

INTELLIA THERAPEUTICS Corporate Overview January 2026

Intellia Therapeutics’ Legal Disclaimer This presentation contains “forward-looking statements” of Intellia Therapeutics, Inc. (“Intellia”, “we” or “our”) within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, express or implied statements about Intellia’s beliefs and expectations regarding: our ability to successfully develop and commercialize nexiguran ziclumeran (“nex-z”), also known as NTLA-2001, for the treatment of transthyretin (“ATTR”) amyloidosis and lonvoguran ziclumeran (“lonvo-z”), also known as NTLA-2002, for the treatment of hereditary angioedema (“HAE”) to address the significant unmet needs of patients and prescribers in ATTR amyloidosis and HAE, respectively; our ability to achieve upcoming objectives, including presenting topline data from the Phase 3 HAELO of lonvo-z by mid-2026, submitting a biologics license application for lonvo-z for the treatment of HAE in the second half of 2026, successfully launching lonvo-z for the treatment of HAE in the U.S. in the first half of 2027, and resolving the clinical holds on the MAGNITUDE and MAGNITUDE-2 trials of nex-z for the treatment of ATTR amyloidosis and finalizing a plan with regulators on the path forward; our ability to optimize the impact of our collaborations on our development programs, including our collaboration with Regeneron Pharmaceuticals, Inc. ("Regeneron") and our co-development program for ATTR amyloidosis, and to advance additional development candidates; our expectations regarding our uses of capital, expenses, and ability to fund operations into mid-2027; the potential commercial opportunities for our product candidates, including the value and market potential for our product candidates, including the potential of nex-z and lonvo-z to be single-dose treatments administered in an outpatient setting, the potential of nex-z to transform the standard of care for ATTR amyloidosis, provide patients with consistently rapid, deep and durable TTR reduction, stability or improvement in the disease for most patients, improved quality of life, and reduced cardiovascular events and mortality, and represent a meaningful opportunity for significant revenues and healthcare system savings, and the potential of lonvo-z to eliminate HAE attacks and burdensome ongoing therapy for most patients; and our ability to leverage our in vivo and ex vivo technology for pipeline expansion efforts and collaborations. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs of future events, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks related to the ability to develop and commercialize any one or more of Intellia’s product candidates successfully, including risks related to our ability to present a topline data readout from the HAELO study by mid-2026, generate data to support lonvo-z’s potential to be a one-time treatment for HAE, address the clinical hold on the MAGNITUDE and MAGNITUDE-2 trials of nex-z for ATTR amyloidosis and to resume those clinical trials; risks related to Intellia’s ability to protect and maintain its intellectual property position; risks related to Intellia’s relationship with third parties, including our contract manufacturers, licensors and licensees; risks related to the ability of our licensors to protect and maintain their intellectual property position; uncertainties related to the authorization, initiation and conduct of preclinical and clinical studies and other development requirements for our product candidates, including uncertainties related to regulatory approvals to conduct clinical trials; risks related to the results of preclinical studies or clinical studies not being predictive of future results in connection with future studies; the risk that clinical trial results will not be positive; risks related to the development and advancement of in vivo and ex vivo technologies for pipeline expansion and collaborations; risks related to Intellia’s future financial condition and our ability to fund our operations; risks related to Intellia’s collaborations with Regeneron or our other collaborations not continuing or not being successful; and risks related to Intellia's ability to execute its strategic plans, including completing pivotal clinical trials and commercial launch of its product candidates. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause Intellia’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intellia’s most recent Annual Report of Form 10-K and Quarterly Report on Form 10-Q as well as discussions of potential risks, uncertainties, and other important factors in Intellia’s other filings with the Securities and Exchange Commission. All information in this presentation is as of the date on its cover page, and Intellia undertakes no duty to update this information unless required by law.
Our Vision: Transform the Lives of Patients Leveraging Gene Editing Technology Treat patients at the root cause of their disease 1x Single dose treatment with potential lifelong benefit Reduce burden for the patient and the healthcare system Best outcomes for patients
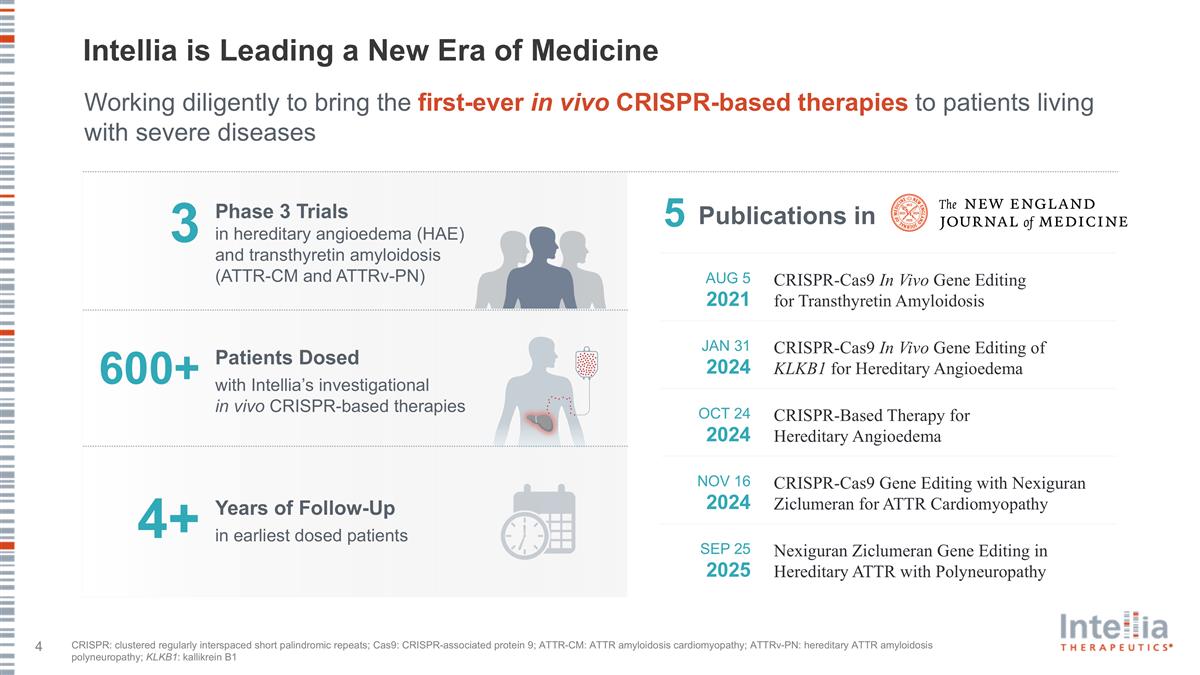
Intellia is Leading a New Era of Medicine 3 Phase 3 Trials in hereditary angioedema (HAE) and transthyretin amyloidosis (ATTR-CM and ATTRv-PN) Patients Dosed with Intellia’s investigational in vivo CRISPR-based therapies 600+ Working diligently to bring the first-ever in vivo CRISPR-based therapies to patients living with severe diseases 4+ Publications in 5 AUG 5 2021 CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis JAN 31 2024 CRISPR-Cas9 In Vivo Gene Editing of KLKB1 for Hereditary Angioedema OCT 24 2024 CRISPR-Based Therapy for Hereditary Angioedema NOV 16 2024 CRISPR-Cas9 Gene Editing with Nexiguran Ziclumeran for ATTR Cardiomyopathy SEP 25 2025 Nexiguran Ziclumeran Gene Editing in Hereditary ATTR with Polyneuropathy Years of Follow-Up in earliest dosed patients CRISPR: clustered regularly interspaced short palindromic repeats; Cas9: CRISPR-associated protein 9; ATTR-CM: ATTR amyloidosis cardiomyopathy; ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy; KLKB1: kallikrein B1
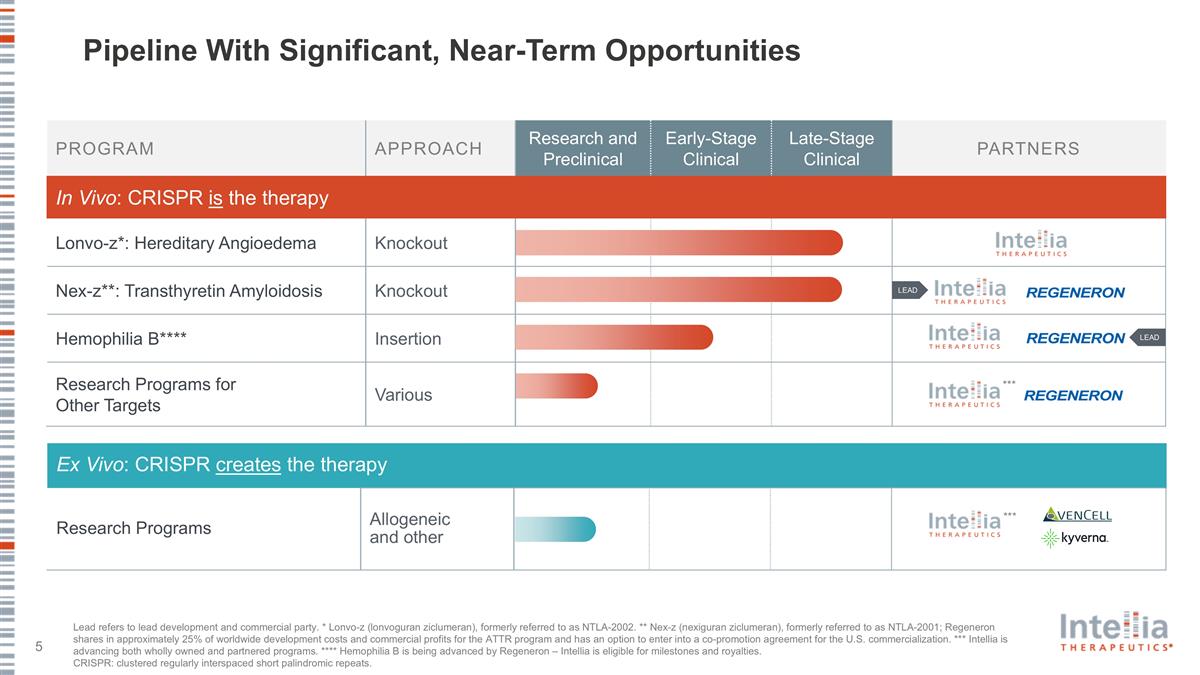
Pipeline With Significant, Near-Term Opportunities PROGRAM APPROACH Research and Preclinical Early-Stage Clinical Late-Stage Clinical PARTNERS In Vivo: CRISPR is the therapy Lonvo-z*: Hereditary Angioedema Knockout Nex-z**: Transthyretin Amyloidosis Knockout Hemophilia B**** Insertion Research Programs for Other Targets Various Ex Vivo: CRISPR creates the therapy Research Programs Allogeneic and other *** LEAD LEAD *** Lead refers to lead development and commercial party. * Lonvo-z (lonvoguran ziclumeran), formerly referred to as NTLA-2002. ** Nex-z (nexiguran ziclumeran), formerly referred to as NTLA-2001; Regeneron shares in approximately 25% of worldwide development costs and commercial profits for the ATTR program and has an option to enter into a co-promotion agreement for the U.S. commercialization. *** Intellia is advancing both wholly owned and partnered programs. **** Hemophilia B is being advanced by Regeneron – Intellia is eligible for milestones and royalties. CRISPR: clustered regularly interspaced short palindromic repeats.
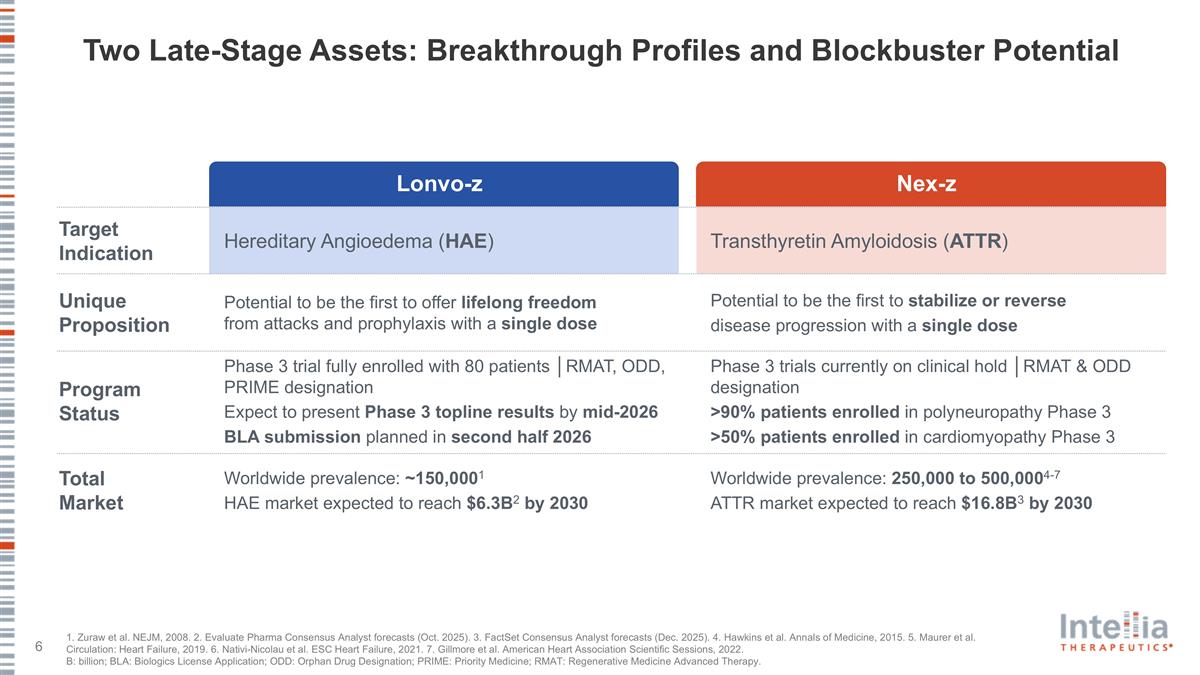
Two Late-Stage Assets: Breakthrough Profiles and Blockbuster Potential Hereditary Angioedema (HAE) Potential to be the first to offer lifelong freedom from attacks and prophylaxis with a single dose Phase 3 trial fully enrolled with 80 patients │RMAT, ODD, PRIME designation Expect to present Phase 3 topline results by mid-2026 BLA submission planned in second half 2026 Worldwide prevalence: ~150,0001 HAE market expected to reach $6.3B2 by 2030 Transthyretin Amyloidosis (ATTR) Potential to be the first to stabilize or reverse disease progression with a single dose Phase 3 trials currently on clinical hold │RMAT & ODD designation >90% patients enrolled in polyneuropathy Phase 3 >50% patients enrolled in cardiomyopathy Phase 3 Worldwide prevalence: 250,000 to 500,0004-7 ATTR market expected to reach $16.8B3 by 2030 Target Indication Unique Proposition Program Status Total Market Lonvo-z Nex-z 1. Zuraw et al. NEJM, 2008. 2. Evaluate Pharma Consensus Analyst forecasts (Oct. 2025). 3. FactSet Consensus Analyst forecasts (Dec. 2025). 4. Hawkins et al. Annals of Medicine, 2015. 5. Maurer et al. Circulation: Heart Failure, 2019. 6. Nativi-Nicolau et al. ESC Heart Failure, 2021. 7. Gillmore et al. American Heart Association Scientific Sessions, 2022. B: billion; BLA: Biologics License Application; ODD: Orphan Drug Designation; PRIME: Priority Medicine; RMAT: Regenerative Medicine Advanced Therapy.
