

Corporate Presentation January 2026

This presentation contains forward-looking statements about Sana Biotechnology, Inc. (the “Company,” “we,” “us,” or “our”) within the meaning of the federal securities laws. All statements other than statements of historical facts contained in this presentation, including, among others, statements regarding the Company’s strategy, expectations, cash runway and future financial condition, future operations, and prospects, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,” “predict,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. The Company has based these forward-looking statements largely on its current expectations, estimates, forecasts and projections about future events and financial trends that it believes may affect its financial condition, results of operations, business strategy and financial needs. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. These statements are subject to risks and uncertainties that could cause the actual results to vary materially, including, among others, the risks inherent in drug development such as those associated with the initiation, cost, timing, progress and results of the Company’s current and future research and development programs, preclinical studies, and clinical trials. For a detailed discussion of the risk factors that could affect the Company’s actual results, please refer to the risk factors identified in the Company’s SEC reports, including its Quarterly Report on Form 10-Q dated November 6, 2025. Except as required by law, the Company undertakes no obligation to update publicly any forward-looking statements for any reason. Cautionary note regarding forward-looking statements
Type 1 diabetes represents a significant opportunity with validated biology ~10M people WW live with type 1 diabetes; current standard treatment remains exogenous insulin Disease impact remains significant, and patients want new alternatives Sana made significant progress in 2025 with immune evasion, regulatory interactions, and manufacturing 2026 goal of filing SC451 IND and starting Phase 1 trial Rapid potential clinical proof of concept with demonstration of immune evasion, beta cell function, and glucose