.2

[Graphic Appears Here] November 2 0 2 5 Corporate Presentation 87050-004-Part-503Nov25 13:39Page 22[Graphic Appears Here]
.2

[Graphic Appears Here] November 2 0 2 5 Corporate Presentation 87050-004-Part-503Nov25 13:39Page 22[Graphic Appears Here]

Cautionary Note Regarding Forward Looking Statements statements This presentation regarding contains future “forward expectations, -looking plans statements” and prospects within the for the meaning company; of the the Private ability Securities to successfully Litigation achieve Reform and Act execute of 1995, on the including company’s 601 goals, programs; priorities expectations and key clinical for additional and preclinical site activations, milestones; planned strategies enrollment, and expectations planned for regulatory the company’s interactions SGT and -003, the SGT potential -212, SGT approval -501 and pathways SGT- for “could,” SGT -“estimate,” 003; timing “expect,” of planned “intend,” clinical “may,” trials of “plan,” SGT -“potential,” 212 and SGT “predict,” -501; and “project,” other statements “should,” “target,” containing “would,” the words “working” “anticipate,” and similar “believe,” expressions “continue,” . Any that forward could -looking cause statements actual results are to based differ on materially management’s and adversely current from expectations those set of forth future in, events or implied and by, are such subject forward to a -number looking statements of risks and. uncertainties These risks other and uncertainties preclinical programs, include, but capsid are not libraries limited and to, other risks enabling associated technologies with the company’s on the timelines ability to expected advance or SGT at all; -003, obtain SGT and -212, maintain SGT-501, necessary SGT-601 and approvals trials of the from company’s the FDA product and other candidates; regulatory obtain, authorities; maintain replicate or protect in clinical intellectual trials positive property results rights related found in to preclinical its product studies candidates; and early replicate -stage clinical preliminary to develop Duchenne, or interim data FA, CPVT from early and- stage other neuromuscular clinicals trials in and the cardiac final data treatments of such trials; and gene compete therapies; successfully manage with expenses; other companies and raise that the are substantial seeking achieve additional its capital other business needed, on objectives the timeline and necessary, continue as to a continue going concern development . For a discussion of SGT-003, of other SGT -risks 212, SGT and uncertainties, -501, SGT-601 and and other other important candidates, factors, any section, of which as well could as discussions cause the company’s of potential actual risks, results uncertainties to differ and from other those important contained factors, in the in forward the company’s -looking statements, most recent see filings the with “Risk the Factors” Securities and hereof Exchange and should Commission not be relied . In addition, upon as representing the forward-looking the company’s statements views included as of any in this date press subsequent release represent to the date the hereof company’s . The company views as anticipates of the date that forward subsequent -looking statements events and at developments some point in will the cause future, the the company’s company views specifically to change disclaims . However, any obligation while the to company do so. may elect to update these our This industry presentation . This contains data involves estimates a number and other of assumptions statistical data and limitations, made by independent and you are parties cautioned and not by us to relating give undue to market weight size to such and data other and data about estimates operate are . In necessarily addition, projections, subject to a assumptions high degree and of uncertainty estimates of and our risk future . performance and the future performance of the markets in which we 87050-004-Part-5 03Nov25 13:39 Page 23 2 © 2025 Solid Biosciences [Graphic Appears Here]

[Graphic Appears Here] [Graphic Appears Here] Ty Howton, J.D. Gabriel Brooks, M.D. Paul Herzich Shuli Kulak, M.D. Chief Operating Officer Chief Medical Officer Chief Technology Officer Head of Corporate Strategy & Business Development [Graphic Appears Here]
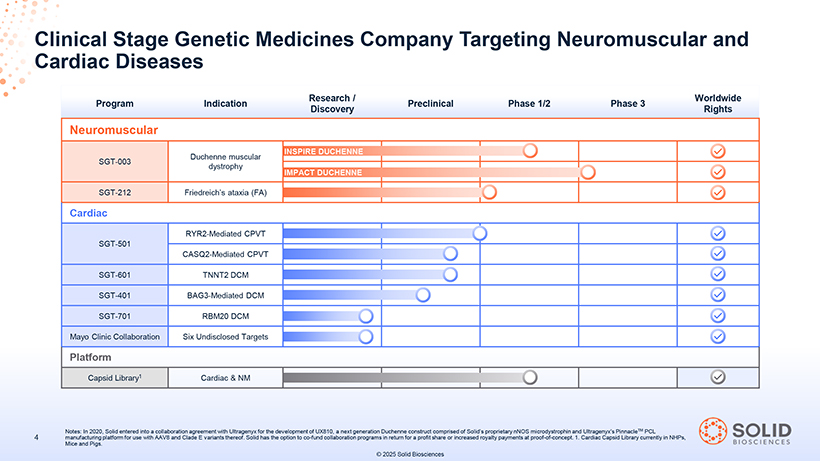
Clinical Stage Genetic Medicines Company Targeting Neuromuscular and Cardiac Diseases Research / Worldwide Program Indication Preclinical Phase 1/2 Phase 3 Discovery Rights Neuromuscular INSPIRE DUCHENNE SGT-003 Duchenne muscular dystrophy IMPACT DUCHENNE SGT-212 Friedreich’s ataxia (FA) Cardiac RYR2-Mediated CPVT SGT-501 CASQ2-Mediated CPVT SGT-601 TNNT2 DCM SGT-401 BAG3-Mediated DCM SGT-701 RBM20 DCM Mayo Clinic Collaboration Six Undisclosed Targets Platform Capsid Library1 Cardiac & NM 87050-004-Part-5 03Nov25 13:39 Page 25 Notes: In 2020, Solid entered into a collaboration agreement with Ultragenyx for the development of UX810, a next generation Duchenne construct comprised of Solid’s proprietary nNOS microdystrophin and Ultragenyx’s PinnacleTM PCL 4 manufacturing platform for use with AAV8 and Clade E variants thereof. Solid has the option to co-fund collaboration programs in return for a profit share or increased royalty payments at proof-of-concept. 1. Cardiac Capsid Library currently in NHPs, Mice and Pigs. © 2025 Solid Biosciences
