

.2 Cyclerion: Pioneering a New Era in Neuropsychiatric Therapies th Investor Call: January 6 , 2026

Legal Disclaimer 2 This presentation has been prepared by Cyclerion Therapeutics, Inc. and its consolidated subsidiaries (“the Company,” “Cyclerion,” “we,” “us,” and “our”). Certain matters discussed in this presentation are “forward-looking statements.” We may, in some cases, use terms such as “potential,” “may,” “expects,” “plans,” “could,” “opportunity,” “intends,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements involve risks, uncertainties and other factors that may cause actual results, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. Forward-looking statements in this presentation include, but are not limited to, statements about the timing of development and commercialization of our product candidates, our ability to develop product candidates, the timing of initiation our Phase 2 Proof-of-Concept study, the design of our Phase 2 POC study, the expected timing of milestone payments related to Akebia clinical trials, our anticipated capital requirements, and the timing of related milestones, availability of clinical data, creation of shareholder value, and adoption of our product candidates once commercialized, ongoing discussions with potential business partners and expected addressable market size. We cannot assure you that the forward-looking statements in this presentation will prove to be accurate. Some of the factors that could cause actual performance and results to differ materially from those projected or suggested in the forward-looking statements due to various risks and uncertainties, include, but are not limited to, the substantial doubt regarding the our ability to continue as a going concern, our ability to raise additional funding, our ability to enroll patients in future clinical studies, our ability to obtain regulatory approval for our product candidates, unanticipated changes to our nonclinical or clinical study protocols due to regulatory reasons or unanticipated events, which could lead to increased costs to us and could delay our development timeline, our reliance on third parties to conduct clinical studies and to manufacture drug supplies for our product candidates, our ability to adequately protect our intellectual property, and the other important risk factors discussed under the heading “Risk Factors” in our Annual Report on Form 10-K filed with the SEC on March 4, 2025. