| © 2026 Scholar Rock, Inc. All rights reserved. 44th Annual J.P. Morgan Healthcare Conference Scholar Rock Investor Presentation JANUARY 12, 2026 .2 |
| © 2026 Scholar Rock, Inc. All rights reserved. 2 Forward-Looking Statements Various statements in this presentation concerning the future expectations, plans and prospects of Scholar Rock Holding Corporation and Scholar Rock, Inc. (collectively, “Scholar Rock”), including without limitation, Scholar Rock’s expectations regarding its growth, strategy, progress and timing of its clinical trials for apitegromab, SRK-439 and its preclinical programs, and indication selection and development timing, including the timing of any regulatory submissions and anticipated approvals, the therapeutic potential, clinical benefits and safety of any product candidates, its ability to address the observations identified in the complete response letter, its cash runway into 2027, expectations regarding commercial launch timing in the US and in Europe, expectations regarding a new fill finish facility and the achievement of important milestones, the ability of any product candidate to perform in humans in a manner consistent with earlier nonclinical, preclinical or clinical trial data, and the potential of its product candidates and proprietary platform. The use of words such as “may,” “could,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify such forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. All such forward-looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, without limitation, that preclinical and clinical data are not predictive of, may be inconsistent with, or more favorable than, data generated from future or ongoing clinical trials of the same product candidate; the results from the Phase 3 SAPPHIRE trial will be sufficient to support regulatory approval; Scholar Rock’s ability to provide the financial support, resources and expertise necessary to identify and develop product candidates on the expected timeline; the data generated from Scholar Rock’s nonclinical and preclinical studies and clinical trials, including from the EMBRAZE clinical trial; information provided or decisions made by regulatory authorities; competition from third parties that are developing products for similar uses; Scholar Rock’s ability to obtain, maintain and protect its intellectual property; the success of Scholar Rock’s current and potential future collaborations; Scholar Rock’s dependence on third parties for development and manufacture of product candidates including, without limitation, to supply any clinical trials; Scholar Rock’s ability to manage expenses and to obtain additional funding when needed to support its business activities; its ability to establish or maintain strategic business alliances; its ability to receive priority or expedited regulatory review or to obtain regulatory approval of apitegromab; its ability to expand globally and the anticipated commercial launch in the United States of apitegromab in 2026; as well as those risks more fully discussed in the section entitled "Risk Factors" in Scholar Rock’s Form 10-K for the year ended December 31, 2024, and Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 as well as discussions of potential risks, uncertainties, and other important factors