

.2 Cyclerion: Pioneering a New Era in Neuropsychiatric Therapies Investor Call th September 24 , 2025, 10:30am EST

2 Legal Disclaimer This presentation has been prepared by Cyclerion Therapeutics, Inc. and its consolidated subsidiaries (“the Company,” “Cyclerion,” “we,” “us,” and “our”). Certain matters discussed in this presentation are “forward-looking statements.” We may, in some cases, use terms such as “potential,” “may,” “expects,” “plans,” “could,” “opportunity,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements involve risks, uncertainties and other factors that may cause actual results, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. Forward-looking statements in this presentation include, but are not limited to, statements about the timing of development and commercialization of our product candidates, our ability to develop product candidates and the timing of related milestones, creation of shareholder value, adoption of our product candidates once commercialized, ongoing discussions with potential business partners and expected addressable market size. We cannot assure you that the forward-looking statements in this presentation will prove to be accurate. Some of the factors that could cause actual performance and results to differ materially from those projected or suggested in the forward-looking statements due to various risks and uncertainties, include, but are not limited to, the substantial doubt regarding the our ability to continue as a going concern, our ability to raise additional funding, our ability to enroll patients in future clinical studies, our ability to obtain regulatory approval for our product candidates, unanticipated changes to our nonclinical