
Dianthus Therapeutics Licensing of DNTH212 (BDCA2 and BAFF/APRIL) from Leads Biolabs October 16, 2025

Forward-looking statements Certain statements in this presentation, other than purely historical information, may constitute “forward-looking statements” within the meaning of the federal securities laws, including for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995, express or implied statements regarding future plans and prospects, including statements regarding the expectations or plans for discovery, preclinical studies, clinical trials and research and development programs, in particular with respect to claseprubart and DNTH212, and any developments or results in connection therewith, including the target product profile and administration of claseprubart and DNTH212; the anticipated timing of the initiation and results from those studies and trials; expectations regarding the clinical trial designs or indications; expectations regarding the time period over which the Company’s capital resources are expected to be sufficient to fund its anticipated operations; and expectations regarding market size, patient population size, and potential opportunities for complement therapies, in particular with respect to claseprubart and DNTH212. Claseprubart and DNTH212 are investigational agents that are not approved as therapies in any indication in any jurisdiction worldwide. The words “opportunity,” “potential,” “milestones,” “runway,” “will,” “anticipate,” “achieve,” “near-term,” “catalysts,” “pursue,” “pipeline,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “predict,” “project,” “should,” “strive,” “would,” “aim,” “target,” “commit,” and similar expressions (including the negatives of these terms or variations of them) generally identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Actual results could differ materially from those included in the forward-looking statements due to various factors, risks and uncertainties, including, but not limited