
Claseprubart (DNTH103) Top-line Ph. 2 MaGic Results in Generalized Myasthenia Gravis September 8, 2025 .2

Forward-looking statements Certain statements in this presentation (“Presentation”), other than purely historical information, may constitute “forward-looking statements” within the meaning of the federal securities laws, including for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995, concerning Dianthus Therapeutics, Inc. (the “Company”). These forward-looking statements include statements regarding the Company’s future plans and prospects, including statements regarding the expectations or plans for discovery, preclinical studies, clinical trials and research and development programs, in particular with respect to claseprubart, and any developments or results in connection therewith, including the target product profile and administration of claseprubart; the anticipated timing of the results from those studies and trials; expectations regarding the clinical trial design for the Phase 3 trial for claseprubart; expectations regarding the use of proceeds and the time period over which the Company’s capital resources will be sufficient to fund its anticipated operations; and expectations regarding market size, patient population size and potential opportunities for complement therapies, in particular with respect to claseprubart. Claseprubart is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide. The words “opportunity,” “potential,” “milestones,” “runway,” “will,” “anticipate,” “achieve,” “near-term,” “catalysts,” “pursue,” “pipeline,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “predict,” “project,” “should,” “strive,” “would,” “aim,” “target,” “commit,” and similar expressions (including the negatives of these terms or variations of them) generally identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Actual results could differ materially from those included in the forward-looking statements due to various factors, risks and uncertainties, including, but not limited to, that preclinical testing of claseprubart and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that the development of claseprubart or the Company's compounds may take longer and/or cost more than planned, that the Company may be unable to successfully complete the clinical development of the Company’s compounds, that the Company may be delayed in initiating, enrolling or completing any clinical trials, and that the Company's compounds may not receive regulatory approval or become commercially successful products. These and other risks and uncertainties are identified under the heading "Risk Factors" included in the Company’s Annual Report on Form 10-K for the period ended December 31, 2024, and other filings that the Company has made and may make with the SEC in the future. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. Dianthus undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.

Introduction Marino Garcia, Chief Executive Officer MaGic Phase 2 Top-line Results Simrat Randhawa, MD, Chief Medical Officer Claseprubart Summary Remarks Marino Garcia, Chief Executive Officer Analyst Q&A Marino Garcia, Chief Executive Officer Simrat Randhawa, MD, Chief Medical Officer John King, Chief Commercial Officer Ryan Savitz, Chief Financial Officer & Chief Business Officer Agenda

Introduction Marino Garcia, CEO
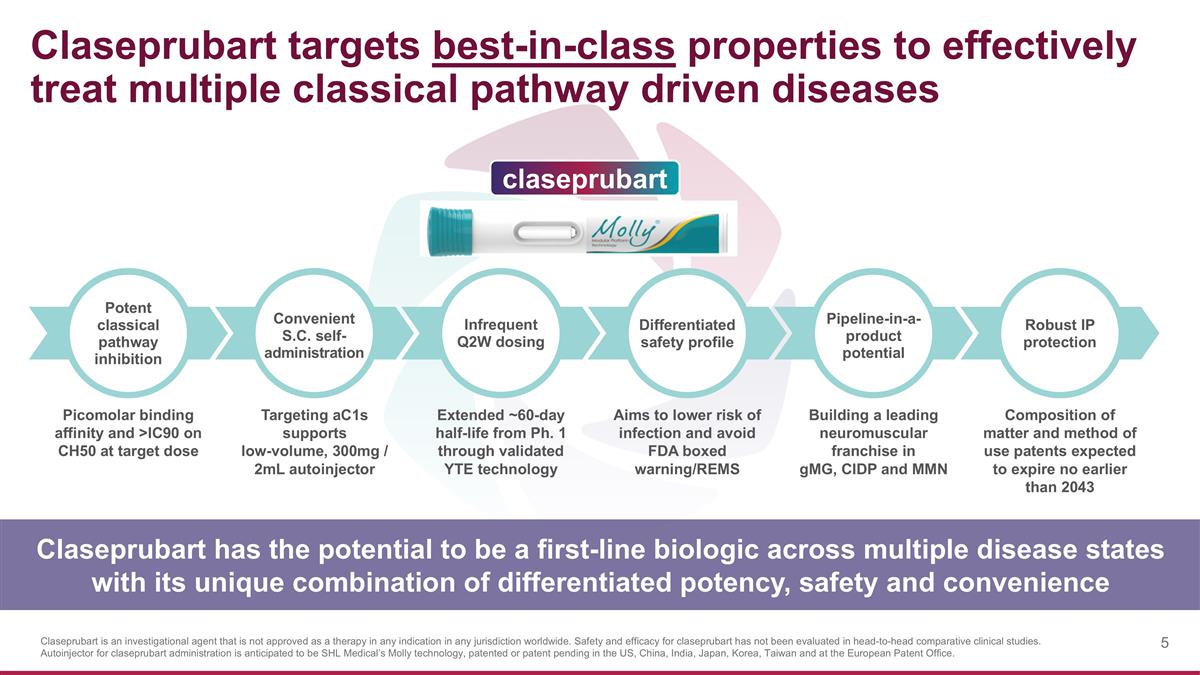
Claseprubart targets best-in-class properties to effectively treat multiple classical pathway driven diseases Picomolar binding affinity and >IC90 on CH50 at target dose Extended ~60-day half-life from Ph. 1 through validated YTE technology Targeting aC1s supports low-volume, 300mg / 2mL autoinjector Aims to lower risk of infection and avoid FDA boxed warning/REMS Building a leading neuromuscular franchise in gMG, CIDP and MMN Composition of matter and method of use patents expected to expire no earlier than 2043 Infrequent Q2W dosing Convenient S.C. self- administration Potent classical pathway inhibition Differentiated safety profile Pipeline-in-a-product potential Robust IP protection Claseprubart has the potential to be a first-line biologic across multiple disease states with its unique combination of differentiated potency, safety and convenience claseprubart Claseprubart is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide. Safety and efficacy for claseprubart has not been evaluated in head-to-head comparative clinical studies. Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office.
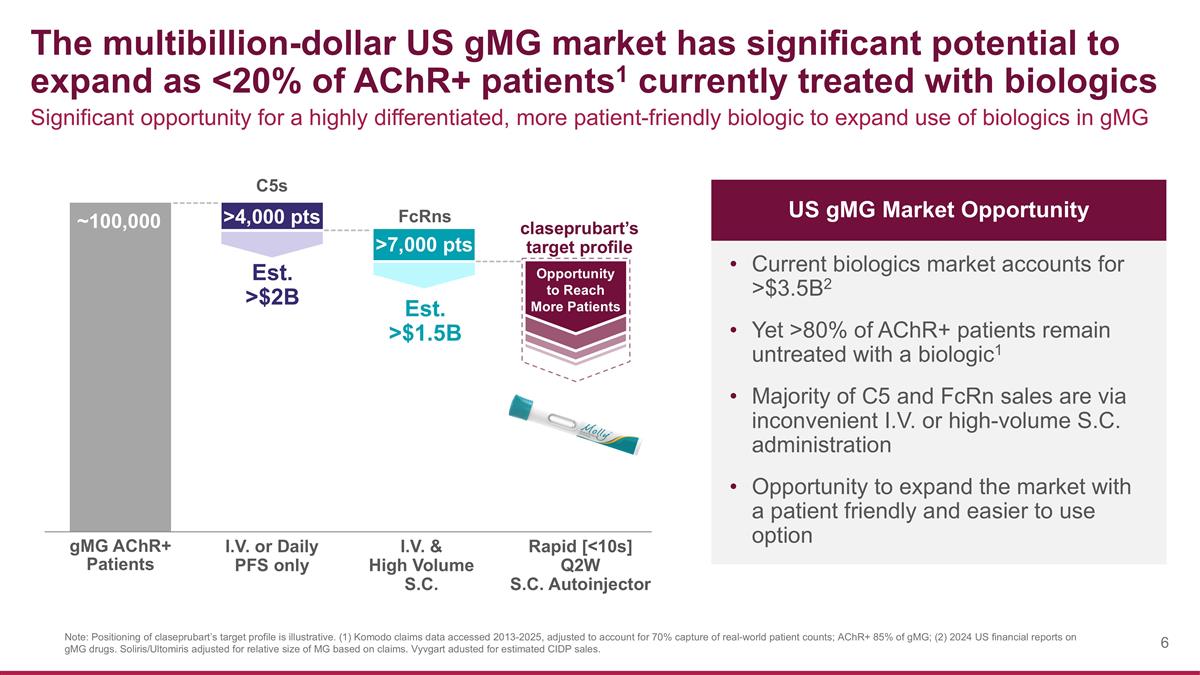
The multibillion-dollar US gMG market has significant potential to expand as <20% of AChR+ patients1 currently treated with biologics Note: Positioning of claseprubart’s target profile is illustrative. (1) Komodo claims data accessed 2013-2025, adjusted to account for 70% capture of real-world patient counts; AChR+ 85% of gMG; (2) 2024 US financial reports on gMG drugs. Soliris/Ultomiris adjusted for relative size of MG based on claims. Vyvgart adusted for estimated CIDP sales. ~100,000 Significant opportunity for a highly differentiated, more patient-friendly biologic to expand use of biologics in gMG Current biologics market accounts for >$3.5B2 Yet >80% of AChR+ patients remain untreated with a biologic1 Majority of C5 and FcRn sales are via inconvenient I.V. or high-volume S.C. administration Opportunity to expand the market with a patient friendly and easier to use option I.V. or Daily PFS only I.V. & High Volume S.C. Rapid [<10s] Q2W S.C. Autoinjector C5s FcRns claseprubart’s target profile Est. >$2B Est. >$1.5B Opportunity to Reach More Patients >4,000 pts >7,000 pts US gMG Market Opportunity
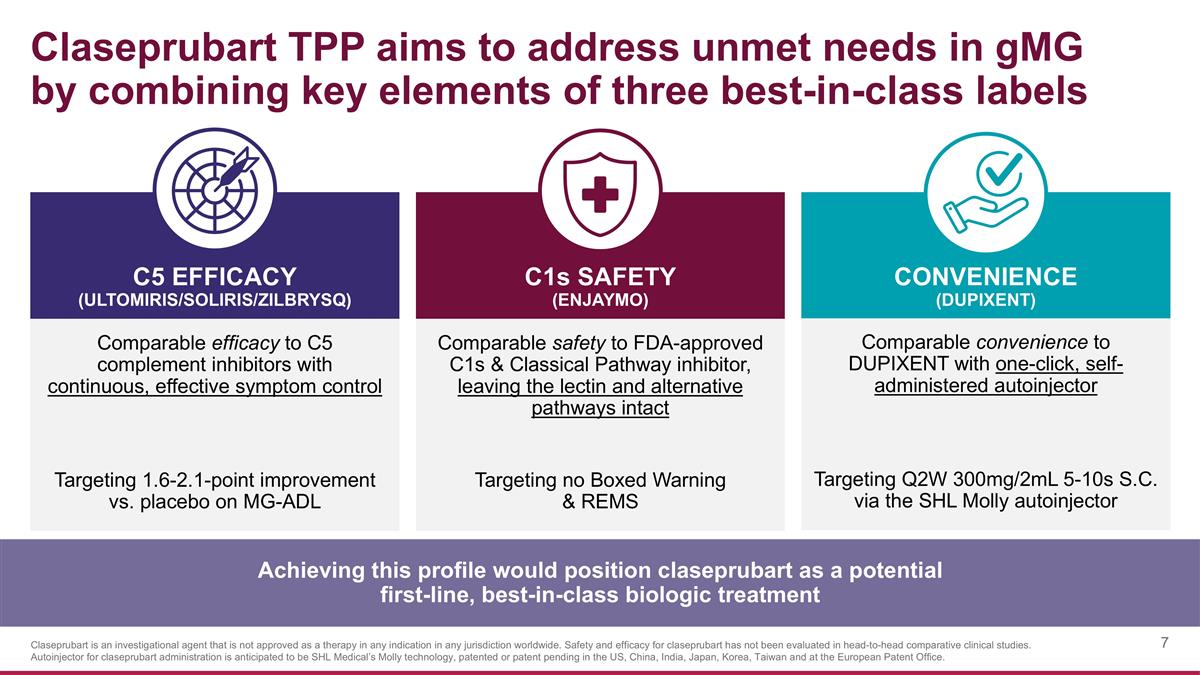
Claseprubart TPP aims to address unmet needs in gMG by combining key elements of three best-in-class labels Comparable efficacy to C5 complement inhibitors with continuous, effective symptom control Targeting 1.6-2.1-point improvement vs. placebo on MG-ADL C1s SAFETY (Enjaymo) C5 EFFICACY (ULTOMIRIS/SOLIRIS/ZILBRYSQ) CONVENIENCE (DUPIXENT) Comparable safety to FDA-approved C1s & Classical Pathway inhibitor, leaving the lectin and alternative pathways intact Targeting no Boxed Warning & REMS Comparable convenience to DUPIXENT with one-click, self-administered autoinjector Targeting Q2W 300mg/2mL 5-10s S.C. via the SHL Molly autoinjector Achieving this profile would position claseprubart as a potential first-line, best-in-class biologic treatment Claseprubart is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide. Safety and efficacy for claseprubart has not been evaluated in head-to-head comparative clinical studies. Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office.
