

.2 TH103 Phase 1a Initial Data Release December 2025

Forward-Looking Statements & Disclaimer This presentation contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, that involve substantial risk and uncertainties. All statements, other than statements of historical fact, contained in this presentation, including statements regarding the strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management of Kalaris, the therapeutic potential of TH103 for neovascular Age-related Macular Degeneration and other exudative and neovascular retinal diseases, the anticipated timeline for reporting data from the ongoing Phase 1a clinical trial of TH103 and the ongoing Phase 1b/2 clinical trial of TH103, plans to advance TH103 into Phase 3 clinical trials and to develop TH103 for additional indications and the sufficiency of Kalaris’ cash resources for the period anticipated, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements are based on current expectations and beliefs of the management of Kalaris as well as assumptions made by, and information currently available to, the management of Kalaris and are subject to risks and uncertainties. There can be no assurance that future developments affecting Kalaris will be those that it has anticipated. Forward-looking statements include, but are not limited to, statements concerning the following: the future operations of Kalaris, including research and development activities; the nature, strategy and focus of Kalaris; the development and commercial potential and potential benefits of any product candidate of Kalaris, including expectations around intellectual property protection; anticipated clinical drug development activities and related timelines, including the expected timing for announcement of data and other clinical results; the uncertainties associated with Kalaris’ product candidate, as well as risks associated with the clinical development and regulatory approval of its product candidate, including potential delays in the completion of clinical trials; expectations regarding the therapeutic benefits, clinical potential and clinical development of TH103; risks related to the inability of Kalaris to obtain sufficient additional capital to continue to advance its product candidate; uncertainties in obtaining successful clinical results for product candidates and unexpected costs that may result therefrom; risks related to the failure to realize any value from any product candidates being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; the ability to obtain, maintain, and protect intellectual property rights related to product candidates; changes in regulatory requirements and government incentives; Kalaris’ competitive position and expectations regarding developments and projections relating to its competitors and any competing therapies that are or become available; potential adverse reactions or changes to business relationships resulting from the completion of Kalaris’ merger with AlloVir, Inc. in March 2025; risks associated