normalization in vivo CAR T cells provide a second potential transformative platform CAR T cells are transformative for many people with blood cancers and autoimmune diseases, but have clear limitations In vivo CAR T cells have potential for no conditioning chemotherapy, comparable efficacy, off-the-shelf availability In vivo non-human primate (NHP) data: potent CAR T cells with cell-specific delivery SG293 – potential for first-in-human data in 2026 Changing the Possible for Patients Sana Biotechnology
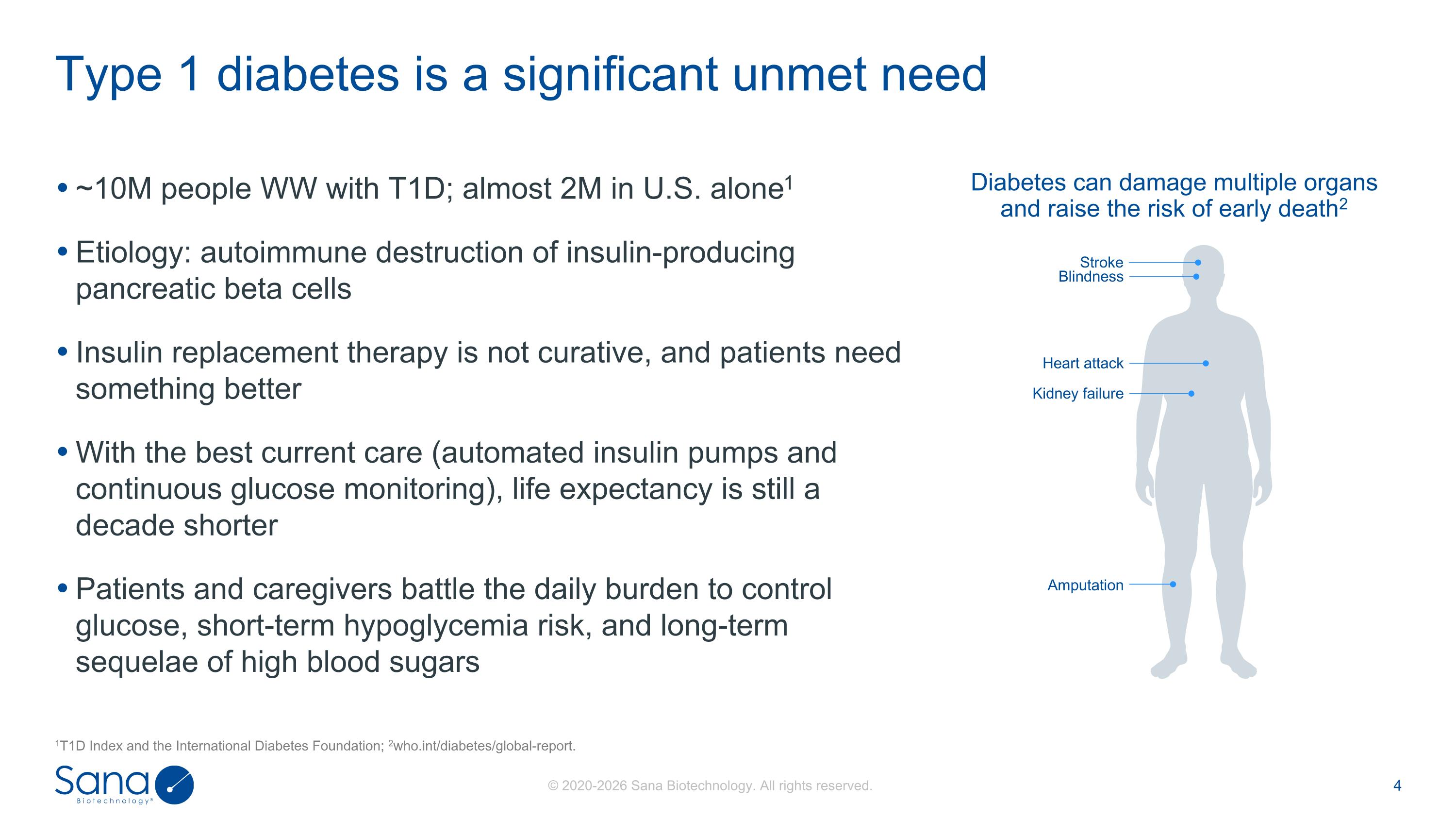
~10M people WW with T1D; almost 2M in U.S. alone1 Etiology: autoimmune destruction of insulin-producing pancreatic beta cells Insulin replacement therapy is not curative, and patients need something better With the best current care (automated insulin pumps and continuous glucose monitoring), life expectancy is still a decade shorter Patients and caregivers battle the daily burden to control glucose, short-term hypoglycemia risk, and long-term sequelae of high blood sugars Type 1 diabetes is a significant unmet need 1T1D Index and the International Diabetes Foundation; 2who.int/diabetes/global-report. Diabetes can damage multiple organs and raise the risk of early death2 Stroke Blindness Heart attack Kidney failure Amputation
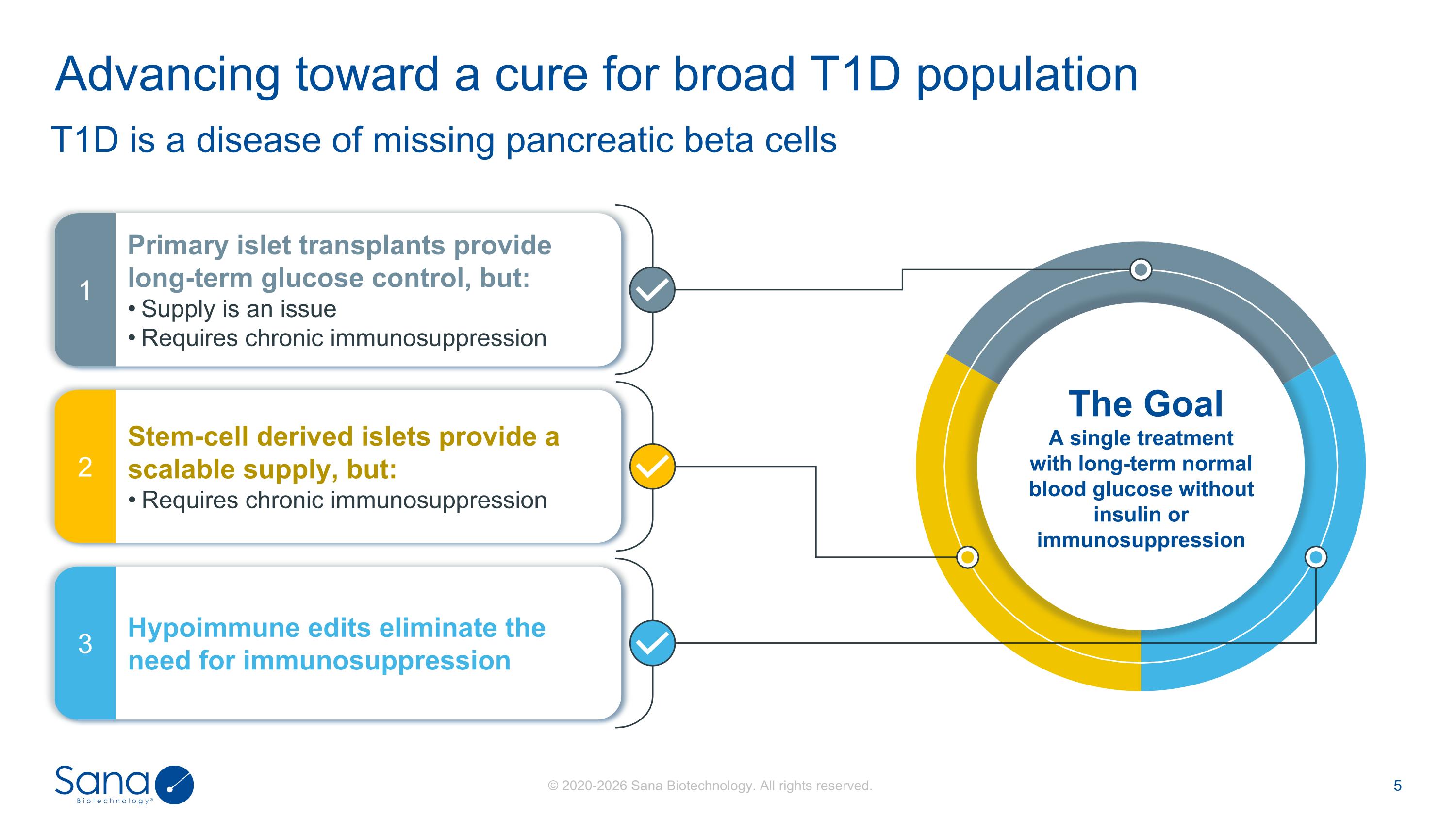
T1D is a disease of missing pancreatic beta cells Advancing toward a cure for broad T1D population Primary islet transplants provide long-term glucose control, but: Supply is an issue Requires chronic immunosuppression 1 Stem-cell derived islets provide a scalable supply, but: Requires chronic immunosuppression 2 Hypoimmune edits eliminate the need for immunosuppression 3 The Goal A single treatment with long-term normal blood glucose without insulin or immunosuppression
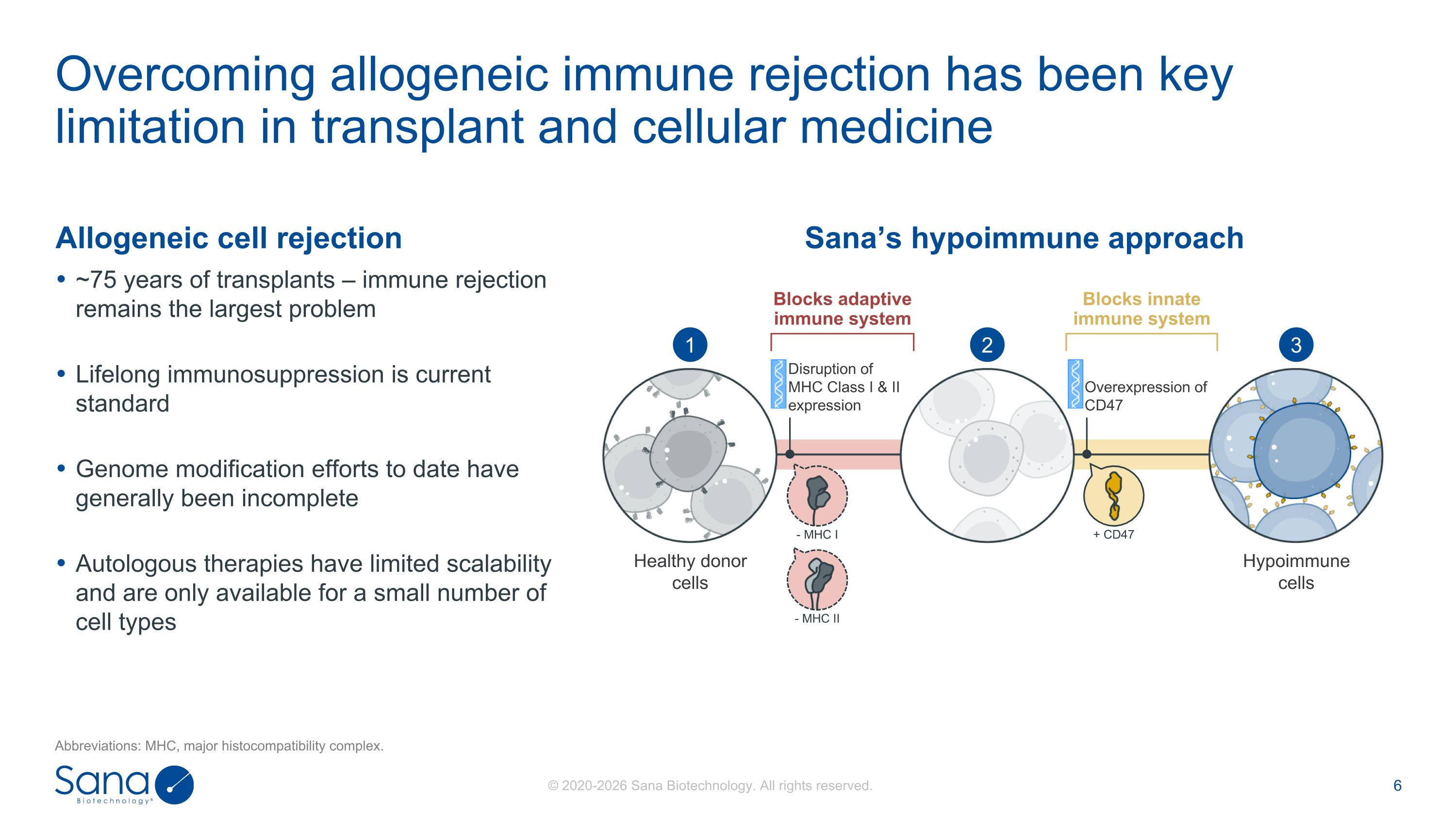
Allogeneic cell rejection ~75 years of transplants – immune rejection remains the largest problem Lifelong immunosuppression is current standard Genome modification efforts to date have generally been incomplete Autologous therapies have limited scalability and are only available for a small number of cell types Overcoming allogeneic immune rejection has been key limitation in transplant and cellular medicine Abbreviations: MHC, major histocompatibility complex. Sana’s hypoimmune approach + CD47 - MHC I - MHC II Healthy donor cells Hypoimmune cells Disruption of MHC Class I & II expression Overexpression of CD47 1 2 3 Blocks adaptive immune system Blocks innate immune system
