

Phase 2 Trial and Open-Label Extension Top-Line Results: Once-Weekly Canvuparatide in Adults with Hypoparathyroidism September 22, 2025 .2

Disclaimer This presentation includes forward looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our product candidates, preclinical study and/or clinical trial timelines, including projected data announcements, future results of operations and financial position, strategy and plans, industry environment, potential growth opportunities, and our expectations for future operations, are forward looking statements The words “ believe,” “ may,” “will,” “ estimate,” “ continue,” “ anticipate,” “ design,” “ “ expect,” “ could,” “ plan,” “ potential,” “ predict,” “ seek,” “ should,” “would,” or the negative version of these words and similar expressions are intended to identify forward looking statements. We have based these forward-looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including but not limited to, our ability to develop and advance our programs and product candidates, our regulatory approvals and filings, and other risks, uncertainties and assumptions identified in our filings with the Securities and Exchange Commission. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time, and it is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, unless required by law. This presentation contains estimates and other information concerning our industry, our business and the markets for our product candidates. Information that is based on estimates, market research or similar methodologies, including prevalence studies which are extrapolated to broader populations, is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. While we are responsible for the accuracy of such information and believe our internal company research as to such matters is reliable and the market definitions are appropriate, neither such research nor these definitions have been verified by any independent source.

Agenda Once-Weekly Canvuparatide for the Potential Treatment of Hypoparathyroidism Kent Hawryluk President & CEO Sam Azoulay, MD Chief Medical Officer Rick Bartram, CPA Chief Financial Officer Top-Line Results Summary Disease Overview and Current Landscape Avail™ and Open-Label Extension Overview Clinical Results and Safety Data Differentiation and Next Steps Q&A Session Kent Hawryluk Sam Azoulay, MD Kent Hawryluk All
Once-Weekly Canvuparatide Achieved Primary Endpoint at Week 12; 79% Responder Rate at 6 Months in Open-Label Extension 63% of patients receiving once-weekly canvuparatide achieved the primary endpoint at Week 12 vs 31% on placebo (p<0.05) All patients completed Avail™ and 94% entered the Open-Label Extension (OLE) Responder status increased to 79% at 6 months in the OLE At Week 12, urine calcium excretion was reduced in canvuparatide-treated patients with elevated baseline values Bone biomarkers increased in canvuparatide-treated patients at Week 12 and continued to improve at 6 months Once-weekly canvuparatide was well-tolerated, with no treatment related serious adverse events or discontinuations during the 12-week trial Results support development for once-weekly dosing and progression into Phase 3
