
Protein degraded. Disease targeted. Lives transformed. October 2025
Legal Disclaimer Statements and Intellectual Property LEGAL DISCLAIMER STATEMENTS The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products; expectations for timing, progress and results from ongoing and planned preclinical and clinical development activities; our ability to successfully manufacture and supply our product candidates for clinical trials; our ability to replicate results achieved in our preclinical studies or clinical trials in any future studies or trials; expectations for future regulatory submissions and potential approvals; the anticipated timing and content of presentations of data from our clinical trials; and our ability to fund our future operations.. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward- looking statements, which speak only as of the date hereof. The forward-looking statements included in this presentation are subject to a variety of risks and uncertainties, including those set forth in our most recent and future filings with the Securities and Exchange Commission. Our actual results could vary significantly from those anticipated in this presentation, and our financial condition and results of operations could be materially adversely affected. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. This presentation also contains estimates, projections and other information concerning the markets for C4 Therapeutics, Inc.’s product candidates, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions and patient use of medicines. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties and actual events, and circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, the Company obtained this industry, business, market and other data from reports, research surveys, clinical trials studies and similar data prepared by market research firms and other third parties, from industry, medical and general publications, from other publicly available information, and from government data and similar sources. INTELLECTUAL PROPERTY C4 Therapeutics, Inc. owns various registered and unregistered trademarks and service marks in the U.S. and internationally, including, without limitation, C4 THERAPEUTICS, our housemark logo, the name of our TORPEDO platform, and the names of our BIDAC and MONODAC degrader products. All trademarks, service marks, or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks, service marks, and trade names in this presentation are referred to without the symbols ®, SM and , but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights to. 2 © 2025 C4 Therapeutics, Inc.
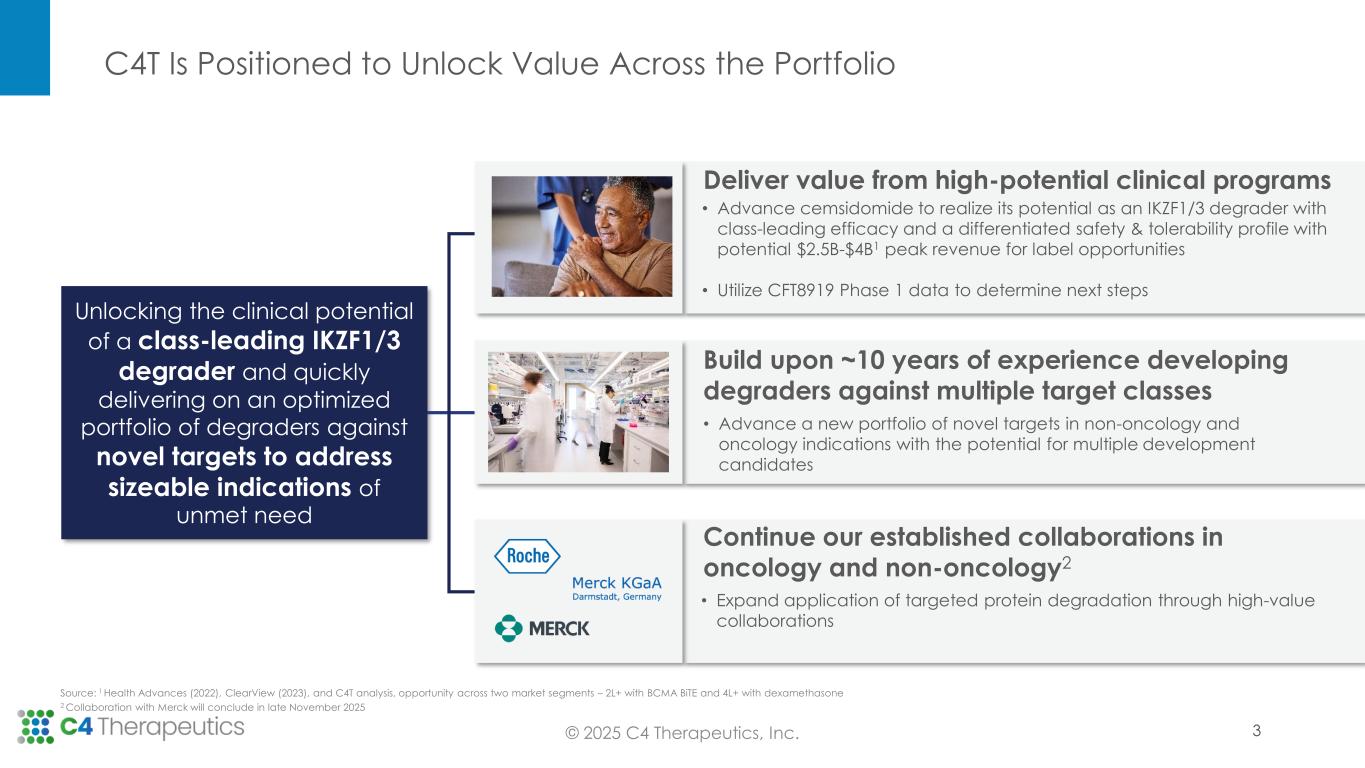
C4T Is Positioned to Unlock Value Across the Portfolio © 2025 C4 Therapeutics, Inc. Source: 1 Health Advances (2022), ClearView (2023), and C4T analysis, opportunity across two market segments – 2L+ with BCMA BiTE and 4L+ with dexamethasone 2 Collaboration with Merck will conclude in late November 2025 Unlocking the clinical potential of a class-leading IKZF1/3 degrader and quickly delivering on an optimized portfolio of degraders against novel targets to address sizeable indications of unmet need Deliver value from high-potential clinical programs Build upon ~10 years of experience developing degraders against multiple target classes • Advance a new portfolio of novel targets in non-oncology and oncology indications with the potential for multiple development candidates Continue our established collaborations in oncology and non-oncology2 • Expand application of targeted protein degradation through high-value collaborations • Advance cemsidomide to realize its potential as an IKZF1/3 degrader with class-leading efficacy and a differentiated safety & tolerability profile with potential $2.5B-$4B1 peak revenue for label opportunities • Utilize CFT8919 Phase 1 data to determine next steps 3
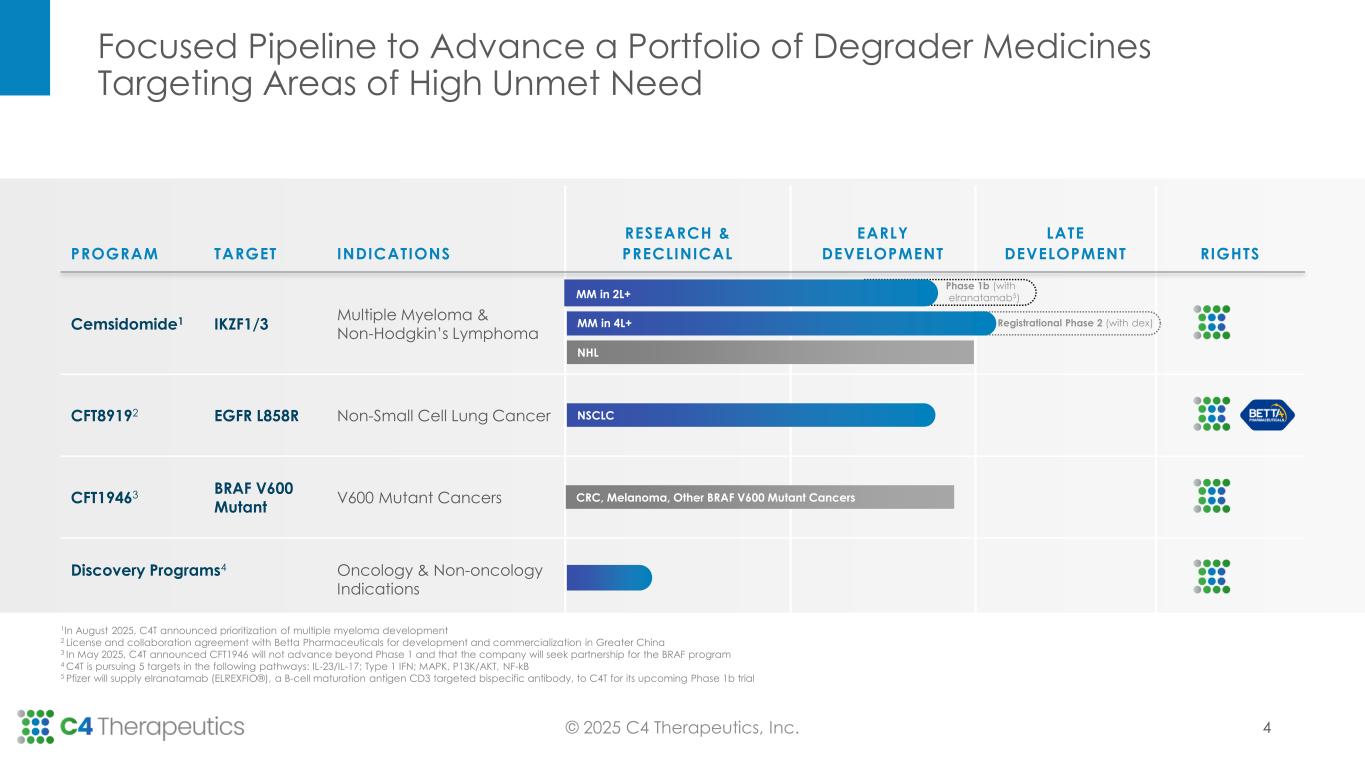
PROGRAM TARGET INDICATIONS RESEARCH & PRECLINICAL EARLY DEVELOPMENT LATE DEVELOPMENT RIGHTS Cemsidomide1 IKZF1/3 Multiple Myeloma & Non-Hodgkin’s Lymphoma CFT89192 EGFR L858R Non-Small Cell Lung Cancer CFT19463 BRAF V600 Mutant V600 Mutant Cancers Discovery Programs4 Oncology & Non-oncology Indications 1In August 2025, C4T announced prioritization of multiple myeloma development 2 License and collaboration agreement with Betta Pharmaceuticals for development and commercialization in Greater China 3 In May 2025, C4T announced CFT1946 will not advance beyond Phase 1 and that the company will seek partnership for the BRAF program 4 C4T is pursuing 5 targets in the following pathways: IL-23/IL-17; Type 1 IFN; MAPK, P13K/AKT, NF-kB 5 Pfizer will supply elranatamab (ELREXFIO®), a B-cell maturation antigen CD3 targeted bispecific antibody, to C4T for its upcoming Phase 1b trial CRC, Melanoma, Other BRAF V600 Mutant Cancers NHL NSCLC Focused Pipeline to Advance a Portfolio of Degrader Medicines Targeting Areas of High Unmet Need © 2025 C4 Therapeutics, Inc. Registrational Phase 2 (with dex)MM in 4L+ Phase 1b (with elranatamab5) 4 MM in 2L+