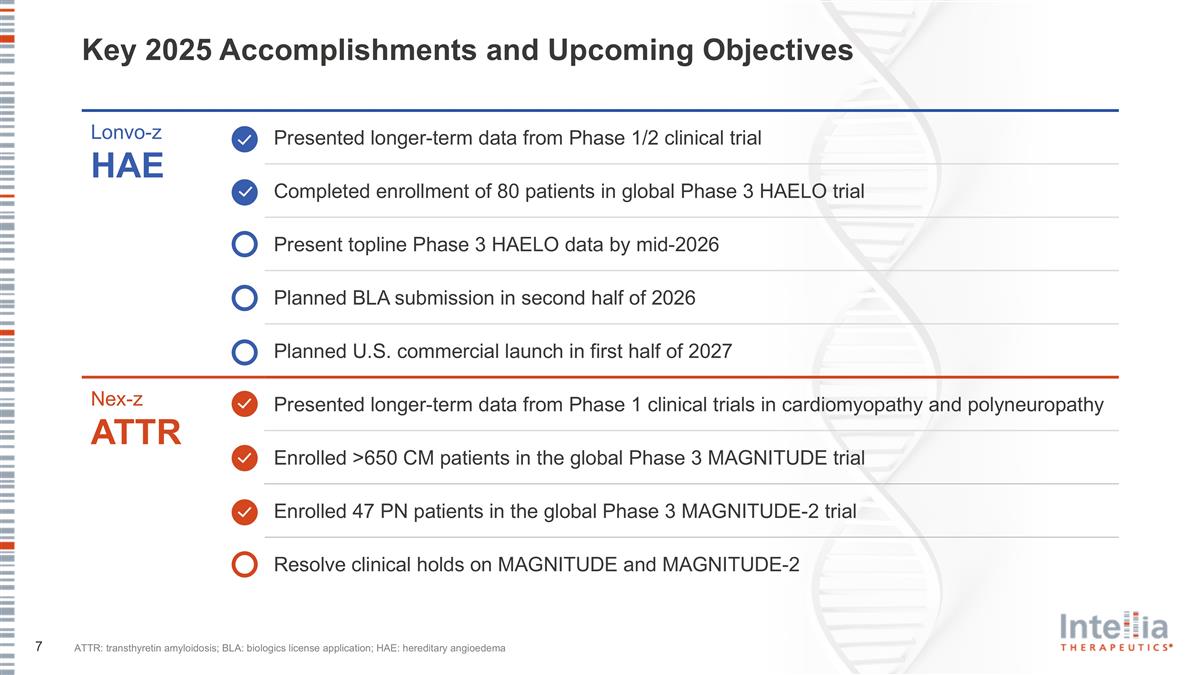
Key 2025 Accomplishments and Upcoming Objectives Lonvo-z HAE Presented longer-term data from Phase 1/2 clinical trial Completed enrollment of 80 patients in global Phase 3 HAELO trial Present topline Phase 3 HAELO data by mid-2026 Planned BLA submission in second half of 2026 Planned U.S. commercial launch in first half of 2027 Nex-z ATTR Presented longer-term data from Phase 1 clinical trials in cardiomyopathy and polyneuropathy Enrolled >650 CM patients in the global Phase 3 MAGNITUDE trial Enrolled 47 PN patients in the global Phase 3 MAGNITUDE-2 trial Resolve clinical holds on MAGNITUDE and MAGNITUDE-2 ATTR: transthyretin amyloidosis; BLA: biologics license application; HAE: hereditary angioedema

Lonvoguran Ziclumeran (lonvo-z) for Hereditary Angioedema

Hereditary Angioedema (HAE): Currently a Life-Long Genetic Condition with Significant Burden Rare, genetic and life-threatening disease Patients have unpredictable, recurrent, painful and potentially life-threatening swelling attacks1,2 Average age of diagnosis is 20 years old3 Symptoms often begin in the first decade of life and typically worsen in puberty4,5 Attacks can be triggered by stress, trauma, infection, fatigue and hormones2 Approximately 7,000 patients treated in the U.S.6 Despite available treatments, significant unmet need persists Many patients only achieve partial clinical control7,8,9 Patients make lifestyle modifications to manage fear and anxiety10 Treatment burden negatively affects patients, especially those taking injectable medications11 Insurance delays and denials associated with maintaining access have significant impacts on individuals with HAE12 1. Zuraw et al. NEJM, 2008. 2. Busse and Christiansen, NEJM, 2020. 3. Farkas et al. Allergy, 2017. 4. Norris et al. Allergy Asthma Proc., 2022. 5. Pancholy et al. Curr Opin. Pediatr., 2019. 6. Castaldo et al. Ann Allergy Asthma Immunol, 2025. 7. Banerji et al. JAMA, 2018. 8. Zuraw et al. Allergy Clin. Immunol, 2021. 9. Longhurst et al. NEJM, 2017. 10. Bork et al. Allergy Asthma Clin. Immunol, 2021. 11. Radojicic et al. Allergy Asthma Proc., 2021. 12. Arora et al. JACI In Practice, 2023. KIM Living with HAE “The fear is always there — a tickle in your throat, and you think, ‘Do I have a cold, or is this a swell?’”
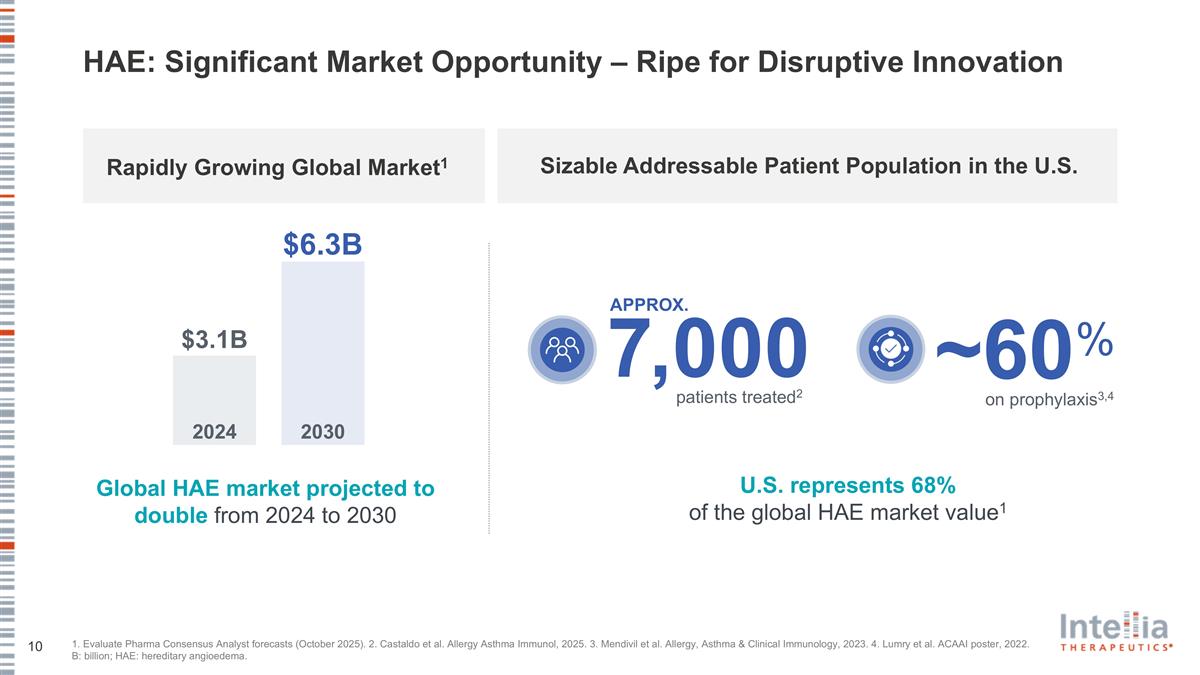
HAE: Significant Market Opportunity – Ripe for Disruptive Innovation 1. Evaluate Pharma Consensus Analyst forecasts (October 2025). 2. Castaldo et al. Allergy Asthma Immunol, 2025. 3. Mendivil et al. Allergy, Asthma & Clinical Immunology, 2023. 4. Lumry et al. ACAAI poster, 2022. B: billion; HAE: hereditary angioedema. 7,000 APPROX. Global HAE market projected to double from 2024 to 2030 patients treated2 U.S. represents 68% of the global HAE market value1 Sizable Addressable Patient Population in the U.S. ~60 % on prophylaxis3,4 2024 2030 $3.1B $6.3B Rapidly Growing Global Market1

Lonvo-z Has the Potential to be a One-Time Treatment for Hereditary Angioedema First and only investigational therapy that targets the KLKB1 gene, to reduce production of kallikrein protein at its source Observations from Phase 1/2 trial: Consistently rapid, deep and durable kallikrein reductions Freedom from HAE attacks and ongoing therapy for most patients Well-tolerated safety profile Lonvo-z is being developed as a one-time IV infusion administered in an outpatient setting Based on interim lonvo-z Phase 1 data as of data cutoff February 12, 2025. Studies are ongoing to assess this investigational product. IV: intravenous; KLKB1: kallikrein B1. KIM Living with HAE
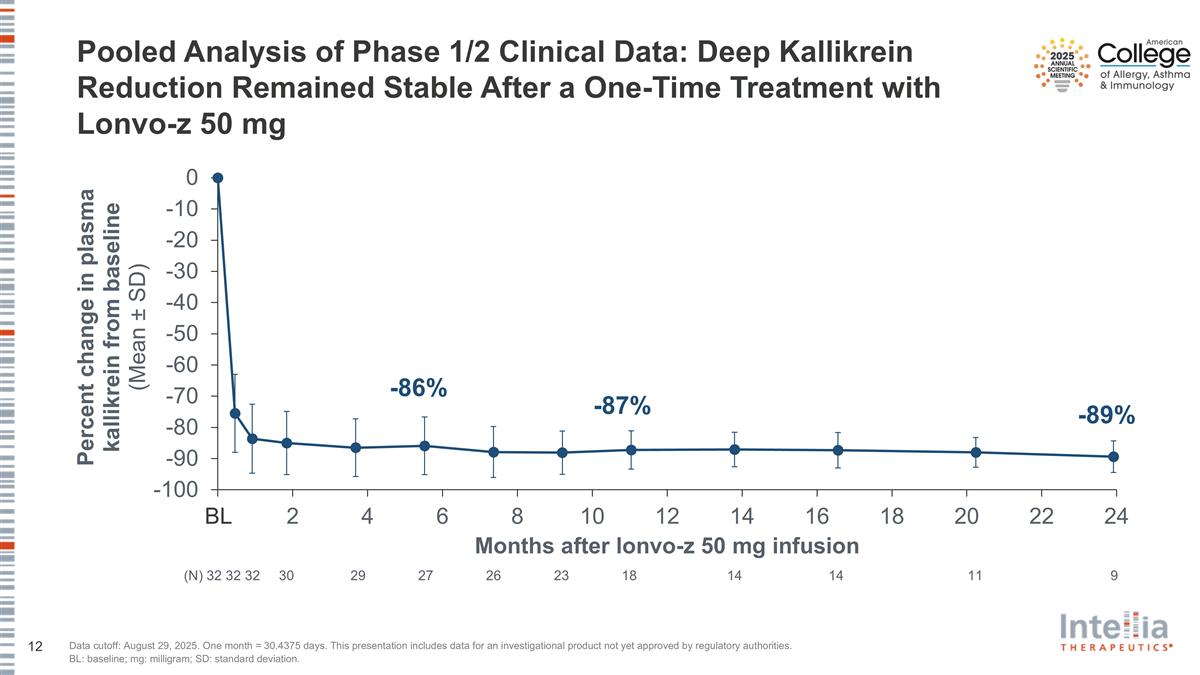
Pooled Analysis of Phase 1/2 Clinical Data: Deep Kallikrein Reduction Remained Stable After a One-Time Treatment with Lonvo-z 50 mg Data cutoff: August 29, 2025. One month = 30.4375 days. This presentation includes data for an investigational product not yet approved by regulatory authorities. BL: baseline; mg: milligram; SD: standard deviation. (N) 32 32 32 30 29 27 26 23 18 14 14 11 9 BL
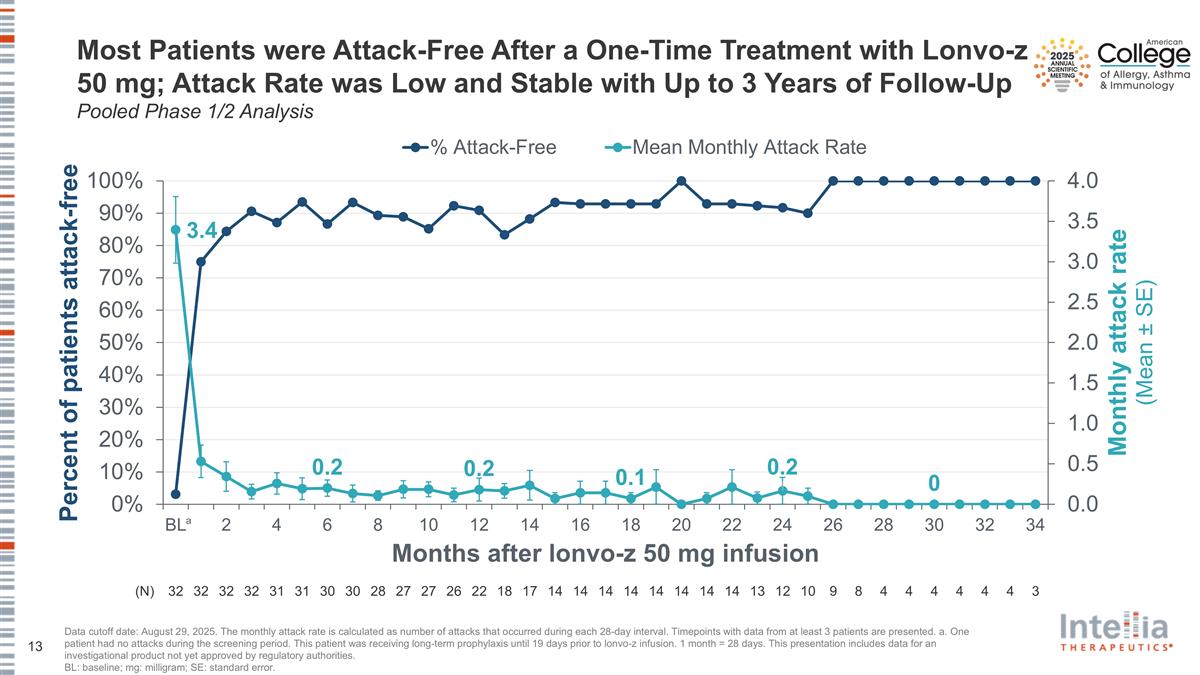
