

Corporate Presentation January 2026

Disclaimers and forward-looking statements This presentation and the accompanying discussion contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding the Company’s plans, progress, and timing relating to the Company’s clinical programs and the presentation of data, the Company’s current and future research and development plans or expectations, the structure, timing and success of the Company’s planned preclinical development, submission of INDs, and clinical trials, the potential benefits of any of the Company’s proprietary platforms or current or future product candidates in treating patients, the Company's ability to fund its operating expenses and capital expenditure requirements with its existing cash and cash equivalents, and the Company’s goals and strategy. TScan intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan,” “on track,” or similar expressions or the negative of those terms. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. The express or implied forward-looking statements included in this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: the beneficial characteristics, safety, efficacy, therapeutic effects and potential advantages of TScan’s TCR-T therapy candidates; TScan’s expectations regarding its preclinical studies being predictive of clinical trial results; the timing of the initiation, progress and expected results of TScan’s preclinical studies, clinical trials and its research and development programs; TScan’s plans relating to developing and commercializing its TCR-T therapy candidates, if approved, including sales strategy; estimates of the size of the addressable market for TScan’s TCR-T therapy candidates; TScan’s manufacturing capabilities and the scalable nature of its manufacturing process; TScan’s estimates regarding expenses, future milestone payments and revenue, capital requirements and needs for additional financing; TScan’s expectations regarding competition; TScan’s anticipated growth strategies; TScan’s ability to attract or retain key personnel; TScan’s ability to establish and maintain development partnerships and collaborations; TScan’s expectations regarding federal, state and foreign regulatory requirements; TScan’s ability to obtain and maintain intellectual property protection for its proprietary platform technology and our product candidates; the sufficiency of TScan’s existing capital resources to fund its future operating expenses and capital expenditure requirements; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of TScan’s most recent Annual Report on Form 10-K and any other filings that TScan has made or may make with the SEC in the future. Any forward-looking statements contained in this presentation represent TScan’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, TScan explicitly disclaims any obligation to update any forward-looking statements.
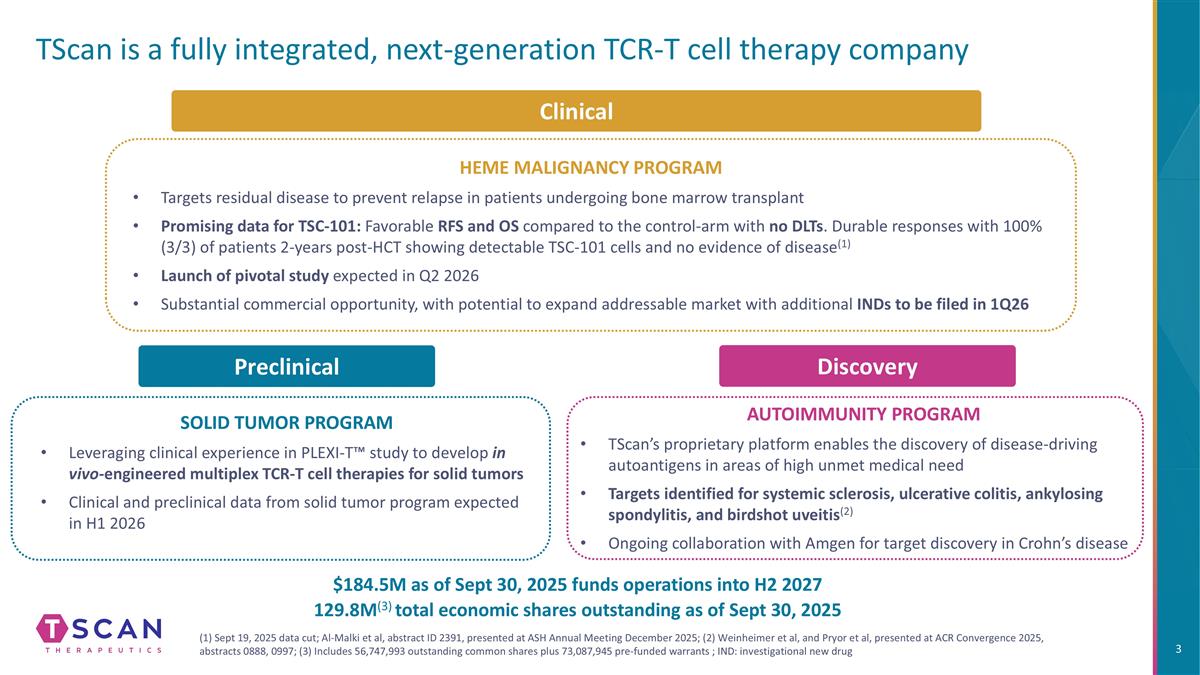