Strong Execution Across Pipeline Positions Us for Continued Advancement © 2025 C4 Therapeutics, Inc. Cemsidomide IKZF1/3 Completed Phase 1 dose escalation in MM Present data from full Phase 1 dose escalation in MM at IMS Annual Meeting Present data from Phase 1 dose escalation data in NHL Initiate Phase 2 trial of cemsidomide + dex Initiate Phase 1b trial of cemsidomide + elranatamab1 CFT8919 EGFR L858R Utilize data from Phase 1 dose escalation trial in Greater China to inform ex-China clinical development Advanced two programs to preclinical milestones through the Roche collaboration Advanced one project to preclinical milestones through the MKDG collaboration Advanced internal and collaboration programs to key discovery milestones Present and publish preclinical work from internal pipeline and TORPEDO platform Discovery 1Pfizer will supply elranatamab (ELREXFIO®), a B-cell maturation antigen CD3 targeted bispecific antibody, to C4T for its upcoming Phase 1b trial Multiple myeloma (MM); peripheral T-cell lymphoma (PTCL), a subtype of NHL; International Myeloma Society (IMS); non-Hodgkin’s Lymphoma (NHL) Complete monotherapy Phase 1 dose escalation trial in BRAF V600 mutant solid tumors Generate sufficient data from Phase 1 cohorts to inform next stepsCFT1946 BRAF V600 Mutant 5

Cemsidomide IKZF1/3 Degrader Multiple Myeloma & Non-Hodgkin’s Lymphoma
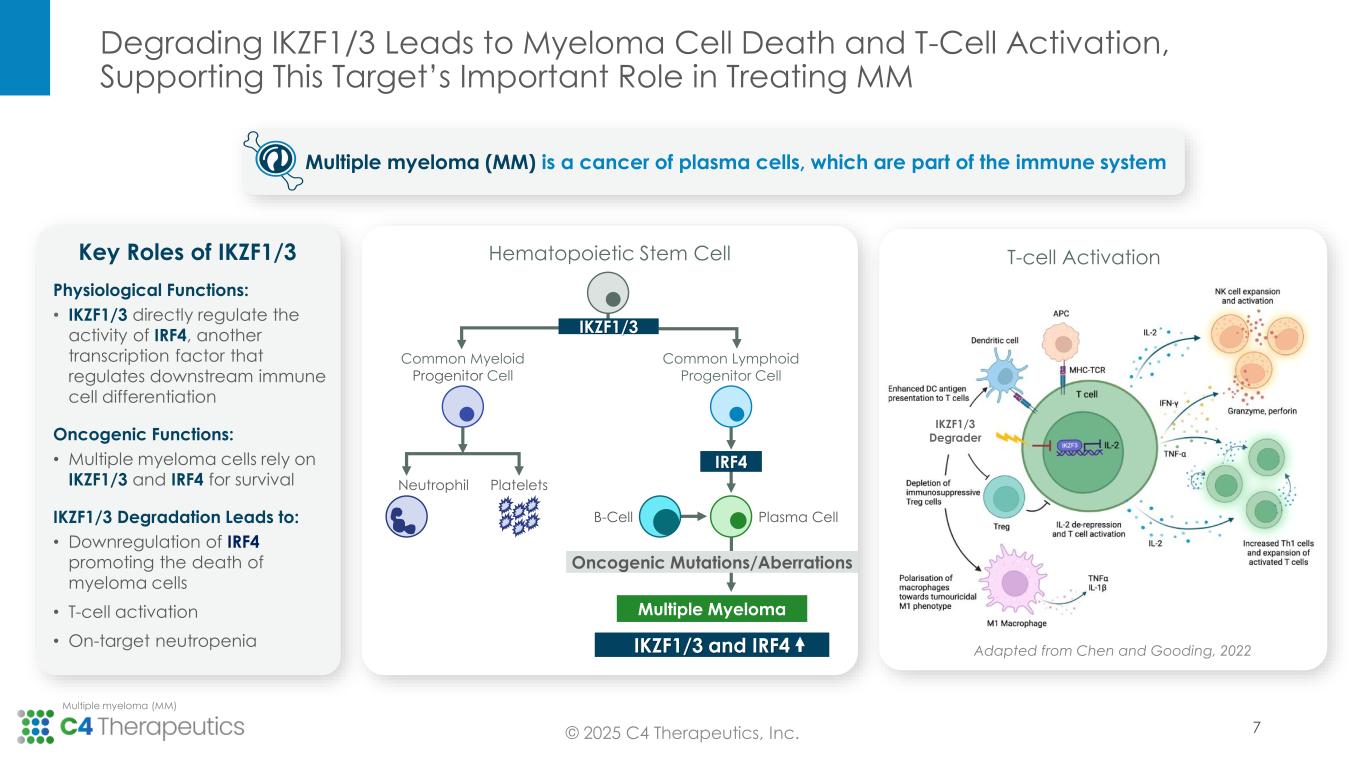
Degrading IKZF1/3 Leads to Myeloma Cell Death and T-Cell Activation, Supporting This Target’s Important Role in Treating MM © 2025 C4 Therapeutics, Inc. Multiple myeloma (MM) Common Myeloid Progenitor Cell Common Lymphoid Progenitor Cell Neutrophil Platelets B-Cell Plasma Cell Oncogenic Mutations/Aberrations IKZF1/3 and IRF4 Multiple Myeloma IRF4 Physiological Functions: • IKZF1/3 directly regulate the activity of IRF4, another transcription factor that regulates downstream immune cell differentiation Oncogenic Functions: • Multiple myeloma cells rely on IKZF1/3 and IRF4 for survival IKZF1/3 Degradation Leads to: • Downregulation of IRF4 promoting the death of myeloma cells • T-cell activation • On-target neutropenia Key Roles of IKZF1/3 IKZF1/3 Hematopoietic Stem Cell Multiple myeloma (MM) is a cancer of plasma cells, which are part of the immune system T-cell Activation Adapted from Chen and Gooding, 2022 IKZF1/3 Degrader 7
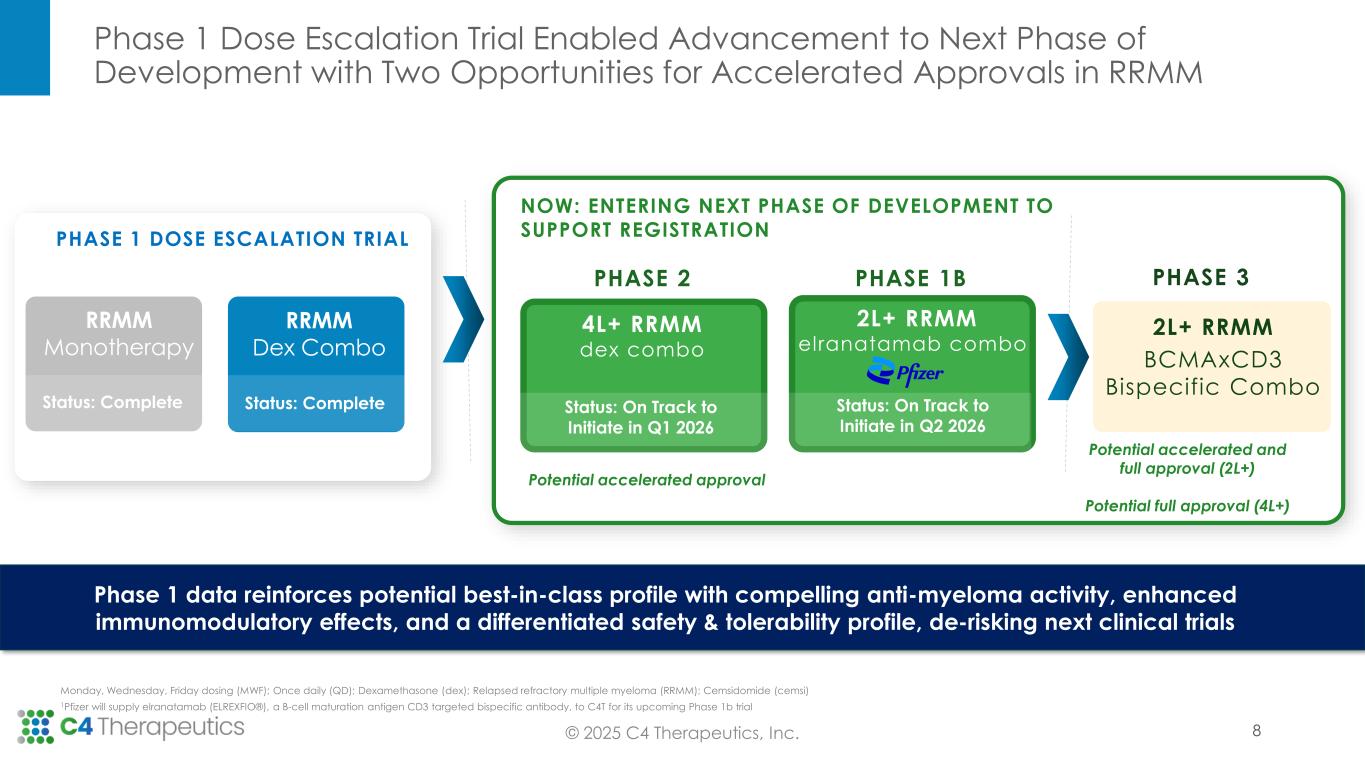
Phase 1 Dose Escalation Trial Enabled Advancement to Next Phase of Development with Two Opportunities for Accelerated Approvals in RRMM © 2025 C4 Therapeutics, Inc. NOW: ENTERING NEXT PHASE OF DEVELOPMENT TO SUPPORT REGISTRATION Monday, Wednesday, Friday dosing (MWF); Once daily (QD); Dexamethasone (dex); Relapsed refractory multiple myeloma (RRMM); Cemsidomide (cemsi) 1Pfizer will supply elranatamab (ELREXFIO®), a B-cell maturation antigen CD3 targeted bispecific antibody, to C4T for its upcoming Phase 1b trial Phase 1 data reinforces potential best-in-class profile with compelling anti-myeloma activity, enhanced immunomodulatory effects, and a differentiated safety & tolerability profile, de-risking next clinical trials Potential accelerated approval PHASE 1 DOSE ESCALATION TRIAL Status: Complete R/R MM Dex Combo Potential accelerated and full approval (2L+) Potential full approval (4L+) Status: Complete RRMM Monotherapy Status: Complete RM Dex Combo PHASE 2 PHASE 1B 4L+ RRMM dex combo 2L+ RRMM elranatamab combo1 Status: On Track to Initiate in Q1 2026 Status: On Track to Initiate in Q2 2026 2L+ RRMM BCMAxCD3 Bispecific Combo PHASE 3 8
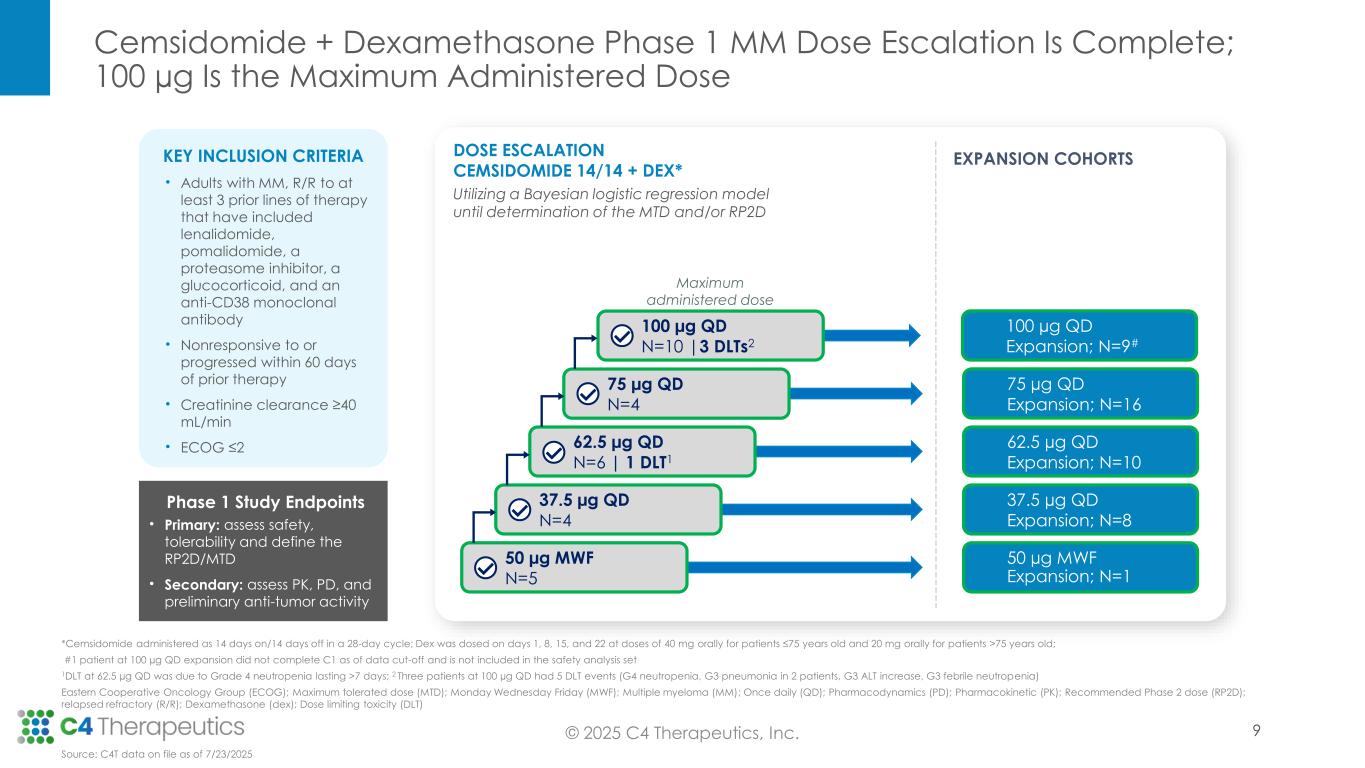
Cemsidomide + Dexamethasone Phase 1 MM Dose Escalation Is Complete; 100 µg Is the Maximum Administered Dose © 2025 C4 Therapeutics, Inc. *Cemsidomide administered as 14 days on/14 days off in a 28-day cycle; Dex was dosed on days 1, 8, 15, and 22 at doses of 40 mg orally for patients ≤75 years old and 20 mg orally for patients >75 years old; #1 patient at 100 µg QD expansion did not complete C1 as of data cut-off and is not included in the safety analysis set 1DLT at 62.5 µg QD was due to Grade 4 neutropenia lasting >7 days; 2 Three patients at 100 µg QD had 5 DLT events (G4 neutropenia, G3 pneumonia in 2 patients, G3 ALT increase, G3 febrile neutropenia) Eastern Cooperative Oncology Group (ECOG); Maximum tolerated dose (MTD); Monday Wednesday Friday (MWF); Multiple myeloma (MM); Once daily (QD); Pharmacodynamics (PD); Pharmacokinetic (PK); Recommended Phase 2 dose (RP2D); relapsed refractory (R/R); Dexamethasone (dex); Dose limiting toxicity (DLT) DOSE ESCALATION CEMSIDOMIDE 14/14 + DEX* Utilizing a Bayesian logistic regression model until determination of the MTD and/or RP2D 50 µg MWF Expansion; N=1 37.5 µg QD Expansion; N=8 62.5 µg QD Expansion; N=10 75 µg QD Expansion; N=16 50 µg MWF N=5 37.5 µg QD N=4 75 µg QD N=4 62.5 µg QD N=6 | 1 DLT1 KEY INCLUSION CRITERIA • Adults with MM, R/R to at least 3 prior lines of therapy that have included lenalidomide, pomalidomide, a proteasome inhibitor, a glucocorticoid, and an anti-CD38 monoclonal antibody • Nonresponsive to or progressed within 60 days of prior therapy • Creatinine clearance ≥40 mL/min • ECOG ≤2 Phase 1 Study Endpoints • Primary: assess safety, tolerability and define the RP2D/MTD • Secondary: assess PK, PD, and preliminary anti-tumor activity EXPANSION COHORTS 100 µg QD N=10 |3 DLTs2 100 µg QD Expansion; N=9# Maximum administered dose Source: C4T data on file as of 7/23/2025 9
