

NASDAQ: RFL October 2025 | Investor Presentation

Forward - Looking Statements Some of the information in this presentation relates to future events or future business and financial performance . Such statements constitute forward - looking information within the meaning of the Private Securities Litigation Act of 1995 . Such statements can be only predictions and the actual events or results may differ from those discussed due to, among other things, the risks described in the public filings and other publications of Rafael Holdings, Inc . Forward - looking statements are identified by words such as “anticipates”, “projects”, “expects”, “plans”, “intends”, “believes”, “estimates”, “target”, and other similar expressions that indicate trends and future events . The market data and certain other statistical information used throughout this presentation are based on independent industry publications, governmental publications, reports by market research firms or other independent sources . Some data are also based on the Company’s good faith estimates . In addition, this presentation includes summaries of scientific activities and outcomes that have been condensed to aid the reader in gaining general understanding . The information about Rafael Holdings, Inc . and its subsidiaries is solely for information purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy any security in any state . Factors that could cause the Company’s results to differ materially from those expressed in forward looking statements include, without limitation, the Company’s need for additional capital ; the Company’s reliance on its Trappsol ® Cyclo product, which may never receive regulatory approval ; the Company’s ability to commercialize any of its proposed drug products if it receives regulatory approval ; the outcome of the Company’s clinical trials, which may not support the Company’s product claims or may result in adverse side effects ; the cost and timing of the Company’s clinical trials ; the Company’s reliance on third parties to conduct clinical trials and to produce its products ; other risks associated with being a clinical stage biotechnology company ; a small number of the Company’s customers account for a substantial portion of our revenue, and the loss of any of these customers would materially decrease our revenues ; and the Company may be negatively affected by currency exchange rate fluctuations . This presentation is not to be copied, transmitted, displayed, distributed (for compensation or otherwise), or altered in any way without the prior written consent of Rafael Holdings, Inc . 2
Clinical Drug Development Trappsol ® Cyclo 3 Focused on Bringing Trappsol ® Cyclo to Market Real Estate Holding Medical Devices Rafael Holdings, Inc. NYSE: RFL / NYSE American: RFLW
Investment Highlights 4 Experienced team with strong track record of success Lead program Trappsol ® Cyclo in Phase 3 trial for the treatment of Niemann - Pick Disease Type C 1 High unmet medical need in NPC; global market expected to reach >$920m by 2031 Independent unblinded Data Monitoring Committee (DMC) performed Interim analysis in June 2025 and recommended continuation of the study through final analysis at 96 - weeks. Strong balance sheet with sufficient capital to fund through multiple potentially value creating milestones Late clinical - stage biotechnology company dedicated to developing life - changing medicines through science and innovation for patients and families living with challenging diseases

5 Leadership Team Howard S. Jonas Chairman of the Board Karen Mullen, FFPM Interim Chief Medical Officer David Polinsky Chief Financial Officer Joshua Fine Chief Operating Officer
6 Trappsol® Cyclo Is Being Evaluated in the Fully Enrolled TransportNPC Phase 3 Clinical Trial for Patients With Niemann - Pick Disease Type C1 48 - week interim analysis (IA) from Phase 3 Trial completed June 13 Data monitoring committee recommended continuing study to the 96 - week final analysis Final analysis targeted for 1H 2026, potential NDA submission to FDA and MAA to EMA by end of 2026 Potential to be a market leading treatment in patients newborn and above treating both systemic and neurological manifestations of NPC Orphan Drug Designation in US and EU, Fast Track in US, Rare Pediatric Disease Designated in US allowing for Priority Review Voucher eligibility

