

Corporate Presentation January 2026

This presentation includes forward-looking statements, including forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation are forward looking statements, including statements regarding our cash runway, strategy and plans, industry environment, potential growth opportunities, and the therapeutic potential of our programs. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “design,” “expect,” “could,” “plan,” “potential,” “predict,” “seek,” “should,” “would,” or the negative version of these words and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including but not limited to, our ability to develop and advance our programs and product candidates, our ability to maintain and establish collaborations or strategic partnerships, our regulatory approvals and filings, and other risks, uncertainties and assumptions identified in our filings with the Securities and Exchange Commission (the “SEC”), including our most recent Form 10-K and Form 10-Q filed with the SEC, and any subsequent filings with the SEC. Moreover, we operate in a very competitive and rapidly changing environment, and it is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking statements and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, unless required by law. This presentation contains estimates and other information concerning our industry, our business and the markets for our products. Information that is based on estimates, market research or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. These sources include government and industry sources. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. While we believe our internal company estimates and research as to such matters is reliable and the market definitions are appropriate, neither such research nor these definitions have been verified by any independent source, and no reliance should be placed on or should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. Forward-looking statements
From novel genome editing systems to curative therapies An in vivo genome editing company capitalizing on its proprietary technologies to create curative genetic medicines Focusing on wholly owned programs in hemophilia A and secreted protein disorders, and partnered assets targeting cardiometabolic indications
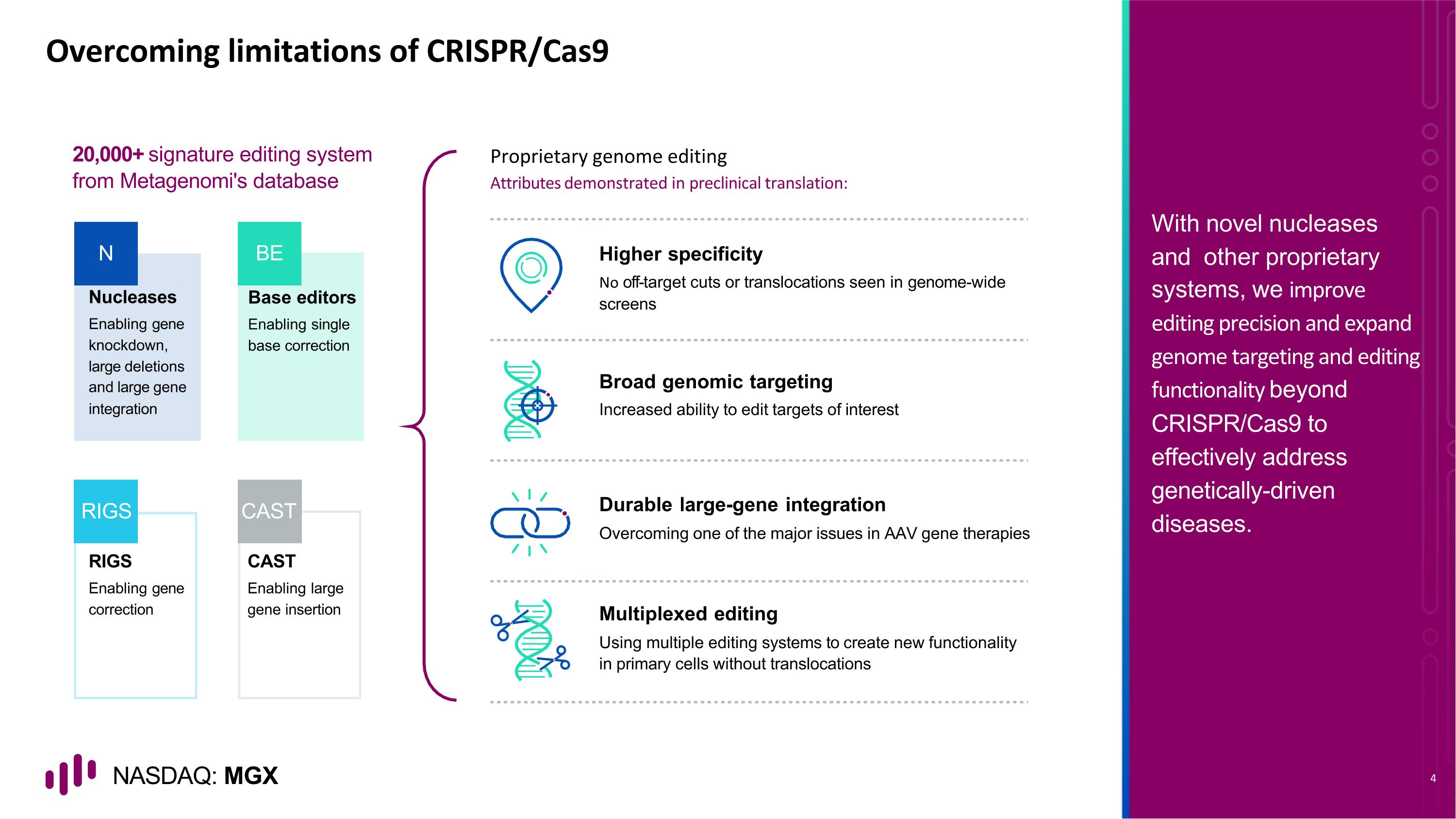
Overcoming limitations of CRISPR/Cas9 Nucleases Enabling gene knockdown, large deletions and large gene integration RIGS Enabling gene correction CAST Enabling large gene insertion Higher specificity No off-target cuts or translocations seen in genome-wide screens Multiplexed editing Using multiple editing systems to create new functionality in primary cells without translocations Durable large-gene integration Overcoming one of the major issues in AAV gene therapies Broad genomic targeting Increased ability to edit targets of interest N RIGS CAST 20,000+ signature editing system from Metagenomi's database Base editors Enabling single base correction BE Proprietary genome editing Attributes demonstrated in preclinical translation: With novel nucleases and other proprietary systems, we improve editing precision and expand genome targeting and editing functionality beyond CRISPR/Cas9 to effectively address genetically-driven diseases.
