
Corporate Presentation November 2025

Forward-looking statements Certain statements in this presentation, other than purely historical information, may constitute “forward-looking statements” within the meaning of the federal securities laws, including for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995, express or implied statements regarding future plans and prospects, including statements regarding the expectations or plans for discovery, preclinical studies, clinical trials and research and development programs, in particular with respect to claseprubart and DNTH212, and any developments or results in connection therewith, including the target product profile and administration of claseprubart and DNTH212; the anticipated timing of the initiation and results from those studies and trials; expectations regarding the clinical trial designs or indications; expectations regarding the time period over which the Company’s capital resources are expected to be sufficient to fund its anticipated operations; and expectations regarding market size, patient population size, and potential opportunities for complement therapies, in particular with respect to claseprubart and DNTH212. Claseprubart and DNTH212 are investigational agents that are not approved as therapies in any indication in any jurisdiction worldwide. The words “opportunity,” “potential,” “milestones,” “runway,” “will,” “anticipate,” “achieve,” “near-term,” “catalysts,” “pursue,” “pipeline,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “predict,” “project,” “should,” “strive,” “would,” “aim,” “target,” “commit,” and similar expressions (including the negatives of these terms or variations of them) generally identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Actual results could differ materially from those included in the forward-looking statements due to various factors, risks and uncertainties, including, but not limited to, that preclinical testing of claseprubart and DNTH212 and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that the development of claseprubart or DNTH212 may take longer and/or cost more than planned, that the Company or its partner may be unable to successfully complete the clinical development of the Company’s compounds, that the Company or its partner may be delayed in initiating, enrolling or completing its planned clinical trials, and that the Company's compounds may not receive regulatory approval or become commercially successful products. These and other risks and uncertainties are identified under the heading "Risk Factors" included in the Company’s Annual Report on Form 10-K for the period ended December 31, 2024, and other filings that the Company has made and may make with the SEC in the future. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. Dianthus undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
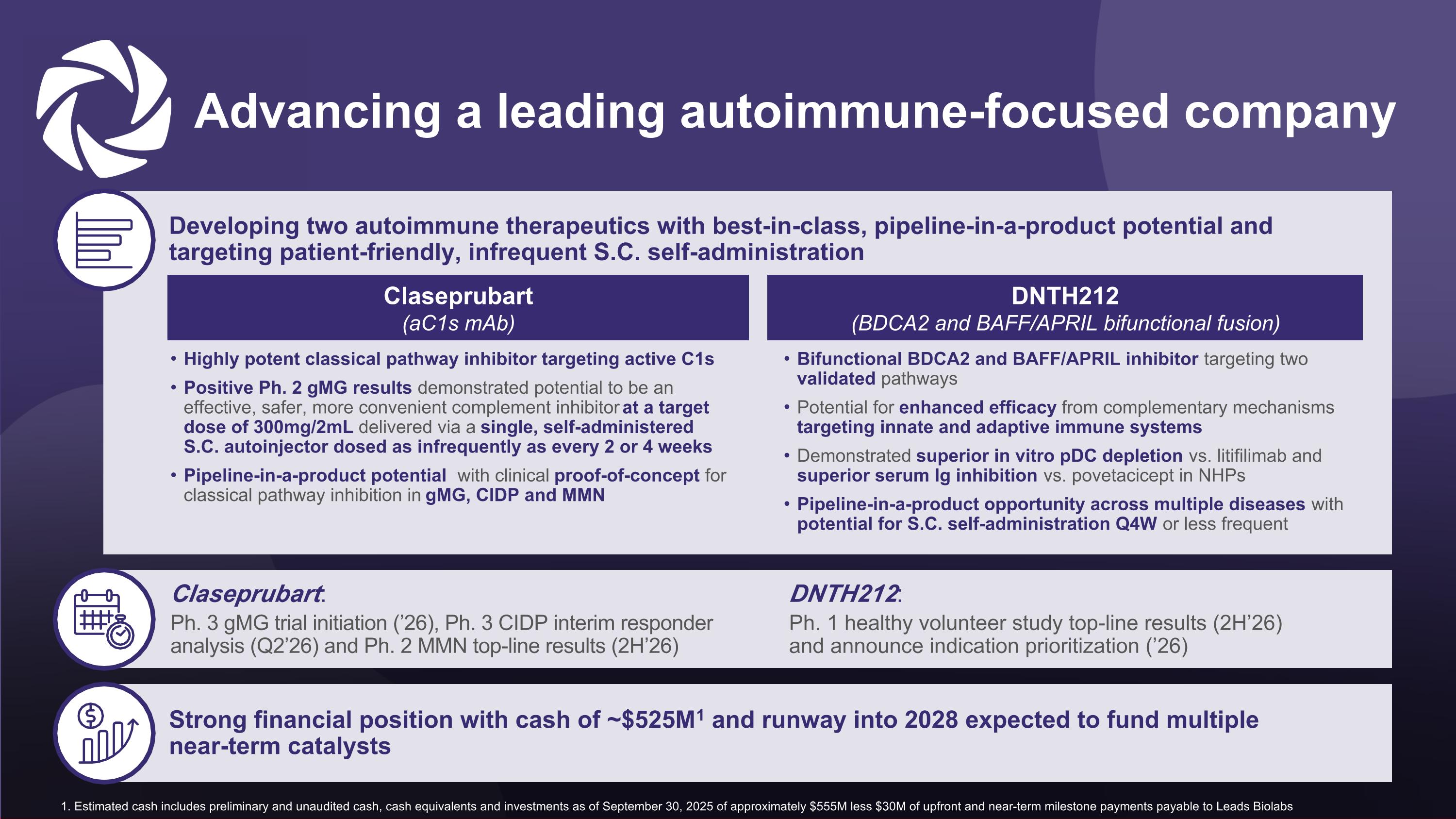
Developing two autoimmune therapeutics with best-in-class, pipeline-in-a-product potential and targeting patient-friendly, infrequent S.C. self-administration Advancing a leading autoimmune-focused company Highly potent classical pathway inhibitor targeting active C1s Positive Ph. 2 gMG results demonstrated potential to be an effective, safer, more convenient complement inhibitor at a target dose of 300mg/2mL delivered via a single, self-administered S.C. autoinjector dosed as infrequently as every 2 or 4 weeks Pipeline-in-a-product potential with clinical proof-of-concept for classical pathway inhibition in gMG, CIDP and MMN Claseprubart Bifunctional BDCA2 and BAFF/APRIL inhibitor targeting two validated pathways Potential for enhanced efficacy from complementary mechanisms targeting innate and adaptive immune systems Demonstrated superior in vitro pDC depletion vs. litifilimab and superior serum Ig inhibition vs. povetacicept in NHPs Pipeline-in-a-product opportunity across multiple diseases with potential for S.C. self-administration Q4W or less frequent DNTH212 (BDCA2 and BAFF/APRIL bifunctional fusion) Strong financial position with cash of ~$525M1 and runway into 2028 expected to fund multiple near-term catalysts Claseprubart (aC1s mAb) 1. Estimated cash includes preliminary and unaudited cash, cash equivalents and investments as of September 30, 2025 of approximately $555M less $30M of upfront and near-term milestone payments payable to Leads Biolabs Claseprubart: Ph. 3 gMG trial initiation (’26), Ph. 3 CIDP interim responder analysis (Q2’26) and Ph. 2 MMN top-line results (2H’26) DNTH212: Ph. 1 healthy volunteer study top-line results (2H’26) and announce indication prioritization (’26)
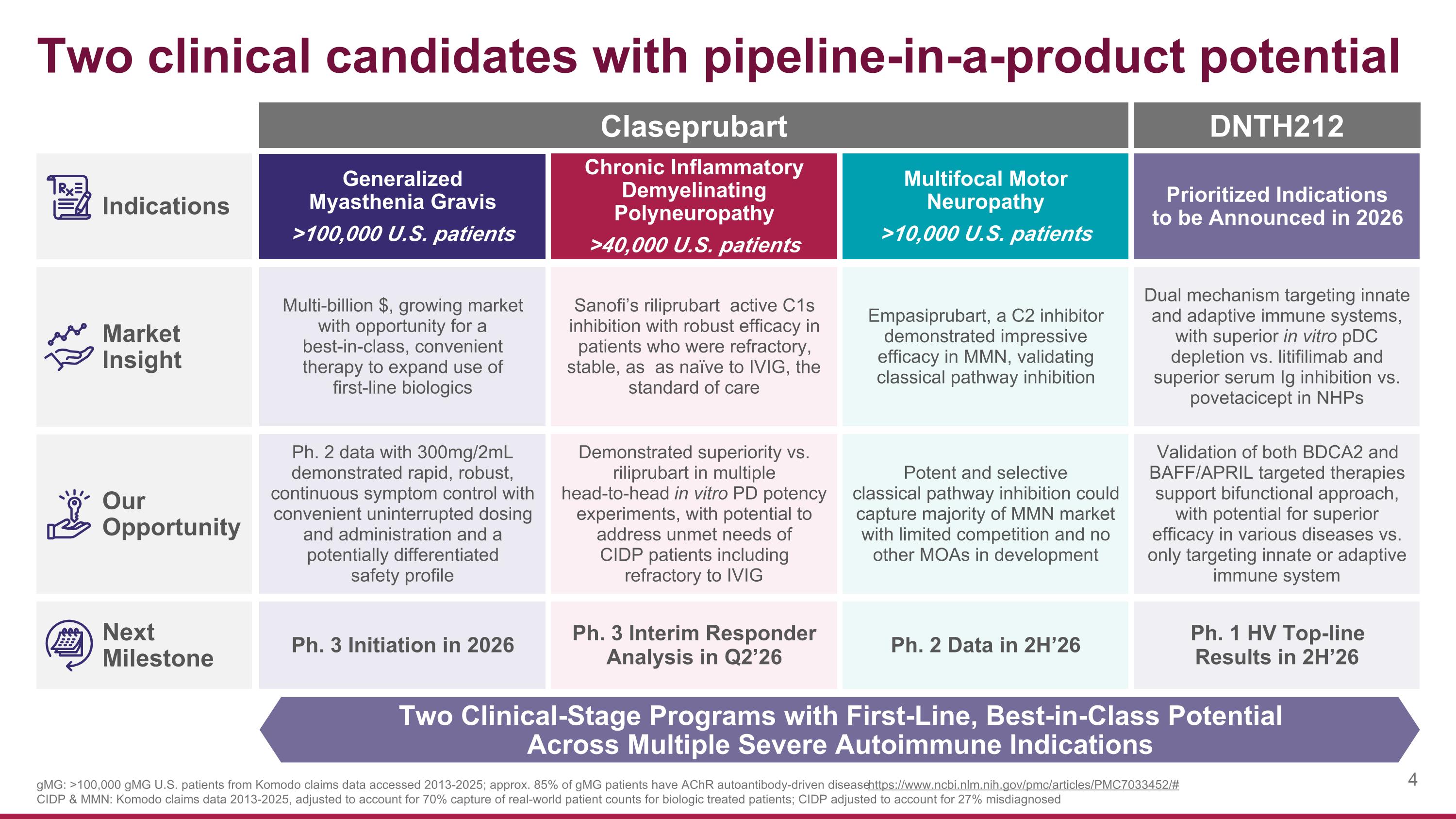
Two clinical candidates with pipeline-in-a-product potential Two Clinical-Stage Programs with First-Line, Best-in-Class Potential Across Multiple Severe Autoimmune Indications Indications Market Insight Our Opportunity Next Milestone gMG: >100,000 gMG U.S. patients from Komodo claims data accessed 2013-2025; approx. 85% of gMG patients have AChR autoantibody-driven disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/# CIDP & MMN: Komodo claims data 2013-2025, adjusted to account for 70% capture of real-world patient counts for biologic treated patients; CIDP adjusted to account for 27% misdiagnosed Generalized Myasthenia Gravis >100,000 U.S. patients Chronic Inflammatory Demyelinating Polyneuropathy >40,000 U.S. patients Multifocal Motor Neuropathy >10,000 U.S. patients Multi-billion $, growing market with opportunity for a best-in-class, convenient therapy to expand use of first-line biologics Sanofi’s riliprubart active C1s inhibition with robust efficacy in patients who were refractory, stable, as as naïve to IVIG, the standard of care Empasiprubart, a C2 inhibitor demonstrated impressive efficacy in MMN, validating classical pathway inhibition Ph. 2 data with 300mg/2mL demonstrated rapid, robust, continuous symptom control with convenient uninterrupted dosing and administration and a potentially differentiated safety profile Demonstrated superiority vs. riliprubart in multiple head-to-head in vitro PD potency experiments, with potential to address unmet needs of CIDP patients including refractory to IVIG Potent and selective classical pathway inhibition could capture majority of MMN market with limited competition and no other MOAs in development Ph. 3 Initiation in 2026 Ph. 3 Interim Responder Analysis in Q2’26 Ph. 2 Data in 2H’26 Prioritized Indications to be Announced in 2026 Dual mechanism targeting innate and adaptive immune systems, with superior in vitro pDC depletion vs. litifilimab and superior serum Ig inhibition vs. povetacicept in NHPs Validation of both BDCA2 and BAFF/APRIL targeted therapies support bifunctional approach, with potential for superior efficacy in various diseases vs. only targeting innate or adaptive immune system Ph. 1 HV Top-line Results in 2H’26 Claseprubart DNTH212

Claseprubart: Building a Best-in-Class Neuromuscular Franchise
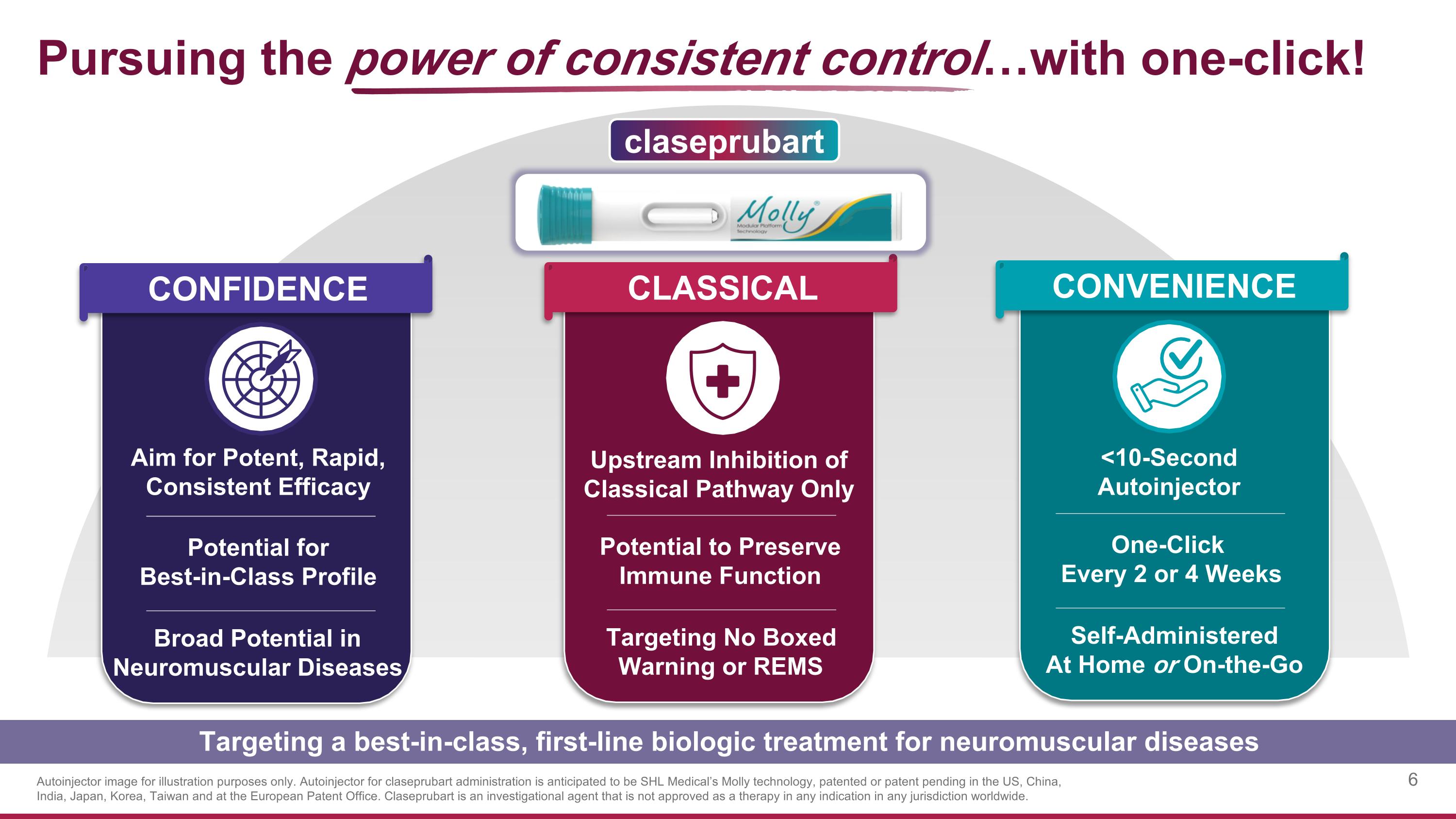
Pursuing the power of consistent control…with one-click! Targeting a best-in-class, first-line biologic treatment for neuromuscular diseases CLASSICAL CONVENIENCE Upstream Inhibition of Classical Pathway Only <10-Second Autoinjector Targeting No Boxed Warning or REMS Self-Administered At Home or On-the-Go Potential to Preserve Immune Function One-Click Every 2 or 4 Weeks claseprubart CONFIDENCE Aim for Potent, Rapid, Consistent Efficacy Broad Potential in Neuromuscular Diseases Potential for Best-in-Class Profile Autoinjector image for illustration purposes only. Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office. Claseprubart is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide.
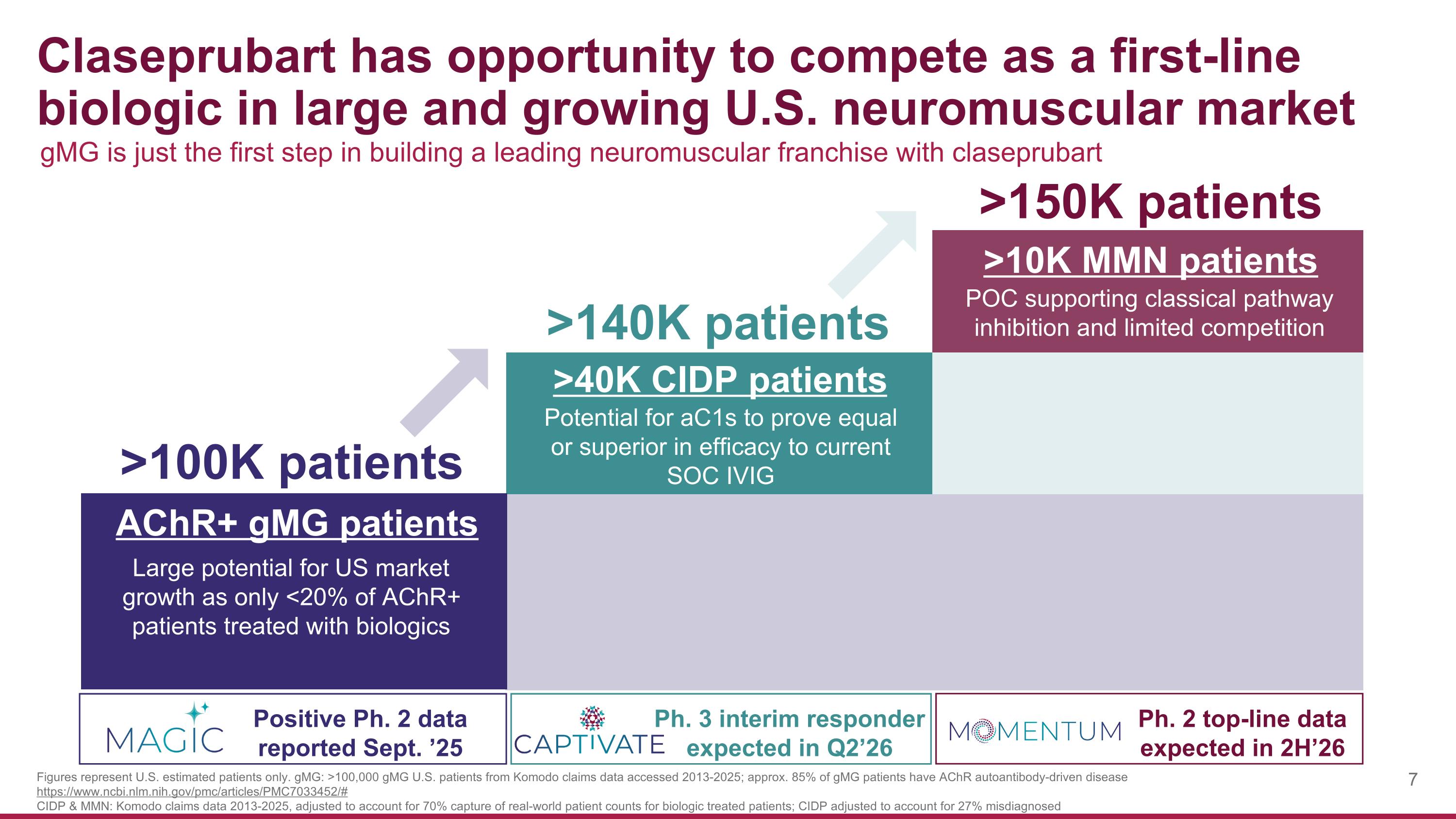
Claseprubart has opportunity to compete as a first-line biologic in large and growing U.S. neuromuscular market >100K patients >140K patients >150K patients AChR+ gMG patients Positive Ph. 2 data reported Sept. ’25 >40K CIDP patients Ph. 3 interim responder expected in Q2’26 Ph. 2 top-line data expected in 2H’26 Large potential for US market growth as only <20% of AChR+ patients treated with biologics >10K MMN patients Potential for aC1s to prove equal or superior in efficacy to current SOC IVIG POC supporting classical pathway inhibition and limited competition gMG is just the first step in building a leading neuromuscular franchise with claseprubart Figures represent U.S. estimated patients only. gMG: >100,000 gMG U.S. patients from Komodo claims data accessed 2013-2025; approx. 85% of gMG patients have AChR autoantibody-driven disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/# CIDP & MMN: Komodo claims data 2013-2025, adjusted to account for 70% capture of real-world patient counts for biologic treated patients; CIDP adjusted to account for 27% misdiagnosed

Claseprubart Opportunity in Generalized Myasthenia Gravis
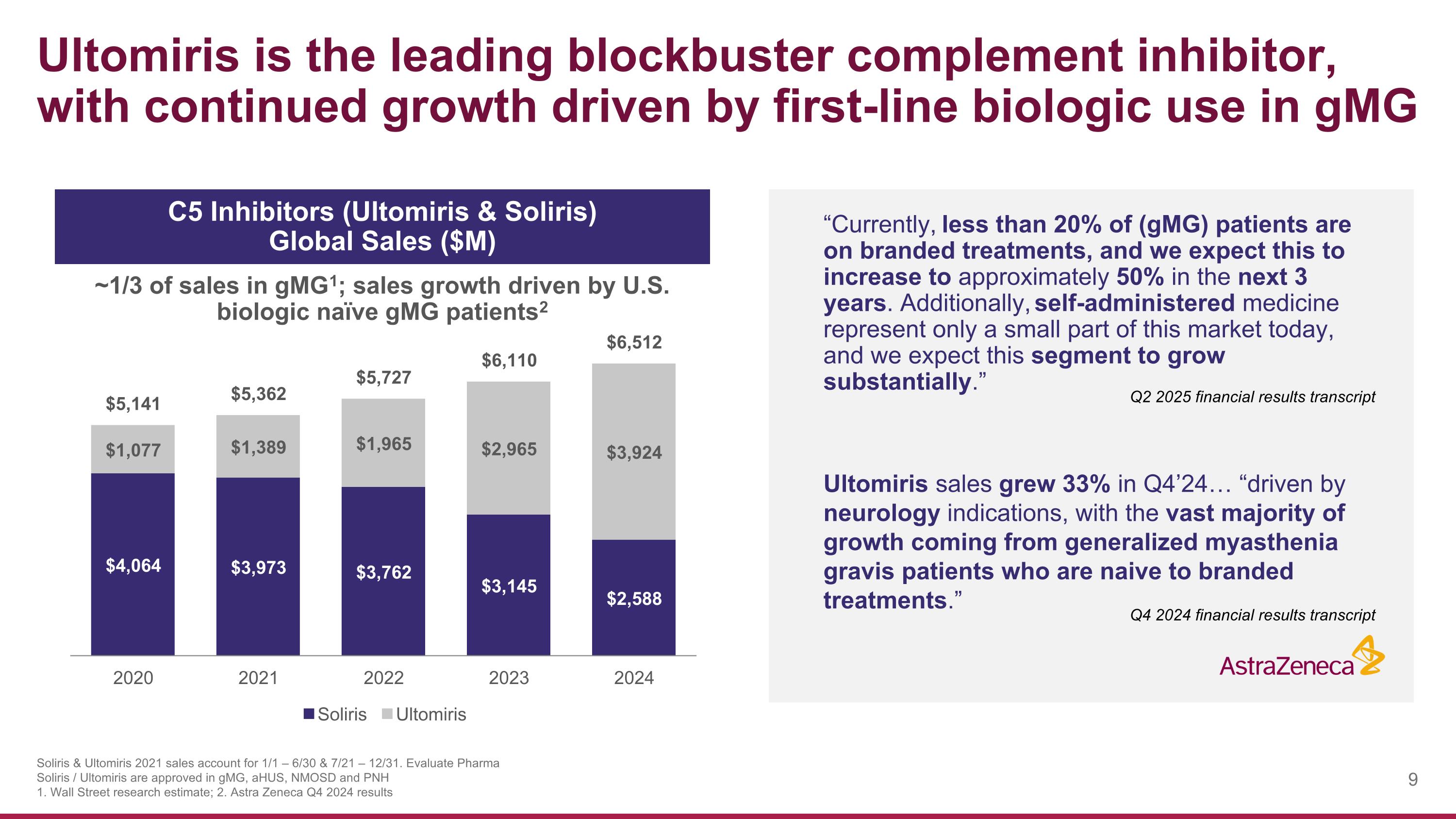
“Currently, less than 20% of (gMG) patients are on branded treatments, and we expect this to increase to approximately 50% in the next 3 years. Additionally, self-administered medicine represent only a small part of this market today, and we expect this segment to grow substantially.” Ultomiris sales grew 33% in Q4’24… “driven by neurology indications, with the vast majority of growth coming from generalized myasthenia gravis patients who are naive to branded treatments.” Ultomiris is the leading blockbuster complement inhibitor, with continued growth driven by first-line biologic use in gMG ~1/3 of sales in gMG1; sales growth driven by U.S. biologic naïve gMG patients2 C5 Inhibitors (Ultomiris & Soliris) Global Sales ($M) Soliris & Ultomiris 2021 sales account for 1/1 – 6/30 & 7/21 – 12/31. Evaluate Pharma Soliris / Ultomiris are approved in gMG, aHUS, NMOSD and PNH 1. Wall Street research estimate; 2. Astra Zeneca Q4 2024 results Q4 2024 financial results transcript Q2 2025 financial results transcript
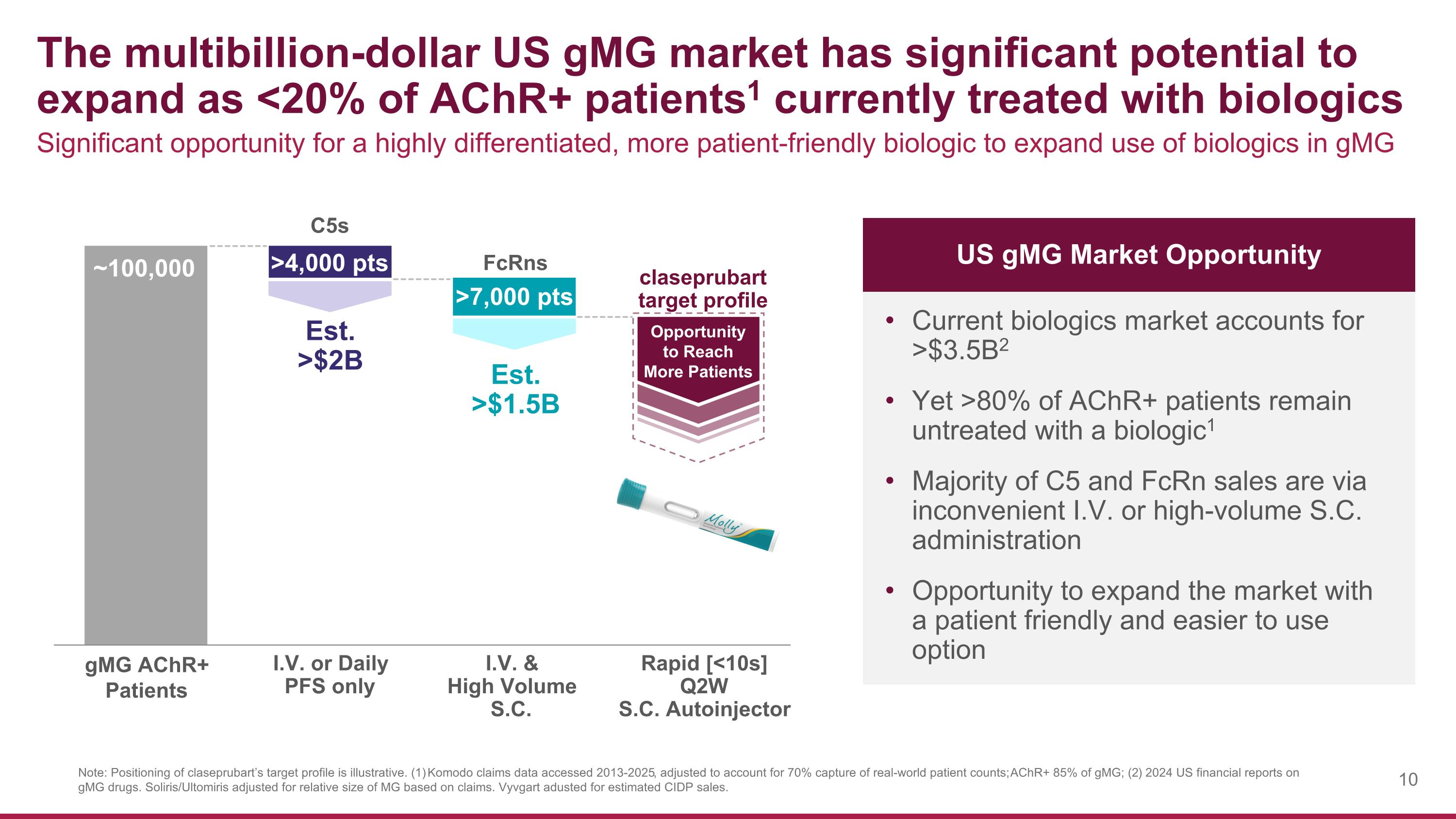
The multibillion-dollar US gMG market has significant potential to expand as <20% of AChR+ patients1 currently treated with biologics Note: Positioning of claseprubart’s target profile is illustrative. (1) Komodo claims data accessed 2013-2025, adjusted to account for 70% capture of real-world patient counts; AChR+ 85% of gMG; (2) 2024 US financial reports on gMG drugs. Soliris/Ultomiris adjusted for relative size of MG based on claims. Vyvgart adusted for estimated CIDP sales. ~100,000 Significant opportunity for a highly differentiated, more patient-friendly biologic to expand use of biologics in gMG Current biologics market accounts for >$3.5B2 Yet >80% of AChR+ patients remain untreated with a biologic1 Majority of C5 and FcRn sales are via inconvenient I.V. or high-volume S.C. administration Opportunity to expand the market with a patient friendly and easier to use option I.V. or Daily PFS only I.V. & High Volume S.C. Rapid [<10s] Q2W S.C. Autoinjector C5s FcRns claseprubart target profile Est. >$2B Est. >$1.5B Opportunity to Reach More Patients >4,000 pts >7,000 pts US gMG Market Opportunity
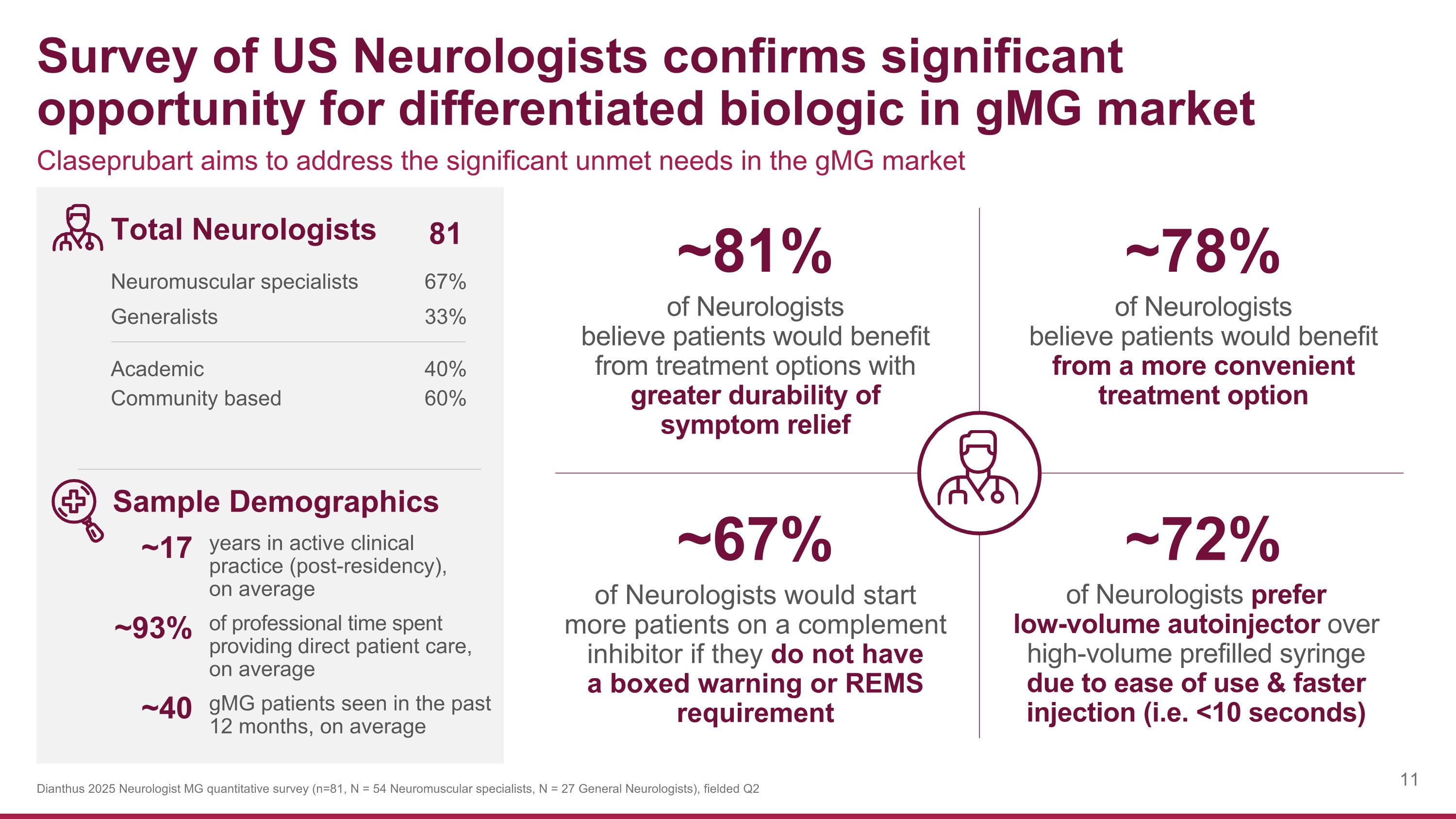
Claseprubart aims to address the significant unmet needs in the gMG market Survey of US Neurologists confirms significant opportunity for differentiated biologic in gMG market Dianthus 2025 Neurologist MG quantitative survey (n=81, N = 54 Neuromuscular specialists, N = 27 General Neurologists), fielded Q2 of Neurologists would start more patients on a complement inhibitor if they do not have a boxed warning or REMS requirement ~67% of Neurologists prefer low-volume autoinjector over high-volume prefilled syringe due to ease of use & faster injection (i.e. <10 seconds) ~72% of Neurologists believe patients would benefit from treatment options with greater durability of symptom relief ~81% of Neurologists believe patients would benefit from a more convenient treatment option ~78% Total Neurologists 81 Neuromuscular specialists 67% Generalists 33% Academic 40% Community based 60% Sample Demographics ~17 years in active clinical practice (post-residency), on average ~93% of professional time spent providing direct patient care, on average ~40 gMG patients seen in the past 12 months, on average

MaGic Ph. 2 Results
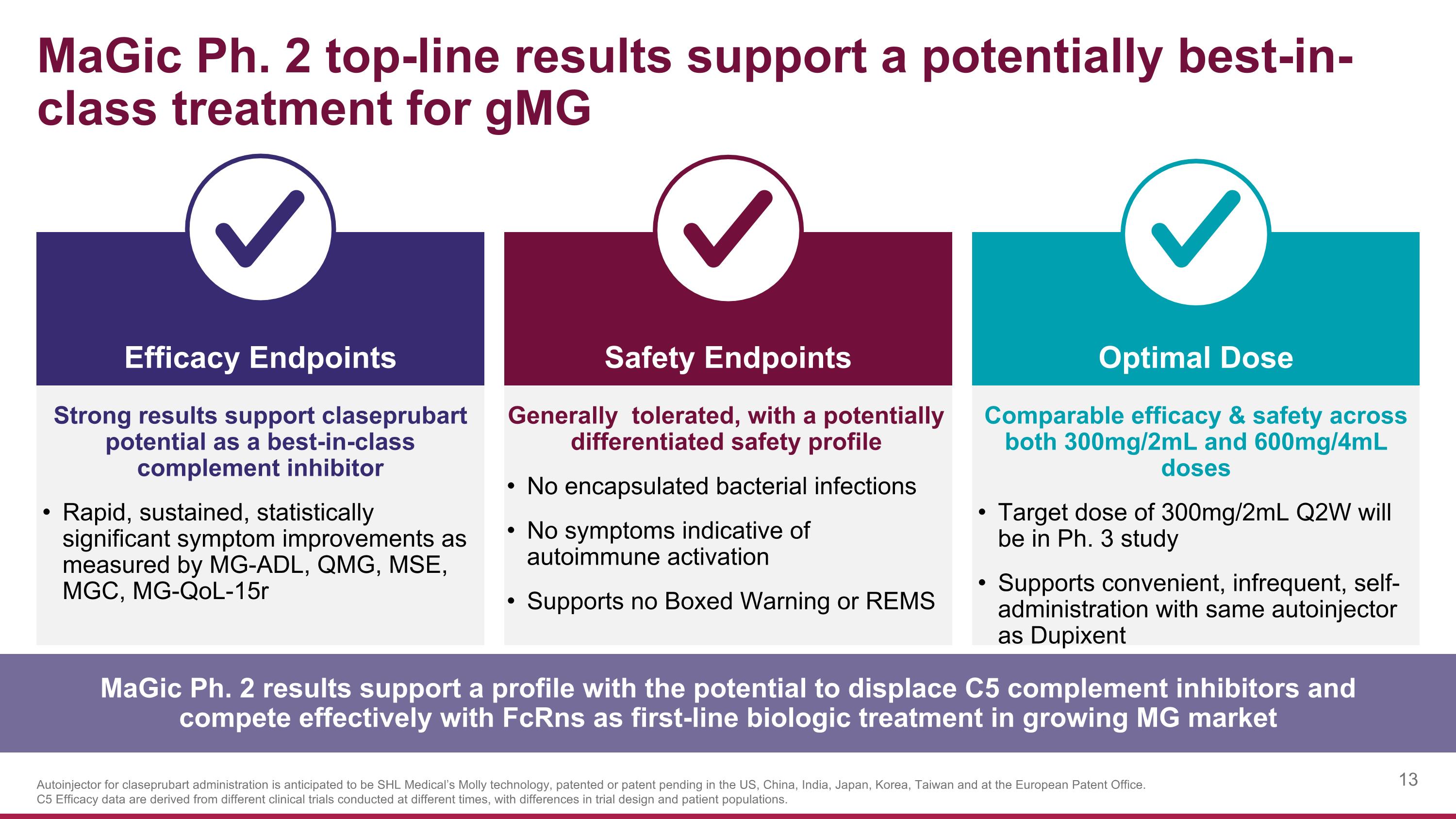
MaGic Ph. 2 top-line results support a potentially best-in-class treatment for gMG Strong results support claseprubart potential as a best-in-class complement inhibitor Rapid, sustained, statistically significant symptom improvements as measured by MG-ADL, QMG, MSE, MGC, MG-QoL-15r Safety Endpoints Efficacy Endpoints Optimal Dose Generally tolerated, with a potentially differentiated safety profile No encapsulated bacterial infections No symptoms indicative of autoimmune activation Supports no Boxed Warning or REMS Comparable efficacy & safety across both 300mg/2mL and 600mg/4mL doses Target dose of 300mg/2mL Q2W will be in Ph. 3 study Supports convenient, infrequent, self-administration with same autoinjector as Dupixent MaGic Ph. 2 results support a profile with the potential to displace C5 complement inhibitors and compete effectively with FcRns as first-line biologic treatment in growing MG market Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office. C5 Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations.