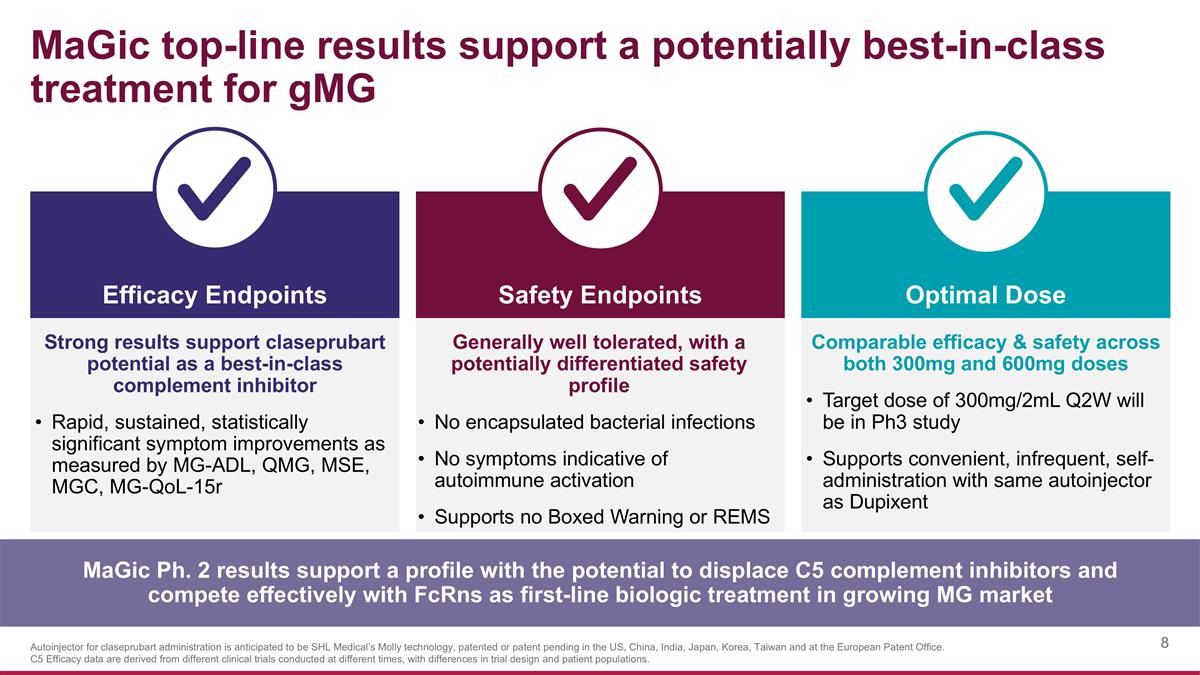
MaGic top-line results support a potentially best-in-class treatment for gMG Strong results support claseprubart potential as a best-in-class complement inhibitor Rapid, sustained, statistically significant symptom improvements as measured by MG-ADL, QMG, MSE, MGC, MG-QoL-15r Safety Endpoints Efficacy Endpoints Optimal Dose Generally well tolerated, with a potentially differentiated safety profile No encapsulated bacterial infections No symptoms indicative of autoimmune activation Supports no Boxed Warning or REMS Comparable efficacy & safety across both 300mg and 600mg doses Target dose of 300mg/2mL Q2W will be in Ph3 study Supports convenient, infrequent, self-administration with same autoinjector as Dupixent MaGic Ph. 2 results support a profile with the potential to displace C5 complement inhibitors and compete effectively with FcRns as first-line biologic treatment in growing MG market Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office. C5 Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations.

MaGic Phase 2 Top-line Results Simrat Randhawa, MD, CMO
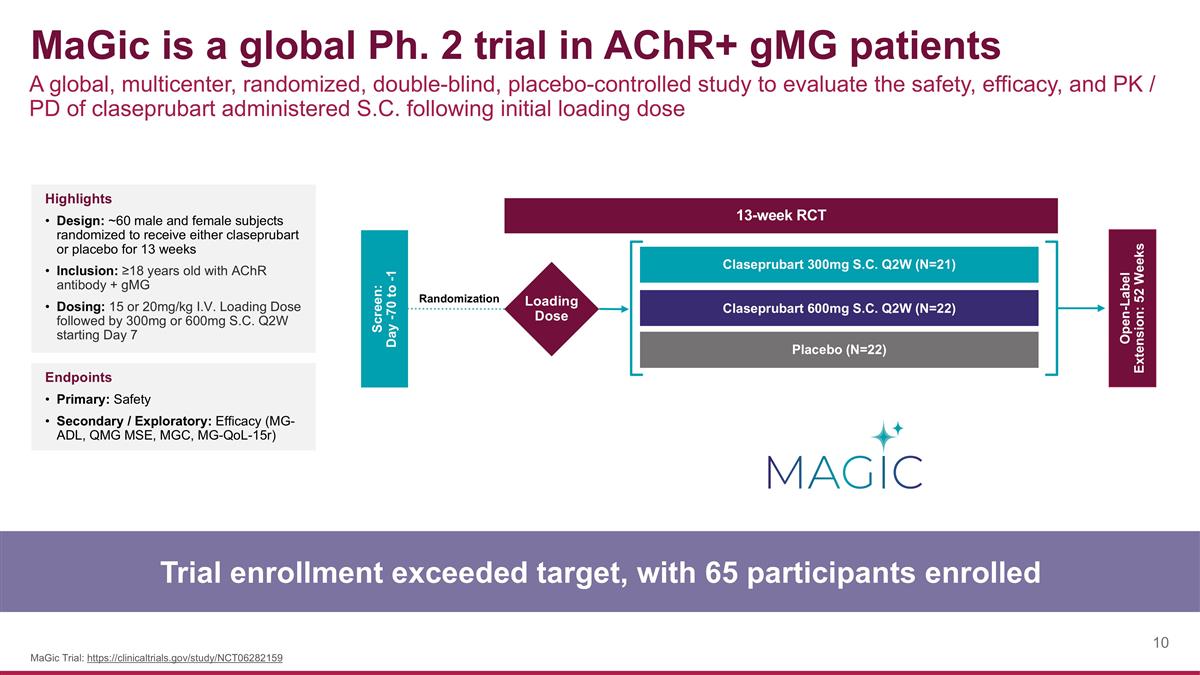
MaGic is a global Ph. 2 trial in AChR+ gMG patients A global, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety, efficacy, and PK / PD of claseprubart administered S.C. following initial loading dose Trial enrollment exceeded target, with 65 participants enrolled MaGic Trial: https://clinicaltrials.gov/study/NCT06282159 Highlights Design: ~60 male and female subjects randomized to receive either claseprubart or placebo for 13 weeks Inclusion: ≥18 years old with AChR antibody + gMG Dosing: 15 or 20mg/kg I.V. Loading Dose followed by 300mg or 600mg S.C. Q2W starting Day 7 Endpoints Primary: Safety Secondary / Exploratory: Efficacy (MG-ADL, QMG MSE, MGC, MG-QoL-15r) Screen: Day -70 to -1 Open-Label Extension: 52 Weeks Claseprubart 300mg S.C. Q2W (N=21) Claseprubart 600mg S.C. Q2W (N=22) Placebo (N=22) 13-week RCT Loading Dose Randomization
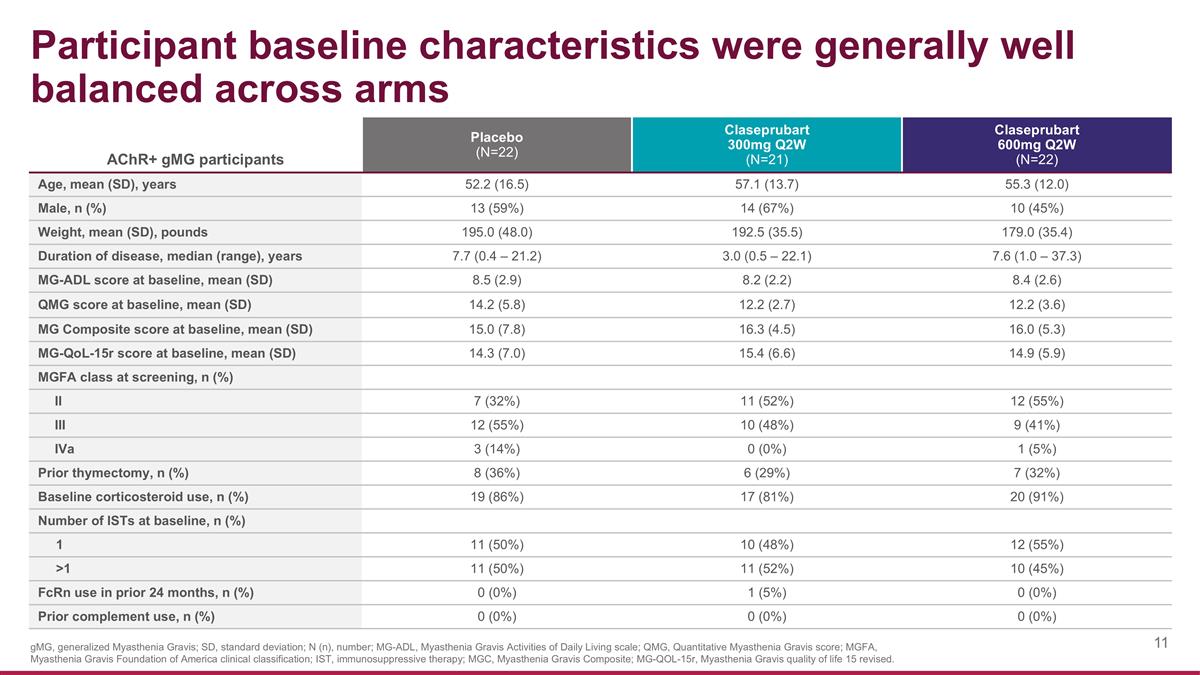