Niemann - Pick Disease Type C 1 Ongoing Pivotal Phase 3 Study 7
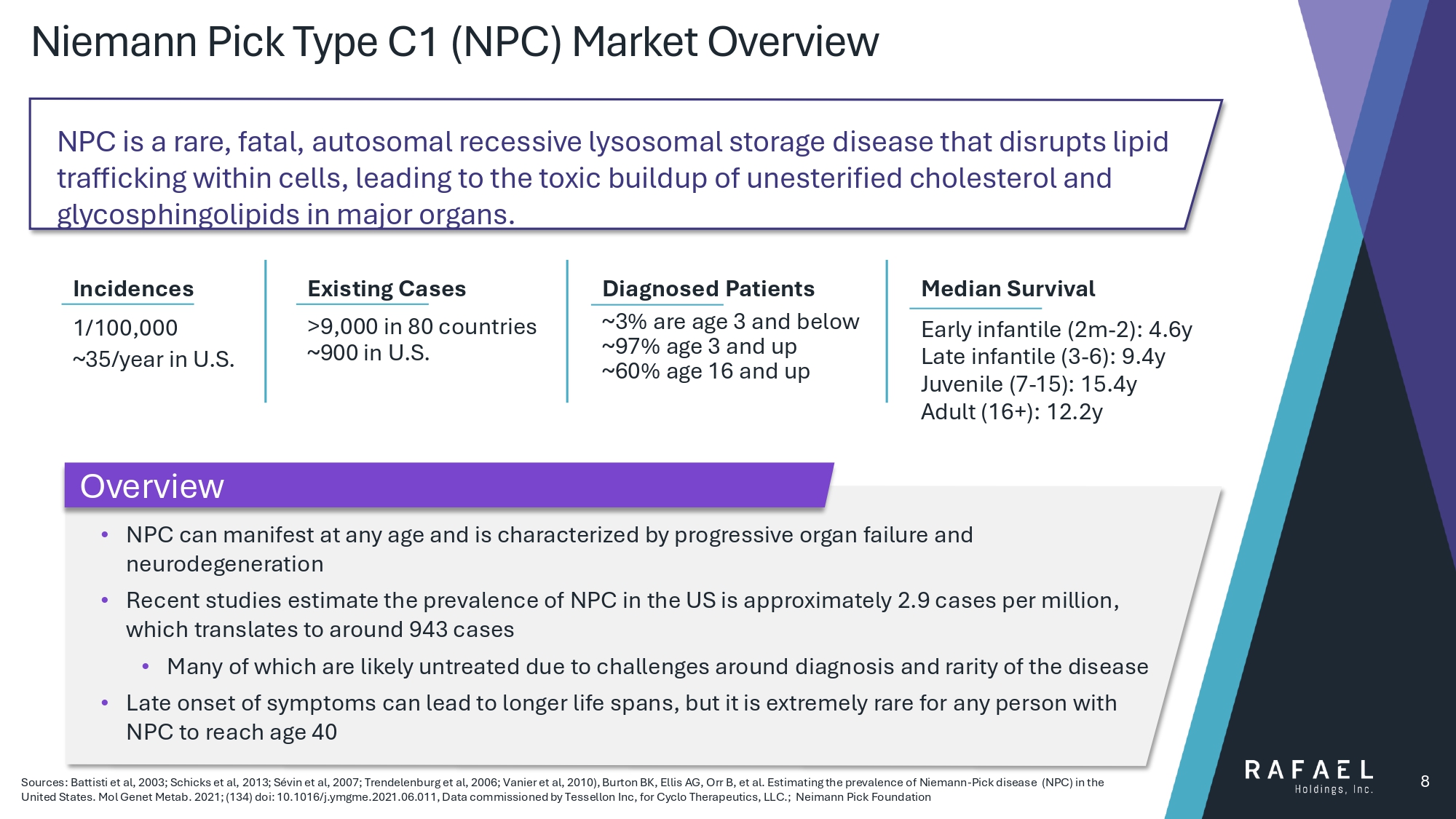
Niemann Pick Type C1 (NPC) Market Overview 8 Incidences 1/100,000 ~35/year in U.S. ~3% are age 3 and below Diagnosed Patients ~97% age 3 and up ~60% age 16 and up Median Survival Early infantile (2m - 2): 4.6y Late infantile (3 - 6): 9.4y Juvenile (7 - 15): 15.4y Adult (16+): 12.2y Existing Cases >9,000 in 80 countries ~900 in U.S. • NPC can manifest at any age and is characterized by progressive organ failure and neurodegeneration • Recent studies estimate the prevalence of NPC in the US is approximately 2.9 cases per million, which translates to around 943 cases • Many of which are likely untreated due to challenges around diagnosis and rarity of the disease • Late onset of symptoms can lead to longer life spans, but it is extremely rare for any person with NPC to reach age 40 Sources: Battisti et al, 2003; Schicks et al, 2013; Sévin et al, 2007; Trendelenburg et al, 2006; Vanier et al, 2010), Burton BK, Ellis AG, Orr B, et al. Estimating the prevalence of Ni emann - Pick disease (NPC) in the United States. Mol Genet Metab . 2021; (134) doi : 10.1016/j.ymgme.2021.06.011, Data commissioned by Tessellon Inc, for Cyclo Therapeutics, LLC.; Neimann Pick Foundation NPC is a rare, fatal, autosomal recessive lysosomal storage disease that disrupts lipid trafficking within cells, leading to the toxic buildup of unesterified cholesterol and glycosphingolipids in major organs. Overview
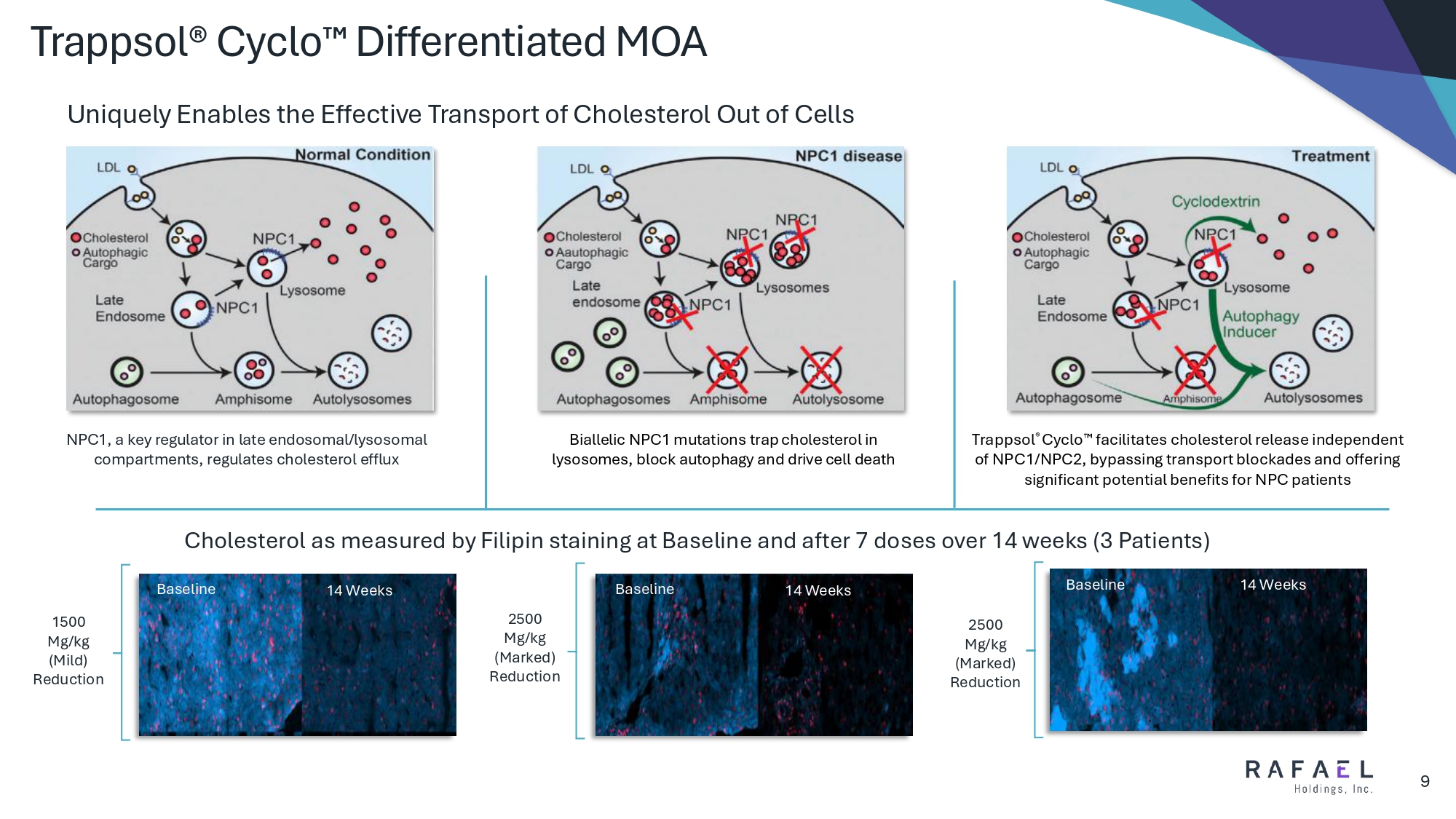
Trappsol® Cyclo Differentiated MOA 9 NPC1, a key regulator in late endosomal/lysosomal compartments, regulates cholesterol efflux Biallelic NPC1 mutations trap cholesterol in lysosomes, block autophagy and drive cell death Trappsol ® Cyclo facilitates cholesterol release independent of NPC1/NPC2, bypassing transport blockades and offering significant potential benefits for NPC patients Uniquely Enables the Effective Transport of Cholesterol Out of Cells Baseline 14 Weeks Baseline 14 Weeks Baseline 14 Weeks Cholesterol as measured by Filipin staining at Baseline and after 7 doses over 14 weeks (3 Patients) 1500 Mg/kg (Mild) Reduction 2500 Mg/kg (Marked) Reduction 2500 Mg/kg (Marked) Reduction

10 Trappsol ® Cyclo Summary of Completed Studies and Open - Label Extension Trappsol ® Cyclo reaches the cerebrospinal fluid within hours of IV infusion, modulating cholesterol metabolism and clearing cellular cholesterol, mirroring preclinical NPC model effects Study 101 1 Study 102 Demonstrated Trappsol ® Cyclo was well - tolerated and may slow disease progression, with potential long - term stabilization compared to the natural course of NPC Study 201 2 Phase 1 study in NPC patients ages 18 years and older showed Trappsol ® Cyclo was well - tolerated with an acceptable safety and tolerability profile Consistent pharmacodynamic effects and safety profile observed in a 48 - week Phase 1/2 study in NPC patients aged 2 years and older Potential to treat both the systemic and neurological manifestations of NPC and is well - tolerated with acceptable safety and tolerability profile 1. Molecular Genetics and Metabolism Report: Intravenous 2 - hydroxypropyl - β - cyclodextrin (Trappsol ® Cyclo ) demonstrates biological activity and impacts cholesterol metabolism in the central nervous system and peripheral tissues in adult subjects with Niemann - Pick Disease Type C1: Results of a phase 1 trial (2 022) 2. Molecular Genetics and Metabolism Report: The Long - term administration of intravenous Trappsol ® Cyclo (HP β CD) results in clinical benefits and stabilization or slowing of disease progression in patients with Niemann - Pick disease Type C1: Results of an international 48 - week Phase I/II trial (2023) 9 of 9 (100%) completer patients exhibited stabilized or improved in CGI - I ratings, a clear indicator of efficacy in NPC - 1 patients. 8 of 9 (89%) completer patients showed Improvements over disease progression (2.15 points per annum) in the 5D - NPC - SS.