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to update such forward-looking statements to reflect subsequent events or circumstances. Certain market data, study data and industry data used in this presentation were obtained from reports of governmental agencies and industry publications, studies and surveys. Management has not independently verified such data and as such, make no guarantees as to its accuracy, completeness or timeliness.

Today’s Agenda 1. Cyclerion and CYC-126 Overview 2. CYC-126 in Treatment Resistant Depression (TRD): Unmet Need and Planned Phase 2 Proof- of-Concept (POC) Study 3. Provide Updates on Path Toward Initiating CYC-126’s Phase 2 Proof-of-Concept Study in 2026 • Product development including the recently announced Medsteer collaboration • Regulatory • Financial 4. Timelines and Milestones 3 3

Cyclerion and CYC-126 Overview 4 4
Cyclerion: a new, pioneering neuropsychiatric-focused company • Lean, nimble team with world-class neuropsychiatric, anesthesia, intelligent medical systems and biopharma experience Platform • Potential first-in-class platform for tech-enhanced therapeutics aiming to optimize patient benefit • Potential first anesthetic-based therapy in a patient feedback loop for neuropsychiatric indications • Differentiated treatment for 3M+ patients with TRD desperate for new options CYC-126: • Known Mode of Action and compelling clinical precedent could help de-risk therapeutic use of propofol for TRD Initial Therapy • Fits within existing treatment paradigm, with the opportunity to be the preferred treatment for patients, physicians, and hospitals • Today’s announcement: Medsteer collaboration provides agreement to incorporate key aspects of closed-loop anesthetic delivery for clinical applications within and well beyond neuropsychiatric diseases, such as TRD Milestones • Capital-efficient path to initiate POC study in 2H 2026 with initial POC data expected in 2027, and full POC data expected in 2028 5 5