with the possible failure to realize, or that it may take longer to realize than expected, certain anticipated benefits of the merger, including with respect to future financial and operating results; the risk of involvement in current and future litigation, including securities class action litigation, that could divert the attention of the management of Kalaris, harm Kalaris’ business and for which Kalaris may not have sufficient insurance coverage to cover all costs and damages; and such other factors as are set forth in Kalaris’ public filings with the U.S. Securities and Exchange Commission, including, but not limited to, those described under the heading “Risk Factors”. Kalaris may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on its forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements Kalaris makes. The forward-looking statements contained in this presentation are made as of the date of this presentation, and Kalaris does not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. This presentation also contains estimates, projections and other statistical data made by independent parties and by Kalaris relating to market size and growth and other data about Kalaris’ industry and business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Kalaris has not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of Kalaris’ future performance and the future performance of the market in which Kalaris operates are necessarily subject to high degree of uncertainty and risk. 2

Your Vision Our Mission Kalaris is a clinical stage biopharmaceutical company dedicated to the development and commercialization of treatments for prevalent retinal diseases Our lead asset, TH103, was invented by Dr. Napoleone Ferrara, whose pioneering research established the anti-VEGF class of therapies for retinal and oncology diseases TH103 is an anti-VEGF therapeutic specifically engineered to achieve extended intraocular retention with enhanced VEGF inhibition, to address major unmet needs Napoleone Ferrara, MD Source Photography: Life Science Breakthrough Prize 3
TH103 Engineered to improve upon a drug class that has helped millions of patients, with optimization built directly into the molecule itself. Fully humanized, recombinant fusion protein designed for intravitreal delivery, with potential to be best-in-class for neovascular and exudative retinal diseases. Targets VEGF as a soluble decoy receptor with high affinity for both VEGF and HSPG, engineered for increased and longer-acting activity. 4

TH103 Preclinical Review & Phase 1a Initial Data Summary 5
TH103: Dual-targeting, next generation drug engineered by anti-VEGF pioneer Dr. Napoleone Ferrara to address major unmet needs in retina disease Optimized VEGF Binding: 1 Leverages higher-affinity VEGFR1 , potentially leading to enhanced VEGF inhibition Extended Ocular Retention: 2 Leverages high-affinity binding to HSPG , potentially providing prolonged retinal retention and driving enhanced efficacy and/or durability Source: 1) Holash, J., Davis, S., Papadopoulos, N., Croll, S. D., Ho, L., Russell, M., ... & Rudge, J. S. (2002). VEGF- Trap: a VEGF blocker with potent antitumor effects. Proceedings of the National Academy of Sciences, 99(17), 11393-11398. 2) Xin H, Biswas N, Li P, et al. 2021. 'Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders', Proc Natl Acad Sci U S A, 118. 6 6
