

Pyxis Oncology MICVO R/M HNSCC Clinical Update December 2025 .2

Forward Looking Statement This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements other than statements of historical facts contained in this presentation, including without limitation statements regarding the Company’s plans to develop, manufacture and commercialize its product candidate, including micvotabart pelidotin (‘MICVO’); preliminary data, timing and progress of the Company’s ongoing clinical trials; the expected results of the Company’s clinical trials; the ability of preliminary, initial and topline clinical data to de-risk MICVO and be confirmed with clinical trial progression, including the safety, tolerability, and potential efficacy of MICVO; the potential differentiation, advantage or effectiveness of MICVO compared to other approved products or products in development; the dosage and treatment potential of MICVO; the size and future of the market; the plans and objectives of management, and the future results of operations and financial position of the Company, are forward-looking statements. These statements are neither promises nor guarantees, but are statements that involve known and unknown risks, uncertainties and other important factors that are in some cases beyond the Company’s control that may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in drug research and development, the Company’s projected cash runway and potential needs for additional funding; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; the Company’s reliance on third parties and collaborators to conduct clinical trials, manufacture their product candidate, and develop and commercialize their product candidate; and the Company’s ability to compete successfully against other drug candidate. Accordingly, investors should not rely upon forward-looking statements as predictions of future events. Except as required by applicable law, the Company undertakes no obligation to update publicly or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Additionally, investors should read risk factors in the section titled “Risk Factors” set forth in Part II, Item 1A. of the Company’s Quarterly Report on Form 10-Q filed on November 3, 2025, and our other filings, each of which is on file with the Securities and Exchange Commission.
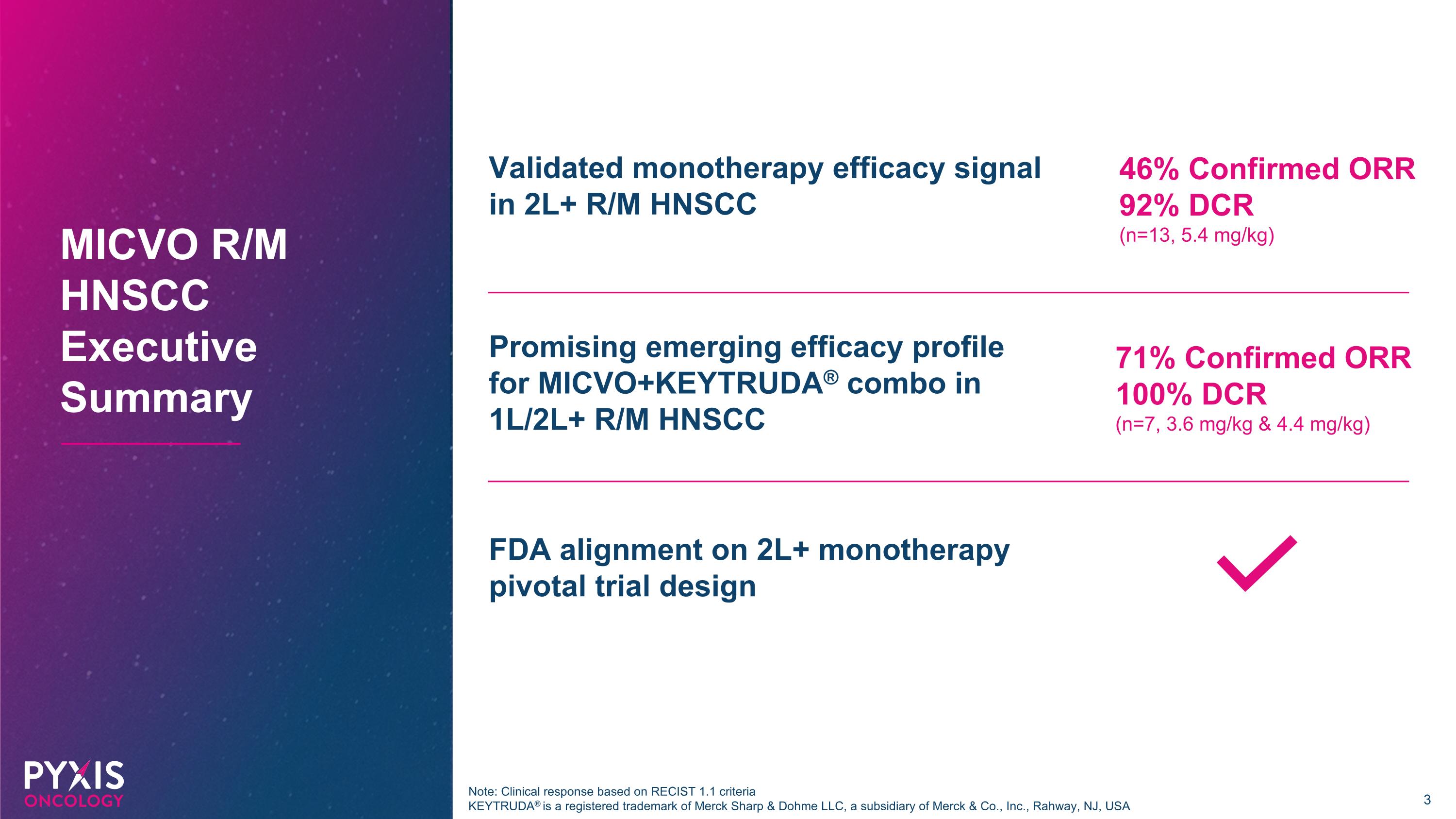
Promising emerging efficacy profile for MICVO+KEYTRUDA® combo in 1L/2L+ R/M HNSCC FDA alignment on 2L+ monotherapy pivotal trial design MICVO R/M HNSCC Executive Summary 46% Confirmed ORR 92% DCR (n=13, 5.4 mg/kg) 71% Confirmed ORR 100% DCR (n=7, 3.6 mg/kg & 4.4 mg/kg) Validated monotherapy efficacy signal in 2L+ R/M HNSCC Note: Clinical response based on RECIST 1.1 criteria KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
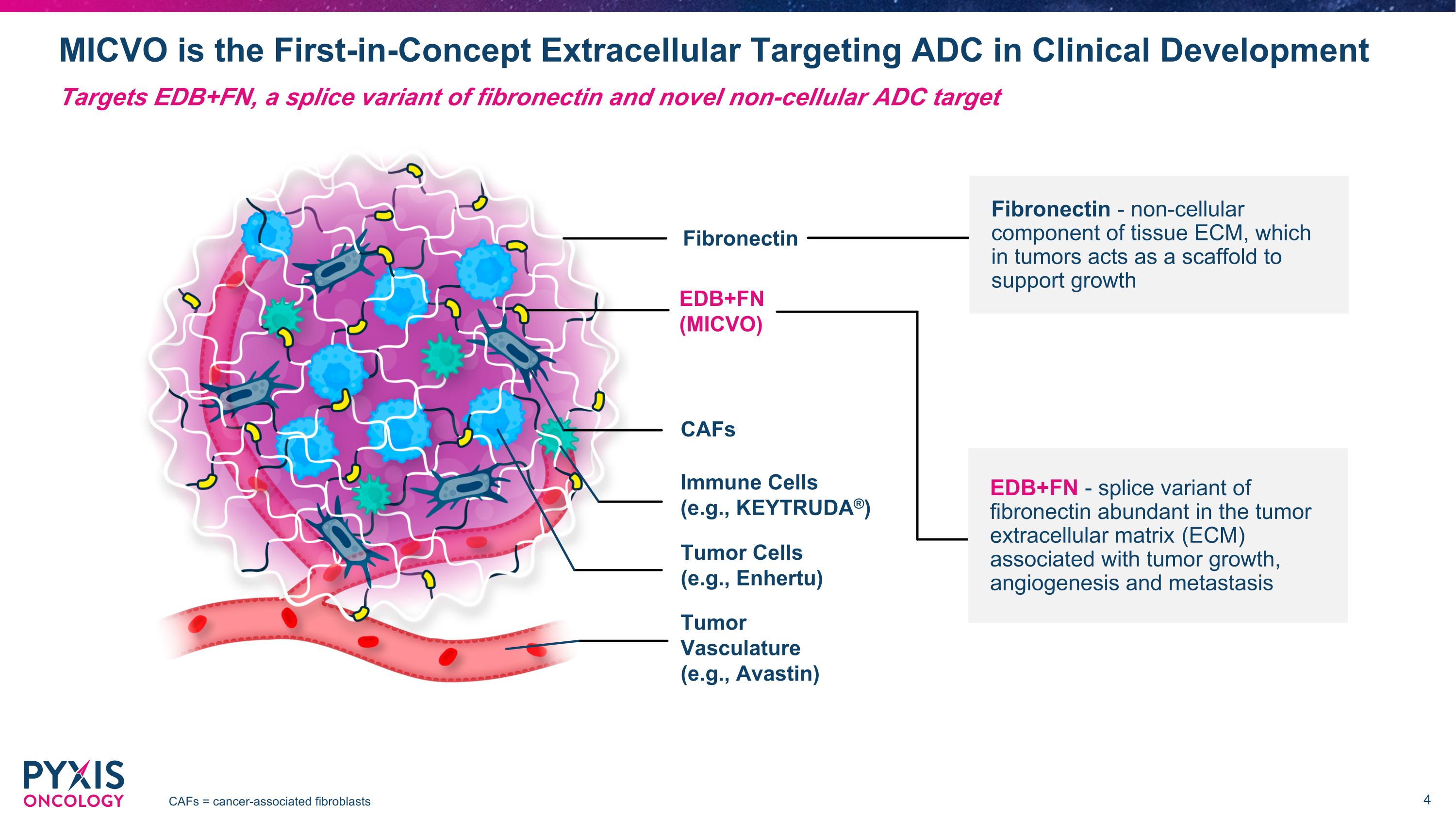
