

The Power of Predictability JANUARY 2026 NASDAQ: BEAM

Cautionary note regarding forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding: the therapeutic applications and potential of our technology, including with respect to SCD, AATD, GSDIa and beta-thalassemia; our plans, and anticipated timing, to advance our programs, including the clinical trial designs and expectations for risto-cel, BEAM-103, BEAM-301 and BEAM-302; our plans and anticipated timing to present data from ongoing clinical trials; our anticipated regulatory interactions and filings; our current expectations and anticipated results of operations, including our expected use of capital; the sufficiency of our capital resources to fund operating expenses and capital expenditure requirements and the period in which such resources are expected to be available; and the therapeutic applications and potential of our technology, including our potential to develop lifelong, curative, precision genetic medicines for patients through base editing, including potential safety advantages, all of which are subject to known and unknown important risks, uncertainties and other factors that may cause our actual results, performance or achievements, market trends, or industry results to differ materially from those expressed or implied by such forward-looking statements. Therefore, any statements contained herein that are not statements of historical fact may be forward-looking statements and should be evaluated as such. Without limiting the foregoing, the words "anticipate," "expect," "suggest," "plan," "vision," “strategy,” “possibility,” “promise,” "believe," "intend," "project," "forecast," "estimates," "targets," "projections," "potential," "should," "could," "would," "may," "might," "will," and the negative thereof and similar words and expressions are intended to identify forward-looking statements. Each forward-looking statement is subject to important risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement, including, without limitation, risks and uncertainties related to: our ability to develop, obtain regulatory approval for, and commercialize our product candidates, which may take longer or cost more than planned; our ability to raise additional