to, that preclinical testing of claseprubart and DNTH212 and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that the development of claseprubart or DNTH212 may take longer and/or cost more than planned, that the Company or its partner may be unable to successfully complete the clinical development of the Company’s compounds, that the Company or its partner may be delayed in initiating, enrolling or completing its planned clinical trials, and that the Company's compounds may not receive regulatory approval or become commercially successful products. These and other risks and uncertainties are identified under the heading "Risk Factors" included in the Company’s Annual Report on Form 10-K for the period ended December 31, 2024, and other filings that the Company has made and may make with the SEC in the future. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. Dianthus undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.

Introduction Marino Garcia, Chief Executive Officer DNTH212 Opportunity Overview Simrat Randhawa, MD, Head of Research & Development Deal Terms & Strategic Perspectives Ryan Savitz, Chief Financial Officer & Chief Business Officer Marino Garcia, Chief Executive Officer Q&A Marino Garcia, Chief Executive Officer Simrat Randhawa, MD, Head of Research & Development Ryan Savitz, Chief Financial Officer & Chief Business Officer Agenda

DNTH212 (BDCA2 and BAFF/APRIL inhibitor) expands leadership in next-generation therapeutics Entered into an exclusive license agreement with Leads Biolabs for global rights (ex-Greater China1) for DNTH212, a first and potentially best-in-class, Ph. 1 ready bifunctional BDCA2 and BAFF/APRIL inhibitor IND cleared by FDA with Ph. 1 SAD study in healthy volunteers and patients with SLE expected to initiate in Q4’25 in China with top-line HV results expected in 2H’26 Pro forma estimated cash of ~$525M2 maintains runway into 2028, expected to fund multiple catalysts Builds on favorable safety and clinical PoC for