

ACR-368 AND ACR-2316 CLINICAL DATA AND AP3 PIPELINE UPDATES ACRIVON CONFERENCE CALL January 8, 2026

Forward-looking statements Certain information contained in this presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 regarding our future results of operations or financial condition, business strategy and plans and objectives of management for future operations. In some cases, you can identify forward-looking statements because they contain words such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or the negative of these words or other similar terms or expressions. Our forward-looking statements are based primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and results of operations. The outcome of the events described in the forward-looking statements is subject to risks and uncertainties, including the factors described in our filings with the U.S. Securities and Exchange Commission. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this presentation. The results, events, and circumstances reflected in the forward-looking statements may not be achieved or occur, and actual results, events, or circumstances could differ materially from those described in the forward-looking statements. You are cautioned not to place undue reliance on these forward-looking statements, which are made only as of the date of this presentation. We undertake no obligation to update any forward-looking statements or to reflect new information or the occurrence of unanticipated events, except as required by law.

Peter Blume-Jensen M.D., Ph.D. CEO, President, and Co-Founder Inventor of the AP3 Platform Acrivon TEAM Participants Adam Levy Ph.D., M.B.A. Chief Financial Officer Mansoor Raza Mirza M.D. Chief Medical Officer

agenda Introduction and Review of the Agenda (Adam Levy) Brief Overview of Acrivon and Generative Phosphoproteomics: Transforming Drug Discovery with Proteome-Wide SAR (Peter Blume-Jensen) ACR-368 Phase 2b Clinical Data and Program Updates (Mansoor Raza Mirza) ACR-2316 Phase 1 Initial Clinical Data (Mansoor Raza Mirza and Peter Blume-Jensen) Nomination of Cell Cycle Program Development Candidate (Adam Levy) Closing remarks (Adam Levy and Peter Blume-Jensen) Q&A (Team)
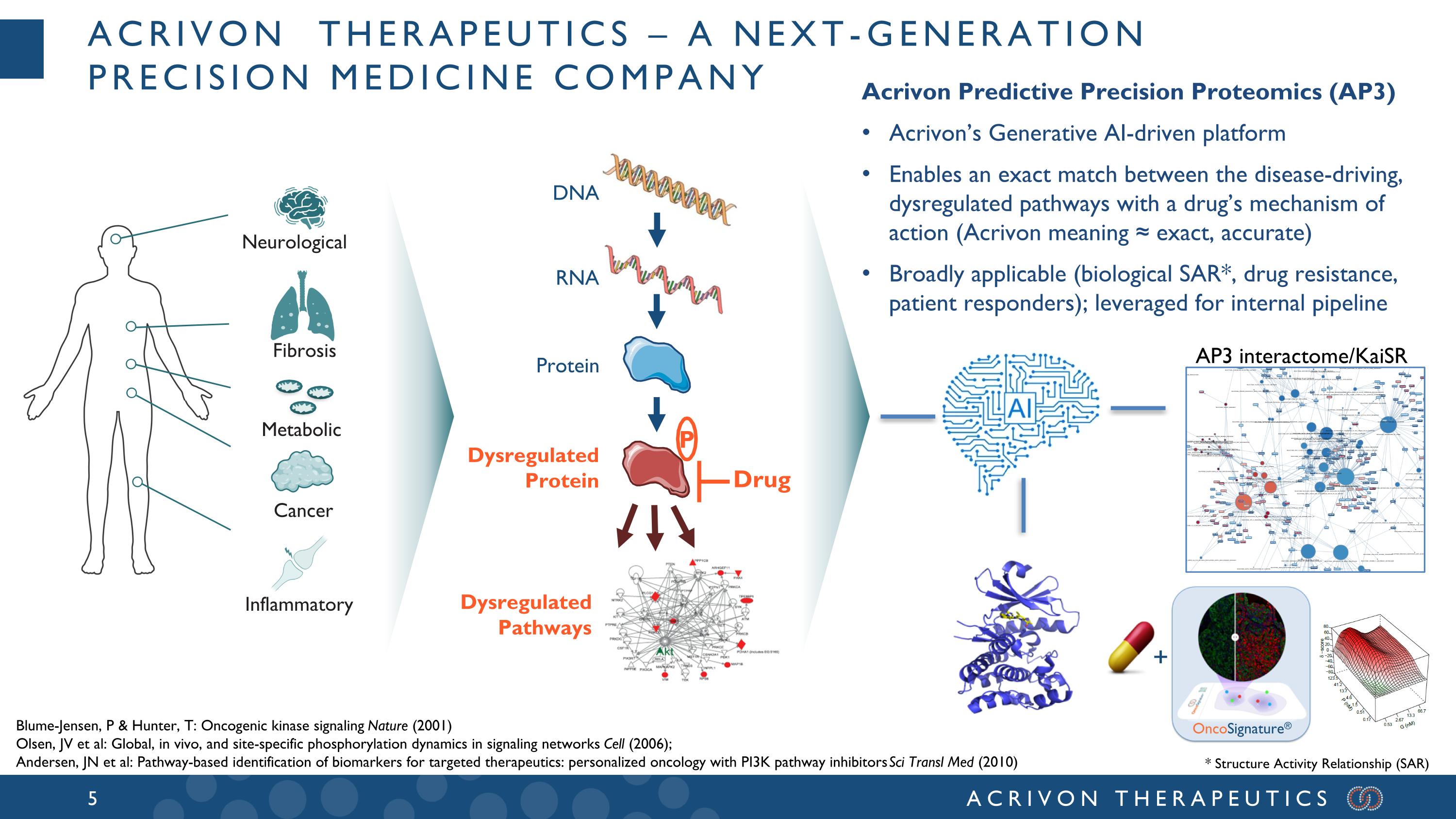
Acrivon Therapeutics – a next-generation precision medicine company Neurological Cancer Inflammatory Fibrosis Drug P Metabolic Acrivon Predictive Precision Proteomics (AP3) Acrivon’s Generative AI-driven platform Enables an exact match between the disease-driving, dysregulated pathways with a drug’s mechanism of action (Acrivon meaning ≈ exact, accurate) Broadly applicable (biological SAR*, drug resistance, patient responders); leveraged for internal pipeline DNA RNA Protein Dysregulated Protein Dysregulated Pathways + OncoSignature® Blume-Jensen, P & Hunter, T: Oncogenic kinase signaling Nature (2001) Olsen, JV et al: Global, in vivo, and site-specific phosphorylation dynamics in signaling networks Cell (2006); Andersen, JN et al: Pathway-based identification of biomarkers for targeted therapeutics: personalized oncology with PI3K pathway inhibitors Sci Transl Med (2010) AP3 interactome/KaiSR * Structure Activity Relationship (SAR)
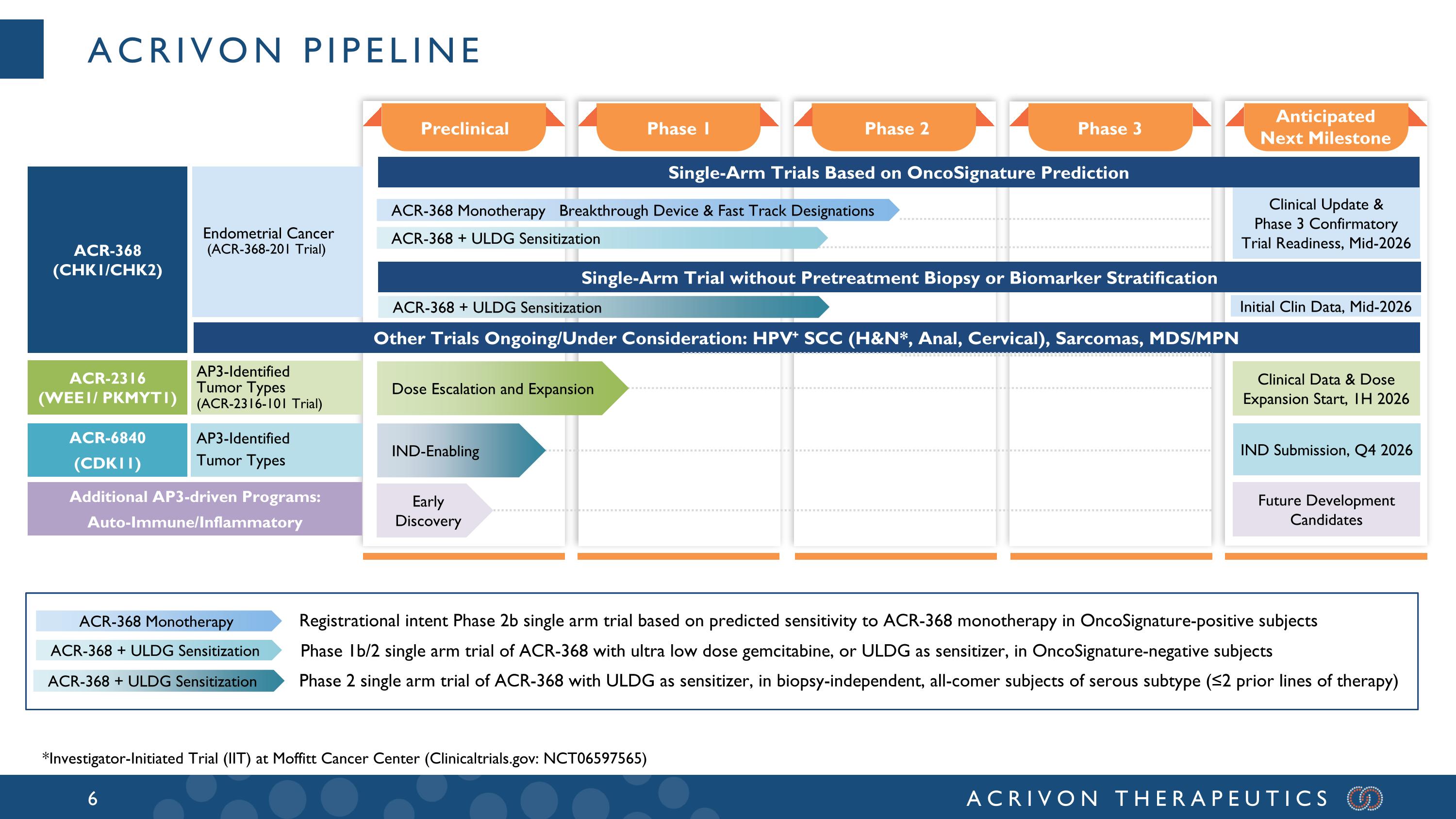
