

.2 J A N U A R Y 2 0 2 6 Corporate Update

Forward Looking Statements and Legal Disclaimers Forward Looking Statements: This presentation contains forward-looking statements. Crinetics Pharmaceuticals, Inc. (“Crinetics,” the “company,” “we,” “us,” or “our”) cautions you that all statements other than statements of historical facts contained in this presentation are forward-looking statements. Such forward-looking statements include, but are not limited to, statements regarding: estimates relating to market size, or our ability to drive diagnosis and treatment for undiagnosed patients; our ability to effectively commercialize PALSONIFY, the expected timing of initiation of a Phase 3 program for atumelnant for CAH and for a Phase 2/3 program of atumelnant for ACTH-dependent Cushing’s syndrome; the plans and timelines for the clinical development of our drug candidates, including the therapeutic potential and clinical benefits or safety profile thereof; and the expected timing for the initiation of clinical trials or the potential benefits of our development candidates in patients across multiple indications; and the expected timing of additional research pipeline updates or the expected timing of the advancement of those programs. In some cases, you can identify forward-looking statements by terms such as “may,” “believe,” “anticipate,” “could,” “should,” “estimate,” “expect,” “intend,” “plan,” “project,” “will,” “contemplate,” “predict,” “continue,” “forecast,” “aspire,” “lead to,” “designed to,” “goal,” “aim,” “potential,” “target,” or other similar terms or the negatives thereof. These statements speak only as of the date of this presentation, involve known and unknown risks, uncertainties, assumptions, and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, without limitation: estimates relating to market size and growth potential, which involve a number of assumptions and limitations, particularly about any projections, assumptions, and estimates of our future performance; the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk; the possibility of unfavorable new clinical data and further analyses of existing clinical data; potential delays in the commencement, enrollment and completion of clinical trials and the reporting of data therefrom; our dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; the success of our clinical trials and nonclinical studies; regulatory developments or political changes, including the ongoing US government shutdown, policies related to pricing and pharmaceutical drug reimbursement in the United States and foreign countries; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval and/or commercialization; our ability to obtain and maintain intellectual property protection for our product candidates; we may use our capital resources sooner than we expect or our cash burn rate may accelerate; and other risks described under the heading “Risk Factors” in documents we file from time to time with the Securities and Exchange Commission. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and, except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Legal Disclaimers: This presentation contains a preliminary and unaudited estimate of our net product revenue from PALSONIFY as of December 31, 2025. This preliminary and unaudited estimate remains subject to completion of our financial closing procedures, including the completion of management’s reviews and related internal controls over financial reporting. Accordingly, such amount reflects our preliminary and unaudited estimate with respect to such information, based on information currently available to management, and may vary from our actual financial position as of December 31, 2025. Further, this preliminary and unaudited estimate is not a comprehensive statement or estimate of our financial results or financial condition as of December 31, 2025. The preliminary and unaudited estimate included in this presentation has been prepared by, and is the responsibility of, our management. In addition, BDO USA, P.C., our independent registered public accounting firm, has not audited, reviewed, examined, compiled, nor applied agreed-upon procedures with respect to the preliminary and unaudited estimate set forth herein. Accordingly, BDO USA, P.C. does not express an opinion or any other form of assurance with respect thereto. It is possible that we may identify items that require us to make adjustments to the preliminary and unaudited estimate set forth herein. This preliminary estimate should not be viewed as a substitute for financial statements prepared in accordance with generally accepted accounting principles in the United States and is not necessarily indicative of the results to be achieved in any future period. Additional information and disclosure is required for a more complete understanding of our financial position and results of operations as of December 31, 2025. Accordingly, you should not place undue reliance on this preliminary and unaudited estimate. CRINETICS PHARMACEUTICALS | 2 CRINETICS PHARMACEUTICALS | 2
Today’s Key Takeaways Strong commercial execution on PALSONIFY 1 demonstrated by robust metrics New atumelnant data demonstrate 2 promising profile for treatment of CAH Crinetics has multiple levers to drive long-term 3 value CRINETICS PHARMACEUTICALS | 3

Palsonify Launch Update CRINETICS PHARMACEUTICALS | 4
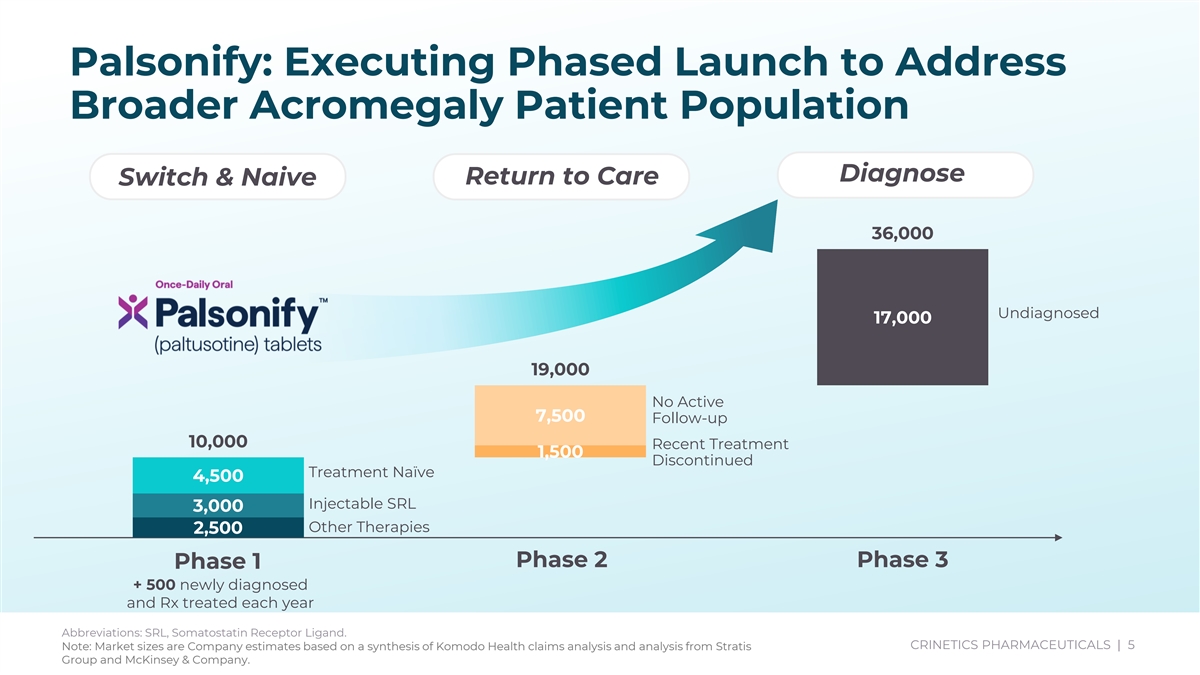
Palsonify: Executing Phased Launch to Address Broader Acromegaly Patient Population Diagnose Return to Care Switch & Naive 36,000 Undiagnosed 17,000 19,000 No Active 7,500 Follow-up 10,000 Recent Treatment 1,500 Discontinued Treatment Naïve 4,500 Injectable SRL 3,000 Other Therapies 2,500 Phase 2 Phase 3 Phase 1 + 500 newly diagnosed and Rx treated each year Abbreviations: SRL, Somatostatin Receptor Ligand. CRINETICS PHARMACEUTICALS | 5 Note: Market sizes are Company estimates based on a synthesis of Komodo Health claims analysis and analysis from Stratis Group and McKinsey & Company.
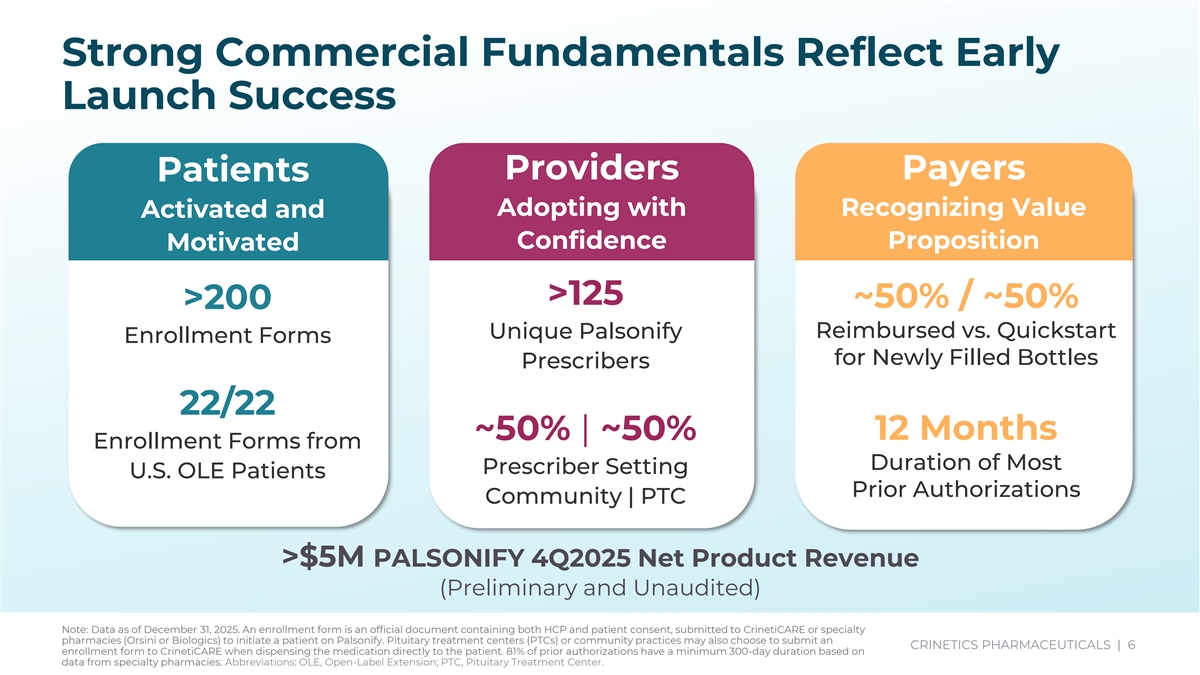
Strong Commercial Fundamentals Reflect Early Launch Success Providers Payers Patients Adopting with Recognizing Value Activated and Confidence Proposition Motivated >125 ~50% / ~50% >200 Unique Palsonify Reimbursed vs. Quickstart Enrollment Forms for Newly Filled Bottles Prescribers 22/22 ~50% | ~50% 12 Months Enrollment Forms from Duration of Most Prescriber Setting U.S. OLE Patients Prior Authorizations Community | PTC >$5M PALSONIFY 4Q2025 Net Product Revenue (Preliminary and Unaudited) Note: Data as of December 31, 2025. An enrollment form is an official document containing both HCP and patient consent, submitted to CrinetiCARE or specialty pharmacies (Orsini or Biologics) to initiate a patient on Palsonify. Pituitary treatment centers (PTCs) or community practices may also choose to submit an CRINETICS PHARMACEUTICALS | 6 enrollment form to CrinetiCARE when dispensing the medication directly to the patient. 81% of prior authorizations have a minimum 300-day duration based on CRINETICS PHARMACEUTICALS | 6 data from specialty pharmacies. Abbreviations: OLE, Open-Label Extension; PTC, Pituitary Treatment Center.

