
PURETECH HEALTH PLC – ANNUAL REPORT AND ACCOUNTS 2024 Exhibit 15.1

BOSTON MA Headquarters PRTC Nasdaq and LSE Relations with Stakeholders 91 Directors’ Report 93 Report of the Nomination Committee 98 Report of the Audit Committee 99 Directors’ Remuneration Report 102 Directors’ Remuneration Policy 106 Annual Report on Remuneration 110 Financial statements Independent Auditor’s Report to the Members of PureTech Health plc 121 Consolidated Statements of Comprehensive Income/(Loss) 126 Consolidated Statements of Financial Position 127 Consolidated Statements of Changes in Equity 128 Consolidated Statements of Cash Flows 129 Notes to the Consolidated Financial Statements 130 PureTech Health plc Statement of Financial Position 176 PureTech Health plc Statements of Changes in Equity 177 Notes to the Financial Statements 178 Additional information History and Development of the Company 181 Risk Factor Annex 182 Directors, Secretary and Advisors to PureTech Health plc 220 Overview Highlights of the Year 1 Letter from the Chair 2 Strategic report Letter from the Chief Executive Officer 4 2025 Strategy to Deliver Shareholder Value 6 Our Key Components of Value 8 Our R&D Approach 9 Our Hub-and-Spoke Model 10 Our Programs 11 ESG report Building and Maintaining a Sustainable Business 22 Governance Risk Management 60 Viability 65 Key Performance Indicators 67 Financial Review 68 Chair’s Overview 81 Board of Directors 82 Management Team 85 The Board 86
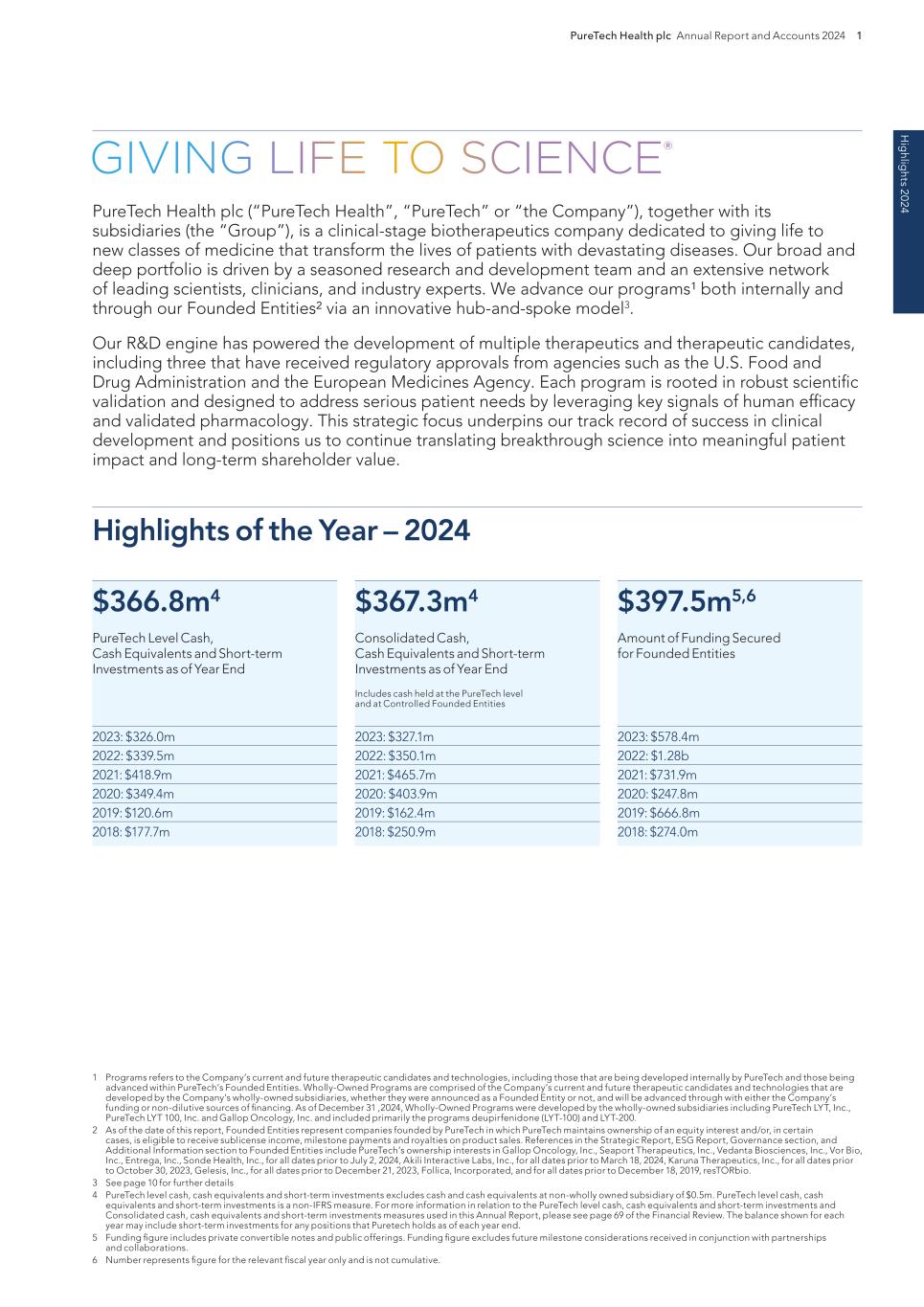
PureTech Health plc Annual Report and Accounts 2024 1 PureTech Health plc (“PureTech Health”, “PureTech” or “the Company”), together with its subsidiaries (the “Group”), is a clinical-stage biotherapeutics company dedicated to giving life to new classes of medicine that transform the lives of patients with devastating diseases. Our broad and deep portfolio is driven by a seasoned research and development team and an extensive network of leading scientists, clinicians, and industry experts. We advance our programs¹ both internally and through our Founded Entities² via an innovative hub-and-spoke model3. Our R&D engine has powered the development of multiple therapeutics and therapeutic candidates, including three that have received regulatory approvals from agencies such as the U.S. Food and Drug Administration and the European Medicines Agency. Each program is rooted in robust scientific validation and designed to address serious patient needs by leveraging key signals of human efficacy and validated pharmacology. This strategic focus underpins our track record of success in clinical development and positions us to continue translating breakthrough science into meaningful patient impact and long-term shareholder value. 1 Programs refers to the Company’s current and future therapeutic candidates and technologies, including those that are being developed internally by PureTech and those being advanced within PureTech’s Founded Entities. Wholly-Owned Programs are comprised of the Company’s current and future therapeutic candidates and technologies that are developed by the Company’s wholly-owned subsidiaries, whether they were announced as a Founded Entity or not, and will be advanced through with either the Company’s funding or non-dilutive sources of financing. As of December 31 ,2024, Wholly-Owned Programs were developed by the wholly-owned subsidiaries including PureTech LYT, Inc., PureTech LYT 100, Inc. and Gallop Oncology, Inc. and included primarily the programs deupirfenidone (LYT-100) and LYT-200. 2 As of the date of this report, Founded Entities represent companies founded by PureTech in which PureTech maintains ownership of an equity interest and/or, in certain cases, is eligible to receive sublicense income, milestone payments and royalties on product sales. References in the Strategic Report, ESG Report, Governance section, and Additional Information section to Founded Entities include PureTech’s ownership interests in Gallop Oncology, Inc., Seaport Therapeutics, Inc., Vedanta Biosciences, Inc., Vor Bio, Inc., Entrega, Inc., Sonde Health, Inc., for all dates prior to July 2, 2024, Akili Interactive Labs, Inc., for all dates prior to March 18, 2024, Karuna Therapeutics, Inc., for all dates prior to October 30, 2023, Gelesis, Inc., for all dates prior to December 21, 2023, Follica, Incorporated, and for all dates prior to December 18, 2019, resTORbio. 3 See page 10 for further details 4 PureTech level cash, cash equivalents and short-term investments excludes cash and cash equivalents at non-wholly owned subsidiary of $0.5m. PureTech level cash, cash equivalents and short-term investments is a non-IFRS measure. For more information in relation to the PureTech level cash, cash equivalents and short-term investments and Consolidated cash, cash equivalents and short-term investments measures used in this Annual Report, please see page 69 of the Financial Review. The balance shown for each year may include short-term investments for any positions that Puretech holds as of each year end. 5 Funding figure includes private convertible notes and public offerings. Funding figure excludes future milestone considerations received in conjunction with partnerships and collaborations. 6 Number represents figure for the relevant fiscal year only and is not cumulative. H ig hlig hts 2024 $397.5m5,6 Amount of Funding Secured for Founded Entities $367.3m4 Consolidated Cash, Cash Equivalents and Short-term Investments as of Year End Includes cash held at the PureTech level and at Controlled Founded Entities $366.8m4 PureTech Level Cash, Cash Equivalents and Short-term Investments as of Year End Highlights of the Year – 2024 2023: $578.4m 2022: $1.28b 2021: $731.9m 2020: $247.8m 2019: $666.8m 2018: $274.0m 2023: $327.1m 2022: $350.1m 2021: $465.7m 2020: $403.9m 2019: $162.4m 2018: $250.9m 2023: $326.0m 2022: $339.5m 2021: $418.9m 2020: $349.4m 2019: $120.6m 2018: $177.7m

2 PureTech Health plc Annual Report and Accounts 2024 A standout achievement was the U.S. FDA approval of KarXT—now marketed by Bristol Myers Squibb (BMS) as Cobenfy™—for the treatment of schizophrenia in adults. Invented and initially developed at PureTech, Cobenfy represents the first drug with a novel mechanism of action for schizophrenia in over 50 years, underscoring our scientific invention and leadership. Complementing this historic approval was a major financial milestone: the acquisition of Karuna Therapeutics, our Founded Entity that shepherded Cobenfy through late-stage development, by BMS for $14 billion. Through the monetization of our equity holdings—including proceeds from the BMS acquisition and a strategic royalty agreement—PureTech has generated approximately $1.1 billion in cash from the $18.5 million it initially invested in the program. Together, these achievements highlight the power of our proven hub-and-spoke model (see page 10) to advance science, build value, and deliver meaningful outcomes. Expanding on this success, we launched Seaport Therapeutics—our latest Founded Entity. Seaport builds on our leadership in neuroscience, a field where we reignited broader investment interest through the success of Karuna. Several key team members from Karuna are now involved at Seaport, leveraging their expertise to advance a promising pipeline of neuropsychiatric medicines. With over $325 million raised across two oversubscribed Series A and Series B financings, Seaport is now advancing multiple drugs developed at PureTech using the Glyph platform that PureTech validated and advanced. 2024 was a landmark year for PureTech— one defined by breakthrough achievements that created long-term value for both patients and shareholders. These accomplishments reflect not only the power of our innovation engine but also the dedication, discipline, and excellence of the PureTech team. From bold scientific bets to smart capital decisions, this year demonstrated what’s possible when vision meets execution. We reached major milestones throughout the year, including the third FDA approval for a therapeutic invented at PureTech, transformative financings for Seaport Therapeutics (a PureTech Founded Entity), and unprecedented clinical results for deupirfenidone (LYT-100), an asset fully owned by PureTech. These achievements, supported by a strong year-end balance sheet of $367 million1, underscore the strength of our capital-efficient and disciplined approach. A Year of Successes for PureTech Innovation These achievements highlight the power of our proven hub-and-spoke model to advance science, build value, and deliver meaningful outcomes. Raju Kucherlapati, Ph.D. Chair of the Board of Directors Le tt er fr o m th e C ha ir Letter from the Chair

PureTech Health plc Annual Report and Accounts 2024 3 The Board has been a steadfast partner throughout this journey—providing strategic oversight, financial discipline, and an unwavering commitment to our long-term mission. As part of this commitment, I traveled to the UK in 2024 to meet directly with several shareholders, reflecting the Board’s active engagement and dedication to maintaining strong, direct relationships with our investor base. I am proud to serve alongside such a thoughtful and forward-looking group. Their counsel has been instrumental in navigating complexity and driving results. On behalf of the Board, I extend my deepest gratitude to our shareholders for their continued support. Your confidence empowers us to pursue life-changing therapies and deliver on our vision. To the entire PureTech team—thank you. Your scientific excellence, operational rigor, and relentless drive have made this year possible. Looking ahead, we remain grounded in the disciplined approach that has long defined PureTech—prioritizing capital efficiency, thoughtful resource allocation, and strategic agility and flexibility. The momentum we have built in 2024 has positioned us for a future of continued impact, and we remain steadfast in our mission to deliver novel medicines that transform patient outcomes. Raju Kucherlapati, Ph.D. Board Chair April 30, 2025 Perhaps the most defining moment of 2024 came in December with the announcement of positive results from ELEVATE IPF, our global Phase 2b trial of deupirfenidone in idiopathic pulmonary fibrosis (IPF). The trial met its primary and key secondary endpoints, demonstrating the potential of deupirfenidone to stabilize lung function decline and meaningfully improve patient outcomes—an advance that could redefine the standard of care for IPF. These results again prove the strength of our scientific platform and our team’s ability to translate bold ideas into patient-impacting innovation. Advancing deupirfenidone into Phase 3 is now a strategic priority for PureTech, which we aim to accomplish with financial partners. Recognizing the significant cash realizations made from our success with Karuna, we also were able to return significant levels of cash to our shareholders during the year against a challenging macroeconomic backdrop. We returned $100 million through a Tender Offer and completed a $50 million share buyback program, which was initiated in 2022. Notably, we accomplished these returns without raising capital from public equity markets for seven consecutive years—all while driving significant patient progress, advancing our pipeline, and maintaining a very strong balance sheet. These actions reflect our confidence in PureTech’s intrinsic value and our commitment to delivering returns for shareholders. At the same time, the Board recognizes that there remains a disconnect between the value of PureTech’s assets and our share price. We are working closely with the CEO and management team to explore all strategic options to address this gap— including recent take-private discussions—with the goal of unlocking value in a manner that is in the best interest of all shareholders. Letter fro m the C hair Note: Certain third-party trademarks are included here; PureTech does not claim any rights to any third-party trademarks. COBENFY™ (xanomeline and trospium chloride) is indicated for the treatment of schizophrenia in adults. For Important Safety Information, see U.S. Full Prescribing Information, including Patient Information on COBENFY.com. Following the acquisition of Karuna, KarXT is now under the stewardship of Bristol Myers Squibb and will be marketed as Cobenfy. 1 This amount represents PureTech level cash, cash equivalents and short-term investments and excludes cash and cash equivalents at non-wholly owned subsidiary of $0.5m. PureTech level cash, cash equivalents and short-term investments is a non-IFRS measure. For more information in relation to the PureTech level cash, cash equivalents and short- term investments and Consolidated cash, cash equivalents and short-term investments measures used in this Annual Report, please see page 69 of the Financial Review. Letter from the Chair continued

4 PureTech Health plc Annual Report and Accounts 2024 Le tt er fr o m th e C hi ef E xe cu ti ve O ffi ce r lung function decline over 26 weeks. To our knowledge, this is an achievement unmatched by any other investigational IPF therapeutic to date. Notably, this higher dose also showed an effect size that was 50% greater than that seen in our trial with pirfenidone (80.9% vs. 54.1%, respectively), further underscoring its potential for superior efficacy. Importantly, deupirfenidone was generally well- tolerated at this higher dose, overcoming the tolerability limitations that constrain current standard-of-care therapies and limit their effectiveness. Furthermore, I’m pleased that we continue to see strong preliminary data from our ongoing open label extension (OLE) trial. As of March 14, 2025, 140 patients have continued in the OLE, with 85 patients having received at least 52 weeks of treatment with deupirfenidone. Preliminary data from those receiving deupirfenidone 825 mg TID indicate that the significant slowing of lung function decline observed in Part A of the trial has been sustained through 52 weeks of treatment, supporting the durability of the treatment effect with this dose and its potential to stabilize lung function decline over time. Detailed OLE results will be presented at an upcoming scientific forum. These results suggest the potential for deupirfenidone to offer improved efficacy without compromising safety and position it as a potential new standard-of-care, not only in IPF, but also potentially in other underserved fibrotic lung diseases. We intend to discuss these results with the FDA before the end of the third quarter of 2025 to align on a potential registrational pathway, with the goal of initiating a Phase 3 trial by the end of the year. We anticipate providing further guidance later this year following the finalization of the trial design and FDA interactions. We will also be presenting details from the Phase 2b ELEVATE IPF trial at the American Thoracic Society International Conference in May 2025. We are committed to advancing deupirfenidone while maintaining capital efficiency, in line with our proven strategy. Subject to feedback from the FDA with respect to trial design, as well as historical data from other Phase 3 IPF studies, we don’t believe our current cash balance would be sufficient to fully fund a Phase 3 trial. We have therefore initiated discussions to explore a range of funding mechanisms—including a potential spin-out of the program into a new Founded Entity and accessing external equity financing, similar to our approach with Karuna and Seaport; project or royalty- based financing; and strategic partnerships—which may be used in combination, to support the program’s continued development as we don’t intend to fully fund a Phase 3 trial on our own. We will, however, continue to fund the program in the interim to maintain development momentum. While deupirfenidone represents our next wave of innovation, we also saw the full potential of our model realized through the FDA approval of Cobenfy™, formerly KarXT, which became the first new mechanism approved for schizophrenia in over 50 years. Invented at PureTech and advanced by our Founded Entity Karuna Therapeutics, Cobenfy’s approval by the FDA in 2024, following Karuna’s acquisition by BMS for approximately $14 billion, marked the culmination of years of scientific, clinical, and strategic execution. Through our equity and royalty interest in Karuna, we not only delivered shareholder returns, but also reinforced the self- 2024 was a defining year for PureTech— one in which the programs we cultivated through our R&D engine came to fruition in ways that delivered meaningful impact for patients and showcased the strength of our innovation engine. We saw the full arc of our strategy on display: from unprecedented clinical results with our wholly-owned program that could reshape the standard of care in a major disease area, to the FDA approval of a first-in-class therapy for schizophrenia that began with our team, to the launch and successful financing of a new Founded Entity in neuropsychiatry. These moments weren’t isolated wins—they were outcomes of a deliberate and disciplined model that translates scientifically validated biology into therapies for areas of high unmet need. Among the most significant milestones of the year was the progress of our wholly- owned program, deupirfenidone (LYT-100), which delivered transformative results in our Phase 2b ELEVATE IPF trial. This randomized, double-blind, placebo- and active-controlled study evaluated two dose levels of deupirfenidone in patients with idiopathic pulmonary fibrosis (IPF), a progressive and fatal lung disease. The trial met its primary and key secondary endpoints, with the higher dose demonstrating the potential to stabilize Delivering on Our Strategy We remain deeply focused on executing a strategy that maximizes value for our shareholders while advancing our mission to improve patients’ lives. Bharatt Chowrira, Ph.D., J.D. Chief Executive Officer and Member of the Board of Directors Letter from the Chief Executive Officer

PureTech Health plc Annual Report and Accounts 2024 5 We remain deeply focused on executing a strategy that maximizes value for our shareholders while advancing our mission to improve patients’ lives, and we will carefully consider any opportunity that arises to create value for our shareholders. Our balance sheet remains strong, with $367 million as of December 31, 2024,1 and we are committed to maintaining financial discipline by allocating capital efficiently to high-impact programs while actively pursuing external funding opportunities. This measured approach allows us to protect our balance sheet while preserving flexibility in a volatile market environment. Our model has always emphasized capital efficiency, and we remain confident in our ability to build value through disciplined execution and strategic agility. I want to thank each member of the PureTech team for their contributions to our work and culture—what we’ve accomplished together is rare and meaningful. I’m also grateful to our Board of Directors for their steadfast guidance and partnership. Finally, to our broader community of collaborators—patients, advocates, clinicians, partners—and to our shareholders, thank you. Your trust and support have been essential to our journey, especially over the past year as I stepped into the role of CEO. We’re deeply grateful for the belief you’ve placed in our vision, our model, and our team. It is a privilege to pursue this mission with you, and we are committed to delivering value to all of our stakeholders. We are proud of what we have achieved together—and we are energized by the impact our science continues to make in the world. Bharatt Chowrira, Ph.D., J.D. Chief Executive Officer and Director April 30, 2025 progression tends to be less than one month and whose overall survival averages 1.7-2.4 months with standard-of-care therapy. We’re also pleased to share topline results from the head and neck cancer study, which showed a favorable safety profile in all cohorts, disease control, and initial efficacy signals, including one CR lasting more than two years. Additional details from both studies are available on pages 14-15. Our Founded Entity Vedanta Biosciences initiated its pivotal Phase 3 program for VE303 in recurrent C. difficile infection, and Vor Bio continued to make clinical progress with trem-cel (VOR33), a promising shielded transplant platform for patients with AML. Taken together, these milestones reflect a robust innovation engine that spans the biotech lifecycle from discovery through commercialization and delivers impact across multiple therapeutic areas. Our hub-and-spoke model has enabled us to achieve this with scientific rigor, executional discipline, and capital efficiency. Despite the strength of our innovation engine and the significant milestones we have achieved, our market capitalization has not reflected the underlying value of the business for some time. This persistent disconnect has remained despite meaningful efforts over the past several years—including the return of $150 million to shareholders via share buybacks and a Tender Offer, attaining a dual listing on Nasdaq, engaging in significant investor outreach and capital market activities, and making strategic shifts in our model—all while delivering meaningful scientific, clinical, and financial milestones that we believe demonstrate the inherent strength of our business. In response, we have been evaluating a range of potential pathways to better align our market value with the strength of our underlying assets and long-term potential. These efforts are grounded in a clear objective: to address structural challenges and deliver value to shareholders in a way that reflects both the maturity of our business and the opportunity ahead. funded cycle that fuels our broader pipeline. Another example of our flexible funding model in motion is Seaport Therapeutics, launched in 2024 to develop neuropsychiatric candidates based on the Glyph platform validated and advanced by PureTech. The rapid growth of Seaport—including more than $325 million raised across its Series A and B rounds in just six months— demonstrates continued external conviction in our R&D engine and our ability to build high-quality companies around transformational programs. Several other programs had important developments this year. Our newest Founded Entity, Gallop Oncology, is advancing LYT-200 for the potential treatment of hematological malignancies and solid tumors. LYT-200, which targets galectin-9, received FDA Fast Track designation for both acute myeloid leukemia (AML) and head and neck cancers, was granted Orphan Drug Designation for AML, and delivered encouraging data across its two clinical trials. The ongoing Phase 1b trial in AML and high-risk myelodysplastic syndromes (MDS) has shown clinical activity and disease stabilization in heavily pretreated patients, both as a monotherapy and in combination with venetoclax/ hypomethylating agents (HMA), along with a favorable safety profile. Data were presented at the American Society for Hematology in 2024, and – since then – the trial has continued to demonstrate robust efficacy and safety. As of April 28, 2025, treatment with LYT-200 has resulted in one complete response (CR), three partial responses (PRs) and more than 50% of patients treated experienced stable disease. When administered in combination with venetoclax/HMA, results as of April 28, 2025, demonstrate that LYT-200 may enhance the efficacy of standard-of-care therapies, resulting in 6 CRs, 1 morphological leukemia-free state, and 50% of patients experiencing stable disease. The average time on combination therapy was four months as of the data cutoff, which is meaningful in a patient population whose time to Letter from the Chief Executive Officer continued Letter fro m the C hief E xecutive O fficer Note: Certain third-party trademarks are included here; PureTech does not claim any rights to any third-party trademarks. COBENFY™ (xanomeline and trospium chloride) is indicated for the treatment of schizophrenia in adults. For Important Safety Information, see U.S. Full Prescribing Information, including Patient Information on COBENFY.com. Following the acquisition of Karuna, KarXT is now under the stewardship of Bristol Myers Squibb and will be marketed as Cobenfy. 1 This amount represents PureTech level cash, cash equivalents and short-term investments and excludes cash and cash equivalents at non-wholly owned subsidiary of $0.5m. PureTech level cash, cash equivalents and short-term investments is a non-IFRS measure. For more information in relation to the PureTech level cash, cash equivalents and short- term investments and Consolidated cash, cash equivalents and short-term investments measures used in this Annual Report, please see page 69 of the Financial Review.
6 PureTech Health plc Annual Report and Accounts 2024 PureTech is at an important strategic juncture. Our priority in 2025 is to address the persistent value disconnect and deliver shareholder returns through potential strategic initiatives and disciplined capital allocation. Our hub-and-spoke model (see page 10) enables us to derive meaningful economics from our Founded Entities—via equity holdings, milestones, and royalties—while supporting the continued development of our programs. This model has allowed us to self-fund our operations for more than seven years without raising capital from public equity markets, preserving shareholder value and ownership. It has also enabled us to return $150 million to shareholders, while continuing to fuel a highly productive and validated R&D engine – one that consistently generates innovative programs with the potential for significant patient impact. At the core of this track record is our ongoing commitment to scientific rigor, high ethical and quality standards, efficient execution, and disciplined capital allocation. Addressing the Value Disconnect Strategy PureTech’s core mission has always been - and continues to be - to deliver transformative treatments to patients with serious diseases and to translate that patient impact into meaningful value for shareholders. Over the past decade, we have achieved significant scientific, clinical, and financial milestones, underpinned by our capital- efficient hub-and-spoke model. However, despite this progress, our market capitalization has not consistently reflected the intrinsic value of our business. We have taken a number of actions in light of this disconnect over time—including share buybacks, a Tender Offer, attaining a dual listing on Nasdaq, engaging in significant investor outreach and capital market activities, and strategic shifts in our R&D model. We continue to believe there is an opportunity to better align our valuation with the underlying strength of our platform, pipeline, and track record of execution. This has been recognized by external parties – as highlighted by the possible cash offer that was recently made public – and the Board actively engages with such approaches as appropriate. At the same time, we remain focused on executing our business plan, advancing our pipeline, and generating future monetization opportunities. Value impact We remain committed to delivering value in a way that reflects both the maturity of our business, the strength of our assets and financial position, and the opportunity ahead. We will carefully consider any opportunity that arises to create value for our shareholders. We will also continue to operate with capital discipline and strategic focus— deploying resources toward our highest-impact programs and protecting our balance sheet, especially against the current macroeconomic backdrop. Our model allows for ongoing progress across multiple fronts, and our Board remains actively engaged in assessing how best to maximize long-term shareholder value. Wholly-Owned Programs Strategy In December 2024, we announced robust Phase 2b data from our Wholly-Owned Program, deupirfenidone (LYT-100), in idiopathic pulmonary fibrosis (IPF). See pages 11-13. These data have been met with enthusiasm from key stakeholders, including key opinion leaders, based on both their clinical strength and the potential to meaningfully improve the standard of care. Strong safety and efficacy data support meaningful differentiation from current therapies and underscore the potential for significant patient impact of this program. We are targeting a meeting with the FDA before the end of Q3 2025 to align on a potential registrational pathway, with the goal of initiating a Phase 3 trial by the end of the year. We anticipate providing further guidance later this year following the finalization of the trial design and FDA interactions. As we advance toward this next phase, we are actively exploring a variety of strategic funding mechanisms—including a potential spin-out of the program into a new Founded Entity and accessing external equity financing, similar to our approach with Karuna and Seaport; project or royalty-based financing and strategic partnerships—which may be used in combination—to support continued development in a capital-efficient manner. We do not intend to fully fund the Phase 3 trial independently; moreover, we don’t believe our current cash reserves would be sufficient to fully fund a Phase 3 trial. We will, however, continue to fund the program in the interim to maintain development momentum. We are also pursuing continued publication and presentation of the deupirfenidone Phase 2b trial data to support external engagement with potential investors, partners, and collaborators. In parallel, we are continuing to support the development of LYT-200 through our Founded Entity, Gallop Oncology, which is currently led by PureTech’s Entrepreneur-In-Residence, Luba Greenwood, J.D. We anticipate additional clinical data in Q3 2025 and are targeting to secure external funding later this year. PureTech is actively pursuing third-party financing to support Gallop’s next phase of growth and will continue to fund the program in the interim to maintain development momentum. Value impact Our R&D approach is designed to efficiently progress the most promising programs in a disciplined and efficient manner. We apply rigorous internal de-risking, set a high threshold for advancement, and explore multiple paths to value creation—including partnerships and external funding opportunities—to reduce risk and optimize capital deployment. Our goal is to deliver high-impact therapies while maximizing shareholder value through thoughtful, and disciplined program execution and capital deployment. 2025 Strategy to Deliver Shareholder Value 20 25 S tr at eg y to D el iv er S ha re ho ld er V al ue

PureTech Health plc Annual Report and Accounts 2024 7 Founded Entities Strategy Consistent with our hub-and-spoke model, we plan to conduct additional sourcing activities and may launch new Founded Entities to house select programs – balancing risk, cost, and potential reward. Initial expenditures associated with any innovation and sourcing activities would be relatively low. In some cases, we may participate in financing rounds to preserve or enhance our ownership position, when we believe it will generate long-term value. Value impact Our Founded Entities offer multiple advantages: — Enabling the advancement of therapeutic candidates through capital-intensive development — Reducing PureTech’s financial exposure — Creating future monetization opportunities — Driving shareholder returns while avoiding dilution at the PureTech level — Providing a diversified portfolio that reduces binary risk and offers multiple shots on goal This structure also attracts specialized management teams and external capital, allowing PureTech to maintain upside potential with reduced operational burden. See page 16 for our Karuna Therapeutics case study. Shareholder Returns Strategy The Board is committed to maximizing value for our shareholders, and – in 2024 alone – PureTech returned $100 million to shareholders through a Tender Offer. The Board may evaluate additional capital return opportunities —particularly in connection with monetization events—and will consider such actions as part of its broader strategy to maximize shareholder value. Value impact While our primary focus remains on building long-term value through scientific and operational execution, we recognize the importance of capital discipline and direct returns to shareholders when appropriate. Our strong balance sheet, capital-efficient model, and potential monetization events provide us with multiple tools to deliver value—both in the near term and over time. 2025 Strateg y to D eliver Shareho ld er Value

8 PureTech Health plc Annual Report and Accounts 2024 O ur K ey C o m p o ne nt s of V al ue Our Key Components of Value Maximizing stakeholder value Note: Certain third-party trademarks are included here; PureTech does not claim any rights to any third-party trademarks. COBENFY™ (xanomeline and trospium chloride) is indicated for the treatment of schizophrenia in adults. For Important Safety Information, see U.S. Full Prescribing Information, including Patient Information on COBENFY.com. Following the acquisition of Karuna, KarXT is now under the stewardship of Bristol Myers Squibb and will be marketed as Cobenfy. 1 As of December 31, 2024. PureTech level cash, cash equivalents and short-term investments excludes cash and cash equivalents at non-wholly owned subsidiary of $0.5m. PureTech level cash, cash equivalents and short-term investments is a non-IFRS measure. 2 This percentage includes number of successful trials out of all trials run for all therapeutic candidates advanced through at least Phase 1 by PureTech or its Founded Entities from 2009 onward. $366.8M 2024YE1 1. Strong Balance Sheet Deupirfenidone (LYT-100) for IPF (entering Phase 3 clinical development) LYT-200 for AML (poised to enter mid-stage clinical development) 2. Wholly-Owned Programs e.g., Seaport, Vedanta 3. Founded Entity Equity Value e.g., Cobenfy™, Seaport progress 4. Royalties, milestone and sublicense income $150M returned to date via Tender Offer & share buyback (additional capital returns possible) 5. Capital Returns 3 FDA approvals and a strong track record of clinical success (>80% of clinical trials successful2) 6. People/R&D Engine

Conduct rigorous, early de-risking Change the lives of patients with devastating diseases Develop solution driven by validated pharmacology Identify significant patient need PureTech Health plc Annual Report and Accounts 2024 9 O ur Innovative R& D A p p ro ach Our Innovative R&D Approach Accelerating Momentum & Delivering Results Key milestones in recent years Deupirfenidone ELEVATE IPF Study PureTech completed successful Phase 2b trial of deupirfenidone in IPF Vedanta Biosciences PureTech’s Founded Entity Vedanta Biosciences iniated Phase 3 trial of VE303 Seaport Therapeutics PureTech launched Founded Entity Seaport Therapeutics; >$325m raised in 2024 Cobenfy™ (formerly KarXT) BMS/Karuna received FDA Approval for Cobenfy™ Gallop Oncology PureTech’s LYT-200 granted Orphan Drug and Fast Track Designations Royalty Pharma PureTech and Royalty Pharma entered into Cobenfy (KarXT) royalty transaction for up to $500 million Karuna Therapeutics and Bristol Myers Squibb PureTech’s Founded Entity Karuna Therapeutics acquired by Bristol Myers Squibb for $14 billion

10 PureTech Health plc Annual Report and Accounts 2024 O ur H ub -a nd -S p o ke M o d el Relevant ownership interests were calculated on a partially diluted basis (as opposed to a voting basis) as of December 31, 2024, including outstanding shares, options and warrants, but excluding unallocated shares authorized to be issued pursuant to equity incentive plans. PureTech controls Gallop Oncology, Inc. Vor Biopharma ownership was calculated on a beneficial ownership basis in accordance with SEC rules as of March 13, 2025. Our Diversified Hub-and-Spoke Model: Robust pipeline of new medicines balances risk with potential for tremendous growth Gallop Oncology 100% equity AML Deupirfenidone (LYT-100) 100% equity IPF Seaport Therapeutics 35.6% equity Neuropsychiatric conditions Vedanta 35.8% equity C. Difficile Ulcerative colitis Entrega 73.8% equity Peptide therapeutics VOR (Nasdaq: VOR) 2.1% equity AML Karuna Therapeutics Acquired by Bristol Myers Squibb for $14B Sonde 34.8% equity Voice-based AI platform PURETECH’S EXPERIENCED R&D TEAM AND EXTENSIVE NETWORK P16 P11P14 P17 P18 P20 P21 P19

Pro g ram s PureTech Health plc Annual Report and Accounts 2024 11 — We acquired deupirfenidone in July 2019 based on insights gained internally and via unpublished findings through our network of collaborators. Deupirfenidone was originally developed by Auspex Pharmaceuticals, Inc. (Auspex), where our Chief Executive Officer, Bharatt Chowrira, Ph.D., J.D., served as Chief Operating Officer. Auspex (now a wholly owned subsidiary of Teva Pharmaceuticals) pioneered deuteration technology and successfully developed deutetrabenazine (Austedo®), the first deuterated drug to ever receive FDA approval.4 — Deupirfenidone is a deuterated form of pirfenidone, one of the two FDA-approved SOC treatments. The strategic replacement of three hydrogen atoms with deuterium at the site of metabolism has been shown to enhance the beneficial pharmacology and clinically-validated efficacy of pirfenidone while maintaining a favorable tolerability profile. This enhancement allows greater levels of drug exposure to be achieved with deupirfenidone compared to the highest approved dose of pirfenidone, without sacrificing tolerability. Program discovery process by the PureTech team Deupirfenidone (LYT-100) Programs — In December 2024, we announced positive topline results from the ELEVATE IPF Phase 2b clinical trial evaluating deupirfenidone in patients with IPF. – ELEVATE IPF was a global, randomized, double-blind, active- and placebo-controlled, dose-ranging trial that evaluated deupirfenidone at two dose levels (550 mg and 825 mg) three times a day (TID) over 26 weeks in patients with IPF. (Figure 1). – The trial achieved its primary endpoint based on the prespecified Bayesian analysis, with a 98.5% posterior probability. This means there is a 98.5% probability that the pooled deupirfenidone arms were superior to placebo in slowing the rate of lung function decline in people with IPF at 26 weeks, as measured by forced vital capacity (FVC), a measurement of the maximum amount of air a person can forcefully exhale from their lungs. • Deupirfenidone 825 mg TID demonstrated the potential to stabilize lung function decline. Patients treated with deupirfenidone 825 mg TID experienced an FVC decline of -21.5 mL over 26 weeks, which is comparable to the expected natural lung function decline in healthy adults aged 60 and older. (Figure 2). • The deupirfenidone arms, 550mg TID and 825mg TID, exhibited dose-dependent efficacy with an FVC reduction from baseline of -80.7mL and -21.5mL, respectively. The placebo and pirfenidone arms responded as reported in previous studies with an FVC reduction from baseline of -112.5mL and -51.6mL, respectively. The performance of these control arms and the dose-dependent response of the deupirfenidone arms suggests that the efficacy of deupirfenidone was not likely a chance observation. (Figure 3). – The deupirfenidone 825 mg TID arm had an effect size, compared to placebo, that was 50% greater than that seen with pirfenidone (80.9% vs. 54.1%, respectively). Additionally, preliminary pharmacokinetic results indicate that deupirfenidone 825 mg TID achieved ~50% higher exposure than pirfenidone 801 mg TID, corresponding with the greater efficacy results demonstrated with deupirfenidone 825 mg TID. – Deupirfenidone was generally well-tolerated with a favorable adverse event profile at both doses studied. (Figure 4). Key milestones achieved and development status PureTech Ownership 100% equity Deupirfenidone (LYT-100) is being developed at PureTech as a potential new standard of care (SOC) for the treatment of idiopathic pulmonary fibrosis (IPF), a progressive and fatal lung disease estimated to affect over 230,000 people across the U.S. and EU51. Only about 25% of IPF patients have ever been treated with either FDA-approved therapeutic (pirfenidone and nintedanib)2, yet combined sales of these medications in 2022 were more than $4 billion3, representing a significant market opportunity in IPF and other fibrotic lung diseases. Despite achieving blockbuster status, the current SOC treatments only modestly slow lung function decline, as their effectiveness is limited by poor tolerability at higher doses. This results in suboptimal efficacy, reduced patient uptake, and poor adherence—all due to a tolerability ceiling that prevents dosing levels that could significantly improve patient outcomes. Our studies have shown that deupirfenidone may overcome these limitations and – to our knowledge – is the only investigational therapeutic for IPF that has demonstrated the potential to stabilize lung function decline over at least 26 weeks while maintaining safety and tolerability. Beyond IPF, deupirfenidone could also address multiple underserved fibrotic diseases, including progressive fibrosing interstitial lung diseases (ILDs) and other fibrotic conditions.

Pr o g ra m s 12 PureTech Health plc Annual Report and Accounts 2024 Programs continued Key milestones achieved and development status FIGURE 1: ELEVATE: Global, Phase 2b, Multicenter, Randomized, Double-blind Clinical Trial FIGURE 3: Deupirfenidone Demonstrated Potential to Serve as a New Standard-of-Care Treatment for IPF FIGURE 2: Deupirfenidone 825 mg TID Significantly Slowed and Stabilized Lung Function Decline FVC decline for deupirfenidone 825 mg TID at 26 weeks in ELEVATE approached the level of natural lung function decline expected in healthy adults aged 60 and older. Note: Patients in all arms were permitted to decrease and re-increase their assigned dose as tolerated Note: Data pulled from separate studies; outputs do not represent data from a head-to-head study * Reflects outputs obtained via frequentist analysis. ** FVC decline at 6 months was estimated assuming linear decline over time. Valenzuela, C., Bonella, F., Moor, C., Weimann, G., Miede, C., Stowasser, S., & Maher, T. (2024). Decline in forced vital capacity (FVC) in subjects with idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) compared with healthy references. Poster presented at the European Respiratory Society International Congress, Vienna, Austria; and Luoto, J., Pihlsgård, M., Wollmer, P., & Elmståhl, S. (2019). Relative and absolute lung function change in a general population aged 60-102 years. The European Respiratory Journal, 53(3), 1701812. https://doi. org/10.1183/13993003.01812-2017. Note: Change from baseline FVC is not adjusted for patient characteristics such as height, age, race, or sex. Note: Efficacy analyses used a random coefficient regression model with absolute FVC or FVCpp including baseline as response variable and week, treatment and interaction between week and treatment as fixed effect. The analyses were performed based on the predefined Full Analysis Set. p values are two-sided and have not been corrected for multiplicity. Change from baseline FVC is not adjusted for patient characteristics such as height, age, race, or sex. FVC = Forced Vital Capacity; TID = three times a day
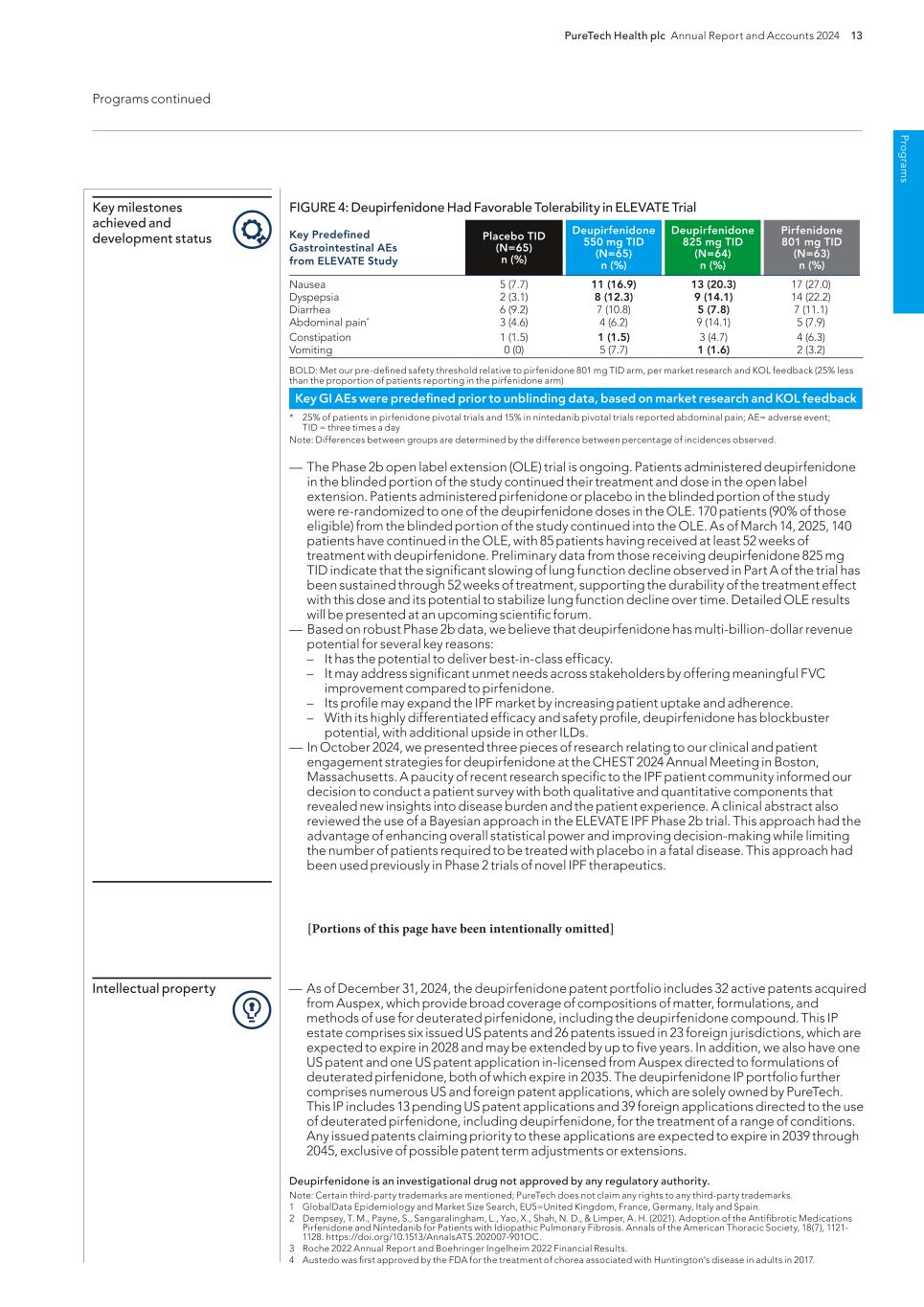
Pro g ram s PureTech Health plc Annual Report and Accounts 2024 13 Programs continued — As of December 31, 2024, the deupirfenidone patent portfolio includes 32 active patents acquired from Auspex, which provide broad coverage of compositions of matter, formulations, and methods of use for deuterated pirfenidone, including the deupirfenidone compound. This IP estate comprises six issued US patents and 26 patents issued in 23 foreign jurisdictions, which are expected to expire in 2028 and may be extended by up to five years. In addition, we also have one US patent and one US patent application in-licensed from Auspex directed to formulations of deuterated pirfenidone, both of which expire in 2035. The deupirfenidone IP portfolio further comprises numerous US and foreign patent applications, which are solely owned by PureTech. This IP includes 13 pending US patent applications and 39 foreign applications directed to the use of deuterated pirfenidone, including deupirfenidone, for the treatment of a range of conditions. Any issued patents claiming priority to these applications are expected to expire in 2039 through 2045, exclusive of possible patent term adjustments or extensions. Intellectual property Deupirfenidone is an investigational drug not approved by any regulatory authority. Note: Certain third-party trademarks are mentioned; PureTech does not claim any rights to any third-party trademarks. 1 GlobalData Epidemiology and Market Size Search, EU5=United Kingdom, France, Germany, Italy and Spain. 2 Dempsey, T. M., Payne, S., Sangaralingham, L., Yao, X., Shah, N. D., & Limper, A. H. (2021). Adoption of the Antifibrotic Medications Pirfenidone and Nintedanib for Patients with Idiopathic Pulmonary Fibrosis. Annals of the American Thoracic Society, 18(7), 1121- 1128. https://doi.org/10.1513/AnnalsATS.202007-901OC. 3 Roche 2022 Annual Report and Boehringer Ingelheim 2022 Financial Results. 4 Austedo was first approved by the FDA for the treatment of chorea associated with Huntington’s disease in adults in 2017. FIGURE 4: Deupirfenidone Had Favorable Tolerability in ELEVATE Trial BOLD: Met our pre-defined safety threshold relative to pirfenidone 801 mg TID arm, per market research and KOL feedback (25% less than the proportion of patients reporting in the pirfenidone arm) * 25% of patients in pirfenidone pivotal trials and 15% in nintedanib pivotal trials reported abdominal pain; AE= adverse event; TID = three times a day Note: Differences between groups are determined by the difference between percentage of incidences observed. — The Phase 2b open label extension (OLE) trial is ongoing. Patients administered deupirfenidone in the blinded portion of the study continued their treatment and dose in the open label extension. Patients administered pirfenidone or placebo in the blinded portion of the study were re-randomized to one of the deupirfenidone doses in the OLE. 170 patients (90% of those eligible) from the blinded portion of the study continued into the OLE. As of March 14, 2025, 140 patients have continued in the OLE, with 85 patients having received at least 52 weeks of treatment with deupirfenidone. Preliminary data from those receiving deupirfenidone 825 mg TID indicate that the significant slowing of lung function decline observed in Part A of the trial has been sustained through 52 weeks of treatment, supporting the durability of the treatment effect with this dose and its potential to stabilize lung function decline over time. Detailed OLE results will be presented at an upcoming scientific forum. — Based on robust Phase 2b data, we believe that deupirfenidone has multi-billion-dollar revenue potential for several key reasons: – It has the potential to deliver best-in-class efficacy. – It may address significant unmet needs across stakeholders by offering meaningful FVC improvement compared to pirfenidone. – Its profile may expand the IPF market by increasing patient uptake and adherence. – With its highly differentiated efficacy and safety profile, deupirfenidone has blockbuster potential, with additional upside in other ILDs. — In October 2024, we presented three pieces of research relating to our clinical and patient engagement strategies for deupirfenidone at the CHEST 2024 Annual Meeting in Boston, Massachusetts. A paucity of recent research specific to the IPF patient community informed our decision to conduct a patient survey with both qualitative and quantitative components that revealed new insights into disease burden and the patient experience. A clinical abstract also reviewed the use of a Bayesian approach in the ELEVATE IPF Phase 2b trial. This approach had the advantage of enhancing overall statistical power and improving decision-making while limiting the number of patients required to be treated with placebo in a fatal disease. This approach had been used previously in Phase 2 trials of novel IPF therapeutics. Key milestones achieved and development status Key GI AEs were predefined prior to unblinding data, based on market research and KOL feedback Key Predefined Gastrointestinal AEs from ELEVATE Study Placebo TID (N=65) n (%) Deupirfenidone 550 mg TID (N=65) n (%) Deupirfenidone 825 mg TID (N=64) n (%) Pirfenidone 801 mg TID (N=63) n (%) Nausea 5 (7.7) 11 (16.9) 13 (20.3) 17 (27.0) Dyspepsia 2 (3.1) 8 (12.3) 9 (14.1) 14 (22.2) Diarrhea 6 (9.2) 7 (10.8) 5 (7.8) 7 (11.1) Abdominal pain* 3 (4.6) 4 (6.2) 9 (14.1) 5 (7.9) Constipation 1 (1.5) 1 (1.5) 3 (4.7) 4 (6.3) Vomiting 0 (0) 5 (7.7) 1 (1.6) 2 (3.2) [Portions of this page have been intentionally omitted]

Pr o g ra m s 14 PureTech Health plc Annual Report and Accounts 2024 AML — The Phase 1b trial evaluating LYT-200 as a monotherapy and in combination with venetoclax/ HMA for AML and MDS is ongoing. LYT-200 has shown a favorable safety profile across both arms and all dose levels with no dose limiting toxicities, as well as clinical efficacy, hematological improvement, and sustained disease management. – As of April 28, 2025, patients have received LYT-200 at five dose levels (2.0 mg/kg to 16.0 mg/ kg) in the monotherapy arm. Across all dose levels, LYT-200 has demonstrated clinical benefit and responses in heavily pre-treated, relapsed/refractory AML/MDS patients, even in those with complex cytogenetics and mutations such as KRAS, NRAS, BRAF, as well as patients previously fully refractory to standard of care. At dose levels of 7.5mg/kg and above, treatment with LYT-200 has resulted in 1 complete response (CR), 3 partial responses (PRs), and more than 50% of patients treated experienced stable disease. The average treatment duration with the single agent was 3.5 months as of the data cutoff. This is meaningful in the heavily pretreated patient population who have already exhausted all standard-of-care options. – When administered in combination with venetoclax/hypomethylating agents (HMA), results as of April 28, 2025, demonstrate that LYT-200 may enhance the efficacy of standard-of-care therapies, even in relapsed or refractory patients. In the combination arm, patients received LYT-200 across three dose levels (4.0 mg/kg, 7.5 mg/kg, and 12.0 mg/kg) with venetoclax/ HMA, resulting in 6 CRs, 1 morphological leukemia-free state (MLFS), and 50% of patients experienced stable disease. The average time on combination therapy is 4 months as of the data cutoff, which is meaningful in a patient population whose time to progression tends to be less than 1 month and whose overall survival averages 1.7-2.4 months with standard-of- care therapy. Patients who benefit show hematological improvement as well as achieve transfusion independence. — In the January 2025 post-period, the FDA granted Fast Track designation to LYT-200 for the treatment of AML. Fast Track designation is a process designed to streamline the development and accelerate the assessment of drugs that target serious conditions with unmet medical need. — In December 2024, we presented data from the dose escalation phase of the ongoing Phase 1b trial evaluating LYT-200 as a monotherapy and in combination with venetoclax/HMA for AML and MDS at the 2024 American Society of Hematology (ASH) Annual Meeting. — In February 2024, the FDA granted orphan drug designation to LYT-200 for the treatment of AML. This designation is given to novel products for the treatment of conditions affecting fewer than 200,000 persons in the U.S, and it qualifies the company for incentives including tax credits for some clinical trials and eligibility for seven years of market exclusivity in the U.S., if the drug is approved for AML. Key milestones achieved and development status — With a focus on providing significant therapeutic benefit to cancer patients, we opportunistically identified a foundational immunosuppressive/pro-tumor mechanism(s) involving galectin-9, which was the basis of certain intellectual property that we licensed from New York University prior to its publication in Nature Medicine. Galectin-9 promotes multiple immunosuppressive pathways in the context of solid tumors and blocking galectin-9 results in tumor cell death in the context of AML and other hematological malignancies. High levels of galectin-9 expression in tumor tissue, on leukemia cells as well as in patients’ blood are linked to more advanced disease and worse outcomes. LYT-200 is a fully human IgG4 monoclonal antibody designed to inhibit the activity of galectin-9. We believe that LYT-200 is the most advanced clinical program against this target. It has the potential to be used as a single agent and in combination with other anti-cancer therapies, depending on the cancer type, treatment setting, and line of treatment. In pre-clinical models, LYT-200 has also demonstrated direct cytotoxic, anti-leukemic effects through multiple mechanisms, as well as synergy with standard of care. Program discovery process by the PureTech team Programs continued Gallop Oncology / LYT-200 PureTech Ownership 100% equity Gallop Oncology™ is advancing a first-in-class, mechanistically differentiated approach to cancer treatment by targeting a novel, pro-tumor and immunosuppressive molecule. Gallop’s LYT-200 is an anti-galectin-9 monoclonal antibody (mAb) being developed for the treatment of hematological malignancies, including acute myeloid leukemia (AML) and high-risk myelodysplastic syndromes (MDS), as well as solid tumors, including head and neck cancers, with a focus on metastatic disease.

Pro g ram s PureTech Health plc Annual Report and Accounts 2024 15 Locally advanced/metastatic solid tumors — The Phase 1b trial evaluating LYT-200 as a monotherapy and in combination with tislelizumab for the treatment of locally advanced/metastatic, relapsed/refractory solid tumors, including head and neck cancers, has successfully completed. LYT-200 demonstrated a favorable safety profile in all cohorts and showed disease control and initial efficacy signals. The LYT-200 solid tumor study enrolled 44 relapsed/refractory solid tumor patients across 13 sites in the United States. – The single agent (SA) cohorts dosed LYT-200 between 0.2 – 16 mg/kg every two weeks (Q2W) or 10 mg/kg every week (QW). The combination cohorts dosed LYT-200 at 6.3mg/kg QW and 16mg/kg QW with an anti-PD-1 antibody, tislelizumab. The SA cohorts enrolled 20 all-comer patients, while the combination cohorts enrolled 24 patients, including 19 with head and neck cancer and five with urothelial cancer. The median number of prior lines of treatment was four in the single agent cohorts and three in the combination cohorts. There were no dose limiting toxicities in this study. There were no LYT-200 related serious adverse events in the SA cohorts. Eight patients (40%) experienced grade 3 or higher adverse events, none of which were related to LYT-200. In combination with tislelizumab, three patients (12.5%) experienced an immune mediated adverse reaction (IMAR), all of which were related to tislelizumab (grade 2 hypothyroidism, grade 1 hyperthyroidism, and grade 3 cholangitis). There was one LYT-200- related grade 3 serious adverse event, an asymptomatic elevation of the protein troponin, that occurred with 16 mg/kg QW LYT-200 plus tislelizumab, led to treatment discontinuation, but resolved fully on steroid treatment. 14 patients (58.3%) experienced grade 3 or higher adverse events of which one (the elevated troponin) was related to LYT-200. In the SA cohorts, three patients (16% of evaluable patients) achieved stable disease. Disease control has been noted from dose levels of 6.3 mg/kg and above. – In the combination cohorts, two urothelial cancer patients (out of 4 evaluable patients) treated with 6.3 mg/kg QW LYT-200 plus tislelizumab had stable disease. In head and neck patients treated with 6.3 mg/kg QW LYT-200 plus tislelizumab, one patient experienced a complete response lasting more than 2 years; two patients had a partial response; and two patients had stable disease, resulting in an overall response rate of 33% and a disease control rate of 50%. At 16 mg/kg plus tislelizumab, three patients (out of seven evaluable) had stable disease, giving a disease control rate of 43%. For patients achieving a response as per RECIST 1.1 criteria, the six-month progression-free survival (PFS) is 67%, and the 12-month PFS is 33%. — In March 2024, the FDA granted Fast Track designation for LYT-200 in combination with anti-PD1 therapy for the treatment of recurrent/metastatic head and neck cancers. Fast Track designation is a process designed to streamline the development and accelerate the assessment of drugs that target serious conditions with unmet need. Key milestones achieved and development status — The intellectual property portfolio for LYT-200 provides broad intellectual property coverage for antibody-based immunotherapy technologies. As of December 31, 2024, there are 16 families of intellectual property within this patent portfolio, including eight (8) families of patent filings that are co-owned with and/or exclusively licensed from New York University which cover antibodies that target galectin-9, including LYT-200, and methods of using these antibodies in various immuno-oncology technologies and other therapeutic methods. In addition, the intellectual property portfolio includes seven (7) families of company-owned patent applications covering the use of anti-galectin-9 antibodies in the diagnosis and treatment of various cancers, including solid tumors and hematological cancers, and one family of patent applications co-owned with BeiGene directed to combination therapies for the treatment of solid tumors. This intellectual property portfolio comprises four (4) issued U.S. patents which are expected to expire in 2038, 14 pending U.S. patent applications, which if issued, are expected to expire 2037 through 2045, one (1) international PCT application, 82 pending foreign applications and 20 issued patents in foreign jurisdictions. Intellectual property Programs continued LYT-200 is an investigational drug not approved by any regulatory authority. [Portions of this page have been intentionally omitted]

Pr o g ra m s 16 PureTech Health plc Annual Report and Accounts 2024 Note: Certain third-party trademarks are included here; PureTech does not claim any rights to any third-party trademarks. COBENFY™ (xanomeline and trospium chloride) is indicated for the treatment of schizophrenia in adults. For Important Safety Information, see U.S. Full Prescribing Information, including Patient Information on COBENFY.com. Following the acquisition of Karuna, KarXT is now under the stewardship of Bristol Myers Squibb and will be marketed as Cobenfy. 1 As of 22 March 2023, PureTech has sold its right to receive a 3 percent royalty from Karuna to Royalty Pharma on net sales up to $2 billion annually, after which threshold PureTech will receive 67 percent of the royalty payments and Royalty Pharma will receive 33 percent. Additionally, under its license agreement with Karuna, PureTech retains the right to also receive certain sublicense income. The acquisition of Karuna by Bristol Meyers Squibb (NYSE: BMY), had no impact on our rights or obligations under the Exclusive Patent License Agreement with Karuna, which remains in full force and effect. — In March 2024, Bristol Myers Squibb (NYSE: BMY) announced the completion of its acquisition of Karuna for $330.00 per share, for a total equity value of approximately $14 billion. With the acquisition’s completion, Karuna is now a wholly owned subsidiary of BMS. Through monetization of equity holdings in Karuna, including gross proceeds from the BMS acquisition and a strategic royalty agreement, PureTech has generated approximately $1.1 billion to date from an initial allocation of $18.5 million in Karuna’s founding. — In September 2024, BMS announced that Cobenfy (formerly known as KarXT) had received U.S. Food and Drug Administration approval for the treatment of schizophrenia in adults. The FDA approval triggered two separate milestone payments to PureTech totaling $29 million under agreements with Royalty Pharma and PureTech’s Founded Entity, Karuna Therapeutics (now BMS). Under these agreements, PureTech is also entitled to potential future payments related to additional milestones as well as approximately 2% royalties on net annual sales over $2 billion. Key milestones achieved and development status — We and our collaborators, including leading schizophrenia experts, were excited about efficacy data generated in schizophrenia and Alzheimer’s disease by Eli Lilly with xanomeline, which had notable efficacy stemming from its activation of muscarinic receptors (M1 and M4) but had been held back by gastrointestinal tolerability issues. To overcome this, we invented KarXT, an oral M1/ M4-preferring muscarinic agonist, by combining xanomeline (a muscarinic agonist) with trospium (a peripherally acting muscarinic antagonist that doesn’t cross the blood brain barrier). This enabled the beneficial effects of M1/M4 activation in the brain without the peripheral side effects. We conducted key human tolerability proof-of-concept studies with KarXT that allowed Karuna, and subsequently BMS to advance it further in schizophrenia patients. In September 2024, Cobenfy (formerly known as KarXT) received U.S. FDA approval for the treatment of schizophrenia in adults. Program discovery process by the PureTech team — Phase 3 trials, ADEPT-2 and ADEPT-3, are ongoing and continue to evaluate Cobenfy for the treatment of psychosis in Alzheimer’s disease. Programs continued Karuna Therapeutics A wholly owned subsidiary of Bristol Myers Squibb PureTech Ownership PureTech is entitled to milestone payments, royalties and up to $400 million in milestone payments under its agreement with Royalty Pharma. Karuna Therapeutics is a wholly owned subsidiary of Bristol Myers Squibb (BMS) driven to create and deliver transformative medicines for people living with psychiatric and neurological conditions. Cobenfy (formerly known as KarXT), is the first new drug with a novel mechanism to treat schizophrenia in more than 50 years. Cobenfy was invented and initially developed by PureTech. Consistent with its unique model of drug development, PureTech advanced Cobenfy by founding Karuna Therapeutics, which progressed Cobenfy through Phase 3 development before being acquired by BMS for $14 billion in March 2024. [Portions of this page have been intentionally omitted]

Pro g ram s PureTech Health plc Annual Report and Accounts 2024 17 — In April 2024, we launched Seaport Therapeutics with a $100 million oversubscribed Series A financing round to advance novel neuropsychiatric medicines. Daphne Zohar was appointed as a Founder, Chief Executive Officer and a member of the Board, and Steven M. Paul, M.D., as a Founder and Chair of the Board of Directors. — In October 2024, Seaport raised a total of $226 million in its oversubscribed Series B financing round. This financing brought the total capital raised by Seaport to $326 million since the company’s launch in April 2024. — In May 2024, Seaport presented two posters detailing the results from multiple clinical trials of SPT-300 at the Society of Biological Psychiatry (SOBP) Annual Meeting in Austin, TX. Together, these data summarize some of the evidence supporting the core mechanisms of SPT-300 as Seaport advances to later-stage clinical studies. – The first poster provided data from the first-in-human, multi-part Phase 1 trial of SPT-300 from which topline results were first reported in December 2022. Overall, the Phase 1 trial was well tolerated and evaluated oral bioavailability, safety, tolerability, pharmacokinetics and GABAA target engagement. – The second poster provided details from the Phase 2a trial of SPT-300 in the Trier Social Stress Test (TSST), a validated clinical model of anxiety in healthy volunteers. Topline results from the trial, demonstrating SPT-300 substantially reduced salivary cortisol at all post-TSST timepoints compared to placebo, meeting the study’s primary endpoint and demonstrating regulation of the hypothalamic-pituitary-adrenal axis reactivity to acute stress, were first reported in November 2023. — In December 2024, Seaport presented additional data from its first-in-human, multi-part Phase 1 study of SPT-300 in healthy volunteers at the American College of Neuropsychopharmacology (ACNP) Annual Meeting, held in Phoenix, Arizona. New data presented at the conference include further safety analyses and pharmacokinetic and pharmacodynamic data. In the Phase 1 study, increases in EEG beta frequency power and reduction in saccadic eye velocity were observed at approximately 4 hours post-dose. Somnolence peaked in this same timeframe and diminished by 6 to 8 hours post-dose, consistent with both pharmacodynamic markers and blood levels of allopregnanolone. Based on these results, SPT-300 is suitable for chronic dosing and oral administration at night in a planned Phase 2b placebo-controlled study in major depressive disorder with or without anxious distress. Key milestones achieved and development status — With intersecting interests in enabling promising neuropsychiatric drugs to reach their full potential and the emerging science around the lymphatic system, we identified a breakthrough technology being developed at Monash University that had the potential to selectively transport therapeutic molecules through the lymphatic system. — Through the Glyph platform, drugs are absorbed like dietary fats through the intestinal lymphatic system and transported into circulation. The Glyph technology has the potential to be widely applied to many therapeutic molecules that have high first-pass metabolism leading to low bioavailability and/or side effects, including hepatotoxicity. We prioritized areas of high unmet patient need where the broad application of treatment options with validated efficacy was untapped due to these issues. The Glyph platform was advanced initially at PureTech and now exclusively licensed and assigned to Seaport and has been applied to efficiently generate multiple therapeutic candidates within Seaport’s pipeline. Program discovery process by the PureTech team Programs continued Seaport Therapeutics PureTech Ownership 35.6% equity, milestone and sublicense payments, and 3-5% royalties Seaport Therapeutics is a clinical-stage biopharmaceutical company advancing the development of novel neuropsychiatric medicines in areas of high unmet patient needs. The company has a proven strategy of advancing clinically validated mechanisms previously held back by limitations that are overcome with its proprietary Glyph™ technology platform. All the therapeutic candidates in its pipeline of first and best-in-class medicines are based on the Glyph platform. Seaport is led by an experienced team that invented and advanced important neuropsychiatric medicines and are guided by an extensive network of renowned scientists, clinicians and key opinion leaders. Seaport’s clinical-stage pipeline includes its most advanced therapeutic candidate, SPT- 300, an oral prodrug of allopregnanolone, which is being advanced for the treatment of major depressive disorder (MDD) with or without anxious distress; SPT-320, a novel oral prodrug of agomelatine, which is being advanced for the treatment of generalized anxiety disorder (GAD); and SPT-348, a prodrug of a non-hallucinogenic neuroplastogen, which is in development for the treatment of mood and other neuropsychiatric disorders. [Portions of this page have been intentionally omitted]

Pr o g ra m s 18 PureTech Health plc Annual Report and Accounts 2024 — In April 2024, Vedanta was awarded $3.9 million from the global nonprofit CARB-X to ready its preclinical candidate VE707 for a first-in-human clinical study to examine its ability to reduce colonization and prevent subsequent infections caused by multi-drug-resistant organisms. The funding was triggered by Vedanta’s completion of certain milestones for VE707, which were supported by previous CARB-X awards totaling $5.7 million. — In May 2024, Vedanta enrolled the first patient in the pivotal Phase 3 RESTORATiVE303 study of VE303 for the prevention of recurrent C. difficile infection. This study is intended to form the basis for a Biologics License Application to be filed with the U.S. Food and Drug Administration. — In July 2024, Vedanta announced it had expanded its leadership team, including appointing a Head of Commercial in alignment with having initiated the pivotal clinical phase. — In October 2024, Vedanta shared additional analyses from the CONSORTIUM trial at Infectious Diseases Week (IDWeek) 2024. The analyses characterized the benefit of VE303 on antibiotic resistant gene levels in patient microbiomes. Antibiotic resistance can lead to the development of hard-to-treat infections throughout the body, and as VE303 administration appears to correlate with reduced prevalence of organisms carrying these resistance genes, the result describes an additional dimension of VE303’s therapeutic profile. — In the January 2025 post-period, Vedanta published additional results from the VE303 Phase 2 CONSORTIUM clinical trial in Nature Medicine, providing a new level of profiling of the multiple mechanisms by which VE303 may decrease the odds of rCDI. The CONSORTIUM trial showed that the higher dose of VE303 studied was well tolerated and reduced the odds of CDI recurrence by more than 80% compared with placebo. Clinical results from the trial were previously published in the Journal of the American Medical Association (JAMA). Key milestones achieved and development status — We engaged with leading world-renowned experts in immunology and identified and in- licensed intellectual property to pioneer the concept of therapeutically defined consortia of microbes that could modulate the immune system or treat bacterial infections. Program discovery process by the PureTech team Programs continued Vedanta Biosciences PureTech Ownership 35.8% equity Vedanta Biosciences is a clinical-stage biopharmaceutical company developing medicines for the treatment of gastrointestinal diseases. The company’s lead assets are potential first- in-class oral therapies – VE303, in a Phase 3 registrational trial for prevention of recurrent C. difficile infection (rCDI), and VE202, in a Phase 2 trial for treatment of ulcerative colitis. Vedanta’s pipeline has been built using the company’s industry-leading product engine for the development of therapies based on defined consortia of bacteria grown from pure clonal cell banks. The product engine, supported by broad foundational intellectual property, includes one of the largest libraries of bacteria isolated from the human microbiome, vast clinical datasets, proprietary capabilities in consortium design, and end-to-end CGMP manufacturing capabilities at commercial launch scale. [Portions of this page have been intentionally omitted]

Pro g ram s PureTech Health plc Annual Report and Accounts 2024 19 — In 2024, Vor expanded its ongoing Phase 1/2 VBP101 study to include patients diagnosed with myelodysplastic syndrome (MDS) in addition to its original trial population of patients with AML. During the trial, patients received trem-cel, a shielded stem cell transplant lacking CD33 manufactured by Vor, followed by Mylotarg™, a CD33-directed Antibody Drug Conjugate (ADC) therapy. Preliminary data suggest improved relapse-free survival compared to published groups of AML patients at high risk of relapse post-transplant. Trem-cel + MylotargTM also continued to show engraftment, shielding, and a broadened therapeutic window. Vor received supportive feedback from the FDA regarding a registrational clinical trial design. — In 2024, Vor dosed the first patient in VBP301, its Phase 1/2, multicenter, open-label, first-in- human study of VCAR33ALLO in patients with relapsed or refractory AML after standard-of-care transplant or a trem-cel transplant and received both Fast Track designation and Orphan Drug designation from the FDA. Patients treated with the lowest dose of VCAR33ALLO , a CAR-T cell therapy manufactured by Vor from lymphocytes collected from the patient’s original transplant donor, demonstrated promising biomarker data. — In September 2024, Vor disclosed a new preclinical asset, VADC45, an ADC that targets the CD45 protein. CD45 is a well-validated target for a wide variety of blood cancers with clinical proof of concept. Initial data suggest VADC45 has large potential opportunities across oncology, gene therapy, and autoimmune disorders. Key milestones achieved and development status — We were interested in approaches to treat hematological malignancies that currently have poor response rates or poor adverse event profiles despite recent advances in cell therapies and targeted therapies. We worked with Vor Bio Scientific Advisory Board Chair, Siddhartha Mukherjee, M.D., Ph.D., on key intellectual property, which Vor Bio exclusively in-licensed from Columbia University, and on advancing this concept through critical proof-of- concept experiments Program discovery process by the PureTech team Programs continued Vor PureTech Ownership 2.1% equity Vor Bio is a clinical-stage cell and genome engineering company that aims to change the standard of care for patients with blood cancers by engineering hematopoietic stem cells to enable targeted therapies post-transplant. [Portions of this page have been intentionally omitted]

Pr o g ra m s 20 PureTech Health plc Annual Report and Accounts 2024 Programs continued — We were interested in enabling the oral administration of biologics, which has been a long- standing problem in drug development. We engaged with leading experts in drug administration, including Robert Langer, Sc.D., screened over 100 technologies, and the initial platform was licensed from Samir Mitragotri, Ph.D., when he was Professor of Chemical Engineering at UC Santa Barbara (currently Hiller Professor of Bioengineering and Hansjorg Wyss Professor of Biologically Inspired Engineering at Harvard University). We later enhanced this platform with intellectual property developed by our team. Program discovery by the PureTech team Entrega PureTech Ownership 73.8% equity Entrega is unlocking key areas of medicine by enabling oral administration of biologics, vaccines and other drugs that are otherwise not efficiently absorbed when taken orally. The vast majority of biologic drugs, including peptides, proteins and other macromolecules, are currently administered by injection, which can present challenges for healthcare administration and compliance with treatment regimes. For this growing class of therapies, including newly developed treatments for diabetes and weight loss, oral administration may be the ideal route of administration. Entrega’s technology platform is an innovative approach to oral administration, which uses a proprietary, customizable hydrogel dosage form to control local fluid microenvironments in the GI tract to both enhance absorption and reduce the variability of drug exposure. Peptide therapeutics (e.g., the emerging GLP-1 agonist class) are ideally suited to benefit from Entrega’s approach and our platform has demonstrated increased oral peptide availability of two- to three-fold over standard permeation enhancer formulations. [Portions of this page have been intentionally omitted]

Pro g ram s PureTech Health plc Annual Report and Accounts 2024 21 Programs continued — In the February 2025 post-period, Sonde began design and implementation of validation studies with three organizations within the Department of the Air Force (DAF). Two organizations will be studying the impact of shift work on soldiers’ performance beginning in March and the third organization will study the impact of fatigue on soldiers operating in the field beginning in June. — In the February 2025 post-period, Sonde successfully completed a large-scale trial with one of the top oil and gas conglomerates in the world. Over 4,000 workers used Sonde’s Mental Fitness tracking app and insight dashboard over 5 weeks at 60 different sites. Sonde was able to effectively identify at-risk workers, enabling proactive intervention by flagging individuals with high sensitivity and deliver significant positive predictive value for a healthy population, ensuring most workers who needed support were identified. — In the January 2025 post-period, Sonde successfully integrated its voice-based health monitoring technology into Qualcomm’s Snapdragon® S7+ Gen 1 Sound Platform. Building on its longtime partnership with Qualcomm, Sonde optimized its technology to operate within the strict power and processing constraints of audio earbud and headset devices while maintaining its industry- leading accuracy. — In August 2024, Sonde announced that it has been selected by AFWERX, the innovation arm of the Department of the Air Force (DAF), for a Small Business Innovation Research (SBIR) Phase II contract in the amount of $1,215,606 focused on Sonde Mental Fitness. This contract builds on work completed through a Phase 1 contract awarded in January 2024 that identified multiple use cases for employing Sonde Mental Fitness vocal biomarker tracking to promote mental health engagement and resource utilization and to enhance operational decision-making. The customization and integration work planned for this SBIR Phase II effort will enable use and validation of Sonde’s mental fitness tracking technology in DAF settings. — In July 2024, Sonde launched Sonde Cognitive Fitness, which analyses eight vocal characteristics from 30-second voice interactions to provide cognitive effort tracking and insight, helping people manage their mental well-being and productivity effectively. Key milestones achieved and development status — We identified vocal features as a leading non-invasive source of health data, particularly given the evolving technology landscape where voice interactions with devices are rapidly increasing. We developed novel intellectual property around this concept and helped advance the technology from an academic concept to a commercially focused technology. Program discovery by the PureTech team Sonde PureTech Ownership 34.8% equity Sonde Health is the global leader in voice AI health tracking and data insights. Sonde’s vocal biomarker platform serves apps and devices spanning consumer wellness to population health. Leveraging a best-in-class voice data set and clinical research with over 1.2 million samples from 85,000+ individuals on four continents, Sonde uses advanced audio signal processing, speech science, and machine learning to sense and analyze subtle vocal changes due to changes in a person’s physiology to provide key insights into health and well-being. [Portions of this page have been intentionally omitted]

E SG re p o rt 22 PureTech Health plc Annual Report and Accounts 2024 [Pages 22 to 29 have been intentionally omitted]

E SG rep o rt E SG rep o rt PureTech Health plc Annual Report and Accounts 2024 23

24 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt

PureTech Health plc Annual Report and Accounts 2024 25 E SG rep o rt

26 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt

PureTech Health plc Annual Report and Accounts 2024 27 E SG rep o rt

28 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt

PureTech Health plc Annual Report and Accounts 2024 29 E SG rep o rt

PATIENTS 30 PureTech Health plc Annual Report and Accounts 2024 Our ESG framework continued E SG re p o rt Pa ti en ts As pioneers in clinical stage biotherapeutics, our mission remains to address devastating diseases and improve patient health worldwide through innovative medicines. In 2024, we continued to develop our programs through the expertise of our dedicated team and in collaboration with our extensive network of scientists, clinicians and industry leaders. For details on our programs, please see pages 11-21.To accomplish this goal consistently and ethically, we focus our sustainability efforts on three key areas that enable patient support : Commitment #1: Addressing unmet medical needs Commitment #2: Ensuring patient safety Commitment #3: Accelerating our R&D engine to unlock new medicines

75% PureTech Health plc Annual Report and Accounts 2024 31 Our ESG framework continued E SG rep o rt Patients IPF is a fatal disease with clear patient need; 75% in US not on treatment primarily due to efficacy/tolerability trade off with existing treatments Despite the underserved patient population, IPF is multi-billion-dollar market, with ~250,000 patients in USA and EU52 PureTech’s deupirfenidone demonstrated strong, consistent, & durable efficacy with favorable tolerability; advancing into pivotal Phase 3 Deupirfenidone also has the potential to address multiple underserved diseases, including progressive fibrosing interstitial lung diseases (ILDs), a group of lung diseases closely related to IPF, as well as other fibrotic conditions where there is human data with pirfenidone suggestive of clinical benefit. PureTech is committed to continuing the development of deupirfenidone to improving the lives of those impacted by IPF through research, advocacy, and innovation. Our initiatives: We undertake efforts to drive awareness of our investigational treatments. Our initiative create inclusive resources to engage both patients and caregivers in clinical trials. Rare Disease Day In February 2024, we celebrated Rare Disease Day in which employees wore blue to show their support for pulmonary fibrosis awareness. The purpose of this initiative is to help raise awareness for the 6,000+ rare diseases that impact over 300 million people globally, and to advocate for equitable access to diagnosis, treatment, care and social opportunities. Patient Advocacy Partnerships PureTech is a sponsor of the Pulmonary Fibrosis Foundation’s (PFF) Corporate Committee to fulfill a responsibility to the pulmonary fibrosis community. Through this committee, members work together towards solutions to issues that impact patients, supporting the advancement of research, and contributing to the education needs of the patient and medical community. In March 2024, we continued to sponsor PFF annual fundraiser, Broadway Belts! The event raised over $550,000 in 2024 to support the PFF’s programs and have raised over $3 million to date. Commitment #1: Addressing unmet medical needs Our team remains dedicated to providing therapeutics for unmet medical needs. We leverage the substantial groundwork laid by the biopharmaceutical industry, which has dedicated decades to discovering novel modalities and proving efficacy in patients. Despite these advancements, barriers have prevented important new medicines from reaching their full potential. Our R&D approach starts with a problem that has significant patient need and we aim to unlock new classes of medicine by applying innovative approaches and technologies to small molecules and biologics that have demonstrated human efficacy but have fallen short of meeting patients’ needs. With our cutting-edge R&D efforts, we are targeting these gaps while creating long-term value for both patients and shareholders see pages 9-10 for more on our R&D model. Demonstrated track record of inventing groundbreaking treatments: Cobenfy™ 1 During 2024, the PureTech-invented therapeutic, Cobenfy™ (formerly KarXT), received U.S. Food and Drug Administration (FDA) approval for the treatment of schizophrenia in adults. (See page 16 for more on our case study with Cobenfy.) Cobenfy was invented at PureTech to address a tolerability challenge that had held back a potential new class of medicines for the treatment of neuropsychiatric conditions, such as schizophrenia. The FDA approval of Cobenfy is a testament to our unique R&D engine that creates and develops treatments to target unmet patient needs. We apply this approach across our portfolio and will continue to leverage this successful drug development model as we enter our next phase of innovation to offer a positive impact for patients. Making progress in the fight against idiopathic pulmonary fibrosis (IPF) Our most advanced therapeutic candidate, deupirfenidone (LYT-100), is being developed for the potential treatment of IPF. We are excited for the potentially significant impact we can offer patients in need with this program. In December 2024, we announced positive Phase 2b trial data from this program, which highlight its potential to become the new standard-of-care treatment for IPF. (See pages 11-13 for details on PureTech’s deupirfenidone program) 1 Note: Certain third-party trademarks are included here; PureTech does not claim any rights to any third-party trademarks. COBENFY™ (xanomeline and trospium chloride) is indicated for the treatment of schizophrenia in adults. For Important Safety Information, see U.S. Full Prescribing Information, including Patient Information on COBENFY.com. Following the acquisition of Karuna, KarXT is now under the stewardship of Bristol Myers Squibb and will be marketed as Cobenfy. 2 GlobalData Epidemiology and Market Size Search, EU5=United Kingdom, France, Germany, Italy and Spain.

32 PureTech Health plc Annual Report and Accounts 2024 Our ESG framework continued E SG re p o rt Pa ti en ts Patients When sponsoring an Investigational New Drug (IND) application, we acknowledge our responsibility to both participants and the regulatory agencies who put their trust in us to act responsibly. We have a robust governance framework in place to ensure patient oversight which includes effective policies and protocols such as our Safety Management Plans and Medical Monitoring Plans, which helps us to monitor, review and act on any incidents. All protocols are compliant with ICH E6 (R2) per FDA regulations and most of our studies have Independent Data Safety Monitoring Committees. Clinical trial participants are made fully aware of all risks involved prior to participating in a clinical trial. To confirm this, we ensure that every patient has provided informed consent of their willingness to participate through a signed voluntary commitment. Our informed consent requirements are set out in the PureTech Clinical Research Policy. We also rely on the use of human biological specimens to develop our innovative therapies through clinical trials, which require informed consent. Our Human Biological Specimens Policy specifies our commitment to respecting both donors and the specimens they provide and that collecting, obtaining, storing and using human biological samples must be obtained through consent. Our President is responsible for ensuring that PureTech follows all US and applicable international regulatory requirements and standards and applicable bioethics principles. In 2024, there were no FDA sponsored inspections related to clinical trial management and pharmacovigilance that resulted in PureTech receiving Voluntary Action Indicated (VAI) and Official Action Indicated (OAI) from the FDA. Bioethics: R&D Our ethical and quality management standards, allow for continuous improvement through R&D, while helping us to maintain high standards of product quality and safety in compliance with relevant regulations at each phase. In 2024, we spent $69 million on research and development projects to develop new and innovative therapeutics (see page 73 for details on R&D expenses). As we enhance our R&D strategy, we continue to assess and identify areas for improvement across our clinical trial safety, quality and risk management processes. We have robust policies relating to Good Manufacturing Practices (GMP) and regulatory inspections to reinforce ethics into our processes. Environmental factors remain integral in our R&D and further information on our waste data can be found on page 44. We also strive to implement green chemistry and eco-design principles. For example, optimizing large-scale drug substance processes to replace more hazardous solvents that negatively impact the environment. Our SVP of Medical Affairs, Camilla Graham, MD, MPH, plays a vital role in advancing deupirfenidone, and collaborating with advocacy groups to support the IPF community: IPF Awareness Month In September 2024, we continued our efforts to promote Pulmonary Fibrosis Awareness Month to raise awareness of IPF and to serve as inspiration for our employees. We participated in an awareness walk, hosted a webinar, and made a donation of $7,000 to the PFF. On PFF National Walk Day, our team came together to raise awareness and funds for those affected by this devastating disease. We are proud to support organizations like PFF, which is dedicated to accelerating new treatment development for people living with IPF. Commitment #2: Ensuring patient safety Patient safety remains our top priority and informs all aspects of our work. To ensure clinical trial and R&D integrity, our research team works with external partners to adhere to strict procedures, processes and guidelines. Responsible development practices and diligent oversight guide our efforts to develop innovative medicines that have the potential to transform patient lives. Delivering Safe Clinical Trials We conduct all clinical trials according to the highest standards of ethics and safety. All our trials follow the standards of the International Conference on Harmonization (ICH) Good Clinical Practice guidelines and the World Medical Association (WMA) Declaration of Helsinki on the Ethical Principles for Medical Research Involving Human Subjects. To ensure compliance and rigor in our approach, we seek approval from Independent Ethics Committees and local regulatory authorities on all investigative medicine trials. In addition, our employees who are engaged with clinical trials, either as clinical staff or their designees, are responsible for ensuring full compliance with best clinical practice. “Patient advocacy groups play a critical role in ensuring that people living with pulmonary fibrosis are educated about their disease, understand what to expect from medical care, share tips to manage side effects of medications, raise awareness about clinical trials, and provide a space for caregivers to receive needed support. There are fantastic local and national pulmonary fibrosis advocacy organizations that do important work and it’s imperative that we offer our support.”

PureTech Health plc Annual Report and Accounts 2024 33 Our ESG framework continued E SG rep o rt Patients Bioethics: Animal Research Animal research continues to play a vital and irreplaceable role in progressing drug discovery, as it assists scientists in addressing biological uncertainties. PureTech conducts animal testing only when necessary to further the development of therapeutics. This is mandated by regulatory bodies before human trials of new medications can proceed to ensure the safety of clinical trial participants. We follow the guidelines outlined under the USDA Animal Welfare Act and are dedicated to the humane and ethical treatment of animals. Studies involving animals are evaluated and approved by the Executive Team and are carried out at external qualified and certified vendors that fulfil our standards and anticipated practices for animal care, welfare and handling. Whenever we contemplate animal testing, we are devoted to applying the replacement, reduction and refinement of animal studies (3Rs): — Replace We use alternative methods to animal testing wherever possible. — Reduce We use the minimum number of animals in trials. — Refine We minimize pain, suffering and distress, and improve the welfare of animals used in trials. Bioethics: Quality Management We have a robust Quality Management System (QMS) in place to oversee our raw material suppliers. Our QMS consists of various Standard Operating Procedures (SOPs) which describe our controlled processes that result in consistent quality control as per PureTech’s quality system. SOPs include, but are not limited to: — Clinical Quality Audit Management — Clinical Quality Event Management — GMP/GLP Vendor Selection and Qualification — GMP/GLP Vendor Audit Procedure — Clinical Operations Safety Monitoring & Management (via Safety Vigilance Distribution tool) — Nonconformance Procedure for GMP Activities To ensure our QMS is robust and up to date, a risk assessment protocol is built into our procedures for vendor audits, vendor oversight, and data integrity for Chemistry, Manufacturing, and Controls (CMC). This allows us to quickly determine vendor risks and accelerate new vendor onboarding to meet business demands. Ensuring Drug Efficacy and Safety None of PureTech’s wholly-owned programs are currently on the market. In 2024, PureTech received no FDA warning letters, no products were delayed due to a lack of regulatory approval and no product recalls took place. As we continue to advance our therapeutic candidates towards commercialization, we will continue to practice our clinical protocols diligently to ensure ongoing safety and compliance across our operations and clinical trials. Commitment #3: Accelerating our R&D engine to unlock new medicines R&D has been the bedrock of progress in global health and a key component in the successful and innovative discovery and development of our therapeutic candidates. Our strong R&D model - centered on three guiding principles - has generated a robust pipeline to date, enabling us to continue to fulfill our unyielding commitment to delivering potentially life-changing new therapies for patients in need. — Target areas with significant patient need to offer transformative patient benefit — Develop solutions driven by validated efficacy — Advance therapeutics through rigorous and de-risked paths to unlock new classes of medicine We will continue to leverage this model, our scientific insight and our network of scientists, clinicians and industry leaders to unlock new medicines and deliver highly innovative therapeutics for patients.

34 PureTech Health plc Annual Report and Accounts 2024 Our ESG framework continued E SG re p o rt Pe o p le Our employees are predominantly located near our headquarters in Boston, MA, with three individuals based in London. As of December 31, 2024, we had a total of 56 employees. 41% of our employees work in R&D roles. [Portions of this page have been intentionally omitted]

PureTech Health plc Annual Report and Accounts 2024 35 Our ESG framework continued E SG rep o rt Peo p le [This page has been intentionally omitted]
36 PureTech Health plc Annual Report and Accounts 2024 Our ESG framework continued E SG re p o rt People Pe o p le Commitment #2: Promoting employee development to attract and retain the best talent Human capital is vital to a successful business operation to support the identification of new opportunities, and innovations. We depend on our people, their scientific knowledge, skills and commitment to thrive. As such, the personal development, retention and recruitment of industry-leading talent is one of our top priorities at PureTech. This priority is linked to our core business strategy by ensuring we have a strong workforce which remains at the forefront of our industry in developing new therapeutic candidates. Recruitment and Retention As our programs advance and our business rapidly evolves, the PureTech team has evolved with it over the course of years. In 2024, we completed a successful launch of Seaport Therapeutics to house our CNS pipeline, with an oversubscribed Series A (see page 17 for details). As part of this, the team involved with the CNS pipeline transitioned to Seaport Therapeutics to maintain continuity, which is reflected in the figure below. 2022 2023 2024 Total number of employees 111 90 56 Year-over-year growth (%) 16.8% (18%) (37%) Employee turnover (%) 30.62% 44.1% 28% Note: The 2024 YoY growth was -37%, of which 20 percentage-point was attributed to the Seaport Therapeutics transition. [Portions of this page have been intentionally omitted]

PureTech Health plc Annual Report and Accounts 2024 37 E SG rep o rt Peo p le [Pages 37 to 58 have been intentionally omitted]

38 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt Pe o p le

PureTech Health plc Annual Report and Accounts 2024 39 E SG rep o rt Peo p le

40 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt Pl an et

PureTech Health plc Annual Report and Accounts 2024 41 E SG rep o rt Planet

42 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt Pl an et

PureTech Health plc Annual Report and Accounts 2024 43 E SG rep o rt Planet

44 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt Pl an et

PureTech Health plc Annual Report and Accounts 2024 45 E SG rep o rt Planet

46 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt G ov er na nc e

PureTech Health plc Annual Report and Accounts 2024 47 E SG rep o rt G overnance

48 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt G ov er na nc e

PureTech Health plc Annual Report and Accounts 2024 49 E SG rep o rt G overnance

50 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt G ov er na nc e

PureTech Health plc Annual Report and Accounts 2024 51 E SG rep o rt G overnance

52 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt G ov er na nc e

PureTech Health plc Annual Report and Accounts 2024 53 E SG rep o rt G overnance

54 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt TC FD R ep o rt

PureTech Health plc Annual Report and Accounts 2024 55 E SG rep o rt TC FD Rep o rt

56 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt TC FD R ep o rt

PureTech Health plc Annual Report and Accounts 2024 57 E SG rep o rt TC FD Rep o rt

58 PureTech Health plc Annual Report and Accounts 2024 E SG re p o rt TC FD R ep o rt

PureTech Health plc Annual Report and Accounts 2024 59 G overnance Governance Our world class Board of Directors provides strong governance

60 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e The execution of the Group’s strategy is subject to a range of risks and uncertainties. As a clinical-stage biotherapeutics company, the Group operates in an inherently high-risk environment. The Group’s strategic approach seeks to aid the Group’s risk management efforts to achieve an effective balancing of risk and reward. Risk assessment, evaluation and mitigation are integral parts of the Group’s management process. The Group, however, also recognizes that ultimately no strategy provides an assurance against loss, as for example we saw in 2024 with founded-entity Akili Interactive Labs, Inc., which merged with privately-held Virtual Therapeutics and ceased trading as a public company in July 2024. Risks are formally identified by the Board and appropriate internal controls are put in place and tailored to the specific risks to monitor and mitigate them on an ongoing basis. If multiple or an emerging risk event occurs, it is possible that the overall effect of such events would compound the overall effect on the Group. The principal risks that the Board has identified as the key business risks facing the Group are set out in the table below along with the impact and mitigation management plan with respect to each risk. These risks are only a high-level summary of the principal risks affecting our business; any number of these or other risks could have a material adverse effect on the Group or its financial condition, development, results of operations, subsidiary companies and/or future prospects. Further information on the risks facing the Group can be found on pages 182 to 219 which also includes a description of circumstances under which principal and other risks and uncertainties might arise in the course of our business and their potential impact. Risk Impact* Management Plans/Actions 1 Risks related to science and technology failure The science and technology being developed or commercialized by some of our businesses may fail and/or our businesses may not be able to develop their intellectual property into commercially viable therapeutics or technologies. There is also a risk that certain of the businesses may fail or not succeed as anticipated, resulting in significant decline of our value. The failure of any of our businesses could decrease our value. A failure of one of the major businesses could also impact the reputation of PureTech as a developer of high value technologies and possibly make additional fundraising by PureTech or any Founded Entity more difficult or unavailable on acceptable terms at all. Prior to additional steps in the development of any technology, extensive due diligence is carried out that covers all the major business risks, including technological feasibility, competition and technology advances, market size, strategy, adoption and intellectual property protection. A capital efficient approach is employed, which requires the achievement of a level of proof of concept prior to the commitment of substantial capital is committed. Capital deployment is generally tranched to ensure the funding of programs only to their next value milestone. Members of our Board or our management team serve on the board of directors of several of the businesses so as to continue to guide each business’s strategy and to oversee proper execution thereof. We use our extensive network of advisors to ensure that each business has appropriate domain expertise as it develops and executes on its strategy and the R&D Committee of our Board reviews each program at each stage of development and advises our Board on further actions. Additionally, we have a diversified model with numerous assets such that the failure of any one of our businesses or therapeutic candidates would not result in a failure of all of our businesses. Risk management

PureTech Health plc Annual Report and Accounts 2024 61 Risk management continued Risk Impact* Management Plans/Actions 2 Risks related to clinical trial failure Clinical trials and other tests to assess the commercial viability of a therapeutic candidate are typically expensive, complex and time-consuming, and have uncertain outcomes. Conditions in which clinical trials are conducted differ, and results achieved in one set of conditions could be different from the results achieved in different conditions or with different subject populations. If our therapeutic candidates fail to achieve successful outcomes in their respective clinical trials, the therapeutics will not receive regulatory approval and in such event cannot be commercialized. In addition, if we fail to complete or experience delays in completing clinical tests for any of our therapeutic candidates, we may not be able to obtain regulatory approval or commercialize our therapeutic candidates on a timely basis, or at all. A critical failure of a clinical trial may result in termination of the program and a significant decrease in our value. Significant delays in a clinical trial to support the appropriate regulatory approvals could impact the amount of capital required for the business to become fully sustainable on a cash flow basis. We have a diversified model to limit the impact of clinical trial outcomes on our ability to operate as a going concern. We have dedicated internal resources to establish and monitor each of the clinical programs for the purpose of maximising successful outcomes. We also engage outside experts to help create well-designed clinical programs that provide valuable information and mitigate the risk of failure. Significant scientific due diligence and preclinical experiments are conducted prior to a clinical trial to evaluate the odds of the success of the trial. In the event of the outsourcing of these trials, care and attention are given to assure the quality of the vendors used to perform the work. 3 Risks related to regulatory approval The pharmaceutical industry is highly regulated. Regulatory authorities across the world enforce a range of laws and regulations governing the testing, approval, manufacturing, labelling and marketing of pharmaceutical therapeutics. Stringent standards are imposed which relate to the quality, safety and efficacy of these therapeutics. These requirements are a major determinant of the commercial viability of developing a drug substance or medical device given the time, expertise and expense which must be invested. We may not obtain regulatory approval for our therapeutic candidates. Moreover, approval in one territory offers no guarantee that regulatory approval will be obtained in any other territory. Even if therapeutics are approved, subsequent regulatory difficulties may arise, or the conditions relating to the approval may be more onerous or restrictive than we anticipate. The failure of one of our therapeutics to obtain any required regulatory approval, or conditions imposed in connection with any such approval, may result in a significant decrease in our value. We manage our regulatory risk by employing highly experienced clinical managers and regulatory affairs professionals who, where appropriate, will commission advice from external advisors and consult with the regulatory authorities on the design of our preclinical and clinical programs. These experts ensure that high-quality protocols and other documentation are submitted during the regulatory process, and that well-reputed contract research organizations with global capabilities are retained to manage the trials. We also engage with experts, including on our R&D Committee, to help design clinical trials to help provide valuable information and maximize the likelihood of regulatory approval. Additionally, we have a diversified model with numerous assets such that the failure to receive regulatory approval or subsequent regulatory difficulties with respect to any one therapeutic would not adversely impact all of our therapeutics and businesses. G overnance

62 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Risk management continued Risk Impact* Management Plans/Actions 4 Risks related to therapeutic safety There is a risk of adverse reactions with all drugs and medical devices. If any of our therapeutics are found to cause adverse reactions or unacceptable side effects, then therapeutic development may be delayed, additional expenses may be incurred if further studies are required, and, in extreme circumstances, it may prove necessary to suspend or terminate development. This may occur even after regulatory approval has been obtained, in which case additional trials may be required, the approval may be suspended or withdrawn or require product labels to include additional safety warnings. Adverse events or unforeseen side effects may also potentially lead to product liability claims against us as the developer of the therapeutics and sponsor of the relevant clinical trials. These risks are also applicable to our Founded Entities and any trials they conduct or therapeutic candidates they develop. Adverse reactions or unacceptable side effects may result in a smaller market for our therapeutics, or even cause the therapeutics to fail to meet regulatory requirements necessary for sale of the therapeutic. This, as well as any claims for injury or harm resulting from our therapeutics, may result in a significant decrease in our value. Safety is our top priority in the design of our therapeutics. We conduct extensive preclinical and clinical trials which test for and identify any adverse side effects. Despite these steps and precautions, we cannot fully avoid the possibility of unforeseen side effects. To mitigate the risk further we have insurance in place to cover product liability claims which may arise during the conduct of clinical trials. 5 Risks related to therapeutic profitability and competition We may be unable to sell our therapeutics profitably if reimbursement from third-party payers – such as private health insurers and government health authorities – is restricted or not available. If, for example, it proves difficult to build a sufficiently strong economic case based on the burden of illness and population impact. Third-party payers are increasingly attempting to curtail healthcare costs by challenging the prices that are charged for pharmaceutical therapeutics and denying or limiting coverage and the level of reimbursement. Moreover, even if the therapeutics can be sold profitably, they may not be adopted by patients and the medical community. Alternatively, our competitors – many of whom have considerably greater financial and human resources – may develop safer or more effective therapeutics or be able to compete more effectively in the markets targeted by us. New companies may enter these markets and novel therapeutics and technologies may become available which are more commercially successful than those being developed by us. These risks are also applicable to our Founded Entities and could result in a decrease in their value. The failure to obtain reimbursement from third party payers, and competition from other therapeutics, could significantly decrease the amount of revenue we may receive from therapeutic sales for certain therapeutics. This may result in a significant decrease in our value. We engage reimbursement experts to conduct pricing and reimbursement studies for our therapeutics to ensure that a viable path to reimbursement, or direct user payment, is available. We also closely monitor the competitive landscape for our therapeutics and therapeutic candidates and adapt our business plans accordingly. Not all therapeutics that we are developing will rely on reimbursement. Also, while we cannot control outcomes, we seek to design studies to generate data that will help support potential reimbursement.