Acrivon Pipeline Anticipated Next Milestone Phase 3 Phase 2 Phase 1 Preclinical ACR-368 (CHK1/CHK2) Single-Arm Trials Based on OncoSignature Prediction Other Trials Ongoing/Under Consideration: HPV+ SCC (H&N*, Anal, Cervical), Sarcomas, MDS/MPN Clinical Data & Dose Expansion Start, 1H 2026 Clinical Update & Phase 3 Confirmatory Trial Readiness, Mid-2026 Endometrial Cancer (ACR-368-201 Trial) AP3-Identified Tumor Types (ACR-2316-101 Trial) Future Development Candidates Additional AP3-driven Programs: Auto-Immune/Inflammatory ACR-368 Monotherapy ACR-368 + ULDG Sensitization Registrational intent Phase 2b single arm trial based on predicted sensitivity to ACR-368 monotherapy in OncoSignature-positive subjects Phase 1b/2 single arm trial of ACR-368 with ultra low dose gemcitabine, or ULDG as sensitizer, in OncoSignature-negative subjects ACR-368 + ULDG Sensitization ACR-368 Monotherapy Breakthrough Device & Fast Track Designations ACR-2316 (WEE1/ PKMYT1) Early Discovery Dose Escalation and Expansion AP3-Identified Tumor Types ACR-6840 (CDK11) IND Submission, Q4 2026 IND-Enabling *Investigator-Initiated Trial (IIT) at Moffitt Cancer Center (Clinicaltrials.gov: NCT06597565) Single-Arm Trial without Pretreatment Biopsy or Biomarker Stratification ACR-368 + ULDG Sensitization Initial Clin Data, Mid-2026 ACR-368 + ULDG Sensitization Phase 2 single arm trial of ACR-368 with ULDG as sensitizer, in biopsy-independent, all-comer subjects of serous subtype (≤2 prior lines of therapy)
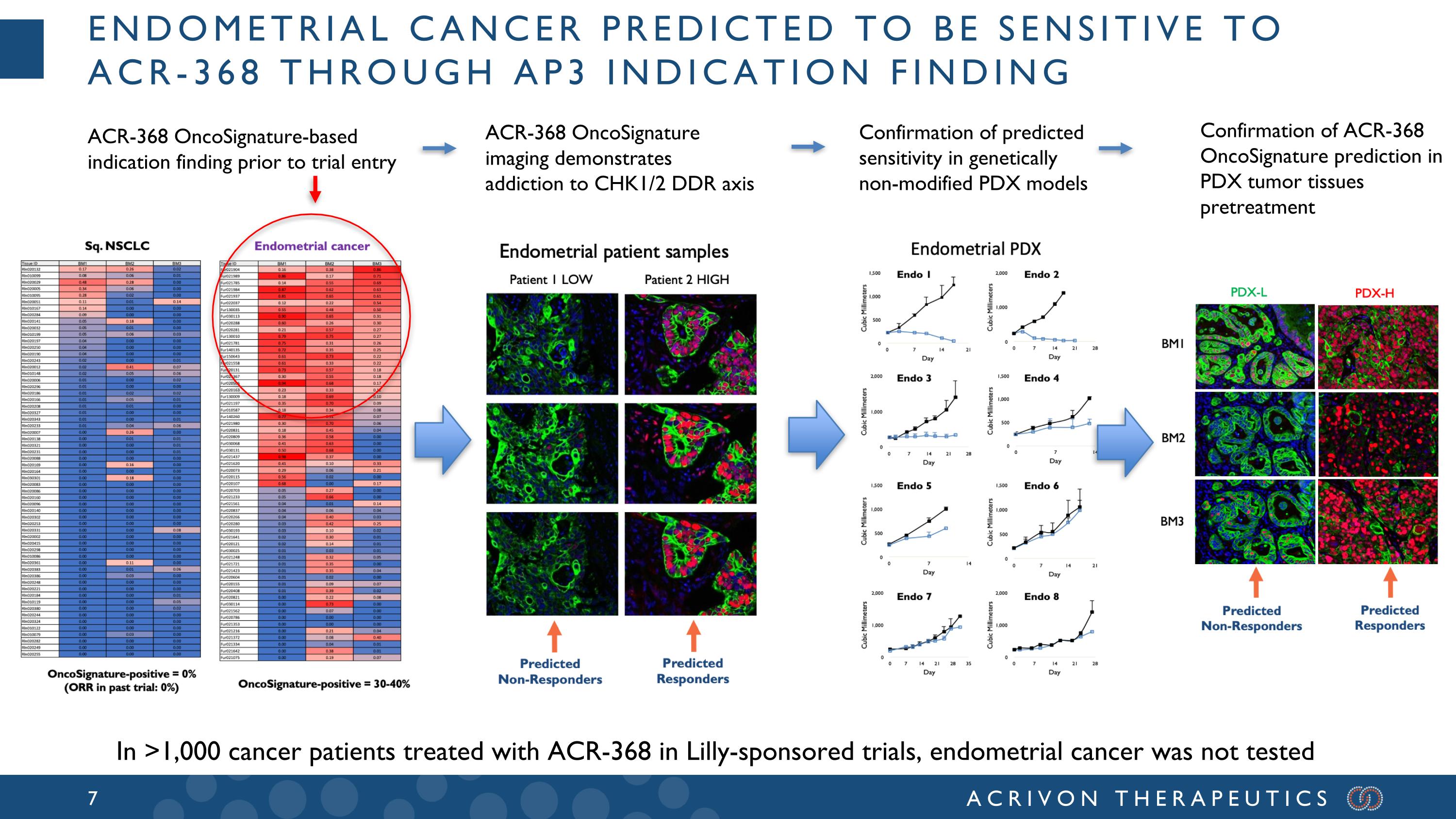
Endometrial cancer predicted to be sensitive to ACR-368 through ap3 indication finding ACR-368 OncoSignature-based indication finding prior to trial entry ACR-368 OncoSignature imaging demonstrates addiction to CHK1/2 DDR axis Confirmation of predicted sensitivity in genetically non-modified PDX models Confirmation of ACR-368 OncoSignature prediction in PDX tumor tissues pretreatment In >1,000 cancer patients treated with ACR-368 in Lilly-sponsored trials, endometrial cancer was not tested
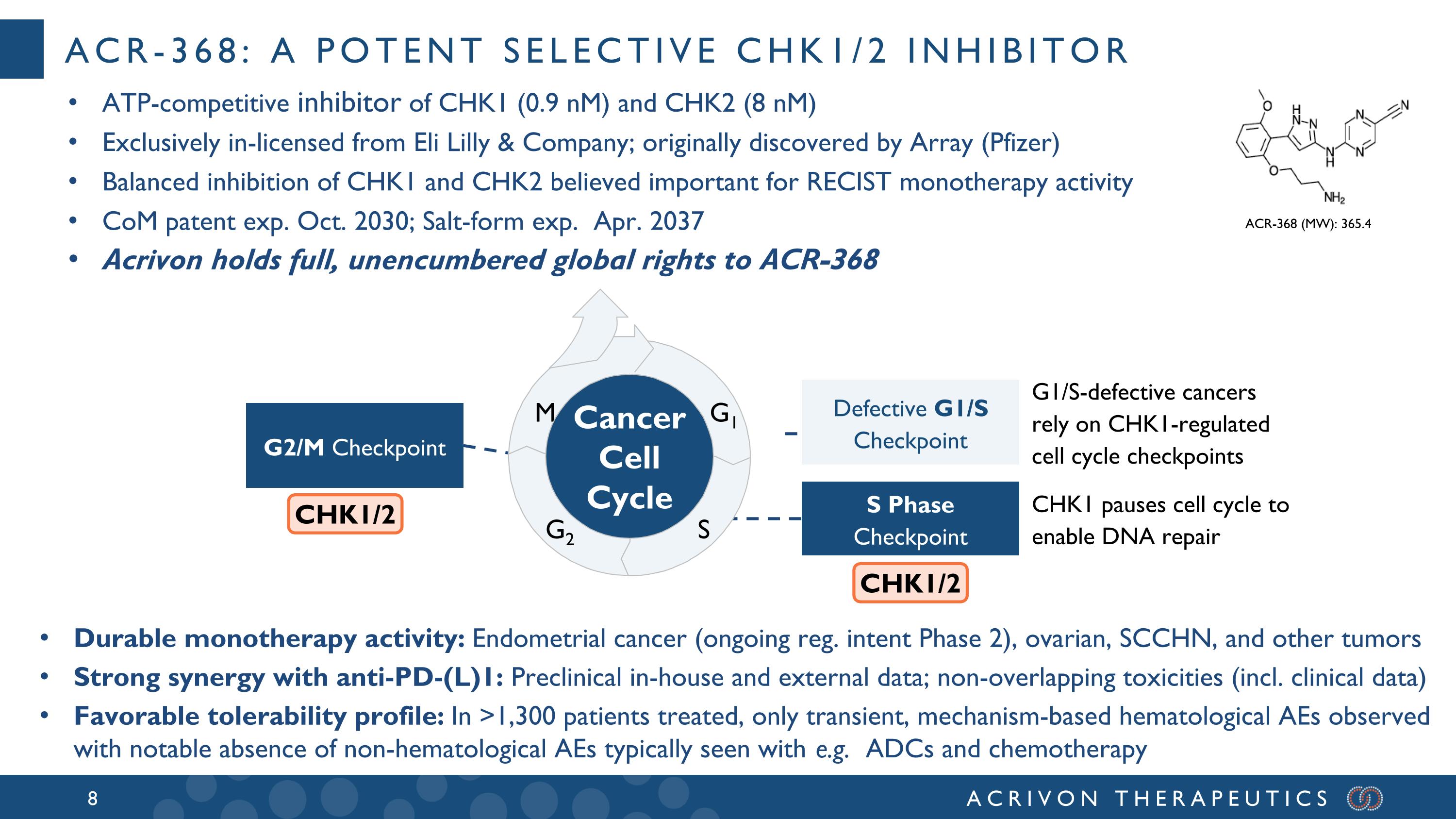
ACR-368: a potent selective CHK1/2 inhibitor ATP-competitive inhibitor of CHK1 (0.9 nM) and CHK2 (8 nM) Exclusively in-licensed from Eli Lilly & Company; originally discovered by Array (Pfizer) Balanced inhibition of CHK1 and CHK2 believed important for RECIST monotherapy activity CoM patent exp. Oct. 2030; Salt-form exp. Apr. 2037 Acrivon holds full, unencumbered global rights to ACR-368 ACR-368 (MW): 365.4 Durable monotherapy activity: Endometrial cancer (ongoing reg. intent Phase 2), ovarian, SCCHN, and other tumors Strong synergy with anti-PD-(L)1: Preclinical in-house and external data; non-overlapping toxicities (incl. clinical data) Favorable tolerability profile: In >1,300 patients treated, only transient, mechanism-based hematological AEs observed with notable absence of non-hematological AEs typically seen with e.g. ADCs and chemotherapy G1/S-defective cancers rely on CHK1-regulated cell cycle checkpoints CHK1 pauses cell cycle to enable DNA repair G2/M Checkpoint S Phase Checkpoint M G1 S Cancer Cell Cycle G2 CHK1/2 CHK1/2 Defective G1/S Checkpoint
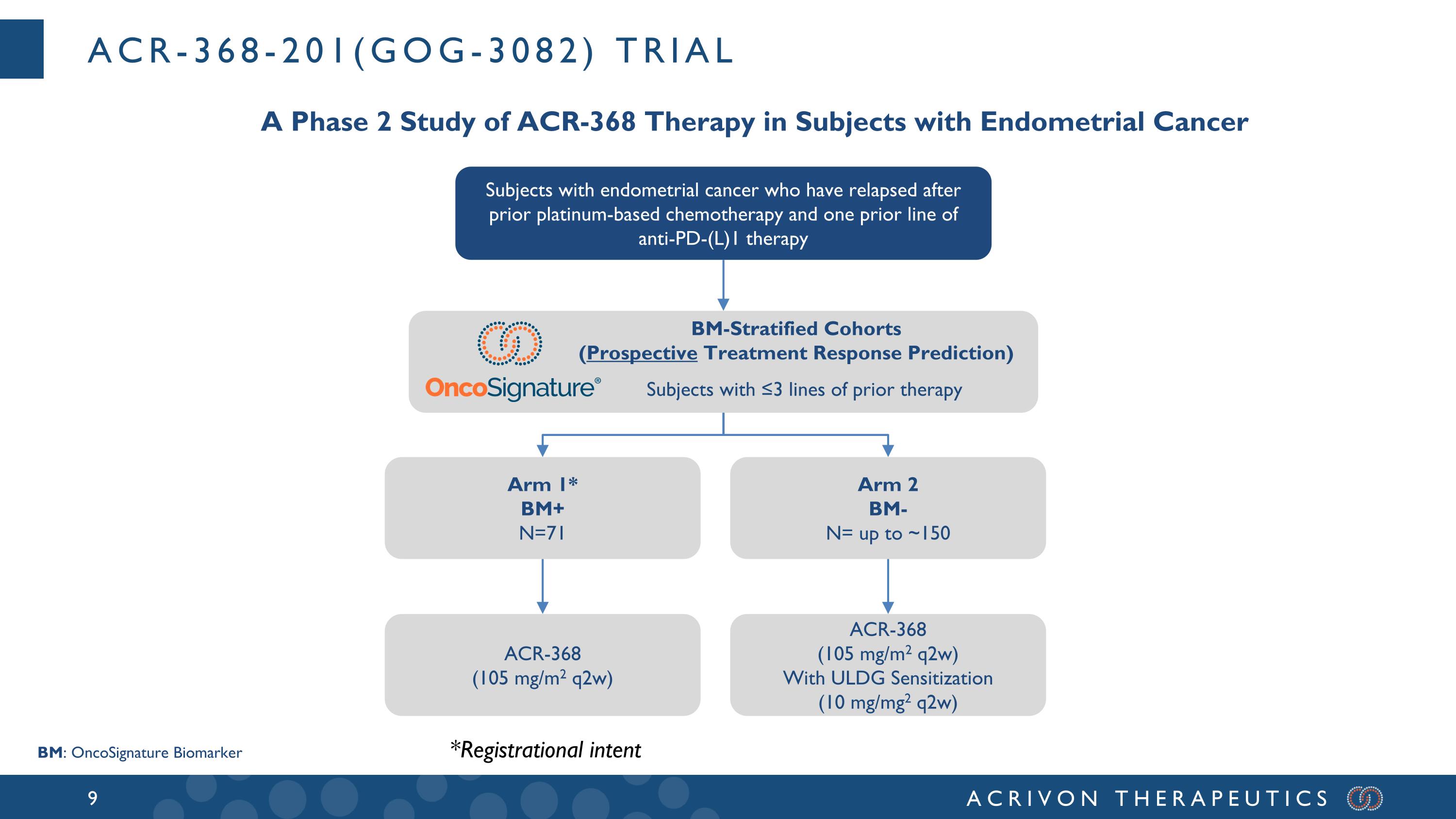