January 2026 CRNX Corporate Update.pptx Palsonify is Delivering Meaningful Patient Impact Megan David Ashleigh David Previously treated with combination of Previously treated with somatuline depot Previously treated with SRL injections, monthly and weekly injections, and is injections, then Mycapssa, and is now and is now on PALSONIFY. now on PALSONIFY. on PALSONIFY. Ashleigh is an OLE patient. “I’ve had some type of pain in my “For the first time in a long time, hands since before 2018. I’d been on “Being on PALSONIFY has been managing my acromegaly feels, PALSONIFY for about a week and a wonderful. I've been waiting for the well, manageable. Now I don't think half. My wife and I were getting clinical trial to be over so I can shout so much about my acromegaly ready for bed. It got quiet. And I it from the rooftops.” medication. I just get up, take my looked down and said ‘Baby my pills, and get ready for the day.” hands don't hurt.’” CRINETICS PHARMACEUTICALS | 7

Atumelnant: Adult CAH Phase 2 & OLE Update CRINETICS PHARMACEUTICALS | 8
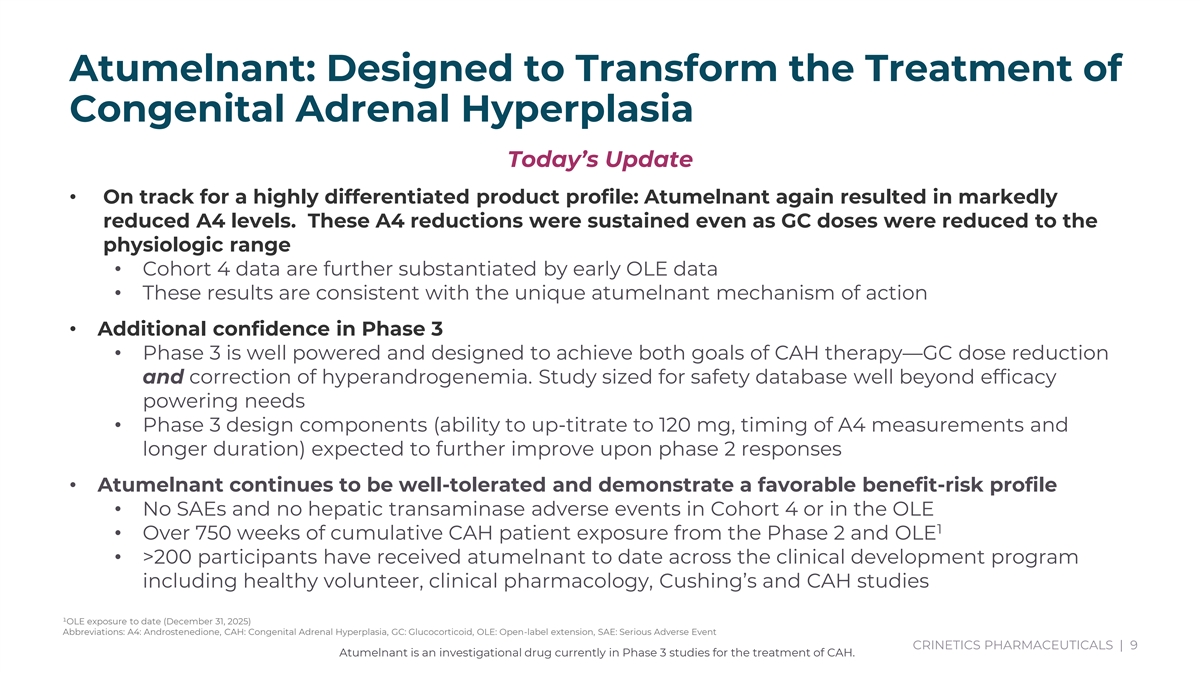
Atumelnant: Designed to Transform the Treatment of Congenital Adrenal Hyperplasia Today’s Update • On track for a highly differentiated product profile: Atumelnant again resulted in markedly reduced A4 levels. These A4 reductions were sustained even as GC doses were reduced to the physiologic range • Cohort 4 data are further substantiated by early OLE data • These results are consistent with the unique atumelnant mechanism of action • Additional confidence in Phase 3 • Phase 3 is well powered and designed to achieve both goals of CAH therapy—GC dose reduction and correction of hyperandrogenemia. Study sized for safety database well beyond efficacy powering needs • Phase 3 design components (ability to up-titrate to 120 mg, timing of A4 measurements and longer duration) expected to further improve upon phase 2 responses • Atumelnant continues to be well-tolerated and demonstrate a favorable benefit-risk profile • No SAEs and no hepatic transaminase adverse events in Cohort 4 or in the OLE 1 • Over 750 weeks of cumulative CAH patient exposure from the Phase 2 and OLE • >200 participants have received atumelnant to date across the clinical development program including healthy volunteer, clinical pharmacology, Cushing’s and CAH studies ¹OLE exposure to date (December 31, 2025) Abbreviations: A4: Androstenedione, CAH: Congenital Adrenal Hyperplasia, GC: Glucocorticoid, OLE: Open-label extension, SAE: Serious Adverse Event CRINETICS PHARMACEUTICALS | 9 Atumelnant is an investigational drug currently in Phase 3 studies for the treatment of CAH.
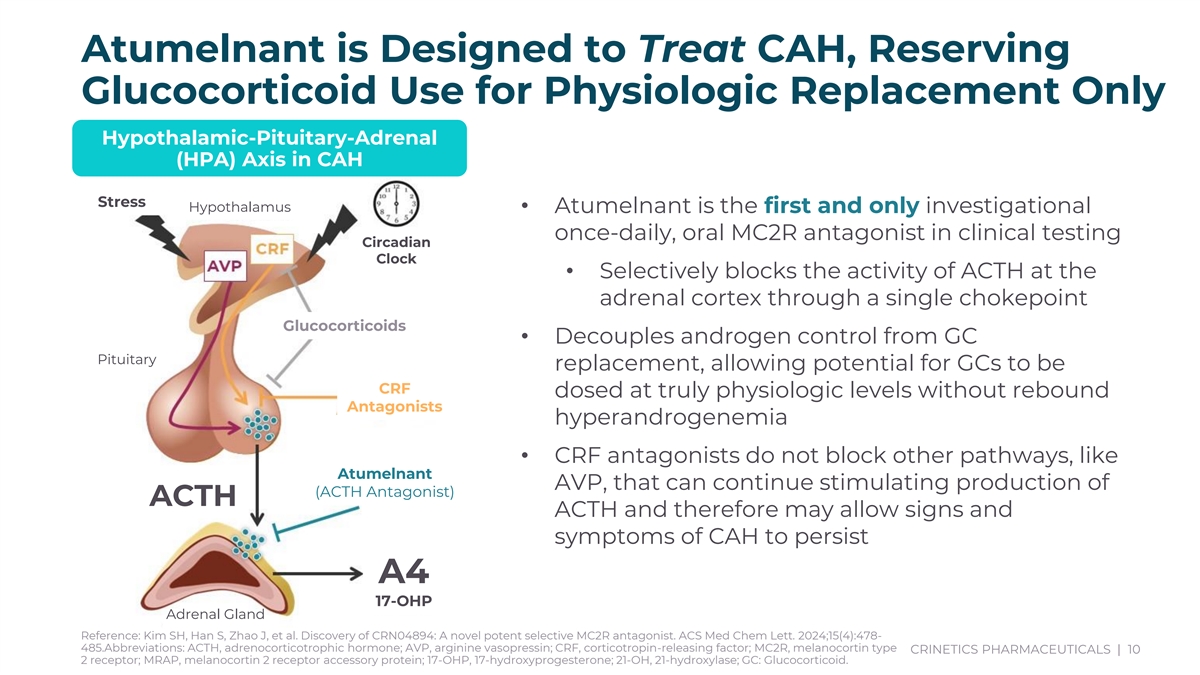
Atumelnant is Designed to Treat CAH, Reserving Glucocorticoid Use for Physiologic Replacement Only Hypothalamic-Pituitary-Adrenal (HPA) Axis in CAH Stress Hypothalamus • Atumelnant is the first and only investigational once-daily, oral MC2R antagonist in clinical testing Circadian Clock • Selectively blocks the activity of ACTH at the adrenal cortex through a single chokepoint Glucocorticoids • Decouples androgen control from GC Pituitary replacement, allowing potential for GCs to be CRF dosed at truly physiologic levels without rebound Antagonists hyperandrogenemia • CRF antagonists do not block other pathways, like Atumelnant AVP, that can continue stimulating production of (ACTH Antagonist) ACTH ACTH and therefore may allow signs and symptoms of CAH to persist A4 17-OHP Adrenal Gland Reference: Kim SH, Han S, Zhao J, et al. Discovery of CRN04894: A novel potent selective MC2R antagonist. ACS Med Chem Lett. 2024;15(4):478- 485.Abbreviations: ACTH, adrenocorticotrophic hormone; AVP, arginine vasopressin; CRF, corticotropin-releasing factor; MC2R, melanocortin type CRINETICS PHARMACEUTICALS | 10 2 receptor; MRAP, melanocortin 2 receptor accessory protein; 17-OHP, 17-hydroxyprogesterone; 21-OH, 21-hydroxylase; GC: Glucocorticoid.