in Scholar Rock’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Scholar Rock’s views only as of today and should not be relied upon as representing its views as of any subsequent date. All information in this press release is as of the date of the release, and Scholar Rock undertakes no duty to update this information unless required by law. This presentation may also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we compete are necessarily subject to a high degree of uncertainty and risk. Apitegromab and SRK-439 are investigational drug candidates under evaluation. Apitegromab and SRK-439 have not been approved for any use by the FDA or any other regulatory agency and the safety and efficacy of apitegromab and SRK-439 have not been established. |
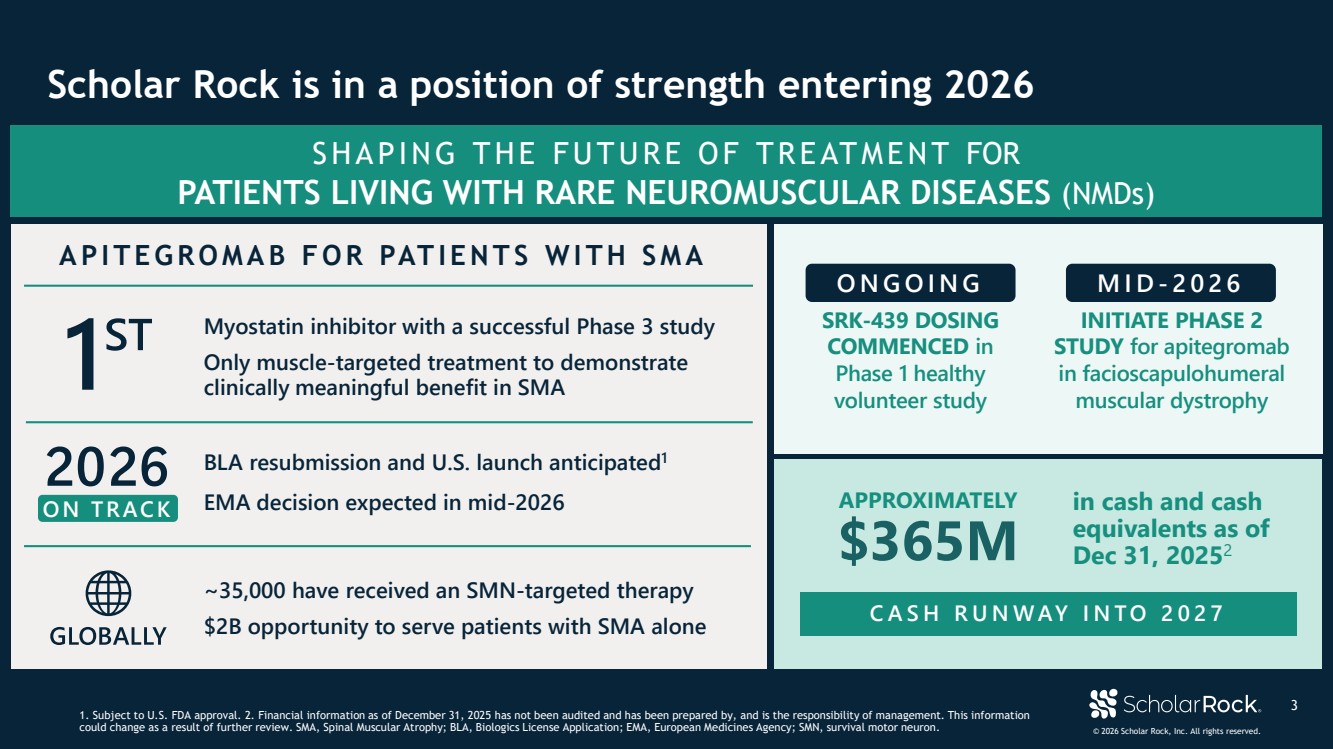
| © 2026 Scholar Rock, Inc. All rights reserved. SHAPING THE FUTURE OF TREATMENT FOR PATIENTS LIVING WITH RARE NEUROMUSCULAR DISEASES (NMDs) INITIATE PHASE 2 STUDY for apitegromab in facioscapulohumeral muscular dystrophy SRK-439 DOSING COMMENCED in Phase 1 healthy volunteer study ONGOING MID - 2026 APITEGROMAB FOR PATIENTS WITH SMA Myostatin inhibitor with a successful Phase 3 study Only muscle-targeted treatment to demonstrate clinically meaningful benefit in SMA ON TRACK BLA resubmission and U.S. launch anticipated1 EMA decision expected in mid-2026 ~35,000 have received an SMN-targeted therapy $2B opportunity to serve patients with SMA alone $365M in cash and cash equivalents as of Dec 31, 20252 CASH RUNWAY INTO 2027 APPROXIMATELY 3 Scholar Rock is in a position of strength entering 2026 1. Subject to U.S. FDA approval. 2. Financial information as of December 31, 2025 has not been audited and has been prepared by, and is the responsibility of management. This information could change as a result of further review. SMA, Spinal Muscular Atrophy; BLA, Biologics License Application; EMA, European Medicines Agency; SMN, survival motor neuron. |
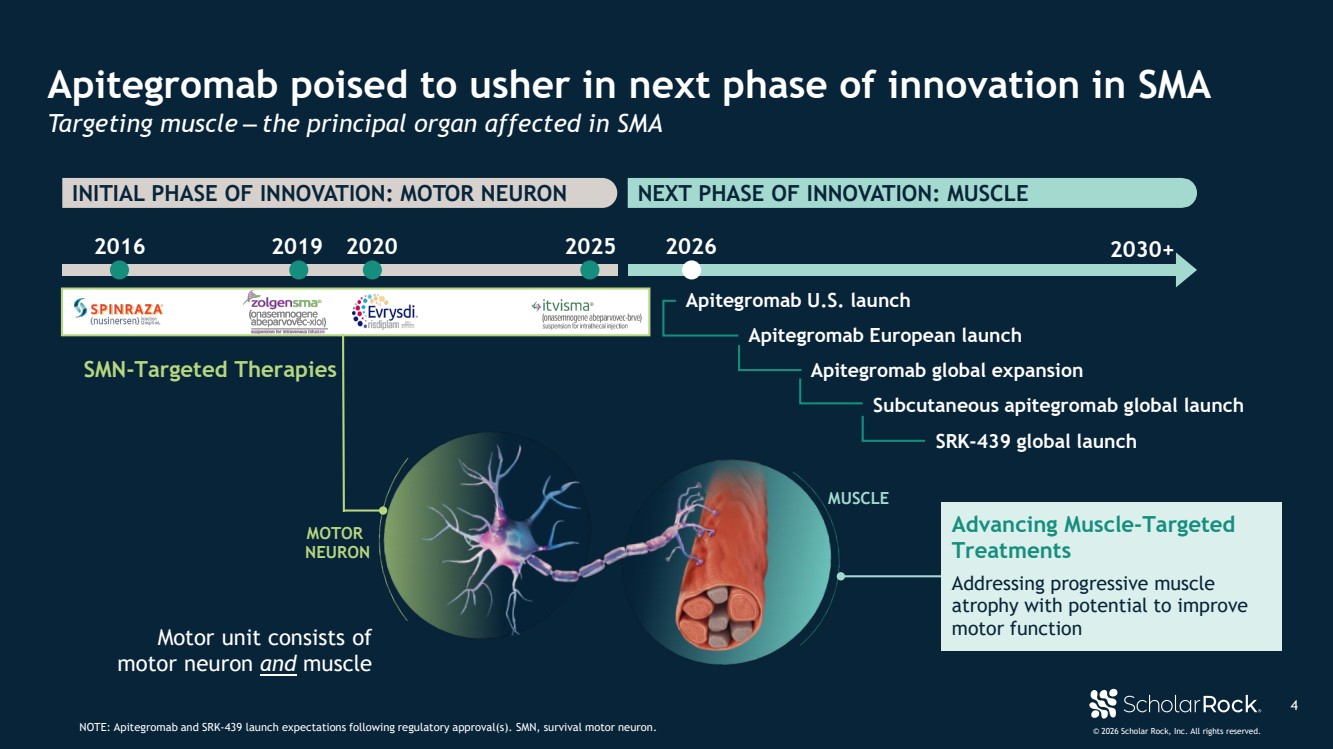
| © 2026 Scholar Rock, Inc. All rights reserved. INITIAL PHASE OF INNOVATION: MOTOR NEURON NEXT PHASE OF INNOVATION: MUSCLE 4 Apitegromab poised to usher in next phase of innovation in SMA Targeting muscle ‒ the principal organ affected in SMA NOTE: Apitegromab and SRK-439 launch expectations following regulatory approval(s). SMN, survival motor neuron. Motor unit consists of motor neuron and muscle 2016 2019 2020 2025 2026 MUSCLE MOTOR NEURON SMN-Targeted Therapies Apitegromab U.S. launch SRK-439 global launch 2030+ Advancing Muscle-Targeted Treatments Addressing progressive muscle atrophy with potential to improve motor function Subcutaneous apitegromab global launch Apitegromab European launch Apitegromab global expansion |
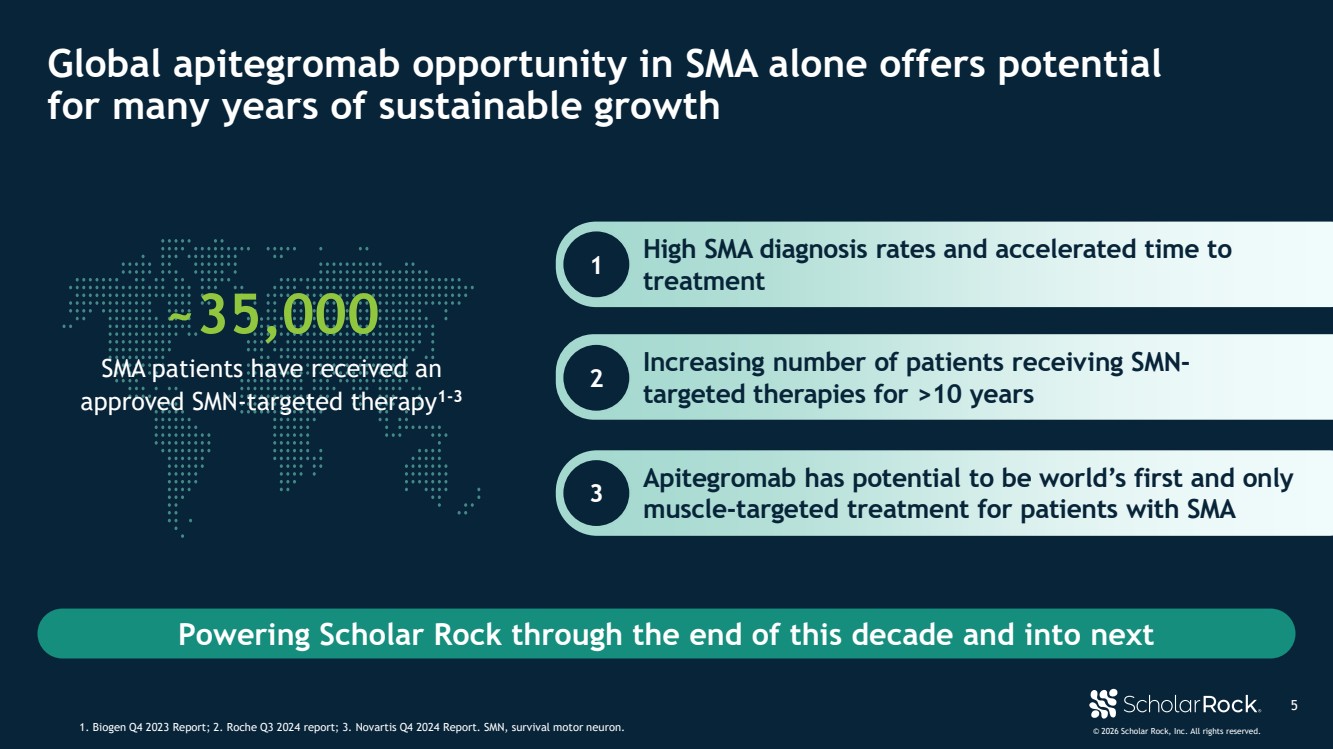
| © 2026 Scholar Rock, Inc. All rights reserved. ~35,000 SMA patients have received an approved SMN-targeted therapy1-3 Powering Scholar Rock through the end of this decade and into next High SMA diagnosis rates and accelerated time to treatment 5 Global apitegromab opportunity in SMA alone offers potential for many years of sustainable growth 1. Biogen Q4 2023 Report; 2. Roche Q3 2024 report; 3. Novartis Q4 2024 Report. SMN, survival motor neuron. 1 Increasing number of patients receiving SMN-targeted therapies for >10 years 2 Apitegromab has potential to be world’s first and only muscle-targeted treatment for patients with SMA 3 |
