
CORPORATE OVERVIEW May, 2025 ®

2 Forward-looking statements This presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to express or implied statements regarding the current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, , including statements regarding the estimated market for our product candidates, if approved, our development plans, our preclinical and clinical results and other future conditions, including our cash runway, and the safety, efficacy, and regulatory and clinical design or progress, potential regulatory submissions, approvals and timing thereof of any of our product candidates. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, risks relating to: (i) the success and timing of our ongoing clinical trials, (ii) the success and timing of our product development activities and initiating clinical trials, (iii) the success and timing of our collaboration partners’ product development activities, (iv) the timing of and our ability to obtain and maintain regulatory approval of any of our product candidates, (v) our plans to research, discover and develop additional product candidates, (vi) our ability to enter into collaborations for the development of new product candidates, (vii) our ability to establish manufacturing capabilities, and our collaboration partners’ abilities to manufacture our product candidates and scale production, (viii) our ability to meet any specific milestones set forth herein, and (ix) the potential addressable market sizes for product candidates. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. For further information regarding the risks, uncertainties and other factors that may cause differences between our expectations and actual results, you should review the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2024 filed with the Securities and Exchange Commission (“SEC”) and our other filings with the SEC. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.
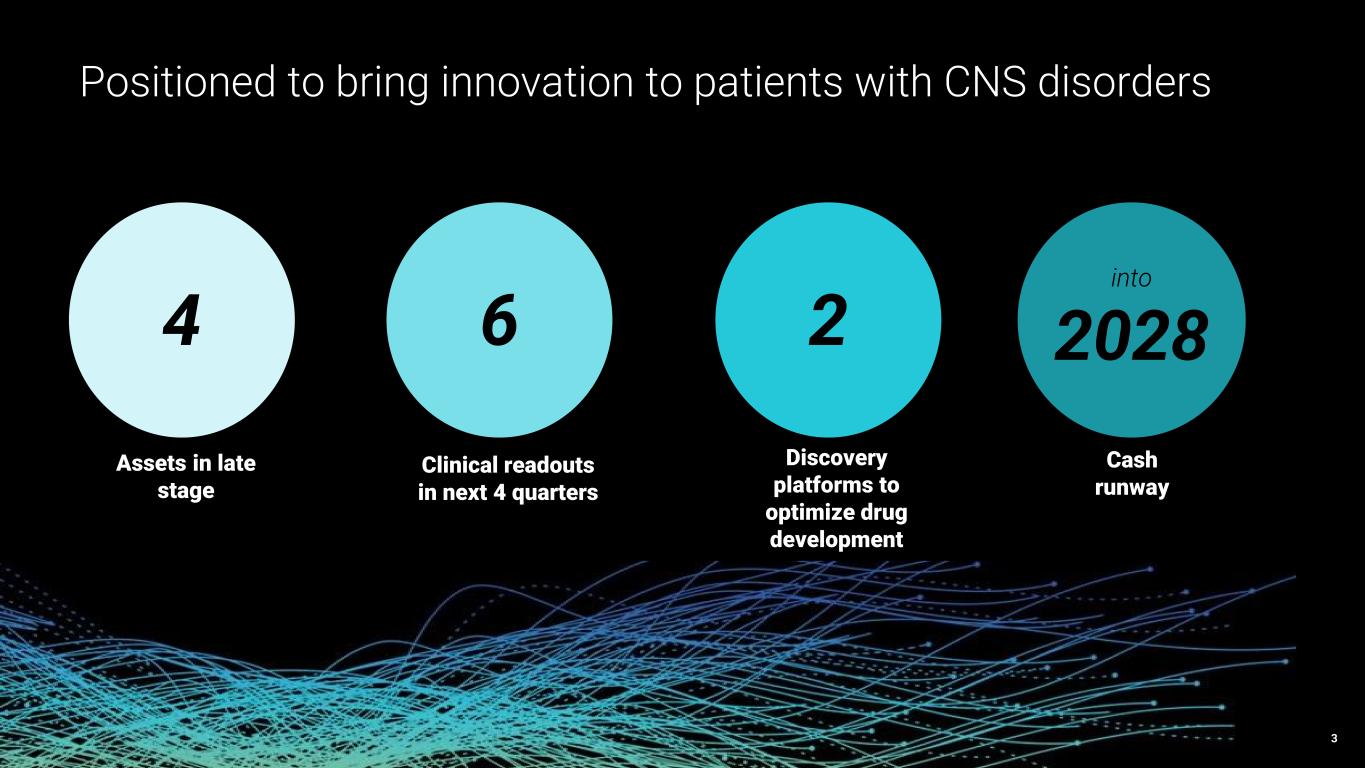
3 4 6 2 2028 Positioned to bring innovation to patients with CNS disorders Assets in late stage Clinical readouts in next 4 quarters Discovery platforms to optimize drug development Cash runway into
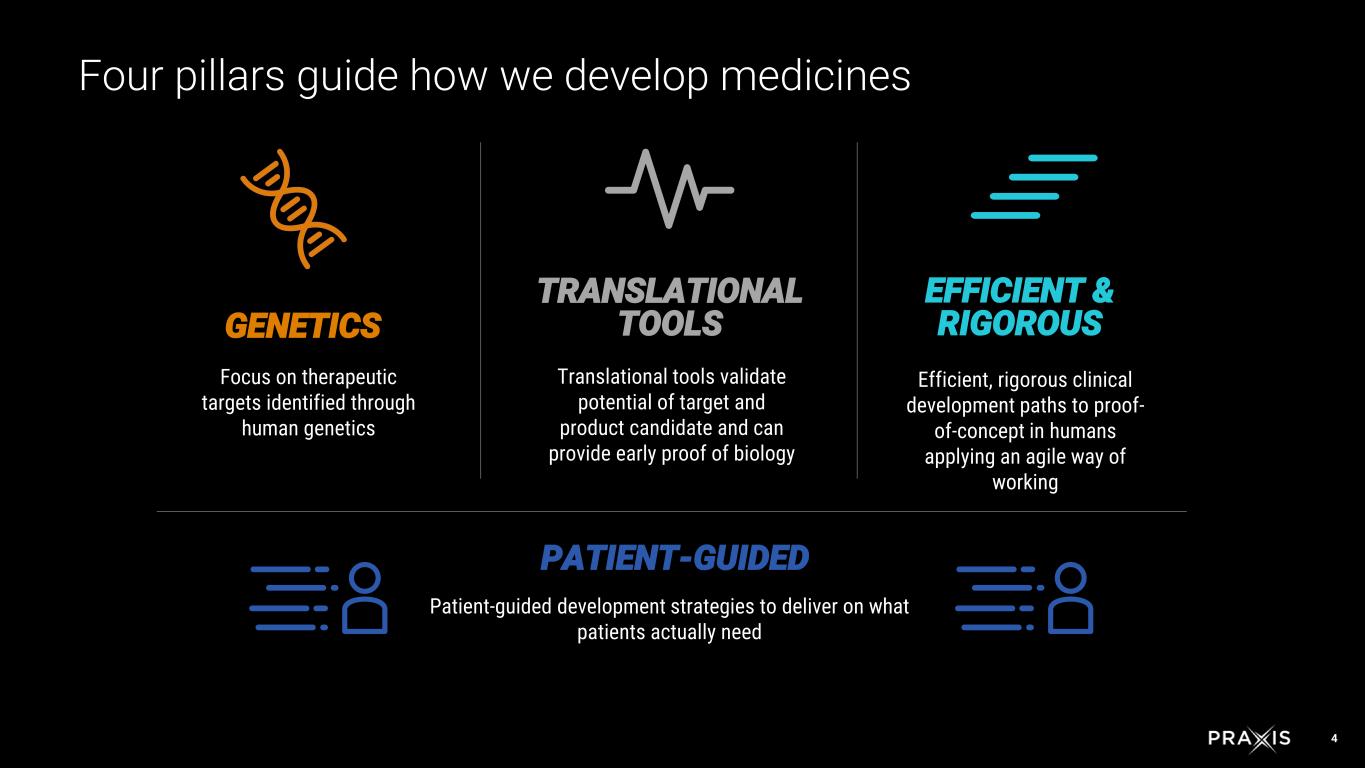
4 GENETICS Focus on therapeutic targets identified through human genetics TRANSLATIONAL TOOLS Translational tools validate potential of target and product candidate and can provide early proof of biology EFFICIENT & RIGOROUS Efficient, rigorous clinical development paths to proof- of-concept in humans applying an agile way of working PATIENT-GUIDED Patient-guided development strategies to deliver on what patients actually need Four pillars guide how we develop medicines
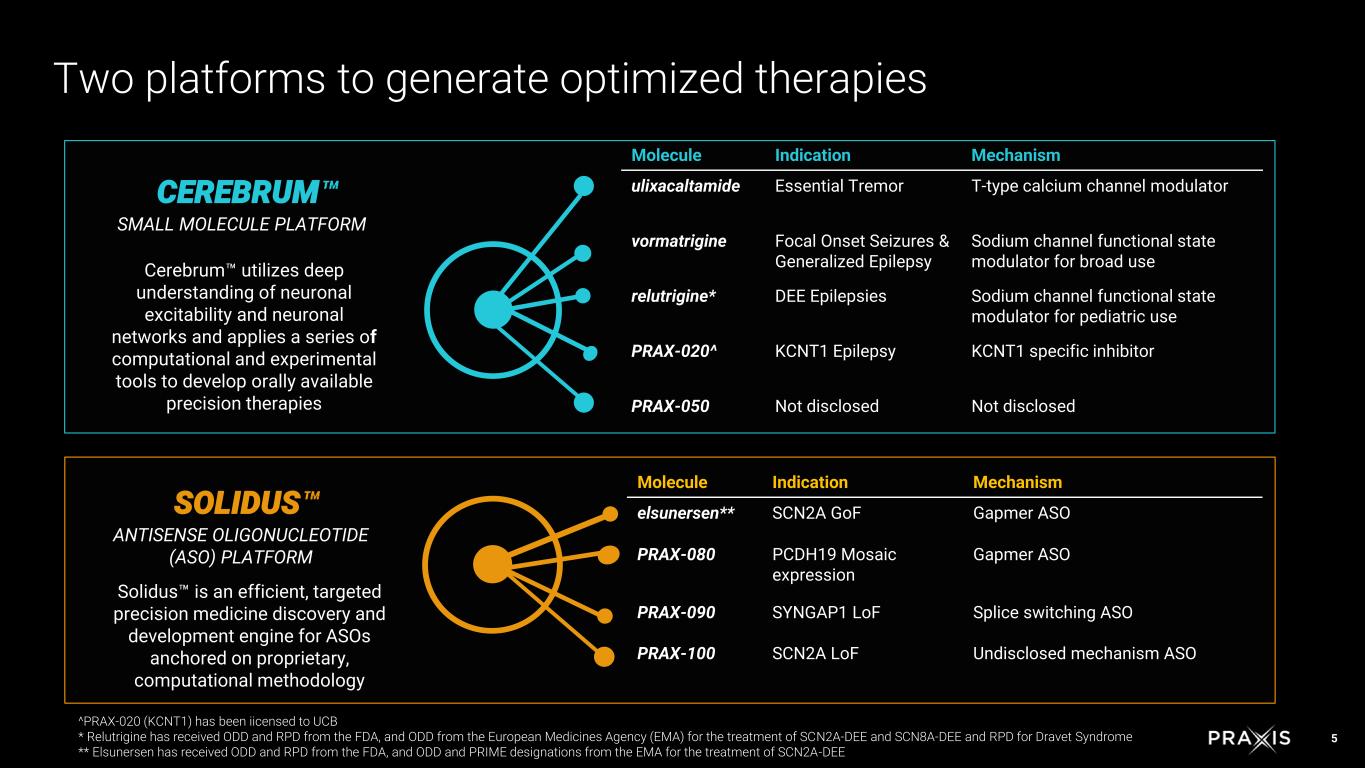
5 CEREBRUM SMALL MOLECULE PLATFORM Cerebrum utilizes deep understanding of neuronal excitability and neuronal networks and applies a series of computational and experimental tools to develop orally available precision therapies SOLIDUS ANTISENSE OLIGONUCLEOTIDE (ASO) PLATFORM Solidus is an efficient, targeted precision medicine discovery and development engine for ASOs anchored on proprietary, computational methodology Two platforms to generate optimized therapies Molecule Indication Mechanism ulixacaltamide Essential Tremor T-type calcium channel modulator vormatrigine Focal Onset Seizures & Generalized Epilepsy Sodium channel functional state modulator for broad use relutrigine* DEE Epilepsies Sodium channel functional state modulator for pediatric use PRAX-020^ KCNT1 Epilepsy KCNT1 specific inhibitor PRAX-050 Not disclosed