

KRRO-121: A Potential First-in-Class Treatment for Ammonia Control January 27th, 2026

Forward-Looking Statements and Disclaimers Forward-Looking Statements Certain statements in this presentation may constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements include, but are not limited to, express or implied statements regarding expectations, hopes, beliefs, intentions or strategies of Korro Bio, Inc. (Korro) regarding the future including, without limitation, express or implied statements regarding: the timing of the regulatory filing for KRRO-121; KRRO-121’s pan-urea cycle disorder (UCD) potential; KRRO-121’s first in class potential as a treatment for ammonia control; KRRO-121’s ability to drive strong patient engagement and recruitment in clinical trials; KRRO-121’s pipeline-in-a-product, blockbuster potential; KRRO-121’s differentiation and potential impact for patients; among others. In addition, any statements that refer to projections, forecasts, or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “would,” “aim,” “target,” “commit,” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Forward-looking statements are based on current expectations and assumptions that, while considered reasonable are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including risks inherent in biopharmaceutical development; risks associated with pre-clinical studies and clinical studies; risks associated with validating in clinical trials observations from pre-clinical studies; along with other risks inherent in biopharmaceutical development; and other risks associated with obtaining regulatory approvals and protecting intellectual property; as well as risks associated with general economic conditions (including recent geopolitical uncertainty and potential supply chain disruptions due to changes in economic policy); and other risks and uncertainties indicated from time to time in Korro’s filings with the SEC, including “Risk Factors” in Korro’s most recent Quarterly Report on Form 10-K or Form 10-Q filed with the SEC, as such may be amended or supplemented by its other filings with the SEC. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements in this presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Except as required by law, Korro does not undertake or accept any duty to release publicly any updates or revisions to any forward-looking statements to reflect any change in their expectations or in the events, conditions or circumstances on which any such statement is based. This presentation does not