
2026 J.P. MORGAN HEALTHCARE CONFERENCE GENERATIVE AI DRUG CREATION

Disclaimers F O R W A R D - L O O K I N G S T A T E M E N T S Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding any or all of the following: (i) Absci’s preclinical studies, clinical trials, as well as partnered and internally developed programs, including, without limitation, manufacturing capabilities, status of such studies and trials and expectations regarding data, safety and efficacy generally; (ii) data included in the above-described oral presentation, as well as the ability to use data from ongoing and planned clinical trials for the design and initiation of further clinical trials; (iii) Absci’s strategy, goals, anticipated financial performance and the sufficiency of its cash resources; (iv) regulatory submissions and authorizations, including timelines for and expectations regarding any anticipated regulatory agency decisions; (v) the expected benefits of its collaborations with partners; and (vi) the therapeutic value, development, and commercial potential of antibody therapies, as well as other technologies. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation, the risks and uncertainties discussed under the heading “Risk Factors” in Absci Corporation’s most recent annual report on Form 10- K and in any other subsequent filings made by Absci Corporation with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. We disclaim any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law. 1 M A R K E T A N D S T A T I S T I C A L I N F O R M A T I O N This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other industry data. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the data generated by independent parties and cannot guarantee their accuracy or completeness. 2 T R A D E M A R K U S A G E This presentation/document/webpage contains references to our trademarks and service marks and to those belonging to third parties. Absci®, ®, SoluPro®, Bionic SoluPro® and SoluPure® are Absci registered trademarks with the U.S. Patent and Trademark Office. We also use various other trademarks, service marks and trade names in our business, including the Absci AI logo mark ( ), the Unlimit with us mark ( ), Denovium, Integrated