PureTech Health plc Annual Report and Accounts 2024 63 G overnance Risk management continued Risk Impact* Management Plans/Actions 6 Risks related to intellectual property protection We may not be able to obtain patent protection for some of our therapeutics or maintain the secrecy of their trade secrets and know-how. If we are unsuccessful in doing so, others may market competitive therapeutics at significantly lower prices. Alternatively, we may be sued for infringement of third-party patent rights. If these actions are successful, then we would have to pay substantial damages and potentially remove our therapeutics from the market. We license certain intellectual property rights from third parties. If we fail to comply with our obligations under these agreements, it may enable the other party to terminate the agreement. This could impair our freedom to operate and potentially lead to third parties preventing us from selling certain of our therapeutics. The failure to obtain patent protection and maintain the secrecy of key information may significantly decrease the amount of revenue we may receive from therapeutic sales. Any infringement litigation against us may result in the payment of substantial damages by us and result in a significant decrease in our value. We spend significant resources in the prosecution of our patent applications and maintenance of our patents, and we have in-house patent counsel and patent group to help with these activities. We also work with experienced external attorneys and law firms to help with the protection, maintenance and enforcement of our patents. Third party patent filings are monitored to ensure the Group continues to have freedom to operate. Confidential information (both our own and information belonging to third parties) is protected through use of confidential disclosure agreements with third parties, and suitable provisions relating to confidentiality and intellectual property exist in our employment and advisory contracts. Licenses are monitored for compliance with their terms. 7 Risks related to enterprise profitability We expect to continue to incur substantial expenditure in further research and development activities. There is no guarantee that we will become operationally profitable, and, even if we do so, we may be unable to sustain operational profitability. The strategic aim of the business is to generate profits for our shareholders through the commercialization of technologies through therapeutic sales, strategic partnerships and sales of businesses or parts thereof. The timing and size of these potential inflows are uncertain. Should revenues from our activities not be achieved, or in the event that they are achieved but at values significantly less than the amount of capital invested, then it would be difficult to sustain our business. We retain significant cash in order to support funding of our Founded Entities and our Wholly-Owned Programs. We have close relationships with a wide group of investors and strategic partners to ensure we can continue to access the capital markets and additional monetization and funding for our businesses. Additionally, our Founded Entities are able to raise money directly from third party investors and strategic partners. 8 Risks related to hiring and retaining qualified employees and key personnel We operate in complex and specialized business domains and require highly qualified and experienced management to implement our strategy successfully. We and many of our businesses are located in the United States which is a very competitive employment market. Moreover, the rapid development which is envisaged by us may place unsupportable demands on our current managers and employees, particularly if we cannot attract sufficient new employees. There is also the risk that we may lose key personnel. The failure to attract highly effective personnel or the loss of key personnel would have an adverse impact on our ability to continue to grow and may negatively affect our competitive advantage. The Board regularly seeks external expertise to assess the competitiveness of the compensation packages of its senior management. Senior management continually monitors and assesses compensation levels to ensure we remain competitive in the employment market. We maintain an extensive recruiting network through our Board members, advisors and scientific community involvement. We also employ an executive as a full- time in-house recruiter and retain outside recruiters when necessary or advisable. Additionally, we are proactive in our retention efforts and include incentive-based compensation in the form of equity awards and annual bonuses, as well as a competitive benefits package. We have a number of employee engagement efforts to strengthen our PureTech community.

64 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Risk management continued Risk Impact* Management Plans/Actions 9 Risks related to business, economic or public health disruptions Business, economic, financial or geopolitical disruptions or global health concerns could seriously harm our development efforts and increase our costs and expenses. Broad-based business, economic, financial or geopolitical disruptions could adversely affect our ongoing or planned research and development activities. Global health concerns, such as a further pandemic, or geopolitical events, like the ongoing consequences of the armed conflicts, could also result in social, economic, and labor instability in the countries in which we operate or the third parties with whom we engage. We consider the risk to be increasing since the prior year and note further risks associated with the banking system and global financial stability. We cannot presently predict the scope and severity of any potential business shutdowns or disruptions, but if we or any of the third parties with whom we engage, including the suppliers, clinical trial sites, regulators, providers of financial services and other third parties with whom we conduct business, were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted. It is also possible that global health concerns or geopolitical events such as these ones could disproportionately impact the hospitals and clinical sites in which we conduct any of our current and/or future clinical trials, which could have a material adverse effect on our business and our results of operation and financial impact. We regularly review the business, economic, financial and geopolitical environment in which we operate. It is possible that we may see further impact as a result of current geopolitical tensions. We monitor the position of our suppliers, clinical trial sites, regulators, providers of financial services and other third parties with whom we conduct business. We develop and execute contingency plans to address risks where appropriate.

G o vernance PureTech Health plc Annual Report and Accounts 2024 65 Viability PureTech Health plc Viability Statement In accordance with the UK Corporate Governance Code (Governance Code) published in July 2018, the Directors have assessed the prospects of the Company with respect to the December 31, 2024 financial position. Based on current projections, the Directors believe that the Company has sufficient available funding to extend operations into at least 2027. This period is deemed appropriate having assessed the financial health as of December 31, 2024. We expect our Wholly-Owned Programs3 to significantly progress during this period and our key Controlled Founded Entities2 to reach significant development milestones over the period of the assessment. As we advance our Wholly-Owned Programs and Controlled Founded Entities, our future decisions will be driven by the data of our programs and access to capital from external sources to fund these programs. Our current projections are consistent with our disciplined R&D approach to advance our Wholly-Owned Programs and Controlled Founded Entities through the development process and not commit resources to further development unless specific thresholds for advancement are met, including access to sources of external funding. The Directors have evaluated our cash and cash equivalents and short-term investment of $367.3 million as of December 31, 2024. The Directors have determined that these amounts are sufficient for the advancement of our Wholly-Owned Programs in the near term, to support our existing Founded Entities1, should they require it, and our strategy around creating and supporting new Founded Entities. Additionally, the Directors have determined that these amounts are also sufficient to provide reasonable returns for our shareholders and to fund the Company’s operating costs into at least 2027. The Directors' review has considered all of the principal and emerging risks identified and focused on the pathway to regulatory approval of each therapeutic candidate being developed within our Wholly-Owned Programs as well as those of our Founded Entities. The Directors reviewed the near-term liquidity and considered funding plans of our Wholly-Owned Programs and Founded Entities in our assessment of long-term cash flow projections. It should be noted that the majority of funding has been allocated to support the Company’s strategy around Founded Entities, alongside the advancement of the Wholly-Owned Programs which could become Founded Entities themselves. The Directors confirm that they have a reasonable expectation that we will continue to operate and meet our obligations as they become due over the period of the assessment. In making this statement, the Directors carried out a robust assessment of the principal and emerging risks, including those that would threaten our business model, future performance, solvency or liquidity and evaluated plausible scenarios that included these risks. This assessment was made in consideration of our strong financial position, current strategy, and management of principal and emerging risks. The following facts support the Directors’ view of the viability: — We have a cash, cash equivalents and short-term investments position of $367.3 million as of December 31, 2024. — Our cash, cash equivalents and short-term investments are highly liquid and readily available. — We have control over the spending and strategic direction of our Wholly-Owned Programs and Controlled Founded Entities. — We do not intend to fully fund our LYT-100 program's Phase 3 trial or LYT 200's Phase 2 trial on our own. — Our business model is structured so that we are not reliant on the successful outcomes of any one therapeutic or technology within the Wholly-Owned Programs, or any Founded Entities. In addition, the fact that our Wholly-Owned Programs and Founded Entities are currently in the research and development stage means that these therapeutics, technologies and entities are not reliant on cash inflows from product sales or services during the period of this assessment. This also means that we are not highly susceptible to conditions in one or more market sectors in this time frame. The utilization of existing cash, cash equivalents and short- term investments to advance these therapeutics, technologies and entities is within our control, and the spending and investment decisions are largely discretionary. Therefore, there is management control on reducing discretionary spending if unforeseen liquidity risks arise. Although engaging with collaboration partners is highly valuable from a validation and, in some cases, funding perspective, we are not solely reliant on cash flows from such sources over the period of assessment. Further, the Directors have considered milestone and royalty funding based on existing arrangements, milestone payments from the Royalty Purchase Agreement with Royalty Pharma, the ability of the Wholly-Owned Programs and each Controlled Founded Entity to enter into new collaboration agreements, all of which could be expected to generate cash in-flows but were not included in the assessment.

G ov er na nc e 66 PureTech Health plc Annual Report and Accounts 2024 The Directors note that our ownership stakes in the Founded Entities are expected to be illiquid in nature, with the exception of our ownership stakes in the entity which is publicly traded on Nasdaq. While we anticipate holding these ownership stakes through the achievement of significant milestones or other events, we will continue to be diligent in exploring monetization opportunities after key value accretion has occurred similar to the execution of the sale of 1,750,000 common shares of Karuna for an aggregate proceeds of $218.1 million in 2021, the sale of 602,100 common shares of Karuna for an aggregate proceeds of $115.5 million in 2022, the sale of 535,400 common shares of Vor for an aggregate proceeds of $3.3 million in 2022, the sale of 167,579 common shares of Karuna for an aggregate proceeds of $33.3 million in 2023, the sale of 886,885 common shares of Karuna for an aggregate proceeds of $292.7 million in 2024, and the sale of 12,527,476 common shares of Akili for an aggregate proceeds of $5.4 million in 2024. We also expect that certain of these Founded Entities may not be successful, and this could result in a loss of the amounts previously invested. For example, Gelesis was listed on the New York Stock Exchange as of December 31, 2022 and was delisted from the New York Stock Exchange in April 2023. On October 30, 2023, Gelesis ceased operations and filed a voluntary petition for relief under the United States bankruptcy code. However, even if certain Founded Entities are not successful, our liquidity is expected to remain sufficient to achieve the remaining milestone events, fund operational costs and provide returns for our shareholders over the period of assessment. The Directors have concluded, based on our strong financial position and readily available cash, cash equivalents and short- term investments, that we are highly likely to be able to fund our infrastructure requirements, advance our Wholly-Owned Programs in the near term, and contribute amounts necessary for the Founded Entities to reach significant development milestones over the period of the assessment. Therefore, there is a reasonable expectation that we have adequate resources and will continue to operate and meet our obligations over the period of the assessment. Viability continued 1. Founded Entities are comprised of the entities which the Company incorporated and announced the incorporation as a Founded Entity externally. It includes certain of the Company’s wholly-owned subsidiaries which have been announced by the Company as Founded Entities, Controlled Founded Entities2 and deconsolidated Founded Entities. As of December 31, 2024, deconsolidated Founded Entities included Vor Biopharma, Inc., Gelesis, Inc., Sonde Health, Inc., Vedanta Biosciences, Inc., and Seaport Therapeutics, Inc. 2. Controlled Founded Entities are comprised of the Company’s consolidated operational subsidiaries that currently have already raised third-party dilutive capital. As of December 31, 2024, Entrega was the only entity under this definition. 3. Wholly-Owned Programs are comprised of the Company’s current and future therapeutic candidates and technologies that are developed by the Company's wholly-owned subsidiaries, whether they were announced as a Founded Entity or not, and will be advanced through with either the Company's funding or non-dilutive sources of financing. As of December 31, 2024, Wholly-Owned Programs were developed by the wholly-owned subsidiaries including PureTech LYT, Inc., PureTech LYT 100, Inc., and Gallop Oncology, Inc., and included primarily the programs deupirfenidone (LYT-100) and LYT-200.

G overnance PureTech Health plc Annual Report and Accounts 2024 67 Note: Certain third-party trademarks are included here; PureTech does not claim any rights to any third-party trademarks.COBENFY™ (xanomeline and trospium chloride) is indicated for the treatment of schizophrenia in adults. For Important Safety Information, see U.S. Full Prescribing Information, including Patient Information on COBENFY.com. Following the acquisition of Karuna, KarXT is now under the stewardship of Bristol Myers Squibb and will be marketed as Cobenfy. 1 Funding figure includes private convertible notes and public offerings. Funding figure excludes future milestone considerations received in conjunction with partnerships and collaborations. 2 Number represents figure for the relevant fiscal year only and is not cumulative. Key Performance Indicators – 2024 The key performance indicators (KPIs) below measure our performance against our strategy. As PureTech’s strategy has evolved, new KPIs have replaced older metrics that are no longer representative of our progress. 2023: $578.4m 2022: $1.28b 2021: $731.9m 2020: $247.8m 2019: $666.8m 2018: $274.0m 2017: $102.9m Progress Seaport, Vedanta and Vor raised funds in the form of financings in 2024, including $339.1 million by third party financial and strategic investors. $397.5m1,2 Amount of funding secured for Founded Entities $351.1m (>88%) came from third parties 2023: $133.3m 2022: $115.4m 2021: $218.1m 2020: $350.6m 2019: $9.3m Progress A key component of our strategy is to derive value from the equity growth of our Founded Entities. In 2024, we generated cash proceeds of approximately $327 million from the acquisitions of two of our Founded Entities (Karuna Therapeutics and Akili Interactive) and milestone payments related to the FDA approval of PureTech-invented CobenfyTM (formerly known as KarXT) under agreements with Royalty Pharma and Karuna Therapeutics (now a wholly-owned subsidiary of Bristol Myers Squibb). $327.4m2 Proceeds generated from Founded Entity monetization events 2023: 5 2022: 4 2021: 11 2020: 6 2019: 6 Progress Vedanta initiated one clinical trial in 2024, and at least 5 additional clinical trials continue to progress in 2025 across the Wholly- Owned and Founded Entity pipelines. 12 Number of clinical trial initiations 2023: 1 2022: 1 2021: 2 2020: 3 2019: 1 2018: 1 2017: 1 Progress In 2024, we advanced a new therapeutic candidate from our GlyphTM platform. This candidate, now known as SPT-348, is a non-hallucinogenic neuroplastogen in development at our Founded Entity Seaport Therapeutics for the treatment of mood and neuropsychiatric disorders. 12 Number of programs created by PureTech 2023: 1 2022: 1 2021: 1 2020: 3 2019: 0 Progress In 2024, PureTech completed a multiyear Phase 2b clinical trial of deupirfenidone (LYT-100) in patients with idiopathic pulmonary fibrosis. PureTech intends to discuss these results with the FDA before the end of the third quarter of 2025 to align on a potential registrational pathway, with the goal of initiating a Phase 3 trial by the end of the year. The Company anticipates providing further guidance later this year following the finalization of the trial design and FDA interactions. 02 Number of programs advanced internally through clinical phases 2023: 5 2022: 6 2021: 6 2020: 5 2019: 5 Progress PureTech completed the multiyear Phase 2b clinical trial of deupirfenidone (LYT-100) in patients with idiopathic pulmonary fibrosis (IPF) in 2024. 12 Number of clinical trial readouts

G ov er na nc e 68 PureTech Health plc Annual Report and Accounts 2024 Reporting Framework You should read the following discussion and analysis together with our Consolidated Financial Statements, including the notes thereto, set forth elsewhere in this report. Some of the information contained in this discussion and analysis or set forth elsewhere in this report, including information with respect to our plans and strategy for our business and financing our business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including the risks set forth on pages 60 to 64 and in the Additional Information section from pages 182 to 219, our actual results could differ materially from the results described in or implied by these forward-looking statements. Our audited Consolidated Financial Statements as of December 31, 2024 and 2023, and for the years ended December 31, 2024, 2023 and 2022, have been prepared in accordance with UK-adopted International Financial Reporting Standards ("IFRSs"). The Consolidated Financial Statements also comply fully with IFRSs as issued by the International Accounting Standards Board ("IASB"). The following discussion contains references to the Consolidated Financial Statements of PureTech Health plc (the "Parent") and its consolidated subsidiaries, together "the Group". These financial statements consolidate PureTech Health plc’s subsidiaries and include the Group’s interest in associates by way of equity method, as well as investments held at fair value. Subsidiaries are those entities over which the Group maintains control. Associates are those entities in which the Group does not have control for financial accounting purposes but maintains significant influence over financial and operating policies. Where the Group has neither control nor significant influence for financial accounting purposes, or when the investment in associates is not in instruments that would be considered equity for accounting purposes, we recognize our holdings in such entity as an investment at fair value with changes in fair value being recorded in the Consolidated Statement of Comprehensive Income/(Loss). For purposes of our Consolidated Financial Statements, each of our Founded Entities1 are considered to be either a “subsidiary", an “associate” or an "investment held at fair value" depending on whether the Group controls or maintains significant influence over the financial and operating policies of the respective entity at the respective period end date, and depending on the form of the investment. For additional information regarding the accounting treatment of these entities, see Note 1. Material Accounting Policies to our Consolidated Financial Statements included in this report. For additional information regarding our operating structure, see “Basis of Presentation and Consolidation” below. Business Background and Results Overview The business background is discussed above from pages 1 to 21, which describes the business development of our Wholly- Owned Programs3 and Founded Entities. Our ability to generate product revenue sufficient to achieve profitability will depend on the successful development and eventual commercialization of one or more therapeutic candidates of our wholly-owned or Controlled Founded Entities2, which may or may not occur. Historically, certain of our Founded Entities' therapeutics received marketing authorization from the FDA, but our Wholly-Owned Programs have not generated revenue from product sales to date. Furthermore, our ability to achieve profitability will largely rely on successfully monetizing our investment in Founded Entities, including the sale of rights to royalties, entering into strategic partnerships, and other related business development activities. We deconsolidated a number of our Founded Entities, specifically Seaport Therapeutics, Inc. ("Seaport") in October 2024, Vedanta Biosciences, Inc. ("Vedanta") in 2023, Sonde Health Inc. ("Sonde") in 2022, Karuna Therapeutics, Inc. ("Karuna"), Vor Biopharma Inc. ("Vor") and Gelesis in 2019, and Akili in 2018. Any deconsolidation affects our financials in the following manner: — our ownership interest does not provide us with a controlling financial interest; — we no longer control the Founded Entity's assets and liabilities, and as a result, we derecognize the assets, liabilities and non-controlling interests related to the Founded Entity from our Consolidated Financial Statements; — we record our retained investment in the Founded Entity at fair value; and — the resulting amount of any gain or loss is recognized. Whilst we do not plan to fully fund our LYT-100 or LYT-200 programs, we anticipate providing certain level of funding in 2025 while we seek external sources of funding. Consequently, we anticipate our expenses to increase in the short term as we continue to advance these Wholly-Owned Programs. However, we anticipate a decrease in our expenses in the mid- and long- term in connection with execution of our current strategy of housing these Wholly-Owned Programs in Founded Entities and accessing external sources of funding at the Founded Entity level, which, over time, could lead to the deconsolidation of the Founded Entities. The increase in our expenses and capital requirements in the near term will involve: — continued research and development efforts to advance our clinical programs through development; and — addition of clinical, scientific, operational, financial and management information systems and maintaining appropriate levels of personnel to execute on our strategic initiatives. Financial Review 1. Founded Entities are comprised of the entities which the Company incorporated and announced the incorporation as a Founded Entity externally. It includes certain of the Company’s wholly-owned subsidiaries which have been announced by the Company as Founded Entities, Controlled Founded Entities2 and deconsolidated Founded Entities. As of December 31, 2024, deconsolidated Founded Entities included Vor Biopharma, Inc., Gelesis, Inc., Sonde Health, Inc., Vedanta Biosciences, Inc., and Seaport Therapeutics, Inc. 2. Controlled Founded Entities are comprised of the Company’s consolidated operational subsidiaries that currently have already raised third-party dilutive capital. As of December 31, 2024, Controlled Founded Entities included only Entrega. Inc. 3. Wholly-Owned Programs are comprised of the Company’s current and future therapeutic candidates and technologies that are developed by the Company's wholly-owned subsidiaries, whether they were announced as a Founded Entity or not, and will be advanced through with either the Company's funding or non-dilutive sources of financing. As of December 31 ,2024, Wholly-Owned Programs were developed by the wholly-owned subsidiaries including PureTech LYT, Inc., PureTech LYT 100, Inc. and Gallop Oncology, Inc. and included primarily the programs deupirfenidone (LYT-100), and LYT-200.

G overnance PureTech Health plc Annual Report and Accounts 2024 69 In addition, with respect to our Founded Entities’ programs, we anticipate that we will continue to fund a small portion of development costs by strategically participating in such companies’ financings when we believe participation in such financings is in the best interests of our shareholders. The form of any such participation may include investment in public or private financings, collaboration, partnership arrangements, and/or licensing arrangements, among others. Our management and strategic decision makers consider the future funding needs of our Founded Entities and evaluate rigorously the needs and opportunities for returns with respect to each of these Founded Entities routinely and on a case-by-case basis. As a result, we may need access to substantial additional funding in the future at the PureTech level, following the period described below in the Funding Requirements section, to support our continuing operations and pursue our growth strategy, including participating in financing activities at the Founded Entity level. We expect to finance our operations through a combination of monetization of our interests in our Founded Entities, collaborations with third parties, or other sources. We may be unable to access additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we are unable to raise capital or enter into such agreements, as and when needed, we may have to delay, scale back or discontinue our continuing operations and pursuit of our growth strategy, including participating in financing activities at the Founded Entity level. Further, if we are unable to obtain external funding for our LYT-100 and LYT-200 Wholly-Owned programs, we may have to delay, scale back or discontinue the development and commercialization of one or more of these Wholly-Owned programs. Measuring Performance The Financial Review discusses our operating and financial performance, our cash flows and liquidity as well as our financial position and our resources. The results of current period are compared with the results of the comparative period in the prior year. Reported Performance Reported performance considers all factors that have affected the results of our business, as reflected in our Consolidated Financial Statements. Core Performance Core performance measures are alternative performance measures, which are adjusted and non-IFRS measures. These measures cannot be derived directly from our Consolidated Financial Statements. We believe that these non-IFRS performance measures, when provided in combination with reported performance, will provide investors, analysts and other stakeholders with helpful complementary information to better understand our financial performance and our financial position from period to period. The measures are also used by management for planning and reporting purposes. The measures are not substitutable for IFRS financial information and should not be considered superior to financial information presented in accordance with IFRS. Cash flow and liquidity PureTech Level cash, cash equivalents and short-term investments Measure type: Core performance Definition: Cash and cash equivalents and short-term investments held at PureTech Health plc and our wholly-owned subsidiaries. Why we use it: PureTech Level cash, cash equivalents and short- term investments is a measure that provides valuable additional information with respect to cash, cash equivalents and short-term investments available to fund the Wholly-Owned Programs and make certain investments in Founded Entities. Recent Developments (subsequent to December 31, 2024) The Group has evaluated subsequent events after December 31, 2024 up to the date of issuance, April 30, 2025, of the Consolidated Financial Statements, and has not identified any recordable or disclosable events not otherwise reported in these Consolidated Financial Statements or notes thereto. Financial Highlights The following is the reconciliation of the amounts appearing in our Consolidated Statement of Financial Position to the alternative performance measure described above: (in thousands) December 31, 2024 December 31, 2023 Cash and cash equivalents 280,641 191,081 Short-term investments 86,666 136,062 Consolidated cash, cash equivalents and short-term investments 367,307 327,143 Less: cash and cash equivalents held at non-wholly owned subsidiaries (493) (1,097) PureTech Level cash, cash equivalents and short-term investments $366,813 $326,046 Basis of Presentation and Consolidation Our Consolidated Financial Information consolidates the financial information of PureTech Health plc, as well as its subsidiaries, and includes our interest in associates and investments held at fair value and is reported in reportable segments as described below. Basis for Segmentation Our Directors are our strategic decision-makers. Our operating segments are determined based on the financial information provided to our Directors periodically for the purposes of allocating resources and assessing performance. We have determined each of our Wholly-Owned Programs represents an operating segment, and we have aggregated each of these operating segments into one reportable segment, the Wholly-Owned Programs segment. Each of our Controlled Founded Entities represents an operating segment. We aggregate each Controlled Founded Entity operating segment into one reportable segment, the Controlled Founded Entities segment. The aggregation is based on the high level of operational and financial similarities of the operating segments. For our entities that do not meet the definition of an operating segment, we present this information in the Parent Company and Other column in our segment footnote to reconcile the information in this footnote to our Consolidated Financial Statements. Substantially all of our revenue and profit generating activities are generated within the United States and, accordingly, no geographical disclosures are provided. Financial Review continued
G ov er na nc e 70 PureTech Health plc Annual Report and Accounts 2024 Following is the description of our reportable segments: Wholly-Owned Programs The Wholly-Owned Programs segment is advancing Wholly- Owned Programs which are focused on treatments for patients with devastating diseases. The Wholly-Owned Programs segment is comprised of the technologies that are wholly- owned and will be advanced through with either the Group's funding or non-dilutive sources of financing. The operational management of the Wholly-Owned Programs segment is conducted by the PureTech Health team, which is responsible for the strategy, business development, and research and development. Controlled Founded Entities The Controlled Founded Entities segment is comprised of the Group’s consolidated operational subsidiaries as of December 31, 2024 that either have, or have plans to hire, independent management teams and currently have already raised third- party dilutive capital. These subsidiaries have active research and development programs and have an equity or debt investment partner, who will provide additional industry knowledge and access to networks, as well as additional funding to continue the pursued growth of the entity. The Group’s entities that were determined not to meet the definition of an operating segment are included in the Parent Company and Other column to reconcile the segment information to the Consolidated Financial Statements. This column captures activities not directly attributable to the Group’s operating segment and includes the activities of the Parent, corporate support functions, certain research and development support functions that are not directly attributable to a strategic business segment as well as the elimination of intercompany transactions. This column also captures the operating results for our deconsolidated entities through the date of deconsolidation (e.g. Seaport in 2024, Vedanta in 2023, and Sonde in 2022), and accounting for our holdings in Founded Entities for which control has been lost, which primarily represent: the activity associated with deconsolidating an entity we no longer control, the gain or loss on our investments accounted for at fair value (e.g. our ownership stakes in Seaport, Sonde, Vedanta, and Vor) and our net income or loss of associates accounted for using the equity method. There was no change to the reportable segments in 2024, except for the changes to the composition of the reportable segments as described below. In January 2024, we launched two new Founded Entities (Seaport Therapeutics "Seaport" and Gallop Oncology "Gallop") to advance certain programs from the Wholly- Owned Programs segment. The financial results of these programs were included in the Wholly-Owned Programs segment as of and for the year ended December 31, 2023. Seaport was deconsolidated on October 18, 2024 upon completion of its Series B preferred share financing. The financial results of Seaport through the date of deconsolidation are included within the Parent Company and Other column as of December 31, 2024. As of December 31, 2024, Alivio, a wholly-owned subsidiary of the Group, was dormant and did not meet the definition of operating segment. The financial results of this entity were removed from the Wholly-Owned Programs segment and are included in the Parent Company and Other column. The corresponding information for 2023 and 2022 has been restated to include Alivio in the Parent Company and Other column so that the segment disclosures are presented on a comparable basis. The table below summarizes the entities that comprised each of our segments as of December 31, 2024: Wholly-Owned Programs Segment Ownership Percentage PureTech LYT 100.0% PureTech LYT-100, Inc. 100.0% Gallop Oncology, Inc. (Indirectly Held through PureTech LYT) 100.0% Controlled Founded Entities Segment Entrega, Inc. 77.3% Parent Company and Other1 Alivio Therapeutics, Inc. 100.0% Follica, LLC 85.4% Gelesis, Inc.2 —% Seaport Therapeutics, Inc.3 42.9% Sonde Health, Inc.4 40.2% Vedanta Biosciences, Inc.5 46.9% PureTech Health plc 100.0% PureTech Health LLC 100.0% PureTech Securities Corporation 100.0% PureTech Securities II Corporation 100.0% PureTech Management, Inc. 100.0% 1 Includes dormant, inactive and shell entities as well as Founded Entities that were deconsolidated prior to 2024. 2 Gelesis filed for bankruptcy in October 2023. 3 Seaport Therapeutics, Inc. was deconsolidated on October 18, 2024. 4 Sonde Health, Inc was deconsolidated on May 25, 2022. 5 Vedanta Biosciences, Inc. was deconsolidated on March 1, 2023. Components of Our Results of Operations Revenue To date, we have not generated any revenue from product sales and we do not expect to generate any meaningful revenue from product sales in the near future. We derive our revenue from the following: Contract revenue We generate revenue primarily from licenses, services and collaboration agreements, including amounts that are recognized related to upfront payments, milestone payments, royalties and amounts due to us for research and development services. In the future, revenue may include additional milestone payments and royalties on any net product sales under our licensing agreements. We expect that any revenue we generate will fluctuate from period to period as a result of the timing and amount of license, research and development services and milestone and other payments. Grant Revenue Grant revenue is derived from grant awards we receive from governmental agencies and non-profit organizations for certain qualified research and development expenses. We recognize grants from governmental agencies and non-profit organizations as grant revenue in the Consolidated Statement of Comprehensive Income/(Loss), gross of the expenditures that were related to obtaining the grant, when there is reasonable assurance that we will comply with the conditions within the grant agreement and there is reasonable assurance that payments under the grants will be received. We evaluate the conditions of each grant as of each reporting date to ensure that we have reasonable assurance of meeting the conditions of each grant arrangement, and it is expected that the grant payment will be received as a result of meeting the necessary conditions. Financial Review continued

G overnance PureTech Health plc Annual Report and Accounts 2024 71 Operating Expenses Research and Development Expenses Research and development expenses consist primarily of costs incurred for our research activities, including our discovery efforts, and the development of our wholly-owned and our Controlled Founded Entities’ therapeutic candidates, which include: — employee-related expenses, including salaries, related benefits and equity-based compensation; — expenses incurred in connection with the preclinical and clinical development of our wholly-owned and our Founded Entities’ therapeutic candidates, including our agreements with contract research organizations; — expenses incurred under agreements with consultants who supplement our internal capabilities; — the cost of lab supplies and acquiring, developing and manufacturing preclinical study materials and clinical trial materials; — costs related to compliance with regulatory requirements; and — facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other operating costs. We expense all research costs in the periods in which they are incurred and development costs are capitalized only if certain criteria are met. For the periods presented, we have not capitalized any development costs since we have not met the necessary criteria required for capitalization. Research and development activities are central to our business model. Whilst we do not plan to fully fund our LYT-100 or LYT-200 programs, we anticipate providing certain level of funding in 2025 while we seek external sources of funding. Consequently, we anticipate that our research and development expenses will increase in the short term as we continue to advance these Wholly-Owned Programs. However, we anticipate a decrease in our research and development expenses in the mid- and long-term in connection with execution of our current strategy of housing these Wholly-Owned Programs in Founded Entities and accessing external sources of funding at the Founded Entity level, which, over time, could lead to the deconsolidation of the Founded Entities. The successful development of and external funding for our wholly-owned and our Founded Entities’ therapeutic candidates are highly uncertain. As such, at this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the remainder of the development of these therapeutic candidates through our funding or in conjunction with our external partners. We do not anticipate fully-funding either the programs at the Founded Entities or the Wholly-Owned Programs and in the absence of access to adequate funding from external sources, we may have to delay, scale back or discontinue one or more of these therapeutic candidates. We are also unable to predict when, if ever, material net cash inflows will commence from our wholly- owned or our Founded Entities’ therapeutic candidates. This is due to the numerous risks and uncertainties associated with developing therapeutics, including the uncertainty of: — progressing research and development of our Wholly- Owned Programs and Founded Entities and continuing to progress our various technology platforms and other potential therapeutic candidates based on previous human efficacy and clinically validated biology within our Wholly- Owned Programs and Founded Entities; — establishing an appropriate safety profile with investigational new drug application; — the success of our Founded Entities and their need for additional capital; — identifying new therapeutic candidates to add to our existing Wholly-Owned Programs or Founded Entities; — successful enrollment in, and the initiation and completion of, clinical trials; — the timing, receipt and terms of any marketing approvals from applicable regulatory authorities; — establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; — addressing any competing technological and market developments, as well as any changes in governmental regulations; — negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter and performing our obligations under such arrangements; — maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how, as well as obtaining and maintaining regulatory exclusivity for our wholly-owned and our Founded Entities’ therapeutic candidates; — continued acceptable safety profile of our therapeutics, if any, following approval; and — attracting, hiring and retaining qualified personnel. A change in the outcome of any of these variables with respect to the development of a therapeutic candidate could mean a significant change in the costs and timing associated with the development of that therapeutic candidate. For example, the FDA, the EMA, or another comparable foreign regulatory authority may require us to conduct clinical trials beyond those that we anticipate will be required for the completion of clinical development of a therapeutic candidate, or we may experience significant trial delays due to patient enrollment or other reasons, in which case we would be required to expend significant additional financial resources and time on the completion of clinical development. In addition, we may obtain unexpected results from our clinical trials, and we may elect to discontinue, delay or modify clinical trials of some therapeutic candidates or focus on others. Identifying potential therapeutic candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our wholly- owned and our Founded Entities’ therapeutic candidates, if approved, may not achieve commercial success. General and Administrative Expenses General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in our executive, finance, corporate and business development and administrative functions. General and administrative expenses also include professional fees for legal, patent, accounting, auditing, tax and consulting services, travel expenses and facility-related expenses, which include direct depreciation costs and allocated expenses for rent and maintenance of facilities and other operating costs. Financial Review continued

G ov er na nc e 72 PureTech Health plc Annual Report and Accounts 2024 Other Income (Expense) Other income (expense) consists primarily of gains and losses on financial instruments. Finance Income/(Costs) Finance costs consist of loan interest expense, interest expense due to accretion of and adjustment to the sale of future royalties liability as well as the changes in the fair value of certain liabilities associated with financing transactions, mainly subsidiary preferred share liability in respect of preferred shares issued by our non-wholly owned subsidiaries to third parties. Finance income consists of interest income on funds invested in money market funds and U.S. treasuries. Share of Net Income (Loss) of Associates Accounted for Using the Equity Method, Gain on Dilution of Ownership Interest and Impairment of Investment in Associates Associates (or equity accounted investees) are accounted for using the equity method and are initially recognized at cost, or if recognized upon deconsolidation, they are initially recorded at fair value at the date of deconsolidation. The Consolidated Financial Statements include our share of the total comprehensive income/(loss) of equity accounted investees, from the date that significant influence commences until the date that significant influence ceases. When the share of losses exceeds the net investment in the investee, including the investment considered long-term interests, the carrying amount is reduced to nil and recognition of further losses is discontinued except to the extent that we have incurred legal or constructive obligations or made payments on behalf of an investee. We compare the recoverable amount of the investment to its carrying amount on a go-forward basis and determine the need for impairment. When our share in the equity of the investee changes as a result of equity transactions in the investee (related to financing events of the investee), we calculate a gain or loss on such change in ownership and related share in the investee's equity. In 2023, we recorded our share of the net loss of Gelesis which reduced the carrying amount of our investment in Gelesis to zero. On October 30, 2023, Gelesis ceased operations and our significant influence in Gelesis ceased. In 2024, we recorded our share of the net losses of Sonde which reduced the carrying amount of our investment in Sonde to zero. Income Tax The amount of taxes currently payable or refundable is accrued, and deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred tax assets are also recognized for realizable loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using substantively enacted tax rates in effect for the year in which those temporary differences are expected to be recovered or settled. Net deferred tax assets are not recorded if we do not assess their realization as probable. The effect on deferred tax assets and liabilities of a change in income tax rates is recognized in our financial statements in the period that includes the substantive enactment date or the change in tax status. We expect that our general and administrative expenses in support of our research and development efforts will increase in the short-term while we seek funding from external sources for the Wholly-Owned Programs. However, we anticipate a decrease in our general and administrative expenses in the mid- and long-term in connection with execution of our current strategy as we do not intend to fully fund our LYT-100 program’s Phase 3 trial or LYT-200’s Phase 2 trial on our own, and as we seek to fund future development of the clinical programs within our Wholly-Owned Programs with external sources of funding at the Founded Entity level, which, over time, could lead to the deconsolidation of the Founded Entities that house these programs. Total Other Income/(Expense) Gain on Deconsolidation of Subsidiary Upon losing control over a subsidiary, the assets and liabilities are derecognized along with any related non-controlling interest (“NCI”). Any interest retained in the former subsidiary is measured at fair value when control is lost. Any resulting gain or loss is recognized as profit or loss in the Consolidated Statement of Comprehensive Income/(Loss). Gain/(Loss) on Investments Held at Fair Value Investments held at fair value include both unlisted and listed securities held by us, which include investments in Seaport, Vedanta, and other insignificant investments. We account for investments in convertible preferred shares in accordance with IFRS 9 as investments held at fair value when the preferred shares do not provide their holders with access to returns associated with a residual equity interest. Under IFRS 9, the preferred share investments are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest. Realized Gain/(Loss) on Sale of Investments Realized gain/(loss) on sale of investments held at fair value relates to realized differences in the per share disposal price of a listed security as compared to the per share exchange quoted price at the time of disposal. The realized loss in 2022 is attributable to the settlement of call options written by the Group on Karuna stock. The amounts in 2023 and 2024 are not significant. Gain/(Loss) on Investments in Notes from Associates Gain/(loss) on investments in notes from associates relates to our investment in the notes from Gelesis and Vedanta. We account for these notes in accordance with IFRS 9 as investments held at fair value, with changes in fair value recognized through the Consolidated Statement of Comprehensive Income/(Loss). The loss in 2023 is primarily attributable to a decrease in the fair value of our notes from Gelesis as Gelesis ceased operations and filed a voluntary petition for relief under the provisions of Chapter 7 of Title 11 of the United States Bankruptcy Code in October 2023. In 2024, the Bankruptcy Court approved an executed agreement for a third party to acquire the remaining net assets of Gelesis for $15.0 million. As the only senior secured creditor, we expect to receive a majority of the proceeds from the sale after deduction of Bankruptcy Court related legal and administrative costs. We recorded a gain of $11.4 million in 2024 for the changes in the fair value of these notes. Financial Review continued
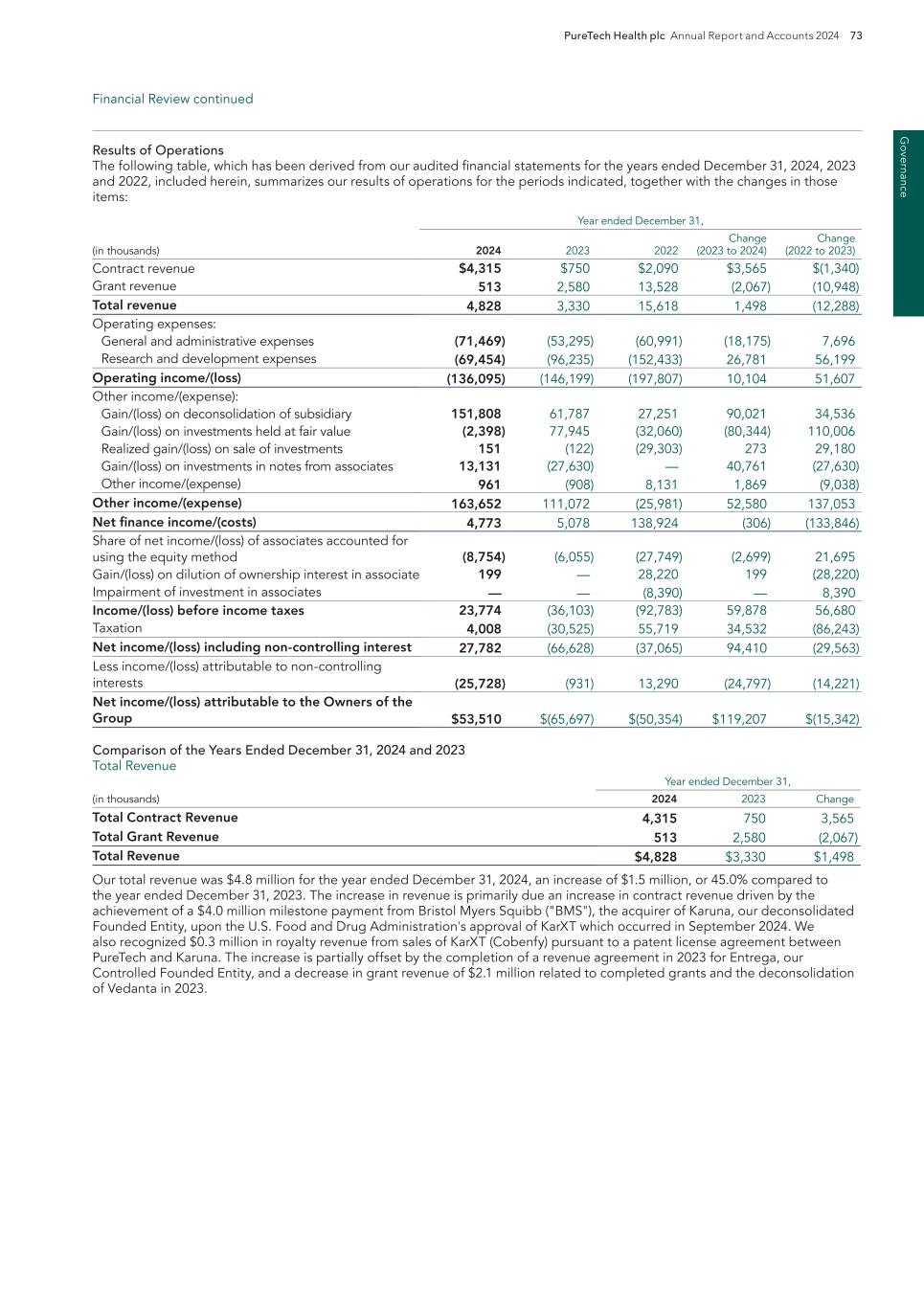
G overnance PureTech Health plc Annual Report and Accounts 2024 73 Results of Operations The following table, which has been derived from our audited financial statements for the years ended December 31, 2024, 2023 and 2022, included herein, summarizes our results of operations for the periods indicated, together with the changes in those items: Year ended December 31, (in thousands) 2024 2023 2022 Change (2023 to 2024) Change (2022 to 2023) Contract revenue $4,315 $750 $2,090 $3,565 $(1,340) Grant revenue 513 2,580 13,528 (2,067) (10,948) Total revenue 4,828 3,330 15,618 1,498 (12,288) Operating expenses: General and administrative expenses (71,469) (53,295) (60,991) (18,175) 7,696 Research and development expenses (69,454) (96,235) (152,433) 26,781 56,199 Operating income/(loss) (136,095) (146,199) (197,807) 10,104 51,607 Other income/(expense): Gain/(loss) on deconsolidation of subsidiary 151,808 61,787 27,251 90,021 34,536 Gain/(loss) on investments held at fair value (2,398) 77,945 (32,060) (80,344) 110,006 Realized gain/(loss) on sale of investments 151 (122) (29,303) 273 29,180 Gain/(loss) on investments in notes from associates 13,131 (27,630) — 40,761 (27,630) Other income/(expense) 961 (908) 8,131 1,869 (9,038) Other income/(expense) 163,652 111,072 (25,981) 52,580 137,053 Net finance income/(costs) 4,773 5,078 138,924 (306) (133,846) Share of net income/(loss) of associates accounted for using the equity method (8,754) (6,055) (27,749) (2,699) 21,695 Gain/(loss) on dilution of ownership interest in associate 199 — 28,220 199 (28,220) Impairment of investment in associates — — (8,390) — 8,390 Income/(loss) before income taxes 23,774 (36,103) (92,783) 59,878 56,680 Taxation 4,008 (30,525) 55,719 34,532 (86,243) Net income/(loss) including non-controlling interest 27,782 (66,628) (37,065) 94,410 (29,563) Less income/(loss) attributable to non-controlling interests (25,728) (931) 13,290 (24,797) (14,221) Net income/(loss) attributable to the Owners of the Group $53,510 $(65,697) $(50,354) $119,207 $(15,342) Comparison of the Years Ended December 31, 2024 and 2023 Total Revenue Year ended December 31, (in thousands) 2024 2023 Change Total Contract Revenue 4,315 750 3,565 Total Grant Revenue 513 2,580 (2,067) Total Revenue $4,828 $3,330 $1,498 Our total revenue was $4.8 million for the year ended December 31, 2024, an increase of $1.5 million, or 45.0% compared to the year ended December 31, 2023. The increase in revenue is primarily due an increase in contract revenue driven by the achievement of a $4.0 million milestone payment from Bristol Myers Squibb ("BMS"), the acquirer of Karuna, our deconsolidated Founded Entity, upon the U.S. Food and Drug Administration's approval of KarXT which occurred in September 2024. We also recognized $0.3 million in royalty revenue from sales of KarXT (Cobenfy) pursuant to a patent license agreement between PureTech and Karuna. The increase is partially offset by the completion of a revenue agreement in 2023 for Entrega, our Controlled Founded Entity, and a decrease in grant revenue of $2.1 million related to completed grants and the deconsolidation of Vedanta in 2023. Financial Review continued
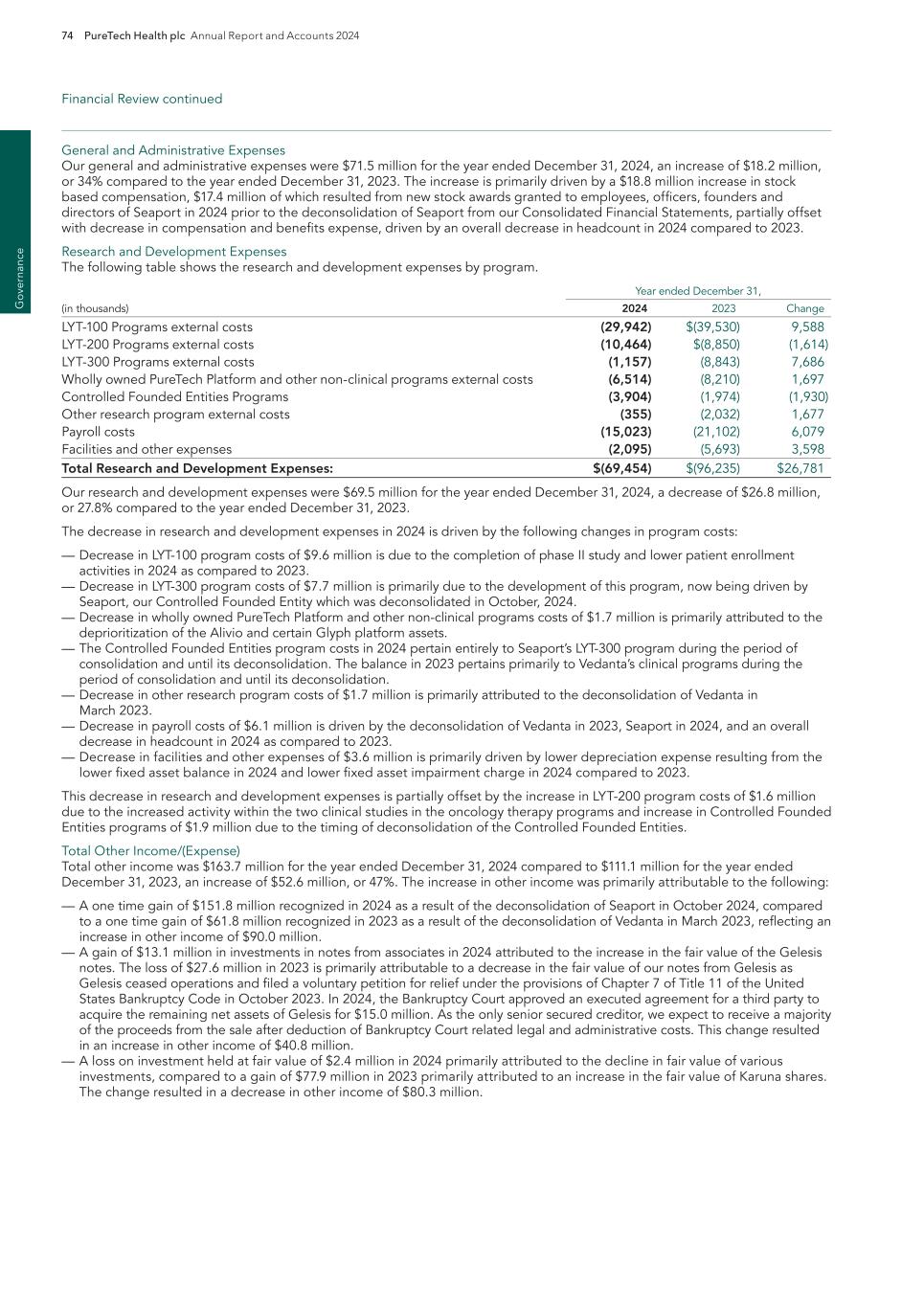
G ov er na nc e 74 PureTech Health plc Annual Report and Accounts 2024 General and Administrative Expenses Our general and administrative expenses were $71.5 million for the year ended December 31, 2024, an increase of $18.2 million, or 34% compared to the year ended December 31, 2023. The increase is primarily driven by a $18.8 million increase in stock based compensation, $17.4 million of which resulted from new stock awards granted to employees, officers, founders and directors of Seaport in 2024 prior to the deconsolidation of Seaport from our Consolidated Financial Statements, partially offset with decrease in compensation and benefits expense, driven by an overall decrease in headcount in 2024 compared to 2023. Research and Development Expenses The following table shows the research and development expenses by program. Year ended December 31, (in thousands) 2024 2023 Change LYT-100 Programs external costs (29,942) $(39,530) 9,588 LYT-200 Programs external costs (10,464) $(8,850) (1,614) LYT-300 Programs external costs (1,157) (8,843) 7,686 Wholly owned PureTech Platform and other non-clinical programs external costs (6,514) (8,210) 1,697 Controlled Founded Entities Programs (3,904) (1,974) (1,930) Other research program external costs (355) (2,032) 1,677 Payroll costs (15,023) (21,102) 6,079 Facilities and other expenses (2,095) (5,693) 3,598 Total Research and Development Expenses: $(69,454) $(96,235) $26,781 Our research and development expenses were $69.5 million for the year ended December 31, 2024, a decrease of $26.8 million, or 27.8% compared to the year ended December 31, 2023. The decrease in research and development expenses in 2024 is driven by the following changes in program costs: — Decrease in LYT-100 program costs of $9.6 million is due to the completion of phase II study and lower patient enrollment activities in 2024 as compared to 2023. — Decrease in LYT-300 program costs of $7.7 million is primarily due to the development of this program, now being driven by Seaport, our Controlled Founded Entity which was deconsolidated in October, 2024. — Decrease in wholly owned PureTech Platform and other non-clinical programs costs of $1.7 million is primarily attributed to the deprioritization of the Alivio and certain Glyph platform assets. — The Controlled Founded Entities program costs in 2024 pertain entirely to Seaport’s LYT-300 program during the period of consolidation and until its deconsolidation. The balance in 2023 pertains primarily to Vedanta’s clinical programs during the period of consolidation and until its deconsolidation. — Decrease in other research program costs of $1.7 million is primarily attributed to the deconsolidation of Vedanta in March 2023. — Decrease in payroll costs of $6.1 million is driven by the deconsolidation of Vedanta in 2023, Seaport in 2024, and an overall decrease in headcount in 2024 as compared to 2023. — Decrease in facilities and other expenses of $3.6 million is primarily driven by lower depreciation expense resulting from the lower fixed asset balance in 2024 and lower fixed asset impairment charge in 2024 compared to 2023. This decrease in research and development expenses is partially offset by the increase in LYT-200 program costs of $1.6 million due to the increased activity within the two clinical studies in the oncology therapy programs and increase in Controlled Founded Entities programs of $1.9 million due to the timing of deconsolidation of the Controlled Founded Entities. Total Other Income/(Expense) Total other income was $163.7 million for the year ended December 31, 2024 compared to $111.1 million for the year ended December 31, 2023, an increase of $52.6 million, or 47%. The increase in other income was primarily attributable to the following: — A one time gain of $151.8 million recognized in 2024 as a result of the deconsolidation of Seaport in October 2024, compared to a one time gain of $61.8 million recognized in 2023 as a result of the deconsolidation of Vedanta in March 2023, reflecting an increase in other income of $90.0 million. — A gain of $13.1 million in investments in notes from associates in 2024 attributed to the increase in the fair value of the Gelesis notes. The loss of $27.6 million in 2023 is primarily attributable to a decrease in the fair value of our notes from Gelesis as Gelesis ceased operations and filed a voluntary petition for relief under the provisions of Chapter 7 of Title 11 of the United States Bankruptcy Code in October 2023. In 2024, the Bankruptcy Court approved an executed agreement for a third party to acquire the remaining net assets of Gelesis for $15.0 million. As the only senior secured creditor, we expect to receive a majority of the proceeds from the sale after deduction of Bankruptcy Court related legal and administrative costs. This change resulted in an increase in other income of $40.8 million. — A loss on investment held at fair value of $2.4 million in 2024 primarily attributed to the decline in fair value of various investments, compared to a gain of $77.9 million in 2023 primarily attributed to an increase in the fair value of Karuna shares. The change resulted in a decrease in other income of $80.3 million. Financial Review continued

G overnance PureTech Health plc Annual Report and Accounts 2024 75 Net Finance Income/(Costs) Net finance income/costs) was $4.8 million for the year ended December 31, 2024, compared to $5.1 million for the year ended December 31, 2023, a decrease of $0.3 million or 6%. The reduction in net finance income is primarily attributed to an increase in the fair value of subsidiary preferred share liability offset by various other changes. Share of Net Income/(loss) of Associates Accounted for Using the Equity Method For the year ended December 31, 2024, the share in net loss of associates reported under the equity method was $8.8 million as compared to the share in net loss of associates of $6.1 million for the year ended December 31, 2023, an increase in loss of $2.7 million or 45%. The increase in loss was primarily attributable to the increase in loss from Sonde and Group's share of loss from Seaport accounted for under the equity method upon deconsolidation in October, 2024. Taxation For the year ended December 31, 2024, the income tax benefit was $4.0 million, compared to an income tax expense of $30.5 million for the year ended December 31, 2023, a decrease in income tax expense of $34.5 million or 113%. This decrease in tax expense was primarily attributable to the recognition of previously unrecognized deferred tax assets and related tax benefits in 2024, compared to the income tax expense recognized in 2023 due to an increase in unrecognized deferred tax assets that were not expected to be utilized in the future as well as certain discrete events and transactions from 2023, such as the tax effects from the sale of future royalties to Royalty Pharma. The income tax benefits in 2024 were partially offset by an increase in pre-tax income in the tax-consolidated U.S. group and an increase in Massachusetts income tax expense. Comparison of the Years Ended December 31, 2023 and 2022 For the comparison of 2023 to 2022, refer to Part I, Item 5 “Operating and Financial Review and Prospects” of our Annual Report on Form 20-F for the year ended December 31, 2023. Material Accounting Policies and Significant Judgments and Estimates Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with UK-adopted International Financial Reporting Standards ("IFRSs"). The Consolidated Financial Statements also comply fully with IFRSs as issued by the International Accounting Standards Board ("IASB"). In the preparation of these financial statements, we are required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates under different assumptions or conditions. Our estimates and assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revisions and future periods if the revision affects both current and future periods. While our significant accounting policies are described in more detail in the notes to our Consolidated Financial Statements appearing at the end of this report, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements. See Note 1. Material Accounting Policies to our Consolidated Financial Statements for a further detailed description of our material accounting policies. Financial instruments We account for our financial instruments according to IFRS 9. In accordance with IFRS 9, we carry certain financial assets and financial liabilities at fair value, with changes in fair value through profit and loss ("FVTPL"). Valuation of these financial instruments includes determining the appropriate valuation methodology and making certain estimates such as the future expected returns on the financial instrument in different scenarios, appropriate discount rate, volatility, and term to exit. In accordance with IFRS 9, when issuing preferred shares in our subsidiaries, we determine the classification of financial instruments in terms of liability or equity. Such determination involves judgement. These judgements include an assessment of whether the financial instruments include any embedded derivative features, whether they include contractual obligations upon us to deliver cash or other financial assets or to exchange financial assets or financial liabilities with another party at any point in the future prior to liquidation, and whether that obligation will be settled by exchanging a fixed amount of cash or other financial assets for a fixed number of the Group's equity instruments. Consolidation The Consolidated Financial Statements include the financial statements of the Group and the entities it controls. Based on the applicable accounting rules, we control an investee when we are exposed, or have rights, to variable returns from our involvement with the investee and have the ability to affect those returns through our power over the investee. Therefore, an assessment is required to determine whether we have (i) power over the investee; (ii) exposure, or rights, to variable returns from our involvement with the investee; and (iii) the ability to use our power over the investee to affect the amount of our returns. Judgement is required to perform such assessment, and it requires that we consider, among others, activities that most significantly affect the returns of the investee, our voting shares, representation on the board, rights to appoint board members and management, shareholders agreements, de facto power and other contributing factors. Sale of Future Royalties Liability We account for the sale of future royalties liability as a financial liability, as we continue to hold the rights under the royalty bearing licensing agreement and have a contractual obligation to deliver cash to an investor for a portion of the royalty we receive. This liability is tied to the future royalties we may receive from product sales. We have no obligation to pay any amounts to the counterparty if we do not receive any royalties in the future. Interest on the sale of future royalties liability is recognized using the effective interest rate over the life of the related royalty stream. The sale of future royalties liability and the related interest expense are based on our current estimates of future royalties expected to be paid over the life of the arrangement. Forecasts are updated periodically as new data is obtained. Any increases, decreases or a shift in timing of estimated cash flows require us to re-calculate the amortized cost of the sale of future royalties liability as the present value of the estimated future contractual cash flows that are discounted at the liability’s original effective interest rate. The adjustment is recognized immediately in profit or loss as income or expense. In determining the appropriate accounting treatment for the Royalty Purchase Agreement during 2023, management applied significant judgement. Financial Review continued

G ov er na nc e 76 PureTech Health plc Annual Report and Accounts 2024 Investment in Associates When we do not control an investee but maintain significant influence over the financial and operating policies of the investee, the investee is an associate. Significant influence is presumed to exist when we hold 20 % or more of the voting power of an entity, unless it can be clearly demonstrated that this is not the case. We evaluate if we maintain significant influence over associates by assessing if we have the power to participate in the financial and operating policy decisions of the associate. Associates are accounted for using the equity method (equity accounted investees) and are initially recognized at cost, or if recognized upon deconsolidation, they are initially recorded at fair value at the date of deconsolidation. The Consolidated Financial Statements include our share of the total comprehensive income or loss of equity accounted investees, from the date that significant influence commences until the date that significant influence ceases. When our share of losses exceeds the net investment in an equity accounted investee, including investments considered to be long-term interests ("LTI"), the carrying amount is reduced to zero and recognition of further losses is discontinued except to the extent that we have incurred legal or constructive obligations or made payments on behalf of an investee. To the extent we hold interests in associates that are not providing access to returns underlying ownership interests, the instrument held by us is accounted for in accordance with IFRS 9. Judgement is required in order to determine whether we have significant influence over financial and operating policies of investees. This judgement includes, among others, an assessment whether we have representation on the board of the investee, whether we participate in the policy-making processes of the investee, whether there is any interchange of managerial personnel, whether there is any essential technical information provided to the investee, and if there are any transactions between us and the investee. Judgement is also required to determine which instruments we hold in the investee form part of the investment in associates, which is accounted for under IAS 28 and scoped out of IFRS 9, and which instruments are separate financial instruments that fall under the scope of IFRS 9. This judgement includes an assessment of the characteristics of the financial instrument of the investee held by us and whether such financial instrument provides access to returns underlying an ownership interest. Where the Group has other investments in an equity accounted investee that are not accounted for under IAS 28, judgement is required in determining if such investments constitute long-term interests for the purposes of IAS 28. This determination is based on the individual facts and circumstances and characteristics of each investment, but is driven, among other factors, by the intention and likelihood to settle the instrument through redemption or repayment in the foreseeable future, and whether or not the investment is likely to be converted to common stock or other equity instruments. Recent Accounting Pronouncements For information on recent accounting pronouncements, see Note 2. New Standards and Interpretations to our Consolidated Financial Statements. Cash Flow and Liquidity Our cash flows may fluctuate and are difficult to forecast and will depend on many factors, including: — the expenses incurred in the development of wholly-owned and Controlled Founded Entities' therapeutic candidates; — the revenue, if any, generated by wholly-owned and Controlled-Founded Entities' therapeutic candidates; — the revenue, if any, generated from licensing and royalty agreements with Founded Entities; — the financing requirements of the Wholly-Owned Programs and our Founded Entities; and — the investing activities including the monetization, through sale, of shares held in our public Founded Entities. As of December 31, 2024, we had cash and cash equivalents of $280.6 million and short-term investments of $86.7 million. As of December 31, 2024, we had PureTech Level cash, cash equivalents and short-term investments of $366.8 million. PureTech Level cash, cash equivalents and short-term investments is a non-IFRS measure (for a definition of PureTech Level cash, cash equivalents and short-term investments and a reconciliation with the IFRS number, see the section Measuring Performance earlier in this Financial Review). In March 2024, we received total proceeds of $292.7 million before income tax in exchange for our holding of 886,885 shares of Karuna common stock as a result of the completion of Karuna acquisition by Bristol Myers Squibb (“BMS”). Cash Flows The following table summarizes our cash flows for each of the periods presented: Year ended December 31, (in thousands) 2024 2023 2022 Net cash used in operating activities $(134,369) $(105,917) $(178,792) Net cash provided by (used in) investing activities 240,888 68,991 (107,223) Net cash provided by (used in) financing activities (16,958) 78,141 (29,827) Net increase (decrease) in cash and cash equivalents $89,560 $41,215 $(315,842) Financial Review continued

G overnance PureTech Health plc Annual Report and Accounts 2024 77 Operating Activities Net cash used in operating activities was $134.4 million for the year ended December 31, 2024, as compared to $105.9 million for the year ended December 31, 2023, resulting in an increase of $28.5 million in net cash used in operating activities. The increase in cash outflows is primarily attributable to $37.8 million increase in tax payments related to the sale of the Karuna shares, offset by a net increase in interest receipts and decrease in interest payment of $9.5 million. Net cash used in operating activities was $105.9 million for the year ended December 31, 2023, as compared to $178.8 million for the year ended December 31, 2022, resulting in a decrease of $72.9 million in net cash used in operating activities. The decrease in outflows is primarily attributable to our lower operating loss mainly due to a decrease in research and development activities in the Wholly-Owned Programs and Controlled Founded Entities and a decrease of operating cash flows as a result of the deconsolidation of Vedanta on March 1, 2023. Investing Activities Net cash provided by investing activities was $240.9 million for the year ended December 31, 2024, as compared to net cash provided by investing activities of $69.0 million for the year ended December 31, 2023, resulting in an increase of $171.9 million in cash provided by investing activities. The increase in net cash provided by investing activities was primarily attributable to an increase in proceeds from the sale of investments held at fair value of $264.8 million, partially offset by an increase in cash outflow from short-term investment activities (redemptions, net of purchases) amounting to $17.2 million, and the derecognition of cash balances of $91.6 million upon deconsolidation of Seaport in 2024, compared to $13.8 million from the deconsolidation of Vedanta in 2023, a net increase in cash outflow of $77.8 million. Net cash provided by investing activities was $69.0 million for the year ended December 31, 2023, as compared to net cash outflow of $107.2 for the year ended December 31, 2022, resulting in an increase of $176.2 million in net cash from investing activities. The increase in net cash from investing activities was primarily attributable to increased cash inflow from short-term investment activities (redemptions, net of purchases) amounting to $264.4 million, partially offset by a reduction in proceeds from the sale of investments held at fair value of $85.4 million. Financing Activities Net cash used in financing activities was $17.0 million for the year ended December 31, 2024, as compared to net cash provided by financing activities of $78.1 million for the year ended December 31, 2023, resulting in an increase of $95.1 million in net cash used in financing activities. The increase in net cash used in financing activities was primarily attributable to a $87.9 million increase in share repurchase activities, related primarily to the repurchase of $100.0 million of shares in the June 2024 tender offer, and a $75.0 million decrease in cash inflow from Royalty Pharma under Royalty Purchase Agreement, partially offset by a $68.1 million proceeds from issuance of subsidiary preferred shares in 2024 as compared to 2023. Net cash provided by financing activities was $78.1 million for the year ended December 31, 2023, as compared to net cash used in financing activities of $29.8 million for the year ended December 31, 2022, resulting in an increase of $108.0 million in the net cash provided by financing activities. The increase in the net cash provided by financing activities was primarily attributable to the receipts of $100.0 million upfront payment from Royalty Pharma upon execution of Royalty Purchase Agreement in March 2023, and a $6.8 million decrease in treasury stock purchase in 2023 as compared to 2022. Funding Requirements We have incurred operating losses since inception. Based on our current plans, we believe our existing financial assets as of December 31, 2024, will be sufficient to fund our operations and capital expenditure requirements into at least 2027. We expect to incur substantial additional expenditures in the near term to support our ongoing and future activities. We anticipate to continue to incur net operating losses for the foreseeable future to support our existing Founded Entities and our strategy around creating and supporting other Founded Entities, should they require it, to reach significant development milestones over the period of the assessment in conjunction with our external partners. We also expect to incur significant costs to advance our Wholly-Owned Programs, although we do not intend to fully fund our LYT-100 program's Phase 3 trial or LYT-200 program's Phase 2 trial, on our own, to continue research and development efforts, to discover and progress new therapeutic candidates and to fund the Group’s operating costs into at least 2027. Our ability to fund our therapeutic development and clinical operations as well as ability to fund our existing and future Founded Entities will depend on the amount and timing of cash received from financings at the Founded Entity level, monetization of shares of public Founded Entities and potential business development activities. Our future capital requirements will depend on many factors, including: — the costs, timing and outcomes of clinical trials and regulatory reviews associated with our wholly-owned therapeutic candidates; — the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property related claims; — the emergence of competing technologies and products and other adverse marketing developments; — the effect on our therapeutic and product development activities of actions taken by the U.S. Food and Drug Administration (“FDA”), the European Medicines Agency (“EMA”) or other regulatory authorities; — the number and types of future therapeutics we develop and support with the goal of commercialization; — The costs, timing and outcomes of identifying, evaluating, and investing in technologies and drug candidates to develop as Wholly-Owned Programs or as Founded Entities; and — the success of our Founded Entities and their need for additional capital. A change in the outcome of any of these or other variables with respect to the development of any of our wholly-owned therapeutic candidates could significantly change the costs and timing associated with the development of that therapeutic candidate. Further, our operating plans may change, and we may need additional funds to meet operational needs and capital requirements for clinical trials and other research and development activities. We currently have no credit facility or other committed sources of capital beyond our existing financial assets. Because of the numerous risks and uncertainties associated with the development and commercialization of our wholly-owned therapeutic candidates, we have only a general estimate of the amounts of increased capital outlays and operating expenditures associated with our current and anticipated therapeutic development programs and these may change in the future. Financial Review continued
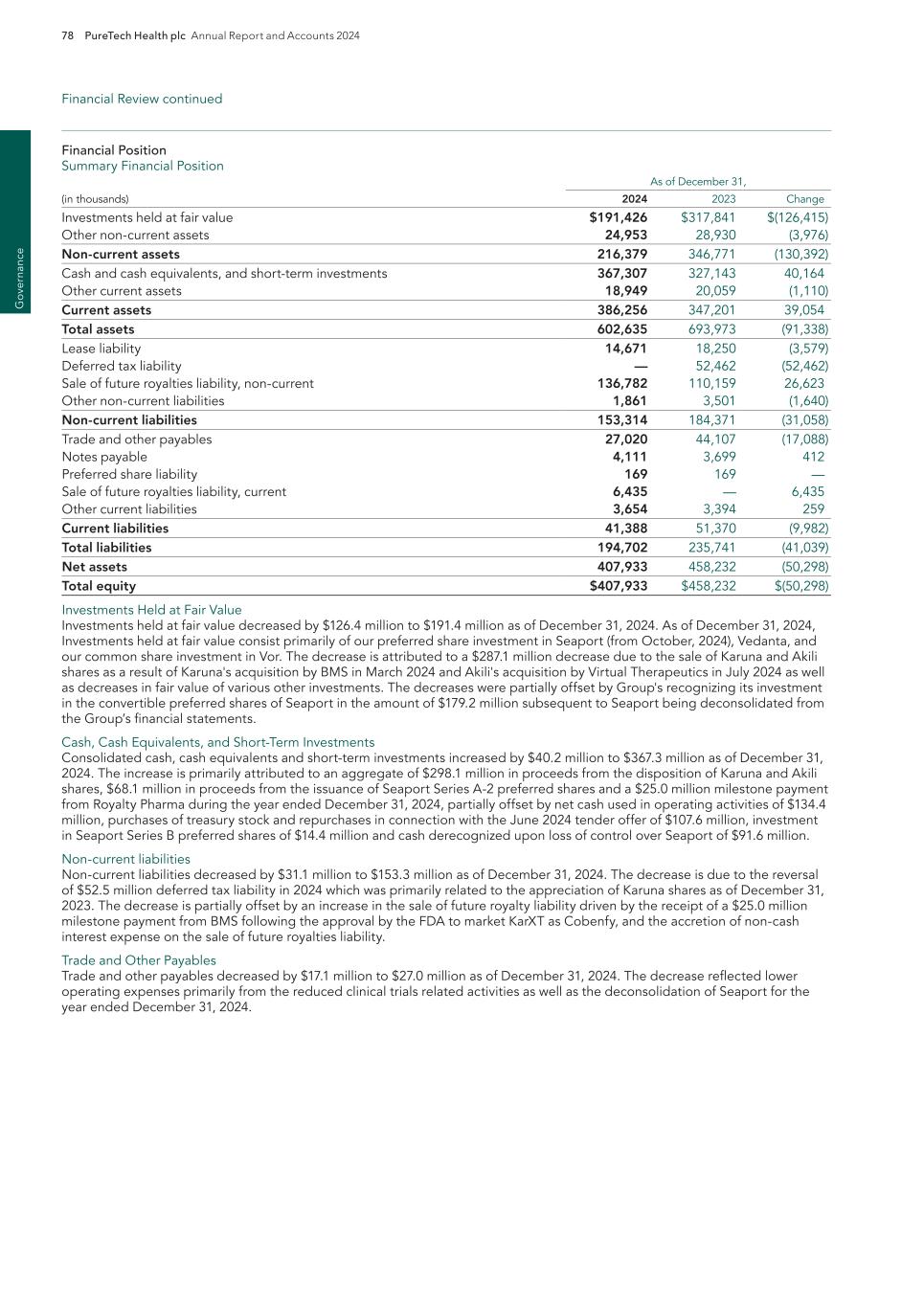
G ov er na nc e 78 PureTech Health plc Annual Report and Accounts 2024 Financial Position Summary Financial Position As of December 31, (in thousands) 2024 2023 Change Investments held at fair value $191,426 $317,841 $(126,415) Other non-current assets 24,953 28,930 (3,976) Non-current assets 216,379 346,771 (130,392) Cash and cash equivalents, and short-term investments 367,307 327,143 40,164 Other current assets 18,949 20,059 (1,110) Current assets 386,256 347,201 39,054 Total assets 602,635 693,973 (91,338) Lease liability 14,671 18,250 (3,579) Deferred tax liability — 52,462 (52,462) Sale of future royalties liability, non-current 136,782 110,159 26,623 Other non-current liabilities 1,861 3,501 (1,640) Non-current liabilities 153,314 184,371 (31,058) Trade and other payables 27,020 44,107 (17,088) Notes payable 4,111 3,699 412 Preferred share liability 169 169 — Sale of future royalties liability, current 6,435 — 6,435 Other current liabilities 3,654 3,394 259 Current liabilities 41,388 51,370 (9,982) Total liabilities 194,702 235,741 (41,039) Net assets 407,933 458,232 (50,298) Total equity $407,933 $458,232 $(50,298) Investments Held at Fair Value Investments held at fair value decreased by $126.4 million to $191.4 million as of December 31, 2024. As of December 31, 2024, Investments held at fair value consist primarily of our preferred share investment in Seaport (from October, 2024), Vedanta, and our common share investment in Vor. The decrease is attributed to a $287.1 million decrease due to the sale of Karuna and Akili shares as a result of Karuna's acquisition by BMS in March 2024 and Akili's acquisition by Virtual Therapeutics in July 2024 as well as decreases in fair value of various other investments. The decreases were partially offset by Group's recognizing its investment in the convertible preferred shares of Seaport in the amount of $179.2 million subsequent to Seaport being deconsolidated from the Group’s financial statements. Cash, Cash Equivalents, and Short-Term Investments Consolidated cash, cash equivalents and short-term investments increased by $40.2 million to $367.3 million as of December 31, 2024. The increase is primarily attributed to an aggregate of $298.1 million in proceeds from the disposition of Karuna and Akili shares, $68.1 million in proceeds from the issuance of Seaport Series A-2 preferred shares and a $25.0 million milestone payment from Royalty Pharma during the year ended December 31, 2024, partially offset by net cash used in operating activities of $134.4 million, purchases of treasury stock and repurchases in connection with the June 2024 tender offer of $107.6 million, investment in Seaport Series B preferred shares of $14.4 million and cash derecognized upon loss of control over Seaport of $91.6 million. Non-current liabilities Non-current liabilities decreased by $31.1 million to $153.3 million as of December 31, 2024. The decrease is due to the reversal of $52.5 million deferred tax liability in 2024 which was primarily related to the appreciation of Karuna shares as of December 31, 2023. The decrease is partially offset by an increase in the sale of future royalty liability driven by the receipt of a $25.0 million milestone payment from BMS following the approval by the FDA to market KarXT as Cobenfy, and the accretion of non-cash interest expense on the sale of future royalties liability. Trade and Other Payables Trade and other payables decreased by $17.1 million to $27.0 million as of December 31, 2024. The decrease reflected lower operating expenses primarily from the reduced clinical trials related activities as well as the deconsolidation of Seaport for the year ended December 31, 2024. Financial Review continued

G overnance PureTech Health plc Annual Report and Accounts 2024 79 Quantitative and Qualitative Disclosures about Financial Risks Interest Rate Sensitivity As of December 31, 2024, we had cash and cash equivalents of $280.6 million and short-term investments of $86.7 million, while we had PureTech Level cash, cash equivalents and short-term investments of $366.8 million. PureTech Level cash, cash equivalents and short-term investments is a non- IFRS measure (for a definition of PureTech Level cash, cash equivalents and short-term investments and a reconciliation with the IFRS number, see the section Measuring Performance earlier in this Financial review). Our exposure to interest rate sensitivity is impacted by changes in the underlying U.K. and U.S. bank interest rates. We have not entered into investments for trading or speculative purposes. Due to the conservative nature of our investment portfolio, which is predicated on capital preservation and investments in short duration, high- quality U.S. Treasury Bills and related money market accounts, we do not believe a change in interest rates would have a material effect on the fair market value of our portfolio, and therefore, we do not expect our operating results or cash flows to be significantly affected by changes in market interest rates. Foreign Currency Exchange Risk We maintain our Consolidated Financial Statements in our functional currency, which is the U.S. dollar. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Non-monetary assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the date of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income (loss) for the respective periods. Such foreign currency gains or losses were not material for all reported periods. Controlled Founded Entity Investments We maintain investments in certain Controlled Founded Entities. Our investments in Controlled Founded Entities are eliminated as intercompany transactions upon financial consolidation. We are exposed to a subsidiary preferred share liability owing to the terms of existing preferred shares and the ownership of Controlled Founded Entities preferred shares by third parties. The liability of preferred shares is maintained at fair value through profit and loss. We view our exposure to third-party subsidiary preferred share liability as low as of December 31, 2024 as the liability is not significant. Please refer to Note 17. Subsidiary Preferred Shares to our Consolidated Financial Statements for further information regarding our exposure to Controlled Founded Entity investments. Deconsolidated Founded Entity Investments We maintain certain debt or equity holdings in Founded Entities which have been deconsolidated. These holdings are deemed either as investments carried at fair value under IFRS 9 with changes in fair value recorded through profit and loss or as associates accounted for under IAS 28 using the equity method. Our exposure to investments held at fair value and investments in notes from associates was $191.4 million and $17.7 million, respectively, as of December 31, 2024, and we may or may not be able to realize the value in the future. Accordingly, we view the risk as high. Our exposure to investments in associates is limited to the carrying amount of the investment. We are not exposed to further contractual obligations or contingent liabilities beyond the value of initial investment. Accordingly, we do not view this risk as high. Equity Price Risk As of December 31, 2024, we held 2,671,800 common shares of Vor with a fair value of $3.0 million. As of December 31, 2023, we held 886,885 common shares of Karuna, 2,671,800 common shares of Vor, and 12,527,476 common shares of Akili with fair value of $280.7 million, $6.0 million, and $6.1 million, respectively. The common shares of Karuna and Akili were disposed of in 2024 as part of Karuna's acquisition by BMS in March 2024 and Akili's acquisition by Virtual Therapeutics in July 2024. The investment in Vor is exposed to fluctuations in the market price of Vor's common shares. We view the exposure to equity price risk as low. Liquidity Risk We do not believe we will encounter difficulty in meeting the obligations associated with our financial liabilities that are settled by delivering cash or another financial asset. While we believe our cash and cash equivalents and short-term investments do not contain excessive risk, we cannot provide absolute assurance that in the future, our investments will not be subject to adverse changes or decline in value based on market conditions. Financial Review continued

80 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Credit Risk We maintain an investment portfolio in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity and meet operating needs. Although our investments are subject to credit risk, our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure from any single issue, issuer or type of investment. We do not own derivative financial instruments. Accordingly, we do not believe that there is any material market risk exposure with respect to derivative or other financial instruments. Credit risk is also the risk of financial loss if a customer or counterparty to a financial instrument fails to meet its contractual obligations. We are potentially subject to concentrations of credit risk in accounts receivable. Concentrations of credit risk with respect to receivables is owed to the limited number of companies comprising our receivable base. However, our exposure to credit losses is currently low due to the immateriality of the outstanding receivable balance, a small number of counterparties and the high credit quality or healthy financial conditions of these counterparties. Foreign Private Issuer Status Owing to our U.S. listing on the Nasdaq Global Market, we report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. As long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including: — the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; — sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; — the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events; and — Regulation FD, which regulates selective disclosures of material information by issuers. Financial Review continued

PureTech Health plc Annual Report and Accounts 2024 81 G overnance Since June 20, 2024, I am excited to now serve as Chair to continue the strong governance practices at PureTech. Under my leadership, the Board has been focused on exploring every avenue for maximising shareholder value. This year the Nomination Committee, with assistance from the rest of the Board and the Company’s management, continued to explore adding new non-executive directors to strengthen the Board’s skillsets and reinforce the strong governance that has been a hallmark of the Company’s Board and broader operations. As a testament to these efforts, I am delighted to welcome Dr. Michele Holcomb, Ph.D. to the Board. Dr. Holcomb joined the Board in September 2024 and brings more than 30 years of experience at leading global healthcare companies, and the Board is excited to have her as we work to identify and develop medicines with the potential to transform lives. The Board looks forward to being able to discuss these matters with our shareholders in connection with our AGM or indeed at any other time during the year. Dr. Raju Kucherlapati, Ph.D. Chair April 30, 2025 Dear Shareholder, I am pleased to introduce our Corporate Governance Report. This Report sets out our governance framework and the work of the Board and its committees. As a Board, we are responsible for ensuring there is an effective governance framework in place. This includes setting the Company’s strategic objectives, ensuring the right leadership and resources are in place to achieve these objectives, monitoring performance, ensuring that sufficient internal controls and protections are in place and reporting to shareholders. An effective governance framework is also designed to ensure accountability, fairness and transparency in the Company’s relationships with all of its stakeholders, whether shareholders, employees, partners, the government or the wider patient community. We believe that good corporate governance is essential for building a successful and sustainable business. The Board is committed to the highest standards of corporate governance and undertakes to maintain a sound framework for our control and management. In this Report, we provide details of that framework. The key constituents necessary to deliver a robust structure are in place and, accordingly, this report includes a description of how the Company has applied the principles and provisions of the Governance Code and how it intends to apply those principles in the future. Chair’s overview “We believe that good corporate governance is essential for building a successful and sustainable business.”

82 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Sharon Barber-Lui Independent Non-Executive Director, Transaction Committee Member Michele Holcomb, Ph.D. Independent Non-Executive Director , Transaction Committee Member Sharon Barber-Lui has served as a member of our Board since March 2022 and became the Chair of the Audit Committee in April 2022. Ms. Barber-Lui has been the Chief Financial Officer and Senior Vice President, North America at Teva Pharmaceutical Industries Ltd. since July 2023. Prior to joining Teva, Ms. Barber-Lui worked as Senior Vice President of Global Finance at EQRx and at Merck for over twenty years in roles of advancing responsibility, including most recently as the Head of Portfolio Market Strategy, Operations and Business Analytics from 2019 through 2021 and Chief Financial Officer from 2014 through 2018 for Merck’s U.S. oncology business. Prior to that Ms. Barber-Lui held a number of other roles with Merck including Treasurer of U.S. Region, Head of U.S. Treasury Operations, and Head of Legal Entity Integration and Global Treasury Services, among others. Ms. Barber-Lui began her career as an accountant for KPMG LLP, and she received her bachelor’s degree as well as her M.B.A. from Lehigh University. Ms. Barber-Lui is a member of the American Institute of Certified Public Accountants. She is also the recipient of Merck & Co. Inc.’s Top Talent Designation, Women’s Leadership Recognition and Oncology Women’s Leader Recognition. Michele Holcomb, Ph.D. has served as a member of our Board since September 2024 and is a member of the Audit Committee and Transaction Committee. Dr. Holcomb is also a member of the board of directors and chair of the Nominating and ESG (NESG) committee of Kimball Electronics Inc (Nasdaq: KE). Dr. Holcomb previously worked as Executive Vice President, Chief Strategy and Business Development Officer at Cardinal Health from January 2017 until September 2022. Prior to joining Cardinal Health, Dr. Holcomb was the Chief Operating Officer of Global R&D and SVP of Strategy, Portfolio, Search and Partnerships at Teva Pharmaceuticals. She also spent 15 years at McKinsey & Company and was a Partner of the Global Pharmaceutical Practice. She also serves on the board of the Abigail Wexner Research Institute at Nationwide Children’s Hospital in Columbus, the BalletMet of Columbus, where she chairs the long-range planning committee and the Liberty Science Center in New Jersey. Dr. Holcomb received a B.S. in chemistry from Stanford University and a Ph.D. in chemistry from the University of California, Berkeley, and previously worked as an R&D chemist at Ciba-Geigy and Syntex Pharmaceuticals. Dr. Holcomb is also a member of the editorial advisory board of Pharmaceutical Executive and has lectured on healthcare strategy at Kellogg (Northwestern), Columbia and Fuqua (Duke) business schools. PureTech Health is led by a seasoned and accomplished Board of Directors and management team with extensive experience in maximising shareholder value, discovering scientific breakthroughs, and delivering therapeutics to market. Board of Directors (alphabetically)* * The biography for executive director Bharatt Chowrira can be found on page 85.

PureTech Health plc Annual Report and Accounts 2024 83 G overnance Board of Directors continued Kiran Mazumdar-Shaw Independent Non-Executive Director John LaMattina, Ph.D. Senior Independent Director, Transaction Committee Member, R&D Committee Member Robert Langer, Sc.D. Co-Founder and Non-Executive Director, R&D Committee Member John LaMattina, Ph.D., has served as a member of our Board since 2009, and assumed the role of Senior Independent Director in April 2025. Dr. LaMattina previously worked at Pfizer in different roles from 1977 to 2007, including vice president of U.S. Discovery Operations in 1993, senior vice president of worldwide discovery operations in 1998, senior vice president of worldwide development in 1999 and president of global research and development from 2003 to 2007. Dr. LaMattina serves on the board of directors of Ligand Pharmaceuticals and Vedanta Biosciences, Inc. Dr. LaMattina previously served on the boards of Immunome Inc. until October 2023 and Zafgen, Inc. until April 2020. He is also a trustee associate of Boston College. During Dr. LaMattina’s leadership tenure, Pfizer discovered and/or developed a number of important new medicines including Tarceva, Chantix, Zoloft, Selzentry and Lyrica, along with a number of other medicines currently in late stage development for cancer, rheumatoid arthritis and pain. He is the author of numerous scientific publications and U.S. patents. Dr. LaMattina received the 1998 Boston College Alumni Award of Excellence in Science and the 2004 American Diabetes Association Award for Leadership and Commitment in the Fight Against Diabetes. He was awarded an Honorary Doctor of Science degree from the University of New Hampshire in 2007. In 2010, he was the recipient of the American Chemical Society’s Earle B. Barnes Award for Leadership in Chemical Research Management. He is the author of “Devalued and Distrusted—Can the Pharmaceutical Industry Restore its Broken Image,” “Drug Truths: Dispelling the Myths About Pharma R&D,” “Pharma and Profits: Balancing Innovation, Medicine, and Drug Prices” and an author of the Drug Truths blog at Forbes. com. Dr. LaMattina received a B.S. in Chemistry from Boston College and received a Ph.D. in Organic Chemistry from the University of New Hampshire. He then moved on to Princeton University as a National Institutes of Health postdoctoral fellow in the laboratory of professor E. C. Taylor. Robert S. Langer, Sc.D., is a co- founder, member of PureTech’s R&D Committee and has served as a member of the board of directors since our founding. Dr. Langer has served as the David H. Koch Institute professor at MIT since 2005. He served as a member of the FDA’s science board from 1995 to 2002 and as its chairman from 1999 to 2002. Dr. Langer serves on the board of directors of Seer Bio. Dr. Langer previously served on the boards of Moderna, Inc., which he co-founded, until August 2024, Abpro Korea until February 2024 and Frequency Therapeutics, Inc. until November 2023. Dr. Langer has received over 250 major awards, including the 2006 U.S. National Medal of Science, the Charles Stark Draper Prize in 2002 and the 2012 Priestley Medal. He is also the first engineer to receive the Gairdner Foundation International Award. Dr. Langer has received the Dickson Prize for Science, Heinz Award, Harvey Prize, John Fritz Award, General Motors Kettering Prize for Cancer Research, Dan David Prize in Materials Science, Breakthough Prize in Life Sciences, National Medal of Science, National Medal of Technology and Innovation, Kyoto Prize, Wolf Prize, Albany Medical Center Prize in Medicine and Biomedical Research and the Lemelson-MIT prize. In 2006, he was inducted into the National Inventors Hall of Fame. In January 2015, Dr. Langer was awarded the 2015 Queen Elizabeth Prize for Engineering. Dr. Langer received his bachelor’s degree in Chemical Engineering from Cornell University and his Sc.D. in Chemical Engineering from MIT. Kiran Mazumdar-Shaw has served as a member of our Board since September 2020. Ms. Mazumdar- Shaw has been the executive chairperson of Biocon Limited, which she founded in 1978, since April 2020, and she served as managing director of Biocon Limited from 1995 to 2020. Ms. Mazumdar-Shaw holds key positions in various industry, educational, government and professional bodies globally. She served as a full-term member of the board of trustees of Massachusetts Institute of Technology until June 2023. She has been elected as a member of the prestigious U.S.-based National Academy of Engineering. She also serves as a director on the board of United Breweries Limited, and non- executive director on the board of Narayana Health. Ms. Mazumdar- Shaw previously served as the lead independent member of the board of Infosys Ltd until March 2023. Ms. Mazumdar-Shaw has received two of India’s highest civilian honors, the Padma Shri in 1989 and the Padma Bhushan in 2005. She was also honored with the Order of Australia, Australia’s highest civilian honor in January 2020. In 2016, she was conferred with the highest French distinction – Knight of the Legion of Honour – and in 2014 received the Othmer Gold Medal in 2014 from the U.S.-based Chemical Heritage Foundation for her pioneering efforts in biotechnology. Ms. Mazumdar- Shaw has been ranked as one of the world’s top 20 inspirational leaders in the field of biopharmaceuticals by The Medicine Maker Power List 2020, and she was the winner of EY World Entrepreneur of the Year™ 2020 Award. She was the first woman business leader from India to sign the Giving Pledge, an initiative of the Gates Foundation, committing to give the majority of her wealth to philanthropic causes. She received a bachelor’s degree in science, Zoology Hons., from Bangalore University and a master’s degree in malting and brewing from Ballarat College, Melbourne University. She has been awarded several honorary degrees from other universities globally. Raju Kucherlapati, Ph.D. Chair of the Board, Transaction Committee Member, R&D Committee Member Raju Kucherlapati, Ph.D., has served as a member of our Board since 2014 and assumed the role of Chair on June 20, 2024, after having served as interim Chair since the June 2023. Dr. Kucherlapati has also served as chair of PureTech’s Nomination Committee since December 31, 2022 and Senior Independent Director from December 31, 2022 to April 2025. He has been the Paul C. Cabot professor of Genetics and a professor of medicine at Harvard Medical School since 2001. Dr. Kucherlapati currently serves on the board of directors of KEW Inc. Dr. Kucherlapati previously served on the board of Gelesis Holdings, Inc. until October 2023. He was a founder and former board member of Abgenix (acquired by Amgen for $2.2 billion), Cell Genesys and Millennium Pharmaceuticals (acquired by Takeda for $8.8 billion). He was the first scientific director of the Harvard-Partners Center for Genetics and Genomics. He is a fellow of the American Association for the Advancement of Science and a member of the National Academy of Medicine. Dr. Kucherlapati received his Ph.D. from the University of Illinois. He trained at Yale and has held faculty positions at Princeton University, University of Illinois College of Medicine and the Albert Einstein College of Medicine. He served on the editorial board of the New England Journal of Medicine and was Editor in Chief of the journal Genomics. He was a member of the presidential commission for the study of bioethical issues during the Obama administration. His laboratory at Harvard Medical School is involved in cloning and characterization of human disease genes with a focus on human syndromes with a significant cardiovascular involvement, use of genetic/genomic approaches to understand the biology of cancer and the generation and characterization of genetically modified mouse models for cancer and other human disorders. His laboratory was a part of the Human Genome Program that was responsible for mapping and sequencing the human genome. Dr. Kucherlapati developed methods for modifying mammalian genes that lead to gene targeting in mice. He has developed many mouse models for human disease, including a large set of models for human colorectal cancer. His laboratory was a part of The Cancer Genome Atlas (TCGA) program that uses genetic/genomic approaches to understand the biology of cancer. He is a promoter of personalized/precision medicine.

84 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Board of Directors continued H. Robert Horvitz, Ph.D.** Board Advisor, R&D Committee Chair Dennis Ausiello, M.D.** Board Advisor, R&D Committee Member Dennis Ausiello, M.D., is a board advisor and member of the PureTech R&D Committee. He is the Jackson Distinguished Professor of Clinical Medicine and was previously director, emeritus of the M.D./Ph.D. Program at Harvard Medical School. Dr. Ausiello is chairman of medicine, emeritus and director of the Center for Assessment Technology and Continuous Health (CATCH) at Massachusetts General Hospital (MGH). This center is a partnership among MGH, MIT and Harvard University with a mission to develop real-time assessment of human traits in wellness and disease. In partnership with industry, it is creating tools for measurements of traditional and novel phenotypes. Understanding the need for partnerships between the academy and industry, Dr. Ausiello served on the board of directors of Pfizer Pharmaceuticals, where he was their former lead director. He currently serves as a member of the board of directors of Seres Therapeutics, Inc. and Alnylam Pharmaceuticals, Inc. Dr. Ausiello is also a member of the board of directors of several non-public biotech companies and is a consultant to Verily (formerly Google Life Sciences) and Pfizer Pharmaceuticals. Dr. Ausiello is a nationally recognized leader in academic medicine who was elected to the National Academy of Medicine in 1999 and the American Academy of Arts and Sciences in 2003. He has published numerous articles, book chapters and textbooks and has served as an editor of Cecil’s Textbook of Medicine. Dr. Ausiello received his BA from Harvard College and an M.D. from the University of Pennsylvania. H. Robert Horvitz, Ph.D., is a board observer and Chair of the R&D Committee at PureTech. He received the Nobel Prize in Physiology or Medicine and is the David H. Koch Professor of Biology at Massachusetts Institute of Technology, an investigator of the Howard Hughes Medical Institute, neurobiologist (Neurology) at Massachusetts General Hospital, a member of the MIT McGovern Institute for Brain Research and the MIT Koch Institute for Integrative Cancer Research. He is cofounder of multiple life science companies, including Epizyme (EPZM), Mitobridge (acquired by Astellas) and Idun Pharmaceuticals (acquired by Pfizer) and was a member of the Scientific Advisory Board of the Novartis Institutes for BioMedical Research. Dr. Horvitz was a member of the board of trustees of the Massachusetts General Hospital. He also previously served as Chairman of the Board of Trustees of the Society for Science and the Public and as President of the Genetics Society of America. Dr. Horvitz is a member of the U.S. National Academy of Sciences, the U.S. National Academy of Medicine and the American Philosophical Society and is a foreign member of the Royal Society of London. He is a fellow of the American Academy of Arts and Sciences and of the American Academy of Microbiology. Dr. Horvitz received the U.S. National Academies of Science Award in Molecular Biology; the Charles A. Dana Award for Pioneering Achievements in Health; the Ciba-Drew Award for Biomedical Science; the General Motors Cancer Research Foundation Alfred P. Sloan, Jr. Prize; the Gairdner Foundation International Award; the March of Dimes Prize in Developmental Biology; the Genetics Society of America Medal; the Bristol-Myers Squibb Award for Distinguished Achievement in Neuroscience; the Wiley Prize in the Biomedical Sciences; the Peter Gruber Foundation Genetics Prize; the American Cancer Society Medal of Honor; the Alfred G. Knudson Award of the National Cancer Institute; and the UK Genetics Society Mendel Medal. He has received honorary doctoral degrees from the University of Rome, Cambridge University, Pennsylvania State University and the University of Miami. Daphne Zohar** Founder and Board Advisor Daphne Zohar is a board observer and senior advisor. A founder of PureTech, Ms. Zohar served as chief executive officer and a member of the board of directors since our formation and UK main market listing in 2015 until her departure in April 2024, to become founder, chief executive officer and a board member of PureTech founded entity, Seaport Therapeutics, Inc. A successful entrepreneur, Ms. Zohar created PureTech, assembling a leading team to help implement her vision for the company, and was a key driver in fundraising, business development and establishing the underlying programs and platforms that have resulted in PureTech’s productive R & D engine, which led to 29 new medicines being advanced via its Wholly Owned Pipeline and Founded Entities, including Cobenfy (KarXT) that has received US FDA approval. As the founding CEO of PureTech, she also co-founded PureTech’s entities, including Karuna Therapeutics, which was acquired by Bristol Myers Squibb for $14 billion. Ms. Zohar has been recognized as a top leader and innovator in biotechnology by a number of sources, including EY, Fierce Pharma, BioWorld, MIT Technology Review, The Boston Globe and Scientific American including recent recognition as one of Goldman Sachs’ most influential entrepreneurs in 2024 and being named to STAT’s 2025 STATUS list, the ultimate list of leaders in life sciences. She serves on the BIO (Biotechnology Innovation Organization) Board Executive Committee as well as the Health Section Committee. Ms. Zohar is a member of the Duke-Margolis Center Policy Roundtable on the Inflation Reduction Act (IRA) and the Health Affairs IRA Observatory. She is also a co-founder and host of the Biotech Hangout podcast, a weekly discussion of biotech news with a group of industry leaders and experts.

PureTech Health plc Annual Report and Accounts 2024 85 G overnance Eric Elenko, Ph.D. Co-Founder and President Bharatt Chowrira, Ph.D., J.D. Chief Executive Officer, Member of the Board of Directors Bharatt Chowrira, Ph.D., J.D., has been our chief executive officer since his appointment by the Board in April 2024. He was formerly president and chief business, finance and operating officer since September 2022, president and chief business, legal and operating officer from January 2022 through September 2022 and our president and chief of business and strategy from March 2017 through December 2021. Dr. Chowrira has served as a member of PureTech’s Board since February 2021 and also serves on the board of directors of Seaport Therapeutics. Inc. Prior to joining PureTech, Dr. Chowrira was the president of Synlogic, Inc., a biopharmaceutical company focused on developing synthetic microbiome-based therapeutics, from September 2015 to February 2017, where he oversaw and managed corporate and business development, alliance management, financial, human resources, intellectual property and legal operations. Prior to that, Dr. Chowrira was the chief operating officer of Auspex Pharmaceuticals, Inc. from October 2013 to July 2015, which was acquired by Teva Pharmaceutical Industries Ltd. in the spring of 2015. Previously, he was president and chief executive officer of Addex Therapeutics Ltd., a biotechnology company publicly-traded on the SIX Swiss Exchange, from August 2011 to July 2013. Prior to that Dr. Chowrira held various leadership and management positions at Nektar Therapeutics (chief operating officer), Merck & Co, or Merck (vice president), Sirna Therapeutics (general counsel; acquired by Merck) and Ribozyme Pharmaceuticals (chief patent counsel). Dr. Chowrira previously served on the board of directors of Vedanta Biosciences, Inc. from September 2018 to February 2023, Akili Interactive Labs, Inc. from November 2017 to September 2019 and June 2021 to October 2022, Vor Biopharma from August 2018 to June 2020, and Karuna Therapeutics, Inc. from March 2017 to December 2019. Dr. Chowrira received a J.D. from the University of Denver’s Sturm College of Law, a Ph.D. in Molecular Biology from the University of Vermont College of Medicine, a M.S. in Molecular Biology from Illinois State University and a B.S. in Microbiology from the UAS, Bangalore, India. Eric Elenko, Ph.D., has served as our president since his appointment by the Board in April 2024. Prior to his current role, Dr. Elenko served as chief innovation officer since June 2015 and held various other positions at PureTech prior thereto. While at PureTech, Dr. Elenko has led the development of a number of programs, including Akili Interactive Labs, Inc., Gelesis, Inc., Karuna Therapeutics, Inc. (acquired by Bristol Myers Squibb for $14.0 billion) and Sonde Health, Inc. Dr. Elenko is a founder and serves on the board of directors of Seaport Therapeutics. Inc. and Sonde Health, Inc. Prior to joining PureTech, Dr. Elenko was a consultant with McKinsey & Company from February 2002 to September 2005, where he advised senior executives of both Fortune 500 and specialty pharmaceutical companies on a range of issues such as product licensing, mergers and acquisitions, research and development strategy and marketing. Dr. Elenko received a B.A. in Biology from Swarthmore College and his Ph.D. in Biomedical Sciences from University of California, San Diego. Management Team (alphabetically)* Michael Inbar, CPA, MBA Chief Accounting Officer Robert Lyne Chief Portfolio Officer Charles (Chip) Sherwood, J.D. General Counsel and Company Secretary Michael Inbar, CPA, MBA, is the chief accounting officer at PureTech where he leads all aspects of accounting, compliance, and finance operations. Prior to joining PureTech in 2023, Mr. Inbar was the chief financial officer of Acronis Inc., a private multinational software company, and interim chief financial officer at Wallarm, Inc., a private cyber-security company. He has held several leadership roles in other technology and biotechnology companies, including Solid Biosciences, Inc., Syros Pharmaceuticals, Inc., and GlassHouse Technologies, Inc. Mr. Inbar started his career in public accounting and spent 11 years in the audit and assurance practice, mostly with EY. Mr. Inbar has over 20 years of experience in accounting and finance, with expertise in scaling up businesses to support organic growth or acquisitions, debt and equity fundraising, and building high performing finance teams to support companies’ objectives and success. Robert Lyne is the chief portfolio officer at PureTech. Prior to joining PureTech, Mr. Lyne was the Chief Executive Officer at Arix Bioscience plc, a transatlantic venture capital company focused on investing in innovative biotechnology companies. He began his career as a lawyer at international law firm Bird & Bird LLP in London before moving to Touchstone Innovations, a London listed biotech and technology investor, which was acquired in 2017. He has worked on over 80 venture capital financings in Europe and North America as well as multiple trade exits and IPOs. As an experienced UK plc executive, Mr. Lyne has broad experience formulating and implementing corporate strategy. Mr. Lyne has a BA from the University of Oxford and an LLB from Oxford Brookes University. Charles Sherwood, J.D., is the general counsel and company secretary at PureTech, where he leads the company’s corporate legal function, including corporate governance and compliance. Mr. Sherwood also serves on the board of directors of Vedanta Biosciences, Inc. Prior to joining PureTech in August 2021, he was the Vice President, Corporate Legal Counsel at Anika Therapeutics, a small-cap NASDAQ-listed biotechnology company. During his time at Anika, Charles built and led the legal department, where he served as a strategic advisor to management and the Board and developed extensive subject matter expertise involving strategic transactions, intellectual property, product and brand marketing, financing and other financial matters and securities compliance and other compliance matters. Mr. Sherwood received a B.A. in economics from Middlebury College and a J.D. from Vanderbilt University Law School. He is admitted to the Massachusetts Bar. ** Dr. Horvitz, Dr. Ausiello and Ms. Zohar are not members of the PureTech Board. As Board Observers, Dr. Horvitz, and Ms. Zohar attend the majority of Board meetings. Dr. Horvitz and Dr. Ausiello are members of PureTech’s R&D Committee, of which Dr. Horvitz is the Chair.