| © 2026 Scholar Rock, Inc. All rights reserved. 6 Poised for 2026 U.S. launch of apitegromab, followed by EMA approval and launch in Europe, beginning with Germany NOTE: Following regulatory approval(s). Building a 50-country platform to serve patients with rare, severe neuromuscular diseases United States Europe Expansion to Asia Pacific, LATAM Countries |
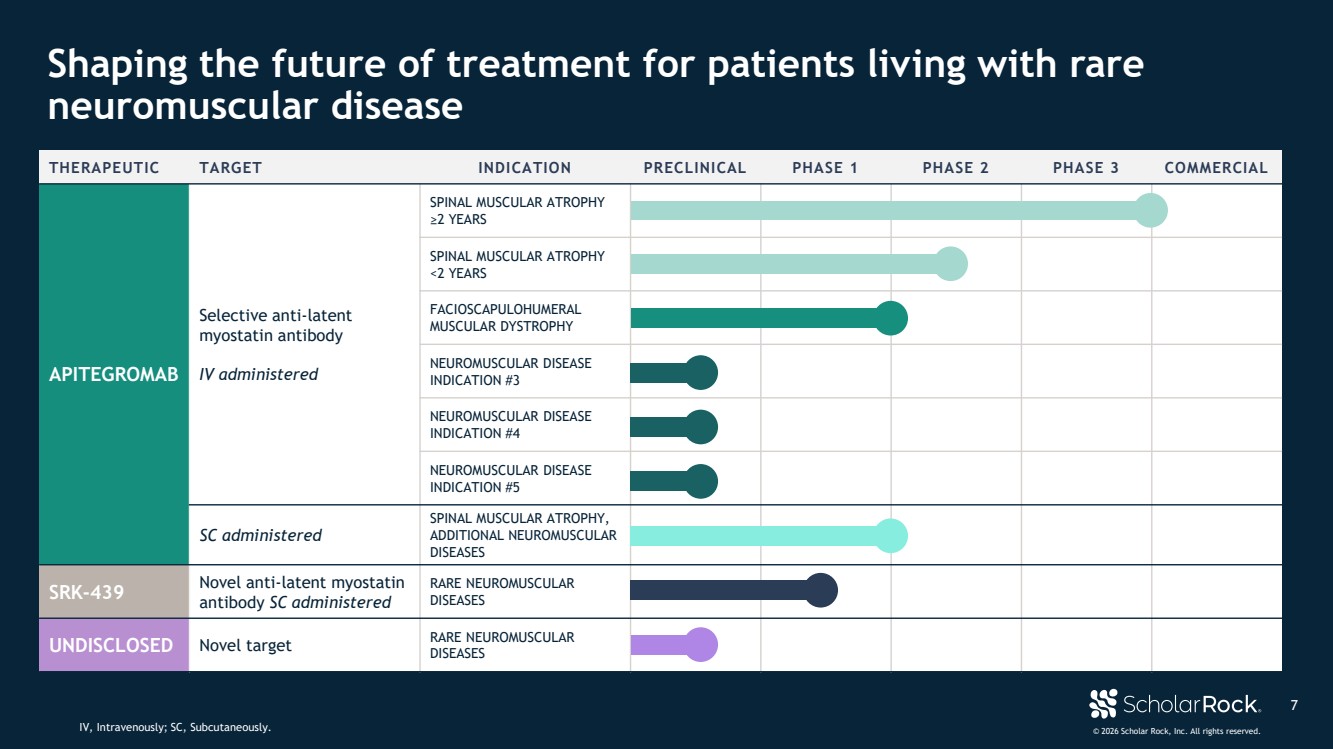
| © 2026 Scholar Rock, Inc. All rights reserved. 7 Shaping the future of treatment for patients living with rare neuromuscular disease IV, Intravenously; SC, Subcutaneously. THERAPEUTIC TARGET INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 COMMERCIAL APITEGROMAB Selective anti-latent myostatin antibody IV administered SPINAL MUSCULAR ATROPHY ≥2 YEARS SPINAL MUSCULAR ATROPHY <2 YEARS FACIOSCAPULOHUMERAL MUSCULAR DYSTROPHY NEUROMUSCULAR DISEASE INDICATION #3 NEUROMUSCULAR DISEASE INDICATION #4 NEUROMUSCULAR DISEASE INDICATION #5 SC administered SPINAL MUSCULAR ATROPHY, ADDITIONAL NEUROMUSCULAR DISEASES SRK-439 Novel anti-latent myostatin antibody SC administered RARE NEUROMUSCULAR DISEASES UNDISCLOSED Novel target RARE NEUROMUSCULAR DISEASES |
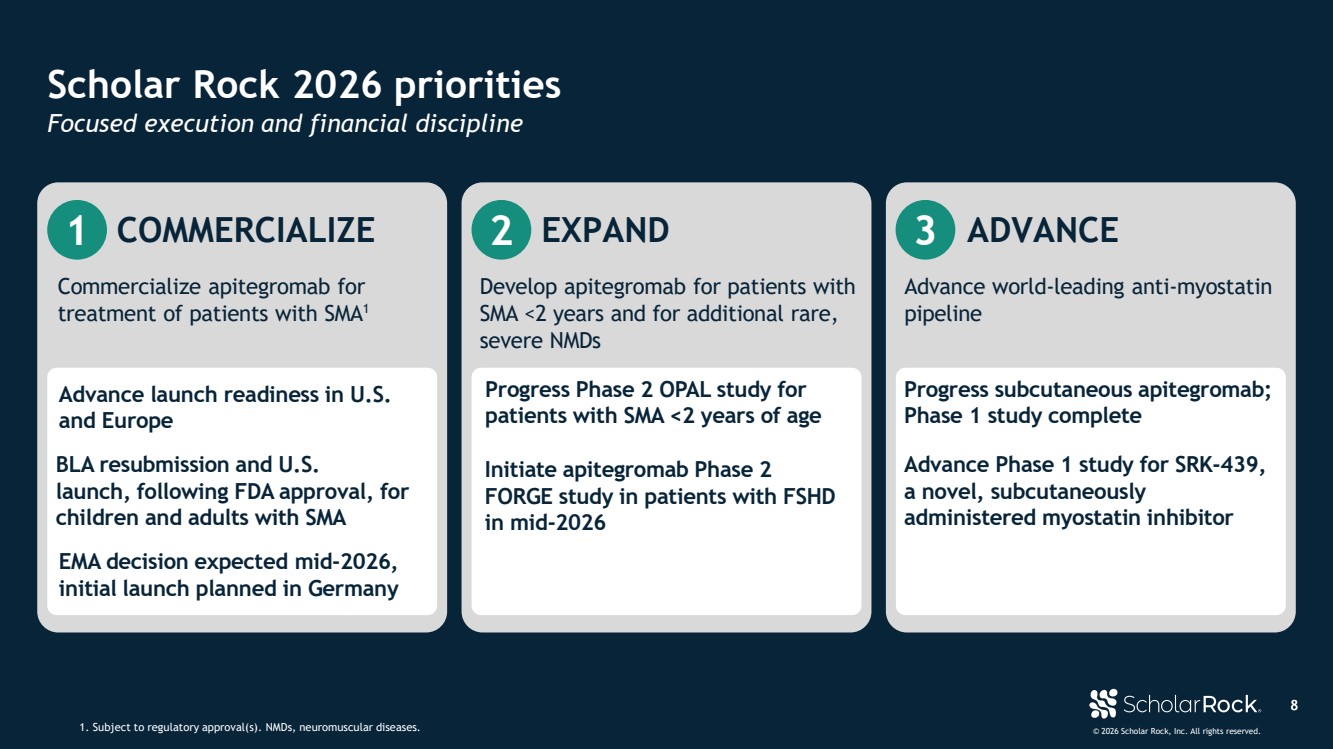
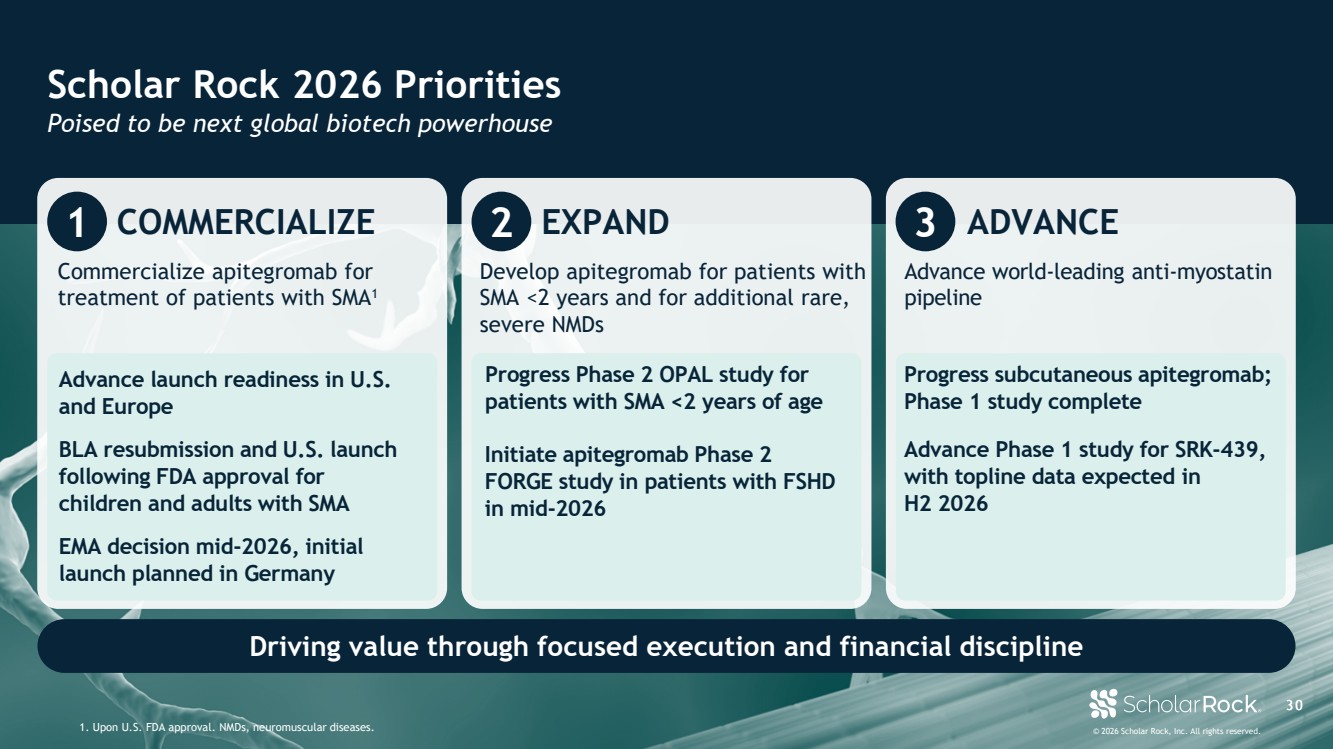
| © 2026 Scholar Rock, Inc. All rights reserved. 8 Scholar Rock 2026 priorities 1. Subject to regulatory approval(s). NMDs, neuromuscular diseases. Focused execution and financial discipline 1 COMMERCIALIZE Commercialize apitegromab for treatment of patients with SMA1 BLA resubmission and U.S. launch, following FDA approval, for children and adults with SMA Advance launch readiness in U.S. and Europe 2 EXPAND Develop apitegromab for patients with SMA <2 years and for additional rare, severe NMDs Initiate apitegromab Phase 2 FORGE study in patients with FSHD in mid-2026 Progress Phase 2 OPAL study for patients with SMA <2 years of age EMA decision expected mid-2026, initial launch planned in Germany 3 ADVANCE Advance world-leading anti-myostatin pipeline Progress subcutaneous apitegromab; Phase 1 study complete Advance Phase 1 study for SRK-439, a novel, subcutaneously administered myostatin inhibitor |
| © 2026 Scholar Rock, Inc. All rights reserved. Commercialize Apitegromab for Treatment of Patients with SMA Transforming the treatment of SMA with muscle-targeted therapy 9 |
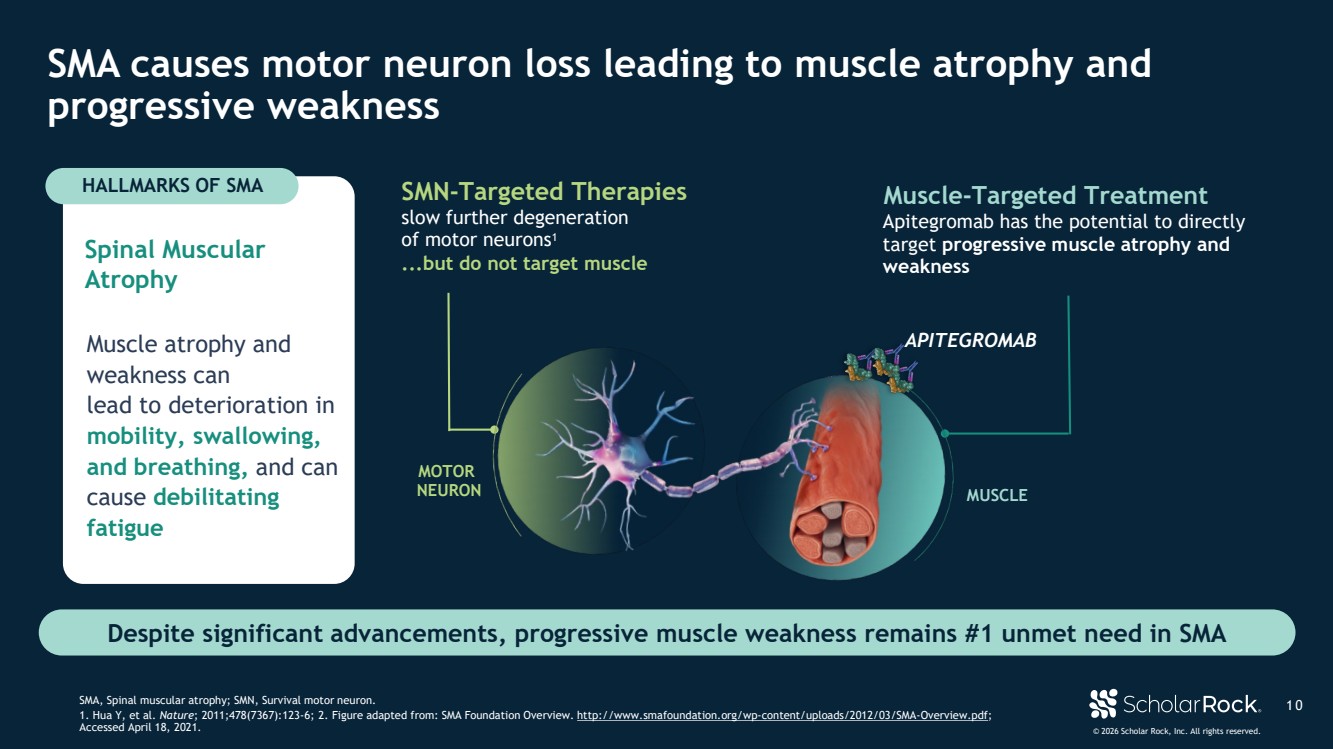
| © 2026 Scholar Rock, Inc. All rights reserved. ...but do not target muscle SMN-Targeted Therapies slow further degeneration of motor neurons1 Apitegromab has the potential to directly target progressive muscle atrophy and weakness Muscle-Targeted Treatment Spinal Muscular Atrophy Muscle atrophy and weakness can lead to deterioration in mobility, swallowing, and breathing, and can cause debilitating fatigue 1 0 SMA causes motor neuron loss leading to muscle atrophy and progressive weakness SMA, Spinal muscular atrophy; SMN, Survival motor neuron. 1. Hua Y, et al. Nature; 2011;478(7367):123-6; 2. Figure adapted from: SMA Foundation Overview. http://www.smafoundation.org/wp-content/uploads/2012/03/SMA-Overview.pdf; Accessed April 18, 2021. Despite significant advancements, progressive muscle weakness remains #1 unmet need in SMA HALLMARKS OF SMA MUSCLE MOTOR NEURON APITEGROMAB |
