

AUGUST 2025 Innovating GPCR-Targeted Therapies to Reach Large Untapped Market Opportunities .2

Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Each of these forward-looking statements involves substantial risks and uncertainties that could cause actual results to differ significantly from those expressed or implied by such forward-looking statements. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding: the design, objectives, initiation, timing, progress and results of current and future preclinical studies and clinical trials of our product candidates, including the ongoing Phase 1b and Phase 2 clinical trials for TX45, in Group 2 Pulmonary Hypertension; the proposed initiation of a Phase 2 trial for the treatment of PH-ILD, the proposed initiation of a Phase 1 clinical trial for our second program in Hereditary Hemorrhagic Telangiectasia; the expected timing of program updates and data disclosures; the timing of filing INDs and other regulatory documents; the timing and likelihood of seeking regulatory approval for our product candidates including TX45 and TX2100; the competitive landscape for and market potential of our product candidates; our expected cash runway; and our ability to identify and develop additional product candidates as well as pursue additional indications. These forward-looking statements reflect our current beliefs and expectations. Many factors may cause differences between current expectations and actual results, including the early stage of our development efforts; success in preclinical testing and earlier clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidates; clinical site activation rates or clinical trial enrollment rates that are lower than expected; changes in expected or existing competition; changes in the regulatory environment; the uncertainties and timing of the regulatory approval process; the impact of macroeconomic conditions, including the conflict in Ukraine and the conflict in the Middle East, heightened inflation and uncertain credit and financial markets, on our business, clinical trials and financial position; and unexpected litigation or other disputes. These and other risks are described more fully in our filings with the Securities and Exchange Commission (“SEC”), including the risks detailed in our Quarterly Report on Form 10-Q filed with the SEC on August 7, 2025, and other documents we subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. DISCLAIMER

Tectonic Tx: GPCR-Targeted Therapies for High-Value Opportunities TECX focused on discovery & development of GPCR-target biologics with significant unmet need Founded in 2019 by Tim Springer and Andrew Kruse Executive team with numerous accomplishments, resulting in 20 “first” approvals Long-acting relaxin in Phase 2 trial, Phase 1 results support best-in-class potential Initial indication targeting Group 2 Pulmonary Hypertension (PH) associated with Heart Failure with Preserved Ejection Fraction (HFpEF), or PH-HFpEF, with Phase 2 trial enriched for CpcPH (combined pre- and post-capillary PH) Relaxin physiologic and hemodynamic effects demonstrated preclinically and in Phase 1b study Positive Phase 1b Part A clinical trial results achieved or exceeded all hemodynamic targets, supporting Phase 2 study ~1.4M+ Group 2 PH-HFpEF patients in the U.S. with no approved therapy*; high 5-year mortality Potential peak multi-billion-dollar * revenue potential for Group 2 PH-HFpEF patients with EF > 40% Astra Zeneca also pursuing a Group 2 PH relaxin program targeting both HFpEF and HFrEF patients TX45 Phase 1b Part B hemodynamic topline results in PH-HFrEF expected early Q4’25 Positive PH-HFrEF results could potentially expand the addressable TX45 patient population by ~1.1M patients in the U.S. * TX45 PH-ILD (PH associated with Interstitial Lung Disease) Phase 2 trial initiation expected in 2026 Positive PH-ILD (WHO Group 3) Phase 2 results could potentially expand TX45 into a new $1B+ indication Targeting rare bleeding disorder called Hereditary Hemorrhagic Telangiectasia (HHT) Significant market potential, no approved therapies for HHT, estimated ~75K patients in the U.S. alone (15-20% severe) TX2100 Phase 1 clinical trial initiation in healthy volunteers expected in Q1’26 Cash runway into Q4’28 with $287.4 million in cash and cash equivalents as of 06/30/25 Clinical-Stage Biotech Tenured Team TX45 Lead Pipeline Asset Relaxin Potentially Ideal for PH-HFpEF PH-HFpEF Significant Market Potential Well-Capitalized Potential to Expand TX45 to PH-HFrEF * Estimates based on company sponsored market analysis conducted by Health Advances TX2100 Second Pipeline Asset Potential to Expand TX45 to PH-ILD AUGUST 2025

This Accomplished Team Has Delivered for Patients and Investors Alise Reicin, M.D. CEO, Director Daniel Lochner CFO Peter McNamara, Ph.D. CSO Anthony Muslin, M.D. CDO Marcella Ruddy, M.D. CMO Marc Schwabish, Ph.D. CBO Timothy Springer, Ph.D. Co-Founder FOUNDED MULTIPLE SUCCESSFUL COMPANIES GPCR EXPERT, FORBES ”30 under 30” LeukoSite Andrew Kruse, Ph.D. Co-Founder Multiple Awards and Fellowships (Biomedical Research, NIH, Amgen, Sloan Research) 2022 Lasker Award AUGUST 2025
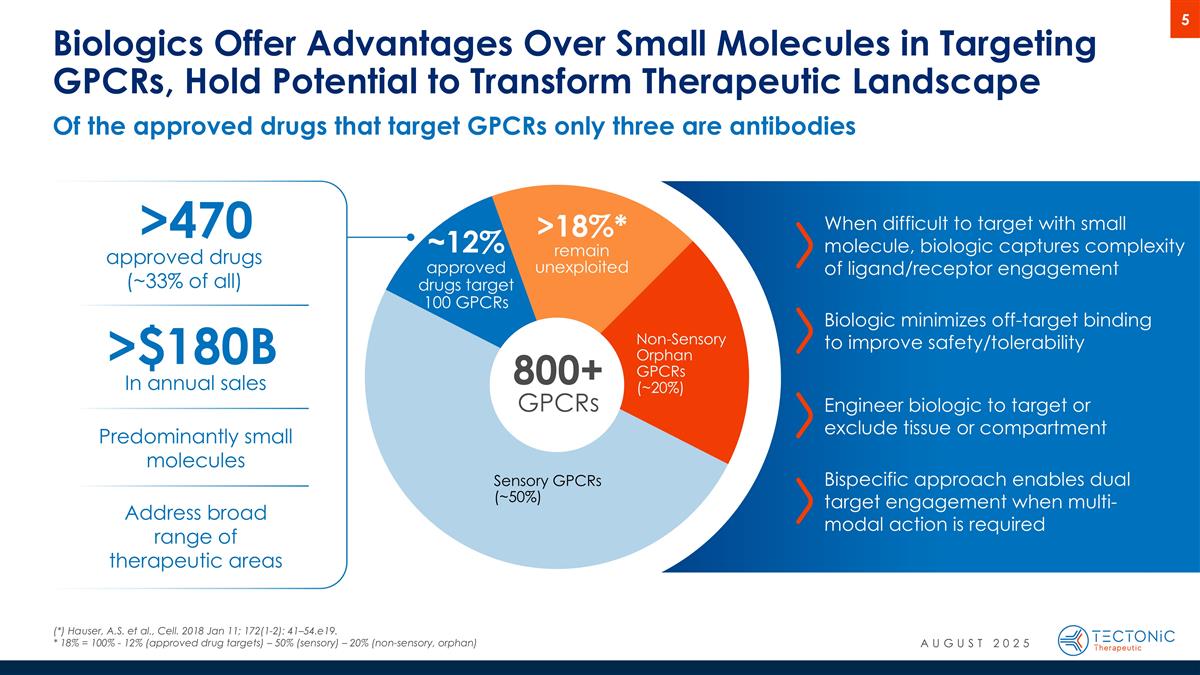
