

Mereo BioPharma J.P. Morgan Conference .2

Disclaimer This presentation has been prepared by Mereo BioPharma Group plc (the “Company”) solely for your information and for the purpose of providing background information on the Company, its business and the industry in which it operates or any particular aspect thereof. For the purposes of this notice, “presentation” means this document, any oral presentation, any question and answer session and any written or oral material discussed or distributed during any related presentation meeting. This presentation has not been independently verified and no representation or warranty, express or implied, is made or given by or on behalf of the Company or any of its subsidiaries, or any of any such person’s directors, officers, employees, agents, affiliates or advisers, as to, and no reliance should be placed on, the accuracy, completeness or fairness of the information or opinions contained in this presentation and no responsibility or liability is assumed by any such persons for any such information or opinions or for any errors or omissions. All information presented or contained in this presentation is subject to verification, correction, completion and change without notice. In giving this presentation, none of the Company or any of its subsidiaries, or any of any such person’s directors, officers, employees, agents, affiliates or advisers, undertakes any obligation to amend, correct or update this presentation or to provide the recipient with access to any additional information that may arise in connection with it. To the extent available, the data contained in this presentation has come from official or third-party sources. Third party industry publications, studies and surveys generally state that the data contained therein have been obtained from sources believed to be reliable, but that there is no guarantee of the accuracy or completeness of such data. While the Company believes that each of these publications, studies and surveys has been prepared by a reputable source, the Company has not independently verified the data contained therein. In addition, certain of the data contained in this presentation come from the Company’s own internal research and estimates based on the knowledge and experience of the Company’s management in the market in which the Company operates. Further, certain of the data has been provided to the Company by contract research organizations that the Company retains to conduct clinical trials, or by other third parties contracted by the Company. While the Company believes that such internal research and estimates and such other data are reasonable and reliable, they, and, where applicable, their underlying methodology and assumptions, have not been verified by any independent source for accuracy