MICVO is the First-in-Concept Extracellular Targeting ADC in Clinical Development CAFs = cancer-associated fibroblasts Tumor Vasculature (e.g., Avastin) Fibronectin CAFs EDB+FN (MICVO) Immune Cells (e.g., KEYTRUDA®) Tumor Cells (e.g., Enhertu) EDB+FN - splice variant of fibronectin abundant in the tumor extracellular matrix (ECM) associated with tumor growth, angiogenesis and metastasis Targets EDB+FN, a splice variant of fibronectin and novel non-cellular ADC target Fibronectin - non-cellular component of tissue ECM, which in tumors acts as a scaffold to support growth
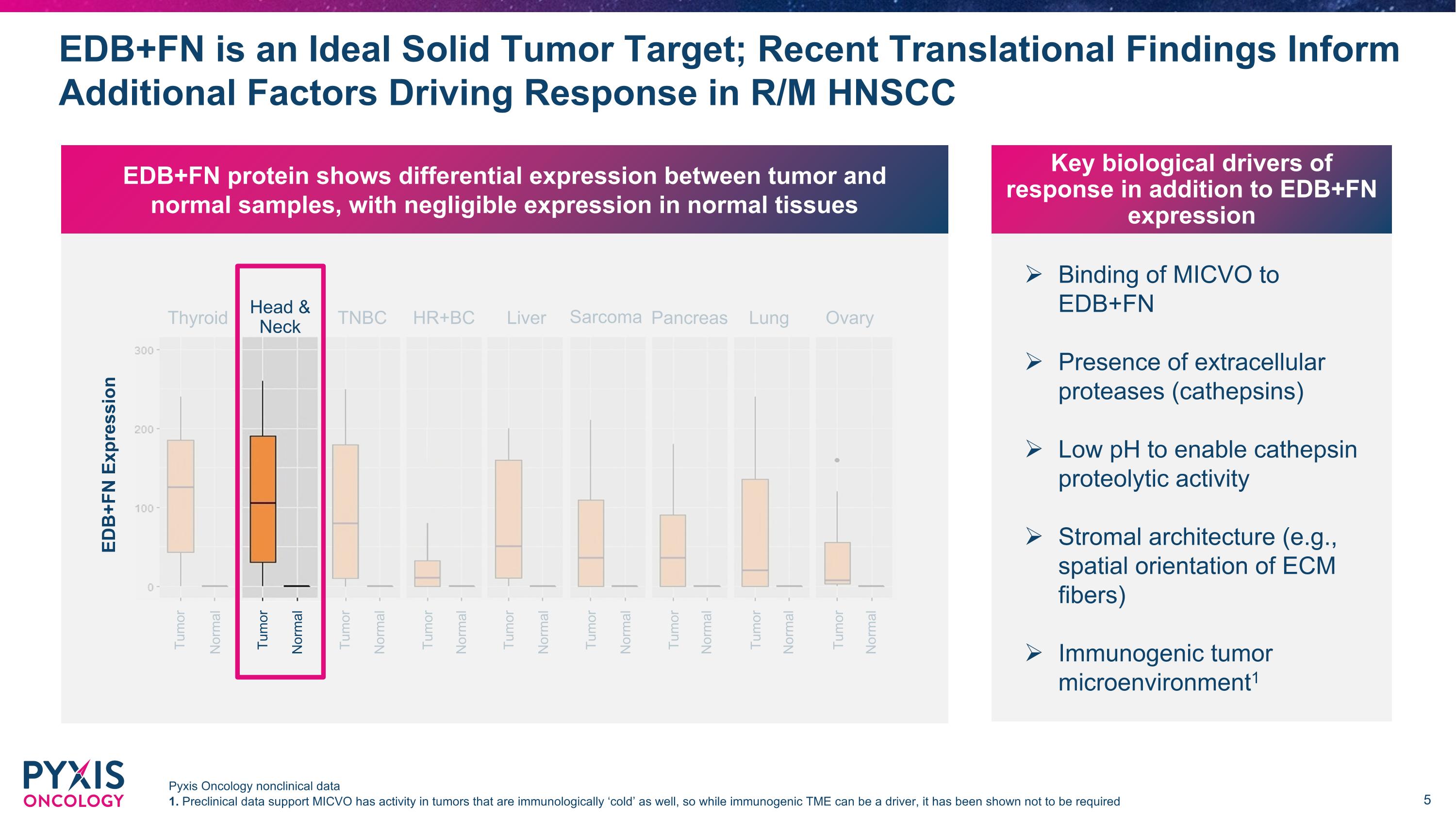
Binding of MICVO to EDB+FN Presence of extracellular proteases (cathepsins) Low pH to enable cathepsin proteolytic activity Stromal architecture (e.g., spatial orientation of ECM fibers) Immunogenic tumor microenvironment1 Key biological drivers of response in addition to EDB+FN expression EDB+FN is an Ideal Solid Tumor Target; Recent Translational Findings Inform Additional Factors Driving Response in R/M HNSCC Pyxis Oncology nonclinical data 1. Preclinical data support MICVO has activity in tumors that are immunologically ‘cold’ as well, so while immunogenic TME can be a driver, it has been shown not to be required EDB+FN protein shows differential expression between tumor and normal samples, with negligible expression in normal tissues EDB+FN Expression Head & Neck Thyroid TNBC Liver Sarcoma Lung Ovary Pancreas HR+BC Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal
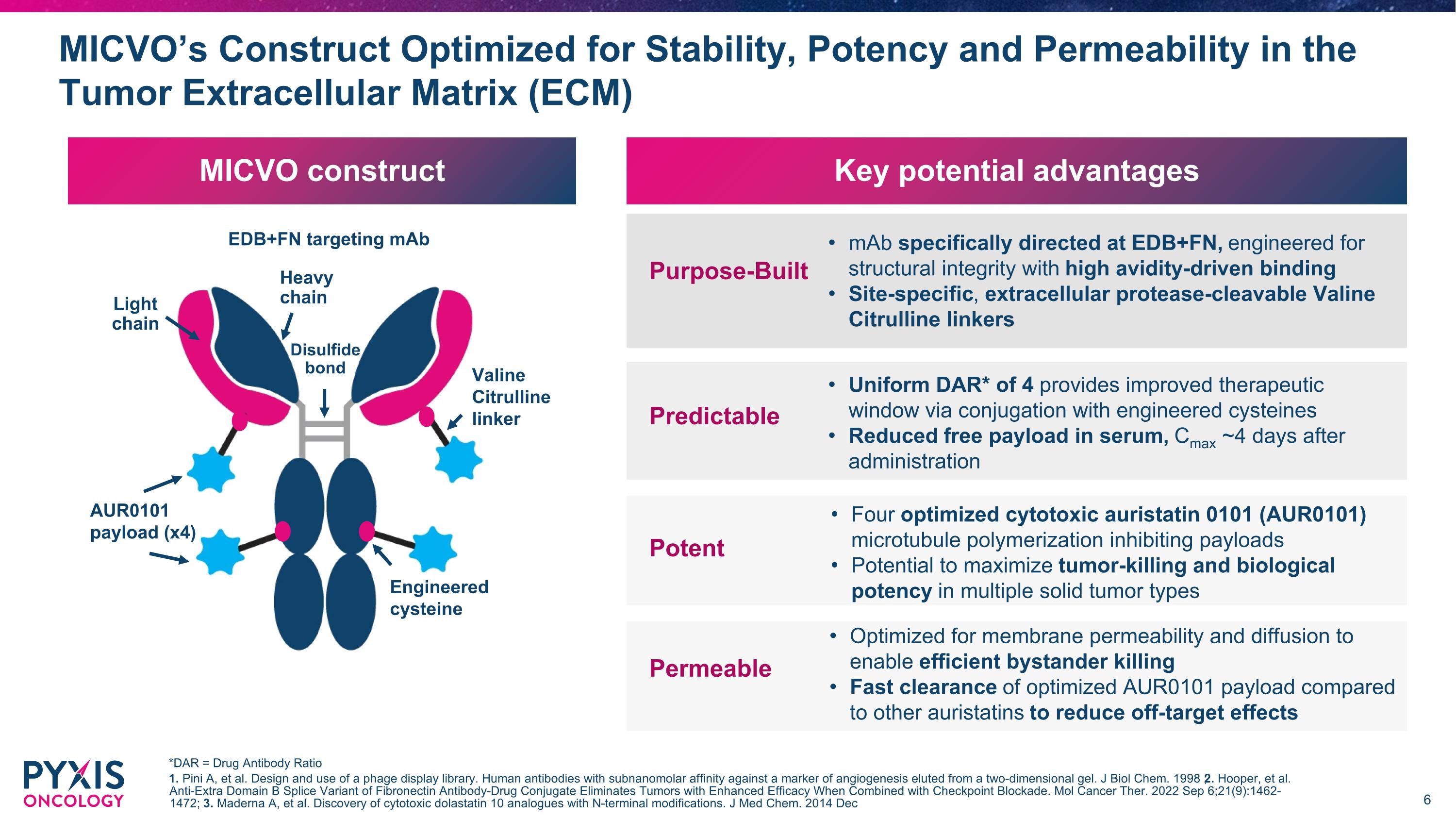
MICVO construct MICVO’s Construct Optimized for Stability, Potency and Permeability in the Tumor Extracellular Matrix (ECM) EDB+FN targeting mAb AUR0101 payload (x4) Valine Citrulline linker Light chain Heavy chain Engineered cysteine Disulfide bond Key potential advantages mAb specifically directed at EDB+FN, engineered for structural integrity with high avidity-driven binding Site-specific, extracellular protease-cleavable Valine Citrulline linkers Uniform DAR* of 4 provides improved therapeutic window via conjugation with engineered cysteines Reduced free payload in serum, Cmax ~4 days after administration Four optimized cytotoxic auristatin 0101 (AUR0101) microtubule polymerization inhibiting payloads Potential to maximize tumor-killing and biological potency in multiple solid tumor types *DAR = Drug Antibody Ratio 1. Pini A, et al. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998 ; 2. Hooper, et al. Anti-Extra Domain B Splice Variant of Fibronectin Antibody-Drug Conjugate Eliminates Tumors with Enhanced Efficacy When Combined with Checkpoint Blockade. Mol Cancer Ther. 2022 Sep 6;21(9):1462-1472; 3. Maderna A, et al. Discovery of cytotoxic dolastatin 10 analogues with N-terminal modifications. J Med Chem. 2014 Dec Purpose-Built Predictable Optimized for membrane permeability and diffusion to enable efficient bystander killing Fast clearance of optimized AUR0101 payload compared to other auristatins to reduce off-target effects Potent Permeable
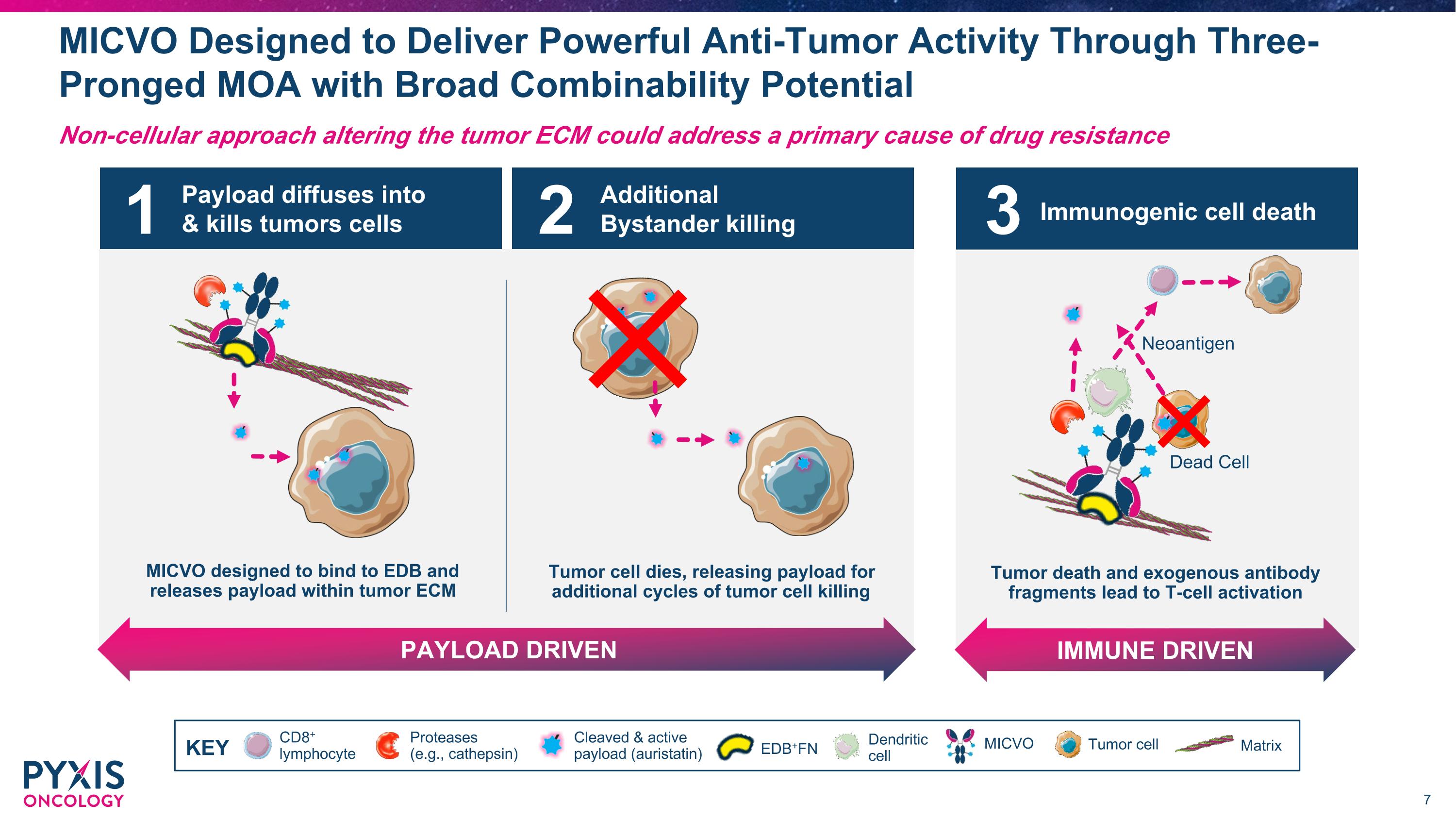
