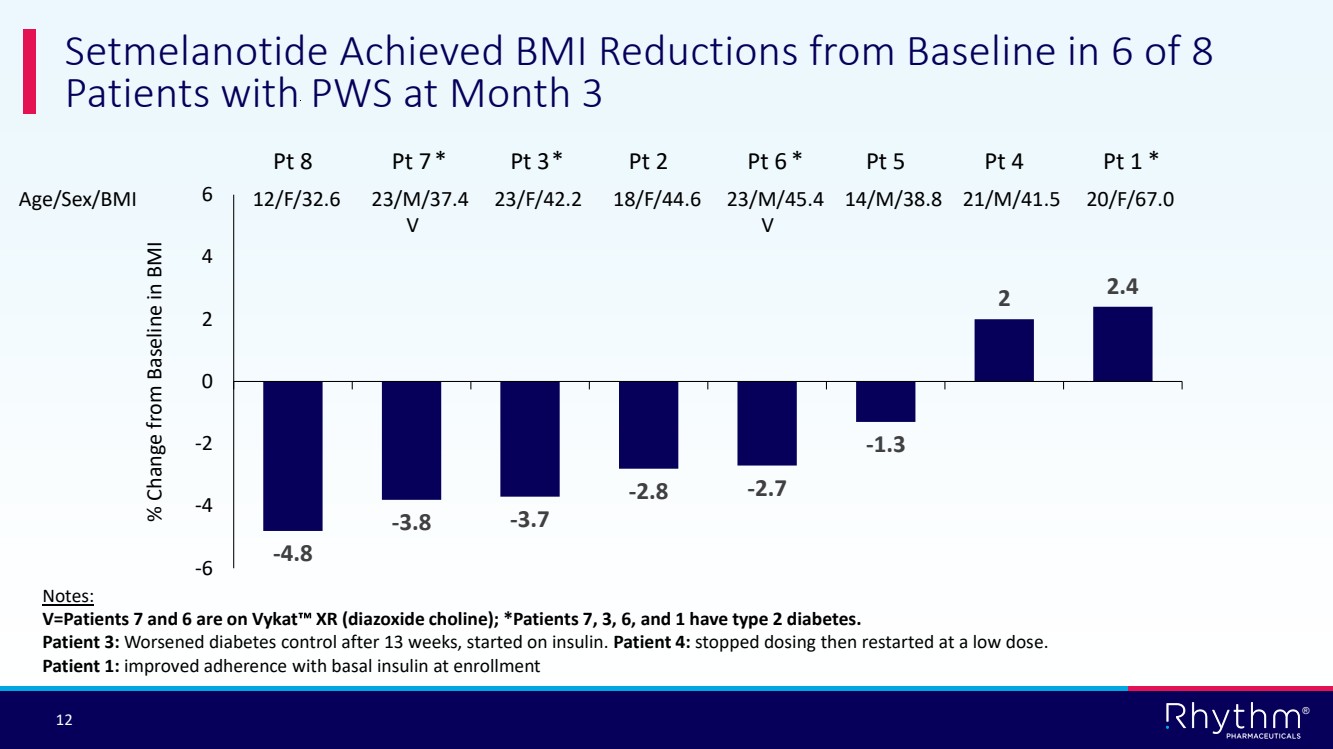
| 12 ® Setmelanotide Achieved BMI Reductions from Baseline in 6 of 8 Patients with PWS at Month 3 -4.8 -3.8 -3.7 -2.8 -2.7 -1.3 2 2.4 -6 -4 -2 0 2 4 6 Pt 8 Pt 7 Pt 3 Pt 2 Pt 6 Pt 5 Pt 4 Pt 1 Age/Sex/BMI 12/F/32.6 23/M/37.4 23/F/42.2 18/F/44.6 23/M/45.4 14/M/38.8 21/M/41.5 20/F/67.0 V V % Change from Baseline in BMI Notes: V=Patients 7 and 6 are on Vykat XR (diazoxide choline); *Patients 7, 3, 6, and 1 have type 2 diabetes. Patient 3: Worsened diabetes control after 13 weeks, started on insulin. Patient 4: stopped dosing then restarted at a low dose. Patient 1: improved adherence with basal insulin at enrollment * * * * |