Of the approved drugs that target GPCRs only three are antibodies Biologics Offer Advantages Over Small Molecules in Targeting GPCRs, Hold Potential to Transform Therapeutic Landscape When difficult to target with small molecule, biologic captures complexity of ligand/receptor engagement Biologic minimizes off-target binding to improve safety/tolerability Engineer biologic to target or exclude tissue or compartment Bispecific approach enables dual target engagement when multi-modal action is required 800+ GPCRs Non-Sensory Orphan GPCRs (~20%) Sensory GPCRs (~50%) >470 >18%* remain unexploited ~12% approved drugs target 100 GPCRs Predominantly small molecules approved drugs (~33% of all) >$180B In annual sales Address broad range of therapeutic areas (*) Hauser, A.S. et al., Cell. 2018 Jan 11; 172(1-2): 41–54.e19. * 18% = 100% - 12% (approved drug targets) – 50% (sensory) – 20% (non-sensory, orphan) AUGUST 2025
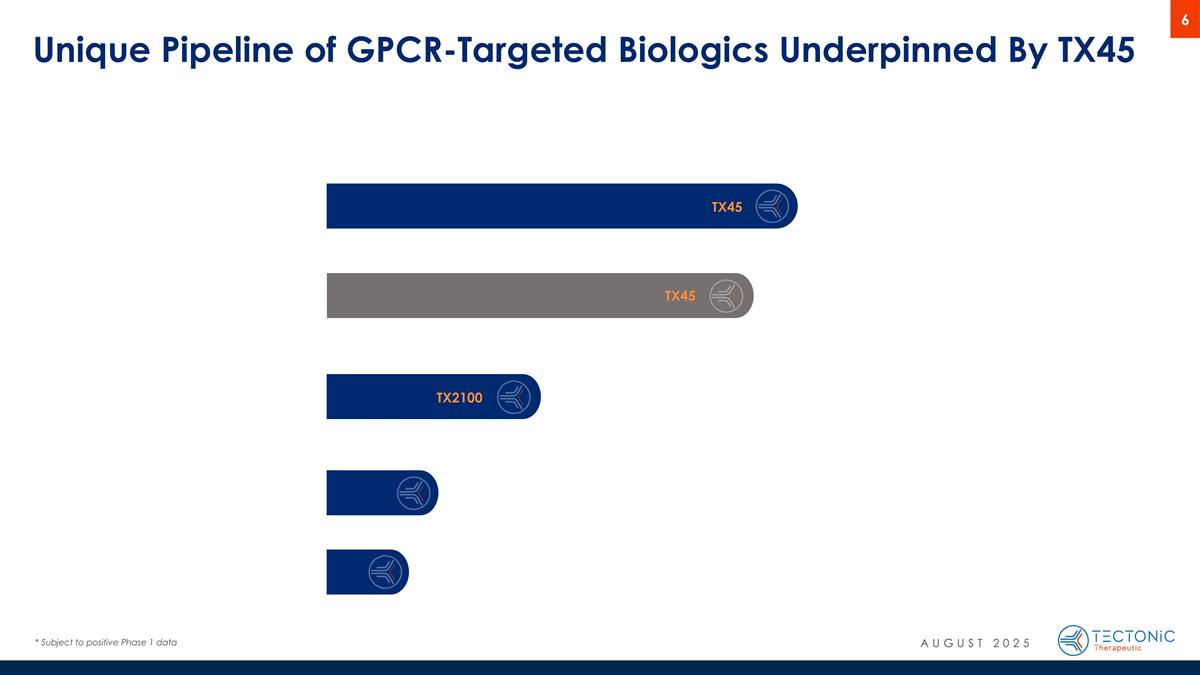
Unique Pipeline of GPCR-Targeted Biologics Underpinned By TX45 TX45 TX2100 TX45 AUGUST 2025 * Subject to positive Phase 1 data

TX45: Long-acting relaxin to address large, unmet need in Group 2 PH RXFP1 agonist with differentiated profile

Mechanism appears ideal to address disease pathology TX45: Potential Best-in-class Treatment for Group 2 PH-HFpEF High unmet need Streamlined and differentiated clinical strategy Potential to expand opportunity Relaxin with optimized PK Protein engineering has extended pharmacologic half-life to support monthly dosing Rigorous Phase 1 PK/PD model enabled robust Phase 2 dose selection Group 2 PH-HFpEF* has no approved therapy >1M+ patients in US** and high 5-year mortality Pulmonary and systemic vasodilator; improves cardiac relaxation during diastole Reversal of fibrosis in pulmonary vasculature and heart Anti-inflammatory Phase 1b Part A hemodynamic data in PH-HFpEF demonstrated improvement in left heart function and pulmonary hemodynamics Clear benefit observed with TX45 in rodent PH and congestive heart failure models Enrichment strategy for CpcPH patients where there is the greatest unmet need Expected 6 min walk test for Phase 3 endpoint, no outcome study needed for approval Potential early launch and premium pricing relative to broad heart failure indication Group 2 PH-HFrEF, PH-ILD, Other PH Groups Supporting clinical and pre-clinical data * Heart Failure with preserved Ejection Fraction, ** US prevalence numbers for Class 2 and 3, estimates based on company sponsored market analysis conducted by Health Advances AUGUST 2025
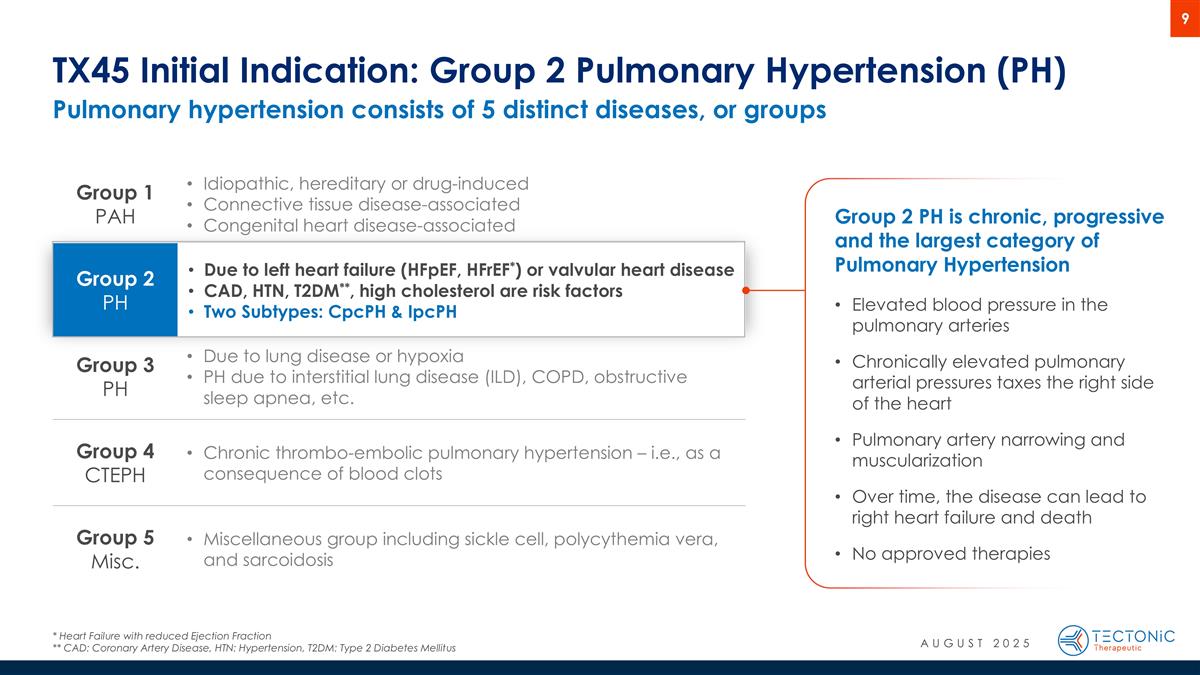
Pulmonary hypertension consists of 5 distinct diseases, or groups TX45 Initial Indication: Group 2 Pulmonary Hypertension (PH) * Heart Failure with reduced Ejection Fraction ** CAD: Coronary Artery Disease, HTN: Hypertension, T2DM: Type 2 Diabetes Mellitus Group 1 PAH Idiopathic, hereditary or drug-induced Connective tissue disease-associated Congenital heart disease-associated Group 2 PH Due to left heart failure (HFpEF, HFrEF*) or valvular heart disease CAD, HTN, T2DM**, high cholesterol are risk factors Two Subtypes: CpcPH & IpcPH Group 3 PH Due to lung disease or hypoxia PH due to interstitial lung disease (ILD), COPD, obstructive sleep apnea, etc. Group 4 CTEPH Chronic thrombo-embolic pulmonary hypertension – i.e., as a consequence of blood clots Group 5 Misc. Miscellaneous group including sickle cell, polycythemia vera, and sarcoidosis Group 2 PH is chronic, progressive and the largest category of Pulmonary Hypertension Elevated blood pressure in the pulmonary arteries Chronically elevated pulmonary arterial pressures taxes the right side of the heart Pulmonary artery narrowing and muscularization Over time, the disease can lead to right heart failure and death No approved therapies AUGUST 2025
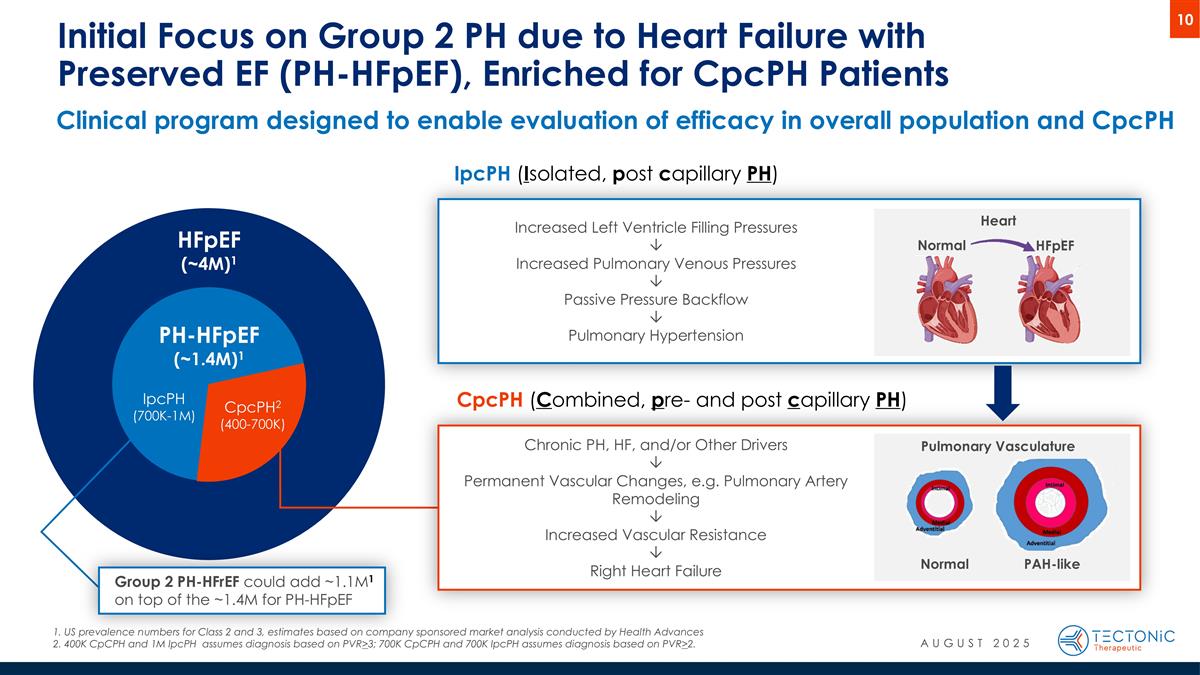
Clinical program designed to enable evaluation of efficacy in overall population and CpcPH Initial Focus on Group 2 PH due to Heart Failure with Preserved EF (PH-HFpEF), Enriched for CpcPH Patients PH-HFpEF (~1.4M)1 HFpEF (~4M)1 IpcPH (700K-1M) CpcPH2 (400-700K) Normal HFpEF Heart IpcPH (Isolated, post capillary PH) US prevalence numbers for Class 2 and 3, estimates based on company sponsored market analysis conducted by Health Advances 400K CpCPH and 1M IpcPH assumes diagnosis based on PVR>3; 700K CpCPH and 700K IpcPH assumes diagnosis based on PVR>2. CpcPH (Combined, pre- and post capillary PH) Normal PAH-like Pulmonary Vasculature Chronic PH, HF, and/or Other Drivers Ô Permanent Vascular Changes, e.g. Pulmonary Artery Remodeling Ô Increased Vascular Resistance Ô Right Heart Failure Increased Left Ventricle Filling Pressures Ô Increased Pulmonary Venous Pressures Ô Passive Pressure Backflow Ô Pulmonary Hypertension Group 2 PH-HFrEF could add ~1.1M1 on top of the ~1.4M for PH-HFpEF AUGUST 2025
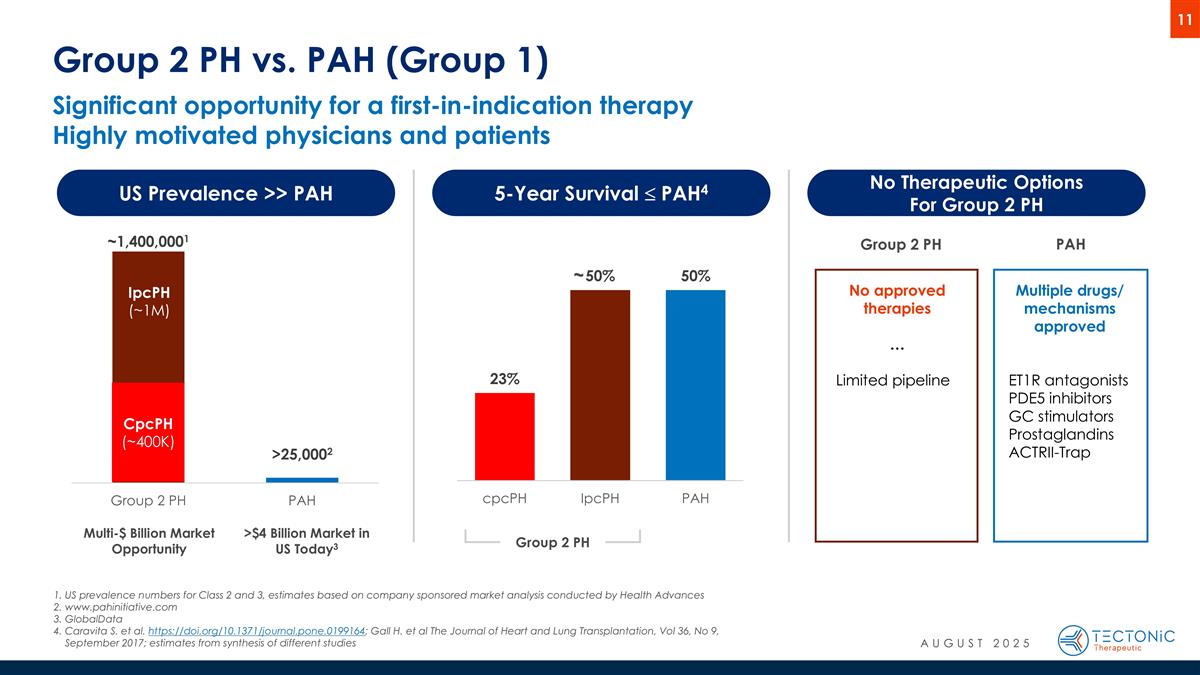
Significant opportunity for a first-in-indication therapy Highly motivated physicians and patients Group 2 PH vs. PAH (Group 1) US Prevalence >> PAH 5-Year Survival £ PAH4 No Therapeutic Options For Group 2 PH Multiple drugs/ mechanisms approved ET1R antagonists PDE5 inhibitors GC stimulators Prostaglandins ACTRII-Trap No approved therapies … Limited pipeline Group 2 PH PAH CpcPH (~400K) IpcPH (~1M) Group 2 PH Multi-$ Billion Market Opportunity >$4 Billion Market in US Today3 US prevalence numbers for Class 2 and 3, estimates based on company sponsored market analysis conducted by Health Advances www.pahinitiative.com GlobalData Caravita S. et al. https://doi.org/10.1371/journal.pone.0199164; Gall H. et al The Journal of Heart and Lung Transplantation, Vol 36, No 9, September 2017; estimates from synthesis of different studies ~ AUGUST 2025