Payload diffuses into & kills tumors cells MICVO designed to bind to EDB and releases payload within tumor ECM Additional Bystander killing PAYLOAD DRIVEN Tumor cell dies, releasing payload for additional cycles of tumor cell killing 1 2 MICVO Designed to Deliver Powerful Anti-Tumor Activity Through Three-Pronged MOA with Broad Combinability Potential Non-cellular approach altering the tumor ECM could address a primary cause of drug resistance CD8+ lymphocyte Dendritic cell MICVO Cleaved & active payload (auristatin) Tumor cell Matrix Proteases (e.g., cathepsin) KEY EDB+FN Immunogenic cell death IMMUNE DRIVEN Tumor death and exogenous antibody fragments lead to T-cell activation Dead Cell Neoantigen 3
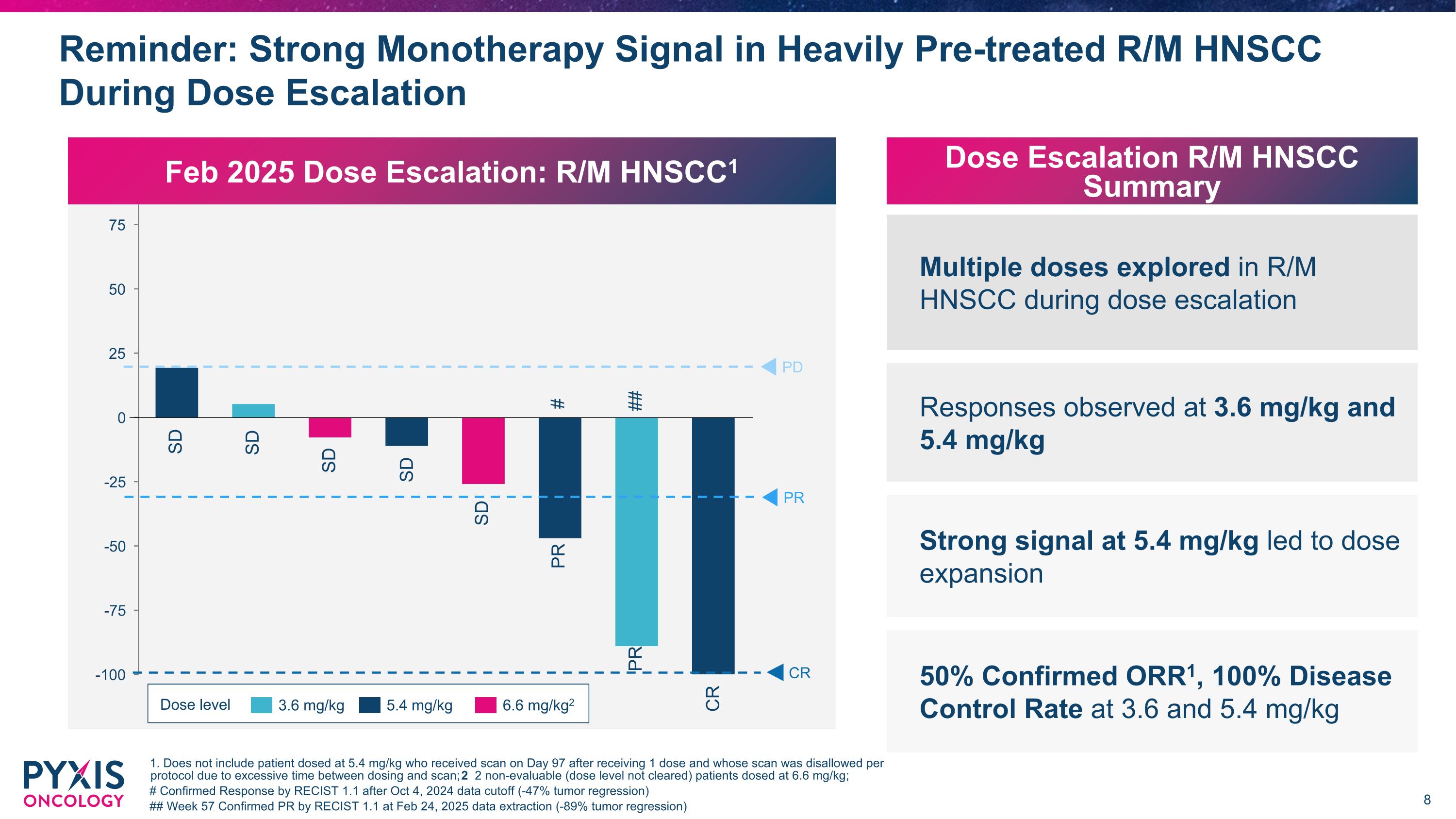
Reminder: Strong Monotherapy Signal in Heavily Pre-treated R/M HNSCC During Dose Escalation 1. Does not include patient dosed at 5.4 mg/kg who received scan on Day 97 after receiving 1 dose and whose scan was disallowed per protocol due to excessive time between dosing and scan; 2 2 non-evaluable (dose level not cleared) patients dosed at 6.6 mg/kg; # Confirmed Response by RECIST 1.1 after Oct 4, 2024 data cutoff (-47% tumor regression) ## Week 57 Confirmed PR by RECIST 1.1 at Feb 24, 2025 data extraction (-89% tumor regression) Dose level 3.6 mg/kg 5.4 mg/kg Feb 2025 Dose Escalation: R/M HNSCC1 PD SD SD SD PR CR SD PR CR SD PR Multiple doses explored in R/M HNSCC during dose escalation Responses observed at 3.6 mg/kg and 5.4 mg/kg Strong signal at 5.4 mg/kg led to dose expansion Dose Escalation R/M HNSCC Summary 50% Confirmed ORR1, 100% Disease Control Rate at 3.6 and 5.4 mg/kg # ## 6.6 mg/kg2
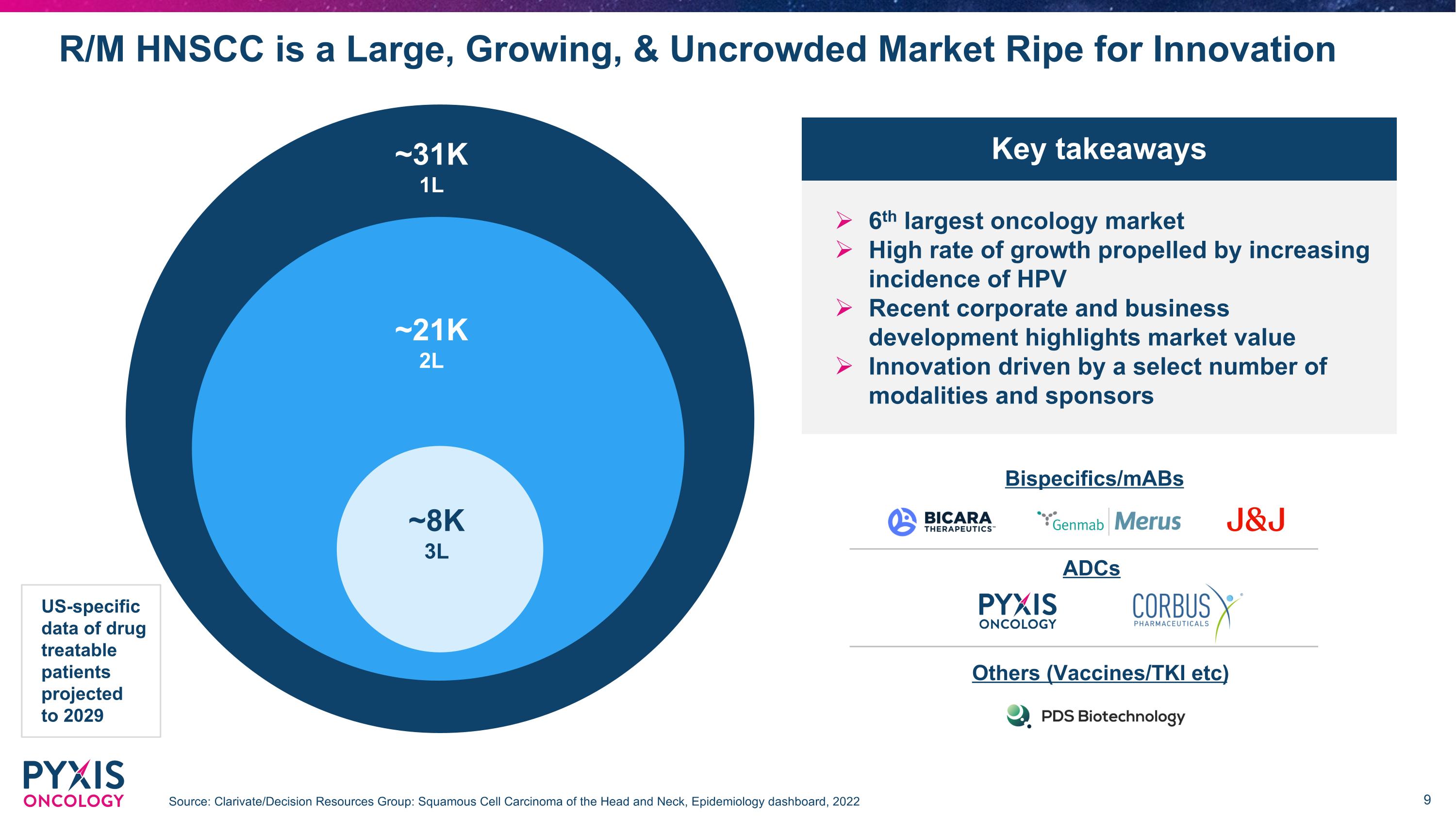
R/M HNSCC is a Large, Growing, & Uncrowded Market Ripe for Innovation Source: Clarivate/Decision Resources Group: Squamous Cell Carcinoma of the Head and Neck, Epidemiology dashboard, 2022 ~31K 1L ~21K 2L ~8K 3L US-specific data of drug treatable patients projected to 2029 6th largest oncology market High rate of growth propelled by increasing incidence of HPV Recent corporate and business development highlights market value Innovation driven by a select number of modalities and sponsors Key takeaways Bispecifics/mABs ADCs Others (Vaccines/TKI etc)
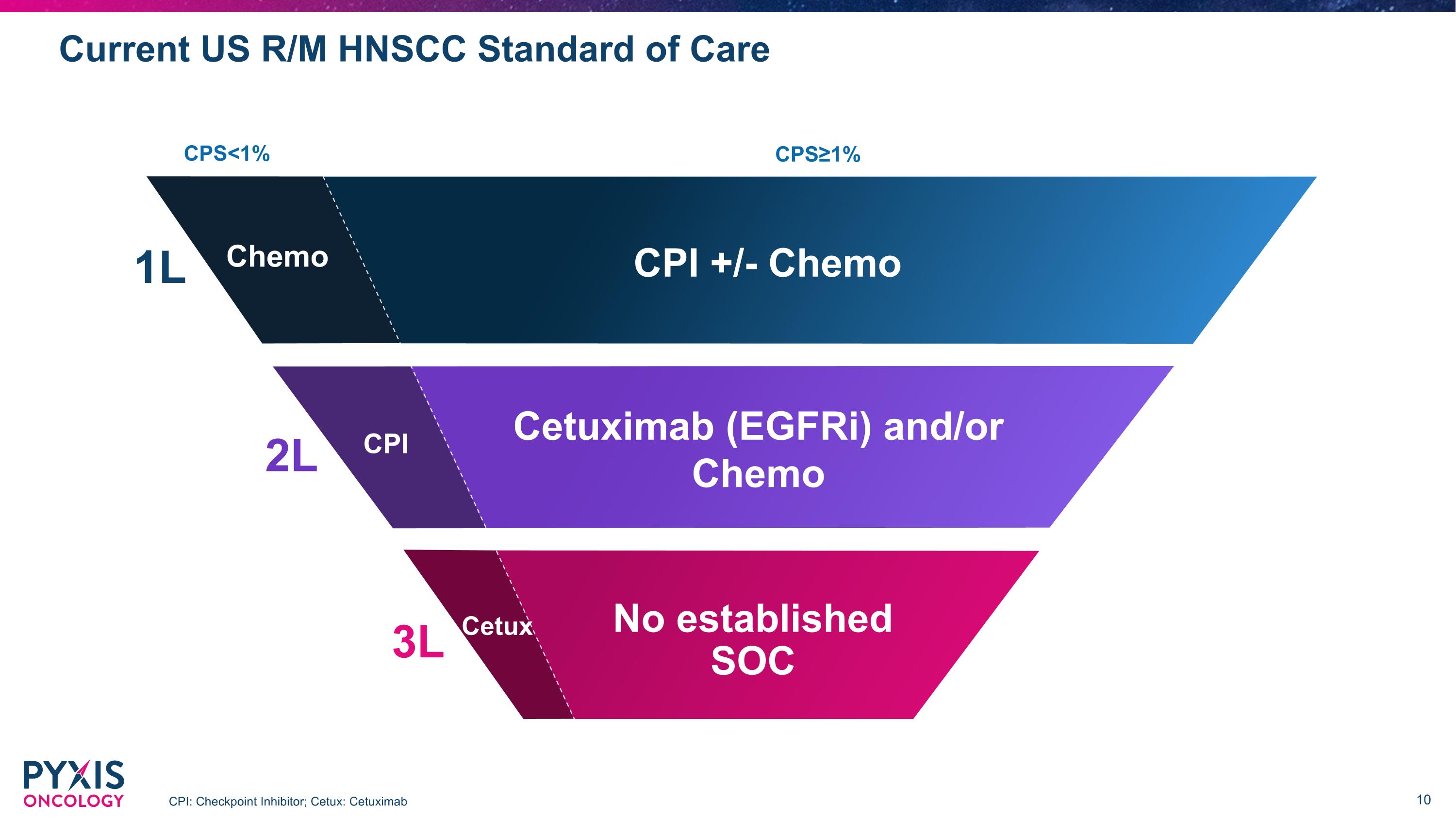
Current US R/M HNSCC Standard of Care CPI: Checkpoint Inhibitor; Cetux: Cetuximab CPI 3.0K 1L CPS<1% CPS≥1% CPI +/- Chemo Chemo 2L Cetuximab (EGFRi) and/or Chemo CPI 3L Cetux No established SOC
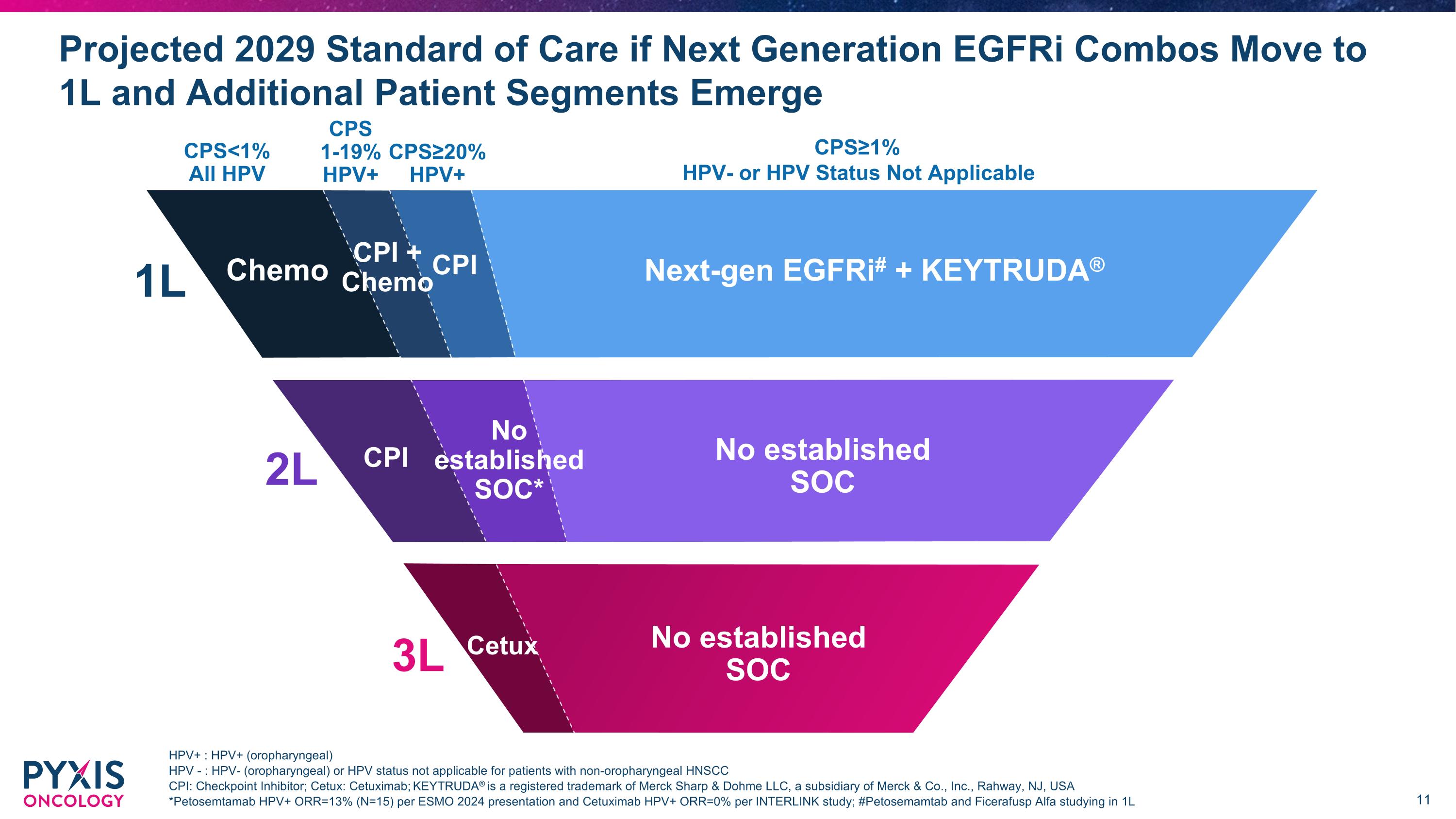
Projected 2029 Standard of Care if Next Generation EGFRi Combos Move to 1L and Additional Patient Segments Emerge CPI 3.0K 1L CPS<1% All HPV CPS≥20% HPV+ CPS 1-19% HPV+ CPS≥1% HPV- or HPV Status Not Applicable Next-gen EGFRi# + KEYTRUDA® Chemo CPI CPI + Chemo 2L No established SOC* No established SOC CPI 3L No established SOC HPV+ : HPV+ (oropharyngeal) HPV - : HPV- (oropharyngeal) or HPV status not applicable for patients with non-oropharyngeal HNSCC CPI: Checkpoint Inhibitor; Cetux: Cetuximab; KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA *Petosemtamab HPV+ ORR=13% (N=15) per ESMO 2024 presentation and Cetuximab HPV+ ORR=0% per INTERLINK study; #Petosemamtab and Ficerafusp Alfa studying in 1L Cetux