funding, which may not be available; our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates; that preclinical testing of our product candidates and preliminary or interim data from preclinical studies and clinical trials may not be predictive of the results or success of ongoing or later clinical trials; that initiation and enrollment of our clinical trials may take longer than expected; that our product candidates or the delivery modalities we rely on to administer them may cause serious adverse events; the uncertainty that our product candidates will receive regulatory approval necessary to initiate or continue human clinical trials, that our product candidates may experience manufacturing or supply interruptions or failures; risks related to competitive products; and the other risks and uncertainties identified under the headings "Risk Factors Summary" and "Risk Factors" and elsewhere in our annual report on Form 10-K for the year ended December 31, 2024, our quarterly reports on Form 10-Q, and in any subsequent filings with the Securities and Exchange Commission (the "SEC") which are available on the SEC's website at www.sec.gov. Additional information will be made available by our annual and quarterly reports and other filings that we make from time to time with the SEC. These forward-looking statements speak only as of the date of this presentation. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by applicable law. .
Our vision is to provide lifelong cures for patients suffering from serious diseases POTENTIAL FOR one-time, curative therapies PLATFORM FOR rapidly programmable precision medicines GENE EDITING FOR rare and common diseases
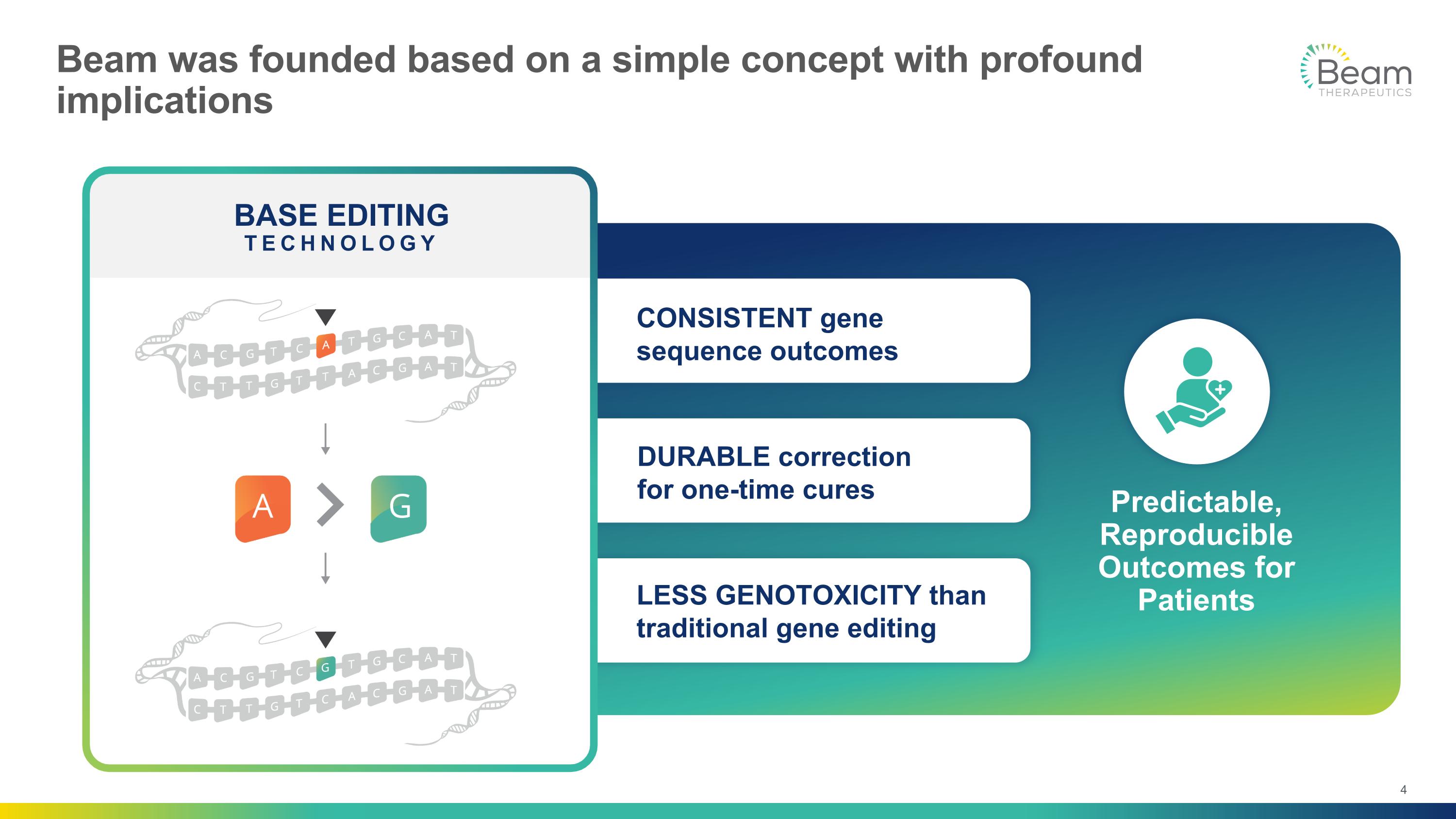
LESS GENOTOXICITY than traditional gene editing DURABLE correction for one-time cures CONSISTENT gene sequence outcomes Predictable, Reproducible Outcomes for Patients Beam was founded based on a simple concept with profound implications BASE EDITING TECHNOLOGY
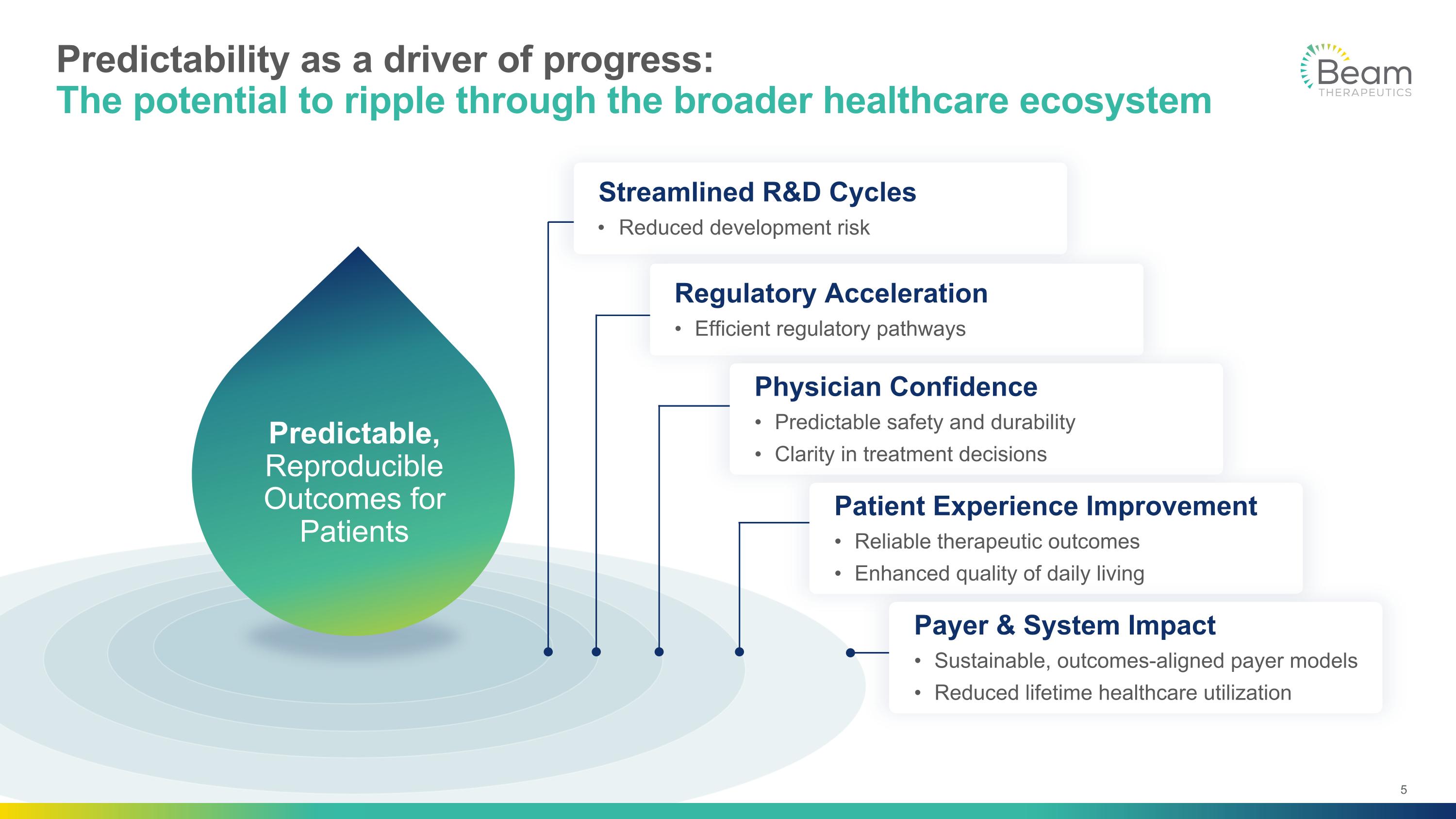