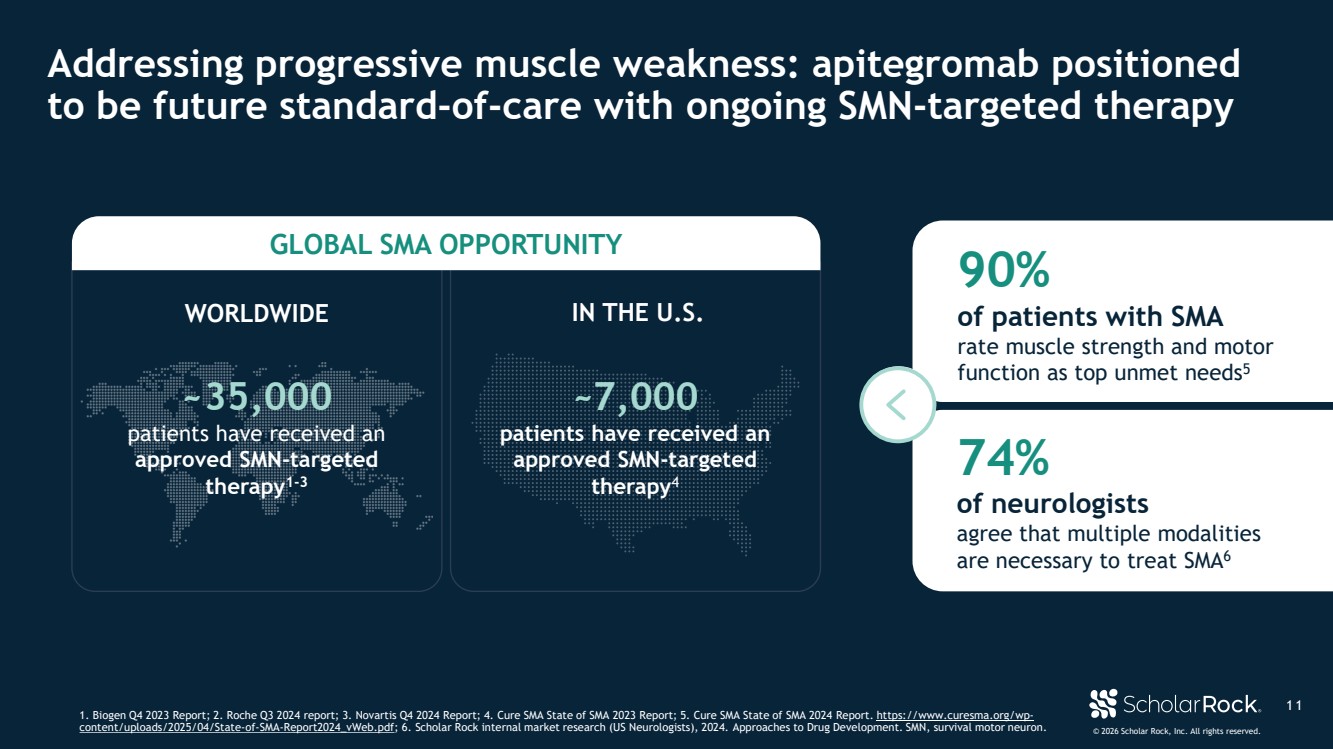
| © 2026 Scholar Rock, Inc. All rights reserved. ~35,000 patients have received an approved SMN-targeted therapy1-3 ~7,000 patients have received an approved SMN-targeted therapy4 GLOBAL SMA OPPORTUNITY 90% of patients with SMA rate muscle strength and motor function as top unmet needs5 1 1 Addressing progressive muscle weakness: apitegromab positioned to be future standard-of-care with ongoing SMN-targeted therapy 1. Biogen Q4 2023 Report; 2. Roche Q3 2024 report; 3. Novartis Q4 2024 Report; 4. Cure SMA State of SMA 2023 Report; 5. Cure SMA State of SMA 2024 Report. https://www.curesma.org/wp-content/uploads/2025/04/State-of-SMA-Report2024_vWeb.pdf; 6. Scholar Rock internal market research (US Neurologists), 2024. Approaches to Drug Development. SMN, survival motor neuron. WORLDWIDE IN THE U.S. 74% of neurologists agree that multiple modalities are necessary to treat SMA6 |
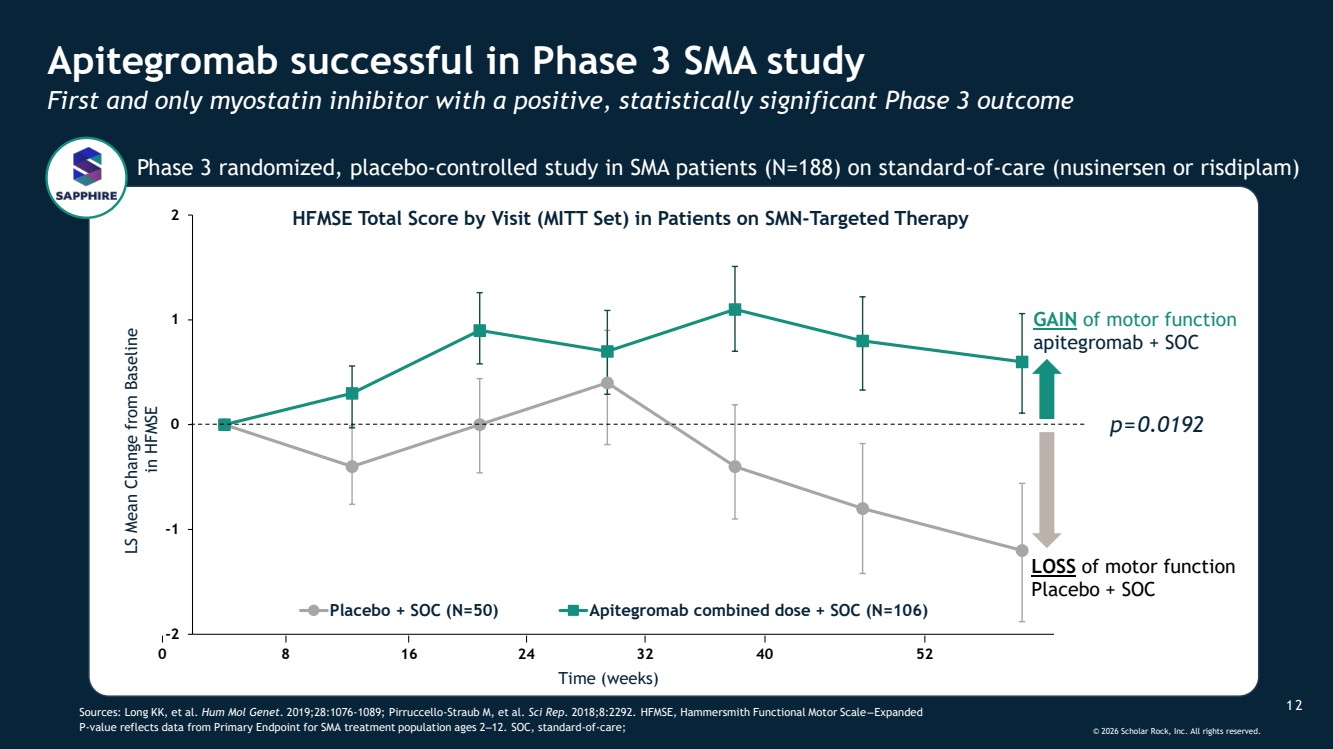
| © 2026 Scholar Rock, Inc. All rights reserved. 1 2 Apitegromab successful in Phase 3 SMA study First and only myostatin inhibitor with a positive, statistically significant Phase 3 outcome Sources: Long KK, et al. Hum Mol Genet. 2019;28:1076-1089; Pirruccello-Straub M, et al. Sci Rep. 2018;8:2292. HFMSE, Hammersmith Functional Motor Scale ‒ Expanded P-value reflects data from Primary Endpoint for SMA treatment population ages 2‒12. SOC, standard-of-care; GAIN of motor function apitegromab + SOC -2 -1 0 1 2 Placebo + SOC (N=50) Apitegromab combined dose + SOC (N=106) 0 8 16 24 32 40 Time (weeks) 52 LS Mean Change from Baseline in HFMSE LOSS of motor function Placebo + SOC HFMSE Total Score by Visit (MITT Set) in Patients on SMN-Targeted Therapy p=0.0192 Phase 3 randomized, placebo-controlled study in SMA patients (N=188) on standard-of-care (nusinersen or risdiplam) GAIN of motor function apitegromab + SOC |
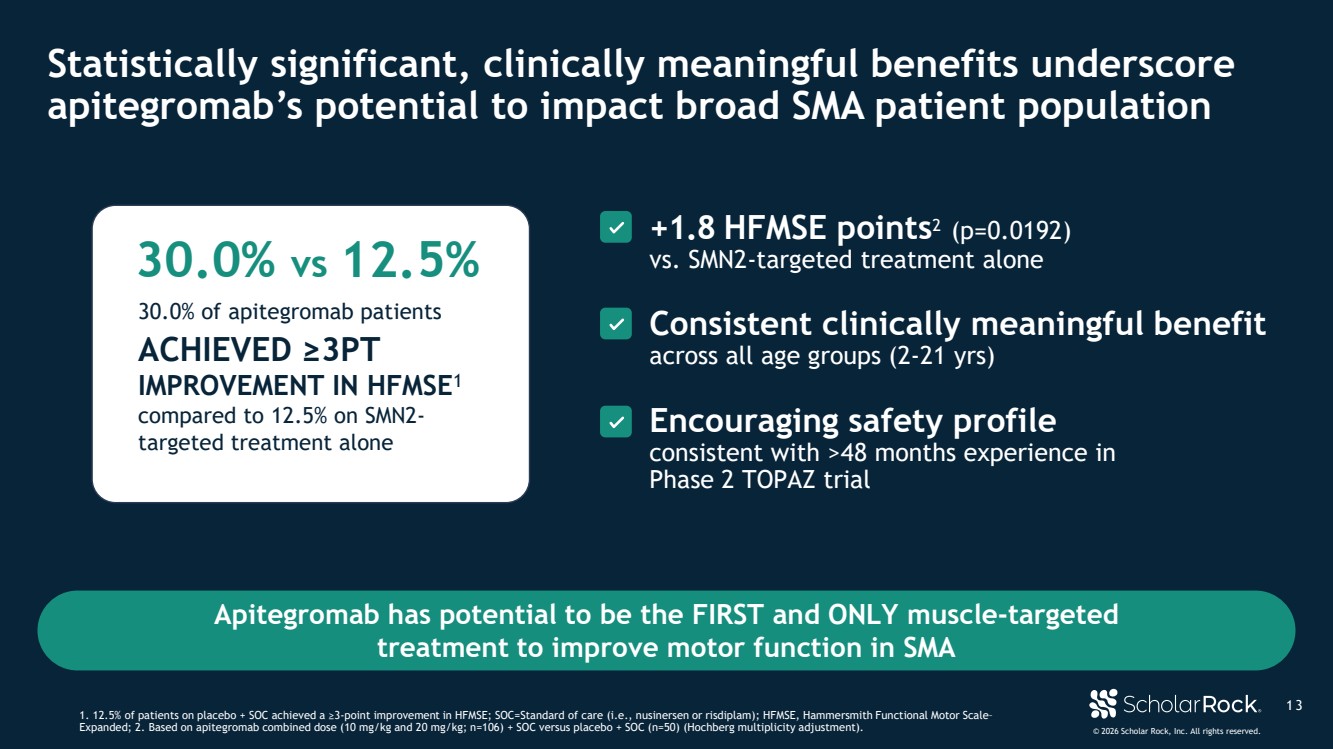
| © 2026 Scholar Rock, Inc. All rights reserved. Apitegromab has potential to be the FIRST and ONLY muscle-targeted treatment to improve motor function in SMA 30.0% vs 12.5% 30.0% of apitegromab patients ACHIEVED ≥3PT IMPROVEMENT IN HFMSE1 compared to 12.5% on SMN2- targeted treatment alone +1.8 HFMSE points2 (p=0.0192) vs. SMN2-targeted treatment alone Consistent clinically meaningful benefit across all age groups (2-21 yrs) Encouraging safety profile consistent with >48 months experience in Phase 2 TOPAZ trial 1 3 Statistically significant, clinically meaningful benefits underscore apitegromab’s potential to impact broad SMA patient population 1. 12.5% of patients on placebo + SOC achieved a ≥3-point improvement in HFMSE; SOC=Standard of care (i.e., nusinersen or risdiplam); HFMSE, Hammersmith Functional Motor Scale– Expanded; 2. Based on apitegromab combined dose (10 mg/kg and 20 mg/kg; n=106) + SOC versus placebo + SOC (n=50) (Hochberg multiplicity adjustment). |
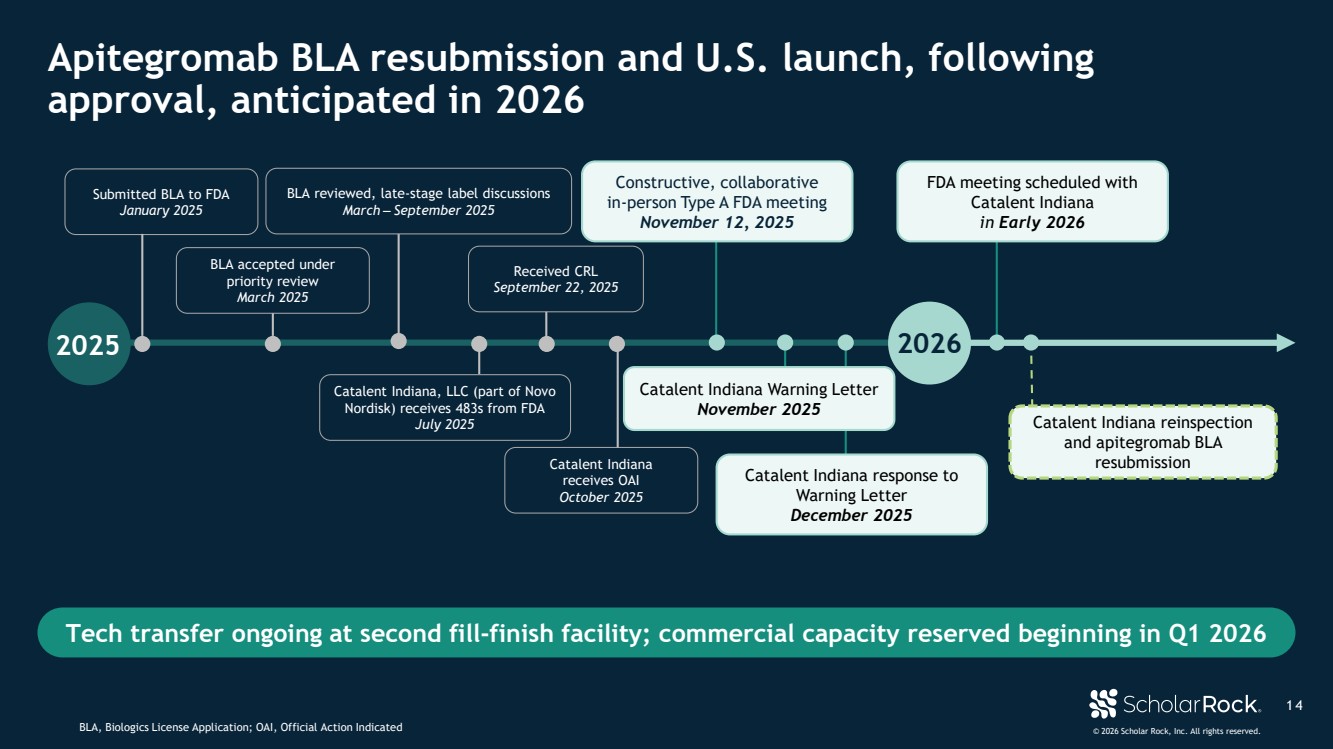