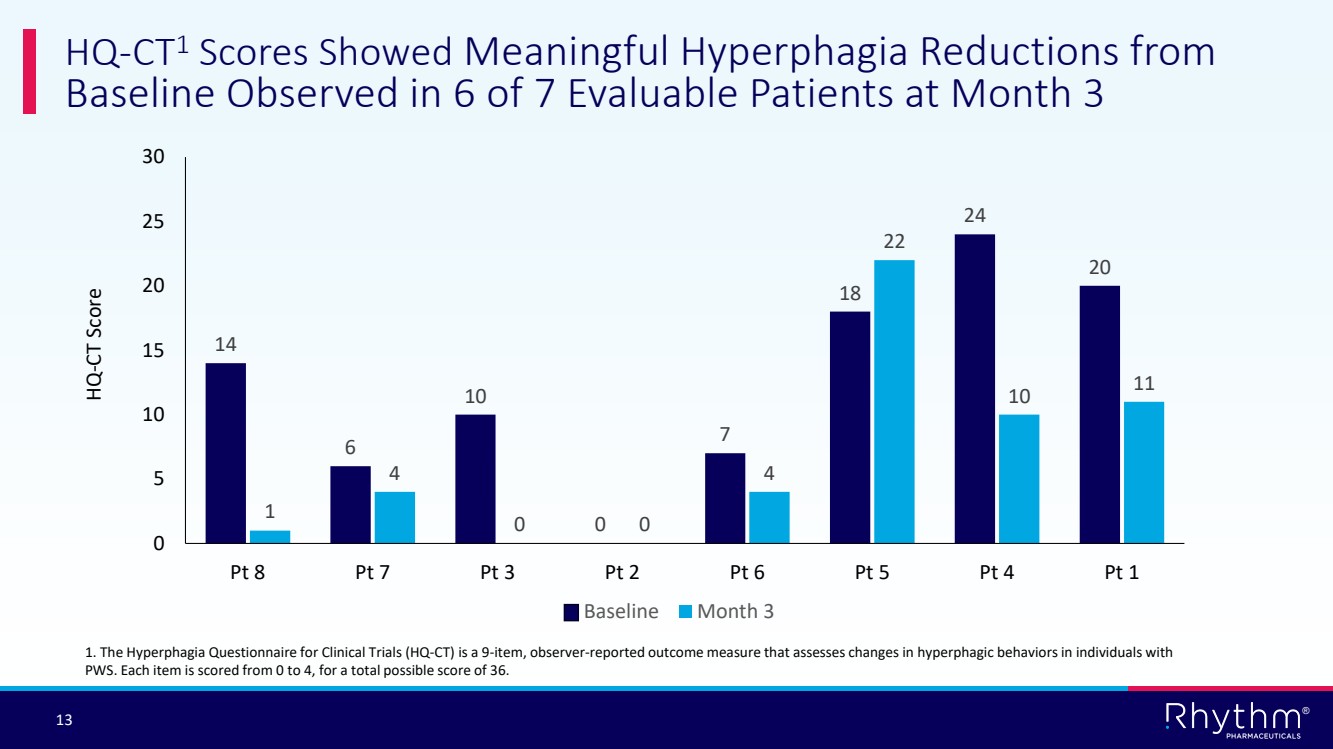
| 13 ® HQ-CT1 Scores Showed Meaningful Hyperphagia Reductions from Baseline Observed in 6 of 7 Evaluable Patients at Month 3 14 6 10 0 7 18 24 20 1 4 0 0 4 22 10 11 0 5 10 15 20 25 30 Pt 8 Pt 7 Pt 3 Pt 2 Pt 6 Pt 5 Pt 4 Pt 1 Baseline Month 3 HQ-CT Score 1. The Hyperphagia Questionnaire for Clinical Trials (HQ-CT) is a 9-item, observer-reported outcome measure that assesses changes in hyperphagic behaviors in individuals with PWS. Each item is scored from 0 to 4, for a total possible score of 36. |