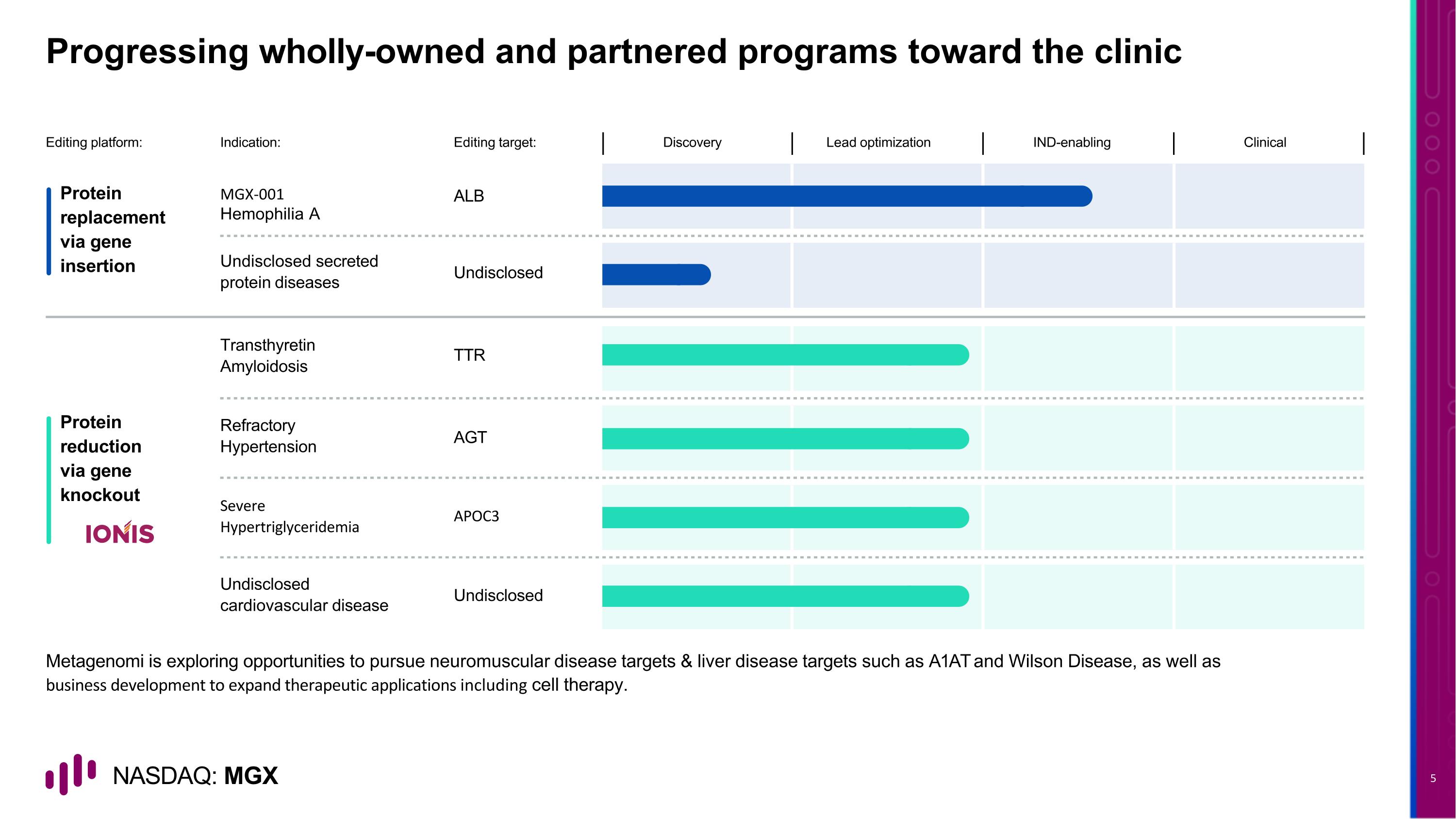
Progressing wholly-owned and partnered programs toward the clinic Protein replacement via gene insertion Protein reduction via gene knockout ALB MGX-001 Hemophilia A Editing platform: Editing target: Discovery Lead optimization IND-enabling Clinical Undisclosed Undisclosed secreted protein diseases TTR Transthyretin Amyloidosis AGT Refractory Hypertension APOC3 Severe Hypertriglyceridemia Undisclosed Undisclosed cardiovascular disease Metagenomi is exploring opportunities to pursue neuromuscular disease targets & liver disease targets such as A1AT and Wilson Disease, as well as business development to expand therapeutic applications including cell therapy. Indication:
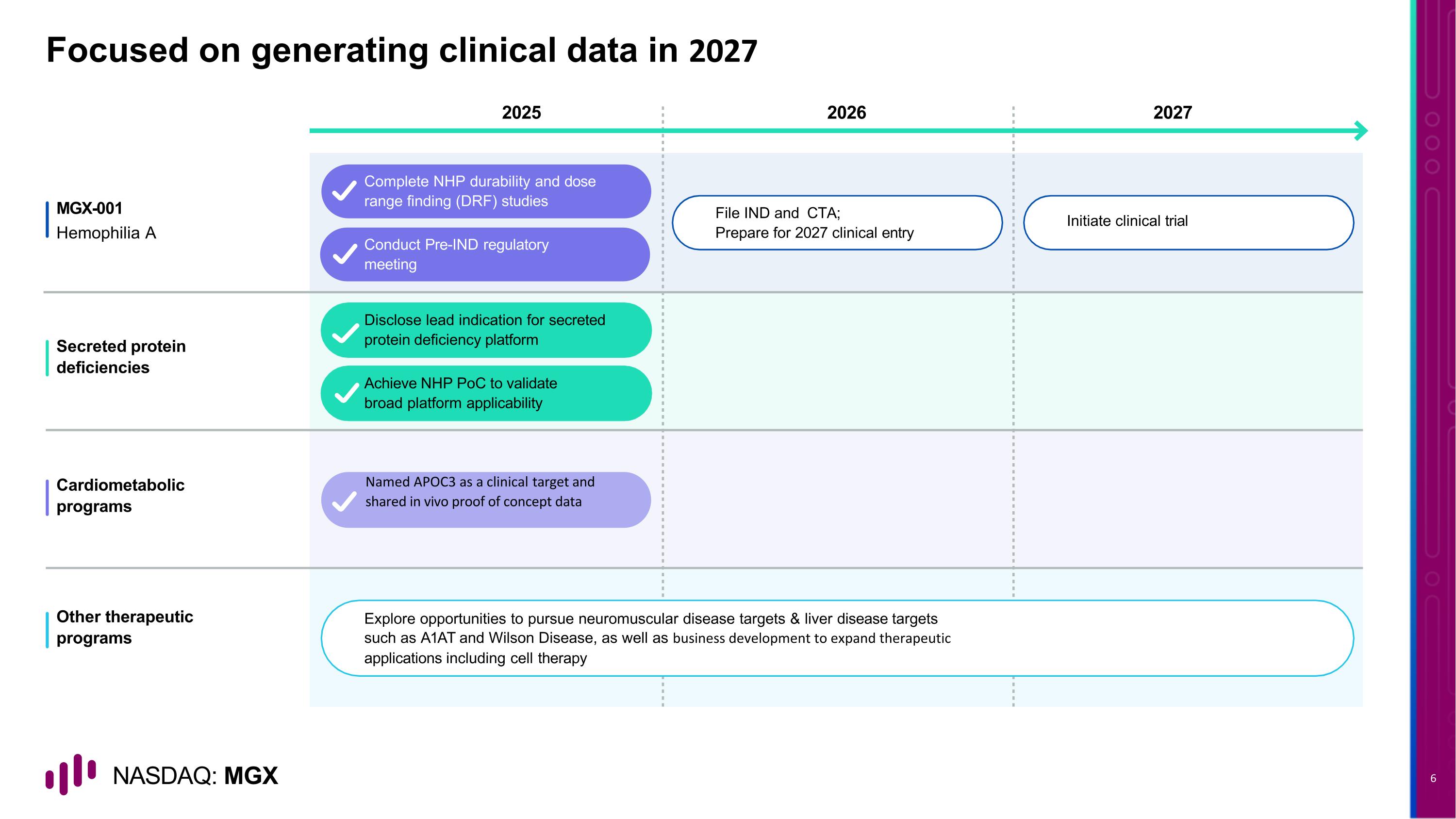
Focused on generating clinical data in 2027 2026 2027 2025 MGX-001 Hemophilia A Secreted protein deficiencies Cardiometabolic programs Other therapeutic programs Complete NHP durability and dose range finding (DRF) studies Disclose lead indication for secreted protein deficiency platform File IND and CTA; Prepare for 2027 clinical entry Initiate clinical trial Conduct Pre-IND regulatory meeting Achieve NHP PoC to validate broad platform applicability Named APOC3 as a clinical target and