| © 2026 Scholar Rock, Inc. All rights reserved. 1 4 Apitegromab BLA resubmission and U.S. launch, following approval, anticipated in 2026 BLA, Biologics License Application; OAI, Official Action Indicated Tech transfer ongoing at second fill-finish facility; commercial capacity reserved beginning in Q1 2026 Submitted BLA to FDA January 2025 Catalent Indiana receives OAI October 2025 2025 2026 BLA accepted under priority review March 2025 Catalent Indiana, LLC (part of Novo Nordisk) receives 483s from FDA July 2025 Received CRL September 22, 2025 Catalent Indiana Warning Letter November 2025 FDA meeting scheduled with Catalent Indiana in Early 2026 Constructive, collaborative in-person Type A FDA meeting November 12, 2025 Catalent Indiana response to Warning Letter December 2025 BLA reviewed, late-stage label discussions March ‒ September 2025 Catalent Indiana reinspection and apitegromab BLA resubmission |
| © 2026 Scholar Rock, Inc. All rights reserved. >2,600 SMA Prescribers Prescribers and SMA Treatment Centers 1 5 U.S. commercial field team focused on engagement, disease education across SMA stakeholder landscape Broaden and deepen engagement Patient Advocacy National & Regional Payers Educating on unmet need and potential benefit of apitegromab Market access team engaging with national and regional payers Building lasting relationships one patient, one caregiver, one family at a time Strong collaboration with advocacy groups 140 SMA Centers Understand patient journey and roles of treatment team |
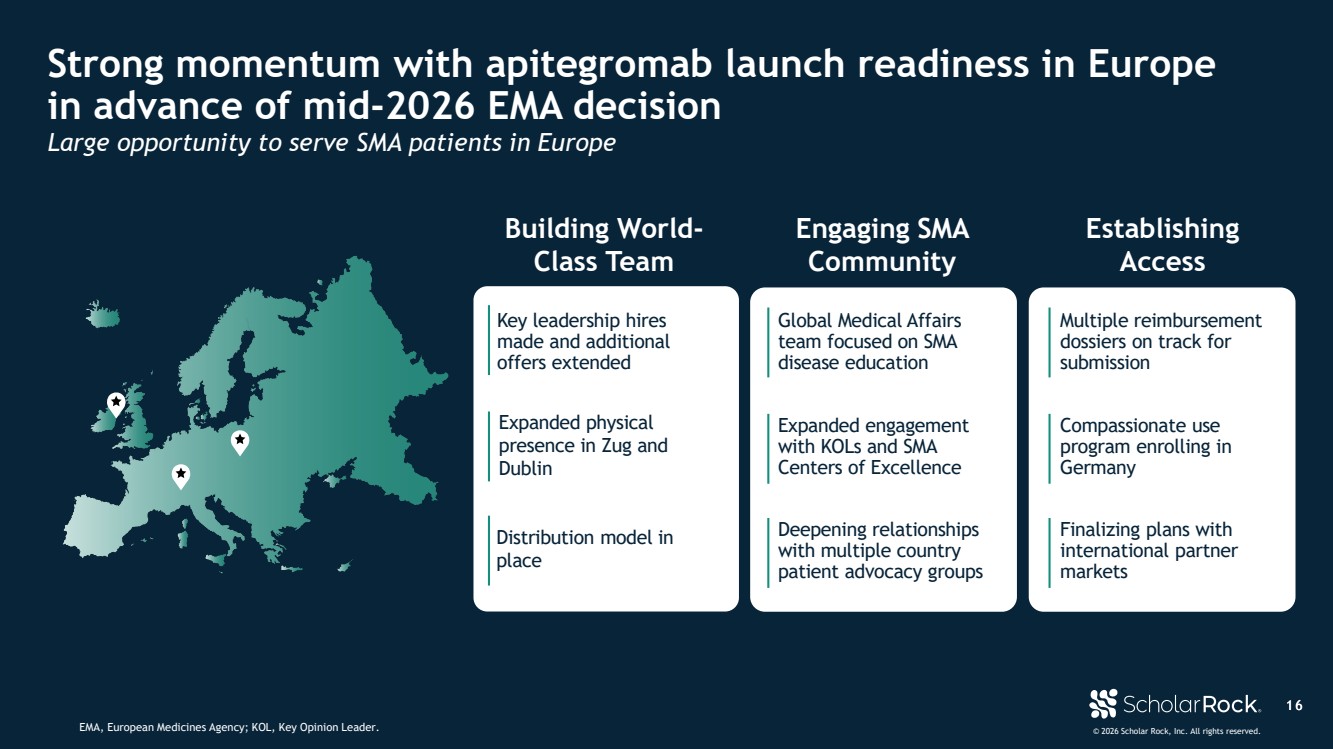
| © 2026 Scholar Rock, Inc. All rights reserved. 1 6 Strong momentum with apitegromab launch readiness in Europe in advance of mid-2026 EMA decision EMA, European Medicines Agency; KOL, Key Opinion Leader. Large opportunity to serve SMA patients in Europe Key leadership hires made and additional offers extended Distribution model in place Expanded physical presence in Zug and Dublin Building World-Class Team Engaging SMA Community Expanded engagement with KOLs and SMA Centers of Excellence Deepening relationships with multiple country patient advocacy groups Global Medical Affairs team focused on SMA disease education Establishing Access Multiple reimbursement dossiers on track for submission Compassionate use program enrolling in Germany Finalizing plans with international partner markets |
| © 2026 Scholar Rock, Inc. All rights reserved. Apitegromab in SMA represents a large global opportunity to serve patients 1. Revenue as of Biogen 3Q25 financial update, Roche 3Q25 financial update, and Novartis 3Q25 financial update; 2. Scholar Rock internal estimates as of December 2025. SMN, survival motor neuron; SMA, spinal muscular atrophy. SMA community demanding the first and only muscle-targeted therapy ~$5B1 muscle-targeted treatment to show clinical benefit in SMA Apitegromab global revenue potential $2B+2 Estimated 2025 global revenue for SMN-targeted therapies 1st & Only 1 7 |
| © 2026 Scholar Rock, Inc. All rights reserved. Develop Apitegromab for Patients with SMA Under 2 and for Additional Neuromuscular Diseases (NMDs) Building a pipeline -in - a -product to reach more patients with SMA and with additional rare, severe NMDs 1 8 |
| © 2026 Scholar Rock, Inc. All rights reserved. Evaluating PK, PD, efficacy, safety, and tolerability of apitegromab over 48 weeks 1 9 Furthering our commitment to broad SMA community Ongoing Phase 2 OPAL study evaluating apitegromab in infants and toddlers with SMA PK, Pharmacokinetic, PD, Pharmacodynamic. Patient enrollment and dosing underway in Phase 2 OPAL study including evaluation of apitegromab in patients who received SMN1-targeted gene therapy Expanding our potential impact to reach patients earlier Addressing the needs of children under 2 years of age with SMA seeking to address the motor neuron and muscle in youngest patients Time is muscle |