purport to summarize all of the conditions, risks and other attributes of an investment in Korro. Industry and Market Data Certain information contained in this presentation relates to or is based on studies, publications, surveys and Korro’s own internal estimates and research. In this presentation, Korro relies on, and refers to, publicly available information and statistics regarding market participants in the sector in which Korro competes and other industry data. Any comparison of Korro to any other entity assumes the reliability of the information available to Korro. Korro obtained this information and statistics from third-party sources, including reports by market research firms and company filings. In addition, all of the market data included in this presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Korro believes its internal research is reliable, such research has not been verified by any independent source and Korro has not independently verified the information. Trademarks This presentation may contain trademarks, service marks, trade names and copyrights of Korro or other third parties, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this presentation may be listed without the TM, SM © or ® symbols, but Korro will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to its trademarks, service marks, trade names and copyrights.

Expanding to New Biological Frontiers with RNA Editing Ram Aiyar, PhD, MBA Chief Executive Officer
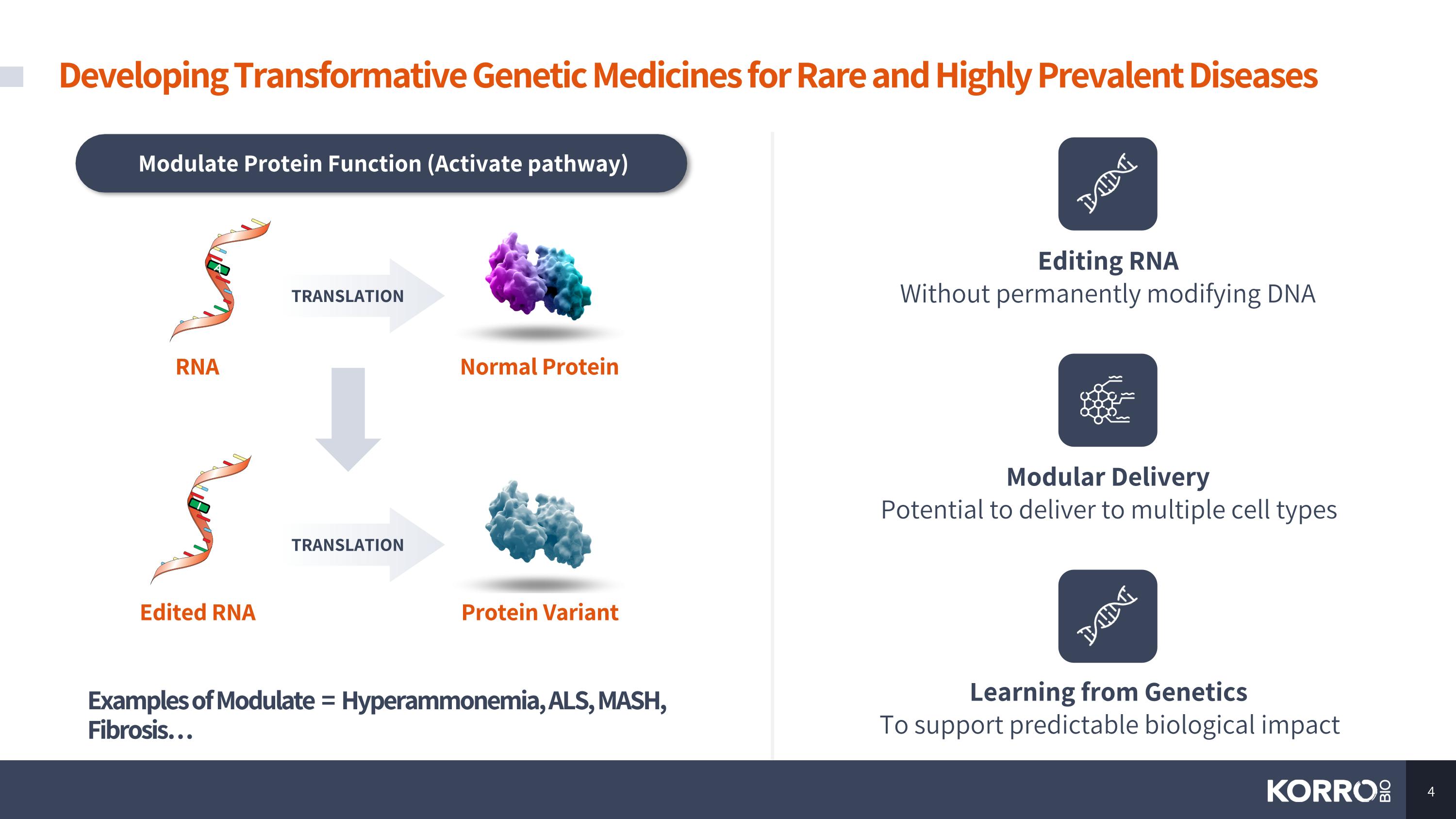
Modulate Protein Function (Activate pathway) Examples of Modulate = Hyperammonemia, ALS, MASH, Fibrosis… RNA TRANSLATION A Normal Protein TRANSLATION I Protein Variant Edited RNA Editing RNA Without permanently modifying DNA Modular Delivery Potential to deliver to multiple cell types Learning from Genetics To support predictable biological impact Developing Transformative Genetic Medicines for Rare and Highly Prevalent Diseases

KRRO-121 Scientific Overview and Preclinical Data Loïc Vincent, PhD Chief Scientific Officer

Glutamine Synthetase (GS) is a critical ammonia clearing mechanism Genetic evidence uncovers a key amino acid modification that can augment GS protein stability Ammonia-lowering benefits of stabilized GS activity may address substantial unmet need in patients with poor ammonia control, including UCD and hepatic encephalopathy KRRO-121 is a GalNAc-conjugated ASO that edits GS mRNA to generate a stable, de novo GS variant specifically in the liver KRRO-121 demonstrates potential to enable robust ammonia clearance, supporting a pan-UCD approach that may enable dietary liberalization as well as clinical activity in other ammonia-driven diseases, such as HE Mechanism: Stabilizing Glutamine Synthetase to Clear Ammonia KRRO-121 regulatory submission to enable commencement of FIH trial is anticipated in the 2nd half of 2026