Most Patients were Attack-Free After a One-Time Treatment with Lonvo-z 50 mg; Attack Rate was Low and Stable with Up to 3 Years of Follow-Up Pooled Phase 1/2 Analysis Data cutoff date: August 29, 2025. The monthly attack rate is calculated as number of attacks that occurred during each 28-day interval. Timepoints with data from at least 3 patients are presented. a. One patient had no attacks during the screening period. This patient was receiving long-term prophylaxis until 19 days prior to lonvo-z infusion. 1 month = 28 days. This presentation includes data for an investigational product not yet approved by regulatory authorities. BL: baseline; mg: milligram; SE: standard error. (N) 32 32 32 32 31 31 30 30 28 27 27 26 22 18 17 14 14 14 14 14 14 14 14 13 12 10 9 8 4 4 4 4 4 4 3 a
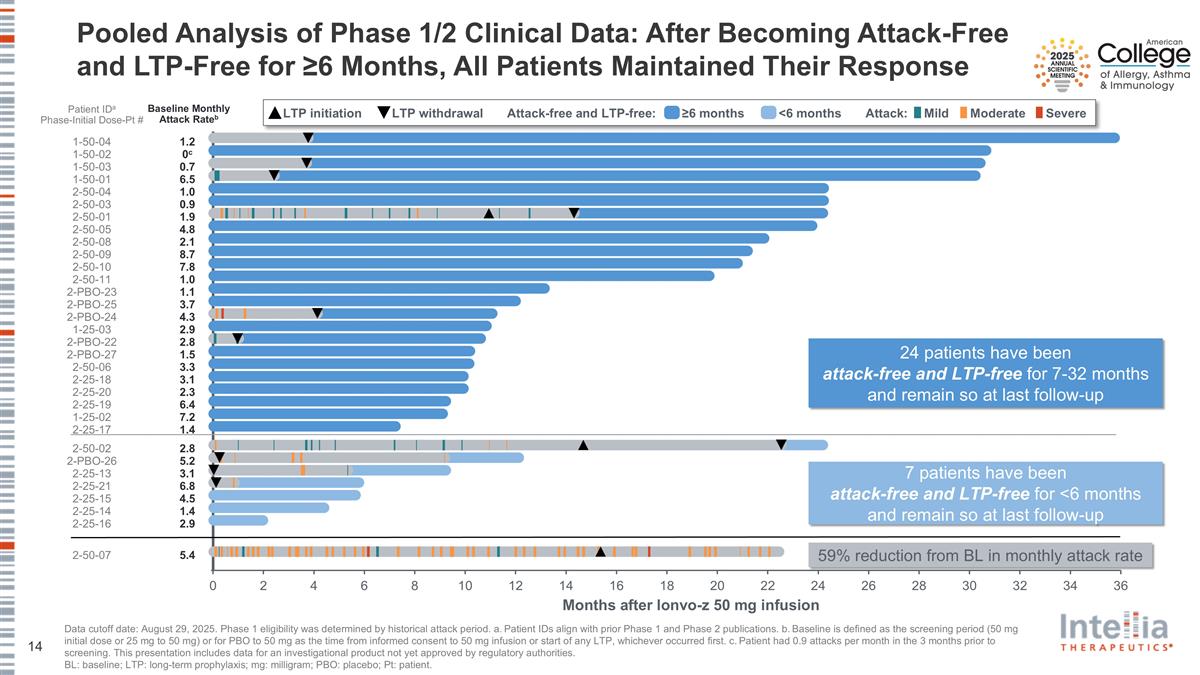
Pooled Analysis of Phase 1/2 Clinical Data: After Becoming Attack-Free and LTP-Free for ≥6 Months, All Patients Maintained Their Response Data cutoff date: August 29, 2025. Phase 1 eligibility was determined by historical attack period. a. Patient IDs align with prior Phase 1 and Phase 2 publications. b. Baseline is defined as the screening period (50 mg initial dose or 25 mg to 50 mg) or for PBO to 50 mg as the time from informed consent to 50 mg infusion or start of any LTP, whichever occurred first. c. Patient had 0.9 attacks per month in the 3 months prior to screening. This presentation includes data for an investigational product not yet approved by regulatory authorities. BL: baseline; LTP: long-term prophylaxis; mg: milligram; PBO: placebo; Pt: patient. Patient IDa Phase-Initial Dose-Pt # Baseline Monthly Attack Rateb 1.2 1-50-04 0c 1-50-02 6.5 1-50-01 0.7 1-50-03 1.0 2-50-04 0.9 2-50-03 2.1 2-50-08 7.8 2-50-10 1.1 2-PBO-23 1.0 2-50-11 3.7 2-PBO-25 4.3 2-PBO-24 6.4 2-25-19 7.2 1-25-02 3.3 2-50-06 1.9 2-50-01 3.1 2-25-18 2.3 2-25-20 5.2 2-PBO-26 1.4 2-25-17 3.1 2-25-13 1.5 2-PBO-27 2.8 2-PBO-22 6.8 2-25-21 1.4 2-25-14 2.9 2-25-16 4.5 2-25-15 2.8 2-50-02 5.4 2-50-07 4.8 2-50-05 2.9 1-25-03 8.7 2-50-09 Months after lonvo-z 50 mg infusion 24 patients have been attack-free and LTP-free for 7-32 months and remain so at last follow-up 7 patients have been attack-free and LTP-free for <6 months and remain so at last follow-up 59% reduction from BL in monthly attack rate Mild Moderate Severe LTP initiation LTP withdrawal Attack-free and LTP-free: ≥6 months <6 months Attack:
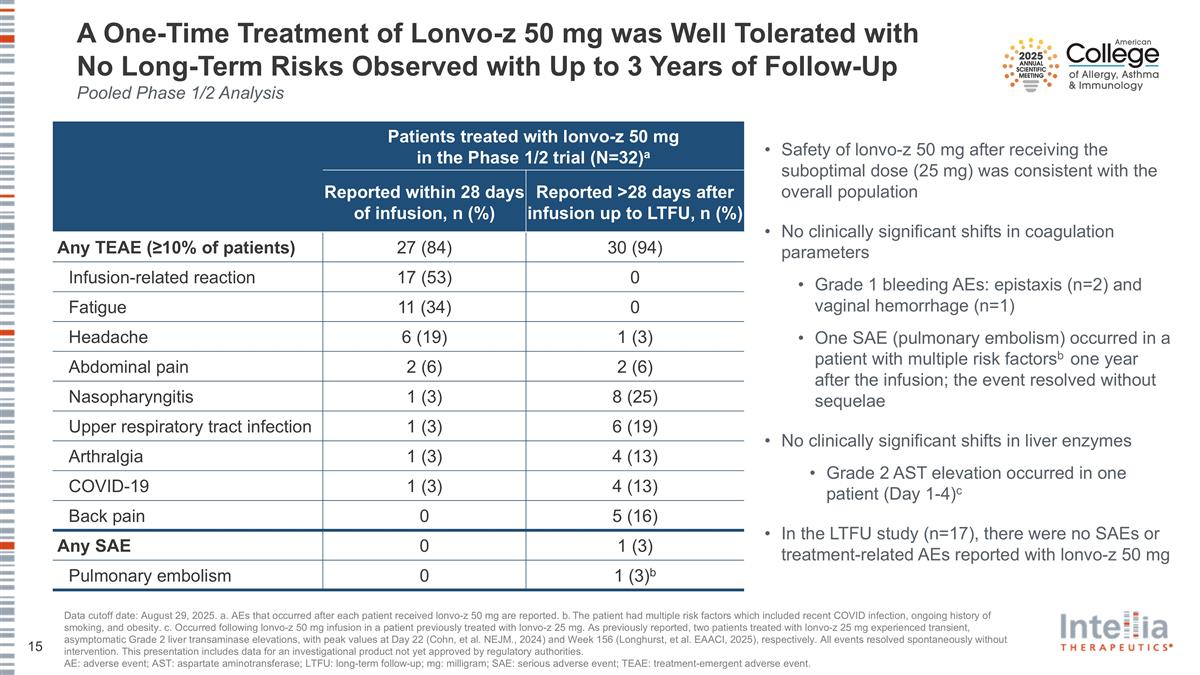
A One-Time Treatment of Lonvo-z 50 mg was Well Tolerated with No Long-Term Risks Observed with Up to 3 Years of Follow-Up Pooled Phase 1/2 Analysis Data cutoff date: August 29, 2025. a. AEs that occurred after each patient received lonvo-z 50 mg are reported. b. The patient had multiple risk factors which included recent COVID infection, ongoing history of smoking, and obesity. c. Occurred following lonvo-z 50 mg infusion in a patient previously treated with lonvo-z 25 mg. As previously reported, two patients treated with lonvo-z 25 mg experienced transient, asymptomatic Grade 2 liver transaminase elevations, with peak values at Day 22 (Cohn, et al. NEJM., 2024) and Week 156 (Longhurst, et al. EAACI, 2025), respectively. All events resolved spontaneously without intervention. This presentation includes data for an investigational product not yet approved by regulatory authorities. AE: adverse event; AST: aspartate aminotransferase; LTFU: long-term follow-up; mg: milligram; SAE: serious adverse event; TEAE: treatment-emergent adverse event. Patients treated with lonvo-z 50 mg in the Phase 1/2 trial (N=32)a Reported within 28 days of infusion, n (%) Reported >28 days after infusion up to LTFU, n (%) Any TEAE (≥10% of patients) 27 (84) 30 (94) Infusion-related reaction 17 (53) 0 Fatigue 11 (34) 0 Headache 6 (19) 1 (3) Abdominal pain 2 (6) 2 (6) Nasopharyngitis 1 (3) 8 (25) Upper respiratory tract infection 1 (3) 6 (19) Arthralgia 1 (3) 4 (13) COVID-19 1 (3) 4 (13) Back pain 0 5 (16) Any SAE 0 1 (3) Pulmonary embolism 0 1 (3)b Safety of lonvo-z 50 mg after receiving the suboptimal dose (25 mg) was consistent with the overall population No clinically significant shifts in coagulation parameters Grade 1 bleeding AEs: epistaxis (n=2) and vaginal hemorrhage (n=1) One SAE (pulmonary embolism) occurred in a patient with multiple risk factorsb one year after the infusion; the event resolved without sequelae No clinically significant shifts in liver enzymes Grade 2 AST elevation occurred in one patient (Day 1-4)c In the LTFU study (n=17), there were no SAEs or treatment-related AEs reported with lonvo-z 50 mg
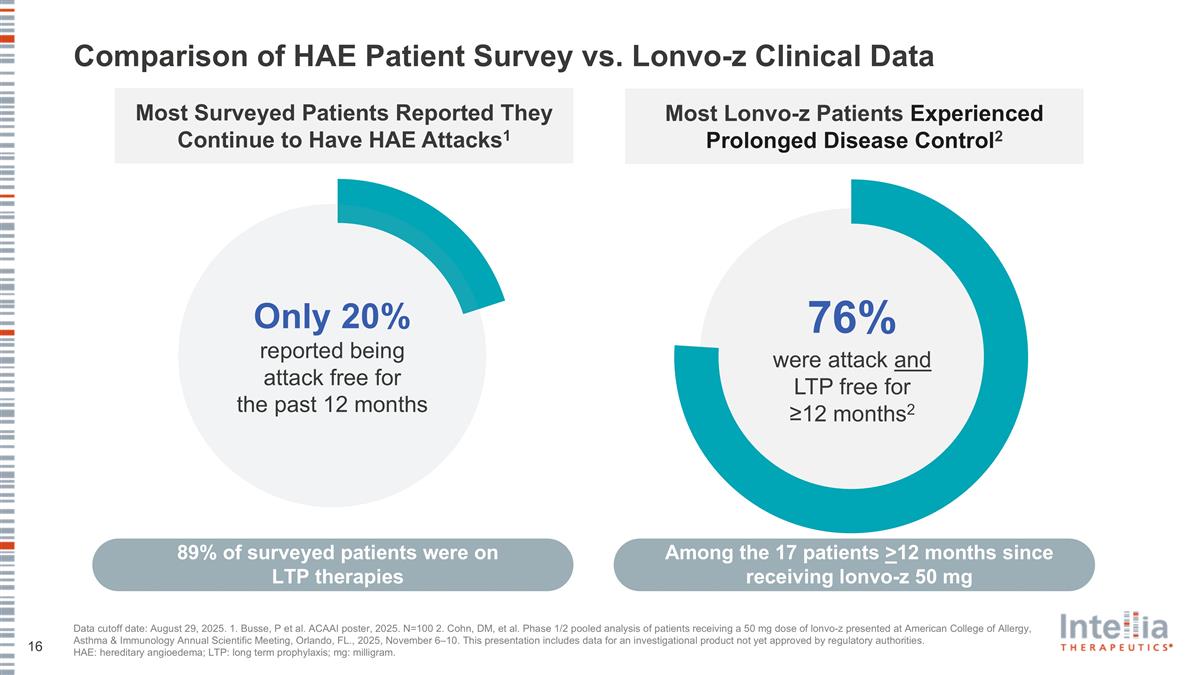