or clinical study protocols due to regulatory reasons or unanticipated events, which could lead to increased costs to us and could delay our development timeline, our reliance on third parties to conduct clinical studies and to manufacture drug supplies for our product candidates, our ability to adequately protect our intellectual property, and the other important risk factors discussed under the heading “Risk Factors” in our Annual Report on Form 10-K filed with the SEC on March 4, 2025 as well as other risks and uncertainties which may be described in any subsequent quarterly report on Form 10-Q filed by the Company and the other reports the Company files with the Securities Exchange Commission. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward- looking statements are made only as of the date of this presentation and the Company undertakes no obligation to update such forward-looking statements to reflect subsequent events or circumstances. Certain market data, study data and industry data used in this presentation were obtained from reports of governmental agencies and industry publications, studies and surveys. Management has not independently verified such data and as such, make no guarantees as to its accuracy, completeness or timeliness.
33 Relaunching Cyclerion: A new, pioneering neuropsychiatric-focused company Strong Company Fundamentals Strategic Pipeline Focus ü Built on opportunity for patient impact and ü Tech-enhanced therapies to optimize patient shareholder value creation benefit ü Driven by lean, nimble team with world-class ü Significant neuropsychiatric market potential neuropsychiatric and biopharma experience with patients requiring new treatment options ü Plan