
August 2025 Corporate Presentation

Corporate Presentation Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives, expected results and timing of its preclinical studies and clinical trials for KER-065, including its regulatory plans; the potential of Keros’ proprietary discovery approach; and Keros’ ability to drive long-term growth. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its product candidates, KER-065 and elritercept; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; and Keros’ dependence on third parties in connection with manufacturing, clinical trials and preclinical studies. These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 6, 2025, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Disclaimer 2
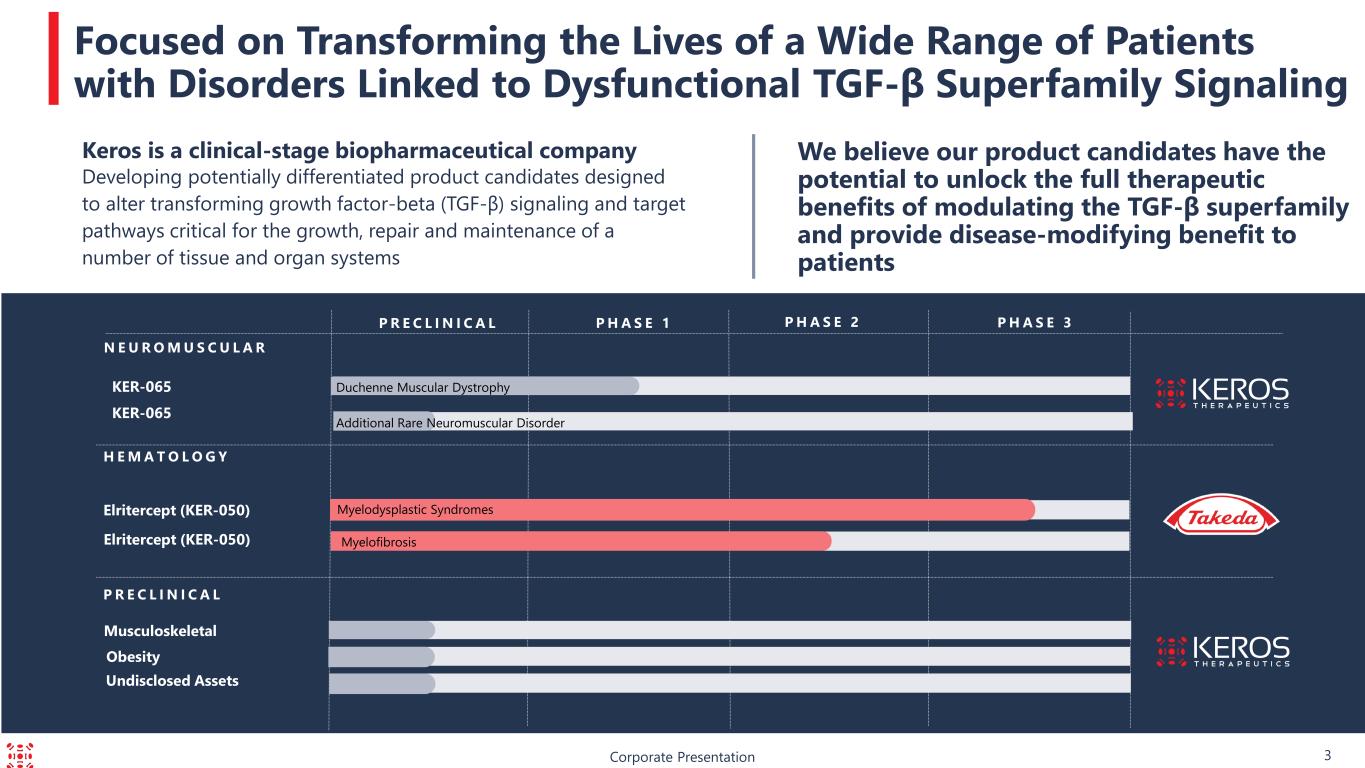
KER-065 P R E C L I N I C A L Corporate Presentation Focused on Transforming the Lives of a Wide Range of Patients with Disorders Linked to Dysfunctional TGF-β Superfamily Signaling We believe our product candidates have the potential to unlock the full therapeutic benefits of modulating the TGF-β superfamily and provide disease-modifying benefit to patients P H A S E 3P R E C L I N I C A L P H A S E 1 P H A S E 2 Elritercept (KER-050) N E U R O M U S C U L A R Elritercept (KER-050) H E M A T O L O G Y Musculoskeletal Keros is a clinical-stage biopharmaceutical company Developing potentially differentiated product candidates designed to alter transforming growth factor-beta (TGF-β) signaling and target pathways critical for the growth, repair and maintenance of a number of tissue and organ systems Undisclosed Assets Obesity 3 Myelodysplastic Syndromes Myelofibrosis KER-065 Duchenne Muscular Dystrophy Additional Rare Neuromuscular Disorder

Corporate Presentation KER-065: Neuromuscular Diseases 4

Corporate Presentation 5 KER-065 KER-065 is an investigational modified activin receptor IIA (ActRIIA) and activin receptor IIB (ActRIIB) ligand trap • ~50% amino acids derived from each activin receptor KER-065 is designed to bind and inhibit activin A and myostatin to: • Improve muscle regeneration to increase muscle size and strength • Inhibit and reverse fibrosis • Inhibit inflammation • Reduce fat • Improve bone health through bone anabolic mechanisms KER-065 is designed differently from other activin receptor ligand traps: • Reduced binding to bone morphogenic proteins (BMPs) to avoid the vascular/bleeding events observed with ActRIIb-Fc derived from the native sequences
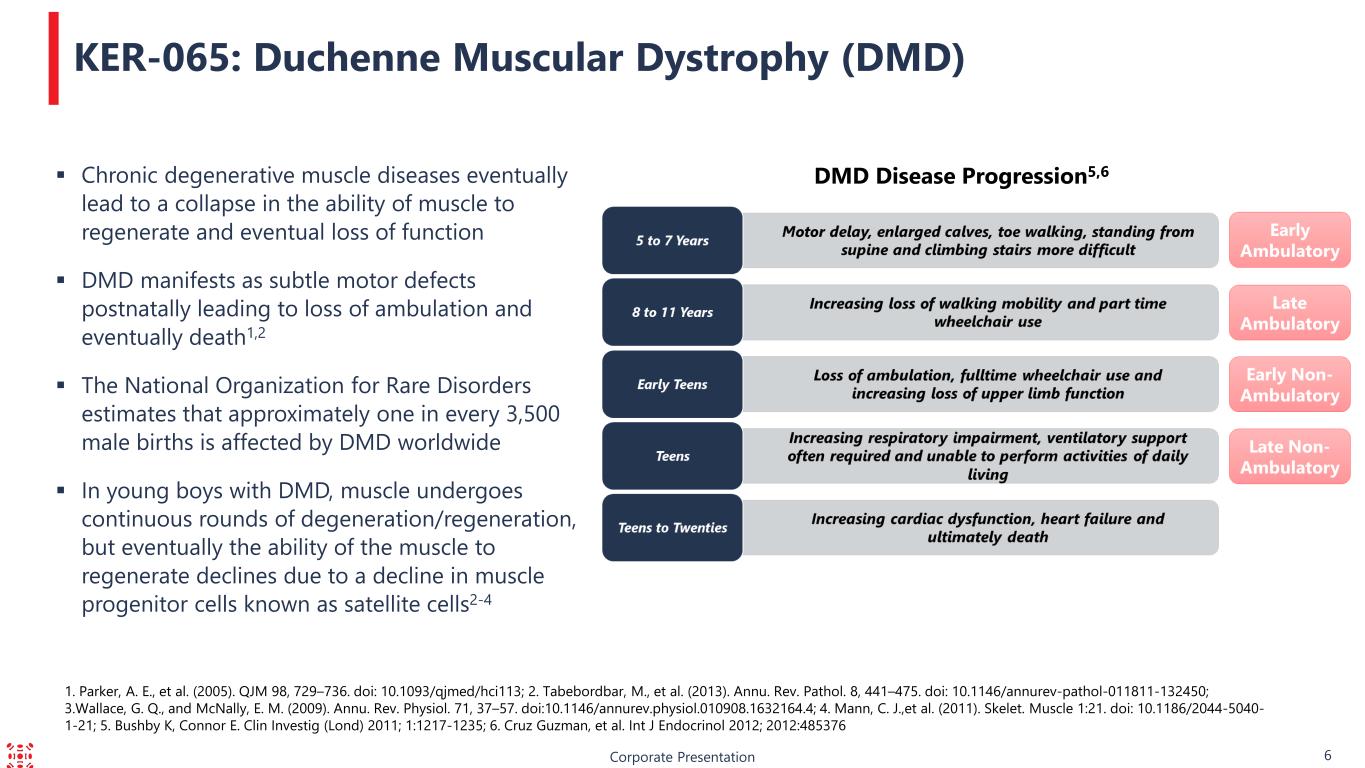
1. Parker, A. E., et al. (2005). QJM 98, 729–736. doi: 10.1093/qjmed/hci113; 2. Tabebordbar, M., et al. (2013). Annu. Rev. Pathol. 8, 441–475. doi: 10.1146/annurev-pathol-011811-132450; 3.Wallace, G. Q., and McNally, E. M. (2009). Annu. Rev. Physiol. 71, 37–57. doi:10.1146/annurev.physiol.010908.1632164.4; 4. Mann, C. J.,et al. (2011). Skelet. Muscle 1:21. doi: 10.1186/2044-5040- 1-21; 5. Bushby K, Connor E. Clin Investig (Lond) 2011; 1:1217-1235; 6. Cruz Guzman, et al. Int J Endocrinol 2012; 2012:485376 Corporate Presentation 6 Chronic degenerative muscle diseases eventually lead to a collapse in the ability of muscle to regenerate and eventual loss of function DMD manifests as subtle motor defects postnatally leading to loss of ambulation and eventually death1,2 The National Organization for Rare Disorders estimates that approximately one in every 3,500 male births is affected by DMD worldwide In young boys with DMD, muscle undergoes continuous rounds of degeneration/regeneration, but eventually the ability of the muscle to regenerate declines due to a decline in muscle progenitor cells known as satellite cells2-4 KER-065: Duchenne Muscular Dystrophy (DMD) DMD Disease Progression5,6