BDCA2 and BAFF/APRIL targeted biologics, with potential for enhanced efficacy from complementary mechanisms targeting innate and adaptive immune systems Continues Dianthus’ strategy of pursuing clinically validated MoAs with next-generation best-in-class therapeutics and a pipeline-in-a-product potential DNTH212 demonstrated superior in vitro inhibition of pDCs vs. litifilimab and superior Ig reductions in NHPs vs. povetacicept highlighting potential for improved clinical benefit in severe autoimmune diseases Note: DNTH212 is being developed in China by Leads Biolabs as LBL-047 1. Greater China includes Mainland China, Hong Kong, Macau, and Taiwan Targeting patient-friendly convenience with S.C. self-administration and Q4W or less frequent dosing 2. Estimated pro forma cash includes preliminary and unaudited cash, cash equivalents and investments as of September 30, 2025 of approximately $555 million less $30 million of upfront and near-term milestone payments

DNTH212 Opportunity Overview
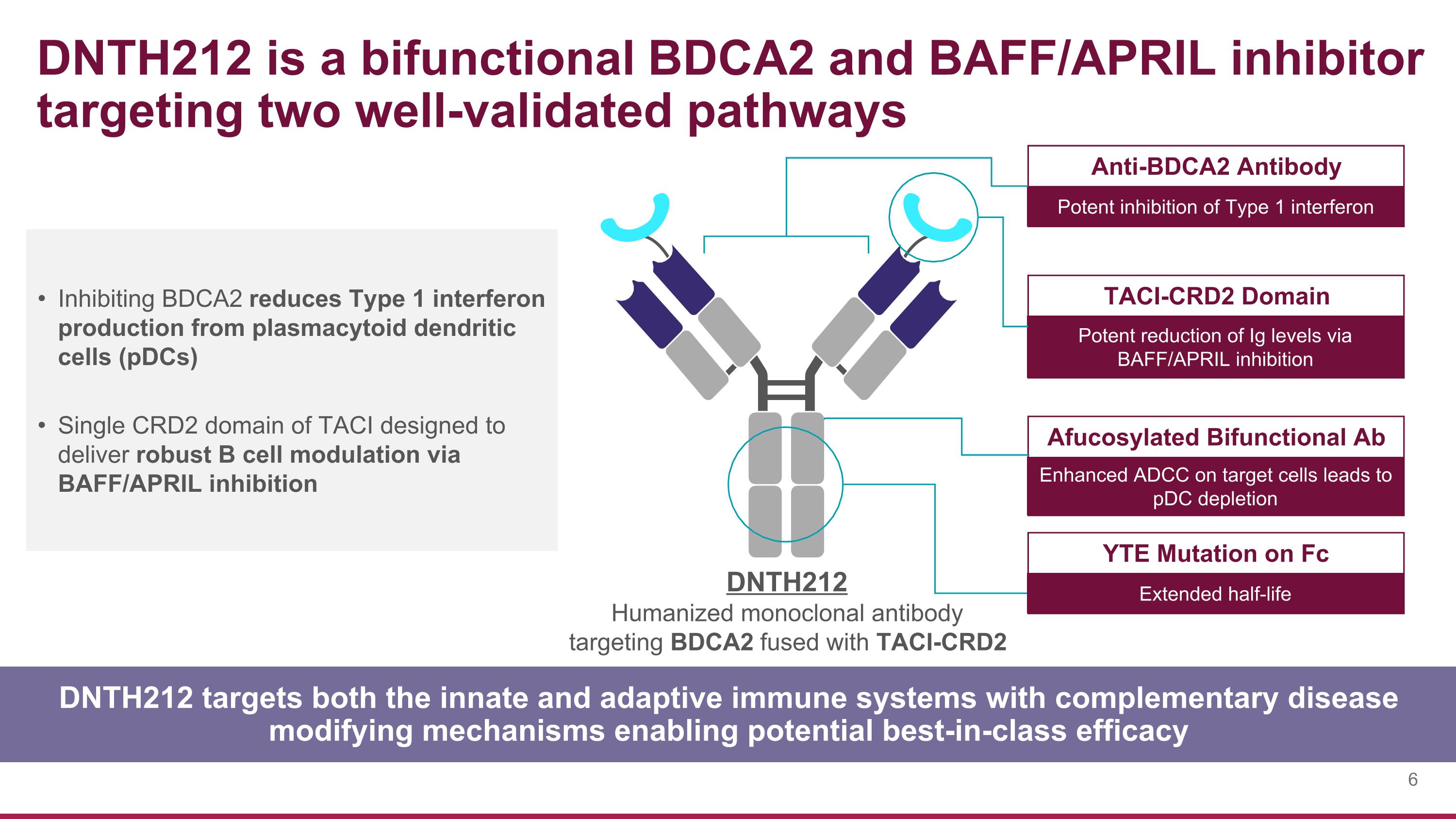
Inhibiting BDCA2 reduces Type 1 interferon production from plasmacytoid dendritic cells (pDCs) Single CRD2 domain of TACI designed to deliver robust B cell modulation via BAFF/APRIL inhibition DNTH212 targets both the innate and adaptive immune systems with complementary disease modifying mechanisms enabling potential best-in-class efficacy DNTH212 is a bifunctional BDCA2 and BAFF/APRIL inhibitor targeting two well-validated pathways Anti-BDCA2 Antibody Potent inhibition of Type 1 interferon TACI-CRD2 Domain Potent reduction of Ig levels via BAFF/APRIL inhibition Afucosylated Bifunctional Ab Enhanced ADCC on target cells leads to pDC depletion YTE Mutation on Fc Extended half-life DNTH212 Humanized monoclonal antibody targeting BDCA2 fused with TACI-CRD2
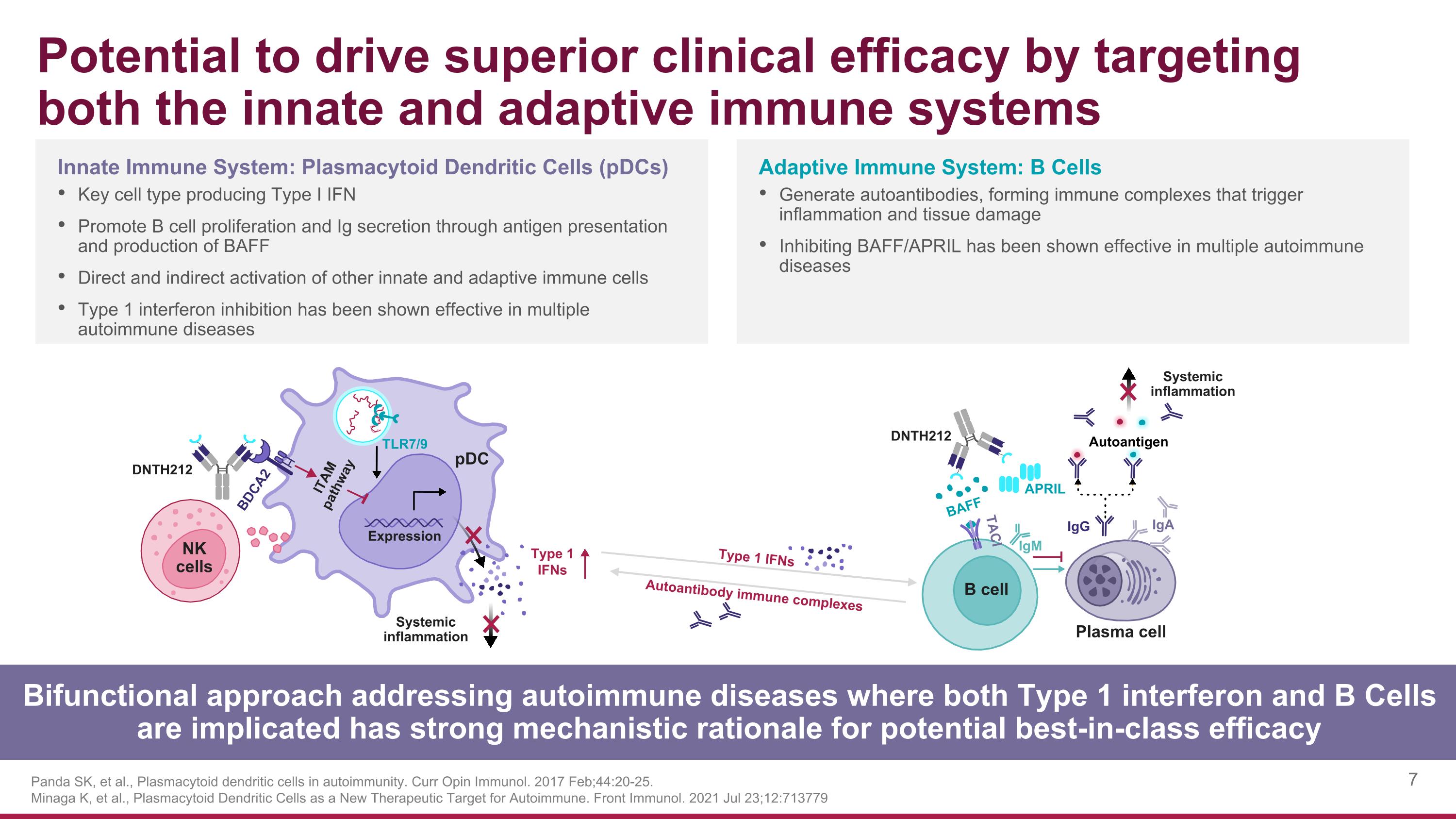