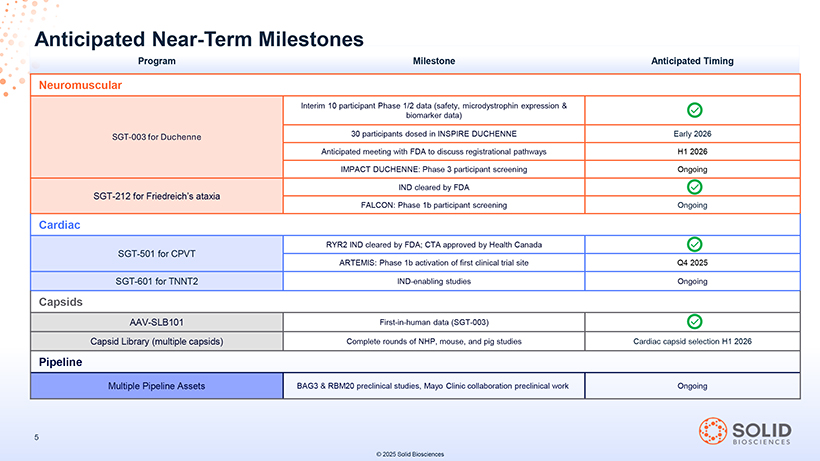
Anticipated Near-Term Milestones Program Milestone Anticipated Timing Neuromuscular Interim 10 participant Phase 1/2 data (safety, microdystrophin expression & biomarker data) SGT-003 for Duchenne 30 participants dosed in INSPIRE DUCHENNE Early 2026 Anticipated meeting with FDA to discuss registrational pathways H1 2026 IMPACT DUCHENNE: Phase 3 participant screening Ongoing SGT-212 for Friedreich’s ataxia IND cleared by FDA FALCON: Phase 1b participant screening Ongoing Cardiac SGT-501 for CPVT RYR2 IND cleared by FDA; CTA approved by Health Canada ARTEMIS: Phase 1b activation of first clinical trial site Q4 2025 SGT-601 for TNNT2 IND-enabling studies Ongoing Capsids AAV-SLB101 First-in-human data (SGT-003) Capsid Library (multiple capsids) Complete rounds of NHP, mouse, and pig studies Cardiac capsid selection H1 2026 Pipeline Multiple Pipeline Assets BAG3 & RBM20 preclinical studies, Mayo Clinic collaboration preclinical work Ongoing © 2025 Solid Biosciences

[Graphic Appears Here] D u c h e n n e M u s c u l a r D y s t r o p h y ( D u c h e n n e ) 87050-004-Part-5 03Nov25 13:39 Page 27 [Graphic Appears Here]
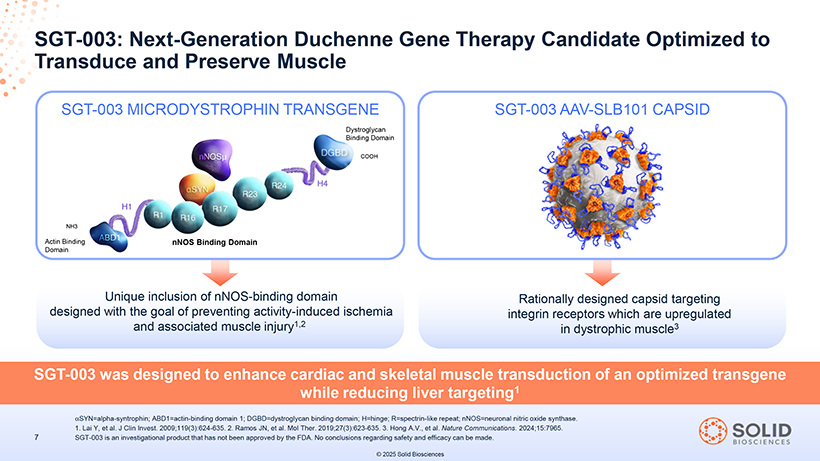
[Graphic Appears Here] SGT Transduce -003: Next and- Generation Preserve Muscle Duchenne Gene Therapy Candidate Optimized to SGT-003 MICRODYSTROPHIN TRANSGENE SGT-003 AAV-SLB101 CAPSID Dystroglycan Binding Domain COOH NH3 Actin Binding nNOS Binding Domain Domain Unique inclusion of nNOS-binding domain Rationally designed capsid targeting designed with the goal of preventing activity-induced ischemia integrin receptors which are upregulated and associated muscle injury1,2 in dystrophic muscle3 SGT-003 was designed to enhance cardiac and skeletal muscle transduction of an optimized transgene while reducing liver targeting1 87050-004-Part-5 03Nov25 13:39 Page 28 áSYN=alpha-syntrophin; ABD1=actin-binding domain 1; DGBD=dystroglycan binding domain; H=hinge; R=spectrin-like repeat; nNOS=neuronal nitric oxide synthase. 1. Lai Y, et al. J Clin Invest. 2009;119(3):624-635. 2. Ramos JN, et al. Mol Ther. 2019;27(3):623-635. 3. Hong A.V., et al. Nature Communications. 2024;15:7965. 7 SGT-003 is an investigational product that has not been approved by the FDA. No conclusions regarding safety and efficacy can be made. © 2025 Solid Biosciences [Graphic Appears Here]
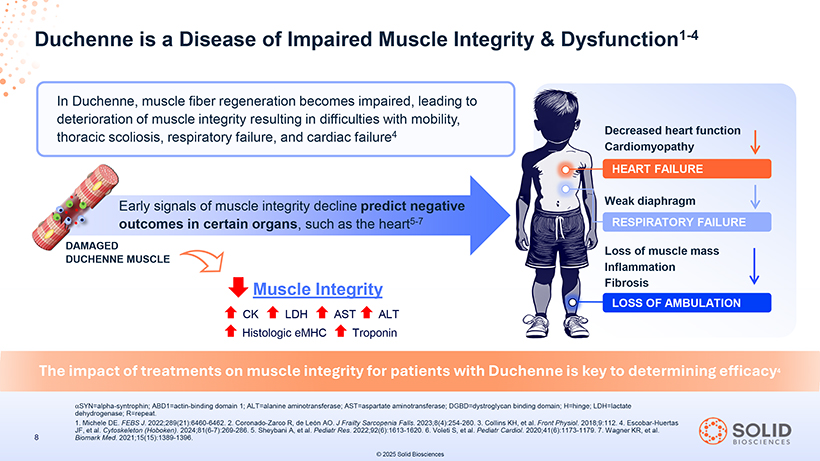
Duchenne is a Disease of Impaired Muscle Integrity & Dysfunction1-4 In Duchenne, muscle fiber regeneration becomes impaired, leading to deterioration of muscle integrity resulting in difficulties with mobility, thoracic scoliosis, respiratory failure, and cardiac failure4 Decreased heart function Cardiomyopathy HEART FAILURE Early signals of muscle integrity decline predict negative Weak diaphragm outcomes in certain organs, such as the heart5-7 RESPIRATORY FAILURE DAMAGED Loss of muscle mass DUCHENNE MUSCLE Inflammation Muscle Integrity Fibrosis LOSS OF AMBULATION CK LDH AST ALT Histologic eMHC Troponin The impact of treatments on muscle integrity for patients with Duchenne is key to determining efficacy4 áSYN=alpha-syntrophin; dehydrogenase; 87050-004-Part-5 R=repeat. ABD1=actin-binding domain 1; ALT=alanine 03Nov25 aminotransferase; 13:39 AST=aspartate aminotransferase; Page DGBD=dystroglycan 29 binding domain; H=hinge; LDH=lactate 1. JF, Michele et al. Cytoskeleton DE. FEBS J. (Hoboken) 2022;289(21):6460-6462. . 2024;81(6-7):269-286. 2. Coronado-Zarco 5. Sheybani R, A, de et al. León Pediatr AO. J Res. Frailty 2022;92(6):1613-1620. Sarcopenia Falls. 2023;8(4):254-260. 6. Voleti S, et al. Pediatr 3. Collins Cardiol. KH, et 2020;41(6):1173-1179. al. Front Physiol. 2018;9:112. 7. Wagner 4. Escobar-Huertas KR, et al. 8 Biomark Med. 2021;15(15):1389-1396.
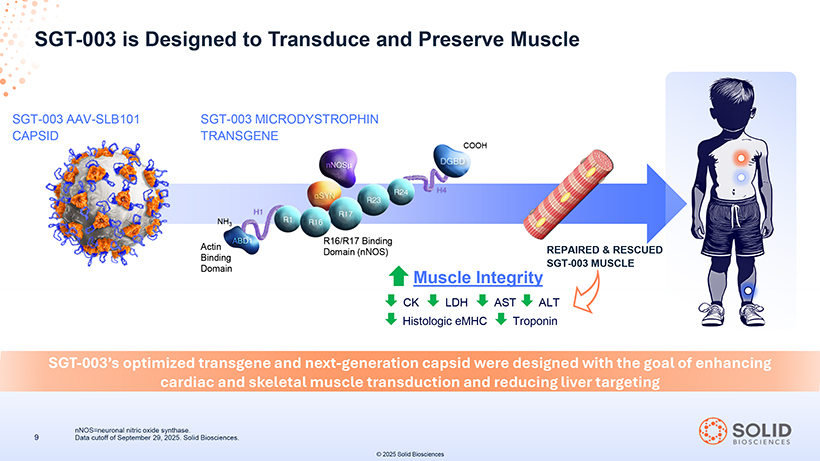
[Graphic Appears Here] SGT-003 AAV-SLB101 SGT-003 MICRODYSTROPHIN CAPSID TRANSGENE [Graphic Appears Here][Graphic Appears Here] SGT-003’s optimized transgene and next-generation capsid were designed with the goal of enhancing cardiac and skeletal muscle transduction and reducing liver targeting 87050-004-Part-5 03Nov25 13:39 Page 30 nNOS=neuronal nitric oxide synthase. 9 Data cutoff of September 29, 2025. Solid Biosciences. © 2025 Solid Biosciences [Graphic Appears Here]
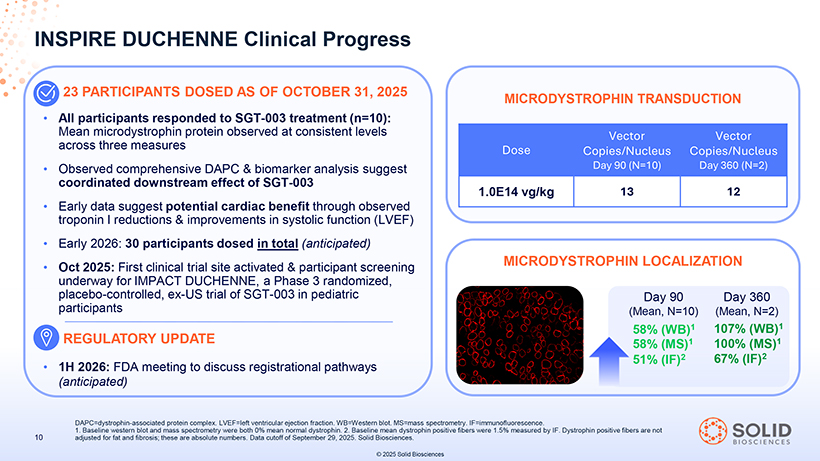