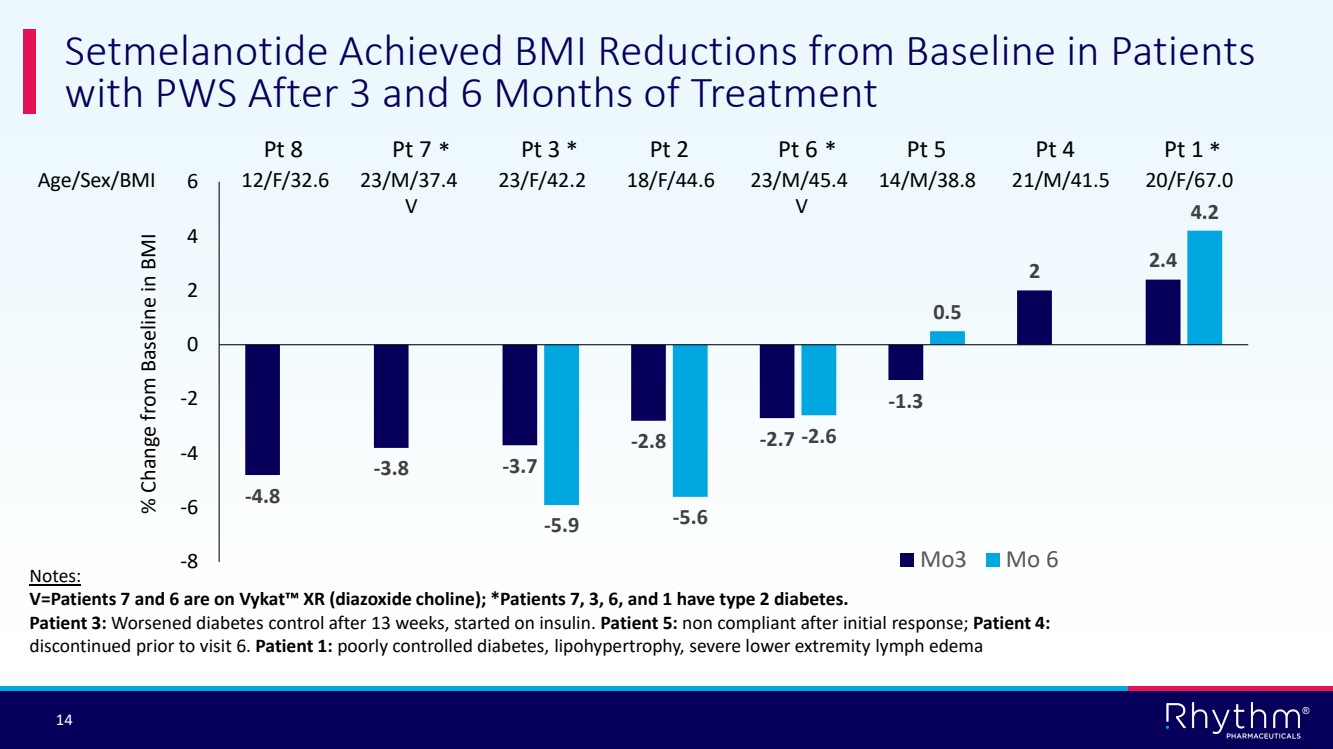
| 14 ® -4.8 -3.8 -3.7 -2.8 -2.7 -1.3 2 2.4 -5.9 -5.6 -2.6 0.5 4.2 -8 -6 -4 -2 0 2 4 6 Pt 8 Pt 7 Pt 3 Pt 2 Pt 6 Pt 5 Pt 4 Pt 1 Mo3 Mo 6 Setmelanotide Achieved BMI Reductions from Baseline in Patients with PWS After 3 and 6 Months of Treatment Age/Sex/BMI 12/F/32.6 23/M/37.4 23/F/42.2 18/F/44.6 23/M/45.4 14/M/38.8 21/M/41.5 20/F/67.0 V V % Change from Baseline in BMI Notes: V=Patients 7 and 6 are on Vykat XR (diazoxide choline); *Patients 7, 3, 6, and 1 have type 2 diabetes. Patient 3: Worsened diabetes control after 13 