
CO PY RI GH T © A BC EL LE RA January 14, 2025 AbCellera Corporate Overview

These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors are described under "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and elsewhere in the documents we file with the Securities and Exchange Commission from time to time. We caution you that forward-looking statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent our views as of the date hereof. We undertake no obligation to update any forward-looking statements for any reason, except as required by law. DISCLAIMER This presentation contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. The forward-looking statements are based on management’s beliefs and assumptions and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including statements regarding our ability to develop, commercialize and achieve market acceptance of our current and planned products and services, our research and development efforts, and other matters regarding our business strategies, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. 2 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew

3 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew We are a clinical-stage biotech company focused on developing novel antibody medicines. Founded: 2012 Employees: ~600 Locations: Vancouver & Montreal, Canada Sydney, Australia IPO: December 2020 Liquidity: $680M* Partnerships* 100+ Programs 40+ Partners Platform $500M+ In total platform investments We have built a fully integrated antibody drug platform from discovery to clinical manufacturing and development. We form strategic partnerships with companies that bring novel biology or technology. *As of September 30, 2025 1. AbCellera-led programs 2. Partner-led programs, including under Trianni licenses, to have reached the clinic 16 Molecules in the clinic2 We are advancing an internal pipeline of programs. 20+ Internal program starts 2 Molecules in the clinic1 Programs 2 Molecules in IND-enabling activities 300K+sq ft of research and manufacturing facilities