Predictability as a driver of progress: The potential to ripple through the broader healthcare ecosystem Predictable, Reproducible Outcomes for Patients Streamlined R&D Cycles Reduced development risk Regulatory Acceleration Efficient regulatory pathways Physician Confidence Predictable safety and durability Clarity in treatment decisions Patient Experience Improvement Reliable therapeutic outcomes Enhanced quality of daily living Payer & System Impact Sustainable, outcomes-aligned payer models Reduced lifetime healthcare utilization
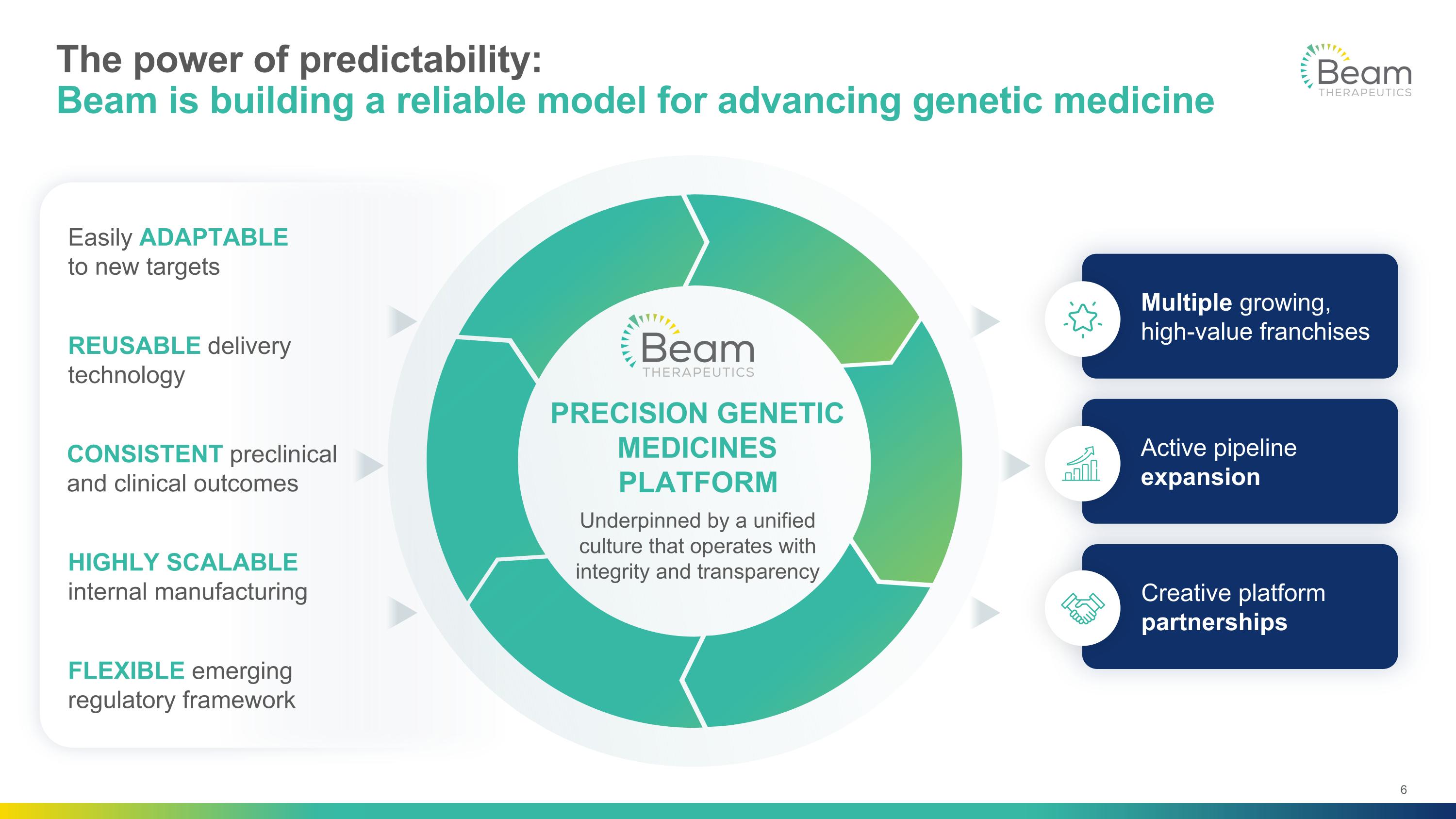
PRECISION GENETIC MEDICINES PLATFORM Underpinned by a unified culture that operates with integrity and transparency The power of predictability: Beam is building a reliable model for advancing genetic medicine Multiple growing, high-value franchises Active pipeline expansion Creative platform partnerships REUSABLE delivery technology CONSISTENT preclinical and clinical outcomes HIGHLY SCALABLE internal manufacturing FLEXIBLE emerging regulatory framework Easily ADAPTABLE to new targets
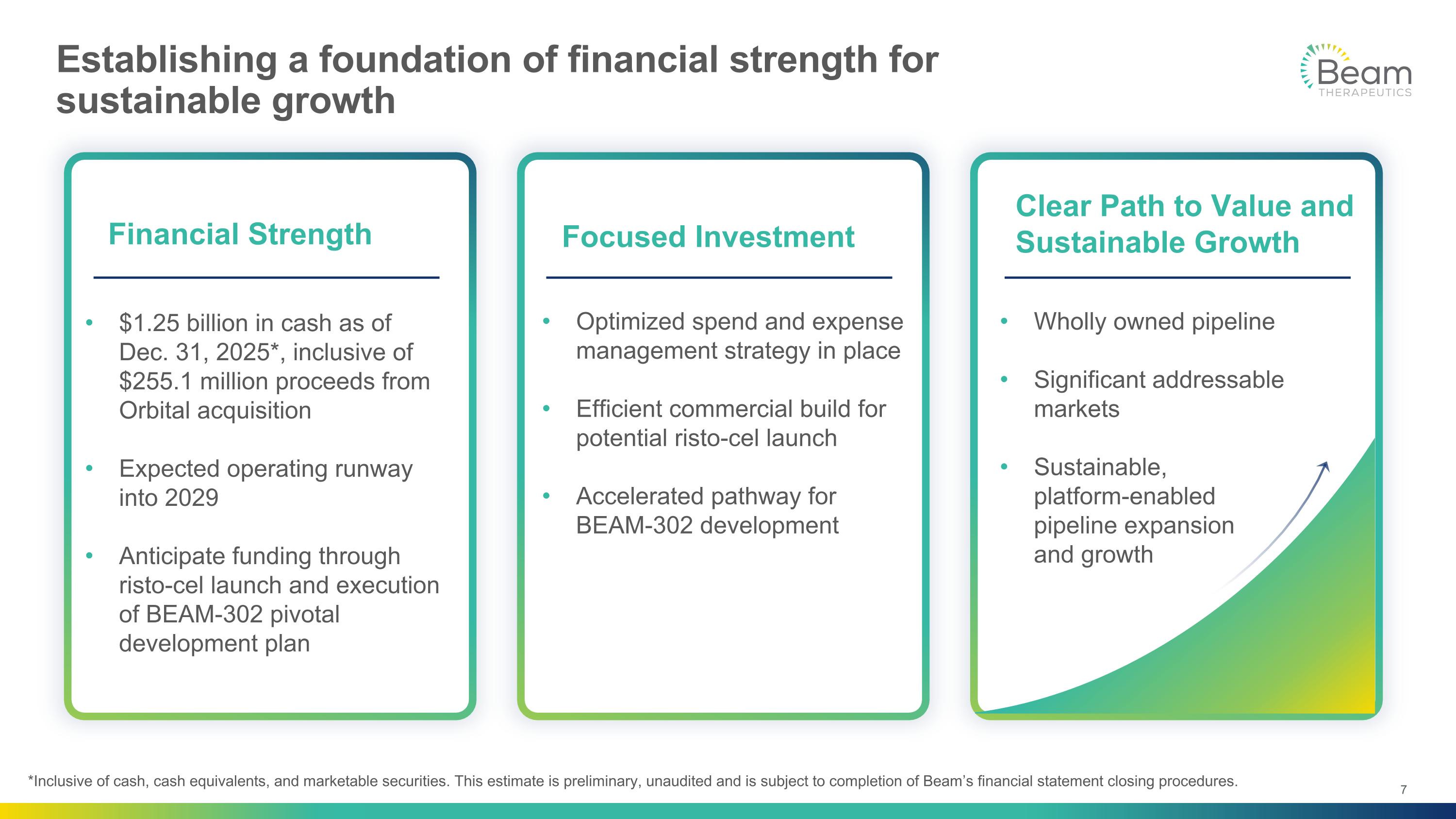
Establishing a foundation of financial strength for sustainable growth Wholly owned pipeline Significant addressable markets Sustainable, platform-enabled pipeline expansion and growth $1.25 billion in cash as of Dec. 31, 2025*, inclusive of $255.1 million proceeds from Orbital acquisition Expected operating runway into 2029 Anticipate funding through risto-cel launch and execution of BEAM-302 pivotal development plan Financial Strength Focused Investment Clear Path to Value and Sustainable Growth Optimized spend and expense management strategy in place Efficient commercial build for potential risto-cel launch Accelerated pathway for BEAM-302 development *Inclusive of cash, cash equivalents, and marketable securities. This estimate is preliminary, unaudited and is subject to completion of Beam’s financial statement closing procedures.
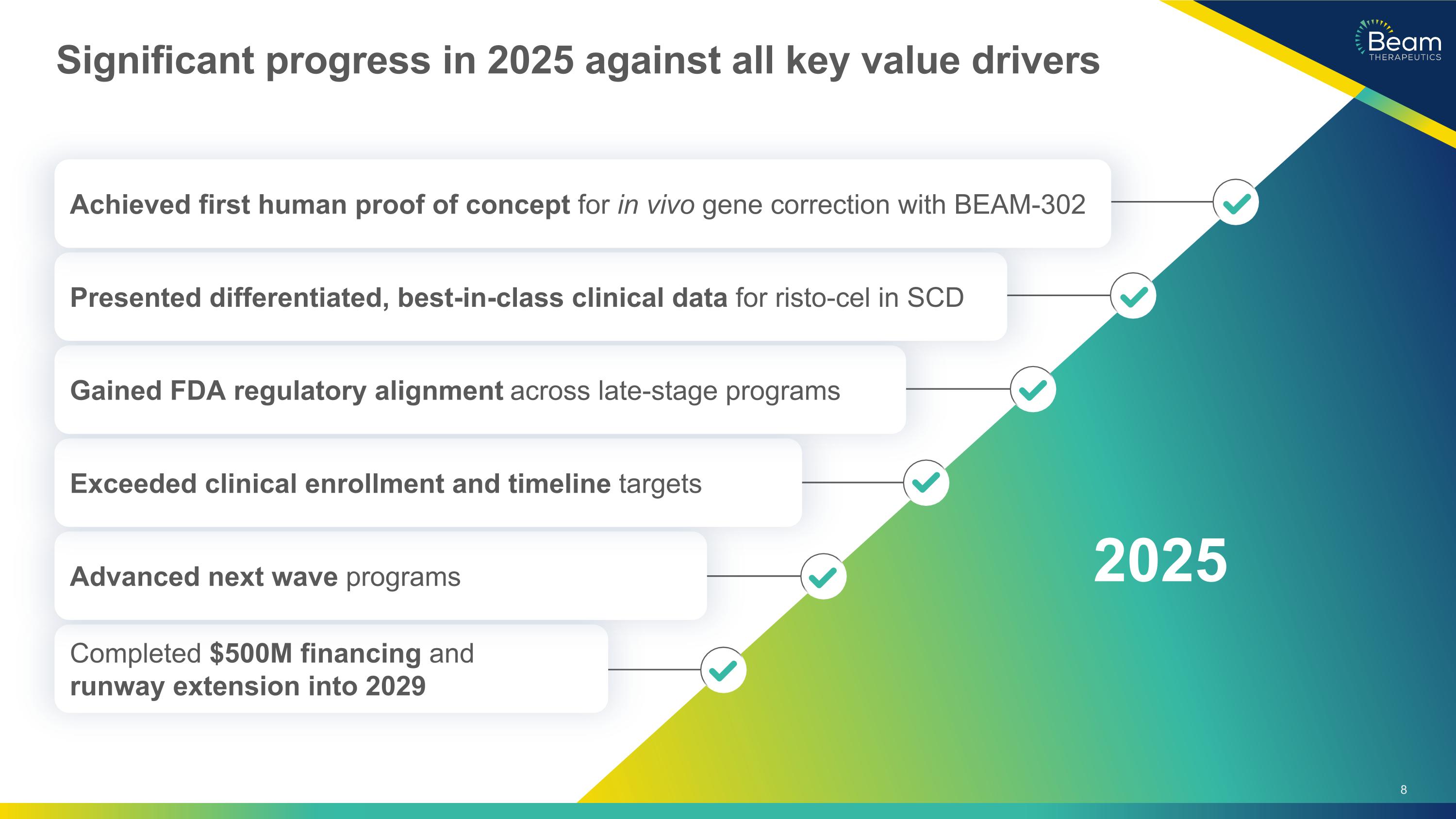
Significant progress in 2025 against all key value drivers Completed $500M financing and runway extension into 2029 Advanced next wave programs Exceeded clinical enrollment and timeline targets Gained FDA regulatory alignment across late-stage programs Presented differentiated, best-in-class clinical data for risto-cel in SCD Achieved first human proof of concept for in vivo gene correction with BEAM-302 2025

Liver-targeted Genetic Disease Franchise
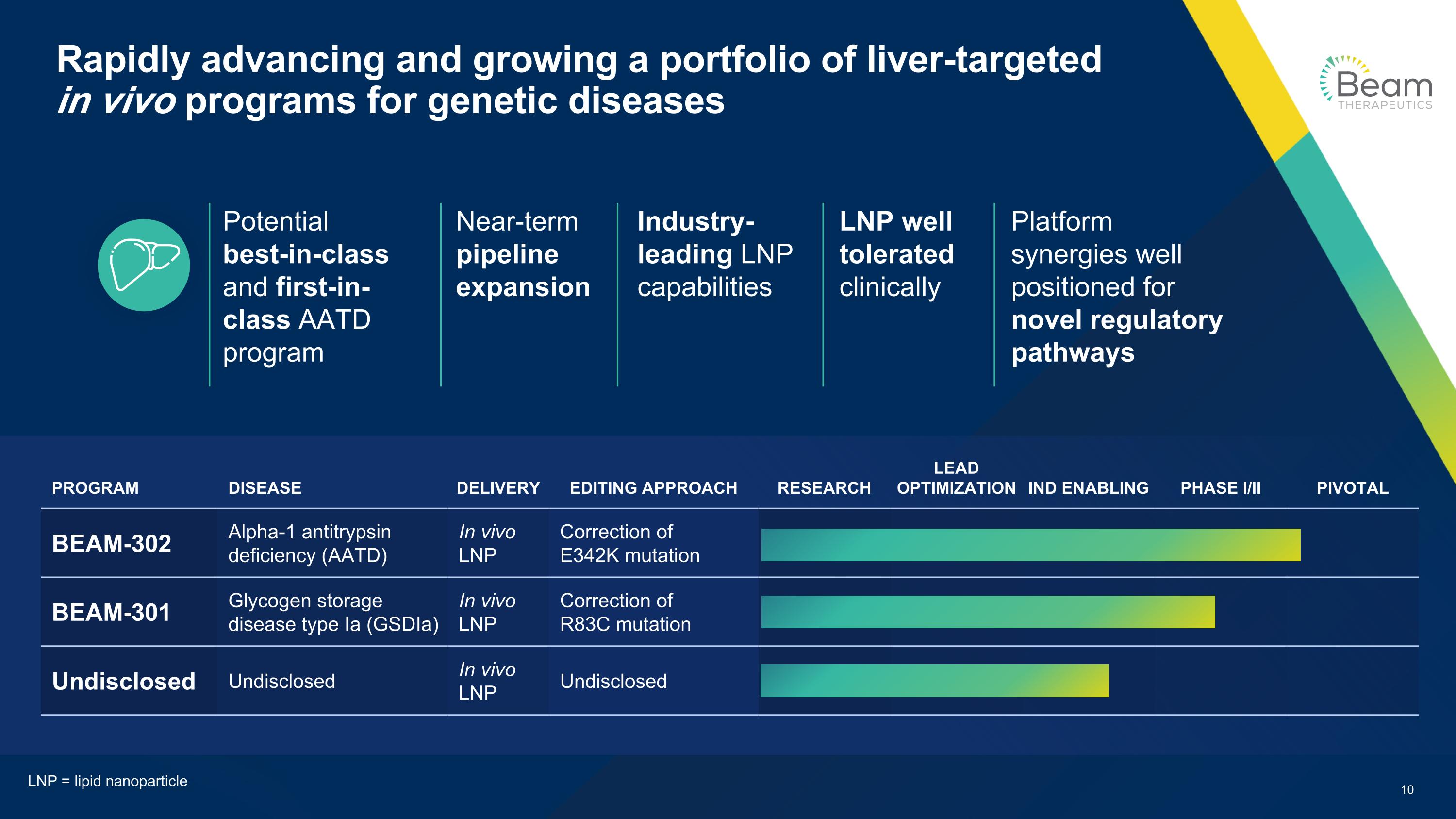
