| NASDAQ: NXTC SIM0505 LNCB74 J A N U A R Y 2 0 2 6 Corporate Presentation |
| Forward-Looking Statements 2 To the extent that statements contained in this presentation are not descriptions of historical facts, they may be deemed to be forward-looking statements under the Private Securities Litigation Reform Act of 1995. These statements are based on current expectations, forecasts, assumptions and other information available to NextCure as of the date hereof. Forward-looking statements include statements regarding NextCure’s expectations, beliefs, intentions or strategies regarding the future and can be identified by forward-looking words such as “may,” “will,” “potential,” “expects,” “believes,” “intends,” “hope,” “towards,” “forward,” “later” and similar expressions. Examples of forward-looking statements in this presentation include, among others, statements about our licensing agreement with Simcere Zaiming, statements about the development plans for our products, statements about the progress and evaluation and expected timing of results of NextCure’s ongoing or planned clinical trials, expectations regarding the potential benefits, activity, effectiveness and safety of our research stage, preclinical stage, and clinical stage therapeutic candidates, NextCure’s financial guidance, expected upcoming milestones, and NextCure’s plans, objectives and intentions with respect to the discovery and development of therapeutic products. Forward-looking statements involve substantial risks and uncertainties that could cause actual results to differ materially from those projected in any forward-looking statement. Such risks and uncertainties include, among others: positive results in preclinical studies may not be predictive of the results of clinical trials; NextCure’s limited operating history and no products approved for commercial sale; NextCure’s history of significant losses; NextCure’s need to obtain additional financing; risks related to clinical development, marketing approval and commercialization; the unproven approach to the discovery and development of product candidates based on NextCure’s discovery platform; and dependence on key