CYC-126: Potential to be the first individualized treatment for TRD 1 Generic IV anesthetics (propofol + dexmedetomidine) with extensive clinical safety experience, and a personalized delivery system operating as a co-pilot to anesthesiologist We believe sedation could recalibrate brain region communications that are dysregulated in INDUCTION MAINTENANCE duration TBD by clinical evidence patients with TRD. MONTH 1 MONTH 2 MONTH 3 MONTH 4 MONTH 5 Potentially two stages of treatment: Induction 3 Procedures a week for first 3 weeks and then one procedure a month thereafter. and maintenance, treatment duration estimated to be 2-3 hours A clear unmet need with <10% of the 3M TRD patients currently being treated with approved therapies We believe CYC-126 could provide a new therapeutic layer that could address all TRD patients 1 Intravenous 6 6 WHY HOW WHAT
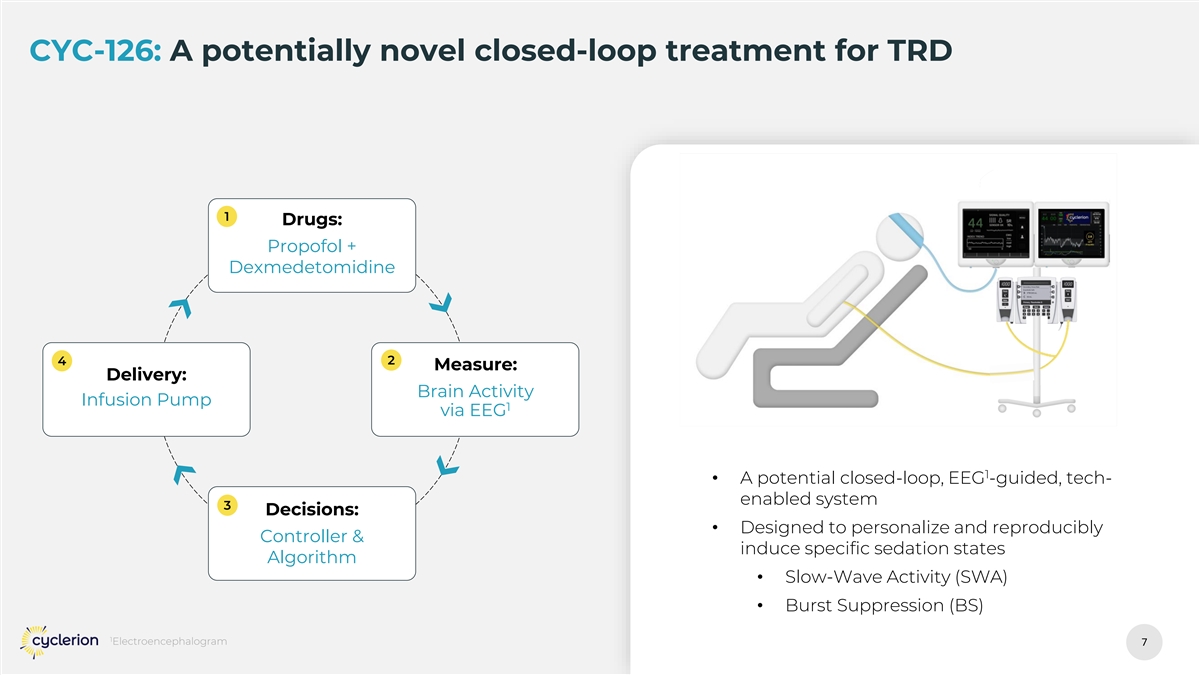
CYC-126: A potentially novel closed-loop treatment for TRD 1 Drugs: Propofol + Dexmedetomidine 4 2 Measure: Delivery: Brain Activity Infusion Pump 1 via EEG 1 • A potential closed-loop, EEG -guided, tech- enabled system 3 Decisions: • Designed to personalize and reproducibly Controller & induce specific sedation states Algorithm • Slow-Wave Activity (SWA) • Burst Suppression (BS) 1 Electroencephalogram 7 7

CYC-126 in Treatment Resistant Depression: Unmet Need and Phase 2 Proof-of-Concept Study 8 8

Burden of the disease: Unmet need to be addressed by novel therapeutics 1 Major depression: #1 cause of disability worldwide ~1 in 3 patients do not achieve adequate relief using 2 current treatments 3 • ~3+ million US adults with treatment-resistant depression • Of TRD patients who respond to a current treatment, ~1/2 relapse after only 3 months of maintenance treatment Suicide rate is 7-fold higher among hospitalized TRD 7x 5 patients than in treatment responsive MDD patients • Current treatments require 3-6 weeks to become 4 effective – too slow to aid acutely suicidal patients 1 2 World Health Organization, “Global Burden of Mental Disorders”. Cleveland Clinic Journal of 3 4 Medicine. Volume 75 • Number 1 January 2008. IMS and Truven Health. National Center for Injury 5 9 9 Prevention and Control CDC, 2014. Major Depressive Disorder
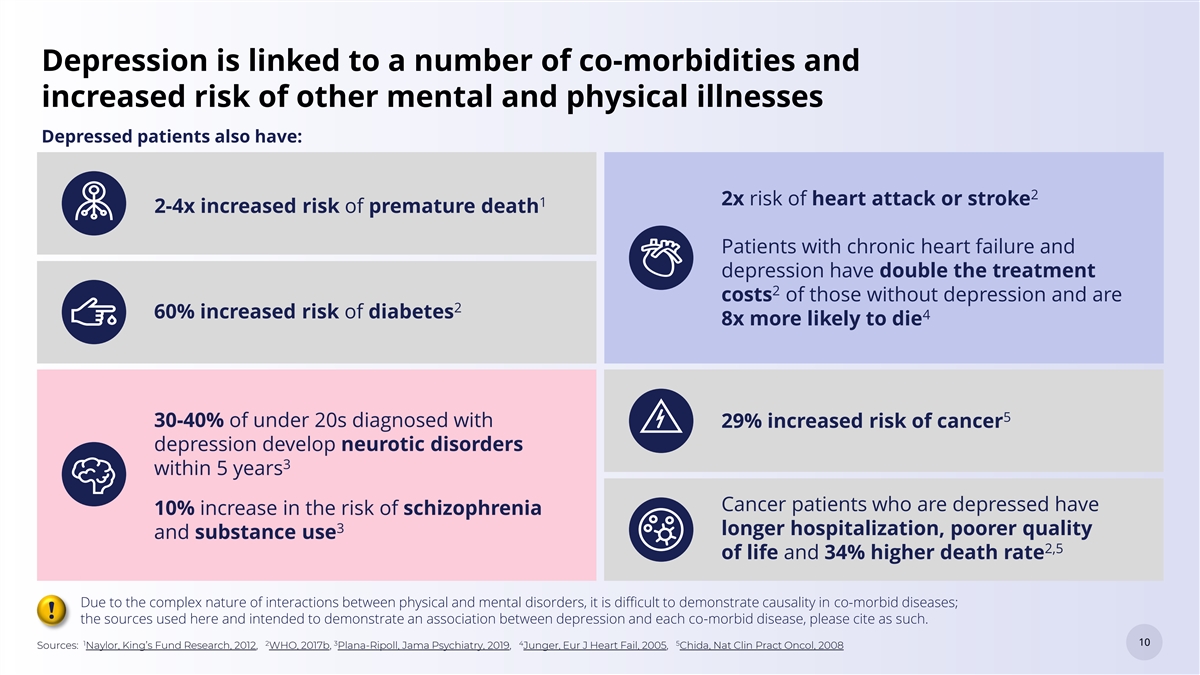