TScan is a fully integrated, next-generation TCR-T cell therapy company HEME MALIGNANCY PROGRAM Targets residual disease to prevent relapse in patients undergoing bone marrow transplant Promising data for TSC-101: Favorable RFS and OS compared to the control-arm with no DLTs. Durable responses with 100% (3/3) of patients 2-years post-HCT showing detectable TSC-101 cells and no evidence of disease(1) Launch of pivotal study expected in Q2 2026 Substantial commercial opportunity, with potential to expand addressable market with additional INDs to be filed in 1Q26 SOLID TUMOR PROGRAM Leveraging clinical experience in PLEXI-T™ study to develop in vivo-engineered multiplex TCR-T cell therapies for solid tumors Clinical and preclinical data from solid tumor program expected in H1 2026 Discovery AUTOIMMUNITY PROGRAM TScan’s proprietary platform enables the discovery of disease-driving autoantigens in areas of high unmet medical need Targets identified for systemic sclerosis, ulcerative colitis, ankylosing spondylitis, and birdshot uveitis(2) Ongoing collaboration with Amgen for target discovery in Crohn’s disease (1) Sept 19, 2025 data cut; Al-Malki et al, abstract ID 2391, presented at ASH Annual Meeting December 2025; (2) Weinheimer et al, and Pryor et al, presented at ACR Convergence 2025, abstracts 0888, 0997; (3) Includes 56,747,993 outstanding common shares plus 73,087,945 pre-funded warrants ; IND: investigational new drug Clinical Preclinical $184.5M as of Sept 30, 2025 funds operations into H2 2027 129.8M(3) total economic shares outstanding as of Sept 30, 2025
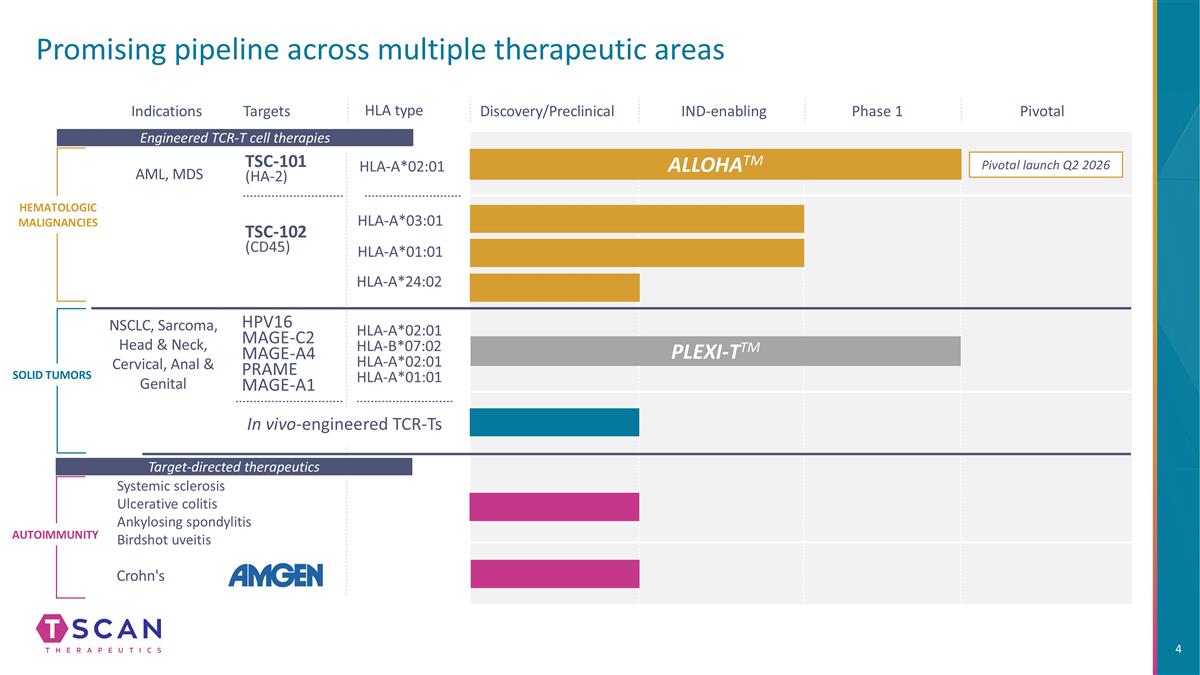
Promising pipeline across multiple therapeutic areas HLA type Indications Targets HLA-A*02:01 HLA-A*02:01 HLA-B*07:02 HLA-A*02:01 HLA-A*01:01 TSC-101 (HA-2) HEMATOLOGIC MALIGNANCIES SOLID TUMORS AML, MDS NSCLC, Sarcoma, Head & Neck, Cervical, Anal & Genital Autoimmunity IND-enabling Phase 1 Pivotal HLA-A*03:01 ALLOHATM PLEXI-TTM Target-directed therapeutics Engineered TCR-T cell therapies Pivotal launch Q2 2026 Crohn's Systemic sclerosis Ulcerative colitis Ankylosing spondylitis Birdshot uveitis TSC-102 (CD45) HPV16 MAGE-C2 MAGE-A4 PRAME MAGE-A1 Discovery/Preclinical In vivo-engineered TCR-Ts HLA-A*01:01 HLA-A*24:02
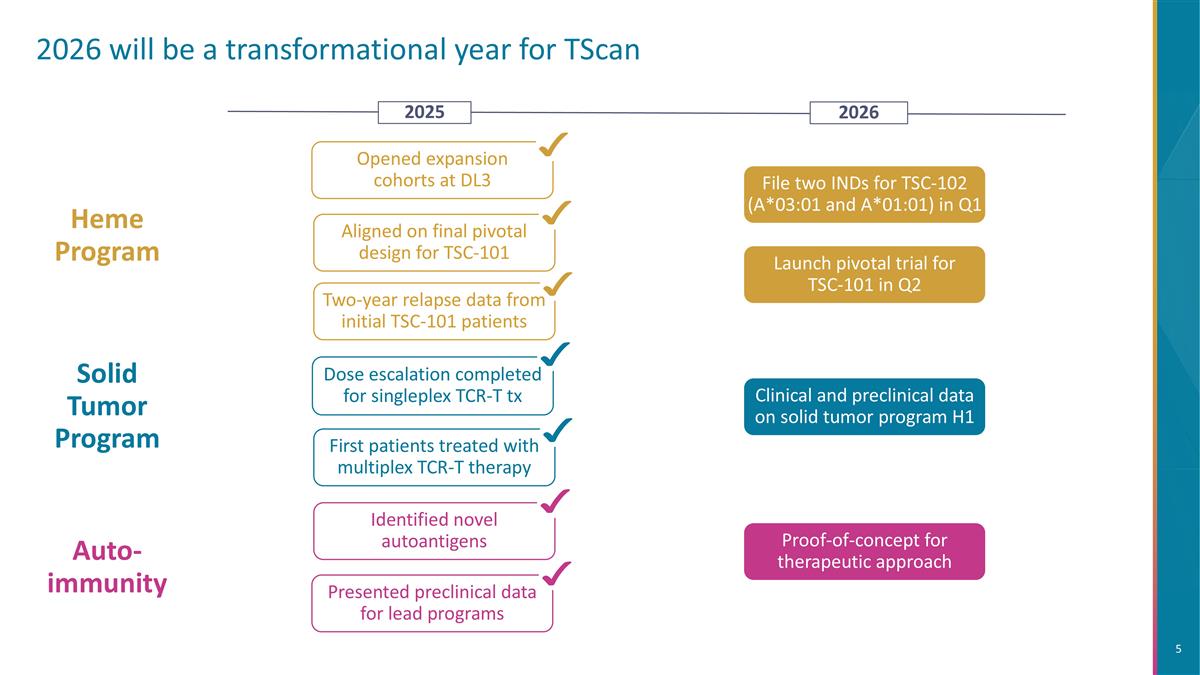
2026 will be a transformational year for TScan Aligned on final pivotal design for TSC-101 Two-year relapse data from initial TSC-101 patients Heme Program File two INDs for TSC-102 (A*03:01 and A*01:01) in Q1 Opened expansion cohorts at DL3 Auto-immunity Identified novel autoantigens Presented preclinical data for lead programs Clinical and preclinical data on solid tumor program H1 Solid Tumor Program First patients treated with multiplex TCR-T therapy Dose escalation completed for singleplex TCR-T tx 2026 2025 Proof-of-concept for therapeutic approach Launch pivotal trial for TSC-101 in Q2

Heme Malignancies: Targeting residual disease to prevent relapse in patients undergoing allogeneic HCT
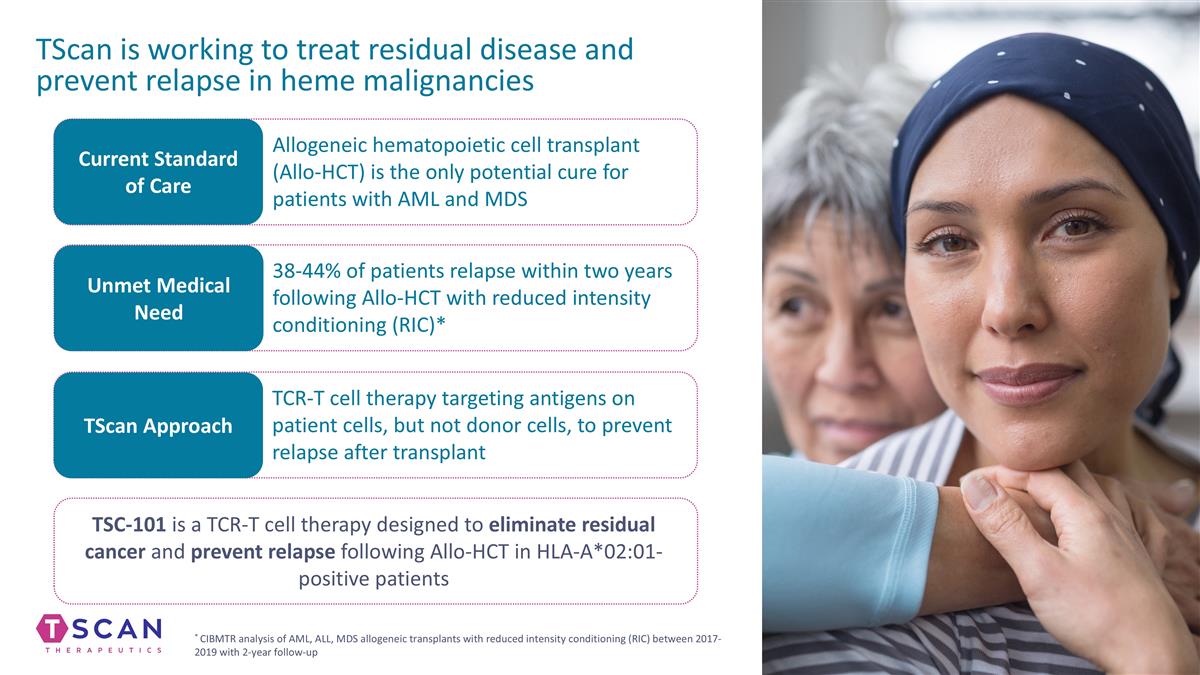
TScan is working to treat residual disease and prevent relapse in heme malignancies Current Standard of Care Unmet Medical Need TScan Approach Allogeneic hematopoietic cell transplant (Allo-HCT) is the only potential cure for patients with AML and MDS 38-44% of patients relapse within two years following Allo-HCT with reduced intensity conditioning (RIC)* TCR-T cell therapy targeting antigens on patient cells, but not donor cells, to prevent relapse after transplant TSC-101 is a TCR-T cell therapy designed to eliminate residual cancer and prevent relapse following Allo-HCT in HLA-A*02:01-positive patients * CIBMTR analysis of AML, ALL, MDS allogeneic transplants with reduced intensity conditioning (RIC) between 2017-2019 with 2-year follow-up