shared in vivo proof of concept data Explore opportunities to pursue neuromuscular disease targets & liver disease targets such as A1AT and Wilson Disease, as well as business development to expand therapeutic applications including cell therapy

MGX-001 in hemophilia A advancing toward clinic Demonstrated curative Factor VIII activity with best-in-class treatment potential Dose-dependent efficacy of both AAV and LNP Therapeutically relevant FVIII activity in each animal treated in all but the lowest dose Data informs clinical dose regimen strategy Potential competitive advantages of MGX-001 Enables endogenous production of FVIII for hemostatic regulation Potential to effectively treat both adults and children One-time potentially curative therapy allowing patients a hemophilia free mindset Builds on previous data Durable FVIII activity over an approximately 19-month study Encouraging safety profile, with minimal steroid use at the time of dosing No off-target editing IND submission on track for Q4 2026 Completed pre-IND meeting in December 2025 Ongoing KOL and patient advocacy engagement
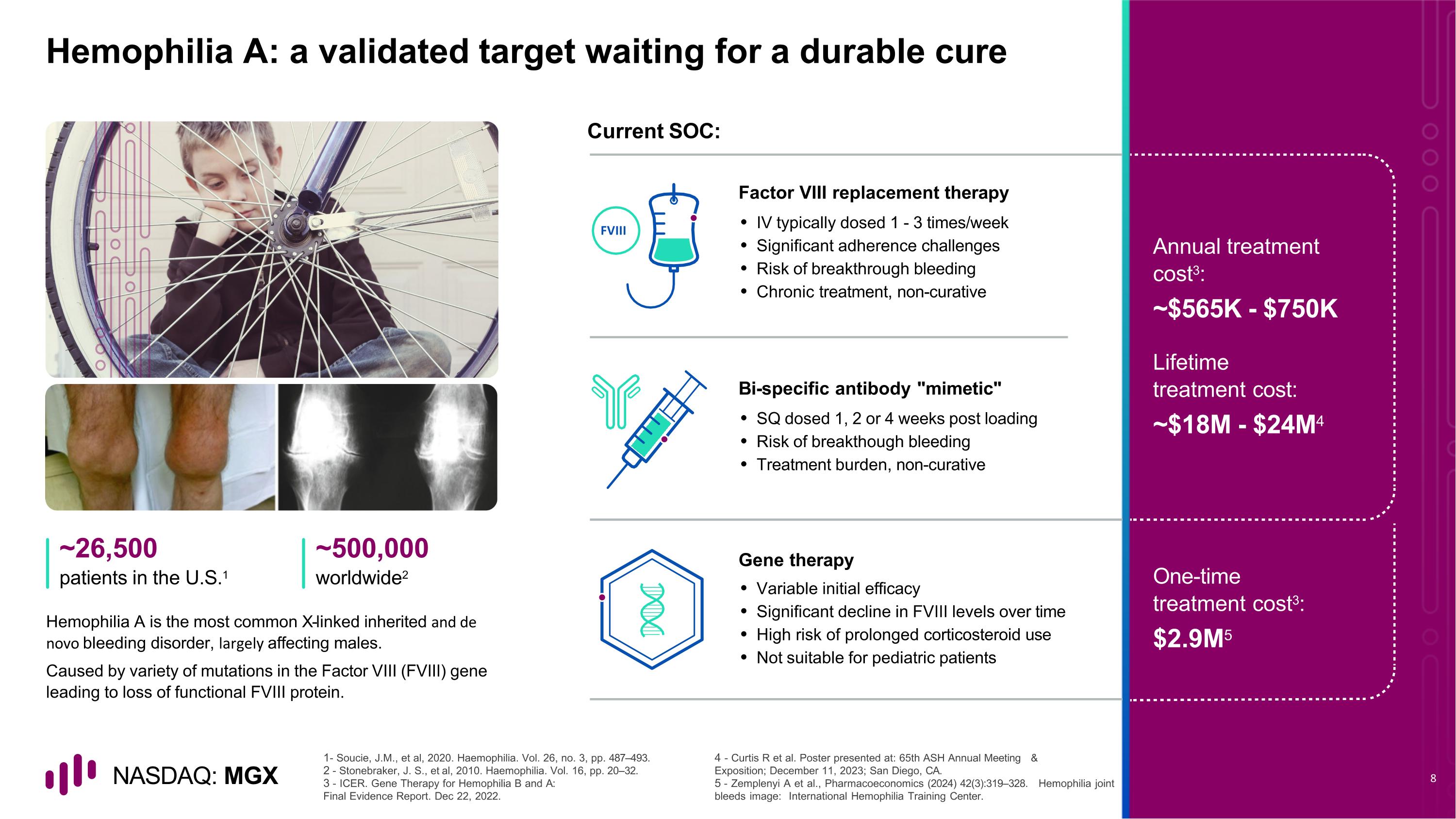