4 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Our platform was built through 10+ years of drug discovery partnerships. Since 2014, we have partnered with some of the industry’s most innovative pharma and biotech companies. Partnerships were a driver for R&D, and provided near-term revenue in the form of research payments and long-term potential revenue in the form of royalty stakes in those drug programs. In 2023, we shifted our focus from partnerships to advancing a pipeline of internal and co-developed programs. partnered-initiated therapeutic programs with downstreams* molecules from partnered-led programs have reached the clinic* 100+ 16 AbbVie *As of September 30, 2025

5 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Use our competitive advantage in antibody drug creation to build a pipeline of differentiated assets. STRATEGY Discovery for GPCR and ion channel targets Novel modalities, including multi-specifics and ADCs Indication agnostic
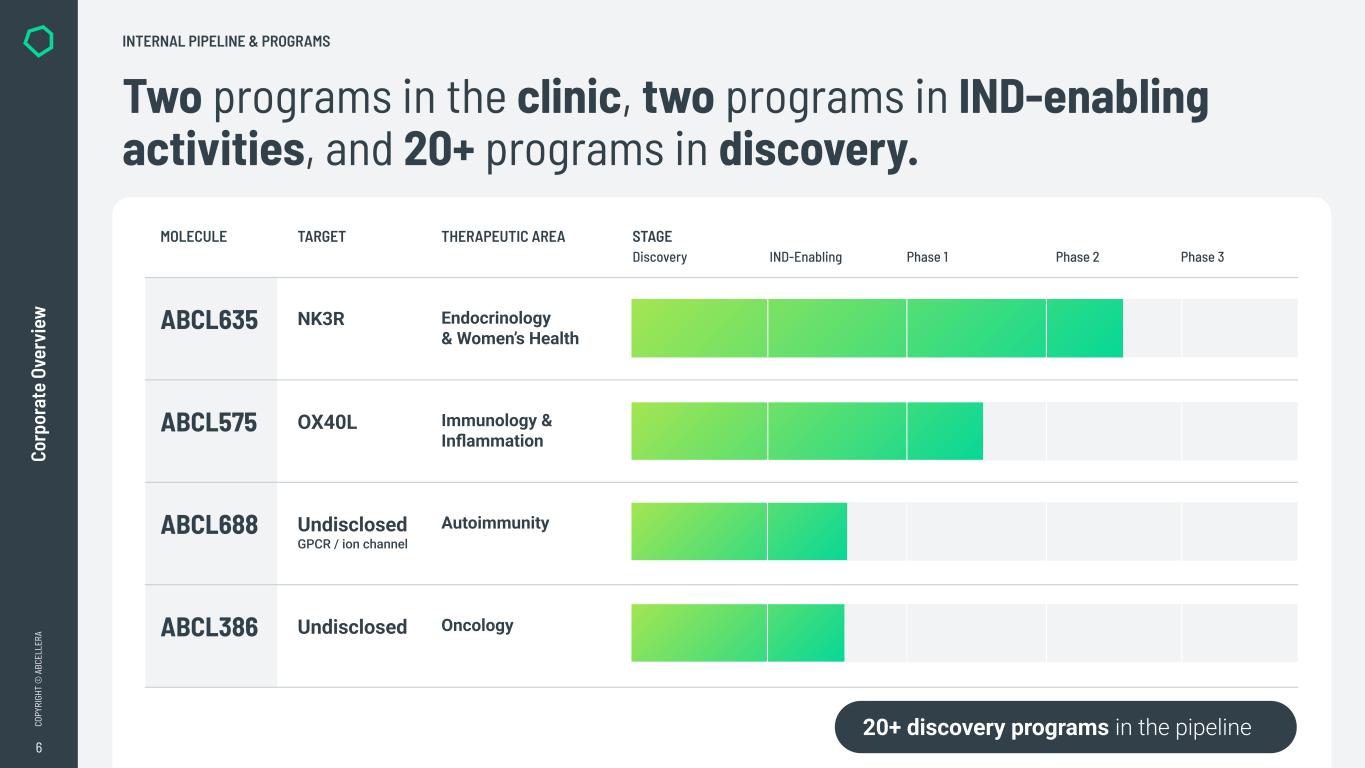
6 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Two programs in the clinic, two programs in IND-enabling activities, and 20+ programs in discovery. INTERNAL PIPELINE & PROGRAMS 20+ discovery programs in the pipeline MOLECULE TARGET THERAPEUTIC AREA STAGE Discovery IND-Enabling Phase 1 Phase 2 Phase 3 ABCL635 NK3R Endocrinology & Women’s Health ABCL575 OX40L Immunology & Inflammation ABCL688 Undisclosed GPCR / ion channel Autoimmunity ABCL386 Undisclosed Oncology
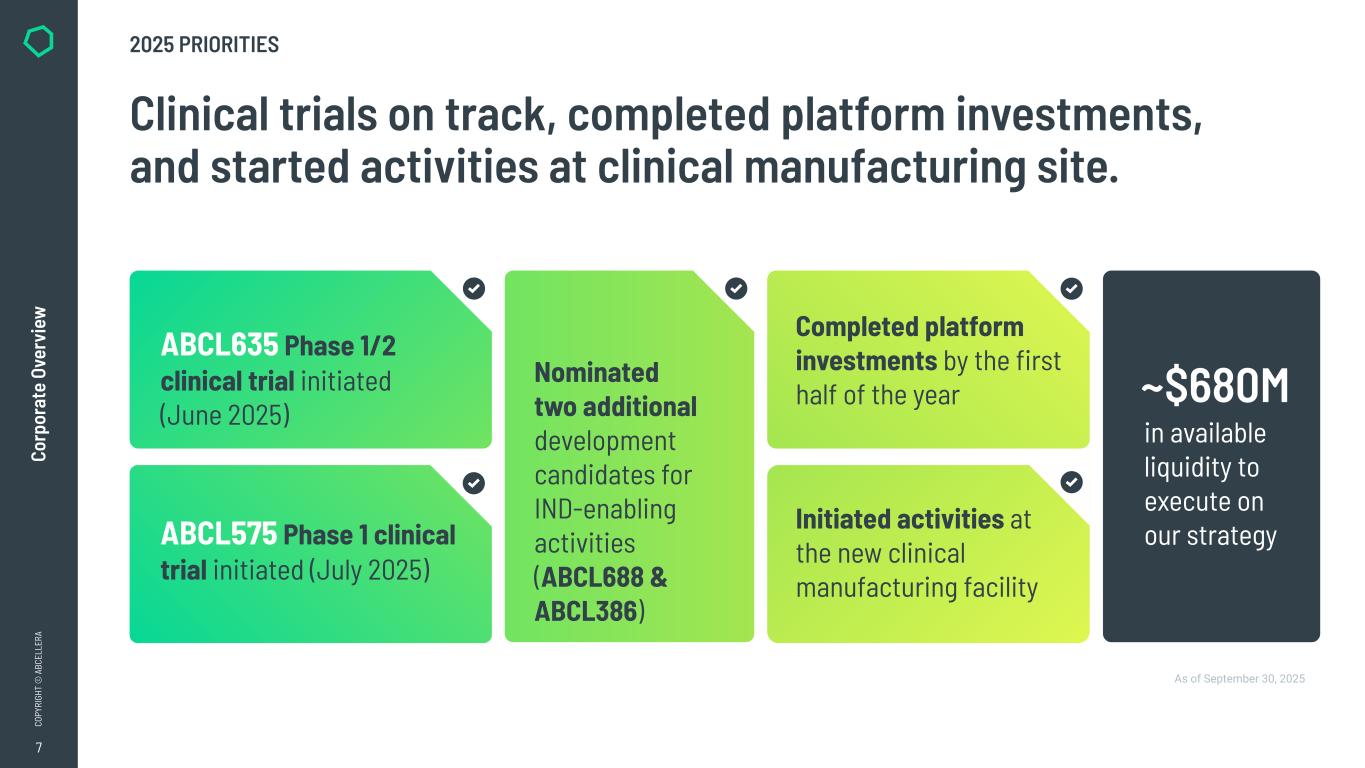
7 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew 2025 PRIORITIES As of September 30, 2025 Clinical trials on track, completed platform investments, and started activities at clinical manufacturing site. ABCL635 Phase 1/2 clinical trial initiated (June 2025) ABCL575 Phase 1 clinical trial initiated (July 2025) Completed platform investments by the first half of the year Initiated activities at the new clinical manufacturing facility Nominated two additional development candidates for IND-enabling activities (ABCL688 & ABCL386) in available liquidity to execute on our strategy ~$680M
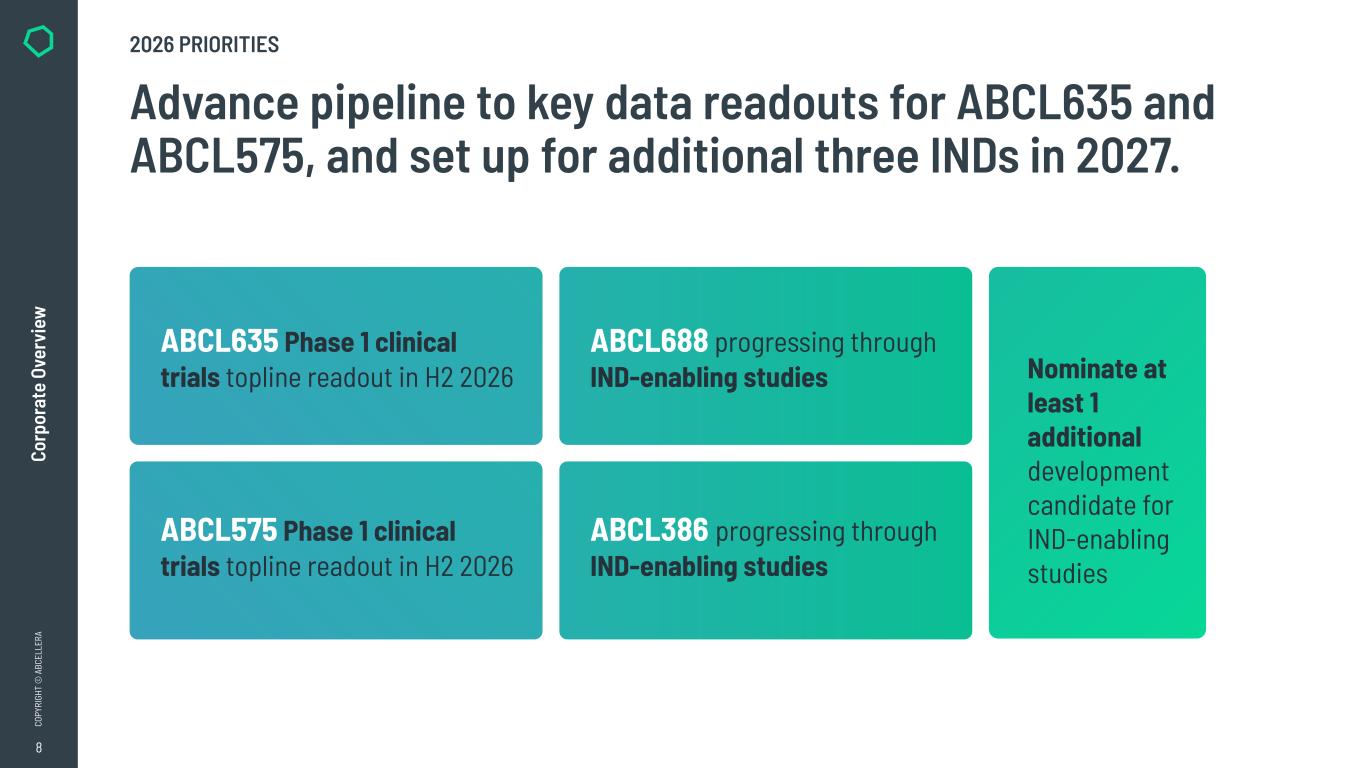
8 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Advance pipeline to key data readouts for ABCL635 and ABCL575, and set up for additional three INDs in 2027. Nominate at least 1 additional development candidate for IND-enabling studies 2026 PRIORITIES ABCL635 Phase 1 clinical trials topline readout in H2 2026 ABCL575 Phase 1 clinical trials topline readout in H2 2026 ABCL688 progressing through IND-enabling studies ABCL386 progressing through IND-enabling studies
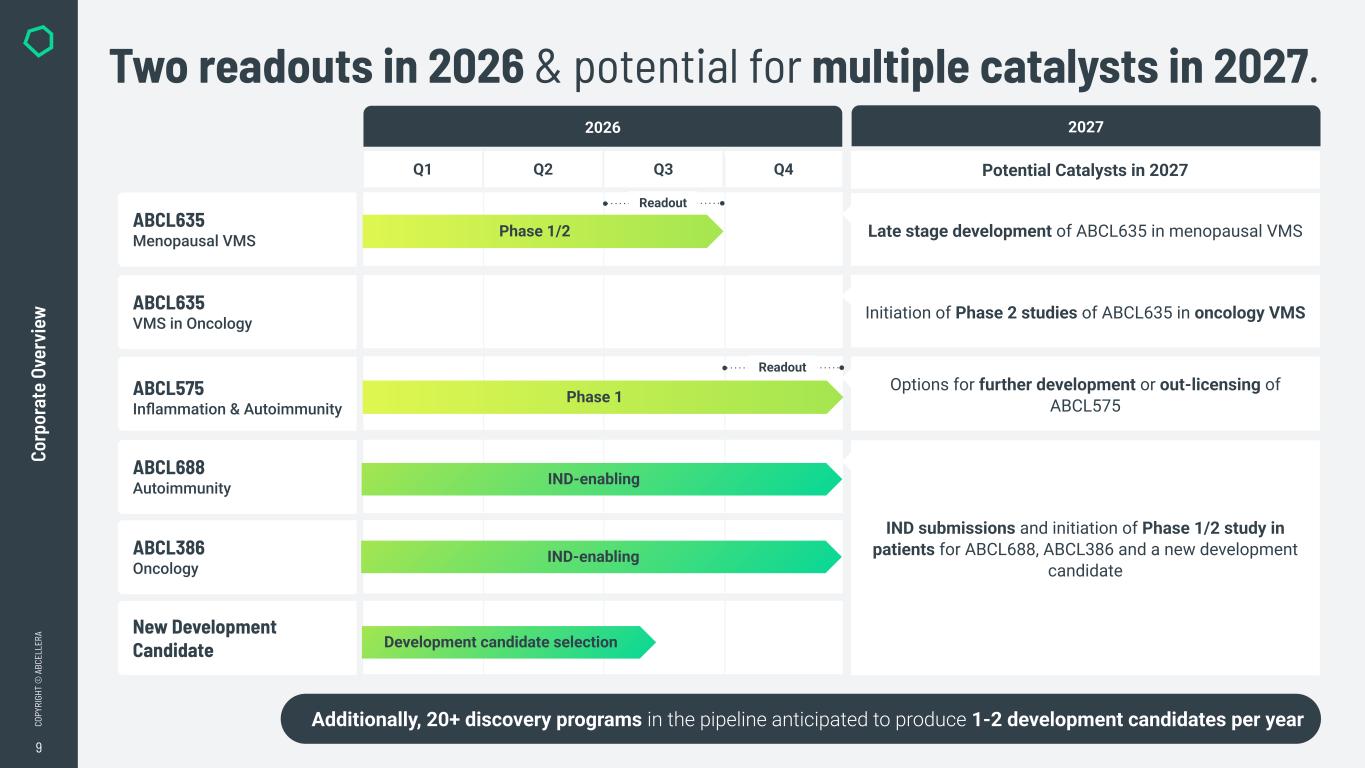