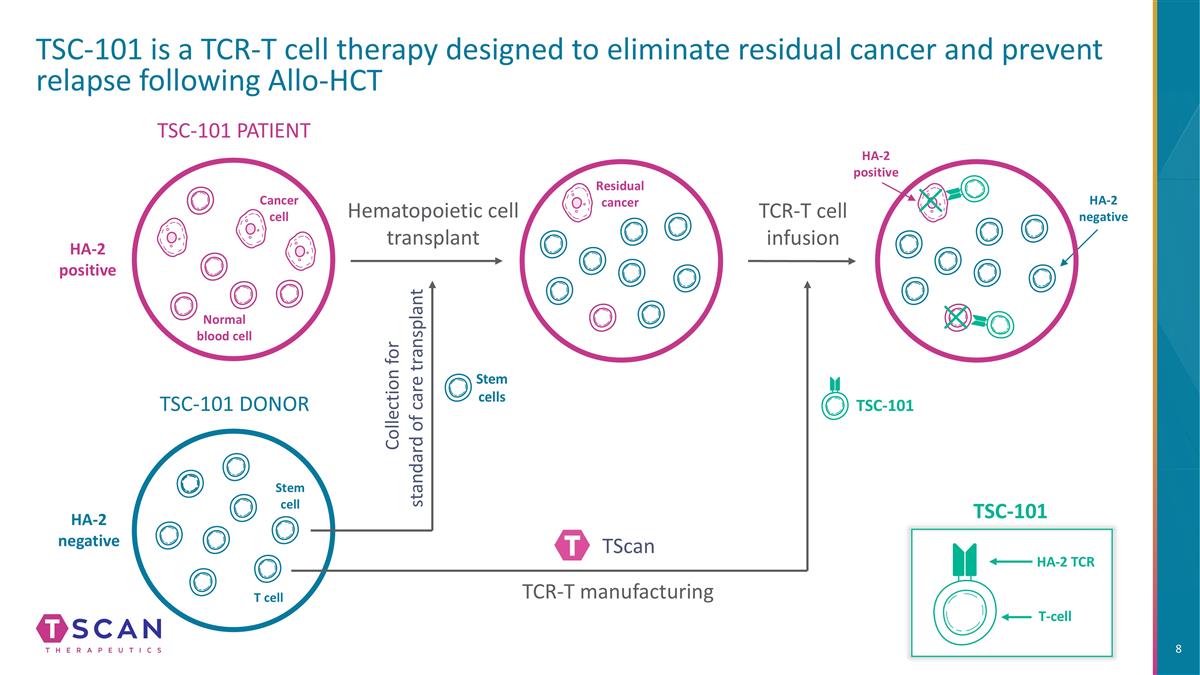
TSC-101 is a TCR-T cell therapy designed to eliminate residual cancer and prevent relapse following Allo-HCT TSC-101 PATIENT TSC-101 DONOR Hematopoietic cell transplant Cancer cell Normal blood cell Stem cell T cell HA-2 positive HA-2 negative HA-2 negative HA-2 positive TSC-101 Residual cancer Stem cells TCR-T cell infusion T-cell HA-2 TCR TSC-101 Collection for standard of care transplant TScan TCR-T manufacturing
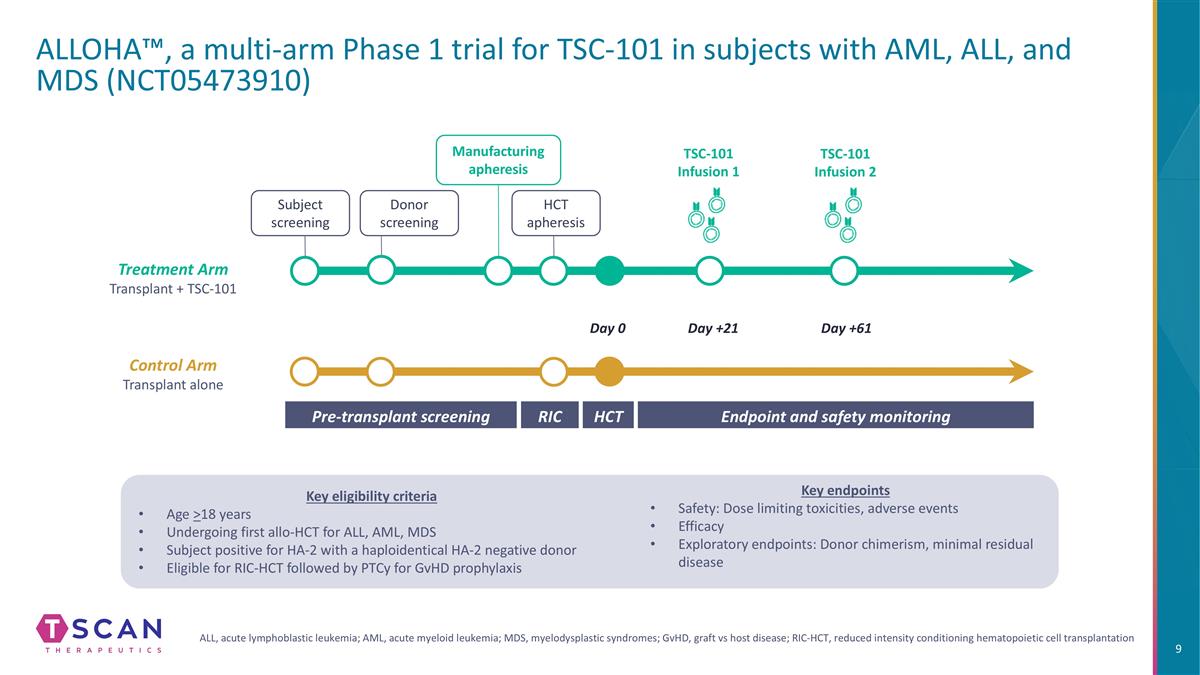
ALLOHA™, a multi-arm Phase 1 trial for TSC-101 in subjects with AML, ALL, and MDS (NCT05473910) HCT Pre-transplant screening Endpoint and safety monitoring RIC Day 0 Day +21 Day +61 Donor screening Manufacturing apheresis HCT apheresis TSC-101 Infusion 1 TSC-101 Infusion 2 Subject screening Control Arm Transplant alone Treatment Arm Transplant + TSC-101 Key eligibility criteria Age >18 years Undergoing first allo-HCT for ALL, AML, MDS Subject positive for HA-2 with a haploidentical HA-2 negative donor Eligible for RIC-HCT followed by PTCy for GvHD prophylaxis Key endpoints Safety: Dose limiting toxicities, adverse events Efficacy Exploratory endpoints: Donor chimerism, minimal residual disease ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; GvHD, graft vs host disease; RIC-HCT, reduced intensity conditioning hematopoietic cell transplantation
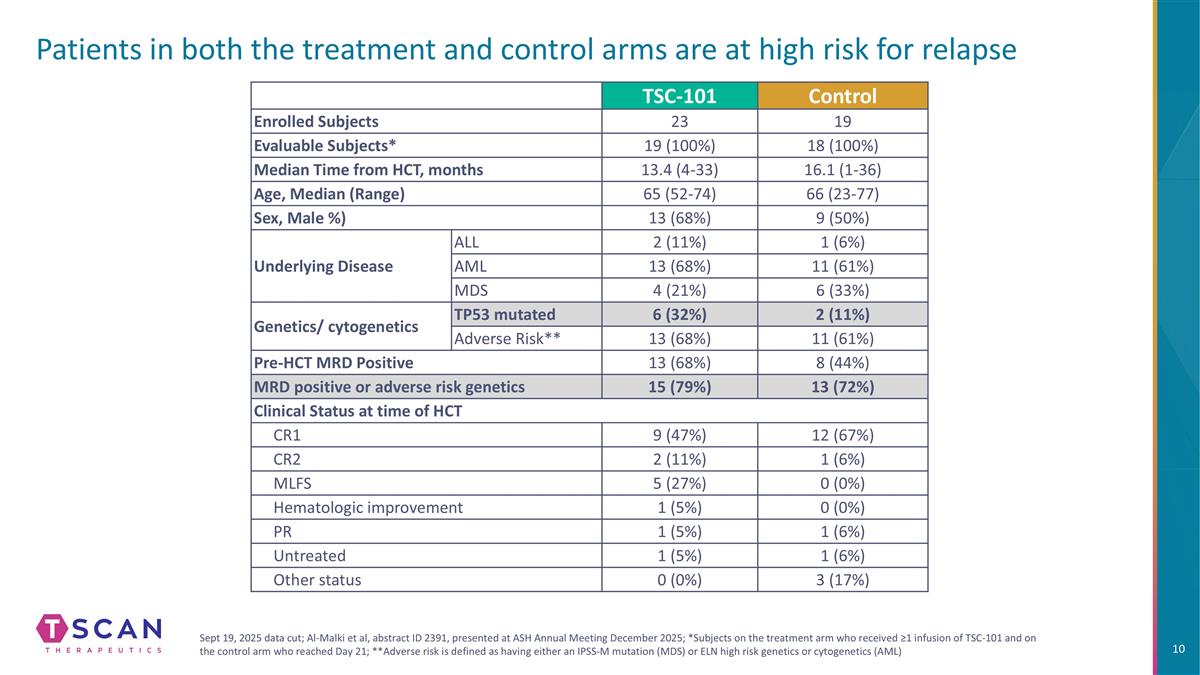
Patients in both the treatment and control arms are at high risk for relapse TSC-101 Control Enrolled Subjects 23 19 Evaluable Subjects* 19 (100%) 18 (100%) Median Time from HCT, months 13.4 (4-33) 16.1 (1-36) Age, Median (Range) 65 (52-74) 66 (23-77) Sex, Male %) 13 (68%) 9 (50%) Underlying Disease ALL 2 (11%) 1 (6%) AML 13 (68%) 11 (61%) MDS 4 (21%) 6 (33%) Genetics/ cytogenetics TP53 mutated 6 (32%) 2 (11%) Adverse Risk** 13 (68%) 11 (61%) Pre-HCT MRD Positive 13 (68%) 8 (44%) MRD positive or adverse risk genetics 15 (79%) 13 (72%) Clinical Status at time of HCT CR1 9 (47%) 12 (67%) CR2 2 (11%) 1 (6%) MLFS 5 (27%) 0 (0%) Hematologic improvement 1 (5%) 0 (0%) PR 1 (5%) 1 (6%) Untreated 1 (5%) 1 (6%) Other status 0 (0%) 3 (17%) Sept 19, 2025 data cut; Al-Malki et al, abstract ID 2391, presented at ASH Annual Meeting December 2025; *Subjects on the treatment arm who received ≥1 infusion of TSC-101 and on the control arm who reached Day 21; **Adverse risk is defined as having either an IPSS-M mutation (MDS) or ELN high risk genetics or cytogenetics (AML)
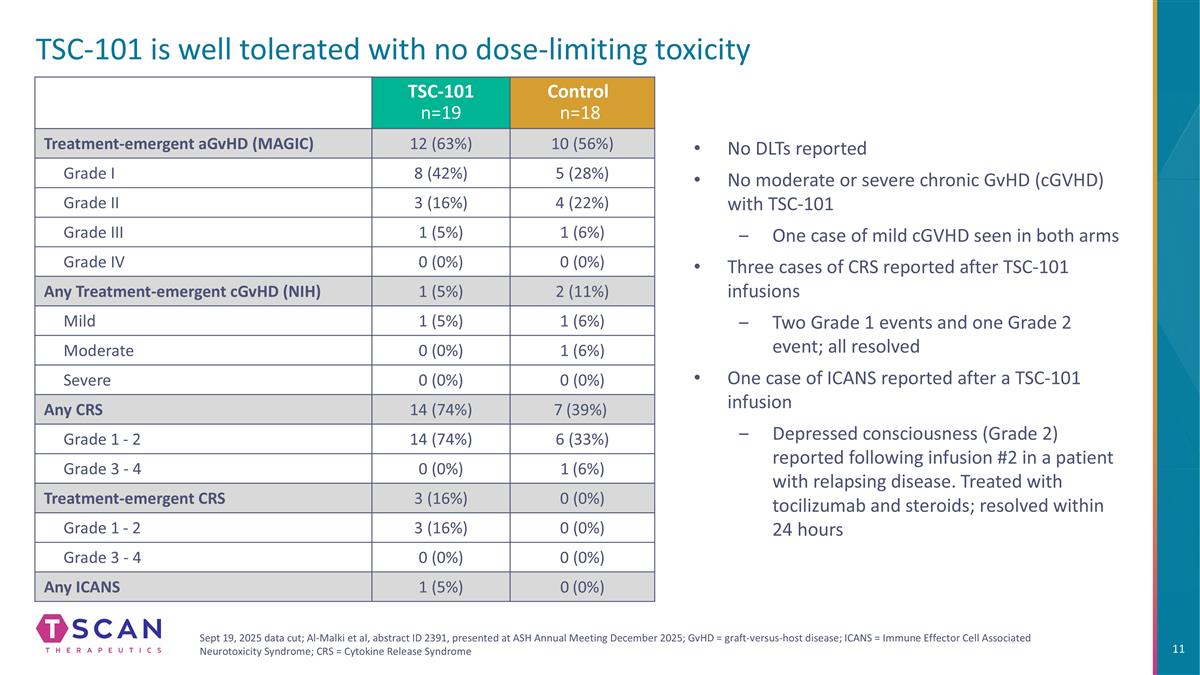
TSC-101 is well tolerated with no dose-limiting toxicity TSC-101 n=19 Control n=18 Treatment-emergent aGvHD (MAGIC) 12 (63%) 10 (56%) Grade I 8 (42%) 5 (28%) Grade II 3 (16%) 4 (22%) Grade III 1 (5%) 1 (6%) Grade IV 0 (0%) 0 (0%) Any Treatment-emergent cGvHD (NIH) 1 (5%) 2 (11%) Mild 1 (5%) 1 (6%) Moderate 0 (0%) 1 (6%) Severe 0 (0%) 0 (0%) Any CRS 14 (74%) 7 (39%) Grade 1 - 2 14 (74%) 6 (33%) Grade 3 - 4 0 (0%) 1 (6%) Treatment-emergent CRS 3 (16%) 0 (0%) Grade 1 - 2 3 (16%) 0 (0%) Grade 3 - 4 0 (0%) 0 (0%) Any ICANS 1 (5%) 0 (0%) No DLTs reported No moderate or severe chronic GvHD (cGVHD) with TSC-101 One case of mild cGVHD seen in both arms Three cases of CRS reported after TSC-101 infusions Two Grade 1 events and one Grade 2 event; all resolved One case of ICANS reported after a TSC-101 infusion Depressed consciousness (Grade 2) reported following infusion #2 in a patient with relapsing disease. Treated with tocilizumab and steroids; resolved within 24 hours Sept 19, 2025 data cut; Al-Malki et al, abstract ID 2391, presented at ASH Annual Meeting December 2025; GvHD = graft-versus-host disease; ICANS = Immune Effector Cell Associated Neurotoxicity Syndrome; CRS = Cytokine Release Syndrome