Hypoparathyroidism: A Chronic, Serious Endocrine Disease Affects >250,000 patients in the U.S. and E.U., representing a significant unmet need and market opportunity1,2 PTH, parathyroid hormone. 1. Clarke B et al JCEM 2016, 2. Powers J et al JBMR 2013, 3. Khan A et al JBMR 2022, 4. Yao L et al Complications Symptoms JBMR 2022. Hypocalcemia – low calcium: Tetany, muscle cramping/spasms/twitching, numbness, tingling, seizures Deficiency in parathyroid hormone (PTH) Etiology: Inadvertent removal of parathyroid during thyroid surgery and less commonly due to autoimmune disease and genetic disorders Cardiovascular: Arrythmias, ischemic heart disease SYMPTOMS3,4 COMPLICATIONS3,4 Cognition: Cognitive impairment, confusion Depression / Infections CAUSE Hypercalcemia – high calcium: Polyuria, nausea, vomiting, constipation, weakness Renal: Kidney stones, chronic kidney disease, nephrocalcinosis
Managing Hypoparathyroidism: A Significant Treatment Opportunity PTH, parathyroid hormone; QoL=quality of life. 1. Khan A et al. JBMR 2022. Once-weekly canvuparatide designed to overcome key limitations of current therapies Calcium and active vitamin D1 supplementation Daily PTH replacement therapy Investigational once-weekly PTH replacement therapy STANDARD OF CARE Does not address underlying pathophysiology Significant pill burden Serum calcium fluctuations Contributes to renal complications Palopegteriparatide Approved in U.S. and E.U. for the treatment of hypoparathyroidism in adults Eneboparatide In Phase 3 development Designed to: Lower daily peak-to-trough PTH exposures vs. daily injectables Normalize serum and urine calcium Eliminate pill burden Convenient weekly dosing Improve QoL Reduce complications DAILY INJECTIONS Canvuparatide WEEKLY INJECTION
Avail™ Phase 2 Trial and OLE Overview
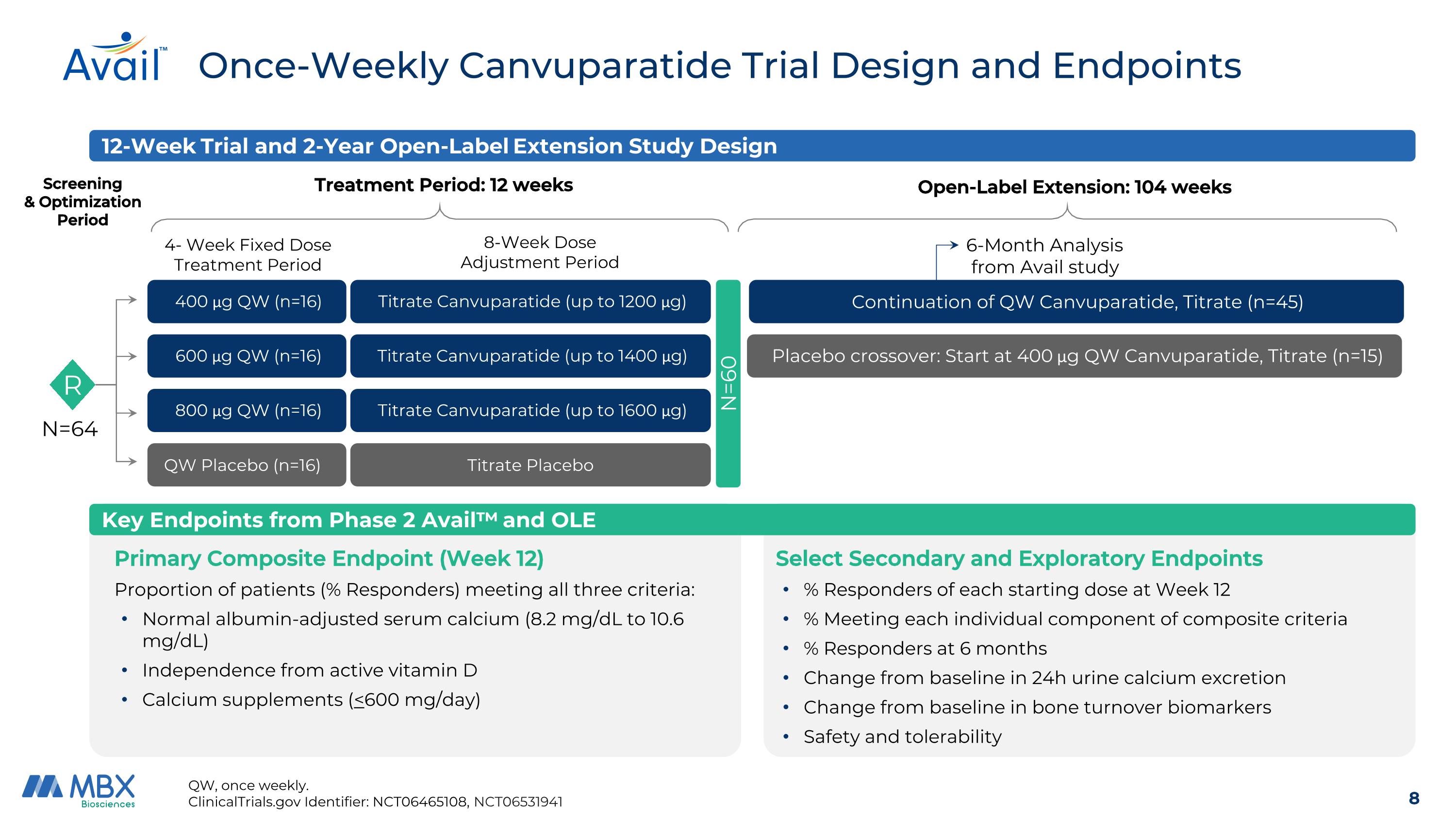
Once-Weekly Canvuparatide Trial Design and Endpoints QW, once weekly. ClinicalTrials.gov Identifier: NCT06465108, NCT06531941 Key Endpoints from Phase 2 Avail™ and OLE Screening & Optimization Period Treatment Period: 12 weeks 4- Week Fixed Dose Treatment Period 8-Week Dose Adjustment Period 800 μg QW (n=16) N=64 600 μg QW (n=16) 400 μg QW (n=16) QW Placebo (n=16) Titrate Canvuparatide (up to 1600 μg) Titrate Canvuparatide (up to 1400 μg) Titrate Canvuparatide (up to 1200 μg) Titrate Placebo R 12-Week Trial and 2-Year Open-Label Extension Study Design Open-Label Extension: 104 weeks Placebo crossover: Start at 400 μg QW Canvuparatide, Titrate (n=15) Continuation of QW Canvuparatide, Titrate (n=45) N=60 6-Month Analysis from Avail study Primary Composite Endpoint (Week 12) Proportion of patients (% Responders) meeting all three criteria: Normal albumin-adjusted serum calcium (8.2 mg/dL to 10.6 mg/dL) Independence from active vitamin D Calcium supplements (<600 mg/day) Select Secondary and Exploratory Endpoints % Responders of each starting dose at Week 12 % Meeting each individual component of composite criteria % Responders at 6 months Change from baseline in 24h urine calcium excretion Change from baseline in bone turnover biomarkers Safety and tolerability
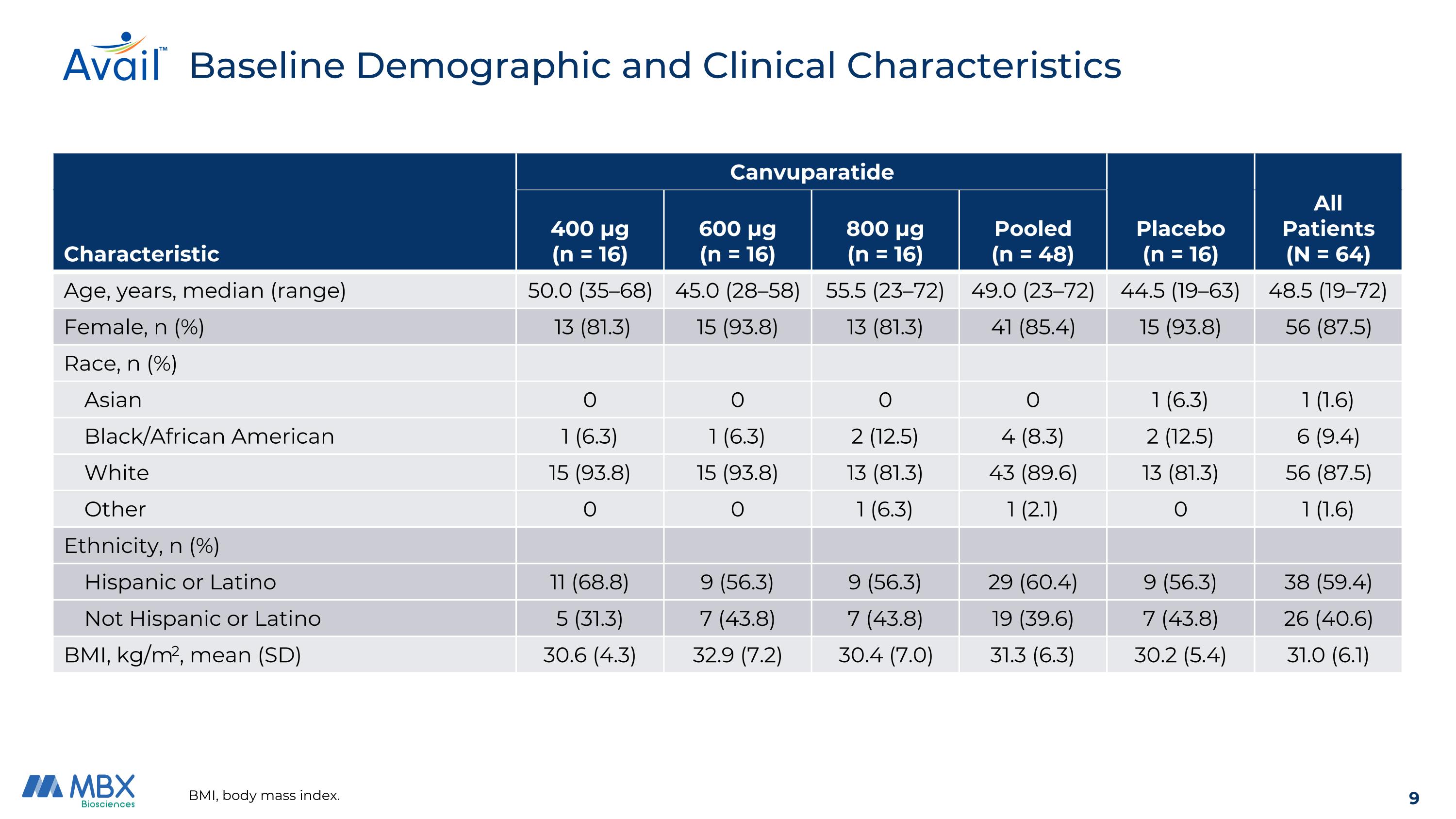
BMI, body mass index. Baseline Demographic and Clinical Characteristics Canvuparatide Characteristic 400 μg (n = 16) 600 μg (n = 16) 800 μg (n = 16) Pooled (n = 48) Placebo (n = 16) All Patients (N = 64) Age, years, median (range) 50.0 (35–68) 45.0 (28–58) 55.5 (23–72) 49.0 (23–72) 44.5 (19–63) 48.5 (19–72) Female, n (%) 13 (81.3) 15 (93.8) 13 (81.3) 41 (85.4) 15 (93.8) 56 (87.5) Race, n (%) Asian 0 0 0 0 1 (6.3) 1 (1.6) Black/African American 1 (6.3) 1 (6.3) 2 (12.5) 4 (8.3) 2 (12.5) 6 (9.4) White 15 (93.8) 15 (93.8) 13 (81.3) 43 (89.6) 13 (81.3) 56 (87.5) Other 0 0 1 (6.3) 1 (2.1) 0 1 (1.6) Ethnicity, n (%) Hispanic or Latino 11 (68.8) 9 (56.3) 9 (56.3) 29 (60.4) 9 (56.3) 38 (59.4) Not Hispanic or Latino 5 (31.3) 7 (43.8) 7 (43.8) 19 (39.6) 7 (43.8) 26 (40.6) BMI, kg/m2, mean (SD) 30.6 (4.3) 32.9 (7.2) 30.4 (7.0) 31.3 (6.3) 30.2 (5.4) 31.0 (6.1)