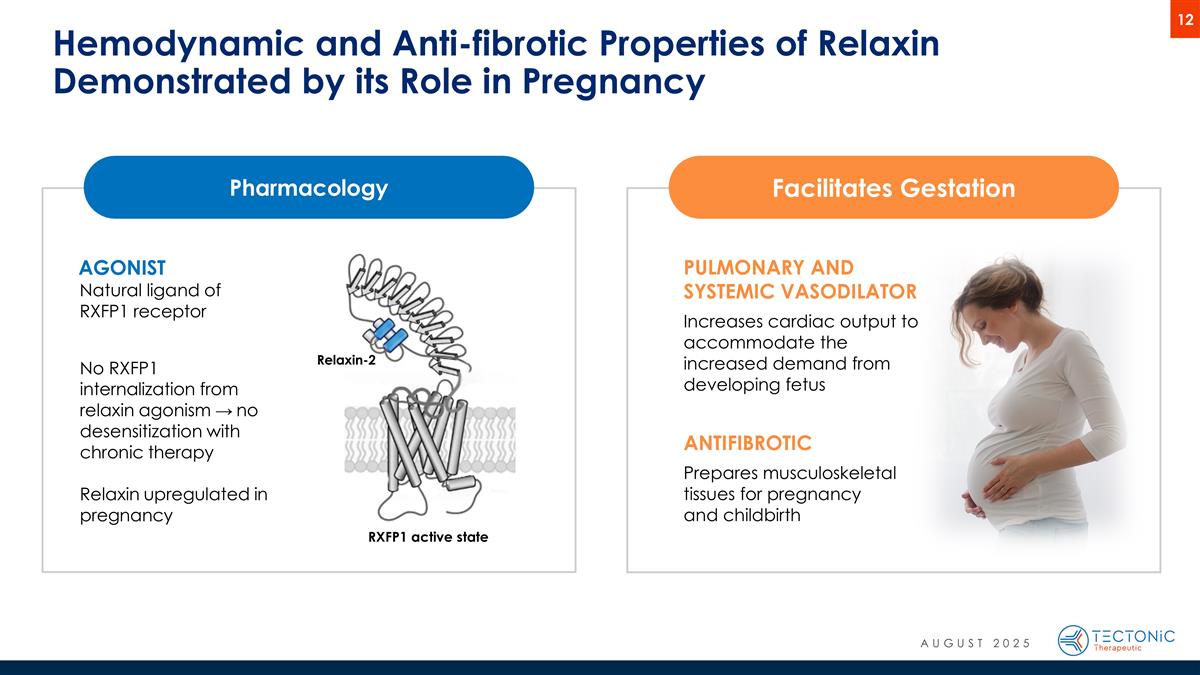
Hemodynamic and Anti-fibrotic Properties of Relaxin Demonstrated by its Role in Pregnancy Facilitates Gestation PULMONARY AND SYSTEMIC VASODILATOR Increases cardiac output to accommodate the increased demand from developing fetus ANTIFIBROTIC Prepares musculoskeletal tissues for pregnancy and childbirth Pharmacology AGONIST Natural ligand of RXFP1 receptor No RXFP1 internalization from relaxin agonism → no desensitization with chronic therapy Relaxin upregulated in pregnancy Relaxin-2 RXFP1 active state AUGUST 2025
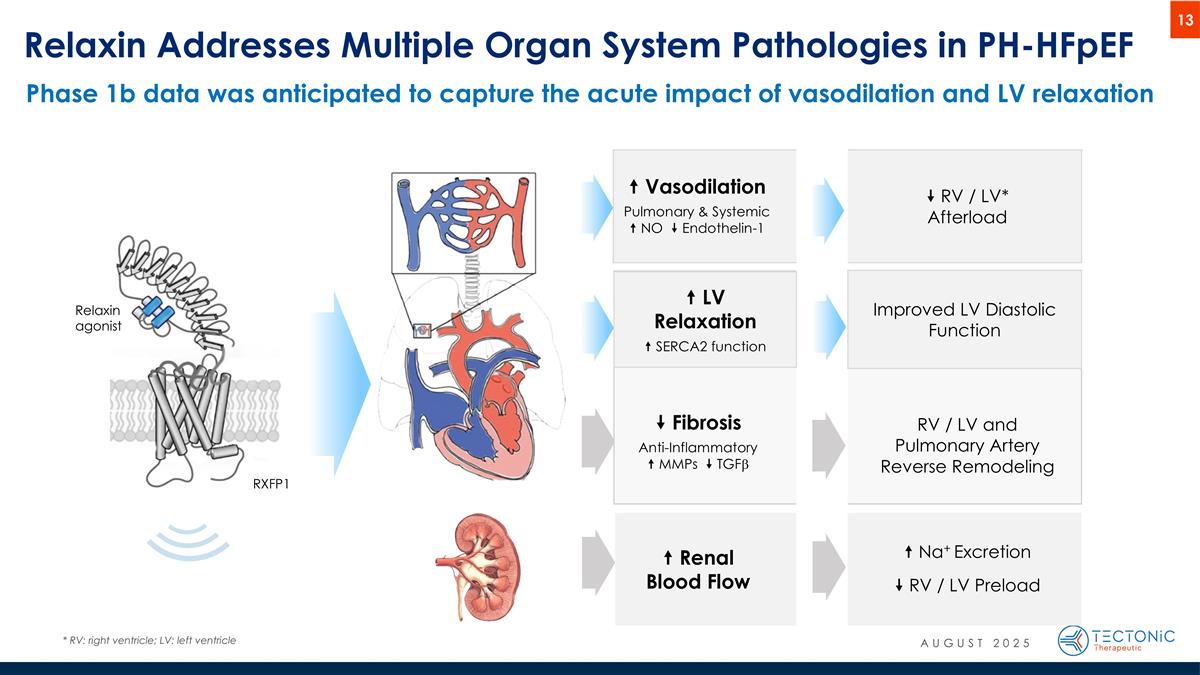
“ Vasodilation Pulmonary & Systemic “ NO ” Endothelin-1 ” Fibrosis Anti-Inflammatory “ MMPs ” TGFb “ Renal Blood Flow ” RV / LV* Afterload RV / LV and Pulmonary Artery Reverse Remodeling “ Na+ Excretion ” RV / LV Preload Relaxin Addresses Multiple Organ System Pathologies in PH-HFpEF * RV: right ventricle; LV: left ventricle Relaxin agonist RXFP1 “ LV Relaxation “ SERCA2 function Improved LV Diastolic Function Phase 1b data was anticipated to capture the acute impact of vasodilation and LV relaxation AUGUST 2025
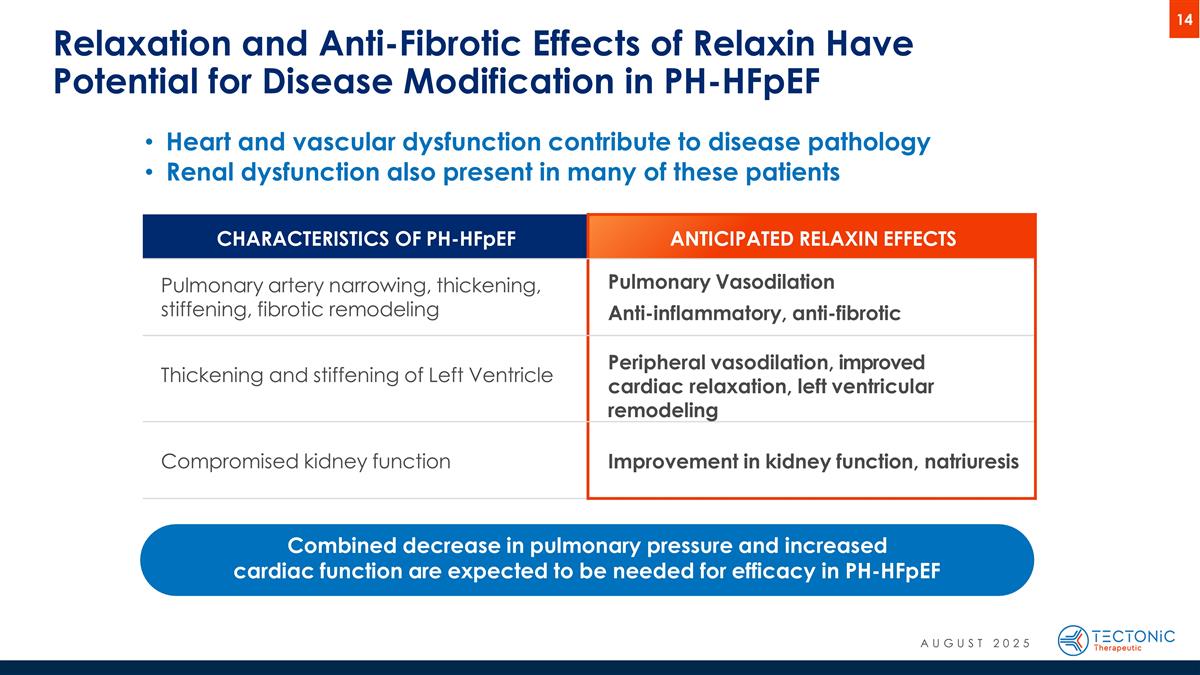
Heart and vascular dysfunction contribute to disease pathology Renal dysfunction also present in many of these patients Relaxation and Anti-Fibrotic Effects of Relaxin Have Potential for Disease Modification in PH-HFpEF CHARACTERISTICS OF PH-HFpEF ANTICIPATED RELAXIN EFFECTS Pulmonary artery narrowing, thickening, stiffening, fibrotic remodeling Pulmonary Vasodilation Anti-inflammatory, anti-fibrotic Thickening and stiffening of Left Ventricle Peripheral vasodilation, improved cardiac relaxation, left ventricular remodeling Compromised kidney function Improvement in kidney function, natriuresis Combined decrease in pulmonary pressure and increased cardiac function are expected to be needed for efficacy in PH-HFpEF AUGUST 2025
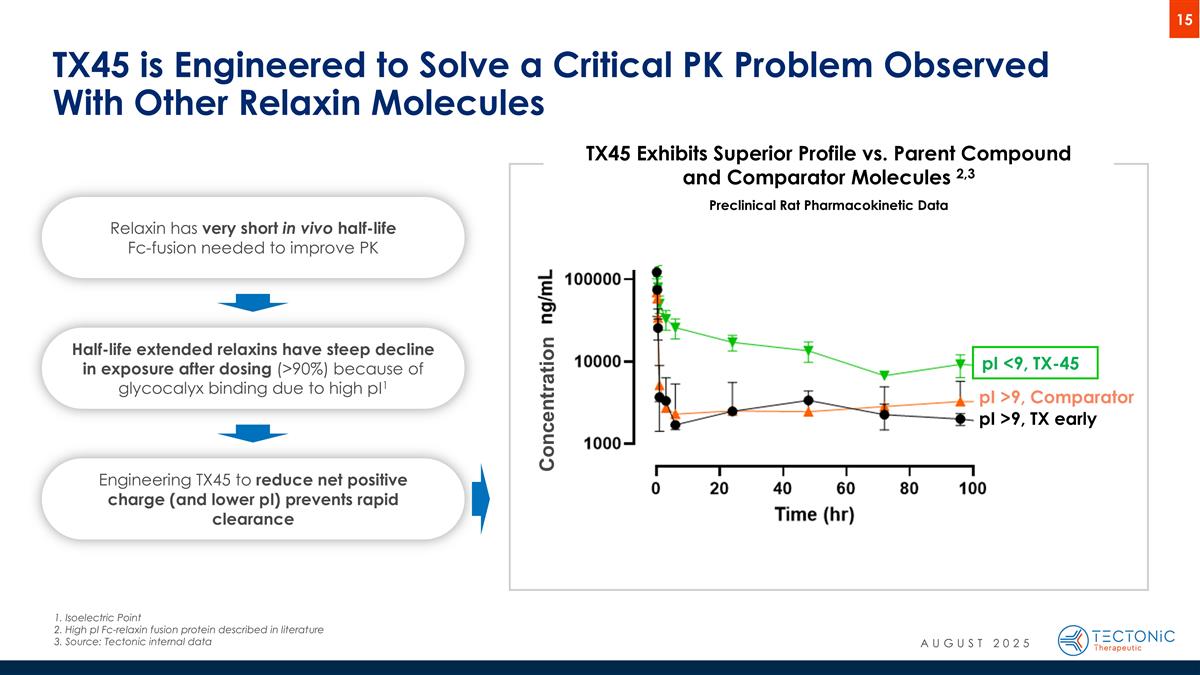
TX45 is Engineered to Solve a Critical PK Problem Observed With Other Relaxin Molecules pI >9, Comparator pI >9, TX early pI <9, TX-45 TX45 Exhibits Superior Profile vs. Parent Compound and Comparator Molecules 2,3 Preclinical Rat Pharmacokinetic Data Half-life extended relaxins have steep decline in exposure after dosing (>90%) because of glycocalyx binding due to high pI1 Engineering TX45 to reduce net positive charge (and lower pI) prevents rapid clearance Relaxin has very short in vivo half-life Fc-fusion needed to improve PK Concentration 1. Isoelectric Point 2. High pI Fc-relaxin fusion protein described in literature 3.Source: Tectonic internal data AUGUST 2025
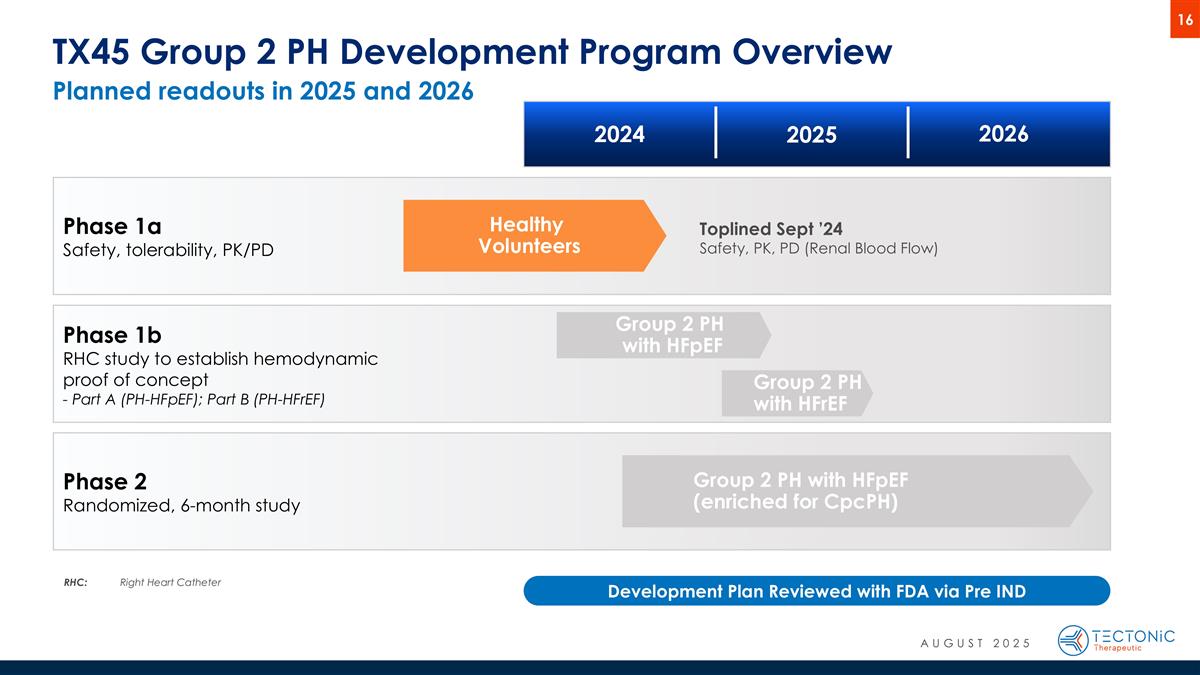
Phase 1b RHC study to establish hemodynamic proof of concept - Part A (PH-HFpEF); Part B (PH-HFrEF) Group 2 PH with HFpEF (enriched for CpcPH) Group 2 PH with HFpEF Healthy Volunteers TX45 Group 2 PH Development Program Overview Phase 1a Safety, tolerability, PK/PD Phase 2 Randomized, 6-month study 2024 2025 2026 RHC:Right Heart Catheter Development Plan Reviewed with FDA via Pre IND Group 2 PH with HFrEF Toplined Sept ’24 Safety, PK, PD (Renal Blood Flow) AUGUST 2025 Planned readouts in 2025 and 2026
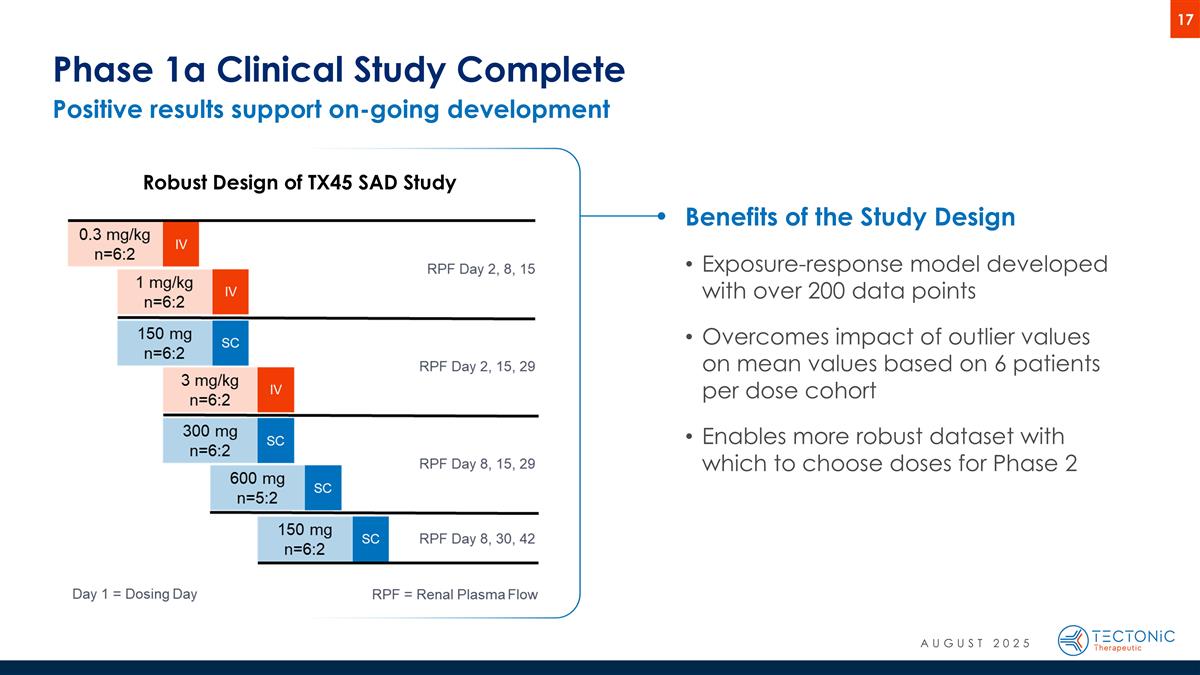
Positive results support on-going development Phase 1a Clinical Study Complete Robust Design of TX45 SAD Study Benefits of the Study Design Exposure-response model developed with over 200 data points Overcomes impact of outlier values on mean values based on 6 patients per dose cohort Enables more robust dataset with which to choose doses for Phase 2 AUGUST 2025
TX45 Shows Favorable Safety Profile No discontinuations or treatment-related SAEs No observed injection site reactions, immunogenicity, or detection of anti-drug antibodies Most common AE was transient orthostatic tachycardia (increases in heart rate with standing) Placebo: 17% vs TX45: 23% Not associated with drops in blood pressure No clinically meaningful changes in vital signs or labs AUGUST 2025
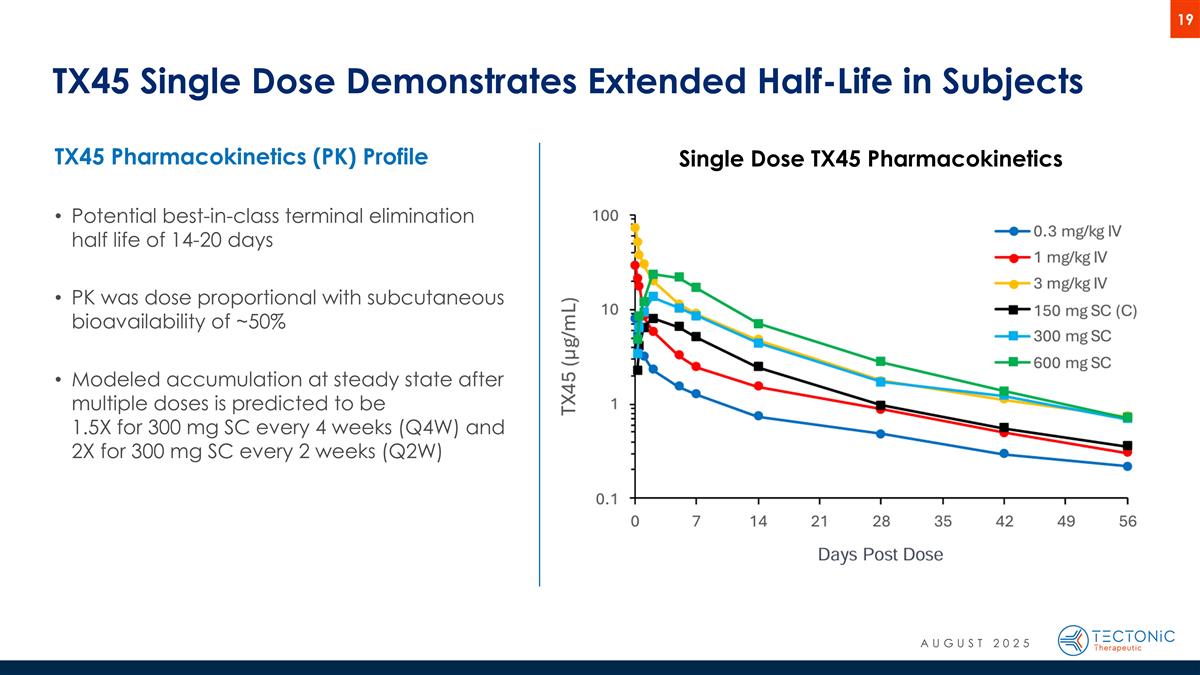
TX45 Single Dose Demonstrates Extended Half-Life in Subjects Single Dose TX45 Pharmacokinetics TX45 Pharmacokinetics (PK) Profile Potential best-in-class terminal elimination half life of 14-20 days PK was dose proportional with subcutaneous bioavailability of ~50% Modeled accumulation at steady state after multiple doses is predicted to be 1.5X for 300 mg SC every 4 weeks (Q4W) and 2X for 300 mg SC every 2 weeks (Q2W) AUGUST 2025
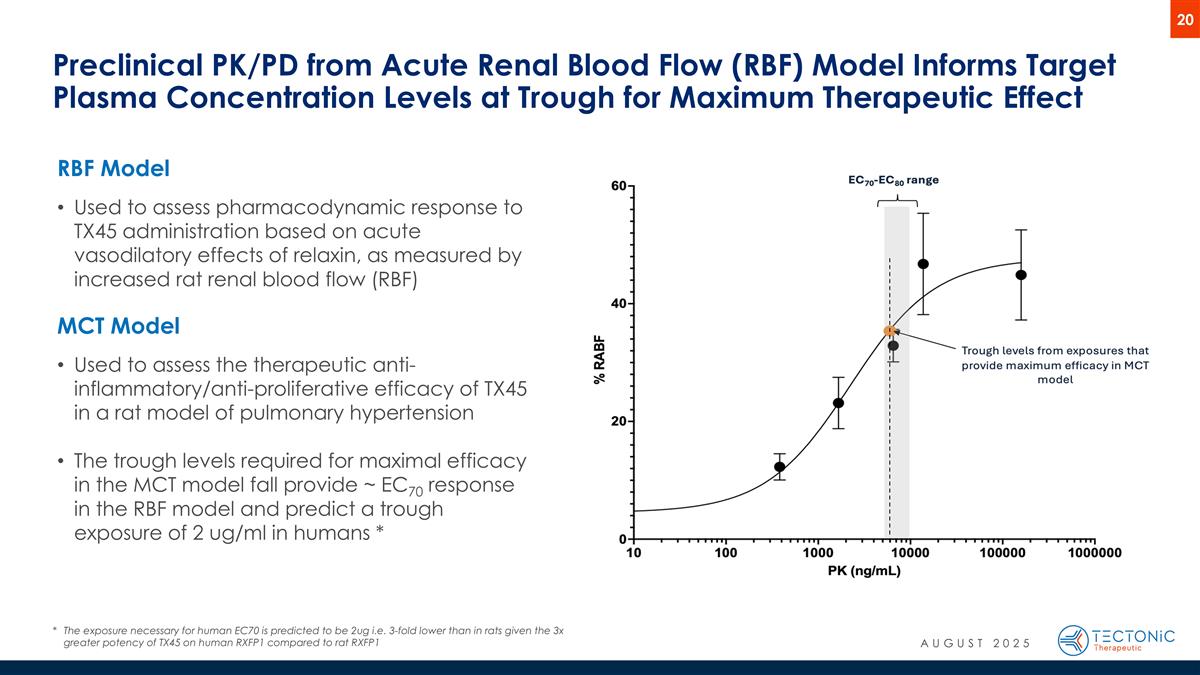
Preclinical PK/PD from Acute Renal Blood Flow (RBF) Model Informs Target Plasma Concentration Levels at Trough for Maximum Therapeutic Effect RBF Model Used to assess pharmacodynamic response to TX45 administration based on acute vasodilatory effects of relaxin, as measured by increased rat renal blood flow (RBF) MCT Model Used to assess the therapeutic anti-inflammatory/anti-proliferative efficacy of TX45 in a rat model of pulmonary hypertension The trough levels required for maximal efficacy in the MCT model fall provide ~ EC70 response in the RBF model and predict a trough exposure of 2 ug/ml in humans * *The exposure necessary for human EC70 is predicted to be 2ug i.e. 3-fold lower than in rats given the 3x greater potency of TX45 on human RXFP1 compared to rat RXFP1 AUGUST 2025