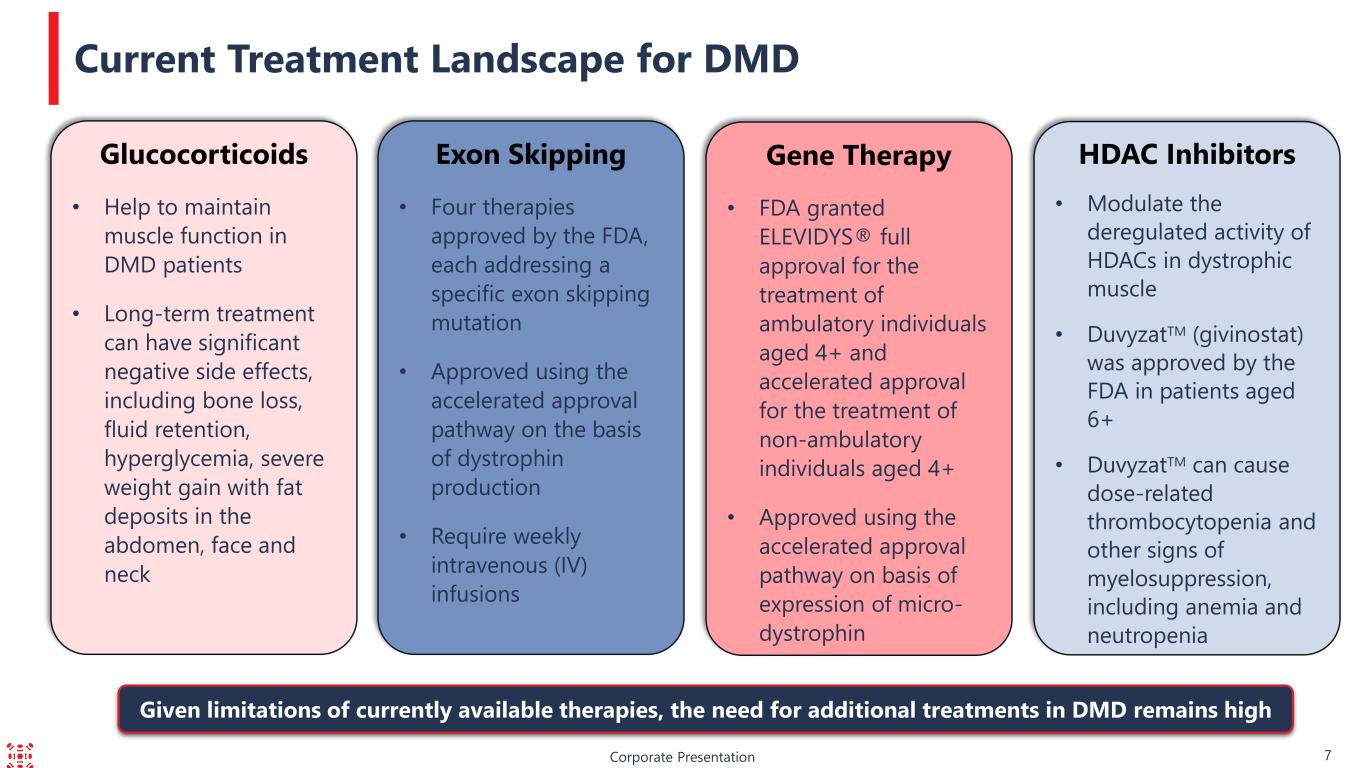
HDAC Inhibitors • Modulate the deregulated activity of HDACs in dystrophic muscle • DuvyzatTM (givinostat) was approved by the FDA in patients aged 6+ • DuvyzatTM can cause dose-related thrombocytopenia and other signs of myelosuppression, including anemia and neutropenia Corporate Presentation 7 Current Treatment Landscape for DMD Gene Therapy • FDA granted ELEVIDYS® full approval for the treatment of ambulatory individuals aged 4+ and accelerated approval for the treatment of non-ambulatory individuals aged 4+ • Approved using the accelerated approval pathway on basis of expression of micro- dystrophin Exon Skipping • Four therapies approved by the FDA, each addressing a specific exon skipping mutation • Approved using the accelerated approval pathway on the basis of dystrophin production • Require weekly intravenous (IV) infusions Glucocorticoids • Help to maintain muscle function in DMD patients • Long-term treatment can have significant negative side effects, including bone loss, fluid retention, hyperglycemia, severe weight gain with fat deposits in the abdomen, face and neck Given limitations of currently available therapies, the need for additional treatments in DMD remains high
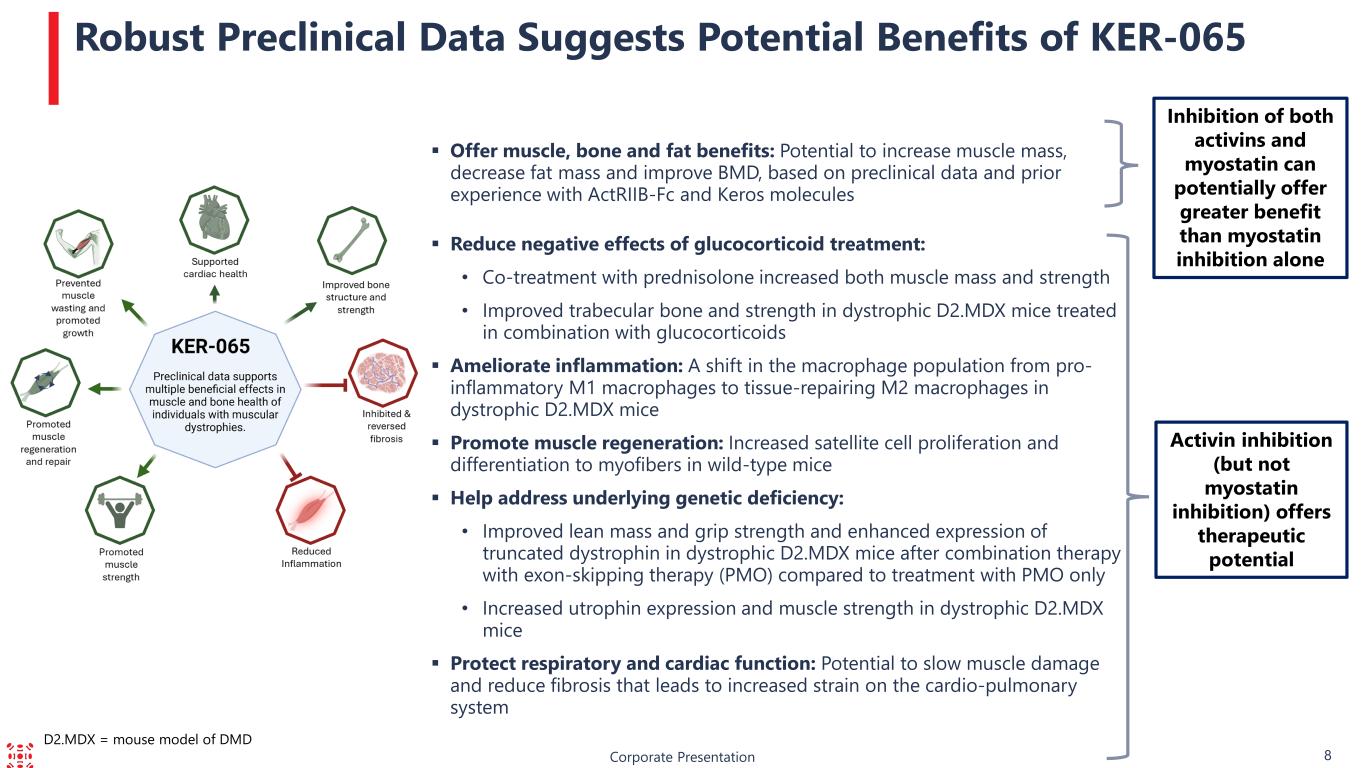
Corporate Presentation 8 Robust Preclinical Data Suggests Potential Benefits of KER-065 Activin inhibition (but not myostatin inhibition) offers therapeutic potential Offer muscle, bone and fat benefits: Potential to increase muscle mass, decrease fat mass and improve BMD, based on preclinical data and prior experience with ActRIIB-Fc and Keros molecules Reduce negative effects of glucocorticoid treatment: • Co-treatment with prednisolone increased both muscle mass and strength • Improved trabecular bone and strength in dystrophic D2.MDX mice treated in combination with glucocorticoids Ameliorate inflammation: A shift in the macrophage population from pro- inflammatory M1 macrophages to tissue-repairing M2 macrophages in dystrophic D2.MDX mice Promote muscle regeneration: Increased satellite cell proliferation and differentiation to myofibers in wild-type mice Help address underlying genetic deficiency: • Improved lean mass and grip strength and enhanced expression of truncated dystrophin in dystrophic D2.MDX mice after combination therapy with exon-skipping therapy (PMO) compared to treatment with PMO only • Increased utrophin expression and muscle strength in dystrophic D2.MDX mice Protect respiratory and cardiac function: Potential to slow muscle damage and reduce fibrosis that leads to increased strain on the cardio-pulmonary system Inhibition of both activins and myostatin can potentially offer greater benefit than myostatin inhibition alone D2.MDX = mouse model of DMD
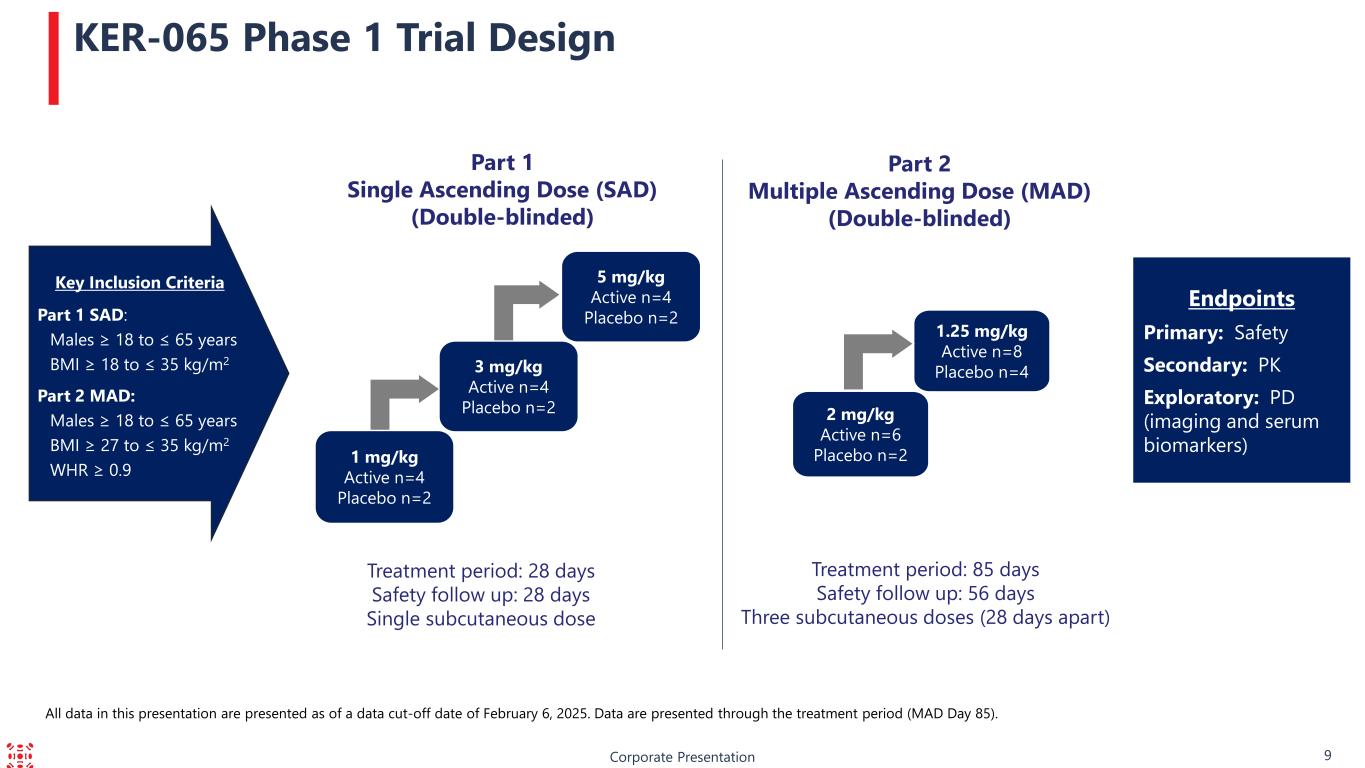
Corporate Presentation 9 KER-065 Phase 1 Trial Design Part 1 Single Ascending Dose (SAD) (Double-blinded) Treatment period: 28 days Safety follow up: 28 days Single subcutaneous dose Part 2 Multiple Ascending Dose (MAD) (Double-blinded) Treatment period: 85 days Safety follow up: 56 days Three subcutaneous doses (28 days apart) Endpoints Primary: Safety Secondary: PK Exploratory: PD (imaging and serum biomarkers)1 mg/kg Active n=4 Placebo n=2 5 mg/kg Active n=4 Placebo n=2 3 mg/kg Active n=4 Placebo n=2 2 mg/kg Active n=6 Placebo n=2 1.25 mg/kg Active n=8 Placebo n=4 Key Inclusion Criteria Part 1 SAD: Males ≥ 18 to ≤ 65 years BMI ≥ 18 to ≤ 35 kg/m2 Part 2 MAD: Males ≥ 18 to ≤ 65 years BMI ≥ 27 to ≤ 35 kg/m2 WHR ≥ 0.9 All data in this presentation are presented as of a data cut-off date of February 6, 2025. Data are presented through the treatment period (MAD Day 85).