Sana has pioneered hypoimmune technology
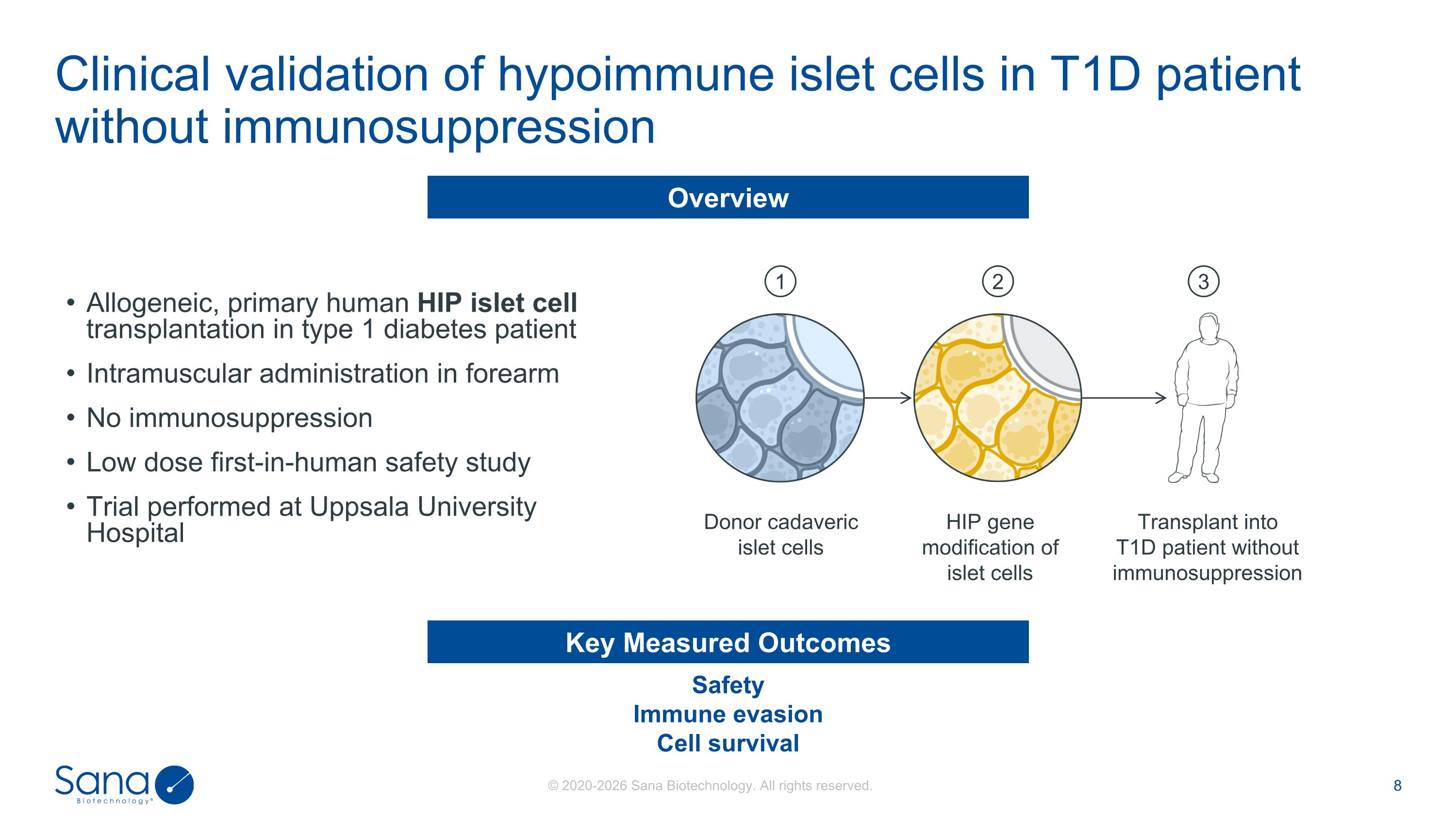
Transplant into T1D patient without immunosuppression HIP gene modification of islet cells Donor cadaveric islet cells 1 2 3 Safety Immune evasion Cell survival Key Measured Outcomes Allogeneic, primary human HIP islet cell transplantation in type 1 diabetes patient Intramuscular administration in forearm No immunosuppression Low dose first-in-human safety study Trial performed at Uppsala University Hospital Overview Clinical validation of hypoimmune islet cells in T1D patient without immunosuppression
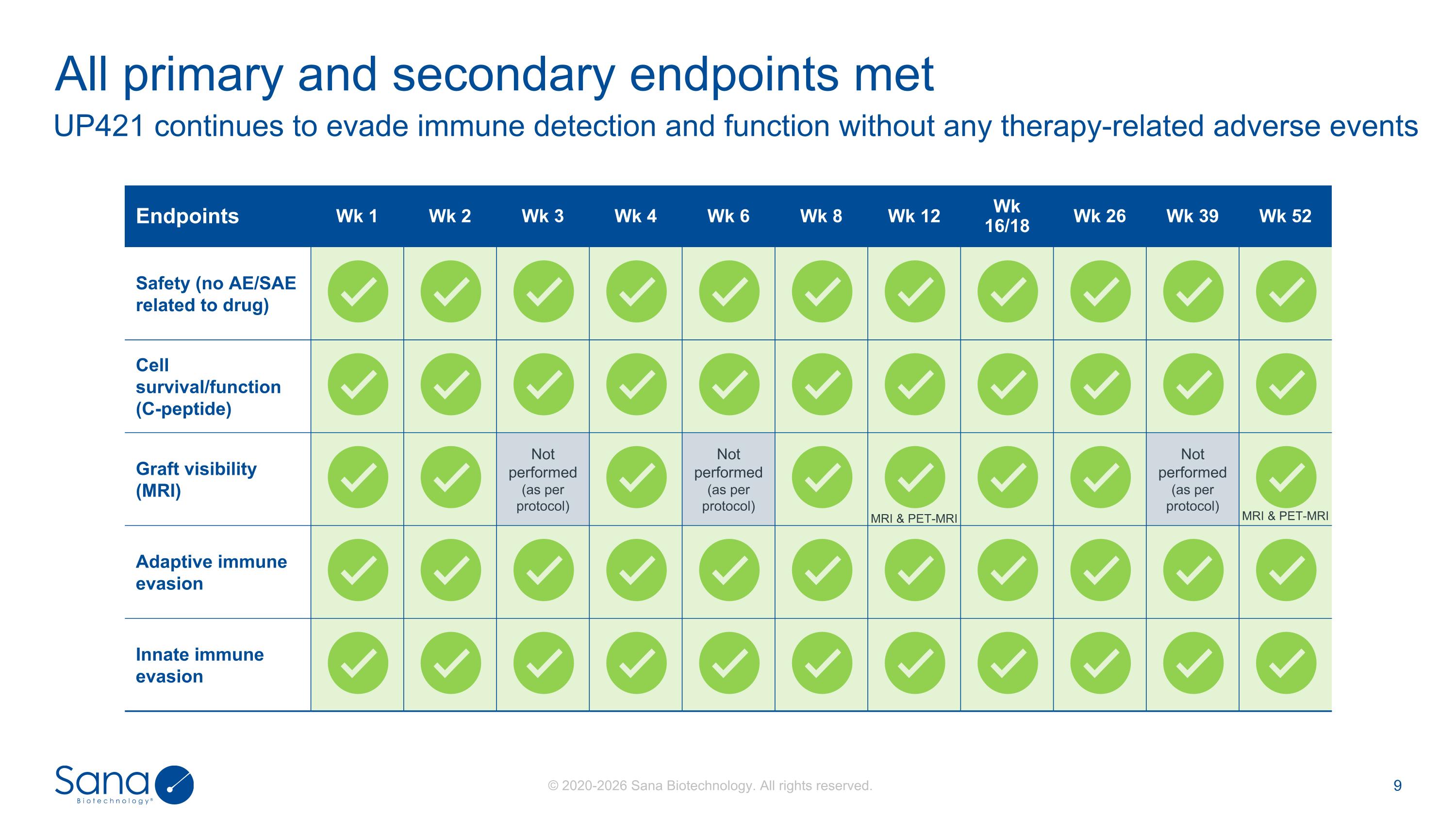
UP421 continues to evade immune detection and function without any therapy-related adverse events All primary and secondary endpoints met Endpoints Wk 1 Wk 2 Wk 3 Wk 4 Wk 6 Wk 8 Wk 12 Wk 16/18 Wk 26 Wk 39 Wk 52 Safety (no AE/SAE related to drug) Cell survival/function (C-peptide) Graft visibility (MRI) Not performed (as per protocol) Not performed (as per protocol) MRI & PET-MRI Not performed (as per protocol) MRI & PET-MRI Adaptive immune evasion Innate immune evasion
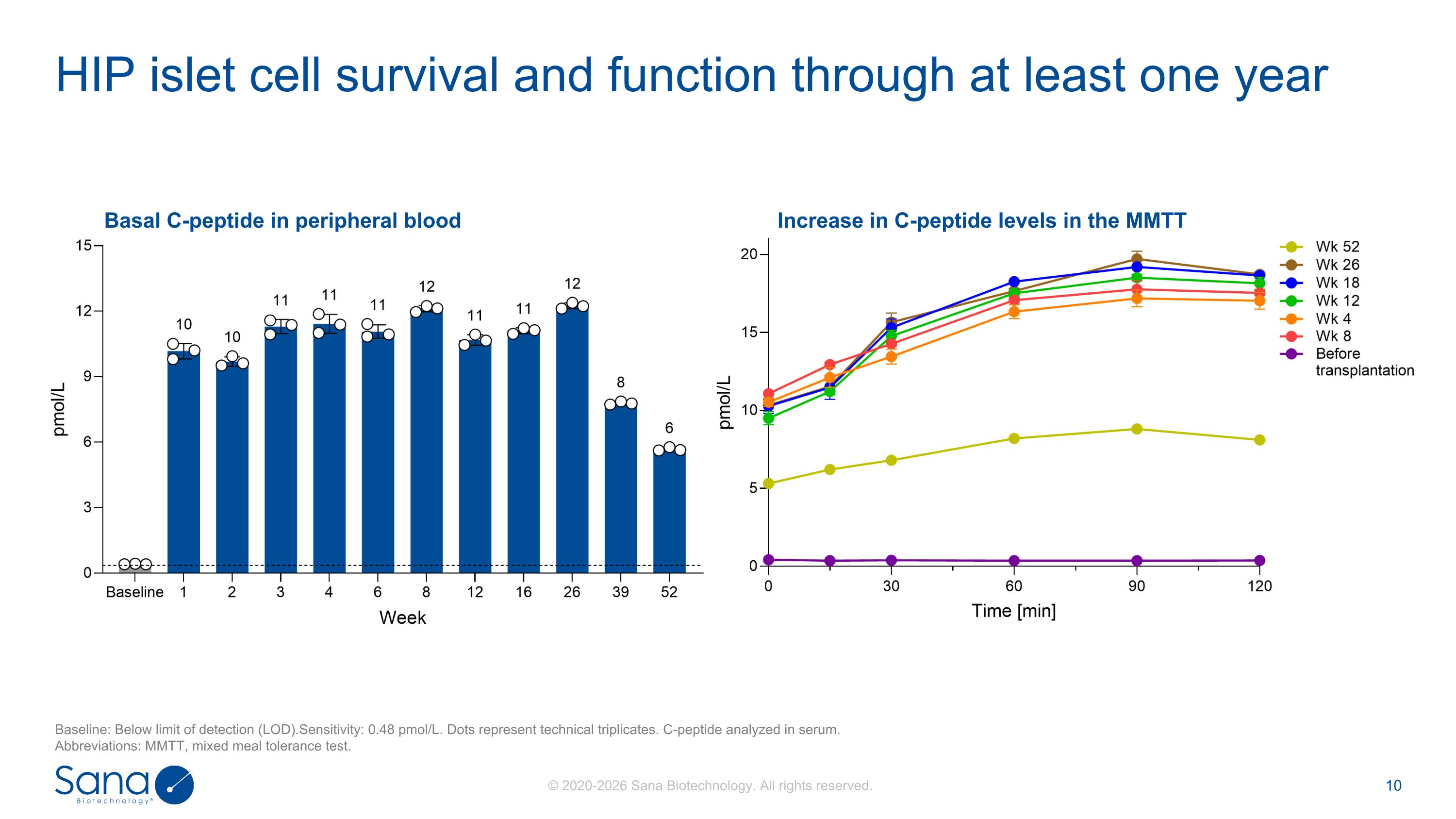
Basal C-peptide in peripheral blood HIP islet cell survival and function through at least one year Baseline: Below limit of detection (LOD).Sensitivity: 0.48 pmol/L. Dots represent technical triplicates. C-peptide analyzed in serum. Abbreviations: MMTT, mixed meal tolerance test. Increase in C-peptide levels in the MMTT
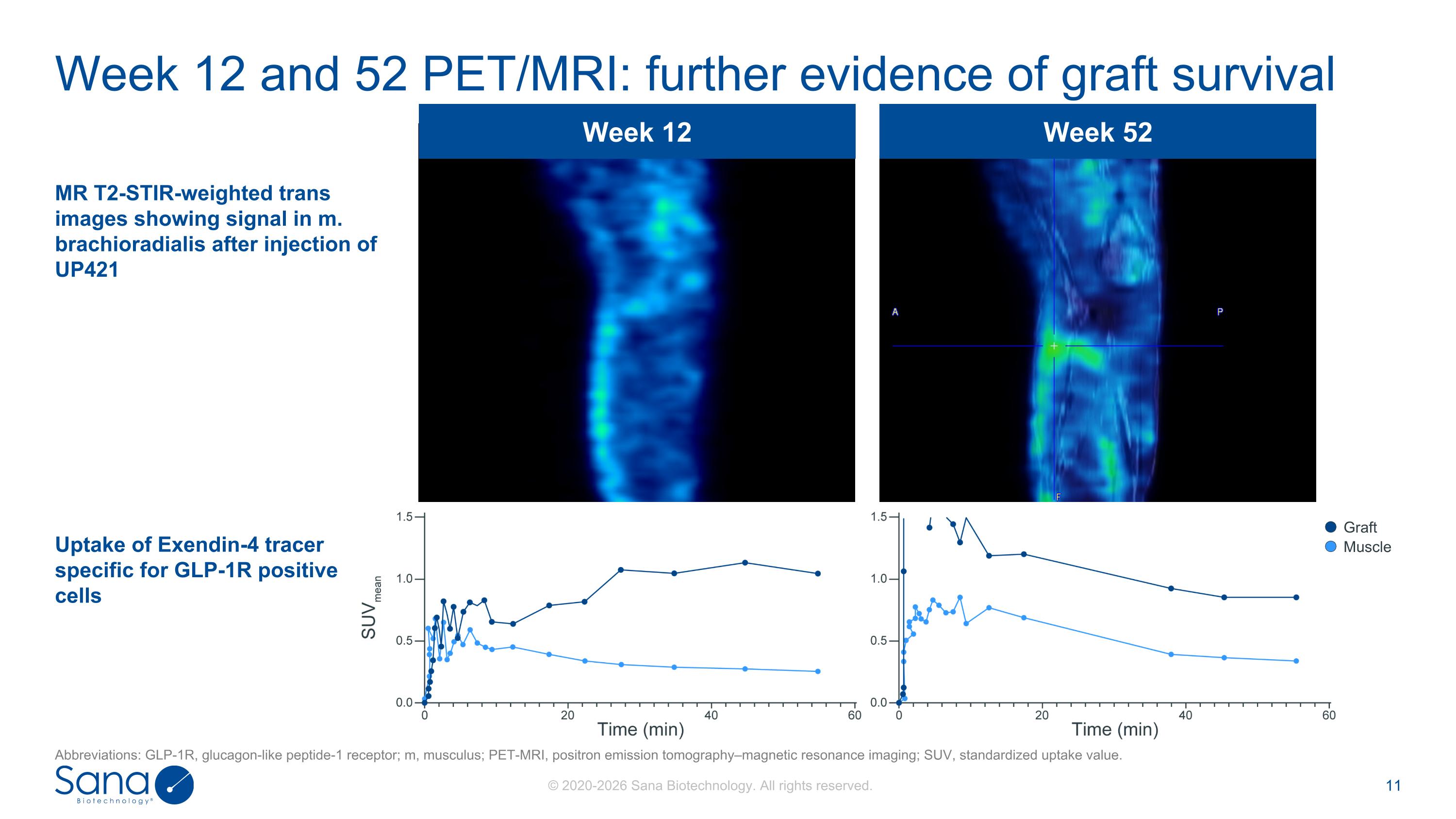
Week 12 Week 52 Week 12 and 52 PET/MRI: further evidence of graft survival MR T2-STIR-weighted trans images showing signal in m. brachioradialis after injection of UP421 Uptake of Exendin-4 tracer specific for GLP-1R positive cells Graft Muscle Abbreviations: GLP-1R, glucagon-like peptide-1 receptor; m, musculus; PET-MRI, positron emission tomography–magnetic resonance imaging; SUV, standardized uptake value.