86 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e The Company’s schedule of matters reserved for the Board includes the following matters: — approval and monitoring of our strategic aims and objectives; — approval of the annual operating and capital expenditure budget; — changes to our capital structure, the issue of any of our securities and material borrowings; — approval of the annual report and half-year results statement, accounting policies and practices or any matter having a material impact on our future financial performance; — ensuring a sound system of internal control and risk management; — approving Board appointments and removals, and approving policies relating to directors’ remuneration; — strategic acquisitions; — major disposals of our assets or subsidiaries; — approval of all circulars, prospectuses and other documents issued to shareholders governed by the Financial Conduct Authority’s (FCA) Listing Rules, Disclosure Guidance and Transparency Rules or the City Code on Takeovers and Mergers; — approval of terms of reference and membership of Board committees; — considering and, where appropriate, approving directors’ conflicts of interest; and — approval, subject to shareholder approval, of the appointment and remuneration of the auditors. The schedule of matters reserved to the Board is available on request from the Company Secretary or within the Investors section of our website at www.puretechhealth.com. The Board delegates specific responsibilities to certain committees that assist the Board in carrying out its functions and ensure independent oversight of internal control and risk management. The three principal Board committees (Audit, Remuneration and Nomination) play an essential role in supporting the Board in fulfilling its responsibilities and ensuring that we maintain the highest standards of corporate governance. Each committee has its own terms of reference which set out the specific matters for which delegated authority has been given by the Board. The terms of reference for each of the committees are fully compliant with the provisions of the Governance Code. All of these are available on request from the Company Secretary or within the Investors section of our website at www. puretechhealth.com. Roles and responsibilities of the Board The Board is responsible to shareholders for our overall management as a whole. The main roles of the Board are: — creating value for shareholders; — providing business and scientific leadership; — approving our strategic objectives; — ensuring that the necessary financial and human resources are in place to meet strategic objectives; — overseeing our system of risk management; and — setting the values and standards for both our business conduct and governance matters. The Directors are also responsible for ensuring that obligations to shareholders and other stakeholders are understood and met and that communication with shareholders is maintained. The responsibility of the Directors is collective, taking into account their respective roles as Executive Directors and Non-Executive Directors. All Directors are equally accountable to the Company’s shareholders for the proper stewardship of its affairs and our long- term success. The Board reviews strategic issues on a regular basis. During the past year the Board has played an active role on a variety of strategic initiatives of the Company. Members served as subject matter experts, advised on asset evaluation strategy and reviewed potential transactions. In addition, several members served on an independent transactions committee, led by the interim Chair. As a result, certain members have devoted substantial time and effort to the Company, above and beyond what would typically be expected of Non-Executive Directors. The Board has also exercised control over our performance by agreeing on budgetary and operational targets and monitoring performance against those targets. The Board has overall responsibility for our system of internal controls and risk management. Any decisions made by the Board on policies and strategy to be adopted by us or changes to current policies and strategy are made following presentations by the Executive Director and other members of management, and only after a detailed process of review and challenge by the Board. Once made, the Executive Director and other members of management are fully empowered to implement those decisions. Except for a formal schedule of matters which are reserved for decision and approval by the Board, the Board has delegated our day-to-day management to the Chief Executive Officer who is supported by other members of the senior management team. The schedule of matters reserved for Board decision and approval are those significant to us as a whole due to their strategic, financial or reputational implications. The Board

PureTech Health plc Annual Report and Accounts 2024 87 G overnance The Board continued Senior Independent Director The Company’s Senior Independent Director is Dr. John LaMattina, who was appointed to the role in April 2025. During 2024, Dr. Raju Kucherlapati served in the role. The Board has considered the position of Senior Independent Director, following Dr. Kucherlapati’s appointment as Chair, and has determined that the functions of the role would be best fulfilled by Dr. LaMattina. Following Dr. LaMattina’s appointment, a key responsibility of the Senior Independent Director is to be available to shareholders in the event that they may feel it inappropriate to relay views through the Chair or Chief Executive Officer. In addition, the Senior Independent Director is to serve as an intermediary between the rest of the Board and the Chair where necessary. Further, the Senior Independent Director will lead the Board in its deliberations on any matters on which the Chair is conflicted. The Board has considered the position of Senior Independent Director, following Dr. Kucherlapati’s appointment as Chair, and has determined that the functions of the role would be best fulfilled by Dr. LaMattina. The roles of Chair and Chief Executive Officer The Company’s Chair is Dr. Raju Kucherlapati. He assumed the role of Chair of the Board on June 20, 2024, after having served as interim Chair since the June 2023. The Nomination Committee carefully considered Dr. Kucherlapati’s skills, knowledge and expertise, as well as his long-term involvement in the evolution of PureTech and his exemplary leadership during his tenure as interim Chair in their decision to appoint him to the role of Chair. There is and will remain a clear division of responsibilities between the Chair and the Chief Executive Officer. The Chair is responsible for the leadership and conduct of the Board and for ensuring effective communication with shareholders. The Chair facilitates the full and effective contribution of Non- Executive Directors at Board and Committee meetings, ensures that they are kept well informed and ensures a constructive relationship between the Executive Directors and Non-Executive Directors. The Chair also ensures that the Board committees carry out their duties, including reporting back to the Board either orally or in writing following their meetings at the next Board meeting. In the role of the Chief Executive Officer, Dr. Bharatt Chowrira, is to lead the execution of the Company’s strategy and the executive management of PureTech. He is responsible, among other things, for the development and implementation of strategy and processes which enable us to meet the requirements of shareholders, for delivering the operating plans and budgets for our businesses, for monitoring business performance against key performance indicators (KPIs) and reporting on these to the Board and for providing the appropriate environment to recruit, engage, retain and develop the high-quality personnel needed to deliver our strategy. Board size and composition As of December 31, 2024, there were seven Directors on the Board: the Non-Executive Chair, one Executive Director and five Non-Executive Directors. The biographies of these Directors are provided on pages 82 to 85. Raju Kucherlapati, Ph.D., assumed the role of Chair of the Board on June 20, 2024, after having served as interim Chair since the June 2023. Michele Holcomb, Ph.D. joined the Board as a Non-Executive Director on September 23, 2024. Additionally, former Executive Director Daphne Zohar departed from the Board on April 8, 2024 to become chief executive officer of PureTech founded entity, Seaport Therapeutics, Inc. There were no other changes to the composition of the Board during 2024. The Company’s policy relating to the terms of appointment and the remuneration of both Executive and Non-Executive Directors is detailed in the Directors’ Remuneration Report on pages 102 to 122. The size and composition of the Board is regularly reviewed by the Nomination Committee to ensure there is an appropriate and diverse mix of skills and experience on the Board. The Board may appoint any person to serve as a Director, either to fill a vacancy or as an addition to the existing Board. Any Director so appointed by the Board shall hold office only until the following AGM and then shall be eligible for election by the shareholders. In accordance with the Governance Code, all of the Directors will be offering themselves for election at the AGM to be held on June 4, 2025, full details of which are set out in the notice of meeting accompanying this Annual Report. Non-Executive Directors The Company’s Non-Executive Directors are Dr. Raju Kucherlapati (Chair), Ms. Sharon Barber-Lui, Dr. Michele Holcomb, Dr. John LaMattina, Dr. Robert Langer, and Ms. Kiran Mazumdar-Shaw. The Non-Executive Directors provide us with a wide range of skills and experience. Each Non-Executive Director has significant senior level experience as well as an extensive network in each of their own fields, an innovative mindset and independent judgement on issues of strategy, performance and risk, and is well placed to constructively challenge and scrutinize the performance of management. In addition, certain of our Non- Executive Directors also serve as members of one or more boards of directors of our Founded Entities and are key drivers for our Wholly-Owned Programs.

88 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e The Board continued Board meetings and decisions The Board meets regularly during the year, as well as on an ad hoc basis as required by business need. The Board had eight scheduled meetings in 2024, and details on attendance are set forth in the table below: Director Number of Board Meetings Attended Raju Kucherlapati 8/8 Sharon Barber-Lui 8/8 Michele Holcomb* 2/2 John LaMattina 8/8 Robert Langer 7/8 Kiran Mazumdar-Shaw 5/8 Bharatt Chowrira 8/8 Daphne Zohar** 3/3 * Dr. Holcomb joined the Company’s Board in September 2024. ** Ms. Zohar joined all three meetings as an Executive Director prior to her resignation from the role on April 8, 2024. While each current director was able to attend the vast majority of meetings in 2024, in the event of any unavoidable absence, the impacted Director would review with management the topics and materials to be discussed at the meeting, and provide appropriate feedback to be conveyed at such meeting, as was the case with respect to the meetings any director was unable to attend. The Board also acted by unanimous written consent eleven times in 2024. On occasion it was more expedient for the Board to approve matters, especially administrative matters, by unanimous written consent rather than to convene a meeting for the purpose. Directors were, however, provided with an opportunity to discuss any concerns they had with the written resolution before its issue for signature. At each quarterly meeting of the Board, there was a closed session held in which only the Chair and the other Non-Executive Directors participated. In certain meetings held to discuss a specific topic or topics, a closed session was not held due to limited time allocated for such meeting or the nature of the topic being considered. The schedule of Board and Committee meetings each year is, so far as is possible, determined before the commencement of that year and all Directors or, if applicable, all Committee members, are expected to attend each meeting. Supplementary meetings of the Board and/or the Committees are held as and when necessary. Each member of the Board receives in advance of each scheduled meeting detailed Board packages, which include an agenda based upon matters to be addressed and appropriate presentation and background materials. If a Director is unable to attend a meeting due to exceptional circumstances, he or she will nonetheless receive the meeting materials and discuss the materials with the Chief Executive Officer. Independence The Governance Code requires that at least 50 percent of the Board of a UK premium listed company, excluding the Chair, consists of Non-Executive Directors determined by the Board to be independent in character and judgement and free from relationships or circumstances which may affect, or could appear to affect, the Directors’ judgement. The Board regards Ms. Barber-Lui, Dr. Holcomb, Dr. LaMattina and Ms. Mazumdar-Shaw as Independent Non-Executive Directors for the purposes of the Governance Code. In reaching this determination, the Board duly considered (i) their directorships and links with other Directors through their involvement in other subsidiary companies; (ii) their equity interests in PureTech and/or the Founded Entities, including equity grants of restricted stock units made to Non- Executive Directors by the Company under its Performance Share Plan; and (iii) in respect of Dr. LaMattina, the length of his tenure as a Directors of the Company. The Board is satisfied that the judgement, experience and challenging approach adopted by each of these Directors should ensure that they each make a significant contribution to the work of the Board and its committees. Therefore, the Board has determined that Ms. Barber-Lui, Dr. Holcomb, Dr. LaMattina, and Ms. Mazumdar-Shaw are of independent character and judgement, notwithstanding the circumstances described at (i), (ii) and (iii) above. During 2024, the Nomination Committee, with assistance from the rest of the Board and the Company’s management, welcomed Dr. Holcomb to the Board. The Committee continues to evaluate potentially adding additional independent non-executive directors to strengthen the Board’s skillsets and reinforce the strong governance that has been a hallmark of the Company’s Board and broader operations. The Nomination Committee and the Company intend to conduct a thorough and expeditious process to identify the best candidates. Progress updates will be provided in due course. Board support, indemnity and insurance The Company Secretary, Mr. Charles Sherwood, is responsible to the Board for ensuring Board procedures are followed, applicable rules and regulations are complied with and that the Board is advised on governance and relevant regulatory matters. All Directors have access to the impartial advice and services of the Company Secretary. There is also an agreed procedure for Directors to take independent professional advice at the Company’s expense. In accordance with the Company’s Articles of Association and a contractual Deed of Indemnity, the Directors have been granted an indemnity issued by the Company to the extent permitted by law in respect of liabilities incurred to third parties as a result of their office. The indemnity would not provide any coverage where a Director is proved to have acted fraudulently or with wilful misconduct. The Company has also arranged appropriate insurance cover in respect of legal action against its Directors and officers.

PureTech Health plc Annual Report and Accounts 2024 89 G overnance The Board continued Induction, awareness and development In preparation for the Company’s initial public offering (IPO), and upon joining the Board subsequent to the IPO, Directors received an induction briefing from the Company’s legal advisors on their duties and responsibilities as Directors of a publicly quoted company. The Directors also received presentations from the Company’s corporate brokers prior to the IPO. In addition, in order to ensure that the Directors continue to further their understanding of the challenges facing our Founded Entities and Wholly-Owned Programs, the Board periodically receives the presentations and reports covering the business and operations of each of our Founded Entities as well as its Wholly- Owned Programs. We have put in place a comprehensive induction plan for any new Directors, including following the recent appointment of Dr. Michele Holcomb to the Company’s Board in September 2024. This program is tailored to the needs of each individual Director and agreed with him or her so that he or she can gain a better understanding of us and our businesses. In addition, the Company facilitates sessions as appropriate with our advisors, as well as appropriate governance specialists, to ensure that any new Directors are fully aware of, and understand, their responsibilities and obligations of a publicly quoted company and of the governance framework within which they must operate. Board effectiveness and performance evaluation The Board periodically reviews its effectiveness and performance. The Board seeks the assistance of an independent third-party provider at least once every three years in its evaluation in compliance with the Governance Code, and will otherwise carry out an internally facilitated Board evaluation led by the Senior Independent Director, assisted by the Company Secretary, covering the effectiveness of the Board as a whole, its individual Directors and its Committees. For 2024, internal evaluations of the Board demonstrated that the Board and its Committees fulfil their responsibilities, operate effectively and demonstrate a clear structure and division of responsibilities between the Board and its Committees. The increased quality of Board materials and presentations and advances in the process for evaluating strategic transactions were favourably viewed. The Board will continue to perform internal evaluations to ensure the effectiveness of the Board and ensure alignment with the interests of stakeholders. In addition to the above, the Non-Executive Directors, led by the Senior Independent Director, will periodically appraise the Chair’s performance, following which the Senior Independent Director will provide any feedback to the Chair. The performance of each of the Directors on the Board and the performance of the committees of the Board will be reviewed by the Chair as deemed necessary. The performance of Executive Directors will be reviewed by the Board on an ongoing basis, as deemed necessary, in the absence of the Executive Director under review. The Chair, Chief Executive Officer and senior management team work together to ensure that the Directors receive relevant information to enable them to discharge their duties and that such information is accurate, timely and clear. This information includes quarterly management accounts containing analysis of performance against budget as well as a summary of the operational performance of each of our businesses against its goals. Additional information is provided as appropriate for the topics being addressed at the meeting. At each meeting, the Board receives presentations from the Chief Executive Officer and, by invitation, other members of senior management as required. This ensures that all Directors are in a position to effectively monitor our overall performance, and to contribute to the development and implementation of its strategy. Company Board meetings are held either in our offices in Boston, Massachusetts, U.S., or by videoconference. This practice began during the onset of the COVID-19 pandemic for the safety of the Board and has continued in recent years. The venue of Board meetings varies depending on the schedules and health of our directors. The Board endeavours to hold at least two in-person meetings during the year, as they give members of the Company’s senior management team, as well as the senior management of the Founded Entities, the opportunity to formally present to the Board on new technology development and business strategies. Certain Directors also serve on the boards of directors of our Founded Entities. These Founded Entity boards of directors meet regularly during the year, as well as on an ad hoc basis as required by business need. This service enables the Directors to have deep understanding of the businesses and contribute significantly to the strategy and oversight of these businesses. Directors’ conflicts of interest Each Director has a statutory duty under the Companies Act 2006 (the CA 2006) to avoid a situation in which he or she has or can have a direct or indirect interest that conflicts or may potentially conflict with the interests of the Company. This duty is in addition to the continuing duty that a Director owes to the Company to disclose to the Board any transaction or arrangement under consideration by the Company in which he or she is interested. The Company’s Articles of Association permit the Board to authorize conflicts or potential conflicts of interest. The Board has established procedures for managing and, where appropriate, authorizing any such conflicts or potential conflicts of interest. In deciding whether to authorize any conflict, the Directors must have regard to their general duties under the CA 2006 and their overriding obligation to act in a way they consider, in good faith, will be most likely to promote the Company’s success. In addition, the Directors are able to impose limits or conditions when giving authorization to a conflict or potential conflict of interest if they think this is appropriate. The authorization of any conflict matter, and the terms of any authorization, may be reviewed by the Board at any time. The Board believes that the procedures established to deal with conflicts of interest are operating effectively.

90 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Identification and evaluation of risks The Board actively identifies and evaluates the risks inherent in the business and ensures that appropriate controls and procedures are in place to manage these risks. The Board obtains an update regarding our Wholly-Owned Programs and all Founded Entities on a regular basis, and reviews our performance and the performance of our Wholly-Owned Programs and Founded Entities on a quarterly basis. However, the performance and structuring of business units may be reviewed more frequently if deemed appropriate. The key risks and uncertainties we face, as well as the relevant mitigations, are set out on pages 60-64 and in the Additional Information section from pages 182 to 219. Information and financial reporting systems We evaluate and manage significant risks associated with the process for preparing consolidated accounts by having in place systems and internal controls that ensure adequate accounting records are maintained and transactions are recorded accurately and fairly to permit the preparation of financial statements in accordance with IFRS. The Board approves the annual operating budgets and regularly receives details of actual performance measured against the budget. Principal risks and uncertainties Our operations and the implementation of our objectives and strategy are subject to a number of key risks and uncertainties. Principal and emerging risks are formally reviewed by the Board at least annually and appropriate procedures are put in place to monitor and, to the extent possible, mitigate these risks. A summary of the key risks affecting us and the steps taken to manage these risks are set out on pages 60-64 and in the Additional Information section from pages 182 to 219. Political expenditure It is the Board’s policy not to incur political expenditure or otherwise make cash contributions to political parties and it has no intention of changing that policy. 2025 Annual General Meeting The Notice of the AGM, which will be held at 11:00 am EDT (4:00 pm BST) on June 16, 2025 at the Company’s corporate headquarters at 6 Tide Street, in Boston, Massachusetts, U.S., is enclosed with this report. Details of the resolutions and the explanatory notes thereto are included with the Notice. To ensure compliance with the Governance Code, the Board proposes separate resolutions for each issue and proxy forms allow shareholders who are unable to attend the AGM to vote for or against or to withhold their vote on each resolution. In addition, to encourage shareholders to participate in the AGM process, the Company proposes to offer electronic proxy voting through the Registrar’s website and through the CREST service. The results of all proxy voting will be published on our website after the AGM. Our website at www.puretechhealth.com is the primary source of information on us. The website includes an overview of our activities, details of our businesses, and details of all of our recent announcements. Committees of the Board The Board has three principal committees: the Nomination Committee, the Audit Committee and the Remuneration Committee. The composition of the three principal committees of the Board and the attendance of the members throughout the year is set out in the respective committee reports contained in this Annual Report. In addition to the principal committees there are two other committees, the Transaction and R&D Committees, on which non-executive directors participate. Transaction Committee Since early 2023, the Board has maintained a standing Transaction Committee. The committee meets ad hoc and more formally when actively evaluating a transaction. During 2024, the Transaction Committee met formally twelve times, in consideration of the Seaport asset transfer and financings and a number of other matters the Board assessed and evaluated. R&D Committee The R&D Committee meets quarterly to discuss continued progress of ongoing Wholly-Owned Programs and evaluate opportunities for new programs. During 2024, the R&D Committee met formally four times. The terms of reference of each committee are available on request from the Company Secretary and within the Investors section of our website at www.puretechhealth.com. Internal Control The Board fully recognizes the importance of the guidance contained in the Guidance on Risk Management, Internal Control and Related Financial and Business Reporting. Our internal controls were in place during the whole of 2024 and we are satisfied that we have adequate controls and that our internal control over financial reporting was effective for the year ended December 31, 2024. The Board is responsible for establishing and monitoring internal control systems and for reviewing the effectiveness of these systems. The Board views the effective operation of a rigorous system of internal control as critical to our success; however, it recognizes that such systems are designed to manage rather than eliminate risk of failure and can provide only reasonable and not absolute assurance against material misstatement or loss. The key elements of our internal control system, all of which have been in place during the financial year and up to the date these financial statements were approved, are as follows: Control environment and procedures We have a clear organizational structure with defined responsibilities and accountabilities. It adopts the highest values surrounding quality, integrity and ethics, and these values are communicated clearly throughout the whole organization. Detailed written policies and procedures have been established covering key operating and compliance risk areas. These policies and procedures are reviewed and the effectiveness of the systems of internal control is assessed periodically by the Board. The Board continued

PureTech Health plc Annual Report and Accounts 2024 91 G overnance The Board recognizes its duties under Section 172 of the Companies Act 2006 and continuously has regard to how the Company’s activities and decisions will impact investors, employees, those with whom it has a business relationship, the community and environment and its reputation for high standards of business conduct. In weighing all of the relevant factors, the Board, acting in good faith and fairly between members, makes decisions and takes actions that it considers will best lead to the long- term success of the Company. In accordance with Section 172, it is the responsibility of the Board as a whole to ensure that a satisfactory dialogue takes place and that the Board considers the potential impact on the Company’s key stakeholders when making decisions. The Board is committed to understanding and engaging with shareholders and other key stakeholder groups of the Company in order to maximize value and promote long-term Company success in line with our strategic objectives, as well as to promote and ensure fairness between our stakeholders. The Board believes that appropriate steps and considerations have been taken during the year so that each Director has an understanding of the various key stakeholders of the Company. The Board recognizes its responsibility to contemplate all such stakeholder needs and concerns as part of its discussions, decision-making, and in the course of taking actions and will continue to make stakeholder engagement a top priority in the coming years. During the year, the Board assessed its current activities between the Board and its stakeholders, which demonstrated that the Board actively engages with its stakeholders and takes their various objectives into consideration when making decisions. Stakeholder How we engage Key matters identified Further information Investors – Our shareholders are the owners and investors in our business. We make significant efforts to engage with our shareholders and understand their objectives. We engage with our shareholders through a number of mechanisms to ensure that shareholder views are brought into the boardroom and considered in our decision-making. – The Board’s primary shareholder contact is through the Chief Executive Officer. The Chair, the Senior Independent Director and other Directors, as appropriate, make themselves available for contact with major shareholders and other stakeholders in order to understand their issues and concerns. – Stakeholder engagement will often take place by the Executive Directors and senior management through investor meetings and investor roadshows, including participation at healthcare conferences and participating in fireside chats at those events, with the Board receiving regular updates by way of analysis reports on stakeholder views. – Meetings were held throughout the year with institutional shareholders. Key shareholder publications including the annual report, the full year and half year results announcements and press releases and the information for investors are available on the Company’s website: www.puretechhealth.com. – Our Board keeps its Strategy and Business Model under regular review. During the past year, the Board has engaged to carefully consider its strategy for future growth and development, in particular devoting attention to the future prospects of its business model and its listing venues and the risks and opportunities this would give to the Company’s stakeholders. – The company carefully manages its expenditure and anticipates future capital needs through careful capital management and capital allocation to its Wholly-Owned Programs and clinical trials as well as opportunities to secure financing from third parties, for example we monetized PureTech’s royalty in Bristol Myers Squibbs’ Cobenfy® for up to $500 million, with $100 million in cash paid up front. Our Board also carefully considers opportunities for disposal of shares in our Founded Entities, which have generated over $815 million in non-dilutive proceeds to advance our pipeline and growth since 2020. – The Board seeks to ensure appropriate board structure and the Nomination Committee continues to actively evaluate seasoned candidates with extensive experience suitable for a Company of PureTech’s size. – Governance Section of ARA (Pages 59 to 101) – ESG Report (Pages 22 to 58) – Remuneration Report (Pages 102 to 119) – Our Key Components of Value (Page 8) Relations with Stakeholders – Section 172 Statement [Portions of this page have been intentionally omitted]

92 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Stakeholder How we engage Key matters identified Further information Our People – Our employees are crucial to the success of our business and many key decisions made by our Board have an impact on them. It is important to understand the employee perspective and ensure that we maintain an engaged workforce, as we believe that this will lead to better business results. We engage with our employees in various ways to ensure that their voice is heard in the management of our business including: – The conduct of regular town hall meetings, email briefings to employees on key events as well as communication through the company intranet site and an engagement survey – The implementation of regular appraisals and personal development programs – The Board recognizes the importance of an incentivized and engaged workforce, especially in the highly competitive biotechnology cluster of the greater Boston area. While the Board recognized the three methods suggested in the Code for workforce engagement, the Board opted for a more informal approach given the Company’s number of employees. The Board is responsive to the views of employees, and regularly seeks feedback from the Executive Directors on the overall culture of the Company which is aligned to the purpose, values and strategy of the organization. Executive Directors provide insights based on the feedback from routine employee engagement, such as through surveys and Town Hall Meetings. – The Board aims to attract and retain employees. This is attained through a combination of competitive remuneration and benefit packages and an established personal management and development program. This program is implemented with a view to development of the individual in an inclusive environment where employees from diverse backgrounds can thrive. – We are proud to be a company dedicated to giving life to new classes of medicine to improve the lives of patients with devastating diseases and believe we have established a business where our employees are proud to work. – ESG Report (Pages 22 to 58) – Remuneration Report (Pages 102 to 120) – Strategic Report (Pages 4 to 21) Community & Environment – We are committed to supporting the communities in which we operate and the wider public. To that end, we have developed various mechanisms for engagement including: – Internships/partnerships with local universities and programs – Charitable giving – Building Certifications – Therapeutic Focus – We are committed to improving our practices to ensure our business operates on a sustainable basis. In particular, we have created an ESG committee chaired by one of our Non-Executive Directors to guide our sustainability initiatives. Our business operates with low carbon emissions, and we are committed to delivering long-term environmental sustainability. – We partner with local universities and programs to offer paid internship and externship programs, generally within technical fields in our development organization. – The company engages with local community and supports charitable causes. In particular, in 2024, PureTech made charitable contributions to the Pulmonary Fibrosis Foundation, Young Man with a Plan and The Greater Boston Food Bank. – ESG Report (Pages 22 to 58) Suppliers/ Business Partners – Our business model creates value through partnerships and relationships with various key collaborators, and we continually evaluate how to strengthen relationships and arrangements with these institutions and individuals. Our engagement in 2024 included: – Quality updates and quality audits – Meetings with key surgeons to understand/identify potential indications and applications for therapeutics – Partnerships – BeiGene – We aim to build clear and reliable supply arrangements with our contract manufacturers for clinical product supply, in particular with an emphasis on quality, especially in relation to a clinical environment. – We seek partnerships with other life sciences organizations to secure non-dilutive funding, access to development opportunities and access to materials for our clinical trials. – Our Diversified Hub-and-Spoke Model (Page 10) – Gallop Oncology (Page 14) – Seaport Therapeutics (Page 17) – Vedanta Biosciences (Page 18) Relations with Stakeholders – Section 172 Statement continued

PureTech Health plc Annual Report and Accounts 2024 93 G overnance The Company’s issued ordinary share capital comprises a single class of ordinary shares. Details on movements in issued share capital can be found in Note 16 to the financial statements, page 160. Rights of ordinary shares All of the Company’s issued ordinary shares are fully paid up and rank pari passu in all respects and there are no special rights with regard to control of the Company. There are no restrictions on the transfer of ordinary shares or on the exercise of voting rights attached to them, which are governed by the Articles of Association and relevant UK legislation. The Directors are not aware of any agreements between holders of the Company’s shares that may result in restrictions on the transfer of securities or in voting rights. Substantial shareholders As of March 31, 2025, the Company had been advised that the shareholders listed below hold interests of 3 percent or more in its ordinary share capital (other than interests of the Directors which are detailed on page 116 of the Directors’ Remuneration Report). Other than as shown, so far as the Company (and its Directors) are aware, no other person holds or is beneficially interested in a disclosable interest in the Company. Shareholder % Invesco Asset Management Limited 17.07 Baillie Gifford & Co 6.30 Lansdowne Partners International Limited 6.01 Citigroup as principal 5.14 Recordati SPA Pharmaceutical Company 3.98 Vanguard Group 3.95 Fidelity International Limited 3.41 Daphne Zohar 3.22 Powers of the Directors Subject to the Company’s Articles of Association, UK legislation and any directions given by special resolution, the business of the Company is managed by the Board of Directors. Details of the matters reserved for the Board can be found in the Corporate Governance Report on page 86. The Directors present their report and the audited consolidated financial statements for the financial year ended December 31, 2024. Certain disclosure requirements for inclusion in this report have been incorporated by way of cross reference to the Strategic Report, the Directors’ Remuneration Report and the ESG Report which should be read in conjunction with this report. The Company was incorporated on May 8, 2015 as a public company limited by shares in the UK and has a registered office situated at 13th Floor, One Angel Court, London, EC2R 7HJ, United Kingdom. The Company was admitted to the premium listing segment of the Official List of the UK Listing Authority and to trading on the main market of the London Stock Exchange on June 24, 2015. The Company’s American Depository Shares, each representing 10 ordinary shares, began trading on the Nasdaq Global Market on November 16, 2020. Directors The membership of the Board can be found below, and biographical details of the directors can be found on pages 82 to 85 and are deemed to be incorporated into this report. Descriptions of the terms of the directors’ service contracts are set forth on page 110 and page 117 of this report. All current directors shall retire from office and will offer themselves for reappointment by the members at the Company’s upcoming AGM. Details of the interests of directors in the share capital of the Company as of December 31, 2024 are set out in the Annual Report on Remuneration on page 116 and Note 26 to the financial statements, located on page 172. There have been no changes in such interests from December 31, 2024 to March 31, 2025, except as specifically set forth in those sections. Results and dividends We generated a gain for the year ended December 31, 2024 of $27.8 million (2023: Loss of $66.6 million). The Directors do not recommend the payment of a dividend for the year ended December 31, 2024 (2023: nil). Share capital As of December 31, 2024, the ordinary issued share capital of the Company stood at 257,927,489 shares of £0.01 each, including shares issuable upon conversion of outstanding ADSs, with 17,738,040 shares held in treasury by the Company. Details on share capital are set out in Note 16 to the financial statements, page 160. Directors’ Report for the year ended December 31, 2024

94 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Articles of Association The Articles of Association of the Company can only be amended by special resolution at a general meeting of the shareholders. No amendments are proposed at the 2025 AGM. The following have served as Directors of the Company during the 2024 financial year. Name Role Age (as of December 31, 2024) Dr. Raju Kucherlapati Chair, Independent Non-Executive Director 81 Dr. Bharatt Chowrira Chief Executive Officer 59 Dr. Michele Holcomb Independent Non-Executive Director 56 Dr. Robert Langer Non-Executive Director 76 Dr. John LaMattina Senior Independent Non-Executive Director 74 Ms. Kiran Mazumdar-Shaw Independent Non-Executive Director 71 Ms. Sharon Barber-Lui Independent Non-Executive Director 51 Ms. Daphne Zohar Former Chief Executive Officer (departed the Board in April 2024) 54 The Directors consider that the Company has, throughout the year ended December 31, 2024, applied the main principles and complied with the provisions set out in the Governance Code with the following exceptions: — Dr. Raju Kucherlapati, the Chair, is also Chair of the Nomination Committee, which is not aligned with provision 17 of the Governance Code. In making the determination for maintaining Dr. Kucherlapati as Chair of the Nomination Committee the Board duly considered (i) his professional background (ii) his tenure on the Board and experience. The Board deemed this to be relevant experience making his role as Chair of Committee in the best interest of the Company’s shareholders. — Dr. Raju Kucherlapati, served as Senior Independent Director while acting as Chair which is not aligned with provision 12 of the Governance Code. The Company has since remedied this position by the appointment of Dr. John LaMattina the role of Senior Independent Director in April 2025. Further explanation as to how the provisions set out in the Governance Code have been applied by the Company is provided in this Report, the Report of the Nomination Committee and the Report of the Audit Committee. Financial instruments The financial risk management and internal control processes and policies, and exposure to the risks associated with financial instruments can be found in Note 18 to the financial statements and the Corporate Governance section of the Annual Report on page 100. Sustainable development and environmental matters Details of the Company’s policies and performance, as well as disclosures concerning GHG emissions, are provided in the ESG Report on pages 22 to 58. Directors’ liabilities (Directors’ indemnities) As at the date of this report, the Company has granted qualifying third party indemnities to each of its Directors against any liability that attaches to them in defending proceedings brought against them, to the extent permitted by the Companies Act. In addition, Directors and officers of the Company and its controlled-Founded Entities have been and continue to be covered by Directors’ and officers’ liability insurance. See further description of indemnity and insurance on page 88. Political donations No political contributions/donations for political purposes were made by the Company or any of our affiliate companies to any political party, politician, elected official or candidate for public office during the financial year ended December 31, 2024 (2023: nil). Significant agreements There are no agreements between the Company or any of our affiliate companies and any of its employees or any Director which provide for compensation to be paid to an employee or a Director for loss of office as a consequence of a takeover of the Company. Compliance with the UK Corporate Governance Code The Directors are committed to a high standard of corporate governance and compliance with the best practice of the UK Corporate Governance Code (Governance Code) published in July 2018. The Governance Code is available at the Financial Reporting Council website at www.frc.org.uk. Directors’ Report for the year ended December 31, 2024 continued

PureTech Health plc Annual Report and Accounts 2024 95 G overnance were purchased by the company and held as treasury shares. Such treasury shares do not receive dividend rights and may not exercise voting rights. Future business developments Information on the Company and its Wholly-Owned Programs and Founded Entities’ future developments can be found in the Strategic Report on pages 11 to 21. Risk and internal controls The principal risks we face are set out on pages 60 to 64 and in the Additional Information section from pages 182 to 216. The Audit Committee’s assessment of internal controls is laid out on page 95. Subsequent Events Information related to events occurring after December 31, 2024, can be found in footnote 28 to the consolidated financial statements. Research and Development Information on our research and development activities can be found in the Strategic Report on pages 11 to 13. Going concern As of December 31, 2024, the directors had a reasonable expectation that we had adequate resources to continue in operational existence into 2027. Annual General Meeting The Notice of the AGM, which will be held at 11:00 am EDT (4:00 pm BST) on June 16, 2025, at the Company’s corporate headquarters at 6 Tide Street, in Boston, Massachusetts, U.S. is enclosed with this report. Details of the resolutions and the explanatory notes thereto are included with the Notice. To ensure compliance with the Governance Code, the Board proposes separate resolutions for each issue and proxy forms allow shareholders who are unable to attend the AGM to vote for or against or to withhold their vote on each resolution. In addition, to encourage shareholders to participate in the AGM process, the Company proposes to offer electronic proxy voting through the Registrar’s website and through the CREST service. The results of all proxy voting will be published on our website after the AGM. The Notice of the Meeting, together with an explanation of the items of business, will be contained in a circular to shareholders to be dated April 30, 2025. Pension schemes Information on the Company’s 401K Plan can be found in the Annual Report on Remuneration on page 106. Related party transactions Details of related party transactions can be found in Note 26 of the financial statements on pages 171 to 172. Tender Offer On May 20, 2024, the Company launched a proposed $100 million Tender Offer, which was approved by shareholders on June 4, 2024, with 99.9% of the ordinary shares cast voting in favour of the proposal. The Tender Offer was completed on June 24, 2024, resulting in the delivery and cancellation of 31,540,670 ordinary shares. The Company is pleased with the support shown for the 2024 tender offer, which illustrated the successful execution of the Company’s business model to generate excess cash and our commitment to ongoing evaluations of capital return activities for our shareholders. The Board carefully considered various options for returning cash to shareholders and determined that the tender offer would be the most appropriate means of returning cash to shareholders, allowing return of cash in a quick and efficient manner, taking account of the relative costs, complexity and timeframes of the possible methods available. In particular, the Tender Offer provided the shareholders participating in the tender opportunity to sell ordinary shares (including ordinary shares represented by ADSs) at a market-driven price with a premium as at the latest practicable date while also enabling those shareholders who did not wish to participate to maintain their full investment in the Company. The Tender Offer was available to all shareholders regardless of the size of their shareholdings and allowed the Company to broaden the scope of the return of capital to include ordinary shares held by those shareholders whose ordinary shares (including ordinary shares represented by ADSs) may not have been purchased by the Company through a share purchase programme. Share buyback At the 2023 AGM and the 2024 AGM, shareholders gave the Company authority to purchase shares from the market up to an amount equal to 10% of the Company’s issued share capital at that time. On May 9, 2022, the Company commenced a $50 million Share Buyback Programme. The Company executed the Programme in two equal tranches, the first of which was completed on October 26, 2022, and the second which was completed on February 7, 2024. Between May 9, 2022, and February 7, 2024, the Company repurchased an aggregate of 20,182,863 ordinary shares under the Share Buyback Programme, which represents approximately 7% of the Company’s issued share capital at the time the programme commenced. The authority granted from the 2023 AGM expired as of the end of the 2024 AGM, and the authority from the 2024 AGM expires as of the earlier of the end of the 2025 AGM or close of business on 15 September 2025. During 2024, 1,903,990 ordinary shares Directors’ Report for the year ended December 31, 2024 continued

96 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Disclosure of information under Listing Rule 9.8.4R For the purposes of LR 9.8.4R, the information required to be disclosed can be found in the sections of the Annual Report and Financial Statements listed in the table below. Listing Rule Requirement Location in Annual Report A statement of the amount of interest capitalized during the period under review and details of any related tax relief. N/A Information required in relation to the publication of unaudited financial information. N/A Details of any long-term incentive schemes. Directors’ Remuneration Report, page 102 Details of any arrangements under which a Director has waived emoluments, or agreed to waive any future emoluments, from the Company. N/A Details of any non-pre-emptive issues of equity for cash. N/A Details of any non-pre-emptive issues of equity for cash by any unlisted major subsidiary undertaking. Directors’ Report, page 93 Details of parent participation in a placing by a listed subsidiary. N/A Details of any contract of significance in which a Director is or was materially interested. N/A Details of any contract of significance between the Company (or one of its subsidiaries) and a controlling shareholder. N/A Details of any provision of services by a controlling shareholder. N/A Details of waiver of dividends or future dividends by a shareholder. N/A Where a shareholder has agreed to waive dividends, details of such waiver, together with those relating to dividends which are payable during the period under review. N/A Board statements in respect of relationship agreement with the controlling shareholder. N/A Disclosure of information to auditor The Directors who held office at the date of approval of this Directors’ report confirm that: — so far as the Director is aware, there is no relevant audit information of which the Company’s Auditor is unaware; and — the Director has taken all steps that he/she ought to have taken as a Director in order to make himself/herself aware of any relevant audit information and to establish that the Company’s Auditor is aware of that information. This confirmation is given and should be interpreted in accordance with the provisions of Section 418 of the CA 2006. Whistleblowing, anti-bribery and corruption We seek at all times to conduct our business with the highest standards of integrity and honesty. We also have an anti- bribery and corruption policy which prohibits our employees from engaging in bribery or any other form of corruption. In addition, we have a whistleblowing policy under which staff are encouraged to report to the Chief Executive Officer or the President any alleged wrongdoing, breach of a legal obligation or improper conduct by or on the part of us or any of our officers, Directors, employees, consultants or advisors. In the event of a communication to the Executive Directors or others, including via the Company’s Whistleblower hotline, pursuant to these policies, this information will be shared with the Audit Committee who will evaluate the claims and in turn report to the rest of the Board. Directors’ Report for the year ended December 31, 2024 continued

PureTech Health plc Annual Report and Accounts 2024 97 G overnance Under applicable law and regulations, the Directors are also responsible for preparing a Strategic Report, Directors’ Report, Directors’ Remuneration Report and Corporate Governance Statement that complies with that law and those regulations. The Directors are responsible for the maintenance and integrity of the corporate and financial information included on the Company’s website. Legislation in the UK governing the preparation and dissemination of financial statements may differ from legislation in other jurisdictions. Responsibility statement of the Directors in respect of the annual financial report We confirm that to the best of our knowledge: — the financial statements, prepared in accordance with the applicable set of accounting standards, give a true and fair view of the assets, liabilities, financial position and profit or loss of the Company and the undertakings included in the consolidation taken as a whole; and — the strategic report includes a fair review of the development and performance of the business and the position of the issuer and the undertakings included in the consolidation taken as a whole, together with a description of the principal risks and uncertainties that they face. We consider the annual report and accounts, taken as a whole, is fair, balanced and understandable and provides the information necessary for shareholders to assess the Group’s position and performance, business model and strategy. By Order of the Board Bharatt Chowrira, Ph.D., J.D. Chief Executive Officer and Director April 30, 2025 Statement of Directors’ responsibilities in respect of the Annual Report and the financial statements The Directors are responsible for preparing the Annual Report and the Group and parent Company financial statements in accordance with applicable law and regulations. Company law requires the directors to prepare Group and parent Company financial statements for each financial year. Under that law they are required to prepare the Group financial statements in accordance with UK-adopted international accounting standards and applicable law and have elected to prepare the parent Company financial statements on the same basis. In addition, the Group financial statements are required under the UK Disclosure Guidance and Transparency Rules to be prepared in accordance with the UK-adopted international accounting standards. Under Company law the Directors must not approve the financial statements unless they are satisfied that they give a true and fair view of the state of affairs of the Group and parent Company and of the Group’s profit or loss for that period. In preparing each of the Group and parent Company financial statements, the directors are required to: — select suitable accounting policies and then apply them consistently; — make judgements and estimates that are reasonable, relevant and reliable; — state whether they have been prepared in accordance with the UK-adopted international accounting standards; — assess the Group and parent Company’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern; and — use the going concern basis of accounting unless they either intend to liquidate the Group or the parent Company or to cease operations, or have no realistic alternative but to do so. The Directors are responsible for keeping adequate accounting records that are sufficient to show and explain the parent Company’s transactions and disclose with reasonable accuracy at any time the financial position of the parent Company and enable them to ensure that its financial statements comply with the Companies Act 2006. They are responsible for such internal control as they determine is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error, and have general responsibility for taking such steps as are reasonably open to them to safeguard the assets of the Group and to prevent and detect fraud and other irregularities. Directors’ Report for the year ended December 31, 2024 continued

98 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e After such consideration, the Board has determined Dr. Langer and Ms. Mazumdar-Shaw to be independent in character and judgement and free from relationships or circumstances which might affect, or appear to affect, the Directors’ judgement in their service on the Nomination Committee. While the Board has not deemed Dr. Langer independent for the purposes of overall Board composition, he is independent in the context of his service on the Nomination Committee. The Board duly considered (i) his involvement in other Founded Entities and (ii) the exceptional circumstance that Dr. Langer is a founding Director of the Company. The Nomination Committee meets as required to initiate the selection process of, and make recommendations to, the Board with regard to the appointment of new Directors. During 2024, the Nomination Committee met one time to review the structure, size and composition of the Board in light of the requirements of the Governance Code. Dr. Kucherlapati and Dr. Langer participated in the meeting, and Ms. Mazumdar-Shaw was unable to attend. The Chief Executive Officer and the President were invited to and attended the meeting. After conducting a thorough search and internal evaluation, the Nomination Committee made two appointments during the year. Dr. Kucherlapati assumed the role of Chair of the Board on June 20, 2024, after having served as interim Chair since the June 2023. Additionally, Dr. Holcomb, joined the Board as a Non-Executive Director on September 23, 2024. The Committee is pleased with these appointments, and the Company will provide additional updates in due course. Diversity policy Diversity within the Company’s Board and the Management Team is essential in maximizing its effectiveness, as it enriches debates, business planning and problem-solving. The Company approaches diversity in its widest sense so as to recruit and develop the best talent available, based on merit and assessed against objective criteria of skills, knowledge, independence and experience as well as other criteria such as gender, age and ethnicity. This approach is also applied to ensuring diversity within the Board and the Remuneration, Audit and Nomination committees. The Company will adhere to a strategy of recruiting individuals who meet these criteria as it searches for additional independent Non-Executive Directors to the Board, as discussed below. The Committee’s primary objective is to ensure that the Company maintains the strongest possible leadership across both the Board and the Management Team. Information regarding the Company’s diversity efforts can be found in the ESG Report on pages 22 to 58. Board and Committee evaluation Information regarding the evaluation of the Board and its Committees can be found on page 90. Committee responsibilities The Nomination Committee assists the Board in discharging its responsibilities relating to the composition and make-up of the Board and any Committees of the Board. It is also responsible for periodically reviewing the Board’s structure and identifying potential candidates to be appointed as Directors or Committee members as the need may arise. The Nomination Committee is responsible for evaluating the balance of skills, knowledge and experience and the size, structure and composition of the Board and Committees of the Board, retirements and appointments of additional and replacement Directors and Committee members, and makes appropriate recommendations to the Board on such matters. A full copy of the Committee’s Terms of Reference is available on request from the Company Secretary and within the Investor’s section on Company’s website at www. puretechhealth.com. Committee membership The Nomination Committee consisted of Dr. Raju Kucherlapati, who served as the committee’s Chair, Dr. Robert Langer, and Ms. Kiran Mazumdar-Shaw during 2024. The biographies of the Nomination Committee members can be found on pages 82 to 83. The Governance Code requires that a majority of the members of a nomination committee should be independent Non- Executive Directors. In making their determination for the year 2024, the Board regarded Dr. Raju Kucherlapati, Dr. Langer and Ms. Mazumdar- Shaw as meeting the independence criteria set out in the Governance Code as it is applied to their service on the Nomination Committee. In reaching this determination, the Board duly considered (i) their directorships and links with other Directors through their involvement in other Founded Entities; (ii) their equity interests in PureTech Health and/or the Founded Entities. The Board also duly considered the extent to which these matters may impact their service on the Nomination Committee. Report of the Nomination Committee Raju Kucherlapati, Ph.D. Chair, Nomination Committee

PureTech Health plc Annual Report and Accounts 2024 99 G overnance The Governance Code requires that the audit committee be comprised of independent Non-Executive Directors, with the chair of the Board refraining from serving on the Committee. In making the independence determination for the Chair, Dr. Kucherlapati prior to Dr. Holcomb joining the Board and the Committeee, the Board considered his (i) his prior service on the Board (ii) relevant leadership positions within the sector and (iii) ongoing progress towards adding a new non-executive director with relevant audit committee experience. The Board deemed this to be recent and relevant financial experience, qualifying Dr. Kucherlapati to serve on the Committee. Ms. Barber-Lui has served as Chair of the Committee since April 26, 2022. Ms. Barber-Lui has experience as a Chartered Accountant and has held numerous senior executive positions in her career. The Board has deemed this to be recent and relevant financial experience, qualifying her to be Chair of the Committee. Ms. Barber-Lui has accounting experience, is currently the Chief Financial Officer and Senior Vice President, North America at Teva Pharmaceutical Industries Ltd., a publicly-traded Israeli company (NYSE and TASE: TEVA), and has held a number of senior finance and executive leadership positions in her career. The Board has deemed this to be recent and relevant financial experience qualifying her to be Chair of the Committee. Both Dr. LaMattina, and Dr. Kucherlapati prior to Dr. Holcomb replacing him on the committee, have also been deemed to have recent and relevant financial experience qualifying them to serve on the Committee. The Board based this determination based on (i) their numerous senior leadership positions and (ii) their competence in the sector in which the company operates. The biographies of the Committee members can be found on pages 82 to 83. The Committee met three times during the year, with Ms. Barber- Lui and Dr. LaMattina each attending all three meetings, Dr. Kucherlapati attending two meetings prior to his departure from the Committee and Dr. Holcomb attending one meeting after replacing Dr. Kucherlapati on the Committee. In 2024, the Chief Executive Officer was invited to and attended all of the meetings, and the President attended two of the meetings. The Auditor was invited to and attended all three of the meetings. When appropriate, the Committee met with the Auditor without any members of the executive management team being present. Activities during the year During the year, the Committee also undertook the normal recurring items, the most important of which are noted below. Committee responsibilities The Audit Committee monitors the integrity of our financial statements and reviews all proposed annual and half-yearly results announcements to be made by us with consideration being given to any significant financial reporting judgements contained in them. The Committee also advises the Board on whether it believes the annual report and accounts, taken as a whole, are fair, balanced and understandable and provide the information necessary for shareholders to assess the Company’s position and performance, business model and strategy. The Committee also considers internal controls and has complied with the provisions of the Competition and Markets Authority Order. Additionally we are in compliance with legal requirements, including the provisions of the, FCA’s Listing Rules, Disclosure Guidance and Transparency Rules, and reviews any recommendations from the Group’s Auditor regarding improvements to internal controls and the adequacy of resources within our finance function. A full copy of the Committee’s Terms of Reference is available on request from the Company Secretary and within the Investor’s section on the Company’s website at www.puretechhealth.com. Committee membership The Committee consists of three independent Non-Executive Directors, Ms. Sharon Barber-Lui, Dr. Michele Holcomb and Dr. John LaMattina. During 2024, Dr. Kucherlapati also served as of member of the Committee prior to Dr. Holcomb joining the Board on September 23, 2024. Report of the Audit Committee Ms. Sharon Barber-Lui Chair, Audit Committee

100 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e The main driver of the year-over-year change was activities related to the deconsolidation and new investment in Seaport. We considered the underlying economics of the valuations and sought external expertise in determining the appropriate valuation of the financial investments. These valuations rely, in large part, on the capital structure, values of recent transactions and market movement. These values also determine the amount of gain (loss) on the financial instruments. The Committee believes that we considered the pertinent terms and underlying economics of each of the financial instruments, as well as the advice of external experts, and as such concluded that the financial Instruments were appropriately recorded. Regulatory compliance Ensuring compliance for FCA regulated businesses also represents an important control risk from the perspective of the Committee. We engage with outside counsel and other advisors on a regular basis to ensure compliance with legal requirements. Review of Annual Report and Accounts and Half- yearly Report The Committee carried out a thorough review of our 2024 Annual Report and Accounts and our 2024 Half-yearly Report resulting in the recommendation of both for approval by the Board. In carrying out its review, the Committee gave particular consideration to whether the Annual Report, taken as a whole, was fair, balanced and understandable, concluding that it was. It did this primarily through consideration of the reporting of our business model and strategy, the competitive landscape in which it operates, the significant risks it faces, the progress made against its strategic objectives and the progress made by, and changes in fair value of, its Founded Entities during the year. Going concern At least annually, the Committee considers the going concern principle on which the financial statements are prepared. As a business which seeks to fund the development of its Wholly- Owned Programs, as well as support its Founded Entities with further capital, the business model is currently inherently cash consuming. As of December 31, 2024, we had sufficient funding to extend operations into 2027 based on the Company’s strategic operating plan. Therefore, while an inability of the Wholly-Owned Programs and Founded Entities to raise funds through equity financings with outside investors, strategic arrangements, licensing deals or debt facilities may require us to modify our level of capital deployment into our Wholly-Owned Programs and Founded Entities or to more actively seek to monetize one or more Founded Entities, it would not threaten our ability to continue as a going concern. Significant issues considered in relation to the financial statements The Committee considered, in conjunction with management and the external auditor, the significant areas of estimation, judgement and possible error in preparing the financial statements and disclosures, discussed how these were addressed and approved the conclusions of this work. The principal areas of focus in this regard were the deconsolidation of Seaport Therapeutics, and valuation of Level 3 financial instruments, including those related to Seaport Therapeutics, Vedanta Biosciences and Sonde Health, as well as the valuation of the investement in the subsidiary companies within the Parent Company financial statements. Accounting treatment for the sale of future royalties An area of judgment in our financial statements and, therefore audit risk, relates to the determination of the appropriate accounting treatment for the Royalty Agreement. In order to determine the amortized cost of the sale of future royalties liability, management is required to estimate the total amount of future receipts from and payments to Royalty Pharma under the Royalty Purchase Agreement over the life of the agreement. At year end, this analysis resulted in a sale of future royalty liability of $143.2 million (2023 – $110.2 million). We considered the pertinent terms and underlying economics of the agreement in determining the appropriate accounting treatment. Valuation of investment in subsidiary companies An area of judgement in our financial statements and, therefore audit risk, relates to the valuation of investment in subsidiary companies which at year end had a carrying value totaling $462.7 million (2023 – $456.9 million). The main driver of the year-over-year change in risk is a result of the carrying amount of the net assets of the parent company exceeding the implied market capitalisation at various points throughout the year that constituted an impairment trigger. As of December 31, 2024, an impairment assessment of the investment in subsidiaries was conducted using the fair value less costs to sell method. The carrying amount of the investment in subsidiaries was approximately 1% lower than the implied market capitalization. After applying an estimated control premium, it was determined that the investment in subsidiaries was not impaired as of December 31, 2024. The Committee believes that the application of a control premium and the level of premium applied are reasonable and thus concluded that the investment in subsidiaries was appropriately recorded. Valuation of financial instruments An area of judgement in our financial statements and, therefore audit risk, relates to the valuation of investments held at fair value that do not have a quoted active market price which at year end had a carrying value totaling $177 million (2023 – $25 million). Report of the Audit Committee continued

PureTech Health plc Annual Report and Accounts 2024 101 G overnance External audit We have engaged PricewaterhouseCoopers LLP (UK) as our Auditor since 2023. The current audit partner is Sam Taylor who has been our audit partner since June 2023. The effectiveness of the external audit process is dependent on appropriate risk identification. In November 2024, the Committee discussed the Auditor’s audit plan for 2024. This included a summary of the proposed audit scope and a summary of what the Auditor considered to be the most significant financial reporting risks facing us together with the Auditor’s proposed audit approach to these significant risk areas. The main areas of audit focus for the year were (a) Deconsolidation of Founded Entities and (b) Valuation of financial instruments. Appointment and independence The Committee advises the Board on the appointment of the external Auditor and on its remuneration both for audit and non-audit work, and discusses the nature, scope and results of the audit with the external Auditor. The Committee keeps under review the cost-effectiveness and the independence and objectivity of the external Auditor. Controls in place to ensure this include monitoring the independence and effectiveness of the audit, a policy on the engagement of the external Auditor to supply non-audit services, and a review of the scope of the audit and fee and performance of the external Auditor. Non-audit work The Committee approves all fees paid to the Auditor for non-audit work. Where appropriate, the Committee sanctions the use of PricewaterhouseCoopers LLP for non-audit services in accordance with our non-audit services policy. With the exception of fees paid in connection with access to the firm’s accounting research and disclosure database and fees in respect of the auditors’ review of the Group’s interim financial statements, there were no non-audit fees received by PwC in 2024. The non-audit fees policy is compliant with ethical Standards for Auditors. In 2024, PwC received total fees of $2.8 million (2023: $2.7 million). Fees paid to PwC are set out in note 9 to the financial statements. The Committee is satisfied with the independence of PricewaterhouseCoopers. Sharon Barber-Lui Chair of Audit Committee April 30, 2025 Compliance The Committee has had a role in supporting our compliance with the Governance Code, which applies to us for the 2024 financial year. The Board has included a statement regarding our longer-term viability on page 65. The Committee worked with management and assessed that there is a robust process in place to support the statement made by the Board. Similarly, the Committee worked with management to ensure that the current processes underpinning its oversight of internal controls provide appropriate support for the Board’s statement on the effectiveness of risk management and internal controls. Risk and internal controls The principal risks we face are set out on pages 60 to 64 and in the Additional Information section from pages 182 to 216. The Committee has directed that management engage in a continuous process to review internal controls around financial reporting and safeguarding of assets. Management has engaged external advisors to complete internal control testing on behalf of management for the 2024 financial year and the results were presented to the Committee. Based on the above, we have satisfied ourselves that we have adequate controls and that our internal control over financial reporting is effective for the year ended December 31, 2024. We have a formal whistleblowing policy. The Committee is satisfied that the policy has been designed to encourage staff to report suspected wrongdoing as soon as possible, to provide staff with guidance on how to raise those concerns, and to ensure staff that they should be able to raise genuine concerns without fear of reprisals, even if they turn out to be mistaken. Internal audit We do not maintain a separate internal audit function. This is principally due to our size, where close control over operations is exercised by a small number of executives. In assessing the need for an internal audit function, the Committee considered the risk assessment performed by management to identify key areas of assurance and the whole system of internal financial and operational controls. The Company achieves internal assurance by performing the risk assessment of the key areas of assurance and maintaining related key internal controls, as well as engaging external advisors to perform internal control testing, as described above. Report of the Audit Committee continued

102 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Committee responsibilities The Remuneration Committee’s primary purpose is to assist the Board in determining the Company’s remuneration policies. The Remuneration Committee has the responsibility for setting the remuneration policy for all Executive Directors and the Chairman of the Company, with the objective of attracting, retaining and motivating executive management. In determining such policy, the Remuneration Committee takes into account all factors which it deems necessary including regulatory requirements, the views of shareholders and stakeholders, the risk appetite of the Company, and alignment to the Company’s long term goals and strategic plan. The Remuneration Committee is also responsible for determining the total individual remuneration package of each Executive Director, including share awards, as well as recommending and monitoring the level and structure of remuneration for senior management, and reviewing the design of all share incentive plans and determining awards under such plans. In carrying out its duties, the Remuneration Committee has regard to current information for remuneration in other companies of comparable scale and complexity and can appoint remuneration consultants to assist in such process. A full copy of the Remuneration Committee’s Terms of Reference is available on request from the Company Secretary and within the Investors section of the Company’s website at www.puretechhealth.com. Committee membership The Remuneration Committee consists of Dr. LaMattina, Dr. Kucherlapati and Ms. Mazumdar-Shaw, with Dr. LaMattina serving as Chair of the Committee. The biographies of the Committee members can be found on pages 82 to 83. The Committee met five times during the year, with Dr. LaMattina and Dr. Kucherlapati in attendance for all five meetings and Ms. Mazumdar-Shaw in attendance for three of the five meetings. The Committee also acted by unanimous written consent three times during the year. During 2024, the Chief Executive Officer and the President were invited to all of the meetings, with Ms. Zohar attending two of the three meetings prior to her resignation from the CEO role, and Dr. Chowrira attending all five meetings, first as President and then as CEO following his appointment to the role in April 2024. However, no Executive Director was permitted to participate in discussions or decisions about their personal remuneration. The Directors’ Remuneration Report is split into three sections, namely: — This Annual Statement: summarizing and explaining the major decisions on Directors’ remuneration in the year; — The Directors’ Remuneration Policy: setting out the framework for remuneration for our Directors on pages 106 to 109; and — The Annual Report on Remuneration: setting out the implementation of the Remuneration Policy in the year ended December 31, 2024 and the continued implementation for the year ending December 31, 2025 on pages 110 to 120. The current Directors’ Remuneration Policy was approved at the June 2024 AGM, and such approval is effective for three years from that date. The Directors’ Remuneration Report (excluding that part of the report containing the Directors’ Remuneration Policy on pages 106 to 109) is subject to a shareholder vote at this year’s AGM. The vote to approve the Directors’ Remuneration Report is advisory only and does not affect the actual historical remuneration paid to any individual Director. Directors’ Remuneration Report for the year ended December 31, 2024 Dr. John LaMattina Chair, Remuneration Committee

PureTech Health plc Annual Report and Accounts 2024 103 G overnance The Committee remains comfortable that our remuneration packages are consistent with the principles of the UK Corporate Governance Code and best practice. The key aims of our Remuneration Policy and the Code principles to which they relate are as follows: — promote our long-term success (Code principle: Proportionality); — attract, retain and motivate high caliber senior management and focus them on the delivery of our long-term strategic and business objectives (Proportionality, alignment to culture and risk); — be simple and understandable, both externally and internally (Clarity, simplicity, predictability and proportionality); — achieve consistency of approach across senior management to the extent appropriate and informed by relevant market benchmarks (Clarity and alignment to culture); and — encourage widespread equity ownership across the executive team to ensure a long-term focus and alignment of interest with shareholders (Alignment to culture, risk). A summary of the 2024 Directors’ Remuneration Policy is set out on pages 106 to 109. Our Remuneration Policy The success of PureTech depends on the motivation and retention of our highly skilled workforce with significant expertise across a range of science and technology disciplines, as well as our highly-experienced management team and seasoned Directors. PureTech’s Remuneration Policy is therefore an important part of our business strategy. Our guiding principle is to provide market competitive remuneration packages, including with respect to cash compensation in the form of base salary, annual bonuses and benefits as well as share-based compensation, benchmarked against data generated from our local markets to enable us to put together and retain a top tier team. The Directors’ Remuneration Policy was approved by shareholders at the 2024 AGM with 64.5% support, and the Remuneration Report was approved by shareholders at the 2024 AGM with 56.0% support. As detailed in the Remuneration Policy section of this Report, following these results, the Committee engaged with shareholders to address investor concerns and explain our approach of balancing UK standards on remuneration with practices designed to ensure that PureTech can remain competitive against U.S. peer companies. As part of this shareholder engagement, our Board Chair and Remuneration Committee member, Dr. Kucherlapati, traveled to the UK in 2024 to meet directly with several shareholders. Whilst the Committee recognises that our hybrid approach of offering both performance shares and restricted shares is still somewhat unusual in the context of the UK environment, we believe it is key to enabling us to effectively compete for talent with other companies in the biotech cluster around Boston. We face increasing challenges to retain key people in a market where competitor organisations are offering large equity grants to senior employees without long- term performance conditions, so it is crucial that our Policy allows us to offer similar packages. Many of our larger shareholders understand and recognise the commercial realities within which we operate, although we do appreciate that some investors were not supportive at last year’s AGM. We will continue to consult and engage with shareholders going forward to understand specific issues of concern. Directors’ Remuneration Report continued

104 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Directors’ Remuneration Report continued In relation to the PSP, PureTech’s performance over the last three financial years was very strong in terms of the achievement of strategic objectives despite such performance not translating to growth in the Company’s share price. Overall, the share price declined from an average price of 324 pence during the last three months of 2021 to an average price of 159 pence during the last three months of 2024. Strong strategic performance over the three-year performance period resulted in PSP awards granted to the executive management team in 2022 vesting at a level of 35.3 percent after the end of the 2024 financial year. The Committee considered holistic business performance over the period, as well as the individual contribution of the Executive Director in that time, and determined that no discretion should be exercised in respect of the vesting outcome. Full details of payments to the Executive Director in 2024 can be found later in this report. The Committee believes the Remuneration Policy operated as intended during the year and that remuneration outcomes are appropriate, taking into account outcomes throughout the business, company and individual performance and the stakeholder experience. The year ahead For 2025, the following key decisions have been made in relation to how the Policy will be implemented: — Base salary for the Executive Director was increased by 5.5 percent, which is consistent with the midpoint of the general range of increases for the workforce of between 4.0 and 7.0 percent. The Committee is comfortable that the increase awarded to the Executive Director is appropriate, taking into consideration a number of factors and with particular regard to the retention of top talent as part of the continued advancement of the Company’s Wholly- Owned Programs. — The annual bonus target and maximum will remain at 50 percent and 100 percent of base salary respectively for the Chief Executive Officer. — The grant of PSP awards in 2025 will remain at the level of 600 percent of base salary for the Chief Executive Officer, in line with the limits as set out in the Policy, with half of the awards granted as performance shares and half as time-vesting restricted shares. — For the performance share element, a mix of performance measures linked to absolute TSR, relative TSR and key strategic metrics which are tied to business progress over the three-year performance period will be retained. For the 2025 award, the weightings will be based 50 percent on TSR and 50 percent on strategic metrics, the same approach as adopted in 2024. Performance and reward in 2024 During 2024, PureTech delivered strong execution and achievement of key strategic and financial goals, which has been reflected in the annual bonus outcome. The Company delivered substantial growth and generated momentum to support future growth in the coming years as our balance sheet, Founded Entities equity and royalty stakes, and Wholly-Owned Programs position PureTech with the strength to build substantial value for shareholders in the current environment. This growth is due in large part to (i) significant development and advancement of our Wholly-Owned Programs and activities initiated or progressed to potentially bring these innovative therapies to market, (ii) key support provided to the Founded Entities as their businesses progress and, in certain cases, execution of key transactions or financings, (iii) substantial development and expansion of the Company’s intellectual property portfolio, and (iv) completion of various strategic sourcing and strategic planning initiatives with the forward looking goal to enhance shareholder value. This increase in value, together with management’s operational performance at PureTech and within Wholly-Owned Programs and Founded Entities, resulted in the Remuneration Committee approving an outcome of 128% of the target performance goals based on pre-specified targets. In line with our standard approach, the Committee then reviewed the overall performance of the Company and the individual Executive Director before determining the final bonus payout. The Committee considered operational performance, the overall growth of the business during the year, the extent to which the target performance goals had in some cases been exceeded, and the individual contribution of the Executive Director. The Committee focused on the remarkable LYT-100 Phase 2B clinical trial data results as an exceptional achievement, and also considered at length the successful activities of certain Founded Entities and the value created for PureTech, especially the successful spin-off and Series A and Series B financing transactions for Seaport Therapeutics, which raised over $325 million during the year. See further details of our performance highlights in 2024 on pages 1 to 5. Following this exercise, the Committee determined that no discretion should be exercised and that a bonus equal to 64% of base salary (representing 128% of target) was to be awarded to the Executive Director.

PureTech Health plc Annual Report and Accounts 2024 105 G overnance Directors’ Remuneration Report continued Remuneration for other Colleagues In addition to matters relating to Executive Director remuneration, the Committee also reviews the compensation policies for the wider employee base, with a particular focus on the use of equity compensation throughout the whole organization. PureTech grants its employees awards of performance shares and restricted shares as well as market-value stock options, helping to ensure a degree of competitiveness against other U.S. companies operating in the same sector. Following shareholder approval of the new Performance Share Plan in 2023, we have greater flexibility in operating the plan given the dilution limits agreed at the time. Closing comments The Committee is comfortable that the operation of the Policy for 2024 has demonstrated a robust link between performance and reward given the successes recorded during the year. The Committee believes the Remuneration Policy, and the proposed operation of the Policy for 2025, is appropriate and continues to strike a suitable balance between UK investor expectations and the realities of operating in a competitive U.S. market. The Committee looks forward to shareholders’ support at the 2025 Annual General Meeting for the advisory resolution covering this Annual Statement and the Annual Report on Remuneration. Non-Executive Director compensation Currently, Non-Executive Directors receive a mixture of cash and ordinary shares in PureTech. The fee levels payable to date have been significantly below the levels typically payable for experienced Non-Executive Directors at U.S. companies in our sector. During 2024, we reviewed the level of cash compensation to ensure that it was consistent with the very significant contributions made by the Non-Executive Directors. Based on this review, we approved the award of annual payments of $25,000 to Non-Executive Directors serving on the Transaction and R&D Committees. These payments comply with the 2024 Remuneration Policy, and are appropriate in light of the significant time commitment required to serve on these committees. Full details of these payments are set out on page 112 of the Directors’ Remuneration Report. For 2025, the equity portion of fees for Non-Executive Directors remains at a maximum of $150,000. Levels of base salary cash compensation for each Non-Executive Director will remain at $75,000, with additional cash compensation tied to director service on the principal committees, and the Transaction and R&D Committees. A further review of the cash element will be undertaken later this year in the interests of ensuring ongoing competitiveness. We retain the flexibility to grant a portion of the equity part of the fees for Non-Executive Directors in the form of equity in subsidiary entities in which PureTech has a controlling interest, including Founded Entities. This provides a cash-efficient way for Director pay to become competitive, while enabling Directors to be directly aligned with the success of subsidiary companies as well as with PureTech as a whole. As you will appreciate, in some cases individual Directors have been instrumental in Board deliberations on subsidiary company matters (including those relating to our Founded Entities) and this is one way in which this can be recognized.
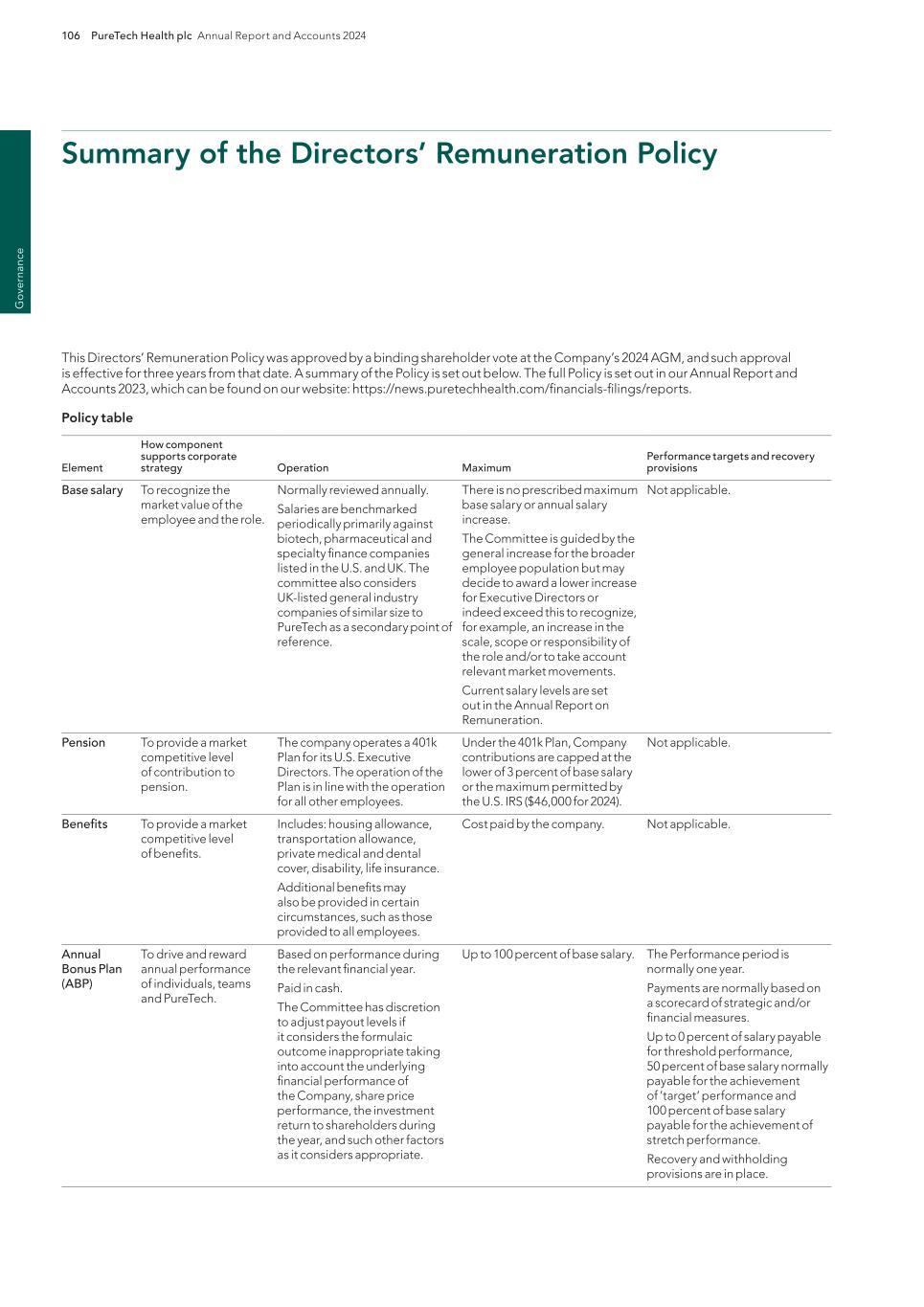
106 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e This Directors’ Remuneration Policy was approved by a binding shareholder vote at the Company’s 2024 AGM, and such approval is effective for three years from that date. A summary of the Policy is set out below. The full Policy is set out in our Annual Report and Accounts 2023, which can be found on our website: https://news.puretechhealth.com/financials-filings/reports. Policy table Element How component supports corporate strategy Operation Maximum Performance targets and recovery provisions Base salary To recognize the market value of the employee and the role. Normally reviewed annually. Salaries are benchmarked periodically primarily against biotech, pharmaceutical and specialty finance companies listed in the U.S. and UK. The committee also considers UK-listed general industry companies of similar size to PureTech as a secondary point of reference. There is no prescribed maximum base salary or annual salary increase. The Committee is guided by the general increase for the broader employee population but may decide to award a lower increase for Executive Directors or indeed exceed this to recognize, for example, an increase in the scale, scope or responsibility of the role and/or to take account relevant market movements. Current salary levels are set out in the Annual Report on Remuneration. Not applicable. Pension To provide a market competitive level of contribution to pension. The company operates a 401k Plan for its U.S. Executive Directors. The operation of the Plan is in line with the operation for all other employees. Under the 401k Plan, Company contributions are capped at the lower of 3 percent of base salary or the maximum permitted by the U.S. IRS ($46,000 for 2024). Not applicable. Benefits To provide a market competitive level of benefits. Includes: housing allowance, transportation allowance, private medical and dental cover, disability, life insurance. Additional benefits may also be provided in certain circumstances, such as those provided to all employees. Cost paid by the company. Not applicable. Annual Bonus Plan (ABP) To drive and reward annual performance of individuals, teams and PureTech. Based on performance during the relevant financial year. Paid in cash. The Committee has discretion to adjust payout levels if it considers the formulaic outcome inappropriate taking into account the underlying financial performance of the Company, share price performance, the investment return to shareholders during the year, and such other factors as it considers appropriate. Up to 100 percent of base salary. The Performance period is normally one year. Payments are normally based on a scorecard of strategic and/or financial measures. Up to 0 percent of salary payable for threshold performance, 50 percent of base salary normally payable for the achievement of ’target’ performance and 100 percent of base salary payable for the achievement of stretch performance. Recovery and withholding provisions are in place. Summary of the Directors’ Remuneration Policy

PureTech Health plc Annual Report and Accounts 2024 107 G overnance Element How component supports corporate strategy Operation Maximum Performance targets and recovery provisions Long-term incentives To drive and reward our sustained performance, promote the retention of the leaders of the business and to align executive interests with those of shareholders. The Company can make long- term incentive awards of either performance shares or time- vesting restricted shares. For performance shares, vesting is dependent on the satisfaction of performance targets and continued service. Performance and vesting periods are normally three years. For time-vesting restricted shares, vesting is dependent on continued service and Remuneration Committee confirmation that Company and individual performance has been satisfactory over the vesting period. Vesting normally takes place in three equal annual tranches over a three-year period following grant. All awards will be subject to a two-year post-vesting holding period during which vested shares cannot be sold other than to settle tax. This post-vesting period continues post-cessation of employment. The Committee also has the discretion to adjust vesting levels of performance-related awards to override formulaic outcomes, taking into account similar factors as apply in relation to annual bonus awards, but by reference to the performance period. For the Chief Executive Officer, 600 percent of base salary. This will normally be split 300 percent of base salary in performance shares and 300 percent of base salary in time-vesting restricted shares. For other Executive Directors, 300 percent of base salary. This will normally be split 150 percent of salary in performance shares and 150 percent in time-vesting restricted shares. Participants may benefit from the value of dividends paid over the vesting period to the extent that awards vest. This benefit is delivered in the form of cash or additional shares at the time that awards vest. For performance shares, the performance period is normally three years. Up to 25 percent of a performance share award vests at threshold performance (0 percent vests below this), increasing to 100 percent pro-rata for maximum performance. Normally at least half of any performance share award will be measured against TSR targets with the remainder measured against relevant financial or strategic measures. Performance conditions are agreed by the Committee on an annual basis. For time-vesting restricted shares, there are no performance conditions other than the requirement for the Remuneration Committee to confirm a satisfactory level of Company and individual performance over the vesting period. Recovery and withholding provisions are in place for both performance and time-vesting restricted shares. Share ownership/ Holding Period Further aligns executives with investors, while encouraging employee share ownership. The Committee requires that Executive Directors who participate in a long-term incentive plan operated by the Company retain half of the net shares vesting under any long-term incentive plan until a shareholding requirement is met. Minimum of 400 percent of base salary for the Chief Executive Officer and a minimum of 200 percent of base salary for the other Executive Directors. None. Summary of the Directors’ Remuneration Policy continued

108 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Element How component supports corporate strategy Operation Maximum Performance targets and recovery provisions Post- cessation holding period Aligns executives with investors and promotes long-term decision making Executive Directors must hold shares for two years after the date of termination of their employment. Lower of (i) 400 percent of base salary for the Chief Executive Officer and 200 percent of base salary for the other Executive Directors and (ii) the Executive Director’s shareholding at the date that notice is served. None. Non- Executive Directors To provide fee levels and structure reflecting time commitments and responsibilities of each role, in line with those provided by similarly- sized companies and companies operating in our sector. Remuneration provided to Non- Executive Directors is operated in line with the terms set out in the Articles of Association. Cash fees, normally paid on a quarterly basis, are comprised of the following elements: – Base fee. – Additional fees. A portion of the compensation to Non-Executive Directors is in the form of PureTech Health plc ordinary shares.Additional remuneration is payable for additional services to PureTech such as the Chairship of a Committee or membership on a Committee. Additional remuneration is also payable for services provided beyond those services traditionally provided as a director , including membership of the Transaction and R&D Committees. Taxable benefits may be provided and may be grossed up where appropriate. Any remuneration provided to a Non-Executive Director will be in line with the limits set out in the Articles of Association. The fee levels of the Non- Executive Directors are reviewed on an annual basis. Subject to the limits set out in the Articles of Association, fees may be increased to reflect changes in responsibility or time commitment, and/or to maintain fees at appropriate levels relative to other companies operating in the sector. None. Notes: 1 In the event that the Company elects any non-U.S. Executive Directors, the 401k Plan may not be an appropriate pension arrangement. In such cases an alternative pension arrangement may be offered. Any such arrangement would not be higher than the pension rate operated for the majority of employees in that jurisdiction. 2 For those below Board level, a lower annual bonus opportunity and equity award size may apply. In general, these differences arise from the development of remuneration arrangements that are market competitive for the various categories of individuals, together with the fact that remuneration of the Executive Directors and senior executives places significant emphasis on performance-related pay. 3 The choice of the performance metrics for the annual bonus scheme reflects the Committee’s belief that incentive compensation should be appropriately challenging and linked to the delivery of the Company’s strategy. Further information on the choice of performance measures and targets is set out in the Annual Report on Remuneration. 4 The performance conditions applicable to the performance shares (see Annual Report on Remuneration) are selected by the Remuneration Committee on the basis that they reward the delivery of long-term returns to shareholders and are consistent with the Company’s objective of delivering superior levels of long-term value to shareholders while providing the Company with tools to successfully recruit and retain employees in the U.S. 5 For the avoidance of doubt, the Company reserves the right to honour any commitments entered into in the past with current or former Directors (such as the vesting/exercise of share awards) notwithstanding that these may not be in line with this Remuneration Policy. Details of any payments to former Directors will be set out in the Annual Report on Remuneration as they arise. Summary of the Directors’ Remuneration Policy continued

PureTech Health plc Annual Report and Accounts 2024 109 G overnance Recovery and withholding provisions Recovery and withholding provisions (’’clawback and malus’’) may be operated at the discretion of the Remuneration Committee in respect of awards granted under the Performance Share Plan and in certain circumstances under the Annual Bonus Plan (including where there has been a material misstatement of accounts, or in the event of fraud, gross misconduct or conduct having a materially detrimental effect on the Company’s reputation). The issue giving rise to the recovery and withholding must be discovered within three years of vesting or payment and there is flexibility to recover overpayments by withholding future incentive payments and recovering the amount directly from the employee. In compliance with U.S. Securities and Exchange Commission reporting and Nasdaq listing standards, effective as of November 8, 2023, the Committee adopted a Policy for Recovery of Erroneously Awarded Compensation. This policy requires that the Remuneration Committee clawback excess incentive compensation from executive officers following a required accounting restatement where, based on the restated financials, executives would have missed the portion of the award tied to a specific financial performance metrics. The policy covers restatements involving the financial measures within the Performance Share Plan and Annual Bonus Plan and is intended to apply in addition to and in concert with the Company’s existing clawback and malus provisions. Service contracts Executive Directors’ service contracts do not provide for liquidated damages, longer periods of notice on a change of control of the Company or additional compensation on an Executive Director’s cessation of employment with us, except as discussed below. The Committee’s Policy is to offer service contracts for Executive Directors with notice periods of no more than 12 months, and typically between 60 to 180 days. Service contracts provide for severance pay following termination in the case that employment is terminated by the Company without ‘cause’, or by the employee for ‘good reason’. In this case severance pay as set out in the contract is no greater than 12-months’ base salary and is aligned to the duration of any restrictive covenants placed on the employee. Service contracts may also provide for the continuation of benefits but for no longer than a 12-month period post termination. Service contracts also provide for the payment of international tax in non-U.S. jurisdictions if applicable to the Executive Director. They also can provide for garden leave and, if required by applicable law, the recovery and withholding of incentive payments. Service contracts are available for inspection at the company’s registered office. Consideration of shareholder views following the 2024 AGM In light of the voting outcome on the remuneration resolutions at the 2024 AGM, the Board has taken steps to understand the views of shareholders. Specifically, the Company wrote to shareholders representing more than 70% of the Company’s issued share capital to offer engagement with the Chair of the Board. Following that initial outreach, meetings were held with shareholders representing more than 50% of issued share capital during the months of July and August 2024. As part of this shareholder engagement, our Board Chair and Remuneration Committee member, Dr. Kucherlapati, traveled to the UK in 2024 to meet directly with several shareholders. While there were a variety of topics discussed during these engagements, there was broad recognition from shareholders regarding the rationale for the changes made to the Remuneration Policy and the implementation of the previous policy, particularly in relation to the challenges the Company faces as a UK-listed business competing for talent in the U.S. where its operations are based. The Board would like to thank the shareholders who have engaged with the Company during this process. The Board will continue to engage openly and constructively with shareholders as it continues to develop the Company’s approach to governance, remuneration and reporting in the periods ahead. The Company will seek to engage directly with major shareholders and their representative bodies should any material changes be proposed to the Remuneration Policy or its implementation. Summary of the Directors’ Remuneration Policy continued

110 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Implementation of the Remuneration Policy for the year ending December 31, 2025 Base salary The Committee reviewed the base salary levels for the Executive Director in early 2025 and an increase of 5.5 percent was awarded. This increase was in line with the midpoint of the general range of increases across the workforce of between 4.0 and 7.0 percent. The Committee is comfortable that this level of increase is appropriate in the context of the need to retain top talent as part of the continued advancement of the Company’s Wholly-Owned Programs. 2024 Base salary 2025 Base salary Bharatt Chowrira* Chief Executive Officer $850,000 $896,750 * Dr. Chowrira’s base salary for 2024 increased upon execution of his new employment agreement to reflect his appointment to Chief Executive Officer from President on April 8, 2024. Due to the timing of his appointment during the year, in 2024 he received a blended base salary totaling $781,008. Pension We will continue to contribute under the 401k Plan subject to the maximum set out in the Policy table. Benefits Benefits provided will continue to include, as appropriate, housing allowance, transportation allowance, private medical, disability and dental coverage. Benefits payments in 2025 to the Executive Director are expected to generally be in-line with those provided in 2024 and will not include amounts for housing allowance or transportation allowance. Annual bonus For 2025, the operation of the annual bonus plan will be similar to the plan’s operation in 2024. The maximum annual bonus will continue to be 100 percent of base salary for the Executive Director with a target annual bonus to be 50 percent of base salary. The 2025 annual bonus will be based on business development goals (including the strategic development of our Wholly-Owned Programs), as well as financial and capital markets based goals. The performance metrics and targets will be disclosed in the FY2025 Annual Report and Accounts given that they are considered commercially sensitive at the current time. Long-term incentives Awards under the PSP will be made to the Executive Director in 2025. The Chief Executive Officer will receive a performance share award with a face value of 300 percent of base salary and a restricted share award with a face value of 300 percent of base salary. The performance share awards will be subject to the performance conditions described below, measured over the three-year period ended 31 December 2027. As a clinical-stage therapeutics company, the Company believes that TSR is an appropriate and objective measure of the Company’s performance. In addition, measuring TSR on both an absolute and relative basis rewards our management team for absolute value creation for our shareholders whilst also incentivizing outperformance of the market. To provide a balance to the TSR performance conditions that is more directly based on Management’s long term strategic performance, TSR is complemented by measures linked to strategic delivery. There will be a robust assessment of the achievement of the strategic targets over the three-year period with full disclosure in the Directors’ Remuneration Report following the end of the performance period. Further detail of the performance conditions is set out below: — 30 percent based on the achievement of absolute TSR targets. — 20 percent based on the achievement of a relative TSR performance condition, 10 percent each against two benchmarks (explained below). — 50 percent based on the achievement of strategic targets. The minimum performance target for the absolute TSR portion of the performance share award will be TSR equal to 10 percent per annum, whilst the maximum target will be TSR equal to 20 percent per annum. Annual Report on Remuneration

PureTech Health plc Annual Report and Accounts 2024 111 G overnance Relative TSR will be measured against the constituent companies of the FTSE 250 Index (excluding Investment Trusts) and the MSCI Europe Health Care Index (each benchmark applying to 10 percent of the performance share award, respectively). The minimum performance target will be achievement of TSR equal to the median company in the Index and the maximum performance target will be achievement of upper quartile TSR performance. 25 percent of each element of the TSR targets will vest for threshold performance. Strategic measures will be based on the achievement of milestones and other qualitative measures of performance over the performance period. Strategic targets will be set at the outset based on development of Wholly-Owned Programs, financial achievements, including monetization of Founded Entities, product pipeline growth, operational excellence, strategic development or transaction related goals and other shareholder value enhancing metrics in line with our strategic plan. Full disclosure of the measures, weightings and strategic targets will be made retrospectively. Any performance shares which vest will be subject to a two-year post-vesting holding period. The restricted shares to be granted to the Executive Director will vest subject to continued employment and a Remuneration Committee assessment that Company and individual performance has been satisfactory. In line with normal practice in the United States, vesting will take place in three equal annual tranches over three years. For each tranche, there will be a two-year post-vesting holding period. Non-Executive Directors Fees for our Board of Directors have been reviewed for 2025. The level of cash compensation is not being increased for 2025 although, as noted in the Annual Statement from the Chair of the Remuneration Committee, a further review will be undertaken later this year in the interests of ensuring ongoing competitiveness. At the 2024 AGM, the Board increased the equity component of compensation from $50,000 to $150,000. At least $50,000 of this amount will continue to be paid in PureTech Health ordinary shares. . FY2025 Chair fee $125,000 Basic fee $75,000 Equity-based Component $150,000 Additional fees: Chair of a committee $10,000 Membership of the Transaction Committee $25,000 Membership of the R&D Committee $25,000 Membership of the Nominating, Governance, and Audit Committees $5,000 Membership of a subsidiary board $0 to $10,000 As our Board of Directors consists of leading experts with the experience of successfully developing technologies and bringing them to market, this gives rise to the possibility that the intellectual property we seek to acquire has been developed by one of our Non-Executive Directors and/or that our Non-Executive Directors provide technical or otherwise specialized advisory services to the Company above and beyond the services typically provided by a Non-Executive Director. In such exceptional circumstances, our Remuneration Policy provides us with the flexibility to remunerate them with equity in the relevant subsidiary company as we would any other inventor of the intellectual property or provider of technical advisory services. This practice is in line with other companies in the life sciences sector. If the Company is unable to offer market-competitive remuneration in these circumstances, it risks forfeiting opportunities to obtain intellectual property developed by our Non-Executive Directors and/or foregoing valuable advisory services. The Company believes foregoing such intellectual property and/or advisory services would not be in the long-term interest of our shareholders. Accordingly, subsidiary equity grants may be made to Non-Executive Directors upon the occurrence of the exceptional circumstances set out above. Annual Report on Remuneration continued
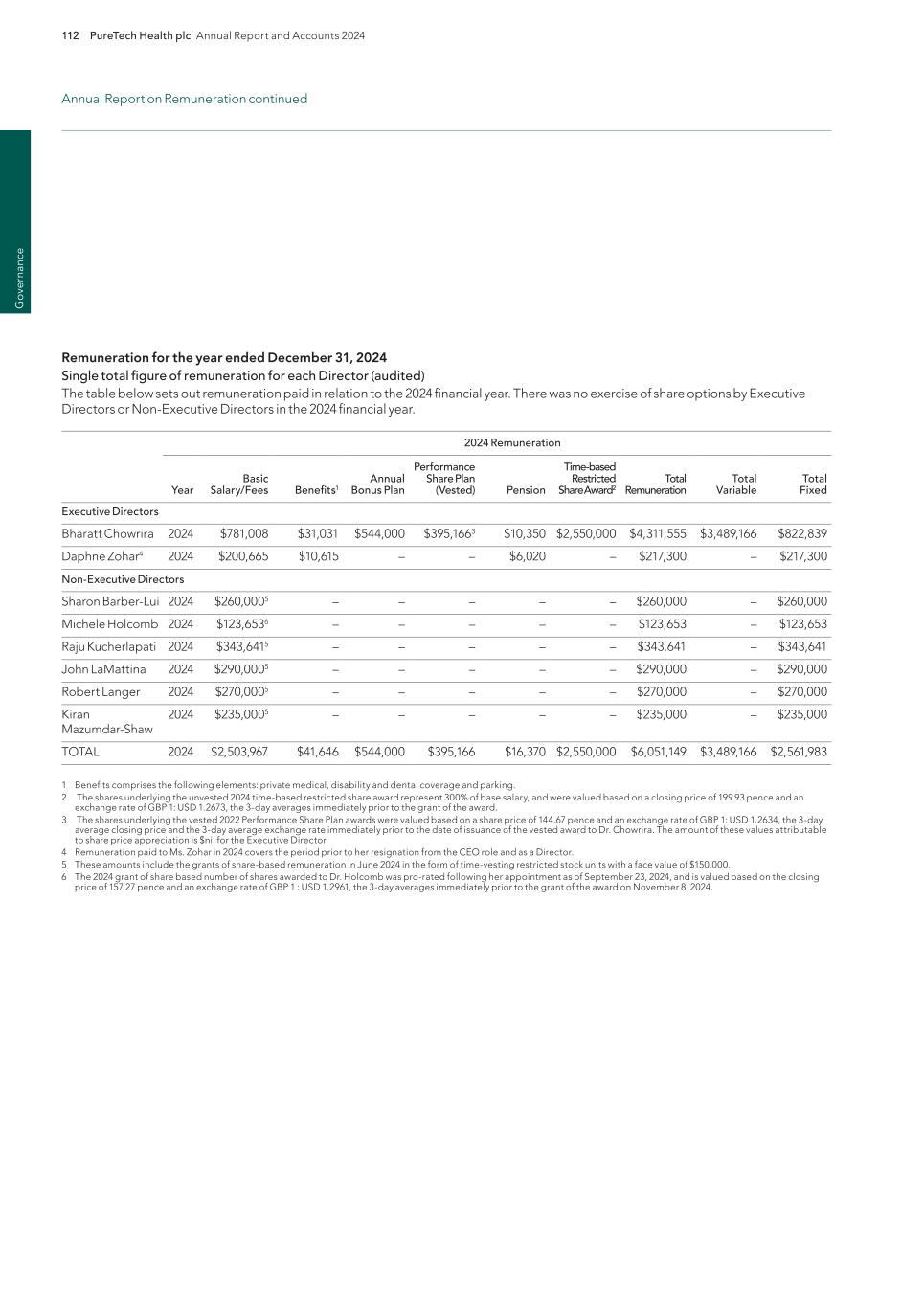
112 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Remuneration for the year ended December 31, 2024 Single total figure of remuneration for each Director (audited) The table below sets out remuneration paid in relation to the 2024 financial year. There was no exercise of share options by Executive Directors or Non-Executive Directors in the 2024 financial year. 2024 Remuneration Year Basic Salary/Fees Benefits1 Annual Bonus Plan Performance Share Plan (Vested) Pension Time-based Restricted Share Award2 Total Remuneration Total Variable Total Fixed Executive Directors Bharatt Chowrira 2024 $781,008 $31,031 $544,000 $395,1663 $10,350 $2,550,000 $4,311,555 $3,489,166 $822,839 Daphne Zohar4 2024 $200,665 $10,615 – – $6,020 – $217,300 – $217,300 Non-Executive Directors Sharon Barber-Lui 2024 $260,0005 – – – – – $260,000 – $260,000 Michele Holcomb 2024 $123,6536 – – – – – $123,653 – $123,653 Raju Kucherlapati 2024 $343,6415 – – – – – $343,641 – $343,641 John LaMattina 2024 $290,0005 – – – – – $290,000 – $290,000 Robert Langer 2024 $270,0005 – – – – – $270,000 – $270,000 Kiran Mazumdar-Shaw 2024 $235,0005 – – – – – $235,000 – $235,000 TOTAL 2024 $2,503,967 $41,646 $544,000 $395,166 $16,370 $2,550,000 $6,051,149 $3,489,166 $2,561,983 1 Benefits comprises the following elements: private medical, disability and dental coverage and parking. 2 The shares underlying the unvested 2024 time-based restricted share award represent 300% of base salary, and were valued based on a closing price of 199.93 pence and an exchange rate of GBP 1: USD 1.2673, the 3-day averages immediately prior to the grant of the award. 3 The shares underlying the vested 2022 Performance Share Plan awards were valued based on a share price of 144.67 pence and an exchange rate of GBP 1: USD 1.2634, the 3-day average closing price and the 3-day average exchange rate immediately prior to the date of issuance of the vested award to Dr. Chowrira. The amount of these values attributable to share price appreciation is $nil for the Executive Director. 4 Remuneration paid to Ms. Zohar in 2024 covers the period prior to her resignation from the CEO role and as a Director. 5 These amounts include the grants of share-based remuneration in June 2024 in the form of time-vesting restricted stock units with a face value of $150,000. 6 The 2024 grant of share based number of shares awarded to Dr. Holcomb was pro-rated following her appointment as of September 23, 2024, and is valued based on the closing price of 157.27 pence and an exchange rate of GBP 1 : USD 1.2961, the 3-day averages immediately prior to the grant of the award on November 8, 2024. Annual Report on Remuneration continued
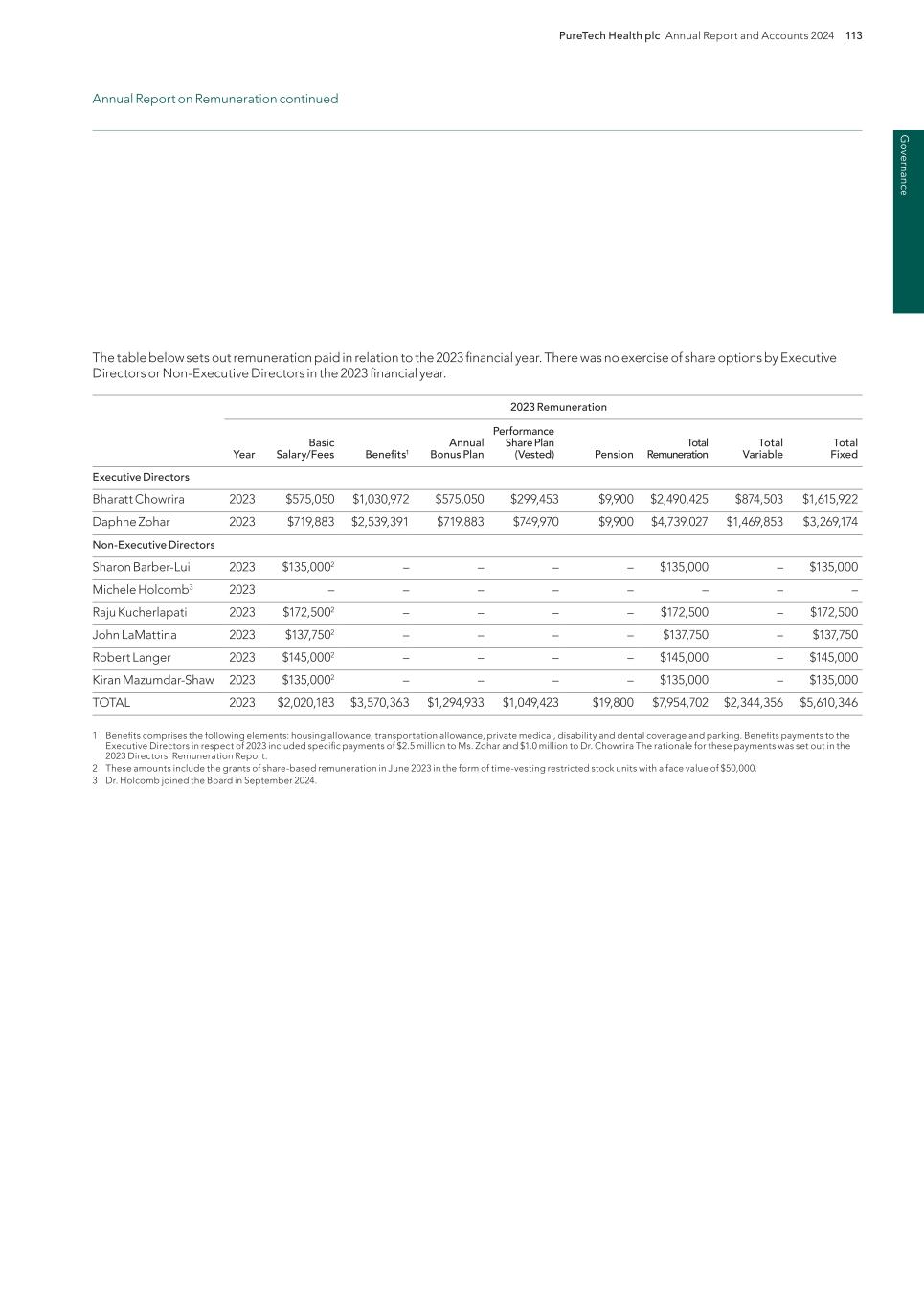
PureTech Health plc Annual Report and Accounts 2024 113 G overnance The table below sets out remuneration paid in relation to the 2023 financial year. There was no exercise of share options by Executive Directors or Non-Executive Directors in the 2023 financial year. 2023 Remuneration Year Basic Salary/Fees Benefits1 Annual Bonus Plan Performance Share Plan (Vested) Pension Total Remuneration Total Variable Total Fixed Executive Directors Bharatt Chowrira 2023 $575,050 $1,030,972 $575,050 $299,453 $9,900 $2,490,425 $874,503 $1,615,922 Daphne Zohar 2023 $719,883 $2,539,391 $719,883 $749,970 $9,900 $4,739,027 $1,469,853 $3,269,174 Non-Executive Directors Sharon Barber-Lui 2023 $135,0002 – – – – $135,000 – $135,000 Michele Holcomb3 2023 – – – – – – – – Raju Kucherlapati 2023 $172,5002 – – – – $172,500 – $172,500 John LaMattina 2023 $137,7502 – – – – $137,750 – $137,750 Robert Langer 2023 $145,0002 – – – – $145,000 – $145,000 Kiran Mazumdar-Shaw 2023 $135,0002 – – – – $135,000 – $135,000 TOTAL 2023 $2,020,183 $3,570,363 $1,294,933 $1,049,423 $19,800 $7,954,702 $2,344,356 $5,610,346 1 Benefits comprises the following elements: housing allowance, transportation allowance, private medical, disability and dental coverage and parking. Benefits payments to the Executive Directors in respect of 2023 included specific payments of $2.5 million to Ms. Zohar and $1.0 million to Dr. Chowrira The rationale for these payments was set out in the 2023 Directors’ Remuneration Report. 2 These amounts include the grants of share-based remuneration in June 2023 in the form of time-vesting restricted stock units with a face value of $50,000. 3 Dr. Holcomb joined the Board in September 2024. Annual Report on Remuneration continued