Depression is linked to a number of co-morbidities and increased risk of other mental and physical illnesses Depressed patients also have: 2 2x risk of heart attack or stroke 1 2-4x increased risk of premature death Patients with chronic heart failure and depression have double the treatment 2 costs of those without depression and are 2 60% increased risk of diabetes 4 8x more likely to die 5 30-40% of under 20s diagnosed with 29% increased risk of cancer depression develop neurotic disorders 3 within 5 years Cancer patients who are depressed have 10% increase in the risk of schizophrenia 3 longer hospitalization, poorer quality and substance use 2,5 of life and 34% higher death rate Due to the complex nature of interactions between physical and mental disorders, it is difficult to demonstrate causality in co-morbid diseases; ! the sources used here and intended to demonstrate an association between depression and each co-morbid disease, please cite as such. 1 2 3 4 5 10 10 Sources: Naylor, King’s Fund Research, 2012, WHO, 2017b, Plana-Ripoll, Jama Psychiatry, 2019, Junger, Eur J Heart Fail, 2005, Chida, Nat Clin Pract Oncol, 2008
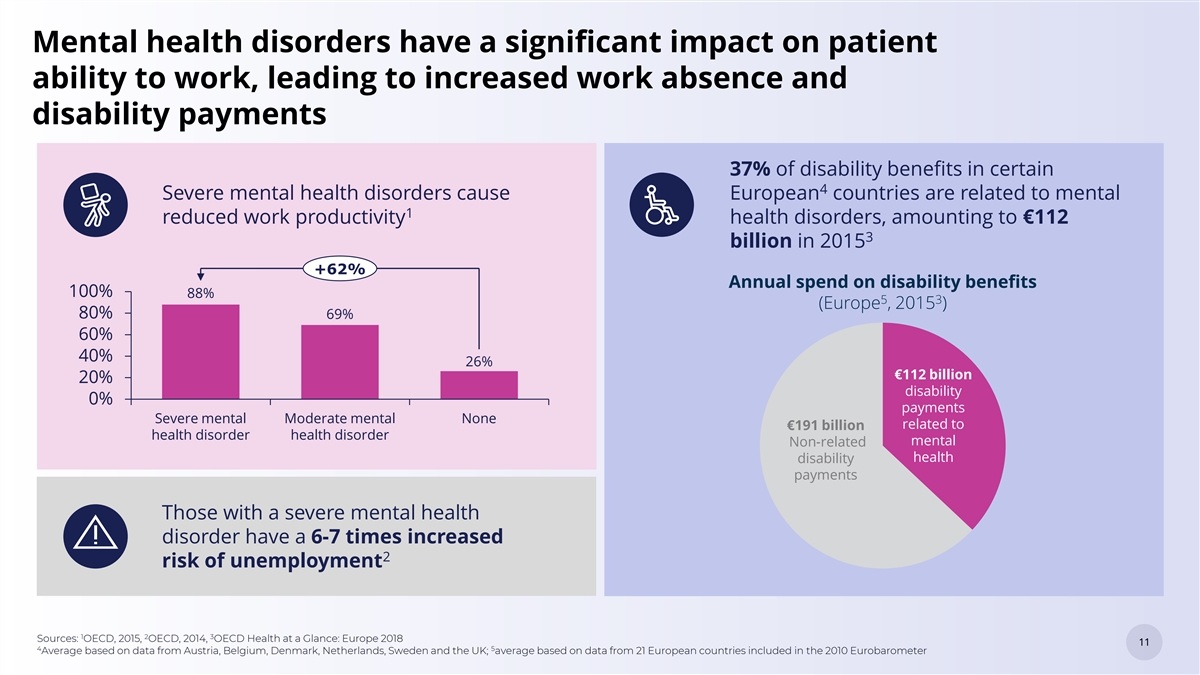
Mental health disorders have a significant impact on patient ability to work, leading to increased work absence and disability payments 37% of disability benefits in certain 4 Severe mental health disorders cause European countries are related to mental 1 reduced work productivity health disorders, amounting to €112 3 billion in 2015 +62% Annual spend on disability benefits 100% 88% 5 3 (Europe , 2015 ) 80% 69% 60% 40% 26% €112 billion 20% disability 0% payments Severe mental Moderate mental None related to €191 billion health disorder health disorder mental Non-related health disability payments Those with a severe mental health disorder have a 6-7 times increased 2 risk of unemployment 1 2 3 Sources: OECD, 2015, OECD, 2014, OECD Health at a Glance: Europe 2018 11 11 4 5 Average based on data from Austria, Belgium, Denmark, Netherlands, Sweden and the UK; average based on data from 21 European countries included in the 2010 Eurobarometer
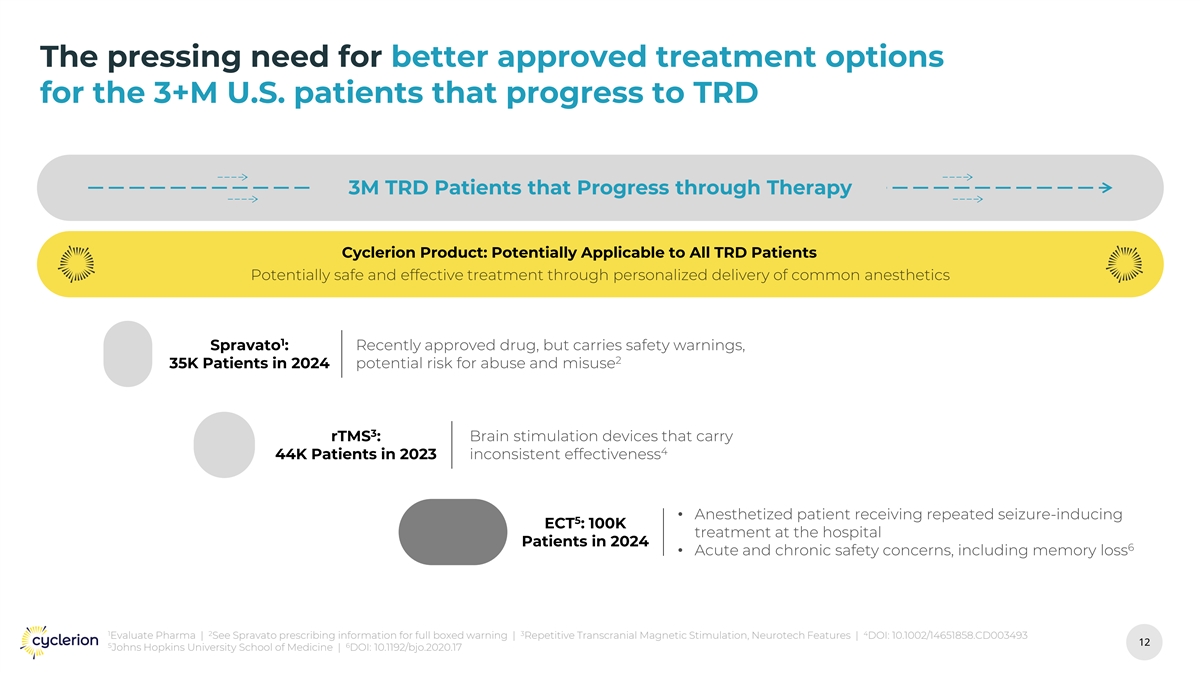