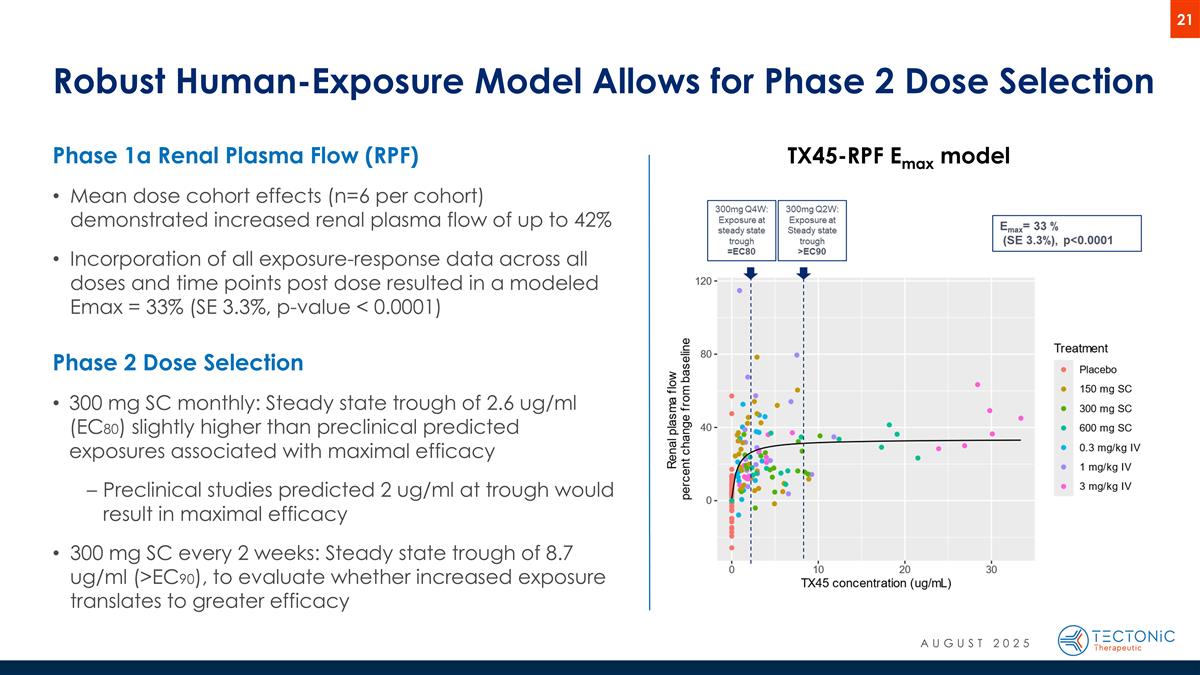
Robust Human-Exposure Model Allows for Phase 2 Dose Selection Phase 1a Renal Plasma Flow (RPF) Mean dose cohort effects (n=6 per cohort) demonstrated increased renal plasma flow of up to 42% Incorporation of all exposure-response data across all doses and time points post dose resulted in a modeled Emax = 33% (SE 3.3%, p-value < 0.0001) Phase 2 Dose Selection 300 mg SC monthly: Steady state trough of 2.6 ug/ml (EC80) slightly higher than preclinical predicted exposures associated with maximal efficacy Preclinical studies predicted 2 ug/ml at trough would result in maximal efficacy 300 mg SC every 2 weeks: Steady state trough of 8.7 ug/ml (>EC90), to evaluate whether increased exposure translates to greater efficacy TX45-RPF Emax model AUGUST 2025
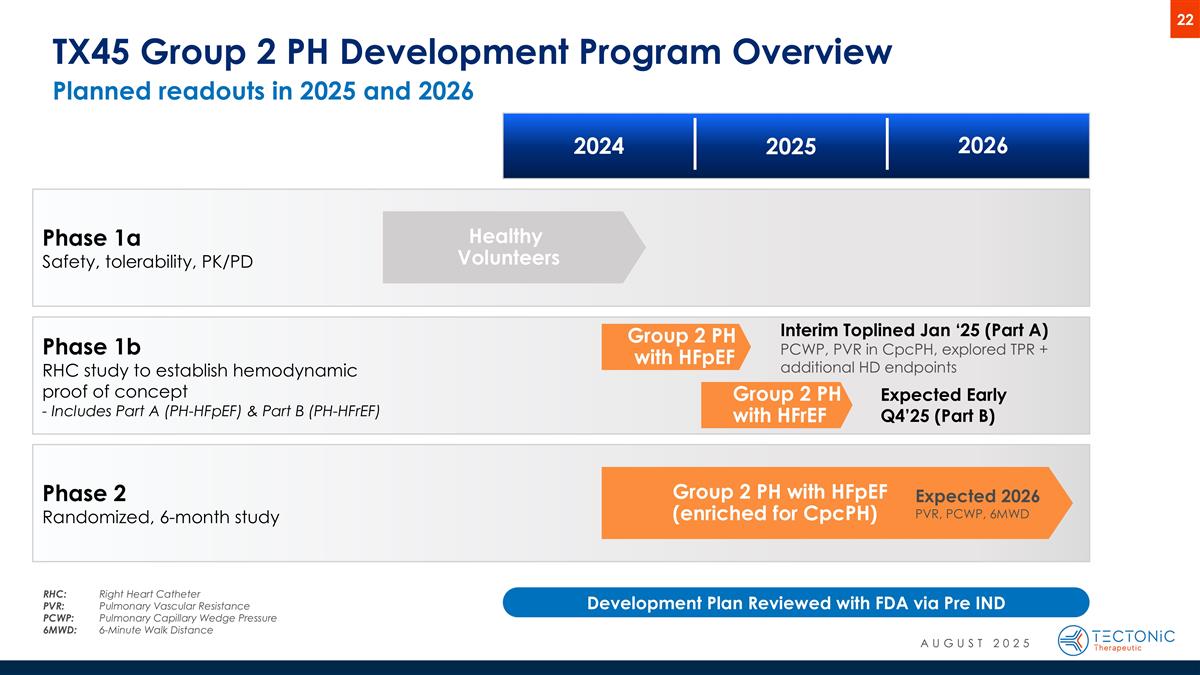
Phase 1b RHC study to establish hemodynamic proof of concept - Includes Part A (PH-HFpEF) & Part B (PH-HFrEF) Group 2 PH with HFpEF (enriched for CpcPH) Group 2 PH with HFpEF Healthy Volunteers Planned readouts in 2025 and 2026 TX45 Group 2 PH Development Program Overview Phase 1a Safety, tolerability, PK/PD Phase 2 Randomized, 6-month study Interim Toplined Jan ‘25 (Part A) PCWP, PVR in CpcPH, explored TPR + additional HD endpoints Expected 2026 PVR, PCWP, 6MWD 2024 2025 2026 RHC:Right Heart Catheter PVR: Pulmonary Vascular Resistance PCWP: Pulmonary Capillary Wedge Pressure 6MWD: 6-Minute Walk Distance Development Plan Reviewed with FDA via Pre IND Group 2 PH with HFrEF Expected Early Q4’25 (Part B) AUGUST 2025
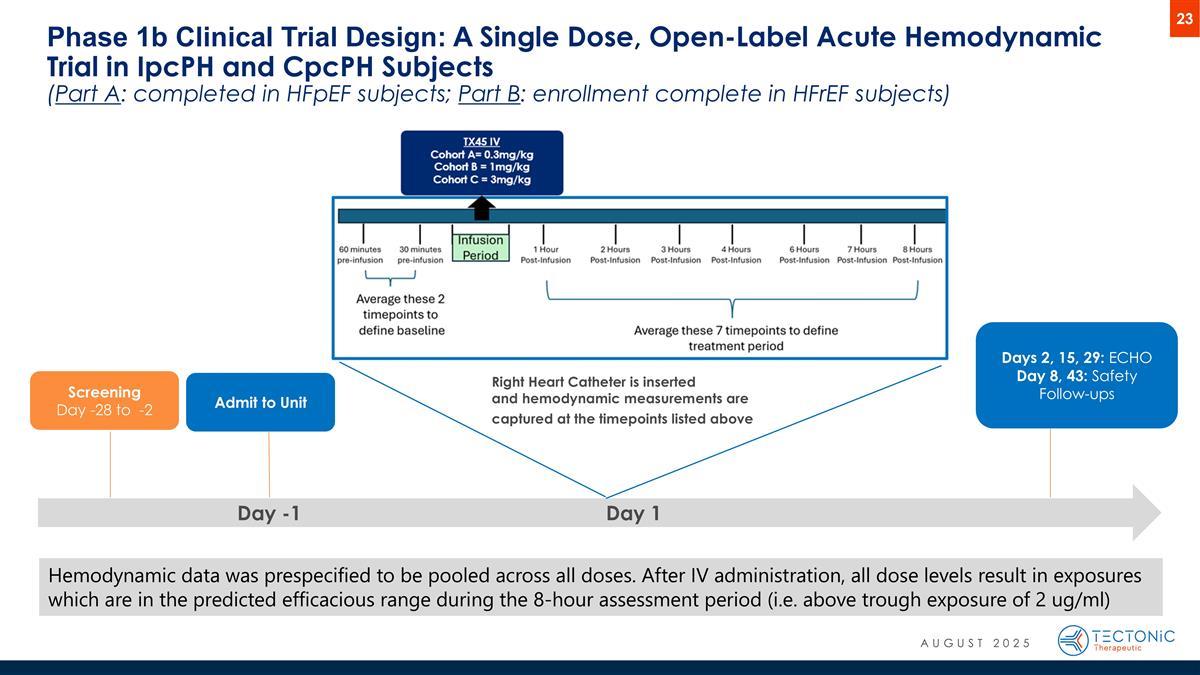
Hemodynamic data was prespecified to be pooled across all doses. After IV administration, all dose levels result in exposures which are in the predicted efficacious range during the 8-hour assessment period (i.e. above trough exposure of 2 ug/ml) Phase 1b Clinical Trial Design: A Single Dose, Open-Label Acute Hemodynamic Trial in IpcPH and CpcPH Subjects (Part A: completed in HFpEF subjects; Part B: enrollment complete in HFrEF subjects) Day -1 Day 1 Screening Day -28 to -2 Admit to Unit Days 2, 15, 29: ECHO Day 8, 43: Safety Follow-ups Right Heart Catheter is inserted and hemodynamic measurements are captured at the timepoints listed above AUGUST 2025
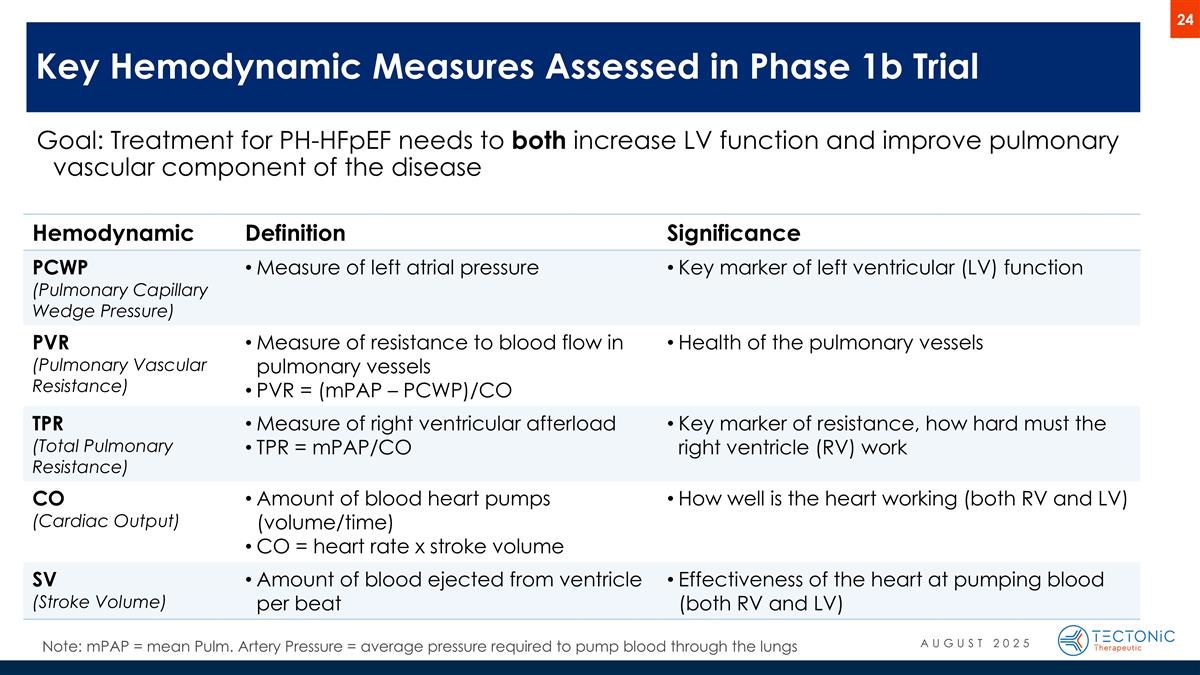
Phase 1b trial results that help predict APEX trial success Goal: Treatment for PH-HFpEF needs to both increase LV function and improve pulmonary vascular component of the disease Key Hemodynamic Measures Assessed in Phase 1b Trial Hemodynamic Definition Significance PCWP (Pulmonary Capillary Wedge Pressure) Measure of left atrial pressure Key marker of left ventricular (LV) function PVR (Pulmonary Vascular Resistance) Measure of resistance to blood flow in pulmonary vessels PVR = (mPAP – PCWP)/CO Health of the pulmonary vessels TPR (Total Pulmonary Resistance) Measure of right ventricular afterload TPR = mPAP/CO Key marker of resistance, how hard must the right ventricle (RV) work CO (Cardiac Output) Amount of blood heart pumps (volume/time) CO = heart rate x stroke volume How well is the heart working (both RV and LV) SV (Stroke Volume) Amount of blood ejected from ventricle per beat Effectiveness of the heart at pumping blood (both RV and LV) Note: mPAP = mean Pulm. Artery Pressure = average pressure required to pump blood through the lungs AUGUST 2025
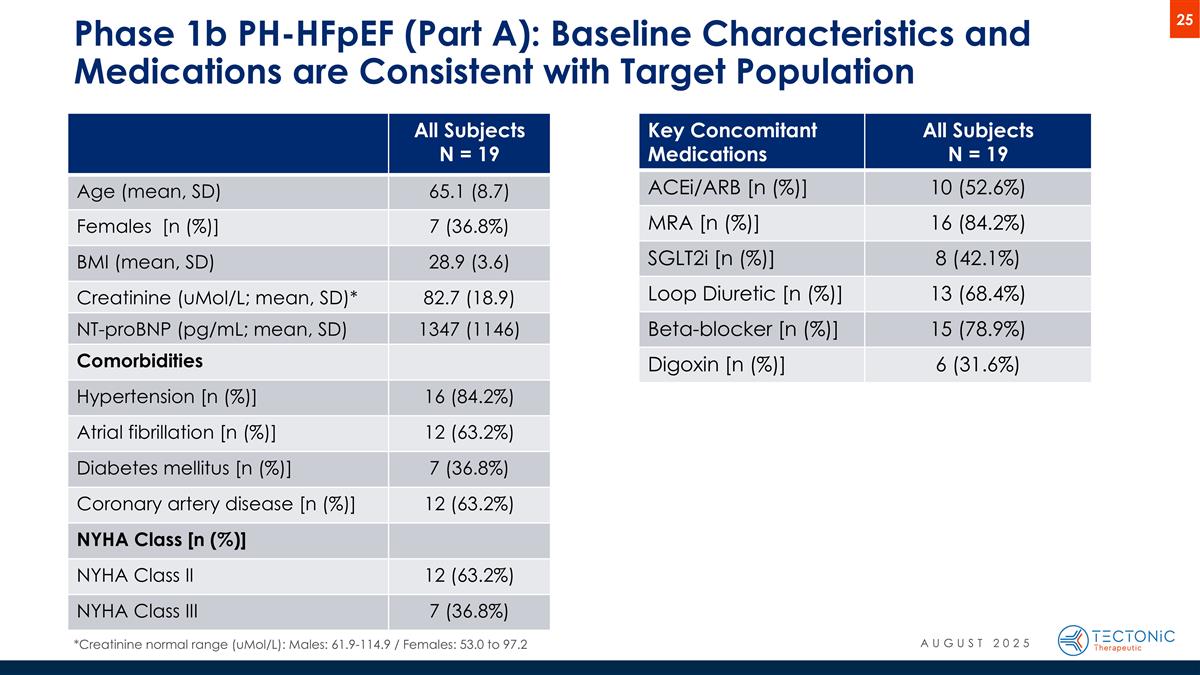
Phase 1b PH-HFpEF (Part A): Baseline Characteristics and Medications are Consistent with Target Population All Subjects N = 19 Age (mean, SD) 65.1 (8.7) Females [n (%)] 7 (36.8%) BMI (mean, SD) 28.9 (3.6) Creatinine (uMol/L; mean, SD)* 82.7 (18.9) NT-proBNP (pg/mL; mean, SD) 1347 (1146) Comorbidities Hypertension [n (%)] 16 (84.2%) Atrial fibrillation [n (%)] 12 (63.2%) Diabetes mellitus [n (%)] 7 (36.8%) Coronary artery disease [n (%)] 12 (63.2%) NYHA Class [n (%)] NYHA Class II 12 (63.2%) NYHA Class III 7 (36.8%) Key Concomitant Medications All Subjects N = 19 ACEi/ARB [n (%)] 10 (52.6%) MRA [n (%)] 16 (84.2%) SGLT2i [n (%)] 8 (42.1%) Loop Diuretic [n (%)] 13 (68.4%) Beta-blocker [n (%)] 15 (78.9%) Digoxin [n (%)] 6 (31.6%) AUGUST 2025 *Creatinine normal range (uMol/L): Males: 61.9-114.9 / Females: 53.0 to 97.2
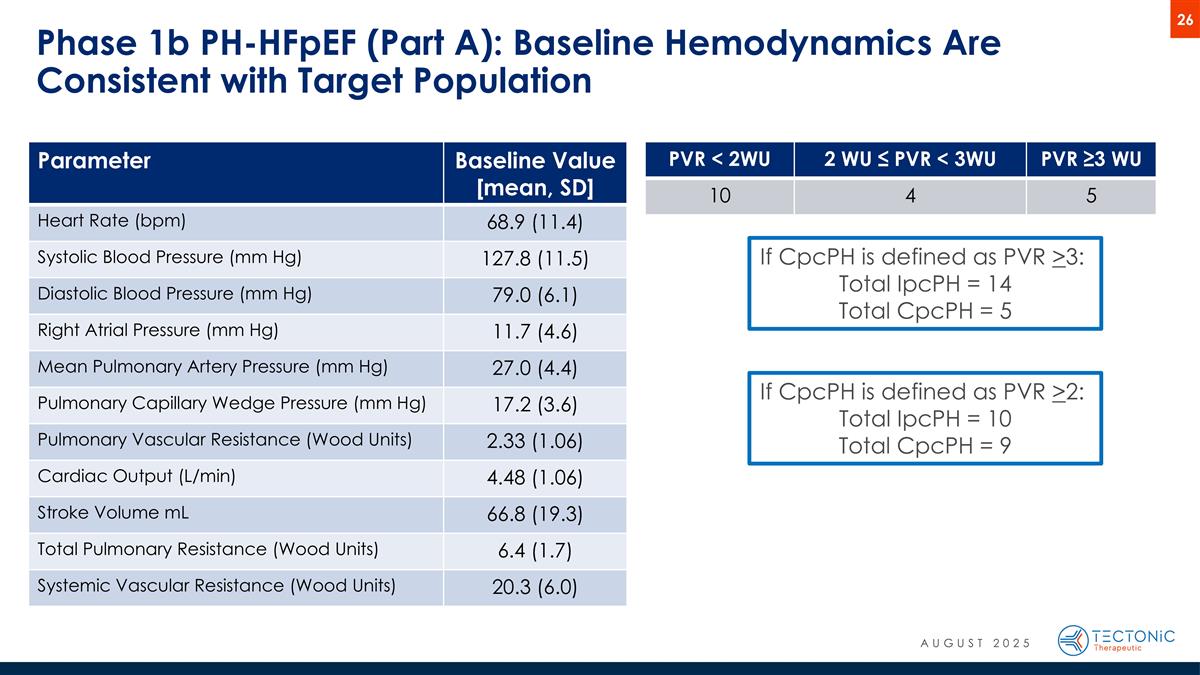
Phase 1b PH-HFpEF (Part A): Baseline Hemodynamics Are Consistent with Target Population PVR < 2WU 2 WU ≤ PVR < 3WU PVR ≥3 WU 10 4 5 If CpcPH is defined as PVR >2: Total IpcPH = 10 Total CpcPH = 9 If CpcPH is defined as PVR >3: Total IpcPH = 14 Total CpcPH = 5 Parameter Baseline Value [mean, SD] Heart Rate (bpm) 68.9 (11.4) Systolic Blood Pressure (mm Hg) 127.8 (11.5) Diastolic Blood Pressure (mm Hg) 79.0 (6.1) Right Atrial Pressure (mm Hg) 11.7 (4.6) Mean Pulmonary Artery Pressure (mm Hg) 27.0 (4.4) Pulmonary Capillary Wedge Pressure (mm Hg) 17.2 (3.6) Pulmonary Vascular Resistance (Wood Units) 2.33 (1.06) Cardiac Output (L/min) 4.48 (1.06) Stroke Volume mL 66.8 (19.3) Total Pulmonary Resistance (Wood Units) 6.4 (1.7) Systemic Vascular Resistance (Wood Units) 20.3 (6.0) AUGUST 2025
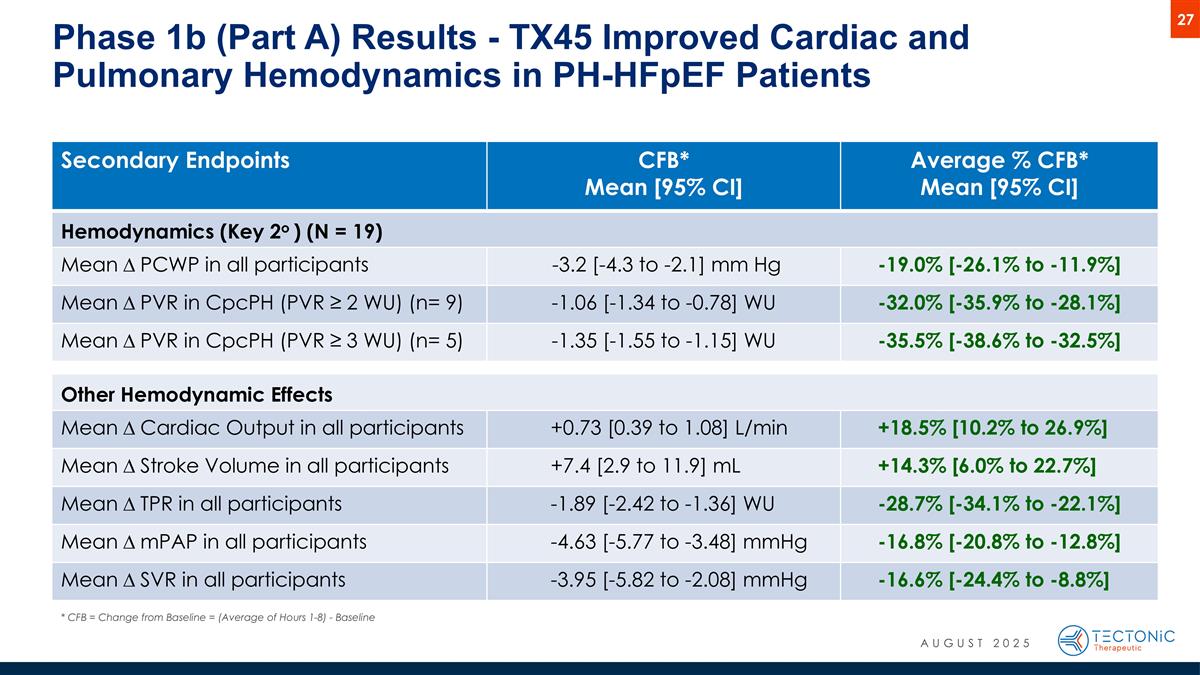