114 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Annual bonus outcome for 2024 (audited) For the 2024 annual bonus, targets were set for a balanced scorecard at the beginning of the year. The 2024 targets were focused on (i) development goals designed to incentivize the team to continue development of the Company’s Programs, generate valuable clinical data in support of the Company’s Wholly-Owned Programs, create innovative Programs, publish key results and achieve patent protection for the Company’s Wholly-Owned Programs; and (ii) strategic goals designed to incentivize the team to complete important deals, execute strategic partnerships, monetize Founded Entity holdings or otherwise strengthen the Company’s balance sheet, strengthen the Company’s investor base and provide support for Founded Entity transactions and financings. Both categories contained pre-specified stetch goals for exceptional achievement within each performance category. The Remuneration Committee did not account for the achievement of any previously un-specified goals or other achievements by the management team in determining the final bonus payouts. The table below sets out the performance assessment and associated bonus outcomes: Target Goals – Achievement (audited) Performance Measures Category Achievement Percentage of Target Attained Program Development (60%) The Program Development Goals were 150 percent achieved in 2024. The management team’s performance resulted in an achievement outcome of 90 percent which was equal to the pre-specified target of 60 percent, along with 30% for additional stretch goals for this category. A description of performance in 2024 is set out below: The Company completed enrollment and announced topline data of the Phase 2b multiple ascending dose studies for LYT-100 in healthy older adults to support proceeding in IPF while additionally satisfying the stretch goals of achieving primary endpoint with a favourable safety profile and superior efficacy at the higher dose of LYT-100. The Company also completed enrollment of Phase 1b/2a study results with LYT-200 while additionally satisfying the stretch goal of demonstrating proof of concept in AML cancer patients and receiving orphan drug designation for LYT-200. 90% Strategic Goals (40%) The Strategic Goals were 95 percent achieved in 2024. The management team’s performance resulted in an achievement outcome of 38 percent out of a pre-specified cap of 40 percent for this category of the goals. A description of performance in 2024 is set out below: The Company successfully spun out the CNS programs and Glyph platform into Seaport Therapeutics, which raised over $325 million during the year, completed a $100 million tender offer, completed various strategic sourcing initiatives for new programs, and extensively evaluated certain strategic transactions and options to enhance shareholder value. 38% Total based on Pre- Specified Targets 128% Accordingly, the Committee determined a bonus payout of 64 percent of base salary representing Company achievement of 128 percent of its target goals for 2024. Each of the above target categories are subject to maximum percentage achievement limits that aggregate to 100 percent of the target bonus (i.e. 50 percent of salary), provided that the Committee may pre-specify certain stretch targets that would increase the ultimate bonus payout above 100 percent of target (i.e. 50 percent of base salary) if achieved. In this case, the Committee determined that payouts at 64 percent of base salary (i.e. 128 percent of target) were appropriate taking into account the overall performance of the Executive Director, the pre-specified goals and stretch targets, and the achievements of the business as set forth above. For 2024, the annual bonus payment percentage awarded to Executive Directors was substantially below the prior year, and no discretion was exercised by the Committee related to the annual bonus or any other aspect of compensation. Annual Report on Remuneration continued
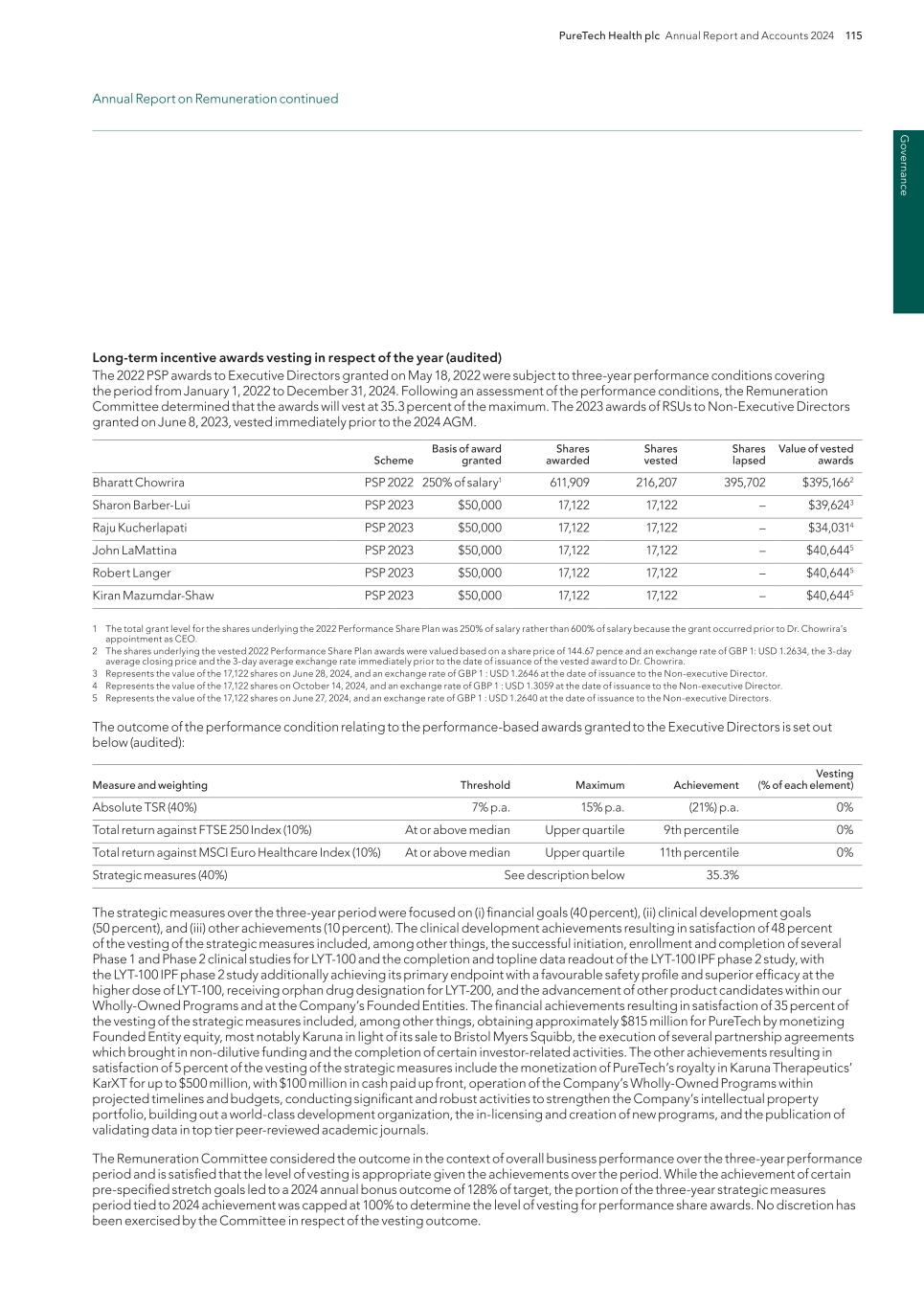
PureTech Health plc Annual Report and Accounts 2024 115 G overnance Long-term incentive awards vesting in respect of the year (audited) The 2022 PSP awards to Executive Directors granted on May 18, 2022 were subject to three-year performance conditions covering the period from January 1, 2022 to December 31, 2024. Following an assessment of the performance conditions, the Remuneration Committee determined that the awards will vest at 35.3 percent of the maximum. The 2023 awards of RSUs to Non-Executive Directors granted on June 8, 2023, vested immediately prior to the 2024 AGM. Scheme Basis of award granted Shares awarded Shares vested Shares lapsed Value of vested awards Bharatt Chowrira PSP 2022 250% of salary1 611,909 216,207 395,702 $395,1662 Sharon Barber-Lui PSP 2023 $50,000 17,122 17,122 – $39,6243 Raju Kucherlapati PSP 2023 $50,000 17,122 17,122 – $34,0314 John LaMattina PSP 2023 $50,000 17,122 17,122 – $40,6445 Robert Langer PSP 2023 $50,000 17,122 17,122 – $40,6445 Kiran Mazumdar-Shaw PSP 2023 $50,000 17,122 17,122 – $40,6445 1 The total grant level for the shares underlying the 2022 Performance Share Plan was 250% of salary rather than 600% of salary because the grant occurred prior to Dr. Chowrira’s appointment as CEO. 2 The shares underlying the vested 2022 Performance Share Plan awards were valued based on a share price of 144.67 pence and an exchange rate of GBP 1: USD 1.2634, the 3-day average closing price and the 3-day average exchange rate immediately prior to the date of issuance of the vested award to Dr. Chowrira. 3 Represents the value of the 17,122 shares on June 28, 2024, and an exchange rate of GBP 1 : USD 1.2646 at the date of issuance to the Non-executive Director. 4 Represents the value of the 17,122 shares on October 14, 2024, and an exchange rate of GBP 1 : USD 1.3059 at the date of issuance to the Non-executive Director. 5 Represents the value of the 17,122 shares on June 27, 2024, and an exchange rate of GBP 1 : USD 1.2640 at the date of issuance to the Non-executive Directors. The outcome of the performance condition relating to the performance-based awards granted to the Executive Directors is set out below (audited): Measure and weighting Threshold Maximum Achievement Vesting (% of each element) Absolute TSR (40%) 7% p.a. 15% p.a. (21%) p.a. 0% Total return against FTSE 250 Index (10%) At or above median Upper quartile 9th percentile 0% Total return against MSCI Euro Healthcare Index (10%) At or above median Upper quartile 11th percentile 0% Strategic measures (40%) See description below 35.3% The strategic measures over the three-year period were focused on (i) financial goals (40 percent), (ii) clinical development goals (50 percent), and (iii) other achievements (10 percent). The clinical development achievements resulting in satisfaction of 48 percent of the vesting of the strategic measures included, among other things, the successful initiation, enrollment and completion of several Phase 1 and Phase 2 clinical studies for LYT-100 and the completion and topline data readout of the LYT-100 IPF phase 2 study, with the LYT-100 IPF phase 2 study additionally achieving its primary endpoint with a favourable safety profile and superior efficacy at the higher dose of LYT-100, receiving orphan drug designation for LYT-200, and the advancement of other product candidates within our Wholly-Owned Programs and at the Company’s Founded Entities. The financial achievements resulting in satisfaction of 35 percent of the vesting of the strategic measures included, among other things, obtaining approximately $815 million for PureTech by monetizing Founded Entity equity, most notably Karuna in light of its sale to Bristol Myers Squibb, the execution of several partnership agreements which brought in non-dilutive funding and the completion of certain investor-related activities. The other achievements resulting in satisfaction of 5 percent of the vesting of the strategic measures include the monetization of PureTech’s royalty in Karuna Therapeutics’ KarXT for up to $500 million, with $100 million in cash paid up front, operation of the Company’s Wholly-Owned Programs within projected timelines and budgets, conducting significant and robust activities to strengthen the Company’s intellectual property portfolio, building out a world-class development organization, the in-licensing and creation of new programs, and the publication of validating data in top tier peer-reviewed academic journals. The Remuneration Committee considered the outcome in the context of overall business performance over the three-year performance period and is satisfied that the level of vesting is appropriate given the achievements over the period. While the achievement of certain pre-specified stretch goals led to a 2024 annual bonus outcome of 128% of target, the portion of the three-year strategic measures period tied to 2024 achievement was capped at 100% to determine the level of vesting for performance share awards. No discretion has been exercised by the Committee in respect of the vesting outcome. Annual Report on Remuneration continued
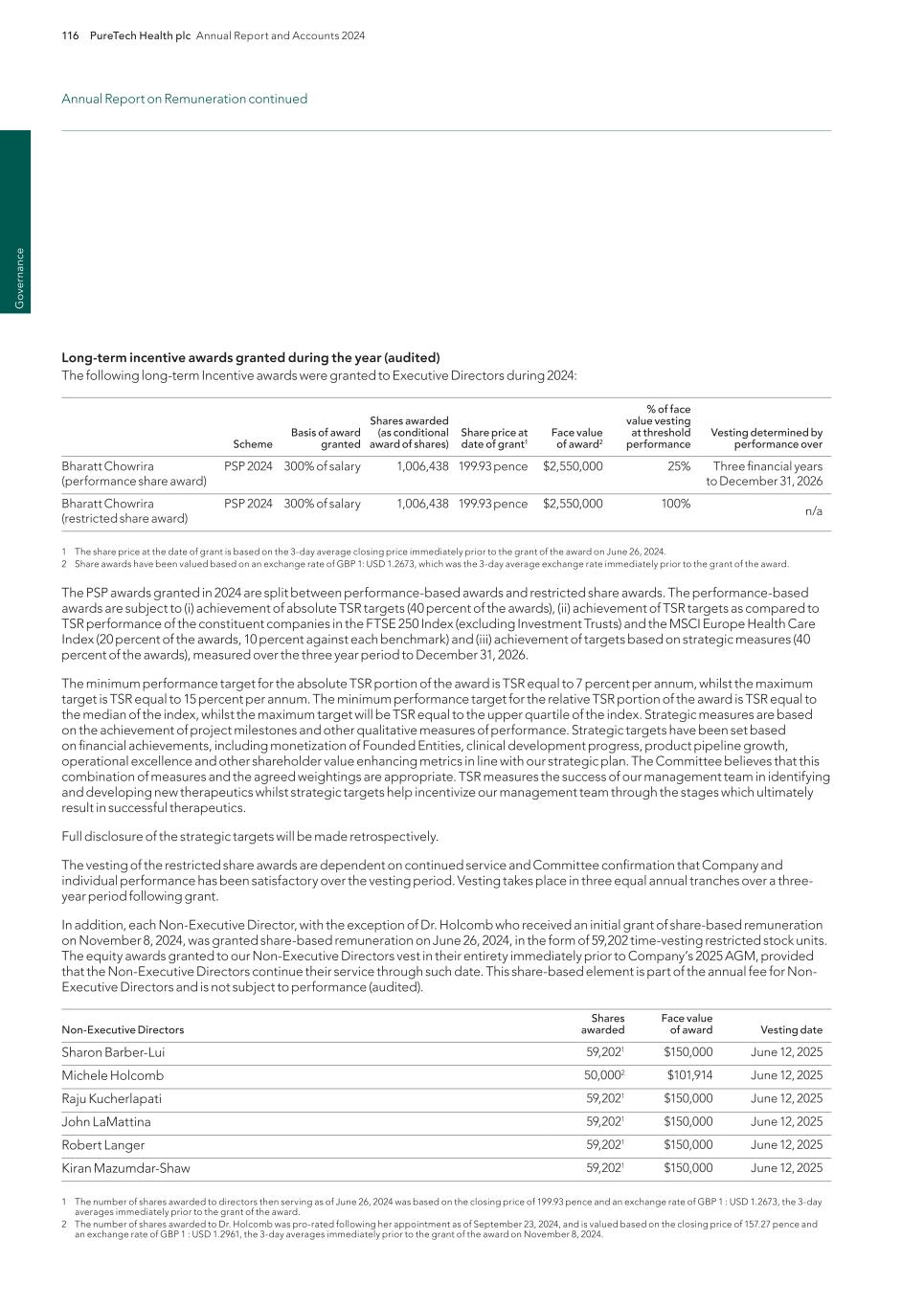
116 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Long-term incentive awards granted during the year (audited) The following long-term Incentive awards were granted to Executive Directors during 2024: Scheme Basis of award granted Shares awarded (as conditional award of shares) Share price at date of grant1 Face value of award2 % of face value vesting at threshold performance Vesting determined by performance over Bharatt Chowrira (performance share award) PSP 2024 300% of salary 1,006,438 199.93 pence $2,550,000 25% Three financial years to December 31, 2026 Bharatt Chowrira (restricted share award) PSP 2024 300% of salary 1,006,438 199.93 pence $2,550,000 100% n/a 1 The share price at the date of grant is based on the 3-day average closing price immediately prior to the grant of the award on June 26, 2024. 2 Share awards have been valued based on an exchange rate of GBP 1: USD 1.2673, which was the 3-day average exchange rate immediately prior to the grant of the award. The PSP awards granted in 2024 are split between performance-based awards and restricted share awards. The performance-based awards are subject to (i) achievement of absolute TSR targets (40 percent of the awards), (ii) achievement of TSR targets as compared to TSR performance of the constituent companies in the FTSE 250 Index (excluding Investment Trusts) and the MSCI Europe Health Care Index (20 percent of the awards, 10 percent against each benchmark) and (iii) achievement of targets based on strategic measures (40 percent of the awards), measured over the three year period to December 31, 2026. The minimum performance target for the absolute TSR portion of the award is TSR equal to 7 percent per annum, whilst the maximum target is TSR equal to 15 percent per annum. The minimum performance target for the relative TSR portion of the award is TSR equal to the median of the index, whilst the maximum target will be TSR equal to the upper quartile of the index. Strategic measures are based on the achievement of project milestones and other qualitative measures of performance. Strategic targets have been set based on financial achievements, including monetization of Founded Entities, clinical development progress, product pipeline growth, operational excellence and other shareholder value enhancing metrics in line with our strategic plan. The Committee believes that this combination of measures and the agreed weightings are appropriate. TSR measures the success of our management team in identifying and developing new therapeutics whilst strategic targets help incentivize our management team through the stages which ultimately result in successful therapeutics. Full disclosure of the strategic targets will be made retrospectively. The vesting of the restricted share awards are dependent on continued service and Committee confirmation that Company and individual performance has been satisfactory over the vesting period. Vesting takes place in three equal annual tranches over a three- year period following grant. In addition, each Non-Executive Director, with the exception of Dr. Holcomb who received an initial grant of share-based remuneration on November 8, 2024, was granted share-based remuneration on June 26, 2024, in the form of 59,202 time-vesting restricted stock units. The equity awards granted to our Non-Executive Directors vest in their entirety immediately prior to Company’s 2025 AGM, provided that the Non-Executive Directors continue their service through such date. This share-based element is part of the annual fee for Non- Executive Directors and is not subject to performance (audited). Non-Executive Directors Shares awarded Face value of award Vesting date Sharon Barber-Lui 59,2021 $150,000 June 12, 2025 Michele Holcomb 50,0002 $101,914 June 12, 2025 Raju Kucherlapati 59,2021 $150,000 June 12, 2025 John LaMattina 59,2021 $150,000 June 12, 2025 Robert Langer 59,2021 $150,000 June 12, 2025 Kiran Mazumdar-Shaw 59,2021 $150,000 June 12, 2025 1 The number of shares awarded to directors then serving as of June 26, 2024 was based on the closing price of 199.93 pence and an exchange rate of GBP 1 : USD 1.2673, the 3-day averages immediately prior to the grant of the award. 2 The number of shares awarded to Dr. Holcomb was pro-rated following her appointment as of September 23, 2024, and is valued based on the closing price of 157.27 pence and an exchange rate of GBP 1 : USD 1.2961, the 3-day averages immediately prior to the grant of the award on November 8, 2024. Annual Report on Remuneration continued
PureTech Health plc Annual Report and Accounts 2024 117 G overnance Payments for Loss of Office (audited) There were no payments for Loss of Office during 2024. Payments to past Directors (audited) Daphne Zohar resigned from her roles as the Company’s Chief Executive Officer and a member of the Company’s Board of Directors on April 9, 2024 in connection with the founding of Seaport Therapeutics, Inc. (Seaport). Ms. Zohar was paid base salary, benefits and pension as Chief Executive Officer of the Company through April 8, 2024. Following Ms. Zohar’s resignation, her outstanding PSP awards continue to vest for the duration of her service as senior advisor and observer to the Board. As of result of her continued service during 2024, Ms. Zohar’s 2022 PSP award of 1,532,051 shares vested as of December 31, 2024. Based on performance during the period, the Board determined that 35.3% of the award vested, and in February 2025 Ms. Zohar received 541,324 ordinary shares pursuant to this award, valued at $989,388 based on the 3-day average share price and exchange rate (GBP 1: USD 1.2634) immediately prior to the issuance of the award. These shares are subject to the applicable holding period. One additional PSPS award from 2023 of 1,678,971 shares will continue to vest while Ms. Zohar is providing services to the Company. Ms. Zohar did not receive any payments for loss of office. Ms. Zohar received payments totalling $100,000 for her service as a senior advisor and board observer from April 9, 2024 through April 8, 2025. The term of Ms. Zohar’s service as a senior advisor and board observer has been extended to April 8, 2026. The post-employment shareholding policy will apply to Ms. Zohar, requiring a shareholding worth 400 percent of base salary to be retained for two years following the cessation of her employment. Directors’ shareholdings (audited) Executive Directors are required to maintain share ownership equal to a minimum of 400 percent of base salary for the Chief Executive Officer and a minimum of 200 percent of base salary for any other Executive Directors. Post-employment shareholding requirements apply, requiring the retention of a minimum share ownership based on a multiple of their salary for a two year period. The table below sets out current Directors’ shareholdings which are beneficially owned, subject to a performance condition, subject to a service condition and interests of connected persons. Director Directors’ Share Interests Shares Owned Outright Vested But Unexercised Options Options Subject To Service RSUs Subject To Performance Conditions RSUs Subject To Service Conditions Total December 31, 2024 Bharatt Chowrira 1,000,0011 1,950,000 – 1,677,0282 1,006,4383 5,633,467 Sharon Barber-Lui 38,629 – – – 59,2024 97,831 Michele Holcomb5 – – – – 50,0006 50,000 Raju Kucherlapati 2,509,650 – – – 59,2024 2,568,852 John LaMattina7 1,324,130 – – – 59,2024 1,383,332 Robert Langer8 2,760,854 – – – 59,2024 2,820,056 Kiran Mazumdar-Shaw 49,819 – – – 59,2024 109,021 1 Does not include 108,103 shares which were issued in February 2025 pursuant to the PSP award granted to Dr. Chowrira covering the financial years 2022, 2023 and 2024. The performance conditions related to this award were measured as of the close of business on December 31, 2024. As of March 31, 2025, Dr. Chowrira owned 1,108,104 shares outright. 2 Includes the following PSP awards, which are subject to performance conditions: 670,590 (2023) and 1,006,438 (2024). 3 Includes the following PSP awards, which are subject to service conditions: 1,006,438 (2024). 4 Denotes RSUs, which are subject to continued service, that were granted in June 2024 and vest immediately prior to the 2025 Annual General Meeting. 5 Dr. Holcomb joined the Board in September 2024. 6 Denotes RSUs, which are subject to continued service, that were granted in November 2024 following Dr. Holcomb’s appointment to the Board, and vest immediately prior to the 2025 Annual General Meeting. 7 A portion of Dr. LaMattina’s shareholding in the Company is indirect. As of December 31, 2024, an aggregate of 1,324,130 ordinary shares are split between (i) 1,184,774 shares held by the John L LaMattina Revocable Trust and (ii) 139,356 shares held by the LaMattina Charitable Trust. During 2024, Dr. LaMattina’s ownership decreased by an aggregate of 107,522 shares, with a decrease of 54,882 ordinary shares as a result of his participation in the Company’s 2024 tender offer, and a decrease of 52,640 ordinary shares as a result of certain charitable donations. 8 A portion of Dr. Langer’s shareholding in the Company is indirect. As of December 31, 2024, an aggregate of 2,760,854 ordinary shares are split between (i) 2,332,578 shares held by Dr. Langer directly, (ii) 419,867 shares held by the Langer Family 2020 Trust, and (iii) 8,409 shares held by Dr. Langer and Laura Langer jointly. During 2024, Dr. Langer’s ownership decreased by 233,098 ordinary shares as a result of his participation in the Company’s 2024 tender offer. Annual Report on Remuneration continued
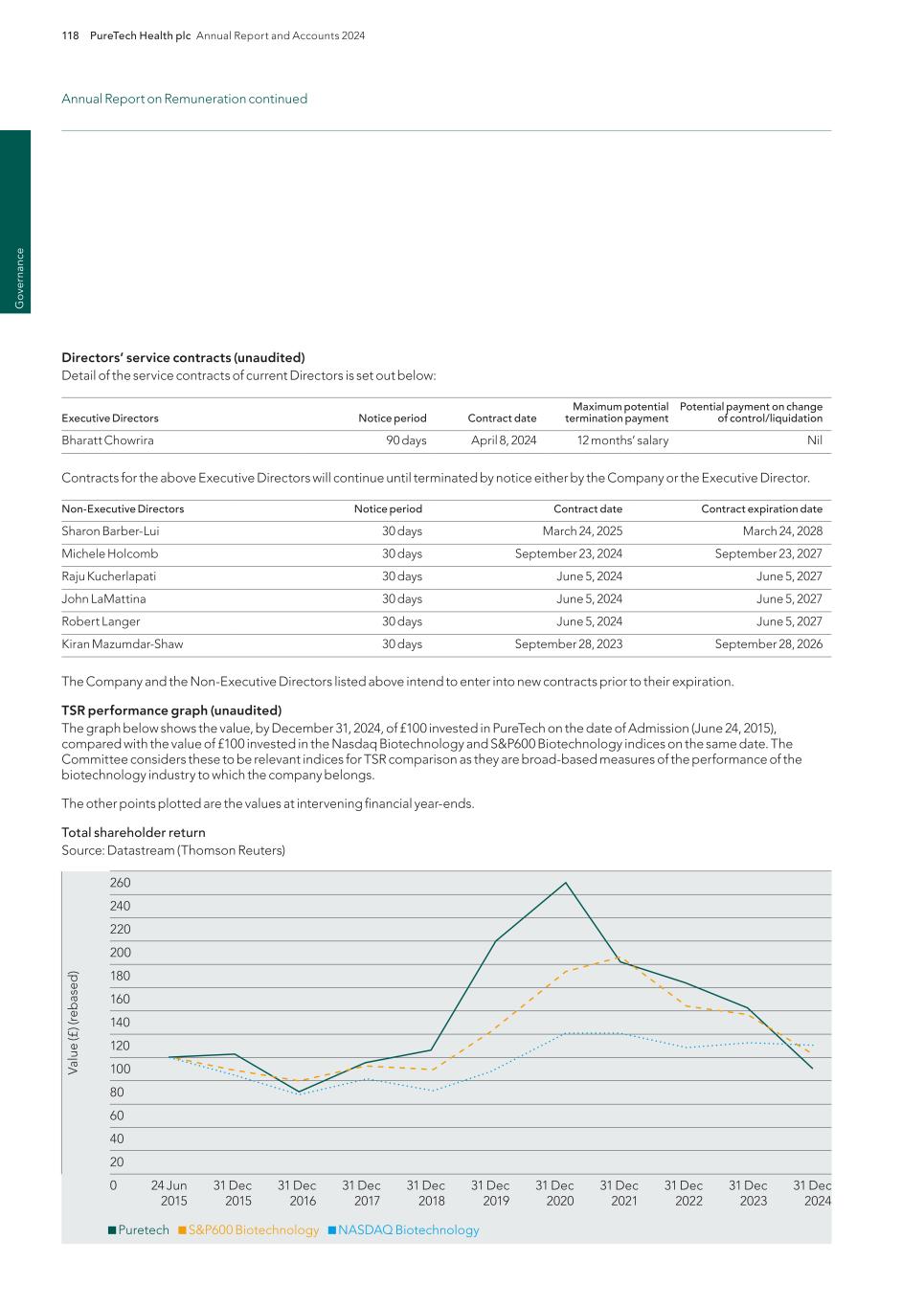
260 240 220 200 180 160 140 120 100 80 60 40 20 0 24 Jun 2015 31 Dec 2016 31 Dec 2015 31 Dec 2017 31 Dec 2018 31 Dec 2019 31 Dec 2020 31 Dec 2021 31 Dec 2024 31 Dec 2023 31 Dec 2022 Va lu e (£ ) ( re b as ed ) Puretech S&P600 Biotechnology NASDAQ Biotechnology 118 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Directors’ service contracts (unaudited) Detail of the service contracts of current Directors is set out below: Executive Directors Notice period Contract date Maximum potential termination payment Potential payment on change of control/liquidation Bharatt Chowrira 90 days April 8, 2024 12 months’ salary Nil Contracts for the above Executive Directors will continue until terminated by notice either by the Company or the Executive Director. Non-Executive Directors Notice period Contract date Contract expiration date Sharon Barber-Lui 30 days March 24, 2025 March 24, 2028 Michele Holcomb 30 days September 23, 2024 September 23, 2027 Raju Kucherlapati 30 days June 5, 2024 June 5, 2027 John LaMattina 30 days June 5, 2024 June 5, 2027 Robert Langer 30 days June 5, 2024 June 5, 2027 Kiran Mazumdar-Shaw 30 days September 28, 2023 September 28, 2026 The Company and the Non-Executive Directors listed above intend to enter into new contracts prior to their expiration. TSR performance graph (unaudited) The graph below shows the value, by December 31, 2024, of £100 invested in PureTech on the date of Admission (June 24, 2015), compared with the value of £100 invested in the Nasdaq Biotechnology and S&P600 Biotechnology indices on the same date. The Committee considers these to be relevant indices for TSR comparison as they are broad-based measures of the performance of the biotechnology industry to which the company belongs. The other points plotted are the values at intervening financial year-ends. Total shareholder return Source: Datastream (Thomson Reuters) Annual Report on Remuneration continued
PureTech Health plc Annual Report and Accounts 2024 119 G overnance Chief Executive Officer’s Remuneration History (unaudited) Year Incumbent Role Single figure of total remuneration Annual bonus pay-out against maximum PSP Vesting against maximum opportunity 2015 Daphne Zohar Chief Executive Officer $955,599 100% n/a 2016 Daphne Zohar Chief Executive Officer $747,634 38.75% n/a 2017 Daphne Zohar Chief Executive Officer $821,898 50% n/a 2018 Daphne Zohar Chief Executive Officer $2,139,870 65% 50% 2019 Daphne Zohar Chief Executive Officer $5,783,682 100% 100% 2020 Daphne Zohar Chief Executive Officer $7,194,841 100% 100% 2021 Daphne Zohar Chief Executive Officer $2,472,800 75% 95.8% 2022 Daphne Zohar Chief Executive Officer $1,487,964 45% 24.2% 2023 Daphne Zohar Chief Executive Officer $4,739,027 100% 35.3% 2024 Bharatt Chowrira Chief Executive Officer $4,311,5551 64% 35.3% 1 Dr. Chowrira’s single-figure remuneration for 2024 includes $2,550,000, or 300% of base salary, the value underlying the unvested 2024 time-based restricted share award. This award was valued based on a closing price of 199.93 pence and an exchange rate of GBP 1: USD 1.2673, the 3-day averages immediately prior to the grant of the award. Percentage change in remuneration of Directors and employees (unaudited) The table below shows the change in the Directors’ remuneration compared to the change in remuneration of all of our full-time employees who were employed throughout the same periods: 2023 to 2024 2022 to 2023 2021 to 2022 2020 to 2021 2019 to 2020 Base salary/ fees1 Bene- fits2 Annual bonus Base salary/ fees Bene- fits Annual bonus Base salary/ fees Bene- fits Annual bonus Base salary/ fees Bene- fits Annual bonus Base salary/ fees Bene- fits Annual bonus Bharatt Chowrira (CEO)3 35.8% (97%) (5.4%) 8.5% 3790% 141% 6% (10%) (36%) N/A N/A N/A 3% 0% 3% Sharon Barber-Lui4 92.6% N/A N/A 17.3% N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A Michele Holcomb5 N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A Raju Kucherlapati 99.2% N/A N/A 27.8% N/A N/A (7%) N/A N/A 38.1% N/A N/A 11% N/A N/A John LaMattina 111% N/A N/A (5%) N/A N/A 0% N/A N/A 16% N/A N/A 19% N/A N/A Robert Langer 86.2% N/A N/A 0% N/A N/A 0% N/A N/A 16% N/A N/A 13% N/A N/A Kiran Mazumdar-Shaw 74.1% N/A N/A 0% N/A N/A 0% N/A N/A 635% N/A N/A N/A N/A N/A Employees6 5% 2% 33% 9% 12% 77% 12% 6% (22%) 9% 7% 1% 45% N/A N/A 1 Fee amounts for Non-Executive Directors in 2023 include grants of share based remuneration in the form of time-vesting restricted stock units with a face value of $50,000, while 2024 amounts include grants of share based remuneration in the form of time-vesting restricted stock units with a face value of $150,000. 2 This segment includes: housing allowance, transportation allowance, private medical and dental cover, disability and life insurance. Benefits payments in 2023 included a payment of $1.0m to Bharatt Chowrira, as explained in last year’s Directors’ Remuneration Report. 3 Joined the Board effective February 2021. 4 Joined the Board effective March 2022. 5 Joined the Board effective September 2024. 6 Does not include employees of Founded Entities. Annual Report on Remuneration continued
120 PureTech Health plc Annual Report and Accounts 2024 G ov er na nc e Relative importance of spend on pay (unaudited) The following table sets out the percentage change in overall spend on pay and distributions to shareholders in 2024 compared to 2023: 2024 2023 % change Staff costs1 $32,522,471 $37,913,231 (14.2%) Distributions to Shareholders $104,703,4972 $19,067,6603 449.1% 1 Excludes Non-Controlled Founded Entities. 2 Represents the value of the 31,540,670 ordinary shares repurchased pursuant to the terms of the Company’s $100 million tender offer, and 1,903,990 ordinary shares repurchased under the Company’s share repurchase programme during 2024. 3 Represents the value of the 7,683,526 ordinary shares repurchased under the Company’s share repurchase programme during 2023. Details of the Remuneration Committee, advisors to the Committee and their fees The Remuneration Committee consists of Dr. LaMattina, Ms. Mazumdar-Shaw and Dr. Kucherlapati, with Dr. LaMattina serving as the Chair of the Committee. In 2024 the Committee received independent remuneration advice from Korn Ferry (UK) Limited, who was appointed by and is accountable to the Committee. A separate practice within Korn Ferry provides certain other candidate placement services to the Company. The terms of engagement between the Committee and Korn Ferry are available from the Company Secretary on request. The Committee also consults with Executive Directors. However, no Director is permitted to participate in discussions or decisions about their personal remuneration. During the year, fees in respect of remuneration advice from Korn Ferry amounted to £22,200. Korn Ferry is a founder member of the Remuneration Consultants’ Group and complies with its Code of Conduct which sets out guidelines to ensure that its advice is independent and free of undue influence. Statement of voting at general meeting (unaudited) The table below sets out the proxy results of the vote on our Remuneration Report at our 2024 AGM: Resolutions For % Against % Withheld Total votes cast To approve the Directors’ Remuneration Report 72,296,583 55.95% 56,922,574 44.05% 51,706,134 129,219,157 The table below sets out the proxy results of the vote on our Remuneration Policy at our 2024 AGM: Resolutions For % Against % Withheld Total votes cast To approve the Directors’ Remuneration Policy 83,722,702 64.46% 46,157,643 35.54% 51,044,946 129,880,345 2025 AGM The Company’s AGM will be held at 11:00 am EDT (4:00 pm BST) on June 16, 2025 at the Company’s headquarters at 6 Tide Street, Boston, Massachusetts. Information regarding the voting outcome will be disclosed in next year’s Annual Report on Remuneration. This report has been prepared by the Remuneration Committee and has been approved by the Board. It complies with the UK Companies Act 2006 and related regulations. This report will be put to shareholders for approval at the forthcoming AGM. On behalf of the Board of Directors Charles Sherwood, J.D. Company Secretary April 30, 2025 Annual Report on Remuneration continued

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 121 [Pages 121 to 125 have been intentionally omitted]

Fi na nc ia l s ta te m en ts 122 PureTech Health plc Annual Report and Accounts 2024

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 123

Fi na nc ia l s ta te m en ts 124 PureTech Health plc Annual Report and Accounts 2024

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 125
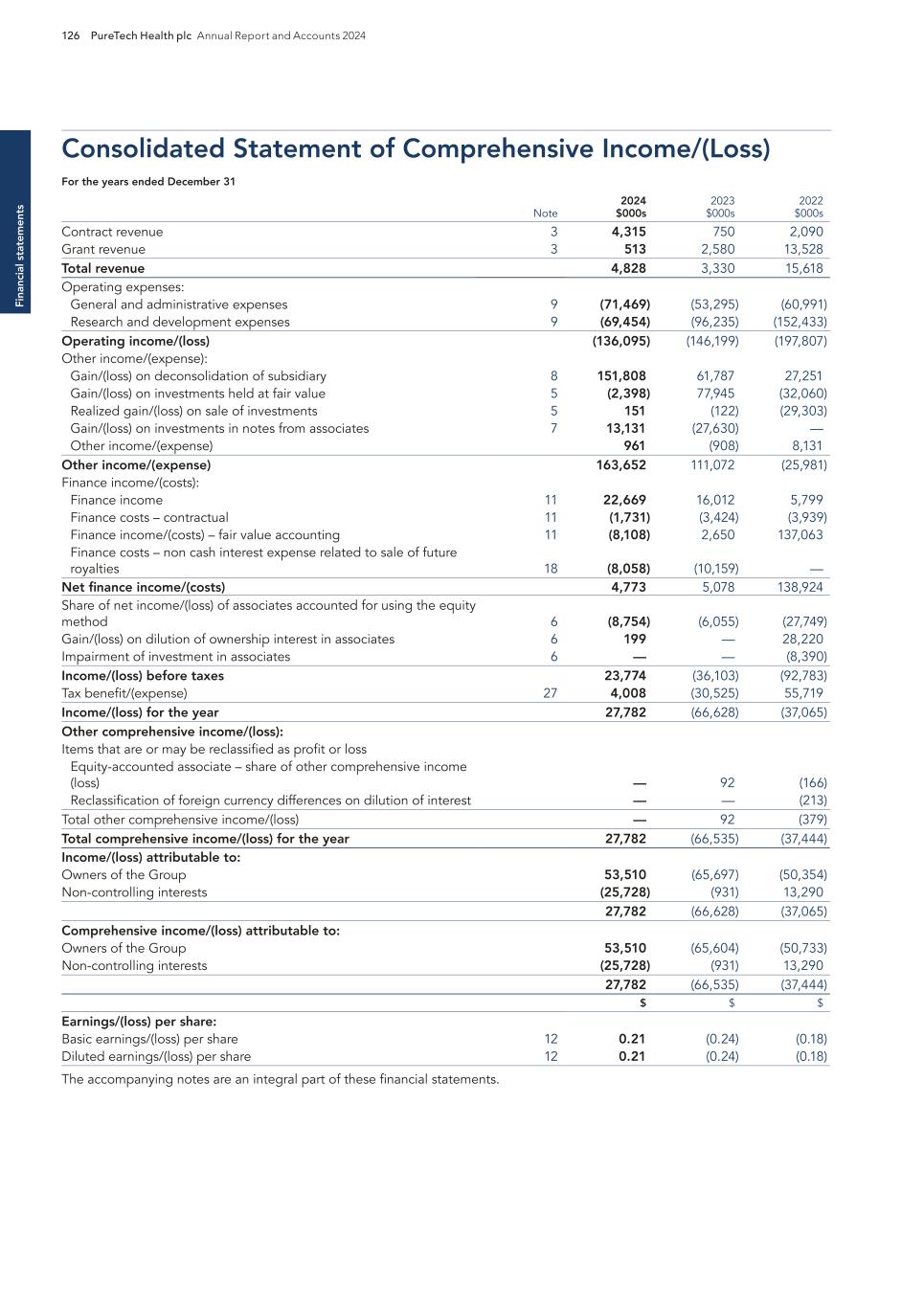
Fi na nc ia l s ta te m en ts 126 PureTech Health plc Annual Report and Accounts 2024 Consolidated Statement of Comprehensive Income/(Loss) For the years ended December 31 Note 2024 $000s 2023 $000s 2022 $000s Contract revenue 3 4,315 750 2,090 Grant revenue 3 513 2,580 13,528 Total revenue 4,828 3,330 15,618 Operating expenses: General and administrative expenses 9 (71,469) (53,295) (60,991) Research and development expenses 9 (69,454) (96,235) (152,433) Operating income/(loss) (136,095) (146,199) (197,807) Other income/(expense): Gain/(loss) on deconsolidation of subsidiary 8 151,808 61,787 27,251 Gain/(loss) on investments held at fair value 5 (2,398) 77,945 (32,060) Realized gain/(loss) on sale of investments 5 151 (122) (29,303) Gain/(loss) on investments in notes from associates 7 13,131 (27,630) — Other income/(expense) 961 (908) 8,131 Other income/(expense) 163,652 111,072 (25,981) Finance income/(costs): Finance income 11 22,669 16,012 5,799 Finance costs – contractual 11 (1,731) (3,424) (3,939) Finance income/(costs) – fair value accounting 11 (8,108) 2,650 137,063 Finance costs – non cash interest expense related to sale of future royalties 18 (8,058) (10,159) — Net finance income/(costs) 4,773 5,078 138,924 Share of net income/(loss) of associates accounted for using the equity method 6 (8,754) (6,055) (27,749) Gain/(loss) on dilution of ownership interest in associates 6 199 — 28,220 Impairment of investment in associates 6 — — (8,390) Income/(loss) before taxes 23,774 (36,103) (92,783) Tax benefit/(expense) 27 4,008 (30,525) 55,719 Income/(loss) for the year 27,782 (66,628) (37,065) Other comprehensive income/(loss): Items that are or may be reclassified as profit or loss Equity-accounted associate – share of other comprehensive income (loss) — 92 (166) Reclassification of foreign currency differences on dilution of interest — — (213) Total other comprehensive income/(loss) — 92 (379) Total comprehensive income/(loss) for the year 27,782 (66,535) (37,444) Income/(loss) attributable to: Owners of the Group 53,510 (65,697) (50,354) Non-controlling interests (25,728) (931) 13,290 27,782 (66,628) (37,065) Comprehensive income/(loss) attributable to: Owners of the Group 53,510 (65,604) (50,733) Non-controlling interests (25,728) (931) 13,290 27,782 (66,535) (37,444) $ $ $ Earnings/(loss) per share: Basic earnings/(loss) per share 12 0.21 (0.24) (0.18) Diluted earnings/(loss) per share 12 0.21 (0.24) (0.18) The accompanying notes are an integral part of these financial statements.
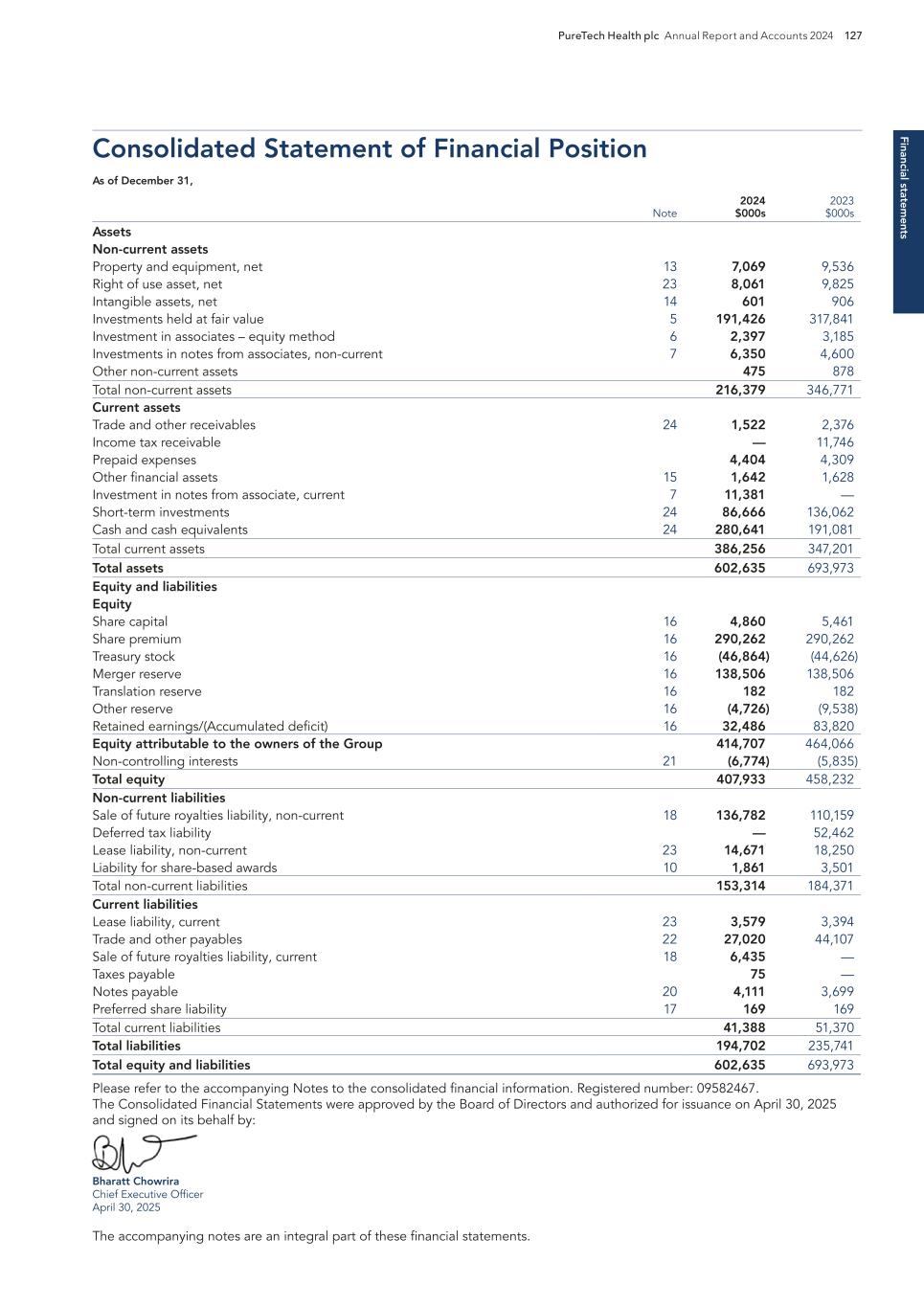
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 127 Consolidated Statement of Financial Position As of December 31, Note 2024 $000s 2023 $000s Assets Non-current assets Property and equipment, net 13 7,069 9,536 Right of use asset, net 23 8,061 9,825 Intangible assets, net 14 601 906 Investments held at fair value 5 191,426 317,841 Investment in associates – equity method 6 2,397 3,185 Investments in notes from associates, non-current 7 6,350 4,600 Other non-current assets 475 878 Total non-current assets 216,379 346,771 Current assets Trade and other receivables 24 1,522 2,376 Income tax receivable — 11,746 Prepaid expenses 4,404 4,309 Other financial assets 15 1,642 1,628 Investment in notes from associate, current 7 11,381 — Short-term investments 24 86,666 136,062 Cash and cash equivalents 24 280,641 191,081 Total current assets 386,256 347,201 Total assets 602,635 693,973 Equity and liabilities Equity Share capital 16 4,860 5,461 Share premium 16 290,262 290,262 Treasury stock 16 (46,864) (44,626) Merger reserve 16 138,506 138,506 Translation reserve 16 182 182 Other reserve 16 (4,726) (9,538) Retained earnings/(Accumulated deficit) 16 32,486 83,820 Equity attributable to the owners of the Group 414,707 464,066 Non-controlling interests 21 (6,774) (5,835) Total equity 407,933 458,232 Non-current liabilities Sale of future royalties liability, non-current 18 136,782 110,159 Deferred tax liability — 52,462 Lease liability, non-current 23 14,671 18,250 Liability for share-based awards 10 1,861 3,501 Total non-current liabilities 153,314 184,371 Current liabilities Lease liability, current 23 3,579 3,394 Trade and other payables 22 27,020 44,107 Sale of future royalties liability, current 18 6,435 — Taxes payable 75 — Notes payable 20 4,111 3,699 Preferred share liability 17 169 169 Total current liabilities 41,388 51,370 Total liabilities 194,702 235,741 Total equity and liabilities 602,635 693,973 Please refer to the accompanying Notes to the consolidated financial information. Registered number: 09582467. The Consolidated Financial Statements were approved by the Board of Directors and authorized for issuance on April 30, 2025 and signed on its behalf by: Bharatt Chowrira Chief Executive Officer April 30, 2025 The accompanying notes are an integral part of these financial statements.
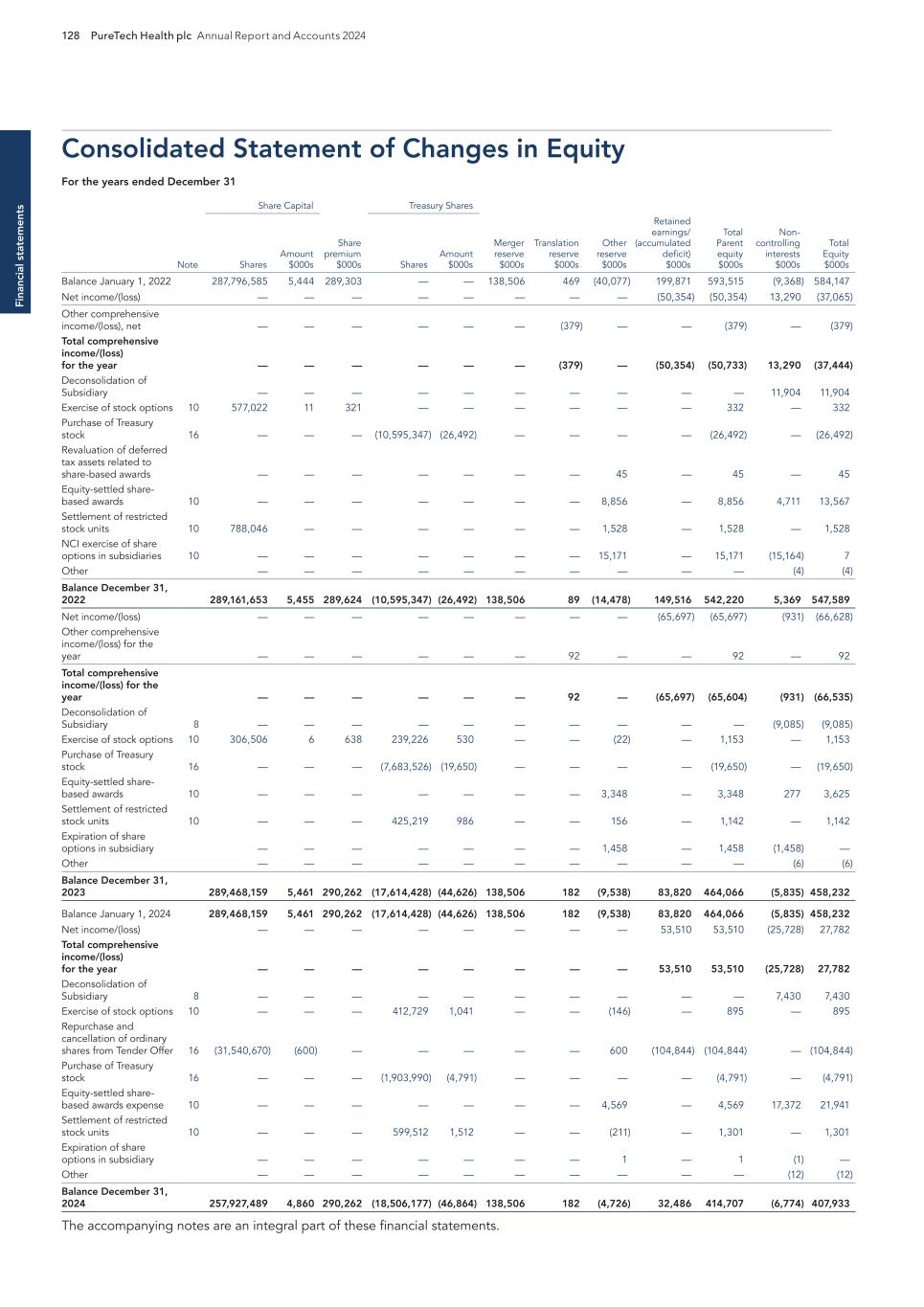
Fi na nc ia l s ta te m en ts 128 PureTech Health plc Annual Report and Accounts 2024 Consolidated Statement of Changes in Equity For the years ended December 31 Share Capital Treasury Shares Note Shares Amount $000s Share premium $000s Shares Amount $000s Merger reserve $000s Translation reserve $000s Other reserve $000s Retained earnings/ (accumulated deficit) $000s Total Parent equity $000s Non- controlling interests $000s Total Equity $000s Balance January 1, 2022 287,796,585 5,444 289,303 — — 138,506 469 (40,077) 199,871 593,515 (9,368) 584,147 Net income/(loss) — — — — — — — — (50,354) (50,354) 13,290 (37,065) Other comprehensive income/(loss), net — — — — — — (379) — — (379) — (379) Total comprehensive income/(loss) for the year — — — — — — (379) — (50,354) (50,733) 13,290 (37,444) Deconsolidation of Subsidiary — — — — — — — — — — 11,904 11,904 Exercise of stock options 10 577,022 11 321 — — — — — — 332 — 332 Purchase of Treasury stock 16 — — — (10,595,347) (26,492) — — — — (26,492) — (26,492) Revaluation of deferred tax assets related to share-based awards — — — — — — — 45 — 45 — 45 Equity-settled share- based awards 10 — — — — — — — 8,856 — 8,856 4,711 13,567 Settlement of restricted stock units 10 788,046 — — — — — — 1,528 — 1,528 — 1,528 NCI exercise of share options in subsidiaries 10 — — — — — — — 15,171 — 15,171 (15,164) 7 Other — — — — — — — — — — (4) (4) Balance December 31, 2022 289,161,653 5,455 289,624 (10,595,347) (26,492) 138,506 89 (14,478) 149,516 542,220 5,369 547,589 Net income/(loss) — — — — — — — — (65,697) (65,697) (931) (66,628) Other comprehensive income/(loss) for the year — — — — — — 92 — — 92 — 92 Total comprehensive income/(loss) for the year — — — — — — 92 — (65,697) (65,604) (931) (66,535) Deconsolidation of Subsidiary 8 — — — — — — — — — — (9,085) (9,085) Exercise of stock options 10 306,506 6 638 239,226 530 — — (22) — 1,153 — 1,153 Purchase of Treasury stock 16 — — — (7,683,526) (19,650) — — — — (19,650) — (19,650) Equity-settled share- based awards 10 — — — — — — — 3,348 — 3,348 277 3,625 Settlement of restricted stock units 10 — — — 425,219 986 — — 156 — 1,142 — 1,142 Expiration of share options in subsidiary — — — — — — — 1,458 — 1,458 (1,458) — Other — — — — — — — — — — (6) (6) Balance December 31, 2023 289,468,159 5,461 290,262 (17,614,428) (44,626) 138,506 182 (9,538) 83,820 464,066 (5,835) 458,232 Balance January 1, 2024 289,468,159 5,461 290,262 (17,614,428) (44,626) 138,506 182 (9,538) 83,820 464,066 (5,835) 458,232 Net income/(loss) — — — — — — — — 53,510 53,510 (25,728) 27,782 Total comprehensive income/(loss) for the year — — — — — — — — 53,510 53,510 (25,728) 27,782 Deconsolidation of Subsidiary 8 — — — — — — — — — — 7,430 7,430 Exercise of stock options 10 — — — 412,729 1,041 — — (146) — 895 — 895 Repurchase and cancellation of ordinary shares from Tender Offer 16 (31,540,670) (600) — — — — — 600 (104,844) (104,844) — (104,844) Purchase of Treasury stock 16 — — — (1,903,990) (4,791) — — — — (4,791) — (4,791) Equity-settled share- based awards expense 10 — — — — — — — 4,569 — 4,569 17,372 21,941 Settlement of restricted stock units 10 — — — 599,512 1,512 — — (211) — 1,301 — 1,301 Expiration of share options in subsidiary — — — — — — — 1 — 1 (1) — Other — — — — — — — — — — (12) (12) Balance December 31, 2024 257,927,489 4,860 290,262 (18,506,177) (46,864) 138,506 182 (4,726) 32,486 414,707 (6,774) 407,933 The accompanying notes are an integral part of these financial statements.
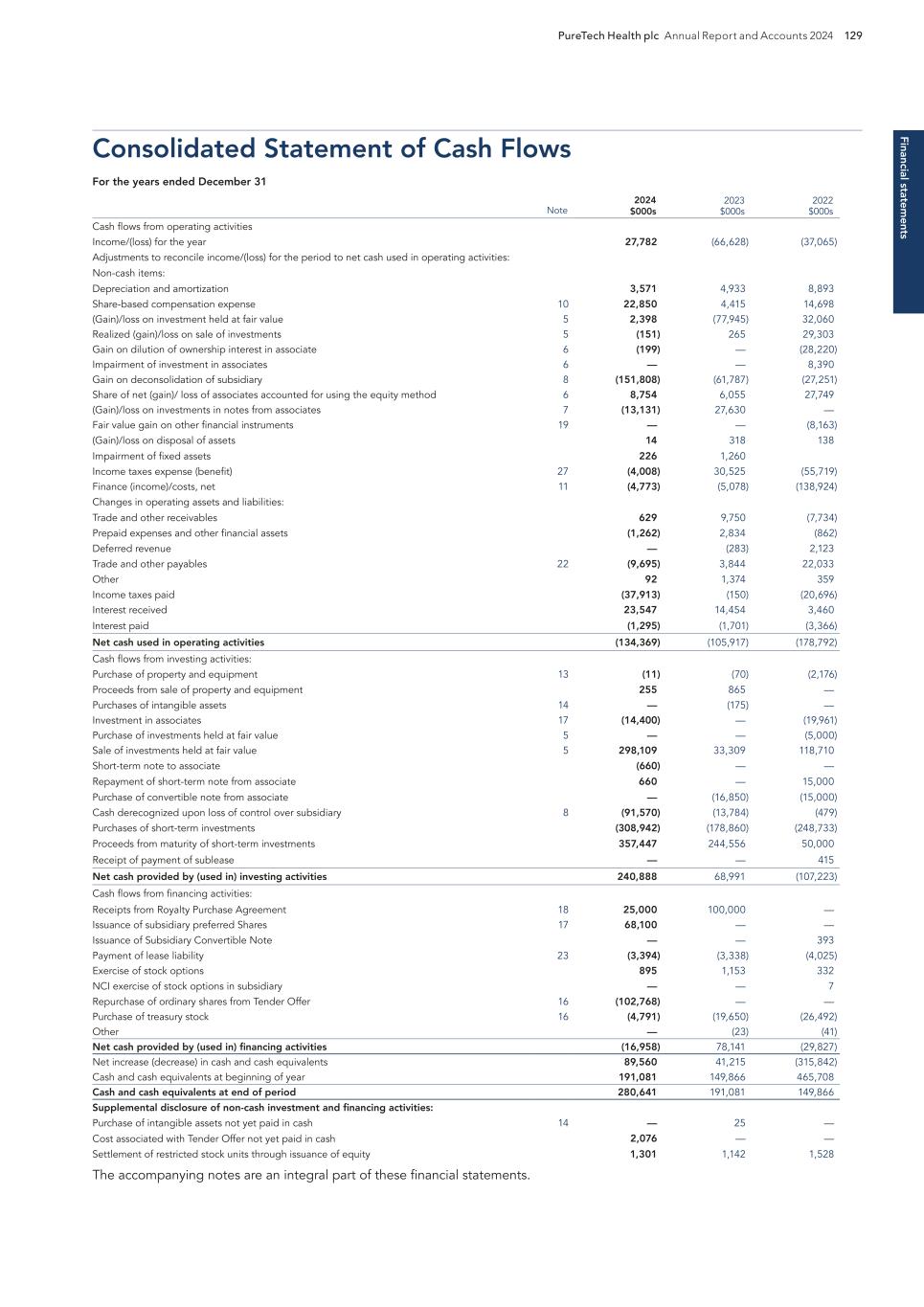
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 129 Consolidated Statement of Cash Flows For the years ended December 31 Note 2024 $000s 2023 $000s 2022 $000s Cash flows from operating activities Income/(loss) for the year 27,782 (66,628) (37,065) Adjustments to reconcile income/(loss) for the period to net cash used in operating activities: Non-cash items: Depreciation and amortization 3,571 4,933 8,893 Share-based compensation expense 10 22,850 4,415 14,698 (Gain)/loss on investment held at fair value 5 2,398 (77,945) 32,060 Realized (gain)/loss on sale of investments 5 (151) 265 29,303 Gain on dilution of ownership interest in associate 6 (199) — (28,220) Impairment of investment in associates 6 — — 8,390 Gain on deconsolidation of subsidiary 8 (151,808) (61,787) (27,251) Share of net (gain)/ loss of associates accounted for using the equity method 6 8,754 6,055 27,749 (Gain)/loss on investments in notes from associates 7 (13,131) 27,630 — Fair value gain on other financial instruments 19 — — (8,163) (Gain)/loss on disposal of assets 14 318 138 Impairment of fixed assets 226 1,260 Income taxes expense (benefit) 27 (4,008) 30,525 (55,719) Finance (income)/costs, net 11 (4,773) (5,078) (138,924) Changes in operating assets and liabilities: Trade and other receivables 629 9,750 (7,734) Prepaid expenses and other financial assets (1,262) 2,834 (862) Deferred revenue — (283) 2,123 Trade and other payables 22 (9,695) 3,844 22,033 Other 92 1,374 359 Income taxes paid (37,913) (150) (20,696) Interest received 23,547 14,454 3,460 Interest paid (1,295) (1,701) (3,366) Net cash used in operating activities (134,369) (105,917) (178,792) Cash flows from investing activities: Purchase of property and equipment 13 (11) (70) (2,176) Proceeds from sale of property and equipment 255 865 — Purchases of intangible assets 14 — (175) — Investment in associates 17 (14,400) — (19,961) Purchase of investments held at fair value 5 — — (5,000) Sale of investments held at fair value 5 298,109 33,309 118,710 Short-term note to associate (660) — — Repayment of short-term note from associate 660 — 15,000 Purchase of convertible note from associate — (16,850) (15,000) Cash derecognized upon loss of control over subsidiary 8 (91,570) (13,784) (479) Purchases of short-term investments (308,942) (178,860) (248,733) Proceeds from maturity of short-term investments 357,447 244,556 50,000 Receipt of payment of sublease — — 415 Net cash provided by (used in) investing activities 240,888 68,991 (107,223) Cash flows from financing activities: Receipts from Royalty Purchase Agreement 18 25,000 100,000 — Issuance of subsidiary preferred Shares 17 68,100 — — Issuance of Subsidiary Convertible Note — — 393 Payment of lease liability 23 (3,394) (3,338) (4,025) Exercise of stock options 895 1,153 332 NCI exercise of stock options in subsidiary — — 7 Repurchase of ordinary shares from Tender Offer 16 (102,768) — — Purchase of treasury stock 16 (4,791) (19,650) (26,492) Other — (23) (41) Net cash provided by (used in) financing activities (16,958) 78,141 (29,827) Net increase (decrease) in cash and cash equivalents 89,560 41,215 (315,842) Cash and cash equivalents at beginning of year 191,081 149,866 465,708 Cash and cash equivalents at end of period 280,641 191,081 149,866 Supplemental disclosure of non-cash investment and financing activities: Purchase of intangible assets not yet paid in cash 14 — 25 — Cost associated with Tender Offer not yet paid in cash 2,076 — — Settlement of restricted stock units through issuance of equity 1,301 1,142 1,528 The accompanying notes are an integral part of these financial statements.

Fi na nc ia l s ta te m en ts 130 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements (Amounts in thousands, except share and per share data, or exercise price and conversion price) 1. Material Accounting Policies Description of Business PureTech Health plc (the “Parent”) is a public company incorporated, domiciled and registered in the United Kingdom (“UK”). The registered number is 09582467 and the registered address is 13th Floor, One Angel Court, London, EC2R 7HJ, United Kingdom. The Parent and its subsidiaries are together referred to as the “Group”. The Parent company financial statements present financial information about the Parent as a separate entity and not about its Group. The accounting policies set out below have, unless otherwise stated, been applied consistently to all periods presented in these group financial statements. Basis of Presentation The consolidated financial statements of the Group (the "Consolidated Financial Statements") are presented as of December 31, 2024 and 2023, and for the years ended December 31, 2024, 2023 and 2022. The Consolidated Financial Statements have been approved by the Directors on April 30, 2025, and are prepared in accordance with UK-adopted International Financial Reporting Standards ("IFRSs"). The Consolidated Financial Statements also comply fully with IFRSs as issued by the International Accounting Standards Board ("IASB"). UK-adopted IFRSs differs in certain respects from IFRSs as issued by the IASB. However, the differences have no impact for the periods presented. For presentation of the Consolidated Statement of Comprehensive Income/(Loss), the Group uses a classification based on the function of expenses, rather than based on their nature, as it is more representative of the format used for internal reporting and management purposes and is consistent with international practice. Certain amounts in the Consolidated Financial Statements and accompanying notes may not add due to rounding. All percentages have been calculated using unrounded amounts. Basis of Measurement The Consolidated Financial Statements are prepared on the historical cost basis except that the following assets and liabilities are stated at their fair value: investments held at fair value, investments in notes from associates and liabilities classified as fair value through the profit or loss. Use of Judgments and Estimates In preparing the Consolidated Financial Statements, management has made judgements, estimates and assumptions that affect the application of the Group’s accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an on-going basis. Significant estimation is applied in determining the following: — Financial instruments (see Note 19. Financial Instruments): In accordance with IFRS 9, Financial Instruments ("IFRS 9"), the Group carries certain financial assets and financial liabilities at fair value, with changes in fair value through profit and loss ("FVTPL"). Valuation of the aforementioned financial instruments includes determining the appropriate valuation methodology and making certain estimates such as the equity value of an entity, volatility, and term to liquidity. Significant judgement is also applied in determining the following: — Whether financial instruments should be classified as liability or equity (see Note 17. Subsidiary Preferred Shares.). The judgement includes an assessment of whether the financial instruments include contractual obligations of the Group to deliver cash or other financial assets or to exchange financial assets or financial liabilities with another party, and whether those obligations could be settled by the Group exchanging a fixed amount of cash or other financial assets for a fixed number of its own equity instruments. Further information about these critical judgements and estimates is included below under Financial Instruments. — Whether the power to control investees exists (see Note 5. Investments Held at Fair Value, Note 6. Investments in Associates and Note 8. Gain/(loss) on Deconsolidation of Subsidiary and accounting policy with regard to Subsidiaries below). The judgement includes an assessment of whether the Group has (i) power over the investee; (ii) exposure, or rights, to variable returns from its involvement with the investee; and (iii) the ability to use its power over the investee to affect the amount of its own returns. The Group considers among others its voting shares, shareholder agreements, ability to appoint board members, representation on the board, rights to appoint management, de facto control, investee dependence on the Group, etc. If the power to control the investee exists, it consolidates the financial statements of such investee in the Consolidated Financial Statements of the Group. Upon issuance of new shares in an investee and/or a change in any shareholders or governance agreements, the Group reassesses its ability to control the investee based on the revised voting interest, revised board composition and revised subsidiary governance and management structure. When such new circumstances result in the Group losing its power to control the investee, the investee is deconsolidated.

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 131 1. Accounting policies continued Notes to the Consolidated Financial Statements continued — Whether the Group has significant influence over financial and operating policies of investees in order to determine if the Group should account for its investment as an associate based on IAS 28 Investments in Associates and Joint Ventures ("IAS 28") or a financial instrument based on IFRS 9 (refer to Note 5. Investments Held at Fair Value and Note 6. Investments in Associates ). This judgement includes, among others, an assessment whether the Group has representation on the board of directors of the investee, whether the Group participates in the policy making processes of the investee, whether there is any interchange of managerial personnel, whether there is any essential technical information provided to the investee and if there are any transactions between the Group and the investee. — Upon determining that the Group does have significant influence over the financial and operating policies of an investee, if the Group holds more than a single instrument issued by its equity-accounted investee, judgement is required to determine whether the additional instrument forms part of the investment in the associate, which is accounted for under IAS 28 and scoped out of IFRS 9, or it is a separate financial instrument that falls in the scope of IFRS 9. This judgement includes an assessment of the characteristics of the financial instrument of the investee held by the Group and whether such financial instrument provides access to returns underlying an ownership interest. — When the Group has other investments in an equity accounted investee that are not accounted for under IAS 28, judgement is required in determining if such investments constitute long-term interests ("LTI") for the purposes of IAS 28. This determination is based on the individual facts and circumstances and characteristics of each investment, but is driven, among other factors, by the intention and likelihood to settle the instrument through redemption or repayment in the foreseeable future, and whether or not the investment is likely to be converted to common stock or other equity instruments. After considering the individual facts and circumstances of the Group’s investment in its associate's preferred stock in the manner described above, including the long-term nature of such investment, the ability of the Group to convert its preferred stock investment to an investment in common shares and the likelihood of such conversion, the Group concluded that such investment was considered a long-term interest. — In determining the appropriate accounting treatment for the Royalty Purchase Agreement during 2023, management applied significant judgement (refer to Note 18. Sale of Future Royalties Liability). As of December 31, 2024, the Group had cash and cash equivalents of $280,641 and short-term investments of $86,666. Considering the Group’s financial position as of December 31, 2024, and its principal risks and opportunities, the Group prepared a going concern analysis covering a period of at least the twelve-month period from the date of signing the Consolidated Financial Statements ("the going concern period") utilizing realistic scenarios and applying a severe but plausible downside scenario. Even under the downside scenario, the analysis demonstrates the Group continues to maintain sufficient liquidity headroom and continues to comply with all financial obligations. The Board of Directors believe the Group and the Parent is adequately resourced to continue in operational existence for at least the twelve-month period from the date of signing the Consolidated Financial Statements. Accordingly, the Board of Directors considered it appropriate to adopt the going concern basis of accounting in preparing the Consolidated Financial Statements and the PureTech Health plc Financial Statements. Basis of consolidation The Consolidated Financial Statements as of December 31, 2024 and 2023, and for each of the years ended December 31, 2024, 2023 and 2022, comprise PureTech Health plc and its consolidated subsidiaries. Intra-group balances and transactions, and any unrealized income and expenses arising from intra-group transactions, are eliminated. Subsidiaries As used in these financial statements, the term subsidiaries refers to entities that are controlled by the Group. Under applicable accounting rules, the Group controls an entity when it is exposed to, or has the rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. In assessing control, the Group takes into consideration potential voting rights, board representation, shareholders' agreements, ability to appoint board of directors and management, de facto control and other related factors. The financial statements of subsidiaries are included in the Consolidated Financial Statements from the date that control commences until the date that control ceases. Losses applicable to the non-controlling interests ("NCI") in a subsidiary are allocated to the non-controlling interests even if doing so causes the non- controlling interests to have a deficit balance. A list of all current and former subsidiaries organized with respect to classification as of December 31, 2024, and the Group’s total voting percentage, based on outstanding voting common and preferred shares as of December 31, 2024, 2023 and 2022, is outlined below. All current subsidiaries are domiciled within the United States and conduct business activities solely within the United States.
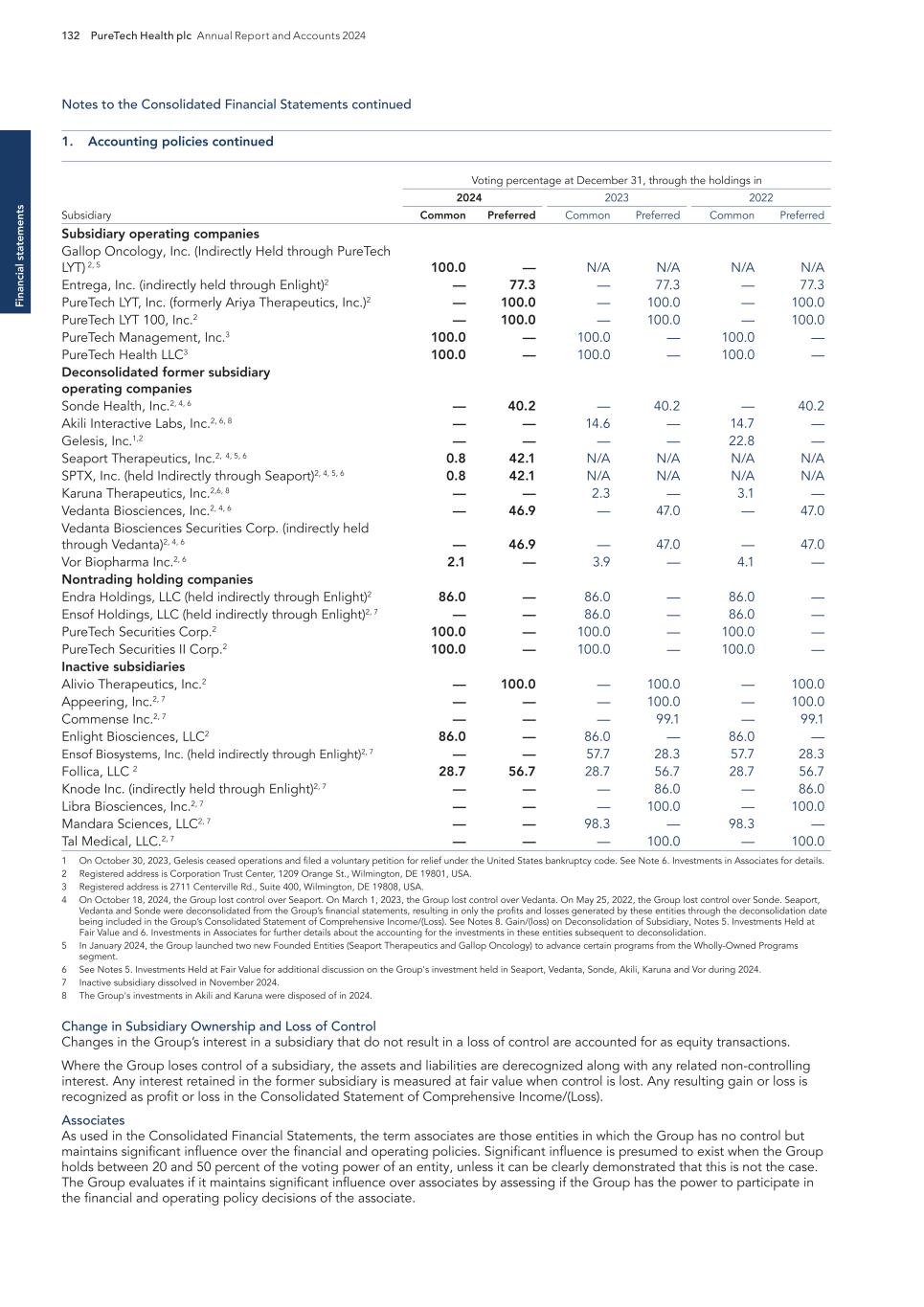
Fi na nc ia l s ta te m en ts 132 PureTech Health plc Annual Report and Accounts 2024 1. Accounting policies continued Notes to the Consolidated Financial Statements continued Voting percentage at December 31, through the holdings in 2024 2023 2022 Subsidiary Common Preferred Common Preferred Common Preferred Subsidiary operating companies Gallop Oncology, Inc. (Indirectly Held through PureTech LYT) 2, 5 100.0 — N/A N/A N/A N/A Entrega, Inc. (indirectly held through Enlight)2 — 77.3 — 77.3 — 77.3 PureTech LYT, Inc. (formerly Ariya Therapeutics, Inc.)2 — 100.0 — 100.0 — 100.0 PureTech LYT 100, Inc.2 — 100.0 — 100.0 — 100.0 PureTech Management, Inc.3 100.0 — 100.0 — 100.0 — PureTech Health LLC3 100.0 — 100.0 — 100.0 — Deconsolidated former subsidiary operating companies Sonde Health, Inc.2, 4, 6 — 40.2 — 40.2 — 40.2 Akili Interactive Labs, Inc.2, 6, 8 — — 14.6 — 14.7 — Gelesis, Inc.1,2 — — — — 22.8 — Seaport Therapeutics, Inc.2, 4, 5, 6 0.8 42.1 N/A N/A N/A N/A SPTX, Inc. (held Indirectly through Seaport)2, 4, 5, 6 0.8 42.1 N/A N/A N/A N/A Karuna Therapeutics, Inc.2,6, 8 — — 2.3 — 3.1 — Vedanta Biosciences, Inc.2, 4, 6 — 46.9 — 47.0 — 47.0 Vedanta Biosciences Securities Corp. (indirectly held through Vedanta)2, 4, 6 — 46.9 — 47.0 — 47.0 Vor Biopharma Inc.2, 6 2.1 — 3.9 — 4.1 — Nontrading holding companies Endra Holdings, LLC (held indirectly through Enlight)2 86.0 — 86.0 — 86.0 — Ensof Holdings, LLC (held indirectly through Enlight)2, 7 — — 86.0 — 86.0 — PureTech Securities Corp.2 100.0 — 100.0 — 100.0 — PureTech Securities II Corp.2 100.0 — 100.0 — 100.0 — Inactive subsidiaries Alivio Therapeutics, Inc.2 — 100.0 — 100.0 — 100.0 Appeering, Inc.2, 7 — — — 100.0 — 100.0 Commense Inc.2, 7 — — — 99.1 — 99.1 Enlight Biosciences, LLC2 86.0 — 86.0 — 86.0 — Ensof Biosystems, Inc. (held indirectly through Enlight)2, 7 — — 57.7 28.3 57.7 28.3 Follica, LLC 2 28.7 56.7 28.7 56.7 28.7 56.7 Knode Inc. (indirectly held through Enlight)2, 7 — — — 86.0 — 86.0 Libra Biosciences, Inc.2, 7 — — — 100.0 — 100.0 Mandara Sciences, LLC2, 7 — — 98.3 — 98.3 — Tal Medical, LLC.2, 7 — — — 100.0 — 100.0 1 On October 30, 2023, Gelesis ceased operations and filed a voluntary petition for relief under the United States bankruptcy code. See Note 6. Investments in Associates for details. 2 Registered address is Corporation Trust Center, 1209 Orange St., Wilmington, DE 19801, USA. 3 Registered address is 2711 Centerville Rd., Suite 400, Wilmington, DE 19808, USA. 4 On October 18, 2024, the Group lost control over Seaport. On March 1, 2023, the Group lost control over Vedanta. On May 25, 2022, the Group lost control over Sonde. Seaport, Vedanta and Sonde were deconsolidated from the Group’s financial statements, resulting in only the profits and losses generated by these entities through the deconsolidation date being included in the Group’s Consolidated Statement of Comprehensive Income/(Loss). See Notes 8. Gain/(loss) on Deconsolidation of Subsidiary, Notes 5. Investments Held at Fair Value and 6. Investments in Associates for further details about the accounting for the investments in these entities subsequent to deconsolidation. 5 In January 2024, the Group launched two new Founded Entities (Seaport Therapeutics and Gallop Oncology) to advance certain programs from the Wholly-Owned Programs segment. 6 See Notes 5. Investments Held at Fair Value for additional discussion on the Group's investment held in Seaport, Vedanta, Sonde, Akili, Karuna and Vor during 2024. 7 Inactive subsidiary dissolved in November 2024. 8 The Group's investments in Akili and Karuna were disposed of in 2024. Change in Subsidiary Ownership and Loss of Control Changes in the Group’s interest in a subsidiary that do not result in a loss of control are accounted for as equity transactions. Where the Group loses control of a subsidiary, the assets and liabilities are derecognized along with any related non-controlling interest. Any interest retained in the former subsidiary is measured at fair value when control is lost. Any resulting gain or loss is recognized as profit or loss in the Consolidated Statement of Comprehensive Income/(Loss). Associates As used in the Consolidated Financial Statements, the term associates are those entities in which the Group has no control but maintains significant influence over the financial and operating policies. Significant influence is presumed to exist when the Group holds between 20 and 50 percent of the voting power of an entity, unless it can be clearly demonstrated that this is not the case. The Group evaluates if it maintains significant influence over associates by assessing if the Group has the power to participate in the financial and operating policy decisions of the associate.

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 133 1. Accounting policies continued Notes to the Consolidated Financial Statements continued Application of the Equity Method to Associates Associates are accounted for using the equity method (equity accounted investees) and are initially recognized at cost, or if recognized upon deconsolidation, they are initially recorded at fair value at the date of deconsolidation. The Consolidated Financial Statements include the Group’s share of the total comprehensive income or loss of equity accounted investees, from the date that significant influence commences until the date that significant influence ceases. To the extent the Group holds interests in associates that are not providing access to returns underlying ownership interests, the instrument is accounted for in accordance with IFRS 9 as investments held at fair value. When the Group’s share of losses exceeds its equity method investment in the investee, losses are applied against long-term interests, which are investments accounted for under IFRS 9. Investments are determined to be long-term interests when they are long-term in nature and in substance they form part of the Group's net investment in that associate. This determination is impacted by many factors, among others, whether settlement by the investee through redemption or repayment is planned or likely in the foreseeable future, whether the investment can be converted and/or is likely to be converted to common stock or other equity instrument and other factors regarding the nature of the investment. Whilst this assessment is dependent on many specific facts and circumstances of each investment, typically conversion features whereby the investment is likely to convert to common stock or other equity instruments would point to the investment being a long-term interest. Similarly, where the investment is not planned or likely to be settled through redemption or repayment in the foreseeable future, this would indicate that the investment is a long-term interest. When the net investment in the associate, which includes the Group’s investments in other long-term interests, is reduced to nil, recognition of further losses is discontinued except to the extent that the Group has incurred legal or constructive obligations or made payments on behalf of an investee. The Group has adopted the amendments to IAS 28 that addresses the dual application of IAS 28 and IFRS 9 when equity method losses are applied against long-term interests. The amendments provide the annual sequence in which both standards are to be applied in such a case. The Group has applied the equity method losses to the long-term interests presented as part of Investments held at fair value subsequent to remeasuring such investments to their fair value at balance sheet date. Sale of Future Royalties Liability The Group accounts for the sale of future royalties liability as a financial liability, as it continues to hold the rights under the royalty bearing licensing agreement and has a contractual obligation to deliver cash to an investor for a portion of the royalty it receives. Interest on the sale of future royalties liability is recognized using the effective interest rate over the life of the related royalty stream. The sale of future royalties liability and the related interest expense are based on the Group’s current estimates of future royalties expected to be paid over the life of the arrangement. Forecasts are updated periodically as new data is obtained. Any increases, decreases or a shift in timing of estimated cash flows require the Group to re-calculate the amortized cost of the sale of future royalties liability as the present value of the estimated future contractual cash flows that are discounted at the liability’s original effective interest rate. The adjustment is recognized immediately in profit or loss as income or expense. Financial Instruments Classification The Group classifies its financial assets in the following measurement categories: — Those to be measured subsequently at fair value either through other comprehensive income "FVOCI", or through profit or loss "FVTPL", and — Those to be measured at amortized cost. The classification depends on the Group’s business model for managing the financial assets and the contractual terms of the cash flows. For assets measured at fair value, gains and losses are recorded in profit or loss. Measurement At initial recognition, the Group measures a financial asset at its fair value plus, in the case of a financial asset not at FVTPL, transaction costs that are directly attributable to the acquisition of the financial asset. Transaction costs of financial assets that are carried at FVTPL are expensed. Impairment The Group assesses on a forward-looking basis the expected credit losses associated with its debt instruments carried at amortized cost. For trade receivables, the Group applies the simplified approach permitted by IFRS 9, which requires expected lifetime losses to be recognized from initial recognition of the receivables. Financial Assets The Group’s financial assets consist of cash and cash equivalents, investments in debt securities, trade and other receivables, investments in notes from associates, restricted cash deposits and investments in equity securities. The Group’s financial assets are virtually all classified into the following categories: investments held at fair value, investments in notes from associates, trade and other receivables, short-term investments and cash and cash equivalents. The Group determines the classification of financial assets at initial recognition depending on the purpose for which the financial assets were acquired. Investments held at fair value are investments in equity instruments. Such investments consist of the Group's minority interest holdings where the Group has no significant influence or preferred share investments that are not providing access to returns underlying ownership interests and are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest. These financial assets are initially measured at fair value and subsequently re-measured at fair value at each reporting date. The Group has elected to record the changes in fair values for the financial assets falling under this category through profit and loss. Please refer to Note 5. Investments Held at Fair Value.

Fi na nc ia l s ta te m en ts 134 PureTech Health plc Annual Report and Accounts 2024 1. Accounting policies continued Notes to the Consolidated Financial Statements continued Changes in the fair value of financial assets at FVTPL are recognized in other income/(expense) in the Consolidated Statement of Comprehensive Income/(Loss) as applicable. The investments in notes from associates, since their contractual terms do not consist solely of cash flow payments of principal and interest on the principal amount outstanding, are initially and subsequently measured at fair value, with changes in fair value recognized through profit and loss. Cash and cash equivalents consist of demand deposits with banks and other financial institutions and highly liquid instruments with original maturities of three months or less at the date of purchase. Cash and cash equivalents are carried at cost, which approximates their fair value. Short-term investments consist of short-term US treasury bills that are held to maturity. The contractual terms consist solely of payment of the principal and interest and the Group's business model is to hold the treasury bills to maturity. As such, such short- term investments are recorded at amortized cost. As of balance sheet date, amortized cost approximated the fair value of such short-term investments. Trade and other receivables are non-derivative financial assets with fixed and determinable payments that are not quoted on active markets. These financial assets are carried at the amounts expected to be received less any expected lifetime losses. Such losses are determined taking into account previous experience, credit rating and economic stability of counterparty and economic conditions. When a trade receivable is determined to be uncollectible, it is written off against the available provision. As of balance sheet date, the Group did not record any such expected lifetime losses related to the outstanding trade and other receivable balances. Trade and other receivables are included in current assets, unless maturities are greater than 12 months after the end of the reporting period. Financial Liabilities The Group’s financial liabilities primarily consist of trade and other payables, and preferred shares. The majority of the Group’s subsidiaries have preferred shares and certain notes payable with embedded derivatives, which are classified as current liabilities. When the Group has preferred shares and notes with embedded derivatives that qualify for bifurcation, the Group has elected to account for the entire instrument as FVTPL after determining under IFRS 9 that the instrument qualifies to be accounted for under such FVTPL method. The Group derecognizes a financial liability when its contractual obligations are discharged, cancelled or expire. Equity Instruments Issued by the Group Financial instruments issued by the Group are treated as equity only to the extent that they meet the following two conditions, in accordance with IAS 32: 1. They include no contractual obligations upon the Group to deliver cash or other financial assets or to exchange financial assets or financial liabilities with another party under conditions that are potentially unfavorable to the Group; and 2. Where the instrument will or may be settled in the Group’s own equity instruments, it is either a non-derivative that includes no obligation to deliver a variable number of the Group’s own equity instruments or is a derivative that will be settled by the Group exchanging a fixed amount of cash or other financial assets for a fixed number of its own equity instruments. To the extent that this definition is not met, the financial instrument is classified as a financial liability. Where the instrument so classified takes the legal form of the Group’s own shares, the amounts presented in the Group's shareholders' equity exclude amounts in relation to those shares. Changes in the fair value of liabilities at FVTPL are recognized in net finance income /(costs) in the Consolidated Statement of Comprehensive Income/(Loss) as applicable. IFRS 15, Revenue from Contracts with Customers The standard establishes a five-step principle-based approach for revenue recognition and is based on the concept of recognizing an amount that reflects the consideration for performance obligations only when they are satisfied, and the control of goods or services is transferred. The majority of the Group’s contract revenue is generated from licenses and services, some of which are part of collaboration arrangements. Management reviewed contracts where the Group received consideration in order to determine whether or not they should be accounted for in accordance with IFRS 15. To date, the Group has entered into transactions that generate revenue and meet the scope of either IFRS 15 or IAS 20 Accounting for Government Grants. Contract revenue is recognized at either a point-in-time or over time, depending on the nature of the performance obligations. The Group accounts for agreements that meet the definition of IFRS 15 by applying the following five step model: — Identify the contract(s) with a customer – A contract with a customer exists when (i) the Group enters into an enforceable contract with a customer that defines each party’s rights regarding the goods or services to be transferred and identifies the payment terms related to those goods or services, (ii) the contract has commercial substance and, (iii) the Group determines that collection of substantially all consideration for goods or services that are transferred is probable based on the customer’s intent and ability to pay the promised consideration. — Identify the performance obligations in the contract – Performance obligations promised in a contract are identified based on the goods or services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the good or service either on its own or together with other resources that are readily available from third parties or from the Group, and are distinct in the context of the contract, whereby the transfer of the goods or services is separately identifiable from other promises in the contract.

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 135 1. Accounting policies continued Notes to the Consolidated Financial Statements continued — Determine the transaction price – The transaction price is determined based on the consideration to which the Group will be entitled in exchange for transferring goods or services to the customer. To the extent the transaction price includes variable consideration, the Group estimates the amount of variable consideration that should be included in the transaction price utilizing either the expected value method or the most likely amount method depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in the Group’s judgement, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. — Allocate the transaction price to the performance obligations in the contract – If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative standalone selling price basis. — Recognize revenue when (or as) the Group satisfies a performance obligation – The Group satisfies performance obligations either over time or at a point in time as discussed in further detail below. Revenue is recognized at the time the related performance obligation is satisfied by transferring a promised good or service to a customer. Revenue generated from services agreements (typically where licenses and related services were combined into one performance obligation) is determined to be recognized over time when it can be determined that the services meet one of the following: (a) the customer simultaneously receives and consumes the benefits provided by the entity’s performance as the entity performs; (b) the entity’s performance creates or enhances an asset that the customer controls as the asset is created or enhanced; or (c) the entity’s performance does not create an asset with an alternative use to the entity and the entity has an enforceable right to payment for performance completed to date. It was determined that the Group has contracts that meet criteria (a), since the customer simultaneously receives and consumes the benefits provided by the Group’s performance as the Group performs. Therefore, revenue is recognized over time using the input method based on costs incurred to date as compared to total contract costs. The Group believes that in research and development service type agreements using costs incurred to date represents the most faithful depiction of the entity’s performance towards complete satisfaction of a performance obligation. Revenue from licenses that are not part of a combined performance obligation are recognized at a point in time. Such licenses relate to intellectual property that has significant stand-alone functionality and as such represent a right to use the entity's intellectual property as it exists at the point in time at which the license is granted. Royalty revenue received in respect of licensing agreements when the license of intellectual property is the predominant item in the arrangement is recognized as the related third-party sales in the licensee occur. Amounts that are receivable or have been received per contractual terms but have not been recognized as revenue since performance has not yet occurred or has not yet been completed are recorded as deferred revenue. The Group classifies as non- current deferred revenue amounts received for which performance is expected to occur beyond one year or one operating cycle. Grant Revenue The Group recognizes grants from governmental agencies as grant revenue in the Consolidated Statement of Comprehensive Income/(Loss), gross of the expenditures that were related to obtaining the grant, when there is reasonable assurance that the Group will comply with the conditions within the grant agreement and there is reasonable assurance that payments under the grants will be received. The Group evaluates the conditions of each grant as of each reporting date to ensure that the Group has reasonable assurance of meeting the conditions of each grant arrangement and that it is expected that the grant payment will be received as a result of meeting the necessary conditions. The Group submits qualifying expenses for reimbursement after the Group has incurred the research and development expense. The Group records an unbilled receivable upon incurring such expenses. In cases in which the grant revenue is received prior to the expenses being incurred or recognized, the amounts received are deferred until the related expense is incurred and/or recognized. Grant revenue is recognized in the Consolidated Statement of Comprehensive Income/(Loss) at the time in which the Group recognizes the related reimbursable expense for which the grant is intended to compensate. Functional and Presentation Currency The Consolidated Financial Statements are presented in United States dollars (“US dollars”). The functional currency of all members of the Group is the U.S. dollar. The Group's share in foreign exchange differences in associates were reported in other comprehensive income/(loss). Foreign Currency Transactions in foreign currencies are translated to the respective functional currencies of Group entities at the foreign exchange rate ruling at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies at the balance sheet date are retranslated to the functional currency at the foreign exchange rate ruling at that date. Foreign exchange differences arising on remeasurement are recognized in the Consolidated Statement of Comprehensive Income/(Loss). Non-monetary assets and liabilities that are measured in terms of historical cost in a foreign currency are translated using the exchange rate at the date of the transaction. Share Capital Ordinary shares are classified as equity. The Group's equity is comprised of share capital, share premium, merger reserve, other reserve, translation reserve, and retained earnings/accumulated deficit. Treasury Shares Treasury shares acquired as a result of repurchasing shares are recognized at cost and are deducted from shareholders' equity. No gain or loss is recognized in profit and loss for the purchase, sale, re-issue or cancellation of the Group's own equity shares. The nominal value related to shares that are repurchased and cancelled are reduced from share capital and transferred to a capital redemption reserve.

Fi na nc ia l s ta te m en ts 136 PureTech Health plc Annual Report and Accounts 2024 1. Accounting policies continued Notes to the Consolidated Financial Statements continued Property and Equipment Property and equipment is stated at cost less accumulated depreciation and any accumulated impairment losses. Cost includes expenditures that are directly attributable to the acquisition of the asset. Assets under construction represent leasehold improvements and machinery and equipment to be used in operations or research and development activities. When parts of an item of property and equipment have different useful lives, they are accounted for as separate items (major components) of property and equipment. Depreciation is calculated using the straight-line method over the estimated useful life of the related asset: Laboratory and manufacturing equipment 2-8 years Furniture and fixtures 7 years Computer equipment and software 1-5 years Leasehold improvements 5-10 years, or the remaining term of the lease, if shorter Depreciation methods, useful lives and residual values are reviewed at each balance sheet date. Intangible Assets Intangible assets, which include purchased patents and licenses with finite useful lives, are carried at historical cost less accumulated amortization, if amortization has commenced. Intangible assets with finite lives are amortized from the time they are available for their intended use. Amortization is calculated using the straight-line method to allocate the costs of patents and licenses over their estimated useful lives. Research and development intangible assets, which are still under development and have accordingly not yet obtained marketing approval, are presented as In-Process Research and Development (IPR&D). The cost of IPR&D represents upfront payments as well as additional contingent payments based on development, regulatory and sales milestones related to certain license agreement where the Group licenses IP from a third party. These milestones are capitalized as the milestone is triggered. See Note 25. Commitments and Contingencies. IPR&D is not amortized since it is not yet available for its intended use, but it is evaluated for potential impairment on an annual basis or more frequently when facts and circumstances warrant. Impairment of Non-Financial Assets The Group reviews the carrying amounts of its property and equipment and intangible assets at each reporting date to determine whether there are indicators of impairment. If any such indicators of impairment exist, then an asset’s recoverable amount is estimated. The recoverable amount is the higher of an asset’s fair value less cost of disposal and value in use. The Group’s IPR&D intangible assets are not yet available for their intended use. As such, they are tested for impairment at least annually. An impairment loss is recognized when an asset’s carrying amount exceeds its recoverable amount. For the purposes of impairment testing, assets are grouped at the lowest levels for which there are largely independent cash flows. If a non-financial asset instrument is impaired, an impairment loss is recognized in the Consolidated Statement of Comprehensive Income/(Loss). Investments in associates are considered impaired if, and only if, objective evidence indicates that one or more events, which occurred after the initial recognition, have had an impact on the future cash flows from the net investment and that impact can be reliably estimated. If an impairment exists, the Group measures an impairment by comparing the carrying value of the net investment in the associate to its recoverable amount and recording any excess as an impairment loss. See Note 6. Investments in Associates for impairment recorded in respect of an investment in associate during the year ended December 31, 2022. Employee Benefits Short-Term Employee Benefits Short-term employee benefit obligations are measured on an undiscounted basis and expensed as the related service is provided. A liability is recognized for the amount expected to be paid if the Group has a present legal or constructive obligation due to past service provided by the employee, and the obligation can be estimated reliably. Defined Contribution Plans A defined contribution plan is a post-employment benefit plan under which an entity pays fixed contributions into a separate entity and has no legal or constructive obligation to pay further amounts. Obligations for contributions to defined contribution plans are recognized as an employee benefit expense in the periods during which related services are rendered by employees. Share-based Payments Share-based payment arrangements, in which the Group receives goods or services as consideration for its own equity instruments, are accounted for as equity-settled share-based payment transactions (except certain restricted stock units – see below) in accordance with IFRS 2. The grant date fair value of employee share-based payment awards is recognized as an expense with a corresponding increase in equity over the requisite service period related to the awards. The amount recognized as an expense is adjusted to reflect the actual number of awards for which the related service and non-market performance conditions are expected to be met, such that the amount ultimately recognized as an expense is based on the number of awards that do meet the related service and non-market performance conditions at the vesting date. For share-based payment awards with market conditions, the grant date fair value is measured to reflect such conditions and there is no true-up for differences between expected and actual outcomes. Certain restricted stock units are treated as liability settled awards starting in 2021. Such awards are remeasured at every reporting date until settlement date and are recognized as compensation expense over the requisite service period. Differences in remeasurement are recognized in profit and loss. The cumulative cost that will ultimately be recognized in respect of these awards will equal to the amount at settlement. The fair value of the awards is measured using option pricing models and other appropriate models, which take into account the terms and conditions of the awards granted.

