
.3

1 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Precision Oncology Through Synthetic Lethality Transforming DDR Inhibition into Patient Benefit January 2026 Confidential

2 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Forward - Looking Statements Certain information contained in this presentation includes “forward - looking statements”, within the meaning of Section 27A of t he Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended related to our study analyses, clinical trials, r egu latory submissions, and projected cash position. We may, in some cases use terms such as “future,” “predicts,” “believes,” “potential,” “continue,” “anticipate s,” “estimates,” “expects,” “plans,” “intends,” “targeting,” “confidence,” “may,” “could,” “might,” “likely,” “will,” “should” or other words that convey uncertai nty of the future events or outcomes to identify these forward - looking statements. Our forward - looking statements are based on current beliefs and expectations of our management team and on information currently available to management that involve risks, potential changes in circumstances, assumptions, and uncertainties. All st atements contained in this presentation other than statements of historical fact are forward - looking statements, including statements regarding our ability to develop, commercialize, and achieve market acceptance of our current and planned products and services, our research and development efforts, including t imi ng considerations and other matters regarding our business strategies, use of capital, results of operations and financial position, and plans and object ive s for future operations. Any or all of the forward - looking statements may turn out to be wrong or be affected by inaccurate assumptions we might make or by known or un known risks and uncertainties. These forward - looking statements are subject to risks and uncertainties including, without limitation, risks rela ted to the success, timing, and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regardin g t he timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material chang es to our currently projected expenses), futility analyses, presentations at conferences and data reported in an abstract, and receipt of interim or preliminary resul ts (including, without limitation, any preclinical results or data), which are not necessarily indicative of the final results of our ongoing clinical trials, our u nde rstanding of product candidates mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs and our ability to predict clinical outcomes based on such preclinical and early clinical result, and our ability to continue as a going concern, and the other risks, uncert ainties, and other factors described under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere i n t he documents we file with the U.S. Securities and Exchange Commission. For all these reasons, actual results and developments could be materially differen t from those expressed in or implied by our forward - looking statements. You are cautioned not to place undue reliance on these forward - looking statements, wh ich are made only as of the date of this presentation. We undertake no obligation to update such forward - looking statements for any reason, except as required by law. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of any securit ies in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such st ate or jurisdiction. This presentation may not be reproduced, forwarded to any person or published, in whole or in part. Private placements are speculative, illiquid, carry a high degree of risk and should only be purchased by persons who can afford the loss of their entire investment.