Secondary Endpoints CFB* Mean [95% CI] Average % CFB* Mean [95% CI] Hemodynamics (Key 2o ) (N = 19) Mean D PCWP in all participants -3.2 [-4.3 to -2.1] mm Hg -19.0% [-26.1% to -11.9%] Mean D PVR in CpcPH (PVR ≥ 2 WU) (n= 9) -1.06 [-1.34 to -0.78] WU -32.0% [-35.9% to -28.1%] Mean D PVR in CpcPH (PVR ≥ 3 WU) (n= 5) -1.35 [-1.55 to -1.15] WU -35.5% [-38.6% to -32.5%] Other Hemodynamic Effects Mean D Cardiac Output in all participants +0.73 [0.39 to 1.08] L/min +18.5% [10.2% to 26.9%] Mean D Stroke Volume in all participants +7.4 [2.9 to 11.9] mL +14.3% [6.0% to 22.7%] Mean D TPR in all participants -1.89 [-2.42 to -1.36] WU -28.7% [-34.1% to -22.1%] Mean D mPAP in all participants -4.63 [-5.77 to -3.48] mmHg -16.8% [-20.8% to -12.8%] Mean D SVR in all participants -3.95 [-5.82 to -2.08] mmHg -16.6% [-24.4% to -8.8%] Phase 1b (Part A) Results - TX45 Improved Cardiac and Pulmonary Hemodynamics in PH-HFpEF Patients * CFB = Change from Baseline = (Average of Hours 1-8) - Baseline AUGUST 2025
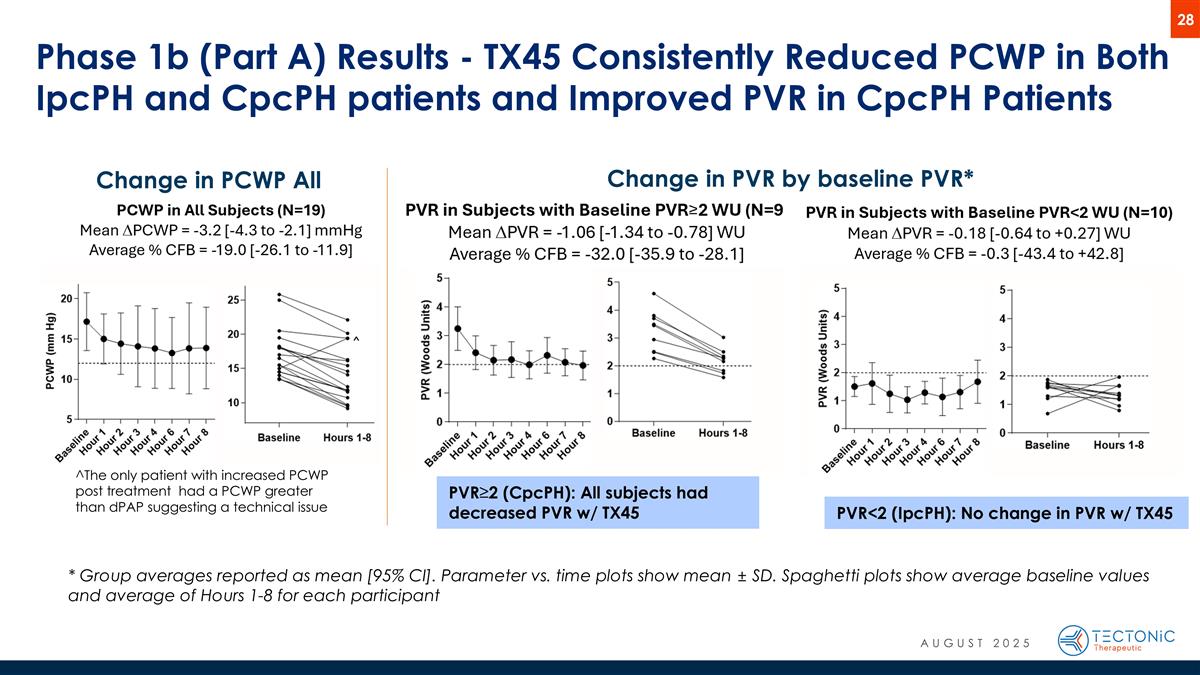
Phase 1b (Part A) Results - TX45 Consistently Reduced PCWP in Both IpcPH and CpcPH patients and Improved PVR in CpcPH Patients * Group averages reported as mean [95% CI]. Parameter vs. time plots show mean ± SD. Spaghetti plots show average baseline values and average of Hours 1-8 for each participant Change in PCWP All Subjects* Change in PVR by baseline PVR* PVR<2 (IpcPH): No change in PVR w/ TX45 PVR≥2 (CpcPH): All subjects had decreased PVR w/ TX45 ^The only patient with increased PCWP post treatment had a PCWP greater than dPAP suggesting a technical issue AUGUST 2025
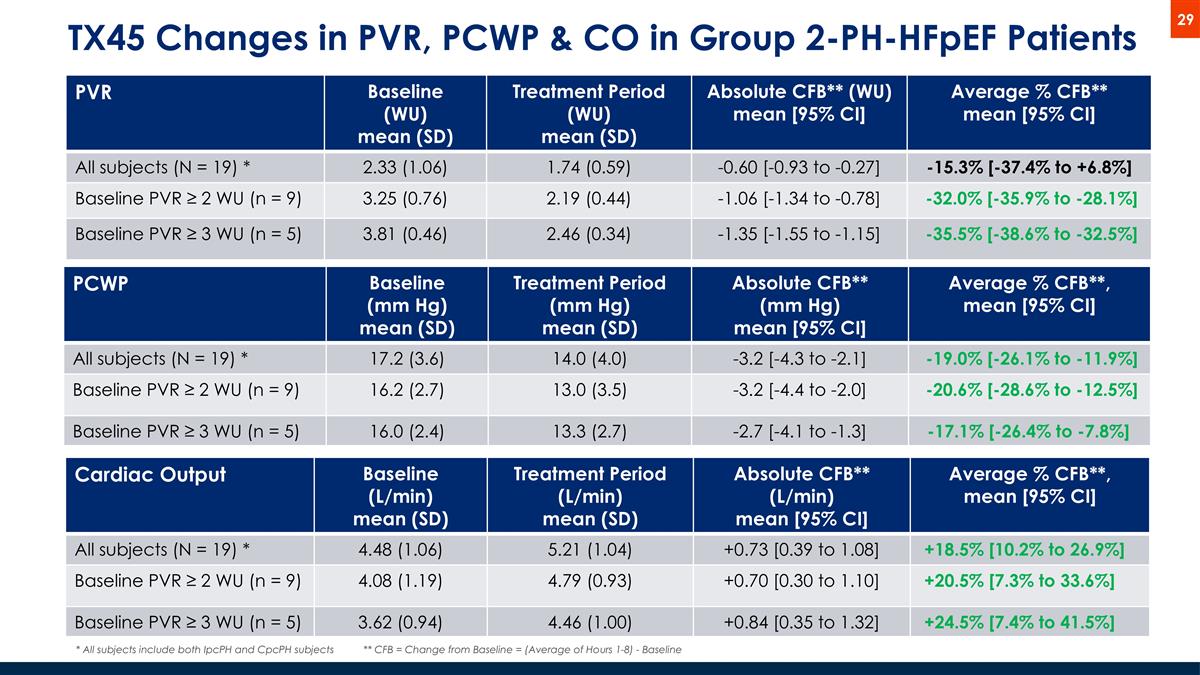
TX45 Changes in PVR, PCWP & CO in Group 2-PH-HFpEF Patients PVR Baseline (WU) mean (SD) Treatment Period (WU) mean (SD) Absolute CFB** (WU) mean [95% CI] Average % CFB** mean [95% CI] All subjects (N = 19) * 2.33 (1.06) 1.74 (0.59) -0.60 [-0.93 to -0.27] -15.3% [-37.4% to +6.8%] Baseline PVR ≥ 2 WU (n = 9) 3.25 (0.76) 2.19 (0.44) -1.06 [-1.34 to -0.78] -32.0% [-35.9% to -28.1%] Baseline PVR ≥ 3 WU (n = 5) 3.81 (0.46) 2.46 (0.34) -1.35 [-1.55 to -1.15] -35.5% [-38.6% to -32.5%] * All subjects include both IpcPH and CpcPH subjects PCWP Baseline (mm Hg) mean (SD) Treatment Period (mm Hg) mean (SD) Absolute CFB** (mm Hg) mean [95% CI] Average % CFB**, mean [95% CI] All subjects (N = 19) * 17.2 (3.6) 14.0 (4.0) -3.2 [-4.3 to -2.1] -19.0% [-26.1% to -11.9%] Baseline PVR ≥ 2 WU (n = 9) 16.2 (2.7) 13.0 (3.5) -3.2 [-4.4 to -2.0] -20.6% [-28.6% to -12.5%] Baseline PVR ≥ 3 WU (n = 5) 16.0 (2.4) 13.3 (2.7) -2.7 [-4.1 to -1.3] -17.1% [-26.4% to -7.8%] Cardiac Output Baseline (L/min) mean (SD) Treatment Period (L/min) mean (SD) Absolute CFB** (L/min) mean [95% CI] Average % CFB**, mean [95% CI] All subjects (N = 19) * 4.48 (1.06) 5.21 (1.04) +0.73 [0.39 to 1.08] +18.5% [10.2% to 26.9%] Baseline PVR ≥ 2 WU (n = 9) 4.08 (1.19) 4.79 (0.93) +0.70 [0.30 to 1.10] +20.5% [7.3% to 33.6%] Baseline PVR ≥ 3 WU (n = 5) 3.62 (0.94) 4.46 (1.00) +0.84 [0.35 to 1.32] +24.5% [7.4% to 41.5%] ** CFB = Change from Baseline = (Average of Hours 1-8) - Baseline
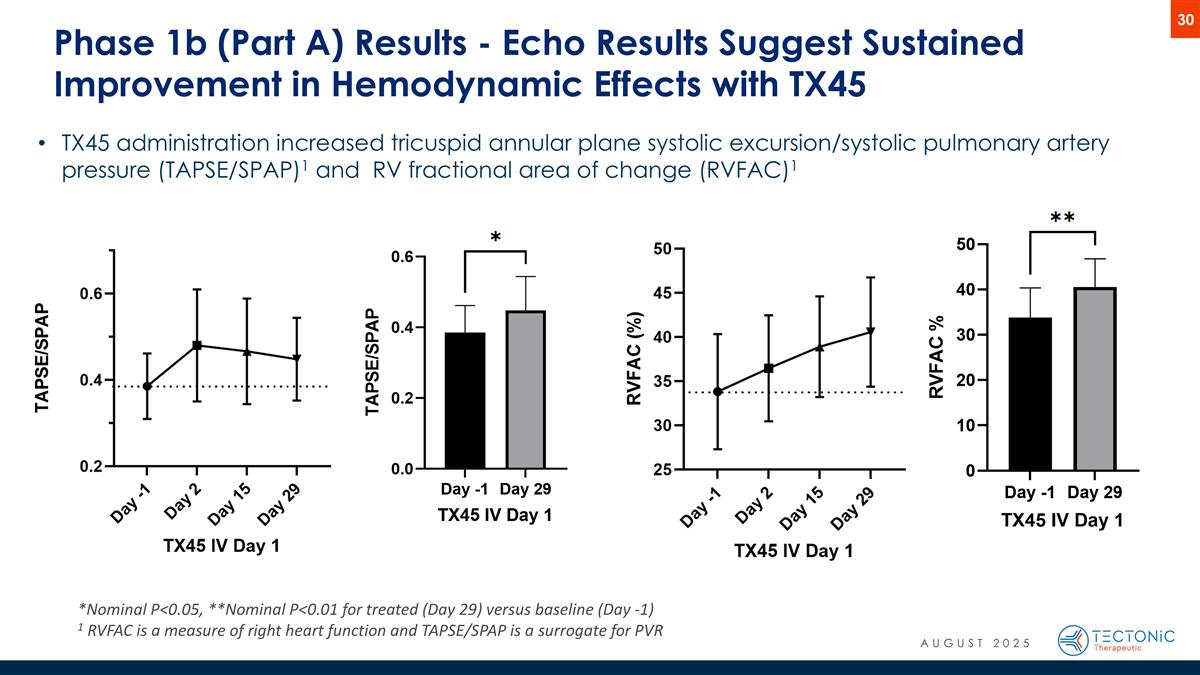
Phase 1b (Part A) Results - Echo Results Suggest Sustained Improvement in Hemodynamic Effects with TX45 *Nominal P<0.05, **Nominal P<0.01 for treated (Day 29) versus baseline (Day -1) 1 RVFAC is a measure of right heart function and TAPSE/SPAP is a surrogate for PVR TX45 administration increased tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/SPAP)1 and RV fractional area of change (RVFAC)1 AUGUST 2025
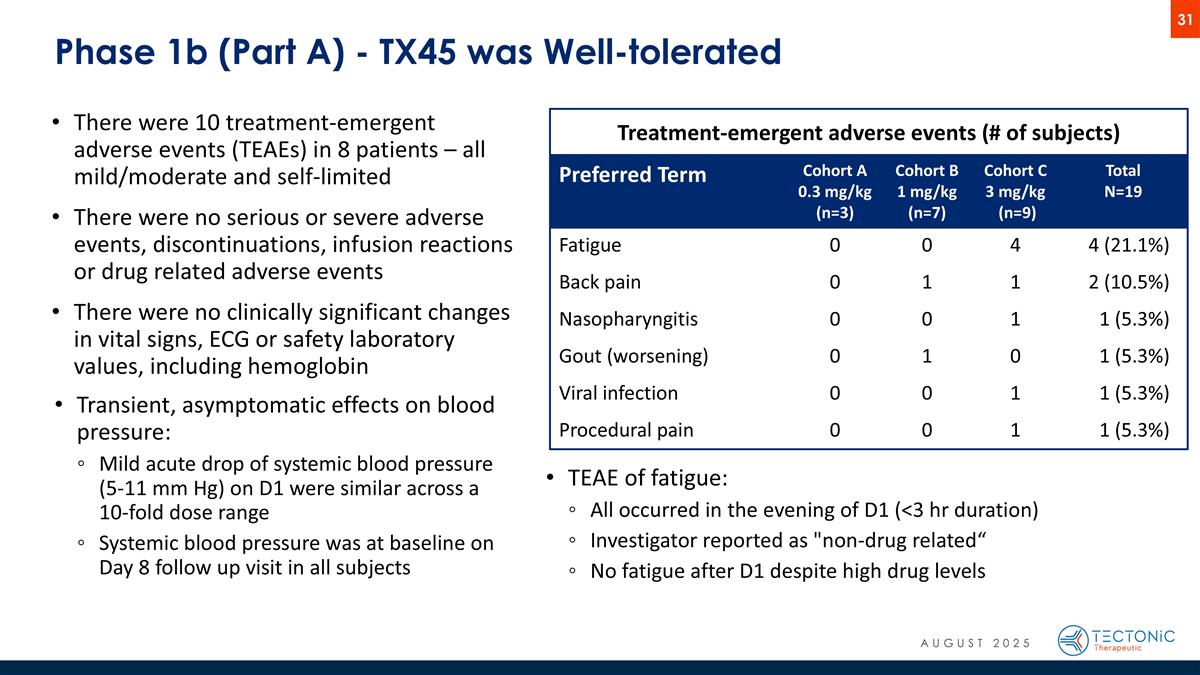
Phase 1b (Part A) - TX45 was Well-tolerated Preferred Term Cohort A 0.3 mg/kg (n=3) Cohort B 1 mg/kg (n=7) Cohort C 3 mg/kg (n=9) Total N=19 Fatigue 0 0 4 4 (21.1%) Back pain 0 1 1 2 (10.5%) Nasopharyngitis 0 0 1 1 (5.3%) Gout (worsening) 0 1 0 1 (5.3%) Viral infection 0 0 1 1 (5.3%) Procedural pain 0 0 1 1 (5.3%) Treatment-emergent adverse events (# of subjects) There were 10 treatment-emergent adverse events (TEAEs) in 8 patients – all mild/moderate and self-limited There were no serious or severe adverse events, discontinuations, infusion reactions or drug related adverse events There were no clinically significant changes in vital signs, ECG or safety laboratory values, including hemoglobin Transient, asymptomatic effects on blood pressure: Mild acute drop of systemic blood pressure (5-11 mm Hg) on D1 were similar across a 10-fold dose range Systemic blood pressure was at baseline on Day 8 follow up visit in all subjects TEAE of fatigue: All occurred in the evening of D1 (<3 hr duration) Investigator reported as "non-drug related“ No fatigue after D1 despite high drug levels AUGUST 2025
Combined Decrease in PCWP and PVR Appears to Enhance Improvement in Exercise Capacity Decreasing PCWP alone is expected to improve exercise capacity PCWP provides insight into left ventricular function and correlates with exercise capacity in HFpEF, HFrEF1 and Group 2 pulmonary hypertension (CpcPH)2 SGLT2 inhibitor dapagliflozin decreased resting PCWP 20%3 and increased 6MWD by 20m in HFpEF4 Decreasing both PCWP and PVR in CpcPH appears to further increase in exercise capacity In PAH, lowering of PVR by 20% is associated with clinically significant improvements in 6MWD5 In Group 2 PH (CpcPH), lowering of PCWP and PVR is correlated with increased 6MWD2 CpcPH patients undergoing pulmonary artery denervation surgery achieved a treatment-adjusted average decrease of 19% in PCWP and 32% in PVR, and increased 6MWT distance 69m6 [NOTE: This was a severe population of CpcPH patients (mean PVR>6 WU) and we expect impact on 6MWD will be clinically important but not as large as demonstrated in this study] 1 Wolsk E et al. Eur. J. Heart Fail. 2018 2 Zotter-Tufaro C et al. J. Am. Coll. Cardiol. H.F. 2015 3 Borlaug B et al. Circulation 2023 4 Lewis GD et al. Circ. Heart Failure 2023 5 www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209279Orig1s000MedR.pdf 6 Zhang H et al. JACC Cardiovasc. Interv. 2019 AUGUST 2025