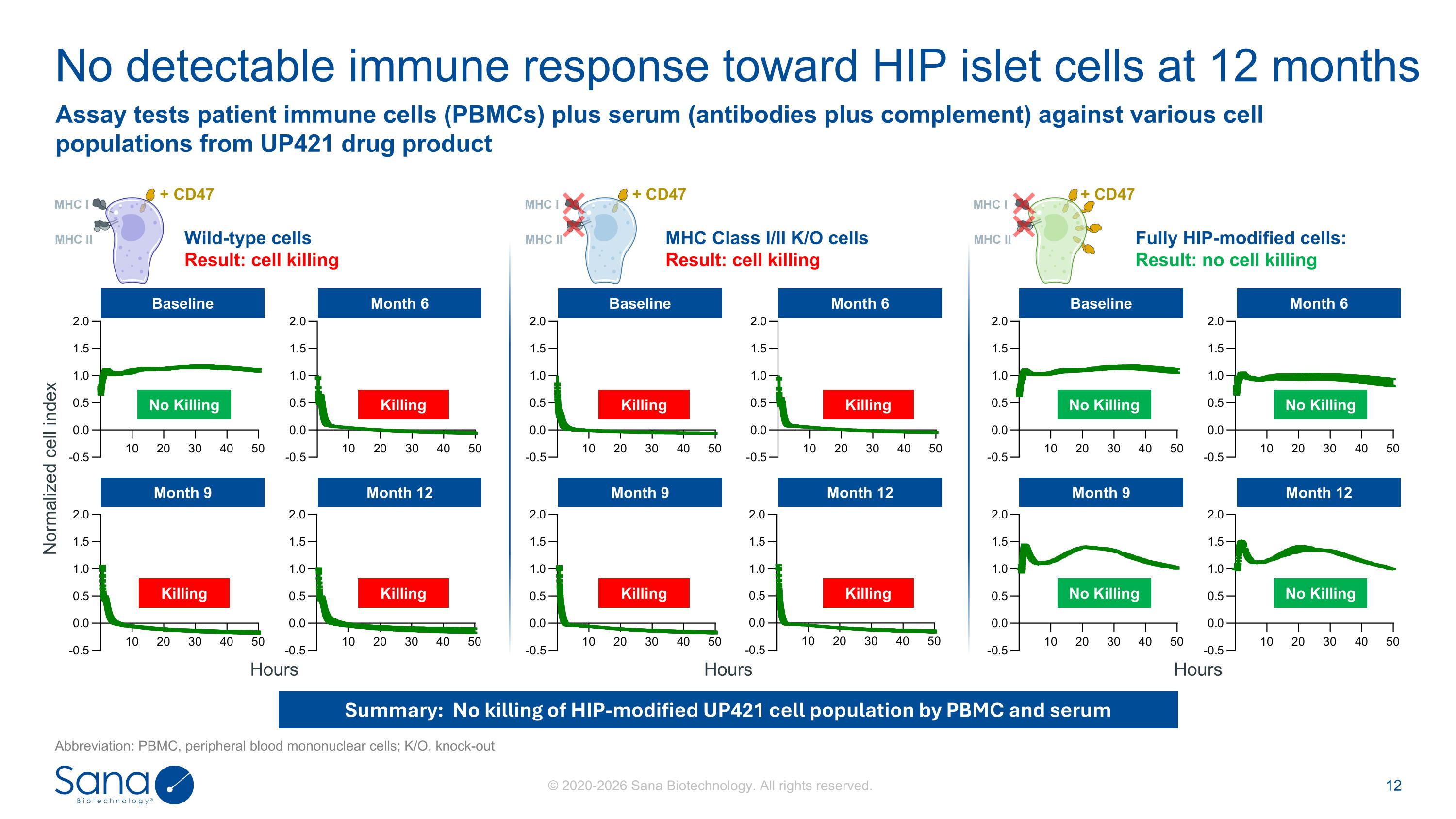
Wild-type cells Result: cell killing MHC Class I/II K/O cells Result: cell killing Fully HIP-modified cells: Result: no cell killing No detectable immune response toward HIP islet cells at 12 months Abbreviation: PBMC, peripheral blood mononuclear cells; K/O, knock-out Summary: No killing of HIP-modified UP421 cell population by PBMC and serum Assay tests patient immune cells (PBMCs) plus serum (antibodies plus complement) against various cell populations from UP421 drug product Month 9 Month 12 Month 9 Month 12 Month 9 Month 12 Baseline Month 6 Baseline Month 6 Baseline Month 6 No Killing Killing Killing Killing Killing Killing Killing Killing No Killing No Killing No Killing No Killing Normalized cell index Hours Hours Hours + CD47 MHC I MHC II + CD47 MHC I MHC II + CD47 MHC I MHC II
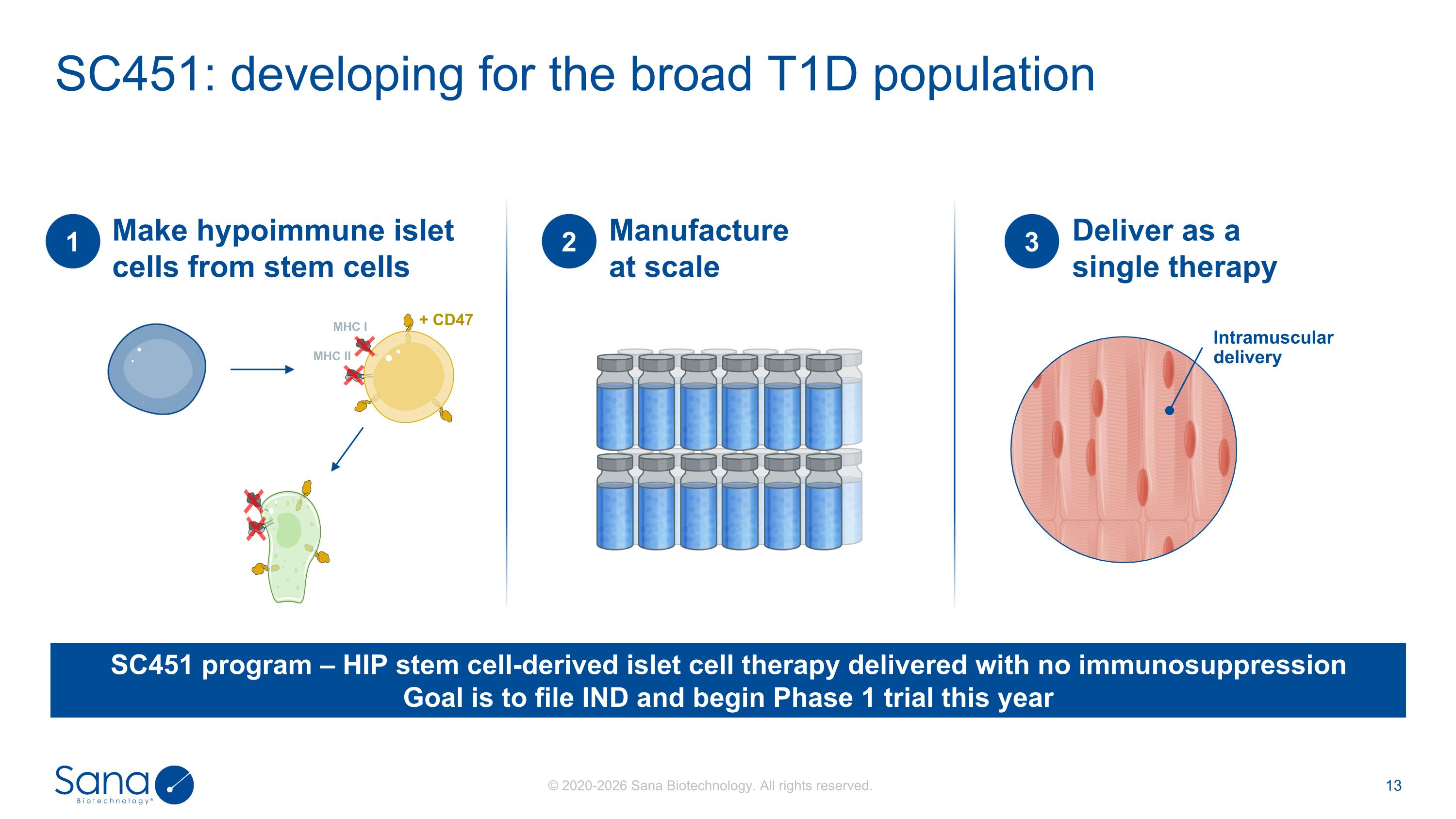
SC451: developing for the broad T1D population Make hypoimmune islet cells from stem cells 1 + CD47 MHC I MHC II Manufacture at scale 2 Deliver as a single therapy 3 SC451 program – HIP stem cell-derived islet cell therapy delivered with no immunosuppression Goal is to file IND and begin Phase 1 trial this year Intramuscular delivery
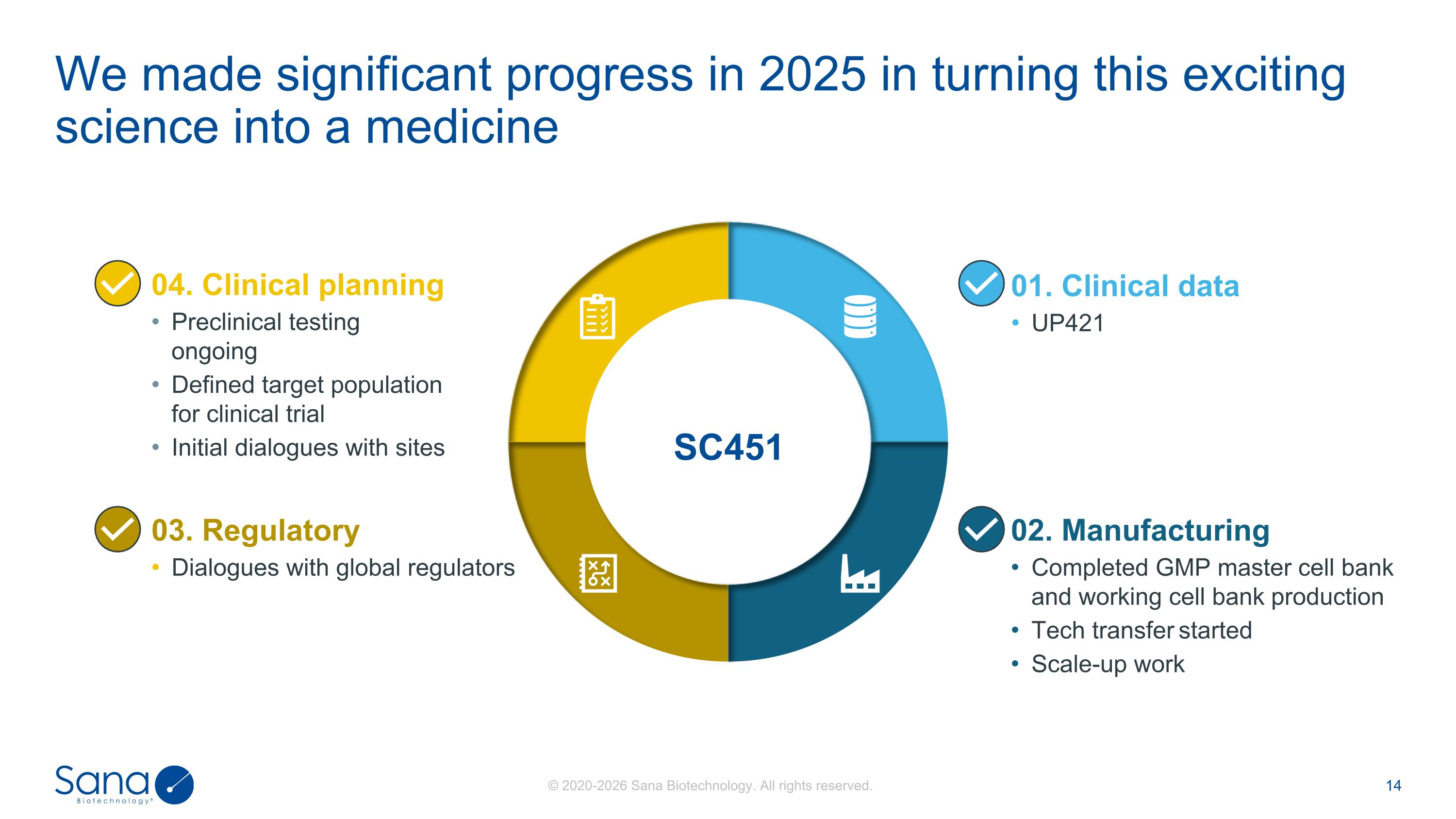