The pressing need for better approved treatment options for the 3+M U.S. patients that progress to TRD 3M TRD Patients that Progress through Therapy Cyclerion Product: Potentially Applicable to All TRD Patients 1 Spravato : 35K Patients in 2024 3 rTMS : 44K Patients in 2023 5 ECT : 100K Patients in 2024 1 2 3 4 Evaluate Pharma | See Spravato prescribing information for full boxed warning | Repetitive Transcranial Magnetic Stimulation, Neurotech Features | DOI: 10.1002/14651858.CD003493 12 12 5 6 Johns Hopkins University School of Medicine | DOI: 10.1192/bjo.2020.17
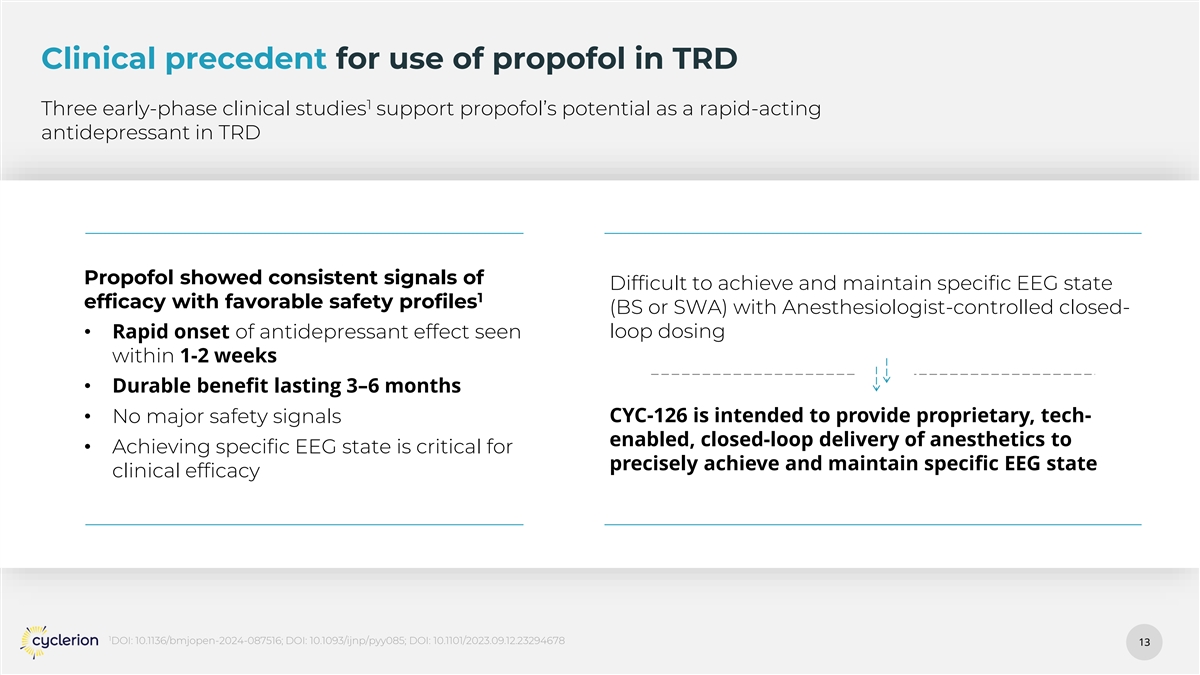
Clinical precedent for use of propofol in TRD 1 Three early-phase clinical studies support propofol’s potential as a rapid-acting antidepressant in TRD Propofol showed consistent signals of Difficult to achieve and maintain specific EEG state 1 efficacy with favorable safety profiles (BS or SWA) with Anesthesiologist-controlled closed- • Rapid onset of antidepressant effect seen loop dosing within 1-2 weeks • Durable benefit lasting 3–6 months CYC-126 is intended to provide proprietary, tech- • No major safety signals enabled, closed-loop delivery of anesthetics to • Achieving specific EEG state is critical for precisely achieve and maintain specific EEG state clinical efficacy 1 DOI: 10.1136/bmjopen-2024-087516; DOI: 10.1093/ijnp/pyy085; DOI: 10.1101/2023.09.12.23294678 13 13
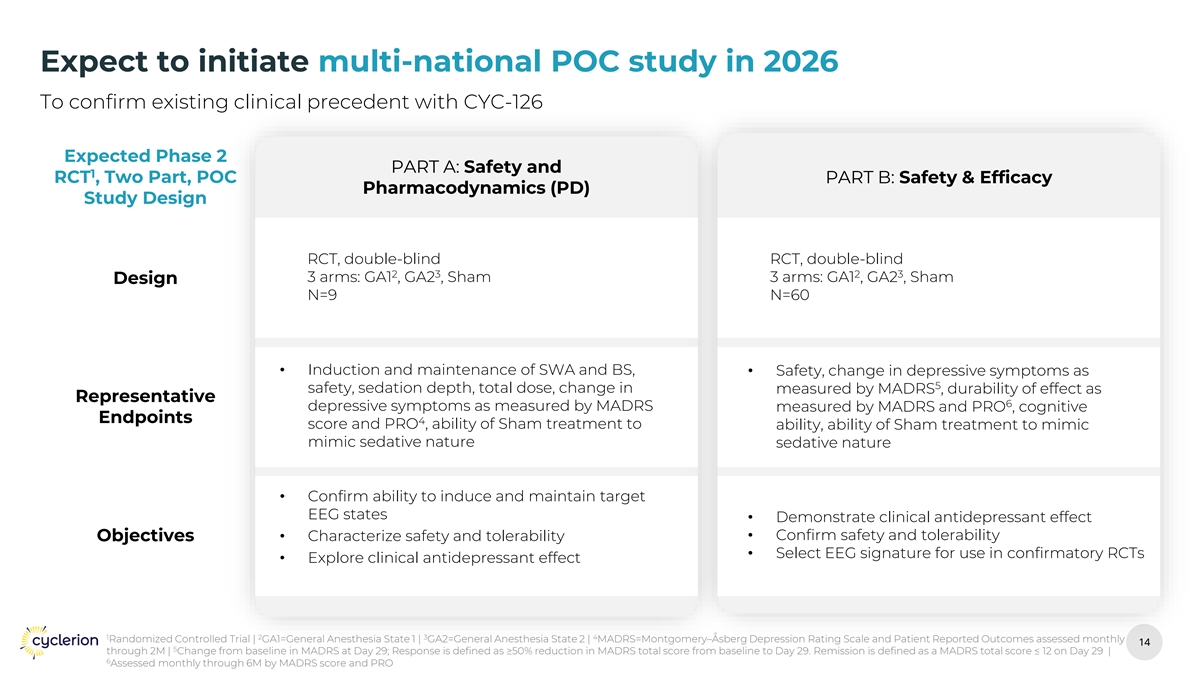
Expect to initiate multi-national POC study in 2026 To confirm existing clinical precedent with CYC-126 Expected Phase 2 PART A: Safety and 1 RCT , Two Part, POC PART B: Safety & Efficacy Pharmacodynamics (PD) Study Design RCT, double-blind RCT, double-blind 2 3 2 3 3 arms: GA1 , GA2 , Sham 3 arms: GA1 , GA2 , Sham Design N=9 N=60 • Induction and maintenance of SWA and BS, • Safety, change in depressive symptoms as 5 safety, sedation depth, total dose, change in measured by MADRS , durability of effect as Representative 6 depressive symptoms as measured by MADRS measured by MADRS and PRO , cognitive Endpoints 4 score and PRO , ability of Sham treatment to ability, ability of Sham treatment to mimic mimic sedative nature sedative nature • Confirm ability to induce and maintain target EEG states • Demonstrate clinical antidepressant effect • Confirm safety and tolerability Objectives• Characterize safety and tolerability • Select EEG signature for use in confirmatory RCTs • Explore clinical antidepressant effect 1 2 3 4 Randomized Controlled Trial | GA1=General Anesthesia State 1 | GA2=General