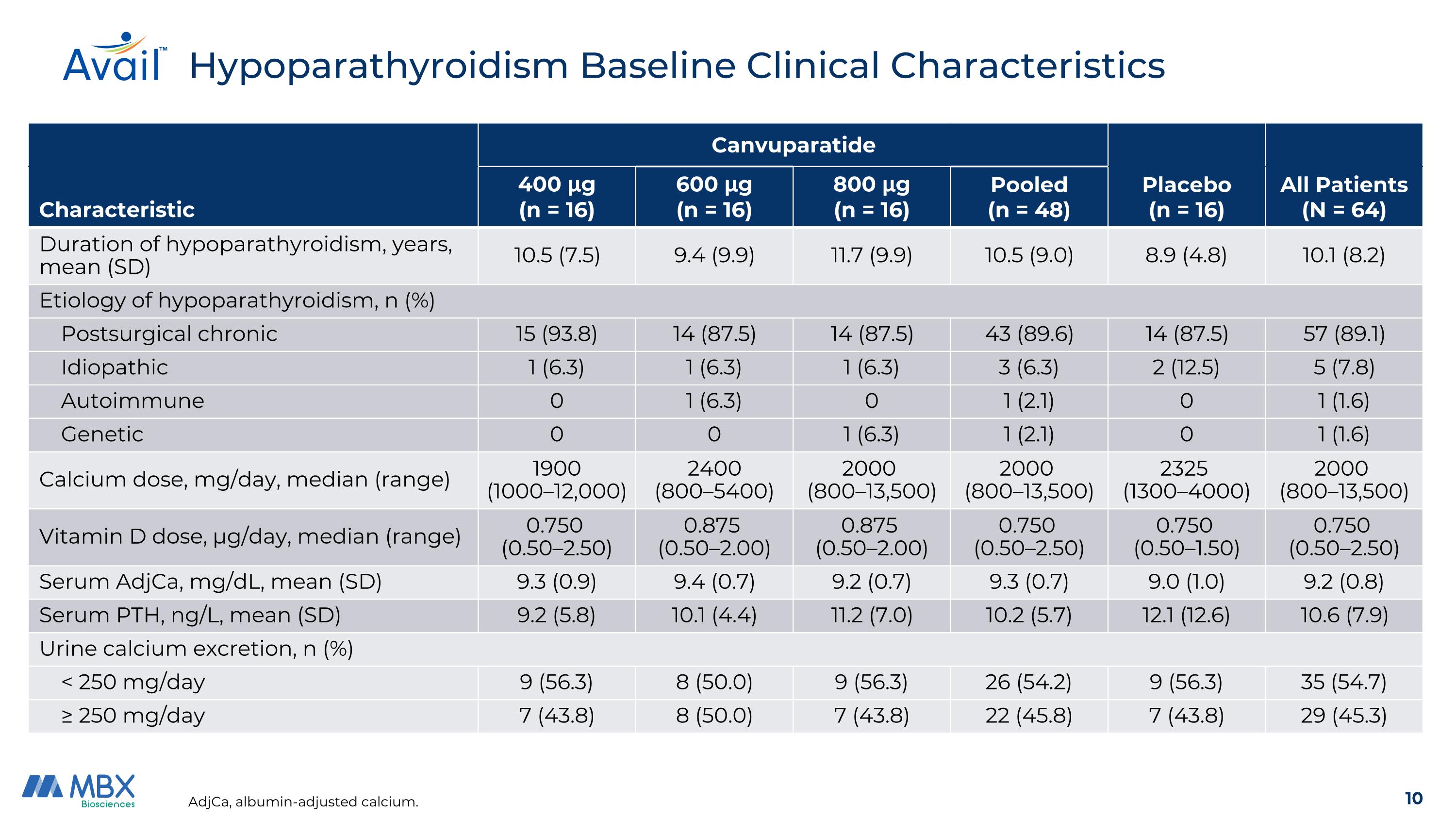
Hypoparathyroidism Baseline Clinical Characteristics AdjCa, albumin-adjusted calcium. Canvuparatide Characteristic 400 μg (n = 16) 600 μg (n = 16) 800 μg (n = 16) Pooled (n = 48) Placebo (n = 16) All Patients (N = 64) Duration of hypoparathyroidism, years, mean (SD) 10.5 (7.5) 9.4 (9.9) 11.7 (9.9) 10.5 (9.0) 8.9 (4.8) 10.1 (8.2) Etiology of hypoparathyroidism, n (%) Postsurgical chronic 15 (93.8) 14 (87.5) 14 (87.5) 43 (89.6) 14 (87.5) 57 (89.1) Idiopathic 1 (6.3) 1 (6.3) 1 (6.3) 3 (6.3) 2 (12.5) 5 (7.8) Autoimmune 0 1 (6.3) 0 1 (2.1) 0 1 (1.6) Genetic 0 0 1 (6.3) 1 (2.1) 0 1 (1.6) Calcium dose, mg/day, median (range) 1900 (1000–12,000) 2400 (800–5400) 2000 (800–13,500) 2000 (800–13,500) 2325 (1300–4000) 2000 (800–13,500) Vitamin D dose, μg/day, median (range) 0.750 (0.50–2.50) 0.875 (0.50–2.00) 0.875 (0.50–2.00) 0.750 (0.50–2.50) 0.750 (0.50–1.50) 0.750 (0.50–2.50) Serum AdjCa, mg/dL, mean (SD) 9.3 (0.9) 9.4 (0.7) 9.2 (0.7) 9.3 (0.7) 9.0 (1.0) 9.2 (0.8) Serum PTH, ng/L, mean (SD) 9.2 (5.8) 10.1 (4.4) 11.2 (7.0) 10.2 (5.7) 12.1 (12.6) 10.6 (7.9) Urine calcium excretion, n (%) < 250 mg/day 9 (56.3) 8 (50.0) 9 (56.3) 26 (54.2) 9 (56.3) 35 (54.7) ≥ 250 mg/day 7 (43.8) 8 (50.0) 7 (43.8) 22 (45.8) 7 (43.8) 29 (45.3)
Avail™ Phase 2: Clinical Results
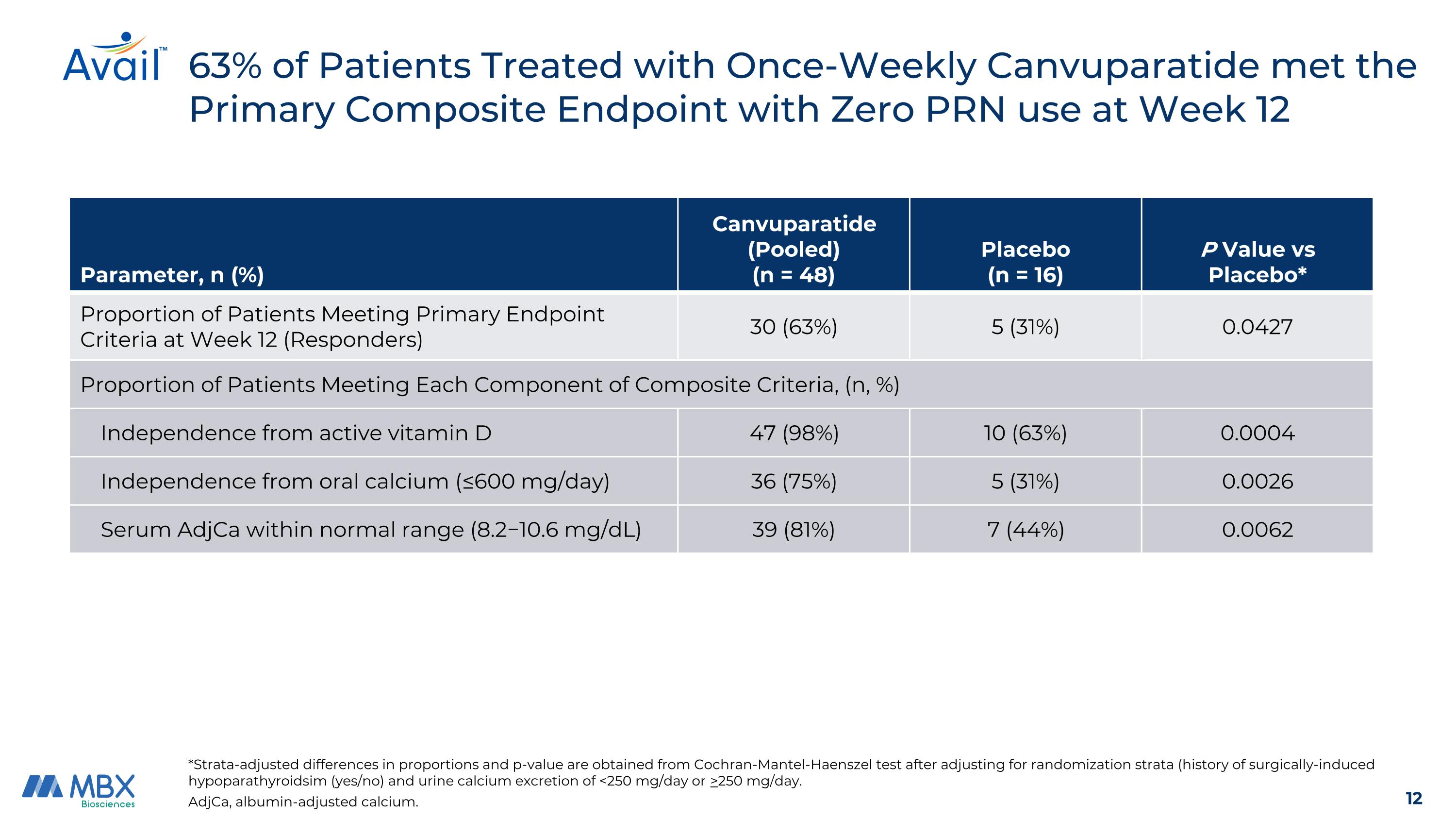
63% of Patients Treated with Once-Weekly Canvuparatide met the Primary Composite Endpoint with Zero PRN use at Week 12 AdjCa, albumin-adjusted calcium. Parameter, n (%) Canvuparatide (Pooled) (n = 48) Placebo (n = 16) P Value vs Placebo* Proportion of Patients Meeting Primary Endpoint Criteria at Week 12 (Responders) 30 (63%) 5 (31%) 0.0427 Proportion of Patients Meeting Each Component of Composite Criteria, (n, %) Independence from active vitamin D 47 (98%) 10 (63%) 0.0004 Independence from oral calcium (≤600 mg/day) 36 (75%) 5 (31%) 0.0026 Serum AdjCa within normal range (8.2−10.6 mg/dL) 39 (81%) 7 (44%) 0.0062 *Strata-adjusted differences in proportions and p-value are obtained from Cochran-Mantel-Haenszel test after adjusting for randomization strata (history of surgically-induced hypoparathyroidsim (yes/no) and urine calcium excretion of <250 mg/day or >250 mg/day.
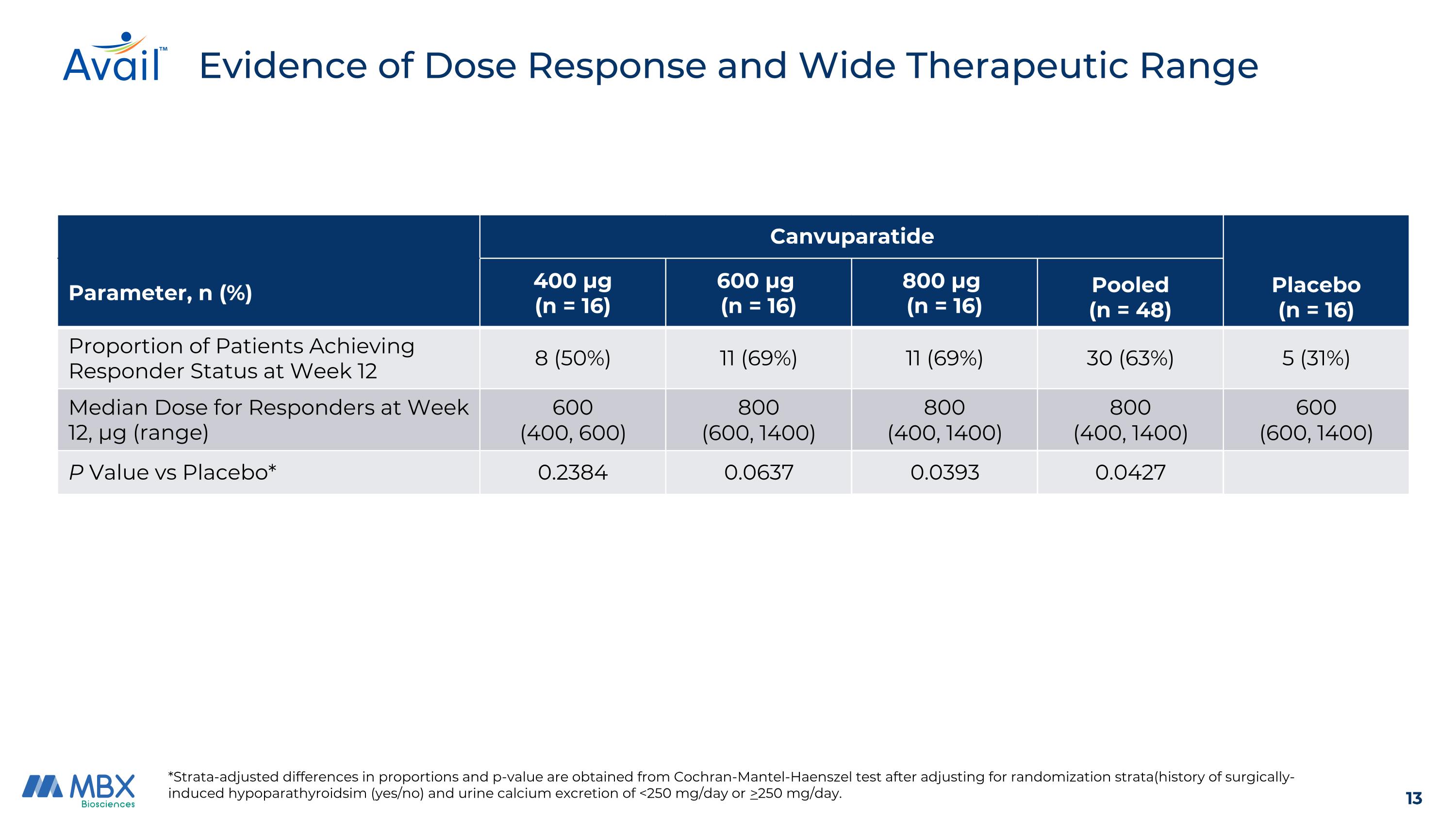
Evidence of Dose Response and Wide Therapeutic Range Canvuparatide Placebo (n = 16) Parameter, n (%) 400 μg (n = 16) 600 μg (n = 16) 800 μg (n = 16) Pooled (n = 48) Proportion of Patients Achieving Responder Status at Week 12 8 (50%) 11 (69%) 11 (69%) 30 (63%) 5 (31%) Median Dose for Responders at Week 12, μg (range) 600 (400, 600) 800 (600, 1400) 800 (400, 1400) 800 (400, 1400) 600 (600, 1400) P Value vs Placebo* 0.2384 0.0637 0.0393 0.0427 *Strata-adjusted differences in proportions and p-value are obtained from Cochran-Mantel-Haenszel test after adjusting for randomization strata(history of surgically-induced hypoparathyroidsim (yes/no) and urine calcium excretion of <250 mg/day or >250 mg/day.