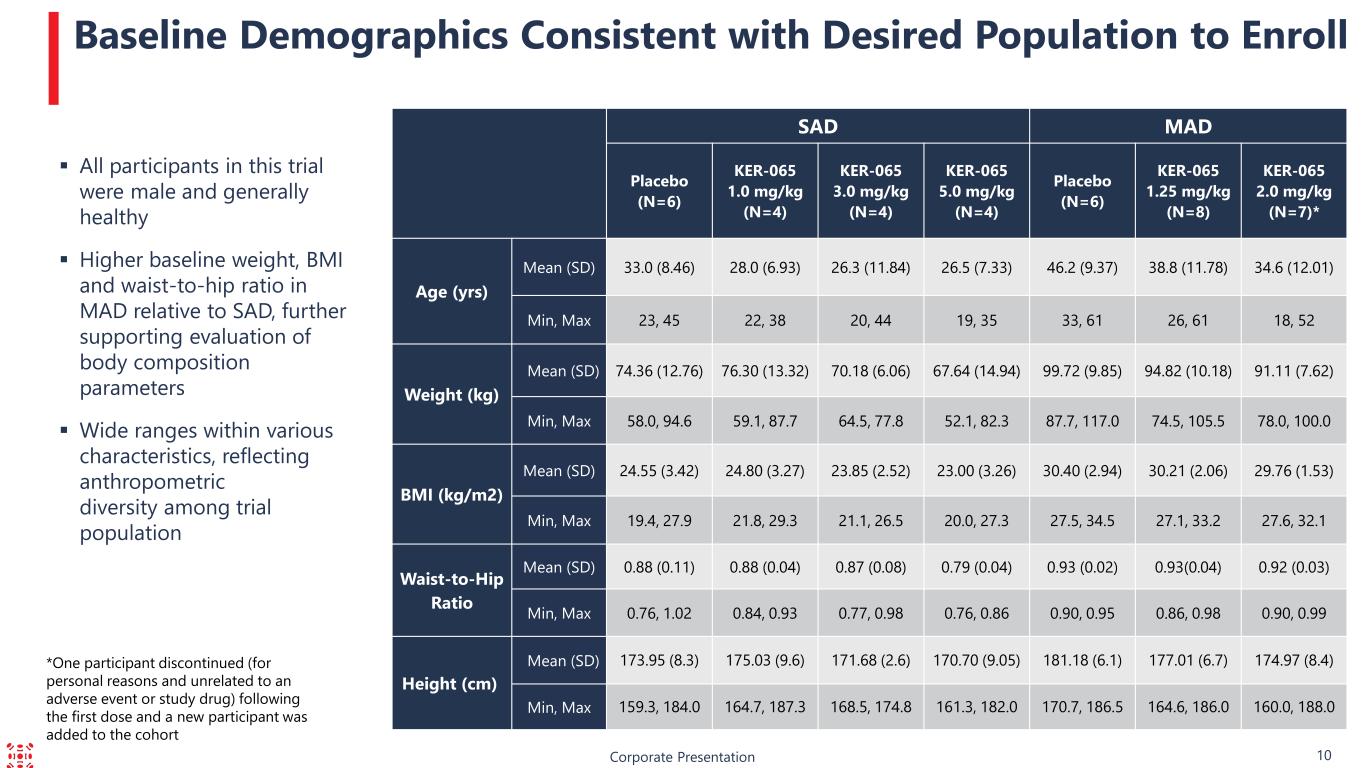
Corporate Presentation 10 Baseline Demographics Consistent with Desired Population to Enroll All participants in this trial were male and generally healthy Higher baseline weight, BMI and waist-to-hip ratio in MAD relative to SAD, further supporting evaluation of body composition parameters Wide ranges within various characteristics, reflecting anthropometric diversity among trial population SAD MAD Placebo (N=6) KER-065 1.0 mg/kg (N=4) KER-065 3.0 mg/kg (N=4) KER-065 5.0 mg/kg (N=4) Placebo (N=6) KER-065 1.25 mg/kg (N=8) KER-065 2.0 mg/kg (N=7)* Age (yrs) Mean (SD) 33.0 (8.46) 28.0 (6.93) 26.3 (11.84) 26.5 (7.33) 46.2 (9.37) 38.8 (11.78) 34.6 (12.01) Min, Max 23, 45 22, 38 20, 44 19, 35 33, 61 26, 61 18, 52 Weight (kg) Mean (SD) 74.36 (12.76) 76.30 (13.32) 70.18 (6.06) 67.64 (14.94) 99.72 (9.85) 94.82 (10.18) 91.11 (7.62) Min, Max 58.0, 94.6 59.1, 87.7 64.5, 77.8 52.1, 82.3 87.7, 117.0 74.5, 105.5 78.0, 100.0 BMI (kg/m2) Mean (SD) 24.55 (3.42) 24.80 (3.27) 23.85 (2.52) 23.00 (3.26) 30.40 (2.94) 30.21 (2.06) 29.76 (1.53) Min, Max 19.4, 27.9 21.8, 29.3 21.1, 26.5 20.0, 27.3 27.5, 34.5 27.1, 33.2 27.6, 32.1 Waist-to-Hip Ratio Mean (SD) 0.88 (0.11) 0.88 (0.04) 0.87 (0.08) 0.79 (0.04) 0.93 (0.02) 0.93(0.04) 0.92 (0.03) Min, Max 0.76, 1.02 0.84, 0.93 0.77, 0.98 0.76, 0.86 0.90, 0.95 0.86, 0.98 0.90, 0.99 Height (cm) Mean (SD) 173.95 (8.3) 175.03 (9.6) 171.68 (2.6) 170.70 (9.05) 181.18 (6.1) 177.01 (6.7) 174.97 (8.4) Min, Max 159.3, 184.0 164.7, 187.3 168.5, 174.8 161.3, 182.0 170.7, 186.5 164.6, 186.0 160.0, 188.0 *One participant discontinued (for personal reasons and unrelated to an adverse event or study drug) following the first dose and a new participant was added to the cohort
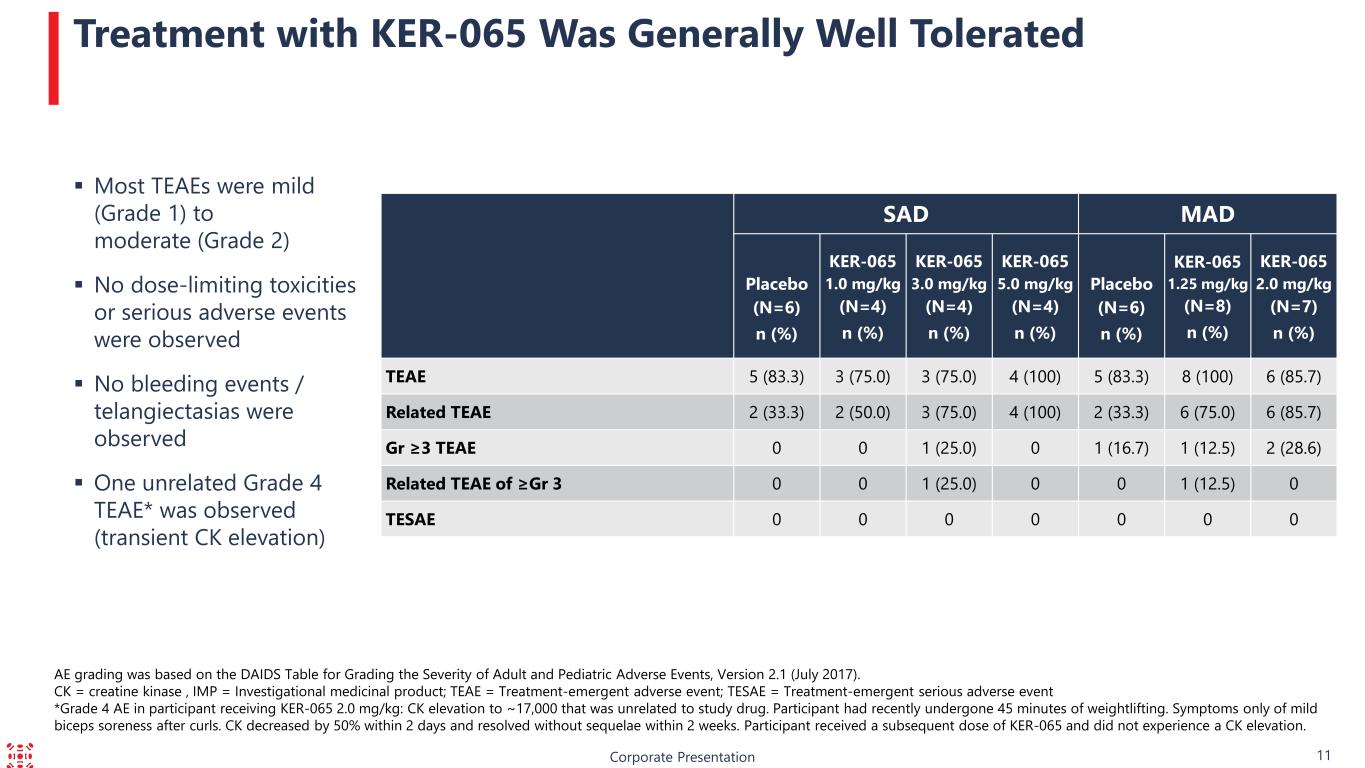
Corporate Presentation 11 Treatment with KER-065 Was Generally Well Tolerated Most TEAEs were mild (Grade 1) to moderate (Grade 2) No dose-limiting toxicities or serious adverse events were observed No bleeding events / telangiectasias were observed One unrelated Grade 4 TEAE* was observed (transient CK elevation) AE grading was based on the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.1 (July 2017). CK = creatine kinase , IMP = Investigational medicinal product; TEAE = Treatment-emergent adverse event; TESAE = Treatment-emergent serious adverse event *Grade 4 AE in participant receiving KER-065 2.0 mg/kg: CK elevation to ~17,000 that was unrelated to study drug. Participant had recently undergone 45 minutes of weightlifting. Symptoms only of mild biceps soreness after curls. CK decreased by 50% within 2 days and resolved without sequelae within 2 weeks. Participant received a subsequent dose of KER-065 and did not experience a CK elevation. SAD MAD Placebo (N=6) n (%) KER-065 1.0 mg/kg (N=4) n (%) KER-065 3.0 mg/kg (N=4) n (%) KER-065 5.0 mg/kg (N=4) n (%) Placebo (N=6) n (%) KER-065 1.25 mg/kg (N=8) n (%) KER-065 2.0 mg/kg (N=7) n (%) TEAE 5 (83.3) 3 (75.0) 3 (75.0) 4 (100) 5 (83.3) 8 (100) 6 (85.7) Related TEAE 2 (33.3) 2 (50.0) 3 (75.0) 4 (100) 2 (33.3) 6 (75.0) 6 (85.7) Gr ≥3 TEAE 0 0 1 (25.0) 0 1 (16.7) 1 (12.5) 2 (28.6) Related TEAE of ≥Gr 3 0 0 1 (25.0) 0 0 1 (12.5) 0 TESAE 0 0 0 0 0 0 0
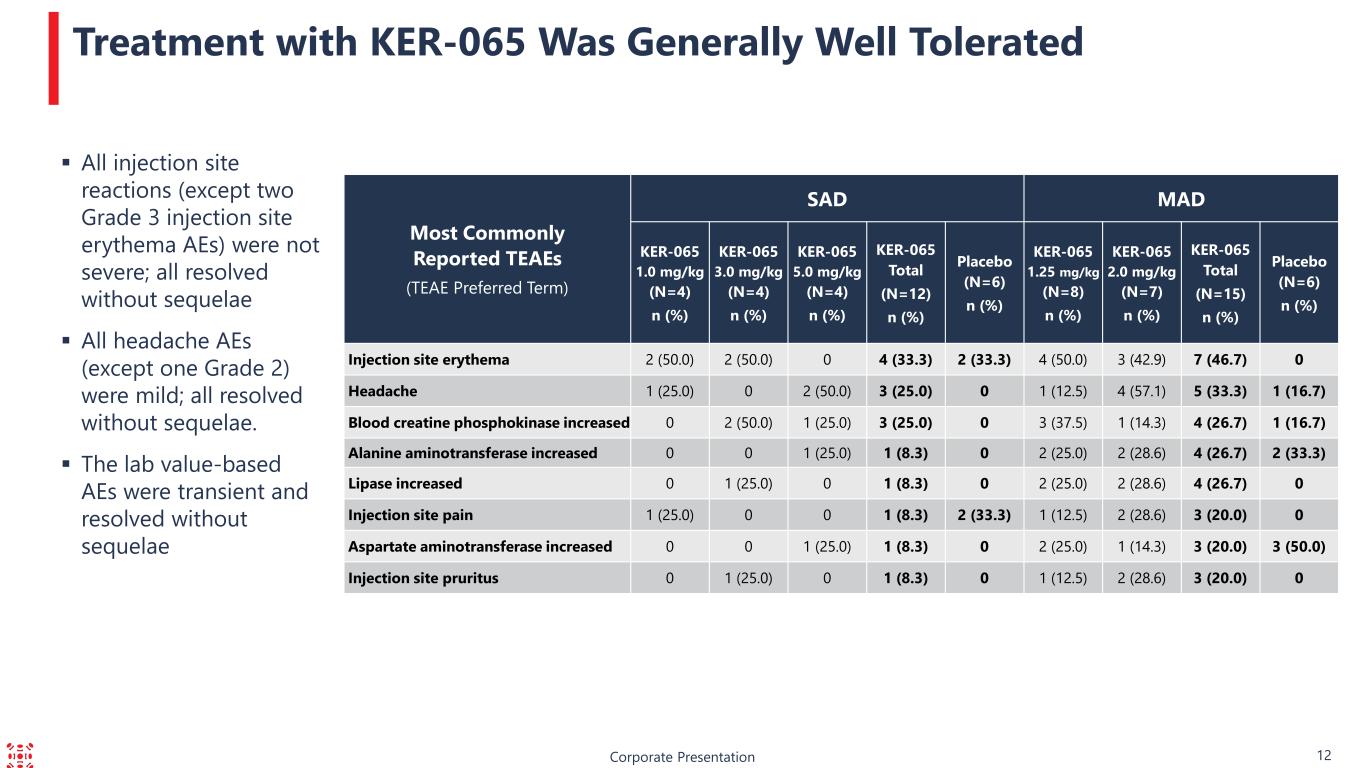
Corporate Presentation 12 Treatment with KER-065 Was Generally Well Tolerated Most Commonly Reported TEAEs (TEAE Preferred Term) SAD MAD KER-065 1.0 mg/kg (N=4) n (%) KER-065 3.0 mg/kg (N=4) n (%) KER-065 5.0 mg/kg (N=4) n (%) KER-065 Total (N=12) n (%) Placebo (N=6) n (%) KER-065 1.25 mg/kg (N=8) n (%) KER-065 2.0 mg/kg (N=7) n (%) KER-065 Total (N=15) n (%) Placebo (N=6) n (%) Injection site erythema 2 (50.0) 2 (50.0) 0 4 (33.3) 2 (33.3) 4 (50.0) 3 (42.9) 7 (46.7) 0 Headache 1 (25.0) 0 2 (50.0) 3 (25.0) 0 1 (12.5) 4 (57.1) 5 (33.3) 1 (16.7) Blood creatine phosphokinase increased 0 2 (50.0) 1 (25.0) 3 (25.0) 0 3 (37.5) 1 (14.3) 4 (26.7) 1 (16.7) Alanine aminotransferase increased 0 0 1 (25.0) 1 (8.3) 0 2 (25.0) 2 (28.6) 4 (26.7) 2 (33.3) Lipase increased 0 1 (25.0) 0 1 (8.3) 0 2 (25.0) 2 (28.6) 4 (26.7) 0 Injection site pain 1 (25.0) 0 0 1 (8.3) 2 (33.3) 1 (12.5) 2 (28.6) 3 (20.0) 0 Aspartate aminotransferase increased 0 0 1 (25.0) 1 (8.3) 0 2 (25.0) 1 (14.3) 3 (20.0) 3 (50.0) Injection site pruritus 0 1 (25.0) 0 1 (8.3) 0 1 (12.5) 2 (28.6) 3 (20.0) 0 All injection site reactions (except two Grade 3 injection site erythema AEs) were not severe; all resolved without sequelae All headache AEs (except one Grade 2) were mild; all resolved without sequelae. The lab value-based AEs were transient and resolved without sequelae