9 CO PY RI GH T © A BC EL LE RA Q1 Q2 Q3 Q4 Co rp or at e Ov er vi ew Two readouts in 2026 & potential for multiple catalysts in 2027. 2026 ABCL635 Menopausal VMS ABCL635 VMS in Oncology ABCL575 Inflammation & Autoimmunity ABCL386 Oncology ABCL688 Autoimmunity New Development Candidate Development candidate selection Phase 1/2 Phase 1 Additionally, 20+ discovery programs in the pipeline anticipated to produce 1-2 development candidates per year IND-enabling IND-enabling 2027 Late stage development of ABCL635 in menopausal VMS Potential Catalysts in 2027 Initiation of Phase 2 studies of ABCL635 in oncology VMS Options for further development or out-licensing of ABCL575 IND submissions and initiation of Phase 1/2 study in patients for ABCL688, ABCL386 and a new development candidate Readout Readout

10 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Internal Programs
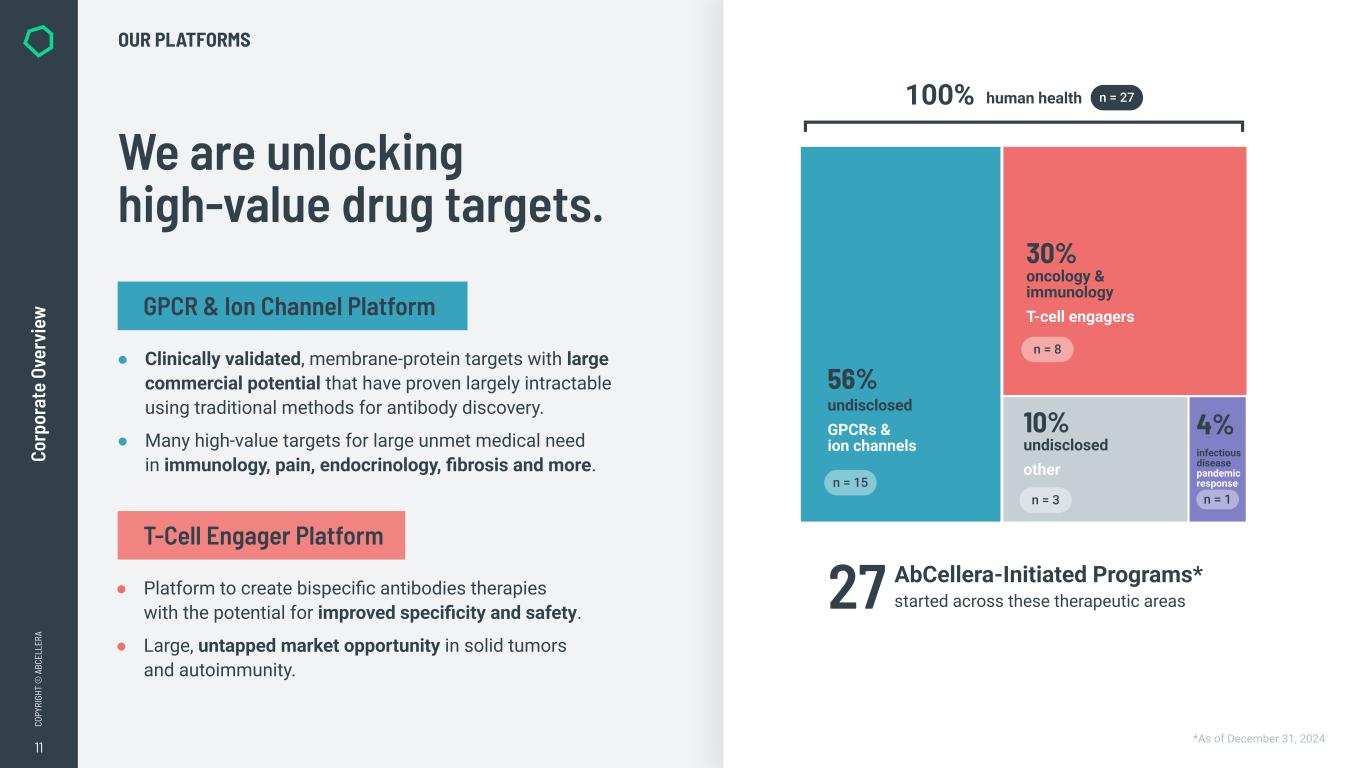
11 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew *As of December 31, 2024 We are unlocking high-value drug targets. OUR PLATFORMS ● Clinically validated, membrane-protein targets with large commercial potential that have proven largely intractable using traditional methods for antibody discovery. ● Many high-value targets for large unmet medical need in immunology, pain, endocrinology, fibrosis and more. ● Platform to create bispecific antibodies therapies with the potential for improved specificity and safety. ● Large, untapped market opportunity in solid tumors and autoimmunity. GPCR & Ion Channel Platform T-Cell Engager Platform AbCellera-Initiated Programs* started across these therapeutic areas27 100% human health n = 27 30% oncology & immunology T-cell engagers 56% undisclosed GPCRs & ion channels n = 8 n = 15 infectious disease pandemic response 4%10% undisclosed other n = 3 n = 1

12 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Internal Programs ABCL635
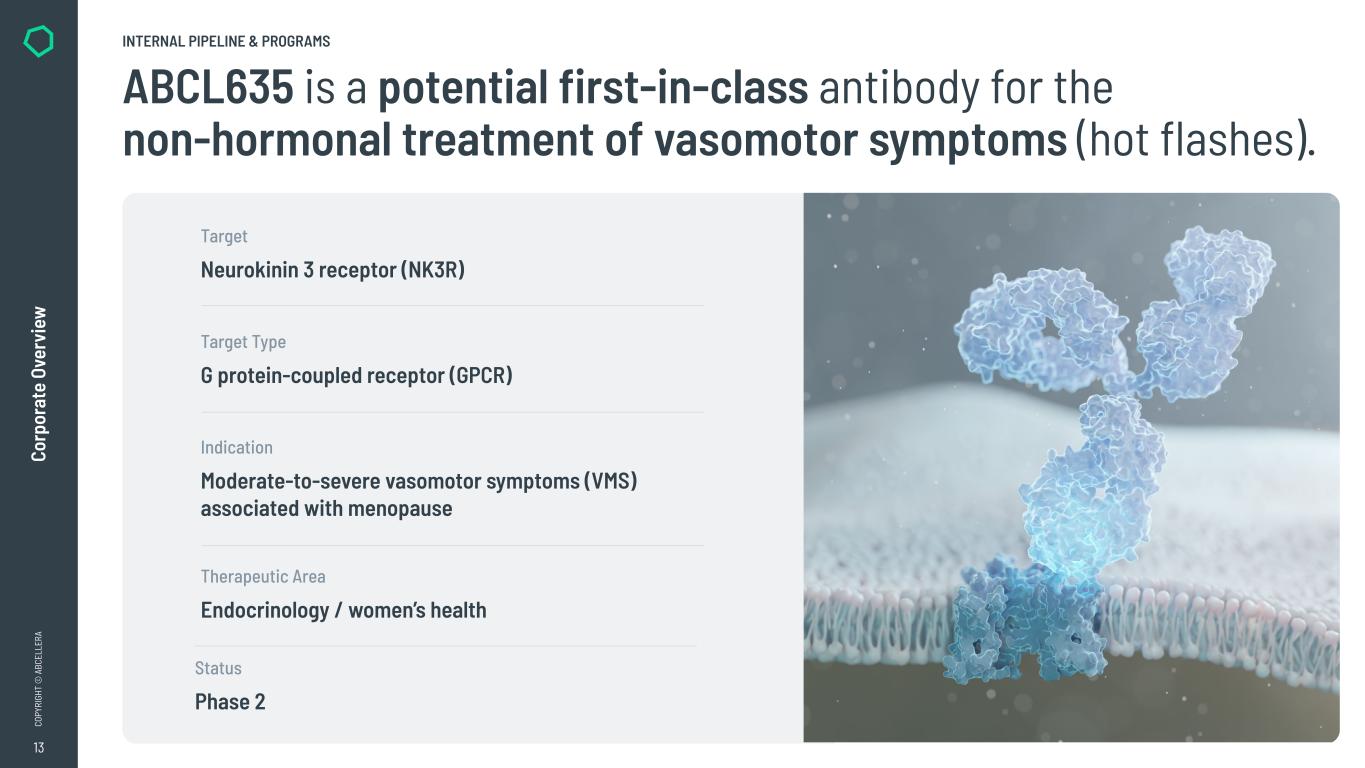
13 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew INTERNAL PIPELINE & PROGRAMS Neurokinin 3 receptor (NK3R) Target G protein-coupled receptor (GPCR) Target Type Moderate-to-severe vasomotor symptoms (VMS) associated with menopause Indication Endocrinology / women’s health Therapeutic Area Phase 2 Status ABCL635 is a potential first-in-class antibody for the non-hormonal treatment of vasomotor symptoms (hot flashes).
