
January 2026 Corporate Presentation

Corporate Presentation Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives, expected results and timing of its preclinical studies and clinical trials for rinvatercept (KER-065), including its regulatory plans; and the potential of Keros’ proprietary discovery approach, including additional indications which we may pursue. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its product candidates, rinvatercept and elritercept; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; and Keros’ dependence on third parties in connection with manufacturing, clinical trials and preclinical studies. These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 5, 2025, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Disclaimer 2
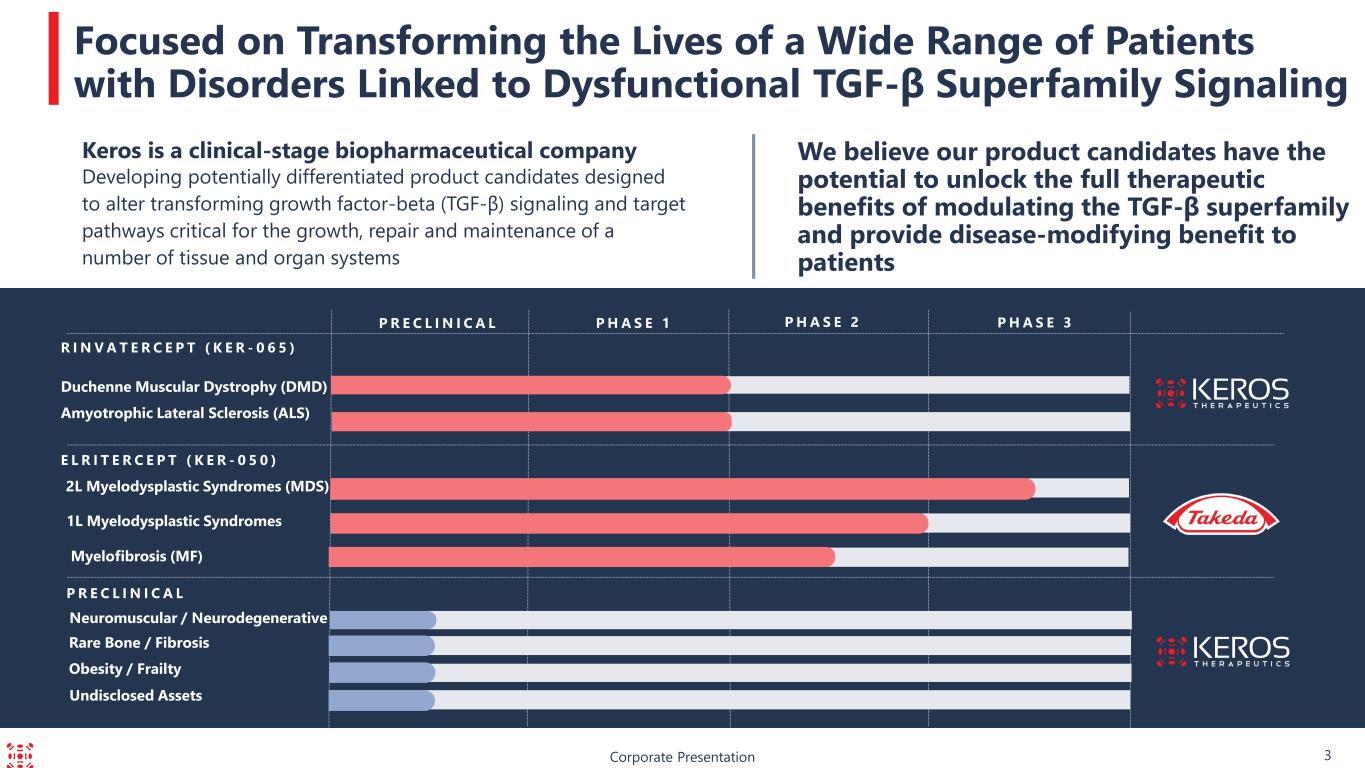
Duchenne Muscular Dystrophy (DMD) P R E C L I N I C A L Corporate Presentation Focused on Transforming the Lives of a Wide Range of Patients with Disorders Linked to Dysfunctional TGF-β Superfamily Signaling We believe our product candidates have the potential to unlock the full therapeutic benefits of modulating the TGF-β superfamily and provide disease-modifying benefit to patients P H A S E 3P R E C L I N I C A L P H A S E 1 P H A S E 2 R I N V A T E R C E P T ( K E R - 0 6 5 ) 2L Myelodysplastic Syndromes (MDS) E L R I T E R C E P T ( K E R - 0 5 0 ) Neuromuscular / Neurodegenerative Keros is a clinical-stage biopharmaceutical company Developing potentially differentiated product candidates designed to alter transforming growth factor-beta (TGF-β) signaling and target pathways critical for the growth, repair and maintenance of a number of tissue and organ systems Undisclosed Assets Rare Bone / Fibrosis 3 Amyotrophic Lateral Sclerosis (ALS) 1L Myelodysplastic Syndromes Myelofibrosis (MF) Obesity / Frailty

Corporate Presentation Rinvatercept (KER-065) 4
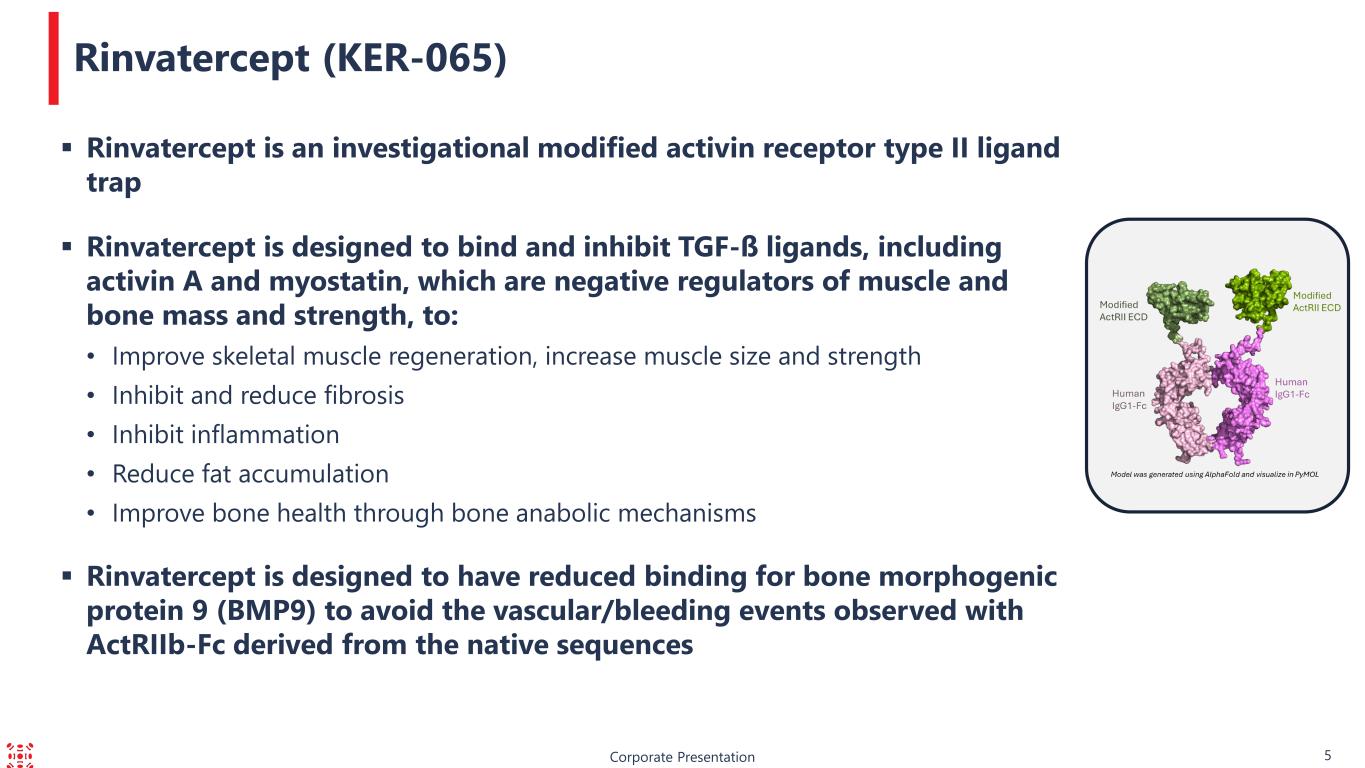
Corporate Presentation 5 Rinvatercept (KER-065) Rinvatercept is an investigational modified activin receptor type II ligand trap Rinvatercept is designed to bind and inhibit TGF-ß ligands, including activin A and myostatin, which are negative regulators of muscle and bone mass and strength, to: • Improve skeletal muscle regeneration, increase muscle size and strength • Inhibit and reduce fibrosis • Inhibit inflammation • Reduce fat accumulation • Improve bone health through bone anabolic mechanisms Rinvatercept is designed to have reduced binding for bone morphogenic protein 9 (BMP9) to avoid the vascular/bleeding events observed with ActRIIb-Fc derived from the native sequences
