

Corporate Presentation January 2026

2 Forward - Looking Statements This presentation and any accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the pres ent er or ADC Therapeutics or any officer, director, employee, agent or advisor of ADC Therapeutics. This presentation does not purport to be all - inclusive or to contain all of the information you may desire. Information provided in this presentation and any accompanying or al presentation speak only as of the date hereof. This presentation contains forward - looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. In some cases you can identify forward - looking statements by terminology such as "may", "will", "should", "would", "expect", "intend", "plan", "anticipate", "believe", "est ima te", "predict", "potential", "seem", "seek", "future", "continue", or "appear" or the negative of these terms or similar expressions, although not all forward - looking statements contain these identifying words. For ward - looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: changes to the preliminary unaudited Q4 and full year 2025 net product revenue and cash and cash equivalents as of December 31, 2025; expected cash runway at least to 2028 which assumes us e o f minimum liquidity amount required to be maintained under its loan agreement covenants; whether future LOTIS - 7 clinical trial results will be consistent with or different from the LOTIS - 7 data pr esented by the Company on December 3, 2025, the timing, publication and outcome of the full LOTIS - 7 trial, compendia inclusion and regulatory strategy and the commercial opportunity; the timing of th e PFS events and topline data release for LOTIS - 5 and the results of the trial, the timing for the sBLA submission and full FDA approval; the Company's ability to grow ZYNLONTA® revenue in the United States and potential peak rev en ue; the ability of our partners to commercialize ZYNLONTA® in foreign markets, the timing and amount of future revenue and payments to us from such partnerships and their abi lit y to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions; the timing and results of the Company's clinical trials; the timing, publication and results of investigator - initiated trials includ ing those studying FL and MZL and the potential regulatory and/or compendia strategy and the future opportunity; the timing and outcome of regulatory submissions for the Company's products or product c and idates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Company's indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities , a nd the restrictions imposed on the Company's activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cas h required to service such indebtedness; and the Company's ability to obtain financial and other resources for its research, development, clinical, and commercial activities; and the uncertaintie s o f international trade policies, including tariffs, sanctions, trade barriers and most favored nation drug pricing and the potential impact they may have on our business, financial condition, and results of ope rations. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward - looking statements is contained in the "Risk Fac tors" section of the Company's Annual Report on Form 10 - K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements i nvo lve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, ac hievements or prospects expressed in or implied by such forward - looking statements. The Company cautions investors not to place undue reliance on the forward - looking statements contained in this docum ent. Forward - looking statements are based on our management’s beliefs and assumptions and on information currently available to our m anagement. No assurance can be given that such future results will be achieved. Such forward - looking statements contained in this presentation speak only as of the date of this presentation. The Com pany expressly disclaim any obligation or undertaking to update these forward - looking statements contained in this presentation to reflect any change in our expectations or any change in events, con ditions, or circumstances on which such statements are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of a ny such forward - looking statements. The revenue and cash and cash equivalents figures are preliminary and unaudited and reflect the Company’s estimated financial re sults. In preparing this information, management made a number of complex and subjective judgments and estimates about the appropriateness of certain reported amounts and disclosures. The Com pan y’s actual financial results for the quarter and year ended December 31, 2025 have not yet been finalized by management or audited or reviewed by the Company’s independent auditors. The prelimin ary financial information is not a comprehensive statement of all financial results for the quarter or year ended December 31, 2025. Subsequent information or events may lead to material differences be twe en the foregoing preliminary financial results and those reported in the Company’s subsequent SEC filings. Accordingly, investors should not place undue reliance on these preliminary financial resul ts. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data d eri ved from third - party sources and our own internal estimates and research. While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verifie d, and we make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this present ation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, although we believe our own internal research is re lia ble, such research has not been verified by any independent source.