01. Clinical data UP421 03. Regulatory Dialogues with global regulators 02. Manufacturing Completed GMP master cell bank and working cell bank production Tech transfer started Scale-up work 04. Clinical planning Preclinical testing ongoing Defined target population for clinical trial Initial dialogues with sites SC451 We made significant progress in 2025 in turning this exciting science into a medicine
SC451: next steps for value creation Complete GLP toxicology study & non-clinical testing package Complete GMP tech transfer & manufacture clinical trial material Make significant progress on commercial scale manufacturing process File IND and equivalent in at least one other geography Begin Phase 1 testing
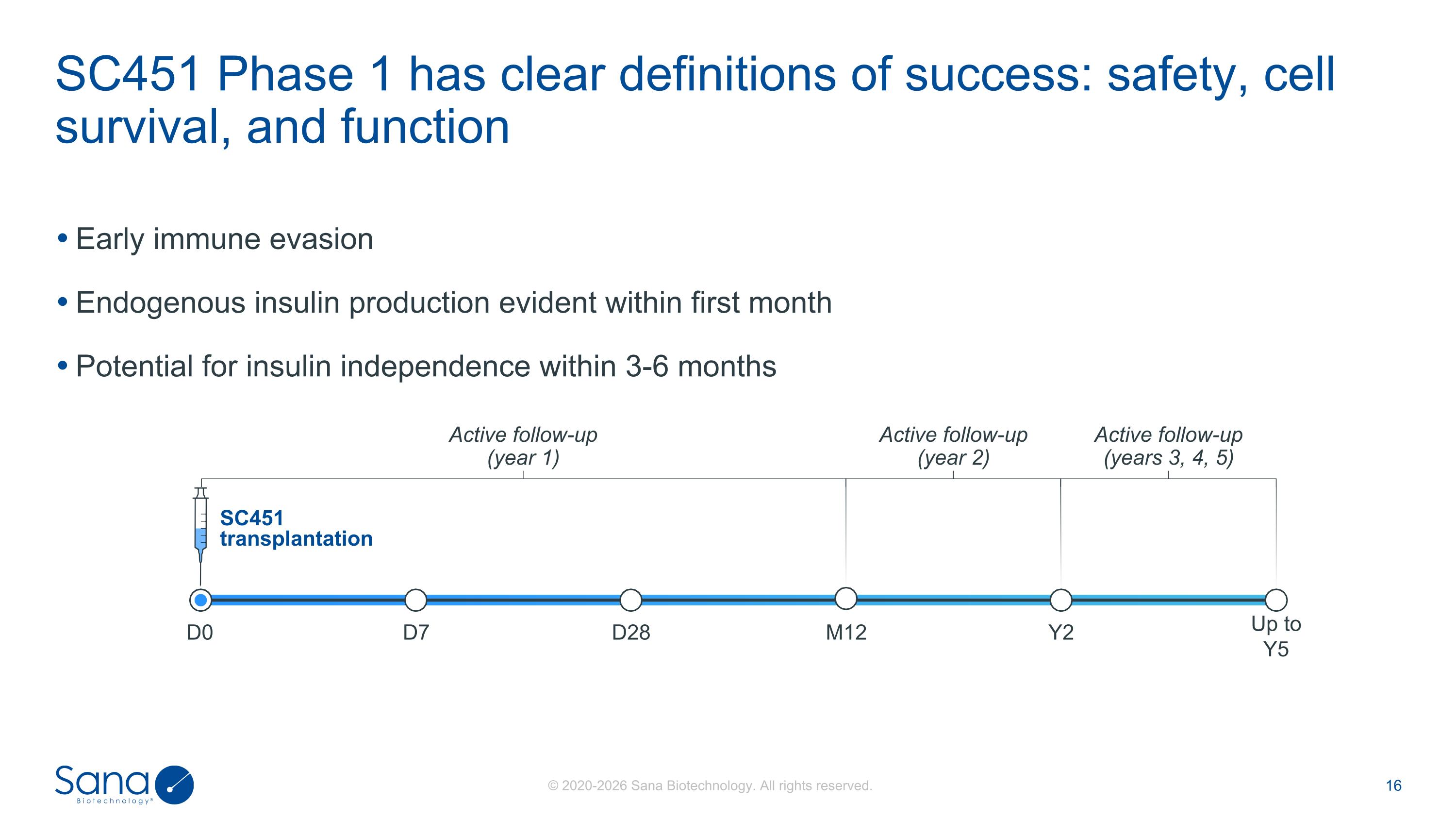
Early immune evasion Endogenous insulin production evident within first month Potential for insulin independence within 3-6 months SC451 Phase 1 has clear definitions of success: safety, cell survival, and function D7 D0 D28 SC451 transplantation Up to Y5 Y2 M12 Active follow-up (year 1) Active follow-up (year 2) Active follow-up (years 3, 4, 5)
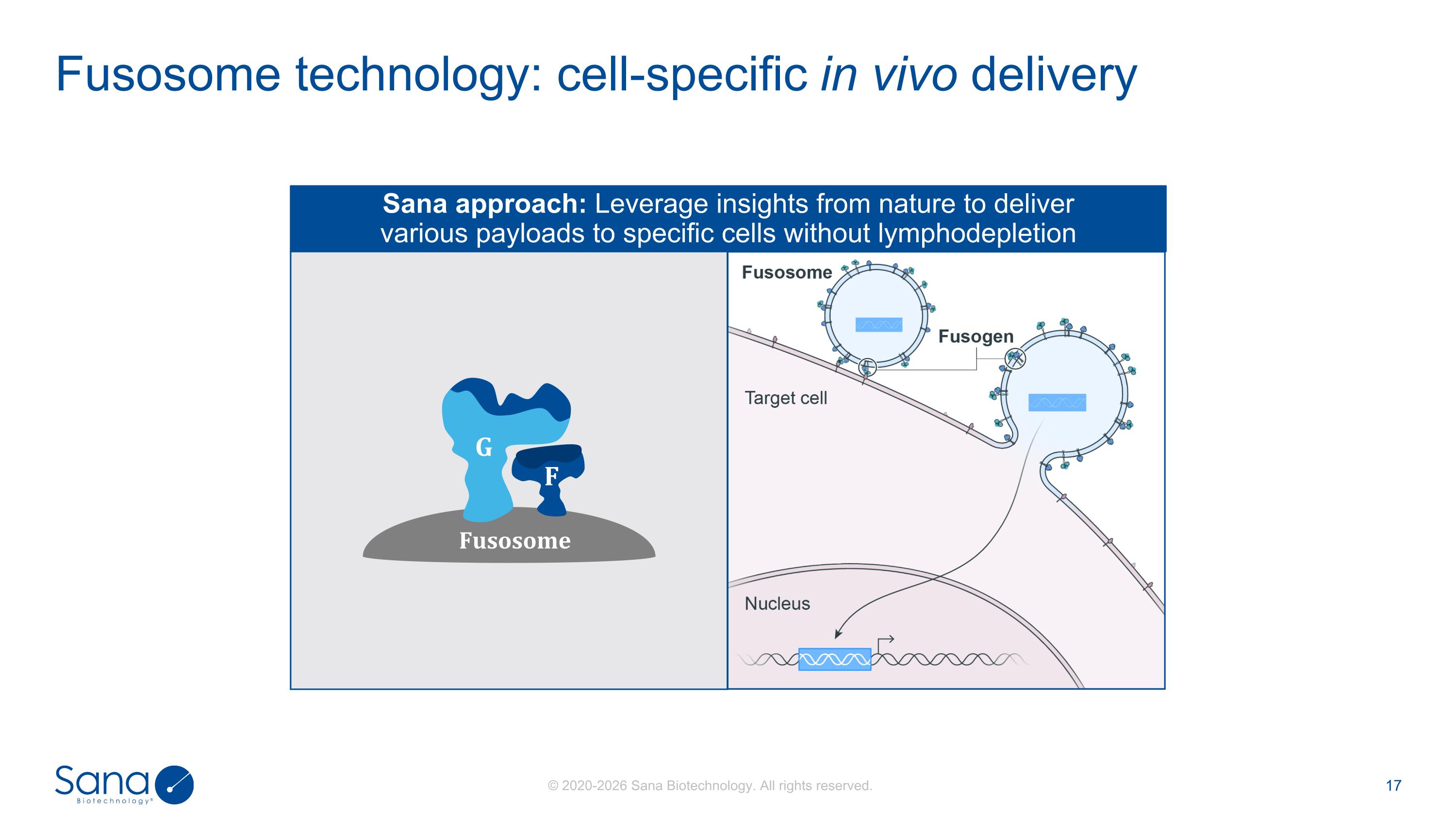
Fusosome technology: cell-specific in vivo delivery F G Fusosome Sana approach: Leverage insights from nature to deliver various payloads to specific cells without lymphodepletion
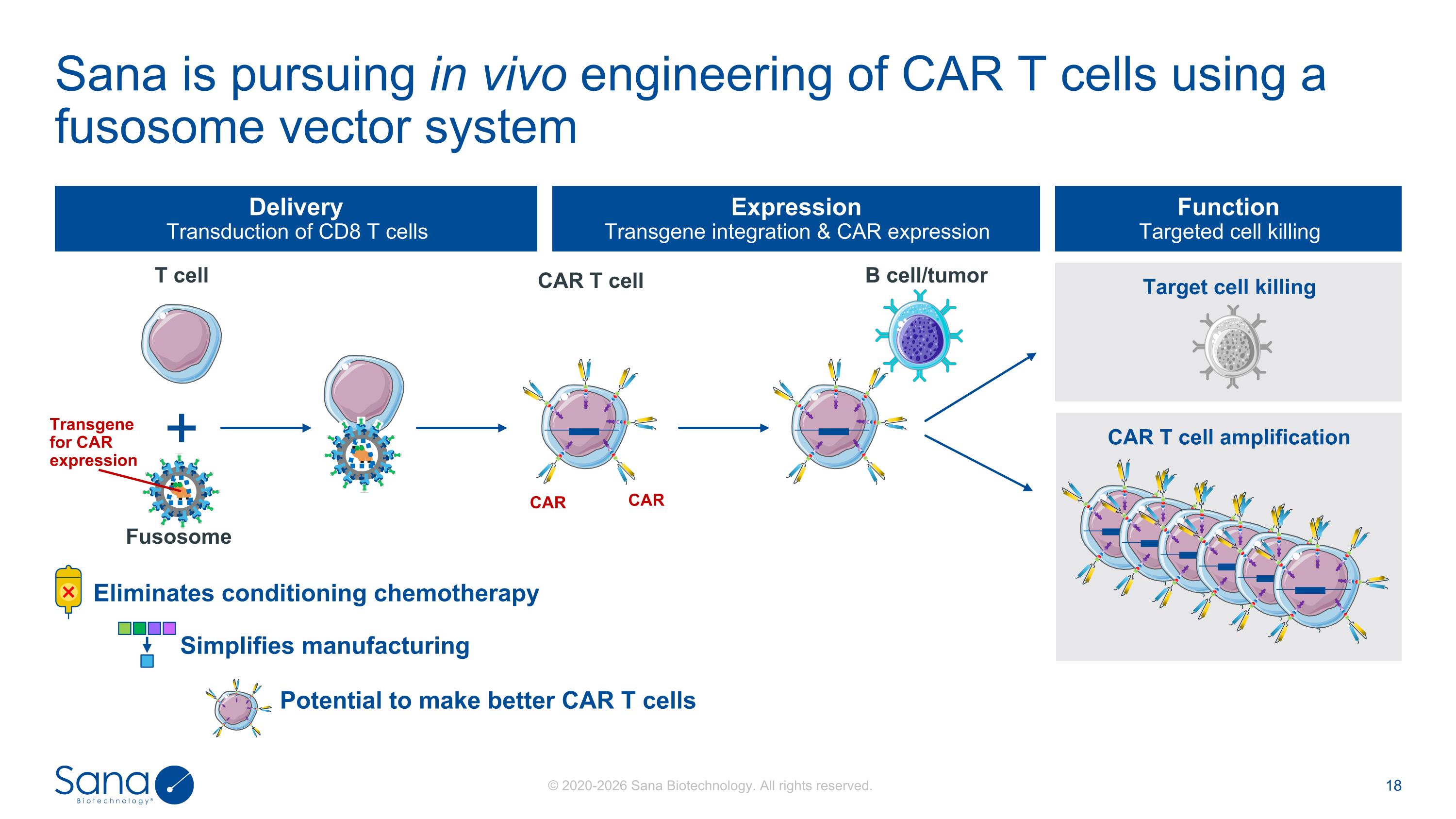
Target cell killing CAR T cell amplification Delivery Transduction of CD8 T cells Expression Transgene integration & CAR expression Function Targeted cell killing T cell Fusosome B cell/tumor CAR T cell Transgene for CAR expression CAR CAR Sana is pursuing in vivo engineering of CAR T cells using a fusosome vector system Potential to make better CAR T cells Eliminates conditioning chemotherapy Simplifies manufacturing