or completeness and are subject to change without notice. Accordingly, undue reliance should not be placed on any of the data contained in this presentation. Forward-Looking Statements This presentation contains “forward-looking” statements that involve substantial risks and uncertainties. All statements other than statements of historical fact contained in this presentation are forward-looking statements within the meaning of Section 27A of the United States Securities Act of 1933, as amended, and Section 21E of the United States Securities Exchange Act of 1934, as amended. Forward-looking statements relate to future events, including, but not limited to, statements regarding future clinical development, efficacy, safety and therapeutic potential of clinical product candidates, including expectations as to reporting of data, conduct and timing and potential future clinical activity and milestones and expectations regarding the initiation, design and reporting of data from clinical trials. Forward-looking statements are often identified by the words “believe,” “expect,” “anticipate,” “plan,” “intend,” “foresee,” “should,” “would,” “could,” “may,” “estimate,” “outlook” and similar expressions, including the negative thereof. The absence of these words, however, does not mean that the statements are not forward-looking. These forward-looking statements are based on the Company’s current expectations, beliefs and assumptions concerning future developments and involve risks and uncertainties that could cause actual results, performance, or events to differ materially from those expressed or implied in such statements. Such risks and uncertainties include, among others, the uncertainties inherent in the clinical development process; the Company’s reliance on third parties to conduct its clinical trials and provide funding for its clinical trials; the Company’s dependence on enrollment of patients in its clinical trials; and the Company’s dependence on its key executives. You should carefully consider the foregoing factors and the other risks and uncertainties that affect the Company’s business, including those described in the “Risk Factors” section of its Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission (“SEC”) on March 26, 2025, as well as discussions of potential risks, uncertainties, and other important factors in the Company’s subsequent filings with the SEC. You should not place undue reliance on any forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to publicly update or revise any forward-looking statements after the date they are made, whether as a result of new information, future events or otherwise, except to the extent required by law. This presentation also contains estimates, projections and other information concerning the Company’s business and