[Graphic Appears Here] All participants responded to SGT-003 treatment (n=10): Mean microdystrophin protein observed at consistent levels across three measures Observed comprehensive DAPC & biomarker analysis suggest coordinated downstream effect of SGT-003 Early data suggest potential cardiac benefit through observed troponin I reductions & improvements in systolic function (LVEF) Early 2026: 30 participants dosed in total (anticipated) Oct 2025: First clinical trial site activated & participant screening underway for IMPACT DUCHENNE, a Phase 3 randomized, placebo-controlled, ex-US trial of SGT-003 in pediatric participants REGULATORY UPDATE 1H 2026: FDA meeting to discuss registrational pathways (anticipated) 87050-004-Part-5 03Nov25 13:39 MICRODYSTROPHIN TRANSDUCTION Vector Vector Dose Copies/Nucleus Copies/Nucleus Day 90 (N=10) Day 360 (N=2) 1.0E14 vg/kg 13 12 MICRODYSTROPHIN LOCALIZATION [Graphic Appears Here] Day 90 Day 360 (Mean, N=10) (Mean, N=2) 58% (WB)1 107% (WB)1 58% (MS)1 100% (MS)1 51% (IF)2 67% (IF)2 DAPC=dystrophin 1. Baseline western -associated blot and mass protein spectrometry complex. LVEF=left were both ventricular 0% mean ejection normal dystrophin fraction. WB=Western . 2. Baseline blot mean . MS=mass dystrophin spectrometry positive fibers . IF=immunofluorescence were 1.5% measured .by IF. Dystrophin positive fibers are not 10 adjusted for fat and fibrosis; these are absolute numbers. Data cutoff of September 29, 2025. Solid Biosciences. © 2025 Solid Biosciences [Graphic Appears Here]
[Graphic Appears Here] 87050-004-Part-5 03Nov25 13:39 Page 32 M[Graphic Appears Here] 87050-004-Part-5 03Nov25 13:39 Page 32 Microdystrophin positive fibers are not adjusted for fat and fibrosis; this is an absolute number. Representative images are shown. 11 1. Data cutoff of September 29, 2025. Solid Biosciences. 2. Anastasi G, et al. Cells Tissues Organs. 2003;173(1):54-63. © 2025 Solid Biosciences [Graphic Appears Here] icrodystrophin positive fibers are not adjusted for fat and fibrosis; this is an absolute number. Representative images are shown. 11 1. Data cutoff of September 29, 2025. Solid Biosciences. 2. Anastasi G, et al. Cells Tissues Organs. 2003;173(1):54-63. © 2025 Solid Biosciences [Graphic Appears Here]
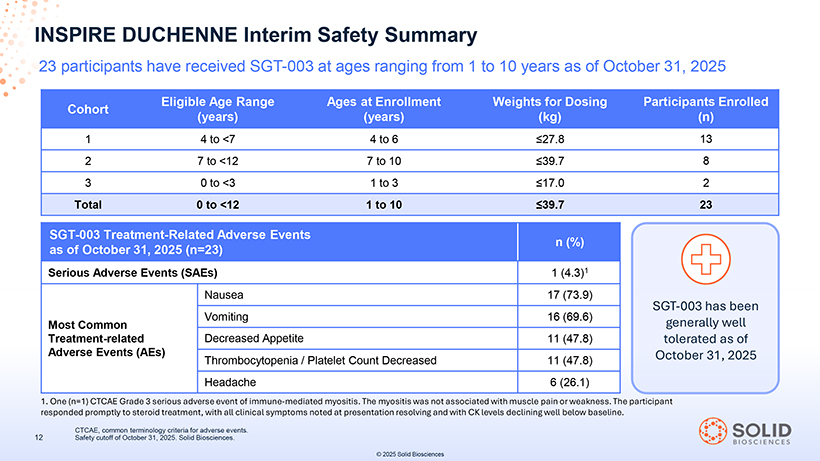
INSPIRE DUCHENNE Interim Safety Summary 23 participants have received SGT-003 at ages ranging from 1 to 10 years as of October 31, 2025 [Graphic Appears Here] Eligible Age Range Ages at Enrollment Weights for Dosing Participants Enrolled Cohort (years) (years) (kg) (n) 1 4 to <7 4 to 6 ≤27.8 13 2 7 to <12 7 to 10 ≤39.7 8 3 0 to <3 1 to 3 ≤17.0 2 Total 0 to <12 1 to 10 ≤39.7 23 SGT-003 Treatment-Related Adverse Events as of October 31, 2025 (n=23) n (%) Serious Adverse Events (SAEs) 1 (4.3)1 Nausea 17 (73.9) SGT-003 has been Most Common Vomiting 16 (69.6) generally well Treatment-related Decreased Appetite 11 (47.8) tolerated as of Adverse Events (AEs) Thrombocytopenia / Platelet Count Decreased 11 (47.8) October 31, 2025 Headache 6 (26.1) 1. One (n=1) CTCAE Grade 3 serious adverse event of immune-mediated myositis. The myositis was not associated with muscle pain or weakness. The participant responded 87050 promptly - to 004 steroid -Part e ment, -5 with all clinical sympt 03Nov25 ms n ted at 13:39 presentation resolving andPage with CK levels 33declining well below baseline. CTCAE, common terminology criteria for adverse events. 12 Safety cutoff of October 31, 2025. Solid Biosciences. [Graphic Appears Here] © 2025 Solid Biosciences
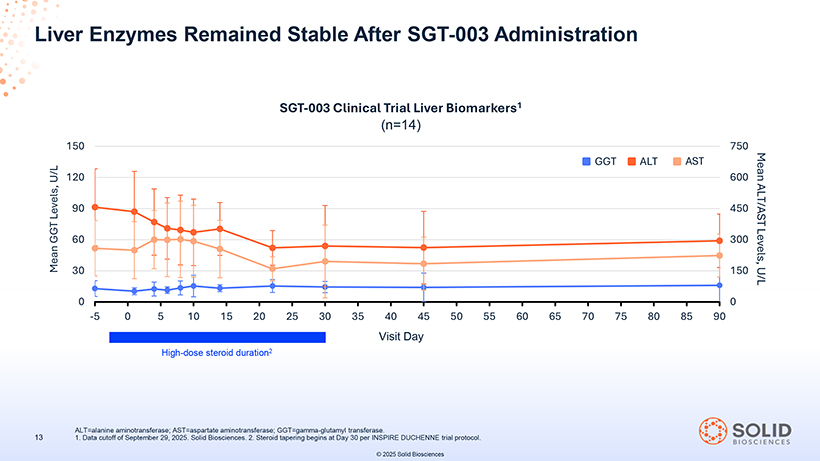
Liver Enzymes Remained Stable After SGT-003 Administration SGT-003 Clinical Trial Liver Biomarkers1 (n=14) 1 ALT=alanine . Data cutoff aminotransferase; of September 29, 2025 AST=aspartate . Solid Biosciences aminotransferase; . 2. Steroid GGT=gamma tapering begins -glutamyl at Day transferase 30 per INSPIRE . DUCHENNE trial protocol. © 2025 Solid Biosciences
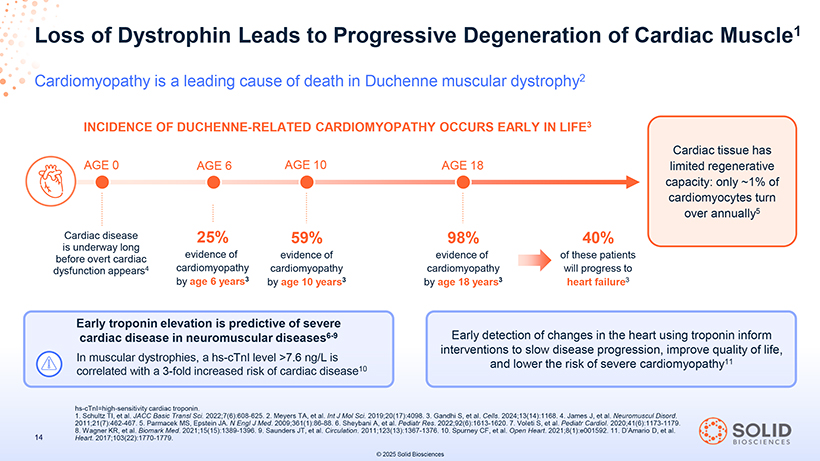
Loss of Dystrophin Leads to Progressive Degeneration of Cardiac Muscle1 Cardiomyopathy is a leading cause of death in Duchenne muscular dystrophy2 Cardiac disease 25% 59% 98% 40% is underway long before overt cardiac evidence of evidence of evidence of of these patients dysfunction appears4 cardiomyopathy cardiomyopathy cardiomyopathy will progress to by age 6 years3 by age 10 years3 by age 18 years3 heart failure3 Early troponin elevation is predictive of severe cardiac disease in neuromuscular diseases6-9 Early detection of changes in the heart using troponin inform interventions to slow disease progression, improve quality of life, In muscular dystrophies, a hs-cTnI level >7.6 ng/L is and lower the risk of severe cardiomyopathy11 correlated with a 3-fold increased risk of cardiac disease10 hs 187050 . - Schultz cTnI=high TI, -et sensitivity - al 004 . JACCcardiac Basic -Part Transl oponin - Sci 5 . . 2022;7(6):608-625. 203Nov25 . Meyers TA, et al. Int J 13:39 Mol Sci. 2019;20(17):4098 . 3. Gandhi Page S, et al. Cells 35 . 2024;13(14):1168 . 4. James J, et al. Neuromuscul Disord. 2011;21(7):462 8. Wagner KR, et -467 al.. Biomark 5. Parmacek Med .MS, 2021;15(15):1389 Epstein JA. N Engl -1396 J. Med 9. Saunders . 2009;361(1):86 JT, et al. - 88 Circulation . 6. Sheybani . 2011;123(13):1367 A, et al. Pediatr- 1376 Res. .2022;92(6):1613 10. Spurney CF, -1620 et al.. Open 7. Voleti Heart S, et . 2021;8(1):e001592 al. Pediatr Cardiol.. 11 2020;41(6):1173 . D’Amario D, et -1179 al. . Heart. 2017;103(22):1770-1779.
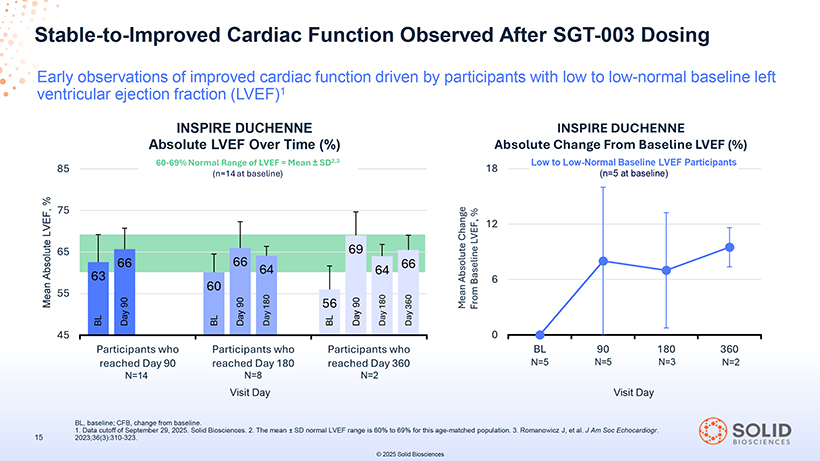