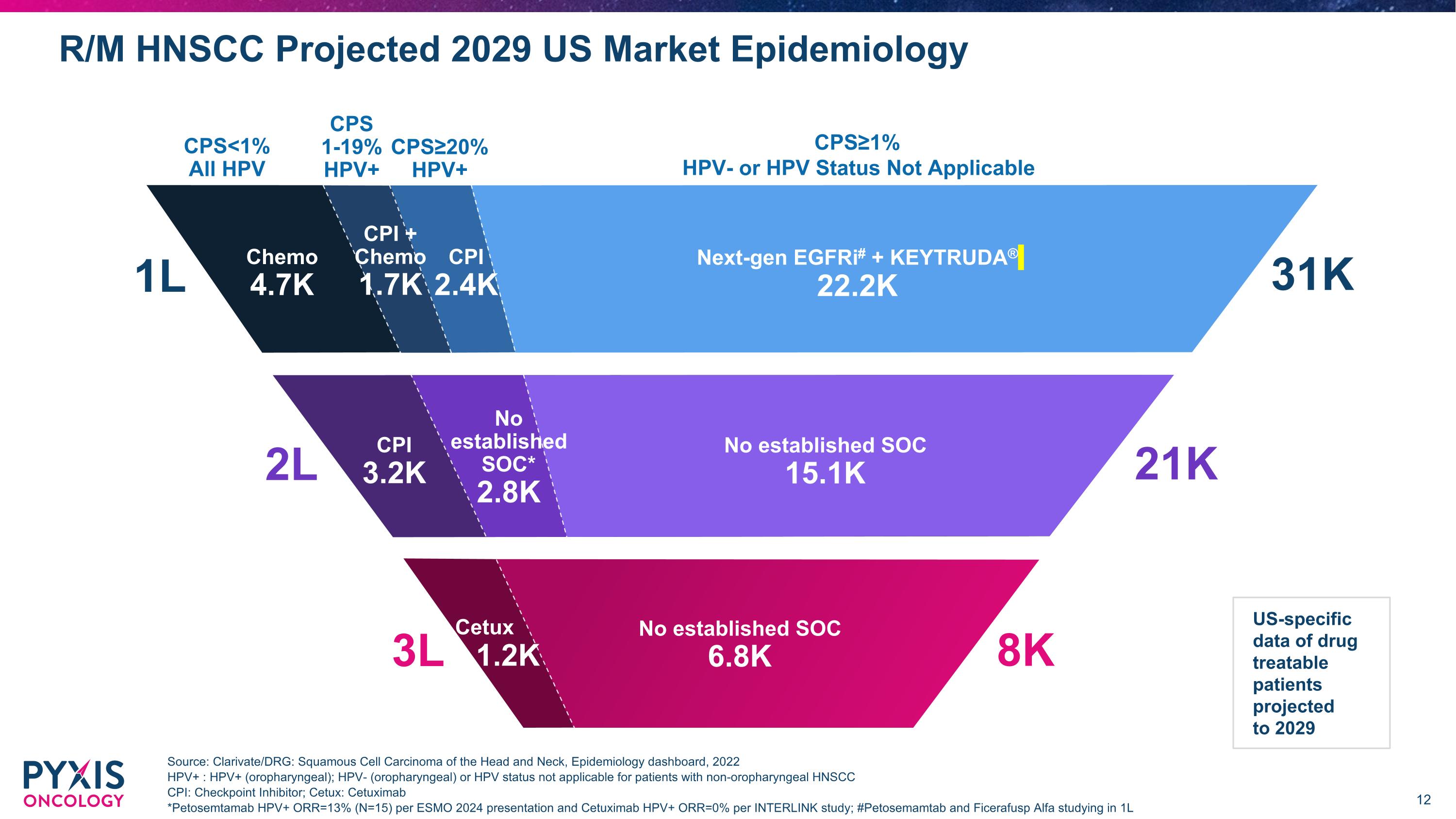
R/M HNSCC Projected 2029 US Market Epidemiology Source: Clarivate/DRG: Squamous Cell Carcinoma of the Head and Neck, Epidemiology dashboard, 2022 HPV+ : HPV+ (oropharyngeal); HPV- (oropharyngeal) or HPV status not applicable for patients with non-oropharyngeal HNSCC CPI: Checkpoint Inhibitor; Cetux: Cetuximab *Petosemtamab HPV+ ORR=13% (N=15) per ESMO 2024 presentation and Cetuximab HPV+ ORR=0% per INTERLINK study; #Petosemamtab and Ficerafusp Alfa studying in 1L US-specific data of drug treatable patients projected to 2029 CPI 3.0K 1L CPS<1% All HPV CPS≥20% HPV+ CPS 1-19% HPV+ CPS≥1% HPV- or HPV Status Not Applicable Next-gen EGFRi# + KEYTRUDA® 22.2K Chemo 4.7K CPI 2.4K CPI + Chemo 1.7K 31K 2L No established SOC* 2.8K No established SOC 15.1K 21K CPI 3.2K 8K 3L Cetux 1.2K No established SOC 6.8K
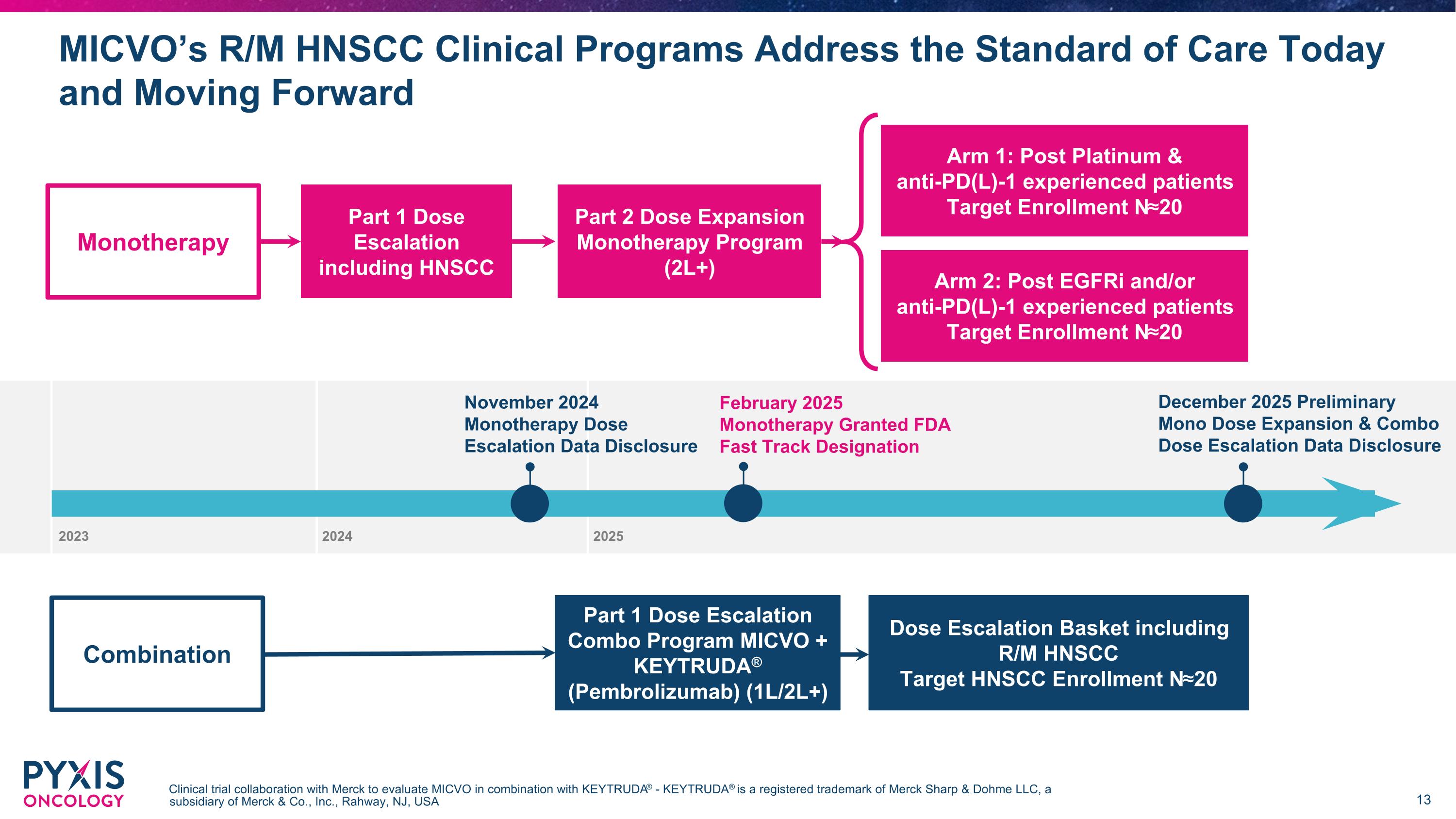
November 2024 Monotherapy Dose Escalation Data Disclosure December 2025 Preliminary Mono Dose Expansion & Combo Dose Escalation Data Disclosure MICVO’s R/M HNSCC Clinical Programs Address the Standard of Care Today and Moving Forward Clinical trial collaboration with Merck to evaluate MICVO in combination with KEYTRUDA® - KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA Arm 1: Post Platinum & anti-PD(L)-1 experienced patients Target Enrollment N≈20 Part 2 Dose Expansion Monotherapy Program (2L+) Part 1 Dose Escalation Combo Program MICVO + KEYTRUDA® (Pembrolizumab) (1L/2L+) Part 1 Dose Escalation including HNSCC Dose Escalation Basket including R/M HNSCC Target HNSCC Enrollment N≈20 Monotherapy Combination Arm 2: Post EGFRi and/or anti-PD(L)-1 experienced patients Target Enrollment N≈20 2023 2024 2025 February 2025 Monotherapy Granted FDA Fast Track Designation
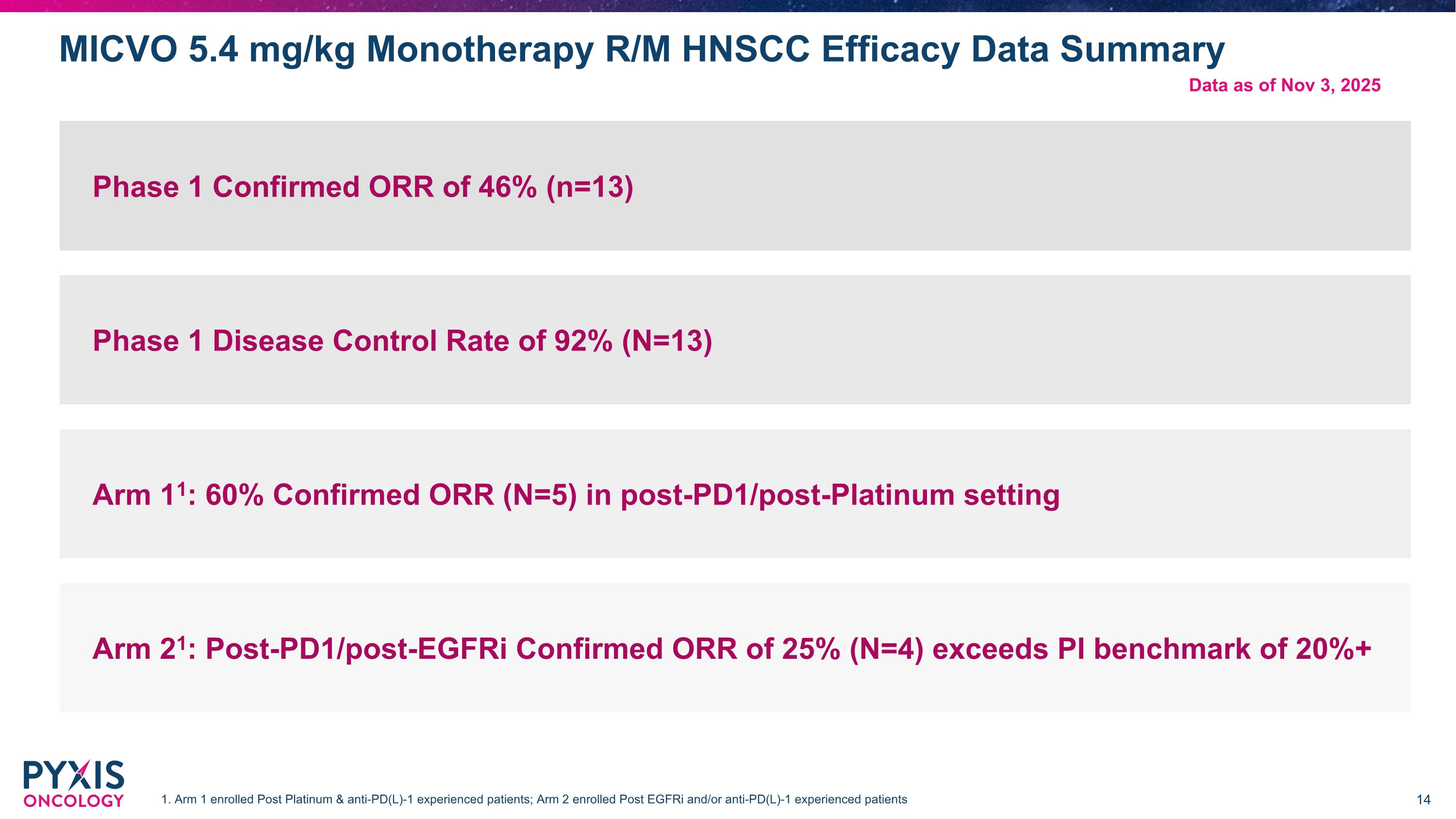
MICVO 5.4 mg/kg Monotherapy R/M HNSCC Efficacy Data Summary Phase 1 Confirmed ORR of 46% (n=13) Phase 1 Disease Control Rate of 92% (N=13) Arm 11: 60% Confirmed ORR (N=5) in post-PD1/post-Platinum setting Arm 21: Post-PD1/post-EGFRi Confirmed ORR of 25% (N=4) exceeds PI benchmark of 20%+ 1. Arm 1 enrolled Post Platinum & anti-PD(L)-1 experienced patients; Arm 2 enrolled Post EGFRi and/or anti-PD(L)-1 experienced patients Data as of Nov 3, 2025
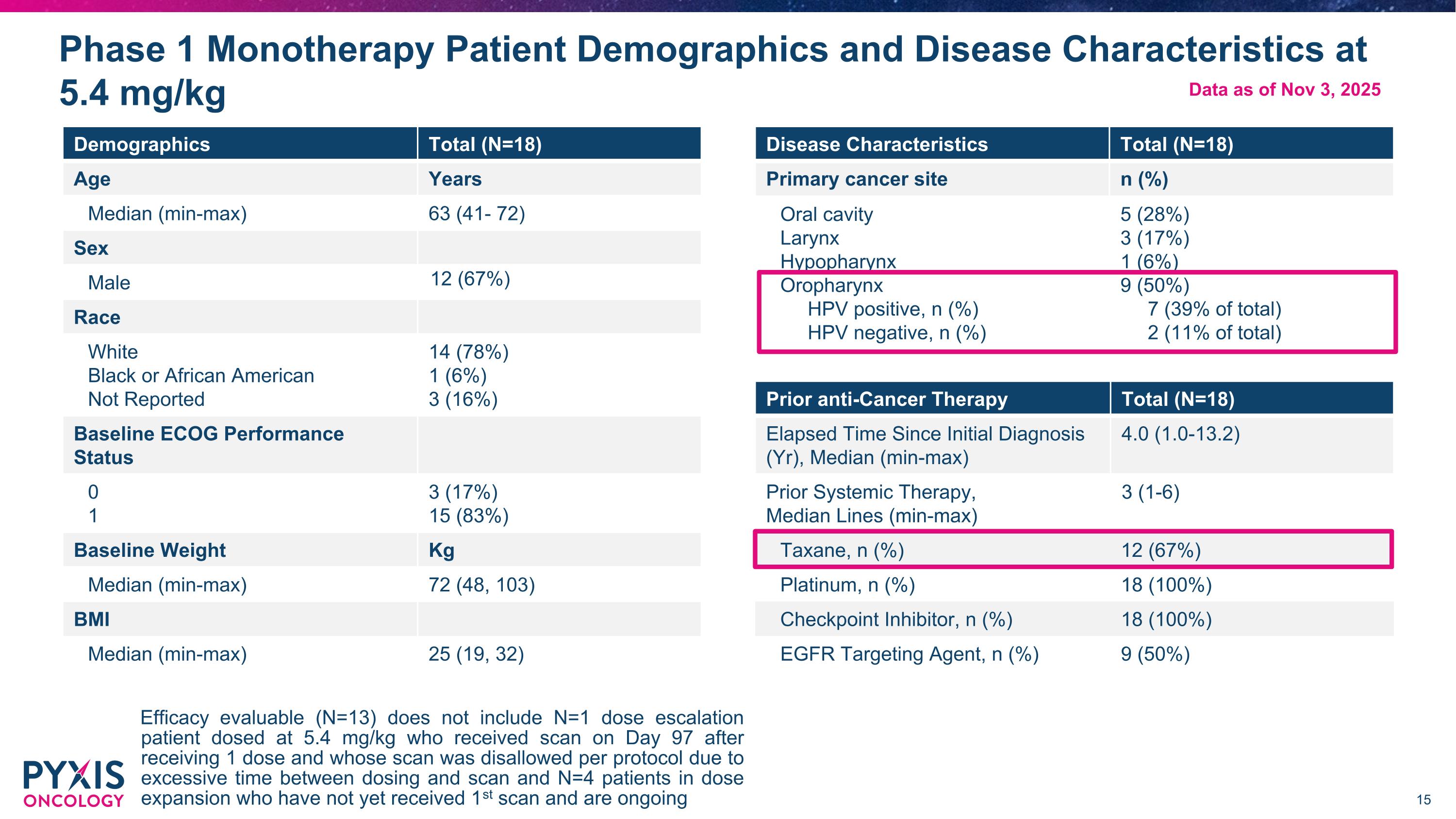
Phase 1 Monotherapy Patient Demographics and Disease Characteristics at 5.4 mg/kg Efficacy evaluable (N=13) does not include N=1 dose escalation patient dosed at 5.4 mg/kg who received scan on Day 97 after receiving 1 dose and whose scan was disallowed per protocol due to excessive time between dosing and scan and N=4 patients in dose expansion who have not yet received 1st scan and are ongoing Demographics Total (N=18) Age Years Median (min-max) 63 (41- 72) Sex Male 12 (67%) Race White Black or African American Not Reported 14 (78%) 1 (6%) 3 (16%) Baseline ECOG Performance Status 0 1 3 (17%) 15 (83%) Baseline Weight Kg Median (min-max) 72 (48, 103) BMI Median (min-max) 25 (19, 32) Prior anti-Cancer Therapy Total (N=18) Elapsed