Rapidly advancing and growing a portfolio of liver-targeted in vivo programs for genetic diseases PROGRAM DISEASE DELIVERY EDITING APPROACH RESEARCH LEAD OPTIMIZATION IND ENABLING PHASE I/II PIVOTAL BEAM-302 Alpha-1 antitrypsin deficiency (AATD) In vivo LNP Correction of E342K mutation BEAM-301 Glycogen storage disease type Ia (GSDIa) In vivo LNP Correction of R83C mutation Undisclosed Undisclosed In vivo LNP Undisclosed Industry-leading LNP capabilities Platform synergies well positioned for novel regulatory pathways Potential best-in-class and first-in-class AATD program Near-term pipeline expansion LNP well tolerated clinically LNP = lipid nanoparticle
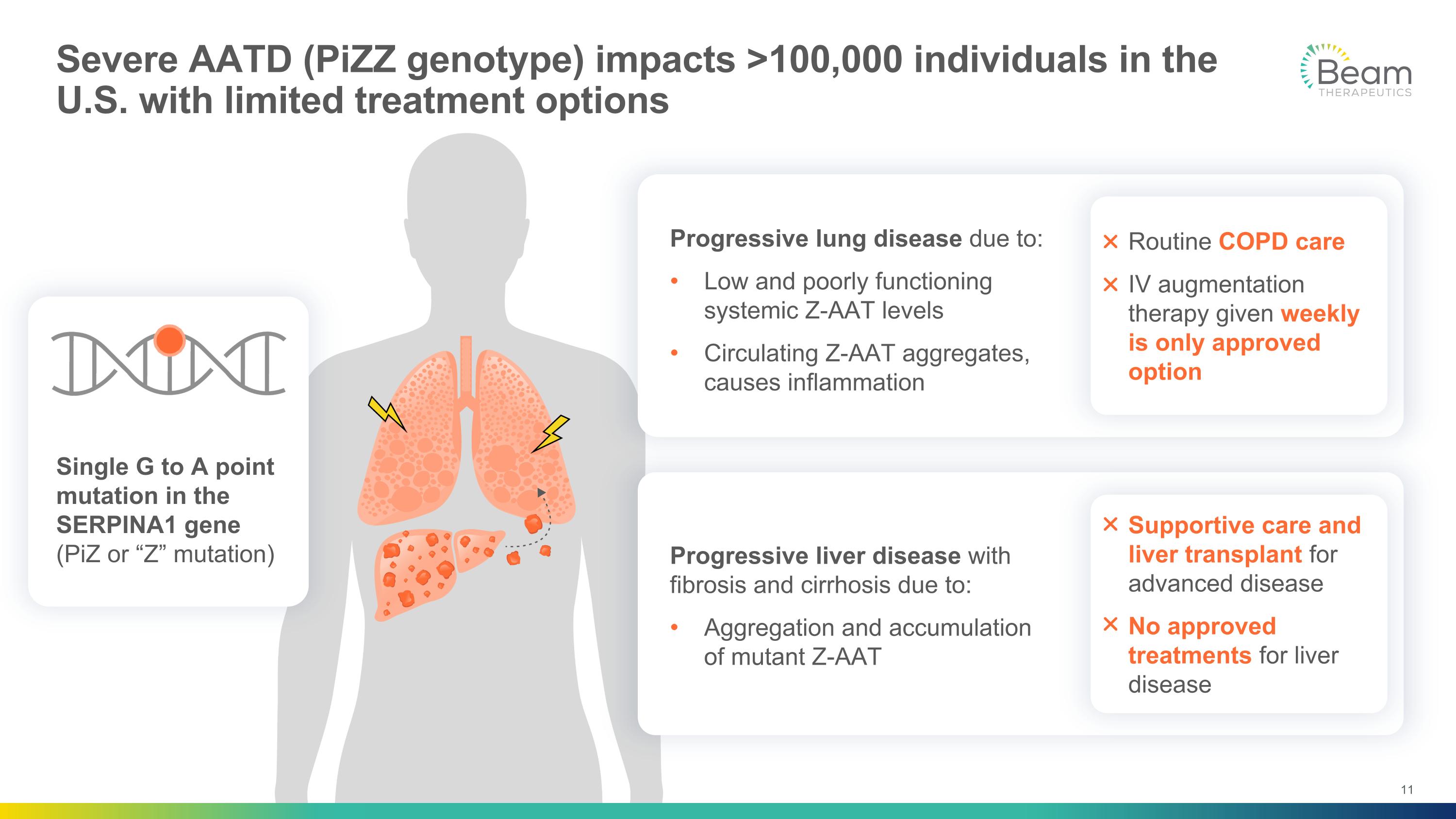
Severe AATD (PiZZ genotype) impacts >100,000 individuals in the U.S. with limited treatment options Single G to A point mutation in the SERPINA1 gene (PiZ or “Z” mutation) Routine COPD care IV augmentation therapy given weekly is only approved option Supportive care and liver transplant for advanced disease No approved treatments for liver disease Progressive liver disease with fibrosis and cirrhosis due to: Aggregation and accumulation of mutant Z-AAT Progressive lung disease due to: Low and poorly functioning systemic Z-AAT levels Circulating Z-AAT aggregates, causes inflammation
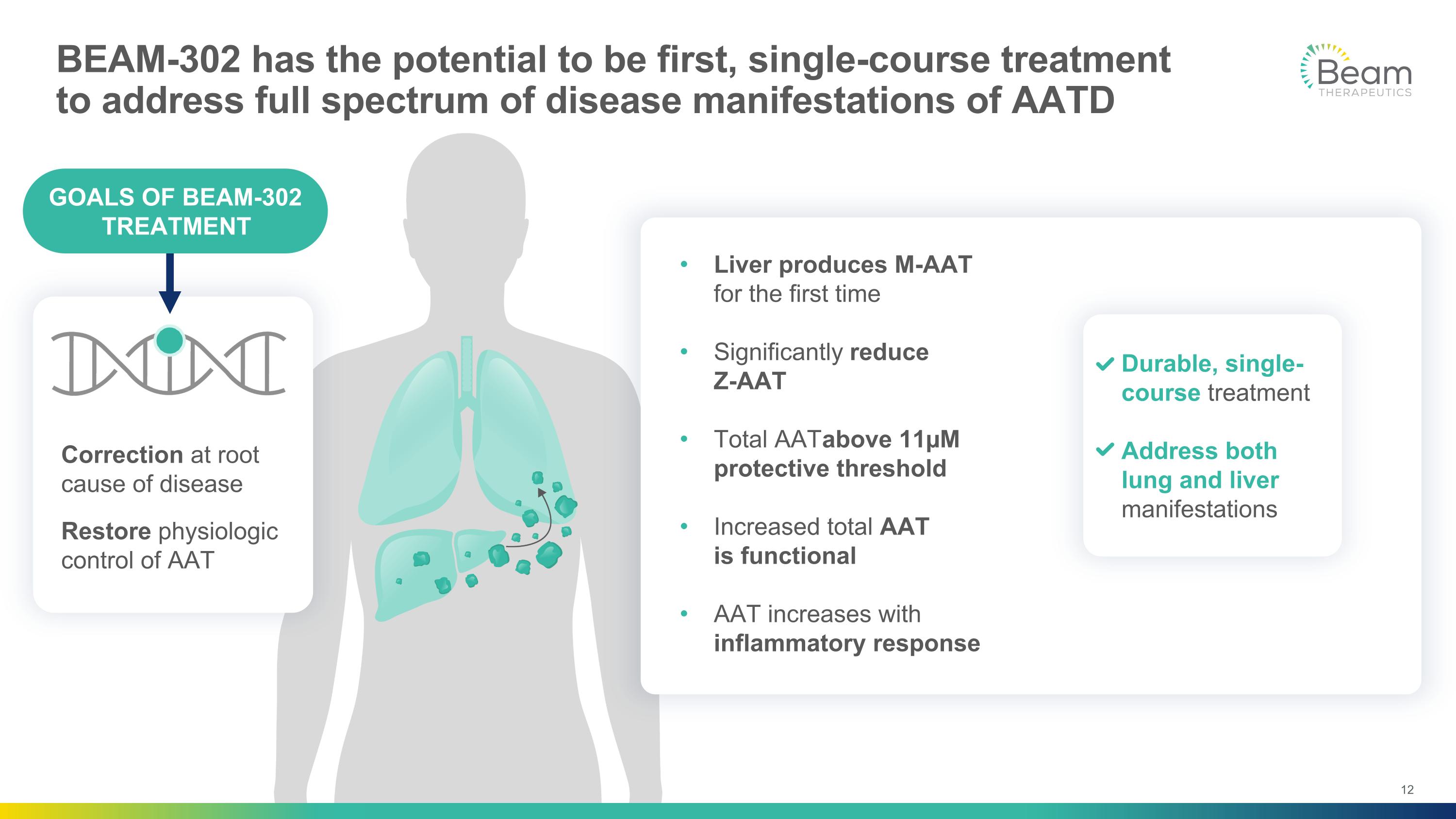
BEAM-302 has the potential to be first, single-course treatment to address full spectrum of disease manifestations of AATD Durable, single-course treatment Address both lung and liver manifestations Liver produces M-AAT for the first time Significantly reduce Z-AAT Total AAT above 11µM protective threshold Increased total AAT is functional AAT increases with inflammatory response Correction at root cause of disease Restore physiologic control of AAT GOALS OF BEAM-302 TREATMENT
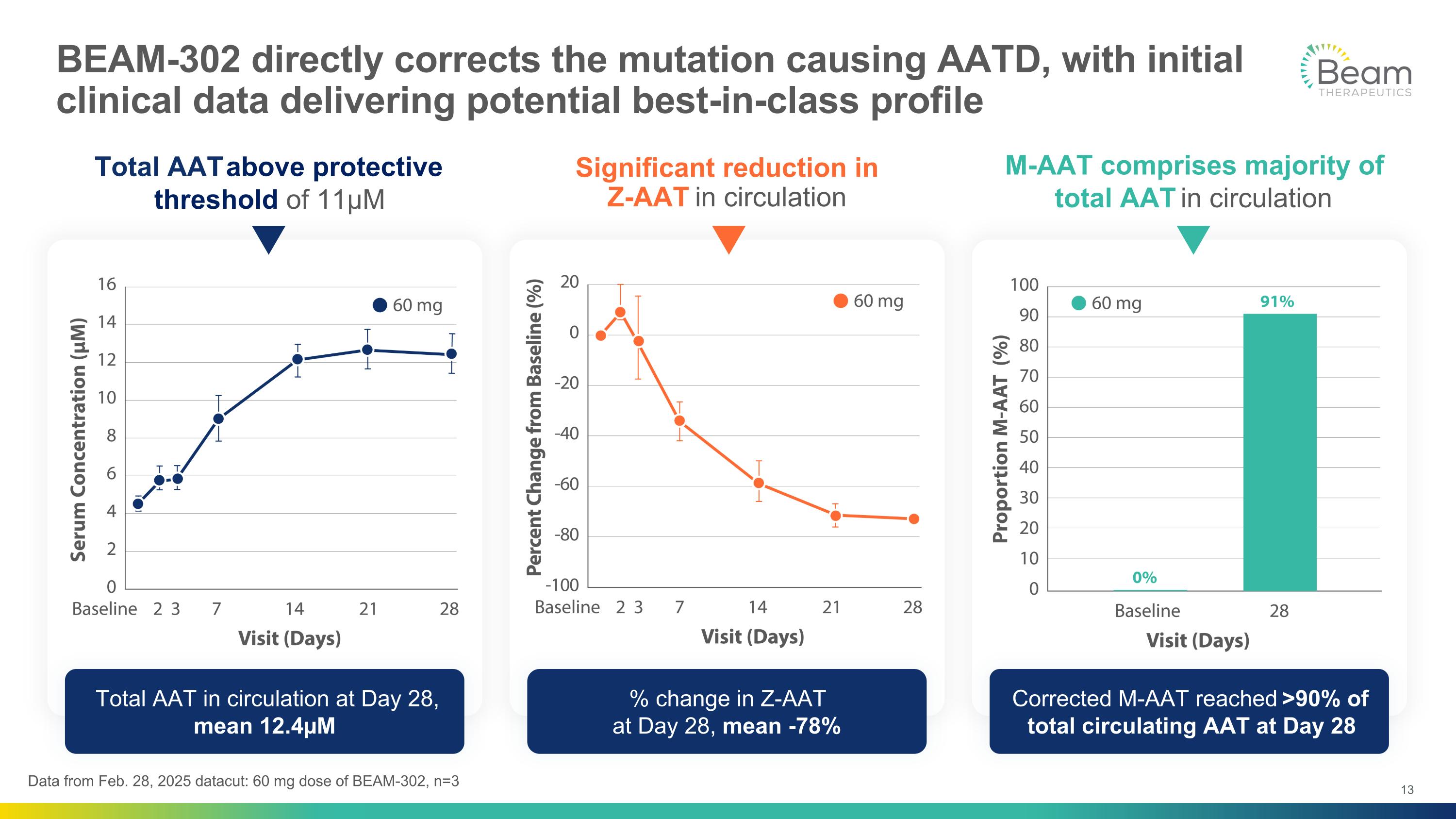
BEAM-302 directly corrects the mutation causing AATD, with initial clinical data delivering potential best-in-class profile Total AAT in circulation at Day 28, mean 12.4µM % change in Z-AAT at Day 28, mean -78% Corrected M-AAT reached >90% of total circulating AAT at Day 28 Significant reduction in Z-AAT in circulation M-AAT comprises majority of total AAT in circulation Total AAT above protective threshold of 11µM Data from Feb. 28, 2025 datacut: 60 mg dose of BEAM-302, n=3
