

Investor call November 3, 2025 .2

Disclaimers and forward-looking statements This presentation and accompanying discussion contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding the Company’s plans, progress, expectations, and timing relating to the Company’s hematologic malignancies program, including updated manufacturing process resulting in shortened manufacturing times, lower cost of goods, improved chimerism or relapse, and commercial-ready process, clinical updates of the ALLOHA Phase 1 heme trial, presentation of data, dosing of patients, filing of an new IND applications and initiation of Phase 1 development, and clinical trial design and initiation of a pivotal trial for TSC-101; the Company’s plans, progress, expectations, and timing relating to the Company’s solid tumor program, including clinical updates of the PLEXI-T Phase 1 solid tumor trial, development of in vivo manufacturing, and presentation of data; the Company’s plans, progress, expectations, and timing relating to the Company’s autoimmunity programs, including identification of novel targets; the progress of the hematologic malignancies, solid tumor, and autoimmunity programs being indicative or predictive of the success of each program; the Company’s current and future research and development plans or expectations; the structure, timing and success of the Company’s planned preclinical development, submission of INDs, and clinical trials; the potential benefits of any of the Company’s proprietary platforms, multiplexing, or current or future product candidates in treating patients; the Company’s ability to fund its operating plan into the second half of 2027 with its existing cash, cash equivalents, and marketable securities; the expected charges, cost reductions and savings, and capital preservation associated with the strategic prioritization; and the Company’s goals, strategy and anticipated financial performance. TScan intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan,” “on track,” or similar expressions or the negative of those terms. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. The express or implied forward-looking statements included in this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: the beneficial characteristics, safety, efficacy, therapeutic effects and potential advantages of TScan’s TCR-T therapy product candidates; TScan’s expectations regarding its preclinical studies being predictive of clinical trial results; TScan’s approved INDs being indicative or predictive of bringing TScan closer to its goal of providing customized TCR-T therapies to treat patients with cancer; the timing of the launch, initiation, progress, expected results and announcements of TScan’s preclinical studies, clinical trials and its research and development programs; TScan’s ability to enroll patients for its clinical trials within its expected timeline; TScan’s plans relating to developing and commercializing its TCR-T therapy product candidates, if approved, including sales strategy; estimates of the size of the addressable market for TScan’s TCR-T therapy product candidates; TScan’s manufacturing capabilities and the scalable nature of its manufacturing process; TScan’s estimates regarding expenses, future milestone payments and revenue, capital requirements and needs for additional financing; TScan’s expectations regarding competition; TScan’s anticipated growth strategies; TScan’s ability to attract or retain key personnel; TScan’s ability to establish and maintain development partnerships and collaborations; TScan’s expectations regarding federal, state and foreign regulatory requirements; TScan’s ability to obtain and maintain intellectual property protection for its proprietary platform technology and our product candidates; the sufficiency of TScan’s existing capital resources to fund its future operating expenses and capital expenditure requirements; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of TScan’s most recent Annual Report on Form 10-K and any other filings that TScan has made or may make with the SEC in the future. Any forward-looking statements contained in this presentation represent TScan’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, TScan explicitly disclaims any obligation to update any forward-looking statements. TScan Therapeutics, Investor Call November 3, 2025
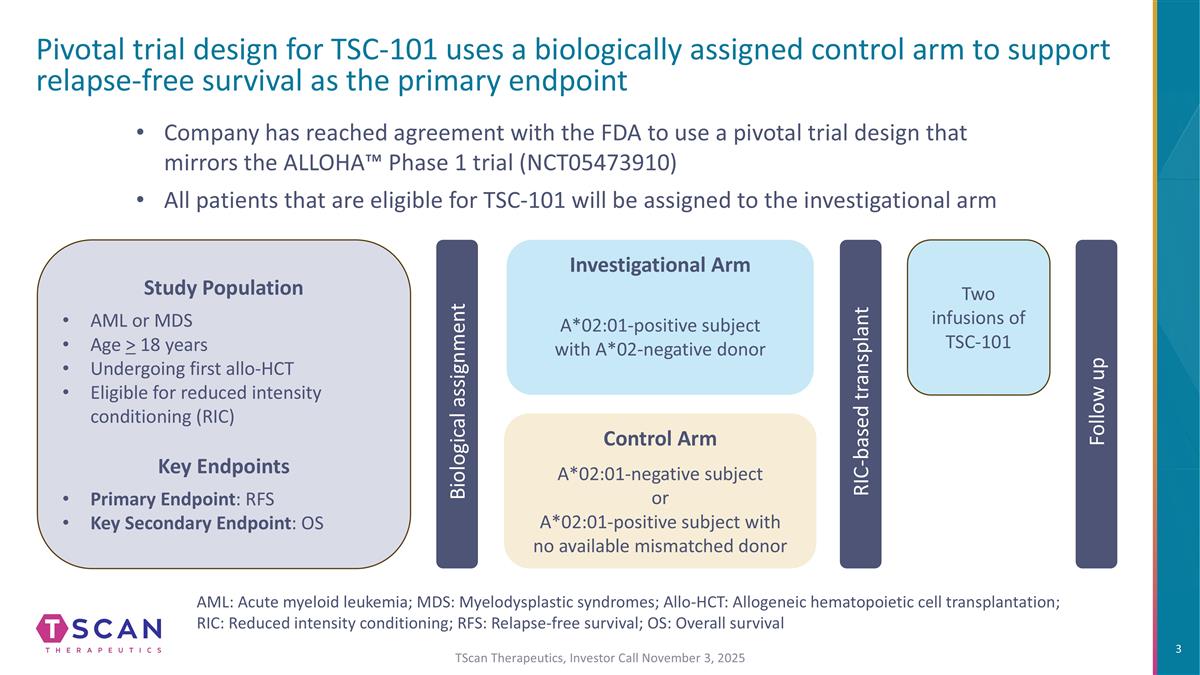
Pivotal trial design for TSC-101 uses a biologically assigned control arm to support relapse-free survival as the primary endpoint Biological assignment Company has reached agreement with the FDA to use a pivotal trial design that mirrors the ALLOHA™ Phase 1 trial (NCT05473910) All patients that are eligible for TSC-101 will be assigned to the investigational arm Two infusions of TSC-101 RIC-based transplant Follow up Study Population AML or MDS Age > 18 years Undergoing first allo-HCT Eligible for reduced intensity conditioning (RIC) Key Endpoints Primary Endpoint: RFS Key Secondary Endpoint: OS AML: Acute myeloid leukemia; MDS: Myelodysplastic syndromes; Allo-HCT: Allogeneic hematopoietic cell transplantation; RIC: Reduced intensity conditioning; RFS: Relapse-free survival; OS: Overall survival Investigational Arm A*02:01-positive subject with A*02-negative donor Control Arm A*02:01-negative subject or A*02:01-positive subject with no available mismatched donor TScan Therapeutics, Investor Call November 3, 2025

Investor call November 3, 2025