Time Since Initial Diagnosis (Yr), Median (min-max) 4.0 (1.0-13.2) Prior Systemic Therapy, Median Lines (min-max) 3 (1-6) Taxane, n (%) 12 (67%) Platinum, n (%) 18 (100%) Checkpoint Inhibitor, n (%) 18 (100%) EGFR Targeting Agent, n (%) 9 (50%) Disease Characteristics Total (N=18) Primary cancer site n (%) Oral cavity Larynx Hypopharynx Oropharynx HPV positive, n (%) HPV negative, n (%) 5 (28%) 3 (17%) 1 (6%) 9 (50%) 7 (39% of total) 2 (11% of total) Data as of Nov 3, 2025
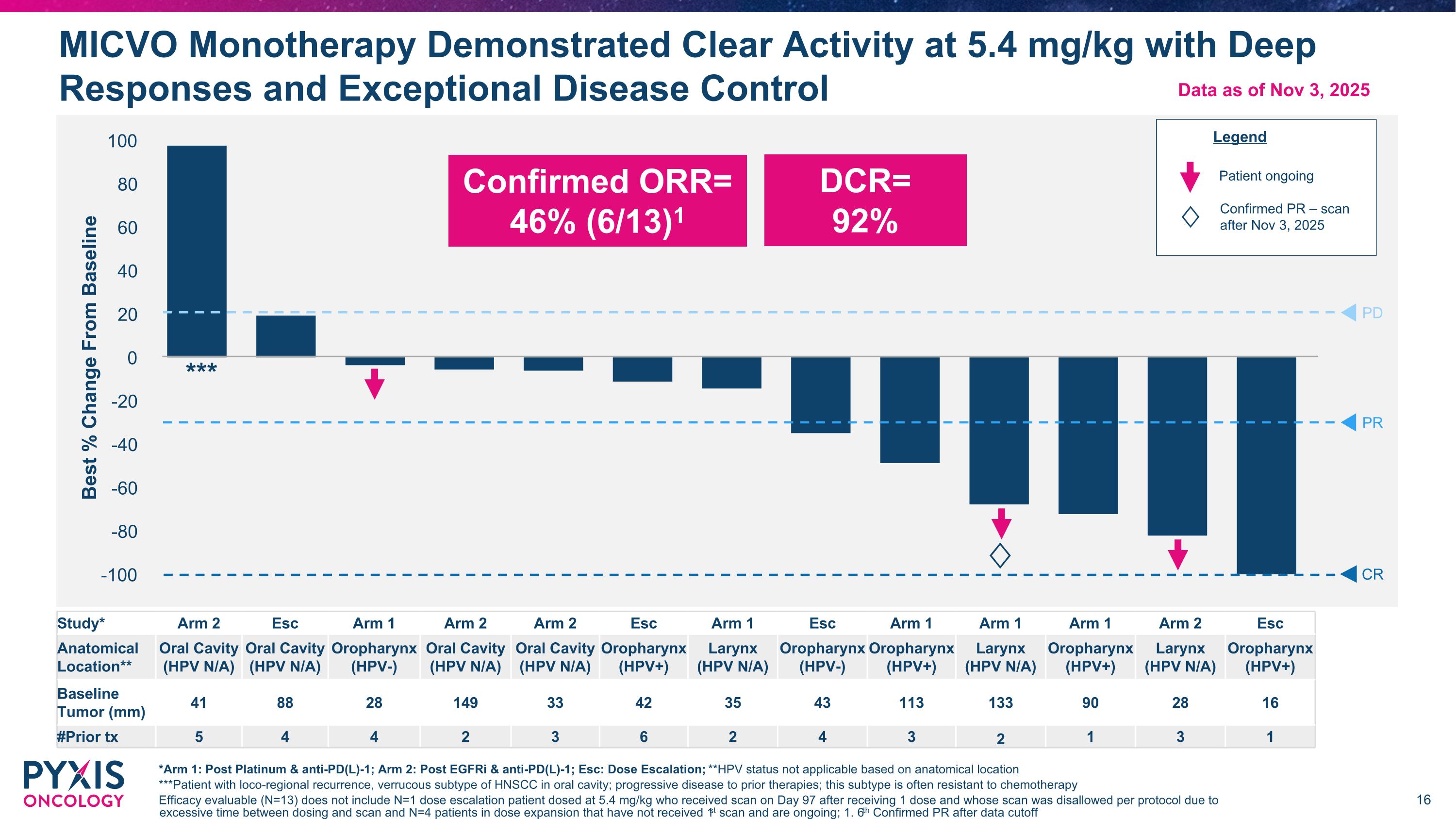
MICVO Monotherapy Demonstrated Clear Activity at 5.4 mg/kg with Deep Responses and Exceptional Disease Control *Arm 1: Post Platinum & anti-PD(L)-1; Arm 2: Post EGFRi & anti-PD(L)-1; Esc: Dose Escalation; **HPV status not applicable based on anatomical location ***Patient with loco-regional recurrence, verrucous subtype of HNSCC in oral cavity; progressive disease to prior therapies; this subtype is often resistant to chemotherapy Efficacy evaluable (N=13) does not include N=1 dose escalation patient dosed at 5.4 mg/kg who received scan on Day 97 after receiving 1 dose and whose scan was disallowed per protocol due to excessive time between dosing and scan and N=4 patients in dose expansion that have not received 1st scan and are ongoing; 1. 6th Confirmed PR after data cutoff PD PR CR ♢ Study* Arm 2 Esc Arm 1 Arm 2 Arm 2 Esc Arm 1 Esc Arm 1 Arm 1 Arm 1 Arm 2 Esc Anatomical Location** Oral Cavity (HPV N/A) Oral Cavity (HPV N/A) Oropharynx (HPV-) Oral Cavity (HPV N/A) Oral Cavity (HPV N/A) Oropharynx (HPV+) Larynx (HPV N/A) Oropharynx (HPV-) Oropharynx (HPV+) Larynx (HPV N/A) Oropharynx (HPV+) Larynx (HPV N/A) Oropharynx (HPV+) Baseline Tumor (mm) 41 88 28 149 33 42 35 43 113 133 90 28 16 #Prior tx 5 4 4 2 3 6 2 4 3 2 1 3 1 Legend Patient ongoing Confirmed ORR= 46% (6/13)1 DCR= 92% Data as of Nov 3, 2025 Confirmed PR – scan after Nov 3, 2025 ♢ ***
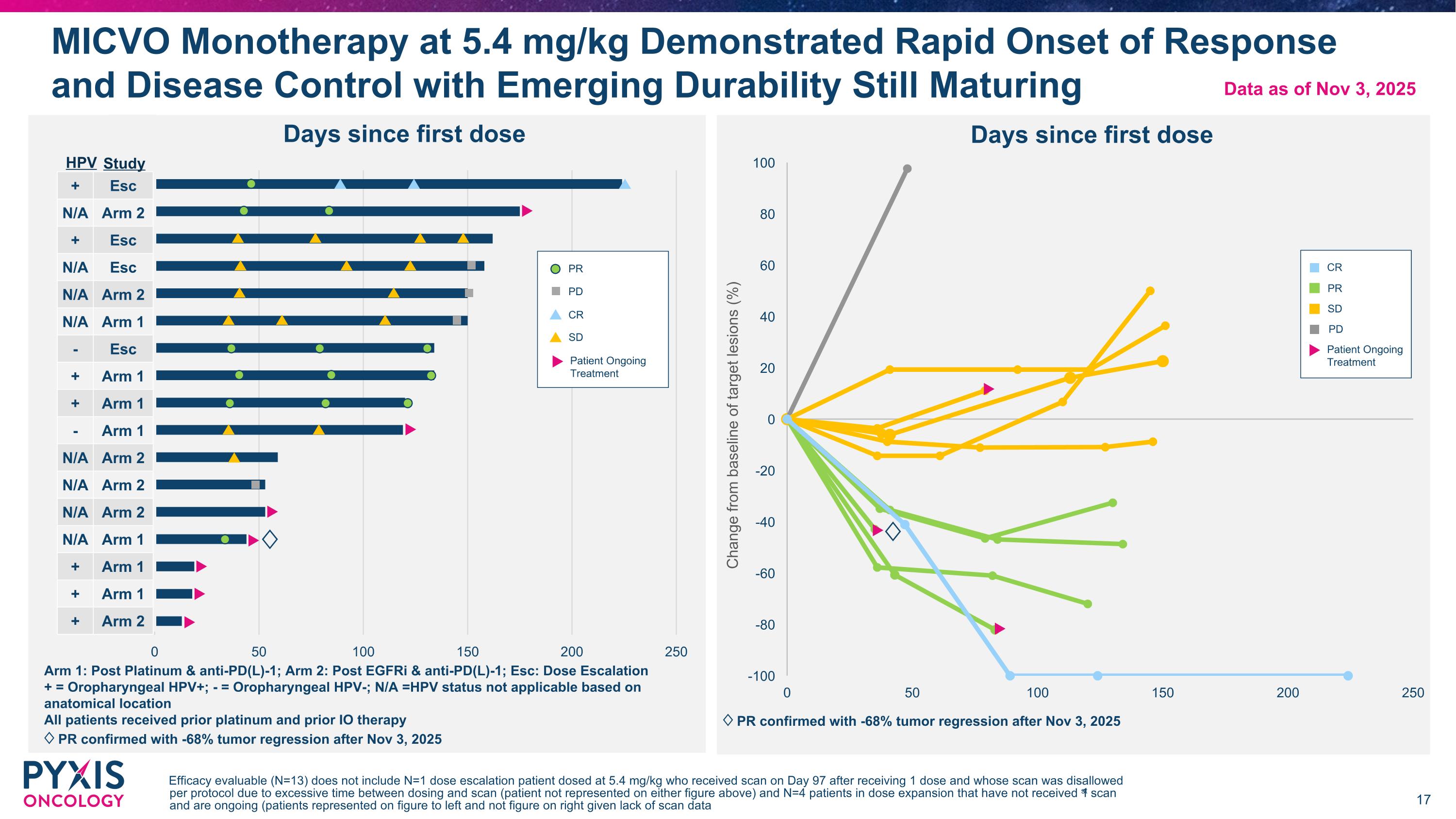
MICVO Monotherapy at 5.4 mg/kg Demonstrated Rapid Onset of Response and Disease Control with Emerging Durability Still Maturing Efficacy evaluable (N=13) does not include N=1 dose escalation patient dosed at 5.4 mg/kg who received scan on Day 97 after receiving 1 dose and whose scan was disallowed per protocol due to excessive time between dosing and scan (patient not represented on either figure above) and N=4 patients in dose expansion that have not received 1st scan and are ongoing (patients represented on figure to left and not figure on right given lack of scan data Arm 1: Post Platinum & anti-PD(L)-1; Arm 2: Post EGFRi & anti-PD(L)-1; Esc: Dose Escalation + = Oropharyngeal HPV+; - = Oropharyngeal HPV-; N/A =HPV status not applicable based on anatomical location All patients received prior platinum and prior IO therapy ♢ PR confirmed with -68% tumor regression after Nov 3, 2025 HPV + Esc N/A Arm 2 + Esc N/A Esc N/A Arm 2 N/A Arm 1 - Esc + Arm 1 + Arm 1 - Arm 1 N/A Arm 2 N/A Arm 2 N/A Arm 2 N/A Arm 1 + Arm 1 + Arm 1 + Arm 2 PR PD CR SD Patient Ongoing Treatment PR PD SD CR Patient Ongoing Treatment Study Data as of Nov 3, 2025 ♢ ♢ ♢ PR confirmed with -68% tumor regression after Nov 3, 2025