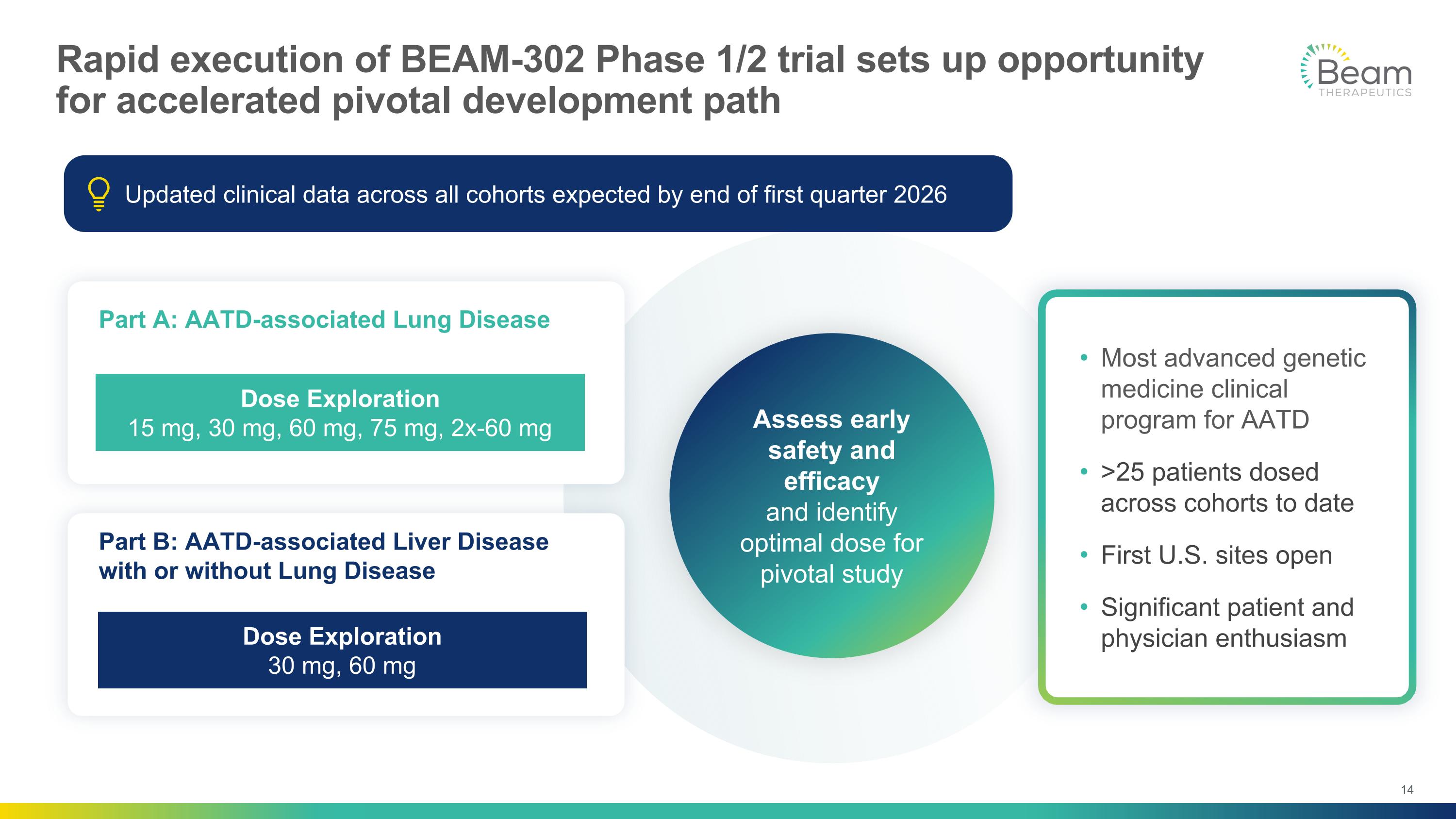
Rapid execution of BEAM-302 Phase 1/2 trial sets up opportunity for accelerated pivotal development path Most advanced genetic medicine clinical program for AATD >25 patients dosed across cohorts to date First U.S. sites open Significant patient and physician enthusiasm Part A: AATD-associated Lung Disease Part B: AATD-associated Liver Disease with or without Lung Disease Assess early safety and efficacy and identify optimal dose for pivotal study Dose Exploration Dose Exploration 30 mg, 60 mg Dose Exploration 15 mg, 30 mg, 60 mg, 75 mg, 2x-60 mg Updated clinical data across all cohorts expected by end of first quarter 2026
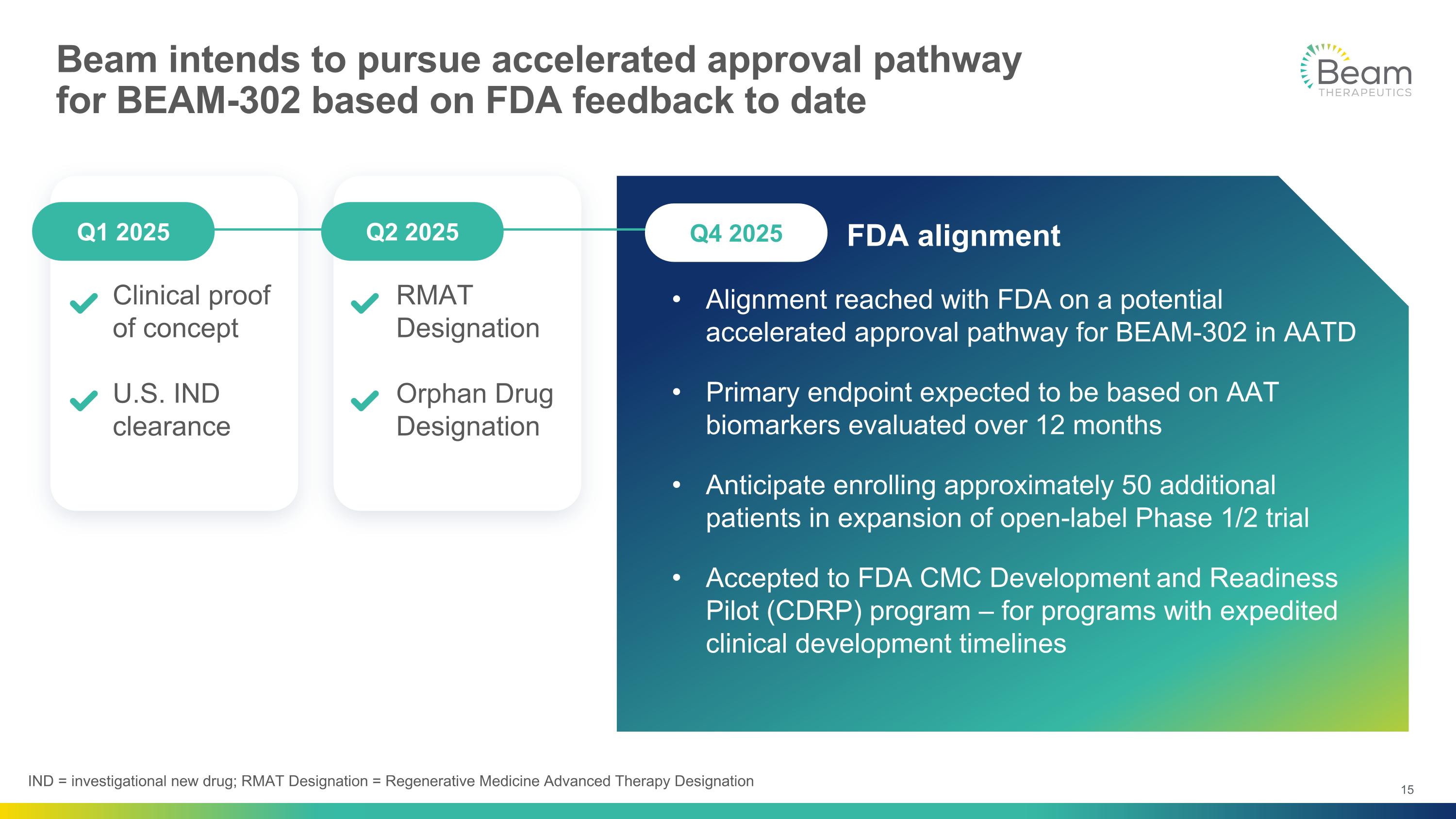
Beam intends to pursue accelerated approval pathway for BEAM-302 based on FDA feedback to date Alignment reached with FDA on a potential accelerated approval pathway for BEAM-302 in AATD Primary endpoint expected to be based on AAT biomarkers evaluated over 12 months Anticipate enrolling approximately 50 additional patients in expansion of open-label Phase 1/2 trial Accepted to FDA CMC Development and Readiness Pilot (CDRP) program – for programs with expedited clinical development timelines Clinical proof of concept U.S. IND clearance INTEREST RMAT Designation Orphan Drug Designation FDA alignment Q2 2025 Q1 2025 Q4 2025 IND = investigational new drug; RMAT Designation = Regenerative Medicine Advanced Therapy Designation
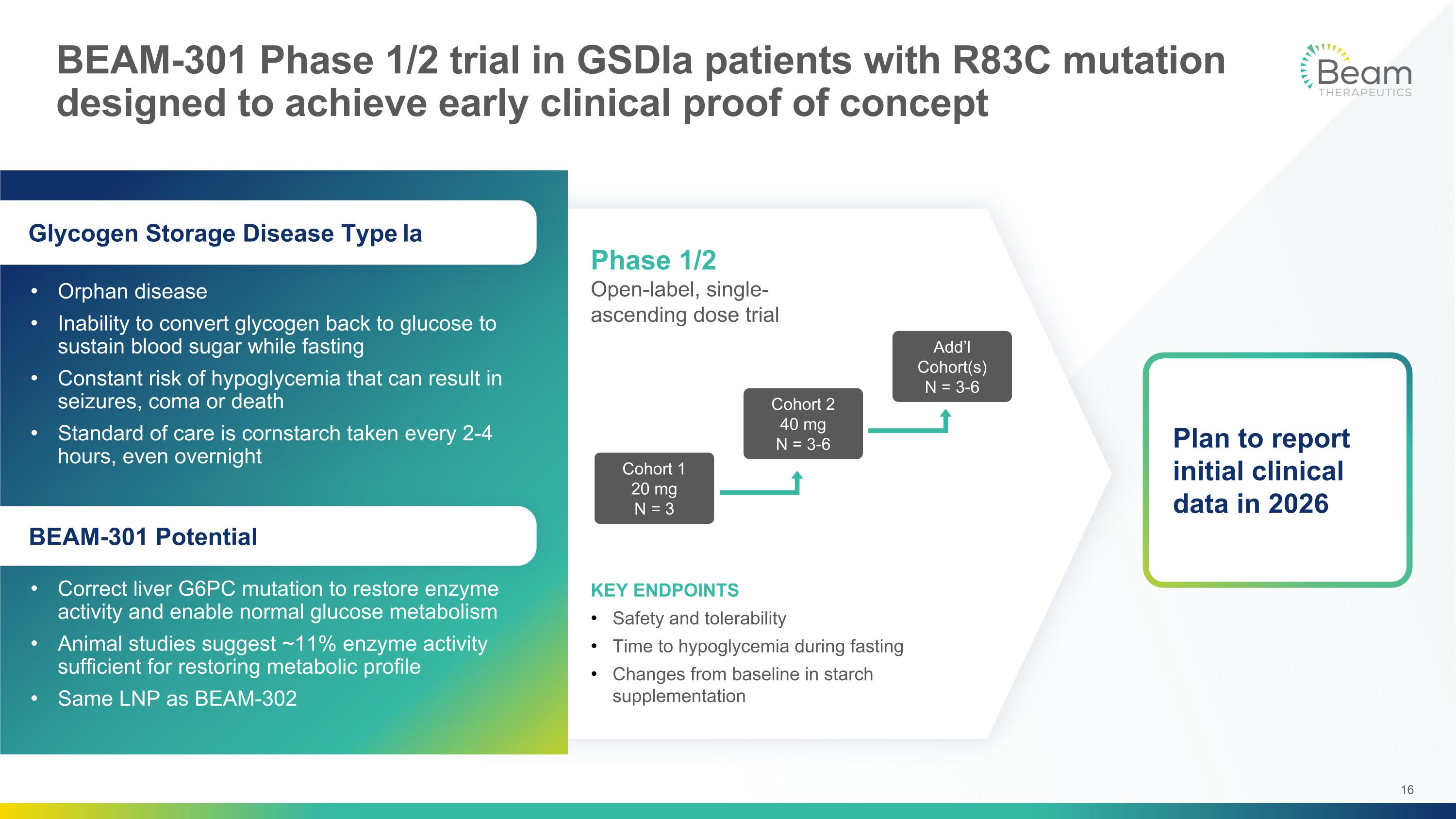
KEY ENDPOINTS Safety and tolerability Time to hypoglycemia during fasting Changes from baseline in starch supplementation Phase 1/2 Open-label, single-ascending dose trial Cohort 1 20 mg N = 3 Cohort 2 40 mg N = 3-6 Add’l Cohort(s) N = 3-6 BEAM-301 Phase 1/2 trial in GSDIa patients with R83C mutation designed to achieve early clinical proof of concept Orphan disease Inability to convert glycogen back to glucose to sustain blood sugar while fasting Constant risk of hypoglycemia that can result in seizures, coma or death Standard of care is cornstarch taken every 2-4 hours, even overnight Correct liver G6PC mutation to restore enzyme activity and enable normal glucose metabolism Animal studies suggest ~11% enzyme activity sufficient for restoring metabolic profile Same LNP as BEAM-302 Glycogen Storage Disease Type Ia BEAM-301 Potential Plan to report initial clinical data in 2026