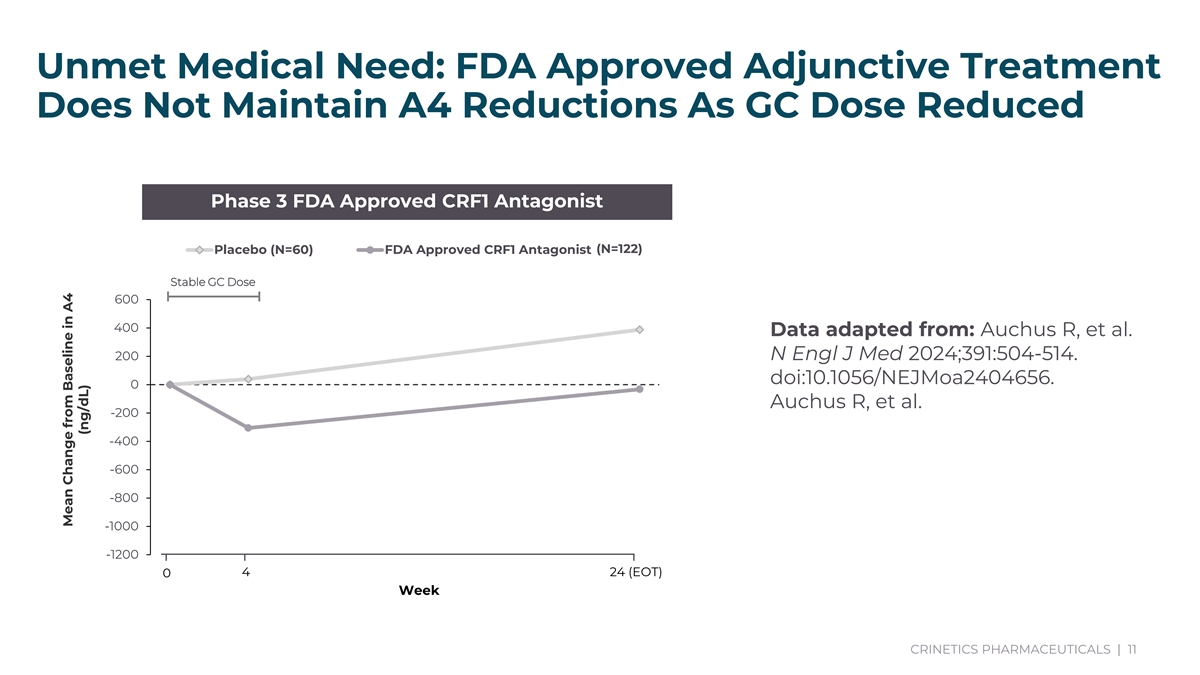
Unmet Medical Need: FDA Approved Adjunctive Treatment Does Not Maintain A4 Reductions As GC Dose Reduced Phase 3 FDA Approved CRF1 Antagonist Placebo (N=60) FDA Approved CRF1 Antagonist (N=122) Stable GC Dose 600 400 Data adapted from: Auchus R, et al. 200 N Engl J Med 2024;391:504-514. doi:10.1056/NEJMoa2404656. 0 Auchus R, et al. -200 -400 -600 -800 -1000 -1200 4 24 (EOT) 0 Week CRINETICS PHARMACEUTICALS | 11 Data adapted from: Auchus R, et al. N Engl J Med 2024;391:504-514. doi:10.1056/NEJMoa2404656. Auchus R, et al. Journal of Clinical Endocrinology & Metabolism. 2021;107(3):801–812. doi:10.1210/clinem/dgab749. Mean Change from Baseline in A4 (ng/dL)
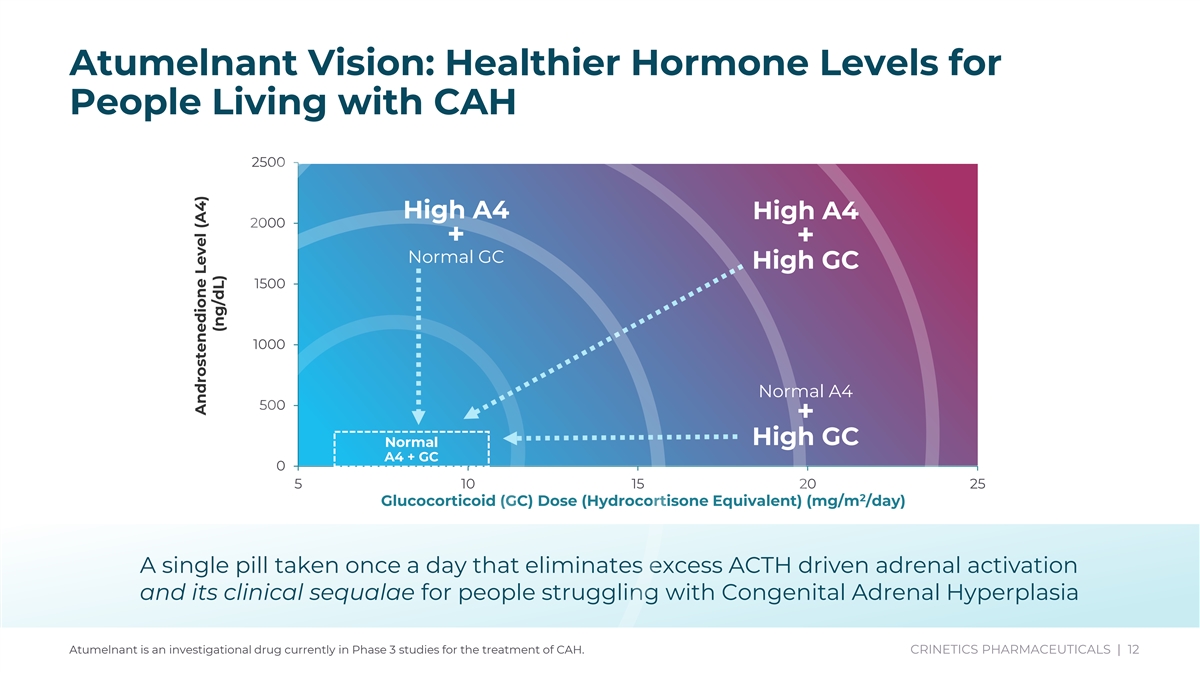
Atumelnant Vision: Healthier Hormone Levels for People Living with CAH 2500 High A4 High A4 2000 + + Normal GC High GC 1500 1000 Normal A4 500 + High GC Normal A4 + GC 0 5 10 15 20 25 2 Glucocorticoid (GC) Dose (Hydrocortisone Equivalent) (mg/m /day) A single pill taken once a day that eliminates excess ACTH driven adrenal activation and its clinical sequalae for people struggling with Congenital Adrenal Hyperplasia Atumelnant is an investigational drug currently in Phase 3 studies for the treatment of CAH. CRINETICS PHARMACEUTICALS | 12 Androstenedione Level (A4) (ng/dL)
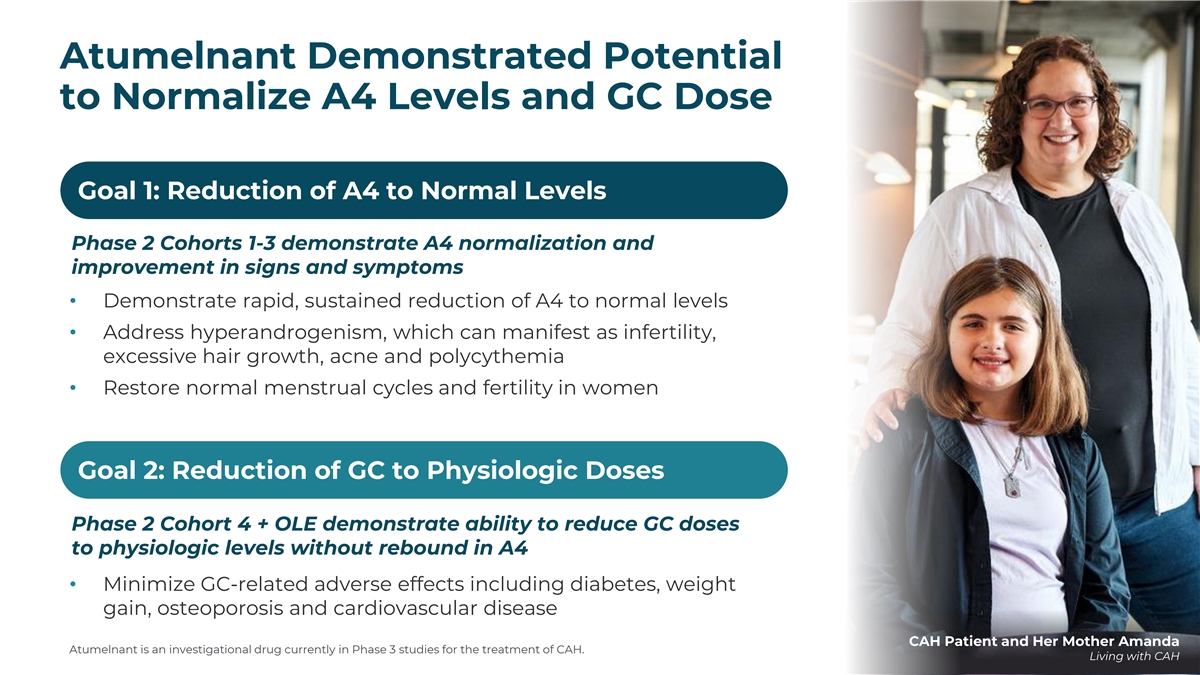
Atumelnant Demonstrated Potential to Normalize A4 Levels and GC Dose Goal 1: Reduction of A4 to Normal Levels Phase 2 Cohorts 1-3 demonstrate A4 normalization and improvement in signs and symptoms • Demonstrate rapid, sustained reduction of A4 to normal levels • Address hyperandrogenism, which can manifest as infertility, excessive hair growth, acne and polycythemia • Restore normal menstrual cycles and fertility in women Goal 2: Reduction of GC to Physiologic Doses Phase 2 Cohort 4 + OLE demonstrate ability to reduce GC doses to physiologic levels without rebound in A4 • Minimize GC-related adverse effects including diabetes, weight gain, osteoporosis and cardiovascular disease CAH Patient and Her Mother Amanda Atumelnant is an investigational drug currently in Phase 3 studies for the treatment of CAH. Living with CAH
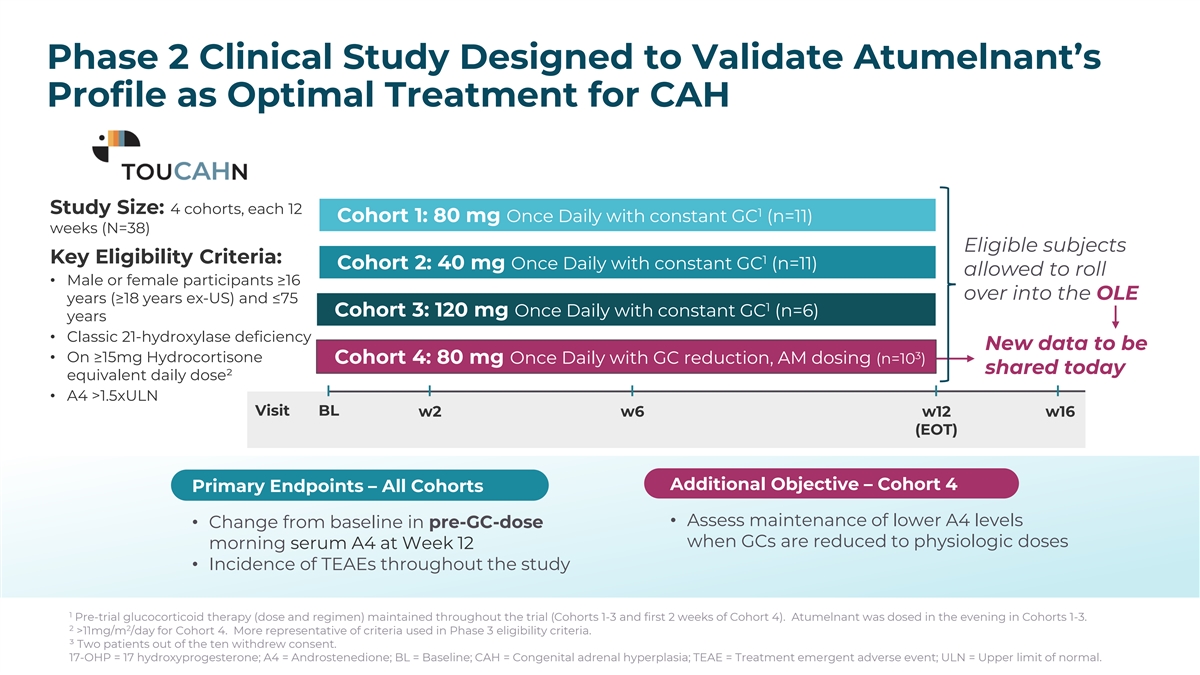
Treatment Arms: Key Eligibility Criteria • Completed cohorts, each 12 weeks (n=28) • Male or female participants ≥16 years (≥18 years ex-US) and ≤75 40 mg Once Daily (n=11) years • Classic 21-hydroxylase 80 mg Once Daily (n=11) deficiency • On ≥15mg Hydrocortisone 120 mg Once Daily (n=6) equivalent daily dose • A4 >1.5xULN Primary Endpoint: Change from baseline in pre-GC morning serum A4 at week 12 Secondary Endpoint: Change from baseline in pre-GC morning serum 17-OHP at week 12 Primary Safety Assessment: Incidence of TEAEs throughout the study Phase 2 Clinical Study Designed to Validate Atumelnant’s Pre-trial glucocorticoid therapy (dose and regimen) maintained throughout the trial Profile as Optimal Treatment for CAH A4: Androstenedione; ULN: Upper limit of normal; 17-OHP: 17 hydroxyprogesterone; TEAE: Treatment emergent adverse event. PK: pharmacokinetics Treatment Arms: Study Size: 4 cohorts, each 12 1 Cohort 1: 80 mg Once Daily with constant GC (n=11) Key Eligibility Criteria weeks (N=38) • 4 cohorts, each 12 weeks (N=28 + 6-12 cohort 4) Eligible subjects N=28-40 Key Eligibility Criteria: 1 Cohort 2: 40 mg Once Daily with constant GC (n=11) allowed to roll • Male or female 40 mg Once Daily (PM) (n=11) • Male