Comparison of HAE Patient Survey vs. Lonvo-z Clinical Data Most Surveyed Patients Reported They Continue to Have HAE Attacks1 Data cutoff date: August 29, 2025. 1. Busse, P et al. ACAAI poster, 2025. N=100 2. Cohn, DM, et al. Phase 1/2 pooled analysis of patients receiving a 50 mg dose of lonvo-z presented at American College of Allergy, Asthma & Immunology Annual Scientific Meeting, Orlando, FL., 2025, November 6–10. This presentation includes data for an investigational product not yet approved by regulatory authorities. HAE: hereditary angioedema; LTP: long term prophylaxis; mg: milligram. Only 20% reported being attack free for the past 12 months 89% of surveyed patients were on LTP therapies 76% were attack and LTP free for ≥12 months2 Most Lonvo-z Patients Experienced Prolonged Disease Control2 Among the 17 patients >12 months since receiving lonvo-z 50 mg
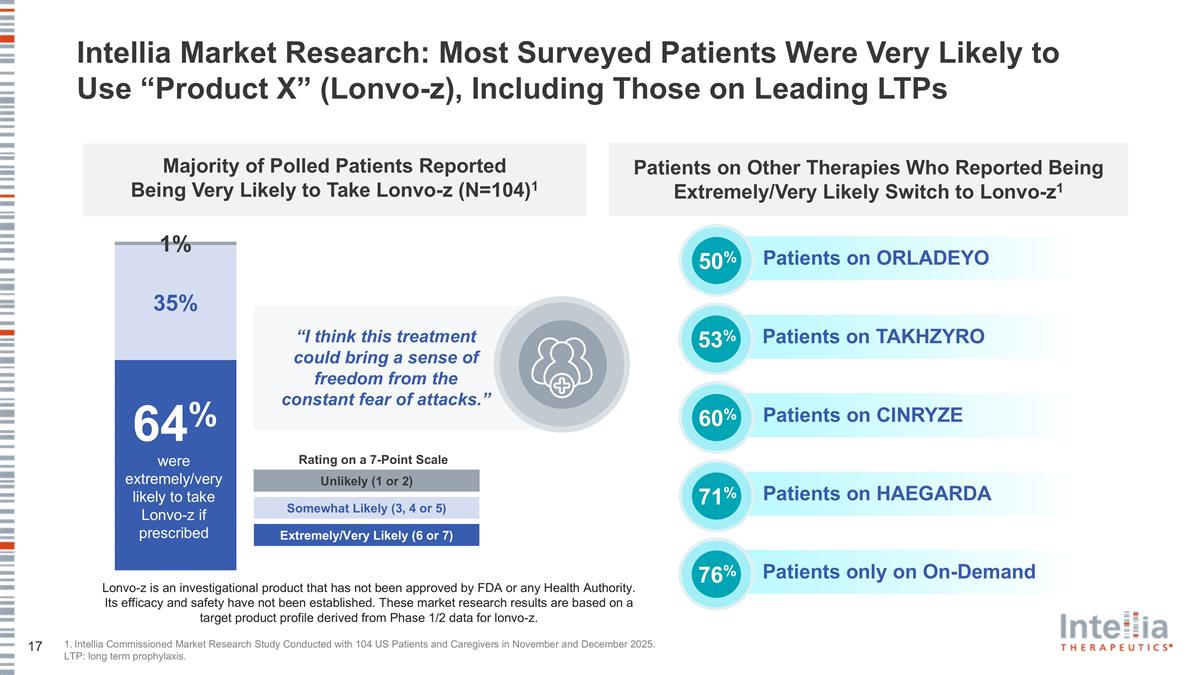
Intellia Market Research: Most Surveyed Patients Were Very Likely to Use “Product X” (Lonvo-z), Including Those on Leading LTPs 1. Intellia Commissioned Market Research Study Conducted with 104 US Patients and Caregivers in November and December 2025. LTP: long term prophylaxis. Unlikely (1 or 2) Somewhat Likely (3, 4 or 5) Extremely/Very Likely (6 or 7) % “I think this treatment could bring a sense of freedom from the constant fear of attacks.” Lonvo-z is an investigational product that has not been approved by FDA or any Health Authority. Its efficacy and safety have not been established. These market research results are based on a target product profile derived from Phase 1/2 data for lonvo-z. Patients on ORLADEYO 50% % 64% were extremely/very likely to take Lonvo-z if prescribed Patients on Other Therapies Who Reported Being Extremely/Very Likely Switch to Lonvo-z1 Majority of Polled Patients Reported Being Very Likely to Take Lonvo-z (N=104)1 Patients on TAKHZYRO 53% Patients on CINRYZE 60% Patients only on On-Demand 76% Patients on HAEGARDA 71% Rating on a 7-Point Scale
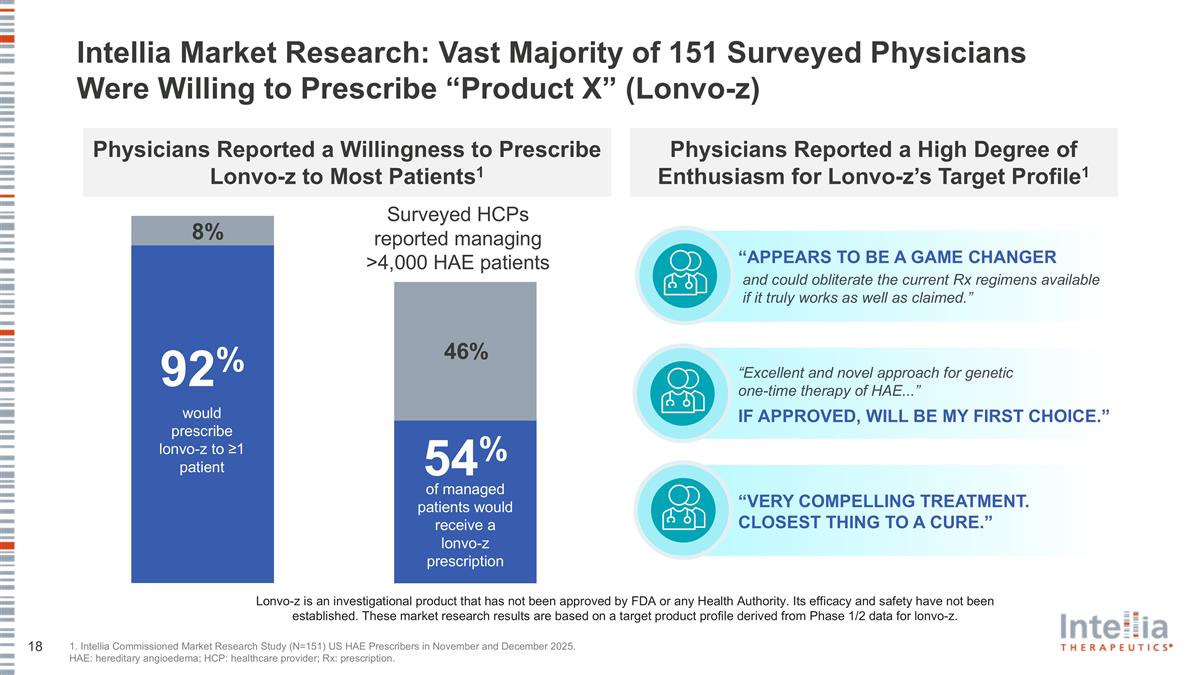
Physicians Reported a High Degree of Enthusiasm for Lonvo-z’s Target Profile1 Physicians Reported a Willingness to Prescribe Lonvo-z to Most Patients1 Intellia Market Research: Vast Majority of 151 Surveyed Physicians Were Willing to Prescribe “Product X” (Lonvo-z) 1. Intellia Commissioned Market Research Study (N=151) US HAE Prescribers in November and December 2025. HAE: hereditary angioedema; HCP: healthcare provider; Rx: prescription. and could obliterate the current Rx regimens available if it truly works as well as claimed.” “APPEARS TO BE A GAME CHANGER “VERY COMPELLING TREATMENT. CLOSEST THING TO A CURE.” “Excellent and novel approach for genetic one-time therapy of HAE...” IF APPROVED, WILL BE MY FIRST CHOICE.” 8% would prescribe lonvo-z to ≥1 patient 92% Lonvo-z is an investigational product that has not been approved by FDA or any Health Authority. Its efficacy and safety have not been established. These market research results are based on a target product profile derived from Phase 1/2 data for lonvo-z. 46% of managed patients would receive a lonvo-z prescription 54% Surveyed HCPs reported managing >4,000 HAE patients
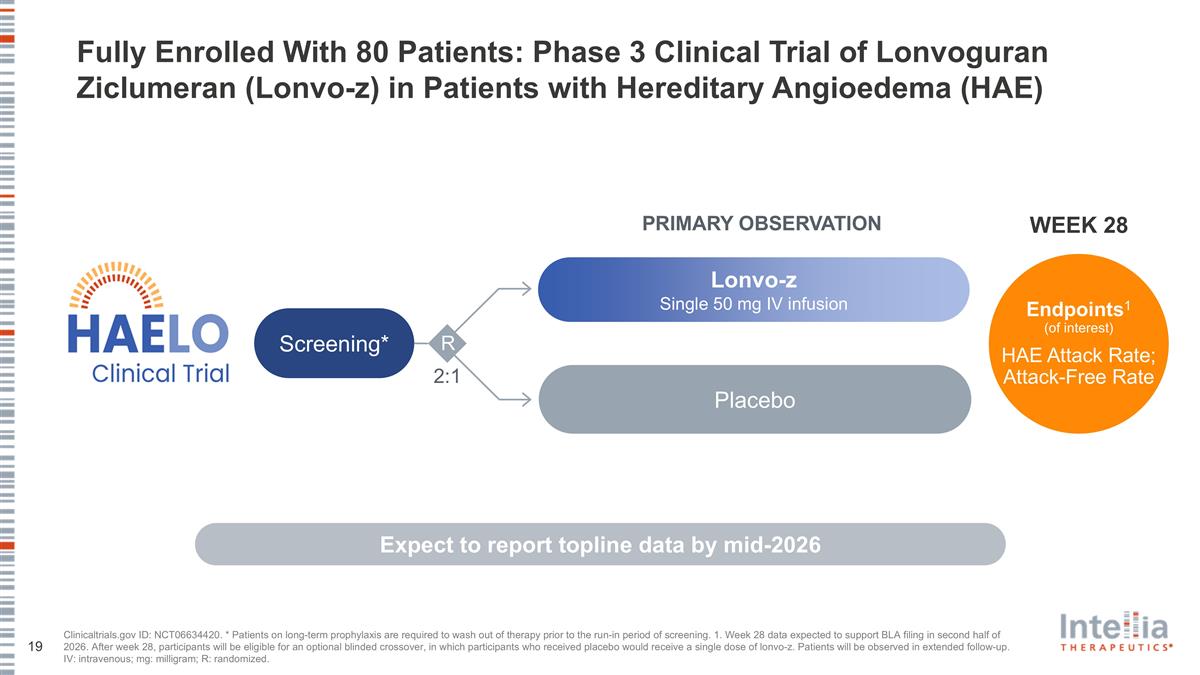
Clinicaltrials.gov ID: NCT06634420. * Patients on long-term prophylaxis are required to wash out of therapy prior to the run-in period of screening. 1. Week 28 data expected to support BLA filing in second half of 2026. After week 28, participants will be eligible for an optional blinded crossover, in which participants who received placebo would receive a single dose of lonvo-z. Patients will be observed in extended follow-up. IV: intravenous; mg: milligram; R: randomized. 2:1 Placebo Lonvo-z Single 50 mg IV infusion Screening* PRIMARY OBSERVATION R Endpoints1 (of interest) HAE Attack Rate; Attack-Free Rate WEEK 28 Fully Enrolled With 80 Patients: Phase 3 Clinical Trial of Lonvoguran Ziclumeran (Lonvo-z) in Patients with Hereditary Angioedema (HAE) Expect to report topline data by mid-2026

Preparing for a Successful Planned Commercial Launch in 1H 2027 Core commercialization team in place Scale field sales and reimbursement teams Finalize distribution model Identify treatment centers Finalize pricing Contracting strategy Priorities in 2026… Overall launch strategy in place Field medical team deployed and engaging with KOLs Relationships established with HAEA and medical societies Payer engagement underway HAEA: Hereditary Angioedema Association; KOL: key opinion leader.