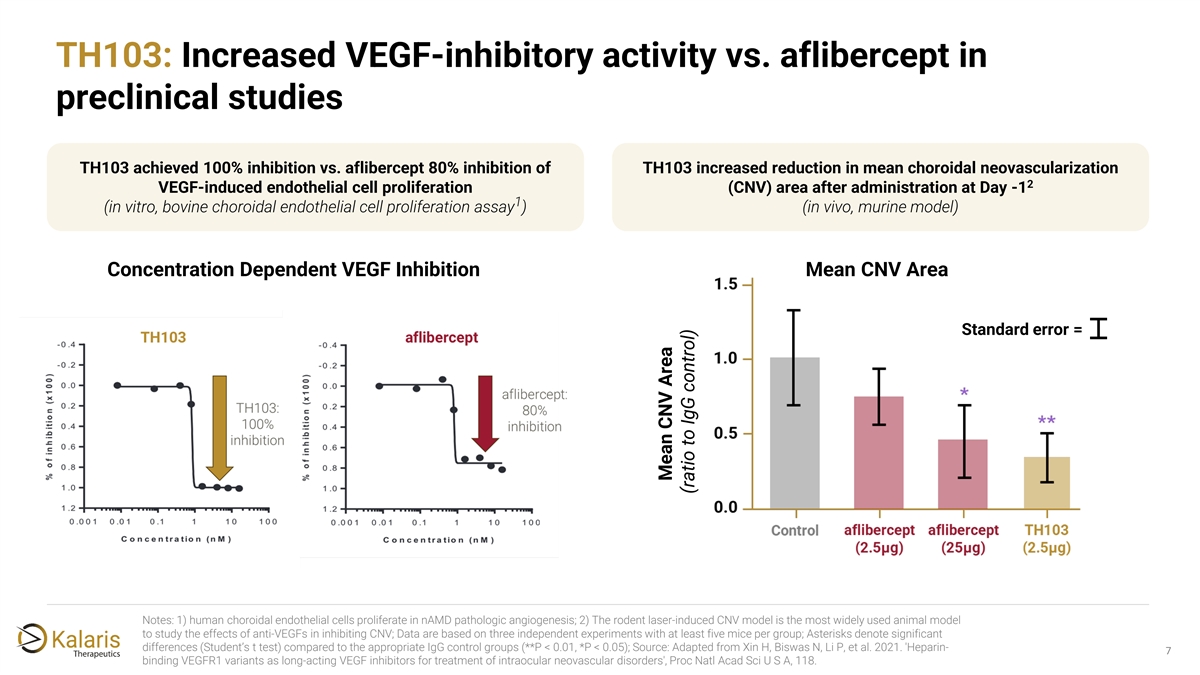
TH103: Increased VEGF-inhibitory activity vs. aflibercept in preclinical studies TH103 achieved 100% inhibition vs. aflibercept 80% inhibition of TH103 increased reduction in mean choroidal neovascularization 2 VEGF-induced endothelial cell proliferation (CNV) area after administration at Day -1 1 (in vitro, bovine choroidal endothelial cell proliferation assay ) (in vivo, murine model) Concentration Dependent VEGF Inhibition Mean CNV Area TH103 Standard error = aflibercept Notes: 1) human choroidal endothelial cells proliferate in nAMD pathologic angiogenesis; 2) The rodent laser-induced CNV model is the most widely used animal model to study the effects of anti-VEGFs in inhibiting CNV; Data are based on three independent experiments with at least five mice per group; Asterisks denote significant differences (Student’s t test) compared to the appropriate IgG control groups (**P < 0.01, *P < 0.05); Source: Adapted from Xin H, Biswas N, Li P, et al. 2021. 'Heparin- 7 binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders', Proc Natl Acad Sci U S A, 118. Mean CNV Area (ratio to IgG control)
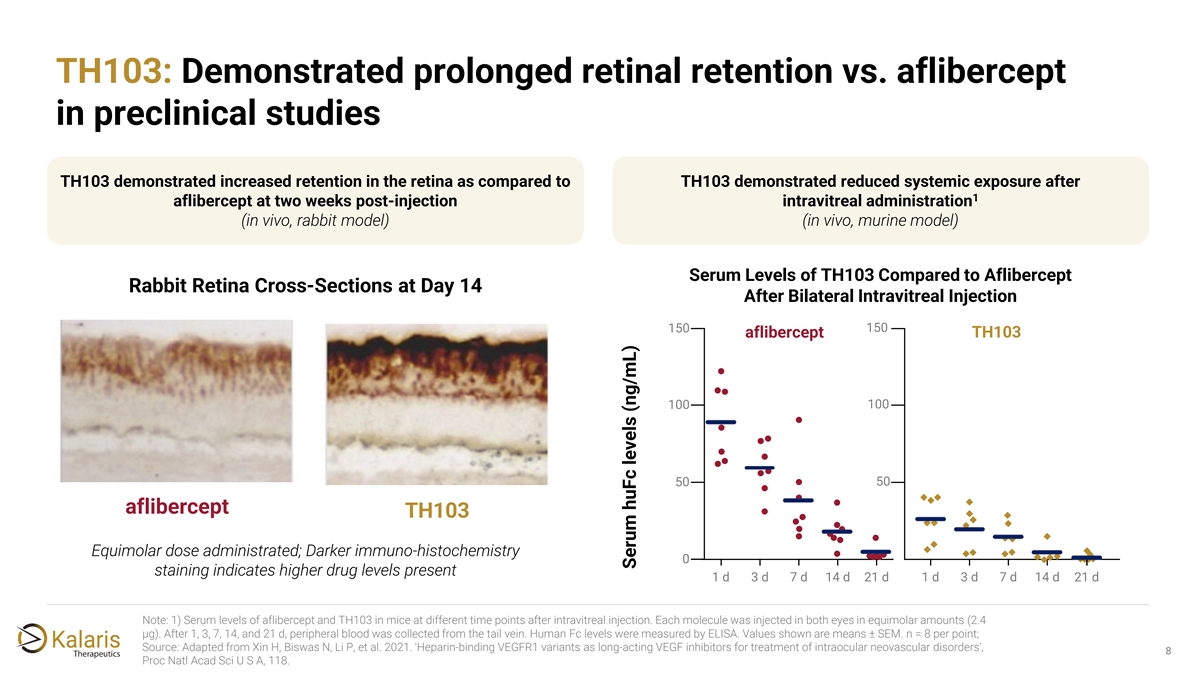
TH103: Demonstrated prolonged retinal retention vs. aflibercept in preclinical studies TH103 demonstrated increased retention in the retina as compared to TH103 demonstrated reduced systemic exposure after 1 aflibercept at two weeks post-injection intravitreal administration (in vivo, rabbit model) (in vivo, murine model) Serum Levels of TH103 Compared to Aflibercept Rabbit Retina Cross-Sections at Day 14 After Bilateral Intravitreal Injection 150 150 aflibercept TH103 100 100 50 50 aflibercept TH103 Equimolar dose administrated; Darker immuno-histochemistry 0 staining indicates higher drug levels present 1 d 3 d 7 d 14 d 21 d 1 d 3 d 7 d 14 d 21 d Note: 1) Serum levels of aflibercept and TH103 in mice at different time points after intravitreal injection. Each molecule was injected in both eyes in equimolar amounts (2.4 μg). After 1, 3, 7, 14, and 21 d, peripheral blood was collected from the tail vein. Human Fc levels were measured by ELISA. Values shown are means ± SEM. n = 8 per point; Source: Adapted from Xin H, Biswas N, Li P, et al. 2021. 'Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders', 8 Proc Natl Acad Sci U S A, 118. Serum huFc levels (ng/mL)
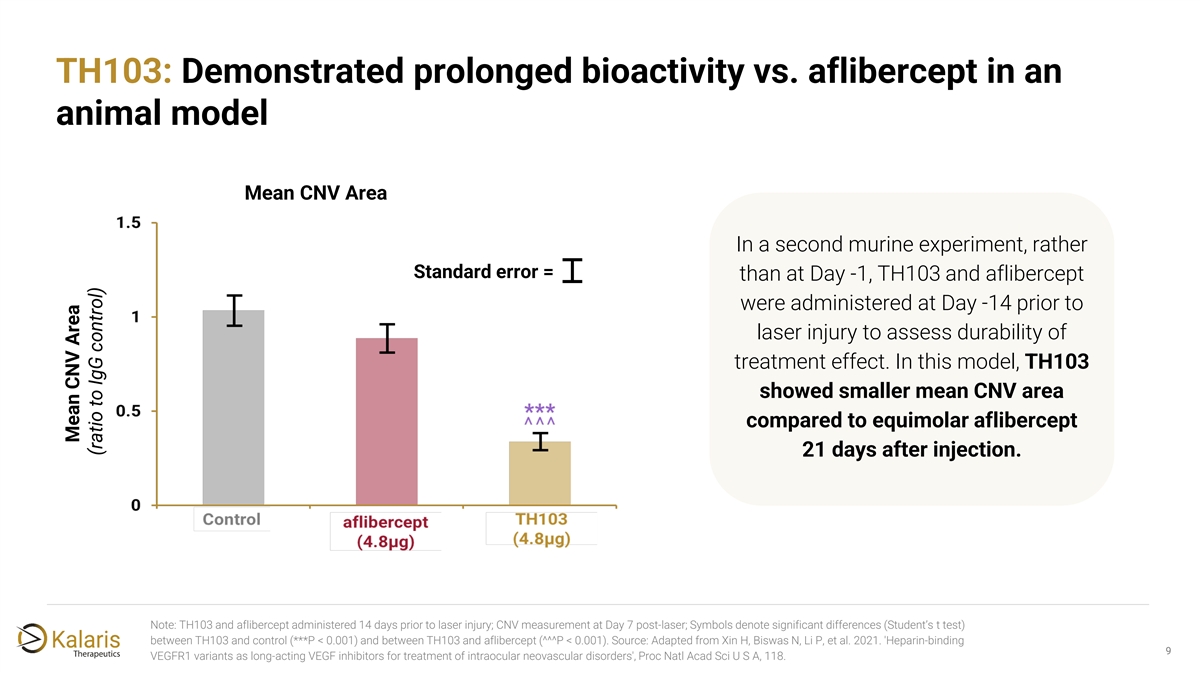