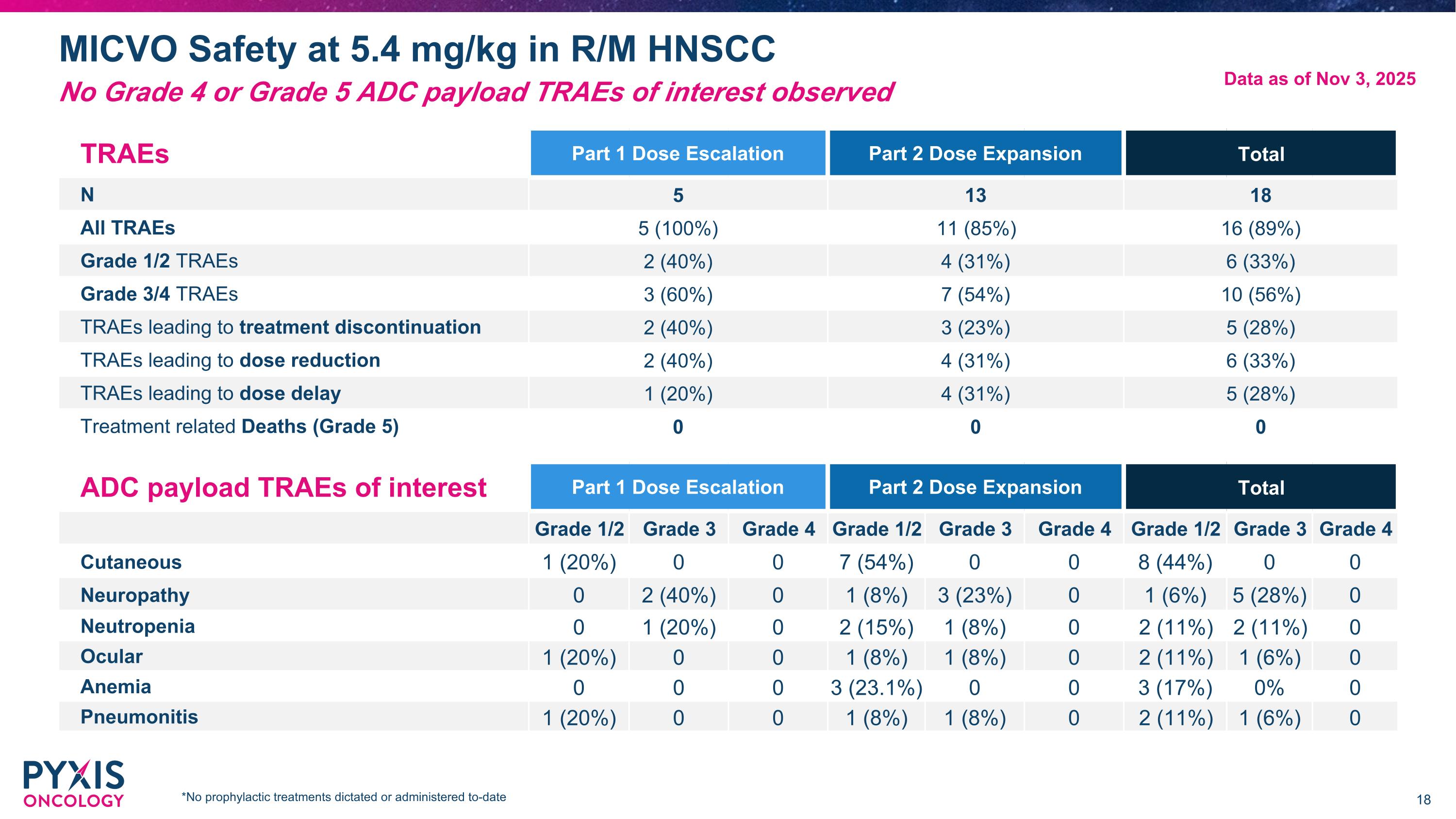
MICVO Safety at 5.4 mg/kg in R/M HNSCC TRAEs Part 1 Dose Escalation Part 2 Dose Expansion Total N 5 13 18 All TRAEs 5 (100%) 11 (85%) 16 (89%) Grade 1/2 TRAEs 2 (40%) 4 (31%) 6 (33%) Grade 3/4 TRAEs 3 (60%) 7 (54%) 10 (56%) TRAEs leading to treatment discontinuation 2 (40%) 3 (23%) 5 (28%) TRAEs leading to dose reduction 2 (40%) 4 (31%) 6 (33%) TRAEs leading to dose delay 1 (20%) 4 (31%) 5 (28%) Treatment related Deaths (Grade 5) 0 0 0 ADC payload TRAEs of interest Part 1 Dose Escalation Part 2 Dose Expansion Total Grade 1/2 Grade 3 Grade 4 Grade 1/2 Grade 3 Grade 4 Grade 1/2 Grade 3 Grade 4 Cutaneous 1 (20%) 0 0 7 (54%) 0 0 8 (44%) 0 0 Neuropathy 0 2 (40%) 0 1 (8%) 3 (23%) 0 1 (6%) 5 (28%) 0 Neutropenia 0 1 (20%) 0 2 (15%) 1 (8%) 0 2 (11%) 2 (11%) 0 Ocular 1 (20%) 0 0 1 (8%) 1 (8%) 0 2 (11%) 1 (6%) 0 Anemia 0 0 0 3 (23.1%) 0 0 3 (17%) 0% 0 Pneumonitis 1 (20%) 0 0 1 (8%) 1 (8%) 0 2 (11%) 1 (6%) 0 Data as of Nov 3, 2025 No Grade 4 or Grade 5 ADC payload TRAEs of interest observed *No prophylactic treatments dictated or administered to-date
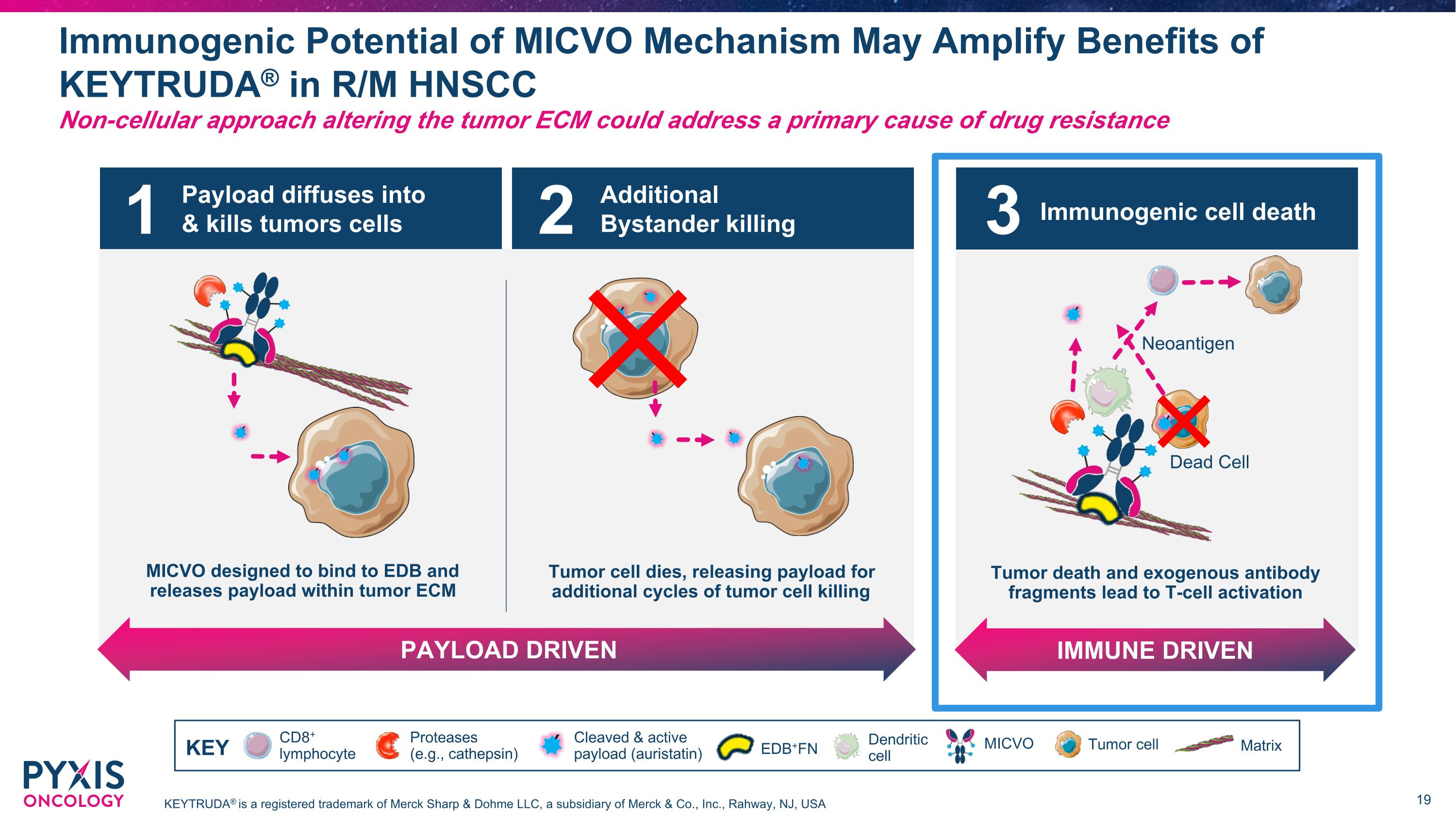
Payload diffuses into & kills tumors cells MICVO designed to bind to EDB and releases payload within tumor ECM Additional Bystander killing PAYLOAD DRIVEN Tumor cell dies, releasing payload for additional cycles of tumor cell killing 1 2 Immunogenic Potential of MICVO Mechanism May Amplify Benefits of KEYTRUDA® in R/M HNSCC Non-cellular approach altering the tumor ECM could address a primary cause of drug resistance CD8+ lymphocyte Dendritic cell MICVO Cleaved & active payload (auristatin) Tumor cell Matrix Proteases (e.g., cathepsin) KEY EDB+FN Immunogenic cell death IMMUNE DRIVEN Tumor death and exogenous antibody fragments lead to T-cell activation Dead Cell Neoantigen 3 KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
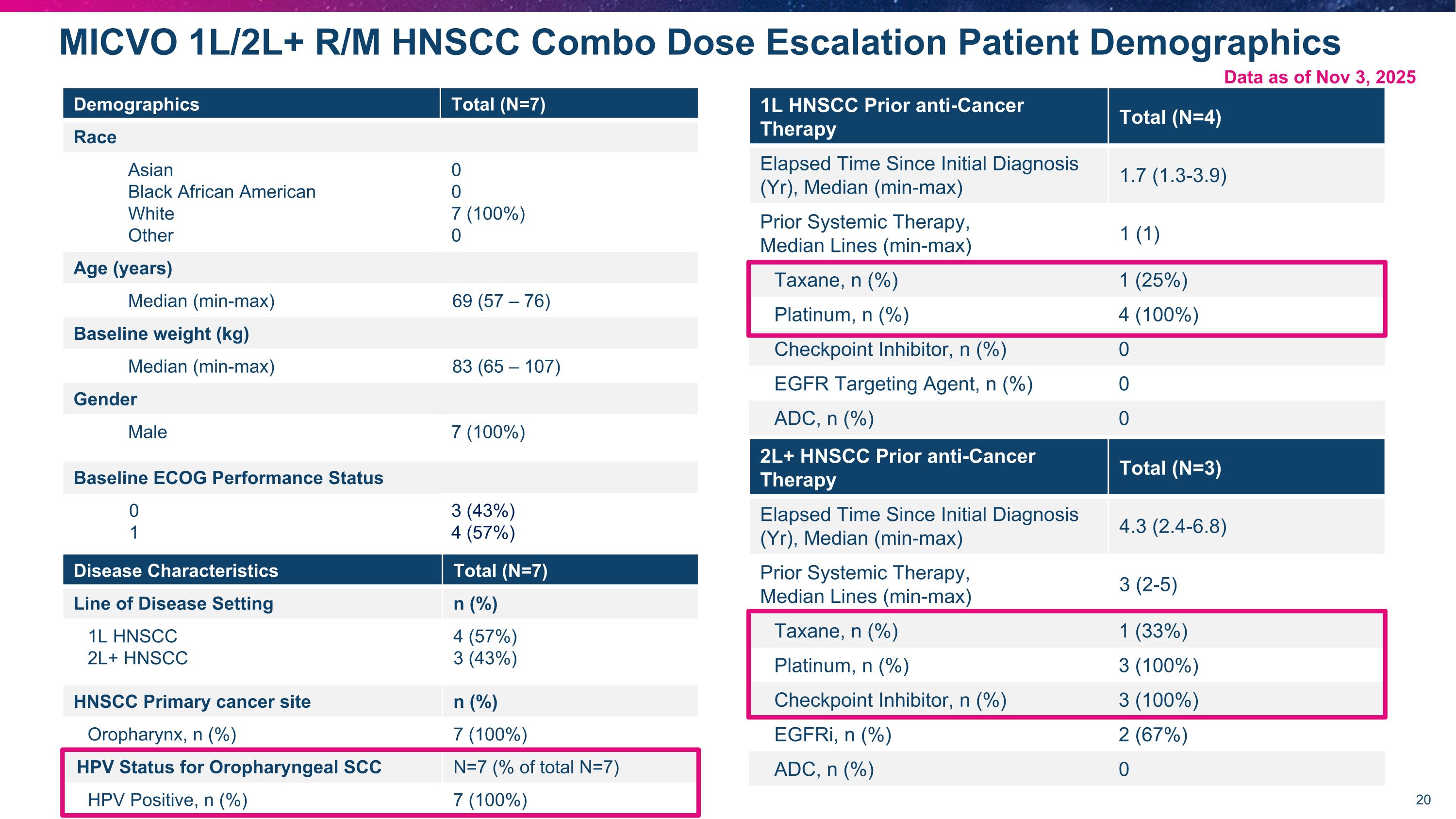
Demographics Total (N=7) Race Asian Black African American White Other 0 0 7 (100%) 0 Age (years) Median (min-max) 69 (57 – 76) Baseline weight (kg) Median (min-max) 83 (65 – 107) Gender Male 7 (100%) Baseline ECOG Performance Status 0 1 3 (43%) 4 (57%) MICVO 1L/2L+ R/M HNSCC Combo Dose Escalation Patient Demographics Data as of Nov 3, 2025 Disease Characteristics Total (N=7) Line of Disease Setting n (%) 1L HNSCC 2L+ HNSCC 4 (57%) 3 (43%) HNSCC Primary cancer site n (%) Oropharynx, n (%) 7 (100%) HPV Status for Oropharyngeal SCC N=7 (% of total N=7) HPV Positive, n (%) 7 (100%) 2L+ HNSCC Prior anti-Cancer Therapy Total (N=3) Elapsed Time Since Initial Diagnosis (Yr), Median (min-max) 4.3 (2.4-6.8) Prior Systemic Therapy, Median Lines (min-max) 3 (2-5) Taxane, n (%) 1 (33%) Platinum, n (%) 3 (100%) Checkpoint Inhibitor, n (%) 3 (100%) EGFRi, n (%) 2 (67%) ADC, n (%) 0 1L HNSCC Prior anti-Cancer Therapy Total (N=4) Elapsed Time Since Initial Diagnosis (Yr), Median (min-max) 1.7 (1.3-3.9) Prior Systemic Therapy, Median Lines (min-max) 1 (1) Taxane, n (%) 1 (25%) Platinum, n (%) 4 (100%) Checkpoint Inhibitor, n (%) 0 EGFR Targeting Agent, n (%) 0 ADC, n (%) 0 Data as of Nov 3, 2025