3 ADC Therapeutics: A Pioneer in ADCs with Significant Growth Opportunity from ZYNLONTA Corporate Capability Strategy P ioneer in the field of ADCs with specialized capabilities across development, technical operations, and commercialization Pursuing expansion of FDA - approved ZYNLONTA® to become backbone for powerful combination therapies in earlier lines of DLBCL as well as indolent lymphomas Delivering on our strategy supported by an accomplished and multidisciplinary team with an expected cash runway at least to 2028
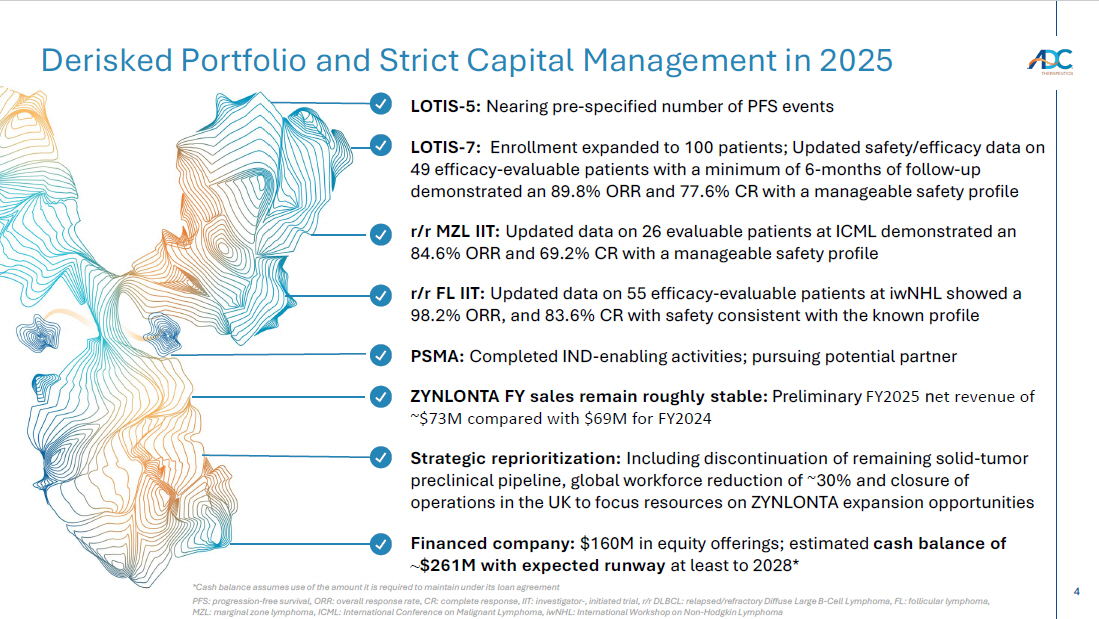
4 Derisked Portfolio and Strict Capital Management in 2025 *Cash balance assumes use of the amount it is required to maintain under its loan agreement PFS: progression - free survival, ORR: overall response rate, CR: complete response, IIT: investigator - , initiated trial, r/r DLBC L: relapsed/refractory Diffuse Large B - Cell Lymphoma, FL: follicular lymphoma, MZL: marginal zone lymphoma, ICML: International Conference on Malignant Lymphoma, iwNHL : International Workshop on Non - Hodgkin Lymphoma LOTIS - 5: Nearing pre - specified number of PFS events LOTIS - 7: Enrollment expanded to 100 patients; Updated safety/efficacy data on 49 efficacy - evaluable patients with a minimum of 6 - months of follow - up demonstrated an 89.8% ORR and 77.6% CR with a manageable safety profile r/r MZL IIT: Updated data on 26 evaluable patients at ICML demonstrated an 84.6% ORR and 69.2% CR with a manageable safety profile r/r FL IIT: Updated data on 55 efficacy - evaluable patients at iwNHL showed a 98.2% ORR, and 83.6% CR with safety consistent with the known profile PSMA: Completed IND - enabling activities; pursuing potential partner ZYNLONTA FY sales remain roughly stable: Preliminary FY2025 n et revenue of ~$73M compared with $69M for FY2024 Strategic reprioritization: Including discontinuation of remaining solid - tumor preclinical pipeline, global workforce reduction of ~ 30% and closure of operations in the UK to focus resources on ZYNLONTA expansion opportunities Financed company: $160M in equity offerings; estimated cash balance of ~ $261M with expected runway at least to 2028*
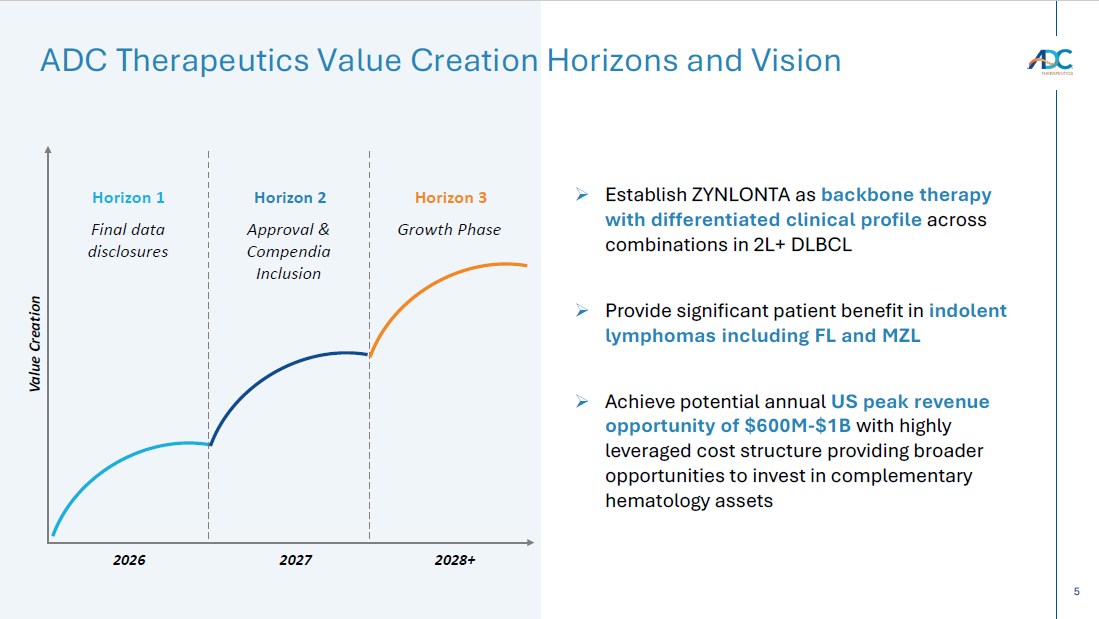
5 ADC Therapeutics Value Creation Horizons and Vision Value Creation Final data disclosures Horizon 2 Approval & Compendia Inclusion Horizon 3 Growth Phase Horizon 1 2026 2027 2028+ » Establish ZYNLONTA as backbone therapy with differentiated clinical profile across combinations in 2L+ DLBCL » Provide significant patient benefit in indolent lymphomas including FL and MZL » Achieve potential annual US peak revenue opportunity of $600M - $1B with highly leveraged cost structure providing broader opportunities to invest in complementary hematology assets

6 CORPORATE Please visit ZYNLONTA.com for complete prescribing information including indication, warnings and precautions. → ZYNLONTA is a CD19 - directed ADC indicated as monotherapy for the treatment of adult patients with relapsed or refractory large B - cell lymphoma after two or more lines of systemic therapy → Rapid, deep, and durable efficacy – Median time to CR 1.5 months – 48.3% ORR and 24.8% CR – Median duration of response not yet reached for patients in CR at 2 - year follow - up → Manageable safety profile – No CRS or ICANS and no cumulative irreversible toxicities → Accessibility – Simple Q3W dosing with no REMS or inpatient stay requirements ZYNLONTA is Ideally Suited Across Care Settings for Patients with r/r DLBCL
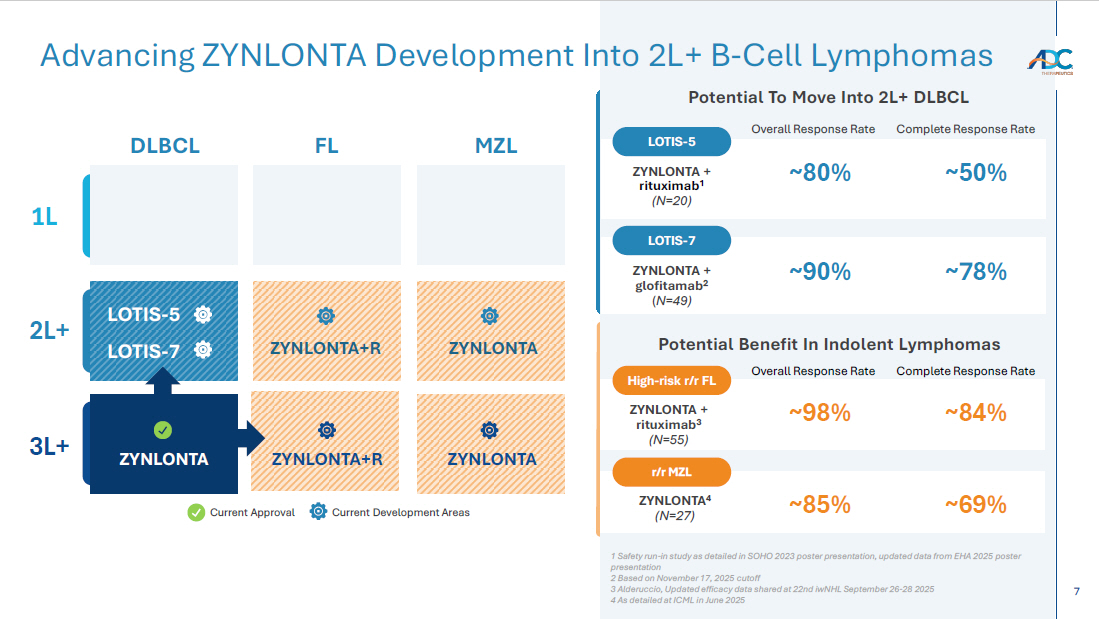
7 Advancing ZYNLONTA Development Into 2L+ B - Cell Lymphomas Current Approval Current Development Areas DLBCL FL MZL 1L 2L+ 3L+ ZYNLONTA+R ZYNLONTA ZYNLONTA+R ZYNLONTA ZYNLONTA Potential Benefit In Indolent Lymphomas LOTIS - 5 LOTIS - 7 Potential To Move Into 2L+ DLBCL ~50% ~80% ZYNLONTA + rituximab 1 (N=20) LOTIS - 5 Overall Response Rate Complete Response Rate ZYNLONTA + rituximab 3 (N= 55 ) High - risk r/r FL Overall Response Rate Complete Response Rate ~84% ~98% ZYNLONTA + glofitamab 2 ( N=49) LOTIS - 7 ~ 78 % ~90 % ZYNLONTA 4 (N=27) r/r MZL ~ 69 % ~ 85 % 1 Safety run - in study as detailed in SOHO 2023 poster presentation, updated data from EHA 2025 poster presentation 2 Based on November 17, 2025 cutoff 3 Alderuccio, Updated efficacy data shared at 22nd iwNHL September 26 - 28 2025 4 As detailed at ICML in June 2025
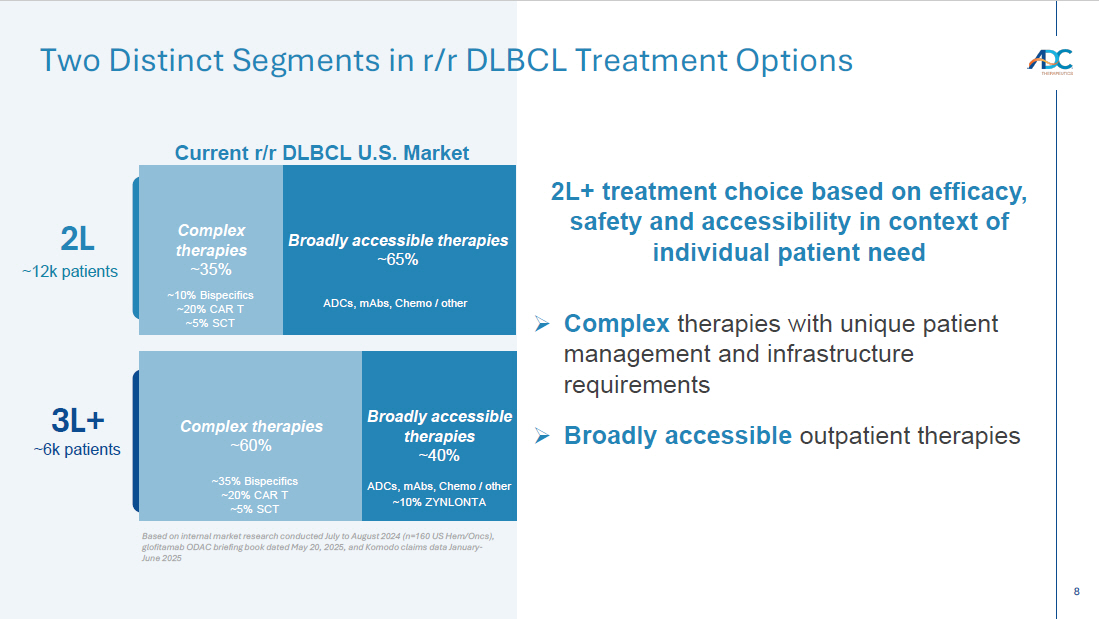
8 Broadly accessible therapies ~65% 2L 3L+ Complex therapies ~60% ~12k patients ~6k patients Two Distinct Segments in r/r DLBCL Treatment Options Complex therapies ~35% 2L+ treatment choice based on efficacy, safety and accessibility in context of individual patient need » Complex therapies with unique patient management and infrastructure requirements » Broadly accessible outpatient therapies Broadly accessible therapies ~40% ~10% Bispecifics ~20% CAR T ~ 5 % SCT ~ 35% Bispecifics ~20% CAR T ~5% SCT Current r/r DLBCL U.S. Market ~10% ZYNLONTA ADCs, mAbs , Chemo / other ADCs, mAbs , Chemo / other Based on internal market research conducted July to August 2024 (n=160 US Hem/ Oncs ), glofitamab ODAC briefing book dated May 20, 2025, and Komodo claims data January - June 2025
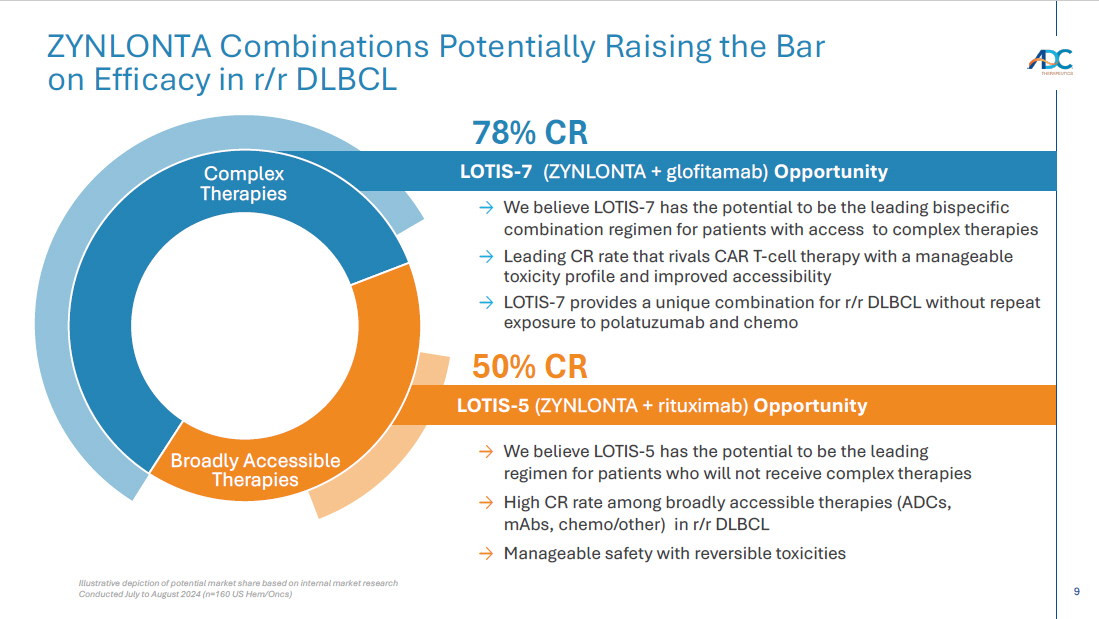