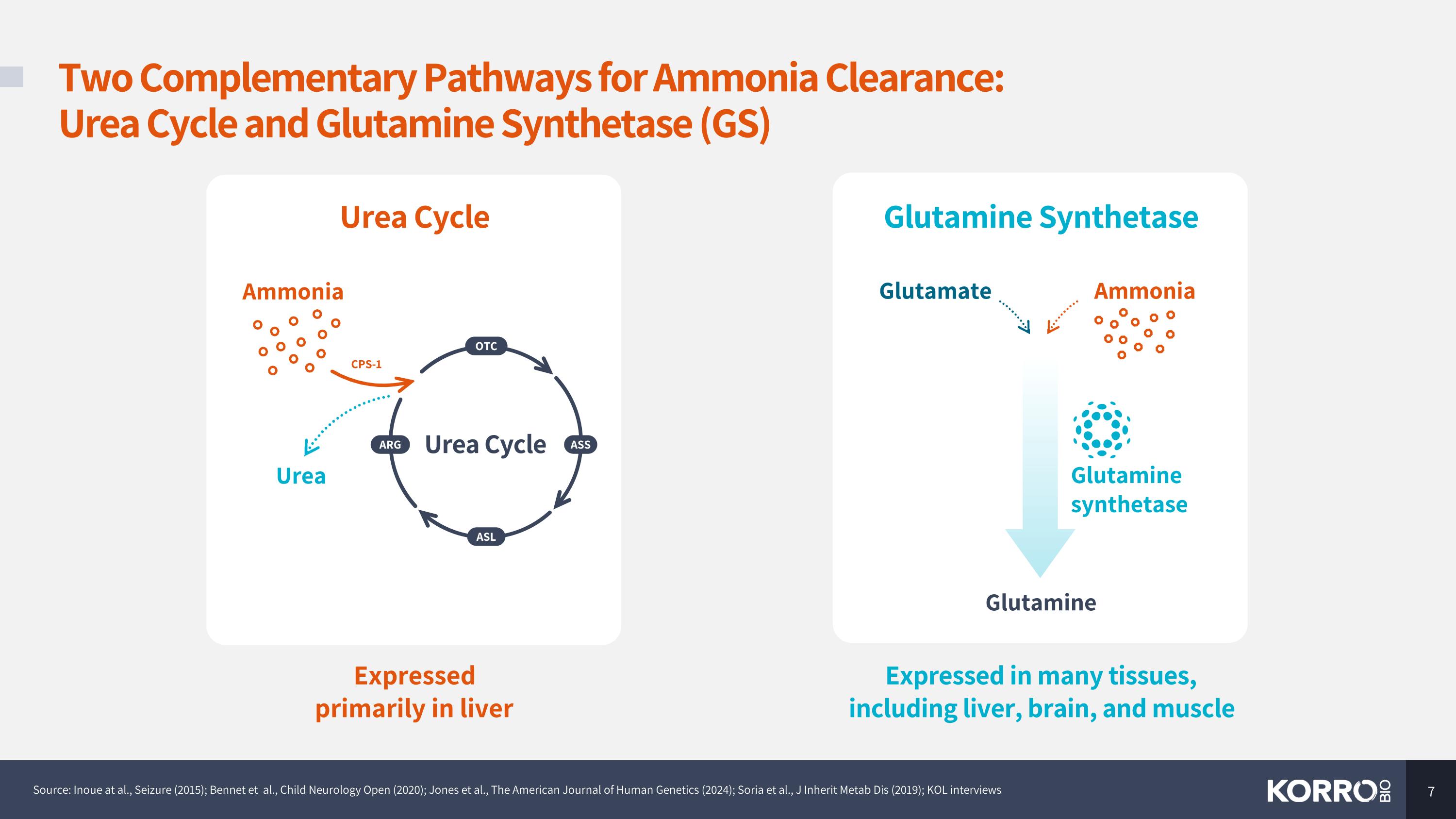
Two Complementary Pathways for Ammonia Clearance: Urea Cycle and Glutamine Synthetase (GS) Source: Inoue at al., Seizure (2015); Bennet et al., Child Neurology Open (2020); Jones et al., The American Journal of Human Genetics (2024); Soria et al., J Inherit Metab Dis (2019); KOL interviews Glutamine Glutamine synthetase Glutamine Synthetase Glutamate Ammonia Expressed in many tissues, including liver, brain, and muscle Urea Cycle Expressed primarily in liver Ammonia Urea Urea Cycle OTC ASL ARG ASS CPS-1
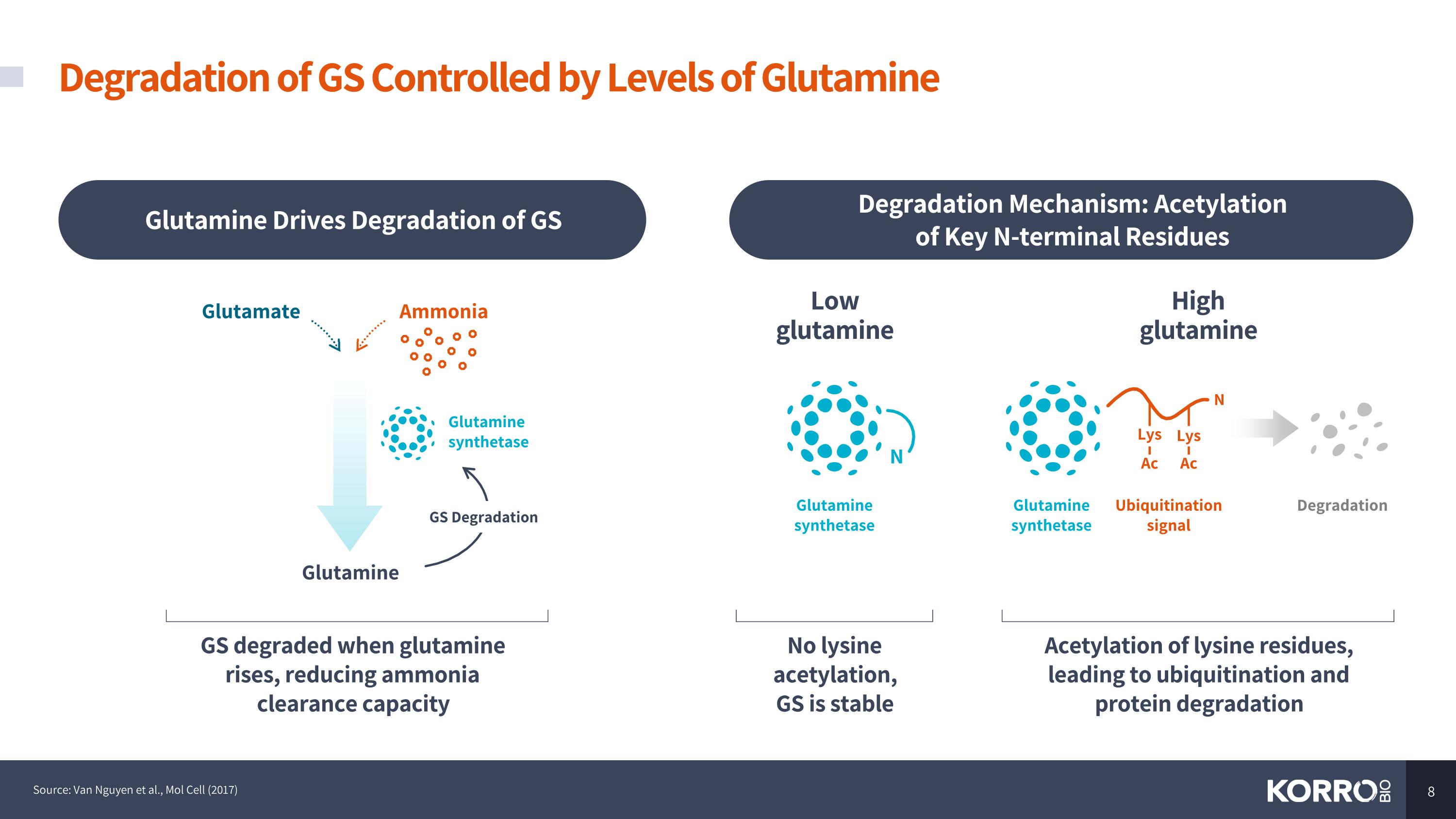
Degradation of GS Controlled by Levels of Glutamine Source: Van Nguyen et al., Mol Cell (2017) GS degraded when glutamine rises, reducing ammonia clearance capacity Glutamine Drives Degradation of GS Degradation Mechanism: Acetylation of Key N-terminal Residues Glutamine Glutamine synthetase Glutamate Ammonia GS Degradation High glutamine Acetylation of lysine residues, leading to ubiquitination and protein degradation Ubiquitination signal Glutamine synthetase N Lys Lys Ac Ac Low glutamine No lysine acetylation, GS is stable N Glutamine synthetase Degradation
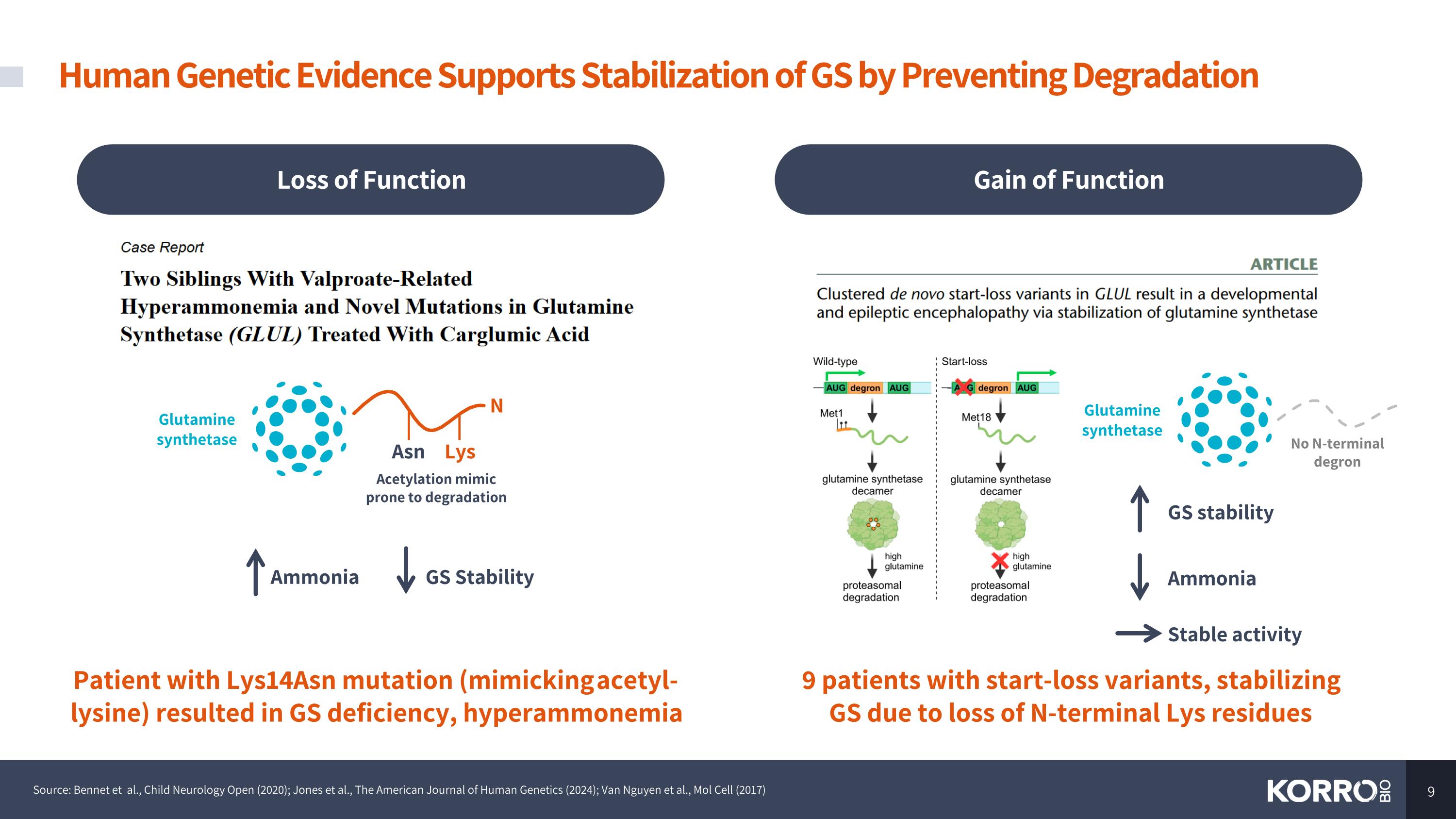
9 patients with start-loss variants, stabilizing GS due to loss of N-terminal Lys residues GS stability Stable activity Patient with Lys14Asn mutation (mimicking acetyl-lysine) resulted in GS deficiency, hyperammonemia GS Stability Ammonia Acetylation mimic prone to degradation Human Genetic Evidence Supports Stabilization of GS by Preventing Degradation Source: Bennet et al., Child Neurology Open (2020); Jones et al., The American Journal of Human Genetics (2024); Van Nguyen et al., Mol Cell (2017) N Asn Lys Glutamine synthetase No N-terminal degron Glutamine synthetase Gain of Function Loss of Function Ammonia
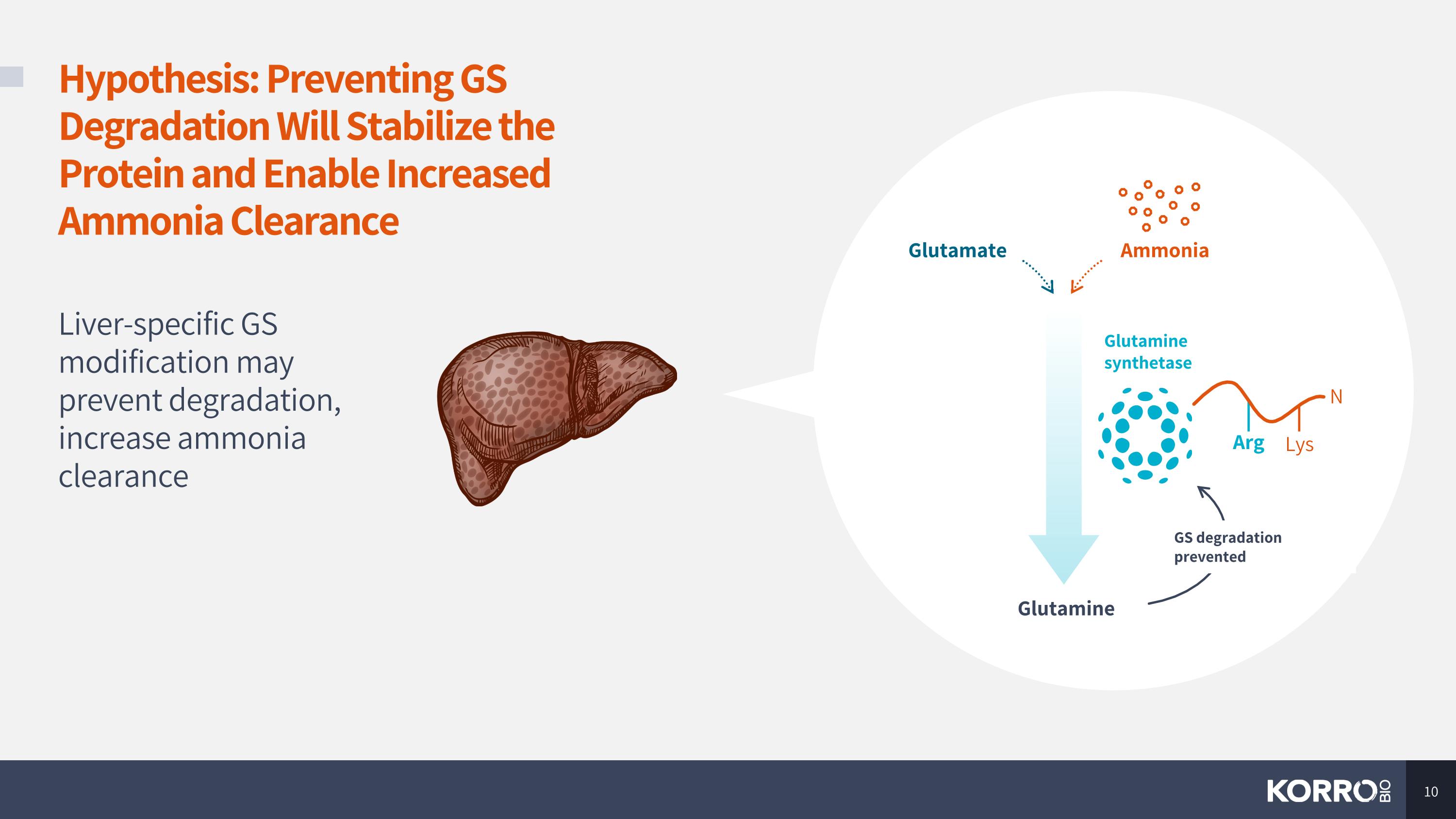
Hypothesis: Preventing GS Degradation Will Stabilize the Protein and Enable Increased Ammonia Clearance Liver-specific GS modification may prevent degradation, increase ammonia clearance Glutamine Glutamine synthetase N Arg Lys Glutamate Ammonia GS degradation prevented
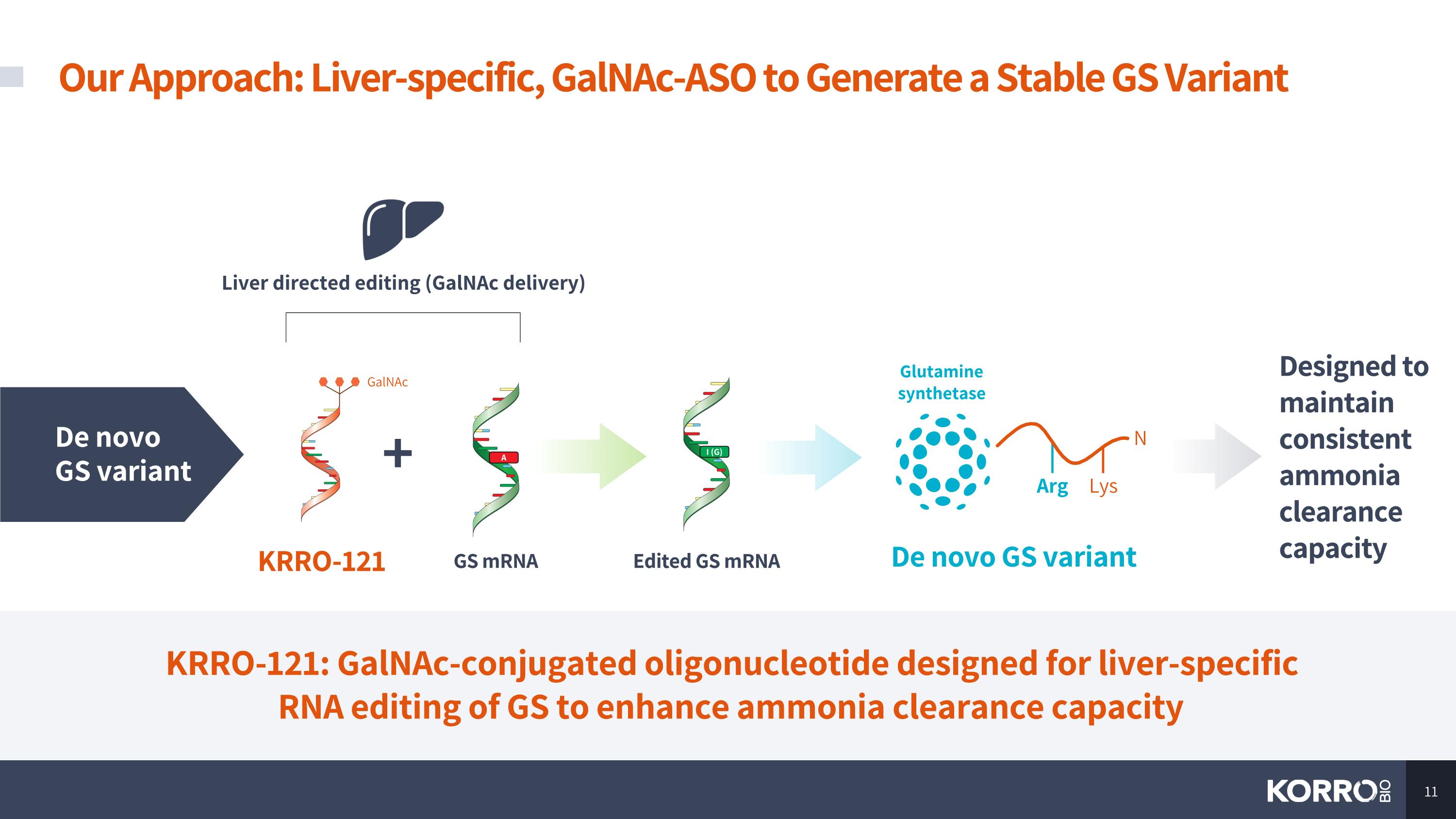