Lowers risk of off-target toxicity Lowers immunogenicity risk, potentially improving safety, persistence, and ability to re-dose Improves manufacturability CAR T cells typically undergo multi-logarithmic expansion inside the patient in order to clear target cells Integrated DNA replicates with cell division; mRNA does not Sana made two critical assumptions in developing this program Cell specificity of delivery will be important Integration into T cell DNA is important
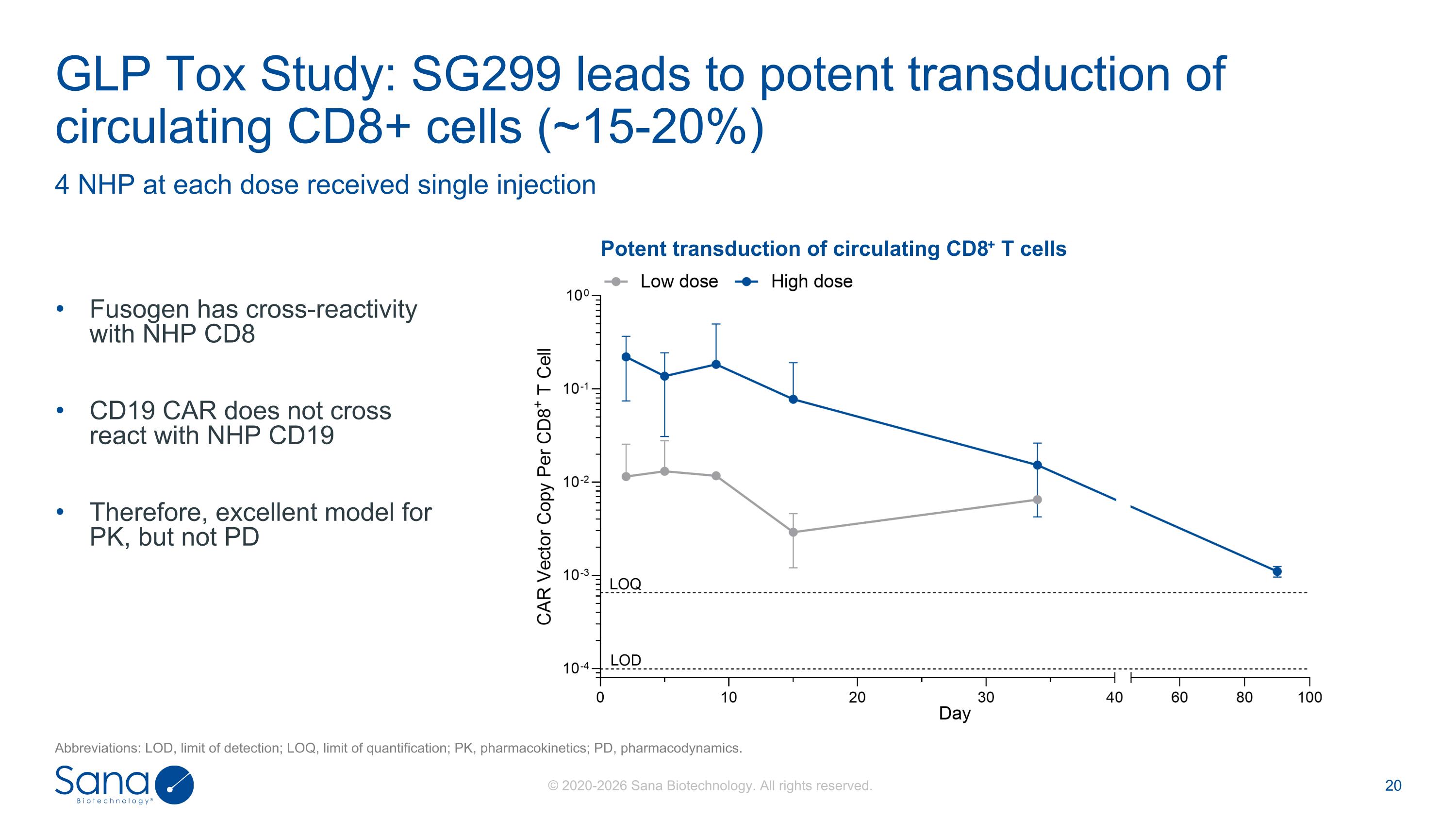
Fusogen has cross-reactivity with NHP CD8 CD19 CAR does not cross react with NHP CD19 Therefore, excellent model for PK, but not PD 4 NHP at each dose received single injection GLP Tox Study: SG299 leads to potent transduction of circulating CD8+ cells (~15-20%) Potent transduction of circulating CD8+ T cells Abbreviations: LOD, limit of detection; LOQ, limit of quantification; PK, pharmacokinetics; PD, pharmacodynamics.
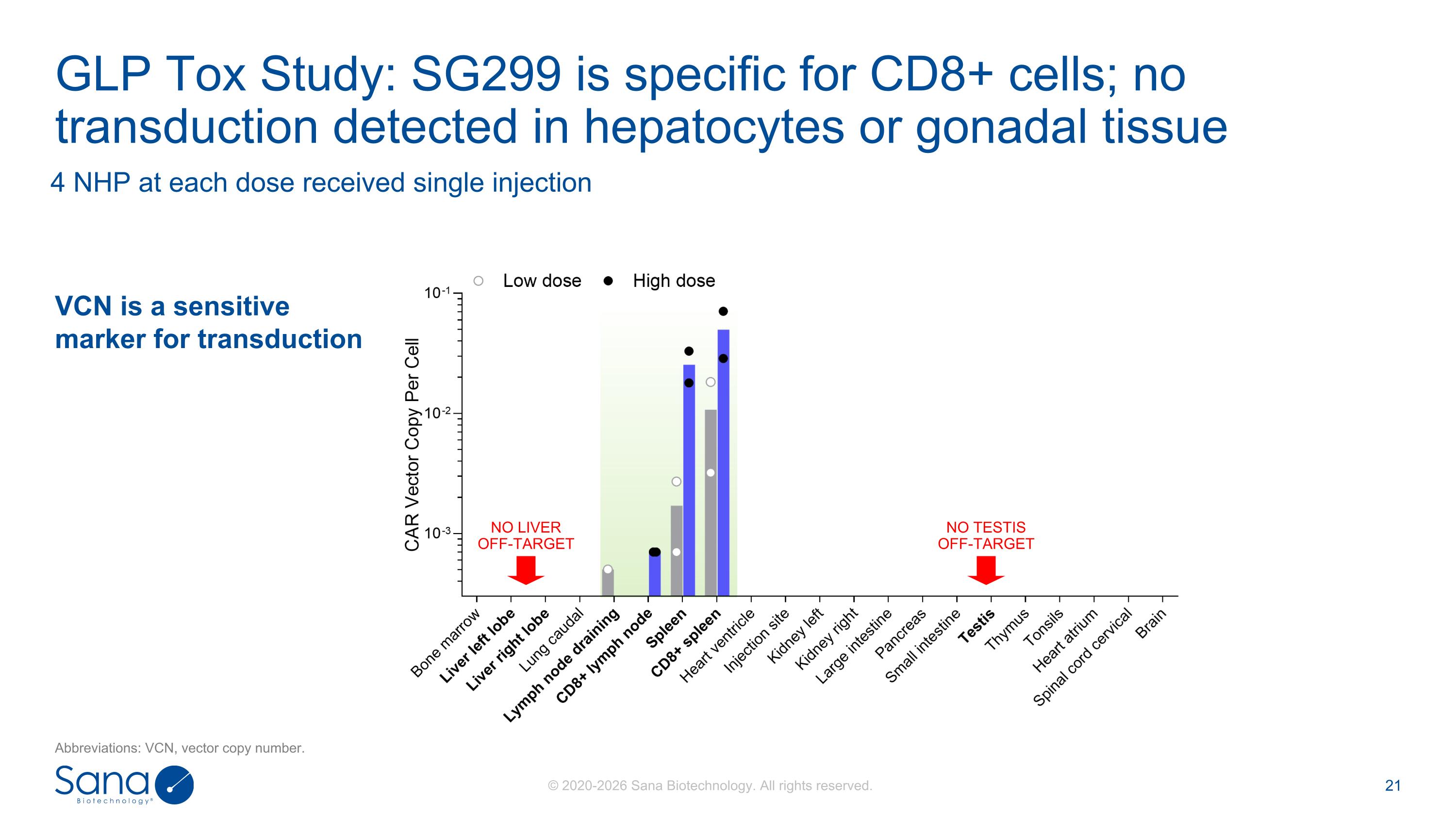
4 NHP at each dose received single injection GLP Tox Study: SG299 is specific for CD8+ cells; no transduction detected in hepatocytes or gonadal tissue NO LIVER OFF-TARGET NO TESTIS OFF-TARGET VCN is a sensitive marker for transduction Abbreviations: VCN, vector copy number.