9 LOTIS - 7 (ZYNLONTA + glofitamab) Opportunity LOTIS - 5 (ZYNLONTA + rituximab) Opportunity Illustrative depiction of potential market share based on internal market research Conducted July to August 2024 (n=160 US Hem/ Oncs ) ZYNLONTA Combinations Potentially Raising the Bar on Efficacy in r/r DLBCL → We believe LOTIS - 7 has the potential to be the leading bispecific combination regimen for patients with access to complex therapies → Leading CR rate that rivals CAR T - cell therapy with a manageable toxicity profile and improved accessibility → L OTIS - 7 provides a unique combination for r/r DLBCL without repeat exposure to polatuzumab and chemo → We believe LOTIS - 5 has the potential to be the leading regimen for patients who will not receive complex therapies → High CR rate among broadly accessible therapies (ADCs, mAbs , chemo/other) in r/r DLBCL → Manageable safety with reversible toxicities Complex Therapies 50% CR Broadly Accessible Therapies 78 % CR
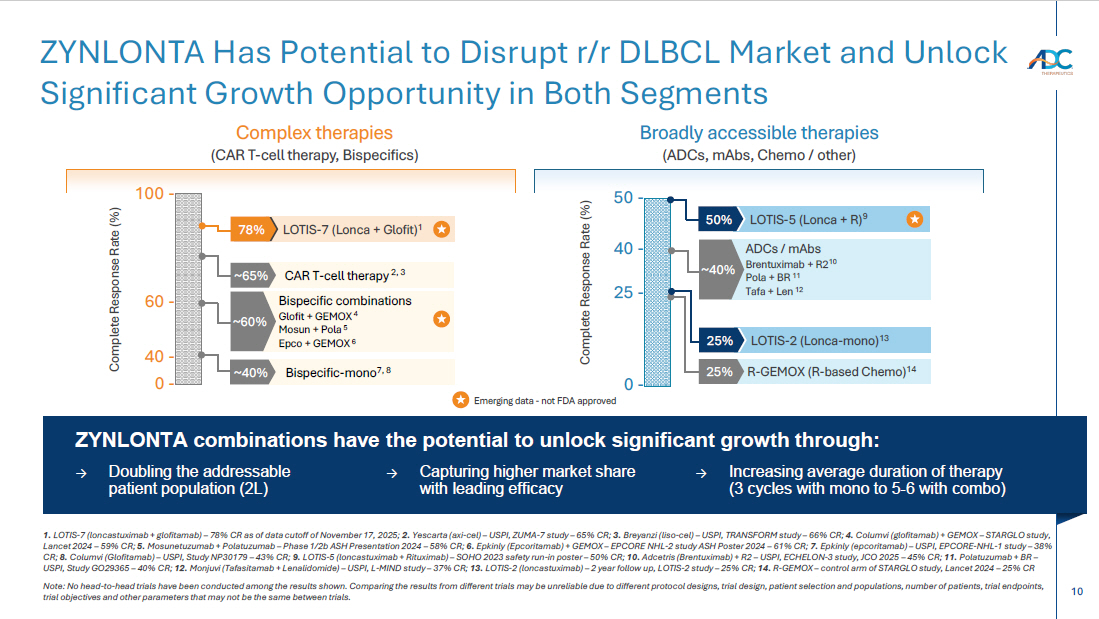
10 1. LOTIS - 7 (loncastuximab + glofitamab) – 78% CR as of data cutoff of November 17, 2025; 2. Yescarta (axi - cel) – USPI, ZUMA - 7 study – 65% CR; 3. Breyanzi ( liso - cel) – USPI, TRANSFORM study – 66% CR; 4. Columvi ( g lofitamab ) + GEMOX – STARGLO study, Lancet 2024 – 59% CR; 5. Mosunetuzumab + Polatuzumab – Phase 1/2b ASH Presentation 2024 – 58% CR; 6. Epkinly ( Epcoritamab ) + GEMOX – EPCORE NHL - 2 study ASH Poster 2024 – 61% CR; 7. Epkinly ( epcoritamab ) – USPI, EPCORE - NHL - 1 study – 38% CR; 8. Columvi ( Glofitamab ) – USPI, Study NP30179 – 43% CR; 9. LOTIS - 5 (loncastuximab + Rituximab) – SOHO 2023 safety run - in poster – 50% CR; 10. Adcetris (Brentuximab) + R2 – USPI, ECHELON - 3 study, JCO 2025 – 45% CR; 11. Polatuzumab + BR – USPI, Study GO29365 – 40% CR; 12. Monjuvi ( Tafasitamab + Lenalidomide) – USPI, L - MIND study – 37% CR; 13. LOTIS - 2 ( l oncastuximab ) – 2 year follow up, LOTIS - 2 study – 25% CR; 14. R - GEMOX – control arm of STARGLO study, Lancet 2024 – 25% CR Note: No head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unr eliable due to different protocol designs, trial design, patient selection and populations, number of patients, trial endpoin ts, trial objectives and other parameters that may not be the same between trials. ZYNLONTA Has Potential to Disrupt r/r DLBCL Market and Unlock Significant Growth Opportunity in Both Segments Emerging data - not FDA approved 100 - 0 - CAR T - cell therapy 2, 3 ~65% Bispecific combinations Glofit + GEMOX 4 Mosun + Pola 5 Epco + GEMOX 6 ~60% LOTIS - 7 (Lonca + Glofit) 1 78% Bispecific - mono 7, 8 ~40% Complete Response Rate (%) 40 - 60 - ZYNLONTA combinations have the potential to unlock significant growth through: → Doubling the addressable patient population (2L) → Capturing higher market share with leading efficacy → Increasing average duration of therapy (3 cycles with mono to 5 - 6 with combo) 50 - 0 - LOTIS - 5 (Lonca + R) 9 50% ADCs / mAbs Brentuximab + R2 10 Pola + BR 11 Tafa + Len 12 ~40% LOTIS - 2 ( Lonca - mono) 13 25% R - GEMOX (R - based Chemo) 14 25% Complete Response Rate (%) 25 - 40 - Complex therapies (CAR T - cell therapy, Bispecifics ) Broadly accessible therapies (ADCs, mAbs , Chemo / other)
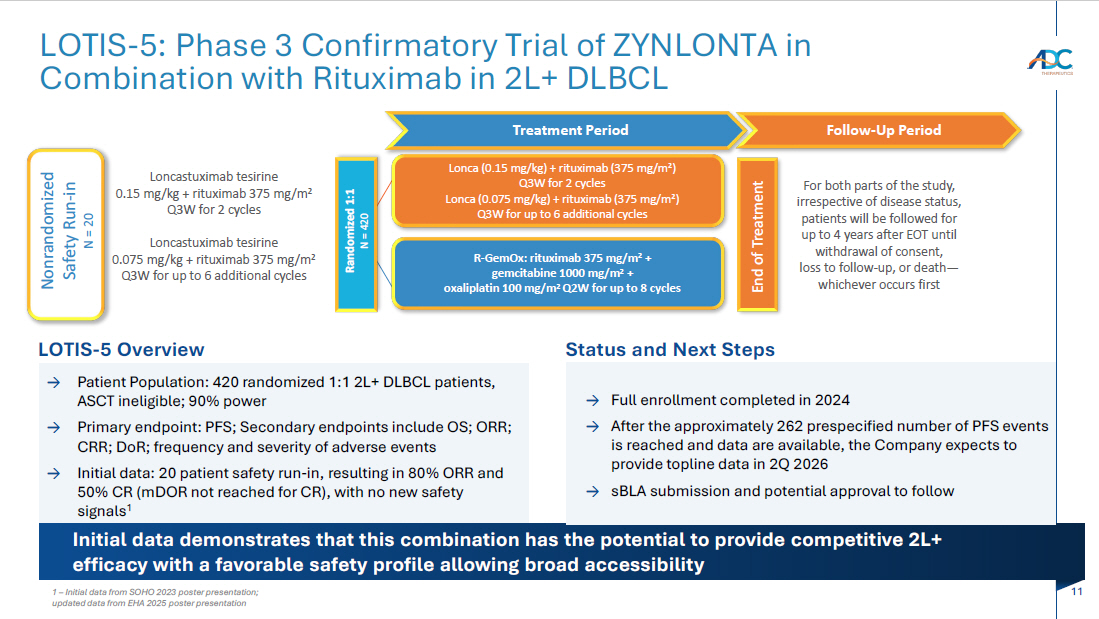
11 LOTIS - 5: Phase 3 Confirmatory Trial of ZYNLONTA in Combination with Rituximab in 2L+ DLBCL Initial data demonstrates that this combination has the potential to provide competitive 2L+ efficacy with a favorable safety profile allowing broad accessibility Status and Next Steps → Full enrollment completed in 2024 → A fter the approximately 262 prespecified number of PFS events is reached and data are available, the Company expects to provide topline data in 2Q 2026 → sBLA submission and potential approval to follow LOTIS - 5 Overview → Patient Population: 420 randomized 1:1 2L+ DLBCL patients, ASCT ineligible; 90% power → Primary endpoint: PFS; Secondary endpoints include OS; ORR; CRR; DoR ; frequency and severity of adverse events → Initial data: 20 patient safety run - in, resulting in 80% ORR and 50% CR ( mDOR not reached for CR), with no new safety signals 1 Treatment Period Follow - Up Period End of Treatment Randomized 1:1 N = 420 Loncastuximab tesirine 0.15 mg/kg + rituximab 375 mg/m 2 Q3W for 2 cycles Loncastuximab tesirine 0.075 mg/kg + rituximab 375 mg/m 2 Q3W for up to 6 additional cycles Lonca (0.15 mg/kg) + rituximab (375 mg/m 2 ) Q3W for 2 cycles Lonca (0.075 mg/kg) + rituximab (375 mg/m 2 ) Q3W for up to 6 additional cycles R - GemOx : rituximab 375 mg/m 2 + gemcitabine 1000 mg/m 2 + oxaliplatin 100 mg/m 2 Q2W for up to 8 cycles For both parts of the study, irrespective of disease status, patients will be followed for up to 4 years after EOT until withdrawal of consent, loss to follow - up, or death — whichever occurs first Nonrandomized Safety Run - in N = 20 1 – Initial data from SOHO 2023 poster presentation; updated data from EHA 2025 poster presentation
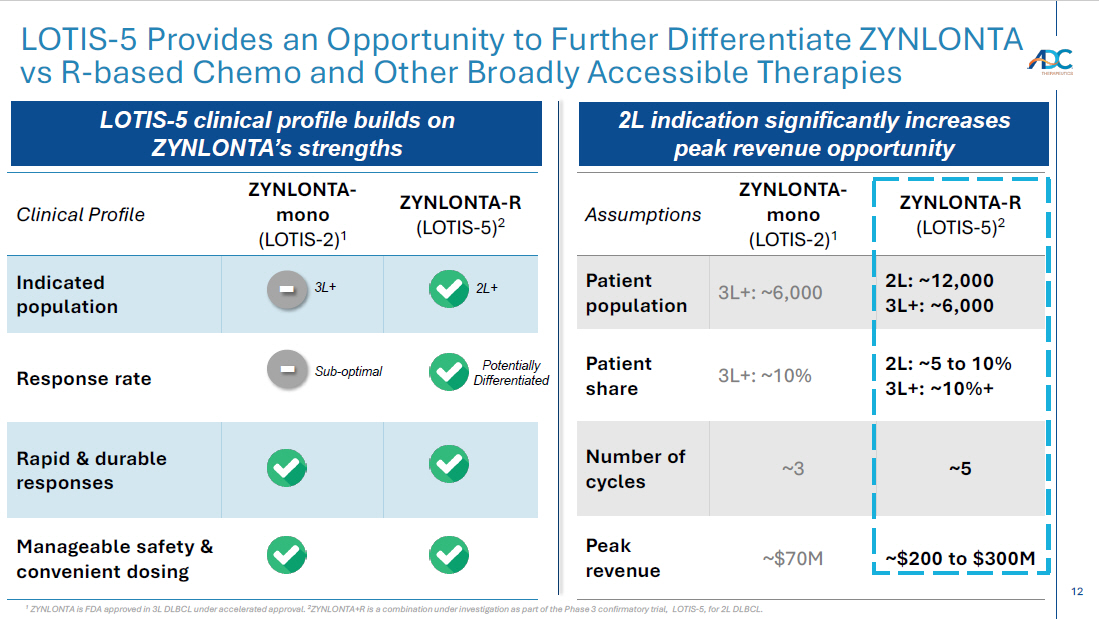
12 LOTIS - 5 Provides an Opportunity to Further Differentiate ZYNLONTA vs R - based Chemo and Other Broadly Accessible Therapies ZYNLONTA - R (LOTIS - 5) 2 ZYNLONTA - mono (LOTIS - 2) 1 Clinical Profile Indicated population Response rate Rapid & durable responses Manageable safety & convenient dosing ZYNLONTA - R (LOTIS - 5) 2 ZYNLONTA - mono (LOTIS - 2) 1 Assumptions 2L: ~12,000 3L+: ~6,000 3L+: ~6,000 Patient population 2L: ~5 to 10% 3L+: ~10%+ 3L+: ~10% Patient share ~5 ~3 Number of cycles ~$200 to $300M ~$70M Peak revenue 2L+ Potentially Differentiated 3L+ - Sub - optimal - LOTIS - 5 clinical profile builds on ZYNLONTA’s strengths 2L indication significantly increases peak revenue opportunity 1 ZYNLONTA is FDA approved in 3L DLBCL under accelerated approval. 2 ZYNLONTA+R is a combination under investigation as part of the Phase 3 confirmatory trial, LOTIS - 5, for 2L DLBCL.