Our Approach: Liver-specific, GalNAc-ASO to Generate a Stable GS Variant De novo GS variant Liver directed editing (GalNAc delivery) KRRO-121 GS mRNA + I (G) A Edited GS mRNA De novo GS variant N Arg Lys Glutamine synthetase Designed to maintain consistent ammonia clearance capacity GalNAc KRRO-121: GalNAc-conjugated oligonucleotide designed for liver-specific RNA editing of GS to enhance ammonia clearance capacity
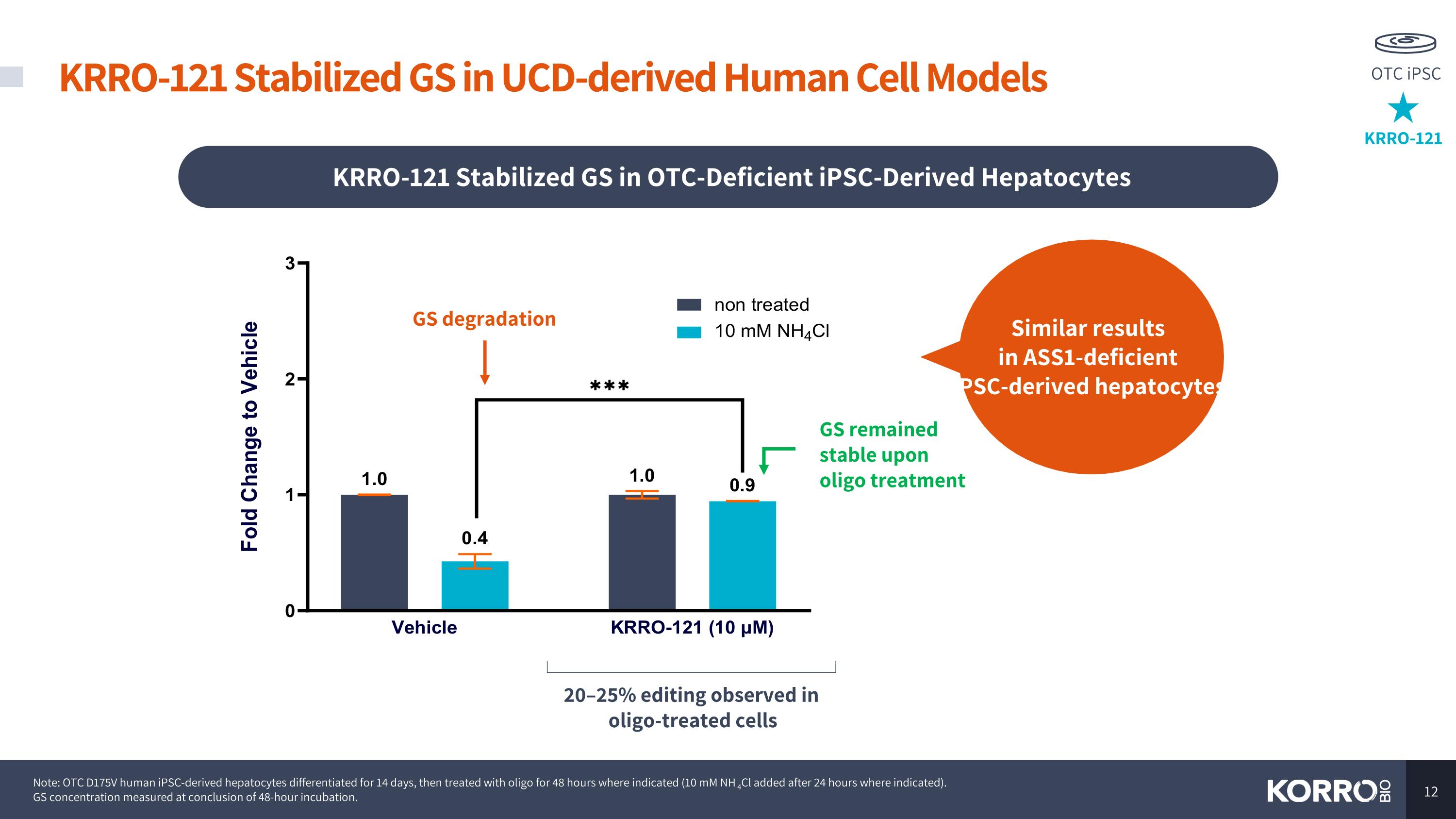
KRRO-121 Stabilized GS in OTC-Deficient iPSC-Derived Hepatocytes GS degradation GS remained stable upon oligo treatment Similar results in ASS1-deficient iPSC-derived hepatocytes 20–25% editing observed in oligo-treated cells Note: OTC D175V human iPSC-derived hepatocytes differentiated for 14 days, then treated with oligo for 48 hours where indicated (10 mM NH4Cl added after 24 hours where indicated). GS concentration measured at conclusion of 48-hour incubation. KRRO-121 Stabilized GS in UCD-derived Human Cell Models KRRO-121 OTC iPSC
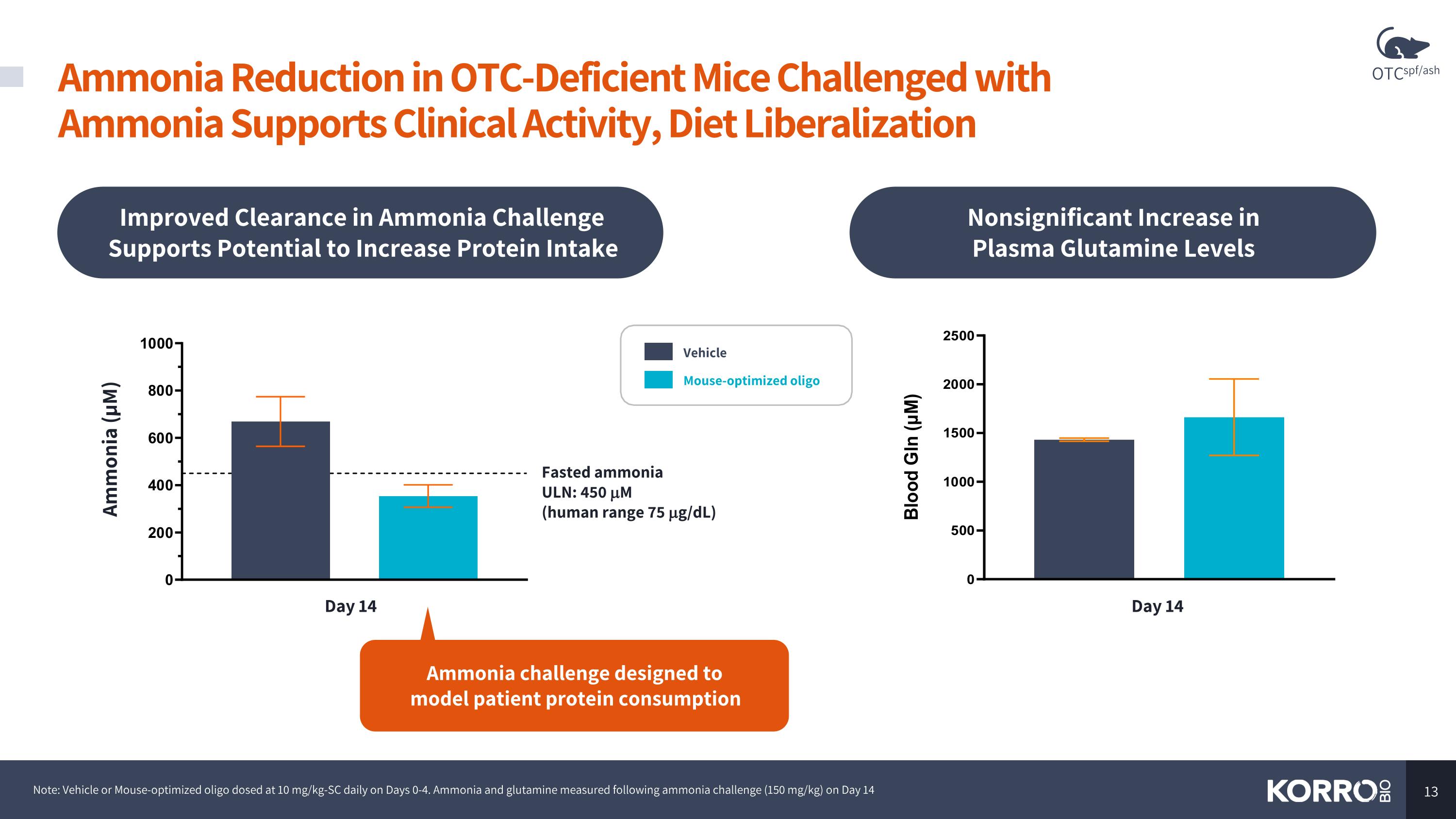
Improved Clearance in Ammonia Challenge Supports Potential to Increase Protein Intake Note: Vehicle or Mouse-optimized oligo dosed at 10 mg/kg-SC daily on Days 0-4. Ammonia and glutamine measured following ammonia challenge (150 mg/kg) on Day 14 Ammonia challenge designed to model patient protein consumption Nonsignificant Increase in Plasma Glutamine Levels Fasted ammonia ULN: 450 mM (human range 75 mg/dL) Ammonia (µM) Day 14 Day 14 Vehicle Mouse-optimized oligo Ammonia Reduction in OTC-Deficient Mice Challenged with Ammonia Supports Clinical Activity, Diet Liberalization OTCspf/ash
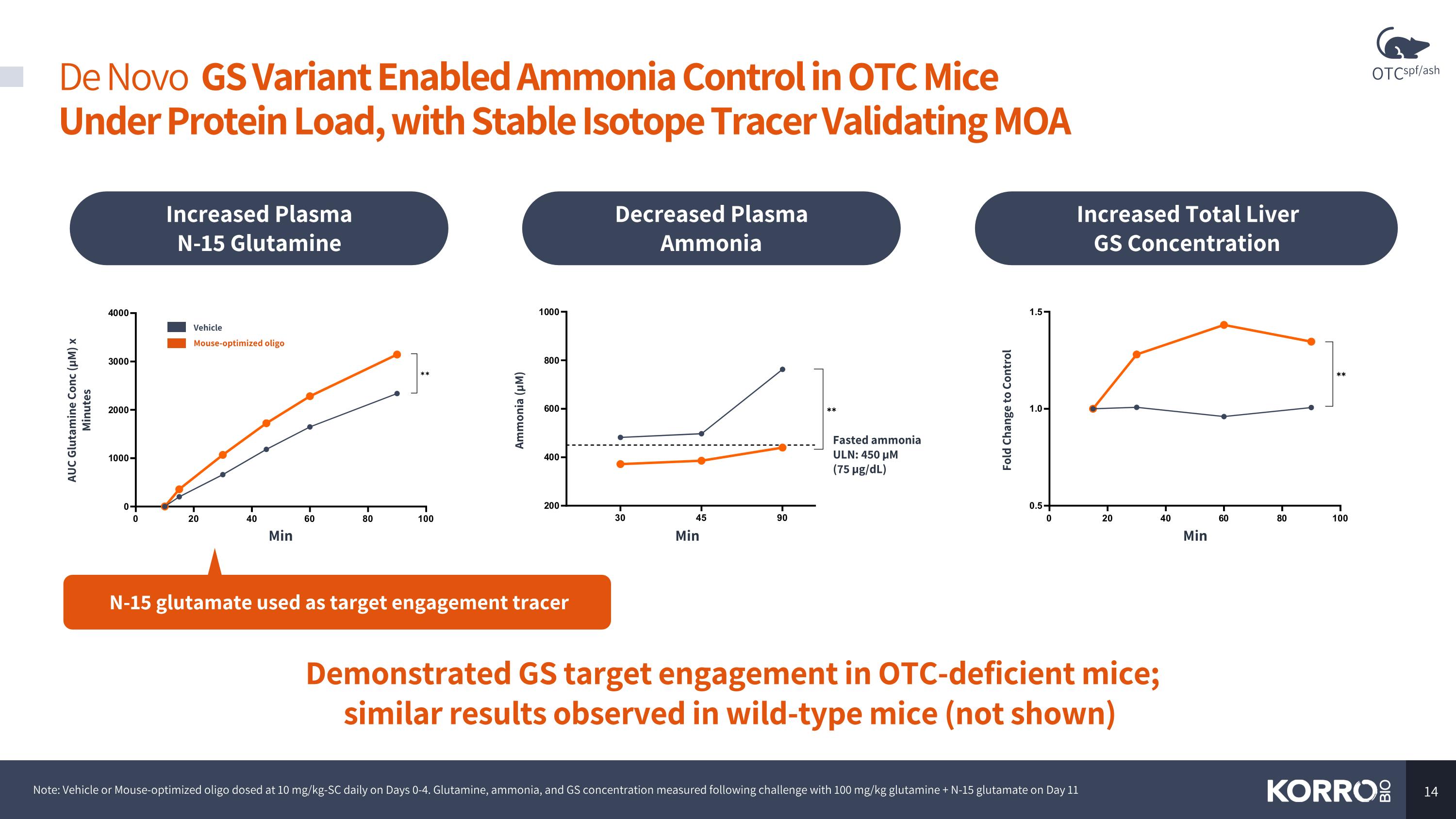
De Novo GS Variant Enabled Ammonia Control in OTC Mice Under Protein Load, with Stable Isotope Tracer Validating MOA Note: Vehicle or Mouse-optimized oligo dosed at 10 mg/kg-SC daily on Days 0-4. Glutamine, ammonia, and GS concentration measured following challenge with 100 mg/kg glutamine + N-15 glutamate on Day 11 Demonstrated GS target engagement in OTC-deficient mice; similar results observed in wild-type mice (not shown) Min Ammonia (µM) Min AUC Glutamine Conc (µM) x Minutes Min Decreased Plasma Ammonia Increased Plasma N-15 Glutamine Increased Total Liver GS Concentration N-15 glutamate used as target engagement tracer Fasted ammonia ULN: 450 µM (75 µg/dL) Vehicle Mouse-optimized oligo Fold Change to Control OTCspf/ash
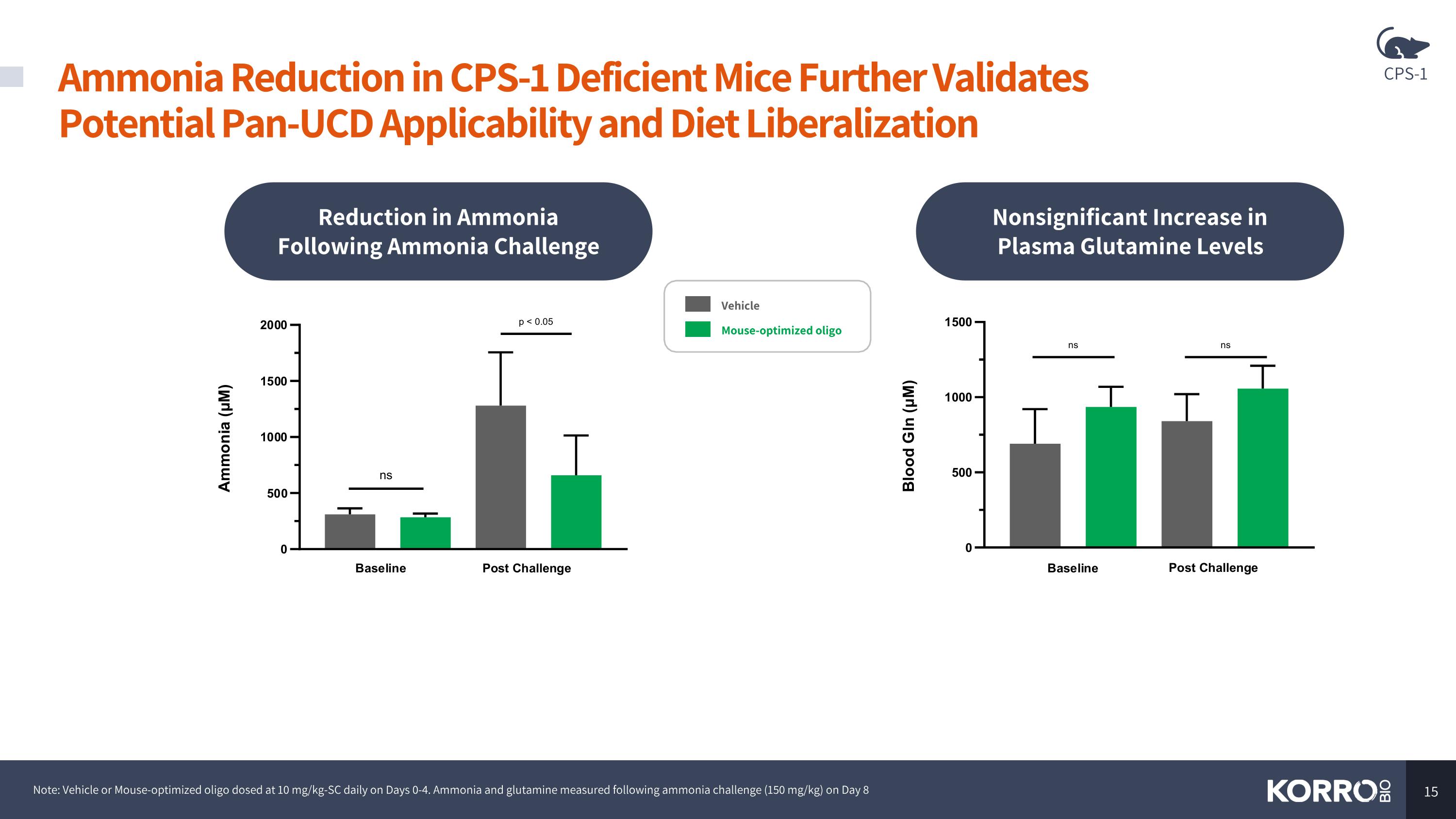