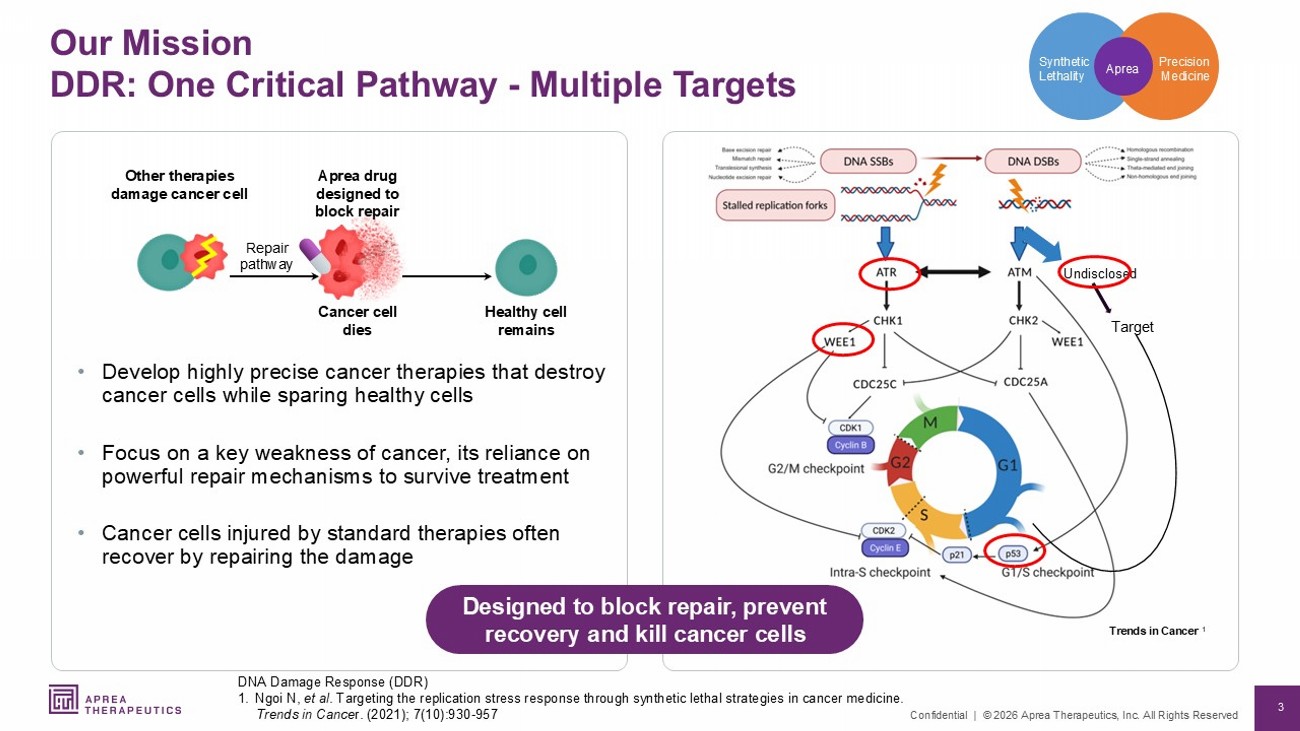
3 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Our Mission DDR: One Critical Pathway - Multiple Targets DNA Damage Response (DDR) 1. Ngoi N, et al . Targeting the replication stress response through synthetic lethal strategies in cancer medicine. Trends in Cance r. (2021); 7(10):930 - 957 Undisclosed Target Precision Medicine Synthetic Lethality Aprea • Develop highly precise cancer therapies that destroy cancer cells while sparing healthy cells • Focus on a key weakness of cancer, its reliance on powerful repair mechanisms to survive treatment • Cancer cells injured by standard therapies often recover by repairing the damage Designed to block repair, prevent recovery and kill cancer cells Repair pathway Other therapies damage cancer cell Healthy cell remains Aprea drug designed to block repair Cancer cell dies Trends in Cancer 1
4 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Ushering in a New Phase of Precision Oncology - Synthetic Lethality Synthetic Lethality • A clinically validated way to kill cancer 1 • Exploits tumor specific genetic weaknesses • Spares healthy cells • Biomarker driven identification of right patients • Potential for improved efficacy with better safety Potential fundamental transformation in cancer treatment • Shift from toxic chemotherapy to potentially safer, targeted agents • Attack cancer more precisely • Better quality of life and longer survival 1 . Previtali V, et al. New Horizons of Synthetic Lethality in Cancer: Current Development and Future Perspectives. J Med Chem. (2024);67:11488 - 11521
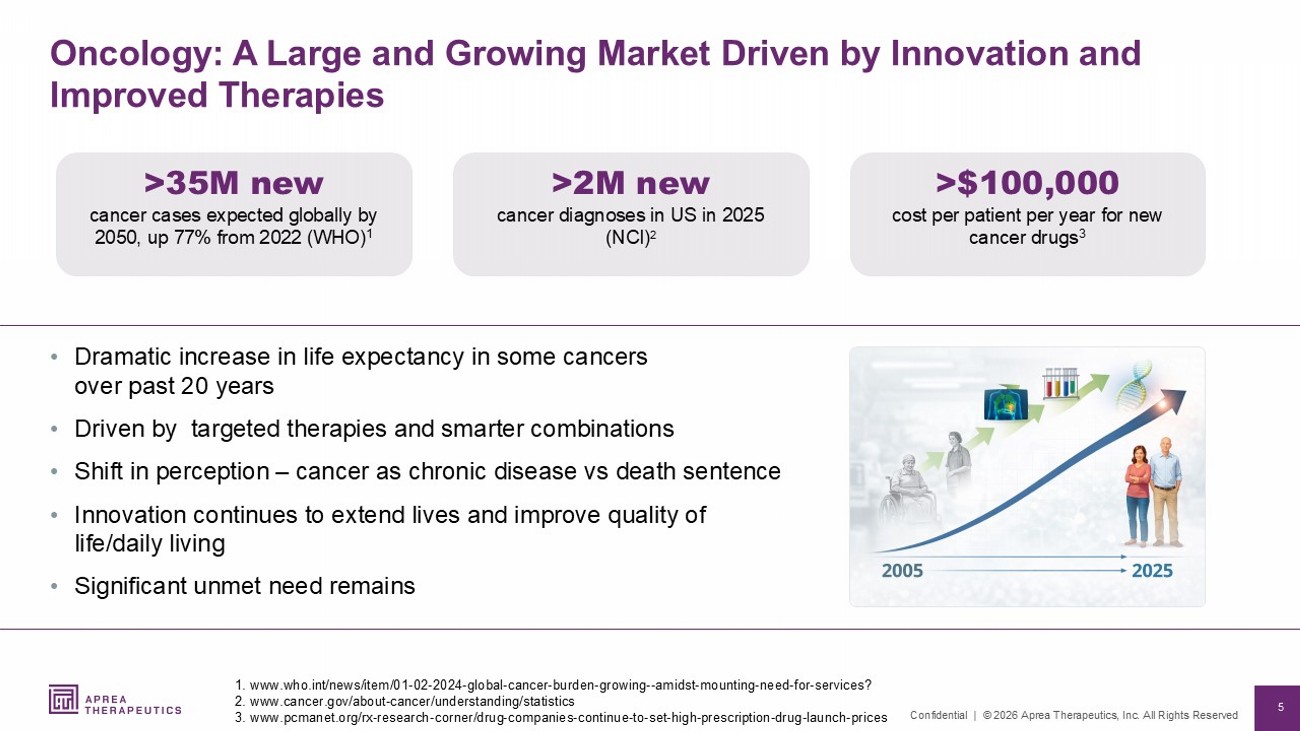
5 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Oncology: A Large and Growing Market Driven by Innovation and Improved Therapies • Dramatic increase in life expectancy in some cancers over past 20 years • Driven by targeted therapies and smarter combinations • Shift in perception – cancer as chronic disease vs death sentence • Innovation continues to extend lives and improve quality of life/daily living • Significant unmet need remains 1. www.who.int /news/item/01 - 02 - 2024 - global - cancer - burden - growing -- amidst - mounting - need - for - services? 2. www.cancer.gov /about - cancer/understanding/statistics 3. www.pcmanet.org /rx - research - corner/drug - companies - continue - to - set - high - prescription - drug - launch - prices >35M new cancer cases expected globally by 2050, up 77% from 2022 (WHO) 1 >2M new cancer diagnoses in US in 2025 (NCI) 2 >$100,000 cost per patient per year for new cancer drugs 3
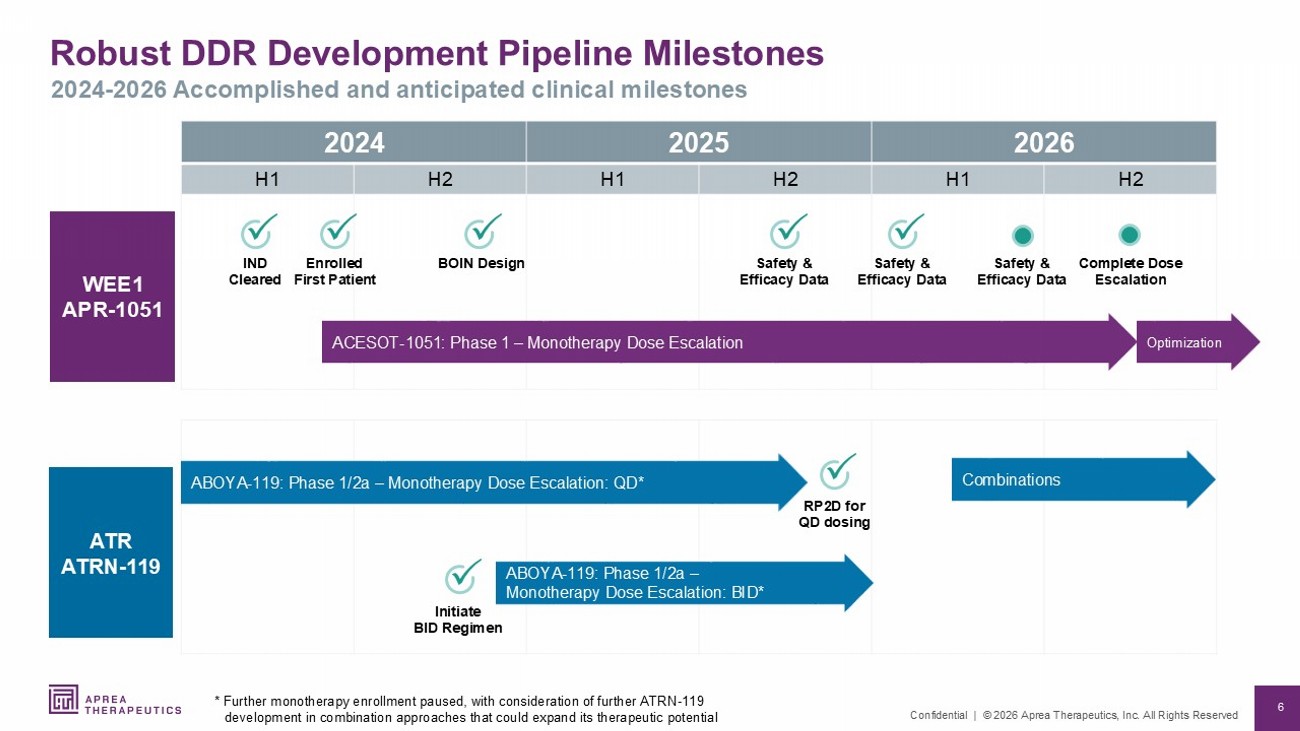
6 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Robust DDR Development Pipeline Milestones 2024 - 2026 Accomplished and anticipated clinical milestones 2026 2025 2024 H2 H1 H2 H1 H2 H1 ATR ATRN - 119 WEE1 APR - 1051 RP2D for QD dosing ACESOT - 1051: Phase 1 – Monotherapy Dose Escalation Enrolled First Patient Complete Dose Escalation IND Cleared ABOYA - 119: Phase 1/2a – Monotherapy Dose Escalation: QD* BOIN Design Initiate BID Regimen Optimization ABOYA - 119: Phase 1/2a – Monotherapy Dose Escalation: BID* * Further monotherapy enrollment paused, with consideration of further ATRN - 119 development in combination approaches that could expand its therapeutic potential Safety & Efficacy Data Safety & Efficacy Data Combinations Safety & Efficacy Data