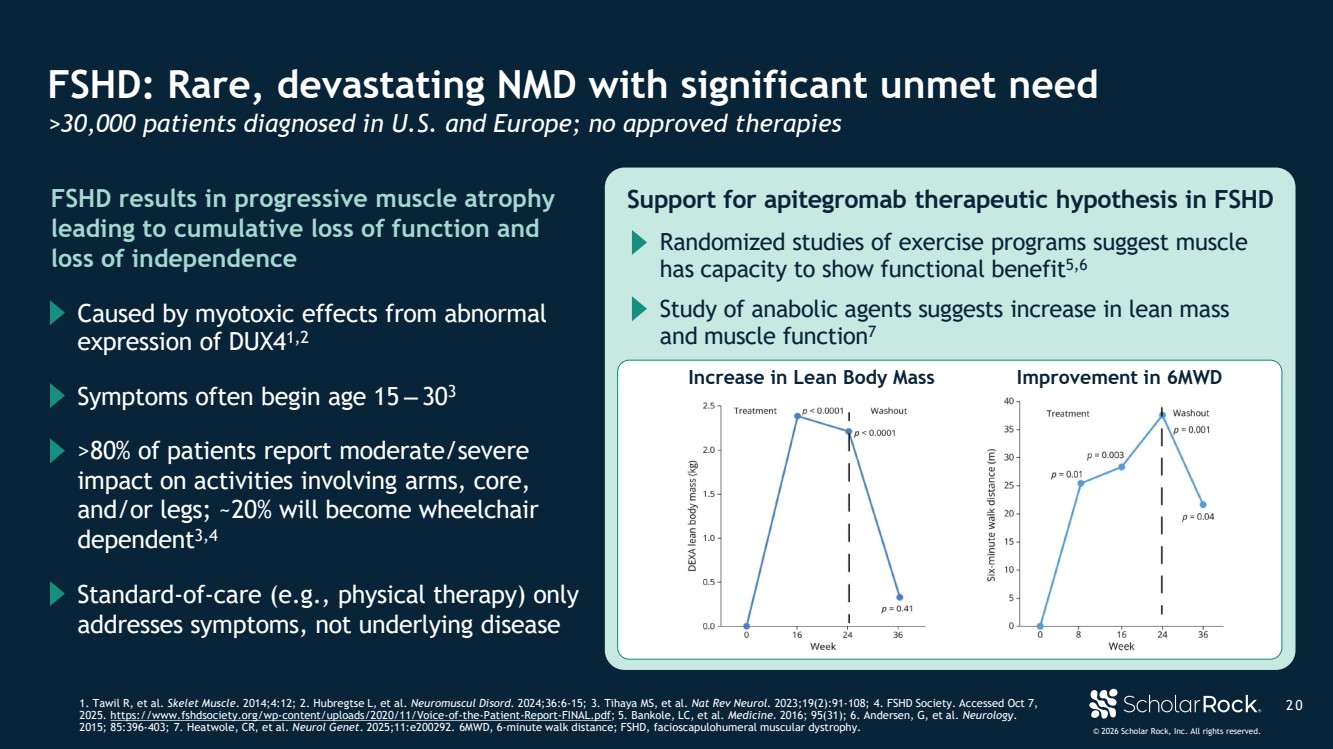
| © 2026 Scholar Rock, Inc. All rights reserved. 2 0 FSHD: Rare, devastating NMD with significant unmet need >30,000 patients diagnosed in U.S. and Europe; no approved therapies 1. Tawil R, et al. Skelet Muscle. 2014;4:12; 2. Hubregtse L, et al. Neuromuscul Disord. 2024;36:6-15; 3. Tihaya MS, et al. Nat Rev Neurol. 2023;19(2):91-108; 4. FSHD Society. Accessed Oct 7, 2025. https://www.fshdsociety.org/wp-content/uploads/2020/11/Voice-of-the-Patient-Report-FINAL.pdf; 5. Bankole, LC, et al. Medicine. 2016; 95(31); 6. Andersen, G, et al. Neurology. 2015; 85:396-403; 7. Heatwole, CR, et al. Neurol Genet. 2025;11:e200292. 6MWD, 6-minute walk distance; FSHD, facioscapulohumeral muscular dystrophy. FSHD results in progressive muscle atrophy leading to cumulative loss of function and loss of independence Symptoms often begin age 15 ‒ 303 Caused by myotoxic effects from abnormal expression of DUX41,2 Standard-of-care (e.g., physical therapy) only addresses symptoms, not underlying disease >80% of patients report moderate/severe impact on activities involving arms, core, and/or legs; ~20% will become wheelchair dependent3,4 Support for apitegromab therapeutic hypothesis in FSHD Randomized studies of exercise programs suggest muscle has capacity to show functional benefit5,6 Study of anabolic agents suggests increase in lean mass and muscle function7 Increase in Lean Body Mass Improvement in 6MWD |
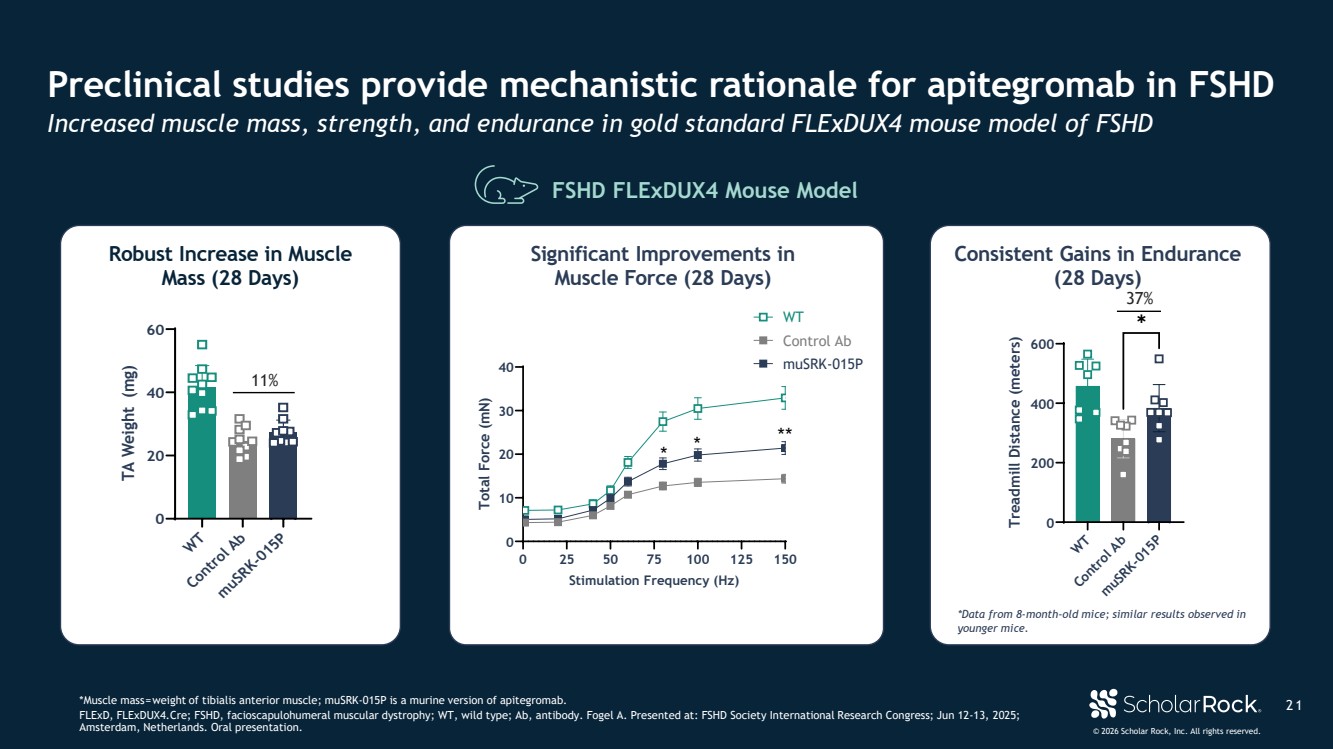
| © 2026 Scholar Rock, Inc. All rights reserved. 2 1 Preclinical studies provide mechanistic rationale for apitegromab in FSHD Increased muscle mass, strength, and endurance in gold standard FLExDUX4 mouse model of FSHD *Muscle mass=weight of tibialis anterior muscle; muSRK-015P is a murine version of apitegromab. FLExD, FLExDUX4.Cre; FSHD, facioscapulohumeral muscular dystrophy; WT, wild type; Ab, antibody. Fogel A. Presented at: FSHD Society International Research Congress; Jun 12-13, 2025; Amsterdam, Netherlands. Oral presentation. FSHD FLExDUX4 Mouse Model Robust Increase in Muscle Mass (28 Days) WT Control Ab muSRK-015P 0 20 40 60 TA Weight (mg) 11% Significant Improvements in Muscle Force (28 Days) 0 25 50 75 100 125 150 0 10 20 30 40 Stimulation Frequency (Hz) Total Force (mN) Control Ab muSRK-015P * * ** WT Consistent Gains in Endurance (28 Days) WT Control Ab muSRK-015P 0 200 400 600 Treadmill Distance (meters) ✱ *Data from 8-month-old mice; similar results observed in younger mice. 37% |
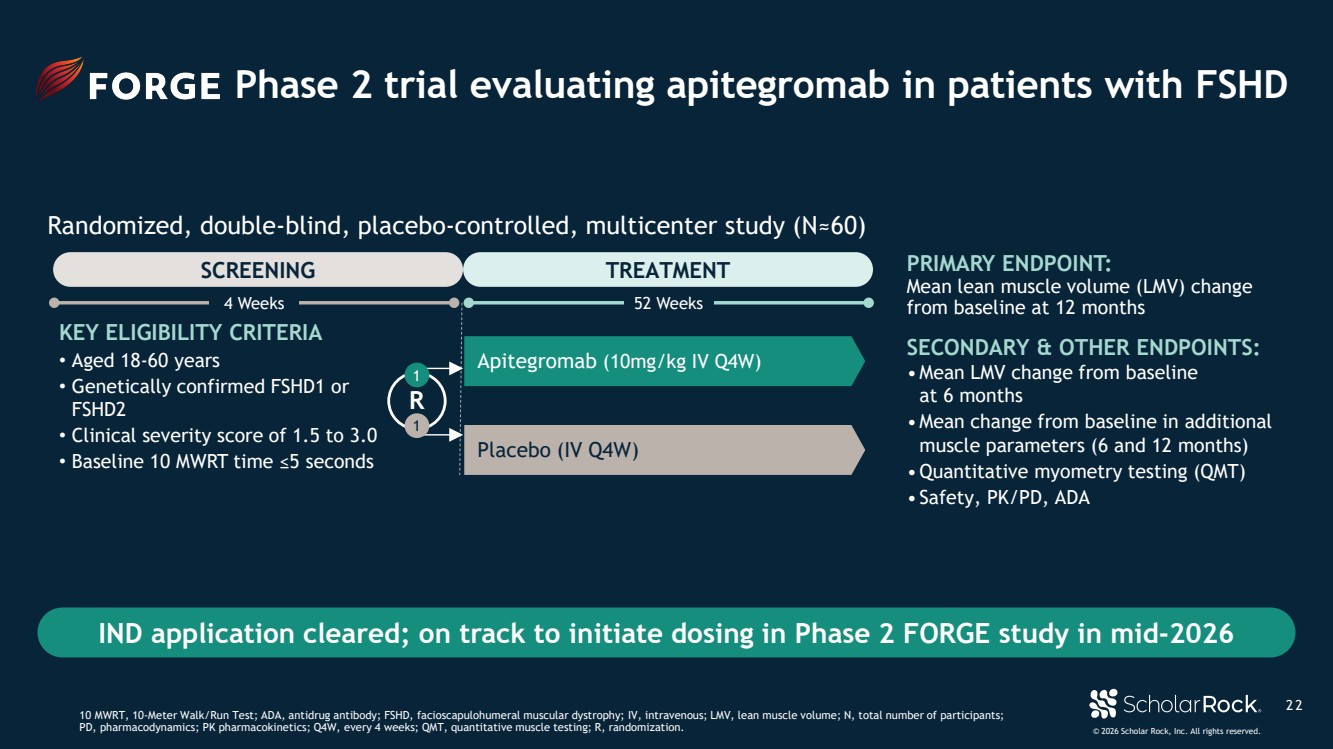