to focus on disciplined advancement ü Novel, improved or first-in-class therapies through de-risked value inflection points ü Validated modes of action with potentially rapid path to clinical Proof-of-Concept (POC) Clear strategic plan to advance programs through de-risked inflection points to drive shareholder value

44 Cyclerion’s neuropsychiatric expertise and agility in action Deep Industry Expertise with Strong Relationships from early research to late commercialization Officers Key Advisors Regina Graul, PhD; CEO Rhonda Chicko; CFO Scholar Rock, Editas, EQRx, Ironwood, Ironwood Cyclerion Laeben Lester, MD Linda Carpenter, Husseini Manji, Anesthesiology leader; MD MD, FRCPC Johns Hopkins Neuropsychiatry Development leader; Board of Directors leader; Brown, Yale, Oxford, J&J, Director of Director NIMH, UK Errol De Souza, Steven Hyman, MD BRaIN*** Govt PhD, Chair Broad Institute of Neurocrine, MIT* and Harvard, Lisa Loram, PhD Lawrence Olanoff, Royalty Pharma Erika De Boever, Provost of Harvard Regulatory Affairs; U of MD, PhD DDS, MPH, PhD NIMH** Director Colorado, Boulder R&D; MUSC, Forest, Clinical Development; Celsion U of Michigan Michael Higgins Peter Hecht, PhD Voyager Tisento, Cyclerion, Therapeutics, Ironwood Michael Madden Andy Parratt Jessica Duda Camp4, Polaris Medical Device New Product BD Advisor; Partners, Ironwood Development; Planning & Red Sky Mountain Medical Commercialization; Regina Graul, PhD Dina Katabi, PhD Karuna, AlgavitaBio EQRx, Ironwood, MIT, Emerald Cyclerion Innovations, MacArthur Fellow *Massachusetts Institute of Technology **National Institute of Mental Health *** Brain Research and Interventional Neurotherapeutics (BRaIN) program at Butler Hospital