TX45 was well-tolerated Transient asymptomatic decreases in BP were observed over the first 24 hours after an IV dose TX45 observed to improve left heart function and pulmonary hemodynamics, which together should increase probability of success of APEX Phase 2 Trial TX45 has a differentiated profile compared with PAH drugs which improved pulmonary hemodynamics without an improvement in left heart function and failed in PH-HFpEF Echocardiographic analyses suggest sustained improvements in hemodynamics with single dose TX45 These data support our focus on PH-HFpEF as the first indication for TX45, with enrichment in our Phase 2 trial for subjects with CpcPH where the benefit could be the greatest TX45 Phase 1b Part A Results in PH-HFpEF Met/Exceeded Expectations, Expected to Increase Phase 2 Probability of Success AUGUST 2025
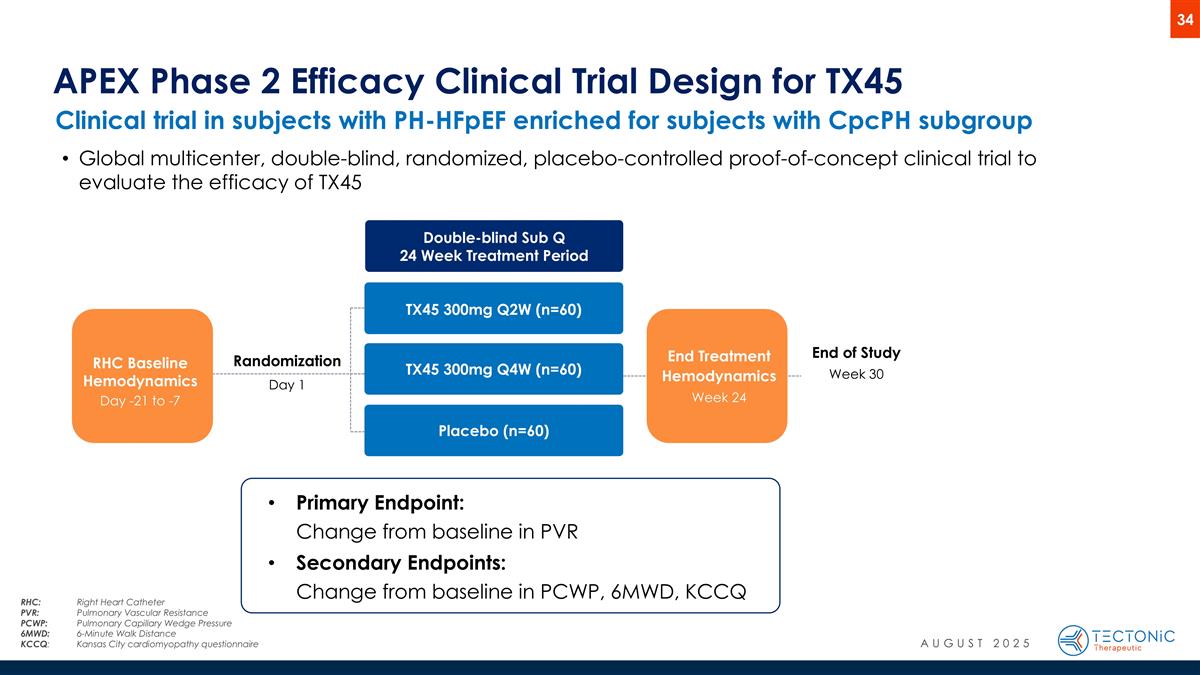
End of Study Week 30 End Treatment Hemodynamics Week 24 Clinical trial in subjects with PH-HFpEF enriched for subjects with CpcPH subgroup APEX Phase 2 Efficacy Clinical Trial Design for TX45 Randomization Day 1 RHC Baseline Hemodynamics Day -21 to -7 Double-blind Sub Q 24 Week Treatment Period TX45 300mg Q2W (n=60) TX45 300mg Q4W (n=60) Placebo (n=60) Primary Endpoint: Change from baseline in PVR Secondary Endpoints: Change from baseline in PCWP, 6MWD, KCCQ Global multicenter, double-blind, randomized, placebo-controlled proof-of-concept clinical trial to evaluate the efficacy of TX45 AUGUST 2025 RHC:Right Heart Catheter PVR: Pulmonary Vascular Resistance PCWP: Pulmonary Capillary Wedge Pressure 6MWD: 6-Minute Walk Distance KCCQ:Kansas City cardiomyopathy questionnaire
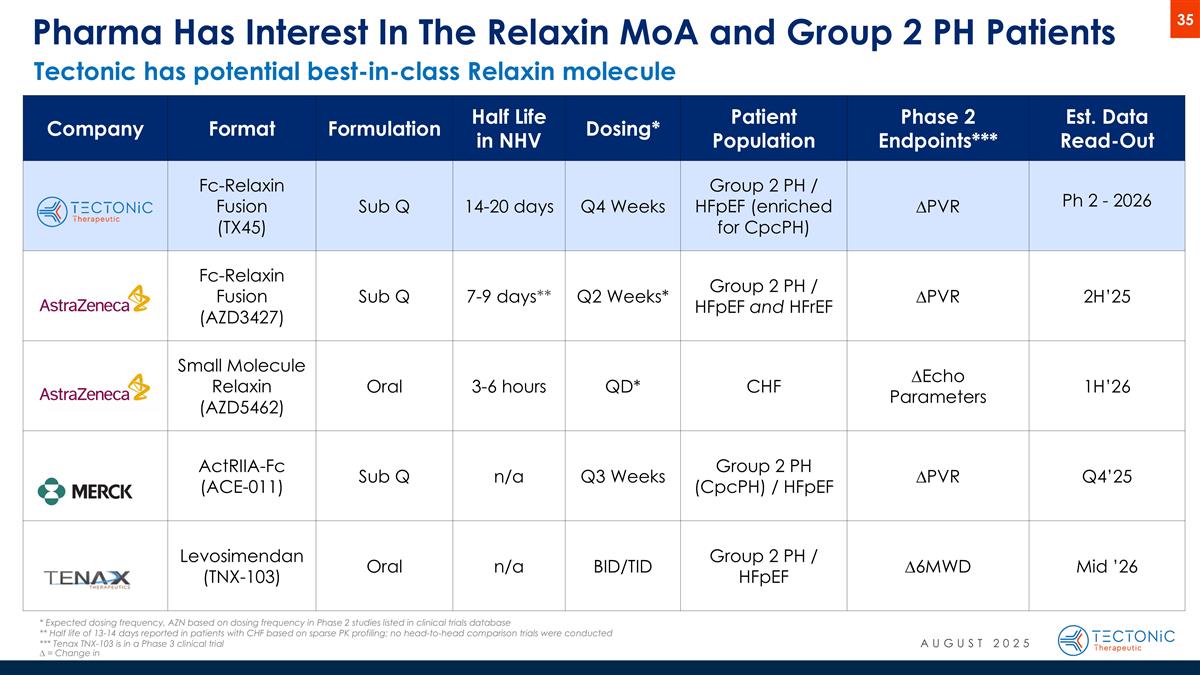
Pharma Has Interest In The Relaxin MoA and Group 2 PH Patients Company Format Formulation Half Life in NHV Dosing* Patient Population Phase 2 Endpoints*** Est. Data Read-Out Fc-Relaxin Fusion (TX45) Sub Q 14-20 days Q4 Weeks Group 2 PH / HFpEF (enriched for CpcPH) ∆PVR Ph 2 - 2026 Fc-Relaxin Fusion (AZD3427) Sub Q 7-9 days** Q2 Weeks* Group 2 PH / HFpEF and HFrEF ∆PVR 2H’25 Small Molecule Relaxin (AZD5462) Oral 3-6 hours QD* CHF ∆Echo Parameters 1H’26 ActRIIA-Fc (ACE-011) Sub Q n/a Q3 Weeks Group 2 PH (CpcPH) / HFpEF ∆PVR Q4’25 Levosimendan (TNX-103) Oral n/a BID/TID Group 2 PH / HFpEF ∆6MWD Mid ’26 * Expected dosing frequency, AZN based on dosing frequency in Phase 2 studies listed in clinical trials database ** Half life of 13-14 days reported in patients with CHF based on sparse PK profiling; no head-to-head comparison trials were conducted *** Tenax TNX-103 is in a Phase 3 clinical trial ∆ = Change in Tectonic has potential best-in-class Relaxin molecule AUGUST 2025

TX45 PH-ILD Program Potential differentiated treatment of Pulmonary Hypertension associated with Interstitial Lung Disease (PH-ILD)
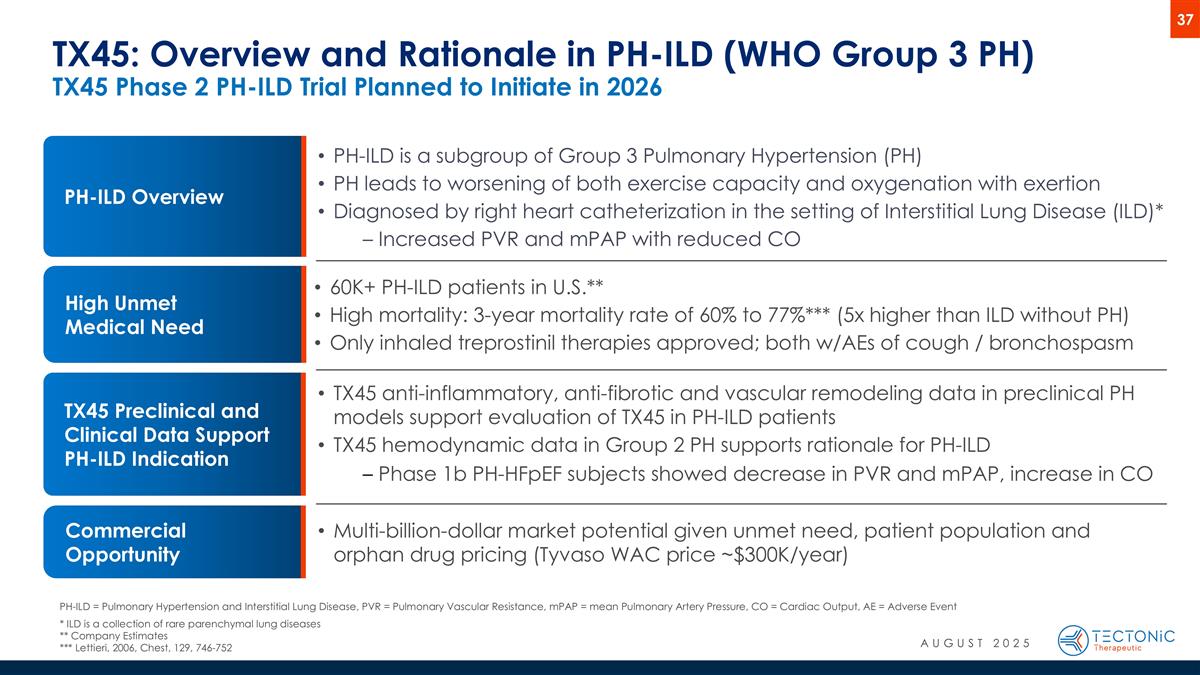
TX45 Preclinical and Clinical Data Support PH-ILD Indication TX45: Overview and Rationale in PH-ILD (WHO Group 3 PH) TX45 Phase 2 PH-ILD Trial Planned to Initiate in 2026 High Unmet Medical Need 60K+ PH-ILD patients in U.S.** High mortality: 3-year mortality rate of 60% to 77%*** (5x higher than ILD without PH) Only inhaled treprostinil therapies approved; both w/AEs of cough / bronchospasm TX45 anti-inflammatory, anti-fibrotic and vascular remodeling data in preclinical PH models support evaluation of TX45 in PH-ILD patients TX45 hemodynamic data in Group 2 PH supports rationale for PH-ILD Phase 1b PH-HFpEF subjects showed decrease in PVR and mPAP, increase in CO Multi-billion-dollar market potential given unmet need, patient population and orphan drug pricing (Tyvaso WAC price ~$300K/year) Commercial Opportunity PH-ILD Overview PH-ILD is a subgroup of Group 3 Pulmonary Hypertension (PH) PH leads to worsening of both exercise capacity and oxygenation with exertion Diagnosed by right heart catheterization in the setting of Interstitial Lung Disease (ILD)* Increased PVR and mPAP with reduced CO PH-ILD = Pulmonary Hypertension and Interstitial Lung Disease, PVR = Pulmonary Vascular Resistance, mPAP = mean Pulmonary Artery Pressure, CO = Cardiac Output, AE = Adverse Event * ILD is a collection of rare parenchymal lung diseases ** Company Estimates *** Lettieri, 2006, Chest, 129, 746-752 AUGUST 2025
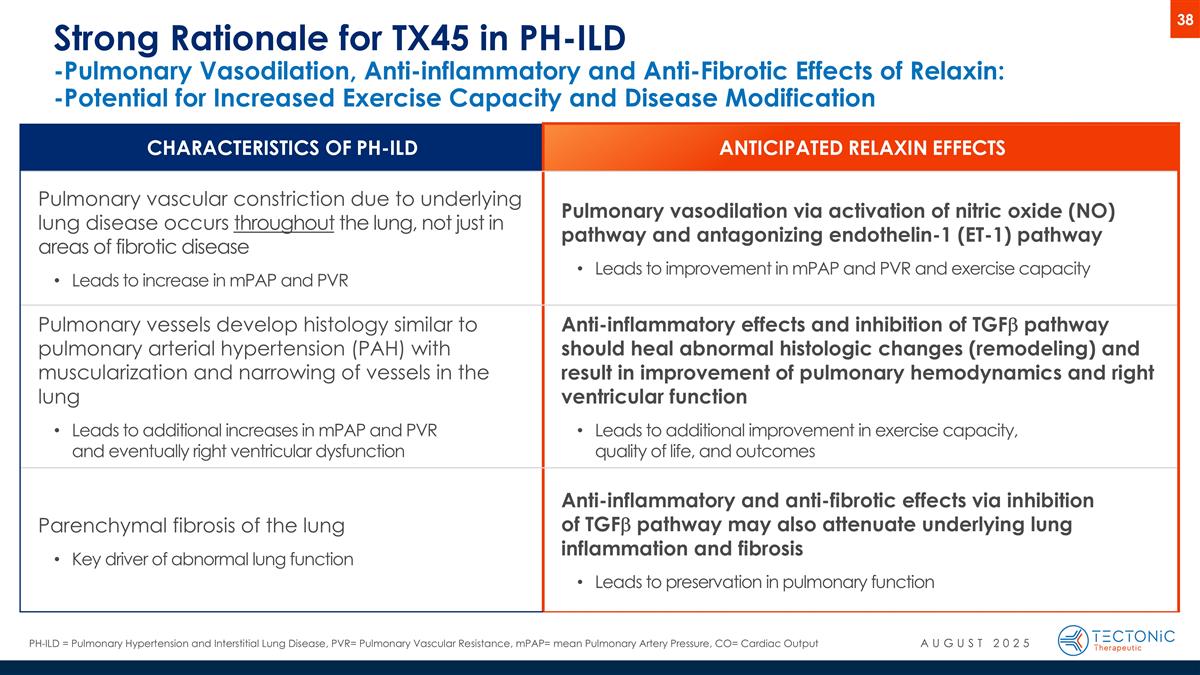