7 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Strong Drug Development and Commercial Expertise Experienced team in synthetic lethality and targeted therapy Management Board of Directors Jean - Pierre Bizzari, M.D. Director Oren Gilad, Ph.D. President and CEO Richard Peters, M.D., Ph.D. Chairman of the Board Gabriela Gruia, M.D. Director Michael Grissinger Director Marc Duey Director Bernd R. Seizinger , M.D., Ph.D. Director Rifat Pamukcu , M.D. Director John Henneman Director Oren Gilad, Ph.D. President and CEO John P. Hamill Sr. Vice President and CFO Philippe Pultar, MD Sr. Medical Advisor Ze’ev Weiss, CPA, B.Sc. Chief Business Advisor Mike Carleton, Ph.D. Translational Medicine Advisor Brian Wiley SVP, Corporate Strategy

8 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved 8 WEE1 Inhibitor: APR - 1051 ACESOT - 1051: Clinical Proof - Of - Concept Confidential
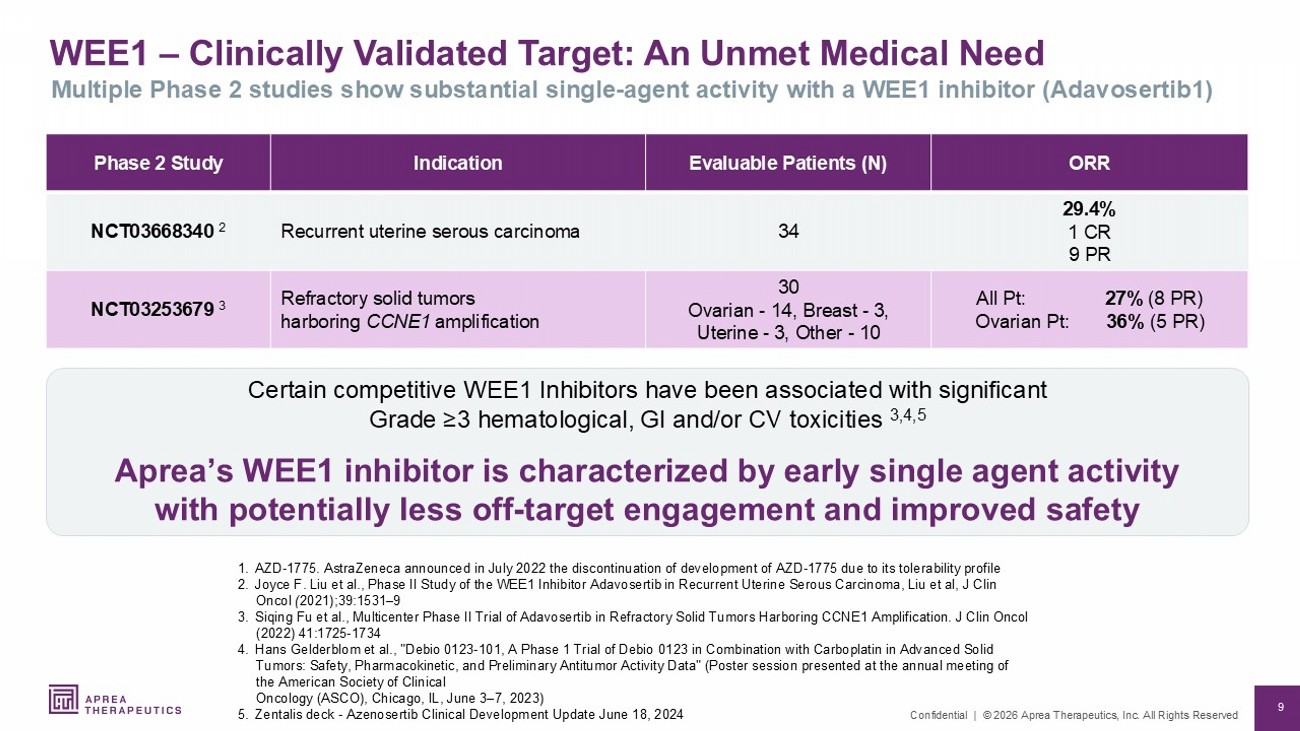
9 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved WEE1 – Clinically Validated Target: An Unmet Medical Need 1. AZD - 1775. AstraZeneca announced in July 2022 the discontinuation of development of AZD - 1775 due to its tolerability profile 2. Joyce F. Liu et al., Phase II Study of the WEE1 Inhibitor Adavosertib in Recurrent Uterine Serous Carcinoma, Liu et al, J Clin Oncol ( 2021);39:1531 – 9 3. Siqing Fu et al., Multicenter Phase II Trial of Adavosertib in Refractory Solid Tumors Harboring CCNE1 Amplification. J Clin Oncol (2022) 41:1725 - 1734 4. Hans Gelderblom et al., " Debio 0123 - 101, A Phase 1 Trial of Debio 0123 in Combination with Carboplatin in Advanced Solid Tumors: Safety, Pharmacokinetic, and Preliminary Antitumor Activity Data" (Poster session presented at the annual meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 3 – 7, 2023) 5. Zentalis deck - Azenosertib Clinical Development Update June 18, 2024 Multiple Phase 2 studies show substantial single - agent activity with a WEE1 inhibitor (Adavosertib1) Certain competitive WEE1 Inhibitors have been associated with significant Grade ≥3 hematological, GI and/or CV toxicities 3,4,5 Aprea’s WEE1 inhibitor is characterized by early single agent activity with potentially less off - target engagement and improved safety ORR Evaluable Patients (N) Indication Phase 2 Study 29.4% 1 CR 9 PR 34 Recurrent uterine serous carcinoma NCT03668340 2 All Pt: 27% (8 PR) Ovarian Pt: 36% (5 PR) 30 Ovarian - 14, Breast - 3, Uterine - 3, Other - 10 Refractory solid tumors harboring CCNE1 amplification NCT03253679 3
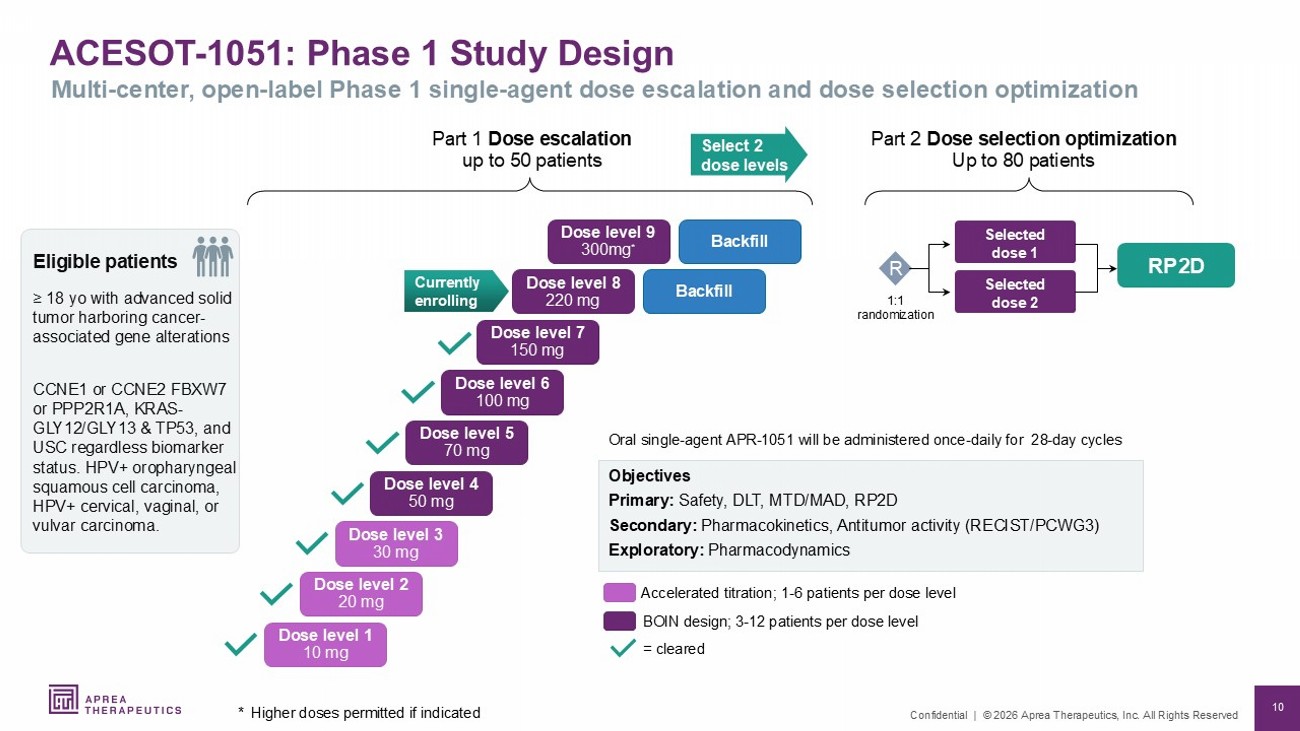
10 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Eligible patients ≥ 18 yo with advanced solid tumor harboring cancer - associated gene alterations CCNE1 or CCNE2 FBXW7 or PPP2R1A, KRAS - GLY12/GLY13 & TP53, and USC regardless biomarker status. HPV+ oropharyngeal squamous cell carcinoma, HPV+ cervical, vaginal, or vulvar carcinoma. ACESOT - 1051: Phase 1 Study Design * Higher doses permitted if indicated Multi - center, open - label Phase 1 single - agent dose escalation and dose selection optimization Part 1 Dose escalation up to 50 patients Select 2 dose levels RP2D Oral single - agent APR - 1051 will be administered once - daily for 28 - day cycles Objectives Primary: Safety, DLT, MTD/MAD, RP2D Secondary: Pharmacokinetics, Antitumor activity (RECIST/PCWG3) Exploratory: Pharmacodynamics = cleared Accelerated titration; 1 - 6 patients per dose level BOIN design; 3 - 12 patients per dose level Currently enrolling Part 2 Dose selection optimization Up to 80 patients Selected dose 2 Selected dose 1 1:1 randomization Dose level 1 10 mg Dose level 2 20 mg Dose level 3 30 mg Dose level 4 50 mg Dose level 5 70 mg Dose level 6 100 mg Dose level 7 150 mg Dose level 8 220 mg Dose level 9 300mg * Backfill Backfill R