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 137 1. Accounting policies continued Notes to the Consolidated Financial Statements continued Development Costs Expenditures on research activities are recognized as incurred in the Consolidated Statement of Comprehensive Income/(Loss). In accordance with IAS 38, development costs are capitalized only if the expenditure can be measured reliably, the product or process is technically and commercially feasible, future economic benefits are probable, the Group can demonstrate its ability to use or sell the intangible asset, the Group intends to and has sufficient resources to complete development and to use or sell the asset, and it is able to measure reliably the expenditure attributable to the intangible asset during its development. The point at which technical feasibility is determined to have been reached is, generally, when regulatory approval has been received where applicable. Management determines that commercial viability has been reached when a clear market and pricing point have been identified, which may coincide with achieving meaningful recurring sales. Otherwise, the development expenditure is recognized as incurred in the Consolidated Statement of Comprehensive Income/(Loss). As of balance sheet date, the Group has not capitalized any development costs. Provisions A provision is recognized in the Consolidated Statement of Financial Position when the Group has a present legal or constructive obligation due to a past event that can be reliably measured, and it is probable that an outflow of economic benefits will be required to settle the obligation. Provisions are determined by discounting the expected future cash flows at a pre-tax rate that reflects risks specific to the liability. Leases The Group leases real estate for use in operations. These leases have lease terms of approximately 10 years. The Group includes options that are reasonably certain to be exercised as part of the determination of the lease term. The group determines if an arrangement is a lease at inception of the contract in accordance with guidance detailed in IFRS 16. Right-of-use ("ROU") assets represent the Group’s right to use an underlying asset for the lease term and lease liabilities represent the Group's obligation to make lease payments arising from the lease. Operating lease ROU assets and lease liabilities are recognized at commencement date based on the present value of the lease payments over the lease term. As most of the Group's leases do not provide an implicit rate, the Group used its estimated incremental borrowing rate, based on information available at commencement date, in determining the present value of future payments. The Group’s leases are virtually all leases of real estate. The Group has elected to account for lease payments as an expense on a straight-line basis over the life of the lease for: — Leases with a term of 12 months or less and containing no purchase options; and — Leases where the underlying asset has a value of less than $5,000. The right-of-use asset is depreciated on a straight-line basis and the related lease liability gives rise to an interest charge. Finance Income and Finance Costs Finance income consists of interest income on funds invested in money market funds and U.S. treasuries. Finance income is recognized as it is earned. Finance costs consist mainly of loan, notes and lease liability interest expenses, interest expense due to accretion of and adjustment to sale of future royalties liability as well as the changes in the fair value of financial liabilities carried at FVTPL (such changes can consist of finance income when the fair value of such financial liabilities decrease). Taxation Tax on the profit or loss for the year comprises current and deferred income tax. In accordance with IAS 12, tax is recognized in the Consolidated Statement of Comprehensive Income/(Loss) except to the extent that it relates to items recognized directly in equity. Current income tax is the expected tax payable or receivable on the taxable income or loss for the year, using tax rates enacted or substantially enacted at the reporting date, and any adjustment to tax payable in respect of previous years. Deferred tax is recognized due to temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax assets are recognized for unused tax losses, unused tax credits and deductible temporary differences to the extent that it is probable that future taxable profits will be available against which they can be used. Deferred tax assets with respect to investments in associates are recognized only to the extent that it is probable the temporary difference will reverse in the foreseeable future and taxable profit will be available against which the temporary difference can be utilized. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realized. Deferred tax is measured at the tax rates that are expected to be applied to temporary differences when they reverse, using tax rates enacted or substantively enacted at the reporting date. Deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when the deferred income tax assets and liabilities relate to income taxes levied by the same taxation authority on either the same taxable entity or different taxable entities where there is an intention to settle the balances on a net basis.

Fi na nc ia l s ta te m en ts 138 PureTech Health plc Annual Report and Accounts 2024 1. Accounting policies continued Notes to the Consolidated Financial Statements continued Fair Value Measurements The Group’s accounting policies require that certain financial assets and certain financial liabilities be measured at their fair value. The Group uses valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, maximizing the use of relevant observable inputs and minimizing the use of unobservable inputs. Fair values are categorized into different levels in a fair value hierarchy based on the inputs used in the valuation techniques as follows: — Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities. — Level 2: inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices). — Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs). The Group recognizes transfers between levels of the fair value hierarchy at the end of the reporting period during which the change has occurred. The carrying amount of cash and cash equivalents, accounts receivable, restricted cash, deposits, accounts payable, accrued expenses and other current liabilities in the Group’s Consolidated Statement of Financial Position approximates their fair value because of the short maturities of these instruments. Operating Segments Operating segments are reported in a manner that is consistent with the internal reporting provided to the chief operating decision maker (“CODM”). The CODM reviews discrete financial information for the operating segments in order to assess their performance and is responsible for making decisions about resources allocated to the segments. The CODM has been identified as the Group’s Board of Directors. 2. New Standards and Interpretations The Group has applied Amendments to IAS 1 Presentation of Financial Statements: Classification of Liabilities as Current or Non- Current for the first time for its reporting period ended December 31, 2024. This amendment did not have any impact on the amounts recognized in prior and current periods. In April 2024, IFRS 18, Presentation and Disclosure in Financial Statements was issued to achieve comparability of the financial performance of similar entities. The standard, which replaces IAS 1 Presentation of Financial Statements, impacts the presentation of primary financial statements and notes, including the statement of earnings where companies will be required to present separate categories of income and expense for operating, investing, and financing activities with prescribed subtotals for each new category. The standard will also require management-defined performance measures to be explained and included in a separate note within the consolidated financial statements. The standard is effective for annual reporting periods beginning on or after January 1, 2027, including interim financial statements, and requires retrospective application. The Group is currently assessing the impact of the new standard. In May 2024, Amendments to IFRS 9 and IFRS 7, Targeted Improvements to Financial Instruments Standards, was issued to clarify the date of recognition and derecognition of some financial assets and liabilities, with a new exception for some financial liabilities settled through an electronic cash transfer system; clarify and add further guidance for assessing whether a financial asset meets the solely payments of principal and interest (SPPI) criterion; add new disclosures for certain instruments with contractual terms that can change cash flows (such as some instruments with features linked to the achievement of environment, social and governance (ESG) targets); and update the disclosures for equity instruments designated at fair value through other comprehensive income (FVOCI). The standard is effective for annual reporting periods beginning on or after January 1, 2026, including interim financial statements, and requires prospective application. The Group is currently assessing the impact of the new standard. In July 2024, the International Accounting Standards Board published the IFRS Interpretations Committee ("Committee")'s agenda decision clarifying certain requirements for disclosure of revenue and expenses for reporting segments under IFRS 8, Operating Segments. Committee agenda decisions do not have an effective date as entities are afforded a sufficient amount of time to implement them. The Group is currently assessing the impact of the Committee agenda decision and plans to apply the new requirements in its annual financial statements for the year ending December 31, 2025. Certain other new accounting standards, interpretations, and amendments to existing standards have been published that are effective for annual periods commencing on or after January 1, 2025 and have not been early adopted by the Group in preparing the Consolidated Financial Statements. These standards, amendments or interpretations are not expected to have a material impact on the Group in the prior, current, or future periods.
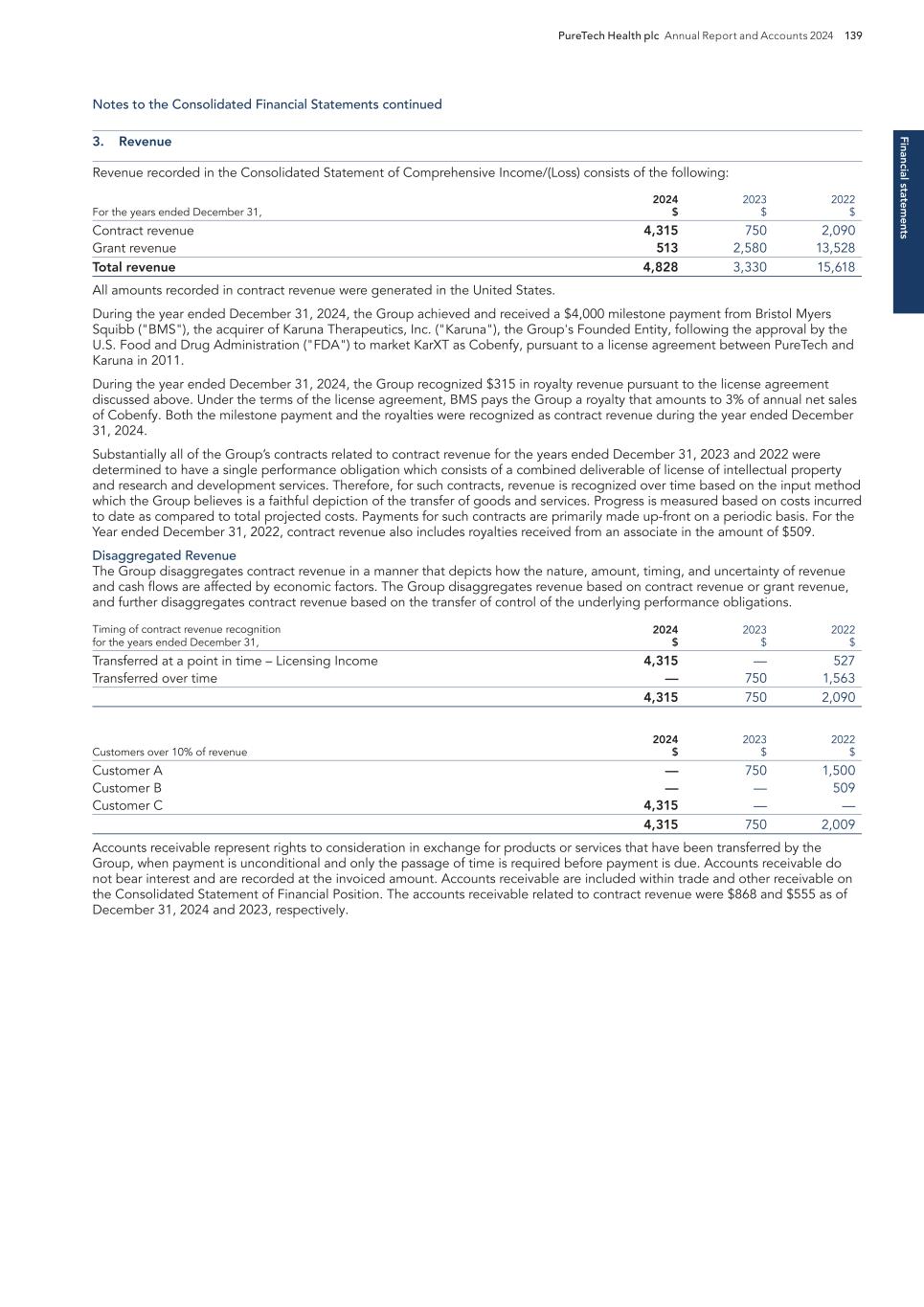
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 139 Notes to the Consolidated Financial Statements continued 3. Revenue Revenue recorded in the Consolidated Statement of Comprehensive Income/(Loss) consists of the following: For the years ended December 31, 2024 $ 2023 $ 2022 $ Contract revenue 4,315 750 2,090 Grant revenue 513 2,580 13,528 Total revenue 4,828 3,330 15,618 All amounts recorded in contract revenue were generated in the United States. During the year ended December 31, 2024, the Group achieved and received a $4,000 milestone payment from Bristol Myers Squibb ("BMS"), the acquirer of Karuna Therapeutics, Inc. ("Karuna"), the Group's Founded Entity, following the approval by the U.S. Food and Drug Administration ("FDA") to market KarXT as Cobenfy, pursuant to a license agreement between PureTech and Karuna in 2011. During the year ended December 31, 2024, the Group recognized $315 in royalty revenue pursuant to the license agreement discussed above. Under the terms of the license agreement, BMS pays the Group a royalty that amounts to 3% of annual net sales of Cobenfy. Both the milestone payment and the royalties were recognized as contract revenue during the year ended December 31, 2024. Substantially all of the Group’s contracts related to contract revenue for the years ended December 31, 2023 and 2022 were determined to have a single performance obligation which consists of a combined deliverable of license of intellectual property and research and development services. Therefore, for such contracts, revenue is recognized over time based on the input method which the Group believes is a faithful depiction of the transfer of goods and services. Progress is measured based on costs incurred to date as compared to total projected costs. Payments for such contracts are primarily made up-front on a periodic basis. For the Year ended December 31, 2022, contract revenue also includes royalties received from an associate in the amount of $509. Disaggregated Revenue The Group disaggregates contract revenue in a manner that depicts how the nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factors. The Group disaggregates revenue based on contract revenue or grant revenue, and further disaggregates contract revenue based on the transfer of control of the underlying performance obligations. Timing of contract revenue recognition for the years ended December 31, 2024 $ 2023 $ 2022 $ Transferred at a point in time – Licensing Income 4,315 — 527 Transferred over time — 750 1,563 4,315 750 2,090 Customers over 10% of revenue 2024 $ 2023 $ 2022 $ Customer A — 750 1,500 Customer B — — 509 Customer C 4,315 — — 4,315 750 2,009 Accounts receivable represent rights to consideration in exchange for products or services that have been transferred by the Group, when payment is unconditional and only the passage of time is required before payment is due. Accounts receivable do not bear interest and are recorded at the invoiced amount. Accounts receivable are included within trade and other receivable on the Consolidated Statement of Financial Position. The accounts receivable related to contract revenue were $868 and $555 as of December 31, 2024 and 2023, respectively.

Fi na nc ia l s ta te m en ts 140 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 4. Segment Information Basis for Segmentation The Directors are the Group’s chief operating decision-makers. The Group’s operating segments are determined based on the financial information provided to the Board of Directors periodically for the purposes of allocating resources and assessing performance. The Group has determined each of its Wholly-Owned Programs represents an operating segment and the Group has aggregated each of these operating segments into one reportable segment, the Wholly-Owned Programs segment. Each of the Group’s Controlled Founded Entities represents an operating segment. The Group aggregates each Controlled Founded Entity operating segment into one reportable segment, the Controlled Founded Entities segment. The aggregation is based on the high level of operational and financial similarities of the operating segments. For the Group’s entities that do not meet the definition of an operating segment, the Group presents this information in the Parent Company and Other column in its segment footnote to reconcile the information in this footnote to the Consolidated Financial Statements. Substantially all of the Group’s revenue and profit generating activities are generated within the United States and, accordingly, no geographical disclosures are provided. Following is the description of the Group's reportable segments: Wholly-Owned Programs The Wholly-Owned Programs segment is advancing Wholly-Owned Programs which are focused on treatments for patients with devastating diseases. The Wholly-Owned Programs segment is comprised of the technologies that are wholly-owned and will be advanced through with either the Group's funding or non-dilutive sources of financing. The operational management of the Wholly-Owned Programs segment is conducted by the PureTech Health team, which is responsible for the strategy, business development, and research and development. Controlled Founded Entities The Controlled Founded Entities segment is comprised of the Group’s consolidated operational subsidiaries as of December 31, 2024 that either have, or have plans to hire, independent management teams and currently have already raised third-party dilutive capital. These subsidiaries have active research and development programs and have an equity or debt investment partner, who will provide additional industry knowledge and access to networks, as well as additional funding to continue the pursued growth of the entity. The Group’s entities that were determined not to meet the definition of an operating segment are included in the Parent Company and Other column to reconcile the information in this footnote to the Consolidated Financial Statements. This column captures activities not directly attributable to the Group's operating segments and includes the activities of the Parent, corporate support functions, certain research and development support functions that are not directly attributable to a strategic business segment as well as the elimination of intercompany transactions. This column also captures the operating results for the deconsolidated entities through the date of deconsolidation (e.g. Seaport in 2024, Vedanta in 2023, and Sonde in 2022) and accounting for the Group's holdings in Founded Entities for which control has been lost, which primarily represent: the activity associated with deconsolidating an entity when the Group no longer controls the entity, the gain or loss on the Group's investments accounted for at fair value (e.g. the Group's ownership stakes in Vor, Vedanta, Sonde and Seaport) and the Group's net income or loss of associates accounted for using the equity method. The term "Founded Entities" refers to entities which the Group incorporated and announced the incorporation as a Founded Entity externally. It includes certain of the Group’s wholly-owned subsidiaries which have been announced by the Group as Founded Entities, Controlled Founded Entities and deconsolidated Founded Entities. In January 2024, the Group launched two new Founded Entities (Seaport Therapeutics "Seaport" and Gallop Oncology "Gallop") to advance certain programs from the Wholly-Owned Programs segment. The financial results of these programs were included in the Wholly-Owned Programs segment as of and for the year ended December 31, 2023. Seaport was deconsolidated on October 18, 2024 upon the completion of its Series B preferred share financing. The financial results of Seaport through the date of deconsolidation are included within the Parent Company and Other column as of December 31, 2024. It is impracticable for the Group to recast its segment results for the years ended December 31, 2023 and 2022 as the cost to develop the information would be excessive. However, as Seaport is a pre-commercial, clinical-stage biopharmaceutical company, it primarily performs research and development activities. Seaport incurred direct research and development expenses of $8,843 for the year ended December 31, 2023, which are included in the Wholly-Owned Program segment. Seaport incurred direct research and development expenses of $5,061 for the year ended December 31, 2024, prior to its deconsolidation from the Group’s Consolidated Financial Statements. As of December 31, 2024, Alivio was dormant and did not meet the definition of operating segment. Therefore, the financial results of Alivio were removed from the Wholly-Owned Programs segment and are included in the Parent Company and Other column. The corresponding information for 2023 and 2022 has been restated to include Alivio in the Parent Company and Other column so that the segment disclosures are presented on a comparable basis.
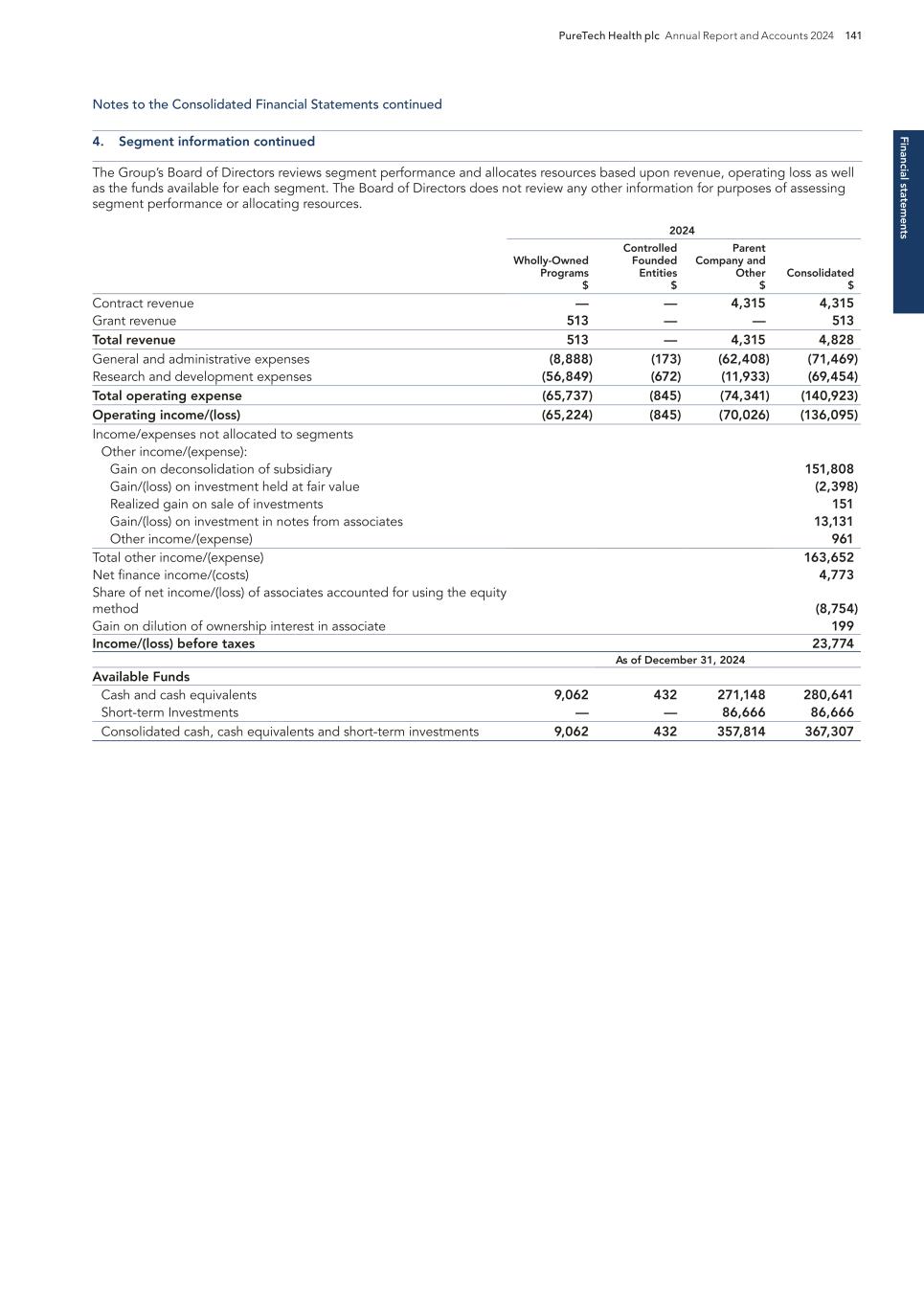
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 141 4. Segment information continued Notes to the Consolidated Financial Statements continued The Group’s Board of Directors reviews segment performance and allocates resources based upon revenue, operating loss as well as the funds available for each segment. The Board of Directors does not review any other information for purposes of assessing segment performance or allocating resources. 2024 Wholly-Owned Programs $ Controlled Founded Entities $ Parent Company and Other $ Consolidated $ Contract revenue — — 4,315 4,315 Grant revenue 513 — — 513 Total revenue 513 — 4,315 4,828 General and administrative expenses (8,888) (173) (62,408) (71,469) Research and development expenses (56,849) (672) (11,933) (69,454) Total operating expense (65,737) (845) (74,341) (140,923) Operating income/(loss) (65,224) (845) (70,026) (136,095) Income/expenses not allocated to segments Other income/(expense): Gain on deconsolidation of subsidiary 151,808 Gain/(loss) on investment held at fair value (2,398) Realized gain on sale of investments 151 Gain/(loss) on investment in notes from associates 13,131 Other income/(expense) 961 Total other income/(expense) 163,652 Net finance income/(costs) 4,773 Share of net income/(loss) of associates accounted for using the equity method (8,754) Gain on dilution of ownership interest in associate 199 Income/(loss) before taxes 23,774 As of December 31, 2024 Available Funds Cash and cash equivalents 9,062 432 271,148 280,641 Short-term Investments — — 86,666 86,666 Consolidated cash, cash equivalents and short-term investments 9,062 432 357,814 367,307
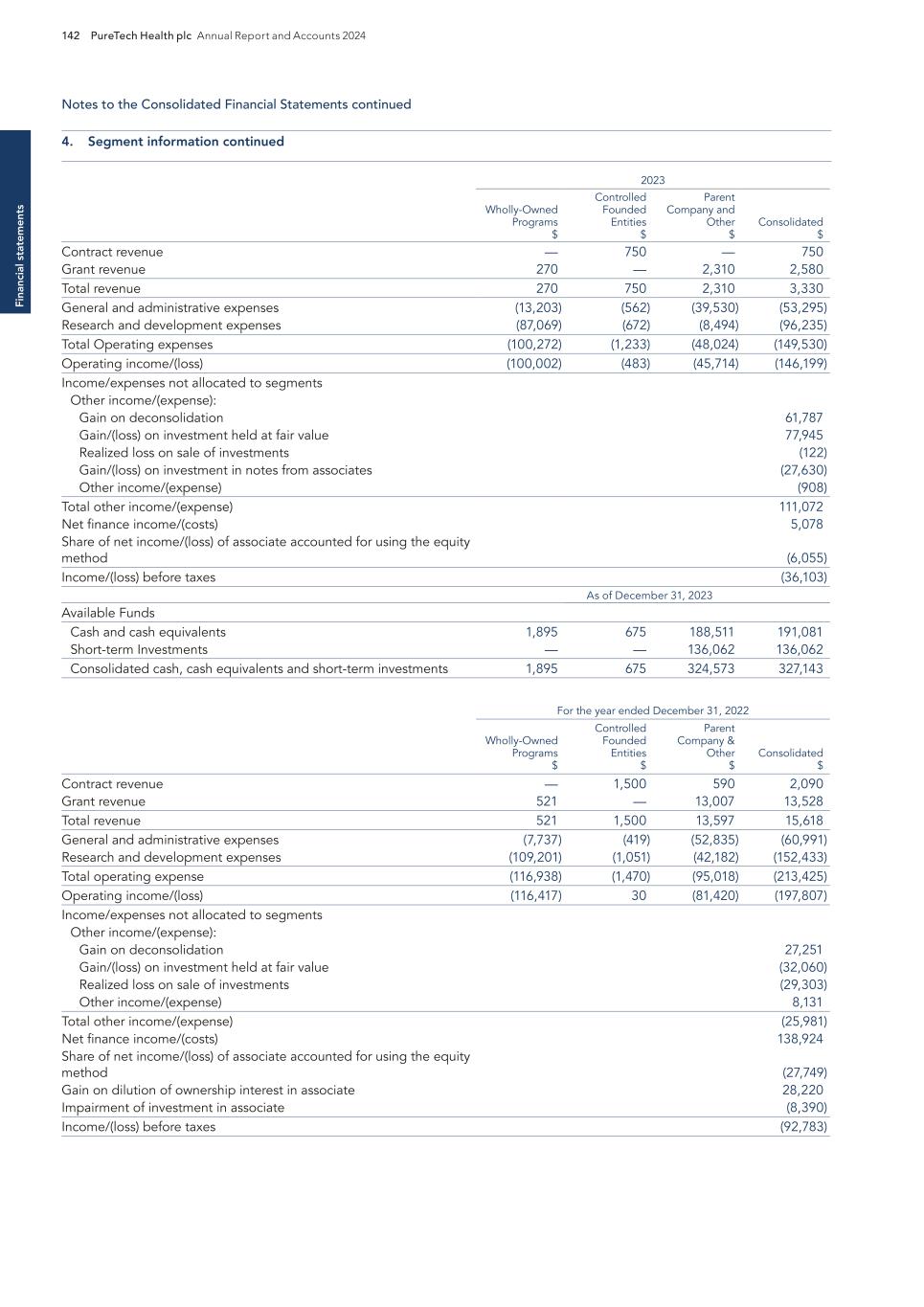
Fi na nc ia l s ta te m en ts 142 PureTech Health plc Annual Report and Accounts 2024 4. Segment information continued Notes to the Consolidated Financial Statements continued 2023 Wholly-Owned Programs $ Controlled Founded Entities $ Parent Company and Other $ Consolidated $ Contract revenue — 750 — 750 Grant revenue 270 — 2,310 2,580 Total revenue 270 750 2,310 3,330 General and administrative expenses (13,203) (562) (39,530) (53,295) Research and development expenses (87,069) (672) (8,494) (96,235) Total Operating expenses (100,272) (1,233) (48,024) (149,530) Operating income/(loss) (100,002) (483) (45,714) (146,199) Income/expenses not allocated to segments Other income/(expense): Gain on deconsolidation 61,787 Gain/(loss) on investment held at fair value 77,945 Realized loss on sale of investments (122) Gain/(loss) on investment in notes from associates (27,630) Other income/(expense) (908) Total other income/(expense) 111,072 Net finance income/(costs) 5,078 Share of net income/(loss) of associate accounted for using the equity method (6,055) Income/(loss) before taxes (36,103) As of December 31, 2023 Available Funds Cash and cash equivalents 1,895 675 188,511 191,081 Short-term Investments — — 136,062 136,062 Consolidated cash, cash equivalents and short-term investments 1,895 675 324,573 327,143 For the year ended December 31, 2022 Wholly-Owned Programs $ Controlled Founded Entities $ Parent Company & Other $ Consolidated $ Contract revenue — 1,500 590 2,090 Grant revenue 521 — 13,007 13,528 Total revenue 521 1,500 13,597 15,618 General and administrative expenses (7,737) (419) (52,835) (60,991) Research and development expenses (109,201) (1,051) (42,182) (152,433) Total operating expense (116,938) (1,470) (95,018) (213,425) Operating income/(loss) (116,417) 30 (81,420) (197,807) Income/expenses not allocated to segments Other income/(expense): Gain on deconsolidation 27,251 Gain/(loss) on investment held at fair value (32,060) Realized loss on sale of investments (29,303) Other income/(expense) 8,131 Total other income/(expense) (25,981) Net finance income/(costs) 138,924 Share of net income/(loss) of associate accounted for using the equity method (27,749) Gain on dilution of ownership interest in associate 28,220 Impairment of investment in associate (8,390) Income/(loss) before taxes (92,783)
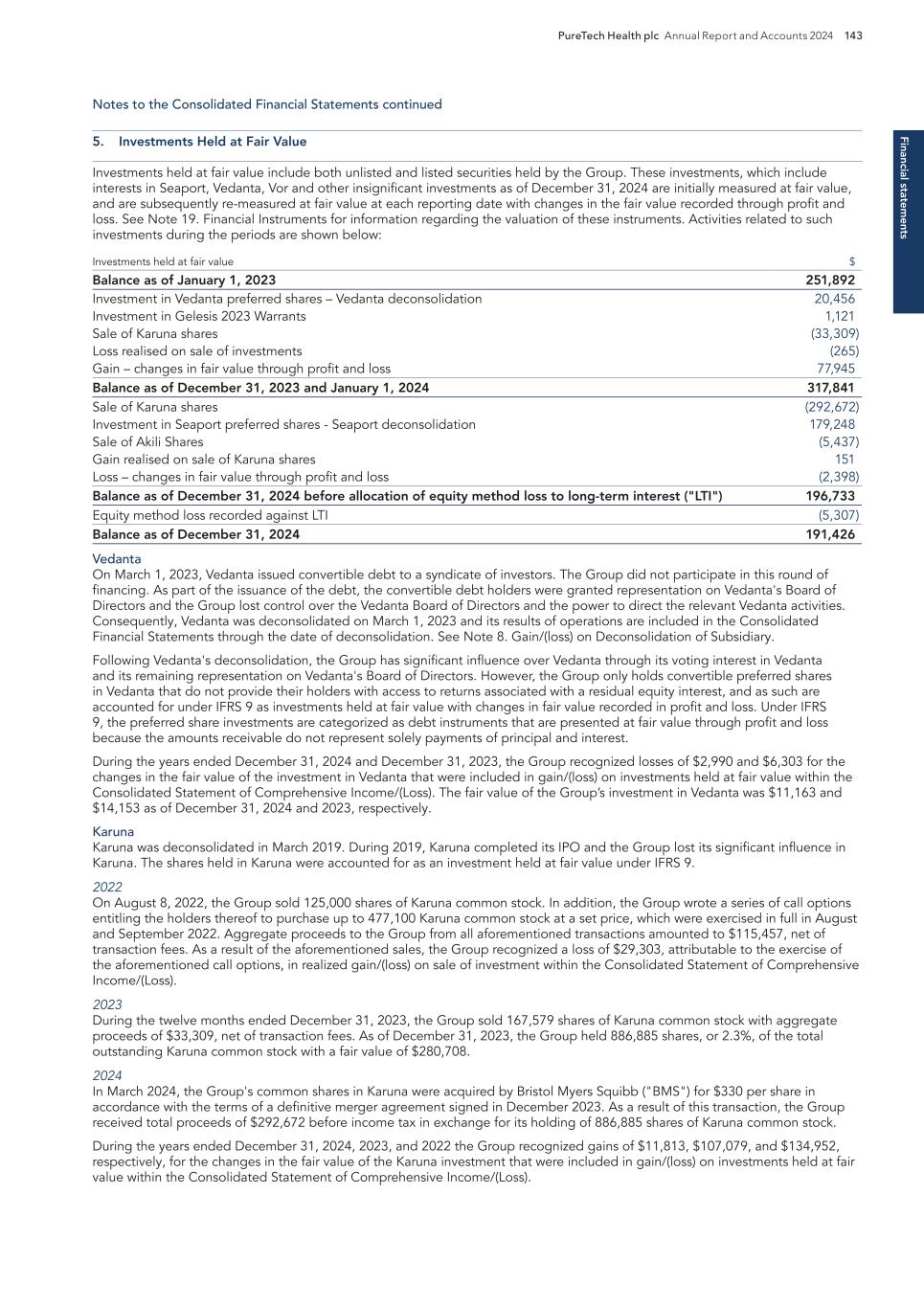
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 143 Notes to the Consolidated Financial Statements continued 5. Investments Held at Fair Value Investments held at fair value include both unlisted and listed securities held by the Group. These investments, which include interests in Seaport, Vedanta, Vor and other insignificant investments as of December 31, 2024 are initially measured at fair value, and are subsequently re-measured at fair value at each reporting date with changes in the fair value recorded through profit and loss. See Note 19. Financial Instruments for information regarding the valuation of these instruments. Activities related to such investments during the periods are shown below: Investments held at fair value $ Balance as of January 1, 2023 251,892 Investment in Vedanta preferred shares – Vedanta deconsolidation 20,456 Investment in Gelesis 2023 Warrants 1,121 Sale of Karuna shares (33,309) Loss realised on sale of investments (265) Gain – changes in fair value through profit and loss 77,945 Balance as of December 31, 2023 and January 1, 2024 317,841 Sale of Karuna shares (292,672) Investment in Seaport preferred shares - Seaport deconsolidation 179,248 Sale of Akili Shares (5,437) Gain realised on sale of Karuna shares 151 Loss – changes in fair value through profit and loss (2,398) Balance as of December 31, 2024 before allocation of equity method loss to long-term interest ("LTI") 196,733 Equity method loss recorded against LTI (5,307) Balance as of December 31, 2024 191,426 Vedanta On March 1, 2023, Vedanta issued convertible debt to a syndicate of investors. The Group did not participate in this round of financing. As part of the issuance of the debt, the convertible debt holders were granted representation on Vedanta's Board of Directors and the Group lost control over the Vedanta Board of Directors and the power to direct the relevant Vedanta activities. Consequently, Vedanta was deconsolidated on March 1, 2023 and its results of operations are included in the Consolidated Financial Statements through the date of deconsolidation. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. Following Vedanta's deconsolidation, the Group has significant influence over Vedanta through its voting interest in Vedanta and its remaining representation on Vedanta's Board of Directors. However, the Group only holds convertible preferred shares in Vedanta that do not provide their holders with access to returns associated with a residual equity interest, and as such are accounted for under IFRS 9 as investments held at fair value with changes in fair value recorded in profit and loss. Under IFRS 9, the preferred share investments are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest. During the years ended December 31, 2024 and December 31, 2023, the Group recognized losses of $2,990 and $6,303 for the changes in the fair value of the investment in Vedanta that were included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss). The fair value of the Group’s investment in Vedanta was $11,163 and $14,153 as of December 31, 2024 and 2023, respectively. Karuna Karuna was deconsolidated in March 2019. During 2019, Karuna completed its IPO and the Group lost its significant influence in Karuna. The shares held in Karuna were accounted for as an investment held at fair value under IFRS 9. 2022 On August 8, 2022, the Group sold 125,000 shares of Karuna common stock. In addition, the Group wrote a series of call options entitling the holders thereof to purchase up to 477,100 Karuna common stock at a set price, which were exercised in full in August and September 2022. Aggregate proceeds to the Group from all aforementioned transactions amounted to $115,457, net of transaction fees. As a result of the aforementioned sales, the Group recognized a loss of $29,303, attributable to the exercise of the aforementioned call options, in realized gain/(loss) on sale of investment within the Consolidated Statement of Comprehensive Income/(Loss). 2023 During the twelve months ended December 31, 2023, the Group sold 167,579 shares of Karuna common stock with aggregate proceeds of $33,309, net of transaction fees. As of December 31, 2023, the Group held 886,885 shares, or 2.3%, of the total outstanding Karuna common stock with a fair value of $280,708. 2024 In March 2024, the Group's common shares in Karuna were acquired by Bristol Myers Squibb ("BMS") for $330 per share in accordance with the terms of a definitive merger agreement signed in December 2023. As a result of this transaction, the Group received total proceeds of $292,672 before income tax in exchange for its holding of 886,885 shares of Karuna common stock. During the years ended December 31, 2024, 2023, and 2022 the Group recognized gains of $11,813, $107,079, and $134,952, respectively, for the changes in the fair value of the Karuna investment that were included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss).

Fi na nc ia l s ta te m en ts 144 PureTech Health plc Annual Report and Accounts 2024 5. Investments Held at Fair Value continued Notes to the Consolidated Financial Statements continued Vor Vor was deconsolidated in February 2019. On February 9, 2021, Vor closed its initial public offering. Subsequent to the closing, the Group held 3,207,200 shares of Vor common stock, representing 8.6% of Vor common stock. In August and December 2022, the Group sold an aggregate of 535,400 shares of Vor common stock for aggregate proceeds of $3,253. During the years ended December 31, 2024, 2023 and 2022, the Group recognized losses of $3,046, $11,756, and $16,247, respectively, for the changes in the fair value of the investment that were included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss). The fair value of the Group’s investment in Vor was $2,966 and $6,012 as of December 31, 2024 and 2023, respectively. Gelesis Gelesis was deconsolidated in July 2019. The common stock held in Gelesis was accounted for under the equity method, while the preferred shares and warrants held by the Group fell under the guidance of IFRS 9 and were treated as financial assets held at fair value, with changes to the fair value of the instruments recorded through the Consolidated Statement of Comprehensive Income/(Loss). Please refer to Note 6. Investments in Associates for information regarding the Group's investment in Gelesis as an associate. 2022 On January 13, 2022, Gelesis completed its business combination with Capstar Special Purpose Acquisition Corp ("Capstar"). As part of the business combination, all shares in Gelesis, common and preferred, including the shares held by the Group, were exchanged for common shares of the merged entity and unvested common shares that will vest upon the stock price of the new combined entity reaching certain target prices (hereinafter "Gelesis Earn-out Shares"). In addition, the Group invested $15,000 in the class A common shares of Capstar as part of the Private Investment in Public Equity ("PIPE") transaction that took place immediately prior to the closing of the business combination and an additional $4,961, as part of the Backstop Agreement signed with Capstar on December 30, 2021 (See Note 6. Investments in Associates). Pursuant to the business combination, Gelesis became a wholly-owned subsidiary of Capstar and Capstar changed its name to Gelesis Holdings, Inc., which began trading on the New York Stock Exchange under the ticker symbol "GLS" on January 14, 2022. The exchange of the preferred stock (including warrants) for common stock (including common stock warrants) represents an additional investment in Gelesis equity investment. The Group recorded the changes in fair value of the preferred stock and warrants through the date of the exchange upon which the preferred shares and warrants were derecognized and recorded as an additional investment in Gelesis equity interest. As part of the aforementioned exchange, the Group received 4,526,622 Gelesis Earn-out Shares, which were valued on the date of the exchange at $14,214. The Group accounted for such Gelesis Earn-out Shares under IFRS 9 as investments held at fair value with changes in fair value recorded through profit and loss. 2023 In February and May 2023, as part of Gelesis' issuance of senior secured promissory notes to the Group, Gelesis also issued to the Group (i) warrants to purchase 23,688,047 shares of Gelesis common stock with an exercise price of $0.2744 per share (ii) warrants to purchase 192,307,692 shares of Gelesis common stock at an exercise price of $0.0182 per share and (iii) warrants to purchase 43,133,803 shares of Gelesis common stock at an exercise price of $0.0142 per share. These warrants expire five years after issuance and are collectively referred to as the Gelesis 2023 Warrants. The Gelesis 2023 Warrants were recorded at their initial fair value of $1,121 and then subsequently re-measured to fair value through the profit and loss. As Gelesis ceased operations in October 2023, the fair value of the Gelesis 2023 Warrants was $0 as of December 31, 2024 and 2023, respectively, and no gain or loss was recognized in 2024. During the years ended December 31, 2023 and 2022, the Group recognized losses of $1,264 and $18,476, respectively, related to the change in the fair value of these instruments that was included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss). Sonde On May 25, 2022, Sonde completed a Series B preferred share financing, which resulted in the Group losing control over Sonde and the deconsolidation of Sonde. Therefore, the results of operations of Sonde are included in the Consolidated Financial Statements through the date of deconsolidation. See 8. Gain/(loss) on Deconsolidation of Subsidiary. Following deconsolidation, the Group had significant influence in Sonde through its 48.2% voting interest in Sonde and its remaining representation on Sonde's Board of Directors. The Group holds Preferred A-1, A-2 and B shares. The Preferred A-1 shares have the same terms as common stock and provide their shareholders with access to returns associated with a residual equity ownership in Sonde. Consequently, the investment in Preferred A-1 shares is accounted for under the equity method. The convertible Preferred A-2 and B shares do not provide their shareholders with access to returns associated with a residual equity interest and as such, are accounted for under IFRS 9 as investments held at fair value with changes in fair value recorded in profit and loss. Under IFRS 9, the A-2 and B preferred share investments are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest.

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 145 5. Investments Held at Fair Value continued Notes to the Consolidated Financial Statements continued During the years ended December 31, 2024, 2023 and 2022, the Group recognized a loss of $5,102, a loss of $994, and a gain of $235, respectively, for the changes in the fair value of the investment in Sonde that were included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss). For the year ended December 31, 2024, the Group’s recognized an additional loss of $5,307 on its investment in Sonde’s Preferred A-2 and B shares. The recognition of the additional loss occurs because the Group’s share of equity method losses, from applying the equity method of accounting to its investment in Sonde’s Preferred A-1 shares, was greater than its equity method investment balance and because the Group’s investment in Sonde’s Preferred A-2 and B shares represents a long-term interest. The additional loss of $5,307 is included in share of net income / (loss) of associates accounted for using the equity method within the Consolidated Statement of Comprehensive Income/(Loss). The fair value of the Group’s investment in Sonde's Preferred A-2 and B shares was $0 and $10,408 as of December 31, 2024 and 2023, respectively. Akili Akili was deconsolidated in 2018. At time of deconsolidation, the Group did not hold common shares in Akili and the preferred shares it held did not have equity-like features. Therefore, the preferred shares held by the Group fell under the guidance of IFRS 9 and were treated as a financial asset held at fair value and changes to the fair value of the preferred shares were recorded through the Consolidated Statement of Comprehensive Income/(Loss), in accordance with IFRS 9. 2022 On August 19, 2022, Akili Interactive merged with Social Capital Suvretta Holdings Corp. I, a special purpose acquisition company. The combined company's securities began trading on August 22, 2022 on the Nasdaq Stock Market under the ticker symbol "AKLI". As part of this transaction, the Akili Interactive shares held by the Group were exchanged for the common stock of the combined company's securities as well as unvested common stock ("Akili Earnout Shares") that will vest when the share price exceeds certain thresholds. In addition, as part of a PIPE transaction that took place concurrently with the closing of the transaction, the Group purchased 500,000 shares for a total consideration of $5,000. Following the closing of the aforementioned transactions, the Group held 12,527,476 shares of the combined entity and 1,433,914 Akili Earn-out Shares, with a total fair value of $15,102 as of December 31, 2022. 2024 On July 2, 2024, Akili was acquired by Virtual Therapeutics, and the Group received total proceeds of $5,437 before income taxes in exchange for its holding of 12,527,476 shares of Akili common stock. As a result, the Group no longer holds any ownership interests in Akili. During the years ended December 31, 2024, 2023 and 2022, the Group recognized losses of $985, $8,681, and $131,419, respectively, for the changes in the fair value of the investment in Akili that were included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss). Seaport On October 18, 2024, Seaport Therapeutics, Inc. ("Seaport") completed a Series B preferred share financing, which resulted in the Group’s voting interest being below 50% and the Group losing control over Seaport Board of Directors. Consequently, the Group no longer had the power to direct the relevant Seaport activities. As a result, Seaport was deconsolidated on this date and its results of operations are included in the Consolidated Financial Statements through the date of deconsolidation. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. Following deconsolidation, the Group still has significant influence in Seaport through its voting interest in Seaport and its remaining representation on Seaport's Board of Directors. The Group also has an investment held at fair value in Seaport through its ownership of Seaport's Series A-1, A-2 and B convertible preferred shares. The Group's preferred shares do not provide their shareholders with access to returns associated with a residual equity interest and as such, are accounted for under IFRS 9 as investments held at fair value with changes in fair value recorded in profit and loss. Under IFRS 9, the preferred share investments are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest. As of October 18, 2024, the closing date of the Series B preferred share financing, the Group owns 3,031,578 of Series B preferred stock, 8,421,052 of Series A-2 preferred stock, and 40,000,000 of Series A-1 preferred stock. These preferred shares had a fair value of $179,248 and $177,288 as of October 18, 2024 and December 31, 2024, respectively. The fair value of the preferred shares is determined by management using a valuation model that utilizes both the market backsolve and probability-weighted expected return methods. The valuation of the investment is categorized as Level 3 in the fair value hierarchy due to the use of significant unobservable inputs, which have a significant effect on the valuation. The significant assumptions in the valuation include the estimated equity value of Seaport, the probability of Seaport entering into an initial public offering and achieving a certain clinical trial development milestone, and the estimated time to liquidity. During the year ended December 31, 2024, the Group recognized a loss of $1,960 for the changes in the fair value of the investment in Seaport that were included in gain/(loss) on investments held at fair value within the Consolidated Statement of Comprehensive Income/(Loss).

Fi na nc ia l s ta te m en ts 146 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 6. Investments in Associates Gelesis (Boston, MA) Gelesis was founded by the Group and raised funding through preferred shares financings as well as issuances of warrants and loans. As of July 1, 2019, Gelesis was deconsolidated from the Group’s financial statements. Upon deconsolidation, the preferred shares and warrants held by the Group fell under the guidance of IFRS 9 Financial Instruments and were treated as financial assets held at fair value and the investment in common shares of Gelesis was subject to IAS 28 Investment in Associates as the Group had significant influence over Gelesis. Backstop agreement – 2022 and 2021 On December 30, 2021, the Group signed an agreement (the "Backstop Agreement") with Capstar and had committed to acquire Capstar class A common shares at $10 per share immediately prior to the closing of the business combination between Gelesis and Capstar, in case, the Available Funds, as defined in the agreement, were less than $15,000. According to the Backstop Agreement, if the Group had to acquire any shares under the agreement, the Group would receive an additional 1,322,500 class A common shares of Capstar at no additional consideration. The Group determined that such agreement meets the definition of a derivative under IFRS 9 and as such, should be recorded at fair value with changes in fair value recorded through profit and loss. The derivative was initially recorded at fair value adjusted to defer the day 1 gain equal to the difference between the fair value of $11,200 and transaction price of zero on the effective date of the Backstop Agreement and as such, was initially recorded at zero. The deferred gain was amortized over the period from the effective date until the settlement date, January 13, 2022. During the years ended December 31, 2022 and 2021, the Group recognized income of $10,400 and $800, respectively, for the amortization of the deferred gain. During the year ended December 31, 2022, the Group recognized a loss of $2,776 in respect of the decrease in the fair value of the derivative until the settlement date, resulting in a net gain of $7,624 recorded during the year ended December 31, 2022 in respect of the Backstop Agreement. The gain was included in other Income/(expense) in the Consolidated Statement of Comprehensive Income/(Loss). The fair value of the derivative on the settlement date in the amount of $8,424 represents an additional investment in Gelesis as part of the SPAC transaction described below. On January 13, 2022, as part of the conclusion of the aforementioned Backstop Agreement, the Group acquired 496,145 class A common shares of Capstar for $4,961 and received an additional 1,322,500 class A common shares of Capstar for no additional consideration. 2022 Share exchange – Capstar On January 13, 2022, Gelesis completed its business combination with Capstar. As part of the business combination, all shares in Gelesis, common and preferred, including the shares held by the Group, were exchanged for common shares of the merged entity and unvested common shares that will vest upon the stock price of the new combined entity reaching certain target prices (the "Gelesis Earn-out Shares"). In addition, the Group invested $15,000 in the class A common shares of Capstar as part of the PIPE transaction that took place immediately prior to the closing of the business combination and an additional $4,961, as part of the Backstop Agreement described above. Pursuant to the business combination, Gelesis became a wholly-owned subsidiary of Capstar and Capstar changed its name to Gelesis Holdings, Inc., which began trading on the New York Stock Exchange under the ticker symbol "GLS" on January 14, 2022. Following the closing of the business combination, the PIPE transaction, the settlement of the aforementioned Backstop Agreement with Capstar, and the exchange of all preferred shares in Gelesis to common shares in the new combined entity, the Group holds 16,727,582 common shares of Gelesis Holdings Inc., which was equal to approximately 23.2% of Gelesis Holdings Inc's outstanding common shares at the time of the exchange. Due to the Group's significant equity holding and voting interest in Gelesis, the Group continued to maintain significant influence in Gelesis and as such, continued to account for its Gelesis equity investment under the equity method. Gelesis was deemed to be the acquirer in Gelesis Holdings Inc. and the financial assets and financial liabilities in Capstar were deemed to be acquired by Gelesis in consideration for the shares held by Capstar legacy shareholders. As such, the Group did not revalue the retained investment in Gelesis but rather treated the exchange as a dilution of its equity interest in Gelesis from 42.0% as of December 31, 2021 to 22.8% as of January 13, 2022 (including warrants that provide its holders access to returns associated with equity holders). After considering the aforementioned additional investments, the exchange of the preferred stock, previously accounted for as an investment held at fair value, to common stock (and representing an additional equity investment in Gelesis), the earn-out shares received in Gelesis (see Note 5. Investments Held at Fair Value) and the offset of previously unrecognized equity method losses, the net gain recorded on the dilution of interest amounted to $28,255. Impairment Following Gelesis’ decline in its market price in 2022 and its lack of liquidity, the Group recorded an impairment loss of $8,390 as of December 31, 2022 in respect of its investment in Gelesis. The recoverable amount of the investment in Gelesis was $4,910 as of December 31, 2022, which was determined based on fair value less costs to sell (which were estimated to be insignificant). Fair value was determined based on level 1 of the fair value hierarchy as Gelesis shares were traded on an active market as of December 31, 2022. The impairment loss was presented separately in the Consolidated Statement of Comprehensive Income/(loss) for the year ended December 31, 2022 in the line item impairment of investment in associates.

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 147 6. Investments in Associates continued Notes to the Consolidated Financial Statements continued 2023 During the year ended December 31, 2023, the Group entered into agreements with Gelesis to purchase senior secured convertible promissory notes and warrants for shares of Gelesis common stock (see Note 7. Investment in Notes from Associates). The warrants to purchase shares of Gelesis common stock represented potential voting rights to the Group and it was therefore necessary to consider whether they were substantive. If these potential voting rights were substantive and the Group had the practical ability to exercise the rights and take control of greater than 50% of Gelesis common stock, the Group would be required to consolidate Gelesis under the accounting standards. In February 2023, the Group obtained warrants to purchase 23,688,047 shares of Gelesis common stock (the “February Warrants”) at an exercise price of $0.2744 per share. The exercise of the February Warrants was subject to the approval of the Gelesis stockholders until May 1, 2023. On May 1, 2023, stockholder approval was no longer required for the Group to exercise the February Warrants. The potential voting rights associated with the February Warrants were not substantive as the exercise price of the February Warrants was at a significant premium to the fair value of the Gelesis common stock. In May 2023, the Group obtained warrants to purchase 235,441,495 shares of Gelesis common stock (the “May Warrants”). The May Warrants were exercisable at the option of the Group and had an exercise price of either $0.0182 or $0.0142. The May Warrants were substantive as the Group would have benefited from exercising such warrants since their exercise price was at the money or at an insignificant premium over the fair value of the Gelesis common stock. However, that benefit from exercising the May Warrants only existed for a short period of time because in June 2023, the potential voting rights associated with the May Warrants were impacted by the terms and conditions of a merger agreement that the Group signed with Gelesis on June 12, 2023 (the "Merger Agreement") and were no longer substantive. On October 12, 2023, the Group terminated the Merger Agreement with Gelesis as certain closing conditions were not satisfied. In October 2023, Gelesis ceased operations and filed a voluntary petition for relief under the provisions of Chapter 7 of Title 11 of the United States Bankruptcy Code. A Chapter 7 trustee has been appointed by the Bankruptcy Court who has control over the assets and liabilities of Gelesis, effectively eliminating the authority and powers of the Board of Directors of Gelesis and its executive officers to act on behalf of Gelesis. The assets of Gelesis are in liquidation and Gelesis no longer has any officers or employees. The Group ceased accounting for Gelesis as an equity method investment as it no longer has significant influence in Gelesis. During the year ended December 31, 2023, the Group recorded $4,910 as its share in the losses of Gelesis and the Group’s balance in this equity method investment was zero as of December 31, 2024 and 2023, respectively. Sonde (Boston, MA) On May 25, 2022, Sonde completed a Series B preferred share financing. As a result of the aforementioned financing, the Group's voting interest was reduced below 50% and the Group lost its control over Sonde, and as such, ceased to consolidate Sonde on the date the round of financing was completed. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. Following deconsolidation, the Group has significant influence in Sonde through its voting interest in Sonde and its remaining representation on Sonde's Board of Directors. The Group's voting interest at the date of deconsolidation was 48.2% and remained at 40.2% subsequently. The Group holds Preferred A-1, A-2 and B shares. The Preferred A-1 shares, in substance, have the same terms as common stock and as such, provide their shareholders with access to returns associated with a residual equity ownership in Sonde. Consequently, the investment in Preferred A-1 shares is accounted for under the equity method. The Preferred A-2 and B shares, however, do not provide their shareholders with access to returns associated with a residual equity interest and as such, are accounted for under IFRS 9, as investments held at fair value. The fair value of the Preferred A-1 shares on the date of deconsolidation amounted to $7,716, which is the initial value of the equity method investment in Sonde. During the years ended December 31, 2024, 2023, and 2022, the Group recorded a loss of $8,492, $1,052 and $3,443, respectively, related to Sonde's equity method of accounting. As of December 31, 2023, the equity method investment in Sonde had a balance of $3,185. The Group’s share in Sonde’s losses in 2024 exceeded the Group’s equity method investment in Sonde. As a result, the Group's equity method investment in Sonde is reduced to $0 as of December 31, 2024. Since the Group’s investment in Sonde’s Preferred A-2 and B shares represents a long- term interest, the Group recognized additional equity method loses, totaling $5,307, against its investment in Sonde's Preferred A-2 and B shares (See Note 5. Investments Held at Fair Value), reducing the balance of the preferred share investment to $0 as of December 31, 2024. Since the Group did not incur legal or constructive obligations or made payments on behalf of Sonde, the Group stopped recognizing additional equity method losses in 2024. As of December 31, 2024, unrecognized equity method losses amounted to $14,447. Seaport (Boston, MA) On October 18, 2024, Seaport completed a Series B preferred share financing. As a result of this financing, the Group's voting interest was reduced below 50%, and the Group no longer controls Seaport's Board of Directors. Consequently, the Group lost control over Seaport, and as such, ceased to consolidate Seaport on the date the round of financing was completed. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. Following deconsolidation, the Group still has significant influence in Seaport through its voting interest in Seaport and its remaining representation on Seaport's Board of Directors. The Group's voting interest as of the date of deconsolidation and as of December 31, 2024 was 43.0% and 42.9%, respectively. The Group holds both common shares and preferred shares in Seaport. The common shares are subject to IAS 28 Investment in Associates and Joint Ventures due to the Group's retained significant influence and are accounted for under the equity method. The preferred shares do not provide their shareholders with access to returns associated with a residual equity interest and as such, are accounted for under IFRS 9 as investments held at fair value.
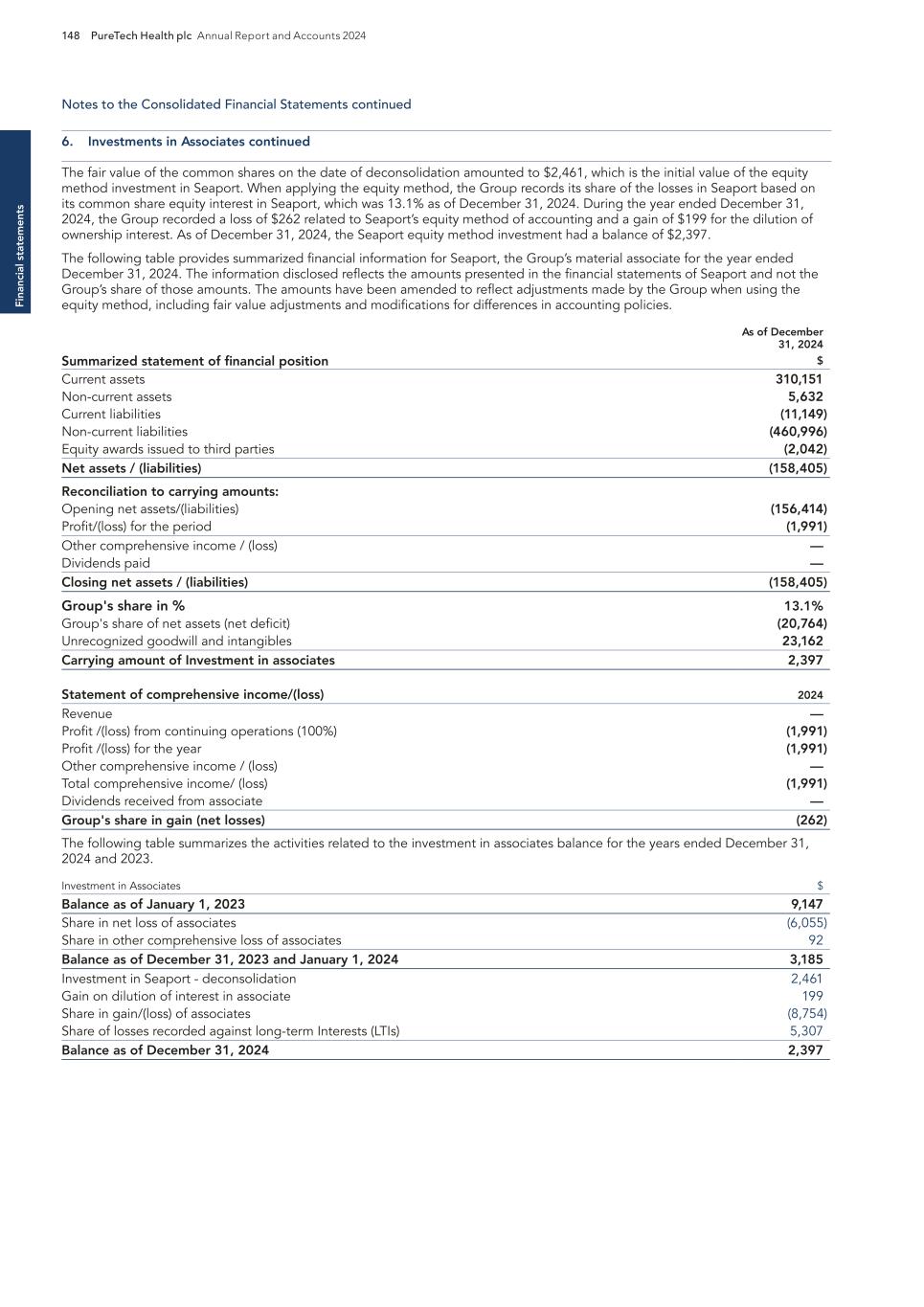
Fi na nc ia l s ta te m en ts 148 PureTech Health plc Annual Report and Accounts 2024 6. Investments in Associates continued Notes to the Consolidated Financial Statements continued The fair value of the common shares on the date of deconsolidation amounted to $2,461, which is the initial value of the equity method investment in Seaport. When applying the equity method, the Group records its share of the losses in Seaport based on its common share equity interest in Seaport, which was 13.1% as of December 31, 2024. During the year ended December 31, 2024, the Group recorded a loss of $262 related to Seaport’s equity method of accounting and a gain of $199 for the dilution of ownership interest. As of December 31, 2024, the Seaport equity method investment had a balance of $2,397. The following table provides summarized financial information for Seaport, the Group’s material associate for the year ended December 31, 2024. The information disclosed reflects the amounts presented in the financial statements of Seaport and not the Group’s share of those amounts. The amounts have been amended to reflect adjustments made by the Group when using the equity method, including fair value adjustments and modifications for differences in accounting policies. As of December 31, 2024 Summarized statement of financial position $ Current assets 310,151 Non-current assets 5,632 Current liabilities (11,149) Non-current liabilities (460,996) Equity awards issued to third parties (2,042) Net assets / (liabilities) (158,405) Reconciliation to carrying amounts: Opening net assets/(liabilities) (156,414) Profit/(loss) for the period (1,991) Other comprehensive income / (loss) — Dividends paid — Closing net assets / (liabilities) (158,405) Group's share in % 13.1% Group's share of net assets (net deficit) (20,764) Unrecognized goodwill and intangibles 23,162 Carrying amount of Investment in associates 2,397 2024Statement of comprehensive income/(loss) Revenue — Profit /(loss) from continuing operations (100%) (1,991) Profit /(loss) for the year (1,991) Other comprehensive income / (loss) — Total comprehensive income/ (loss) (1,991) Dividends received from associate — Group's share in gain (net losses) (262) The following table summarizes the activities related to the investment in associates balance for the years ended December 31, 2024 and 2023. Investment in Associates $ Balance as of January 1, 2023 9,147 Share in net loss of associates (6,055) Share in other comprehensive loss of associates 92 Balance as of December 31, 2023 and January 1, 2024 3,185 Investment in Seaport - deconsolidation 2,461 Gain on dilution of interest in associate 199 Share in gain/(loss) of associates (8,754) Share of losses recorded against long-term Interests (LTIs) 5,307 Balance as of December 31, 2024 2,397

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 149 Notes to the Consolidated Financial Statements continued 7. Investment in Notes from Associates Gelesis On July 27, 2022, the Group, as a lender, entered into an unsecured promissory note (the "Junior Note") with Gelesis, as a borrower, in the amount of $15,000. The Junior Note bears an annual interest rate of 15% per annum. The maturity date of the Junior Note is the earlier of December 31, 2023 or five business days following the consummation of a qualified financing by Gelesis. Based on the terms of the Junior Note, due to the option to convert to a variable amount of shares at the time of default, the Junior Note is required to be measured at fair value with changes in fair value recorded through profit and loss. During the year ended December 31, 2023, the Group entered into multiple agreements with Gelesis to purchase senior secured convertible promissory notes (the "Senior Notes") and warrants for share of Gelesis common stock for a total consideration of $11,850. The Senior Notes are secured by a first-priority lien on substantially all assets of Gelesis and the guarantors (other than the equity interests in, and assets held by Gelesis s.r.l., a subsidiary of Gelesis, and certain other exceptions). The initial fair value of the Senior Notes and warrants was determined to be $10,729 and $1,121, respectively. The Senior Notes represent debt instruments that are presented at fair value through profit and loss as the amounts receivable do not solely represent payments of principal and interest as the Senior Notes are convertible into Gelesis common stock. In October 2023, Gelesis ceased operations and filed a voluntary petition for relief under the provisions of Chapter 7 of Title 11 of the United States Bankruptcy Code. Therefore, the Group determined that the fair value of the Junior Note and the Senior Notes with the warrants was $0 as of December 31, 2023. In June 2024, the Bankruptcy Court approved an executed agreement for a third party to acquire the remaining net assets of Gelesis for $15,000. As the only senior secured creditor, the Group is expected to receive a majority of the proceeds from this sale after deduction of Bankruptcy Court related legal and administrative costs in 2025. As of December 31, 2024, these notes were determined to have a fair value of $11,381. For the years ended December 31, 2024 and 2023, the Group recorded a gain of $11,381 and a loss of $27,230, respectively, for the changes in the fair value of these notes, which were included in gain/(loss) on investments in notes from associates in the Consolidated Statement of Comprehensive Income/(Loss). Vedanta On April 24, 2023, Vedanta closed the second tranche of its convertible debt for additional proceeds of $18,000, of which $5,000 were invested by the Group. The convertible debt carries an interest rate of 9% per annum. The debt has various conversion triggers, and the conversion price is established at the lower of 80% of the equity price of the last financing round, or a certain pre- money valuation cap established in the agreement. If the convertible debt is not earlier converted or repaid, the entire outstanding amount of the convertible debt shall be due and payable upon the earliest to occur of (a) the later of (x) November 1, 2025 and (y) the date which is sixty (60) days after all amounts owed under, or in connection with, the loan Vedanta received from a certain investor have been paid in full, or (b) the consummation of a Deemed Liquidation Event (as defined in Vedanta’s Amended and Restated Certificate of Incorporation). Due to the terms of the convertible debt, the investment in such convertible debt is measured at fair value with changes in the fair value recorded through profit and loss. As of December 31, 2024 and 2023, the Vedanta convertible debt was determined to have a fair value of $6,350 and $4,600, respectively. During the year ended December 31, 2024 and December 31, 2023 Group recorded a gain of $1,750 and a loss of $400, respectively, for the changes in the fair value of the Vedanta convertible debt, which were included in gain/(loss) on investments in notes from associates in the Consolidated Statement of Comprehensive Income/ (Loss). The following is the activity in respect of investments in notes from associates during the period. The fair value of the notes from associates of $17,731 and $4,600 as of December 31, 2024 and 2023, respectively, is determined using unobservable Level 3 inputs. See Note 19. Financial Instruments for additional information. Investment in notes from associates $ Balance as of January 1, 2023 16,501 Investment In Gelesis Notes 10,729 Investment in Vedanta convertible debt 5,000 Changes in the fair value of the notes (27,630) Balance as of December 31, 2023 and January 1, 2024 4,600 Changes in the fair value of the notes 13,131 Balance as of December 31, 2024 17,731

Fi na nc ia l s ta te m en ts 150 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 8. Gain/(loss) on Deconsolidation of Subsidiary Upon the Group losing control over a subsidiary, the assets and liabilities of the subsidiary are derecognized along with any related non-controlling interest. Any interest that the Group retains in the former subsidiary is measured at fair value when control is lost. Any resulting gain or loss is included in gain/(loss) on deconsolidation of subsidiary in the Consolidated Statement of Comprehensive Income/(Loss). Sonde On May 25, 2022, Sonde completed a Series B preferred share financing and amended its Voting Agreement to grant the Series B preferred stockholders representation on Sonde’s Board of Directors. As a result of the Series B preferred share financing and the amendment to the Voting Agreement, the Group's voting interest was reduced below 50%, and the Group no longer controls Sonde's Board of Directors, which is the governance body that has the power to direct the relevant activities of Sonde. Consequently, the Group concluded that it lost control over Sonde, and therefore, Sonde was deconsolidated on May 25, 2022 from the Group’s Consolidated Financial Statements. The results of Sonde’s operations are included in the Group’s Consolidated Financial Statements through the date of deconsolidation. Following deconsolidation, the Group has significant influence over Sonde through its voting interest in Sonde and its remaining representation on Sonde's Board of Directors. The Group holds Preferred A-1, A-2 and B shares. The Preferred A-1 shares have the same terms as common stock and provide their shareholders with access to returns associated with a residual equity ownership in Sonde. Consequently, the investment in Preferred A-1 shares is accounted for under the equity method. The convertible Preferred A-2 and B shares do not provide their shareholders with access to returns associated with a residual equity interest, and, as such, are accounted for under IFRS 9, as investments held at fair value with changes in fair value recorded in profit and loss. Under IFRS 9, the investments in Preferred A-2 and B shares are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest. Upon deconsolidation, the Group derecognized the assets, liabilities and non-controlling interest in respect of Sonde and recorded its aforementioned investment in Sonde at fair value. The deconsolidation resulted in a gain of $27,251. As of the date of deconsolidation, the investment in Sonde convertible preferred shares amounted to $18,848. As of December 31, 2024 and 2023, the Group’s investment in Sonde’s convertible preferred shares held at fair value was $0 and $10,408, respectively, and categorized as Level 3 in the fair value hierarchy. Vedanta On March 1, 2023, Vedanta issued convertible debt to a syndicate of investors. The Group did not participate in this round of financing. As part of the issuance of the debt, the convertible debt holders were granted representation on Vedanta's Board of Directors, and the Group lost control over the Vedanta Board of Directors, which is the governance body that has the power to direct the relevant activities of Vedanta. Consequently, Vedanta was deconsolidated on March 1, 2023 from the Group’s Consolidated Financial Statements. The results of Vedanta’s operations are included in the Group’s Consolidated Financial Statements through the date of deconsolidation. Following deconsolidation, the Group has significant influence over Vedanta through its voting interest in Vedanta and its remaining representation on Vedanta's Board of Directors. The Group only holds convertible preferred shares in Vedanta that do not provide their holders with access to returns associated with a residual equity interest, and as such, are accounted for under IFRS 9, Financial Instruments, as investments held at fair value with changes in fair value recorded in profit and loss. Under IFRS 9, the Group’s preferred share investment is categorized as a debt instrument that is presented at fair value through profit and loss because the amounts receivable does not represent solely payments of principal and interest. Upon deconsolidation, the Group derecognized the assets, liabilities and non-controlling interest in respect of Vedanta and recorded its aforementioned investment in Vedanta at fair value. The deconsolidation resulted in a gain of $61,787. As of the date of deconsolidation, the investment in Vedanta convertible preferred shares held at fair value amounted to $20,456. As of December 31, 2024 and 2023, the Group’s investment in Vedanta convertible preferred shares is held at fair value of $11,163 and $14,153, respectively, and categorized as Level 3 in the fair value hierarchy. The significant unobservable inputs used in the fair value measurement of the Group’s investment in the convertible preferred shares of Vedanta and the sensitivity of the fair value measurement to changes in these significant unobservable inputs are disclosed in Note 19. Financial Instruments.
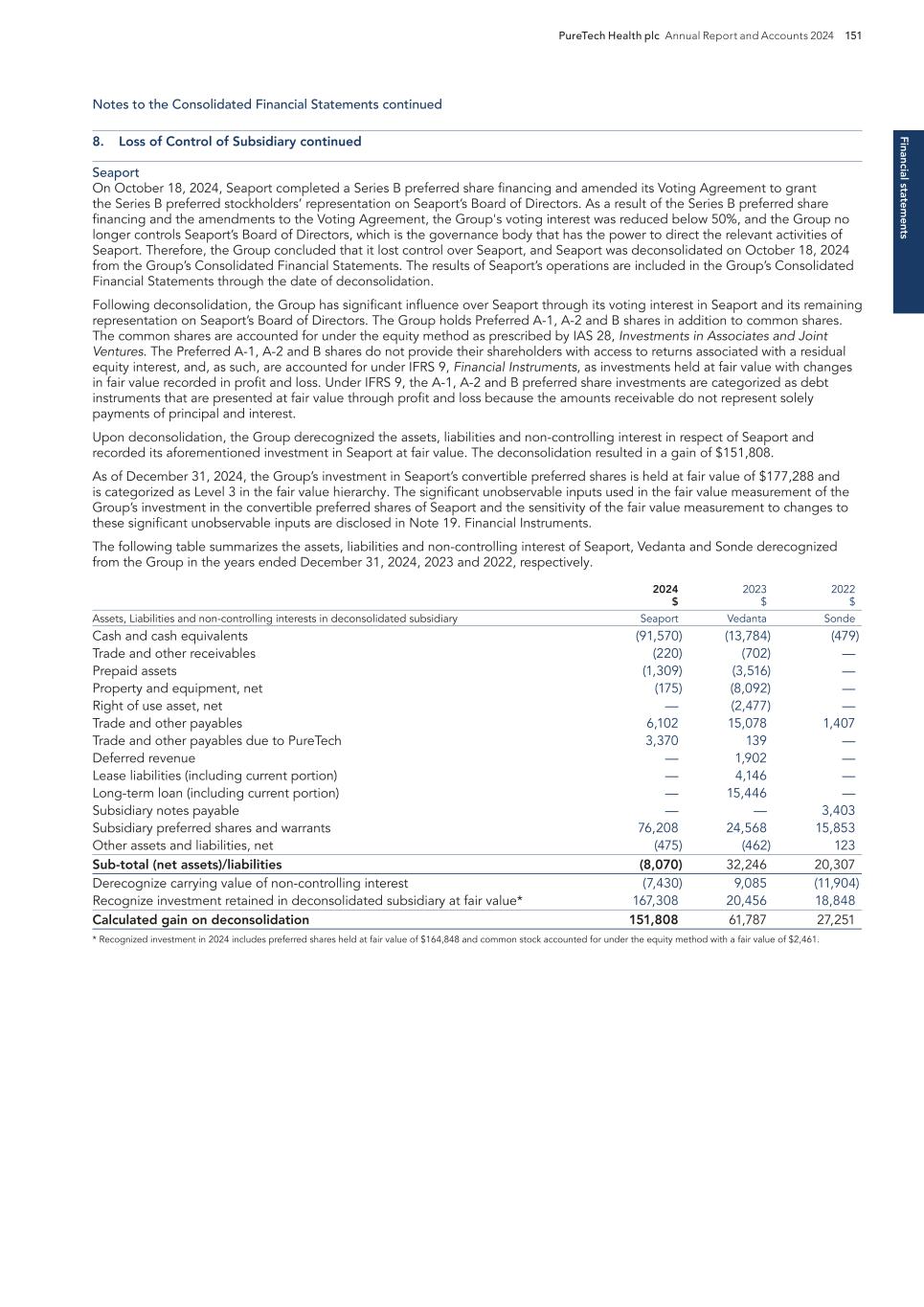
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 151 Notes to the Consolidated Financial Statements continued Seaport On October 18, 2024, Seaport completed a Series B preferred share financing and amended its Voting Agreement to grant the Series B preferred stockholders’ representation on Seaport’s Board of Directors. As a result of the Series B preferred share financing and the amendments to the Voting Agreement, the Group's voting interest was reduced below 50%, and the Group no longer controls Seaport’s Board of Directors, which is the governance body that has the power to direct the relevant activities of Seaport. Therefore, the Group concluded that it lost control over Seaport, and Seaport was deconsolidated on October 18, 2024 from the Group’s Consolidated Financial Statements. The results of Seaport’s operations are included in the Group’s Consolidated Financial Statements through the date of deconsolidation. Following deconsolidation, the Group has significant influence over Seaport through its voting interest in Seaport and its remaining representation on Seaport’s Board of Directors. The Group holds Preferred A-1, A-2 and B shares in addition to common shares. The common shares are accounted for under the equity method as prescribed by IAS 28, Investments in Associates and Joint Ventures. The Preferred A-1, A-2 and B shares do not provide their shareholders with access to returns associated with a residual equity interest, and, as such, are accounted for under IFRS 9, Financial Instruments, as investments held at fair value with changes in fair value recorded in profit and loss. Under IFRS 9, the A-1, A-2 and B preferred share investments are categorized as debt instruments that are presented at fair value through profit and loss because the amounts receivable do not represent solely payments of principal and interest. Upon deconsolidation, the Group derecognized the assets, liabilities and non-controlling interest in respect of Seaport and recorded its aforementioned investment in Seaport at fair value. The deconsolidation resulted in a gain of $151,808. As of December 31, 2024, the Group’s investment in Seaport’s convertible preferred shares is held at fair value of $177,288 and is categorized as Level 3 in the fair value hierarchy. The significant unobservable inputs used in the fair value measurement of the Group’s investment in the convertible preferred shares of Seaport and the sensitivity of the fair value measurement to changes to these significant unobservable inputs are disclosed in Note 19. Financial Instruments. The following table summarizes the assets, liabilities and non-controlling interest of Seaport, Vedanta and Sonde derecognized from the Group in the years ended December 31, 2024, 2023 and 2022, respectively. 2024 $ 2023 $ 2022 $ Assets, Liabilities and non-controlling interests in deconsolidated subsidiary Seaport Vedanta Sonde Cash and cash equivalents (91,570) (13,784) (479) Trade and other receivables (220) (702) — Prepaid assets (1,309) (3,516) — Property and equipment, net (175) (8,092) — Right of use asset, net — (2,477) — Trade and other payables 6,102 15,078 1,407 Trade and other payables due to PureTech 3,370 139 — Deferred revenue — 1,902 — Lease liabilities (including current portion) — 4,146 — Long-term loan (including current portion) — 15,446 — Subsidiary notes payable — — 3,403 Subsidiary preferred shares and warrants 76,208 24,568 15,853 Other assets and liabilities, net (475) (462) 123 Sub-total (net assets)/liabilities (8,070) 32,246 20,307 Derecognize carrying value of non-controlling interest (7,430) 9,085 (11,904) Recognize investment retained in deconsolidated subsidiary at fair value* 167,308 20,456 18,848 Calculated gain on deconsolidation 151,808 61,787 27,251 * Recognized investment in 2024 includes preferred shares held at fair value of $164,848 and common stock accounted for under the equity method with a fair value of $2,461. 8. Loss of Control of Subsidiary continued
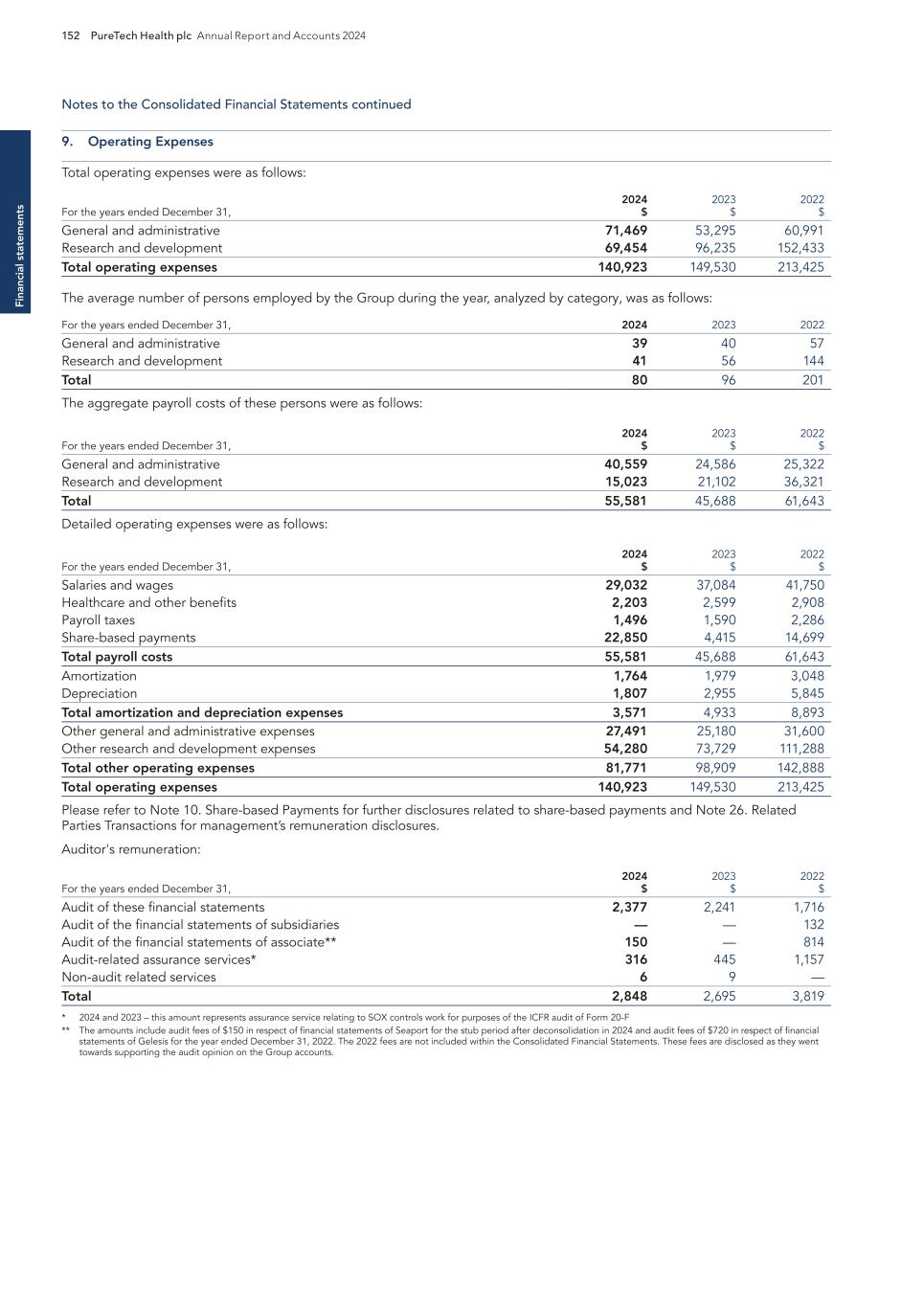
Fi na nc ia l s ta te m en ts 152 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 9. Operating Expenses Total operating expenses were as follows: For the years ended December 31, 2024 $ 2023 $ 2022 $ General and administrative 71,469 53,295 60,991 Research and development 69,454 96,235 152,433 Total operating expenses 140,923 149,530 213,425 The average number of persons employed by the Group during the year, analyzed by category, was as follows: For the years ended December 31, 2024 2023 2022 General and administrative 39 40 57 Research and development 41 56 144 Total 80 96 201 The aggregate payroll costs of these persons were as follows: 2024 $ 2023 $ 2022 $For the years ended December 31, General and administrative 40,559 24,586 25,322 Research and development 15,023 21,102 36,321 Total 55,581 45,688 61,643 Detailed operating expenses were as follows: 2024 $ 2023 $ 2022 $For the years ended December 31, Salaries and wages 29,032 37,084 41,750 Healthcare and other benefits 2,203 2,599 2,908 Payroll taxes 1,496 1,590 2,286 Share-based payments 22,850 4,415 14,699 Total payroll costs 55,581 45,688 61,643 Amortization 1,764 1,979 3,048 Depreciation 1,807 2,955 5,845 Total amortization and depreciation expenses 3,571 4,933 8,893 Other general and administrative expenses 27,491 25,180 31,600 Other research and development expenses 54,280 73,729 111,288 Total other operating expenses 81,771 98,909 142,888 Total operating expenses 140,923 149,530 213,425 Please refer to Note 10. Share-based Payments for further disclosures related to share-based payments and Note 26. Related Parties Transactions for management’s remuneration disclosures. Auditor's remuneration: For the years ended December 31, 2024 $ 2023 $ 2022 $ Audit of these financial statements 2,377 2,241 1,716 Audit of the financial statements of subsidiaries — — 132 Audit of the financial statements of associate** 150 — 814 Audit-related assurance services* 316 445 1,157 Non-audit related services 6 9 — Total 2,848 2,695 3,819 * 2024 and 2023 – this amount represents assurance service relating to SOX controls work for purposes of the ICFR audit of Form 20-F ** The amounts include audit fees of $150 in respect of financial statements of Seaport for the stub period after deconsolidation in 2024 and audit fees of $720 in respect of financial statements of Gelesis for the year ended December 31, 2022. The 2022 fees are not included within the Consolidated Financial Statements. These fees are disclosed as they went towards supporting the audit opinion on the Group accounts.
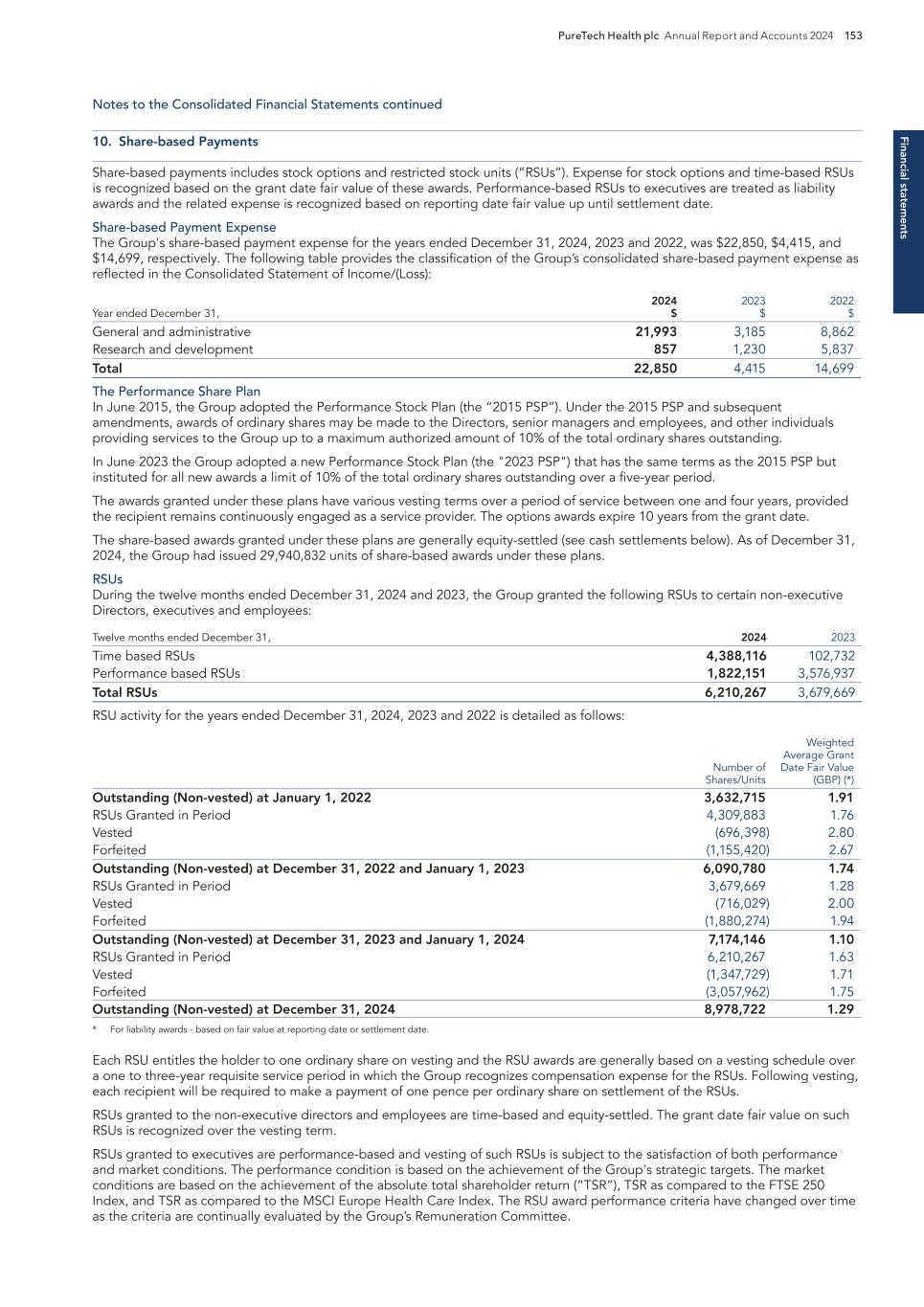
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 153 Notes to the Consolidated Financial Statements continued 10. Share-based Payments Share-based payments includes stock options and restricted stock units (“RSUs”). Expense for stock options and time-based RSUs is recognized based on the grant date fair value of these awards. Performance-based RSUs to executives are treated as liability awards and the related expense is recognized based on reporting date fair value up until settlement date. Share-based Payment Expense The Group's share-based payment expense for the years ended December 31, 2024, 2023 and 2022, was $22,850, $4,415, and $14,699, respectively. The following table provides the classification of the Group’s consolidated share-based payment expense as reflected in the Consolidated Statement of Income/(Loss): Year ended December 31, 2024 $ 2023 $ 2022 $ General and administrative 21,993 3,185 8,862 Research and development 857 1,230 5,837 Total 22,850 4,415 14,699 The Performance Share Plan In June 2015, the Group adopted the Performance Stock Plan (the “2015 PSP”). Under the 2015 PSP and subsequent amendments, awards of ordinary shares may be made to the Directors, senior managers and employees, and other individuals providing services to the Group up to a maximum authorized amount of 10% of the total ordinary shares outstanding. In June 2023 the Group adopted a new Performance Stock Plan (the "2023 PSP") that has the same terms as the 2015 PSP but instituted for all new awards a limit of 10% of the total ordinary shares outstanding over a five-year period. The awards granted under these plans have various vesting terms over a period of service between one and four years, provided the recipient remains continuously engaged as a service provider. The options awards expire 10 years from the grant date. The share-based awards granted under these plans are generally equity-settled (see cash settlements below). As of December 31, 2024, the Group had issued 29,940,832 units of share-based awards under these plans. RSUs During the twelve months ended December 31, 2024 and 2023, the Group granted the following RSUs to certain non-executive Directors, executives and employees: Twelve months ended December 31, 2024 2023 Time based RSUs 4,388,116 102,732 Performance based RSUs 1,822,151 3,576,937 Total RSUs 6,210,267 3,679,669 RSU activity for the years ended December 31, 2024, 2023 and 2022 is detailed as follows: Number of Shares/Units Weighted Average Grant Date Fair Value (GBP) (*) Outstanding (Non-vested) at January 1, 2022 3,632,715 1.91 RSUs Granted in Period 4,309,883 1.76 Vested (696,398) 2.80 Forfeited (1,155,420) 2.67 Outstanding (Non-vested) at December 31, 2022 and January 1, 2023 6,090,780 1.74 RSUs Granted in Period 3,679,669 1.28 Vested (716,029) 2.00 Forfeited (1,880,274) 1.94 Outstanding (Non-vested) at December 31, 2023 and January 1, 2024 7,174,146 1.10 RSUs Granted in Period 6,210,267 1.63 Vested (1,347,729) 1.71 Forfeited (3,057,962) 1.75 Outstanding (Non-vested) at December 31, 2024 8,978,722 1.29 * For liability awards - based on fair value at reporting date or settlement date. Each RSU entitles the holder to one ordinary share on vesting and the RSU awards are generally based on a vesting schedule over a one to three-year requisite service period in which the Group recognizes compensation expense for the RSUs. Following vesting, each recipient will be required to make a payment of one pence per ordinary share on settlement of the RSUs. RSUs granted to the non-executive directors and employees are time-based and equity-settled. The grant date fair value on such RSUs is recognized over the vesting term. RSUs granted to executives are performance-based and vesting of such RSUs is subject to the satisfaction of both performance and market conditions. The performance condition is based on the achievement of the Group's strategic targets. The market conditions are based on the achievement of the absolute total shareholder return (“TSR”), TSR as compared to the FTSE 250 Index, and TSR as compared to the MSCI Europe Health Care Index. The RSU award performance criteria have changed over time as the criteria are continually evaluated by the Group’s Remuneration Committee.
Fi na nc ia l s ta te m en ts 154 PureTech Health plc Annual Report and Accounts 2024 10. Share-based Payments continued Notes to the Consolidated Financial Statements continued The Group recognizes the estimated fair value of performance-based awards with non-market conditions as share-based compensation expense over the performance period based upon its determination of whether it is probable that the performance targets will be achieved. The Group assesses the probability of achieving the performance targets at each reporting period. Cumulative adjustments, if any, are recorded to reflect subsequent changes in the estimated outcome of performance-related conditions. The fair value of the performance-based awards with market conditions is based on the Monte Carlo simulation analysis utilizing a Geometric Brownian Motion process with 100,000 simulations to value those shares. The model considers share price volatility, risk-free rate and other covariance of comparable public companies and other market data to predict distribution of relative share performance. The RSUs to executives are treated as liability awards as the Group has a historical practice of settling these awards in cash, and as such adjusted to fair value at every reporting date until settlement with changes in fair value recorded in earnings as stock based compensation expense. The Group recorded $4,388, $827, and $1,637, respectively, for the years ended December 31, 2024, 2023 and 2022 in respect of all restricted stock units, of which $909, $402, and $1,131, respectively, were in respect of liability settled share-based awards. As of December 31, 2024, the carrying amount of the RSU liability awards was $3,736 with $1,875 current and $1,861 non current, out of which $1,875 related to awards that have met all their performance and market conditions and were settled in February, 2025. As of December 31, 2023, the carrying amount of the RSU liability awards was $4,782 with $1,281 current and $3,501 non- current, out of which $1,281 related to awards that met all their performance and market conditions and were settled in March and May of 2024. Stock Options Stock option activity for the years ended December 31, 2024, 2023 and 2022, is detailed as follows: Number of Options Wtd Average Exercise Price (GBP) Wtd Average of remaining contractual term (in years) Wtd Average Stock Price at Exercise (GBP) Outstanding at January 1, 2022 13,414,118 2.58 8.29 Granted 8,881,000 2.04 Exercised (577,022) 0.50 2.43 Forfeited and expired (3,924,215) 2.89 Options Exercisable at December 31, 2022 and January 1, 2023 6,185,216 2.03 6.21 Outstanding at December 31, 2022 and January 1, 2023 17,793,881 2.31 8.03 Granted 3,120,975 2.22 Exercised (534,034) 1.71 2.46 Forfeited and expired (3,424,232) 2.40 Options Exercisable at December 31, 2023 and January 1, 2024 9,065,830 2.19 6.01 Outstanding at December 31, 2023 and January 1, 2024 16,956,590 2.29 7.20 Granted 2,665,875 1.87 Exercised (412,729) 1.73 2.2 Forfeited and expired (4,725,746) 2.24 Options Exercisable at December 31, 2024 9,534,400 2.33 4.45 Outstanding at December 31, 2024 14,483,990 2.25 5.87 The fair value of the stock options awarded by the Group was estimated on the grant date using the Black-Scholes option valuation model, considering the terms and conditions upon which options were granted, with the following weighted-average assumptions: At December 31, 2024 2023 2022 Expected volatility 44.76% 43.69% 41.70% Expected terms (in years) 6.16 6.16 6.11 Risk-free interest rate 4.31% 4.04% 2.13% Expected dividend yield — — — Exercise price (GBP) 1.87 2.22 2.04 Underlying stock price (GBP) 1.87 2.22 2.04 Expected volatility is based the Group’s historical volatility results. These assumptions resulted in an estimated weighted-average grant-date fair value per share of stock options granted during the years ended December 31, 2024, 2023 and 2022 of $1.18, $1.37 and $1.15, respectively.
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 155 10. Share-based Payments continued Notes to the Consolidated Financial Statements continued The Group incurred share-based payment expense for the stock options of $1,092, $3,310 and $8,351 for the years ended December 31, 2024, 2023 and 2022, respectively. For shares outstanding as of December 31, 2024, the range of exercise prices is detailed as follows: Range of Exercise Prices (GBP) Options Outstanding Wtd Average Exercise Price (GBP) Wtd Average of remaining contractual term (in years) 0.01 89,845 — 4.75 1.00 to 2.00 6,133,522 1.63 6.03 2.00 to 3.00 4,823,373 2.25 6.39 3.00 to 4.00 3,437,250 3.41 4.87 Total 14,483,990 2.25 5.87 Subsidiary Plans For the years ended December 31, 2024, 2023 and 2022, the subsidiaries incurred share-based payment expense of $17,372, $277 and $4,711, respectively. The share-based payment expense for the year ended December 31, 2024, is primarily related to awards granted under the Seaport 2024 Equity Incentive Plan (the "Seaport Plan") approved by the Seaport Board of Directors in 2024. Seaport is deconsolidated from the Group's Consolidated Financial Statements as of October 18, 2024. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. The options granted under the Seaport Plan are equity settled and expire 10 years from the grant date. Typically, the awards vest in four years but vesting conditions can vary based on the discretion of Seaport’s Board of Directors. The estimated grant date fair value of the equity awards is recognized as an expense over the awards’ vesting periods. See tables below for Seaport option- related activities. Before its deconsolidation on October 18, 2024, Seaport granted 7,200,000 shares of restricted stock awards and restricted stock units to certain officers and directors, of which 6,227,778 shares were fully vested as of the deconsolidation date. The fair value of these awards was measured on the date of grant at the estimated fair value of the Seaport common stock using the market backsolve and probability adjusted expected return model. See Note 19. Financial Instruments. The weighted average fair value of these awards was $0.97. As the substantial majority of these awards were fully vested as of the deconsolidation date, the stock- based compensation expense for these awards was recognized in the Group’s Consolidated Statement of Comprehensive Income/ (Loss) for the year ended December 31, 2024. Seaport also granted options to its employees, officers and directors in 2024. The fair value of the stock options awarded by Seaport was estimated on the grant date using the Black-Scholes option valuation model. The weighted average fair value of these awards was $0.92. A summary of stock option activity by number of shares in these subsidiaries is presented in the following table: Outstanding as of January 1, 2024 Granted During the Year Exercised During the Year Expired During the Year Forfeited During the Year Deconsolidation During the Year Outstanding as of December 31, 2024 Entrega 344,500 — — (5,000) (5,000) — 334,500 Seaport — 22,429,780 — — (29,018) (22,400,762) — Outstanding as of January 1, 2023 Granted During the Year Exercised During the Year Expired During the Year Forfeited During the Year Deconsolidation During the Year Outstanding as of December 31, 2023 Entrega 344,500 — — — — — 344,500 Follica 2,776,120 — — (2,170,547) (605,573) — — Vedanta 1,824,576 — — (1,313) (29,607) (1,793,656) — Outstanding as of January 1, 2022 Granted During the Year Exercised During the Year Expired During the Year Forfeited During the Year Deconsolidation During the Year Outstanding as of December 31, 2022 Entrega 349,500 45,000 — (50,000) — — 344,500 Follica 2,686,120 90,000 — — — — 2,776,120 Sonde 2,049,004 — — — — (2,049,004) — Vedanta 1,991,637 490,506 (400,000) (65,235) (192,332) — 1,824,576
Fi na nc ia l s ta te m en ts 156 PureTech Health plc Annual Report and Accounts 2024 10. Share-based Payments continued Notes to the Consolidated Financial Statements continued The weighted-average exercise prices and remaining contractual life for the options outstanding as of December 31, 2024, were as follows: Outstanding at December 31, 2024 Number of options Weighted- average exercise price $ Weighted- average contractual life outstanding Entrega 334,500 1.96 2.78 There were no grants in 2023 under any of the subsidiary option plans. The weighted average exercise prices for the options granted for the years ended December 31, 2024 and 2022, were as follows: For the years ended December 31, 2024 $ 2023 $ 2022 $ Seaport 1.28 — — Entrega — — 0.02 Follica — — 1.86 Vedanta — — 14.94 The weighted average exercise prices for options forfeited during the year ended December 31, 2024, were as follows: Forfeited during the year ended December 31, 2024 Number of options Weighted- average exercise price $ Entrega 5,000 0.02 Seaport 29,018 0.97 The weighted average exercise prices for options exercisable as of December 31, 2024, were as follows: Exercisable at December 31, 2024 Number of Options Weighted- average exercise price $ Exercise Price Range $ Entrega 334,500 1.96 0.02-2.36 There were no subsidiary options exercised during the year ended December 31, 2024. 11. Finance Income/(Costs), net The following table shows the breakdown of finance income and costs: 2024 $ 2023 $ 2022 $For the years ended December 31, Finance income Interest income from financial assets 22,669 16,012 5,799 Total finance income 22,669 16,012 5,799 Finance costs Contractual interest expense on notes payable (684) (1,422) (212) Interest expense on other borrowings — (363) (1,759) Interest expense on lease liability (1,295) (1,544) (1,982) Gain on forgiveness of debt 273 — — Gain/(loss) on foreign currency exchange (25) (94) 14 Total finance costs – contractual (1,731) (3,424) (3,939) Gain/(loss) from changes in fair value of warrant liability — 33 6,740 Gain/(loss) from changes in fair value of preferred shares (8,108) 2,617 130,825 Gain/(loss) from changes in fair value of convertible debt — — (502) Total finance income/(costs) – fair value accounting (8,108) 2,650 137,063 Total finance costs - non cash interest expense related to sale of future royalties (8,058) (10,159) — Finance income/(costs), net 4,773 5,078 138,924
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 157 Notes to the Consolidated Financial Statements continued 12. Earnings/(Loss) per Share Basic earnings/(loss) per share is calculated by dividing the Group's net income or loss for the period attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding, net of treasury shares. Diluted earnings/(loss) per share is calculated by dividing the Group's net income or loss for the period attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding, net of treasury shares, plus the weighted average number of ordinary shares that would be issued at conversion of all the dilutive potential ordinary shares into ordinary shares. Dilutive effects arise from equity-settled shares from the Group's share-based plans. For the years ended December 31, 2023 and 2022, the Group incurred a net loss, and therefore, all outstanding potential securities were considered anti-dilutive. The amount of potential securities that were excluded from the diluted calculation amounted to 1,509,900 and 3,134,131 shares, respectively. Earnings/(Loss) Attributable to Owners of the Group: 2024 2023 2022 Basic $ Diluted $ Basic $ Diluted $ Basic $ Diluted $ Income/(loss) for the year, attributable to the owners of the Group 53,510 53,510 (65,697) (65,697) (50,354) (50,354) Weighted-Average Number of Ordinary Shares: 2024 2023 2022 Basic Diluted Basic Diluted Basic Diluted Issued ordinary shares at January 1, 271,853,731 271,853,731 278,566,306 278,566,306 287,796,585 287,796,585 Effect of shares issued & treasury shares purchased (17,397,423) (17,397,423) (2,263,773) (2,263,773) (3,037,150) (3,037,150) Effect of dilutive shares — 1,571,612 — — — — Weighted average number of ordinary shares at December 31, 254,456,308 256,027,920 276,302,533 276,302,533 284,759,435 284,759,435 Earnings/(Loss) per Share: 2024 2023 2022 Basic $ Diluted $ Basic $ Diluted $ Basic $ Diluted $ Basic and diluted earnings/(loss) per share 0.21 0.21 (0.24) (0.24) (0.18) (0.18)
Fi na nc ia l s ta te m en ts 158 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 13. Property and Equipment Cost Laboratory and Manufacturing Equipment $ Furniture and Fixtures $ Computer Equipment and Software $ Leasehold Improvements $ Construction in process $ Total $ Balance as of January 1, 2023 13,341 1,510 1,419 23,964 2,803 43,037 Additions, net of transfers — — — — 87 87 Disposals (2,886) — (137) — — (3,023) Deconsolidation of subsidiaries (5,092) (438) (365) (8,799) (2,871) (17,565) Reclassifications — — — — (18) (18) Balance as of December 31, 2023 5,363 1,072 917 15,165 1 22,518 Additions, net of transfers 246 — 11 — — 256 Disposals/Impairment (2,215) — (387) — (1) (2,602) Deconsolidation of subsidiaries (246) — (11) — — (256) Reclassifications — — — — — — Balance as of December 31, 2024 3,148 1,072 530 15,165 — 19,916 Accumulated depreciation and impairment loss Laboratory and Manufacturing Equipment $ Furniture and Fixtures $ Computer Equipment and Software $ Leasehold Improvements $ Construction in process $ Total $ Balance as of January 1, 2023 (7,711) (875) (1,244) (10,250) — (20,080) Depreciation (892) (162) (45) (1,856) — (2,955) Disposals 543 — 38 — — 581 Deconsolidation of subsidiaries 3,917 339 357 4,858 — 9,472 Balance as of December 31, 2023 (4,142) (698) (894) (7,248) — (12,982) Depreciation (139) (153) (13) (1,503) — (1,807) Disposals 1,485 — 376 — — 1,861 Deconsolidation of subsidiaries 81 — — — — 81 Balance as of December 31, 2024 (2,715) (851) (530) (8,751) — (12,847) Property and Equipment, net Laboratory and Manufacturing Equipment $ Furniture and Fixtures $ Computer Equipment and Software $ Leasehold Improvements $ Construction in process $ Total $ Balance as of December 31, 2023 1,221 375 23 7,917 1 9,536 Balance as of December 31, 2024 433 221 — 6,414 — 7,069 Depreciation of property and equipment is included in the general and administrative expenses and research and development expenses in the Consolidated Statement of Comprehensive Income/(Loss). The Group recorded depreciation expense of $1,807, $2,955 and $5,845 for the years ended December 31, 2024, 2023 and 2022, respectively.
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 159 Notes to the Consolidated Financial Statements continued 14. Intangible Assets Intangible assets consist of licenses of intellectual property acquired by the Group through various agreements with third parties and are recorded at the value of the consideration transferred. Information regarding the cost and accumulated amortization of intangible assets is as follows: Cost Licenses $ Balance as of January 1, 2023 831 Additions 200 Write-off (105) Deconsolidation of subsidiary (19) Balance as of December 31, 2023 906 Write-off (80) Deconsolidation of subsidiary (225) Balance as of December 31, 2024 601 All the intangible asset licenses represent in-process-research-and-development assets that are currently still being developed and not ready for their intended use. As such, these assets are not amortized but tested for impairment annually. During the year ended December 31, 2024, the Group wrote off one of its research intangible assets for which research was ceased in the amount of $80. During the year ended December 31, 2024, Seaport Therapeutics, Inc. was deconsolidated and as such, $225 in net intangible assets were derecognized. During the year ended December 31, 2023, the Group wrote off two of its research intangible assets for which research was ceased in the amount of $105. During the year ended December 31, 2023, Vedanta, Inc. was deconsolidated and as such, $19 in net intangible assets were derecognized. The Group tested all intangible assets for impairment as of the balance sheet date and concluded that none of such assets were impaired. 15. Other Financial Assets Other financial assets consist primarily of restricted cash reserved as collateral against a letter of credit with a bank that is issued for the benefit of a landlord in lieu of a security deposit for office space leased by the Group. The restricted cash was $1,642 and $1,628 as of December 31, 2024 and 2023, respectively.
Fi na nc ia l s ta te m en ts 160 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 16. Equity Total equity for the Group as of December 31, 2024, and 2023, was as follows: December 31, 2024 $ December 31, 2023 $Equity Share capital, £0.01 par value, issued and paid 257,927,489 and 289,468,159 as of December 31, 2024 and 2023, respectively 4,860 5,461 Share premium 290,262 290,262 Treasury shares, 18,506,177 and 17,614,428 as of December 31, 2024 and 2023, respectively (46,864) (44,626) Merger Reserve 138,506 138,506 Translation reserve 182 182 Other reserves (4,726) (9,538) Retained earnings/(accumulated deficit) 32,486 83,820 Equity attributable to owners of the Group 414,707 464,066 Non-controlling interests (6,774) (5,835) Total equity 407,933 458,232 Shareholders are entitled to vote on all matters submitted to shareholders for a vote. Each ordinary share is entitled to one vote and is entitled to receive dividends when and if declared by the Group’s Directors. On June 18, 2015, the Group acquired the entire issued share capital of PureTech LLC in return for 159,648,387 ordinary shares. This was accounted for as a common control transaction at cost. It was deemed that the share capital was issued in line with movements in share capital as shown prior to the transaction taking place. In addition, the merger reserve records amounts previously recorded as share premium. Other reserves comprise the cumulative credit to share-based payment reserves corresponding to share-based payment expenses recognized through Consolidated Statement of Comprehensive Income/(Loss), settlements of vested stock awards as well as other additions that flow directly through equity such as the excess or deficit from changes in ownership of subsidiaries while control is maintained by the Group. On May 9, 2022, the Group announced the commencement of a $50,000 share repurchase program (the "Program") of its ordinary shares of one pence each. The Group executed the Program in two equal tranches. It entered into an irrevocable non-discretionary instruction with Jefferies International Limited (“Jefferies”) in relation to the purchase by Jefferies of the ordinary shares for an aggregate consideration (excluding expenses) of no greater than $25,000 for each tranche and the simultaneous on-sale of such ordinary shares by Jefferies to the Group, subject to certain volume and price restrictions. In February 2024, the Group completed the Program and has repurchased an aggregate of 20,182,863 ordinary shares under the Program. These shares have been held as treasury shares and are being used to settle the vesting of restricted stock units or exercise of stock options. In March 2024, the Group announced a proposed capital return of $100,000 to its shareholders by way of a tender offer (the "Tender Offer"). The proposed Tender Offer was approved by shareholders at the Annual General Meeting of Stockholders held on June 6, 2024, to acquire a maximum number of 33,500,000 ordinary shares (including ordinary shares represented by American Depository Shares (''ADSs'')) for a fixed price of 250 pence per ordinary share (equivalent to £25.00 per ADS) for a maximum aggregate amount of $100,000 excluding expenses. The Tender Offer was completed on June 24, 2024. The Group repurchased 31,540,670 ordinary shares under the Tender Offer. Following such repurchase, the Group cancelled these shares repurchased. As a result of the cancellation, the nominal value of $600 related to the cancelled shares was reduced from share capital and transferred to a capital redemption reserve, increasing the capital redemption reserve balance to $600 which was included within other reserves in the Consolidated Statement of Changes in Equity. As of December 31, 2024 and December 31, 2023, the Group’s issued share capital was 257,927,489 shares and 289,468,159 shares, respectively, including 18,506,177 shares and 17,614,428 shares repurchased under the share repurchase program, and were held by the Group in treasury, respectively. The Group does not have a limited amount of authorized share capital.
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 161 Notes to the Consolidated Financial Statements continued 17. Subsidiary Preferred Shares Preferred shares issued by subsidiaries often contain redemption and conversion features that are assessed under IFRS 9 in conjunction with the host preferred share instrument. This balance represents subsidiary preferred shares issued to third parties. The subsidiary preferred shares are redeemable upon the occurrence of a contingent event, other than full liquidation of the subsidiaries, that is not considered to be within the control of the subsidiaries. Therefore, these subsidiary preferred shares are classified as liabilities. These liabilities are measured at fair value through profit and loss. The preferred shares are convertible into ordinary shares of the subsidiaries at the option of the holders and are mandatorily convertible into ordinary shares under certain circumstances. Under certain scenarios, the number of ordinary shares receivable on conversion will change and therefore, the number of shares that will be issued is not fixed. As such, the conversion feature is considered to be an embedded derivative that normally would require bifurcation. However, since the subsidiary preferred share liability is measured at fair value through profit and loss, as mentioned above, no bifurcation is required. The preferred shares are entitled to vote with holders of common shares on an as converted basis. In April 2024, Seaport closed a Series A-2 preferred share financing with aggregate proceeds of $100,100 of which $68,100 was from outside investors and $32,000 was from the Group. The $68,100 received from the outside investors was recorded as a subsidiary preferred share liability within the Group’s balance sheet. In October 2024, Seaport closed a Series B preferred share financing with aggregate proceeds of $226,000 of which $211,600 was from outside investors and $14,400 was from the Group. As a result of the Series B preferred share financing, the Group lost control of Seaport, and the Group derecognized the assets, liabilities and non-controlling interest in respect of Seaport from its Consolidated Financial Statements. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. As such, the balance of subsidiary preferred share liability in Seaport is reduced to $0 upon deconsolidation. The fair value of all subsidiary preferred shares as of December 31, 2024 and December 31, 2023, is as follows: 2024 $ 2023 $Balance as of December 31, 2024 and December 31, 2023 Entrega 169 169 Total subsidiary preferred share balance 169 169 As is customary, in the event of any voluntary or involuntary liquidation, dissolution or winding up of a subsidiary, the holders of outstanding subsidiary preferred shares shall be entitled to be paid out of the assets of the subsidiary available for distribution to shareholders and before any payment shall be made to holders of ordinary shares. A merger, acquisition, sale of voting control or other transaction of a subsidiary in which the shareholders of the subsidiary immediately before the transaction do not own a majority of the outstanding shares of the surviving company shall be deemed to be a liquidation event. Additionally, a sale, lease, transfer or other disposition of all or substantially all of the assets of the subsidiary shall also be deemed a liquidation event. As of December 31, 2024 and December 31, 2023, the minimum liquidation preference reflecting the amounts that would be payable to the subsidiary preferred holders upon a liquidation event of the subsidiaries, is as follows: 2024 $ 2023 $Balance as of December 31, 2024 and December 31, 2023 Entrega 2,216 2,216 Follica 6,405 6,405 Total minimum liquidation preference 8,621 8,621 For the years ended December 31, 2024 and 2023, the Group recognized the following changes in the value of subsidiary preferred shares: $ Balance as of January 1, 2023 27,339 Decrease in value of preferred shares measured at fair value – finance costs (income) (2,617) Deconsolidation of subsidiary - (Vedanta) (24,554) Balance as of December 31, 2023 and January 1, 2024 169 Issuance of Seaport A-2 preferred shares - financing cash flow 68,100 Increase in value of preferred shares measured at fair value – finance costs (income) 8,108 Deconsolidation of subsidiary – (Seaport) (76,208) Balance as of December 31, 2024 169