14 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew ● Potential for: ○ First-in-class antibody therapy ○ Enhanced efficacy ○ Differentiated safety profile ○ Monthly (Q4W) subcutaneous dosing schedule, preferred by women with VMS Science DifferentiationCommercial Opportunity Development Path ● NK3R is a GPCR involved in endocrine homeostasis and thermoregulation ● Pathway is clinically validated with small molecules ● Primary scientific risk is in achieving sufficient target engagement ● Approximately 6 million women with moderate-to- severe VMS in US2 ● Novel non-hormonal treatments for VMS are estimated to become a $2B+ market opportunity ● Well-established clinical development path ● Biomarkers enable assessment of target engagement in Phase 1 ● Safety and early efficacy data readouts in 2026 1. US Census Bureau. Women age 45-64. 2. Nappi RE, et. al. Menopause. 2021 May 24;28(8):875-882. doi: 10.1097/GME.0000000000001793. In Phase 2 clinical trial with readout anticipated in Q3 2026. ABCL635 NK3R Antagonist

15 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew VMS are highly prevalent, significantly impact health and well-being, and are the most common reason for seeking treatment for menopause. 1. Avis NE, et al. JAMA Intern Med. 2015 Apr;175(4):531-9. doi: 10.1001/jamainternmed.2014.8063.. 2. Thurston RC, et al. Obstet Gynecol Clin North Am. 2011 Sep;38(3):489-501. doi: 10.1016/j.ogc.2011.05.006. 3. Faubion SS, et al. Mayo Clin Proc. 2023 Jun;98(6):833-845. doi: 10.1016/j.mayocp.2023.02.025. 4. O'Neill MT, et al. Occup Med (Lond). 2023 Sep 29;73(6):332-338. doi: 10.1093/occmed/kqad078. Millions of women seek treatment VMS are a significant burden Commercial Opportunity ABCL635 VMS are the most common symptoms of menopause, persisting for a median of 7.4 years.1 They have a significant impact on quality of life, are associated with cardiovascular disease risk,2 and result in lost productivity, career advancement, and income.3,4,5 Approximately 40 million women are of menopausal age in the US.6 ~30% of women experience moderate-to-severe VMS,7 and it is estimated that more than half seek treatment for menopausal symptoms.8 5. Ko J, et al. Menopause Foundation of Canada; October 16, 2023. Accessed April 24, 2025. https://menopausefoundationcanada.ca/menopause-and-work-in-canada-report/ 6. US Census Bureau. Women age 45-64. 7. Nappi RE, et. al. Menopause. 2021 May 24;28(8):875-882. doi: 10.1097/GME.0000000000001793. 8. Todorova L, et al. Menopause. 2023 Dec 1;30(12):1179-1189. doi: 10.1097/GME.0000000000002265.
16 CO PY RI GH T © A BC EL LE RA In a global study, 57% of women were eligible for MHT, but against using it.1 Co rp or at e Ov er vi ew 1. Stute P, et al. Maturitas. 2022 Oct;164:38-45. doi: 10.1016/j.maturitas.2022.06.008. 2. “The 2023 Nonhormone Therapy Position Statement of The North American Menopause Society” Advisory Panel. 2023 Jun 1;30(6):573-590. doi: 10.1097/GME.0000000000002200. ✝ AbCellera estimate. Despite effective treatments, there remains a large unmet need for many women suffering from VMS. ~12% of women are contraindicated.1 Presently there are contraindications to MHT for estrogen-dependent cancers and cardiovascular disease.2 Menopause Hormone Therapy (MHT) is an effective treatment for VMS, and the current standard of care. However, there are many women who are contraindicated, have complications, or who choose not to take MHT. ABCL635 ~8% of women discontinue MHT within 12 months.1✝ Commercial Opportunity