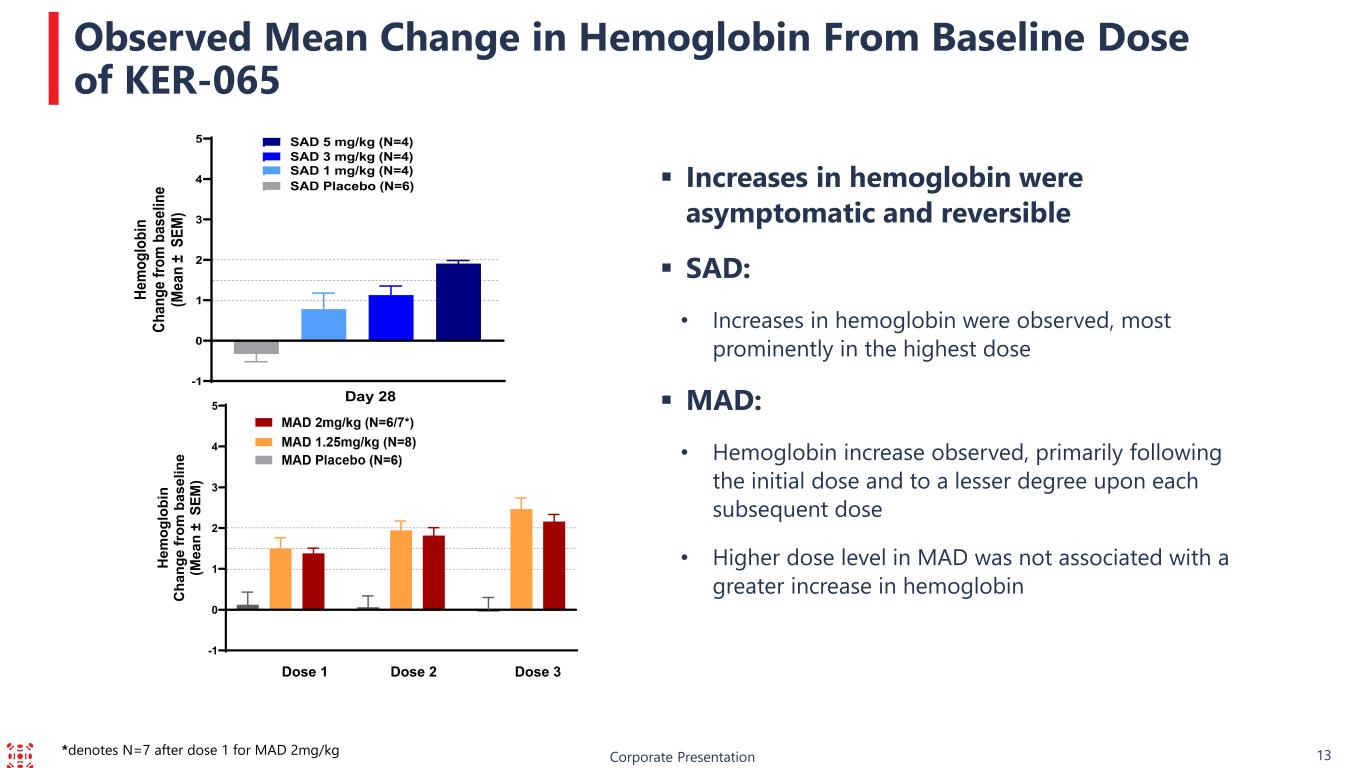
Corporate Presentation 13 Observed Mean Change in Hemoglobin From Baseline Dose of KER-065 Increases in hemoglobin were asymptomatic and reversible SAD: • Increases in hemoglobin were observed, most prominently in the highest dose MAD: • Hemoglobin increase observed, primarily following the initial dose and to a lesser degree upon each subsequent dose • Higher dose level in MAD was not associated with a greater increase in hemoglobin -1 0 1 2 3 4 5 H em og lo bi n C ha ng e fr om b as el in e (M ea n ± S EM ) Dose 1 Dose 2 Dose 3 -1 0 1 2 3 4 5 He m og lo bi n Ch an ge fr om b as el in e (M ea n ± S EM ) SAD Placebo (N=6) SAD 1 mg/kg (N=4) SAD 3 mg/kg (N=4) Day 28 SAD 5 mg/kg (N=4) *denotes N=7 after dose 1 for MAD 2mg/kg
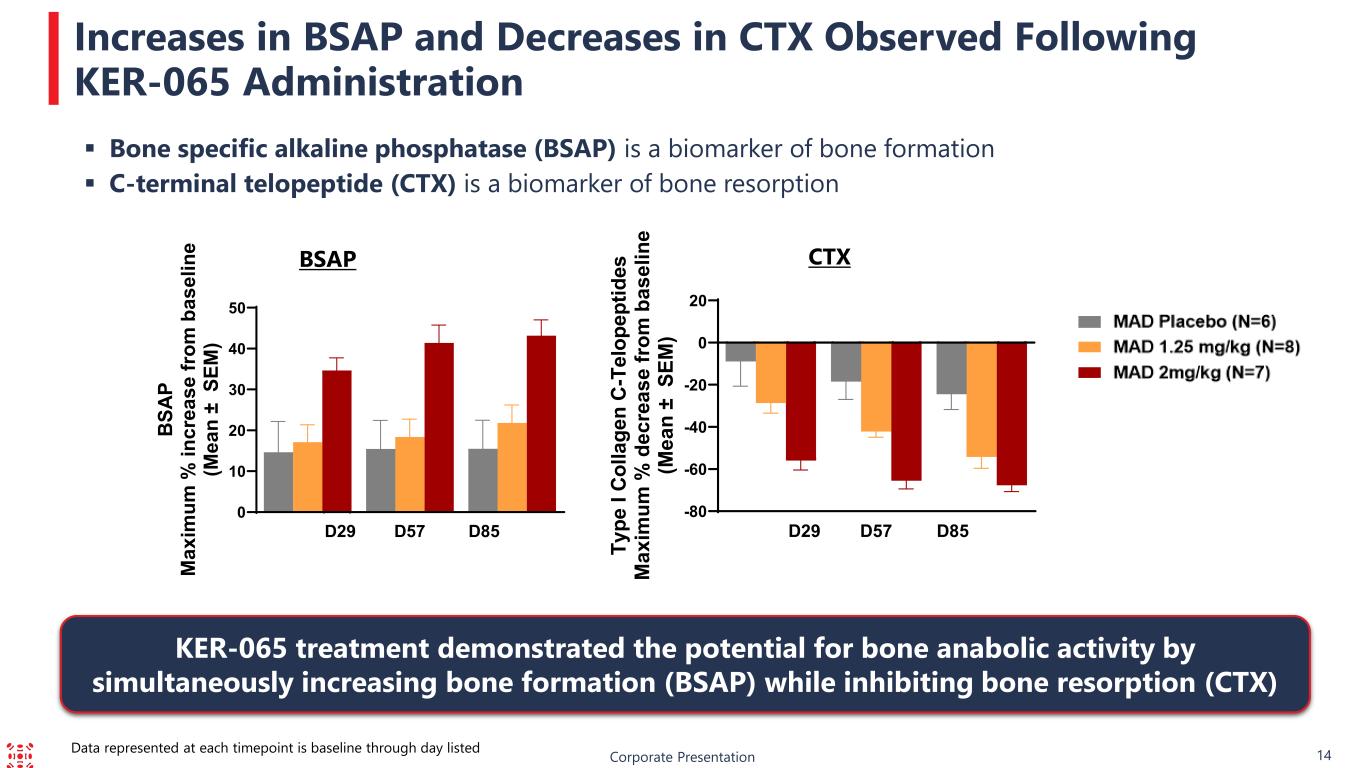
14 BSAP CTX Bone specific alkaline phosphatase (BSAP) is a biomarker of bone formation C-terminal telopeptide (CTX) is a biomarker of bone resorption Increases in BSAP and Decreases in CTX Observed Following KER-065 Administration Corporate Presentation KER-065 treatment demonstrated the potential for bone anabolic activity by simultaneously increasing bone formation (BSAP) while inhibiting bone resorption (CTX) Data represented at each timepoint is baseline through day listed 0 10 20 30 40 50 D29 D57 D85 BS AP M ax im um % in cr ea se fr om b as el in e (M ea n ± S EM ) -80 -60 -40 -20 0 20 D29 D57 D85 Ty pe I Co lla ge n C- Te lo pe pt id es M ax im um % d ec re as e fr om b as el in e (M ea n ± S EM )
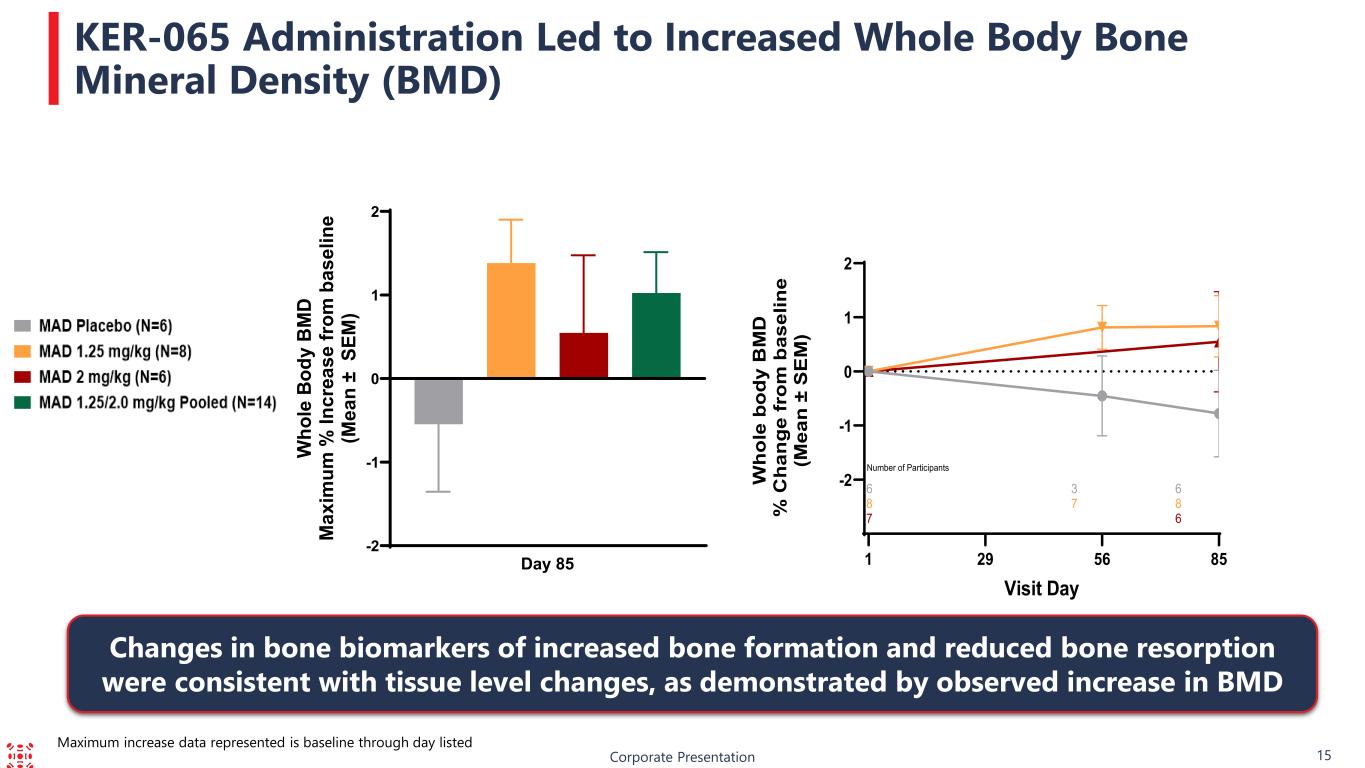
Corporate Presentation 15 KER-065 Administration Led to Increased Whole Body Bone Mineral Density (BMD) Changes in bone biomarkers of increased bone formation and reduced bone resorption were consistent with tissue level changes, as demonstrated by observed increase in BMD Maximum increase data represented is baseline through day listed -2 -1 0 1 2 Day 85 W ho le B od y B M D M ax im um % In cr ea se fr om b as el in e (M ea n ± S EM ) -2 -1 0 1 2 Visit Day W ho le b od y B M D % C ha ng e fr om b as el in e (M ea n ± S E M ) 6 3 6 8 7 8 7 6 Number of Participants 1 56 8529
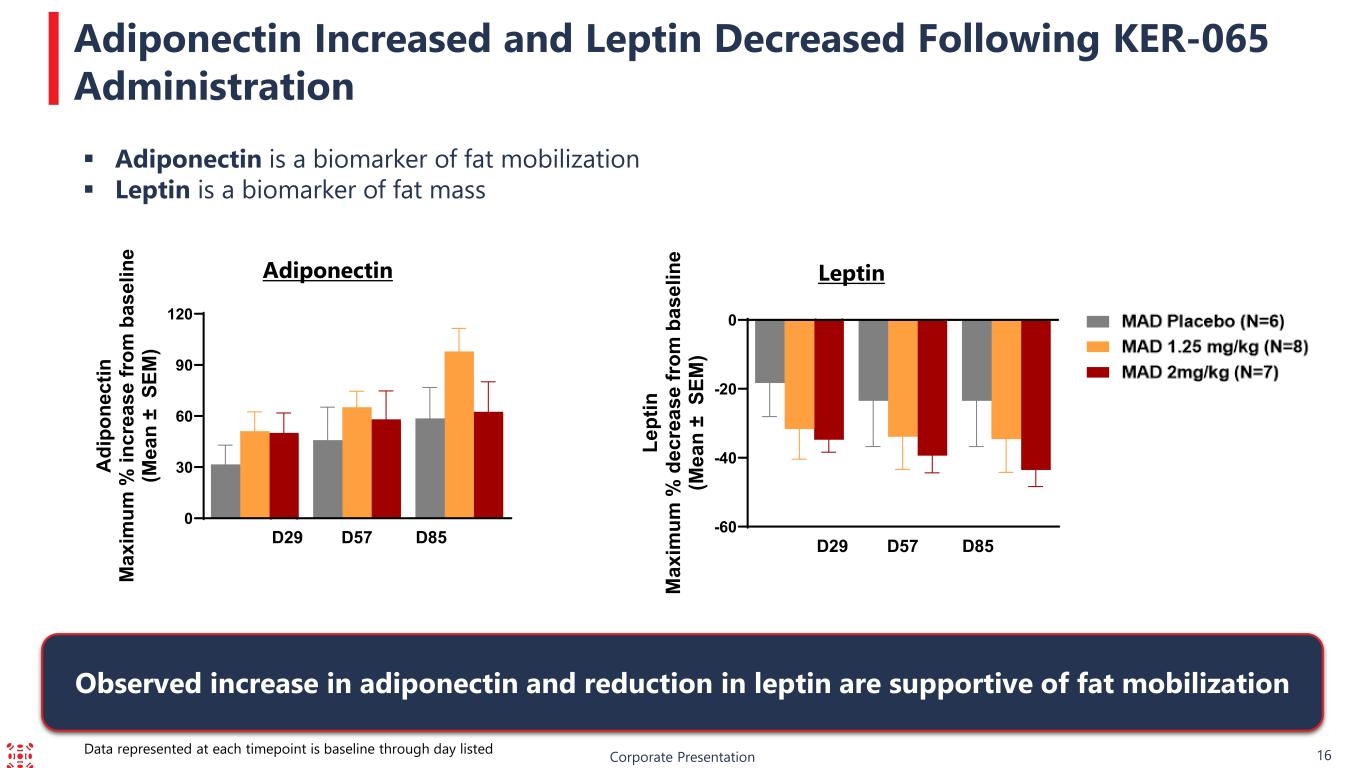
16 Adiponectin Leptin Adiponectin is a biomarker of fat mobilization Leptin is a biomarker of fat mass Adiponectin Increased and Leptin Decreased Following KER-065 Administration Corporate Presentation Observed increase in adiponectin and reduction in leptin are supportive of fat mobilization Data represented at each timepoint is baseline through day listed 0 30 60 90 120 D29 D57 D85 A di po ne ct in M ax im um % in cr ea se fr om b as el in e (M ea n ± S EM ) -60 -40 -20 0 D29 D57 D85 Le pt in M ax im um % d ec re as e fr om b as el in e (M ea n ± S EM )
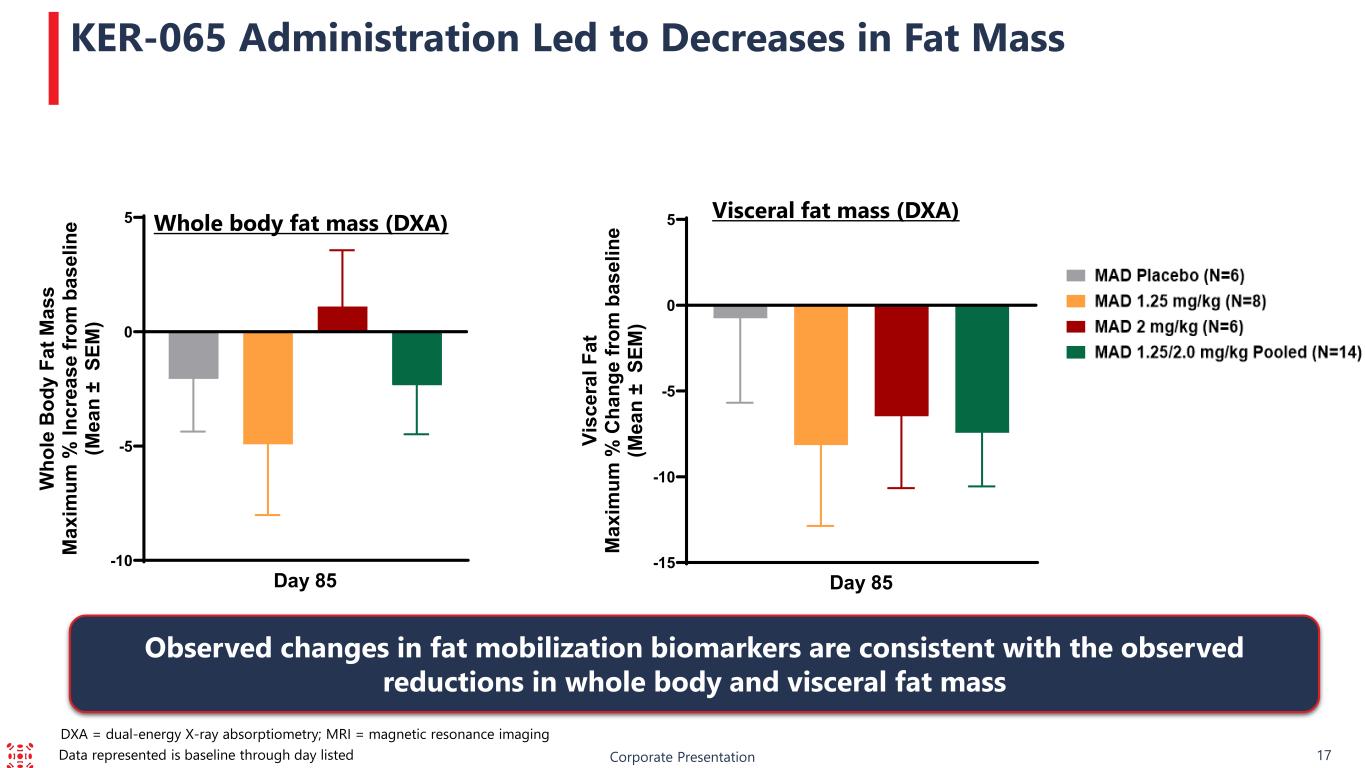
-15 -10 -5 0 5 Day 85 Vi sc er al F at M ax im um % C ha ng e fr om b as el in e (M ea n ± S EM ) -10 -5 0 5 Day 85 W ho le B od y Fa t M as s M ax im um % In cr ea se fr om b as el in e (M ea n ± S EM ) DXA = dual-energy X-ray absorptiometry; MRI = magnetic resonance imaging Corporate Presentation 17 KER-065 Administration Led to Decreases in Fat Mass Whole body fat mass (DXA) Visceral fat mass (DXA) Observed changes in fat mobilization biomarkers are consistent with the observed reductions in whole body and visceral fat mass Data represented is baseline through day listed
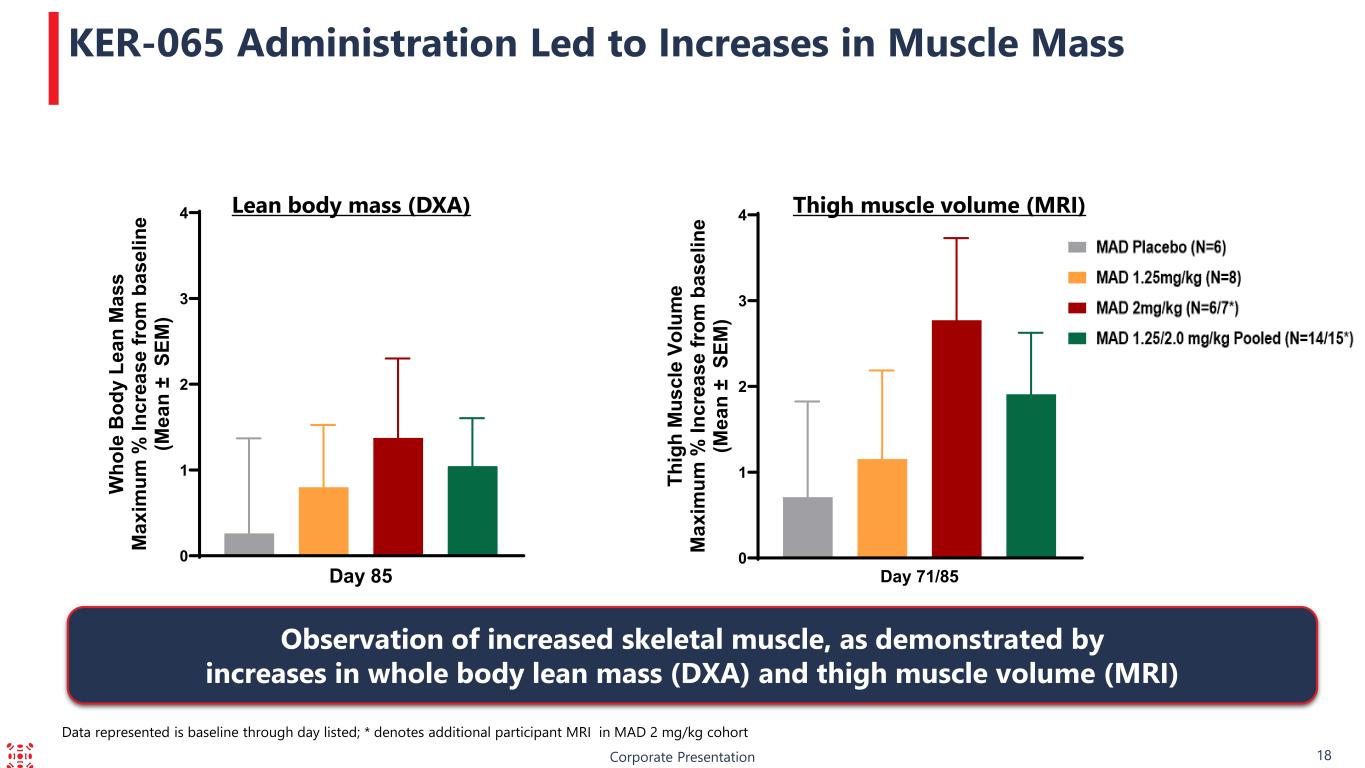
Corporate Presentation 18 KER-065 Administration Led to Increases in Muscle Mass Lean body mass (DXA) Thigh muscle volume (MRI) Observation of increased skeletal muscle, as demonstrated by increases in whole body lean mass (DXA) and thigh muscle volume (MRI) Data represented is baseline through day listed; * denotes additional participant MRI in MAD 2 mg/kg cohort 0 1 2 3 4 Day 85 W ho le B od y Le an M as s M ax im um % In cr ea se fr om b as el in e (M ea n ± S EM ) 0 1 2 3 4 Day 71/85 Th ig h M us cl e Vo lu m e M ax im um % In cr ea se fr om b as el in e (M ea n ± S EM )