Not disclosed Molecule Indication Mechanism elsunersen** SCN2A GoF Gapmer ASO PRAX-080 PCDH19 Mosaic expression Gapmer ASO PRAX-090 SYNGAP1 LoF Splice switching ASO PRAX-100 SCN2A LoF Undisclosed mechanism ASO ^PRAX-020 (KCNT1) has been iicensed to UCB * Relutrigine has received ODD and RPD from the FDA, and ODD from the European Medicines Agency (EMA) for the treatment of SCN2A-DEE and SCN8A-DEE and RPD for Dravet Syndrome ** Elsunersen has received ODD and RPD from the FDA, and ODD and PRIME designations from the EMA for the treatment of SCN2A-DEE
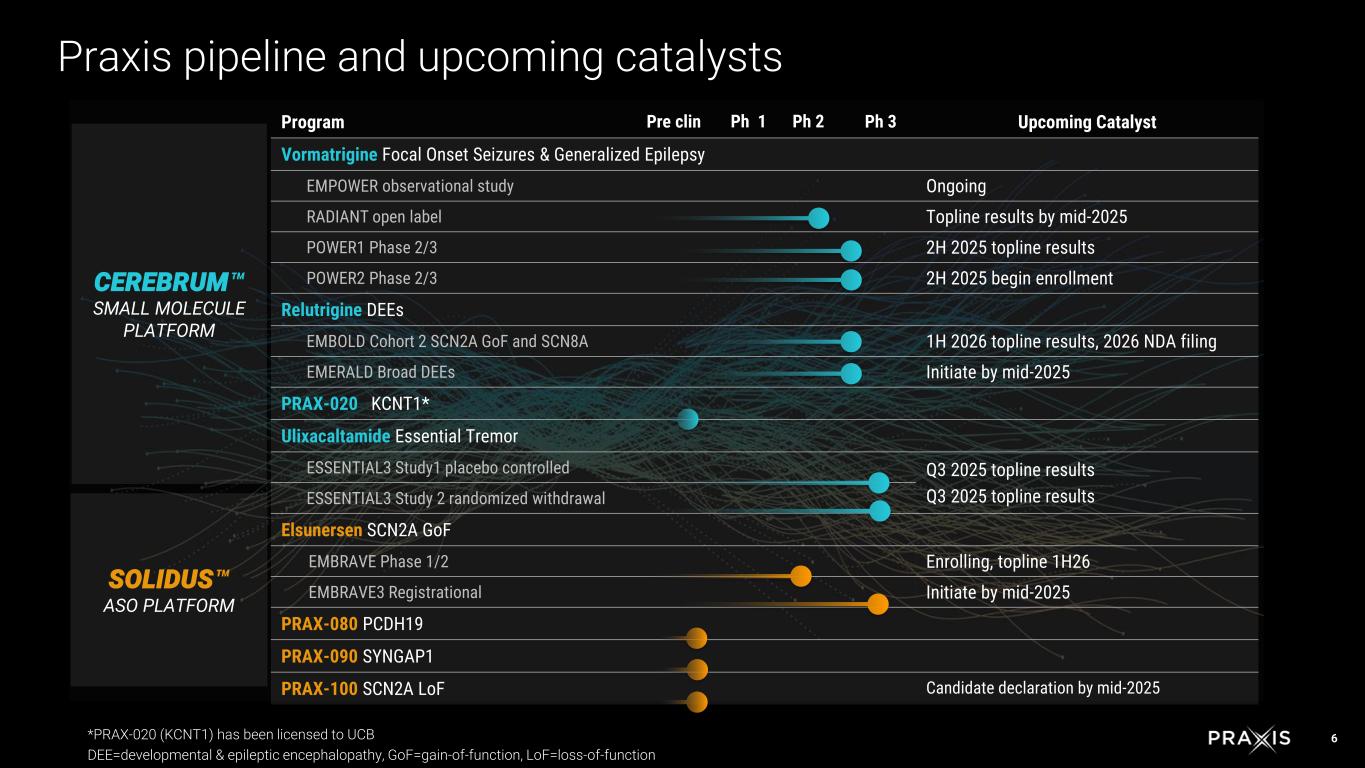
6 Program Pre clin Ph 1 Ph 2 Ph 3 Upcoming Catalyst Vormatrigine Focal Onset Seizures & Generalized Epilepsy EMPOWER observational study Ongoing RADIANT open label Topline results by mid-2025 POWER1 Phase 2/3 2H 2025 topline results POWER2 Phase 2/3 2H 2025 begin enrollment Relutrigine DEEs EMBOLD Cohort 2 SCN2A GoF and SCN8A 1H 2026 topline results, 2026 NDA filing EMERALD Broad DEEs Initiate by mid-2025 PRAX-020 KCNT1* Ulixacaltamide Essential Tremor ESSENTIAL3 Study1 placebo controlled Q3 2025 topline results Q3 2025 topline resultsESSENTIAL3 Study 2 randomized withdrawal Elsunersen SCN2A GoF EMBRAVE Phase 1/2 Enrolling, topline 1H26 EMBRAVE3 Registrational Initiate by mid-2025 PRAX-080 PCDH19 PRAX-090 SYNGAP1 PRAX-100 SCN2A LoF Candidate declaration by mid-2025 CEREBRUM SMALL MOLECULE PLATFORM SOLIDUS ASO PLATFORM Praxis pipeline and upcoming catalysts *PRAX-020 (KCNT1) has been licensed to UCB DEE=developmental & epileptic encephalopathy, GoF=gain-of-function, LoF=loss-of-function

EPILEPSY
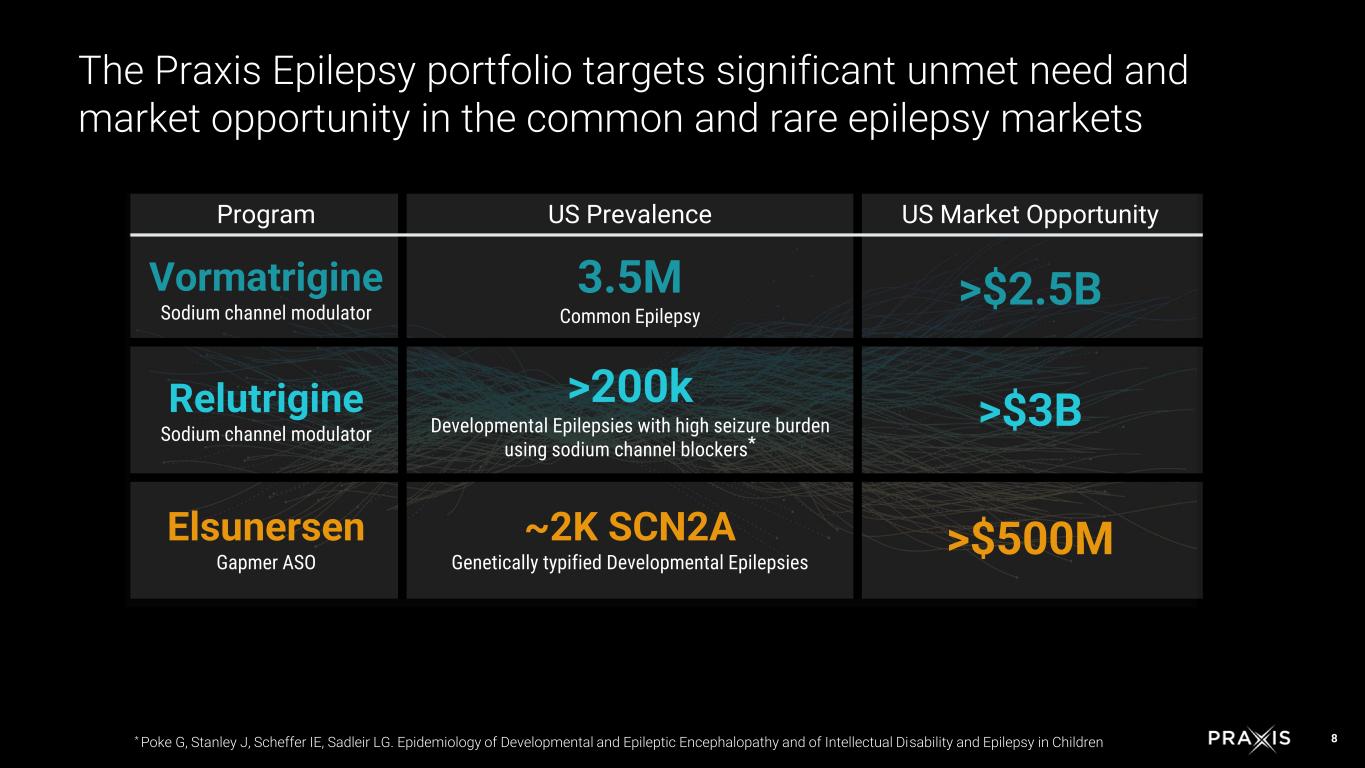
8 The Praxis Epilepsy portfolio targets significant unmet need and market opportunity in the common and rare epilepsy markets Program US Prevalence US Market Opportunity Vormatrigine Sodium channel modulator 3.5M Common Epilepsy >$2.5B Relutrigine Sodium channel modulator >200k Developmental Epilepsies with high seizure burden using sodium channel blockers* >$3B Elsunersen Gapmer ASO ~2K SCN2A Genetically typified Developmental Epilepsies >$500M * Poke G, Stanley J, Scheffer IE, Sadleir LG. Epidemiology of Developmental and Epileptic Encephalopathy and of Intellectual Disability and Epilepsy in Children
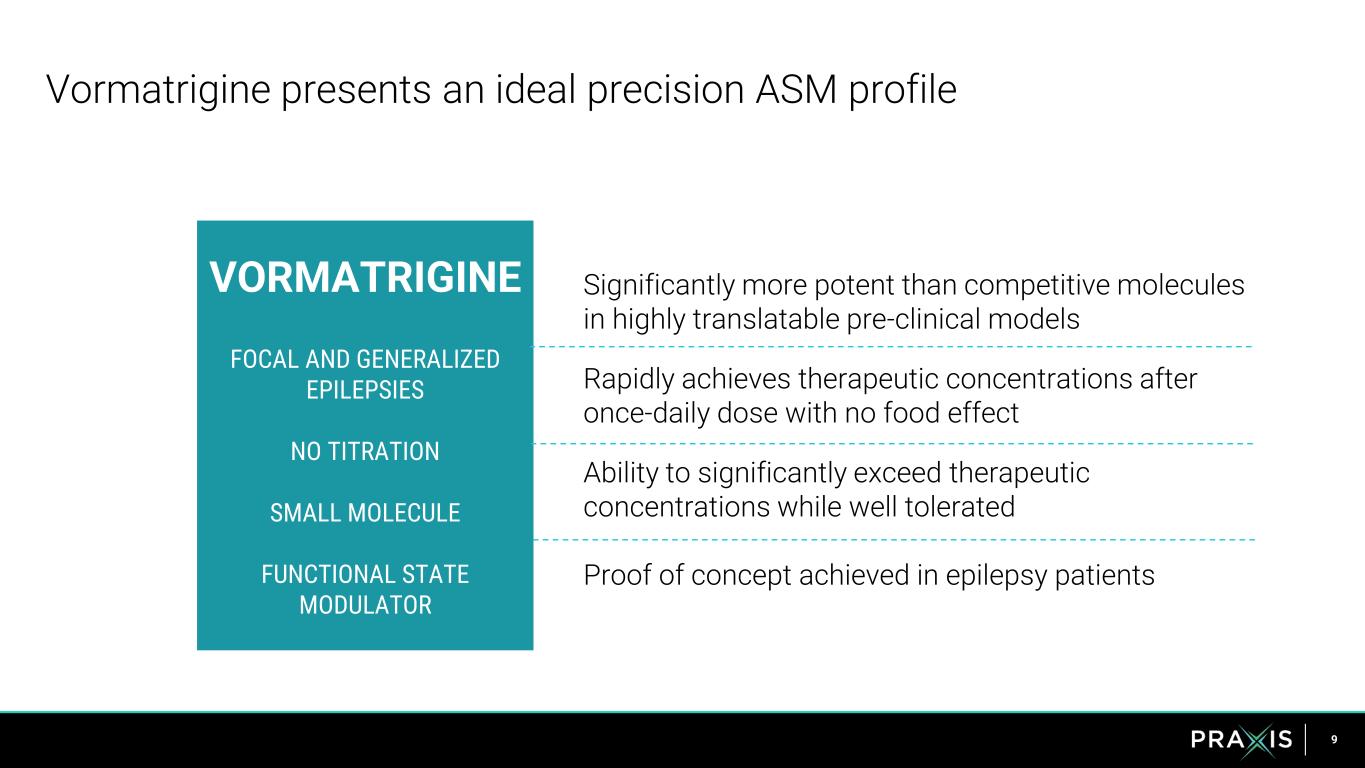
9 Significantly more potent than competitive molecules in highly translatable pre-clinical models Rapidly achieves therapeutic concentrations after once-daily dose with no food effect Ability to significantly exceed therapeutic concentrations while well tolerated Proof of concept achieved in epilepsy patients Vormatrigine presents an ideal precision ASM profile VORMATRIGINE FOCAL AND GENERALIZED EPILEPSIES NO TITRATION SMALL MOLECULE FUNCTIONAL STATE MODULATOR
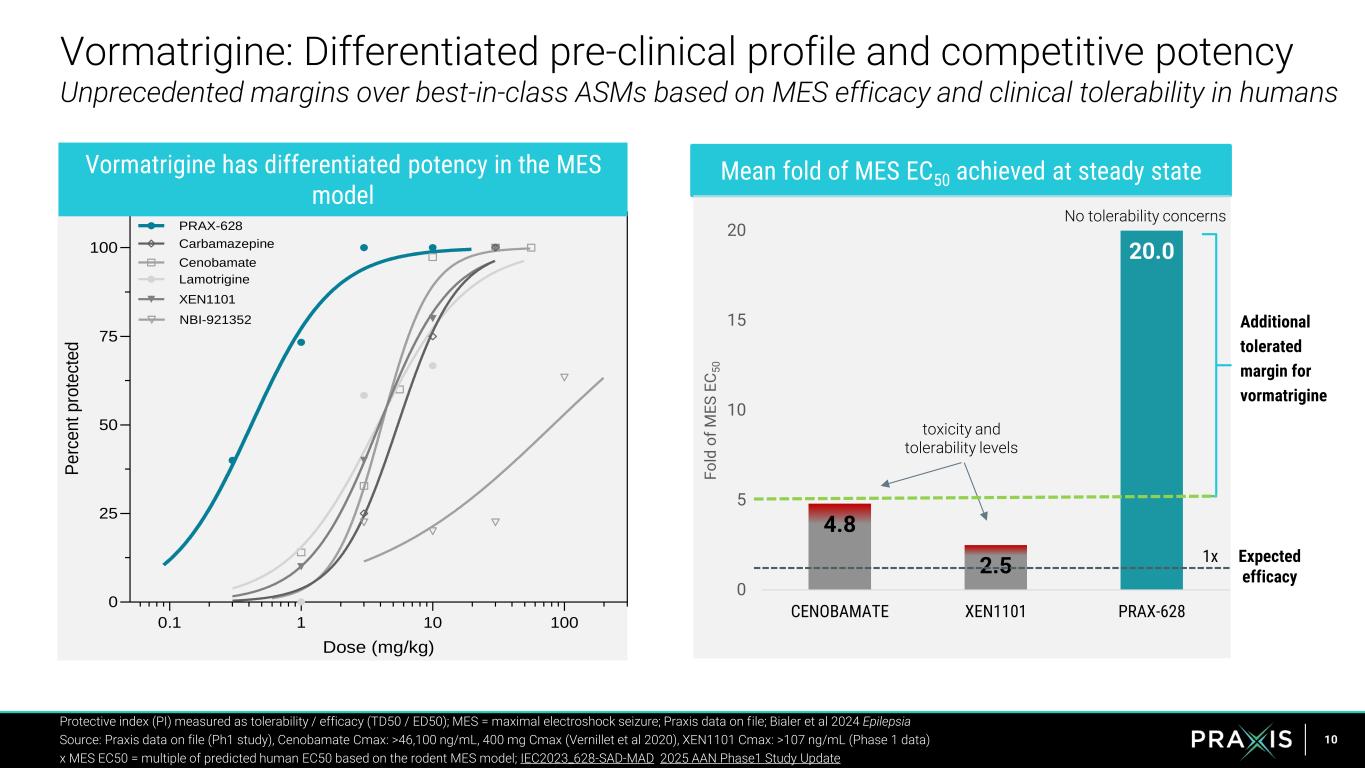
10 0.1 1 10 100 0 25 50 75 100 Dose (mg/kg) P e rc e n t p ro te ct e d PRAX-628 Cenobamate Lamotrigine XEN1101 Carbamazepine NBI-921352 Protective index (PI) measured as tolerability / efficacy (TD50 / ED50); MES = maximal electroshock seizure; Praxis data on file; Bialer et al 2024 Epilepsia Source: Praxis data on file (Ph1 study), Cenobamate Cmax: >46,100 ng/mL, 400 mg Cmax (Vernillet et al 2020), XEN1101 Cmax: >107 ng/mL (Phase 1 data) x MES EC50 = multiple of predicted human EC50 based on the rodent MES model; IEC2023_628-SAD-MAD 2025 AAN Phase1 Study Update Vormatrigine has differentiated potency in the MES model Mean fold of MES EC50 achieved at steady state 4.8 2.5 20.0 0 5 10 15 20 CENOBAMATE XEN1101 PRAX-628 F o ld o f M E S E C 5 0 Additional tolerated margin for vormatrigine toxicity and tolerability levels No tolerability concerns Expected efficacy 1x Vormatrigine: Differentiated pre-clinical profile and competitive potency Unprecedented margins over best-in-class ASMs based on MES efficacy and clinical tolerability in humans