the markets for the Company’s product candidates, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties and actual events, or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, the Company obtained this industry, business, market and other data from reports, research surveys, clinical trials studies and similar data prepared by market research firms and other third parties, from industry, medical and general publications, and from government data and similar sources.

Our vision We are working toward a future where people and families living with rare diseases, especially those with few or no treatment options, have access to therapies that can transform their lives.

Two pivotal rare disease programs and a capital efficient model Achievements and fundamentals Two rare disease programs in-licensed and progressed to pivotal stage: Setrusumab for Osteogenesis Imperfecta (OI) Phase 3 results reported around the end of 2025, partnered with rare disease leader, Ultragenyx - determining path forward Alvelestat for Alpha-1 Antitrypsin Deficiency-associated Lung Disease (AATD-LD) activities to support initiation of the Phase 3 ongoing, following agreement in principle of the primary endpoints Additional clinical stage program – out-licensed to āshibio with EU rights retained Vantictumab for osteopetrosis – clinical stage program with IND planned H2 2026 Financial discipline delivers cash runway into mid-2027 ~$41 million of cash and cash equivalents as of December 31, 2025 Management team with a proven track record in corporate development
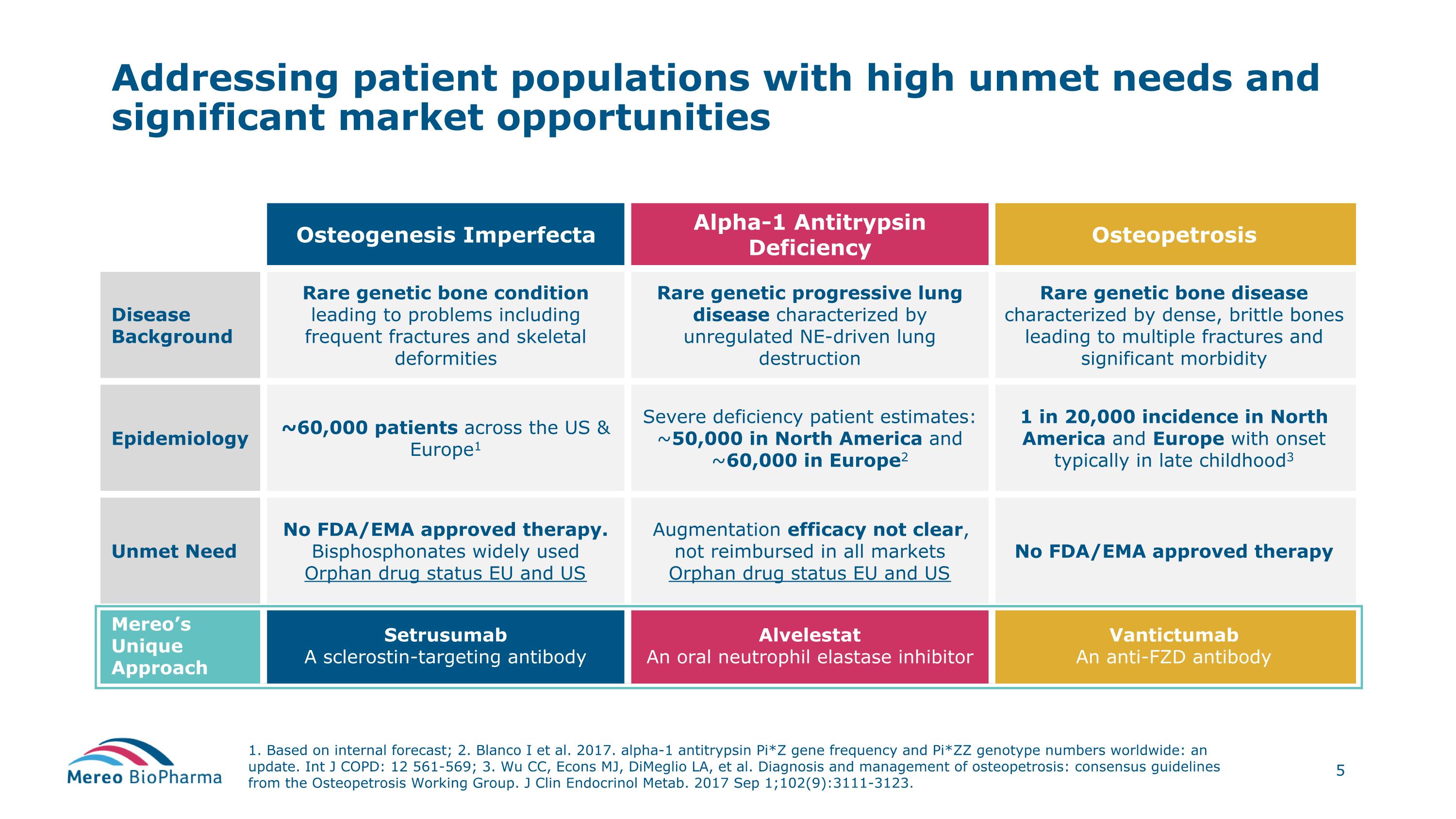
Addressing patient populations with high unmet needs and significant market opportunities 1. Based on internal forecast; 2. Blanco I et al. 2017. alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J COPD: 12 561-569; 3. Wu CC, Econs MJ, DiMeglio LA, et al. Diagnosis and management of osteopetrosis: consensus guidelines from the Osteopetrosis Working Group. J Clin Endocrinol Metab. 2017 Sep 1;102(9):3111-3123. Osteogenesis Imperfecta Alpha-1 Antitrypsin Deficiency Osteopetrosis Disease Background Rare genetic bone condition leading to problems including frequent fractures and skeletal deformities Rare genetic progressive lung disease characterized by unregulated NE-driven lung destruction Rare genetic bone disease characterized by dense, brittle bones leading to multiple fractures and significant morbidity Epidemiology ~60,000 patients across the US & Europe1 Severe deficiency patient estimates: ~50,000 in North America and ~60,000 in Europe2 1 in 20,000 incidence in North America and Europe with onset typically in late childhood3 Unmet Need No FDA/EMA approved therapy. Bisphosphonates widely used Orphan drug status EU and US Augmentation efficacy not clear, not reimbursed in all markets Orphan drug status EU and US No FDA/EMA approved therapy Mereo’s Unique Approach Setrusumab A sclerostin-targeting antibody Alvelestat An oral neutrophil elastase inhibitor Vantictumab An anti-FZD antibody