weeks, started on insulin. Patient 5: non compliant after initial response; Patient 4: discontinued prior to visit 6. Patient 1: poorly controlled diabetes, lipohypertrophy, severe lower extremity lymph edema * * * * |
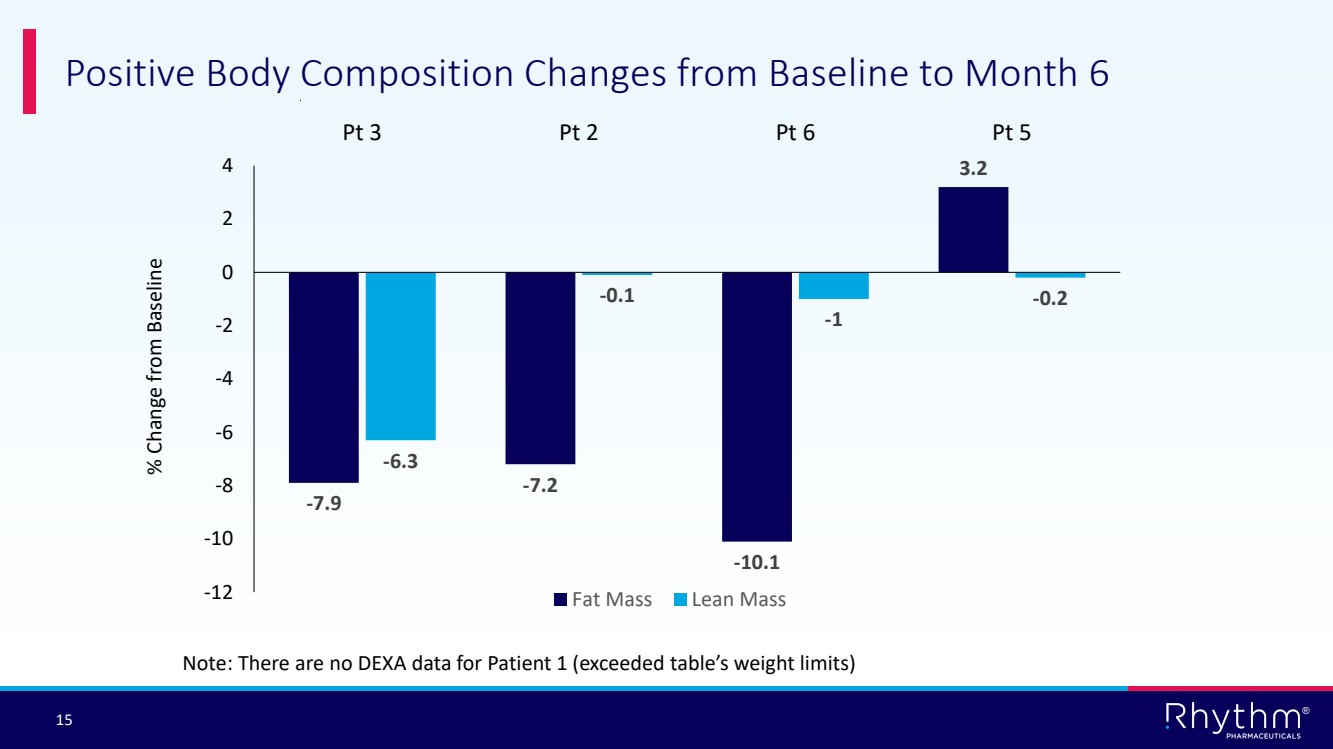
| 15 ® Positive Body Composition Changes from Baseline to Month 6 Note: There are no DEXA data for Patient 1 (exceeded table’s weight limits) -7.9 -7.2 -10.1 3.2 -6.3 -0.1 -1 -0.2 -12 -10 -8 -6 -4 -2 0 2 4 Pt 3 Pt 2 Pt 6 Pt 5 Fat Mass Lean Mass % Change from Baseline |
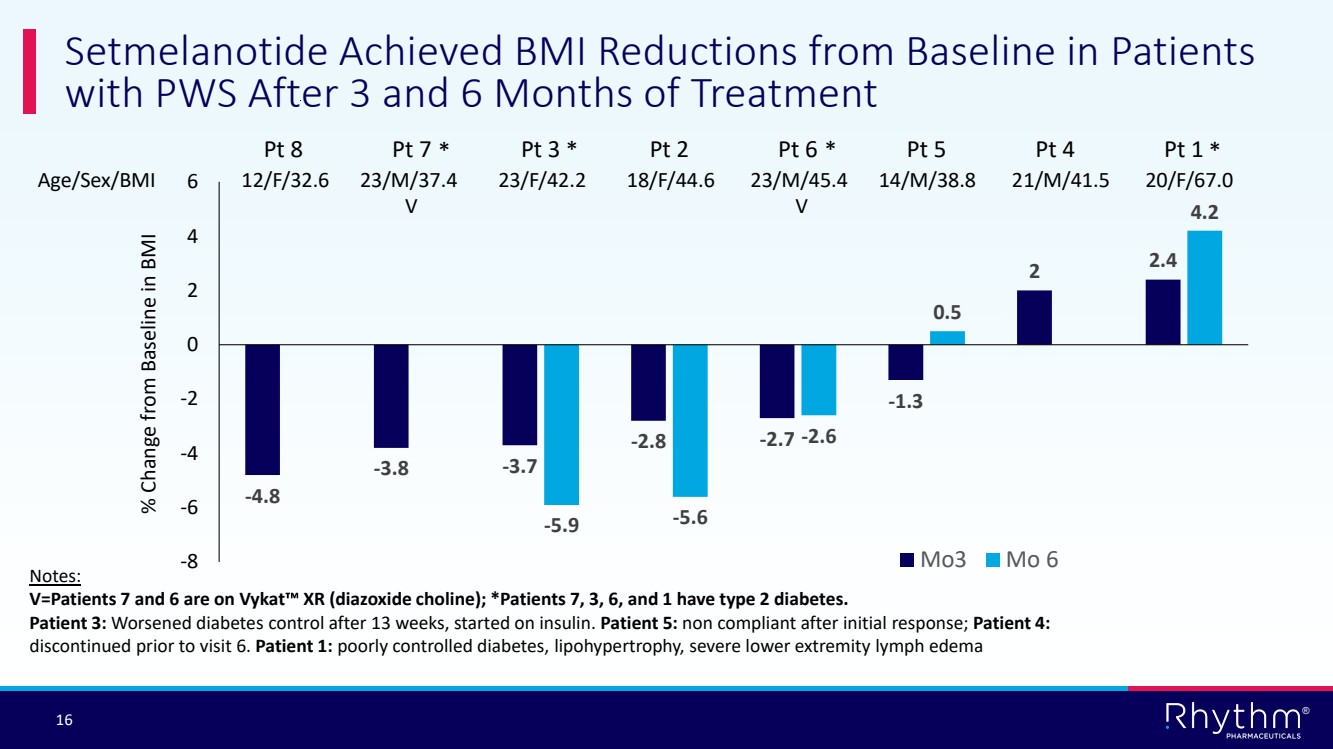