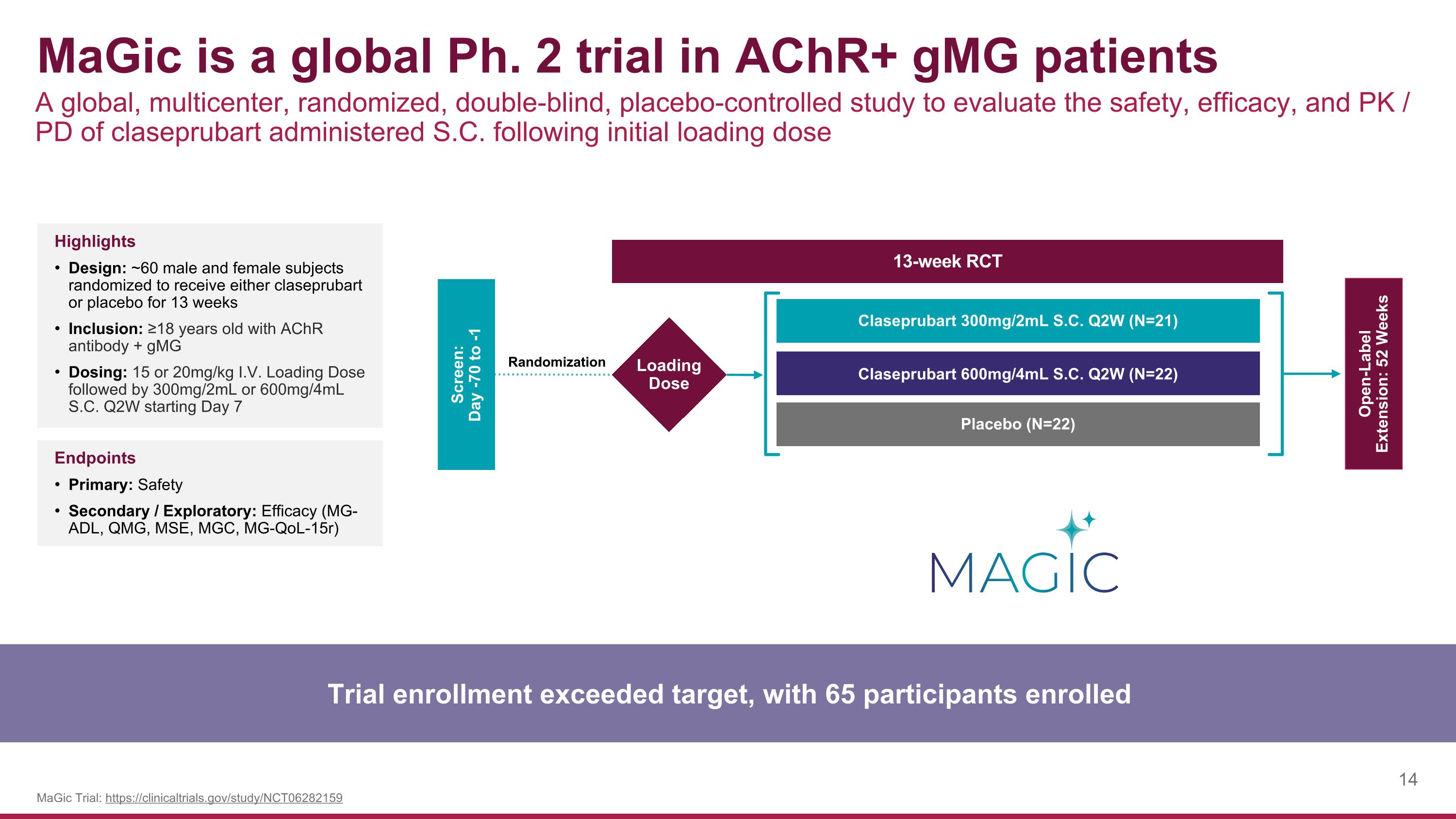
MaGic is a global Ph. 2 trial in AChR+ gMG patients A global, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety, efficacy, and PK / PD of claseprubart administered S.C. following initial loading dose Trial enrollment exceeded target, with 65 participants enrolled MaGic Trial: https://clinicaltrials.gov/study/NCT06282159 Highlights Design: ~60 male and female subjects randomized to receive either claseprubart or placebo for 13 weeks Inclusion: ≥18 years old with AChR antibody + gMG Dosing: 15 or 20mg/kg I.V. Loading Dose followed by 300mg/2mL or 600mg/4mL S.C. Q2W starting Day 7 Endpoints Primary: Safety Secondary / Exploratory: Efficacy (MG-ADL, QMG, MSE, MGC, MG-QoL-15r) Screen: Day -70 to -1 Open-Label Extension: 52 Weeks Claseprubart 300mg/2mL S.C. Q2W (N=21) Claseprubart 600mg/4mL S.C. Q2W (N=22) Placebo (N=22) 13-week RCT Loading Dose Randomization
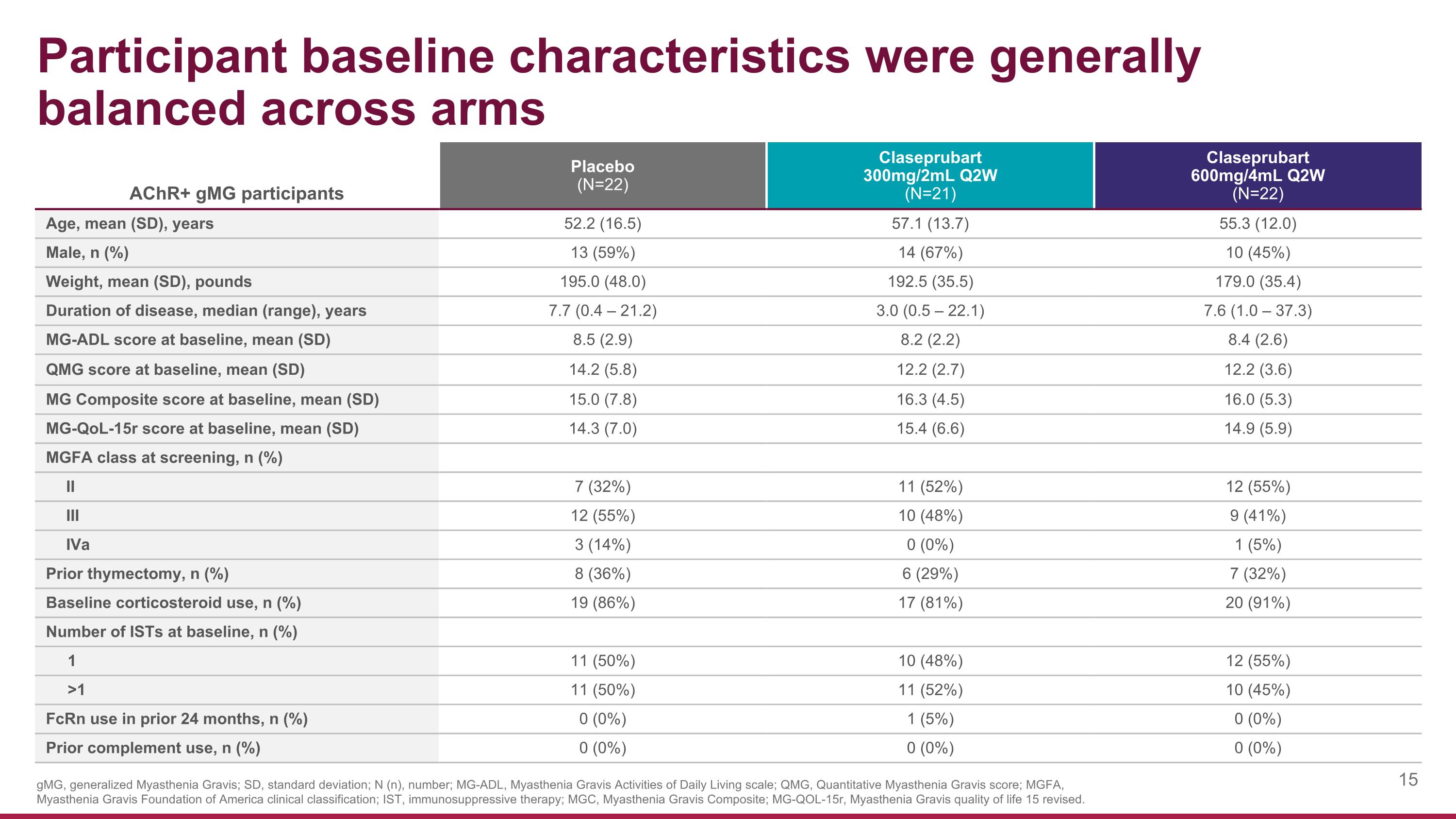
AChR+ gMG participants Placebo (N=22) Claseprubart 300mg/2mL Q2W (N=21) Claseprubart 600mg/4mL Q2W (N=22) Age, mean (SD), years 52.2 (16.5) 57.1 (13.7) 55.3 (12.0) Male, n (%) 13 (59%) 14 (67%) 10 (45%) Weight, mean (SD), pounds 195.0 (48.0) 192.5 (35.5) 179.0 (35.4) Duration of disease, median (range), years 7.7 (0.4 – 21.2) 3.0 (0.5 – 22.1) 7.6 (1.0 – 37.3) MG-ADL score at baseline, mean (SD) 8.5 (2.9) 8.2 (2.2) 8.4 (2.6) QMG score at baseline, mean (SD) 14.2 (5.8) 12.2 (2.7) 12.2 (3.6) MG Composite score at baseline, mean (SD) 15.0 (7.8) 16.3 (4.5) 16.0 (5.3) MG-QoL-15r score at baseline, mean (SD) 14.3 (7.0) 15.4 (6.6) 14.9 (5.9) MGFA class at screening, n (%) II 7 (32%) 11 (52%) 12 (55%) III 12 (55%) 10 (48%) 9 (41%) IVa 3 (14%) 0 (0%) 1 (5%) Prior thymectomy, n (%) 8 (36%) 6 (29%) 7 (32%) Baseline corticosteroid use, n (%) 19 (86%) 17 (81%) 20 (91%) Number of ISTs at baseline, n (%) 1 11 (50%) 10 (48%) 12 (55%) >1 11 (50%) 11 (52%) 10 (45%) FcRn use in prior 24 months, n (%) 0 (0%) 1 (5%) 0 (0%) Prior complement use, n (%) 0 (0%) 0 (0%) 0 (0%) Participant baseline characteristics were generally balanced across arms gMG, generalized Myasthenia Gravis; SD, standard deviation; N (n), number; MG-ADL, Myasthenia Gravis Activities of Daily Living scale; QMG, Quantitative Myasthenia Gravis score; MGFA, Myasthenia Gravis Foundation of America clinical classification; IST, immunosuppressive therapy; MGC, Myasthenia Gravis Composite; MG-QOL-15r, Myasthenia Gravis quality of life 15 revised.
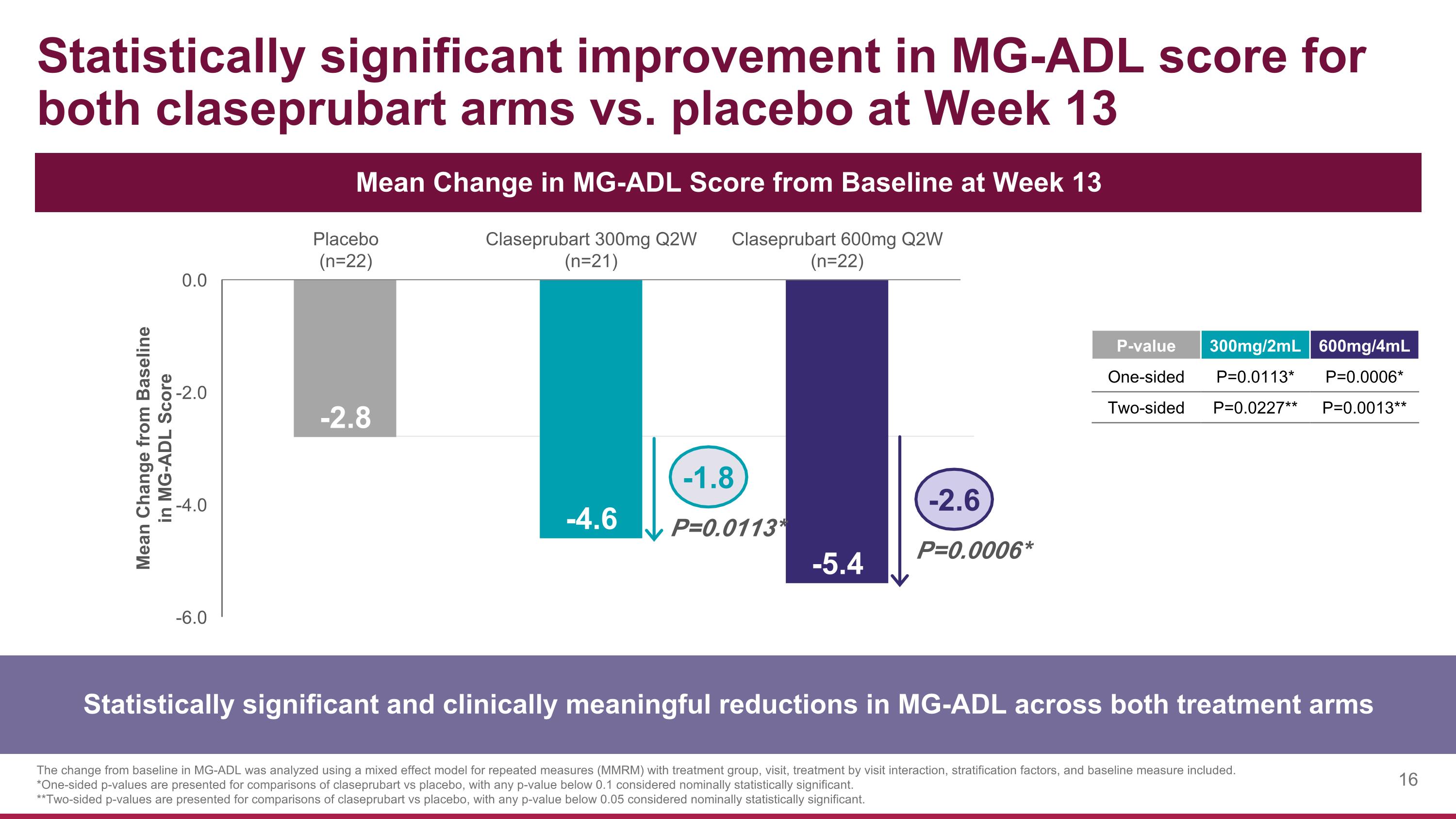
Statistically significant improvement in MG-ADL score for both claseprubart arms vs. placebo at Week 13 Statistically significant and clinically meaningful reductions in MG-ADL across both treatment arms Mean Change in MG-ADL Score from Baseline at Week 13 The change from baseline in MG-ADL was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. **Two-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.05 considered nominally statistically significant. P=0.0113* P=0.0006* -1.8 -2.6 P-value 300mg/2mL 600mg/4mL One-sided P=0.0113* P=0.0006* Two-sided P=0.0227** P=0.0013**
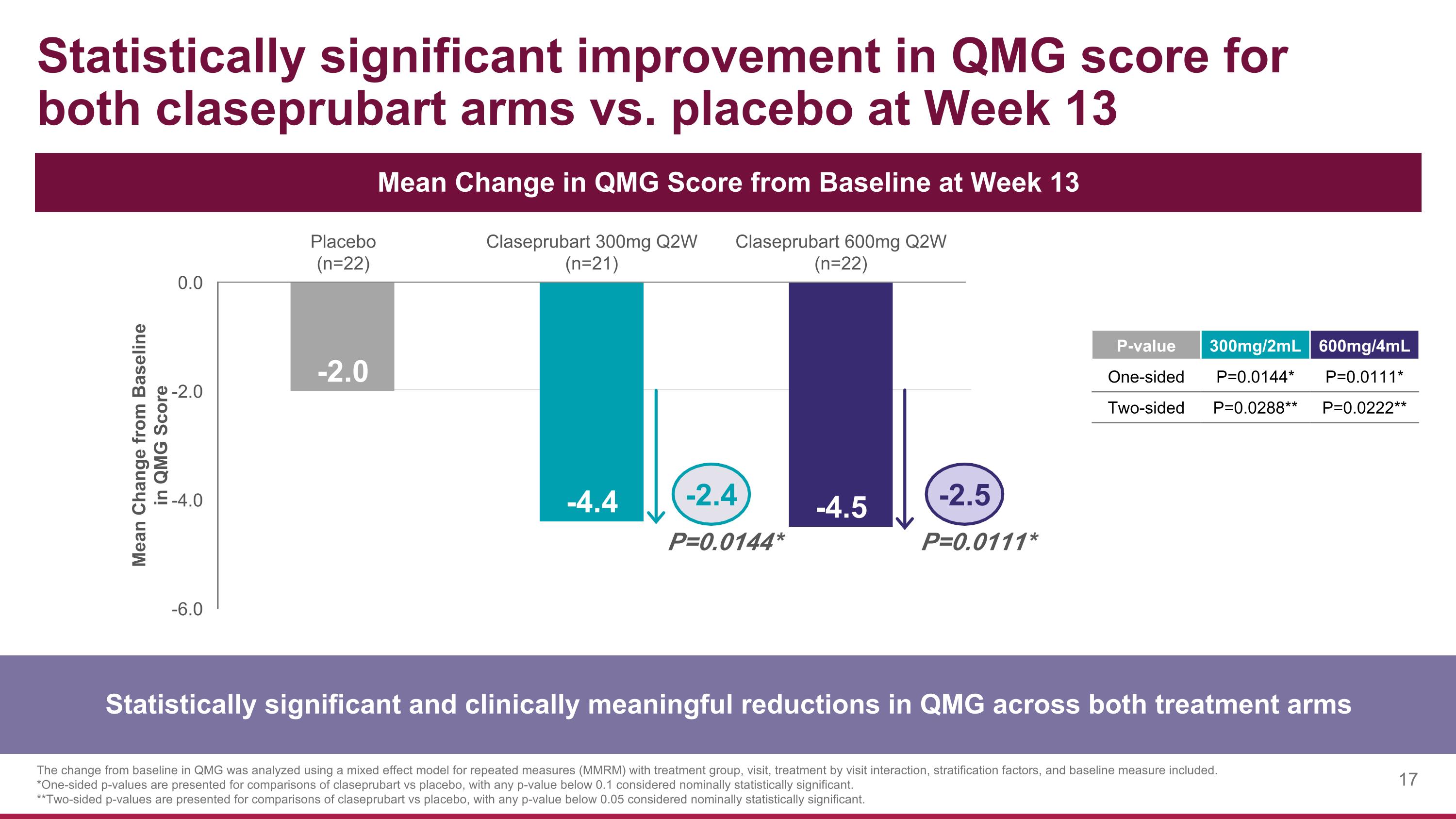
Statistically significant improvement in QMG score for both claseprubart arms vs. placebo at Week 13 Statistically significant and clinically meaningful reductions in QMG across both treatment arms The change from baseline in QMG was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. **Two-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.05 considered nominally statistically significant. Mean Change in QMG Score from Baseline at Week 13 P=0.0144* P=0.0111* -2.4 -2.5 P-value 300mg/2mL 600mg/4mL One-sided P=0.0144* P=0.0111* Two-sided P=0.0288** P=0.0222**
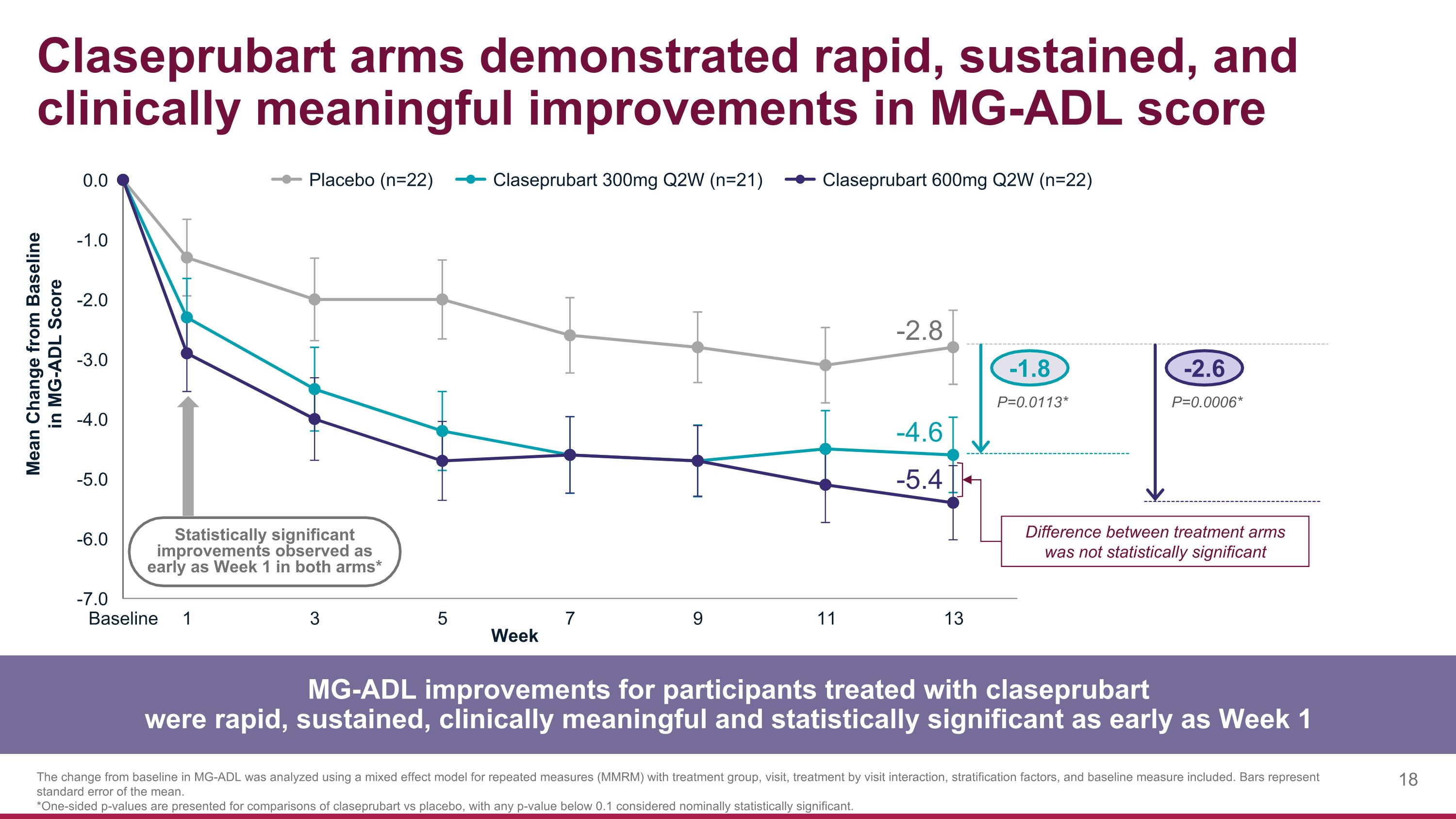
-1.8 -2.6 P=0.0113* P=0.0006* Claseprubart arms demonstrated rapid, sustained, and clinically meaningful improvements in MG-ADL score MG-ADL improvements for participants treated with claseprubart were rapid, sustained, clinically meaningful and statistically significant as early as Week 1 The change from baseline in MG-ADL was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. Bars represent standard error of the mean. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. Statistically significant improvements observed as early as Week 1 in both arms* -2.8 -4.6 -5.4 Difference between treatment arms was not statistically significant
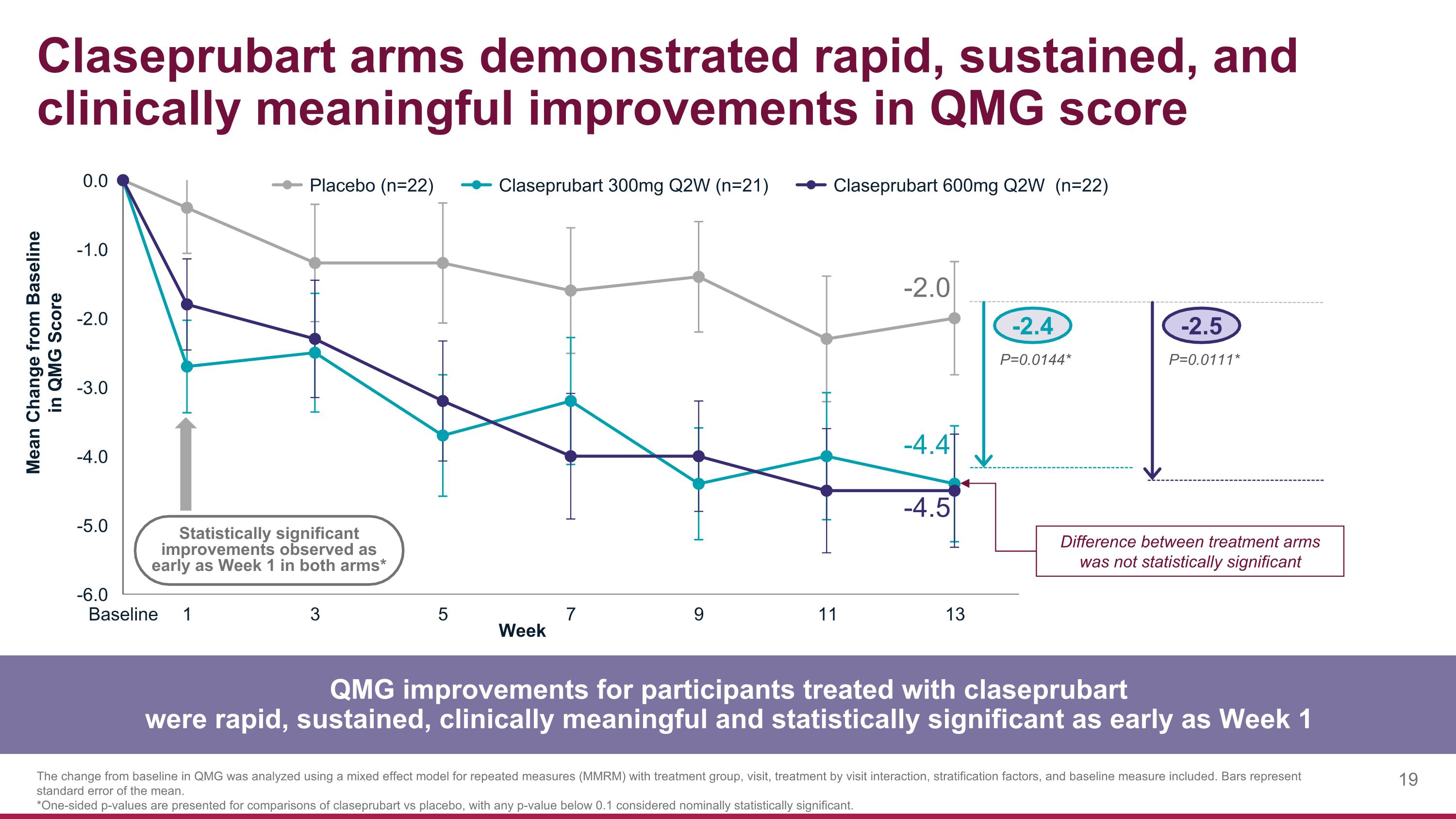
Claseprubart arms demonstrated rapid, sustained, and clinically meaningful improvements in QMG score QMG improvements for participants treated with claseprubart were rapid, sustained, clinically meaningful and statistically significant as early as Week 1 The change from baseline in QMG was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. Bars represent standard error of the mean. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. -2.4 -2.5 P=0.0144* P=0.0111* -2.0 -4.4 -4.5 Statistically significant improvements observed as early as Week 1 in both arms* Difference between treatment arms was not statistically significant
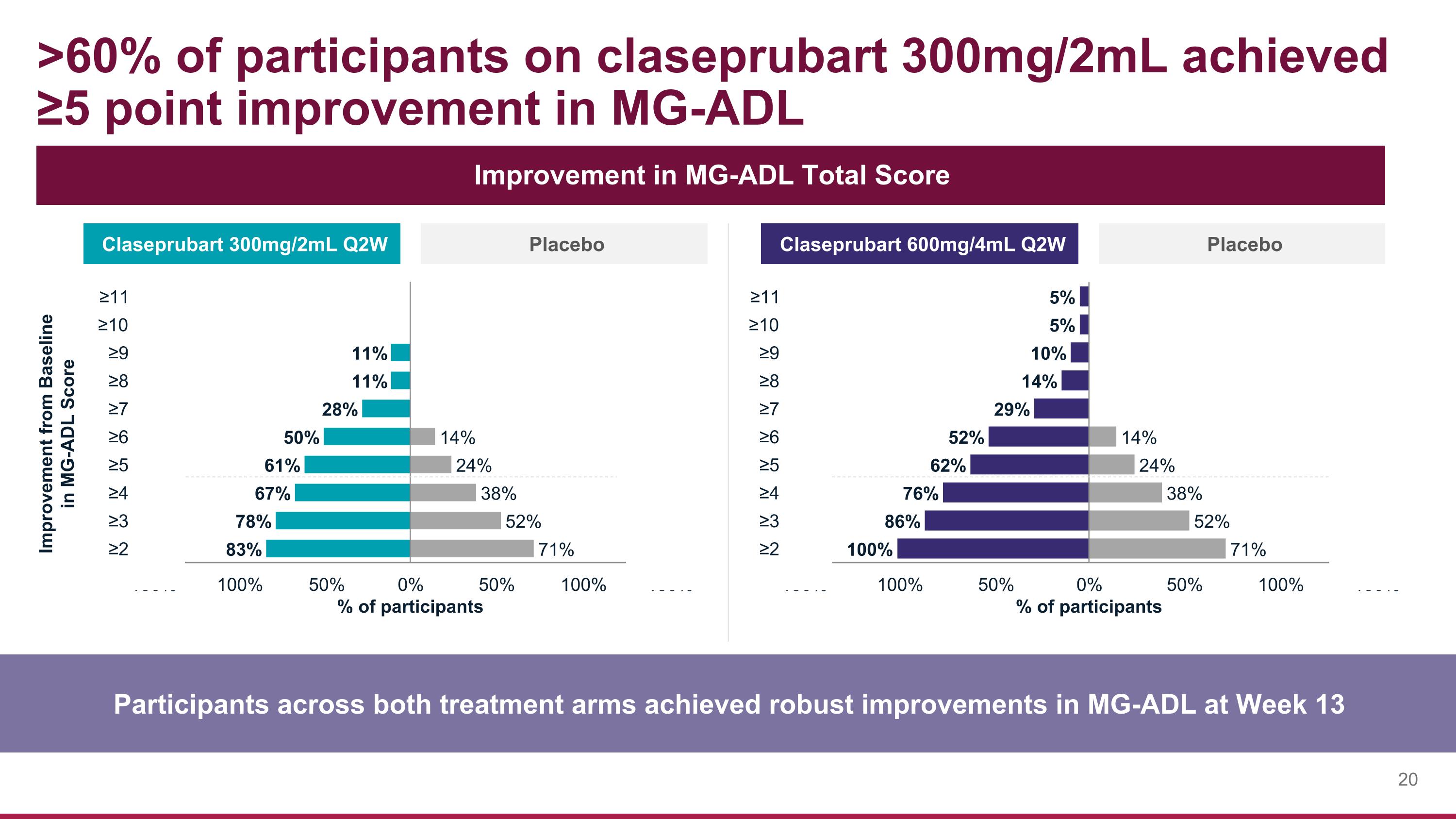
>60% of participants on claseprubart 300mg/2mL achieved ≥5 point improvement in MG-ADL Participants across both treatment arms achieved robust improvements in MG-ADL at Week 13 Improvement in MG-ADL Total Score Claseprubart 300mg/2mL Q2W Placebo Claseprubart 600mg/4mL Q2W Placebo
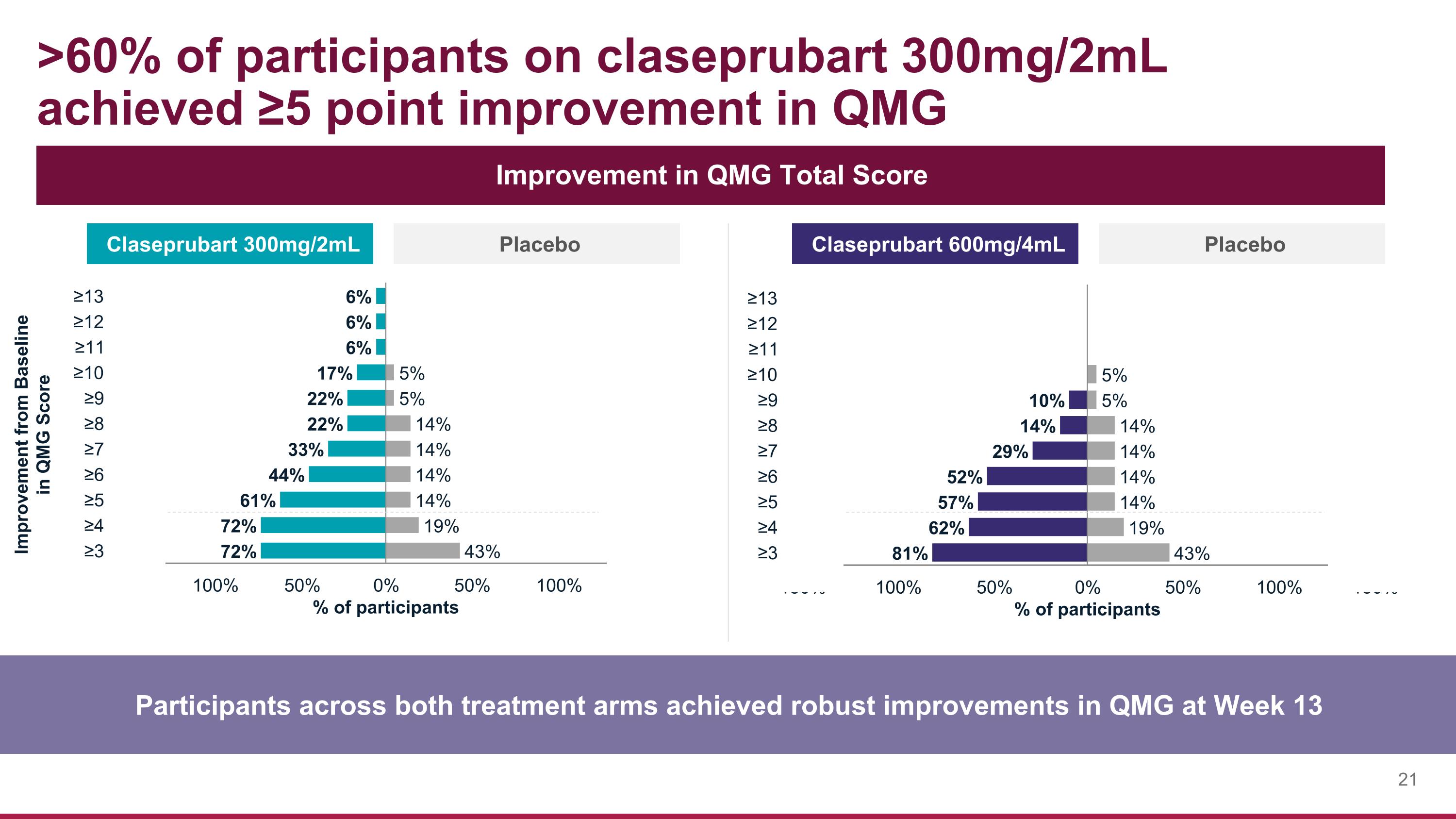
>60% of participants on claseprubart 300mg/2mL achieved ≥5 point improvement in QMG Participants across both treatment arms achieved robust improvements in QMG at Week 13 Improvement in QMG Total Score Claseprubart 300mg/2mL Placebo Claseprubart 600mg/4mL Placebo
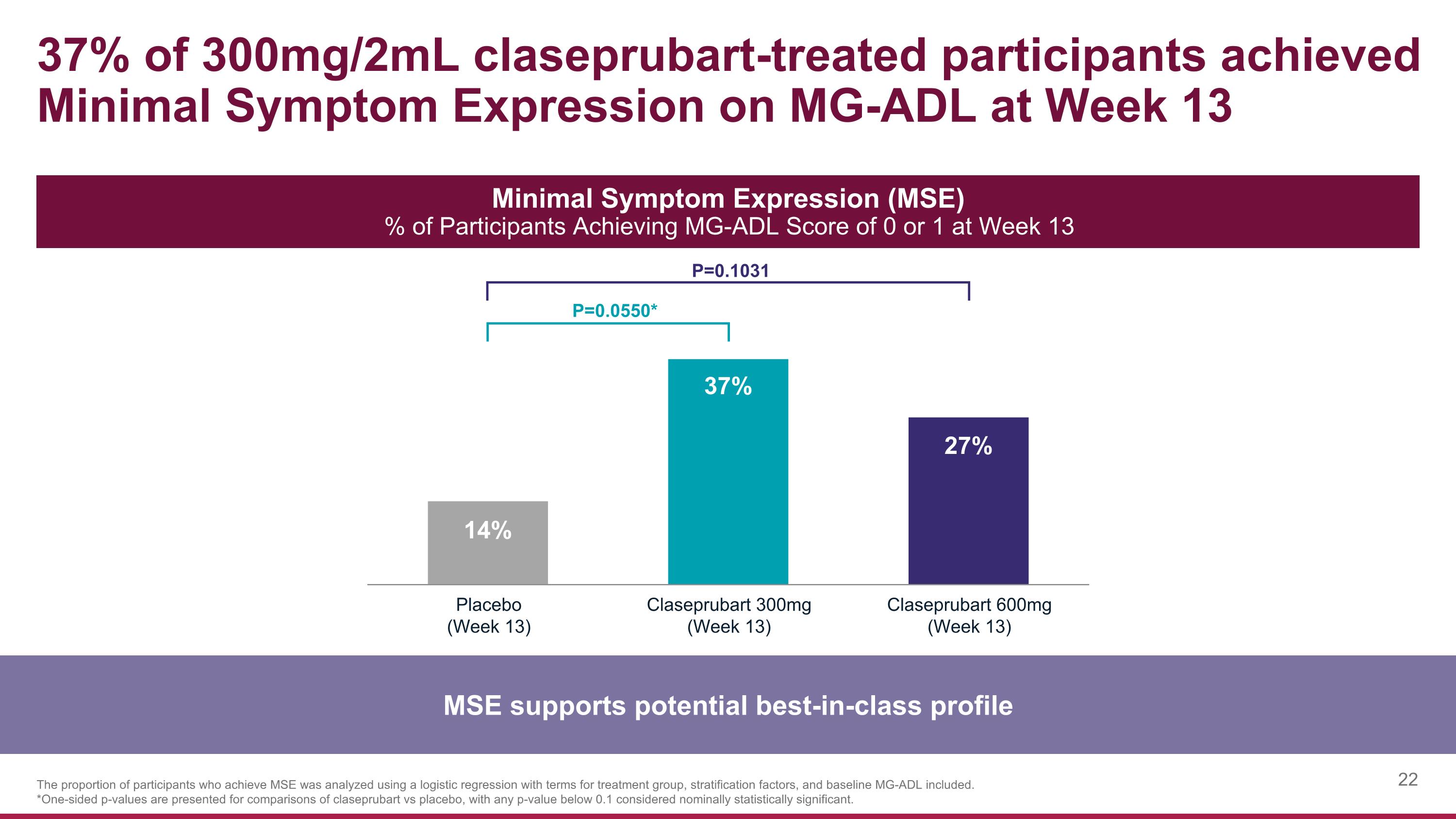
37% of 300mg/2mL claseprubart-treated participants achieved Minimal Symptom Expression on MG-ADL at Week 13 MSE supports potential best-in-class profile Minimal Symptom Expression (MSE) % of Participants Achieving MG-ADL Score of 0 or 1 at Week 13 P=0.0550* P=0.1031 The proportion of participants who achieve MSE was analyzed using a logistic regression with terms for treatment group, stratification factors, and baseline MG-ADL included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant.