or female participants ≥16 participants ≥18 to 75 over into the OLE years (≥18 years ex-US) and ≤75 years. Age: ≥16 years (US) 1 Cohort 3: 120 mg Once Daily with constant GC (n=6) years 80 mg Once Daily (PM) (n=11) • Classic 21-hydroxylase • Classic 21-hydroxylase deficiency deficiency New data to be 3 • On ≥15mg Hydrocortisone Cohort 4: 80 mg Once Daily with GC reduction, AM dosing (n=10 ) • On ≥15mg shared today 120 mg Once Daily (PM) (n=6) equivalent daily dose² 2 (11 mg/m in Cohort 4) • A4 >1.5xULN Hydrocortisone Visit BL w2 w6 w12 w16 equivalent daily dose COHORT 4 - 80 mg Once Daily (AM and GC reduction) (n=6-12) (EOT) • A4 >1.5xULN Additional Objective – Cohort 4 Objectives: Evaluate the Safety, Efficacy, and Pharmacokinetics of Atumelnant Primary Endpoints – All Cohorts • Assess maintenance of lower A4 levels • Change from baseline in pre-GC-dose Primary Endpoint: Change from baseline in morning serum A4 at week 12 when GCs are reduced to physiologic doses morning serum A4 at Week 12 Secondary Endpoint: Change from baseline in morning serum 17-OHP at week 12 • Incidence of TEAEs throughout the study Primary Safety Assessment: Incidence of TEAEs throughout the study A4: Androstenedione; ULN: Upper limit of normal; 17-OHP: 17 hydroxyprogesterone; TEAE: Treatment emergent adverse event. 1 Pre-trial glucocorticoid therapy (dose and regimen) maintained throughout the trial (Cohorts 1-3 and first 2 weeks of Cohort 4). Atumelnant was dosed in the evening in Cohorts 1-3. 2 2 >11mg/m /day for Cohort 4. More representative of criteria used in Phase 3 eligibility criteria. ³ Two patients out of the ten withdrew consent. 17-OHP = 17 hydroxyprogesterone; A4 = Androstenedione; BL = Baseline; CAH = Congenital adrenal hyperplasia; TEAE = Treatment emergent adverse event; ULN = Upper limit of normal. Phase 2 Atumelnant in Congenital Adrenal Hyperplasia (CAH) (TouCAHn) Pre-trial Glucocorticoid Therapy (Dose and Regimen) Maintained Throughout the Trial Treatment Arms: Key Eligibility Criteria • 3 cohorts, each 12 weeks (N=28) N=28 • Male or female participants ≥18 to 75 40 mg Once Daily (n=11) years. Age: ≥16 years (US) • Classic 21-hydroxylase deficiency 80 mg Once Daily (n=11) • On >15mg Hydrocortisone equivalent daily dose 120 mg Once Daily (n=6) • A4 >1.5xULN Objectives: Evaluate the Safety, Efficacy, and Pharmacokinetics of atumelnant Primary Endpoint: Change from baseline in morning serum A4 at week 12 Secondary Endpoint: Change from baseline in morning serum 17-OHP at week 12 Primary Safety Assessment: Incidence of TEAEs throughout the study A4: Androstenedione; ULN: Upper limit of normal; 17-OHP: 17 hydroxyprogesterone; TEAE: Treatment emergent adverse event. Baseline is defined as the last morning window value (i.e. the average of any early morning samples on or after 06:00 but prior to 11:00) prior to the first dose of atumelnant.
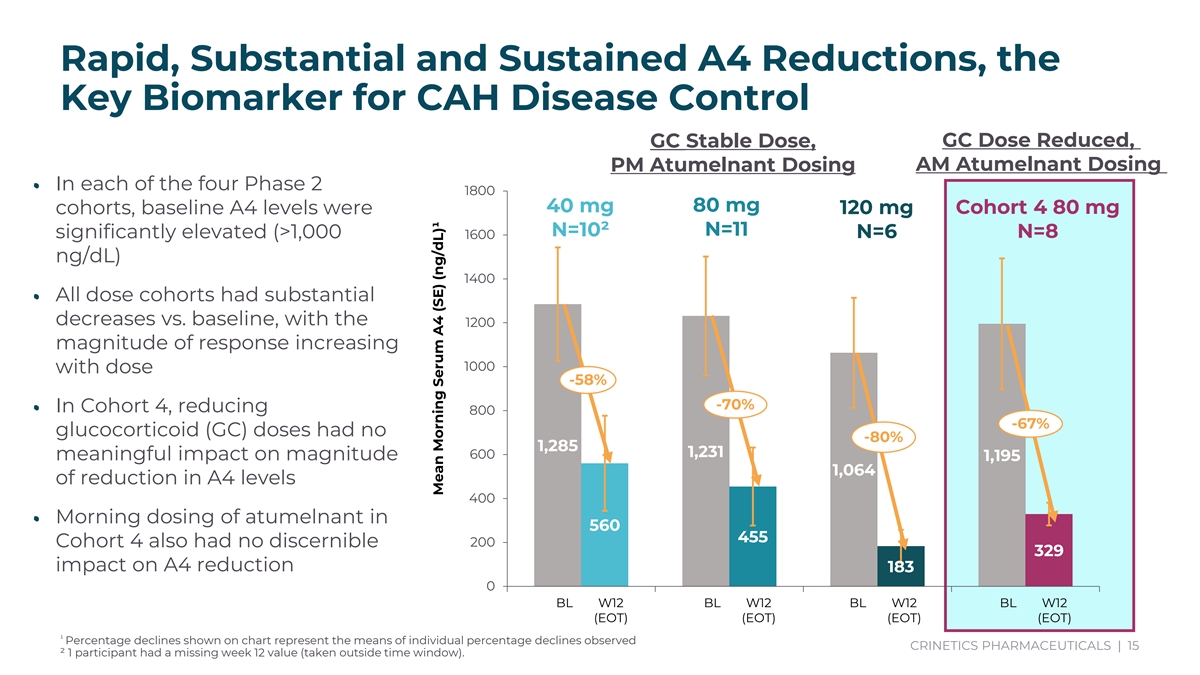
Rapid, Substantial and Sustained A4 Reductions, the Key Biomarker for CAH Disease Control GC Stable Dose, GC Dose Reduced, PM Atumelnant Dosing AM Atumelnant Dosing • In each of the four Phase 2 1800 40 mg 80 mg 120 mg Cohort 4 80 mg cohorts, baseline A4 levels were N=10² N=11 N=8 significantly elevated (>1,000 1600 N=6 ng/dL) 1400 • All dose cohorts had substantial decreases vs. baseline, with the 1200 magnitude of response increasing 1000 with dose -58% -70% • In Cohort 4, reducing 800 -67% glucocorticoid (GC) doses had no -80% 1,285 1,231 600 meaningful impact on magnitude 1,195 1,064 of reduction in A4 levels 400 • Morning dosing of atumelnant in 560 455 200 Cohort 4 also had no discernible 329 impact on A4 reduction 183 0 BL W12 BL W12 BL W12 BL W12 (EOT) (EOT) (EOT) (EOT) ¹ Percentage declines shown on chart represent the means of individual percentage declines observed CRINETICS PHARMACEUTICALS | 15 ² 1 participant had a missing week 12 value (taken outside time window). 