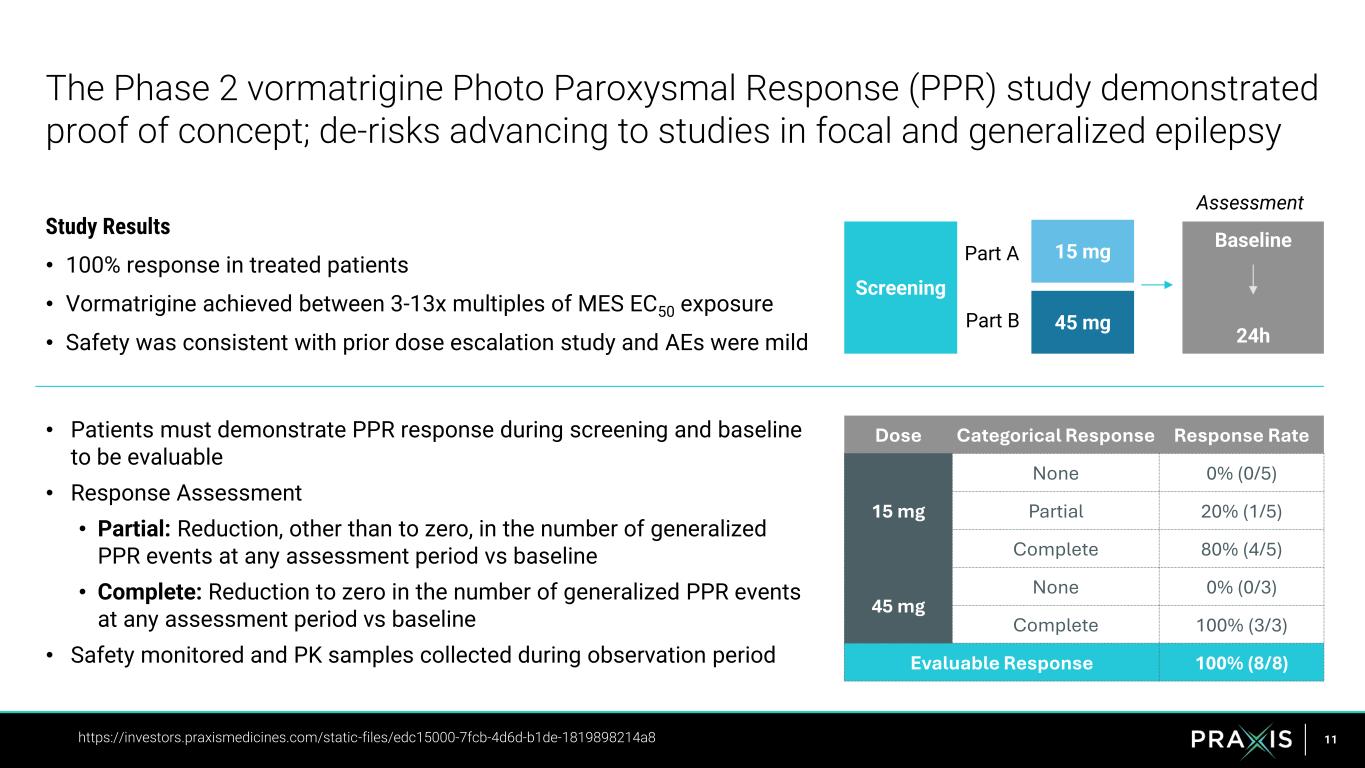
11 • Patients must demonstrate PPR response during screening and baseline to be evaluable • Response Assessment • Partial: Reduction, other than to zero, in the number of generalized PPR events at any assessment period vs baseline • Complete: Reduction to zero in the number of generalized PPR events at any assessment period vs baseline • Safety monitored and PK samples collected during observation period The Phase 2 vormatrigine Photo Paroxysmal Response (PPR) study demonstrated proof of concept; de-risks advancing to studies in focal and generalized epilepsy https://investors.praxismedicines.com/static-files/edc15000-7fcb-4d6d-b1de-1819898214a8 15 mg 45 mg Part A Part B Assessment Screening Baseline 24h Dose Categorical Response Response Rate 15 mg None 0% (0/5) Partial 20% (1/5) Complete 80% (4/5) 45 mg None 0% (0/3) Complete 100% (3/3) Evaluable Response 100% (8/8) Study Results • 100% response in treated patients • Vormatrigine achieved between 3-13x multiples of MES EC50 exposure • Safety was consistent with prior dose escalation study and AEs were mild
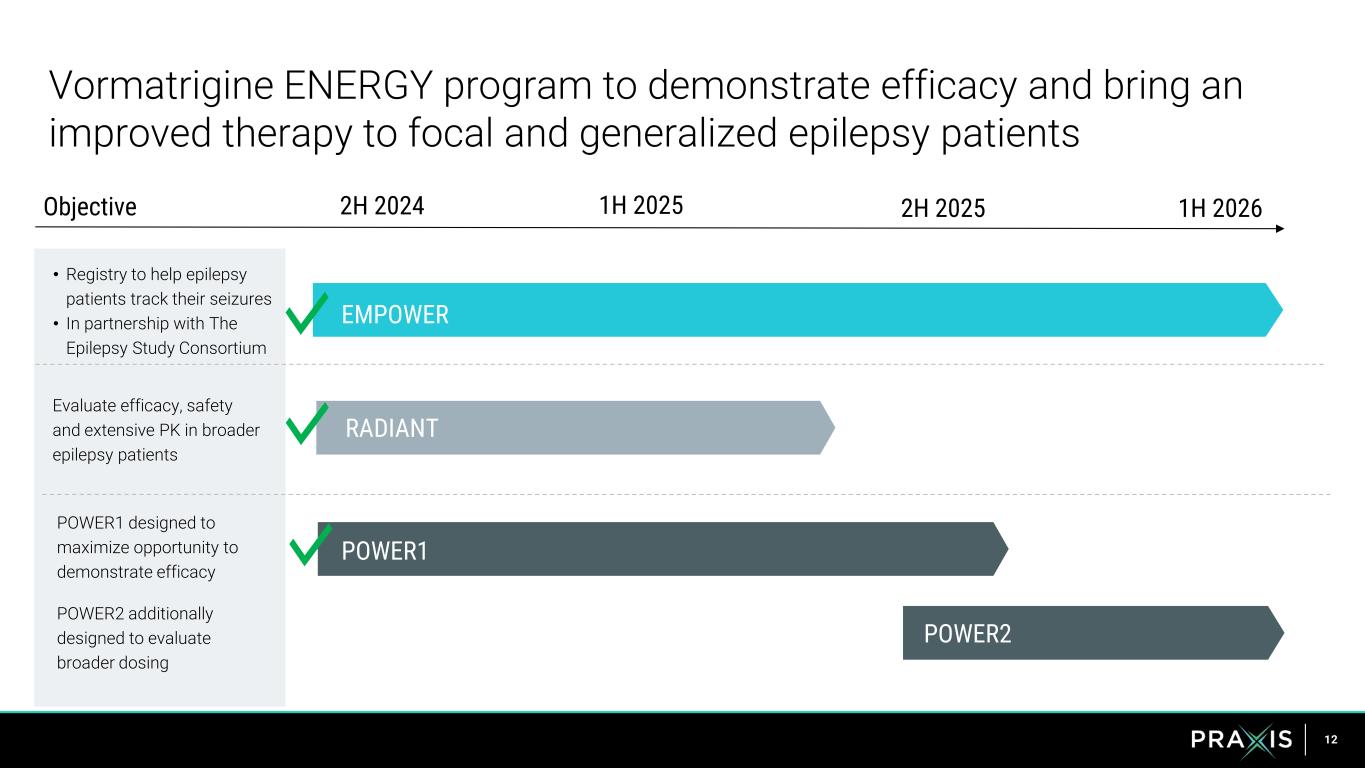
12 Vormatrigine ENERGY program to demonstrate efficacy and bring an improved therapy to focal and generalized epilepsy patients • Registry to help epilepsy patients track their seizures • In partnership with The Epilepsy Study Consortium POWER1 designed to maximize opportunity to demonstrate efficacy POWER2 additionally designed to evaluate broader dosing Evaluate efficacy, safety and extensive PK in broader epilepsy patients EMPOWER POWER1 POWER2 RADIANT 2H 2024 1H 20261H 2025 2H 2025Objective
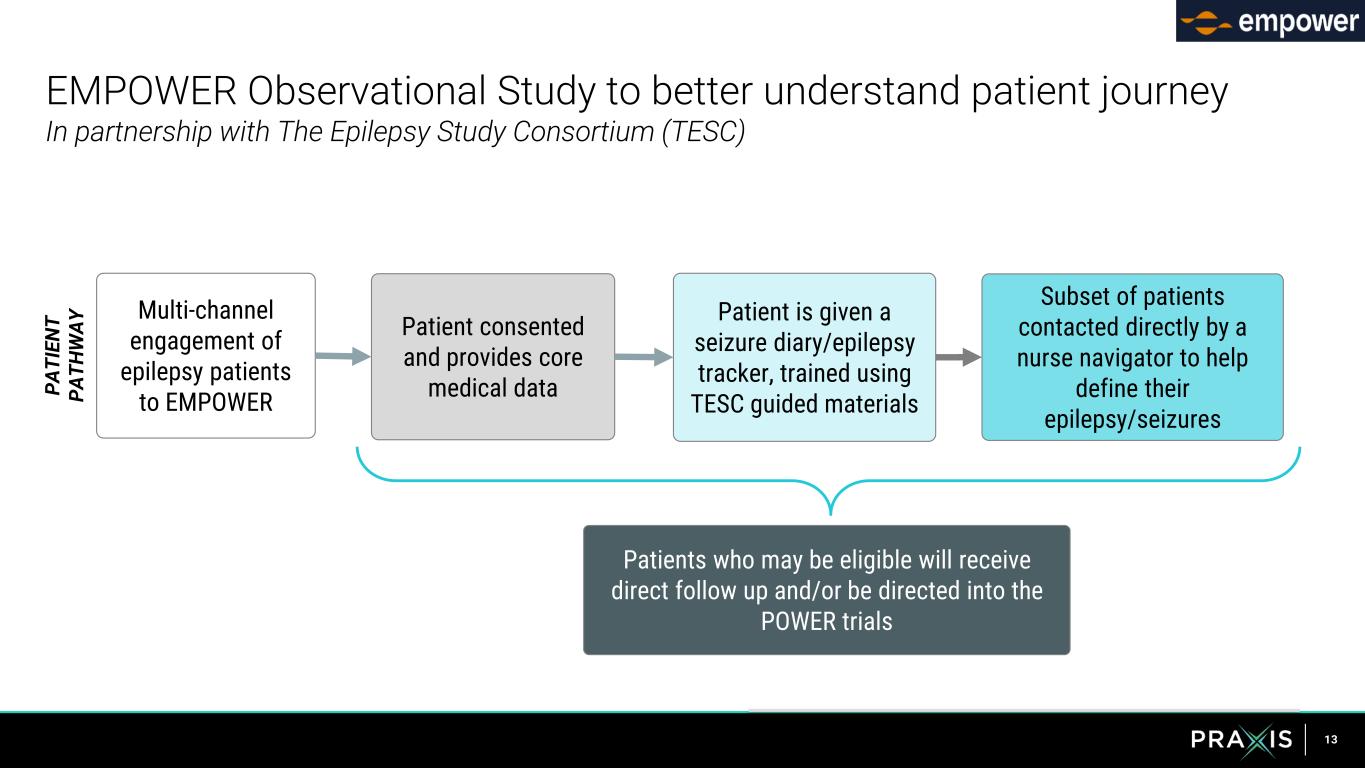
13 EMPOWER Observational Study to better understand patient journey In partnership with The Epilepsy Study Consortium (TESC) P A T IE N T P A T H W A Y Multi-channel engagement of epilepsy patients to EMPOWER Patient consented and provides core medical data Patient is given a seizure diary/epilepsy tracker, trained using TESC guided materials Subset of patients contacted directly by a nurse navigator to help define their epilepsy/seizures Patients who may be eligible will receive direct follow up and/or be directed into the POWER trials
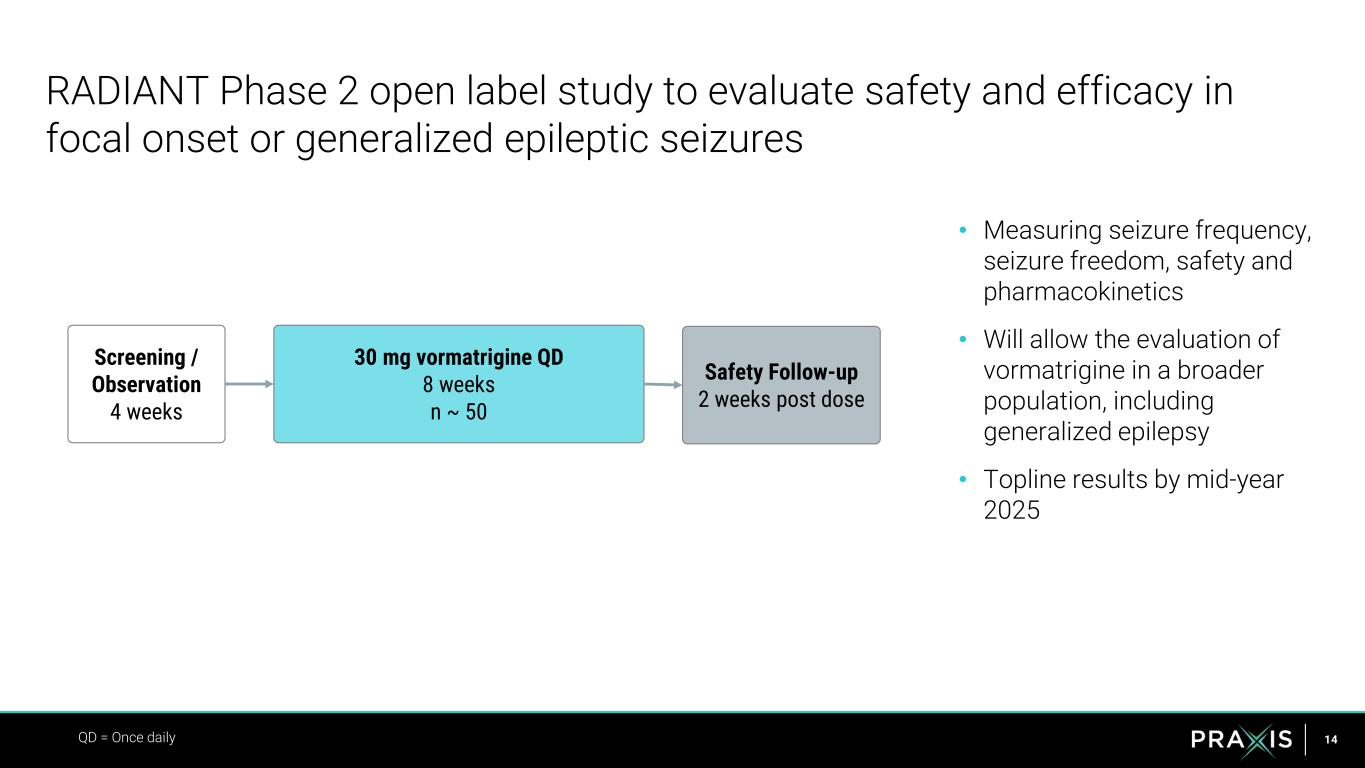
14 • Measuring seizure frequency, seizure freedom, safety and pharmacokinetics • Will allow the evaluation of vormatrigine in a broader population, including generalized epilepsy • Topline results by mid-year 2025 RADIANT Phase 2 open label study to evaluate safety and efficacy in focal onset or generalized epileptic seizures QD = Once daily Screening / Observation 4 weeks 30 mg vormatrigine QD 8 weeks n ~ 50 Safety Follow-up 2 weeks post dose
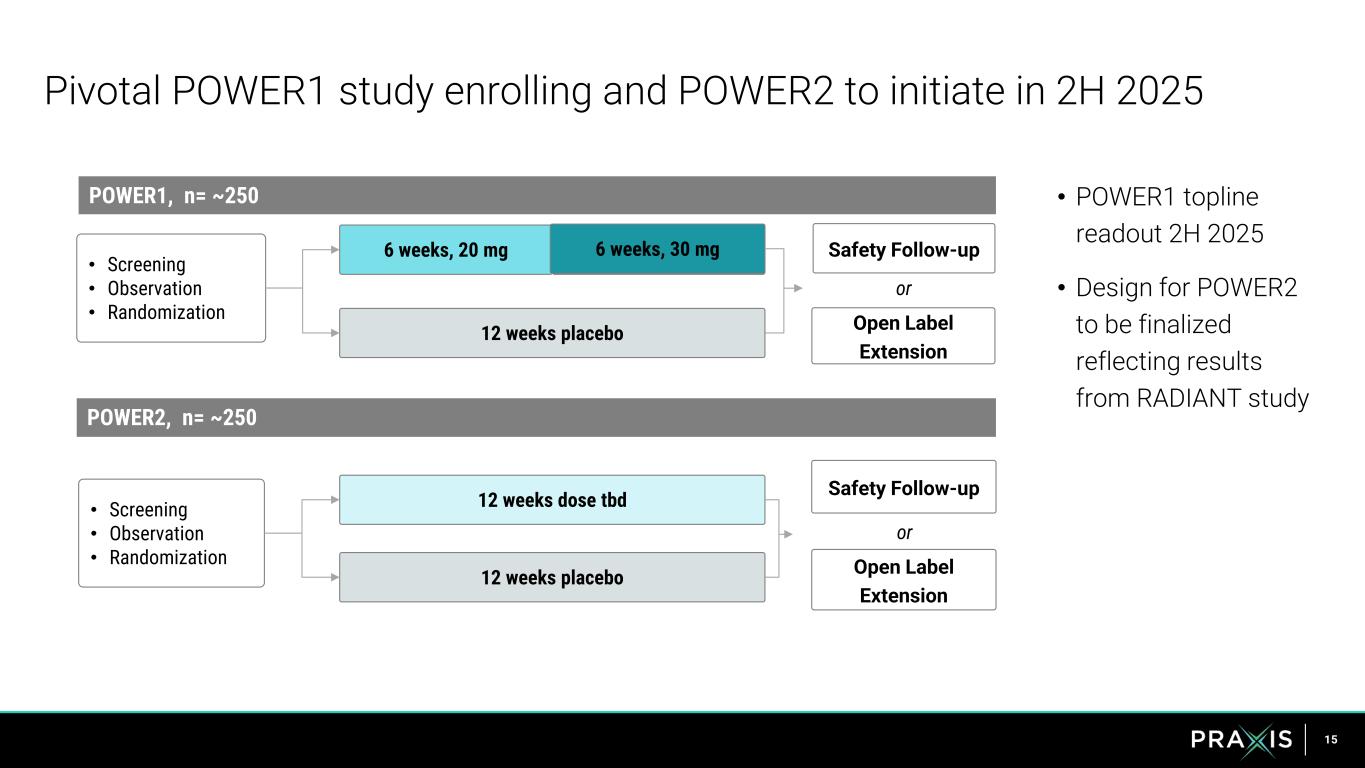
15 Pivotal POWER1 study enrolling and POWER2 to initiate in 2H 2025 6 weeks, 30mg 12 weeks placebo • Screening • Observation • Randomization POWER1, n= ~250 Safety Follow-up • POWER1 topline readout 2H 2025 • Design for POWER2 to be finalized reflecting results from RADIANT study 6 weeks, 20 mg POWER2, n= ~250 • Screening • Observation • Randomization 12 weeks dose tbd 12 weeks placebo 6 s, 30 g Safety Follow-up Open Label Extension or Open Label Extension or
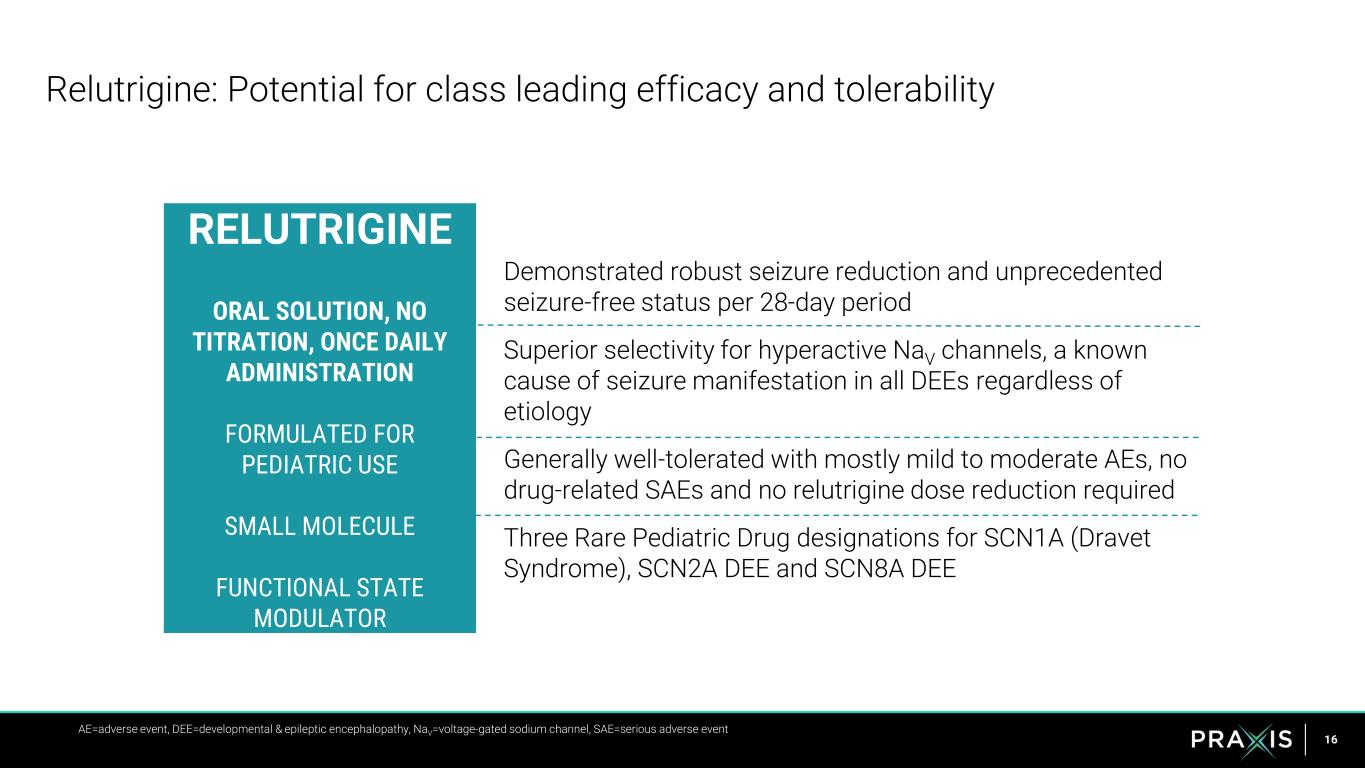