| 16 ® -4.8 -3.8 -3.7 -2.8 -2.7 -1.3 2 2.4 -5.9 -5.6 -2.6 0.5 4.2 -8 -6 -4 -2 0 2 4 6 Pt 8 Pt 7 Pt 3 Pt 2 Pt 6 Pt 5 Pt 4 Pt 1 Mo3 Mo 6 Setmelanotide Achieved BMI Reductions from Baseline in Patients with PWS After 3 and 6 Months of Treatment Age/Sex/BMI 12/F/32.6 23/M/37.4 23/F/42.2 18/F/44.6 23/M/45.4 14/M/38.8 21/M/41.5 20/F/67.0 V V % Change from Baseline in BMI Notes: V=Patients 7 and 6 are on Vykat XR (diazoxide choline); *Patients 7, 3, 6, and 1 have type 2 diabetes. Patient 3: Worsened diabetes control after 13 weeks, started on insulin. Patient 5: non compliant after initial response; Patient 4: discontinued prior to visit 6. Patient 1: poorly controlled diabetes, lipohypertrophy, severe lower extremity lymph edema * * * * |
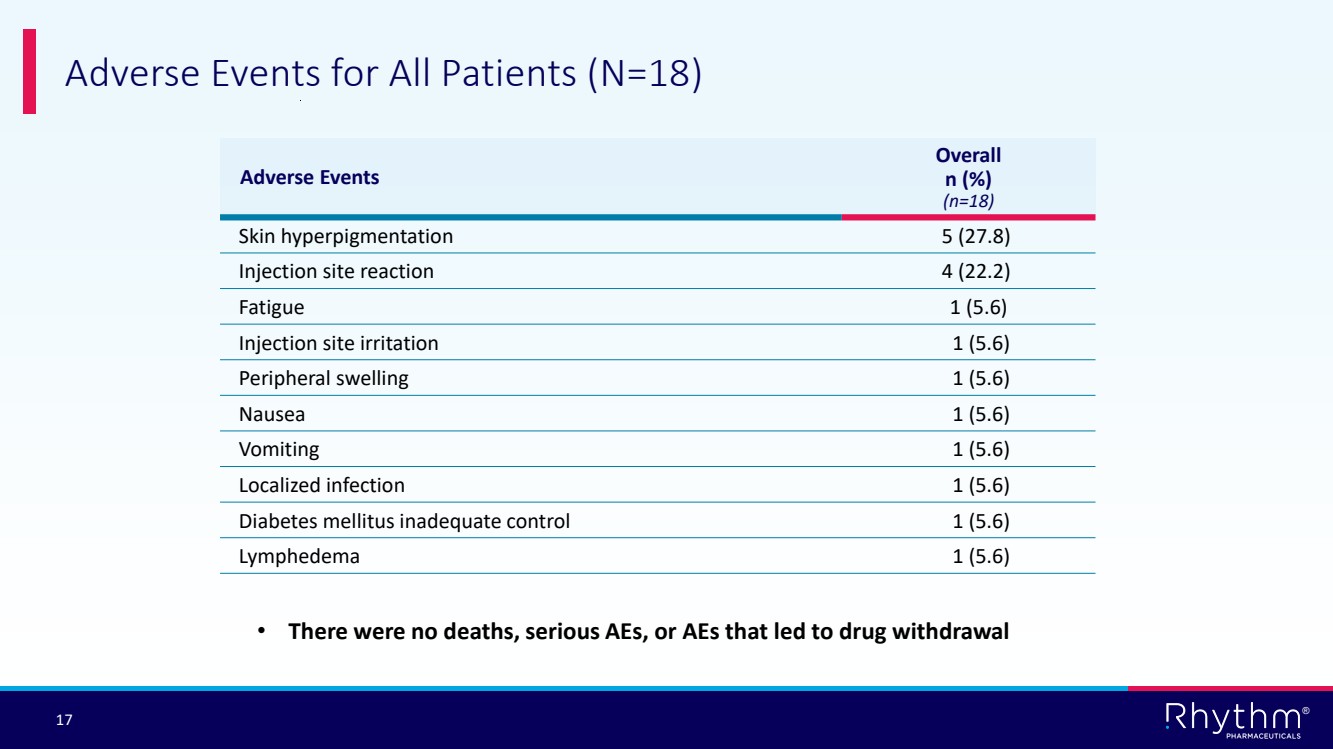
| 17 ® Adverse Events Overall n (%) (n=18) Skin hyperpigmentation 5 (27.8) Injection site reaction 4 (22.2) Fatigue 1 (5.6) Injection site irritation 1 (5.6) Peripheral swelling 1 (5.6) Nausea 1 (5.6) Vomiting 1 (5.6) Localized infection 1 (5.6) Diabetes mellitus inadequate control 1 (5.6) Lymphedema 1 (5.6) Adverse Events for All Patients (N=18) • There were no deaths, serious AEs, or AEs that led to drug withdrawal |