Stable-to-Improved Cardiac Function Observed After SGT-003 Dosing Early observations of improved cardiac function driven by participants with low to low-normal baseline left ventricular ejection fraction (LVEF)1 Participants who Participants who Participants who reached Day 90 reached Day 180 reached Day 360 N=14 N=8 N=2 Visit Day BL, 1. Data baseline; cutoff CFB, of September change from 29, 2025 baseline . Solid . Biosciences. 2. The mean ± SD normal LVEF range is 60% to 69% for this age-matched population. 3. Romanowicz J, et al. J Am Soc Echocardiogr 2023;36(3):310-323. INSPIRE DUCHENNE Absolute LVEF Over Time (%) 60-69% Normal Range of LVEF = Mean ± SD2,3 (n=14 at baseline) INSPIRE DUCHENNE Absolute Change From Baseline LVEF (%) 18 Low to Low-Normal Baseline LVEF Participants (n=5 at baseline)
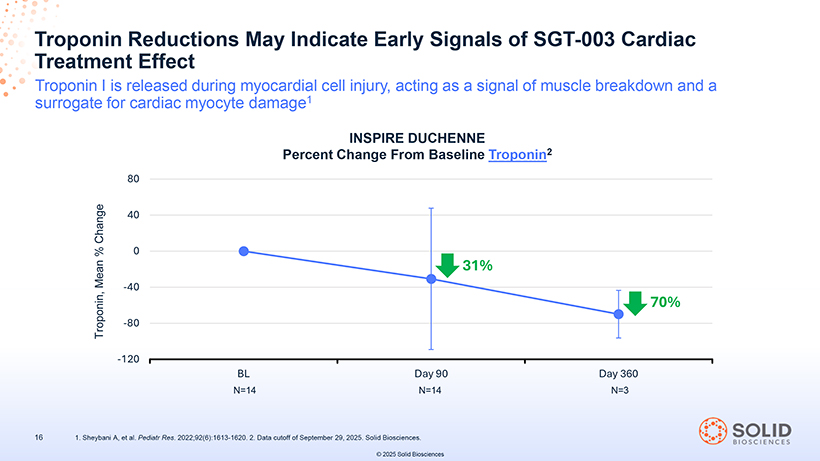
Troponin Treatment Reductions Effect May Indicate Early Signals of SGT-003 Cardiac Troponin I is released during myocardial cell injury, acting as a signal of muscle breakdown and a surrogate for cardiac myocyte damage1 INSPIRE DUCHENNE Percent Change From Baseline Troponin2 80 Change 40 % 0 Mean -40 Troponin, -80 -120 [Graphic Appears Here] BL Day 90 Day 360 N=14 N=14 N=3 87050-004-Part-7 03Nov25 14:52 Page 70 16 1. Sheybani A, et al. Pediatr Res. 2022;92(6):1613-1620. 2. Data cutoff of September 29, 2025. Solid Biosciences. © 2025 Solid Biosciences
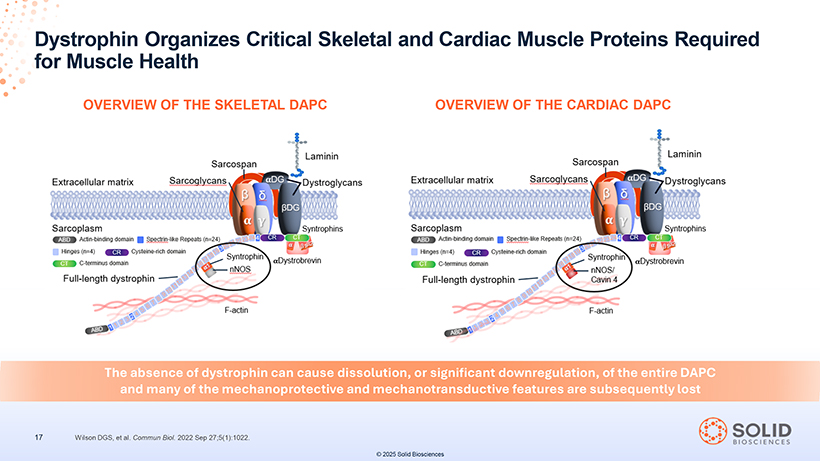
for Dystrophin Muscle Health Organizes Critical Skeletal and Cardiac Muscle Proteins Required OVERVIEW OF THE SKELETAL DAPC OVERVIEW OF THE CARDIAC DAPC The absence of dystrophin can cause dissolution, or significant downregulation, of the entire DAPC and many of the mechanoprotective and mechanotransductive features are subsequently lost
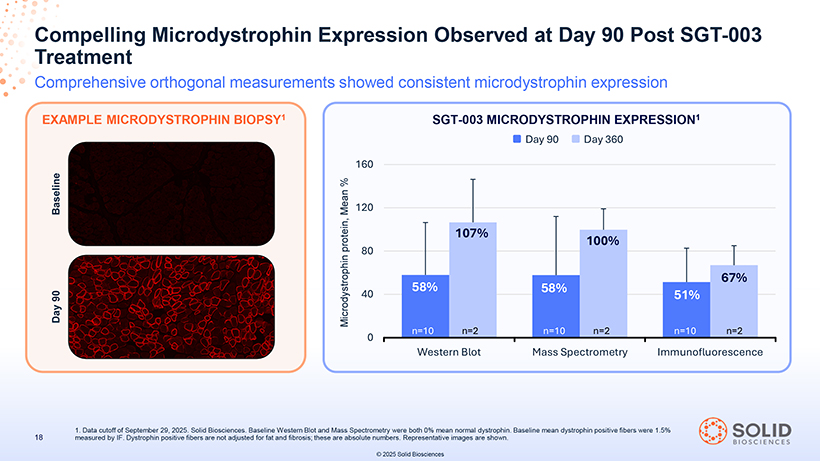
Compelling Treatment Microdystrophin Expression Observed at Day 90 Post SGT-003 Comprehensive orthogonal measurements showed consistent microdystrophin expression EXAMPLE MICRODYSTROPHIN BIOPSY1 1 measured . Data cutoff by IF of. September Dystrophin 29, positive 2025 fibers . Solid are Biosciences not adjusted . Baseline for fat and Western fibrosis; Blot these and Mass are absolute Spectrometry numbers were . Representative both 0% mean images normal are dystrophin shown. . Baseline mean dystrophin positive fibers were 1.5% © 2025 Solid Biosciences SGT-003 MICRODYSTROPHIN EXPRESSION1
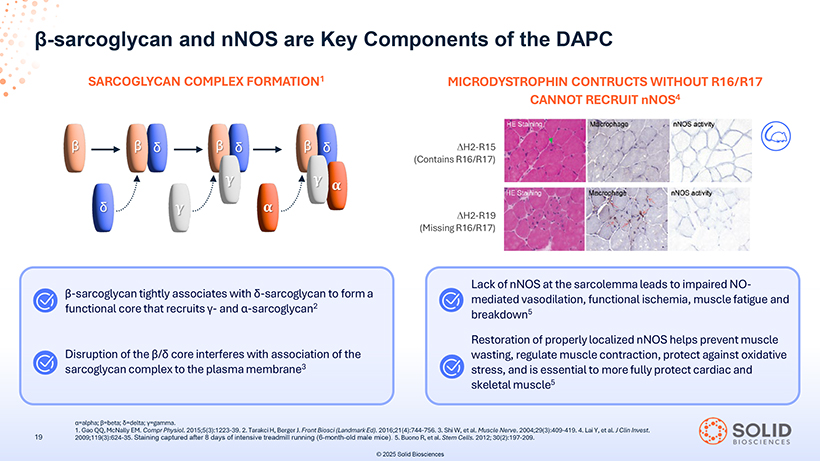
â-sarcoglycan and nNOS are Key Components of the DAPC SARCOGLYCAN COMPLEX FORMATION1 MICRODYSTROPHIN CONTRUCTS WITHOUT R16/R17 CANNOT RECRUIT nNOS4 â-sarcoglycan tightly associates with ä-sarcoglycan to form a functional core that recruits ã- and á-sarcoglycan2 Disruption of the â/ä core interferes with association of the sarcoglycan complex to the plasma membrane3 Lack of nNOS at the sarcolemma leads to impaired NO-mediated vasodilation, functional ischemia, muscle fatigue and breakdown5 Restoration of properly localized nNOS helps prevent muscle wasting, regulate muscle contraction, protect against oxidative stress, and is essential to more fully protect cardiac and skeletal muscle5 1 á= . Gao alpha; QQ, â=beta; McNally ä=delta; EM. Compr ã=gamma Physiol . . 2015;5(3):1223-39. 2. Tarakci H, Berger J. Front Biosci (Landmark Ed). 2016;21(4):744-756. 3. Shi W, et al. Muscle Nerve. 2004;29(3):409-419. 4. Lai Y, et al. J Clin Invest. 2009;119(3):624-35. Staining captured after 8 days of intensive treadmill running (6-month-old male mice). 5. Buono R, et al. Stem Cells. 2012; 30(2):197-209. © 2025 Solid Biosciencesp
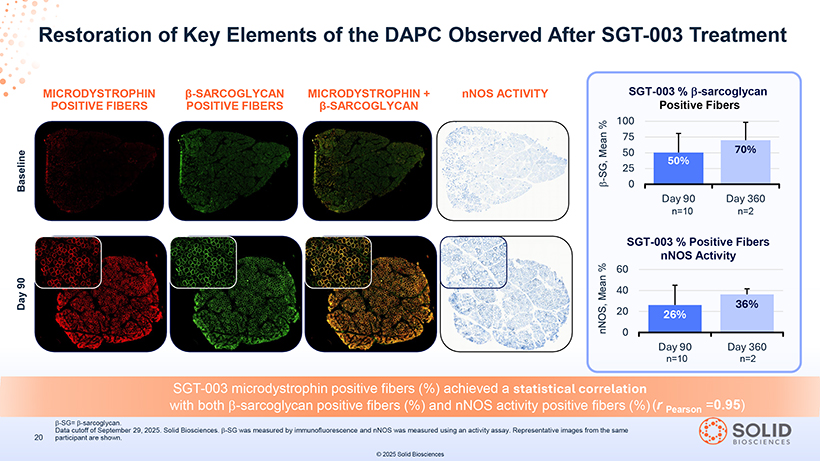
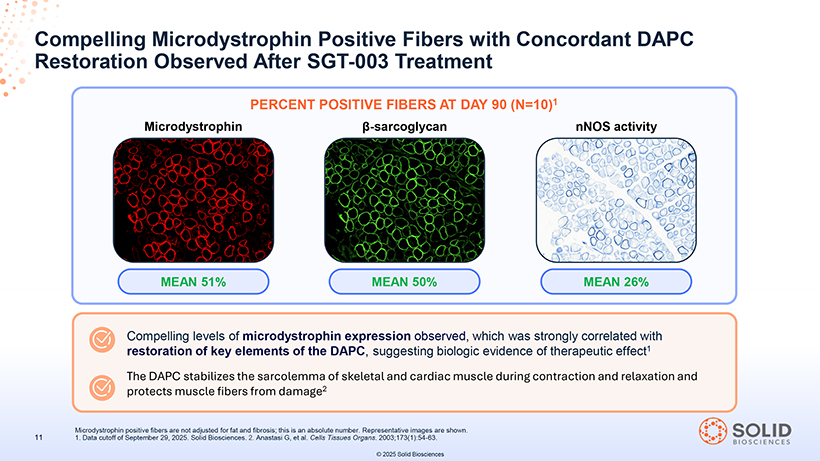
Restoration of Key Elements of the DAPC Observed After SGT-003 Treatment MICRODYSTROPHIN POSITIVE FIBERS â POSITIVE -SARCOGLYCAN FIBERS MICRODYSTROPHIN â-SARCOGLYCAN + nNOS ACTIVITY Positive Fibers SGT-003 microdystrophin positive fibers (%) achieved a statistical correlation with both â-sarcoglycan positive fibers (%) and nNOS activity positive fibers (%) (r Pearson =0.95) -Part-5 03Nov25 13:39 Page 41 participant Data cutoff are of September shown. 29, 2025. Solid Biosciences. â-SG was measured by immunofluorescence and nNOS was measured using an activity assay. Representative images from the same © 2025 Solid Biosciences SGT-003 % Positive Fibers nNOS Activity
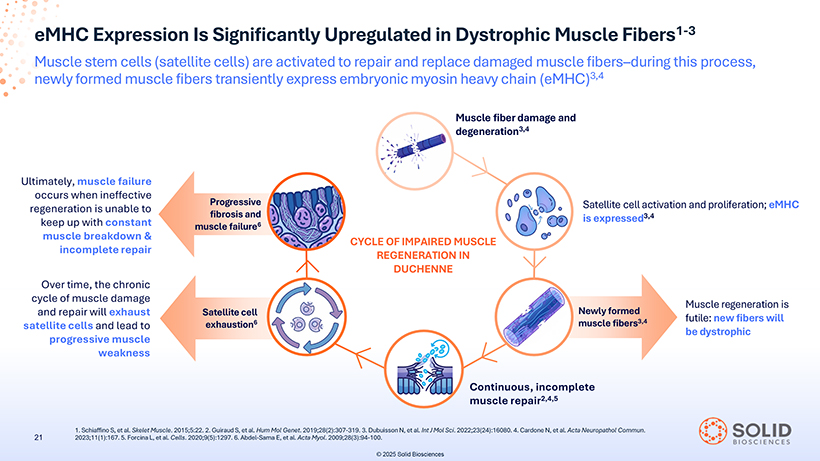
eMHC Expression Is Significantly Upregulated in Dystrophic Muscle Fibers1-3 Muscle stem cells (satellite cells) are activated to repair and replace damaged muscle fibers–during this process, newly formed muscle fibers transiently express embryonic myosin heavy chain (eMHC)3,4 Ultimately, muscle failure occurs when ineffective regeneration is unable to keep up with constant muscle breakdown & incomplete repair Over time, the chronic cycle of muscle damage and repair will exhaust satellite cells and lead to progressive muscle weakness Satellite cell activation and proliferation; eMHC is expressed3,4 Muscle regeneration is futile: new fibers will be dystrophic 1 2023;11(1):167 . Schiaffino S, et . 5 al . Forcina . Skelet L, Muscle et al. Cells . 2015;5:22 . 2020;9(5):1297 . 2. Guiraud. 6S,. Abdel et al. Hum -Sama Mol E, et Genet al. Acta . 2019;28(2):307 Myol. 2009;28(3):94 -319. 3. - Dubuisson 100. N, et al. Int J Mol Sci. 2022;23(24):16080. 4. Cardone N, et al. Acta Neuropathol Commun.