Drug Creation, HiPrBind, and IgDesign. All other trademarks, service marks or trade names referred to in this presentation/document/webpage are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation/document/webpage may be referred to with or without the trademark symbols, but references which omit the symbols should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto 3 COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 2 G E N E R A T IV E A I D R U G C R E A T I O N
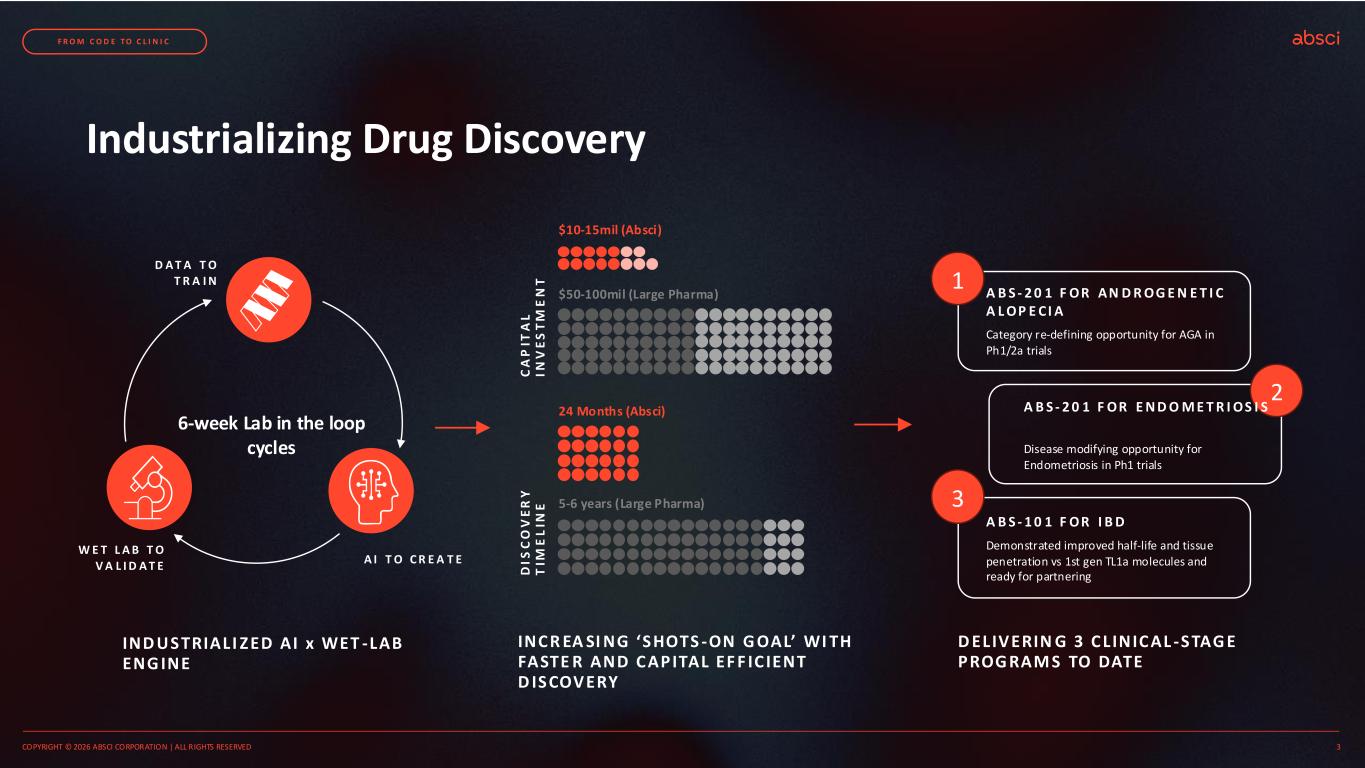
Industrializing Drug Discovery INC R EA SI NG ‘S HOTS -ON G OAL’ WI TH FASTE R AND C APITAL EF F IC IENT D ISCOVERY $10-15mil (Absci) $50-100mil (Large Pharma) 24 Months (Absci) 5-6 years (Large Pharma) C A P IT A L IN V E S T M E N T D IS C O V E R Y T IM E L IN E D ELIV ERIN G 3 C L INIC AL -S TAG E PROG RAM S TO DATE 1 2 3 A B S - 2 0 1 F OR AN D R OG E N E T I C A L OP E CI A A B S - 2 0 1 F OR E N D O M E T R I OS I S A B S - 1 0 1 F OR I B D Category re-defining opportunity for AGA in Ph1/2a trials Disease modifying opportunity for Endometriosis in Ph1 trials Demonstrated improved half-life and tissue penetration vs 1st gen TL1a molecules and ready for partnering COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 3 F R O M C O D E T O C L I N I C IND USTRIALI ZED AI x WET -LAB ENG INE 6-week Lab in the loop cycles D A T A T O T R A I N W E T L A B T O V A L I D A T E A I T O C R E A T E

R A I S I N G T H E B A R O N D E N O V O D E S I G N Absci is the first validated platform to de novo design full length antibodies against ‘zero- prior’ epitopes with atomic accuracy COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 4 SCAN QR CODE TO DOWNLOAD THE MANUSCRIPT F R O M C O D E T O C L I N I C
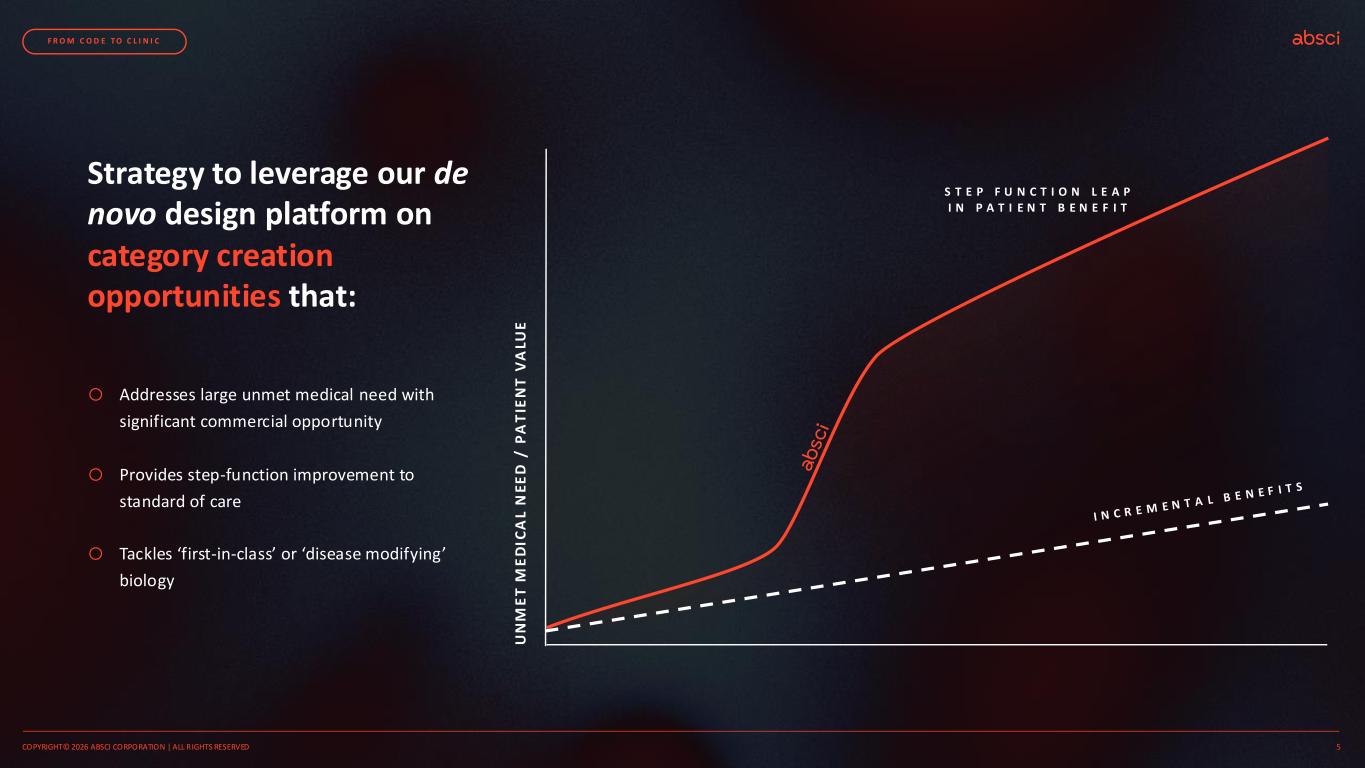
U N M E T M E D IC A L N E E D / P A T IE N T V A LU E Strategy to leverage our de novo design platform on category creation opportunities that: S T E P F U N C T I O N L E A P I N P A T I E N T B E N E F I T o Addresses large unmet medical need with significant commercial opportunity o Provides step-function improvement to standard of care o Tackles ‘first-in-class’ or ‘disease modifying’ biology COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 5 F R O M C O D E T O C L I N I C