| 18 ® Discussion with Dr. Jennifer Miller |
| 19 ® Next Steps |
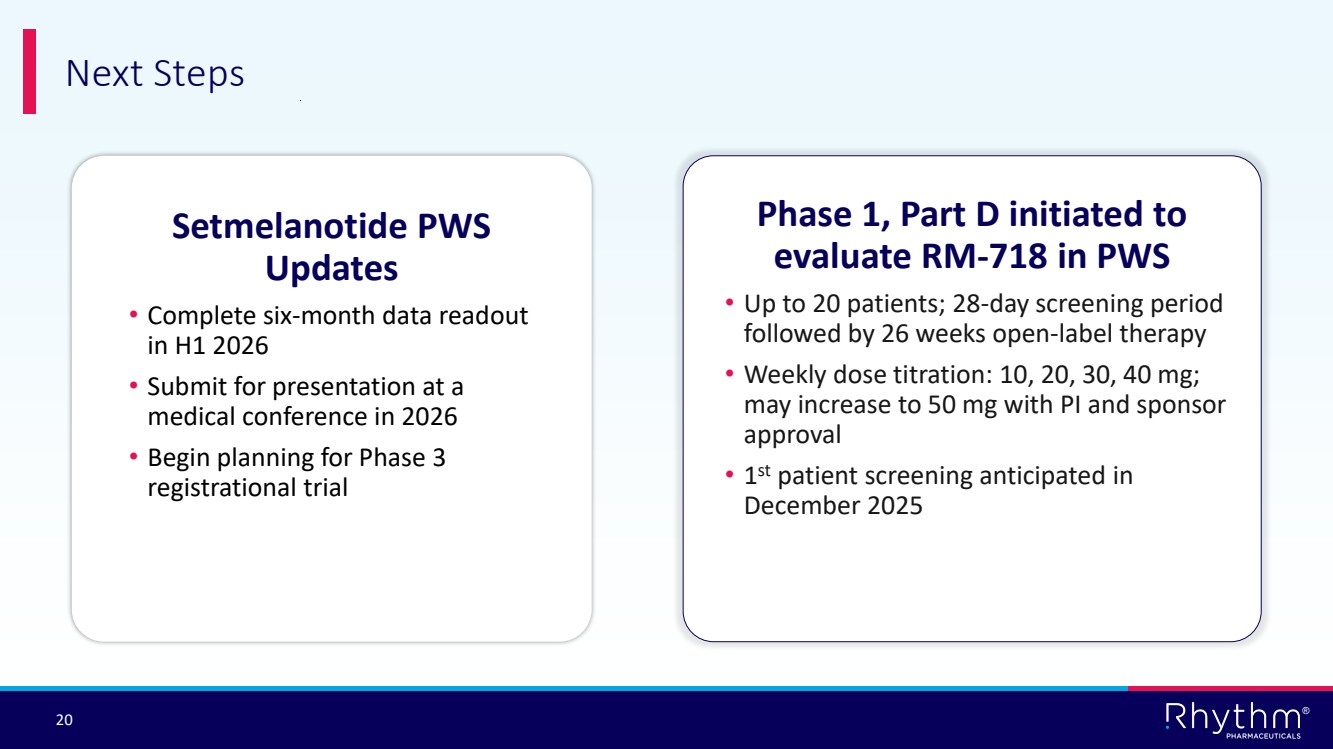
| 20 ® Next Steps Setmelanotide PWS Updates • Complete six-month data readout in H1 2026 • Submit for presentation at a medical conference in 2026 • Begin planning for Phase 3 registrational trial Phase 1, Part D initiated to evaluate RM-718 in PWS • Up to 20 patients; 28-day screening period followed by 26 weeks open-label therapy • Weekly dose titration: 10, 20, 30, 40 mg; may increase to 50 mg with PI and sponsor approval • 1 st patient screening anticipated in December 2025 |
| 21 ® Questions? |