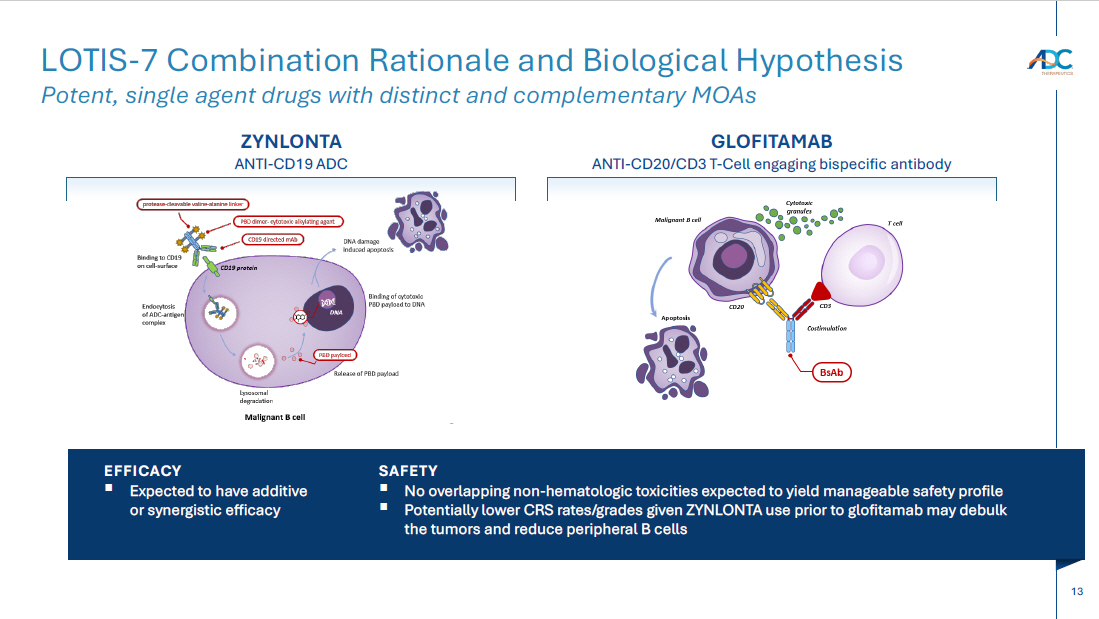
13 LOTIS - 7 Combination Rationale and Biological Hypothesis Potent, single agent drugs with distinct and complementary MOAs ZYNLONTA ANTI - CD19 ADC GLOFITAMAB ANTI - CD20/CD3 T - Cell engaging bispecific antibody EFFICACY ▪ Expected to have additive or synergistic efficacy SAFETY ▪ No overlapping non - hematologic toxicities expected to yield manageable safety profile ▪ Potentially lower CRS rates/grades given ZYNLONTA use prior to glofitamab may debulk the tumors and reduce peripheral B cells
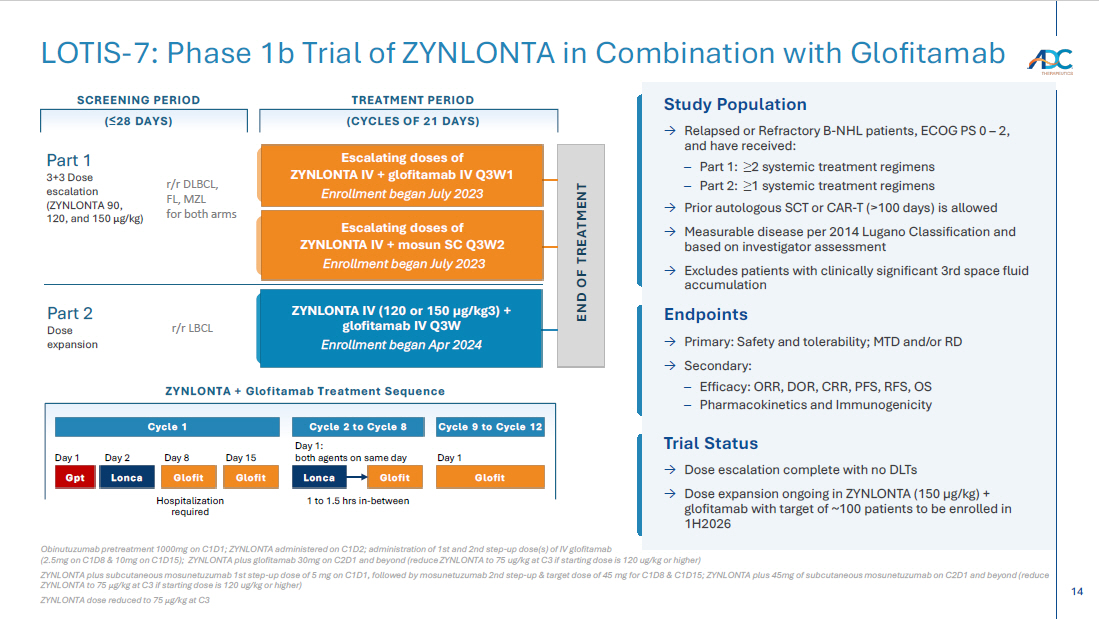
14 END OF TREATMENT Study Population → Relapsed or Refractory B - NHL patients, ECOG PS 0 – 2, and have received: – Part 1: ≥ 2 systemic treatment regimens – Part 2: ≥ 1 systemic treatment regimens → Prior autologous SCT or CAR - T (>100 days) is allowed → Measurable disease per 2014 Lugano Classification and based on investigator assessment → Excludes patients with clinically significant 3rd space fluid accumulation ZYNLONTA IV (120 or 150 µg/kg3) + glofitamab IV Q3W Enrollment began Apr 2024 Escalating doses of ZYNLONTA IV + mosun SC Q3W2 Enrollment began July 2023 Escalating doses of ZYNLONTA IV + glofitamab IV Q3W1 Enrollment began July 2023 SCREENING PERIOD ( ≤ 28 DAYS) TREATMENT PERIOD (CYCLES OF 21 DAYS) r/r DLBCL, FL, MZL for both arms r/r LBCL Part 1 3+3 Dose escalation (ZYNLONTA 90, 120, and 150 μ g /kg) Part 2 Dose expansion Endpoints → Primary: Safety and tolerability; MTD and/or RD → Secondary: – Efficacy: ORR, DOR, CRR, PFS, RFS, OS – Pharmacokinetics and Immunogenicity Trial Status → Dose escalation complete with no DLTs → Dose expansion ongoing in ZYNLONTA (150 µg/kg) + glofitamab with target of ~100 patients to be enrolled in 1H2026 ZYNLONTA + Glofitamab Treatment Sequence Cycle 1 Cycle 2 to Cycle 8 Cycle 9 to Cycle 12 Day 1 Day 2 Day 8 Day 15 Day 1: both agents on same day Day 1 Hospitalization required 1 to 1.5 hrs in - between Glofit Glofit Lonca Glofit Glofit Lonca Gpt Obinutuzumab pretreatment 1000mg on C1D1; ZYNLONTA administered on C1D2; administration of 1st and 2nd step - up dose(s) of IV glo fitamab (2.5mg on C1D8 & 10mg on C1D15); ZYNLONTA plus glofitamab 30mg on C2D1 and beyond (reduce ZYNLONTA to 75 ug/kg at C3 if startin g dose is 120 ug/kg or higher) ZYNLONTA plus subcutaneous mosunetuzumab 1st step - up dose of 5 mg on C1D1, followed by mosunetuzumab 2nd step - up & target dose o f 45 mg for C1D8 & C1D15; ZYNLONTA plus 45mg of subcutaneous mosunetuzumab on C2D1 and beyond (reduce ZYNLONTA to 75 μ g /kg at C3 if starting dose is 120 ug/kg or higher) ZYNLONTA dose reduced to 75 μ g /kg at C3 LOTIS - 7: Phase 1b Trial of ZYNLONTA in Combination with Glofitamab
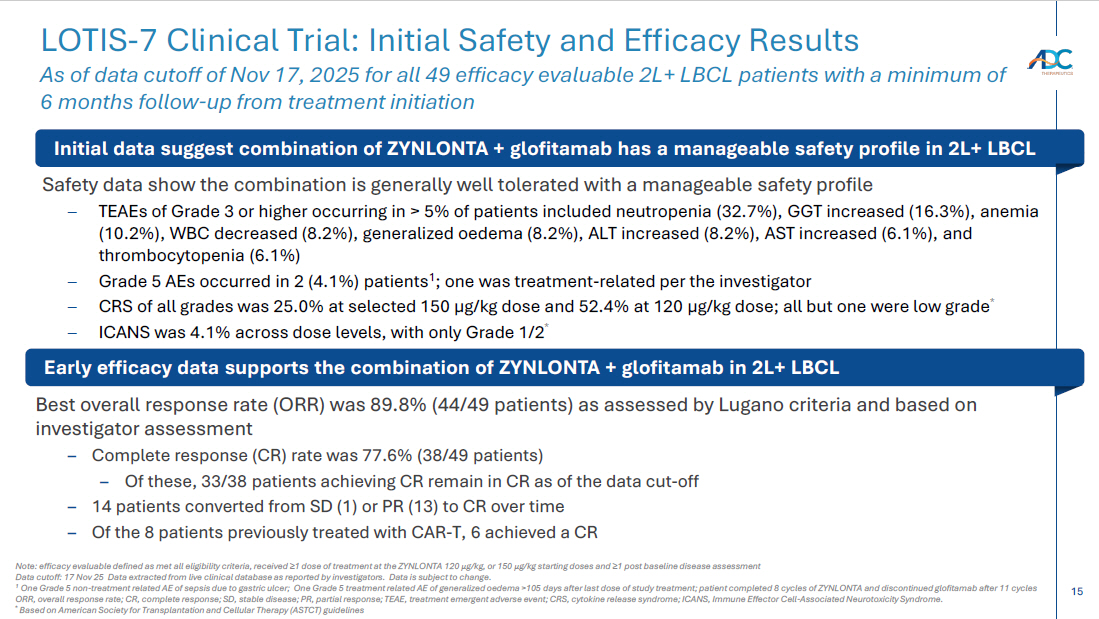
15 Safety data show the combination is generally well tolerated with a manageable safety profile – TEAEs of Grade 3 or higher occurring in > 5% of patients included neutropenia (32.7%), GGT increased (16.3%), anemia (10.2%), WBC decreased (8.2%), generalized oedema (8.2%), ALT increased (8.2%), AST increased (6.1%), and thrombocytopenia (6.1%) – Grade 5 AEs occurred in 2 (4.1%) patients 1 ; one was treatment - related per the investigator – CRS of all grades was 25.0% at selected 150 µg/kg dose and 52.4% at 120 µg/kg dose; all but one were low grade * – ICANS was 4.1% across dose levels, with only Grade 1/2 * Best overall response rate (ORR) was 89.8% (44/49 patients) as assessed by Lugano criteria and based on investigator assessment – Complete response (CR) rate was 77.6% (38/49 patients) – Of these, 33/38 patients achieving CR remain in CR as of the data cut - off – 14 patients converted from SD (1) or PR (13) to CR over time – Of the 8 patients previously treated with CAR - T, 6 achieved a CR LOTIS - 7 Clinical Trial: Initial Safety and Efficacy Results As of data cutoff of Nov 17, 2025 for all 49 efficacy evaluable 2L+ LBCL patients with a minimum of 6 months follow - up from treatment initiation Note: efficacy evaluable defined as met all eligibility criteria, received ≥ 1 dose of treatment at the ZYNLONTA 120 μ g /kg, or 150 μ g /kg starting doses and ≥ 1 post baseline disease assessment Data cutoff: 17 Nov 25 Data extracted from live clinical database as reported by investigators. Data is subject to change. 1 One Grade 5 non - treatment related AE of sepsis due to gastric ulcer; One Grade 5 treatment related AE of generalized oedema >105 da ys after last dose of study treatment; patient completed 8 cycles of ZYNLONTA and discontinued glofitamab after 11 cycles ORR, overall response rate; CR, complete response; SD, stable disease; PR, partial response; TEAE, treatment emergent adverse ev ent; CRS, cytokine release syndrome; ICANS, Immune Effector Cell - Associated Neurotoxicity Syndrome. * Based on American Society for Transplantation and Cellular Therapy (ASTCT) guidelines Initial data suggest combination of ZYNLONTA + glofitamab has a manageable safety profile in 2L+ LBCL Early efficacy data supports the combination of ZYNLONTA + glofitamab in 2L+ LBCL
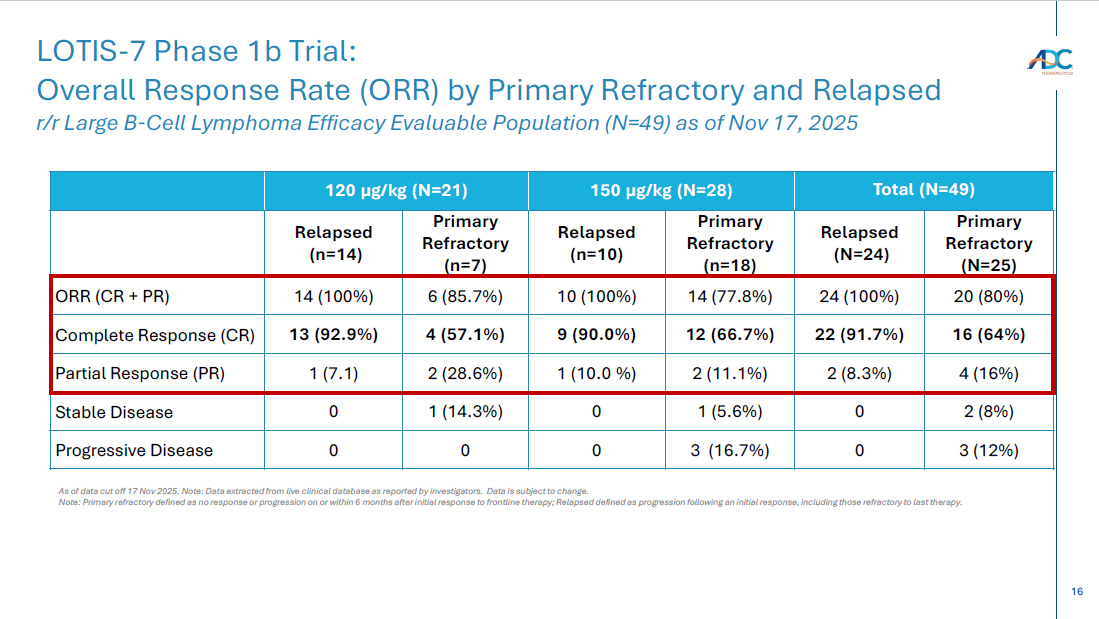
16 Total (N=49) 150 µg/kg (N=28) 120 µg/kg (N=21) Primary Refractory (N=25) Relapsed (N=24) Primary Refractory (n=18) Relapsed (n=10) Primary Refractory (n=7) Relapsed (n=14) 20 (80%) 24 (100%) 14 (77.8%) 10 (100%) 6 (85.7%) 14 (100%) ORR (CR + PR) 16 (64%) 22 (91.7%) 12 (66.7%) 9 (90.0%) 4 (57.1%) 13 (92.9%) Complete Response (CR) 4 (16%) 2 (8.3%) 2 (11.1%) 1 (10.0 %) 2 (28.6%) 1 (7.1) Partial Response (PR) 2 (8%) 0 1 (5.6%) 0 1 (14.3%) 0 Stable Disease 3 (12%) 0 3 (16.7%) 0 0 0 Progressive Disease LOTIS - 7 Phase 1b Trial: Overall Response Rate (ORR ) by Primary Refractory and Relapsed r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of Nov 17, 2025 As of data cut off 17 Nov 2025. Note: Data extracted from live clinical database as reported by investigators. Data is subject to change. Note: Primary refractory defined as no response or progression on or within 6 months after initial response to frontline ther apy ; Relapsed defined as progression following an initial response, including those refractory to last therapy.