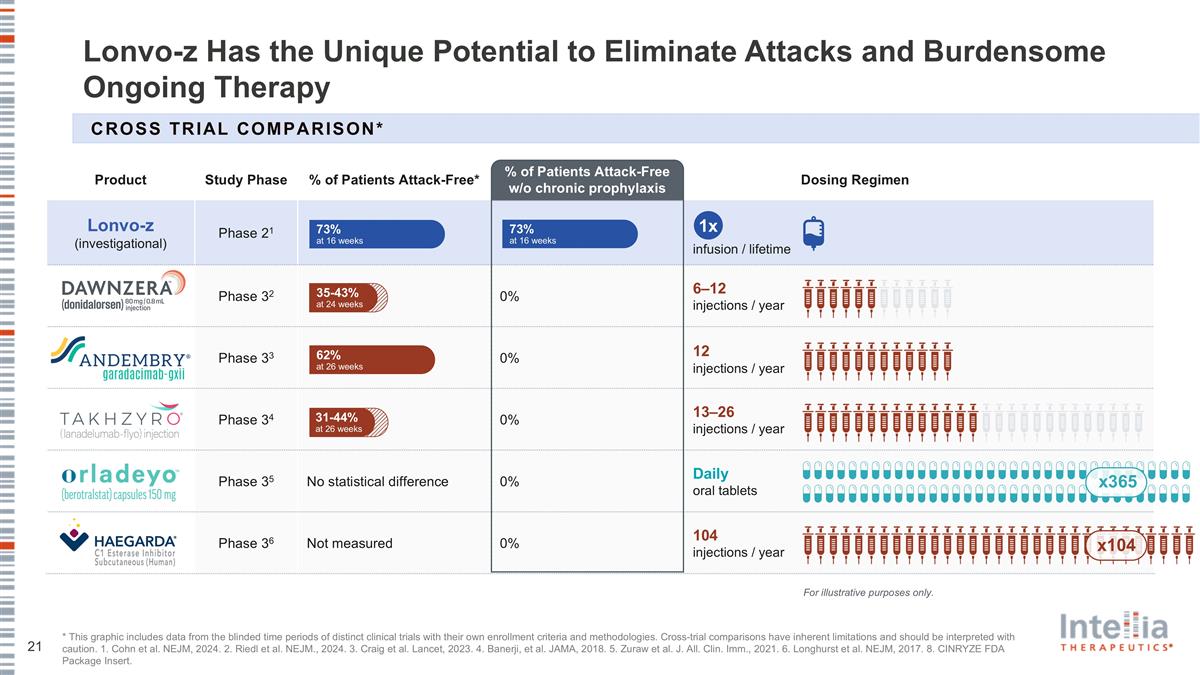
Lonvo-z Has the Unique Potential to Eliminate Attacks and Burdensome Ongoing Therapy * This graphic includes data from the blinded time periods of distinct clinical trials with their own enrollment criteria and methodologies. Cross-trial comparisons have inherent limitations and should be interpreted with caution. 1. Cohn et al. NEJM, 2024. 2. Riedl et al. NEJM., 2024. 3. Craig et al. Lancet, 2023. 4. Banerji, et al. JAMA, 2018. 5. Zuraw et al. J. All. Clin. Imm., 2021. 6. Longhurst et al. NEJM, 2017. 8. CINRYZE FDA Package Insert. Product Study Phase % of Patients Attack-Free* % of Patients Attack-Free w/o chronic prophylaxis Dosing Regimen Lonvo-z (investigational) Phase 21 infusion / lifetime Phase 32 0% 6–12 injections / year Phase 33 0% 12 injections / year Phase 34 0% 13–26 injections / year Phase 35 No statistical difference 0% Daily oral tablets Phase 36 Not measured 0% 104 injections / year 73% at 16 weeks 62% at 26 weeks 35-43% at 24 weeks 31-44% at 26 weeks CROSS TRIAL Comparison* 1x x365 x104 For illustrative purposes only. 73% at 16 weeks

Nexiguran Ziclumeran (nex-z) for ATTR Cardiomyopathy and ATTR Polyneuropathy

Transthyretin Amyloidosis (ATTR): Large and Growing Market with Significant Unmet Need Severe, fatal, progressive disease with shortened life expectancy CM patients have debilitating shortness of breath, arrythmias, reduced mobility and quality of life, as well as a high rate of hospitalization Wild-type disease, the most common form, occurs with aging and manifests as heart failure; inherited TTR mutations lead to rapidly progressive heart failure and/or polyneuropathy 250,000 to 500,000 ATTR patients worldwide1-4; increasing rate of diagnosis due to an aging population and improved disease awareness PN presents as motor and sensory dysfunction, muscle wasting, weight loss, as well as autonomic neuropathy with severe GI symptoms Despite available treatments, significant unmet need persists Inconsistent and slow TTR lowering response observed with silencers5 In Phase 3 trials of silencer or stabilizer therapies for CM, the annual rate of CV events or death is high at ~15% of enrolled patients in the first year5,6 Even on existing therapies, CM patients have a marked decline in quality of life and functional capacity as measured by 6MWT5,6 Treatment adherence due to frequent administration/polypharmacy remains an issue Living with ATTR amyloidosis with polyneuropathy I look at my dad and think, Is that what’s going to happen to me in the future? NANCY 1. Hawkins et al, Annals of Medicine, 2015. 2. Maurer et al, Circulation: Heart Failure, 2019. 3. Nativi-Nicolau et al, ESC Heart Failure, 2021. 4. Gillmore et al, American Heart Association Scientific Sessions, 2022. 5. Fontana et al, NEJM, 2024. 6. Gillmore et al, NEJM, 2024. CM: cardiomyopathy; CV: cardiovascular; GI: gastrointestinal; PN: polyneuropathy; TTR: transthyretin; 6MWT: 6-Minute Walk Test.
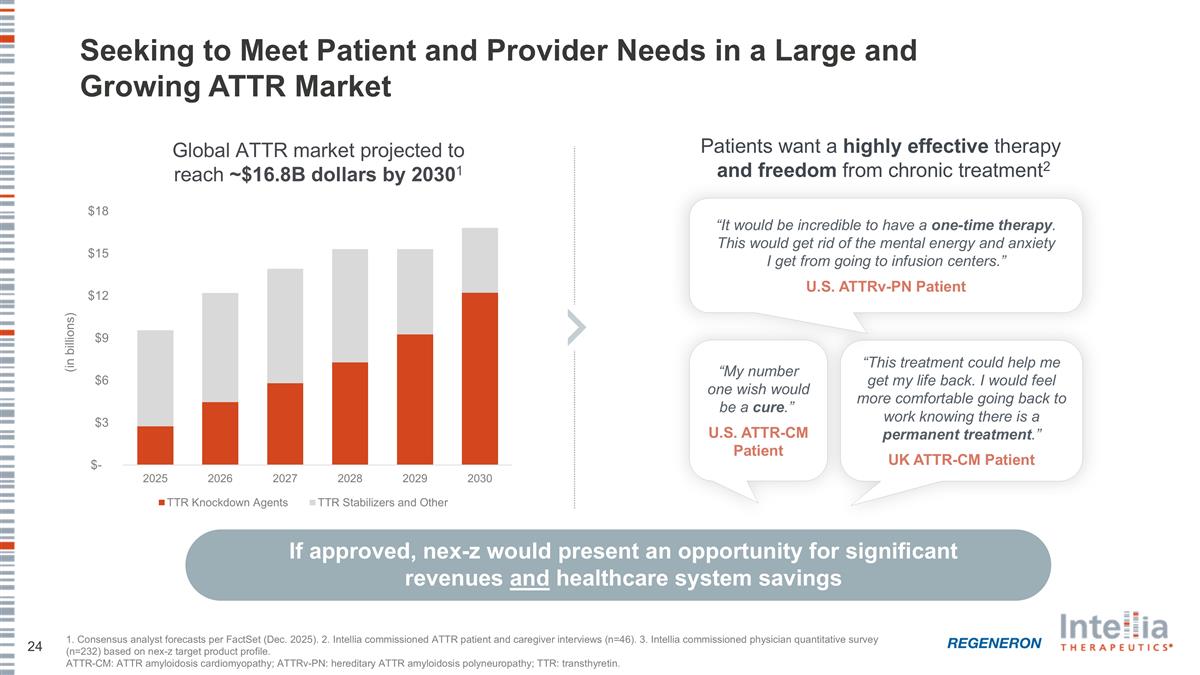
Seeking to Meet Patient and Provider Needs in a Large and Growing ATTR Market If approved, nex-z would present an opportunity for significant revenues and healthcare system savings 1. Consensus analyst forecasts per FactSet (Dec. 2025). 2. Intellia commissioned ATTR patient and caregiver interviews (n=46). 3. Intellia commissioned physician quantitative survey (n=232) based on nex-z target product profile. ATTR-CM: ATTR amyloidosis cardiomyopathy; ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy; TTR: transthyretin. Patients want a highly effective therapy and freedom from chronic treatment2 “My number one wish would be a cure.” U.S. ATTR-CM Patient “This treatment could help me get my life back. I would feel more comfortable going back to work knowing there is a permanent treatment.” UK ATTR-CM Patient “It would be incredible to have a one-time therapy. This would get rid of the mental energy and anxiety I get from going to infusion centers.” U.S. ATTRv-PN Patient Global ATTR market projected to reach ~$16.8B dollars by 20301 (in billions)

Nex-z Has the Potential to Transform the Standard of Care for ATTR Amyloidosis First and only investigational therapy that targets the TTR gene, to reduce production of TTR protein at its source Potential to provide patients with: Consistently rapid, deep and durable TTR reduction Stability or improvement in disease measures Improved quality of life Reduced CV events and mortality Nex-z is being developed as a one-time IV infusion administered in an outpatient setting Based on interim nex-z Phase 1 data as of data cutoff August 21, 2024. Studies are ongoing to assess this investigational product. ATTR amyloidosis: transthyretin amyloidosis; CV: cardiovascular; IV: intravenous. NANCY Living with ATTR amyloidosis with polyneuropathy