Ammonia Reduction in CPS-1 Deficient Mice Further Validates Potential Pan-UCD Applicability and Diet Liberalization Note: Vehicle or Mouse-optimized oligo dosed at 10 mg/kg-SC daily on Days 0-4. Ammonia and glutamine measured following ammonia challenge (150 mg/kg) on Day 8 Reduction in Ammonia Following Ammonia Challenge Nonsignificant Increase in Plasma Glutamine Levels Vehicle Mouse-optimized oligo CPS-1
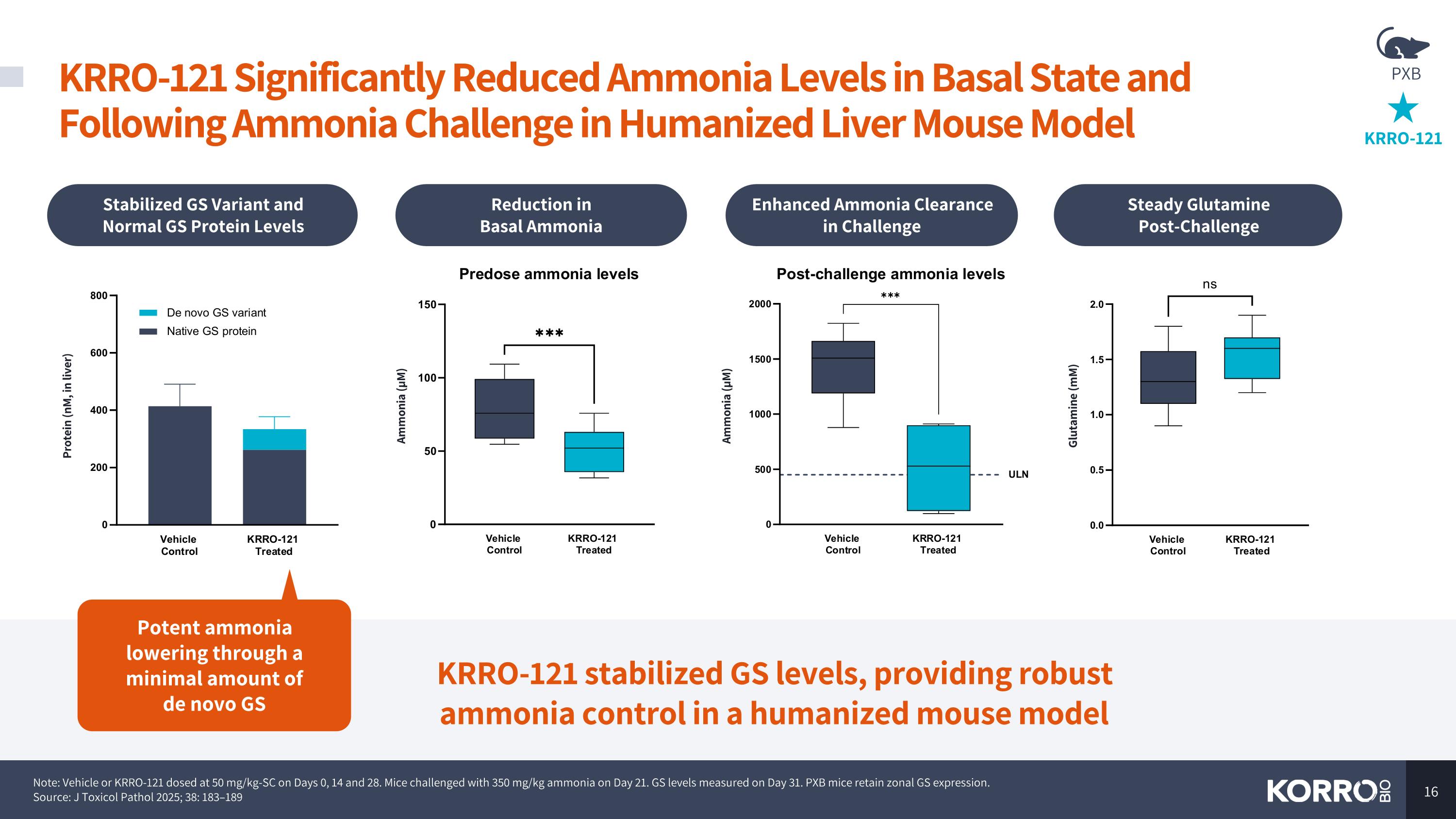
Note: Vehicle or KRRO-121 dosed at 50 mg/kg-SC on Days 0, 14 and 28. Mice challenged with 350 mg/kg ammonia on Day 21. GS levels measured on Day 31. PXB mice retain zonal GS expression. Source: J Toxicol Pathol 2025; 38: 183–189 KRRO-121 Significantly Reduced Ammonia Levels in Basal State and Following Ammonia Challenge in Humanized Liver Mouse Model KRRO-121 stabilized GS levels, providing robust ammonia control in a humanized mouse model Stabilized GS Variant and Normal GS Protein Levels Reduction in Basal Ammonia Enhanced Ammonia Clearance in Challenge Steady Glutamine Post-Challenge Ammonia (µM) Ammonia (µM) Protein (nM, in liver) Glutamine (mM) Potent ammonia lowering through a minimal amount of de novo GS KRRO-121 PXB
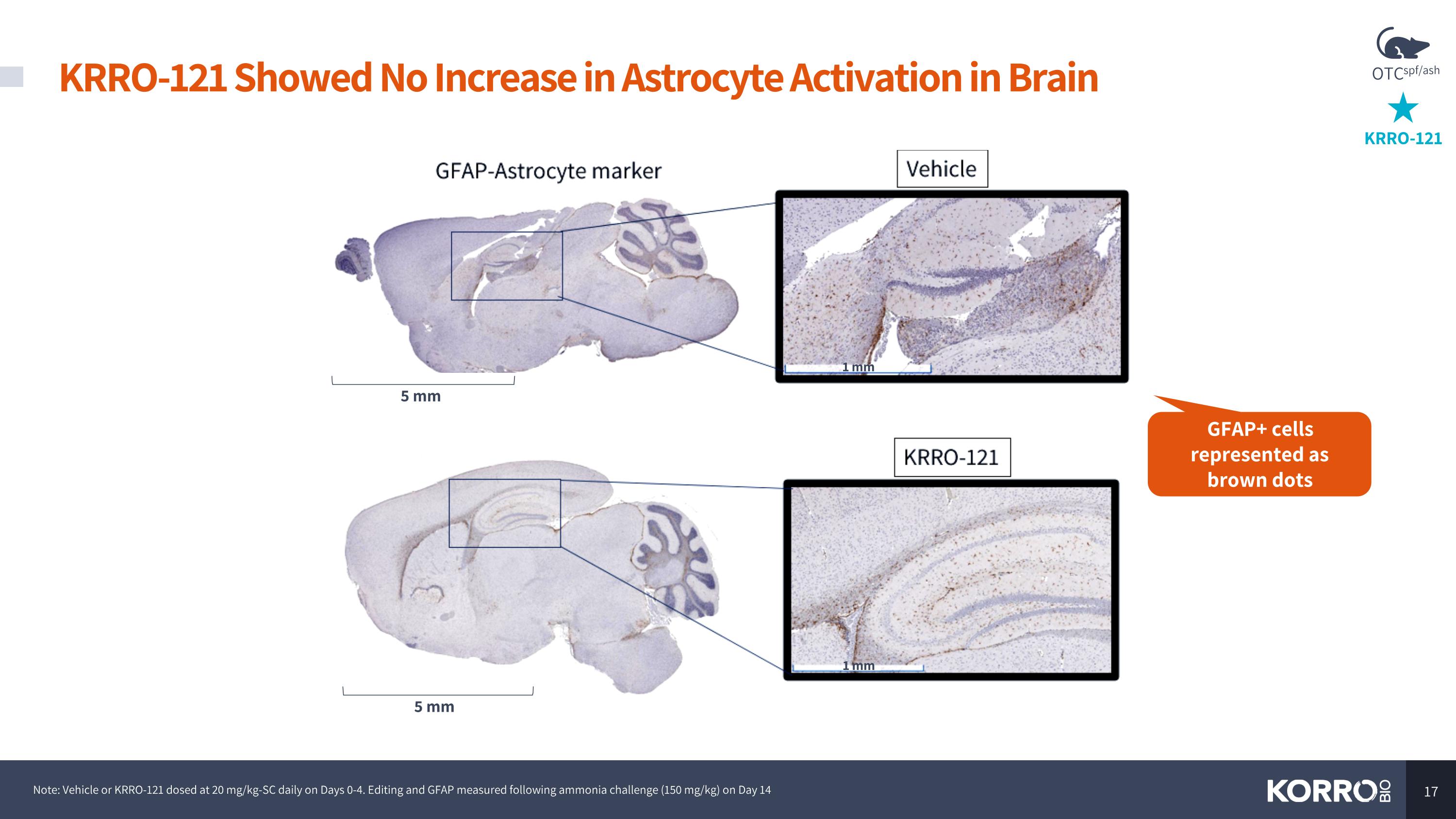
KRRO-121 OTCspf/ash KRRO-121 Showed No Increase in Astrocyte Activation in Brain Note: Vehicle or KRRO-121 dosed at 20 mg/kg-SC daily on Days 0-4. Editing and GFAP measured following ammonia challenge (150 mg/kg) on Day 14 GFAP+ cells represented as brown dots 1 mm 5 mm 5 mm 1 mm
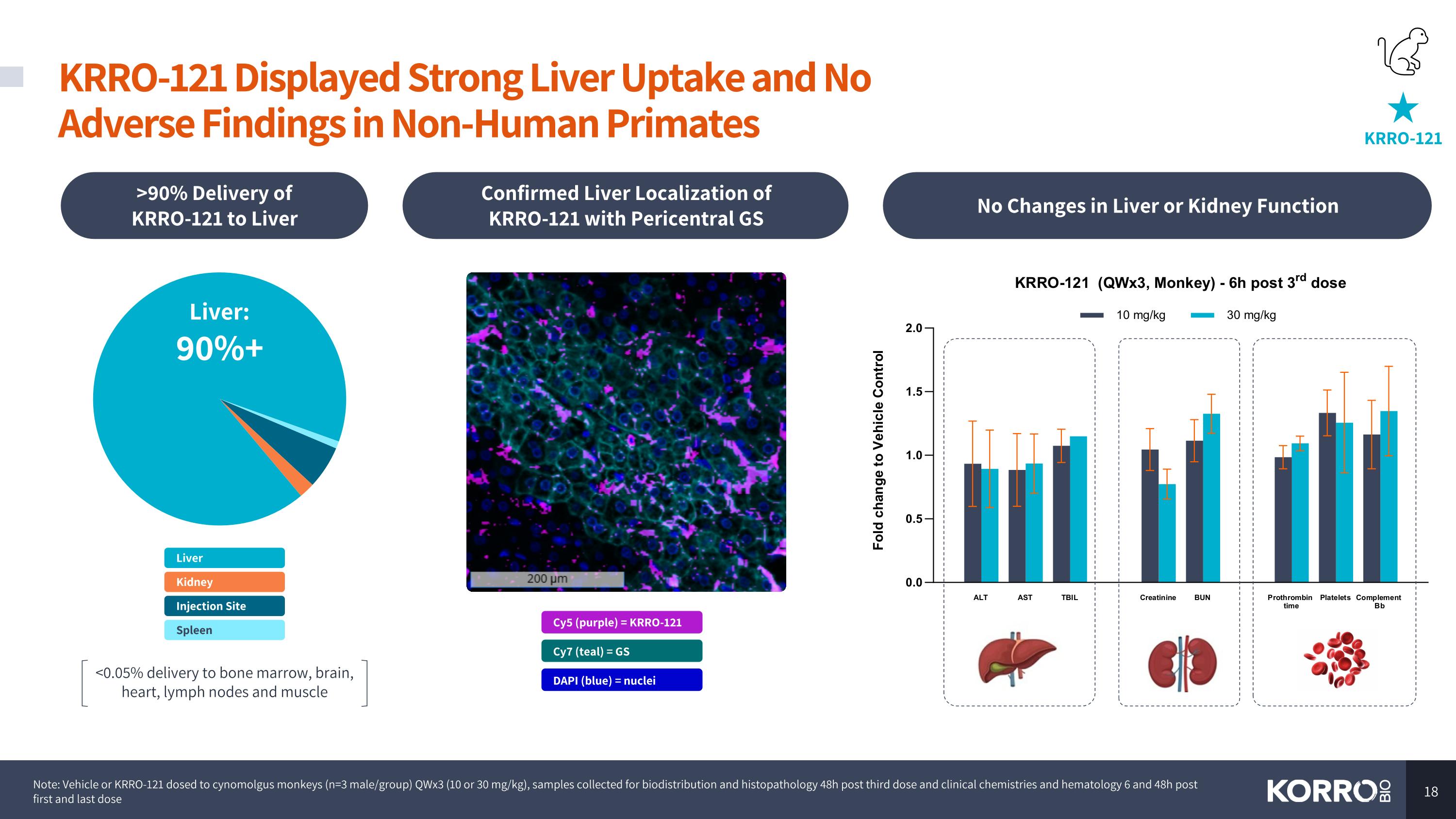
KRRO-121 Displayed Strong Liver Uptake and No Adverse Findings in Non-Human Primates KRRO-121 Note: Vehicle or KRRO-121 dosed to cynomolgus monkeys (n=3 male/group) QWx3 (10 or 30 mg/kg), samples collected for biodistribution and histopathology 48h post third dose and clinical chemistries and hematology 6 and 48h post first and last dose >90% Delivery of KRRO-121 to Liver No Changes in Liver or Kidney Function Confirmed Liver Localization of KRRO-121 with Pericentral GS Cy5 (purple) = KRRO-121 Cy7 (teal) = GS DAPI (blue) = nuclei Injection Site Kidney Liver: 90%+ Spleen Liver <0.05% delivery to bone marrow, brain, heart, lymph nodes and muscle
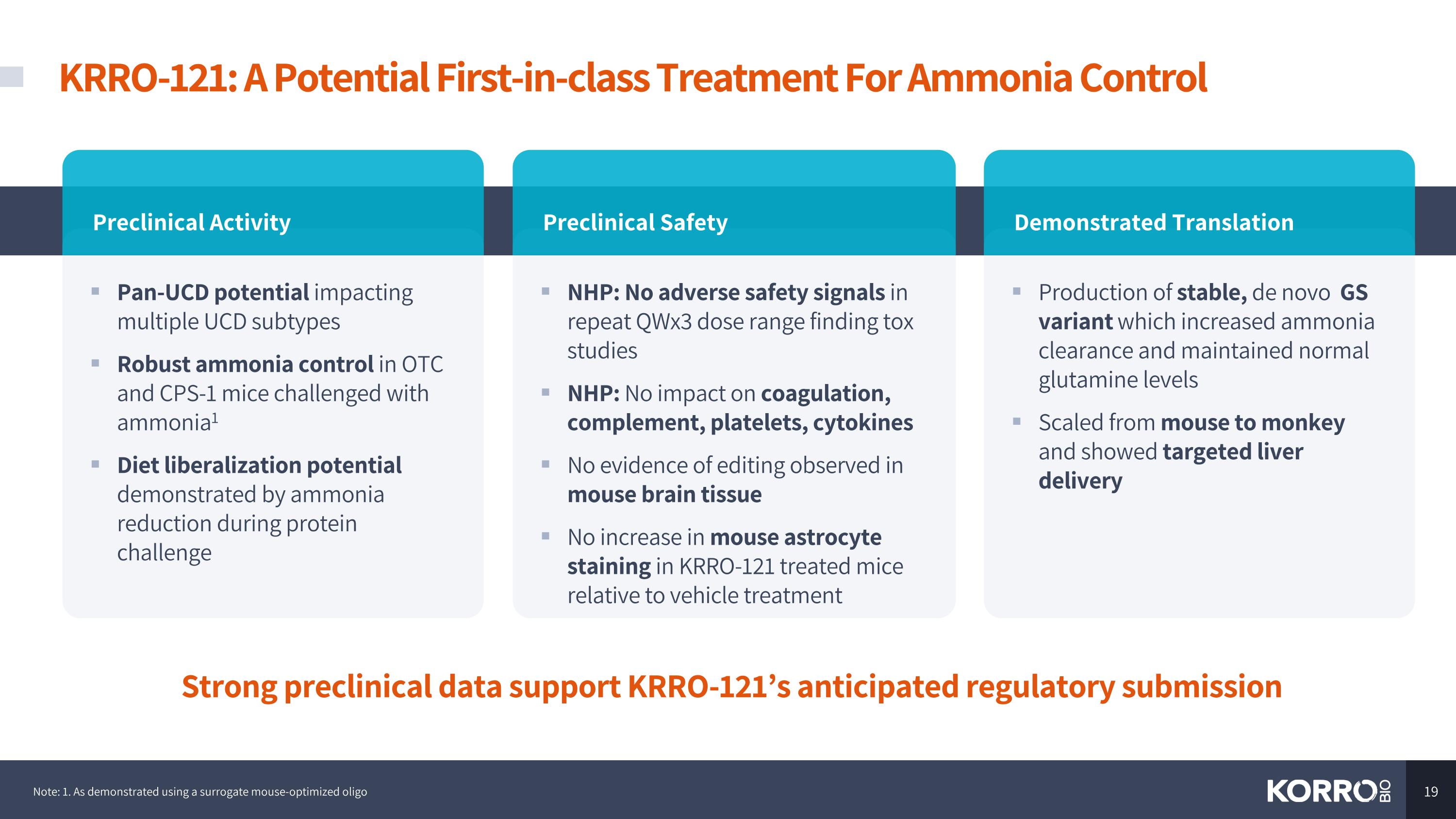
KRRO-121: A Potential First-in-class Treatment For Ammonia Control Pan-UCD potential impacting multiple UCD subtypes Robust ammonia control in OTC and CPS-1 mice challenged with ammonia1 Diet liberalization potential demonstrated by ammonia reduction during protein challenge Preclinical Activity NHP: No adverse safety signals in repeat QWx3 dose range finding tox studies NHP: No impact on coagulation, complement, platelets, cytokines No evidence of editing observed in mouse brain tissue No increase