16 Demonstrated robust seizure reduction and unprecedented seizure-free status per 28-day period Superior selectivity for hyperactive NaV channels, a known cause of seizure manifestation in all DEEs regardless of etiology Generally well-tolerated with mostly mild to moderate AEs, no drug-related SAEs and no relutrigine dose reduction required Three Rare Pediatric Drug designations for SCN1A (Dravet Syndrome), SCN2A DEE and SCN8A DEE Relutrigine: Potential for class leading efficacy and tolerability RELUTRIGINE ORAL SOLUTION, NO TITRATION, ONCE DAILY ADMINISTRATION FORMULATED FOR PEDIATRIC USE SMALL MOLECULE FUNCTIONAL STATE MODULATOR AE=adverse event, DEE=developmental & epileptic encephalopathy, NaV=voltage-gated sodium channel, SAE=serious adverse event
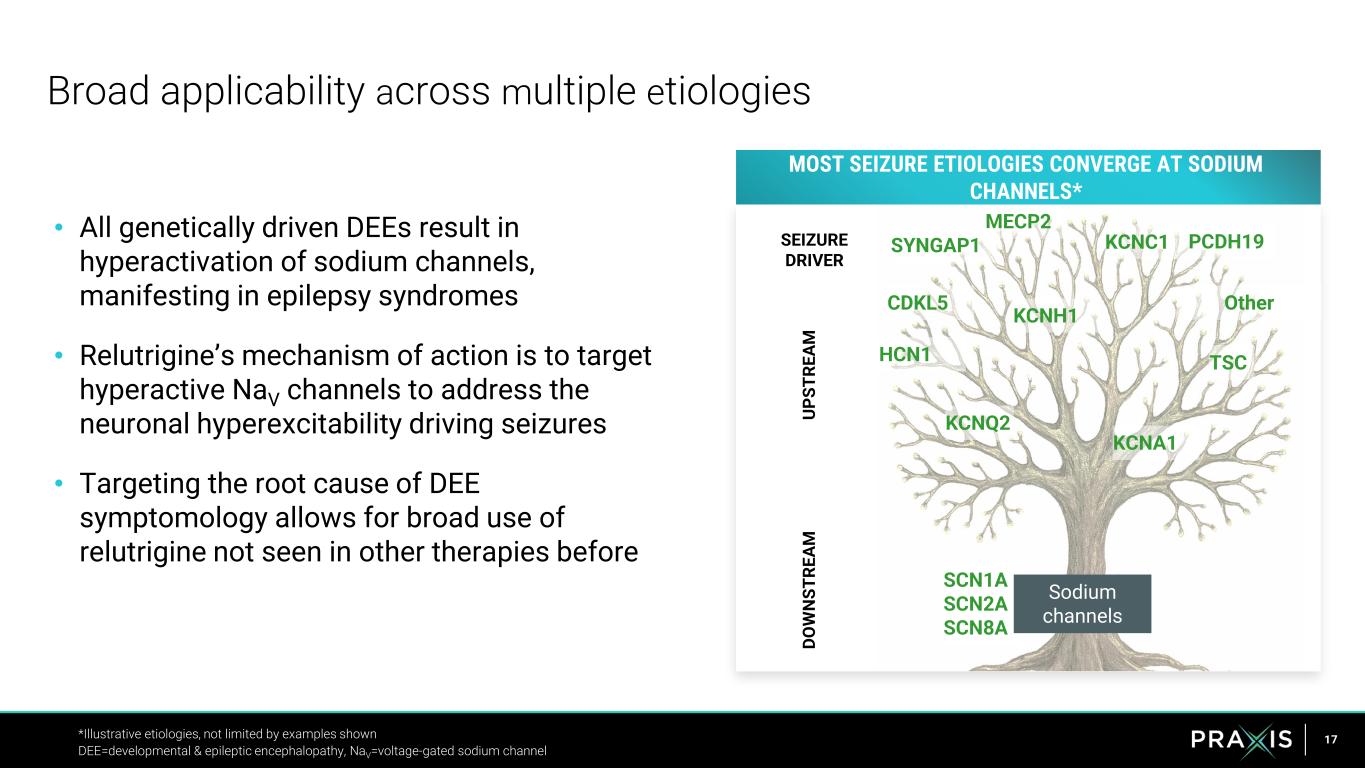
17 • All genetically driven DEEs result in hyperactivation of sodium channels, manifesting in epilepsy syndromes • Relutrigine’s mechanism of action is to target hyperactive NaV channels to address the neuronal hyperexcitability driving seizures • Targeting the root cause of DEE symptomology allows for broad use of relutrigine not seen in other therapies before Broad applicability across multiple etiologies *Illustrative etiologies, not limited by examples shown DEE=developmental & epileptic encephalopathy, NaV=voltage-gated sodium channel OtherCDKL5 KCNQ2 SCN1A SCN2A SCN8A TSC MECP2 MOST SEIZURE ETIOLOGIES CONVERGE AT SODIUM CHANNELS* U P S T R E A M D O W N S T R E A M Sodium channels KCNH1 KCNC1 KCNA1 HCN1 PCDH19SYNGAP1SEIZURE DRIVER
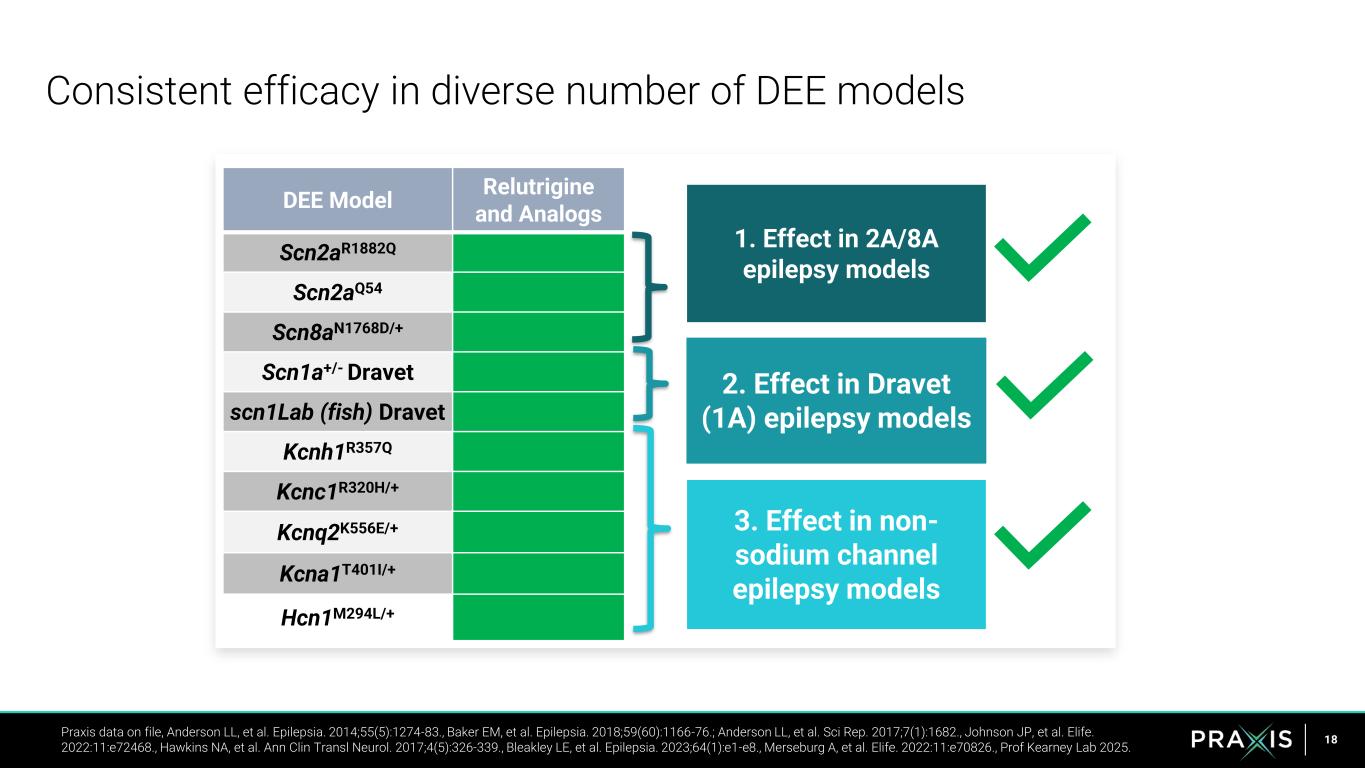
18 Consistent efficacy in diverse number of DEE models Praxis data on file, Anderson LL, et al. Epilepsia. 2014;55(5):1274-83., Baker EM, et al. Epilepsia. 2018;59(60):1166-76.; Anderson LL, et al. Sci Rep. 2017;7(1):1682., Johnson JP, et al. Elife. 2022:11:e72468., Hawkins NA, et al. Ann Clin Transl Neurol. 2017;4(5):326-339., Bleakley LE, et al. Epilepsia. 2023;64(1):e1-e8., Merseburg A, et al. Elife. 2022:11:e70826., Prof Kearney Lab 2025. 1. Effect in 2A/8A epilepsy models 2. Effect in Dravet (1A) epilepsy models 3. Effect in non- sodium channel epilepsy models DEE Model Relutrigine and Analogs Scn2aR1882Q Scn2aQ54 Scn8aN1768D/+ Scn1a+/- Dravet scn1Lab (fish) Dravet Kcnh1R357Q Kcnc1R320H/+ Kcnq2K556E/+ Kcna1T401I/+ Hcn1M294L/+
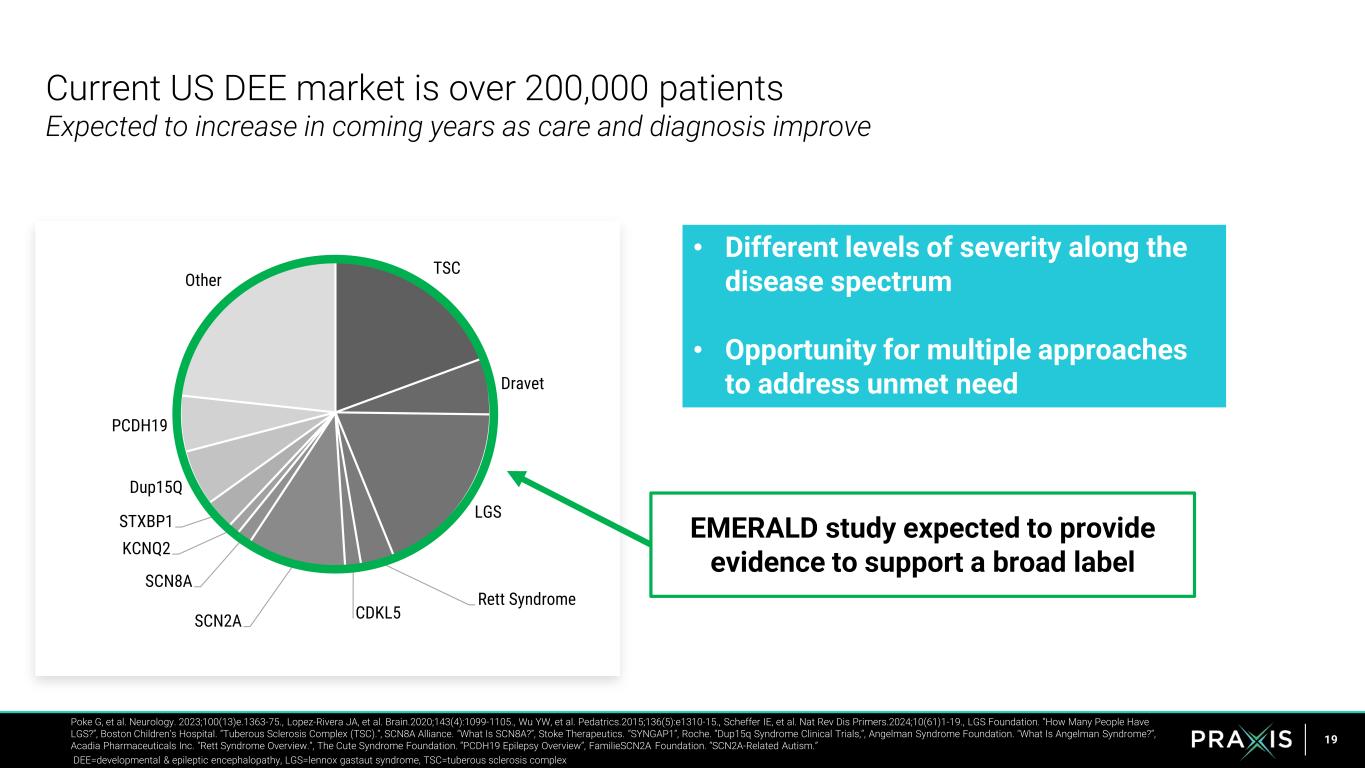
19 Current US DEE market is over 200,000 patients Expected to increase in coming years as care and diagnosis improve Poke G, et al. Neurology. 2023;100(13)e.1363-75., Lopez-Rivera JA, et al. Brain.2020;143(4):1099-1105., Wu YW, et al. Pedatrics.2015;136(5):e1310-15., Scheffer IE, et al. Nat Rev Dis Primers.2024;10(61)1-19., LGS Foundation. “How Many People Have LGS?”, Boston Children’s Hospital. “Tuberous Sclerosis Complex (TSC).”, SCN8A Alliance. “What Is SCN8A?”, Stoke Therapeutics. “SYNGAP1”, Roche. “Dup15q Syndrome Clinical Trials,”, Angelman Syndrome Foundation. "What Is Angelman Syndrome?“, Acadia Pharmaceuticals Inc. "Rett Syndrome Overview.“, The Cute Syndrome Foundation. “PCDH19 Epilepsy Overview”, FamilieSCN2A Foundation. “SCN2A-Related Autism.” DEE=developmental & epileptic encephalopathy, LGS=lennox gastaut syndrome, TSC=tuberous sclerosis complex • Different levels of severity along the disease spectrum • Opportunity for multiple approaches to address unmet need TSC Dravet LGS Rett Syndrome CDKL5SCN2A SCN8A KCNQ2 STXBP1 Dup15Q PCDH19 Other EMERALD study expected to provide evidence to support a broad label
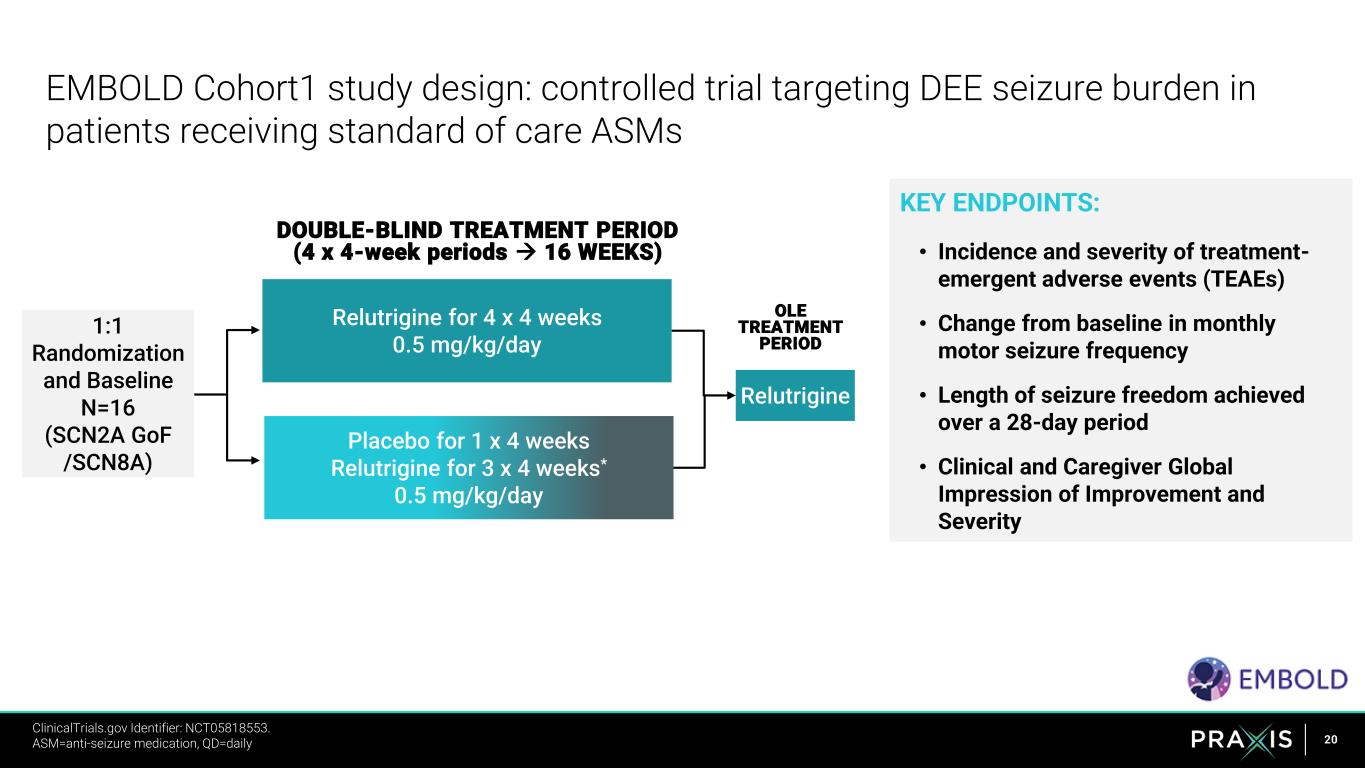