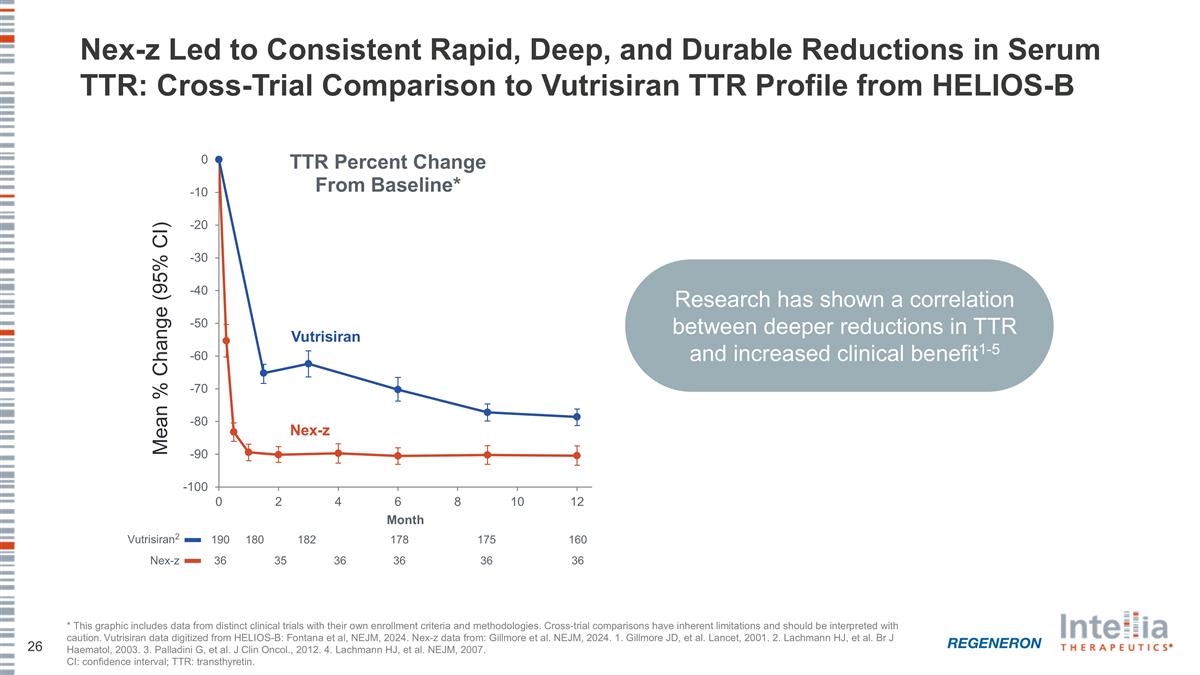
Nex-z Led to Consistent Rapid, Deep, and Durable Reductions in Serum TTR: Cross-Trial Comparison to Vutrisiran TTR Profile from HELIOS-B Vutrisiran2 190 180 182 178 160 175 Nex-z 36 35 36 36 36 36 Nex-z Vutrisiran Mean % Change (95% CI) * This graphic includes data from distinct clinical trials with their own enrollment criteria and methodologies. Cross-trial comparisons have inherent limitations and should be interpreted with caution. Vutrisiran data digitized from HELIOS-B: Fontana et al, NEJM, 2024. Nex-z data from: Gillmore et al. NEJM, 2024. 1. Gillmore JD, et al. Lancet, 2001. 2. Lachmann HJ, et al. Br J Haematol, 2003. 3. Palladini G, et al. J Clin Oncol., 2012. 4. Lachmann HJ, et al. NEJM, 2007. CI: confidence interval; TTR: transthyretin. Research has shown a correlation between deeper reductions in TTR and increased clinical benefit1-5
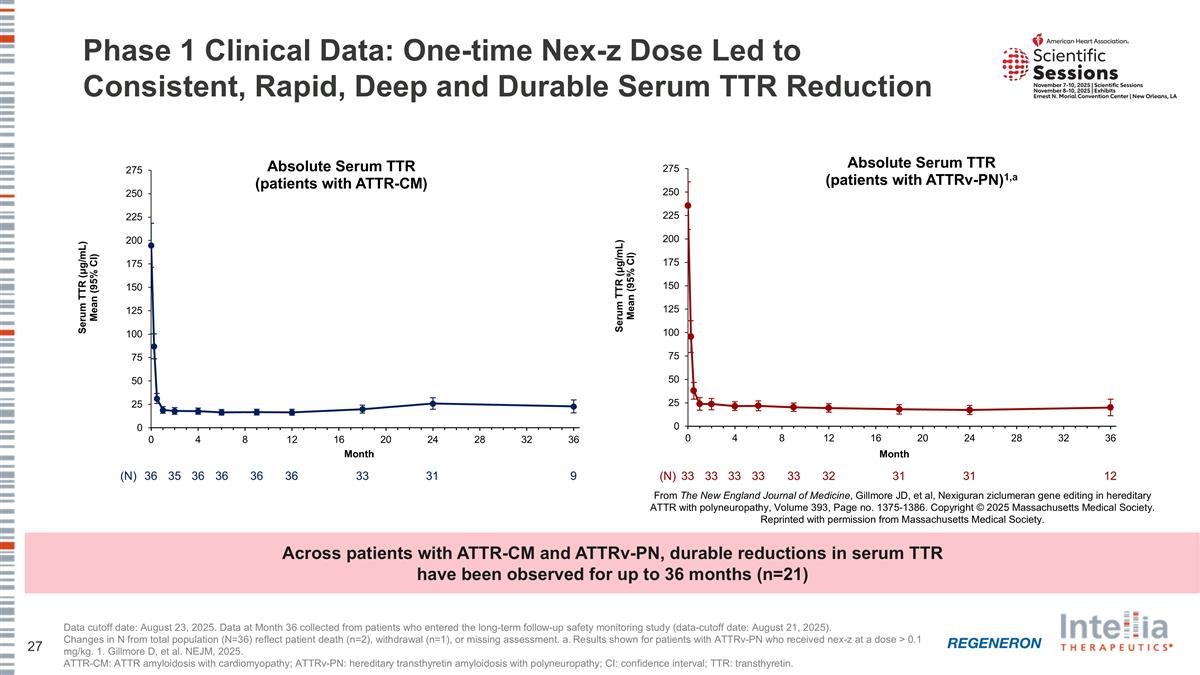
Phase 1 Clinical Data: One-time Nex-z Dose Led to Consistent, Rapid, Deep and Durable Serum TTR Reduction Data cutoff date: August 23, 2025. Data at Month 36 collected from patients who entered the long-term follow-up safety monitoring study (data-cutoff date: August 21, 2025). Changes in N from total population (N=36) reflect patient death (n=2), withdrawal (n=1), or missing assessment. a. Results shown for patients with ATTRv-PN who received nex-z at a dose > 0.1 mg/kg. 1. Gillmore D, et al. NEJM, 2025. ATTR-CM: ATTR amyloidosis with cardiomyopathy; ATTRv-PN: hereditary transthyretin amyloidosis with polyneuropathy; CI: confidence interval; TTR: transthyretin. (N) 36 35 36 36 36 36 33 31 9 From The New England Journal of Medicine, Gillmore JD, et al, Nexiguran ziclumeran gene editing in hereditary ATTR with polyneuropathy, Volume 393, Page no. 1375-1386. Copyright © 2025 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. (N) 33 33 33 33 32 33 31 31 12 Across patients with ATTR-CM and ATTRv-PN, durable reductions in serum TTR have been observed for up to 36 months (n=21)
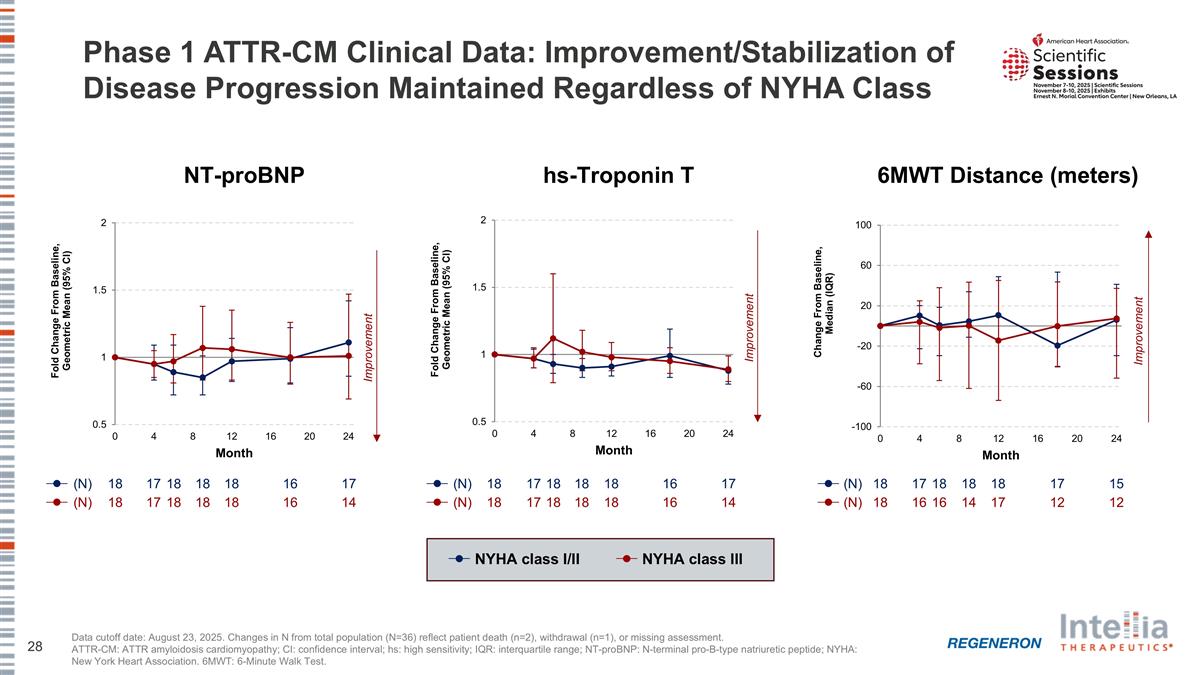
Phase 1 ATTR-CM Clinical Data: Improvement/Stabilization of Disease Progression Maintained Regardless of NYHA Class NT-proBNP hs-Troponin T 6MWT Distance (meters) Data cutoff date: August 23, 2025. Changes in N from total population (N=36) reflect patient death (n=2), withdrawal (n=1), or missing assessment. ATTR-CM: ATTR amyloidosis cardiomyopathy; CI: confidence interval; hs: high sensitivity; IQR: interquartile range; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association. 6MWT: 6-Minute Walk Test. NYHA class I/II NYHA class III (N) 18 17 18 18 18 17 16 (N) 18 17 18 18 18 14 16 (N) 18 17 18 18 17 18 16 (N) 18 17 18 18 14 18 16 (N) 18 17 18 18 18 17 15 (N) 18 16 16 14 17 12 12 Improvement Improvement Improvement
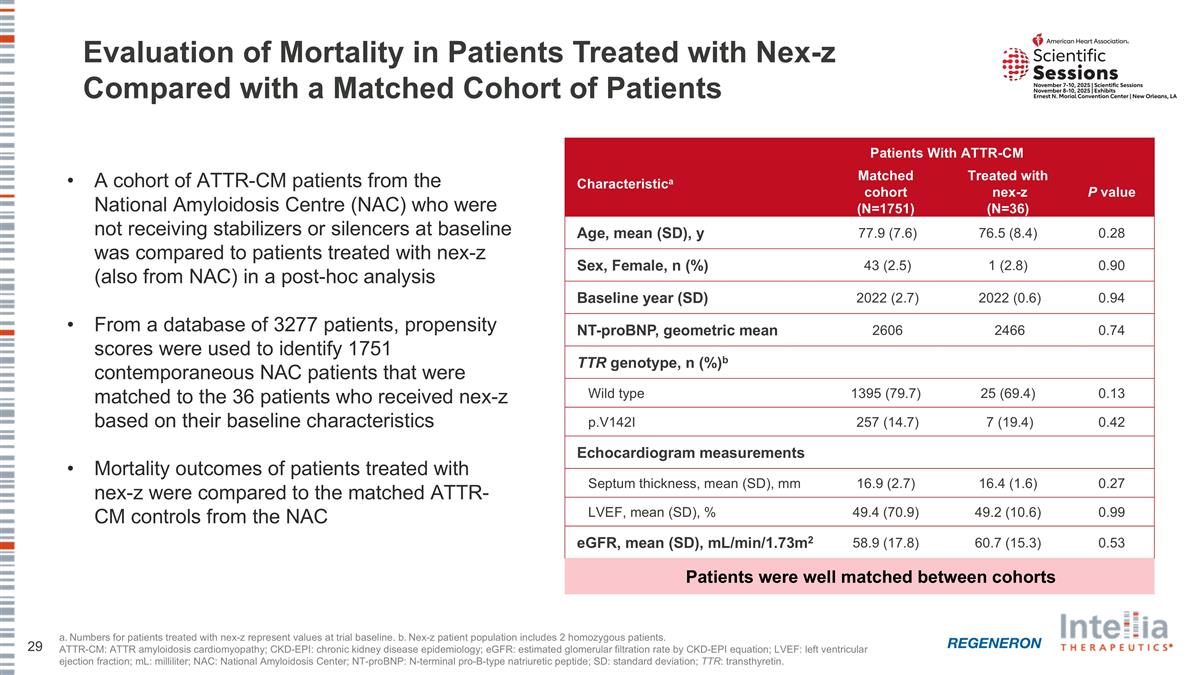
Evaluation of Mortality in Patients Treated with Nex-z Compared with a Matched Cohort of Patients a. Numbers for patients treated with nex-z represent values at trial baseline. b. Nex-z patient population includes 2 homozygous patients. ATTR-CM: ATTR amyloidosis cardiomyopathy; CKD-EPI: chronic kidney disease epidemiology; eGFR: estimated glomerular filtration rate by CKD-EPI equation; LVEF: left ventricular ejection fraction; mL: milliliter; NAC: National Amyloidosis Center; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SD: standard deviation; TTR: transthyretin. A cohort of ATTR-CM patients from the National Amyloidosis Centre (NAC) who were not receiving stabilizers or silencers at baseline was compared to patients treated with nex-z (also from NAC) in a post-hoc analysis From a database of 3277 patients, propensity scores were used to identify 1751 contemporaneous NAC patients that were matched to the 36 patients who received nex-z based on their baseline characteristics Mortality outcomes of patients treated with nex-z were compared to the matched ATTR-CM controls from the NAC Patients With ATTR-CM Characteristica Matched cohort (N=1751) Treated with nex-z (N=36) P value Age, mean (SD), y 77.9 (7.6) 76.5 (8.4) 0.28 Sex, Female, n (%) 43 (2.5) 1 (2.8) 0.90 Baseline year (SD) 2022 (2.7) 2022 (0.6) 0.94 NT-proBNP, geometric mean 2606 2466 0.74 TTR genotype, n (%)b Wild type 1395 (79.7) 25 (69.4) 0.13 p.V142I 257 (14.7) 7 (19.4) 0.42 Echocardiogram measurements Septum thickness, mean (SD), mm 16.9 (2.7) 16.4 (1.6) 0.27 LVEF, mean (SD), % 49.4 (70.9) 49.2 (10.6) 0.99 eGFR, mean (SD), mL/min/1.73m2 58.9 (17.8) 60.7 (15.3) 0.53 Patients were well matched between cohorts