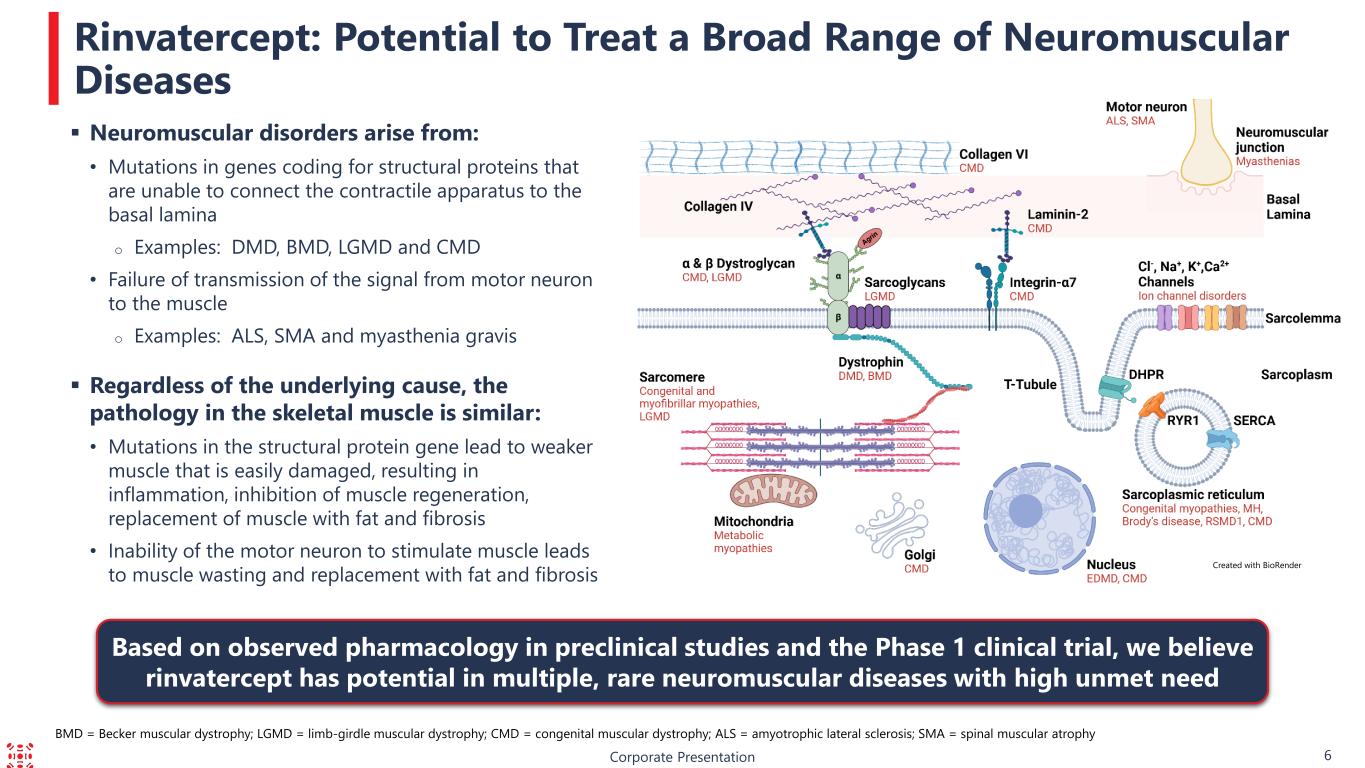
Corporate Presentation 6 Rinvatercept: Potential to Treat a Broad Range of Neuromuscular Diseases Created with BioRender Based on observed pharmacology in preclinical studies and the Phase 1 clinical trial, we believe rinvatercept has potential in multiple, rare neuromuscular diseases with high unmet need BMD = Becker muscular dystrophy; LGMD = limb-girdle muscular dystrophy; CMD = congenital muscular dystrophy; ALS = amyotrophic lateral sclerosis; SMA = spinal muscular atrophy Neuromuscular disorders arise from: • Mutations in genes coding for structural proteins that are unable to connect the contractile apparatus to the basal lamina o Examples: DMD, BMD, LGMD and CMD • Failure of transmission of the signal from motor neuron to the muscle o Examples: ALS, SMA and myasthenia gravis Regardless of the underlying cause, the pathology in the skeletal muscle is similar: • Mutations in the structural protein gene lead to weaker muscle that is easily damaged, resulting in inflammation, inhibition of muscle regeneration, replacement of muscle with fat and fibrosis • Inability of the motor neuron to stimulate muscle leads to muscle wasting and replacement with fat and fibrosis

Corporate Presentation Rinvatercept: Duchenne Muscular Dystrophy (DMD) 7
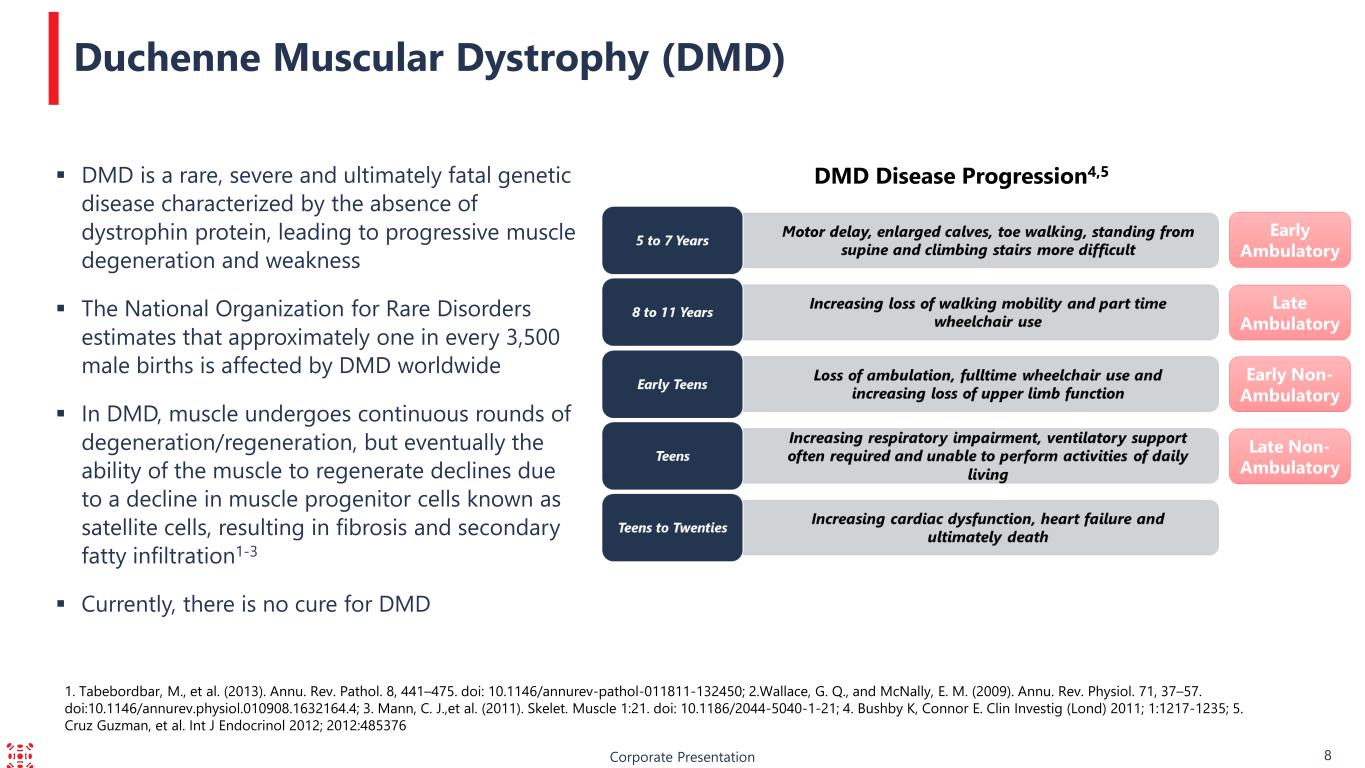
1. Tabebordbar, M., et al. (2013). Annu. Rev. Pathol. 8, 441–475. doi: 10.1146/annurev-pathol-011811-132450; 2.Wallace, G. Q., and McNally, E. M. (2009). Annu. Rev. Physiol. 71, 37–57. doi:10.1146/annurev.physiol.010908.1632164.4; 3. Mann, C. J.,et al. (2011). Skelet. Muscle 1:21. doi: 10.1186/2044-5040-1-21; 4. Bushby K, Connor E. Clin Investig (Lond) 2011; 1:1217-1235; 5. Cruz Guzman, et al. Int J Endocrinol 2012; 2012:485376 Corporate Presentation 8 DMD is a rare, severe and ultimately fatal genetic disease characterized by the absence of dystrophin protein, leading to progressive muscle degeneration and weakness The National Organization for Rare Disorders estimates that approximately one in every 3,500 male births is affected by DMD worldwide In DMD, muscle undergoes continuous rounds of degeneration/regeneration, but eventually the ability of the muscle to regenerate declines due to a decline in muscle progenitor cells known as satellite cells, resulting in fibrosis and secondary fatty infiltration1-3 Currently, there is no cure for DMD Duchenne Muscular Dystrophy (DMD) DMD Disease Progression4,5