IMS Data 7/23/2025 Data Cutoff
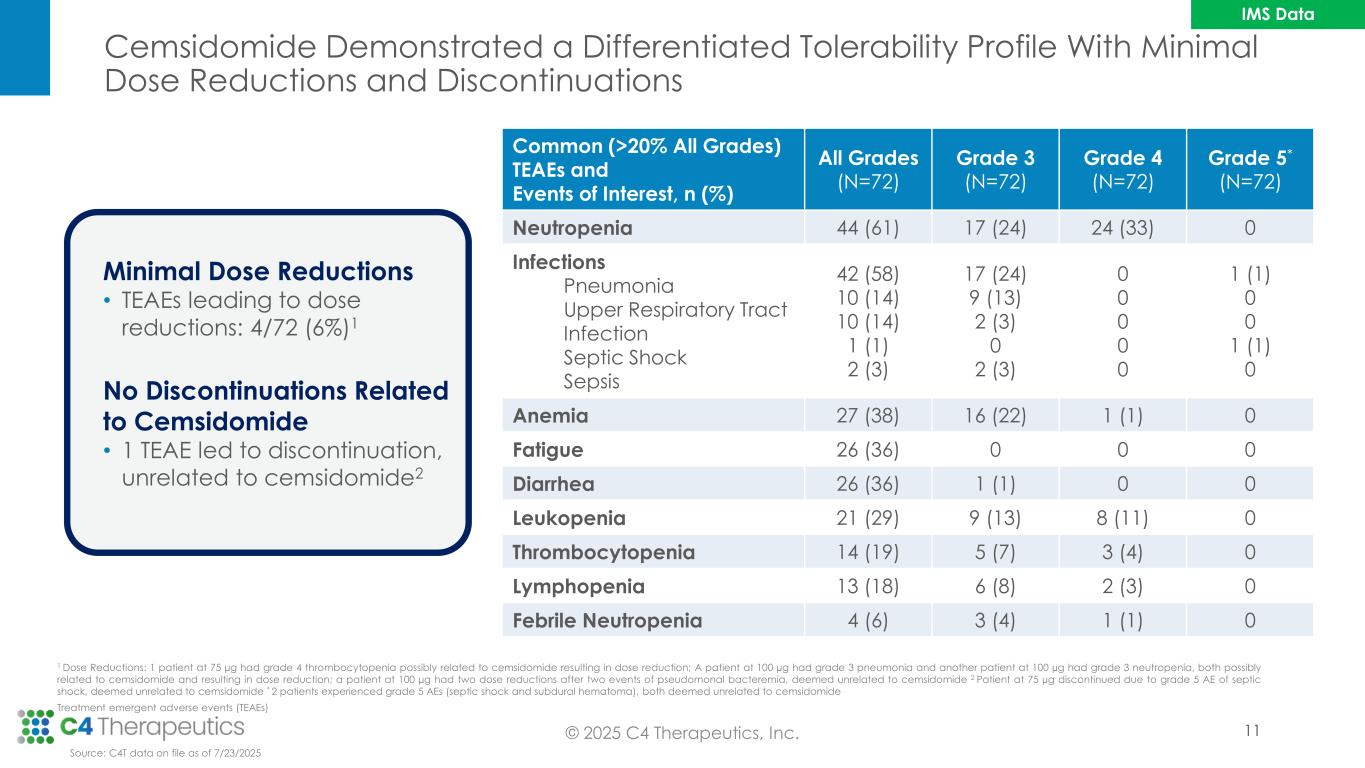
Cemsidomide Demonstrated a Differentiated Tolerability Profile With Minimal Dose Reductions and Discontinuations © 2025 C4 Therapeutics, Inc. Minimal Dose Reductions • TEAEs leading to dose reductions: 4/72 (6%)1 No Discontinuations Related to Cemsidomide • 1 TEAE led to discontinuation, unrelated to cemsidomide2 1 Dose Reductions: 1 patient at 75 µg had grade 4 thrombocytopenia possibly related to cemsidomide resulting in dose reduction; A patient at 100 µg had grade 3 pneumonia and another patient at 100 µg had grade 3 neutropenia, both possibly related to cemsidomide and resulting in dose reduction; a patient at 100 µg had two dose reductions after two events of pseudomonal bacteremia, deemed unrelated to cemsidomide 2 Patient at 75 µg discontinued due to grade 5 AE of septic shock, deemed unrelated to cemsidomide * 2 patients experienced grade 5 AEs (septic shock and subdural hematoma), both deemed unrelated to cemsidomide Treatment emergent adverse events (TEAEs) Common (>20% All Grades) TEAEs and Events of Interest, n (%) All Grades (N=72) Grade 3 (N=72) Grade 4 (N=72) Grade 5* (N=72) Neutropenia 44 (61) 17 (24) 24 (33) 0 Infections Pneumonia Upper Respiratory Tract Infection Septic Shock Sepsis 42 (58) 10 (14) 10 (14) 1 (1) 2 (3) 17 (24) 9 (13) 2 (3) 0 2 (3) 0 0 0 0 0 1 (1) 0 0 1 (1) 0 Anemia 27 (38) 16 (22) 1 (1) 0 Fatigue 26 (36) 0 0 0 Diarrhea 26 (36) 1 (1) 0 0 Leukopenia 21 (29) 9 (13) 8 (11) 0 Thrombocytopenia 14 (19) 5 (7) 3 (4) 0 Lymphopenia 13 (18) 6 (8) 2 (3) 0 Febrile Neutropenia 4 (6) 3 (4) 1 (1) 0 Source: C4T data on file as of 7/23/2025 11 IMS Data
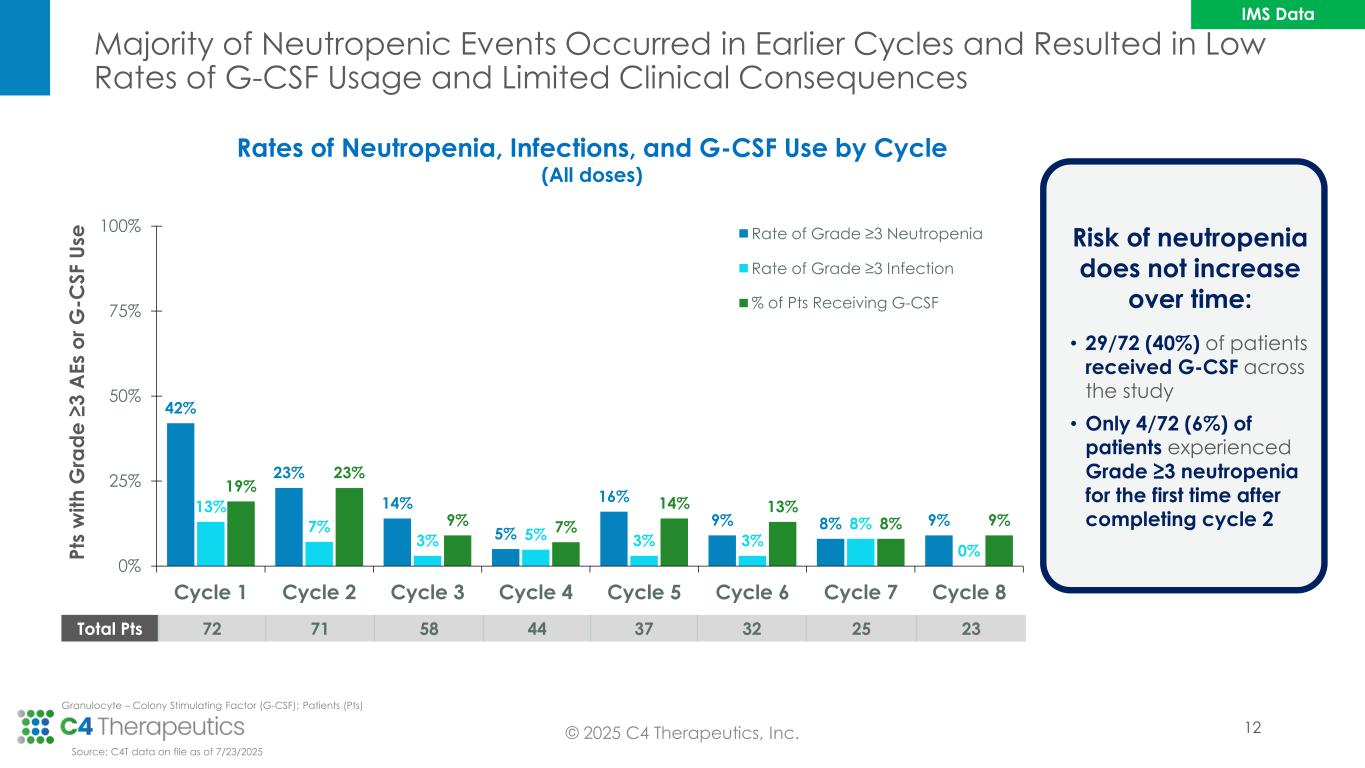
Majority of Neutropenic Events Occurred in Earlier Cycles and Resulted in Low Rates of G-CSF Usage and Limited Clinical Consequences © 2025 C4 Therapeutics, Inc. 42% 23% 14% 5% 16% 9% 8% 9% 13% 7% 3% 5% 3% 3% 8% 0% 19% 23% 9% 7% 14% 13% 8% 9% 0% 25% 50% 75% 100% Cycle 1 Cycle 2 Cycle 3 Cycle 4 Cycle 5 Cycle 6 Cycle 7 Cycle 8 P ts w it h G ra d e ≥ 3 A E s o r G -C S F U se Rate of Grade ≥3 Neutropenia Rate of Grade ≥3 Infection % of Pts Receiving G-CSF Rates of Neutropenia, Infections, and G-CSF Use by Cycle (All doses) Total Pts 72 71 58 44 37 32 25 23 Risk of neutropenia does not increase over time: • 29/72 (40%) of patients received G-CSF across the study • Only 4/72 (6%) of patients experienced Grade ≥3 neutropenia for the first time after completing cycle 2 Source: C4T data on file as of 7/23/2025 Granulocyte – Colony Stimulating Factor (G-CSF); Patients (Pts) IMS Data 12
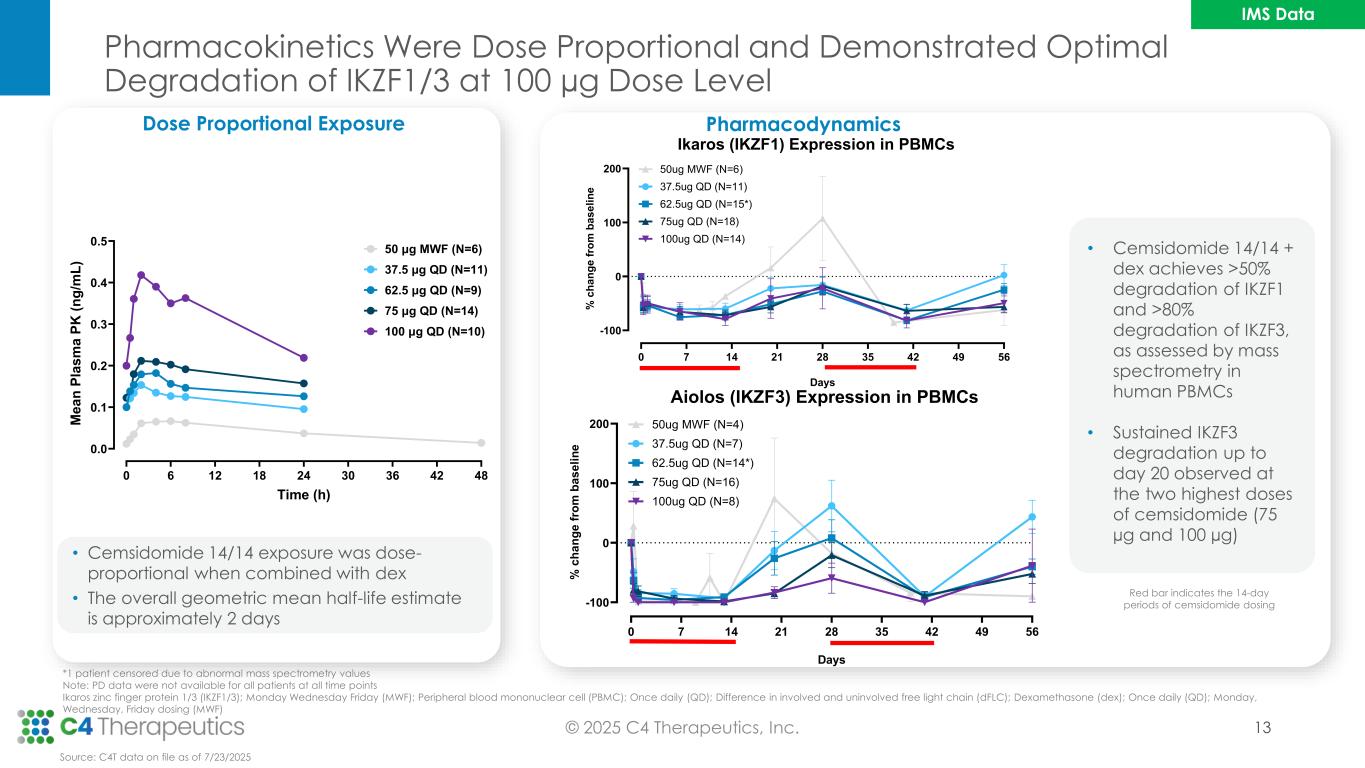
Pharmacokinetics Were Dose Proportional and Demonstrated Optimal Degradation of IKZF1/3 at 100 µg Dose Level 13 *1 patient censored due to abnormal mass spectrometry values Note: PD data were not available for all patients at all time points Ikaros zinc finger protein 1/3 (IKZF1/3); Monday Wednesday Friday (MWF); Peripheral blood mononuclear cell (PBMC); Once daily (QD); Difference in involved and uninvolved free light chain (dFLC); Dexamethasone (dex); Once daily (QD); Monday, Wednesday, Friday dosing (MWF) • Cemsidomide 14/14 exposure was dose- proportional when combined with dex • The overall geometric mean half-life estimate is approximately 2 days Dose Proportional Exposure 0 6 12 18 24 30 36 42 48 0.0 0.1 0.2 0.3 0.4 0.5 Time (h) M e a n P la s m a P K ( n g /m L ) 62.5 μg QD (N=9) 37.5 μg QD (N=11) 50 μg MWF (N=6) 75 μg QD (N=14) 100 μg QD (N=10) Source: C4T data on file as of 7/23/2025 • Cemsidomide 14/14 + dex achieves >50% degradation of IKZF1 and >80% degradation of IKZF3, as assessed by mass spectrometry in human PBMCs • Sustained IKZF3 degradation up to day 20 observed at the two highest doses of cemsidomide (75 µg and 100 µg) Pharmacodynamics 0 7 14 21 28 35 42 49 56 -100 0 100 200 Aiolos (IKZF3) Expression in PBMCs Days % c h a n g e f ro m b a s e li n e 100ug QD (N=8) 75ug QD (N=16) 62.5ug QD (N=14*) 37.5ug QD (N=7) 50ug MWF (N=4) 0 7 14 21 28 35 42 49 56 -100 0 100 200 Ikaros (IKZF1) Expression in PBMCs Days % c h a n g e f ro m b a s e li n e 37.5ug QD (N=11) 50ug MWF (N=6) 100ug QD (N=14) 75ug QD (N=18) 62.5ug QD (N=15*) Red bar indicates the 14-day periods of cemsidomide dosing © 2025 C4 Therapeutics, Inc. IMS Data
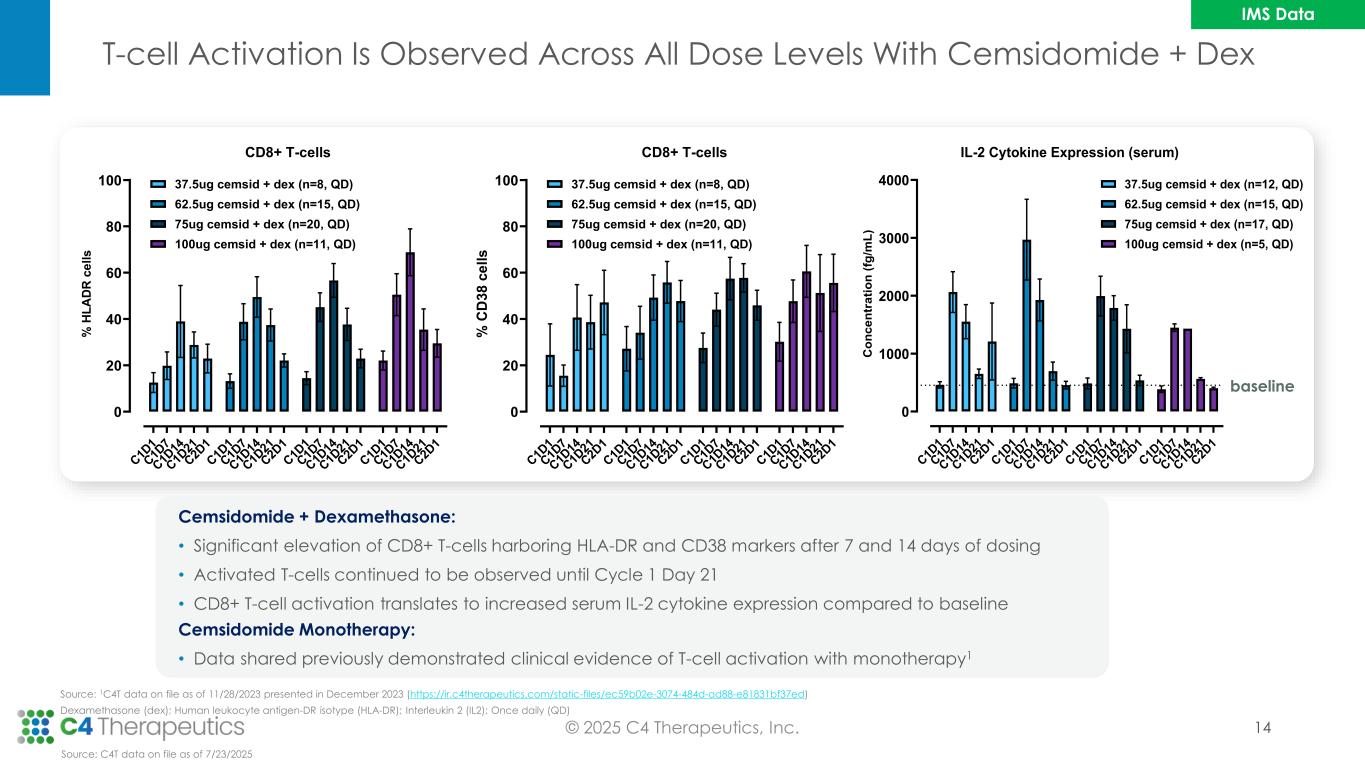