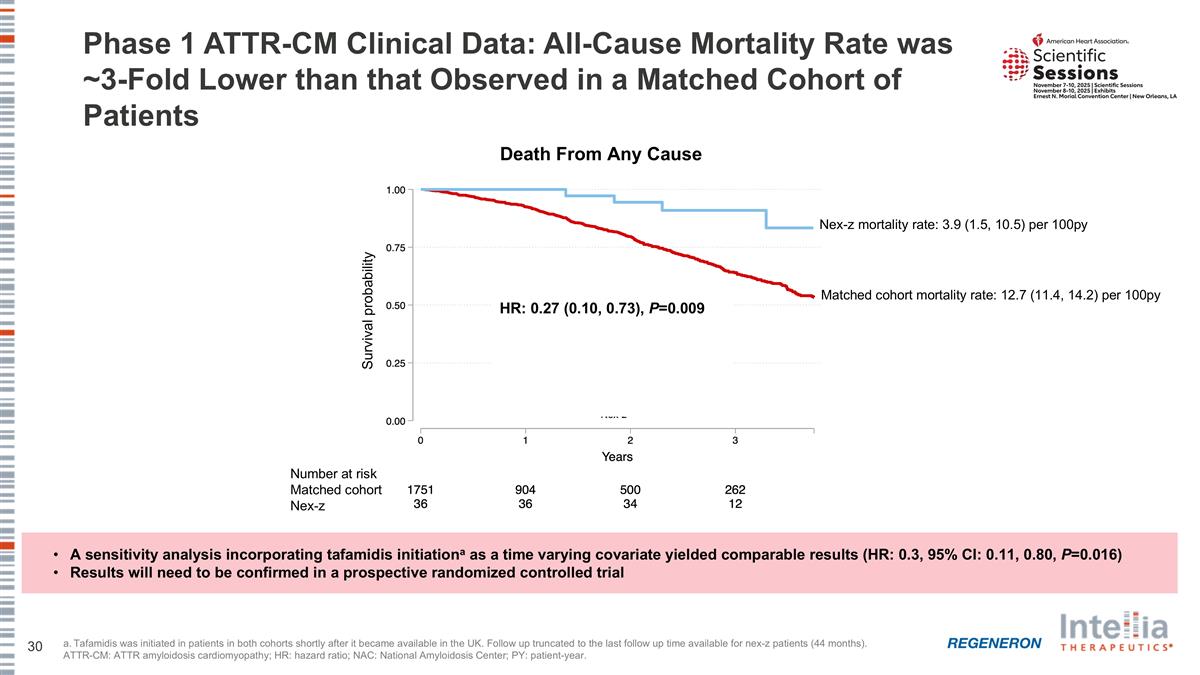
Phase 1 ATTR-CM Clinical Data: All-Cause Mortality Rate was ~3-Fold Lower than that Observed in a Matched Cohort of Patients a. Tafamidis was initiated in patients in both cohorts shortly after it became available in the UK. Follow up truncated to the last follow up time available for nex-z patients (44 months). ATTR-CM: ATTR amyloidosis cardiomyopathy; HR: hazard ratio; NAC: National Amyloidosis Center; PY: patient-year. A sensitivity analysis incorporating tafamidis initiationa as a time varying covariate yielded comparable results (HR: 0.3, 95% CI: 0.11, 0.80, P=0.016) Results will need to be confirmed in a prospective randomized controlled trial Death From Any Cause HR: 0.27 (0.10, 0.73), P=0.009 Survival probability Matched cohort mortality rate: 12.7 (11.4, 14.2) per 100py Nex-z mortality rate: 3.9 (1.5, 10.5) per 100py Number at risk Matched cohort Nex-z
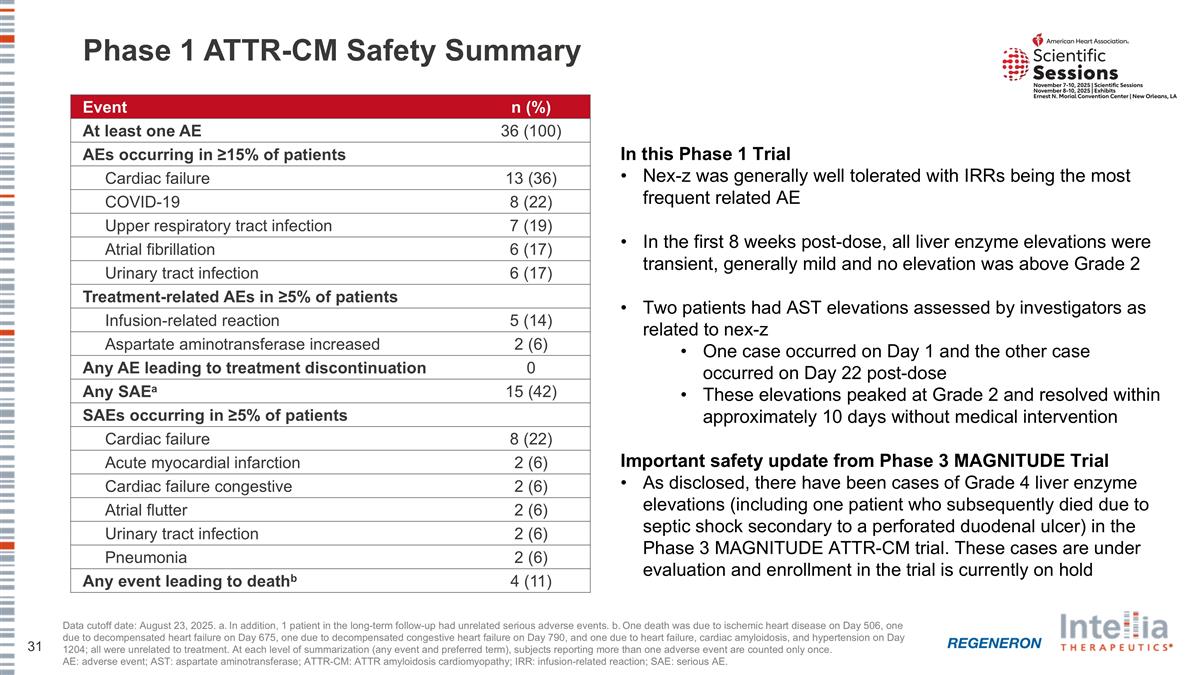
Phase 1 ATTR-CM Safety Summary Data cutoff date: August 23, 2025. a. In addition, 1 patient in the long-term follow-up had unrelated serious adverse events. b. One death was due to ischemic heart disease on Day 506, one due to decompensated heart failure on Day 675, one due to decompensated congestive heart failure on Day 790, and one due to heart failure, cardiac amyloidosis, and hypertension on Day 1204; all were unrelated to treatment. At each level of summarization (any event and preferred term), subjects reporting more than one adverse event are counted only once. AE: adverse event; AST: aspartate aminotransferase; ATTR-CM: ATTR amyloidosis cardiomyopathy; IRR: infusion-related reaction; SAE: serious AE. Event n (%) At least one AE 36 (100) AEs occurring in ≥15% of patients Cardiac failure 13 (36) COVID-19 8 (22) Upper respiratory tract infection 7 (19) Atrial fibrillation 6 (17) Urinary tract infection 6 (17) Treatment-related AEs in ≥5% of patients Infusion-related reaction 5 (14) Aspartate aminotransferase increased 2 (6) Any AE leading to treatment discontinuation 0 Any SAEa 15 (42) SAEs occurring in ≥5% of patients Cardiac failure 8 (22) Acute myocardial infarction 2 (6) Cardiac failure congestive 2 (6) Atrial flutter 2 (6) Urinary tract infection 2 (6) Pneumonia 2 (6) Any event leading to deathb 4 (11) In this Phase 1 Trial Nex-z was generally well tolerated with IRRs being the most frequent related AE In the first 8 weeks post-dose, all liver enzyme elevations were transient, generally mild and no elevation was above Grade 2 Two patients had AST elevations assessed by investigators as related to nex-z One case occurred on Day 1 and the other case occurred on Day 22 post-dose These elevations peaked at Grade 2 and resolved within approximately 10 days without medical intervention Important safety update from Phase 3 MAGNITUDE Trial As disclosed, there have been cases of Grade 4 liver enzyme elevations (including one patient who subsequently died due to septic shock secondary to a perforated duodenal ulcer) in the Phase 3 MAGNITUDE ATTR-CM trial. These cases are under evaluation and enrollment in the trial is currently on hold
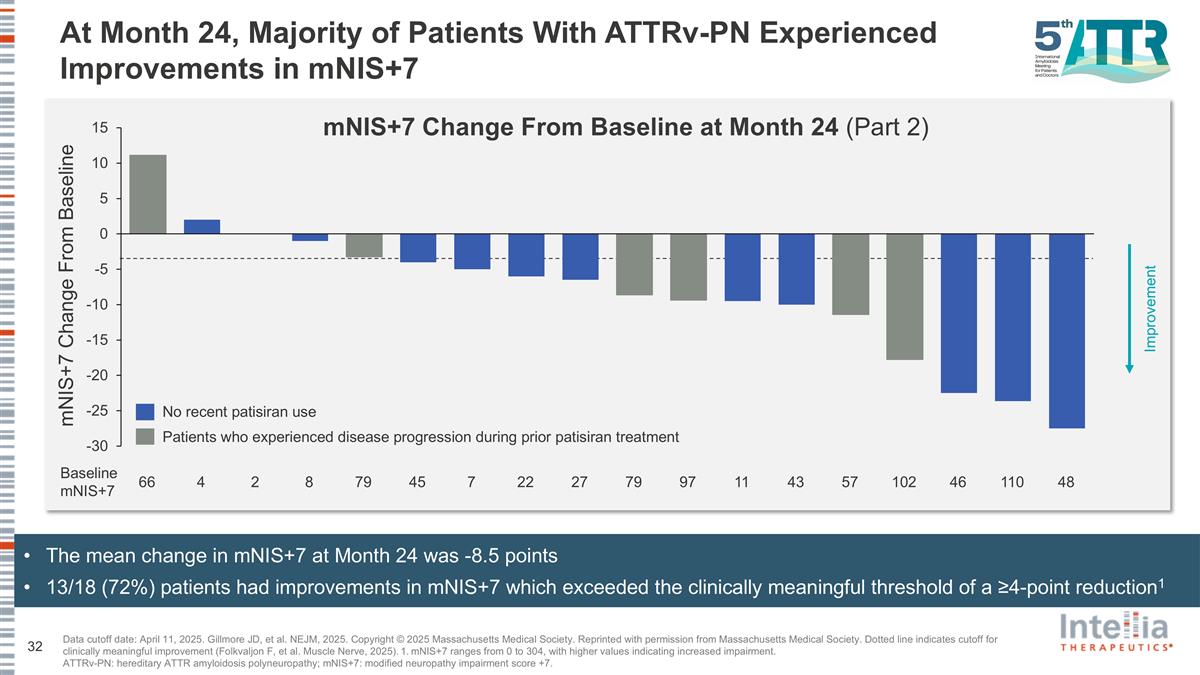
At Month 24, Majority of Patients With ATTRv-PN Experienced Improvements in mNIS+7 mNIS+7 Change From Baseline No recent patisiran use Patients who experienced disease progression during prior patisiran treatment mNIS+7 Change From Baseline at Month 24 (Part 2) Improvement 66 4 2 8 79 45 7 22 27 79 97 11 43 57 102 46 110 48 Baseline mNIS+7 The mean change in mNIS+7 at Month 24 was -8.5 points 13/18 (72%) patients had improvements in mNIS+7 which exceeded the clinically meaningful threshold of a ≥4-point reduction1 Data cutoff date: April 11, 2025. Gillmore JD, et al. NEJM, 2025. Copyright © 2025 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. Dotted line indicates cutoff for clinically meaningful improvement (Folkvaljon F, et al. Muscle Nerve, 2025). 1. mNIS+7 ranges from 0 to 304, with higher values indicating increased impairment. ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy; mNIS+7: modified neuropathy impairment score +7.