Fi na nc ia l s ta te m en ts 162 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 18. Sale of Future Royalties Liability On March 4, 2011, the Group entered into a license agreement (the “License Agreement”) with Karuna, according to which the Group granted Karuna an exclusive license to research, develop and sell KarXT in exchange for a royalty on annual net sales, development and regulatory milestones and a fixed portion of sublicensing income, if any. On March 22, 2023, the Group signed an agreement with Royalty Pharma (the "Royalty Purchase Agreement"), according to which the Group sold Royalty Pharma a partial right to receive royalty payments made by Karuna in respect of net sales of KarXT, if and when received. According to the Royalty Purchase Agreement, all royalties due to the Group under the License Agreement will be paid to Royalty Pharma up to an annual royalties threshold of $60,000, while all royalties above such annual threshold in a given year will be split 33% to Royalty Pharma and 67% to the Group. Under the terms of the Royalty Purchase Agreement, the Group received a non-refundable initial payment of $100,000 at the execution of the Royalty Purchase Agreement and is eligible to receive additional payments in the aggregate of up to an additional $400,000 based on the achievement of certain regulatory and commercial milestones. The Group continues to hold the rights under the License Agreement and has a contractual obligation to deliver cash to Royalty Pharma for a portion of the royalties it receives. Therefore, the Group will continue to account for any royalties and milestones due to the Group under the License Agreement as revenue in its Consolidated Statement of Comprehensive Income/(Loss) and record the proceeds from the Royalty Purchase Agreement as a financial liability on its Consolidated Statement of Financial Position. In determining the appropriate accounting treatment for the Royalty Purchase Agreement, management applied significant judgement. The acquisition of Karuna by Bristol Myers Squibb ("BMS"), which closed on March 18, 2024, had no impact on the Group's rights or obligations under the License Agreement or the Royalty Purchase Agreement, each of which remains in full force and effect. In order to determine the amortized cost of the sale of future royalties liability, management is required to estimate the total amount of future receipts from and payments to Royalty Pharma under the Royalty Purchase Agreement over the life of the agreement. The $100,000 liability, recorded at execution of the Royalty Purchase Agreement, is accreted to the total of these receipts and payments as interest expense over the life of the Royalty Purchase Agreement. These estimates contain assumptions that impact both the amortized cost of the liability and the interest expense that are recognized in each reporting period. Additional proceeds received from Royalty Pharma increase the Group’s financial liability. As royalty payments are made to Royalty Pharma, the balance of the liability is effectively repaid over the life of the Royalty Purchase Agreement. The estimated timing and amount of royalty payments to and proceeds from Royalty Pharma are likely to change over the life of the Royalty Purchase Agreement. A significant increase or decrease in estimated royalty payments, or a significant shift in the timing of cash flows, will materially impact the sale of future royalties liability, interest expense and the time period for repayment. The Group periodically assesses the expected payments to, or proceeds from, Royalty Pharma. Any such changes in amount or timing of cash flows requires the Group to re-calculate the amortized cost of the sale of future royalties liability as the present value of the estimated future cash flows from the Royalty Purchase Agreement that are discounted at the liability’s original effective interest rate. The adjustment is recognized immediately in profit or loss as income or expense. On October 1, 2024, the Group received $25,000 from Royalty Pharma upon the FDA's approval for BMS to market KarXT as Cobenfy. The Group paid Royalty Pharma $315 in the first quarter of 2025 for the royalties received from BMS for the sale of Cobenfy in the fourth quarter of 2024. The following shows the activity in respect of the sale of future royalties liability: Sale of future royalties liability $ Balance as of January 1, 2023 — Amounts received at closing 100,000 Non cash interest expense recognized 10,159 Balance as of December 31, 2023 and January 1, 2024 110,159 Payment from Royalty Pharma – regulatory milestone 25,000 Non cash interest expense recognized 8,058 Balance as of December 31, 2024 143,217 Sale of future royalties liability, current 6,435 Sale of future royalties liability, non-current 136,782
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 163 Notes to the Consolidated Financial Statements continued 19. Financial Instruments The Group’s financial instruments consist of financial assets in the form of notes, convertible notes and investment in shares, and financial liabilities, including preferred shares. Many of these financial instruments are presented at fair value, with changes in fair value recorded through profit and loss. Fair Value Process For financial instruments measured at fair value under IFRS 9, the change in the fair value is reflected through profit and loss. Using the guidance in IFRS 13, the total business enterprise value and allocable equity of each entity being valued can be determined using a market backsolve approach through a recent arm’s length financing round (or a future probable arm's length transaction), market/asset probability-weighted expected return method ("PWERM") approach, discounted cash flow approach, or hybrid approaches. The approaches, in order of strongest fair value evidence, are detailed as follows: Valuation Method Description Market – Backsolve The market backsolve approach benchmarks the original issue price (OIP) of the company’s latest funding transaction as current value. Market/Asset – PWERM Under a PWERM, the company value is based upon the probability-weighted present value of expected future investment returns, considering each of the possible future outcomes available to the enterprise. Possible future outcomes can include IPO scenarios, potential SPAC transactions, merger and acquisition transactions as well as other similar exit transactions of the investee. Income Based – DCF The income approach is used to estimate fair value based on the income streams, such as cash flows or earnings, that an asset or business can be expected to generate. At each measurement date, investments held at fair value (that are not publicly traded) as well as the fair value of subsidiary preferred share liability, including embedded conversion rights that are not bifurcated, were determined using the following allocation methods: option pricing model (“OPM”), PWERM, or hybrid allocation framework. The methods are detailed as follows: Allocation Method Description OPM The OPM model treats preferred stock as call options on the enterprise’s equity value, with exercise prices based on the liquidation preferences of the preferred stock. PWERM Under a PWERM, share value is based upon the probability-weighted present value of expected future investment returns, considering each of the possible future outcomes available to the enterprise, as well as the rights of each share class. Hybrid The hybrid method is a combination of the PWERM and OPM. Under the hybrid method, multiple liquidity scenarios are weighted based on the probability of the scenario's occurrence, similar to the PWERM, while also utilizing the OPM to estimate the allocation of value in one or more of the scenarios. Valuation policies and procedures are regularly monitored by the Group. Fair value measurements, including those categorized within Level 3, are prepared and reviewed for reasonableness and compliance with the fair value measurements guidance under IFRS accounting standards. The Group measures fair value using the following fair value hierarchy that reflects the significance of the inputs used in making the measurements: Fair Value Hierarchy Level Description Level 1 Inputs that are quoted market prices (unadjusted) in active markets for identical instruments. Level 2 Inputs other than quoted prices included within Level 1 that are observable either directly (i.e. as prices) or indirectly (i.e. derived from prices). Level 3 Inputs that are unobservable. This category includes all instruments for which the valuation technique includes inputs not based on observable data and the unobservable inputs have a significant effect on the instruments' valuation. Whilst the Group considers the methodologies and assumptions adopted in fair value measurements as supportable and reasonable, because of the inherent uncertainty of valuation, those estimated values may differ significantly from the values that would have been used had a ready market for the investment existed. Subsidiary Preferred Share Liability As of December 31, 2024 and 2023, the fair value of subsidiary preferred share liability was $169 and $169, respectively. See Note 17. Subsidiary Preferred Shares for the changes in the Group’s subsidiary preferred share liability measured at fair value, which are categorized as Level 3 in the fair value hierarchy. The changes in fair value of subsidiary preferred share liability is recorded in finance income/(costs) – fair value accounting in the Consolidated Statement of Comprehensive Income/(Loss).
Fi na nc ia l s ta te m en ts 164 PureTech Health plc Annual Report and Accounts 2024 19. Financial Instruments continued Notes to the Consolidated Financial Statements continued Investments Held at Fair Value Vor Valuation Vor (Nasdaq: VOR) and additional immaterial investments are listed entities on an active exchange, and as such, the fair value as of December 31, 2024 was calculated utilizing the quoted common share price which is categorized as Level 1 in the fair value hierarchy. Seaport, Vedanta and Sonde As of December 31, 2024, the Group accounts for the following investments under IFRS 9 as investments held at fair value with changes in fair value through profit and loss: Seaport preferred A-1, A-2, and B shares, Vedanta preferred A-1, B, C, and D shares, Sonde preferred A-2 and B shares and other immaterial investment. The valuations of the aforementioned investments are categorized as Level 3 in the fair value hierarchy due to the use of significant unobservable inputs to value such assets. During the year ended December 31, 2024, the Group recorded such investments at fair value and recognized a loss of $10,361 for the changes in fair value of the investments. The following table summarizes the changes in all the Group’s investments held at fair value categorized as Level 3 in the fair value hierarchy: Balance as of January 1, 2023 12,593 Deconsolidation of Vedanta - new investment in Vedanta preferred shares 20,456 Investment in Gelesis 2023 Warrants 1,121 Gain/(loss) on changes in fair value (9,299) Balance as of December 31, 2023 and January 1, 2024 24,872 Deconsolidation of Seaport - new investment in Seaport preferred shares 179,248 Gain/(loss) on changes in fair value (10,361) Balance as of December 31, 2024 193,758 Equity method loss recorded against LTI (5,307) Balance as of December 31, 2024 after allocation of equity method loss to LTI 188,452 The changes in fair value of investments held at fair value is recorded in gain/(loss) on investments held at fair value in the Consolidated Statement of Comprehensive Income/(Loss). As of December 31, 2024, the Group’s material investments held at fair value categorized as Level 3 in the fair value hierarchy include the preferred shares of Seaport, and Vedanta, with fair value of $177,288, and $11,163, respectively. The significant unobservable inputs used at December 31, 2024 in the fair value measurement of these investments and the sensitivity of the fair value measurements for these investments to changes of these significant unobservable inputs are summarized in the tables below. As of December 31, 2024 Investment (Seaport) Measured through Market Backsolve & PWERM Unobservable Inputs (Seaport) Input Value Sensitivity Range Fair Value Increase/ (Decrease) $ Equity Value 538,635 -10% (22,099) +10% 21,716 Time to Liquidity 0.5 -3 months 5,753 +3 months (4,247) Probability of achieving a certain clinical trial development milestone 80% -10% (7,871) +10% 7,871 Probability of entering into an initial public offering 25% and 20% -10% (4,754) +10% 4,754 The unobservable inputs outlined within the table above were used to determine the fair value of our investment in the convertible preferred shares of a private company as of December 31, 2024. Whilst the Group considers the methodologies and assumptions used in the fair value measurement to be supportable and reasonable, because of the inherent uncertainties associated with the valuation, the estimated value may differ significantly from the values that would have been used had a ready market for the investment existed. The fair value measurement of our investment in the convertible preferred shares will be updated at each reporting date. As of December 31, 2024 Investment (Vedanta) Measured through Market Backsolve that Leverages a Monte Carlo Simulation Unobservable Inputs (Vedanta) Input Value Sensitivity Range Fair Value Increase/ (Decrease) $ Equity Value 30,845 -5% (1,617) +5% 1,515 Time to Liquidity 0.27 -3 Months n.a. +3 Months 5,238 Volatility 155% -10% (2,976) +10% 518
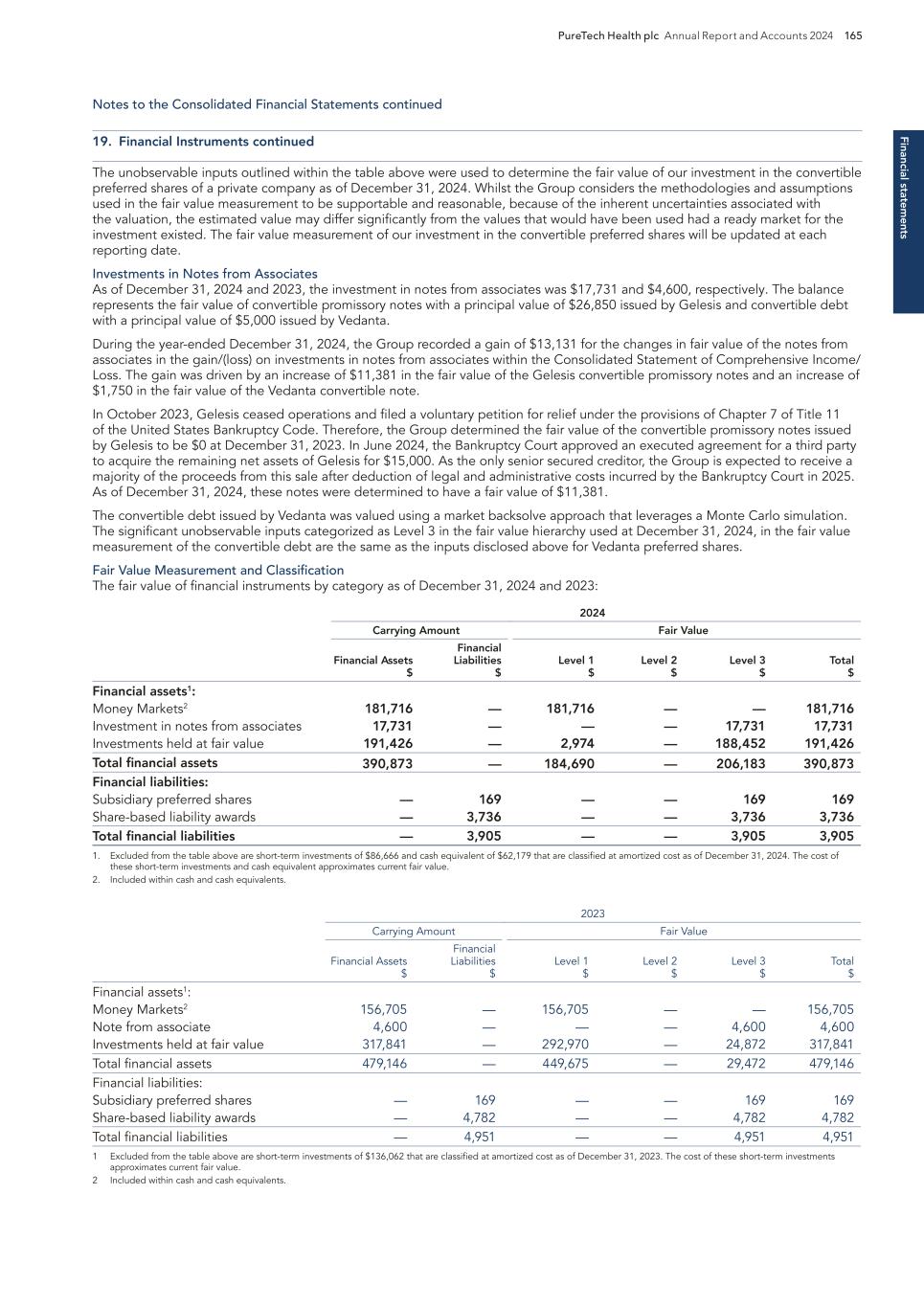
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 165 19. Financial Instruments continued Notes to the Consolidated Financial Statements continued The unobservable inputs outlined within the table above were used to determine the fair value of our investment in the convertible preferred shares of a private company as of December 31, 2024. Whilst the Group considers the methodologies and assumptions used in the fair value measurement to be supportable and reasonable, because of the inherent uncertainties associated with the valuation, the estimated value may differ significantly from the values that would have been used had a ready market for the investment existed. The fair value measurement of our investment in the convertible preferred shares will be updated at each reporting date. Investments in Notes from Associates As of December 31, 2024 and 2023, the investment in notes from associates was $17,731 and $4,600, respectively. The balance represents the fair value of convertible promissory notes with a principal value of $26,850 issued by Gelesis and convertible debt with a principal value of $5,000 issued by Vedanta. During the year-ended December 31, 2024, the Group recorded a gain of $13,131 for the changes in fair value of the notes from associates in the gain/(loss) on investments in notes from associates within the Consolidated Statement of Comprehensive Income/ Loss. The gain was driven by an increase of $11,381 in the fair value of the Gelesis convertible promissory notes and an increase of $1,750 in the fair value of the Vedanta convertible note. In October 2023, Gelesis ceased operations and filed a voluntary petition for relief under the provisions of Chapter 7 of Title 11 of the United States Bankruptcy Code. Therefore, the Group determined the fair value of the convertible promissory notes issued by Gelesis to be $0 at December 31, 2023. In June 2024, the Bankruptcy Court approved an executed agreement for a third party to acquire the remaining net assets of Gelesis for $15,000. As the only senior secured creditor, the Group is expected to receive a majority of the proceeds from this sale after deduction of legal and administrative costs incurred by the Bankruptcy Court in 2025. As of December 31, 2024, these notes were determined to have a fair value of $11,381. The convertible debt issued by Vedanta was valued using a market backsolve approach that leverages a Monte Carlo simulation. The significant unobservable inputs categorized as Level 3 in the fair value hierarchy used at December 31, 2024, in the fair value measurement of the convertible debt are the same as the inputs disclosed above for Vedanta preferred shares. Fair Value Measurement and Classification The fair value of financial instruments by category as of December 31, 2024 and 2023: 2024 Carrying Amount Fair Value Financial Assets $ Financial Liabilities $ Level 1 $ Level 2 $ Level 3 $ Total $ Financial assets1: Money Markets2 181,716 — 181,716 — — 181,716 Investment in notes from associates 17,731 — — — 17,731 17,731 Investments held at fair value 191,426 — 2,974 — 188,452 191,426 Total financial assets 390,873 — 184,690 — 206,183 390,873 Financial liabilities: Subsidiary preferred shares — 169 — — 169 169 Share-based liability awards — 3,736 — — 3,736 3,736 Total financial liabilities — 3,905 — — 3,905 3,905 1. Excluded from the table above are short-term investments of $86,666 and cash equivalent of $62,179 that are classified at amortized cost as of December 31, 2024. The cost of these short-term investments and cash equivalent approximates current fair value. 2. Included within cash and cash equivalents. 2023 Carrying Amount Fair Value Financial Assets $ Financial Liabilities $ Level 1 $ Level 2 $ Level 3 $ Total $ Financial assets1: Money Markets2 156,705 — 156,705 — — 156,705 Note from associate 4,600 — — — 4,600 4,600 Investments held at fair value 317,841 — 292,970 — 24,872 317,841 Total financial assets 479,146 — 449,675 — 29,472 479,146 Financial liabilities: Subsidiary preferred shares — 169 — — 169 169 Share-based liability awards — 4,782 — — 4,782 4,782 Total financial liabilities — 4,951 — — 4,951 4,951 1 Excluded from the table above are short-term investments of $136,062 that are classified at amortized cost as of December 31, 2023. The cost of these short-term investments approximates current fair value. 2 Included within cash and cash equivalents.
Fi na nc ia l s ta te m en ts 166 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued 20. Subsidiary Notes Payable The subsidiary notes payable is comprised of loans and convertible notes. These instruments do not contain embedded derivatives, and therefore, are held at amortized cost. As of December 31, 2024 and December 31, 2023, the balance of notes payable consists of the following: 2024 $ 2023 $Balance as of December 31, Loans 4,111 3,439 Convertible notes — 260 Total subsidiary notes payable 4,111 3,699 Loans In October 2010, Follica entered into a loan and security agreement with Lighthouse Capital Partners VI, L.P. The loan is secured by Follica’s assets, including Follica’s intellectual property and bears interest at a rate of 5.0% in the interest only period and 12.0% in the repayment period. Convertible Notes The activities of the convertible notes were as follows: Knode $ Appeering $ Sonde $ Total $ January 1, 2022 94 141 2,461 2,696 Gross principal - issuance of notes - financing activity — — 393 393 Accrued interest on convertible notes - finance costs 5 8 48 60 Changes in fair value - finance costs — — 502 502 Deconsolidation — — (3,403) (3,403) Balance as of January 1, 2023 99 149 — 248 Accrued interest on convertible notes - finance costs 5 8 — 13 Balance as of December 31, 2023 and January 1, 2024 104 156 — 260 Accrued interest on convertible notes - finance costs 5 7 — 12 Forgiveness of debt – entity dissolution – finance income (109) (164) — (273) Balance as of December 31, 2024 — — — — In November 2024, the Group dissolved Knode and Appeering as they were no longer operational entities. As a result, the principal and interest on these notes outstanding were written off in full as of the dissolution date. 21. Non-Controlling Interest As of December 31, 2024 and 2023, non-controlling interests included Entrega and Follica. Ownership interests of the non- controlling interests in these entities as of December 31, 2024 were 11.7%, and 19.9%, respectively. There was no change from December 31, 2023 in the ownership interests of the non-controlling interests in these two entities. As of December 31, 2022, non- controlling interests included Entrega, Follica, and Vedanta. Ownership interests of the non-controlling interests in these entities were 11.7%, 19.9%, and 12.2%, respectively. Non-controlling interests include the amounts recorded for subsidiary stock awards. See Note 10. Share-based Payments. For the year ended December 31, 2024, Seaport issued 950,000 shares of fully vested common stock to the Group and 3,450,000 shares of common stock to certain officers and directors, of which 2,455,555 shares were fully vested before Seaport's deconsolidation from the Group's Consolidated Financial Statements on October 18, 2024. Ownership interest of non-controlling interests was 61.3% immediately before Seaport's deconsolidation. During the year ended December 31, 2023, Vedanta Biosciences, Inc was deconsolidated. During the year ended December 31, 2022, Sonde Health, Inc was deconsolidated. See Note 8. Gain/(loss) on Deconsolidation of Subsidiary. On February 15, 2022, option holders in Vedanta exercised options into shares of common stock, increasing the NCI interest held from 3.7% to 12.2%. The exercise of the options resulted in an increase in the NCI share in Vedanta shareholder's deficit of $15,164.
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 167 Notes to the Consolidated Financial Statements continued The following table summarizes the changes in the non-controlling ownership interest in subsidiaries: Non-Controlling Interest $ Balance as of January 1, 2022 (9,368) Share of comprehensive income (loss) 13,290 Deconsolidation of subsidiary (Sonde) 11,904 NCI exercise of share-based awards in subsidiaries - change in NCI interest (15,164) Equity settled share-based payments 4,711 Other (4) Balance as of December 31, 2022 and January 1, 2023 5,369 Share of comprehensive income (loss) (931) Deconsolidation of subsidiary (Vedanta) (9,085) Equity settled share-based payments 277 Expiration of share options in subsidiary (1,458) Other (6) Balance as of December 31, 2023 and January 1, 2024 (5,835) Share of comprehensive income (loss) (25,728) Equity settled share-based payments - See Note 10. Share-based Payments 17,372 Deconsolidation of subsidiary (Seaport) 7,430 Other (13) Balance as of December 31, 2024 (6,774) 22. Trade and Other Payables Information regarding Trade and other payables was as follows: Balance as of December 31, 2024 $ 2023 $ Trade payables 5,522 14,637 Accrued expenses 18,705 28,187 Liability for share-based awards- short term 1,875 1,281 Other 917 3 Total trade and other payables 27,020 44,107 Trade and other payables decreased by $17,088 to $27,020 as of December 31, 2024. The decrease reflected lower operating expenses primarily from the reduced clinical trials related activities as well as the deconsolidation of Seaport for the year ended December 31, 2024. 23. Leases and subleases The activity related to the Group’s right of use asset and lease liability for the years ended December 31, 2024 and 2023 is as follows: Right of use asset, net 2024 $ 2023 $ Balance as of January 1, 9,825 14,281 Additions — — Depreciation (1,764) (1,979) Deconsolidated — (2,477) Balance as of December 31, 8,061 9,825 21. Non-Controlling Interest continued
Fi na nc ia l s ta te m en ts 168 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued Total lease liability 2024 $ 2023 $ Balance as of January 1, 21,644 29,128 Additions — — Cash paid for rent - principal - financing cash flow (3,394) (3,338) Cash paid for rent - interest (1,295) (1,544) Interest expense 1,295 1,544 Deconsolidated — (4,146) Balance as of December 31, 18,250 21,644 Depreciation of the right-of-use assets, which virtually all consist of leased real estate, is included in the general and administrative expenses and research and development expenses line items in the Statement of Comprehensive Income/(Loss). The Group recorded depreciation expense of $1,764, $1,979 and $3,047 for the years ended December 31, 2024, 2023 and 2022, respectively. The following table details the short-term and long-term portion of the lease liability as of December 31, 2024 and 2023: Total lease liability 2024 $ 2023 $ Short-term portion of lease liability 3,579 3,394 Long-term portion of lease liability 14,671 18,250 Total lease liability 18,250 21,644 The following table details the future maturities of the lease liability, showing the undiscounted lease payments to be paid after the reporting date: 2024 $ Less than one year 4,644 One to two years 4,419 Two to three years 4,551 Three to four years 4,687 Four to five years 2,796 More than five years — Total undiscounted lease maturities 21,096 Interest 2,846 Total lease liability 18,250 During the year ended December 31, 2019, the Group entered into a lease agreement for certain premises consisting of 50,858 rentable square feet of space located at 6 Tide Street, Boston, Massachusetts. The lease commenced on April 26, 2019 for an initial term consisting of ten years and three months, and there is an option to extend the lease for two consecutive periods of five years each. The Group assessed at the lease commencement date whether it was reasonably certain to exercise the extension options, and deemed such options were not reasonably certain to be exercised. The Group will reassess whether it is reasonably certain to exercise the options only if there is a significant event or significant change in circumstances within its control. On June 26, 2019, the Group executed a sublease agreement with Gelesis. The lease is for 9,446 rentable square feet located on the sixth floor of the Group’s former office at 501 Boylston Street, Boston, Massachusetts. The sublease was set to expire on August 31, 2025, and was determined to be a finance lease. Gelesis ceased operations and filed for bankruptcy on October 30, 2023. As a result, the Group wrote off its receivable in the lease of $1,266 in 2023. On January 23, 2023, the Group executed a sublease agreement with Allonnia, LLC (“Allonnia”). The sublease is for approximately 11,000 rentable square feet located on the third floor of the 6 Tide Street building where the Group’s offices are currently located. Allonnia obtained possession of the premises on February 17, 2023 with a rent commencement date of May 17, 2023. The lease term is two years from the rent commencement date, and Allonnia has the option to extend the sublease for an additional year at the same terms. The annual lease fee is $1,111 per year. The sublease was determined to be an operating lease, and as such, the total lease payments under the sublease agreement are recognized over the lease term on a straight-line basis. In February 2024, Allonnia exercised the option and extended the lease term through May 31, 2026. The annual lease fee increased to $1,279 per year. Rental income recognized by the Group during the year ended December 31, 2024 and 2023 was $1,053 and $781, respectively, which was included in the other income/(expense) line item in the Consolidated Statement of Comprehensive Income/(Loss). 23. Leases and subleases continued
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 169 Notes to the Consolidated Financial Statements continued 24. Capital and Financial Risk Management Capital Risk Management The Group's capital and financial risk management policy is to maintain a strong capital base to support its strategic priorities, maintain investor, creditor and market confidence as well as sustain the future development of the business. The Group’s objectives when managing capital are to safeguard its ability to continue as a going concern, to provide returns for shareholders and benefits for other stakeholders, and to maintain an optimal capital structure to reduce the cost of capital. To maintain or adjust the capital structure, the Group may issue new shares or incur new debt. The Group has no material externally imposed capital requirements. The Group’s share capital is set out in Note 16. Equity. Management continuously monitors the level of capital deployed and available for deployment in the Wholly-Owned Programs segment and at Founded Entities. The Directors seek to maintain a balance between the higher returns that might be possible with higher levels of deployed capital and the advantages and security afforded by a sound capital position. The Group’s Directors have overall responsibility for the establishment and oversight of the Group's capital and risk management framework. The Group is exposed to certain risks through its normal course of operations. The Group’s main objective in using financial instruments is to promote the development and commercialization of intellectual property through the raising and investing of funds for this purpose. The nature, amount and timing of investments are determined by planned future investment activity. Due to the nature of activities and with the aim to maintain the investors’ funds as secure and protected, the Group’s policy is to hold any excess funds in highly liquid and readily available financial instruments and maintain minimal exposure to other financial risks. The Group has exposure to the following risks arising from financial instruments: Credit Risk Credit risk is the risk of financial loss to the Group if a customer or counterparty to a financial instrument fails to meet its contractual obligations. Financial instruments that potentially subject the Group to concentrations of credit risk consist principally of cash and cash equivalents, short-term investments, and trade and other receivables. The Group held the following balances: 2024 $ 2023 $Balance as of December 31 Cash and cash equivalents 280,641 191,081 Short-term investments 86,666 136,062 Trade and other receivables 1,522 2,376 Total 368,828 329,518 The Group invests its excess cash in U.S. Treasury Bills (presented as short-term investments), and money market accounts, which the Group believes are of high credit quality. Further, the Group's cash and cash equivalents and short-term investments are held at diverse, investment-grade financial institutions. The Group assesses the credit quality of customers on an ongoing basis. The credit quality of financial assets is assessed by historical and recent payment history, counterparty financial position, and reference to credit ratings (if available) or to historical information about counterparty default rates. The Group does not have expected credit losses due to the high credit quality or healthy financial conditions of these counterparties. As of December 31, 2024 and 2023, none of the trade and other receivables were impaired. Liquidity Risk Liquidity risk is the risk that the Group will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Group actively manages its liquidity risk by closely monitoring the maturity of its financial assets and liabilities and projected cash flows from operations, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Group’s reputation. Due to the nature of these financial liabilities, the funds are available on demand to provide optimal financial flexibility. The table below summarizes the maturity profile of the Group’s financial liabilities, including subsidiary preferred shares that have customary liquidation preferences, as of December 31, 2024 and 2023, based on contractual undiscounted payments: Balance as of December 31 2024 Carrying Amount $ Within Three Months $ Three to Twelve Months $ One to Five Years $ Total $ (*) Subsidiary notes payable 4,111 4,111 — — 4,111 Trade and other payables 27,020 27,020 — — 27,020 Taxes Payable 75 75 — — 75 Subsidiary preferred shares (Note 17)1 169 169 — — 169 Total 31,375 31,375 — — 31,375 Balance as of December 31 2023 Carrying Amount $ Within Three Months $ Three to Twelve Months $ One to Five Years $ Total $ (*) Subsidiary notes payable 3,699 3,699 — — 3,699 Trade and other payables 44,107 44,107 — — 44,107 Subsidiary preferred shares (Note 17)1 169 169 — — 169 Total 47,975 47,975 — — 47,975 1 Redeemable only upon a liquidation or deemed liquidation event, as defined in the applicable shareholder documents. * Does not include payments in respect of lease obligations nor payments on sale of future royalties liability. For the contractual future payments related to lease obligations, see Note 23. Leases and subleases. For contractual future payments related to sale of future royalties, see Note 18. Sale of Future Royalties Liability

Fi na nc ia l s ta te m en ts 170 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued Interest Rate Sensitivity As of December 31, 2024, the Group had cash and cash equivalents of $280,641, and short-term investments of $86,666. The Group's exposure to interest rate sensitivity is impacted by changes in the underlying U.K. and U.S. bank interest rates. The Group has not entered into investments for trading or speculative purposes. Due to the conservative nature of the Group's investment portfolio, which is predicated on capital preservation and investments in short duration, high-quality U.S. Treasury Bills and related money market accounts, a change in interest rates would not have a material effect on the fair market value of the Group's portfolio, and therefore, the Group does not expect operating results or cash flows to be significantly affected by changes in market interest rates. Controlled Founded Entity Investments The Group maintains investments in certain Controlled Founded Entities. The Group’s investments in Controlled Founded Entities are eliminated as intercompany transactions upon financial consolidation. The Group is, however, exposed to a subsidiary preferred share liability owing to the terms of existing preferred shares and the ownership of Controlled Founded Entities preferred shares by third parties. As discussed in Note 17. Subsidiary Preferred Shares, certain of the Group’s subsidiaries have issued preferred shares that include the right to receive a payment in the event of any voluntary or involuntary liquidation, dissolution or winding up of a subsidiary, including in the event of "deemed liquidation" as defined in the incorporation documents of the entities, which shall be paid out of the assets of the subsidiary available for distribution to shareholders, and before any payment shall be made to holders of ordinary shares. The liability of preferred shares is maintained at fair value through profit and loss and was insignificant as of December 31, 2024. The Group’s cash position supports the business activities of the Controlled Founded Entities. Accordingly, the Group views exposure to the third party subsidiary preferred share liability as low. Deconsolidated Founded Entity Investments The Group maintains certain debt or equity holdings in Founded Entities that are deconsolidated. These holdings are deemed either as investments carried at fair value under IFRS 9 with changes in fair value recorded through profit and loss or as associates accounted for under IAS 28 using the equity method. The Group's exposure to investments held at fair value and investments in notes from associates was $191,426 and $17,731, respectively, as of December 31, 2024, and the Group may or may not be able to realize the value in the future. Accordingly, the Group views the risk as high. The Group’s exposure to investments in associates is limited to the carrying amount of the investment in an associate. The Group is not exposed to further contractual obligations or contingent liabilities beyond the value of the initial investments. As of December 31, 2024, the investments in associates include Sonde and Seaport, and the carrying amounts of the investments under the equity method were $0 and $2,397, respectively. Accordingly, the Group does not view this risk as high. Equity Price Risk As of December 31, 2024, the Group held 2,671,800 common shares of Vor with a fair value of $2,966. As of December 31, 2023, the Group held 886,885 common shares of Karuna, 2,671,800 common shares of Vor, and 12,527,476 common shares of Akili with fair value of $280,708, $6,012, and $6,112, respectively. The common shares of Karuna and Akili were disposed of in 2024 as part of the Karuna's acquisition by BMS in March 2024 and Akili's acquisition by Virtual Therapeutics in July 2024. The investment in Vor is exposed to fluctuations in the market price of Vor's common shares. The Group views the exposure to equity price risk as low. Foreign Exchange Risk The Group maintains Consolidated Financial Statements in the Group's functional currency, which is the U.S. dollar. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at exchange rates prevailing at the balance sheet dates. Non-monetary assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the date of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income (loss) for the respective periods. Such foreign currency gains or losses were not material for all reported periods. The Group does not currently engage in currency hedging activities since its foreign currency risk is limited, but the Group may begin to do so in the future if and when its foreign currency risk exposure changes. 24. Capital and Financial Risk Management continued
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 171 Notes to the Consolidated Financial Statements continued 25. Commitments and Contingencies The Group is a party to certain licensing agreements where the Group is licensing IP from third parties. In consideration for such licenses, the Group has made upfront payments and may be required to make additional contingent payments based on developmental and sales milestones and/or royalty on future sales. As of December 31, 2024, certain milestone events have not yet occurred, and therefore, the Group does not have a present obligation to make the related payments in respect of the licenses. Such milestones are dependent on events that are outside of the control of the Group, and many of these milestone events are remote of occurring. Payments in respect of developmental milestones that are dependent on events that are outside the control of the Group but are reasonably possible to occur amounted to approximately $7,121 and $7,371, respectively, as of December 31, 2024 and December 31, 2023. These milestone amounts represent an aggregate of multiple milestone payments depending on different milestone events in multiple agreements. The probability that all such milestone events will occur in the aggregate is remote. Payments made to license IP represent the acquisition cost of intangible assets. The Group is a party to arrangements with contract manufacturing and contract research organizations, whereby the counterparty provides the Group with research and/or manufacturing services. As of December 31, 2024 and December 31, 2023, the noncancellable commitments in respect of such contracts amounted to approximately $8,395 and $16,422, respectively. In March 2024, a complaint was filed in Massachusetts District Court against the Group alleging breach of contract with respect to certain payments alleged to be owed to a previous employee of a Group's subsidiary based on purported terms of a contract between such individual and the Group. As of December 31, 2024, the Group recognized a provision of $900, which represents management's best estimate of the expected settlement related to the financial obligation associated with the lawsuit, considering the likelihood of settlement. The final settlement amount could vary depending on the outcome of the ongoing negotiations or litigation. The timing for resolution remains uncertain. The Group is involved from time-to-time in various legal proceedings arising in the normal course of business. Although the outcomes of these legal proceedings are inherently difficult to predict, the Group does not expect the resolution of such legal proceedings to have a material adverse effect on its financial position or results of operations. The Group did not book any provisions and did not identify any contingent liabilities requiring disclosure for any legal proceedings other than already included above for the years ended December 31, 2024 and 2023. 26. Related Parties Transactions Related Party Subleases During 2019, the Group executed a sublease agreement with a related party, Gelesis. During 2023, the sublease receivable was written down to zero as Gelesis ceased operations and filed for bankruptcy. The Group recorded $0, $23 and $89 of interest income with respect to the sublease during the years ended December 31, 2024, 2023, and 2022 respectively, which is presented within finance income in the Consolidated Statement of Comprehensive Income/ (Loss). Related Party Royalties The Group received $509 in royalties from Gelesis on its product sales for the year ended December 31, 2022 and recorded such royalty receipt as royalty revenue which was included in contract revenue in the Consolidated Statement of Comprehensive Income/(Loss) for the year ended December 31, 2022. The Group did not record any royalty revenue from Gelesis for the years ended December 31, 2024, and 2023. Key Management Personnel Compensation Key management includes executive directors and members of the executive management team of the Group (not including non- executive directors and not including subsidiary directors). The key management personnel compensation of the Group was as follows for the years ended December 31: 2024 $ 2023 $ 2022 $As of December 31 Short-term employee benefits 5,166 9,714 4,162 Post-employment benefits 61 41 55 Termination benefits 395 417 152 Share-based payment expense 2,540 599 2,741 Total 8,161 10,772 7,109 Short-term employee benefits include salaries, health care and other non-cash benefits. Post-employment benefits include 401K contributions from the Group. Termination benefits include severance pay. Share-based payments are generally subject to vesting terms over future periods. See Note 10. Share-based Payments. As of December 31, 2024, and 2023 the payable due to the key management employees was $1,509, and $4,732, respectively. In addition the Group paid remuneration to non-executive directors in the amounts of $670, $475, and $655 for the years ended December 31, 2024, 2023 and 2022, respectively. Also, the Group incurred $501, $373 and $365, of stock based compensation expense for such non-executive directors for the years ended December 31, 2024, 2023, and 2022 respectively. During the years ended December 31, 2024, 2023 and 2022, the Group incurred $34, $46 and $51, respectively, of expenses paid to related parties.
Fi na nc ia l s ta te m en ts 172 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued Convertible Notes Issued to Directors During the year ended December 31, 2024, the Group dissolved an inactive subsidiary, which held a convertible note issued to a related party. As a result of the entity's dissolution, the convertible note's outstanding balance on the day of dissolution was written down to $0 and a gain of $108 was recorded and included in finance income/ (costs) within the Consolidated Statement of Comprehensive Income/(Loss). As of December 31, 2023, the outstanding related party notes payable was $104, including principal and interest. Directors’ and Senior Managers’ Shareholdings and Share Incentive Awards The Directors and senior managers hold beneficial interests in shares in the following businesses as of December 31, 2024: Business name (share class) Number of shares held as of December 31, 2024 Number of options held as of December 31, 2024 Number of RSUs held as of December 31, 2024 Ownership interest¹ Directors: Dr Robert Langer Entrega (Common) 250,000 82,500 — 4.29% Dr Raju Kucherlapati Enlight (Class B Common) — 30,000 — 3.00% Seaport Therapeutics (Preferred B) 21,052 — — 0.01% Dr John LaMattina Vedanta Biosciences (Common) 25,000 15,000 — 0.25% Seaport Therapeutics (Preferred B)2 21,052 — — 0.01% Michele Holcomb Seaport Therapeutics (Preferred B) 21,052 — — 0.01% Sharon Barber-Lui Seaport Therapeutics (Preferred B) 21,052 — — 0.01% Senior Managers: Eric Elenko Seaport Therapeutics (Common) 950,000 — — 0.64% 1 Ownership interests as of December 31, 2024 are calculated on a diluted basis, including issued and outstanding shares, warrants and options (and written commitments to issue options) but excluding unallocated shares authorized to be issued pursuant to equity incentive plans and any shares issuable upon conversion of outstanding convertible promissory notes. 2 Dr. John and Ms. Mary LaMattina hold 21,052 Series B preferred shares of Seaport Therapeutics. Directors and senior managers hold 10,294,322 ordinary shares and 4.3% voting rights of the Group as of December 31, 2024. This amount excludes options to purchase 2,155,915 ordinary shares. This amount also excludes 3,517,248 shares, which are issuable based on the terms of performance based RSU awards granted to certain senior managers covering the financial years 2024, 2023 and 2022, 1,822,151 shares of time based RSUs to senior managers, which vest over 3 years, and 346,010 shares, which are issuable to directors immediately prior to the Group's 2025 Annual General Meeting of Stockholders, based on the terms of the RSU awards granted to non-executive directors in 2024. Such shares will be issued to such senior managers and non-executive directors in future periods provided that performance and/or service conditions are met, and certain of the shares will be withheld for payment of customary withholding taxes. During the year ended December 31, 2024, certain officers and directors participated in the Tender Offer. See Note 16. Equity for details on the program. Consequently, the Group repurchased a total of 767,533 ordinary shares at 250 pence per ordinary share from these related parties. Other See Note 7. Investment in Notes from Associates for details on the notes issued by Gelesis and Vedanta to the Group. As of December 31, 2024, the Group has a receivable from Seaport in the amount of $408. See Note 6. Investments in Associates for details on the execution and termination of the Merger Agreement with Gelesis in 2023. 26. Related Parties Transactions continued
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 173 Notes to the Consolidated Financial Statements continued 27. Taxation Tax on the profit or loss for the year comprises current and deferred income tax. Tax is recognized in the Consolidated Statement of Comprehensive Income/(Loss) except to the extent that it relates to items recognized directly in equity. For the years ended December 31, 2024, 2023 and 2022, the Group filed a consolidated U.S. federal income tax return which included all subsidiaries in which the Group owned greater than 80% of the vote and value. For the years ended December 31, 2024, 2023 and 2022, the Group filed certain consolidated state income tax returns which included all subsidiaries in which the Group owned greater than 50% of the vote and value. The remaining subsidiaries file separate U.S. tax returns. Amounts recognized in Consolidated Statement of Comprehensive Income/(Loss): 2024 $ 2023 $ 2022 $For the year ended December 31 Income/(loss) for the year 27,782 (66,628) (37,065) Income tax expense/(benefit) (4,008) 30,525 (55,719) Income/(loss) before taxes 23,774 (36,103) (92,783) Recognized Income Tax Expense/(Benefit): 2024 $ 2023 $ 2022 $For the year ended December 31 Federal - current 35,310 (2,246) 13,065 State - current 13,144 (46) 1,336 Total current income tax expense/(benefit) 48,454 (2,292) 14,401 Federal - deferred (46,442) 29,294 (48,240) State - deferred (6,020) 3,523 (21,880) Total deferred income tax expense/(benefit) (52,462) 32,817 (70,120) Total income tax expense/(benefit), recognized (4,008) 30,525 (55,719) The income tax expense/(benefit) was $(4,008), $30,525 and $(55,719) for the tax years ended December 31, 2024, 2023 and 2022, respectively. The income tax benefit recognized in 2024 was primarily attributable to the recognition of a deferred tax asset, generated in 2024 from the sale of the Group’s investment in Akili common stock that was used to offset income generated from the sale of the Group’s investment in Karuna common shares, partially offset with state income tax expense. The income tax expense recognized in 2023 was primarily due to income from the sale of future royalties to Royalty Pharma and the recognition of deferred tax liabilities. Reconciliation of Effective Tax Rate The Group is primarily subject to taxation in the U.S. A reconciliation of the U.S. federal statutory tax rate to the effective tax rate is as follows: 2024 2023 2022 For the year ended December 31 $ % $ % $ % US federal statutory rate 4,994 21.00 (7,573) 21.00 (19,486) 21.00 State taxes, net of federal effect 1,026 4.32 (3,974) 11.01 (8,043) 8.67 Tax credits (2,517) (10.59) (9,167) 25.39 (6,876) 7.41 Stock-based compensation 2,123 8.93 589 (1.63) 788 (0.85) Finance income/(costs) – fair value accounting 1,640 6.90 (556) 1.54 (28,783) 31.02 Loss with respect to associate for which no deferred tax asset is recognized 210 0.88 249 (0.69) 1,413 (1.52) Revaluation of deferred due to rate change (3,419) (14.38) — — (8,856) 9.54 Nondeductible compensation 1,534 6.45 872 (2.42) 300 (0.32) Recognition of deferred tax assets and tax benefits not previously recognized (12,396) (52.14) (433) 1.20 (184) 0.20 Unrecognized deferred tax asset — — 83,984 (232.63) 17,287 (18.63) Deconsolidation of subsidiary 3,863 16.25 (17,506) 48.49 (3,572) 3.85 Cancellation of Debt Income (987) (4.15) — — — — Other 755 3.16 1,321 (3.65) 293 (0.32) Worthless stock deduction (833) (3.50) (17,281) 47.87 — — (4,008) (16.86) 30,525 (84.52) (55,719) 60.05 The Group is also subject to taxation in the UK, but to date, no taxable income has been generated in the UK. Changes in corporate tax rates can change both the current tax expense (benefit) as well as the deferred tax expense (benefit).
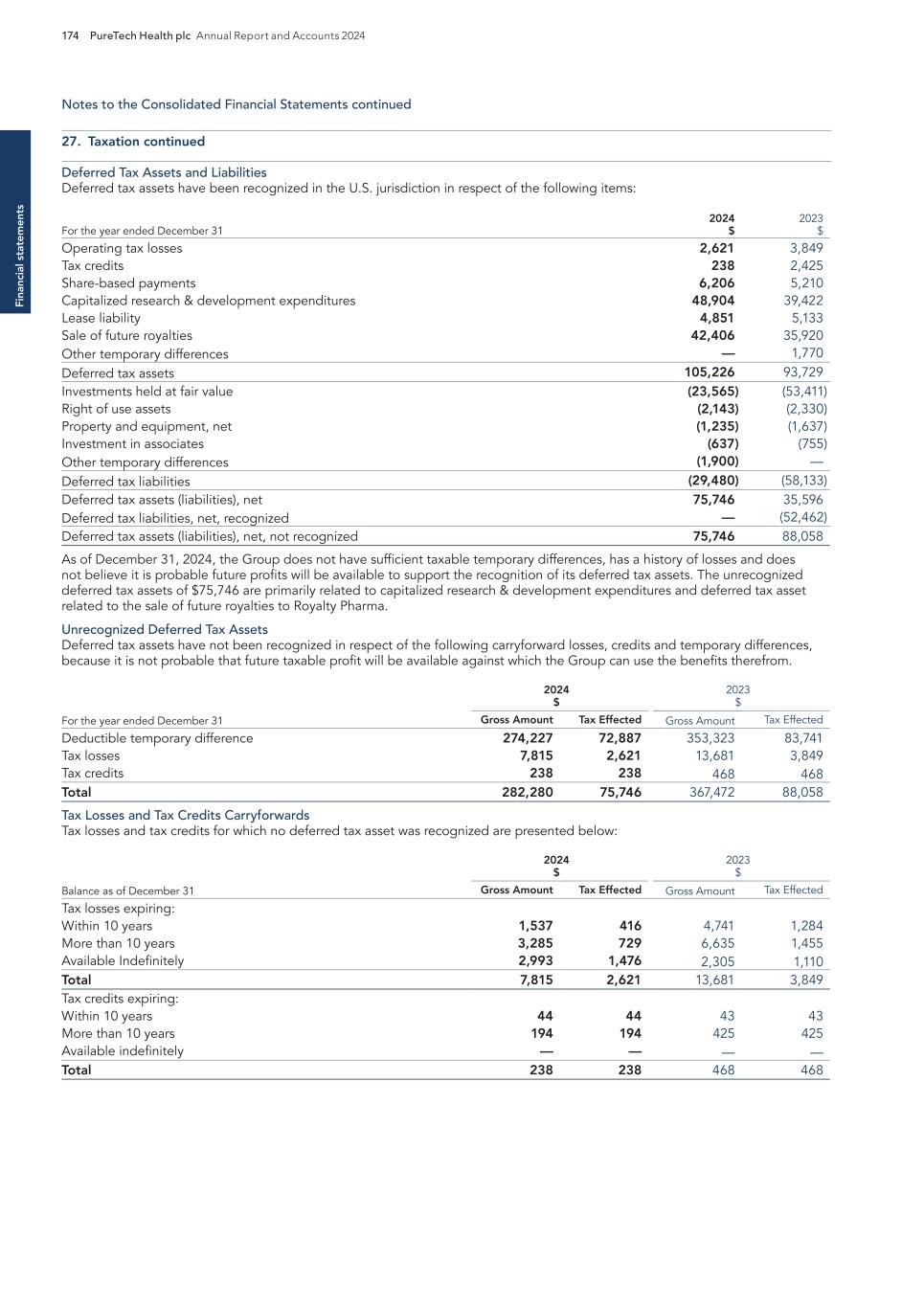
Fi na nc ia l s ta te m en ts 174 PureTech Health plc Annual Report and Accounts 2024 Notes to the Consolidated Financial Statements continued Deferred Tax Assets and Liabilities Deferred tax assets have been recognized in the U.S. jurisdiction in respect of the following items: 2024 $ 2023 $For the year ended December 31 Operating tax losses 2,621 3,849 Tax credits 238 2,425 Share-based payments 6,206 5,210 Capitalized research & development expenditures 48,904 39,422 Lease liability 4,851 5,133 Sale of future royalties 42,406 35,920 Other temporary differences — 1,770 Deferred tax assets 105,226 93,729 Investments held at fair value (23,565) (53,411) Right of use assets (2,143) (2,330) Property and equipment, net (1,235) (1,637) Investment in associates (637) (755) Other temporary differences (1,900) — Deferred tax liabilities (29,480) (58,133) Deferred tax assets (liabilities), net 75,746 35,596 Deferred tax liabilities, net, recognized — (52,462) Deferred tax assets (liabilities), net, not recognized 75,746 88,058 As of December 31, 2024, the Group does not have sufficient taxable temporary differences, has a history of losses and does not believe it is probable future profits will be available to support the recognition of its deferred tax assets. The unrecognized deferred tax assets of $75,746 are primarily related to capitalized research & development expenditures and deferred tax asset related to the sale of future royalties to Royalty Pharma. Unrecognized Deferred Tax Assets Deferred tax assets have not been recognized in respect of the following carryforward losses, credits and temporary differences, because it is not probable that future taxable profit will be available against which the Group can use the benefits therefrom. 2024 $ 2023 $ For the year ended December 31 Gross Amount Tax Effected Gross Amount Tax Effected Deductible temporary difference 274,227 72,887 353,323 83,741 Tax losses 7,815 2,621 13,681 3,849 Tax credits 238 238 468 468 Total 282,280 75,746 367,472 88,058 Tax Losses and Tax Credits Carryforwards Tax losses and tax credits for which no deferred tax asset was recognized are presented below: Balance as of December 31 2024 $ 2023 $ Gross Amount Tax Effected Gross Amount Tax Effected Tax losses expiring: Within 10 years 1,537 416 4,741 1,284 More than 10 years 3,285 729 6,635 1,455 Available Indefinitely 2,993 1,476 2,305 1,110 Total 7,815 2,621 13,681 3,849 Tax credits expiring: Within 10 years 44 44 43 43 More than 10 years 194 194 425 425 Available indefinitely — — — — Total 238 238 468 468 27. Taxation continued

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 175 Notes to the Consolidated Financial Statements continued The Group had U.S. federal net operating losses carry forwards (“NOLs”) of $7,815, $13,681 and $219,466 as of December 31, 2024, 2023 and 2022, respectively, which are available to offset future taxable income. These NOLs expire through 2037 with the exception of $2,993 which is not subject to expiration, and can be utilized up to 80% of annual taxable income. The Group had U.S. federal research and development tax credits of approximately $238, $1,396 and $4,500 as of December 31, 2024, 2023 and 2022, respectively, which are available to offset future taxes that expire at various dates through 2037. The Group also had Federal Orphan Drug credits of approximately $0 and $930 as of December 31, 2024, and 2023. A portion of these federal NOLs and credits can only be used to offset the profits from the Group’s subsidiaries who file separate federal tax returns. These NOLs and credits are subject to review and possible adjustment by the Internal Revenue Service. The Group had state net operating losses carry forwards (“NOLs”) of approximately $125,322, $111,446 and $71,700 for the years ended December 31, 2024, 2023 and 2022, respectively, which are available to offset future taxable income. These NOLs expire at various dates beginning in 2030. The Group had Massachusetts research and development tax credits of approximately $0, $98 and $600 for the years ended December 31, 2024, 2023 and 2022, respectively. These NOLs and credits are subject to review and possible adjustment by state taxing authority. Utilization of the NOLs and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of NOL and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. The Group has performed a Section 382 analysis through December 31, 2024. The results of this analysis concluded that certain net operating losses were subject to limitation under Section 382 of the Internal Revenue Code. None of the Group’s net operating losses, which are subject to a Section 382 limitation, has been recognized in the financial statements. Tax Balances The tax related balances presented in the Statement of Financial Position are as follows: For the year ended December 31 2024 $ 2023 $ Income tax receivable – current — 11,746 Income tax payable – current (75) — Uncertain Tax Positions The Group has no uncertain tax positions as of December 31, 2024. U.S. corporations are routinely subject to audit by federal and state tax authorities in the normal course of business. 28. Subsequent Events The Group has evaluated subsequent events after December 31, 2024, up to the date of issuance, April 30, 2025, of the Consolidated Financial Statements, and has not identified any recordable or disclosable events not otherwise reported in these Consolidated Financial Statements or notes thereto. 27. Taxation continued

Fi na nc ia l s ta te m en ts 176 PureTech Health plc Annual Report and Accounts 2024 Parent Company Statement of Financial Position For the years ended December 31 2024 $000s 2023 $000sNote Assets Non-current assets Investment in subsidiary 2 462,734 456,864 Total non-current assets 462,734 456,864 Current assets Cash and cash equivalents 26,323 20,425 Total current assets 26,323 20,425 Total assets 489,057 477,289 Equity and liabilities Equity Share capital 3 4,860 5,461 Share premium 3 290,262 290,262 Treasury stock 3 (46,864) (44,626) Merger reserve 3 138,506 138,506 Other reserve 3 26,407 21,596 Retained earnings - (Income of $107,421 and loss of $3,178 for 2024 and 2023, respectively) 3 44,574 41,997 Total equity 457,746 453,196 Current liabilities Trade and other payables 3,661 2,033 Intercompany payables 4 27,650 22,061 Total current liabilities 31,311 24,093 Total equity and liabilities 489,057 477,289 Please refer to the accompanying notes to the PureTech Health plc financial information ("Notes"). Registered number: 09582467. The PureTech Health plc financial statements were approved by the Board of Directors and authorized for issuance on April 30, 2025 and signed on its behalf by: Bharatt Chowrira Chief Executive Officer April 30, 2025 The accompanying Notes are an integral part of these financial statements.
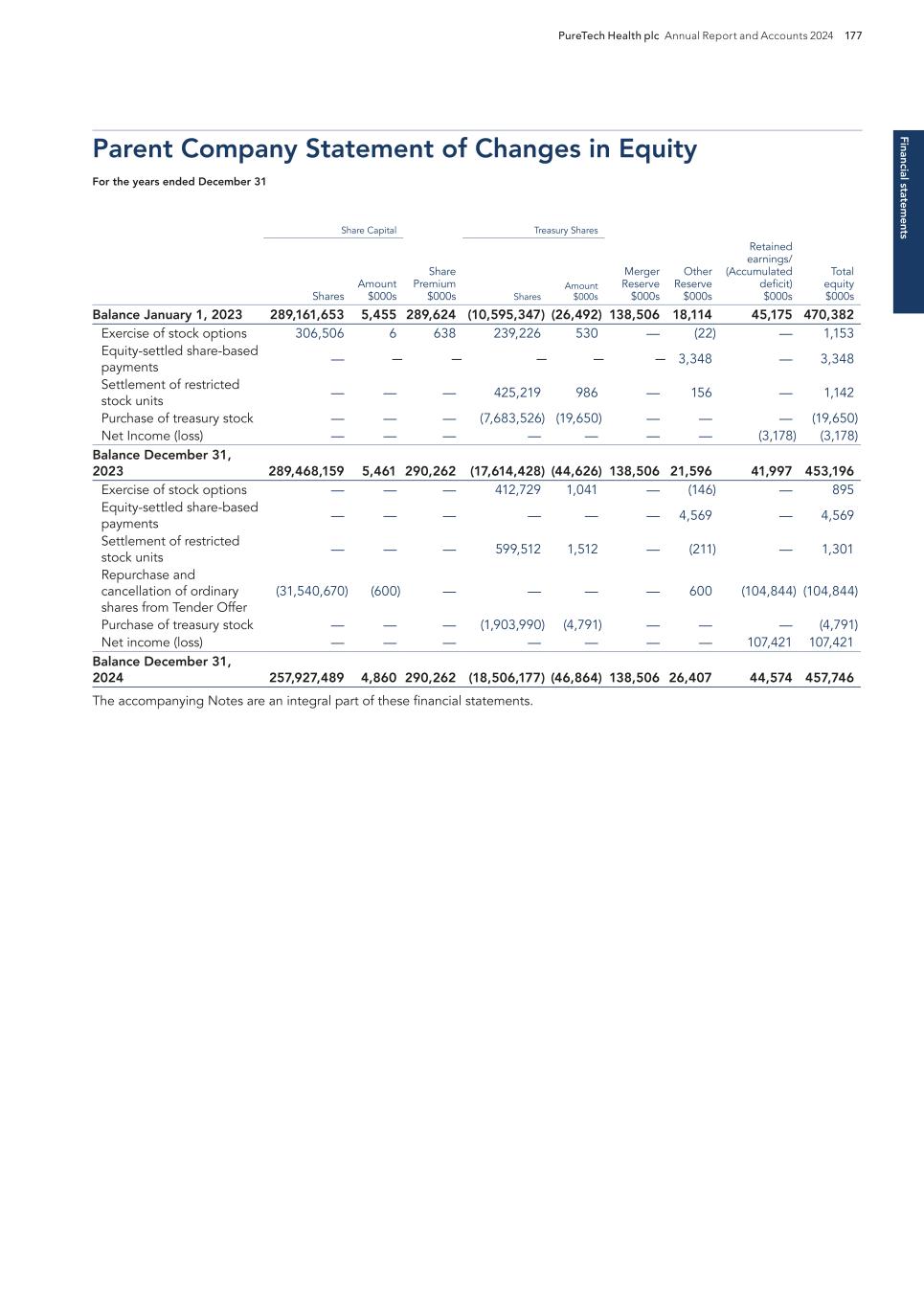
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 177 Parent Company Statement of Changes in Equity For the years ended December 31 Share Capital Treasury Shares Shares Amount $000s Share Premium $000s Shares Amount $000s Merger Reserve $000s Other Reserve $000s Retained earnings/ (Accumulated deficit) $000s Total equity $000s Balance January 1, 2023 289,161,653 5,455 289,624 (10,595,347) (26,492) 138,506 18,114 45,175 470,382 Exercise of stock options 306,506 6 638 239,226 530 — (22) — 1,153 Equity-settled share-based payments — — — — — — 3,348 — 3,348 Settlement of restricted stock units — — — 425,219 986 — 156 — 1,142 Purchase of treasury stock — — — (7,683,526) (19,650) — — — (19,650) Net Income (loss) — — — — — — — (3,178) (3,178) Balance December 31, 2023 289,468,159 5,461 290,262 (17,614,428) (44,626) 138,506 21,596 41,997 453,196 Exercise of stock options — — — 412,729 1,041 — (146) — 895 Equity-settled share-based payments — — — — — — 4,569 — 4,569 Settlement of restricted stock units — — — 599,512 1,512 — (211) — 1,301 Repurchase and cancellation of ordinary shares from Tender Offer (31,540,670) (600) — — — — 600 (104,844) (104,844) Purchase of treasury stock — — — (1,903,990) (4,791) — — — (4,791) Net income (loss) — — — — — — — 107,421 107,421 Balance December 31, 2024 257,927,489 4,860 290,262 (18,506,177) (46,864) 138,506 26,407 44,574 457,746 The accompanying Notes are an integral part of these financial statements.

Fi na nc ia l s ta te m en ts 178 PureTech Health plc Annual Report and Accounts 2024 Notes to the Financial Statements (amounts in thousands, except share and per share data) 1. Accounting policies Basis of Preparation and Measurement The financial statements of PureTech Health plc (the “Parent”) are presented as of December 31, 2024 and 2023, and for the years ended December 31, 2024 and 2023, and have been prepared under the historical cost convention in accordance with FRS 101 ‘Reduced Disclosure Framework’ and in accordance with the Companies Act 2006 as applicable to companies using FRS 101. As permitted by FRS 101, the Parent has taken advantage of the disclosure exemptions available under that standard in relation to: — a cash flow statement A summary of the material accounting policies that have been applied consistently throughout the year is set out below. Certain amounts in the Parent Company Financial Statements and accompanying notes may not add due to rounding. All percentages have been calculated using unrounded amounts. Functional and Presentation Currency The functional currency of the Parent is United States ("U.S.”) Dollars and the financial statements are presented in U.S. Dollars. Investments Investments are stated at historical cost less any provision for impairment in value, and are held for long-term investment purposes. Provisions are based upon an assessment of events or changes in circumstances that indicate that an impairment has occurred, such as the performance and/or prospects (including the financial prospects) of the investee company being significantly below the expectations on which the investment was based, a significant adverse change in the markets in which the investee company operates, or a deterioration in general market conditions. Impairment If there is an indication that an asset might be impaired, the Parent would perform an impairment review. An asset is impaired if the recoverable amount, being the higher of fair value less cost to sell and value in use, is less than its carrying amount. Value in use is measured based on future discounted cash flows attributable to the asset. In such cases, the carrying value of the asset is reduced to its recoverable amount with a corresponding charge recognized in the profit and loss statement. Dividend Income Dividend received from the Parent's subsidiary is recorded as dividend income in the profit and loss statement. Financial Instruments Currently the Parent does not have derivative financial instruments. Financial assets and financial liabilities are recognized and cease to be recognized on the basis of when the related titles pass to or from the Parent. Share-Based Payments Share-based payment awards granted in subsidiaries to employees, Board of Directors and consultants to be settled in Parent's equity instruments are accounted for as equity-settled share-based payment transactions in accordance with IFRS 2. Restricted stock units granted in subsidiaries to the executives are accounted for as share-based liability awards in accordance with IFRS 2 as they can be cash-settled at PureTech's discretion and have a history of being cash-settled. The grant date fair value of equity-settled share-based payment awards and the settlement date fair value of the share-based liability awards are recognized as an increase to the investment with a corresponding increase in equity. For equity-settled restricted stock units, the grant date fair value is the grant date share price. For share-based liability awards, the fair value at each reporting date is measured using the Monte Carlo simulation analysis considering share price volatility, risk-free rate, and other covariance of comparable public companies and other market data to predict distribution of relative share performance. For stock options, the fair value is measured using an option pricing model, which takes into account the terms and conditions of the options granted. When the subsidiary settles the equity awards other than by the Parent's equity, the settlement is recorded as a decrease in equity against a corresponding decrease to the investment account.
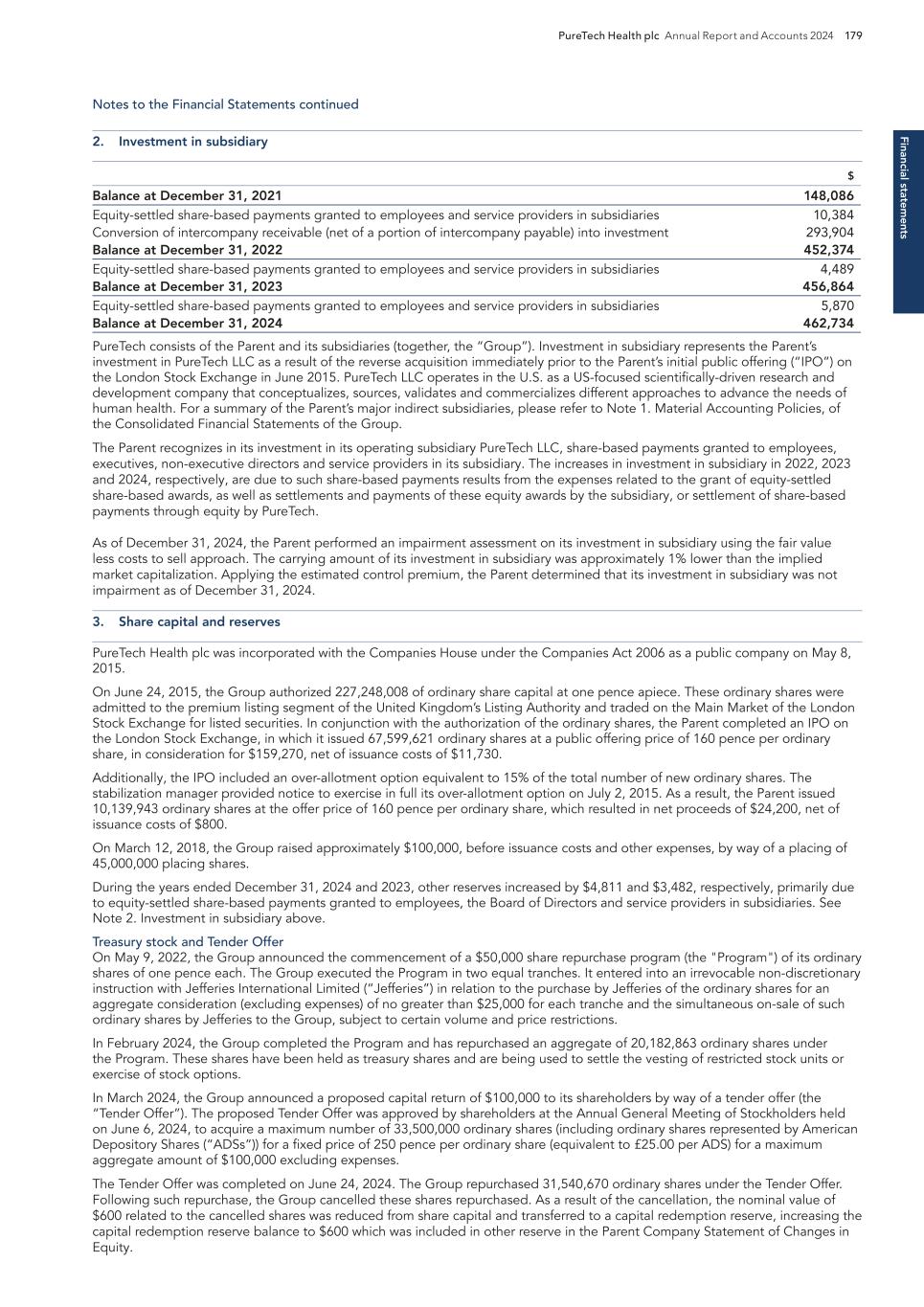
Financial statem ents PureTech Health plc Annual Report and Accounts 2024 179 Notes to the Financial Statements continued 2. Investment in subsidiary $ Balance at December 31, 2021 148,086 Equity-settled share-based payments granted to employees and service providers in subsidiaries 10,384 Conversion of intercompany receivable (net of a portion of intercompany payable) into investment 293,904 Balance at December 31, 2022 452,374 Equity-settled share-based payments granted to employees and service providers in subsidiaries 4,489 Balance at December 31, 2023 456,864 Equity-settled share-based payments granted to employees and service providers in subsidiaries 5,870 Balance at December 31, 2024 462,734 PureTech consists of the Parent and its subsidiaries (together, the “Group”). Investment in subsidiary represents the Parent’s investment in PureTech LLC as a result of the reverse acquisition immediately prior to the Parent’s initial public offering (“IPO”) on the London Stock Exchange in June 2015. PureTech LLC operates in the U.S. as a US-focused scientifically-driven research and development company that conceptualizes, sources, validates and commercializes different approaches to advance the needs of human health. For a summary of the Parent’s major indirect subsidiaries, please refer to Note 1. Material Accounting Policies, of the Consolidated Financial Statements of the Group. The Parent recognizes in its investment in its operating subsidiary PureTech LLC, share-based payments granted to employees, executives, non-executive directors and service providers in its subsidiary. The increases in investment in subsidiary in 2022, 2023 and 2024, respectively, are due to such share-based payments results from the expenses related to the grant of equity-settled share-based awards, as well as settlements and payments of these equity awards by the subsidiary, or settlement of share-based payments through equity by PureTech. As of December 31, 2024, the Parent performed an impairment assessment on its investment in subsidiary using the fair value less costs to sell approach. The carrying amount of its investment in subsidiary was approximately 1% lower than the implied market capitalization. Applying the estimated control premium, the Parent determined that its investment in subsidiary was not impairment as of December 31, 2024. 3. Share capital and reserves PureTech Health plc was incorporated with the Companies House under the Companies Act 2006 as a public company on May 8, 2015. On June 24, 2015, the Group authorized 227,248,008 of ordinary share capital at one pence apiece. These ordinary shares were admitted to the premium listing segment of the United Kingdom’s Listing Authority and traded on the Main Market of the London Stock Exchange for listed securities. In conjunction with the authorization of the ordinary shares, the Parent completed an IPO on the London Stock Exchange, in which it issued 67,599,621 ordinary shares at a public offering price of 160 pence per ordinary share, in consideration for $159,270, net of issuance costs of $11,730. Additionally, the IPO included an over-allotment option equivalent to 15% of the total number of new ordinary shares. The stabilization manager provided notice to exercise in full its over-allotment option on July 2, 2015. As a result, the Parent issued 10,139,943 ordinary shares at the offer price of 160 pence per ordinary share, which resulted in net proceeds of $24,200, net of issuance costs of $800. On March 12, 2018, the Group raised approximately $100,000, before issuance costs and other expenses, by way of a placing of 45,000,000 placing shares. During the years ended December 31, 2024 and 2023, other reserves increased by $4,811 and $3,482, respectively, primarily due to equity-settled share-based payments granted to employees, the Board of Directors and service providers in subsidiaries. See Note 2. Investment in subsidiary above. Treasury stock and Tender Offer On May 9, 2022, the Group announced the commencement of a $50,000 share repurchase program (the "Program") of its ordinary shares of one pence each. The Group executed the Program in two equal tranches. It entered into an irrevocable non-discretionary instruction with Jefferies International Limited (“Jefferies”) in relation to the purchase by Jefferies of the ordinary shares for an aggregate consideration (excluding expenses) of no greater than $25,000 for each tranche and the simultaneous on-sale of such ordinary shares by Jefferies to the Group, subject to certain volume and price restrictions. In February 2024, the Group completed the Program and has repurchased an aggregate of 20,182,863 ordinary shares under the Program. These shares have been held as treasury shares and are being used to settle the vesting of restricted stock units or exercise of stock options. In March 2024, the Group announced a proposed capital return of $100,000 to its shareholders by way of a tender offer (the “Tender Offer”). The proposed Tender Offer was approved by shareholders at the Annual General Meeting of Stockholders held on June 6, 2024, to acquire a maximum number of 33,500,000 ordinary shares (including ordinary shares represented by American Depository Shares (“ADSs”)) for a fixed price of 250 pence per ordinary share (equivalent to £25.00 per ADS) for a maximum aggregate amount of $100,000 excluding expenses. The Tender Offer was completed on June 24, 2024. The Group repurchased 31,540,670 ordinary shares under the Tender Offer. Following such repurchase, the Group cancelled these shares repurchased. As a result of the cancellation, the nominal value of $600 related to the cancelled shares was reduced from share capital and transferred to a capital redemption reserve, increasing the capital redemption reserve balance to $600 which was included in other reserve in the Parent Company Statement of Changes in Equity.

Fi na nc ia l s ta te m en ts 180 PureTech Health plc Annual Report and Accounts 2024 4. Intercompany payables The Parent had a balance due to its operating subsidiary PureTech LLC of $27,650 as of December 31, 2024, which is related to IPO costs and operating expenses. These intercompany payables do not bear any interest and are repayable upon demand. 5. Profit and loss account As permitted by Section 408 of the Companies Act 2006, the Parent’s profit and loss account has not been included in these financial statements. The Parent’s net income for the year was $107,421. During the year ended December 31, 2024, the Parent recorded income of $110,500 in respect of dividend received from its subsidiary. 6. Directors’ remuneration, employee information and share-based payments The remuneration of the executive Directors of the Parent company is disclosed in Note 26. Related Parties Transactions, of the Group's Consolidated Financial Statements. Full details of Directors’ remuneration can be found in the audited sections of the Directors’ Remuneration Report. Full detail of the share-based payment charge and the related disclosures can be found in Note 10. Share-based Payments, of the Group's Consolidated Financial Statements. The Parent had no employees during 2024 or 2023. Notes to the Financial Statements continued

Financial statem ents PureTech Health plc Annual Report and Accounts 2024 181 History and Development of the Company We were incorporated and registered under the laws of England and Wales with the Registrar of Companies of England and Wales, United Kingdom in May 2015 as “PureTech Health plc.” Our predecessor entity, PureTech Health LLC (the "Predecessor Entity"), commenced formal operations and began engaging in initial sourcing activities in 2004, raising its first financing round greater than $5 million in the same year. The Predecessor Entity was acquired by PureTech Health plc on June 18, 2015 in a reorganization completed in connection with our initial public offering on the London Stock Exchange. The Predecessor Entity is now a wholly- owned subsidiary of PureTech Health plc. Our registered office is situated at 13th Floor, One Angel Court, London, EC2R 7HJ, United Kingdom, and our telephone number is +(1) 617 482 2333. Our U.S. operations are conducted by our wholly-owned subsidiary PureTech Health LLC, a Delaware limited liability company. Our ordinary shares have traded on the main market of the London Stock Exchange since June 2015, and our ADSs have traded on the Nasdaq Global Market since November 2020. Our agent for service of process in the United States is PureTech Health LLC located at 6 Tide Street, Suite 400, Boston, Massachusetts 02210 where our corporate headquarters and laboratories are located. Our website address is http://www.puretechhealth.com. The reference to our website is an inactive textual reference only, and information contained in, or that can be accessed through our website or any other website cited in this annual report is not part of hereof.

182 PureTech Health plc Annual Report and Accounts 2024 A d d it io na l i nf o rm at io n Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may undertake. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as may be required by law, we have no plans to update our forward- looking statements to reflect events or circumstances after the date of this annual report on Form 20-F. We qualify all of our forward-looking statements by these cautionary statements. This Annual Report and Accounts and our associated Annual Report on Form 20-F include statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Additionally, certain information we may disclose (either herein or elsewhere) is informed by the expectations of various stakeholders or third-party frameworks and, as such, may not necessarily be material for purposes of our filings under U.S. federal securities laws, even if we use “material” or similar language in discussing such matters. Risks Related to our Financial Position and Need for Additional Capital We are a clinical-stage biotherapeutics company and have incurred significant operating losses since our inception. We may continue to incur significant operating losses for the foreseeable future. Investment in biotechnology, including therapeutic development and medical device development, is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential therapeutic candidate will be unable to demonstrate effectiveness or an acceptable safety profile, gain regulatory approval or certification (where applicable) and become commercially viable. To date, only three of our Founded Entities’ therapeutic candidates, Karuna Therapeutics, Inc.’s (now a wholly owned subsidiary of Bristol Myers Squibb, Inc.) Cobenfy® received U.S. Food and Drug Administration, or FDA, approval, and both Gelesis, Inc.’s Plenity® and Akili Interactive Labs, Inc.’s EndeavorRx® have received marketing authorization from the FDA and have been CE Marked in the European Union, or EU. All of the therapeutic candidates in our Wholly-Owned Programs and the majority of our Founded Entities’ therapeutic candidates may require substantial additional development time, including extensive clinical research, and resources before we would be able to apply for or receive regulatory clearances, certifications or approvals and begin generating revenue from therapeutic sales. Since our inception, we have invested most of our resources in developing our technology and therapeutic candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, raising capital and providing general and administrative support for these operations, including with respect to our Founded Entities. We are not operationally profitable and have incurred operating losses in each year since our inception. Our operating losses for the years ended December 31, 2022, 2023 and 2024 were $197.8 million, $146.2 million and $136.1 million, respectively. We have no therapeutics developed in our Wholly-Owned Programs approved for commercial sale and have not generated any revenues from therapeutic sales, and we and our Founded Entities have financed operations solely through the sale of equity securities, revenue from strategic alliances and government funding and, with respect to certain of our Founded Entities, debt financings. We continue to incur significant research and development, or R&D, and other expenses related to ongoing operations and expect to incur losses for the foreseeable future. We anticipate continued losses for the foreseeable future. Due to risks and uncertainties associated with the development of drugs, biologics and medical devices, we are unable to predict the timing or amount of our expenses, or when we will be able to generate any meaningful revenue or achieve or maintain profitability, if ever. In addition, our expenses could increase beyond our current expectations if we are required by the FDA, the European Medicines Agency, or the EMA, or other comparable foreign regulatory authorities and notified bodies in the EU to perform preclinical studies or clinical trials in addition to those that we currently anticipate, or if there are any delays in any of our or our future collaborators’ clinical trials or the development of our existing therapeutic candidates and any other therapeutic candidates that we may identify. Even if our existing therapeutic candidates or any future therapeutic candidates that we may identify are approved for commercial sale, we anticipate incurring significant costs associated with commercializing any approved therapeutic and ongoing compliance efforts. Our business faces significant risks. You should carefully consider all of the information set forth in this Annual Report and Accounts, including the following risk factors which we face and which are faced by our industry. These risks are not listed in any particular order of priority and are intended to supplement the risks identified elsewhere. Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occur. This Annual Report and Accounts and our associated Annual Report on Form 20-F also contain forward-looking statements that involve risks and uncertainties. Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain important factors including the risks described below and elsewhere. All statements contained in this Annual Report and Accounts and our associated Annual Report on Form 20-F, other than statements of historical fact, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “would,” “could,” “should,” “continue” and similar expressions are intended to identify forward- looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements in this Annual Report and Accounts and associated Annual Report on Form 20-F include, among other things, statements about: — our ability to realize value from our Founded Entities, which may be impacted if we reduce our ownership to a minority interest or otherwise cede control to other investors through contractual agreements or otherwise; — the success, cost and timing of our clinical development within our Wholly-Owned Programs and our Founded Entities, including the progress of, and results from, our Wholly-Owned Programs ’ and our Founded Entities’ preclinical and clinical trials of deupirfenidone (LYT-100), LYT-200, or our therapeutics candidates, and our technology platforms and other potential therapeutic candidates within our Wholly- Owned Programs and therapeutic candidates being developed by our Founded Entities; — our ability to obtain and maintain regulatory clearance, certification, authorization, or approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities, and any related restrictions, limitations or warnings in the label of any of the therapeutic candidates, if cleared, certified, authorized, or approved; — our ability to compete with companies currently marketing or engaged in the development of treatments for indications within our Wholly-Owned Programs or our Founded Entities are designed to target; — our plans to pursue research and development of other future therapeutic candidates; — the potential advantages of the therapeutic candidates within our Wholly-Owned Programs and the therapeutic candidates developed by our Founded Entities; — the rate and degree of market acceptance and clinical utility of our therapeutic candidates; — the success of our collaborations and partnerships with third parties; — our estimates regarding the potential market opportunity for the therapeutic candidates within our Wholly-Owned Programs and the therapeutic candidates being developed by our Founded Entities; — our sales, marketing and distribution capabilities and strategy; — our ability to establish and maintain arrangements for manufacture of the therapeutic candidates within our Wholly-Owned Programs and therapeutic candidates being developed by our Founded Entities; — our intellectual property position; — our expectations related to the use of capital; — our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; — the impact of government laws and regulations; and — our competitive position. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements, which speak only as of the date made. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. You should refer to the below for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Risk Factor Annex

PureTech Health plc Annual Report and Accounts 2024 183 Risk Factor Annex continued A d d itio nal info rm atio n As of December 31, 2024, we had cash, cash equivalents and short term investments of $367.3 million at the PureTech Health plc level. Based on current projections, the Directors believe that the company has sufficient available funding to extend operations into at least 2027. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, sales of assets or programs, other sources, such as strategic collaborations or license and development agreements, or a combination of these approaches. Even if we believe we have sufficient funds for our current or future operating plans, we may opportunistically seek additional capital if market conditions are favorable or if we have specific strategic considerations. Our spending will vary based on new and ongoing therapeutic development and corporate activities. Our future funding requirements, both short-term and long-term, will depend on many factors, including, but not limited to: — the time and cost necessary to complete ongoing, planned and future unplanned clinical trials (such term to include clinical studies in these Risk Factors where context requires and the item being studied or subject of a potential study may be regulated as a medical device in the EU), including our ongoing clinical trials for certain of our therapeutic candidates, and potential future clinical trials for certain of our therapeutic candidates; — the outcome, timing and cost of meeting regulatory requirements established by the FDA, the EMA and other comparable foreign regulatory authorities; — the progress, timing, scope and costs of our preclinical studies, clinical trials and other related activities for our ongoing and planned clinical trials, and potential future clinical trials; — the costs of obtaining clinical and commercial supplies of raw materials and drug products for the therapeutic candidates within our Wholly- Owned Programs , as applicable, and any other therapeutic candidates we may identify and develop; — our ability to successfully identify and negotiate acceptable terms for third-party supply and contract manufacturing agreements with contract manufacturing organizations, or CMOs; — the costs of commercialization activities for any of the therapeutic candidates within our Wholly-Owned Programs that receive marketing approval, including the costs and timing of establishing therapeutic sales, marketing, distribution and manufacturing capabilities, or entering into strategic collaborations with third parties to leverage or access these capabilities; — the amount and timing of sales and other revenues from the therapeutic candidates within our Wholly-Owned Programs , if approved, including the sales price and the availability of coverage and adequate third-party reimbursement; — the cash requirements of our Founded Entities and our ability and willingness to provide them with financing; — the cash requirements of any future acquisitions or discovery of therapeutic candidates; — the time and cost necessary to respond to technological and market developments, including other therapeutics that may compete with one or more of our Wholly-Owned Programs or those of our Founded Entities; — the costs of acquiring, licensing or investing in intellectual property rights, therapeutics, therapeutic candidates and businesses; — our ability to attract, hire and retain qualified personnel as we expand R&D and establish a commercial infrastructure; — the costs of maintaining, expanding and protecting our intellectual property portfolio; — the costs of operating as a public company in the United Kingdom, or UK, and the United States, or US, and maintaining listings on both the London Stock Exchange, or the LSE, and The Nasdaq Global Market, or Nasdaq; and — costs associated with any adverse market conditions or other macroeconomic factors. We cannot be certain that additional funding will be available on acceptable terms, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit or terminate one or more research or development programs or the potential commercialization of any approved therapeutics or be unable to expand operations or otherwise capitalize on business opportunities, as desired, which could materially affect our business, prospects, financial condition and results of operations. As of December 31, 2024, we had never generated revenue from the therapeutic candidates within our Wholly-Owned Programs , and we may never be operationally profitable. We may never be able to develop or commercialize marketable therapeutics or achieve operational profitability. Revenue from the sale of any therapeutic candidate for which regulatory clearance, certification, authorization or approval is obtained will be dependent, in part, upon the size of the markets in the territories for which we gain regulatory clearance, certification, authorization or approval, the accepted price for the therapeutic, the ability to obtain reimbursement at any price and whether we own the commercial rights for that territory. Our growth strategy depends on our ability to generate revenue. In addition, if the number of addressable patients is not as anticipated, the indication or intended use cleared, certified, authorized or approved by regulatory authorities or notified bodies is narrower than expected, or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of such therapeutics, even if cleared, certified, authorized or approved. Even if we are able to generate revenue from the sale of any cleared, certified, authorized or approved therapeutics, we may not become operationally profitable and may need to obtain additional funding to continue operations. Even if we achieve operational profitability in the future, we may not be able to sustain profitability in subsequent periods. If we are unable to achieve sustained profitability, it would depress the value of our company and could impair our ability to raise capital, expand our business, diversify our R&D pipeline, market the therapeutic candidates within our Wholly-Owned Programs , if cleared or approved, and pursue or continue our operations. Our prior losses, combined with expected future losses, have had and may continue to have an adverse effect on our shareholders’ equity and working capital. We may require substantial additional funding to achieve our business goals. If we are unable to obtain this funding when needed and on acceptable terms, we could be forced to delay, limit or terminate certain of our therapeutic development efforts. Certain of our Founded Entities will similarly require substantial additional funding to achieve their business goals. Across our Wholly-Owned Programs and our Founded Entities, we established the underlying platforms that have resulted in the development of 29 therapeutics and therapeutic candidates, including three (Cobenfy, Plenity and EndeavorRx) that have commercial approval, with Cobenfy receiving U.S. FDA approval, and both Plenity and EndeavorRx receiving both U.S. FDA approval and European marketing authorization. Developing biotherapeutics is expensive and time- consuming, and with respect to the therapeutic candidates within our Wholly-Owned Programs , we expect to require substantial additional capital to conduct research, preclinical studies and clinical trials for our current and future programs, establish pilot scale and commercial scale manufacturing processes and facilities, seek regulatory approvals for the therapeutic candidates within our Wholly-Owned Programs and launch and commercialize any therapeutics for which we receive regulatory approval, including building our own commercial sales, marketing and distribution organization. With respect to our Founded Entities’ programs, we anticipate that we will continue to fund a small portion of development costs by strategically participating in such companies’ financings when doing so would be in the interests of our shareholders. We expect to finance our future cash needs through a combination of public and private equity offerings, debt financings, strategic partnerships, sales of assets and alliances and licensing arrangements, among others. We, and indirectly, our shareholders, may bear the cost of issuing and servicing any such securities and of entering into and maintaining any such strategic partnerships or other arrangements. Because any decision by us to issue debt or equity securities in the future will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of any future financing transactions. Our management and strategic decision makers have not made decisions regarding the future allocation of certain of our resources among our Founded Entities, but evaluate the needs and opportunities with respect to each of these Founded Entities routinely and on a case-by-case basis. In connection with any collaboration agreements relating to our Wholly- Owned Programs , we are also responsible for the payments to third parties of expenses that may include milestone payments, license maintenance fees and royalties, including in the case of certain of our agreements with academic institutions or other companies from whom intellectual property rights underlying their respective programs have been in-licensed or acquired. Because the outcome of any preclinical or clinical development and regulatory approval process is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development, regulatory approval or certification process and potential commercialization of our Wholly-Owned Programs and any future therapeutic candidates we may identify.