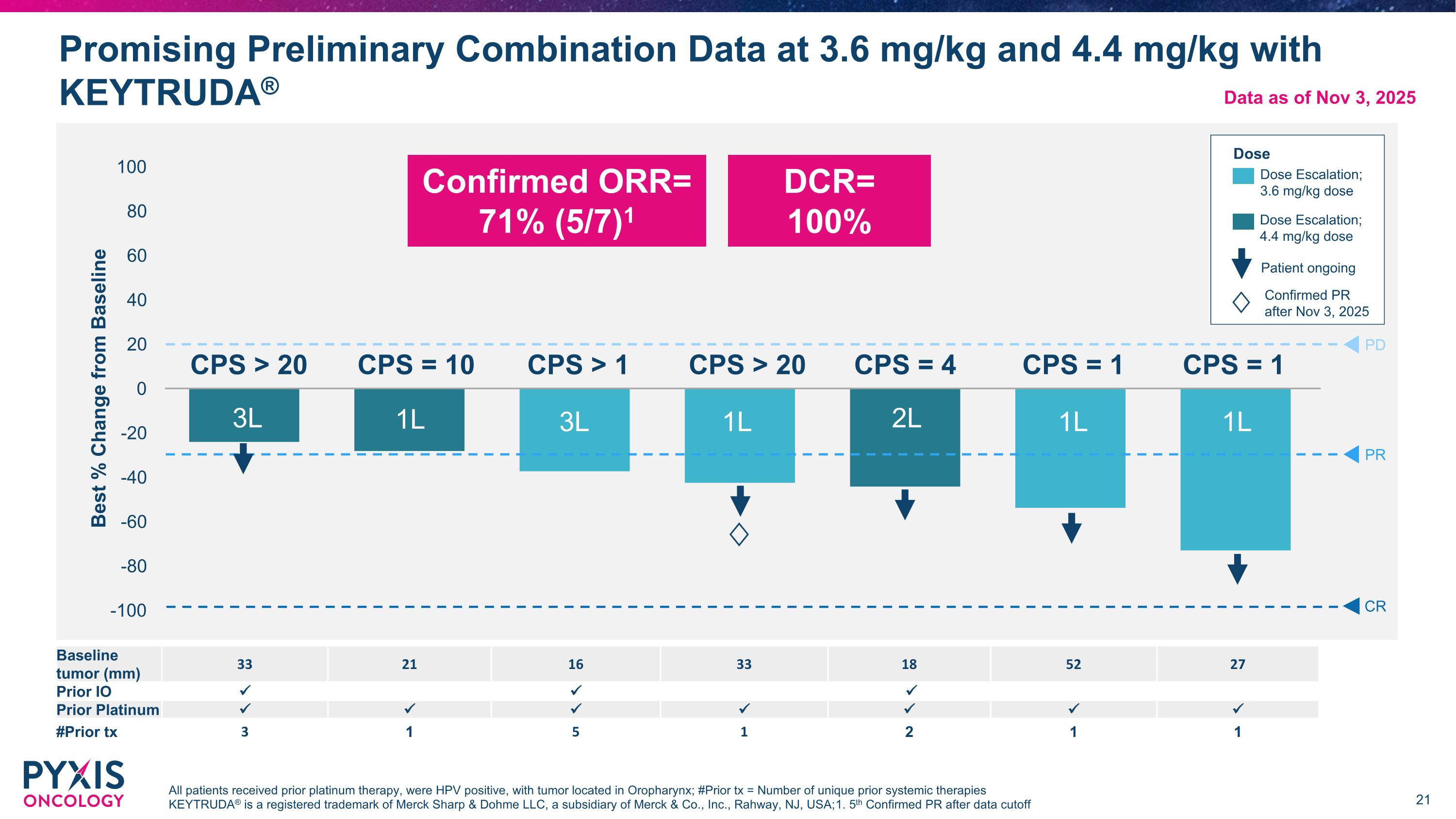
Promising Preliminary Combination Data at 3.6 mg/kg and 4.4 mg/kg with KEYTRUDA® All patients received prior platinum therapy, were HPV positive, with tumor located in Oropharynx; #Prior tx = Number of unique prior systemic therapies KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; 1. 5th Confirmed PR after data cutoff PD PR CR CPS = 1 CPS = 1 CPS = 10 3L 1L 3L 1L 2L 1L 1L Baseline tumor (mm) 33 21 16 33 18 52 27 Prior IO Prior Platinum #Prior tx 3 1 5 1 2 1 1 Dose Dose Escalation; 3.6 mg/kg dose Dose Escalation; 4.4 mg/kg dose DCR= 100% CPS > 20 CPS > 1 CPS > 20 CPS = 4 Data as of Nov 3, 2025 Confirmed ORR= 71% (5/7)1 Patient ongoing Confirmed PR after Nov 3, 2025 ♢ ♢
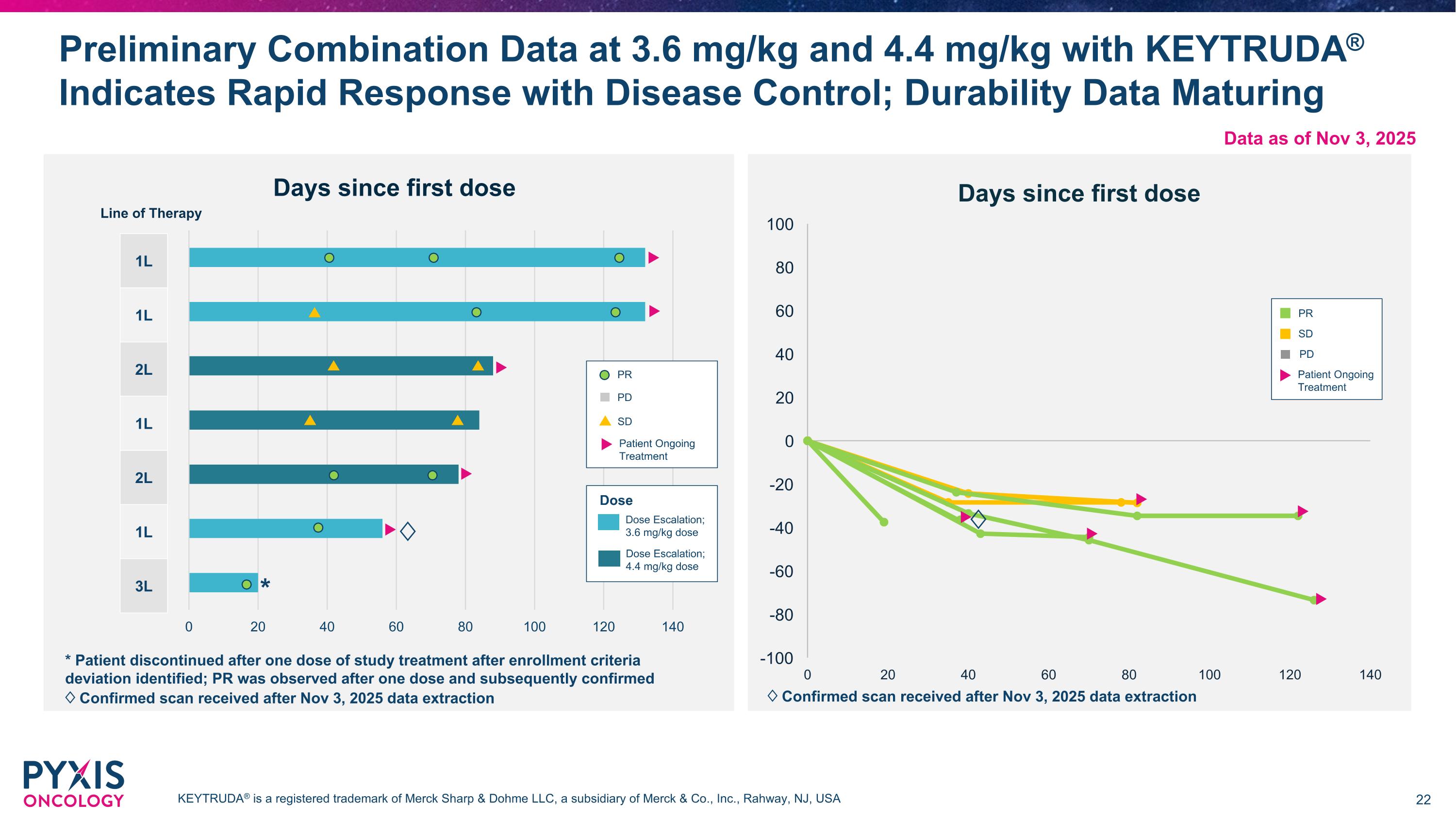
Preliminary Combination Data at 3.6 mg/kg and 4.4 mg/kg with KEYTRUDA® Indicates Rapid Response with Disease Control; Durability Data Maturing * Patient discontinued after one dose of study treatment after enrollment criteria deviation identified; PR was observed after one dose and subsequently confirmed ♢ Confirmed scan received after Nov 3, 2025 data extraction Dose Dose Escalation; 3.6 mg/kg dose Dose Escalation; 4.4 mg/kg dose 1L 1L 2L 1L 2L 1L 3L PR PD SD Patient Ongoing Treatment Line of Therapy PR PD SD Patient Ongoing Treatment KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA * Data as of Nov 3, 2025 ♢ ♢ ♢ Confirmed scan received after Nov 3, 2025 data extraction
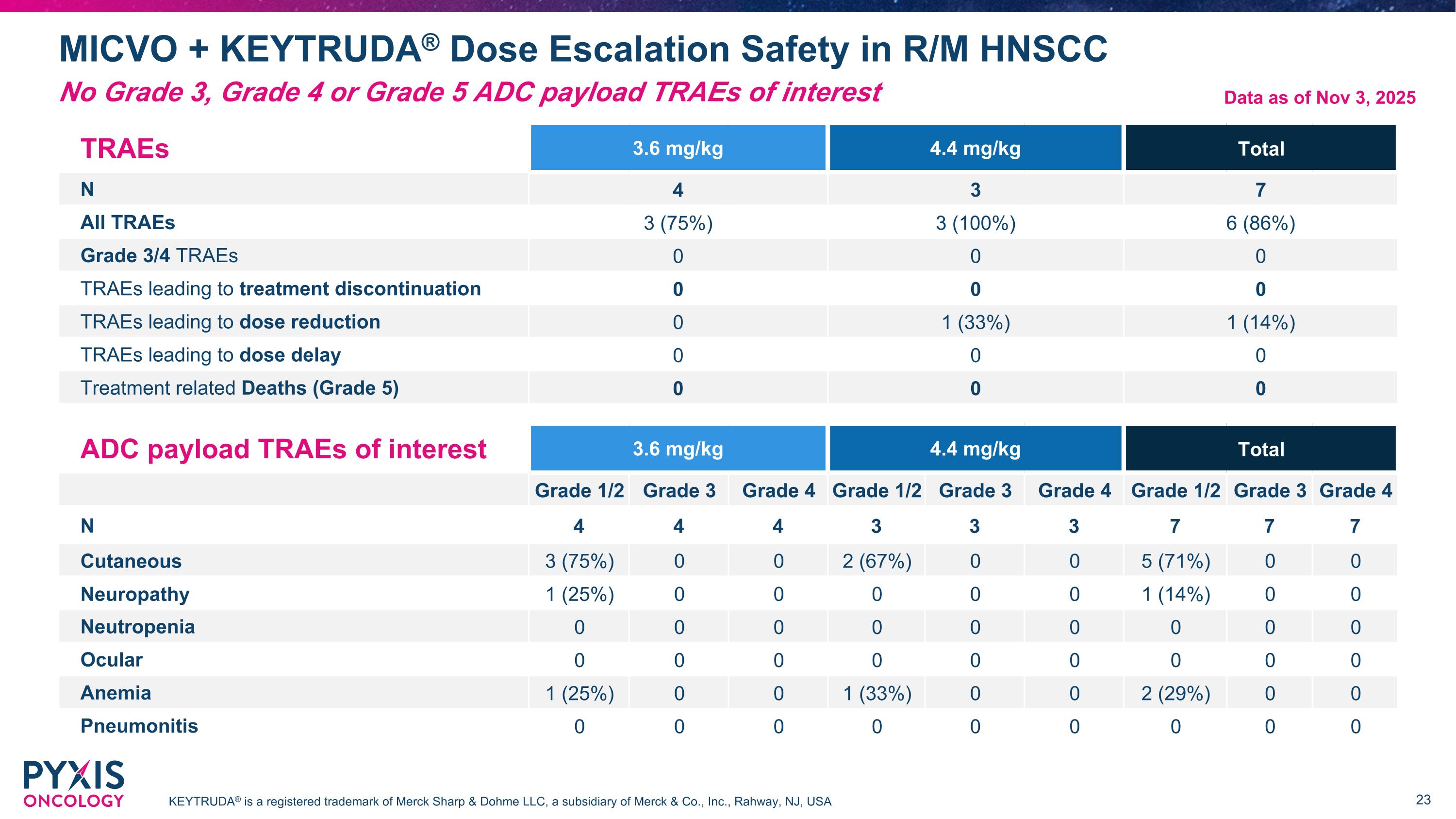
MICVO + KEYTRUDA® Dose Escalation Safety in R/M HNSCC KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA TRAEs 3.6 mg/kg 4.4 mg/kg Total N 4 3 7 All TRAEs 3 (75%) 3 (100%) 6 (86%) Grade 3/4 TRAEs 0 0 0 TRAEs leading to treatment discontinuation 0 0 0 TRAEs leading to dose reduction 0 1 (33%) 1 (14%) TRAEs leading to dose delay 0 0 0 Treatment related Deaths (Grade 5) 0 0 0 ADC payload TRAEs of interest 3.6 mg/kg 4.4 mg/kg Total Grade 1/2 Grade 3 Grade 4 Grade 1/2 Grade 3 Grade 4 Grade 1/2 Grade 3 Grade 4 N 4 4 4 3 3 3 7 7 7 Cutaneous 3 (75%) 0 0 2 (67%) 0 0 5 (71%) 0 0 Neuropathy 1 (25%) 0 0 0 0 0 1 (14%) 0 0 Neutropenia 0 0 0 0 0 0 0 0 0 Ocular 0 0 0 0 0 0 0 0 0 Anemia 1 (25%) 0 0 1 (33%) 0 0 2 (29%) 0 0 Pneumonitis 0 0 0 0 0 0 0 0 0 Data as of Nov 3, 2025 No Grade 3, Grade 4 or Grade 5 ADC payload TRAEs of interest
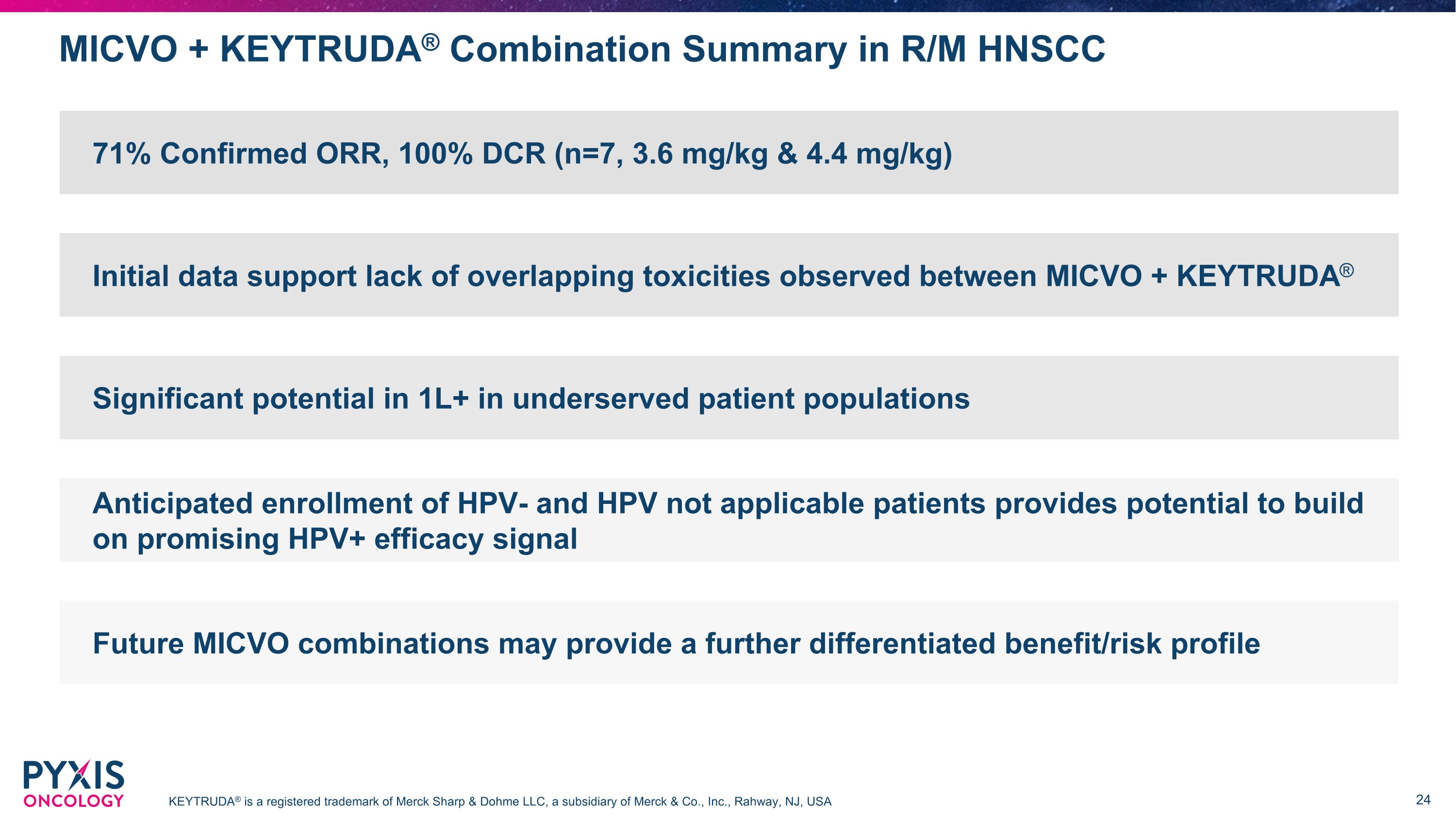
MICVO + KEYTRUDA® Combination Summary in R/M HNSCC KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA 71% Confirmed ORR, 100% DCR (n=7, 3.6 mg/kg & 4.4 mg/kg) Initial data support lack of overlapping toxicities observed between MICVO + KEYTRUDA® Significant potential in 1L+ in underserved patient populations Anticipated enrollment of HPV- and HPV not applicable patients provides potential to build on promising HPV+ efficacy signal Future MICVO combinations may provide a further differentiated benefit/risk profile