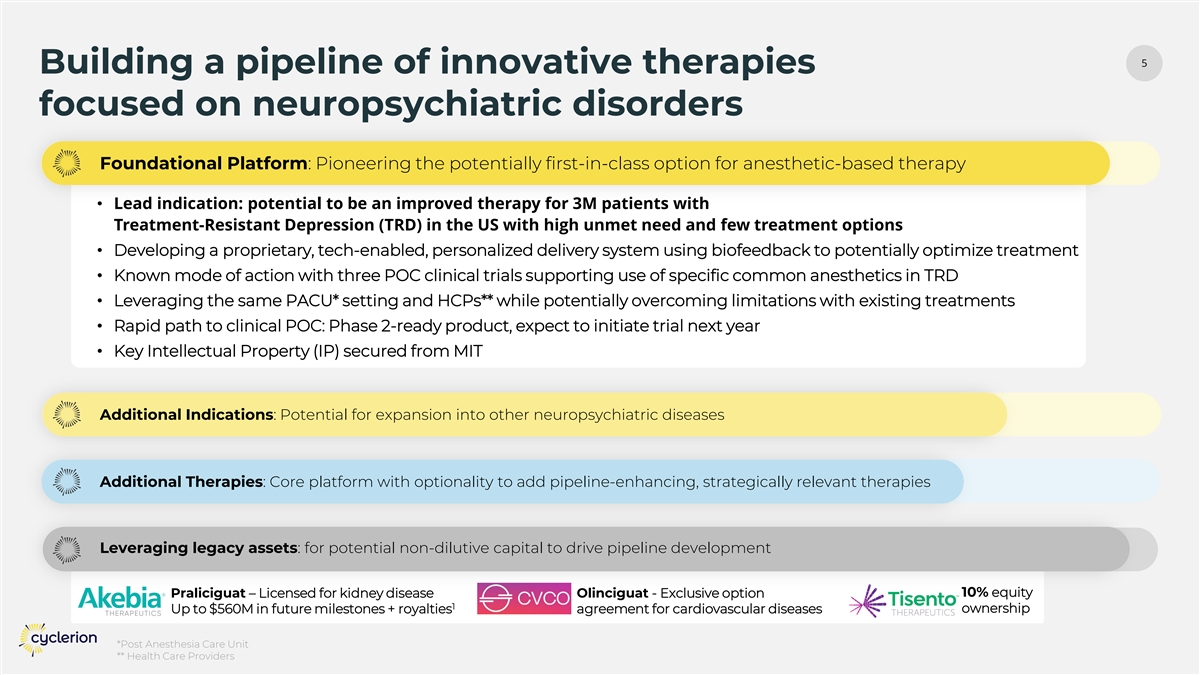
5 Building a pipeline of innovative therapies focused on neuropsychiatric disorders Foundational Platform: Pioneering the potentially first-in-class option for anesthetic-based therapy • Lead indication: potential to be an improved therapy for 3M patients with Treatment-Resistant Depression (TRD) in the US with high unmet need and few treatment options • Developing a proprietary, tech-enabled, personalized delivery system using biofeedback to potentially optimize treatment • Known mode of action with three POC clinical trials supporting use of specific common anesthetics in TRD • Leveraging the same PACU* setting and HCPs** while potentially overcoming limitations with existing treatments • Rapid path to clinical POC: Phase 2-ready product, expect to initiate trial next year • Key Intellectual Property (IP) secured from MIT Additional Indications: Potential for expansion into other neuropsychiatric diseases Additional Therapies: Core platform with optionality to add pipeline-enhancing, strategically relevant therapies Leveraging legacy assets: for potential non-dilutive capital to drive pipeline development Praliciguat – Licensed for kidney disease Olinciguat - Exclusive option 10% equity 1 ownership Up to $560M in future milestones + royalties agreement for cardiovascular diseases *Post Anesthesia Care Unit ** Health Care Providers
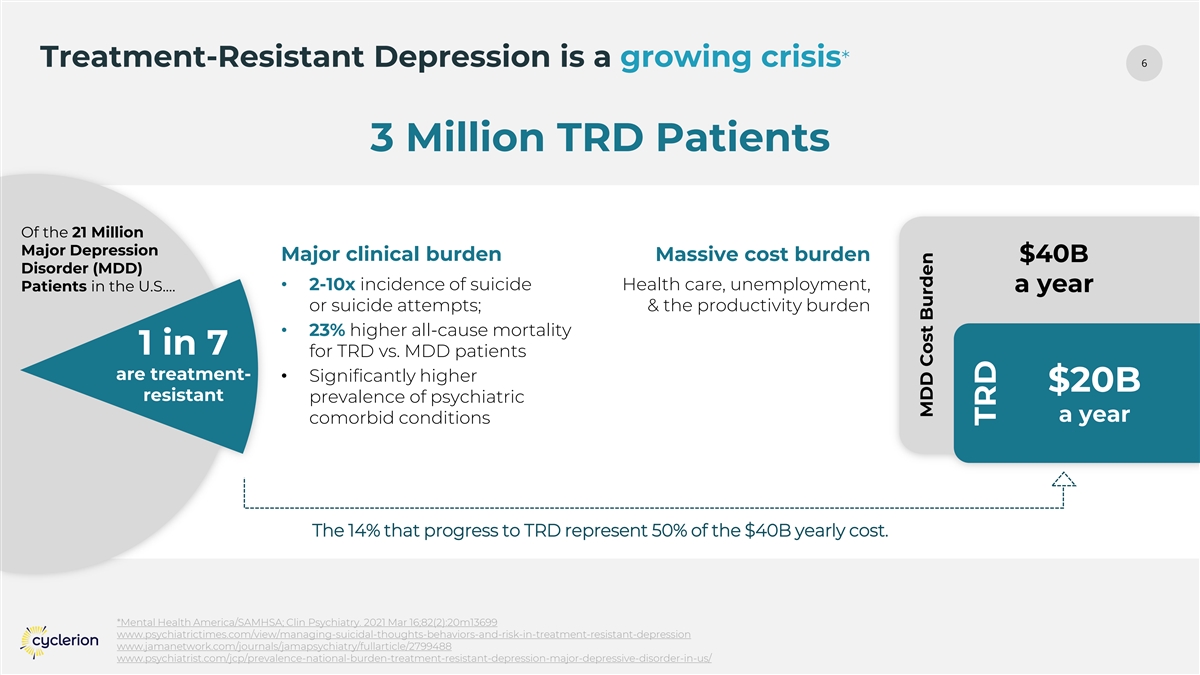
6 Treatment-Resistant Depression is a growing crisis* 3 Million TRD Patients Of the 21 Million Major Depression Major clinical burden Massive cost burden $40B Disorder (MDD) • 2-10x incidence of suicide Health care, unemployment, Patients in the U.S.