40 mg 80 mg Cohort 4 120 mg -60% -70% 67% -80% F ULN M ULN BL W12 BL W12 BL W12 BL W12 (EOT) (EOT) (EOT) (EOT) Mean Morning Serum A4 (SE) (ng/dL)¹ Mean Morning Serum A4 (SE) (ng/dL)
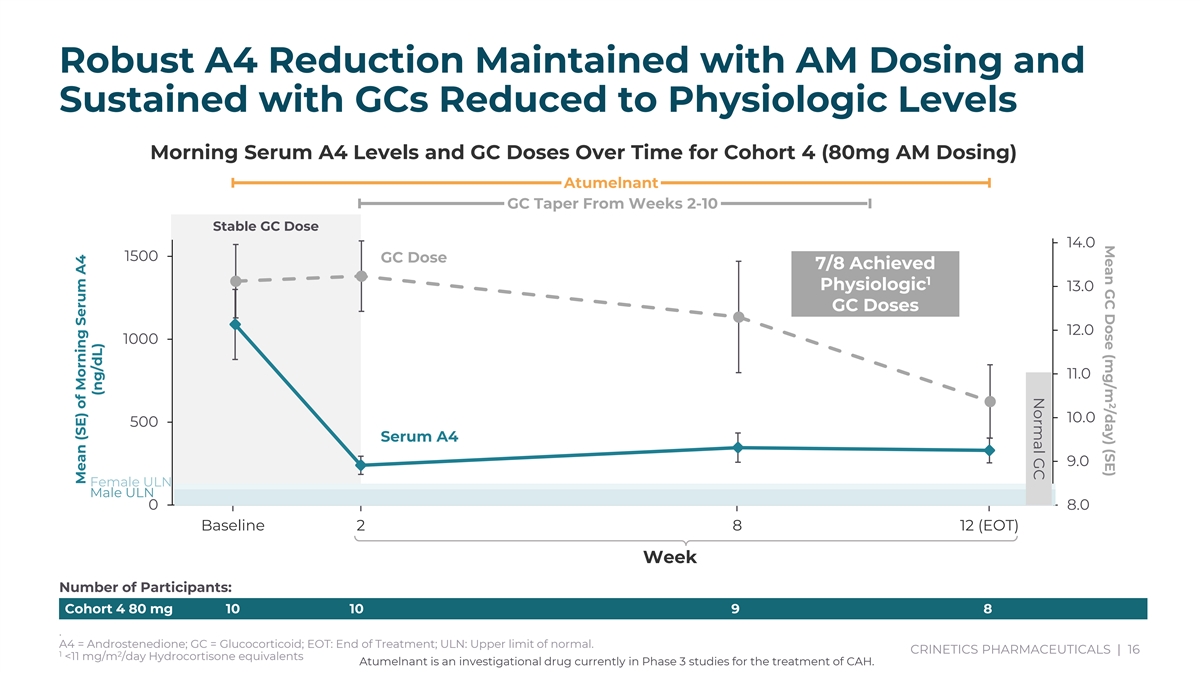
2 Mean GC Dose (mg/m /day) (SE) Normal GC Robust A4 Reduction Maintained with AM Dosing and Sustained with GCs Reduced to Physiologic Levels Morning Serum A4 Levels and GC Doses Over Time for Cohort 4 (80mg AM Dosing) Atumelnant GC Taper From Weeks 2-10 Stable GC Dose 14.0 1500 GC Dose 7/8 Achieved 1 Physiologic 13.0 GC Doses 12.0 1000 11.0 10.0 500 Serum A4 9.0 Female ULN Male ULN 0 8.0 Baseline 2 8 12 (EOT) Week Number of Participants: Cohort 4 80 mg 10 10 9 8 . A4 = Androstenedione; GC = Glucocorticoid; EOT: End of Treatment; ULN: Upper limit of normal. CRINETICS PHARMACEUTICALS | 16 1 2 <11 mg/m /day Hydrocortisone equivalents Atumelnant is an investigational drug currently in Phase 3 studies for the treatment of CAH. Mean (SE) of Morning Serum A4 (ng/dL)
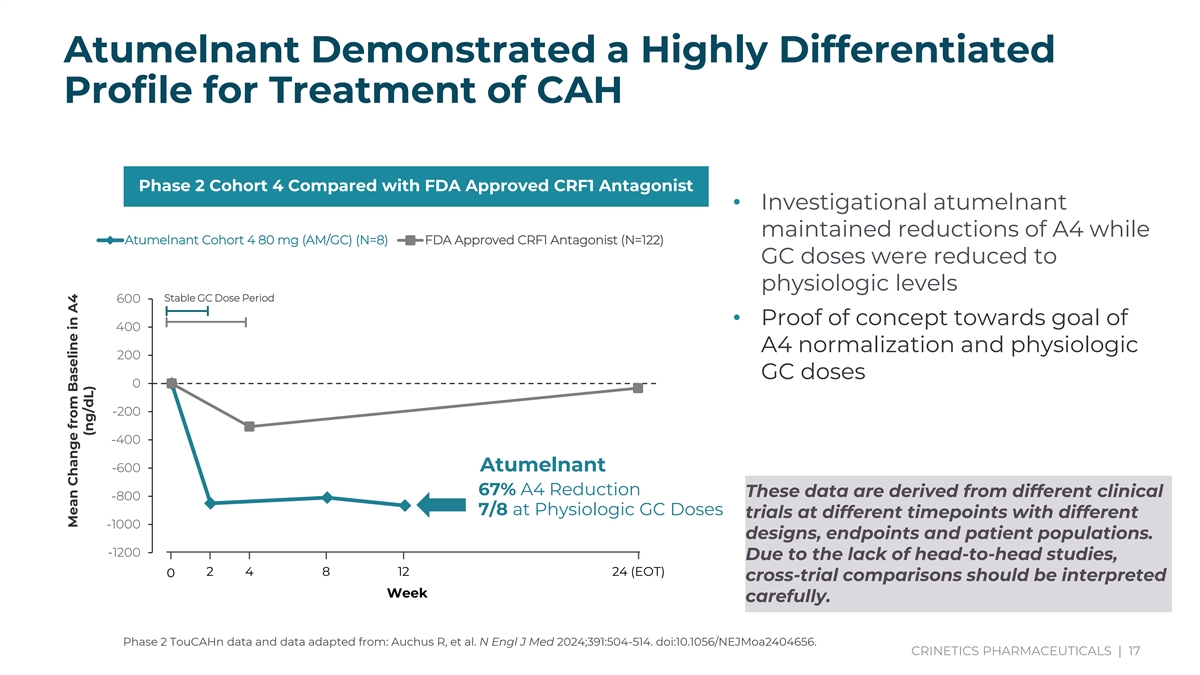
Atumelnant Demonstrated a Highly Differentiated Profile for Treatment of CAH Phase 2 Cohort 4 Compared with FDA Approved CRF1 Antagonist • Investigational atumelnant maintained reductions of A4 while Atumelnant Cohort 4 80 mg (AM/GC) (N=8) FDA Approved CRF1 Antagonist (N=122) GC doses were reduced to physiologic levels Stable GC Dose Period 600 • Proof of concept towards goal of 400 A4 normalization and physiologic 200 GC doses 0 -200 -400 Baseline 2 4 8 12 (EOT) 24 (EOT) Atumelnant -600 Week 67% A4 Reduction These data are derived from different clinical -800 7/8 at Physiologic GC Doses trials at different timepoints with different -1000 designs, endpoints and patient populations. -1200 Due to the lack of head-to-head studies, 2 4 8 12 24 (EOT) 0 cross-trial comparisons should be interpreted Week carefully. Phase 2 TouCAHn data and data adapted from: Auchus R, et al. N Engl J Med 2024;391:504-514. doi:10.1056/NEJMoa2404656. CRINETICS PHARMACEUTICALS | 17 * 7/8 patients achieved physiologic doses by EOT. A4 = Androstenedione; GC = Glucocorticoid; EOT: End of Treatment; ULN: Upper limit of normal. Cohort 4 80 mg 10 10 9 8 Mean Change from Baseline (SE) of Morning Serum A4 (ng/dL) Mean Change from Baseline in A4 (ng/dL)
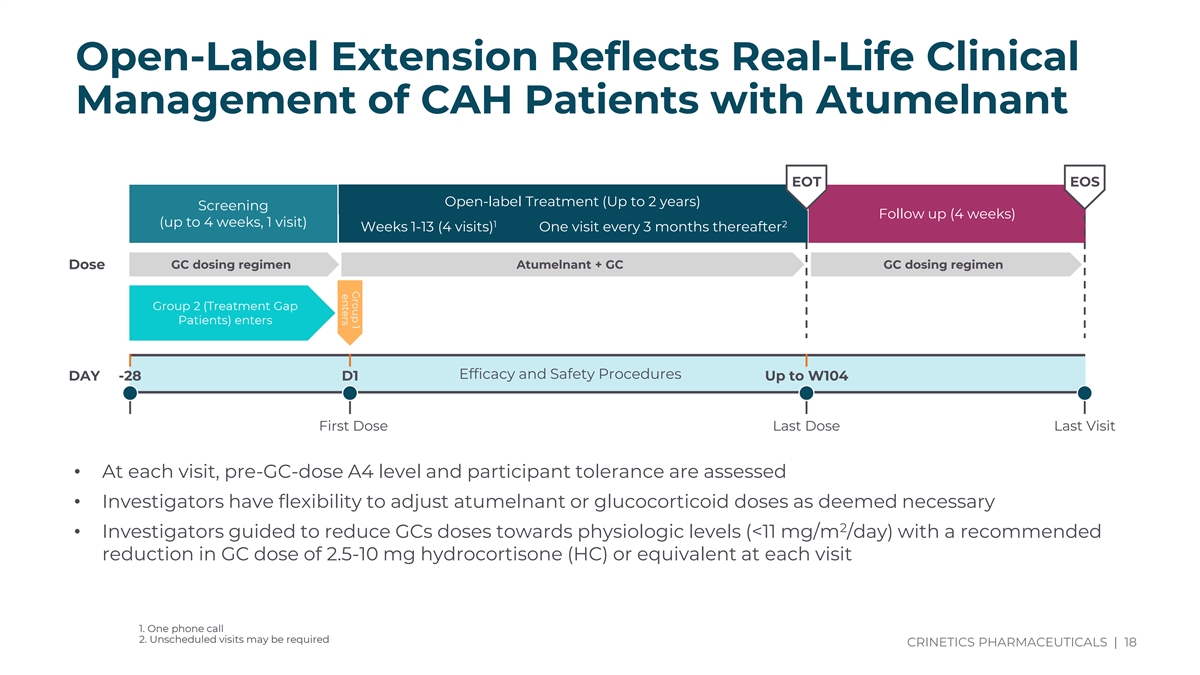
Group 1 enters Open-Label Extension Reflects Real-Life Clinical Management of CAH Patients with Atumelnant EOT EOS Open-label Treatment (Up to 2 years) Screening Follow up (4 weeks) (up to 4 weeks, 1 visit) 1 2 Weeks 1-13 (4 visits) One visit every 3 months thereafter GC dosing regimen Atumelnant + GC GC dosing regimen Dose Group 2 (Treatment Gap Patients) enters Efficacy and Safety Procedures DAY -28 D1 Up to W104 First Dose Last Dose Last Visit • At each visit, pre-GC-dose A4 level and participant tolerance are assessed • Investigators have flexibility to adjust atumelnant or glucocorticoid doses as deemed necessary 2 • Investigators guided to reduce GCs doses towards physiologic levels (<11 mg/m /day) with a recommended reduction in GC dose of 2.5-10 mg hydrocortisone (HC) or equivalent at each visit 1. One phone call 2. Unscheduled visits may be required CRINETICS PHARMACEUTICALS | 18