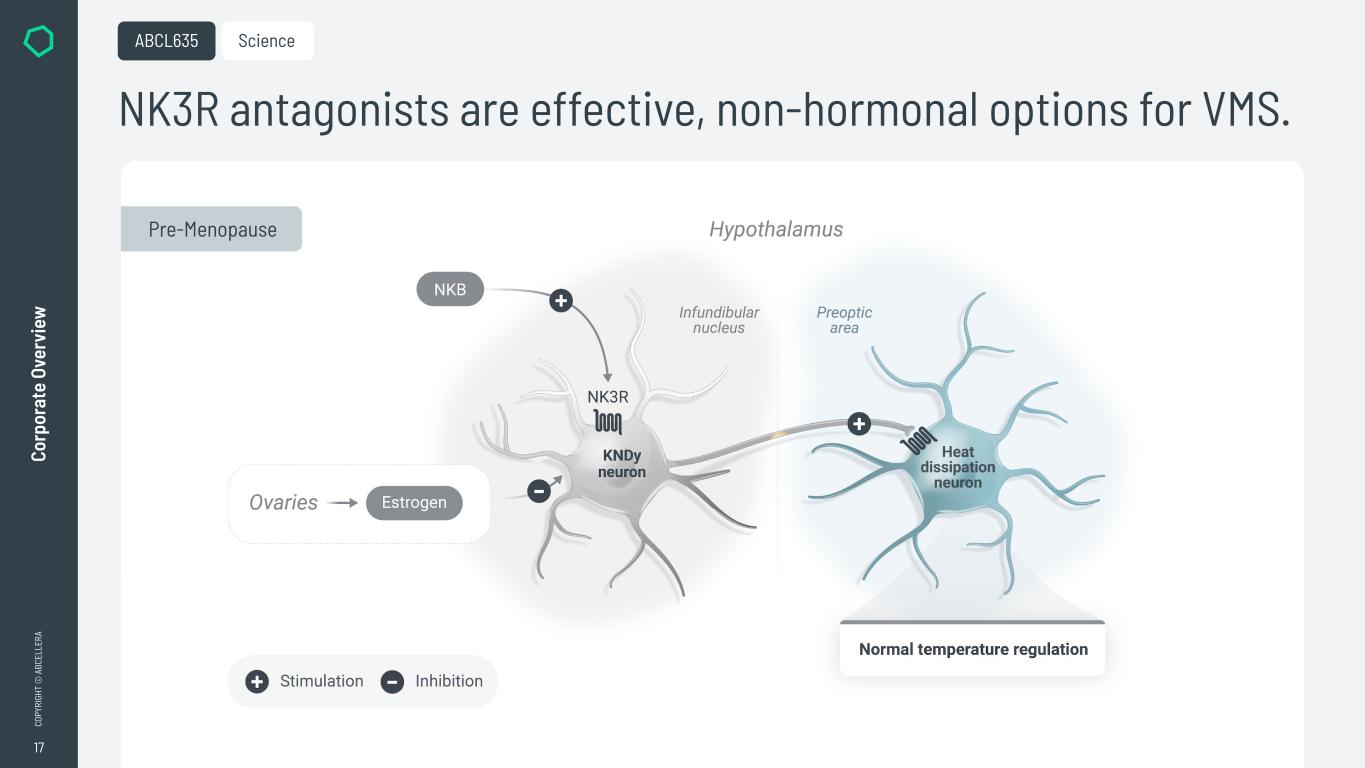
17 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew NK3R antagonists are effective, non-hormonal options for VMS. ABCL635 Science Pre-Menopause
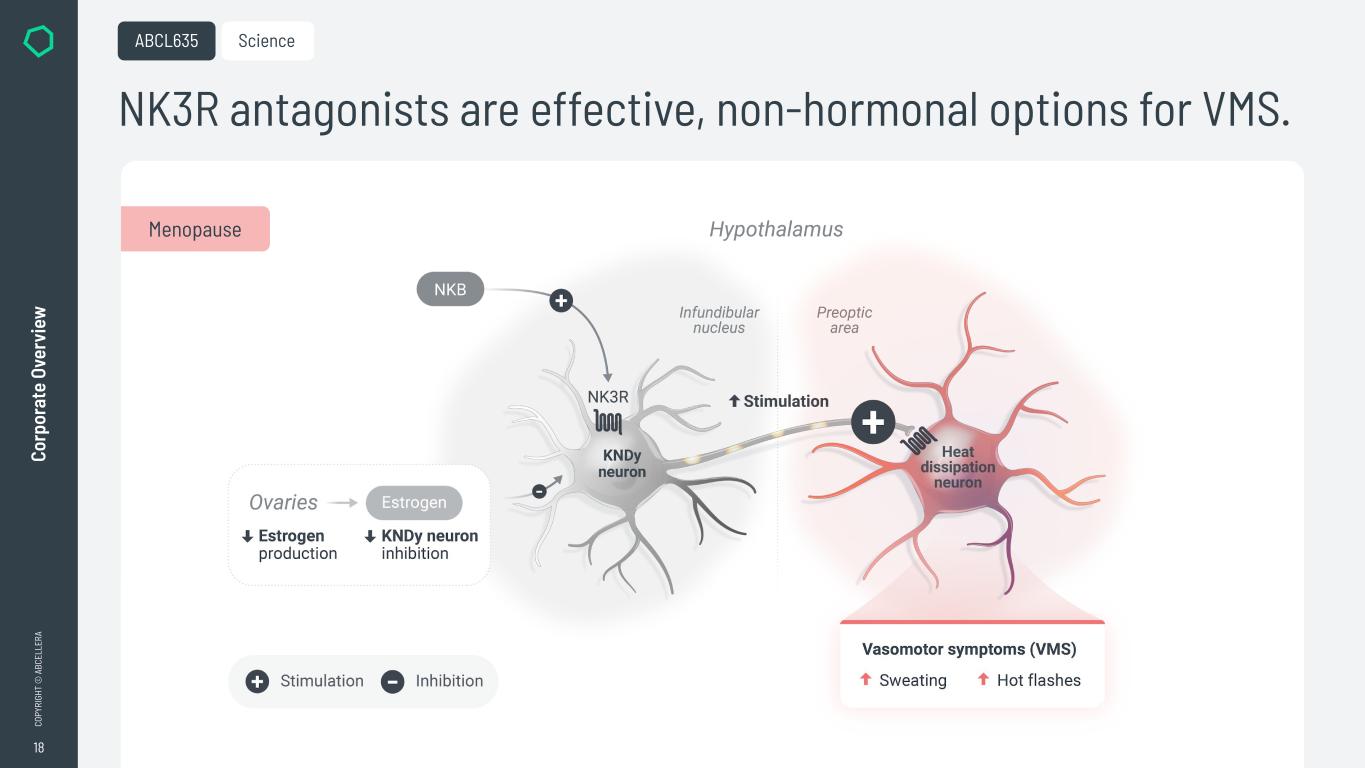
18 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew NK3R antagonists are effective, non-hormonal options for VMS. ABCL635 Menopause Science
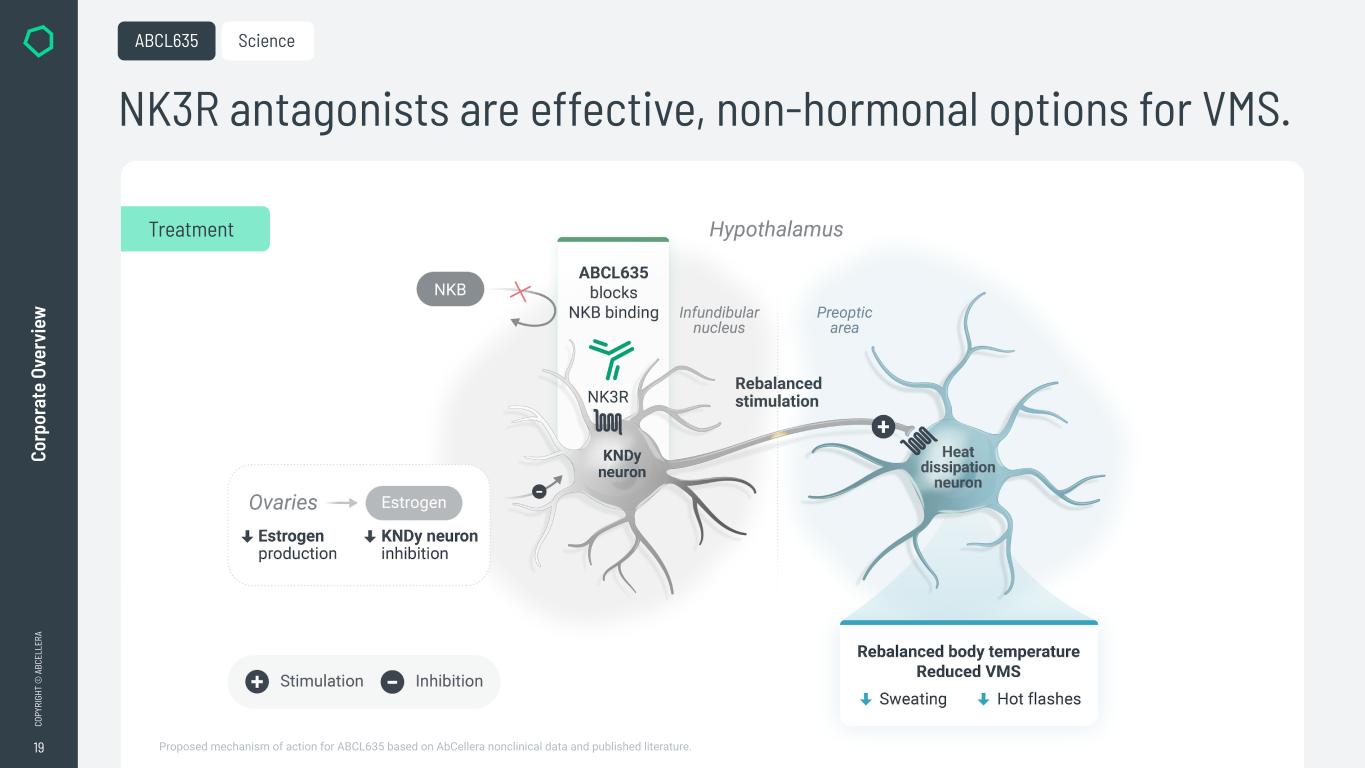
19 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew NK3R antagonists are effective, non-hormonal options for VMS. ABCL635 Treatment Science Proposed mechanism of action for ABCL635 based on AbCellera nonclinical data and published literature.

20 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Recently approved NK3R therapies are building the market. ABCL635 Small molecule NK3R antagonist Approved by US FDA on May 12, 2023 Stage Daily oral treatment Dosing ● Effective in reducing severity and frequency of VMS. ● Boxed warning for liver toxicity. Requires liver monitoring. Fezolinetant (Veozah®) by Astellas Small molecule NK3R and NK1R antagonist Approved by US FDA October 24, 2025 Stage Daily oral treatment Dosing ● Effective in reducing severity and frequency of VMS. ● Warnings for CNS depressant effect, daytime impairment, and liver enzyme elevation. Requires liver monitoring. Elinzanetant (LynkuetTM) by Bayer Differentiation CNS: Central nervous system
21 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew ABCL635 is designed to offer an improved treatment option for women with moderate-to-severe VMS due to menopause. 1. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Monoclonal Antibodies. [Updated 2024 Dec 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548844/. 2. Pinkerton JV, et al. JAMA. 2024 Aug 22;332(16):1343–54. doi: 10.1001/jama.2024.14618. 3. Lederman S,et al. Lancet. 2023 Apr 1;401(10382):1091-1102. doi: 10.1016/S0140-6736(23)00085-5. An antibody-based therapeutic may provide several benefits over current non-hormonal treatments: ABCL635 Differentiation 4. Johnson KA, et al. J Clin Endocrinol Metab. 2023 Jul 14;108(8):1981-1997. doi: 10.1210/clinem/dgad058. 5. Panay N., et al. Poster presentation at the North American Menopause Society (NAMS) Annual Meeting, [September 10 – 14, 2024]. Poster number P-121. 6. AbCellera. Sponsored primary market research, 2024. Survey question: If you were presented with two products that were equally efficacious and safe, with similar side effect profiles, which of the following would you prefer to take? Reduced toxicities & side-effects Antibodies are generally not associated with drug-related liver toxicity.1 ABCL635 does not antagonize NK1R, and is therefore not expected to induce fatigue or somnolence.2, 3, 4, 5 Dosing flexibility Over 50% of women with VMS would prefer an injectable every 4 weeks over a daily oral treatment.6 Increasing use of GLP-1 agonists is significantly increasing the autoinjector-experienced population. Enhanced efficacy Wider therapeutic index and longer half-life may enable better target engagement.