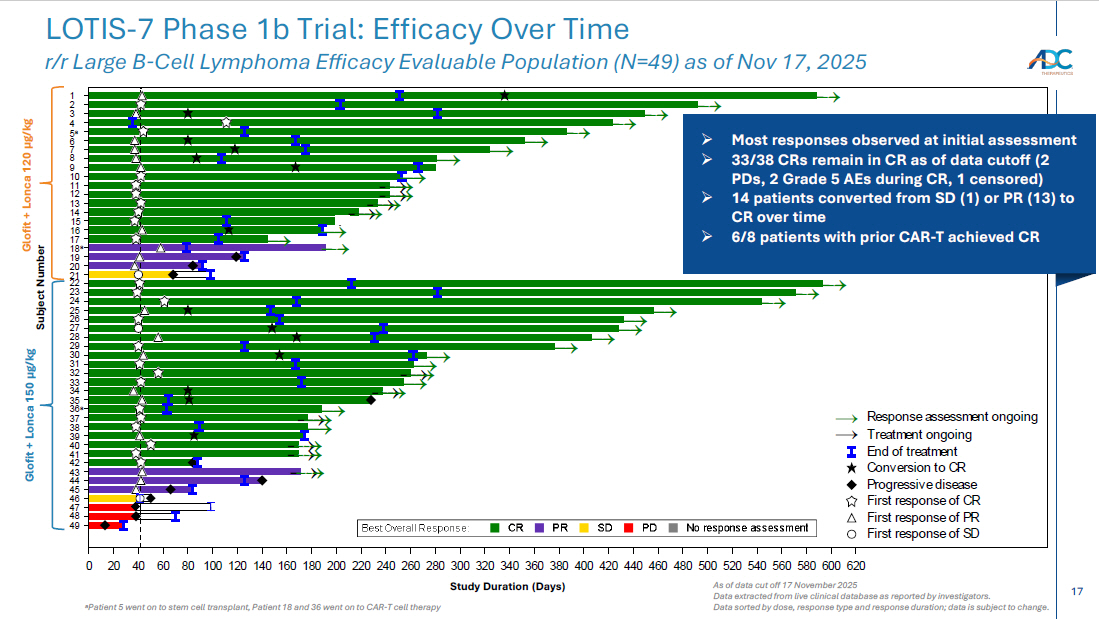
17 a Patient 5 went on to stem cell transplant, Patient 18 and 36 went on to CAR - T cell therapy As of data cut off 17 November 2025 Data extracted from live clinical database as reported by investigators . Data sorted by dose, response type and response duration; data is subject to change. LOTIS - 7 Phase 1b Trial: Efficacy Over Time r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of Nov 17 , 2025 → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → 0 20 40 60 80 100120140160180200220240260280300320340360380400420440460480500520540560580600620 Study Duration (Days) 197-702 196-701 125-701 207-701 203-702 203-709 405-705 404-702 401-703 405-706 109-702 121-716 203-708 203-704 335-703 177-704 332-703 405-703 121-713 500-707 500-706 404-704 332-702 121-707 121-708 121-704 371-701* (150 ug/kg) 121-702* 415-703 203-705 197-701 203-701 195-702 383-704 121-712 203-706 405-704 121-714 203-703 198-701 404-703 107-705 121-709 121-710 177-703 121-706 164-704 335-702 (120 ug/kg) 184-705* S u b j e c t First response of SD First response of PR First response of CR Progressive disease Conversion to CR End of treatment Treatment ongoing → Response assessment ongoing → No response assessmentPDSDPRCRBest Overall Response: First scheduled assessment at 6 weeks → → 25 22 21 20 23 6 Subject Number Glofit + Lonca 120 µg/kg Glofit + Lonca 150 µg/kg 26 27 28 29 30 31 32 33 34 35 36 a 37 5 a 7 8 9 10 11 12 13 14 15 1 24 49 Study Duration (Days) 2 3 4 16 17 18 a 19 38 39 40 41 42 43 44 45 46 47 48 » Most responses observed at initial assessment » 33/38 CRs remain in CR as of data cutoff (2 PDs, 2 Grade 5 AEs during CR, 1 censored) » 14 patients converted from SD (1) or PR (13) to CR over time » 6/8 patients with prior CAR - T achieved CR
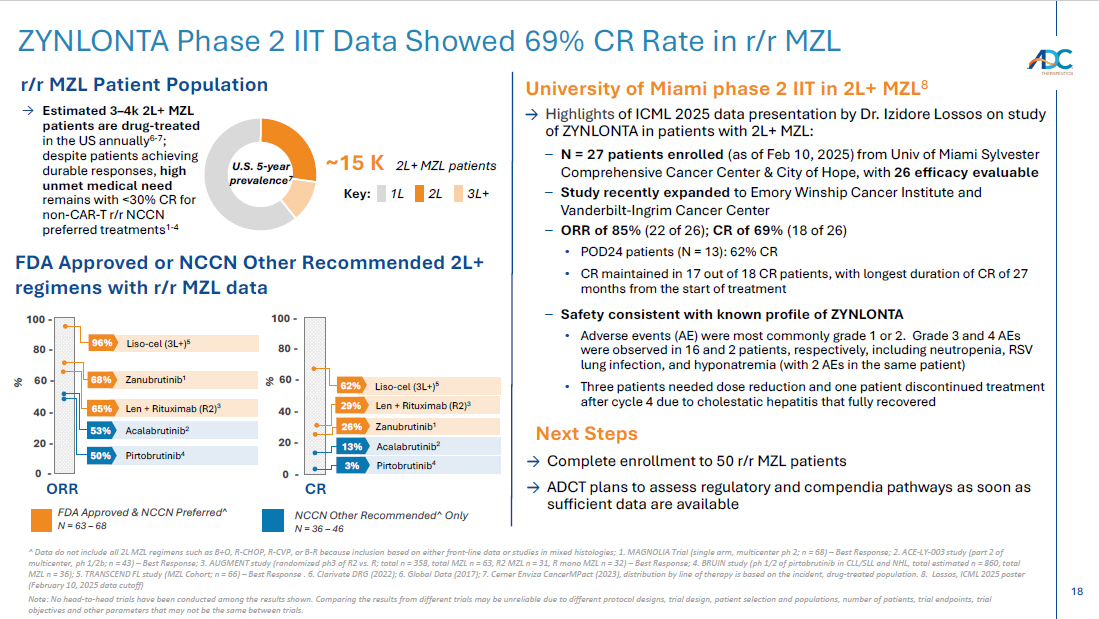
18 ^ Data do not include all 2L MZL regimens such as B+O, R - CHOP, R - CVP, or B - R because inclusion based on either front - line data o r studies in mixed histologies ; 1. MAGNOLIA Trial (single arm, multicenter ph 2; n = 68) – Best Response; 2. ACE - LY - 003 study (part 2 of multicenter, ph 1/2b; n = 43) – Best Response; 3. AUGMENT study (randomized ph3 of R2 vs. R; total n = 358, total MZL n = 63, R2 MZL n = 31, R mono MZL n = 32) – Best Response; 4. BRUIN study ( ph 1/2 of pirtobrutinib in CLL/SLL and NHL, total estimated n = 860, total MZL n = 36) ; 5. TRANSCEND FL study (MZL Cohort; n = 66) – Best Response . 6. Clarivate DRG (2022); 6. Global Data (2017); 7. Cerner Enviza CancerMPact (2023), distribution by line of therapy is based on the incident, drug - treated population. 8. Lossos, ICML 2025 poster (February 10, 2025 data cutoff) Note: No head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unr eliable due to different protocol designs, trial design, patient selection and populations, number of patients, trial endpoin ts, trial objectives and other parameters that may not be the same between trials. ZYNLONTA Phase 2 IIT Data Showed 69 % CR Rate in r/r MZL Zanubrutinib 1 68% % Len + Rituximab (R2) 3 65% Acalabrutinib 2 53% Pirtobrutinib 4 50% Acalabrutinib 2 13% Pirtobrutinib 4 3% 0 - 80 - % 60 - 40 - 20 - Len + Rituximab (R2) 3 29% Zanubrutinib 1 → Highlights of ICML 2025 data presentation by Dr. Izidore Lossos on study of ZYNLONTA in patients with 2L+ MZL: – N = 27 patients enrolled (as of Feb 10, 2025) from Univ of Miami Sylvester Comprehensive Cancer Center & City of Hope, with 26 efficacy evaluable – Study recently expanded to Emory Winship Cancer Institute and Vanderbilt - Ingrim Cancer Center – ORR of 85% (22 of 26); CR of 69% (18 of 26) • POD24 patients (N = 13): 62% CR • CR maintained in 17 out of 18 CR patients, with longest duration of CR of 27 months from the start of treatment – Safety consistent with known profile of ZYNLONTA • Adverse events (AE) were most commonly grade 1 or 2. Grade 3 and 4 AEs were observed in 16 and 2 patients, respectively, including neutropenia, RSV lung infection, and hyponatremia (with 2 AEs in the same patient) • Three patients needed dose reduction and one patient discontinued treatment after cycle 4 due to cholestatic hepatitis that fully recovered ~15 K 2L+ MZL patients U.S. 5 - year prevalence 7 → Estimated 3 – 4k 2L+ MZL patients are drug - treated in the US annually 6 - 7 ; despite patients achieving durable responses, high unmet medical need remains with <30% CR for non - CAR - T r/r NCCN preferred treatments 1 - 4 3L+ Key: 2L 1L Next Steps FDA Approved or NCCN Other Recommended 2L+ regimens with r/r MZL data FDA Approved & NCCN Preferred^ N = 63 – 68 NCCN Other Recommended^ Only N = 36 – 46 r/r MZL Patient Population University of Miami phase 2 IIT in 2L+ MZL 8 → Complete enrollment to 50 r/r MZL patients → ADCT plans to assess regulatory and compendia pathways as soon as sufficient data are available 26% Liso - cel (3L+) 5 96% Liso - cel (3L+) 5 62% 100 - 0 - 80 - 60 - 40 - 20 - 100 - ORR CR