in mouse astrocyte staining in KRRO-121 treated mice relative to vehicle treatment Preclinical Safety Production of stable, de novo GS variant which increased ammonia clearance and maintained normal glutamine levels Scaled from mouse to monkey and showed targeted liver delivery Demonstrated Translation Strong preclinical data support KRRO-121’s anticipated regulatory submission Note: 1. As demonstrated using a surrogate mouse-optimized oligo
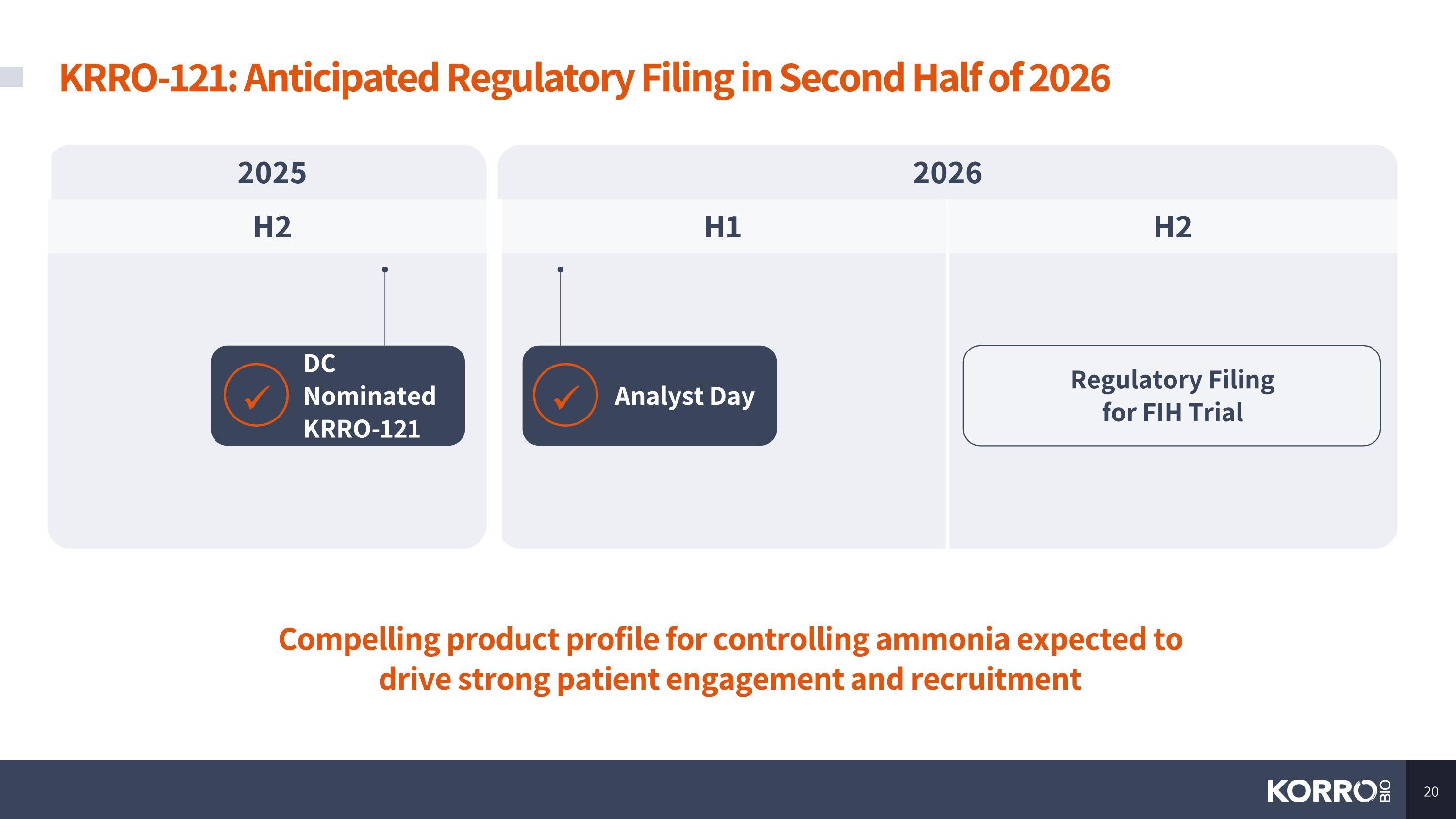
KRRO-121: Anticipated Regulatory Filing in Second Half of 2026 Compelling product profile for controlling ammonia expected to drive strong patient engagement and recruitment 2025 2026 H2 H1 H2 Regulatory Filing for FIH Trial DC Nominated KRRO-121 Analyst Day

KRRO-121 Market Opportunity Todd Chappell, MBA Chief Operating Officer
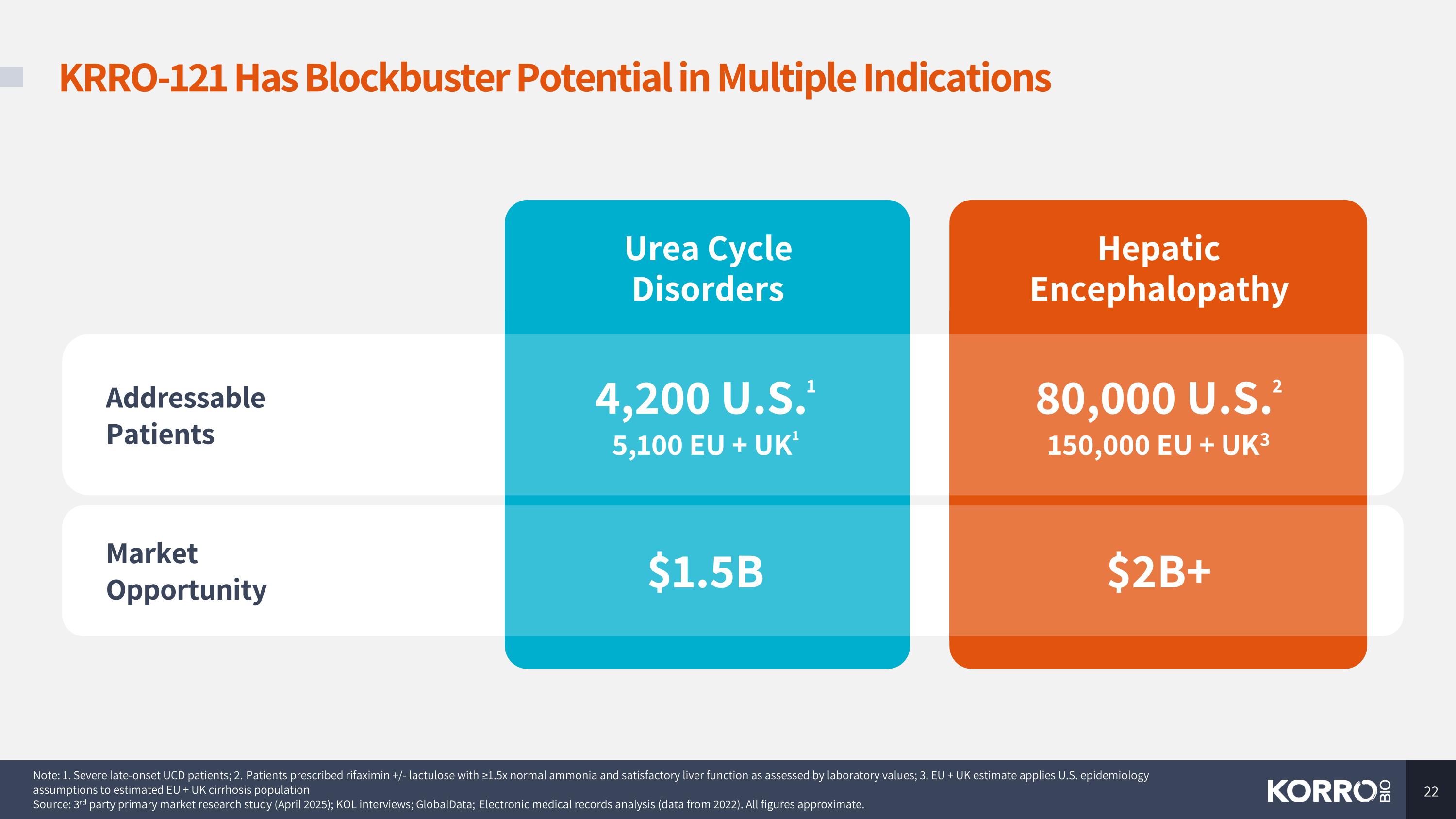
Urea Cycle Disorders Hepatic Encephalopathy KRRO-121 Has Blockbuster Potential in Multiple Indications Note: 1. Severe late-onset UCD patients; 2. Patients prescribed rifaximin +/- lactulose with ≥1.5x normal ammonia and satisfactory liver function as assessed by laboratory values; 3. EU + UK estimate applies U.S. epidemiology assumptions to estimated EU + UK cirrhosis population Source: 3rd party primary market research study (April 2025); KOL interviews; GlobalData; Electronic medical records analysis (data from 2022). All figures approximate. Addressable Patients 4,200 U.S.1 5,100 EU + UK1 80,000 U.S.2 150,000 EU + UK3 Market Opportunity $1.5B $2B+
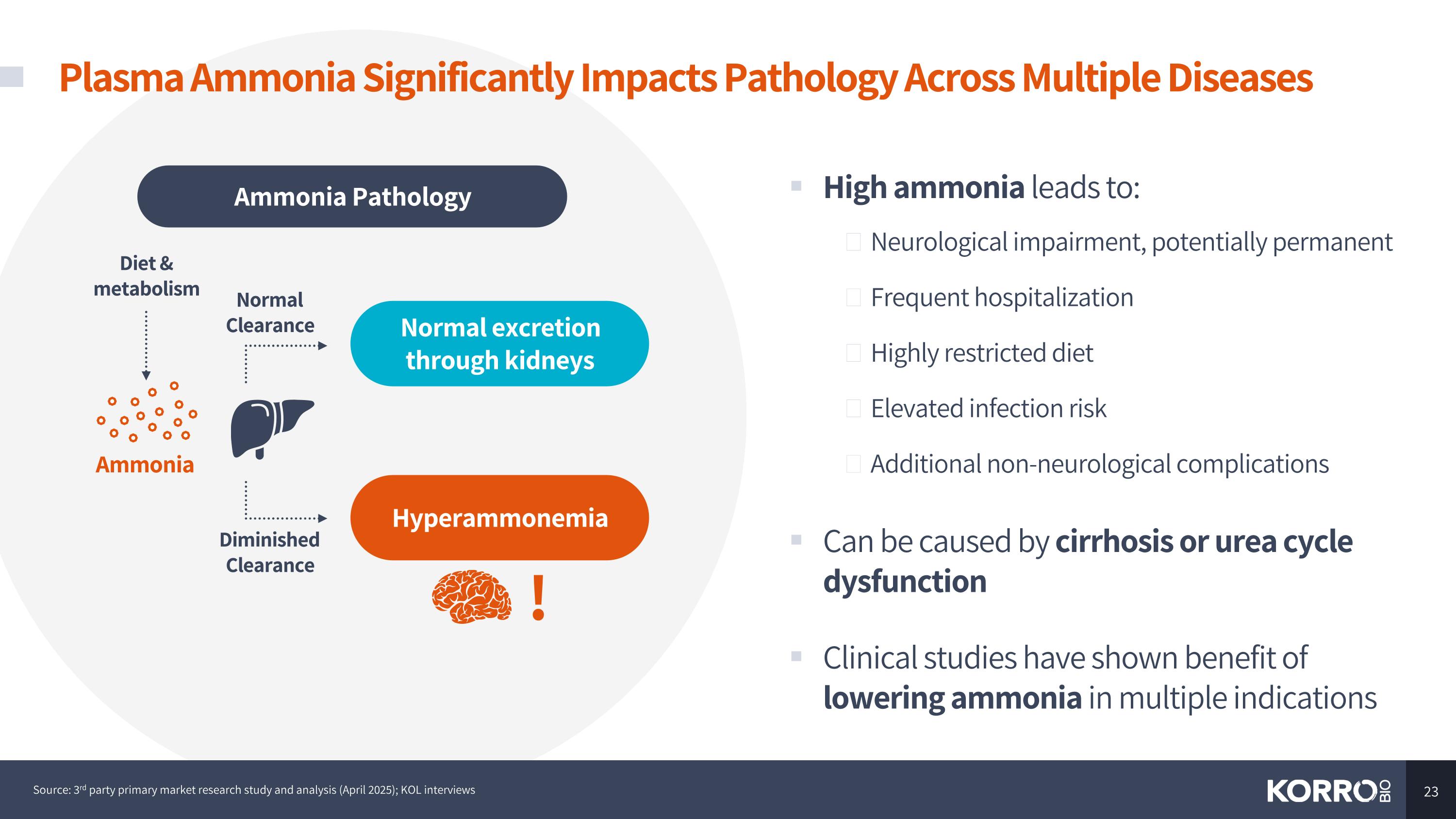
Plasma Ammonia Significantly Impacts Pathology Across Multiple Diseases High ammonia leads to: Neurological impairment, potentially permanent Frequent hospitalization Highly restricted diet Elevated infection risk Additional non-neurological complications Can be caused by cirrhosis or urea cycle dysfunction Clinical studies have shown benefit of lowering ammonia in multiple indications Ammonia Diet & metabolism Normal Clearance Diminished Clearance Normal excretion through kidneys Hyperammonemia Ammonia Pathology ! Source: 3rd party primary market research study and analysis (April 2025); KOL interviews
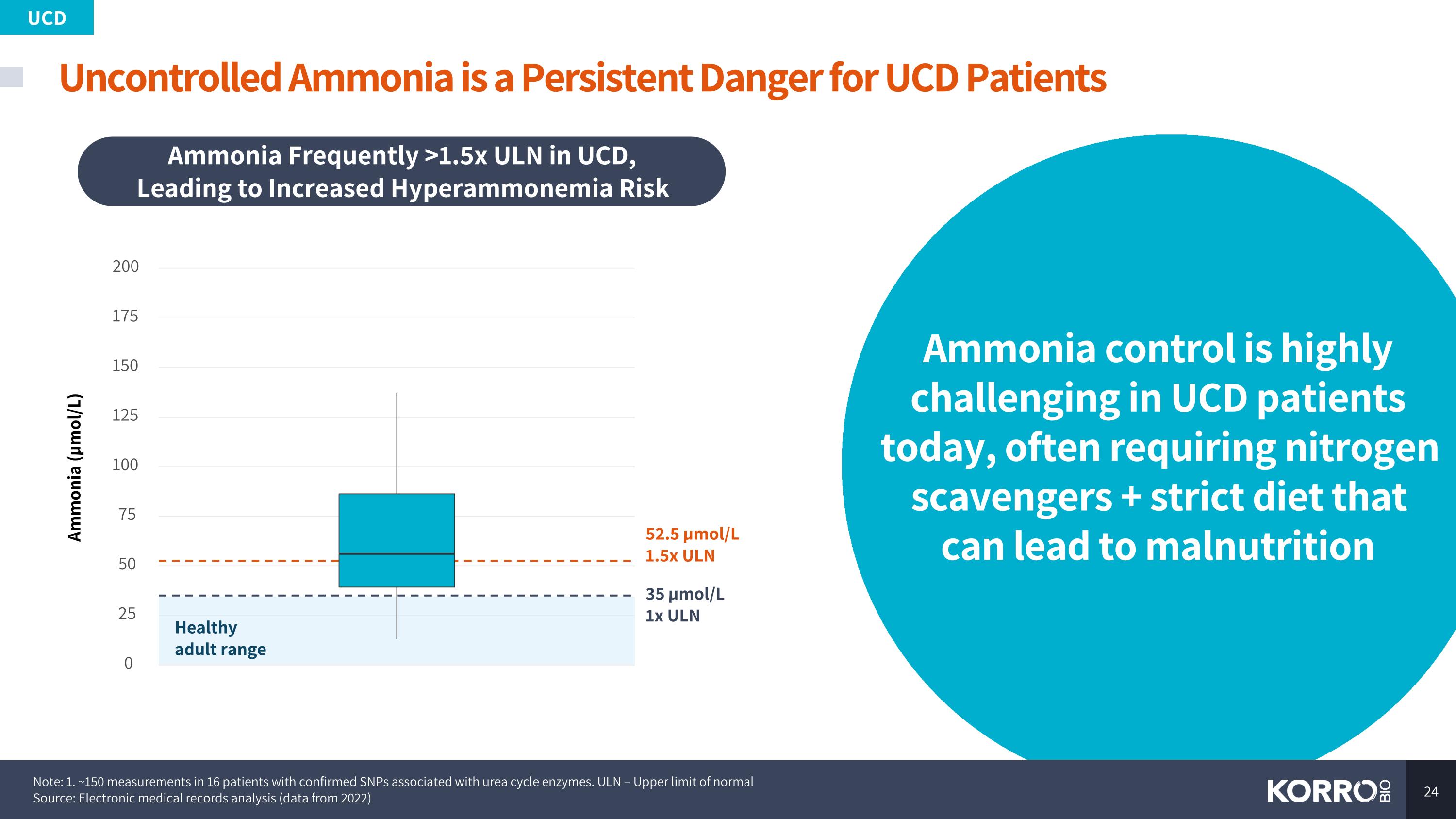
Uncontrolled Ammonia is a Persistent Danger for UCD Patients UCD 35 µmol/L 1x ULN 52.5 µmol/L 1.5x ULN 0 25 50 75 100 125 150 175 200 Ammonia (µmol/L) Healthy adult range Ammonia Frequently >1.5x ULN in UCD, Leading to Increased Hyperammonemia Risk Ammonia control is highly challenging in UCD patients today, often requiring nitrogen scavengers + strict diet that can lead to malnutrition Note: 1. ~150 measurements in 16 patients with confirmed SNPs associated with urea cycle enzymes. ULN – Upper limit of normal Source: Electronic medical records analysis (data from 2022)