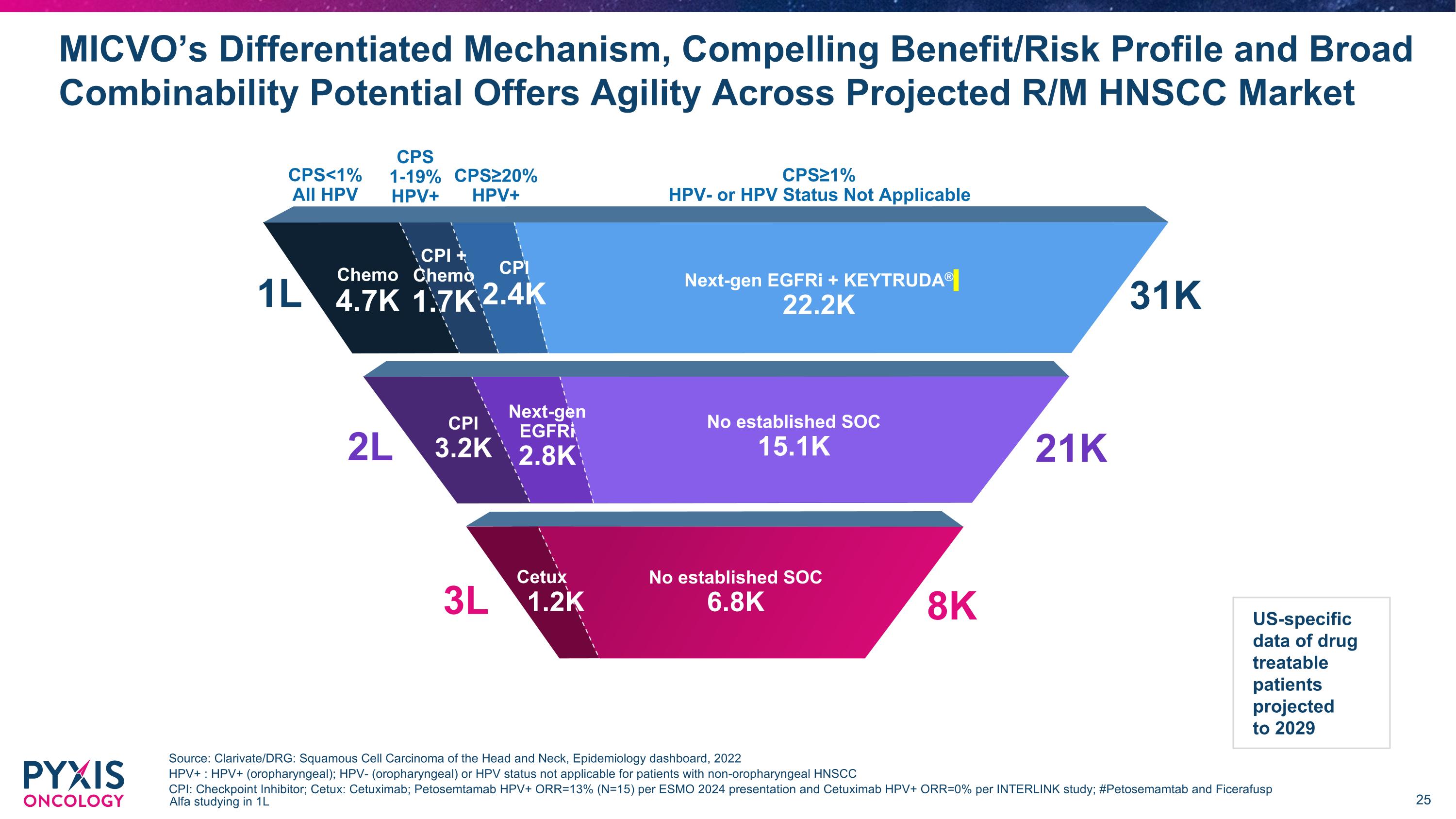
MICVO’s Differentiated Mechanism, Compelling Benefit/Risk Profile and Broad Combinability Potential Offers Agility Across Projected R/M HNSCC Market Source: Clarivate/DRG: Squamous Cell Carcinoma of the Head and Neck, Epidemiology dashboard, 2022 HPV+ : HPV+ (oropharyngeal); HPV- (oropharyngeal) or HPV status not applicable for patients with non-oropharyngeal HNSCC CPI: Checkpoint Inhibitor; Cetux: Cetuximab; Petosemtamab HPV+ ORR=13% (N=15) per ESMO 2024 presentation and Cetuximab HPV+ ORR=0% per INTERLINK study; #Petosemamtab and Ficerafusp Alfa studying in 1L CPI 3.0K 1L CPS<1% All HPV CPS≥20% HPV+ CPS 1-19% HPV+ CPS≥1% HPV- or HPV Status Not Applicable Next-gen EGFRi + KEYTRUDA® 22.2K Chemo 4.7K CPI 2.4K CPI + Chemo 1.7K 31K 2L Next-gen EGFRi 2.8K No established SOC 15.1K 21K CPI 3.2K 8K 3L Cetux 1.2K No established SOC 6.8K US-specific data of drug treatable patients projected to 2029
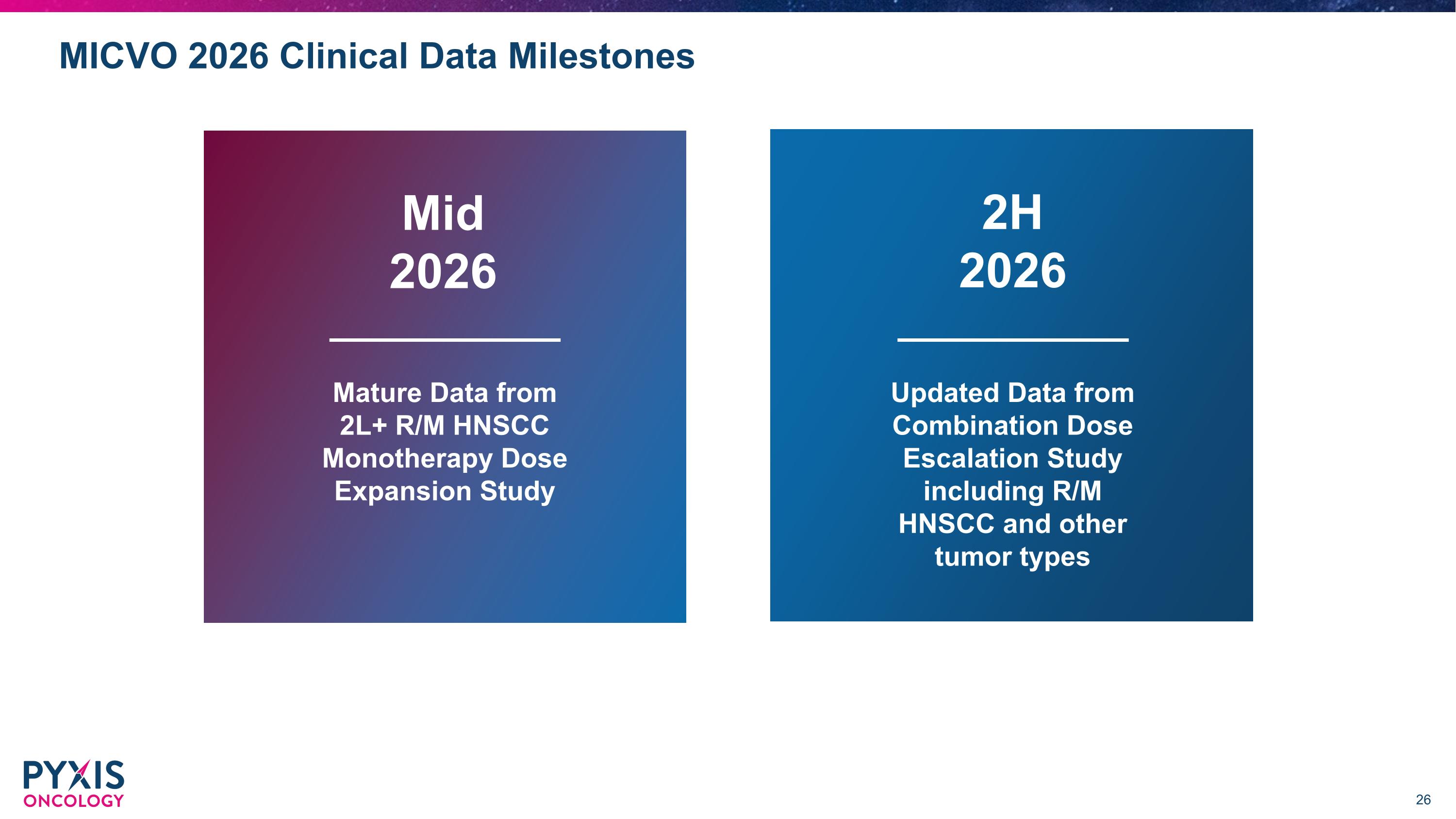
MICVO 2026 Clinical Data Milestones Mature Data from 2L+ R/M HNSCC Monotherapy Dose Expansion Study 2H 2026 Updated Data from Combination Dose Escalation Study including R/M HNSCC and other tumor types Mid 2026

Appendix
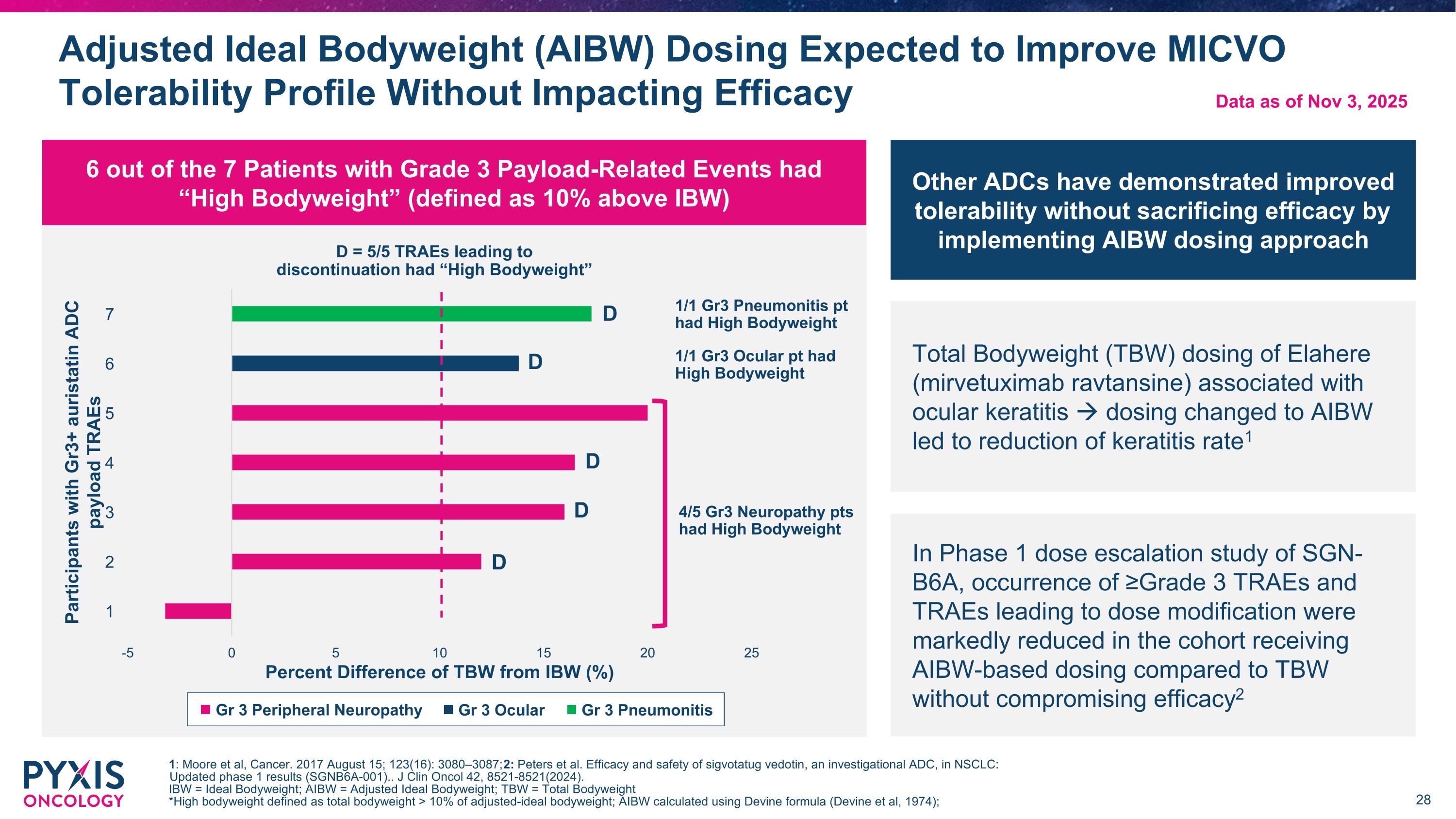
Adjusted Ideal Bodyweight (AIBW) Dosing Expected to Improve MICVO Tolerability Profile Without Impacting Efficacy 1: Moore et al, Cancer. 2017 August 15; 123(16): 3080–3087; 2: Peters et al. Efficacy and safety of sigvotatug vedotin, an investigational ADC, in NSCLC: Updated phase 1 results (SGNB6A-001).. J Clin Oncol 42, 8521-8521(2024). IBW = Ideal Bodyweight; AIBW = Adjusted Ideal Bodyweight; TBW = Total Bodyweight *High bodyweight defined as total bodyweight > 10% of adjusted-ideal bodyweight; AIBW calculated using Devine formula (Devine et al, 1974); Gr 3 Ocular Gr 3 Pneumonitis Gr 3 Peripheral Neuropathy Data as of Nov 3, 2025 Other ADCs have demonstrated improved tolerability without sacrificing efficacy by implementing AIBW dosing approach Total Bodyweight (TBW) dosing of Elahere (mirvetuximab ravtansine) associated with ocular keratitis dosing changed to AIBW led to reduction of keratitis rate1 In Phase 1 dose escalation study of SGN-B6A, occurrence of ≥Grade 3 TRAEs and TRAEs leading to dose modification were markedly reduced in the cohort receiving AIBW-based dosing compared to TBW without compromising efficacy2 1/1 Gr3 Pneumonitis pt had High Bodyweight 1/1 Gr3 Ocular pt had High Bodyweight 4/5 Gr3 Neuropathy pts had High Bodyweight D D D D D D = 5/5 TRAEs leading to discontinuation had “High Bodyweight” 6 out of the 7 Patients with Grade 3 Payload-Related Events had “High Bodyweight” (defined as 10% above IBW)
2L+ R/M HNSCC Monotherapy Pivotal Trial Design Key Design Elements FDA aligned with 2L+ R/M HNSCC Monotherapy pivotal study design Control arm to be current commercially available standard(s) of care1 Study expected to implement Adjusted Ideal Bodyweight (AIBW) dosing 1. Investigator’s choice in comparator arm expected to be Cetuximab, Methotrexate, or Docetaxel