Hemophilia A: a validated target waiting for a durable cure Hemophilia A is the most common X-linked inherited and de novo bleeding disorder, largely affecting males. Caused by variety of mutations in the Factor VIII (FVIII) gene leading to loss of functional FVIII protein. Current SOC: ~26,500 patients in the U.S.1 ~500,000 worldwide2 - Soucie, J.M., et al, 2020. Haemophilia. Vol. 26, no. 3, pp. 487–493. - Stonebraker, J. S., et al, 2010. Haemophilia. Vol. 16, pp. 20–32. - ICER. Gene Therapy for Hemophilia B and A: Final Evidence Report. Dec 22, 2022. - Curtis R et al. Poster presented at: 65th ASH Annual Meeting & Exposition; December 11, 2023; San Diego, CA. - Zemplenyi A et al., Pharmacoeconomics (2024) 42(3):319–328. Hemophilia joint bleeds image: International Hemophilia Training Center. Factor VIII replacement therapy IV typically dosed 1 - 3 times/week Significant adherence challenges Risk of breakthrough bleeding Chronic treatment, non-curative Bi-specific antibody "mimetic" SQ dosed 1, 2 or 4 weeks post loading Risk of breakthough bleeding Treatment burden, non-curative Gene therapy Variable initial efficacy Significant decline in FVIII levels over time High risk of prolonged corticosteroid use Not suitable for pediatric patients Annual treatment cost3: ~$565K - $750K Lifetime treatment cost: ~$18M - $24M4 One-time treatment cost3: $2.9M5 FVIII