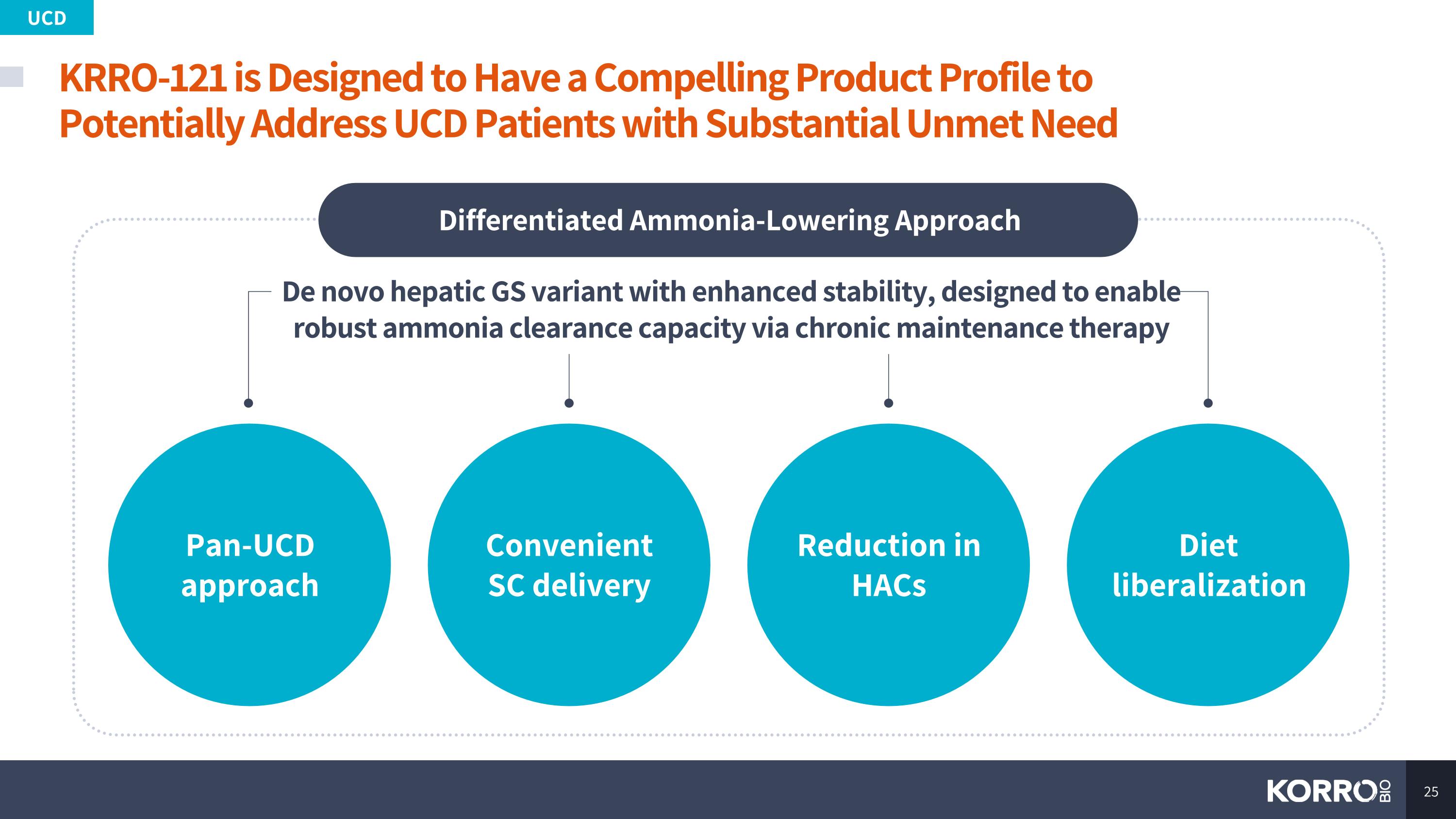
KRRO-121 is Designed to Have a Compelling Product Profile to Potentially Address UCD Patients with Substantial Unmet Need UCD De novo hepatic GS variant with enhanced stability, designed to enable robust ammonia clearance capacity via chronic maintenance therapy Differentiated Ammonia-Lowering Approach Pan-UCD approach Convenient SC delivery Diet liberalization Reduction in HACs
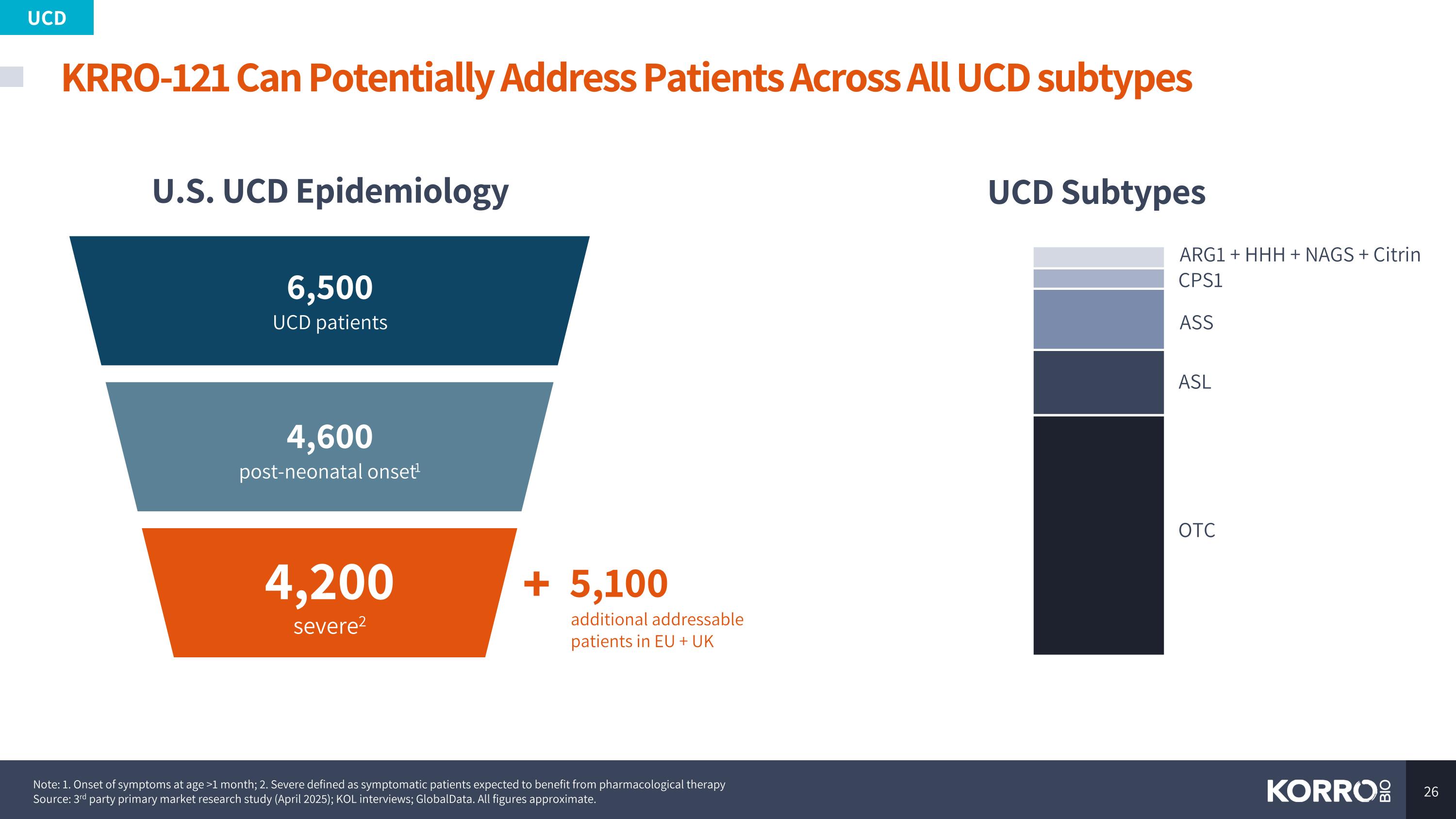
KRRO-121 Can Potentially Address Patients Across All UCD subtypes Note: 1. Onset of symptoms at age >1 month; 2. Severe defined as symptomatic patients expected to benefit from pharmacological therapy Source: 3rd party primary market research study (April 2025); KOL interviews; GlobalData. All figures approximate. U.S. UCD Epidemiology UCD Subtypes UCD OTC ASL ASS CPS1 ARG1 + HHH + NAGS + Citrin 6,500 UCD patients 4,600 post-neonatal onset1 4,200 severe2 5,100 additional addressable patients in EU + UK
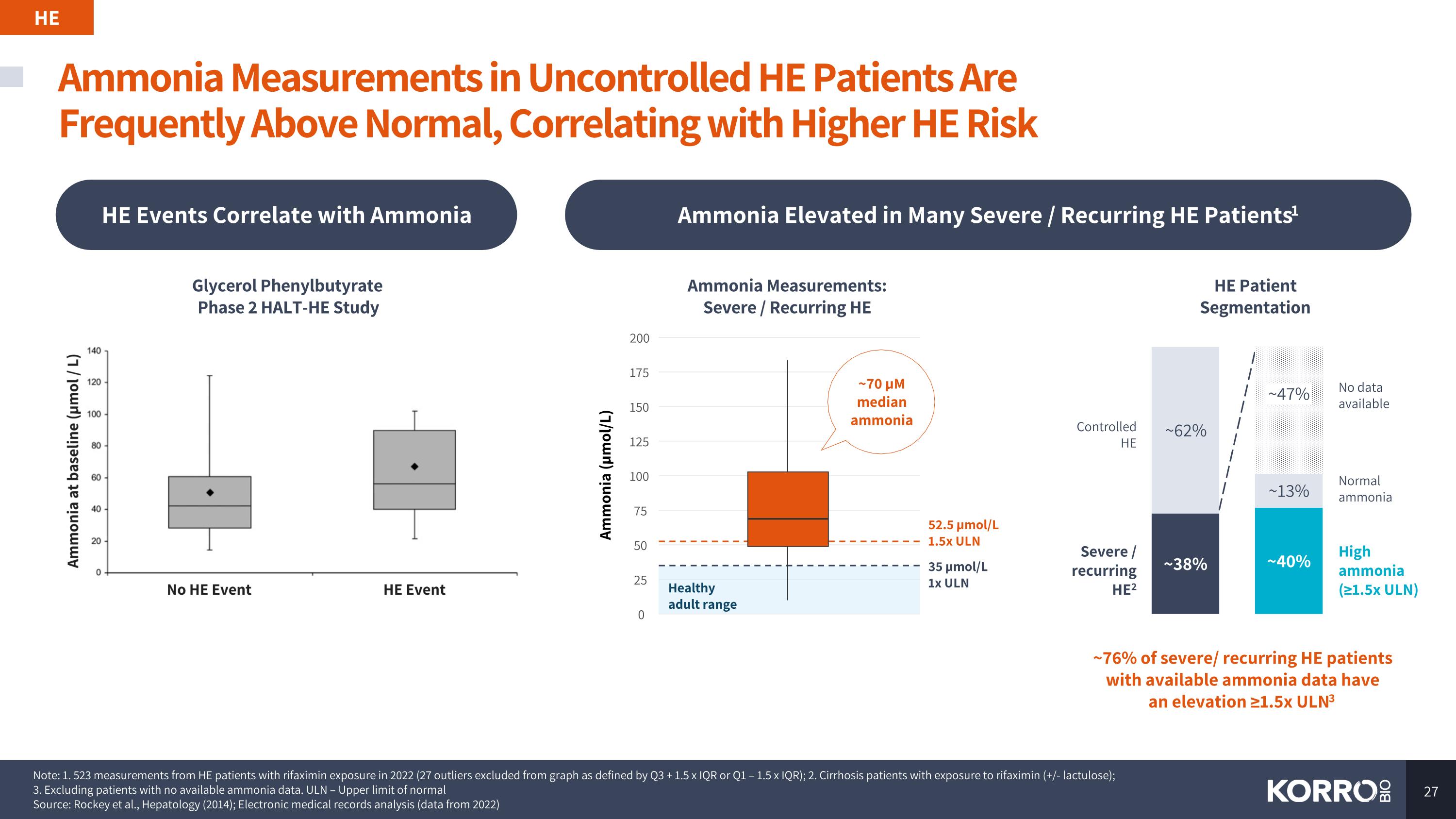
Ammonia Measurements in Uncontrolled HE Patients Are Frequently Above Normal, Correlating with Higher HE Risk HE HE Events Correlate with Ammonia Ammonia Elevated in Many Severe / Recurring HE Patients1 Note: 1. 523 measurements from HE patients with rifaximin exposure in 2022 (27 outliers excluded from graph as defined by Q3 + 1.5 x IQR or Q1 – 1.5 x IQR); 2. Cirrhosis patients with exposure to rifaximin (+/- lactulose); 3. Excluding patients with no available ammonia data. ULN – Upper limit of normal Source: Rockey et al., Hepatology (2014); Electronic medical records analysis (data from 2022) ~47% No data available Controlled HE Severe / recurring HE2 High ammonia (≥1.5x ULN) Normal ammonia 35 µmol/L 1x ULN 52.5 µmol/L 1.5x ULN 0 25 50 75 100 125 150 175 200 Ammonia (µmol/L) Healthy adult range Ammonia Measurements: Severe / Recurring HE HE Patient Segmentation ~70 µM median ammonia ~76% of severe/ recurring HE patients with available ammonia data have an elevation ≥1.5x ULN3 Glycerol Phenylbutyrate Phase 2 HALT-HE Study
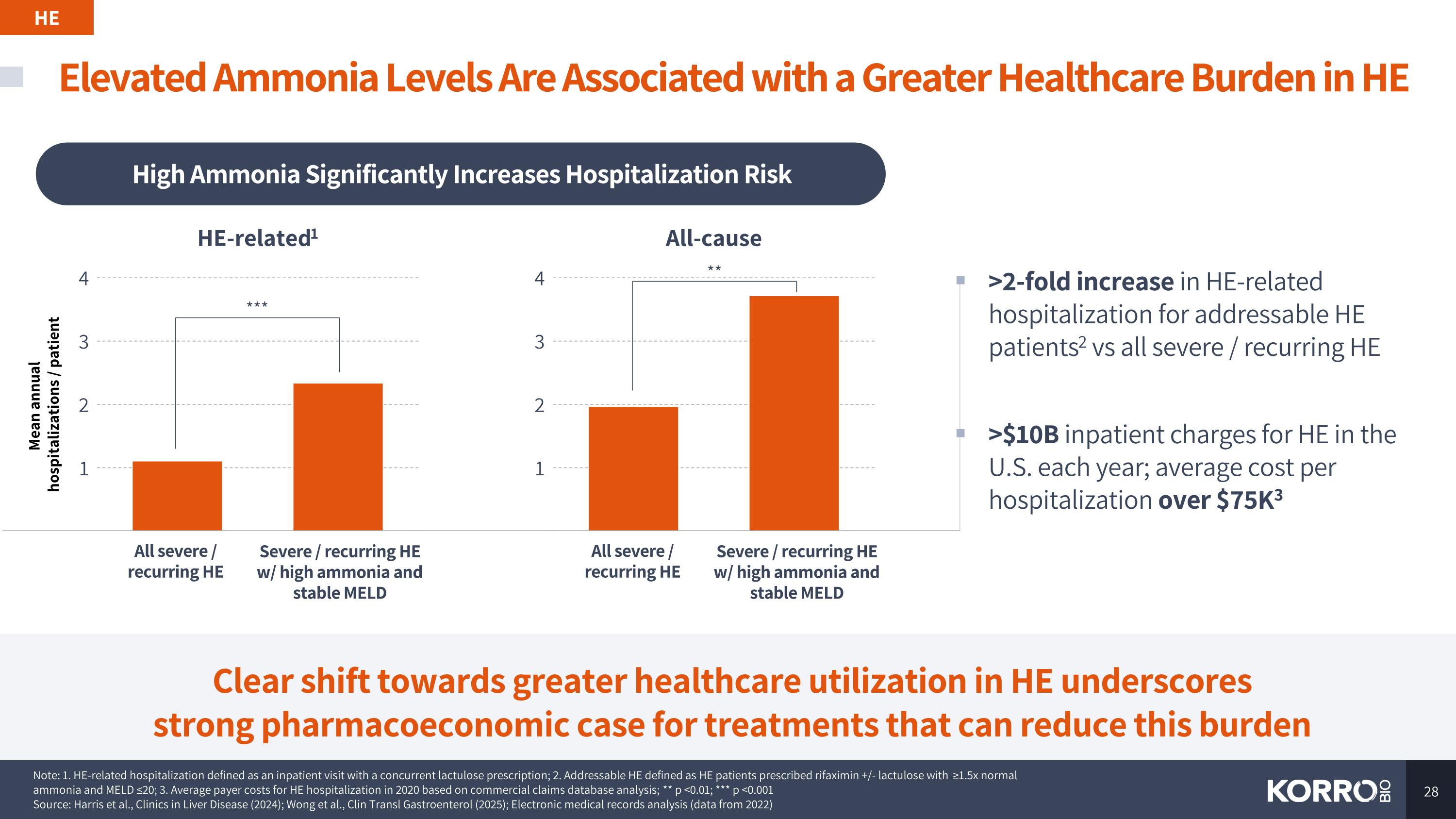