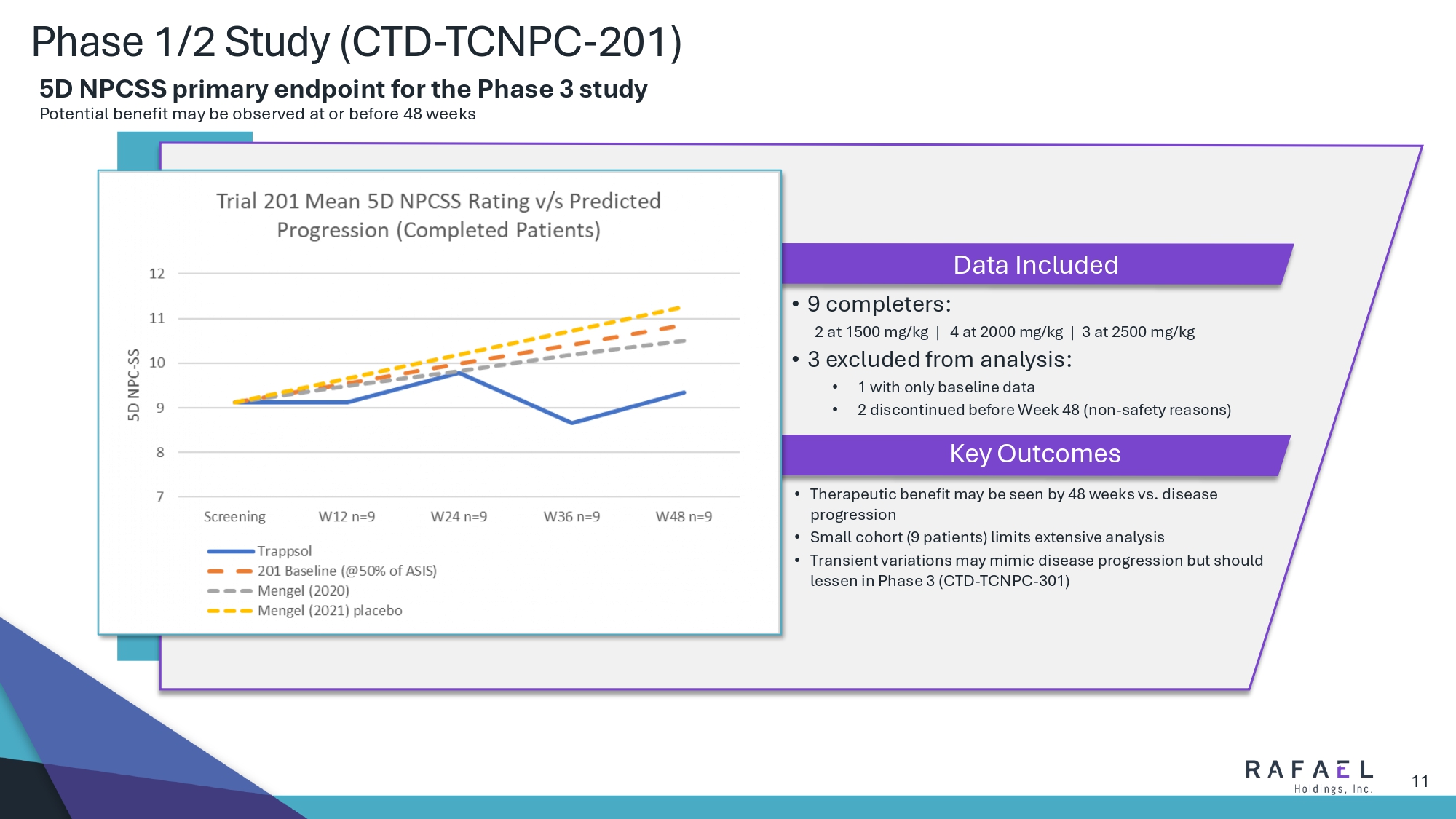
11 Phase 1/2 Study (CTD - TCNPC - 201) • Therapeutic benefit may be seen by 48 weeks vs. disease progression • Small cohort (9 patients) limits extensive analysis • Transient variations may mimic disease progression but should lessen in Phase 3 (CTD - TCNPC - 301) • 9 completers : 2 at 1500 mg/kg | 4 at 2000 mg/kg | 3 at 2500 mg/kg • 3 excluded from analysis : • 1 with only baseline data • 2 discontinued before Week 48 (non - safety reasons) Data Included Key Outcomes 5D NPCSS primary endpoint for the Phase 3 study Potential benefit may be observed at or before 48 weeks
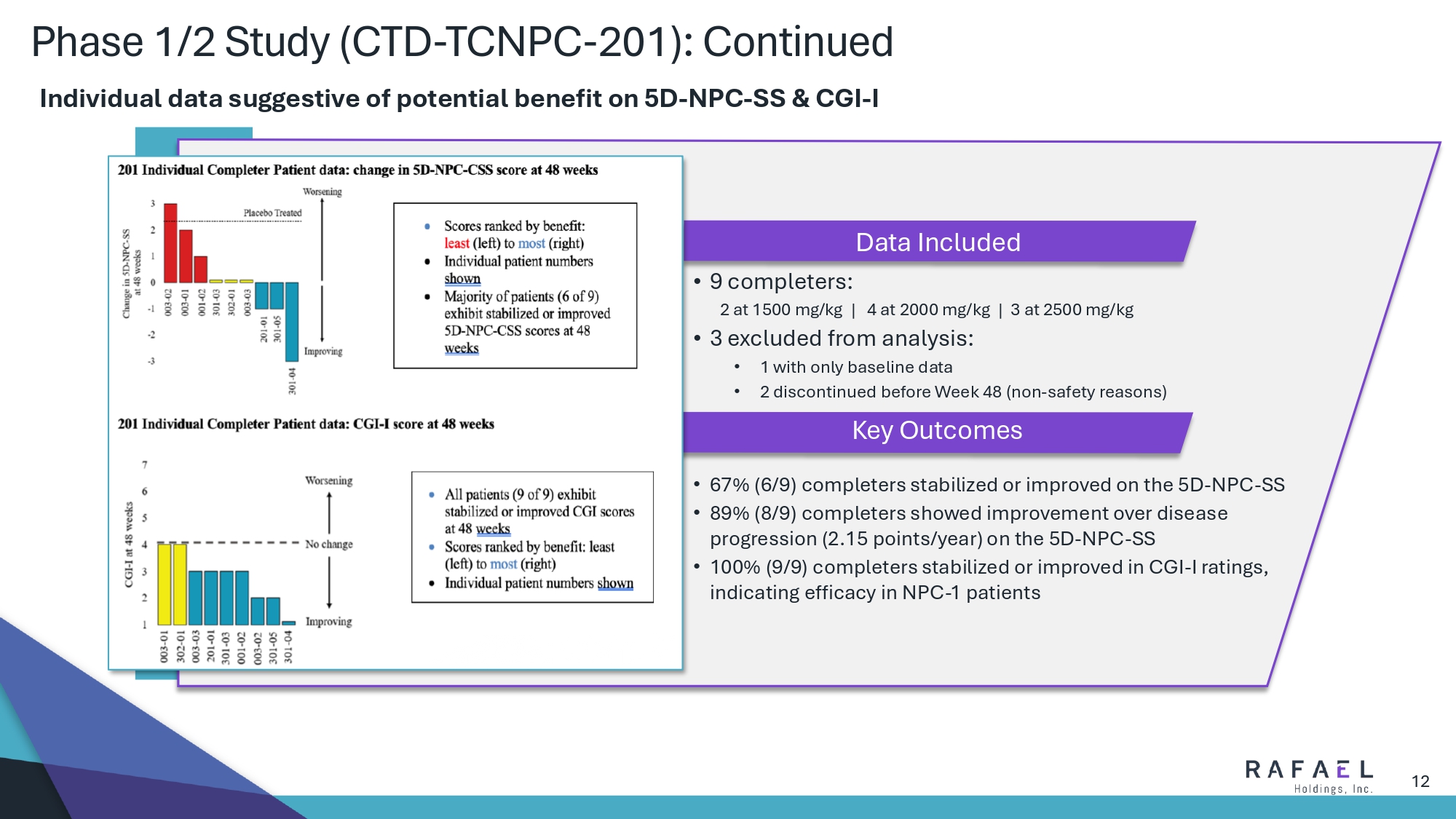
12 Phase 1/2 Study (CTD - TCNPC - 201): Continued • 9 completers : 2 at 1500 mg/kg | 4 at 2000 mg/kg | 3 at 2500 mg/kg • 3 excluded from analysis : • 1 with only baseline data • 2 discontinued before Week 48 (non - safety reasons) Data Included Key Outcomes Individual data suggestive of potential benefit on 5D - NPC - SS & CGI - I • 67% (6/9) completers stabilized or improved on the 5D - NPC - SS • 89% (8/9) completers showed improvement over disease progression (2.15 points/year) on the 5D - NPC - SS • 100% (9/9) completers stabilized or improved in CGI - I ratings, indicating efficacy in NPC - 1 patients
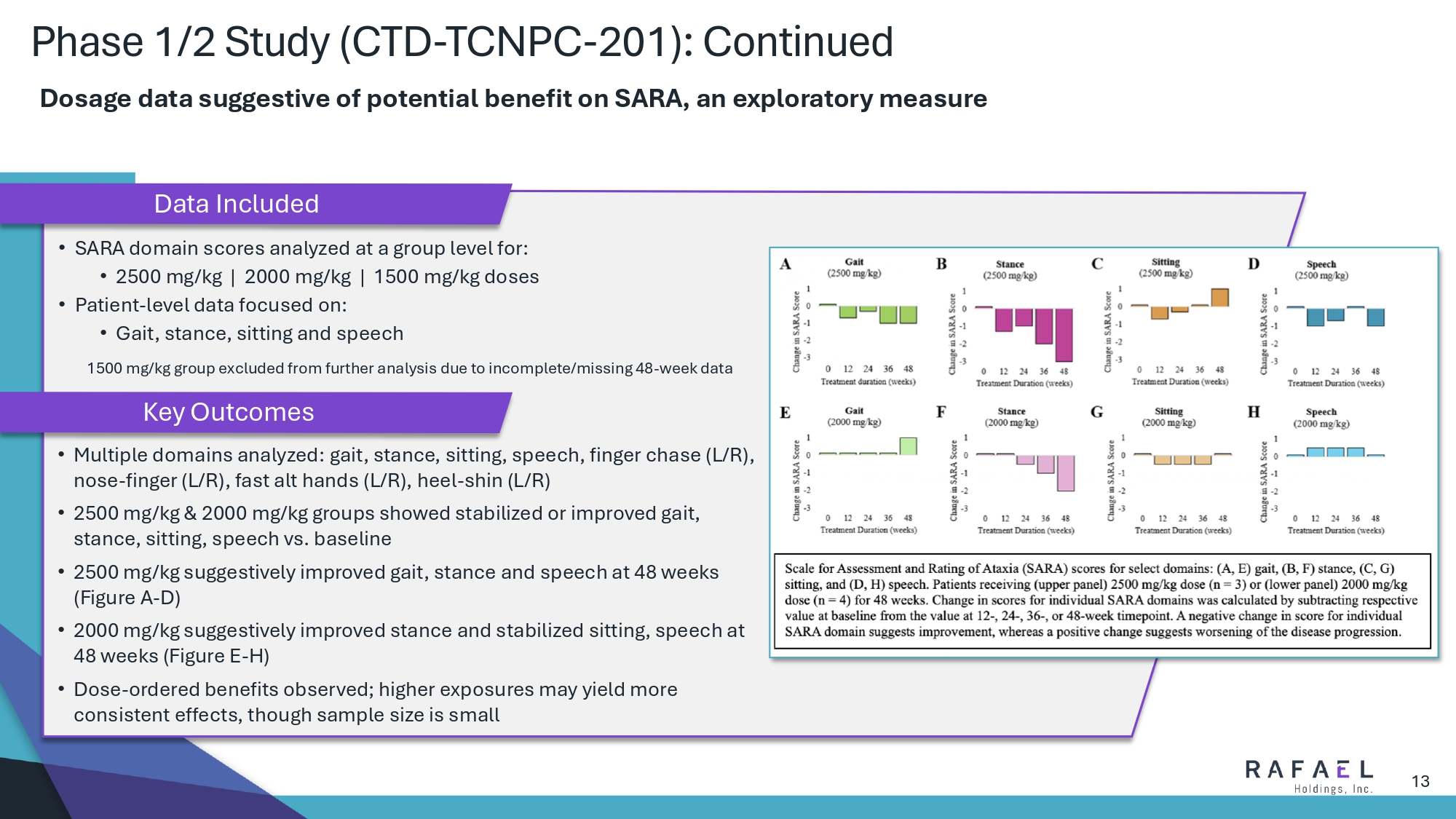
13 Phase 1/2 Study (CTD - TCNPC - 201): Continued Dosage data suggestive of potential benefit on SARA, an exploratory measure • Multiple domains analyzed: gait, stance, sitting, speech, finger chase (L/R), nose - finger (L/R), fast alt hands (L/R), heel - shin (L/R) • 2500 mg/kg & 2000 mg/kg groups showed stabilized or improved gait, stance, sitting, speech vs. baseline • 2500 mg/kg suggestively improved gait, stance and speech at 48 weeks (Figure A - D) • 2000 mg/kg suggestively improved stance and stabilized sitting, speech at 48 weeks (Figure E - H) • Dose - ordered benefits observed; higher exposures may yield more consistent effects, though sample size is small • SARA domain scores analyzed at a group level for: • 2500 mg/kg | 2000 mg/kg | 1500 mg/kg doses • Patient - level data focused on: • Gait, stance, sitting and speech 1500 mg/kg group excluded from further analysis due to incomplete/missing 48 - week data Data Included Key Outcomes
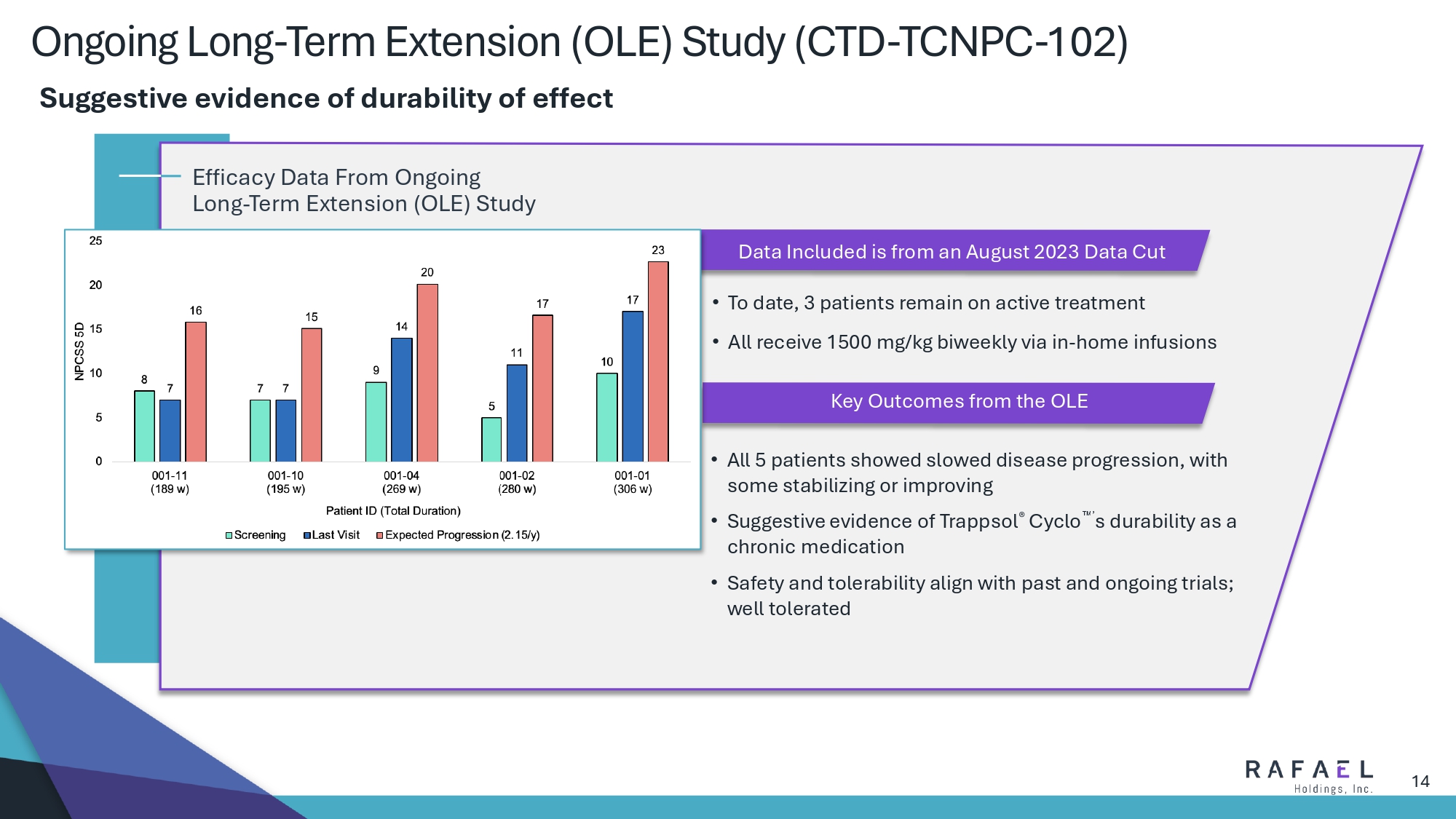
14 Ongoing Long - Term Extension (OLE) Study (CTD - TCNPC - 102) Data Included is from an August 2023 Data Cut Key Outcomes from the OLE • To date, 3 patients remain on active treatment • All receive 1500 mg/kg biweekly via in - home infusions • All 5 patients showed slowed disease progression, with some stabilizing or improving • Suggestive evidence of Trappsol ® Cyclo ’ s durability as a chronic medication • Safety and tolerability align with past and ongoing trials; well tolerated Efficacy Data From Ongoing Long - Term Extension (OLE) Study Suggestive evidence of durability of effect