ACR-368-201(GOG-3082) trial A Phase 2 Study of ACR-368 Therapy in Subjects with Endometrial Cancer Subjects with endometrial cancer who have relapsed after prior platinum-based chemotherapy and one prior line of anti-PD-(L)1 therapy BM: OncoSignature Biomarker BM-Stratified Cohorts (Prospective Treatment Response Prediction) Subjects with ≤3 lines of prior therapy Arm 1* BM+ N=71 Arm 2 BM- N= up to ~150 ACR-368 (105 mg/m2 q2w) With ULDG Sensitization (10 mg/mg2 q2w) ACR-368 (105 mg/m2 q2w) *Registrational intent
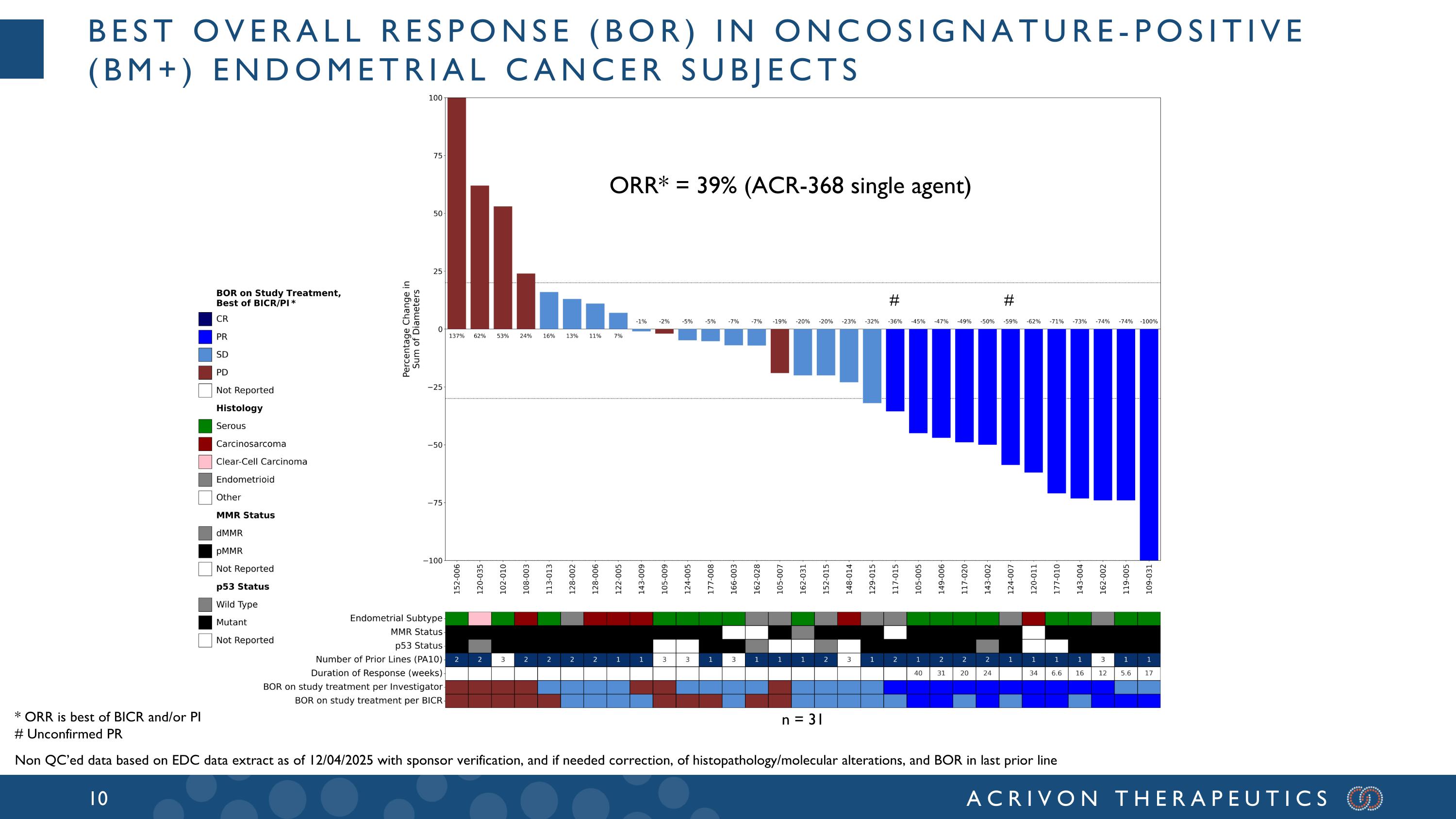
* ORR is best of BICR and/or PI # Unconfirmed PR Non QC’ed data based on EDC data extract as of 12/04/2025 with sponsor verification, and if needed correction, of histopathology/molecular alterations, and BOR in last prior line # # ORR* = 39% (ACR-368 single agent) n = 31 Best overall response (BOR) in oncosignature-positive (BM+) endometrial cancer subjects
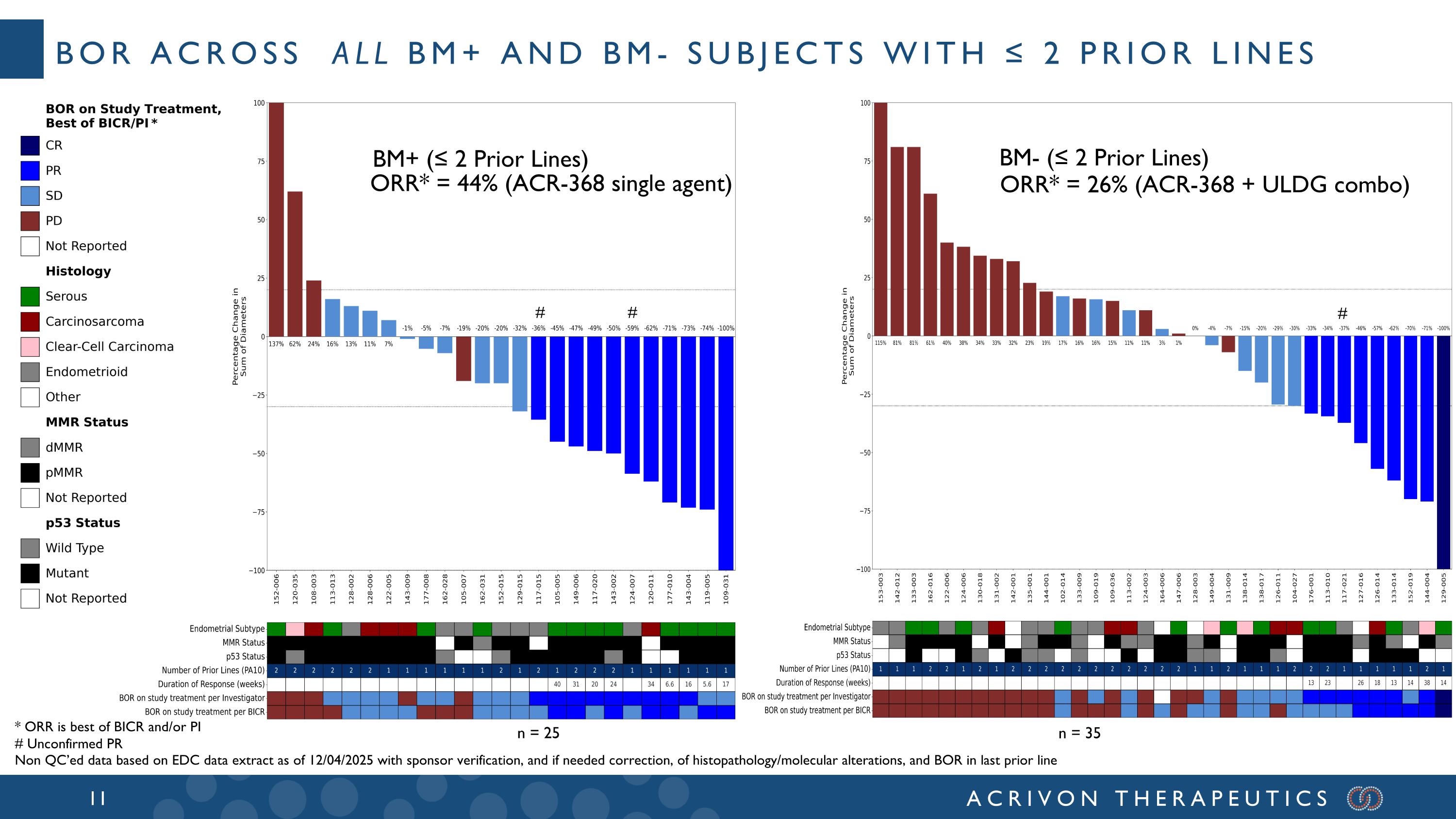
BOR across all BM+ and BM- subjects with ≤ 2 Prior lines n = 25 n = 35 ORR* = 26% (ACR-368 + ULDG combo) ORR* = 44% (ACR-368 single agent) # # # BM+ (≤ 2 Prior Lines) BM- (≤ 2 Prior Lines) * ORR is best of BICR and/or PI # Unconfirmed PR Non QC’ed data based on EDC data extract as of 12/04/2025 with sponsor verification, and if needed correction, of histopathology/molecular alterations, and BOR in last prior line
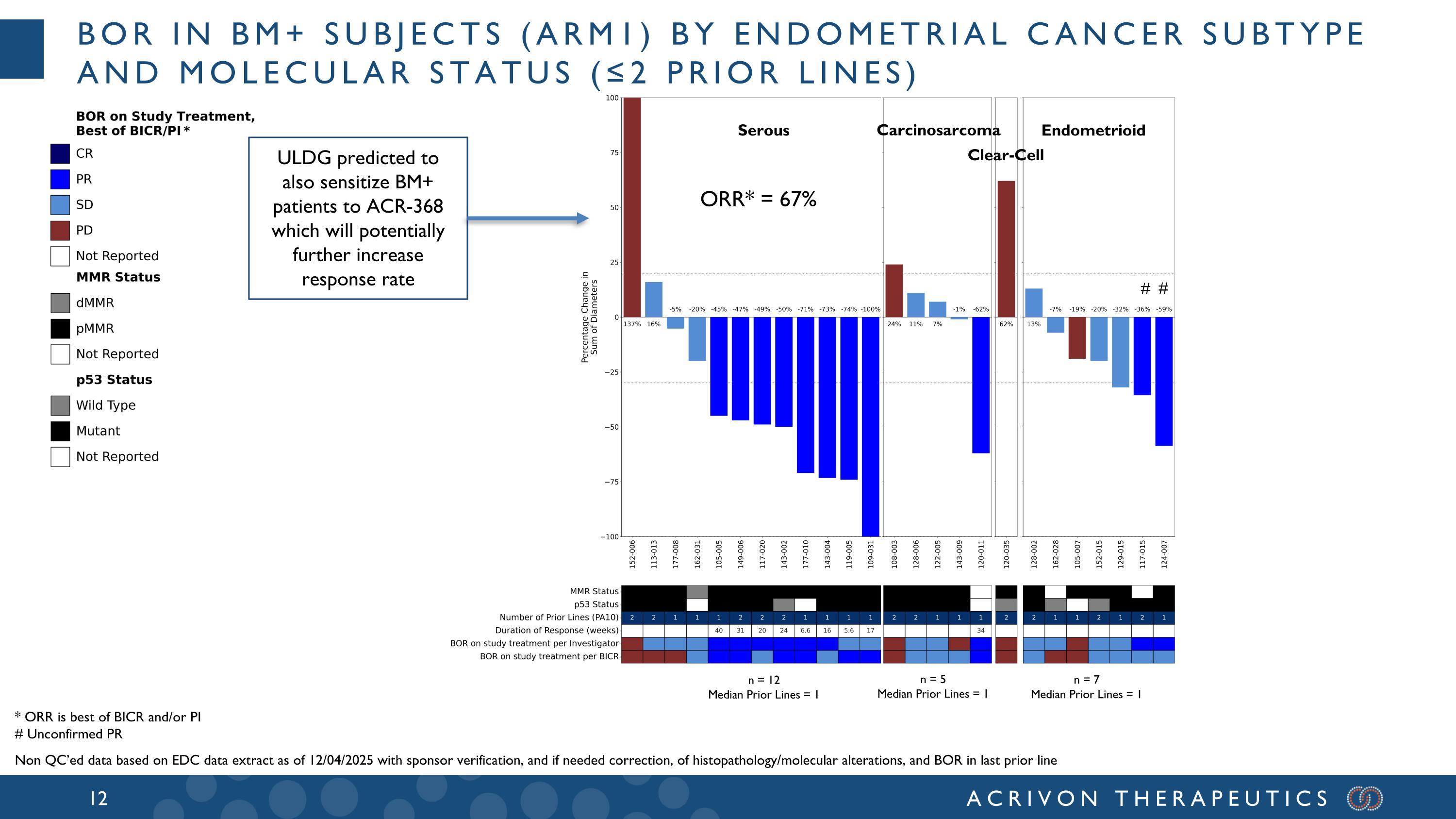
n = 12 Median Prior Lines = 1 n = 5 Median Prior Lines = 1 n = 7 Median Prior Lines = 1 Serous Carcinosarcoma Endometrioid Clear-Cell # # ORR* = 67% BoR in BM+ subjects (arm1) by endometrial cancer subtype and molecular status (≤2 prior lines) ULDG predicted to also sensitize BM+ patients to ACR-368 which will potentially further increase response rate * ORR is best of BICR and/or PI # Unconfirmed PR Non QC’ed data based on EDC data extract as of 12/04/2025 with sponsor verification, and if needed correction, of histopathology/molecular alterations, and BOR in last prior line
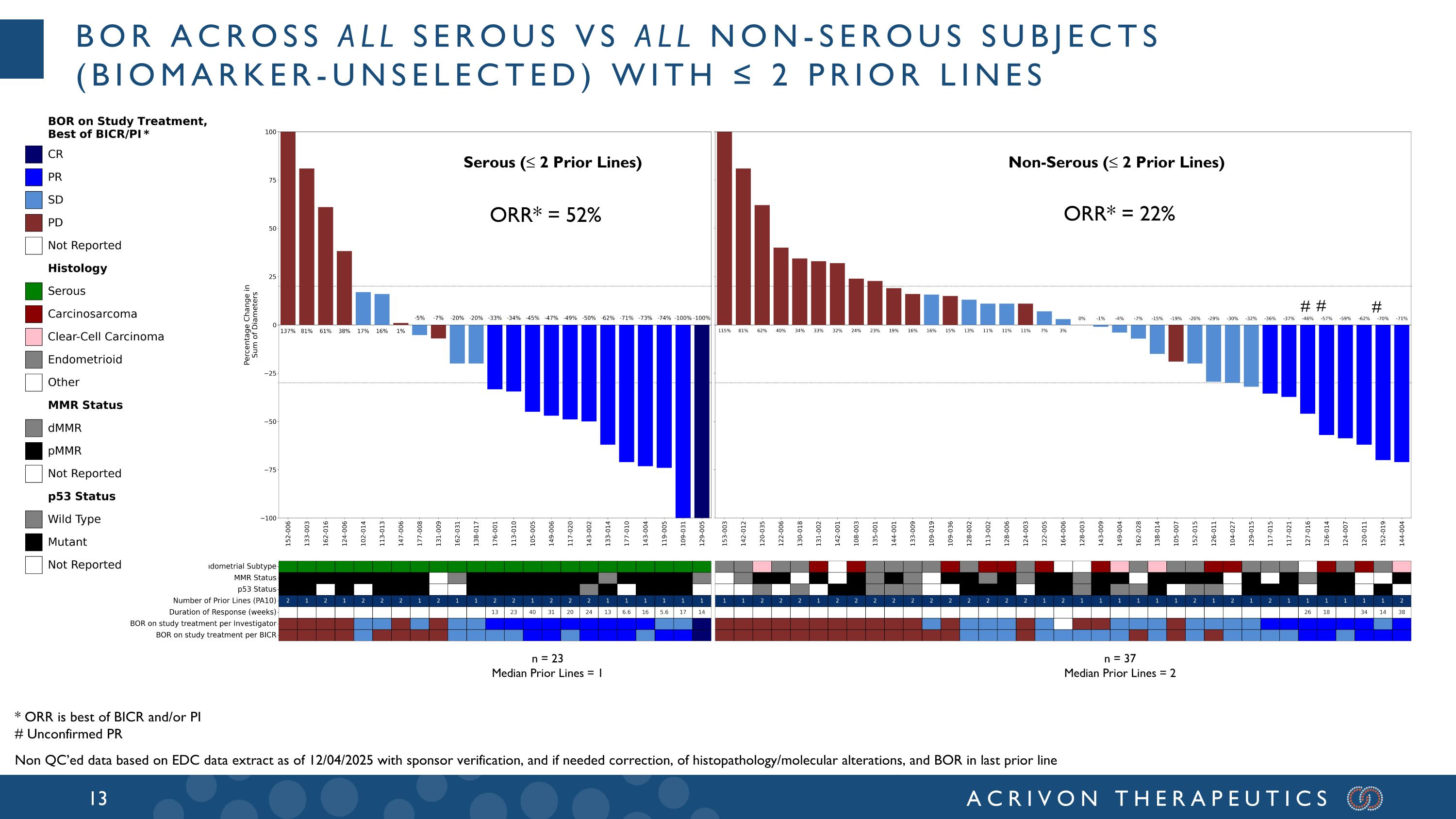
# BOR across all serous vs all non-serous subjects (biomarker-unselected) with ≤ 2 Prior lines n = 23 Median Prior Lines = 1 n = 37 Median Prior Lines = 2 Serous (≤ 2 Prior Lines) Non-Serous (≤ 2 Prior Lines) # # ORR* = 22% ORR* = 52% * ORR is best of BICR and/or PI # Unconfirmed PR Non QC’ed data based on EDC data extract as of 12/04/2025 with sponsor verification, and if needed correction, of histopathology/molecular alterations, and BOR in last prior line
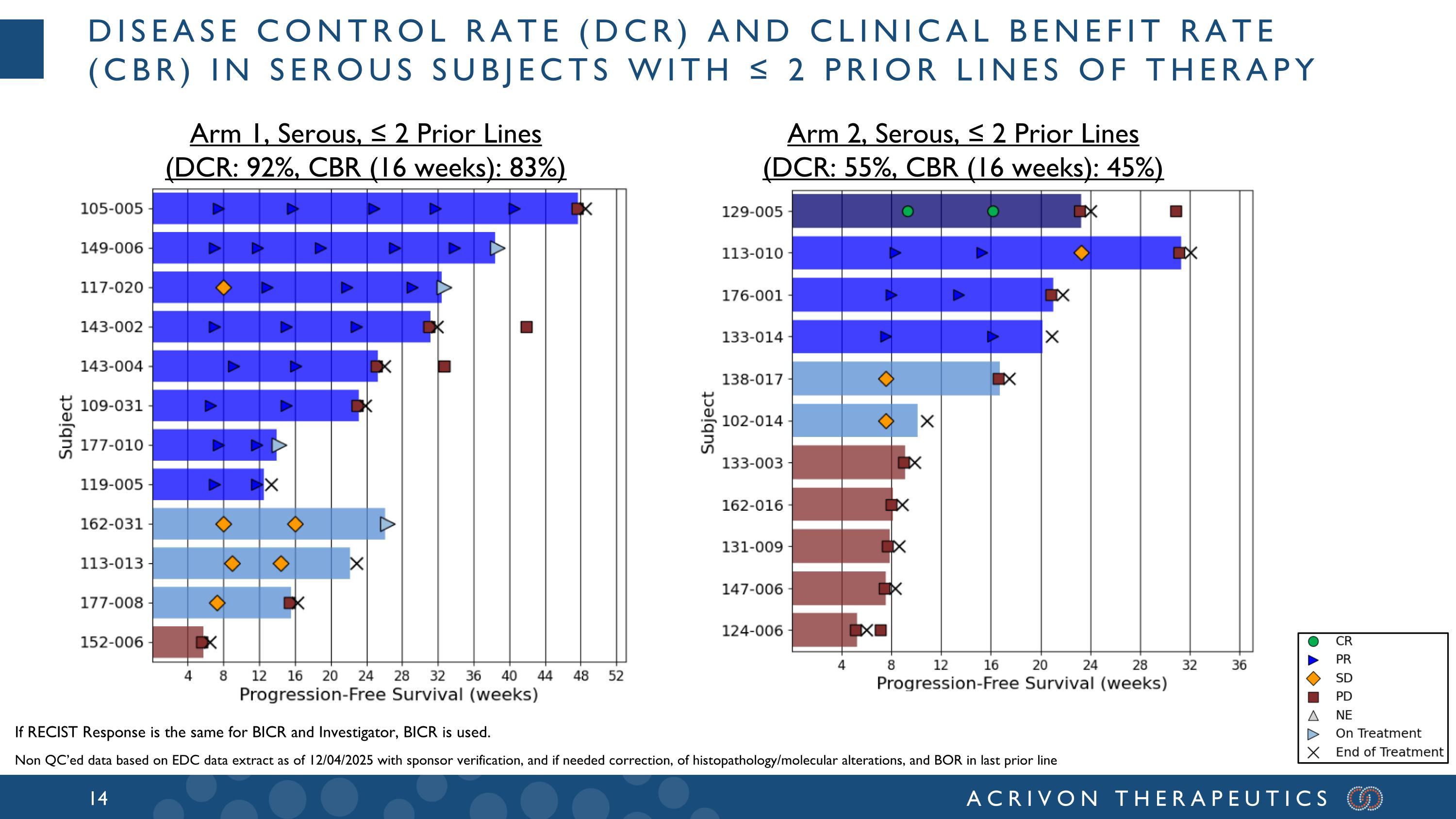
Arm 2, Serous, ≤ 2 Prior Lines (DCR: 55%, CBR (16 weeks): 45%) Arm 1, Serous, ≤ 2 Prior Lines (DCR: 92%, CBR (16 weeks): 83%) If RECIST Response is the same for BICR and Investigator, BICR is used. Disease control rate (DCR) and clinical benefit rate (CBR) in serous subjects with ≤ 2 prior lines of therapy Non QC’ed data based on EDC data extract as of 12/04/2025 with sponsor verification, and if needed correction, of histopathology/molecular alterations, and BOR in last prior line
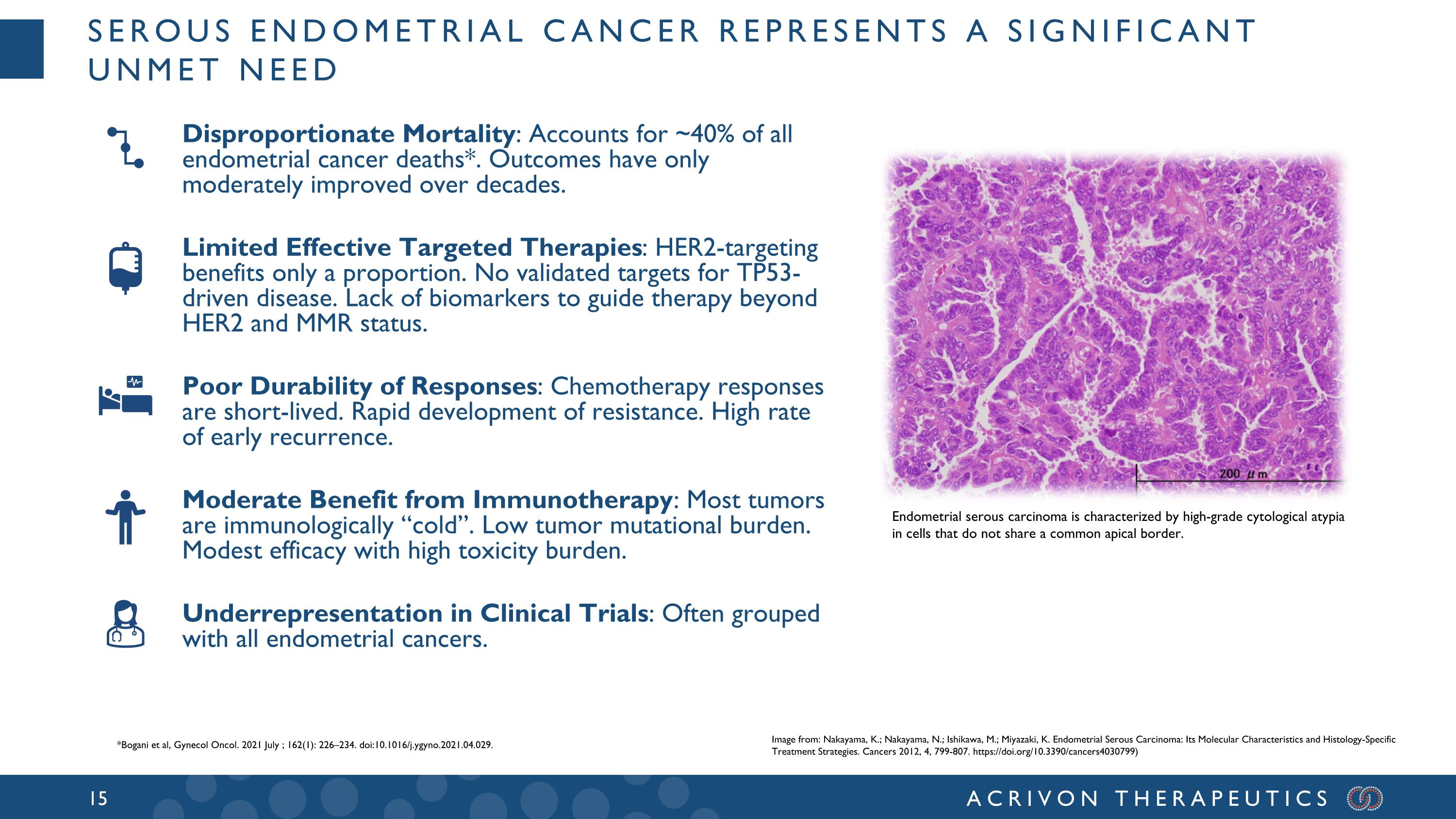