Potential to drive superior clinical efficacy by targeting both the innate and adaptive immune systems Panda SK, et al., Plasmacytoid dendritic cells in autoimmunity. Curr Opin Immunol. 2017 Feb;44:20-25. Minaga K, et al., Plasmacytoid Dendritic Cells as a New Therapeutic Target for Autoimmune. Front Immunol. 2021 Jul 23;12:713779 Bifunctional approach addressing autoimmune diseases where both Type 1 interferon and B Cells are implicated has strong mechanistic rationale for potential best-in-class efficacy Adaptive Immune System: B Cells Generate autoantibodies, forming immune complexes that trigger inflammation and tissue damage Inhibiting BAFF/APRIL has been shown effective in multiple autoimmune diseases Innate Immune System: Plasmacytoid Dendritic Cells (pDCs) Key cell type producing Type I IFN Promote B cell proliferation and Ig secretion through antigen presentation and production of BAFF Direct and indirect activation of other innate and adaptive immune cells Type 1 interferon inhibition has been shown effective in multiple autoimmune diseases DNTH212 Systemic inflammation DNTH212 Systemic inflammation Expression BDCA2 ITAM pathway pDC Type 1 IFNs BAFF TACI IgM APRIL IgG IgA B cell NK cells TLR7/9 Autoantigen Plasma cell Type 1 IFNs Autoantibody immune complexes
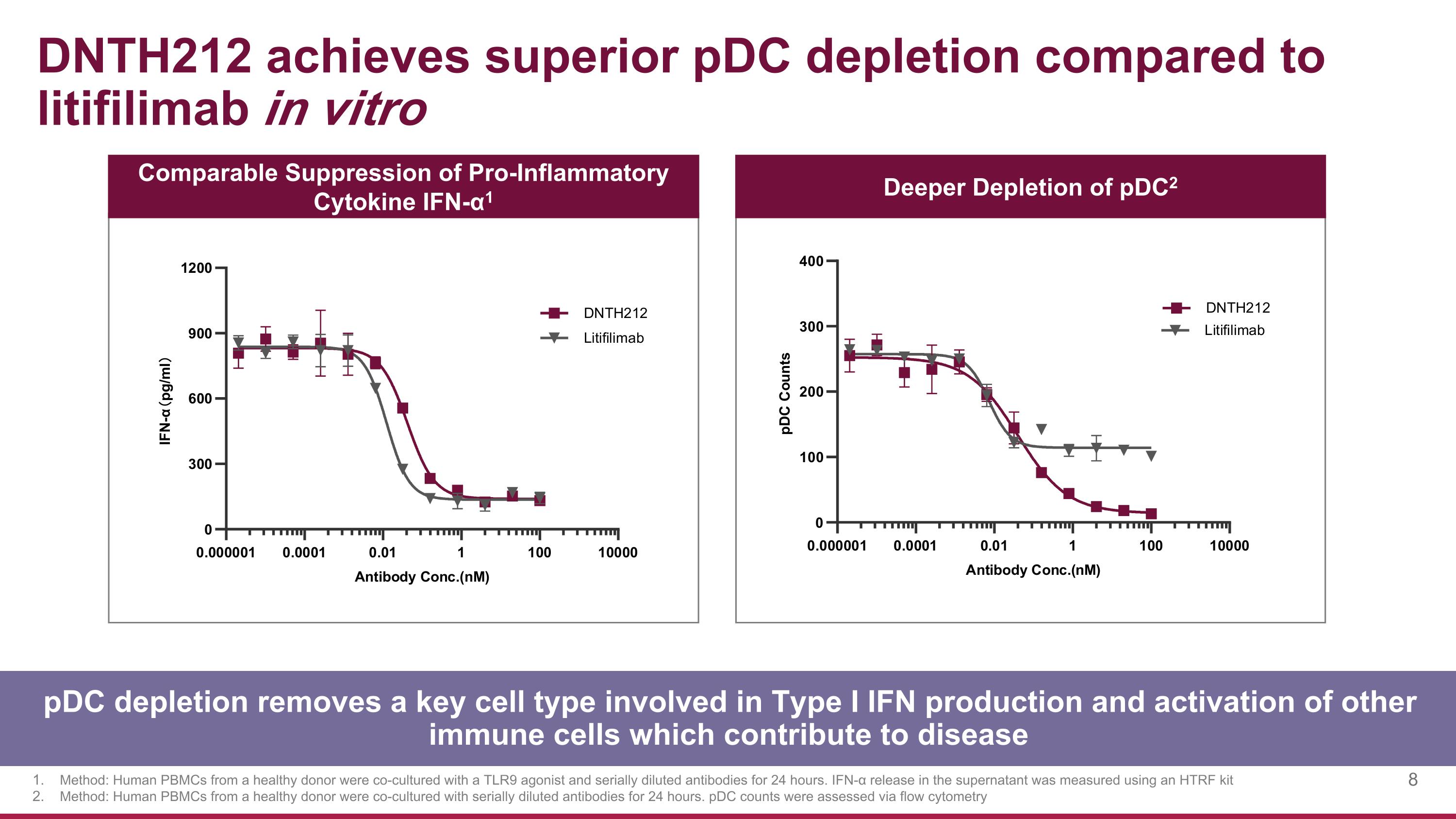
DNTH212 achieves superior pDC depletion compared to litifilimab in vitro Comparable Suppression of Pro-Inflammatory Cytokine IFN-α1 Deeper Depletion of pDC2 Method: Human PBMCs from a healthy donor were co-cultured with a TLR9 agonist and serially diluted antibodies for 24 hours. IFN-α release in the supernatant was measured using an HTRF kit Method: Human PBMCs from a healthy donor were co-cultured with serially diluted antibodies for 24 hours. pDC counts were assessed via flow cytometry pDC depletion removes a key cell type involved in Type I IFN production and activation of other immune cells which contribute to disease
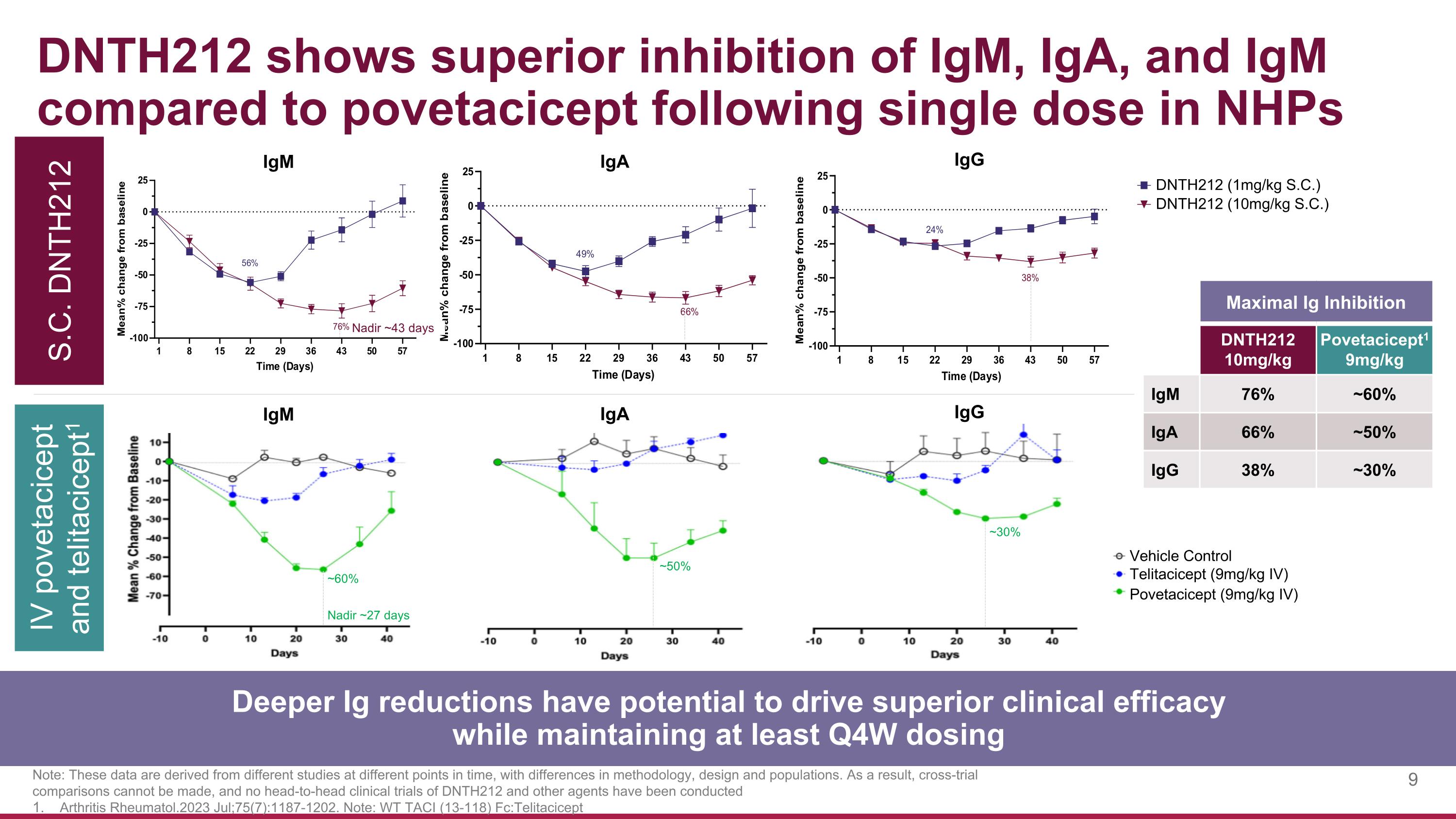