15 Safety From Completed and Ongoing Long - Term Treatment Treatment - Emergent Adverse Events majority mild to moderate in severity, manageable and monitorable and most considered unrelated to Trappsol ® Cyclo No evidence of any untoward effects of Trappsol ® Cyclo on core organ systems (cardiovascular, respiratory, renal, hepatic, gastrointestinal systems or CNS) Safety and tolerability profile consistent across studies and treatment duration, irrespective of age spectrum and disease severity Hearing loss and infusion reactions (most localized) are adverse events of interest Events of hearing loss resolved in most patients, with hearing returning to baseline levels or improved and stabilized while patients continued on study drug A degree of hearing impairment remained at the last available auditory assessment in a limited number of patients The effect on hearing will continue to be monitored closely in the ongoing studies
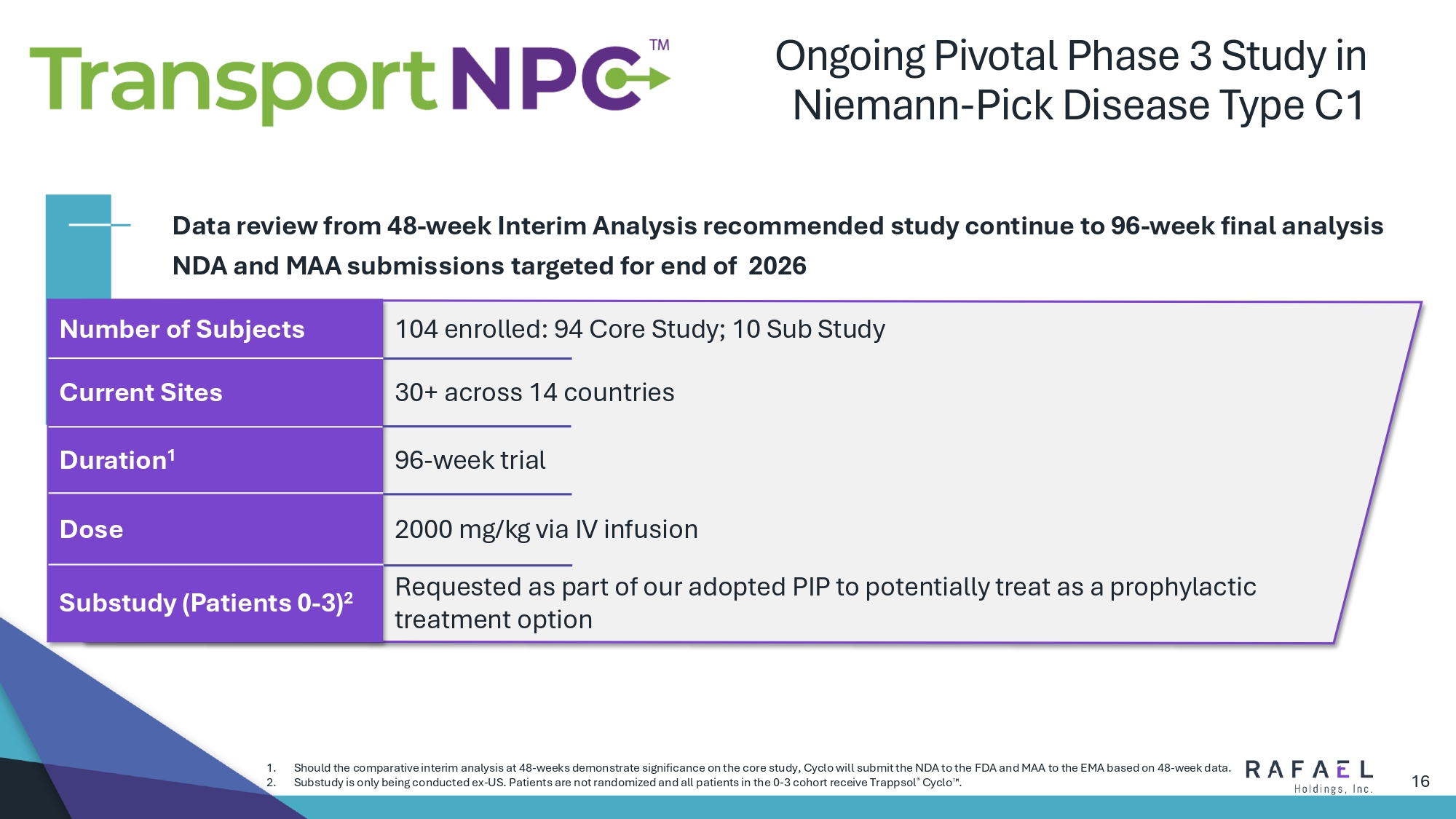
16 Ongoing Pivotal Phase 3 Study in Niemann - Pick Disease Type C1 104 enrolled: 94 Core Study; 10 Sub Study Number of Subjects 30+ across 14 countries Current Sites 96 - week trial Duration 1 2000 mg/kg via IV infusion Dose Requested as part of our adopted PIP to potentially treat as a prophylactic treatment option Substudy (Patients 0 - 3) 2 Data review from 48 - week Interim Analysis recommended study continue to 96 - week final analysis NDA and MAA submissions targeted for end of 2026 1. Should the comparative interim analysis at 48 - weeks demonstrate significance on the core study, Cyclo will submit the NDA to the FDA and MAA to the EMA based on 48 - week data. 2. Substudy is only being conducted ex - US. Patients are not randomized and all patients in the 0 - 3 cohort receive Trappsol ® Cyclo .