… a year or suicide attempts; & the productivity burden • 23% higher all-cause mortality 1 in 7 for TRD vs. MDD patients are treatment- • Significantly higher $20B resistant prevalence of psychiatric a year comorbid conditions The 14% that progress to TRD represent 50% of the $40B yearly cost. *Mental Health America/SAMHSA; Clin Psychiatry. 2021 Mar 16;82(2):20m13699 www.psychiatrictimes.com/view/managing-suicidal-thoughts-behaviors-and-risk-in-treatment-resistant-depression www.jamanetwork.com/journals/jamapsychiatry/fullarticle/2799488 www.psychiatrist.com/jcp/prevalence-national-burden-treatment-resistant-depression-major-depressive-disorder-in-us/ MDD Cost Burden TRD
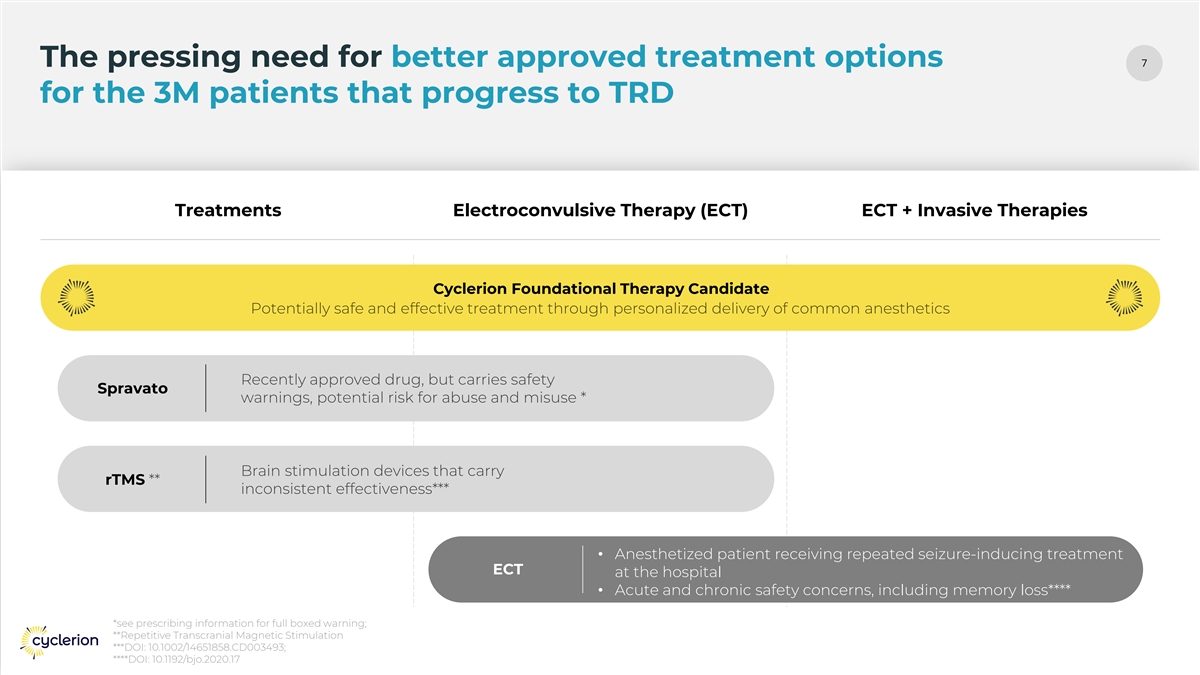
7 The pressing need for better approved treatment options for the 3M patients that progress to TRD Treatments Electroconvulsive Therapy (ECT) ECT + Invasive Therapies Cyclerion Foundational Therapy Candidate Spravato rTMS • Anesthetized patient receiving repeated seizure-inducing treatment ECT at the hospital • Acute and chronic safety concerns, including memory loss**** *see prescribing information for full boxed warning; **Repetitive Transcranial Magnetic Stimulation ***DOI: 10.1002/14651858.CD003493; ****DOI: 10.1192/bjo.2020.17
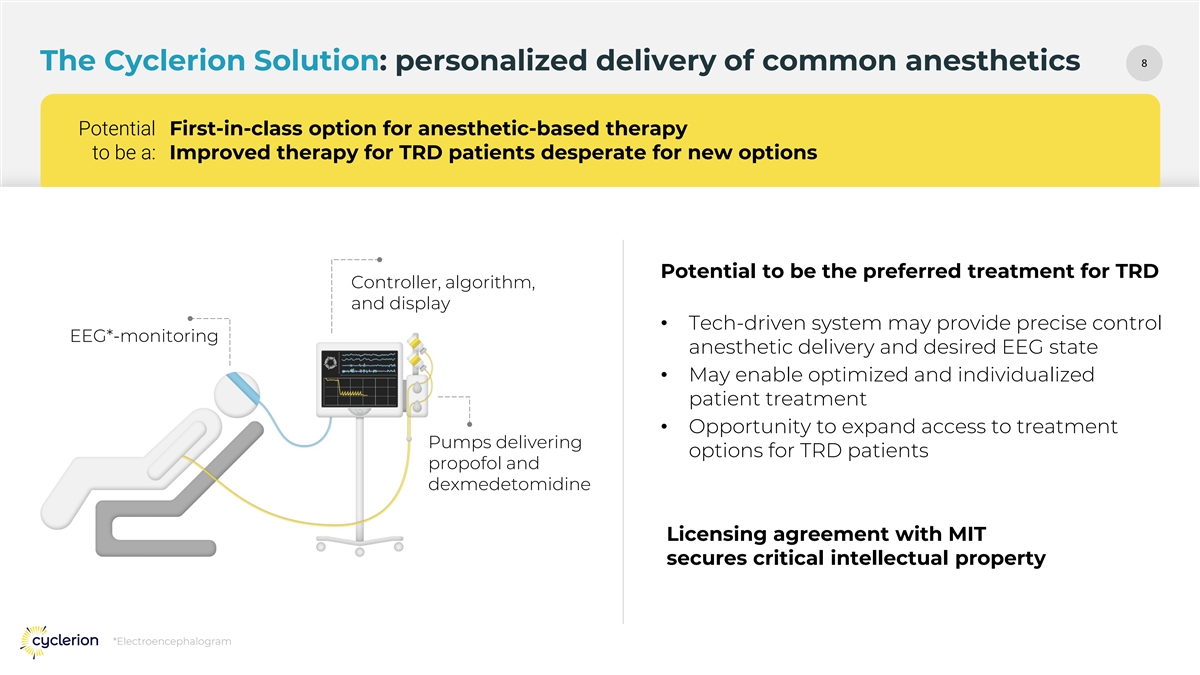
88 The Cyclerion Solution: personalized delivery of common anesthetics Potential First-in-class option for anesthetic-based therapy to be a: Improved therapy for TRD patients desperate for new options Potential to be the preferred treatment for TRD Controller, algorithm, and display • Tech-driven system may provide precise control EEG*-monitoring anesthetic delivery and desired EEG state • May enable optimized and individualized patient treatment • Opportunity to expand access to treatment Pumps delivering options for TRD patients propofol and dexmedetomidine Licensing agreement with MIT secures critical intellectual property *Electroencephalogram