Strong Rationale for TX45 in PH-ILD -Pulmonary Vasodilation, Anti-inflammatory and Anti-Fibrotic Effects of Relaxin: -Potential for Increased Exercise Capacity and Disease Modification CHARACTERISTICS OF PH-ILD ANTICIPATED RELAXIN EFFECTS Pulmonary vascular constriction due to underlying lung disease occurs throughout the lung, not just in areas of fibrotic disease Leads to increase in mPAP and PVR Pulmonary vasodilation via activation of nitric oxide (NO) pathway and antagonizing endothelin-1 (ET-1) pathway Leads to improvement in mPAP and PVR and exercise capacity Pulmonary vessels develop histology similar to pulmonary arterial hypertension (PAH) with muscularization and narrowing of vessels in the lung Leads to additional increases in mPAP and PVR and eventually right ventricular dysfunction Anti-inflammatory effects and inhibition of TGFb pathway should heal abnormal histologic changes (remodeling) and result in improvement of pulmonary hemodynamics and right ventricular function Leads to additional improvement in exercise capacity, quality of life, and outcomes Parenchymal fibrosis of the lung Key driver of abnormal lung function Anti-inflammatory and anti-fibrotic effects via inhibition of TGFb pathway may also attenuate underlying lung inflammation and fibrosis Leads to preservation in pulmonary function PH-ILD = Pulmonary Hypertension and Interstitial Lung Disease, PVR= Pulmonary Vascular Resistance, mPAP= mean Pulmonary Artery Pressure, CO= Cardiac Output 38 AUGUST 2025
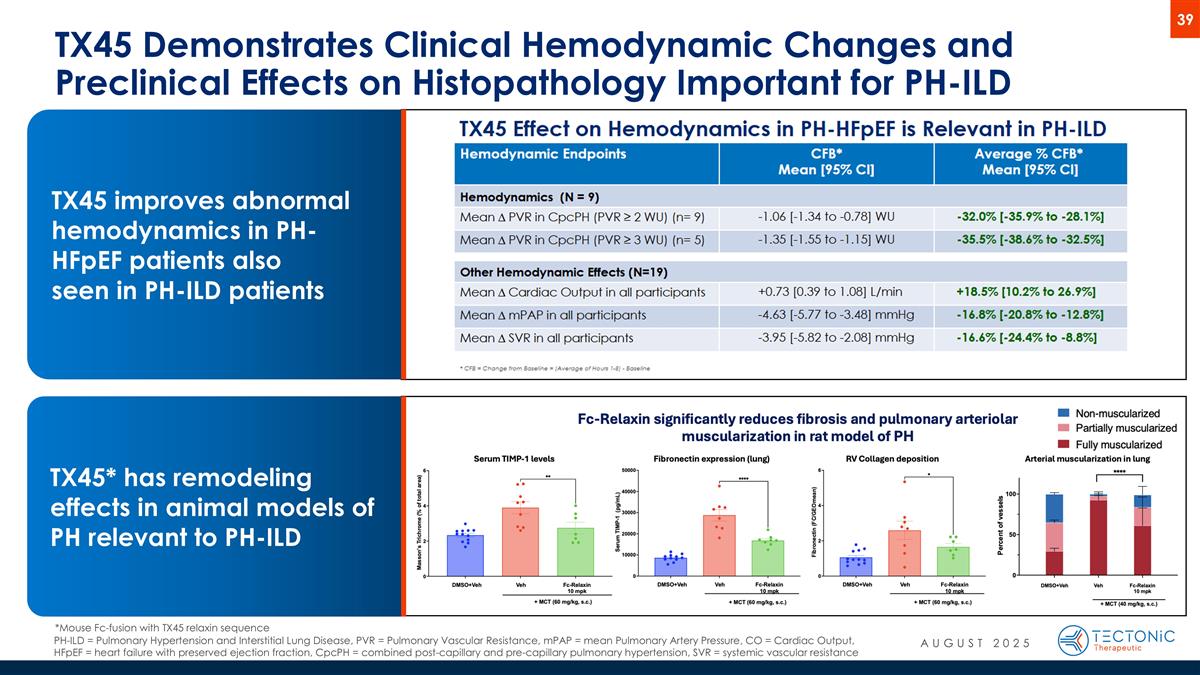
TX45 Demonstrates Clinical Hemodynamic Changes and Preclinical Effects on Histopathology Important for PH-ILD TX45 improves abnormal hemodynamics in PH-HFpEF patients also seen in PH-ILD patients *Mouse Fc-fusion with TX45 relaxin sequence TX45* has remodeling effects in animal models of PH relevant to PH-ILD PH-ILD = Pulmonary Hypertension and Interstitial Lung Disease, PVR = Pulmonary Vascular Resistance, mPAP = mean Pulmonary Artery Pressure, CO = Cardiac Output, HFpEF = heart failure with preserved ejection fraction, CpcPH = combined post-capillary and pre-capillary pulmonary hypertension, SVR = systemic vascular resistance AUGUST 2025
Why Have Inhaled Therapies Succeeded in PH-ILD And Systemic Pulmonary Vasodilatory Compounds Have Failed? Success of inhaled treprostinil treatments: Targeted pulmonary hemodynamic effects while avoiding prostacyclin-specific systemic side effects Clinical trials in focused population of confirmed PH-ILD with 6MWD and QoL as endpoints Inhaled approach resulted in: 1) lowering of PVR and mPAP without potent systemic BP effects, 2) avoided ventilation/perfusion (VQ) mismatch and resultant worsening oxygenation seen with systemic prostacyclin* Multi-factorial reasons for failure of oral pulmonary arterial hypertension (PAH) therapies include selected patient population, toxicity of specific mechanisms and study design – not due to worsening hypoxemia Promising data in PDE5 inhibitors* – Sildenafil, which acts to enhance the nitric oxide (NO) pathway, demonstrated promising clinical data in PH-ILD subjects with positive trends in 6MWD, oxygenation and QoL. Larger trials evaluated ILD subjects and failed TX45 has the potential to improve pulmonary hemodynamics without systemic hypotension or worsening hypoxemia * Sholbin 2024, Kattih, 2025 AUGUST 2025 PH-ILD = Pulmonary Hypertension and Interstitial Lung Disease, PVR = Pulmonary Vascular Resistance, mPAP = mean Pulmonary Artery Pressure, CO = Cardiac Output, 6MWD = Six Minute Walk Distance
TX45 Phase 2 PH-ILD Study: Overview of Design and Rationale Size: ~20 subjects with PH-ILD Baseline right heart catheterization (RHC) to diagnose PH Dosing: 300mg Q2 weeks subcutaneous Endpoints: ∆ from baseline in the following: Safety: oxygenation and systolic blood pressure (sBP) Primary Efficacy: PVR Secondary and exploratory: mPAP and CO, 6MWD, quality of life (QoL) TX45 Open Label, Repeat Dose, 16 Week, Phase 2 Trial Potential improvement in efficacy endpoints over 16 weeks in open-label trial should replicate in placebo-controlled trial PVR and 6MWD have not improved over 16 weeks in placebo arms of randomized controlled trials in PH-ILD* Adequate treatment time to assess improvement in fibrotic/remodeled vessels and to address maintenance of effect Rationale for Approach * Corte 2014, Vachiery 2018, Waxman , 2021 PH-ILD = Pulmonary Hypertension and Interstitial Lung Disease, PVR = Pulmonary Vascular Resistance, mPAP = mean Pulmonary Artery Pressure, CO = Cardiac Output, 6MWD = Six Minute Walk Distance AUGUST 2025
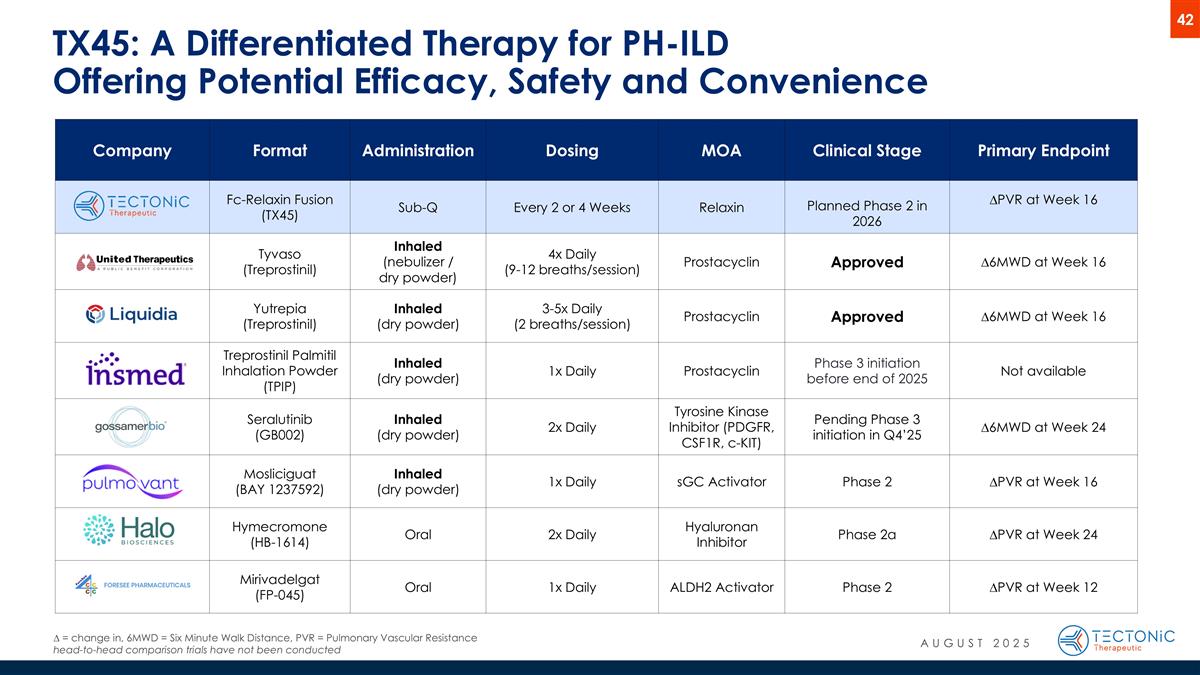
TX45: A Differentiated Therapy for PH-ILD Offering Potential Efficacy, Safety and Convenience ∆ = change in, 6MWD = Six Minute Walk Distance, PVR = Pulmonary Vascular Resistance head-to-head comparison trials have not been conducted Company Format Administration Dosing MOA Clinical Stage Primary Endpoint Fc-Relaxin Fusion (TX45) Sub-Q Every 2 or 4 Weeks Relaxin Planned Phase 2 in 2026 ∆PVR at Week 16 Tyvaso (Treprostinil) Inhaled (nebulizer / dry powder) 4x Daily (9-12 breaths/session) Prostacyclin Approved ∆6MWD at Week 16 Yutrepia (Treprostinil) Inhaled (dry powder) 3-5x Daily (2 breaths/session) Prostacyclin Approved ∆6MWD at Week 16 Treprostinil Palmitil Inhalation Powder (TPIP) Inhaled (dry powder) 1x Daily Prostacyclin Phase 3 initiation before end of 2025 Not available Seralutinib (GB002) Inhaled (dry powder) 2x Daily Tyrosine Kinase Inhibitor (PDGFR, CSF1R, c-KIT) Pending Phase 3 initiation in Q4’25 ∆6MWD at Week 24 Mosliciguat (BAY 1237592) Inhaled (dry powder) 1x Daily sGC Activator Phase 2 ∆PVR at Week 16 Hymecromone (HB-1614) Oral 2x Daily Hyaluronan Inhibitor Phase 2a ∆PVR at Week 24 Mirivadelgat (FP-045) Oral 1x Daily ALDH2 Activator Phase 2 ∆PVR at Week 12 AUGUST 2025