personnel. More detailed information on these and additional factors that could affect NextCure’s actual results are described in NextCure’s filings with the Securities and Exchange Commission (the “SEC”), including in Item 1A of NextCure’s most recent Form 10-K, subsequent Forms 10-Q and elsewhere in the Company’s filings with the SEC. You should not place undue reliance on any forward-looking statements. Forward-looking statements speak only as of the date of this press release, and NextCure assumes no obligation to update any forward-looking statements, except as required by law, even if expectations change. |
| 3 In the Fight Against Cancer Harnessing the Power of Targeted Therapy 3 |
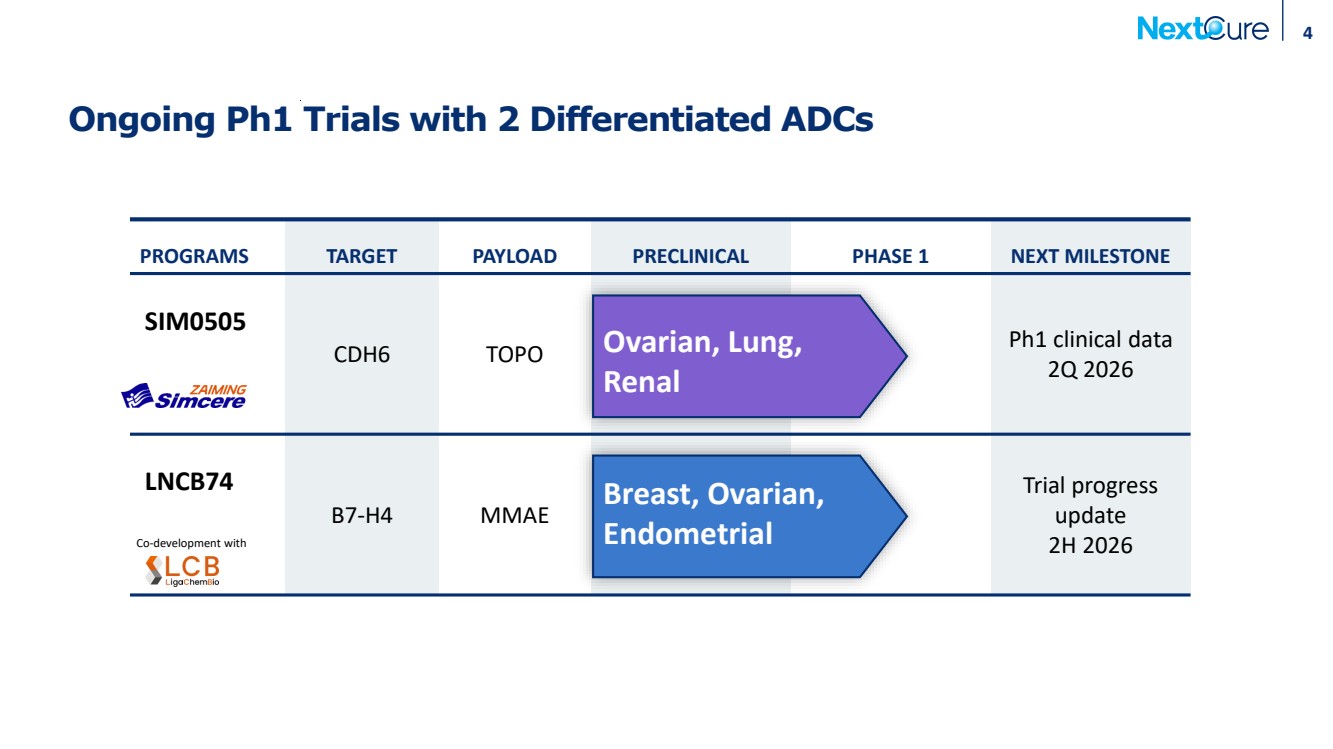
| Ongoing Ph1 Trials with 2 Differentiated ADCs 4 PROGRAMS TARGET PAYLOAD PRECLINICAL PHASE 1 NEXT MILESTONE SIM0505 CDH6 TOPO Ph1 clinical data 2Q 2026 LNCB74 B7-H4 MMAE Trial progress update 2H 2026 Co-development with Breast, Ovarian, Endometrial Ovarian, Lung, Renal |
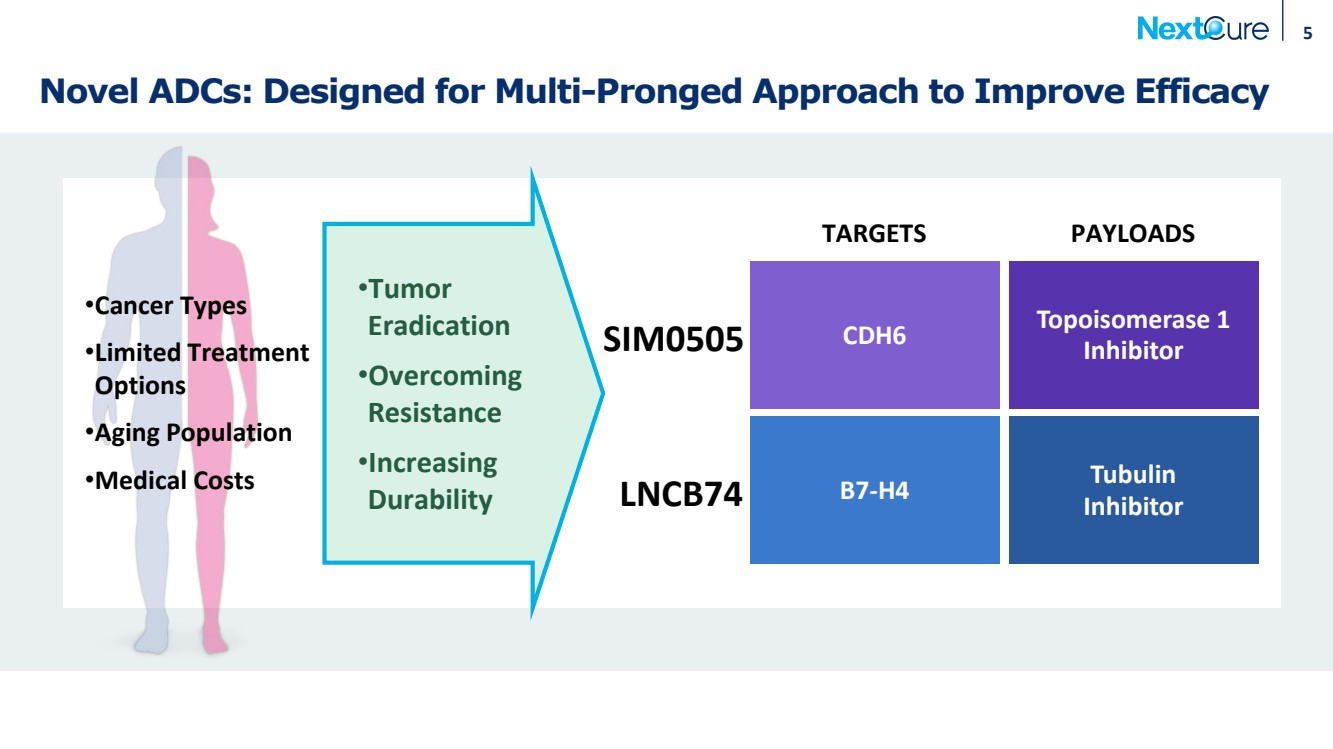
| Novel ADCs: Designed for Multi-Pronged Approach to Improve Efficacy 5 TARGETS PAYLOADS B7-H4 Tubulin Inhibitor LNCB74 CDH6 Topoisomerase 1 SIM0505 Inhibitor •Tumor Eradication •Overcoming Resistance •Increasing Durability •Cancer Types •Limited Treatment Options •Aging Population •Medical Costs |
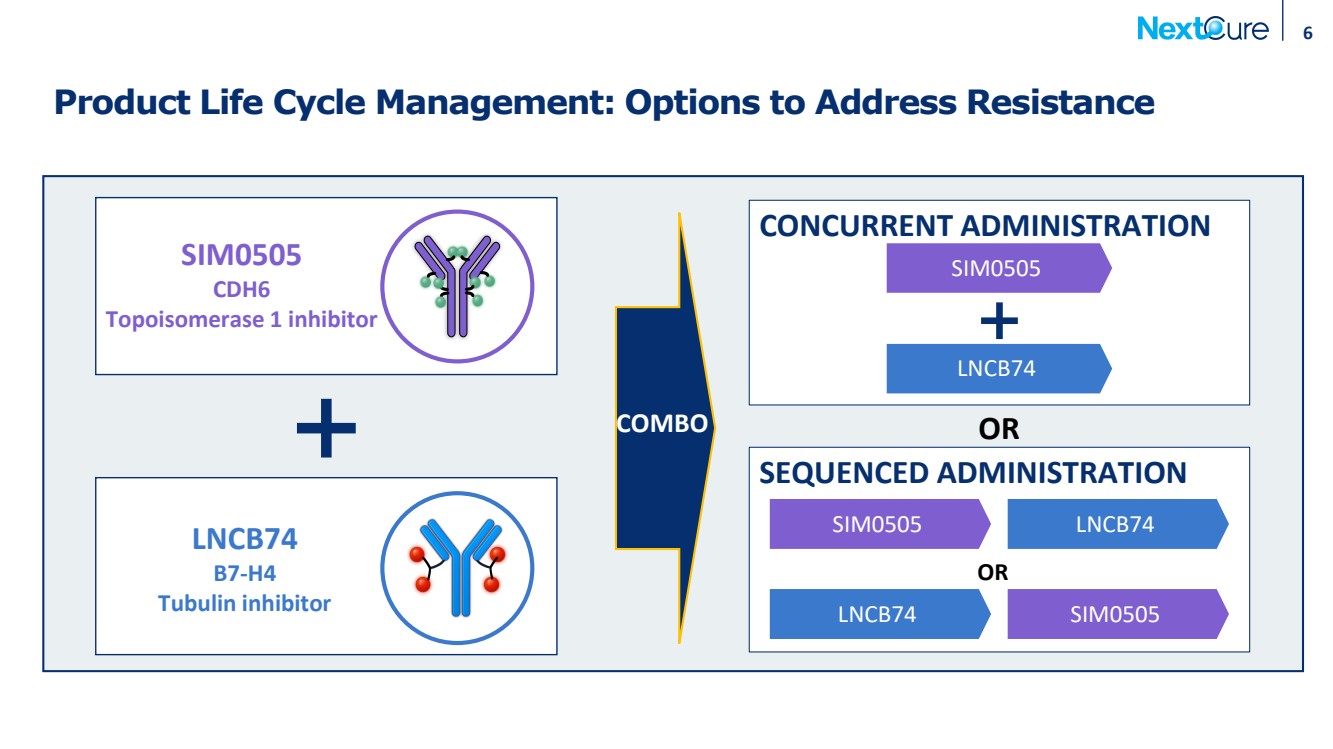
| 6 Product Life Cycle Management: Options to Address Resistance LNCB74 B7-H4 Tubulin inhibitor SIM0505 CDH6 Topoisomerase 1 inhibitor CONCURRENT ADMINISTRATION COMBO SEQUENCED ADMINISTRATION OR LNCB74 SIM0505 LNCB74 SIM0505 SIM0505 LNCB74 OR |
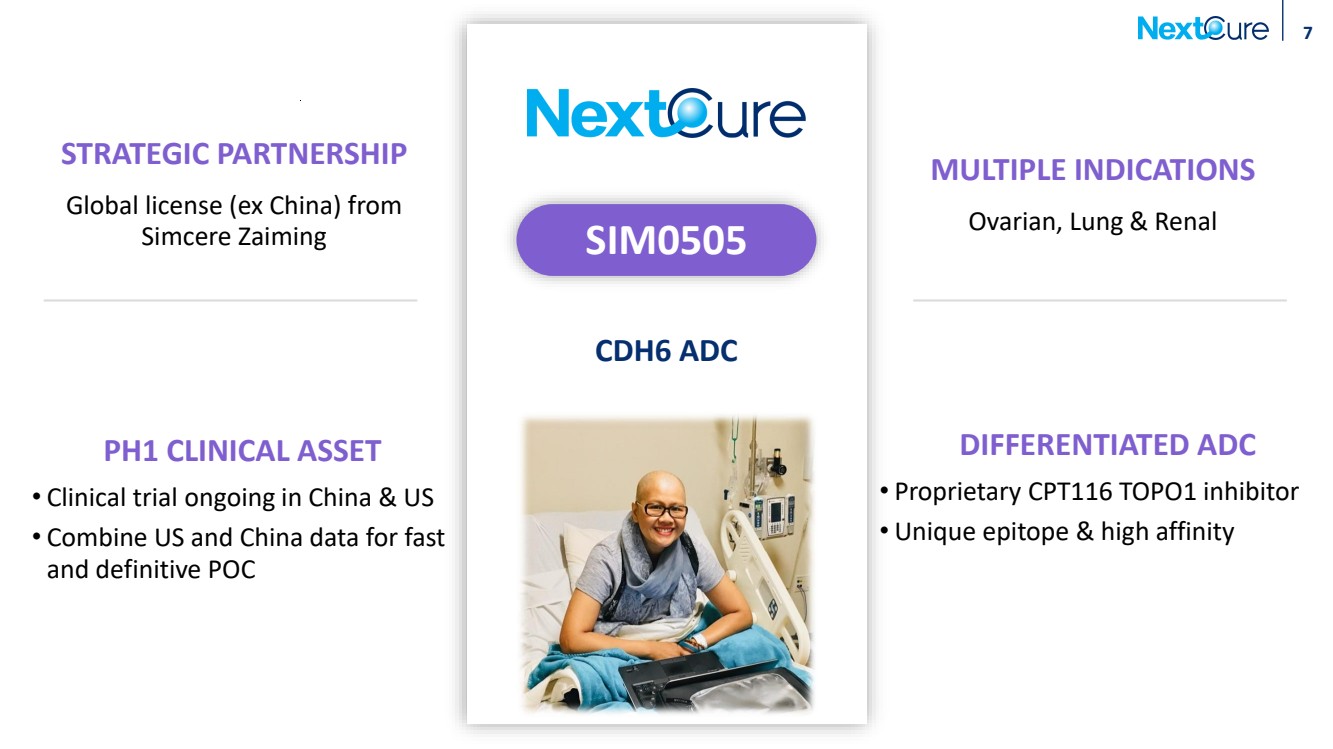
| 7 STRATEGIC PARTNERSHIP Global license (ex China) from Simcere Zaiming DIFFERENTIATED ADC • Proprietary CPT116 TOPO1 inhibitor • Unique epitope & high affinity PH1 CLINICAL ASSET • Clinical trial ongoing in China & US • Combine US and China data for fast and definitive POC MULTIPLE INDICATIONS Ovarian, Lung & Renal CDH6 ADC SIM0505 |
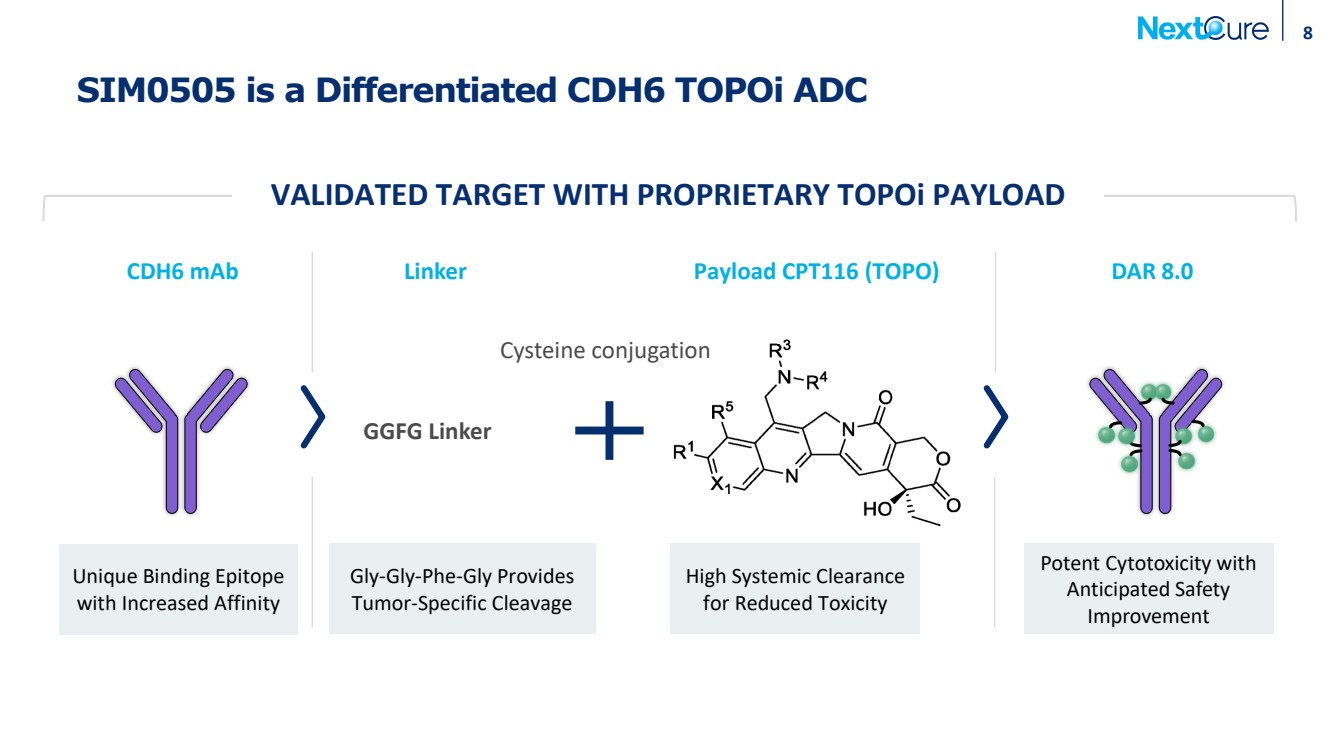