Anesthesia State 2 | MADRS=Montgomery–Åsberg Depression Rating Scale and Patient Reported Outcomes assessed monthly 14 14 5 through 2M | Change from baseline in MADRS at Day 29; Response is defined as ≥50% reduction in MADRS total score from baseline to Day 29. Remission is defined as a MADRS total score ≤ 12 on Day 29 | 6 Assessed monthly through 6M by MADRS score and PRO
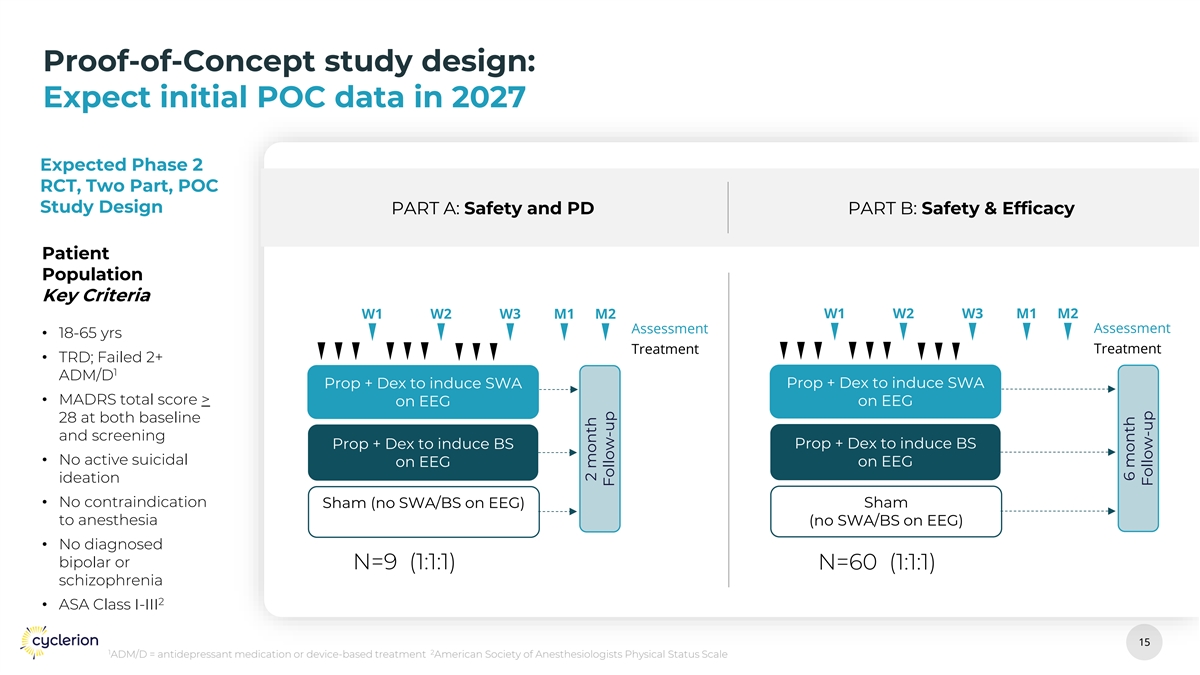
Proof-of-Concept study design: Expect initial POC data in 2027 Expected Phase 2 RCT, Two Part, POC Study Design PART A: Safety and PD PART B: Safety & Efficacy Patient Population Key Criteria W1 W2 W3 M1 M2 W1 W2 W3 M1 M2 Assessment Assessment • 18-65 yrs Treatment Treatment • TRD; Failed 2+ 1 ADM/D Prop + Dex to induce SWA Prop + Dex to induce SWA • MADRS total score > on EEG on EEG 28 at both baseline and screening Prop + Dex to induce BS Prop + Dex to induce BS • No active suicidal on EEG on EEG ideation • No contraindication Sham Sham (no SWA/BS on EEG) to anesthesia (no SWA/BS on EEG) • No diagnosed bipolar or N=9 (1:1:1) N=60 (1:1:1) schizophrenia 2 • ASA Class I-III 15 15 1 2 ADM/D = antidepressant medication or device-based treatment American Society of Anesthesiologists Physical Status Scale 2 month Follow-up 6 month Follow-up
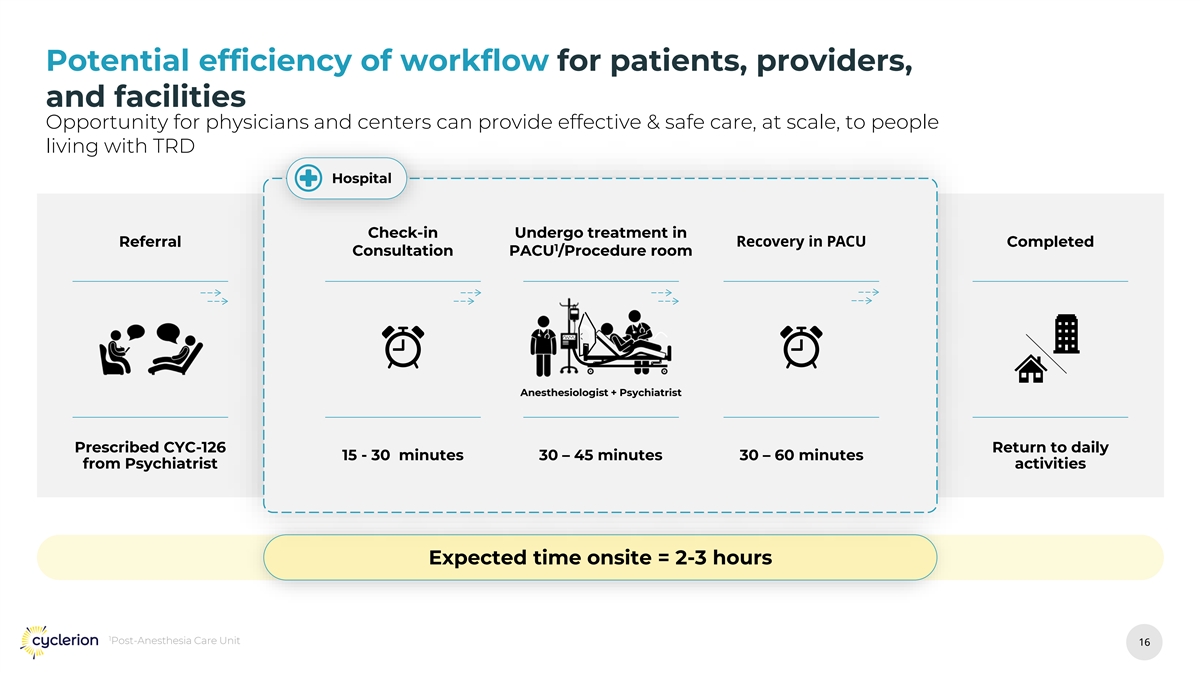
Potential efficiency of workflow for patients, providers, and facilities Opportunity for physicians and centers can provide effective & safe care, at scale, to people living with TRD Hospital Check-in Undergo treatment in Recovery in PACU Referral Completed 1 Consultation PACU /Procedure room Anesthesiologist + Psychiatrist Prescribed CYC-126 Return to daily 15 - 30 minutes 30 – 45 minutes 30 – 60 minutes from Psychiatrist activities Expected time onsite = 2-3 hours 1 Post-Anesthesia Care Unit 16 16
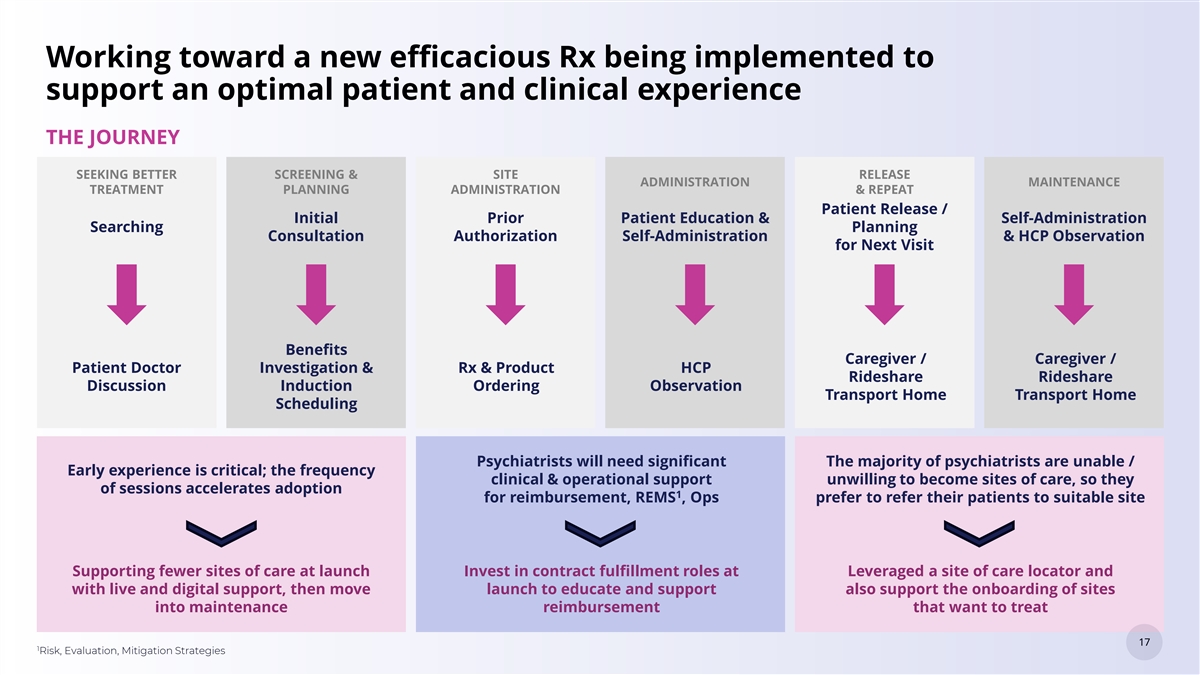
Working toward a new efficacious Rx being implemented to support an optimal patient and clinical experience THE JOURNEY SEEKING BETTER SCREENING & SITE RELEASE ADMINISTRATION MAINTENANCE TREATMENT PLANNING ADMINISTRATION & REPEAT Patient Release / Initial Prior Patient Education & Self-Administration Searching Planning Consultation Authorization Self-Administration & HCP Observation for Next Visit Benefits Caregiver / Caregiver / Patient