ABS-201 has the potential to unlock a wholly new category of therapy in hair “re-growth” Significant clinical and commercial unmet need in androgenetic alopecia1. Strong scientific rationale, with validated target, de-risked Mode of Action, and pharmacology2. 3. COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 6 A B S - 2 0 1 Straightforward development path with objective endpoints
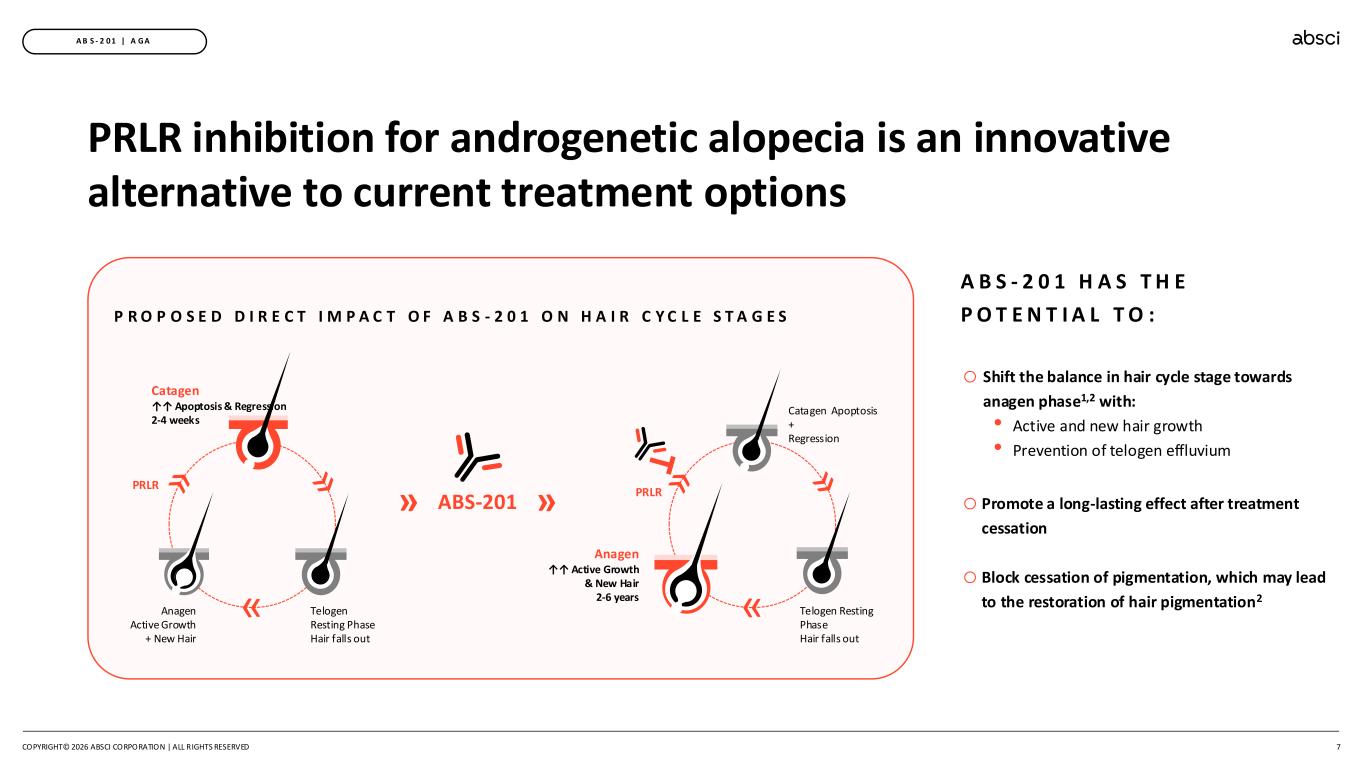
7 A B S - 2 0 1 | A GA PRLR inhibition for androgenetic alopecia is an innovative alternative to current treatment options P R O P O S E D D I R E C T I M P A C T O F A B S - 2 0 1 O N H A I R C Y C L E S T A G E S Telogen Resting Phase Hair falls out Catagen ↑↑ Apoptosis & Regression 2-4 weeks Anagen Active Growth + New Hair » PRLR ABS-201 »» Anagen ↑↑ Active Growth & New Hair 2-6 years Catagen Apoptosis + Regression Telogen Resting Phase Hair falls out » PRLR o Shift the balance in hair cycle stage towards anagen phase1,2 with: • Active and new hair growth • Prevention of telogen effluvium o Promote a long-lasting effect after treatment cessation o Block cessation of pigmentation, which may lead to the restoration of hair pigmentation2 A B S - 2 0 1 H A S T H E P O T E N T I A L T O : COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED
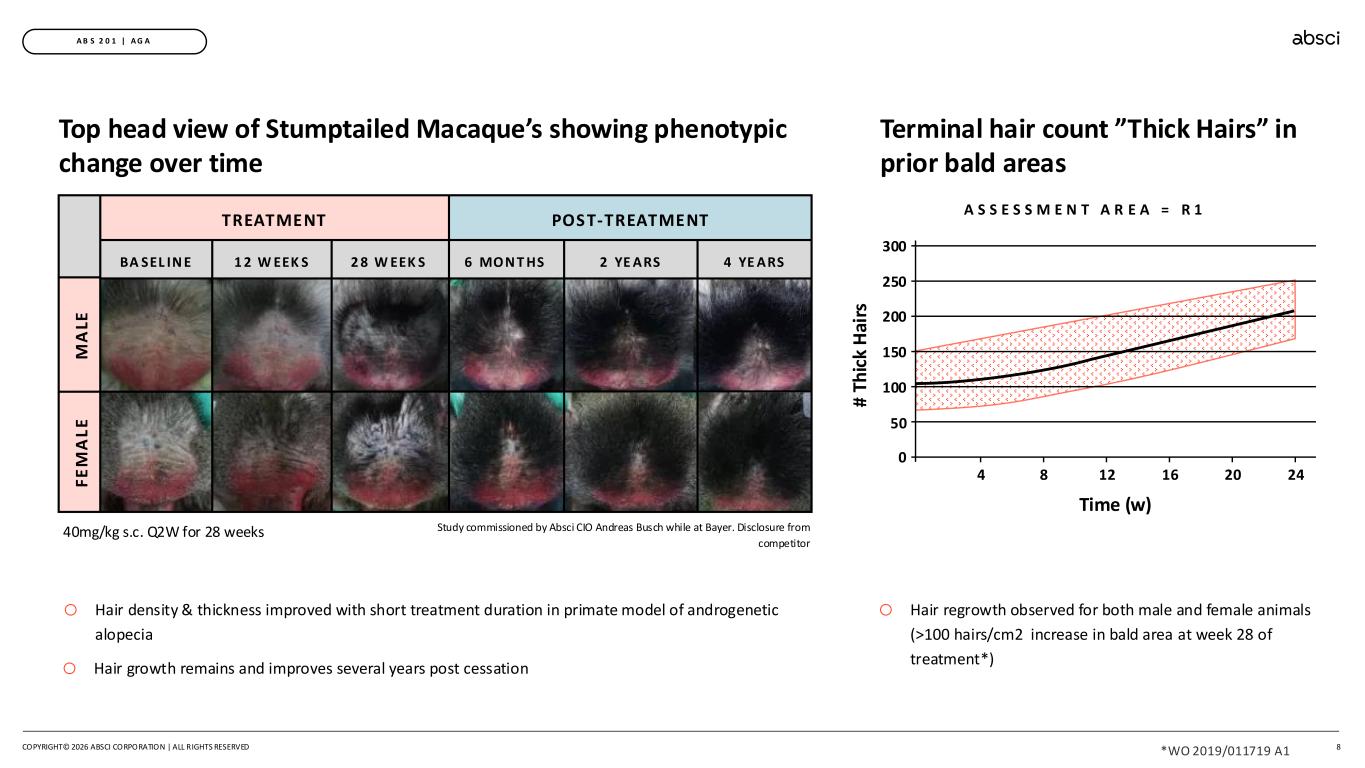
8 A B S 2 0 1 | A G A Terminal hair count ”Thick Hairs” in prior bald areas o Hair regrowth observed for both male and female animals (>100 hairs/cm2 increase in bald area at week 28 of treatment*) # Th ic k H ai rs Time (w) 200 100 0 250 150 50 300 4 8 12 16 20 24 A S S E S S M E N T A R E A = R 1 TREATMENT POST-TREATMENT 1 2 W EEK SBA SEL IN E 2 8 W EEK S 6 MON T HS 2 YE ARS 4 YE ARS F E M A L E M A LE Top head view of Stumptailed Macaque’s showing phenotypic change over time o Hair density & thickness improved with short treatment duration in primate model of androgenetic alopecia o Hair growth remains and improves several years post cessation 40mg/kg s.c. Q2W for 28 weeks Study commissioned by Absci CIO Andreas Busch while at Bayer. Disclosure from competitor COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED *WO 2019/011719 A1
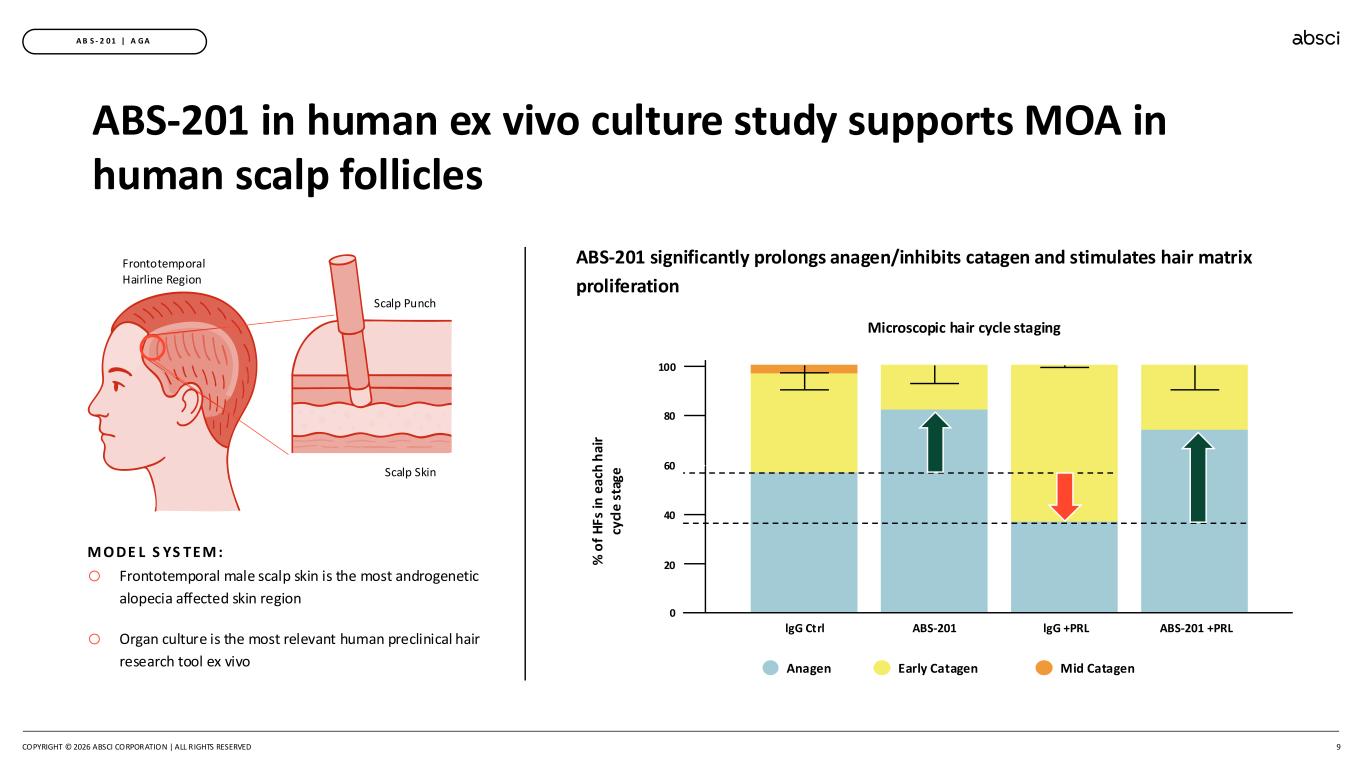
COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 9 A B S - 2 0 1 | A GA ABS-201 in human ex vivo culture study supports MOA in human scalp follicles ABS-201 significantly prolongs anagen/inhibits catagen and stimulates hair matrix proliferation Anagen Early Catagen Mid Catagen lgG Ctrl ABS-201 lgG +PRL ABS-201 +PRL 80 40 0 100 60 20 Frontotemporal Hairline Region Scalp Punch Scalp Skin o Frontotemporal male scalp skin is the most androgenetic alopecia affected skin region o Organ culture is the most relevant human preclinical hair research tool ex vivo M O DE L S YS TEM : Microscopic hair cycle staging % o f H Fs in e ac h h ai r cy cl e s ta g e
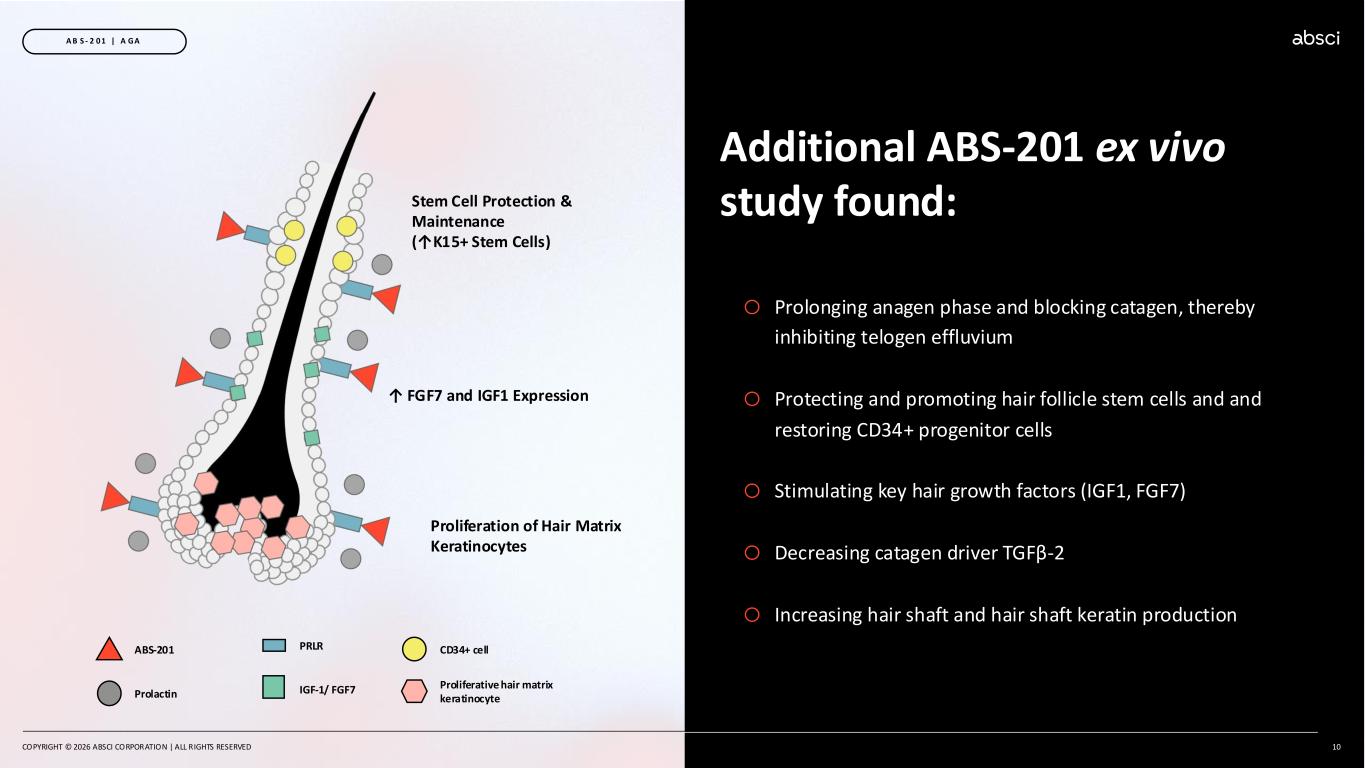
COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 10 A B S - 2 0 1 | A GA Additional ABS-201 ex vivo study found: o Prolonging anagen phase and blocking catagen, thereby inhibiting telogen effluvium o Protecting and promoting hair follicle stem cells and and restoring CD34+ progenitor cells o Stimulating key hair growth factors (IGF1, FGF7) o Decreasing catagen driver TGFβ-2 o Increasing hair shaft and hair shaft keratin production IGF-1/ FGF7 PRLRABS-201 CD34+ cell Proliferative hair matrix keratinocyteProlactin Stem Cell Protection & Maintenance (↑K15+ Stem Cells) ↑ FGF7 and IGF1 Expression Proliferation of Hair Matrix Keratinocytes
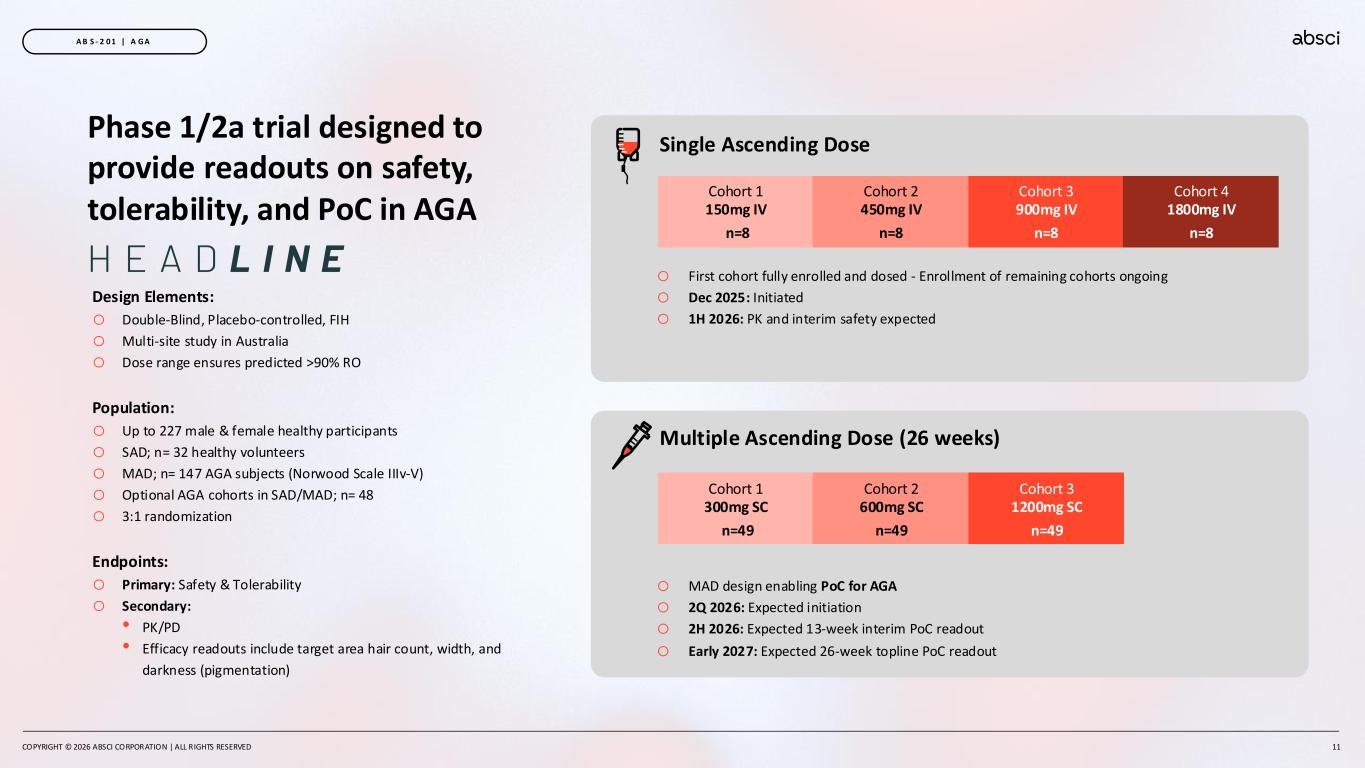
Phase 1/2a trial designed to provide readouts on safety, tolerability, and PoC in AGA Design Elements: o Double-Blind, Placebo-controlled, FIH o Multi-site study in Australia o Dose range ensures predicted >90% RO Population: o Up to 227 male & female healthy participants o SAD; n= 32 healthy volunteers o MAD; n= 147 AGA subjects (Norwood Scale IIIv-V) o Optional AGA cohorts in SAD/MAD; n= 48 o 3:1 randomization Endpoints: o Primary: Safety & Tolerability o Secondary: • PK/PD • Efficacy readouts include target area hair count, width, and darkness (pigmentation) COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 11 A B S - 2 0 1 | A GA Single Ascending Dose Cohort 1 150mg IV n=8 Cohort 2 450mg IV n=8 Cohort 3 900mg IV n=8 Cohort 4 1800mg IV n=8 Cohort 1 300mg SC n=49 Cohort 2 600mg SC n=49 Cohort 3 1200mg SC n=49 Multiple Ascending Dose (26 weeks) o First cohort fully enrolled and dosed - Enrollment of remaining cohorts ongoing o Dec 2025: Initiated o 1H 2026: PK and interim safety expected o MAD design enabling PoC for AGA o 2Q 2026: Expected initiation o 2H 2026: Expected 13-week interim PoC readout o Early 2027: Expected 26-week topline PoC readout