TH103: Demonstrated prolonged bioactivity vs. aflibercept in an animal model Mean CNV Area In a second murine experiment, rather Standard error = than at Day -1, TH103 and aflibercept were administered at Day -14 prior to laser injury to assess durability of treatment effect. In this model, TH103 showed smaller mean CNV area compared to equimolar aflibercept 21 days after injection. Note: TH103 and aflibercept administered 14 days prior to laser injury; CNV measurement at Day 7 post-laser; Symbols denote significant differences (Student’s t test) between TH103 and control (***P < 0.001) and between TH103 and aflibercept (^^^P < 0.001). Source: Adapted from Xin H, Biswas N, Li P, et al. 2021. 'Heparin-binding 9 VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders', Proc Natl Acad Sci U S A, 118. Mean CNV Area (ratio to IgG control)
Phase 1a initial data summary Efficacy: rapid, robust response on BCVA and OCT ✓ parameters observed across dose levels at one month Safety: TH103 generally well tolerated, supporting ✓ exploration of further dose-escalation Durability: ✓ - PK analysis consistent with greater TH103 intraocular retention vs. other leading agents - Single-dose durability signal suggests potential for stronger durability outcomes after standard four- dose loading regimen BCVA = Best Corrected Visual Acuity; CST = Central Subfield Thickness; PK = Pharmacokinetics 10 10
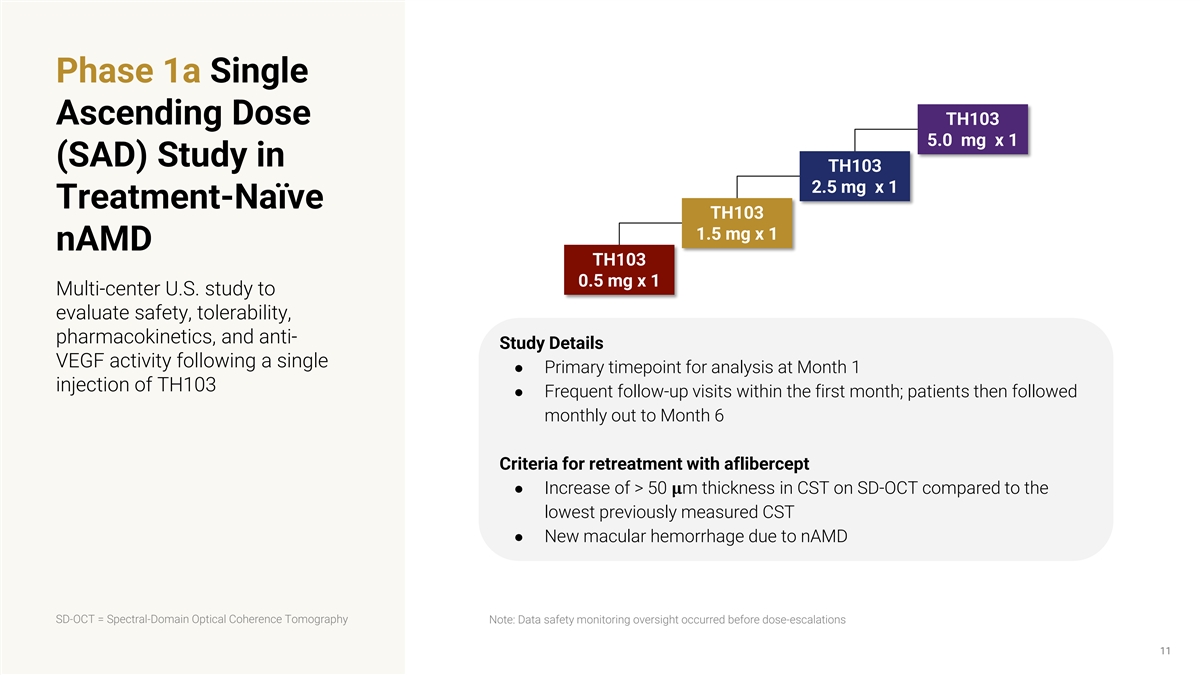
Phase 1a Single Ascending Dose TH103 5.0 mg x 1 (SAD) Study in TH103 2.5 mg x 1 Treatment-Naïve TH103 1.5 mg x 1 nAMD TH103 0.5 mg x 1 Multi-center U.S. study to evaluate safety, tolerability, pharmacokinetics, and anti- Study Details VEGF activity following a single ● Primary timepoint for analysis at Month 1 injection of TH103 ● Frequent follow-up visits within the first month; patients then followed monthly out to Month 6 Criteria for retreatment with aflibercept ● Increase of > 50 ���� m thickness in CST on SD-OCT compared to the lowest previously measured CST ● New macular hemorrhage due to nAMD SD-OCT = Spectral-Domain Optical Coherence Tomography Note: Data safety monitoring oversight occurred before dose-escalations 11
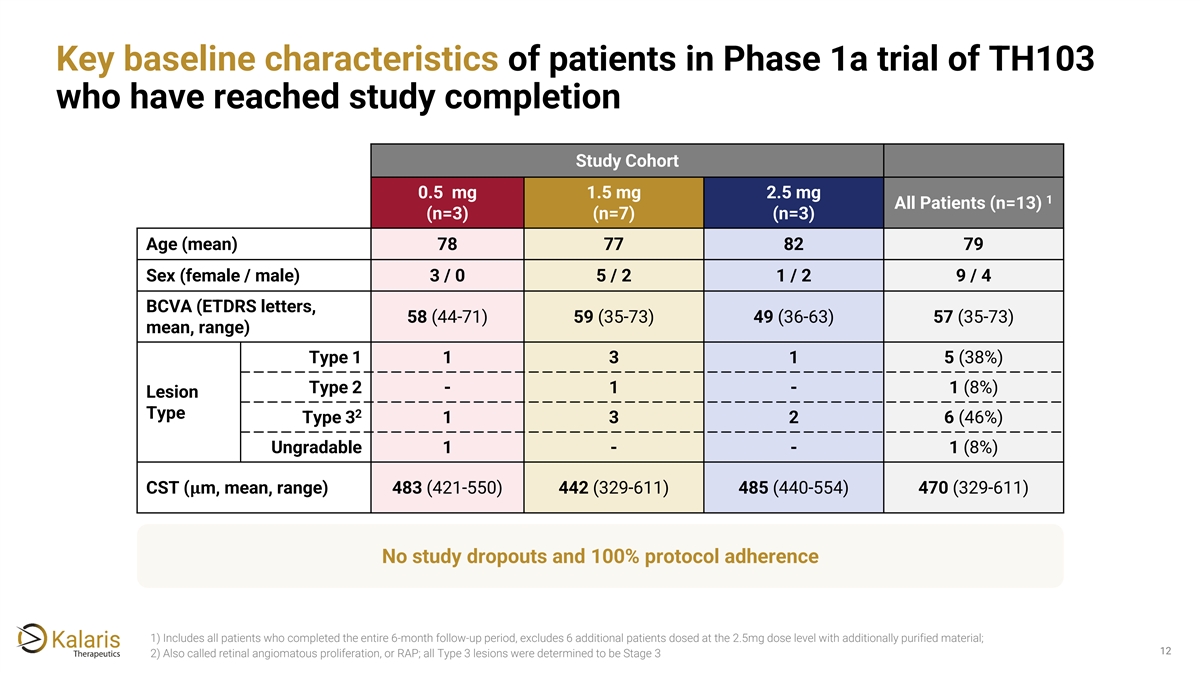
Key baseline characteristics of patients in Phase 1a trial of TH103 who have reached study completion Study Cohort 0.5 mg 1.5 mg 2.5 mg 1 All Patients (n=13) (n=3) (n=7) (n=3) Age (mean) 78 77 82 79 Sex (female / male) 3 / 0 5 / 2 1 / 2 9 / 4 BCVA (ETDRS letters, 58 (44-71) 59 (35-73) 49 (36-63) 57 (35-73) mean, range) Type 1 1 3 1 5 (38%) Type 2 - 1 - 1 (8%) Lesion Type 2 Type 3 1 3 2 6 (46%) Ungradable 1 - - 1 (8%) CST (���� m, mean, range) 483 (421-550) 442 (329-611) 485 (440-554) 470 (329-611) No study dropouts and 100% protocol adherence 1) Includes all patients who completed the entire 6-month follow-up period, excludes 6 additional patients dosed at the 2.5mg dose level with additionally purified material; 12 2) Also called retinal angiomatous proliferation, or RAP; all Type 3 lesions were determined to be Stage 3
Phase 1a initial data summary Efficacy: rapid, robust response on BCVA and OCT ✓ parameters observed across dose levels at one month 13 13
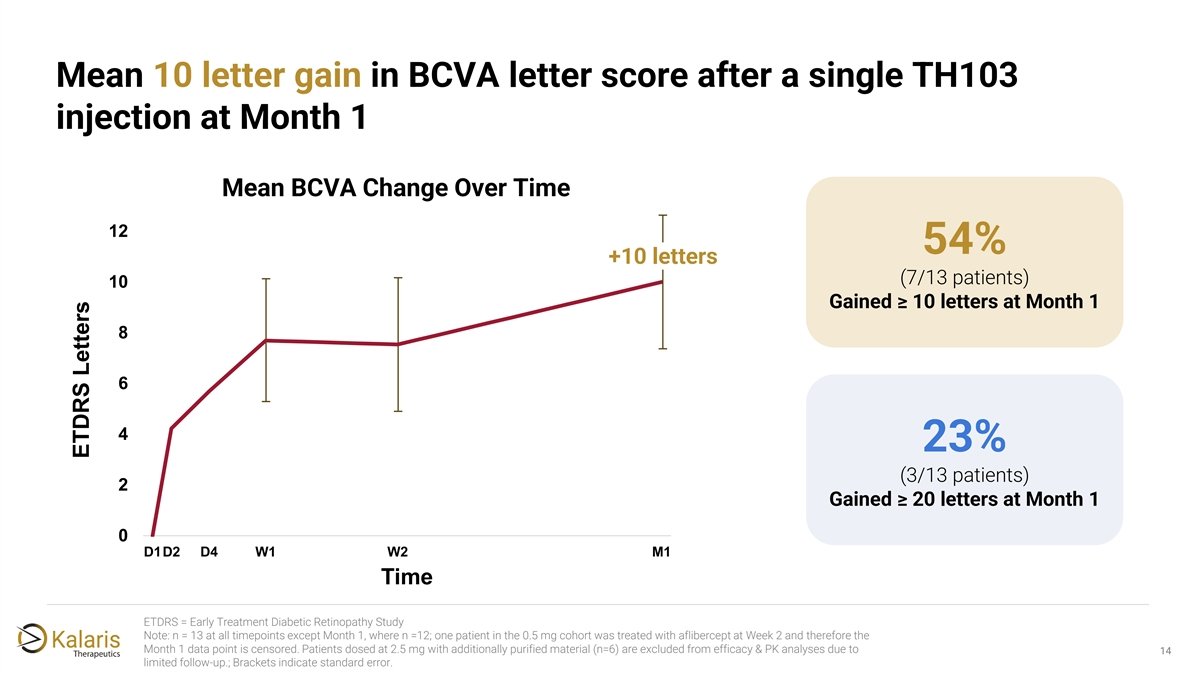