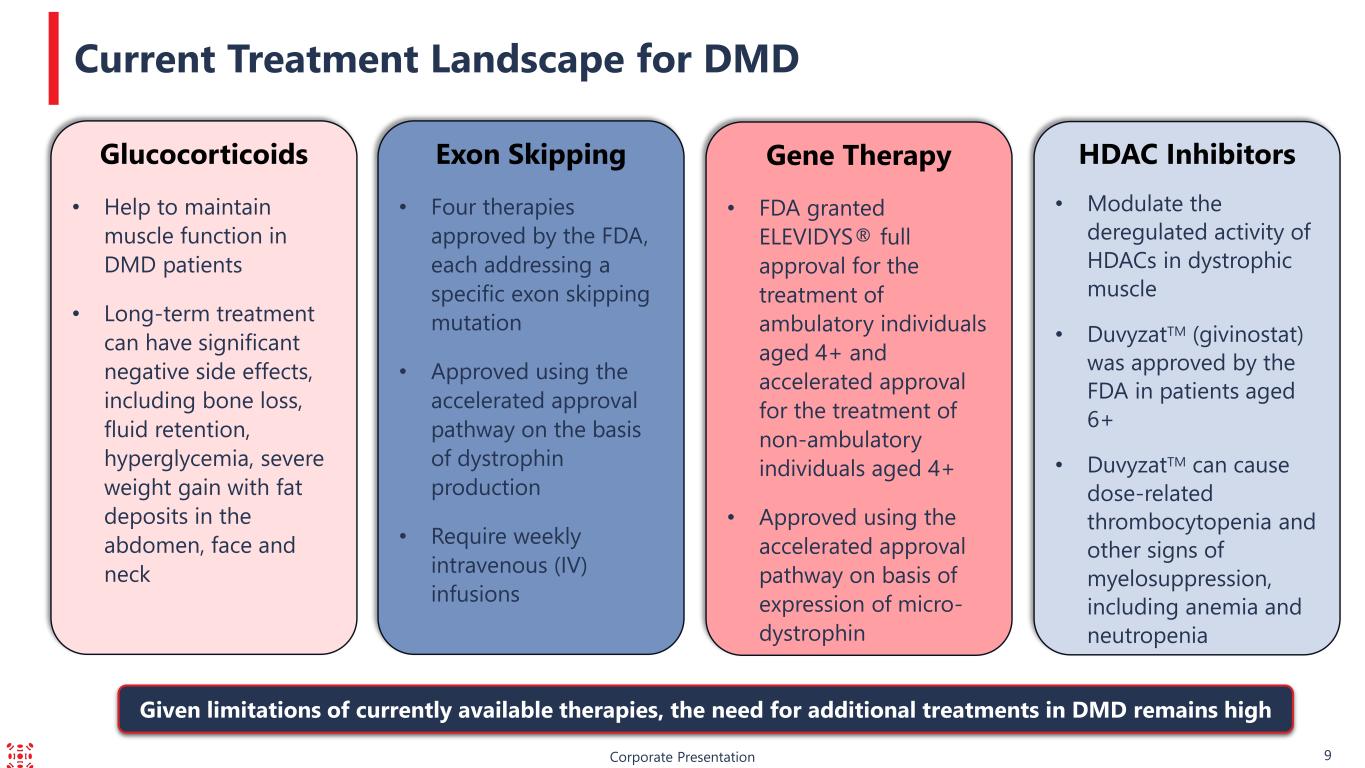
HDAC Inhibitors • Modulate the deregulated activity of HDACs in dystrophic muscle • DuvyzatTM (givinostat) was approved by the FDA in patients aged 6+ • DuvyzatTM can cause dose-related thrombocytopenia and other signs of myelosuppression, including anemia and neutropenia Corporate Presentation 9 Current Treatment Landscape for DMD Gene Therapy • FDA granted ELEVIDYS® full approval for the treatment of ambulatory individuals aged 4+ and accelerated approval for the treatment of non-ambulatory individuals aged 4+ • Approved using the accelerated approval pathway on basis of expression of micro- dystrophin Exon Skipping • Four therapies approved by the FDA, each addressing a specific exon skipping mutation • Approved using the accelerated approval pathway on the basis of dystrophin production • Require weekly intravenous (IV) infusions Glucocorticoids • Help to maintain muscle function in DMD patients • Long-term treatment can have significant negative side effects, including bone loss, fluid retention, hyperglycemia, severe weight gain with fat deposits in the abdomen, face and neck Given limitations of currently available therapies, the need for additional treatments in DMD remains high
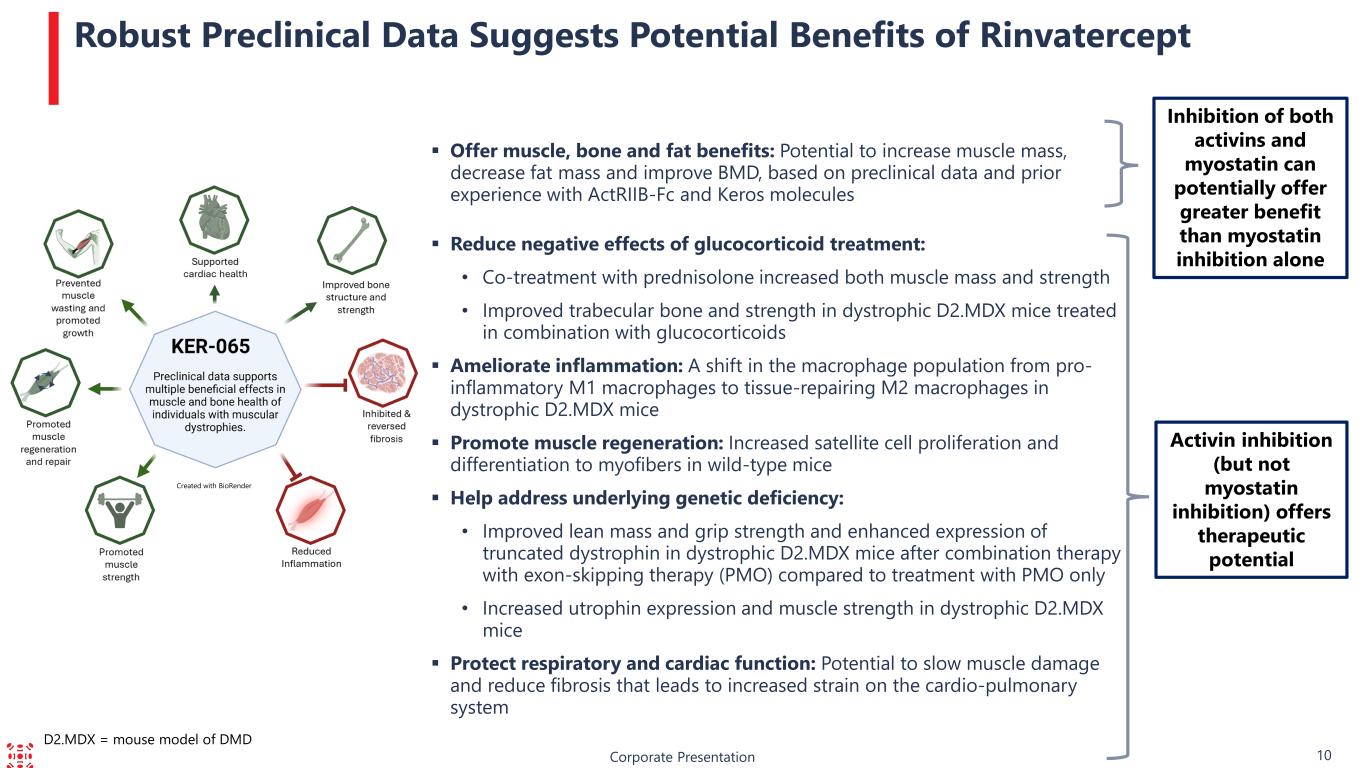
Corporate Presentation 10 Robust Preclinical Data Suggests Potential Benefits of Rinvatercept Activin inhibition (but not myostatin inhibition) offers therapeutic potential Offer muscle, bone and fat benefits: Potential to increase muscle mass, decrease fat mass and improve BMD, based on preclinical data and prior experience with ActRIIB-Fc and Keros molecules Reduce negative effects of glucocorticoid treatment: • Co-treatment with prednisolone increased both muscle mass and strength • Improved trabecular bone and strength in dystrophic D2.MDX mice treated in combination with glucocorticoids Ameliorate inflammation: A shift in the macrophage population from pro- inflammatory M1 macrophages to tissue-repairing M2 macrophages in dystrophic D2.MDX mice Promote muscle regeneration: Increased satellite cell proliferation and differentiation to myofibers in wild-type mice Help address underlying genetic deficiency: • Improved lean mass and grip strength and enhanced expression of truncated dystrophin in dystrophic D2.MDX mice