| SIM0505 is a Differentiated CDH6 TOPOi ADC 8 Payload CPT116 (TOPO) GGFG Linker CDH6 mAb DAR 8.0 Cysteine conjugation Gly-Gly-Phe-Gly Provides Tumor-Specific Cleavage Unique Binding Epitope with Increased Affinity High Systemic Clearance for Reduced Toxicity Linker Potent Cytotoxicity with Anticipated Safety Improvement VALIDATED TARGET WITH PROPRIETARY TOPOi PAYLOAD |
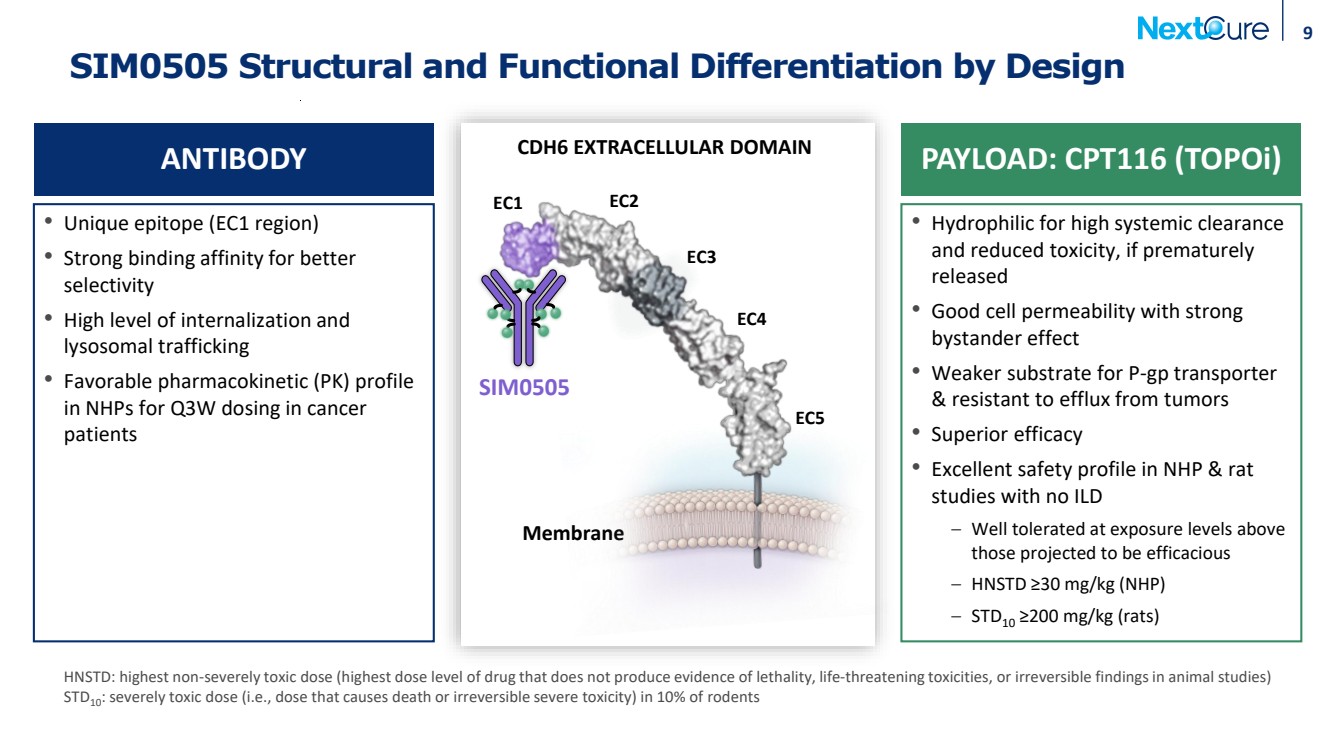
| SIM0505 Structural and Functional Differentiation by Design • Unique epitope (EC1 region) • Strong binding affinity for better selectivity • High level of internalization and lysosomal trafficking • Favorable pharmacokinetic (PK) profile in NHPs for Q3W dosing in cancer patients • Hydrophilic for high systemic clearance and reduced toxicity, if prematurely released • Good cell permeability with strong bystander effect • Weaker substrate for P-gp transporter & resistant to efflux from tumors • Superior efficacy • Excellent safety profile in NHP & rat studies with no ILD – Well tolerated at exposure levels above those projected to be efficacious – HNSTD ≥30 mg/kg (NHP) – STD10 ≥200 mg/kg (rats) 9 ANTIBODY PAYLOAD: CPT116 (TOPOi) HNSTD: highest non-severely toxic dose (highest dose level of drug that does not produce evidence of lethality, life-threatening toxicities, or irreversible findings in animal studies) STD10: severely toxic dose (i.e., dose that causes death or irreversible severe toxicity) in 10% of rodents Membrane EC1 EC2 EC3 EC4 EC5 CDH6 EXTRACELLULAR DOMAIN SIM0505 |
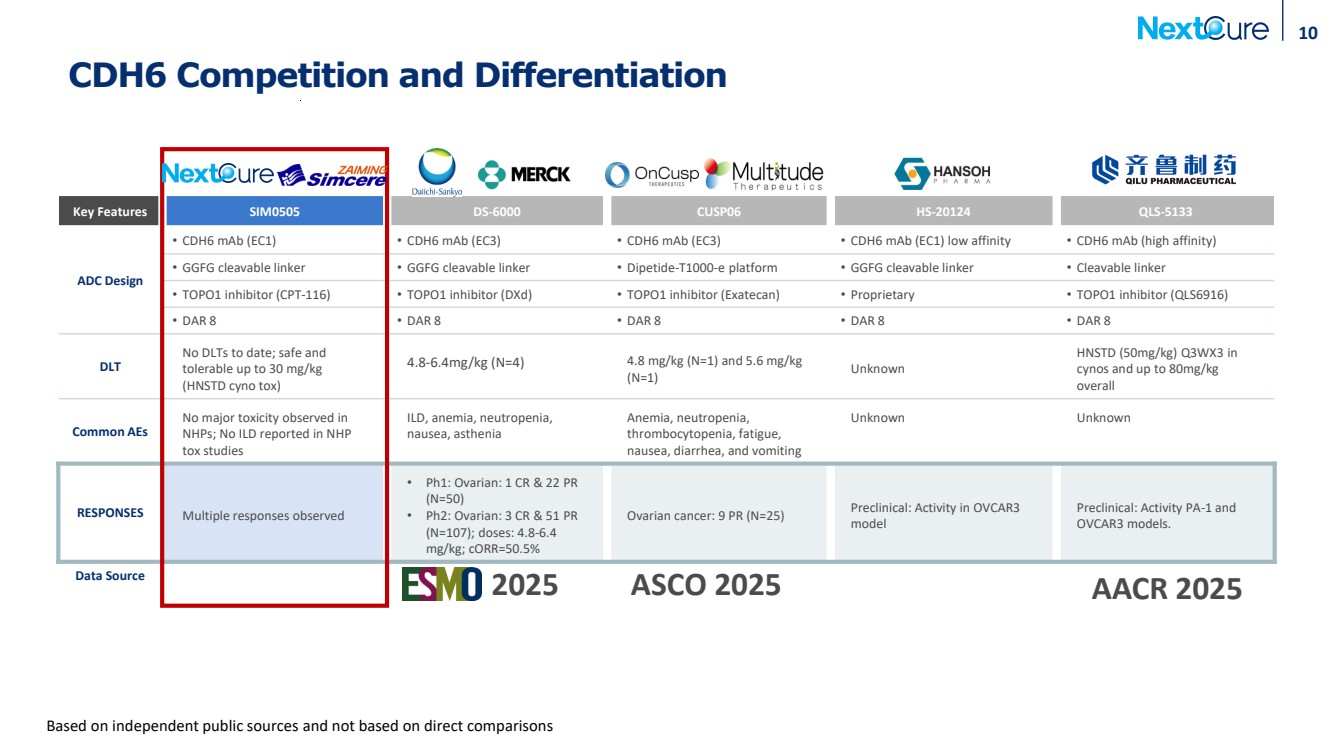
| CDH6 Competition and Differentiation Key Features SIM0505 DS-6000 CUSP06 HS-20124 QLS-5133 ADC Design • CDH6 mAb (EC1) • CDH6 mAb (EC3) • CDH6 mAb (EC3) • CDH6 mAb (EC1) low affinity • CDH6 mAb (high affinity) • GGFG cleavable linker • GGFG cleavable linker • Dipetide-T1000-e platform • GGFG cleavable linker • Cleavable linker • TOPO1 inhibitor (CPT-116) • TOPO1 inhibitor (DXd) • TOPO1 inhibitor (Exatecan) • Proprietary • TOPO1 inhibitor (QLS6916) • DAR 8 • DAR 8 • DAR 8 • DAR 8 • DAR 8 DLT No DLTs to date; safe and tolerable up to 30 mg/kg (HNSTD cyno tox) 4.8-6.4mg/kg (N=4) 4.8 mg/kg (N=1) and 5.6 mg/kg (N=1) Unknown HNSTD (50mg/kg) Q3WX3 in cynos and up to 80mg/kg overall Common AEs No major toxicity observed in NHPs; No ILD reported in