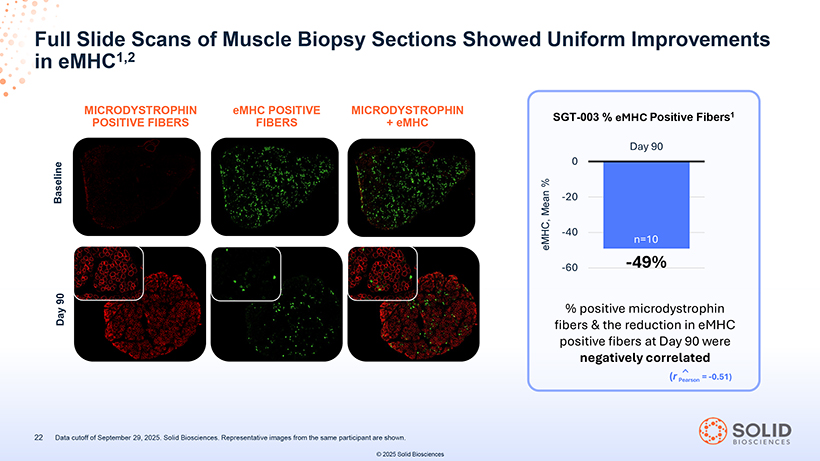
Full Slide Scans of Muscle Biopsy Sections Showed Uniform Improvements in eMHC1,2 MICRODYSTROPHIN POSITIVE FIBERS eMHC FIBERS POSITIVE MICRODYSTROPHIN + eMHC 22 Data cutoff of September 29, 2025. Solid Biosciences. Representative images from the same participant are shown. © 2025 Solid Biosciences SGT-003 % eMHC Positive Fibers1 % positive microdystrophin fibers & the reduction in eMHC positive fibers at Day 90 were negatively correlated (r Pearson = -0.51)
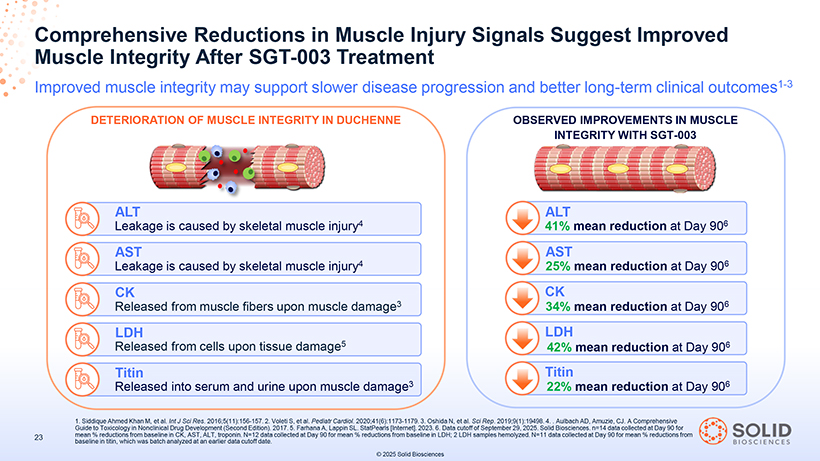
Muscle Comprehensive Integrity Reductions After SGT-003 in Muscle Treatment Injury Signals Suggest Improved Improved muscle integrity may support slower disease progression and better long-term clinical outcomes1-3 DETERIORATION OF MUSCLE INTEGRITY IN DUCHENNE OBSERVED IMPROVEMENTS IN MUSCLE INTEGRITY WITH SGT-003 ALT ALT Leakage is caused by skeletal muscle injury4 41% mean reduction at Day 906 AST AST Leakage is caused by skeletal muscle injury4 25% mean reduction at Day 906 CK CK Released from muscle fibers upon muscle damage3 34% mean reduction at Day 906 LDH LDH Released from cells upon tissue damage5 42% mean reduction at Day 906 Titin Titin Released into serum and urine upon muscle damage3 22% mean reduction at Day 906 Guide 1. Siddique to Toxicology Ahmed Khan in Nonclinical M, et al. Int Drug J Sci Development Res. 2016;5(11):156 (Second Edition) -157. 2.. Voleti 2017. S, 5. et Farhana al. Pediatr A, Lappin Cardiol SL . 2020;41(6):1173 . StatPearls [Internet] -1179 . .2023 3. Oshida . 6. Data N, et cutoff al. Sci of September Rep. 2019;9(1):19498 29, 2025. Solid . 4. . Biosciences Aulbach AD,. n=14 Amuzie, data CJ collected . A Comprehensive at Day 90 for 23 baseline mean % reductions in titin, which from was baseline batch analyzed in CK, AST, at an ALT, earlier troponin data .cutoff N=12 date data . collected at Day 90 for mean % reductions from baseline in LDH; 2 LDH samples hemolyzed. N=11 data collected at Day 90 for mean % reductions from © 2025 Solid Biosciences
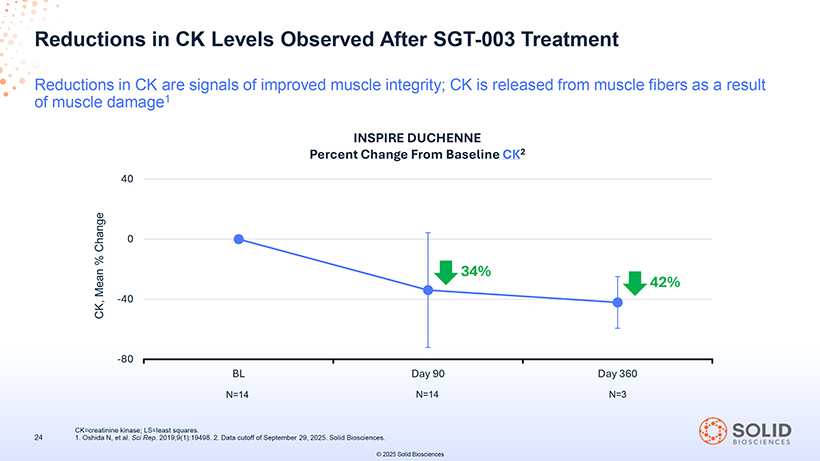
Reductions in CK Levels Observed After SGT-003 Treatment Reductions in CK are signals of improved muscle integrity; CK is released from muscle fibers as a result of muscle damage1 INSPIRE DUCHENNE Percent Change From Baseline CK2 CK=creatinine kinase; LS=least squares. 24 1. Oshida N, et al. Sci Rep. 2019;9(1):19498. 2. Data cutoff of September 29, 2025. Solid Biosciences. © 2025 Solid Biosciences
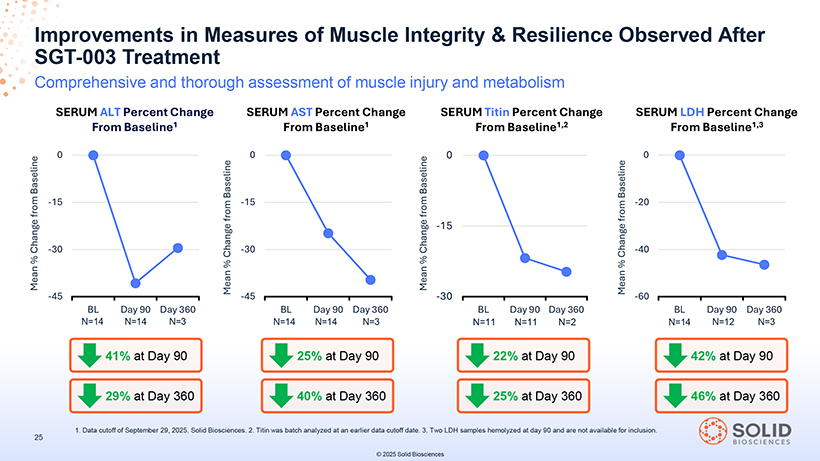
SGT Improvements -003 Treatment in Measures of Muscle Integrity & Resilience Observed After Comprehensive and thorough assessment of muscle injury and metabolism SERUM ALT Percent Change SERUM AST Percent Change SERUM Titin Percent Change SERUM LDH Percent Change From Baseline1 From Baseline1 From Baseline1,2 From Baseline1,3 1. Data cutoff of September 29, 2025. Solid Biosciences. 2. Titin was batch analyzed at an earlier data cutoff date. 3. Two LDH samples hemolyzed at day 90 and are not available for inclusion. 25 © 2025 Solid Biosciences
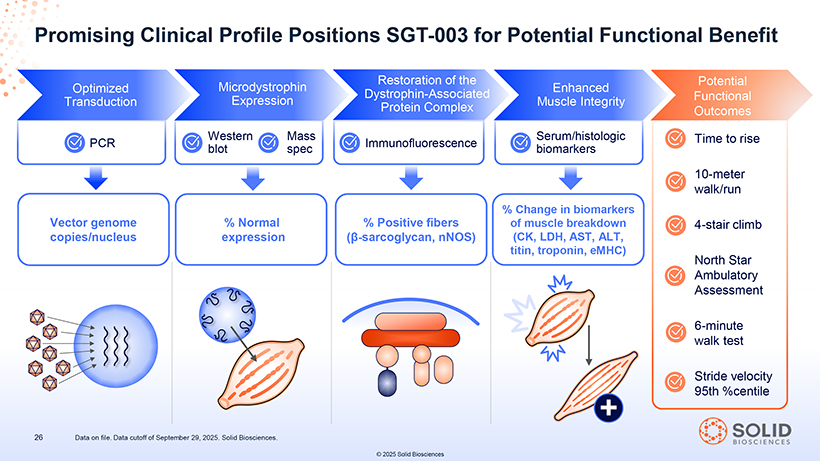
Promising Clinical Profile Positions SGT-003 for Potential Functional Benefit Restoration of the Potential Optimized Microdystrophin Enhanced Dystrophin-Associated Functional Transduction Expression Muscle Integrity Protein Complex Outcomes Western Mass Serum/histologic Time to rise PCR blot spec Immunofluorescence biomarkers 10-meter walk/run % Change in biomarkers Vector genome % Normal % Positive fibers of muscle breakdown 4-stair climb copies/nucleus expression (â-sarcoglycan, nNOS) (CK, LDH, AST, ALT, titin, troponin, eMHC) North Star Ambulatory Assessment 6-minute walk test Stride velocity + 95th %centile 26 Data on file. Data cutoff of September 29, 2025. Solid Biosciences. © 2025 Solid Biosciences

Neuromuscular Pipeline Program F r i e d r e i c h ‘ s A t a x i a ( F A ) © 2025 Solid Biosciences
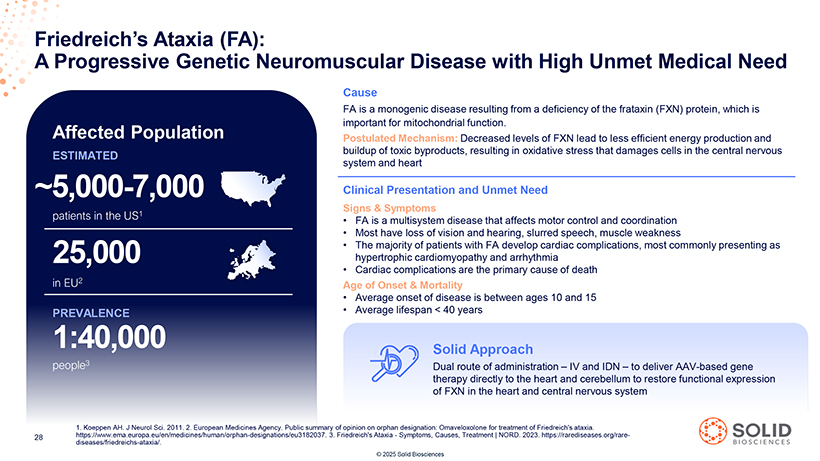
Friedreich’s A Progressive Ataxia Genetic (FA): Neuromuscular Disease with High Unmet Medical Need Cause FA is a monogenic disease resulting from a deficiency of the frataxin (FXN) protein, which is important for mitochondrial function. Affected Population Postulated Mechanism: Decreased levels of FXN lead to less efficient energy production and ESTIMATED buildup of toxic byproducts, resulting in oxidative stress that damages cells in the central nervous system and heart ~5,000-7,000 Clinical Presentation and Unmet Need Signs & Symptoms patients in the US1 • FA is a multisystem disease that affects motor control and coordination • Most have loss of vision and hearing, slurred speech, muscle weakness • The majority of patients with FA develop cardiac complications, most commonly presenting as 25,000 hypertrophic cardiomyopathy and arrhythmia • Cardiac complications are the primary cause of death in EU2 Age of Onset & Mortality • Average onset of disease is between ages 10 and 15 PREVALENCE • Average lifespan < 40 years 1:40,000 Solid Approach [Graphic Appears Here] 1. Koeppen AH. J Neurol Sci. 2011. 2. European Medicines Agency. Public summary of opinion on orphan designation: Omaveloxolone for treatment of Friedreich’s ataxia. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3182037. 3. Friedreich’s Ataxia—Symptoms, Causes, Treatment | NORD. 2023. https://rarediseases.org/rare- diseases/friedreichs-ataxia/. [Graphic Appears Here] © 2025 Solid Biosciences