DNTH212 shows superior inhibition of IgM, IgA, and IgM compared to povetacicept following single dose in NHPs S.C. DNTH212 IV povetacicept and telitacicept1 Note: These data are derived from different studies at different points in time, with differences in methodology, design and populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials of DNTH212 and other agents have been conducted Arthritis Rheumatol.2023 Jul;75(7):1187-1202. Note: WT TACI (13-118) Fc:Telitacicept IgM IgA IgG Deeper Ig reductions have potential to drive superior clinical efficacy while maintaining at least Q4W dosing Maximal Ig Inhibition DNTH212 10mg/kg Povetacicept1 9mg/kg IgM 76% ~60% IgA 66% ~50% IgG 38% ~30% Telitacicept (9mg/kg IV) Vehicle Control Povetacicept (9mg/kg IV) Nadir ~27 days ~60% ~50% ~30% IgM IgA IgG DNTH212 (1mg/kg S.C.) DNTH212 (10mg/kg S.C.) Nadir ~43 days
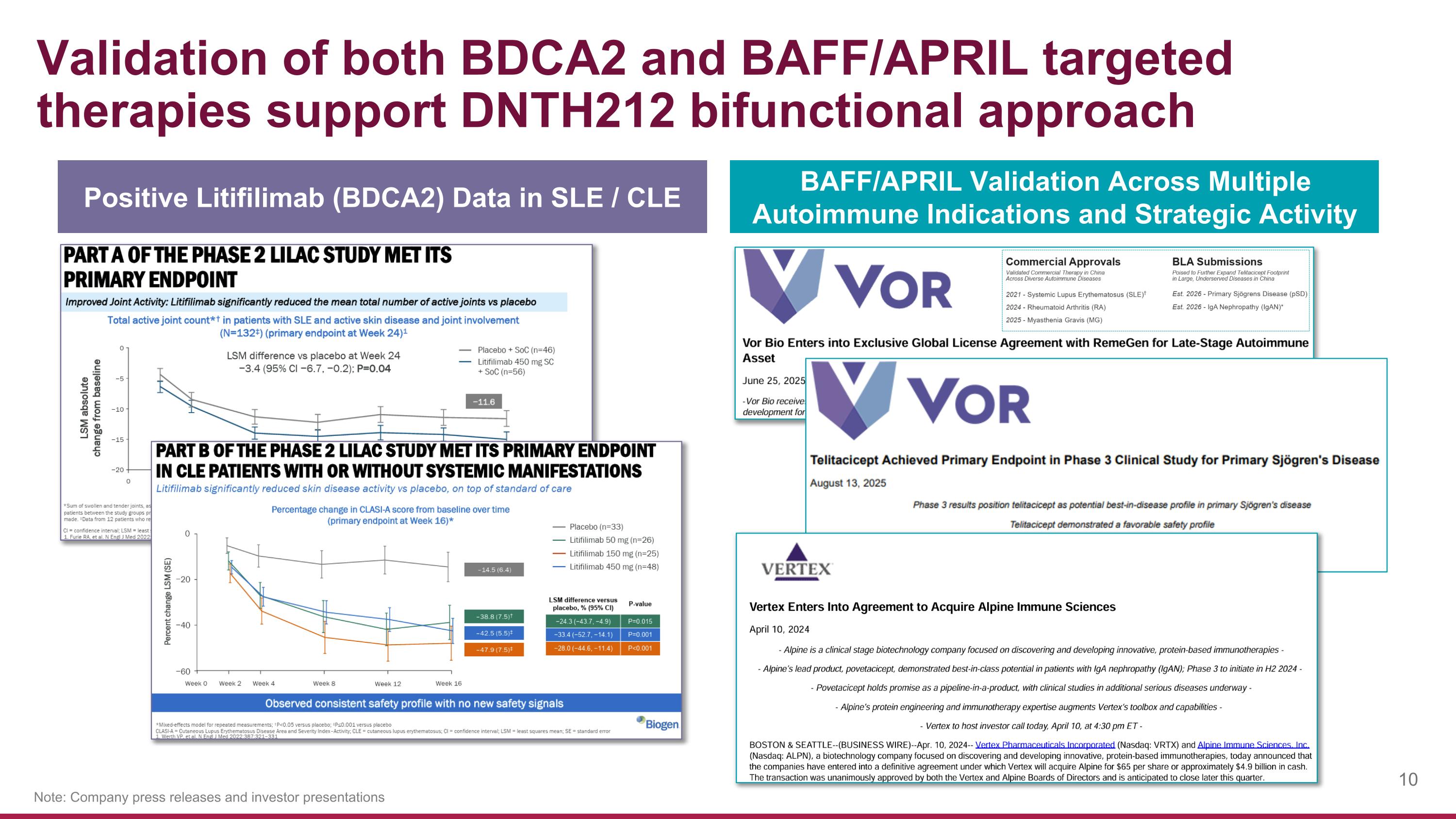
Validation of both BDCA2 and BAFF/APRIL targeted therapies support DNTH212 bifunctional approach Note: Company press releases and investor presentations Positive Litifilimab (BDCA2) Data in SLE / CLE BAFF/APRIL Validation Across Multiple Autoimmune Indications and Strategic Activity
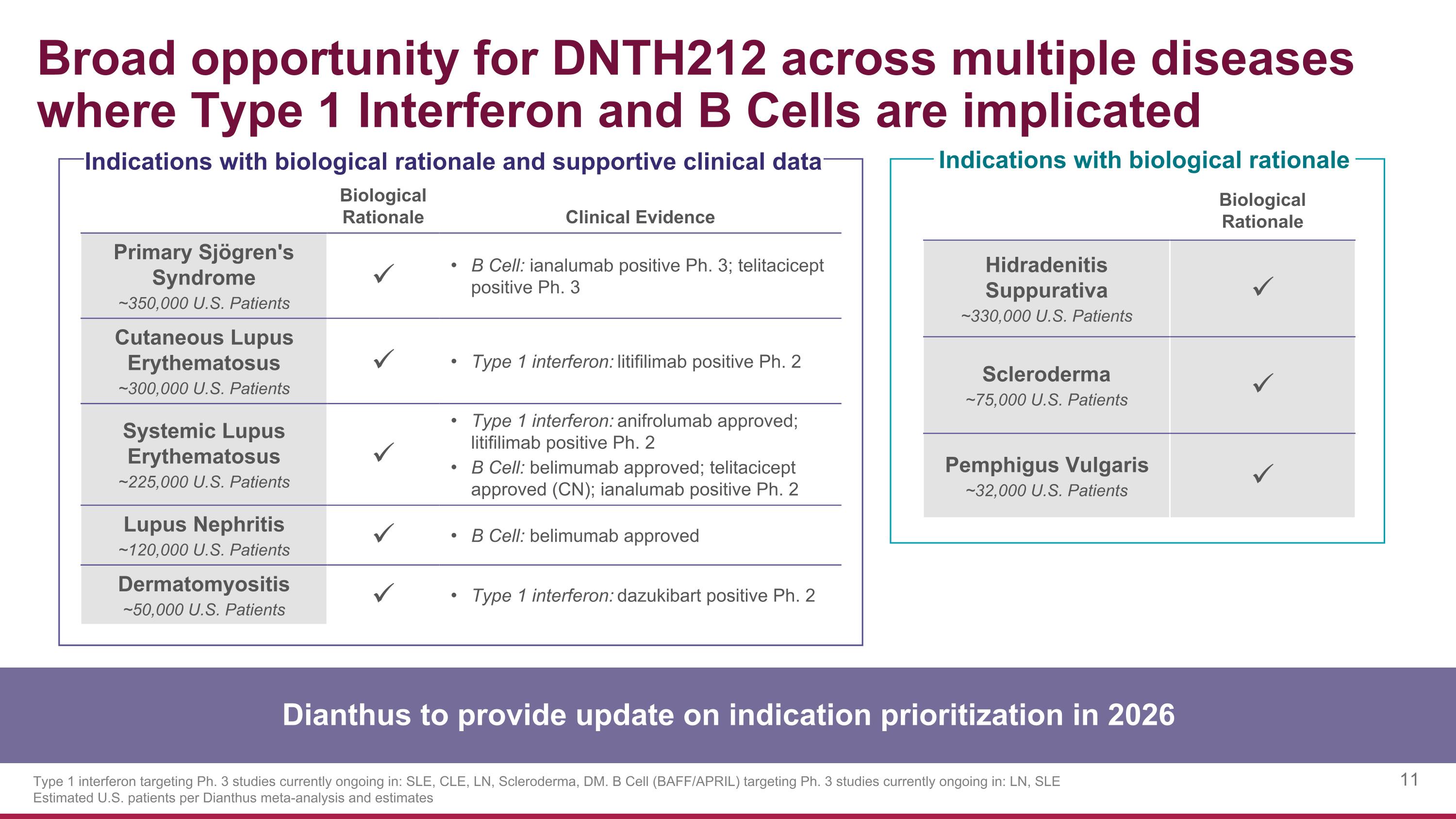
Biological Rationale Clinical Evidence Primary Sjögren's Syndrome ~350,000 U.S. Patients B Cell: ianalumab positive Ph. 3; telitacicept positive Ph. 3 Cutaneous Lupus Erythematosus ~300,000 U.S. Patients Type 1 interferon: litifilimab positive Ph. 2 Systemic Lupus Erythematosus ~225,000 U.S. Patients Type 1 interferon: anifrolumab approved; litifilimab positive Ph. 2 B Cell: belimumab approved; telitacicept approved (CN); ianalumab positive Ph. 2 Lupus Nephritis ~120,000 U.S. Patients B Cell: belimumab approved Dermatomyositis ~50,000 U.S. Patients Type 1 interferon: dazukibart positive Ph. 2 Broad opportunity for DNTH212 across multiple diseases where Type 1 Interferon and B Cells are implicated Type 1 interferon targeting Ph. 3 studies currently ongoing in: SLE, CLE, LN, Scleroderma, DM. B Cell (BAFF/APRIL) targeting Ph. 3 studies currently ongoing in: LN, SLE Estimated U.S. patients per