Mean 10 letter gain in BCVA letter score after a single TH103 injection at Month 1 Mean BCVA Change Over Time 12 54% +10 letters (7/13 patients) 10 Gained ≥ 10 letters at Month 1 8 6 4 23% (3/13 patients) 2 Gained ≥ 20 letters at Month 1 0 D1D2 D4 W1 W2 M1 Time ETDRS = Early Treatment Diabetic Retinopathy Study Note: n = 13 at all timepoints except Month 1, where n =12; one patient in the 0.5 mg cohort was treated with aflibercept at Week 2 and therefore the Month 1 data point is censored. Patients dosed at 2.5 mg with additionally purified material (n=6) are excluded from efficacy & PK analyses due to 14 limited follow-up.; Brackets indicate standard error. ETDRS Letters
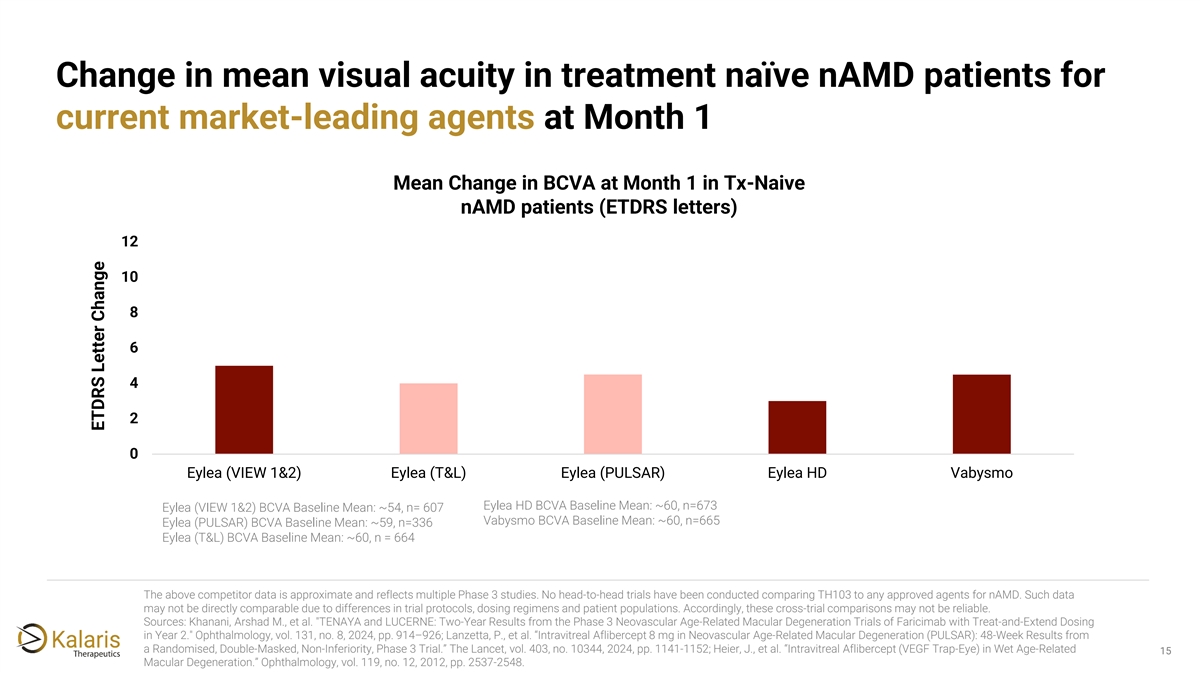
Change in mean visual acuity in treatment naïve nAMD patients for current market-leading agents at Month 1 Mean Change in BCVA at Month 1 in Tx-Naive nAMD patients (ETDRS letters) 12 10 8 6 4 2 0 Eylea (VIEW 1&2) Eylea (T&L) Eylea (PULSAR) Eylea HD Vabysmo Eylea HD BCVA Baseline Mean: ~60, n=673 Eylea (VIEW 1&2) BCVA Baseline Mean: ~54, n= 607 Vabysmo BCVA Baseline Mean: ~60, n=665 Eylea (PULSAR) BCVA Baseline Mean: ~59, n=336 Eylea (T&L) BCVA Baseline Mean: ~60, n = 664 The above competitor data is approximate and reflects multiple Phase 3 studies. No head-to-head trials have been conducted comparing TH103 to any approved agents for nAMD. Such data may not be directly comparable due to differences in trial protocols, dosing regimens and patient populations. Accordingly, these cross-trial comparisons may not be reliable. Sources: Khanani, Arshad M., et al. TENAYA and LUCERNE: Two-Year Results from the Phase 3 Neovascular Age-Related Macular Degeneration Trials of Faricimab with Treat-and-Extend Dosing in Year 2. Ophthalmology, vol. 131, no. 8, 2024, pp. 914–926; Lanzetta, P., et al. “Intravitreal Aflibercept 8 mg in Neovascular Age-Related Macular Degeneration (PULSAR): 48-Week Results from a Randomised, Double-Masked, Non-Inferiority, Phase 3 Trial.” The Lancet, vol. 403, no. 10344, 2024, pp. 1141-1152; Heier, J., et al. “Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-Related 15 Macular Degeneration.” Ophthalmology, vol. 119, no. 12, 2012, pp. 2537-2548. ETDRS Letter Change
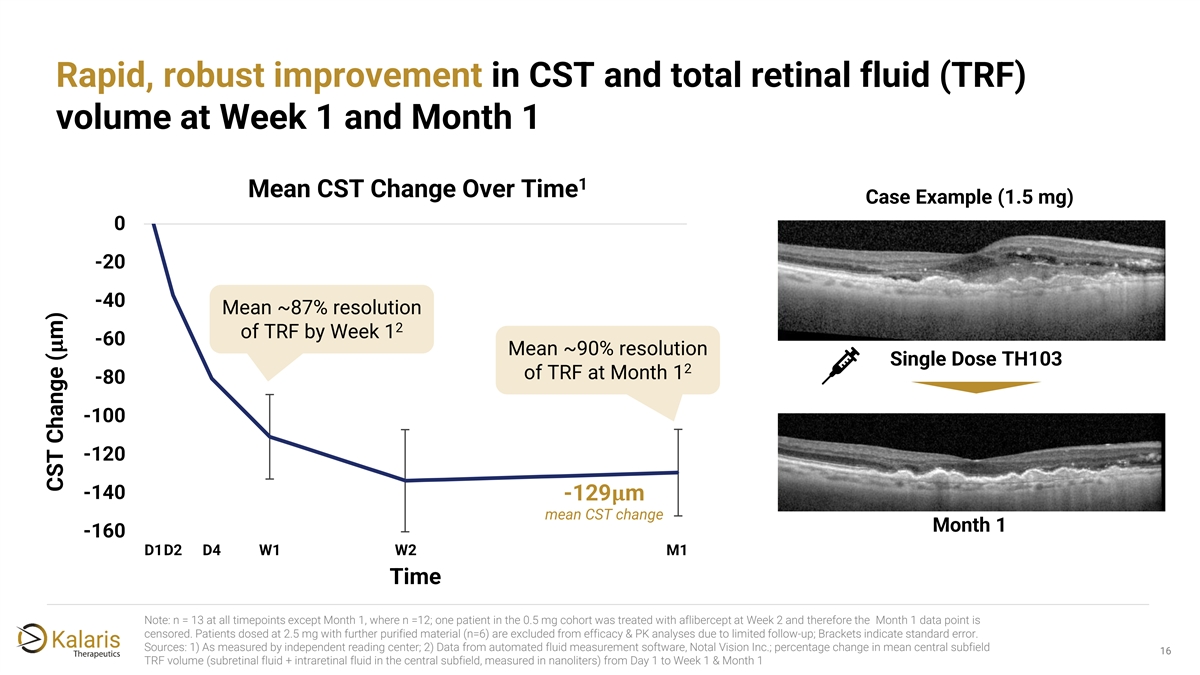
Rapid, robust improvement in CST and total retinal fluid (TRF) volume at Week 1 and Month 1 1 Mean CST Change Over Time Case Example (1.5 mg) 0 -20 -40 Mean ~87% resolution 2 of TRF by Week 1 -60 ���� Mean ~90% resolution Single Dose TH103 2 of TRF at Month 1 -80 -100 -120 -140 -129���� m mean CST change Month 1 -160 D1D2 D4 W1 W2 M1 Time Note: n = 13 at all timepoints except Month 1, where n =12; one patient in the 0.5 mg cohort was treated with aflibercept at Week 2 and therefore the Month 1 data point is censored. Patients dosed at 2.5 mg with further purified material (n=6) are excluded from efficacy & PK analyses due to limited follow-up; Brackets indicate standard error. Sources: 1) As measured by independent reading center; 2) Data from automated fluid measurement software, Notal Vision Inc.; percentage change in mean central subfield 16 1 TRF volume (subretinal fluid + intraretinal fluid in the central subfield, measured in nanoliters) from Day 1 to Week 1 & Month 1 CST Change ( m)