AChR+ gMG participants Placebo (N=22) Claseprubart 300mg Q2W (N=21) Claseprubart 600mg Q2W (N=22) Age, mean (SD), years 52.2 (16.5) 57.1 (13.7) 55.3 (12.0) Male, n (%) 13 (59%) 14 (67%) 10 (45%) Weight, mean (SD), pounds 195.0 (48.0) 192.5 (35.5) 179.0 (35.4) Duration of disease, median (range), years 7.7 (0.4 – 21.2) 3.0 (0.5 – 22.1) 7.6 (1.0 – 37.3) MG-ADL score at baseline, mean (SD) 8.5 (2.9) 8.2 (2.2) 8.4 (2.6) QMG score at baseline, mean (SD) 14.2 (5.8) 12.2 (2.7) 12.2 (3.6) MG Composite score at baseline, mean (SD) 15.0 (7.8) 16.3 (4.5) 16.0 (5.3) MG-QoL-15r score at baseline, mean (SD) 14.3 (7.0) 15.4 (6.6) 14.9 (5.9) MGFA class at screening, n (%) II 7 (32%) 11 (52%) 12 (55%) III 12 (55%) 10 (48%) 9 (41%) IVa 3 (14%) 0 (0%) 1 (5%) Prior thymectomy, n (%) 8 (36%) 6 (29%) 7 (32%) Baseline corticosteroid use, n (%) 19 (86%) 17 (81%) 20 (91%) Number of ISTs at baseline, n (%) 1 11 (50%) 10 (48%) 12 (55%) >1 11 (50%) 11 (52%) 10 (45%) FcRn use in prior 24 months, n (%) 0 (0%) 1 (5%) 0 (0%) Prior complement use, n (%) 0 (0%) 0 (0%) 0 (0%) Participant baseline characteristics were generally well balanced across arms gMG, generalized Myasthenia Gravis; SD, standard deviation; N (n), number; MG-ADL, Myasthenia Gravis Activities of Daily Living scale; QMG, Quantitative Myasthenia Gravis score; MGFA, Myasthenia Gravis Foundation of America clinical classification; IST, immunosuppressive therapy; MGC, Myasthenia Gravis Composite; MG-QOL-15r, Myasthenia Gravis quality of life 15 revised.
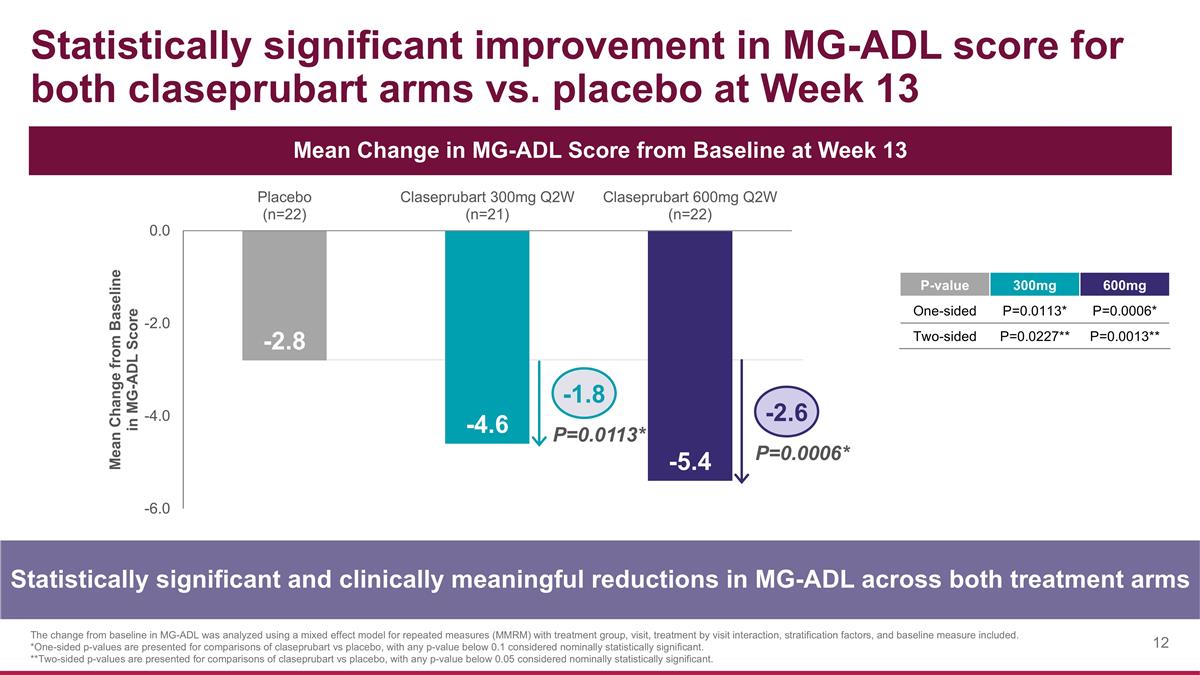
Statistically significant improvement in MG-ADL score for both claseprubart arms vs. placebo at Week 13 Statistically significant and clinically meaningful reductions in MG-ADL across both treatment arms Mean Change in MG-ADL Score from Baseline at Week 13 The change from baseline in MG-ADL was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. **Two-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.05 considered nominally statistically significant. P=0.0113* P=0.0006* -1.8 -2.6 P-value 300mg 600mg One-sided P=0.0113* P=0.0006* Two-sided P=0.0227** P=0.0013**
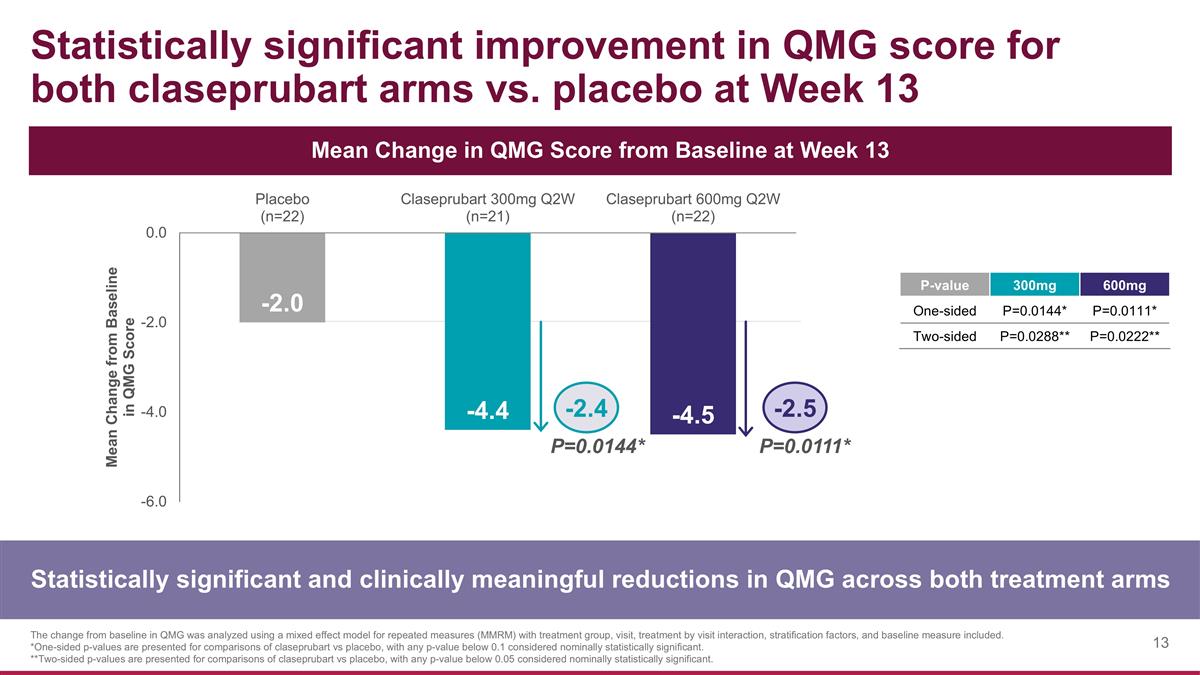
Statistically significant improvement in QMG score for both claseprubart arms vs. placebo at Week 13 Statistically significant and clinically meaningful reductions in QMG across both treatment arms The change from baseline in QMG was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. **Two-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.05 considered nominally statistically significant. Mean Change in QMG Score from Baseline at Week 13 P=0.0144* P=0.0111* -2.4 -2.5 P-value 300mg 600mg One-sided P=0.0144* P=0.0111* Two-sided P=0.0288** P=0.0222**