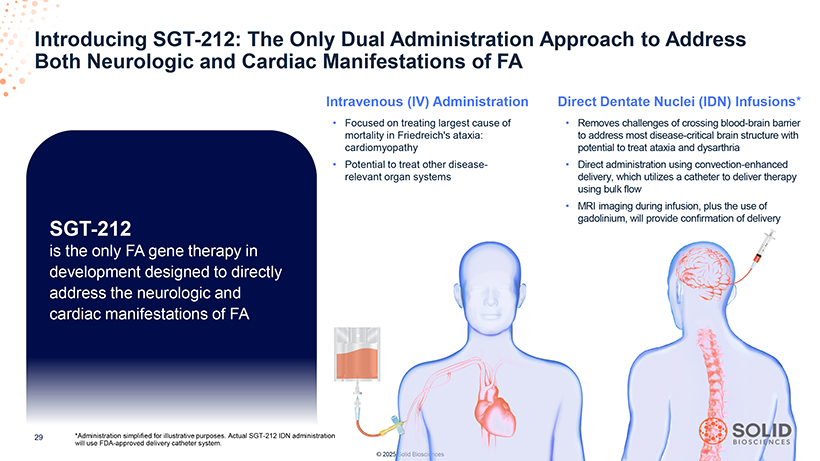
Introducing Both Neurologic SGT -and 212: Cardiac The Only Manifestations Dual Administration of FA Approach to Address Intravenous (IV) Administration Direct Dentate Nuclei (IDN) Infusions* • Focused on treating largest cause of • Removes challenges of crossing blood-brain barrier mortality in Friedreich’s ataxia: to address most disease-critical brain structure with cardiomyopathy potential to treat ataxia and dysarthria • Potential to treat other disease- • Direct administration using convection-enhanced relevant organ systems delivery, which utilizes a catheter to deliver therapy using bulk flow • MRI imaging during infusion, plus the use of SGT-212 gadolinium, will provide confirmation of delivery is the only FA gene therapy in development designed to directly address the neurologic and cardiac manifestations of FA 87050-004-Part-5 03Nov25 13:39 *Administration simplified for illustrative purposes. Actual SGT-212 IDN administration will use FDA-approved delivery catheter system. © 2025 Solid Biosciences [Graphic Appears Here]
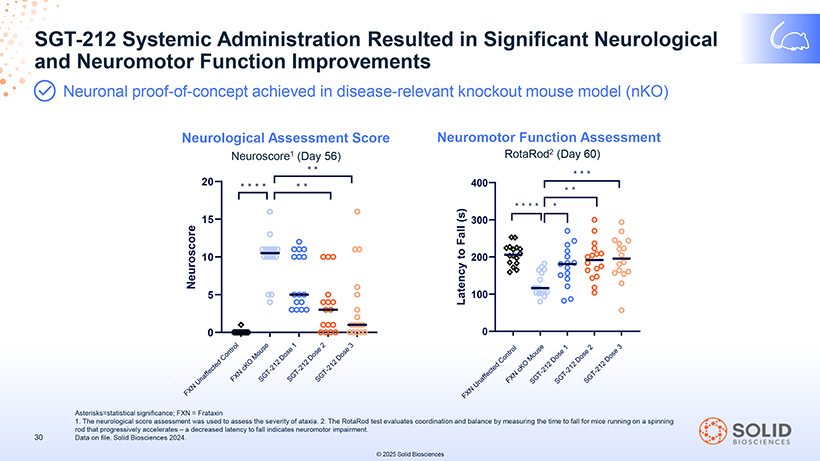
SGT and -Neuromotor 212 Systemic Function Administration Improvements Resulted in Significant Neurological Neuronal proof-of-concept achieved in disease-relevant knockout mouse model (nKO) Neurological Assessment Score Neuroscore1 (Day 56) Neuromotor Function Assessment RotaRod2 (Day 60) Asterisks=statistical significance; FXN = Frataxin 1. The neurological score assessment was used to assess the severity of ataxia. 2. The RotaRod test evaluates coordination and balance by measuring the time to fall for mice running on a spinning rod that progressively accelerates – a decreased latency to fall indicates neuromotor impairment. Data on file. Solid Biosciences 2024. © 2025 Solid Biosciences
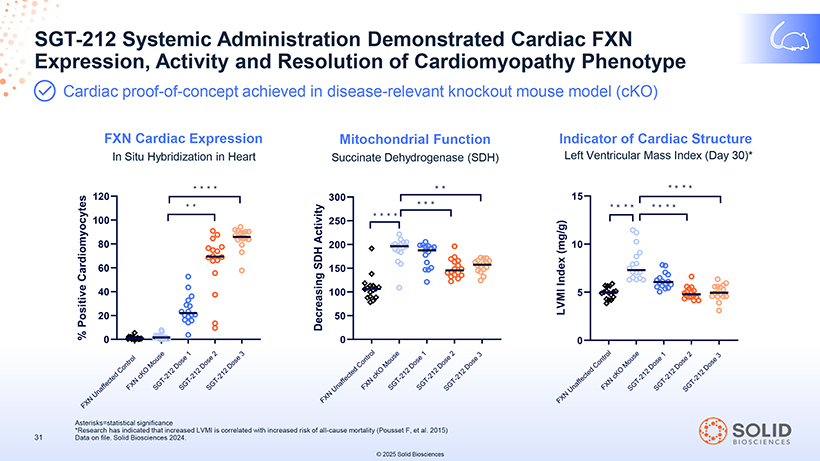
Expression, SGT-212 Systemic Activity Administration and Resolution Demonstrated of Cardiomyopathy Cardiac Phenotype FXN Cardiac proof-of-concept achieved in disease-relevant knockout mouse model (cKO) FXN Cardiac Expression Mitochondrial Function Indicator of Cardiac Structure In Situ Hybridization in Heart Succinate Dehydrogenase (SDH) Left Ventricular Mass Index (Day 30)* *Research Asterisks=statistical has indicated significance that increased LVMI is correlated with increased risk of all-cause mortality (Pousset F, et al. 2015) 31 Data on file. Solid Biosciences 2024. © 2025 Solid Biosciences
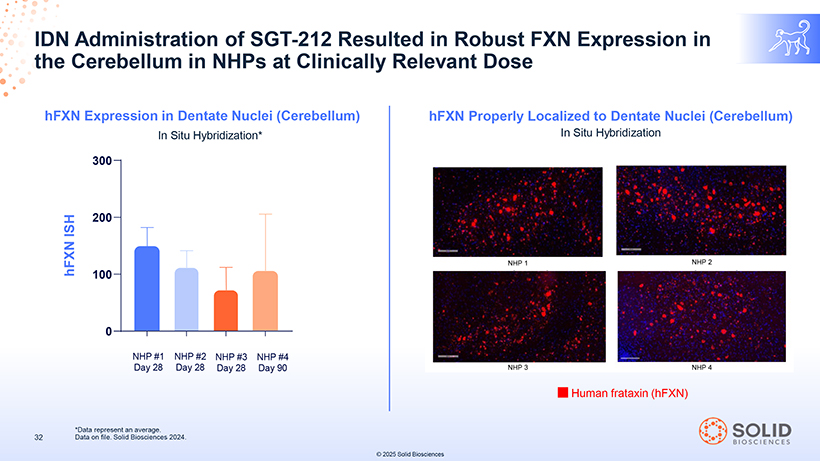
IDN the Cerebellum Administration in NHPs of SGT at -Clinically 212 Resulted Relevant in Robust Dose FXN Expression in hFXN Expression in Dentate Nuclei (Cerebellum) In Situ Hybridization* ISH hFXN NHP #1 NHP #2 NHP #3 NHP #4 Day 28 Day 28 Day 28 Day 90 hFXN Properly Localized to Dentate Nuclei (Cerebellum) In Situ Hybridization NHP 1 NHP 2 NHP 3 NHP 4 Human frataxin (hFXN) 32 *Data Data on represent file. Solid an Biosciences average. 2024. © 2025 Solid Biosciences
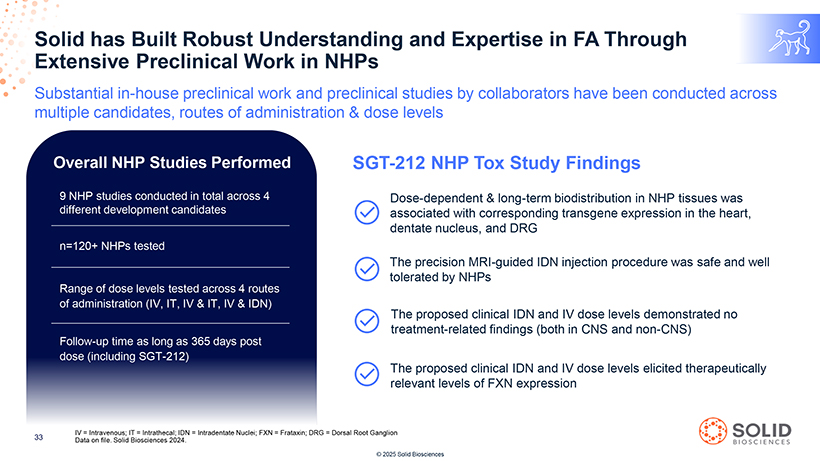
Solid Extensive has Built Preclinical Robust Work Understanding in NHPs and Expertise in FA Through [Graphic Appears Here] Substantial in-house preclinical work and preclinical studies by collaborators have been conducted across multiple candidates, routes of administration & dose levels Overall NHP Studies Performed SGT-212 NHP Tox Study Findings [Graphic Appears Here] [Graphic Appears Here] Dose-dependent & long-term biodistribution in NHP tissues was associated with corresponding transgene expression in the heart, dentate nucleus, and DRG The precision MRI-guided IDN injection procedure was safe and well tolerated by NHPs The proposed clinical IDN and IV dose levels demonstrated no treatment-related findings (both in CNS and non-CNS) The proposed clinical IDN and IV dose levels elicited therapeutically relevant levels of FXN expression Page 54 IV = Intravenous; IT = Intrathecal; IDN = Intradentate Nuclei; FXN = Frataxin; DRG = Dorsal Root Ganglion 33 Data on file. Solid Biosciences 2024. [Graphic Appears Here] © 2025 Solid Biosciences
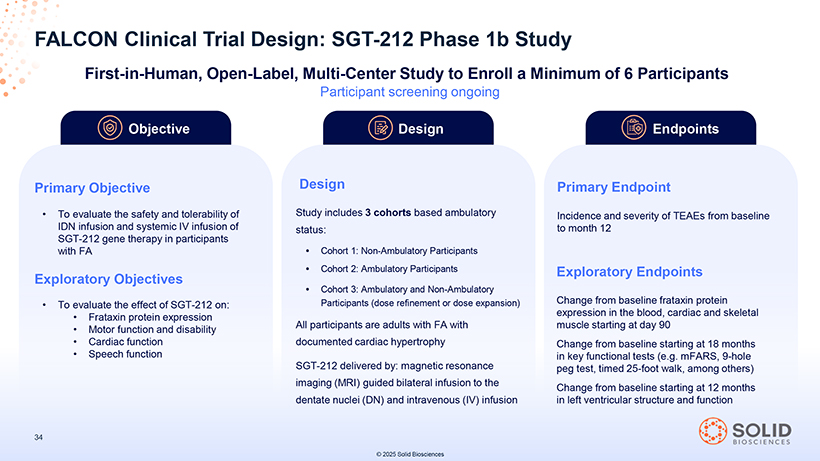