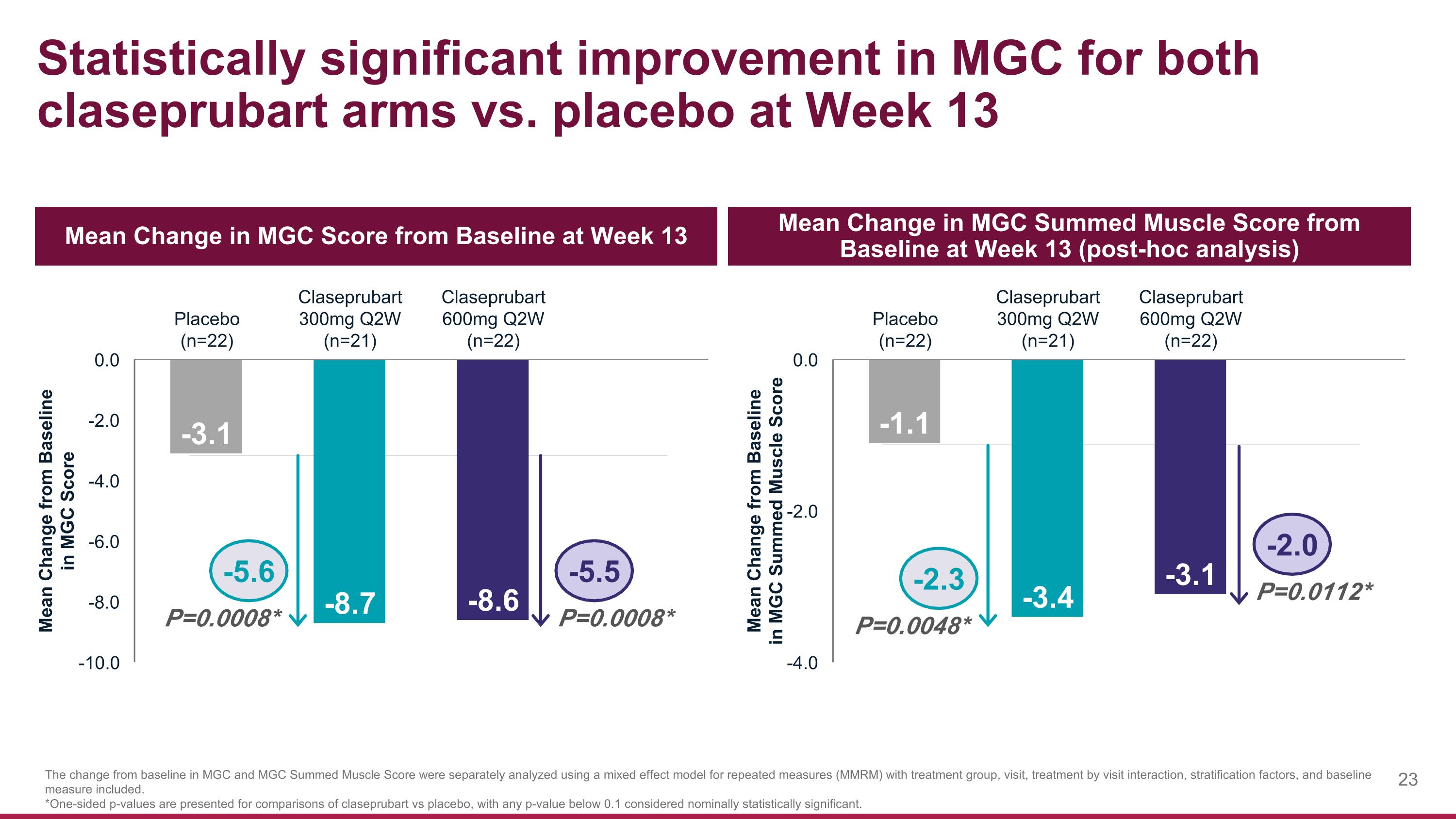
Statistically significant improvement in MGC for both claseprubart arms vs. placebo at Week 13 Mean Change in MGC Score from Baseline at Week 13 Mean Change in MGC Summed Muscle Score from Baseline at Week 13 (post-hoc analysis) The change from baseline in MGC and MGC Summed Muscle Score were separately analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. P=0.0008* -5.5 -5.6 P=0.0008* P=0.0112* -2.0 -2.3 P=0.0048*
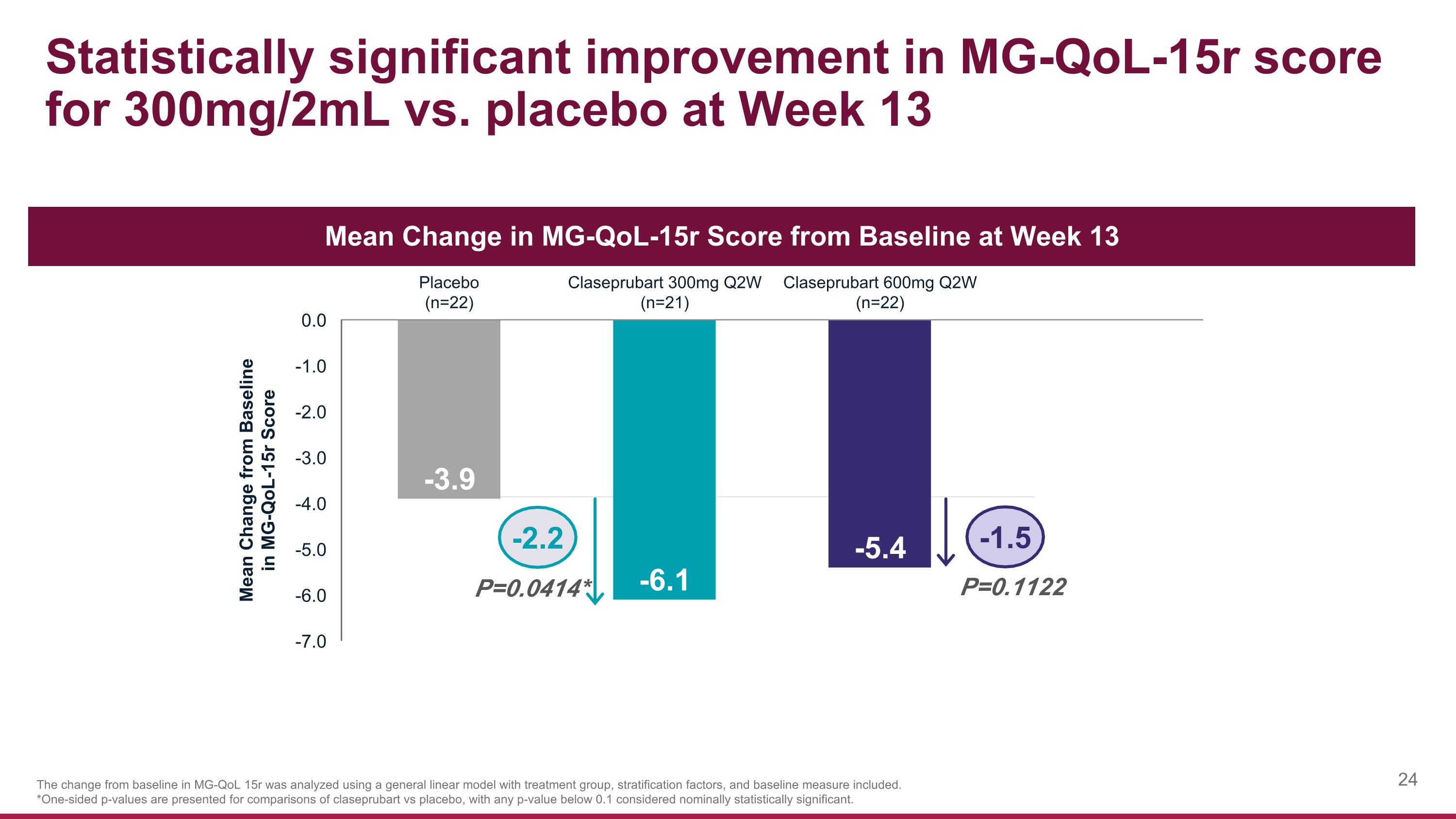
Statistically significant improvement in MG-QoL-15r score for 300mg/2mL vs. placebo at Week 13 The change from baseline in MG-QoL 15r was analyzed using a general linear model with treatment group, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. P=0.0414* P=0.1122 -2.2 -1.5 Mean Change in MG-QoL-15r Score from Baseline at Week 13
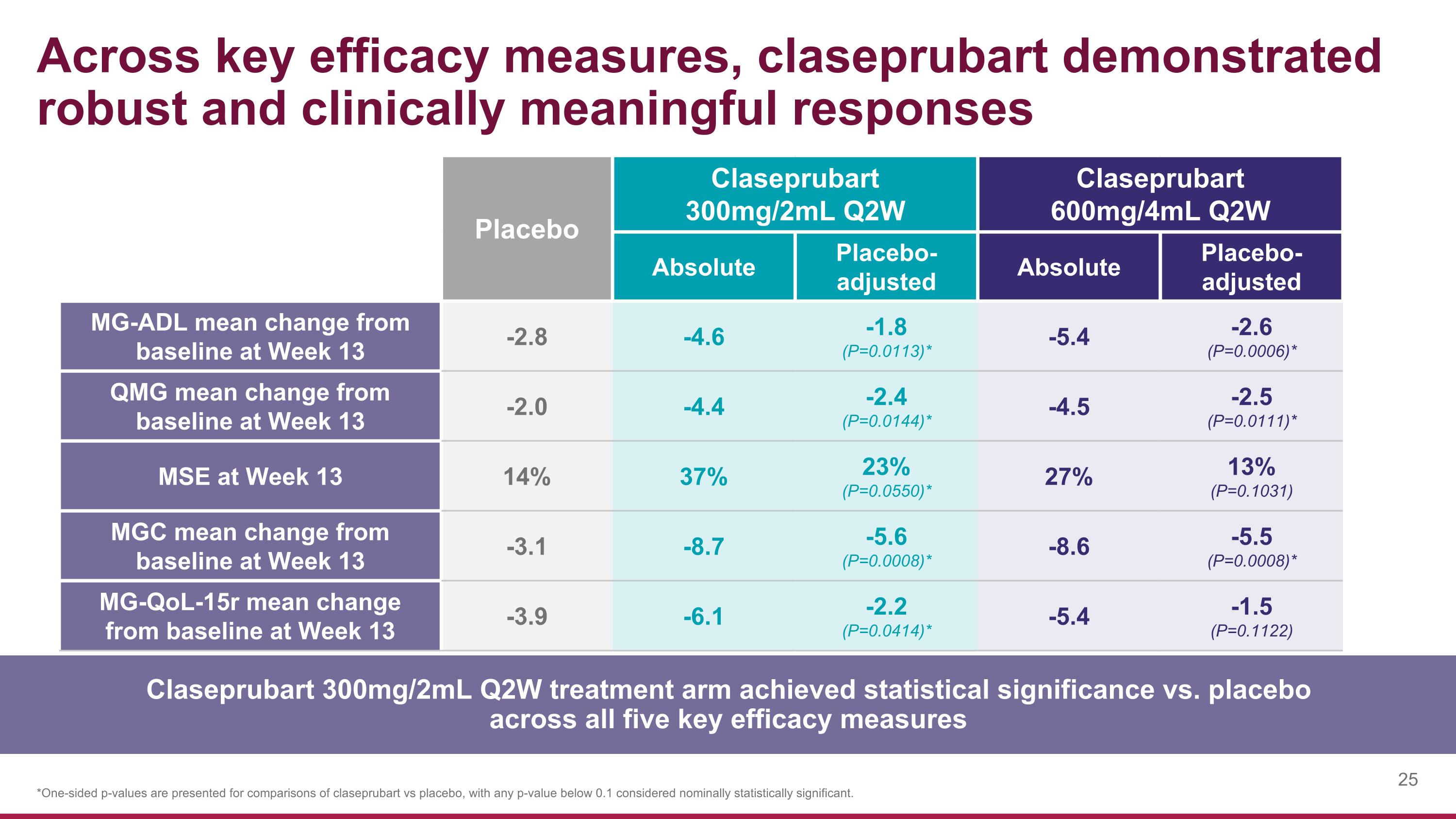
Across key efficacy measures, claseprubart demonstrated robust and clinically meaningful responses Placebo Claseprubart 300mg/2mL Q2W Claseprubart 600mg/4mL Q2W Absolute Placebo-adjusted Absolute Placebo-adjusted MG-ADL mean change from baseline at Week 13 -2.8 -4.6 -1.8 (P=0.0113)* -5.4 -2.6 (P=0.0006)* QMG mean change from baseline at Week 13 -2.0 -4.4 -2.4 (P=0.0144)* -4.5 -2.5 (P=0.0111)* MSE at Week 13 14% 37% 23% (P=0.0550)* 27% 13% (P=0.1031) MGC mean change from baseline at Week 13 -3.1 -8.7 -5.6 (P=0.0008)* -8.6 -5.5 (P=0.0008)* MG-QoL-15r mean change from baseline at Week 13 -3.9 -6.1 -2.2 (P=0.0414)* -5.4 -1.5 (P=0.1122) *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. Claseprubart 300mg/2mL Q2W treatment arm achieved statistical significance vs. placebo across all five key efficacy measures
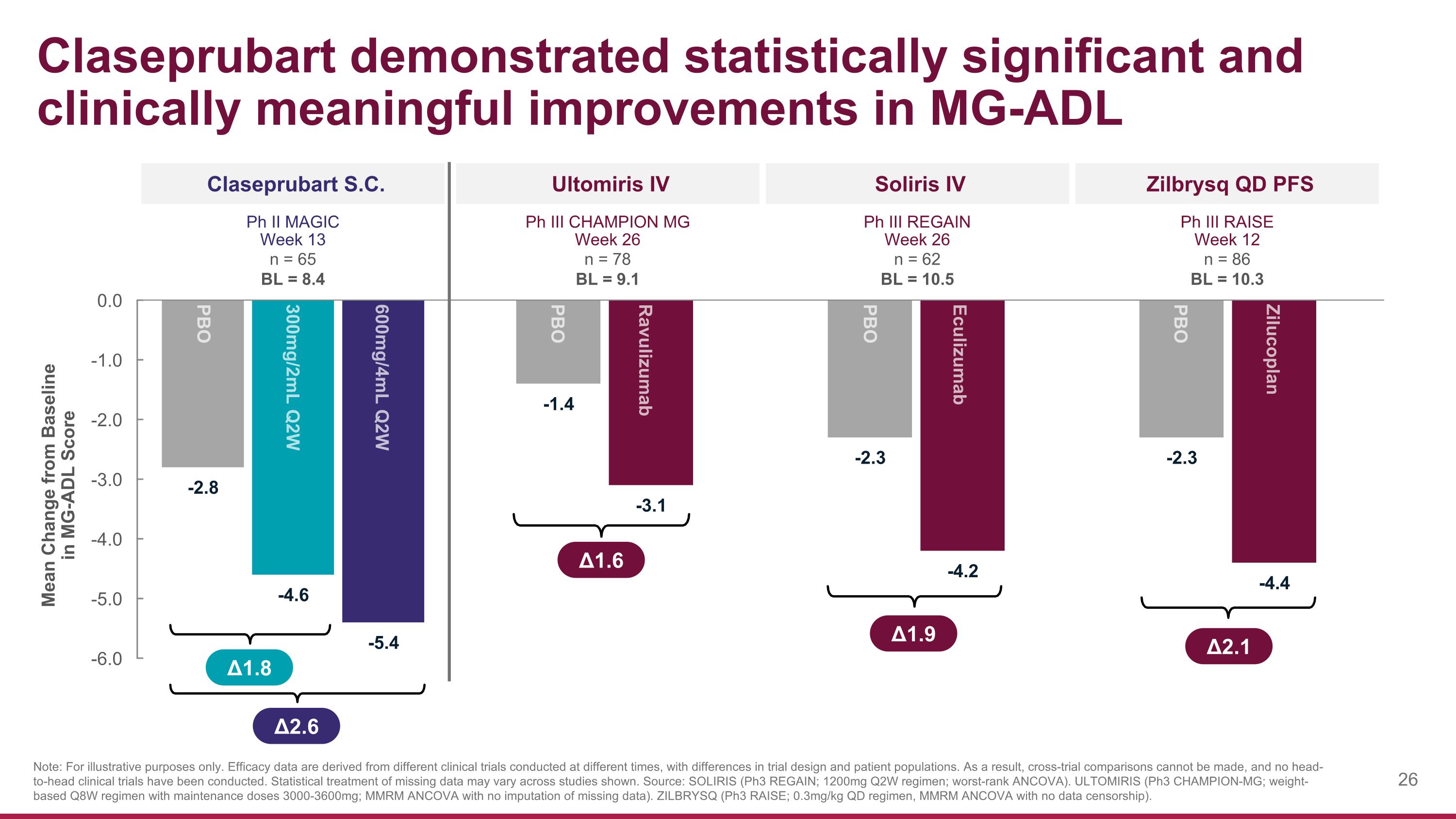
Claseprubart demonstrated statistically significant and clinically meaningful improvements in MG-ADL Claseprubart S.C. Zilbrysq QD PFS Δ1.8 Δ2.6 Note: For illustrative purposes only. Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Statistical treatment of missing data may vary across studies shown. Source: SOLIRIS (Ph3 REGAIN; 1200mg Q2W regimen; worst-rank ANCOVA). ULTOMIRIS (Ph3 CHAMPION-MG; weight-based Q8W regimen with maintenance doses 3000-3600mg; MMRM ANCOVA with no imputation of missing data). ZILBRYSQ (Ph3 RAISE; 0.3mg/kg QD regimen, MMRM ANCOVA with no data censorship). PBO 300mg/2mL Q2W 600mg/4mL Q2W Ph II MAGIC Week 13 n = 65 BL = 8.4 Mean Change from Baseline in MG-ADL Score Soliris IV Ph III REGAIN Week 26 n = 62 BL = 10.5 Ultomiris IV Ph III CHAMPION MG Week 26 n = 78 BL = 9.1 Ph III RAISE Week 12 n = 86 BL = 10.3 Eculizumab PBO Ravulizumab PBO Zilucoplan PBO Δ1.9 Δ1.6 Δ2.1
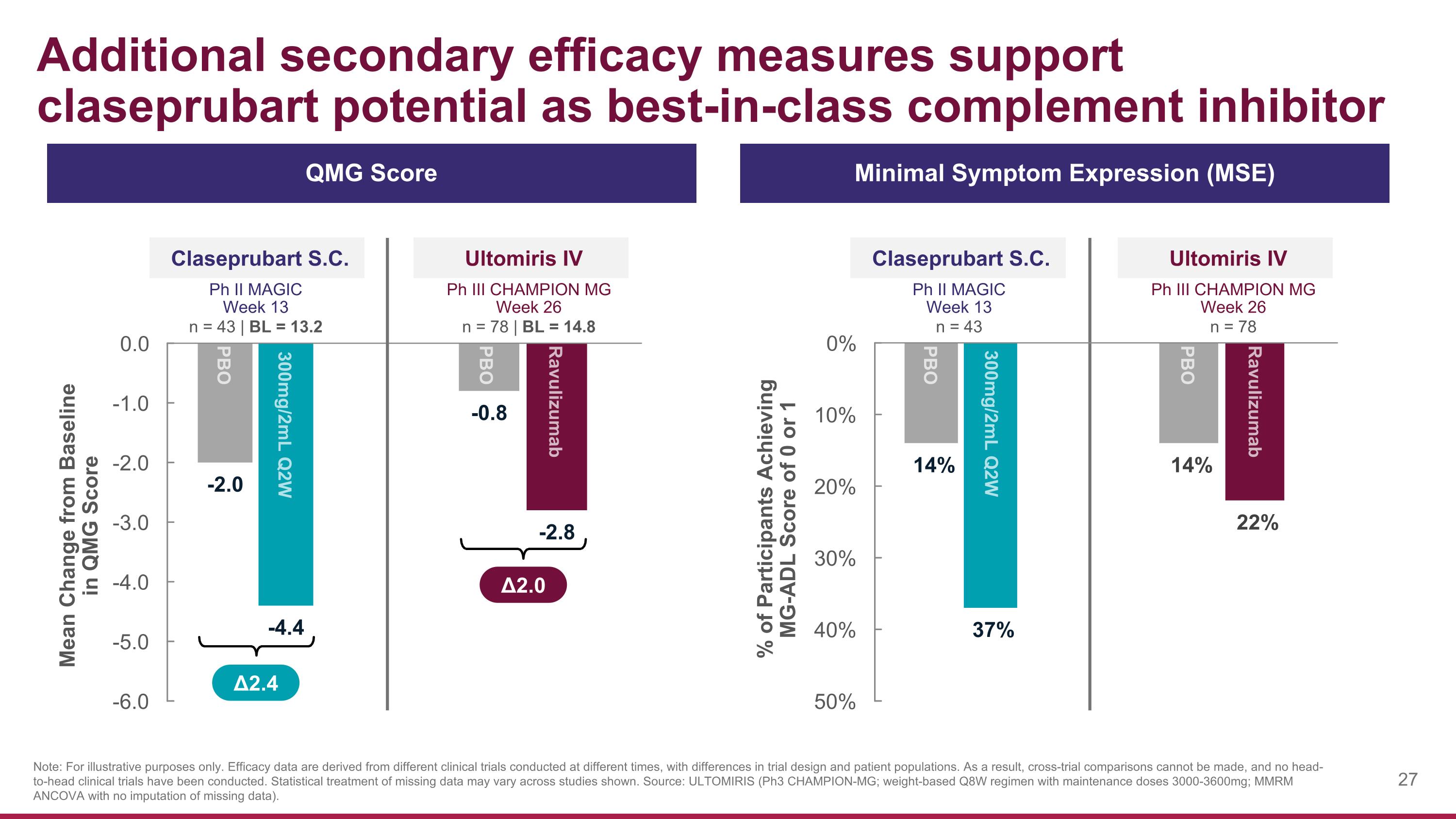
Additional secondary efficacy measures support claseprubart potential as best-in-class complement inhibitor Claseprubart S.C. Ph II MAGIC Week 13 n = 43 | BL = 13.2 Mean Change from Baseline in QMG Score Ultomiris IV Ph III CHAMPION MG Week 26 n = 78 | BL = 14.8 % of Participants Achieving MG-ADL Score of 0 or 1 Claseprubart S.C. Ph II MAGIC Week 13 n = 43 Ultomiris IV Ph III CHAMPION MG Week 26 n = 78 QMG Score Minimal Symptom Expression (MSE) Δ2.4 Note: For illustrative purposes only. Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Statistical treatment of missing data may vary across studies shown. Source: ULTOMIRIS (Ph3 CHAMPION-MG; weight-based Q8W regimen with maintenance doses 3000-3600mg; MMRM ANCOVA with no imputation of missing data). Δ2.0 PBO 300mg/2mL Q2W PBO Ravulizumab Ravulizumab PBO PBO 300mg/2mL Q2W
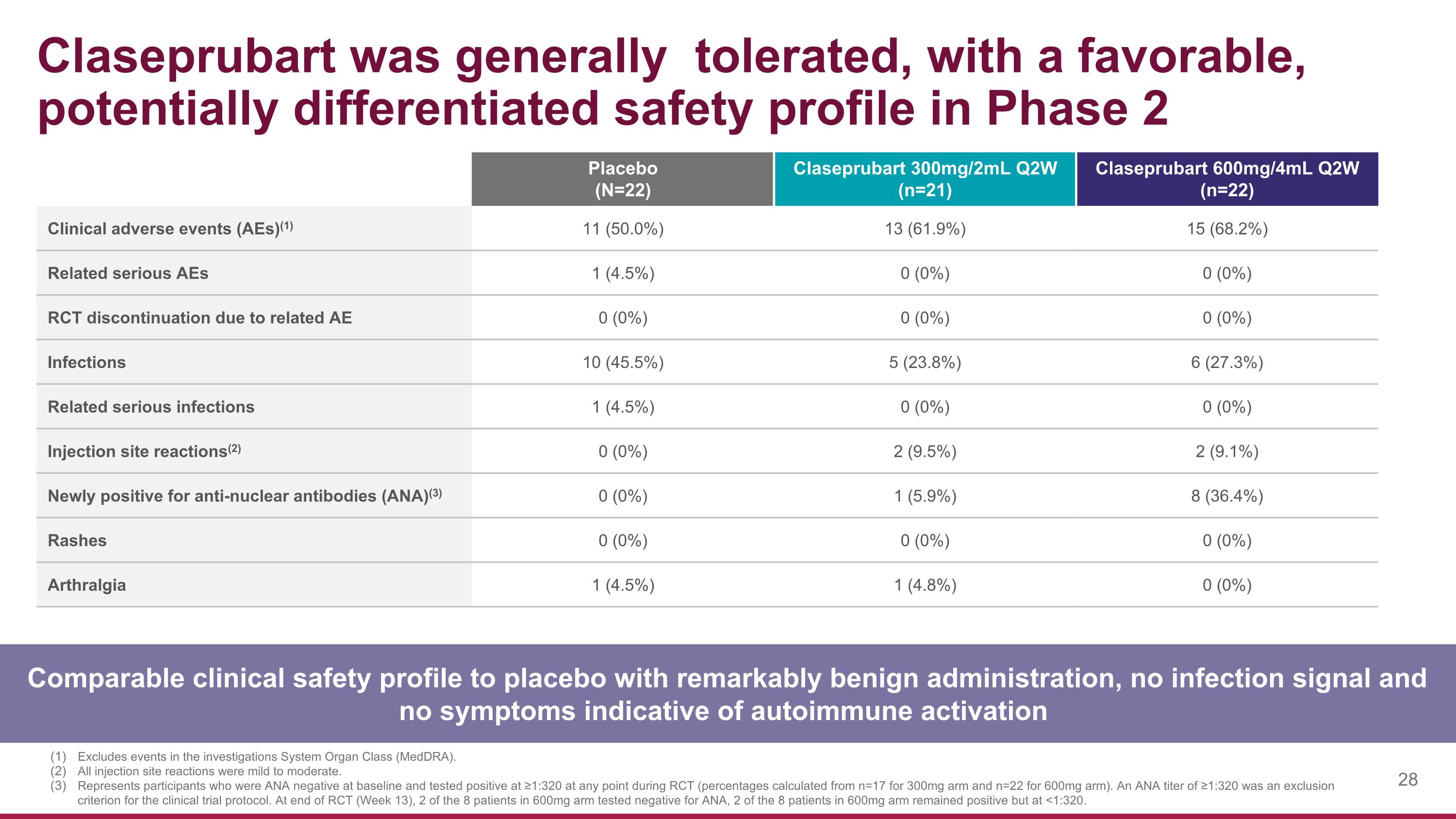
Claseprubart was generally tolerated, with a favorable, potentially differentiated safety profile in Phase 2 Comparable clinical safety profile to placebo with remarkably benign administration, no infection signal and no symptoms indicative of autoimmune activation Placebo (N=22) Claseprubart 300mg/2mL Q2W (n=21) Claseprubart 600mg/4mL Q2W (n=22) Clinical adverse events (AEs)(1) 11 (50.0%) 13 (61.9%) 15 (68.2%) Related serious AEs 1 (4.5%) 0 (0%) 0 (0%) RCT discontinuation due to related AE 0 (0%) 0 (0%) 0 (0%) Infections 10 (45.5%) 5 (23.8%) 6 (27.3%) Related serious infections 1 (4.5%) 0 (0%) 0 (0%) Injection site reactions(2) 0 (0%) 2 (9.5%) 2 (9.1%) Newly positive for anti-nuclear antibodies (ANA)(3) 0 (0%) 1 (5.9%) 8 (36.4%) Rashes 0 (0%) 0 (0%) 0 (0%) Arthralgia 1 (4.5%) 1 (4.8%) 0 (0%) Excludes events in the investigations System Organ Class (MedDRA). All injection site reactions were mild to moderate. Represents participants who were ANA negative at baseline and tested positive at ≥1:320 at any point during RCT (percentages calculated from n=17 for 300mg arm and n=22 for 600mg arm). An ANA titer of ≥1:320 was an exclusion criterion for the clinical trial protocol. At end of RCT (Week 13), 2 of the 8 patients in 600mg arm tested negative for ANA, 2 of the 8 patients in 600mg arm remained positive but at <1:320.