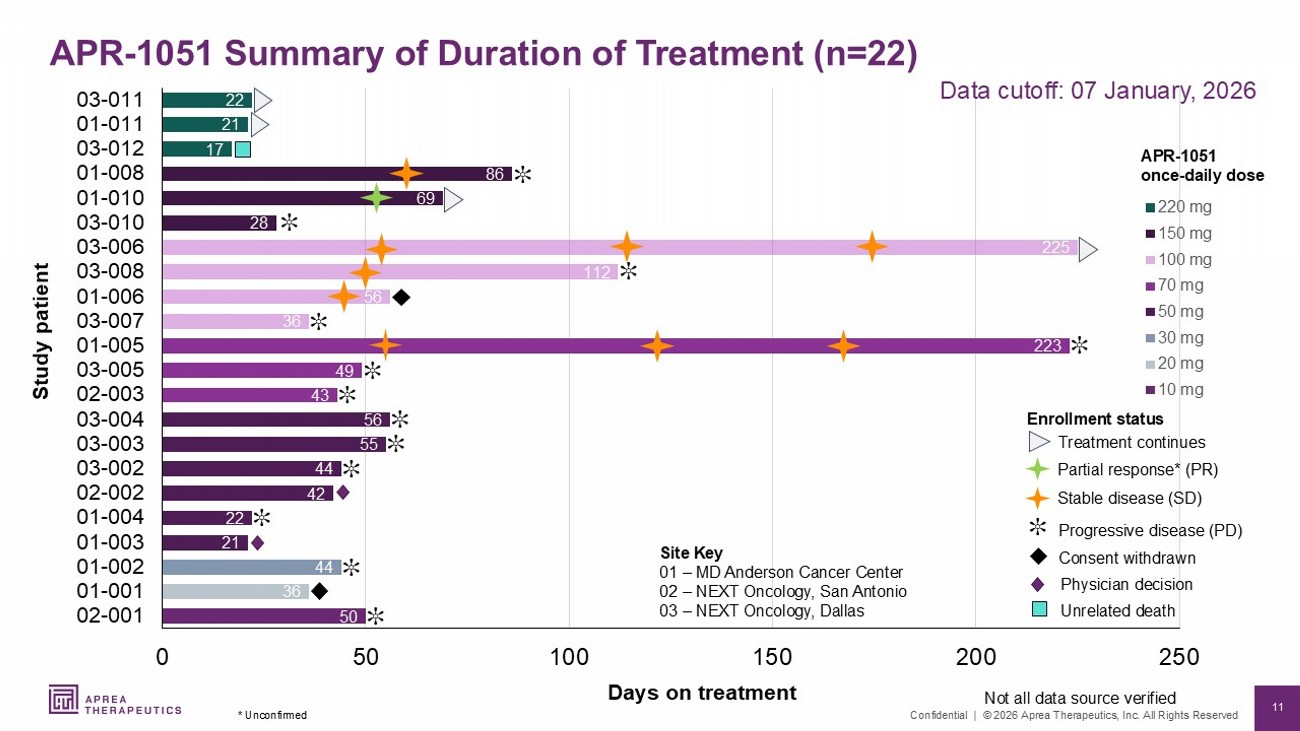
11 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved 50 36 44 21 22 42 44 55 56 43 49 223 36 56 112 225 28 69 86 17 21 22 0 50 100 150 200 250 02-001 01-001 01-002 01-003 01-004 02-002 03-002 03-003 03-004 02-003 03-005 01-005 03-007 01-006 03-008 03-006 03-010 01-010 01-008 03-012 01-011 03-011 220 mg 150 mg 100 mg 70 mg 50 mg 30 mg 20 mg 10 mg Site Key 01 – MD Anderson Cancer Center 02 – NEXT Oncology, San Antonio 03 – NEXT Oncology, Dallas Days on treatment Enrollment status Progressive disease (PD) Stable disease (SD) Study patient APR - 1051 once - daily dose Not all data source verified Consent withdrawn ✼ ✼ ✼ ✼ ✼ ✼ ✼ ✼ Treatment continues ✼ ✼ Physician decision Data cutoff: 07 January, 2026 ✼ ✼ ✼ ✼ Unrelated death Partial response* (PR) APR - 1051 Summary of Duration of Treatment (n=22) * Unconfirmed
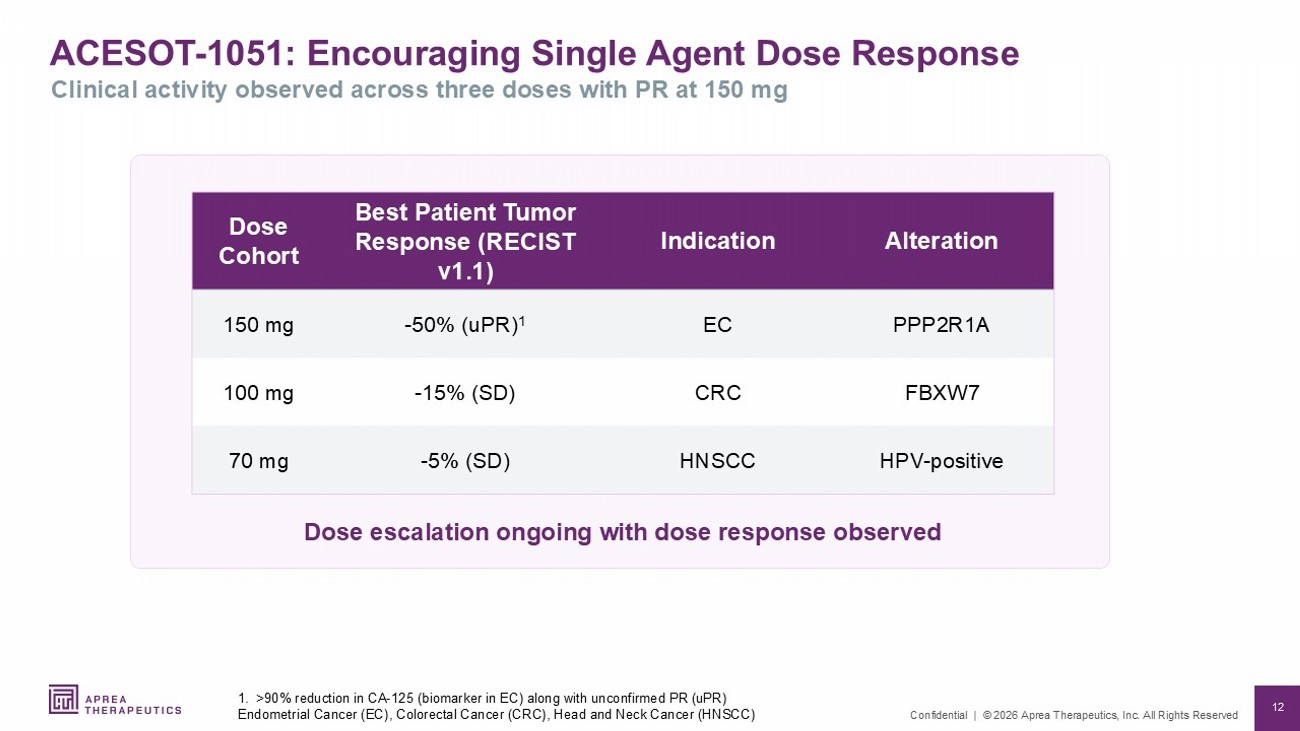
12 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved ACESOT - 1051: Encouraging Single Agent Dose Response 1. >90% reduction in CA - 125 (biomarker in EC) along with unconfirmed PR ( uPR ) Endometrial Cancer (EC), Colorectal Cancer (CRC), Head and Neck Cancer (HNSCC) Clinical activity observed across three doses with PR at 150 mg Alteration Indication Best Patient Tumor Response (RECIST v1.1) Dose Cohort PPP2R1A EC - 50% ( uPR ) 1 150 mg FBXW7 CRC - 15% (SD) 100 mg HPV - positive HNSCC - 5% (SD) 70 mg Dose escalation ongoing with dose response observed
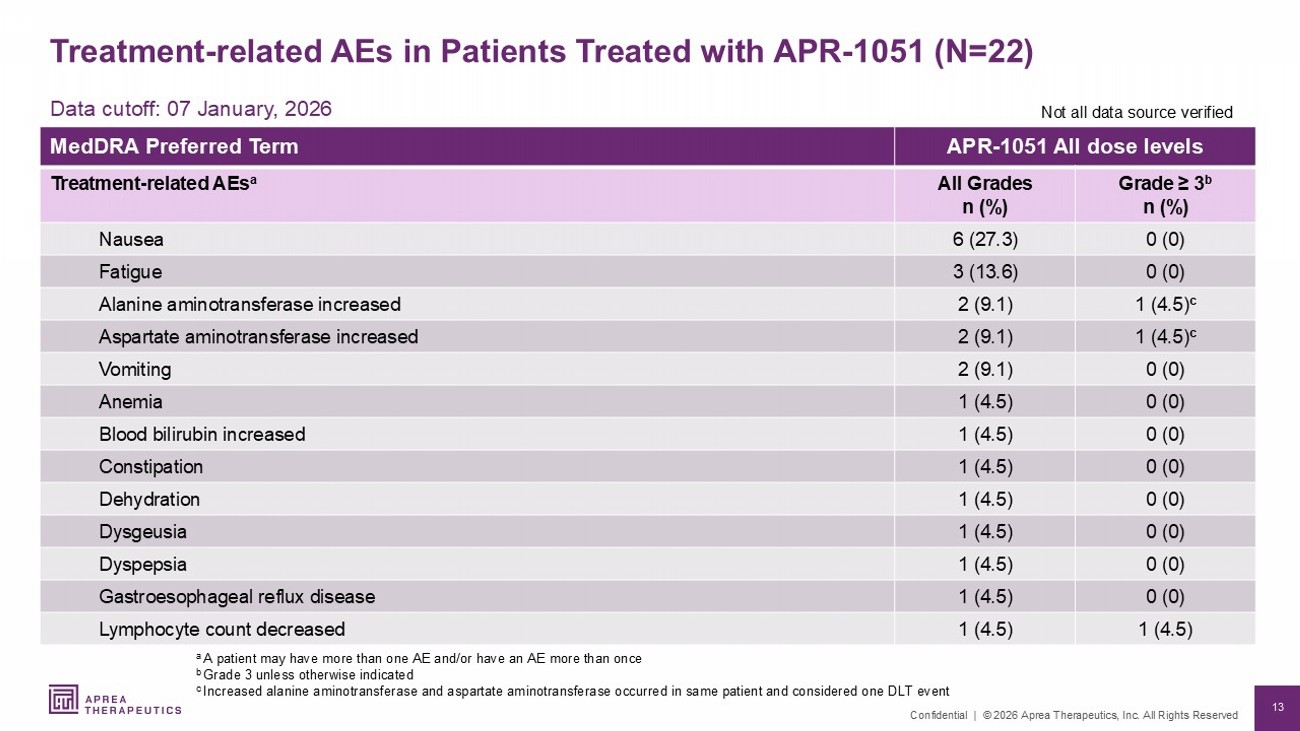
13 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Treatment - related AEs in Patients Treated with APR - 1051 (N=22) APR - 1051 All dose levels MedDRA Preferred Term Grade ≥ 3 b n (%) All Grades n (%) Treatment - related AEs a 0 (0) 6 (27.3) Nausea 0 (0) 3 (13.6) Fatigue 1 (4.5) c 2 (9.1) Alanine aminotransferase increased 1 (4.5) c 2 (9.1) Aspartate aminotransferase increased 0 (0) 2 (9.1) Vomiting 0 (0) 1 (4.5) Anemia 0 (0) 1 (4.5) Blood bilirubin increased 0 (0) 1 (4.5) Constipation 0 (0) 1 (4.5) Dehydration 0 (0) 1 (4.5) Dysgeusia 0 (0) 1 (4.5) Dyspepsia 0 (0) 1 (4.5) Gastroesophageal reflux disease 1 (4.5) 1 (4.5) Lymphocyte count decreased a A patient may have more than one AE and/or have an AE more than once b Grade 3 unless otherwise indicated c I ncreased alanine aminotransferase and aspartate aminotransferase occurred in same patient and considered one DLT event Data cutoff: 07 January, 2026 Not all data source verified
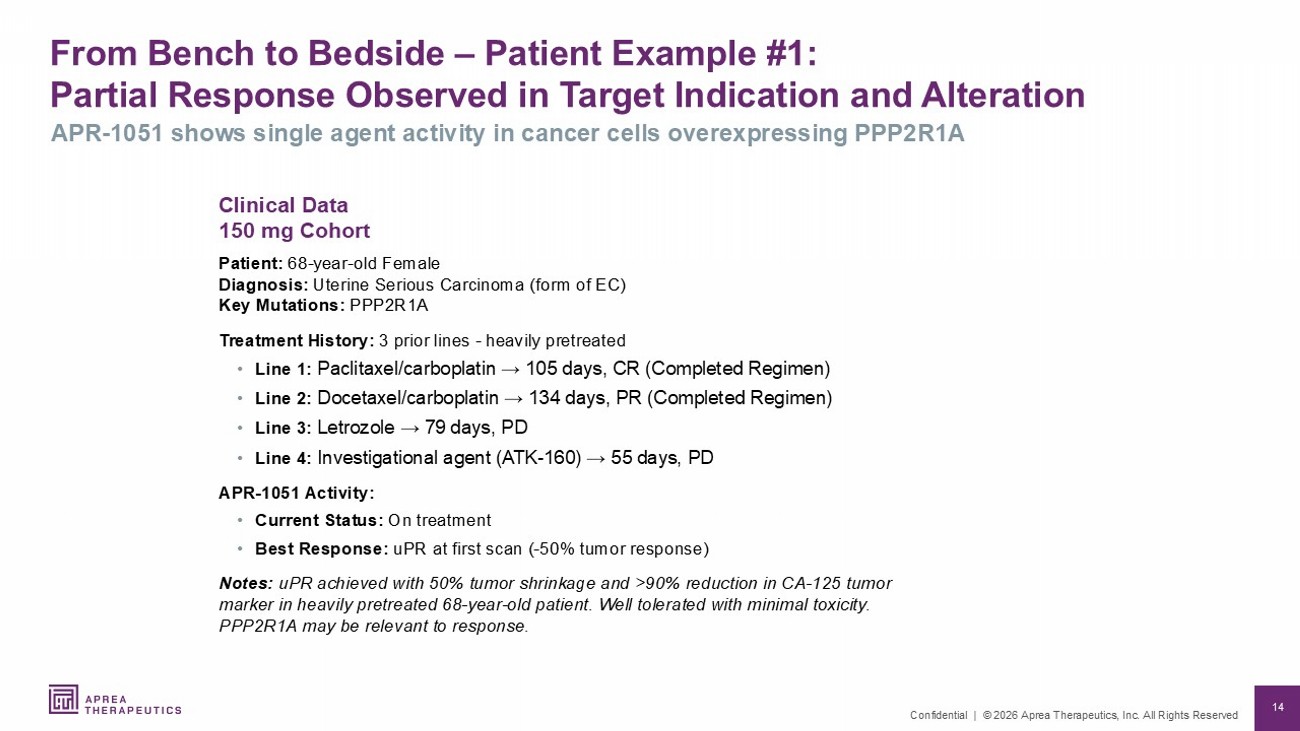
14 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved From Bench to Bedside – Patient Example #1: Partial Response Observed in Target Indication and Alteration APR - 1051 shows single agent activity in cancer cells overexpressing PPP2R1A Clinical Data 150 mg Cohort Patient: 68 - year - old Female Diagnosis: Uterine Serious Carcinoma (form of EC) Key Mutations: PPP2R1A Treatment History: 3 prior lines - heavily pretreated • Line 1: Paclitaxel/carboplatin → 105 days, CR (Completed Regimen) • Line 2: Docetaxel/carboplatin → 134 days, PR (Completed Regimen) • Line 3: Letrozole → 79 days, PD • Line 4: Investigational agent (ATK - 160) → 55 days, PD APR - 1051 Activity: • Current Status: On treatment • Best Response: uPR at first scan ( - 5 0% tumor response) Notes: uPR achieved with 50% tumor shrinkage and >90% reduction in CA - 125 tumor marker in heavily pretreated 68 - year - old patient. Well tolerated with minimal toxicity. PPP2R1A may be relevant to response.