Hematology Franchise
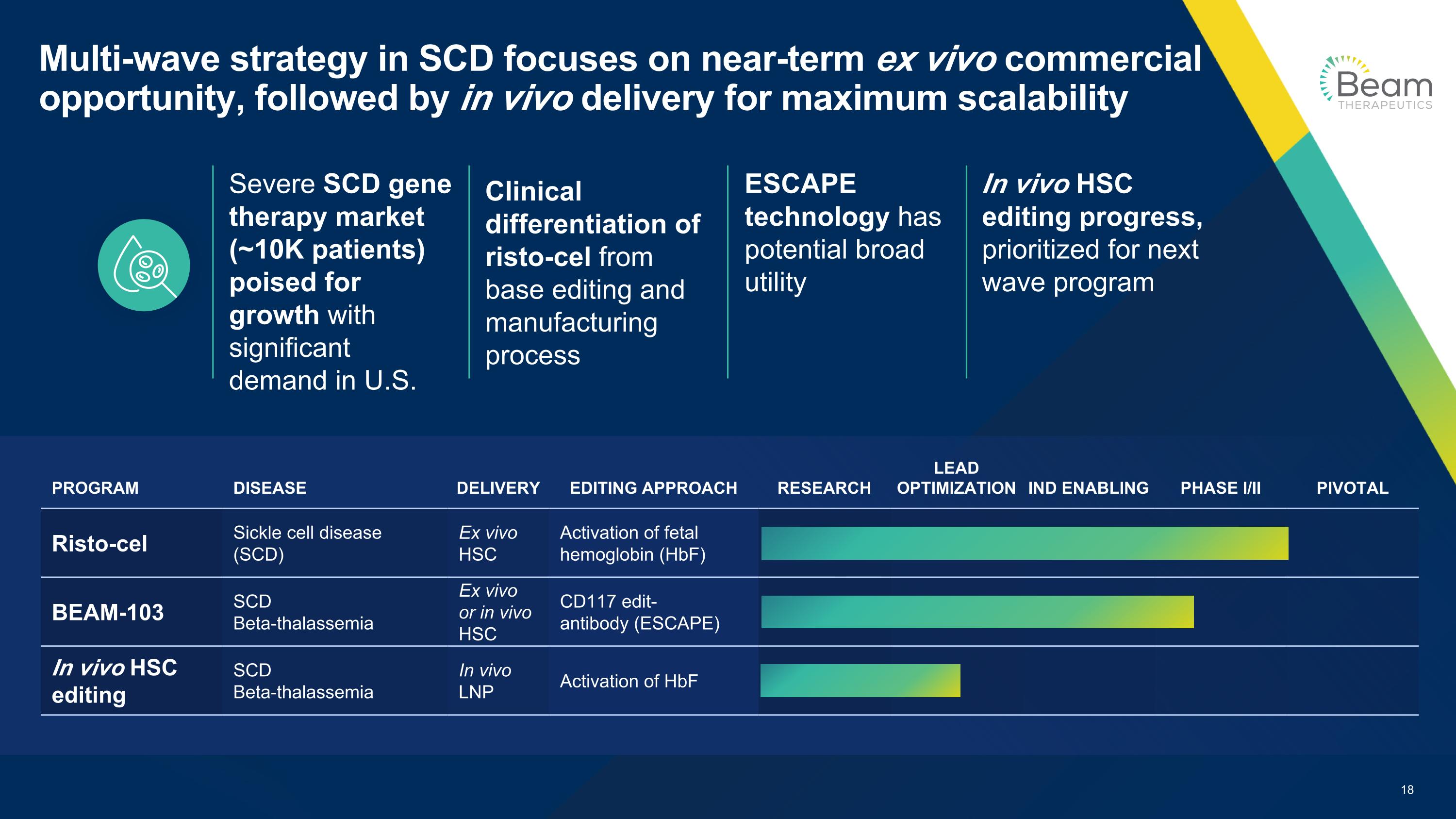
Multi-wave strategy in SCD focuses on near-term ex vivo commercial opportunity, followed by in vivo delivery for maximum scalability ESCAPE technology has potential broad utility Severe SCD gene therapy market (~10K patients) poised for growth with significant demand in U.S. Clinical differentiation of risto-cel from base editing and manufacturing process In vivo HSC editing progress, prioritized for next wave program PROGRAM DISEASE DELIVERY EDITING APPROACH RESEARCH LEAD OPTIMIZATION IND ENABLING PHASE I/II PIVOTAL Risto-cel Sickle cell disease (SCD) Ex vivo HSC Activation of fetal hemoglobin (HbF) BEAM-103 SCD Beta-thalassemia Ex vivo or in vivo HSC CD117 edit- antibody (ESCAPE) In vivo HSC editing SCD Beta-thalassemia In vivo LNP Activation of HbF
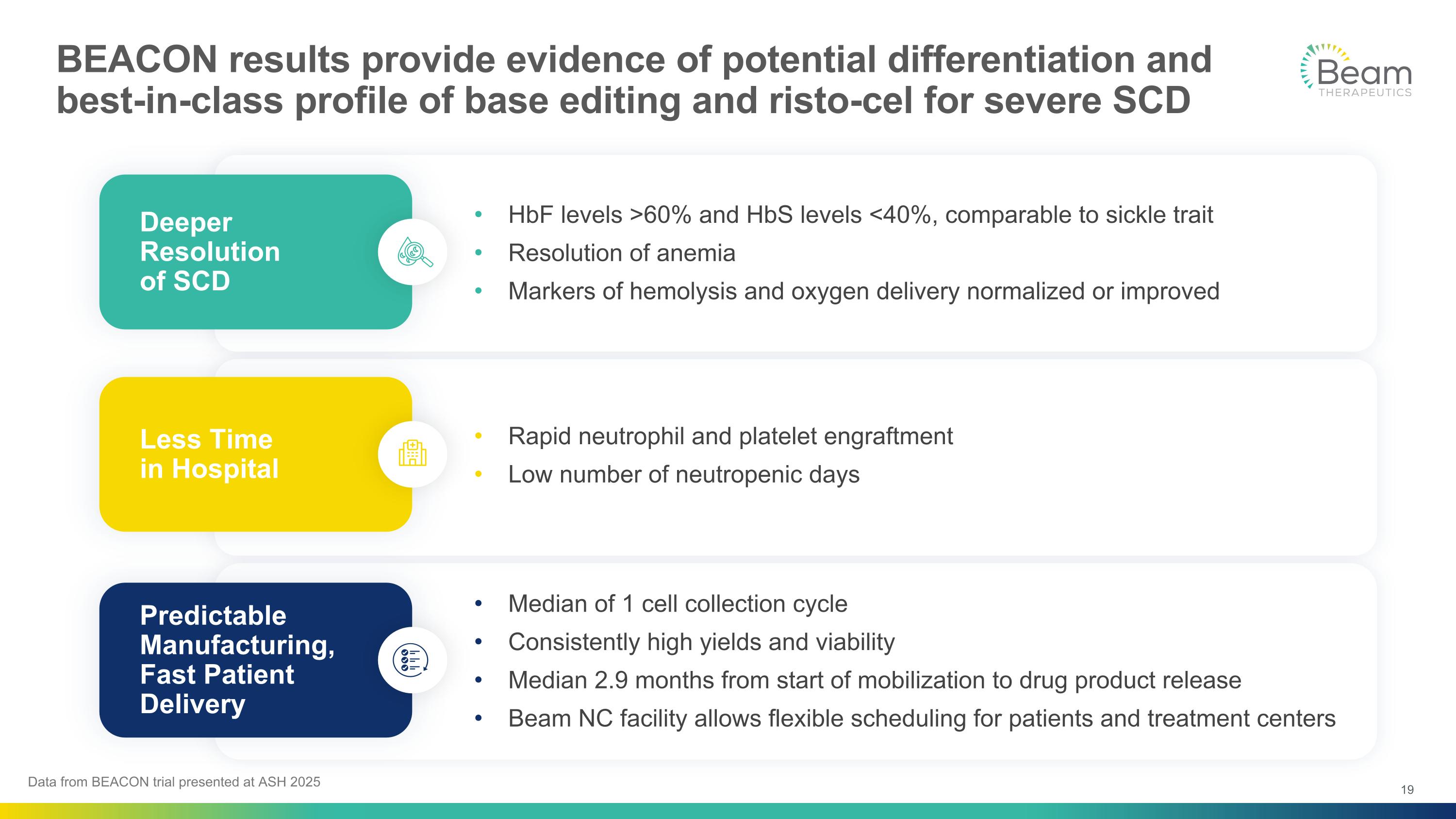
BEACON results provide evidence of potential differentiation and best-in-class profile of base editing and risto-cel for severe SCD Rapid neutrophil and platelet engraftment Low number of neutropenic days Less Time in Hospital HbF levels >60% and HbS levels <40%, comparable to sickle trait Resolution of anemia Markers of hemolysis and oxygen delivery normalized or improved Deeper Resolution of SCD Median of 1 cell collection cycle Consistently high yields and viability Median 2.9 months from start of mobilization to drug product release Beam NC facility allows flexible scheduling for patients and treatment centers Predictable Manufacturing, Fast Patient Delivery Data from BEACON trial presented at ASH 2025
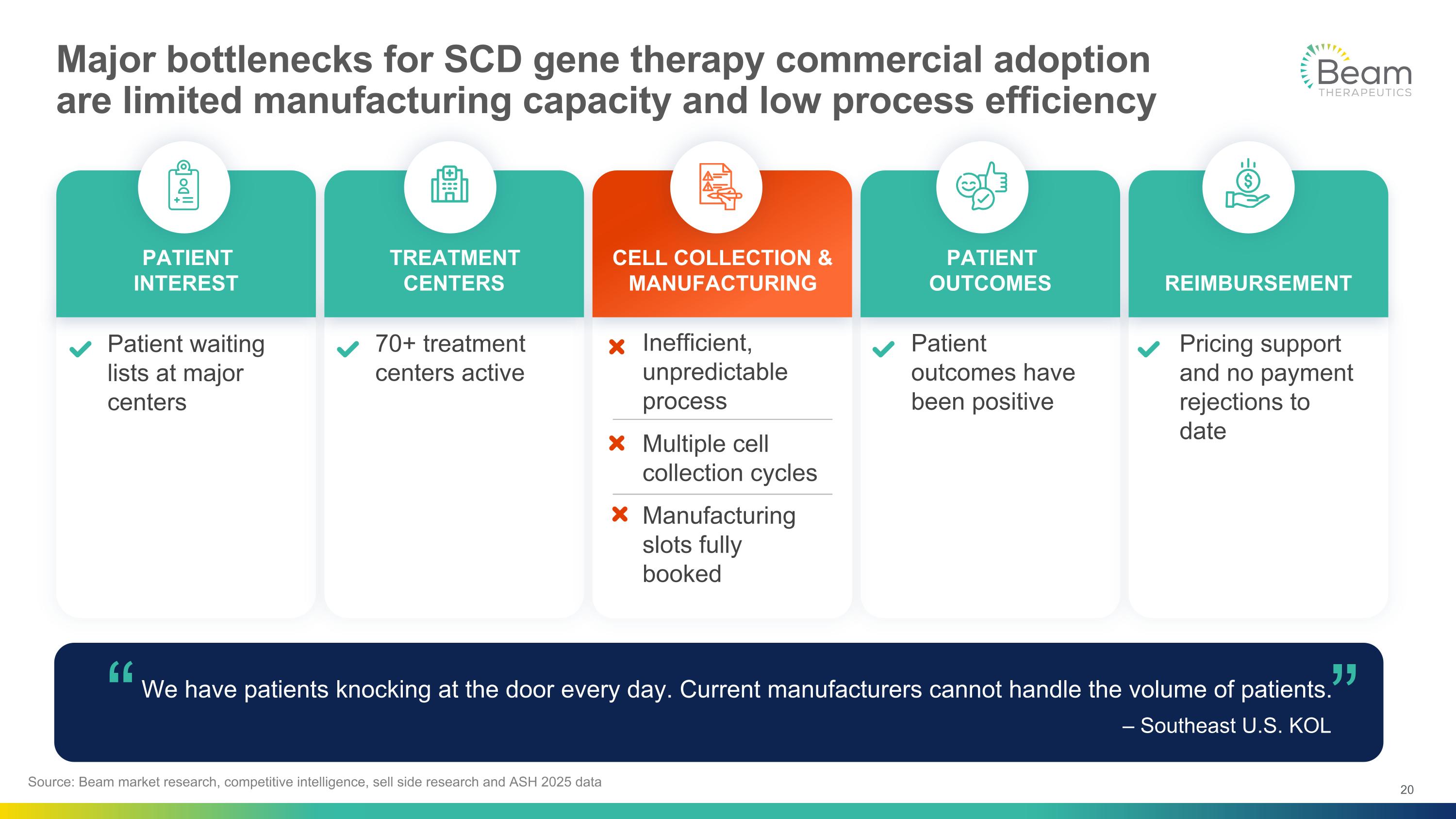
Major bottlenecks for SCD gene therapy commercial adoption are limited manufacturing capacity and low process efficiency We have patients knocking at the door every day. Current manufacturers cannot handle the volume of patients. – Southeast U.S. KOL “ “ Patient waiting lists at major centers 70+ treatment centers active Patient outcomes have been positive Pricing support and no payment rejections to date Inefficient, unpredictable process Multiple cell collection cycles Manufacturing slots fully booked PATIENT INTEREST TREATMENT CENTERS PATIENT OUTCOMES CELL COLLECTION & MANUFACTURING REIMBURSEMENT Source: Beam market research, competitive intelligence, sell side research and ASH 2025 data