Serous endometrial cancer represents a significant unmet need Disproportionate Mortality: Accounts for ~40% of all endometrial cancer deaths*. Outcomes have only moderately improved over decades. Limited Effective Targeted Therapies: HER2-targeting benefits only a proportion. No validated targets for TP53-driven disease. Lack of biomarkers to guide therapy beyond HER2 and MMR status. Poor Durability of Responses: Chemotherapy responses are short-lived. Rapid development of resistance. High rate of early recurrence. Moderate Benefit from Immunotherapy: Most tumors are immunologically “cold”. Low tumor mutational burden. Modest efficacy with high toxicity burden. Underrepresentation in Clinical Trials: Often grouped with all endometrial cancers. Endometrial serous carcinoma is characterized by high-grade cytological atypia in cells that do not share a common apical border. Image from: Nakayama, K.; Nakayama, N.; Ishikawa, M.; Miyazaki, K. Endometrial Serous Carcinoma: Its Molecular Characteristics and Histology-Specific Treatment Strategies. Cancers 2012, 4, 799-807. https://doi.org/10.3390/cancers4030799) *Bogani et al, Gynecol Oncol. 2021 July ; 162(1): 226–234. doi:10.1016/j.ygyno.2021.04.029.
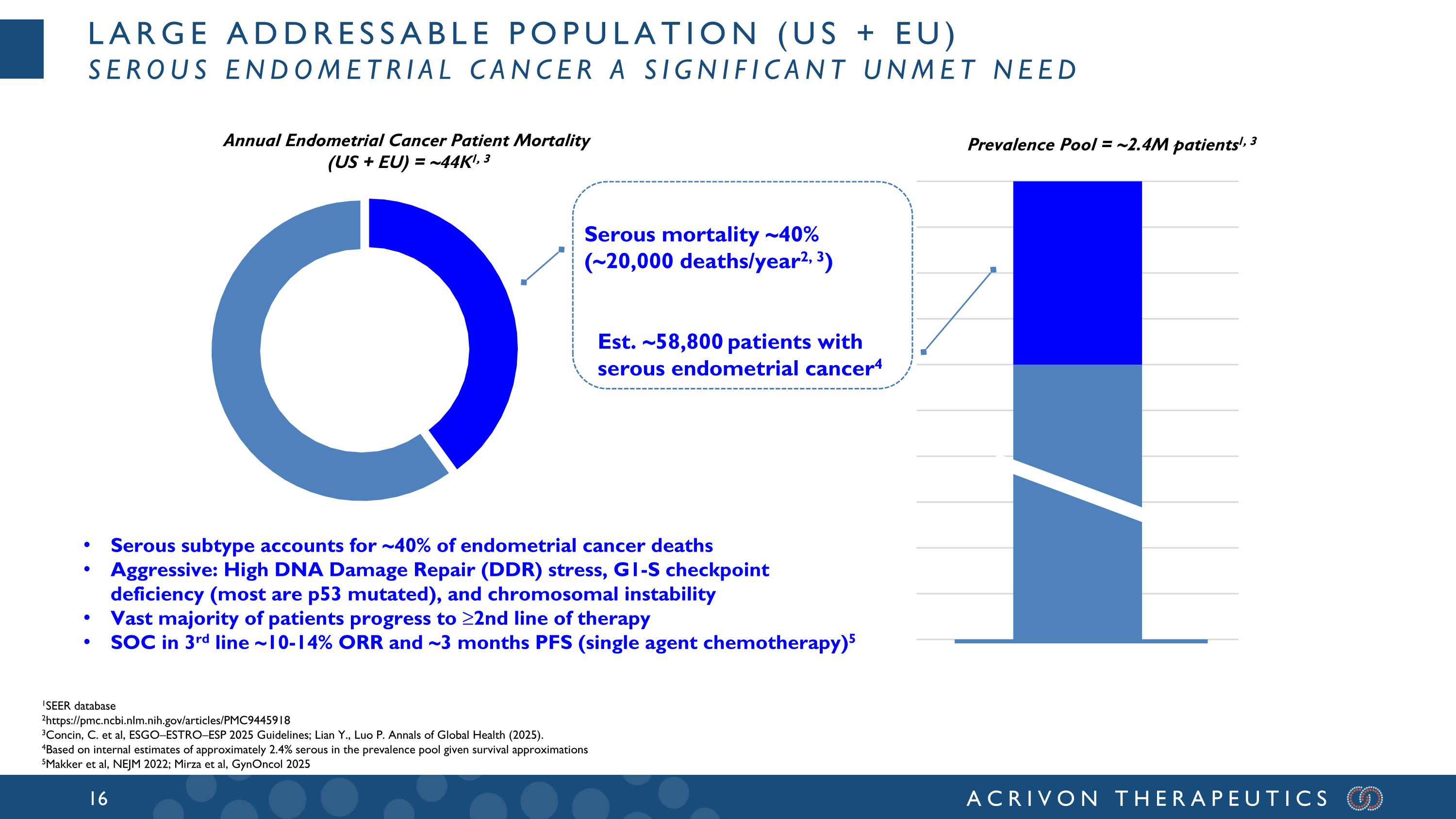
Large addressable population (US + EU) Serous endometrial cancer a significant unmet need Annual Endometrial Cancer Patient Mortality (US + EU) = ~44K1, 3 1SEER database 2https://pmc.ncbi.nlm.nih.gov/articles/PMC9445918 3Concin, C. et al, ESGO–ESTRO–ESP 2025 Guidelines; Lian Y., Luo P. Annals of Global Health (2025). 4Based on internal estimates of approximately 2.4% serous in the prevalence pool given survival approximations 5Makker et al, NEJM 2022; Mirza et al, GynOncol 2025 Serous mortality ~40% (~20,000 deaths/year2, 3) Prevalence Pool = ~2.4M patients1, 3 Est. ~58,800 patients with serous endometrial cancer4 Serous subtype accounts for ~40% of endometrial cancer deaths Aggressive: High DNA Damage Repair (DDR) stress, G1-S checkpoint deficiency (most are p53 mutated), and chromosomal instability Vast majority of patients progress to ≥2nd line of therapy SOC in 3rd line ~10-14% ORR and ~3 months PFS (single agent chemotherapy)5
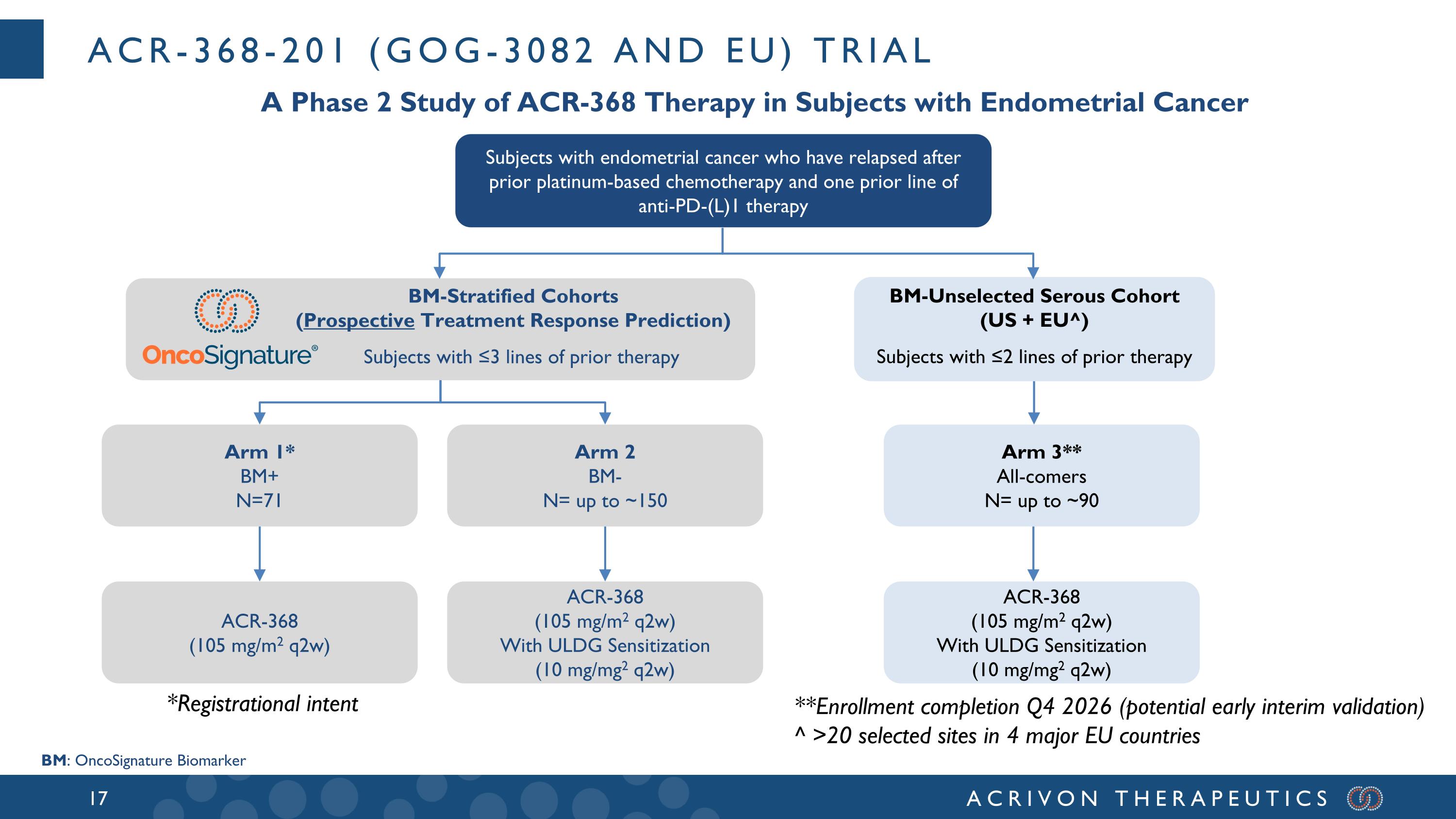
ACR-368-201 (GOG-3082 and EU) trial A Phase 2 Study of ACR-368 Therapy in Subjects with Endometrial Cancer Subjects with endometrial cancer who have relapsed after prior platinum-based chemotherapy and one prior line of anti-PD-(L)1 therapy BM-Stratified Cohorts (Prospective Treatment Response Prediction) Subjects with ≤3 lines of prior therapy Arm 1* BM+ N=71 Arm 2 BM- N= up to ~150 ACR-368 (105 mg/m2 q2w) With ULDG Sensitization (10 mg/mg2 q2w) ACR-368 (105 mg/m2 q2w) *Registrational intent BM: OncoSignature Biomarker BM-Unselected Serous Cohort (US + EU^) Subjects with ≤2 lines of prior therapy Arm 3** All-comers N= up to ~90 ACR-368 (105 mg/m2 q2w) With ULDG Sensitization (10 mg/mg2 q2w) **Enrollment completion Q4 2026 (potential early interim validation) ^ >20 selected sites in 4 major EU countries
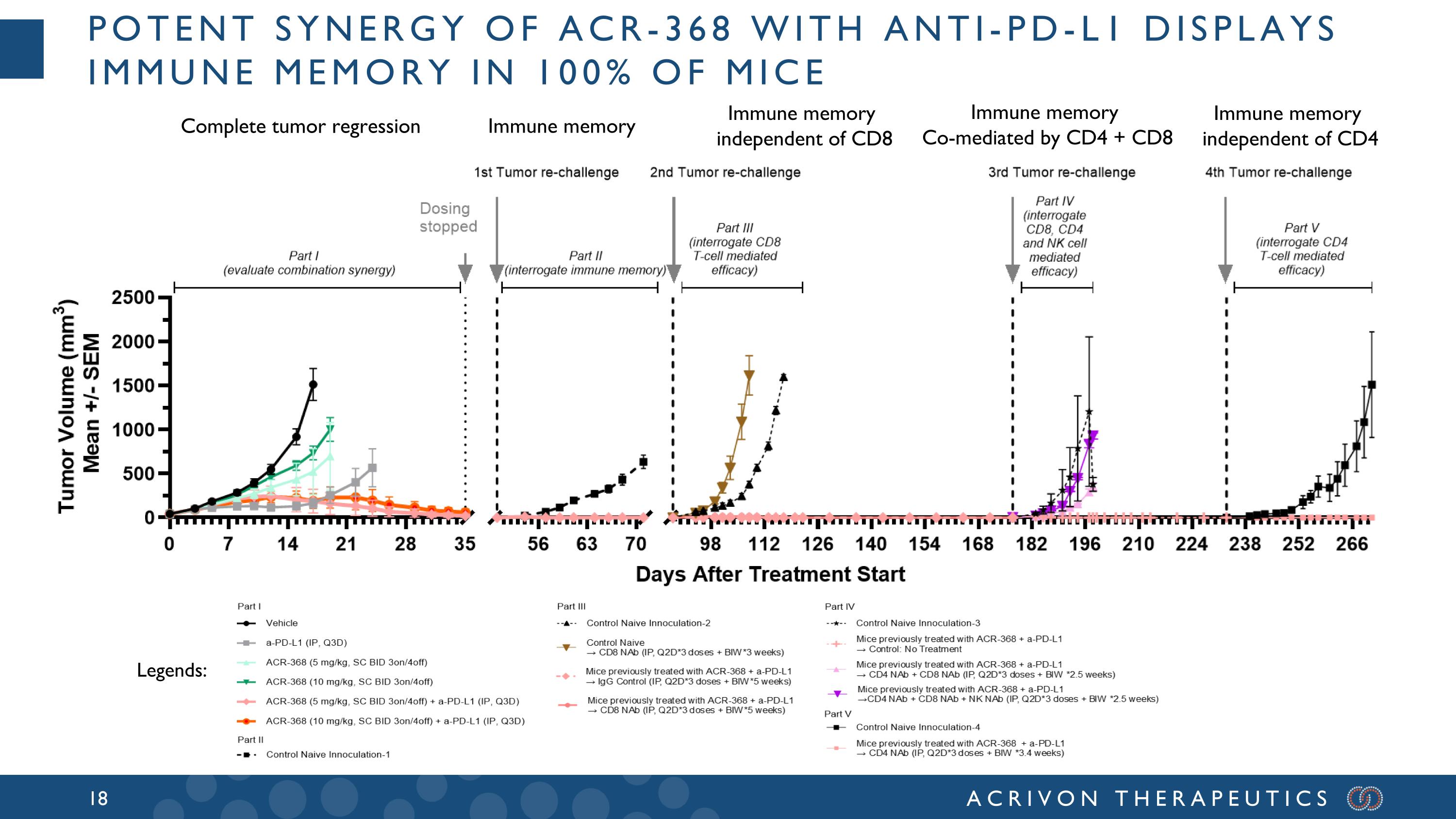
Potent synergy of ACR-368 with anti-PD-L1 displays immune Memory in 100% of Mice Complete tumor regression Immune memory Immune memory independent of CD8 Immune memory independent of CD4 Immune memory Co-mediated by CD4 + CD8 Legends:
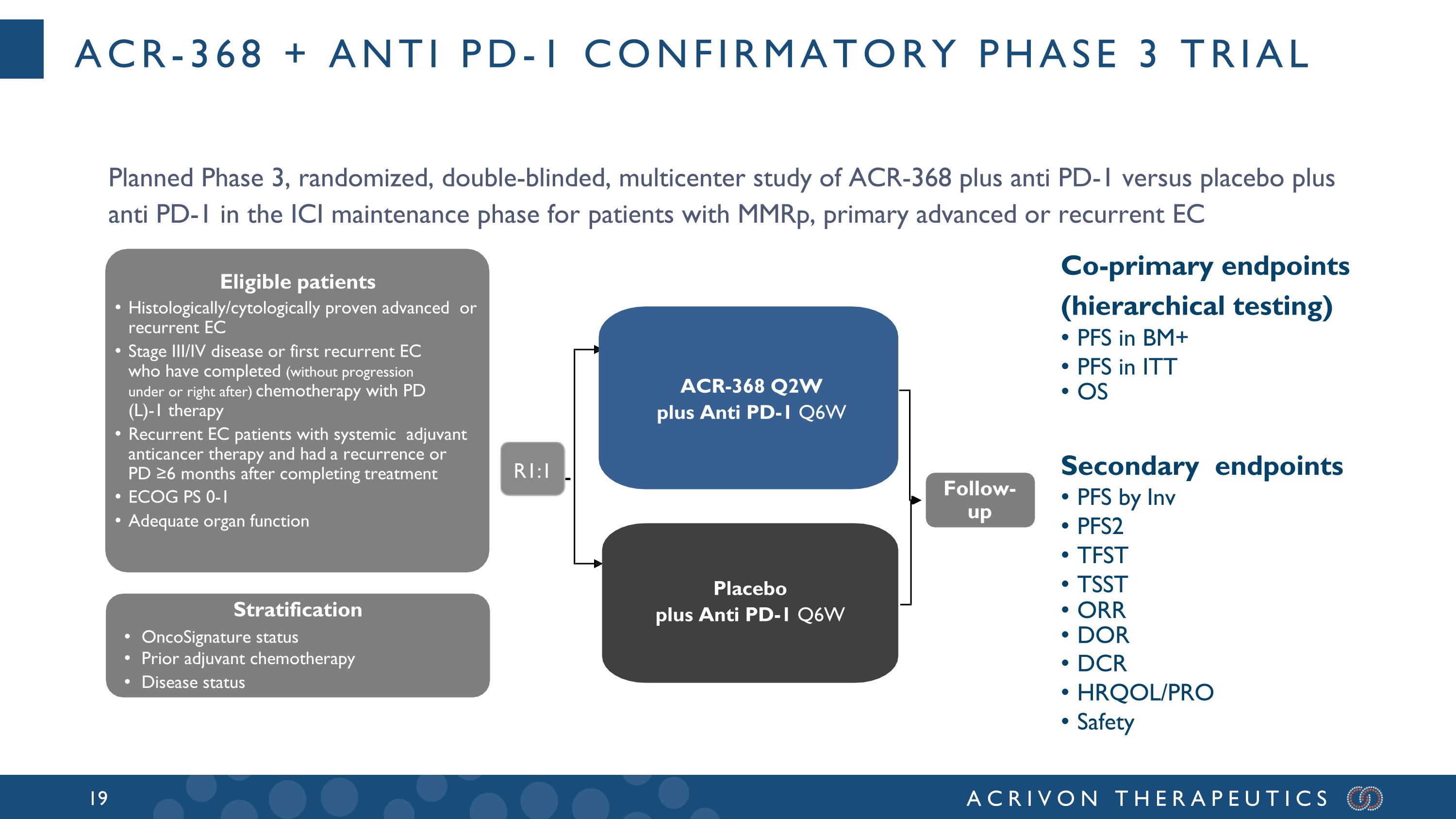