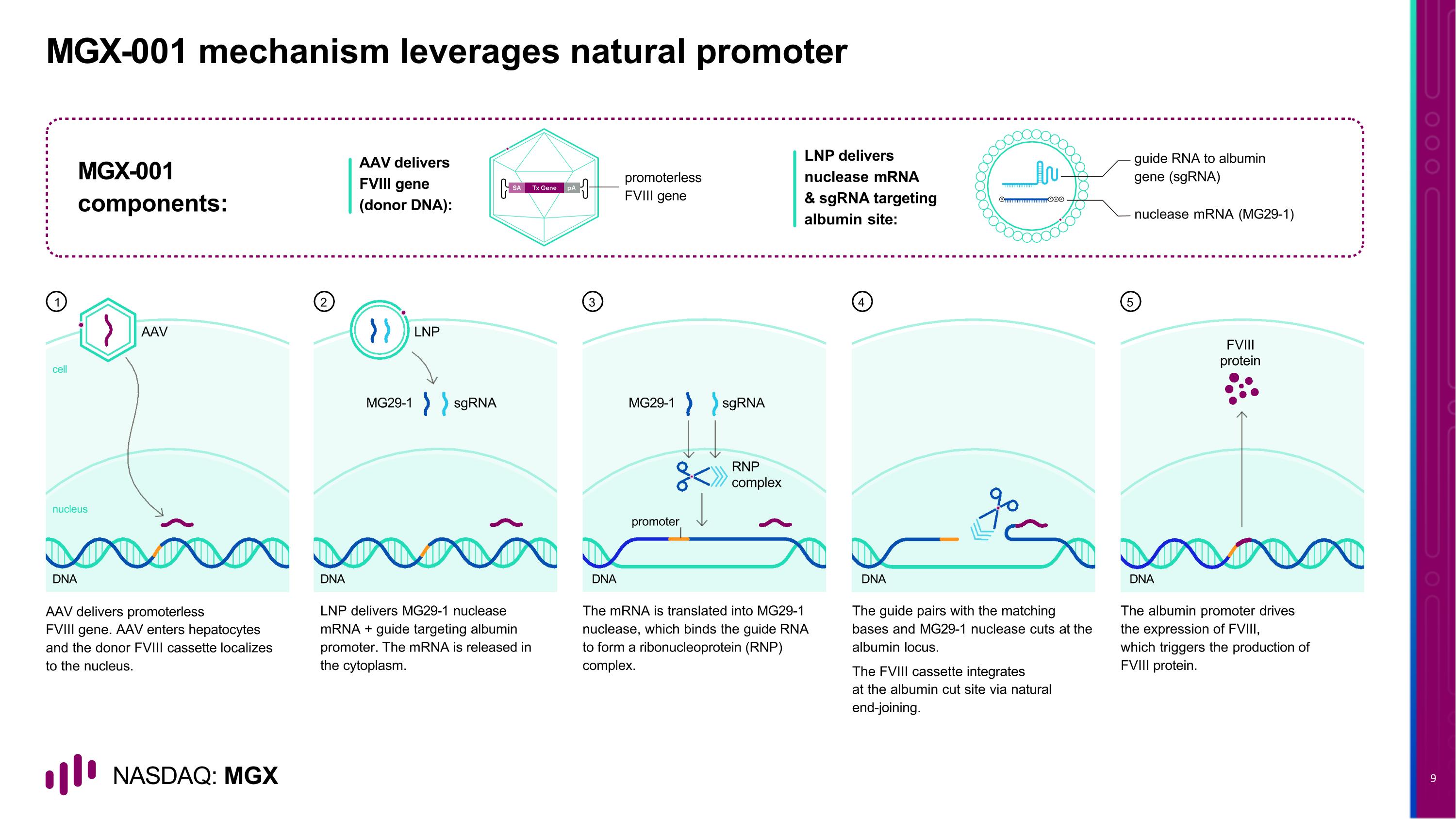
DNA FVIII protein DNA DNA sgRNA promoter MG29-1 RNP complex LNP DNA sgRNA MG29-1 AAV cell nucleus DNA MGX-001 mechanism leverages natural promoter AAV delivers promoterless FVIII gene. AAV enters hepatocytes and the donor FVIII cassette localizes to the nucleus. LNP delivers MG29-1 nuclease mRNA + guide targeting albumin promoter. The mRNA is released in the cytoplasm. The mRNA is translated into MG29-1 nuclease, which binds the guide RNA to form a ribonucleoprotein (RNP) complex. The guide pairs with the matching bases and MG29-1 nuclease cuts at the albumin locus. The FVIII cassette integrates at the albumin cut site via natural end-joining. The albumin promoter drives the expression of FVIII, which triggers the production of FVIII protein. AAV delivers FVIII gene (donor DNA): promoterless FVIII gene MGX-001 components: guide RNA to albumin gene (sgRNA) nuclease mRNA (MG29-1) LNP delivers nuclease mRNA & sgRNA targeting albumin site: 1 2 3 4 5
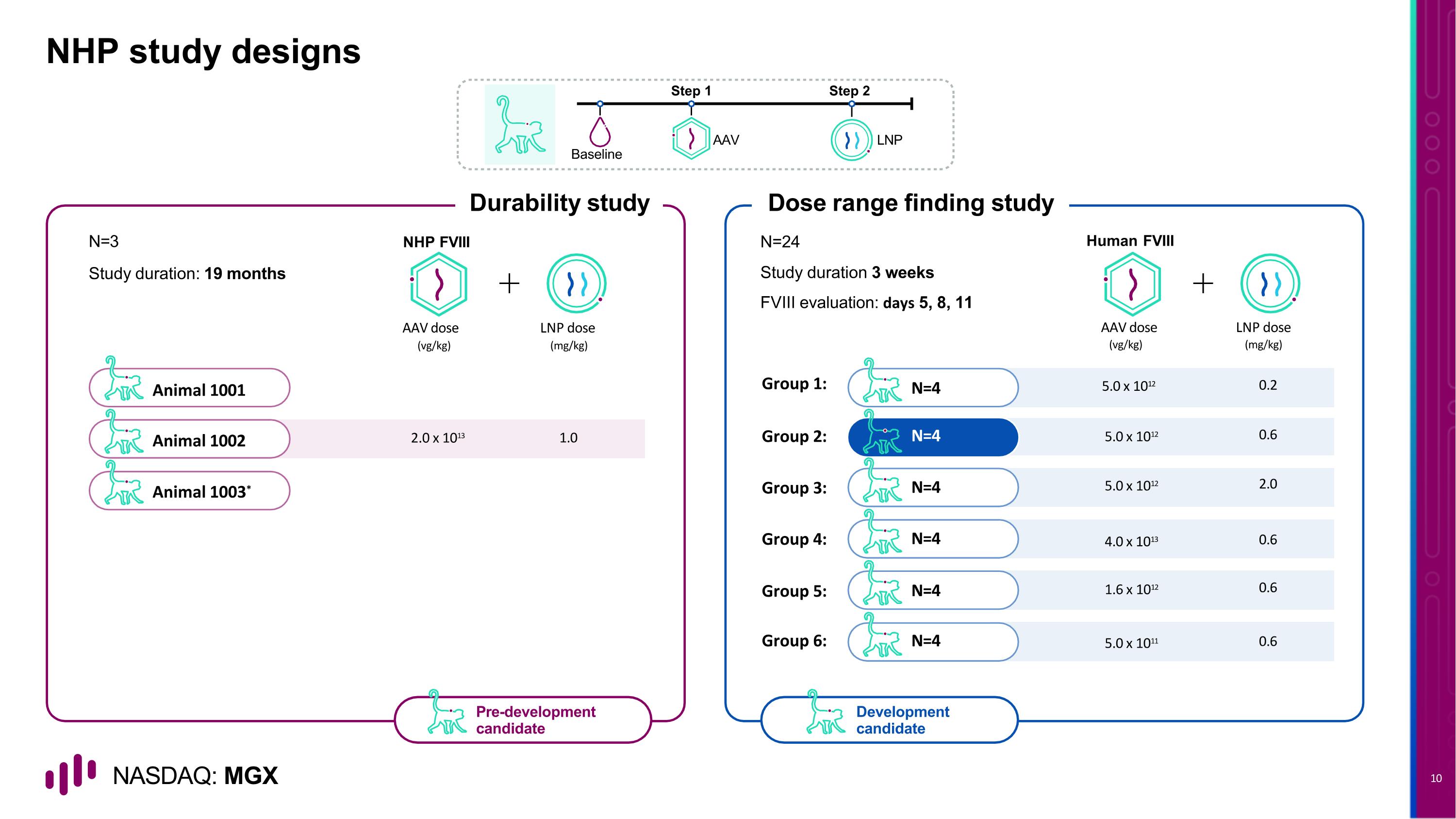