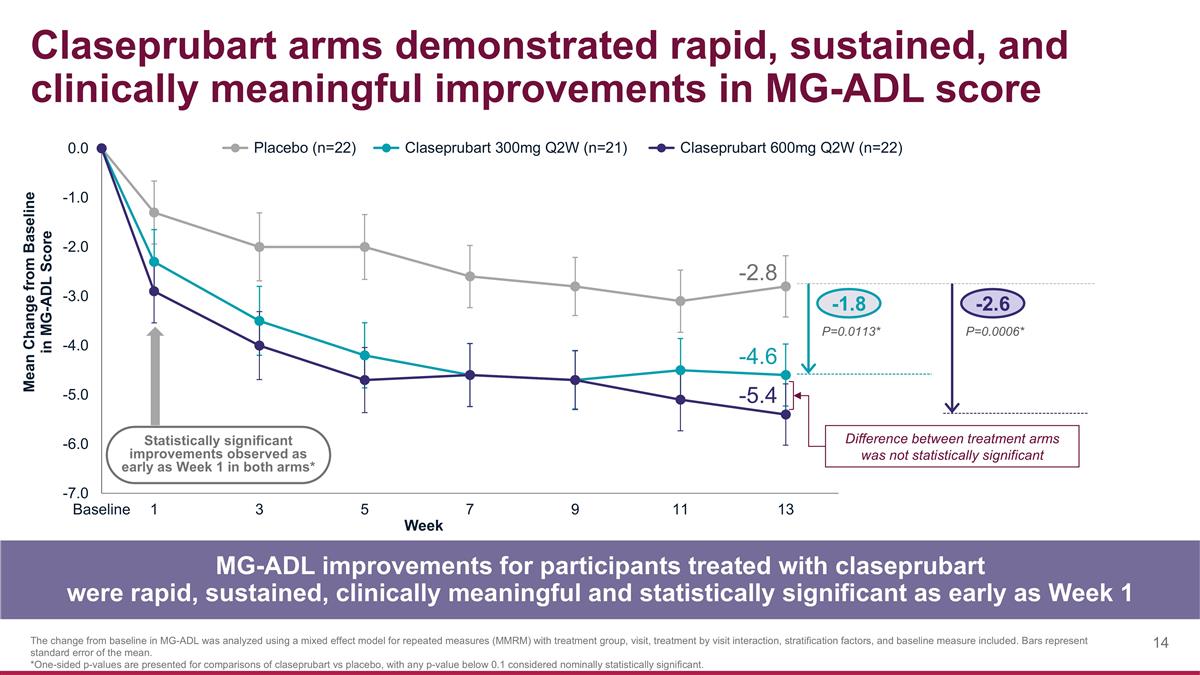
-1.8 -2.6 P=0.0113* P=0.0006* Claseprubart arms demonstrated rapid, sustained, and clinically meaningful improvements in MG-ADL score MG-ADL improvements for participants treated with claseprubart were rapid, sustained, clinically meaningful and statistically significant as early as Week 1 The change from baseline in MG-ADL was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. Bars represent standard error of the mean. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. Statistically significant improvements observed as early as Week 1 in both arms* -2.8 -4.6 -5.4 Difference between treatment arms was not statistically significant
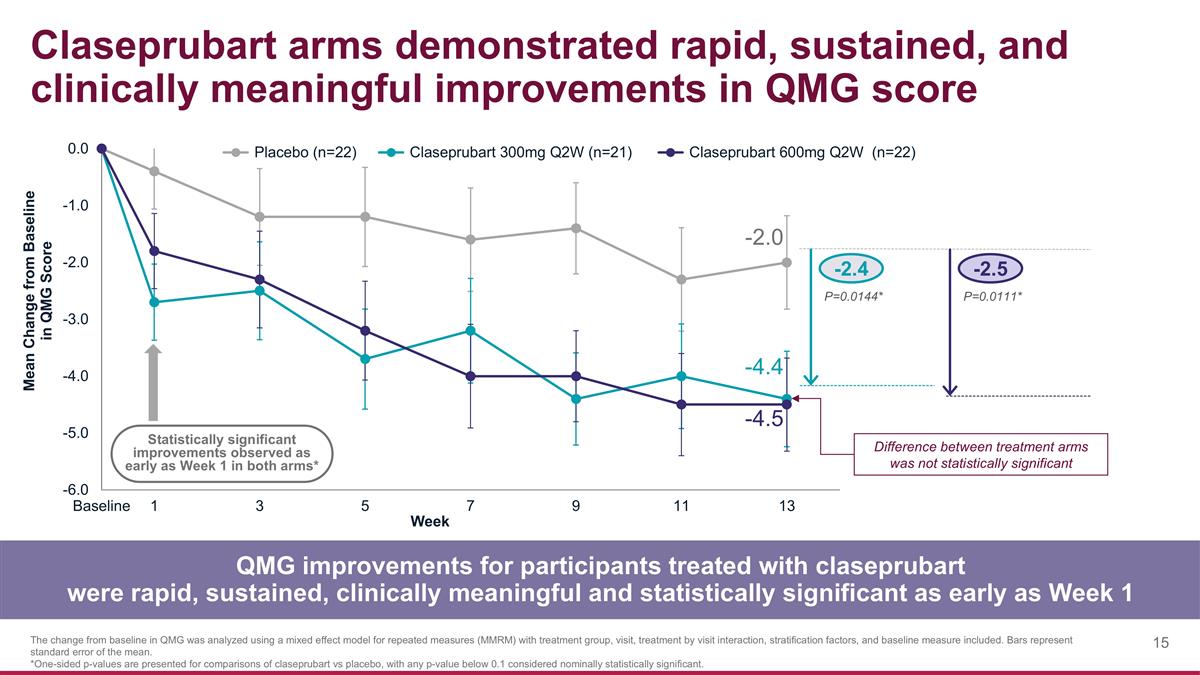
Claseprubart arms demonstrated rapid, sustained, and clinically meaningful improvements in QMG score QMG improvements for participants treated with claseprubart were rapid, sustained, clinically meaningful and statistically significant as early as Week 1 The change from baseline in QMG was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. Bars represent standard error of the mean. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. -2.4 -2.5 P=0.0144* P=0.0111* -2.0 -4.4 -4.5 Statistically significant improvements observed as early as Week 1 in both arms* Difference between treatment arms was not statistically significant
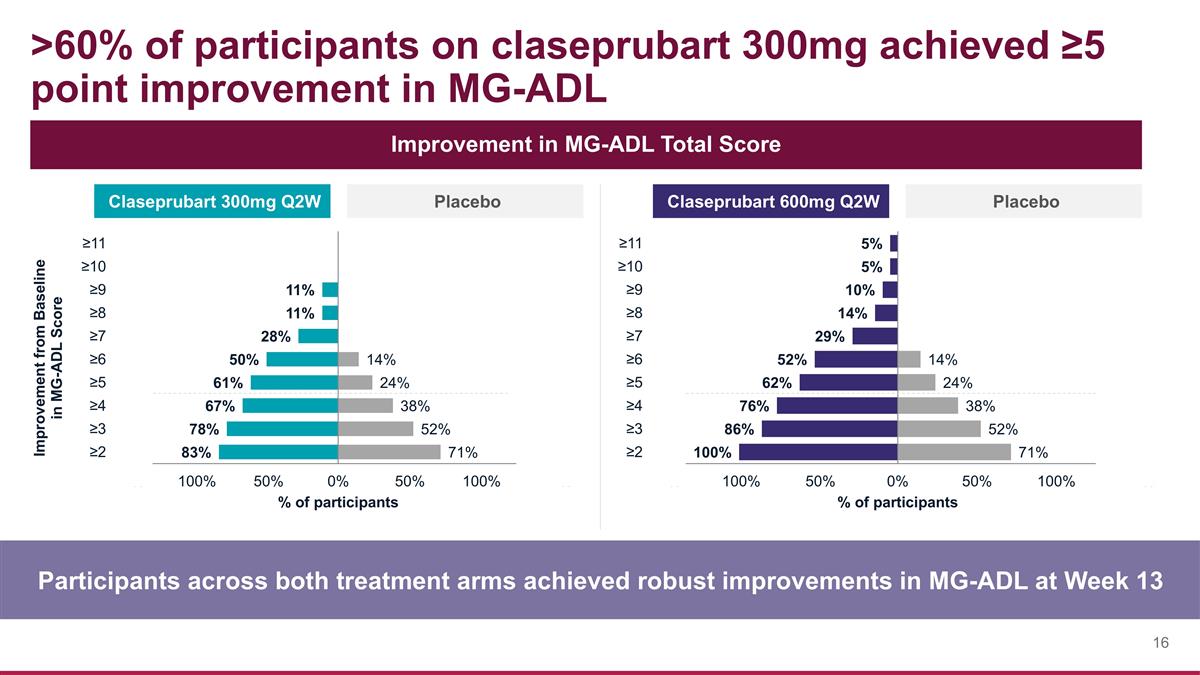
>60% of participants on claseprubart 300mg achieved ≥5 point improvement in MG-ADL Participants across both treatment arms achieved robust improvements in MG-ADL at Week 13 Improvement in MG-ADL Total Score Claseprubart 300mg Q2W Placebo Claseprubart 600mg Q2W Placebo
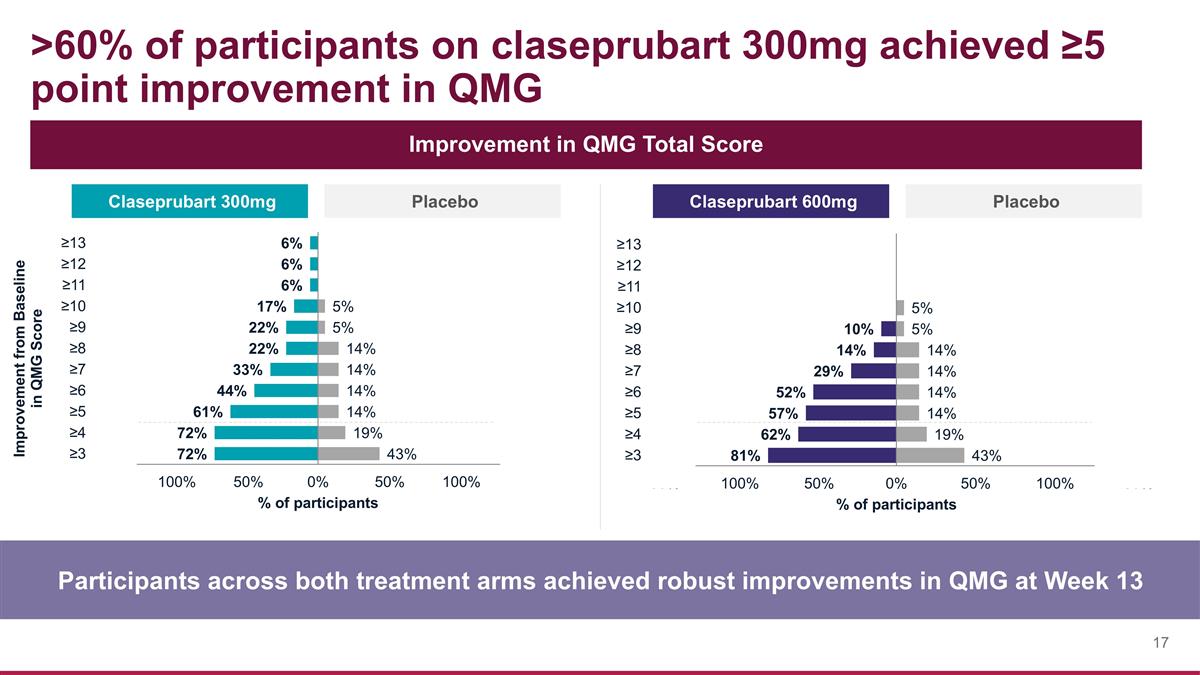
>60% of participants on claseprubart 300mg achieved ≥5 point improvement in QMG Participants across both treatment arms achieved robust improvements in QMG at Week 13 Improvement in QMG Total Score Claseprubart 300mg Placebo Claseprubart 600mg Placebo
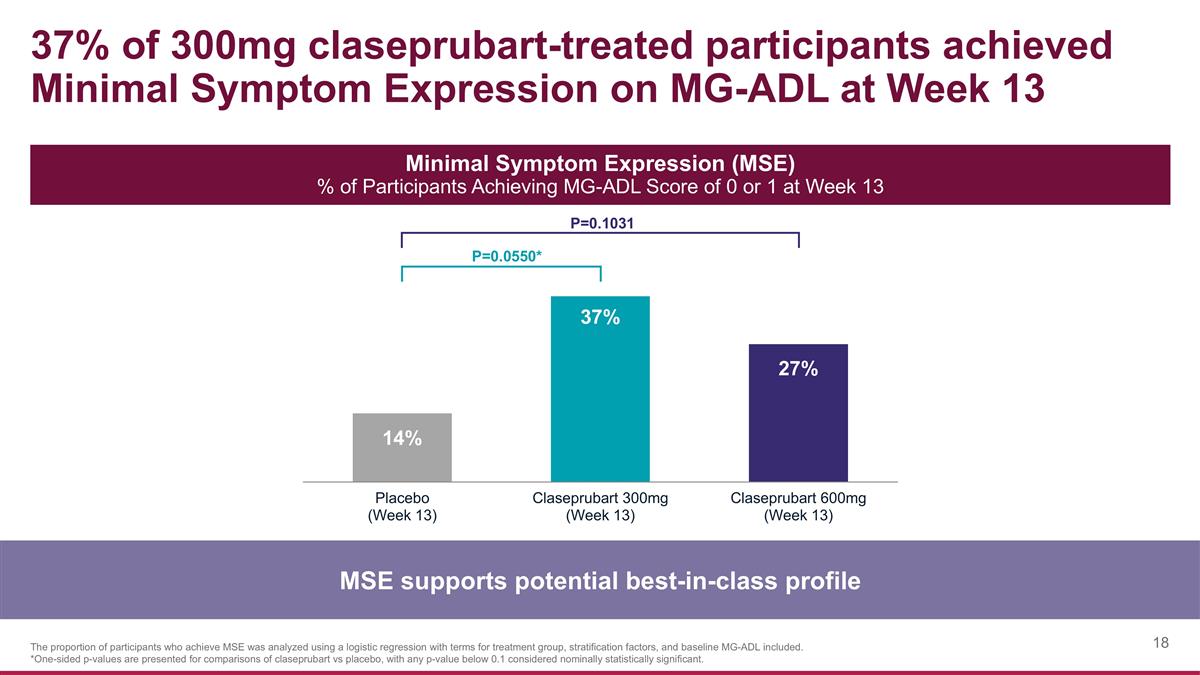
37% of 300mg claseprubart-treated participants achieved Minimal Symptom Expression on MG-ADL at Week 13 MSE supports potential best-in-class profile Minimal Symptom Expression (MSE) % of Participants Achieving MG-ADL Score of 0 or 1 at Week 13 P=0.0550* P=0.1031 The proportion of participants who achieve MSE was analyzed using a logistic regression with terms for treatment group, stratification factors, and baseline MG-ADL included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant.