Rationale for Adding 300mg/2mL Q4W Dosing & Screening Patients for QMG > 10 in Proposed Ph. 3 Trial
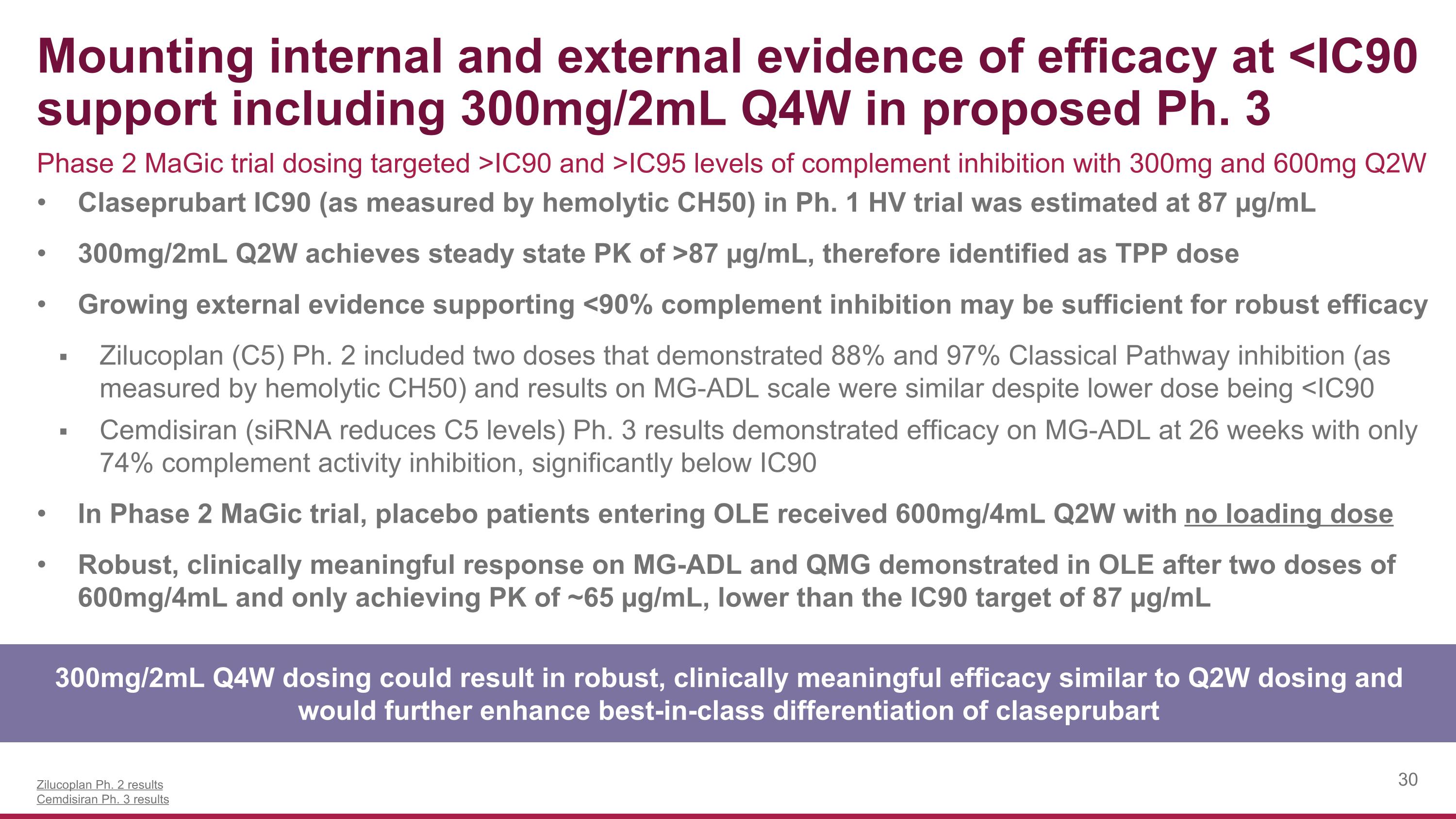
Mounting internal and external evidence of efficacy at <IC90 support including 300mg/2mL Q4W in proposed Ph. 3 300mg/2mL Q4W dosing could result in robust, clinically meaningful efficacy similar to Q2W dosing and would further enhance best-in-class differentiation of claseprubart Claseprubart IC90 (as measured by hemolytic CH50) in Ph. 1 HV trial was estimated at 87 µg/mL 300mg/2mL Q2W achieves steady state PK of >87 µg/mL, therefore identified as TPP dose Growing external evidence supporting <90% complement inhibition may be sufficient for robust efficacy Zilucoplan (C5) Ph. 2 included two doses that demonstrated 88% and 97% Classical Pathway inhibition (as measured by hemolytic CH50) and results on MG-ADL scale were similar despite lower dose being <IC90 Cemdisiran (siRNA reduces C5 levels) Ph. 3 results demonstrated efficacy on MG-ADL at 26 weeks with only 74% complement activity inhibition, significantly below IC90 In Phase 2 MaGic trial, placebo patients entering OLE received 600mg/4mL Q2W with no loading dose Robust, clinically meaningful response on MG-ADL and QMG demonstrated in OLE after two doses of 600mg/4mL and only achieving PK of ~65 µg/mL, lower than the IC90 target of 87 µg/mL Zilucoplan Ph. 2 results Cemdisiran Ph. 3 results Phase 2 MaGic trial dosing targeted >IC90 and >IC95 levels of complement inhibition with 300mg and 600mg Q2W
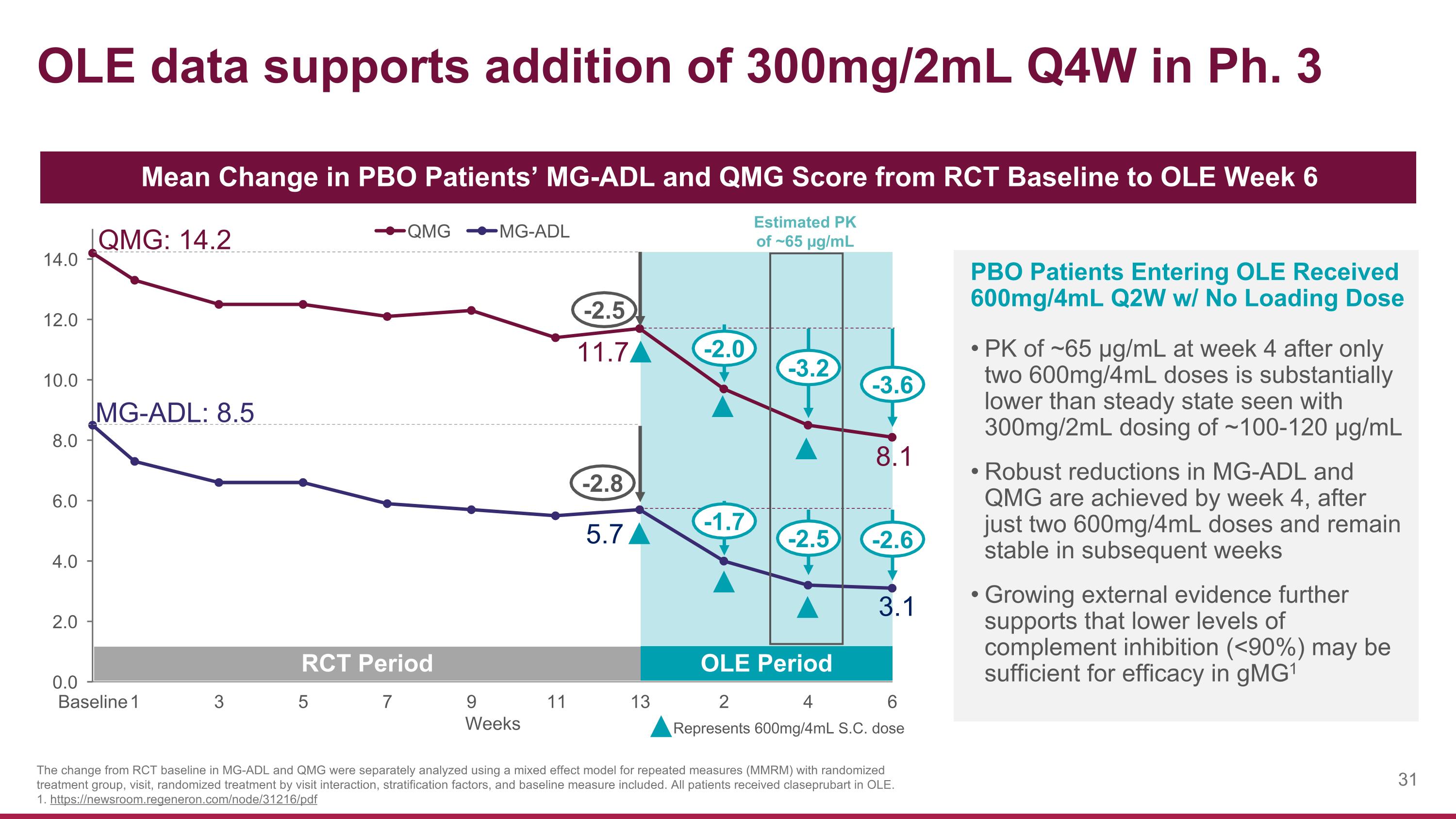
OLE data supports addition of 300mg/2mL Q4W in Ph. 3 Mean Change in PBO Patients’ MG-ADL and QMG Score from RCT Baseline to OLE Week 6 QMG: 14.2 RCT Period OLE Period PBO Patients Entering OLE Received 600mg/4mL Q2W w/ No Loading Dose PK of ~65 µg/mL at week 4 after only two 600mg/4mL doses is substantially lower than steady state seen with 300mg/2mL dosing of ~100-120 µg/mL Robust reductions in MG-ADL and QMG are achieved by week 4, after just two 600mg/4mL doses and remain stable in subsequent weeks Growing external evidence further supports that lower levels of complement inhibition (<90%) may be sufficient for efficacy in gMG1 Represents 600mg/4mL S.C. dose The change from RCT baseline in MG-ADL and QMG were separately analyzed using a mixed effect model for repeated measures (MMRM) with randomized treatment group, visit, randomized treatment by visit interaction, stratification factors, and baseline measure included. All patients received claseprubart in OLE. 1. https://newsroom.regeneron.com/node/31216/pdf 11.7 -2.5 -3.2 -2.0 -3.6 MG-ADL: 8.5 5.7 -2.8 -2.5 -1.7 -2.6 Estimated PK of ~65 µg/mL 3.1 8.1
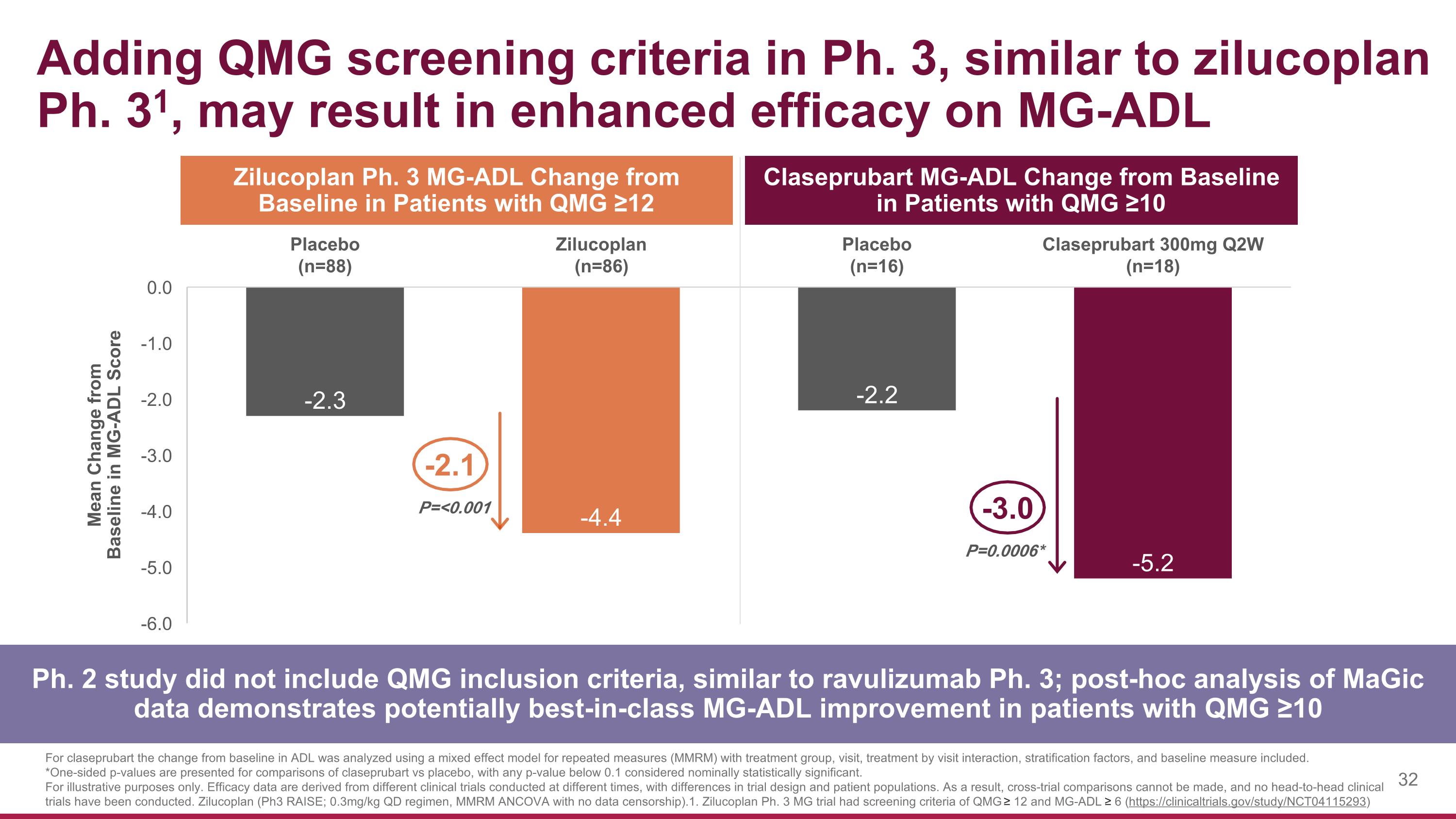
Claseprubart MG-ADL Change from Baseline in Patients with QMG ≥10 Adding QMG screening criteria in Ph. 3, similar to zilucoplan Ph. 31, may result in enhanced efficacy on MG-ADL P=0.0006* -3.0 For claseprubart the change from baseline in ADL was analyzed using a mixed effect model for repeated measures (MMRM) with treatment group, visit, treatment by visit interaction, stratification factors, and baseline measure included. *One-sided p-values are presented for comparisons of claseprubart vs placebo, with any p-value below 0.1 considered nominally statistically significant. For illustrative purposes only. Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Zilucoplan (Ph3 RAISE; 0.3mg/kg QD regimen, MMRM ANCOVA with no data censorship). 1. Zilucoplan Ph. 3 MG trial had screening criteria of QMG ≥ 12 and MG-ADL ≥ 6 (https://clinicaltrials.gov/study/NCT04115293) Ph. 2 study did not include QMG inclusion criteria, similar to ravulizumab Ph. 3; post-hoc analysis of MaGic data demonstrates potentially best-in-class MG-ADL improvement in patients with QMG ≥10 Zilucoplan Ph. 3 MG-ADL Change from Baseline in Patients with QMG ≥12 P=<0.001 -2.1 Mean Change from Baseline in MG-ADL Score
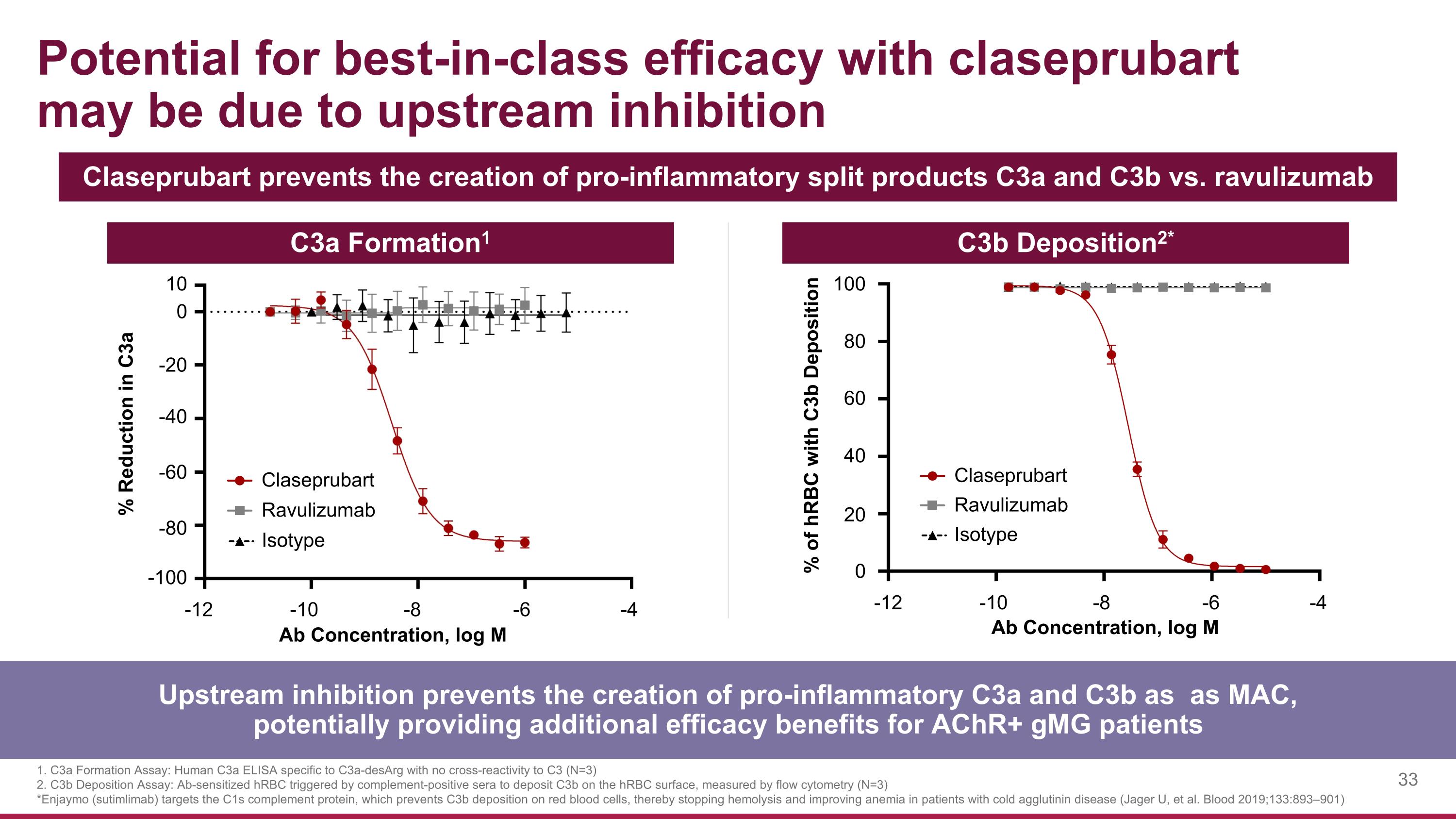
Potential for best-in-class efficacy with claseprubart may be due to upstream inhibition Upstream inhibition prevents the creation of pro-inflammatory C3a and C3b as as MAC, potentially providing additional efficacy benefits for AChR+ gMG patients C3a Formation1 C3b Deposition2* 100 80 60 40 20 0 % of hRBC with C3b Deposition -12 -10 -8 -6 -4 Ab Concentration, log M Claseprubart Isotype Ravulizumab 0 -20 -40 -60 -80 -100 % Reduction in C3a 10 Ab Concentration, log M -12 -10 -8 -6 -4 Claseprubart Isotype Ravulizumab 1. C3a Formation Assay: Human C3a ELISA specific to C3a-desArg with no cross-reactivity to C3 (N=3) 2. C3b Deposition Assay: Ab-sensitized hRBC triggered by complement-positive sera to deposit C3b on the hRBC surface, measured by flow cytometry (N=3) *Enjaymo (sutimlimab) targets the C1s complement protein, which prevents C3b deposition on red blood cells, thereby stopping hemolysis and improving anemia in patients with cold agglutinin disease (Jager U, et al. Blood 2019;133:893–901) Claseprubart prevents the creation of pro-inflammatory split products C3a and C3b vs. ravulizumab
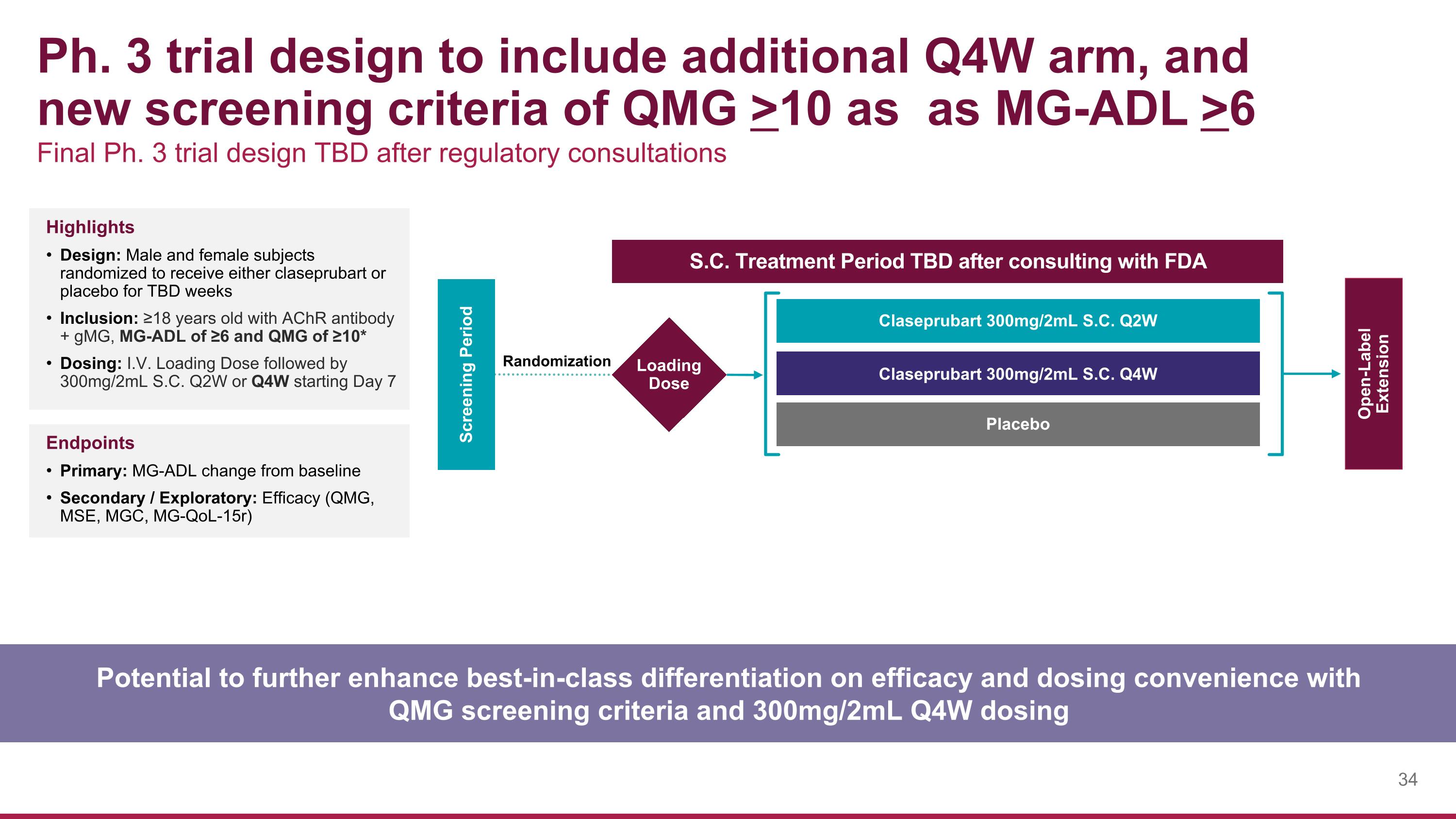
Ph. 3 trial design to include additional Q4W arm, and new screening criteria of QMG >10 as as MG-ADL >6 Potential to further enhance best-in-class differentiation on efficacy and dosing convenience with QMG screening criteria and 300mg/2mL Q4W dosing Final Ph. 3 trial design TBD after regulatory consultations Highlights Design: Male and female subjects randomized to receive either claseprubart or placebo for TBD weeks Inclusion: ≥18 years old with AChR antibody + gMG, MG-ADL of ≥6 and QMG of ≥10* Dosing: I.V. Loading Dose followed by 300mg/2mL S.C. Q2W or Q4W starting Day 7 Endpoints Primary: MG-ADL change from baseline Secondary / Exploratory: Efficacy (QMG, MSE, MGC, MG-QoL-15r) Screening Period Open-Label Extension Claseprubart 300mg/2mL S.C. Q2W Claseprubart 300mg/2mL S.C. Q4W Placebo S.C. Treatment Period TBD after consulting with FDA Loading Dose Randomization
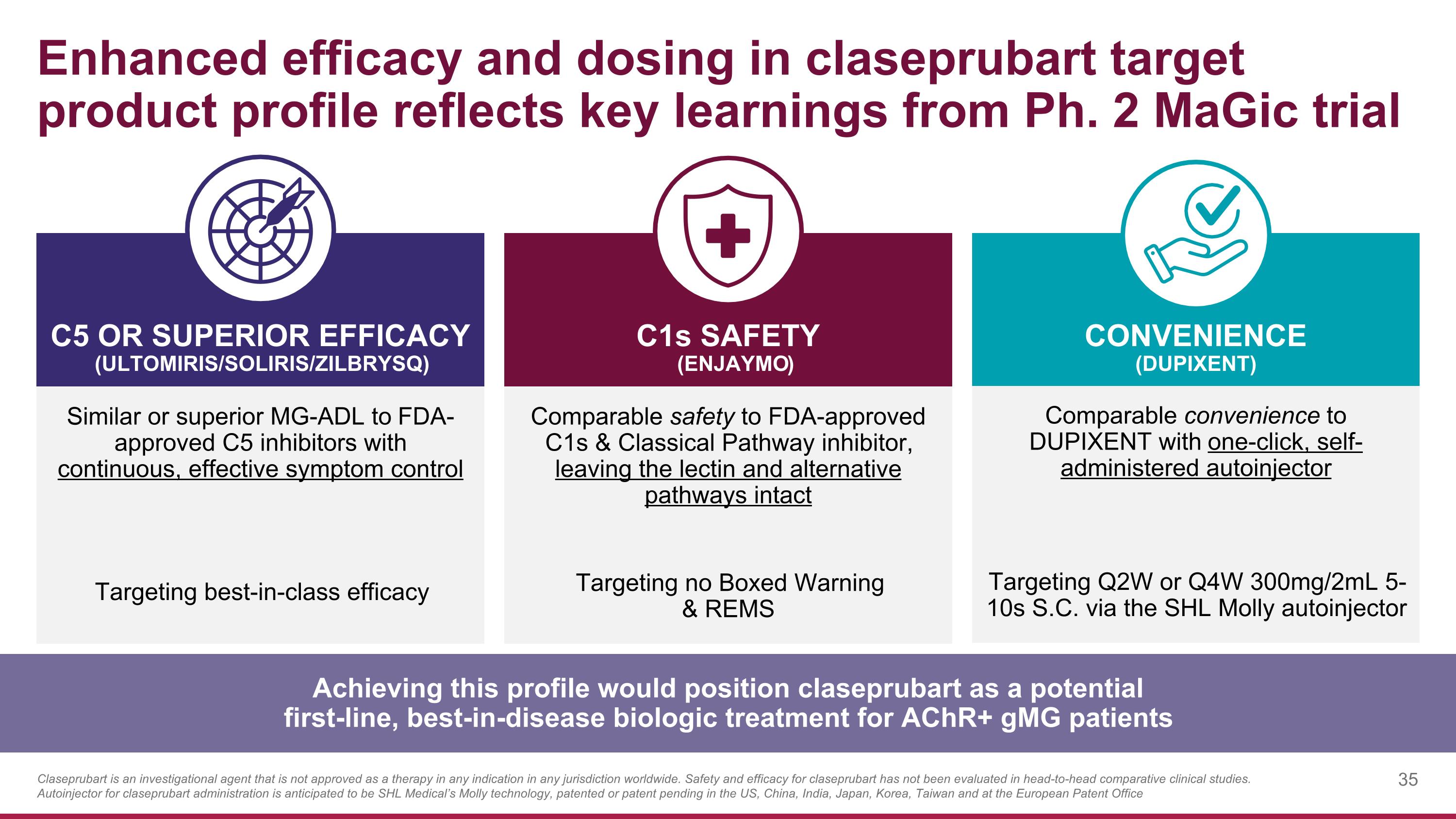
Enhanced efficacy and dosing in claseprubart target product profile reflects key learnings from Ph. 2 MaGic trial Similar or superior MG-ADL to FDA-approved C5 inhibitors with continuous, effective symptom control Targeting best-in-class efficacy C1s SAFETY (Enjaymo) C5 OR SUPERIOR EFFICACY (ULTOMIRIS/SOLIRIS/ZILBRYSQ) CONVENIENCE (DUPIXENT) Comparable safety to FDA-approved C1s & Classical Pathway inhibitor, leaving the lectin and alternative pathways intact Targeting no Boxed Warning & REMS Comparable convenience to DUPIXENT with one-click, self-administered autoinjector Targeting Q2W or Q4W 300mg/2mL 5-10s S.C. via the SHL Molly autoinjector Achieving this profile would position claseprubart as a potential first-line, best-in-disease biologic treatment for AChR+ gMG patients Claseprubart is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide. Safety and efficacy for claseprubart has not been evaluated in head-to-head comparative clinical studies. Autoinjector for claseprubart administration is anticipated to be SHL Medical’s Molly technology, patented or patent pending in the US, China, India, Japan, Korea, Taiwan and at the European Patent Office

Claseprubart Opportunity in Chronic Inflammatory Demyelinating Polyneuropathy
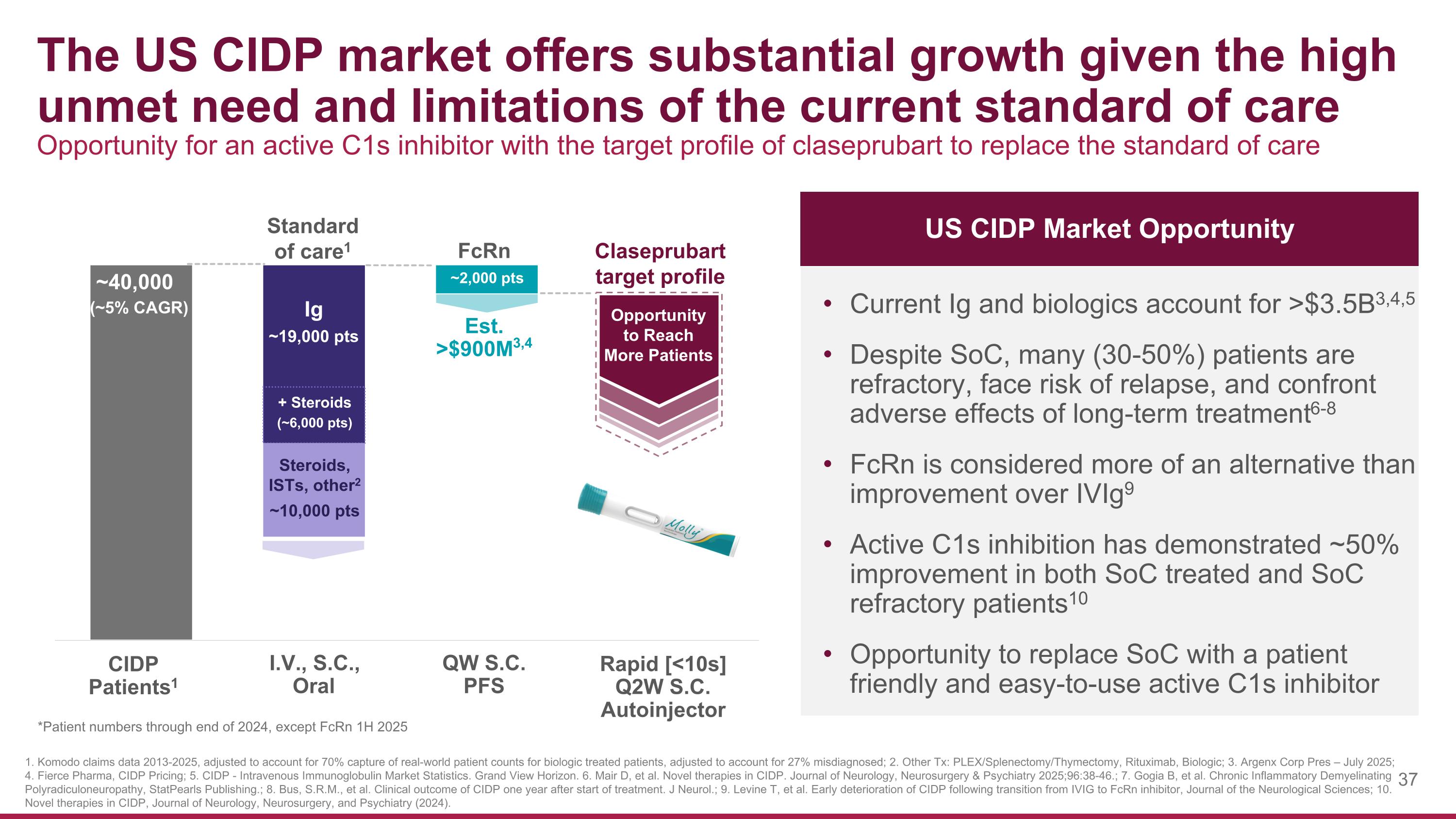