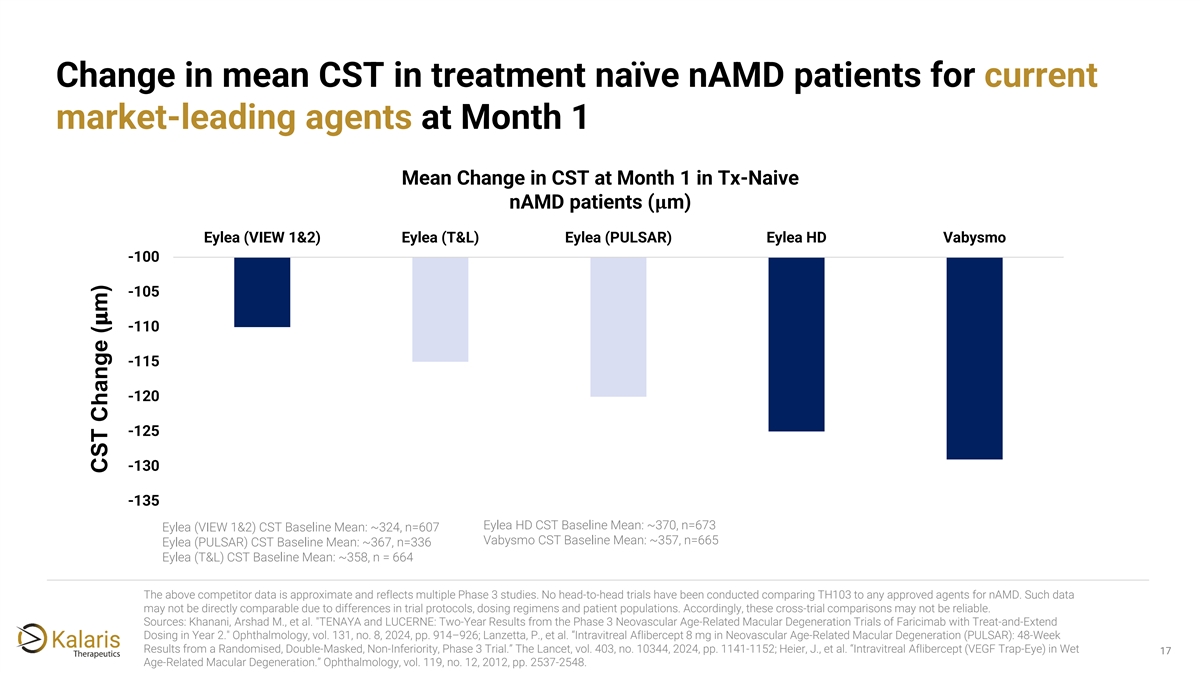
Change in mean CST in treatment naïve nAMD patients for current market-leading agents at Month 1 Mean Change in CST at Month 1 in Tx-Naive nAMD patients (���� m) Eylea (VIEW 1&2) Eylea (T&L) Eylea (PULSAR) Eylea HD Vabysmo -100 -105 ���� -110 -115 -120 -125 -130 -135 Eylea HD CST Baseline Mean: ~370, n=673 Eylea (VIEW 1&2) CST Baseline Mean: ~324, n=607 Vabysmo CST Baseline Mean: ~357, n=665 Eylea (PULSAR) CST Baseline Mean: ~367, n=336 Eylea (T&L) CST Baseline Mean: ~358, n = 664 The above competitor data is approximate and reflects multiple Phase 3 studies. No head-to-head trials have been conducted comparing TH103 to any approved agents for nAMD. Such data may not be directly comparable due to differences in trial protocols, dosing regimens and patient populations. Accordingly, these cross-trial comparisons may not be reliable. Sources: Khanani, Arshad M., et al. TENAYA and LUCERNE: Two-Year Results from the Phase 3 Neovascular Age-Related Macular Degeneration Trials of Faricimab with Treat-and-Extend Dosing in Year 2. Ophthalmology, vol. 131, no. 8, 2024, pp. 914–926; Lanzetta, P., et al. “Intravitreal Aflibercept 8 mg in Neovascular Age-Related Macular Degeneration (PULSAR): 48-Week Results from a Randomised, Double-Masked, Non-Inferiority, Phase 3 Trial.” The Lancet, vol. 403, no. 10344, 2024, pp. 1141-1152; Heier, J., et al. “Intravitreal Aflibercept (VEGF Trap-Eye) in Wet 17 Age-Related Macular Degeneration.” Ophthalmology, vol. 119, no. 12, 2012, pp. 2537-2548. CST Change ( m)
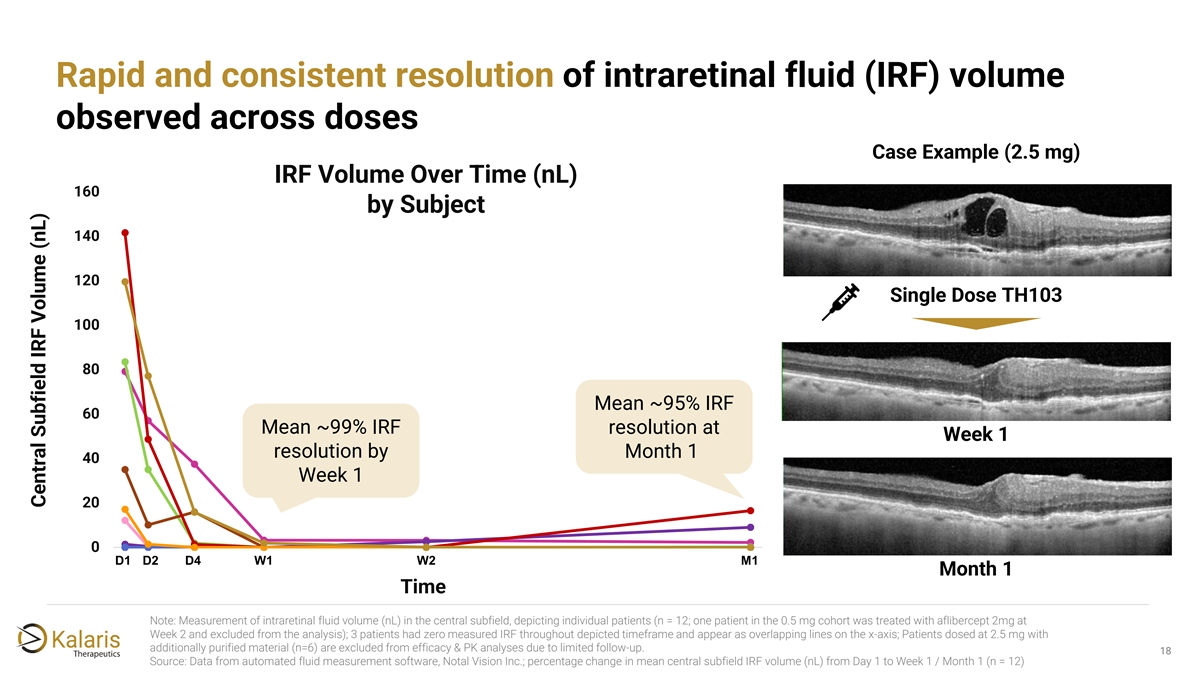
Rapid and consistent resolution of intraretinal fluid (IRF) volume observed across doses Case Example (2.5 mg) IRF Volume Over Time (nL) 160 by Subject 140 120 Single Dose TH103 100 80 Mean ~95% IRF 60 Mean ~99% IRF resolution at Week 1 resolution by Month 1 40 Week 1 20 0 D1 D2 D4 W1 14 W2 M1 1 4 7 10 13 16 19 22 25 28 Month 1 Time Note: Measurement of intraretinal fluid volume (nL) in the central subfield, depicting individual patients (n = 12; one patient in the 0.5 mg cohort was treated with aflibercept 2mg at Week 2 and excluded from the analysis); 3 patients had zero measured IRF throughout depicted timeframe and appear as overlapping lines on the x-axis; Patients dosed at 2.5 mg with additionally purified material (n=6) are excluded from efficacy & PK analyses due to limited follow-up. 18 2 Source: Data from automated fluid measurement software, Notal Vision Inc.; percentage change in mean central subfield IRF volume (nL) from Day 1 to Week 1 / Month 1 (n = 12) Central Subfield IRF Volume (nL)
Phase 1a initial data summary Efficacy: rapid, robust response on BCVA and OCT ✓ parameters observed across dose levels at one month Safety: TH103 generally well tolerated, supporting ✓ exploration of further dose-escalation 19 19
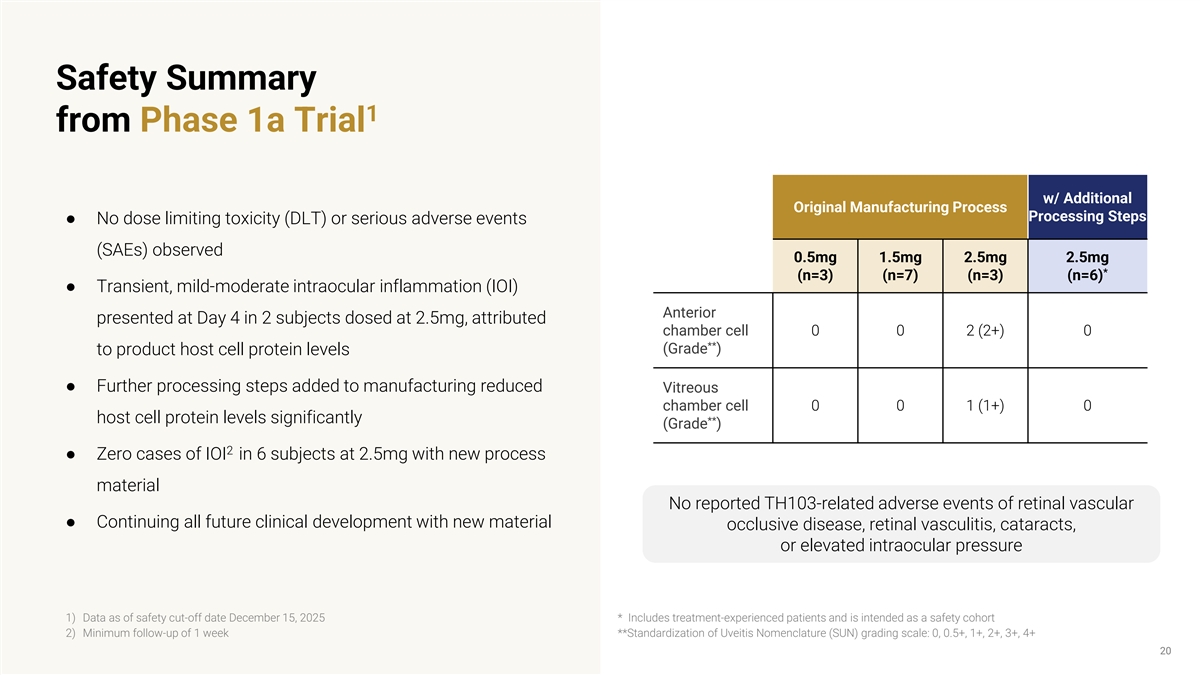