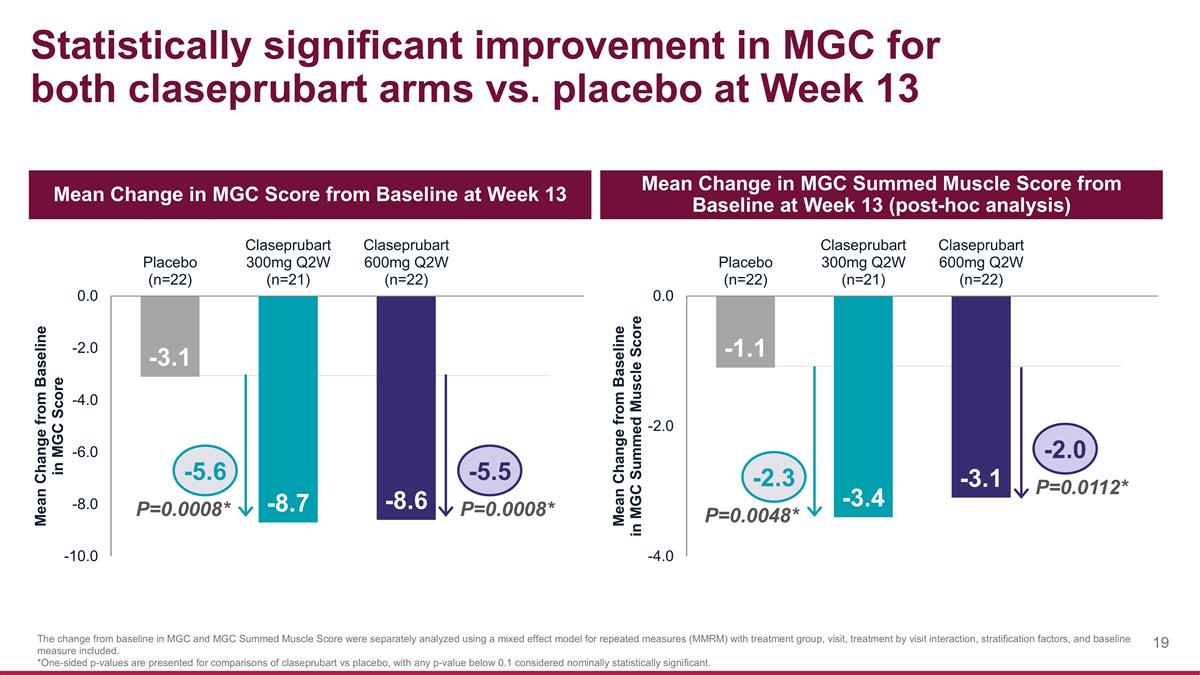
Statistically significant improvement in MGC for both claseprubart arms vs. placebo at Week 13 Mean Change in MGC Score from Baseline at Week 13 Mean Change in MGC Summed Muscle Score from Baseline at Week 13 (post-hoc analysis) The change from baseline in MGC and MGC Summed Muscle Score were separately analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. P=0.0008* -5.5 -5.6 P=0.0008* P=0.0112* -2.0 -2.3 P=0.0048*
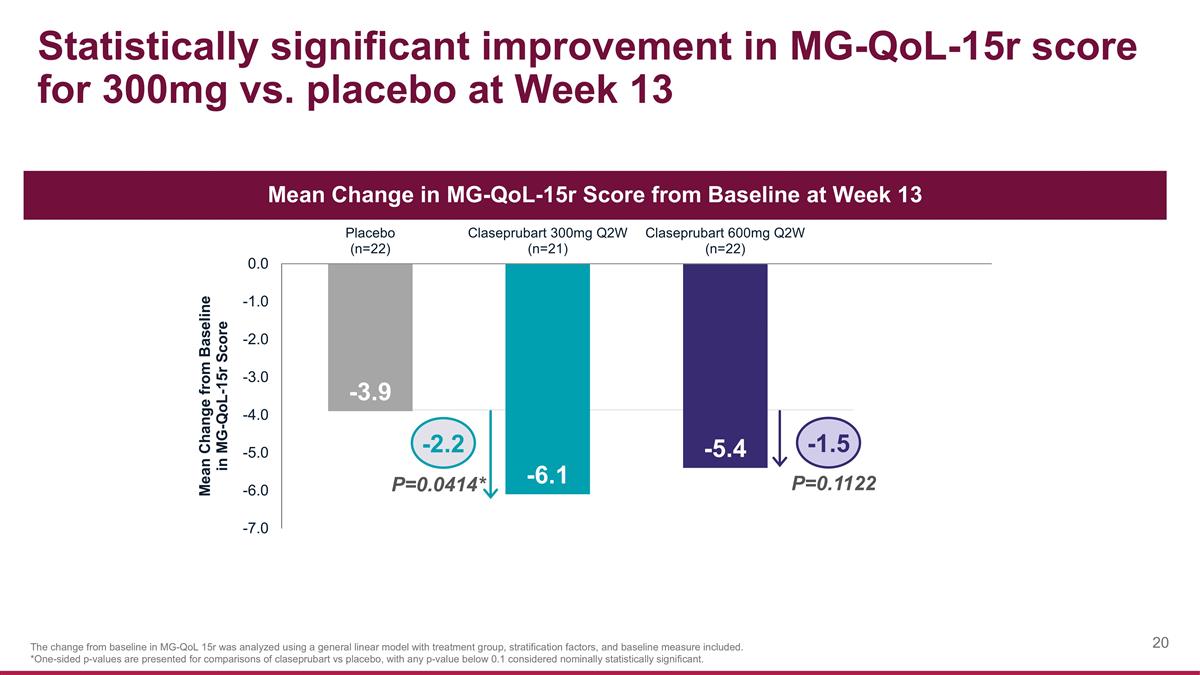
Statistically significant improvement in MG-QoL-15r score for 300mg vs. placebo at Week 13 The change from baseline in MG-QoL 15r was analyzed using a general linear model with treatment group, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. P=0.0414* P=0.1122 -2.2 -1.5 Mean Change in MG-QoL-15r Score from Baseline at Week 13
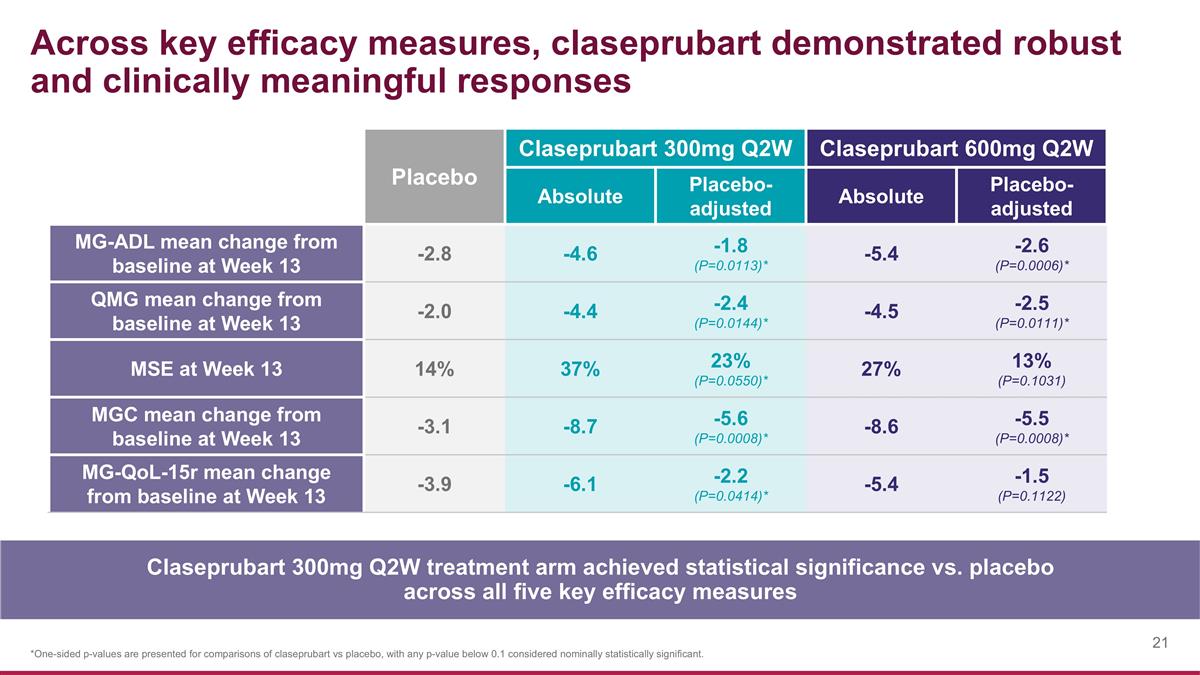
Across key efficacy measures, claseprubart demonstrated robust and clinically meaningful responses Placebo Claseprubart 300mg Q2W Claseprubart 600mg Q2W Absolute Placebo-adjusted Absolute Placebo-adjusted MG-ADL mean change from baseline at Week 13 -2.8 -4.6 -1.8 (P=0.0113)* -5.4 -2.6 (P=0.0006)* QMG mean change from baseline at Week 13 -2.0 -4.4 -2.4 (P=0.0144)* -4.5 -2.5 (P=0.0111)* MSE at Week 13 14% 37% 23% (P=0.0550)* 27% 13% (P=0.1031) MGC mean change from baseline at Week 13 -3.1 -8.7 -5.6 (P=0.0008)* -8.6 -5.5 (P=0.0008)* MG-QoL-15r mean change from baseline at Week 13 -3.9 -6.1 -2.2 (P=0.0414)* -5.4 -1.5 (P=0.1122) *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. Claseprubart 300mg Q2W treatment arm achieved statistical significance vs. placebo across all five key efficacy measures
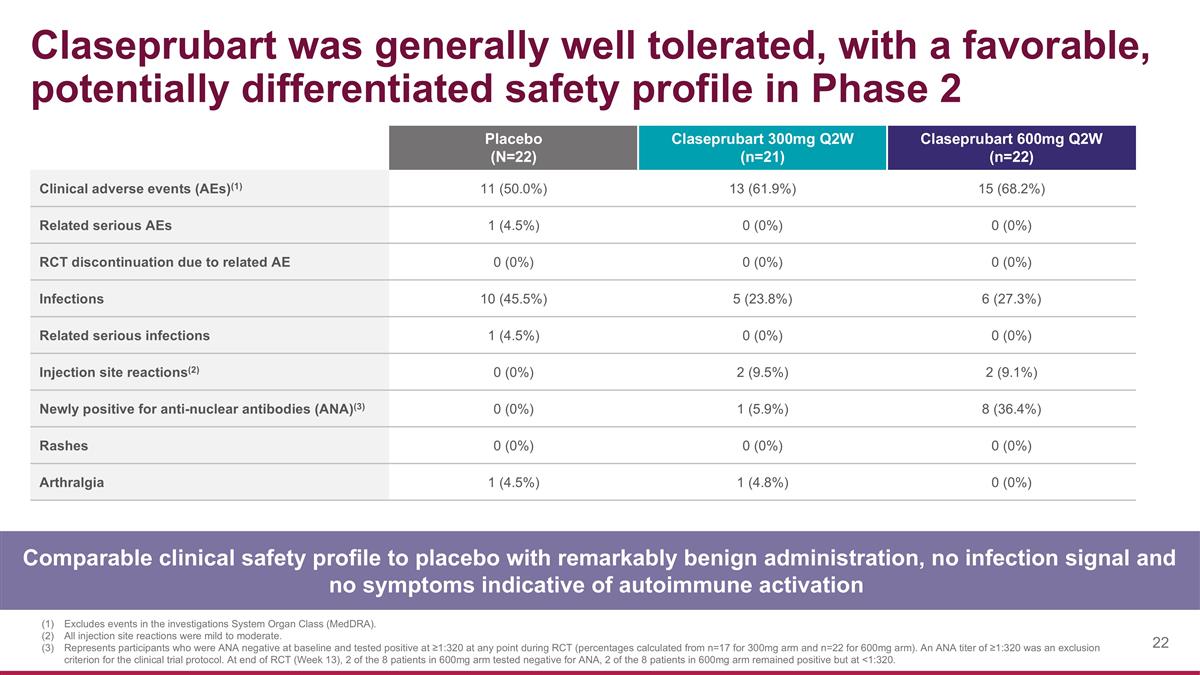
Claseprubart was generally well tolerated, with a favorable, potentially differentiated safety profile in Phase 2 Comparable clinical safety profile to placebo with remarkably benign administration, no infection signal and no symptoms indicative of autoimmune activation Placebo (N=22) Claseprubart 300mg Q2W (n=21) Claseprubart 600mg Q2W (n=22) Clinical adverse events (AEs)(1) 11 (50.0%) 13 (61.9%) 15 (68.2%) Related serious AEs 1 (4.5%) 0 (0%) 0 (0%) RCT discontinuation due to related AE 0 (0%) 0 (0%) 0 (0%) Infections 10 (45.5%) 5 (23.8%) 6 (27.3%) Related serious infections 1 (4.5%) 0 (0%) 0 (0%) Injection site reactions(2) 0 (0%) 2 (9.5%) 2 (9.1%) Newly positive for anti-nuclear antibodies (ANA)(3) 0 (0%) 1 (5.9%) 8 (36.4%) Rashes 0 (0%) 0 (0%) 0 (0%) Arthralgia 1 (4.5%) 1 (4.8%) 0 (0%) Excludes events in the investigations System Organ Class (MedDRA). All injection site reactions were mild to moderate. Represents participants who were ANA negative at baseline and tested positive at ≥1:320 at any point during RCT (percentages calculated from n=17 for 300mg arm and n=22 for 600mg arm). An ANA titer of ≥1:320 was an exclusion criterion for the clinical trial protocol. At end of RCT (Week 13), 2 of the 8 patients in 600mg arm tested negative for ANA, 2 of the 8 patients in 600mg arm remained positive but at <1:320.
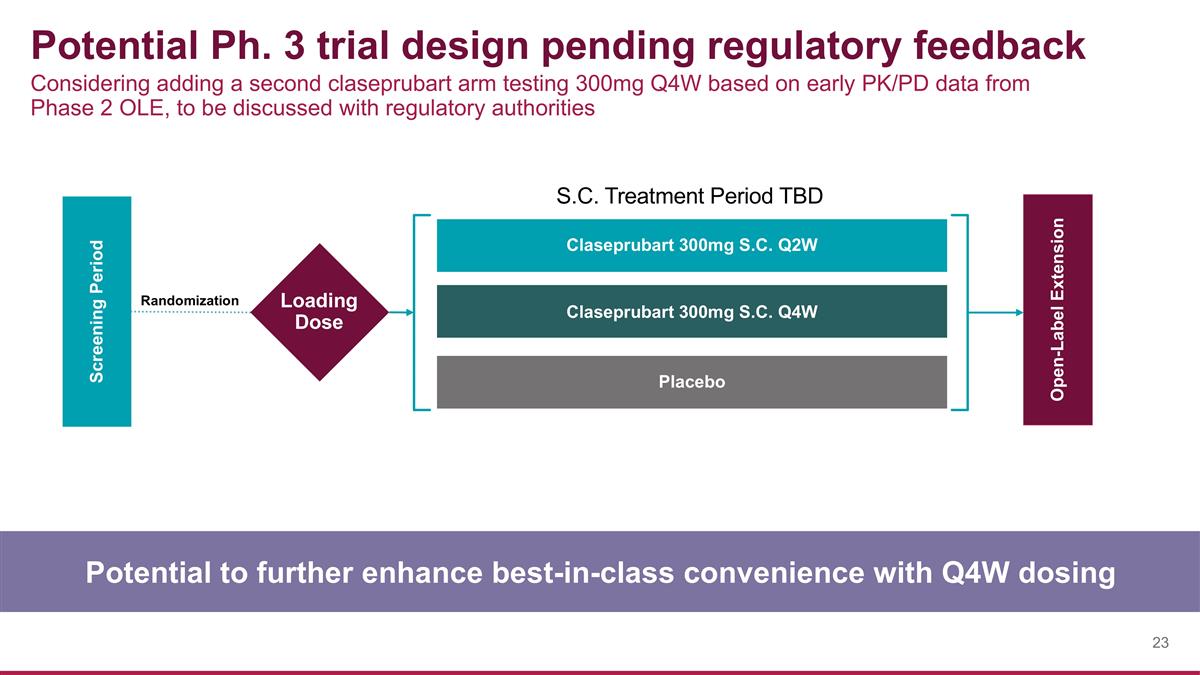
Potential Ph. 3 trial design pending regulatory feedback Potential to further enhance best-in-class convenience with Q4W dosing Screening Period Open-Label Extension Claseprubart 300mg S.C. Q2W Claseprubart 300mg S.C. Q4W Placebo S.C. Treatment Period TBD Loading Dose Randomization Considering adding a second claseprubart arm testing 300mg Q4W based on early PK/PD data from Phase 2 OLE, to be discussed with regulatory authorities

Claseprubart Summary Remarks Marino Garcia, CEO
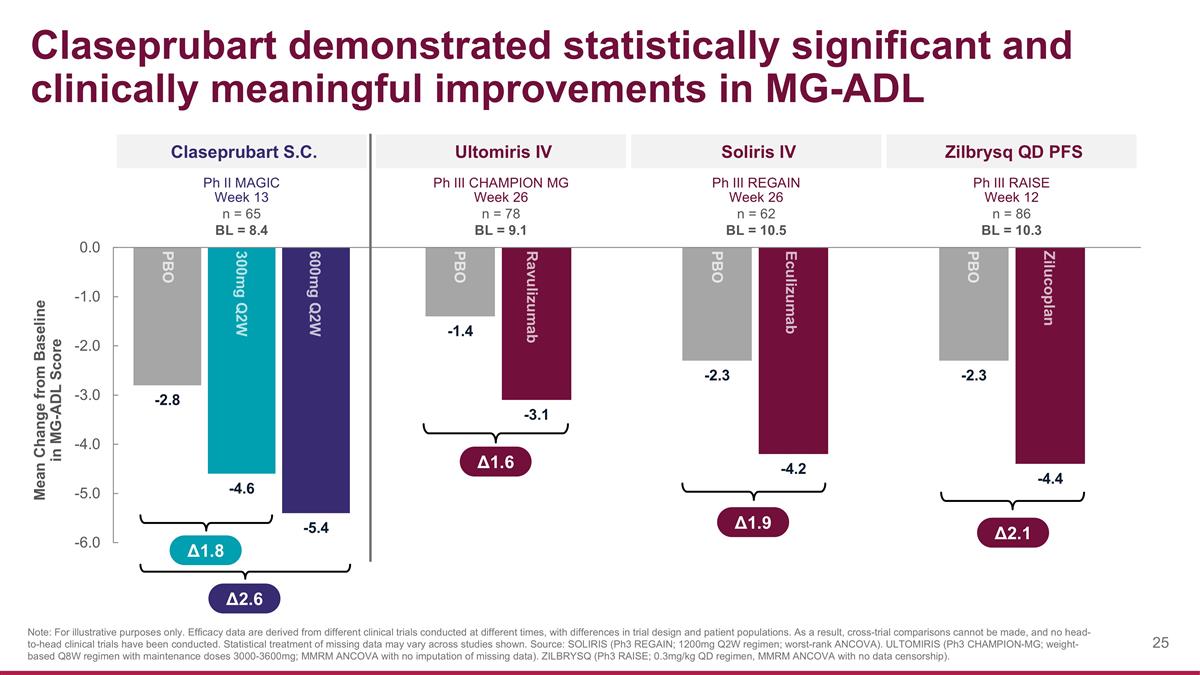
Claseprubart demonstrated statistically significant and clinically meaningful improvements in MG-ADL Claseprubart S.C. Zilbrysq QD PFS Δ1.8 Δ2.6 Note: For illustrative purposes only. Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Statistical treatment of missing data may vary across studies shown. Source: SOLIRIS (Ph3 REGAIN; 1200mg Q2W regimen; worst-rank ANCOVA). ULTOMIRIS (Ph3 CHAMPION-MG; weight-based Q8W regimen with maintenance doses 3000-3600mg; MMRM ANCOVA with no imputation of missing data). ZILBRYSQ (Ph3 RAISE; 0.3mg/kg QD regimen, MMRM ANCOVA with no data censorship). PBO 300mg Q2W 600mg Q2W Ph II MAGIC Week 13 n = 65 BL = 8.4 Mean Change from Baseline in MG-ADL Score Soliris IV Ph III REGAIN Week 26 n = 62 BL = 10.5 Ultomiris IV Ph III CHAMPION MG Week 26 n = 78 BL = 9.1 Ph III RAISE Week 12 n = 86 BL = 10.3 Eculizumab PBO Ravulizumab PBO Zilucoplan PBO Δ1.9 Δ1.6 Δ2.1