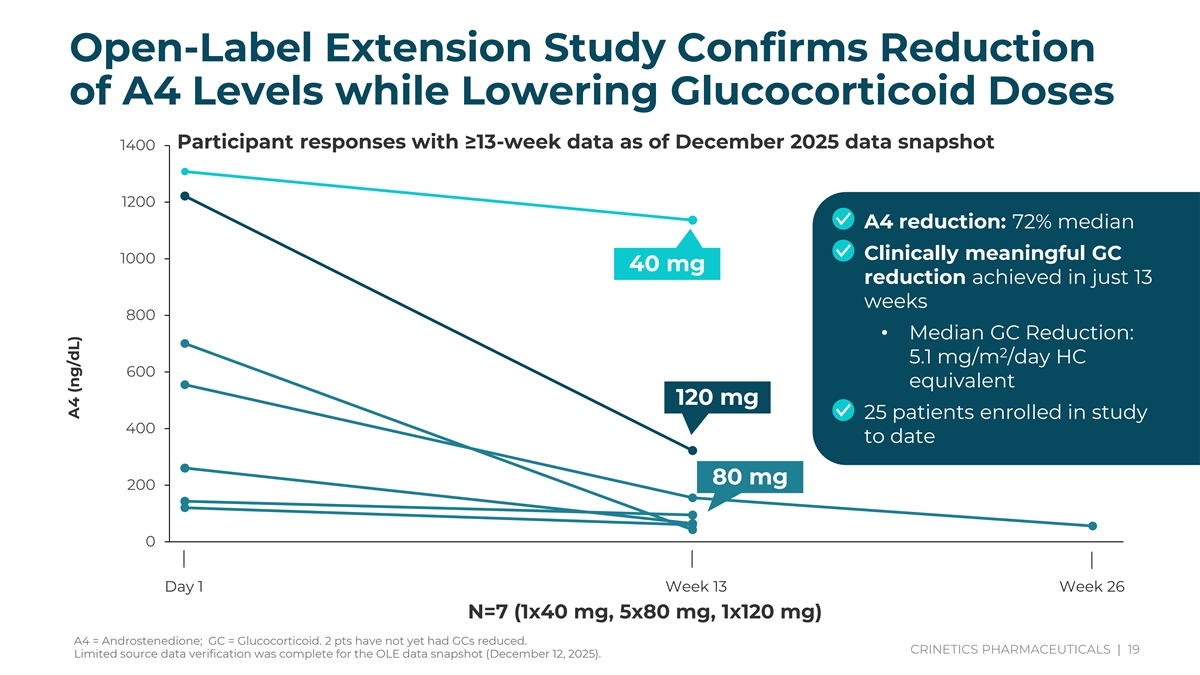
Subject ID Parent Study Subject ID Parent Study Arm Parameter Visit Result AR003-09001 AR003-03001 CRN04894 80 mg A4 (ng/dL) D1 260.715 AR003-09001 AR003-03001 CRN04894 80 mg A4 (ng/dL) W13 64.4625 AR003-09002 AR003-03002 CRN04894 80 mg A4 (ng/dL) D1 143.25 AR003-09002 AR003-03002 CRN04894 80 mg A4 (ng/dL) W13 94.8315 AR003-09003 AR003-03004 CRN04894 40 mg A4 (ng/dL) D1 1308.159 AR003-09003 AR003-03004 CRN04894 40 mg A4 (ng/dL) W13 1136.5455 AR003-09004 AR003-03005 CRN04894 120 mg A4 (ng/dL) D1 1221.9225 Open-Label Extension Study Confirms Reduction AR003-09004 AR003-03005 CRN04894 120 mg A4 (ng/dL) W13 322.3125 BR003-09001 BR003-03004 CRN04894 80 mg A4 (ng/dL) D1 1415.5965 BR003-09002 BR003-03002 A4 (ng/dL) D1 426.885 of A4 Levels while Lowering Glucocorticoid Doses BR017-09001 BR017-03002 CRN04894 80 mg A4 (ng/dL) D1 152.418 UK002-09001 UK002-03001 A4 (ng/dL) D1 751.203 Participant responses with ≥13-week data as of December 2025 data snapshot 1400 US045-09001 US045-03002 A4 (ng/dL) D1 120.33 US045-09001 US045-03002 A4 (ng/dL) W13 59.8785 US047-09001 US047-03001 CRN04894 80 mg A4 (ng/dL) D1 555.237 1200 US047-09001 US047-03001 CRN04894 80 mg A4 (ng/dL) W13 155.5695 • A4 reduction: 72% median US047-09001 US047-03001 CRN04894 80 mg A4 (ng/dL) W26 55.8675 US047-09002 US047-03002 CRN04894 80 mg A4 (ng/dL) D1 700.779 • Clinically meaningful GC 1000 US047-09002 US047-03002 CRN04894 80 mg A4 (ng/dL) W13 42.6885 40 mg reduction achieved in just 13 weeks 800 • Median GC Reduction: 2 5.1 mg/m /day HC 600 equivalent 120 mg • 25 patients enrolled in study 400 to date 80 mg 200 0 Day 1 Week 13 Week 26 N=7 (1x40 mg, 5x80 mg, 1x120 mg) A4 = Androstenedione; GC = Glucocorticoid. 2 pts have not yet had GCs reduced. CRINETICS PHARMACEUTICALS | 19 Limited source data verification was complete for the OLE data snapshot (December 12, 2025). L) A4 (ng/d
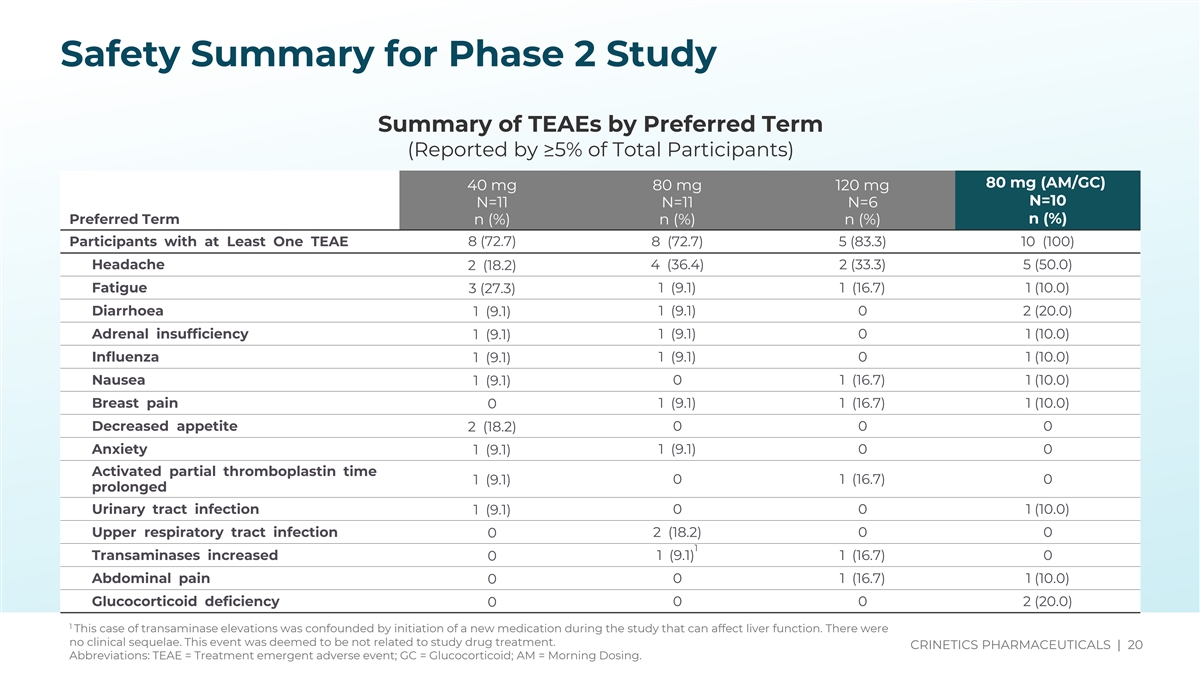
Source(s): t-14-3-1-9-t-teae-5pct-pt Safety Summary for Phase 2 Study Summary of TEAEs by Preferred Term (Reported by ≥5% of Total Participants) 80 mg (AM/GC) 40 mg 80 mg 120 mg N=10 N=11 N=11 N=6 Preferred Term n (%) n (%) n (%) n (%) Participants with at Least One TEAE 8 (72.7) 8 (72.7) 5 (83.3) 10 (100) Headache 2 (18.2) 4 (36.4) 2 (33.3) 5 (50.0) Fatigue 1 (9.1) 1 (16.7) 1 (10.0) 3 (27.3) Diarrhoea 1 (9.1) 1 (9.1) 0 2 (20.0) Adrenal insufficiency 1 (9.1) 0 1 (10.0) 1 (9.1) Influenza 1 (9.1) 0 1 (10.0) 1 (9.1) Nausea 1 (9.1) 0 1 (16.7) 1 (10.0) Breast pain 1 (9.1) 1 (16.7) 1 (10.0) 0 Decreased appetite 2 (18.2) 0 0 0 Anxiety 1 (9.1) 0 0 1 (9.1) Activated partial thromboplastin time 0 1 (16.7) 0 1 (9.1) prolonged Urinary tract infection 0 0 1 (10.0) 1 (9.1) Upper respiratory tract infection 0 2 (18.2) 0 0 1 Transaminases increased 1 (9.1) 1 (16.7) 0 0 Abdominal pain 0 0 1 (16.7) 1 (10.0) Glucocorticoid deficiency 0 0 2 (20.0) 0 1 This case of transaminase elevations was confounded by initiation of a new medication during the study that can affect liver function. There were no clinical sequelae. This event was deemed to be not related to study drug treatment. CRINETICS PHARMACEUTICALS | 20 Abbreviations: TEAE = Treatment emergent adverse event; GC = Glucocorticoid; AM = Morning Dosing.