after combination therapy with exon-skipping therapy (PMO) compared to treatment with PMO only • Increased utrophin expression and muscle strength in dystrophic D2.MDX mice Protect respiratory and cardiac function: Potential to slow muscle damage and reduce fibrosis that leads to increased strain on the cardio-pulmonary system Inhibition of both activins and myostatin can potentially offer greater benefit than myostatin inhibition alone D2.MDX = mouse model of DMD Created with BioRender

Corporate Presentation 11 Treatment with Rinvatercept Was Generally Well Tolerated in Phase 1 Healthy Volunteer Trial Most TEAEs were mild (Grade 1) to moderate (Grade 2) ▸ One unrelated Grade 4 TEAE (transient CK elevation)* was observed No dose-limiting toxicities or serious adverse events were observed ▸No bleeding events/telangiectasias were observed ▸All injection site reactions (except two Grade 3 injection site erythema AEs) were non-severe; all resolved without sequelae ▸All headache AEs (except one Grade 2) were mild; all resolved without sequelae Observed increases in hemoglobin were asymptomatic and reversible Natarajan, H. et al, ASBMR 2025; AE grading was based on the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.1 (July 2017). CK = creatine kinase , TEAE = Treatment-emergent adverse event *Grade 4 AE in participant receiving rinvatercept 2.0 mg/kg: CK elevation to ~17,000 that was unrelated to study drug. Participant had recently undergone 45 minutes of weightlifting. Symptoms only of mild biceps soreness after curls. CK decreased by 50% within 2 days and resolved without sequelae within 2 weeks. Participant received a subsequent dose of rinvatercept and did not experience a CK elevation. We completed a randomized, double-blind, placebo-controlled, two-part Phase 1 clinical trial to evaluate single (1.0 mg/kg, 3.0 mg/kg and 5.0 mg/kg) and multiple (1.25 mg/kg and 2.0 mg/kg) ascending doses of rinvatercept in healthy volunteers. The primary objectives of this trial were to assess safety, tolerability and pharmacokinetics of rinvatercept. Exploratory endpoints included assessments of the pharmacodynamic effect on bone, adipose, muscle, cardiac tissue and fibrosis
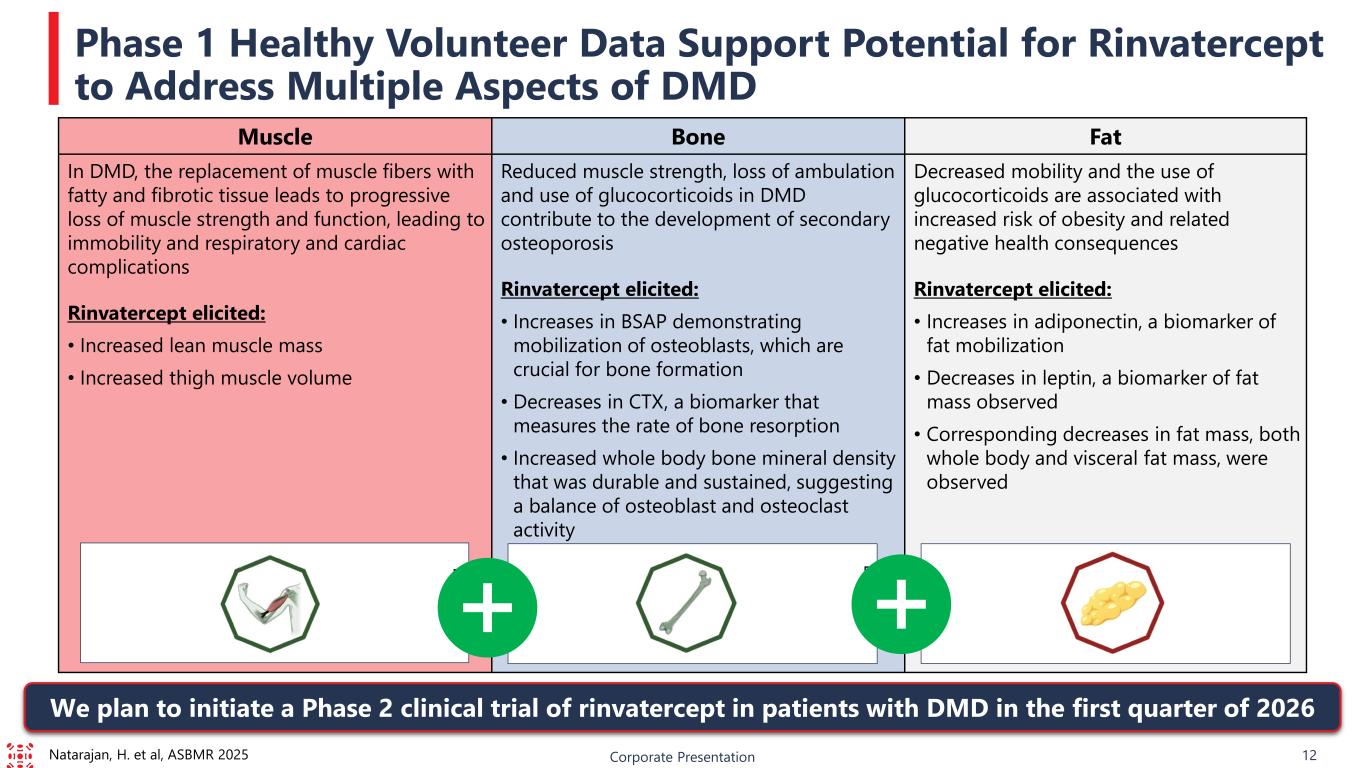