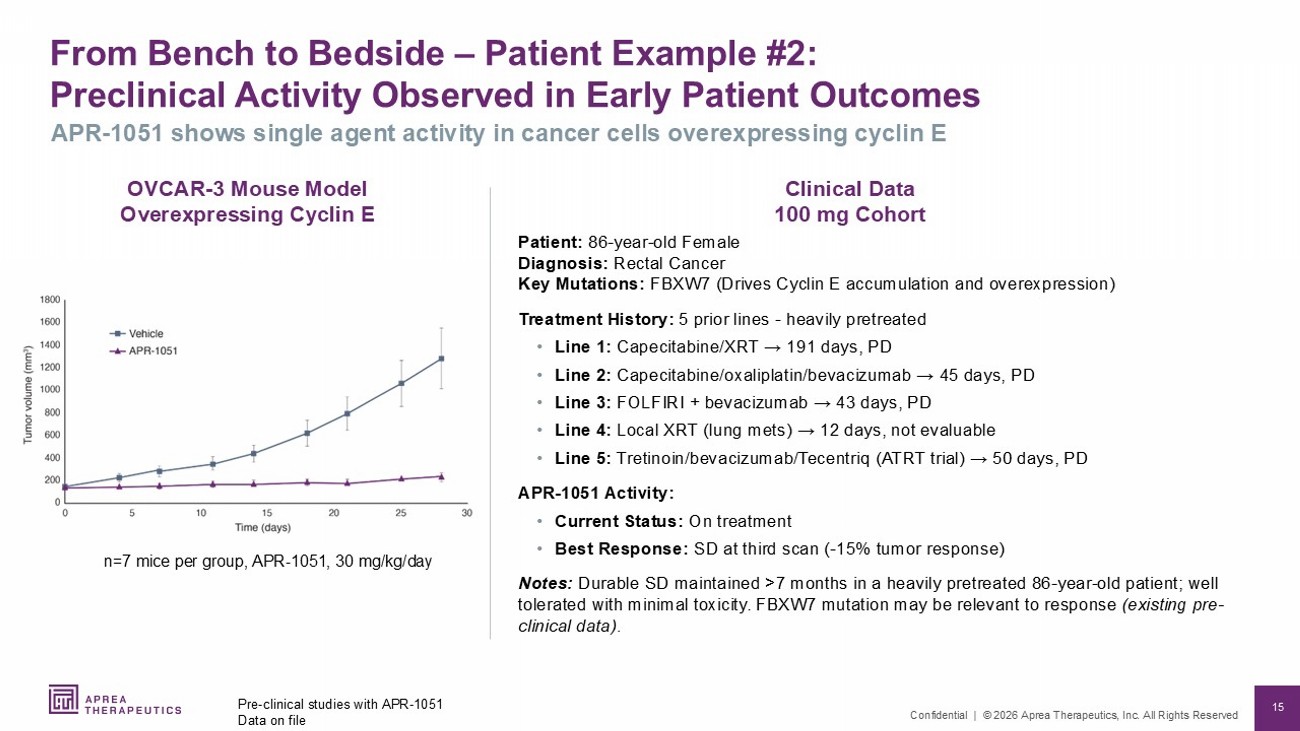
15 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved From Bench to Bedside – Patient Example #2: Preclinical Activity Observed in Early Patient Outcomes Pre - clinical studies with APR - 1051 Data on file APR - 1051 shows single agent activity in cancer cells overexpressing cyclin E n=7 mice per group, APR - 1051, 30 mg/kg/day Clinical Data 100 mg Cohort OVCAR - 3 Mouse Model Overexpressing Cyclin E Patient: 86 - year - old Female Diagnosis: Rectal Cancer Key Mutations: FBXW7 (Drives Cyclin E accumulation and overexpression) Treatment History: 5 prior lines - heavily pretreated • Line 1: Capecitabine/XRT → 191 days, PD • Line 2: Capecitabine/oxaliplatin/bevacizumab → 45 days, PD • Line 3: FOLFIRI + bevacizumab → 43 days, PD • Line 4: Local XRT (lung mets ) → 12 days, not evaluable • Line 5: Tretinoin/bevacizumab/ Tecentriq (ATRT trial) → 50 days, PD APR - 1051 Activity: • Current Status: On treatment • Best Response: SD at third scan ( - 15% tumor response) Notes: Durable SD maintained >7 months in a heavily pretreated 86 - year - old patient; well tolerated with minimal toxicity. FBXW7 mutation may be relevant to response (existing pre - clinical data).
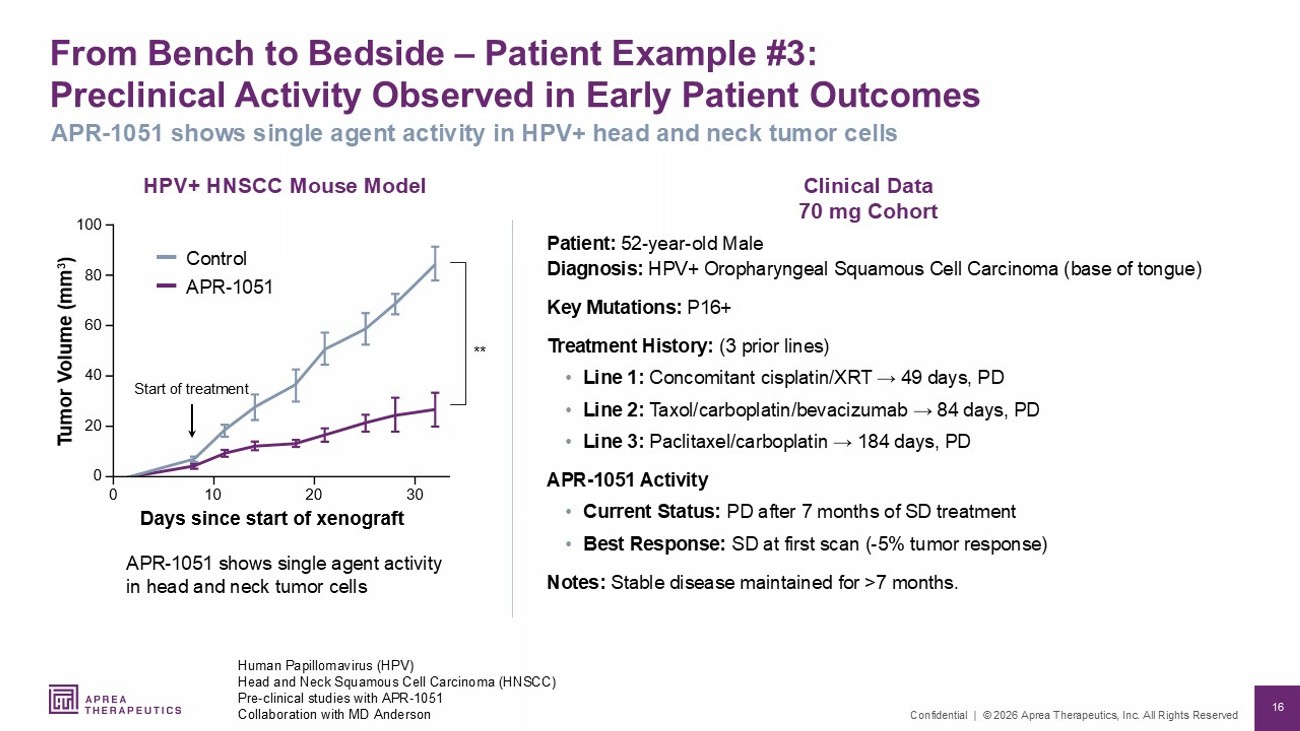
16 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved 'D\VVLQFHVWDUWRI[HQRJUDIW 7 XPRU 9 ROXPH PP Clinical Data 70 mg Cohort HPV+ HNSCC Mouse Model From Bench to Bedside – Patient Example #3: Preclinical Activity Observed in Early Patient Outcomes Patient: 52 - year - old Male Diagnosis: HPV+ Oropharyngeal Squamous Cell Carcinoma (base of tongue) Key Mutations: P16+ Treatment History: (3 prior lines) • Line 1: Concomitant cisplatin/XRT → 49 days, PD • Line 2: Taxol/carboplatin/bevacizumab → 84 days, PD • Line 3: Paclitaxel/carboplatin → 184 days, PD APR - 1051 Activity • Current Status: PD after 7 months of SD treatment • Best Response: SD at first scan ( - 5% tumor response) Notes: Stable disease maintained for >7 months. Human Papillomavirus (HPV) Head and Neck Squamous Cell Carcinoma (HNSCC) Pre - clinical studies with APR - 1051 Collaboration with MD Anderson APR - 1051 shows single agent activity in HPV+ head and neck tumor cells APR - 1051 shows single agent activity in head and neck tumor cells Start of treatment ** Control APR - 1051
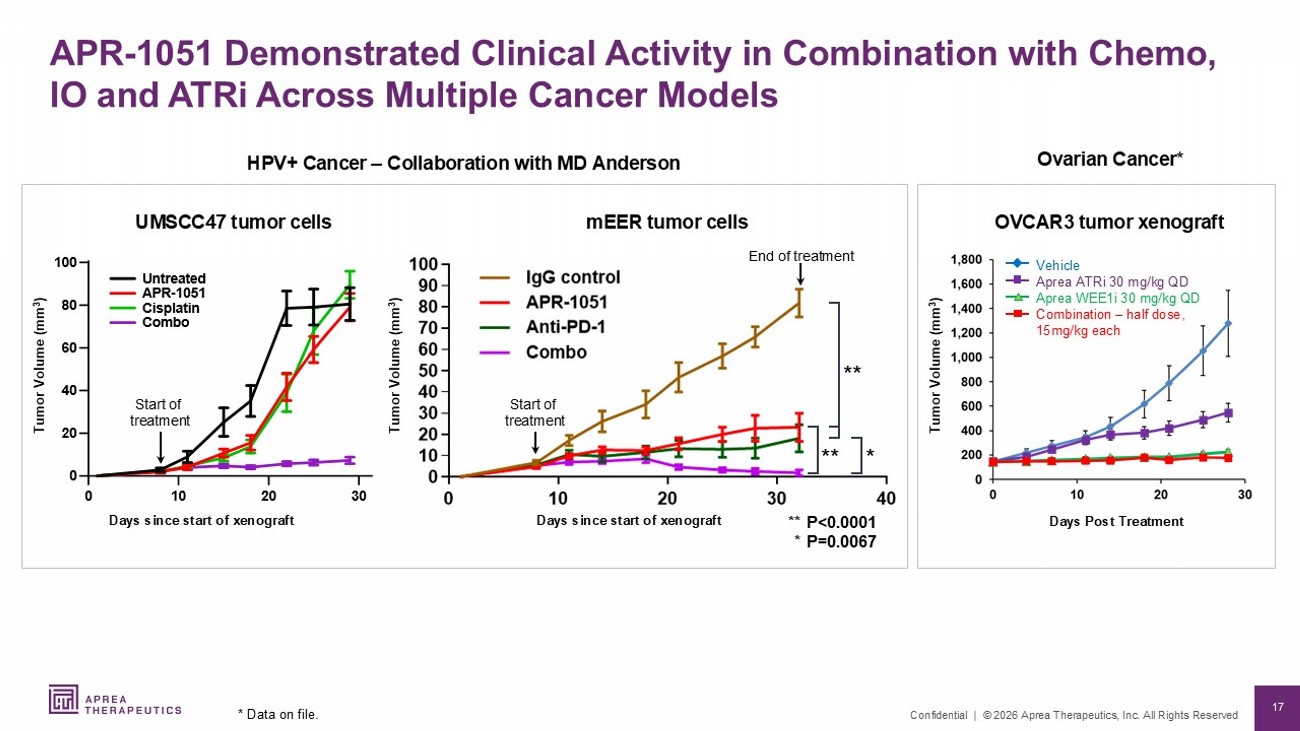