NHP tox studies ILD, anemia, neutropenia, nausea, asthenia Anemia, neutropenia, thrombocytopenia, fatigue, nausea, diarrhea, and vomiting Unknown Unknown RESPONSES Multiple responses observed • Ph1: Ovarian: 1 CR & 22 PR (N=50) • Ph2: Ovarian: 3 CR & 51 PR (N=107); doses: 4.8-6.4 mg/kg; cORR=50.5% Ovarian cancer: 9 PR (N=25) Preclinical: Activity in OVCAR3 model Preclinical: Activity PA-1 and OVCAR3 models. Data Source 10 Based on independent public sources and not based on direct comparisons 2025 ASCO 2025 AACR 2025 |
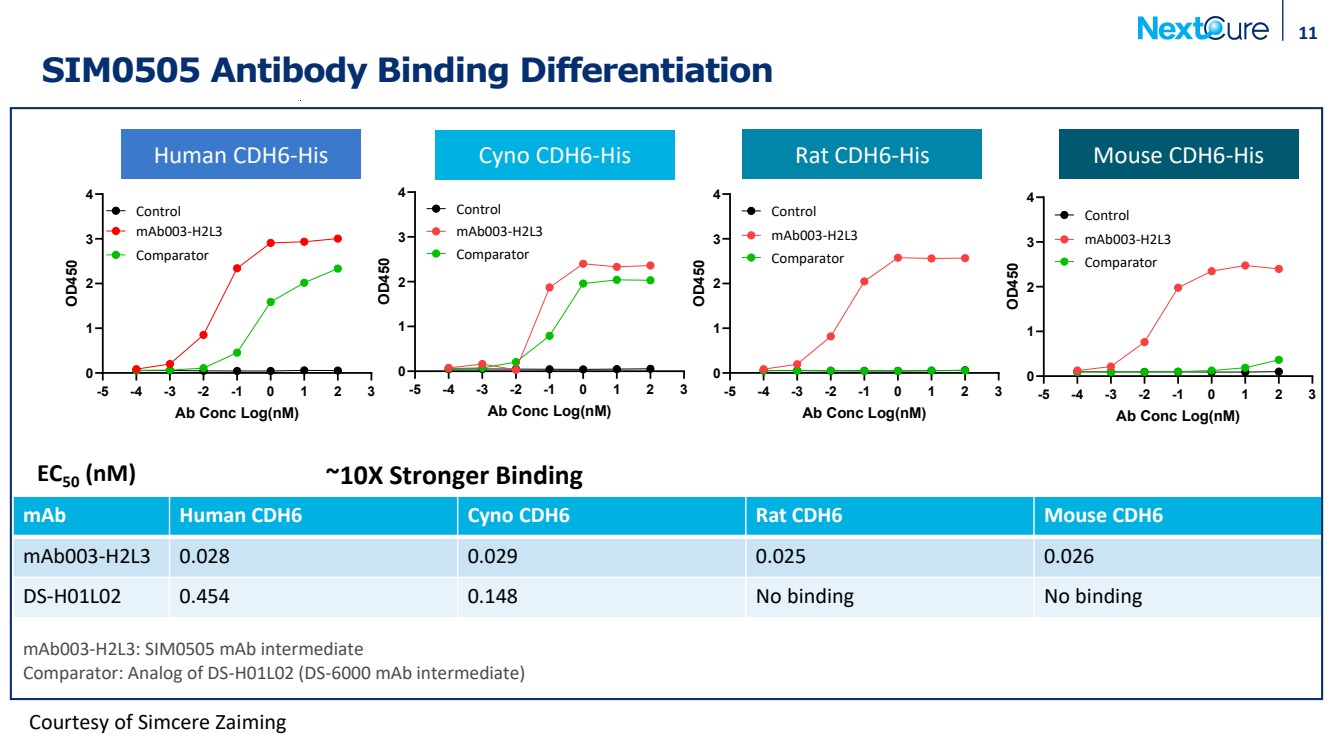
| SIM0505 Antibody Binding Differentiation 11 mAb Human CDH6 Cyno CDH6 Rat CDH6 Mouse CDH6 mAb003-H2L3 0.028 0.029 0.025 0.026 DS-H01L02 0.454 0.148 No binding No binding EC50 (nM) mAb003-H2L3: SIM0505 mAb intermediate Comparator: Analog of DS-H01L02 (DS-6000 mAb intermediate) -5 -4 -3 -2 -1 0 1 2 3 0 1 2 3 4 Human CDH6-His Ab Conc Log(nM) OD450 mAb003-H2L3 Comparator Control -5 -4 -3 -2 -1 0 1 2 3 0 1 2 3 4 Rat CDH6-His Ab Conc Log(nM) OD450 mAb003-H2L3 Comparator Control -5 -4 -3 -2 -1 0 1 2 3 0 1 2 3 4 Mouse CDH6-His Ab Conc Log(nM) OD450 mAb003-H2L3 Comparator Control -5 -4 -3 -2 -1 0 1 2 3 0 1 2 3 4 Cyno CDH6-His Ab Conc Log(nM) OD450 mAb003-H2L3 Comparator Control Courtesy of Simcere Zaiming Human CDH6-His Cyno CDH6-His Rat CDH6-His Mouse CDH6-His ~10X Stronger Binding |
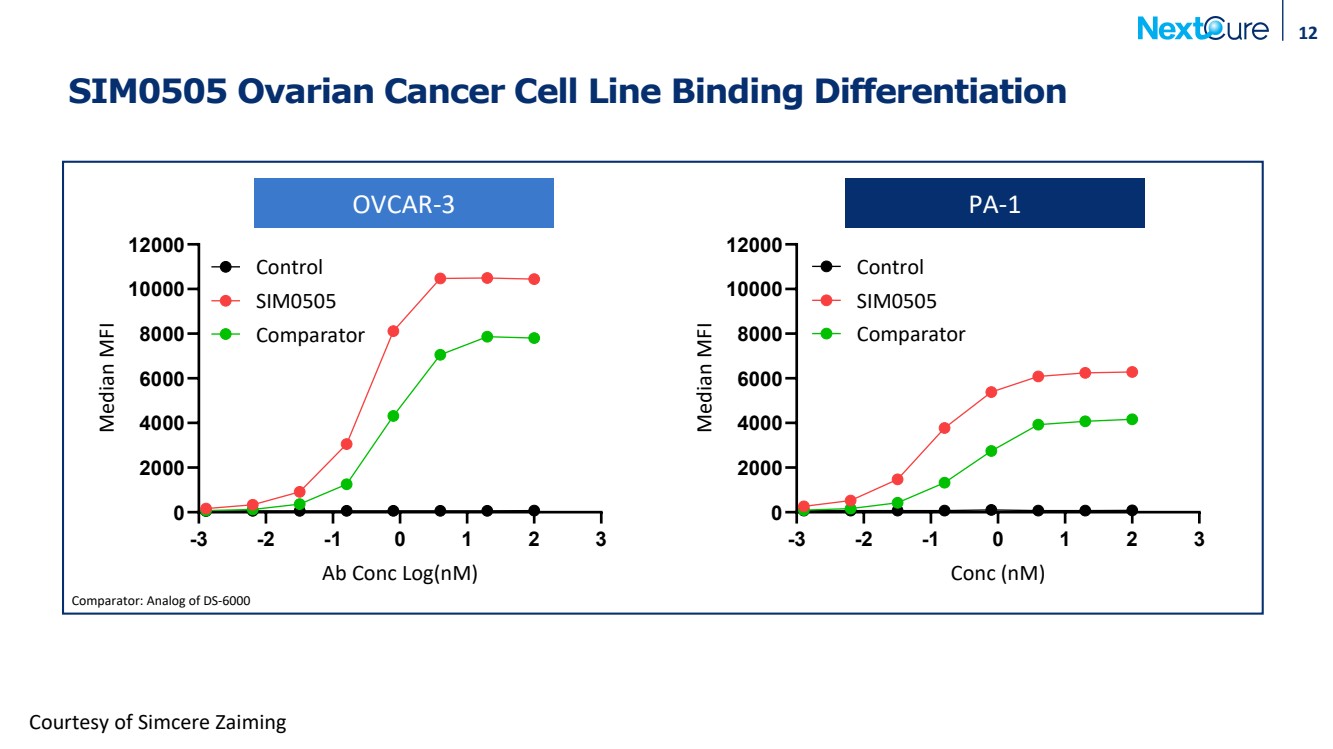
| SIM0505 Ovarian Cancer Cell Line Binding Differentiation 12 -3 -2 -1 0 1 2 3 0 2000 4000 6000 8000 10000 12000 OVCAR3 Ab Conc Log(nM) Median MFI SIM0505 Comparator Control -3 -2 -1 0 1 2 3 0 2000 4000 6000 8000 10000 12000 PA-1 Conc (nM) Median MFI SIM0505 Comparator Control Courtesy of Simcere Zaiming Comparator: Analog of DS-6000 OVCAR-3 PA-1 |
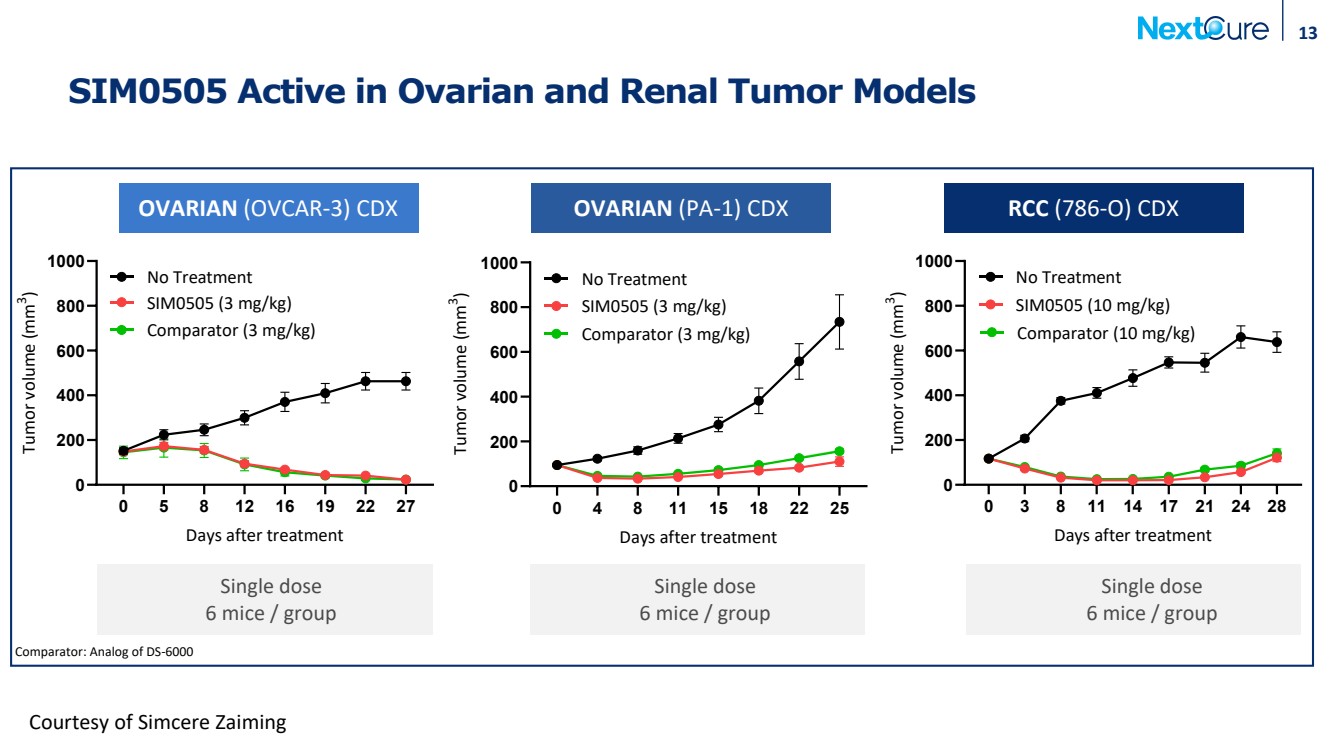
| SIM0505 Active in Ovarian and Renal Tumor Models 13 0 5 8 12 16 19 22 27 0 200 400 600 800 1000 OVCAR3 CDX Days after treatment Tumor volume (mm 3 ) No Treatment SIM0505 (3 mg/kg) Comparator (3 mg/kg) 0 4 8 11 15 18 22 25 0 200 400 600 800 1000 PA-1 CDX Days after treatment Tumor volume (mm 3 ) No Treatment SIM0505 (3 mg/kg) Comparator (3 mg/kg) 0 3 8 11 14 17 21 24 28 0 200 400 600 800 1000 786-O CDX Days after treatment Tumor volume m( m 3 ) No Treatment SIM0505 (10 mg/kg) Comparator (10 mg/kg) OVARIAN (OVCAR-3) CDX OVARIAN (PA-1) CDX RCC (786-O) CDX Courtesy of Simcere Zaiming Comparator: Analog of DS-6000 Single dose 6 mice / group Single dose 6 mice / group Single dose 6 mice / group |