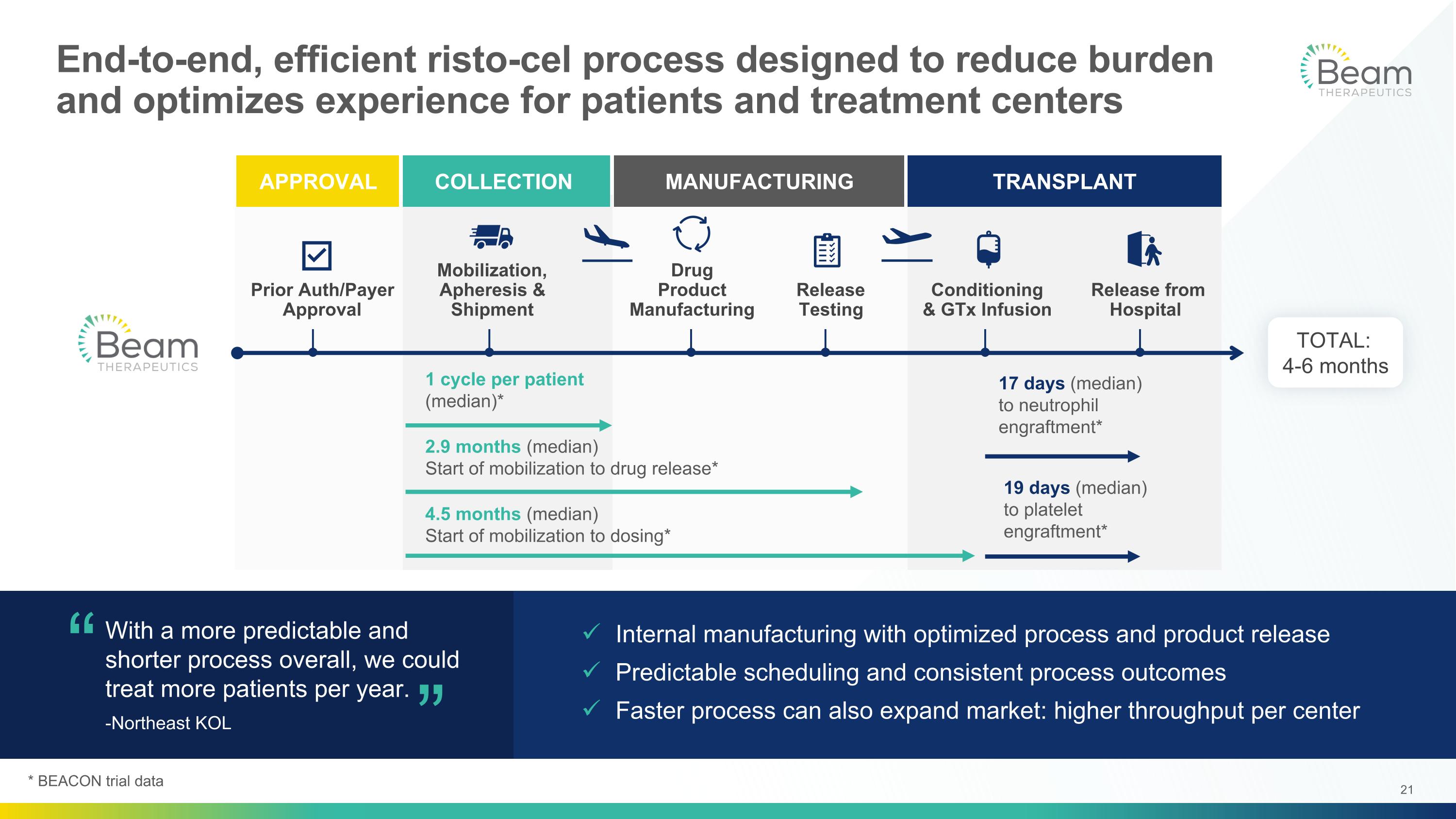
APPROVAL TRANSPLANT COLLECTION MANUFACTURING 17 days (median) to neutrophil engraftment* Internal manufacturing with optimized process and product release Predictable scheduling and consistent process outcomes Faster process can also expand market: higher throughput per center End-to-end, efficient risto-cel process designed to reduce burden and optimizes experience for patients and treatment centers With a more predictable and shorter process overall, we could treat more patients per year. -Northeast KOL “ “ Conditioning & GTx Infusion Drug Product Manufacturing Release Testing Mobilization, Apheresis & Shipment Prior Auth/Payer Approval Release from Hospital 2.9 months (median) Start of mobilization to drug release* 4.5 months (median) Start of mobilization to dosing* 1 cycle per patient (median)* 19 days (median) to platelet engraftment* TOTAL: 4-6 months * BEACON trial data
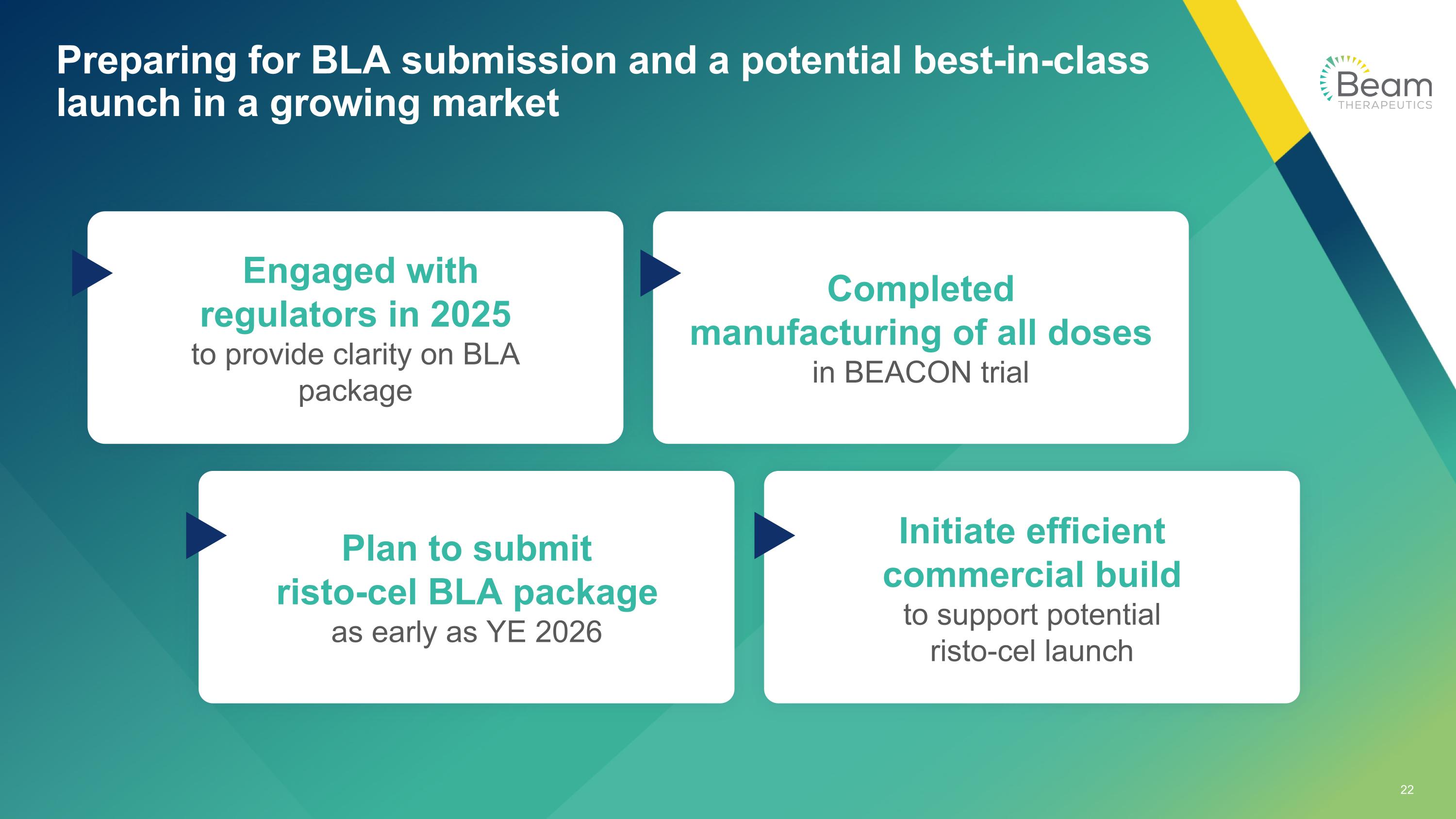
Preparing for BLA submission and a potential best-in-class launch in a growing market Engaged with regulators in 2025 to provide clarity on BLA package Completed manufacturing of all doses in BEACON trial Plan to submit risto-cel BLA package as early as YE 2026 Initiate efficient commercial build to support potential risto-cel launch
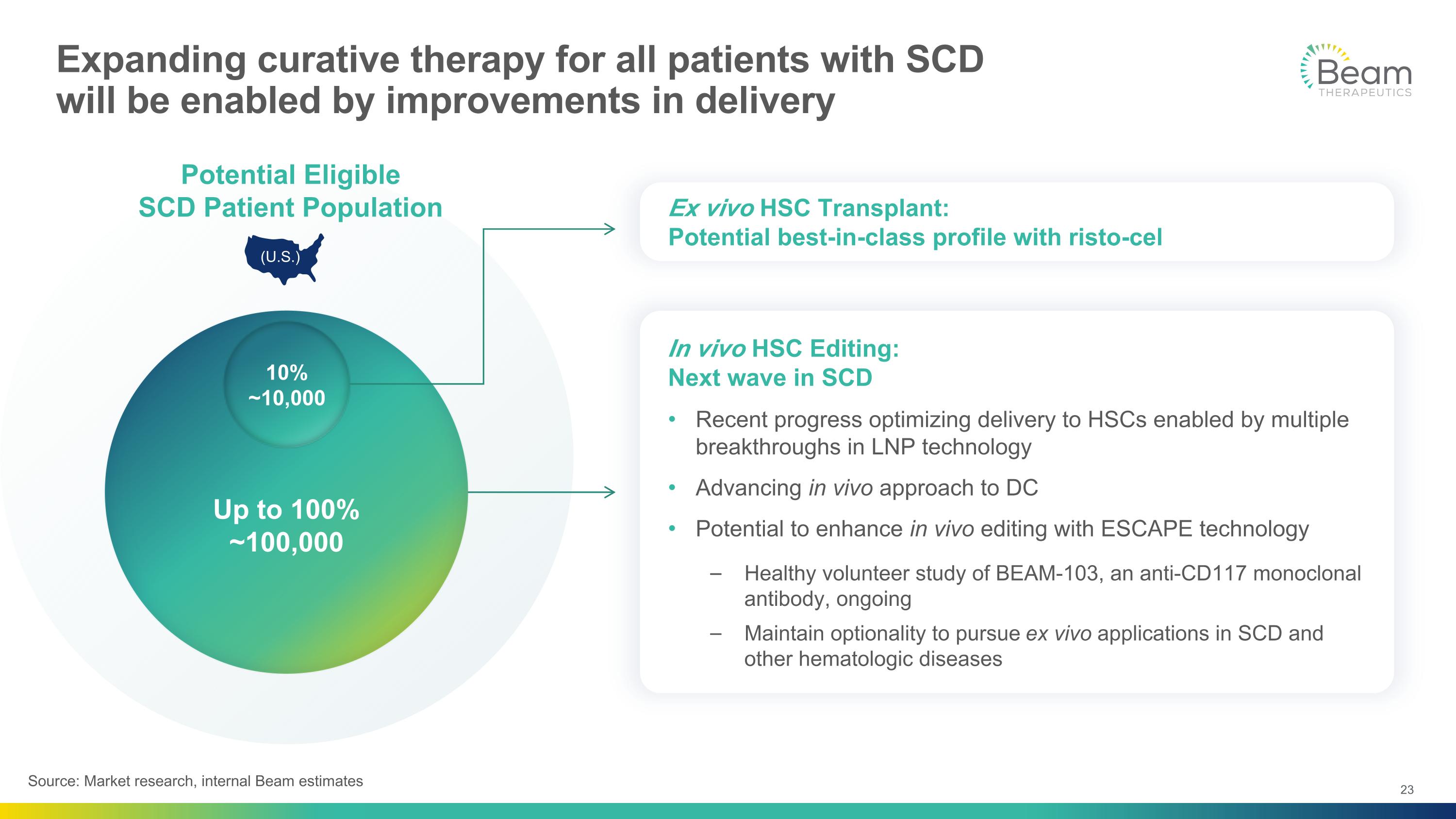
In vivo HSC Editing: Next wave in SCD Recent progress optimizing delivery to HSCs enabled by multiple breakthroughs in LNP technology Advancing in vivo approach to DC Potential to enhance in vivo editing with ESCAPE technology Healthy volunteer study of BEAM-103, an anti-CD117 monoclonal antibody, ongoing Maintain optionality to pursue ex vivo applications in SCD and other hematologic diseases Up to 100% ~100,000 10% ~10,000 Expanding curative therapy for all patients with SCD will be enabled by improvements in delivery Potential Eligible SCD Patient Population (U.S.) Ex vivo HSC Transplant: Potential best-in-class profile with risto-cel Source: Market research, internal Beam estimates