Safety Summary 1 from Phase 1a Trial w/ Additional Original Manufacturing Process Processing Steps ● No dose limiting toxicity (DLT) or serious adverse events (SAEs) observed 0.5mg 1.5mg 2.5mg 2.5mg * (n=3) (n=7) (n=3) (n=6) ● Transient, mild-moderate intraocular inflammation (IOI) Anterior presented at Day 4 in 2 subjects dosed at 2.5mg, attributed chamber cell 0 0 2 (2+) 0 ** (Grade ) to product host cell protein levels ● Further processing steps added to manufacturing reduced Vitreous chamber cell 0 0 1 (1+) 0 host cell protein levels significantly ** (Grade ) 2 ● Zero cases of IOI in 6 subjects at 2.5mg with new process material No reported TH103-related adverse events of retinal vascular ● Continuing all future clinical development with new material occlusive disease, retinal vasculitis, cataracts, or elevated intraocular pressure 1) Data as of safety cut-off date December 15, 2025 * Includes treatment-experienced patients and is intended as a safety cohort 2) Minimum follow-up of 1 week **Standardization of Uveitis Nomenclature (SUN) grading scale: 0, 0.5+, 1+, 2+, 3+, 4+ 20
Phase 1a initial data summary Efficacy: rapid, robust response on BCVA and OCT ✓ parameters observed across dose levels at one month Safety: TH103 generally well tolerated, supporting ✓ exploration of further dose-escalation Durability: ✓ - PK analysis consistent with greater TH103 intraocular retention vs. other leading agents - Single-dose durability signal suggests potential for stronger durability outcomes after standard four- dose loading regimen 21 21
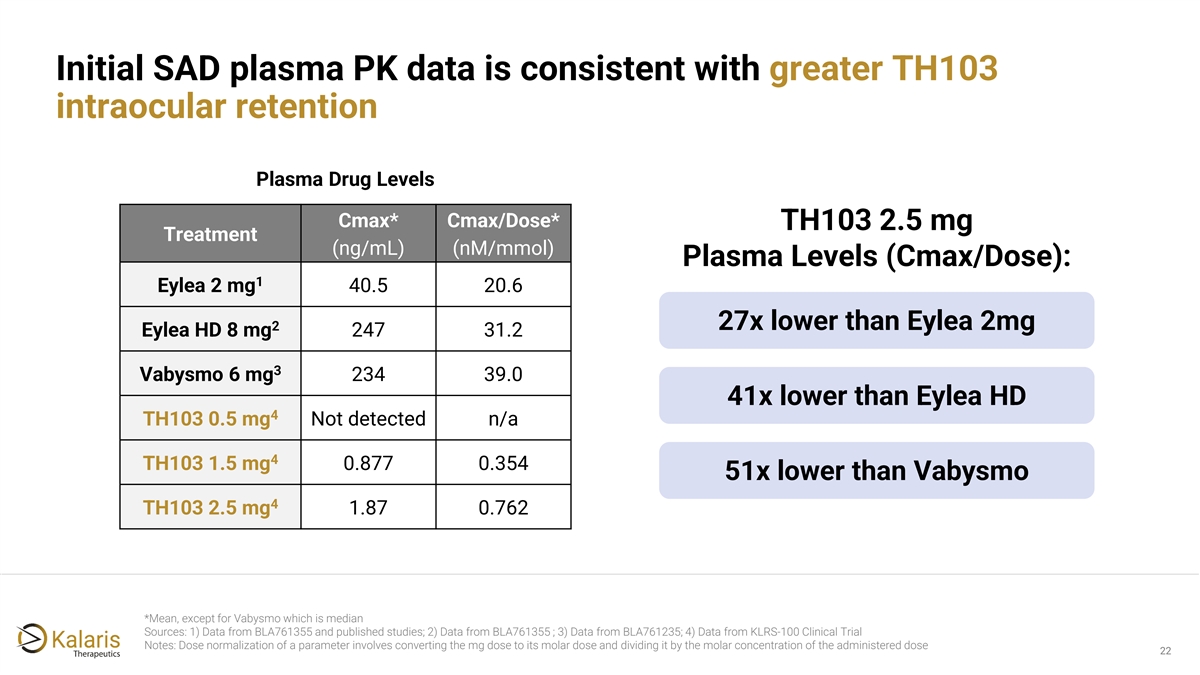
Initial SAD plasma PK data is consistent with greater TH103 intraocular retention Plasma Drug Levels Cmax* Cmax/Dose* TH103 2.5 mg Treatment (ng/mL) (nM/mmol) Plasma Levels (Cmax/Dose): 1 Eylea 2 mg 40.5 20.6 27x lower than Eylea 2mg 2 Eylea HD 8 mg 247 31.2 3 Vabysmo 6 mg 234 39.0 41x lower than Eylea HD 4 TH103 0.5 mg Not detected n/a 4 TH103 1.5 mg 0.877 0.354 51x lower than Vabysmo 4 TH103 2.5 mg 1.87 0.762 *Mean, except for Vabysmo which is median Sources: 1) Data from BLA761355 and published studies; 2) Data from BLA761355 ; 3) Data from BLA761235; 4) Data from KLRS-100 Clinical Trial Notes: Dose normalization of a parameter involves converting the mg dose to its molar dose and dividing it by the molar concentration of the administered dose 22
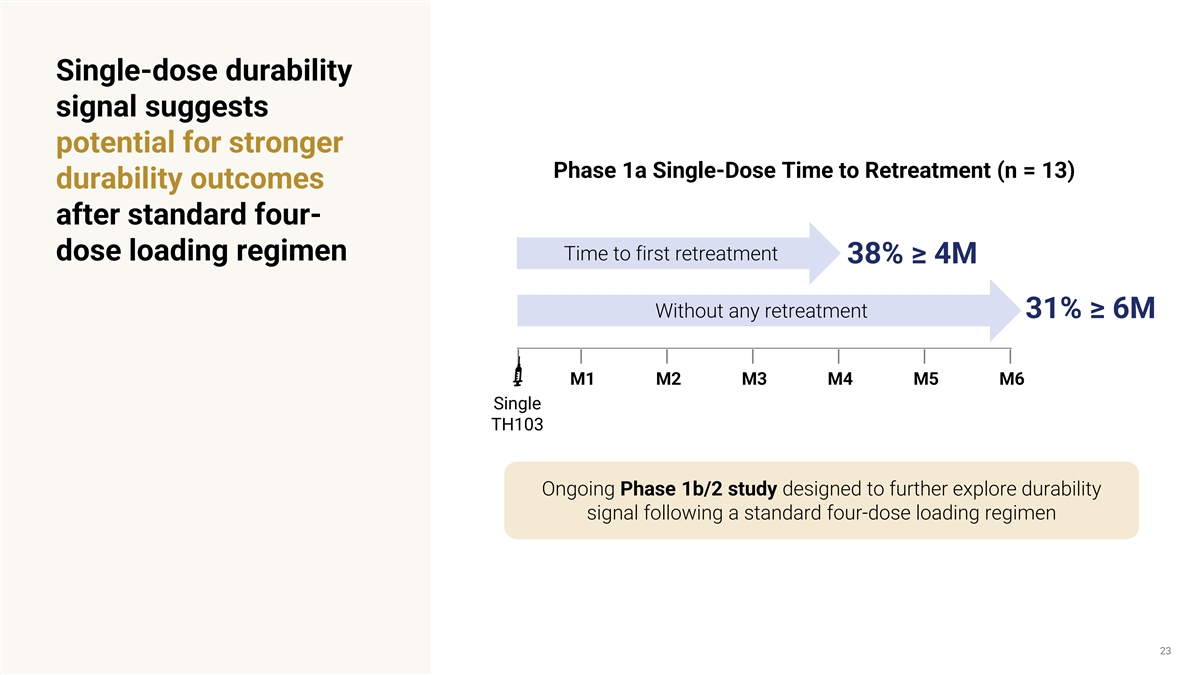
Single-dose durability signal suggests potential for stronger Phase 1a Single-Dose Time to Retreatment (n = 13) durability outcomes after standard four- Time to first retreatment dose loading regimen 38% ≥ 4M Without any retreatment 31% ≥ 6M M1 M2 M3 M4 M5 M6 Single TH103 Ongoing Phase 1b/2 study designed to further explore durability signal following a standard four-dose loading regimen 23
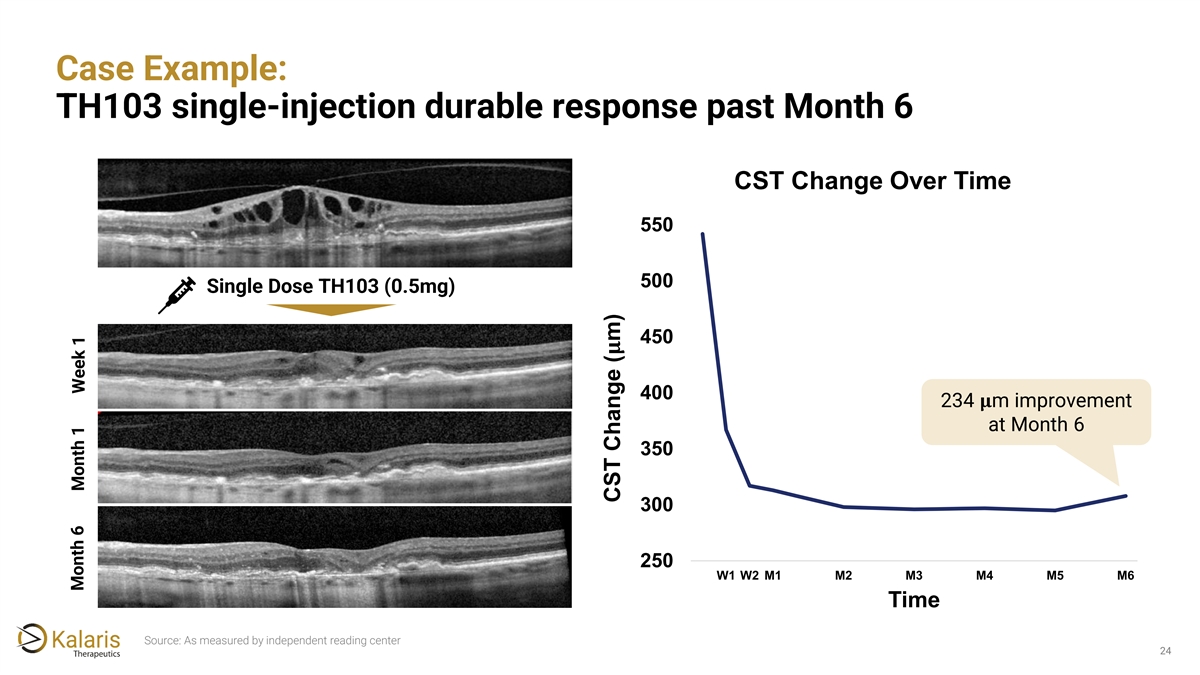
Case Example: TH103 single-injection durable response past Month 6 CST Change Over Time 550 500 Single Dose TH103 (0.5mg) 450 ���� 400 234 ���� m improvement at Month 6 350 300 250 W1 W2 M1 M2 M3 M4 M5 M6 Time Source: As measured by independent reading center 24 Month 6 Month 1 Week 1 CST Change ( m)