Corporate Presentation 19 Encouraging Preclinical and Phase 1 Trial Data Support Rationale for Phase 2 Trial Trial met key objectives for safety, tolerability, pharmacokinetics and pharmacodynamics • Pharmacokinetic profile generally consistent with that of a well-behaved biologic • Dose exposure levels supportive of monthly dosing KER-065 was generally well-tolerated, with no major safety signals observed as of the data cut-off date Pharmacodynamic data offer multiple lines of evidence that we may be achieving sufficient activin inhibition across tissues of interest, at drug exposures that we anticipate targeting in DMD We plan on engaging with regulatory authorities on the KER-065 program in Q3 2025. Subject to the outcome of these regulatory interactions, we expect to: • Initiate a Phase 2 clinical trial in patients with DMD in Q1 2026

Corporate Presentation 20 We Have Received Positive Feedback from Leading DMD Experts on KER-065 Preclinical and Phase 1 Clinical Data “These preclinical data are very exciting, and I will be delighted to see this progressing to clinic .” Laurent Servais, MD PhD, Professor of Paediatric Neuromuscular Diseases, Oxford “Vertebral fractures are a water-shed moment. KER- 065 could help reduce these in patients with DMD.” Emma Ciafaloni, MD, Professor of Neurology and Pediatrics, University of Rochester, and Chair of the Clinical Research Committee MDSTARnet “KER-065 appears promising and may have the potential to benefit a broad spectrum of patients with DMD, from young ambulatory boys to older, non-ambulatory individuals.” Liesbeth De Waele, MD, Deputy Head of Clinics, Dept. of Paediatric Neurology, University Hospitals Leuven in Belgium
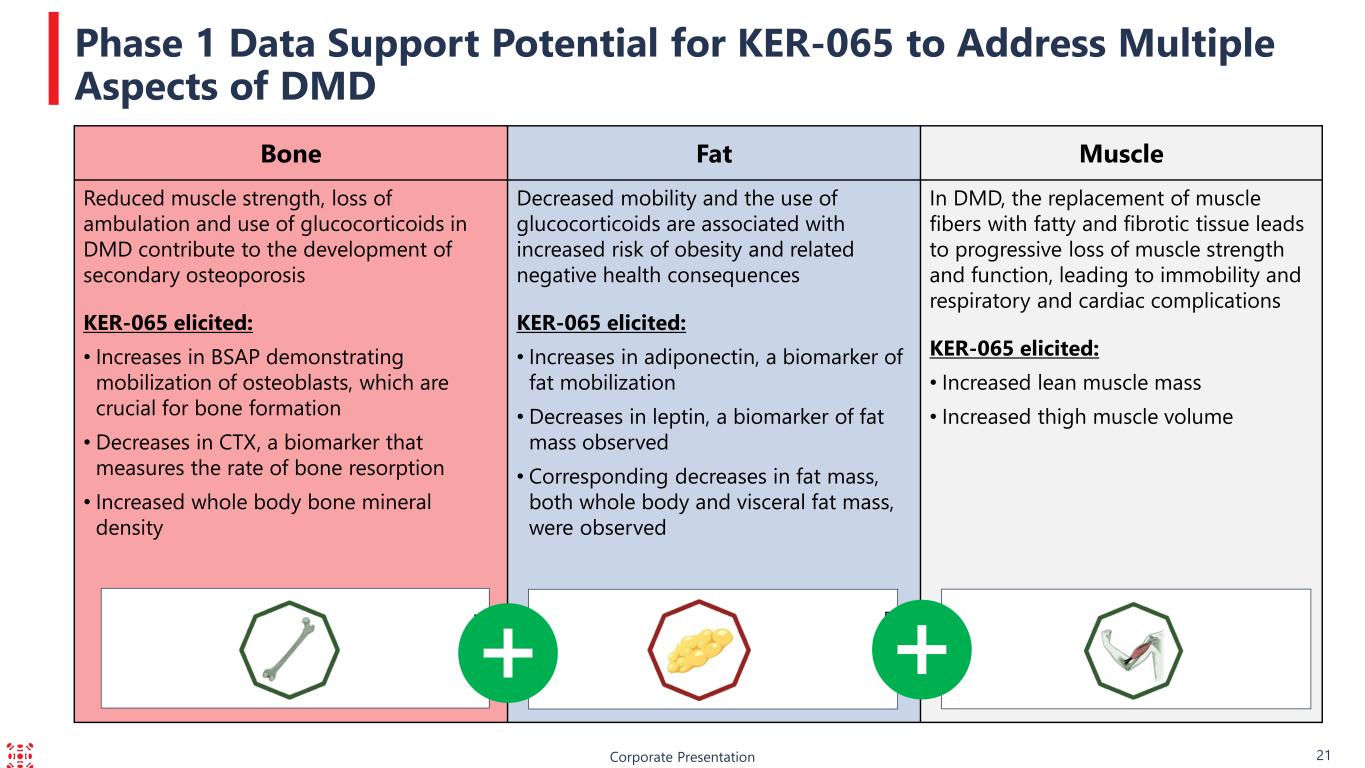
Corporate Presentation 21 Phase 1 Data Support Potential for KER-065 to Address Multiple Aspects of DMD Bone Fat Muscle Reduced muscle strength, loss of ambulation and use of glucocorticoids in DMD contribute to the development of secondary osteoporosis KER-065 elicited: • Increases in BSAP demonstrating mobilization of osteoblasts, which are crucial for bone formation • Decreases in CTX, a biomarker that measures the rate of bone resorption • Increased whole body bone mineral density Decreased mobility and the use of glucocorticoids are associated with increased risk of obesity and related negative health consequences KER-065 elicited: • Increases in adiponectin, a biomarker of fat mobilization • Decreases in leptin, a biomarker of fat mass observed • Corresponding decreases in fat mass, both whole body and visceral fat mass, were observed In DMD, the replacement of muscle fibers with fatty and fibrotic tissue leads to progressive loss of muscle strength and function, leading to immobility and respiratory and cardiac complications KER-065 elicited: • Increased lean muscle mass • Increased thigh muscle volume
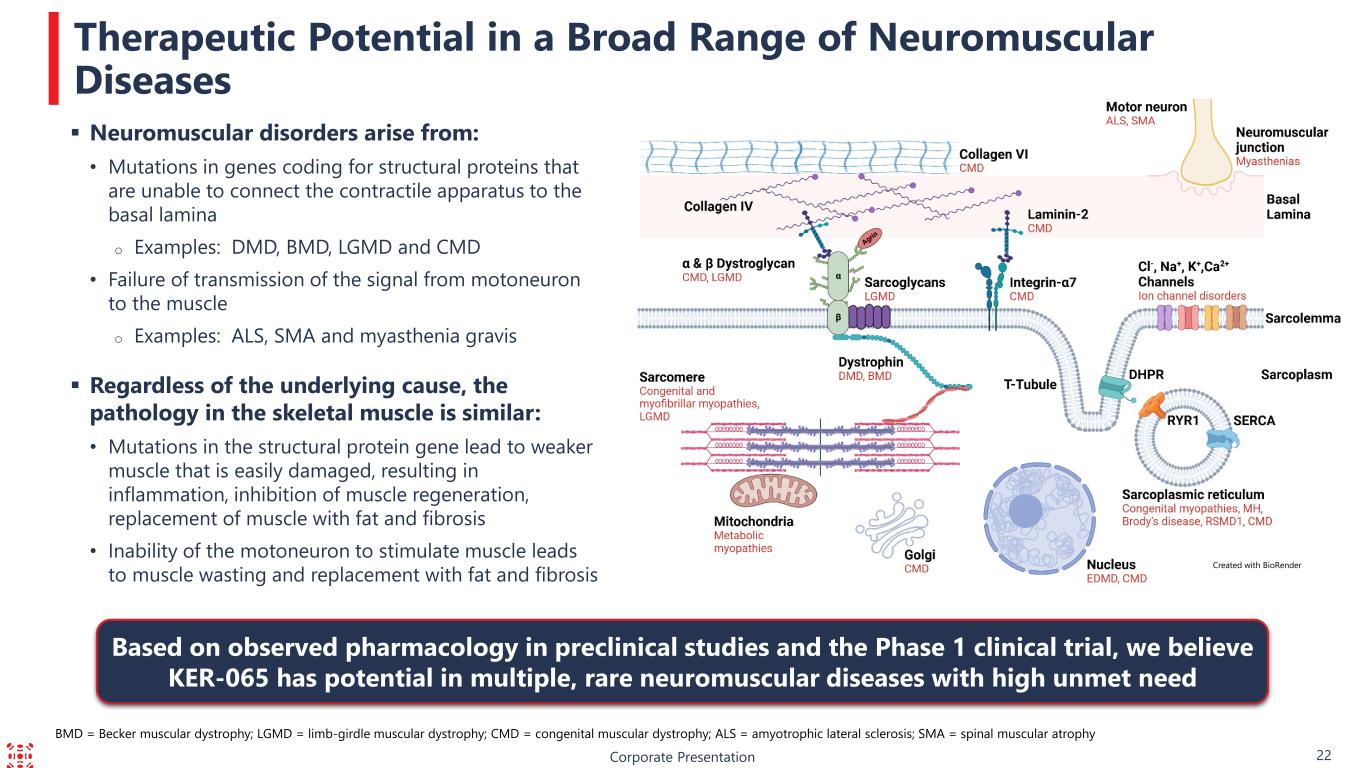
Corporate Presentation 22 Therapeutic Potential in a Broad Range of Neuromuscular Diseases Created with BioRender Based on observed pharmacology in preclinical studies and the Phase 1 clinical trial, we believe KER-065 has potential in multiple, rare neuromuscular diseases with high unmet need BMD = Becker muscular dystrophy; LGMD = limb-girdle muscular dystrophy; CMD = congenital muscular dystrophy; ALS = amyotrophic lateral sclerosis; SMA = spinal muscular atrophy Neuromuscular disorders arise from: • Mutations in genes coding for structural proteins that are unable to connect the contractile apparatus to the basal lamina o Examples: DMD, BMD, LGMD and CMD • Failure of transmission of the signal from motoneuron to the muscle o Examples: ALS, SMA and myasthenia gravis Regardless of the underlying cause, the pathology in the skeletal muscle is similar: • Mutations in the structural protein gene lead to weaker muscle that is easily damaged, resulting in inflammation, inhibition of muscle regeneration, replacement of muscle with fat and fibrosis • Inability of the motoneuron to stimulate muscle leads to muscle wasting and replacement with fat and fibrosis
B Elritercept (KER-050) Investigational Treatment for Anemia and Thrombocytopenia in Patients with Myelodysplastic Syndromes and in Patients with Myelofibrosis Corporate Presentation 23