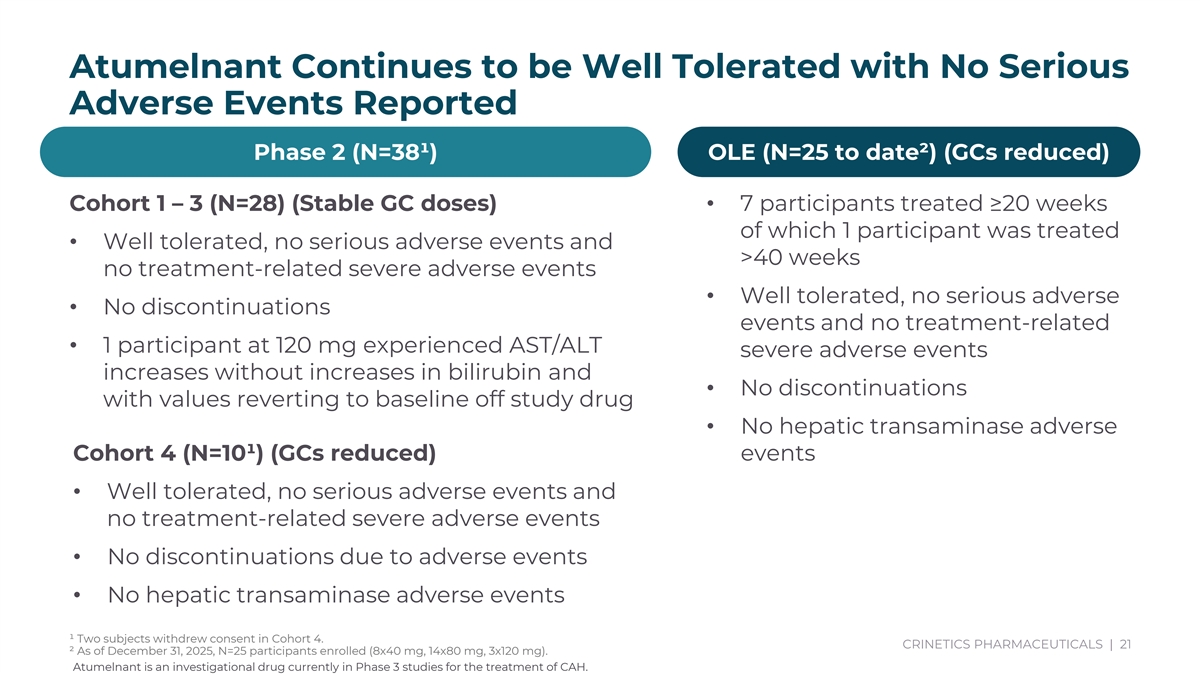
Atumelnant Continues to be Well Tolerated with No Serious Adverse Events Reported Phase 2 (N=38¹) OLE (N=25 to date²) (GCs reduced) Subjects continue to enroll in OLE Study (N=27) Cohort 1 – 3 (N=28) (Stable GC doses)• 7 participants treated ≥20 weeks of which 1 participant was treated • Well tolerated, no serious adverse events and >40 weeks• 7 participants with >20 weeks no treatment-related severe adverse events exposure • Well tolerated, no serious adverse • No discontinuations • 1 participant > 40 weeks events and no treatment-related • Total patient exposure in OLE • 1 participant at 120 mg experienced AST/ALT severe adverse events now at almost 6.5 years increases without increases in bilirubin and • No discontinuations with values reverting to baseline off study drug • No hepatic transaminase adverse Cohort 4 (N=10¹) (GCs reduced) events • Well tolerated, no serious adverse events and no treatment-related severe adverse events • No discontinuations due to adverse events • No hepatic transaminase adverse events ¹ Two subjects withdrew consent in Cohort 4. CRINETICS PHARMACEUTICALS | 21 ² As of December 31, 2025, N=25 participants enrolled (8x40 mg, 14x80 mg, 3x120 mg). Atumelnant is an investigational drug currently in Phase 3 studies for the treatment of CAH.
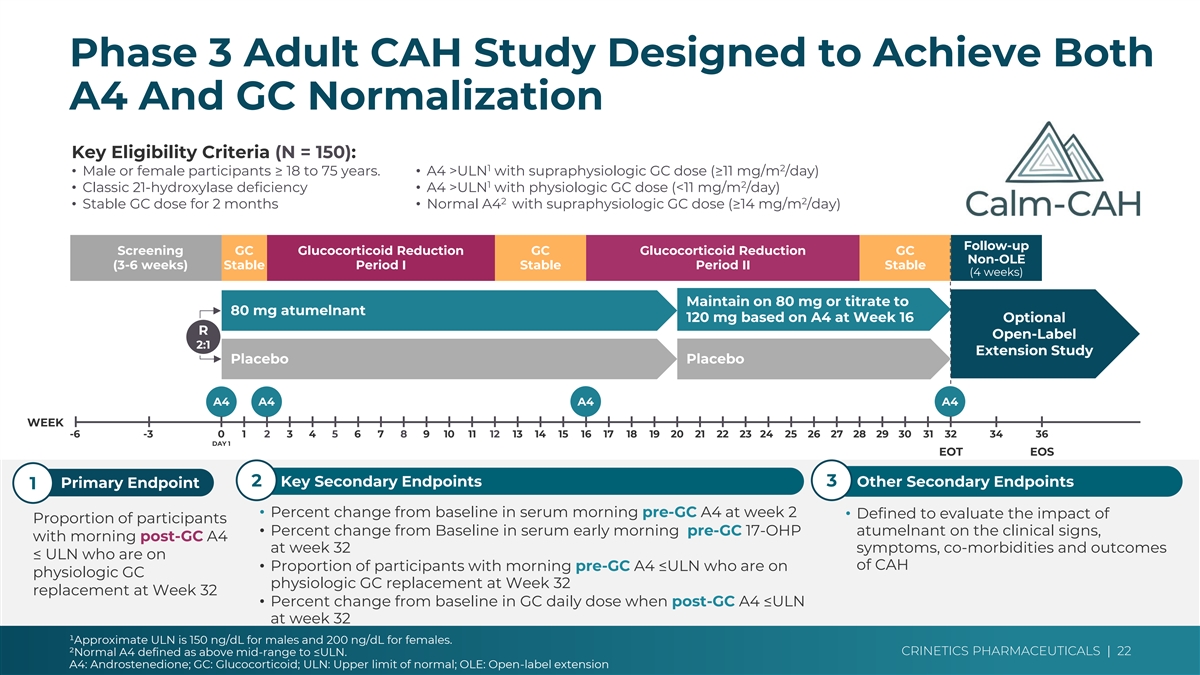
Phase 3 Adult CAH Study Designed to Achieve Both Cohort 4 vs Ph3 ➢ Dose: 80 mgs only vs opportunity to A4 And GC Normalization increase to 120 mg for A4 control ➢ A4: Pre-GC vs Post-GC measurement Key Eligibility Criteria (N = 150): ➢ Timing: AM vs PM dosing 1 2 • Male or female participants ≥ 18 to 75 years. • A4 >ULN with supraphysiologic GC dose (≥11 mg/m /day) 1 2 ➢ Time: 12 weeks vs 32 weeks • Classic 21-hydroxylase deficiency• A4 >ULN with physiologic GC dose (<11 mg/m /day) 2 2 • Stable GC dose for 2 months• Normal A4 with supraphysiologic GC dose (≥14 mg/m /day) Follow-up Screening GC Glucocorticoid Reduction GC Glucocorticoid Reduction GC Non-OLE (3-6 weeks) Stable Period I Stable Period II Stable (4 weeks) Maintain on 80 mg or titrate to 80 mg atumelnant 120 mg based on A4 at Week 16 Optional R Open-Label 2:1 Extension Study Placebo Placebo A4 A4 A4 A4 WEEK -6 -3 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 34 36 Key Differences vs Cohort 4 DAY 1 EOT EOS ➢ Longer timeline/more ➢ Endpoint is post-GC 2 Key Secondary Endpoints 3 Other Secondary Endpoints Primary Endpoint 1 opportunities for GC responder rate reduction • Percent change from baseline in serum morning pre-GC A4 at week 2 • Defined to evaluate the impact of ➢ Larger N Proportion of participants ➢ Opportunity to increase • Percent change from Baseline in serum early morning pre-GC 17-OHP atumelnant on the clinical signs, with morning post-GC A4 ➢ PM dosing at week 32 symptoms, co-morbidities and outcomes atumelnant dose ≤ ULN who are on of CAH • Proportion of participants with morning pre-GC A4 ≤ULN who are on physiologic GC physiologic GC replacement at Week 32 replacement at Week 32 • Percent change from baseline in GC daily dose when post-GC A4 ≤ULN at week 32 ¹Approximate ULN is 150 ng/dL for males and 200 ng/dL for females. CRINETICS PHARMACEUTICALS | 22 ²Normal A4 defined as above mid-range to ≤ULN. A4: Androstenedione; GC: Glucocorticoid; ULN: Upper limit of normal; OLE: Open-label extension
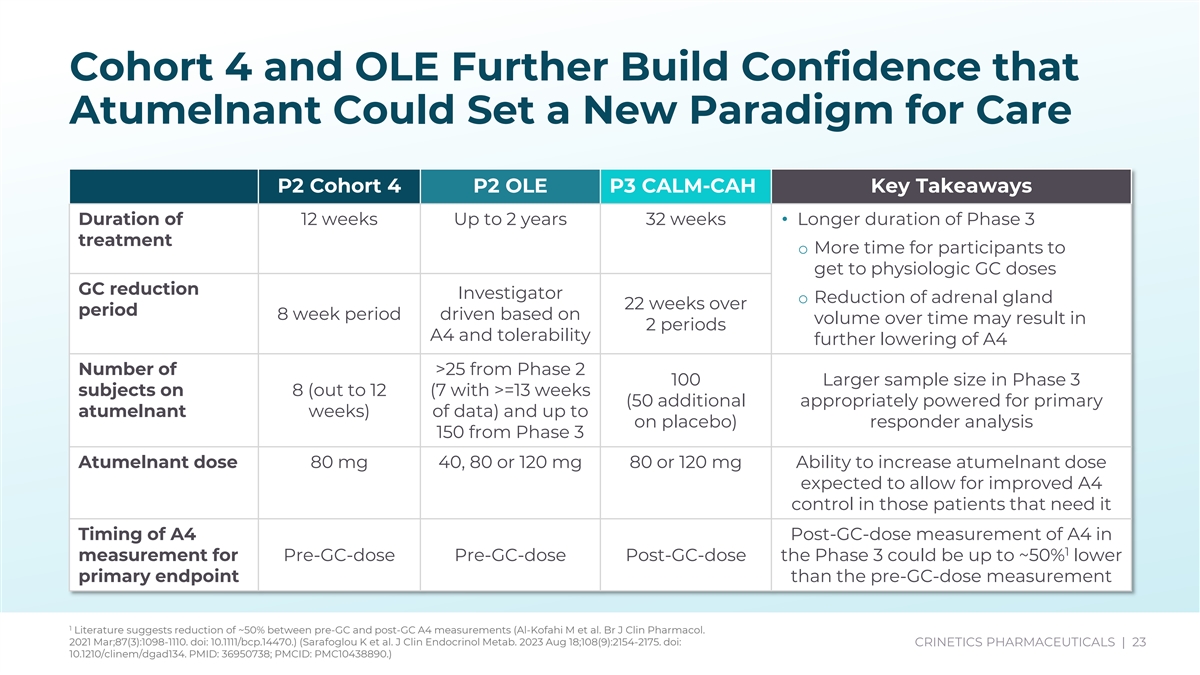
Cohort 4 and OLE Further Build Confidence that Atumelnant Could Set a New Paradigm for Care P2 Cohort 4 P2 OLE - Key Takeaways 80mg 40, 80 or 120 mg 80 or 120 mg Ability to increase atumelnant dose P2 Cohort 4 P2 OLE P3 CALM-CAH Key Takeaways expected to allow for improved A4 control in those patients that need it Duration of 12 weeks Up to 2 years 32 weeks• Longer duration of Phase 3 treatment Post-GC-dose measurement of A4 in o More time for participants to 1 Pre-GC-dose Pre-GC-dose Post-GC-dose get to physiologic GC doses than the pre-GC-dose measurement GC reduction Investigator o Reduction of adrenal gland 22 weeks over period 8 week period driven based on 80mg 40, 80 or 120 mg 80 or 120 mg Ability to increase atumelnant dose volume over time may result in 2 periods A4 and tolerability expected to allow for improved A4 further lowering of A4 control in those patients that need it Number of >25 from Phase 2 100 Larger sample size in Phase 3 12 weeks Up to 2 years 32 weeks• Longer duration of Phase 3 subjects on 8 (out to 12 (7 with >=13 weeks (50 additional appropriately powered for primary atumelnant weeks) of data) and up to o More time for participants to on placebo) responder analysis 150 from Phase 3 get to physiologic GC doses Investigator 22 weeks over Atumelnant dose 80 mg 40, 80 or 120 mg 80 or 120 mg Ability to increase atumelnant dose o Reduction of adrenal gland 8 week period driven based on expected to allow for improved A4 2 periods volume over time may result in control in those patients that need it further lowering of A4 Timing of A4 Post-GC-dose measurement of A4 in >25 from Phase 2 100 (50 Larger sample size in Phase 3 1 measurement for Pre-GC-dose Pre-GC-dose Post-GC-dose the Phase 3 could be up to ~50% lower 8 (out to 12 additional on appropriately powered for primary primary endpoint than the pre-GC-dose measurement weeks) data) and up to placebo) responder analysis 150 from Phase 3 1 Literature suggests reduction of ~50% between pre-GC and post-GC A4 measurements (Al-Kofahi M et al. Br J Clin Pharmacol. No loss of efficacy with AM dosing 2021 Mar;87(3):1098-1110. doi: 10.1111/bcp.14470.) (Sarafoglou K et al. J Clin Endocrinol Metab. 2023 Aug 18;108(9):2154-2175. doi: CRINETICS PHARMACEUTICALS | 23 AM PM PM observed in Cohort 4 10.1210/clinem/dgad134. PMID: 36950738; PMCID: PMC10438890.)