The US CIDP market offers substantial growth given the high unmet need and limitations of the current standard of care Opportunity for an active C1s inhibitor with the target profile of claseprubart to replace the standard of care Current Ig and biologics account for >$3.5B3,4,5 Despite SoC, many (30-50%) patients are refractory, face risk of relapse, and confront adverse effects of long-term treatment6-8 FcRn is considered more of an alternative than improvement over IVIg9 Active C1s inhibition has demonstrated ~50% improvement in both SoC treated and SoC refractory patients10 Opportunity to replace SoC with a patient friendly and easy-to-use active C1s inhibitor US CIDP Market Opportunity 1. Komodo claims data 2013-2025, adjusted to account for 70% capture of real-world patient counts for biologic treated patients, adjusted to account for 27% misdiagnosed; 2. Other Tx: PLEX/Splenectomy/Thymectomy, Rituximab, Biologic; 3. Argenx Corp Pres – July 2025; 4. Fierce Pharma, CIDP Pricing; 5. CIDP - Intravenous Immunoglobulin Market Statistics. Grand View Horizon. 6. Mair D, et al. Novel therapies in CIDP. Journal of Neurology, Neurosurgery & Psychiatry 2025;96:38-46.; 7. Gogia B, et al. Chronic Inflammatory Demyelinating Polyradiculoneuropathy, StatPearls Publishing.; 8. Bus, S.R.M., et al. Clinical outcome of CIDP one year after start of treatment. J Neurol.; 9. Levine T, et al. Early deterioration of CIDP following transition from IVIG to FcRn inhibitor, Journal of the Neurological Sciences; 10. Novel therapies in CIDP, Journal of Neurology, Neurosurgery, and Psychiatry (2024). Claseprubart target profile Est. >$900M3,4 FcRn ~2,000 pts I.V., S.C., Oral QW S.C. PFS Rapid [<10s] Q2W S.C. Autoinjector CIDP Patients1 Opportunity to Reach More Patients Ig ~19,000 pts Steroids, ISTs, other2 ~10,000 pts Standard of care1 ~40,000 (~5% CAGR) + Steroids (~6,000 pts) *Patient numbers through end of 2024, except FcRn 1H 2025
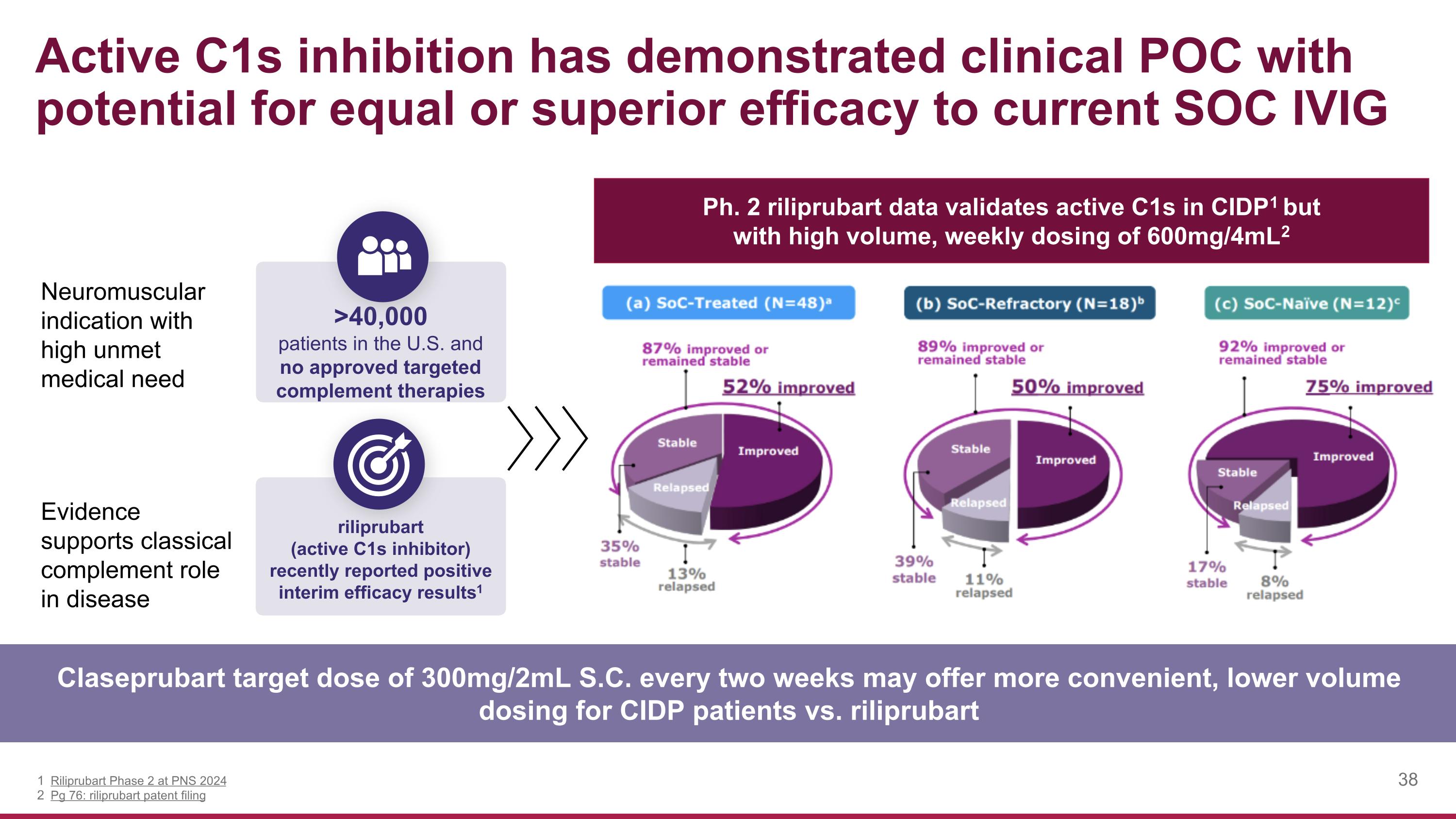
Neuromuscular indication with high unmet medical need Evidence supports classical complement role in disease Active C1s inhibition has demonstrated clinical POC with potential for equal or superior efficacy to current SOC IVIG Claseprubart target dose of 300mg/2mL S.C. every two weeks may offer more convenient, lower volume dosing for CIDP patients vs. riliprubart >40,000 patients in the U.S. and no approved targeted complement therapies riliprubart (active C1s inhibitor) recently reported positive interim efficacy results1 Riliprubart Phase 2 at PNS 2024 Pg 76: riliprubart patent filing Ph. 2 riliprubart data validates active C1s in CIDP1 but with high volume, weekly dosing of 600mg/4mL2
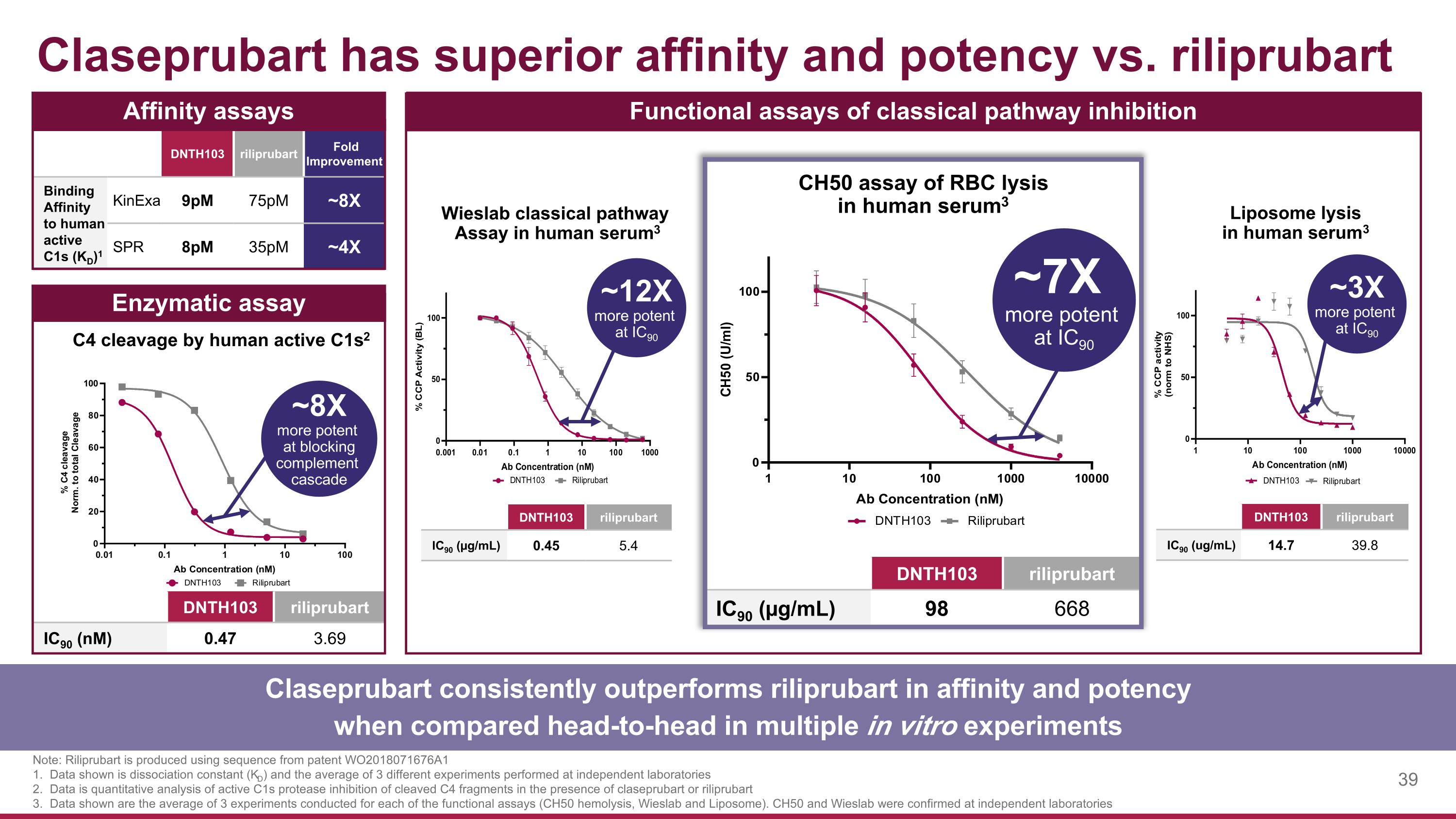
Claseprubart has superior affinity and potency vs. riliprubart Note: Riliprubart is produced using sequence from patent WO2018071676A1 1. Data shown is dissociation constant (KD) and the average of 3 different experiments performed at independent laboratories 2. Data is quantitative analysis of active C1s protease inhibition of cleaved C4 fragments in the presence of claseprubart or riliprubart 3. Data shown are the average of 3 experiments conducted for each of the functional assays (CH50 hemolysis, Wieslab and Liposome). CH50 and Wieslab were confirmed at independent laboratories DNTH103 riliprubart IC90 (nM) 0.47 3.69 C4 cleavage by human active C1s2 Enzymatic assay Functional assays of classical pathway inhibition DNTH103 riliprubart IC90 (µg/mL) 0.45 5.4 Wieslab classical pathway Assay in human serum3 CH50 assay of RBC lysis in human serum3 DNTH103 riliprubart IC90 (µg/mL) 98 668 DNTH103 riliprubart Fold Improvement Binding Affinity to human active C1s (KD)1 KinExa 9pM 75pM ~8X SPR 8pM 35pM ~4X Affinity assays Claseprubart consistently outperforms riliprubart in affinity and potency when compared head-to-head in multiple in vitro experiments ~8X more potent at blocking complement cascade ~12X more potent at IC90 ~7X more potent at IC90 DNTH103 riliprubart IC90 (ug/mL) 14.7 39.8 Liposome lysis in human serum3 ~3X more potent at IC90
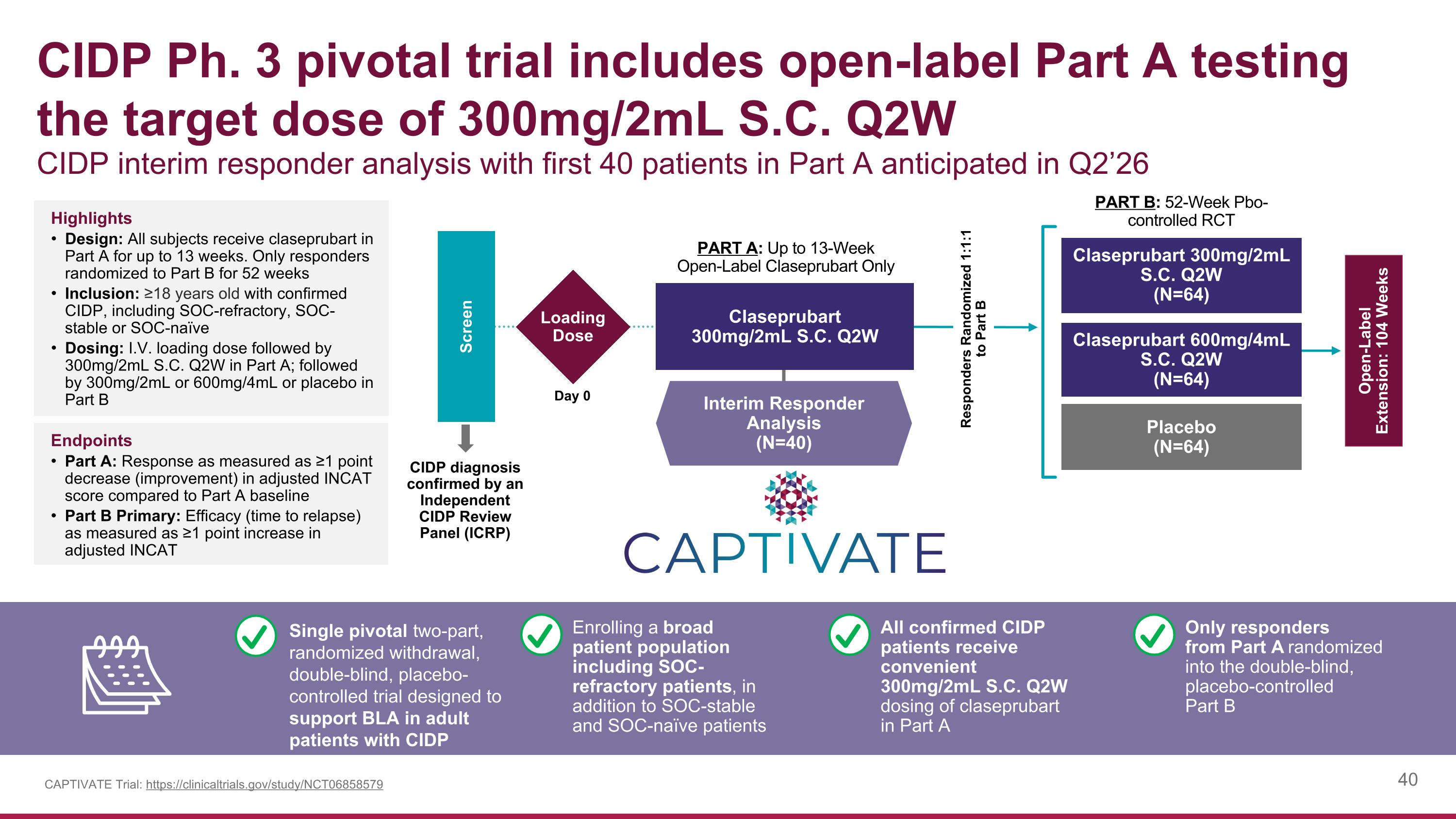
CIDP Ph. 3 pivotal trial includes open-label Part A testing the target dose of 300mg/2mL S.C. Q2W CIDP interim responder analysis with first 40 patients in Part A anticipated in Q2’26 Claseprubart 300mg/2mL S.C. Q2W (N=64) Placebo (N=64) Claseprubart 600mg/4mL S.C. Q2W (N=64) Screen PART A: Up to 13-Week Open-Label Claseprubart Only PART B: 52-Week Pbo- controlled RCT CIDP diagnosis confirmed by an Independent CIDP Review Panel (ICRP) Open-Label Extension: 104 Weeks Loading Dose Day 0 Interim Responder Analysis (N=40) Claseprubart 300mg/2mL S.C. Q2W CAPTIVATE Trial: https://clinicaltrials.gov/study/NCT06858579 Highlights Design: All subjects receive claseprubart in Part A for up to 13 weeks. Only responders randomized to Part B for 52 weeks Inclusion: ≥18 years old with confirmed CIDP, including SOC-refractory, SOC-stable or SOC-naïve Dosing: I.V. loading dose followed by 300mg/2mL S.C. Q2W in Part A; followed by 300mg/2mL or 600mg/4mL or placebo in Part B Endpoints Part A: Response as measured as ≥1 point decrease (improvement) in adjusted INCAT score compared to Part A baseline Part B Primary: Efficacy (time to relapse) as measured as ≥1 point increase in adjusted INCAT Responders Randomized 1:1:1 to Part B Enrolling a broad patient population including SOC-refractory patients, in addition to SOC-stable and SOC-naïve patients All confirmed CIDP patients receive convenient 300mg/2mL S.C. Q2W dosing of claseprubart in Part A Only responders from Part A randomized into the double-blind, placebo-controlled Part B Single pivotal two-part, randomized withdrawal, double-blind, placebo-controlled trial designed to support BLA in adult patients with CIDP

Claseprubart Opportunity in Multifocal Motor Neuropathy
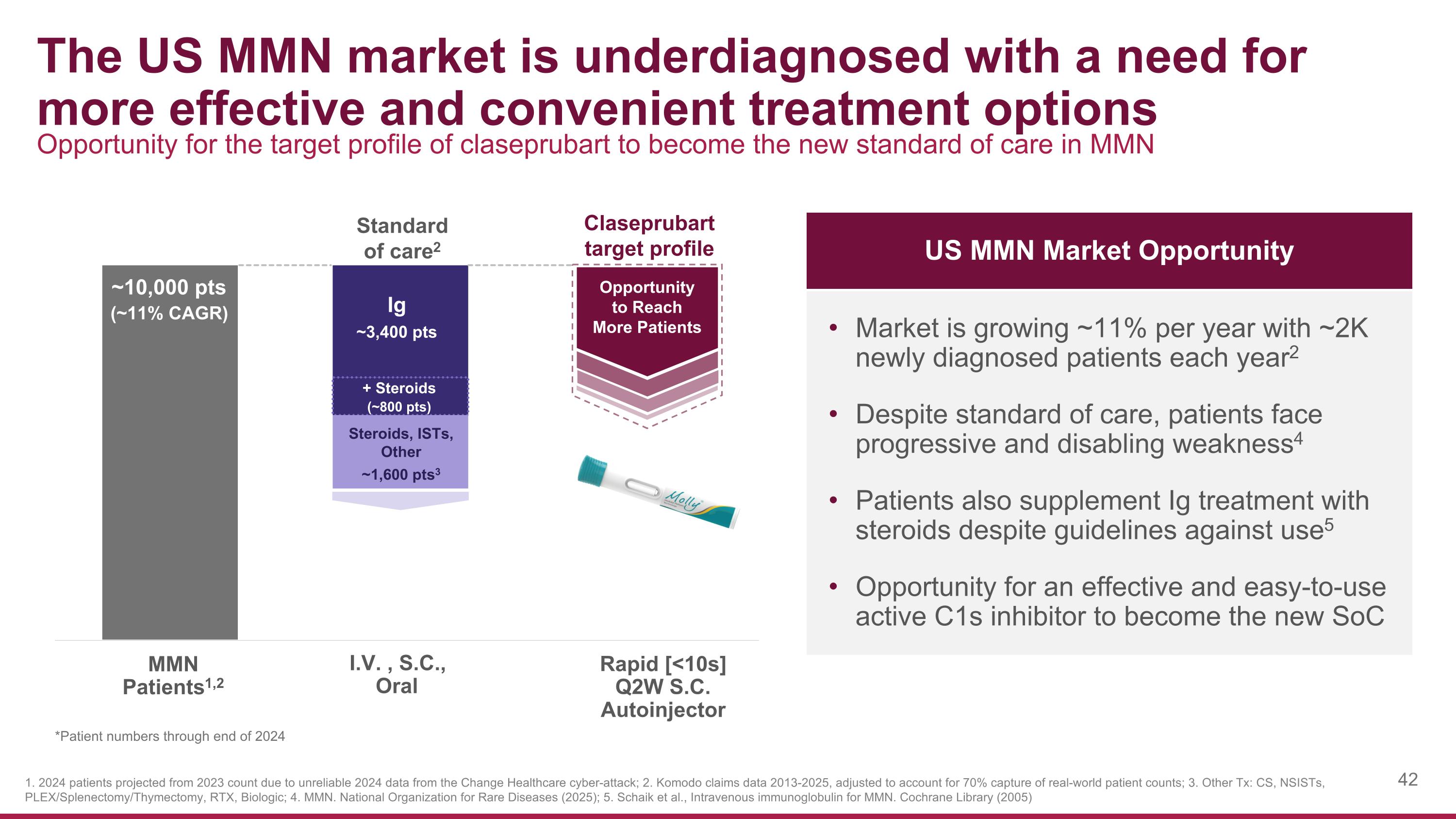
The US MMN market is underdiagnosed with a need for more effective and convenient treatment options US MMN Market Opportunity Opportunity for the target profile of claseprubart to become the new standard of care in MMN 1. 2024 patients projected from 2023 count due to unreliable 2024 data from the Change Healthcare cyber-attack; 2. Komodo claims data 2013-2025, adjusted to account for 70% capture of real-world patient counts; 3. Other Tx: CS, NSISTs, PLEX/Splenectomy/Thymectomy, RTX, Biologic; 4. MMN. National Organization for Rare Diseases (2025); 5. Schaik et al., Intravenous immunoglobulin for MMN. Cochrane Library (2005) Market is growing ~11% per year with ~2K newly diagnosed patients each year2 Despite standard of care, patients face progressive and disabling weakness4 Patients also supplement Ig treatment with steroids despite guidelines against use5 Opportunity for an effective and easy-to-use active C1s inhibitor to become the new SoC Claseprubart target profile I.V. , S.C., Oral Rapid [<10s] Q2W S.C. Autoinjector MMN Patients1,2 Opportunity to Reach More Patients Ig ~3,400 pts Standard of care2 ~10,000 pts (~11% CAGR) Steroids, ISTs, Other ~1,600 pts3 + Steroids (~800 pts) *Patient numbers through end of 2024
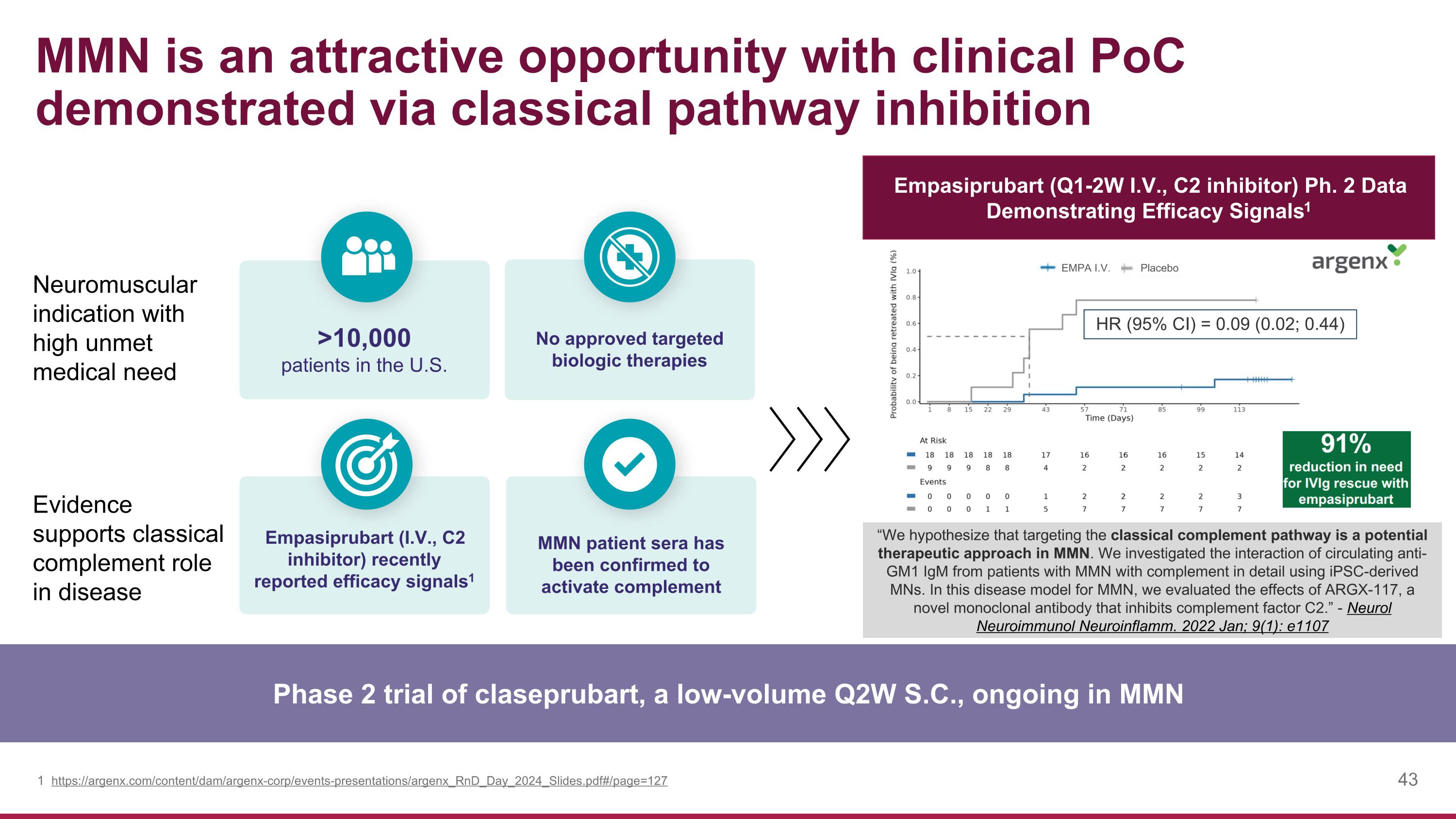
Neuromuscular indication with high unmet medical need Evidence supports classical complement role in disease MMN is an attractive opportunity with clinical PoC demonstrated via classical pathway inhibition Phase 2 trial of claseprubart, a low-volume Q2W S.C., ongoing in MMN No approved targeted biologic therapies Empasiprubart (I.V., C2 inhibitor) recently reported efficacy signals1 MMN patient sera has been confirmed to activate complement >10,000 patients in the U.S. https://argenx.com/content/dam/argenx-corp/events-presentations/argenx_RnD_Day_2024_Slides.pdf#/page=127 Empasiprubart (Q1-2W I.V., C2 inhibitor) Ph. 2 Data Demonstrating Efficacy Signals1 “We hypothesize that targeting the classical complement pathway is a potential therapeutic approach in MMN. We investigated the interaction of circulating anti-GM1 IgM from patients with MMN with complement in detail using iPSC-derived MNs. In this disease model for MMN, we evaluated the effects of ARGX-117, a novel monoclonal antibody that inhibits complement factor C2.” - Neurol Neuroimmunol Neuroinflamm. 2022 Jan; 9(1): e1107 Placebo EMPA I.V.