Setrusumab (UX143) Osteogenesis Imperfecta: a rare genetic bone condition with no FDA or EMA approved therapy
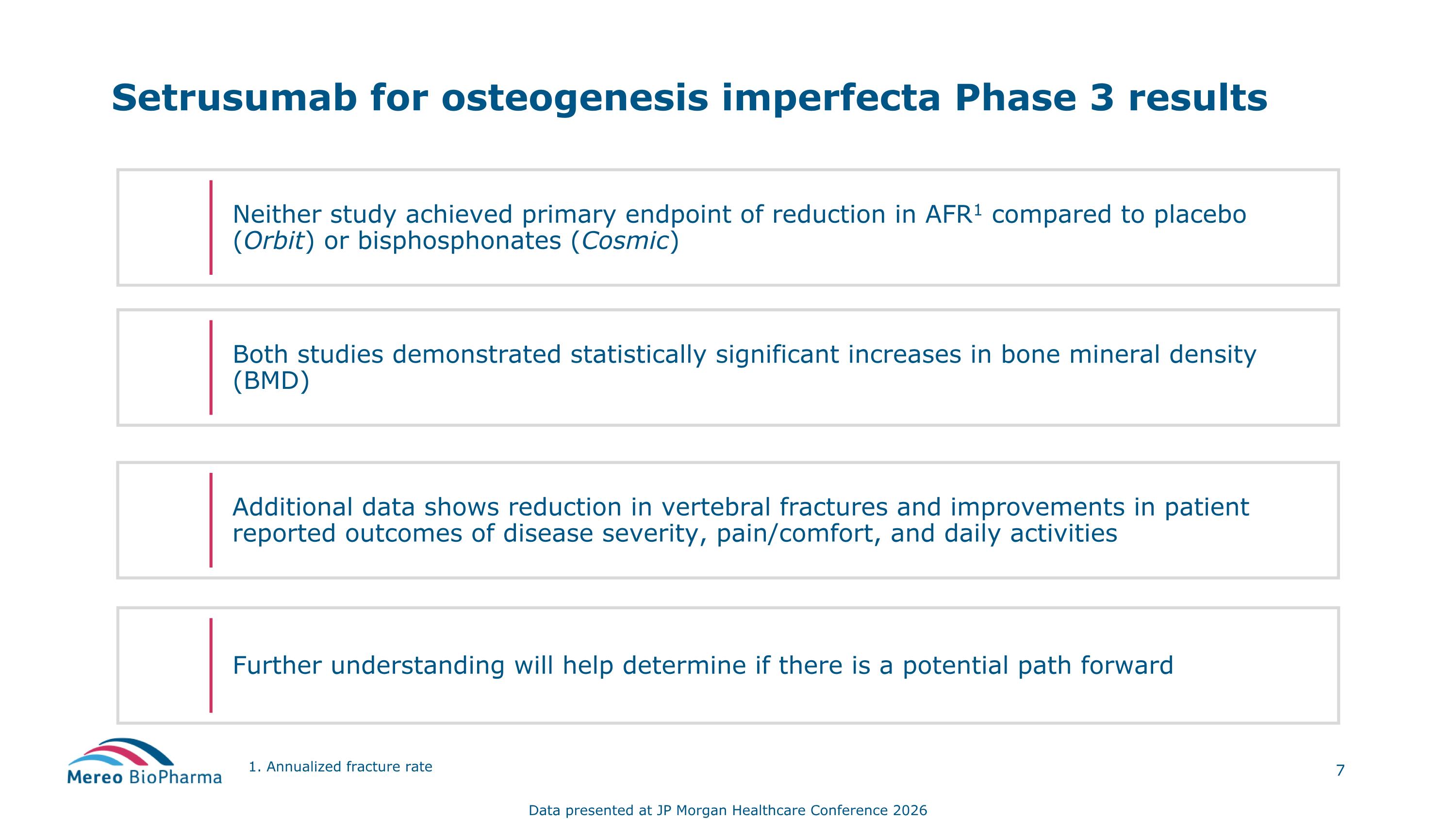
Setrusumab for osteogenesis imperfecta Phase 3 results Neither study achieved primary endpoint of reduction in AFR1 compared to placebo (Orbit) or bisphosphonates (Cosmic) Further understanding will help determine if there is a potential path forward Additional data shows reduction in vertebral fractures and improvements in patient reported outcomes of disease severity, pain/comfort, and daily activities Both studies demonstrated statistically significant increases in bone mineral density (BMD) 1. Annualized fracture rate Data presented at JP Morgan Healthcare Conference 2026
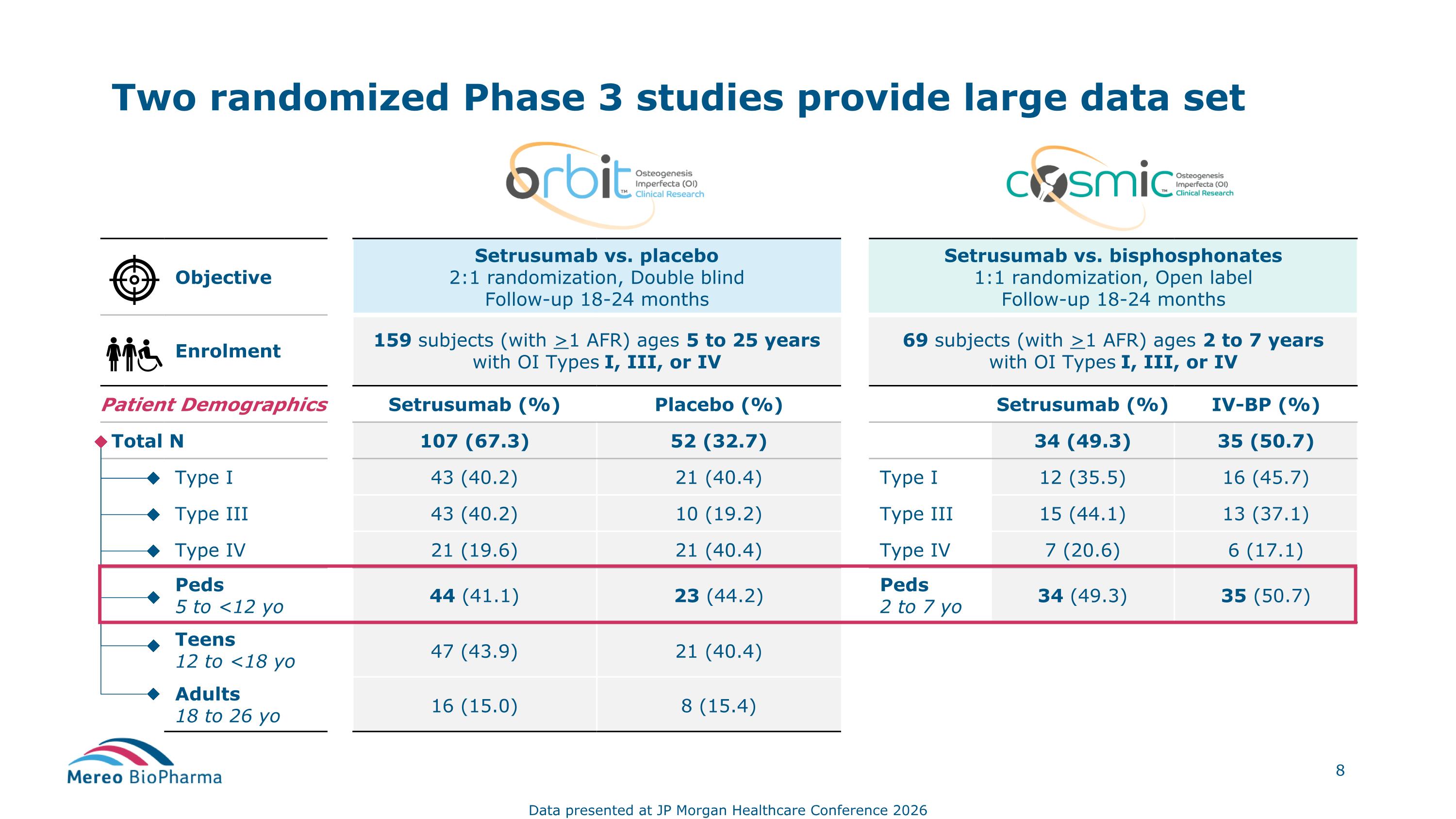
Objective Setrusumab vs. placebo 2:1 randomization, Double blind Follow-up 18-24 months Setrusumab vs. bisphosphonates 1:1 randomization, Open label Follow-up 18-24 months Enrolment 159 subjects (with >1 AFR) ages 5 to 25 years with OI Types I, III, or IV 69 subjects (with >1 AFR) ages 2 to 7 years with OI Types I, III, or IV Patient Demographics Setrusumab (%) Placebo (%) Setrusumab (%) IV-BP (%) Total N ` 107 (67.3) 52 (32.7) 34 (49.3) 35 (50.7) Type I 43 (40.2) 21 (40.4) Type I 12 (35.5) 16 (45.7) Type III 43 (40.2) 10 (19.2) Type III 15 (44.1) 13 (37.1) Type IV 21 (19.6) 21 (40.4) Type IV 7 (20.6) 6 (17.1) Peds 5 to <12 yo 44 (41.1) 23 (44.2) Peds 2 to 7 yo 34 (49.3) 35 (50.7) Teens 12 to <18 yo 47 (43.9) 21 (40.4) Adults 18 to 26 yo 16 (15.0) 8 (15.4) Two randomized Phase 3 studies provide large data set Data presented at JP Morgan Healthcare Conference 2026
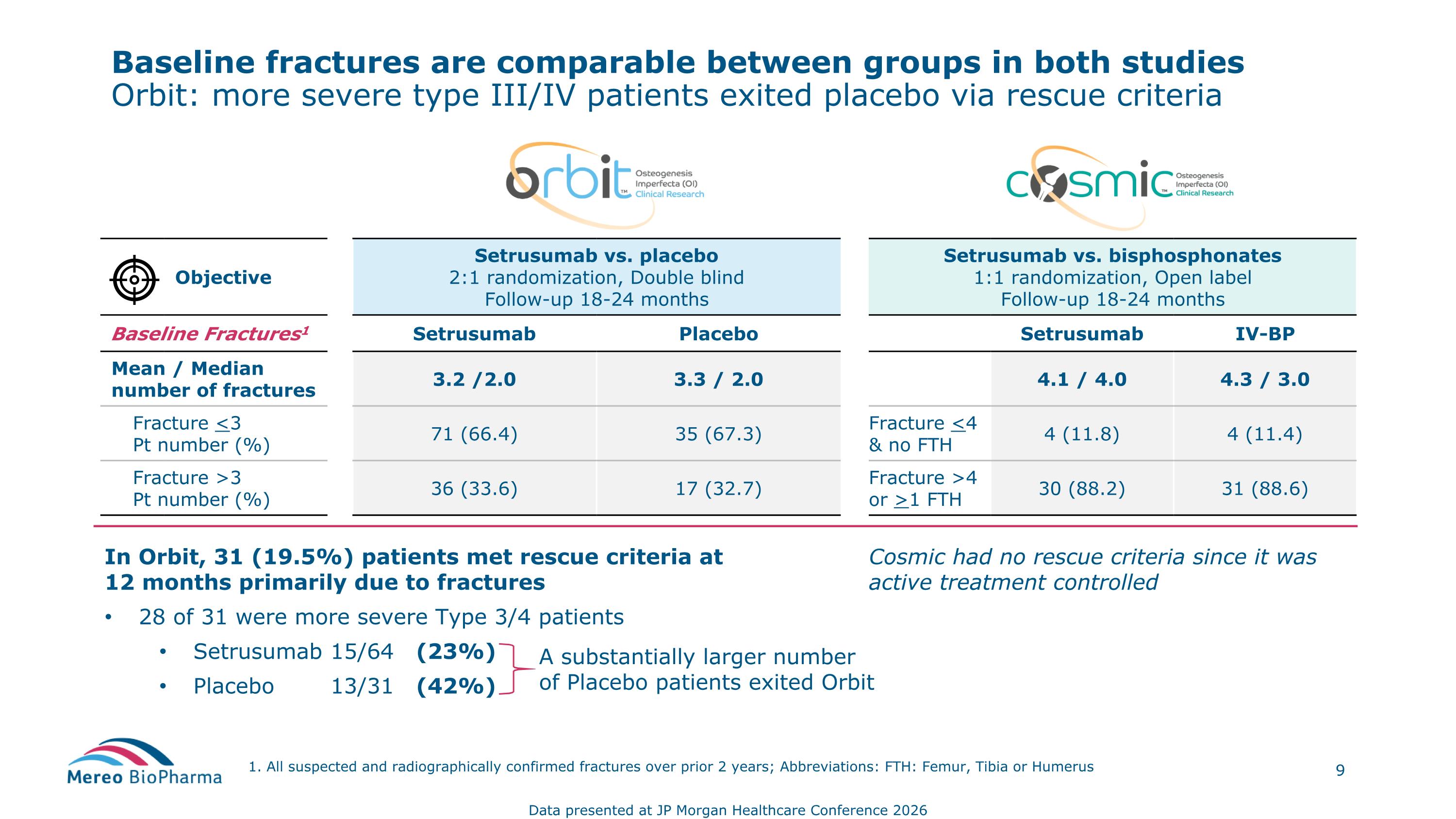
Baseline fractures are comparable between groups in both studies Orbit: more severe type III/IV patients exited placebo via rescue criteria 1. All suspected and radiographically confirmed fractures over prior 2 years; Abbreviations: FTH: Femur, Tibia or Humerus Objective Setrusumab vs. placebo 2:1 randomization, Double blind Follow-up 18-24 months Setrusumab vs. bisphosphonates 1:1 randomization, Open label Follow-up 18-24 months Baseline Fractures1 Setrusumab Placebo Setrusumab IV-BP Mean / Median number of fractures 3.2 /2.0 3.3 / 2.0 4.1 / 4.0 4.3 / 3.0 Fracture <3 Pt number (%) 71 (66.4) 35 (67.3) Fracture <4 & no FTH 4 (11.8) 4 (11.4) Fracture >3 Pt number (%) 36 (33.6) 17 (32.7) Fracture >4 or >1 FTH 30 (88.2) 31 (88.6) In Orbit, 31 (19.5%) patients met rescue criteria at 12 months primarily due to fractures 28 of 31 were more severe Type 3/4 patients Setrusumab 15/64 (23%) Placebo 13/31 (42%) A substantially larger number of Placebo patients exited Orbit Cosmic had no rescue criteria since it was active treatment controlled Data presented at JP Morgan Healthcare Conference 2026