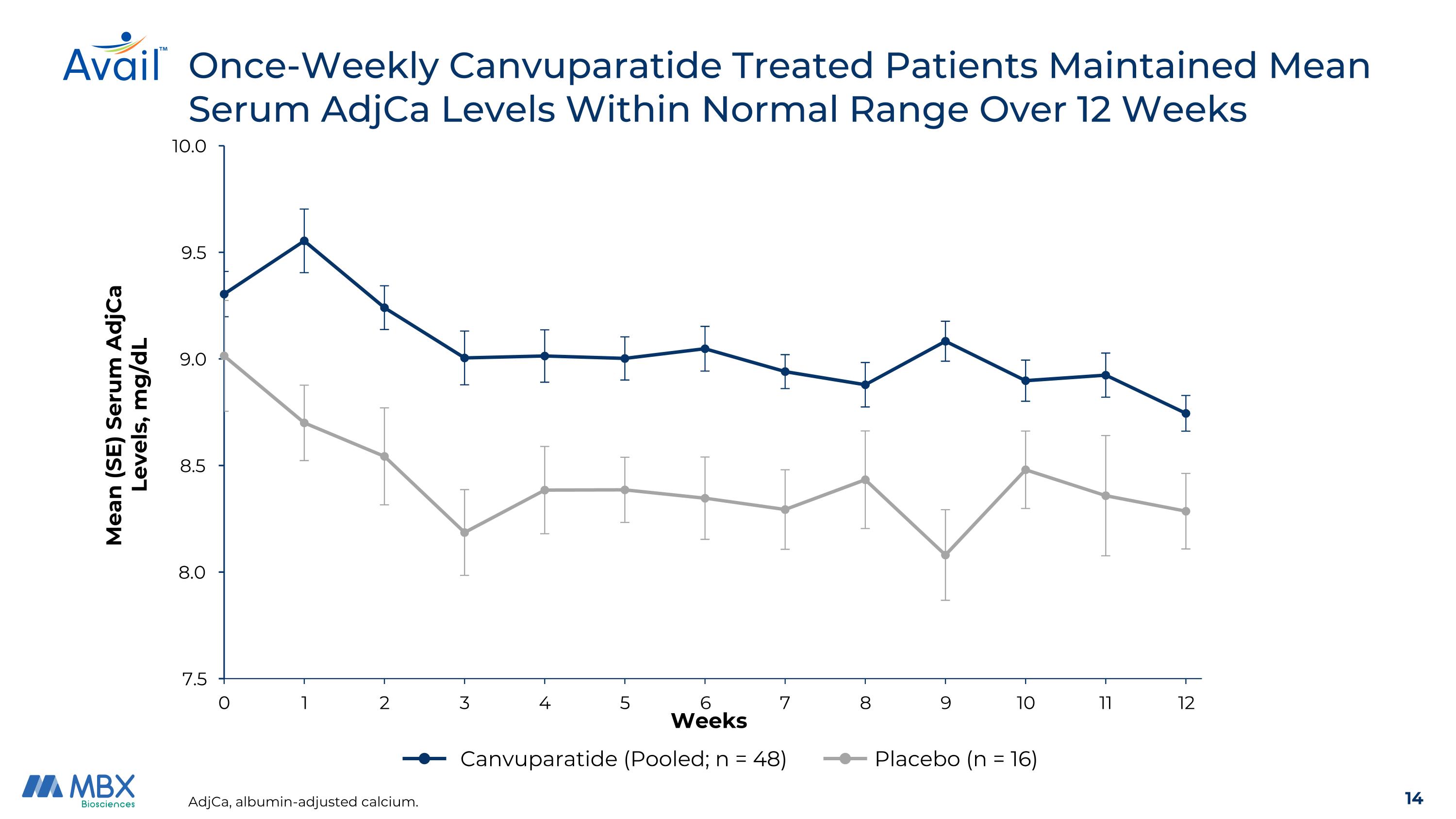
Once-Weekly Canvuparatide Treated Patients Maintained Mean Serum AdjCa Levels Within Normal Range Over 12 Weeks AdjCa, albumin-adjusted calcium. Canvuparatide (Pooled; n = 48) Placebo (n = 16) Mean (SE) Serum AdjCa Levels, mg/dL
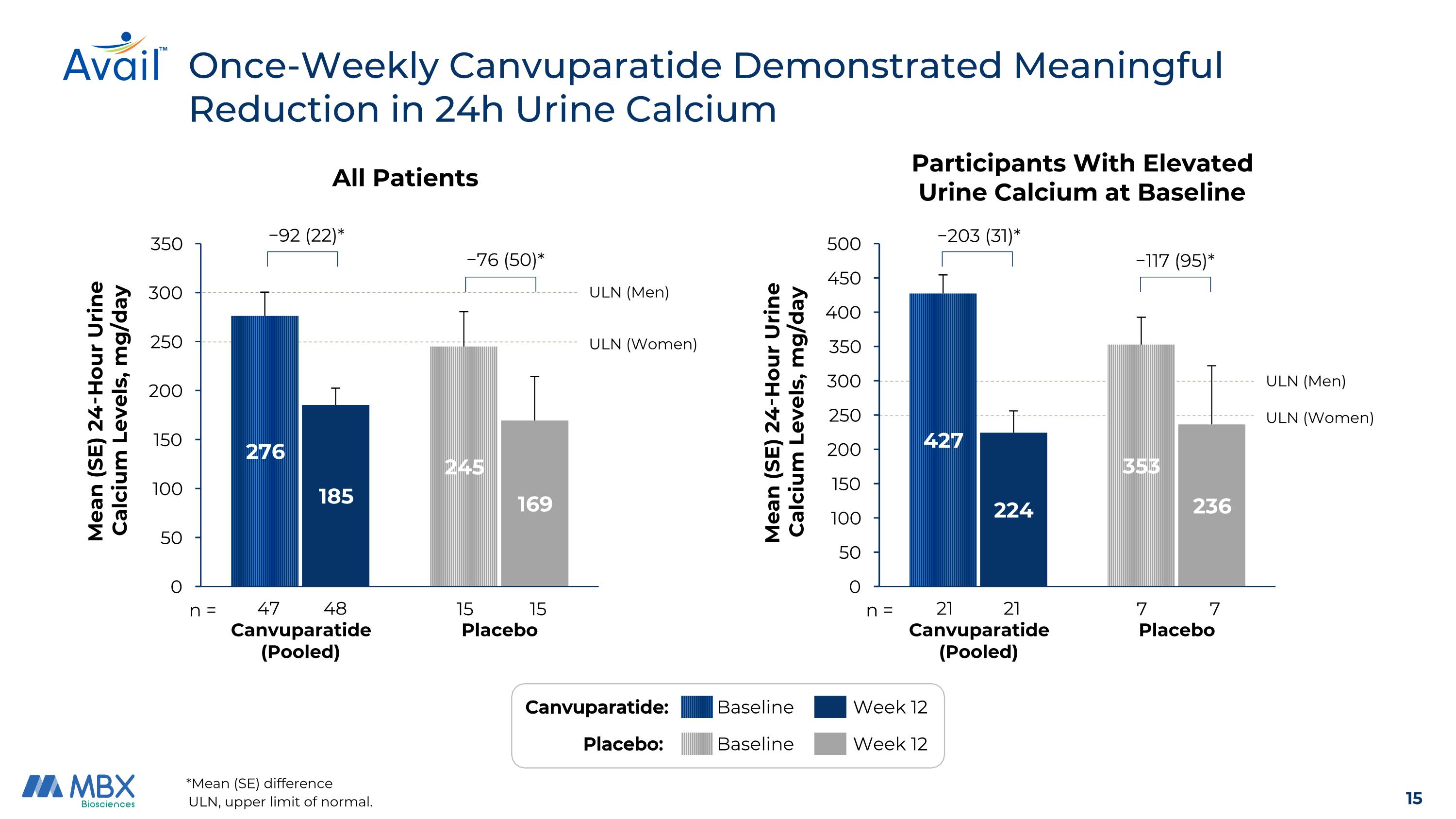
Once-Weekly Canvuparatide Demonstrated Meaningful Reduction in 24h Urine Calcium ULN, upper limit of normal. All Patients ULN (Men) ULN (Women) 15 n = 48 15 47 −92 (22)* −76 (50)* Participants With Elevated Urine Calcium at Baseline ULN (Men) ULN (Women) 7 n = 21 7 21 −203 (31)* −117 (95)* *Mean (SE) difference Canvuparatide: Baseline Week 12 Placebo: Baseline Week 12
OLE: Clinical Results
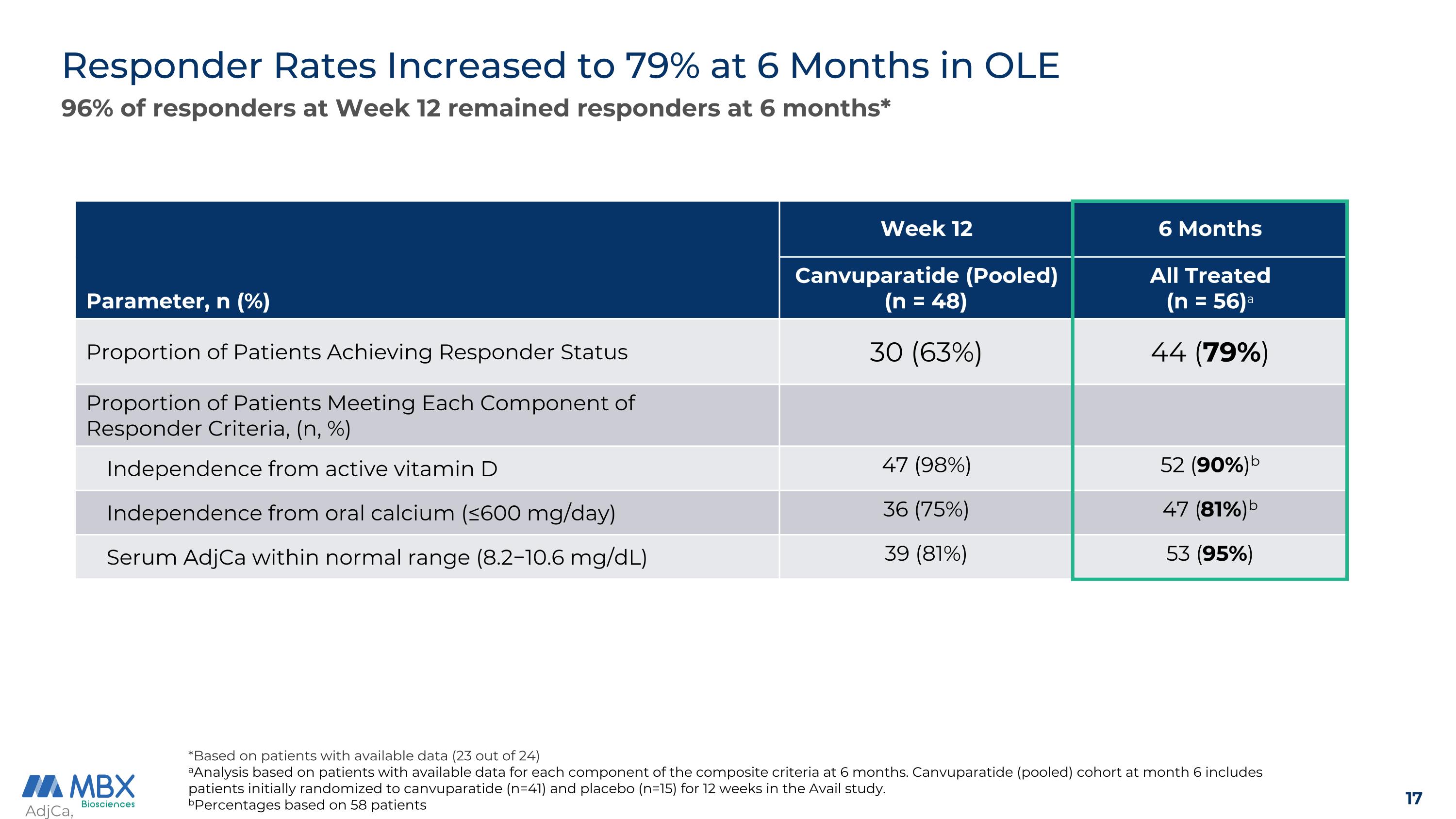
Responder Rates Increased to 79% at 6 Months in OLE AdjCa, albumin-adjusted calcium. *Based on patients with available data (23 out of 24) aAnalysis based on patients with available data for each component of the composite criteria at 6 months. Canvuparatide (pooled) cohort at month 6 includes patients initially randomized to canvuparatide (n=41) and placebo (n=15) for 12 weeks in the Avail study. bPercentages based on 58 patients 96% of responders at Week 12 remained responders at 6 months* Parameter, n (%) Week 12 6 Months Canvuparatide (Pooled) (n = 48) All Treated (n = 56)a Proportion of Patients Achieving Responder Status 30 (63%) 44 (79%) Proportion of Patients Meeting Each Component of Responder Criteria, (n, %) Independence from active vitamin D 47 (98%) 52 (90%)b Independence from oral calcium (≤600 mg/day) 36 (75%) 47 (81%)b Serum AdjCa within normal range (8.2−10.6 mg/dL) 39 (81%) 53 (95%)
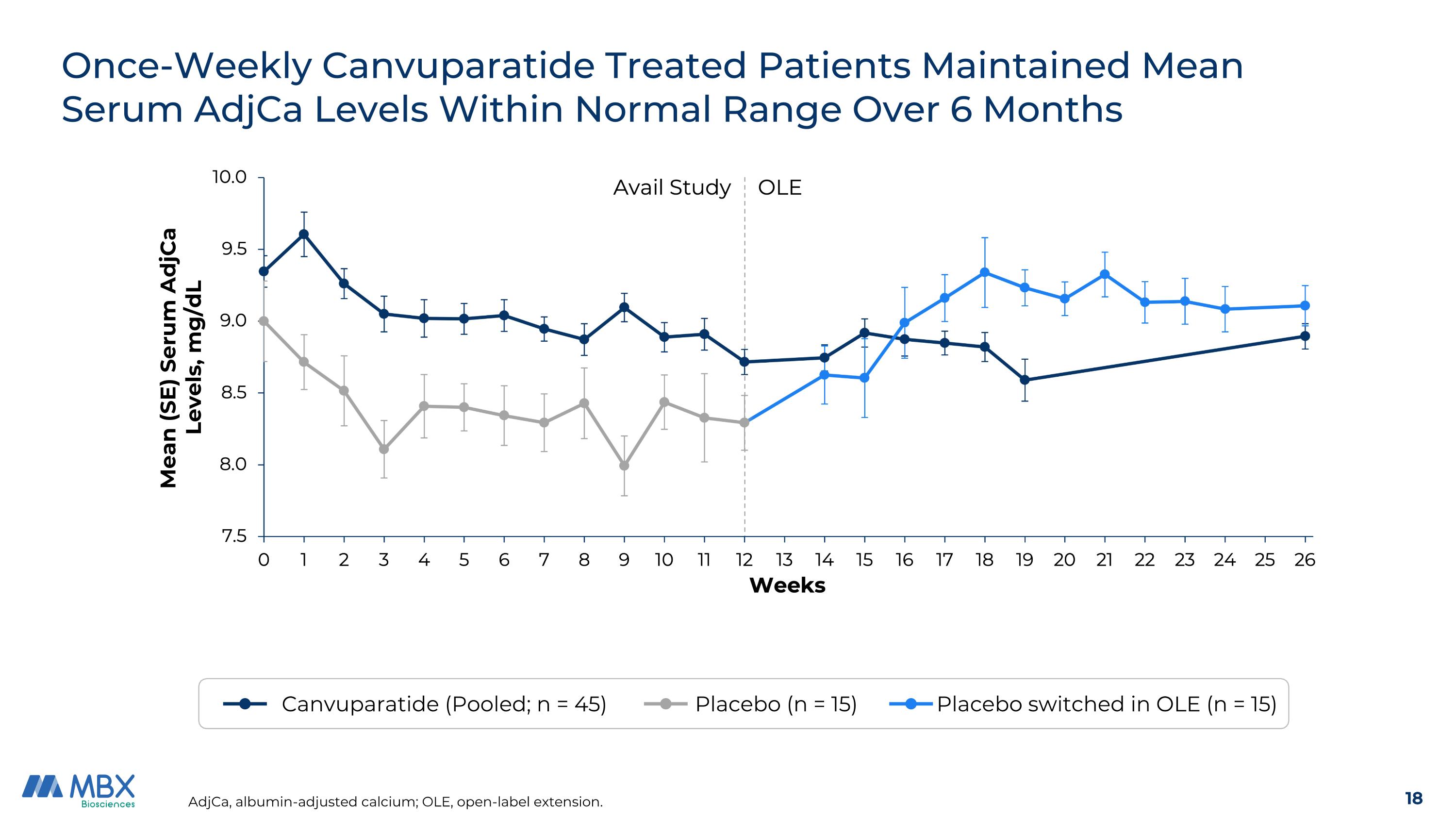
Once-Weekly Canvuparatide Treated Patients Maintained Mean Serum AdjCa Levels Within Normal Range Over 6 Months AdjCa, albumin-adjusted calcium; OLE, open-label extension. Canvuparatide (Pooled; n = 45) Placebo (n = 15) Placebo switched in OLE (n = 15) Mean (SE) Serum AdjCa Levels, mg/dL
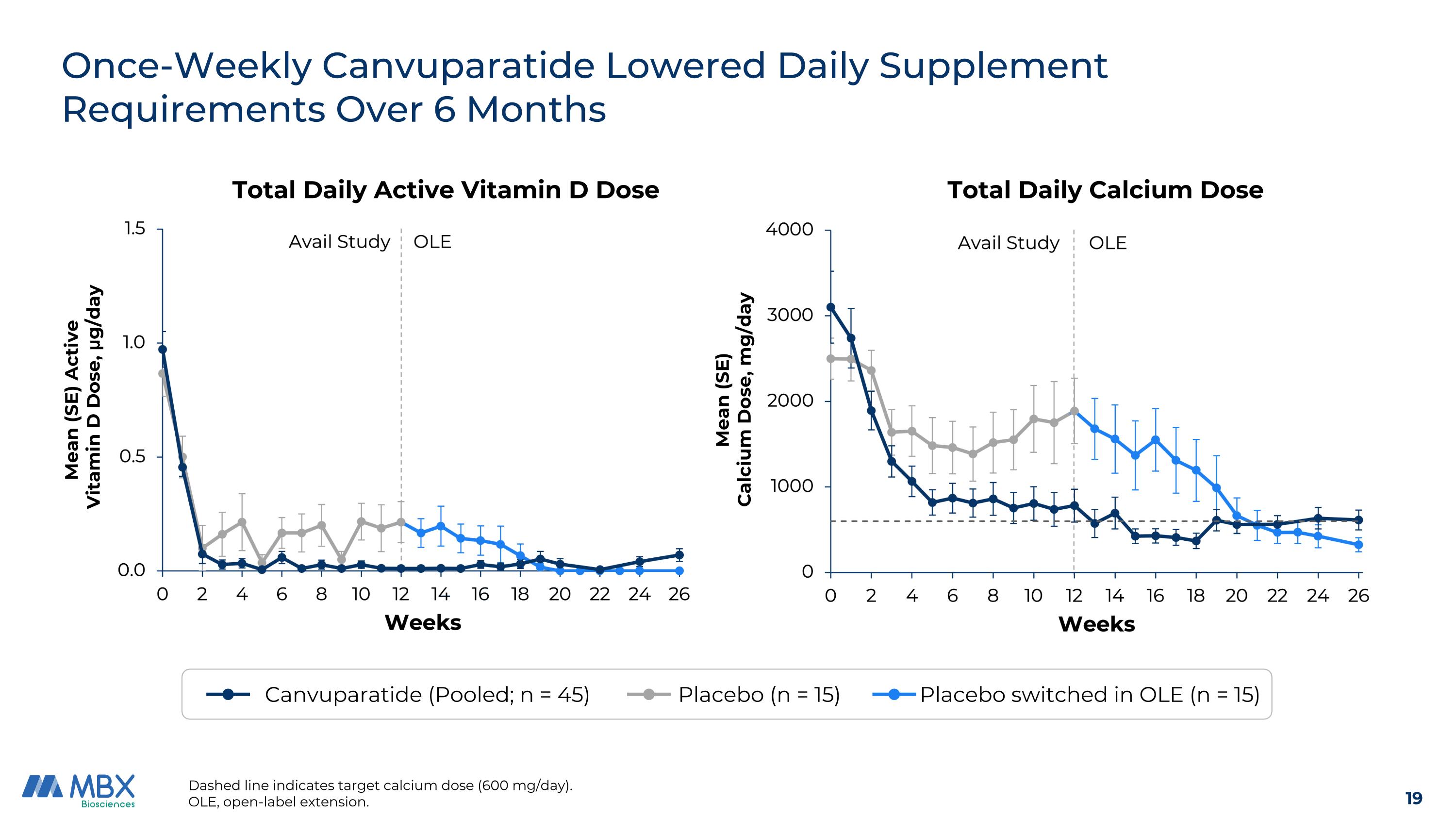
Once-Weekly Canvuparatide Lowered Daily Supplement Requirements Over 6 Months Dashed line indicates target calcium dose (600 mg/day). OLE, open-label extension. Canvuparatide (Pooled; n = 45) Placebo (n = 15) Placebo switched in OLE (n = 15) Mean (SE) Calcium Dose, mg/day Total Daily Calcium Dose Total Daily Active Vitamin D Dose Mean (SE) Active Vitamin D Dose, μg/day
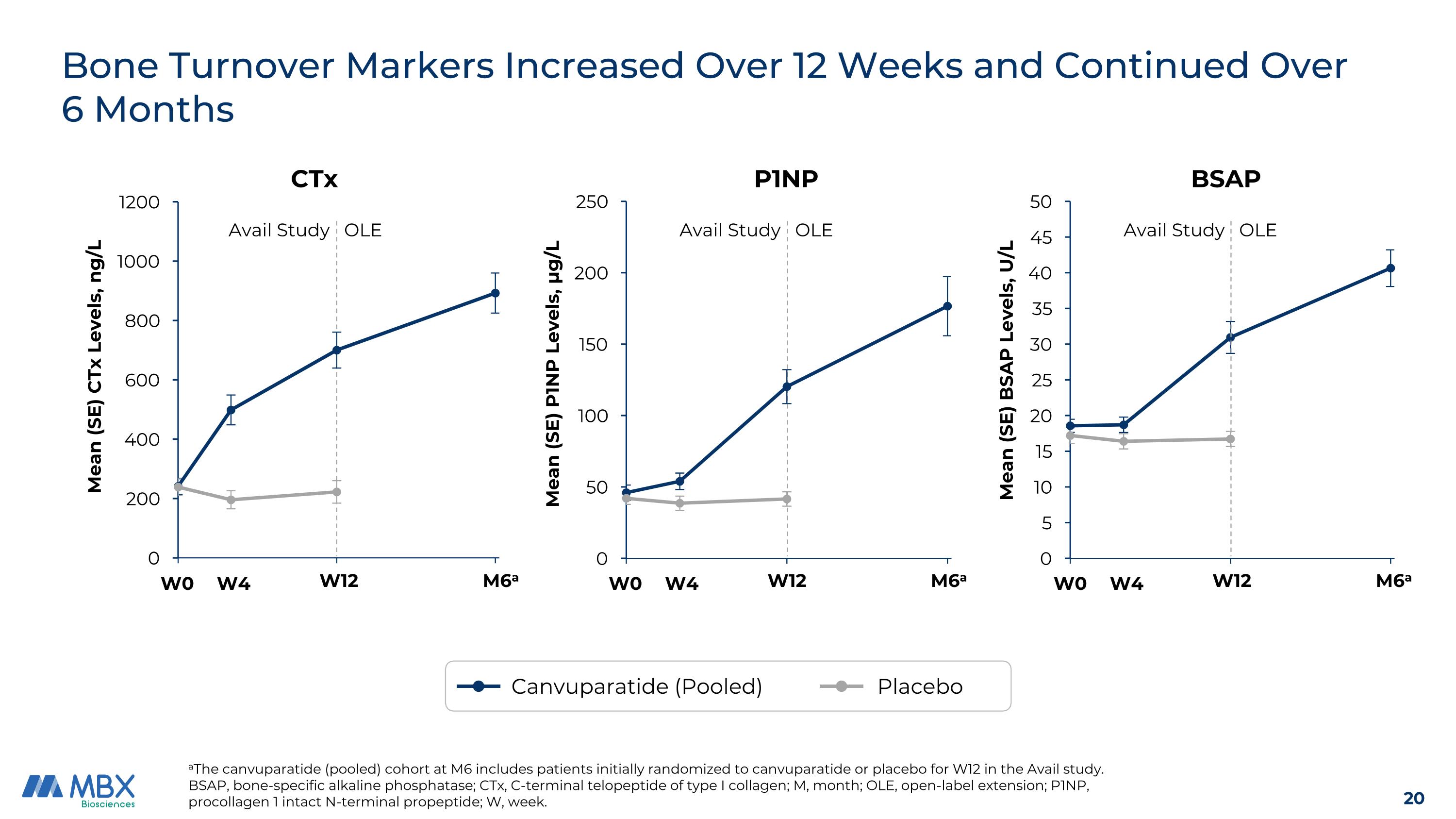