T-cell Activation Is Observed Across All Dose Levels With Cemsidomide + Dex © 2025 C4 Therapeutics, Inc. C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 0 20 40 60 80 100 CD8+ T-cells % H L A D R c e ll s 37.5ug cemsid + dex (n=8, QD) 62.5ug cemsid + dex (n=15, QD) 75ug cemsid + dex (n=20, QD) 100ug cemsid + dex (n=11, QD) C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 0 20 40 60 80 100 CD8+ T-cells % C D 3 8 c e ll s 37.5ug cemsid + dex (n=8, QD) 62.5ug cemsid + dex (n=15, QD) 75ug cemsid + dex (n=20, QD) 100ug cemsid + dex (n=11, QD) C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 C 1D 1 C 1D 7 C 1D 14 C 1D 21 C 2D 1 0 1000 2000 3000 4000 IL-2 Cytokine Expression (serum) C o n c e n tr a ti o n ( fg /m L ) 37.5ug cemsid + dex (n=12, QD) 62.5ug cemsid + dex (n=15, QD) 75ug cemsid + dex (n=17, QD) 100ug cemsid + dex (n=5, QD) Cemsidomide + Dexamethasone: • Significant elevation of CD8+ T-cells harboring HLA-DR and CD38 markers after 7 and 14 days of dosing • Activated T-cells continued to be observed until Cycle 1 Day 21 • CD8+ T-cell activation translates to increased serum IL-2 cytokine expression compared to baseline Source: C4T data on file as of 7/23/2025 baseline Cemsidomide Monotherapy: • Data shared previously demonstrated clinical evidence of T-cell activation with monotherapy1 Source: 1C4T data on file as of 11/28/2023 presented in December 2023 (https://ir.c4therapeutics.com/static-files/ec59b02e-3074-484d-ad88-e81831bf37ed) Dexamethasone (dex); Human leukocyte antigen-DR isotype (HLA-DR); Interleukin 2 (IL2); Once daily (QD) 14 IMS Data
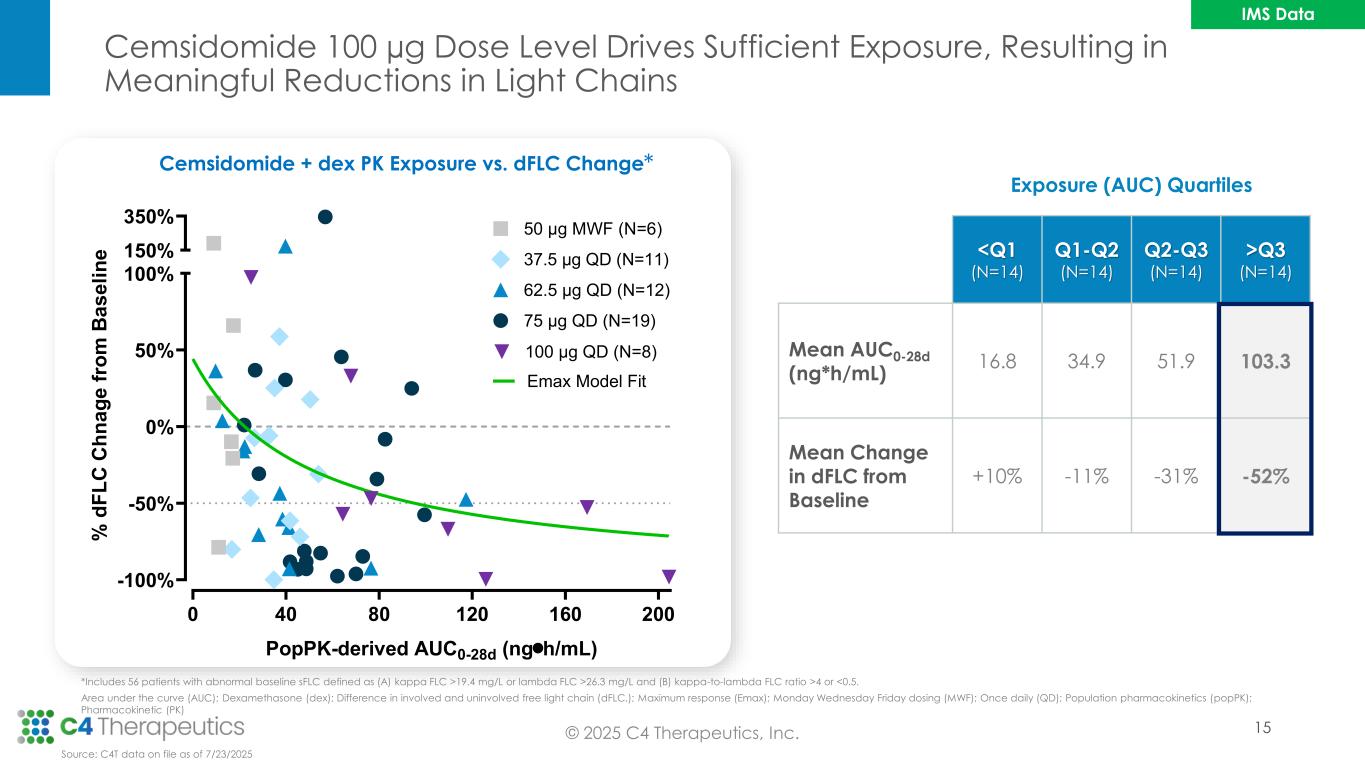
Exposure (AUC) Quartiles <Q1 (N=14) Q1-Q2 (N=14) Q2-Q3 (N=14) >Q3 (N=14) Mean AUC0-28d (ng*h/mL) 16.8 34.9 51.9 103.3 Mean Change in dFLC from Baseline +10% -11% -31% -52% Cemsidomide 100 µg Dose Level Drives Sufficient Exposure, Resulting in Meaningful Reductions in Light Chains 15 Cemsidomide + dex PK Exposure vs. dFLC Change *Includes 56 patients with abnormal baseline sFLC defined as (A) kappa FLC >19.4 mg/L or lambda FLC >26.3 mg/L and (B) kappa-to-lambda FLC ratio >4 or <0.5. Area under the curve (AUC); Dexamethasone (dex); Difference in involved and uninvolved free light chain (dFLC,); Maximum response (Emax); Monday Wednesday Friday dosing (MWF); Once daily (QD); Population pharmacokinetics (popPK); Pharmacokinetic (PK) 0 40 80 120 160 200 -100% -50% 0% 50% 100% 150% 350% PopPK-derived AUC0-28d (ngh/mL) % d F L C C h n a g e f ro m B a s e li n e 50 μg MWF (N=6) 37.5 μg QD (N=11) 62.5 μg QD (N=12) 75 μg QD (N=19) 100 μg QD (N=8) Emax Model Fit * Source: C4T data on file as of 7/23/2025 © 2025 C4 Therapeutics, Inc. IMS Data
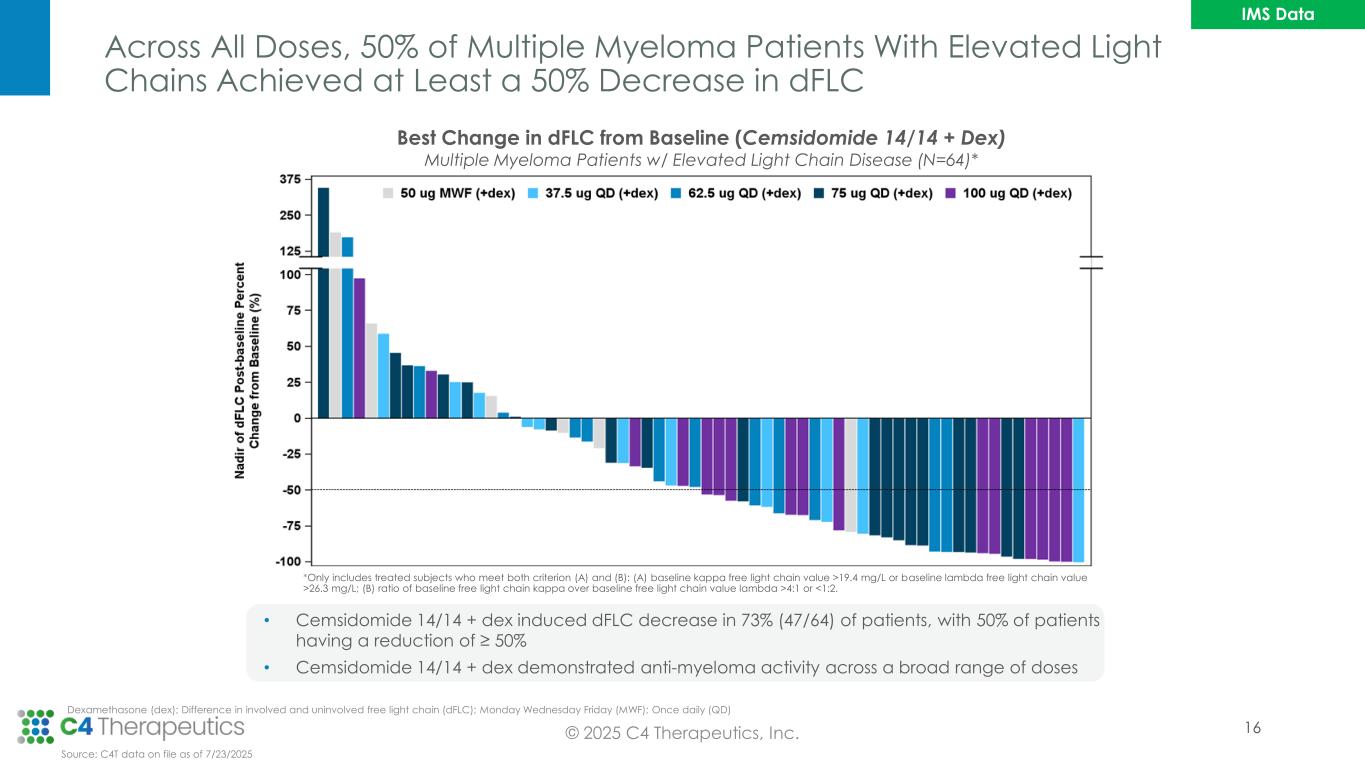
Across All Doses, 50% of Multiple Myeloma Patients With Elevated Light Chains Achieved at Least a 50% Decrease in dFLC © 2025 C4 Therapeutics, Inc. Dexamethasone (dex); Difference in involved and uninvolved free light chain (dFLC); Monday Wednesday Friday (MWF); Once daily (QD) *Only includes treated subjects who meet both criterion (A) and (B): (A) baseline kappa free light chain value >19.4 mg/L or baseline lambda free light chain value >26.3 mg/L; (B) ratio of baseline free light chain kappa over baseline free light chain value lambda >4:1 or <1:2. Best Change in dFLC from Baseline (Cemsidomide 14/14 + Dex) Multiple Myeloma Patients w/ Elevated Light Chain Disease (N=64)* • Cemsidomide 14/14 + dex induced dFLC decrease in 73% (47/64) of patients, with 50% of patients having a reduction of ≥ 50% • Cemsidomide 14/14 + dex demonstrated anti-myeloma activity across a broad range of doses Source: C4T data on file as of 7/23/2025 IMS Data 16
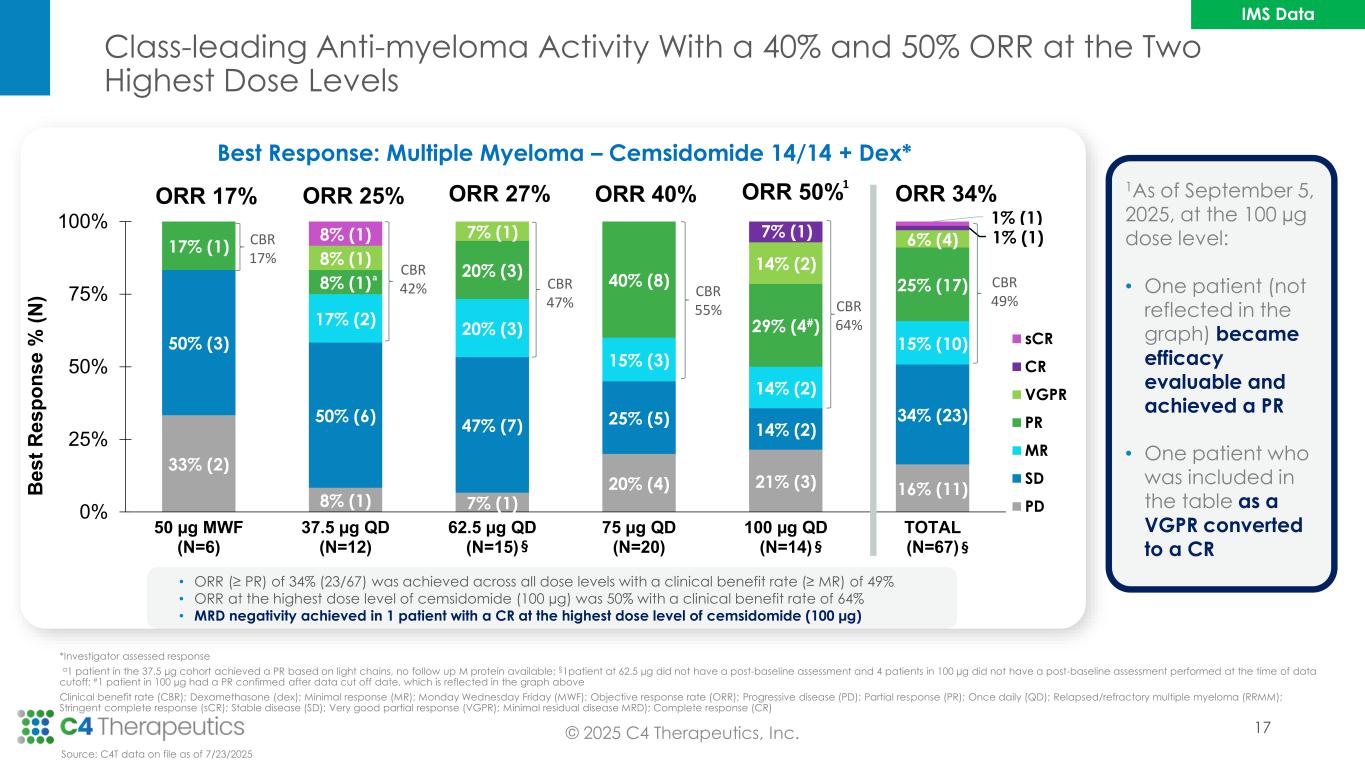
Class-leading Anti-myeloma Activity With a 40% and 50% ORR at the Two Highest Dose Levels 17 *Investigator assessed response a1 patient in the 37.5 µg cohort achieved a PR based on light chains, no follow up M protein available; §1patient at 62.5 µg did not have a post-baseline assessment and 4 patients in 100 µg did not have a post-baseline assessment performed at the time of data cutoff; #1 patient in 100 µg had a PR confirmed after data cut off date, which is reflected in the graph above Clinical benefit rate (CBR); Dexamethasone (dex); Minimal response (MR); Monday Wednesday Friday (MWF); Objective response rate (ORR); Progressive disease (PD); Partial response (PR); Once daily (QD); Relapsed/refractory multiple myeloma (RRMM); Stringent complete response (sCR); Stable disease (SD); Very good partial response (VGPR); Minimal residual disease MRD); Complete response (CR) Best Response: Multiple Myeloma – Cemsidomide 14/14 + Dex* 33% (2) 8% (1) 7% (1) 20% (4) 21% (3) 16% (11) 50% (3) 50% (6) 47% (7) 25% (5) 14% (2) 34% (23) 17% (2) 20% (3) 15% (3) 14% (2) 15% (10) 17% (1) 8% (1) 20% (3) 40% (8) 29% (4#) 25% (17) 8% (1) 7% (1) 14% (2) 6% (4) 7% (1) 1% (1)8% (1) 1% (1) 0% 25% 50% 75% 100% 50 µg MWF (N=6) 37.5 µg QD (N=12) 62.5 µg QD (N=15) 75 µg QD (N=20) 100 µg QD (N=14) TOTAL (N=67) B e s t R e s p o n s e % ( N ) sCR CR VGPR PR MR SD PD CBR 17% CBR 42% CBR 47% ORR 17% ORR 25% ORR 27% ORR 40% ORR 34% CBR 49% a CBR 55% ORR 50% CBR 64% §§ • ORR (≥ PR) of 34% (23/67) was achieved across all dose levels with a clinical benefit rate (≥ MR) of 49% • ORR at the highest dose level of cemsidomide (100 µg) was 50% with a clinical benefit rate of 64% • MRD negativity achieved in 1 patient with a CR at the highest dose level of cemsidomide (100 µg) Source: C4T data on file as of 7/23/2025 § 1 1As of September 5, 2025, at the 100 µg dose level: • One patient (not reflected in the graph) became efficacy evaluable and achieved a PR • One patient who was included in the table as a VGPR converted to a CR © 2025 C4 Therapeutics, Inc. IMS Data