BCorporate Presentation Global License Agreement with Takeda Pharmaceuticals 24 On December 3, 2024, Keros announced it had entered into an exclusive license agreement with Takeda to develop, manufacture and commercialize elritercept globally, other than mainland China, Hong Kong, and Macau Financials: • Keros received an upfront payment of $200 million • Eligible to receive development, approval and commercial milestone payments of over $1.1 billion • Tiered royalties on net sales in the low double-digits to high teens Under the terms of the agreement, in January 2025, Takeda became responsible for all clinical development, manufacturing and commercialization of elritercept in its territory
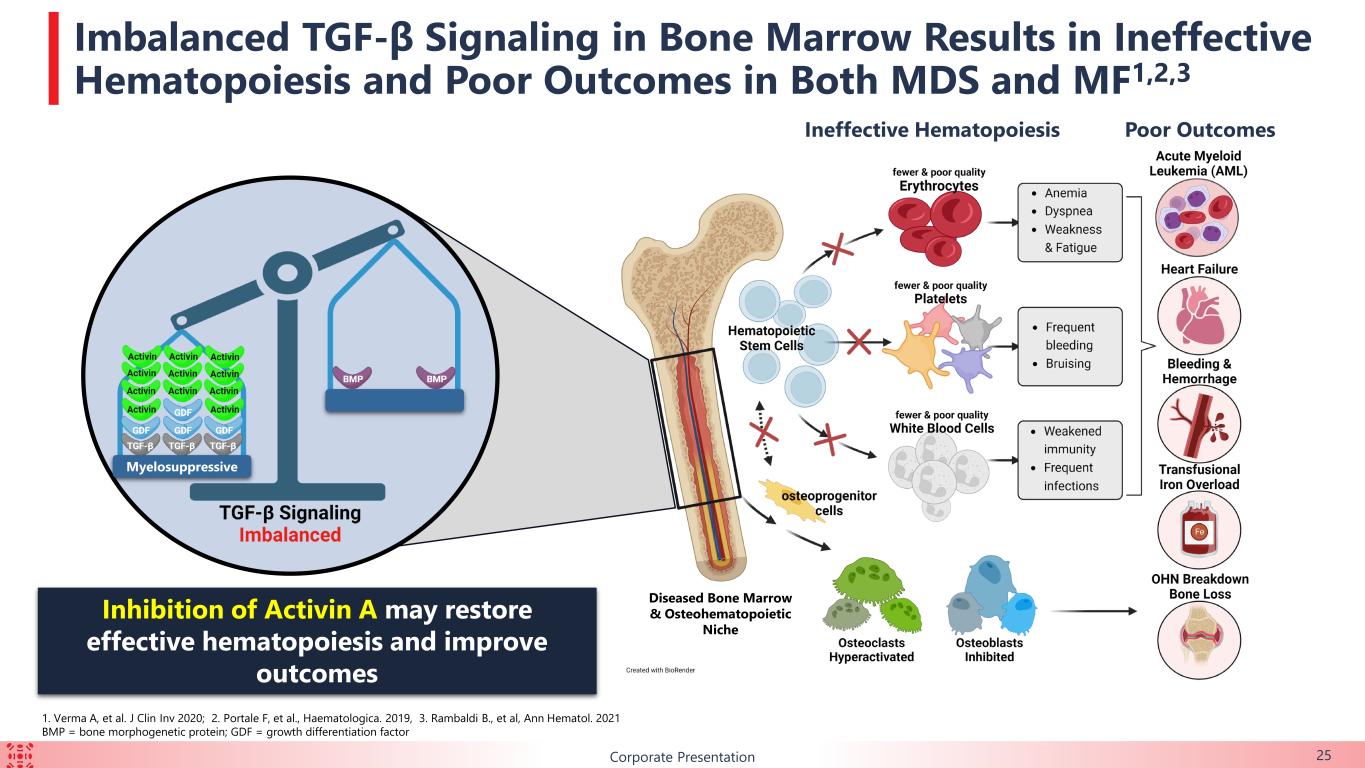
B Imbalanced TGF-β Signaling in Bone Marrow Results in Ineffective Hematopoiesis and Poor Outcomes in Both MDS and MF1,2,3 Inhibition of Activin A may restore effective hematopoiesis and improve outcomes Poor Outcomes 1. Verma A, et al. J Clin Inv 2020; 2. Portale F, et al., Haematologica. 2019, 3. Rambaldi B., et al, Ann Hematol. 2021 BMP = bone morphogenetic protein; GDF = growth differentiation factor Myelosuppressive Ineffective Hematopoiesis Diseased Bone Marrow & Osteohematopoietic Niche Corporate Presentation 25
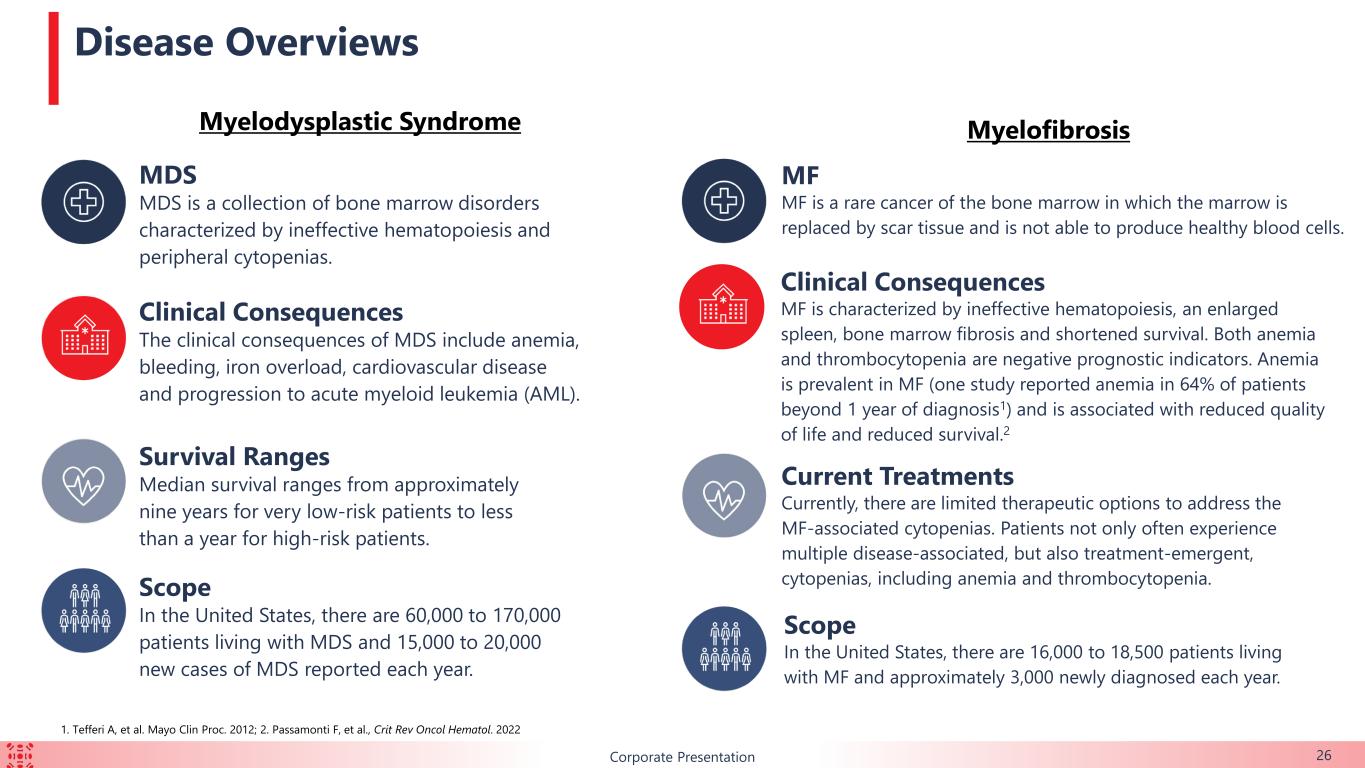
BCorporate Presentation MDS MDS is a collection of bone marrow disorders characterized by ineffective hematopoiesis and peripheral cytopenias. Clinical Consequences The clinical consequences of MDS include anemia, bleeding, iron overload, cardiovascular disease and progression to acute myeloid leukemia (AML). Survival Ranges Median survival ranges from approximately nine years for very low-risk patients to less than a year for high-risk patients. Scope In the United States, there are 60,000 to 170,000 patients living with MDS and 15,000 to 20,000 new cases of MDS reported each year. Disease Overviews 26 MF MF is a rare cancer of the bone marrow in which the marrow is replaced by scar tissue and is not able to produce healthy blood cells. Current Treatments Currently, there are limited therapeutic options to address the MF-associated cytopenias. Patients not only often experience multiple disease-associated, but also treatment-emergent, cytopenias, including anemia and thrombocytopenia. Scope In the United States, there are 16,000 to 18,500 patients living with MF and approximately 3,000 newly diagnosed each year. Clinical Consequences MF is characterized by ineffective hematopoiesis, an enlarged spleen, bone marrow fibrosis and shortened survival. Both anemia and thrombocytopenia are negative prognostic indicators. Anemia is prevalent in MF (one study reported anemia in 64% of patients beyond 1 year of diagnosis1) and is associated with reduced quality of life and reduced survival.2 Myelodysplastic Syndrome Myelofibrosis 1. Tefferi A, et al. Mayo Clin Proc. 2012; 2. Passamonti F, et al., Crit Rev Oncol Hematol. 2022
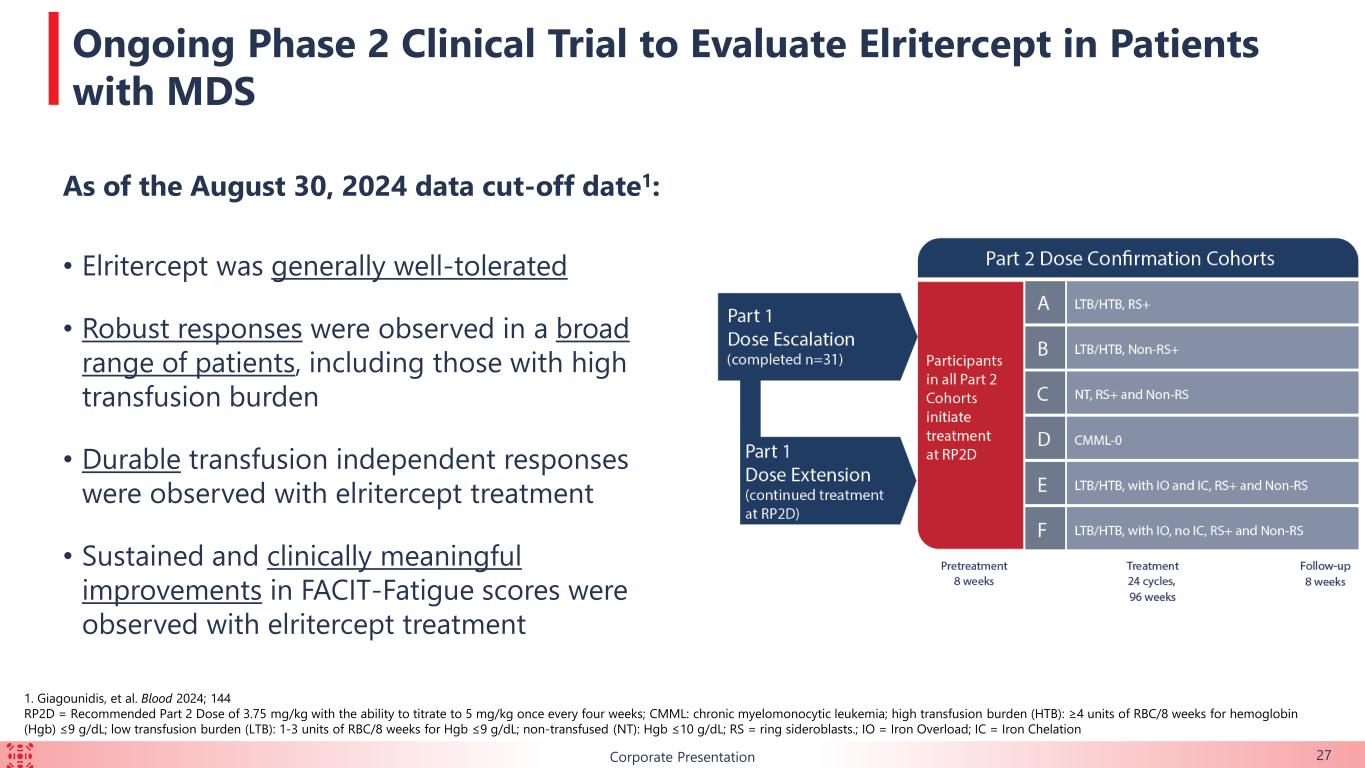
B As of the August 30, 2024 data cut-off date1: • Elritercept was generally well-tolerated • Robust responses were observed in a broad range of patients, including those with high transfusion burden • Durable transfusion independent responses were observed with elritercept treatment • Sustained and clinically meaningful improvements in FACIT-Fatigue scores were observed with elritercept treatment Corporate Presentation 27 Ongoing Phase 2 Clinical Trial to Evaluate Elritercept in Patients with MDS 1. Giagounidis, et al. Blood 2024; 144 RP2D = Recommended Part 2 Dose of 3.75 mg/kg with the ability to titrate to 5 mg/kg once every four weeks; CMML: chronic myelomonocytic leukemia; high transfusion burden (HTB): ≥4 units of RBC/8 weeks for hemoglobin (Hgb) ≤9 g/dL; low transfusion burden (LTB): 1-3 units of RBC/8 weeks for Hgb ≤9 g/dL; non-transfused (NT): Hgb ≤10 g/dL; RS = ring sideroblasts.; IO = Iron Overload; IC = Iron Chelation
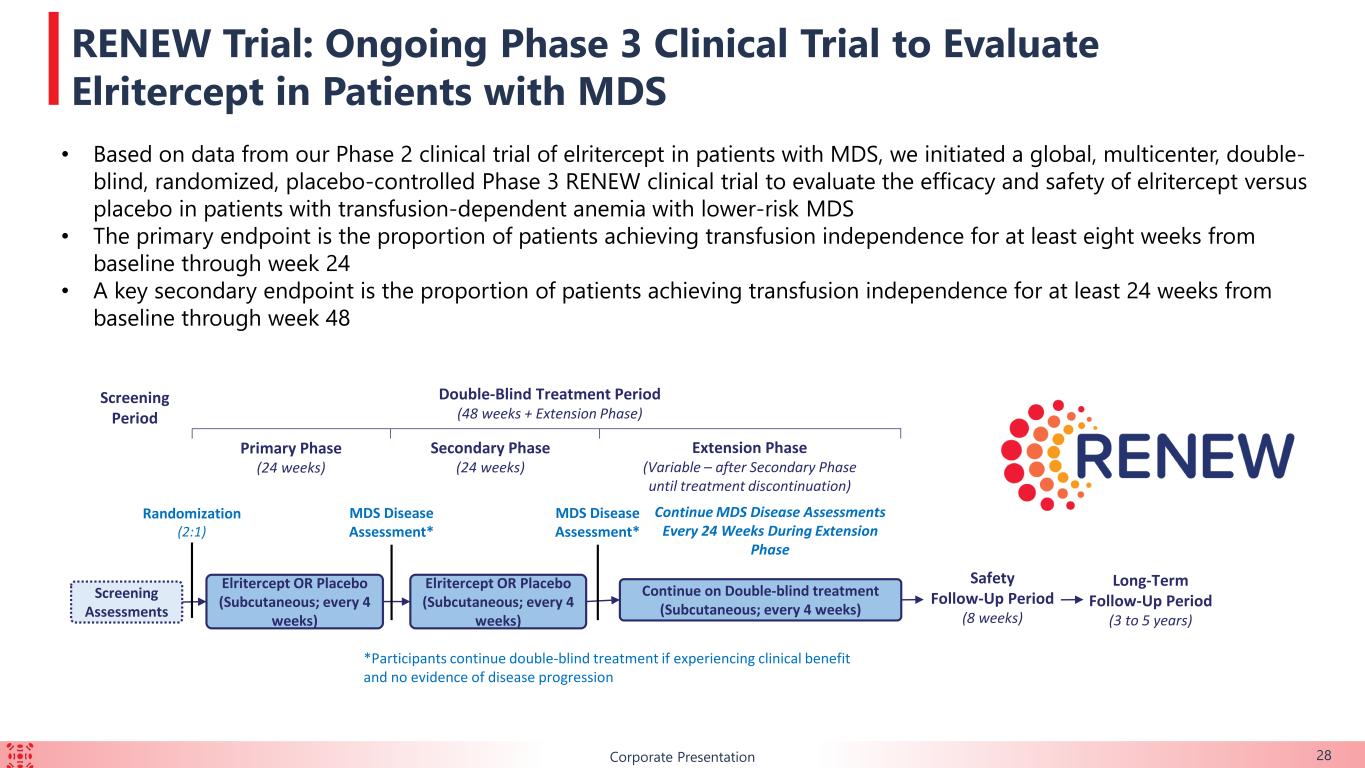