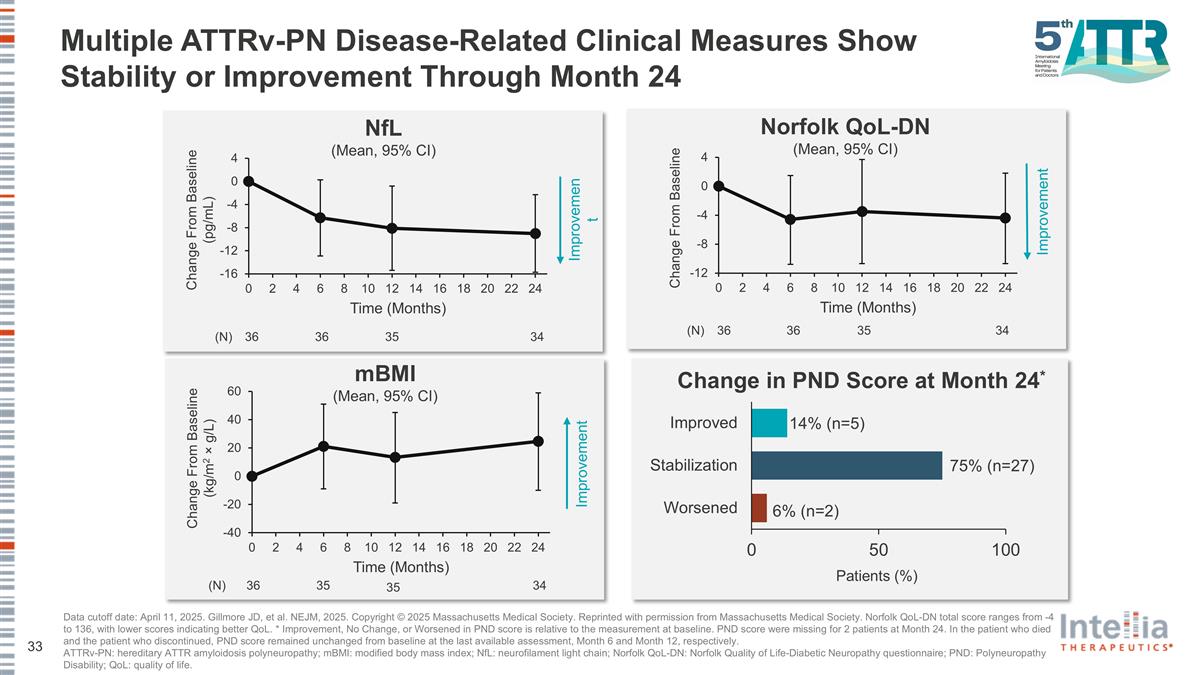
NfL (Mean, 95% CI) 35 (N) 36 36 34 Change From Baseline (pg/mL) Improvement Multiple ATTRv-PN Disease-Related Clinical Measures Show Stability or Improvement Through Month 24 Norfolk QoL-DN (Mean, 95% CI) Change From Baseline 35 (N) 36 36 34 Improvement Change From Baseline (kg/m2 × g/L) mBMI (Mean, 95% CI) 35 (N) 35 36 34 Improvement Change in PND Score at Month 24* Patients (%) 14% (n=5) 75% (n=27) 6% (n=2) Data cutoff date: April 11, 2025. Gillmore JD, et al. NEJM, 2025. Copyright © 2025 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. Norfolk QoL-DN total score ranges from -4 to 136, with lower scores indicating better QoL. * Improvement, No Change, or Worsened in PND score is relative to the measurement at baseline. PND score were missing for 2 patients at Month 24. In the patient who died and the patient who discontinued, PND score remained unchanged from baseline at the last available assessment, Month 6 and Month 12, respectively. ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy; mBMI: modified body mass index; NfL: neurofilament light chain; Norfolk QoL-DN: Norfolk Quality of Life-Diabetic Neuropathy questionnaire; PND: Polyneuropathy Disability; QoL: quality of life.
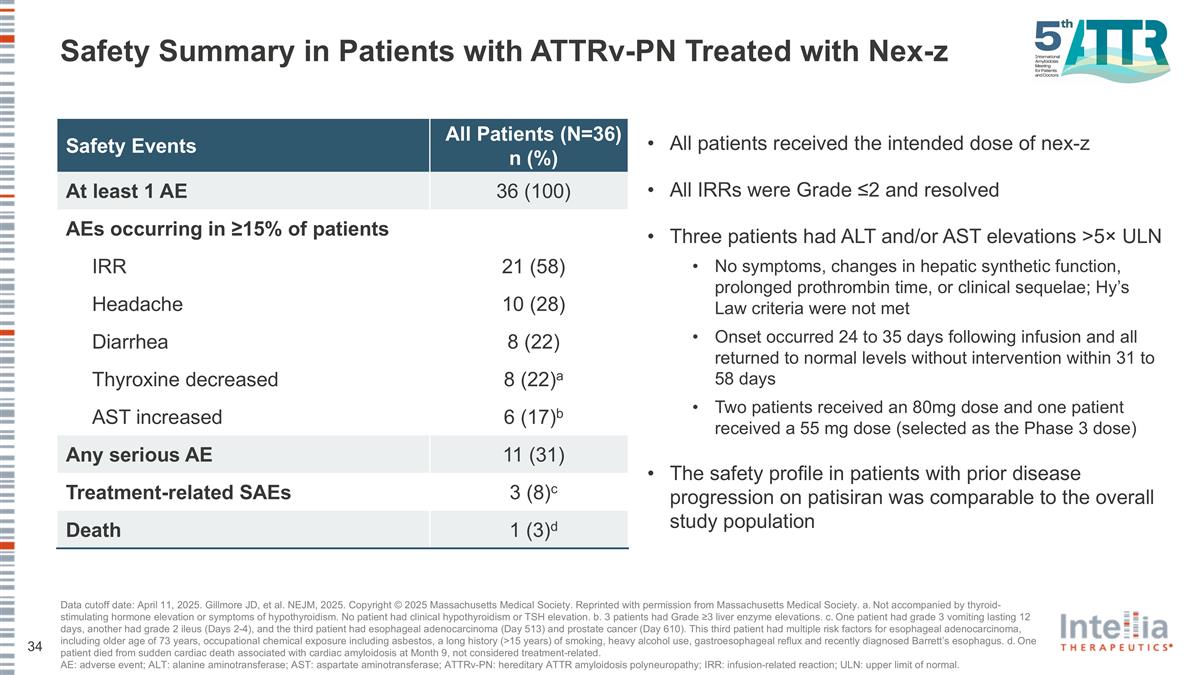
Safety Summary in Patients with ATTRv-PN Treated with Nex-z Safety Events All Patients (N=36) n (%) At least 1 AE 36 (100) AEs occurring in ≥15% of patients IRR 21 (58) Headache 10 (28) Diarrhea 8 (22) Thyroxine decreased 8 (22)a AST increased 6 (17)b Any serious AE 11 (31) Treatment-related SAEs 3 (8)c Death 1 (3)d Data cutoff date: April 11, 2025. Gillmore JD, et al. NEJM, 2025. Copyright © 2025 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. a. Not accompanied by thyroid-stimulating hormone elevation or symptoms of hypothyroidism. No patient had clinical hypothyroidism or TSH elevation. b. 3 patients had Grade ≥3 liver enzyme elevations. c. One patient had grade 3 vomiting lasting 12 days, another had grade 2 ileus (Days 2-4), and the third patient had esophageal adenocarcinoma (Day 513) and prostate cancer (Day 610). This third patient had multiple risk factors for esophageal adenocarcinoma, including older age of 73 years, occupational chemical exposure including asbestos, a long history (>15 years) of smoking, heavy alcohol use, gastroesophageal reflux and recently diagnosed Barrett’s esophagus. d. One patient died from sudden cardiac death associated with cardiac amyloidosis at Month 9, not considered treatment-related. AE: adverse event; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy; IRR: infusion-related reaction; ULN: upper limit of normal. All patients received the intended dose of nex-z All IRRs were Grade ≤2 and resolved Three patients had ALT and/or AST elevations >5× ULN No symptoms, changes in hepatic synthetic function, prolonged prothrombin time, or clinical sequelae; Hy’s Law criteria were not met Onset occurred 24 to 35 days following infusion and all returned to normal levels without intervention within 31 to 58 days Two patients received an 80mg dose and one patient received a 55 mg dose (selected as the Phase 3 dose) The safety profile in patients with prior disease progression on patisiran was comparable to the overall study population
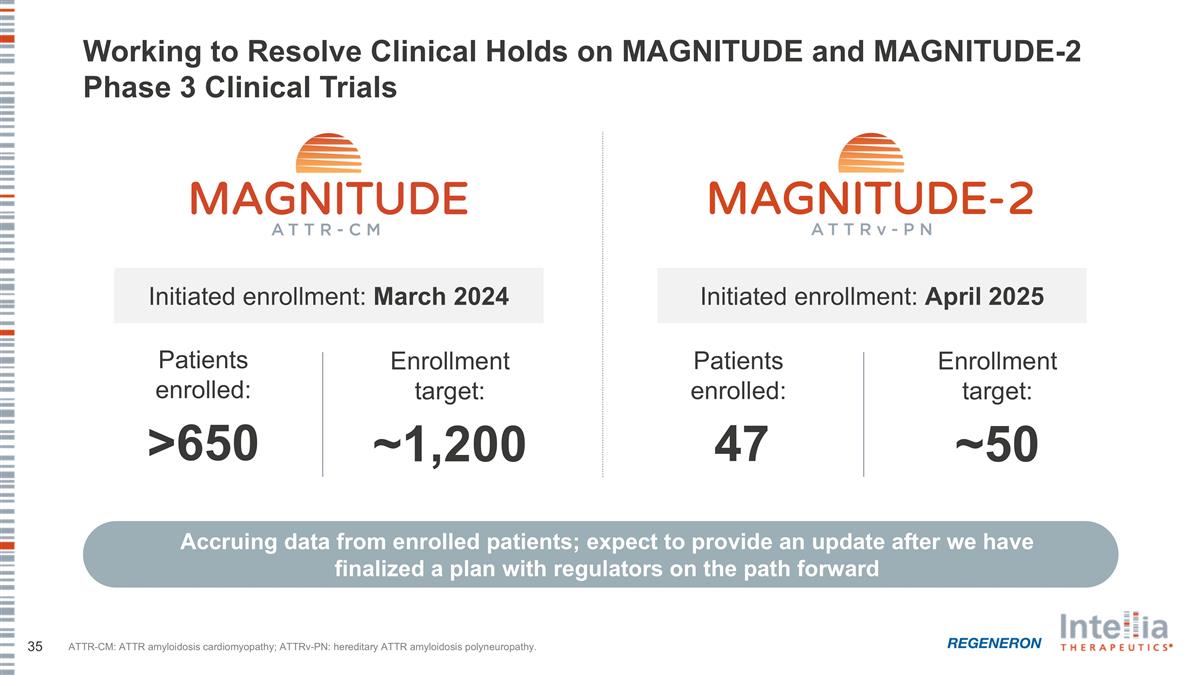
Working to Resolve Clinical Holds on MAGNITUDE and MAGNITUDE-2 Phase 3 Clinical Trials Initiated enrollment: March 2024 Accruing data from enrolled patients; expect to provide an update after we have finalized a plan with regulators on the path forward Patients enrolled: >650 Enrollment target: ~1,200 Initiated enrollment: April 2025 Patients enrolled: 47 Enrollment target: ~50 ATTR-CM: ATTR amyloidosis cardiomyopathy; ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy.
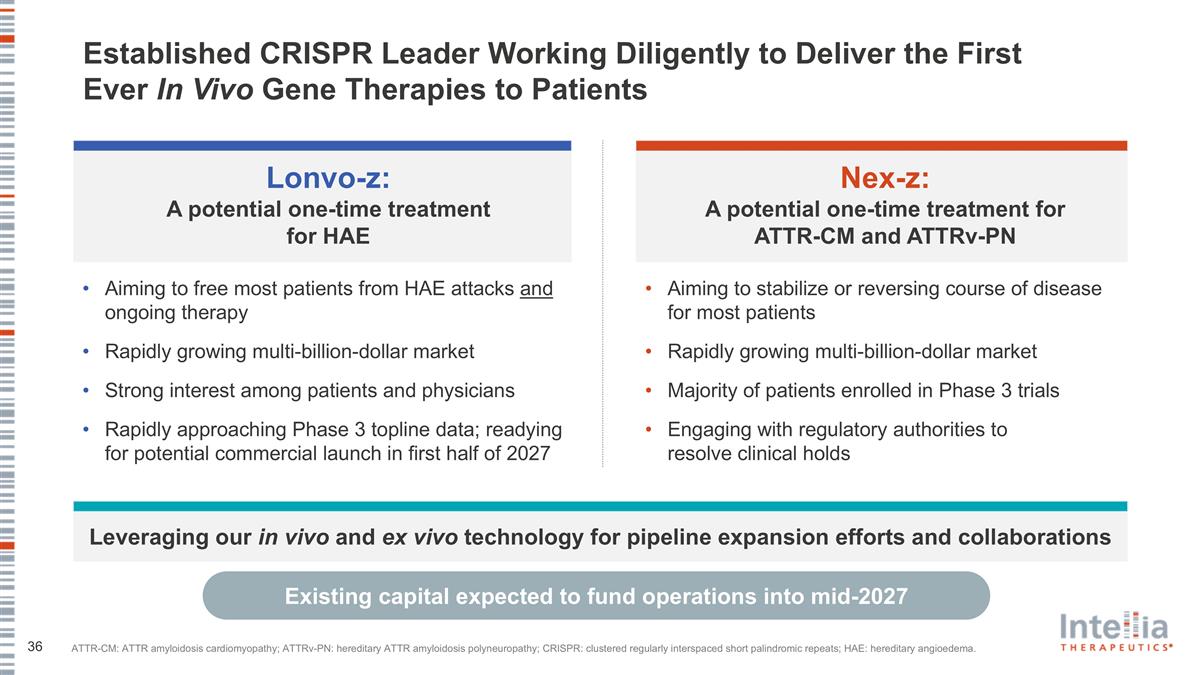
Established CRISPR Leader Working Diligently to Deliver the First Ever In Vivo Gene Therapies to Patients Nex-z: A potential one-time treatment for ATTR-CM and ATTRv-PN Aiming to stabilize or reversing course of disease for most patients Rapidly growing multi-billion-dollar market Majority of patients enrolled in Phase 3 trials Engaging with regulatory authorities to resolve clinical holds Lonvo-z: A potential one-time treatment for HAE Aiming to free most patients from HAE attacks and ongoing therapy Rapidly growing multi-billion-dollar market Strong interest among patients and physicians Rapidly approaching Phase 3 topline data; readying for potential commercial launch in first half of 2027 Leveraging our in vivo and ex vivo technology for pipeline expansion efforts and collaborations Existing capital expected to fund operations into mid-2027 ATTR-CM: ATTR amyloidosis cardiomyopathy; ATTRv-PN: hereditary ATTR amyloidosis polyneuropathy; CRISPR: clustered regularly interspaced short palindromic repeats; HAE: hereditary angioedema.