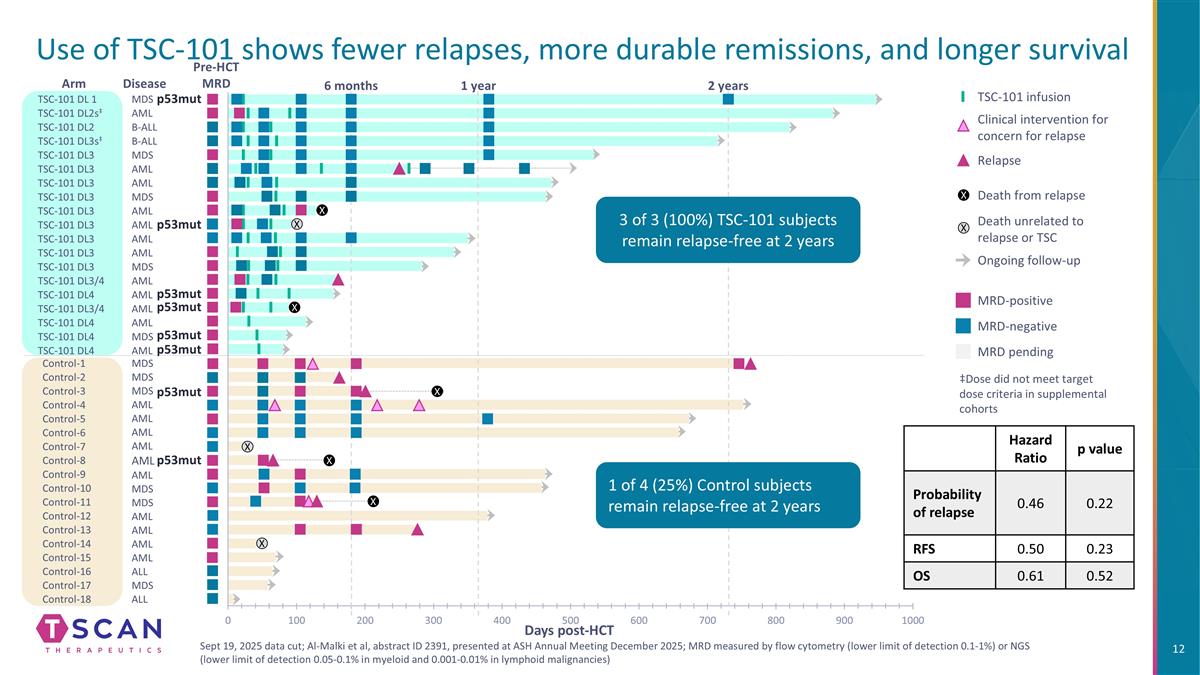
Use of TSC-101 shows fewer relapses, more durable remissions, and longer survival MDS MDS MDS AML AML AML AML AML AML MDS MDS AML Control-1 Control-2 Control-3 Control-7 Control-8 Control-5 Control-4 Control-6 Control-9 Control-10 Control-11 Control-12 Control-13 AML AML Control-14 AML Control-15 ALL Control-16 Control-17 Control-18 MDS ALL Arm B-ALL B-ALL MDS MDS AML AML AML AML AML MDS TSC-101 DL 1 TSC-101 DL2s‡ TSC-101 DL2 TSC-101 DL3s‡ TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 Disease AML AML TSC-101 DL3 MDS TSC-101 DL3/4 AML TSC-101 DL4 TSC-101 DL3/4 TSC-101 DL4 AML AML AML TSC-101 DL4 MDS TSC-101 DL4 AML 2 years 1 year 6 months X X X X X X X X Days post-HCT ‡Dose did not meet target dose criteria in supplemental cohorts MRD-positive MRD-negative MRD pending TSC-101 infusion Clinical intervention for concern for relapse Ongoing follow-up Death from relapse Death unrelated to relapse or TSC Relapse X X p53mut p53mut p53mut p53mut p53mut p53mut p53mut p53mut Hazard Ratio p value Probability of relapse 0.46 0.22 RFS 0.50 0.23 OS 0.61 0.52 Sept 19, 2025 data cut; Al-Malki et al, abstract ID 2391, presented at ASH Annual Meeting December 2025; MRD measured by flow cytometry (lower limit of detection 0.1-1%) or NGS (lower limit of detection 0.05-0.1% in myeloid and 0.001-0.01% in lymphoid malignancies) Pre-HCT MRD 3 of 3 (100%) TSC-101 subjects remain relapse-free at 2 years 1 of 4 (25%) Control subjects remain relapse-free at 2 years
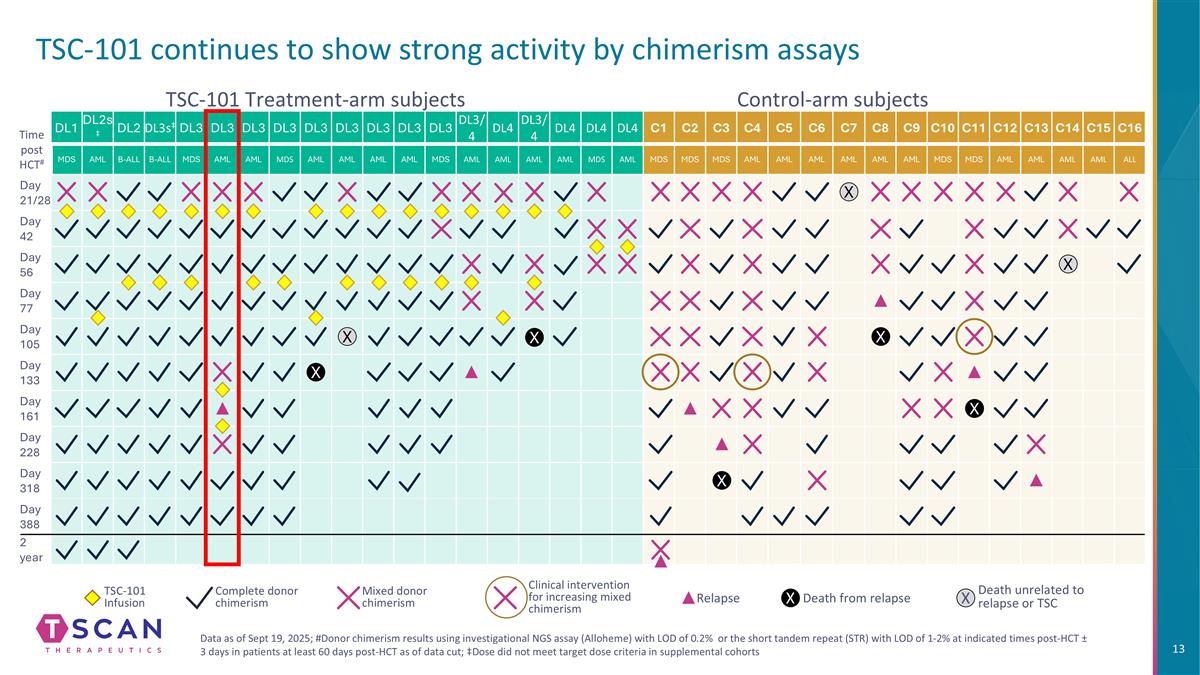
TSC-101 continues to show strong activity by chimerism assays DL1 DL2s‡ DL2 DL3s‡ DL3 DL3 DL3 DL3 DL3 DL3 DL3 DL3 DL3 DL3/4 DL4 DL3/4 DL4 DL4 DL4 C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 C11 C12 C13 C14 C15 C16 MDS AML B-ALL B-ALL MDS AML AML MDS AML AML AML AML MDS AML AML AML AML MDS AML MDS MDS MDS AML AML AML AML AML AML MDS MDS AML AML AML AML ALL Day 21/28 Day 42 Day 56 Day 77 Day 105 Day 133 Day 161 Day 228 Day 318 Day 388 2 year Control-arm subjects TSC-101 Treatment-arm subjects Time post HCT# X X X X X TSC-101 Infusion Complete donor chimerism Mixed donor chimerism Clinical intervention for increasing mixed chimerism Relapse Death from relapse X Death unrelated to relapse or TSC X X X X Data as of Sept 19, 2025; #Donor chimerism results using investigational NGS assay (Alloheme) with LOD of 0.2% or the short tandem repeat (STR) with LOD of 1-2% at indicated times post-HCT ± 3 days in patients at least 60 days post-HCT as of data cut; ‡Dose did not meet target dose criteria in supplemental cohorts
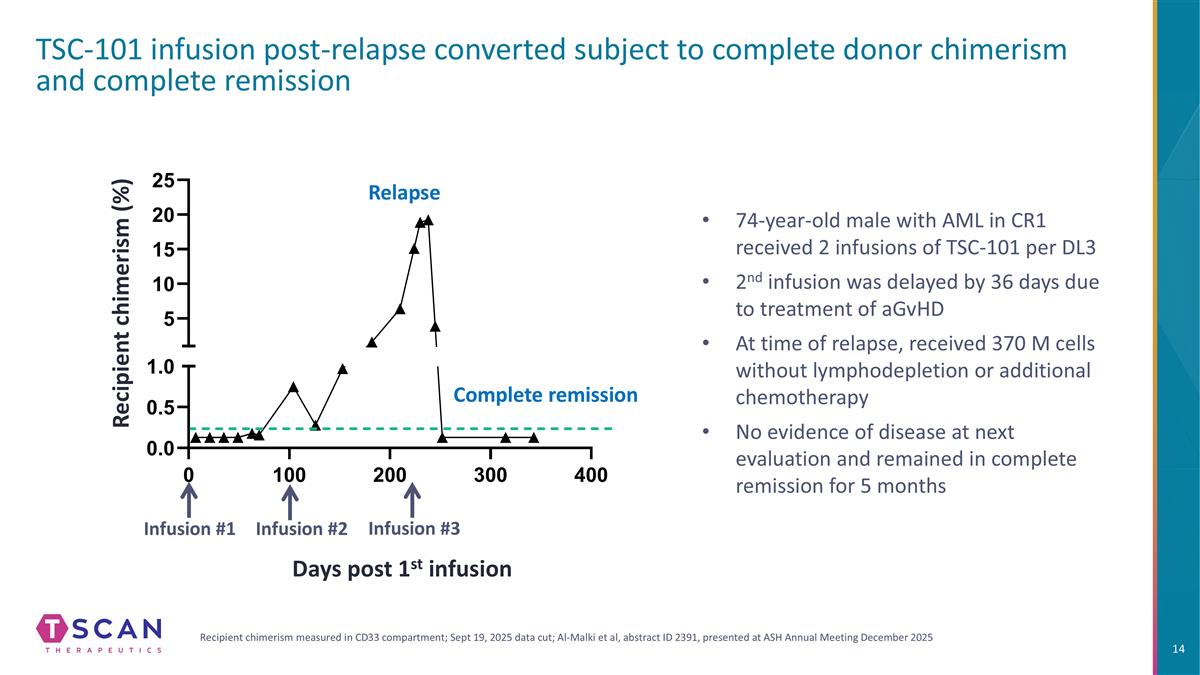