Doctor Investigation & Rx & Product HCP Rideshare Rideshare Discussion Induction Ordering Observation Transport Home Transport Home Scheduling Psychiatrists will need significant The majority of psychiatrists are unable / Early experience is critical; the frequency clinical & operational support unwilling to become sites of care, so they of sessions accelerates adoption 1 for reimbursement, REMS , Ops prefer to refer their patients to suitable site Supporting fewer sites of care at launch Invest in contract fulfillment roles at Leveraged a site of care locator and with live and digital support, then move launch to educate and support also support the onboarding of sites into maintenance reimbursement that want to treat 17 17 1 Risk, Evaluation, Mitigation Strategies

Product Development Update 18 18
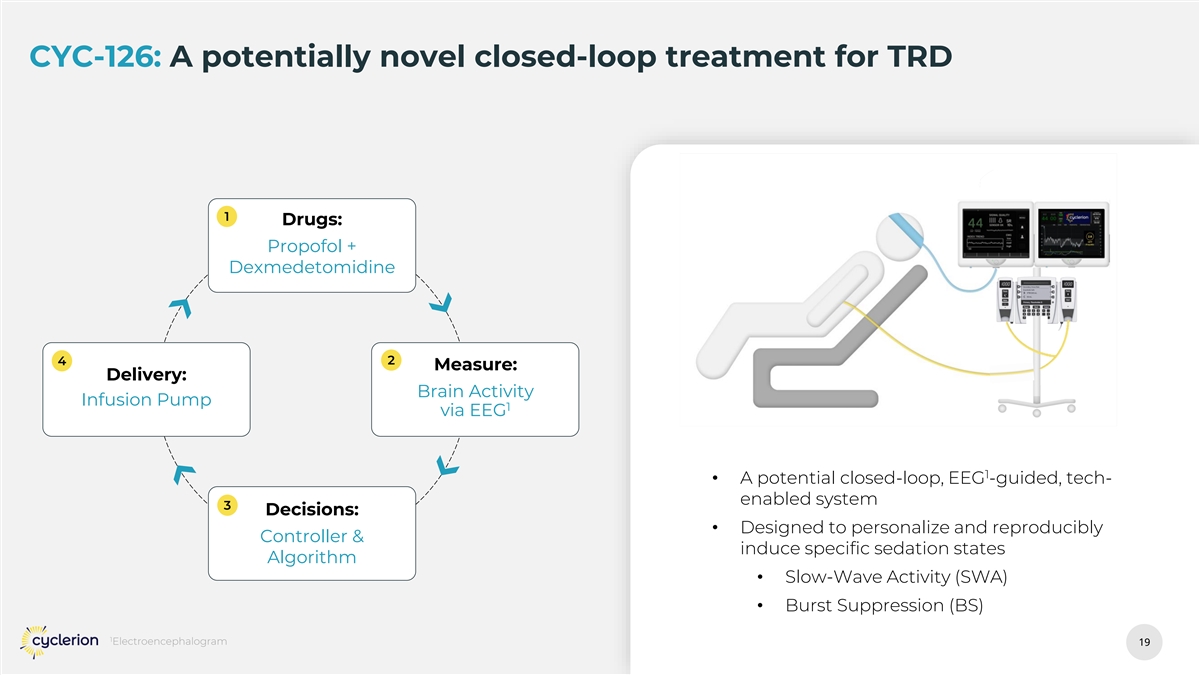
CYC-126: A potentially novel closed-loop treatment for TRD 1 Drugs: Propofol + Dexmedetomidine 4 2 Measure: Delivery: Brain Activity Infusion Pump 1 via EEG 1 • A potential closed-loop, EEG -guided, tech- enabled system 3 Decisions: • Designed to personalize and reproducibly Controller & induce specific sedation states Algorithm • Slow-Wave Activity (SWA) • Burst Suppression (BS) 1 Electroencephalogram 19 19
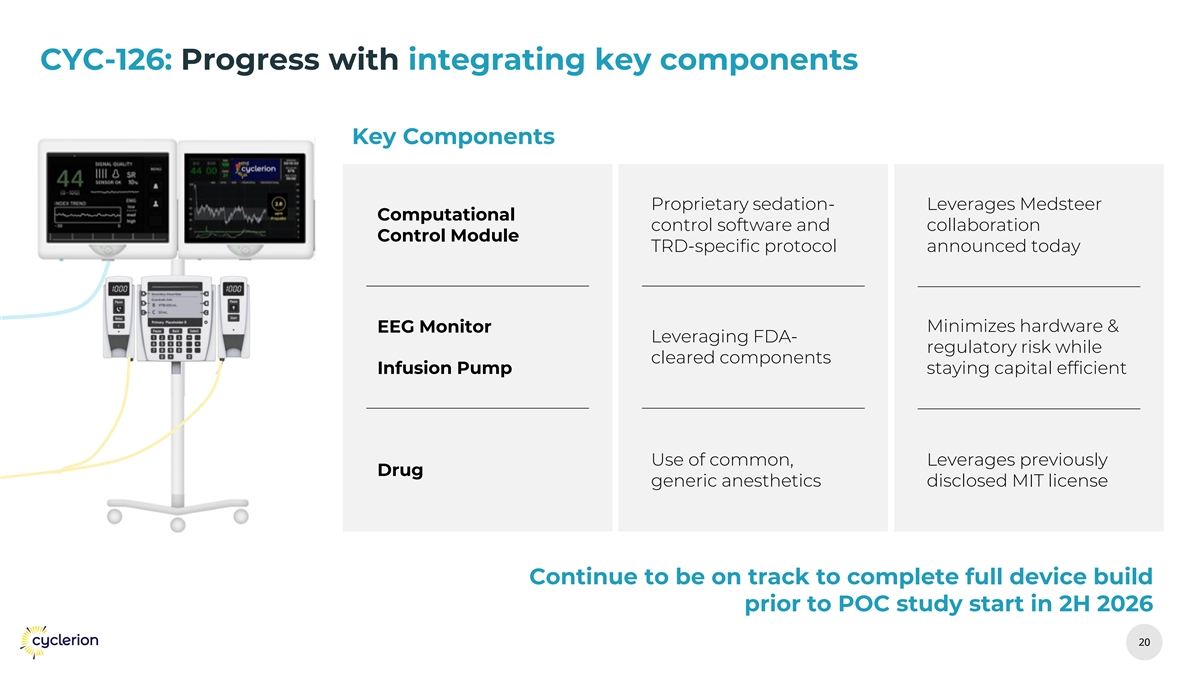
CYC-126: Progress with integrating key components Key Components Proprietary sedation- Leverages Medsteer Computational control software and collaboration Control Module TRD-specific protocol announced today EEG Monitor Minimizes hardware & Leveraging FDA- regulatory risk while cleared components Infusion Pump staying capital efficient Use of common, Leverages previously Drug generic anesthetics disclosed MIT license Continue to be on track to complete full device build prior to POC study start in 2H 2026 20 20
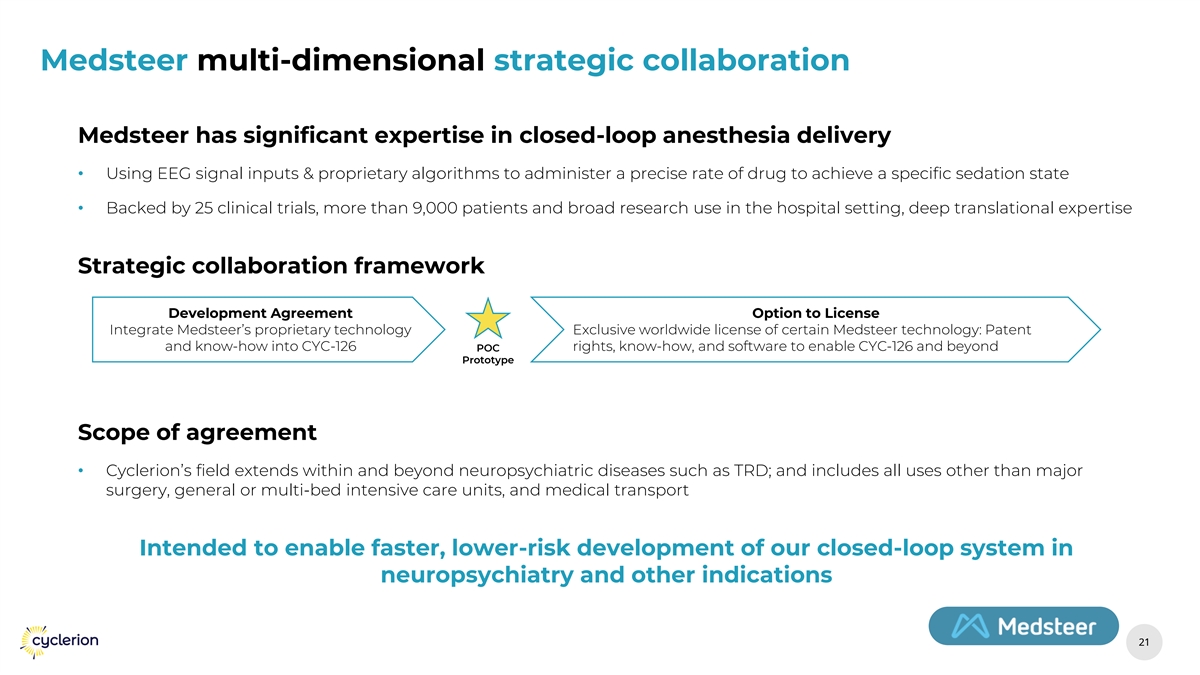
Medsteer multi-dimensional strategic collaboration Medsteer has significant expertise in closed-loop anesthesia delivery • Using EEG signal inputs & proprietary algorithms to administer a precise rate of drug to achieve a specific sedation state • Backed by 25 clinical trials, more than 9,000 patients and broad research use in the hospital setting, deep translational expertise Strategic collaboration framework Development Agreement