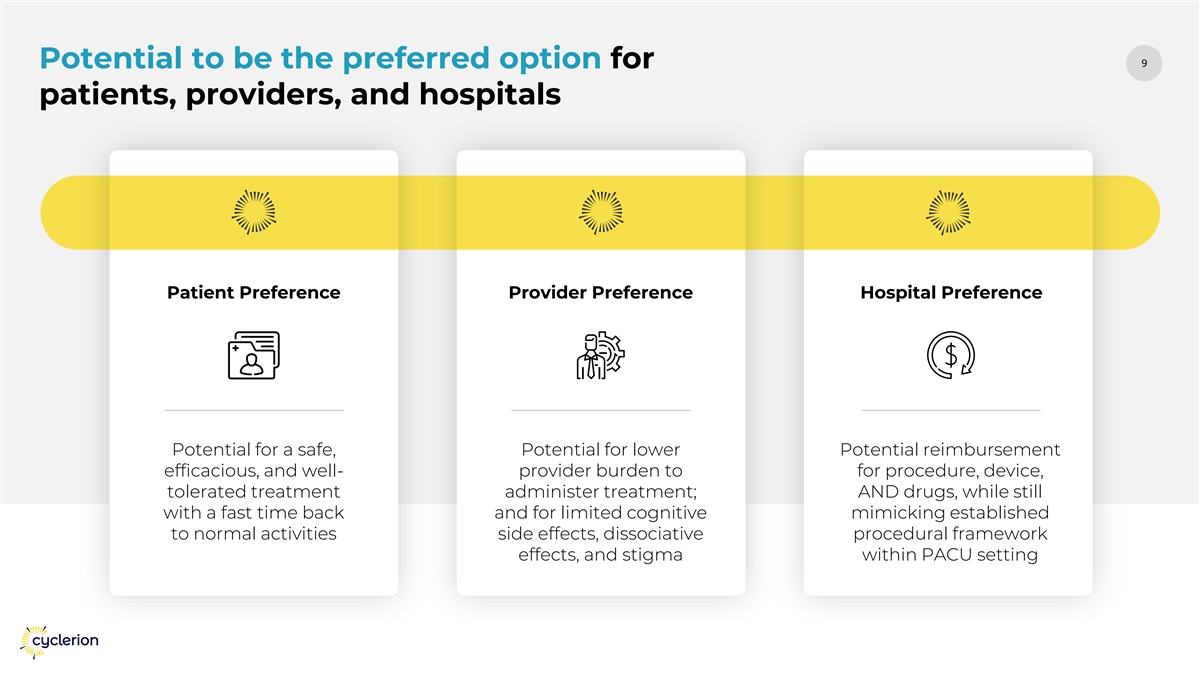
99 Potential to be the preferred option for patients, providers, and hospitals Patient Preference Provider Preference Hospital Preference Potential for a safe, Potential for lower Potential reimbursement efficacious, and well- provider burden to for procedure, device, tolerated treatment administer treatment; AND drugs, while still with a fast time back and for limited cognitive mimicking established to normal activities side effects, dissociative procedural framework effects, and stigma within PACU setting
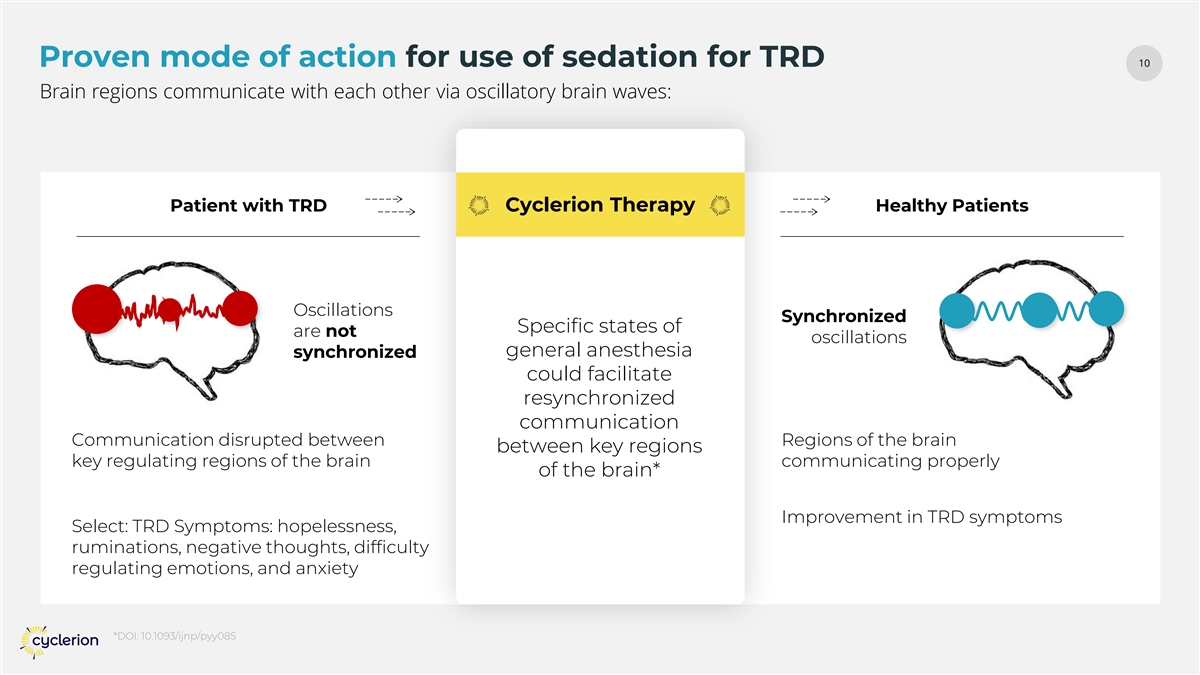
10 Proven mode of action for use of sedation for TRD Brain regions communicate with each other via oscillatory brain waves: Patient with TRD Cyclerion Therapy Healthy Patients Oscillations Synchronized Specific states of are not oscillations general anesthesia synchronized could facilitate resynchronized communication Communication disrupted between Regions of the brain between key regions key regulating regions of the brain communicating properly of the brain* Improvement in TRD symptoms Select: TRD Symptoms: hopelessness, ruminations, negative thoughts, difficulty regulating emotions, and anxiety *DOI: 10.1093/ijnp/pyy085

11 11 Compelling Clinical Precedent for such use of anesthetics for TRD Three early-phase clinical studies support propofol’s potential as a rapid-acting antidepressant in TRD Anesthesiologist-controlled open loop dosing Propofol showed consistent signals of efficacy with favorable safety profiles