| © 2026 Scholar Rock, Inc. All rights reserved. KEY ELIGIBILITY CRITERIA • Aged 18-60 years • Genetically confirmed FSHD1 or FSHD2 • Clinical severity score of 1.5 to 3.0 • Baseline 10 MWRT time ≤5 seconds Apitegromab (10mg/kg IV Q4W) Placebo (IV Q4W) PRIMARY ENDPOINT: Mean lean muscle volume (LMV) change from baseline at 12 months SECONDARY & OTHER ENDPOINTS: •Mean LMV change from baseline at 6 months •Mean change from baseline in additional muscle parameters (6 and 12 months) •Quantitative myometry testing (QMT) • Safety, PK/PD, ADA R 1 1 2 2 Phase 2 trial evaluating apitegromab in patients with FSHD 10 MWRT, 10-Meter Walk/Run Test; ADA, antidrug antibody; FSHD, facioscapulohumeral muscular dystrophy; IV, intravenous; LMV, lean muscle volume; N, total number of participants; PD, pharmacodynamics; PK pharmacokinetics; Q4W, every 4 weeks; QMT, quantitative muscle testing; R, randomization. SCREENING TREATMENT IND application cleared; on track to initiate dosing in Phase 2 FORGE study in mid-2026 4 Weeks 52 Weeks Randomized, double-blind, placebo-controlled, multicenter study (N≈60) |
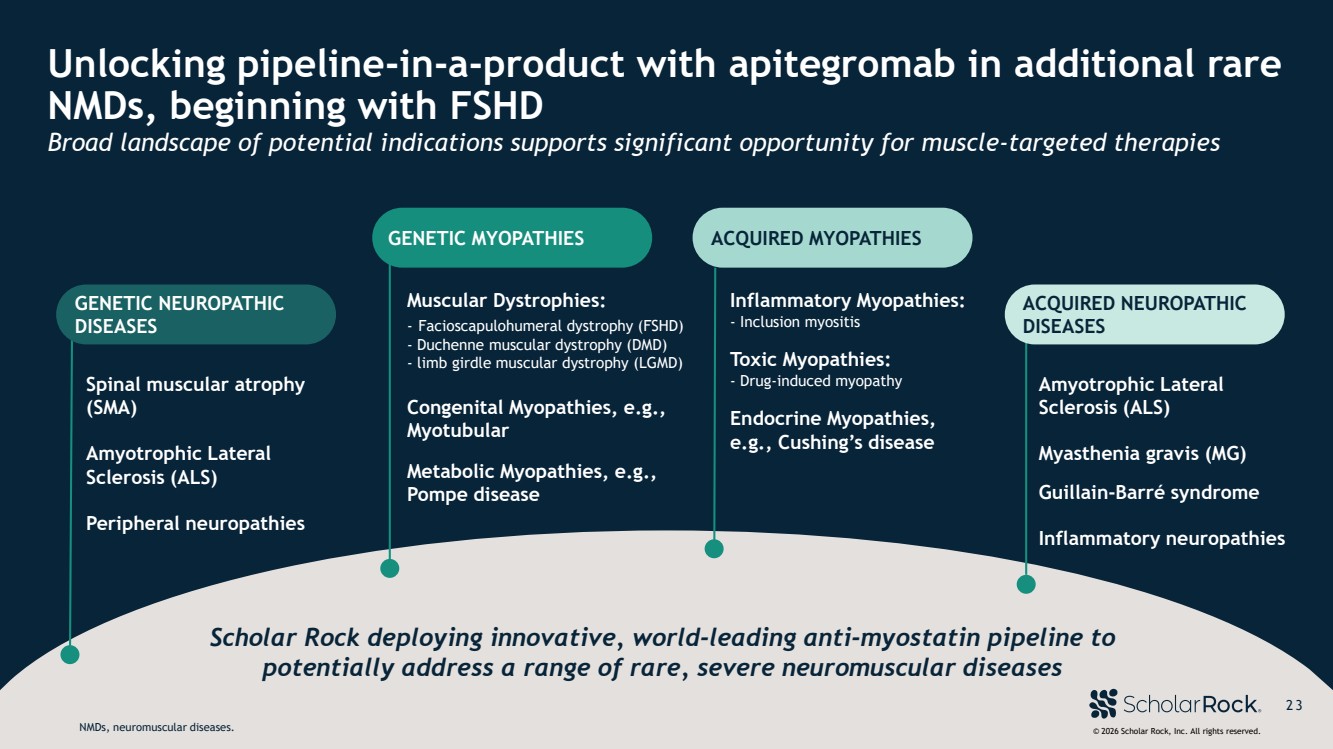
| © 2026 Scholar Rock, Inc. All rights reserved. 2 3 Unlocking pipeline-in-a-product with apitegromab in additional rare NMDs, beginning with FSHD Broad landscape of potential indications supports significant opportunity for muscle-targeted therapies Scholar Rock deploying innovative, world-leading anti-myostatin pipeline to potentially address a range of rare, severe neuromuscular diseases Spinal muscular atrophy (SMA) Amyotrophic Lateral Sclerosis (ALS) Peripheral neuropathies GENETIC MYOPATHIES Muscular Dystrophies: - Facioscapulohumeral dystrophy (FSHD) - Duchenne muscular dystrophy (DMD) - limb girdle muscular dystrophy (LGMD) Congenital Myopathies, e.g., Myotubular Metabolic Myopathies, e.g., Pompe disease ACQUIRED MYOPATHIES Inflammatory Myopathies: - Inclusion myositis Toxic Myopathies: - Drug-induced myopathy Endocrine Myopathies, e.g., Cushing’s disease Amyotrophic Lateral Sclerosis (ALS) Myasthenia gravis (MG) Guillain-Barré syndrome Inflammatory neuropathies ACQUIRED NEUROPATHIC DISEASES © 2026 Scholar Rock, Inc. All rights reserved. 2 3 GENETIC NEUROPATHIC DISEASES NMDs, neuromuscular diseases. |
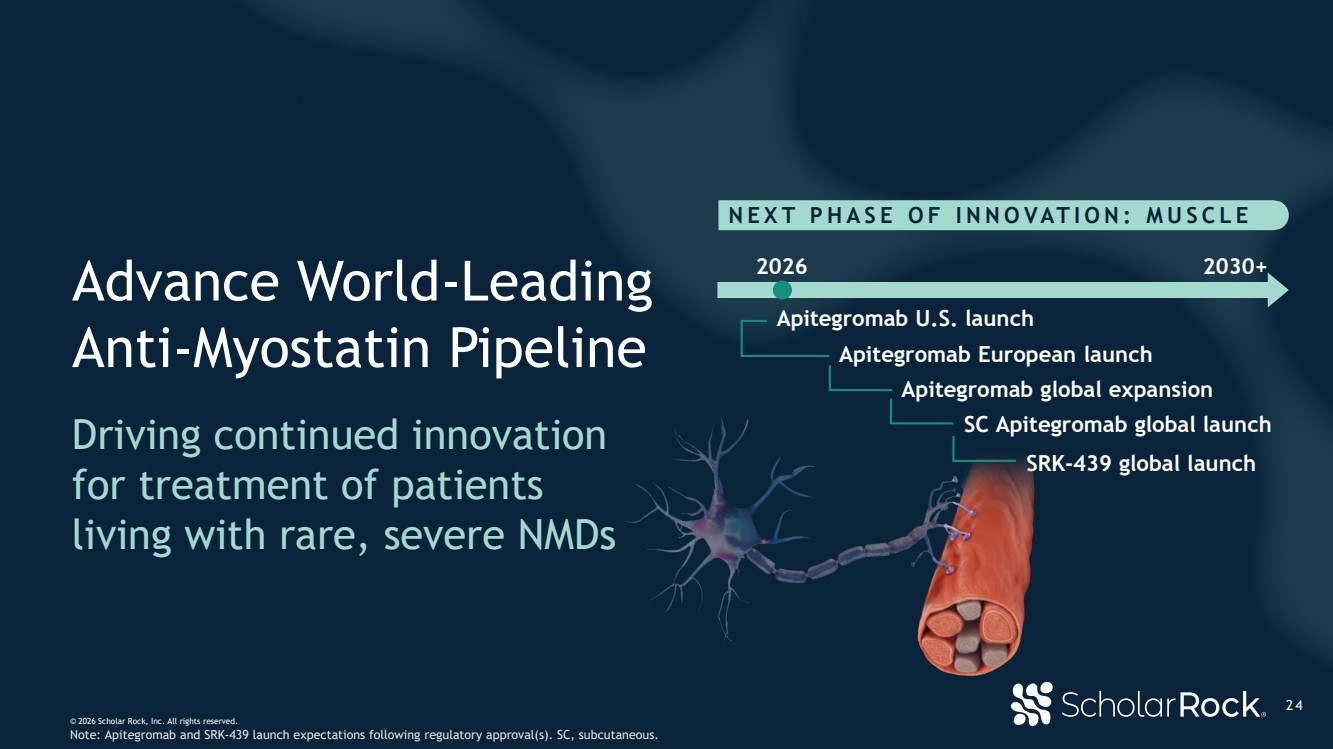
| © 2026 Scholar Rock, Inc. All rights reserved. 2 4 NEXT PHASE OF INNOVATION: MUSCLE 2026 Apitegromab U.S. launch SRK-439 global launch 2030+ SC Apitegromab global launch Apitegromab European launch Apitegromab global expansion Advance World-Leading Anti-Myostatin Pipeline Driving continued innovation for treatment of patients living with rare, severe NMDs Note: Apitegromab and SRK-439 launch expectations following regulatory approval(s). SC, subcutaneous. |
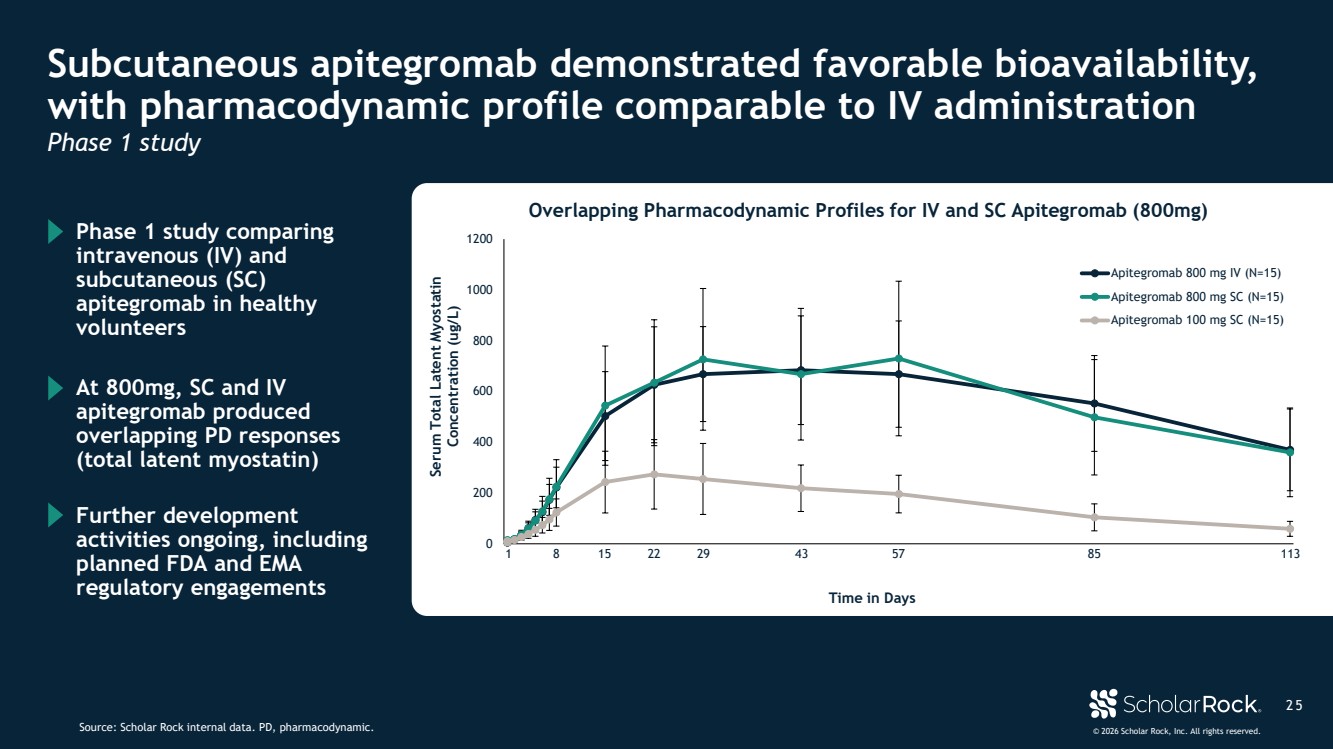
| © 2026 Scholar Rock, Inc. All rights reserved. 2 5 Subcutaneous apitegromab demonstrated favorable bioavailability, with pharmacodynamic profile comparable to IV administration Source: Scholar Rock internal data. PD, pharmacodynamic. Phase 1 study comparing intravenous (IV) and subcutaneous (SC) apitegromab in healthy volunteers Further development activities ongoing, including planned FDA and EMA regulatory engagements At 800mg, SC and IV apitegromab produced overlapping PD responses (total latent myostatin) Phase 1 study 0 200 400 600 800 1000 1200 1 3 5 7 9 1113151719212325272931333537394143454749515355575961636567697173757779818385878991939597991010310510710911113 Apitegromab 800 mg IV (N=15) Apitegromab 800 mg SC (N=15) Apitegromab 100 mg SC (N=15) Serum Total Latent Myostatin Concentration (ug/L) 1 8 15 22 29 43 57 85 113 Time in Days Overlapping Pharmacodynamic Profiles for IV and SC Apitegromab (800mg) |
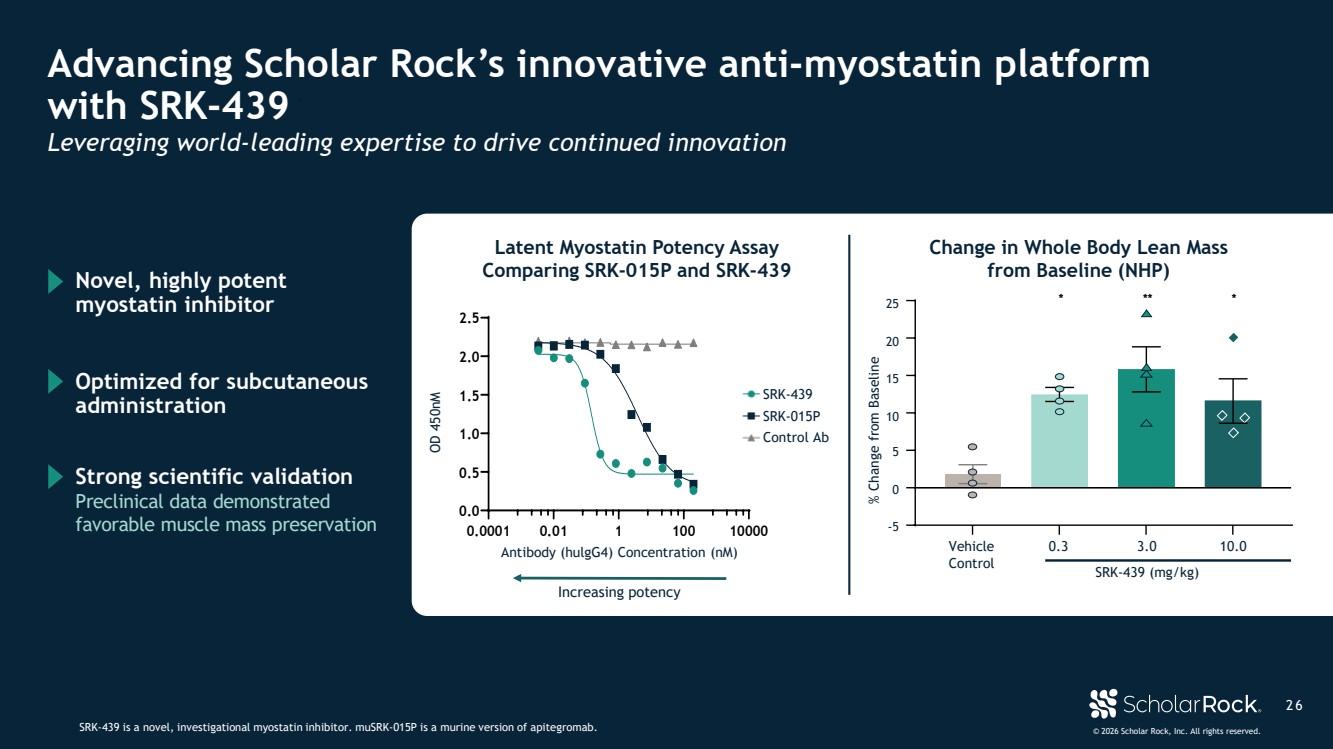