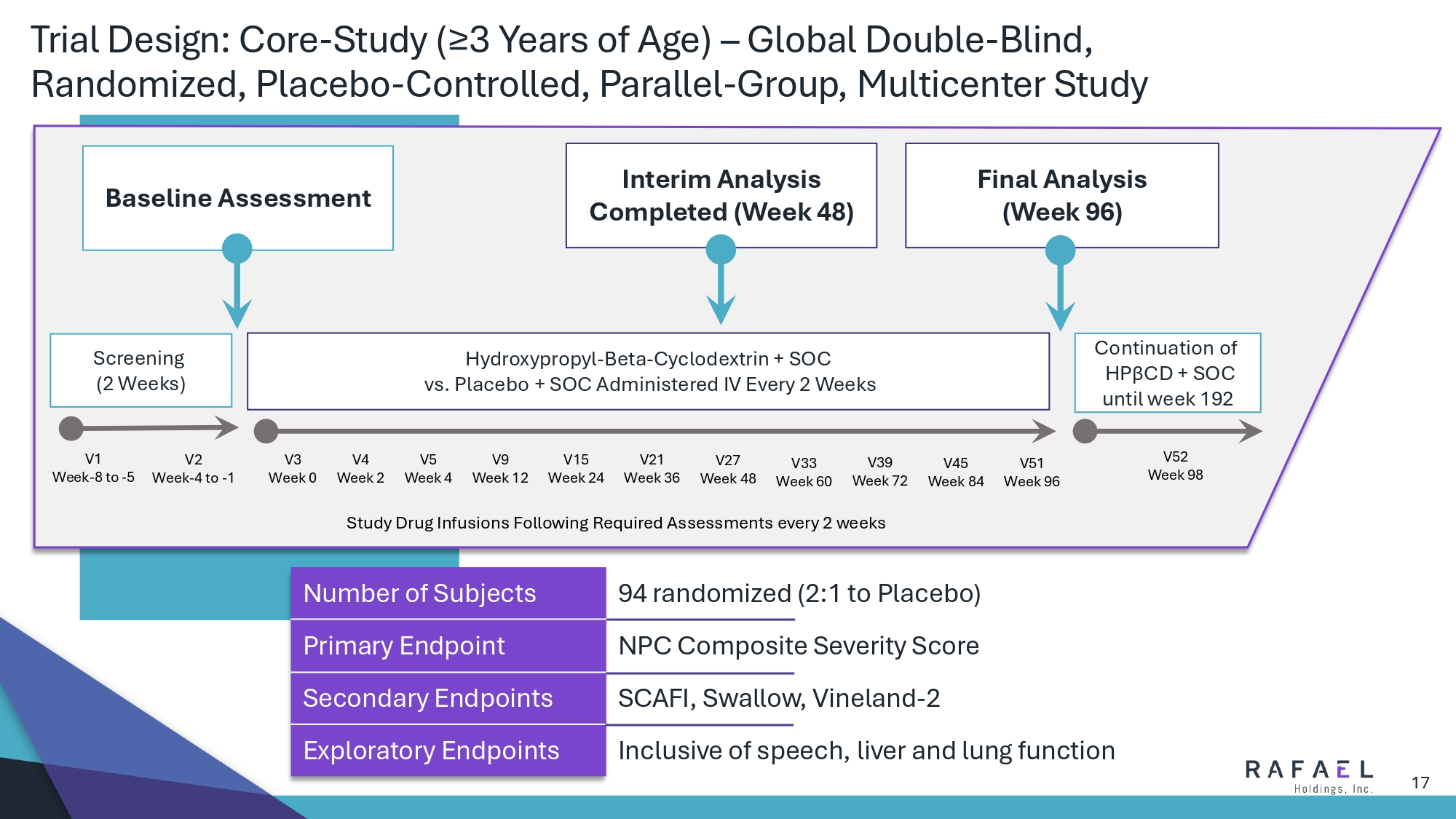
17 Trial Design: Core - Study (≥3 Years of Age) – Global Double - Blind, Randomized, Placebo - Controlled, Parallel - Group, Multicenter Study 94 randomized (2:1 to Placebo) Number of Subjects NPC Composite Severity Score Primary Endpoint SCAFI, Swallow, Vineland - 2 Secondary Endpoints Inclusive of speech, liver and lung function Exploratory Endpoints Baseline Assessment Final Analysis (Week 96) Interim Analysis Completed (Week 48) Screening (2 Weeks) Hydroxypropyl - Beta - Cyclodextrin + SOC vs. Placebo + SOC Administered IV Every 2 Weeks Continuation of HP β CD + SOC until week 192 V1 Week - 8 to - 5 V2 Week - 4 to - 1 V3 Week 0 V4 Week 2 V5 Week 4 V9 Week 12 V15 Week 24 V21 Week 36 V27 Week 48 V33 Week 60 V39 Week 72 V45 Week 84 V51 Week 96 V52 Week 98 Study Drug Infusions Following Required Assessments every 2 weeks
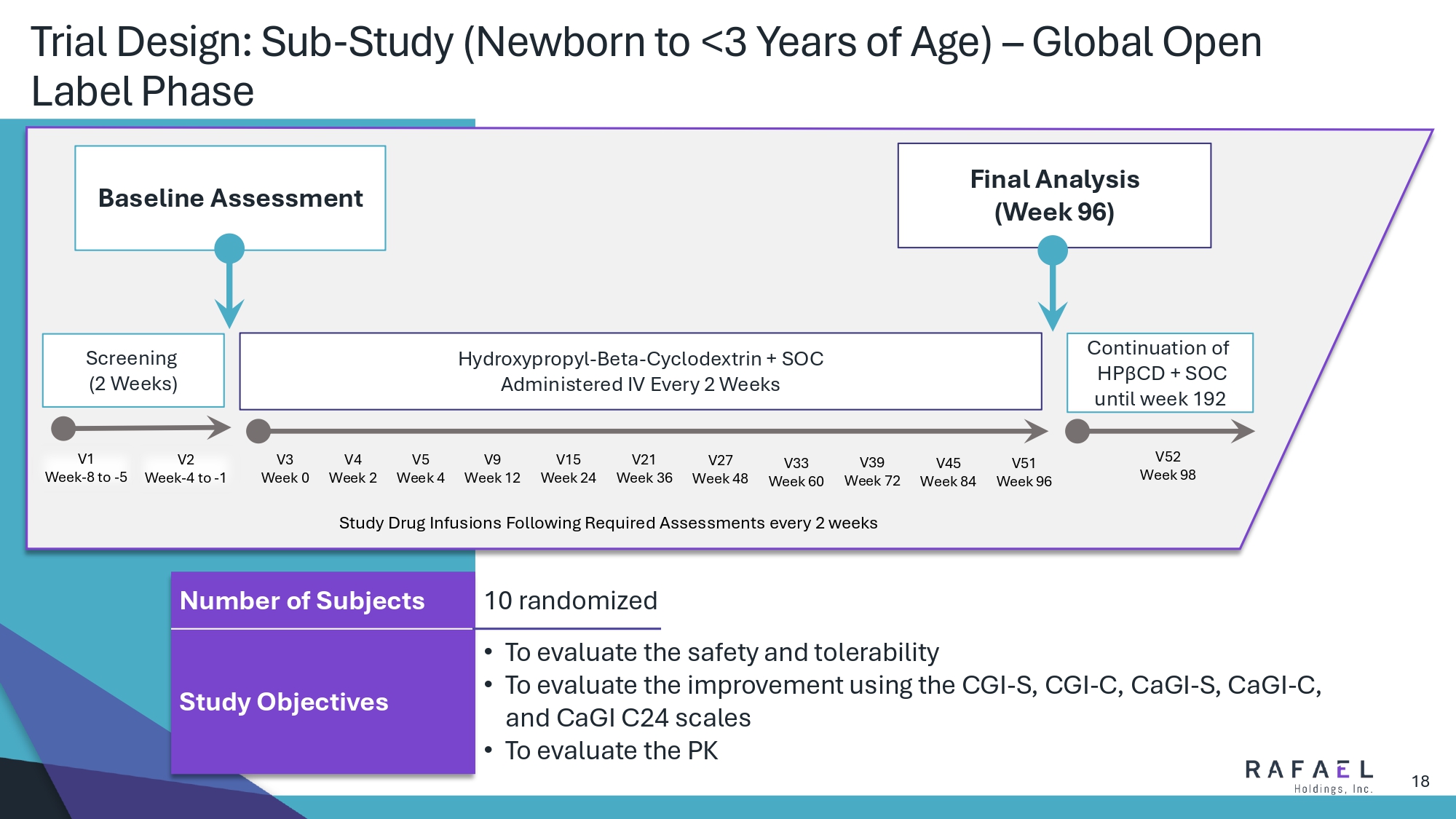
18 Trial Design: Sub - Study (Newborn to <3 Years of Age) – Global Open Label Phase Baseline Assessment Final Analysis (Week 96) Screening (2 Weeks) Hydroxypropyl - Beta - Cyclodextrin + SOC Administered IV Every 2 Weeks Continuation of HP β CD + SOC until week 192 V1 Week - 8 to - 5 V2 Week - 4 to - 1 V3 Week 0 V4 Week 2 V5 Week 4 