Bone Turnover Markers Increased Over 12 Weeks and Continued Over 6 Months aThe canvuparatide (pooled) cohort at M6 includes patients initially randomized to canvuparatide or placebo for W12 in the Avail study. BSAP, bone-specific alkaline phosphatase; CTx, C-terminal telopeptide of type I collagen; M, month; OLE, open-label extension; P1NP, procollagen 1 intact N-terminal propeptide; W, week. BSAP Avail Study OLE W0 W12 M6a W4 P1NP W0 W12 M6a W4 Avail Study OLE M6a CTx W0 W12 W4 Avail Study OLE Canvuparatide (Pooled) Placebo
Avail™ Phase 2: Safety Results
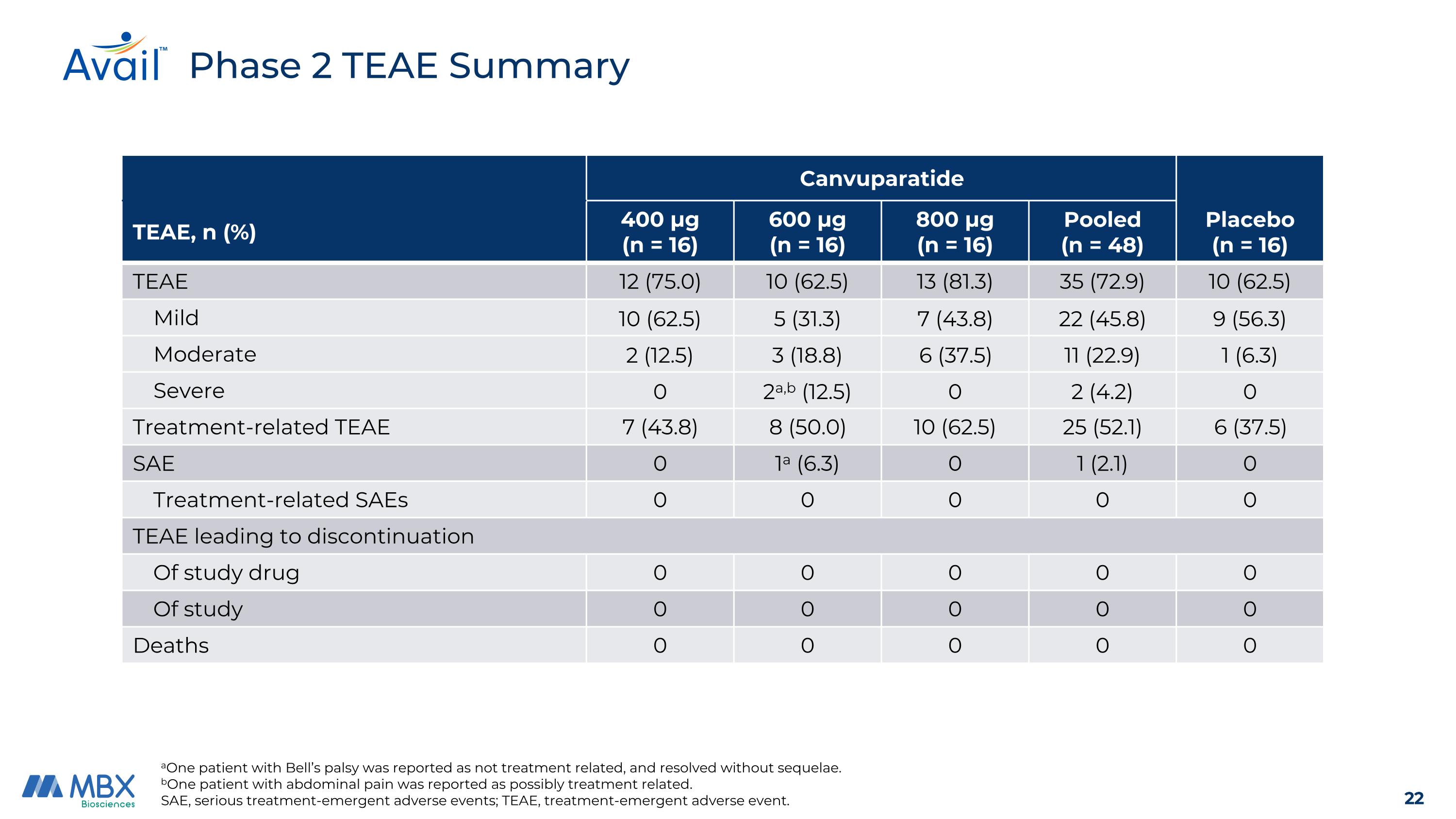
Canvuparatide Placebo (n = 16) TEAE, n (%) 400 μg (n = 16) 600 μg (n = 16) 800 μg (n = 16) Pooled (n = 48) TEAE 12 (75.0) 10 (62.5) 13 (81.3) 35 (72.9) 10 (62.5) Mild 10 (62.5) 5 (31.3) 7 (43.8) 22 (45.8) 9 (56.3) Moderate 2 (12.5) 3 (18.8) 6 (37.5) 11 (22.9) 1 (6.3) Severe 0 2a,b (12.5) 0 2 (4.2) 0 Treatment-related TEAE 7 (43.8) 8 (50.0) 10 (62.5) 25 (52.1) 6 (37.5) SAE 0 1a (6.3) 0 1 (2.1) 0 Treatment-related SAEs 0 0 0 0 0 TEAE leading to discontinuation Of study drug 0 0 0 0 0 Of study 0 0 0 0 0 Deaths 0 0 0 0 0 Phase 2 TEAE Summary aOne patient with Bell’s palsy was reported as not treatment related, and resolved without sequelae. bOne patient with abdominal pain was reported as possibly treatment related. SAE, serious treatment-emergent adverse events; TEAE, treatment-emergent adverse event.