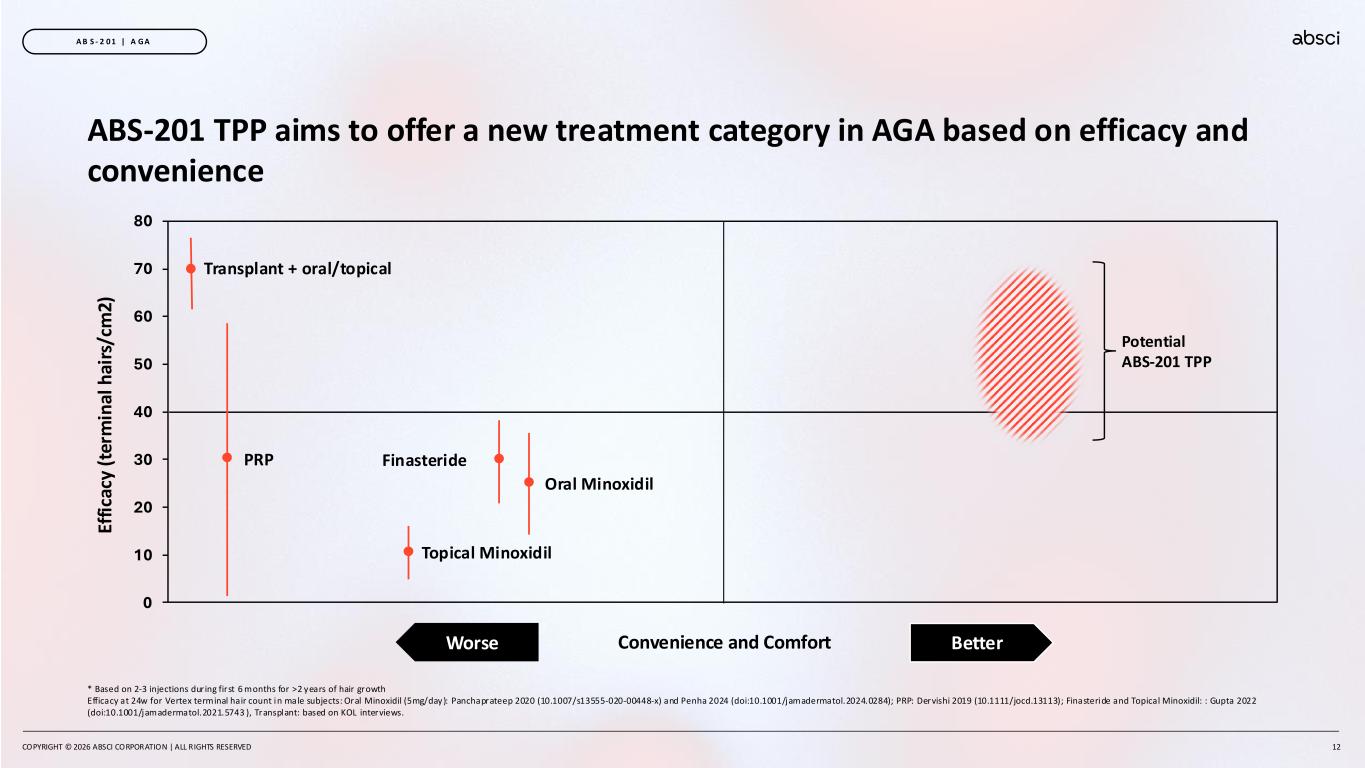
COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 12 A B S - 2 0 1 | A GA ABS-201 TPP aims to offer a new treatment category in AGA based on efficacy and convenience 0 10 20 30 40 50 60 70 80 Ef fi ca cy ( te rm in al h ai rs /c m 2) Topical Minoxidil Oral Minoxidil FinasteridePRP Transplant + oral/topical Potential ABS-201 TPP * Based on 2-3 injections during first 6 months for >2 years of hair growth Efficacy at 24w for Vertex terminal hair count in male subjects: Oral Minoxidil (5mg/day): Panchaprateep 2020 (10.1007/s13555-020-00448-x) and Penha 2024 (doi:10.1001/jamadermatol.2024.0284); PRP: Dervishi 2019 (10.1111/jocd.13113); Finasteride and Topical Minoxidil: : Gupta 2022 (doi:10.1001/jamadermatol.2021.5743 ), Transplant: based on KOL interviews. Convenience and Comfort BetterWorse

Consumer Research Validates Market Potential of ABS-201 Significant Unmet Need: Driven by psycho-social impacts (loss of confidence, self-esteem) from AGA Strong Commercial Demand: Nearly all men and roughly 90% of women are inclined to ask their doctors about ABS-201 High Value Proposition: Significant share of respondents willing to pay a premium for the ABS-201 TPP Disruptive Potential: Over 1/3 of respondents would select ABS-201 before their current treatment, suggesting ABS-201 can effectively compete as first-line therapy 610 Participants: 306 Men | 304 Women COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 13 A B S - 2 0 1 | A GA * A L L P A R T I C I P A N T S E X P E R I E N C I N G H A I R L O S S R E P O R T N EG ATI V E P S Y C H O LO G I C A L I M PA C T 80% MEN 81% WOMEN W OU LD TR Y A B S - 2 0 1 F I R ST (F I R ST L I N E) 37% MEN 36% WOMEN E XT R E M ELY OR VER Y L I K E LY TO AS K H C P A BO U T A B S - 2 0 1 UP TO 97% MEN WOMEN UP TO 88%
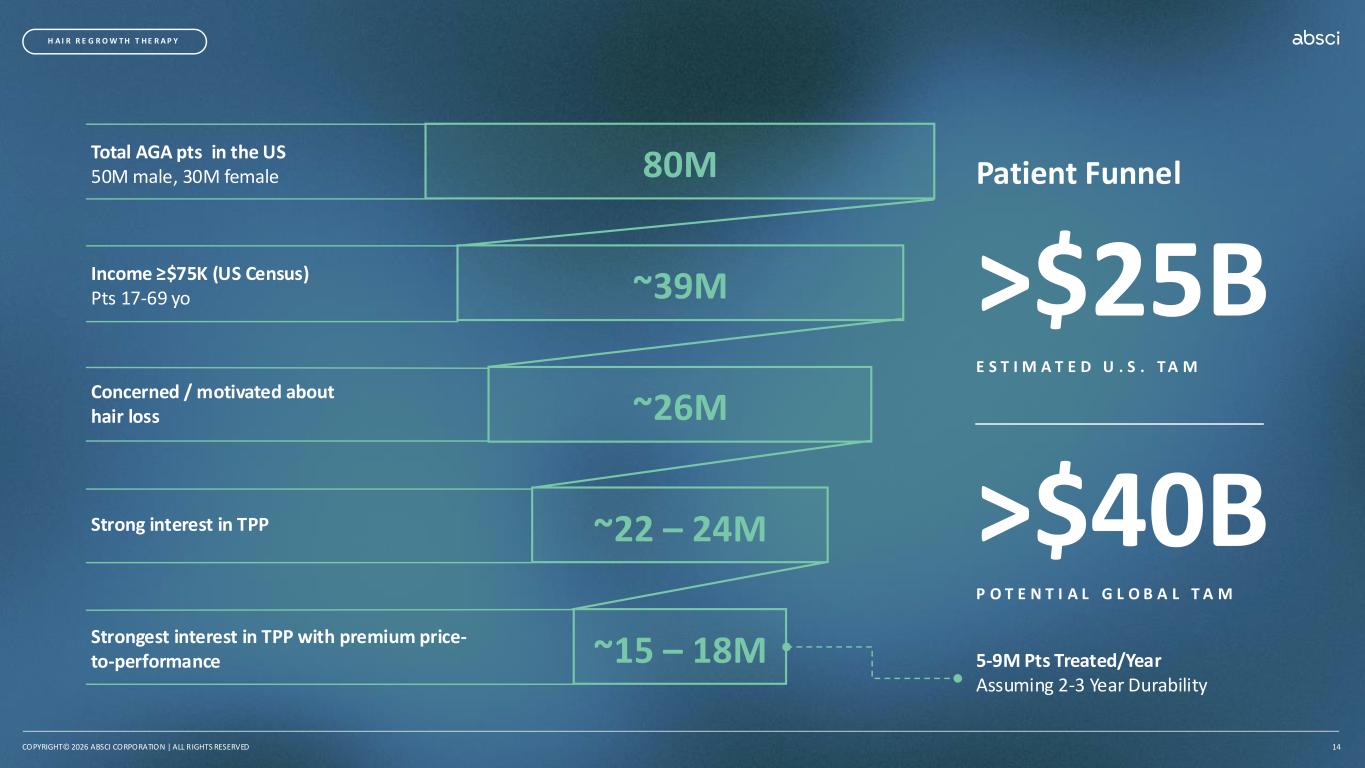