FALCON Clinical Trial Design: SGT-212 Phase 1b Study First-in-Human, Open-Label, Multi-Center Study to Enroll a Minimum of 6 Participants [Graphic Appears Here] To evaluate the safety and tolerability of IDN infusion and systemic IV infusion of SGT-212 gene therapy in participants with FA Exploratory Objectives To evaluate the effect of SGT-212 on: Frataxin protein expression Motor function and disability Cardiac function Speech function 87050-004-Part-5 34 Participant screening ongoing Design Design Study includes 3 cohorts based ambulatory status: Cohort 1: Non-Ambulatory Participants Cohort 2: Ambulatory Participants Cohort 3: Ambulatory and Non-Ambulatory Participants (dose refinement or dose expansion) All participants are adults with FA with documented cardiac hypertrophy SGT-212 delivered by: magnetic resonance imaging (MRI) guided bilateral infusion to the dentate nuclei (DN) and intravenous (IV) infusion 03Nov25 13:39 Page 55 © 2025 Solid Biosciences [Graphic Appears Here] Incidence and severity of TEAEs from baseline to month 12 Exploratory Endpoints Change from baseline frataxin protein expression in the blood, cardiac and skeletal muscle starting at day 90 Change from baseline starting at 18 months in key functional tests (e.g. mFARS, 9-hole peg test, timed 25-foot walk, among others) Change from baseline starting at 12 months in left ventricular structure and function

Cardiac Lead Program C a t e c h o l a m i n e r g i c P o l y m o r p h i c V e n t r i c u l a r T a c h y c a r d i a ( C P V T ) © 2025 Solid Biosciences
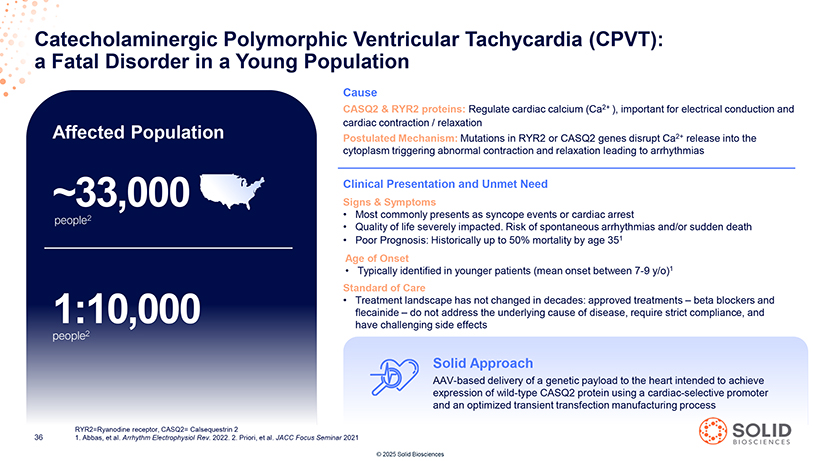
a Catecholaminergic Fatal Disorder in a Polymorphic Young Population Ventricular Tachycardia (CPVT): Cause CASQ2 & RYR2 proteins: Regulate cardiac calcium (Ca2+ ), important for electrical conduction and cardiac contraction / relaxation Affected Population Postulated Mechanism: Mutations in RYR2 or CASQ2 genes disrupt Ca2+ release into the cytoplasm triggering abnormal contraction and relaxation leading to arrhythmias Clinical Presentation and Unmet Need ~33,000 Signs & Symptoms • Most commonly presents as syncope events or cardiac arrest people2 • Quality of life severely impacted. Risk of spontaneous arrhythmias and/or sudden death • Poor Prognosis: Historically up to 50% mortality by age 351 Age of Onset • Typically identified in younger patients (mean onset between 7-9 y/o)1 Standard of Care • Treatment landscape has not changed in decades: approved treatments – beta blockers and 1:10,000 flecainide – do not address the underlying cause of disease, require strict compliance, and have challenging side effects people2 [Graphic Appears Here]
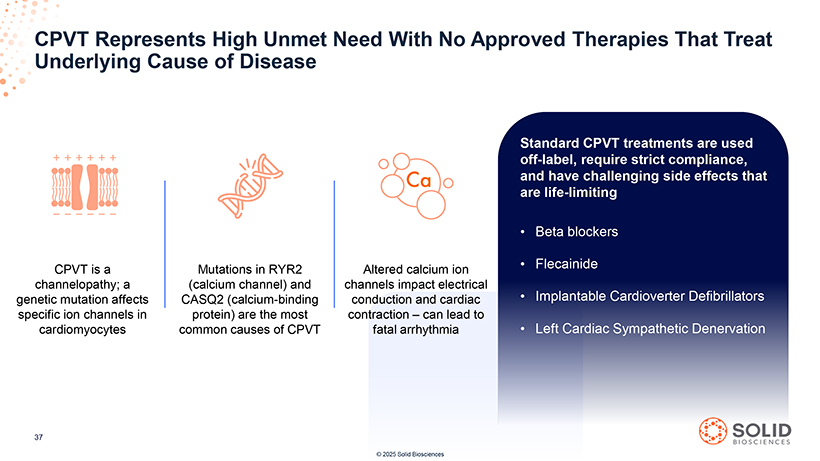
CPVT Underlying Represents Cause High of Disease Unmet Need With No Approved Therapies That Treat [Graphic Appears Here] [Graphic Appears Here] [Graphic Appears Here] [Graphic Appears Here] CPVT is a Mutations in RYR2 Altered calcium ion channelopathy; a (calcium channel) and channels impact electrical genetic mutation affects CASQ2 (calcium-binding conduction and cardiac specific ion channels in protein) are the most contraction – can lead to cardiomyocytes common causes of CPVT fatal arrhythmia 87050-004-Part-5 03Nov25 13:39 Page 58 Standard CPVT treatments are used off-label, require strict compliance, and have challenging side effects that are life-limiting Beta blockers Flecainide Implantable Cardioverter Defibrillators Left Cardiac Sympathetic Denervation 37 [Graphic Appears Here] © 2025 Solid Biosciences
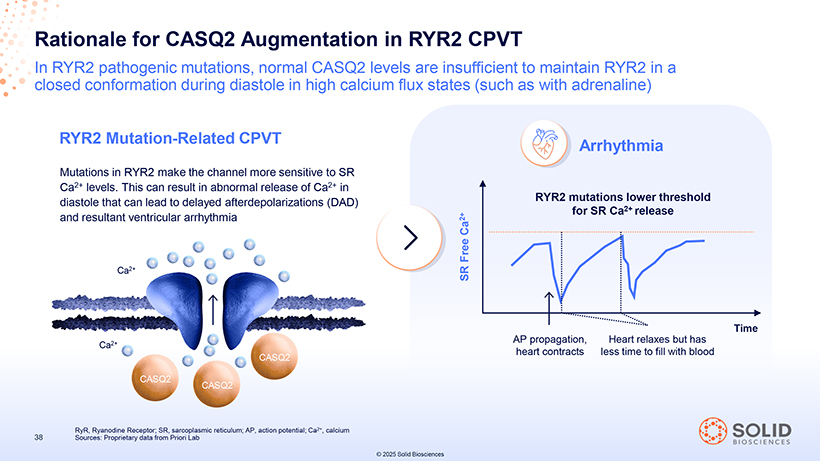
Rationale for CASQ2 Augmentation in RYR2 CPVT In RYR2 pathogenic mutations, normal CASQ2 levels are insufficient to maintain RYR2 in a closed conformation during diastole in high calcium flux states (such as with adrenaline) RYR2 Mutation-Related CPVT Arrhythmia Mutations in RYR2 make the channel more sensitive to SR Ca2+ levels. This can result in abnormal release of Ca2+ in diastole that can lead to delayed afterdepolarizations (DAD) RYR2 mutations lower threshold for SR Ca2+ release and resultant ventricular arrhythmia Ca 2+ Free Ca2+ SR AP propagation, Heart relaxes but has Time Ca2+ heart contracts less time to fill with blood CASQ2 CASQ2 CASQ2 RyR, Ryanodine Receptor; SR, sarcoplasmic reticulum; AP, action potential; Ca2+, calcium 38 Sources: Proprietary data from Priori Lab © 2025 Solid Biosciences
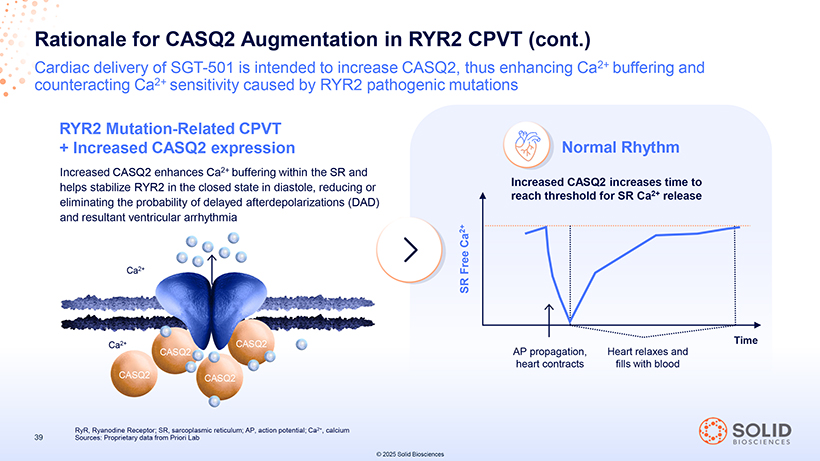
Rationale for CASQ2 Augmentation in RYR2 CPVT (cont.) Cardiac delivery of SGT-501 is intended to increase CASQ2, thus enhancing Ca2+ buffering and counteracting Ca2+ sensitivity caused by RYR2 pathogenic mutations RYR2 Mutation-Related CPVT + Increased CASQ2 expression Normal Rhythm Increased CASQ2 enhances Ca2+ buffering within the SR and helps stabilize RYR2 in the closed state in diastole, reducing or Increased CASQ2 increases time to reach threshold for SR Ca2+ release eliminating the probability of delayed afterdepolarizations (DAD) and resultant ventricular arrhythmia Ca 2+ Ca2+ Free SR Ca2+ CASQ2 AP propagation, Heart relaxes and Time CASQ2 heart contracts fills with blood CASQ2 CASQ2 RyR, Ryanodine Receptor; SR, sarcoplasmic reticulum; AP, action potential; Ca2+, calcium 39 Sources: Proprietary data from Priori Lab © 2025 Solid Biosciences