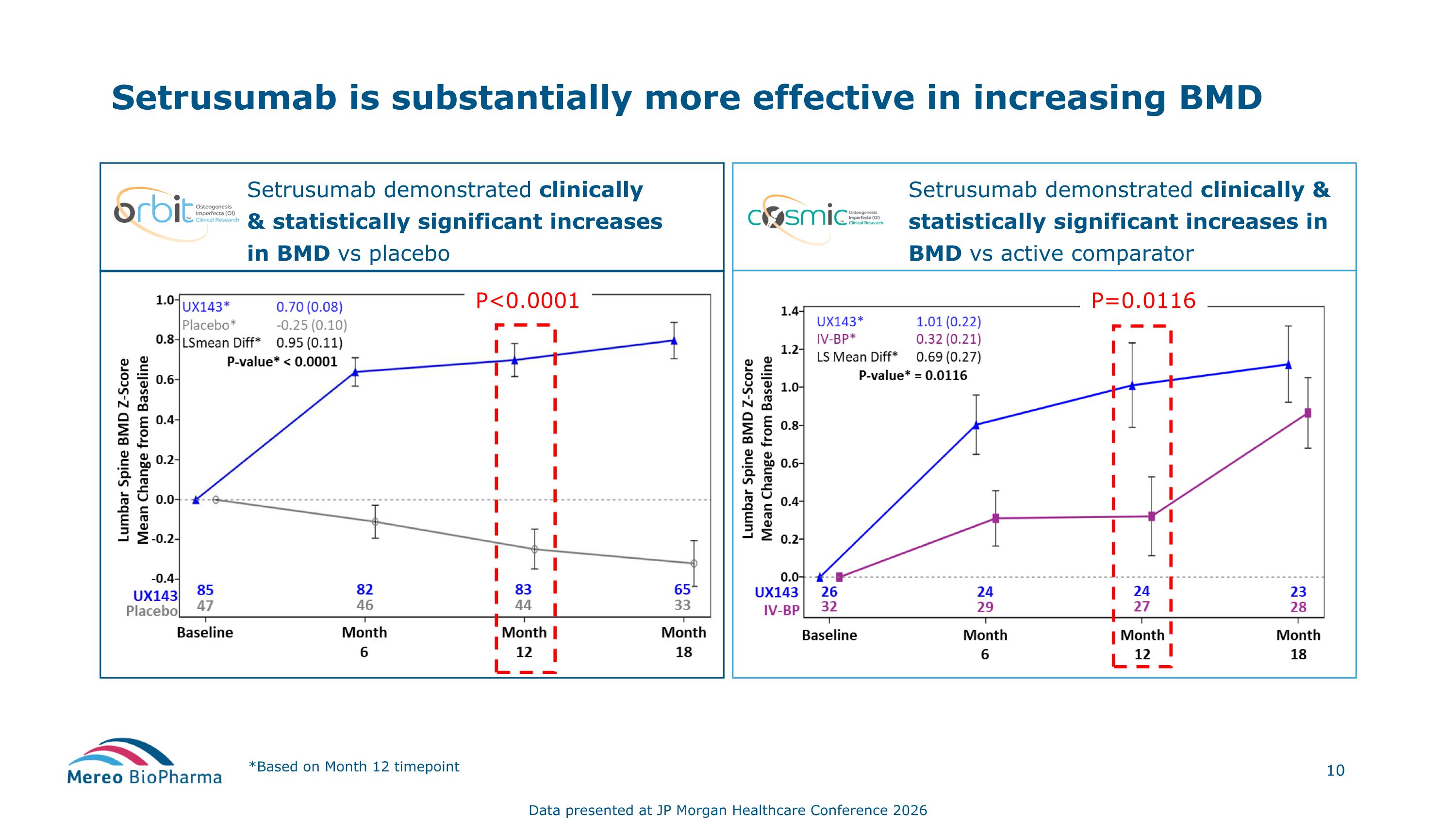
Setrusumab demonstrated clinically & statistically significant increases in BMD vs placebo Setrusumab is substantially more effective in increasing BMD *Based on Month 12 timepoint Setrusumab demonstrated clinically & statistically significant increases in BMD vs active comparator Data presented at JP Morgan Healthcare Conference 2026 P<0.0001 P=0.0116
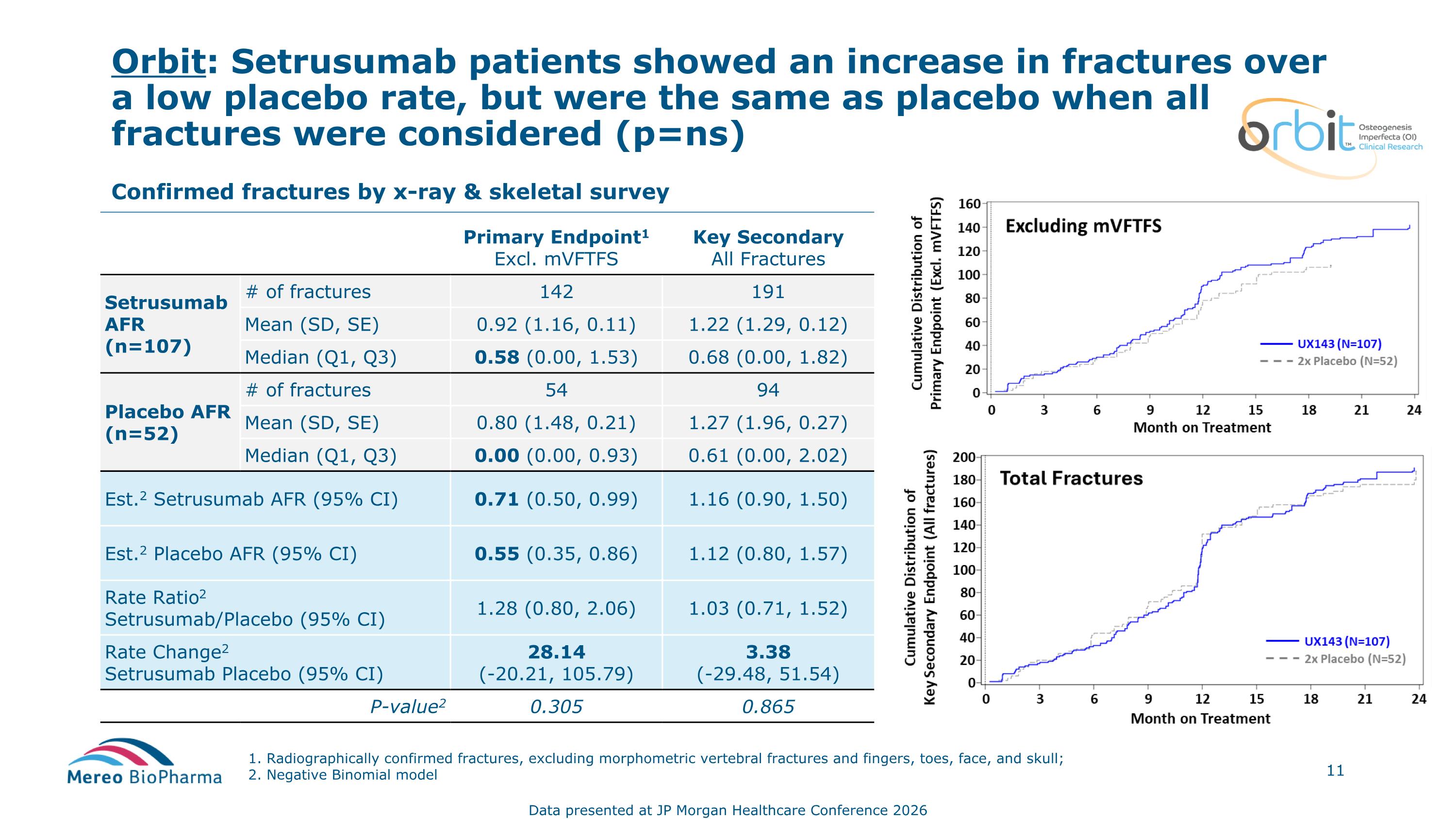
Orbit: Setrusumab patients showed an increase in fractures over a low placebo rate, but were the same as placebo when all fractures were considered (p=ns) 1. Radiographically confirmed fractures, excluding morphometric vertebral fractures and fingers, toes, face, and skull; 2. Negative Binomial model Confirmed fractures by x-ray & skeletal survey Primary Endpoint1 Excl. mVFTFS Key Secondary All Fractures Setrusumab AFR (n=107) # of fractures 142 191 Mean (SD, SE) 0.92 (1.16, 0.11) 1.22 (1.29, 0.12) Median (Q1, Q3) 0.58 (0.00, 1.53) 0.68 (0.00, 1.82) Placebo AFR (n=52) # of fractures 54 94 Mean (SD, SE) 0.80 (1.48, 0.21) 1.27 (1.96, 0.27) Median (Q1, Q3) 0.00 (0.00, 0.93) 0.61 (0.00, 2.02) Est.2 Setrusumab AFR (95% CI) 0.71 (0.50, 0.99) 1.16 (0.90, 1.50) Est.2 Placebo AFR (95% CI) 0.55 (0.35, 0.86) 1.12 (0.80, 1.57) Rate Ratio2 Setrusumab/Placebo (95% CI) 1.28 (0.80, 2.06) 1.03 (0.71, 1.52) Rate Change2 Setrusumab Placebo (95% CI) 28.14 (-20.21, 105.79) 3.38 (-29.48, 51.54) P-value2 0.305 0.865 Data presented at JP Morgan Healthcare Conference 2026
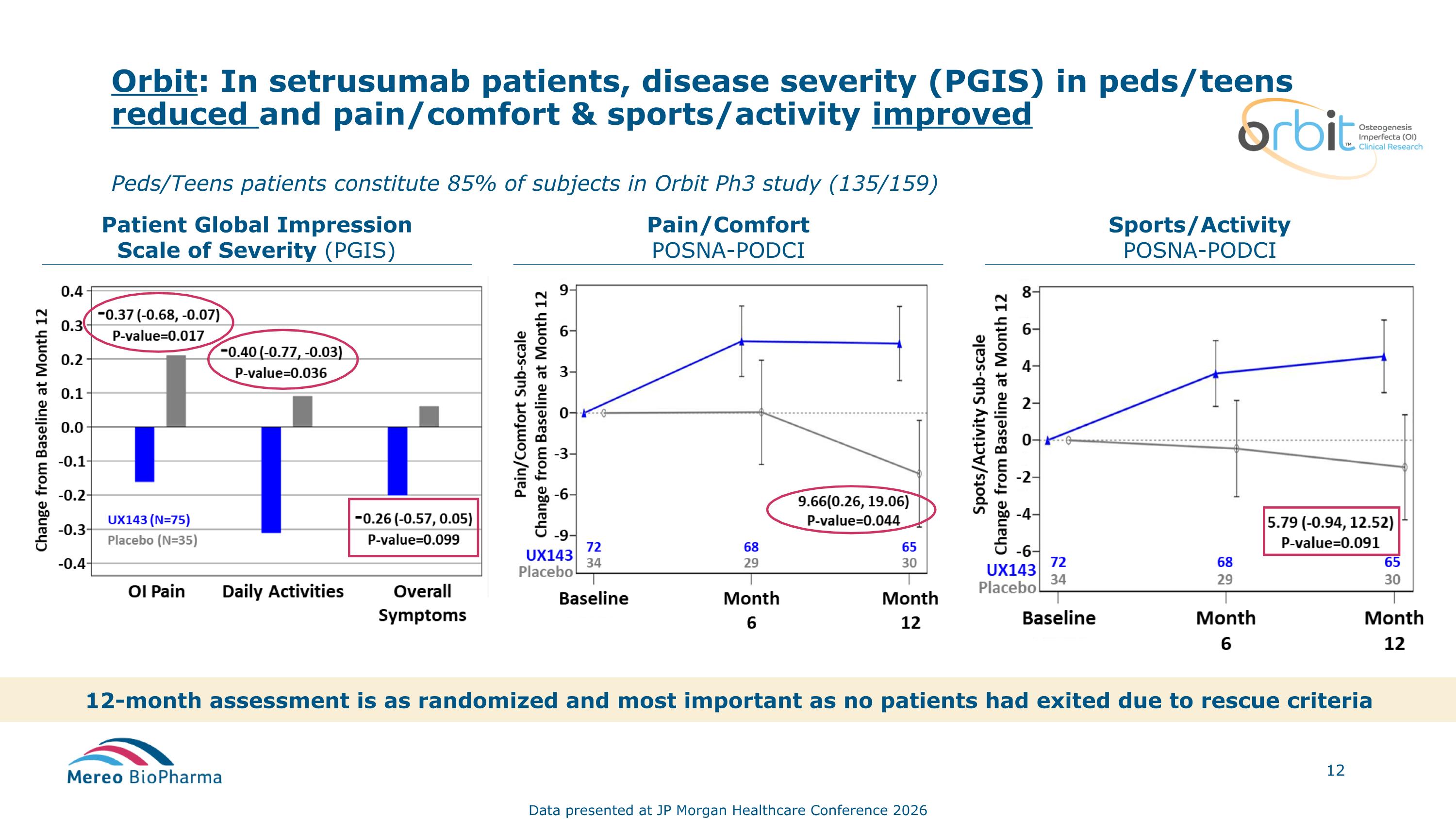
Orbit: In setrusumab patients, disease severity (PGIS) in peds/teens reduced and pain/comfort & sports/activity improved Patient Global Impression Scale of Severity (PGIS) Pain/Comfort POSNA-PODCI Sports/Activity POSNA-PODCI 12-month assessment is as randomized and most important as no patients had exited due to rescue criteria Peds/Teens patients constitute 85% of subjects in Orbit Ph3 study (135/159) Data presented at JP Morgan Healthcare Conference 2026
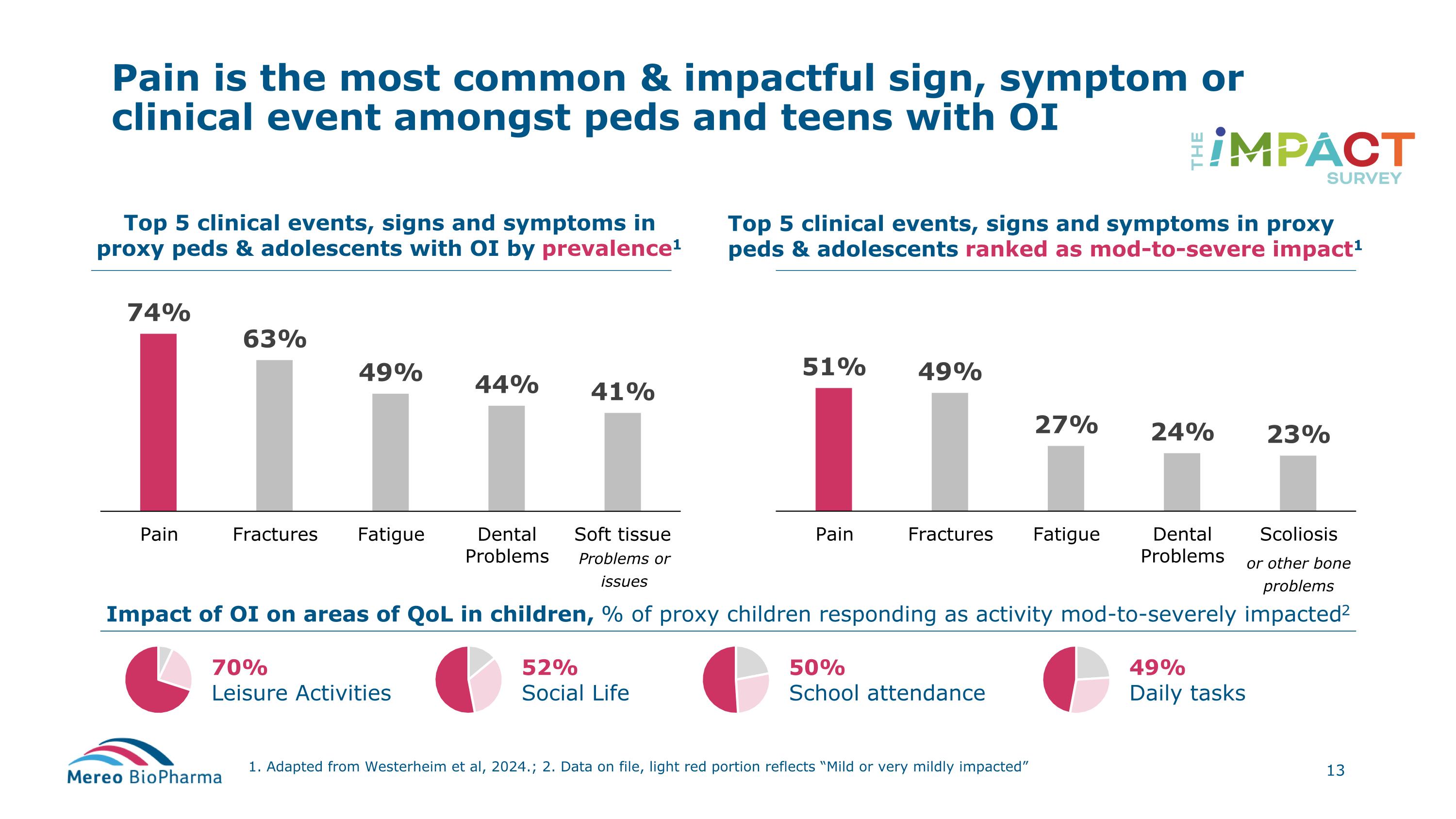
Pain is the most common & impactful sign, symptom or clinical event amongst peds and teens with OI 1. Adapted from Westerheim et al, 2024.; 2. Data on file, light red portion reflects “Mild or very mildly impacted” Top 5 clinical events, signs and symptoms in proxy peds & adolescents with OI by prevalence1 Top 5 clinical events, signs and symptoms in proxy peds & adolescents ranked as mod-to-severe impact1 Impact of OI on areas of QoL in children, % of proxy children responding as activity mod-to-severely impacted2 70% Leisure Activities 52% Social Life 50% School attendance 49% Daily tasks or other bone problems Problems or issues