5-9M Pts Treated/Year Assuming 2-3 Year Durability Patient Funnel >$25B E S T I M A T E D U . S . T A M >$40B P O T E N T I A L G L O B A L T A M Total AGA pts in the US 50M male, 30M female Income ≥$75K (US Census) Pts 17-69 yo Concerned / motivated about hair loss Strong interest in TPP Strongest interest in TPP with premium price- to-performance COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 14 H A I R R E G R O W T H T H E R A P Y 80M ~39M ~26M ~22 – 24M ~15 – 18M

Development of ABS-201 in Endometriosis Addresses Long-standing Unmet Medical Need and Poor standard of care1. Large, untapped market offers significant upside potential 2. Strong Biological And Clinical Rationale: Including POC for PRLR mechanism in humans 3. COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 15 A B S 2 0 1 | E N D O M E T R I O S I S
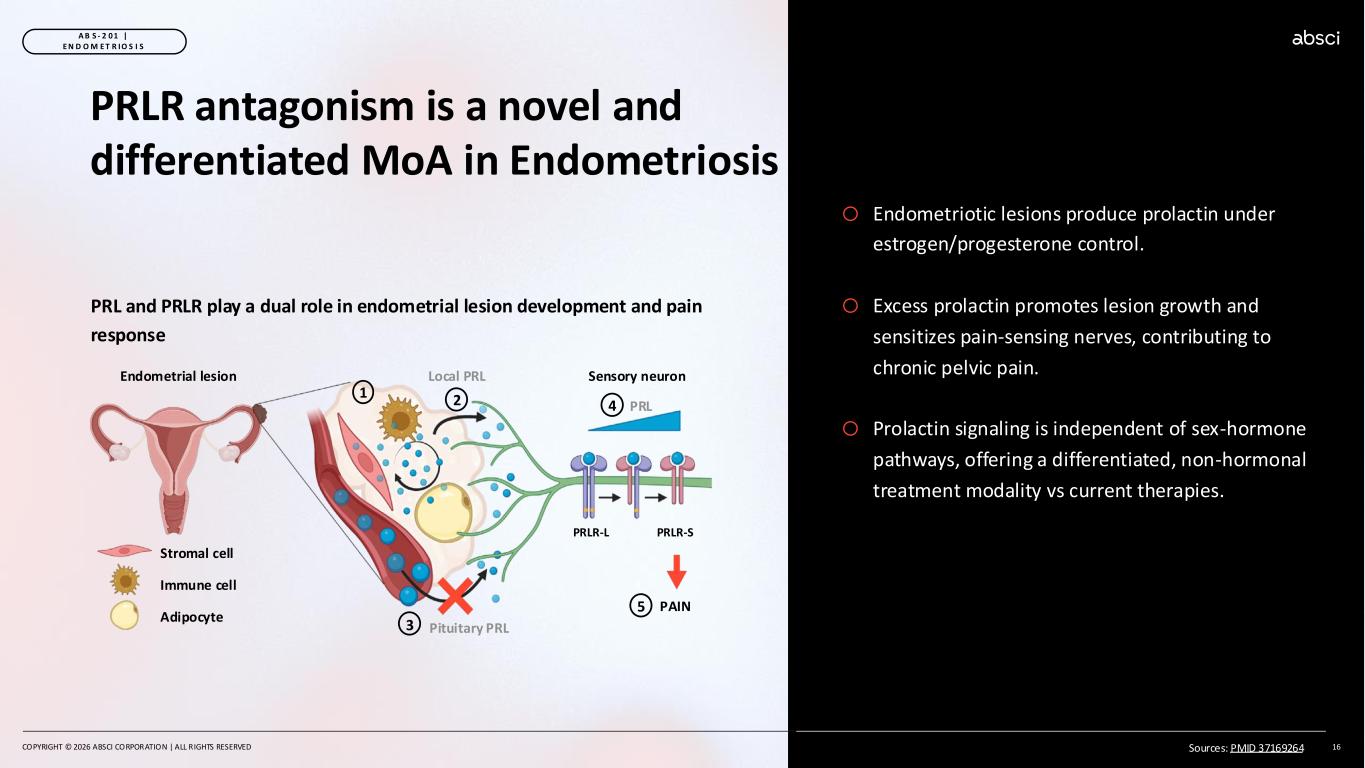
o Endometriotic lesions produce prolactin under estrogen/progesterone control. o Excess prolactin promotes lesion growth and sensitizes pain-sensing nerves, contributing to chronic pelvic pain. o Prolactin signaling is independent of sex-hormone pathways, offering a differentiated, non-hormonal treatment modality vs current therapies. COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 16 A B S - 2 0 1 | E N D O M E T R IO S I S PRLR antagonism is a novel and differentiated MoA in Endometriosis PRL and PRLR play a dual role in endometrial lesion development and pain response Stromal cell Immune cell Adipocyte PRL 1 2 Endometrial lesion Local PRL PRLR-L PRLR-S Sensory neuron PAIN Pituitary PRL3 5 4 Sources: PMID 37169264
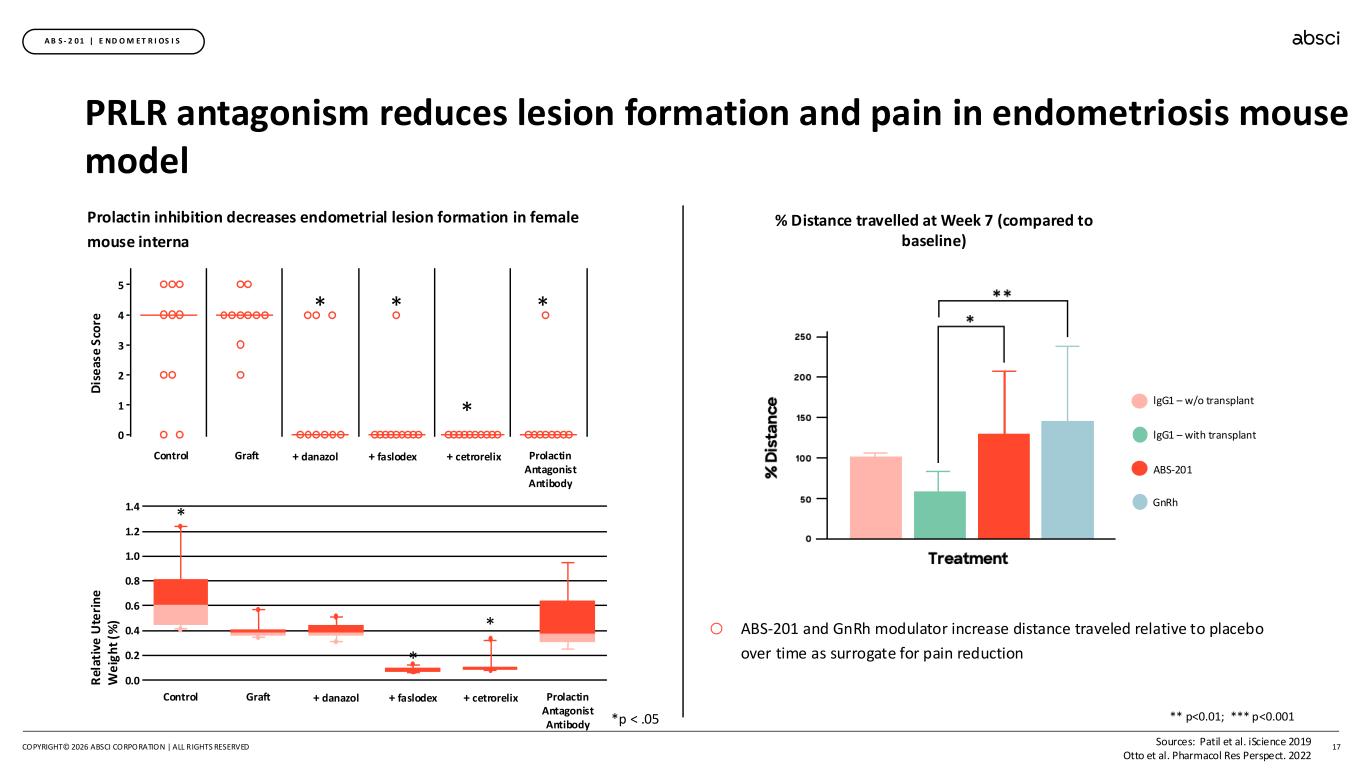
PRLR antagonism reduces lesion formation and pain in endometriosis mouse model 17 A B S - 2 0 1 | E ND O M E T R I OS I S COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED * 1 2 3 4 5 0 * * * D is e as e S co re Control Graft + danazol + faslodex + cetrorelix Prolactin Antagonist Antibody Prolactin inhibition decreases endometrial lesion formation in female mouse interna R el at iv e U te ri n e W e ig h t (% ) 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 * Control Graft + danazol + faslodex + cetrorelix Prolactin Antagonist Antibody * * Sources: Patil et al. iScience 2019 Otto et al. Pharmacol Res Perspect. 2022 % Distance travelled at Week 7 (compared to baseline) lgG1 – w/o transplant lgG1 – with transplant ABS-201 GnRh o ABS-201 and GnRh modulator increase distance traveled relative to placebo over time as surrogate for pain reduction *p < .05 ** p<0.01; *** p<0.001