20 KEY ENDPOINTS: • Incidence and severity of treatment- emergent adverse events (TEAEs) • Change from baseline in monthly motor seizure frequency • Length of seizure freedom achieved over a 28-day period • Clinical and Caregiver Global Impression of Improvement and Severity EMBOLD Cohort1 study design: controlled trial targeting DEE seizure burden in patients receiving standard of care ASMs DOUBLE-BLIND TREATMENT PERIOD (4 x 4-week periods → 16 WEEKS) Relutrigine 1:1 Randomization and Baseline N=16 (SCN2A GoF /SCN8A) OLE TREATMENT PERIOD Relutrigine for 4 x 4 weeks 0.5 mg/kg/day Placebo for 1 x 4 weeks Relutrigine for 3 x 4 weeks* 0.5 mg/kg/day ClinicalTrials.gov Identifier: NCT05818553. https://clinicaltrials.gov/ct2/show/NCT05818553 ASM=anti-seizure medication, QD=daily
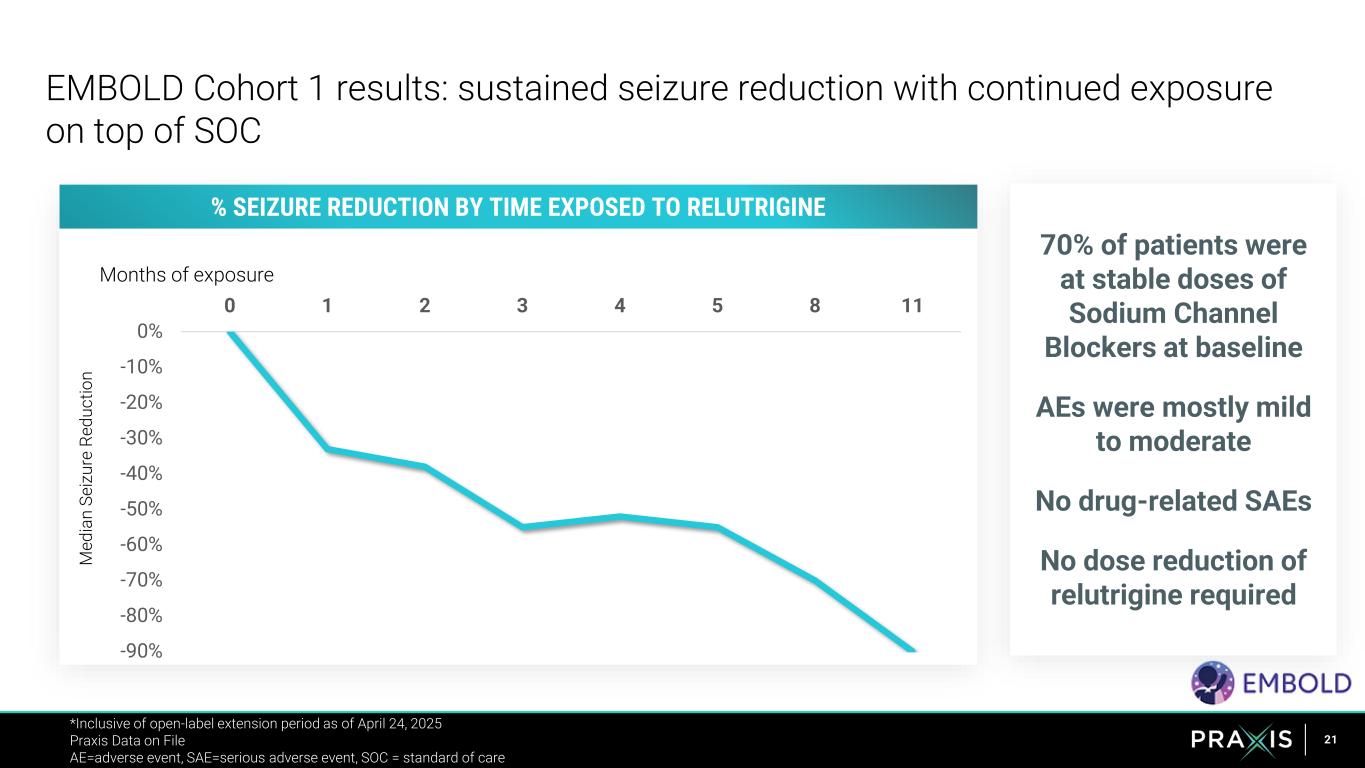
21 -90% -80% -70% -60% -50% -40% -30% -20% -10% 0% 0 1 2 3 4 5 8 11 M e d ia n S e iz u re R e d u c ti o n Months of exposure EMBOLD Cohort 1 results: sustained seizure reduction with continued exposure on top of SOC *Inclusive of open-label extension period as of April 24, 2025 Praxis Data on File AE=adverse event, SAE=serious adverse event, SOC = standard of care % SEIZURE REDUCTION BY TIME EXPOSED TO RELUTRIGINE 70% of patients were at stable doses of Sodium Channel Blockers at baseline AEs were mostly mild to moderate No drug-related SAEs No dose reduction of relutrigine required
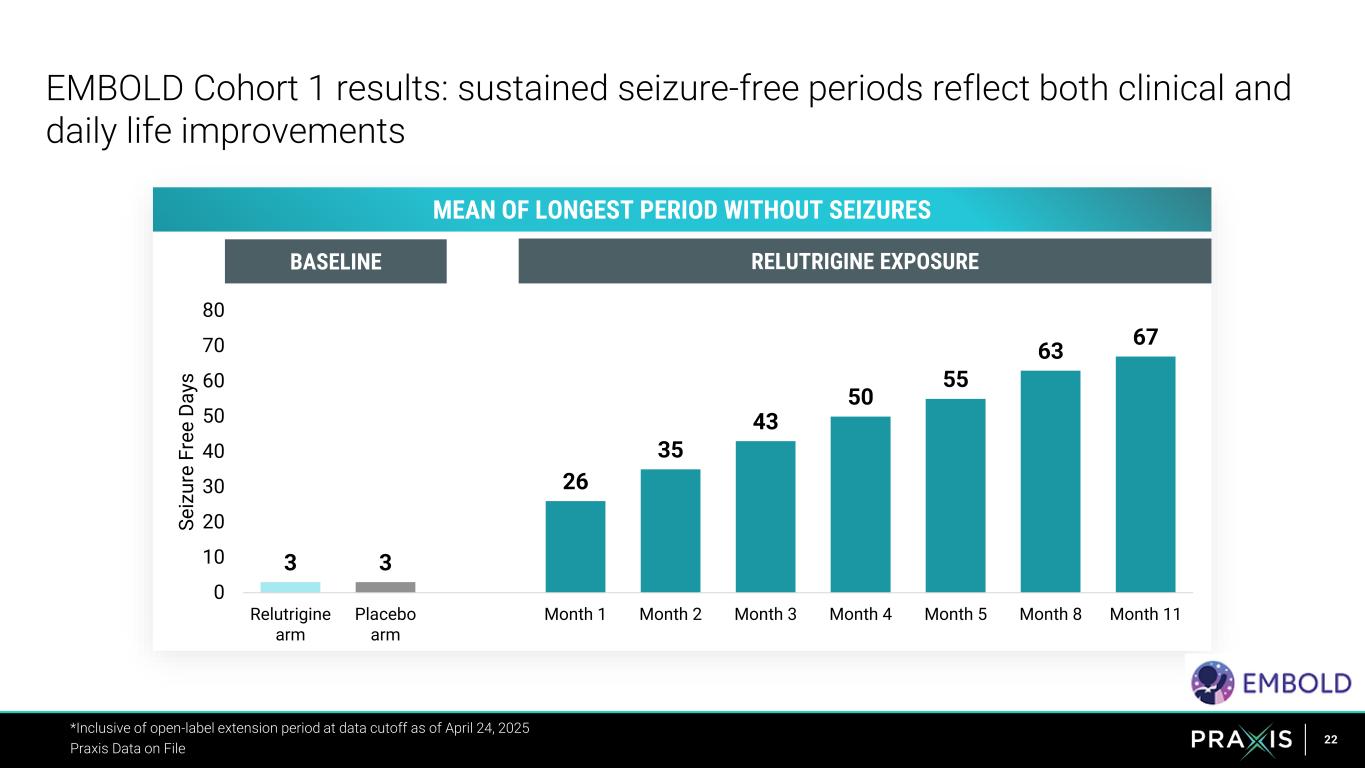
22 EMBOLD Cohort 1 results: sustained seizure-free periods reflect both clinical and daily life improvements *Inclusive of open-label extension period at data cutoff as of April 24, 2025 Praxis Data on File 3 3 26 35 43 50 55 63 67 0 10 20 30 40 50 60 70 80 Relutrigine arm Placebo arm Month 1 Month 2 Month 3 Month 4 Month 5 Month 8 Month 11 S e iz u re F re e D a ys MEAN OF LONGEST PERIOD WITHOUT SEIZURES BASELINE RELUTRIGINE EXPOSURE
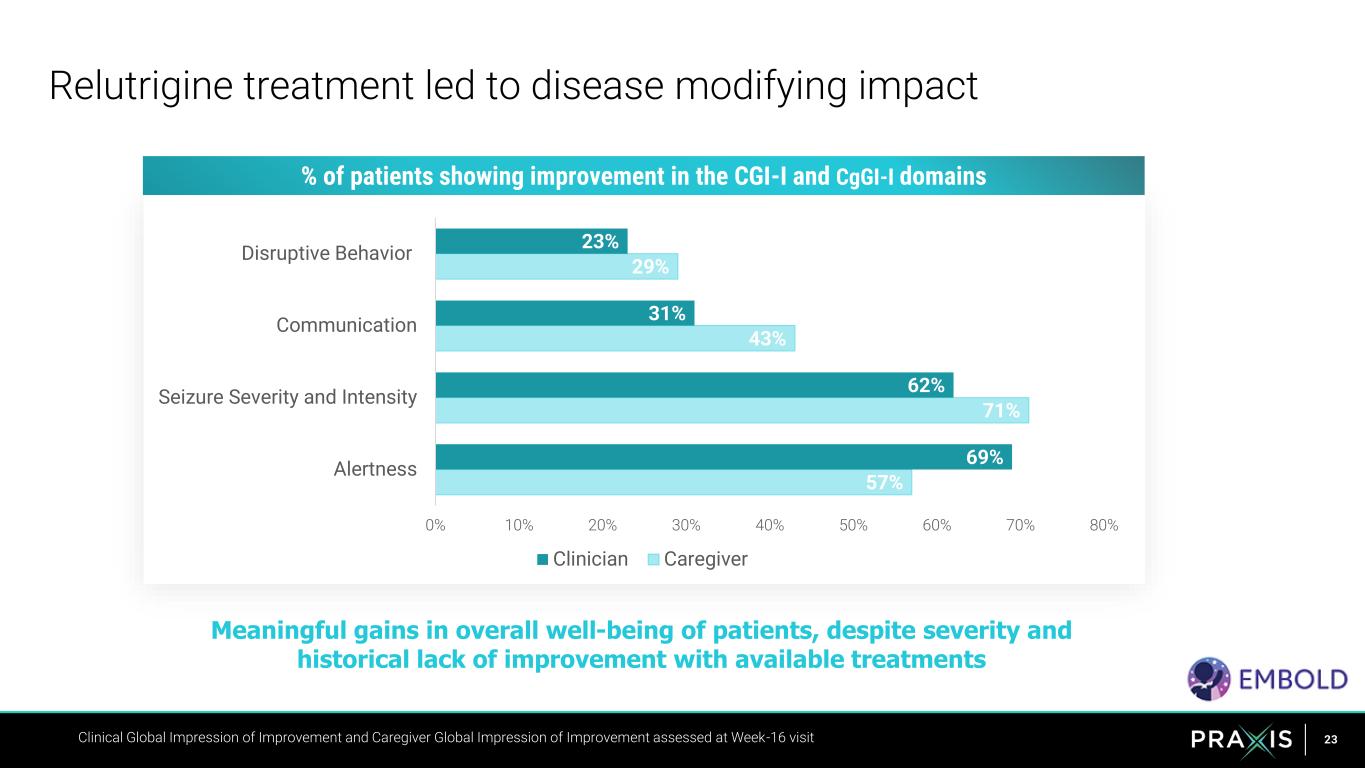
23Clinical Global Impression of Improvement and Caregiver Global Impression of Improvement assessed at Week-16 visit 57% 71% 43% 29% 69% 62% 31% 23% 0% 10% 20% 30% 40% 50% 60% 70% 80% Alertness Seizure Severity and Intensity Communication Disruptive Behavior Clinician Caregiver Relutrigine treatment led to disease modifying impact Meaningful gains in overall well-being of patients, despite severity and historical lack of improvement with available treatments % of patients showing improvement in the CGI-I and CgGI-I domains
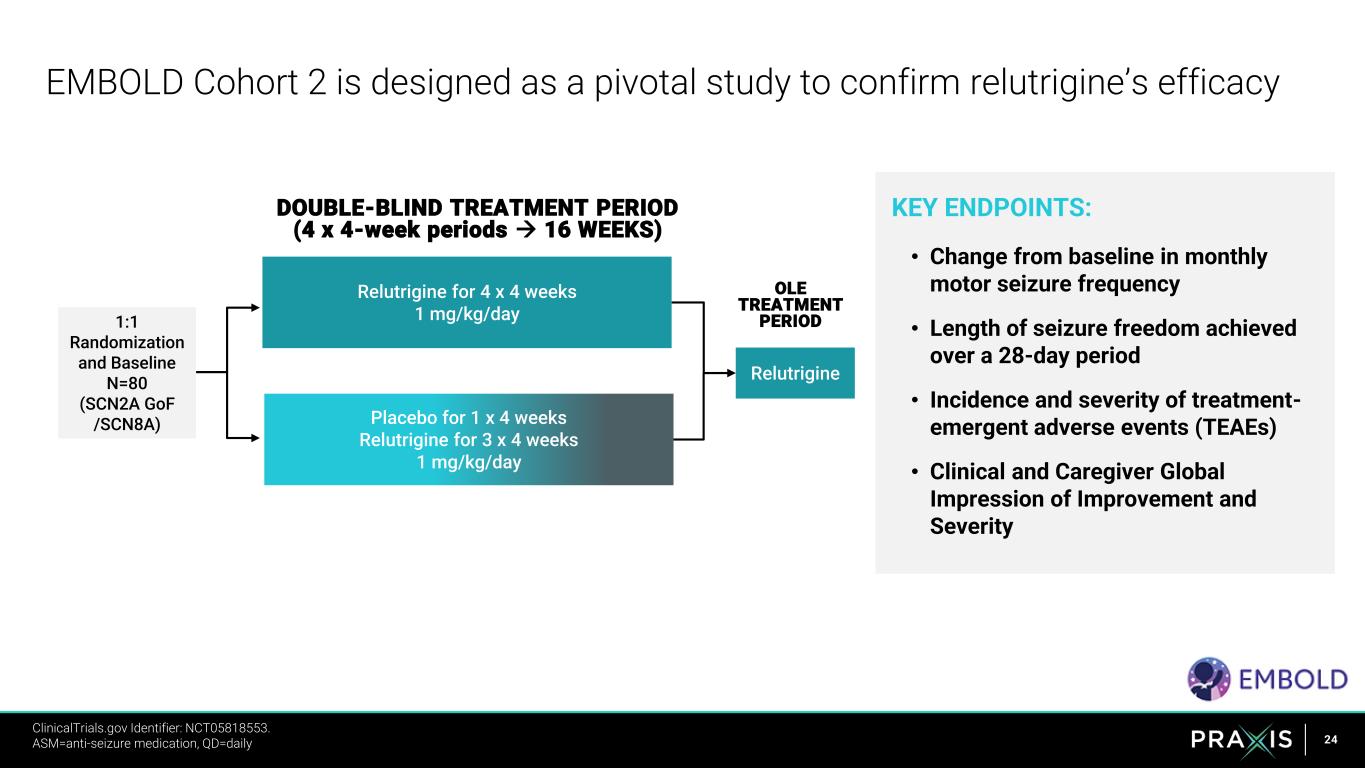
24 KEY ENDPOINTS: • Change from baseline in monthly motor seizure frequency • Length of seizure freedom achieved over a 28-day period • Incidence and severity of treatment- emergent adverse events (TEAEs) • Clinical and Caregiver Global Impression of Improvement and Severity EMBOLD Cohort 2 is designed as a pivotal study to confirm relutrigine’s efficacy DOUBLE-BLIND TREATMENT PERIOD (4 x 4-week periods → 16 WEEKS) Relutrigine 1:1 Randomization and Baseline N=80 (SCN2A GoF /SCN8A) OLE TREATMENT PERIOD Relutrigine for 4 x 4 weeks 1 mg/kg/day Placebo for 1 x 4 weeks Relutrigine for 3 x 4 weeks 1 mg/kg/day ClinicalTrials.gov Identifier: NCT05818553. https://clinicaltrials.gov/ct2/show/NCT05818553 ASM=anti-seizure medication, QD=daily
25 Relutrigine’s clinical profile expanding with EMERALD study Large, unmet needBroad biologic rationale Clinical proof of concept data
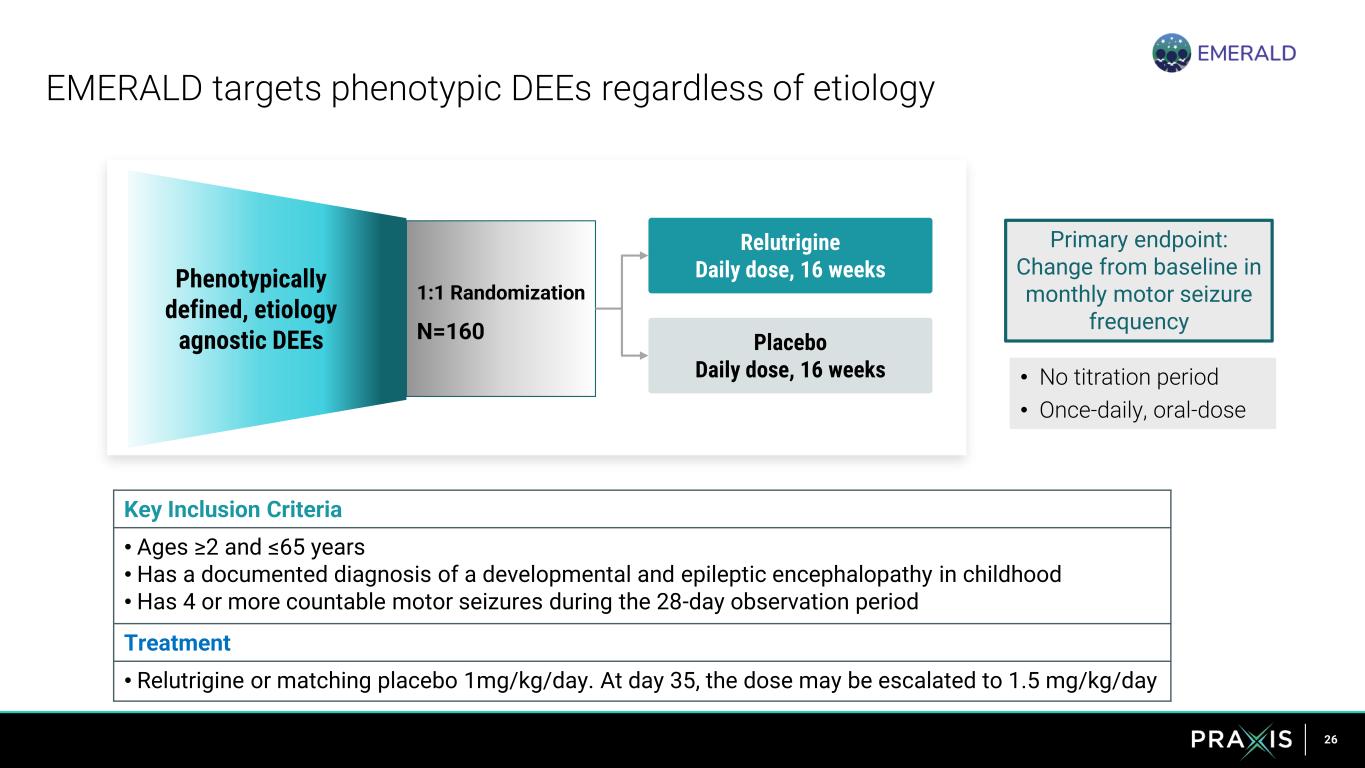