17 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved HPV+ Cancer – Collaboration with MD Anderson Ovarian Cancer * UMSCC47 tumor cells OVCAR3 tumor xenograft Tumor Volume (mm 3 ) Vehicle Aprea ATRi 30 mg/kg QD Aprea WEE1i 30 mg/kg QD Combination – half dose, 15mg/kg each Days Post Treatment Tumor Volume (mm 3 ) Days since start of xenograft mEER tumor cells Tumor Volume (mm 3 ) End of treatment Days since start of xenograft APR - 1051 Demonstrated Clinical Activity in Combination with Chemo, IO and ATRi Across Multiple Cancer Models * Data on file. Start of treatment 8 Q W U HD W H G $ 3 5 & L V S O D W L Q & R P E R Start of treatment ** P<0.0001 * P=0.0067 ** ** *

18 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved 18 APR - 1051: Potentially Differentiated WEE1 Inhibitor Pre - Clinical Confidential
19 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved APR - 1051: Potentially Best - in - Class WEE1 Inhibitor Potent and structurally differentiated, with high selectivity to limit off - target toxicity Aprea APR - 1051 Zentalis Azenosertib (ZN - c3) AstraZeneca Adavosertib (AZD - 1775) Undisclosed
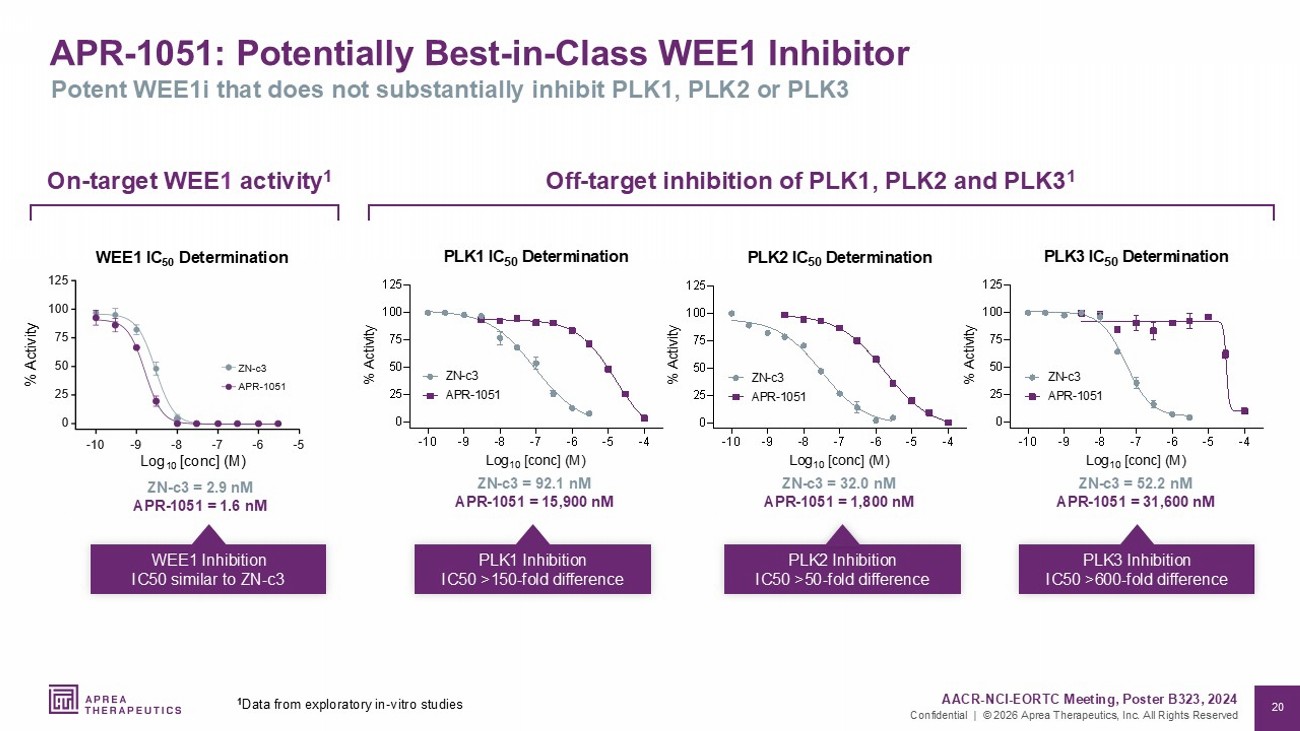
20 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved APR - 1051: Potentially Best - in - Class WEE1 Inhibitor Potent WEE1i that does not substantially inhibit PLK1, PLK2 or PLK3 -10 -9 -8 -7 -6 -5 -4 0 25 50 75 100 125 PLK1 IC 50 Determination Log 10 [conc] (M) % Activity ZN-c3 APR-1051 -10 -9 -8 -7 -6 -5 -4 0 25 50 75 100 125 PLK2 IC 50 Determination Log 10 [conc] (M) % Activity ZN-c3 APR-1051 ZN - c3 = 92.1 nM APR - 1051 = 15,900 nM PLK1 Inhibition IC50 >150 - fold difference ZN - c3 = 32.0 nM APR - 1051 = 1,800 nM PLK2 Inhibition IC50 >50 - fold difference ZN - c3 = 52.2 nM APR - 1051 = 31,600 nM PLK3 Inhibition IC50 >600 - fold difference Off - target inhibition of PLK1, PLK2 and PLK3 1 On - target WEE 1 activity 1 ZN - c3 = 2.9 nM APR - 1051 = 1.6 nM WEE1 Inhibition IC50 similar to ZN - c3 $35 =1 F WEE1 IC 50 Determination % Activity Log 10 [conc] (M) AACR - NCI - EORTC Meeting, Poster B323, 2024 1 Data from exploratory in - vitro studies
21 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Studies Show PLK1 Suppression is Associated with Sepsis - Induced Loss of Intestinal Barrier Function 1 PLK1 protects against sepsis - induced intestinal barrier dysfunction, Cao et al, Scientific Reports (2018). 2 PLK1 protects intestinal barrier function in sepsis: A translational research, Cao et a l, Cytokine (2023). 3 PLK1 protects intestinal barrier function in sepsis: A translational research, Cao et a l, Molecular Medicine (2022). 4 LncRNA DANCR improves the dysfunction of the intestinal barrier and alleviates epithelial injury by targeting the miR - 1306 - 5p/PLK1 axis in sepsis, Wang et al., Cell Biology International (2021).