ACR-368 + Anti PD-1 confirmatory phase 3 trial Placebo Carboplatin AUC 5 mg/mL/min Paclitaxel 175 mg/m2 Q3W for 6 cycles ACR-368 Q2W plus Anti PD-1 Q6W Placebo plus Anti PD-1 Q6W Follow- up R1:1 Eligible patients Histologically/cytologically proven advanced or recurrent EC Stage III/IV disease or first recurrent EC who have completed (without progression under or right after) chemotherapy with PD (L)-1 therapy Recurrent EC patients with systemic adjuvant anticancer therapy and had a recurrence or PD ≥6 months after completing treatment ECOG PS 0-1 Adequate organ function Stratification OncoSignature status Prior adjuvant chemotherapy Disease status Co-primary endpoints (hierarchical testing) PFS in BM+ PFS in ITT OS Secondary endpoints PFS by Inv PFS2 TFST TSST ORR DOR DCR HRQOL/PRO Safety Planned Phase 3, randomized, double-blinded, multicenter study of ACR-368 plus anti PD-1 versus placebo plus anti PD-1 in the ICI maintenance phase for patients with MMRp, primary advanced or recurrent EC
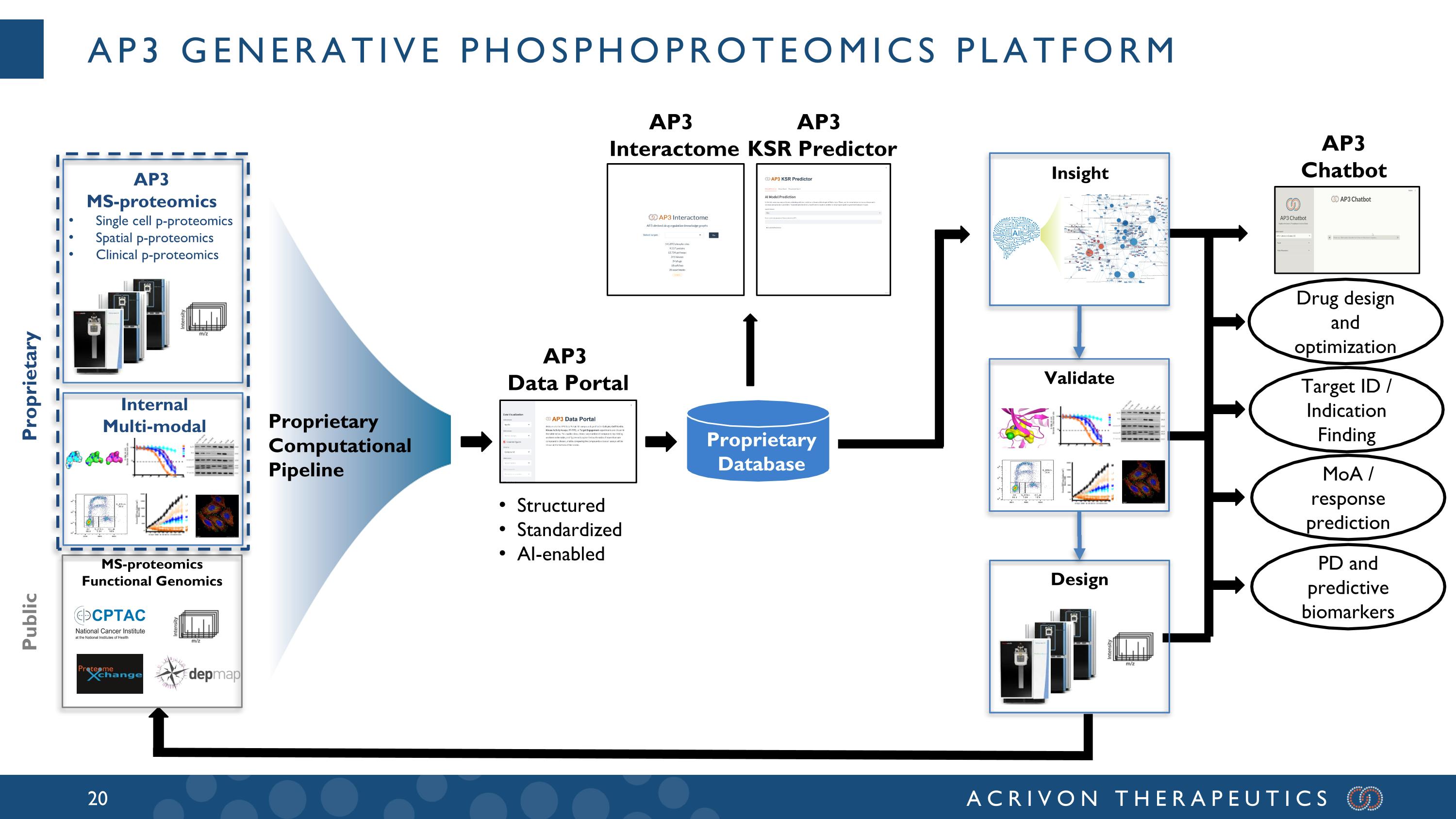
AP3 Generative phosphoproteomics platform Structured Standardized AI-enabled AP3 Interactome AP3 KSR Predictor AP3 Data Portal AP3 Chatbot Proprietary Database Drug design and optimization Target ID / Indication Finding MoA / response prediction PD and predictive biomarkers MS-proteomics Functional Genomics Internal Multi-modal AP3 MS-proteomics Single cell p-proteomics Spatial p-proteomics Clinical p-proteomics Proprietary Public Proprietary Computational Pipeline Validate Design Insight
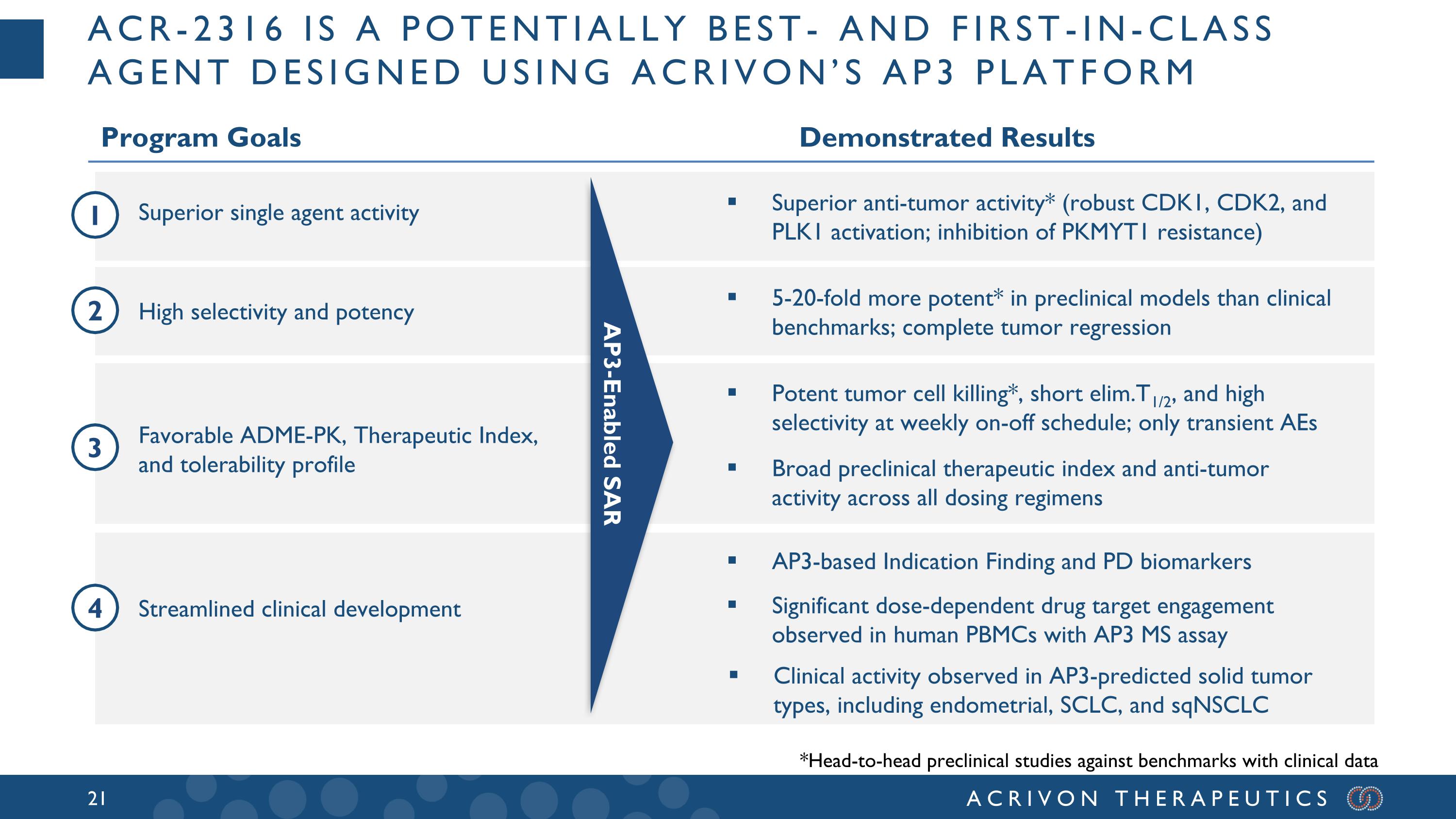
Acr-2316 is a potentially best- and first-in-class agent designed using Acrivon’s ap3 platform Program Goals Demonstrated Results Superior single agent activity High selectivity and potency Streamlined clinical development Favorable ADME-PK, Therapeutic Index, and tolerability profile Broad preclinical therapeutic index and anti-tumor activity across all dosing regimens 5-20-fold more potent* in preclinical models than clinical benchmarks; complete tumor regression Potent tumor cell killing*, short elim.T1/2, and high selectivity at weekly on-off schedule; only transient AEs Superior anti-tumor activity* (robust CDK1, CDK2, and PLK1 activation; inhibition of PKMYT1 resistance) AP3-Enabled SAR AP3-based Indication Finding and PD biomarkers 1 2 3 4 *Head-to-head preclinical studies against benchmarks with clinical data Significant dose-dependent drug target engagement observed in human PBMCs with AP3 MS assay Clinical activity observed in AP3-predicted solid tumor types, including endometrial, SCLC, and sqNSCLC
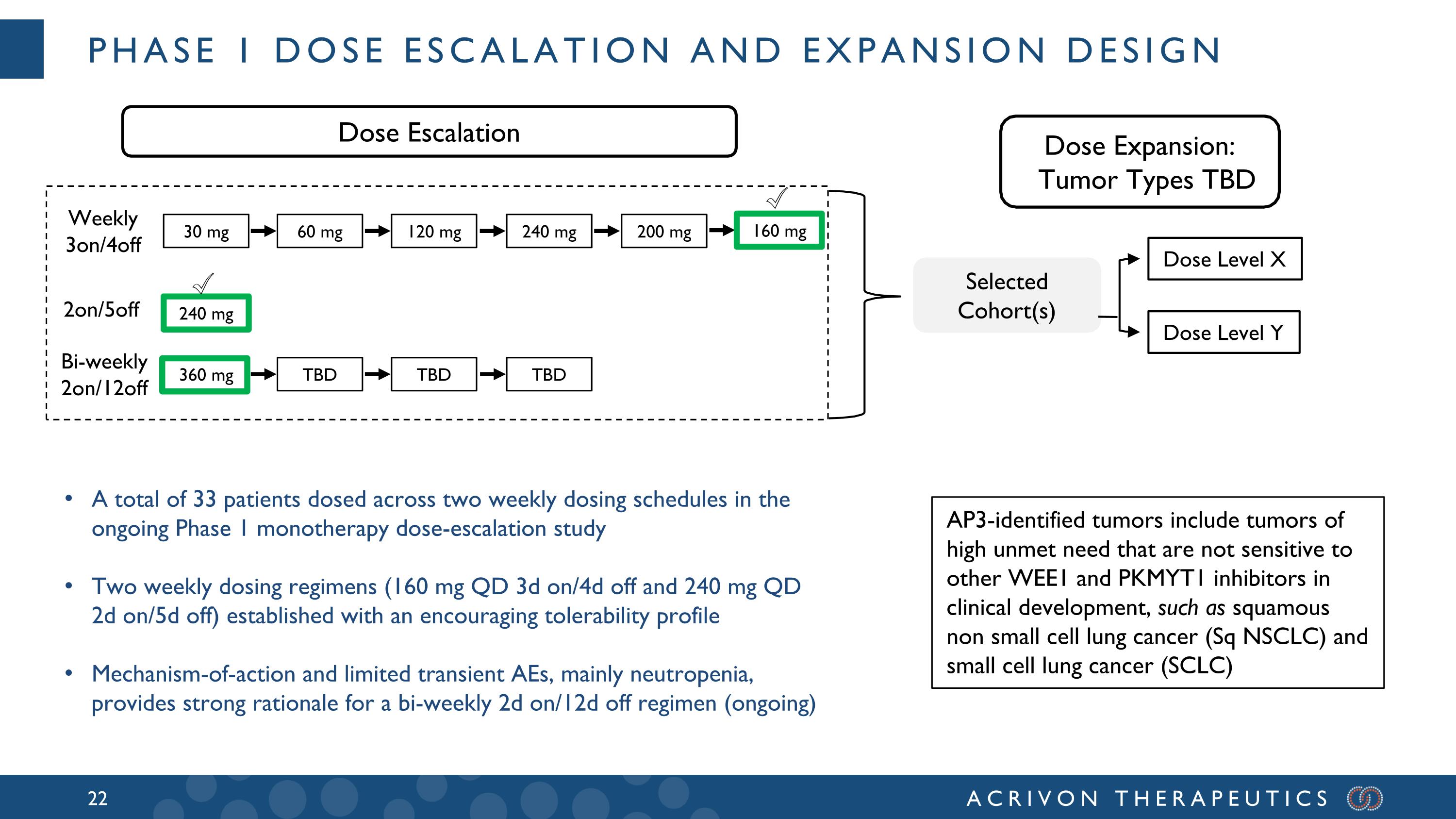
Phase 1 Dose Escalation and Expansion Design Weekly 3on/4off 2on/5off Dose Level X Dose Level Y Selected Cohort(s) Dose Escalation 30 mg 200 mg 240 mg 120 mg 60 mg 240 mg Bi-weekly 2on/12off 360 mg TBD TBD 160 mg TBD Dose Expansion: Tumor Types TBD ✅ AP3-identified tumors include tumors of high unmet need that are not sensitive to other WEE1 and PKMYT1 inhibitors in clinical development, such as squamous non small cell lung cancer (Sq NSCLC) and small cell lung cancer (SCLC) ✅ A total of 33 patients dosed across two weekly dosing schedules in the ongoing Phase 1 monotherapy dose-escalation study Two weekly dosing regimens (160 mg QD 3d on/4d off and 240 mg QD 2d on/5d off) established with an encouraging tolerability profile Mechanism-of-action and limited transient AEs, mainly neutropenia, provides strong rationale for a bi-weekly 2d on/12d off regimen (ongoing)