Dianthus meta-analysis and estimates Indications with biological rationale Indications with biological rationale and supportive clinical data Biological Rationale Hidradenitis Suppurativa ~330,000 U.S. Patients Scleroderma ~75,000 U.S. Patients Pemphigus Vulgaris ~32,000 U.S. Patients Dianthus to provide update on indication prioritization in 2026
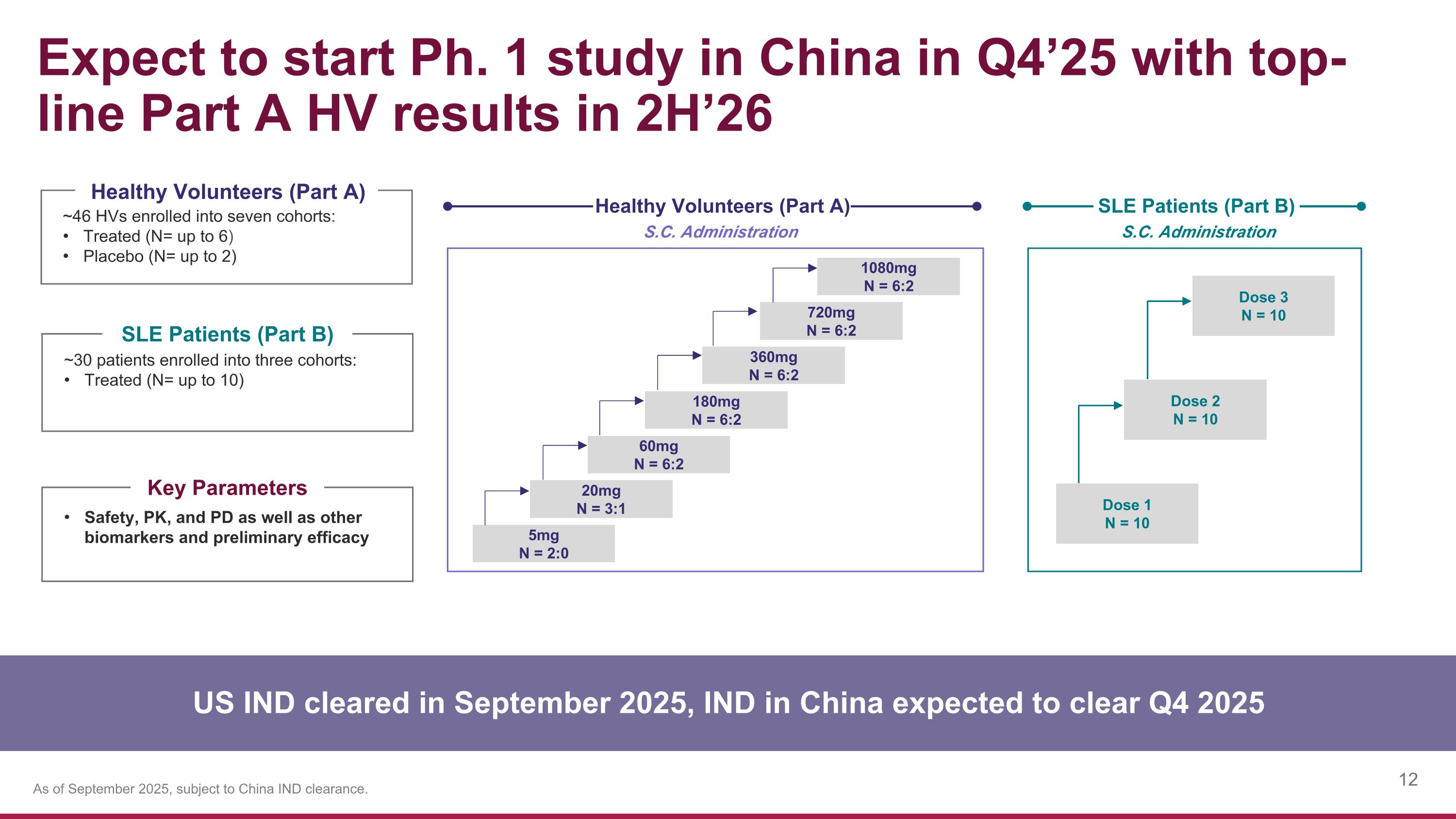
Expect to start Ph. 1 study in China in Q4’25 with top-line Part A HV results in 2H’26 5mg N = 2:0 S.C. Administration Healthy Volunteers (Part A) ~46 HVs enrolled into seven cohorts: Treated (N= up to 6) Placebo (N= up to 2) ~30 patients enrolled into three cohorts: Treated (N= up to 10) Healthy Volunteers (Part A) SLE Patients (Part B) Safety, PK, and PD as well as other biomarkers and preliminary efficacy Key Parameters US IND cleared in September 2025, IND in China expected to clear Q4 2025 S.C. Administration SLE Patients (Part B) 20mg N = 3:1 60mg N = 6:2 180mg N = 6:2 360mg N = 6:2 720mg N = 6:2 1080mg N = 6:2 Dose 1 N = 10 Dose 2 N = 10 Dose 3 N = 10 As of September 2025, subject to China IND clearance.
DNTH212 TPP aims to deliver superior efficacy in a safe and well-tolerated therapy with patient friendly convenience Achieving the TPP would position DNTH212 as a first-line biologic across a range of indications Bifunctional approach has potential for superior efficacy in various disease states versus only targeting innate or adaptive immune system SAFETY EFFICACY CONVENIENCE Inhibiting Type 1 interferon or BAFF/APRIL has been generally safe and well-tolerated Targeting patient friendly S.C. self-administration with Q4W or less frequent dosing

DNTH212 Expands Leadership in Next-generation Therapeutics for Severe Autoimmune Diseases
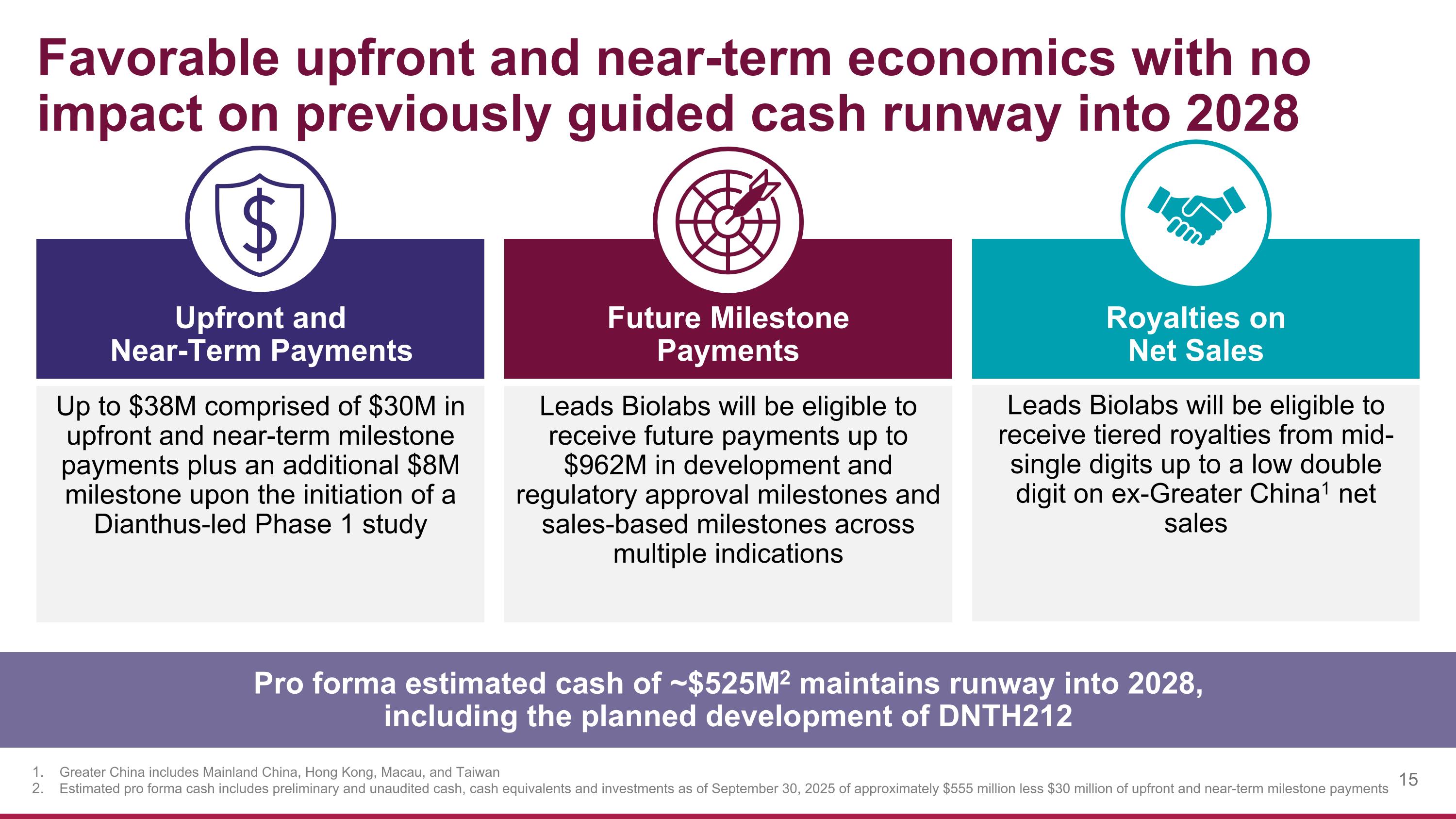