22 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Internal Programs ABCL575
23 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew ABCL575 is a potential best-in-class antibody for the treatment atopic dermatitis. INTERNAL PIPELINE & PROGRAMS OX40 Ligand (OX40L) Target Atopic Dermatitis (AD) Indication Immunology & Inflammation Therapeutic Area Phase 1 Status

24 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew INTERNAL PIPELINE & PROGRAMS ● Competitive space with two late stage programs targeting OX40L (amlitelimab) and OX40 (rocatilimab) ● ABCL575 expected to support Q24W or longer dosing schedule Science DifferentiationCommercial Opportunity Development Path ● OX40L mechanism of action established in atopic dermatitis with a favourable safety profile ● High potential across multiple immunology and inflammation (I&I) indications (asthma, alopecia, HS, celiac etc.) ● Attractive pathway for development of combinations in I&I • Atopic dermatitis is an $11B+* market, growing at over 25% • Need for alternatives beyond IL-13 and IL-4/13 classes in both 1st line and 2nd line (more than 20%** of dupilumab patients discontinue) • Potential of OX40L class across multiple indications is being evaluated ● Well-established clinical development path ● Safety and PK readouts in 2026 Readout of Phase 1 clinical study anticipated in mid 2026 ABCL575 OX40L Antagonist * Cantor Fitzgerald Estimate, September, 2024 ** Spekhorst et al. JAMA Dermatol. 2022; 158(9): 1048
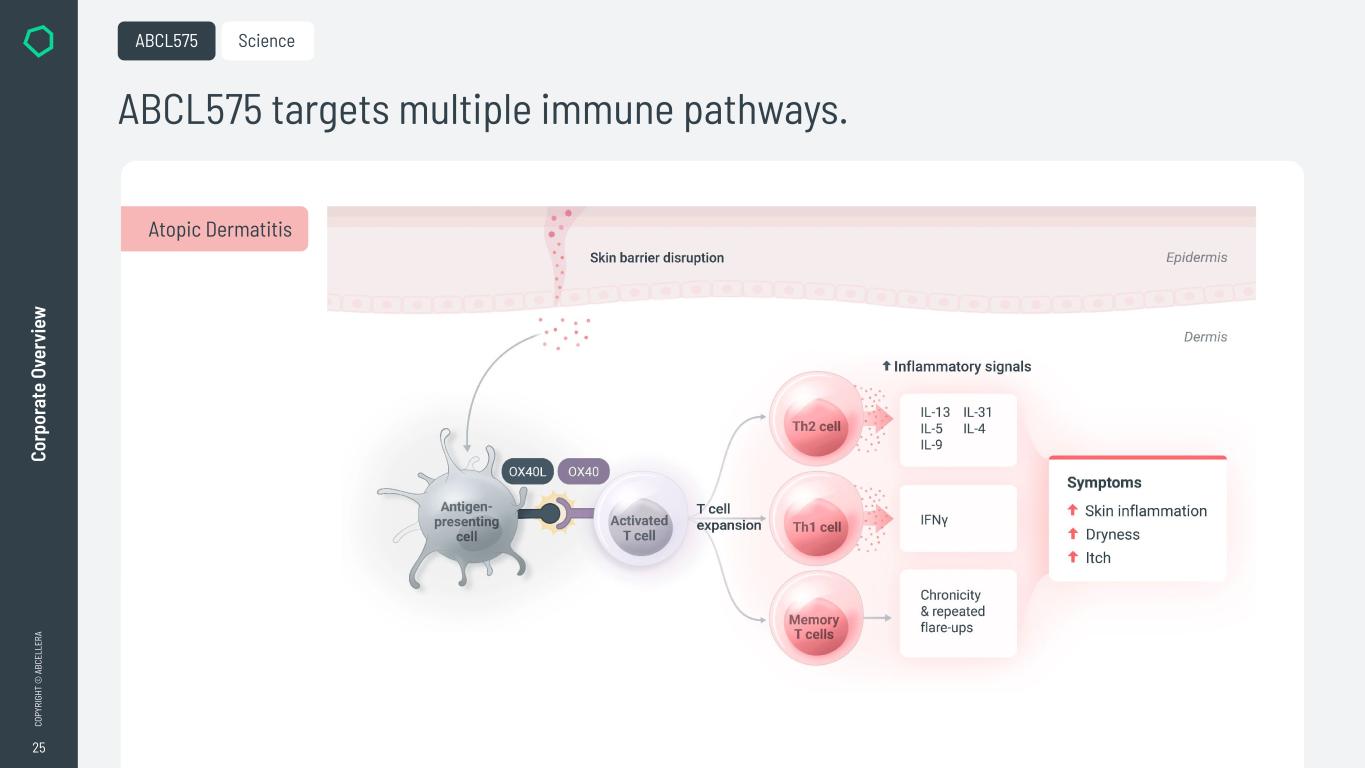
25 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew ABCL575 targets multiple immune pathways. ABCL575 Science Atopic Dermatitis
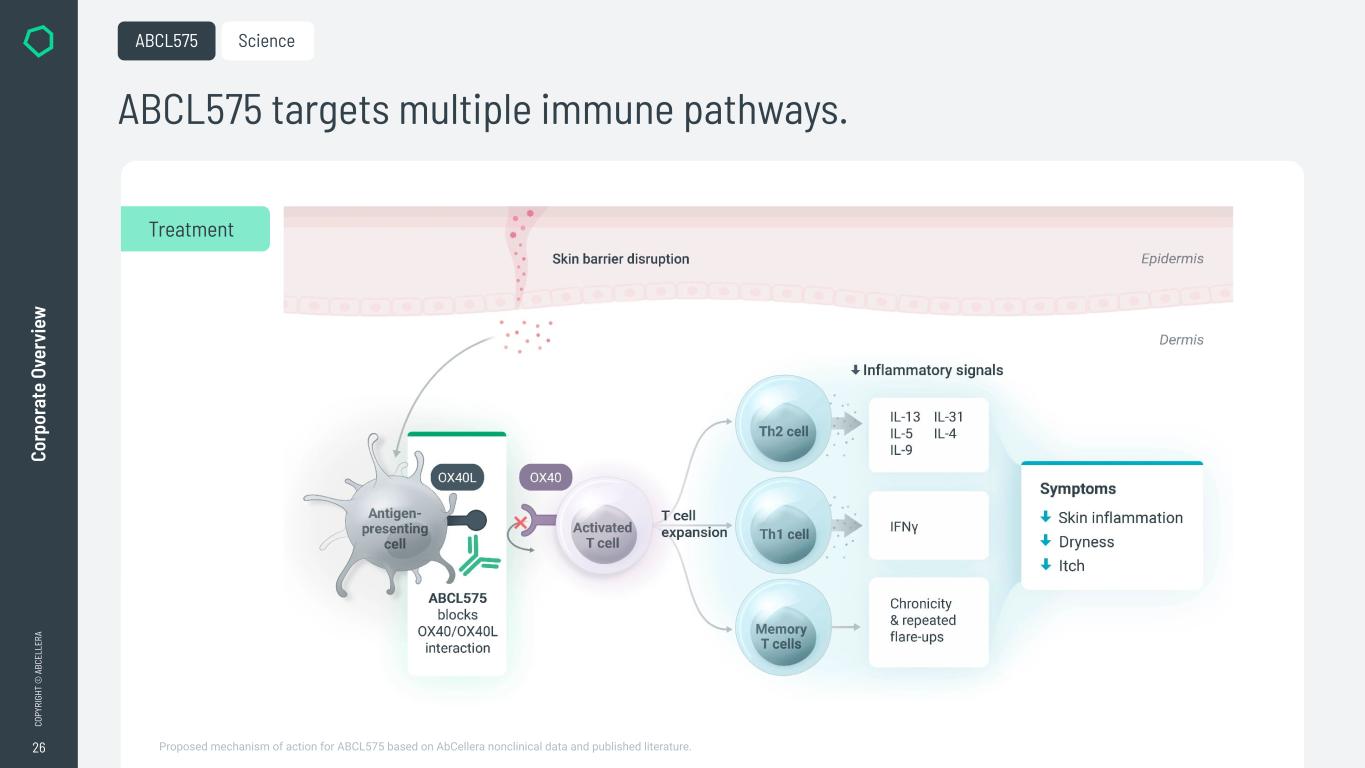
26 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew ABCL575 targets multiple immune pathways. ABCL575 Science Treatment Proposed mechanism of action for ABCL575 based on AbCellera nonclinical data and published literature.