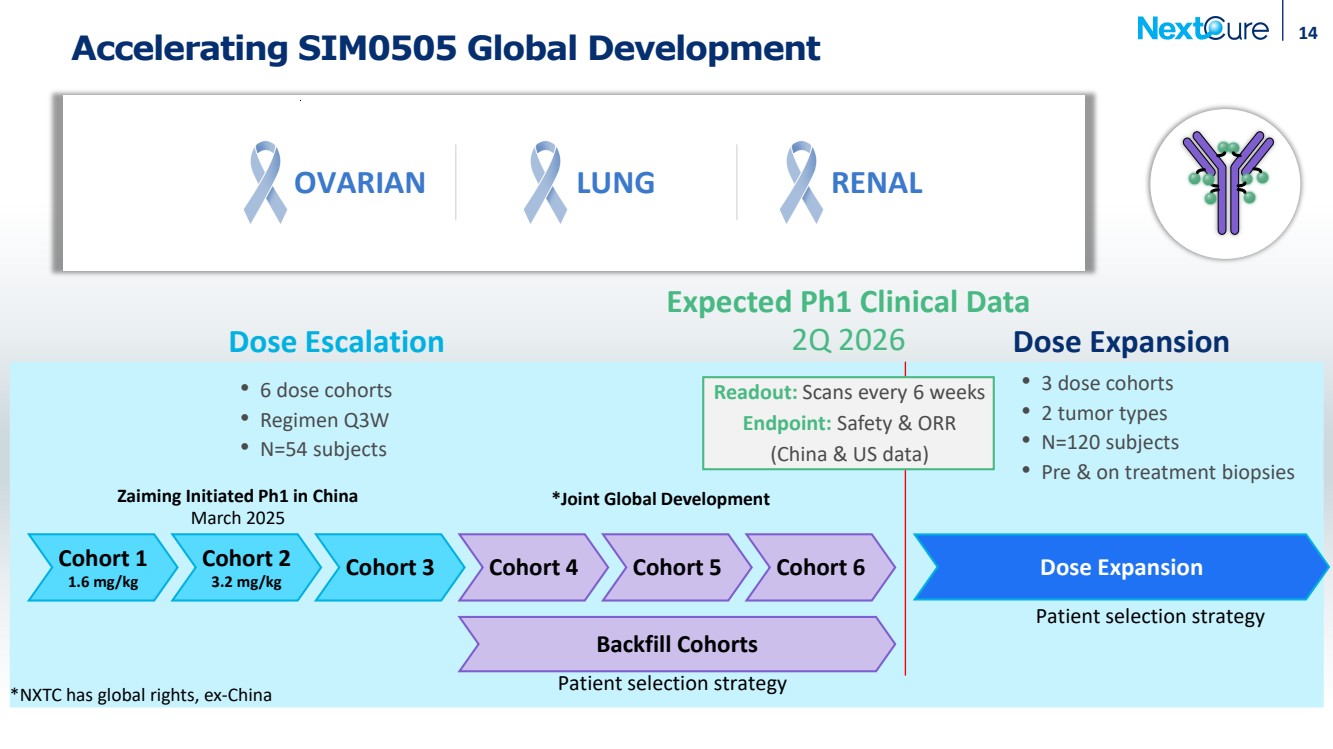
| Accelerating SIM0505 Global Development Expected Ph1 Clinical Data 2Q 2026 Cohort 1 1.6 mg/kg Cohort 2 3.2 mg/kg Cohort 3 Cohort 4 Backfill Cohorts 14 Dose Expansion • 6 dose cohorts • Regimen Q3W • N=54 subjects • 3 dose cohorts • 2 tumor types • N=120 subjects • Pre & on treatment biopsies Patient selection strategy Patient selection strategy Dose Escalation Dose Expansion OVARIAN LUNG RENAL Readout: Scans every 6 weeks Endpoint: Safety & ORR (China & US data) Cohort 5 Cohort 6 Zaiming Initiated Ph1 in China March 2025 *Joint Global Development *NXTC has global rights, ex-China |
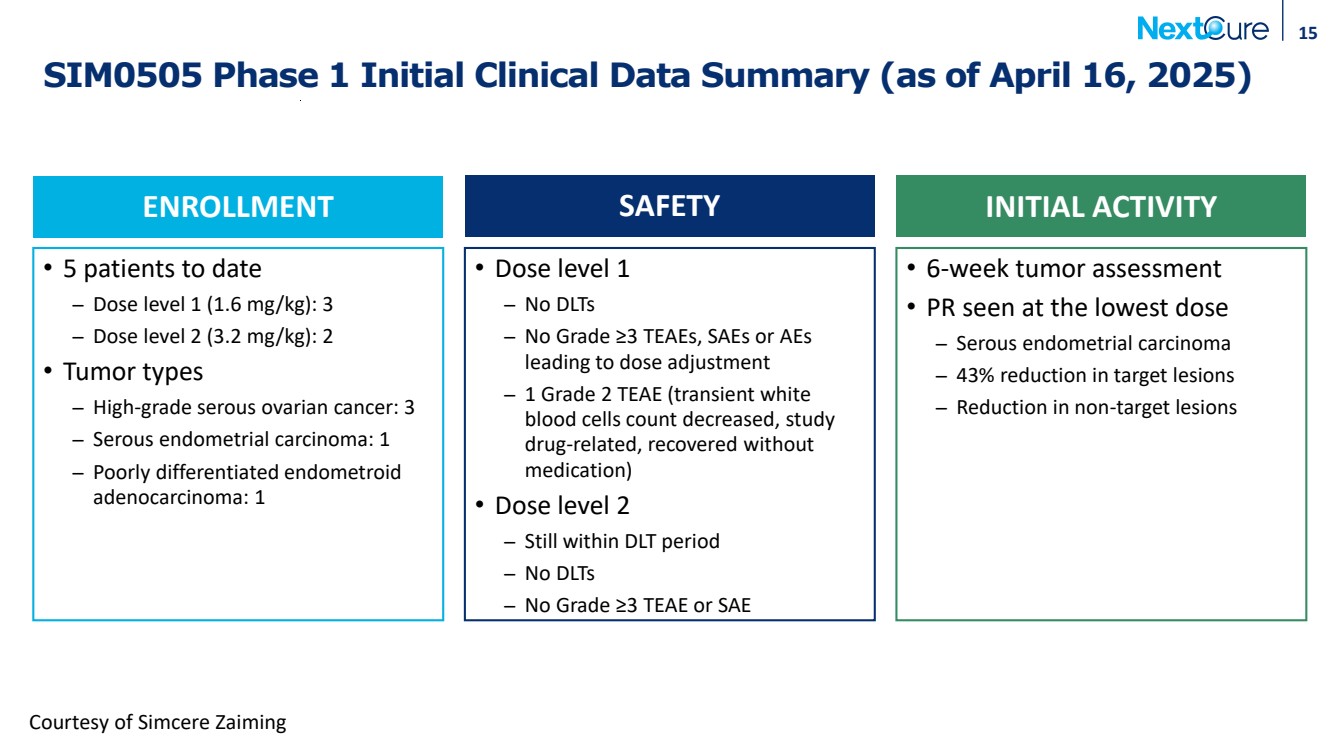
| SIM0505 Phase 1 Initial Clinical Data Summary (as of April 16, 2025) 15 • 5 patients to date ̶ Dose level 1 (1.6 mg/kg): 3 ̶ Dose level 2 (3.2 mg/kg): 2 • Tumor types ̶ High-grade serous ovarian cancer: 3 ̶ Serous endometrial carcinoma: 1 ̶ Poorly differentiated endometroid adenocarcinoma: 1 • Dose level 1 ̶ No DLTs ̶ No Grade ≥3 TEAEs, SAEs or AEs leading to dose adjustment ̶ 1 Grade 2 TEAE (transient white blood cells count decreased, study drug-related, recovered without medication) • Dose level 2 ̶ Still within DLT period ̶ No DLTs ̶ No Grade ≥3 TEAE or SAE • 6-week tumor assessment • PR seen at the lowest dose ̶ Serous endometrial carcinoma ̶ 43% reduction in target lesions ̶ Reduction in non-target lesions ENROLLMENT SAFETY INITIAL ACTIVITY Courtesy of Simcere Zaiming |
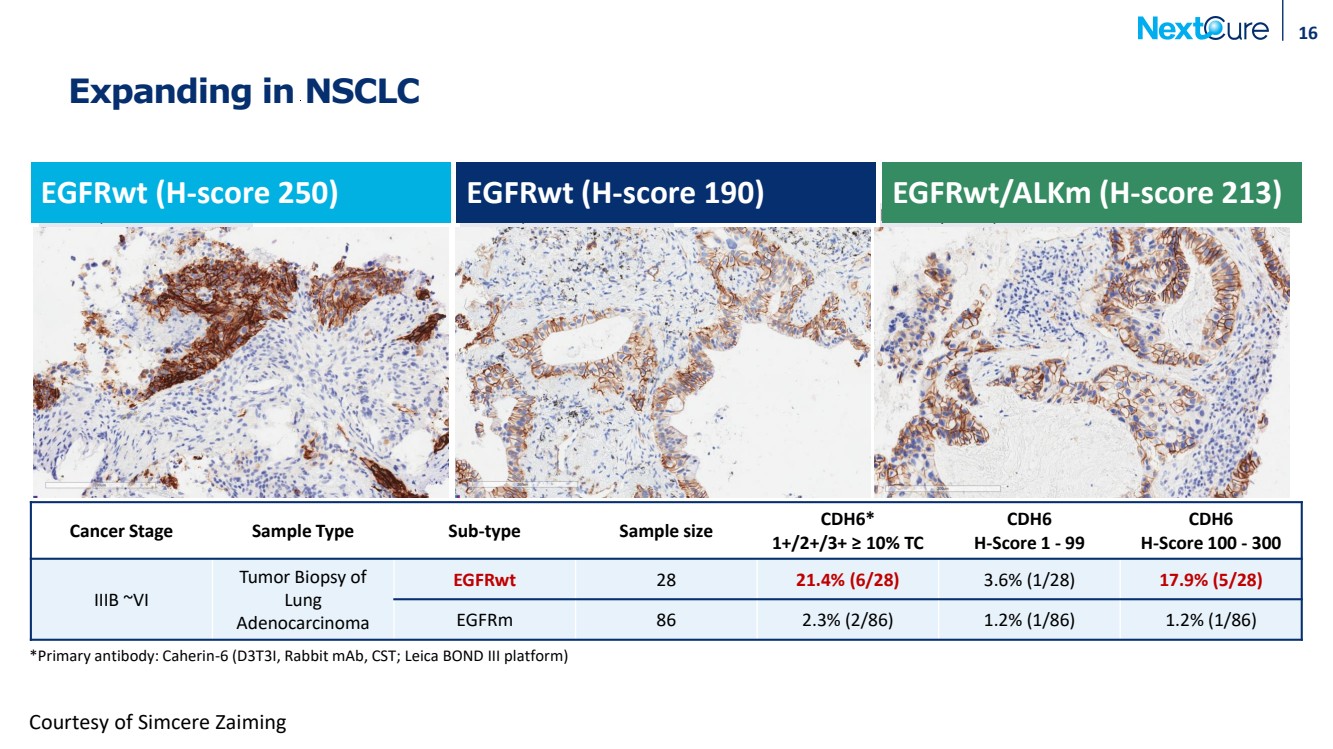
| EGFRwt, CDH6 H-score 250 EGFRwt, CDH6 H-score 190 EGFRwt/ALKm, CDH6 H-score 213 Expanding in NSCLC 16 Courtesy of Simcere Zaiming *Primary antibody: Caherin-6 (D3T3I, Rabbit mAb, CST; Leica BOND III platform) EGFRwt (H-score 250) EGFRwt (H-score 190) EGFRwt/ALKm (H-score 213) Cancer Stage Sample Type Sub-type Sample size CDH6* 1+/2+/3+ ≥ 10% TC CDH6 H-Score 1 - 99 CDH6 H-Score 100 - 300 IIIB ~VI Tumor Biopsy of Lung Adenocarcinoma EGFRwt 28 21.4% (6/28) 3.6% (1/28) 17.9% (5/28) EGFRm 86 2.3% (2/86) 1.2% (1/86) 1.2% (1/86) |