Animal 1001 Animal 1002 Animal 1003* NHP study designs N=3 Durability study AAV LNP Baseline Step 1 Step 2 Dose range finding study Human FVIII Pre-development candidate Development candidate Study duration: 19 months NHP FVIII N=24 Study duration 3 weeks FVIII evaluation: days 5, 8, 11 Group 1: N=4 Group 2: N=4 Group 3: N=4 Group 4: N=4 Group 5: N=4 Group 6: N=4 AAV dose LNP dose (vg/kg) (mg/kg) 2.0 x 1013 1.0 5.0 x 1012 0.6 5.0 x 1012 2.0 4.0 x 1013 0.6 1.6 x 1012 0.6 5.0 x 1011 0.6 5.0 x 1012 0.2 AAV dose LNP dose (vg/kg) (mg/kg)
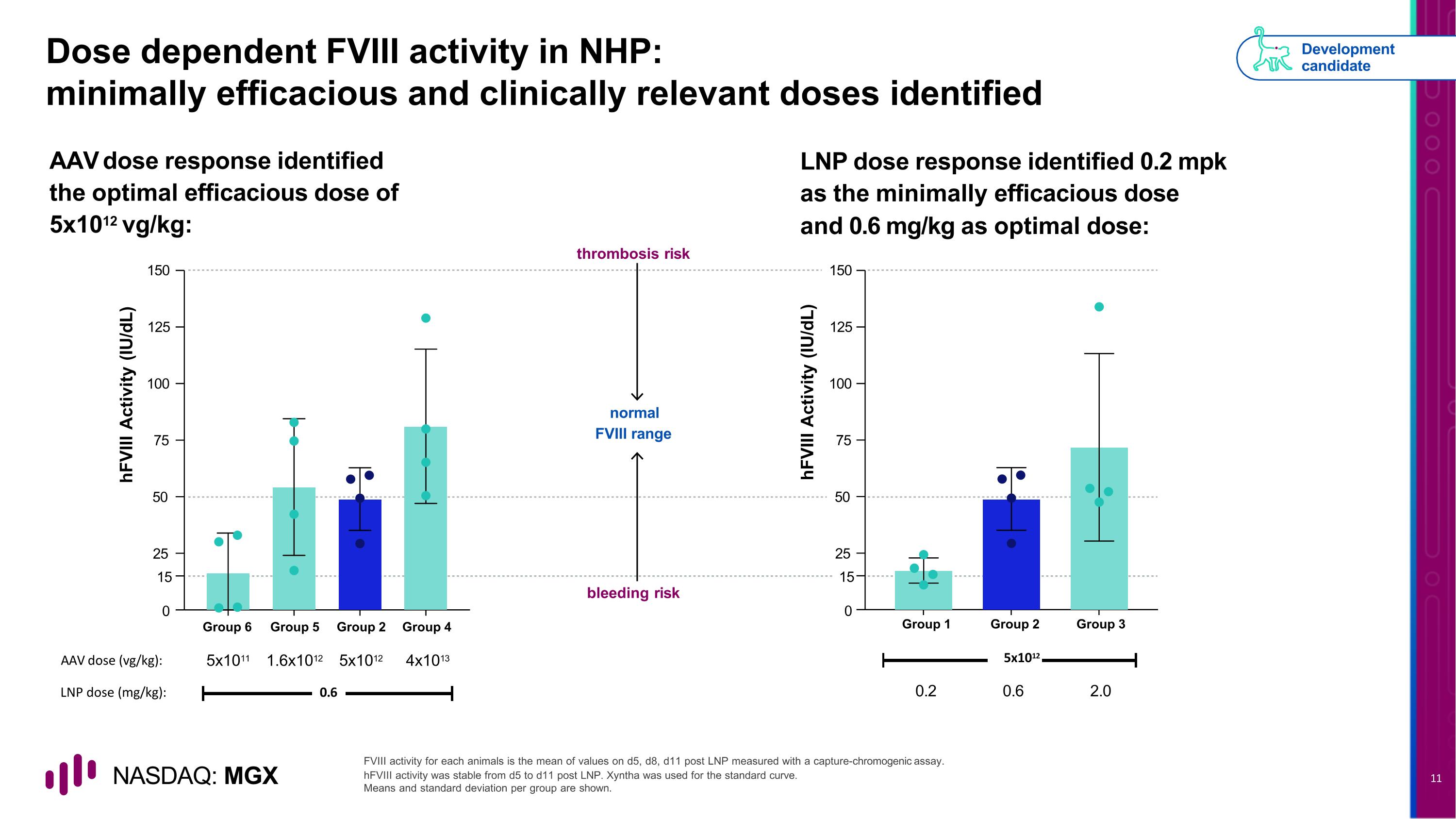
Dose dependent FVIII activity in NHP: minimally efficacious and clinically relevant doses identified bleeding risk normal FVIII range thrombosis risk FVIII activity for each animals is the mean of values on d5, d8, d11 post LNP measured with a capture-chromogenic assay. hFVIII activity was stable from d5 to d11 post LNP. Xyntha was used for the standard curve. Means and standard deviation per group are shown. AAV dose response identified the optimal efficacious dose of 5x1012 vg/kg: LNP dose response identified 0.2 mpk as the minimally efficacious dose and 0.6 mg/kg as optimal dose: Development candidate 25 15 0 50 75 100 125 150 hFVIII Activity (IU/dL) Group 6 Group 5 Group 2 Group 4 AAV dose (vg/kg): 5x1011 1.6x1012 5x1012 4x1013 LNP dose (mg/kg): 0.6 hFVIII Activity (IU/dL) 25 15 0 50 75 100 125 150 Group 2 Group 3 5x1012 0.6 2.0 Group 1 0.2