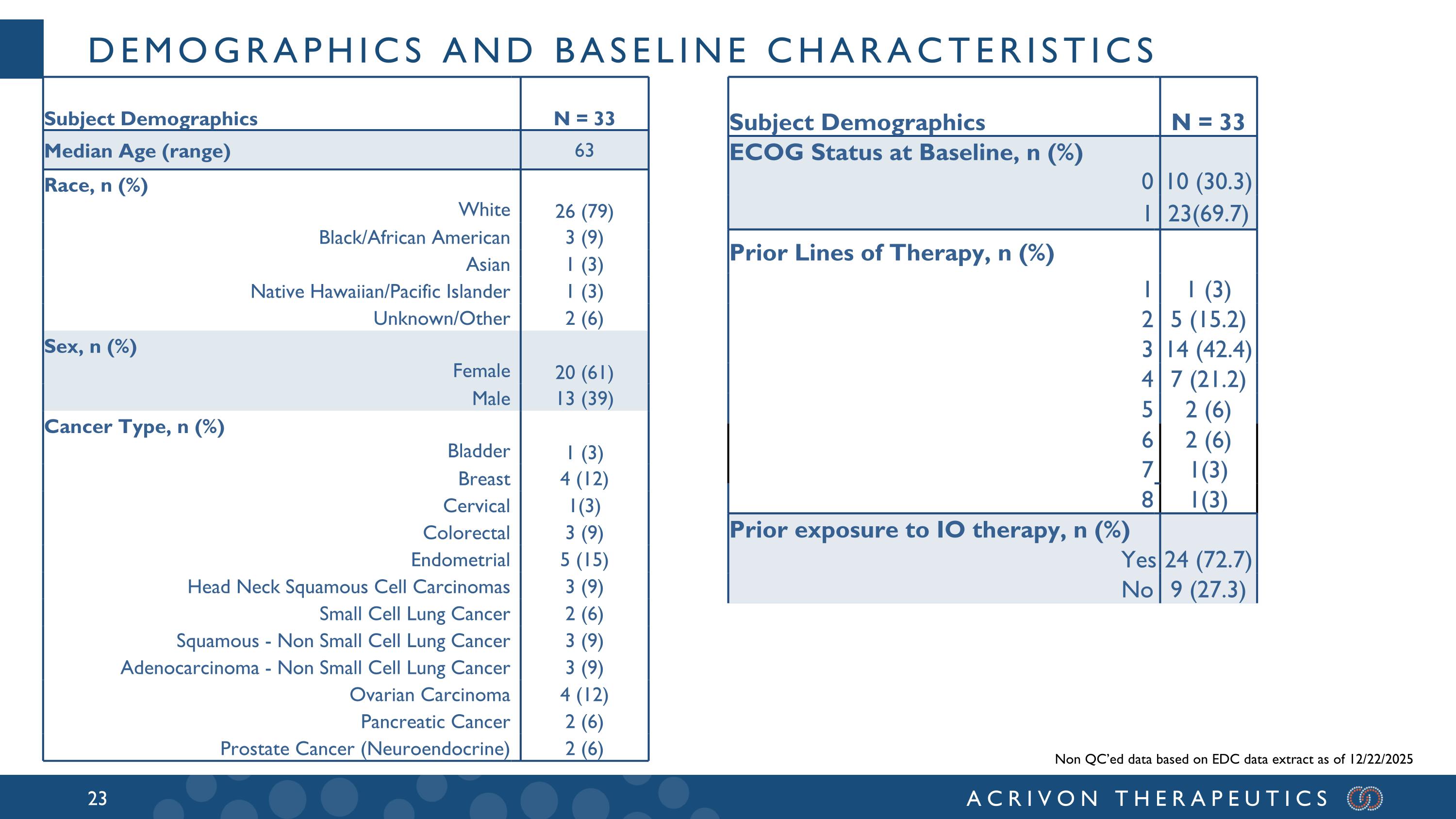
Demographics AND BASELINE CHARACTERISTICS Non QC’ed data based on EDC data extract as of 12/22/2025 Subject Demographics N = 33 ECOG Status at Baseline, n (%) 0 10 (30.3) 1 23(69.7) Prior Lines of Therapy, n (%) 1 1 (3) 2 5 (15.2) 3 14 (42.4) 4 7 (21.2) 5 2 (6) 6 2 (6) 7 1(3) 8 1(3) Prior exposure to IO therapy, n (%) Yes 24 (72.7) No 9 (27.3) Subject Demographics N = 33 Median Age (range) 63 Race, n (%) White 26 (79) Black/African American 3 (9) Asian 1 (3) Native Hawaiian/Pacific Islander 1 (3) Unknown/Other 2 (6) Sex, n (%) Female 20 (61) Male 13 (39) Cancer Type, n (%) Bladder 1 (3) Breast 4 (12) Cervical 1(3) Colorectal 3 (9) Endometrial 5 (15) Head Neck Squamous Cell Carcinomas 3 (9) Small Cell Lung Cancer 2 (6) Squamous - Non Small Cell Lung Cancer 3 (9) Adenocarcinoma - Non Small Cell Lung Cancer 3 (9) Ovarian Carcinoma 4 (12) Pancreatic Cancer 2 (6) Prostate Cancer (Neuroendocrine) 2 (6)
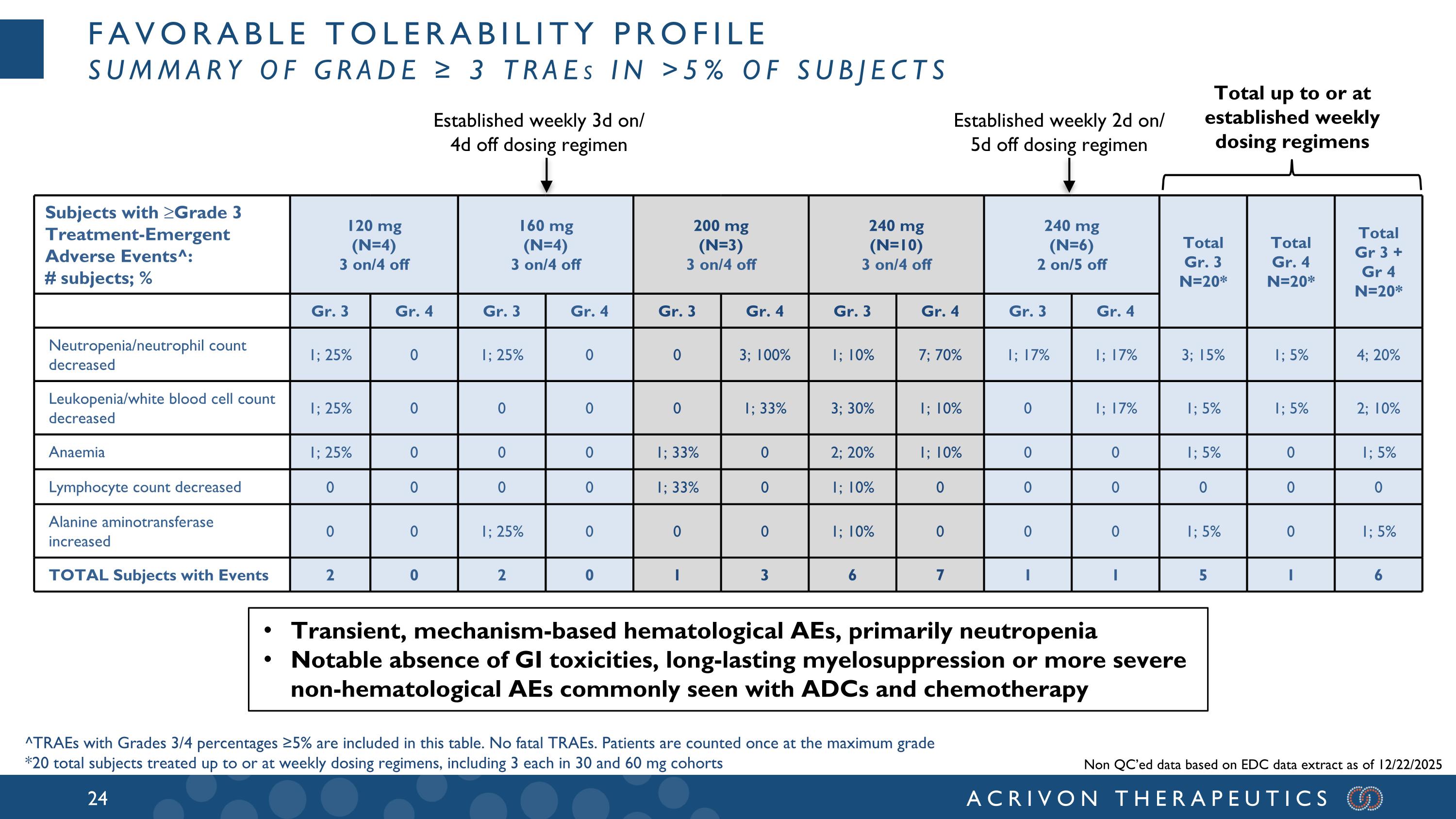
Favorable tolerability profile Summary of Grade ≥ 3 TRAEs in >5% of Subjects Subjects with ≥Grade 3 Treatment-Emergent Adverse Events^: # subjects; % 120 mg (N=4) 3 on/4 off 160 mg (N=4) 3 on/4 off 200 mg (N=3) 3 on/4 off 240 mg (N=10) 3 on/4 off 240 mg (N=6) 2 on/5 off Total Gr. 3 N=20* Total Gr. 4 N=20* Total Gr 3 + Gr 4 N=20* Gr. 3 Gr. 4 Gr. 3 Gr. 4 Gr. 3 Gr. 4 Gr. 3 Gr. 4 Gr. 3 Gr. 4 Neutropenia/neutrophil count decreased 1; 25% 0 1; 25% 0 0 3; 100% 1; 10% 7; 70% 1; 17% 1; 17% 3; 15% 1; 5% 4; 20% Leukopenia/white blood cell count decreased 1; 25% 0 0 0 0 1; 33% 3; 30% 1; 10% 0 1; 17% 1; 5% 1; 5% 2; 10% Anaemia 1; 25% 0 0 0 1; 33% 0 2; 20% 1; 10% 0 0 1; 5% 0 1; 5% Lymphocyte count decreased 0 0 0 0 1; 33% 0 1; 10% 0 0 0 0 0 0 Alanine aminotransferase increased 0 0 1; 25% 0 0 0 1; 10% 0 0 0 1; 5% 0 1; 5% TOTAL Subjects with Events 2 0 2 0 1 3 6 7 1 1 5 1 6 ^TRAEs with Grades 3/4 percentages ≥5% are included in this table. No fatal TRAEs. Patients are counted once at the maximum grade *20 total subjects treated up to or at weekly dosing regimens, including 3 each in 30 and 60 mg cohorts Established weekly 3d on/ 4d off dosing regimen Established weekly 2d on/ 5d off dosing regimen Total up to or at established weekly dosing regimens Transient, mechanism-based hematological AEs, primarily neutropenia Notable absence of GI toxicities, long-lasting myelosuppression or more severe non-hematological AEs commonly seen with ADCs and chemotherapy Non QC’ed data based on EDC data extract as of 12/22/2025