Summary: Strong Rationale for TX45 to Bring a Differentiated Treatment Approach to Address Unmet Needs in PH-ILD PH-ILD is a disease with high morbidity/mortality and insufficient therapeutic options Preclinical PH data and clinical hemodynamic data in PH-HFpEF suggest that TX45 is well suited as a treatment for PH-ILD TX45 offers a potential systemic relaxin therapy for treatment of PH-ILD, enabling expansion of the TX45 program into another large market opportunity Tectonic plans to initiate a Phase 2 study in 2026 to explore safety and efficacy of TX45 treatment in subjects with PH-ILD over 16 weeks Disease Burden Relevant Preclinical / Clinical Data Expand TX45 Potential Phase 2 Planned AUGUST 2025

TX2100 HHT program Potential first-in-class and indication opportunity for Hereditary Hemorrhagic Telangiectasia (HHT), the 2nd most common genetic bleeding disorder
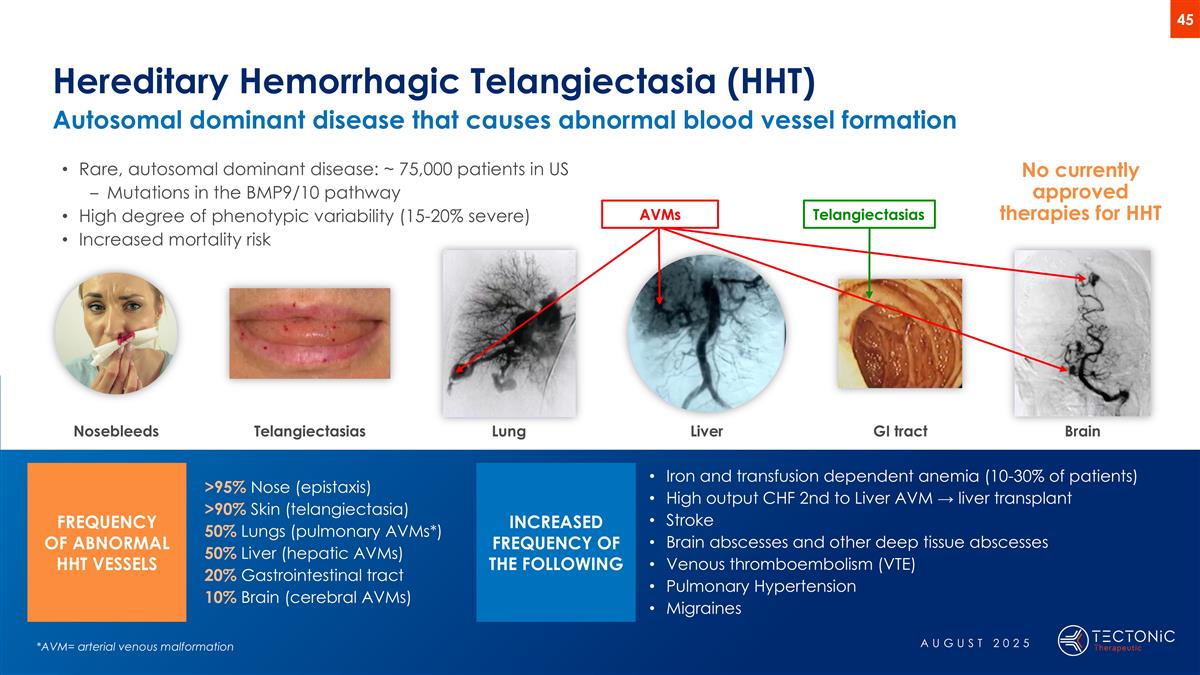
Hereditary Hemorrhagic Telangiectasia (HHT) Autosomal dominant disease that causes abnormal blood vessel formation >95% Nose (epistaxis) >90% Skin (telangiectasia) 50% Lungs (pulmonary AVMs*) 50% Liver (hepatic AVMs) 20% Gastrointestinal tract 10% Brain (cerebral AVMs) Iron and transfusion dependent anemia (10-30% of patients) High output CHF 2nd to Liver AVM → liver transplant Stroke Brain abscesses and other deep tissue abscesses Venous thromboembolism (VTE) Pulmonary Hypertension Migraines Rare, autosomal dominant disease: ~ 75,000 patients in US Mutations in the BMP9/10 pathway High degree of phenotypic variability (15-20% severe) Increased mortality risk No currently approved therapies for HHT *AVM= arterial venous malformation Nosebleeds Lung Liver Brain Telangiectasias GI tract FREQUENCY OF ABNORMAL HHT VESSELS INCREASED FREQUENCY OF THE FOLLOWING Telangiectasias AVMs AUGUST 2025
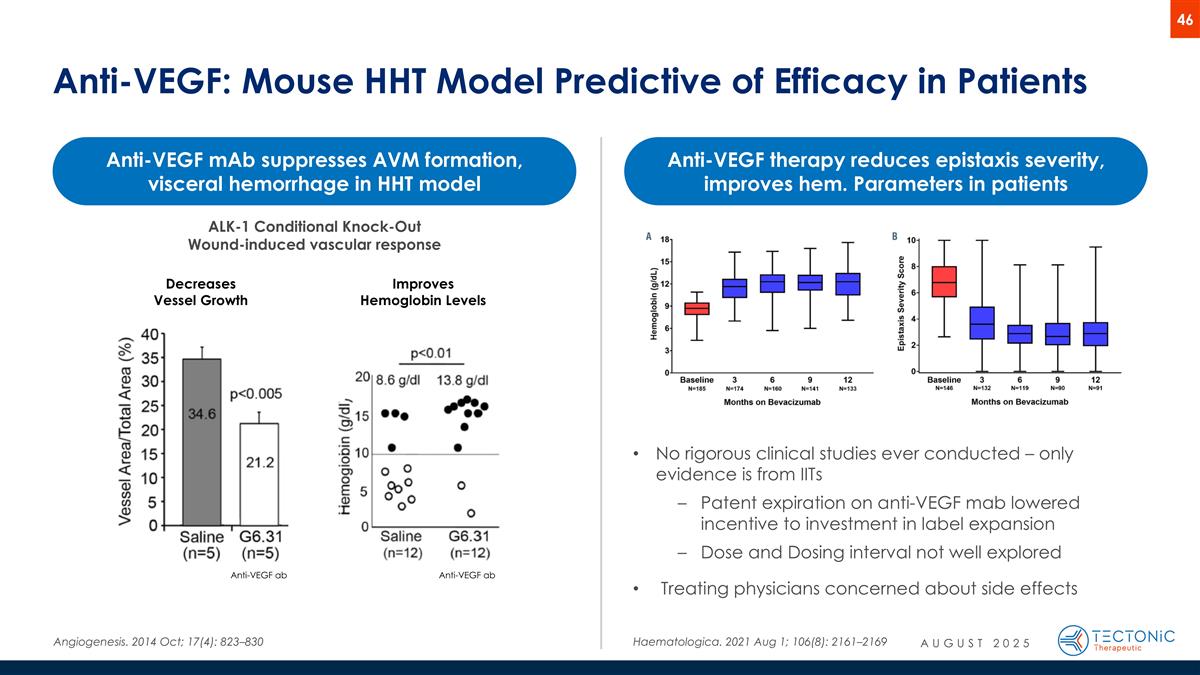
Anti-VEGF: Mouse HHT Model Predictive of Efficacy in Patients Angiogenesis. 2014 Oct; 17(4): 823–830 Incisional wounds Anti-VEGF ab Decreases Vessel Growth Anti-VEGF ab Improves Hemoglobin Levels Anti-VEGF mAb suppresses AVM formation, visceral hemorrhage in HHT model Haematologica. 2021 Aug 1; 106(8): 2161–2169 Anti-VEGF therapy reduces epistaxis severity, improves hem. Parameters in patients ALK-1 Conditional Knock-Out Wound-induced vascular response No rigorous clinical studies ever conducted – only evidence is from IITs Patent expiration on anti-VEGF mab lowered incentive to investment in label expansion Dose and Dosing interval not well explored Treating physicians concerned about side effects AUGUST 2025
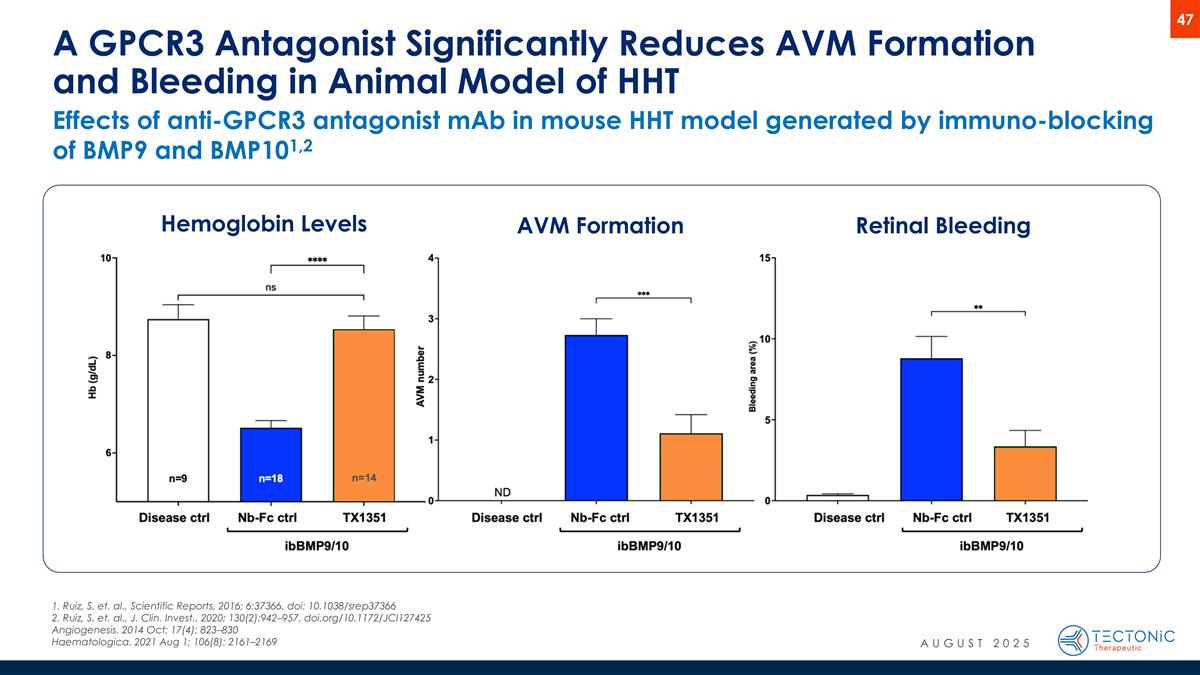
Effects of anti-GPCR3 antagonist mAb in mouse HHT model generated by immuno-blocking of BMP9 and BMP101,2 A GPCR3 Antagonist Significantly Reduces AVM Formation and Bleeding in Animal Model of HHT Hemoglobin Levels AVM Formation Retinal Bleeding n=14 1. Ruiz, S. et. al., Scientific Reports, 2016; 6:37366, doi: 10.1038/srep37366 2. Ruiz, S. et. al., J. Clin. Invest., 2020; 130(2):942–957, doi.org/10.1172/JCI127425 Angiogenesis. 2014 Oct; 17(4): 823–830 Haematologica. 2021 Aug 1; 106(8): 2161–2169 AUGUST 2025
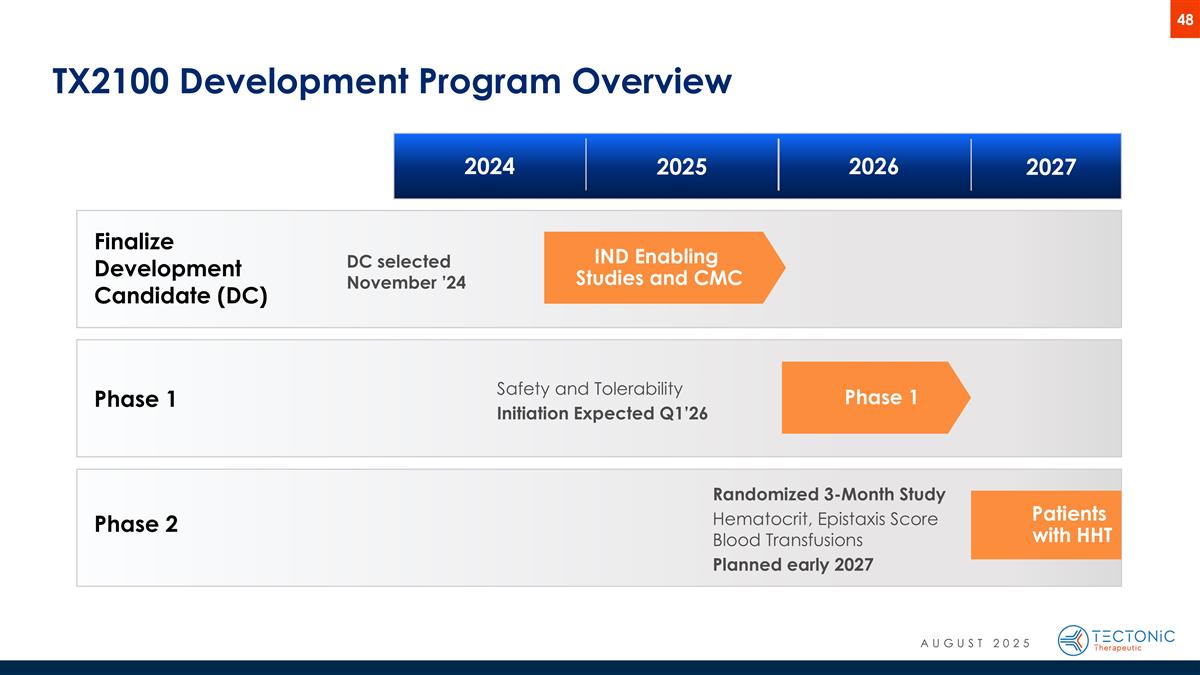
Phase 1 TX2100 Development Program Overview Patients with HHT Phase 1 IND Enabling Studies and CMC Finalize Development Candidate (DC) Phase 2 Safety and Tolerability Initiation Expected Q1’26 Randomized 3-Month Study Hematocrit, Epistaxis Score Blood Transfusions Planned early 2027 2024 2025 2026 2027 DC selected November ’24 AUGUST 2025

Platform Proprietary platform enables reproducible discovery and optimization of GPCR targeted biologics
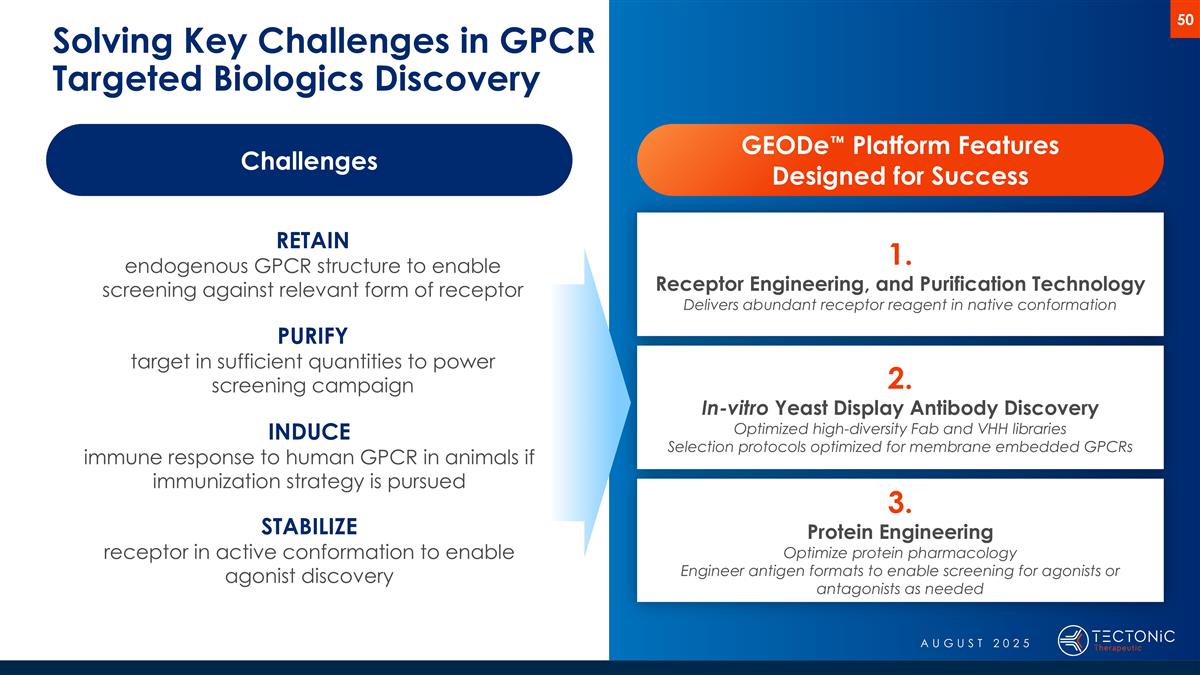
Solving Key Challenges in GPCR Targeted Biologics Discovery Challenges RETAIN endogenous GPCR structure to enable screening against relevant form of receptor PURIFY target in sufficient quantities to power screening campaign INDUCE immune response to human GPCR in animals if immunization strategy is pursued STABILIZE receptor in active conformation to enable agonist discovery GEODe™ Platform Features Designed for Success 1. Receptor Engineering, and Purification Technology Delivers abundant receptor reagent in native conformation 2. In-vitro Yeast Display Antibody Discovery Optimized high-diversity Fab and VHH libraries Selection protocols optimized for membrane embedded GPCRs 3. Protein Engineering Optimize protein pharmacology Engineer antigen formats to enable screening for agonists or antagonists as needed AUGUST 2025
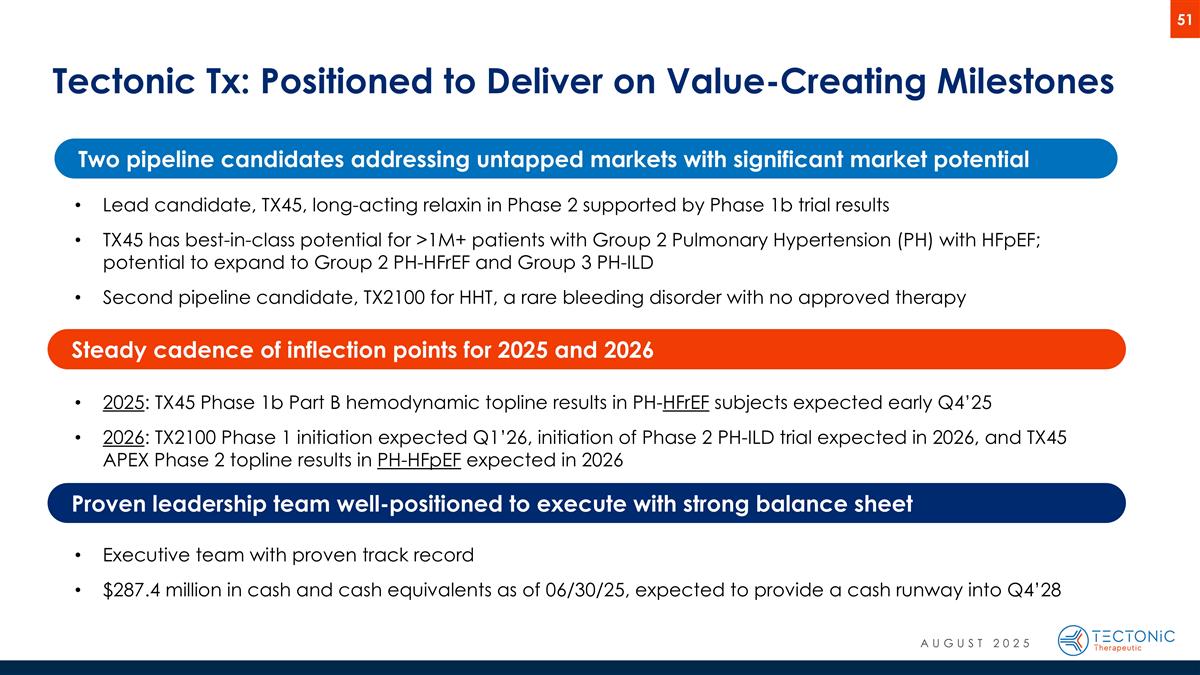
Tectonic Tx: Positioned to Deliver on Value-Creating Milestones Lead candidate, TX45, long-acting relaxin in Phase 2 supported by Phase 1b trial results TX45 has best-in-class potential for >1M+ patients with Group 2 Pulmonary Hypertension (PH) with HFpEF; potential to expand to Group 2 PH-HFrEF and Group 3 PH-ILD Second pipeline candidate, TX2100 for HHT, a rare bleeding disorder with no approved therapy 2025: TX45 Phase 1b Part B hemodynamic topline results in PH-HFrEF subjects expected early Q4’25 2026: TX2100 Phase 1 initiation expected Q1’26, initiation of Phase 2 PH-ILD trial expected in 2026, and TX45 APEX Phase 2 topline results in PH-HFpEF expected in 2026 Executive team with proven track record $287.4 million in cash and cash equivalents as of 06/30/25, expected to provide a cash runway into Q4’28 Two pipeline candidates addressing untapped markets with significant market potential Steady cadence of inflection points for 2025 and 2026 Proven leadership team well-positioned to execute with strong balance sheet AUGUST 2025

Thank You info@tectonictx.com www.tectonictx.com LinkedIn: TectonicTx