184 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Risks Related to Our Founded Entities Our ability to realize value from our Founded Entities may be impacted if we reduce our ownership or otherwise cede control to other investors through contractual agreements or otherwise. We do not have a majority interest in our Non-Controlled Founded Entities. Our interests may be further reduced as such companies raise capital from third-party investors. In addition, we may agree to contractual arrangements for the funding of further developments by one or more of our Founded Entities. As a result, with respect to our Non-Controlled Founded Entities, we may not be able to exercise control over the affairs of such Founded Entity, including that Founded Entity’s governance arrangements and access to management and financial information. We are also party to agreements with certain of our Founded Entities that contain provisions which could force us to exit from that Founded Entity at a time and/or price determined by other investor(s) (for example, by the exercise of drag-along rights). If we were forced to exit out of a Founded Entity, this could have a material adverse effect on our business, financial condition or results of operations and prospects. In addition, if the affairs of one or more Founded Entities in which we hold a minority stake were to be conducted in a manner detrimental to our interests or intentions, our business, reputation and prospects may be adversely affected. As certain of our Founded Entities have completed equity financings, they have entered into certain agreements with the investors participating in such financings, including us. We are party to voting agreements with Entrega, Inc., or Entrega Sonde Health, Inc., or Sonde and Seaport Therapeutics, Inc. or Seaport; investors’ rights agreements with Akili, Vedanta, Entrega, Sonde, Seaport and Vor Biopharma Inc., or Vor, and stockholders’ agreements with Gelesis, Akili, Vedanta, Entrega, and Sonde, pursuant to which we are subject to certain restrictions on the transfer or sale of shares (e.g., pre-emptive rights or drag-along, tag-along rights or lock up agreements), and we may not be able freely to transfer our interest in such Founded Entities or procure the sale of the entire issued share capital of such Founded Entities, similar to other investors who are party to these agreements. In addition, many of our Founded Entities have employee share plans which further dilute our interest in such business. If the affairs of one or more of our Founded Entities were to be conducted or impacted in a manner detrimental to our interests or intentions the value we are able to realize from such entity may be diminished. For example, in October 2023, Gelesis ceased operations and filed a voluntary petition for Chapter 7 bankruptcy liquidation. If we were unable to realize our interest in a Founded Entity or suffer dilution of our shareholding, this could have a material adverse effect on our business, financial condition or results of operation and prospects. Our overall value may be dominated by a single or limited number of our Founded Entities. A large proportion of our overall value may at any time reside in a small proportion of our Founded Entities. Accordingly, there is a risk that if one or more of the intellectual property or commercial rights relevant to a valuable business were impaired, this would have a material adverse impact on our overall value. Furthermore, a large proportion of our overall revenue may at any time be the subject of one, or a small number of, licensed technologies. Should the relevant licenses be terminated or expire this would be likely to have a material adverse effect on the revenue received by us. Any material adverse impact on the value of the business of a Founded Entity could, in the situations described above, or otherwise, have a material adverse effect on our business, financial condition, trading performance and/or prospects. We have limited information about and limited control or influence over our Non-Controlled Founded Entities. While we maintain ownership of equity interests in our Non-Controlled Founded Entities, we do not maintain voting control or direct management and development efforts for these entities. Each of these entities are independently managed, and we do not control the clinical and regulatory development of these Non-Controlled Founded Entities’ therapeutic candidates. Any failure by our Non-Controlled Founded Entities to adhere to regulatory requirements, initiate preclinical studies and clinical trials on schedule or to obtain clearances or approvals for their therapeutic candidates could have an adverse effect on our business, financial condition, results of operation and prospects. The information included in this report about our Non-Controlled Founded Entities is based on (i) our knowledge, which may in some cases be limited, (ii) information that is publicly available, including the public filings of SEC reporting companies, such as Vor, and (iii) information provided to us by our Non-Controlled Founded Entities. Where a date is provided, the information included in Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to current therapeutic candidates or to any future therapeutic candidates on unfavorable terms. To the extent that we or our Founded Entities raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could involve additional restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Additionally, any future collaborations we enter into with third parties may provide capital in the near term, but limit our potential cash flow and revenue in the future. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or therapeutic candidates, or grant licenses or other rights on unfavorable terms. Any such additional fundraising efforts for us may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize therapeutic candidates that we may identify and pursue. Moreover, such financing may result in dilution to shareholders, imposition of debt covenants and repayment obligations, or other restrictions that may affect our business. In addition, if any of our Founded Entities raises funds through the issuance of equity securities, our shareholders’ indirect equity interest in such Founded Entity could be substantially diminished. If any of our Founded Entities raises additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or these therapeutic candidates or grant licenses on terms that are not favorable to us. If we engage in acquisitions or strategic partnerships, this may increase our capital requirements, dilute our shareholders, cause us to incur debt or assume contingent liabilities and subject us to other risks. We may engage in various acquisitions and strategic partnerships in the future, including licensing or acquiring complementary therapeutics, intellectual property rights, technologies or businesses. Any acquisition or strategic partnership may entail numerous risks, including: — increased operating expenses and cash requirements; — the assumption of indebtedness or contingent liabilities; — the issuance of our equity securities which would result in dilution to our shareholders; — assimilation of operations, intellectual property, therapeutics and therapeutic candidates of an acquired company, including difficulties associated with integrating new personnel; — the diversion of our management’s attention from our existing therapeutic programs and initiatives in pursuing such an acquisition or strategic partnership; — retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships; — risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing therapeutics or therapeutic candidates and regulatory approvals; and — our inability to generate revenue from acquired intellectual property, technology and/or therapeutics sufficient to meet our objectives or even to offset the associated transaction and maintenance costs. In addition, if we undertake such a transaction, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense.

PureTech Health plc Annual Report and Accounts 2024 185 Risk Factor Annex continued A d d itio nal info rm atio n Risks Related to the Clinical Development, Regulatory Review and Approval of our and our Founded Entities’ Therapeutic Candidates Risks Related to Clinical Development The therapeutic candidates within our Wholly-Owned Programs and most of our Founded Entities’ therapeutic candidates are in preclinical or clinical development, which is a lengthy and expensive process with uncertain outcomes and the potential for substantial delays. We cannot give any assurance that any of our and our Founded Entities’ therapeutic candidates will receive regulatory clearance, authorization or approval, which is necessary before they can be commercialized. Before obtaining marketing clearance, certification, authorization or approval from regulatory authorities or notified bodies for the sale of our or our Founded Entities’ therapeutic candidates, we or our Founded Entities must conduct extensive clinical trials to demonstrate the safety and efficacy, or with respect to biologics, safety, purity and potency, of the therapeutic candidates in humans. To date, we have focused substantially all of our efforts and financial resources on identifying, acquiring, and developing therapeutic candidates, including conducting lead optimization, preclinical studies and clinical trials, and providing general and administrative support for these operations. To date, only three of our Founded Entities’ products, Karuna’s Cobenfy, Gelesis’ Plenity and Akili’s EndeavorRx, have received commercial approvals, with Cobenfy receiving FDA approval, and both Plenity and EndeavorRx receiving FDA market authorization and European marketing authorization, and we cannot be certain that any of our internal or our Founded Entities’ other therapeutic candidates will receive regulatory clearance, certification, authorization or approval, the timing of such clearance, certification, authorization or approval, if received, or that clinical trials will progress as planned. Our or our Founded Entities’ inability to successfully complete preclinical and clinical development could result in additional costs to us and negatively impact our ability to generate revenue. Our future success is dependent on our and our Founded Entities’ ability to successfully develop, obtain regulatory clearance, certification, authorization or approval for, and then successfully commercialize therapeutic candidates. We and our Founded Entities, with the exceptions of Karuna, Gelesis and Akili, currently have no drugs or biologics approved or devices cleared, certified, authorized or approved for sale and have not generated any revenue from sales of drugs, biologics or devices. We cannot guarantee that we or our Founded Entities will be able in the future to develop or successfully commercialize any of our or their therapeutic candidates. Other than Karuna’s Cobenfy, Gelesis’ Plenity and Akili’s EndeavorRx, all of our Wholly-Owned Programs and our Founded Entities’ therapeutic candidates require additional development; management of preclinical, clinical, and manufacturing activities; and/or regulatory clearances, certification, authorization or approvals. In addition, we or our Founded Entities may need to obtain adequate manufacturing supply; build a commercial organization; commence marketing efforts; and obtain coverage and reimbursement before we generate any significant revenue from commercial therapeutic sales, if ever. Many of the therapeutic candidates in our Wholly-Owned Programs and our Founded Entities’ therapeutic candidates are in early-stage research or translational phases of development, and the risk of failure for these programs is high. We cannot be certain that any of the therapeutic candidates in our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates will be successful in clinical trials or receive regulatory approval, authorization or clearance. Further, our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates may not receive regulatory clearance, certification, authorization or approval even if we believe they are successful in clinical trials. If we or our Founded Entities do not receive regulatory clearance, certification, authorization or approval for our or their therapeutic candidates, we may not be able to continue operations, which may result in dissolution, out-licensing the technology or pursuing an alternative strategy. this report about our Non-Controlled Founded Entities is as of that date and you should not assume that it is accurate as of any other date. As such, there may be developments at our Non-Controlled Founded Entities of which we are unaware that could have an adverse effect on our business, financial condition, results of operation and prospects. For example, on July 2, 2024, Akili Interactive Labs, Inc., merged with privately-held Virtual Therapeutics and ceased trading as a public company. Our Founded Entities are difficult to value given that many of their therapeutic candidates are in the development stage. Investments in early-stage companies, particularly privately held entities, are inherently difficult to value since sales, cash flow and tangible asset values are very limited, which makes the valuation highly dependent on expectations of future development, and any future significant revenues would only arise in the medium to longer terms and are uncertain. Equally, investments in companies just commencing the commercial stage are also difficult to value since sales, cash flow and tangible assets are limited, they have only commenced initial receipts of revenues and valuations are still dependent on expectations of future development. There can be no guarantee that our valuation of our Founded Entities will be considered to be correct in light of the early stage of development for many of these entities and their future performance. As a result, we may not realize the full value of our ownership in such Founded Entities which could adversely affect our business and results of operations. For example, on November 15, 2019, resTORbio, Inc., or resTORbio, announced that its lead therapeutic candidate, RTB101, did not meet its primary endpoint in its Phase 3 study and ceased further development leading to a decline in resTORbio’s stock price from $9.27 to $1.09 and our sale of 7,680,700 common shares of resTORbio. As a result of the foregoing, we recognized a total cash loss of approximately $10 million from our initial investment through sale of shares. Certain of our and our Founded Entities’ therapeutics and therapeutic candidates represent novel therapeutic approaches and negative perception of any therapeutic or therapeutic candidate that we or they develop could adversely affect our ability to conduct our business, obtain and maintain regulatory clearance, authorization or approvals or identify alternate regulatory pathways to market for such therapeutic candidate. Certain of our and our Founded Entities’ therapeutic candidates are considered relatively new and novel therapeutic approaches. Our and their success will depend upon physicians who specialize in the treatment of diseases targeted by our and their therapeutic candidates, prescribing potential treatments that involve the use of our and their therapeutic candidates, if approved, in lieu of, or in addition to, existing treatments with which they are more familiar and for which greater clinical data may be available. Access will also depend on consumer acceptance and adoption of therapeutics that are commercialized. In addition, responses by the U.S., state or foreign governments to negative public perception or ethical concerns may result in new legislation or regulations that could limit our or our Founded Entities’ ability to develop or commercialize any therapeutic candidates, obtain or maintain regulatory approval, identify alternate regulatory pathways to market or otherwise achieve profitability. More restrictive statutory regimes, government regulations or negative public opinion would have an adverse effect on our business, financial condition, results of operations and prospects and may delay or impair the development and commercialization of our or our Founded Entities’ therapeutic candidates or demand for any therapeutics we or they may develop. For example, in the United States and the EU, no therapeutics to date have been approved specifically demonstrating an impact on the microbiome as part of their therapeutic effect. Vedanta is developing a pipeline of microbiome-derived modulators for immune and infectious disease. Microbiome therapies may not be successfully developed or commercialized or gain the acceptance of the public or the medical community. Additionally, adverse events, or AEs, in non-investigational new drug application, or IND, human clinical studies and clinical trials of Vedanta’s therapeutic candidates or in clinical trials of other companies developing similar therapeutics and the resulting publicity, similarly to the AEs publicized with respect to Seres Therapeutics, Inc.’s SER-287 Phase 2 clinical trial, as well as any other AEs in the field of the microbiome, could result in a decrease in demand for any therapeutic that Vedanta may develop. Finally, the FDA, the EMA or other comparable foreign regulatory authorities may lack experience in evaluating the safety and efficacy of therapeutic candidates based on microbiome therapeutics, which could result in a longer than expected regulatory review process, increase expected development costs and delay or prevent potential commercialization of therapeutic candidates.

186 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n — changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; — changes in the standard of care on which a clinical development plan was based, which may require new or additional trials; — the cost of clinical trials of any therapeutic candidates that we may identify and pursue being greater than we anticipate; — clinical trials of any therapeutic candidates that we may identify and pursue producing negative or inconclusive results, which may result in our deciding, or regulators requiring us, to conduct additional clinical trials or abandon therapeutic development programs; — transfer of manufacturing processes to larger-scale facilities operated by a CMO, or by us, and delays or failures by our CMOs or us to make any necessary changes to such manufacturing process; — delays in manufacturing, testing, releasing, validating, or importing/ exporting sufficient stable quantities of therapeutic candidates that we may identify for use in clinical trials or the inability to do any of the foregoing; and — factors we may not be able to control, such as current or potential pandemics or other events that may limit patients, principal investigators or staff or clinical site availability, result in clinical trial protocol deviations, or impact supply of our or our Founded Entities’ therapeutic candidates. Any inability to successfully initiate or complete clinical trials could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our Wholly-Owned Programs , we may be required to or we may elect to conduct additional preclinical studies or clinical trials to bridge data obtained from our modified therapeutic candidates to data obtained from preclinical and clinical research conducted using earlier versions. Clinical trial delays could also shorten any periods during which our therapeutics have patent protection and may allow our competitors to bring therapeutics to market before we do, which could impair our ability to successfully commercialize therapeutic candidates and may harm our business and results of operations. We could also encounter delays if a clinical trial is suspended or terminated by us, by the data safety monitoring board, or DSMB, or by the FDA or other comparable foreign regulatory authorities, or if the IRBs of the institutions in which such trials are being conducted suspend or terminate the participation of their clinical investigators and sites subject to their review. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other comparable foreign regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a therapeutic candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authority may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable foreign regulatory authority may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or comparable foreign regulatory authority, as the case may be, and may ultimately lead to the denial of marketing approval of one or more of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Delays in the initiation, conduct or completion of any clinical trial of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates will increase our costs, slow down the therapeutic candidate development and approval process and delay or potentially jeopardize our ability to commence therapeutic sales and generate revenue. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. In the event we identify any additional therapeutic candidates to pursue, we cannot be sure that submission of an IDE, IND, CTA, or equivalent application, as applicable, will result in the FDA or comparable foreign regulatory authority allowing clinical trials to begin in a timely manner, if at all. Any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations. Preclinical development is uncertain. Our preclinical programs may experience delays or may never advance to clinical trials, which would adversely affect our ability to obtain regulatory clearance, authorization or approvals or commercialize these programs on a timely basis or at all, which would have an adverse effect on our business. Certain of our Wholly-Owned Programs are in the preclinical stage, and their risk of failure is high. Before we can commence clinical trials for a therapeutic candidate, we must complete extensive preclinical testing and studies that support our planned INDs, in the United States, or similar applications in other jurisdictions. We cannot be certain of the timely completion or outcome of our preclinical testing and studies and cannot predict if the FDA or other regulatory authorities will accept our proposed clinical programs or if the outcome of our preclinical testing and studies will ultimately support the further development of our programs. As a result, we cannot be sure that we will be able to submit INDs or similar applications for our preclinical programs on the timelines we expect, if at all, and we cannot be sure that submission of INDs or similar applications will result in the FDA or other regulatory authorities allowing clinical trials to begin. Clinical trials of our or our Founded Entities’ therapeutic candidates may be delayed, and certain programs may never advance in the clinic or may be more costly to conduct than we anticipate, any of which can affect our ability to fund our company and would have a material adverse impact on our platform or our business. Clinical testing is expensive, time-consuming, and subject to uncertainty. We cannot guarantee that any of our ongoing and planned clinical trials will be conducted as planned or completed on schedule, if at all. Moreover, even if these trials are initiated or conducted on a timely basis, issues may arise that could result in the suspension or termination of such clinical trials. A failure of one or more clinical trials can occur at any stage of testing, and our clinical trials may not be successful. Events that may prevent successful or timely initiation or completion of clinical trials include: — inability to generate sufficient preclinical, toxicology, or other in vivo or in vitro data to support the initiation or continuation of clinical trials; — delays in confirming target engagement, patient selection or other relevant biomarkers to be utilized in preclinical and clinical therapeutic candidate development; — delays in reaching a consensus with regulatory agencies as to the design or implementation of our clinical studies; — delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; — delays in identifying, recruiting and training suitable clinical investigators; — delays in obtaining required Institutional Review Board, or IRB, or other reviewing bodies approval or positive opinion at each clinical trial site; — imposition of a temporary or permanent clinical hold by regulatory agencies for a number of reasons, including after review of an IND or amendment, clinical trial application, or CTA, or amendment, investigational device exemption, or IDE, or supplement, or equivalent application or amendment; as a result of a new safety finding that presents unreasonable risk to clinical trial participants; or a negative finding from an inspection of our clinical trial operations or study sites; — developments in trials for other therapeutic candidates with the same targets or related modalities as our or our Founded Entities’ therapeutic candidates conducted by competitors that raise regulatory or safety concerns about risk to patients of the treatment, or if the FDA or similar foreign authorities find that the investigational protocol or plan is clearly deficient to meet its stated objectives; — difficulties in securing access to materials for the comparator arm of certain of our clinical trials; — delays in identifying, recruiting and enrolling suitable patients to participate in clinical trials, and delays caused by patients withdrawing from clinical trials or failing to return for post-treatment follow-up; — difficulties in finding a sufficient number of trial sites, or trial sites deviating from trial protocol or dropping out of a trial; — difficulty collaborating with patient groups and investigators; — failure by CROs, other third parties, or us to adhere to clinical trial requirements; — failure by CROs, other third parties, or us to perform in accordance with the FDA’s or any other regulatory authority’s current good clinical practices, or GCP, requirements, or regulatory guidelines in other countries; — occurrence of AEs or undesirable side effects or other unexpected characteristics associated with the therapeutic candidate that are viewed to outweigh its potential benefits;

PureTech Health plc Annual Report and Accounts 2024 187 Risk Factor Annex continued A d d itio nal info rm atio n If we encounter difficulties enrolling patients in clinical trials, our clinical development activities could be delayed or otherwise adversely affected. Identifying and qualifying trial participants to participate in clinical studies is critical to our success. The timing of our clinical studies depends on the speed at which we can recruit trial participants to participate in testing the therapeutic candidates within our Wholly-Owned Programs . Delays in enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of the therapeutic candidates within our Wholly-Owned Programs . If trial participants are unwilling to participate in our studies because of negative publicity from AEs in our trials or other trials of similar therapeutics, or those related to specific therapeutic area, or for other reasons, including competitive clinical studies for similar patient populations, the timeline for recruiting trial participants, conducting studies, and obtaining regulatory approval of potential therapeutics may be delayed.. Any delays could result in increased costs, delays in advancing our therapeutic candidate development, delays in testing the effectiveness of the therapeutic candidates within our Wholly-Owned Programs , or termination of the clinical studies altogether. We may not be able to identify, recruit and enroll a sufficient number of trial participants, or those with required or desired characteristics to achieve diversity in a study, to complete our clinical studies in a timely manner. Patient and subject enrollment is affected by factors including: — the size and nature of a patient population; — the patient eligibility criteria defined in the applicable clinical trial protocols, which may limit the patient populations eligible for clinical trials to a greater extent than competing clinical trials for the same indication; — the size of the study population required for analysis of the trial’s primary endpoints; — the severity of the disease under investigation; — the proximity of patients to a trial site; — the inclusion and exclusion criteria for the trial in question; — the design of the trial protocol; — the ability to recruit clinical trial investigators with the appropriate competencies and experience; — the availability and efficacy of approved medications or therapies for the disease or condition under investigation; — clinicians’ and patients’ perceptions as to the potential advantages and side effects of the therapeutic candidate being studied in relation to other available therapies and therapeutic candidates; — the ability to obtain and maintain patient consents; and — the risk that patients enrolled in clinical trials will not complete such trials, for any reason. Furthermore, our or our collaborators’ ability to successfully initiate, enroll and conduct a clinical trial outside the United States is subject to numerous additional risks, including: — difficulty in establishing or managing relationships with CROs and physicians; — differing standards for the conduct of clinical trials; — differing standards of care for patients with a particular disease; — an inability to locate qualified local consultants, physicians and partners; and — the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology therapeutics and treatments. If we have difficulty enrolling sufficient numbers of patients to conduct clinical trials as planned, we may need to delay or terminate clinical trials, either of which would have an adverse effect on our business. In addition, the FDA’s and other regulatory authorities’ policies with respect to clinical trials may change and additional government regulations may be enacted. For instance, the regulatory landscape related to clinical trials in the EU recently evolved. The EU Clinical Trials Regulation, or CTR, which was adopted in April 2014 and repeals the EU Clinical Trials Directive, became applicable on January 31, 2022. While the EU Clinical Trials Directive required a separate clinical trial application, or CTA, to be submitted in each member state in which the clinical trial takes place, to both the competent national health authority and an independent ethics committee, the CTR introduces a centralized process and only requires the submission of a single application for multicenter trials. The CTR allows sponsors to make a single submission to both the competent authority and an ethics committee in each member state, leading to a single decision per member state. The assessment procedure of the CTA has been harmonized as well, including a joint assessment by all member states concerned, and a separate assessment by each member state with respect to specific requirements related to its own territory, including ethics rules. Each member state’s decision is communicated to the sponsor via the centralized EU portal. Once the CTA is approved, clinical study development may proceed. The CTR transition period ended on January 31, 2025, and all clinical trials (and related applications) are now fully subject to the provisions of the CTR. Compliance with the CTR requirements by us and our third-party service providers, such as CROs, may impact our developments plans. It is currently unclear to what extent the UK will seek to align its regulations with the EU. The UK regulatory framework in relation to clinical trials is derived from pre-existing EU legislation (as implemented into UK law, through secondary legislation), and after Brexit, EU laws on clinical trials (including the (EU) CTR) have not been directly applicable in Great Britain (i.e., the UK excluding Northern Ireland). The extent to which the regulation of clinical trials in the UK will mirror the (EU) CTR in the long term is not yet certain, however, on December 12, 2024, the UK government introduced a legislative proposal - the Medicines for Human Use (Clinical Trials) Amendment Regulations 2024 - that, if implemented, will replace the current regulatory framework for clinical trials in the UK. The legislative proposal aims to provide a more flexible regime to make it easier to conduct clinical trials in the UK, increase the transparency of clinical trials conducted in the UK and make clinical trials more patient-centered. The UK government has provided the legislative proposal to the UK Parliament for its review and approval. Once the legislative proposal is approved (with or without amendment), it will be adopted into UK law which is expected in early 2026.Under the terms of the Northern Ireland Protocol, provisions of the (EU) CTR which relate to the manufacture and import of investigational medicinal products and auxiliary medicinal products currently apply in Northern Ireland. The results of early-stage clinical trials and preclinical studies may not be predictive of future results. Initial data in clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials. The results of preclinical studies may not be predictive of the results of clinical trials, and the results of any early-stage clinical trials we commence may not be predictive of the results of the later-stage clinical trials. The results of preclinical studies and clinical trials in one set of patients or disease indications, or from preclinical studies or clinical trials that we did not lead, may not be predictive of those obtained in another. In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same therapeutic candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. In addition, preclinical and clinical data are often susceptible to various interpretations and analyses, and many companies that have believed their therapeutic candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval. A number of companies in the pharmaceutical, biopharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development could have a material adverse effect on our business and operating results. Even if early-stage clinical trials are successful, we may need to conduct additional clinical trials of our Wholly-Owned Programs in additional patient populations or under different treatment conditions before we are able to seek approvals or clearances from the FDA or other comparable foreign regulatory authorities to market and sell these therapeutic candidates. Our failure to obtain marketing authorization for the therapeutic candidates within our Wholly-Owned Programs would substantially harm our business, prospects, financial condition and results of operations.

188 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Additionally, if any of the therapeutic candidates within our Wholly- Owned Programs or those of our Founded Entities receives marketing authorization, the FDA could impose contraindications or a boxed warning in the labeling of the therapeutic. For any of our drug or biologic therapeutic candidates receiving marketing authorization, the FDA could require us to adopt a risk evaluation and mitigation strategy, or REMS, and could apply elements to assure safe use to ensure that the benefits of the therapeutic outweigh its risks, which may include, among other things, a Medication Guide outlining the risks of the therapeutic for distribution to patients, a requirement that clinicians or health care settings to become certified prior to prescribing and to participate in additional REMS activities, such as training, patient counseling, and monitoring, and a communication plan to health care practitioners. Furthermore, if we or others later identify undesirable side effects caused by the therapeutic candidates within our Wholly-Owned Programs or those of our Founded Entities , once approved, cleared, certified, or authorized, several potentially significant negative consequences could result, including: — regulatory authorities may suspend or withdraw approvals of such therapeutic candidate, or seek an injunction against its manufacture or distribution; — regulatory authorities may require additional warnings in the labeling, including boxed warnings, or issue safety alerts, Dear Healthcare Provider letters, press releases or other communications containing warnings or other safety information about the therapeutic; — we or our Founded Entities may be required by the FDA to implement a REMS for a marketed drug or biologic or similar risk mitigation measures by foreign regulatory authorities; — we or our Founded Entities may be required to change the way a therapeutic candidate is administered or conduct additional clinical trials; — we or our Founded Entities may be subject to fines, injunctions or the imposition of civil or criminal penalties; — we or our Founded Entities could be sued and held liable for harm caused to patients; and — our or our Founded Entities’ reputations may suffer. Any of these occurrences could prevent us or our Founded Entities from achieving or maintaining market acceptance of the particular therapeutic candidate, if approved, authorized, cleared, or certified, and may harm our business, financial condition and prospects significantly. Risks Related to Regulatory Review and Approval Our clinical trials may fail to demonstrate substantial evidence of the safety and effectiveness of therapeutic candidates that we may identify and pursue for their intended uses, which would prevent, delay or limit the scope of regulatory clearance, certification, authorization or approval and potential commercialization. Before obtaining regulatory approvals for the commercial sale of any of our drug or biological therapeutic candidates, we must demonstrate through lengthy, complex and expensive preclinical studies and clinical trials that the applicable therapeutic candidate is both safe and effective for use in each target indication, and in the case of our Wholly-Owned Programs and Founded Entities’ therapeutic candidates regulated as biological therapeutics in the United States, that the therapeutic candidate is safe, pure and potent for use in its targeted indication. Each therapeutic candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. Similarly, before obtaining regulatory clearances, certifications, authorization or approvals for the commercial sale of any of the device therapeutic candidates of our Founded Entities, our Founded Entities may be required to demonstrate through lengthy, complex and expensive preclinical studies and clinical trials that the applicable therapeutic candidate meets the regulatory standard of clearance, certification, authorization or approval—for example, substantial equivalence to a predicate medical device or a reasonable assurance of safety or effectiveness, as applicable—for its intended use. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical development process. Most therapeutic candidates that begin clinical trials are never approved by regulatory authorities or notified bodies for commercialization. We may be unable to design and execute a clinical trial to support marketing authorization or certification. Use of the therapeutic candidates within our Wholly-Owned Programs or the therapeutic candidates being developed by our Founded Entities could be associated with side effects, AEs or other properties or safety risks, which could delay or halt their clinical development, prevent their regulatory clearance, authorization or approval, cause us to suspend or discontinue clinical trials, abandon a therapeutic candidate, limit their commercial potential, if cleared, authorized or approved, or result in other significant negative consequences that could severely harm our business, prospects, operating results and financial condition. As is the case with pharmaceuticals generally, it is likely that there may be side effects and AEs associated with our and our Founded Entities’ drug or biologic therapeutic candidates’ use. Similarly, investigational devices may also be subject to side effects and AEs. Results of our clinical trials or those being conducted by Founded Entities could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics. Undesirable side effects caused by these therapeutic candidates could cause us, our Founded Entities or regulatory authorities to interrupt, delay or halt clinical trials and could result in more restrictive labeling or the delay or denial of regulatory clearance, certification, authorization or approval by the FDA, the EMA or other comparable foreign regulatory authorities, or notified bodies (when applicable). The side effects related to the therapeutic candidate could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly. Moreover, if therapeutic candidates within our Wholly-Owned Programs are associated with undesirable side effects in preclinical studies or clinical trials or have characteristics that are unexpected, we may elect to abandon their development or limit their development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective, which may limit the commercial expectations for the therapeutic candidate if approved. We may also be required to modify or terminate our study plans based on findings in our preclinical studies or clinical trials. Many therapeutic candidates that initially show promise in early-stage testing may later be found to cause side effects that prevent further development. As we work to advance existing therapeutic candidates and to identify new therapeutic candidates, we cannot be certain that later testing or trials of therapeutic candidates that initially showed promise in early testing will not be found to cause similar or different unacceptable side effects that prevent their further development. It is possible that as we test the therapeutic candidates within our Wholly- Owned Programs in larger, longer and more extensive clinical trials, or as the use of these therapeutic candidates becomes more widespread if they receive regulatory clearance or approval, illnesses, injuries, discomforts and other AEs that were observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, will be reported by subjects. If such side effects become known later in development or upon approval, if any, such findings may harm our business, financial condition and prospects significantly. Additionally, adverse developments in clinical trials of pharmaceutical, biopharmaceutical or biotechnology therapeutics conducted by others may cause the FDA or other regulatory oversight bodies to suspend or terminate our clinical trials or to change the requirements for approval of any of our Wholly-Owned Programs . In addition to side effects caused by the therapeutic candidate, the administration process or related procedures also can cause adverse side effects. If any such AEs occur, our clinical trials could be suspended or terminated. If we are unable to demonstrate that any AEs were not caused by the therapeutic candidate, the FDA, the European Commission, the EMA, or other regulatory authorities or bodies could order us to cease further development of, or deny clearance, certification or approval of, a therapeutic candidate for any or all targeted indications. Even if we can demonstrate that all future serious adverse events, or SAEs, are not therapeutic-related, such occurrences could affect patient recruitment or the ability of enrolled patients to complete the trial. Moreover, if we elect, or are required, to not initiate, delay, suspend or terminate any future clinical trial of any of our Wholly-Owned Programs , the commercial prospects of such therapeutic candidates may be harmed and our ability to generate therapeutic revenues from any of these therapeutic candidates may be delayed or eliminated. Any of these occurrences may harm our ability to develop other therapeutic candidates, and may harm our business, financial condition and prospects significantly.

PureTech Health plc Annual Report and Accounts 2024 189 Risk Factor Annex continued A d d itio nal info rm atio n purity, efficacy and potency. Securing regulatory clearance, authorization or approval also requires the submission of information about the therapeutic manufacturing process to, and inspection of manufacturing facilities by, the relevant regulatory authority. Any therapeutic candidates we or our Founded Entities develop may not be effective, may be only moderately effective, or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing clearance, certification, authorization or approval or prevent or limit commercial use, if cleared, certified, authorized or approved. The process of obtaining marketing clearance, certification, authorization or approval, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if clearance, certification, authorization or approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the therapeutic candidates involved. Changes in marketing authorization policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted therapeutic application, may cause delays in the clearance, authorization, approval or rejection of an application. The FDA, comparable authorities and notified bodies in other countries have substantial discretion in the approval and certification process and may refuse to accept any application or may decide that our data are insufficient for clearance, authorization or approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit, or prevent marketing approval or certification of a therapeutic candidate. Any marketing approval or certification we ultimately obtain may be limited or subject to restrictions or post-market commitments that render the cleared, certified, authorized or approved therapeutic not commercially viable. If we experience delays in obtaining clearance, certification, authorization or approval or if we fail to obtain clearance, certification, authorization or approval of any therapeutic candidates we may develop, the commercial prospects for those therapeutic candidates may be harmed, and our ability to generate revenues will be materially impaired. We have conducted, and may continue to conduct in the future, clinical trials for therapeutic candidates outside the United States, and the FDA, the EMA and comparable foreign regulatory authorities may not accept data from such trials. We have conducted clinical trials outside of the United States in the past, and may in the future choose to conduct one or more clinical trials outside the United States, including in Europe. For example, we have conducted clinical trials in Australia and are conducting or may conduct clinical trials in additional locations outside the United States, including without limitation Argentina, Australia, Brazil, Bulgaria, Chile, Colombia, Czech Republic, Finland, Georgia, Greece, India, Malaysia, Mexico, Moldova, Philippines, Poland, Romania, Spain, South Africa, South Korea, Thailand, Ukraine, and the United Kingdom. The acceptance of study data from clinical trials conducted outside the United States or another jurisdiction by the FDA, the EMA or any comparable foreign regulatory authority may be subject to certain conditions or may not be accepted at all. For example, in cases where data from foreign clinical trials are intended to serve as the sole basis for approval of a drug or biologic in the United States, the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of recognized competence and pursuant to GCP regulations; and (iii) if necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means. In addition, even where the foreign study data are not intended to serve as the sole basis for approval, if the study was not otherwise subject to an IND, the FDA will not accept the data as support for an application for marketing approval unless the study was conducted in accordance with GCP requirements and unless the FDA is able to validate the data from the study through an onsite inspection if deemed necessary. Many foreign regulatory authorities have similar approval requirements. In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that the FDA, the EMA or any comparable foreign regulatory authority will accept data from trials conducted outside of the United States or the applicable jurisdiction. If the FDA, the EMA or any comparable foreign regulatory authority does not accept such data, it would result in the need for additional trials, which would be costly and time-consuming and delay aspects of our business plan, and which may result in therapeutic candidates that we may develop not receiving approval, authorization or clearance for commercialization in the applicable jurisdiction. We cannot be certain that our clinical trials will be successful. Additionally, any safety concerns observed in any one of our clinical trials in our targeted indications could limit the prospects for regulatory clearances, certification, authorization or approval of our therapeutic candidates in those and other indications, which could have a material adverse effect on our business, financial condition and results of operations. In addition, even if such clinical trials are successfully completed, we cannot guarantee that the FDA, the EMA or comparable foreign regulatory authorities or notified bodies (when applicable) will interpret the results as we do, and more trials could be required before we submit our therapeutic candidates for clearance, certification or approval. Even if we believe that our and our Founded Entities’ clinical trials and preclinical studies demonstrate the safety and efficacy of our and their therapeutic candidates, only the FDA and other comparable regulatory agencies may ultimately make such determination. No regulatory agency has made any such determination that any of our Wholly-Owned Programs or those of our Founded Entities are safe or effective for use for any indication. Additionally, we may utilize an “open-label” trial design for some of our future clinical trials. An open-label trial is one where both the patient and investigator know whether the patient is receiving the test article or either an existing approved drug or placebo. Open-label trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label studies are aware that they are receiving treatment. Open- label trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. Patients selected for early clinical studies often include the most severe sufferers and their symptoms may have been bound to improve notwithstanding the new treatment. In addition, open-label trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. The opportunity for bias in clinical trials as a result of open-label design may not be adequately handled and may cause any of our trials that utilize such design to fail or to be considered inadequate and additional trials may be necessary to support future marketing applications. Moreover, results acceptable to support approval in one jurisdiction may be deemed inadequate by another regulatory authority to support regulatory approval in that other jurisdiction. To the extent that the results of the trials are not satisfactory to the FDA, the EMA or comparable foreign regulatory authorities for support of a marketing application, we may be required to expend significant resources, which may not be available to us, to conduct additional trials in support of potential approval of our Wholly-Owned Programs . Even if regulatory approval is secured for a therapeutic candidate, the terms of such approval may limit the scope and use of the specific therapeutic candidate, which may also limit its commercial potential. Even if we complete the necessary preclinical studies and clinical trials, the marketing approval and certification process is expensive, time- consuming and uncertain and may prevent us from obtaining clearance, certification, authorization or approvals for the potential commercialization of therapeutic candidates. Any therapeutic candidate we may develop and the activities associated with their development and potential commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, certification, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other comparable foreign regulatory authorities. Failure to obtain marketing authorization or certification for a therapeutic candidate will prevent us from commercializing the therapeutic candidate in a given jurisdiction. For example, although Karuna, Gelesis and Akili have received commercial approvals, with Cobenfy receiving FDA approval, and both Plenity and EndeavorRx receiving marketing authorization from the FDA and being CE marked in the EU, we and our Founded Entities have not received clearance, certification, authorization or approval to market any of our or their other therapeutic candidates from regulatory authorities in any jurisdiction and it is possible that none of the other therapeutic candidates we and our Founded Entities may seek to develop in the future will ever obtain regulatory clearance, authorization or approval. We have no experience in filing and supporting the applications necessary to gain marketing clearance, certification, authorization or approval and expect to rely on third-party CROs or regulatory consultants to assist us in this process. Securing regulatory clearance, certification, authorization or approval requires the submission of extensive preclinical and clinical data and supporting information to the various regulatory authorities for each therapeutic indication to establish the therapeutic candidate’s safety,

190 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Furthermore, clearance, authorization or approval by the FDA in the United States, if obtained, does not ensure approval or certification by regulatory authorities or notified bodies in other countries or jurisdictions. To market any therapeutics outside of the United States, we or our Founded Entities must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and effectiveness. Clinical trials conducted in one country may not be accepted by regulatory authorities or notified bodies in other countries, and regulatory approval or certification in one country does not mean that regulatory approval or certification will be obtained in any other country. Approval and certification processes vary among countries and can involve additional therapeutic testing and validation and additional or different administrative review periods from those in the United States, including additional preclinical studies or clinical trials, as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities or notified bodies in other jurisdictions. In many jurisdictions outside the United States, a therapeutic candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our therapeutics is also subject to approval. Seeking foreign regulatory approval or certification could result in difficulties and costs for us or our Founded Entities and require additional preclinical studies or clinical trials which could be costly and time-consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our or our Founded Entities’ therapeutics in those countries. The foreign regulatory approval and certification process involves all of the risks associated with FDA approval. We do not have any therapeutics approved for sale in international markets, though two of our Founded Entities, Akili and Gelesis, do. If we or our Founded Entities fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals or certifications in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our therapeutics will be harmed. If the FDA does not conclude that our therapeutic candidates satisfy the requirements for the Section 505(b)(2) regulatory approval pathway, or if the requirements for such therapeutic candidates under Section 505(b) (2) are not as we expect, the approval pathway for those therapeutic candidates will likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated, and in either case may not be successful. We plan to develop one or more therapeutic candidates for which we may plan to seek approval under the 505(b)(2) regulatory pathway. The Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, added Section 505(b)(2) to the FDCA. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Section 505(b)(2), if applicable to us under the FDCA, would allow an NDA we submit to the FDA to rely in part on data in the public domain or the FDA’s prior conclusions regarding the safety and effectiveness of approved compounds, which could expedite the development program for our future therapeutic candidates by potentially decreasing the amount of nonclinical and/or clinical data that we would need to generate in order to obtain FDA approval. If the FDA does not allow us to pursue the Section 505(b)(2) regulatory pathway as anticipated, we may need to conduct additional nonclinical studies and/or clinical trials, provide additional data and information, and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for such therapeutic candidates, and complications and risks associated with such therapeutic candidates, would likely substantially increase. Moreover, inability to pursue the Section 505(b)(2) regulatory pathway could result in new competitive products reaching the market more quickly than any therapeutic candidates we developed, which could adversely impact our competitive position and prospects. Even if we are allowed to pursue the Section 505(b)(2) regulatory pathway, we cannot assure you that any therapeutic candidates we develop will receive the requisite approval for commercialization. In addition, notwithstanding the approval of a number of products by the FDA under Section 505(b)(2), certain pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA’s interpretation of Section 505(b)(2) is successfully challenged, the FDA may change its 505(b)(2) policies and practices, which could delay or even prevent the FDA from approving any NDA that we submit under Section 505(b)(2). In addition, the pharmaceutical industry is highly competitive, and Section 505(b)(2) NDAs are subject to certain requirements designed to protect the patent rights of sponsors of previously approved drugs that are referenced in a Section 505(b)(2) NDA. These requirements may give rise to patent litigation and mandatory delays in approval of our NDAs for If we are unable to obtain regulatory clearance, certification, authorization or approval in one or more jurisdictions for any therapeutic candidates that we may identify and develop, our business could be substantially harmed. We cannot commercialize a therapeutic until the appropriate regulatory authorities or notified bodies have reviewed and cleared, certified, authorized or approved the therapeutic candidate. Clearance, certification, authorization or approval by the FDA, the EMA and comparable foreign regulatory authorities and notified bodies is lengthy and unpredictable, and depends upon numerous factors, including substantial discretion of the regulatory authorities and notified bodies. Clearance, certification, authorization or approval policies, regulations, or the type and amount of preclinical or clinical data necessary to gain clearance, authorization or approval may change during the course of a therapeutic candidate’s development and may vary among jurisdictions, which may cause delays in the clearance, certification, authorization or approval or the decision not to clear, certify, authorize or approve an application. Karuna, Gelesis and Akili have obtained commercial approvals, with Cobenfy receiving FDA approval, and Plenity and EndeavorRx both receiving marketing authorization from the FDA and being CE marked in the EU, but we and our Founded Entities have not obtained regulatory clearance, authorization or approval for any other therapeutic candidates, and it is possible that our current therapeutic candidates and any other therapeutic candidates which we and our Founded Entities may seek to develop in the future will not ever obtain regulatory clearance, certification, authorization or approval. We cannot be certain that any of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates will receive regulatory clearance, certification, authorization or approval or be successfully commercialized even if we or our Founded Entities receive regulatory clearance, certification, authorization or approval. Obtaining marketing clearance, certification, authorization or approval is an extensive, lengthy, expensive and inherently uncertain process, and regulatory authorities and notified bodies may delay, limit or deny clearance , certification, authorization or approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates for many reasons, including but not limited to: — the inability to demonstrate to the satisfaction of the FDA, the EMA or comparable foreign regulatory authorities that the applicable therapeutic candidate is safe, pure, potent or effective as a treatment for our targeted indications or otherwise meets the applicable regulatory standards for clearance, authorization or approval; — the FDA, the EMA or comparable foreign regulatory authorities may disagree with the design, endpoints or implementation of our or our Founded Entities’ clinical trials; — the population studied in the clinical program may not be sufficiently broad or representative to assure safety or efficacy in the full population for which we or our Founded Entities seek clearance, authorization or approval; — the FDA, the EMA or comparable foreign regulatory authorities may require additional preclinical studies or clinical trials beyond those that we or our Founded Entities currently anticipate; — the FDA, the EMA or comparable foreign regulatory authorities may disagree with our or our Founded Entities’ interpretation of data from preclinical studies or clinical trials; — the data collected from clinical trials of therapeutic candidates that we may identify and pursue may not be sufficient to support the submission of an NDA, biologics license application, or BLA, or other submission for regulatory clearance, authorization or approval in the United States or elsewhere; — as applicable, we or our Founded Entities may be unable to demonstrate to the FDA, the EMA or comparable foreign regulatory authorities that a therapeutic candidate’s risk-benefit ratio for its proposed indication is acceptable; — the FDA, the EMA or comparable foreign regulatory authorities may identify deficiencies in the manufacturing processes, test procedures and specifications, or facilities of third-party manufacturers with which we or our Founded Entities contract for clinical and commercial supplies; and — the clearance, certification, authorization or approval policies or regulations of the FDA, the EMA or comparable foreign regulatory authorities may change in a manner that renders the clinical trial design or data insufficient for clearance or approval. The lengthy approval process, as well as the unpredictability of the results of clinical trials and evolving regulatory requirements, may result in our or our Founded Entities’ failure to obtain regulatory clearance, certification, authorization or approval to market therapeutic candidates that we or our Founded Entities may pursue in the United States or elsewhere, which would significantly harm our or our Founded Entities’ business, prospects, financial condition and results of operations.

PureTech Health plc Annual Report and Accounts 2024 191 Risk Factor Annex continued A d d itio nal info rm atio n and advertising, user fees and post-clearance, authorization or approval modifications. Similarly, if applicable, the device components of a combination therapeutic candidate will require any necessary clearances, certifications or approvals or other marketing authorizations in other jurisdictions, which may prove challenging to obtain. The EU regulates medical devices and medicinal products separately, through different legislative instruments, and the applicable requirements will vary depending on the type of drug-device combination product. For instance, drug-delivery products intended to administer a medicinal product where the medicinal product and the device form a single integral product are regulated as medicinal products in the EU. In such a case, the marketing authorization application must include – where available – the results of the assessment of the conformity of the device part with the EU Medical Devices Regulation contained in the manufacturer’s EU declaration of conformity of the device or the relevant certificate issued by a notified body. If the marketing authorization application does not include the results of the conformity assessment and where for the conformity assessment of the device, if used separately, the involvement of a notified body is required, the EMA or the EU member state competent authority must require the applicant to provide a notified body opinion on the conformity of the device. By contrast, in case of drug-delivery products intended to administer a medicinal product where the device and the medicinal product do not form a single integral product (but are e.g., co- packaged), the medicinal product is regulated in accordance with the rules for medicinal products described above while the device part is regulated as a medical device and will have to comply with all the requirements set forth by the Medical Devices Regulation. Certain modifications to our Founded Entities’ device therapeutics may require new 510(k) clearance or other marketing authorizations or certifications and may require our Founded Entities to recall or cease marketing their therapeutics. Akili and Gelesis received de novo classification for EndeavorRx and Plenity, respectively, from the FDA. Once a medical device is permitted to be legally marketed in the United States pursuant to a 510(k) clearance, de novo classification, or a premarket approval, or PMA, a manufacturer may be required to notify the FDA of certain modifications to the device. Manufacturers determine in the first instance whether a change to a medical device requires a new premarket submission, but the FDA may review any manufacturer’s decision. The FDA may not agree with our Founded Entities’ decisions regarding whether new clearances, authorizations or approvals are necessary. They may make modifications or add additional features in the future that they believe do not require a new 510(k) clearance, de novo marketing authorization, or approval of a PMA or PMA amendments or supplements. If the FDA disagrees with their determinations and requires them to submit new 510(k) notifications, requests for de novo classification, or PMAs (or PMA supplements or amendments) for modifications to their previously cleared or authorized therapeutics for which they have concluded that new clearances, authorization or approvals are unnecessary, they may be required to cease marketing or to recall the modified therapeutic until they obtain clearance, authorization or approval, and they may be subject to significant regulatory fines or penalties. In the EU, devices lawfully placed on the market pursuant to the EU Medical Devices Directive prior to May 26, 2021 may generally continue to be made available on the market or put into service, provided that the requirements of the transitional provisions are fulfilled. In particular, no substantial change must be made to the device as such a modification would trigger the obligation to obtain a new certification under the EU Medical Devices Regulation and therefore to have a notified body conducting a new conformity assessment of the devices. Once our devices will be certified under the EU Medical Devices Regulation, we must inform the notified body that carried out the conformity assessment of the medical devices that we market or sell in the EU and the EEA of any planned substantial changes to our quality system or substantial changes to our medical devices that could affect compliance with the general safety and performance requirements laid down in Annex I to the EU Medical Devices Regulation or cause a substantial change to the intended use for which the device has been CE marked. The notified body will then assess the planned changes and verify whether they affect the products’ ongoing conformity with the EU Medical Devices Regulation. If the assessment is favorable, the notified body will issue a new certificate of conformity or an addendum to the existing certificate attesting compliance with the general safety and performance requirements and quality system requirements laid down in the Annexes to the EU Medical Devices Regulation. The notified body may disagree with our proposed changes and product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business. up to 30 months or longer depending on the outcome of any litigation. It is not uncommon for a manufacturer of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements for, pending. competing products. If successful, such petitions can significantly delay, or even prevent, the approval of a new product. Even if the FDA ultimately denies such a petition, the FDA may substantially delay approval while it considers and responds to the petition. In addition, even if we are able to utilize the Section 505(b)(2) regulatory pathway, there is no guarantee this would ultimately lead to streamlined product development or earlier approval. Interim, “top-line,” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available or as additional analyses are conducted, and as the data are subject to audit and verification procedures that could result in material changes in the final data. From time to time, we may publish interim, “top-line,” or preliminary data from our clinical studies, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the interim, top-line, or preliminary results that we report may differ from future results of the same studies or trials, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Data from interim analyses of clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or “top-line” data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim, “top-line,” and preliminary data should be viewed with caution until the final data are available. Material adverse changes between preliminary, “top-line,” or interim data and final data could significantly harm our business prospects. Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular therapeutic candidate or therapeutic and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure. Any information we determine not to disclose may ultimately be deemed significant by you or others with respect to future decisions, conclusions, views, activities or otherwise regarding a particular therapeutic candidate or our business. The complexity of a combination therapeutic that includes a drug or biologic and a medical device presents additional, unique development and regulatory challenges, which may adversely impact our or our Founded Entities’ development plans and our or our Founded Entities’ ability to obtain regulatory clearance, authorization or approval of our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates. We or our Founded Entities may decide to pursue marketing authorization of a combination therapeutic. A combination therapeutic may include, amongst other possibilities, any drug, device, or biologic that is intended for use with another individually specified drug, device, or biologic, where both are required to achieve the intended use, indication, or effect. Developing and obtaining regulatory clearance, authorization or approval in the United States for combination therapeutics pose unique challenges because such therapeutic candidates involve components that are regulated by the FDA under different types of regulatory requirements, and in the United States by different FDA centers. As a result, such therapeutics raise regulatory, policy and review management challenges. For example, because divisions from both FDA’s Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research and FDA’s Center for Devices and Radiological Health must review submissions concerning therapeutic candidates that are combination therapeutics comprised of drug or biologics and devices, respectively, the regulatory review and clearance, authorization or approval process for these therapeutics may be more complex than would otherwise be required for single-agent therapeutics. In addition, differences in regulatory pathways for each component of a combination therapeutic can impact the regulatory processes for all aspects of therapeutic development and management, including clinical investigation, marketing applications, manufacturing and quality control, adverse event reporting, promotion

192 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n (b) the product, without the benefits derived from the orphan status, would not generate sufficient return in the EU to justify the necessary investment; and (3) there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized for marketing in the EU or, if such method exists, the product will be of significant benefit to those affected by that condition. Orphan drug designation entitles a party to financial incentives, such as tax advantages and user fee waivers. Additionally, if a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same disease or condition for seven years, except in certain circumstances, such as a showing of clinical superiority (i.e., another product is safer, more effective or makes a major contribution to patient care) over the product with orphan exclusivity or where the manufacturer is unable to assure sufficient product quantity. Competitors, however, may receive approval of different products for the same disease or condition for which the orphan product has exclusivity, or obtain approval for the same product but for a different disease or condition than that for which the orphan product has exclusivity. In the EU, orphan designation must be requested before submitting a marketing authorization application, or MAA. An EU orphan designation entitles a party to incentives such as reduction of fees or fee waivers, protocol assistance, and access to the centralized procedure. Upon grant of a marketing authorization, orphan medicinal products are entitled to ten years of market exclusivity for the approved indication, which means that the competent authorities cannot accept another MAA, or grant a marketing authorization, or accept an application to extend a marketing authorization for a similar medicinal product for the same indication for a period of ten years. The period of market exclusivity is extended by two years for orphan medicinal products that have also complied with an agreed pediatric investigation plan, or PIP. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications. We have obtained orphan drug designation in the United States for LYT- 200 for the treatment of pancreatic cancer and for the treatment of acute myeloid leukemia, and we may also seek orphan drug designation for other of our therapeutic candidates in the future. We may not be the first to obtain regulatory approval of any therapeutic candidate for its orphan- designated disease or condition and may therefore not obtain orphan drug exclusivity. In addition, exclusive marketing rights in the United States may be limited if we seek approval for an disease or condition broader than the orphan-designated disease or condition or may be lost if the FDA later determines that the request for orphan designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. In the EU, the orphan exclusivity period may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for which it received orphan drug destination, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity or where the prevalence of the condition has increased above the threshold. Additionally, a marketing authorization may be granted to a similar product for the same indication at any time if (i) the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; (ii) the applicant consents to a second orphan medicinal product application; or (iii) the applicant cannot supply enough orphan medicinal product. Orphan drug designation does not ensure that we will receive marketing exclusivity in a particular market, and we cannot assure you that any future application for orphan drug designation with respect to any other therapeutic candidate will be granted. Orphan drug designation neither shortens the development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process. If we or our Founded Entities are unable to successfully validate, develop and obtain regulatory clearance, certification, authorization or approval for companion diagnostic tests for any future drug candidates that require or would commercially benefit from such tests, or experience significant delays in doing so, we or our Founded Entities may not realize the full commercial potential of these drug candidates. In connection with the clinical development of the therapeutic candidates within our Wholly-Owned Programs or Founded Entities’ therapeutic candidates for certain indications, we or our Founded Entities may work with collaborators to develop or obtain access to in vitro companion diagnostic tests to identify patient subsets within a disease category who may derive selective and meaningful benefit from our drug candidates. To be successful, we, our Founded Entities or our collaborators will need to address a number of scientific, technical, regulatory and logistical challenges. The FDA and comparable foreign regulatory authorities regulate in vitro companion diagnostics as medical devices and, under that regulatory framework, will likely require the conduct of clinical trials We may not elect or be able to take advantage of any expedited development or regulatory review and approval processes available to therapeutic candidates granted breakthrough therapy or fast track designation by the FDA. We intend to evaluate and continue ongoing discussions with the FDA on regulatory strategies that could enable us or our Founded Entities to take advantage of expedited development pathways for certain of our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates in the future, although we cannot be certain that our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates will qualify for any expedited development pathways or that regulatory authorities will grant, or allow us or our Founded Entities to maintain, the relevant qualifying designations. Examples of expedited development pathways that we could pursue include breakthrough therapy and fast track designation. The fast track program is intended to expedite or facilitate the process for reviewing therapeutic candidates that meet certain criteria. Specifically, drugs and biologics are eligible for fast track designation if they are intended, alone or in combination with one or more drugs or biologics, to treat serious or life-threatening diseases or conditions and demonstrate the potential to address unmet medical needs for such diseases or conditions. Fast track designation applies to the combination of the therapeutic candidate and the specific indication for which it is being studied. The sponsor of a fast track therapeutic candidate has opportunities for more frequent interactions with the applicable FDA review team during product development and, once a BLA or NDA is submitted, the application may be eligible for priority review. An NDA or BLA submitted for a Fast Track therapeutic candidate may also be eligible for rolling review, where the FDA may consider for review sections of the NDA or BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA or BLA, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application. A “breakthrough therapy” is defined as a drug or biologic that is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening disease or condition, where preliminary clinical evidence indicates that the drug or biologic may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For therapeutic candidates that have been designated as breakthrough therapies, increased interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Drugs and biologics designated as breakthrough therapies also receive the same benefits associated with fast track designation, including eligibility for rolling review of a submitted NDA or BLA, if the relevant criteria are met. Even if we believe a particular therapeutic candidate is eligible for breakthrough therapy or fast track designation, we cannot assure you that the FDA would decide to grant it. Breakthrough therapy designation and fast track designation do not change the standards for approval, and there is no assurance that such designation or eligibility will result in expedited review or approval. Thus, even if we or our Founded Entities do receive breakthrough therapy, fast track designation, or other comparable designation, we or our Founded Entities may not experience a faster development process, review or approval compared to conventional FDA procedures. In addition, the FDA may withdraw either breakthrough therapy or fast track designation if it believes that the therapeutic no longer meets the qualifying criteria. Our business may be harmed if we are unable to avail ourselves of these or any other expedited development and regulatory pathways. We may not be able to obtain or maintain orphan drug designation or Zexclusivity for our therapeutic candidates. Regulatory authorities in some jurisdictions, including the United States, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a drug as an orphan drug if it is intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States, or if the disease or condition affects more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing the drug for the type of disease or condition will be recovered from sales of the product in the United States. The criteria for designating an “orphan medicinal product” in the EU are similar in principle to those in the United States. A medicinal product can be designated as an orphan if its sponsor can establish that: (1) the product is intended for the diagnosis, prevention or treatment of a life threatening or chronically debilitating condition (2) either (a) such condition affects not more than five in 10,000 persons in the EU when the application is made, or

PureTech Health plc Annual Report and Accounts 2024 193 Risk Factor Annex continued A d d itio nal info rm atio n Manufacturers and manufacturers’ facilities are required to comply with extensive requirements imposed by the FDA, the EMA and other comparable foreign regulatory authorities, including ensuring that quality control and manufacturing procedures conform to current good manufacturing practices, or cGMP, or similar foreign regulations. As such, we and our CMOs are subject to continual review and inspections to assess compliance with cGMP, or similar foreign requirements and adherence to commitments made in any marketing authorization, and any future 510(k), de novo classification, certification, PMA, NDA, BLA, MAA, or equivalent application. We and our CMOs are also subject to requirements pertaining to the registration of our manufacturing facilities and the listing of our and our Founded Entities’ therapeutics and therapeutic candidates with the FDA; continued complaint, adverse event and malfunction reporting; corrections and removals reporting; and labeling and promotional requirements. Accordingly, we and others with whom we work must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production and quality control. Karuna’s, Gelesis’ and Akili’s marketing approvals, authorizations and certifications for Cobenfy, Plenity and EndeavorRx, respectively, are and any regulatory clearances, certification, authorization or approvals that we may receive for the therapeutic candidates within our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates will be, subject to limitations on the cleared, certified, authorized or approved indicated uses for which the therapeutic may be marketed and promoted or to the conditions of approval. Any regulatory clearances, certifications, authorizations or approvals that we may receive for the therapeutic candidates within our Wholly-Owned Programs may contain requirements for potentially costly post-marketing testing, such as Phase 4 clinical trials and surveillance to monitor the safety and efficacy of a drug therapeutic. We are required to report certain adverse reactions and production problems, if any, to the FDA and other comparable foreign regulatory authorities. Any new legislation addressing drug or medical safety issues could result in delays in therapeutic development or commercialization, or increased costs to assure compliance. The FDA and other agencies, including the U.S. Department of Justice, and for certain therapeutics, the Federal Trade Commission, closely regulate and monitor the marketing, labeling, advertising and promotion of therapeutics to ensure that they are manufactured, marketed and distributed only for the cleared, certified, authorized or approved indications and in accordance with the provisions of the cleared, certified, authorized or approved labeling. We are, and will be, required to comply with requirements concerning advertising and promotion for the therapeutic candidates within our Wholly-Owned Programs , if cleared, certified, authorized or approved. For example, promotional communications with respect to prescription drugs and medical devices are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the therapeutic’s label or labeling. We may not promote our therapeutics for indications or uses for which they do not have approval, certification, authorization or clearance. The holder of a cleared 510(k), de novo classification, certification or an approved NDA, BLA, PMA, MAA or equivalent marketing authorization must submit new or supplemental applications and obtain clearance, authorization or approval for certain changes to the approved therapeutic, therapeutic labeling, or manufacturing process. For example, any modification to Plenity or EndeavorRx that could significantly affect its safety or effectiveness or that would constitute a major change in its intended use could require a new 510(k) clearance, de novo classification, certification or approval of PMA application. Delays in obtaining required clearances, certifications or approvals would harm our ability to introduce new or enhanced therapeutic in a timely manner, which in turn would harm our or our Founded Entities’ future growth. Failure to submit a new or supplemental application and to obtain approval or certification for certain changes prior to marketing the modified therapeutic may require a recall or to stop selling or distributing the marketed therapeutic as modified, and may lead to significant enforcement actions. Subject to the transitional provisions and in order to sell our products in EU member states, our products must comply with the general safety and performance requirements set forth in the new EU Medical Device Regulation (EU) 2017/745, which repeals and replaces the EU Medical Devices Directive. Compliance with these requirements is a prerequisite to be able to affix the European Conformity, or “CE”, mark to our products, without which they cannot be marketed or sold in the EU. All medical devices placed on the market in the EU must meet the general safety and performance requirements laid down in Annex I to the EU Medical Devices Regulation (EU) 2017/745 including the requirement that a medical device must be designed and manufactured in such a way that, during normal conditions of use, it is suitable for its intended purpose. Medical devices must be safe and effective and must not compromise the clinical condition or safety of patients, or the safety and health of to demonstrate the safety and effectiveness of any diagnostics we or our Founded Entities may develop, which we expect will require separate regulatory clearance, certification, authorization or approval prior to commercialization. In addition, if safe and effective use of a therapeutic product depends on an in vitro companion diagnostic, the FDA generally will require approval, authorization or clearance of that diagnostic, known as a companion diagnostic, before or at the same time that the FDA approves the therapeutic product. In addition, the FDA has historically required approval of a PMA application for companion diagnostics associated with cancer medications. However, in January 2024, the FDA announced its intention to initiate the process to reclassify into Class II most in vitro diagnostic tests that are currently regulated as Class III medical devices, including certain companion diagnostic in-vitro diagnostics. If such reclassification efforts occur, any companion diagnostics that are the subject of the down-classification may no longer require approval of a PMA application, but rather may be marketed pursuant to the generally less burdensome 510(k) clearance process. However, there is no assurance that any companion diagnostic required for therapeutic candidates within our Wholly-Owned Programs or those of our Founded Entities will benefit from the reclassification, or that the reclassification, even if it does occur, will result in a shorter timeline to development or marketing of the companion diagnostic. We or our Founded Entities may rely on third parties for the design, development and manufacture of companion diagnostic tests for our Wholly-Owned Programs ’ or our Founded Entities’ therapeutic candidates that may require such tests. If we or our Founded Entities enter into such collaborative agreements, we will be dependent on the sustained cooperation and effort of our future collaborators in developing and obtaining approval for these companion diagnostics. It may be necessary to resolve issues such as selectivity/specificity, analytical validation, reproducibility, or clinical validation of companion diagnostics during the development and regulatory clearance, certification, authorization or approval processes. Moreover, even if data from preclinical studies and early clinical trials appear to support development of a companion diagnostic for a therapeutic candidate, data generated in later clinical trials may fail to support the analytical and clinical validation of the companion diagnostic. We, our Founded Entities and our future collaborators may encounter difficulties in developing, obtaining regulatory clearance, certification, authorization or approval for, manufacturing and commercializing companion diagnostics similar to those we face with respect to the therapeutic candidates within our Wholly-Owned Programs themselves, including issues with achieving regulatory clearance, certification, authorization or approval, production of sufficient quantities at commercial scale and with appropriate quality standards, and in gaining market acceptance. If we or our Founded Entities are unable to successfully develop companion diagnostics for these therapeutic candidates, or experience delays in doing so, the development of these therapeutic candidates may be adversely affected, these therapeutic candidates may not obtain marketing approval, and we may not realize the full commercial potential of any of these therapeutic candidates that obtain marketing approval. As a result, our business, results of operations and financial condition could be materially harmed. In addition, a diagnostic company with whom we or our Founded Entities contract may decide to discontinue selling or manufacturing the companion diagnostic test that we anticipate using in connection with development and commercialization of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or our relationship with such diagnostic company may otherwise terminate. We or our Founded Entities may not be able to enter into arrangements with another diagnostic company to obtain supplies of an alternative diagnostic test for use in connection with the development and commercialization of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or do so on commercially reasonable terms, which could adversely affect and/or delay the development or commercialization of our or our Founded Entities’ therapeutic candidates. For any cleared, certified, authorized or approved therapeutic, we or our Founded Entities will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and we or our Founded Entities may be subject to penalties if we or our Founded Entities fail to comply with regulatory requirements or experience unanticipated problems with the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Karuna’s Cobenfy, Gelesis’ Plenity and Akili’s EndeavorRx are, and any of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates that are cleared, certified, authorized or approved will be, subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies, and submission of safety, efficacy and other post-market information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.

194 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n In addition, the FDA has historically required approval of a PMA application for companion diagnostics associated with cancer medications. However, in January 2024, the FDA announced its intention to initiate the process to reclassify into Class II most in vitro diagnostic tests that are currently regulated as Class III medical devices, including certain companion diagnostic in-vitro diagnostics. If such reclassification efforts occur, any companion diagnostics that are the subject of the down-classification may no longer require approval of a PMA application, but rather may be marketed pursuant to the generally less burdensome 510(k) clearance process. However, there is no assurance that any companion diagnostic required for therapeutic candidates within our Wholly-Owned Programs or those of our Founded Entities will benefit from the reclassification, or that the reclassification, even if it does occur, will result in a shorter timeline to development or marketing of the companion diagnostic. We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If these legislative or administrative actions impose constraints on the FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted. Outside of the United States, for instance, the EU pharmaceutical legislation is currently undergoing a complete review process, in the context of the Pharmaceutical Strategy for Europe initiative, launched by the European Commission in November 2020. The European Commission’s proposal for revision of several legislative instruments related to medicinal products (potentially reducing the duration of regulatory data protection, revising the eligibility for expedited pathways, etc.) was published on April 26, 2023. The proposed revisions, remain to be agreed and adopted by the European Parliament and European Council, and the proposals may therefore be substantially revised before adoption, which is not anticipated before early 2026. The revisions may, however, have a significant impact on the biopharmaceutical industry in the long term. The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. If, for any of our Wholly-Owned Programs that are cleared or approved, we are found to have improperly promoted off-label uses of those therapeutics, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription therapeutics, if cleared, authorized or approved. In particular, while the FDA permits the dissemination of truthful and non-misleading information about a cleared, authorized or approved therapeutic, a manufacturer may not promote a therapeutic for uses that are not cleared, authorized or approved by the FDA or such other regulatory agencies as reflected in the therapeutic’s cleared, authorized or approved labeling. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees, corporate integrity agreements or permanent injunctions under which specified promotional conduct must be changed or curtailed. If we cannot successfully manage the promotion of the therapeutic candidates within our Wholly-Owned Programs , if cleared, authorized or approved, we could become subject to significant liability, which would materially adversely affect our business and financial condition. Certain of our therapeutic candidates may be regulated as controlled substances, the making, use, sale, importation, exportation, and distribution of which are subject to significant regulation by the U.S. Drug Enforcement Administration, or DEA, and other regulatory agencies. We expect that certain of our therapeutic candidates, if approved, will be regulated as controlled substances, which are subject to state, federal, and foreign laws and regulations regarding their manufacture, use, sale, importation, exportation, and distribution. Among other things, controlled substances are regulated under the federal Controlled Substances Act of 1970, or CSA, and regulations of the DEA. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may not be marketed or sold in the United States. A pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. Certain of our other therapeutic candidates contain Schedule IV substances, which subjects such therapeutic candidates to additional restrictions regarding their manufacture, shipment, storage, sale and use, depending on the scheduling of the active ingredients, and may limit the commercial potential of any of our therapeutic candidates, if approved. users and – where applicable – other persons, provided that any risks which may be associated with their use constitute acceptable risks when weighed against the benefits to the patient and are compatible with a high level of protection of health and safety, taking into account the generally acknowledged state of the art. To demonstrate compliance with the general safety and performance requirements, we or our Founded Entities must undergo a conformity assessment procedure, which varies according to the type of medical device and its (risk) classification. Except for low risk medical devices (Class I), where the manufacturer can self-assess the conformity of its products with the general safety and performance requirements (except for any parts which relate to sterility, metrology or reuse aspects), a conformity assessment procedure requires the intervention of a notified body. The notified body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. If satisfied that the relevant product conforms to the relevant general safety and performance requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE mark to the device, which allows the device to be placed on the market throughout the EU. If we fail to comply with applicable laws and regulations, we would be unable to affix the CE mark to our products, which would prevent us from selling them within the EU. In June 2020, Gelesis received a certification for Plenity as a class III medical device indicated for weight loss in overweight and obese adults with a Body Mass Index of 25-40 kg/m2, when used in conjunction with diet and exercise. Also in June 2020, Akili received a certification for EndeavorRx as a prescription-only digital therapeutic software intended for the treatment of attention and inhibitory control deficits in paediatric patients with ADHD. We or our Founded Entities could also be required to conduct post- marketing clinical trials to verify the safety and efficacy of our or our Founded Entities’ therapeutics in general or in specific patient subsets. If original marketing approval of a drug or biologic was obtained via an accelerated approval pathway, we or our Founded Entities could be required to conduct a successful post-marketing clinical trial to confirm clinical benefit for our or our Founded Entities’ therapeutics. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of marketing clearance, certification, authorization or approval. If a regulatory agency discovers previously unknown problems with a therapeutic, such as AEs of unanticipated severity or frequency, or problems with the facility where the therapeutic is manufactured, or disagrees with the promotion, marketing or labeling of a therapeutic, such regulatory agency may impose restrictions on that therapeutic or us, including requiring withdrawal of the therapeutic from the market. If we or our Founded Entities fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things: — issue warning letters that would result in adverse publicity; — impose civil or criminal penalties; — suspend or withdraw regulatory approvals or certifications; — suspend any of our or our Founded Entities’ ongoing clinical trials; — refuse to approve pending applications or supplements to approved applications submitted by us or our Founded Entities; — impose restrictions on our operations, including closing our CMOs’ facilities; — seize or detain therapeutics; or — require a recall. Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our therapeutics. If regulatory sanctions are applied or if regulatory clearance, authorization or approval is withdrawn, the value of our company and our operating results will be adversely affected. The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory clearance, certification, authorization or approval of the therapeutic candidates within our Wholly-Owned Program or our Founded Entities’ therapeutic candidates.

PureTech Health plc Annual Report and Accounts 2024 195 Risk Factor Annex continued A d d itio nal info rm atio n Risks Related to Manufacturing our Therapeutic Candidates or Those of our Founded Entities Certain of the therapeutic candidates being developed by us or our Founded Entities rely or may rely on third-party manufacturers outside of the United States. Certain of our therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates are currently or may in the future be manufactured outside of the United States. In certain years, the U.S. government has initiated substantial changes in U.S. trade policy and U.S. trade agreements, including the initiation of tariffs on certain foreign goods. In response to these tariffs, certain foreign governments, including Canada, China and Mexico, have instituted or are considering imposing tariffs on certain U.S. goods. If the U.S. imposes additional tariffs on a broader range of imports from certain countries, and in response those countries take further retaliatory trade measures. These actions could impose additional costs on our business. Certain of the therapeutic candidates being developed by us or our Founded Entities are novel, complex and difficult to manufacture. We could experience manufacturing problems that result in delays in our development or commercialization programs or otherwise harm our business. The manufacturing processes our CMOs use to produce our and our Founded Entities’ therapeutic candidates are complex and in certain cases novel. Several factors could cause production interruptions, including inability to develop novel manufacturing processes, equipment malfunctions, facility contamination, raw material shortages or contamination, natural disasters, disruption in utility services, human error or disruptions in the operations of our suppliers, including acquisition of the supplier by a third party or declaration of bankruptcy. For example, Vedanta has its own proprietary cGMP manufacturing facilities for certain therapeutic candidates, including VE202, VE303, VE800 and VE416. Creating defined consortia of live microbial therapeutics for these therapeutic candidates is inherently complex, and therefore can be vulnerable to delays. The expertise required to manufacture these therapeutic candidates is unique to Vedanta, and as a result, it would be difficult and time consuming to find an alternative CMO. In addition, manufacturing of clinical supply for certain of our therapeutic candidates is dependent on third party CMOs, and manufacturing such therapeutic candidates is inherently complex. Some of our and our Founded Entities’ therapeutic candidates include biologics, some of which have physical and chemical properties that cannot be fully characterized. As a result, assays of the finished product may not be sufficient to ensure that the therapeutic candidate is consistent from lot-to-lot or will perform in the intended manner. Accordingly, our CMOs must employ multiple steps to control the manufacturing process to assure that the process is reproducible and the therapeutic candidate is made strictly and consistently in compliance with the process. Problems with the manufacturing process, even minor deviations from the normal process, could result in therapeutic defects or manufacturing failures that result in lot failures, therapeutic recalls, product liability claims or insufficient inventory to conduct clinical trials or supply commercial markets. We or our Founded Entities may encounter problems achieving adequate quantities and quality of clinical-grade materials that meet the FDA, the EMA or other applicable standards or specifications with consistent and acceptable production yields and costs. In addition, the FDA and other foreign regulatory authorities may require us or our Founded Entities to submit samples of any lot of any approved therapeutic together with the protocols showing the results of applicable tests at any time. Under some circumstances, the FDA or other foreign regulatory authorities may require that we or our Founded Entities not distribute a lot until the agency authorizes its release. Slight deviations in the manufacturing process, including those affecting quality attributes and stability, may result in unacceptable changes in the therapeutic that could result in lot failures or therapeutic recalls. Lot failures or therapeutic recalls could cause us or our Founded Entities to delay therapeutic launches or clinical trials, which could be costly to us and otherwise harm our business, financial condition, results of operations and prospects. Various states also independently regulate controlled substances. Though state controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule drugs as well. While some states automatically schedule a drug when the DEA does so, in other states there must be rulemaking or a legislative action. State scheduling may delay commercial sale of any controlled substance drug product for which we obtain federal regulatory approval and adverse scheduling could impair the commercial attractiveness of such product. We or our collaborators must also obtain separate state registrations in order to be able to obtain, handle and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions from the states in addition to those from the DEA or otherwise arising under federal law. For any of our products or therapeutic candidates classified as controlled substances, we and our suppliers, manufacturers, contractors, customers and distributors are required to obtain and maintain applicable registrations from state, federal and foreign law enforcement and regulatory agencies and comply with state, federal and foreign laws and regulations regarding the manufacture, use, sale, importation, exportation and distribution of controlled substances. There is a risk that DEA regulations may limit the supply of the compounds used in clinical trials for our therapeutic candidates, and, in the future, the ability to produce and distribute our products in the volume needed to meet commercial demand. Regulations associated with controlled substances govern manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal. These regulations increase the personnel needs and the expense associated with development and commercialization of therapeutic candidates including controlled substances. The DEA, and some states, conduct periodic inspections of registered establishments that handle controlled substances. Failure to obtain and maintain required registrations or comply with any applicable regulations could delay or preclude us from developing and commercializing our therapeutic candidates containing controlled substances and subject us to enforcement action. The DEA may seek civil penalties, refuse to renew necessary registrations or initiate proceedings to revoke those registrations. In some circumstances, violations could lead to criminal proceedings. Because of their restrictive nature, these regulations could limit commercialization of any of our products or therapeutic candidates that are classified as controlled substances. The EU legislation does not establish different classes of narcotic or psychotropic substances. However, the United Nations, or UN, Single Convention on Narcotic Drugs of 1961 and the UN Convention on Psychotropic Substances of 1971, or the UN Conventions, codify internationally applicable control measures to ensure the availability of narcotic drugs and psychotropic substances for medical and scientific purposes. The individual EU member states are all signatories to these UN Conventions. All signatories have a dual obligation to ensure that these substances are available for medical purposes and to protect populations against abuse and dependence. The UN Conventions regulate narcotic drugs and psychotropic substances as Schedule I, II, III, IV substances with Schedule II substances presenting the lowest relative risk of abuse among such substances and Schedule I and IV substances considered to present the highest risk of abuse. The UN Conventions require signatories to require all persons manufacturing, trading (including exporting and importing) or distributing controlled substances to obtain a license from the relevant authority. Each individual export or import of a controlled substance must also be subject to an authorization. The obligations provided in the UN Conventions and additional requirements are implemented at national level and requirements may vary from one member state to another. In order to develop and commercialize our products in the EU, we need to comply with the national requirements related to controlled substances which is costly and may affect our development plans in the EU.

196 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n The manufacture of pharmaceutical therapeutics is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We and our contract manufacturers must comply with cGMP or similar foreign regulations and guidelines. Manufacturers of pharmaceutical therapeutics often encounter difficulties in production, particularly in scaling up and validating initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if microbial, viral or other contaminations are discovered in our therapeutics or in the manufacturing facilities in which our therapeutic candidate are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot assure you that any stability or other issues relating to the manufacture of any of our therapeutic candidates will not occur in the future. Additionally, our manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to provide any therapeutic candidates to patients in clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. Any adverse developments affecting clinical or potential commercial manufacturing of our therapeutic candidates may result in shipment delays, inventory shortages, lot failures, therapeutic withdrawals or recalls, or other interruptions in the supply of our therapeutic candidates. We may also have to take inventory write-offs and incur other charges and expenses for therapeutic candidates that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. Accordingly, failures or difficulties faced at any level of our supply chain could materially adversely affect our business and delay or impede the development and commercialization of any of our therapeutic candidates and could have a material adverse effect on our business, prospects, financial condition and results of operations. Our or our Founded Entities’ therapeutic candidates must be manufactured in accordance with federal, state and international regulations, and we or our Founded Entities could be forced to recall our or our Founded Entities’ medical devices and therapeutic candidates or terminate production if we or our Founded Entities fail to comply with these regulations. The methods used in, and the facilities used for, the manufacture of medical device therapeutics and therapeutic candidates of our Founded Entities, including Gelesis, Akili, Follica and Sonde, must comply with the FDA’s cGMPs for medical devices, known as the QSR, which is a complex regulatory scheme that covers the procedures and documentation of, among other requirements, the design, testing, validation, verification, complaint handling, production, process controls, quality assurance, labeling, supplier evaluation, packaging, handling, storage, distribution, installation, servicing and shipping of medical devices. Furthermore, we and our Founded Entities are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through, among other oversight methods, periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors, suppliers or CMOs. Our and our Founded Entities’ therapeutics and therapeutic candidates are also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing. Our CMOs also may encounter problems hiring and retaining the experienced scientific, quality assurance, quality-control and manufacturing personnel needed to operate our manufacturing processes, which could result in delays in production or difficulties in maintaining compliance with applicable regulatory requirements. Any problems in our CMOs’ manufacturing process or facilities could result in delays in planned clinical trials and increased costs, and could make us a less attractive collaborator for potential partners, including larger biotechnology companies and academic research institutions, which could limit access to additional attractive development programs. Problems in our manufacturing process could restrict our ability to meet potential future market demand for therapeutics. We do not currently have nor do we plan to acquire the infrastructure or capability internally to manufacture our clinical drug supplies for use in the conduct of our clinical trials, and we lack the resources and the capability to manufacture the therapeutic candidates within our Wholly- Owned Programs on a clinical or commercial scale. Instead, we rely on our third-party manufacturing partners for the production of the active pharmaceutical ingredient, or API, and drug formulation. The facilities used by our third-party manufacturers to manufacture our therapeutic candidates that we may develop must be successfully inspected by the applicable regulatory authorities, including the FDA, after we submit any NDA or BLA to the FDA. We are currently completely dependent on our third-party manufacturers for the production of certain of our therapeutic candidates in accordance with cGMPs or similar foreign requirements, which include, among other things, quality control, quality assurance and the maintenance of records and documentation. Although we have entered into agreements for the manufacture of clinical supplies for such therapeutic candidates, our third-party manufacturers may not perform as agreed, may be unable to comply with these cGMP or similar foreign requirements and with FDA, state and foreign regulatory requirements or may terminate its agreement with us. If any of our third-party manufacturers cannot successfully manufacture material that conforms to our specifications and the applicable regulatory authorities’ strict regulatory requirements, pass regulatory inspection or maintain a compliance status acceptable to the FDA or state or foreign regulatory authorities, our NDAs, BLAs or MAAs will not be approved. In addition, although we are ultimately responsible for ensuring therapeutic quality, we have no direct day-to-day control over our third-party manufacturers’ ability to maintain adequate quality control, quality assurance and qualified personnel. If our third-party manufacturers are unable to satisfy the regulatory requirements for the manufacture of our therapeutics, if approved, or if our suppliers or third-party manufacturers decide they no longer want to manufacture our therapeutics, we will need to find alternative manufacturing facilities, which would be time-consuming and significantly impact our ability to develop, obtain regulatory approval for or market our therapeutics, if approved. If we are required to change contract manufacturers for any reason, we will be required to show that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations. We will also need to verify, such as through a manufacturing comparability study, that any new manufacturing process or procedure will produce our therapeutic candidate according to specifications previously submitted to the FDA or another regulatory authority. We might be unable to identify manufacturers for long-term clinical and commercial supply on acceptable terms or at all. Manufacturers are subject to ongoing periodic announced and unannounced inspection by the FDA and other governmental authorities to ensure compliance with government regulations. As a result, our third-party manufacturers may be subject to increased scrutiny. If we were to experience an unexpected loss of supply for clinical development or commercialization, we could experience delays in our ongoing or planned clinical trials as our third-party manufacturers would need to manufacture additional quantities of our clinical and commercial supply and we may not be able to provide sufficient lead time to enable our third-party manufacturers to schedule a manufacturing slot, or to produce the necessary replacement quantities. This could result in delays in progressing our clinical development activities and achieving regulatory approval for our therapeutics, which could materially harm our business.

PureTech Health plc Annual Report and Accounts 2024 197 Risk Factor Annex continued A d d itio nal info rm atio n If we enter into arrangements with third parties to perform sales, marketing, commercial support, and distribution services, our therapeutic revenue or the profitability of therapeutic revenue may be lower than if we were to market and sell any therapeutics we may develop internally. In addition, we may not be successful in entering into arrangements with third parties to commercialize the therapeutic candidates within our Wholly-Owned Programs or may be unable to do so on terms that are favorable to us or them. We may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our therapeutics effectively or may expose us to legal and regulatory risk by not adhering to regulatory requirements and restrictions governing the sale and promotion of prescription drug therapeutics, including those restricting off-label promotion. If we do not establish commercialization capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing the therapeutic candidates within our Wholly-Owned Programs , if approved. Even if any current or future therapeutic candidate of ours receives regulatory clearance or approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success, in which case we may not generate significant revenues or become profitable. We have never commercialized a therapeutic, and even if any current or future therapeutic candidate of ours is approved by the appropriate regulatory authorities for marketing and sale, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. Physicians may be reluctant to take their patients off their current medications and switch their treatment regimen. Further, patients often acclimate to the treatment regime that they are currently taking and do not want to switch unless their physicians recommend switching therapeutics or they are required to switch due to lack of coverage and adequate reimbursement. In addition, even if we are able to demonstrate our Wholly-Owned Programs ’ safety and efficacy to the FDA and other regulators, safety or efficacy concerns in the medical community may hinder market acceptance. Efforts to educate the medical community and third-party payors on the benefits of the therapeutic candidates within our Wholly-Owned Programs may require significant resources, including management time and financial resources, and may not be successful. The degree of market acceptance of the therapeutic candidates within our Wholly-Owned Programs , if approved for commercial sale, will depend on a number of factors, including: — the efficacy and safety of the therapeutic; — the potential advantages of the therapeutic compared to competitive therapies; — the prevalence and severity of any side effects; — whether the therapeutic is designated under physician treatment guidelines as a first-, second- or third-line therapy; — our ability, or the ability of any future collaborators, to offer the therapeutic for sale at competitive prices; — the therapeutic’s convenience and ease of administration compared to alternative treatments; — the willingness of the target patient population to try, and of physicians to prescribe, the therapeutic; — limitations or warnings, including distribution or use restrictions contained in the therapeutic’s approved labelling; — the strength of sales, marketing and distribution support; — changes in the standard of care for the targeted indications for the therapeutic; and — availability and adequacy of coverage and reimbursement from government payors, managed care plans and other third-party payors. Sales of medical therapeutics also depend on the willingness of physicians to prescribe the treatment, which is likely to be based on a determination by these physicians that the therapeutics are safe, therapeutically effective and cost effective. In addition, the inclusion or exclusion of therapeutics from treatment guidelines established by various physician groups and the viewpoints of influential physicians can affect the willingness of other physicians to prescribe the treatment. We cannot predict whether physicians, physicians’ organizations, hospitals, other healthcare providers, government agencies or private insurers will determine that our therapeutic is safe, therapeutically effective and cost effective as compared with competing treatments. If any therapeutic candidates we develop do not achieve an adequate level of acceptance, we may not generate significant therapeutic revenue, and we may not become profitable. Our or our Founded Entities’ third-party manufacturers may not take the necessary steps to comply with applicable regulations or our or our Founded Entities’ specifications, which could cause delays in the delivery of our therapeutic candidates. In addition, failure to comply with applicable FDA or comparable foreign requirements or later discovery of previously unknown problems with our or our Founded Entities’ therapeutics or therapeutic candidates or manufacturing processes could result in, among other things: warning letters or untitled letters; civil penalties; suspension or withdrawal of approvals or clearances; seizures or recalls of our or our Founded Entities’ therapeutics; total or partial suspension of production or distribution; administrative or judicially imposed sanctions; the FDA’s or foreign regulatory authorities’ refusal to grant pending or future clearances, certifications, authorizations, or approvals for our or our Founded Entities’ therapeutic candidates; clinical holds; refusal to permit the import or export of our or our Founded Entities’ therapeutics or therapeutic candidates; and criminal prosecution of us or our employees. Any of these actions could significantly and negatively impact supply of our or our Founded Entities’ therapeutics or therapeutic candidates. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we or our Founded Entities could lose customers and suffer reduced revenue and increased costs. Risks Related to Commercialization If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any therapeutic candidates we may develop, we may not be successful in commercializing those therapeutic candidates if and when they are approved. We do not have a sales or marketing infrastructure or the capabilities for sale, marketing, or distribution of pharmaceutical therapeutics. To achieve commercial success for any approved therapeutic for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization or outsource these functions to third parties. In the future, we may choose to build a focused sales, marketing, and commercial support infrastructure to market and sell the therapeutic candidates within our Wholly-Owned Programs , if and when they are approved. We may also elect to enter into collaborations or strategic partnerships with third parties to engage in commercialization activities with respect to selected therapeutic candidates, indications or geographic territories, including territories outside the United States, although there is no guarantee we will be able to enter into these arrangements even if the intent is to do so. There are risks involved with both establishing our own commercial capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force or reimbursement specialists is expensive and time consuming and could delay any therapeutic launch. If the commercial launch of a therapeutic candidate for which we recruit a sales force and establish marketing and other commercialization capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition commercialization personnel. Factors that may inhibit our efforts to commercialize any approved therapeutic on our own include: — the inability to recruit and retain adequate numbers of effective sales, marketing, reimbursement, customer service, medical affairs, and other support personnel; — the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future approved therapeutics; — the inability of reimbursement professionals to negotiate arrangements for formulary access, reimbursement, and other acceptance by payors; — the inability to price therapeutics at a sufficient price point to ensure an adequate and attractive level of profitability; — restricted or closed distribution channels that make it difficult to distribute our therapeutics to segments of the patient population; — the lack of complementary therapeutics to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive therapeutic lines; and — unforeseen costs and expenses associated with creating an independent commercialization organization.

198 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n therapeutics. Sales of these or other therapeutic candidates that we may identify will depend substantially, both domestically and abroad, on the extent to which the costs of the therapeutic candidates within our Wholly- Owned Programs will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors. If coverage and adequate reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our therapeutics or therapeutic candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. In many countries, the prices of medical therapeutics are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for the therapeutic candidates within our Wholly-Owned Programs . Accordingly, in markets outside the United States, the reimbursement for therapeutics may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenues and profits. There is also significant uncertainty related to the insurance coverage and reimbursement of newly approved therapeutics and coverage may be more limited than the purposes for which the medicine is approved by the FDA or comparable foreign regulatory authorities. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services. CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. No uniform policy of coverage and reimbursement for therapeutics exists among third-party payors and coverage and reimbursement levels for therapeutics can differ significantly from payor to payor. As a result, the coverage determination process is often a time consuming and costly process that may require us to provide scientific and clinical support for the use of our therapeutics to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel therapeutics such as ours, as there is no body of established practices and precedents for these new therapeutics. Reimbursement agencies in Europe may be more conservative than CMS. For example, a number of cancer drugs have been approved for reimbursement in the United States and have not been approved for reimbursement in certain European countries. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale, and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved therapeutics we may develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize therapeutic candidates, and our overall financial condition. As noted above, in the United States we plan to have various programs to help patients afford our therapeutics, including patient assistance programs and co-pay coupon programs for eligible patients. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Our inability to promptly obtain coverage and profitable reimbursement rates third-party payors for any approved therapeutics that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize therapeutics and our overall financial condition. Any failure by any current or future therapeutic candidate of ours that obtains regulatory approval to achieve market acceptance or commercial success would adversely affect our business prospects. In addition, any negative perception of one of our Founded Entities or any therapeutic candidates marketed or commercialized by them may adversely affect our reputation in the marketplace or among industry participants and our business prospects. The incidence and prevalence for target patient populations of our therapeutic candidates have not been established with precision. If the market opportunities for our therapeutic candidates are smaller than we estimate, or if any approval that we obtain is based on a narrower definition of the patient population, our revenue and ability to achieve profitability may be materially adversely affected. The precise incidence and prevalence for all the conditions we aim to address with our therapeutic candidates are unknown and cannot be precisely determined. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our therapeutic candidates, are based on beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, surveys of clinics, patient foundations or market research, and may prove to be incorrect. Further, new trials may change the estimated incidence or prevalence of these diseases. The total addressable market across all of our therapeutic candidates will ultimately depend upon, among other things, the diagnosis criteria included in the final label for each of our therapeutic candidates approved for sale for these indications, acceptance by the medical community and patient access, drug pricing and reimbursement. The number of patients in the United States and other major markets and elsewhere may turn out to be lower than expected, patients may not be otherwise amenable to treatment with our products or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business. Further, even if we obtain significant market share for our therapeutic candidates, if the potential target populations are very small, we may never achieve profitability despite obtaining such significant market share. The insurance coverage and reimbursement status of newly-approved therapeutics is uncertain. The therapeutic candidates within our Wholly- Owned Programs may become subject to unfavorable pricing regulations, third-party coverage and reimbursement practices, or healthcare reform initiatives, which would harm our business. Failure to obtain or maintain coverage and adequate reimbursement for new or current therapeutics could limit our ability to market those therapeutics and decrease our ability to generate revenue. The regulations that govern marketing approvals, pricing, coverage, and reimbursement for new drugs and other medical therapeutics vary widely from country to country. In the United States, healthcare reform legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a therapeutic before it can be marketed. In many countries, the pricing review period begins after marketing or therapeutic licensing approval is granted. In some foreign markets, pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a therapeutic in a particular country, but then be subject to price regulations that delay our commercial launch of the therapeutic, possibly for lengthy time periods, and negatively impact the revenue we are able to generate from the sale of the therapeutic in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more therapeutics or therapeutic candidates, even if any therapeutic candidates we may develop obtain marketing approval. Our ability to successfully commercialize our therapeutics and therapeutic candidates also will depend in part on the extent to which coverage and adequate reimbursement for these therapeutics and related treatments will be available from government health administration authorities, private health insurers, and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. The availability of coverage and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford treatments such as gene therapy

PureTech Health plc Annual Report and Accounts 2024 199 Risk Factor Annex continued A d d itio nal info rm atio n — federal civil and criminal false claims laws and civil monetary penalty laws, including the False Claims Act, which impose criminal and civil penalties, including through civil “qui tam” or “whistleblower” actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other federal health care programs that are false or fraudulent; knowingly making or causing a false statement material to a false or fraudulent claim or an obligation to pay money to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing such an obligation. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery; — the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA without actual knowledge of the statute or specific intent to violate it; — the federal civil monetary penalties laws, which impose civil fines for, among other things, the offering or transfer or remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies; — the federal Physician Payments Sunshine Act, created under the ACA, and its implementing regulations, which require manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the U.S. Department of Health and Human Services, or HHS, under the Open Payments Program, information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers (physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anaesthetists, anaesthesiologist assistants and certified nurse midwives), and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; — federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; — federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved therapeutics; and — analogous state and foreign laws and regulations, such as state and foreign anti-kickback, false claims, consumer protection and unfair competition laws which may apply to pharmaceutical business practices, including but not limited to, research, distribution, sales and marketing arrangements as well as submitting claims involving healthcare items or services reimbursed by any third-party payer, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government that otherwise restricts payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to file reports with states regarding pricing and marketing information, such as the tracking and reporting of gifts, compensations and other remuneration and items of value provided to healthcare professionals and entities; and state and local laws requiring the registration of pharmaceutical sales representatives. Increasingly, third-party payors are requiring that pharmaceutical companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical therapeutics. We cannot be sure that reimbursement will be available for any therapeutic candidate that we commercialize and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any therapeutic or therapeutic candidate for which we obtain marketing approval. In order to obtain reimbursement, physicians may need to show that patients have superior treatment outcomes with our therapeutics compared to standard of care drugs, including lower-priced generic versions of standard of care drugs. We expect to experience pricing pressures in connection with the sale of any of the therapeutic candidates within our Wholly-Owned Programs , due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new therapeutics. Additionally, we may develop companion diagnostic tests for use with our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. We, or our Founded Entities or our collaborators may be required to obtain coverage and reimbursement for these tests separate and apart from the coverage and reimbursement we seek for our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, once approved. Even if we or our Founded Entities obtain regulatory approval or clearance for such companion diagnostics, there is significant uncertainty regarding our ability to obtain coverage and adequate reimbursement for the same reasons applicable to our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Medicare reimbursement methodologies, whether under Part A, Part B, or clinical laboratory fee schedule may be amended from time to time, and we cannot predict what effect any change to these methodologies would have on any therapeutic candidate or companion diagnostic for which we receive approval. Risks Related to Compliance with Healthcare Laws If we fail to comply with healthcare laws, we could face substantial penalties and our business, operations and financial conditions could be adversely affected. Healthcare providers, physicians and third-party payors in the United States and elsewhere play a primary role in the recommendation and prescription of pharmaceutical therapeutics. Arrangements with healthcare providers, third-party payors and customers can expose pharmaceutical manufacturers to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti- Kickback Statute and the federal False Claims Act, or the FCA, which may constrain the business or financial arrangements and relationships through which such companies sell, market and distribute pharmaceutical therapeutics. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of ownership, pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials. The applicable federal and state healthcare laws and regulations laws that may affect our ability to operate include, but are not limited to: — the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Violations are subject to civil and criminal fines and penalties for each violation, plus up to three times the remuneration involved, imprisonment of up to ten years, and exclusion from government healthcare programs. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers, on the one hand, and prescribers, purchasers and formulary managers, on the other;

200 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Further, in the event we decide to conduct clinical trials or continue to enroll subjects in our ongoing or future clinical trials in the European Economic Area, or EEA, or the United Kingdom, UK, we may be subject to additional privacy restrictions. The EU General Data Protection Regulation 2016/679, or GDPR, and the UK General Data Protection Regulation and the Data Protection Act 2018, or the UK GDPR, could impose comprehensive data privacy compliance obligations in relation to our collection and use of personal data, including a principle of accountability and the obligation to demonstrate compliance through policies, procedures, training and audit, as well as regulating cross-border transfers of personal data out of the EEA and the UK. In relation to data transfers from the EEA to the United States, the EU-US Data Privacy Framework, or DPF, was approved by the European Commission in July 2023 as an effective EU GDPR data transfer mechanism to U.S. entities self-certified under the DPF. The UK Extension to the DPF followed in October 2023, as an effective UK GDPR data transfer mechanism to U.S. entities self-certified under the UK Extension to the DPF. In relation to such cross border transfers of personal data, we expect the existing legal complexity and uncertainty regarding international personal data transfers to continue. In particular, we expect the European Commission approval of the current DPF to be challenged and international transfers to the United States and to other jurisdictions more generally to continue to be subject to enhanced scrutiny by regulators. As the regulatory guidance and enforcement landscape in relation to data transfers continue to develop, we could suffer additional costs, complaints and/or regulatory investigations or fines; we may have to stop using certain tools and vendors and make other operational changes; we may have to implement alternative data transfer mechanisms under the GDPR and/ or take additional compliance and operational measures; and/ or it could otherwise affect the manner in which we provide our services and could adversely affect our business, operations and financial condition. Companies that must comply with the GDPR and UK GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million under the GDPR and £17.5 million under the UK GDPR or 4% of the annual global revenues of the noncompliant undertaking, whichever is greater. The existence of parallel regimes under the GDPR and UK GDPR, and divergence in respect of implementing or supplementary laws across the EEA and UK in certain areas, means that we could be subject to potentially overlapping or divergent enforcement actions for certain actual or perceived violations. Failure to comply with these laws and regulations could result in government enforcement actions (which could include civil, criminal and administrative penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects, employees and other individuals about whom we or our potential collaborators obtain personal information, as well as the providers who share this information with us, may limit our ability to collect, use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business. Healthcare legislative measures aimed at reducing healthcare costs may have a material adverse effect on our business and results of operations. The United States and many foreign jurisdictions have enacted or proposed legislative and regulatory changes affecting the healthcare system that could prevent or delay marketing approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or any future therapeutic candidates, restrict or regulate post-approval activities and affect our or our Founded Entities’ ability to profitably sell any therapeutic for which we or our Founded Entities obtain marketing approval. Changes in regulations, statutes or the interpretation of existing regulations could impact our or our Founded Entities’ business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to therapeutic labeling; (iii) the recall or discontinuation of our therapeutics; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business. In the United States, there have been and continue to be a number of legislative initiatives and judicial challenges to contain healthcare costs. For example, in March 2010, the Affordable Care Act, or the ACA, was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacted the U.S. pharmaceutical industry. The ACA, among other things, subjects biological therapeutics to potential competition by lower-cost biosimilars, addresses Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available, it is possible that some of our business activities, including compensation of physicians with stock or stock options, could, despite efforts to comply, be subject to challenge under one or more of such laws. Additionally, FDA or foreign regulators may not agree that we have mitigated any risk of bias in our clinical trials due to payments or equity interests provided to investigators or institutions which could limit a regulator’s acceptance of those clinical trial data in support of a marketing application. Moreover, efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, disgorgement, monetary fines, exclusion from participation in Medicare, Medicaid and other federal healthcare programs, integrity and oversight agreements to resolve allegations of non-compliance, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. In addition, the approval and commercialization of any of the therapeutic candidates within our Wholly-Owned Programs outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. Failure to comply with data protection laws and regulations could lead to government enforcement actions (which could include civil or criminal penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business. We and any potential collaborators may be subject to federal, state, and foreign data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the United States, numerous federal and state laws and regulations, including federal health information privacy laws, state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators. In addition, we may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA, as amended. Depending on the facts and circumstances, we could be subject to civil, criminal, and administrative penalties if we knowingly obtain, use, or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA. As our operations and business grow, we may become subject to or affected by new or additional data protection laws and regulations and face increased scrutiny or attention from regulatory authorities. In the United States, certain states have adopted data privacy and security laws and regulations, which govern the privacy, processing and protection of health-related and other personal information. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners. For example, the California Consumer Privacy Act of 2018, as amended by the California Privacy Rights Act, or collectively, the CCPA, requires covered businesses that process the personal information of California residents to, among other things: (i) provide certain disclosures to California residents regarding the business’s collection, use, and disclosure of their personal information; (ii) receive and respond to requests from California residents to access, delete, and correct their personal information, or to opt out of certain disclosures of their personal information; and (iii) enter into specific contractual provisions with service providers that process California resident personal information on the business’s behalf. Additional compliance investment and potential business process changes may also be required. Similar laws have passed in other states and are continuing to be proposed at the state and federal level, reflecting a trend toward more stringent privacy legislation in the United States. The enactment of such laws could have potentially conflicting requirements that would make compliance challenging. In the event that we are subject to or affected by HIPAA, the CCPA or other domestic privacy and data protection laws, any liability from failure to comply with the requirements of these laws could adversely affect our financial condition.