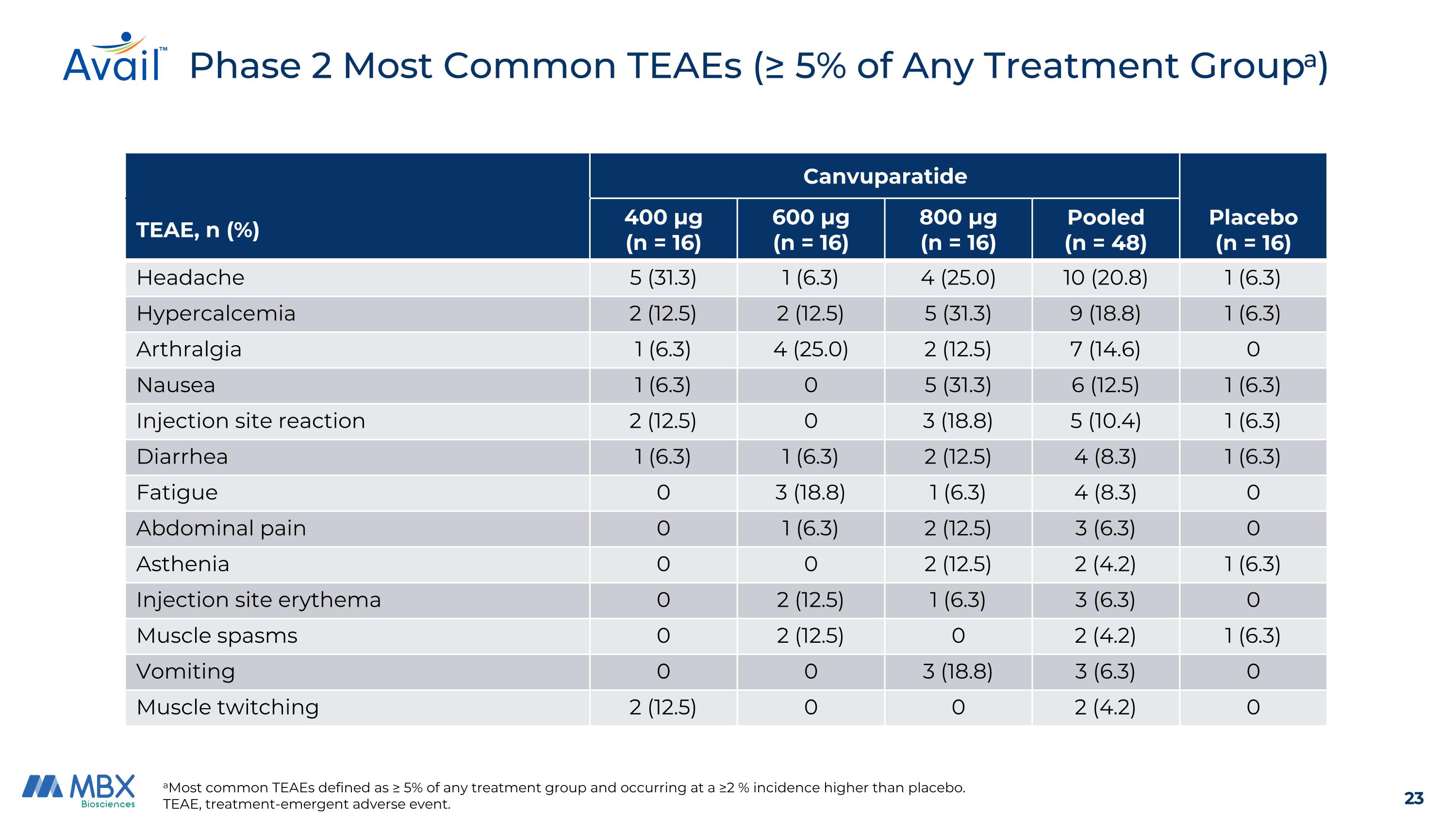
Phase 2 Most Common TEAEs (≥ 5% of Any Treatment Groupa) aMost common TEAEs defined as ≥ 5% of any treatment group and occurring at a ≥2 % incidence higher than placebo. TEAE, treatment-emergent adverse event. Canvuparatide Placebo (n = 16) TEAE, n (%) 400 μg (n = 16) 600 μg (n = 16) 800 μg (n = 16) Pooled (n = 48) Headache 5 (31.3) 1 (6.3) 4 (25.0) 10 (20.8) 1 (6.3) Hypercalcemia 2 (12.5) 2 (12.5) 5 (31.3) 9 (18.8) 1 (6.3) Arthralgia 1 (6.3) 4 (25.0) 2 (12.5) 7 (14.6) 0 Nausea 1 (6.3) 0 5 (31.3) 6 (12.5) 1 (6.3) Injection site reaction 2 (12.5) 0 3 (18.8) 5 (10.4) 1 (6.3) Diarrhea 1 (6.3) 1 (6.3) 2 (12.5) 4 (8.3) 1 (6.3) Fatigue 0 3 (18.8) 1 (6.3) 4 (8.3) 0 Abdominal pain 0 1 (6.3) 2 (12.5) 3 (6.3) 0 Asthenia 0 0 2 (12.5) 2 (4.2) 1 (6.3) Injection site erythema 0 2 (12.5) 1 (6.3) 3 (6.3) 0 Muscle spasms 0 2 (12.5) 0 2 (4.2) 1 (6.3) Vomiting 0 0 3 (18.8) 3 (6.3) 0 Muscle twitching 2 (12.5) 0 0 2 (4.2) 0
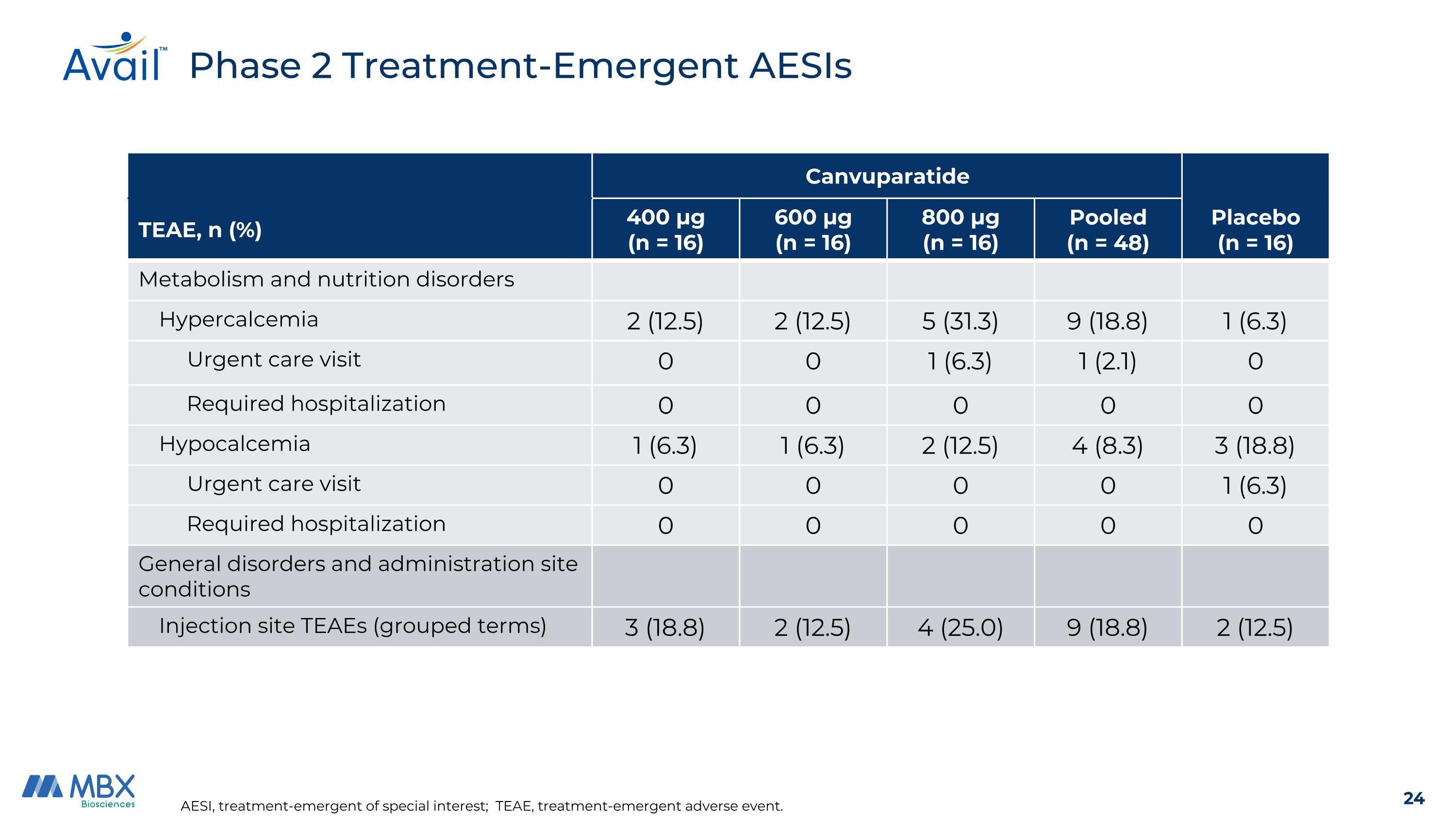
AESI, treatment-emergent of special interest; TEAE, treatment-emergent adverse event. Phase 2 Treatment-Emergent AESIs Canvuparatide Placebo (n = 16) TEAE, n (%) 400 μg (n = 16) 600 μg (n = 16) 800 μg (n = 16) Pooled (n = 48) Metabolism and nutrition disorders Hypercalcemia 2 (12.5) 2 (12.5) 5 (31.3) 9 (18.8) 1 (6.3) Urgent care visit 0 0 1 (6.3) 1 (2.1) 0 Required hospitalization 0 0 0 0 0 Hypocalcemia 1 (6.3) 1 (6.3) 2 (12.5) 4 (8.3) 3 (18.8) Urgent care visit 0 0 0 0 1 (6.3) Required hospitalization 0 0 0 0 0 General disorders and administration site conditions Injection site TEAEs (grouped terms) 3 (18.8) 2 (12.5) 4 (25.0) 9 (18.8) 2 (12.5)
Summary & Next Steps
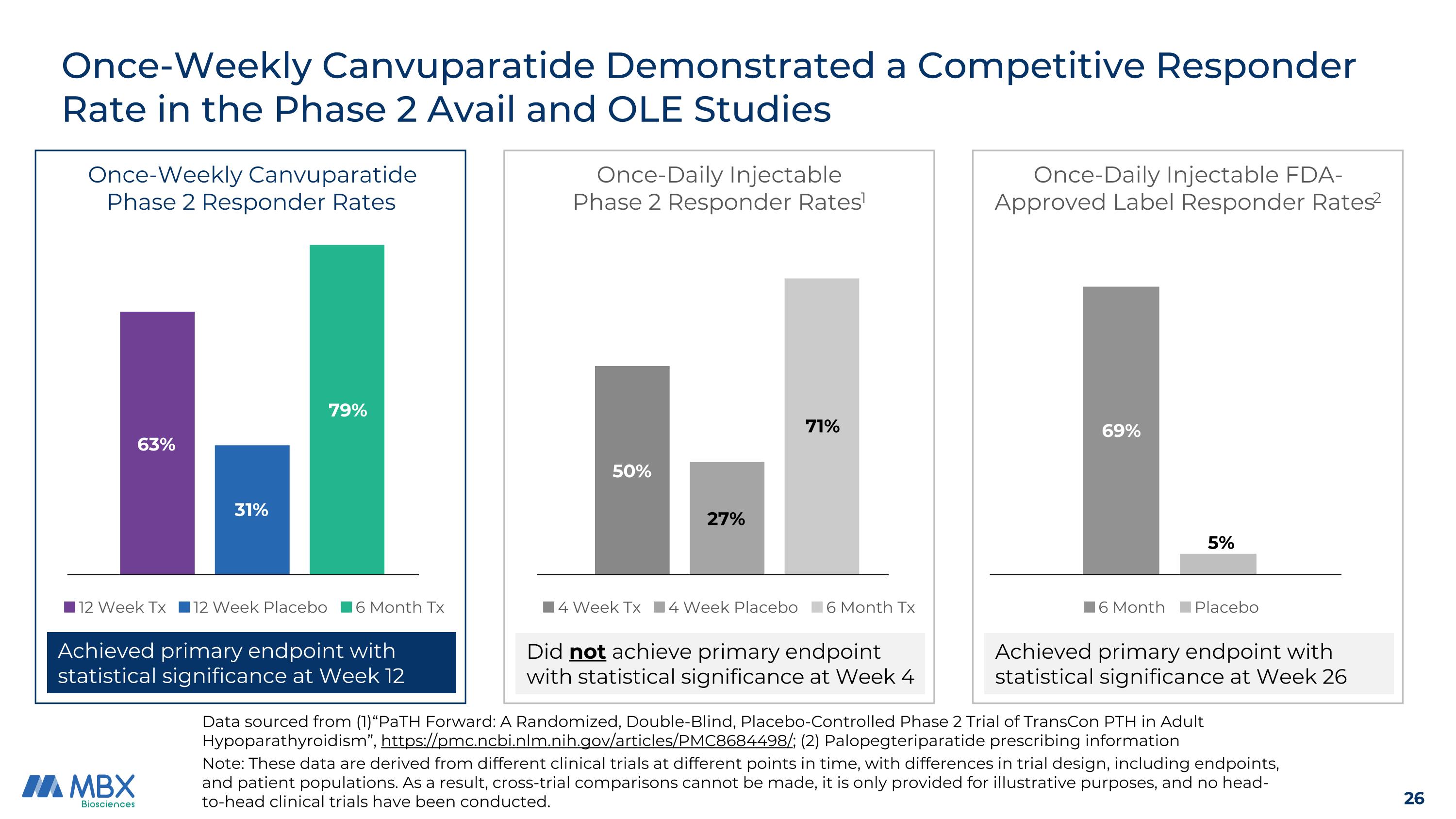