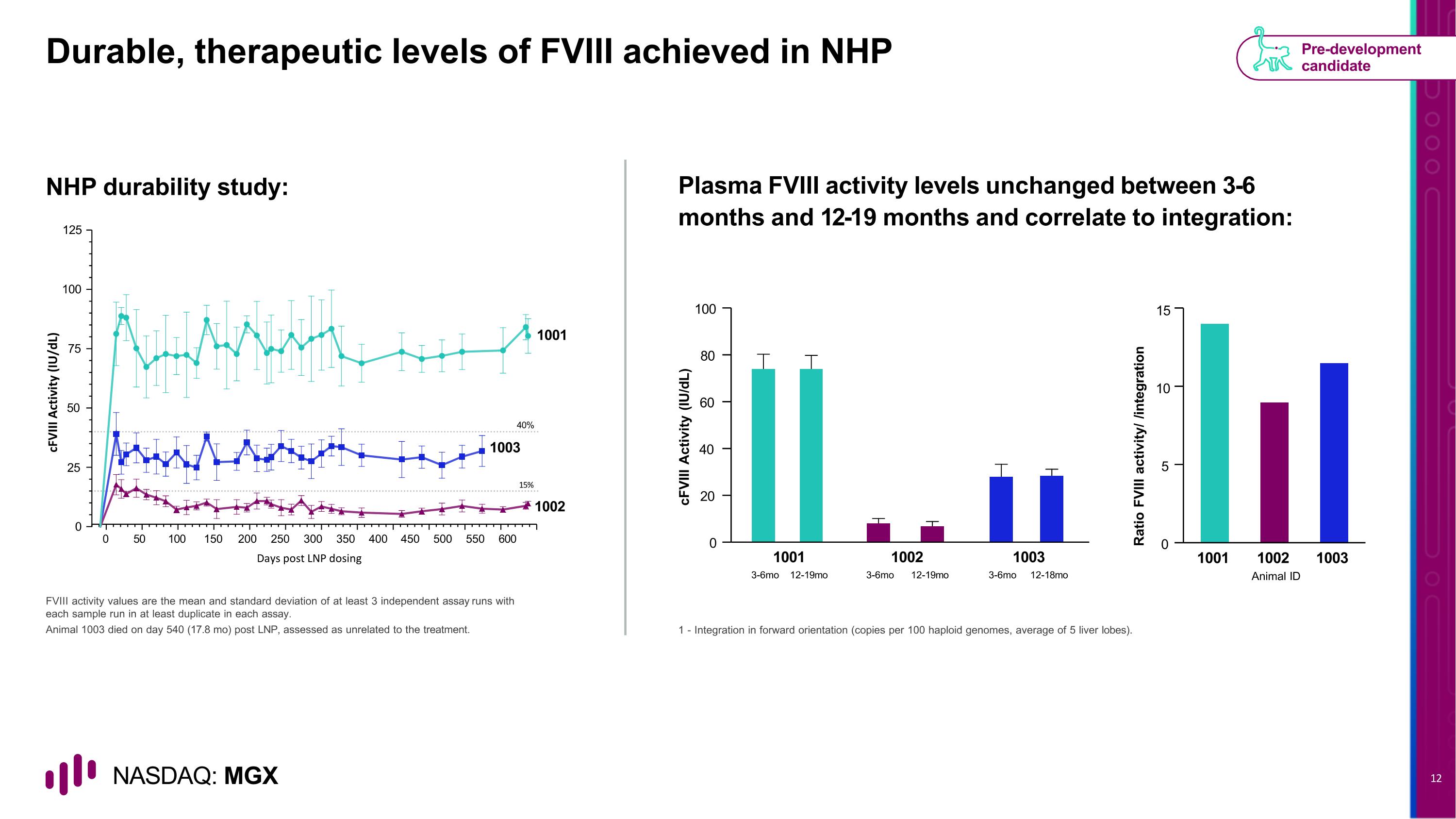
Durable, therapeutic levels of FVIII achieved in NHP NHP durability study: Plasma FVIII activity levels unchanged between 3-6 months and 12-19 months and correlate to integration: FVIII activity values are the mean and standard deviation of at least 3 independent assay runs with each sample run in at least duplicate in each assay. Animal 1003 died on day 540 (17.8 mo) post LNP, assessed as unrelated to the treatment. 1 - Integration in forward orientation (copies per 100 haploid genomes, average of 5 liver lobes). Pre-development candidate 0 20 40 60 80 100 cFVIII Activity (IU/dL) 1001 3-6mo 12-19mo 1002 3-6mo 12-19mo 1003 3-6mo 12-18mo Ratio FVIII activity/ /integration Animal ID 0 5 10 15 1001 1002 1003 0 50 100 150 200 250 300 350 400 450 500 550 600 100 75 50 25 0 125 1001 1003 40% 15% 1002 cFVIII Activity (IU/dL) Days post LNP dosing
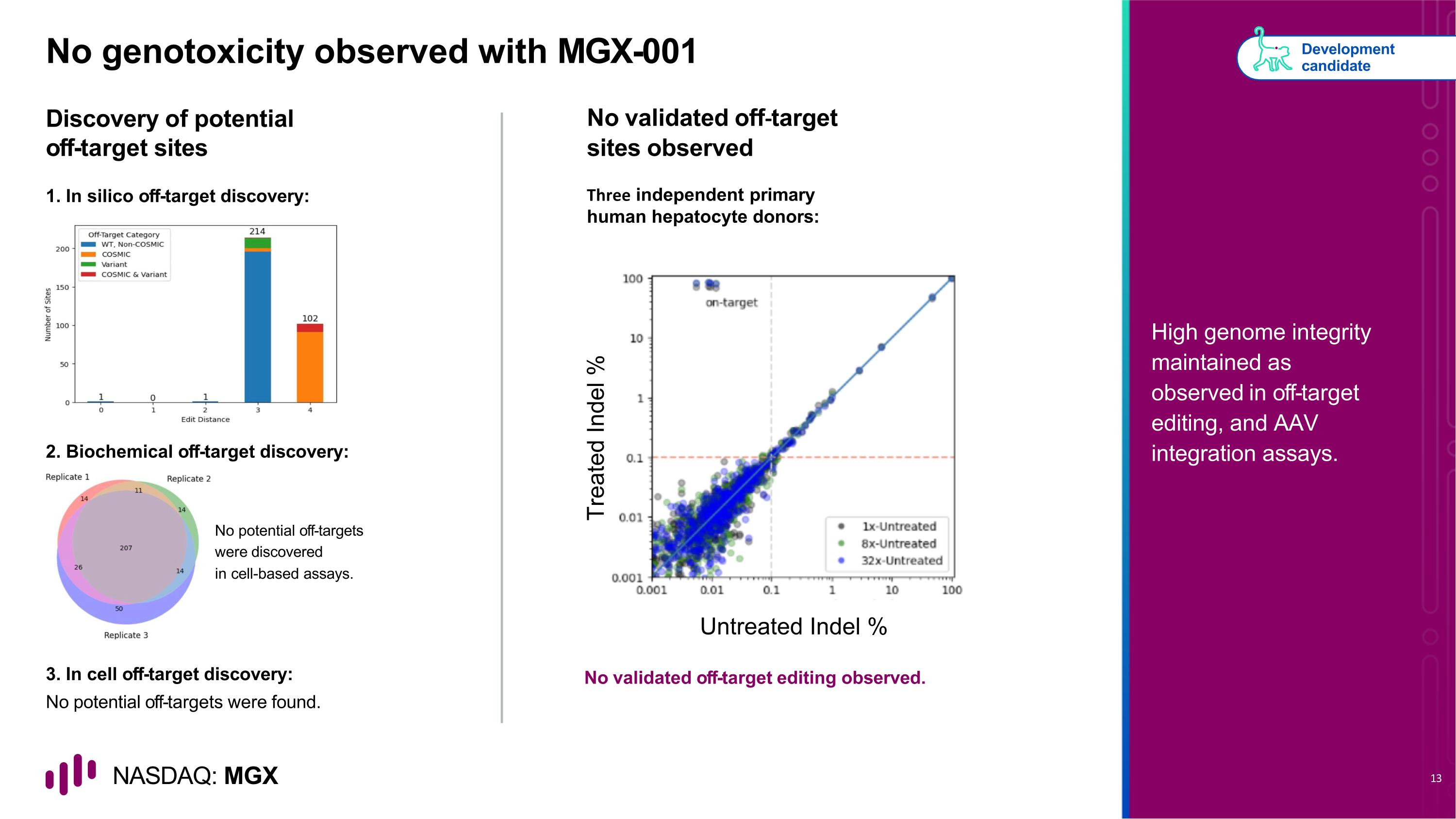
No genotoxicity observed with MGX-001 Discovery of potential off-target sites No validated off-target sites observed 2. Biochemical off-target discovery: 3. In cell off-target discovery: No potential off-targets were found. No potential off-targets were discovered in cell-based assays. No validated off-target editing observed. Development candidate High genome integrity maintained as observed in off-target editing, and AAV integration assays. 