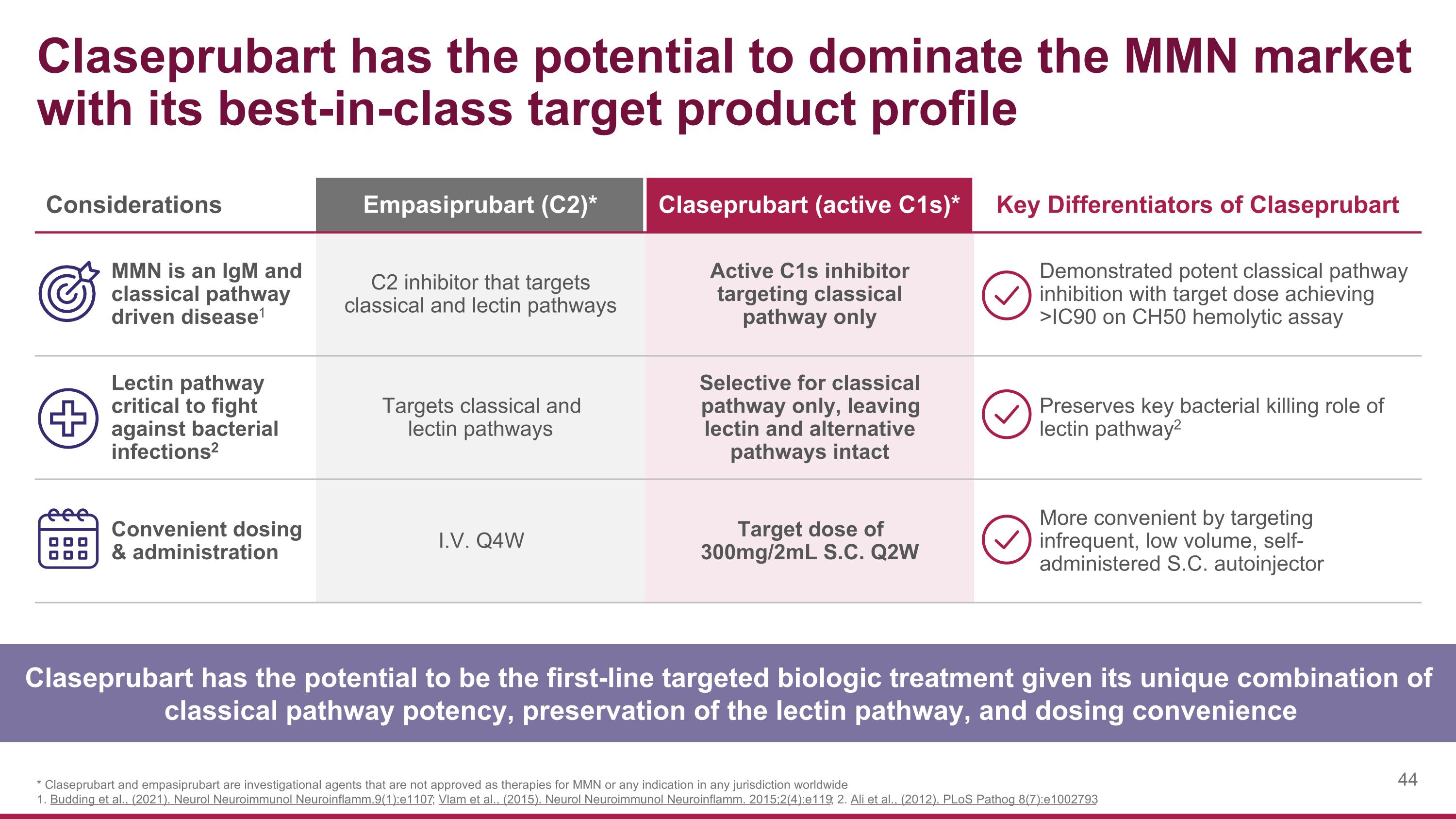
Considerations Empasiprubart (C2)* Claseprubart (active C1s)* Key Differentiators of Claseprubart MMN is an IgM and classical pathway driven disease1 C2 inhibitor that targets classical and lectin pathways Active C1s inhibitor targeting classical pathway only Demonstrated potent classical pathway inhibition with target dose achieving >IC90 on CH50 hemolytic assay Lectin pathway critical to fight against bacterial infections2 Targets classical and lectin pathways Selective for classical pathway only, leaving lectin and alternative pathways intact Preserves key bacterial killing role of lectin pathway2 Convenient dosing & administration I.V. Q4W Target dose of 300mg/2mL S.C. Q2W More convenient by targeting infrequent, low volume, self-administered S.C. autoinjector Claseprubart has the potential to dominate the MMN market with its best-in-class target product profile Claseprubart has the potential to be the first-line targeted biologic treatment given its unique combination of classical pathway potency, preservation of the lectin pathway, and dosing convenience * Claseprubart and empasiprubart are investigational agents that are not approved as therapies for MMN or any indication in any jurisdiction worldwide 1. Budding et al., (2021). Neurol Neuroimmunol Neuroinflamm.9(1):e1107; Vlam et al., (2015). Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e119; 2. Ali et al., (2012). PLoS Pathog 8(7):e1002793
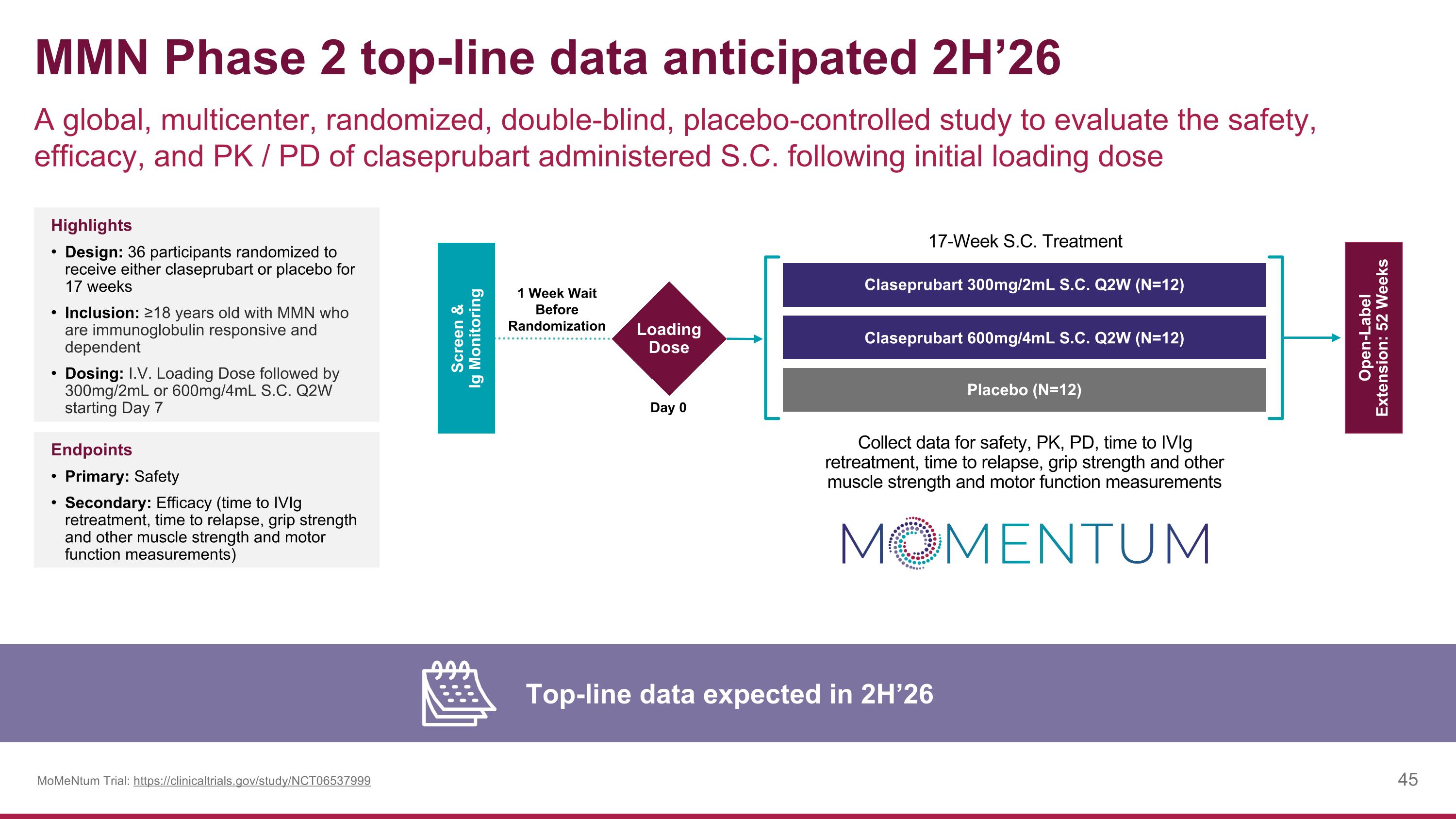
MMN Phase 2 top-line data anticipated 2H’26 A global, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety, efficacy, and PK / PD of claseprubart administered S.C. following initial loading dose Top-line data expected in 2H’26 MoMeNtum Trial: https://clinicaltrials.gov/study/NCT06537999 Highlights Design: 36 participants randomized to receive either claseprubart or placebo for 17 weeks Inclusion: ≥18 years old with MMN who are immunoglobulin responsive and dependent Dosing: I.V. Loading Dose followed by 300mg/2mL or 600mg/4mL S.C. Q2W starting Day 7 Endpoints Primary: Safety Secondary: Efficacy (time to IVIg retreatment, time to relapse, grip strength and other muscle strength and motor function measurements) Screen & Ig Monitoring Day 0 Open-Label Extension: 52 Weeks Claseprubart 300mg/2mL S.C. Q2W (N=12) Claseprubart 600mg/4mL S.C. Q2W (N=12) Placebo (N=12) 17-Week S.C. Treatment Collect data for safety, PK, PD, time to IVIg retreatment, time to relapse, grip strength and other muscle strength and motor function measurements Loading Dose 1 Week Wait Before Randomization

DNTH212: Potential Best-in-Class Bispecific Fusion Protein for Multiple Autoimmune Indications
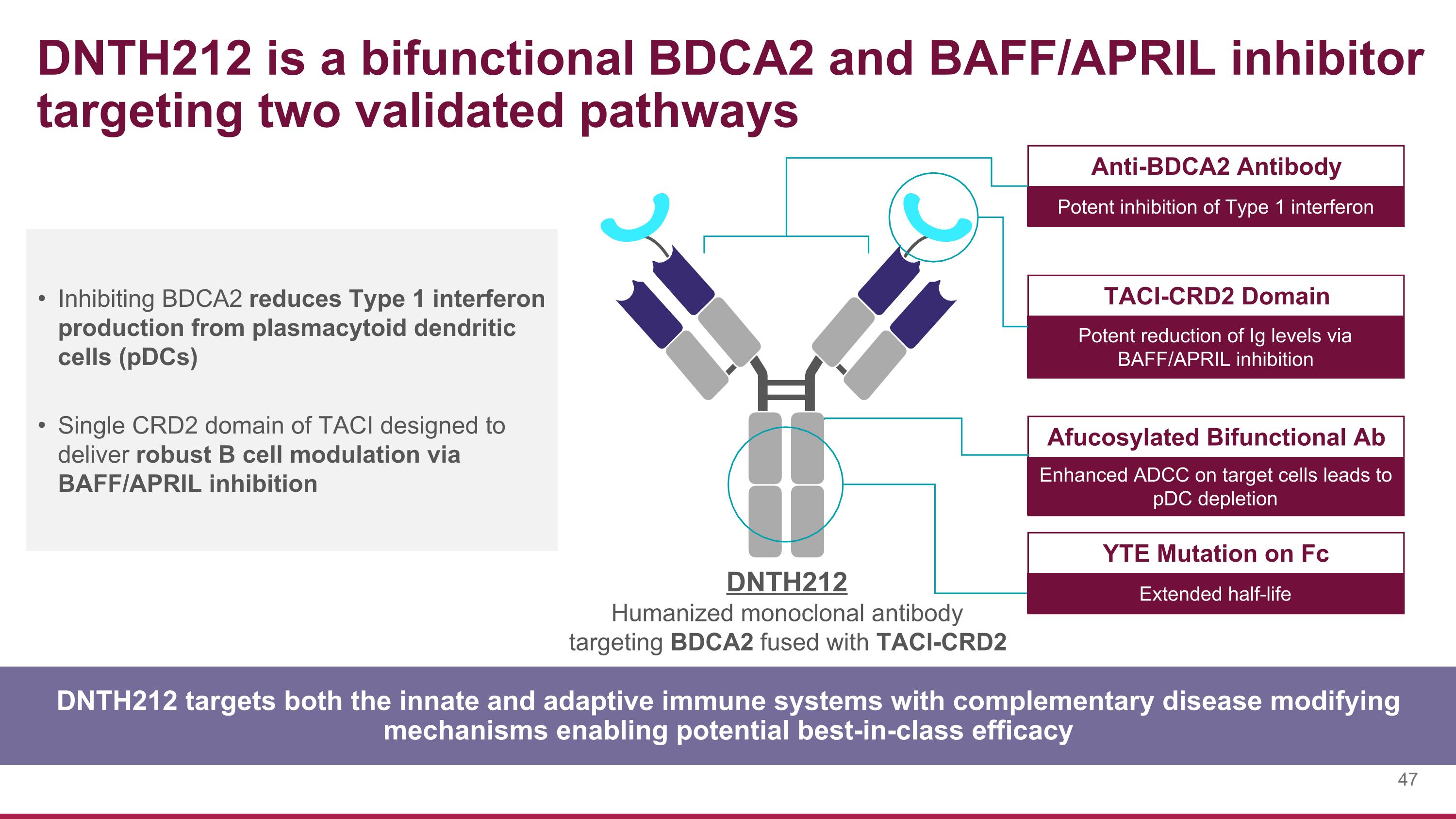
Inhibiting BDCA2 reduces Type 1 interferon production from plasmacytoid dendritic cells (pDCs) Single CRD2 domain of TACI designed to deliver robust B cell modulation via BAFF/APRIL inhibition DNTH212 targets both the innate and adaptive immune systems with complementary disease modifying mechanisms enabling potential best-in-class efficacy DNTH212 is a bifunctional BDCA2 and BAFF/APRIL inhibitor targeting two validated pathways Anti-BDCA2 Antibody Potent inhibition of Type 1 interferon TACI-CRD2 Domain Potent reduction of Ig levels via BAFF/APRIL inhibition Afucosylated Bifunctional Ab Enhanced ADCC on target cells leads to pDC depletion YTE Mutation on Fc Extended half-life DNTH212 Humanized monoclonal antibody targeting BDCA2 fused with TACI-CRD2
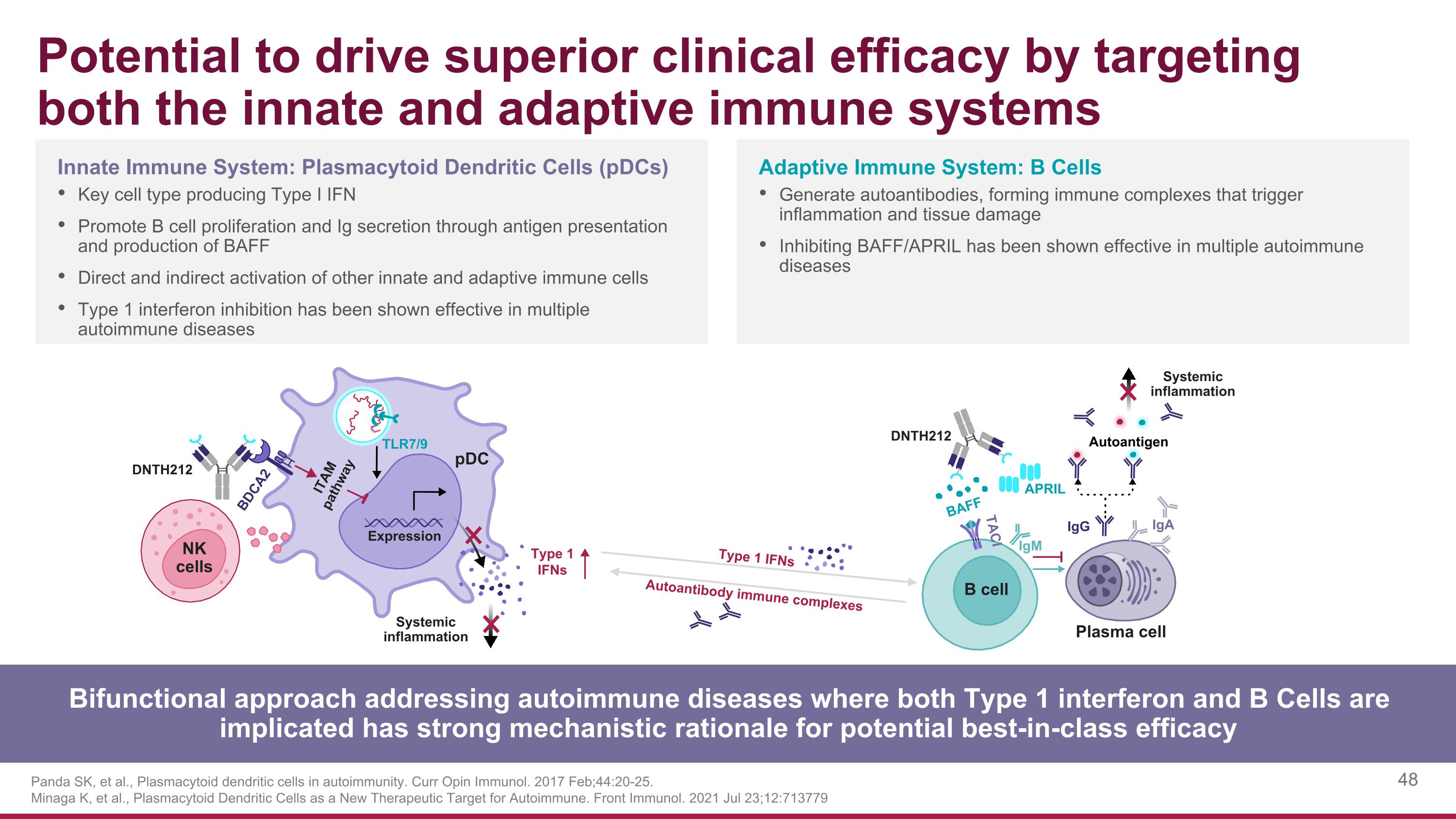
Potential to drive superior clinical efficacy by targeting both the innate and adaptive immune systems Panda SK, et al., Plasmacytoid dendritic cells in autoimmunity. Curr Opin Immunol. 2017 Feb;44:20-25. Minaga K, et al., Plasmacytoid Dendritic Cells as a New Therapeutic Target for Autoimmune. Front Immunol. 2021 Jul 23;12:713779 Bifunctional approach addressing autoimmune diseases where both Type 1 interferon and B Cells are implicated has strong mechanistic rationale for potential best-in-class efficacy Adaptive Immune System: B Cells Generate autoantibodies, forming immune complexes that trigger inflammation and tissue damage Inhibiting BAFF/APRIL has been shown effective in multiple autoimmune diseases Innate Immune System: Plasmacytoid Dendritic Cells (pDCs) Key cell type producing Type I IFN Promote B cell proliferation and Ig secretion through antigen presentation and production of BAFF Direct and indirect activation of other innate and adaptive immune cells Type 1 interferon inhibition has been shown effective in multiple autoimmune diseases DNTH212 Systemic inflammation DNTH212 Systemic inflammation Expression BDCA2 ITAM pathway pDC Type 1 IFNs BAFF TACI IgM APRIL IgG IgA B cell NK cells TLR7/9 Autoantigen Plasma cell Type 1 IFNs Autoantibody immune complexes
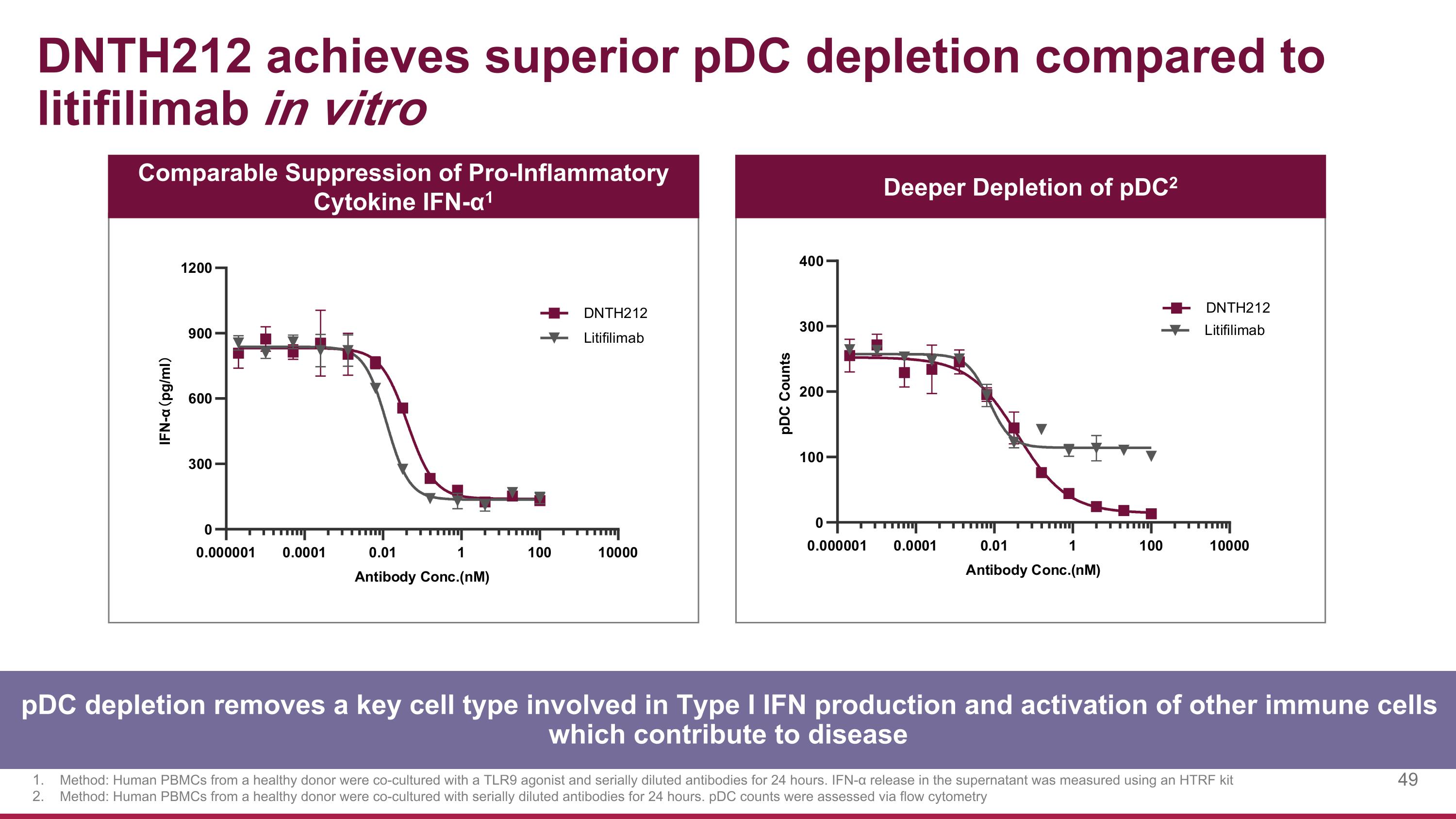
DNTH212 achieves superior pDC depletion compared to litifilimab in vitro Comparable Suppression of Pro-Inflammatory Cytokine IFN-α1 Deeper Depletion of pDC2 Method: Human PBMCs from a healthy donor were co-cultured with a TLR9 agonist and serially diluted antibodies for 24 hours. IFN-α release in the supernatant was measured using an HTRF kit Method: Human PBMCs from a healthy donor were co-cultured with serially diluted antibodies for 24 hours. pDC counts were assessed via flow cytometry pDC depletion removes a key cell type involved in Type I IFN production and activation of other immune cells which contribute to disease
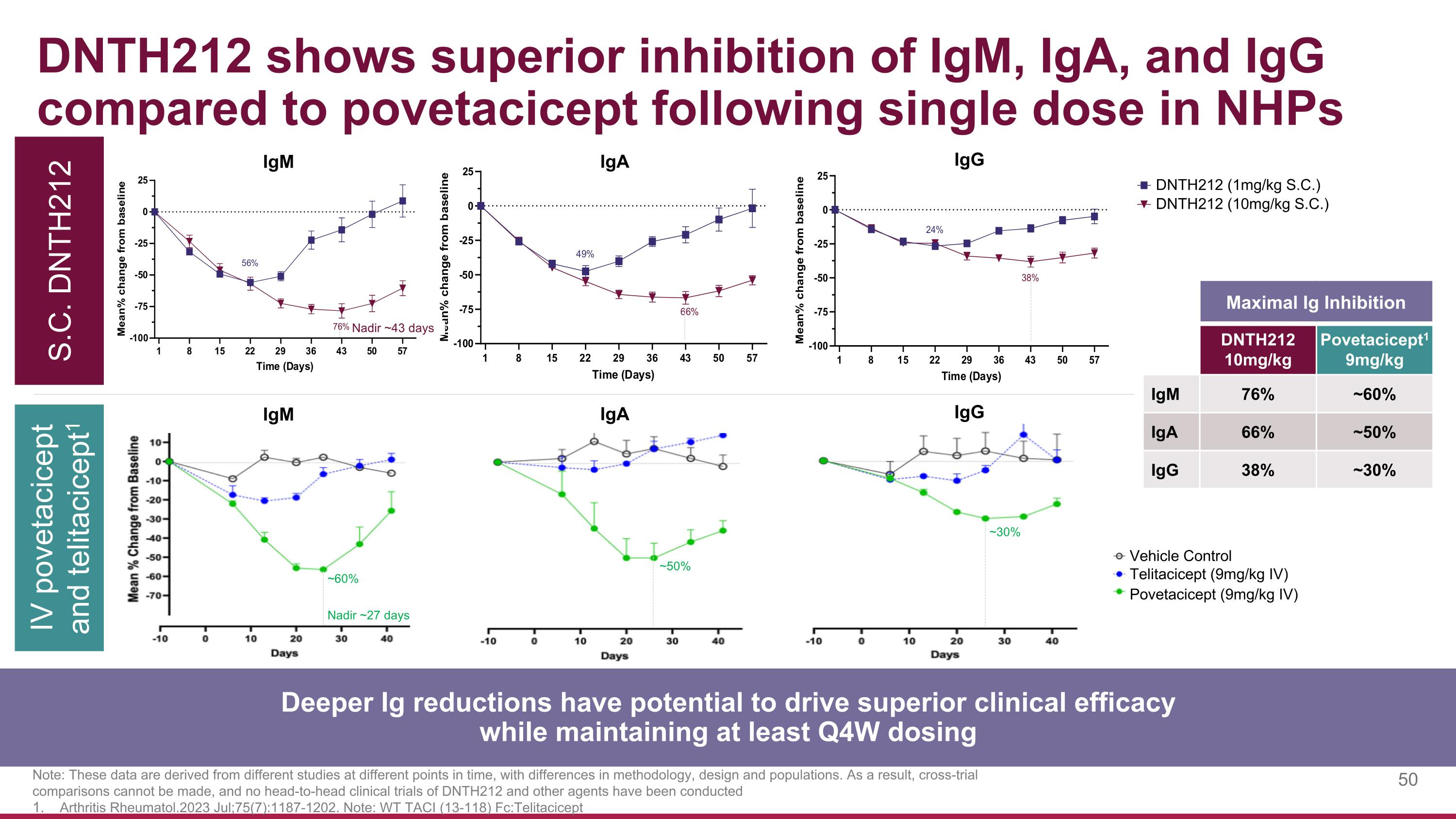
DNTH212 shows superior inhibition of IgM, IgA, and IgG compared to povetacicept following single dose in NHPs S.C. DNTH212 IV povetacicept and telitacicept1 Note: These data are derived from different studies at different points in time, with differences in methodology, design and populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials of DNTH212 and other agents have been conducted Arthritis Rheumatol.2023 Jul;75(7):1187-1202. Note: WT TACI (13-118) Fc:Telitacicept IgM IgA IgG Deeper Ig reductions have potential to drive superior clinical efficacy while maintaining at least Q4W dosing Maximal Ig Inhibition DNTH212 10mg/kg Povetacicept1 9mg/kg IgM 76% ~60% IgA 66% ~50% IgG 38% ~30% Telitacicept (9mg/kg IV) Vehicle Control Povetacicept (9mg/kg IV) Nadir ~27 days ~60% ~50% ~30% IgM IgA IgG DNTH212 (1mg/kg S.C.) DNTH212 (10mg/kg S.C.) Nadir ~43 days
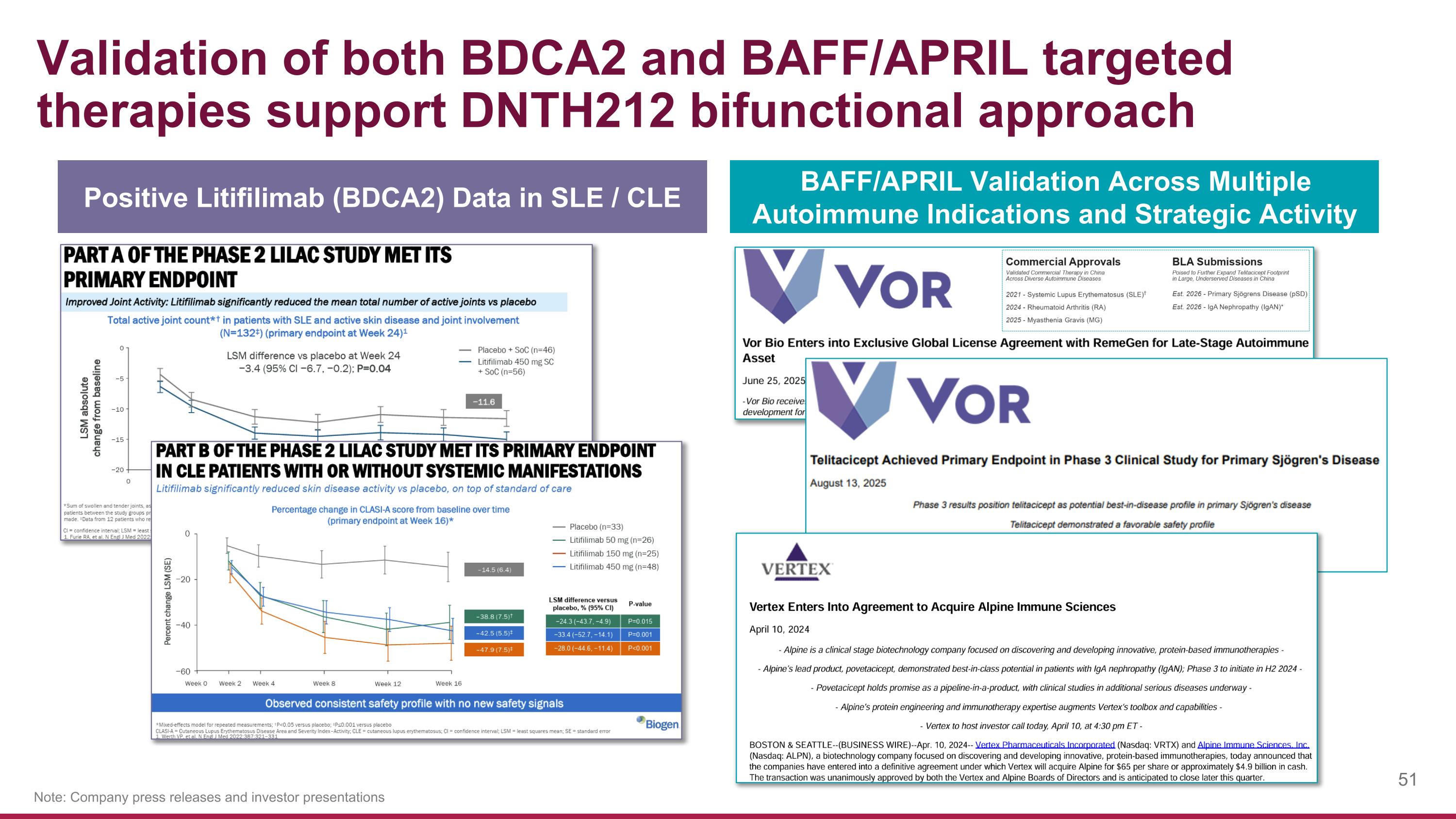
Validation of both BDCA2 and BAFF/APRIL targeted therapies support DNTH212 bifunctional approach Note: Company press releases and investor presentations Positive Litifilimab (BDCA2) Data in SLE / CLE BAFF/APRIL Validation Across Multiple Autoimmune Indications and Strategic Activity
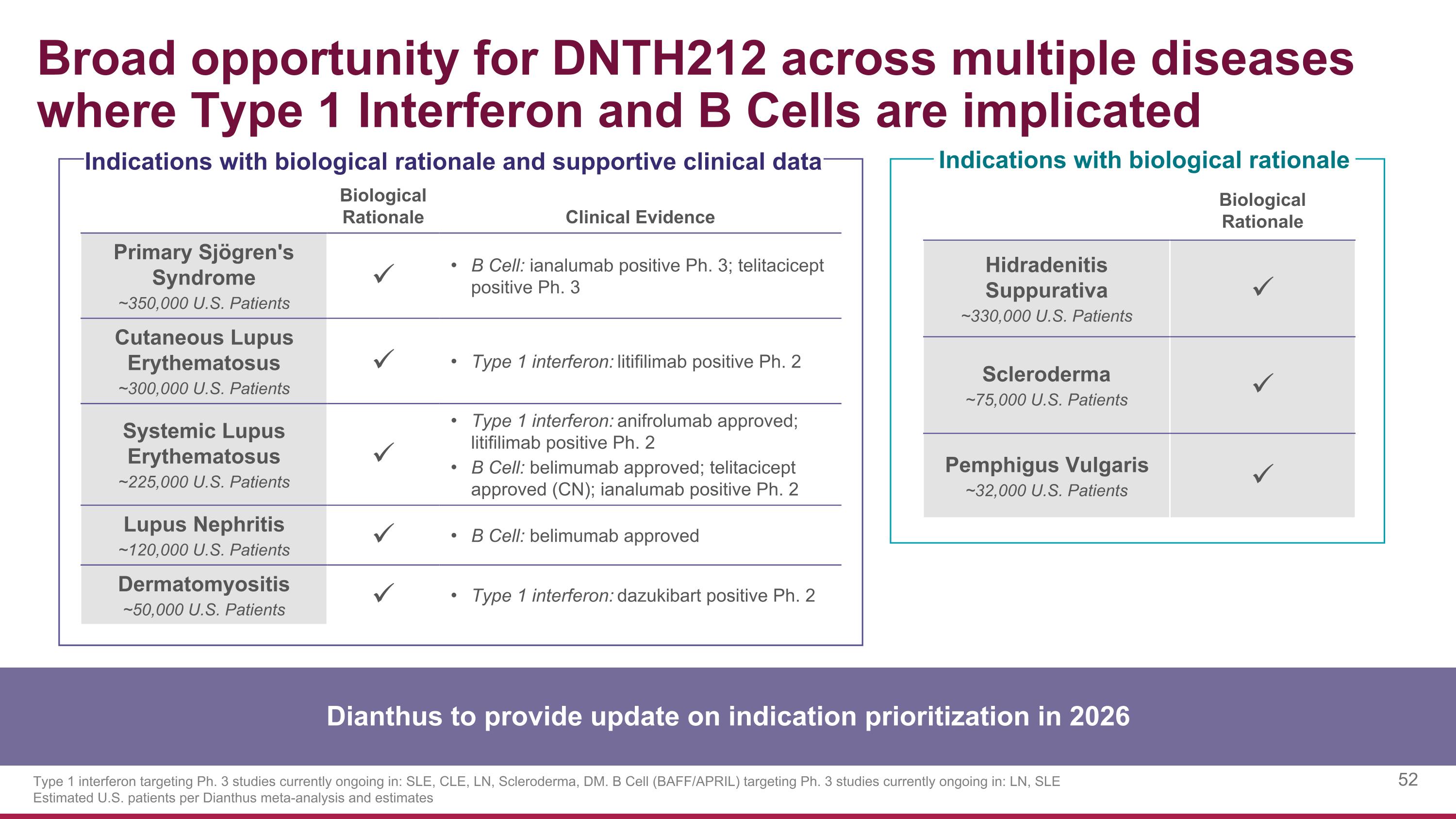
Biological Rationale Clinical Evidence Primary Sjögren's Syndrome ~350,000 U.S. Patients B Cell: ianalumab positive Ph. 3; telitacicept positive Ph. 3 Cutaneous Lupus Erythematosus ~300,000 U.S. Patients Type 1 interferon: litifilimab positive Ph. 2 Systemic Lupus Erythematosus ~225,000 U.S. Patients Type 1 interferon: anifrolumab approved; litifilimab positive Ph. 2 B Cell: belimumab approved; telitacicept approved (CN); ianalumab positive Ph. 2 Lupus Nephritis ~120,000 U.S. Patients B Cell: belimumab approved Dermatomyositis ~50,000 U.S. Patients Type 1 interferon: dazukibart positive Ph. 2 Broad opportunity for DNTH212 across multiple diseases where Type 1 Interferon and B Cells are implicated Type 1 interferon targeting Ph. 3 studies currently ongoing in: SLE, CLE, LN, Scleroderma, DM. B Cell (BAFF/APRIL) targeting Ph. 3 studies currently ongoing in: LN, SLE Estimated U.S. patients per Dianthus meta-analysis and estimates Indications with biological rationale Indications with biological rationale and supportive clinical data Biological Rationale Hidradenitis Suppurativa ~330,000 U.S. Patients Scleroderma ~75,000 U.S. Patients Pemphigus Vulgaris ~32,000 U.S. Patients Dianthus to provide update on indication prioritization in 2026
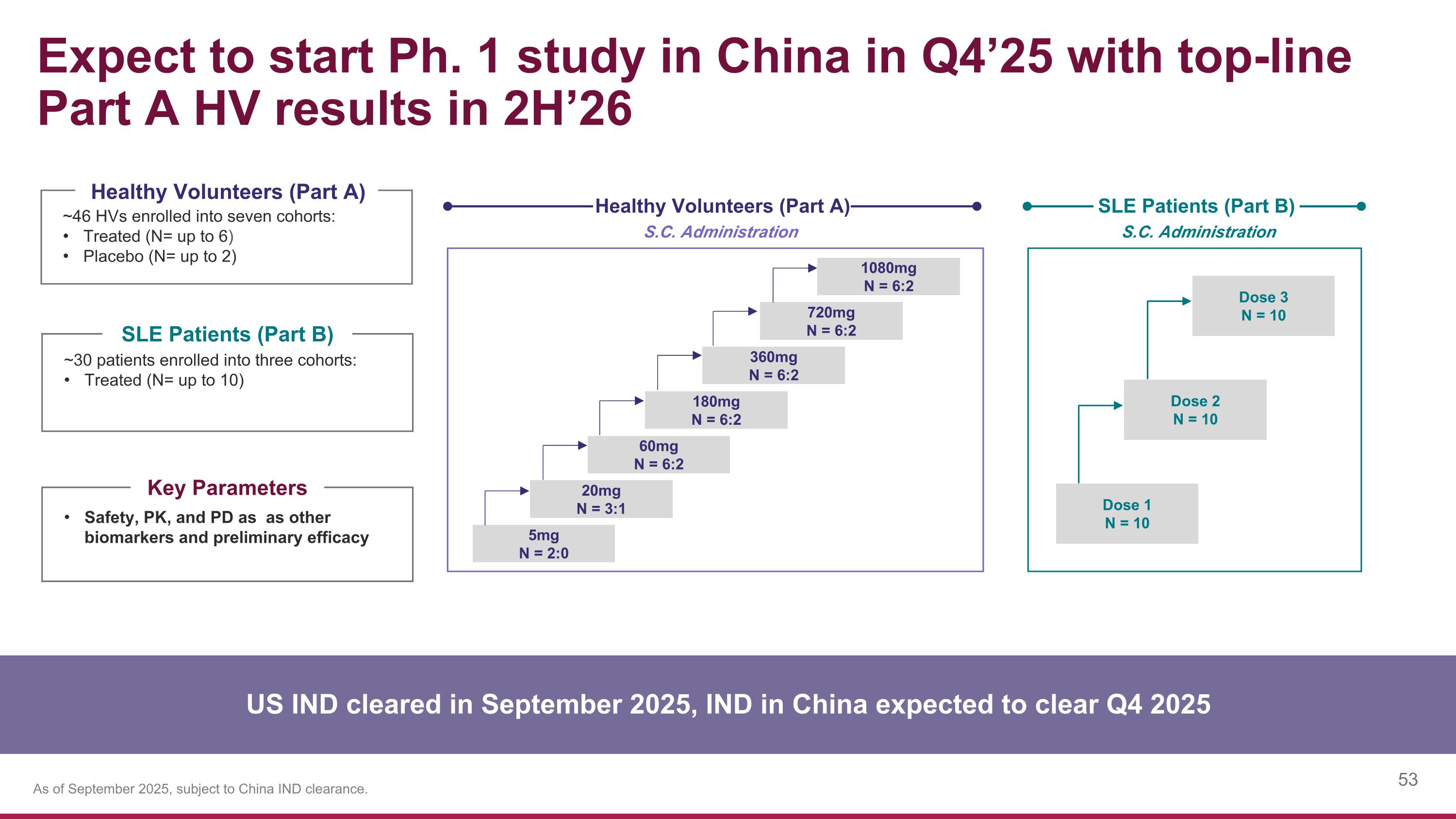
Expect to start Ph. 1 study in China in Q4’25 with top-line Part A HV results in 2H’26 5mg N = 2:0 S.C. Administration Healthy Volunteers (Part A) ~46 HVs enrolled into seven cohorts: Treated (N= up to 6) Placebo (N= up to 2) ~30 patients enrolled into three cohorts: Treated (N= up to 10) Healthy Volunteers (Part A) SLE Patients (Part B) Safety, PK, and PD as as other biomarkers and preliminary efficacy Key Parameters US IND cleared in September 2025, IND in China expected to clear Q4 2025 S.C. Administration SLE Patients (Part B) 20mg N = 3:1 60mg N = 6:2 180mg N = 6:2 360mg N = 6:2 720mg N = 6:2 1080mg N = 6:2 Dose 1 N = 10 Dose 2 N = 10 Dose 3 N = 10 As of September 2025, subject to China IND clearance.