TSC-101 infusion post-relapse converted subject to complete donor chimerism and complete remission Recipient chimerism (%) 74-year-old male with AML in CR1 received 2 infusions of TSC-101 per DL3 2nd infusion was delayed by 36 days due to treatment of aGvHD At time of relapse, received 370 M cells without lymphodepletion or additional chemotherapy No evidence of disease at next evaluation and remained in complete remission for 5 months Days post 1st infusion Infusion #2 Infusion #1 Infusion #3 Recipient chimerism measured in CD33 compartment; Sept 19, 2025 data cut; Al-Malki et al, abstract ID 2391, presented at ASH Annual Meeting December 2025 Relapse Complete remission
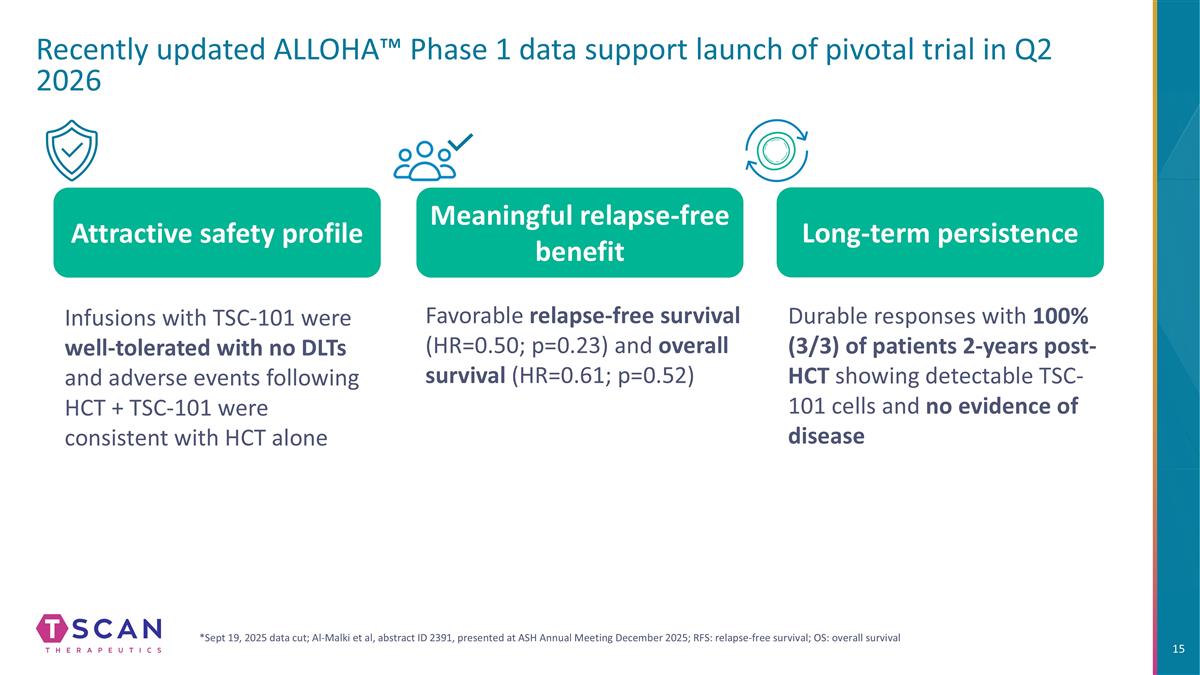
Recently updated ALLOHA™ Phase 1 data support launch of pivotal trial in Q2 2026 Infusions with TSC-101 were well-tolerated with no DLTs and adverse events following HCT + TSC-101 were consistent with HCT alone Durable responses with 100% (3/3) of patients 2-years post-HCT showing detectable TSC-101 cells and no evidence of disease Attractive safety profile Long-term persistence Meaningful relapse-free benefit *Sept 19, 2025 data cut; Al-Malki et al, abstract ID 2391, presented at ASH Annual Meeting December 2025; RFS: relapse-free survival; OS: overall survival Favorable relapse-free survival (HR=0.50; p=0.23) and overall survival (HR=0.61; p=0.52)

Heme Development Strategy Targeting residual disease to prevent relapse in patients undergoing allogeneic HCT
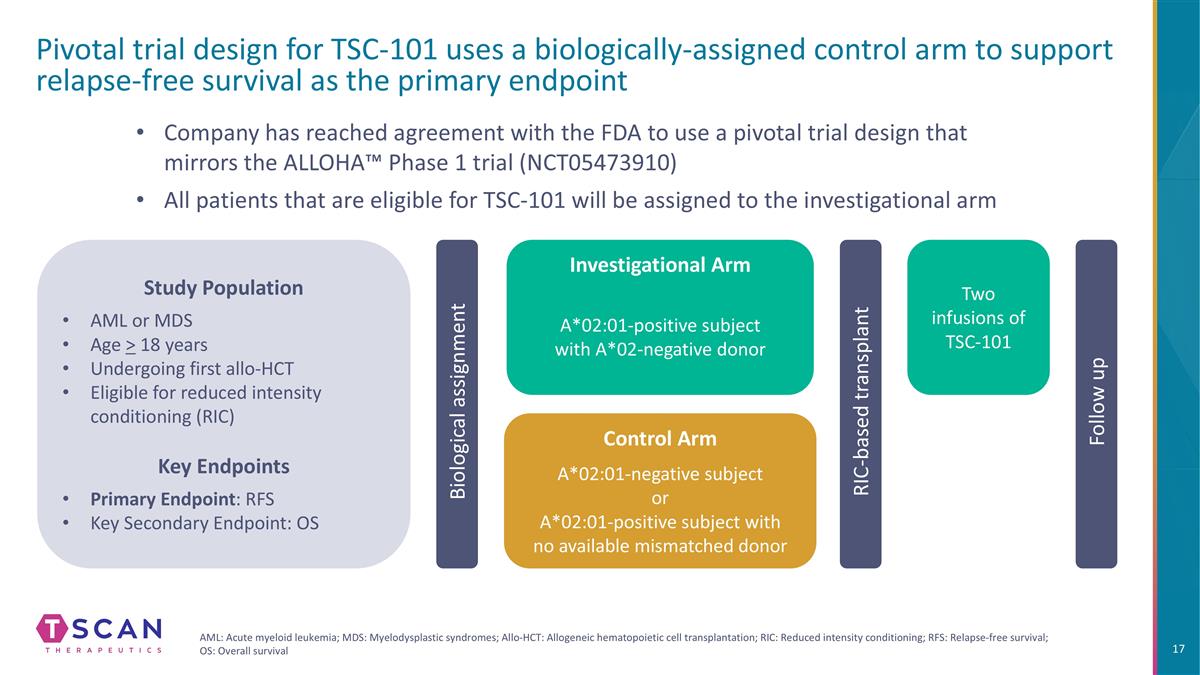
Pivotal trial design for TSC-101 uses a biologically-assigned control arm to support relapse-free survival as the primary endpoint Biological assignment Company has reached agreement with the FDA to use a pivotal trial design that mirrors the ALLOHA™ Phase 1 trial (NCT05473910) All patients that are eligible for TSC-101 will be assigned to the investigational arm Two infusions of TSC-101 RIC-based transplant Follow up Study Population AML or MDS Age > 18 years Undergoing first allo-HCT Eligible for reduced intensity conditioning (RIC) Key Endpoints Primary Endpoint: RFS Key Secondary Endpoint: OS AML: Acute myeloid leukemia; MDS: Myelodysplastic syndromes; Allo-HCT: Allogeneic hematopoietic cell transplantation; RIC: Reduced intensity conditioning; RFS: Relapse-free survival; OS: Overall survival Investigational Arm A*02:01-positive subject with A*02-negative donor Control Arm A*02:01-negative subject or A*02:01-positive subject with no available mismatched donor

Commercial opportunity: Clear unmet need with concentrated market and a broad range of expansion opportunities
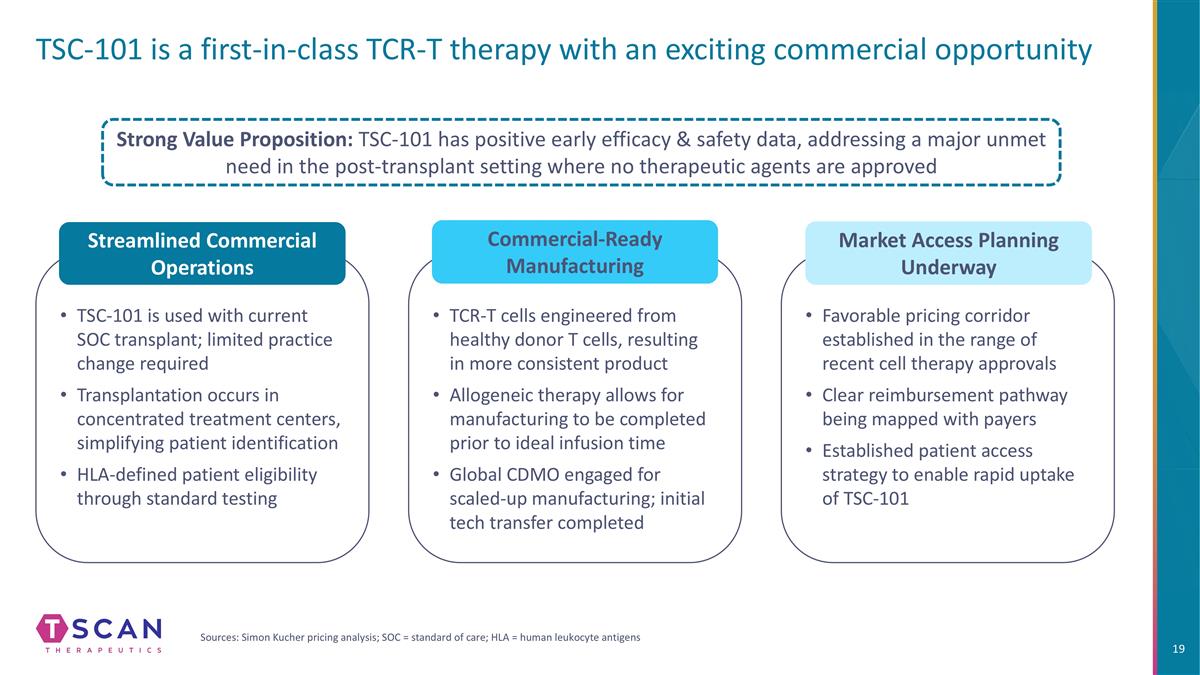
TCR-T cells engineered from healthy donor T cells, resulting in more consistent product Allogeneic therapy allows for manufacturing to be completed prior to ideal infusion time Global CDMO engaged for scaled-up manufacturing; initial tech transfer completed TSC-101 is used with current SOC transplant; limited practice change required Transplantation occurs in concentrated treatment centers, simplifying patient identification HLA-defined patient eligibility through standard testing TSC-101 is a first-in-class TCR-T therapy with an exciting commercial opportunity Streamlined Commercial Operations Commercial-Ready Manufacturing Strong Value Proposition: TSC-101 has positive early efficacy & safety data, addressing a major unmet need in the post-transplant setting where no therapeutic agents are approved Sources: Simon Kucher pricing analysis; SOC = standard of care; HLA = human leukocyte antigens Favorable pricing corridor established in the range of recent cell therapy approvals Clear reimbursement pathway being mapped with payers Established patient access strategy to enable rapid uptake of TSC-101 Market Access Planning Underway