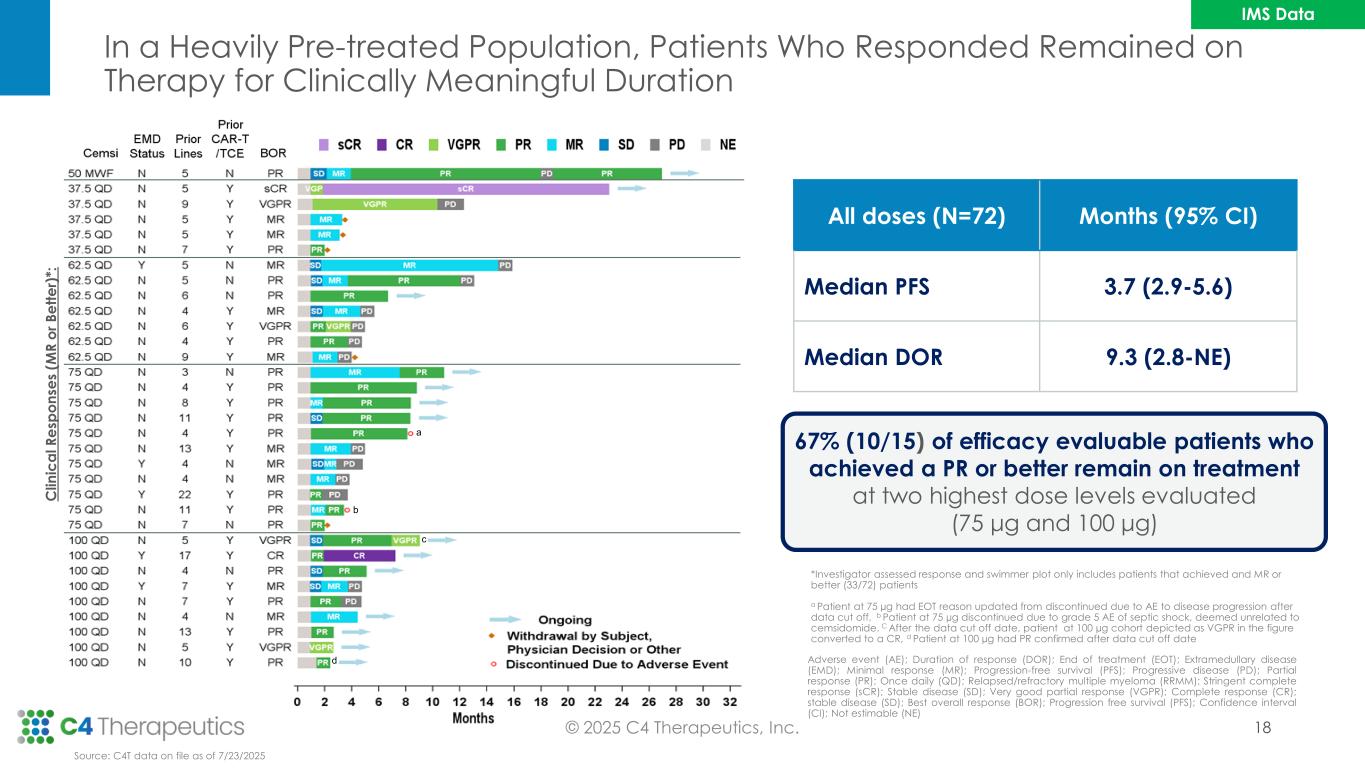
b Adverse event (AE); Duration of response (DOR); End of treatment (EOT); Extramedullary disease (EMD); Minimal response (MR); Progression-free survival (PFS); Progressive disease (PD); Partial response (PR); Once daily (QD); Relapsed/refractory multiple myeloma (RRMM); Stringent complete response (sCR); Stable disease (SD); Very good partial response (VGPR); Complete response (CR); stable disease (SD); Best overall response (BOR); Progression free survival (PFS); Confidence interval (CI); Not estimable (NE) *Investigator assessed response and swimmer plot only includes patients that achieved and MR or better (33/72) patients a Patient at 75 µg had EOT reason updated from discontinued due to AE to disease progression after data cut off, b Patient at 75 µg discontinued due to grade 5 AE of septic shock, deemed unrelated to cemsidomide. C After the data cut off date, patient at 100 µg cohort depicted as VGPR in the figure converted to a CR, d Patient at 100 µg had PR confirmed after data cut off date b a c In a Heavily Pre-treated Population, Patients Who Responded Remained on Therapy for Clinically Meaningful Duration © 2025 C4 Therapeutics, Inc. 67% (10/15) of efficacy evaluable patients who achieved a PR or better remain on treatment at two highest dose levels evaluated (75 μg and 100 μg) Source: C4T data on file as of 7/23/2025 All doses (N=72) Months (95% CI) Median PFS 3.7 (2.9-5.6) Median DOR 9.3 (2.8-NE) C li n ic a l R e sp o n se s (M R o r B e tt e r) *: d 18 IMS Data

Project Optimus Data 9/10/2025 Data Cutoff
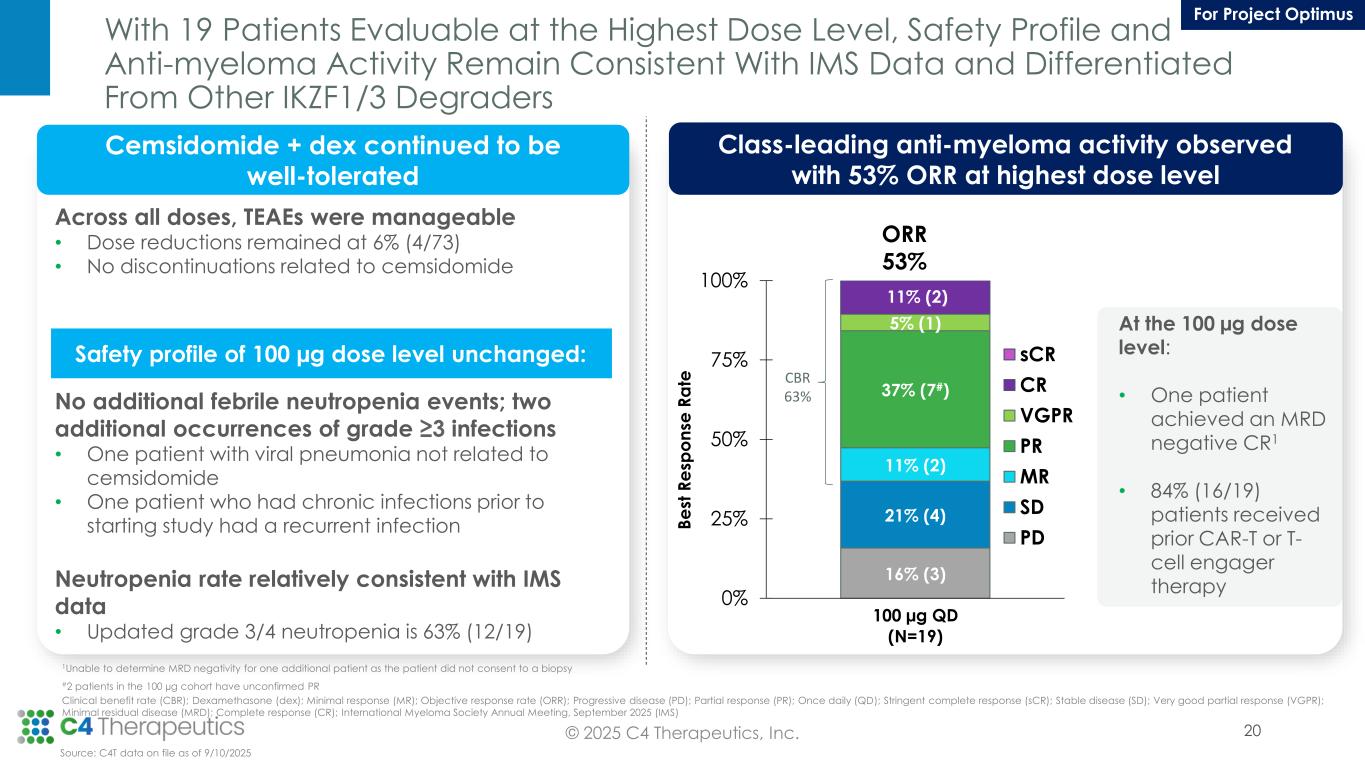
With 19 Patients Evaluable at the Highest Dose Level, Safety Profile and Anti-myeloma Activity Remain Consistent With IMS Data and Differentiated From Other IKZF1/3 Degraders 20© 2025 C4 Therapeutics, Inc. Cemsidomide + dex continued to be well-tolerated Across all doses, TEAEs were manageable • Dose reductions remained at 6% (4/73) • No discontinuations related to cemsidomide No additional febrile neutropenia events; two additional occurrences of grade ≥3 infections • One patient with viral pneumonia not related to cemsidomide • One patient who had chronic infections prior to starting study had a recurrent infection Neutropenia rate relatively consistent with IMS data • Updated grade 3/4 neutropenia is 63% (12/19) Class-leading anti-myeloma activity observed with 53% ORR at highest dose level 16% (3) 21% (4) 11% (2) 37% (7#) 5% (1) 11% (2) 0% 25% 50% 75% 100% 100 µg QD (N=19) sCR CR VGPR PR MR SD PD ORR 53% B e st R e sp o n se R a te At the 100 µg dose level: • One patient achieved an MRD negative CR1 • 84% (16/19) patients received prior CAR-T or T- cell engager therapy 1Unable to determine MRD negativity for one additional patient as the patient did not consent to a biopsy #2 patients in the 100 µg cohort have unconfirmed PR Clinical benefit rate (CBR); Dexamethasone (dex); Minimal response (MR); Objective response rate (ORR); Progressive disease (PD); Partial response (PR); Once daily (QD); Stringent complete response (sCR); Stable disease (SD); Very good partial response (VGPR); Minimal residual disease (MRD); Complete response (CR); International Myeloma Society Annual Meeting, September 2025 (IMS) Source: C4T data on file as of 9/10/2025 CBR 63% For Project Optimus Safety profile of 100 µg dose level unchanged:
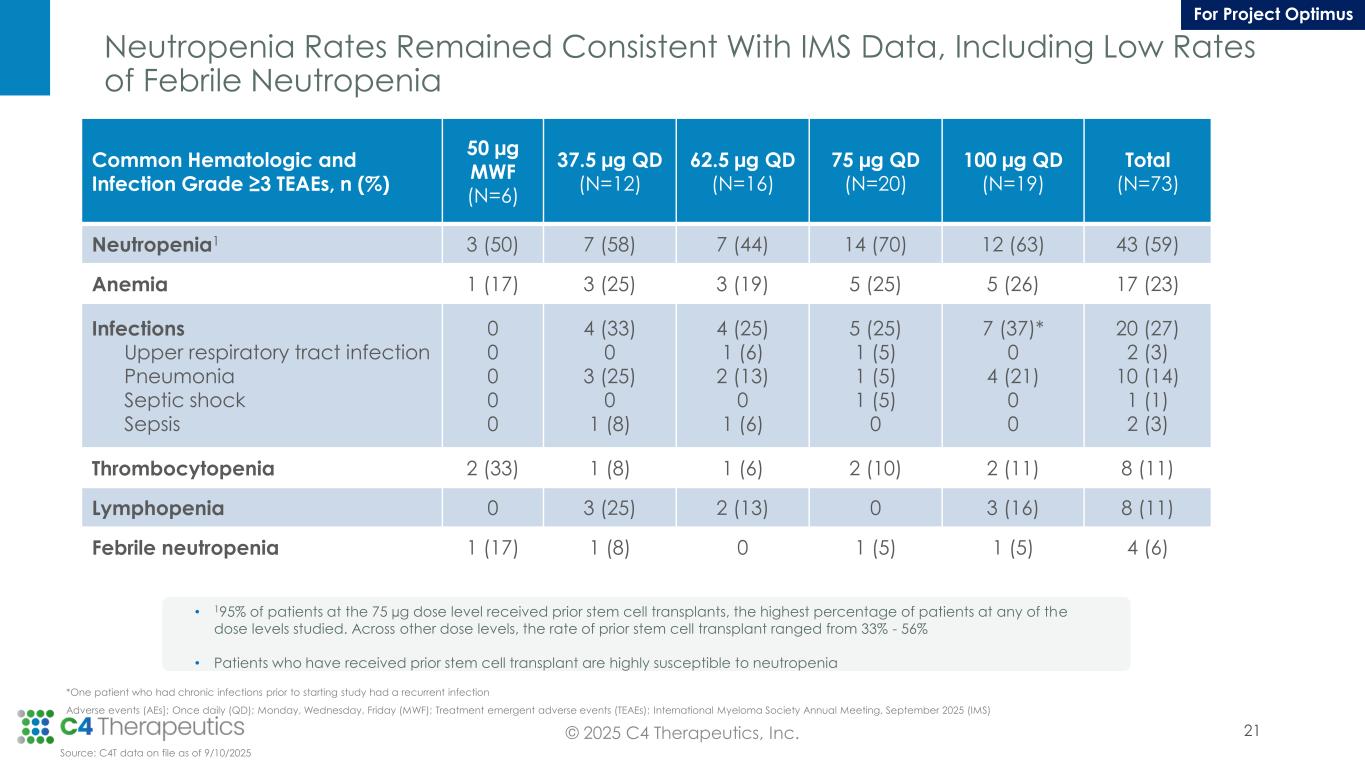
Neutropenia Rates Remained Consistent With IMS Data, Including Low Rates of Febrile Neutropenia *One patient who had chronic infections prior to starting study had a recurrent infection Adverse events (AEs]; Once daily (QD); Monday, Wednesday, Friday (MWF); Treatment emergent adverse events (TEAEs); International Myeloma Society Annual Meeting, September 2025 (IMS) Common Hematologic and Infection Grade ≥3 TEAEs, n (%) 50 µg MWF (N=6) 37.5 µg QD (N=12) 62.5 µg QD (N=16) 75 µg QD (N=20) 100 µg QD (N=19) Total (N=73) Neutropenia1 3 (50) 7 (58) 7 (44) 14 (70) 12 (63) 43 (59) Anemia 1 (17) 3 (25) 3 (19) 5 (25) 5 (26) 17 (23) Infections Upper respiratory tract infection Pneumonia Septic shock Sepsis 0 0 0 0 0 4 (33) 0 3 (25) 0 1 (8) 4 (25) 1 (6) 2 (13) 0 1 (6) 5 (25) 1 (5) 1 (5) 1 (5) 0 7 (37)* 0 4 (21) 0 0 20 (27) 2 (3) 10 (14) 1 (1) 2 (3) Thrombocytopenia 2 (33) 1 (8) 1 (6) 2 (10) 2 (11) 8 (11) Lymphopenia 0 3 (25) 2 (13) 0 3 (16) 8 (11) Febrile neutropenia 1 (17) 1 (8) 0 1 (5) 1 (5) 4 (6) • 195% of patients at the 75 µg dose level received prior stem cell transplants, the highest percentage of patients at any of the dose levels studied. Across other dose levels, the rate of prior stem cell transplant ranged from 33% - 56% • Patients who have received prior stem cell transplant are highly susceptible to neutropenia Source: C4T data on file as of 9/10/2025 21© 2025 C4 Therapeutics, Inc. For Project Optimus