26 Phenotypically defined, etiology agnostic DEEs EMERALD targets phenotypic DEEs regardless of etiology Placebo Daily dose, 16 weeks 1:1 Randomization N=160 Relutrigine Daily dose, 16 weeks • No titration period • Once-daily, oral-dose Key Inclusion Criteria • Ages ≥2 and ≤65 years • Has a documented diagnosis of a developmental and epileptic encephalopathy in childhood • Has 4 or more countable motor seizures during the 28-day observation period Treatment • Relutrigine or matching placebo 1mg/kg/day. At day 35, the dose may be escalated to 1.5 mg/kg/day Primary endpoint: Change from baseline in monthly motor seizure frequency
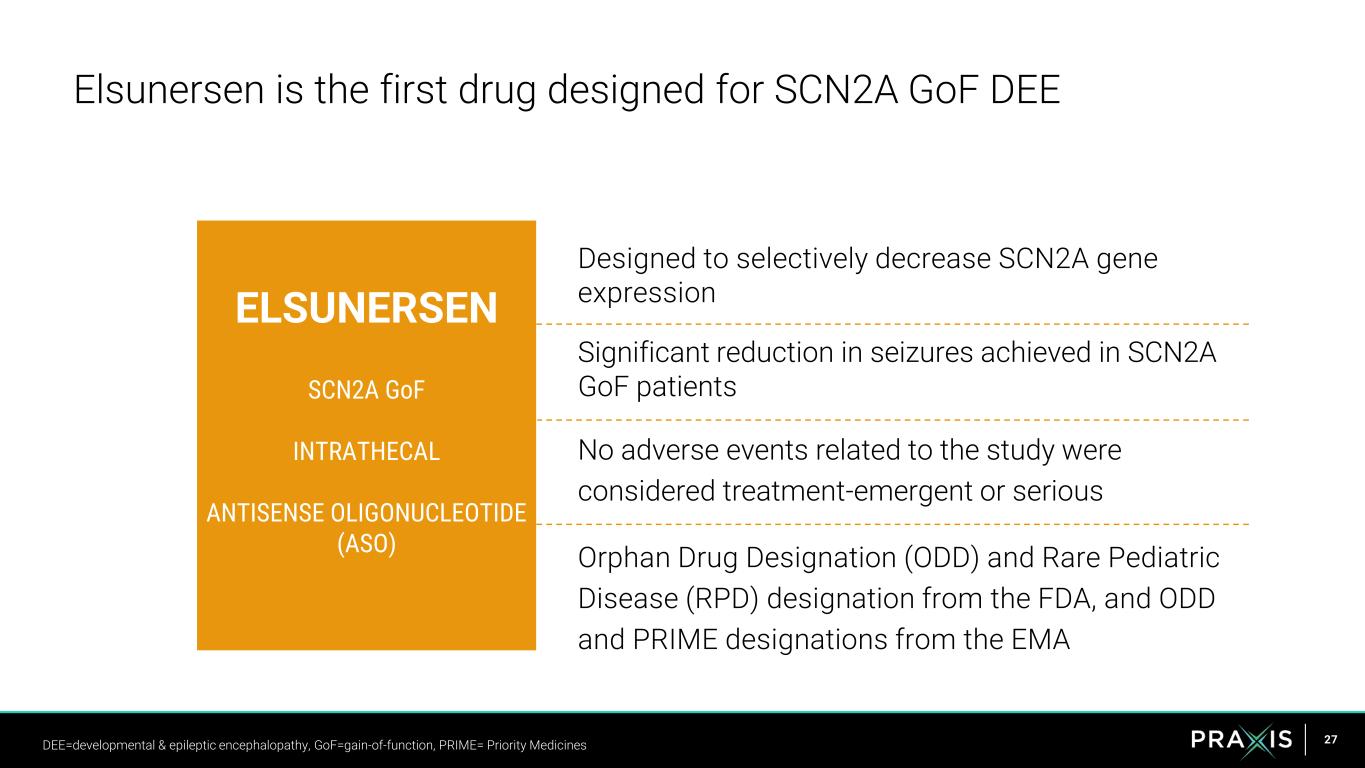
27 Designed to selectively decrease SCN2A gene expression Significant reduction in seizures achieved in SCN2A GoF patients No adverse events related to the study were considered treatment-emergent or serious Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) designation from the FDA, and ODD and PRIME designations from the EMA Elsunersen is the first drug designed for SCN2A GoF DEE ELSUNERSEN SCN2A GoF INTRATHECAL ANTISENSE OLIGONUCLEOTIDE (ASO) DEE=developmental & epileptic encephalopathy, GoF=gain-of-function, PRIME= Priority Medicines
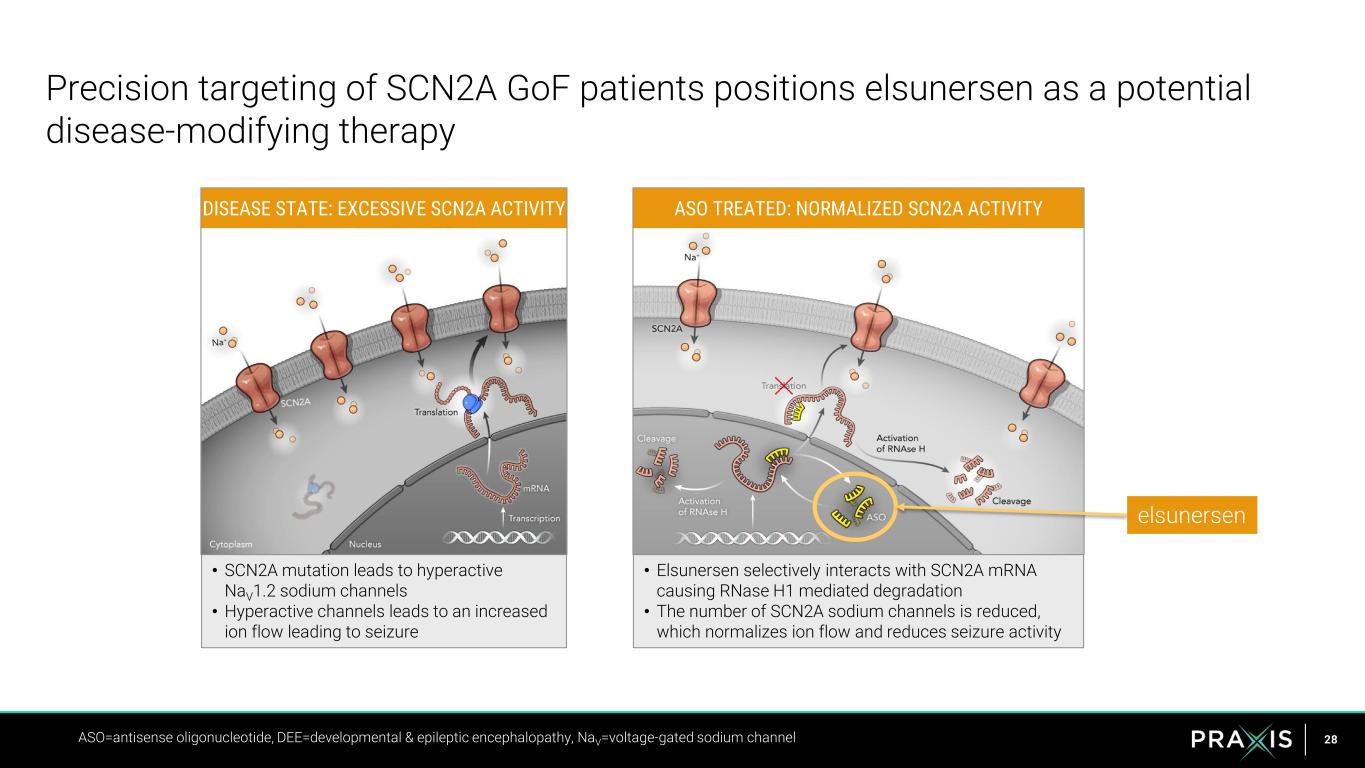
28 Precision targeting of SCN2A GoF patients positions elsunersen as a potential disease-modifying therapy ASO=antisense oligonucleotide, DEE=developmental & epileptic encephalopathy, NaV=voltage-gated sodium channel DISEASE STATE: EXCESSIVE SCN2A ACTIVITY ASO TREATED: NORMALIZED SCN2A ACTIVITY • SCN2A mutation leads to hyperactive NaV1.2 sodium channels • Hyperactive channels leads to an increased ion flow leading to seizure • Elsunersen selectively interacts with SCN2A mRNA causing RNase H1 mediated degradation • The number of SCN2A sodium channels is reduced, which normalizes ion flow and reduces seizure activity elsunersen
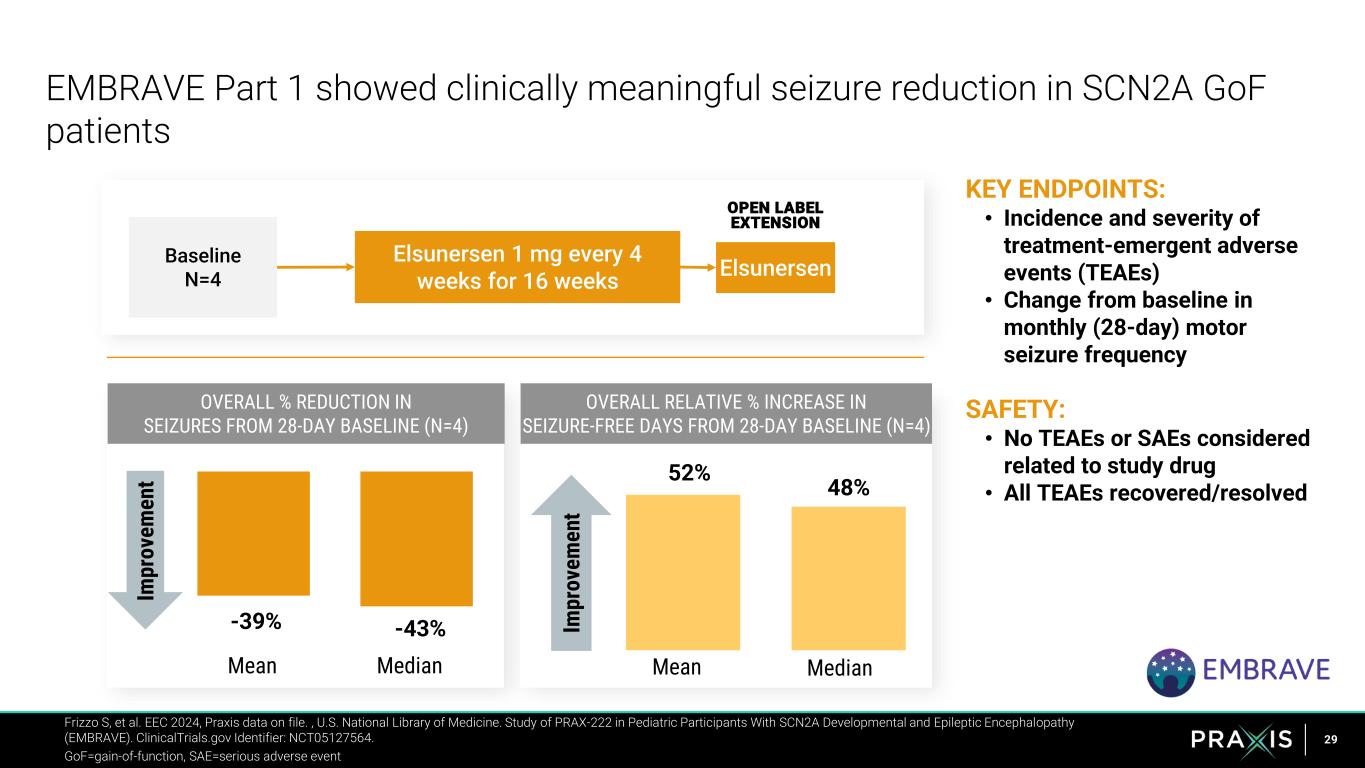
29 KEY ENDPOINTS: • Incidence and severity of treatment-emergent adverse events (TEAEs) • Change from baseline in monthly (28-day) motor seizure frequency SAFETY: • No TEAEs or SAEs considered related to study drug • All TEAEs recovered/resolved EMBRAVE Part 1 showed clinically meaningful seizure reduction in SCN2A GoF patients Elsunersen Baseline N=4 OPEN LABEL EXTENSION Elsunersen 1 mg every 4 weeks for 16 weeks Frizzo S, et al. EEC 2024, Praxis data on file. , U.S. National Library of Medicine. Study of PRAX-222 in Pediatric Participants With SCN2A Developmental and Epileptic Encephalopathy (EMBRAVE). ClinicalTrials.gov Identifier: NCT05127564. GoF=gain-of-function, SAE=serious adverse event -39% -43% Im pr ov em en t Mean Median 52% 48% Mean Median Im pr ov em en t OVERALL % REDUCTION IN SEIZURES FROM 28-DAY BASELINE (N=4) OVERALL RELATIVE % INCREASE IN SEIZURE-FREE DAYS FROM 28-DAY BASELINE (N=4)
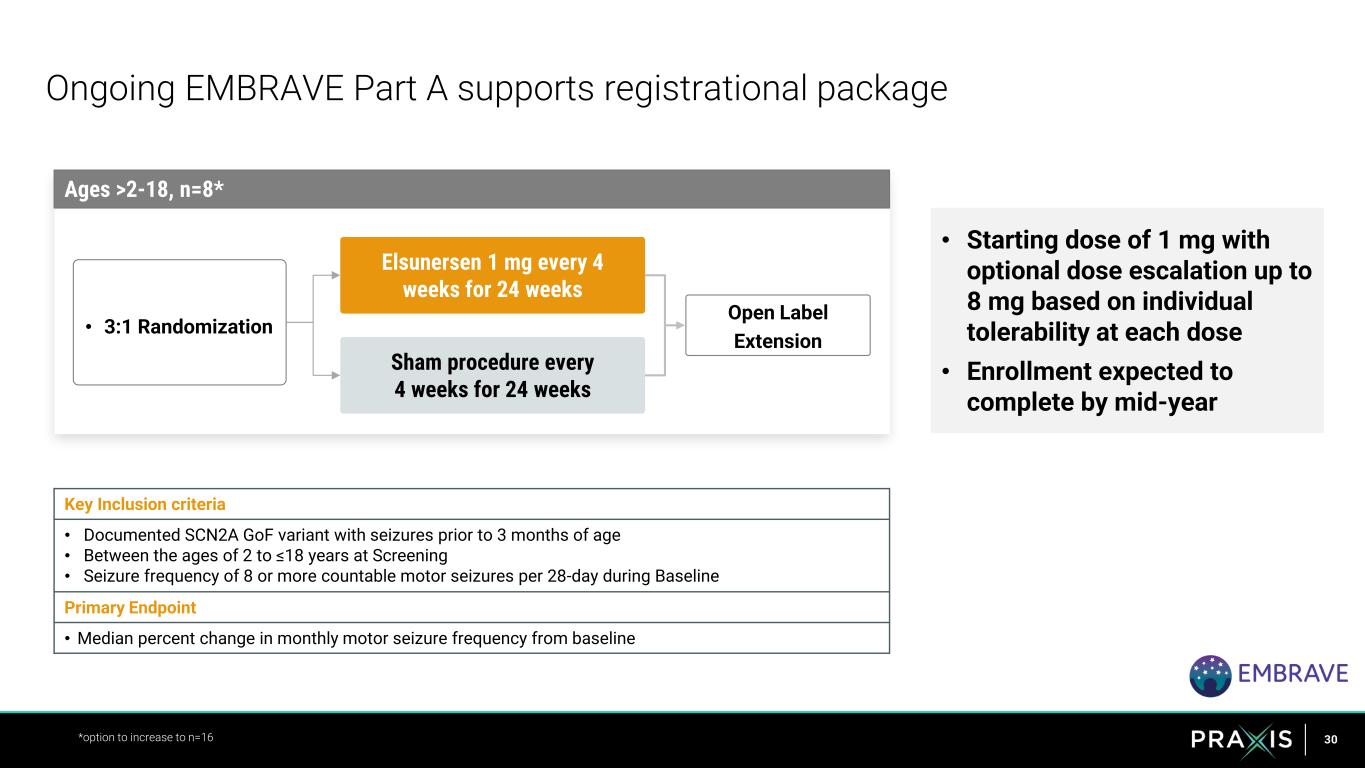
30 • Starting dose of 1 mg with optional dose escalation up to 8 mg based on individual tolerability at each dose • Enrollment expected to complete by mid-year Ongoing EMBRAVE Part A supports registrational package *option to increase to n=16 Sham procedure every 4 weeks for 24 weeks • 3:1 Randomization Ages >2-18, n=8* Elsunersen 1 mg every 4 weeks for 24 weeks Open Label Extension Key Inclusion criteria • Documented SCN2A GoF variant with seizures prior to 3 months of age • Between the ages of 2 to ≤18 years at Screening • Seizure frequency of 8 or more countable motor seizures per 28-day during Baseline Primary Endpoint • Median percent change in monthly motor seizure frequency from baseline
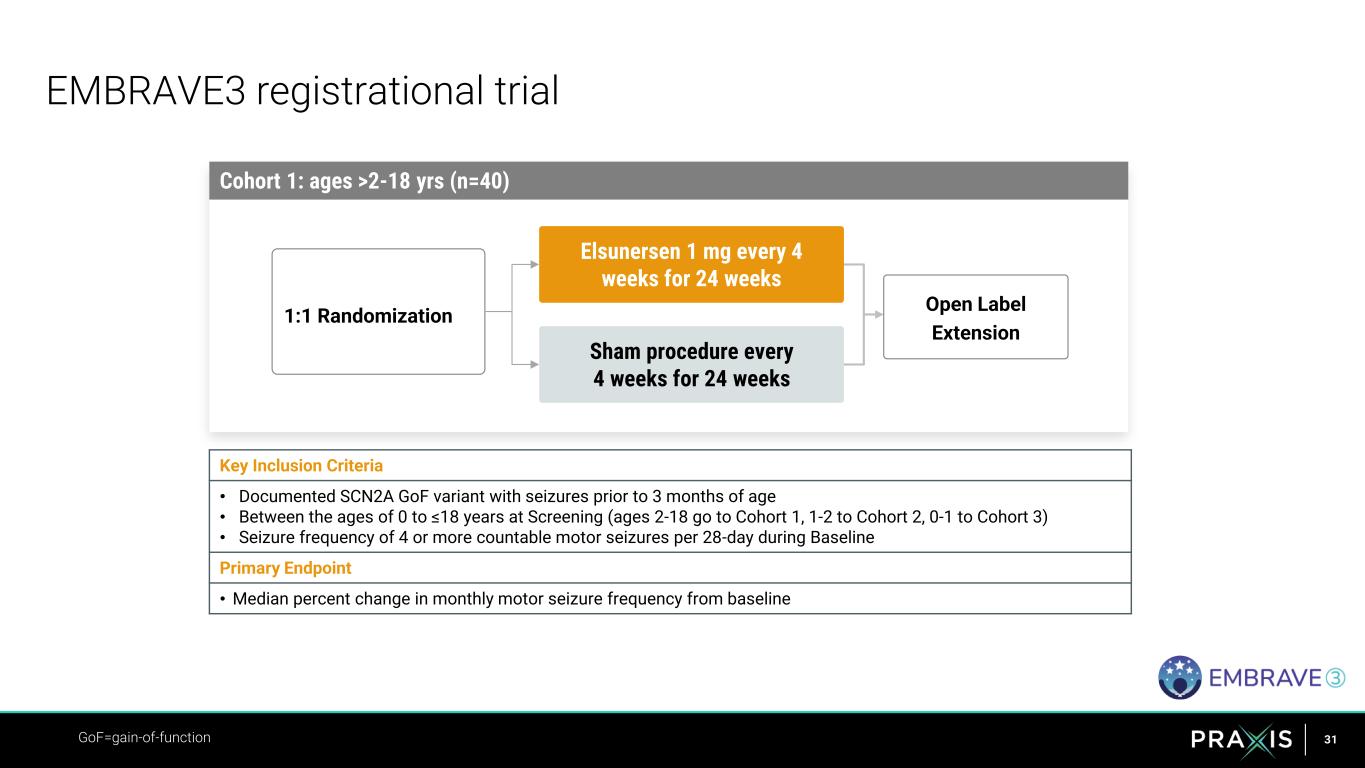