27 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Royalty Portfolio & Partnered Programs
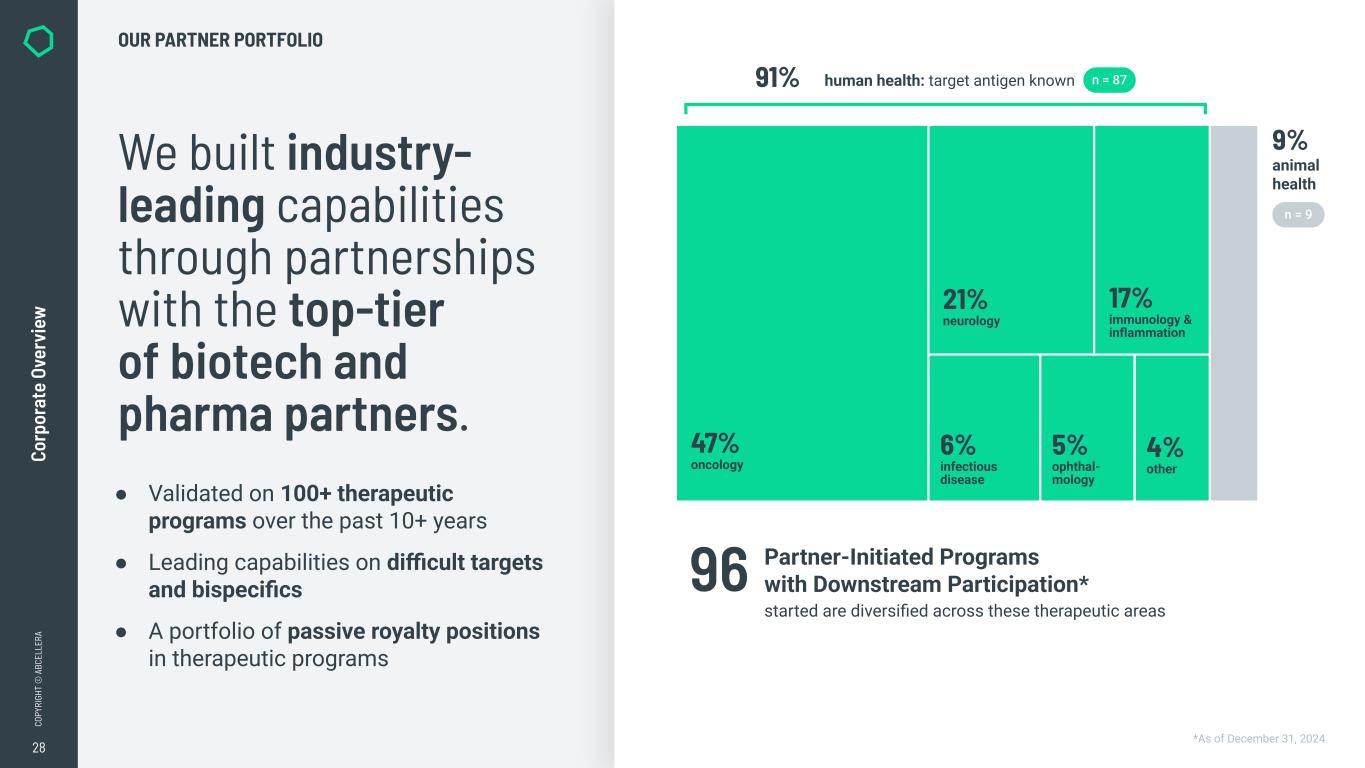
28 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew We built industry- leading capabilities through partnerships with the top-tier of biotech and pharma partners. ● Validated on 100+ therapeutic programs over the past 10+ years ● Leading capabilities on difficult targets and bispecifics ● A portfolio of passive royalty positions in therapeutic programs OUR PARTNER PORTFOLIO *As of December 31, 2024 Partner-Initiated Programs with Downstream Participation* started are diversified across these therapeutic areas 96 91% human health: target antigen known n = 87 9% animal health n = 9 21% neurology 6% infectious disease 5% ophthal- mology 4% other 17% immunology & inflammation 47% oncology
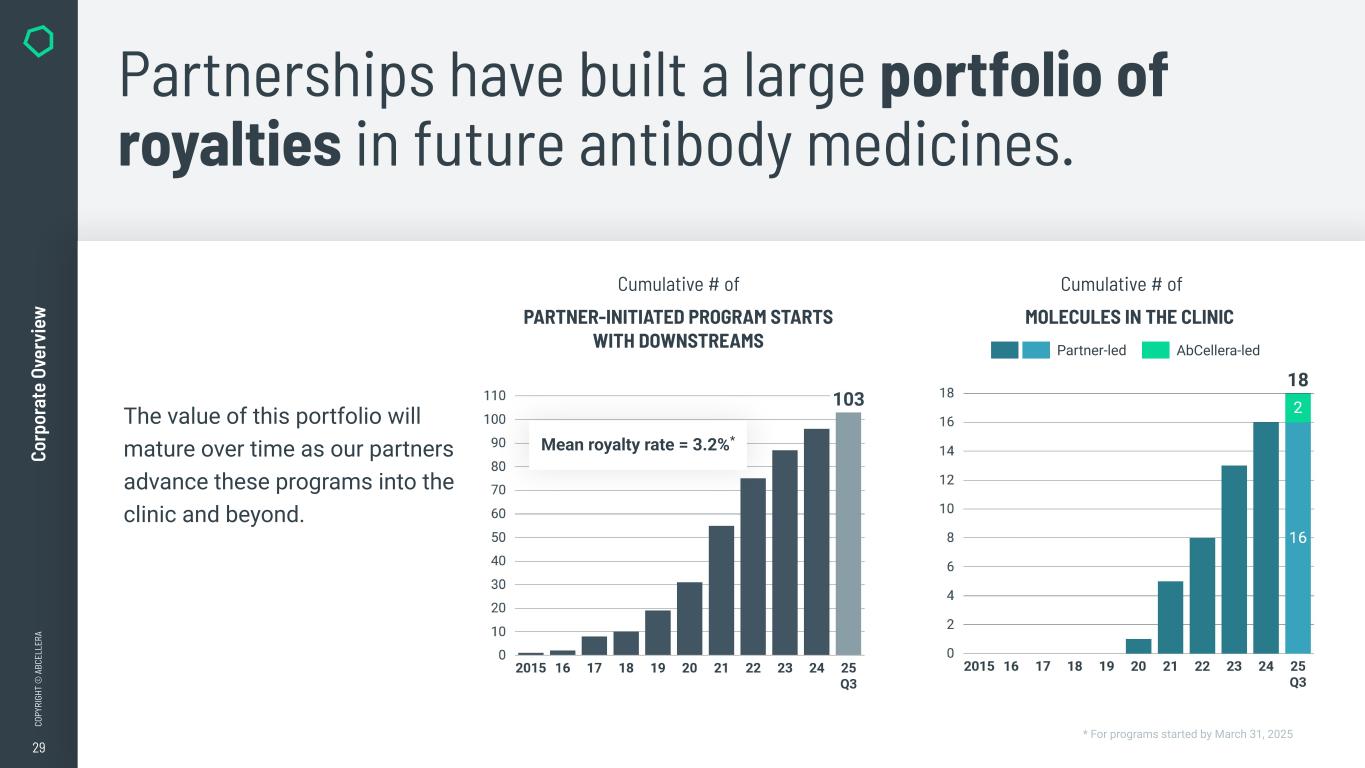
29 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Partnerships have built a large portfolio of royalties in future antibody medicines. The value of this portfolio will mature over time as our partners advance these programs into the clinic and beyond. * For programs started by March 31, 2025 PARTNER-INITIATED PROGRAM STARTS WITH DOWNSTREAMS Cumulative # of MOLECULES IN THE CLINIC Cumulative # of Mean royalty rate = 3.2%* AbCellera-led Partner-led
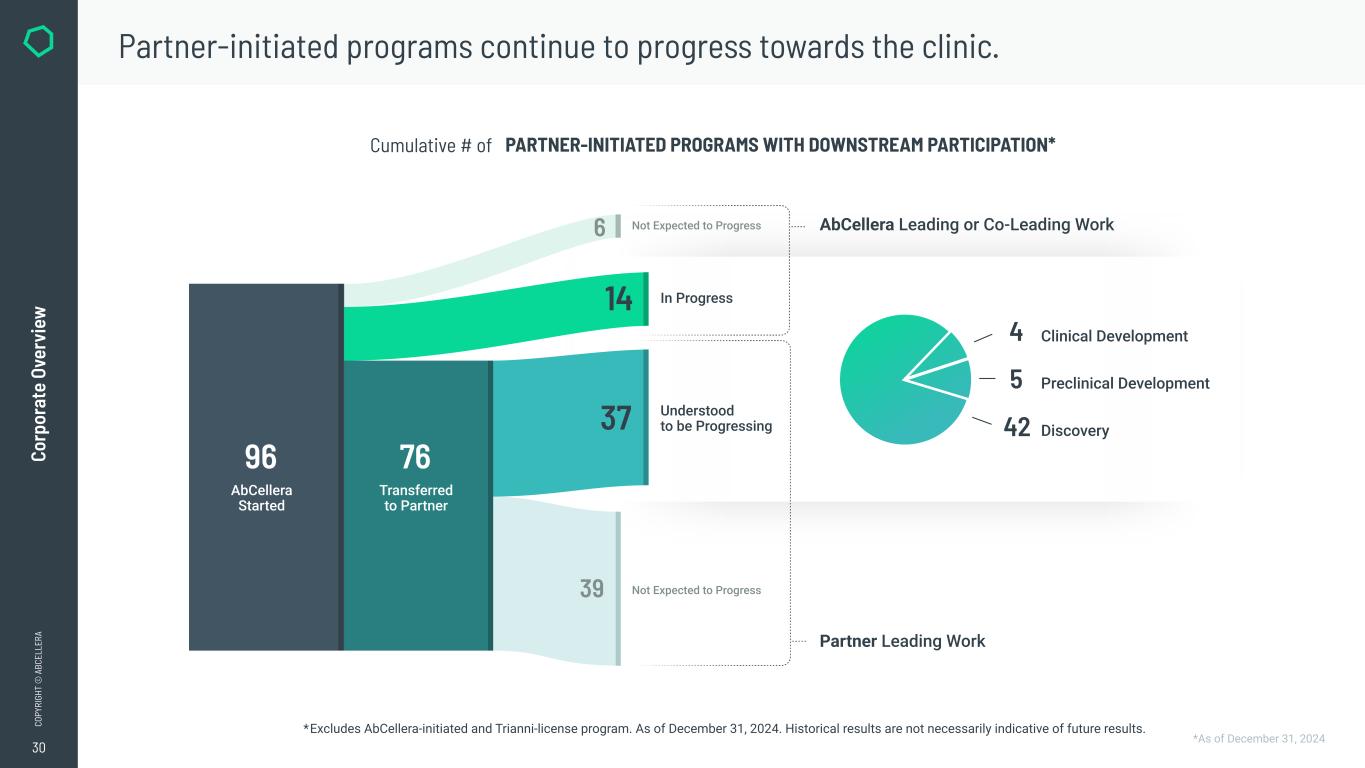
30 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew Partner-initiated programs continue to progress towards the clinic. PARTNER-INITIATED PROGRAMS WITH DOWNSTREAM PARTICIPATION*Cumulative # of *Excludes AbCellera-initiated and Trianni-license program. As of December 31, 2024. Historical results are not necessarily indicative of future results. 