Option to License Integrate Medsteer’s proprietary technology Exclusive worldwide license of certain Medsteer technology: Patent and know-how into CYC-126 POC rights, know-how, and software to enable CYC-126 and beyond Prototype Scope of agreement • Cyclerion’s field extends within and beyond neuropsychiatric diseases such as TRD; and includes all uses other than major surgery, general or multi-bed intensive care units, and medical transport Intended to enable faster, lower-risk development of our closed-loop system in neuropsychiatry and other indications 21 21

Regulatory Update 22 22
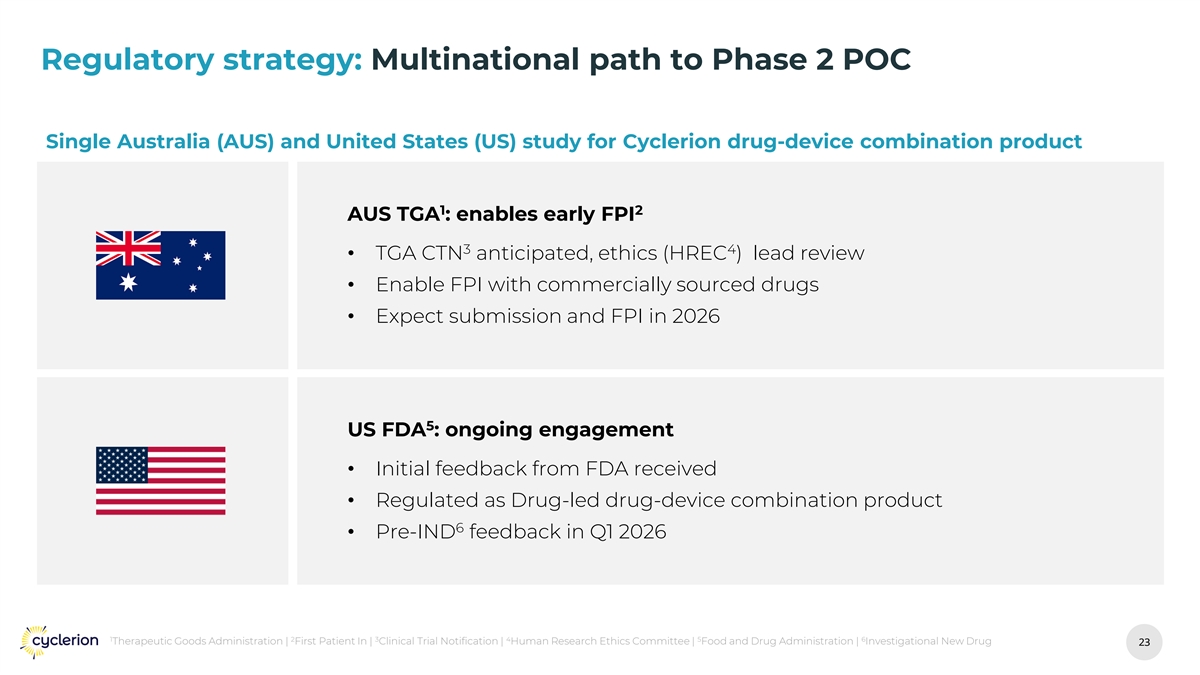
Regulatory strategy: Multinational path to Phase 2 POC Single Australia (AUS) and United States (US) study for Cyclerion drug-device combination product 1 2 AUS TGA : enables early FPI 3 4 • TGA CTN anticipated, ethics (HREC ) lead review • Enable FPI with commercially sourced drugs • Expect submission and FPI in 2026 5 US FDA : ongoing engagement • Initial feedback from FDA received • Regulated as Drug-led drug-device combination product 6 • Pre-IND feedback in Q1 2026 1 2 3 4 5 6 Therapeutic Goods Administration | First Patient In | Clinical Trial Notification | Human Research Ethics Committee | Food and Drug Administration | Investigational New Drug 23 23

Financial Update 24 24

Financial updates th $4.6M Cash Balance as-of September 30 , 2025 Praliciguat Update • Akebia Therapeutics announced initiation of Phase 2 in focal segmental glomerulosclerosis (FSGS) • Expect $1M milestone paid upon first-patient-dosed in 1H 2026 Additional capital needs: • $50M needed to get through full POC data in 2028 • $20M needed to get through initial data in 2027 25 25

Expected Timeline and Milestones 26 26
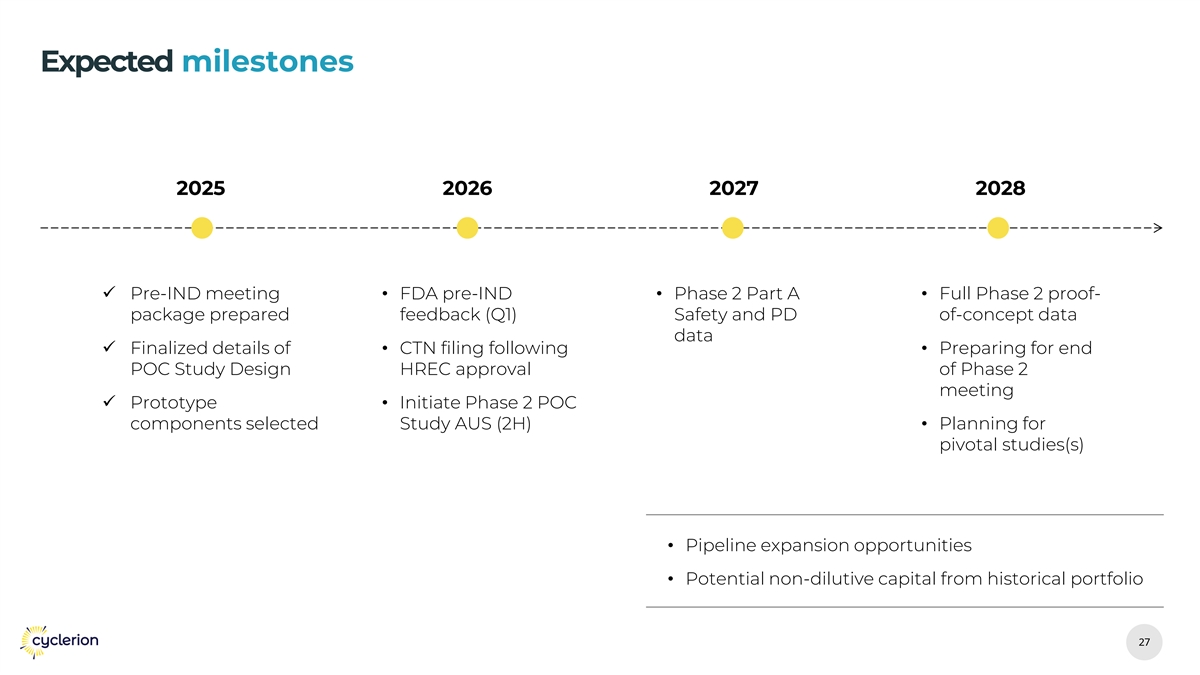
Expected milestones 2025 2026 2027 2028 ✓ Pre-IND meeting • FDA pre-IND • Phase 2 Part A • Full Phase 2 proof- package prepared feedback (Q1) Safety and PD of-concept data data ✓ Finalized details of • CTN filing following • Preparing for end POC Study Design HREC approval of Phase 2 meeting ✓ Prototype • Initiate Phase 2 POC components selected Study AUS (2H)• Planning for pivotal studies(s) • Pipeline expansion opportunities • Potential non-dilutive capital from historical portfolio 27 27

Thank You