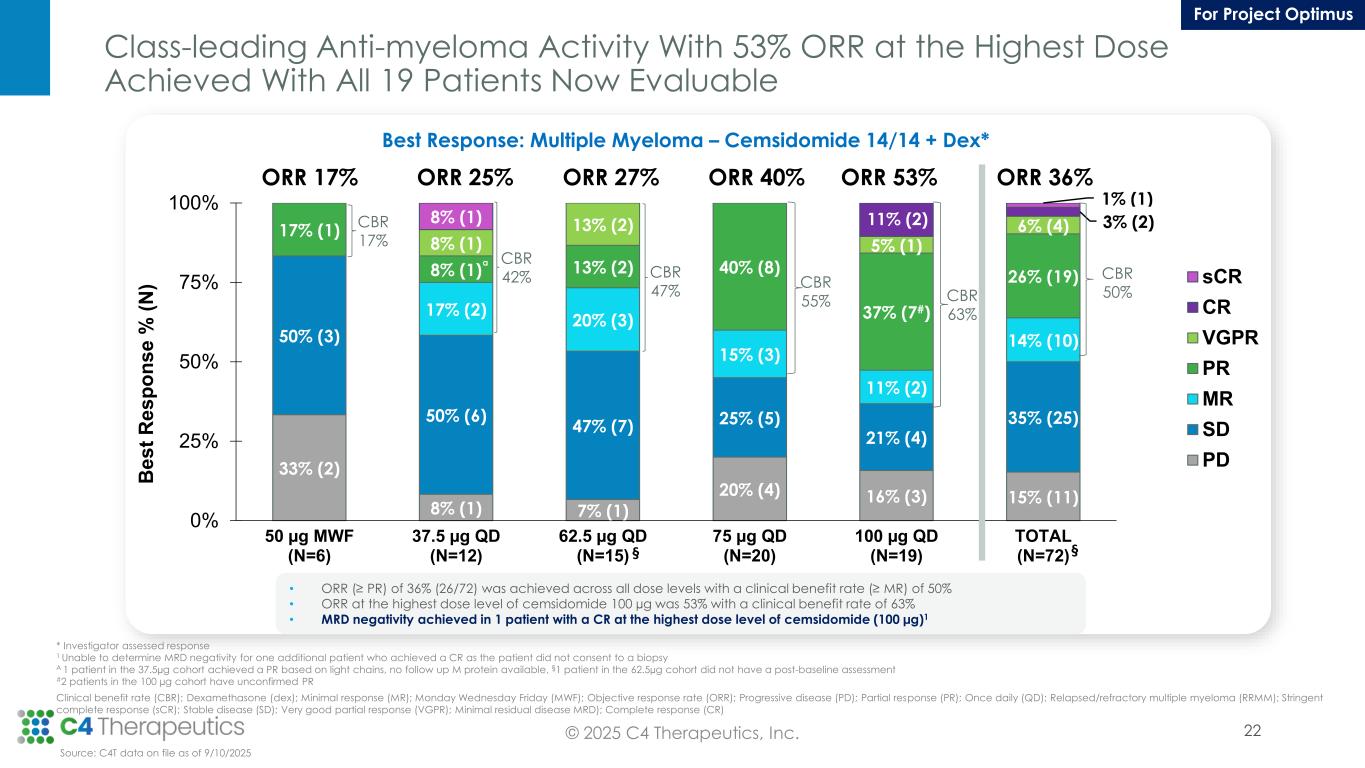
Class-leading Anti-myeloma Activity With 53% ORR at the Highest Dose Achieved With All 19 Patients Now Evaluable Best Response: Multiple Myeloma – Cemsidomide 14/14 + Dex* 33% (2) 8% (1) 7% (1) 20% (4) 16% (3) 15% (11) 50% (3) 50% (6) 47% (7) 25% (5) 21% (4) 35% (25) 17% (2) 20% (3) 15% (3) 11% (2) 14% (10) 17% (1) 8% (1) 13% (2) 40% (8) 37% (7#) 26% (19) 8% (1) 13% (2) 5% (1) 6% (4)11% (2) 3% (2)8% (1) 1% (1) 0% 25% 50% 75% 100% 50 µg MWF (N=6) 37.5 µg QD (N=12) 62.5 µg QD (N=15) 75 µg QD (N=20) 100 µg QD (N=19) TOTAL (N=72) B e s t R e s p o n s e % ( N ) sCR CR VGPR PR MR SD PD CBR 17% CBR 42% CBR 47% ORR 17% ORR 25% ORR 27% ORR 40% ORR 36% CBR 50% a CBR 55% ORR 53% CBR 63% §§ * Investigator assessed response 1 Unable to determine MRD negativity for one additional patient who achieved a CR as the patient did not consent to a biopsy A 1 patient in the 37.5µg cohort achieved a PR based on light chains, no follow up M protein available, §1 patient in the 62.5µg cohort did not have a post-baseline assessment #2 patients in the 100 µg cohort have unconfirmed PR Clinical benefit rate (CBR); Dexamethasone (dex); Minimal response (MR); Monday Wednesday Friday (MWF); Objective response rate (ORR); Progressive disease (PD); Partial response (PR); Once daily (QD); Relapsed/refractory multiple myeloma (RRMM); Stringent complete response (sCR); Stable disease (SD); Very good partial response (VGPR); Minimal residual disease MRD); Complete response (CR) Source: C4T data on file as of 9/10/2025 • ORR (≥ PR) of 36% (26/72) was achieved across all dose levels with a clinical benefit rate (≥ MR) of 50% • ORR at the highest dose level of cemsidomide 100 µg was 53% with a clinical benefit rate of 63% • MRD negativity achieved in 1 patient with a CR at the highest dose level of cemsidomide (100 µg)1 22© 2025 C4 Therapeutics, Inc. For Project Optimus

Clinical Development Plan
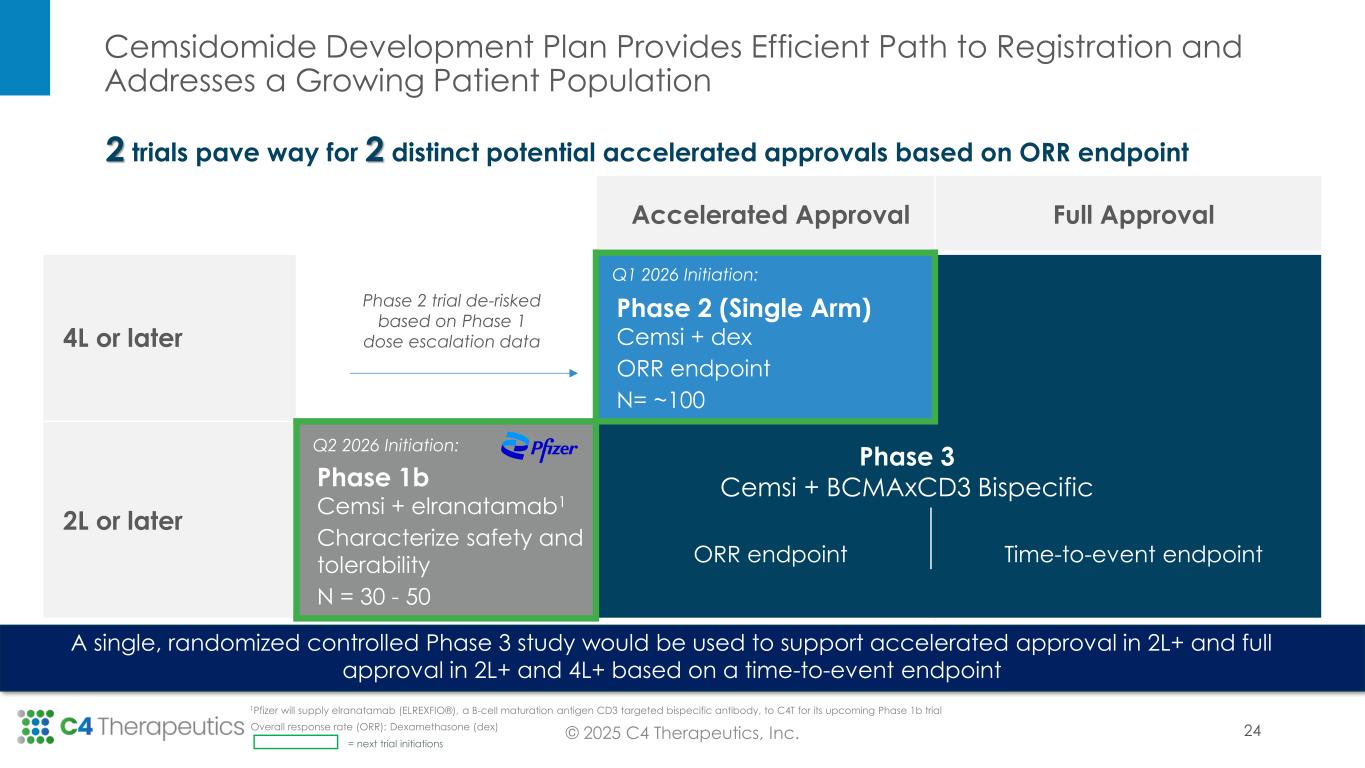
Cemsidomide Development Plan Provides Efficient Path to Registration and Addresses a Growing Patient Population © 2025 C4 Therapeutics, Inc. A single, randomized controlled Phase 3 study would be used to support accelerated approval in 2L+ and full approval in 2L+ and 4L+ based on a time-to-event endpoint Accelerated Approval Full Approval 4L or later Phase 2 (Single Arm) Cemsi + dex ORR endpoint N= ~100 Time-to-event endpoint 2L or later Phase 1b Cemsi + elranatamab1 Characterize safety and tolerability N = 30 - 50 ORR endpoint Phase 2 trial de-risked based on Phase 1 dose escalation data Phase 3 Cemsi + BCMAxCD3 Bispecific Q1 2026 Initiation: Q2 2026 Initiation: 2 trials pave way for 2 distinct potential accelerated approvals based on ORR endpoint = next trial initiations 24Overall response rate (ORR); Dexamethasone (dex) 1Pfizer will supply elranatamab (ELREXFIO®), a B-cell maturation antigen CD3 targeted bispecific antibody, to C4T for its upcoming Phase 1b trial
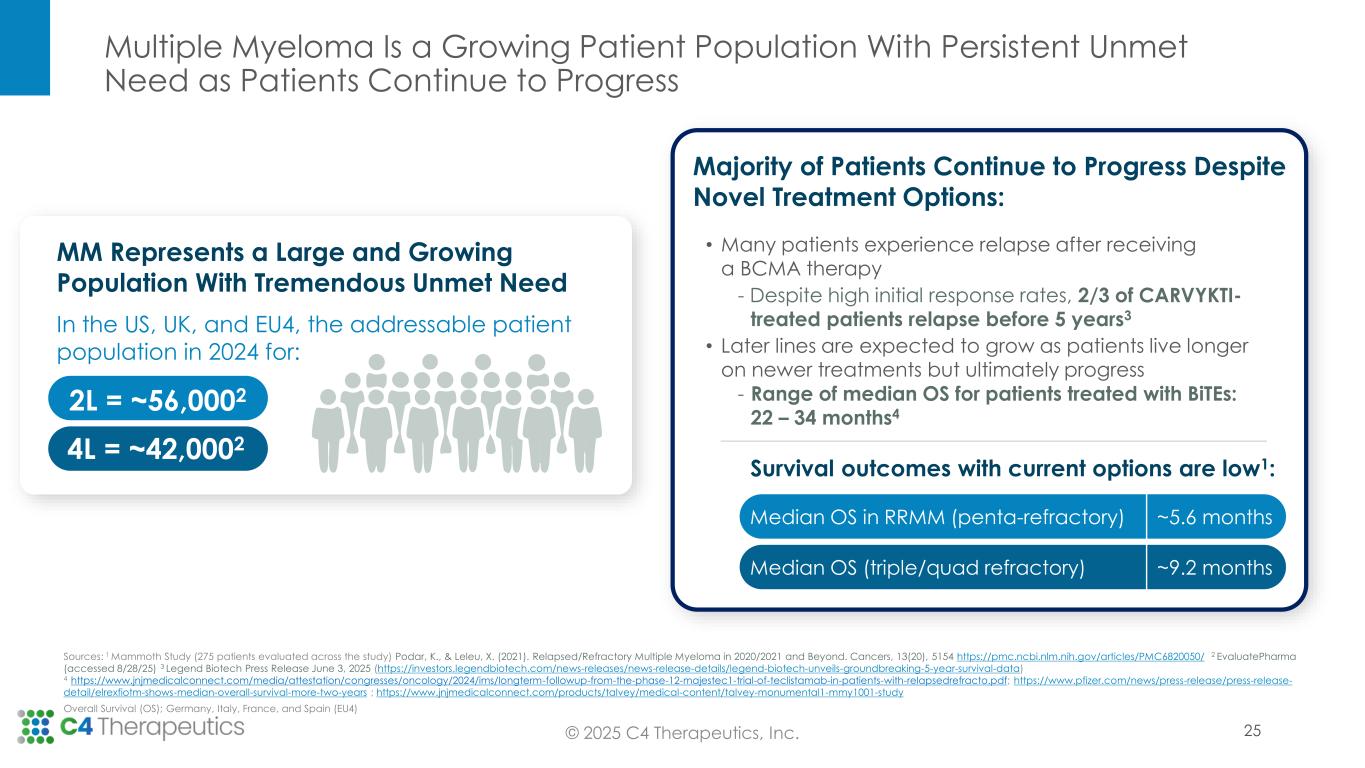
Multiple Myeloma Is a Growing Patient Population With Persistent Unmet Need as Patients Continue to Progress In the US, UK, and EU4, the addressable patient population in 2024 for: Majority of Patients Continue to Progress Despite Novel Treatment Options: MM Represents a Large and Growing Population With Tremendous Unmet Need • Many patients experience relapse after receiving a BCMA therapy - Despite high initial response rates, 2/3 of CARVYKTI- treated patients relapse before 5 years3 • Later lines are expected to grow as patients live longer on newer treatments but ultimately progress - Range of median OS for patients treated with BiTEs: 22 – 34 months4 Survival outcomes with current options are low1: 4L = ~42,0002 2L = ~56,0002 Sources: 1 Mammoth Study (275 patients evaluated across the study) Podar, K., & Leleu, X. (2021). Relapsed/Refractory Multiple Myeloma in 2020/2021 and Beyond. Cancers, 13(20), 5154 https://pmc.ncbi.nlm.nih.gov/articles/PMC6820050/ 2 EvaluatePharma (accessed 8/28/25) 3 Legend Biotech Press Release June 3, 2025 (https://investors.legendbiotech.com/news-releases/news-release-details/legend-biotech-unveils-groundbreaking-5-year-survival-data) 4 https://www.jnjmedicalconnect.com/media/attestation/congresses/oncology/2024/ims/longterm-followup-from-the-phase-12-majestec1-trial-of-teclistamab-in-patients-with-relapsedrefracto.pdf; https://www.pfizer.com/news/press-release/press-release- detail/elrexfiotm-shows-median-overall-survival-more-two-years ; https://www.jnjmedicalconnect.com/products/talvey/medical-content/talvey-monumental1-mmy1001-study Overall Survival (OS); Germany, Italy, France, and Spain (EU4) Median OS in RRMM (penta-refractory) ~5.6 months Median OS (triple/quad refractory) ~9.2 months 25© 2025 C4 Therapeutics, Inc.