Favorable upfront and near-term economics with no impact on previously guided cash runway into 2028 Up to $38M comprised of $30M in upfront and near-term milestone payments plus an additional $8M milestone upon the initiation of a Dianthus-led Phase 1 study Future Milestone Payments Upfront and Near-Term Payments Royalties on Net Sales Leads Biolabs will be eligible to receive future payments up to $962M in development and regulatory approval milestones and sales-based milestones across multiple indications Leads Biolabs will be eligible to receive tiered royalties from mid-single digits up to a low double digit on ex-Greater China1 net sales Pro forma estimated cash of ~$525M2 maintains runway into 2028, including the planned development of DNTH212 Greater China includes Mainland China, Hong Kong, Macau, and Taiwan Estimated pro forma cash includes preliminary and unaudited cash, cash equivalents and investments as of September 30, 2025 of approximately $555 million less $30 million of upfront and near-term milestone payments

DNTH212 expands our leadership in next-generation therapeutics with best-in-class potential Value Proposition Claseprubart DNTH212 Validated Mechanism of Action(s) aC1s: gMG, CIDP Classical Pathway: gMG, CIDP, MMN BDCA2: SLE, CLE BAFF/APRIL: SLE, Sjögren’s, IgAN, MG, RA Best-in-class Potential Superior affinity, potency, and convenience vs. riliprubart Superior convenience and potential potency and safety advantage vs. empasiprubart Dual mechanism, targeting innate and adaptive immune systems Superior in-vitro pDC depletion vs. litifilimab Superior serum Ig inhibition vs. povetacicept in NHPs Convenient Dosing & Administration Targeting self-administered S.C. Q2W or Q4W Targeting self-administered S.C. Q4W or less frequent dosing Pipeline-in-a-product gMG, CIDP, MMN Announce prioritized indications in 2026 DNTH212 illustrates our strategy of pursuing next-generation therapeutics with clinically validated MoAs and best-in-class potential
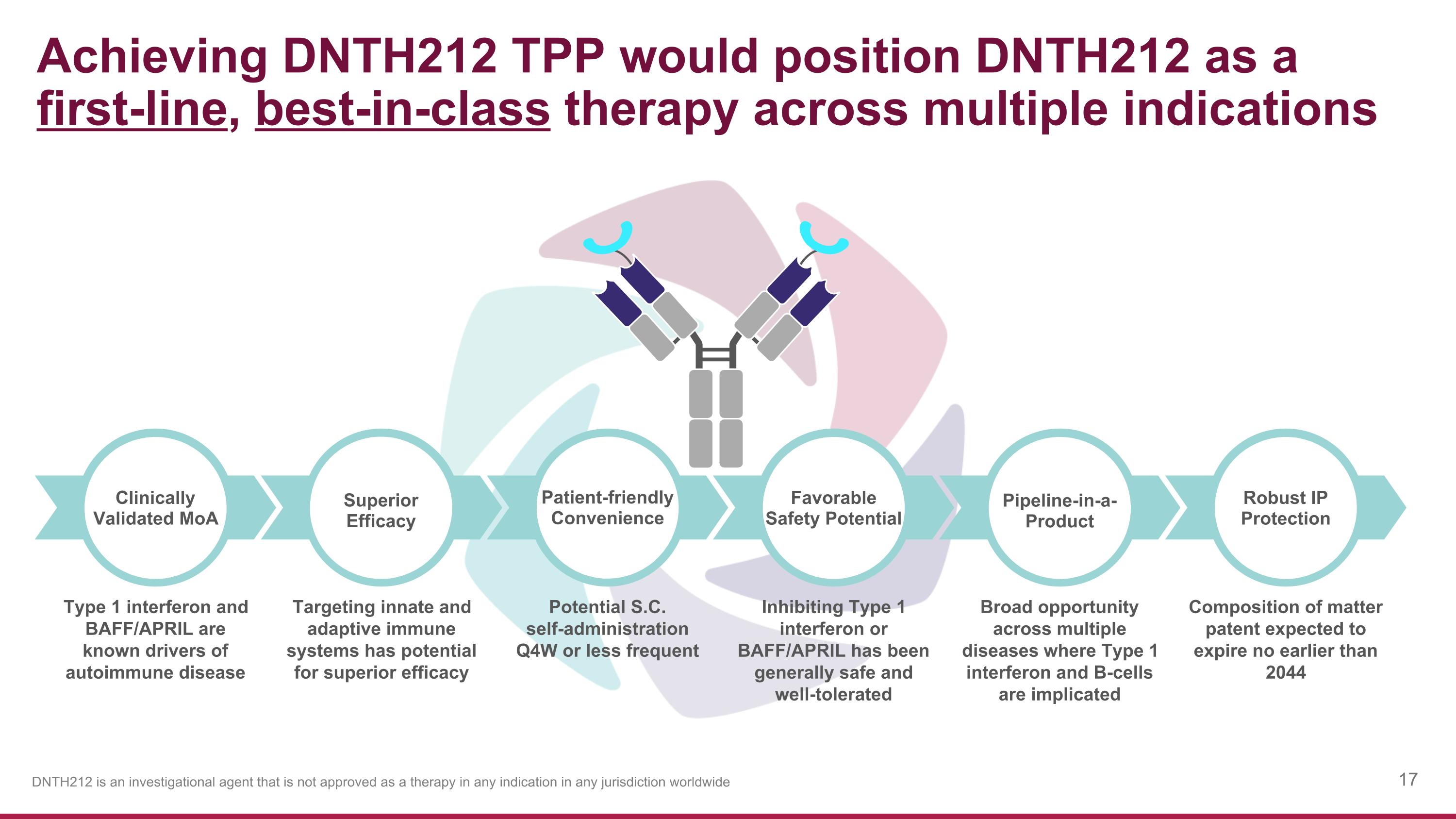