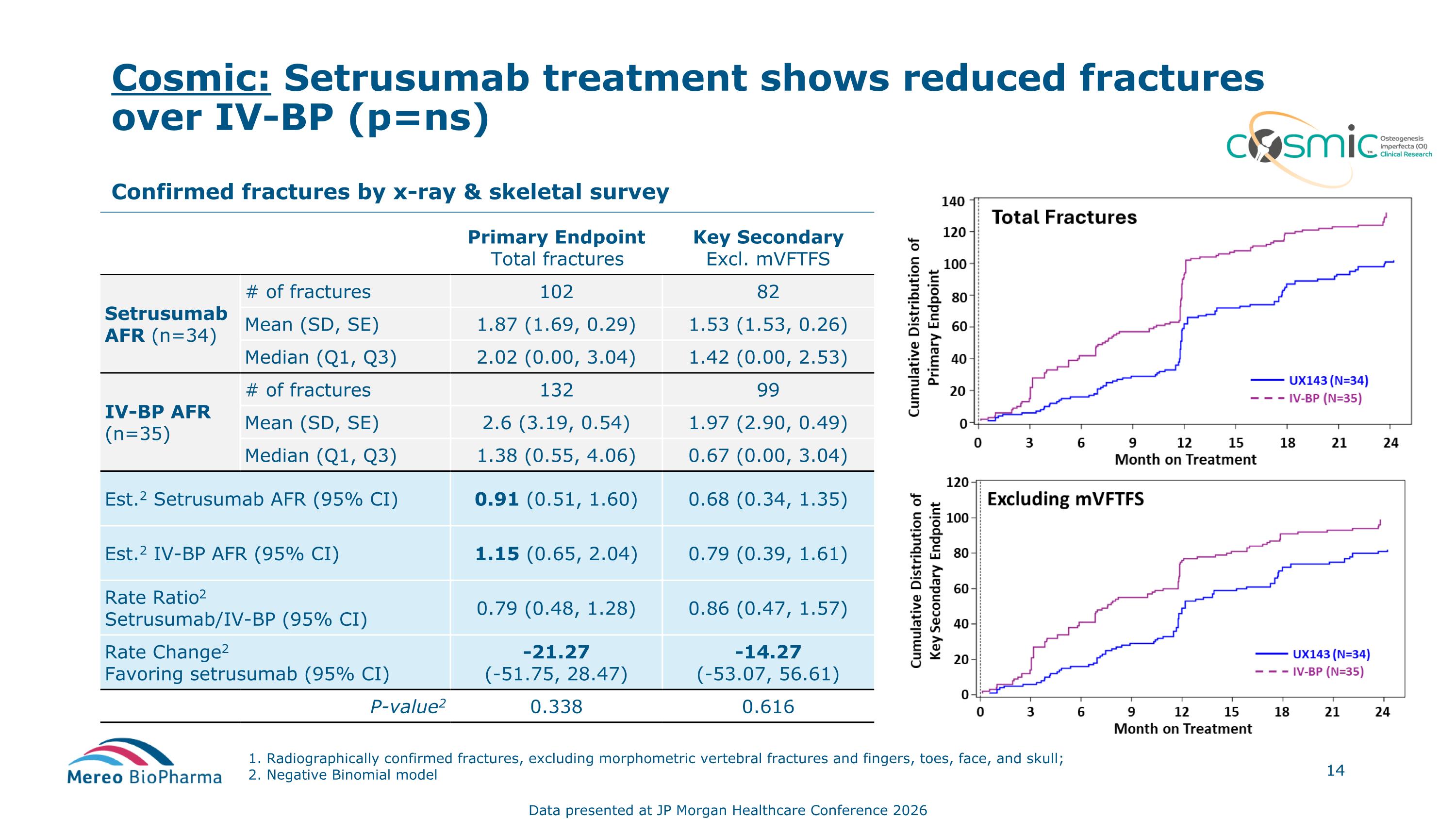
Cosmic: Setrusumab treatment shows reduced fractures over IV-BP (p=ns) Confirmed fractures by x-ray & skeletal survey Primary Endpoint Total fractures Key Secondary Excl. mVFTFS Setrusumab AFR (n=34) # of fractures 102 82 Mean (SD, SE) 1.87 (1.69, 0.29) 1.53 (1.53, 0.26) Median (Q1, Q3) 2.02 (0.00, 3.04) 1.42 (0.00, 2.53) IV-BP AFR (n=35) # of fractures 132 99 Mean (SD, SE) 2.6 (3.19, 0.54) 1.97 (2.90, 0.49) Median (Q1, Q3) 1.38 (0.55, 4.06) 0.67 (0.00, 3.04) Est.2 Setrusumab AFR (95% CI) 0.91 (0.51, 1.60) 0.68 (0.34, 1.35) Est.2 IV-BP AFR (95% CI) 1.15 (0.65, 2.04) 0.79 (0.39, 1.61) Rate Ratio2 Setrusumab/IV-BP (95% CI) 0.79 (0.48, 1.28) 0.86 (0.47, 1.57) Rate Change2 Favoring setrusumab (95% CI) -21.27 (-51.75, 28.47) -14.27 (-53.07, 56.61) P-value2 0.338 0.616 1. Radiographically confirmed fractures, excluding morphometric vertebral fractures and fingers, toes, face, and skull; 2. Negative Binomial model Data presented at JP Morgan Healthcare Conference 2026
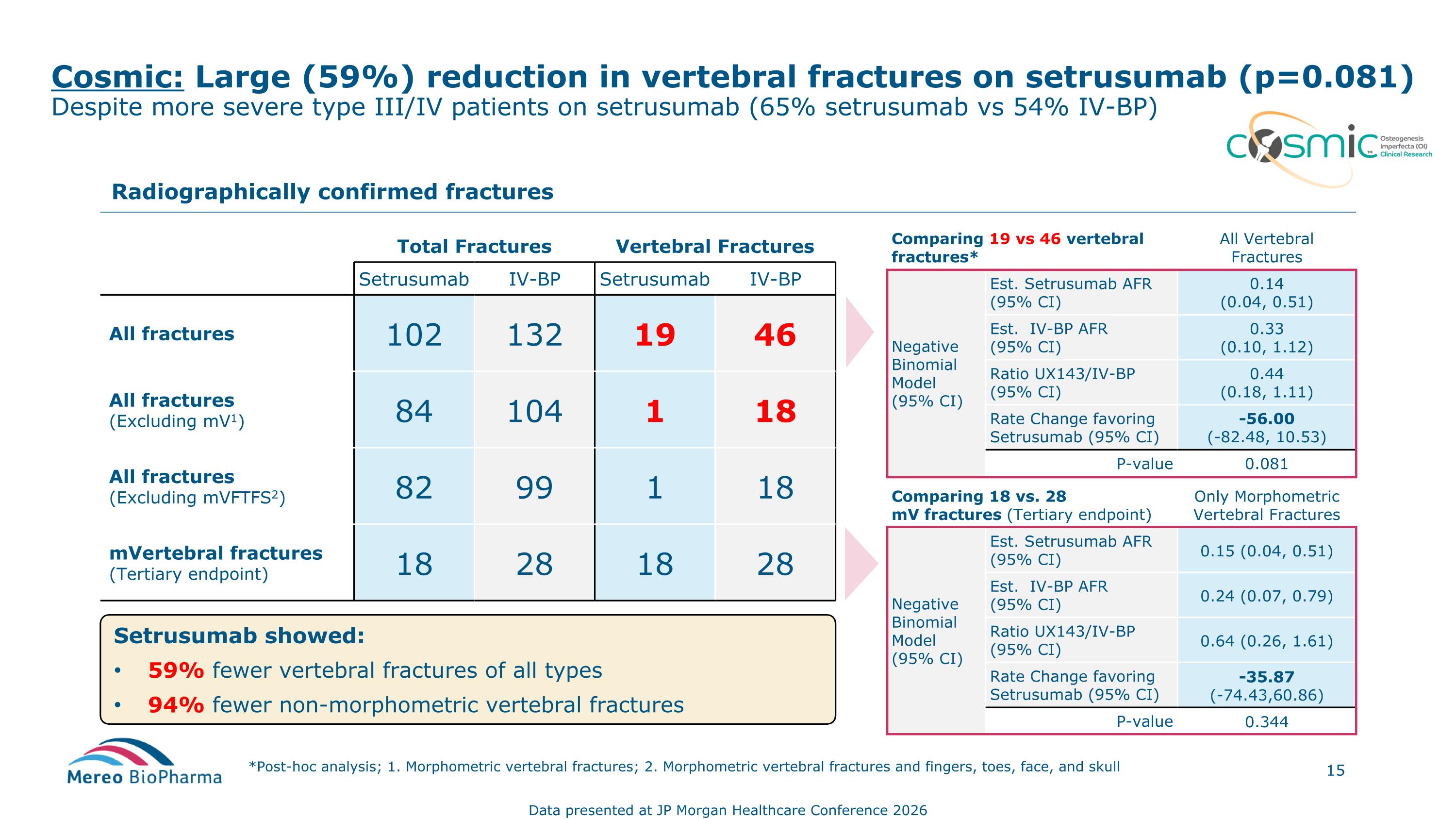
Cosmic: Large (59%) reduction in vertebral fractures on setrusumab (p=0.081) Despite more severe type III/IV patients on setrusumab (65% setrusumab vs 54% IV-BP) *Post-hoc analysis; 1. Morphometric vertebral fractures; 2. Morphometric vertebral fractures and fingers, toes, face, and skull Total Fractures Vertebral Fractures Setrusumab IV-BP Setrusumab IV-BP All fractures 102 132 19 46 All fractures (Excluding mV1) 84 104 1 18 All fractures (Excluding mVFTFS2) 82 99 1 18 mVertebral fractures (Tertiary endpoint) 18 28 18 28 Radiographically confirmed fractures Comparing 19 vs 46 vertebral fractures* All Vertebral Fractures Negative Binomial Model (95% CI) Est. Setrusumab AFR (95% CI) 0.14 (0.04, 0.51) Est. IV-BP AFR (95% CI) 0.33 (0.10, 1.12) Ratio UX143/IV-BP (95% CI) 0.44 (0.18, 1.11) Rate Change favoring Setrusumab (95% CI) -56.00 (-82.48, 10.53) P-value 0.081 Comparing 18 vs. 28 mV fractures (Tertiary endpoint) Only Morphometric Vertebral Fractures Negative Binomial Model (95% CI) Est. Setrusumab AFR (95% CI) 0.15 (0.04, 0.51) Est. IV-BP AFR (95% CI) 0.24 (0.07, 0.79) Ratio UX143/IV-BP (95% CI) 0.64 (0.26, 1.61) Rate Change favoring Setrusumab (95% CI) -35.87 (-74.43,60.86) P-value 0.344 Setrusumab showed: 59% fewer vertebral fractures of all types 94% fewer non-morphometric vertebral fractures Data presented at JP Morgan Healthcare Conference 2026
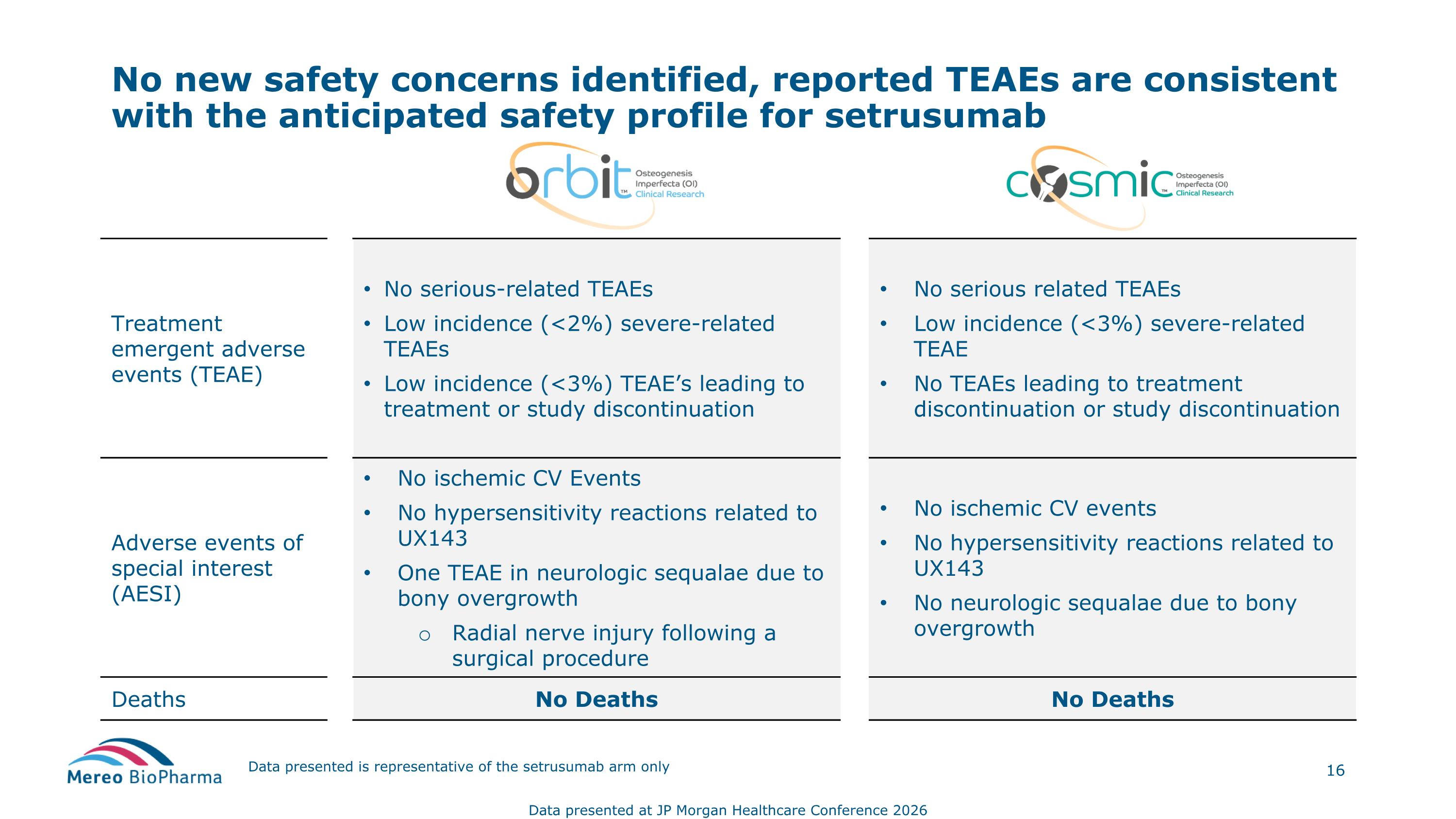