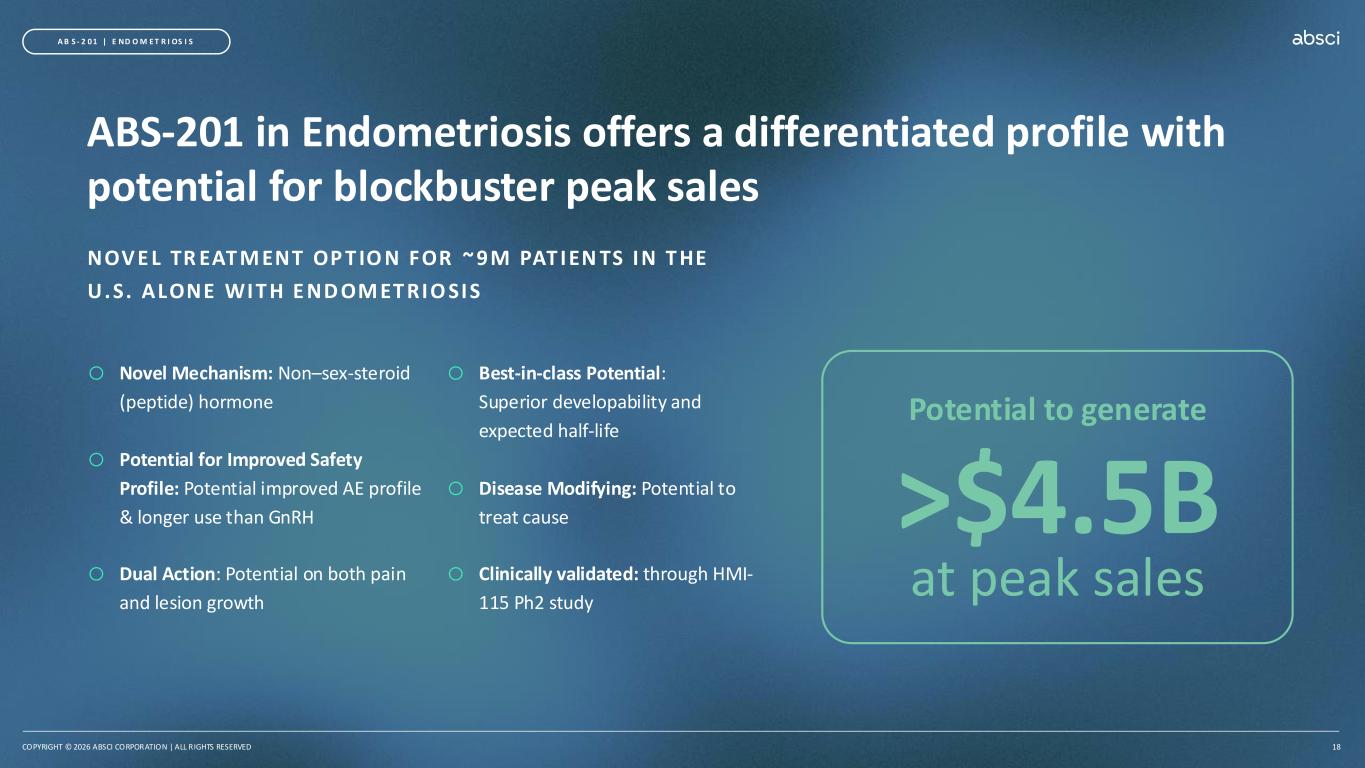
COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 18 >$4.5B at peak sales Potential to generate A B S - 2 0 1 | E ND O M E T R I OS I S ABS-201 in Endometriosis offers a differentiated profile with potential for blockbuster peak sales NOV E L TR EAT M ENT OP T IO N FOR ~ 9M PAT IEN TS IN T HE U.S. ALONE WIT H E ND OM ET RIO SIS o Novel Mechanism: Non–sex-steroid (peptide) hormone o Potential for Improved Safety Profile: Potential improved AE profile & longer use than GnRH o Dual Action: Potential on both pain and lesion growth o Best-in-class Potential: Superior developability and expected half-life o Disease Modifying: Potential to treat cause o Clinically validated: through HMI- 115 Ph2 study
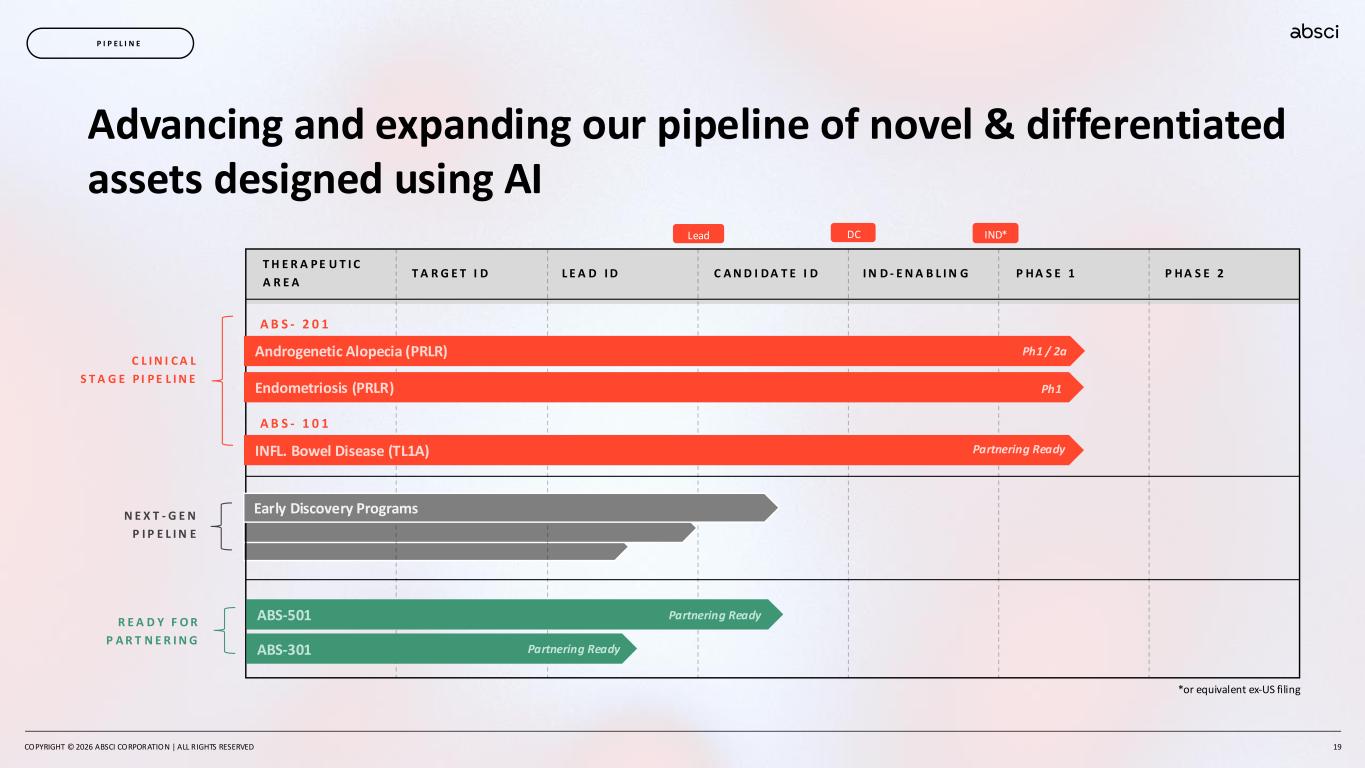
Advancing and expanding our pipeline of novel & differentiated assets designed using AI *or equivalent ex-US filing C L I N I C A L S T A G E P I P E L I N E R E A D Y F O R P A R T N E R I N G N E X T - G E N P I P E L I N E COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 19 P I P E L I N E T H E R A P E U T I C A R E A T A R G E T I D L E A D I D C A N D I D A T E I D I N D - E N A B L I N G P H A S E 1 P H A S E 2 A B S - 2 0 1 Androgenetic Alopecia (PRLR) Endometriosis (PRLR) A B S - 1 0 1 INFL. Bowel Disease (TL1A) Ph1 Partnering Ready ABS-501 ABS-301 Early Discovery Programs Partnering Ready Partnering Ready IND*DCLead Ph1 / 2a

”Multilingual” team with expertise in AI and drug creation COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 20 T E A M Sean McClain Founder, CEO & Board Director Zach Jonasson, PHD Chief Financial Officer & Chief Business Officer Amir Shanehsazzadeh SVP, Chief AI Officer Shelby Walker, JD Chief Legal Officer Karin Wierinck Chief People Officer Christine Lemke SVP, Portfolio & Growth Strategy L E A D E R S H I P T E A M : Sean McClain Founder, CEO & Board Director Sir Mene Pangalos, PhD Former EVP R&D AstraZeneca Mary Szela CEO & President TriSalus Life Sciences Joseph Sirosh, PhD Former CVP AI Microsoft Dan Rabinovitsj VP Hardware Engineering, Meta Karen McGinnis, CPA Former Chief Accounting Officer, Illumina Frans Van Houten Chairman of the Board Former CEO, Royal Phillips B O A R D O F D I R E C T O R S : Andreas Busch, PHD Co-Chair SAB Chief Innovation Officer Ian McInnes, PHD Vice Principal and Head of College University of Glasgow Luis Diaz, MD Head, Division of Solid Tumor Oncology Memorial Sloan Kettering Cancer Center John Wherry, PHD Director, Institute for Immunology & Immune Health, University of Pennsylvania Victor Greiff, PHD Associate Professor University of Oslo Hubert Truebel, MD, PHD, MBA Chief Medical Officer AiCuris Sir Mene Pangalos, PHD Co-Chair SAB Former EVP R&D AstraZeneca S C I E N T I F I C A D V I S O R Y B O A R D : Andreas Busch, PHD Chief Innovation Officer E X P E R T I S E A N D B A C K G R O U N D F R O M :
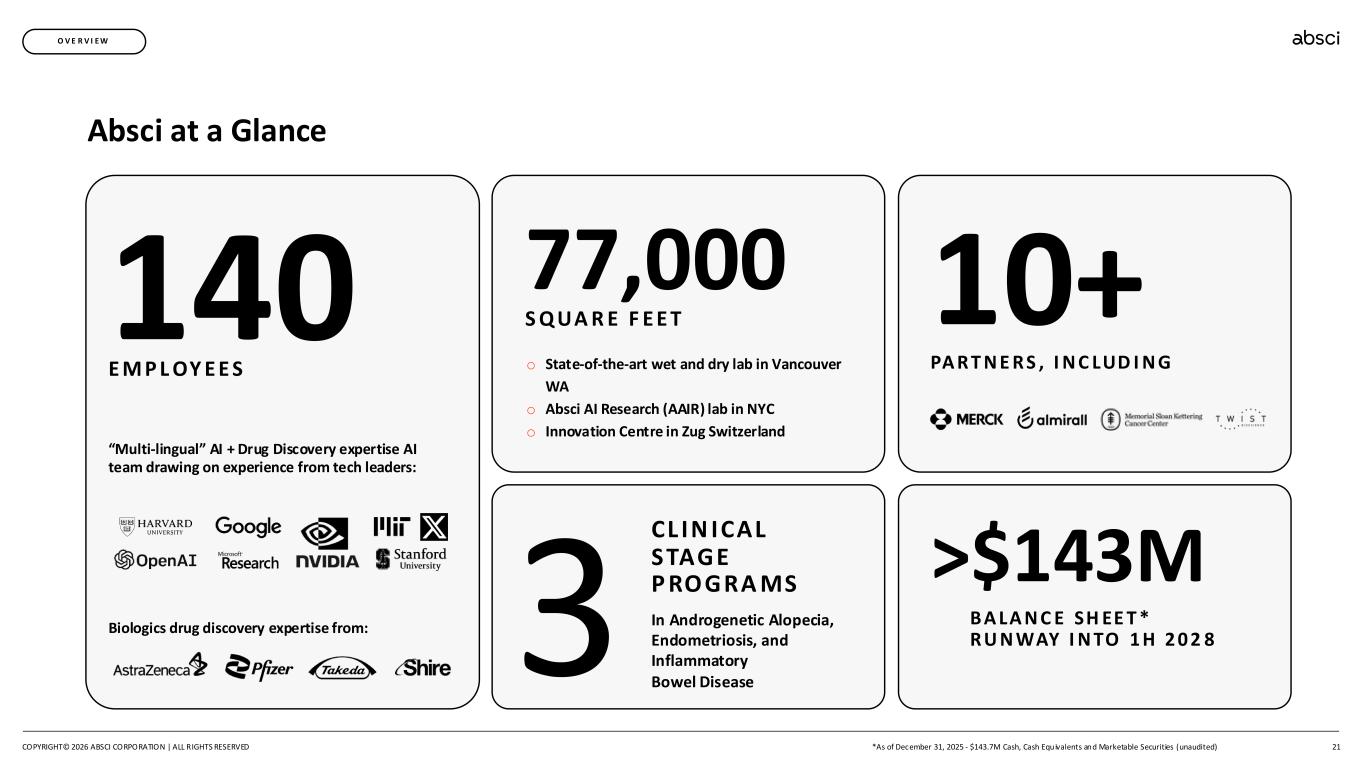
21 140 E M P L OY E E S Biologics drug discovery expertise from: “Multi-lingual” AI + Drug Discovery expertise AI team drawing on experience from tech leaders: 77,000 S Q UA R E F E E T o State-of-the-art wet and dry lab in Vancouver WA o Absci AI Research (AAIR) lab in NYC o Innovation Centre in Zug Switzerland 3 CL IN ICAL STAGE P RO GRA MS In Androgenetic Alopecia, Endometriosis, and Inflammatory Bowel Disease >$143M B A LA N C E SH EE T * R U N WAY I N TO 1 H 2 0 2 8 10+ PA R T N E R S , I N C LUD I N G Absci at a Glance O V E R V I E W COPYRIGHT© 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED *As of December 31, 2025 - $143.7M Cash, Cash Equivalents and Marketable Securities (unaudited)

Leading AI x Bio platform driving 2 Phase 2 readouts in the next 24 months COPYRIGHT © 2026 ABSCI CORPORATION | ALL RIGHTS RESERVED 22 C A T A L Y S T S ABS-20 1 in AGA o Accelerated Ph1/2a Study initiated Dec 2025 o Safety, Tolerability, and PK readout expected 1H 2026 o Interim PoC Readout – anticipated 2H 2026 ABS-20 1 in ENDO o Accelerated Ph1/2a Study initiated Dec 2025 o Phase 2 initiation expected in 2H2026

Generative AI Re(Generative) Biology A B S C I C O R P O R A T I O N 2 0 2 6 A L L R I G H T S R E S E R V E D 2026 J.P. MORGAN HEALTHCARE CONFERENCE