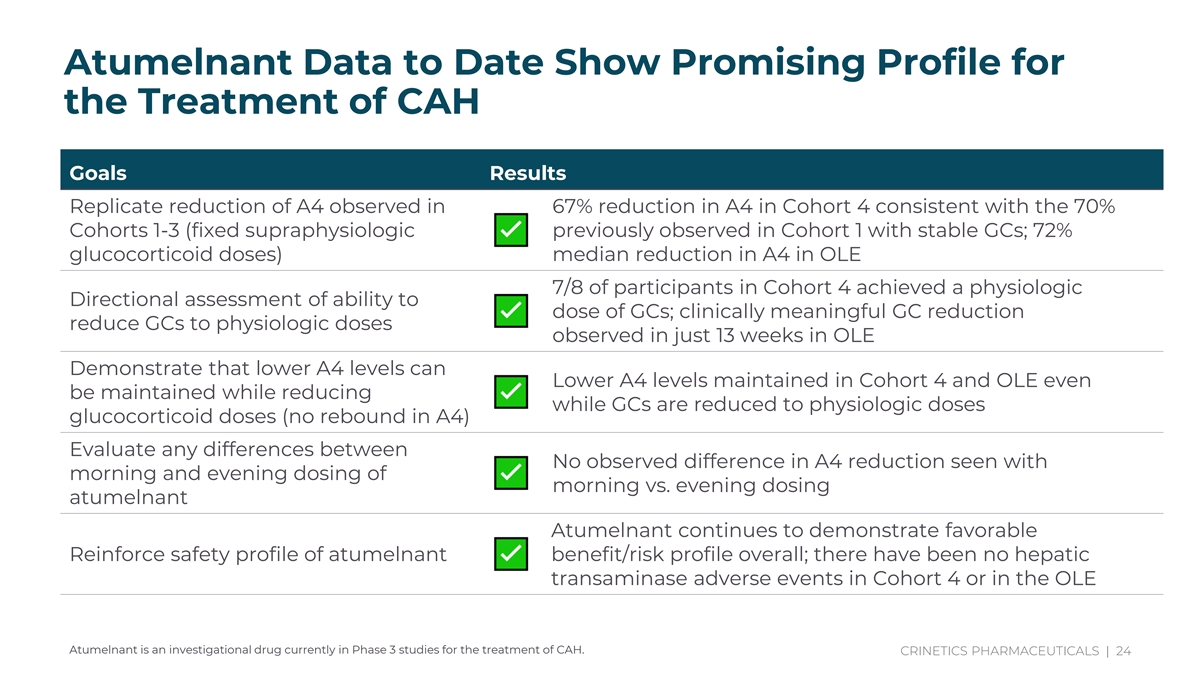
With Atumelnant, Androgen Control is Decoupled from GC Lowering Adjust title And content Atumelnant Data to Date Show Promising Profile for Comprehensive the Treatment of CAH Ph2 data drive confidence in ph3 study design and Goals Results whole new way to Replicate reduction of A4 observed in 67% reduction in A4 in Cohort 4 consistent with the 70% treat CAH, game Cohorts 1-3 (fixed supraphysiologic previously observed in Cohort 1 with stable GCs; 72% changing drug glucocorticoid doses) median reduction in A4 in OLE 7/8 of participants in Cohort 4 achieved a physiologic Directional assessment of ability to dose of GCs; clinically meaningful GC reduction reduce GCs to physiologic doses observed in just 13 weeks in OLE Demonstrate that lower A4 levels can Lower A4 levels maintained in Cohort 4 and OLE even be maintained while reducing while GCs are reduced to physiologic doses glucocorticoid doses (no rebound in A4) Evaluate any differences between No observed difference in A4 reduction seen with morning and evening dosing of morning vs. evening dosing atumelnant Atumelnant continues to demonstrate favorable Reinforce safety profile of atumelnant benefit/risk profile overall; there have been no hepatic transaminase adverse events in Cohort 4 or in the OLE Atumelnant is an investigational drug currently in Phase 3 studies for the treatment of CAH. CRINETICS PHARMACEUTICALS | 24
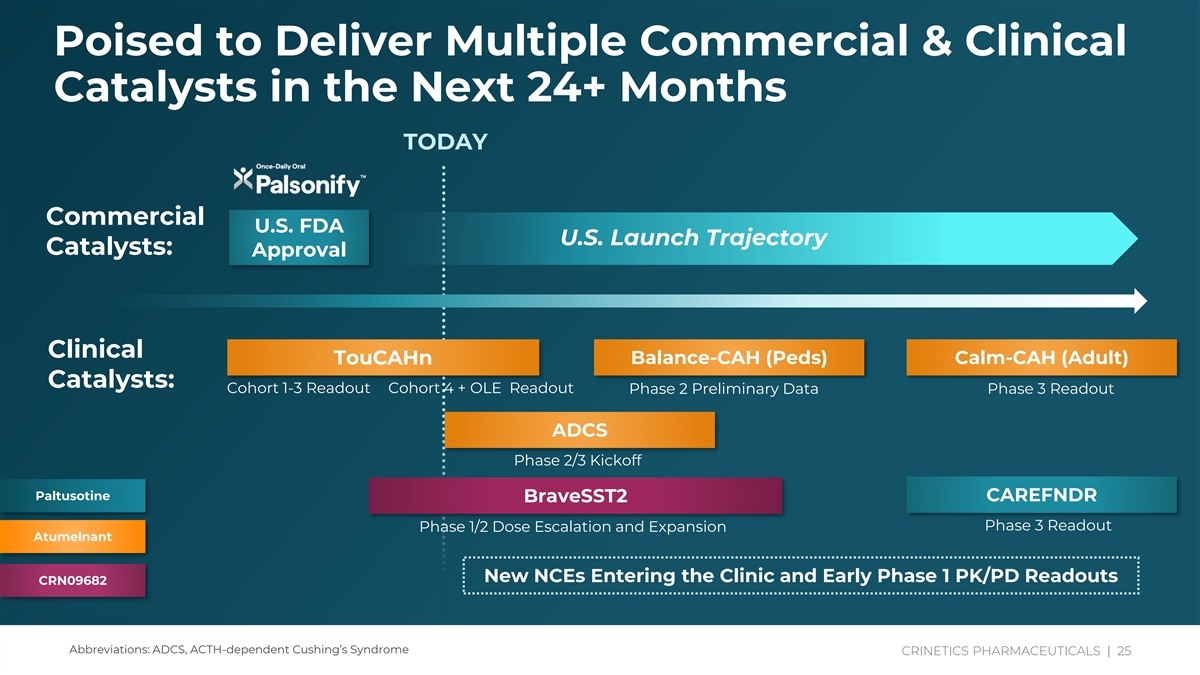
Poised to Deliver Multiple Commercial & Clinical Catalysts in the Next 24+ Months TODAY Commercial U.S. FDA U.S. Launch Trajectory Catalysts: Approval Clinical Balance-CAH (Peds) Calm-CAH (Adult) TouCAHn Catalysts: Cohort 1-3 Readout Cohort 4 + OLE Readout Phase 2 Preliminary Data Phase 3 Readout ADCS Phase 2/3 Kickoff Paltusotine CAREFNDR BraveSST2 Phase 3 Readout Phase 1/2 Dose Escalation and Expansion Atumelnant New NCEs Entering the Clinic and Early Phase 1 PK/PD Readouts CRN09682 Abbreviations: ADCS, ACTH-dependent Cushing’s Syndrome CRINE CRINE TICT SICS PH P A HARM RMAC AC EUT EUT IC ICA AL LS S | | 25 25

Thank You