BCorporate Presentation 28 RENEW Trial: Ongoing Phase 3 Clinical Trial to Evaluate Elritercept in Patients with MDS Screening Assessments Screening Period Double-Blind Treatment Period (48 weeks + Extension Phase) Safety Follow-Up Period (8 weeks) Extension Phase (Variable – after Secondary Phase until treatment discontinuation) Primary Phase (24 weeks) Elritercept OR Placebo (Subcutaneous; every 4 weeks) Continue on Double-blind treatment (Subcutaneous; every 4 weeks) Secondary Phase (24 weeks) Elritercept OR Placebo (Subcutaneous; every 4 weeks) Randomization (2:1) MDS Disease Assessment* MDS Disease Assessment* Continue MDS Disease Assessments Every 24 Weeks During Extension Phase *Participants continue double-blind treatment if experiencing clinical benefit and no evidence of disease progression Long-Term Follow-Up Period (3 to 5 years) • Based on data from our Phase 2 clinical trial of elritercept in patients with MDS, we initiated a global, multicenter, double- blind, randomized, placebo-controlled Phase 3 RENEW clinical trial to evaluate the efficacy and safety of elritercept versus placebo in patients with transfusion-dependent anemia with lower-risk MDS • The primary endpoint is the proportion of patients achieving transfusion independence for at least eight weeks from baseline through week 24 • A key secondary endpoint is the proportion of patients achieving transfusion independence for at least 24 weeks from baseline through week 48
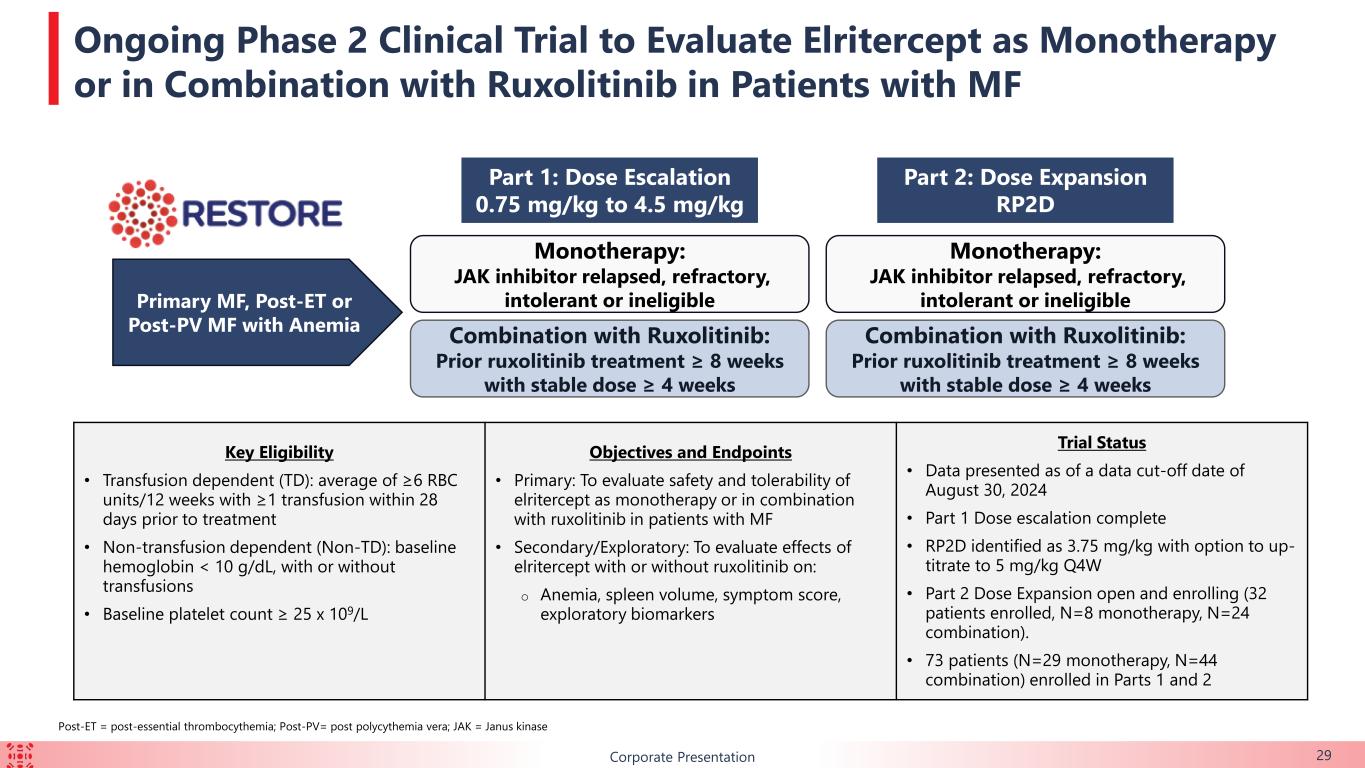
BCorporate Presentation 29 Ongoing Phase 2 Clinical Trial to Evaluate Elritercept as Monotherapy or in Combination with Ruxolitinib in Patients with MF Monotherapy: JAK inhibitor relapsed, refractory, intolerant or ineligible Combination with Ruxolitinib: Prior ruxolitinib treatment ≥ 8 weeks with stable dose ≥ 4 weeks Primary MF, Post-ET or Post-PV MF with Anemia Part 1: Dose Escalation 0.75 mg/kg to 4.5 mg/kg Key Eligibility • Transfusion dependent (TD): average of ≥6 RBC units/12 weeks with ≥1 transfusion within 28 days prior to treatment • Non-transfusion dependent (Non-TD): baseline hemoglobin < 10 g/dL, with or without transfusions • Baseline platelet count ≥ 25 x 109/L Objectives and Endpoints • Primary: To evaluate safety and tolerability of elritercept as monotherapy or in combination with ruxolitinib in patients with MF • Secondary/Exploratory: To evaluate effects of elritercept with or without ruxolitinib on: o Anemia, spleen volume, symptom score, exploratory biomarkers Trial Status • Data presented as of a data cut-off date of August 30, 2024 • Part 1 Dose escalation complete • RP2D identified as 3.75 mg/kg with option to up- titrate to 5 mg/kg Q4W • Part 2 Dose Expansion open and enrolling (32 patients enrolled, N=8 monotherapy, N=24 combination). • 73 patients (N=29 monotherapy, N=44 combination) enrolled in Parts 1 and 2 Monotherapy: JAK inhibitor relapsed, refractory, intolerant or ineligible Combination with Ruxolitinib: Prior ruxolitinib treatment ≥ 8 weeks with stable dose ≥ 4 weeks Part 2: Dose Expansion RP2D Post-ET = post-essential thrombocythemia; Post-PV= post polycythemia vera; JAK = Janus kinase
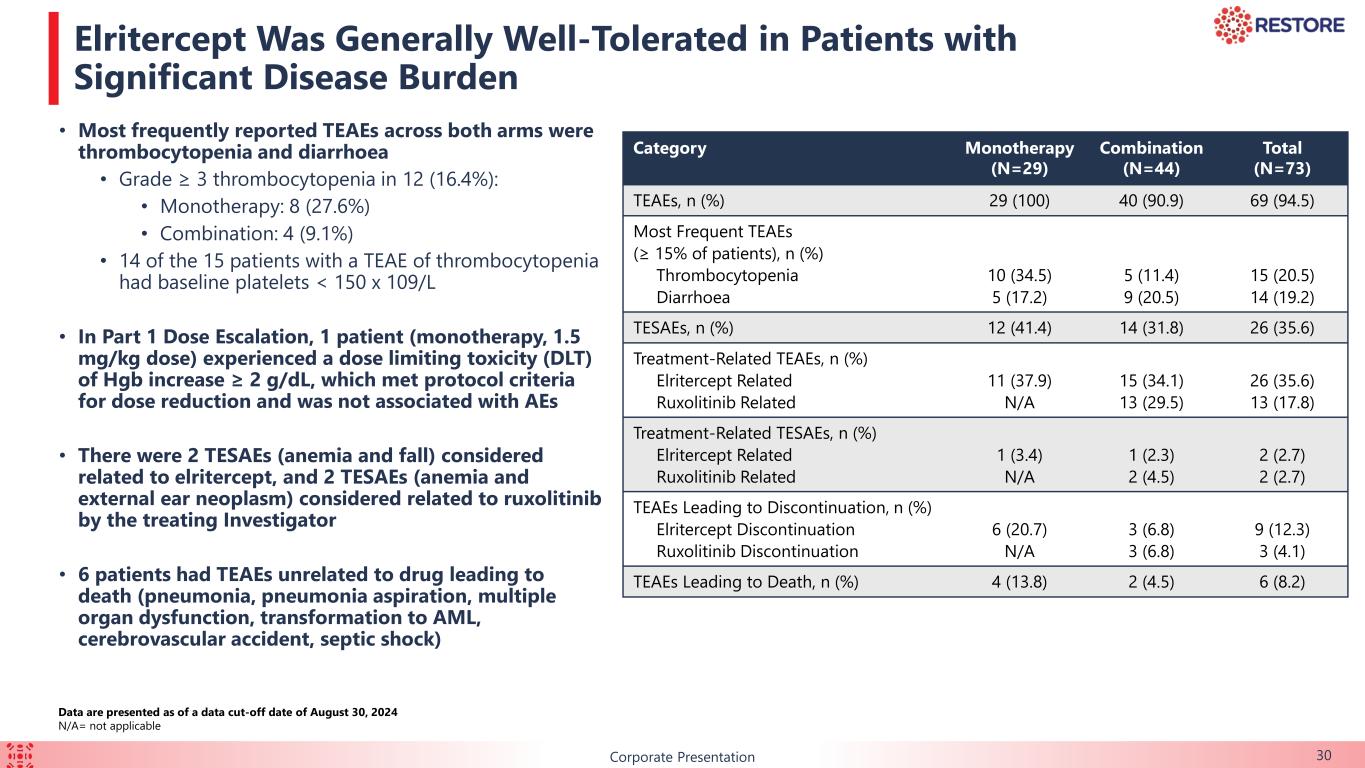
B 30Corporate Presentation Elritercept Was Generally Well-Tolerated in Patients with Significant Disease Burden Data are presented as of a data cut-off date of August 30, 2024 N/A= not applicable Category Monotherapy (N=29) Combination (N=44) Total (N=73) TEAEs, n (%) 29 (100) 40 (90.9) 69 (94.5) Most Frequent TEAEs (≥ 15% of patients), n (%) Thrombocytopenia Diarrhoea 10 (34.5) 5 (17.2) 5 (11.4) 9 (20.5) 15 (20.5) 14 (19.2) TESAEs, n (%) 12 (41.4) 14 (31.8) 26 (35.6) Treatment-Related TEAEs, n (%) Elritercept Related Ruxolitinib Related 11 (37.9) N/A 15 (34.1) 13 (29.5) 26 (35.6) 13 (17.8) Treatment-Related TESAEs, n (%) Elritercept Related Ruxolitinib Related 1 (3.4) N/A 1 (2.3) 2 (4.5) 2 (2.7) 2 (2.7) TEAEs Leading to Discontinuation, n (%) Elritercept Discontinuation Ruxolitinib Discontinuation 6 (20.7) N/A 3 (6.8) 3 (6.8) 9 (12.3) 3 (4.1) TEAEs Leading to Death, n (%) 4 (13.8) 2 (4.5) 6 (8.2) • Most frequently reported TEAEs across both arms were thrombocytopenia and diarrhoea • Grade ≥ 3 thrombocytopenia in 12 (16.4%): • Monotherapy: 8 (27.6%) • Combination: 4 (9.1%) • 14 of the 15 patients with a TEAE of thrombocytopenia had baseline platelets < 150 x 109/L • In Part 1 Dose Escalation, 1 patient (monotherapy, 1.5 mg/kg dose) experienced a dose limiting toxicity (DLT) of Hgb increase ≥ 2 g/dL, which met protocol criteria for dose reduction and was not associated with AEs • There were 2 TESAEs (anemia and fall) considered related to elritercept, and 2 TESAEs (anemia and external ear neoplasm) considered related to ruxolitinib by the treating Investigator • 6 patients had TEAEs unrelated to drug leading to death (pneumonia, pneumonia aspiration, multiple organ dysfunction, transformation to AML, cerebrovascular accident, septic shock)