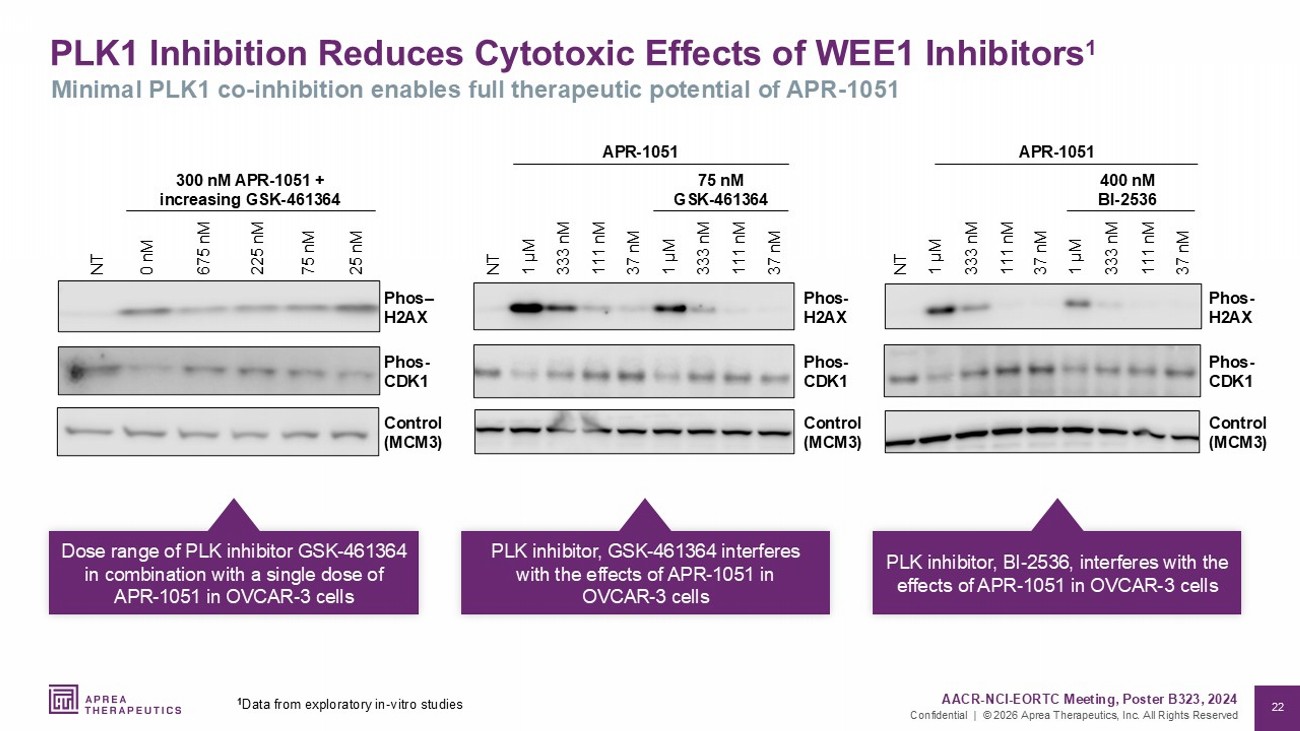
22 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved PLK1 Inhibition Reduces Cytotoxic Effects of WEE1 Inhibitors 1 Minimal PLK1 co - inhibition enables full therapeutic potential of APR - 1051 37 nM 111 nM 333 nM 1 µM 37 nM 111 nM 333 nM 1 µM Phos - H2AX Control (MCM3) NT Phos - CDK1 APR - 1051 75 nM GSK - 461364 0 nM NT 675 nM 25 nM 225 nM 75 nM Phos – H2AX Control (MCM3) Phos - CDK1 300 nM APR - 1051 + increasing GSK - 461364 Control (MCM3) 37 nM 111 nM 333 nM 1 µM 37 nM 111 nM 333 nM 1 µM NT Phos - H2AX 400 nM BI - 2536 APR - 1051 Phos - CDK1 Dose range of PLK inhibitor GSK - 461364 in combination with a single dose of APR - 1051 in OVCAR - 3 cells PLK inhibitor, GSK - 461364 interferes with the effects of APR - 1051 in OVCAR - 3 cells PLK inhibitor, BI - 2536, interferes with the effects of APR - 1051 in OVCAR - 3 cells AACR - NCI - EORTC Meeting, Poster B323, 2024 1 Data from exploratory in - vitro studies

23 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved 23 ATR Inhibitor: ATRN - 119 ABOYA - 119: Clinical Proof - Of - Concept Confidential
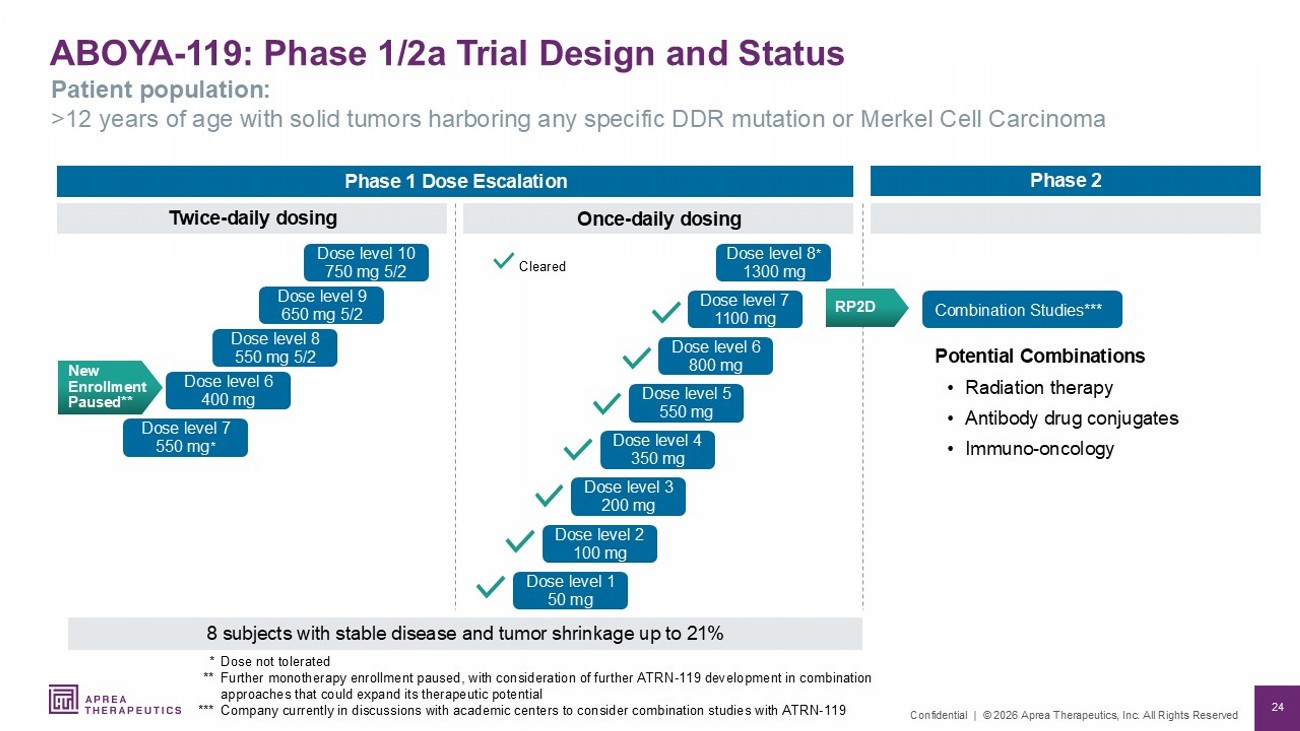
24 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved ABOYA - 119: Phase 1/2a Trial Design and Status Patient population: >12 years of age with solid tumors harboring any specific DDR mutation or Merkel Cell Carcinoma Potential Combinations • Radiation therapy • Antibody drug conjugates • Immuno - oncology Once - daily dosing Twice - daily dosing Cleared New Enrollment Paused** Dose level 6 400 mg Dose level 8 550 mg 5/2 Dose level 9 650 mg 5/2 Dose level 7 550 mg * Dose level 10 750 mg 5/2 Phase 1 Dose Escalation Phase 2 Combination Studies*** * Dose not tolerated ** Further monotherapy enrollment paused, with consideration of further ATRN - 119 development in combination approaches that could expand its therapeutic potential *** Company currently in discussions with academic centers to consider combination studies with ATRN - 119 8 subjects with stable disease and tumor shrinkage up to 21% Dose level 1 50 mg Dose level 2 100 mg Dose level 3 200 mg Dose level 4 350 mg Dose level 5 550 mg Dose level 6 800 mg Dose level 7 1100 mg Dose level 8 * 1300 mg RP2D

25 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Aprea Therapeutics (NASDAQ: APRE) Confidential

26 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved 26 Intellectual Property Portfolio Financial Summary & Capitalization Investment Highlights Confidential
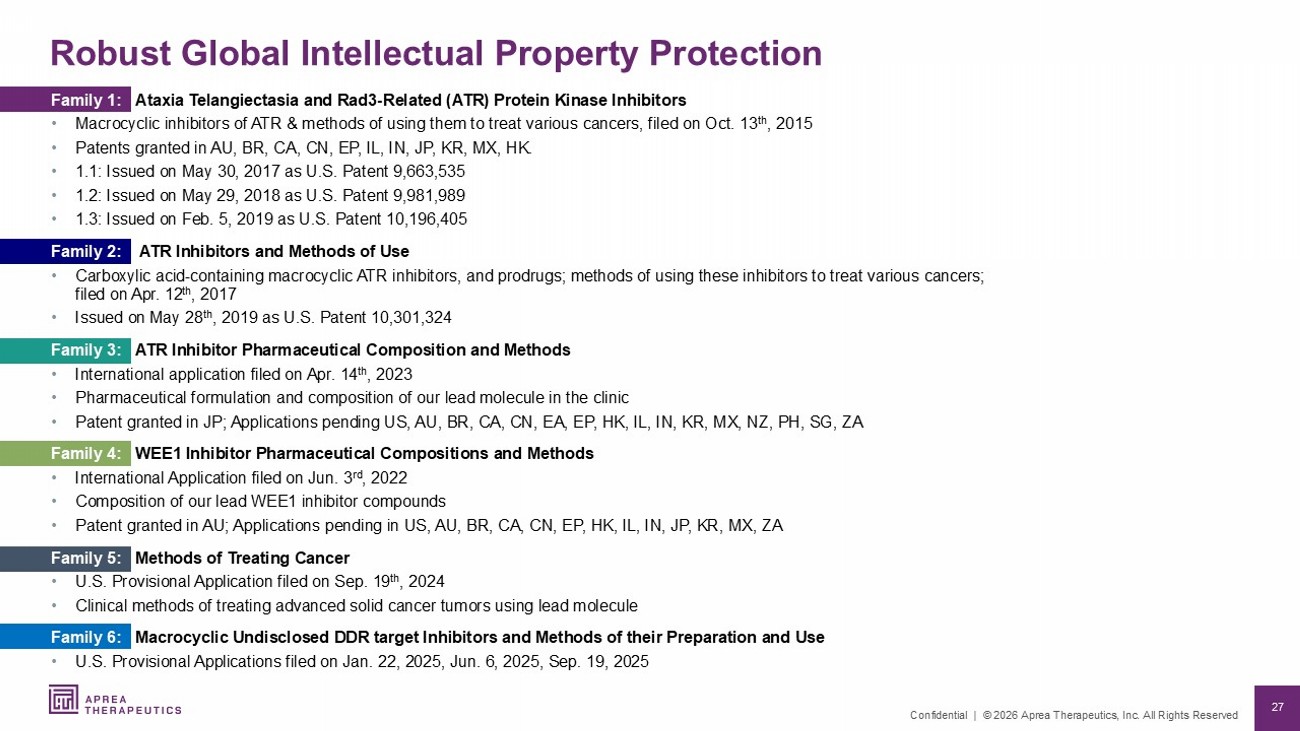
27 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Robust Global Intellectual Property Protection Family 1: Ataxia Telangiectasia and Rad3 - Related (ATR) Protein Kinase Inhibitors • Macrocyclic inhibitors of ATR & methods of using them to treat various cancers, filed on Oct. 13 th , 2015 • Patents granted in AU, BR, CA, CN, EP, IL, IN, JP, KR, MX, HK. • 1.1: Issued on May 30, 2017 as U.S. Patent 9,663,535 • 1.2: Issued on May 29, 2018 as U.S. Patent 9,981,989 • 1.3: Issued on Feb. 5, 2019 as U.S. Patent 10,196,405 Family 2: ATR Inhibitors and Methods of Use • Carboxylic acid - containing macrocyclic ATR inhibitors, and prodrugs; methods of using these inhibitors to treat various cancers; filed on Apr. 12 th , 2017 • Issued on May 28 th , 2019 as U.S. Patent 10,301,324 Family 3: ATR Inhibitor Pharmaceutical Composition and Methods • International