Corporate Presentation 12 Phase 1 Healthy Volunteer Data Support Potential for Rinvatercept to Address Multiple Aspects of DMD Muscle Bone Fat In DMD, the replacement of muscle fibers with fatty and fibrotic tissue leads to progressive loss of muscle strength and function, leading to immobility and respiratory and cardiac complications Rinvatercept elicited: • Increased lean muscle mass • Increased thigh muscle volume Reduced muscle strength, loss of ambulation and use of glucocorticoids in DMD contribute to the development of secondary osteoporosis Rinvatercept elicited: • Increases in BSAP demonstrating mobilization of osteoblasts, which are crucial for bone formation • Decreases in CTX, a biomarker that measures the rate of bone resorption • Increased whole body bone mineral density that was durable and sustained, suggesting a balance of osteoblast and osteoclast activity Decreased mobility and the use of glucocorticoids are associated with increased risk of obesity and related negative health consequences Rinvatercept elicited: • Increases in adiponectin, a biomarker of fat mobilization • Decreases in leptin, a biomarker of fat mass observed • Corresponding decreases in fat mass, both whole body and visceral fat mass, were observed Natarajan, H. et al, ASBMR 2025 We plan to initiate a Phase 2 clinical trial of rinvatercept in patients with DMD in the first quarter of 2026
Corporate Presentation Rinvatercept: Amyotrophic Lateral Sclerosis (ALS) 13
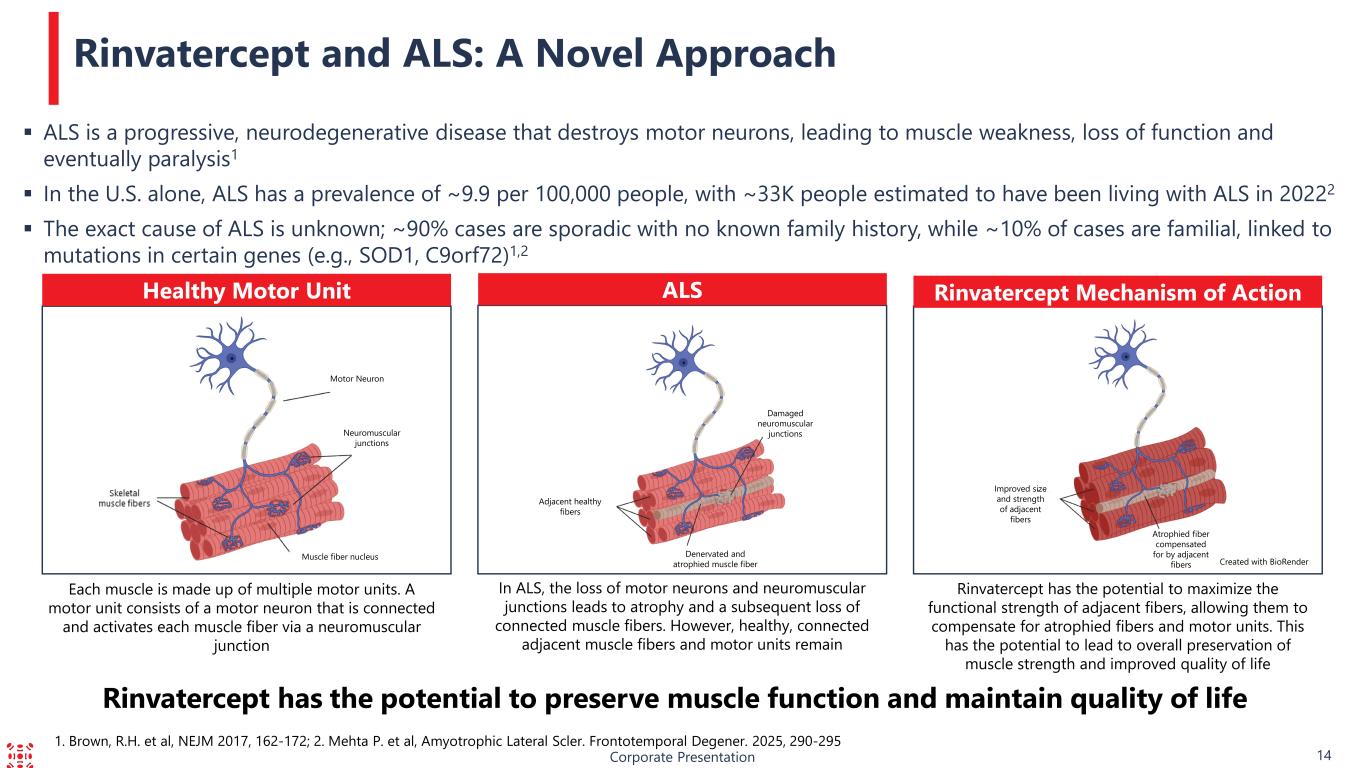
1. Brown, R.H. et al, NEJM 2017, 162-172; 2. Mehta P. et al, Amyotrophic Lateral Scler. Frontotemporal Degener. 2025, 290-295 ALS is a progressive, neurodegenerative disease that destroys motor neurons, leading to muscle weakness, loss of function and eventually paralysis1 In the U.S. alone, ALS has a prevalence of ~9.9 per 100,000 people, with ~33K people estimated to have been living with ALS in 20222 The exact cause of ALS is unknown; ~90% cases are sporadic with no known family history, while ~10% of cases are familial, linked to mutations in certain genes (e.g., SOD1, C9orf72)1,2 Rinvatercept and ALS: A Novel Approach Neuromuscular junctions Healthy Motor Unit ALS Rinvatercept Mechanism of Action Corporate Presentation 14 Rinvatercept has the potential to preserve muscle function and maintain quality of life Damaged neuromuscular junctions Motor Neuron Muscle fiber nucleus Adjacent healthy fibers Denervated and atrophied muscle fiber Improved size and strength of adjacent fibers Atrophied fiber compensated for by adjacent fibers Created with BioRender Each muscle is made up of multiple motor units. A motor unit consists of a motor neuron that is connected