Data presented is representative of the setrusumab arm only Treatment emergent adverse events (TEAE) No serious-related TEAEs Low incidence (<2%) severe-related TEAEs Low incidence (<3%) TEAE’s leading to treatment or study discontinuation No serious related TEAEs Low incidence (<3%) severe-related TEAE No TEAEs leading to treatment discontinuation or study discontinuation Adverse events of special interest (AESI) No ischemic CV Events No hypersensitivity reactions related to UX143 One TEAE in neurologic sequalae due to bony overgrowth Radial nerve injury following a surgical procedure No ischemic CV events No hypersensitivity reactions related to UX143 No neurologic sequalae due to bony overgrowth Deaths No Deaths No Deaths No new safety concerns identified, reported TEAEs are consistent with the anticipated safety profile for setrusumab Data presented at JP Morgan Healthcare Conference 2026
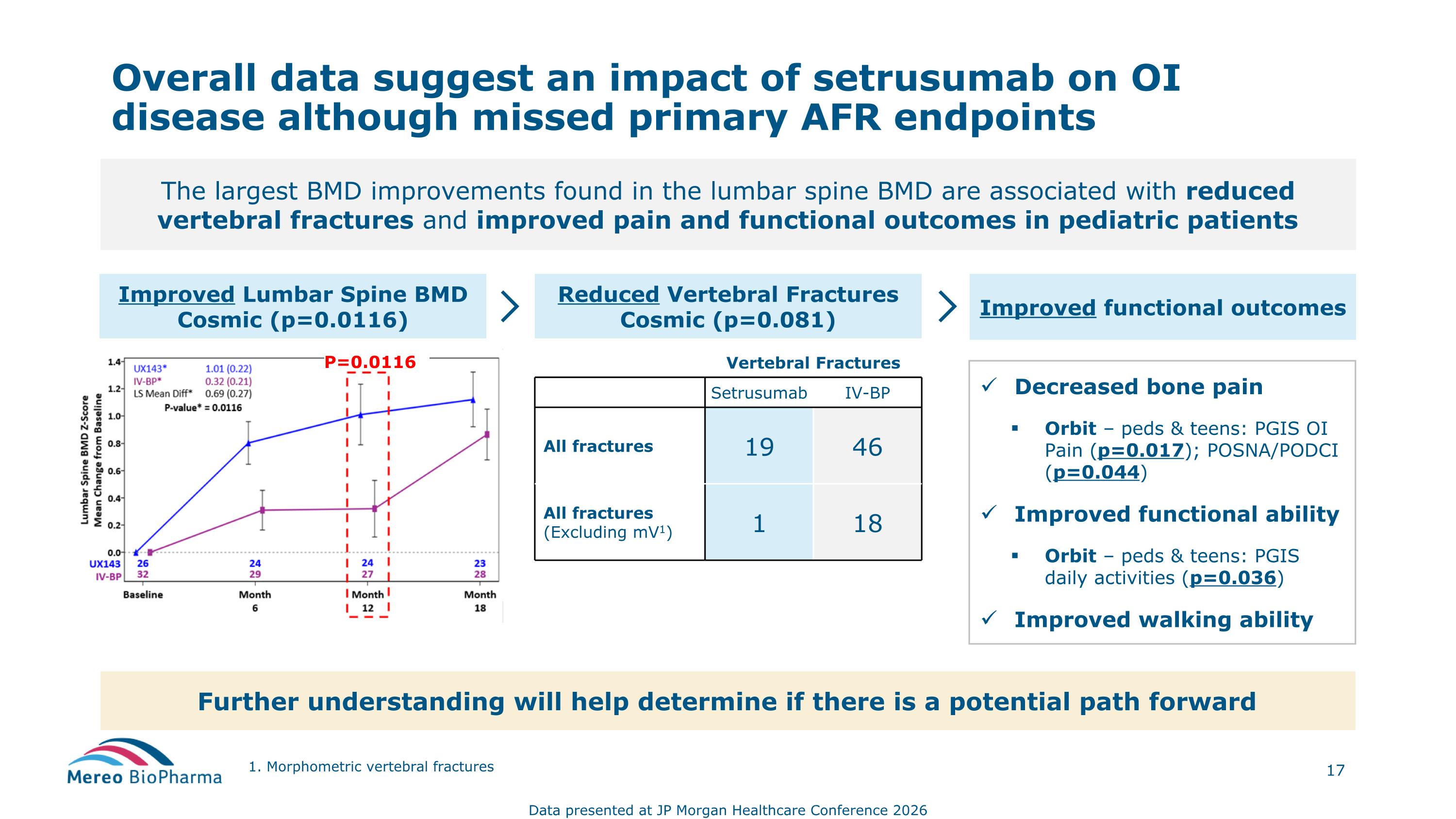
Overall data suggest an impact of setrusumab on OI disease although missed primary AFR endpoints The largest BMD improvements found in the lumbar spine BMD are associated with reduced vertebral fractures and improved pain and functional outcomes in pediatric patients Reduced Vertebral Fractures Cosmic (p=0.081) Improved Lumbar Spine BMD Cosmic (p=0.0116) Improved functional outcomes Vertebral Fractures Setrusumab IV-BP All fractures 19 46 All fractures (Excluding mV1) 1 18 Decreased bone pain Orbit – peds & teens: PGIS OI Pain (p=0.017); POSNA/PODCI (p=0.044) Improved functional ability Orbit – peds & teens: PGIS daily activities (p=0.036) Improved walking ability Further understanding will help determine if there is a potential path forward Data presented at JP Morgan Healthcare Conference 2026 1. Morphometric vertebral fractures P=0.0116

Alvelestat (MPH966) Alpha-1 Antitrypsin Deficiency-associated Lung Disease: a rare progressive lung disease with high unmet need
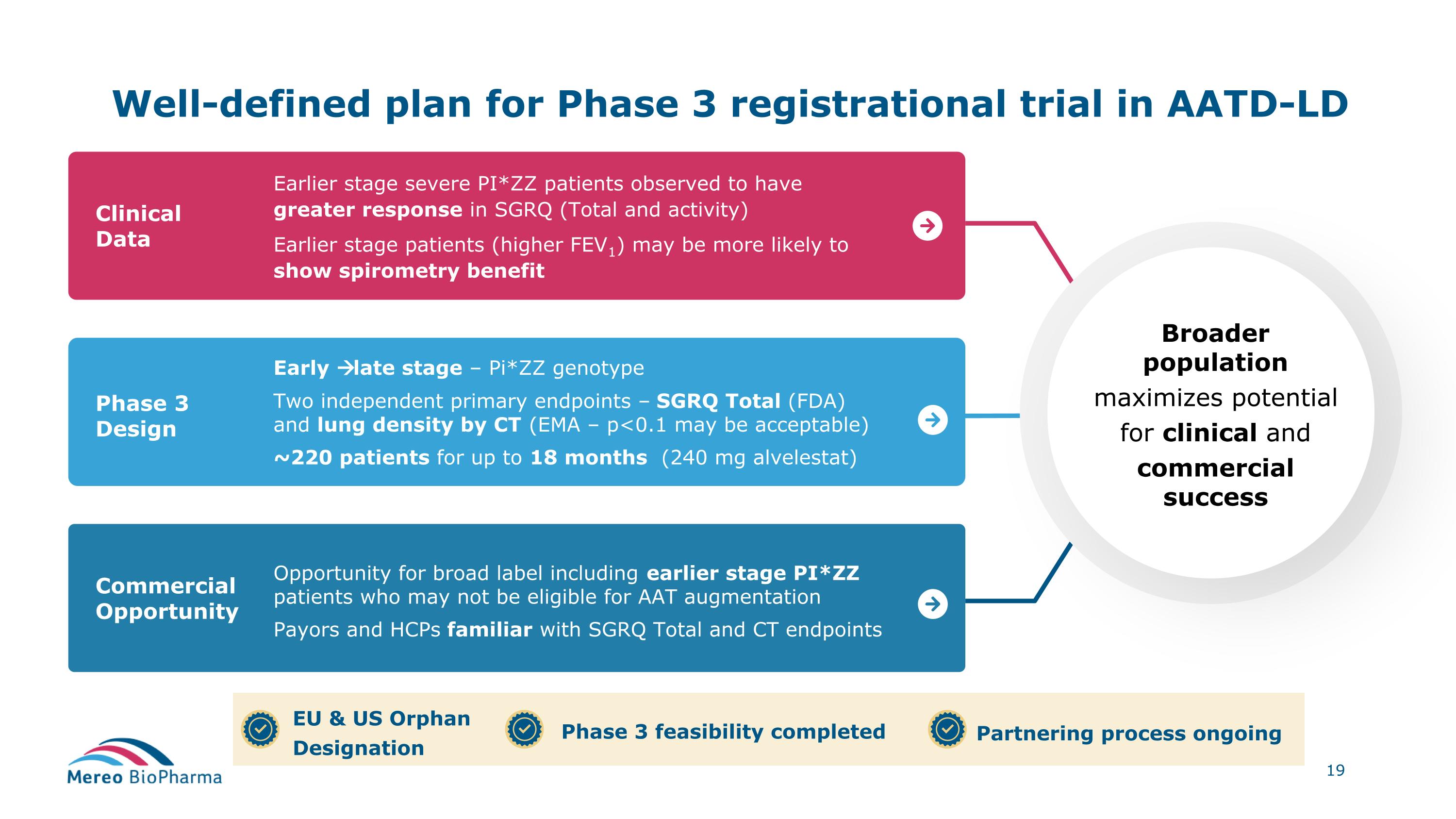
Well-defined plan for Phase 3 registrational trial in AATD-LD Clinical Data Earlier stage severe PI*ZZ patients observed to have greater response in SGRQ (Total and activity) Earlier stage patients (higher FEV1) may be more likely to show spirometry benefit Commercial Opportunity Phase 3 Design Early late stage – Pi*ZZ genotype Two independent primary endpoints – SGRQ Total (FDA) and lung density by CT (EMA – p<0.1 may be acceptable) ~220 patients for up to 18 months (240 mg alvelestat) Opportunity for broad label including earlier stage PI*ZZ patients who may not be eligible for AAT augmentation Payors and HCPs familiar with SGRQ Total and CT endpoints Broader population maximizes potential for clinical and commercial success EU & US Orphan Designation Phase 3 feasibility completed Partnering process ongoing
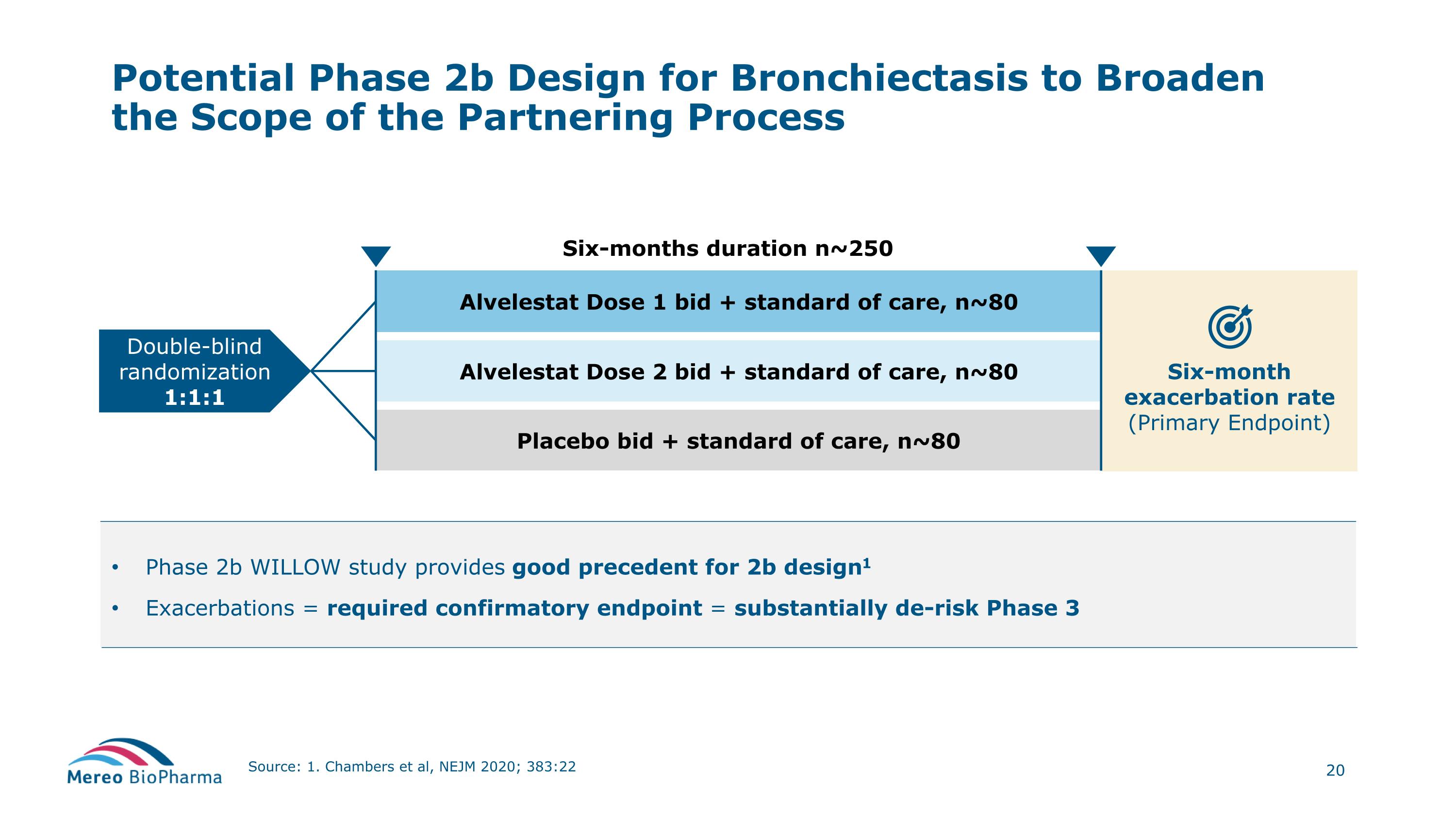
Six-month exacerbation rate (Primary Endpoint) Phase 2b WILLOW study provides good precedent for 2b design1 Exacerbations = required confirmatory endpoint = substantially de-risk Phase 3 Potential Phase 2b Design for Bronchiectasis to Broaden the Scope of the Partnering Process Source: 1. Chambers et al, NEJM 2020; 383:22 Alvelestat Dose 2 bid + standard of care, n~80 Alvelestat Dose 1 bid + standard of care, n~80 Six-months duration n~250 Placebo bid + standard of care, n~80 Double-blind randomization 1:1:1