31 EMBRAVE3 registrational trial GoF=gain-of-function Cohort 1: ages >2-18 yrs (n=40) Sham procedure every 4 weeks for 24 weeks 1:1 Randomization Elsunersen 1 mg every 4 weeks for 24 weeks Open Label Extension Key Inclusion Criteria • Documented SCN2A GoF variant with seizures prior to 3 months of age • Between the ages of 0 to ≤18 years at Screening (ages 2-18 go to Cohort 1, 1-2 to Cohort 2, 0-1 to Cohort 3) • Seizure frequency of 4 or more countable motor seizures per 28-day during Baseline Primary Endpoint • Median percent change in monthly motor seizure frequency from baseline
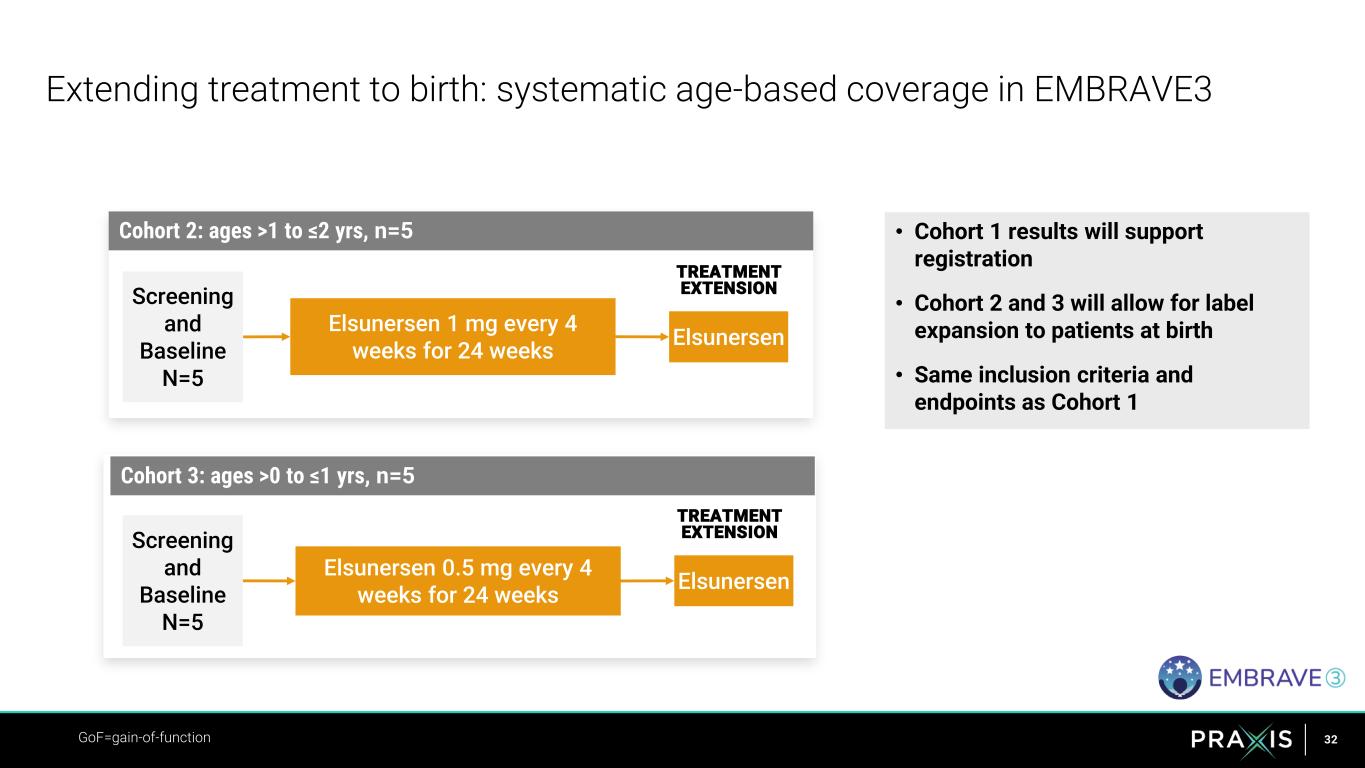
32 • Cohort 1 results will support registration • Cohort 2 and 3 will allow for label expansion to patients at birth • Same inclusion criteria and endpoints as Cohort 1 Extending treatment to birth: systematic age-based coverage in EMBRAVE3 Elsunersen Screening and Baseline N=5 TREATMENT EXTENSION Elsunersen 1 mg every 4 weeks for 24 weeks GoF=gain-of-function Elsunersen Screening and Baseline N=5 Elsunersen 0.5 mg every 4 weeks for 24 weeks Cohort 2: ages >1 to ≤2 yrs, n=5 TREATMENT EXTENSION Cohort 3: ages >0 to ≤1 yrs, n=5
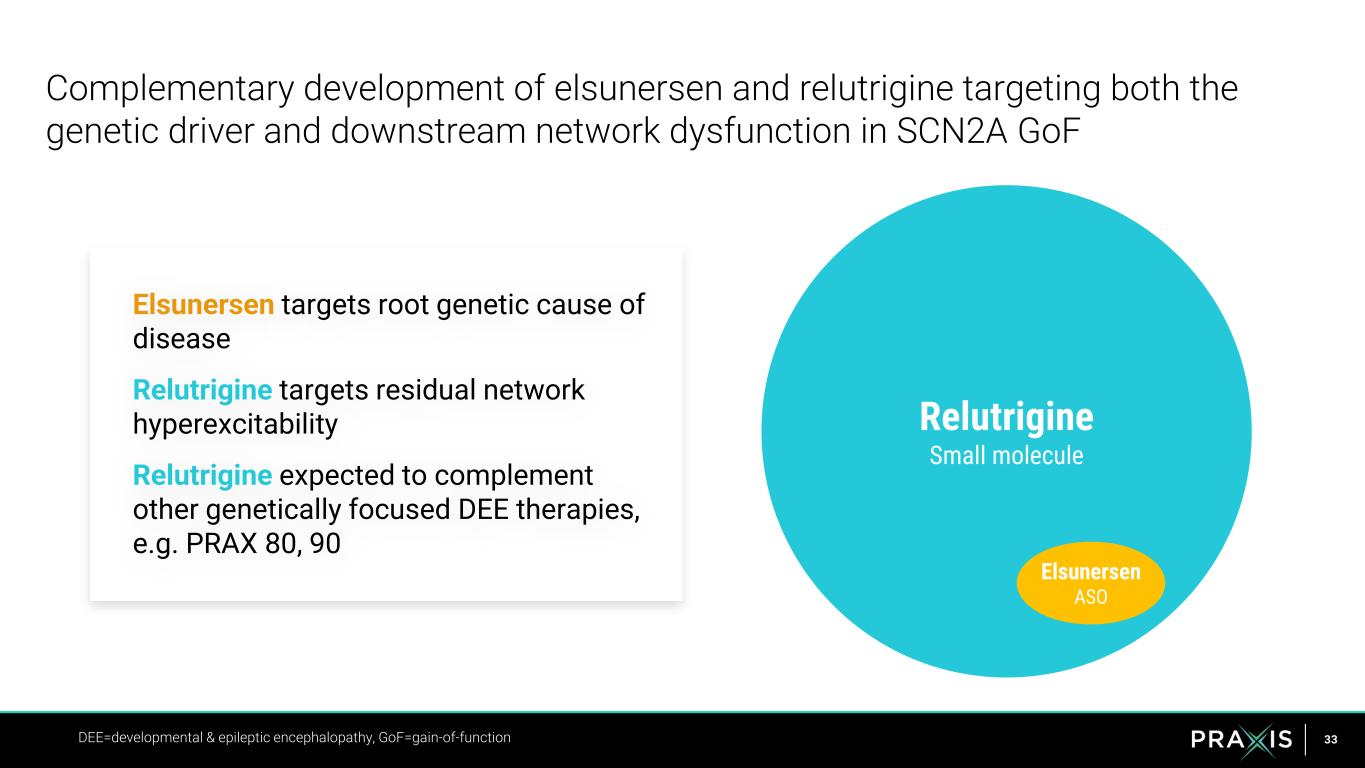
33 Relutrigine Small molecule Elsunersen ASO Complementary development of elsunersen and relutrigine targeting both the genetic driver and downstream network dysfunction in SCN2A GoF DEE=developmental & epileptic encephalopathy, GoF=gain-of-function Elsunersen targets root genetic cause of disease Relutrigine targets residual network hyperexcitability Relutrigine expected to complement other genetically focused DEE therapies, e.g. PRAX 80, 90
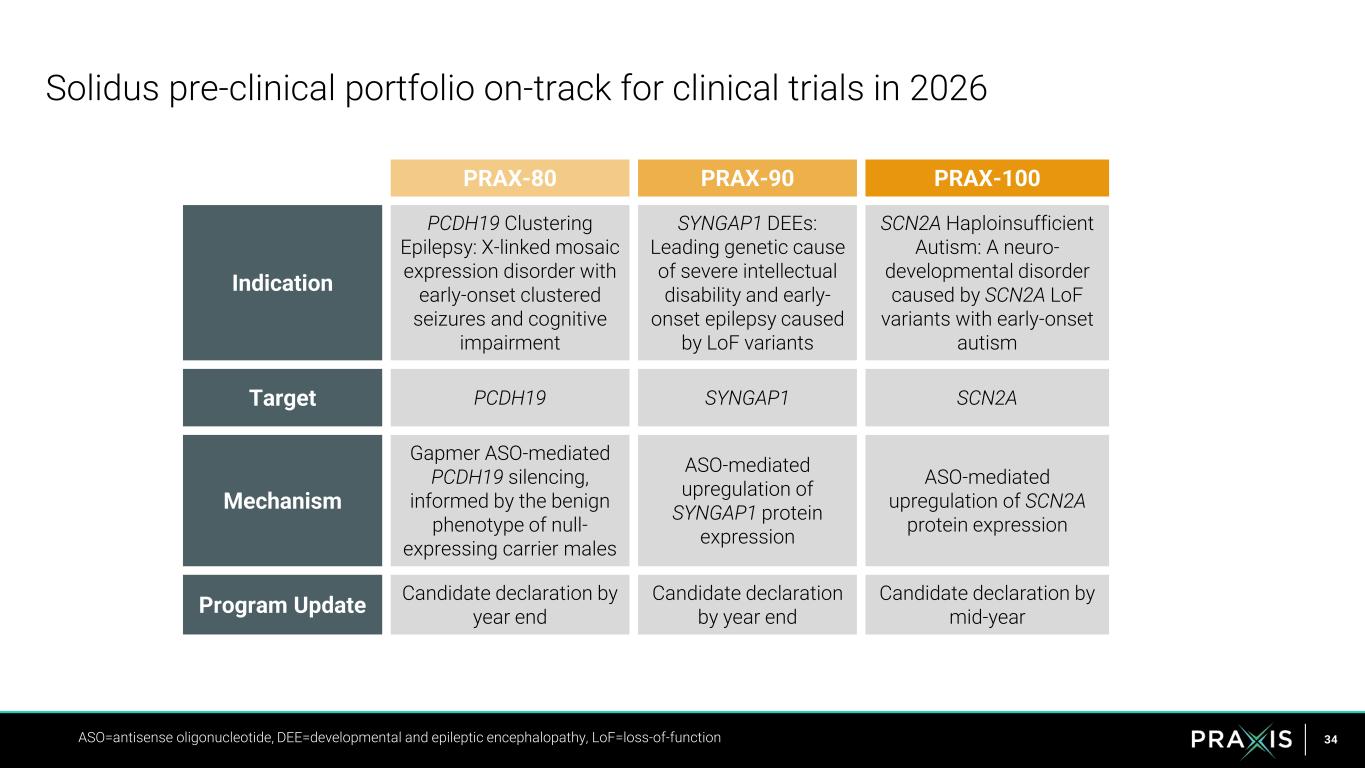
34 Solidus pre-clinical portfolio on-track for clinical trials in 2026 ASO=antisense oligonucleotide, DEE=developmental and epileptic encephalopathy, LoF=loss-of-function PRAX-80 PRAX-90 PRAX-100 Indication PCDH19 Clustering Epilepsy: X-linked mosaic expression disorder with early-onset clustered seizures and cognitive impairment SYNGAP1 DEEs: Leading genetic cause of severe intellectual disability and early- onset epilepsy caused by LoF variants SCN2A Haploinsufficient Autism: A neuro- developmental disorder caused by SCN2A LoF variants with early-onset autism Target PCDH19 SYNGAP1 SCN2A Mechanism Gapmer ASO-mediated PCDH19 silencing, informed by the benign phenotype of null- expressing carrier males ASO-mediated upregulation of SYNGAP1 protein expression ASO-mediated upregulation of SCN2A protein expression Program Update Candidate declaration by year end Candidate declaration by year end Candidate declaration by mid-year

MOVEMENT DISORDERS
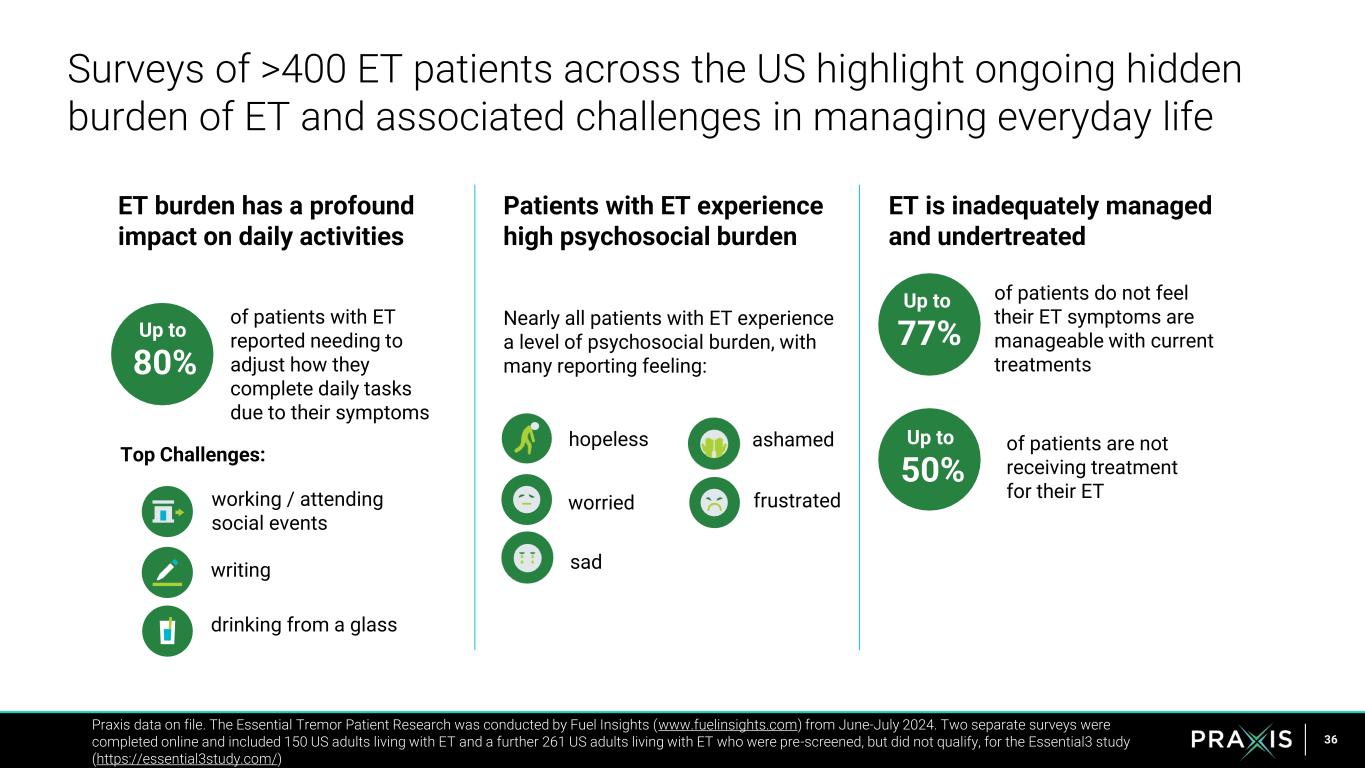
36 Surveys of >400 ET patients across the US highlight ongoing hidden burden of ET and associated challenges in managing everyday life Praxis data on file. The Essential Tremor Patient Research was conducted by Fuel Insights (www.fuelinsights.com) from June-July 2024. Two separate surveys were completed online and included 150 US adults living with ET and a further 261 US adults living with ET who were pre-screened, but did not qualify, for the Essential3 study (https://essential3study.com/) ET burden has a profound impact on daily activities Patients with ET experience high psychosocial burden Nearly all patients with ET experience a level of psychosocial burden, with many reporting feeling: ET is inadequately managed and undertreated Up to 80% working / attending social events writing drinking from a glass frustratedworried Up to 77% Up to 50% of patients do not feel their ET symptoms are manageable with current treatments of patients are not receiving treatment for their ET of patients with ET reported needing to adjust how they complete daily tasks due to their symptoms Top Challenges: ashamed sad hopeless