Achieving DNTH212 TPP would position DNTH212 as a first-line, best-in-class therapy across multiple indications Type 1 interferon and BAFF/APRIL are known drivers of autoimmune disease Potential S.C. self-administration Q4W or less frequent Targeting innate and adaptive immune systems has potential for superior efficacy Inhibiting Type 1 interferon or BAFF/APRIL has been generally safe and well-tolerated Broad opportunity across multiple diseases where Type 1 interferon and B-cells are implicated Composition of matter patent expected to expire no earlier than 2044 Patient-friendly Convenience Superior Efficacy Clinically Validated MoA Favorable Safety Potential Pipeline-in-a-Product Robust IP Protection DNTH212 is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide
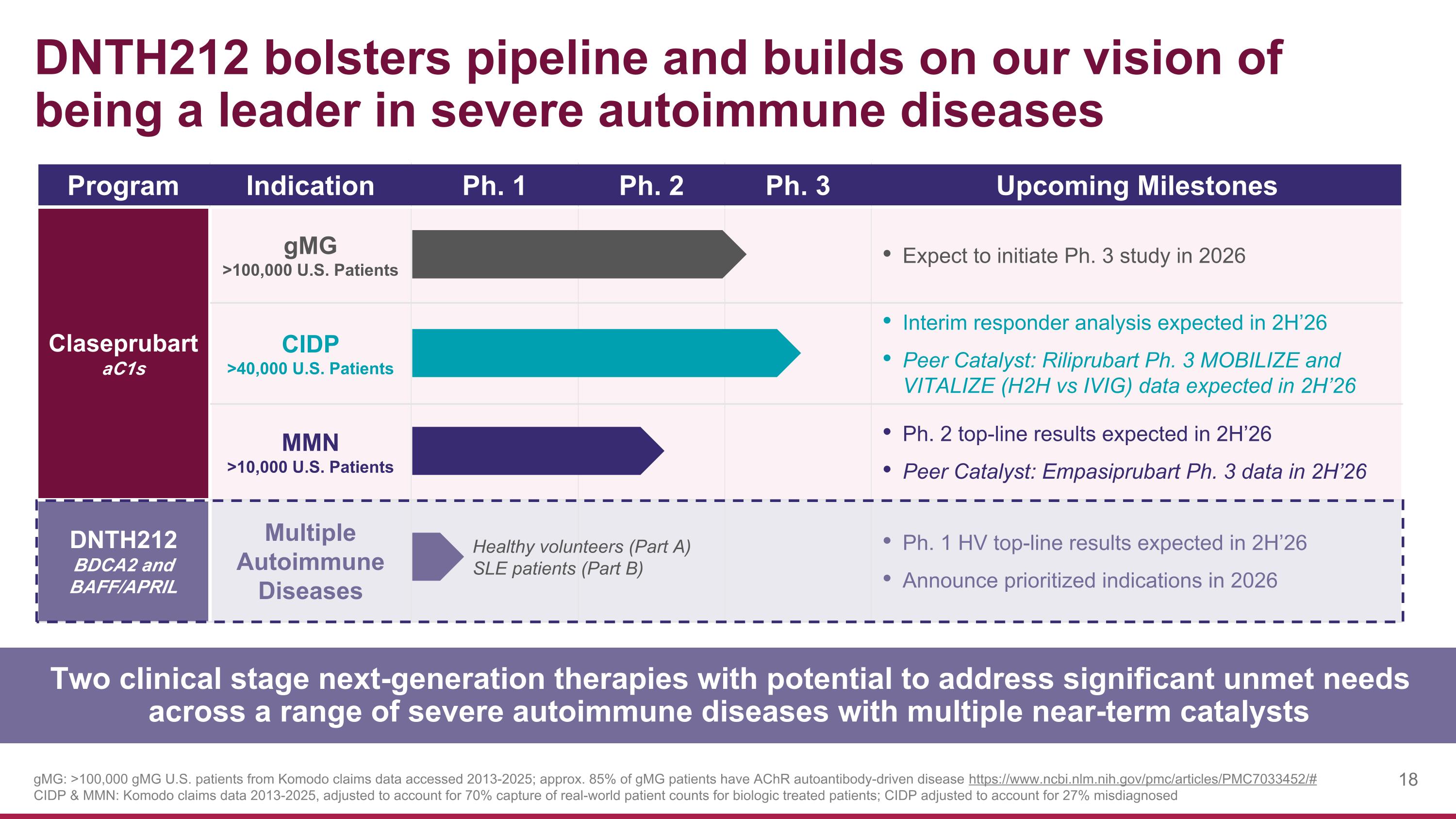
DNTH212 bolsters pipeline and builds on our vision of being a leader in severe autoimmune diseases gMG: >100,000 gMG U.S. patients from Komodo claims data accessed 2013-2025; approx. 85% of gMG patients have AChR autoantibody-driven disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/# CIDP & MMN: Komodo claims data 2013-2025, adjusted to account for 70% capture of real-world patient counts for biologic treated patients; CIDP adjusted to account for 27% misdiagnosed Two clinical stage next-generation therapies with potential to address significant unmet needs across a range of severe autoimmune diseases with multiple near-term catalysts Program Indication Ph. 1 Ph. 2 Ph. 3 Upcoming Milestones Claseprubart aC1s gMG >100,000 U.S. Patients Expect to initiate Ph. 3 study in 2026 CIDP >40,000 U.S. Patients Interim responder analysis expected in 2H’26 Peer Catalyst: Riliprubart Ph. 3 MOBILIZE and VITALIZE (H2H vs IVIG) data expected in 2H’26 MMN >10,000 U.S. Patients Ph. 2 top-line results expected in 2H’26 Peer Catalyst: Empasiprubart Ph. 3 data in 2H’26 DNTH212 BDCA2 and BAFF/APRIL Multiple Autoimmune Diseases Ph. 1 HV top-line results expected in 2H’26 Announce prioritized indications in 2026 Healthy volunteers (Part A) SLE patients (Part B)
October 16, 2025 Q&A Licensing of DNTH212 (BDCA2 and BAFF/APRIL) from Leads Biolabs