and activates each muscle fiber via a neuromuscular junction In ALS, the loss of motor neurons and neuromuscular junctions leads to atrophy and a subsequent loss of connected muscle fibers. However, healthy, connected adjacent muscle fibers and motor units remain Rinvatercept has the potential to maximize the functional strength of adjacent fibers, allowing them to compensate for atrophied fibers and motor units. This has the potential to lead to overall preservation of muscle strength and improved quality of life
Corporate Presentation 15 Third-Party Product Candidate (Apitegromab) Phase 3 Data Demonstrated That Muscle Preservation Can Provide Benefit 1. Crawford, T. et al; Lancet Neurology 2025, v.24, 9, 727-739 HFMSE = Hammersmith Functional Motor Scale Expanded Motor neuron loss is a hallmark of both spinal muscular atrophy (SMA) and ALS ▸ Apitegromab, a third-party product candidate being developed for the treatment of SMA, established proof-of-concept that preserving muscle can provide clinically meaningful benefit in patients with SMA ▸ We believe the data from the SAPPHIRE Phase 3 trial of apitegromab in SMA support that muscle-targeted agents can preserve muscle function even when neuronal degeneration is slowed or persists1 ▸ Functional gains were observed (e.g., significant HFMSE improvement, p=0.0192) ▸ Based on the apitegromab Phase 3 data, we believe rinvatercept could potentially sustain function in still-innervated muscle fibers in patients with ALS ALS trials have generally focused on preserving or reversing the motor neuron loss. With rinvatercept, we plan to target the skeletal muscle to potentially preserve the strength of innervated muscle and provide quality of life benefit
Potential Benefits of Rinvatercept Treatment for Individuals with ALS Corporate Presentation 16 • Skeletal muscle is potentially an active participant in ALS pathology, instead of a passive victim of motor neuron degeneration1 • By promoting muscle growth and function, rinvatercept has the potential to: ▸Counteract muscle atrophy ▸Preserve the neuromuscular junction ▸Reduce inflammation ▸Enhance muscle regeneration Based on the clinical and preclinical data we have generated, we plan to engage regulators on the design of a Phase 2 clinical trial evaluating rinvatercept in patients with ALS in the second half of 2026 1. Duranti, E. The Role of Skeletal Muscle in Amyotrophic Lateral Sclerosis: State of the Art 2025. Muscles 2025 v.4, 22.