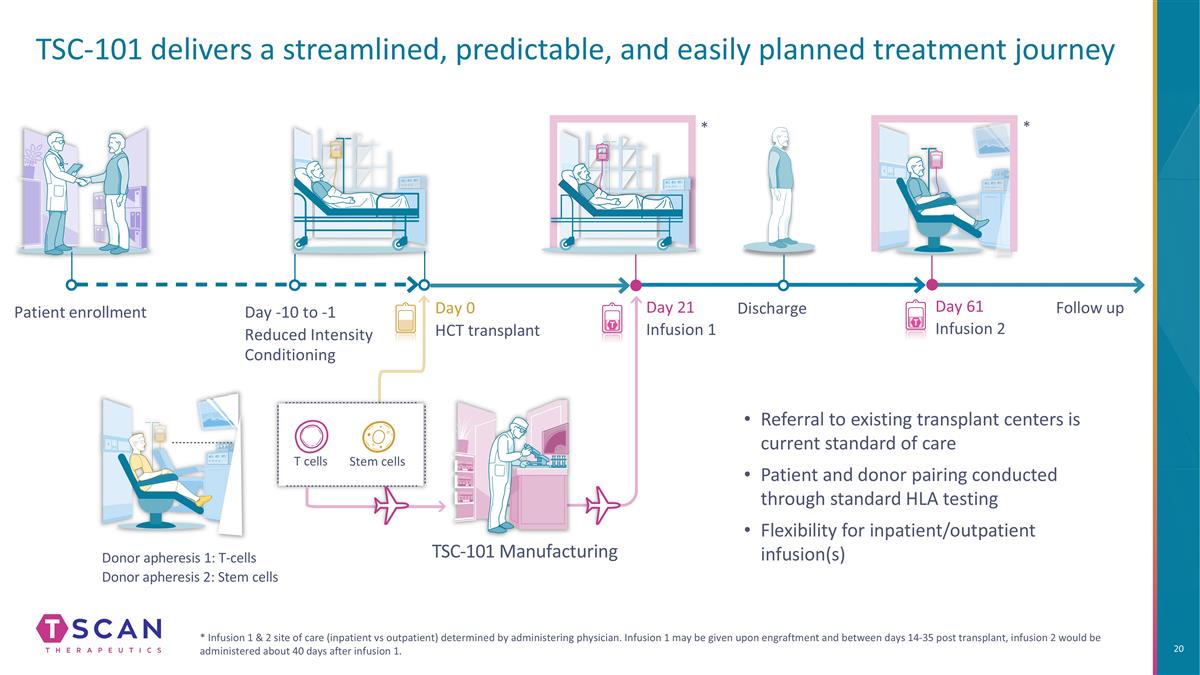
TSC-101 delivers a streamlined, predictable, and easily planned treatment journey * Infusion 1 & 2 site of care (inpatient vs outpatient) determined by administering physician. Infusion 1 may be given upon engraftment and between days 14-35 post transplant, infusion 2 would be administered about 40 days after infusion 1. Patient enrollment Day 0 HCT transplant Donor apheresis 1: T-cells Donor apheresis 2: Stem cells Day 21 Infusion 1 Day 61 Infusion 2 Follow up TSC-101 Manufacturing Day -10 to -1 Reduced Intensity Conditioning Discharge Stem cells T cells * * Referral to existing transplant centers is current standard of care Patient and donor pairing conducted through standard HLA testing Flexibility for inpatient/outpatient infusion(s)
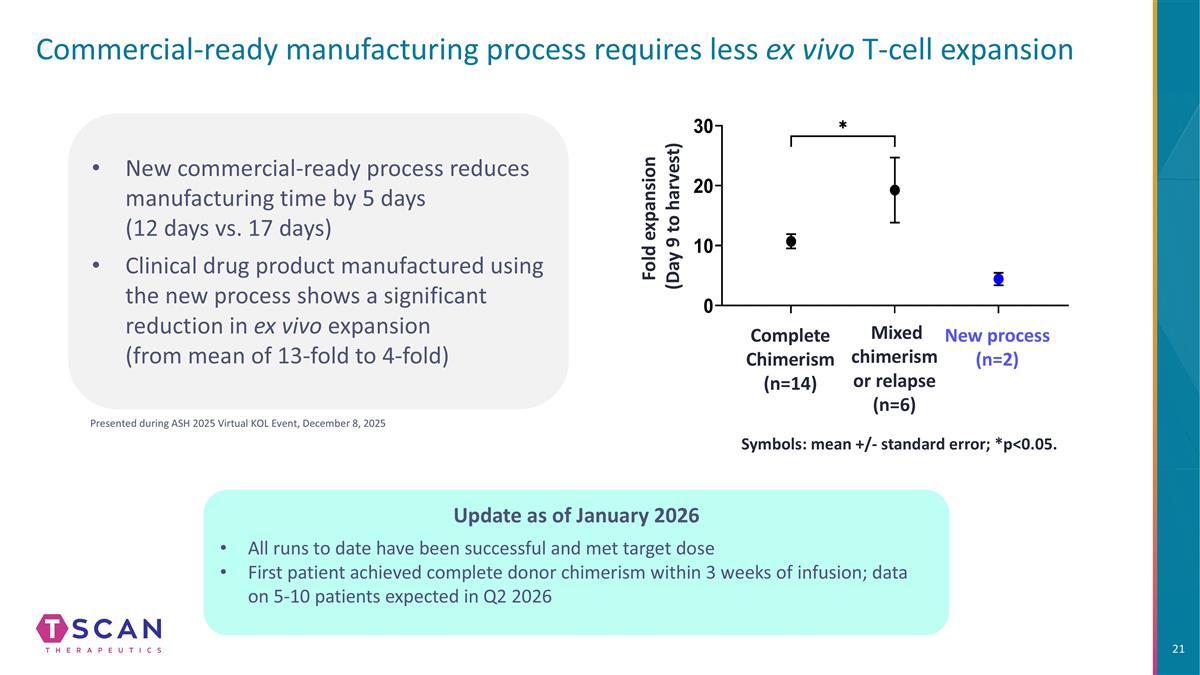
Commercial-ready manufacturing process requires less ex vivo T-cell expansion Symbols: mean +/- standard error; *p<0.05. Mixed chimerism or relapse (n=6) Complete Chimerism (n=14) New process (n=2) Fold expansion (Day 9 to harvest) New commercial-ready process reduces manufacturing time by 5 days (12 days vs. 17 days) Clinical drug product manufactured using the new process shows a significant reduction in ex vivo expansion (from mean of 13-fold to 4-fold) Presented during ASH 2025 Virtual KOL Event, December 8, 2025 Update as of January 2026 All runs to date have been successful and met target dose First patient achieved complete donor chimerism within 3 weeks of infusion; data on 5-10 patients expected in Q2 2026
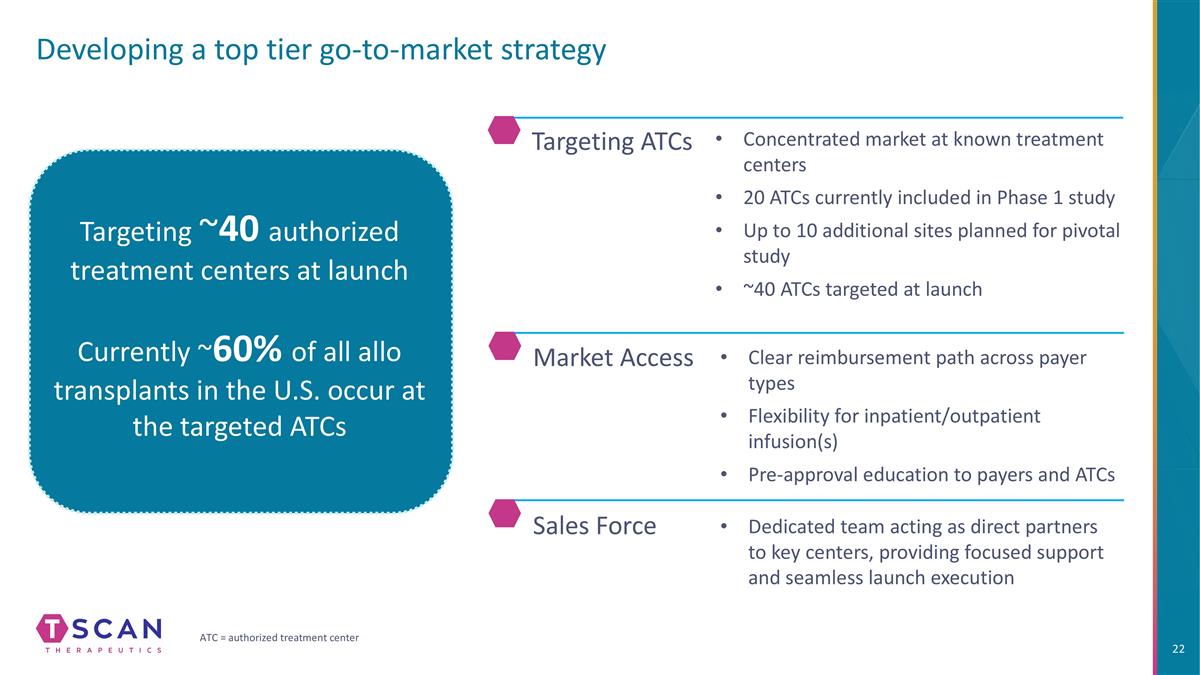
Developing a top tier go-to-market strategy Targeting ~40 authorized treatment centers at launch Currently ~60% of all allo transplants in the U.S. occur at the targeted ATCs Sales Force Market Access Dedicated team acting as direct partners to key centers, providing focused support and seamless launch execution Clear reimbursement path across payer types Flexibility for inpatient/outpatient infusion(s) Pre-approval education to payers and ATCs Targeting ATCs Concentrated market at known treatment centers 20 ATCs currently included in Phase 1 study Up to 10 additional sites planned for pivotal study ~40 ATCs targeted at launch ATC = authorized treatment center
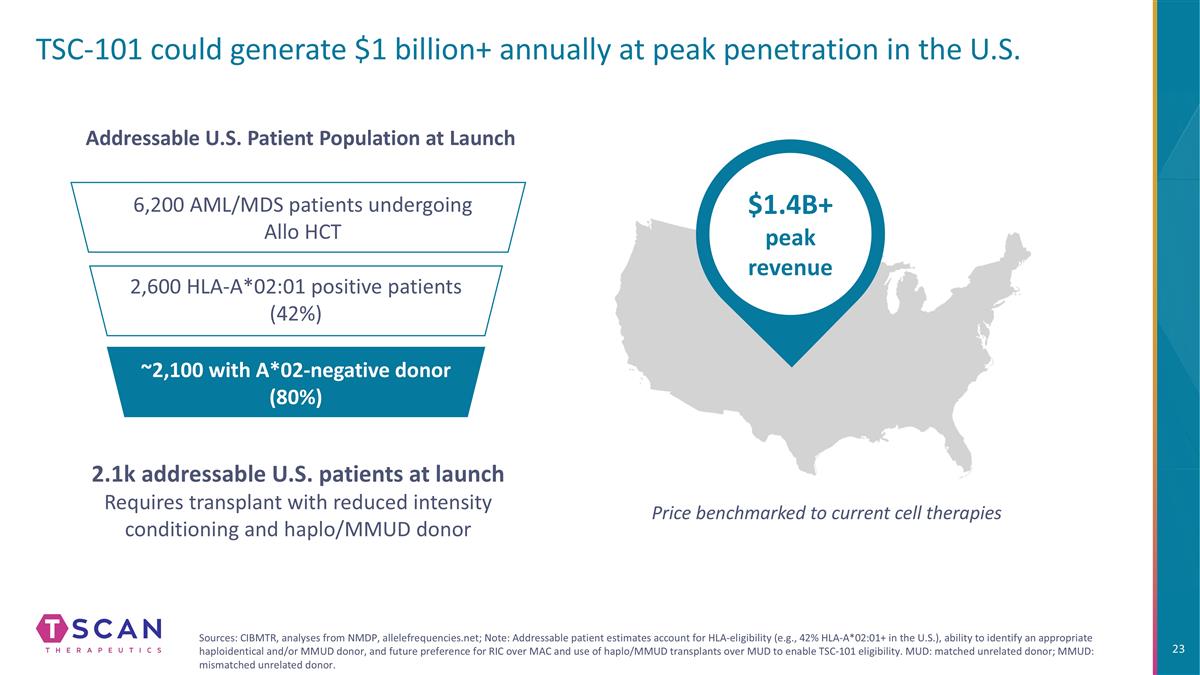
TSC-101 could generate $1 billion+ annually at peak penetration in the U.S. $1.4B+ peak revenue Price benchmarked to current cell therapies 6,200 AML/MDS patients undergoing Allo HCT 2,600 HLA-A*02:01 positive patients (42%) ~2,100 with A*02-negative donor (80%) Addressable U.S. Patient Population at Launch 2.1k addressable U.S. patients at launch Requires transplant with reduced intensity conditioning and haplo/MMUD donor Sources: CIBMTR, analyses from NMDP, allelefrequencies.net; Note: Addressable patient estimates account for HLA-eligibility (e.g., 42% HLA-A*02:01+ in the U.S.), ability to identify an appropriate haploidentical and/or MMUD donor, and future preference for RIC over MAC and use of haplo/MMUD transplants over MUD to enable TSC-101 eligibility. MUD: matched unrelated donor; MMUD: mismatched unrelated donor.