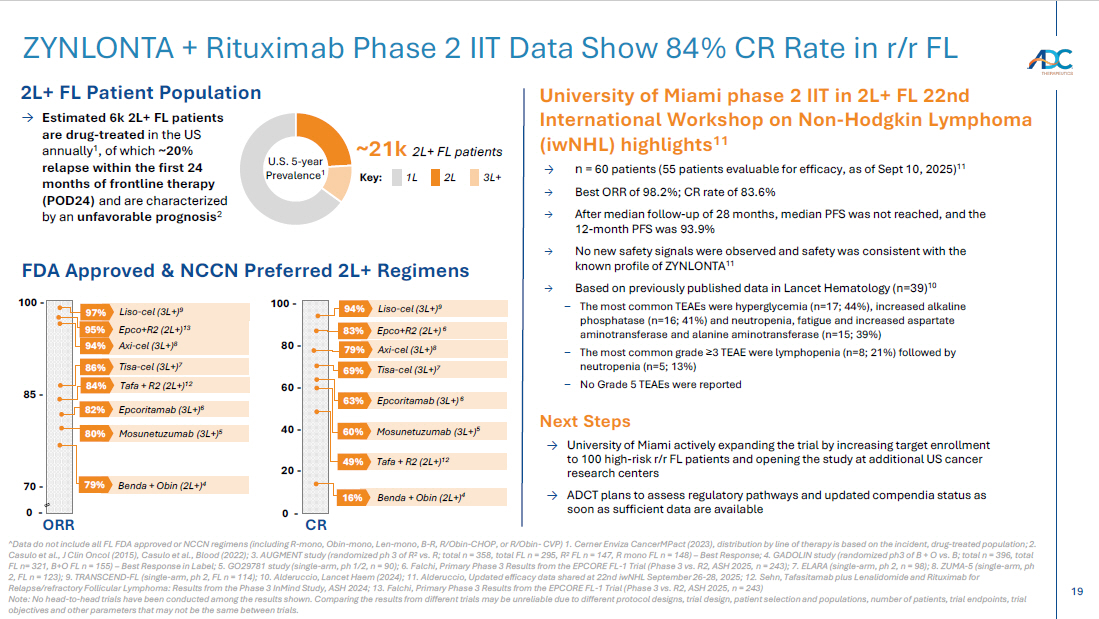
19 ZYNLONTA + Rituximab Phase 2 IIT Data Show 84% CR Rate in r/r FL 2L+ FL Patient Population ~21k 2L+ FL patients U.S. 5 - year Prevalence 1 3L+ Key: 2L 1L → Estimated 6k 2L+ FL patients are drug - treated in the US annually 1 , of which ~20% relapse within the first 24 months of frontline therapy (POD24) and are characterized by an unfavorable prognosis 2 FDA Approved & NCCN Preferred 2L+ Regimens ^Data do not include all FL FDA approved or NCCN regimens (including R - mono, Obin - mono, Len - mono, B - R, R/Obin - CHOP, or R/Obin - C VP) 1. Cerner Enviza CancerMPact (2023), distribution by line of therapy is based on the incident, drug - treated population; 2. Casulo et al., J Clin Oncol (2015), Casulo et al., Blood (2022); 3. AUGMENT study (randomized ph 3 of R 2 vs. R; total n = 358, total FL n = 295, R 2 FL n = 147, R mono FL n = 148) – Best Response; 4. GADOLIN study (randomized ph3 of B + O vs. B; total n = 396, total FL n= 321, B+O FL n = 155) – Best Response in Label; 5. GO29781 study (single - arm, ph 1/2, n = 90); 6. Falchi, Primary Phase 3 Results from the EPCORE FL - 1 Trial (Phase 3 vs. R2, ASH 2025, n = 243); 7. ELARA (sing le - arm, ph 2, n = 98); 8. ZUMA - 5 (single - arm, ph 2, FL n = 123); 9. TRANSCEND - FL (single - arm, ph 2, FL n = 114); 10. Alderuccio, Lancet Haem (2024); 11. Alderuccio, Updated efficacy data shared at 22nd iwNHL September 26 - 28, 2025; 12. Sehn, Tafasitamab plus Lenalidomide and Rituximab for Relapse/refractory Follicular Lymphoma: Results from the Phase 3 InMind Study, ASH 2024; 13. Falchi, Primary Phase 3 Results from the EPCORE FL - 1 Trial (Phase 3 vs. R2, ASH 2025, n = 243) Note: No head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unr eliable due to different protocol designs, trial design, patient selection and populations, number of patients, trial endpoin ts, trial objectives and other parameters that may not be the same between trials. CR Benda + Obin (2L+) 4 16% 80 - 60 - 40 - 20 - 100 - Axi - cel (3L+) 8 79% Liso - cel (3L+) 9 94% Tisa - cel (3L+) 7 69% Epco+R2 (2L+) 6 83% 0 - 85 - ORR 70 - 100 - Axi - cel (3L+) 8 94% Liso - cel (3L+) 9 97% Tisa - cel (3L+) 7 86% Epco+R2 (2L+) 13 95% Mosunetuzumab (3L+) 5 80% Mosunetuzumab (3L+) 5 60% 0 - Tafa + R2 (2L+) 12 84% Tafa + R2 (2L+) 12 49% Benda + Obin (2L+) 4 79% University of Miami phase 2 IIT in 2L+ FL 22nd International Workshop on Non - Hodgkin Lymphoma ( iwNHL ) highlights 11 → University of Miami actively expanding the trial by increasing target enrollment to 100 high - risk r/r FL patients and opening the study at additional US cancer research centers → ADCT plans to assess regulatory pathways and updated compendia status as soon as sufficient data are available Next Steps → n = 60 patients (55 patients evaluable for efficacy, as of Sept 10, 2025) 11 → Best ORR of 98.2%; CR rate of 83.6% → After median follow - up of 28 months, median PFS was not reached, and the 12 - month PFS was 93.9% → No new safety signals were observed and safety was consistent with the known profile of ZYNLONTA 11 → Based on previously published data in Lancet Hematology (n=39) 10 – The most common TEAEs were hyperglycemia (n=17; 44%), increased alkaline phosphatase (n=16; 41%) and neutropenia, fatigue and increased aspartate aminotransferase and alanine aminotransferase (n=15; 39%) – The most common grade ≥ 3 TEAE were lymphopenia (n=8; 21%) followed by neutropenia (n=5; 13%) – No Grade 5 TEAEs were reported Epcoritamab (3L+) 6 82% Epcoritamab (3L+) 6 63%
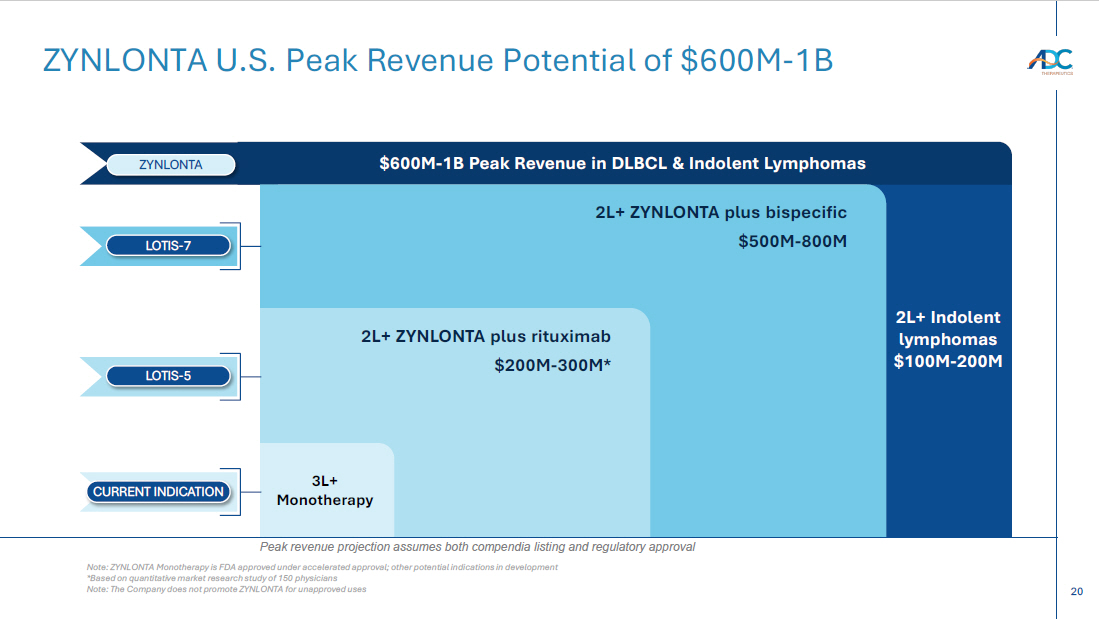
20 2L+ Indolent lymphomas $100M - 200M ZYNLONTA U.S. Peak Revenue Potential of $600M - 1B $600M - 1B Peak Revenue in DLBCL & Indolent Lymphomas ZYNLONTA 2L+ ZYNLONTA plus bispecific $500M - 800M LOTIS - 7 2L+ ZYNLONTA plus rituximab $200M - 300M* LOTIS - 5 3L+ Monotherapy CURRENT INDICATION Note: ZYNLONTA Monotherapy is FDA approved under accelerated approval; other potential indications in development *Based on quantitative market research study of 150 physicians Note: The Company does not promote ZYNLONTA for unapproved uses Peak revenue projection assumes both compendia listing and regulatory approval
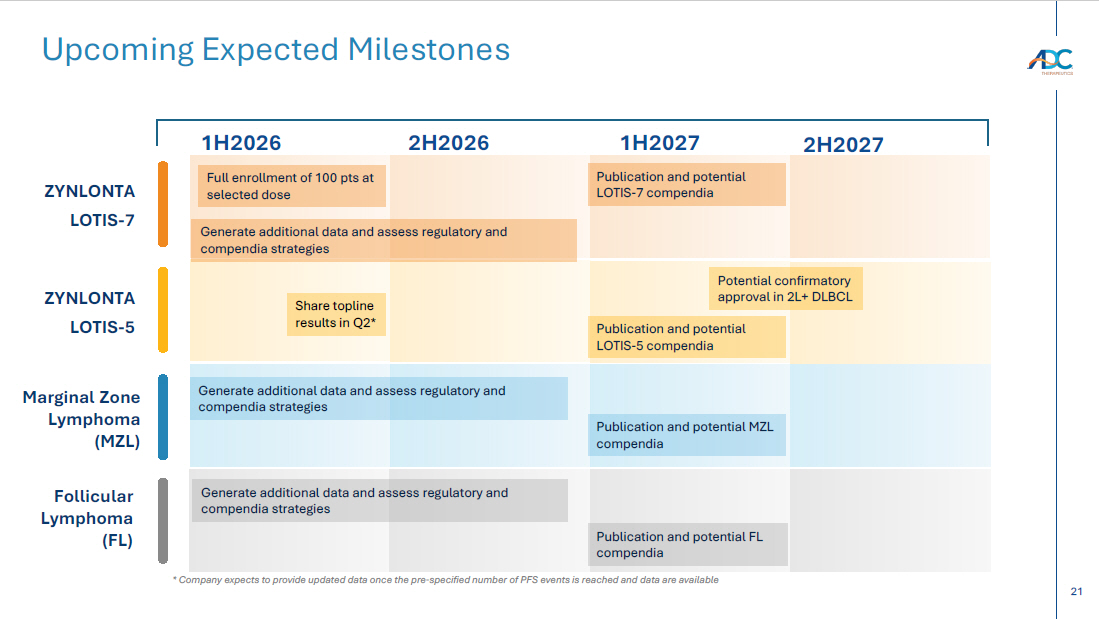