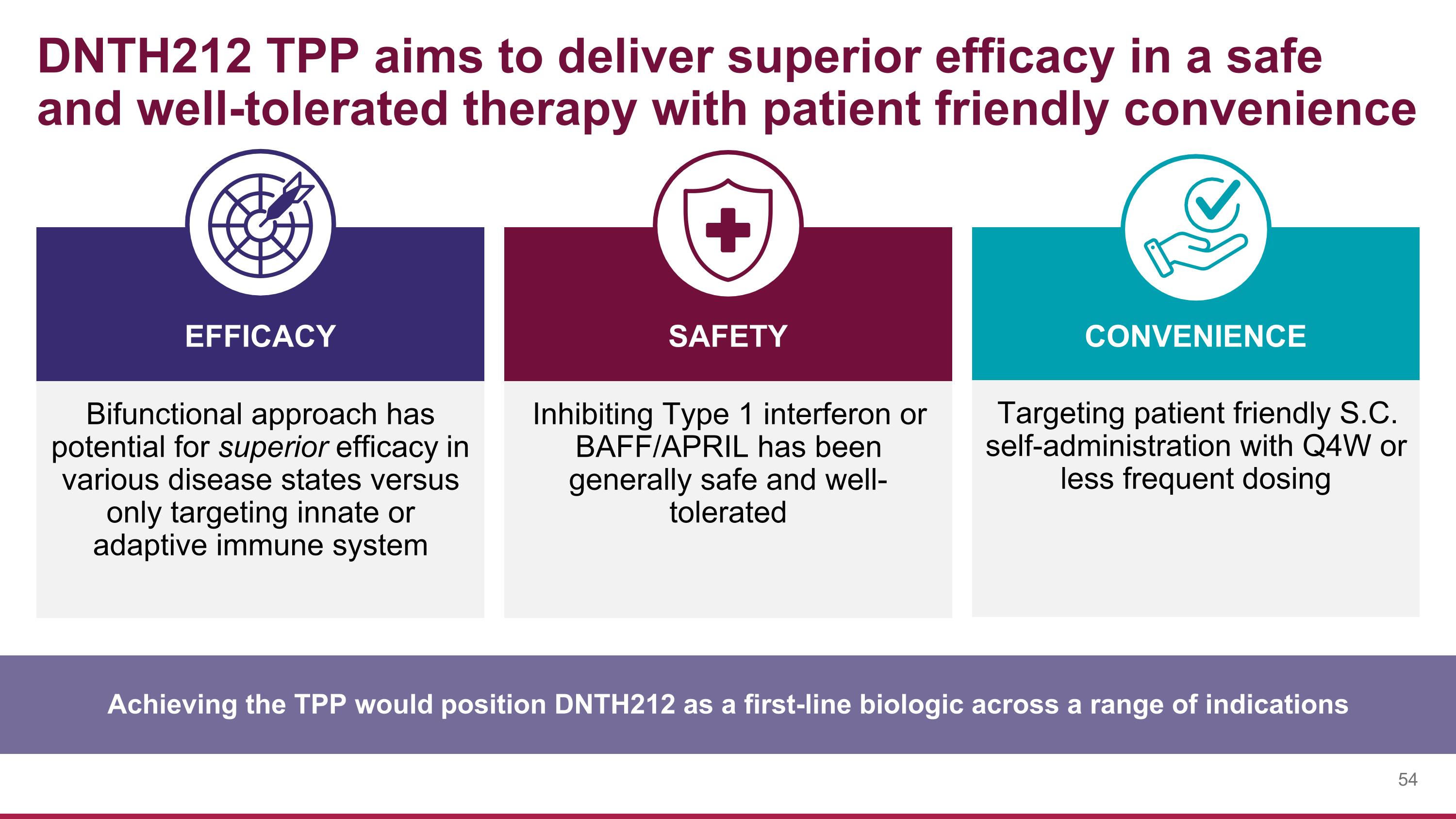
DNTH212 TPP aims to deliver superior efficacy in a safe and well-tolerated therapy with patient friendly convenience Achieving the TPP would position DNTH212 as a first-line biologic across a range of indications Bifunctional approach has potential for superior efficacy in various disease states versus only targeting innate or adaptive immune system SAFETY EFFICACY CONVENIENCE Inhibiting Type 1 interferon or BAFF/APRIL has been generally safe and well-tolerated Targeting patient friendly S.C. self-administration with Q4W or less frequent dosing
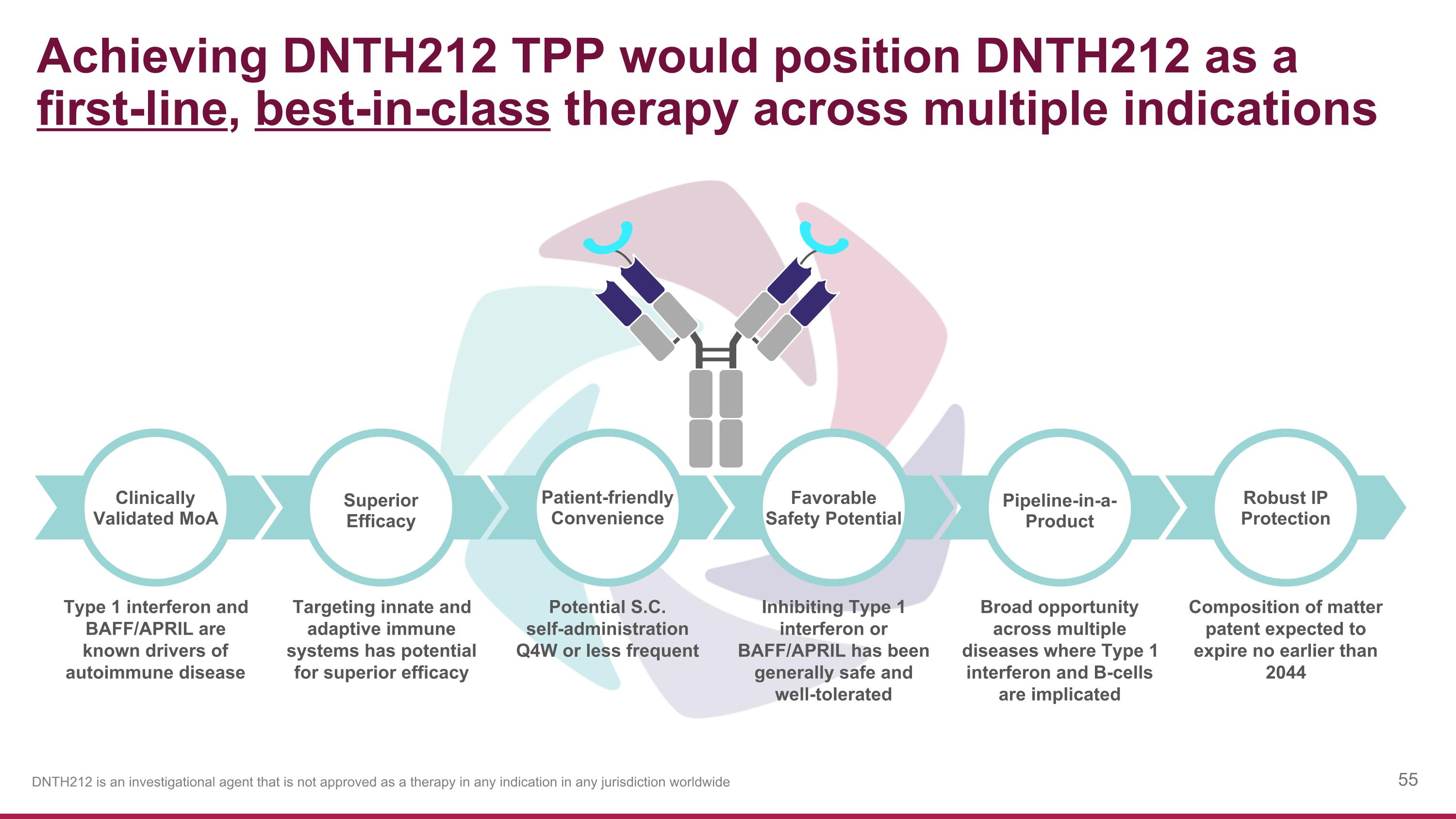
Achieving DNTH212 TPP would position DNTH212 as a first-line, best-in-class therapy across multiple indications Type 1 interferon and BAFF/APRIL are known drivers of autoimmune disease Potential S.C. self-administration Q4W or less frequent Targeting innate and adaptive immune systems has potential for superior efficacy Inhibiting Type 1 interferon or BAFF/APRIL has been generally safe and well-tolerated Broad opportunity across multiple diseases where Type 1 interferon and B-cells are implicated Composition of matter patent expected to expire no earlier than 2044 Patient-friendly Convenience Superior Efficacy Clinically Validated MoA Favorable Safety Potential Pipeline-in-a-Product Robust IP Protection DNTH212 is an investigational agent that is not approved as a therapy in any indication in any jurisdiction worldwide

Recap of Dianthus Leadership in Severe Autoimmune Diseases
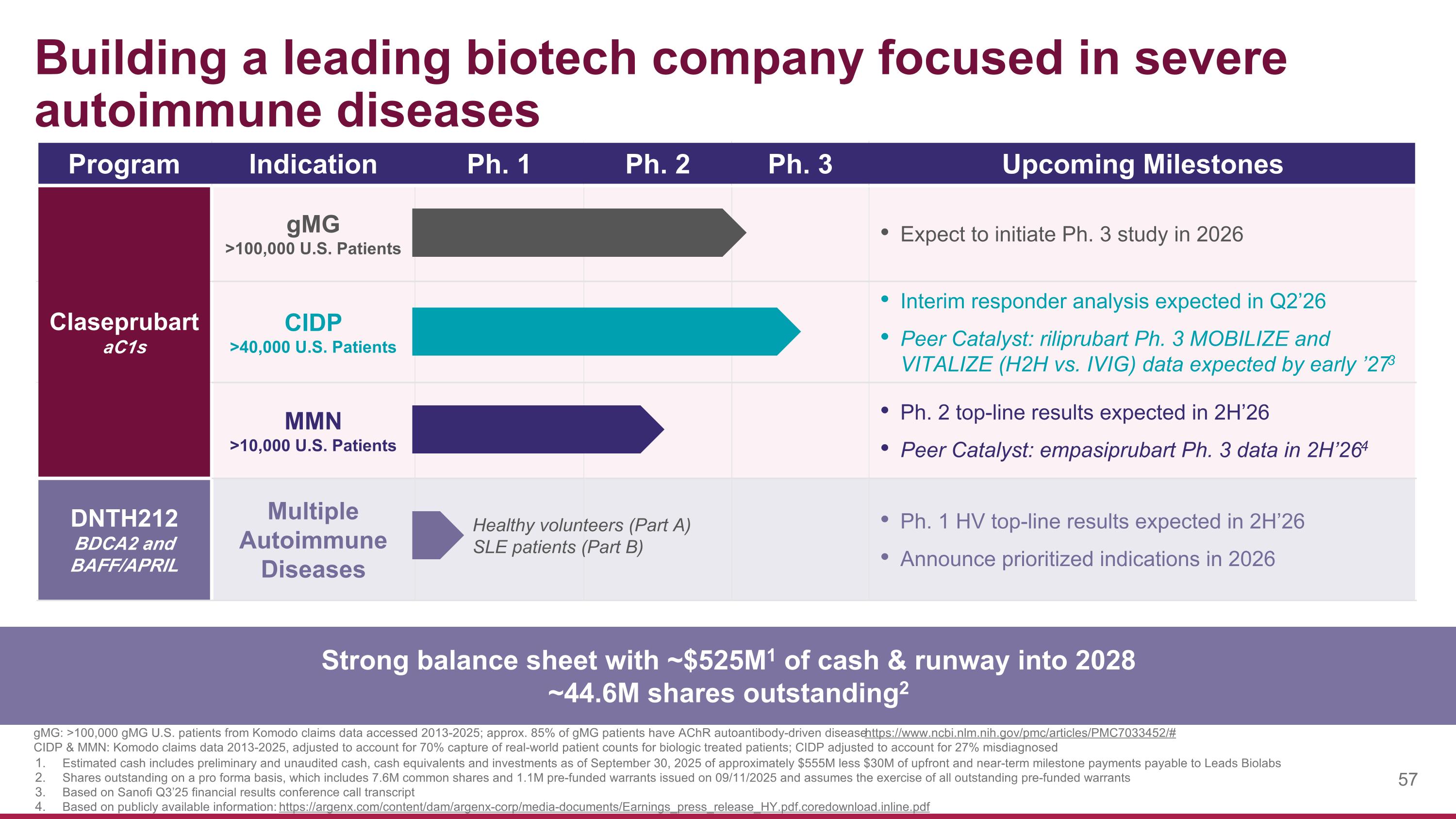
Building a leading biotech company focused in severe autoimmune diseases gMG: >100,000 gMG U.S. patients from Komodo claims data accessed 2013-2025; approx. 85% of gMG patients have AChR autoantibody-driven disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/# CIDP & MMN: Komodo claims data 2013-2025, adjusted to account for 70% capture of real-world patient counts for biologic treated patients; CIDP adjusted to account for 27% misdiagnosed Program Indication Ph. 1 Ph. 2 Ph. 3 Upcoming Milestones Claseprubart aC1s gMG >100,000 U.S. Patients Expect to initiate Ph. 3 study in 2026 CIDP >40,000 U.S. Patients Interim responder analysis expected in Q2’26 Peer Catalyst: riliprubart Ph. 3 MOBILIZE and VITALIZE (H2H vs. IVIG) data expected by early ’273 MMN >10,000 U.S. Patients Ph. 2 top-line results expected in 2H’26 Peer Catalyst: empasiprubart Ph. 3 data in 2H’264 DNTH212 BDCA2 and BAFF/APRIL Multiple Autoimmune Diseases Ph. 1 HV top-line results expected in 2H’26 Announce prioritized indications in 2026 Healthy volunteers (Part A) SLE patients (Part B) Strong balance sheet with ~$525M1 of cash & runway into 2028 ~44.6M shares outstanding2 Estimated cash includes preliminary and unaudited cash, cash equivalents and investments as of September 30, 2025 of approximately $555M less $30M of upfront and near-term milestone payments payable to Leads Biolabs Shares outstanding on a pro forma basis, which includes 7.6M common shares and 1.1M pre-funded warrants issued on 09/11/2025 and assumes the exercise of all outstanding pre-funded warrants Based on Sanofi Q3’25 financial results conference call transcript Based on publicly available information: https://argenx.com/content/dam/argenx-corp/media-documents/Earnings_press_release_HY.pdf.coredownload.inline.pdf

Appendix
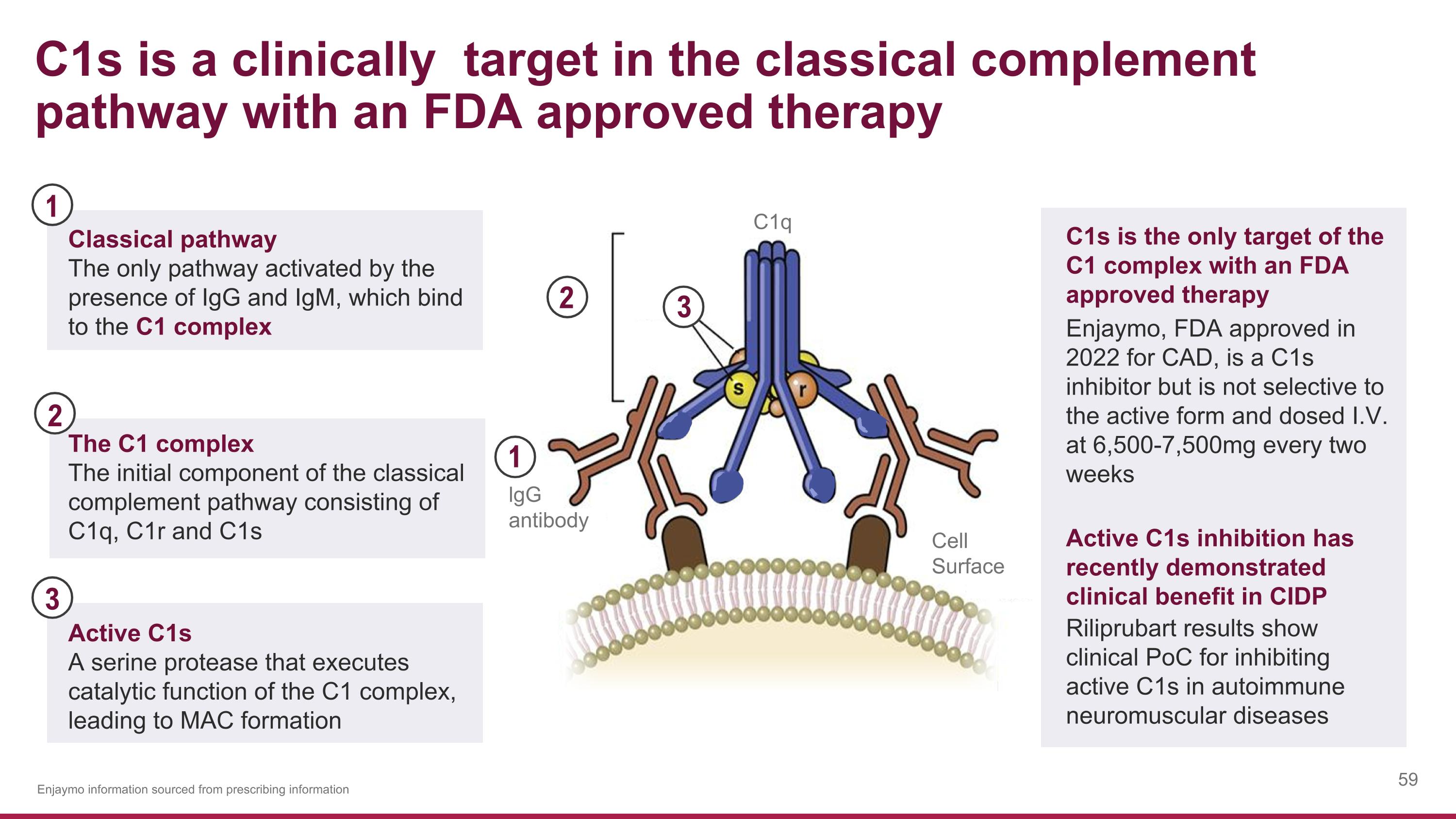
C1q lgG antibody Cell Surface C1s is a clinically target in the classical complement pathway with an FDA approved therapy The C1 complex The initial component of the classical complement pathway consisting of C1q, C1r and C1s 3 2 2 3 Active C1s A serine protease that executes catalytic function of the C1 complex, leading to MAC formation C1s is the only target of the C1 complex with an FDA approved therapy Enjaymo, FDA approved in 2022 for CAD, is a C1s inhibitor but is not selective to the active form and dosed I.V. at 6,500-7,500mg every two weeks Enjaymo information sourced from prescribing information 1 Classical pathway The only pathway activated by the presence of IgG and IgM, which bind to the C1 complex 1 Active C1s inhibition has recently demonstrated clinical benefit in CIDP Riliprubart results show clinical PoC for inhibiting active C1s in autoimmune neuromuscular diseases
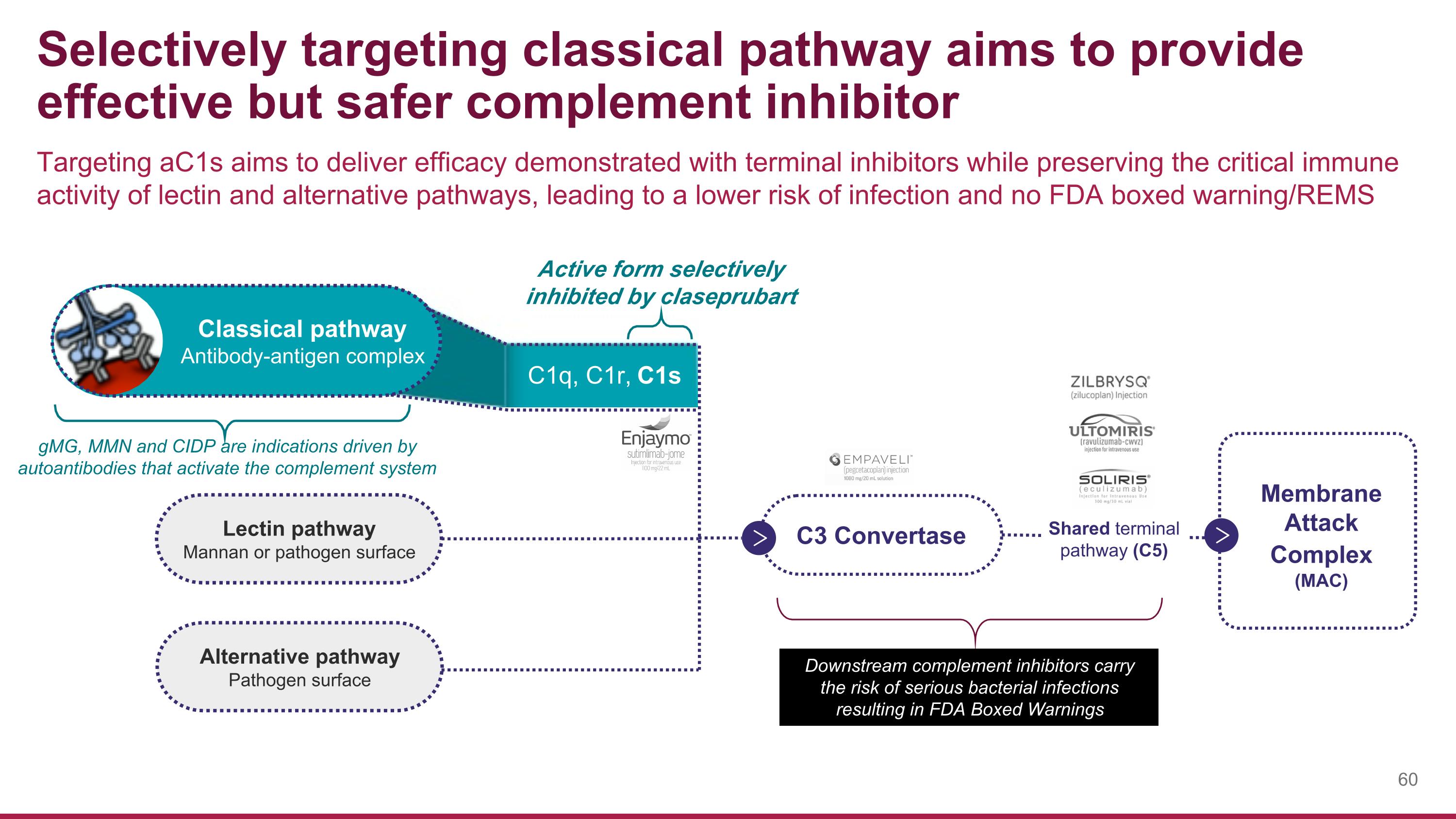
Membrane Attack Complex (MAC) Selectively targeting classical pathway aims to provide effective but safer complement inhibitor Targeting aC1s aims to deliver efficacy demonstrated with terminal inhibitors while preserving the critical immune activity of lectin and alternative pathways, leading to a lower risk of infection and no FDA boxed warning/REMS Downstream complement inhibitors carry the risk of serious bacterial infections resulting in FDA Boxed Warnings Lectin pathway Mannan or pathogen surface Alternative pathway Pathogen surface C3 Convertase Shared terminal pathway (C5) Classical pathway Antibody-antigen complex Active form selectively inhibited by claseprubart C1q, C1r, C1s gMG, MMN and CIDP are indications driven by autoantibodies that activate the complement system
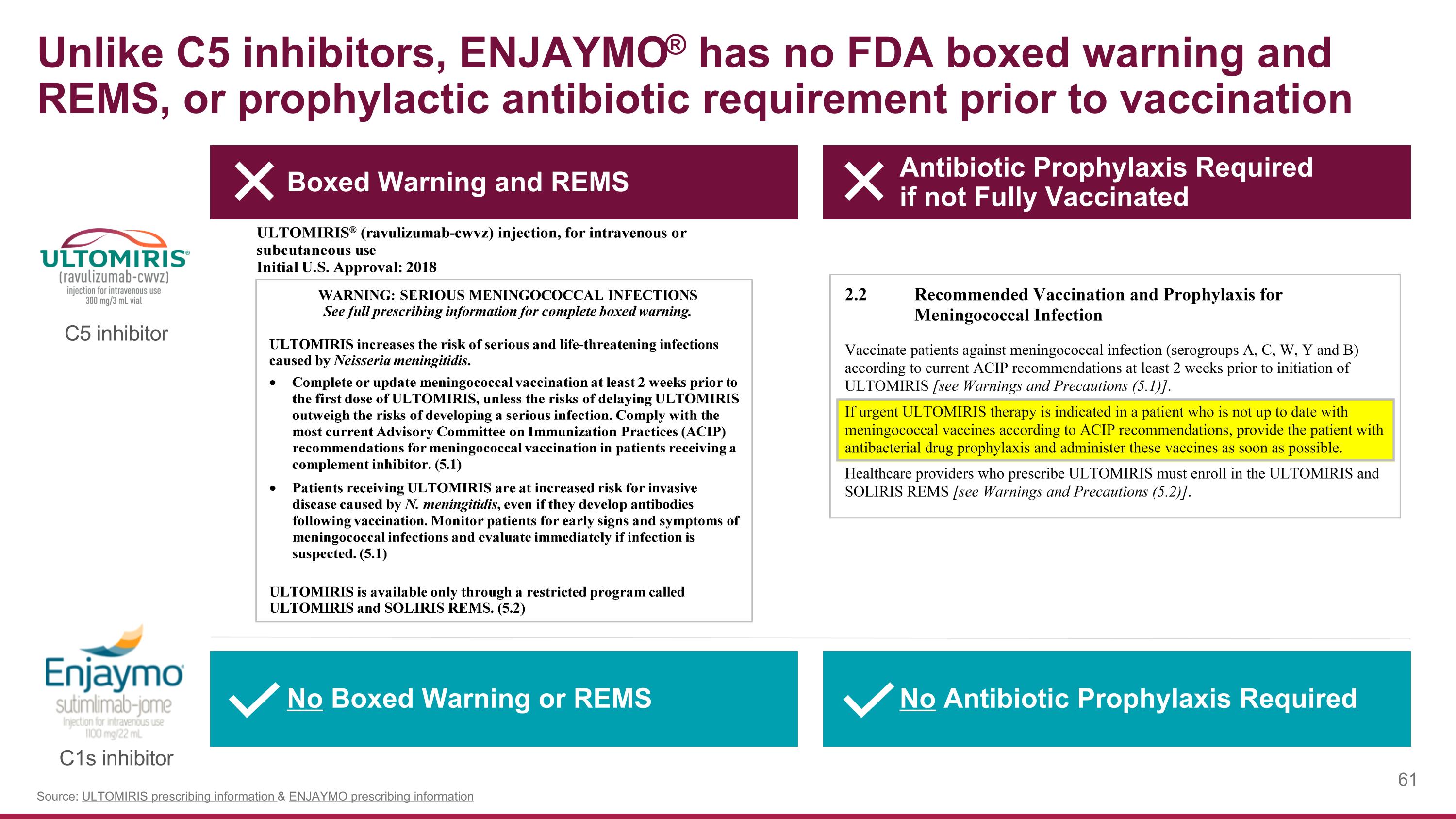
Unlike C5 inhibitors, ENJAYMO® has no FDA boxed warning and REMS, or prophylactic antibiotic requirement prior to vaccination Source: ULTOMIRIS prescribing information & ENJAYMO prescribing information Boxed Warning and REMS Antibiotic Prophylaxis Required if not Fully Vaccinated No Boxed Warning or REMS No Antibiotic Prophylaxis Required C5 inhibitor C1s inhibitor
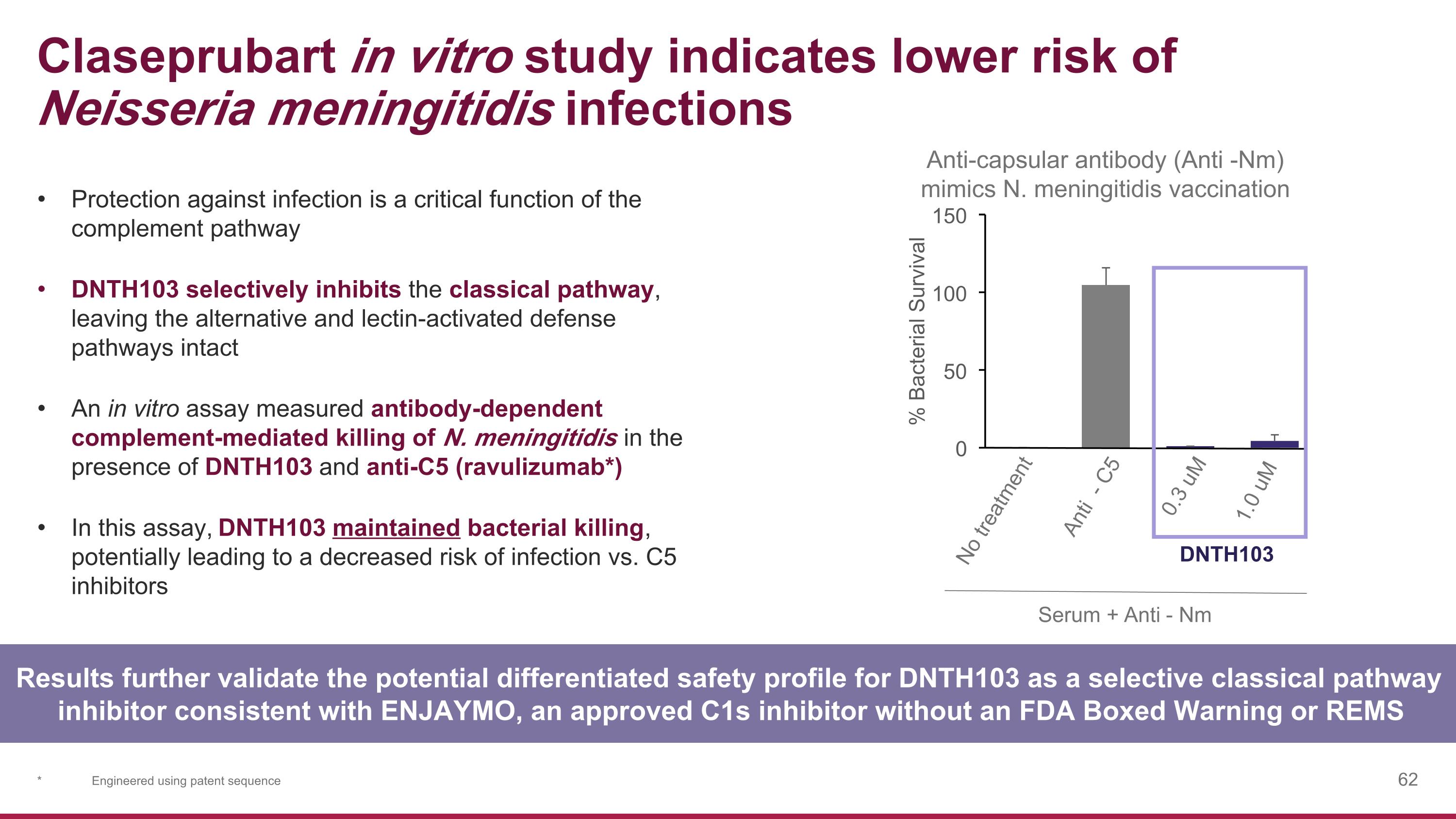
Claseprubart in vitro study indicates lower risk of Neisseria meningitidis infections Anti-capsular antibody (Anti -Nm) mimics N. meningitidis vaccination 0 50 100 150 % Bacterial Survival Serum +Anti - Nm 0.3 uM 1.0 uM DNTH103 Anti - C5 No treatment Results further validate the potential differentiated safety profile for DNTH103 as a selective classical pathway inhibitor consistent with ENJAYMO, an approved C1s inhibitor without an FDA Boxed Warning or REMS Protection against infection is a critical function of the complement pathway DNTH103 selectively inhibits the classical pathway, leaving the alternative and lectin-activated defense pathways intact An in vitro assay measured antibody-dependent complement-mediated killing of N. meningitidis in the presence of DNTH103 and anti-C5 (ravulizumab*) In this assay, DNTH103 maintained bacterial killing, potentially leading to a decreased risk of infection vs. C5 inhibitors * Engineered using patent sequence
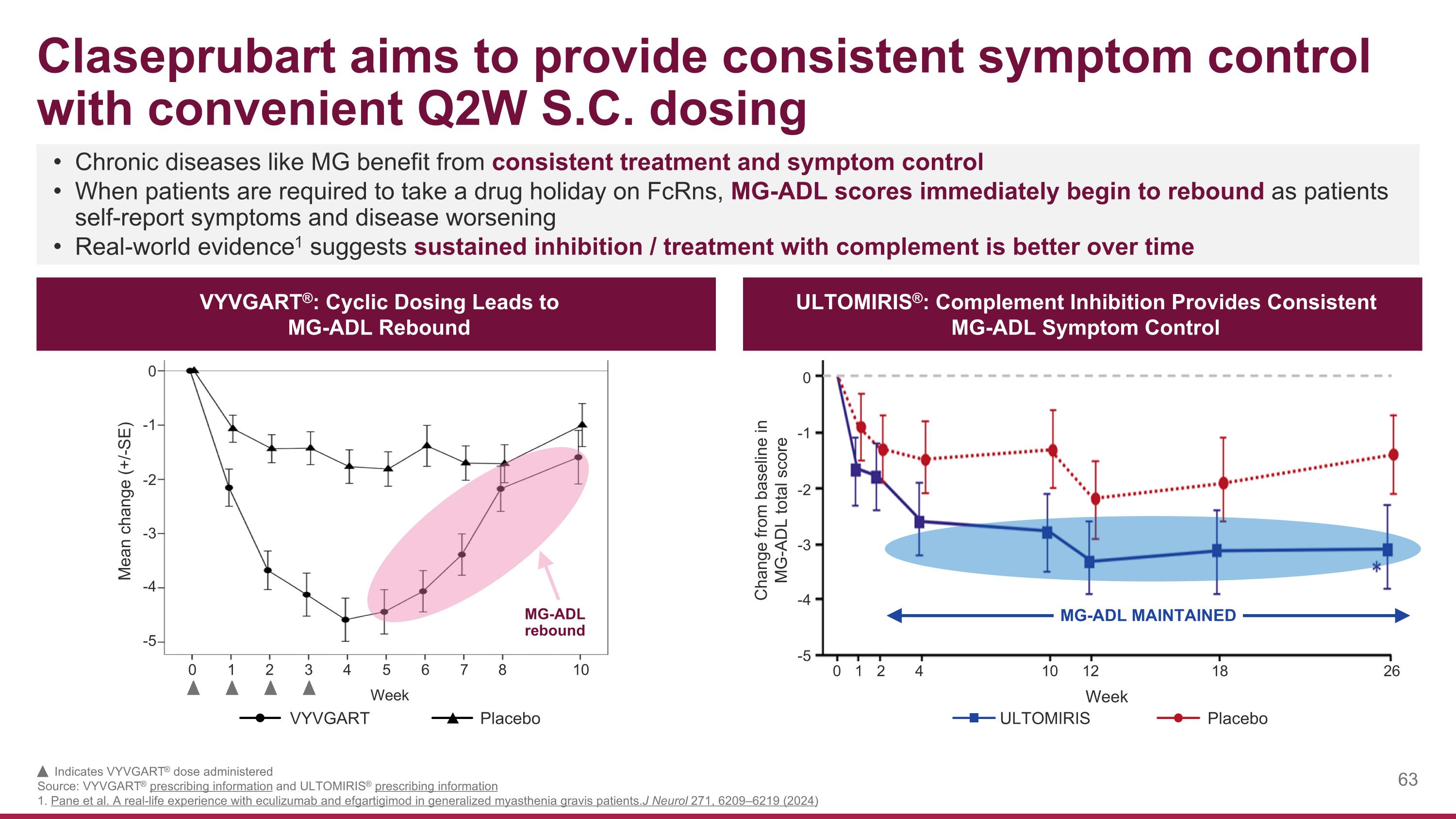
Indicates VYVGART® dose administered Source: VYVGART® prescribing information and ULTOMIRIS® prescribing information 1. Pane et al. A real-life experience with eculizumab and efgartigimod in generalized myasthenia gravis patients. J Neurol 271, 6209–6219 (2024) Claseprubart aims to provide consistent symptom control with convenient Q2W S.C. dosing Chronic diseases like MG benefit from consistent treatment and symptom control When patients are required to take a drug holiday on FcRns, MG-ADL scores immediately begin to rebound as patients self-report symptoms and disease worsening Real-world evidence1 suggests sustained inhibition / treatment with complement is better over time ULTOMIRIS®: Complement Inhibition Provides Consistent MG-ADL Symptom Control VYVGART®: Cyclic Dosing Leads to MG-ADL Rebound Mean change (+/-SE) MG-ADL rebound -5 -4 -3 -2 -1 0 0 1 2 3 4 5 6 7 8 10 VYVGART Placebo Placebo ULTOMIRIS 18 12 10 4 2 1 0 -5 -4 -3 -2 -1 0 26 MG-ADL MAINTAINED Change from baseline in MG-ADL total score Week Week
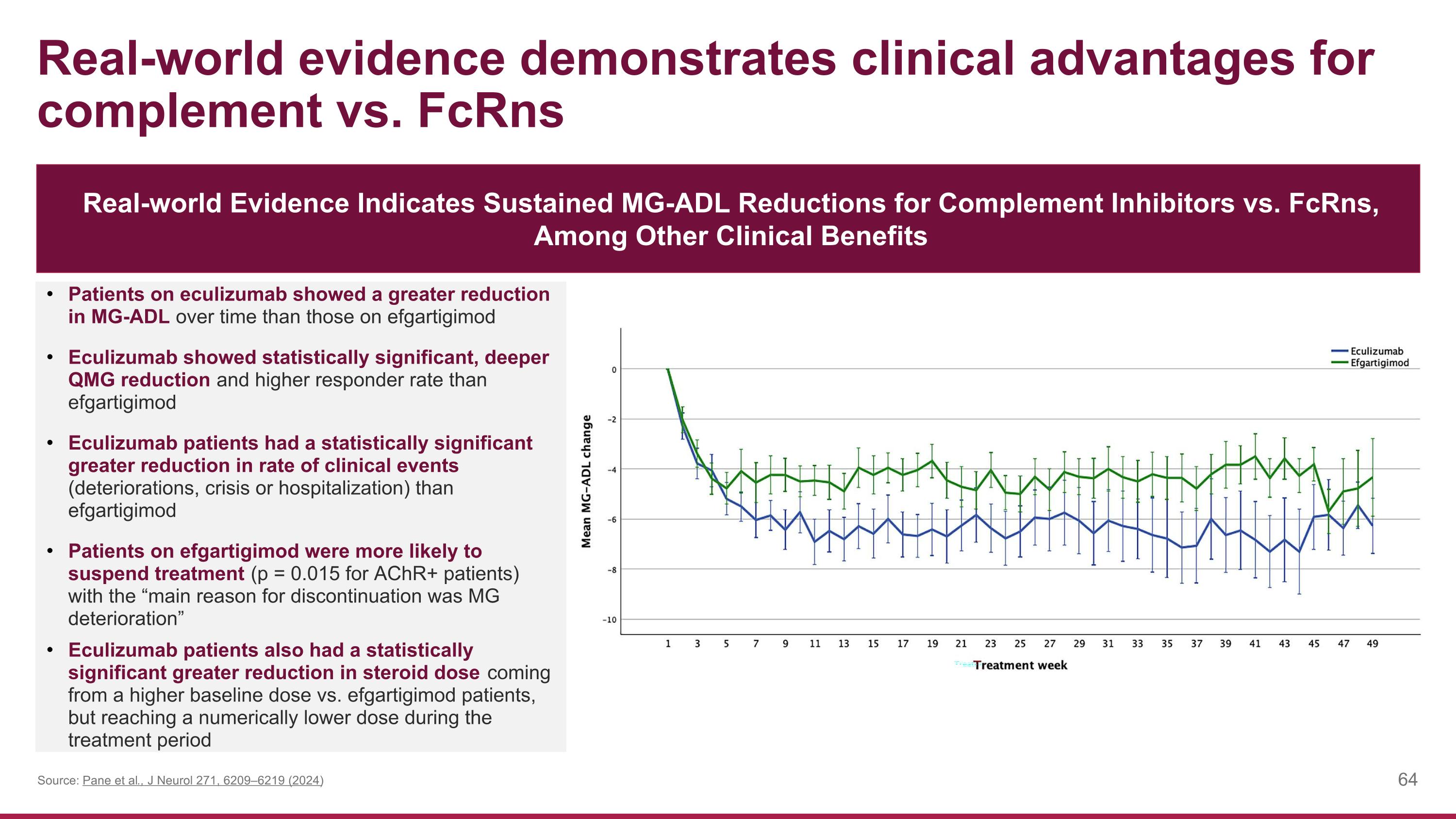
Patients on eculizumab showed a greater reduction in MG-ADL over time than those on efgartigimod Eculizumab showed statistically significant, deeper QMG reduction and higher responder rate than efgartigimod Eculizumab patients had a statistically significant greater reduction in rate of clinical events (deteriorations, crisis or hospitalization) than efgartigimod Patients on efgartigimod were more likely to suspend treatment (p = 0.015 for AChR+ patients) with the “main reason for discontinuation was MG deterioration” Eculizumab patients also had a statistically significant greater reduction in steroid dose coming from a higher baseline dose vs. efgartigimod patients, but reaching a numerically lower dose during the treatment period Real-world Evidence Indicates Sustained MG-ADL Reductions for Complement Inhibitors vs. FcRns, Among Other Clinical Benefits Real-world evidence demonstrates clinical advantages for complement vs. FcRns Source: Pane et al., J Neurol 271, 6209–6219 (2024)