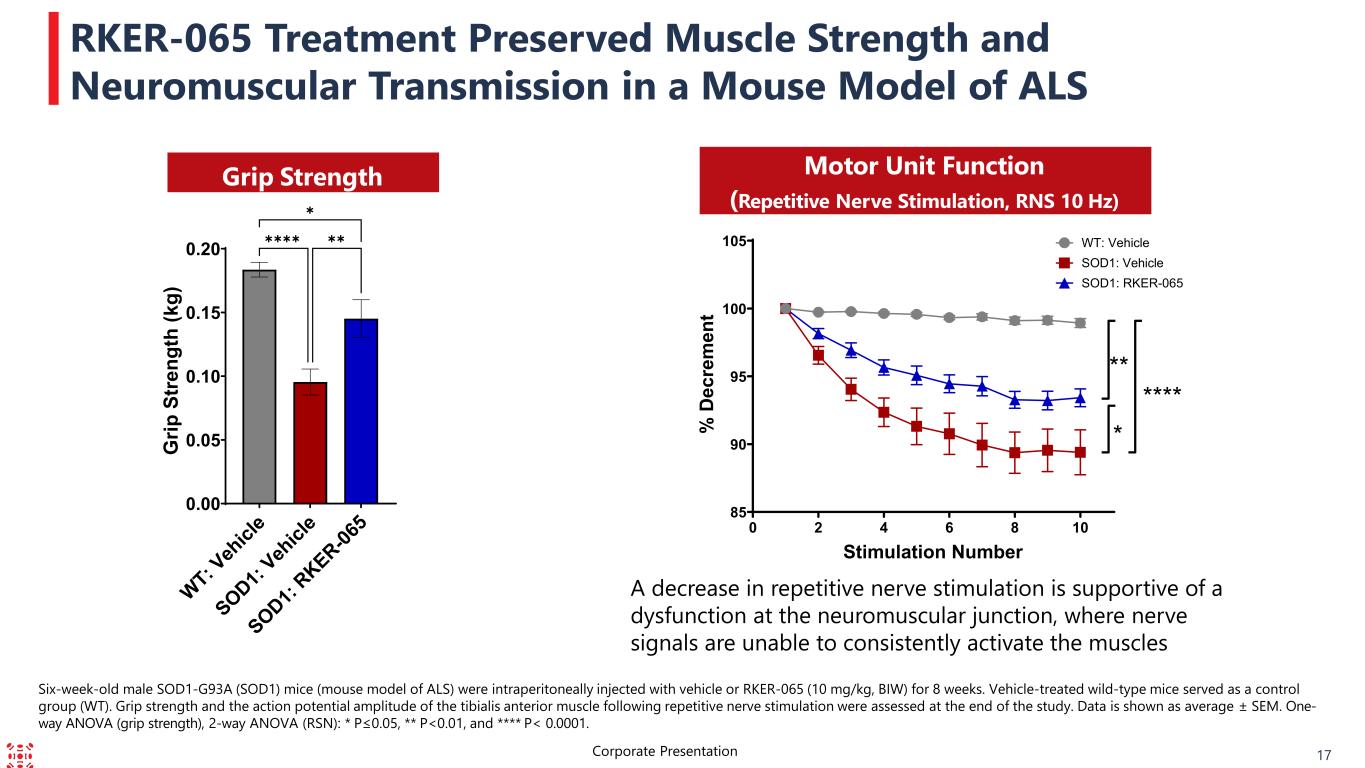
RKER-065 Treatment Preserved Muscle Strength and Neuromuscular Transmission in a Mouse Model of ALS Six-week-old male SOD1-G93A (SOD1) mice (mouse model of ALS) were intraperitoneally injected with vehicle or RKER-065 (10 mg/kg, BIW) for 8 weeks. Vehicle-treated wild-type mice served as a control group (WT). Grip strength and the action potential amplitude of the tibialis anterior muscle following repetitive nerve stimulation were assessed at the end of the study. Data is shown as average ± SEM. One- way ANOVA (grip strength), 2-way ANOVA (RSN): * P≤0.05, ** P<0.01, and **** P< 0.0001. 17Corporate Presentation Grip Strength Motor Unit Function (Repetitive Nerve Stimulation, RNS 10 Hz) WT: V eh icl e SOD1: Veh icl e SOD1: RKER-06 5 0.00 0.05 0.10 0.15 0.20 G rip S tr en gt h (k g) ✱✱✱✱ ✱ ✱✱ 0 2 4 6 8 10 85 90 95 100 105 Stimulation Number % D ec re m en t **** ** * WT: Vehicle SOD1: Vehicle SOD1: RKER-065 A decrease in repetitive nerve stimulation is supportive of a dysfunction at the neuromuscular junction, where nerve signals are unable to consistently activate the muscles
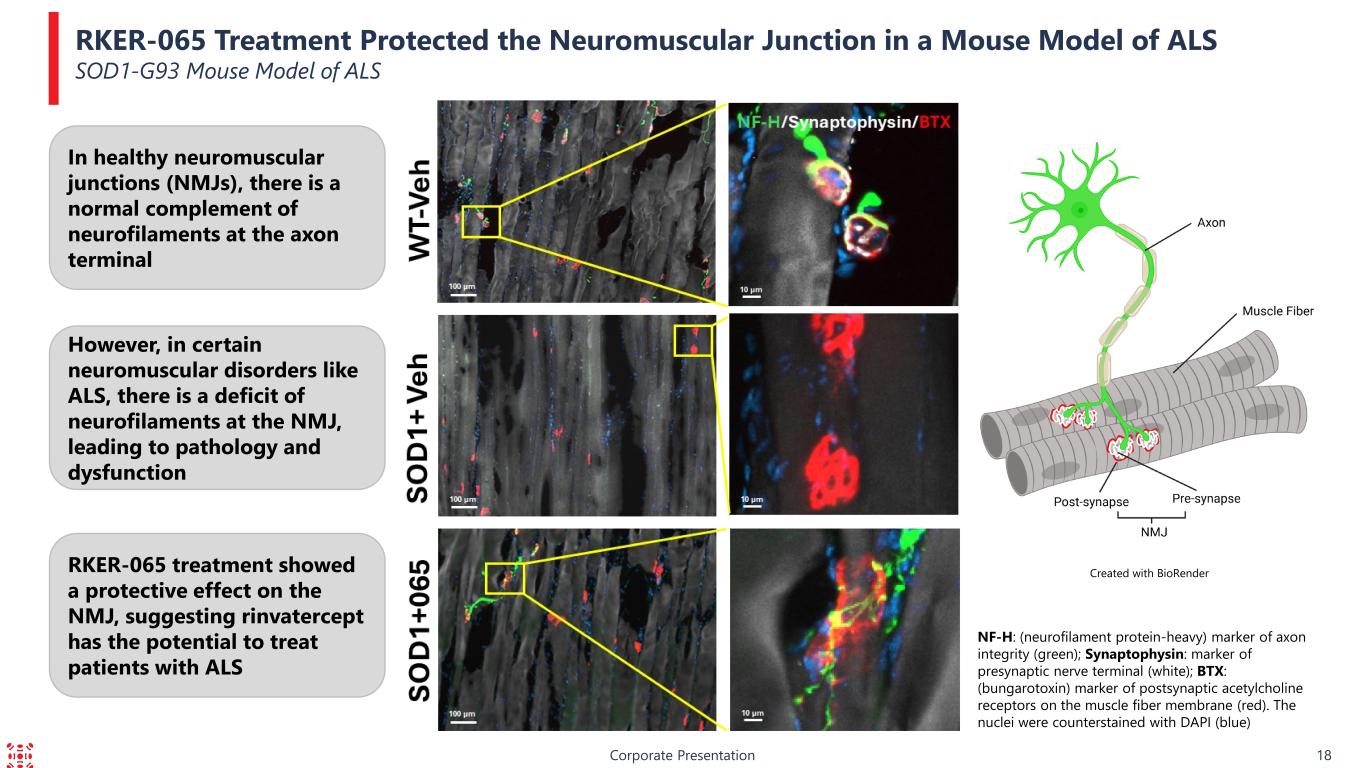
In healthy neuromuscular junctions (NMJs), there is a normal complement of neurofilaments at the axon terminal RKER-065 Treatment Protected the Neuromuscular Junction in a Mouse Model of ALS SOD1-G93 Mouse Model of ALS Corporate Presentation 18 NF-H: (neurofilament protein-heavy) marker of axon integrity (green); Synaptophysin: marker of presynaptic nerve terminal (white); BTX: (bungarotoxin) marker of postsynaptic acetylcholine receptors on the muscle fiber membrane (red). The nuclei were counterstained with DAPI (blue) Created with BioRender However, in certain neuromuscular disorders like ALS, there is a deficit of neurofilaments at the NMJ, leading to pathology and dysfunction RKER-065 treatment showed a protective effect on the NMJ, suggesting rinvatercept has the potential to treat patients with ALS
Corporate Presentation 19 Elritercept (KER-050) Investigational Treatment for Anemia and Thrombocytopenia in Patients with Myelodysplastic Syndromes and in Patients with Myelofibrosis

Corporate Presentation Global License Agreement with Takeda Pharmaceuticals On December 3, 2024, Keros announced it had entered into an exclusive license agreement with Takeda to develop, manufacture and commercialize elritercept globally, other than mainland China, Hong Kong, and Macau Financials: • Keros received an upfront payment of $200 million • Eligible to receive development, approval and commercial milestone payments of over $1.1 billion • Development: $90M ($10M received in 2025 for Phase 3 RENEW clinical trial first patient dosed) • Commercial: $280M • Sales: $740M • Tiered royalties on net sales in the low double-digits to high teens Under the terms of the agreement, in January 2025, Takeda became responsible for all clinical development, manufacturing and commercialization of elritercept in its territory 20