| © 2026 Scholar Rock, Inc. All rights reserved. 2 6 Advancing Scholar Rock’s innovative anti-myostatin platform with SRK-439 SRK-439 is a novel, investigational myostatin inhibitor. muSRK-015P is a murine version of apitegromab. Leveraging world-leading expertise to drive continued innovation 0.0001 0.01 1 100 10000 0.0 0.5 1.0 1.5 2.0 2.5 Antibody (huIgG4) Concentration (nM) OD 450nM SRK-439 SRK-015P Isotype Control Inhibition of Tolloid Cleavage of Latent Myostatin by SRK-015P and SRK-439 Change in Whole Body Lean Mass from Baseline (NHP) OD 450nM Antibody (hulgG4) Concentration (nM) Latent Myostatin Potency Assay Comparing SRK-015P and SRK-439 * ** * Vehicle Control 0.3 3.0 10.0 SRK-439 (mg/kg) -5 0 5 10 15 20 25 % Change from Baseline % Change from Baseline SRK-439 SRK-015P Control Ab Novel, highly potent myostatin inhibitor Optimized for subcutaneous administration Preclinical data demonstrated favorable muscle mass preservation Strong scientific validation Increasing potency |
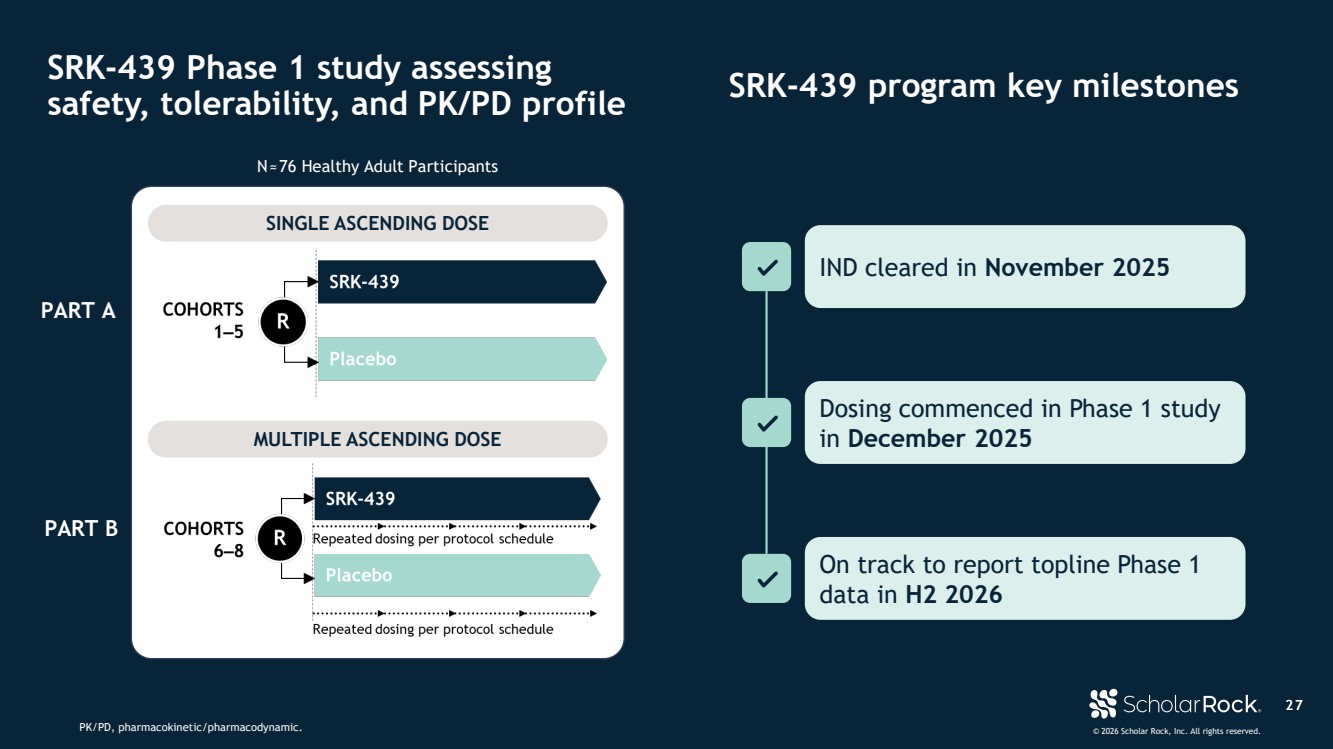
| © 2026 Scholar Rock, Inc. All rights reserved. N≈76 Healthy Adult Participants PART A PART B 2 7 SRK-439 Phase 1 study assessing safety, tolerability, and PK/PD profile SRK-439 program key milestones 2 7 IND cleared in November 2025 Dosing commenced in Phase 1 study in December 2025 On track to report topline Phase 1 data in H2 2026 SINGLE ASCENDING DOSE MULTIPLE ASCENDING DOSE PK/PD, pharmacokinetic/pharmacodynamic. |
| © 2026 Scholar Rock, Inc. All rights reserved. Financials & Upcoming Milestones 2 8 |
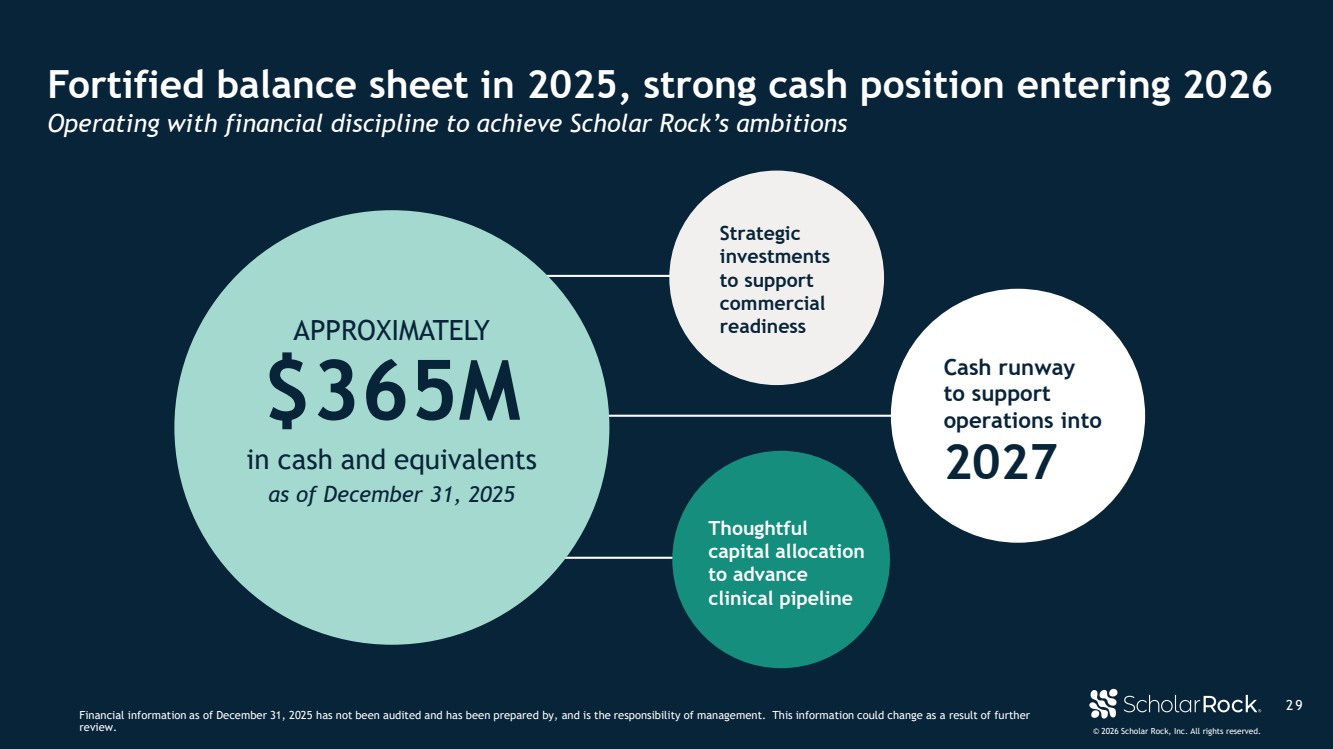
| © 2026 Scholar Rock, Inc. All rights reserved. 2 9 Fortified balance sheet in 2025, strong cash position entering 2026 Operating with financial discipline to achieve Scholar Rock’s ambitions Financial information as of December 31, 2025 has not been audited and has been prepared by, and is the responsibility of management. This information could change as a result of further review. Thoughtful capital allocation to advance clinical pipeline Strategic investments to support commercial readiness Cash runway to support operations into 2027 as of December 31, 2025 $365M in cash and equivalents APPROXIMATELY |
| © 2026 Scholar Rock, Inc. All rights reserved. 3 0 Scholar Rock 2026 Priorities Poised to be next global biotech powerhouse 1. Upon U.S. FDA approval. NMDs, neuromuscular diseases. Driving value through focused execution and financial discipline 1 COMMERCIALIZE Commercialize apitegromab for treatment of patients with SMA1 BLA resubmission and U.S. launch following FDA approval for children and adults with SMA Advance launch readiness in U.S. and Europe 2 EXPAND Develop apitegromab for patients with SMA <2 years and for additional rare, severe NMDs Initiate apitegromab Phase 2 FORGE study in patients with FSHD in mid-2026 Progress Phase 2 OPAL study for patients with SMA <2 years of age EMA decision mid-2026, initial launch planned in Germany 3 ADVANCE Advance world-leading anti-myostatin pipeline Progress subcutaneous apitegromab; Phase 1 study complete Advance Phase 1 study for SRK-439, with topline data expected in H2 2026 |
| © 2026 Scholar Rock, Inc. All rights reserved. For more information, please contact: ir@scholarrock.com media@scholarrock.com Or visit us at www.scholarrock.com 3 1 |