PureTech Health plc Annual Report and Accounts 2024 201 Risk Factor Annex continued A d d itio nal info rm atio n Such reforms could have an adverse effect on anticipated revenue from therapeutic candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop therapeutic candidates. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect: — the demand for the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, if approved; — our ability to receive or set a price that we believe is fair for our therapeutics; — our ability to generate revenue and achieve or maintain profitability; — the amount of taxes that we are required to pay; and — the availability of capital. Other healthcare reform measures may be adopted in the future, and may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, lower reimbursement, and new payment methodologies. This could lower the price that we receive for any approved therapeutic. Any denial in coverage or reduction in reimbursement from Medicare or other government-funded programs may result in a similar denial or reduction in payments from private payors, which may prevent us from being able to generate sufficient revenue, attain profitability or commercialize the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, if approved. Litigation and legislative efforts to change or repeal the ACA are likely to continue, with unpredictable and uncertain results. In the EU, similar developments may affect our ability to profitably commercialize our therapeutic candidates, if approved. On December 13, 2021, Regulation No 2021/2282 on Health Technology Assessment, or HTA, amending Directive 2011/24/EU, was adopted. The Regulation entered into force in January 2022 and has been applicable since January 2025, with phased implementation based on the type of product, i.e. oncology and advanced therapy medicinal products as of 2025, certain high-risk medical devices as of 2026, orphan medicinal products as of 2028, and all other medicinal products by 2030. The Regulation intends to boost cooperation among EU member states in assessing health technologies, including new medicinal products as well as certain high-risk medical devices, and provide the basis for cooperation at the EU level for joint clinical assessments in these areas. It will permit EU member states to use common HTA tools, methodologies, and procedures across the EU, working together in four main areas, including joint clinical assessment of the innovative health technologies with the highest potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU member states will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technology, and making decisions on pricing and reimbursement. Risks Related to Competition We face significant competition in an environment of rapid technological and scientific change, and there is a possibility that our competitors may achieve regulatory approval before us or develop therapies that are safer, more advanced or more effective than ours, which may negatively impact our ability to successfully market or commercialize any therapeutic candidates we may develop and ultimately harm our financial condition. The development and commercialization of new drug therapeutics is highly competitive. We may face competition with respect to any therapeutic candidates that we seek to develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies worldwide. Potential competitors also include academic institutions, government agencies, and other public and private research organizations that conduct research, seek patent protection, and establish collaborative arrangements for research, development, manufacturing, and commercialization. There are a number of major pharmaceutical and biotechnology companies that are currently pursuing the development and commercialization of potential medicines targeting similar treatment areas as we are. If any of our competitors receive FDA or foreign regulatory authorities approval before we do, the therapeutic candidates within our Wholly-Owned Programs would not be the first treatment on the market, and our market share may be limited. In addition to competition from other companies targeting our target indications, any therapeutics we may develop may also face competition from other types of therapies. a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations, and establishes annual fees and taxes on manufacturers of certain branded prescription drugs. Since the enactment of the ACA, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Payment methodologies may be subject to changes in healthcare legislation and regulatory challenges. For example, in order for a drug therapeutic to receive federal reimbursement under the Medicaid or Medicare Part B programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. In December 2018, the CMS published a final rule permitting further collections and payments to and from certain ACA qualified health plans and health insurance issuers under the ACA risk adjustment program in response to the outcome of the federal district court litigation regarding the method CMS uses to determine this risk adjustment. Since then, the ACA risk adjustment program payment parameters have been updated annually. In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, resulted in aggregate reductions of Medicare payments to providers, which went into effect in 2013, and, due to subsequent legislative amendments, will remain in effect through 2032, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022, unless additional Congressional action is taken. The American Taxpayer Relief Act of 2012 further reduced Medicare payments to several types of providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In addition, in March 2021, Congress enacted the American Rescue Plan Act of 2021, which, among other things, eliminated the statutory cap on drug manufacturers’ Medicaid Drug Rebate Program rebate liability, effective January 1, 2024. There has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. On August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023), and replaces the Part D coverage gap discount program with a new manufacturer discounting program (which began in 2025). The IRA permits the Secretary of the Department of Health and Human Services to implement many of these provisions through guidance, as opposed to regulation, for the initial years. CMS has published the negotiated prices for the initial ten drugs, which will first be effective in 2026, and the list of the subsequent 15 drugs that will be subject to negotiation, although the Medicare drug price negotiation program is currently subject to legal challenges. For that and other reasons, it is currently unclear how the IRA will be effectuated. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological therapeutic pricing, including price or patient reimbursement constraints, discounts, restrictions on certain therapeutic access and marketing cost disclosure, drug price reporting and other transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Some states have enacted legislation creating so-called prescription drug affordability boards, which ultimately may attempt to impose price limits on certain drugs in these states. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical therapeutics and which suppliers will be included in their prescription drug and other healthcare programs. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices. There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at containing or lowering the cost of healthcare. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our therapeutic.

202 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n We believe that any of the therapeutic candidates within our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates that are approved as a biological therapeutic under a BLA should qualify for the 12- year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider such therapeutic candidates to be reference therapeutics for competing therapeutics, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar therapeutic, once approved, will be substituted for any one of our, our Founded Entities’ or our collaborators’ reference therapeutics in a way that is similar to traditional generic substitution for non-biologic therapeutics is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. If competitors are able to obtain marketing approval for biosimilars referencing any therapeutics that we or our Founded Entities develop alone or with collaborators that may be approved, such therapeutics may become subject to competition from such biosimilars, with the attendant competitive pressure and potential adverse consequences. Risks Related to Reliance on Third Parties We are currently party to and may seek to enter into additional collaborations, licenses and other similar arrangements and may not be successful in maintaining existing arrangements or entering into new ones, and even if we are, we may not realize the benefits of such relationships, which could cause us to expend significant resources and give rise to substantial business risk with no assurance of financial return. We are currently parties to license and collaboration agreements with a number of universities and pharmaceutical companies and expect to enter into additional agreements as part of our business strategy. Establishing strategic collaborations is difficult and time-consuming. Our discussions with potential collaborators may not lead to the establishment of collaborations on favorable terms, if at all. Potential collaborators may reject collaborations based upon their assessment of our financial, regulatory or intellectual property position. Even if we successfully establish new collaborations, these relationships may never result in the successful development or commercialization of therapeutic candidates or the generation of sales revenue. The success of our current and any future collaboration arrangements will depend heavily on the efforts and activities of our collaborators. Collaborations are subject to numerous risks, which may include risks that: — collaborators may have significant discretion in determining the efforts and resources that they will apply to collaborations; — collaborators may not pursue development and commercialization of the therapeutic candidates within our Wholly-Owned Programs or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in their strategic focus due to their acquisition of competitive therapeutics or their internal development of competitive therapeutics, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities; — collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial, abandon a therapeutic candidate, repeat or conduct new clinical trials or require a new formulation of a therapeutic candidate for clinical testing; — collaborators could independently develop, or develop with third parties, therapeutics that compete directly or indirectly with our therapeutics or therapeutic candidates; — a collaborator with marketing, manufacturing and distribution rights to one or more therapeutics may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities; — we could grant exclusive rights to our collaborators that would prevent us from collaborating with others; — collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; — disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our current or future therapeutic candidates or that results in costly litigation or arbitration that diverts management attention and resources; — collaborations may be terminated, which may result in a need for additional capital to pursue further development or commercialization of the applicable current or future therapeutic candidates; Many of our current or potential competitors, either alone or with their strategic partners, have: — greater financial, technical, and human resources than we have at every stage of the discovery, development, manufacture, and commercialization of therapeutics; — more extensive resources for preclinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing, and selling drug therapeutics; — therapeutics that have been approved or are in late stages of development; and — collaborative arrangements in our target markets with leading companies and research institutions. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize therapeutics that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any therapeutics that we may develop. Furthermore, currently approved therapeutics could be discovered to have application for treatment of our targeted disease indications or similar indications, which could give such therapeutics significant regulatory and market timing advantages over the therapeutic candidates within our Wholly-Owned Programs . Our competitors may also obtain FDA, EMA or other comparable foreign regulatory approval for their therapeutics more rapidly than we may obtain approval for ours and may obtain orphan therapeutic exclusivity from the FDA for indications that we are targeting, which could result in our competitors establishing a strong market position before we are able to enter the market. Additionally, therapeutics or technologies developed by our competitors may render our potential therapeutic candidates uneconomical or obsolete and we may not be successful in marketing any therapeutic candidates we may develop against competitors. In addition, we could face litigation or other proceedings with respect to the scope, ownership, validity and/or enforceability of our patents relating to our competitors’ therapeutics and our competitors may allege that our therapeutics infringe, misappropriate or otherwise violate their intellectual property. The availability of our competitors’ therapeutics could limit the demand, and the price we are able to charge, for any therapeutics that we may develop and commercialize. The therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates for which we or our Founded Entities intend to seek approval as biologic therapeutics may face competition sooner than anticipated. If we or our Founded Entities are successful in achieving regulatory approval to commercialize any biologic therapeutic candidate we or our Founded Entities develop alone or with collaborators, it may face competition from biosimilar therapeutics. In the United States, certain of the therapeutic candidates within our Wholly-Owned Programs and our Founded Entities’ therapeutic candidates are regulated by the FDA as biologic therapeutics subject to approval under the BLA pathway. The Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated pathway for the approval of biosimilar and interchangeable biologic therapeutics following the approval of an original BLA. The abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand therapeutic. Under the BPCIA, an application for a biosimilar therapeutic may not be submitted until four years following the date that the reference therapeutic was first licensed by the FDA. In addition, the approval of a biosimilar therapeutic may not be made effective by the FDA until 12 years after the reference therapeutic was first licensed by the FDA. During this 12-year period of exclusivity, another company may still market a competing version of the reference therapeutic if the FDA approves a full BLA for the competing therapeutic containing the sponsor’s own preclinical data and data from adequate and well- controlled clinical trials to demonstrate the safety, purity and potency of their therapeutic. In the EU, upon receiving a marketing authorization, new biological entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a biosimilar application. During the additional two-year period of market exclusivity, a biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no biosimilar product can be marketed until the expiration of the market exclusivity.

PureTech Health plc Annual Report and Accounts 2024 203 Risk Factor Annex continued A d d itio nal info rm atio n Even if we successfully establish new collaborations, these relationships may never result in the successful development or commercialization of therapeutic candidates or the generation of sales revenue. To the extent that we enter into collaborative arrangements, the related therapeutic revenues are likely to be lower than if we directly marketed and sold therapeutics. Such collaborators may also consider alternative therapeutic candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for any future therapeutic candidate. Management of our relationships with collaborators will require: — significant time and effort from our management team; — coordination of our marketing and R&D programs with the marketing and R&D priorities of our collaborators; and — effective allocation of our resources to multiple projects. We rely on third parties to assist in conducting our clinical trials and some aspects of our research and preclinical testing, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research, or testing. We currently rely and expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions, and clinical investigators, to conduct some aspects of research and preclinical testing and clinical trials. Any of these third parties may terminate their engagements with us or be unable to fulfill their contractual obligations. If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. If we need to enter into alternative arrangements, it would delay therapeutic development activities. Further, although our reliance on these third parties for clinical development activities limits our control over these activities, we remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards. For example, notwithstanding the obligations of a CRO for a trial of one of the therapeutic candidates within our Wholly- Owned Programs , we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA and comparable foreign regulatory authorities require us to comply with requirements, commonly referred to as GCPs, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The FDA and comparable foreign regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators, clinical trial sites and IRBs. If we or our third-party contractors fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving the therapeutic candidates within our Wholly-Owned Programs , which would delay the regulatory approval process. We cannot be certain that, upon inspection, the FDA or comparable foreign regulatory authorities will determine that any of our clinical trials comply with GCPs. We are also required to register certain clinical trials and post the results of completed clinical trials on databases including a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions. Furthermore, the third parties conducting clinical trials on our behalf are not our employees, and except for remedies available to us under our agreements with such contractors, we cannot control whether or not they devote sufficient time, skill and resources to our ongoing development programs. These contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug or medical device development activities, which could impede their ability to devote appropriate time to our clinical programs. If these third parties, including clinical investigators, do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we may not be able to obtain, or may be delayed in obtaining, regulatory approvals for the therapeutic candidates within our Wholly-Owned Programs . If that occurs, we will not be able to, or may be delayed in our efforts to, successfully commercialize the therapeutic candidates within our Wholly-Owned Programs . In such an event, our financial results and the commercial prospects for any therapeutic candidates that we seek to develop could be harmed, our costs could increase and our ability to generate revenues could be delayed, impaired or foreclosed. — collaborators may own or co-own intellectual property covering therapeutics that result from our collaboration with them, and in such cases, we would not have the exclusive right to develop or commercialize such intellectual property; — disputes may arise with respect to the ownership of any intellectual property developed pursuant to our collaborations; and — a collaborator’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings. Additionally, we may seek to enter into additional collaborations, joint ventures, licenses and other similar arrangements for the development or commercialization of the therapeutic candidates within our Wholly-Owned Programs , due to capital costs required to develop or commercialize the therapeutic candidate or manufacturing constraints. We may not be successful in our efforts to establish such collaborations for the therapeutic candidates within our Wholly-Owned Programs because our R&D pipeline may be insufficient, the therapeutic candidates within our Wholly-Owned Programs may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view the therapeutic candidates within our Wholly-Owned Programs as having the requisite potential to demonstrate safety and efficacy or significant commercial opportunity, or collaborators may pursue existing or other development- stage therapeutics or alternative technologies in preference to those being developed in collaboration with us. In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process can be time consuming and complex. Further, any future collaboration agreements may restrict us from entering into additional agreements with potential collaborators. We cannot be certain that, following a strategic transaction or license, we will achieve an economic benefit that justifies such transaction. Even if we are successful in our efforts to establish such collaborations, the terms that we agree upon may not be favorable to us, and we may not be able to maintain such collaborations if, for example, development or approval of a therapeutic candidate is delayed, the safety of a therapeutic candidate is questioned or sales of an approved therapeutic candidate are unsatisfactory. Additionally, if we enter into R&D collaborations during the early phases of therapeutic development, success will in part depend on the performance of research collaborators. We will not directly control the amount or timing of resources devoted by research collaborators to activities related to therapeutic candidates. Research collaborators may not commit sufficient resources to our R&D programs. If any research collaborator fails to commit sufficient resources, the preclinical or clinical development programs related to the collaboration could be delayed or terminated. In addition, any potential future collaborations may be terminable by our strategic partners, and we may not be able to adequately protect our rights under these agreements. Furthermore, strategic partners may negotiate for certain rights to control decisions regarding the development and commercialization of the therapeutic candidates within our Wholly- Owned Programs , if approved, and may not conduct those activities in the same manner as we do. Any termination of collaborations we enter into in the future, or any delay in entering into collaborations related to the therapeutic candidates within our Wholly-Owned Programs , could delay the development and commercialization of the therapeutic candidates within our Wholly-Owned Programs and reduce their competitiveness if they reach the market, which could have a material adverse effect on our business, financial condition and results of operations. We anticipate relying upon strategic collaborations for marketing and commercializing our existing therapeutic candidates, and we may rely even more on strategic collaborations for R&D of other therapeutic candidates or discoveries. We may sell therapeutic offerings through strategic partnerships with pharmaceutical and biotechnology companies. If we are unable to establish or manage such strategic collaborations on terms favorable to us in the future, our R&D efforts and potential to generate revenue may be limited. If we fail to make required milestone or royalty payments to collaborators or to observe other obligations in agreements with them, the collaborators may have the right to terminate or stop performance of those agreements.

204 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n The therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates may compete with other therapeutic candidates and marketed therapeutics for access to manufacturing facilities. Any performance failure on the part of our or our Founded Entities’ existing or future manufacturers could delay clinical development, marketing approval, certification or commercialization. Our and certain of our Founded Entities’ current and anticipated future dependence upon others for the manufacturing of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates may adversely affect our future profit margins and our ability to commercialize any therapeutic candidates that receive marketing clearance or approval on a timely and competitive basis. If the contract manufacturing facilities on which we and certain of our Founded Entities’ rely do not continue to meet regulatory requirements or are unable to meet our or our Founded Entities’ supply demands, our business will be harmed. All entities involved in the preparation of therapeutic candidates for clinical trials or commercial sale, including our and certain of our Founded Entities’ existing CMOs for the therapeutic candidates within our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates, are subject to extensive regulation. Components of a finished drug or biologic therapeutic approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with cGMP, or similar regulatory requirements outside the United States. These regulations govern manufacturing processes and procedures, including recordkeeping, and the implementation and operation of quality systems to control and assure the quality of investigational therapeutics and therapeutics approved for sale. Similarly, medical devices must be manufactured in accordance with QSR and similar foreign requirements. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of Karuna’s Cobenfy, Gelesis’ Plenity, Akili’s EndeavorRx, our Founded Entities’ other therapeutic candidates or the therapeutic candidates within our Wholly-Owned Programs . Our or our Founded Entities’ failure, or the failure of third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us or our Founded Entities, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals or certification, license revocation, suspension of production, seizures or recalls of therapeutic candidates or marketed drugs or devices, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect clinical or commercial supplies of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. We and/or our CMOs must supply all necessary documentation, as applicable, in support of a marketing application, such as an NDA, BLA, PMA or MAA, on a timely basis and must adhere to regulations enforced by the FDA and other regulatory agencies through their facilities inspection program. Some of our CMOs have never produced a commercially approved pharmaceutical therapeutic and therefore have not obtained the requisite regulatory authority approvals to do so. The facilities and quality systems of some or all of our third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or any of our other potential therapeutics. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or our other potential therapeutics or the associated quality systems for compliance with the regulations applicable to the activities being conducted. Although we oversee the CMOs, we cannot control the manufacturing process of, and are completely dependent on, our CMO partners for compliance with the regulatory requirements. If these facilities do not pass a pre-approval plant inspection, regulatory approval of the therapeutics may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever. Our or our Founded Entities’ use of third parties to manufacture the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and other therapeutic candidates that we or our Founded Entities may develop for preclinical studies and clinical trials may increase the risk that we or our Founded Entities will not have sufficient quantities of our or our Founded Entities’ therapeutic candidates, therapeutics, or necessary quantities of such materials on time or at an acceptable cost. With respect to certain of the therapeutic candidates within our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates, we and certain of our Founded Entities do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture drug supplies for our ongoing clinical trials or any future clinical trials that we or our Founded Entities may conduct, and we and our Founded Entities lack the resources to manufacture any therapeutic candidates on a commercial scale. We rely, and expect to continue to rely, on third-party manufacturers to produce our and certain of our Founded Entities’ therapeutic candidates or other therapeutic candidates that we or our Founded Entities may identify for clinical trials, as well as for commercial manufacture if any therapeutic candidates receive marketing authorization. Any significant delay or discontinuity in the supply of a therapeutic candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to replace a third-party manufacturer could considerably delay the clinical development and potential regulatory authorization of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, which could harm our business and results of operations. We or our Founded Entities may be unable to identify and appropriately qualify third-party manufacturers or establish agreements with third-party manufacturers or do so on acceptable terms. Even if we or our Founded Entities are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including: — reliance on the third party for sourcing of raw materials, components, and such other goods as may be required for execution of its manufacturing processes and the oversight by the third party of its suppliers; — reliance on the third party for regulatory compliance and quality assurance for the manufacturing activities each performs; — the possible breach of the manufacturing agreement by the third party; — the possible misappropriation of proprietary information, including trade secrets and know-how; and — the possible termination or non-renewal of the agreement by the third party at a time that is costly or inconvenient for us or our Founded Entities. Furthermore, all of our CMOs are engaged with other companies to supply and/or manufacture materials or therapeutics for such companies, which exposes our manufacturers to regulatory risks for the production of such materials and therapeutics. The facilities used by our contract manufacturers to manufacture our drug, or medical device therapeutic candidates are subject to review by the FDA pursuant to inspections that will be conducted after we submit an NDA, BLA, PMA application or other marketing application to the FDA. We do not control the manufacturing process of, and are to some extent dependent on, our contract manufacturing partners for compliance with the regulatory requirements, known as cGMP requirements for manufacture of drug, biologic and device therapeutics. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, we will not be able to secure or maintain regulatory authorization for the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates manufactured at these manufacturing facilities. We are subject to similar requirements in foreign jurisdictions. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or another comparable foreign regulatory agency does not approve these facilities for the manufacture of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or if any agency withdraws its approval in the future, we or our Founded Entities may need to find alternative manufacturing facilities, which would negatively impact our or our Founded Entities’ ability to develop, obtain regulatory authorization or certification for or market the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, if cleared, certified or approved.

PureTech Health plc Annual Report and Accounts 2024 205 Risk Factor Annex continued A d d itio nal info rm atio n The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal, technological and factual questions and has in recent years been the subject of much litigation. The standards that the U.S. Patent and Trademark Office, or the USPTO, and its foreign counterparts use to grant patents are not always applied predictably or uniformly. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States, or vice versa. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can prevent a patent from issuing from a pending application or later invalidate or narrow the scope of an issued patent. For example, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued that protect our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, in whole or in part, or which effectively prevent others from commercializing competitive therapeutic candidates. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative therapeutic candidates in a non-infringing manner. In addition, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical therapeutic candidates to ours, or limit the duration of the patent protection of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. For example, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, re-examination, inter partes review, post-grant review or interference proceedings challenging our owned or licensed patent rights. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future therapeutic candidates. Furthermore, our and our Founded Entities’ intellectual property rights may be subject to a reservation of rights by one or more third parties. We are party to a license agreement with New York University related to certain intellectual property underlying our LYT-200 therapeutic candidate, which is subject to certain rights of the government, including march-in rights, to such intellectual property due to the fact that the research was funded at least in part by the U.S. government. We are also party to other license agreements for intellectual property underlying certain of our therapeutic candidates and programs. Additionally, our Founded Entities Akili, Follica, Vedanta, Sonde and Vor, are party to license agreements with academic institutions pursuant to which such Founded Entities have in-licensed certain intellectual property underlying various of their therapeutic candidates. While these license agreements are exclusive, they contain provisions pursuant to which the government has certain rights, including march-in rights, to such patents and technologies due to the fact that the research was funded at least in part by the U.S. government. When new technologies are developed with government funding, the government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the government to use the invention or to have others use the invention on its behalf. These rights may permit the government to disclose our information to third parties and to exercise march-in rights to use or allow third parties to use our technology. The regulatory authorities or notified bodies (when applicable) also may, at any time following clearance, certification or approval of a therapeutic for sale, audit the manufacturing facilities of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our therapeutic specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us or a third party to implement, and that may include the temporary or permanent suspension of a clinical study or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business. Additionally, if supply from one approved manufacturer is interrupted, an alternative manufacturer would need to be qualified. For drug and biologic therapeutics, as applicable, an NDA, BLA supplement or MAA variation, or equivalent foreign regulatory filing, is also required, which could result in further delay. Similarly, for medical devices, a new marketing application or supplement may be required. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines. These factors could cause us or our Founded Entities to incur higher costs and could cause the delay or termination of clinical trials, regulatory submissions, required approvals, or commercialization of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Furthermore, if our or our Founded Entities’ suppliers fail to meet contractual requirements and we or our Founded Entities are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our or our Founded Entities’ clinical trials may be delayed or we or our Founded Entities could lose potential revenue. Risks Related to Our Intellectual Property Risks Related to Our Intellectual Property Protection If we or our Founded Entities are unable to obtain and maintain sufficient intellectual property protection for our or our Founded Entities’ existing therapeutic candidates or any other therapeutic candidates that we or they may identify, or if the scope of the intellectual property protection we or they currently have or obtain in the future is not sufficiently broad, our competitors could develop and commercialize therapeutic candidates similar or identical to ours, and our ability to successfully commercialize our existing therapeutic candidates and any other therapeutic candidates that we or they may pursue may be impaired. As is the case with other pharmaceutical and biopharmaceutical companies, our success depends in large part on our ability to obtain and maintain protection of the intellectual property we may own solely and jointly with others, particularly patents, in the United States and other countries with respect to our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and technology. We and our Founded Entities seek to protect our proprietary position by filing patent applications in the United States and abroad related to our and our Founded Entities’ existing therapeutic candidates, our various proprietary technologies, and any other therapeutic candidates or technologies that we or they may identify. Obtaining, maintaining and enforcing pharmaceutical and biopharmaceutical patents is costly, time consuming and complex, and we may not be able to file or prosecute all necessary or desirable patent applications, or maintain, enforce or license patents that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we could fail to identify patentable aspects of our R&D output before it is too late to obtain patent protection. Although we take reasonable measures, we have systems in place to remind us of filing and prosecution deadlines, and we employ outside firms and rely on outside counsel to monitor patent deadlines, we may miss or fail to meet a patent deadline, including in a foreign country, which could negatively impact our patent rights and harm our competitive position, business, and prospects. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed to third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.

206 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n In some jurisdictions including European Union countries, compulsory licensing laws compel patent owners to grant licenses to third parties. In addition, some countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we, our Founded Entities or any of our licensors are forced to grant a license to third parties under patents relevant to our or our Founded Entities’ business, or if we, our Founded Entities or our licensors are prevented from enforcing patent rights against third parties, our competitive position may be substantially impaired in such jurisdictions. Our or our Founded Entities’ proprietary rights may not adequately protect our technologies and therapeutic candidates, and do not necessarily address all potential threats to our competitive advantage. The degree of future protection afforded by our or our Founded Entities’ intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our or our Founded Entities’ business, or permit us to maintain our competitive advantage. The following examples are illustrative: — others may be able to make therapeutics that are the same as or similar to the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates but that are not covered by the claims of the patents that we or our Founded Entities own or have exclusively licensed; — others, including inventors or developers of our or our Founded Entities’ owned or in-licensed patented technologies who may become involved with competitors, may independently develop similar technologies that function as alternatives or replacements for any of our or our Founded Entities’ technologies without infringing our intellectual property rights; — we, our Founded Entities or our licensors or our other collaboration partners might not have been the first to conceive and reduce to practice the inventions covered by the patents or patent applications that we or our Founded Entities own or license or will own or license; — we, our Founded Entities or our licensors or our other collaboration partners might not have been the first to file patent applications covering certain of the patents or patent applications that we or they own or have obtained a license, or will own or will have obtained a license; — we, our Founded Entities or our licensors may fail to meet obligations to the U.S. government with respect to in-licensed patents and patent applications funded by U.S. government grants, leading to the loss of patent rights; — it is possible that our or our Founded Entities’ pending patent applications will not result in issued patents; — it is possible that there are prior public disclosures that could invalidate our, our Founded Entities’ or our licensors’ patents; — issued patents that we or our Founded Entities own or exclusively license may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; — our or our Founded Entities’ competitors might conduct R&D activities in countries where we do not have patent rights, or in countries where R&D safe harbor laws exist, and then use the information learned from such activities to develop competitive therapeutics for sale in our major commercial markets; — ownership, validity or enforceability of our, our Founded Entities’ or our licensors’ patents or patent applications may be challenged by third parties; and — the patents of third parties or pending or future applications of third parties, if issued, may have an adverse effect on our business. Risks Related to Our License Arrangements The failure to maintain our licenses and realize their benefits may harm our business. We have acquired and in-licensed certain of our technologies from third parties. We may in the future acquire, in-license or invest in additional technology that we believe would be beneficial to our business. We are subject to a number of risks associated with our acquisition, in-license or investment in technology, including the following: — diversion of financial and managerial resources from existing operations; — failure to successfully negotiate a proposed acquisition, in-license or investment in a timely manner and at a price or on terms and conditions favorable to us; — failure to successfully combine and integrate a potential acquisition into our existing business to fully realize the benefits of such acquisition; The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, our rights in such inventions may be subject to certain requirements to manufacture therapeutics embodying such inventions in the United States. Any exercise by the government of such rights or by any third party of its reserved rights could harm our competitive position, business, financial condition, results of operations, and prospects. If our or our Founded Entities’ trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected. Our or our Founded Entities’ registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We and our Founded Entities may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we and our Founded Entities are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. We and our Founded Entities may license our trademarks and trade names to third parties, such as distributors. Though these license agreements may provide guidelines for how our or our Founded Entities’ trademarks and trade names may be used, a breach of these agreements or misuse of our trademarks and tradenames by our licensees may jeopardize our rights in or diminish the goodwill associated with our trademarks and trade names. Our or our Founded Entities’ efforts to enforce or protect our proprietary rights related to trademarks, trade names, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our competitive position, business, financial condition, results of operations and prospects. We may not be able to protect our intellectual property rights throughout the world. Filing, prosecuting and defending patents on the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect or enforce intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we and our Founded Entities may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing therapeutics made using our inventions in and into the United States or other jurisdictions. Competitors may use our and our Founded Entities’ technologies in jurisdictions where we have not obtained patent protection to develop their own therapeutics and may also export infringing therapeutics to territories where we have patent protection, but enforcement is not as strong as that in the United States. These therapeutics may compete with our or our Founded Entities’ therapeutics and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology and pharmaceutical therapeutics, which could make it difficult for us to stop the infringement of our or our Founded Entities’ patents or marketing of competing therapeutics in violation of our proprietary rights generally. Proceedings to enforce our or our Founded Entities’ patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our or our Founded Entities’ patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us or our Founded Entities. We may not prevail in any lawsuits that we or our Founded Entities initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.

PureTech Health plc Annual Report and Accounts 2024 207 Risk Factor Annex continued A d d itio nal info rm atio n from our licensors and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensors, including by agreeing to terms that could enable third parties (potentially including our competitors) to receive licenses to a portion of the intellectual property rights that are subject to our or our Founded Entities’ existing licenses. Any of these events could have a material adverse effect on our or our Founded Entities’ competitive position, business, financial conditions, results of operations, and prospects. If we or our Founded Entities fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or these agreements are terminated or we or our Founded Entities otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business. We are party to various agreements that we depend on to develop our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and various proprietary technologies, and our rights to use currently licensed intellectual property, or intellectual property to be licensed in the future, are or will be subject to the continuation of and our and our Founded Entities’ compliance with the terms of these agreements. For example, under certain of our and our Founded Entities’ license agreements we and our Founded Entities are required to use commercially reasonable efforts to develop and commercialize therapeutic candidates covered by the licensed intellectual property rights, maintain the licensed intellectual property rights, and achieve certain development milestones, each of which could result in termination in the event we or our Founded Entities fail to comply. In spite of our efforts, our or our Founded Entities’ licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our or our Founded Entities’ ability to develop and commercialize therapeutics and technology covered by these license agreements. Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including: — the scope of rights granted under the license agreement and other interpretation-related issues; — the extent to which our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; — the sublicensing of patent and other rights under our or our Founded Entities’ collaborative development relationships; — our and our Founded Entities’ diligence obligations under the license agreement and what activities satisfy those diligence obligations; — the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our and our Founded Entities’ licensors and us and our Founded Entities and our partners; and — the priority of invention of patented technology. In addition, certain provisions in our and our Founded Entities’ license agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the agreement, either of which could have a material adverse effect on our or our Founded Entities’ business, financial condition, results of operations and prospects. Moreover, if disputes over intellectual property that we or our Founded Entities have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected therapeutic candidates, which could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects. Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts. Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. However, our research, development and commercialization activities may be subject to claims that we infringe or otherwise violate patents or other intellectual property rights owned or controlled by third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical — the impact of regulatory reviews on a proposed acquisition, in-license or investment; and — the outcome of any legal proceedings that may be instituted with respect to the proposed acquisition, in-license or investment. If we fail to properly evaluate potential acquisitions, in-licenses, investments or other transactions associated with the creation of new R&D programs or the maintenance of existing ones, we might not achieve the anticipated benefits of any such transaction, we might incur costs in excess of what we anticipate, and management resources and attention might be diverted from other necessary or valuable activities. Our or our Founded Entities’ rights to develop and commercialize our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates are subject in part to the terms and conditions of licenses granted to us and our Founded Entities by others, and the patent protection, prosecution and enforcement for some of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates may be dependent on our and our Founded Entities’ licensors. We and our Founded Entities currently are reliant upon licenses of certain intellectual property rights and proprietary technologies from third parties that are important or necessary to the development of our and our Founded Entities’ proprietary technologies, including technologies related to our Wholly-Owned Programs and our Founded Entities’ therapeutic candidates. These licenses, and other licenses we and they may enter into in the future, may not provide adequate rights to use such intellectual property and proprietary technologies in all relevant fields of use or in all territories in which we or our Founded Entities may wish to develop or commercialize technology and therapeutic candidates in the future. Licenses to additional third-party proprietary technology or intellectual property rights that may be required for our or our Founded Entities’ development programs may not be available in the future or may not be available on commercially reasonable terms. In that event, we or our Founded Entities may be required to expend significant time and resources to redesign our proprietary technology or therapeutic candidates or to develop or license replacement technology, which may not be feasible on a technical or commercial basis. If we and our Founded Entities are unable to do so, we may not be able to develop and commercialize technology and therapeutic candidates in fields of use and territories for which we are not granted rights pursuant to such licenses, which could harm our competitive position, business, financial condition, results of operations and prospects significantly. In some circumstances, we and our Founded Entities may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain and enforce the patents, covering technology that we or our Founded Entities license from third parties. In addition, some of our or our Founded Entities’ agreements with our licensors require us to obtain consent from the licensor before we can enforce patent rights, and our licensor may withhold such consent or may not provide it on a timely basis. Therefore, we cannot be certain that our licensors or collaborators will prosecute, maintain, enforce and defend such intellectual property rights in a manner consistent with the best interests of our business, including by taking reasonable measures to protect the confidentiality of know-how and trade secrets, or by paying all applicable prosecution and maintenance fees related to intellectual property registrations for any of our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates and proprietary technologies. We and our Founded Entities also cannot be certain that our licensors have drafted or prosecuted the patents and patent applications licensed to us in compliance with applicable laws and regulations, which may affect the validity and enforceability of such patents or any patents that may issue from such applications. This could cause us to lose rights in any applicable intellectual property that we in-license, and as a result our ability to develop and commercialize therapeutic candidates may be adversely affected and we may be unable to prevent competitors from making, using and selling competing therapeutics. In addition, our or our Founded Entities’ licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, while we cannot currently determine the amount of the royalty obligations we would be required to pay on sales of future therapeutics, if any, the amounts may be significant. The amount of our and our Founded Entities’ future royalty obligations will depend on the technology and intellectual property we and our Founded Entities use in therapeutic candidates that we successfully develop and commercialize, if any. Therefore, even if we or our Founded Entities successfully develop and commercialize therapeutic candidates, we may be unable to achieve or maintain profitability. In addition, we or our Founded Entities may seek to obtain additional licenses

208 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Parties making claims against us or our Founded Entities may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition and prospects. Risks Related to Our Patents Patent terms may be inadequate to protect our competitive position on therapeutic candidates for an adequate amount of time. Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional or international patent application filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates are obtained, once the patent life has expired, we or our Founded Entities may be open to competition from competitive therapeutics, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new therapeutic candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our or our Founded Entities’ owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing therapeutics similar or identical to ours. If we or our Founded Entities are not able to obtain patent term extension or non-patent exclusivity in the United States under the Hatch-Waxman Act and in foreign countries under similar legislation, thereby potentially extending the marketing exclusivity term of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, our business may be materially harmed. Depending upon the timing, duration and specifics of FDA marketing approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, one or more of the U.S. patents covering each of such therapeutic candidates or the use thereof may be eligible for up to five years of patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act allows a maximum of one patent to be extended per new drug application, or NDA, for an FDA approved therapeutic as compensation for the patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of therapeutic approval and only those claims covering such approved drug therapeutic, a method for using it or a method for manufacturing it may be extended. Patent term extension also may be available in certain foreign countries upon regulatory approval of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Nevertheless, we or our Founded Entities may not be granted patent term extension either in the United States or in any foreign country because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the term of extension, as well as the scope of patent protection during any such extension, afforded by the governmental authority could be less than we request. If we or our Founded Entities are unable to obtain patent term extension or restoration, or the term of any such extension is less than our request, the period during which we will have the right to exclusively market our therapeutic may be shortened and our competitors may obtain approval of competing therapeutics following our patent expiration sooner, and our revenue could be reduced, possibly materially. Further, for certain of our and our Founded Entities’ licensed patents, we and our Founded Entities do not have the right to control prosecution, including filing with the USPTO, a petition for patent term extension under the Hatch-Waxman Act. Thus, if one of our or our Founded Entities’ licensed patents is eligible for patent term extension under the Hatch- Waxman Act, we may not be able to control whether a petition to obtain a patent term extension is filed with, or whether a patent term extension is obtained from, the USPTO. industries, including patent infringement lawsuits, interferences, derivation, oppositions, inter partes review and post-grant review before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. Our competitors in both the United States and abroad, many of which have substantially greater resources and have made substantial investments in patent portfolios and competing technologies, may have applied for or obtained or may in the future apply for or obtain, patents that will prevent, limit or otherwise interfere with our ability to make, use and sell, if approved, the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. In addition, many companies in the biotechnology and pharmaceutical industries have employed intellectual property litigation as a means to gain an advantage over their competitors. As the biotechnology and pharmaceutical industries expand and more patents are issued, and as we gain greater visibility and market exposure as a public company, the risk increases that our existing therapeutic candidates and any other therapeutic candidates that we or our Founded Entities may identify may be subject to claims of infringement of the patent rights of third parties. There may be other third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our or our Founded Entities’ existing therapeutic candidates and any other therapeutic candidates that we or they may identify. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our or our Founded Entities’ existing therapeutic candidates and any other therapeutic candidates that we or they may identify may infringe. In addition, third parties may obtain patents in the future and claim that use of our or our Founded Entities’ technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of our or our Founded Entities’ existing therapeutic candidates and any other therapeutic candidates that we or they may identify, any molecules formed during the manufacturing process, or any final therapeutic itself, the holders of any such patents may be able to block our ability to commercialize such therapeutic candidate unless we obtained a license under the applicable patents, or until such patents expire. Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Furthermore, the scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history and can involve other factors such as expert opinion. Our analysis of these issues, including interpreting the relevance or the scope of claims in a patent or a pending application, determining applicability of such claims to our proprietary technologies or therapeutic candidates, predicting whether a third party’s pending patent application will issue with claims of relevant scope, and determining the expiration date of any patent in the United States or abroad that we consider relevant may be incorrect, which may negatively impact our or our Founded Entities’ ability to develop and market the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. We do not always conduct independent reviews of pending patent applications of and patents issued to third parties. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our or our Founded Entities’ formulations, processes for manufacture or methods of use, including any combination therapies, the holders of any such patents may be able to block our or our Founded Entities’ ability to develop and commercialize the applicable therapeutic candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all, or it may be non-exclusive, which could result in our competitors gaining access to the same intellectual property. Parties making claims against us or our Founded Entities may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our or our Founded Entities’ existing therapeutic candidates and any other therapeutic candidates that we may identify. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. In the event of a successful claim of infringement against us or our Founded Entities, we or our Founded Entities may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing therapeutics or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.

PureTech Health plc Annual Report and Accounts 2024 209 Risk Factor Annex continued A d d itio nal info rm atio n The America Invents Act also includes a number of significant changes that affect the way patent applications are prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our or our Founded Entities’ owned or in-licensed patent applications and the enforcement or defense of our or our Founded Entities’ owned or in-licensed issued patents, all of which could have a material adverse effect on our competitive position, business, financial condition, results of operations, and prospects. In addition, the patent positions of companies in the development and commercialization of pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court and Federal Circuit rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future. Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements. Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We and our Founded Entities have systems in place to remind us to pay these fees, and we and our Founded Entities employ outside firms and rely on outside counsel to pay these fees due to the USPTO and non-U.S. patent agencies. However, we and our Founded Entities cannot guarantee that our licensors have similar systems and procedures in place to pay such fees. In addition, the USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non- compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business. Risks Related to Confidentiality If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed. We and our Founded Entities consider proprietary trade secrets, confidential know-how and unpatented know-how to be important to our business. We and our Founded Entities may rely on trade secrets and confidential know-how to protect our technology, especially where patent protection is believed by us to be of limited value. However, trade secrets and confidential know-how are difficult to protect, and we have limited control over the protection of trade secrets and confidential know-how used by our licensors, collaborators and suppliers. Because we have relied in the past on third parties to manufacture the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, because we may continue to do so in the future, and because we expect to collaborate with third parties on the development of our current therapeutic candidates and any future therapeutic candidates we develop, we may, at times, share trade secrets with them. We also conduct joint R&D programs that may require us to share trade secrets under the terms of our R&D partnerships or similar agreements. Under such circumstances, trade secrets and confidential know-how can be difficult to maintain as confidential. Also, there are detailed rules and requirements regarding the patents that may be submitted to the FDA for listing in the Approved Drug Products with Therapeutic Equivalence Evaluations, or the Orange Book. We or our Founded Entities may be unable to obtain patents covering the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates that contain one or more claims that satisfy the requirements for listing in the Orange Book. Even if we or our Founded Entities submit a patent for listing in the Orange Book, the FDA may decline to list the patent, or a manufacturer of generic drugs may challenge the listing. If or when one of the therapeutic candidates within our Wholly- Owned Programs or our Founded Entities’ therapeutic candidates is approved and a patent covering that therapeutic candidate is not listed in the Orange Book, a manufacturer of generic drugs would not have to provide advance notice to us of any abbreviated new drug application, or ANDA, filed with the FDA to obtain permission to sell a generic version of such therapeutic candidate. Issued patents covering our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates could be found invalid or unenforceable if challenged in courts or patent offices. If we, our Founded Entities or one of our licensing partners initiated legal proceedings against a third party to enforce a patent covering one or more of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, the defendant could counterclaim that the patent covering the relevant therapeutic candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including subject matter eligibility, novelty, nonobviousness, written description or enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to our or our Founded Entities’ patents in such a way that they no longer cover our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Such a loss of patent protection could have a material adverse impact on our business. Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our and our Founded Entities’ ability to protect our therapeutics. Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to a patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us and our Founded Entities to be cognizant of the time from invention to filing of a patent application and be diligent in filing patent applications, but circumstances could prevent us from promptly filing patent applications on our inventions. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we, our Founded Entities or our licensors were the first to either (i) file any patent application related to our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or (ii) invent any of the inventions claimed in our, our Founded Entities or our licensor’s patents or patent applications.

210 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Risks Related to Challenges or Lawsuits Related to Intellectual Property We may become involved in lawsuits to protect or enforce our or our Founded Entities’ patents or other intellectual property, which could be expensive, time consuming and unsuccessful. Competitors may infringe our or our Founded Entities’ patents or other intellectual property. Our and our Founded Entities’ ability to enforce our patent or other intellectual property rights depends on our ability to detect infringement. It may be difficult to detect infringers who do not advertise the components or methods that are used in connection with their therapeutics and services. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s therapeutic or service. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded if we were to prevail may not be commercially meaningful. If we were to initiate legal proceedings against a third party to enforce a patent covering one or more of our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates, the defendant could counterclaim that the patent covering our or our Founded Entities’ therapeutic candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including subject matter eligibility, novelty, nonobviousness, written description or enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our or our Founded Entities’ patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same technology. Our defense of litigation or interference or derivation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue clinical trials, continue research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring therapeutic candidates to market. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our or our Founded Entities’ confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could adversely impact the price of our ADSs. Furthermore, any of the foregoing could have a material adverse effect on our financial condition, results of operations, and prospects. We and our Founded Entities may be subject to claims challenging the inventorship of our patents and other intellectual property. Our and our Founded Entities’ agreements with employees and our personnel policies provide that any inventions conceived by an individual in the course of rendering services to us shall be our exclusive property. Although our policy is to have all such individuals complete these agreements, we may not obtain these agreements in all circumstances, and individuals with whom we have these agreements may not comply with their terms. The assignment of intellectual property may not be automatic upon the creation of an invention and despite such agreement, such inventions may become assigned to third parties. In the event of unauthorized use or disclosure of our trade secrets or proprietary information, these agreements, even if obtained, may not provide meaningful protection, particularly for our trade secrets or other confidential information. We, our Founded Entities or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we, our Founded Entities or our licensors may have inventorship disputes arising from conflicting obligations of employees, consultants or others who are involved in developing our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Litigation may be necessary to defend against these and other claims challenging inventorship of our, our We and our Founded Entities seek to protect our confidential proprietary information, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and collaborators. These agreements are designed to protect our proprietary information. However, we cannot be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our and our Founded Entities’ trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose proprietary information, including trade secrets, and we may not be able to obtain adequate remedies for such breaches. We and our Founded Entities also seek to preserve the integrity and confidentiality of our confidential proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. If any of our or our Founded Entities’ confidential proprietary information were to be lawfully obtained or independently developed by a competitor, we or our Founded Entities would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our or our Founded Entities’ therapeutics that we consider proprietary. We or our Founded Entities may not be able to obtain adequate remedies in the event of such unauthorized use. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. Trade secrets will also over time be disseminated within the industry through independent development, the publication of journal articles and the movement of personnel skilled in the art from company to company or academic to industry scientific positions. Though our or our Founded Entities’ agreements with third parties typically restrict the ability of our advisors, employees, collaborators, licensors, suppliers, third-party contractors and consultants to publish data potentially relating to our trade secrets, our agreements may contain certain limited publication rights. In addition, if any of our or our Founded Entities’ trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. Despite employing the contractual and other security precautions described above, the need to share trade secrets increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. If any of these events occurs or if we otherwise lose protection for our trade secrets, the value of such information may be greatly reduced and our competitive position, business, financial condition, results of operations, and prospects would be harmed. We or our Founded Entities may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers. As is common in the biotechnology and pharmaceutical industries, we and our Founded Entities employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we and our Founded Entities try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know- how of others in their work for us, we or our Founded Entities may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If we or our Founded Entities fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we or our Founded Entities are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.

PureTech Health plc Annual Report and Accounts 2024 211 Risk Factor Annex continued A d d itio nal info rm atio n or other key employees could impede the achievement of research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval for and commercialize the therapeutic candidates within our Wholly-Owned Programs . Competition to hire qualified personnel in our industry is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. Furthermore, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited. We will need to expand our organization and we may experience difficulties in managing this growth, which could disrupt our operations. As we mature, we expect to expand our full-time employee base and to hire more consultants and contractors. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time toward managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional therapeutic candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize therapeutic candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth. Because we are developing multiple programs and therapeutic candidates and are pursuing a variety of target indications and treatment modalities, we may expend our limited resources to pursue a particular therapeutic candidate and fail to capitalize on development opportunities or therapeutic candidates that may be more profitable or for which there is a greater likelihood of success. Because we have limited financial and personnel resources, we may forgo or delay pursuit of opportunities with potential target indications or therapeutic candidates that later prove to have greater commercial potential than our current and planned development programs and therapeutic candidates. Our resource allocation decisions may cause us to fail to capitalize on viable commercial therapeutics or profitable market opportunities. Our spending on current and future research and development programs and other future therapeutic candidates for specific indications may not yield any commercially viable future therapeutic candidates. If we do not accurately evaluate the commercial potential or target market for a particular therapeutic candidate, we may be required to relinquish valuable rights to that therapeutic candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such future therapeutic candidates. Additionally, we may pursue additional in-licenses or acquisitions of development-stage assets or programs, which entails additional risk to us. Identifying, selecting and acquiring promising therapeutic candidates requires substantial technical, financial and human resources expertise. Efforts to do so may not result in the actual acquisition or license of a successful therapeutic candidate, potentially resulting in a diversion of our management’s time and the expenditure of our resources with no resulting benefit. For example, if we are unable to identify programs that ultimately result in approved therapeutics, we may spend material amounts of our capital and other resources evaluating, acquiring and developing therapeutics that ultimately do not provide a return on our investment. Founded Entities’ or our licensors’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we, our Founded Entities or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our competitive position, business, financial condition, results of operations and prospects. Risks Related to Our Business and Industry We attempt to distribute our scientific, execution and financing risks across a variety of therapeutic areas, indications, programs and modalities that are driven by our proven innovation and drug development strategy. However, our assessment of, and approach to, risk may not be comprehensive or effectively avoid delays or failures in one or more of our programs. Failures in one or more of our programs could adversely impact other programs and have a material adverse impact on our business, results of operations and ability to fund our business. While we aim to distribute our scientific, execution and financing risks across programs, there may be foreseen and unforeseen risks across the therapeutic candidates within our Wholly-Owned Programs and programs being developed by our Founded Entities in whole or in part. In addition, if any one or more of our clinical programs encounter safety, tolerability, or efficacy problems, developmental delays, regulatory issues, or other problems, our business could be significantly harmed. As our and certain of our Founded Entities’ therapeutic candidates progress through clinical development, we or others may determine that certain of our risk allocation decisions were incorrect or insufficient, that individual programs or our science in general has technology or biology risks that were unknown or underappreciated, or that we have allocated resources across our programs in such a way that did not maximize potential value creation. All of these risks may relate to our current and future programs sharing similar science and infrastructure, and in the event material decisions in any of these areas turn out to have been incorrect or under-optimized, we may experience a material adverse impact on our business and ability to fund our operations. Our business is highly dependent on the clinical advancement of our programs and our success in identifying potential therapeutic candidates. Delay or failure to advance our programs could adversely impact our business. Over time, our and our Founded Entities’ preclinical and clinical work led us to identify potential synergies across target therapeutic indications, generating a broad portfolio of therapeutic candidates across multiple programs. Even if a particular program is successful in any phase of development, such program could fail at a later phase of development, and other programs within the same therapeutic area may still fail at any phase of development including at phases where earlier programs in that therapeutic area were successful. This may be a result of technical challenges unique to that program or due to biology risk, which is unique to every program. As we progress our programs through clinical development, there may be new technical challenges that arise that cause an entire program or a group of programs within an area of focus to fail. Our future success depends on our ability to retain key employees, directors, consultants and advisors and to attract, retain and motivate qualified personnel. Our ability to compete in the highly competitive biotechnology industry depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. We are highly dependent on the management, R&D, clinical, financial and business development expertise of our executive officers, our directors, as well as the other members of our scientific and clinical teams, including Bharatt Chowrira, our chief executive officer, and Eric Elenko, our President. The loss of the services of any of our executive officers and other key personnel, and our inability to find suitable replacements could result in delays in therapeutic development and our financial condition and results of operations could be materially adversely affected. Furthermore, each of our executive officers may terminate their employment with us at any time. Recruiting and retaining qualified scientific and clinical personnel and, if we progress the development of the therapeutic candidates within our Wholly-Owned Programs toward scaling up for commercialization, sales and marketing personnel, will also be critical to our success. The loss of the services of our executive officers

212 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions or incur other harm to our business. Our and our Founded Entities’ employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. We are exposed to the risk of fraud, misconduct or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors as well as the employees, independent contractors, consultants, commercial partners and vendors of our Founded Entities. Misconduct by these parties could include intentional, reckless and negligent conduct that fails to: comply with the laws of the FDA and comparable foreign regulatory authorities; provide true, complete and accurate information to the FDA and comparable foreign regulatory authorities; comply with manufacturing standards we have established; comply with healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws; or report financial information or data accurately or to disclose unauthorized activities. If we or our Founded Entities obtain FDA or comparable foreign regulatory authorities approval, or notified bodies certification, of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and begin commercializing those therapeutics in the United States and abroad, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. In particular, research, sales, marketing, education and other business arrangements in the healthcare industry are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, educating, marketing and promotion, sales and commission, certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions. Employee litigation and unfavorable publicity could negatively affect our future business. Our employees may, from time to time, bring lawsuits against us regarding injury, creating a hostile work place, discrimination, wage and hour disputes, sexual harassment, or other employment issues. In recent years, there has been an increase in the number of discrimination and harassment claims generally. Coupled with the expansion of social media platforms and similar devices that allow individuals access to a broad audience, these claims have had a significant negative impact on some businesses. Certain companies that have faced employment- or harassment-related lawsuits have had to terminate management or other key personnel, and have suffered reputational harm that has negatively impacted their business. If we were to face any employment-related claims, our business could be negatively affected. If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business. We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste therapeutics. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations. Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any therapeutic candidates that we may develop. We face an inherent risk of product liability exposure related to the testing of therapeutic candidates in human clinical trials and will face an even greater risk if we commercially sell any therapeutics that we may develop. If we cannot successfully defend ourselves against claims that the therapeutic candidates within our Wholly-Owned Programs or medicines caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in: — decreased demand for any therapeutic candidates or medicines that we may develop; — injury to our reputation and significant negative media attention; — withdrawal of clinical trial participants; — significant costs to defend the related litigation; — substantial monetary awards to trial participants or patients; — loss of revenue; and — the inability to commercialize the therapeutic candidates within our Wholly-Owned Programs . Although we maintain product liability insurance, including coverage for clinical trials that we sponsor, it may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage as we commence additional clinical trials and if we successfully commercialize any therapeutic candidates. The market for insurance coverage is increasingly expensive, and the costs of insurance coverage will increase as our clinical programs increase in size. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. Litigation against us could be costly and time-consuming to defend and could result in additional liabilities. In March 2024, a complaint was filed against us by a third-party alleging breach of contract with respect to certain payments alleged to be owed to such third party by us. We intend to defend ourselves vigorously though the ultimate outcome of this matter and the timing for resolution remains uncertain. We may from time to time be subject to additional legal proceedings and claims that arise in the ordinary course of business or otherwise, such as claims brought by third parties in connection with commercial disputes and employment claims made by our current or former employees. Claims may also be asserted by or on behalf of a variety of other parties, including government agencies, patients, or stockholders. We could also be subject to securities class action litigation. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business. Any litigation involving us may result in substantial costs, operationally restrict our business, and may divert management’s attention and resources, which may seriously harm our business, overall financial condition, and results of operations. Insurance may not cover existing or future claims, be sufficient to fully compensate us for one or more of such claims, or continue to be available on terms acceptable to us. A claim brought against us that is uninsured or underinsured could result in unanticipated costs, thereby adversely impacting our results of operations. The increasing use of social media platforms presents new risks and challenges. Social media is increasingly being used to communicate about our and our Founded Entities’ clinical development programs and the diseases our therapeutics are being developed to treat, and we intend to utilize appropriate social media in connection with our commercialization efforts following approval of the therapeutic candidates within our Wholly-Owned Programs . Social media practices in the biopharmaceutical industry continue to evolve and regulations relating to such use are not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media channels to comment on their experience in an ongoing blinded clinical study or to report an alleged adverse event. When such disclosures occur, there is a risk that we fail to monitor and comply with applicable adverse event reporting obligations or we may not be able to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about the therapeutic candidates within our Wholly-Owned Programs . There is also a risk of inappropriate

PureTech Health plc Annual Report and Accounts 2024 213 Risk Factor Annex continued A d d itio nal info rm atio n Focus on environmental sustainability and social initiatives could increase our costs, harm our reputation and adversely impact our financial results. There has been public focus by investors, patients, environmental activists, the media, governmental and nongovernmental organizations and other stakeholders on a variety of environmental, social and other sustainability matters. We may experience pressure to make commitments relating to sustainability matters that affect us, including the design and implementation of specific risk mitigation strategic initiatives relating to sustainability. Expectations regarding the management of environmental, social and governance (“ESG”) initiatives continue to evolve. While we may from time to time engage in various initiatives (including but not limited to voluntary disclosures, policies or goals) to improve our ESG profile or respond to stakeholder expectations, we cannot guarantee that these initiatives will have the desired effect. If we do not, or are not perceived to, adequately address ESG matters affecting our business or set and meet relevant sustainability goals, our reputation and financial results may suffer. In addition, even if we are effective at addressing such concerns, we may experience increased costs as a result of executing upon our sustainability goals that may not be offset by any benefit to our reputation, which could have an adverse impact on our business and financial condition. In addition, this emphasis on environmental, social and other sustainability matters has resulted and may result in the adoption of new laws, rules and regulations, including new reporting requirements. If we fail to comply with such laws, rules, regulations or reporting requirements, our reputation and business could be adversely impacted. We may acquire businesses, or therapeutics or therapeutic candidates, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions. We acquire or in-license businesses or therapeutics from other companies or create joint ventures with third parties that we believe will complement or augment our existing business. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture or retain key personnel from the acquired company. We may encounter numerous difficulties in developing, manufacturing and marketing any new therapeutics or therapeutic candidates resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition or license, we will achieve the expected synergies to justify the transaction. Failure to successfully identify, complete, manage and integrate acquisitions could materially and adversely affect our business, financial condition and results of operations and could cause the price of our securities to decline. Changes in funding or staffing for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new therapeutics and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal functions on which the operation of our business may rely, which could negatively impact our business. The ability of the FDA, foreign regulatory authorities and notified bodies to review and approve or certify new therapeutics or take action with respect to other regulatory matters can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept payment of user fees, and statutory, regulatory, and policy changes. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. The priorities of the FDA and foreign regulatory authorities may also influence the ability of the FDA and foreign regulatory authorities to take action on regulatory matters, for example the FDA’s and foreign regulatory authorities’ budget and funding levels and ability to hire and retain key personnel. Disruptions at the FDA and foreign regulatory authorities may also slow the time necessary for new drugs to be reviewed and/or approved, or for other actions to be taken, by relevant government agencies, which would adversely affect our business. For example, in recent years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Similarly, a prolonged government shutdown could prevent the timely review of our patent applications by the USPTO, which could delay the issuance of any U.S. patents to which we might otherwise be entitled. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or therapeutic efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions. Cyberattacks or other failures in our telecommunications or information technology systems, or those of our collaborators, contract research organizations, third-party logistics providers, distributors or other contractors or consultants, could result in information theft, data corruption and significant disruption of our business operations. We collect and maintain information in digital form that is necessary to conduct our business, and we are increasingly dependent on information technology, or IT, systems and infrastructure to operate our business. In the ordinary course of our business, we collect, store, and transmit large amounts of confidential information, including intellectual property, proprietary business information, clinical trial data, and personal information (collectively, “Confidential Information”) of clinical trial participants, employees, and contractors. It is critical that we do so in a secure manner to maintain the confidentiality and integrity of such Confidential Information. As use of digital technologies has increased, cyber incidents, including third parties gaining access to employee accounts using stolen or inferred credentials, computer malware (e.g., ransomware), viruses, misconfigurations, “bugs” or other vulnerabilities, malicious code spamming, phishing attacks or other means, and deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our, our collaborators’, our CROs’, third-party logistics providers’, distributors’ and other contractors’ and consultants’ systems and networks, and the confidentiality, availability and integrity of our data. There can be no assurance that we will be successful in preventing cyberattacks or successfully mitigating their effects. Similarly, there can be no assurance that our collaborators, CROs, third-party logistics providers, distributors and other contractors and consultants will be successful in protecting our clinical and other data that is stored on their systems. We and certain of our service providers are from time to time subject to cyberattacks and security incident. Although to our knowledge we have not experienced any significant system failure, accident or material security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of development programs and business operations. Any cyberattack, data breach or destruction or loss of data could result in a violation of applicable U.S. and international privacy, data protection and other laws, and subject us to litigation and governmental investigations and proceedings by federal, state and local regulatory entities in the United States and by international regulatory entities, resulting in exposure to material civil and/or criminal liability. A security incident could also expose us to risks and could cause management distraction and the obligation to devote significant financial and other resources to mitigate such problems, which would increase our future information security costs, including through organizational changes, deploying additional personnel, reinforcing administrative, physical and technical safeguards, further training of employees, changing third-party vendor control practices, and engaging third-party subject matter experts and consultants and reduce the demand for our technology and services. Any security compromise affecting us, our collaborators, CROs, third-party logistics providers, distributors, and other contractors and consultants, or our industry, whether real or perceived, could harm our reputation, erode confidence in the effectiveness of our security measures and lead to regulatory scrutiny. Further, our general liability insurance and corporate risk program may not cover all potential claims to which we are exposed and may not be adequate to indemnify us for all liability that maybe imposed; and could have a material adverse effect on our business and prospects. For example, the loss of clinical trial data from completed or ongoing clinical trials for any of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates could result in delays in our development and regulatory approval efforts and significantly increase our costs to recover or reproduce the data.

214 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n — differing and changing regulatory requirements; — difficulties in compliance with different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations; — changes in a specific country’s or region’s political or economic environment, including, but not limited to, the implications of one or more of the following occurring the decision of the United Kingdom: — future activities subject to the terms of the Trade and Cooperation Agreement between the United Kingdom and the European Union effective May 1, 2021, which has not impacted our results to-date; — a second referendum on Scottish independence from the United Kingdom; and/or — a snap general election; and — negative consequences from changes in tax laws. In addition, our business strategy incorporates potential international expansion to target patient populations outside the United States. If we or our Founded Entities receive regulatory approval for and commercialize any of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates in patient populations outside the United States, we may hire sales representatives and conduct physician and patient association outreach activities outside of the United States. Doing business internationally involves a number of risks, including, but not limited to: — multiple, conflicting, and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements, and other governmental approvals, permits, and licenses; — failure by us to obtain and maintain regulatory approvals for the use of our therapeutics in various countries; — additional potentially relevant third-party patent rights; — complexities and difficulties in obtaining protection and enforcing our intellectual property; — difficulties in staffing and managing foreign operations; — complexities associated with managing multiple payor reimbursement regimes, government payors, or patient self-pay systems; — limits in our ability to penetrate international markets; — financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our therapeutics, and exposure to foreign currency exchange rate fluctuations; — natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; — certain expenses including, among others, expenses for travel, translation, and insurance; and — regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, its books and records provisions, or its anti-bribery provisions. Any of these factors could significantly harm our potential international expansion and operations and, consequently, our results of operations. Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations. Our ability to invest in and expand our business and meet our financial obligations, to attract and retain third-party contractors and collaboration partners and to raise additional capital depends on our operating and financial performance, which, in turn, is subject to numerous factors, including the prevailing economic and political conditions and financial, business and other factors beyond our control, such as the rate of unemployment, the number of uninsured persons in the United States, political influences and inflationary pressures. For example, an overall decrease in or loss of insurance coverage among individuals in the United States as a result of unemployment, underemployment or the repeal of certain provisions of the ACA, may decrease the demand for healthcare services and pharmaceuticals. If fewer patients are seeking medical care because they do not have insurance coverage, we and our Founded Entities may experience difficulties in any eventual commercialization of the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and our business, results of operations, financial condition and cash flows could be adversely affected. In addition, our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets upon which pharmaceutical and biopharmaceutical companies such as us are dependent for sources of capital. The global economy, including credit and financial markets, has recently experienced extreme volatility and disruptions, including severely diminished liquidity and credit Separately, in response to the global COVID-19 pandemic, the FDA postponed most inspections of domestic and foreign manufacturing facilities at various points. If a prolonged government shutdown, shortages In funding or staffing or other disruption occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Future shutdowns or other disruptions could also affect other government agencies such as the SEC, which may also impact our business by delaying review of our public filings, to the extent such review is necessary, and our ability to access the public markets. Furthermore, in the EU, notified bodies must be officially designated to certify products and services in accordance with the EU Medical Devices Regulation. Their designation process, which is significantly stricter under the new Regulation, has experienced considerable delays due to the COVID-19 pandemic . Despite a recent increase in designations, the current number of notified bodies designated under the new Regulation remains significantly lower than the number of notified bodies designated under the previous regime. The current designated notified bodies are therefore facing a backlog of requests as a consequence of which review times have lengthened. This situation may impact the way we are conducting our business in the EU and the EEA and the ability of our notified body to timely review and process our regulatory submissions and perform its audits. We or the third parties upon whom we depend may be adversely affected by a natural disaster and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster. Natural disasters could severely disrupt our operations, and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as the manufacturing facilities of our third-party CMOs, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place currently are limited and are unlikely to prove adequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which, could have a material adverse effect on our business, financial condition, results of operations and prospects. We will continue to incur increased costs as a result of operating as a U.S.- listed public company, and our management will be required to devote substantial time to new compliance initiatives. As a U.S. public company, and particularly now that we are no longer an emerging growth company, we have incurred and will continue to incur significant legal, accounting and other expenses that we did not incur as a public company listed only on the LSE. In addition, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and rules subsequently implemented by the SEC and Nasdaq have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel continue to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance. We continue to evaluate these rules and regulations and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Risks Related to Our International Operations Our international operations may expose us to business, regulatory, political, operational, financial, pricing and reimbursement and economic risks associated with doing business outside of the United States. As a company based in the United Kingdom, our business is subject to risks associated with being organized outside of the United States. While the majority of our operations are in the United States and our functional currency is the U.S. dollar, our future results could be harmed by a variety of international factors, including: — economic weakness, including rising inflation and interest rates, or political instability in particular non-U.S. economies and markets;

PureTech Health plc Annual Report and Accounts 2024 215 Risk Factor Annex continued A d d itio nal info rm atio n The United Kingdom’s withdrawal from the European Union may have a negative effect on our business. Since the end of the Brexit transition period on January 1, 2021, and the implementation of the Windsor Framework on January 1, 2025, the UK has not been directly subject to EU laws with respect to medicinal products As a result of the Northern Ireland Protocol, different rules applied in Northern Ireland than in Great Britain; broadly, Northern Ireland continued to follow the EU regulatory regime. However, on January 1, 2025, a new arrangement called the “Windsor Agreement” came into effect and reintegrated Northern Ireland under the regulatory authority of the MHRA with respect to medicinal products. The Windsor Framework removes EU licensing processes, and EU labelling and serialization requirements in relation to Northern Ireland, and introduces a UK-wide licensing process for medicinal products. There could be additional uncertainty and risk around what these changes mean to our business. It is currently unclear to what extent the UK Government will seek to align its regulations with the EU. The EU laws that have been transposed into UK law through secondary legislation remain applicable in Great Britain, but new legislation such as the (EU) CTR is not applicable in Great Britain. Whilst the EU-UK Trade and Cooperation Agreement, or TCA, includes the mutual recognition of Good Manufacturing Practice, or GMP, inspections of manufacturing facilities for medicinal products and GMP documents issued, it does not contain wholesale mutual recognition of UK and EU pharmaceutical regulations and product standards. There may be divergent local requirements in Great Britain from the EU in the future, which may impact clinical and development activities that occur in the UK in the future. Similarly, clinical trial submissions in the UK cannot be bundled with those of EU member states within the EMA Clinical Trial Information System, or CTIS, adding further complexity, cost and potential risk to future clinical and development activity in the UK. Exchange rate fluctuations may materially affect our results of operations and financial condition. Although we are based in the United Kingdom, our financial statements are denominated in U.S dollars and many of our business activities are carried out with partners outside the U.S. and United Kingdom and these transactions may be denominated in another currency. As a result, our business and the price of our ADSs may be affected by fluctuations in foreign exchange rates not only between the pound sterling and the U.S. dollar, but also the currencies of other countries, which may have a significant impact on our results of operations and cash flows from period to period. Currently, we do not have any exchange rate hedging arrangements in place. Risks Related to Our Equity Securities and ADSs The market price of our ADSs has been and will likely continue to be highly volatile, and you could lose all or part of your investment. The market price of our ADSs has been and will likely continue to be volatile. The stock market in general, and the market for biopharmaceutical companies in particular, has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your ADSs at or above the purchase price. The market price for our ADSs may be influenced by many factors, including: — adverse results or delays in our preclinical studies or clinical trials; — reports of AEs or other negative results in clinical trials of third parties’ therapeutic candidates that target the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates’ target indications; — an inability for us to obtain additional funding on reasonable terms or at all; — any delay in submitting an IND, BLA or NDA for the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates and any adverse development or perceived adverse development with respect to the FDA’s review of that IND, BLA or NDA; — failure to develop successfully and commercialize the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates; — announcements we make regarding our current therapeutic candidates, acquisition of potential new therapeutic candidates and companies and/ or in-licensing; — failure to maintain our or our Founded Entities’ existing license arrangements or enter into new licensing and collaboration agreements; — failure by us, our Founded Entities or our licensors to prosecute, maintain or enforce our intellectual property rights; — changes in laws or regulations applicable to future therapeutics; availability, fluctuating interest and inflation rates, tariffs and trade wars, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. A severe or prolonged economic downturn could result in a variety of risks to our business, including a reduced ability to raise additional capital when needed on acceptable terms, if at all, and weakened demand for the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates. A weak or declining economy could also strain our suppliers, possibly resulting in supply disruption. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business. Additionally, we maintain the majority of our cash and cash equivalents in accounts with major U.S. and multi-national financial institutions, and our deposits at certain of these institutions exceed insured limits. Market conditions can impact the viability of these institutions. In the event of failure of any of the financial institutions where we maintain our cash and cash equivalents, there can be no assurance that we would be able to access uninsured funds in a timely manner or at all. Any inability to access or delay in accessing these funds could adversely affect our business and financial position. We are subject to the U.K. Bribery Act 2010, or the Bribery Act, the U.S. Foreign Corrupt Practices Act of 1977 (as amended) (“FCPA”) and other anti-corruption laws, as well as export control laws, import and customs laws, trade and economic sanctions laws and other laws governing our operations. Our operations are subject to anti-corruption laws, including the Bribery Act, the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. §201, the U.S. Travel Act, and other anti-corruption laws that apply in countries where we do business. These laws generally prohibit us and our employees and intermediaries acting on our behalf from corruptly authorizing, promising, offering, or providing, directly or indirectly, anything of value, to government officials or other persons to obtain or retain business or gain some other business advantage. The Bribery Act also prohibits: (i) “commercial” bribery of private parties, in addition to bribery involving domestic or foreign officials; (ii) the acceptance of bribes, as well as the giving of bribes, and (iii) “facilitation payments”, meaning generally low level payments designed to secure or expedite routine governmental actions or other conduct to which persons are already under obligations to perform. The Bribery Act also creates an offence applicable corporate entities for failure to prevent bribery by our employees, officers, directors and other third parties acting on our behalf, to which the only defence is to maintain “adequate procedures” designed to prevent such acts of bribery. In the future, we and our strategic partners may operate in jurisdictions that pose a heightened risk of potential Bribery Act or FCPA violations, and we may participate in collaborations and relationships with third parties whose conduct could potentially subject us to liability under the Bribery Act, FCPA or other anti-corruption laws, even if we do not explicitly authorize or have actual knowledge of such activities. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted. We are also subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United Kingdom and the United States, and authorities in the European Union and its member states, including applicable export control regulations, economic sanctions and embargoes on certain countries, regions, and persons, import and customs requirements and currency exchange regulations, collectively referred to as the Trade Control laws. Compliance with Trade Control Laws regarding the import and export of our products may create delays in the introduction of our products in international markets, and, in some cases, prevent the export of our products to some countries altogether. We have policies and procedures designed to promote compliance with anti-corruption laws and Trade Control laws. However, there is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws, including the Bribery Act, the FCPA or other legal requirements, including Trade Control laws. If we are not in compliance with the Bribery Act, the FCPA and other anti-corruption laws or Trade Control laws, we may be subject to criminal and civil penalties, disgorgement, suspension or debarment from government contracts as well as other sanctions and remedial measures, and may also result in collateral litigation. These consequences could have an adverse impact on our business, financial condition, results of operations and liquidity. Likewise, any investigation of any potential violations of the Bribery Act, the FCPA, other anti-corruption laws or Trade Control laws by United Kingdom, United States or other authorities could also have an adverse impact on our reputation, our business, results of operations and financial condition. In addition, responding to any enforcement action may result in a significant diversion of management’s attention and resources and significant defense costs and other professional fees.

216 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n Holders of ADSs are not treated as holders of our ordinary shares. If you purchase an ADS, you will become a holder of ADSs with underlying ordinary shares in a company incorporated under English law. Holders of ADSs are not treated as holders of our ordinary shares, unless they withdraw the ordinary shares underlying their ADSs in accordance with the deposit agreement and applicable laws and regulations. The depositary is the holder of the ordinary shares underlying the ADSs. Holders of ADSs therefore do not have any rights as holders of our ordinary shares, other than the rights that they have pursuant to the deposit agreement. See “Description of Securities Other Than Equity Securities” in our Annual Report on Form 20-F. Holders of ADSs may be subject to limitations on the transfer of their ADSs and the withdrawal of the underlying ordinary shares. ADSs are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason, subject to the right of ADS holders to cancel their ADSs and withdraw the underlying ordinary shares. Temporary delays in the cancellation of your ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting or we are paying a dividend on our ordinary shares. In addition, ADS holders may not be able to cancel their ADSs and withdraw the underlying ordinary shares when they owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities. See “Description of Securities Other Than Equity Securities” in our Annual Report on Form 20-F. ADS holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable outcomes to the plaintiff(s) in any such action. The deposit agreement governing the ADSs representing our ordinary shares provides that, to the fullest extent permitted by law, holders and beneficial owners of ADSs irrevocably waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to the ADSs or the deposit agreement. If this jury trial waiver provision is not permitted by applicable law, an action could proceed under the terms of the deposit agreement with a jury trial. If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. To our knowledge, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the federal securities laws has not been finally adjudicated by the U.S. Supreme Court. However, we believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of New York, which govern the deposit agreement, by a federal or state court in the City of New York, which has non-exclusive jurisdiction over matters arising under the deposit agreement. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the deposit agreement and the ADSs. It is advisable that you consult legal counsel regarding the jury waiver provision before entering into the deposit agreement. If you or any other holders or beneficial owners of ADSs bring a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under federal securities laws, you or such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and/or the depositary. If a lawsuit is brought against us and/or the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in any such action, depending on, among other things, the nature of the claims, the judge or justice hearing such claims, and the venue of the hearing. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with the U.S. federal securities laws and the rules and regulations promulgated thereunder. — inability to obtain adequate clinical or commercial supply for the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates or the inability to do so at acceptable prices; — adverse regulatory decisions, including failure to reach agreement with applicable regulatory authorities on the design or scope of our planned clinical trials; — failure to obtain and maintain regulatory exclusivity for the therapeutic candidates within our Wholly-Owned Programs or our Founded Entities’ therapeutic candidates; — regulatory approval or commercialization of new therapeutics or other methods of treating our target disease indications by our competitors; — failure to meet or exceed financial projections we may provide to the public or to the investment community; — publication of research reports or comments by securities or industry analysts; — the perception of the pharmaceutical and biotechnology industries by the public, legislatures, regulators and the investment community; — announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our Founded Entities our strategic collaboration partners or our competitors; — disputes or other developments relating to proprietary rights, including patents, litigation matters and our or our Founded Entities’ ability to obtain patent protection for our technologies; — additions or departures of our key scientific or management personnel; — significant lawsuits, including patent or shareholder litigation, against us; — changes in the market valuations of similar companies; — adverse developments relating to any of the above or additional factors with respect to our Founded Entities; — sales or potential sales of substantial amounts of our ADSs; and — trading volume of our ADSs. In addition, companies trading in the stock market in general, and Nasdaq, in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our ADSs, regardless of our actual operating performance. Since our ADSs were initially sold in November 2020 at a price of $33.00 per ADS, our ADS price has fluctuated significantly. If the market price of our ADSs does not exceed the price at which you acquired them, you may not realize any return on your investment in us and may lose some or all of your investment. If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our ADS price and trading volume could decline. The trading market for our ADSs and ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If no or few securities or industry analysts cover our company, the trading price for our ADSs and ordinary shares would be negatively impacted. If one or more of the analysts who covers us downgrades our equity securities or publishes incorrect or unfavorable research about our business, the price of our ordinary shares and ADSs would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, or downgrades our securities, demand for our ordinary shares and ADSs could decrease, which could cause the price of our ordinary shares and ADSs or their trading volume to decline. Future sales, or the possibility of future sales, of a substantial number of our securities could adversely affect the price of the shares and dilute shareholders. Sales of a substantial number of our ADSs in the public market could occur at any time, subject to certain restrictions described below. If our existing shareholders sell, or indicate an intent to sell, substantial amounts of our securities in the public market, the trading price of the ADSs could decline significantly and could decline below the original purchase price. As of March 31, 2025, we had 240,189,449 outstanding ordinary shares. Ordinary shares subject to outstanding options under our equity incentive plans and the ordinary shares reserved for future issuance under our equity incentive plans will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations.

PureTech Health plc Annual Report and Accounts 2024 217 Risk Factor Annex continued A d d itio nal info rm atio n One of our principal shareholders has a significant holding in the company which may give them influence in certain matters requiring approval by shareholders, including approval of significant corporate transactions in certain circumstances. As of March 31, 2025, Invesco Asset Management Limited, or Invesco, held approximately 17.07 percent of our ordinary shares. Accordingly, Invesco may, as a practical matter, be able to influence certain matters requiring approval by shareholders, including approval of significant corporate transactions in certain circumstances. Such concentration of ownership may also have the effect of delaying or preventing any future proposed change in control of the company. The trading price of the ordinary shares could be adversely affected if potential new investors are disinclined to invest in the company because they perceive disadvantages to a large shareholding being concentrated in the hands of a single shareholder. The interests of Invesco and the investors that acquire ADSs may not be aligned. Invesco may make acquisitions of, or investments in, other businesses in the same sectors as us or our Founded Entities. These businesses may be, or may become, competitors of us or our Founded Entities. In addition, funds or other entities managed or advised by Invesco may be in direct competition with us or our Founded Entities on potential acquisitions of, or investments in, certain businesses. In addition, Invesco holds equity interests in certain of our Founded Entities where they may exert direct influence. You will not have the same voting rights as the holders of our ordinary shares and may not receive voting materials in time to be able to exercise your right to vote. Except as described in our Annual Report on Form 20-F and the deposit agreement, holders of the ADSs will not be able to exercise voting rights attaching to the ordinary shares represented by the ADSs. Under the terms of the deposit agreement, holders of the ADSs may instruct the depositary to vote the ordinary shares underlying their ADSs. Otherwise, holders of ADSs will not be able to exercise their right to vote unless they withdraw the ordinary shares underlying their ADSs to vote them in person or by proxy in accordance with applicable laws and regulations and our Articles of Association. Even so, ADS holders may not know about a meeting far enough in advance to withdraw those ordinary shares. If we ask for the instructions of holders of the ADSs, the depositary, upon timely notice from us, will notify ADS holders of the upcoming vote and arrange to deliver our voting materials to them. Upon our request, the depositary will mail to holders a shareholder meeting notice that contains, among other things, a statement as to the manner in which voting instructions may be given. We cannot guarantee that ADS holders will receive the voting materials in time to ensure that they can instruct the depositary to vote the ordinary shares underlying their ADSs. A shareholder is only entitled to participate in, and vote at, the meeting of shareholders, provided that it holds our ordinary shares as of the record date set for such meeting and otherwise complies with our Articles of Association. In addition, the depositary’s liability to ADS holders for failing to execute voting instructions or for the manner of executing voting instructions is limited by the deposit agreement. As a result, holders of ADSs may not be able to exercise their right to give voting instructions or to vote in person or by proxy and they may not have any recourse against the depositary or us if their ordinary shares are not voted as they have requested or if their shares cannot be voted. You may not receive distributions on our ordinary shares represented by the ADSs or any value for them if it is illegal or impractical to make them available to holders of ADSs. The depositary for the ADSs has agreed to pay to you any cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of our ordinary shares your ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit distribution on the ADSs, ordinary shares, rights or anything else to holders of the ADSs. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to you. These restrictions may have an adverse effect on the value of your ADSs. Because we do not have immediate plans to pay any cash dividends on our ADSs, capital appreciation, if any, may be your sole source of gains and you may never receive a return on your investment. Under current English law, a company’s accumulated realized profits must exceed its accumulated realized losses (on a non-consolidated basis) before dividends can be declared and paid. Therefore, we must have sufficient distributable profits before declaring and paying a dividend. We have not paid dividends in the past on our ordinary shares. We have not announced any immediate plans to pay any cash dividends. As a result, capital appreciation, if any, on our ADSs will be your sole source of gains for the foreseeable future, and you would suffer a loss on your investment if you were unable to sell your ADSs at or above the price that you initially paid for them. Investors seeking cash dividends should not purchase our ADSs. Risks Related to Our Corporate Status We are not regulated as an “investment company” under the Investment Company Act of 1940, as amended, or the 1940 Act, and if we were deemed an “investment company” under the 1940 Act, applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business. The 1940 Act and the rules thereunder contain detailed parameters for the organization and operation of investment companies. Among other things, the 1940 Act and the rules thereunder limit or prohibit transactions with affiliates, impose limitations on the issuance of debt and equity securities and impose certain governance requirements. We have not been and do not intend to become regulated as an investment company, and we intend to conduct our activities so that we will not be deemed to be an investment company under the 1940 Act. In order to ensure that we are not deemed to be an investment company, we may be limited in the assets that we may continue to own and, further, may need to dispose of or acquire certain assets at such times or on such terms as may be less favorable to us than in the absence of such requirement. If anything were to happen which would cause us to be deemed to be an investment company under the 1940 Act (such as significant changes in the value of our Founded Entities or a change in circumstance that results in a reclassification of our interests in our Founded Entities for purposes of the 1940 Act), the requirements imposed by the 1940 Act could make it impractical for us to continue our business as currently conducted, which would materially adversely affect our business, results of operations and financial condition. In addition, if we were to become inadvertently subject to the 1940 Act, any violation of the 1940 Act could subject us to material adverse consequences, including potentially significant regulatory penalties and the possibility that certain of our contracts could be deemed unenforceable. AAs a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the SEC than a U.S. company. This may limit the information available to holders of ADSs or our ordinary shares. We are a “foreign private issuer,” as defined in the SEC’s rules and regulations and, consequently, we are not subject to all of the disclosure requirements applicable to U.S. domestic public companies. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act, including the U.S. proxy rules under Section 14 of the Exchange Act. In addition, our officers and directors are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, while we currently make annual and semi-annual filings with respect to our listing on the LSE, we will not be required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. domestic issuers and will not be required to file quarterly reports on Form 10-Q or current reports on Form 8-K under the Exchange Act. In addition, “foreign private issuers” are exempt from Regulation FD, which prohibits selective disclosures of material information. Accordingly, there will be less publicly available information concerning our company than there would be if we were not a foreign private issuer. As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from Nasdaq corporate governance listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards. As a foreign private issuer listed on Nasdaq, we are subject to corporate governance listing standards. However, rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the United Kingdom, which is our home country, may differ significantly from corporate governance listing standards. For example, neither the corporate laws of the United Kingdom nor our articles of association require a majority of our directors to be independent and we could include non-independent directors as members of our nomination and remuneration committee, though a majority is required, and our independent directors would not necessarily hold regularly scheduled meetings at which only independent directors are present. Currently, we follow home country practice to the maximum extent possible. Therefore, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers. See “Governance” of this Annual Report and Accounts and “Item 16G—Corporate Governance” of our Annual Report on Form 20-F.

218 PureTech Health plc Annual Report and Accounts 2024 Risk Factor Annex continued A d d it io na l i nf o rm at io n the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected. Risks Related to Tax Matters We are treated as a U.S. domestic corporation for U.S. federal income tax purposes. We are treated as a U.S. domestic corporation for U.S. federal income tax purposes under Section 7874(b) of the Internal Revenue Code of 1986, as amended, or the Code. As a result, we are subject to U.S. income tax on our worldwide income and any dividends paid by us (or deemed to be paid by us for U.S. federal income tax purposes) to Non-U.S. Holders (as defined in the discussion under “Taxation in the United States” in our Annual Report on Form 20-F) will generally be subject to U.S. federal income tax withholding at a 30 percent rate or such lower rate as provided in an applicable treaty. Furthermore, PureTech Health plc is also resident for tax purposes in the U.K. and subject to U.K. corporation tax on its worldwide income and gains. Consequently, we may be liable for both U.S. and U.K. income tax, which could have a material adverse effect on our financial condition and results of operations. This discussion of certain U.S. federal income tax risks is subject in its entirety to the summaries set forth in “Certain United Kingdom Tax Considerations” and “Taxation in the United States” in our Annual Report on Form 20-F. Our ability to use our U.S. net operating losses and certain other tax attributes to offset future U.S. taxable income and income tax liabilities may be subject to certain limitations. As of December 31, 2024, we had U.S. federal and state net operating loss carryforwards, or NOLs, of approximately $7.8 million and $380.8 million, respectively, which, subject to the following discussion, are generally available to be carried forward to offset our future taxable income, if any, until such NOLs are used or expire. Our federal NOLs generated in taxable years beginning after December 31, 2017 are not subject to expiration, but may generally only be used to offset 80% of taxable income in years beginning after December 31, 2020. As of December 31, 2024, we also had U.S. federal research and development and other tax credit carryforwards of approximately $0.2 million, available to reduce our future income tax liabilities, if any. These NOLs and tax credit carryforwards could expire unused, to the extent subject to expiration, and be unavailable to offset future taxable income or income tax liabilities. In general, under Sections 382 and 383 of the Code, a corporation that undergoes an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in its equity ownership by certain shareholders or groups of shareholders over a rolling three year period, is subject to limitations on its ability to utilize its pre-change U.S. federal NOLs and tax credit carryforwards to offset future taxable income and income tax liabilities. Similar rules may apply under state law. Our existing federal NOLs and tax credits may be subject to limitation arising from previous ownership changes. Future changes in our stock ownership, some of which are outside of our control, could result in ownership changes under Sections 382 or 383 of the Code, and our ability to utilize our federal NOLs or tax credit carryforwards could be further limited. Additionally, we may not be able to utilize the NOLs or tax credit carryforwards of our Founded Entities that have been deconsolidated or that will deconsolidate in the future. Furthermore, our ability to utilize NOLs of companies that we have acquired or may acquire in the future may be subject to similar limitations. For these reasons, even if we attain profitability, we may not be able to realize a tax benefit from the use of our NOLs or tax credit carryforwards. We may be unable to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future U.K. tax liabilities. As a U.K. incorporated and tax resident entity, PureTech Health plc is subject to U.K. corporate taxation on its tax-adjusted trading profits. Due to the nature of our business, PureTech Health plc has generated losses since inception and therefore we have not paid any U.K. corporation tax. Subject to numerous utilization criteria and restrictions (including those that limit the percentage of profits that can be reduced by carried forward losses and those that can restrict the use of carried forward losses where there is a change of ownership of more than half the ordinary shares of the We may lose our foreign private issuer status in the future, which could result in significant additional cost and expense. While we currently qualify as a foreign private issuer, the determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, the next determination will be made with respect to us on June 30, 2025. In the future, we would lose our foreign private issuer status if we to fail to meet the requirements necessary to maintain our foreign private issuer status as of the relevant determination date. For example, if more than 50 percent of our securities are held by U.S. residents and more than 50 percent of the members of our executive committee or members of our board of directors are residents or citizens of the United States, we could lose our foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly more than costs we incur as a foreign private issuer. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our financial statements in accordance with U.S. GAAP, rather than IFRS, and modify certain of our policies to comply with corporate governance practices associated with U.S. domestic issuers. Such conversion of our financial statements to U.S. GAAP will involve significant time and cost. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers such as the ones described above and exemptions from procedural requirements related to the solicitation of proxies. Risks Related to Our Internal Controls Failure to maintain effective internal control over financial reporting could have a material adverse effect on our business, financial condition, results of operations, and stock price and may adversely affect investor confidence in our company and, as a result, the value of our ADSs and your investment. Section 404 of the Sarbanes-Oxley Act requires us to evaluate the effectiveness of our internal controls over financial reporting as of the end of each fiscal year, including a management report assessing the effectiveness of our internal controls over financial reporting, and a report issued by our independent registered public accounting firm on that assessment. Our ability to comply with the annual internal control reporting requirements will depend on the effectiveness of our financial reporting and data systems and controls across our company. We expect these systems and controls to require additional investment as we become increasingly more complex and our business grows. To effectively manage this complexity, we will need to continue to maintain and revise our operational, financial and management controls, and our reporting systems and procedures. Certain weaknesses or deficiencies or failures to implement required new or improved controls, or difficulties encountered in the implementation or operation of these controls, could harm our operating results and cause us to fail to meet our financial reporting obligations, or result in material misstatements in our financial statements, which could adversely affect our business and reduce the value of our ADSs. We have in the past and may in the future identify a material weakness in our internal control over financial reporting. If we discover additional material weaknesses in our internal control over financial reporting in the future, we may not successfully remediate any such material weakness on a timely basis or at all. Any failure to remediate any significant deficiencies or material weaknesses identified by us or to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. If we fail to maintain effective internal control over financial reporting, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which could cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets or lead to a decline in the trading price of our securities. We may also be required to restate our financial statements from prior periods. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations, litigation from shareholders and civil or criminal sanctions, which could have a material adverse effect on our business. Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud. We are subject to certain reporting requirements of the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within

PureTech Health plc Annual Report and Accounts 2024 219 Risk Factor Annex continued A d d itio nal info rm atio n company and a major change in the nature, conduct or scale of the trade), we expect these to be eligible for carry forward and utilization against future U.K. operating profits. Future changes to tax laws could materially adversely affect our company and reduce net returns to our shareholders. The tax treatment of the company is subject to changes in tax laws, regulations and treaties, or the interpretation thereof, tax policy initiatives and reforms under consideration and the practices of tax authorities in jurisdictions in which we operate, as well as tax policy initiatives and reforms related to the Organisation for Economic Co-Operation and Development’s, or OECD, Base Erosion and Profit Shifting, or BEPS, Project, the European Commission’s state aid investigations and other initiatives. Such changes may include (but are not limited to) the taxation of operating income, investment income, dividends received or (in the specific context of withholding tax) dividends paid. We are unable to predict what tax reform may be proposed or enacted in the future or what effect such changes would have on our business, but such changes, to the extent they are brought into tax legislation, regulations, policies or practices, could affect our financial position and overall or effective tax rates in the future in countries where we have operations, reduce post-tax returns to our shareholders, and increase the complexity, burden and cost of tax compliance. Tax authorities may disagree with our positions and conclusions regarding certain tax positions, resulting in unanticipated costs, taxes or non- realization of expected benefits. A tax authority may disagree with tax positions that we have taken, which could result in increased tax liabilities. For example, HM Revenue & Customs, or HMRC, the Internal Revenue Service or another tax authority could challenge our allocation of income by tax jurisdiction and the amounts paid between certain of our Founded Entities pursuant to our intercompany arrangements and transfer pricing policies, including amounts paid with respect to our intellectual property development. Similarly, a tax authority could assert that we are subject to tax in a jurisdiction where we believe we have not established a taxable connection, often referred to as a “permanent establishment” under international tax treaties, and such an assertion, if successful, could increase our expected tax liability in one or more jurisdictions. A tax authority may take the position that material income tax liabilities, interest and penalties are payable by us, in which case, we expect that we might contest such assessment. Contesting such an assessment may be lengthy and costly and if we were unsuccessful in disputing the assessment, the implications could increase our anticipated effective tax rate, where applicable. Shareholder protections found in provisions under the U.K. City Code on Takeovers and Mergers, or the Takeover Code, will not apply if our securities are no longer admitted to trading on a regulated market or a multilateral trading facility in the United Kingdom or on any stock exchange in the Channel Islands or the Isle of Man and our place of management and control is considered to change to outside the United Kingdom. We are registered as a public limited company incorporated in England and Wales and have our ordinary shares admitted to trading on a regulated market in the United Kingdom (being the main market of the LSE). Accordingly, we are currently subject to the Takeover Code and, as a result, our shareholders are entitled to the benefit of certain takeover offer protections provided under the Takeover Code. The Takeover Code provides a framework within which takeovers of companies are regulated and conducted. If, at the time of a takeover offer, we have de-listed from the main market of the LSE (and do not maintain a listing of securities on any other regulated market or a multilateral trading facility in the United Kingdom or on any stock exchange in the Channel Islands or the Isle of Man) and the Panel on Takeovers and Mergers determine that we do not have our place of central management and control in the United Kingdom, then the Takeover Code may not apply to us and our shareholders would not be entitled to the benefit of the various protections that the Takeover Code affords. In particular, we would not be subject to the rules regarding mandatory takeover bids. The following is a brief summary of some of the most important rules of the Takeover Code: — when any person acquires, whether by a series of transactions over a period of time or not, an interest in shares which (taken together with shares already held by that person and an interest in shares held or acquired by persons acting in concert with him or her) carry 30 percent or more of the voting rights of a company that is subject to the Takeover Code, that person is generally required to make a mandatory offer to all the holders of any class of equity share capital or other class of transferable securities carrying voting rights in that company to acquire the balance of their interests in the company; — when any person who, together with persons acting in concert with him or her, is interested in shares representing not less than 30 percent but does not hold more than 50 percent of the voting rights of a company that is subject to the Takeover Code, and such person, or any person acting in concert with him or her, acquires an additional interest in shares which increases the percentage of shares carrying voting rights in which he or she is interested, then such person is generally required to make a mandatory offer to all the holders of any class of equity share capital or other class of transferable securities carrying voting rights of that company to acquire the balance of their interests in the company; — a mandatory offer triggered in the circumstances described in the two paragraphs above must be in cash (or be accompanied by a cash alternative) and at not less than the highest price paid within the preceding 12 months to acquire any interest in shares in the company by the person required to make the offer or any person acting in concert with him or her; — in relation to a voluntary offer (i.e. any offer which is not a mandatory offer), when interests in shares representing 10 percent or more of the shares of a class have been acquired for cash by an offeror (i.e., a bidder) and any person acting in concert with it in the offer period and the previous 12 months, the offer must be in cash or include a cash alternative for all shareholders of that class at not less than the highest price paid for any interest in shares of that class by the offeror and by any person acting in concert with it in that period. Further, if an offeror acquires for cash any interest in shares during the offer period, a cash alternative must be made available at not less than the highest price paid for any interest in the shares of that class; — if the offeror acquires an interest in shares in an offeree company (i.e., a target) at a price higher than the value of the offer, the offer must be increased to not less than the highest price paid for the interest in shares so acquired; — the offeree company must obtain competent advice as to whether the terms of any offer are fair and reasonable and the substance of such advice must be made known to all the shareholders, together with the opinion of the board of directors of the offeree company; — special or favorable deals for selected shareholders are not permitted, except in certain circumstances where independent shareholder approval is given and the arrangements are regarded as fair and reasonable in the opinion of the financial adviser to the offeree; — all shareholders must be given the same information; — each document published in connection with an offer by or on behalf of the offeror or offeree must state that the directors of the offeror or the offeree, as the case may be, accept responsibility for the information contained therein; — profit forecasts, quantified financial benefits statements and asset valuations must be made to specified standards and must be reported on by professional advisers; — misleading, inaccurate or unsubstantiated statements made in documents or to the media must be publicly corrected immediately; — actions during the course of an offer by the offeree company, which might frustrate the offer are generally prohibited unless shareholders approve these plans. Frustrating actions would include, for example, lengthening the notice period for directors under their service contract or agreeing to sell off material parts of the target group; — stringent and detailed requirements are laid down for the disclosure of dealings in relevant securities during an offer, including the prompt disclosure of positions and dealing in relevant securities by the parties to an offer and any person who is interested (directly or indirectly) in 1 percent or more of any class of relevant securities; and employees of both the offeror and the offeree company and the trustees of the offeree company’s pension scheme must be informed about an offer. In addition, the offeree company’s employee representatives and pension scheme trustees have the right to have a separate opinion on the effects of the offer on employment appended to the offeree board of directors’ circular or published on a website.

220 PureTech Health plc Annual Report and Accounts 2024 A d d it io na l i nf o rm at io n Joint Corporate Brokers UBS AG 5 Broadgate London EC2M 2QS United Kingdom Tel: +44 207 567 8000 Peel Hunt LLP 100 Liverpool Street London EC2M 2AT United Kingdom Tel: +44 207 418 8900 Registrar Computershare Investor Services PLC The Pavilions Bridgwater Road Bristol BS99 6ZY United Kingdom Tel: +44 (0)370 707 1147 Solicitors DLA Piper UK LLP 160 Aldersgate Street Barbican, London EC1A 4HT United Kingdom Tel: +44 870 011 1111 Company Registration Number 09582467 Registered Office 13th Floor One Angel Court London EC2R 7HJ United Kingdom Website www.puretechhealth.com Board of Directors Dr. Raju Kucherlapati (Chair) Dr. Bharatt Chowrira (Chief Executive Officer) Dr. Robert Langer (Non-Executive Director) Dr. John LaMattina (Senior Independent Director) Dr. Michele Holcomb (Independent Non-Executive Director) Ms. Kiran Mazumdar-Shaw (Independent Non- Executive Director) Ms. Sharon Barber-Lui (Independent Non-Executive Director) Company Secretary Mr. Charles Sherwood Media and Public Relations FTI Consulting, Inc. 200 Aldersgate Street Barbican London EC1A 4HD United Kingdom Tel: +44 203 727 1000 Independent Auditor PricewaterhouseCoopers LLP 3 Forbury Place 23 Forbury Road Reading RG1 3JH United Kingdom Tel: +44 (0) 118 959 7111 Company information Directors, Secretary and Advisors to PureTech

This document is printed on Mohawk Superfine, a carbon neutral paper containing 100% virgin fibre sourced from well managed, responsible, FSC® certified forests and controlled resources. The pulp used in this product is bleached using an elemental chlorine free (ECF) process. Designed and produced by Whitehouse Associates, London. Printed by Donnelley Financial Solutions on FSC® certified paper. Donnelley Financial Solutions is an EMAS certified company and its Environmental Management System is certified to ISO 14001.

PureTech Health 6 Tide Street Suite 400 Boston MA 02210 Tel: +1 617 482 2333 Email: info@puretechhealth.com