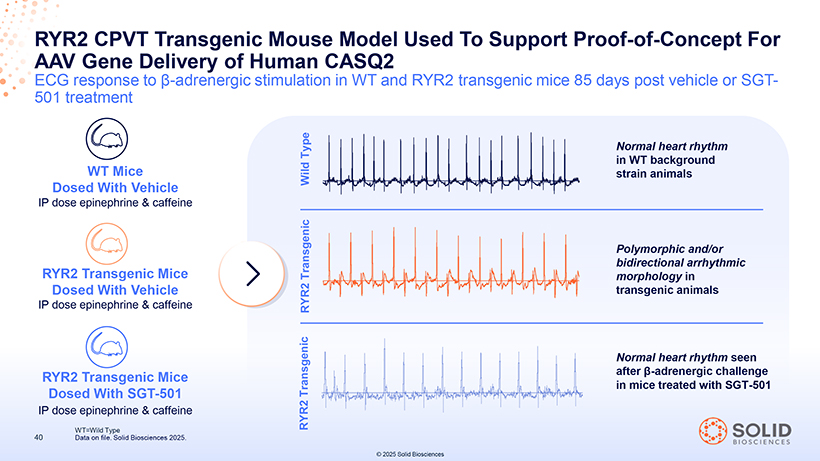
AAV RYR2 Gene CPVT Delivery Transgenic of Human Mouse CASQ2 Model Used To Support Proof-of-Concept For ECG response to â-adrenergic stimulation in WT and RYR2 transgenic mice 85 days post vehicle or SGT-501 treatment Type Normal heart rhythm WT Mice in WT background Dosed With Vehicle Wild strain animals IP dose epinephrine & caffeine Polymorphic and/or bidirectional arrhythmic RYR2 Transgenic Mice Transgenic morphology in Dosed With Vehicle transgenic animals IP dose epinephrine & caffeine RYR2 Normal heart rhythm seen RYR2 Transgenic Mice after â-adrenergic challenge Transgenic in mice treated with SGT-501 Dosed With SGT-501 IP dose epinephrine & caffeine RYR2 WT=Wild Type 40 Data on file. Solid Biosciences 2025. © 2025 Solid Biosciences
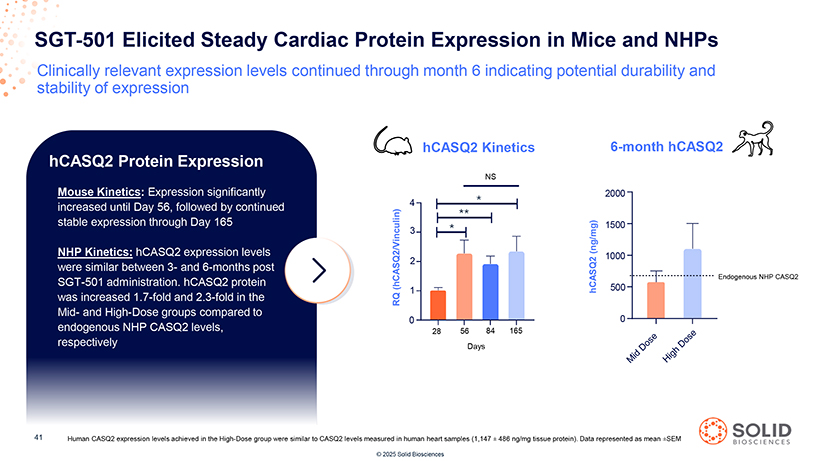
SGT-501 Elicited Steady Cardiac Protein Expression in Mice and NHPs Clinically relevant expression levels continued through month 6 indicating potential durability and stability of expression hCASQ2 Protein Expression Mouse Kinetics: Expression significantly increased until Day 56, followed by continued stable expression through Day 165 NHP Kinetics: hCASQ2 expression levels were similar between 3- and 6-months post SGT-501 administration. hCASQ2 protein was increased 1.7-fold and 2.3-fold in the Mid- and High-Dose groups compared to endogenous NHP CASQ2 levels, respectively [Graphic Appears Here] 87050-004-Part-5 03Nov25 13:39 [Graphic Appears Here] 41 Human CASQ2 expression levels achieved in the High-Dose group were similar to CASQ2 levels measured in human heart samples (1,147 ± 486 ng/mg tissue protein). Data represented as mean ±SEM [Graphic Appears Here] © 2025 Solid Biosciences
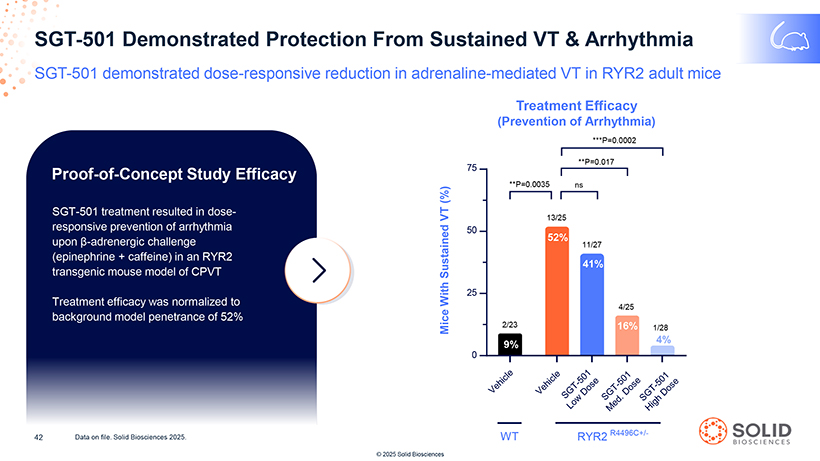
SGT-501 Demonstrated Protection From Sustained VT & Arrhythmia SGT-501 demonstrated dose-responsive reduction in adrenaline-mediated VT in RYR2 adult mice Treatment Efficacy (Prevention of Arrhythmia) ***P=0.0002 **P=0.017 Proof-of-Concept Study Efficacy 75 **P=0.0035 ns SGT-501 treatment resulted in dose- (%) VT 13/25 responsive prevention of arrhythmia 50 upon â-adrenergic challenge 52% 11/27 (epinephrine + caffeine) in an RYR2 41% transgenic mouse model of CPVT Sustained 25 Treatment efficacy was normalized to With 4/25 background model penetrance of 52% Mice 2/23 16% 1/28 9% 4% 0 R4496C+/- 42 Data on file. Solid Biosciences 2025. WT RYR2 © 2025 Solid Biosciences
SGT-501 was Well Tolerated in NHP GLP Toxicology Study FINDINGS • SGT-501 was well tolerated at each evaluated dose level: NHP GLP Tox Study no adverse effects were observed on hematology or serum clinical chemistry in NHPs after treatment. 3- and 6-month timepoints 6 treatment groups across 3 dose • SGT-501 IV administration of SGT-501 resulted in vector levels biodistribution in NHP cardiac tissue, providing confidence Evaluated single and triple in potential for increased cardiac human CASQ2 immunosuppression regimens expression in CPVT patients. N = 4/group • Human CASQ2 transgene protein expression was detected only in the heart. 43 Data on file. Solid Biosciences 2025. © 2025 Solid Biosciences
ARTEMIS Clinical Trial Design: SGT-501 Phase 1b Study First-in-Human, Open-Label, Multi-Center Study to Enroll a Minimum of 6 Participants Activation of first clinical trial site expected Q4 2025 Objective Design Endpoints Primary Objective Design Primary Endpoint • To evaluate the safety and tolerability of a Study includes up to 3 cohorts based on age Incidence of TEAEs through Day 360 single IV infusion of SGT-501 gene and on dose level therapy in participants with CPVT • Cohort 1: Participants ≥ 18, Dose Level 1 Secondary Endpoints • Cohort 21: Participants ≥ 18, Dose Level 22 Secondary Objectives Change from baseline of VAS on exercise • Cohort 3: Participants ≥ 7 to < 18 years of age, treadmill test at Day 180 • To evaluate the efficacy of SGT-501 by: dosed level at or below dose(s) assessed in • Assessing arrhythmia burden during adults2 Exploratory Endpoints exercise • Assessing arrhythmia burden over All participants must have a history of life- Change from baseline in the incidence of time threatening ventricular arrhythmic event with ventricular arrhythmia at Day 180 with ECG documented prior history of a VAS score of ≥ 2, patch and must be on a stable dose of background beta-blocker and/or flecainide TEAEs=treatment-emergent adverse events 44 1. Optional for dose exploration; 2. Based on DSMB recommendation

500 Rutherford Avenue, Third Floor, Charlestown, MA 02129 investors@solidbio.com www.solidbio.com Learn about ways to partner with us: www.solidbio.com/partners/ © 2025 Solid Biosciences