was central to all propofol studies: • Rapid onset of antidepressant effect seen • Burst suppression (RCT* + Pilot) or slow-wave sleep (SWS) optimization (SWIPED**) within 1-2 weeks • Propofol titrated using EEG metrics • Durable benefit lasting 3–6 months (e.g., suppression ratio, SWS density) • No major safety signals *Randomized Control Trial **DOI: 10.1136/bmjopen-2024-087516
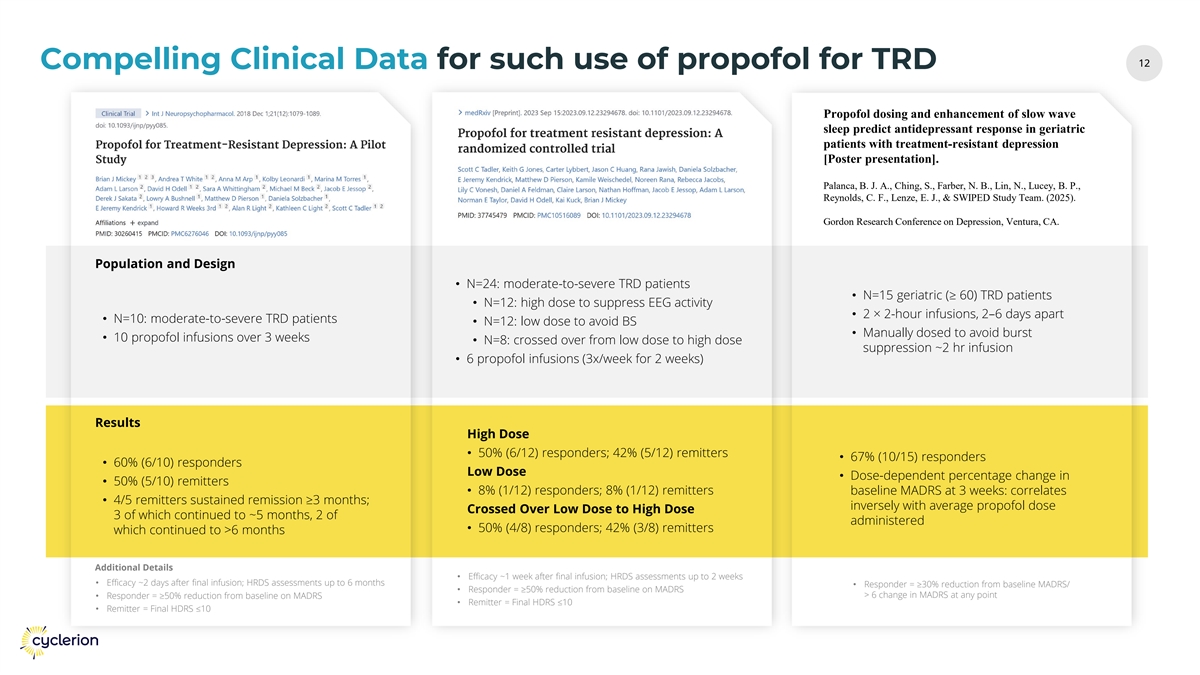
12 12 Compelling Clinical Data for such use of propofol for TRD Propofol dosing and enhancement of slow wave sleep predict antidepressant response in geriatric patients with treatment-resistant depression [Poster presentation]. Palanca, B. J. A., Ching, S., Farber, N. B., Lin, N., Lucey, B. P., Reynolds, C. F., Lenze, E. J., & SWIPED Study Team. (2025). Gordon Research Conference on Depression, Ventura, CA. Population and Design • N=24: moderate-to-severe TRD patients • N=15 geriatric (≥ 60) TRD patients • N=12: high dose to suppress EEG activity • 2 × 2-hour infusions, 2–6 days apart • N=10: moderate-to-severe TRD patients • N=12: low dose to avoid BS • Manually dosed to avoid burst • 10 propofol infusions over 3 weeks • N=8: crossed over from low dose to high dose suppression ~2 hr infusion • 6 propofol infusions (3x/week for 2 weeks) Results High Dose • 50% (6/12) responders; 42% (5/12) remitters • 67% (10/15) responders • 60% (6/10) responders Low Dose • Dose-dependent percentage change in • 50% (5/10) remitters • 8% (1/12) responders; 8% (1/12) remitters baseline MADRS at 3 weeks: correlates • 4/5 remitters sustained remission ≥3 months; inversely with average propofol dose Crossed Over Low Dose to High Dose 3 of which continued to ~5 months, 2 of administered • 50% (4/8) responders; 