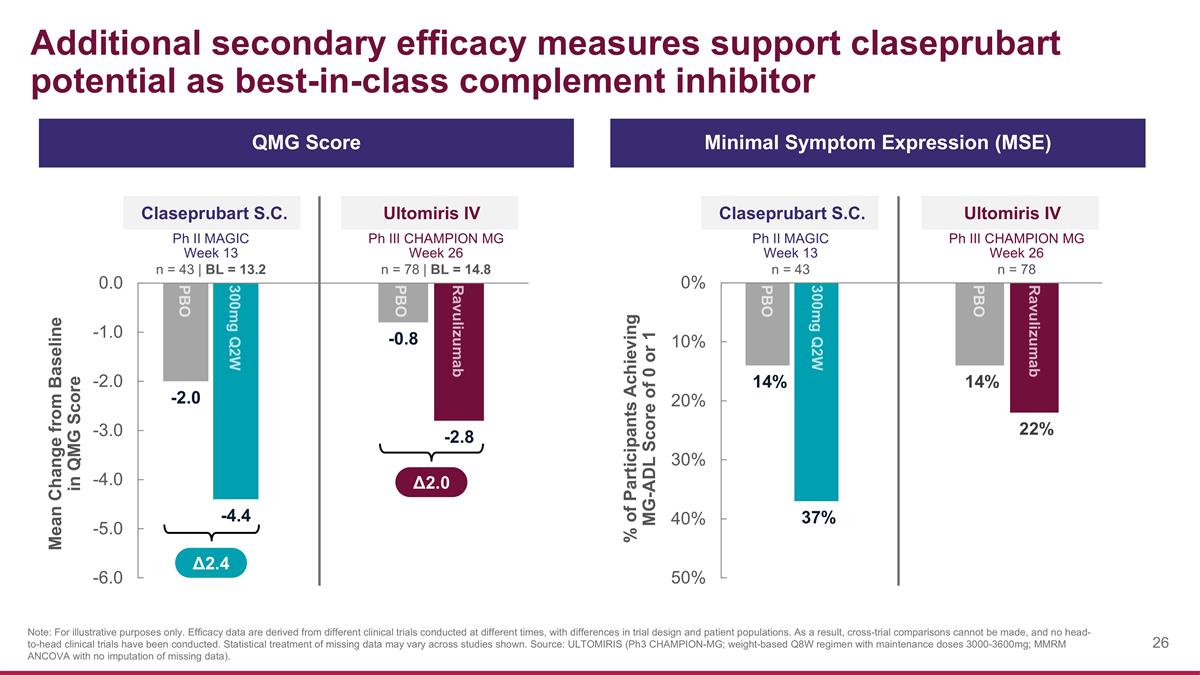
Additional secondary efficacy measures support claseprubart potential as best-in-class complement inhibitor Claseprubart S.C. Ph II MAGIC Week 13 n = 43 | BL = 13.2 Mean Change from Baseline in QMG Score Ultomiris IV Ph III CHAMPION MG Week 26 n = 78 | BL = 14.8 % of Participants Achieving MG-ADL Score of 0 or 1 Claseprubart S.C. Ph II MAGIC Week 13 n = 43 Ultomiris IV Ph III CHAMPION MG Week 26 n = 78 QMG Score Minimal Symptom Expression (MSE) Δ2.4 Note: For illustrative purposes only. Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Statistical treatment of missing data may vary across studies shown. Source: ULTOMIRIS (Ph3 CHAMPION-MG; weight-based Q8W regimen with maintenance doses 3000-3600mg; MMRM ANCOVA with no imputation of missing data). Δ2.0 PBO 300mg Q2W PBO Ravulizumab Ravulizumab PBO PBO 300mg Q2W
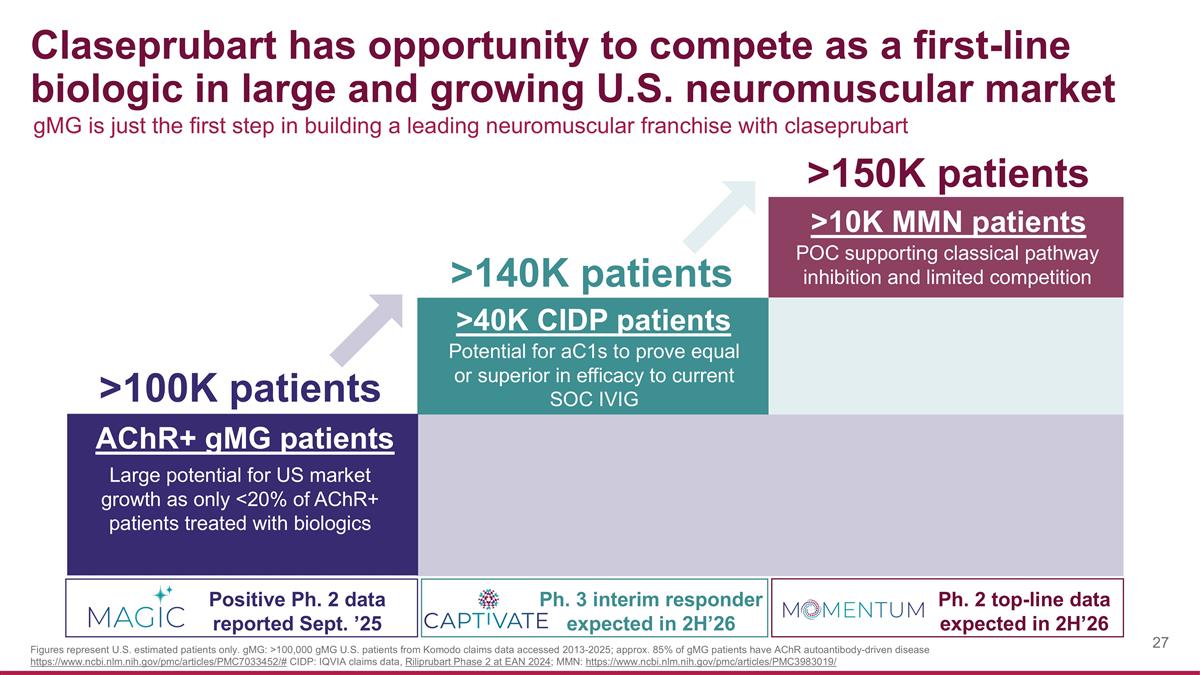
Claseprubart has opportunity to compete as a first-line biologic in large and growing U.S. neuromuscular market >100K patients >140K patients >150K patients AChR+ gMG patients Positive Ph. 2 data reported Sept. ’25 >40K CIDP patients Ph. 3 interim responder expected in 2H’26 Ph. 2 top-line data expected in 2H’26 Large potential for US market growth as only <20% of AChR+ patients treated with biologics >10K MMN patients Potential for aC1s to prove equal or superior in efficacy to current SOC IVIG POC supporting classical pathway inhibition and limited competition gMG is just the first step in building a leading neuromuscular franchise with claseprubart Figures represent U.S. estimated patients only. gMG: >100,000 gMG U.S. patients from Komodo claims data accessed 2013-2025; approx. 85% of gMG patients have AChR autoantibody-driven disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/# CIDP: IQVIA claims data, Riliprubart Phase 2 at EAN 2024; MMN: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983019/
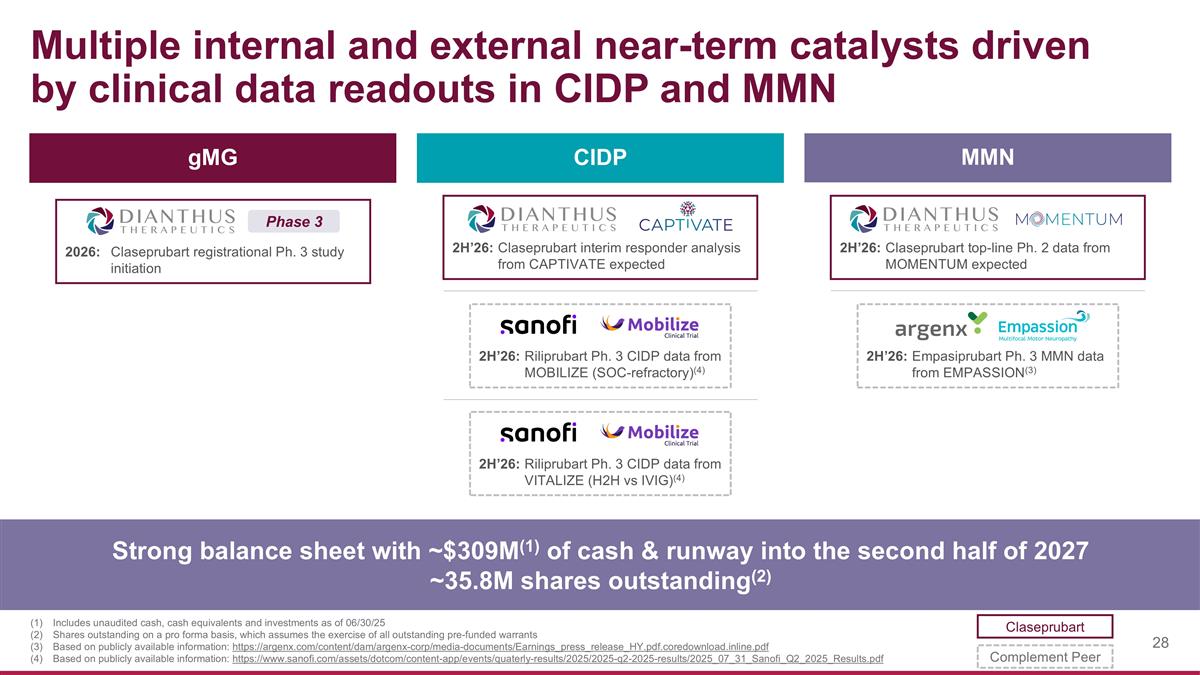