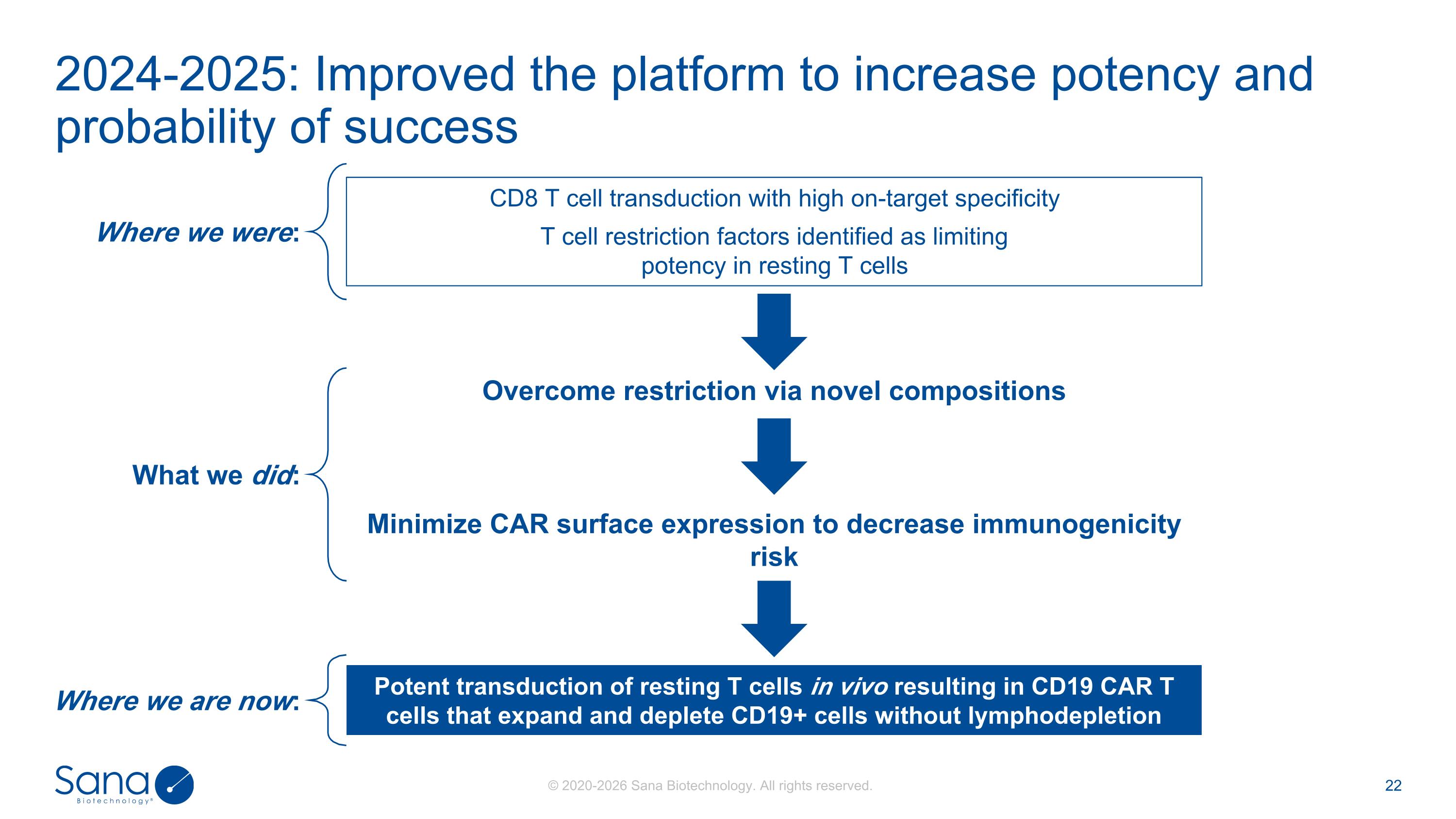
CD8 T cell transduction with high on-target specificity T cell restriction factors identified as limiting potency in resting T cells 2024-2025: Improved the platform to increase potency and probability of success Potent transduction of resting T cells in vivo resulting in CD19 CAR T cells that expand and deplete CD19+ cells without lymphodepletion Overcome restriction via novel compositions Minimize CAR surface expression to decrease immunogenicity risk Where we were: What we did: Where we are now:
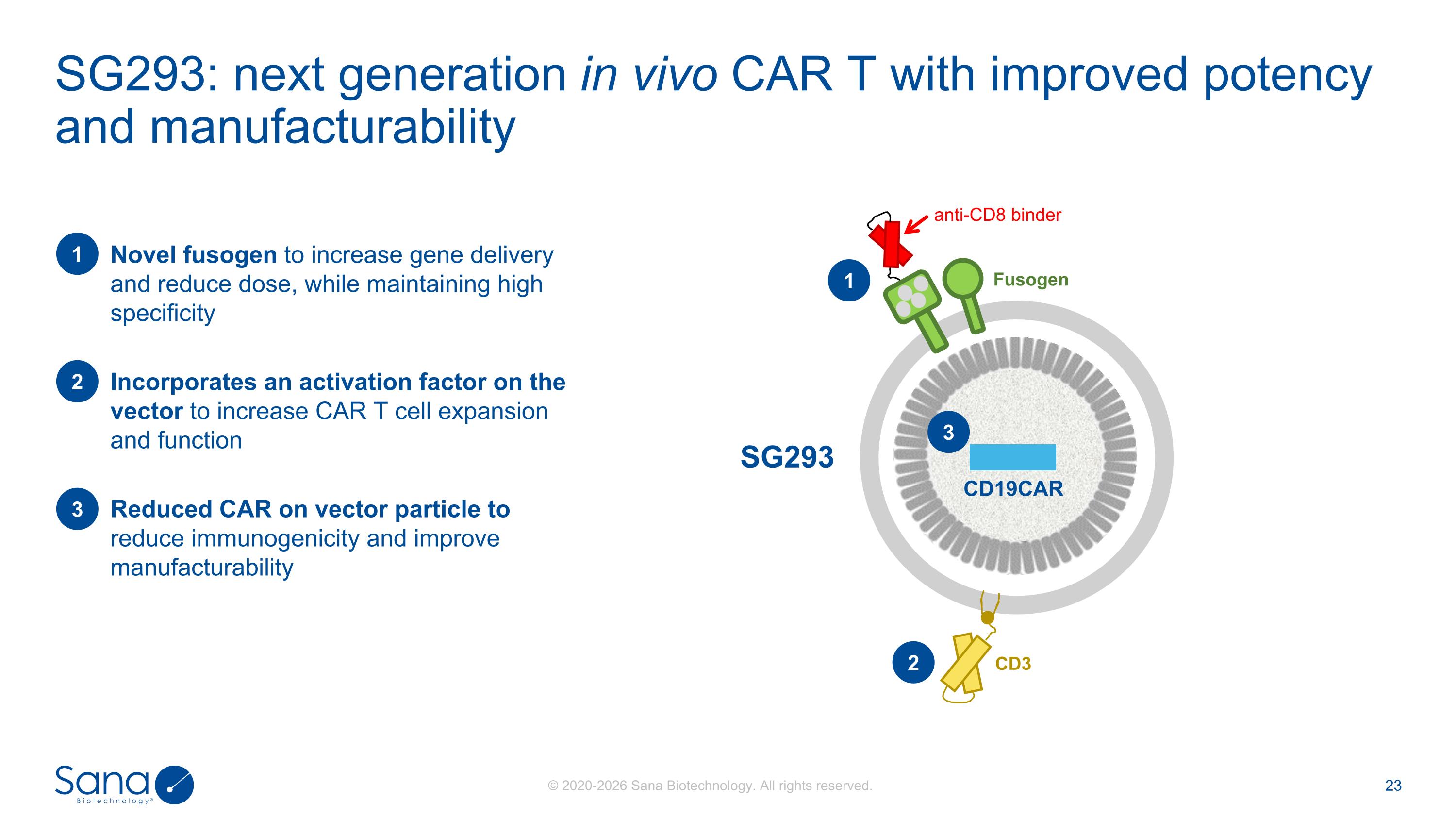
SG293: next generation in vivo CAR T with improved potency and manufacturability SG293 Novel fusogen to increase gene delivery and reduce dose, while maintaining high specificity 1 Reduced CAR on vector particle to reduce immunogenicity and improve manufacturability 2 Incorporates an activation factor on the vector to increase CAR T cell expansion and function 3 CD3 Fusogen anti-CD8 binder CD19CAR 3 1 2
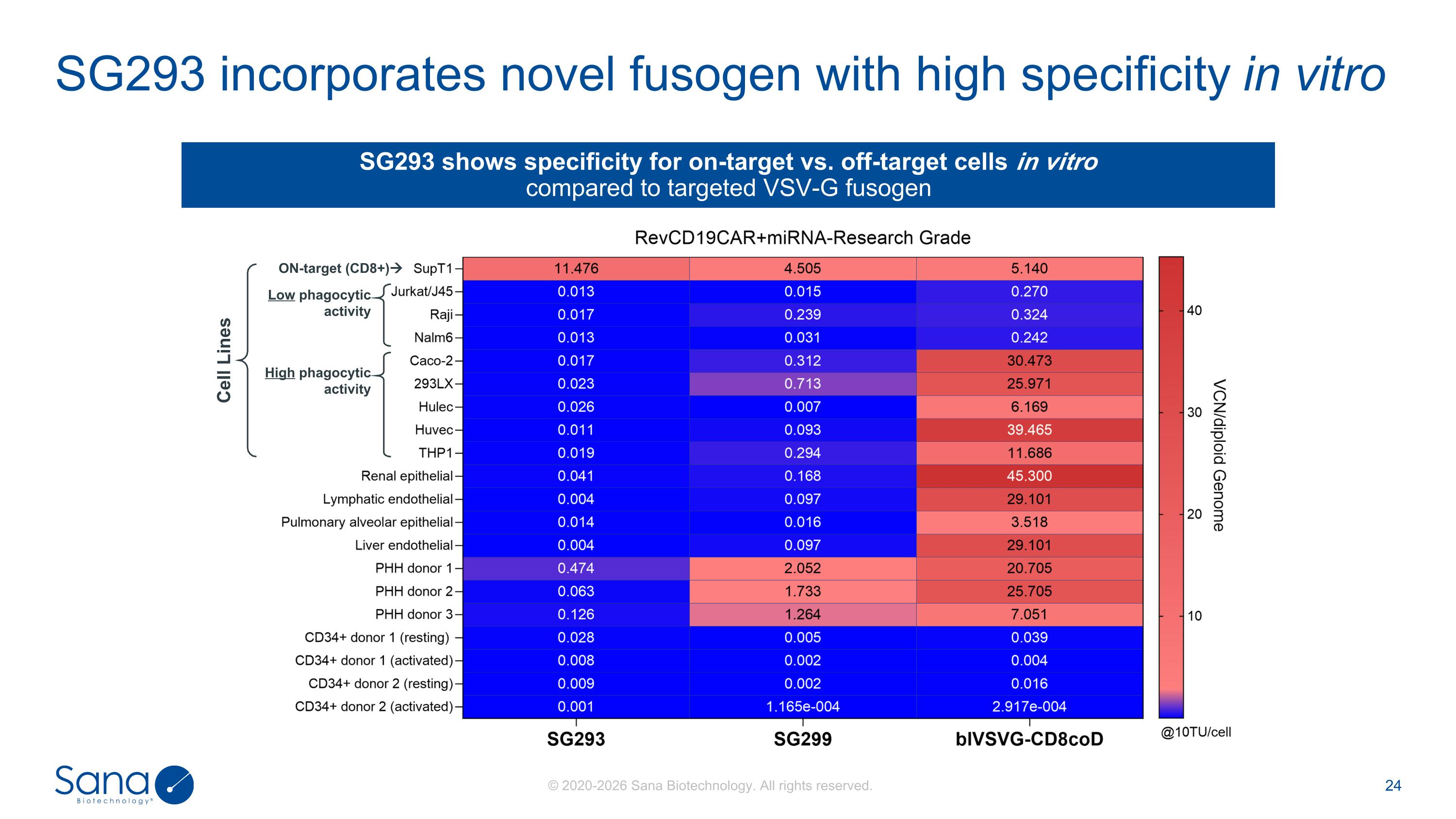
SG293 incorporates novel fusogen with high specificity in vitro SG293 shows specificity for on-target vs. off-target cells in vitro compared to targeted VSV-G fusogen Cell Lines ON-target (CD8+) Low phagocytic activity High phagocytic activity
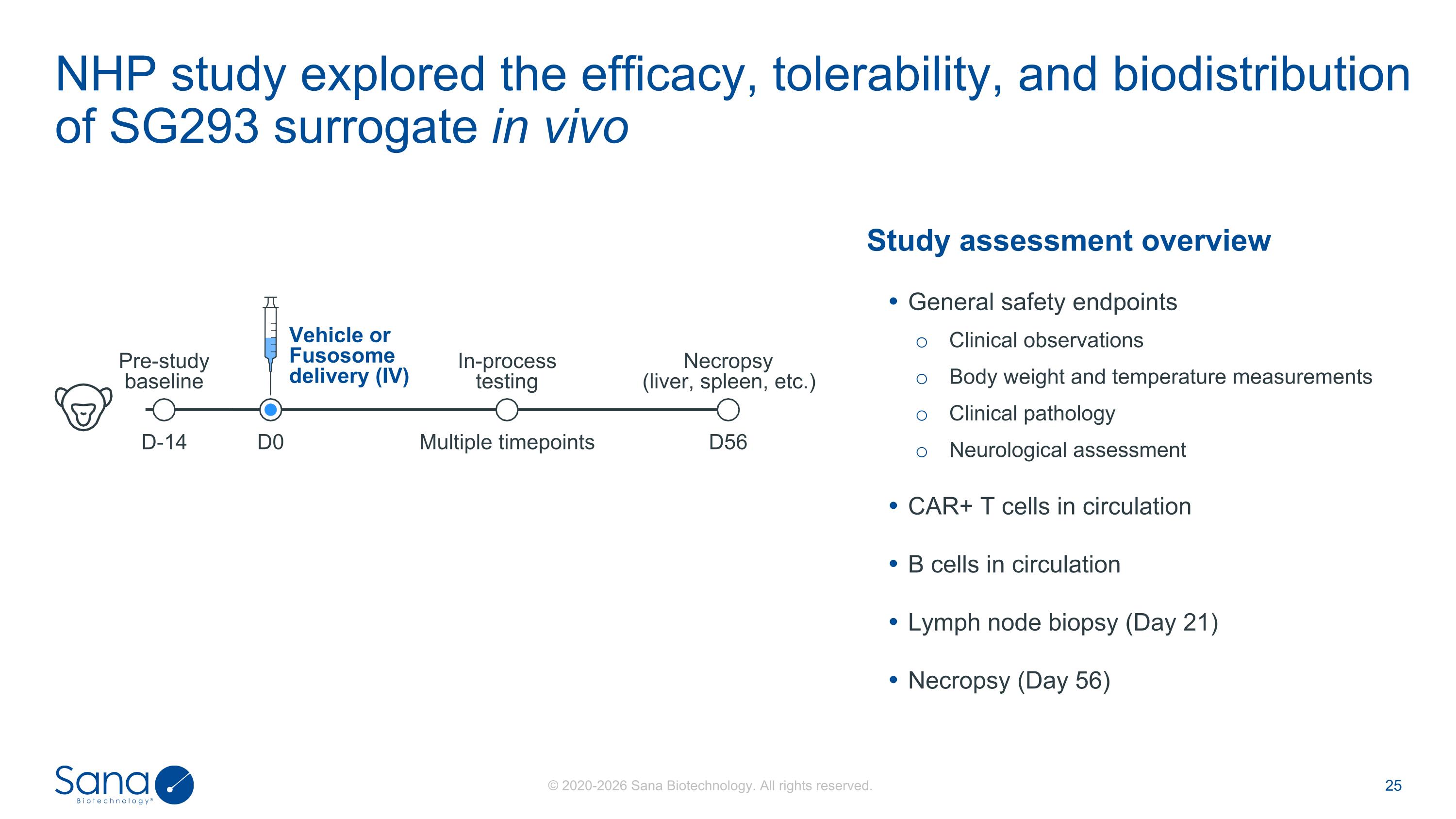
NHP study explored the efficacy, tolerability, and biodistribution of SG293 surrogate in vivo D-14 Multiple timepoints Necropsy (liver, spleen, etc.) Vehicle or Fusosome delivery (IV) In-process testing Pre-study baseline D0 D56 Study assessment overview General safety endpoints Clinical observations Body weight and temperature measurements Clinical pathology Neurological assessment CAR+ T cells in circulation B cells in circulation Lymph node biopsy (Day 21) Necropsy (Day 56)