ACR-2316 initial activity in AP3-predicted tumor types A total of 33 patients dosed across two weekly dosing schedules in the ongoing Phase 1 monotherapy dose-escalation study Clinical activity observed at dose level 120 mg and above, with tumor shrinkage in 9 out of 20 evaluable patients, including SCLC and squamous NSCLC, two tumor types which have not shown sensitivity to previous clinical WEE1 or PKMYT1 inhibitors: Endometrial cancer (Mullerian tumor) at the 120 mg dose level, who had progressed after prior platinum-based chemo and tamoxifen and second-line pembrolizumab-lenvatinib had confirmed PR and was on therapy for 42 weeks Squamous NSCLC (BRCA1mut) receiving 200 mg (enrolled at the 240 mg dose level), who had progressed after prior lines with platinum-based chemo/durvalumab followed by three other immune therapies, had a PR at most recent scan after several scans showing deepening tumor shrinkage** SCLC (MSS) at the established 160 mg dose level, who had progressed after cisplatin/durvalumab, second-line tarlatamab and multi-organ radiation therapy had PR (50% tumor shrinkage) at first scan* *Since EDC data extract a subsequent scan showed further shrinkage (69%) of target lesion, but progression of non-target lesions (liver metastases) **Since EDC data extract a subsequent scan has confirmed the PR Non QC’ed data based on EDC data extract as of 12/22/2025
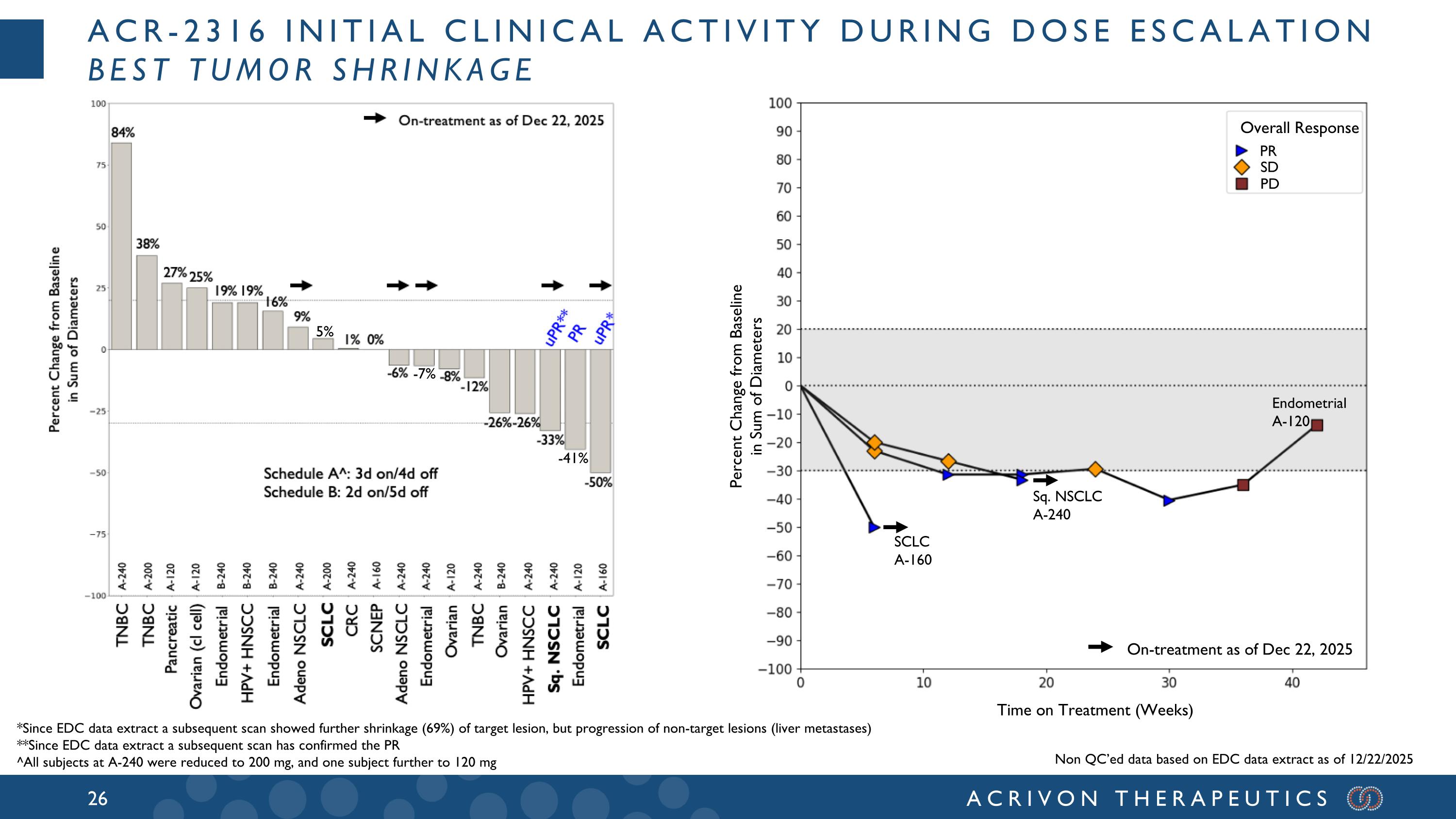
ACR-2316 initial clinical activity during dose escalation best tumor shrinkage *Since EDC data extract a subsequent scan showed further shrinkage (69%) of target lesion, but progression of non-target lesions (liver metastases) **Since EDC data extract a subsequent scan has confirmed the PR ^All subjects at A-240 were reduced to 200 mg, and one subject further to 120 mg Endometrial A-120 Sq. NSCLC A-240 SCLC A-160 Overall Response PR SD PD Percent Change from Baseline in Sum of Diameters Time on Treatment (Weeks) On-treatment as of Dec 22, 2025 Non QC’ed data based on EDC data extract as of 12/22/2025 5% -7% -41%
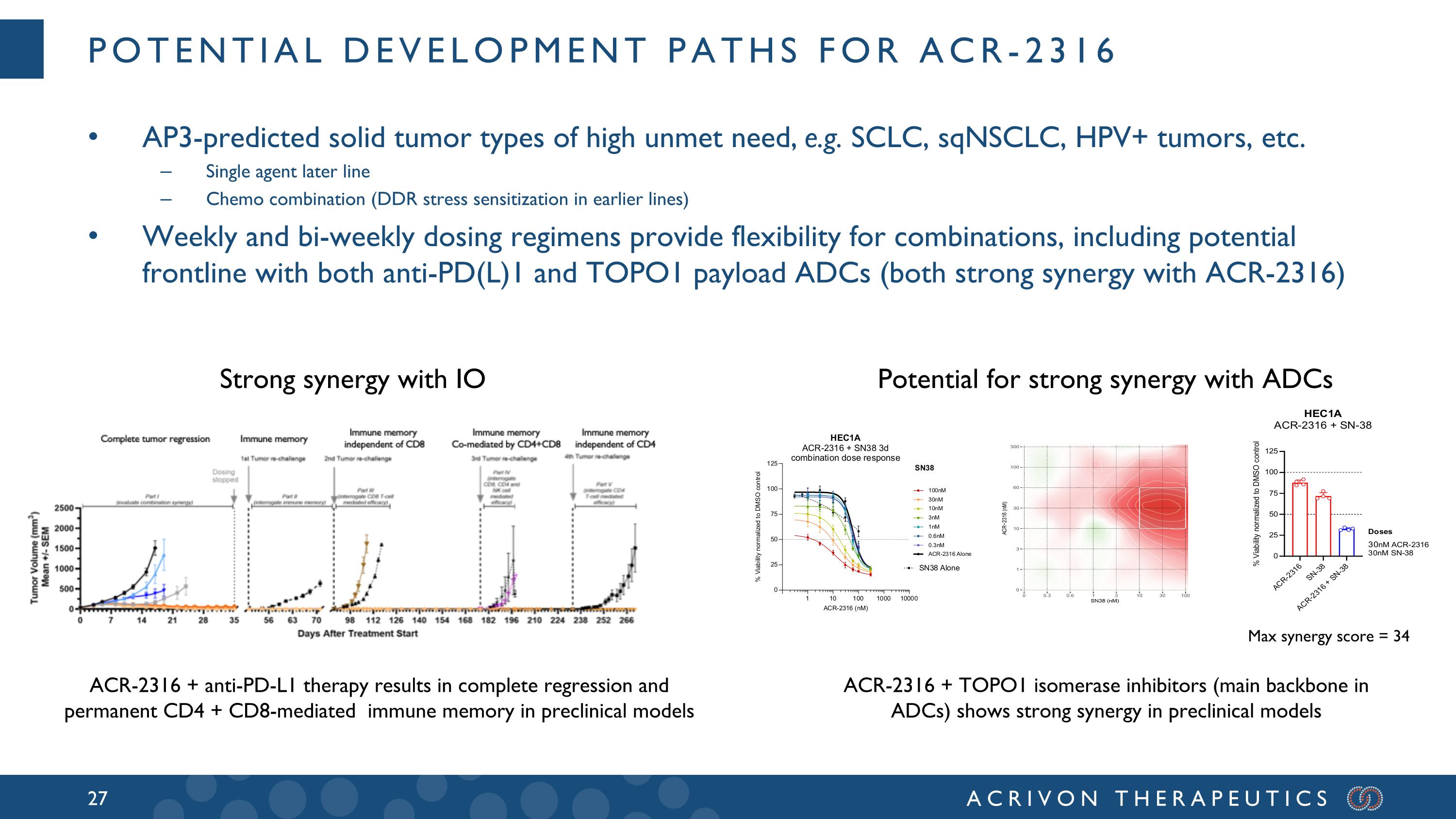
Potential development paths for ACR-2316 AP3-predicted solid tumor types of high unmet need, e.g. SCLC, sqNSCLC, HPV+ tumors, etc. Single agent later line Chemo combination (DDR stress sensitization in earlier lines) Weekly and bi-weekly dosing regimens provide flexibility for combinations, including potential frontline with both anti-PD(L)1 and TOPO1 payload ADCs (both strong synergy with ACR-2316) ACR-2316 + anti-PD-L1 therapy results in complete regression and permanent CD4 + CD8-mediated immune memory in preclinical models ACR-2316 + TOPO1 isomerase inhibitors (main backbone in ADCs) shows strong synergy in preclinical models Max synergy score = 34 Strong synergy with IO Potential for strong synergy with ADCs
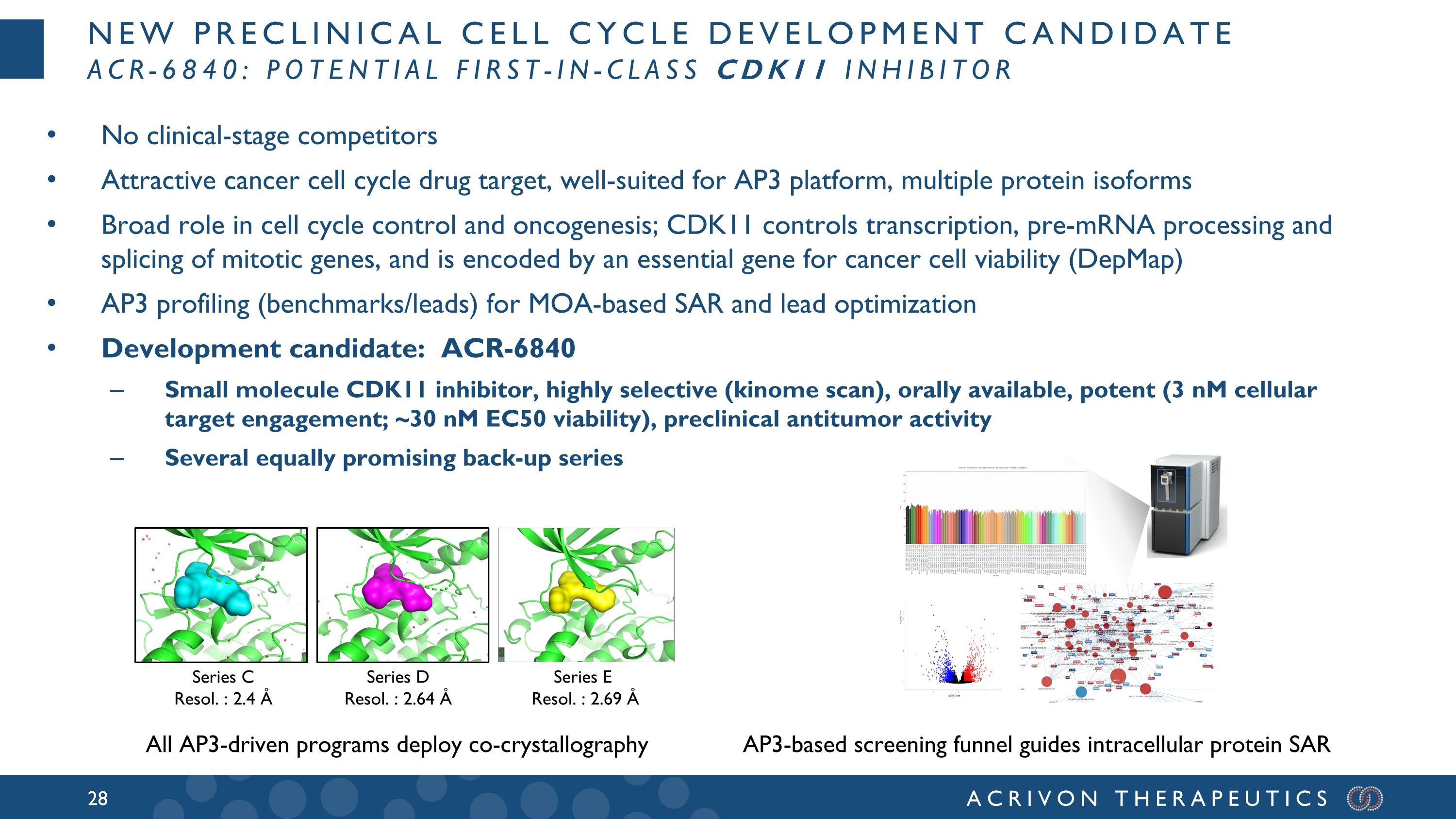
New preclinical cell cycle development candidate ACR-6840: potential first-in-class CDK11 inhibitor No clinical-stage competitors Attractive cancer cell cycle drug target, well-suited for AP3 platform, multiple protein isoforms Broad role in cell cycle control and oncogenesis; CDK11 controls transcription, pre-mRNA processing and splicing of mitotic genes, and is encoded by an essential gene for cancer cell viability (DepMap) AP3 profiling (benchmarks/leads) for MOA-based SAR and lead optimization Development candidate: ACR-6840 Small molecule CDK11 inhibitor, highly selective (kinome scan), orally available, potent (3 nM cellular target engagement; ~30 nM EC50 viability), preclinical antitumor activity Several equally promising back-up series Series D Resol. : 2.64 Å Series C Resol. : 2.4 Å AP3-based screening funnel guides intracellular protein SAR Series E Resol. : 2.69 Å All AP3-driven programs deploy co-crystallography