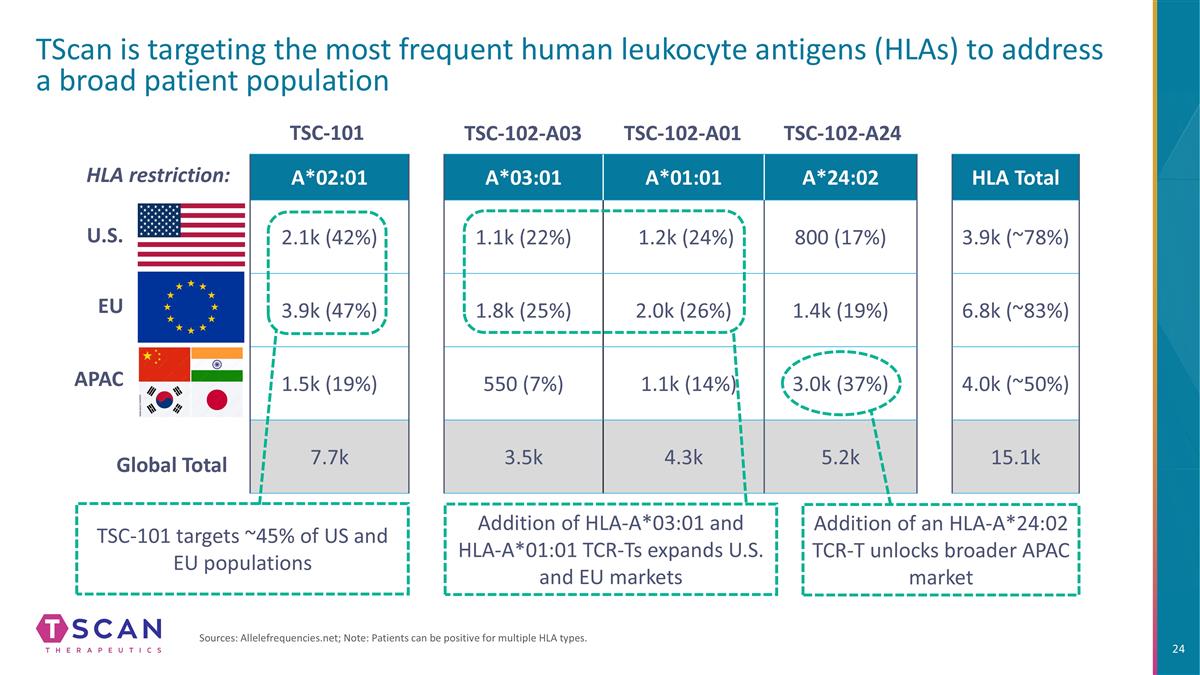
A*03:01 A*01:01 A*24:02 HLA Total 1.1k (22%) 1.2k (24%) 800 (17%) 3.9k (~78%) 1.8k (25%) 2.0k (26%) 1.4k (19%) 6.8k (~83%) 550 (7%) 1.1k (14%) 3.0k (37%) 4.0k (~50%) 3.5k 4.3k 5.2k 15.1k A*02:01 2.1k (42%) 3.9k (47%) 1.5k (19%) 7.7k TScan is targeting the most frequent human leukocyte antigens (HLAs) to address a broad patient population Sources: Allelefrequencies.net; Note: Patients can be positive for multiple HLA types. U.S. EU APAC HLA restriction: Addition of HLA-A*03:01 and HLA-A*01:01 TCR-Ts expands U.S. and EU markets Addition of an HLA-A*24:02 TCR-T unlocks broader APAC market TSC-101 targets ~45% of US and EU populations TSC-101 TSC-102-A03 TSC-102-A01 TSC-102-A24 Global Total
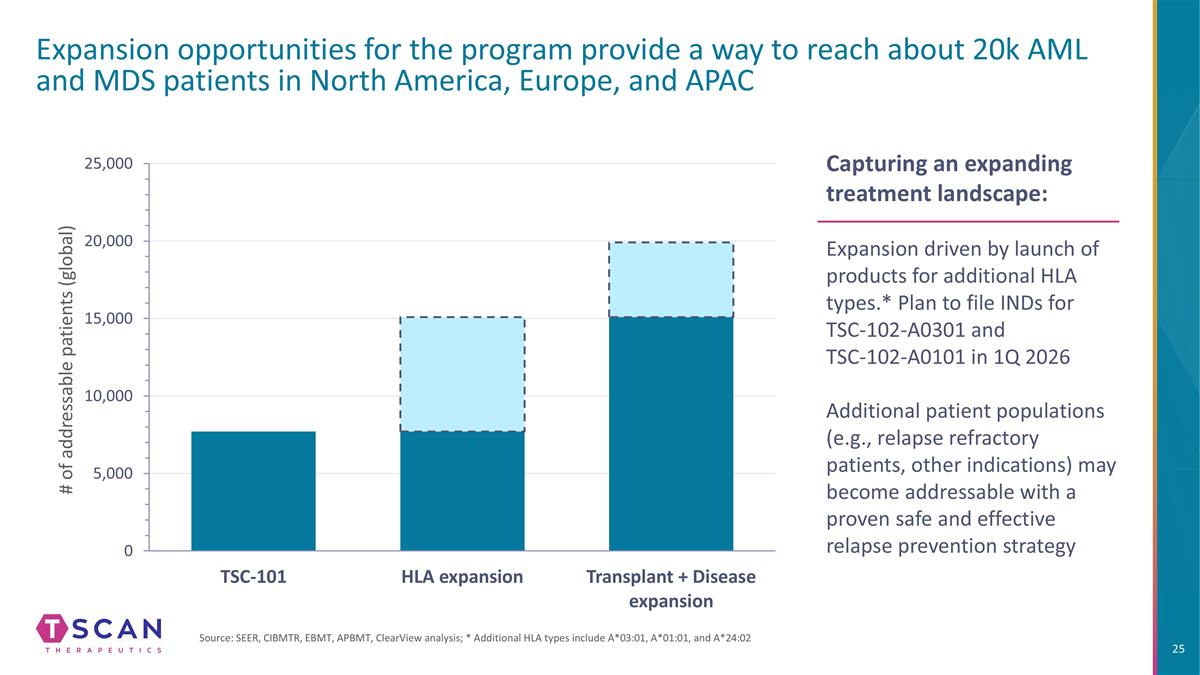
Expansion opportunities for the program provide a way to reach about 20k AML and MDS patients in North America, Europe, and APAC Capturing an expanding treatment landscape: Expansion driven by launch of products for additional HLA types.* Plan to file INDs for TSC-102-A0301 and TSC-102-A0101 in 1Q 2026 Additional patient populations (e.g., relapse refractory patients, other indications) may become addressable with a proven safe and effective relapse prevention strategy # of addressable patients (global) Source: SEER, CIBMTR, EBMT, APBMT, ClearView analysis; * Additional HLA types include A*03:01, A*01:01, and A*24:02
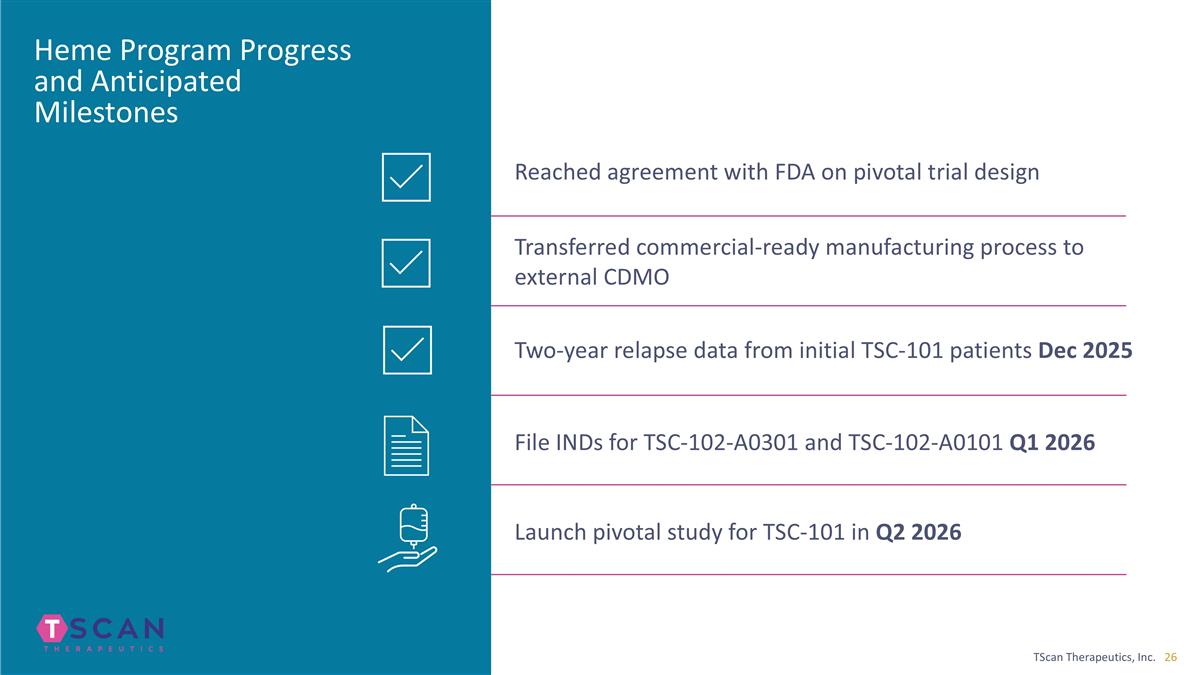
Heme Program Progress and Anticipated Milestones Reached agreement with FDA on pivotal trial design Transferred commercial-ready manufacturing process to external CDMO File INDs for TSC-102-A0301 and TSC-102-A0101 Q1 2026 Launch pivotal study for TSC-101 in Q2 2026 Two-year relapse data from initial TSC-101 patients Dec 2025

Solid Tumors Developing multiplex TCR-T therapy to overcome tumor heterogeneity
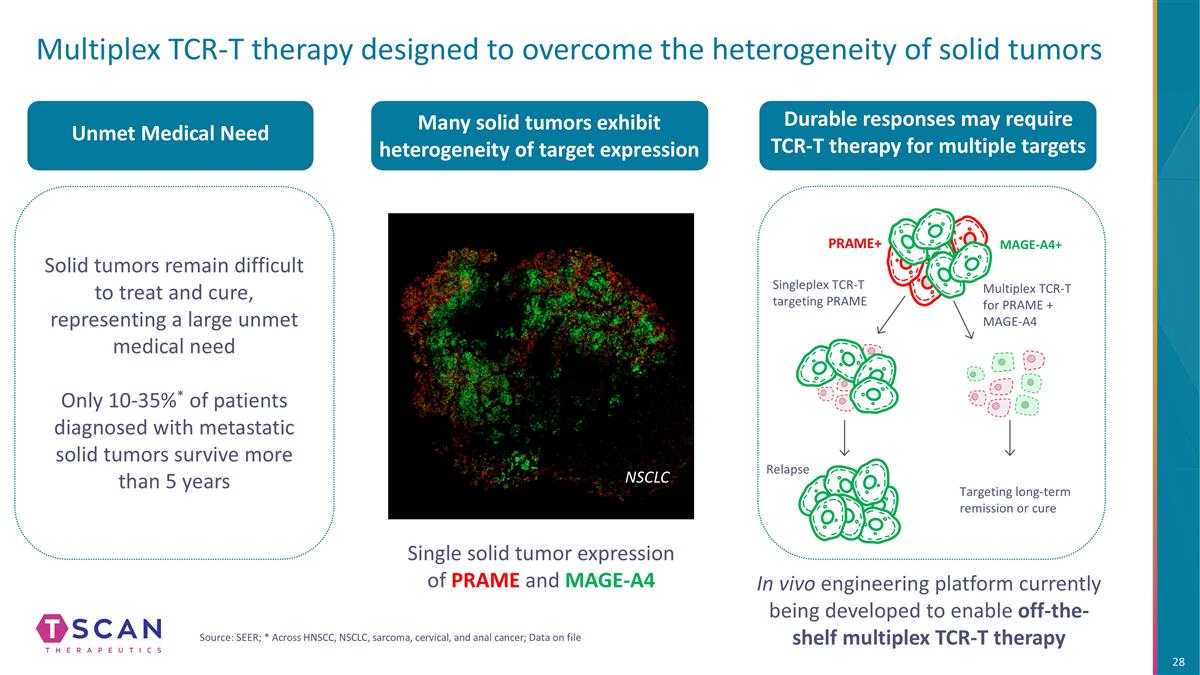
Multiplex TCR-T therapy designed to overcome the heterogeneity of solid tumors Relapse Targeting long-term remission or cure Many solid tumors exhibit heterogeneity of target expression NSCLC Single solid tumor expression of PRAME and MAGE-A4 MAGE-A4+ Singleplex TCR-T targeting PRAME Multiplex TCR-T for PRAME + MAGE-A4 PRAME+ Solid tumors remain difficult to treat and cure, representing a large unmet medical need Only 10-35%* of patients diagnosed with metastatic solid tumors survive more than 5 years Unmet Medical Need Durable responses may require TCR-T therapy for multiple targets In vivo engineering platform currently being developed to enable off-the-shelf multiplex TCR-T therapy Source: SEER; * Across HNSCC, NSCLC, sarcoma, cervical, and anal cancer; Data on file
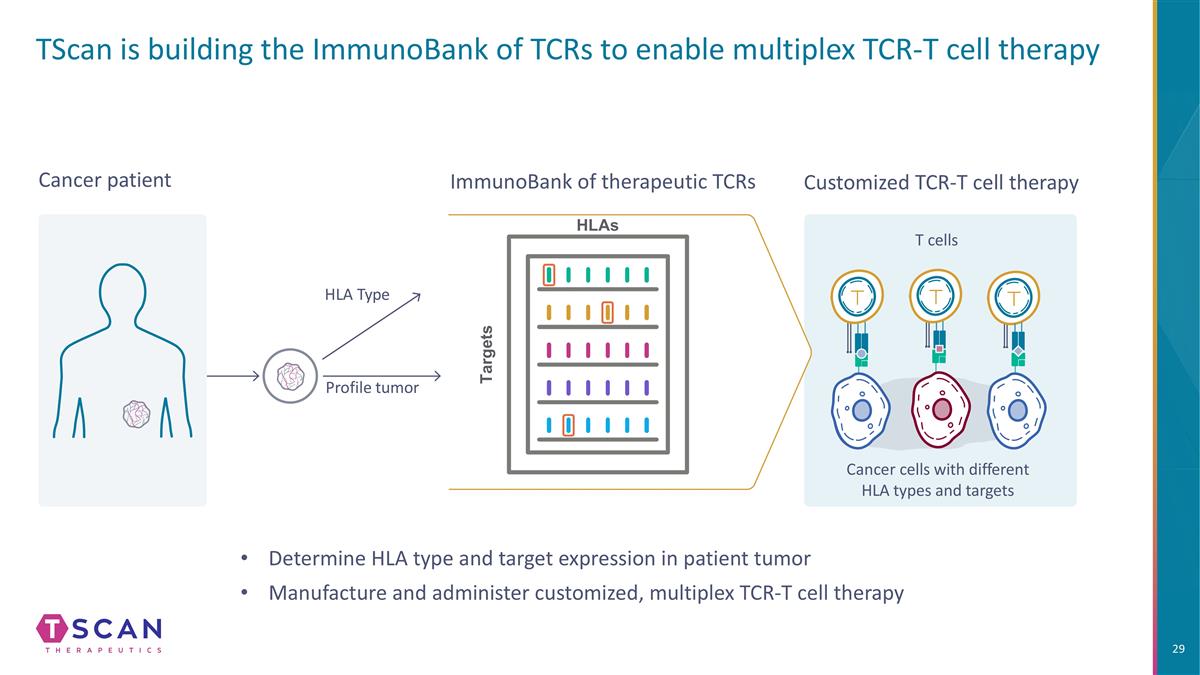
TScan is building the ImmunoBank of TCRs to enable multiplex TCR-T cell therapy Customized TCR-T cell therapy Cancer cells with different HLA types and targets T cells Cancer patient Profile tumor HLA Type ImmunoBank of therapeutic TCRs HLAs Targets Determine HLA type and target expression in patient tumor Manufacture and administer customized, multiplex TCR-T cell therapy
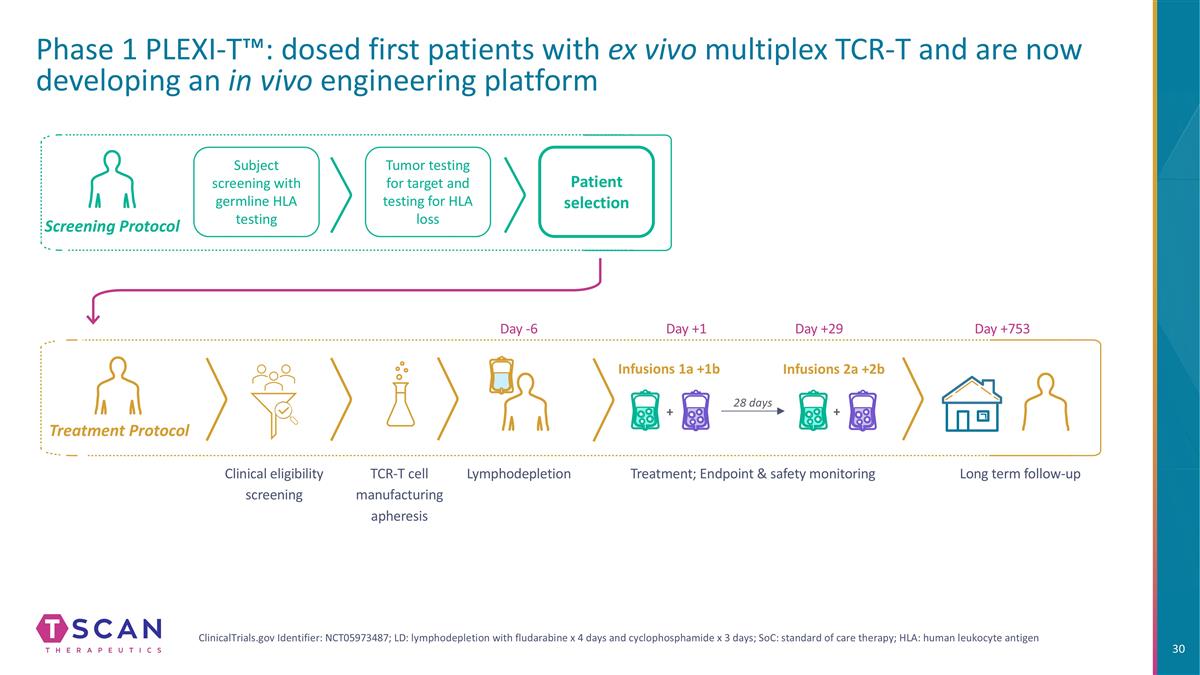