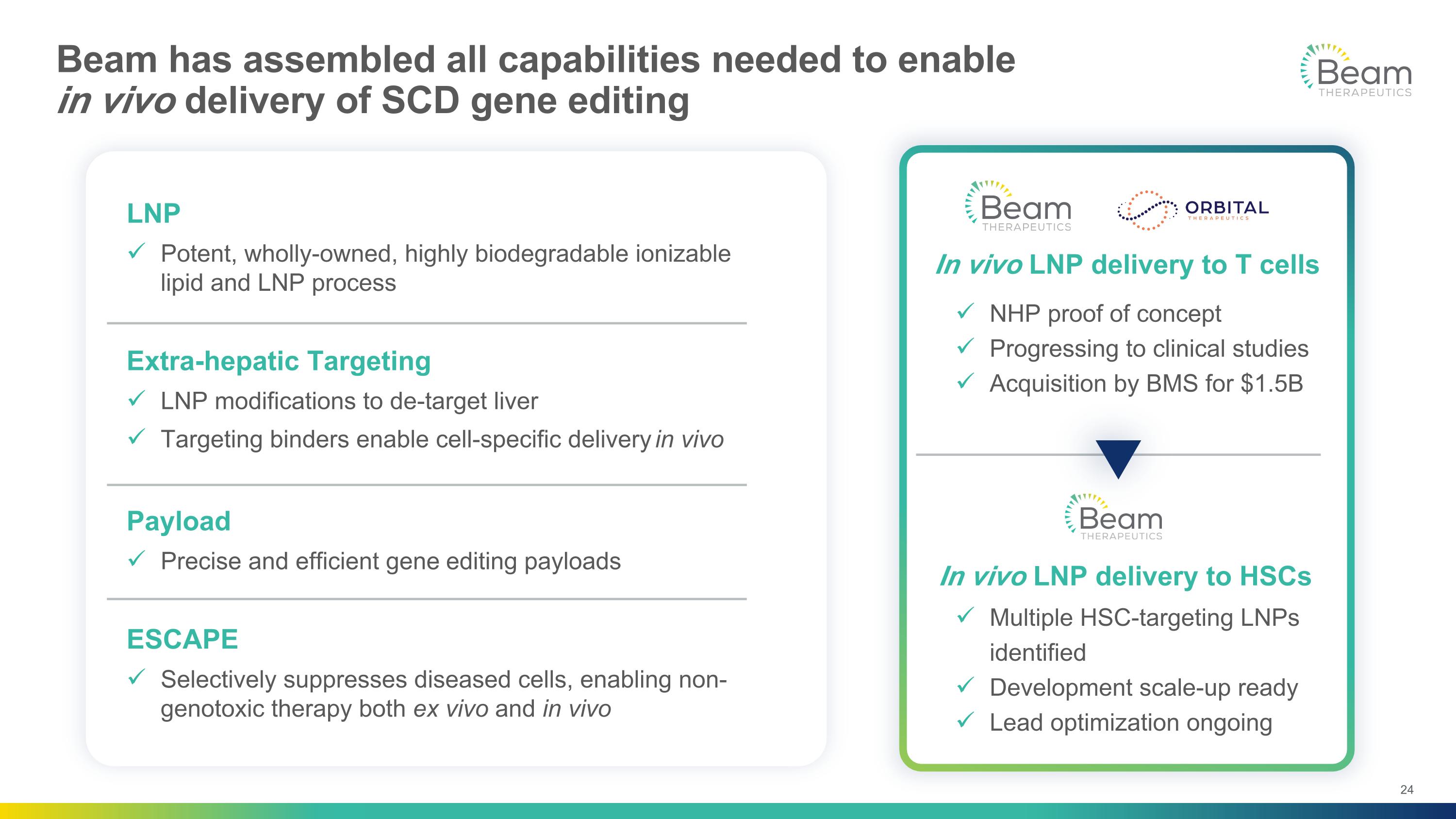
Beam has assembled all capabilities needed to enable in vivo delivery of SCD gene editing LNP Potent, wholly-owned, highly biodegradable ionizable lipid and LNP process Extra-hepatic Targeting LNP modifications to de-target liver Targeting binders enable cell-specific delivery in vivo Payload Precise and efficient gene editing payloads ESCAPE Selectively suppresses diseased cells, enabling non-genotoxic therapy both ex vivo and in vivo In vivo LNP delivery to HSCs In vivo LNP delivery to T cells NHP proof of concept Progressing to clinical studies Acquisition by BMS for $1.5B Multiple HSC-targeting LNPs identified Development scale-up ready Lead optimization ongoing
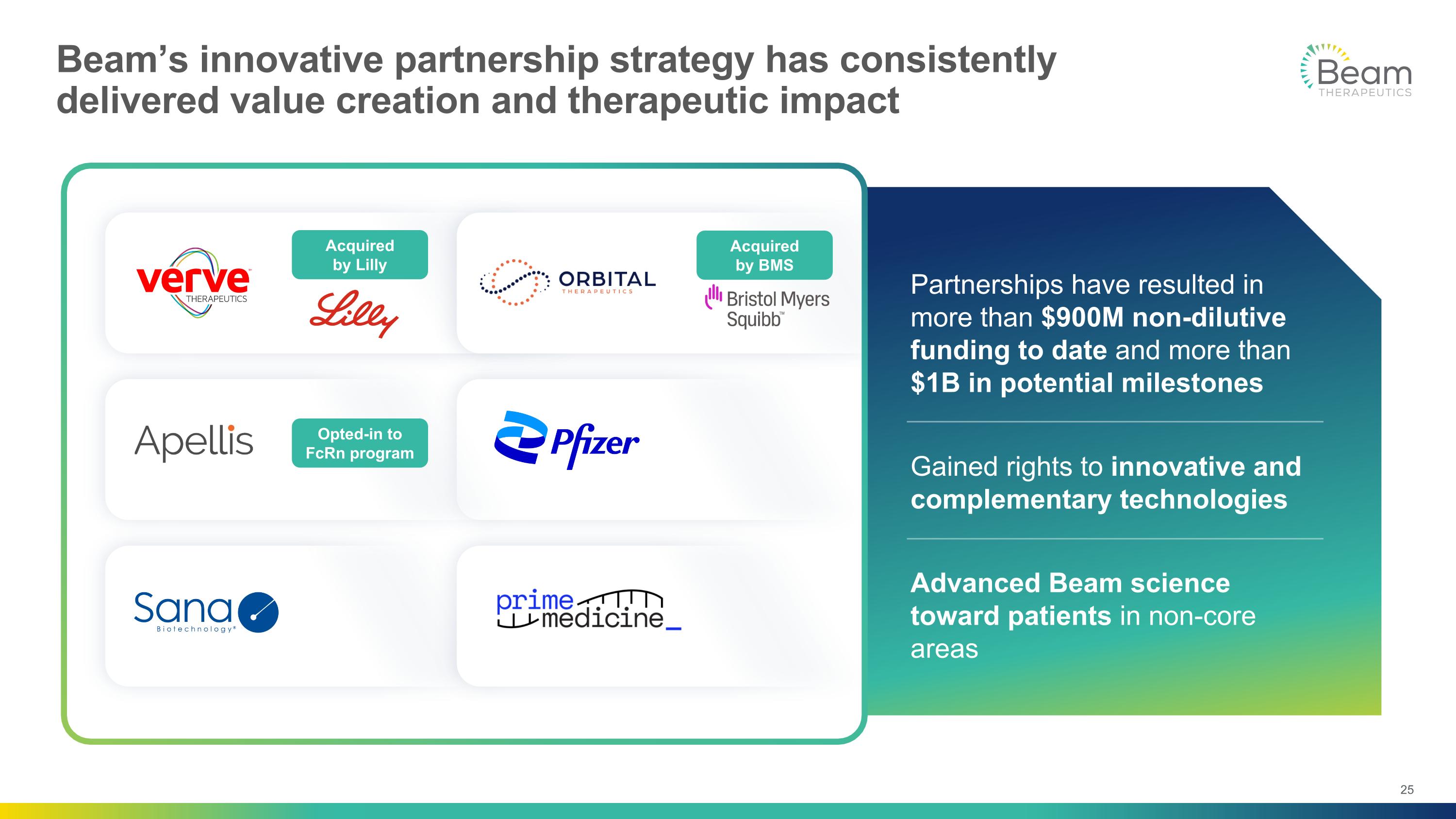
Partnerships have resulted in more than $900M non-dilutive funding to date and more than $1B in potential milestones Gained rights to innovative and complementary technologies Advanced Beam science toward patients in non-core areas Beam’s innovative partnership strategy has consistently delivered value creation and therapeutic impact Acquired by Lilly Opted-in to FcRn program Acquired by BMS
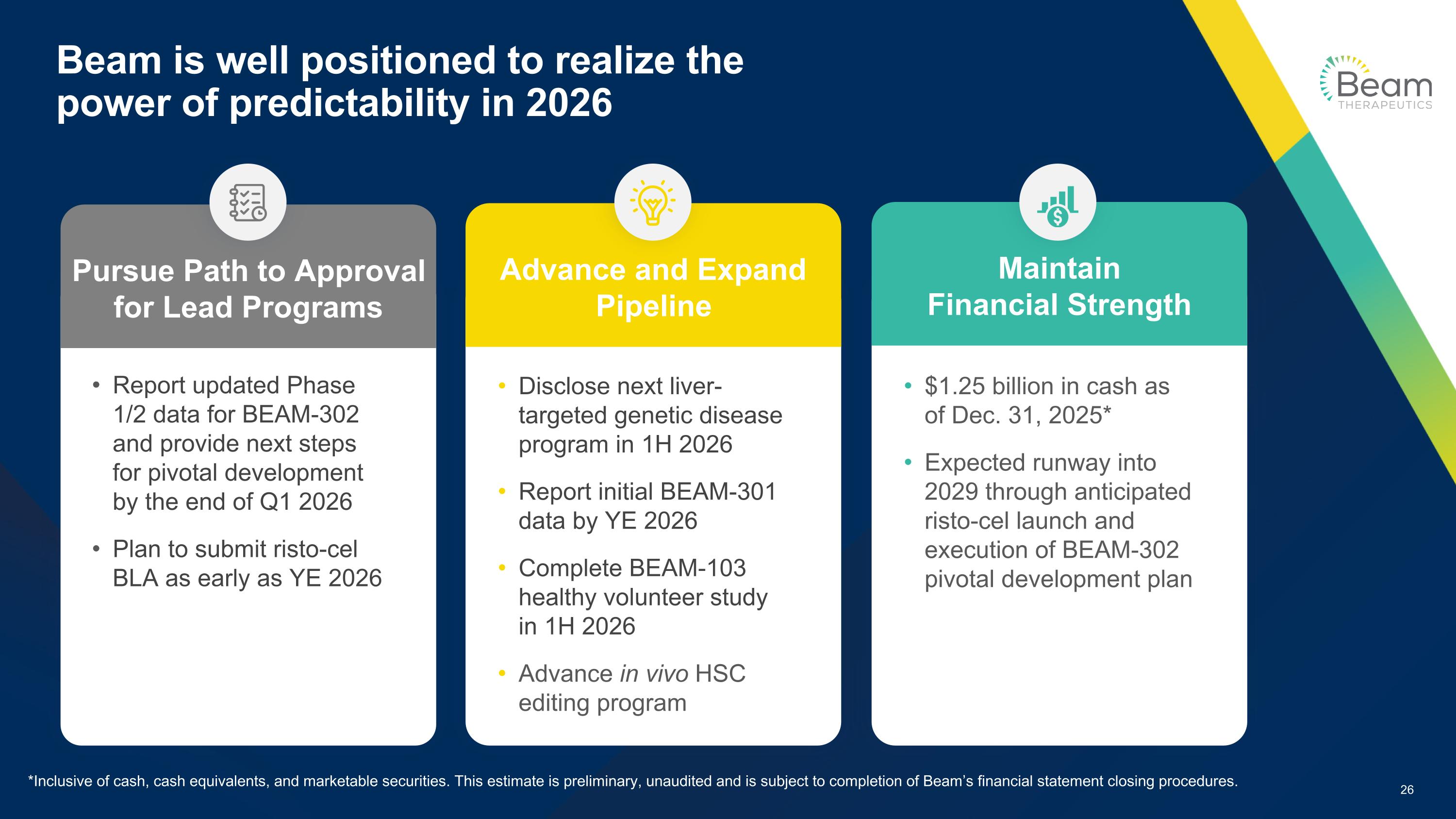
Beam is well positioned to realize the power of predictability in 2026 Report updated Phase 1/2 data for BEAM-302 and provide next steps for pivotal development by the end of Q1 2026 Plan to submit risto-cel BLA as early as YE 2026 Disclose next liver-targeted genetic disease program in 1H 2026 Report initial BEAM-301 data by YE 2026 Complete BEAM-103 healthy volunteer study in 1H 2026 Advance in vivo HSC editing program $1.25 billion in cash as of Dec. 31, 2025* Expected runway into 2029 through anticipated risto-cel launch and execution of BEAM-302 pivotal development plan Pursue Path to Approval for Lead Programs Maintain Financial Strength Advance and Expand Pipeline *Inclusive of cash, cash equivalents, and marketable securities. This estimate is preliminary, unaudited and is subject to completion of Beam’s financial statement closing procedures.

THANK YOU Kyle LIVING WITH SICKLE CELL DISEASE Dan LIVING WITH ALPHA-1 ANTITRYPSIN DEFICIENCY Alyssa and Gayle LIVING WITH GLYCOGEN STORAGE DISEASE TYPE IA