21 Upcoming Expected Milestones 1H2026 ZYNLONTA LOTIS - 7 ZYNLONTA LOTIS - 5 Generate additional data and assess regulatory and compendia strategies Marginal Zone Lymphoma (MZL) Generate additional data and assess regulatory and compendia strategies * Company expects to provide updated data once the pre - specified number of PFS events is reached and data are available 2H2026 1H2027 Publication and potential LOTIS - 7 compendia Potential confirmatory approval in 2L+ DLBCL Publication and potential MZL compendia Full enrollment of 100 pts at selected dose Publication and potential LOTIS - 5 compendia Share topline results in Q2* Publication and potential FL compendia Generate additional data and assess regulatory and compendia strategies Follicular Lymphoma (FL) 2H2027

Thank You
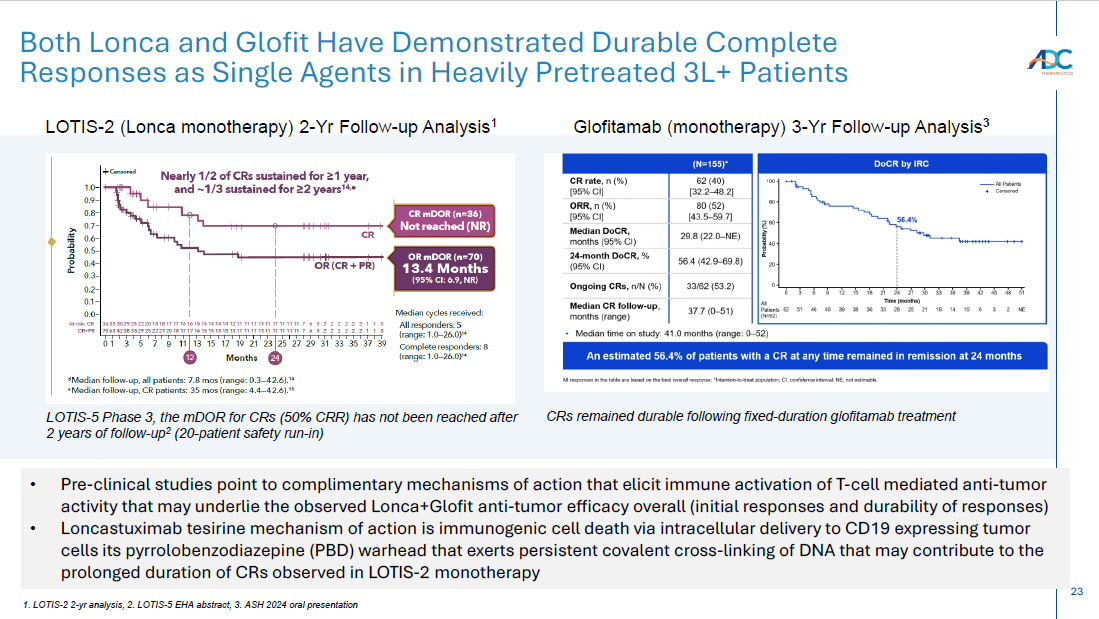
23 Both Lonca and Glofit Have Demonstrated Durable Complete Responses as Single Agents in Heavily Pretreated 3L+ Patients LOTIS - 2 (Lonca monotherapy) 2 - Yr Follow - up Analysis 1 Glofitamab (monotherapy) 3 - Yr Follow - up Analysis 3 1. LOTIS - 2 2 - yr analysis, 2. LOTIS - 5 EHA abstract, 3. ASH 2024 oral presentation • Pre - clinical studies point to complimentary mechanisms of action that elicit immune activation of T - cell mediated anti - tumor activity that may underlie the observed Lonca+Glofit anti - tumor efficacy overall (initial responses and durability of responses) • Loncastuximab tesirine mechanism of action is immunogenic cell death via intracellular delivery to CD19 expressing tumor cells its pyrrolobenzodiazepine (PBD) warhead that exerts persistent covalent cross - linking of DNA that may contribute to the prolonged duration of CRs observed in LOTIS - 2 monotherapy LOTIS - 5 Phase 3, the mDOR for CRs (50% CRR) has not been reached after 2 years of follow - up 2 (20 - patient safety run - in) CRs remained durable following fixed - duration glofitamab treatment
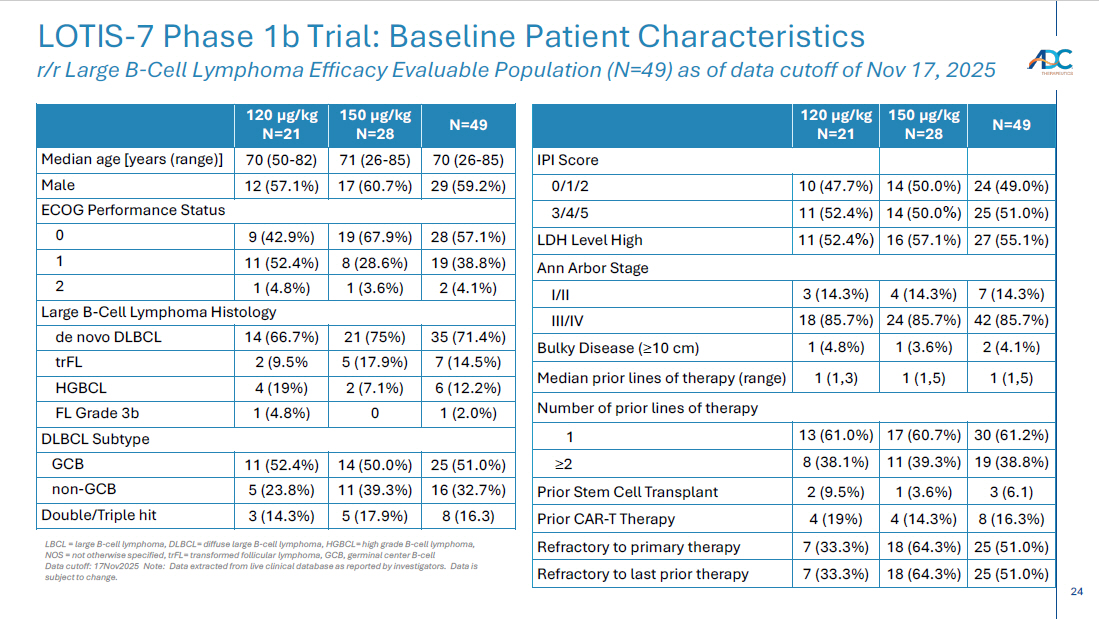
24 CORPORATE LOTIS - 7 Phase 1b Trial: Baseline Patient Characteristics r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of data cutoff of Nov 17, 2025 N=49 150 µg/kg N=28 120 µg/kg N=21 IPI Score 24 (49.0%) 14 (50.0%) 10 (47.7%) 0/1/2 25 (51.0%) 14 (50.0 % ) 11 (52.4%) 3/4/5 27 (55.1%) 16 (57.1%) 11 (52.4 %) LDH Level High Ann Arbor Stage 7 (14.3%) 4 (14.3%) 3 (14.3%) I/II 42 (85.7%) 24 (85.7%) 18 (85.7%) III/IV 2 (4.1%) 1 (3.6%) 1 (4.8%) Bulky Disease ( ≥ 10 cm) 1 (1,5) 1 (1,5) 1 (1,3) Median prior lines of therapy (range) Number of prior lines of therapy 30 (61.2%) 17 (60.7%) 13 (61.0%) 1 19 (38.8%) 11 (39.3%) 8 (38.1%) ≥ 2 3 (6.1) 1 (3.6%) 2 (9.5%) Prior Stem Cell Transplant 8 (16.3%) 4 (14.3%) 4 (19%) Prior CAR - T Therapy 25 (51.0%) 18 (64.3%) 7 (33.3%) Refractory to primary therapy 25 (51.0%) 18 (64.3%) 7 (33.3%) Refractory to last prior therapy N=49 150 µg/kg N=28 120 µg/kg N=21 70 (26 - 85) 71 (26 - 85) 70 (50 - 82) Median age [years (range)] 29 (59.2%) 17 (60.7%) 12 (57.1%) Male ECOG Performance Status 28 (57.1%) 19 (67.9%) 9 (42.9%) 0 19 (38.8%) 8 (28.6%) 11 (52.4%) 1 2 (4.1%) 1 (3.6%) 1 (4.8 % ) 2 Large B - Cell Lymphoma Histology 35 (71.4%) 21 (75%) 14 (66.7%) de novo DLBCL 7 (14.5%) 5 (17.9%) 2 (9.5% trFL 6 (12.2%) 2 (7.1%) 4 (19%) HGBCL 1 (2.0%) 0 1 (4.8%) FL Grade 3b DLBCL Subtype 25 (51.0%) 14 (50.0%) 11 (52.4%) GCB 16 (32.7%) 11 (39.3%) 5 (23.8%) non - GCB 8 (16.3) 5 (17.9%) 3 (14.3%) Double/Triple hit LBCL = large B - cell lymphoma, DLBCL= diffuse large B - cell lymphoma, HGBCL= high grade B - cell lymphoma, NOS = not otherwise specified, trFL = transformed follicular lymphoma, GCB, germinal center B - cell Data cutoff: 17Nov 2025 Note: Data extracted from live clinical database as reported by investigators. Data is subject to change.
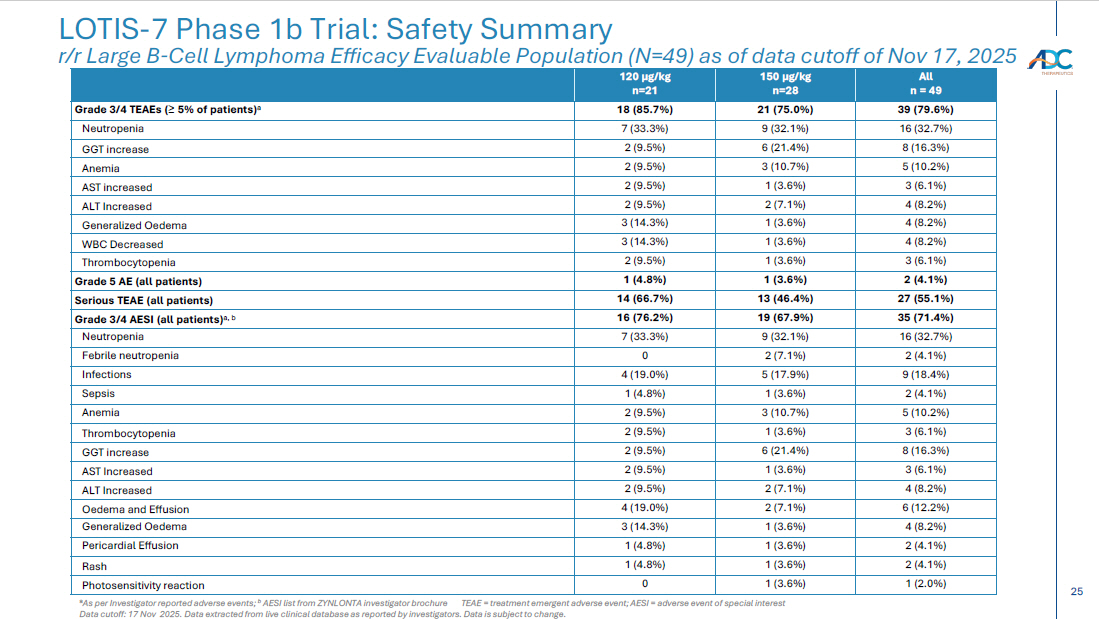
25 CORPORATE LOTIS - 7 Phase 1b Trial: Safety Summary r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of data cutoff of Nov 17, 2025 a As per Investigator reported adverse events; b AESI list from ZYNLONTA investigator brochure TEAE = treatment emergent adverse event; AESI = adverse event of special in terest Data cutoff: 17 Nov 2025. Data extracted from live clinical database as reported by investigators. Data is subject to change. All n = 49 150 µg/kg n=28 120 µg/kg n=21 39 (79.6%) 21 (75.0%) 18 (85.7%) Grade 3/4 TEAEs ( ≥ 5% of patients) a 16 (32.7%) 9 (32.1%) 7 (33.3%) Neutropenia 8 (16.3%) 6 (21.4%) 2 (9.5%) GGT increase 5 (10.2%) 3 (10.7%) 2 (9.5%) Anemia 3 (6.1%) 1 (3.6%) 2 (9.5%) AST increased 4 (8.2%) 2 (7.1%) 2 (9.5%) ALT Increased 4 (8.2%) 1 (3.6%) 3 (14.3%) Generalized Oedema 4 (8.2%) 1 (3.6%) 3 (14.3%) WBC Decreased 