96 6 76 39 14 37 42 5 4 Understood to be Progressing In Progress AbCellera Leading or Co-Leading Work Partner Leading Work AbCellera Started Transferred to Partner Clinical Development Preclinical Development Discovery Not Expected to Progress Not Expected to Progress *As of December 31, 2024
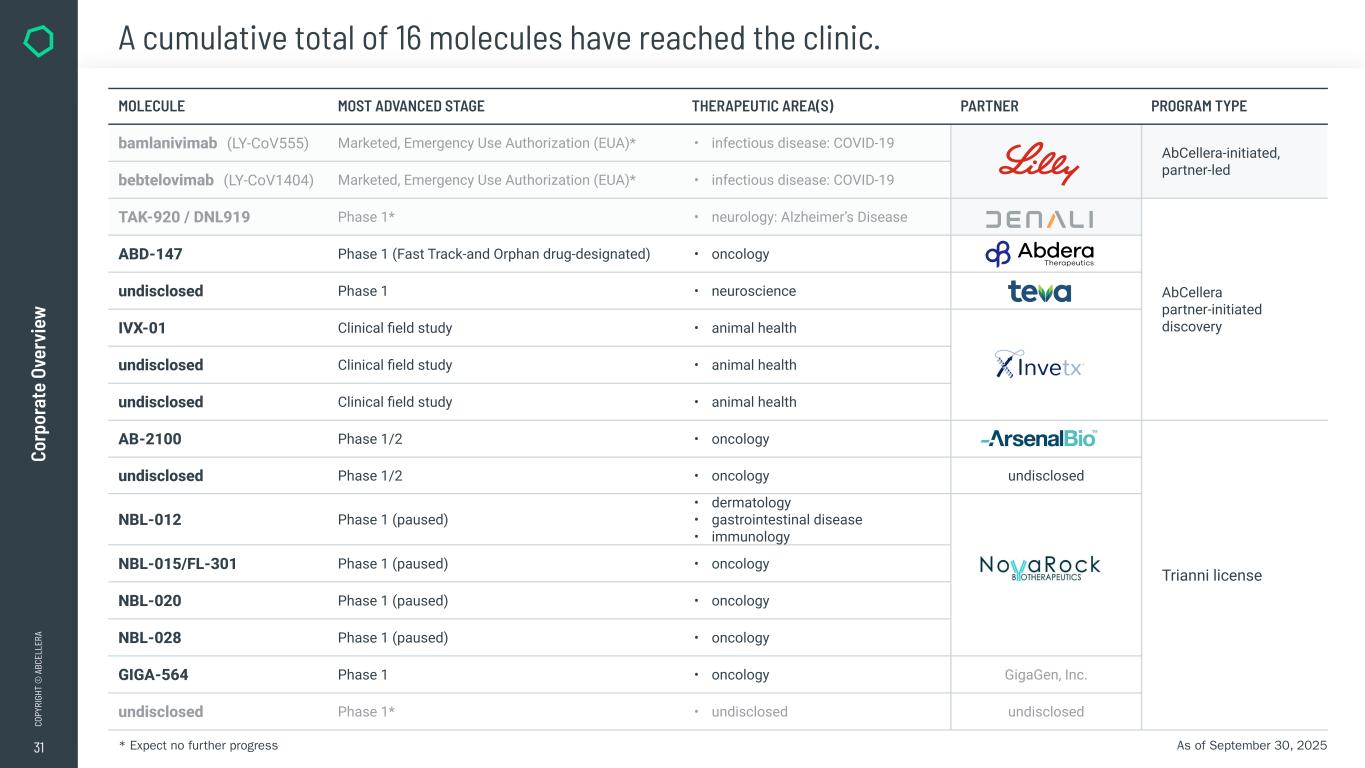
31 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew A cumulative total of 16 molecules have reached the clinic. MOLECULE MOST ADVANCED STAGE THERAPEUTIC AREA(S) PARTNER PROGRAM TYPE bamlanivimab (LY-CoV555) Marketed, Emergency Use Authorization (EUA)* • infectious disease: COVID-19 AbCellera-initiated, partner-led bebtelovimab (LY-CoV1404) Marketed, Emergency Use Authorization (EUA)* • infectious disease: COVID-19 TAK-920 / DNL919 Phase 1* • neurology: Alzheimer’s Disease AbCellera partner-initiated discovery ABD-147 Phase 1 (Fast Track-and Orphan drug-designated) • oncology undisclosed Phase 1 • neuroscience IVX-01 Clinical field study • animal health undisclosed Clinical field study • animal health undisclosed Clinical field study • animal health AB-2100 Phase 1/2 • oncology Trianni license undisclosed Phase 1/2 • oncology undisclosed NBL-012 Phase 1 (paused) • dermatology • gastrointestinal disease • immunology NBL-015/FL-301 Phase 1 (paused) • oncology NBL-020 Phase 1 (paused) • oncology NBL-028 Phase 1 (paused) • oncology GIGA-564 Phase 1 • oncology GigaGen, Inc. undisclosed Phase 1* • undisclosed undisclosed As of September 30, 2025* Expect no further progress

32 CO PY RI GH T © A BC EL LE RA Co rp or at e Ov er vi ew THANK YOU