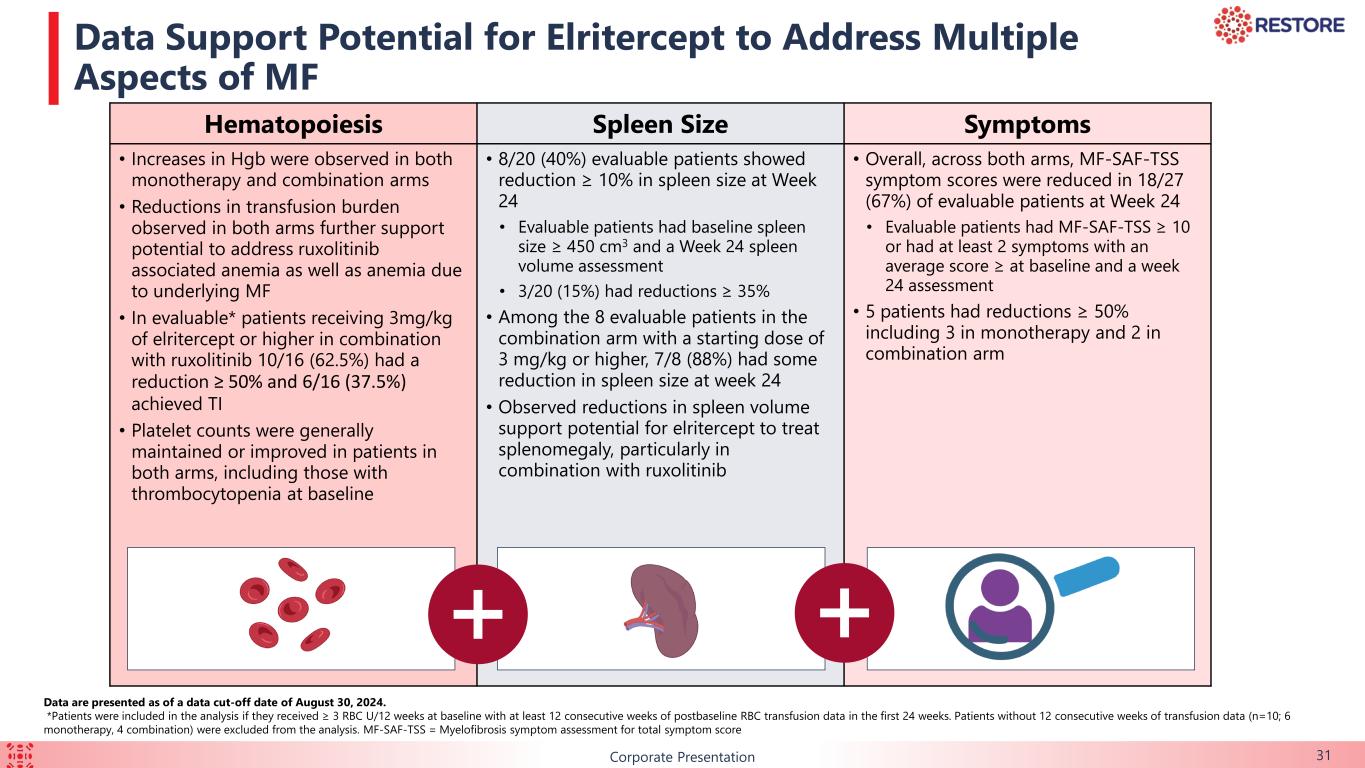
B 31Corporate Presentation Data Support Potential for Elritercept to Address Multiple Aspects of MF Hematopoiesis Spleen Size Symptoms • Increases in Hgb were observed in both monotherapy and combination arms • Reductions in transfusion burden observed in both arms further support potential to address ruxolitinib associated anemia as well as anemia due to underlying MF • In evaluable* patients receiving 3mg/kg of elritercept or higher in combination with ruxolitinib 10/16 (62.5%) had a reduction ≥ 50% and 6/16 (37.5%) achieved TI • Platelet counts were generally maintained or improved in patients in both arms, including those with thrombocytopenia at baseline • 8/20 (40%) evaluable patients showed reduction ≥ 10% in spleen size at Week 24 • Evaluable patients had baseline spleen size ≥ 450 cm3 and a Week 24 spleen volume assessment • 3/20 (15%) had reductions ≥ 35% • Among the 8 evaluable patients in the combination arm with a starting dose of 3 mg/kg or higher, 7/8 (88%) had some reduction in spleen size at week 24 • Observed reductions in spleen volume support potential for elritercept to treat splenomegaly, particularly in combination with ruxolitinib • Overall, across both arms, MF-SAF-TSS symptom scores were reduced in 18/27 (67%) of evaluable patients at Week 24 • Evaluable patients had MF-SAF-TSS ≥ 10 or had at least 2 symptoms with an average score ≥ at baseline and a week 24 assessment • 5 patients had reductions ≥ 50% including 3 in monotherapy and 2 in combination arm Data are presented as of a data cut-off date of August 30, 2024. *Patients were included in the analysis if they received ≥ 3 RBC U/12 weeks at baseline with at least 12 consecutive weeks of postbaseline RBC transfusion data in the first 24 weeks. Patients without 12 consecutive weeks of transfusion data (n=10; 6 monotherapy, 4 combination) were excluded from the analysis. MF-SAF-TSS = Myelofibrosis symptom assessment for total symptom score

Corporate Presentation Proprietary Discovery Approach 32

Corporate Presentation We have developed a proprietary library of ActRII ligand traps by combining sequences from ActRIIA and ActRIIB ▸ We have engineered molecules that are designed to have the therapeutic properties of either or both parent molecules ▸ Our ActRII program has produced a broader pipeline of engineered ligand traps, and we currently have an expansive library of unique variants in preclinical development ▸ KER-065 was nominated out of this proprietary library of ActRII ligand traps for clinical development This discovery approach has the potential to identify additional molecules with differentiated profiles from existing third-party products and product candidates ▸ Pipeline of preclinical assets: musculoskeletal; obesity; other undisclosed indications Proprietary Discovery Approach 33

Corporate Presentation Delivering on Our Priorities to Drive Long-Term Growth 34 Proven record of advancing molecules from discovery to value creation – We discovered elritercept and advanced it into two Phase 2 clinical trials, one in patients with MDS and one in patients with MF, and one Phase 3 clinical trial in patients with MDS. Keros’ December 2024 global license agreement with Takeda included $200 million upfront, future potential milestone payments over $1.1 billion, and tiered royalties on net sales. We have demonstrated a disciplined approach to capital allocation – We manage our clinical programs dynamically, utilizing a data-driven approach to determine which assets to develop. This operating model led to the decision to deprioritize cibotercept. Our extensive knowledge of our assets and drug development informs our decision-making process regarding when to pursue demonstrating proof-of-concept, balanced with the imperative of maintaining an efficient timeframe and cost- effective budget. We believe we are well-positioned to establish proof-of-concept of KER-065 and expand development into additional rare neuromuscular diseases – Based on observed pharmacology in preclinical studies and the Phase 1 clinical trial, we believe KER-065 has a differentiated profile in multiple, rare neuromuscular diseases with high unmet medical need.