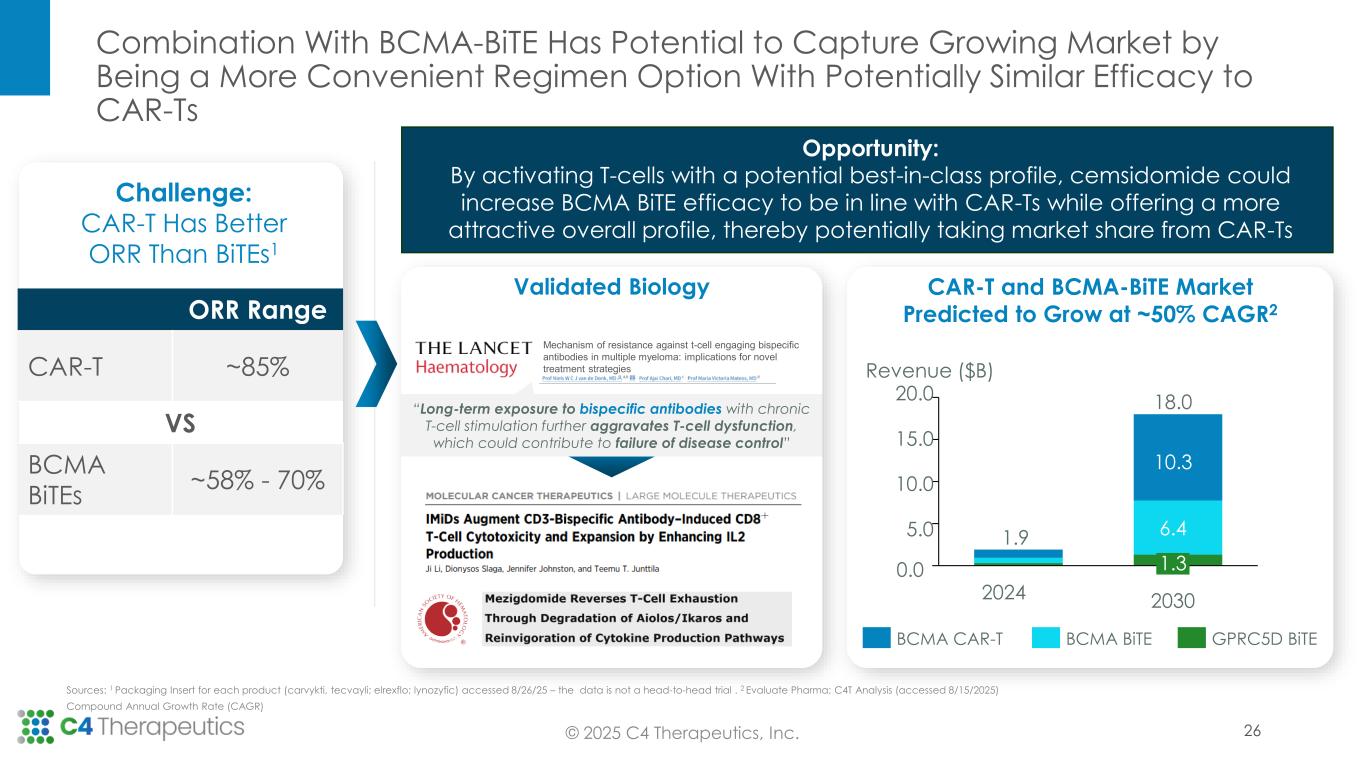
Opportunity: By activating T-cells with a potential best-in-class profile, cemsidomide could increase BCMA BiTE efficacy to be in line with CAR-Ts while offering a more attractive overall profile, thereby potentially taking market share from CAR-Ts 0.0 5.0 10.0 15.0 20.0 Revenue ($B) 2024 1.9 18.0 2030 10.3 6.4 1.3 BCMA CAR-T BCMA BiTE GPRC5D BiTE Sources: 1 Packaging Insert for each product (carvykti, tecvayli; elrexflo; lynozyfic) accessed 8/26/25 – the data is not a head-to-head trial . 2 Evaluate Pharma; C4T Analysis (accessed 8/15/2025) Compound Annual Growth Rate (CAGR) ORR Range CAR-T ~85% VS BCMA BiTEs ~58% - 70% Challenge: CAR-T Has Better ORR Than BiTEs1 Validated Biology Mechanism of resistance against t-cell engaging bispecific antibodies in multiple myeloma: implications for novel treatment strategies “Long-term exposure to bispecific antibodies with chronic T-cell stimulation further aggravates T-cell dysfunction, which could contribute to failure of disease control” CAR-T and BCMA-BiTE Market Predicted to Grow at ~50% CAGR2 Combination With BCMA-BiTE Has Potential to Capture Growing Market by Being a More Convenient Regimen Option With Potentially Similar Efficacy to CAR-Ts 26© 2025 C4 Therapeutics, Inc.
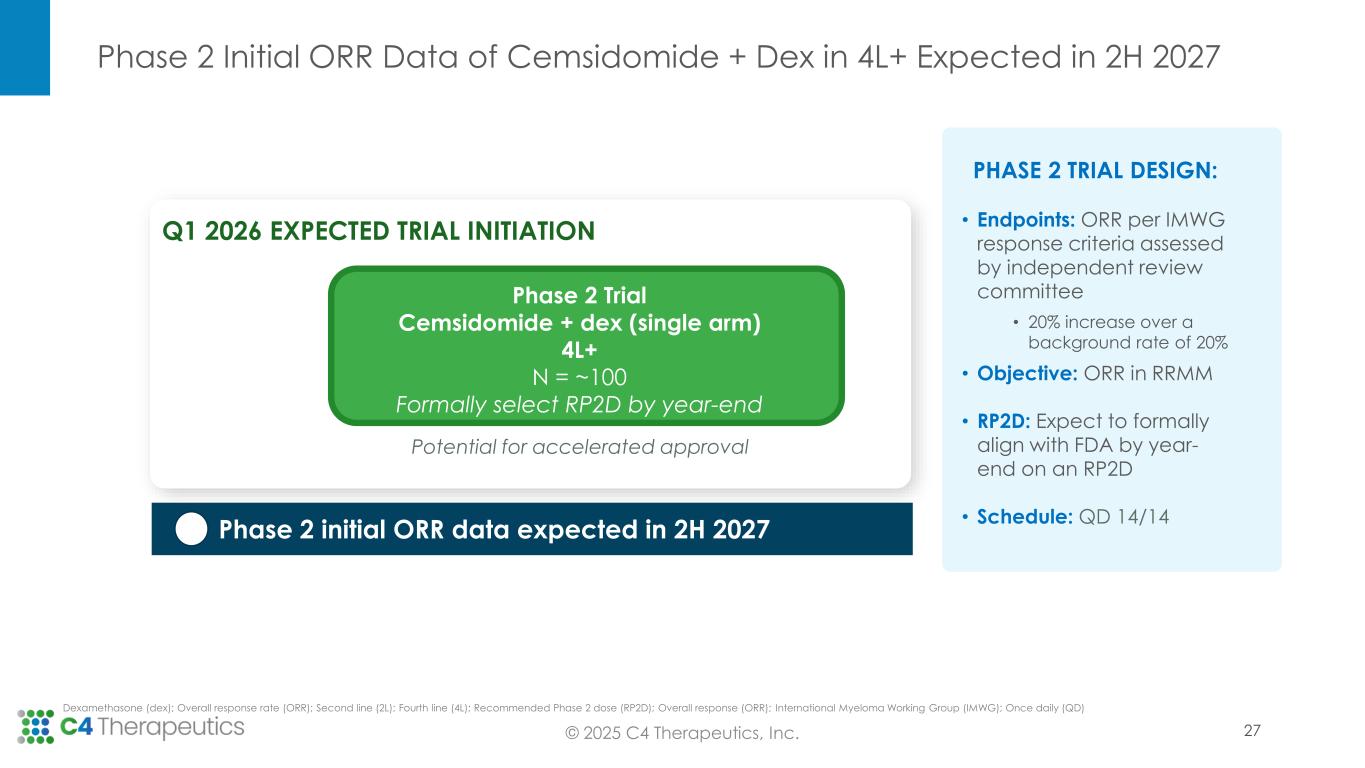
Phase 2 Initial ORR Data of Cemsidomide + Dex in 4L+ Expected in 2H 2027 © 2025 C4 Therapeutics, Inc. PHASE 2 TRIAL DESIGN: • Endpoints: ORR per IMWG response criteria assessed by independent review committee • 20% increase over a background rate of 20% • Objective: ORR in RRMM • RP2D: Expect to formally align with FDA by year- end on an RP2D • Schedule: QD 14/14 Dexamethasone (dex); Overall response rate (ORR); Second line (2L); Fourth line (4L); Recommended Phase 2 dose (RP2D); Overall response (ORR); International Myeloma Working Group (IMWG); Once daily (QD) Phase 2 initial ORR data expected in 2H 2027 Potential for accelerated approval Phase 2 Trial Cemsidomide + dex (single arm) 4L+ N = ~100 Formally select RP2D by year-end Q1 2026 EXPECTED TRIAL INITIATION 27
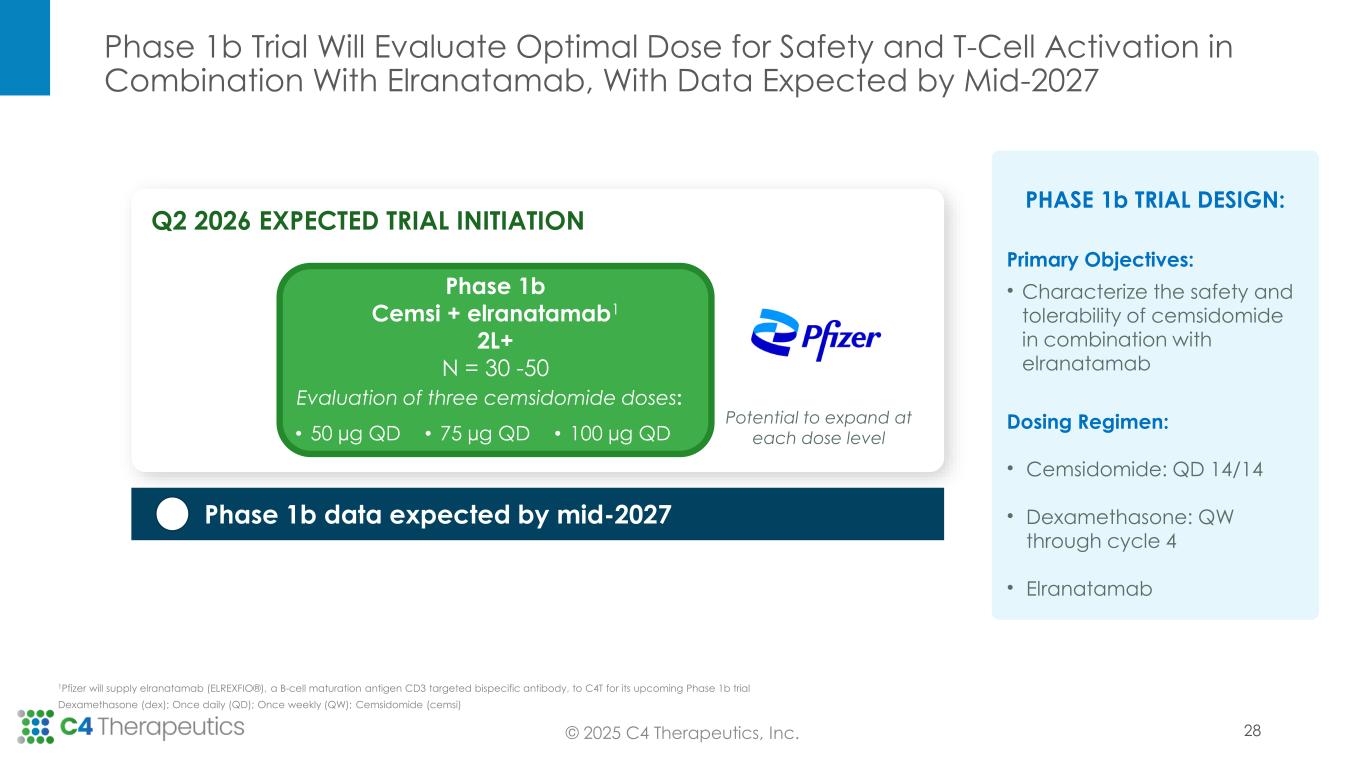
Phase 1b Trial Will Evaluate Optimal Dose for Safety and T-Cell Activation in Combination With Elranatamab, With Data Expected by Mid-2027 © 2025 C4 Therapeutics, Inc. PHASE 1b TRIAL DESIGN: Primary Objectives: • Characterize the safety and tolerability of cemsidomide in combination with elranatamab Dosing Regimen: • Cemsidomide: QD 14/14 • Dexamethasone: QW through cycle 4 • Elranatamab Dexamethasone (dex); Once daily (QD); Once weekly (QW); Cemsidomide (cemsi) Phase 1b data expected by mid-2027 Phase 1b Cemsi + elranatamab1 2L+ N = 30 -50 Evaluation of three cemsidomide doses: • 50 µg QD • 75 µg QD • 100 µg QD Potential to expand at each dose level Q2 2026 EXPECTED TRIAL INITIATION 28 1Pfizer will supply elranatamab (ELREXFIO®), a B-cell maturation antigen CD3 targeted bispecific antibody, to C4T for its upcoming Phase 1b trial

Discovery Portfolio
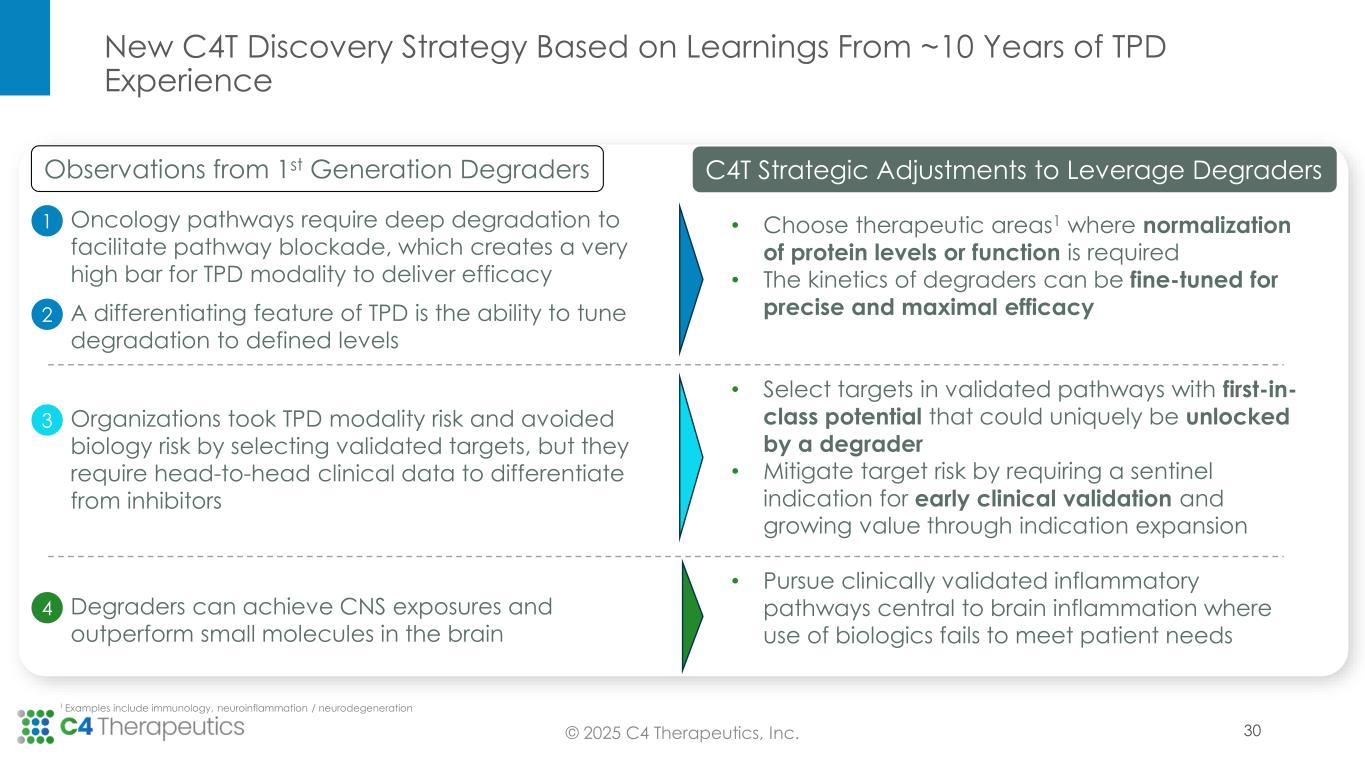
New C4T Discovery Strategy Based on Learnings From ~10 Years of TPD Experience 1 Examples include immunology, neuroinflammation / neurodegeneration • Choose therapeutic areas1 where normalization of protein levels or function is required • The kinetics of degraders can be fine-tuned for precise and maximal efficacy 3 Organizations took TPD modality risk and avoided biology risk by selecting validated targets, but they require head-to-head clinical data to differentiate from inhibitors 4 Degraders can achieve CNS exposures and outperform small molecules in the brain Oncology pathways require deep degradation to facilitate pathway blockade, which creates a very high bar for TPD modality to deliver efficacy 1 2 A differentiating feature of TPD is the ability to tune degradation to defined levels • Select targets in validated pathways with first-in- class potential that could uniquely be unlocked by a degrader • Mitigate target risk by requiring a sentinel indication for early clinical validation and growing value through indication expansion • Pursue clinically validated inflammatory pathways central to brain inflammation where use of biologics fails to meet patient needs Observations from 1st Generation Degraders C4T Strategic Adjustments to Leverage Degraders 30© 2025 C4 Therapeutics, Inc.