| POTENTIAL FOR IMPROVED SAFETY & EFFICACY Opportunity to Develop Differentiated CDH6 ADC Therapeutic 17 CDH6 ADC ONGOING PH1 TRIAL IN CHINA & US PH1 CLINICAL DATA EXPECTED 2Q 2026 SIM0505 |
| 18 LNCB74 B7-H4 ADC DIFFERENTIATED ADC Unique antibody linker STRATEGIC PARTNERSHIP 50/50 co-development partnership with LigaChem Biosciences MULTIPLE INDICATIONS Breast, Ovarian, Endometrial PH1 CLINICAL ASSET • US clinical trial ongoing • CLIA validated IHC biomarker assay |
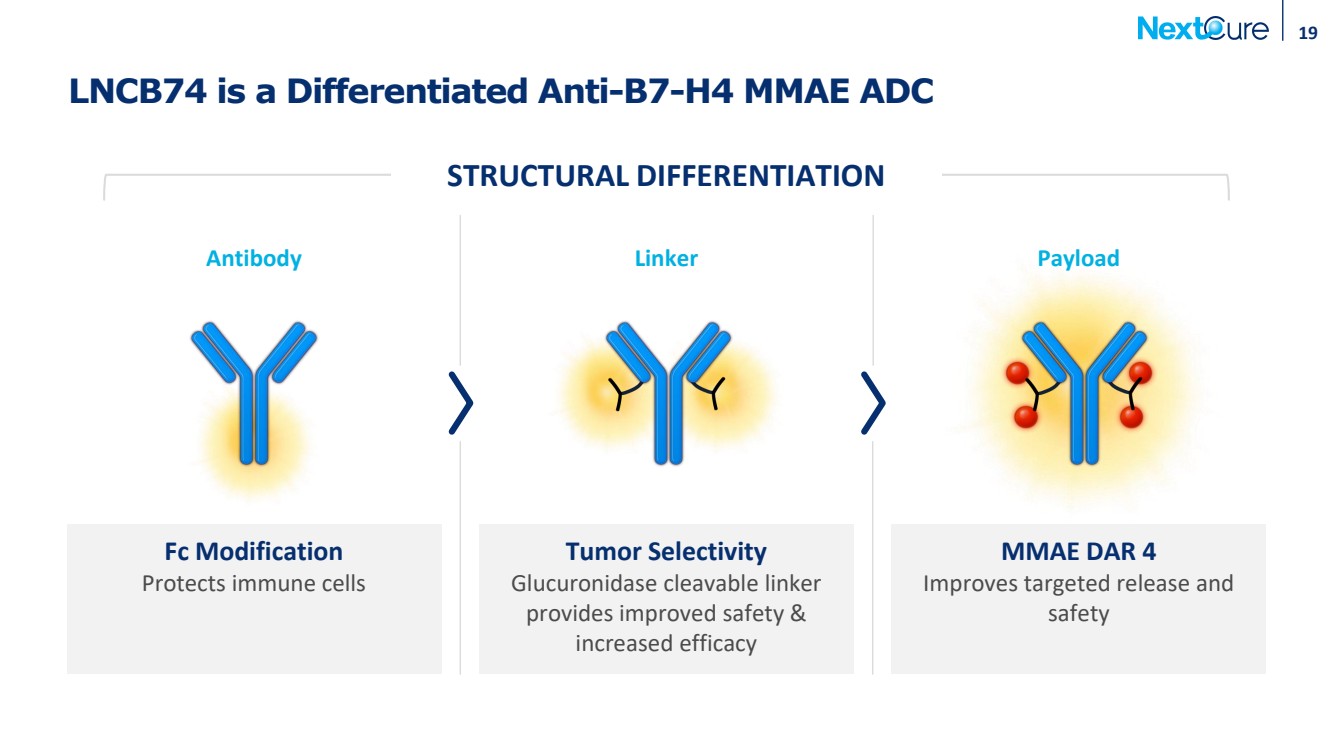
| LNCB74 is a Differentiated Anti-B7-H4 MMAE ADC Fc Modification Protects immune cells Tumor Selectivity Glucuronidase cleavable linker provides improved safety & increased efficacy 19 MMAE DAR 4 Improves targeted release and safety Antibody Linker Payload STRUCTURAL DIFFERENTIATION |
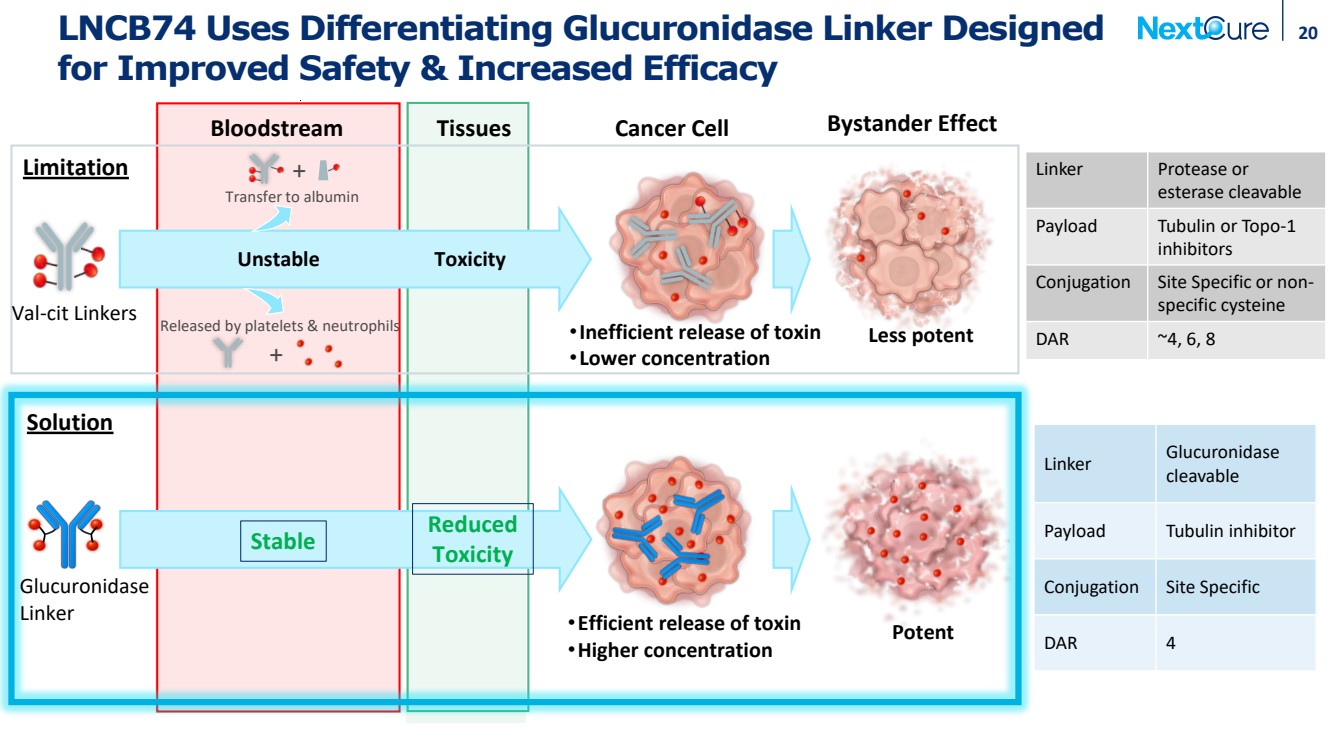
| LNCB74 Uses Differentiating Glucuronidase Linker Designed for Improved Safety & Increased Efficacy 20 Bloodstream Tissues Cancer Cell Bystander Effect Linker Glucuronidase cleavable Payload Tubulin inhibitor Conjugation Site Specific DAR 4 •Efficient release of toxin •Higher concentration Stable Potent Solution Reduced Toxicity + + Transfer to albumin Released by platelets & neutrophils Unstable Toxicity •Inefficient release of toxin •Lower concentration Less potent Limitation Linker Protease or esterase cleavable Payload Tubulin or Topo-1 inhibitors Conjugation Site Specific or non-specific cysteine DAR ~4, 6, 8 Val-cit Linkers Glucuronidase Linker |
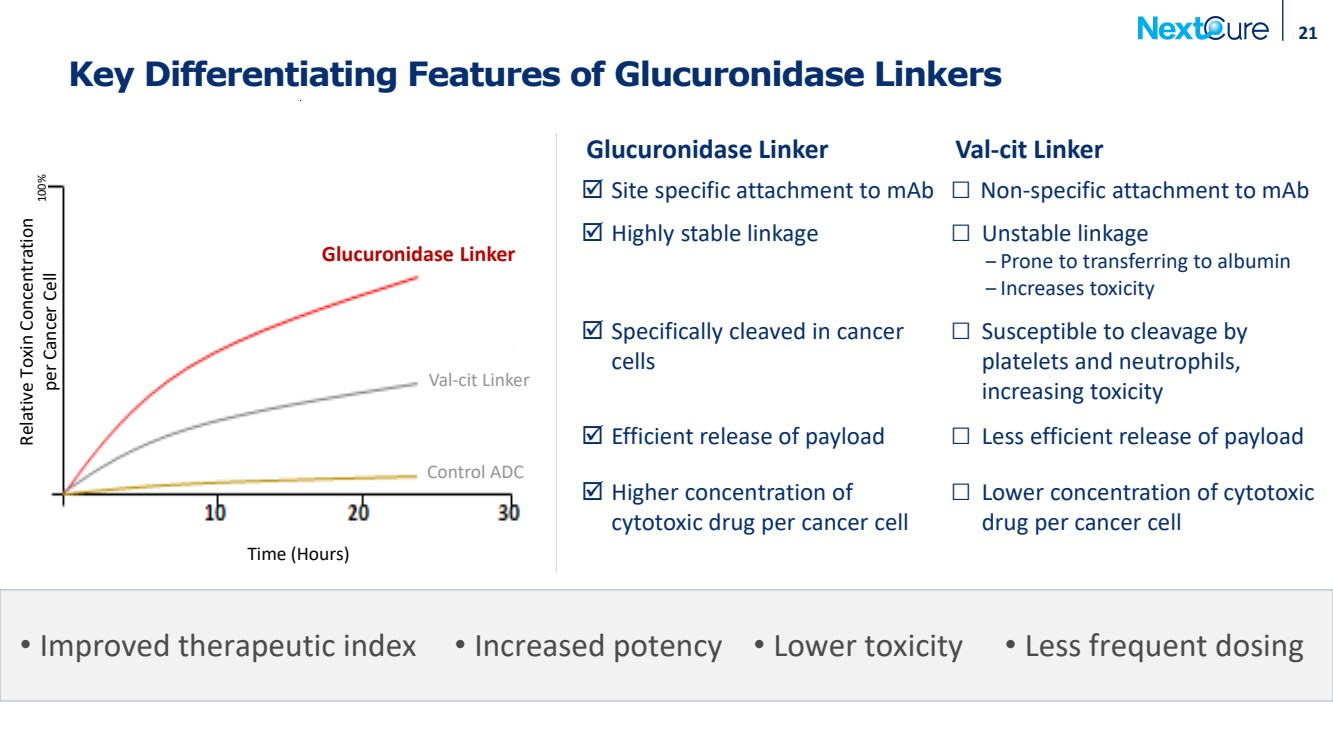
| Key Differentiating Features of Glucuronidase Linkers 21 Time (Hours) Relative Toxin Concentration per Cancer Cell 100% Val-cit Linker Glucuronidase Linker Control ADC Glucuronidase Linker Val-cit Linker Site specific attachment to mAb □ Non-specific attachment to mAb Highly stable linkage □ Unstable linkage ‒ Prone to transferring to albumin ‒ Increases toxicity Specifically cleaved in cancer cells □ Susceptible to cleavage by platelets and neutrophils, increasing toxicity Efficient release of payload □ Less efficient release of payload Higher concentration of cytotoxic drug per cancer cell □ Lower concentration of cytotoxic drug per cancer cell • Improved therapeutic index • Increased potency • Lower toxicity • Less frequent dosing |