Elevated Ammonia Levels Are Associated with a Greater Healthcare Burden in HE HE Clear shift towards greater healthcare utilization in HE underscores strong pharmacoeconomic case for treatments that can reduce this burden High Ammonia Significantly Increases Hospitalization Risk 1 2 3 4 Severe / recurring HE w/ high ammonia and stable MELD All severe / recurring HE Note: 1. HE-related hospitalization defined as an inpatient visit with a concurrent lactulose prescription; 2. Addressable HE defined as HE patients prescribed rifaximin +/- lactulose with ≥1.5x normal ammonia and MELD ≤20; 3. Average payer costs for HE hospitalization in 2020 based on commercial claims database analysis; ** p <0.01; *** p <0.001 Source: Harris et al., Clinics in Liver Disease (2024); Wong et al., Clin Transl Gastroenterol (2025); Electronic medical records analysis (data from 2022) Severe / recurring HE w/ high ammonia and stable MELD All severe / recurring HE HE-related1 All-cause 1 2 3 4 Mean annual hospitalizations / patient *** ** >2-fold increase in HE-related hospitalization for addressable HE patients2 vs all severe / recurring HE >$10B inpatient charges for HE in the U.S. each year; average cost per hospitalization over $75K3
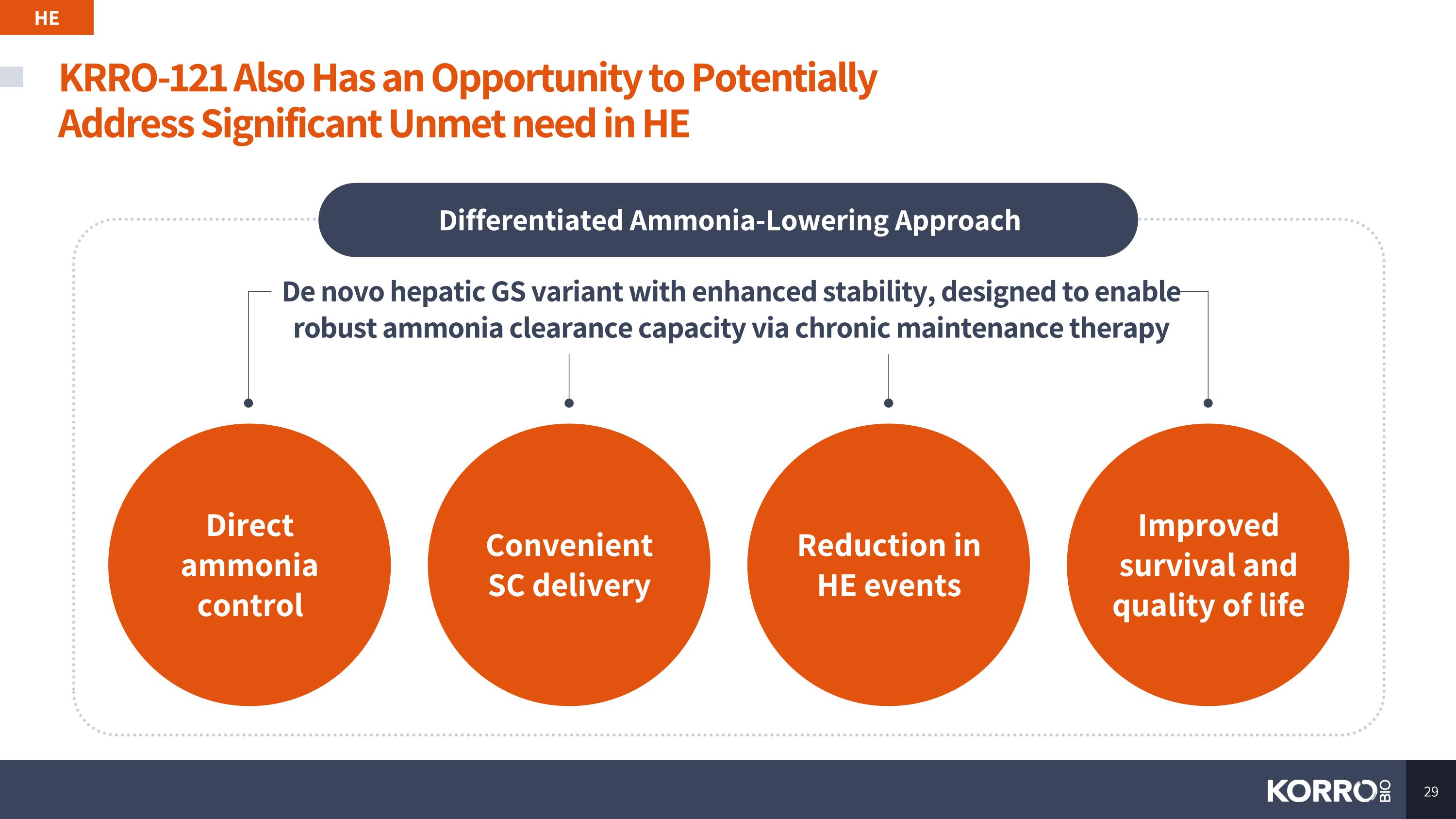
KRRO-121 Also Has an Opportunity to Potentially Address Significant Unmet need in HE HE De novo hepatic GS variant with enhanced stability, designed to enable robust ammonia clearance capacity via chronic maintenance therapy Differentiated Ammonia-Lowering Approach Direct ammonia control Convenient SC delivery Improved survival and quality of life Reduction in HE events
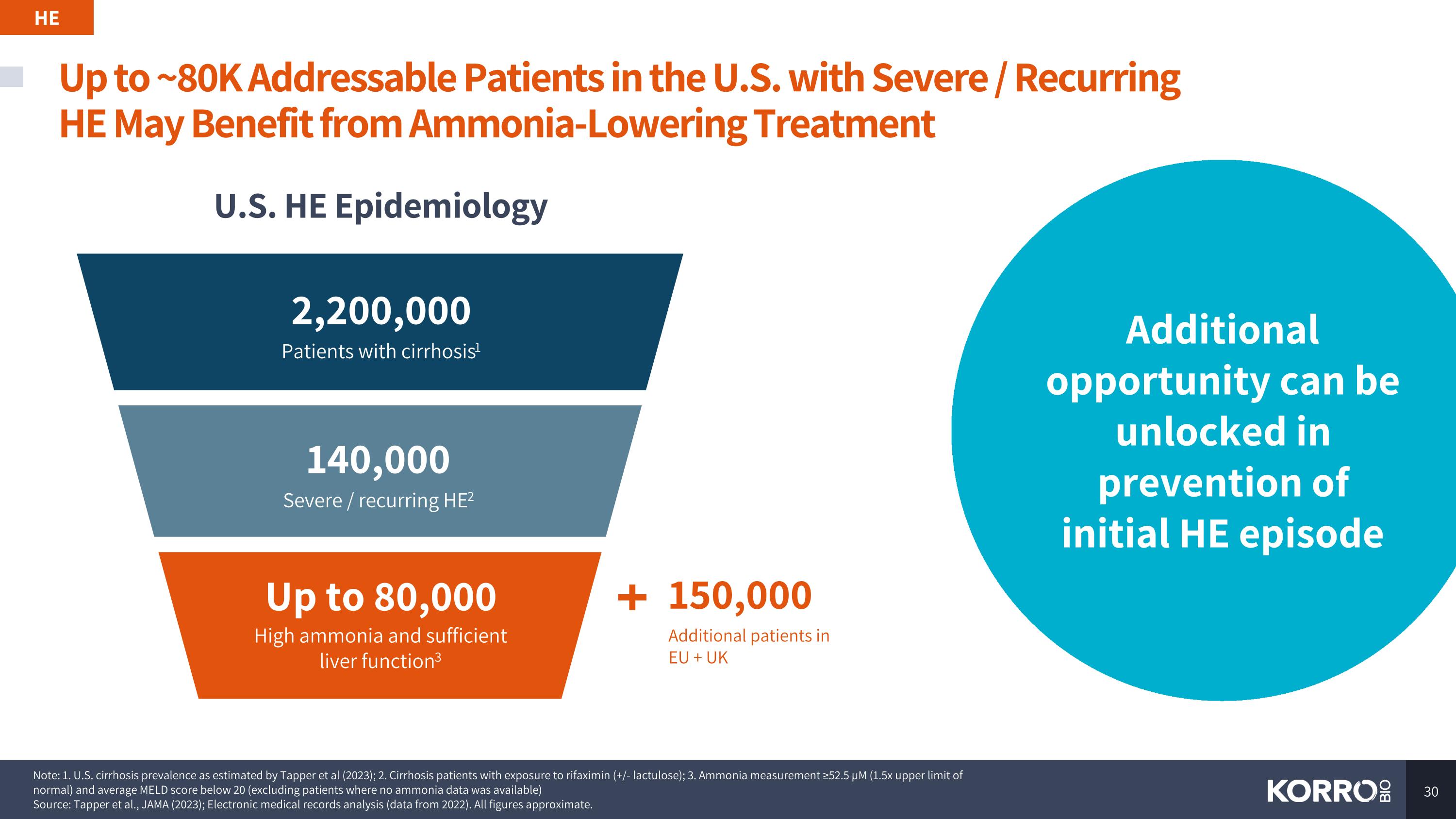
Up to ~80K Addressable Patients in the U.S. with Severe / Recurring HE May Benefit from Ammonia-Lowering Treatment Note: 1. U.S. cirrhosis prevalence as estimated by Tapper et al (2023); 2. Cirrhosis patients with exposure to rifaximin (+/- lactulose); 3. Ammonia measurement ≥52.5 µM (1.5x upper limit of normal) and average MELD score below 20 (excluding patients where no ammonia data was available) Source: Tapper et al., JAMA (2023); Electronic medical records analysis (data from 2022). All figures approximate. HE Additional opportunity can be unlocked in prevention of initial HE episode U.S. HE Epidemiology Up to 80,000 High ammonia and sufficient liver function3 150,000 Additional patients in EU + UK 2,200,000 Patients with cirrhosis1 140,000 Severe / recurring HE2

Ram Aiyar, PhD, MBA Chief Executive Officer Closing remarks

Key Takeaways from KRRO-121 Significant unmet medical need for controlling ammonia Robust scientific / genetic evidence supporting GS stabilization approach Transformative potential to impact patients Vision for the future as a leader in modulating disease biology