Multiple internal and external near-term catalysts driven by clinical data readouts in CIDP and MMN Strong balance sheet with ~$309M(1) of cash & runway into the second half of 2027 ~35.8M shares outstanding(2) Includes unaudited cash, cash equivalents and investments as of 06/30/25 Shares outstanding on a pro forma basis, which assumes the exercise of all outstanding pre-funded warrants Based on publicly available information: https://argenx.com/content/dam/argenx-corp/media-documents/Earnings_press_release_HY.pdf.coredownload.inline.pdf Based on publicly available information: https://www.sanofi.com/assets/dotcom/content-app/events/quaterly-results/2025/2025-q2-2025-results/2025_07_31_Sanofi_Q2_2025_Results.pdf MMN gMG CIDP 2026:Claseprubart registrational Ph. 3 study initiation Phase 3 2H’26:Claseprubart interim responder analysis from CAPTIVATE expected 2H’26:Riliprubart Ph. 3 CIDP data from MOBILIZE (SOC-refractory)(4) 2H’26:Claseprubart top-line Ph. 2 data from MOMENTUM expected 2H’26:Riliprubart Ph. 3 CIDP data from VITALIZE (H2H vs IVIG)(4) 2H’26:Empasiprubart Ph. 3 MMN data from EMPASSION(3) Claseprubart Complement Peer
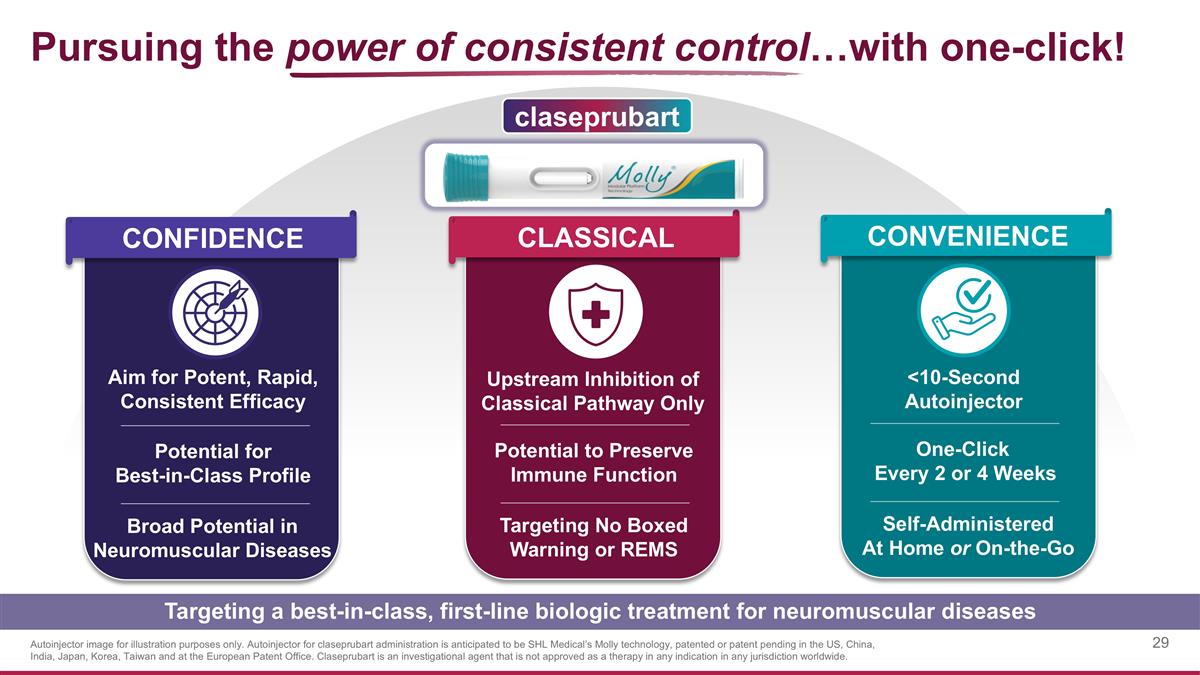
Pursuing the power of consistent control…with one-click! Targeting a best-in-class, first-line biologic treatment for neuromuscular diseases CLASSICAL CONVENIENCE Upstream Inhibition of Classical Pathway Only <10-Second Autoinjector Targeting No Boxed Warning or REMS Self-Administered At Home or On-the-Go Potential to Preserve Immune Function One-Click Every 2 or 4 Weeks claseprubart CONFIDENCE Aim for Potent, Rapid, Consistent Efficacy Broad Potential in Neuromuscular Diseases Potential for Best-in-Class Profile Autoinjector image for illustration purposes only. Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office. Claseprubart is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide.
Q&A Top-line Ph. 2 MaGic Results in Generalized Myasthenia Gravis September 8, 2025

Appendix
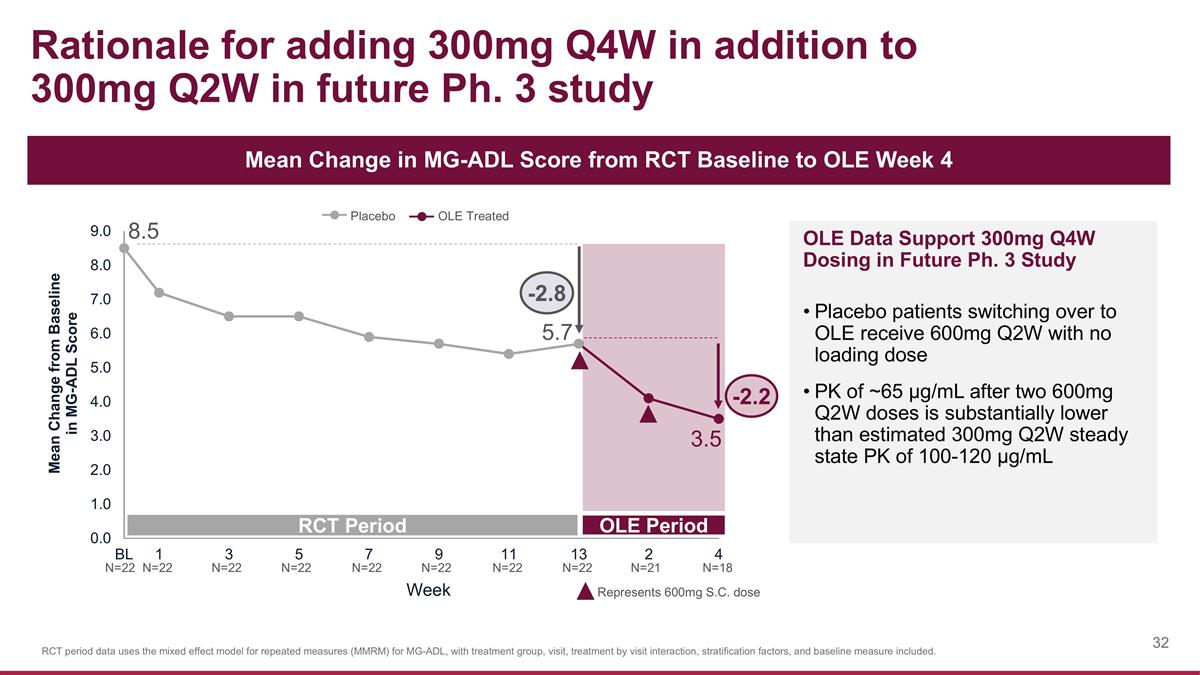
Rationale for adding 300mg Q4W in addition to 300mg Q2W in future Ph. 3 study Mean Change in MG-ADL Score from RCT Baseline to OLE Week 4 8.5 RCT Period OLE Period Placebo OLE Treated Week OLE Data Support 300mg Q4W Dosing in Future Ph. 3 Study Placebo patients switching over to OLE receive 600mg Q2W with no loading dose PK of ~65 µg/mL after two 600mg Q2W doses is substantially lower than estimated 300mg Q2W steady state PK of 100-120 µg/mL N=22 Represents 600mg S.C. dose N=22 N=22 N=22 N=22 N=22 N=22 N=22 N=18 N=21 RCT period data uses the mixed effect model for repeated measures (MMRM) for MG-ADL, with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. 5.7 -2.8 -2.2 3.5