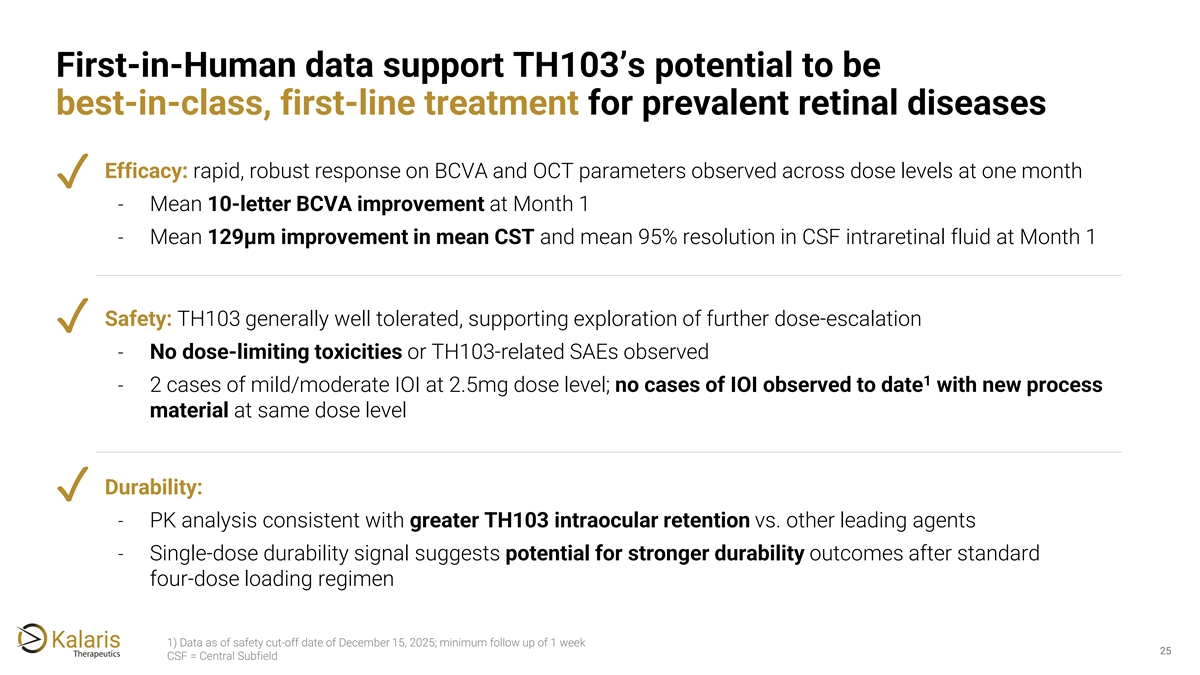
First-in-Human data support TH103’s potential to be best-in-class, first-line treatment for prevalent retinal diseases Efficacy: rapid, robust response on BCVA and OCT parameters observed across dose levels at one month ✓ - Mean 10-letter BCVA improvement at Month 1 - Mean 129μm improvement in mean CST and mean 95% resolution in CSF intraretinal fluid at Month 1 Safety: TH103 generally well tolerated, supporting exploration of further dose-escalation ✓ - No dose-limiting toxicities or TH103-related SAEs observed 1 - 2 cases of mild/moderate IOI at 2.5mg dose level; no cases of IOI observed to date with new process material at same dose level Durability: ✓ - PK analysis consistent with greater TH103 intraocular retention vs. other leading agents - Single-dose durability signal suggests potential for stronger durability outcomes after standard four-dose loading regimen 1) Data as of safety cut-off date of December 15, 2025; minimum follow up of 1 week 25 25 CSF = Central Subfield

Phase 1b/2 & Next Steps 26
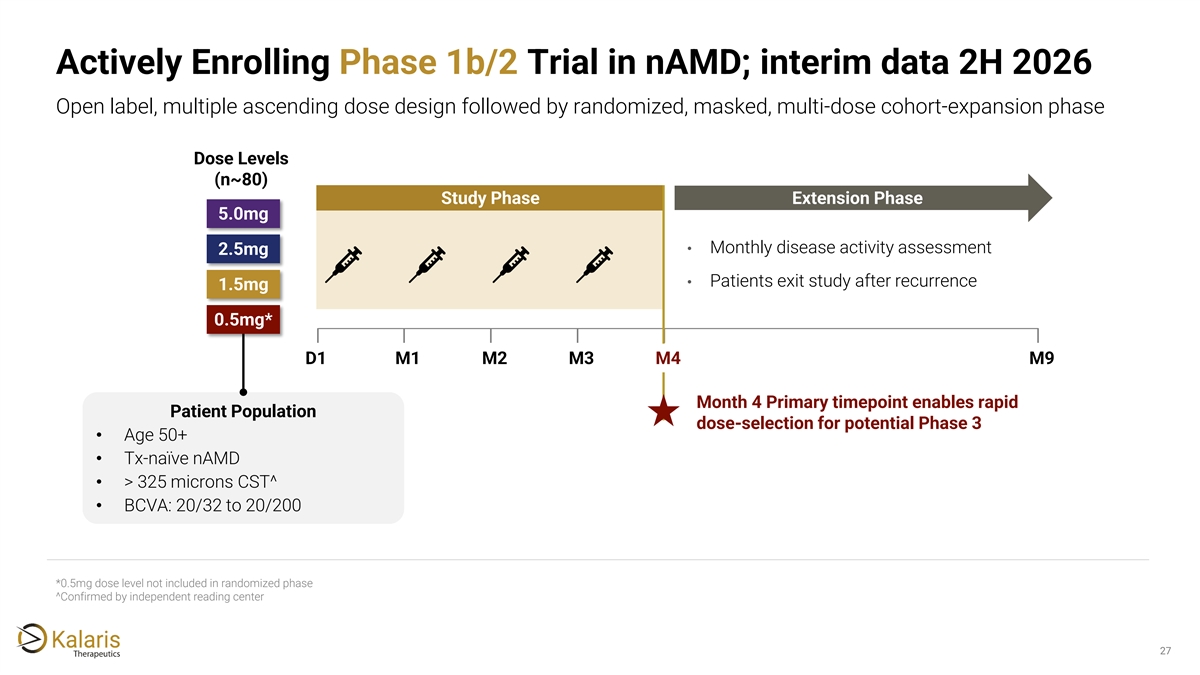
Actively Enrolling Phase 1b/2 Trial in nAMD; interim data 2H 2026 Open label, multiple ascending dose design followed by randomized, masked, multi-dose cohort-expansion phase Dose Levels (n~80) Study Phase Extension Phase 5.0mg • Monthly disease activity assessment 2.5mg • Patients exit study after recurrence 1.5mg 0.5mg* D1 M1 M2 M3 M4 M9 Month 4 Primary timepoint enables rapid Patient Population dose-selection for potential Phase 3 • Age 50+ • Tx-naïve nAMD • > 325 microns CST^ • BCVA: 20/32 to 20/200 *0.5mg dose level not included in randomized phase ^Confirmed by independent reading center 27

Kalaris is accelerating First-in-Human data support TH103's potentially clinically 1 meaningful differentiation and advancement into multi-dose TH103 clinical trials development into later stage studies with preliminary readout expected in 2H 2026 Actively enrolling a Phase 1b/2 multiple ascending dose- finding study in up to 80 nAMD patients; preliminary data 2 from Phase 1b/2 trial is expected in 2H 2026 Planned expansions beyond nAMD into other prevalent VEGF-mediated diseases such as Diabetic Macular Edema / 3 Diabetic Retinopathy, Retinal Vein Occlusion 28

29
Glossary OCT: Optical Coherence BCVA: Best Corrected Visual Acuity DR: Diabetic Retinopathy Tomography Cmax: Maximum Plasma ETDRS: Early Treatment Diabetic PK: Pharmacokinetics Concentration Retinopathy Study HSPG: Heparan Sulfate CNV: choroidal Neovascularization RVO: Retinal Vein Occlusion Proteoglycans CST: Central Subfield Thickness IOI: Intraocular Inflammation SAD: Single Ascending Dose DLT: Dose Limiting Toxicity IOP: Intraocular Pressure SAE: Serious Adverse Events SD-OCT: Spectral-Domain Optical DME: Diabetic Macular Edema IRF: Intraretinal Fluid Coherence Tomography nAMD: neovascular Age-related TRF: Total Retinal Fluid Macular Degeneration 30