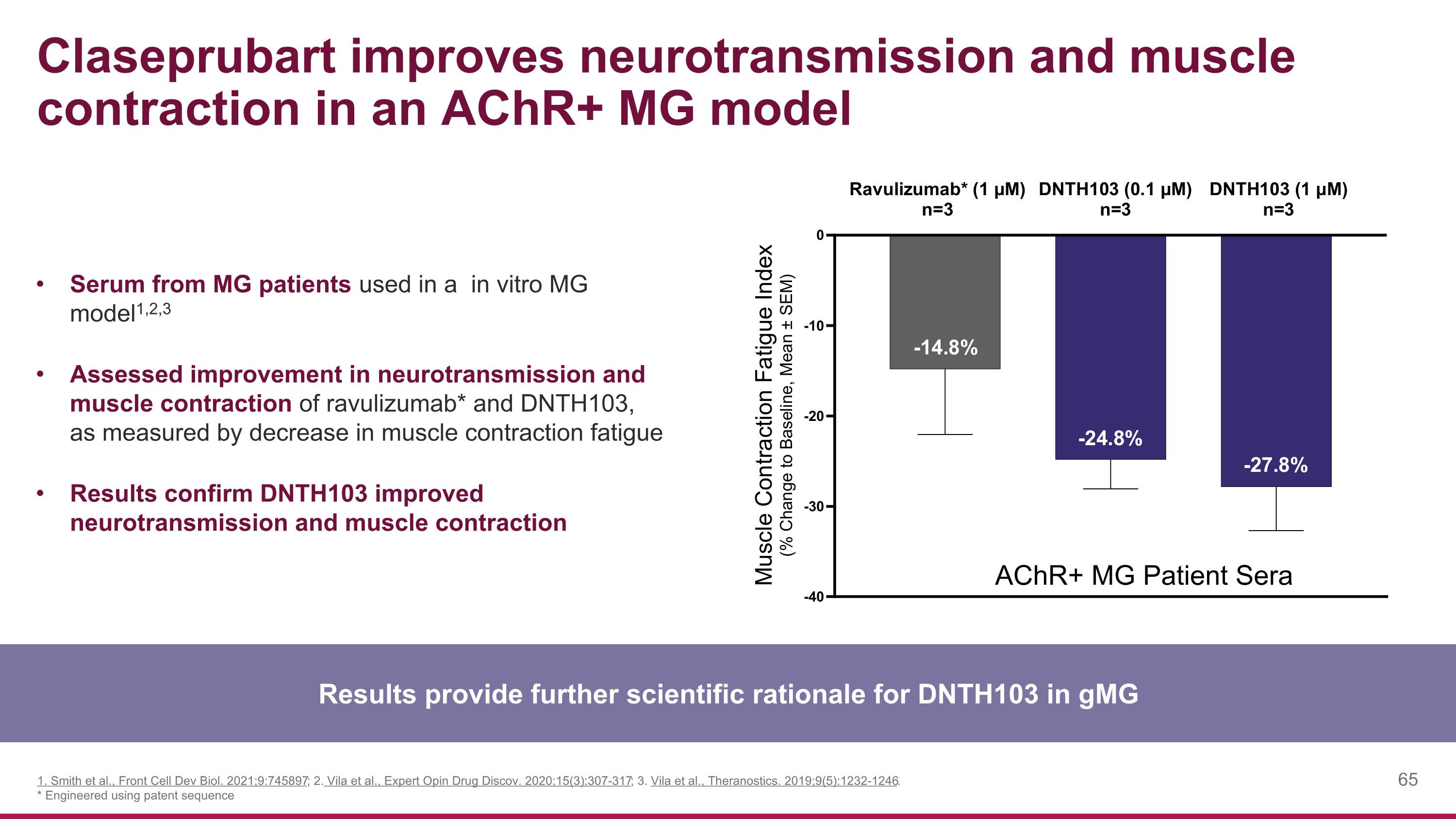
Claseprubart improves neurotransmission and muscle contraction in an AChR+ MG model Results provide further scientific rationale for DNTH103 in gMG Serum from MG patients used in a in vitro MG model1,2,3 Assessed improvement in neurotransmission and muscle contraction of ravulizumab* and DNTH103, as measured by decrease in muscle contraction fatigue Results confirm DNTH103 improved neurotransmission and muscle contraction 1. Smith et al., Front Cell Dev Biol. 2021;9:745897; 2. Vila et al., Expert Opin Drug Discov. 2020;15(3):307-317; 3. Vila et al., Theranostics. 2019;9(5):1232-1246. * Engineered using patent sequence AChR+ MG Patient Sera
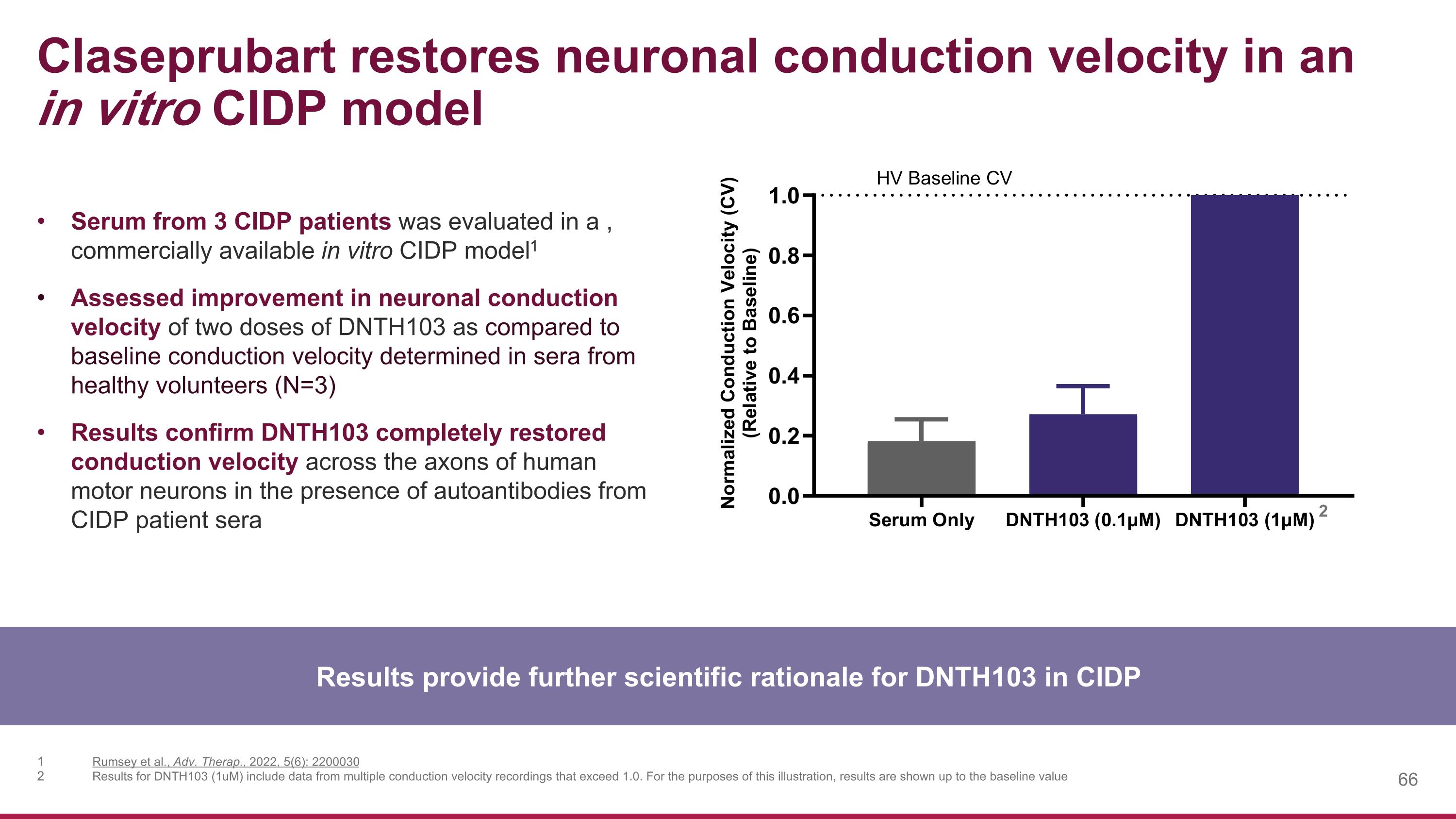
Claseprubart restores neuronal conduction velocity in an in vitro CIDP model Results provide further scientific rationale for DNTH103 in CIDP Serum from 3 CIDP patients was evaluated in a , commercially available in vitro CIDP model1 Assessed improvement in neuronal conduction velocity of two doses of DNTH103 as compared to baseline conduction velocity determined in sera from healthy volunteers (N=3) Results confirm DNTH103 completely restored conduction velocity across the axons of human motor neurons in the presence of autoantibodies from CIDP patient sera Rumsey et al., Adv. Therap., 2022, 5(6): 2200030 Results for DNTH103 (1uM) include data from multiple conduction velocity recordings that exceed 1.0. For the purposes of this illustration, results are shown up to the baseline value 2
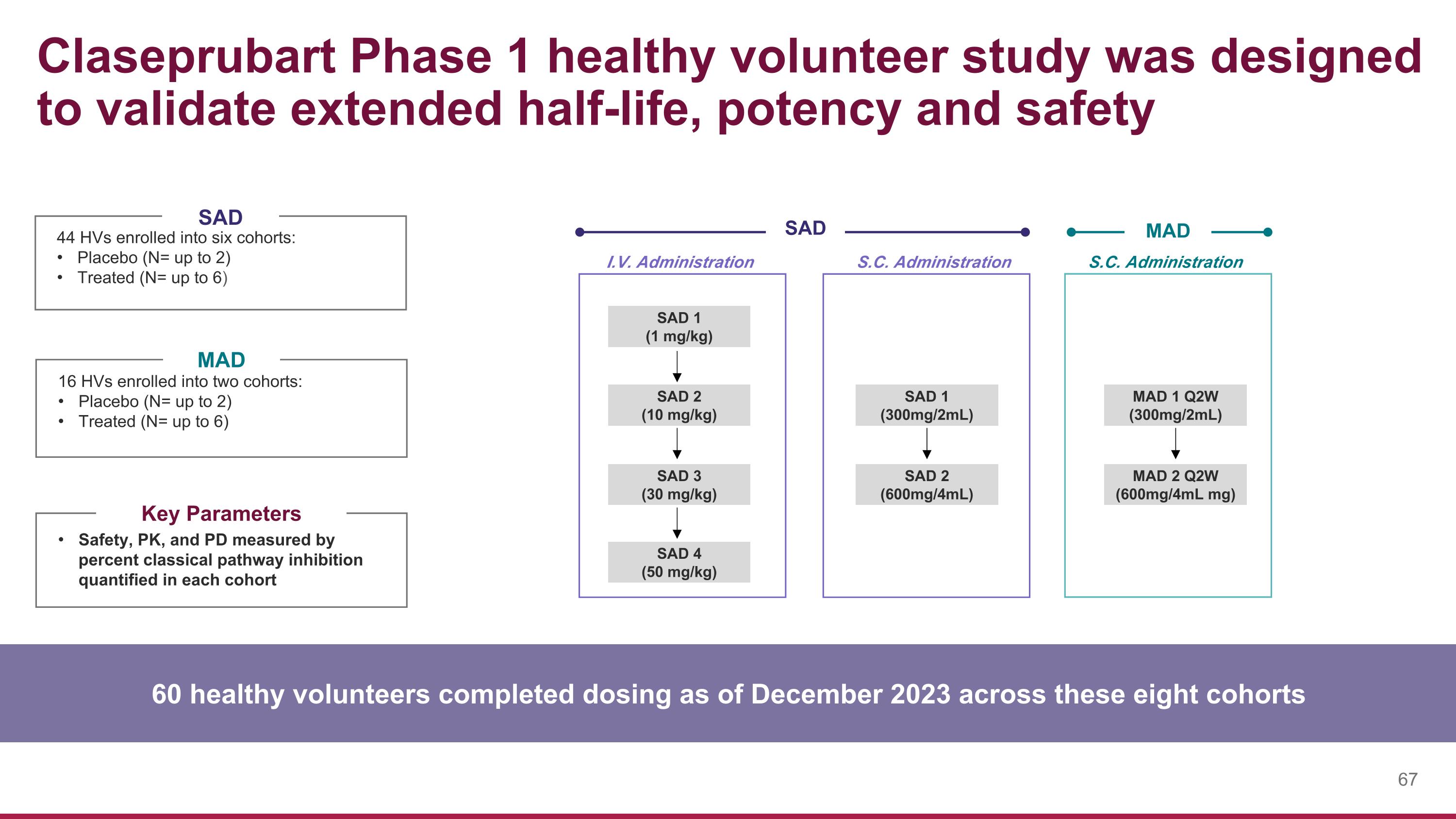
SAD 1 (1 mg/kg) SAD 3 (30 mg/kg) SAD 2 (10 mg/kg) MAD 1 Q2W (300mg/2mL) MAD 2 Q2W (600mg/4mL mg) SAD 1 (300mg/2mL) SAD 2 (600mg/4mL) I.V. Administration S.C. Administration S.C. Administration SAD MAD Claseprubart Phase 1 healthy volunteer study was designed to validate extended half-life, potency and safety 44 HVs enrolled into six cohorts: Placebo (N= up to 2) Treated (N= up to 6) 16 HVs enrolled into two cohorts: Placebo (N= up to 2) Treated (N= up to 6) SAD MAD Safety, PK, and PD measured by percent classical pathway inhibition quantified in each cohort Key Parameters 60 healthy volunteers completed dosing as of December 2023 across these eight cohorts SAD 4 (50 mg/kg)
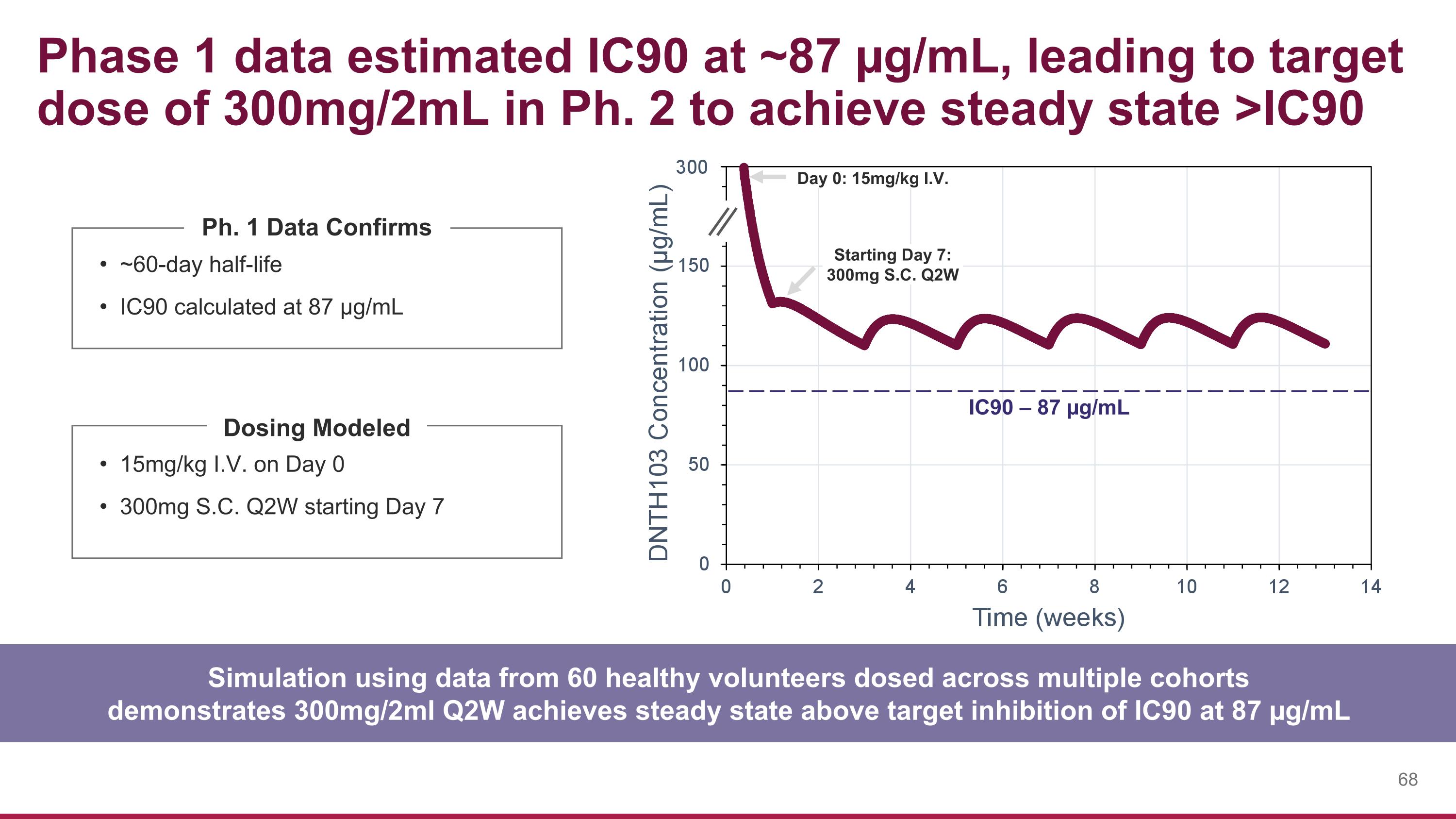
15mg/kg I.V. on Day 0 300mg S.C. Q2W starting Day 7 Phase 1 data estimated IC90 at ~87 µg/mL, leading to target dose of 300mg/2mL in Ph. 2 to achieve steady state >IC90 ~60-day half-life IC90 calculated at 87 µg/mL Ph. 1 Data Confirms Simulation using data from 60 healthy volunteers dosed across multiple cohorts demonstrates 300mg/2ml Q2W achieves steady state above target inhibition of IC90 at 87 µg/mL IC90 – 87 µg/mL Day 0: 15mg/kg I.V. Starting Day 7: 300mg S.C. Q2W Dosing Modeled
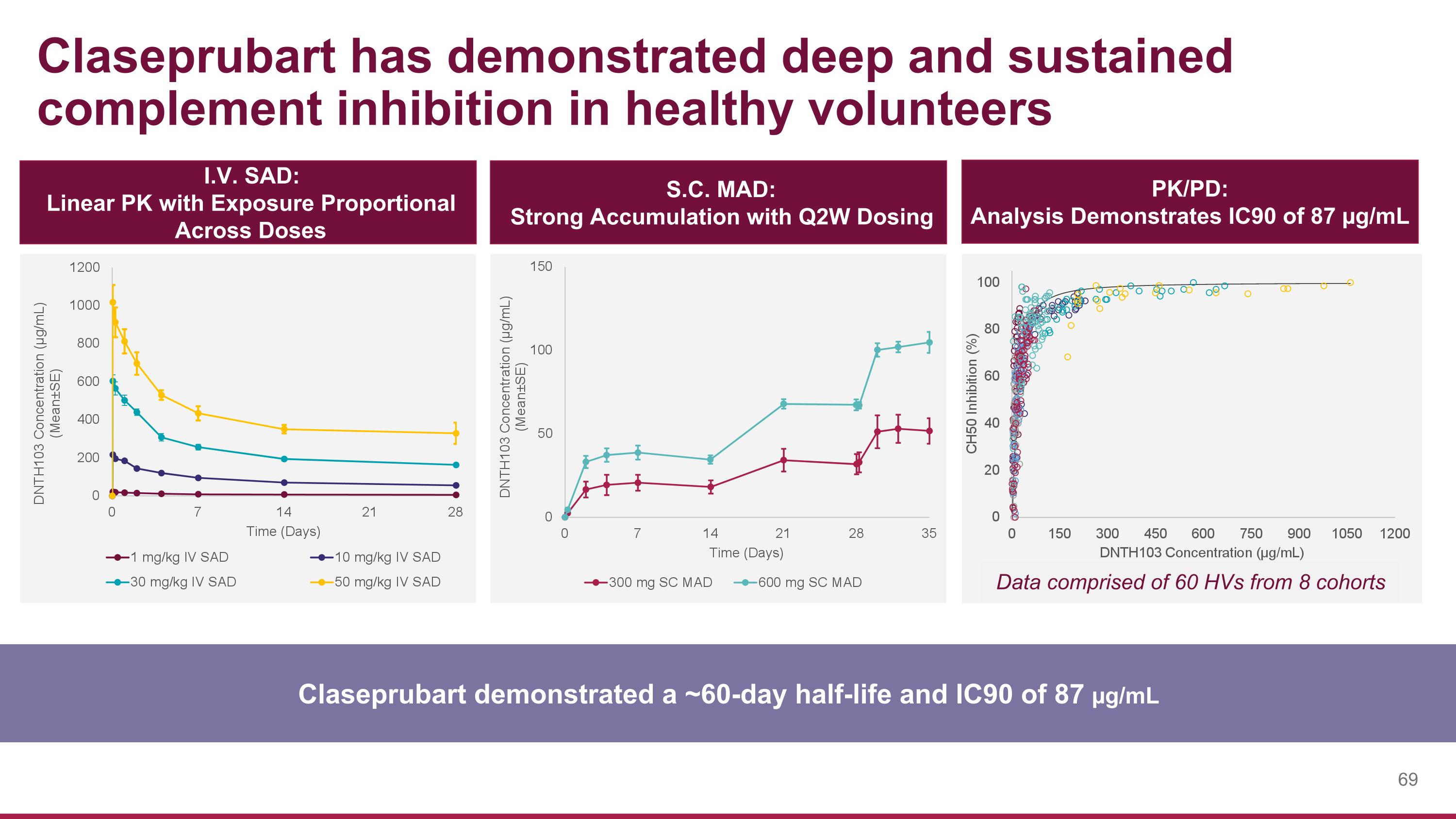
Claseprubart has demonstrated deep and sustained complement inhibition in healthy volunteers Claseprubart demonstrated a ~60-day half-life and IC90 of 87 µg/mL I.V. SAD: Linear PK with Exposure Proportional Across Doses S.C. MAD: Strong Accumulation with Q2W Dosing PK/PD: Analysis Demonstrates IC90 of 87 µg/mL Data comprised of 60 HVs from 8 cohorts
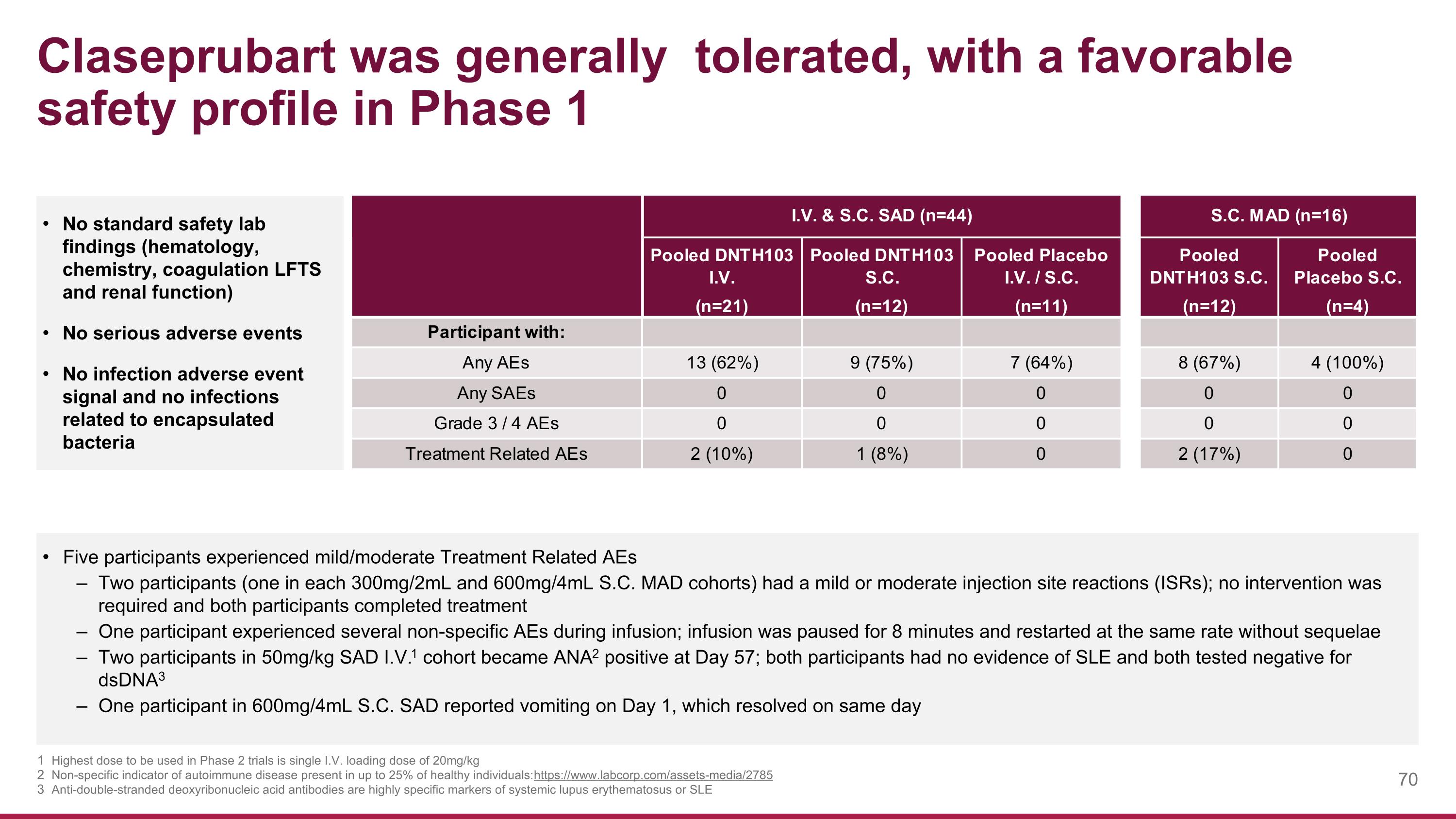
Claseprubart was generally tolerated, with a favorable safety profile in Phase 1 No standard safety lab findings (hematology, chemistry, coagulation LFTS and renal function) No serious adverse events No infection adverse event signal and no infections related to encapsulated bacteria Five participants experienced mild/moderate Treatment Related AEs Two participants (one in each 300mg/2mL and 600mg/4mL S.C. MAD cohorts) had a mild or moderate injection site reactions (ISRs); no intervention was required and both participants completed treatment One participant experienced several non-specific AEs during infusion; infusion was paused for 8 minutes and restarted at the same rate without sequelae Two participants in 50mg/kg SAD I.V.1 cohort became ANA2 positive at Day 57; both participants had no evidence of SLE and both tested negative for dsDNA3 One participant in 600mg/4mL S.C. SAD reported vomiting on Day 1, which resolved on same day Highest dose to be used in Phase 2 trials is single I.V. loading dose of 20mg/kg Non-specific indicator of autoimmune disease present in up to 25% of healthy individuals: https://www.labcorp.com/assets-media/2785 Anti-double-stranded deoxyribonucleic acid antibodies are highly specific markers of systemic lupus erythematosus or SLE
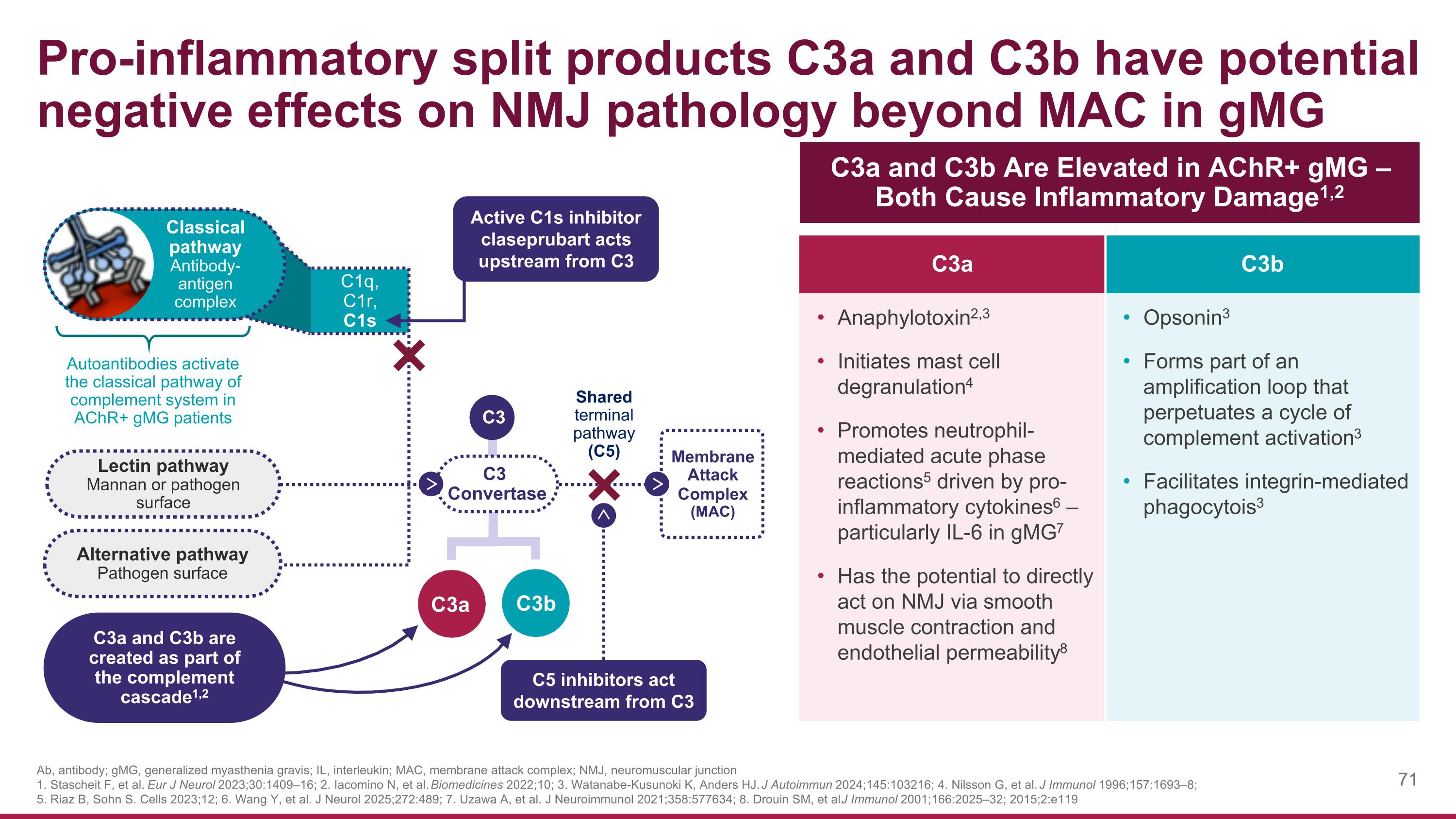
C3a and C3b Are Elevated in AChR+ gMG – Both Cause Inflammatory Damage1,2 C3a C3b Anaphylotoxin2,3 Initiates mast cell degranulation4 Promotes neutrophil-mediated acute phase reactions5 driven by pro-inflammatory cytokines6 – particularly IL-6 in gMG7 Has the potential to directly act on NMJ via smooth muscle contraction and endothelial permeability8 Opsonin3 Forms part of an amplification loop that perpetuates a cycle of complement activation3 Facilitates integrin-mediated phagocytois3 Pro-inflammatory split products C3a and C3b have potential negative effects on NMJ pathology beyond MAC in gMG C1q, C1r, C1s Membrane Attack Complex (MAC) Lectin pathway Mannan or pathogen surface Autoantibodies activate the classical pathway of complement system in AChR+ gMG patients Alternative pathway Pathogen surface C3 Convertase Classical pathway Antibody-antigen complex C3 C3b C3a C3a and C3b are created as part of the complement cascade1,2 Shared terminal pathway (C5) C5 inhibitors act downstream from C3 Active C1s inhibitor claseprubart acts upstream from C3 Ab, antibody; gMG, generalized myasthenia gravis; IL, interleukin; MAC, membrane attack complex; NMJ, neuromuscular junction 1. Stascheit F, et al. Eur J Neurol 2023;30:1409–16; 2. Iacomino N, et al. Biomedicines 2022;10; 3. Watanabe-Kusunoki K, Anders HJ. J Autoimmun 2024;145:103216; 4. Nilsson G, et al. J Immunol 1996;157:1693–8; 5. Riaz B, Sohn S. Cells 2023;12; 6. Wang Y, et al. J Neurol 2025;272:489; 7. Uzawa A, et al. J Neuroimmunol 2021;358:577634; 8. Drouin SM, et al. J Immunol 2001;166:2025–32; 2015;2:e119

SENIOR MANAGEMENT Accomplished team of biotech industry veterans and scientists committed to bringing innovation to market Select Experience Includes: BOARD OF DIRECTORS Alison Lawton Chair of the Board Board Member, ProQR and X4, Prior Chair of Board, Magenta Sujay Kango Board Member, Adanate, Inc. Anne McGeorge Board Member, The Oncology Institute, Board Member, Be the Match Simon Read, Ph.D. Former CEO and Founder, Mariana Oncology, Venture Partner, Atlas Venture, Former CSO, Ra Pharma Steven Romano, M.D. EVP, Chief Research & Development Officer, Silence Therapeutics, Inc. Paula Soteropoulos Venture Partner, 5AM Ventures Jonathan Violin, Ph.D. Venture Partner, Fairmount, Co-founder of Dianthus, Board Member, Astria Therapeutics, and former President/CEO of Viridian Therapeutics Marino Garcia President & CEO, Dianthus Select Autoimmune Drugs Developed by Dianthus Team Polly Hanff Head of Quality Scott Nogi Head of Business Operations Marino Garcia President & CEO Jennifer Davis Ruff Head of Investor Relations & Corporate Affairs Ronny Hashmonay, M.D. Chief Development & Medical Affairs Officer Simrat Randhawa, M.D. EVP, Head of R&D Ryan Savitz Chief Financial Officer & Chief Business Officer John C. King Chief Commercial Officer Kristina Maximenko Chief People Officer Adam Veness, Esq. General Counsel Rivka Gluck, R.N. Head of Clinical Development, Operations Debra Segal Head of Regulatory Affairs Edward Carr Chief Accounting Officer Jud Taylor Head of Technical Operations Jennifer Cross VP, Pipeline Strategy & Research Angel Cooper VP, Program Management