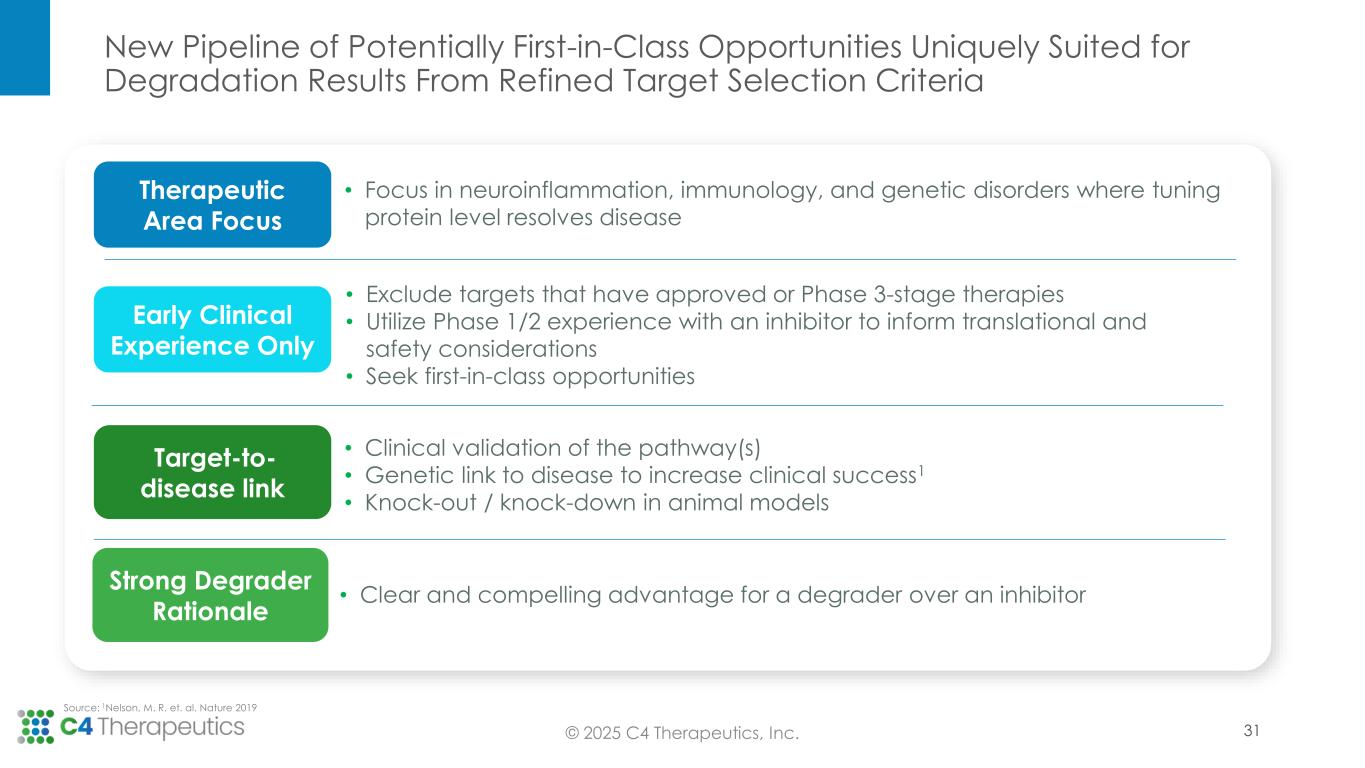
New Pipeline of Potentially First-in-Class Opportunities Uniquely Suited for Degradation Results From Refined Target Selection Criteria • Exclude targets that have approved or Phase 3-stage therapies • Utilize Phase 1/2 experience with an inhibitor to inform translational and safety considerations • Seek first-in-class opportunities Early Clinical Experience Only • Clinical validation of the pathway(s) • Genetic link to disease to increase clinical success1 • Knock-out / knock-down in animal models Target-to- disease link • Clear and compelling advantage for a degrader over an inhibitor Strong Degrader Rationale • Focus in neuroinflammation, immunology, and genetic disorders where tuning protein level resolves disease Therapeutic Area Focus Source: 1Nelson, M. R. et. al. Nature 2019 © 2025 C4 Therapeutics, Inc. 31
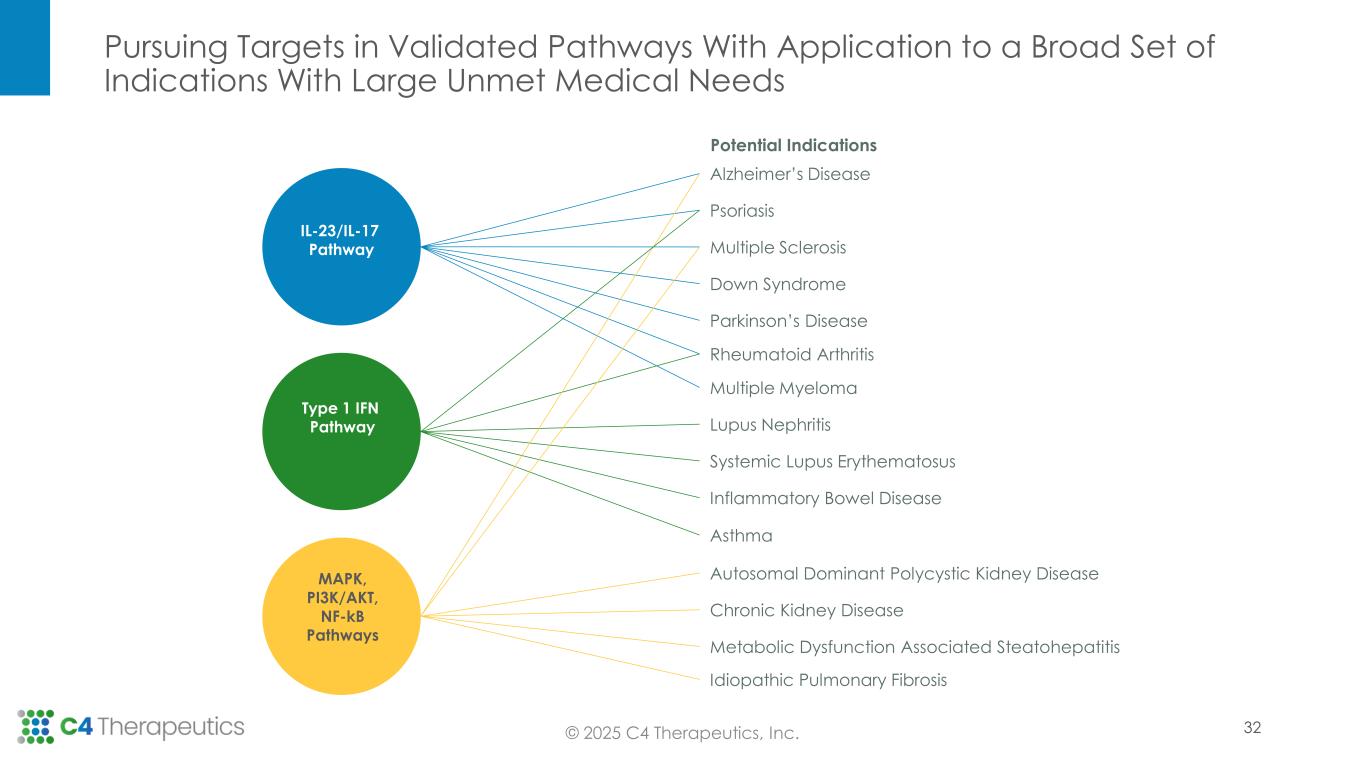
Pursuing Targets in Validated Pathways With Application to a Broad Set of Indications With Large Unmet Medical Needs © 2025 C4 Therapeutics, Inc. Alzheimer’s Disease Psoriasis Multiple Sclerosis Down Syndrome Parkinson’s Disease Rheumatoid Arthritis Multiple Myeloma Lupus Nephritis Systemic Lupus Erythematosus Inflammatory Bowel Disease Asthma Autosomal Dominant Polycystic Kidney Disease Chronic Kidney Disease Metabolic Dysfunction Associated Steatohepatitis Idiopathic Pulmonary Fibrosis IL-23/IL-17 Pathway Type 1 IFN Pathway MAPK, PI3K/AKT, NF-kB Pathways Potential Indications 32
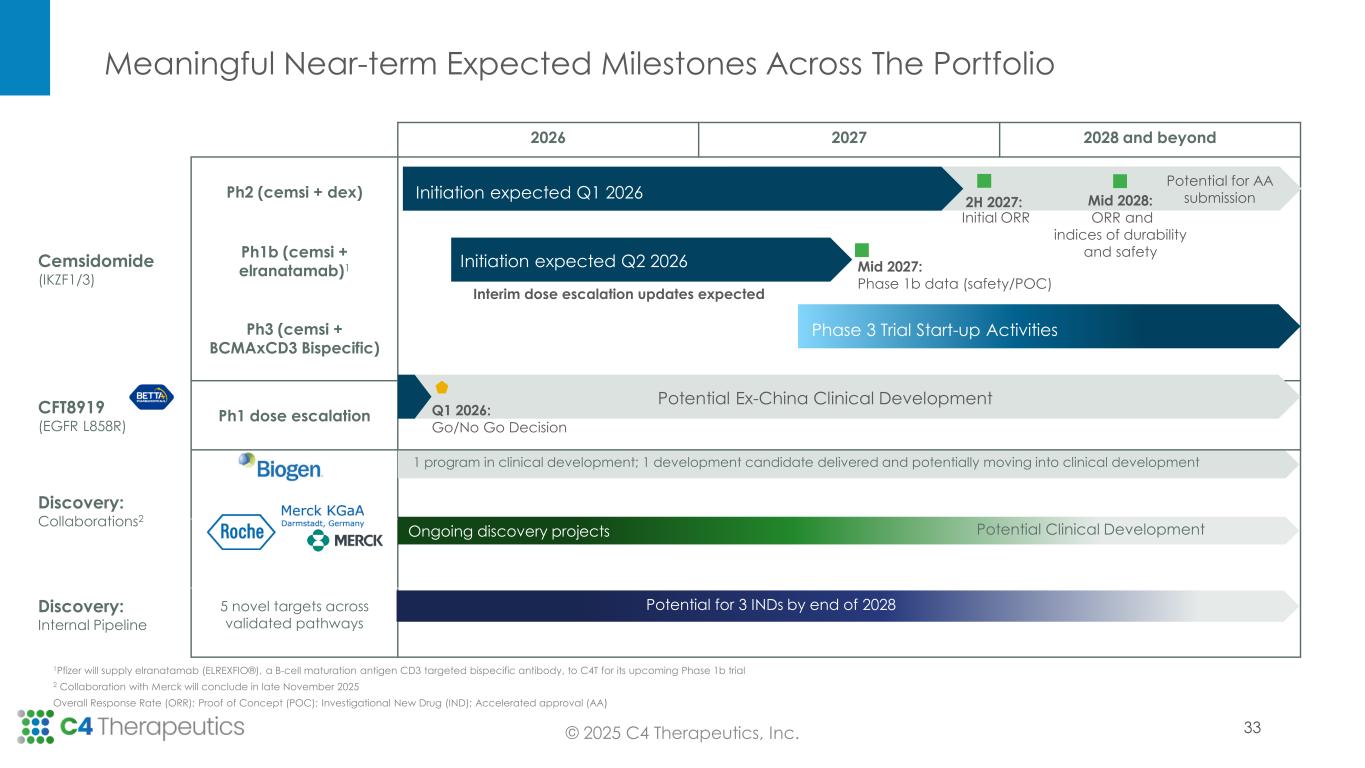
Meaningful Near-term Expected Milestones Across The Portfolio 2026 2027 2028 and beyond Cemsidomide (IKZF1/3) Ph2 (cemsi + dex) Ph1b (cemsi + elranatamab)1 Ph3 (cemsi + BCMAxCD3 Bispecific) CFT8919 (EGFR L858R) Ph1 dose escalation Discovery: Collaborations2 Discovery: Internal Pipeline 5 novel targets across validated pathways Phase 3 Trial Start-up Activities Initiation expected Q1 2026 Initiation expected Q2 2026 Mid 2027: Phase 1b data (safety/POC) Mid 2028: ORR and indices of durability and safety 2H 2027: Initial ORR Potential for AA submission Q1 2026: Go/No Go Decision Potential Ex-China Clinical Development © 2025 C4 Therapeutics, Inc. Interim dose escalation updates expected 1 program in clinical development; 1 development candidate delivered and potentially moving into clinical development Ongoing discovery projects Potential Clinical Development Potential for 3 INDs by end of 2028 1Pfizer will supply elranatamab (ELREXFIO®), a B-cell maturation antigen CD3 targeted bispecific antibody, to C4T for its upcoming Phase 1b trial 2 Collaboration with Merck will conclude in late November 2025 Overall Response Rate (ORR); Proof of Concept (POC); Investigational New Drug (IND); Accelerated approval (AA) 33