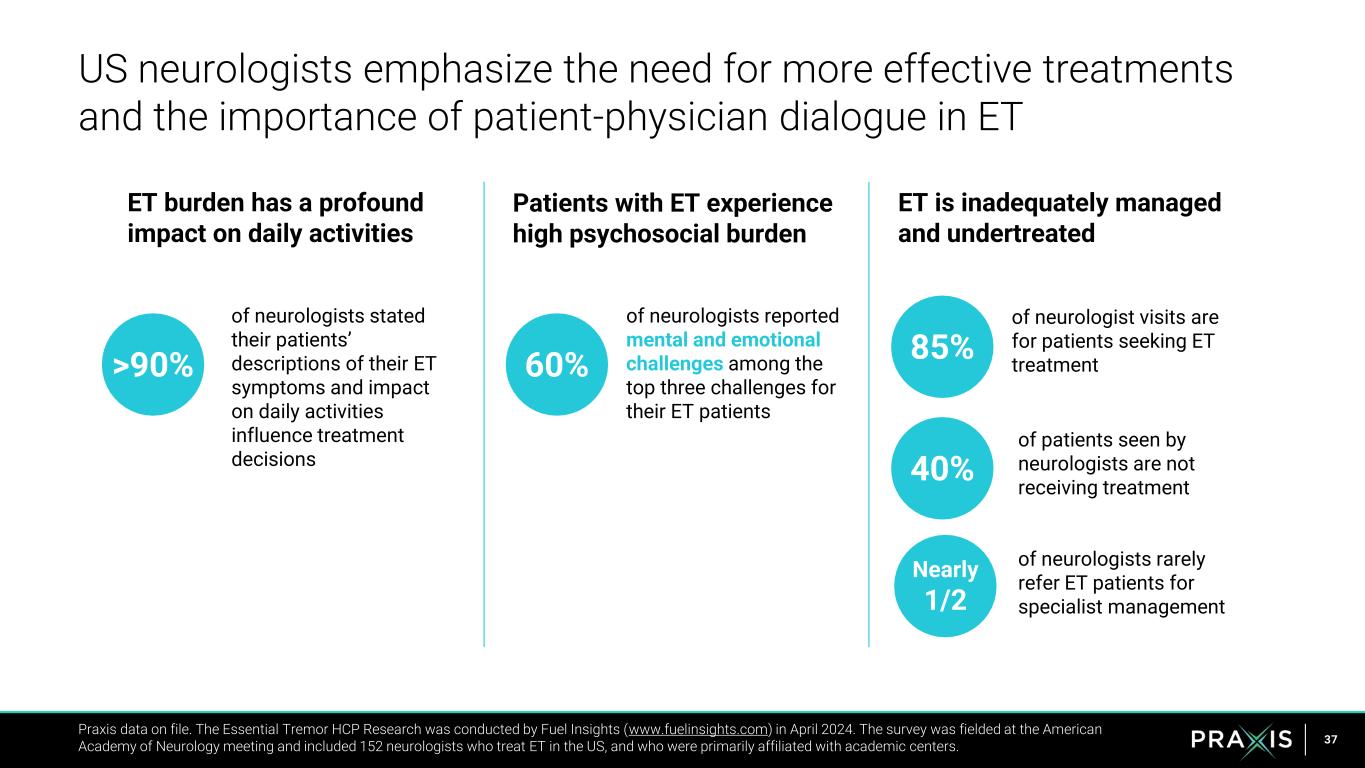
37 US neurologists emphasize the need for more effective treatments and the importance of patient-physician dialogue in ET Praxis data on file. The Essential Tremor HCP Research was conducted by Fuel Insights (www.fuelinsights.com) in April 2024. The survey was fielded at the American Academy of Neurology meeting and included 152 neurologists who treat ET in the US, and who were primarily affiliated with academic centers. ET burden has a profound impact on daily activities Patients with ET experience high psychosocial burden ET is inadequately managed and undertreated 60% of neurologists reported mental and emotional challenges among the top three challenges for their ET patients 40% >90% of patients seen by neurologists are not receiving treatment of neurologists stated their patients’ descriptions of their ET symptoms and impact on daily activities influence treatment decisions 85% of neurologist visits are for patients seeking ET treatment Nearly 1/2 of neurologists rarely refer ET patients for specialist management
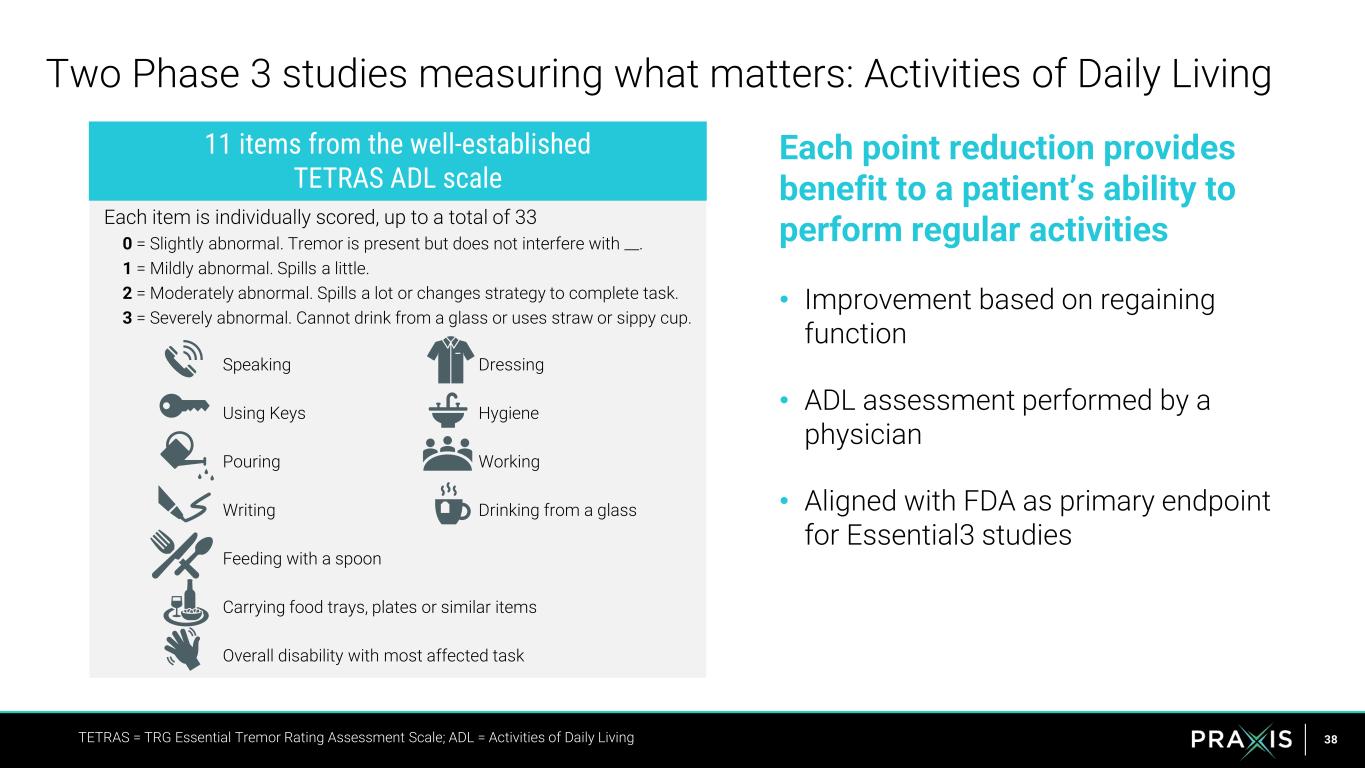
38 Speaking Dressing Using Keys Hygiene Pouring Working Writing Drinking from a glass Feeding with a spoon Carrying food trays, plates or similar items Overall disability with most affected task Each point reduction provides benefit to a patient’s ability to perform regular activities • Improvement based on regaining function • ADL assessment performed by a physician • Aligned with FDA as primary endpoint for Essential3 studies Two Phase 3 studies measuring what matters: Activities of Daily Living TETRAS = TRG Essential Tremor Rating Assessment Scale; ADL = Activities of Daily Living Each item is individually scored, up to a total of 33 0 = Slightly abnormal. Tremor is present but does not interfere with __. 1 = Mildly abnormal. Spills a little. 2 = Moderately abnormal. Spills a lot or changes strategy to complete task. 3 = Severely abnormal. Cannot drink from a glass or uses straw or sippy cup. 11 items from the well-established TETRAS ADL scale
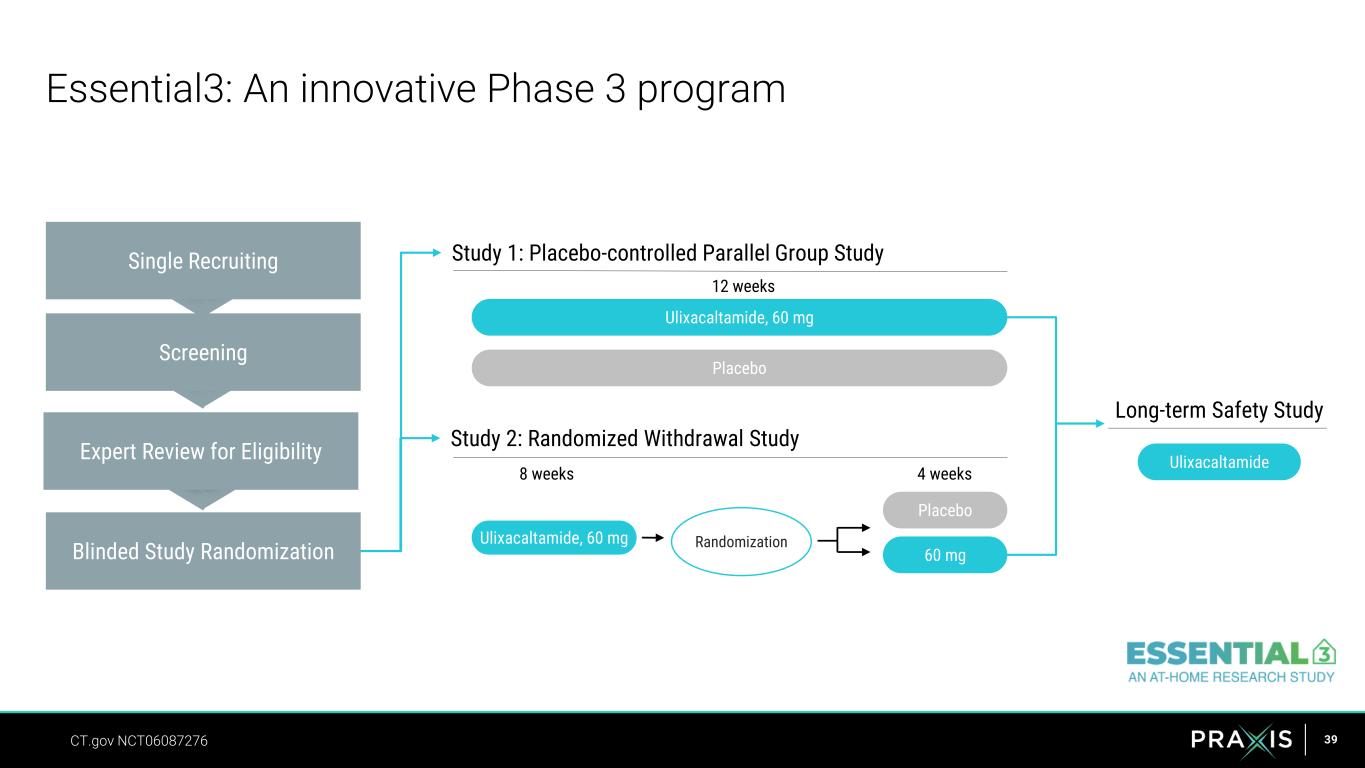
39 Essential3: An innovative Phase 3 program Single Recruiting Expert Review for Eligibility Screening Blinded Study Randomization Study 1: Placebo-controlled Parallel Group Study Ulixacaltamide, 60 mg Placebo Study 2: Randomized Withdrawal Study Ulixacaltamide, 60 mg Randomization 60 mg Placebo Long-term Safety Study Ulixacaltamide CT.gov NCT06087276 8 weeks 4 weeks 12 weeks
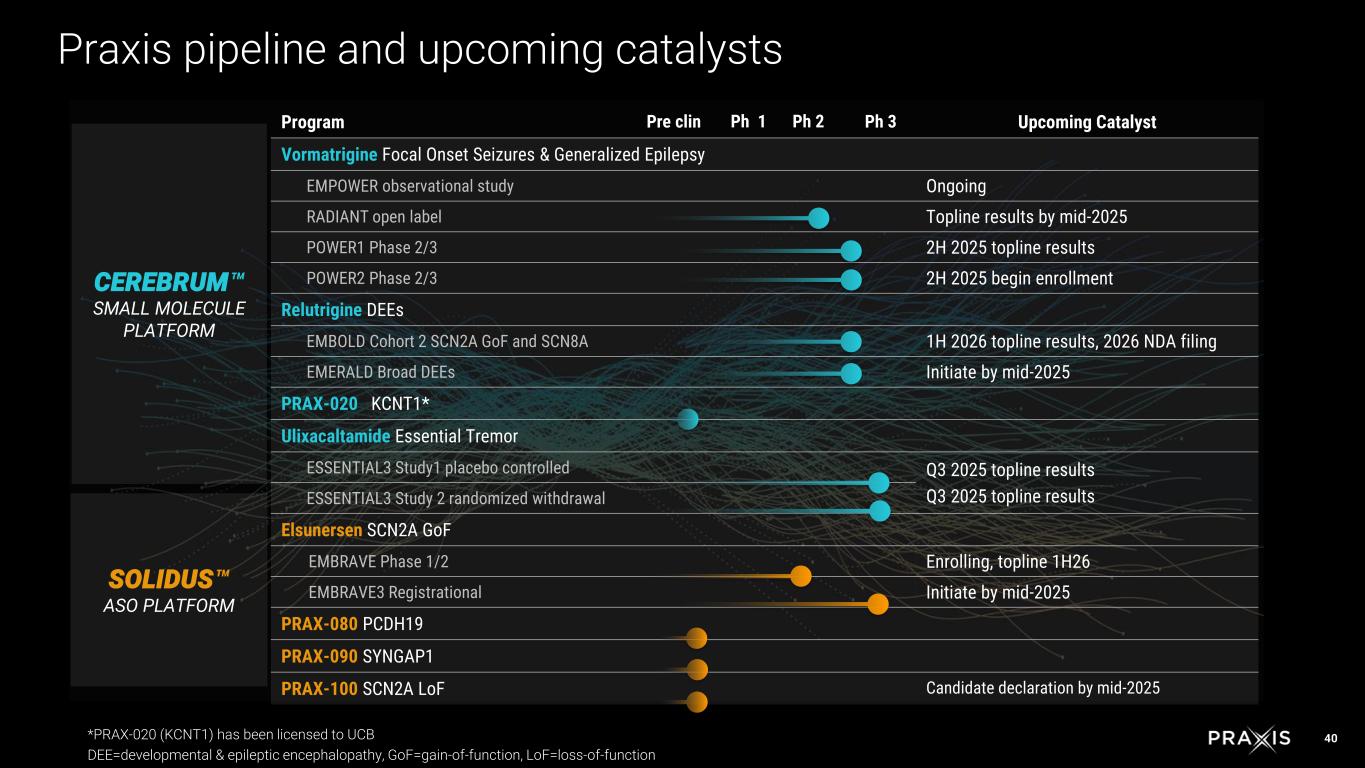
40 Program Pre clin Ph 1 Ph 2 Ph 3 Upcoming Catalyst Vormatrigine Focal Onset Seizures & Generalized Epilepsy EMPOWER observational study Ongoing RADIANT open label Topline results by mid-2025 POWER1 Phase 2/3 2H 2025 topline results POWER2 Phase 2/3 2H 2025 begin enrollment Relutrigine DEEs EMBOLD Cohort 2 SCN2A GoF and SCN8A 1H 2026 topline results, 2026 NDA filing EMERALD Broad DEEs Initiate by mid-2025 PRAX-020 KCNT1* Ulixacaltamide Essential Tremor ESSENTIAL3 Study1 placebo controlled Q3 2025 topline results Q3 2025 topline resultsESSENTIAL3 Study 2 randomized withdrawal Elsunersen SCN2A GoF EMBRAVE Phase 1/2 Enrolling, topline 1H26 EMBRAVE3 Registrational Initiate by mid-2025 PRAX-080 PCDH19 PRAX-090 SYNGAP1 PRAX-100 SCN2A LoF Candidate declaration by mid-2025 CEREBRUM SMALL MOLECULE PLATFORM SOLIDUS ASO PLATFORM Praxis pipeline and upcoming catalysts *PRAX-020 (KCNT1) has been licensed to UCB DEE=developmental & epileptic encephalopathy, GoF=gain-of-function, LoF=loss-of-function

®