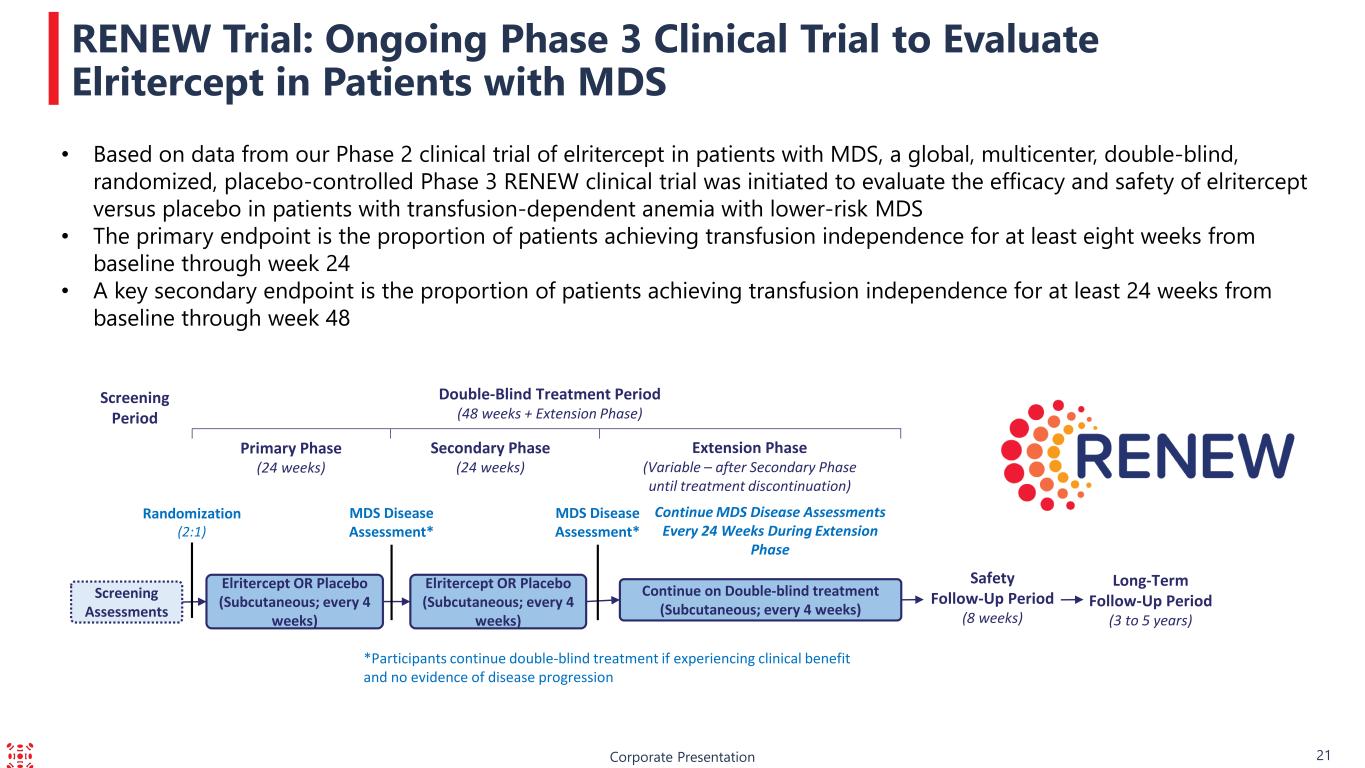
Corporate Presentation RENEW Trial: Ongoing Phase 3 Clinical Trial to Evaluate Elritercept in Patients with MDS Screening Assessments Screening Period Double-Blind Treatment Period (48 weeks + Extension Phase) Safety Follow-Up Period (8 weeks) Extension Phase (Variable – after Secondary Phase until treatment discontinuation) Primary Phase (24 weeks) Elritercept OR Placebo (Subcutaneous; every 4 weeks) Continue on Double-blind treatment (Subcutaneous; every 4 weeks) Secondary Phase (24 weeks) Elritercept OR Placebo (Subcutaneous; every 4 weeks) Randomization (2:1) MDS Disease Assessment* MDS Disease Assessment* Continue MDS Disease Assessments Every 24 Weeks During Extension Phase *Participants continue double-blind treatment if experiencing clinical benefit and no evidence of disease progression Long-Term Follow-Up Period (3 to 5 years) • Based on data from our Phase 2 clinical trial of elritercept in patients with MDS, a global, multicenter, double-blind, randomized, placebo-controlled Phase 3 RENEW clinical trial was initiated to evaluate the efficacy and safety of elritercept versus placebo in patients with transfusion-dependent anemia with lower-risk MDS • The primary endpoint is the proportion of patients achieving transfusion independence for at least eight weeks from baseline through week 24 • A key secondary endpoint is the proportion of patients achieving transfusion independence for at least 24 weeks from baseline through week 48 21

Corporate Presentation Proprietary Discovery Approach 22

Proprietary Discovery Approach • Keros has a broad approach to target the TGF-β superfamily to develop product candidates with the potential to treat a broad range of indications, including neuromuscular and neurodegenerative disorders, rare bone and fibrosis diseases and obesity/frailty • Large library of agonists and antagonists • Proprietary library of modified ActRII ligand traps • Mono, bi and multimodal antagonist • Systemically deliverable ligands • Multiple candidates in preclinical development Rinvatercept and elritercept were both nominated from our proprietary library of modified ActRII ligand traps for clinical development Corporate Presentation 23