3 (6.1%) 1 (3.6%) 2 (9.5%) Thrombocytopenia 2 (4.1%) 1 (3.6%) 1 (4.8%) Grade 5 AE (all patients) 27 (55.1%) 13 (46.4%) 14 (66.7%) Serious TEAE (all patients) 35 (71.4%) 19 (67.9%) 16 (76.2%) Grade 3/4 AESI (all patients) a, b 16 (32.7%) 9 (32.1%) 7 (33.3%) Neutropenia 2 (4.1%) 2 (7.1%) 0 Febrile neutropenia 9 (18.4%) 5 (17.9%) 4 (19.0%) Infections 2 (4.1%) 1 (3.6%) 1 (4.8%) Sepsis 5 (10.2%) 3 (10.7%) 2 (9.5%) Anemia 3 (6.1%) 1 (3.6%) 2 (9.5%) Thrombocytopenia 8 (16.3%) 6 (21.4%) 2 (9.5%) GGT increase 3 (6.1%) 1 (3.6%) 2 (9.5%) AST Increased 4 (8.2%) 2 (7.1%) 2 (9.5%) ALT Increased 6 (12.2%) 2 (7.1%) 4 (19.0%) Oedema and Effusion 4 (8.2%) 1 (3.6%) 3 (14.3%) Generalized Oedema 2 (4.1%) 1 (3.6%) 1 (4.8%) Pericardial Effusion 2 (4.1%) 1 (3.6%) 1 (4.8%) Rash 1 (2.0%) 1 (3.6%) 0 Photosensitivity reaction
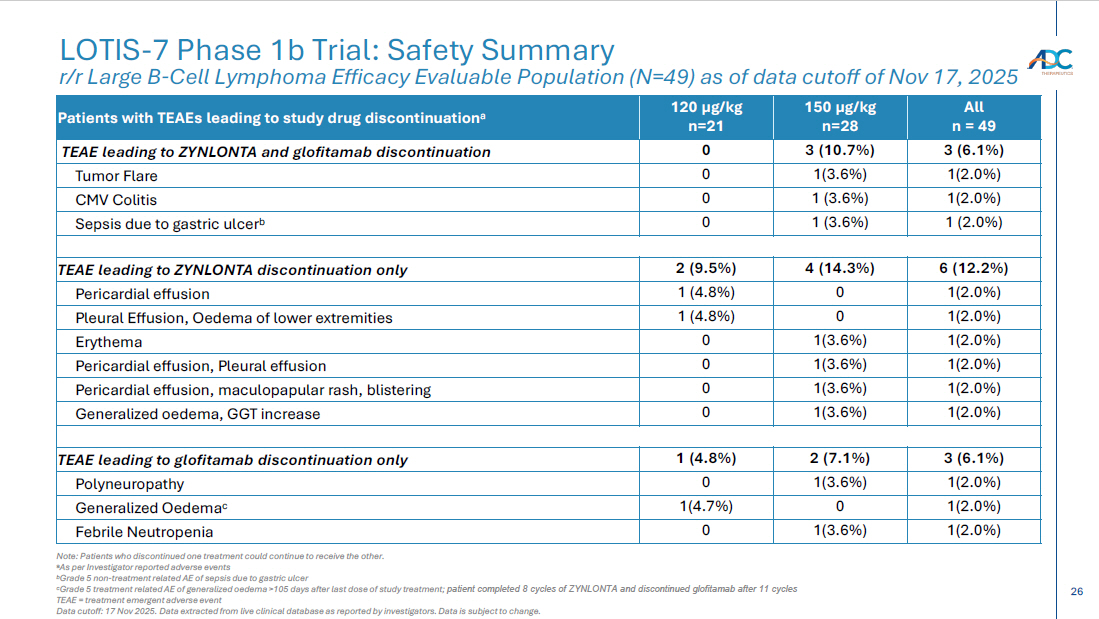
26 CORPORATE LOTIS - 7 Phase 1b Trial: Safety Summary r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of data cutoff of Nov 17, 2025 Note: Patients who discontinued one treatment could continue to receive the other. a As per Investigator reported adverse events b Grade 5 non - treatment related AE of sepsis due to gastric ulcer c Grade 5 treatment related AE of generalized oedema >105 days after last dose of study treatment; patient completed 8 cycles of ZYNLONTA and discontinued glofitamab after 11 cycles TEAE = treatment emergent adverse event Data cutoff: 17 Nov 2025. Data extracted from live clinical database as reported by investigators. Data is subject to change. All n = 49 150 µg/kg n=28 120 µg/kg n=21 Patients with TEAEs leading to study drug discontinuation a 3 (6.1%) 3 (10.7%) 0 TEAE leading to ZYNLONTA and glofitamab discontinuation 1(2.0%) 1(3.6%) 0 Tumor Flare 1(2.0%) 1 (3.6%) 0 CMV Colitis 1 (2.0%) 1 (3.6%) 0 Sepsis due to gastric ulcer b 6 (12.2%) 4 (14.3%) 2 (9.5%) TEAE leading to ZYNLONTA discontinuation only 1(2.0%) 0 1 (4.8%) Pericardial effusion 1(2.0%) 0 1 (4.8%) Pleural Effusion, Oedema of lower extremities 1(2.0%) 1(3.6%) 0 Erythema 1(2.0%) 1(3.6%) 0 Pericardial effusion, Pleural effusion 1(2.0%) 1(3.6%) 0 Pericardial effusion, maculopapular rash, blistering 1(2.0%) 1(3.6%) 0 Generalized oedema, GGT increase 3 (6.1%) 2 (7.1%) 1 (4.8%) TEAE leading to glofitamab discontinuation only 1(2.0%) 1(3.6%) 0 Polyneuropathy 1(2.0%) 0 1(4.7%) Generalized Oedema c 1(2.0%) 1(3.6%) 0 Febrile Neutropenia
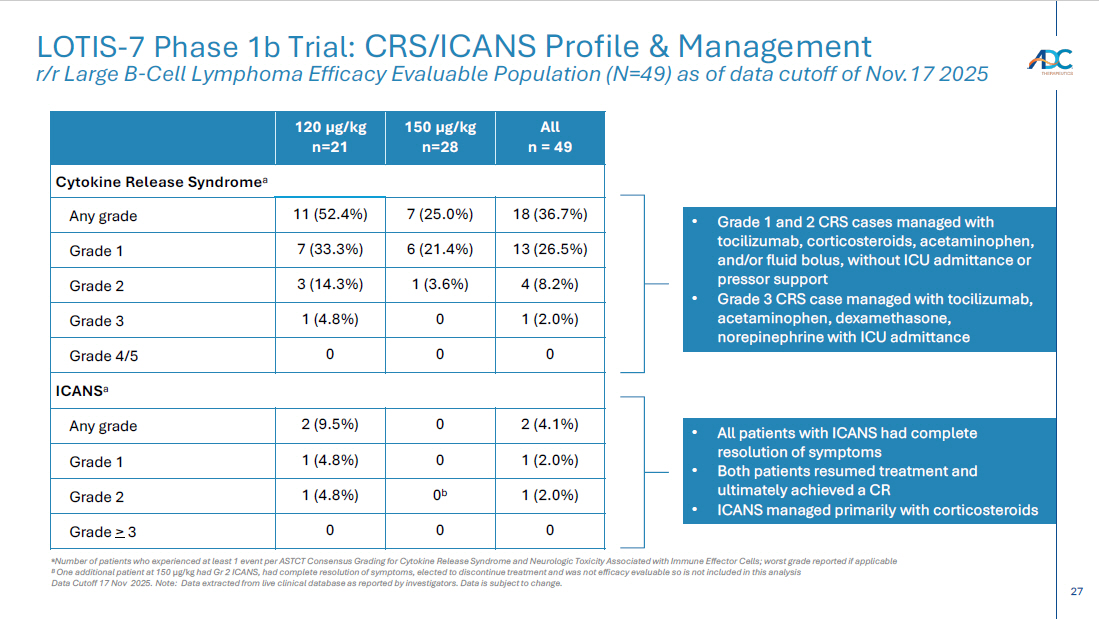
27 CORPORATE LOTIS - 7 Phase 1b Trial: CRS/ICANS Profile & Management r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of data cutoff of Nov.17 2025 a Number of patients who experienced at least 1 event per ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Tox icity Associated with Immune Effector Cells; worst grade reported if applicable B One additional patient at 150 µg/kg had Gr 2 ICANS, had complete resolution of symptoms, elected to discontinue treatment and was not efficacy evaluable so is no t i ncluded in this analysis Data Cutoff 17 Nov 2025. Note: Data extracted from live clinical database as reported by investigators. Data is subject to change. All n = 49 150 µg/kg n=28 120 µg/kg n=21 Cytokine Release Syndrome a 18 (36.7%) 7 (25.0%) 11 (52.4%) Any grade 13 (26.5%) 6 (21.4%) 7 (33.3%) Grade 1 4 (8.2%) 1 (3.6%) 3 (14.3%) Grade 2 1 (2.0%) 0 1 (4.8%) Grade 3 0 0 0 Grade 4/5 ICANS a 2 (4.1%) 0 2 (9.5%) Any grade 1 (2.0%) 0 1 (4.8%) Grade 1 1 (2.0%) 0 b 1 (4.8%) Grade 2 0 0 0 Grade > 3 • Grade 1 and 2 CRS cases managed with tocilizumab, corticosteroids, acetaminophen, and/or fluid bolus, without ICU admittance or pressor support • Grade 3 CRS case managed with tocilizumab, acetaminophen, dexamethasone, norepinephrine with ICU admittance • All patients with ICANS had complete resolution of symptoms • Both patients resumed treatment and ultimately achieved a CR • ICANS managed primarily with corticosteroids
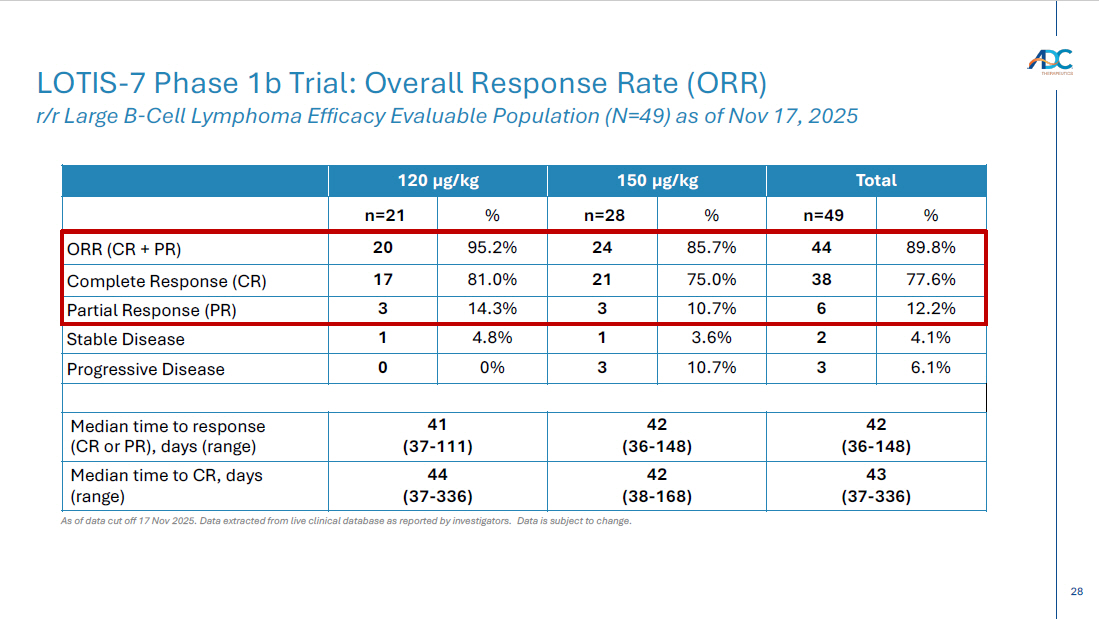
28 Total 150 µg/kg 120 µg/kg % n=49 % n=28 % n=21 89.8% 44 85.7% 24 95.2% 20 ORR (CR + PR) 77.6% 38 75.0% 21 81.0% 17 Complete Response (CR) 12.2% 6 10.7% 3 14.3% 3 Partial Response (PR) 4.1% 2 3.6% 1 4.8% 1 Stable Disease 6.1% 3 10.7% 3 0% 0 Progressive Disease 42 (36 - 148) 42 (36 - 148) 41 (37 - 111) Median time to response (CR or PR), days (range) 43 (37 - 336) 42 (38 - 168) 44 (37 - 336) Median time to CR, days (range) As of data cut off 17 Nov 2025. Data extracted from live clinical database as reported by investigators. Data is subject to change. LOTIS - 7 Phase 1b Trial: Overall Response Rate (ORR) r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=49) as of Nov 17, 2025