application filed on Apr. 14 th , 2023 • Pharmaceutical formulation and composition of our lead molecule in the clinic • Patent granted in JP; Applications pending US, AU, BR, CA, CN, EA, EP, HK, IL, IN, KR, MX, NZ, PH, SG, ZA Family 4: WEE1 Inhibitor Pharmaceutical Compositions and Methods • International Application filed on Jun. 3 rd , 2022 • Composition of our lead WEE1 inhibitor compounds • Patent granted in AU; Applications pending in US, AU, BR, CA, CN, EP, HK, IL, IN, JP, KR, MX, ZA Family 5: Methods of Treating Cancer • U.S. Provisional Application filed on Sep. 19 th , 2024 • Clinical methods of treating advanced solid cancer tumors using lead molecule Family 6: Macrocyclic Undisclosed DDR target Inhibitors and Methods of their Preparation and Use • U.S. Provisional Applications filed on Jan. 22, 2025, Jun. 6, 2025, Sep. 19, 2025
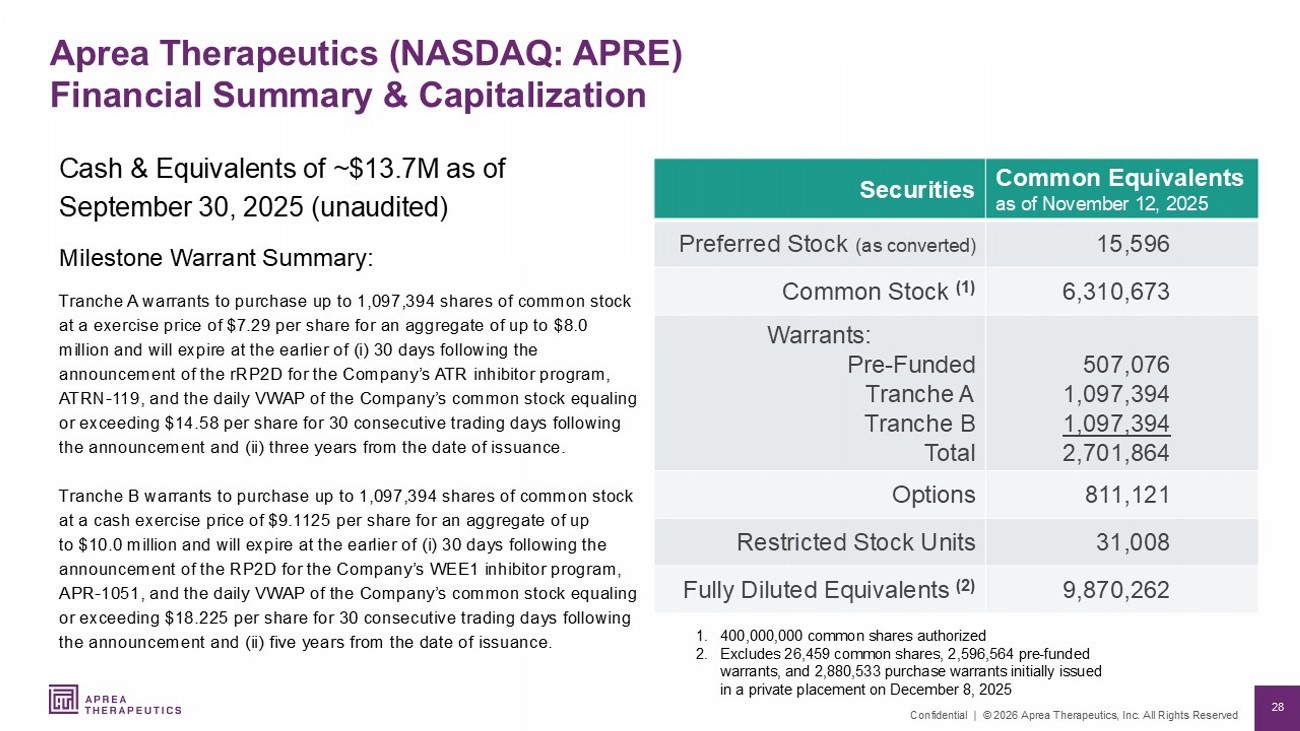
28 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Common Equivalents as of November 12, 2025 Securities 15,596 Preferred Stock (as converted) 6,310,673 Common Stock (1) 507,076 1,097,394 1,097,394 2,701,864 Warrants: Pre - Funded Tranche A Tranche B Total 811,121 Options 31,008 Restricted Stock Units 9,870,262 Fully Diluted Equivalents (2) 1. 400,000,000 common shares authorized 2. Excludes 26,459 common shares, 2,596,564 pre - funded warrants, and 2,880,533 purchase warrants initially issued in a private placement on December 8, 2025 Aprea Therapeutics (NASDAQ: APRE) Financial Summary & Capitalization Cash & Equivalents of ~$13.7M as of September 30, 2025 (unaudited) Milestone Warrant Summary: Tranche A warrants to purchase up to 1,097,394 shares of common stock at a exercise price of $7.29 per share for an aggregate of up to $8.0 million and will expire at the earlier of ( i ) 30 days following the announcement of the rRP2D for the Company’s ATR inhibitor program, ATRN - 119, and the daily VWAP of the Company’s common stock equaling or exceeding $14.58 per share for 30 consecutive trading days following the announcement and (ii) three years from the date of issuance. Tranche B warrants to purchase up to 1,097,394 shares of common stock at a cash exercise price of $9.1125 per share for an aggregate of up to $10.0 million and will expire at the earlier of (i) 30 days following the announcement of the RP2D for the Company’s WEE1 inhibitor program, APR - 1051, and the daily VWAP of the Company’s common stock equaling or exceeding $18.225 per share for 30 consecutive trading days following the announcement and (ii) five years from the date of issuance.
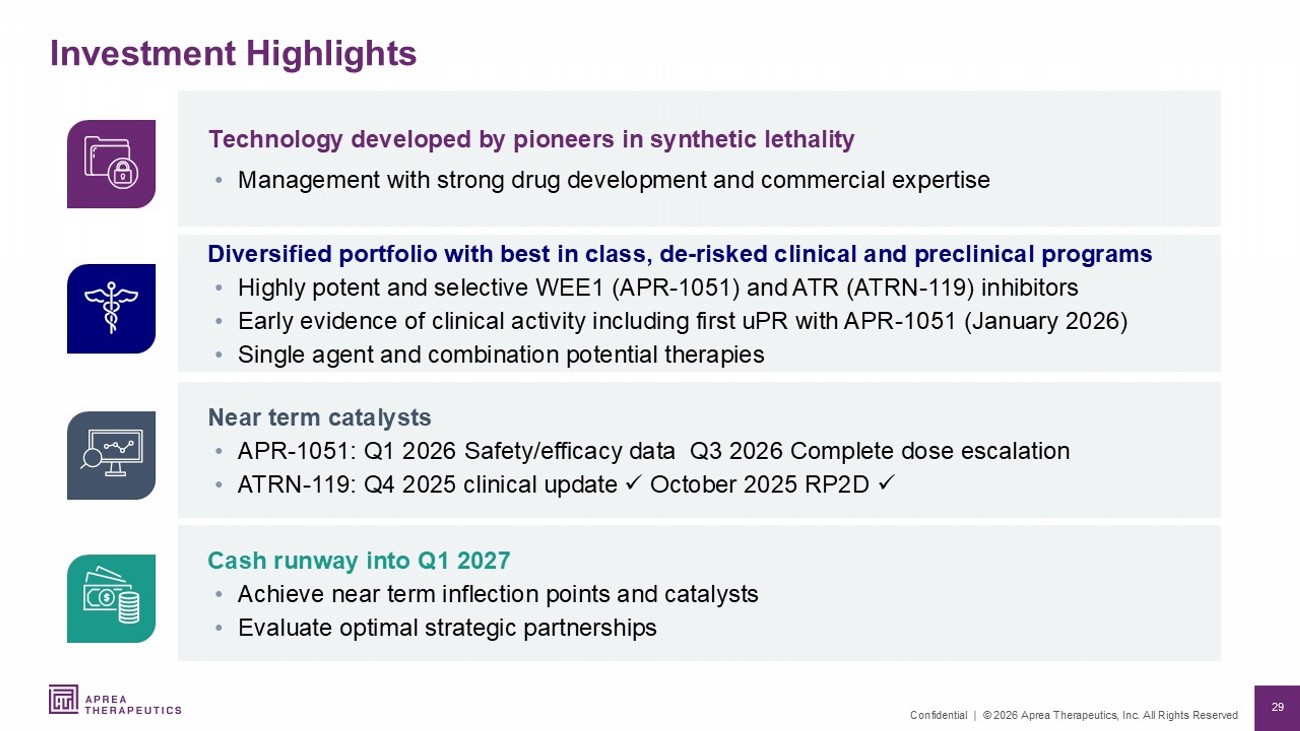
29 Confidential | © 2026 Aprea Therapeutics, Inc. All Rights Reserved Cash runway into Q1 2027 • Achieve near term inflection points and catalysts • Evaluate optimal strategic partnerships Near term catalysts • APR - 1051: Q1 2026 Safety/efficacy data Q3 2026 Complete dose escalation • ATRN - 119: Q4 2025 clinical update x October 2025 RP2D x Diversified portfolio with best in class, de - risked clinical and preclinical programs • Highly potent and selective WEE1 (APR - 1051) and ATR (ATRN - 119) inhibitors • Early evidence of clinical activity including first uPR with APR - 1051 (January 2026) • Single agent and combination potential therapies Technology developed by pioneers in synthetic lethality • Management with strong drug development and commercial expertise Investment Highlights