42% (3/8) remitters which continued to >6 months Additional Details • Efficacy ~1 week after final infusion; HRDS assessments up to 2 weeks • Efficacy ~2 days after final infusion; HRDS assessments up to 6 months • Responder = ≥30% reduction from baseline MADRS/ • Responder = ≥50% reduction from baseline on MADRS > 6 change in MADRS at any point • Responder = ≥50% reduction from baseline on MADRS • Remitter = Final HDRS ≤10 • Remitter = Final HDRS ≤10
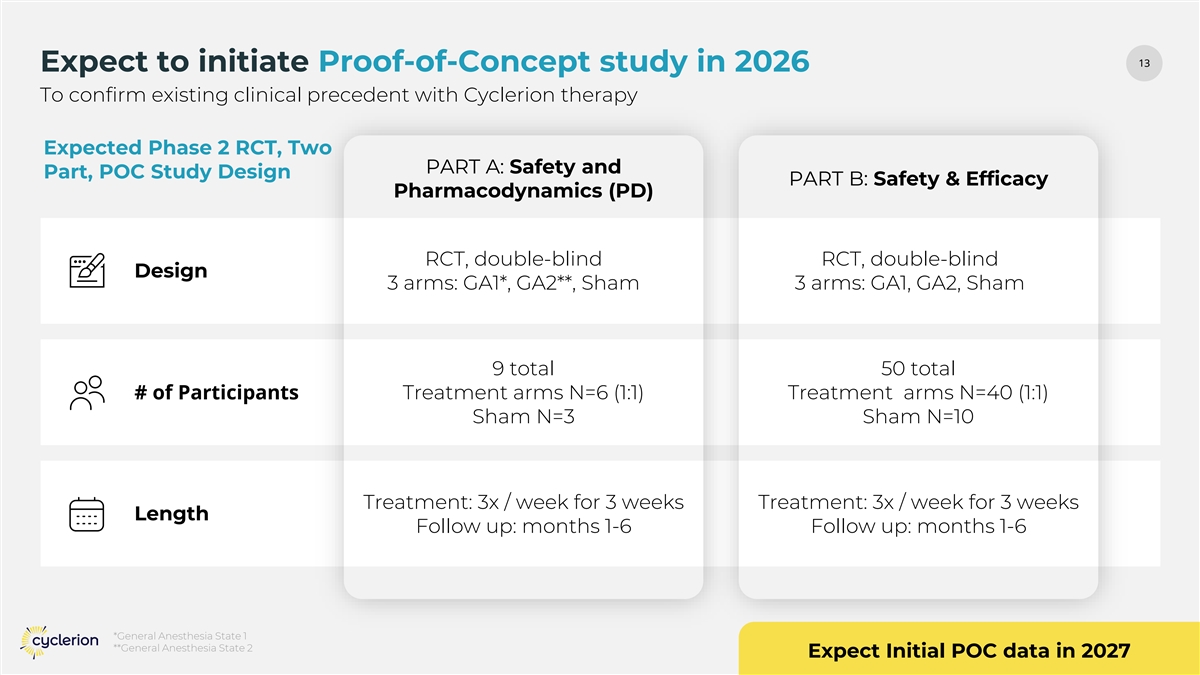
13 13 Expect to initiate Proof-of-Concept study in 2026 To confirm existing clinical precedent with Cyclerion therapy Expected Phase 2 RCT, Two PART A: Safety and Part, POC Study Design PART B: Safety & Efficacy Pharmacodynamics (PD) RCT, double-blind RCT, double-blind Design 3 arms: GA1*, GA2**, Sham 3 arms: GA1, GA2, Sham 9 total 50 total # of Participants Treatment arms N=6 (1:1) Treatment arms N=40 (1:1) Sham N=3 Sham N=10 Treatment: 3x / week for 3 weeks Treatment: 3x / week for 3 weeks Length Follow up: months 1-6 Follow up: months 1-6 *General Anesthesia State 1 **General Anesthesia State 2 Expect Initial POC data in 2027
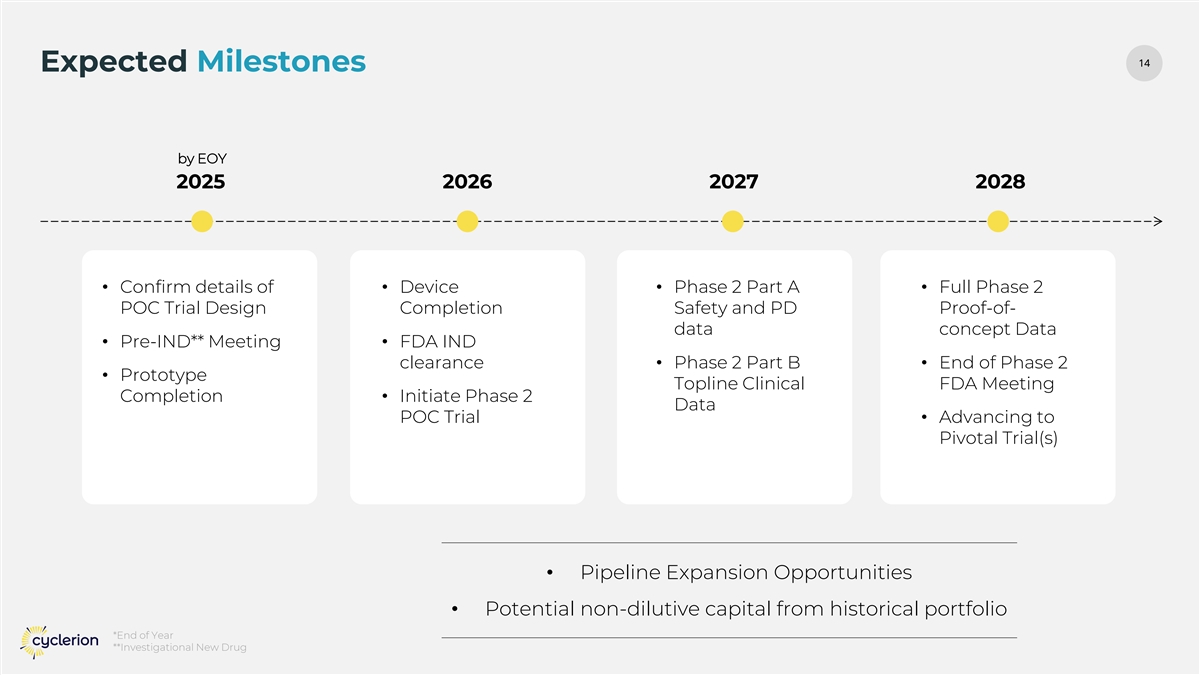
14 14 Expected Milestones by EOY 2025 2026 2027 2028 • Confirm details of • Device • Phase 2 Part A • Full Phase 2 POC Trial Design Completion Safety and PD Proof-of- data concept Data • Pre-IND** Meeting • FDA IND clearance • Phase 2 Part B • End of Phase 2 • Prototype Topline Clinical FDA Meeting Completion • Initiate Phase 2 Data POC Trial • Advancing to Pivotal Trial(s) • Pipeline Expansion Opportunities • Potential non-dilutive capital from historical portfolio *End of Year **Investigational New Drug

Thank You September 2025