Vantictumab Osteopetrosis: a rare bone disease with high unmet need
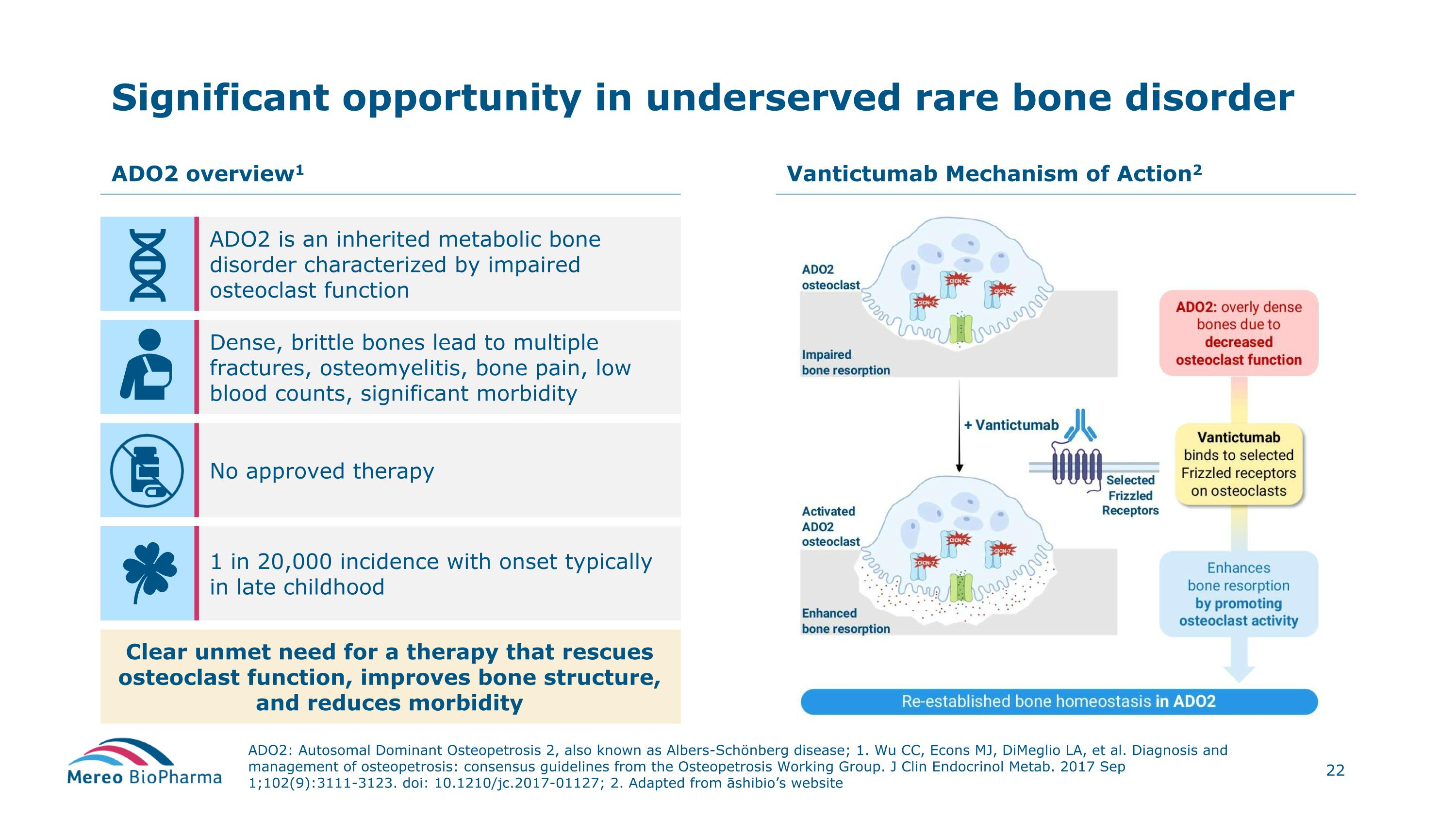
Significant opportunity in underserved rare bone disorder ADO2: Autosomal Dominant Osteopetrosis 2, also known as Albers-Schönberg disease; 1. Wu CC, Econs MJ, DiMeglio LA, et al. Diagnosis and management of osteopetrosis: consensus guidelines from the Osteopetrosis Working Group. J Clin Endocrinol Metab. 2017 Sep 1;102(9):3111-3123. doi: 10.1210/jc.2017-01127; 2. Adapted from āshibio’s website ADO2 overview1 Vantictumab Mechanism of Action2 ADO2 is an inherited metabolic bone disorder characterized by impaired osteoclast function Dense, brittle bones lead to multiple fractures, osteomyelitis, bone pain, low blood counts, significant morbidity No approved therapy 1 in 20,000 incidence with onset typically in late childhood Clear unmet need for a therapy that rescues osteoclast function, improves bone structure, and reduces morbidity
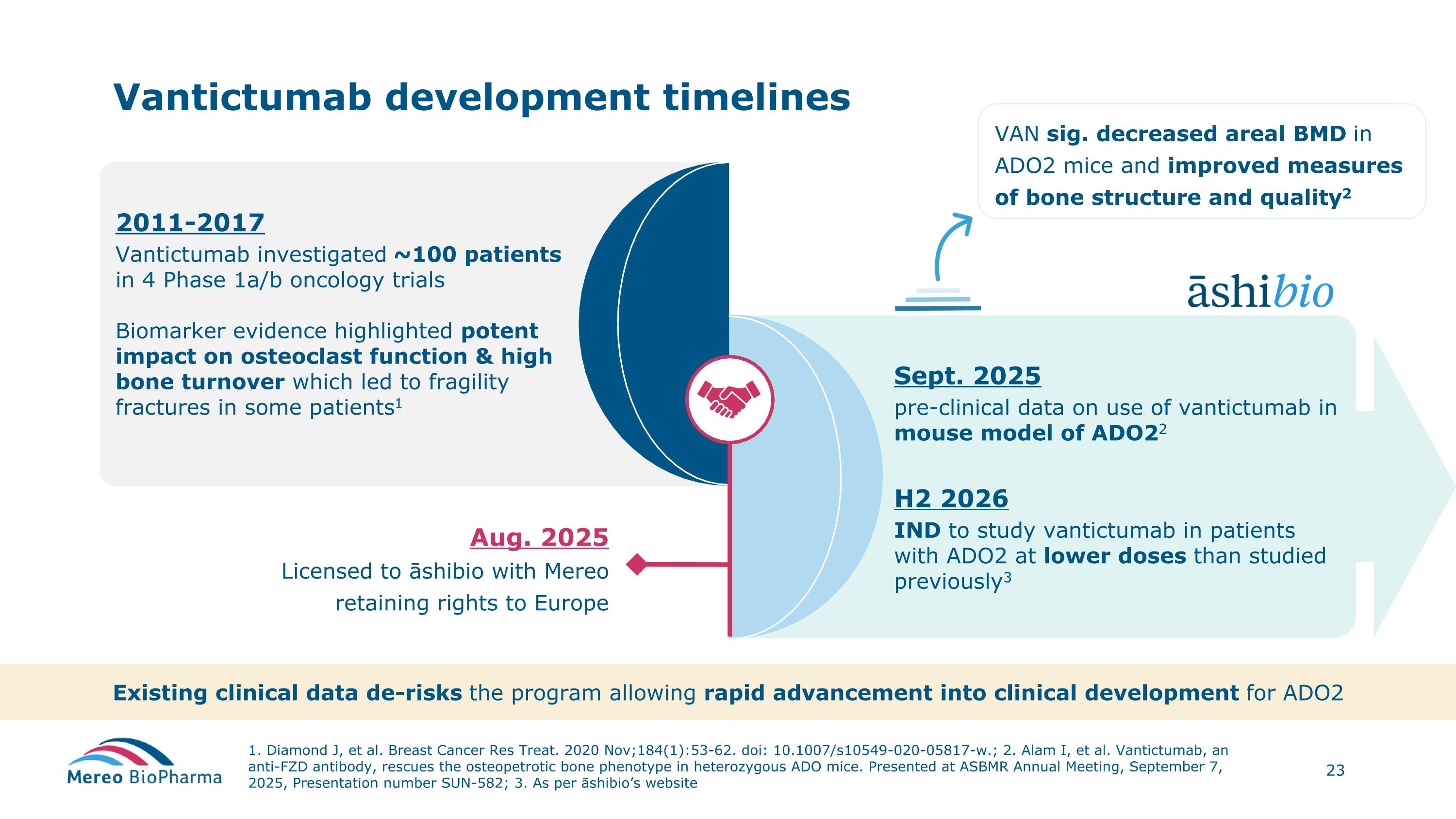
2011-2017 Vantictumab investigated ~100 patients in 4 Phase 1a/b oncology trials Biomarker evidence highlighted potent impact on osteoclast function & high bone turnover which led to fragility fractures in some patients1 Vantictumab development timelines 1. Diamond J, et al. Breast Cancer Res Treat. 2020 Nov;184(1):53-62. doi: 10.1007/s10549-020-05817-w.; 2. Alam I, et al. Vantictumab, an anti-FZD antibody, rescues the osteopetrotic bone phenotype in heterozygous ADO mice. Presented at ASBMR Annual Meeting, September 7, 2025, Presentation number SUN-582; 3. As per āshibio’s website Aug. 2025 Licensed to āshibio with Mereo retaining rights to Europe Sept. 2025 pre-clinical data on use of vantictumab in mouse model of ADO22 H2 2026 IND to study vantictumab in patients with ADO2 at lower doses than studied previously3 VAN sig. decreased areal BMD in ADO2 mice and improved measures of bone structure and quality2 Existing clinical data de-risks the program allowing rapid advancement into clinical development for ADO2

Key milestones
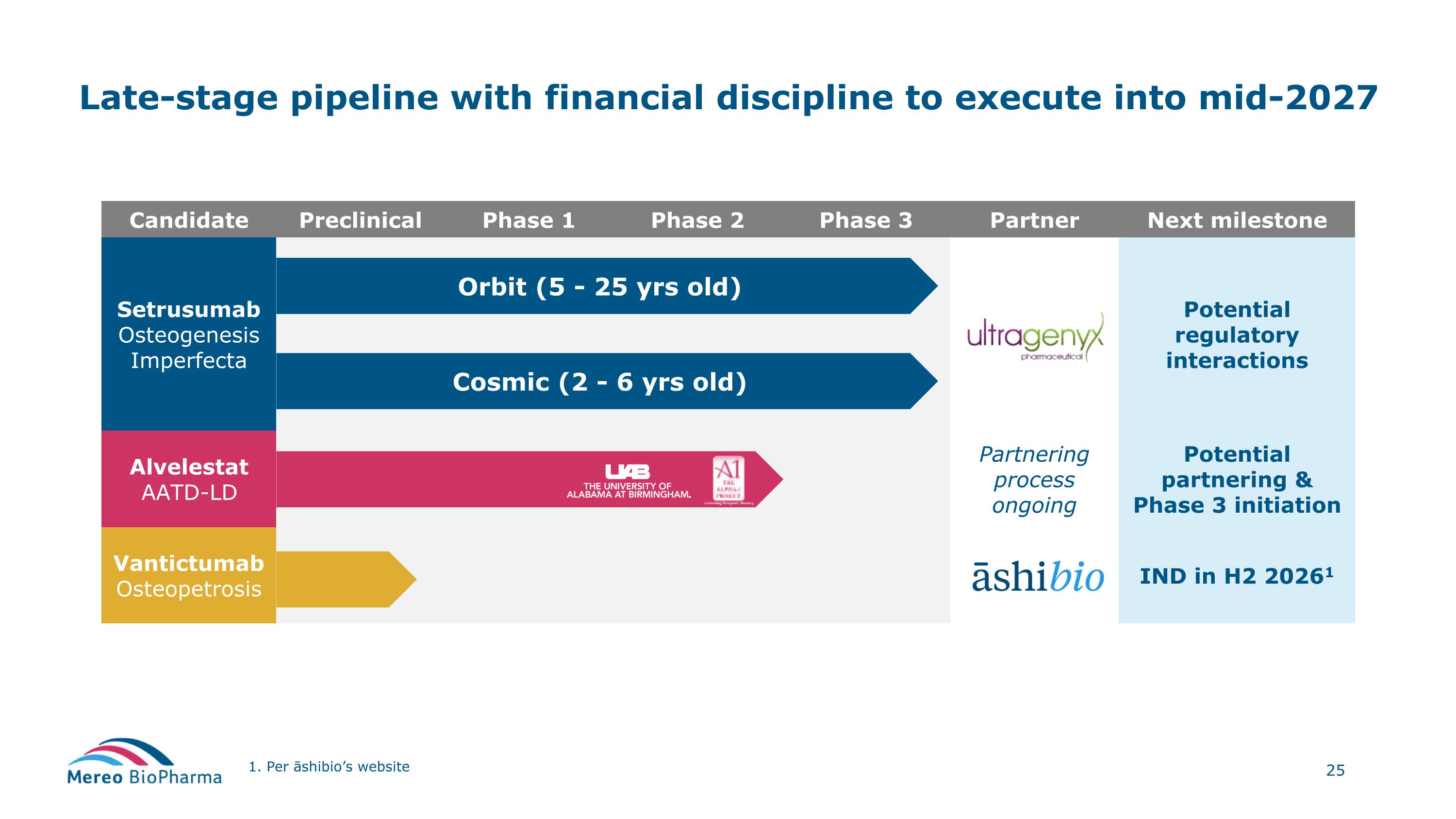
Candidate Preclinical Phase 1 Phase 2 Phase 3 Partner Next milestone Setrusumab Osteogenesis Imperfecta Potential regulatory interactions Alvelestat AATD-LD Partnering process ongoing Potential partnering & Phase 3 initiation Vantictumab Osteopetrosis IND in H2 20261 Late-stage pipeline with financial discipline to execute into mid-2027 1. Per āshibio’s website Orbit (5 - 25 yrs old) Cosmic (2 - 6 yrs old)

Thank you With a special thank you to members of our community, who generously agreed to be featured in this presentation.