Tumor testing for target and testing for HLA loss Patient selection Infusions 1a +1b Infusions 2a +2b Subject screening with germline HLA testing Treatment Protocol Screening Protocol ClinicalTrials.gov Identifier: NCT05973487; LD: lymphodepletion with fludarabine x 4 days and cyclophosphamide x 3 days; SoC: standard of care therapy; HLA: human leukocyte antigen Phase 1 PLEXI-T™: dosed first patients with ex vivo multiplex TCR-T and are now developing an in vivo engineering platform Clinical eligibility screening TCR-T cell manufacturing apheresis Lymphodepletion Treatment; Endpoint & safety monitoring Long term follow-up Day -6 Day +1 Day +29 Day +753 28 days + +
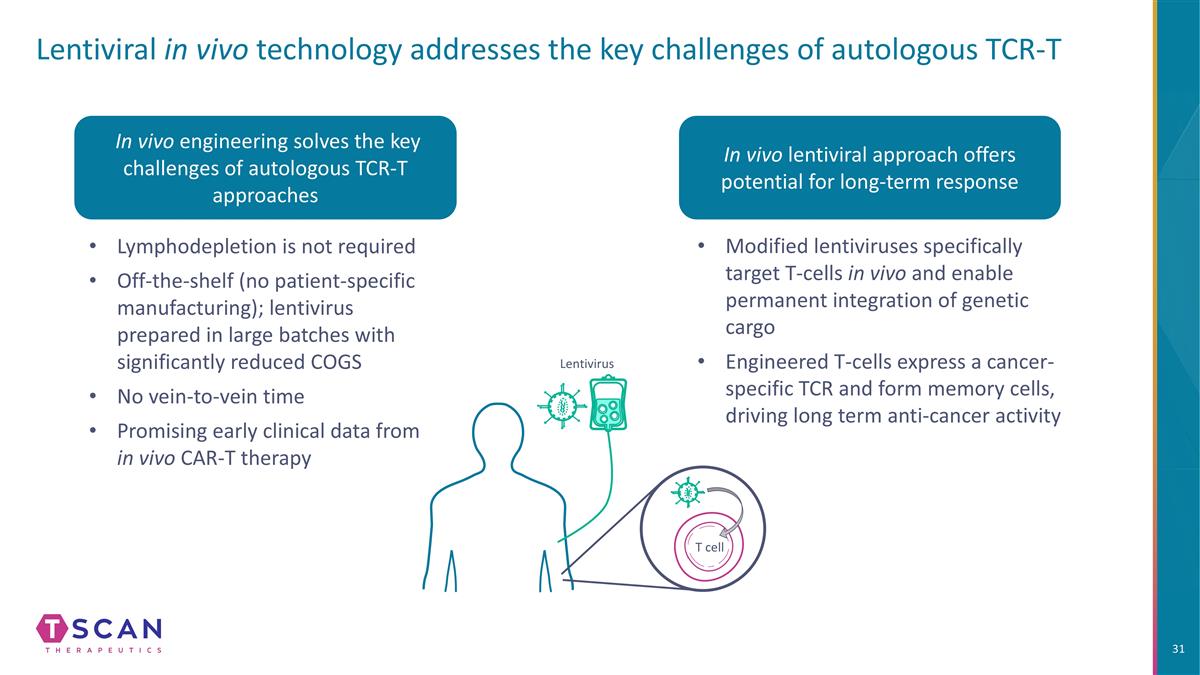
Lentivirus Lentiviral in vivo technology addresses the key challenges of autologous TCR-T In vivo engineering solves the key challenges of autologous TCR-T approaches Lymphodepletion is not required Off-the-shelf (no patient-specific manufacturing); lentivirus prepared in large batches with significantly reduced COGS No vein-to-vein time Promising early clinical data from in vivo CAR-T therapy In vivo lentiviral approach offers potential for long-term response Modified lentiviruses specifically target T-cells in vivo and enable permanent integration of genetic cargo Engineered T-cells express a cancer-specific TCR and form memory cells, driving long term anti-cancer activity T cell

Autoimmunity Deploying TargetScan platform to discover novel T-cell targets in autoimmune disorders
Autoimmunity represents an exciting area of unmet need with few validated targets TScan’s target discovery platform provides a way to identify targets in autoimmune disease, unlocking the development of targeted therapeutics Current therapies typically provide general immune suppression, leading to complications (e.g., increased risk of infection) Target-specific therapies provide a way to address the cause, rather than the symptoms, of autoimmunity Many autoimmune disorders have a substantial T-cell component, but the targets of these pathogenic or protective T-cells are largely unknown
TargetScan platform can be used to identify the shared autoantigens driving T-cell mediated autoimmune diseases, enabling development of first-in-class drugs Multi-year collaboration using TargetScan to identify targets for T cells in patients with Crohn’s disease Identified targets for systemic sclerosis, ulcerative colitis, ankylosing spondylitis, and birdshot uveitis using proprietary platform
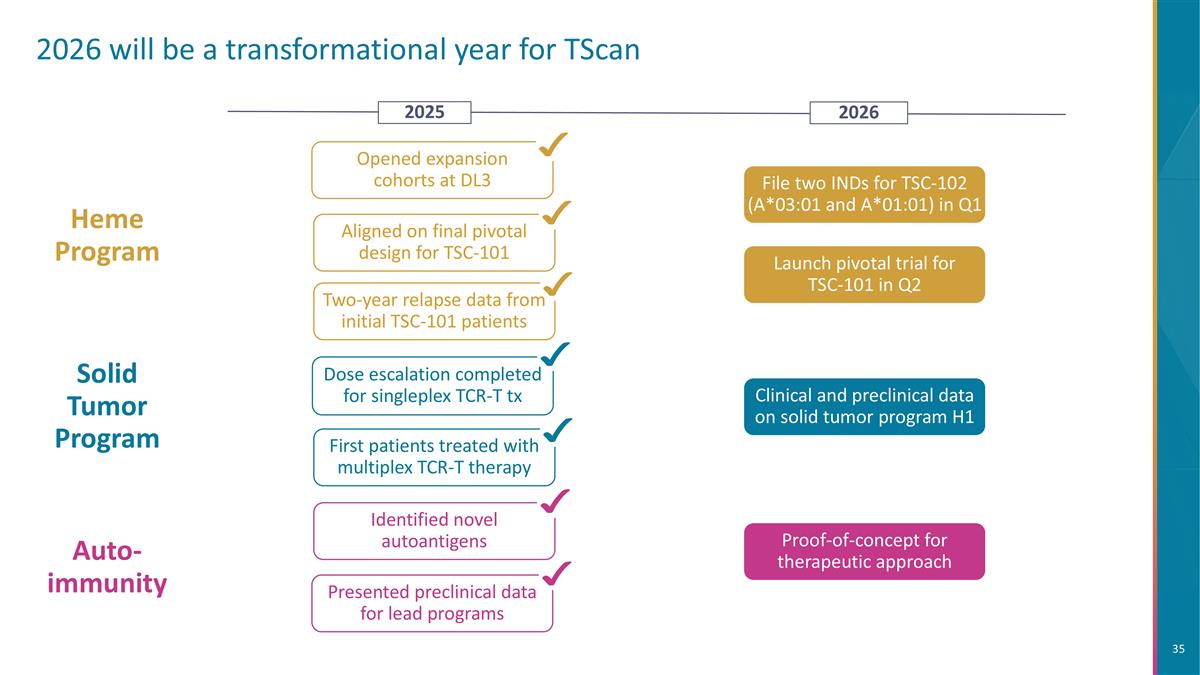
2026 will be a transformational year for TScan Aligned on final pivotal design for TSC-101 Two-year relapse data from initial TSC-101 patients Heme Program File two INDs for TSC-102 (A*03:01 and A*01:01) in Q1 Opened expansion cohorts at DL3 Auto-immunity Identified novel autoantigens Presented preclinical data for lead programs Clinical and preclinical data on solid tumor program H1 Solid Tumor Program First patients treated with multiplex TCR-T therapy Dose escalation completed for singleplex TCR-T tx 2026 2025 Proof-of-concept for therapeutic approach Launch pivotal trial for TSC-101 in Q2

THANK YOU