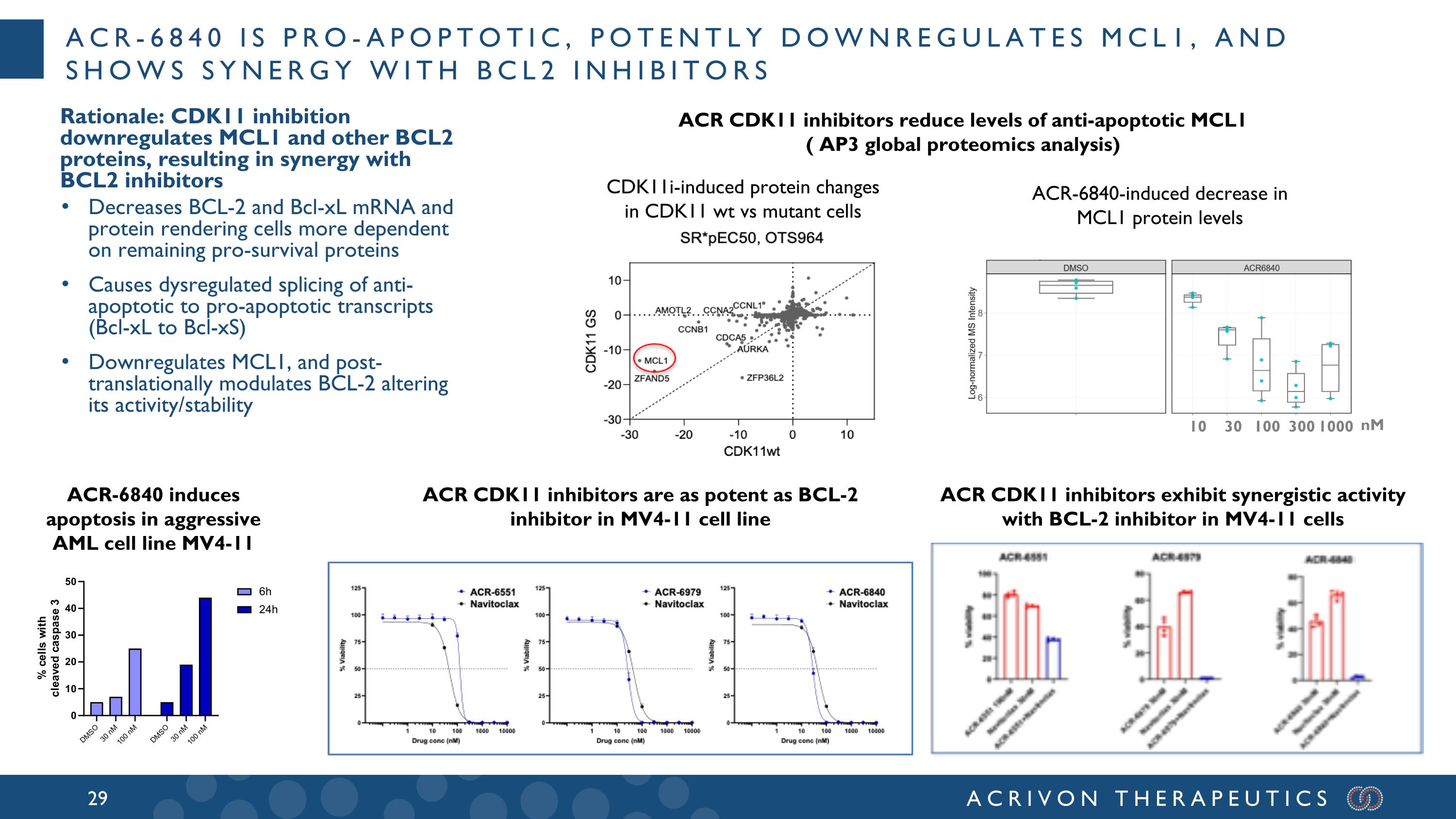
ACR-6840 is pro-apoptotic, potently downregulates MCL1, and shows synergy with BCL2 inhibitors Rationale: CDK11 inhibition downregulates MCL1 and other BCL2 proteins, resulting in synergy with BCL2 inhibitors Decreases BCL-2 and Bcl-xL mRNA and protein rendering cells more dependent on remaining pro-survival proteins Causes dysregulated splicing of anti-apoptotic to pro-apoptotic transcripts (Bcl-xL to Bcl-xS) Downregulates MCL1, and post-translationally modulates BCL-2 altering its activity/stability ACR CDK11 inhibitors exhibit synergistic activity with BCL-2 inhibitor in MV4-11 cells ACR CDK11 inhibitors are as potent as BCL-2 inhibitor in MV4-11 cell line ACR-6840 induces apoptosis in aggressive AML cell line MV4-11 ACR CDK11 inhibitors reduce levels of anti-apoptotic MCL1 ( AP3 global proteomics analysis) 10 30 100 300 1000 nM CDK11i-induced protein changes in CDK11 wt vs mutant cells ACR-6840-induced decrease in MCL1 protein levels

FINANCIAL HIGHLIGHTS Approximate Cash and Investments $119M Projected runway into Q2 2027 Approximate Fully Diluted Shares Outstanding 45.1M Current operating plan, assuming no additional financing Balance sheet 31-Dec-2025 Shares, pre-funded warrants, and equity grants outstanding 31-Dec-2025 Notes: Preliminary, Unaudited. The cash and investments amount is preliminary and subject to completion of financial closing procedures. As a result, it may differ materially from the amount that will be reflected in the Company’s consolidated financial statements for the quarter and year ended December 31, 2025.
Take home messages ACR-368 ACR-2316 AP3-driven Pipeline SUBTITILE HERE Serous EC shows high proportion of BM+ and high sensitivity to ACR-368 (ORR in BM+ = 67% and ORR in all-comer = 52% in ongoing Ph 2b trial), providing for a highly compelling clinical profile Given expansion to ≥20 EU sites and no biopsy requirement, trial enrollment in US+EU all-comer serous EC (Arm 3; N = 90) anticipated to be completed in Q4 2026 (initial data update mid-2026) Arm 3 subjects will receive ACR-368 with ULDG sensitization, which might further boost ORR Phase 3 trial design and protocol submitted to the FDA for frontline treatment with ACR-368 + anti-PD-1 based on strong synergy (complete regression and immune memory) in preclinical studies Weekly oral dosing regimens established with favorable tolerability profile (primarily transient neutropenia) Initial clinical activity observed in AP3-selected solid tumor types, including SCLC and sqNSCLC, tumor types not previously shown sensitive to WEE1 inhibitors currently in clinical development Bi-weekly oral dosing regimen (2d on/12d off) being explored with potential for potent tumor cell killing and maximal dosing flexibility as single agent or as combination therapy Next AP3-driven preclinical cell cycle target disclosed as CDK11, a key cell cycle regulatory molecule ACR-6840 Development Candidate: A potential first-in-class, potent, selective CDK11 oral inhibitor Acrivon AP3 Generative Phosphoproteomics fueling streamlined drug development and clinical deliverables