V9 Week 12 V15 Week 24 V21 Week 36 V27 Week 48 V33 Week 60 V39 Week 72 V45 Week 84 V51 Week 96 V52 Week 98 Study Drug Infusions Following Required Assessments every 2 weeks 10 randomized Number of Subjects • To evaluate the safety and tolerability • To evaluate the improvement using the CGI - S, CGI - C, CaGI - S, CaGI - C, and CaGI C24 scales • To evaluate the PK Study Objectives
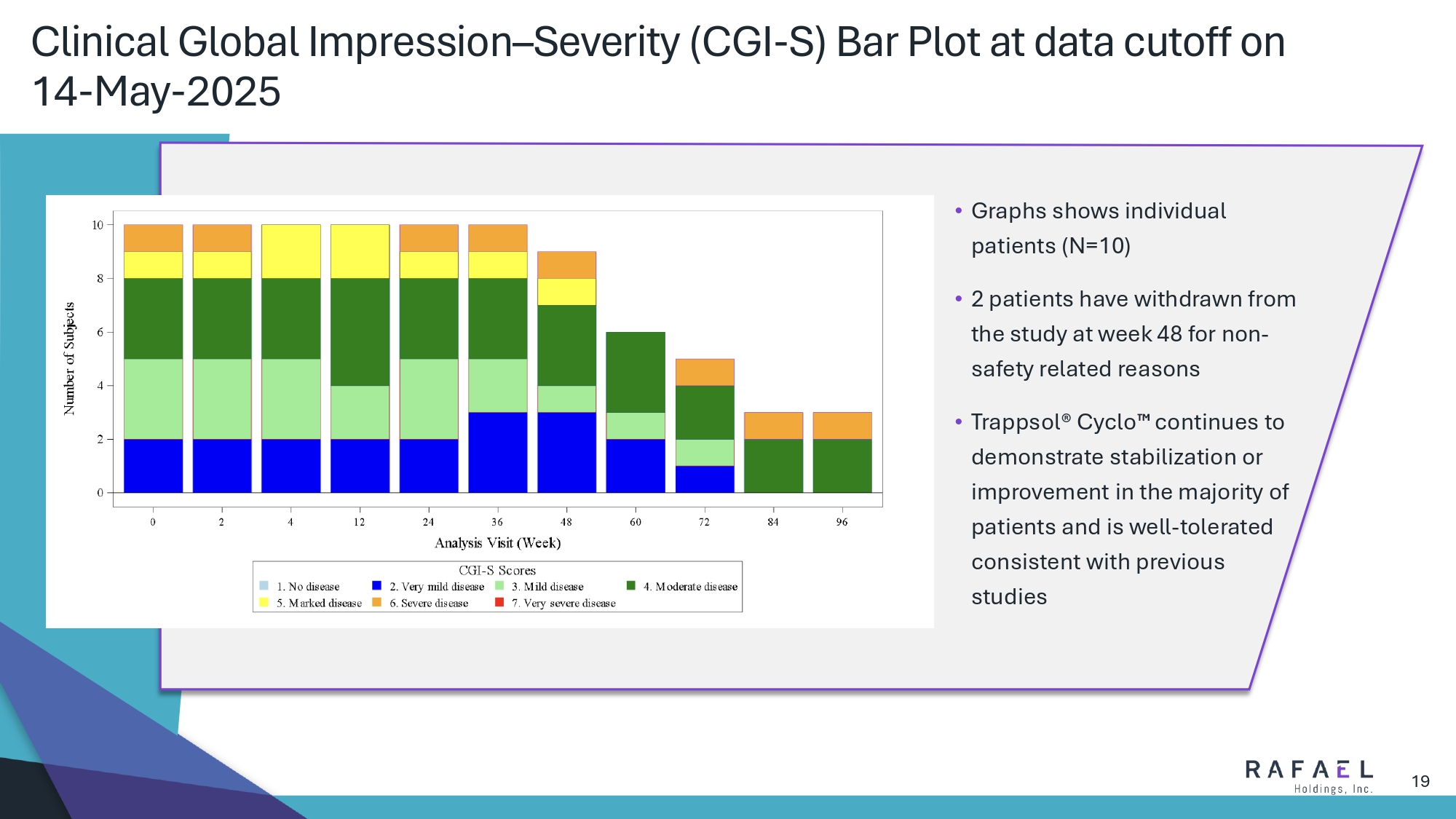
19 Clinical Global Impression – Severity (CGI - S) Bar Plot at data cutoff on 14 - May - 2025 • Graphs shows individual patients (N=10) • 2 patients have withdrawn from the study at week 48 for non - safety related reasons • Trappsol ® Cyclo continues to demonstrate stabilization or improvement in the majority of patients and is well - tolerated consistent with previous studies
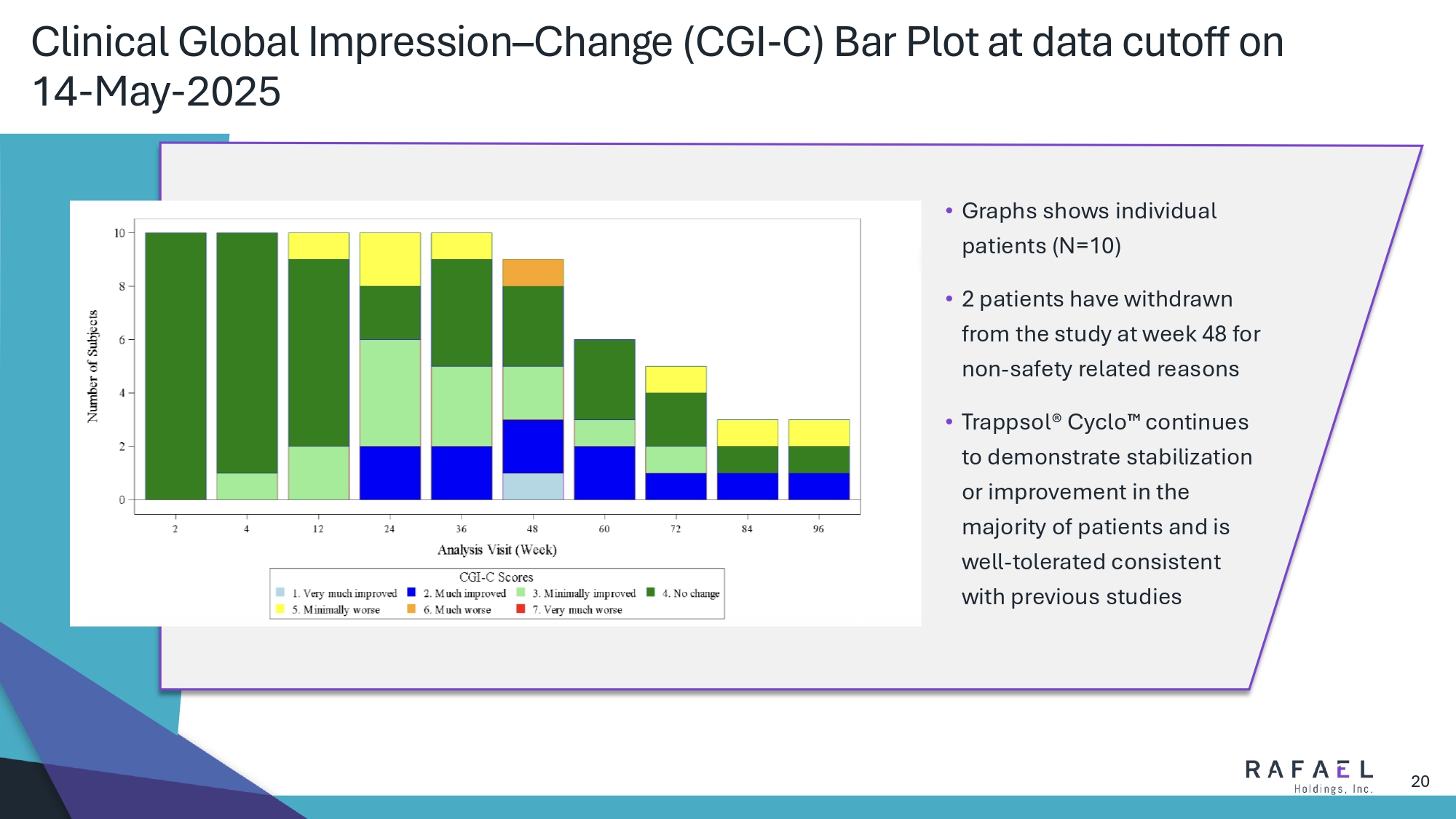
20 Clinical Global Impression – Change (CGI - C) Bar Plot at data cutoff on 14 - May - 2025 • Graphs shows individual patients (N=10) • 2 patients have withdrawn from the study at week 48 for non - safety related reasons • Trappsol ® Cyclo continues to demonstrate stabilization or improvement in the majority of patients and is well - tolerated consistent with previous studies
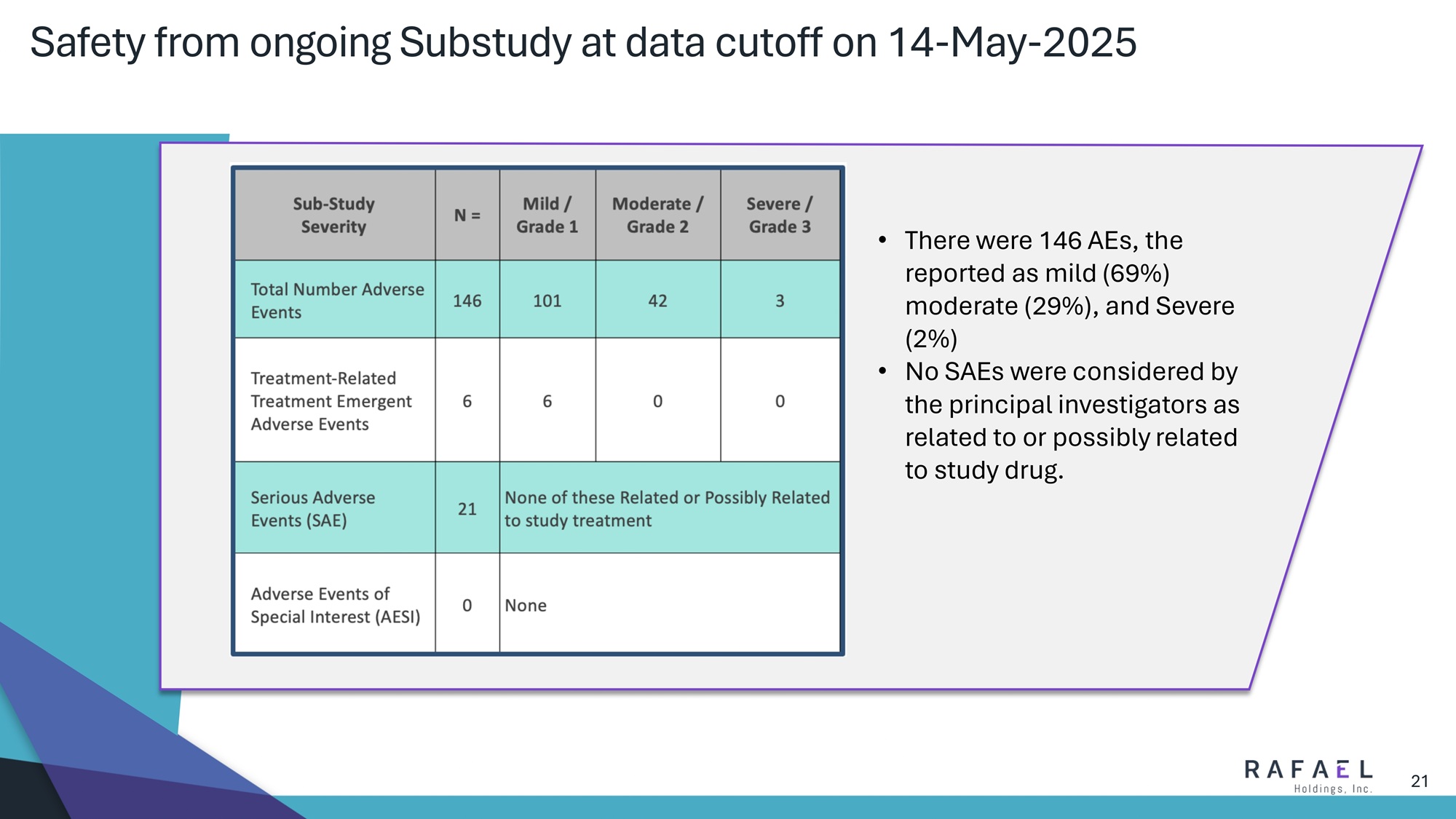
21 Safety from ongoing Substudy at data cutoff on 14 - May - 2025 • T here were 146 AEs, with the majority reported as mild (69%) or moderate (29%), 3 reported as severe and the remainder reported as Serious Adverse Events(SAEs) (14%); and • No SAEs were considered by the principal investigators as related to or possibly related to study drug.
22 Investment Summary Phase 3 Clinical trial to be completed 1H 2026. Potential NDA and MAA submissions to FDA and EMA by end of 2026 1. Intelligence, D. (n.d.). Niemann Pick Disease market size, sustainable insights and growth report 2025 - 2033 . DataMIntelligence 2. As of April 30, 2025; includes net $24.9 million proceeds rights offering Ability to Realize Potential Value Creation Significant Commercial Opportunity ~ $62.8M Cash Balance 2 x Global NPC market expected to reach >$920m by 2033 1 x Recent US approved NCP product pricing is consistent with pricing analogs ranging from $400K to $1.3M x Streamlined company focused on bringing Trappsol ® Cyclo to market x all - in - one Endoscopic Carpal Tunnel Release system, approved by FDA December 2024 x Sufficient capital to fund the company through multiple potentially value creating milestones

NASDAQ: RFL Thank You! 23