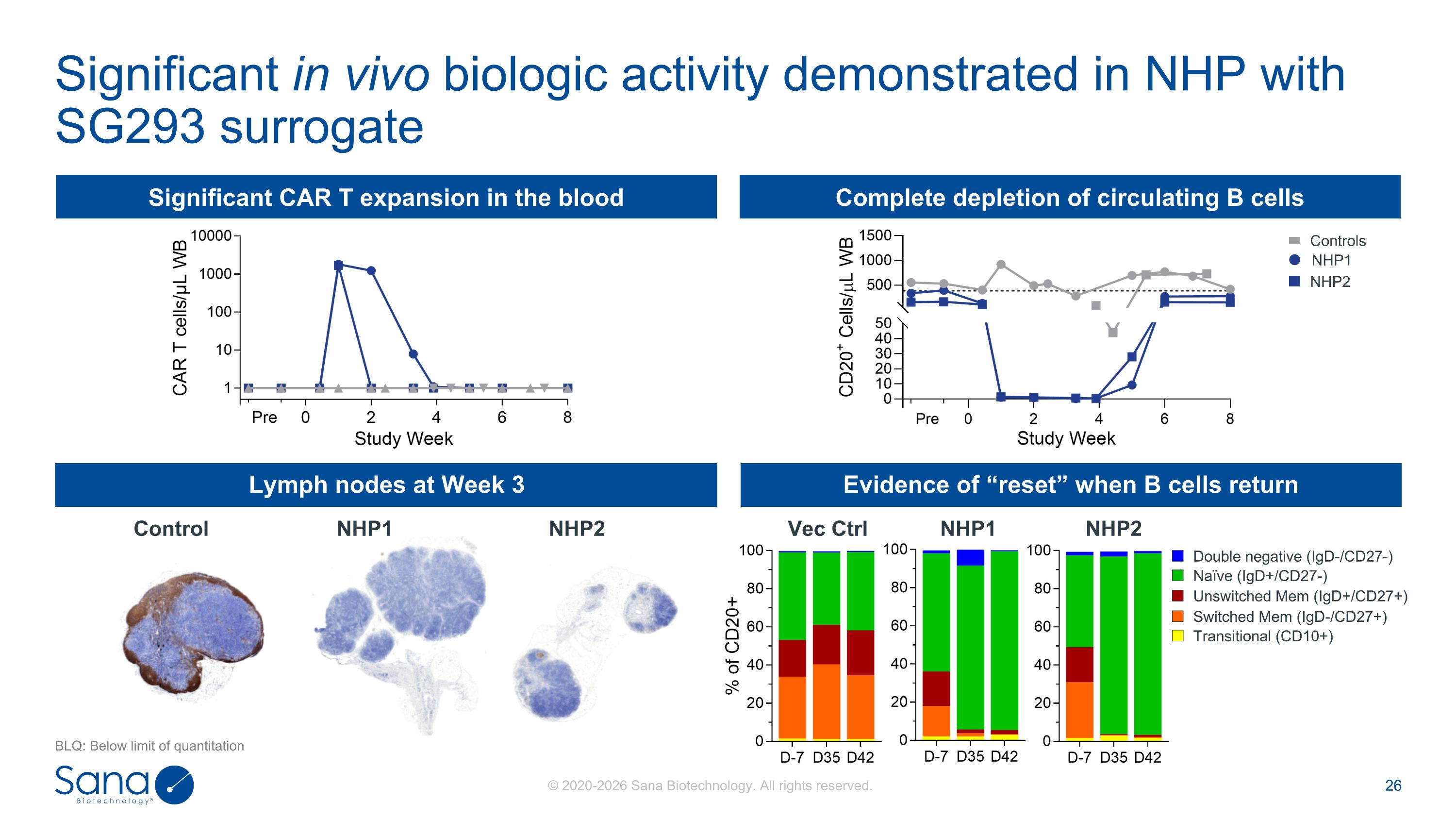
Significant in vivo biologic activity demonstrated in NHP with SG293 surrogate BLQ: Below limit of quantitation Significant CAR T expansion in the blood Complete depletion of circulating B cells Lymph nodes at Week 3 Evidence of “reset” when B cells return Vec Ctrl NHP1 NHP2 Control NHP1 NHP2 Double negative (IgD-/CD27-) Naïve (IgD+/CD27-) Unswitched Mem (IgD+/CD27+) Switched Mem (IgD-/CD27+) Transitional (CD10+) NHP1 NHP2 Controls
Sana’s fusogen platform has the potential for multiple best-in-class therapies SG293: Potential for initial clinical data in 2026 First trial likely in blood cancers With early safety & efficacy data, may be able to move rapidly into autoimmune diseases Recent progress creates potential for best-in-class in vivo CAR T cell platform Beyond CD19, Sana is ready to expand into BCMA, CD22, & more CD22 BCMA etc.
Type 1 diabetes: a disease in need of better alternatives Scientific validation that a functional cure is possible SC451 assembles all components into a scalable platform 2026: Goal is to file IND & start Phase 1 trial Potential for early clinical proof of concept: Immune evasion Endogenous insulin production Glucose control without exogenous insulin Fusogen platform has potential to deliver multiple therapies SG293 has potential for best-in-class profile SG293 goal: Initial first-in-human data in 2026 Sana: next 12-18 months can be transformative