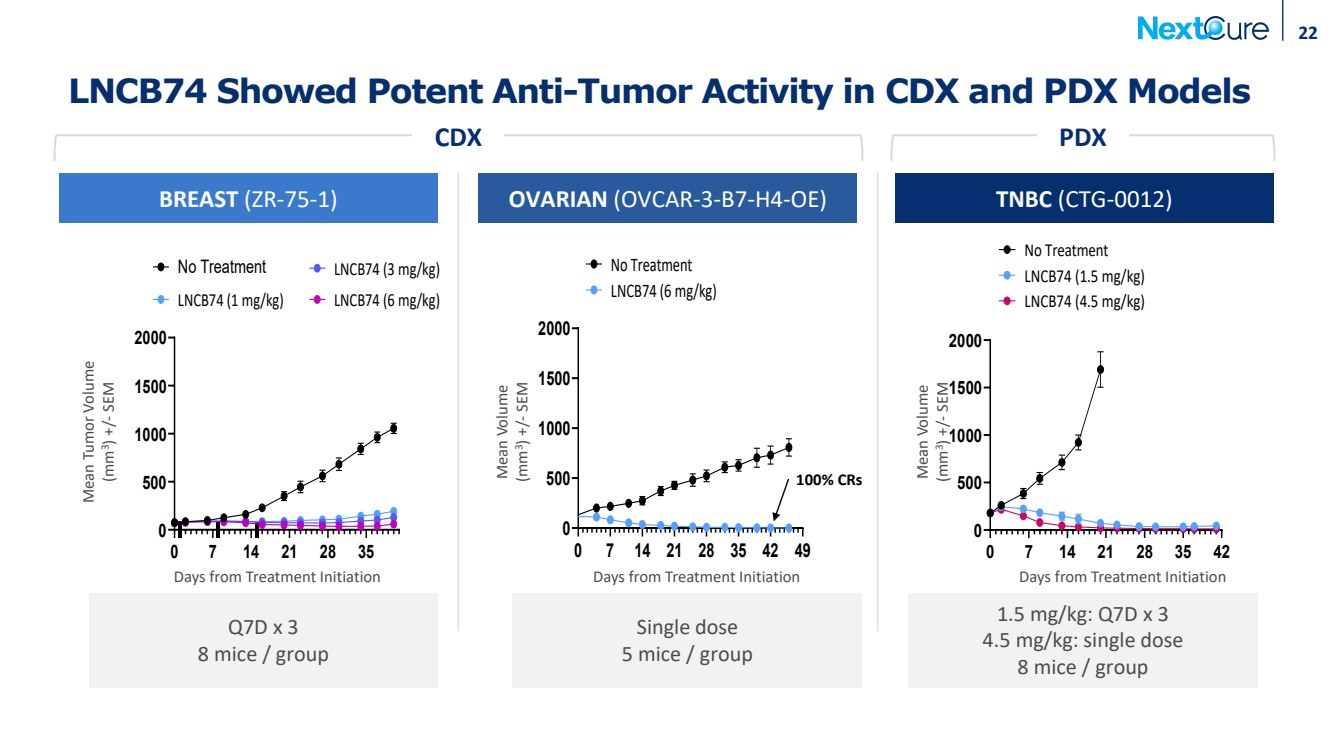
| 22 LNCB74 Showed Potent Anti-Tumor Activity in CDX and PDX Models OVARIAN (OVCAR-3-B7-H4-OE) Days from Treatment Initiation Mean Volume (mm3) +/- SEM TNBC (CTG-0012) Mean Volume (mm3) +/- SEM Days from Treatment Initiation BREAST (ZR-75-1) Days from Treatment Initiation Mean Tumor Volume (mm3) +/- SEM CDX PDX Q7D x 3 8 mice / group 1.5 mg/kg: Q7D x 3 4.5 mg/kg: single dose 8 mice / group Single dose 5 mice / group 0 7 14 21 28 35 0 500 1000 1500 2000 No Treatment LNCB74 (6 mg/kg) LNCB74 (3 mg/kg) LNCB74 (1 mg/kg) 0 7 14 21 28 35 42 49 0 500 1000 1500 2000 No Treatment LNCB74 (6 mg/kg) 100% CRs 0 7 14 21 28 35 42 0 500 1000 1500 2000 No Treatment LNCB74 (1.5 mg/kg) LNCB74 (4.5 mg/kg) |
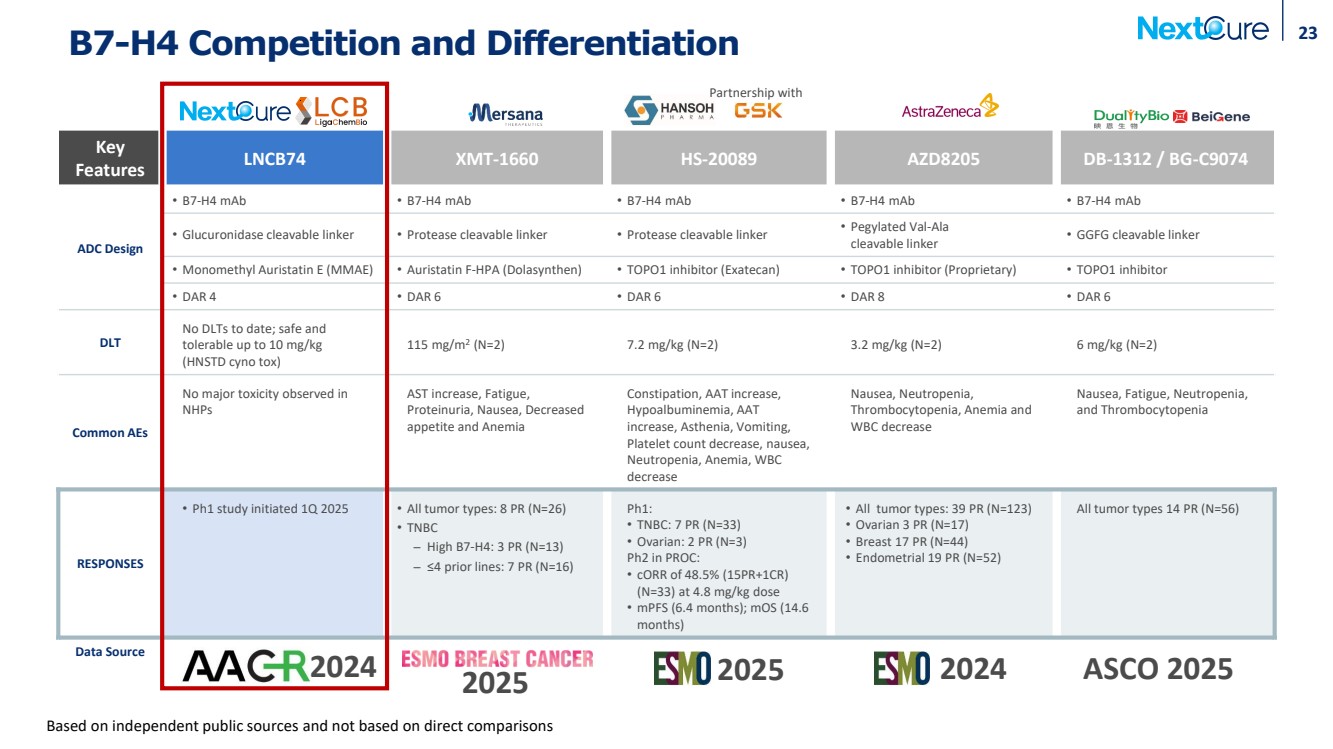
| B7-H4 Competition and Differentiation Key Features LNCB74 XMT-1660 HS-20089 AZD8205 DB-1312 / BG-C9074 ADC Design • B7-H4 mAb • B7-H4 mAb • B7-H4 mAb • B7-H4 mAb • B7-H4 mAb • Glucuronidase cleavable linker • Protease cleavable linker • Protease cleavable linker • Pegylated Val-Ala cleavable linker • GGFG cleavable linker • Monomethyl Auristatin E (MMAE) • Auristatin F-HPA (Dolasynthen) • TOPO1 inhibitor (Exatecan) • TOPO1 inhibitor (Proprietary) • TOPO1 inhibitor • DAR 4 • DAR 6 • DAR 6 • DAR 8 • DAR 6 DLT No DLTs to date; safe and tolerable up to 10 mg/kg (HNSTD cyno tox) 115 mg/m2 (N=2) 7.2 mg/kg (N=2) 3.2 mg/kg (N=2) 6 mg/kg (N=2) Common AEs No major toxicity observed in NHPs AST increase, Fatigue, Proteinuria, Nausea, Decreased appetite and Anemia Constipation, AAT increase, Hypoalbuminemia, AAT increase, Asthenia, Vomiting, Platelet count decrease, nausea, Neutropenia, Anemia, WBC decrease Nausea, Neutropenia, Thrombocytopenia, Anemia and WBC decrease Nausea, Fatigue, Neutropenia, and Thrombocytopenia RESPONSES • Ph1 study initiated 1Q 2025 • All tumor types: 8 PR (N=26) • TNBC ̶ High B7-H4: 3 PR (N=13) ̶ ≤4 prior lines: 7 PR (N=16) Ph1: • TNBC: 7 PR (N=33) • Ovarian: 2 PR (N=3) Ph2 in PROC: • cORR of 48.5% (15PR+1CR) (N=33) at 4.8 mg/kg dose • mPFS (6.4 months); mOS (14.6 months) • All tumor types: 39 PR (N=123) • Ovarian 3 PR (N=17) • Breast 17 PR (N=44) • Endometrial 19 PR (N=52) All tumor types 14 PR (N=56) Data Source 2024 2024 Partnership with 23 2025 Based on independent public sources and not based on direct comparisons 2025 ASCO 2025 |
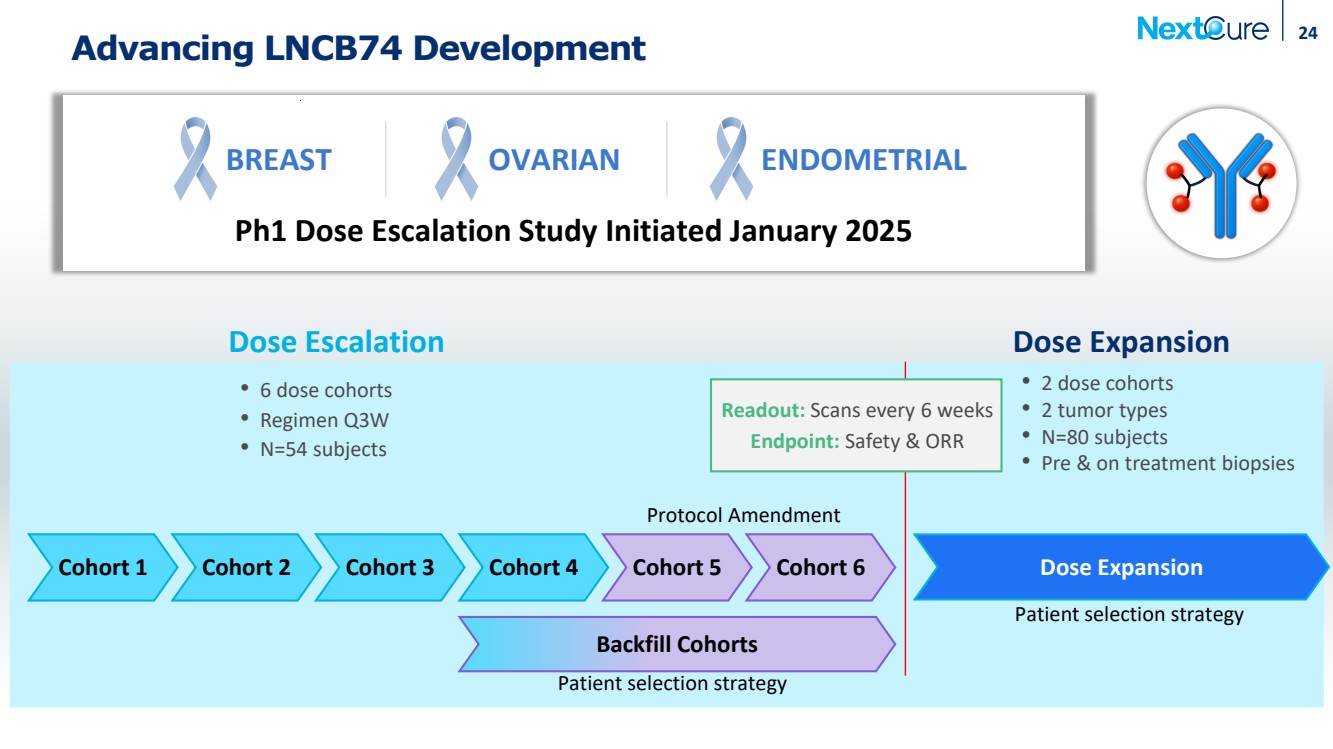
| Advancing LNCB74 Development Cohort 1 Cohort 2 Cohort 3 Cohort 4 Backfill Cohorts 24 Dose Expansion • 6 dose cohorts • Regimen Q3W • N=54 subjects • 2 dose cohorts • 2 tumor types • N=80 subjects • Pre & on treatment biopsies Patient selection strategy Patient selection strategy Dose Escalation Dose Expansion Cohort 5 Cohort 6 BREAST OVARIAN ENDOMETRIAL Ph1 Dose Escalation Study Initiated January 2025 Protocol Amendment Readout: Scans every 6 weeks Endpoint: Safety & ORR |
| IMPROVED SAFETY & INCREASED EFFICACY Opportunity to Develop Differentiated B7-H4 ADC Therapeutic 25 B7-H4 ADC UNMET NEED IN BREAST & GYNECOLOGICAL CANCERS PATIENT SELECTION STRATEGY |
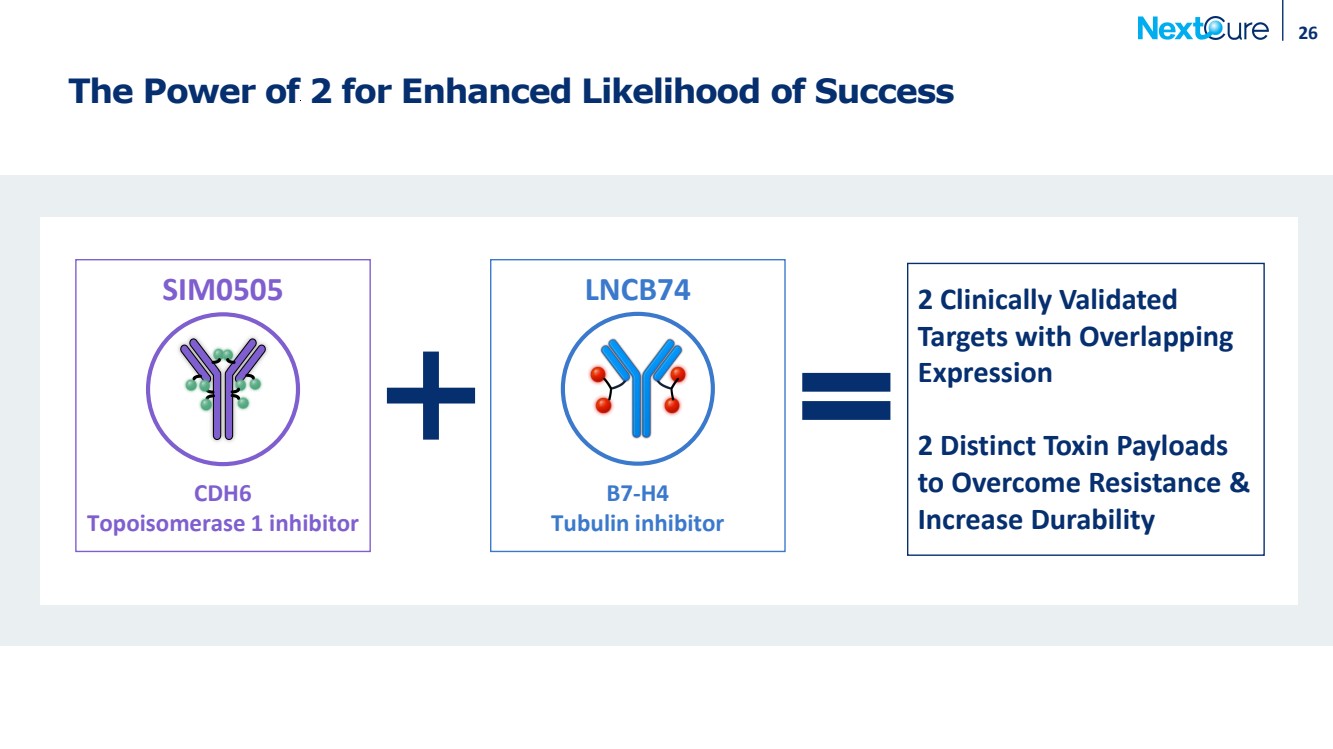
| 26 The Power of 2 for Enhanced Likelihood of Success SIM0505 LNCB74 CDH6 Topoisomerase 1 inhibitor B7-H4 Tubulin inhibitor 2 Clinically Validated Targets with Overlapping Expression 2 Distinct Toxin Payloads to Overcome Resistance & Increase Durability |
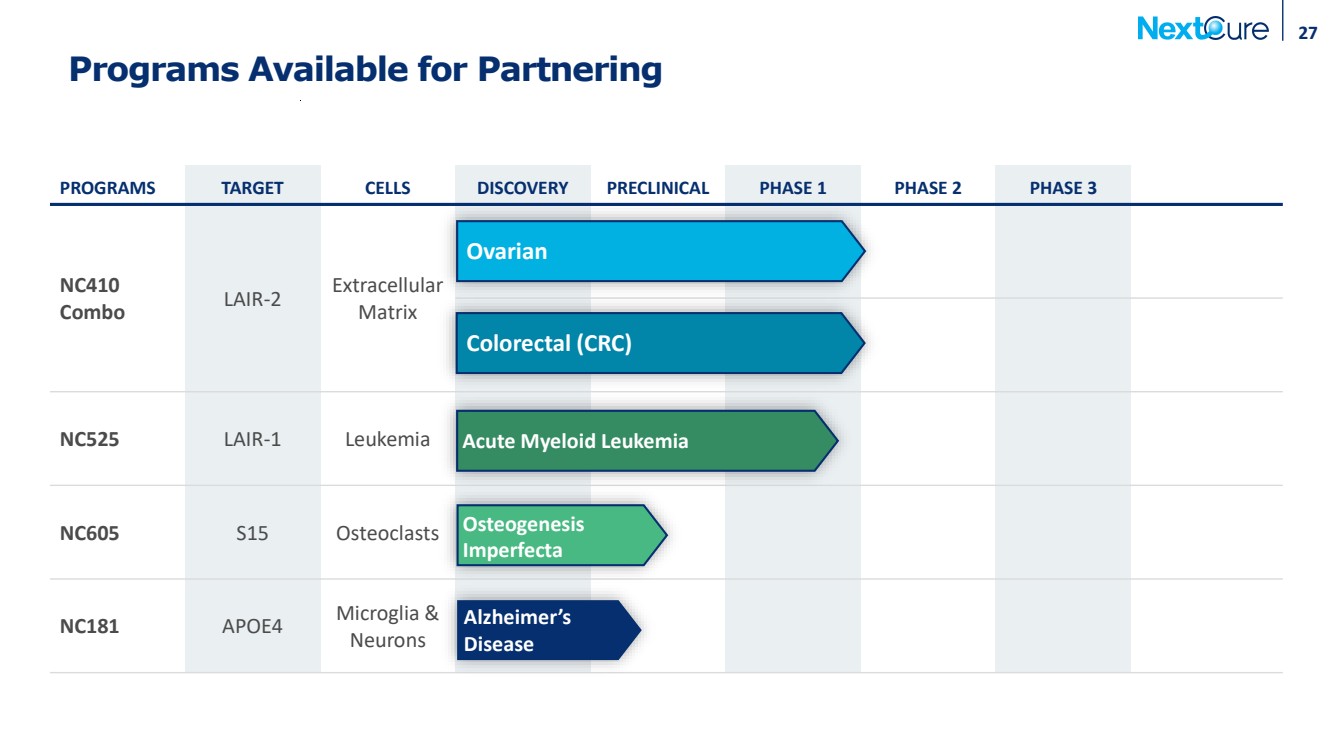
| Programs Available for Partnering 27 PROGRAMS TARGET CELLS DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NC410 Combo LAIR-2 Extracellular Matrix NC525 LAIR-1 Leukemia NC605 S15 Osteoclasts NC181 APOE4 Microglia & Neurons Alzheimer’s Disease Osteogenesis Imperfecta Acute Myeloid Leukemia Colorectal (CRC) Ovarian |
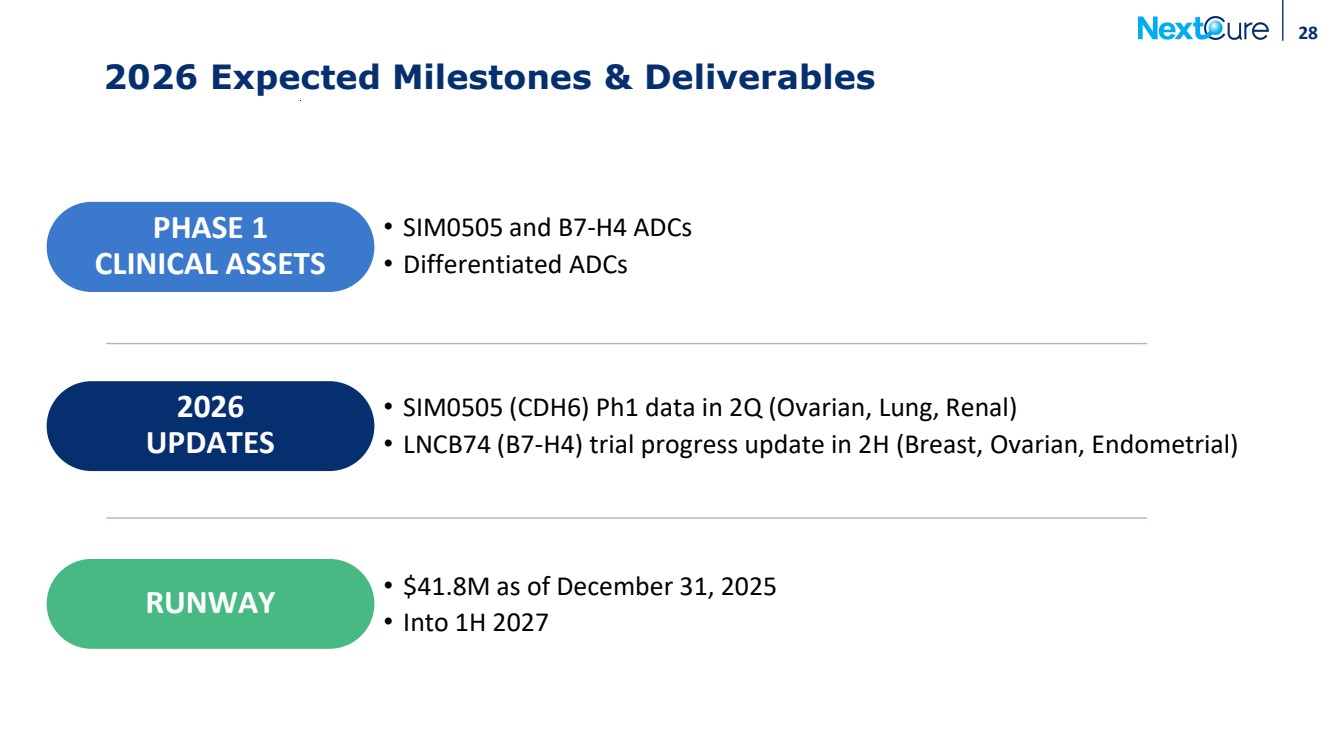
| 28 2026 Expected Milestones & Deliverables • SIM0505 (CDH6) Ph1 data in 2Q (Ovarian, Lung, Renal) • LNCB74 (B7-H4) trial progress update in 2H (Breast, Ovarian, Endometrial) 2026 UPDATES PHASE 1 CLINICAL ASSETS • SIM0505 and B7-H4 ADCs • Differentiated ADCs RUNWAY • $41.8M as of December 31, 2025 • Into 1H 2027 |