Once-Weekly Canvuparatide Demonstrated a Competitive Responder Rate in the Phase 2 Avail and OLE Studies Note: These data are derived from different clinical trials at different points in time, with differences in trial design, including endpoints, and patient populations. As a result, cross-trial comparisons cannot be made, it is only provided for illustrative purposes, and no head-to-head clinical trials have been conducted. Achieved primary endpoint with statistical significance at Week 12 Data sourced from (1)“PaTH Forward: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial of TransCon PTH in Adult Hypoparathyroidism”, https://pmc.ncbi.nlm.nih.gov/articles/PMC8684498/; (2) Palopegteriparatide prescribing information Did not achieve primary endpoint with statistical significance at Week 4 Achieved primary endpoint with statistical significance at Week 26 63% 31% 79% 71% 27% 50% 69% 5% Once-Weekly Canvuparatide Phase 2 Responder Rates Once-Daily Injectable Phase 2 Responder Rates1 Once-Daily Injectable FDA-Approved Label Responder Rates2
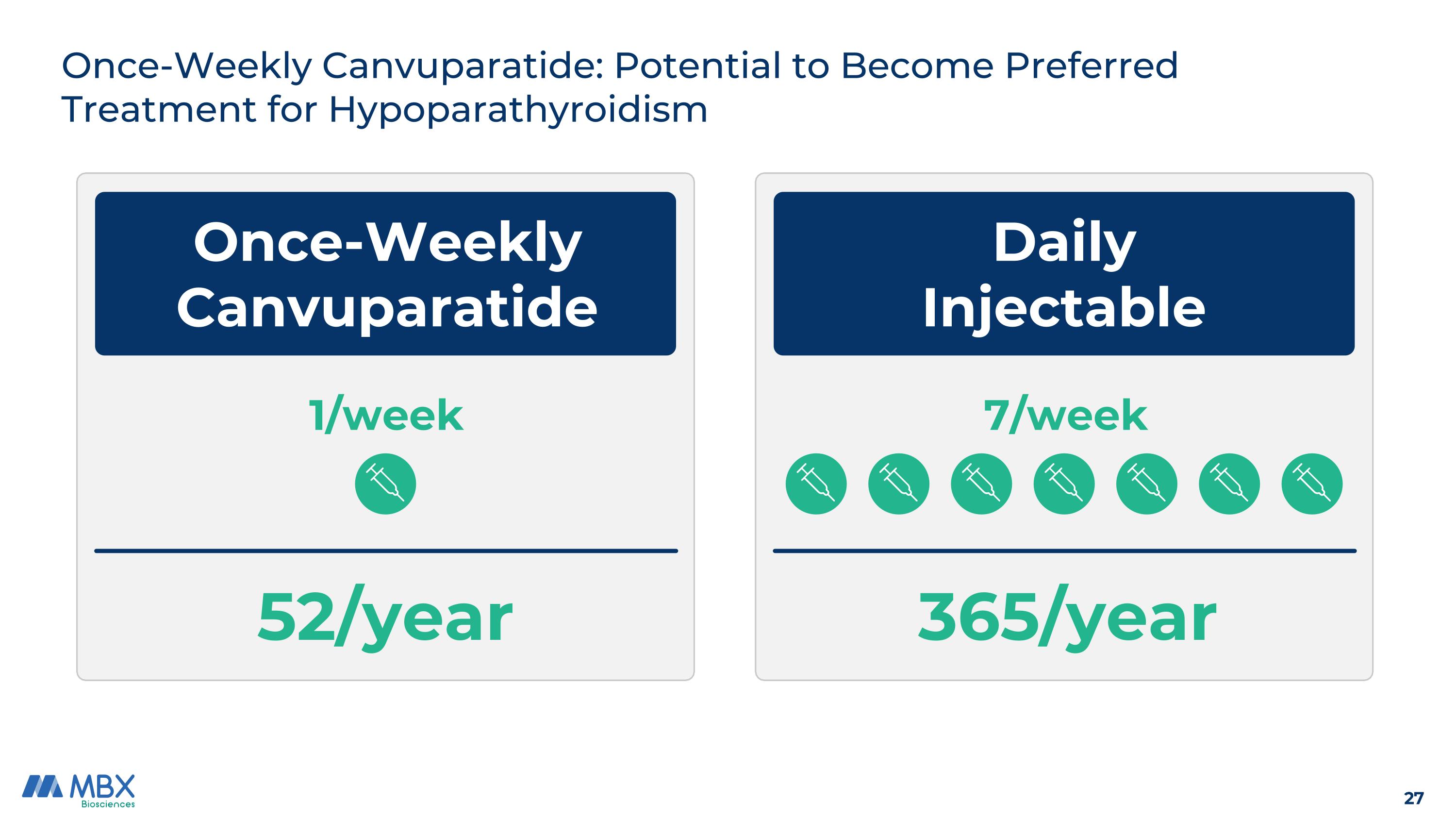
Once-Weekly Canvuparatide Daily Injectable 7/week Once-Weekly Canvuparatide: Potential to Become Preferred Treatment for Hypoparathyroidism 52/year 365/year 1/week

Plans for Advancing Potential Best-in-Class Canvuparatide Program Present Phase 2 topline results at the International HypoPARAthyroidism Conference (Oct 2025) Request End-of-Phase 2 meeting with FDA Share full Phase 2 results at major medical meeting and in publication Report 52-week OLE follow-up data in 2026 Plan to initiate global Phase 3 in 2026

We thank the patients and their caregivers, investigators, partners, and the passionate team at MBX who helped to make Avail™ a success www.mbxbio.com investors@mbxbio.com