1. In silico off-target discovery: Three independent primary human hepatocyte donors: Untreated Indel % Treated Indel %
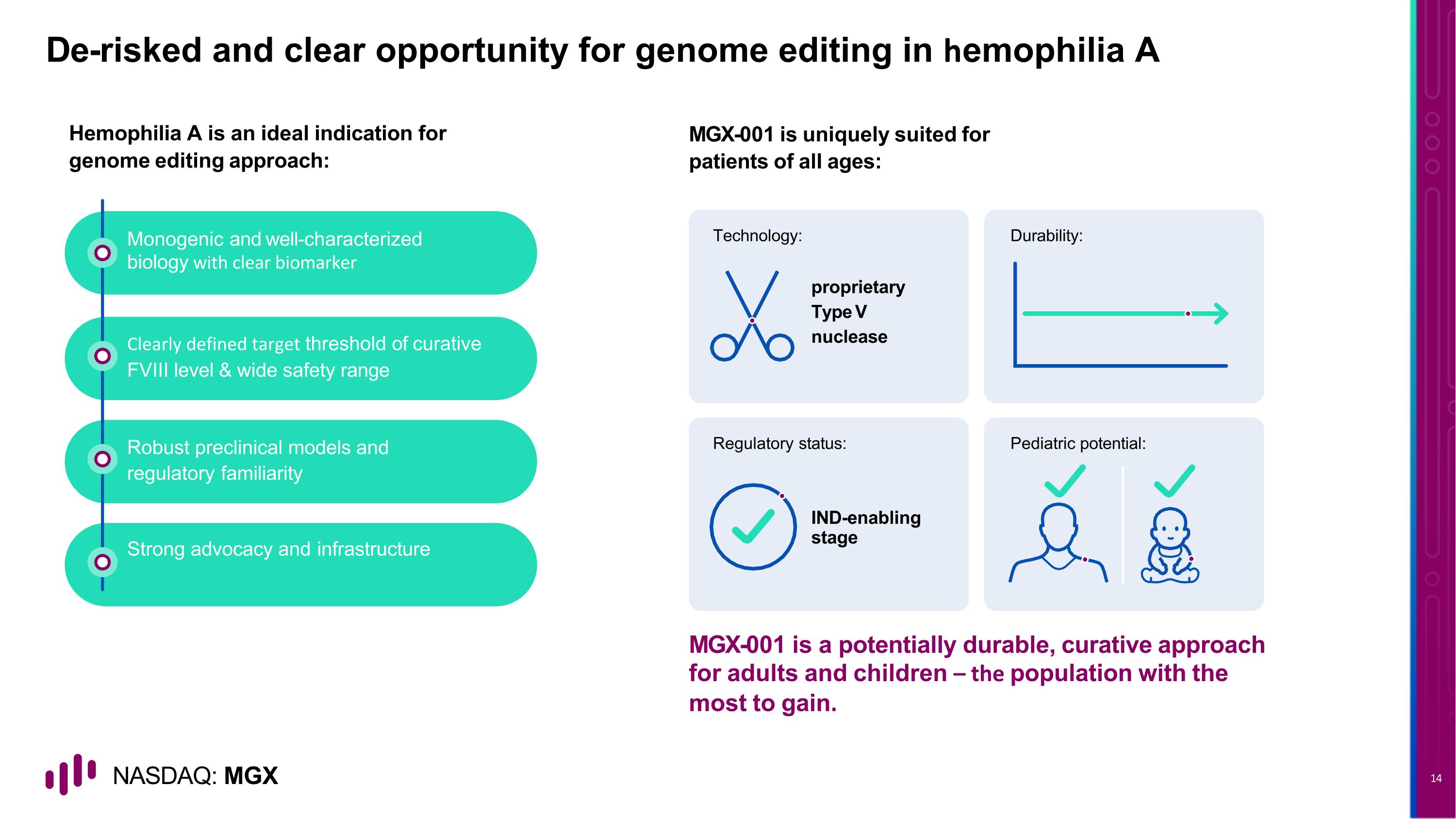
Strong advocacy and infrastructure Robust preclinical models and regulatory familiarity Clearly defined target threshold of curative FVIII level & wide safety range Monogenic and well-characterized biology with clear biomarker De-risked and clear opportunity for genome editing in hemophilia A proprietary Type V nuclease IND-enabling stage Technology: Durability: Regulatory status: Pediatric potential: Hemophilia A is an ideal indication for genome editing approach: MGX-001 is uniquely suited for patients of all ages: MGX-001 is a potentially durable, curative approach for adults and children – the population with the most to gain.

Potential cure for adults and children living with hemophilia A Designed to enable the body's own ability to produce FVIII Expected durable FVIII activity via gene integration Established regulatory and clinical pathways for advancement to pivotal study Our goal: to provide patients with a hemophilia-free mind
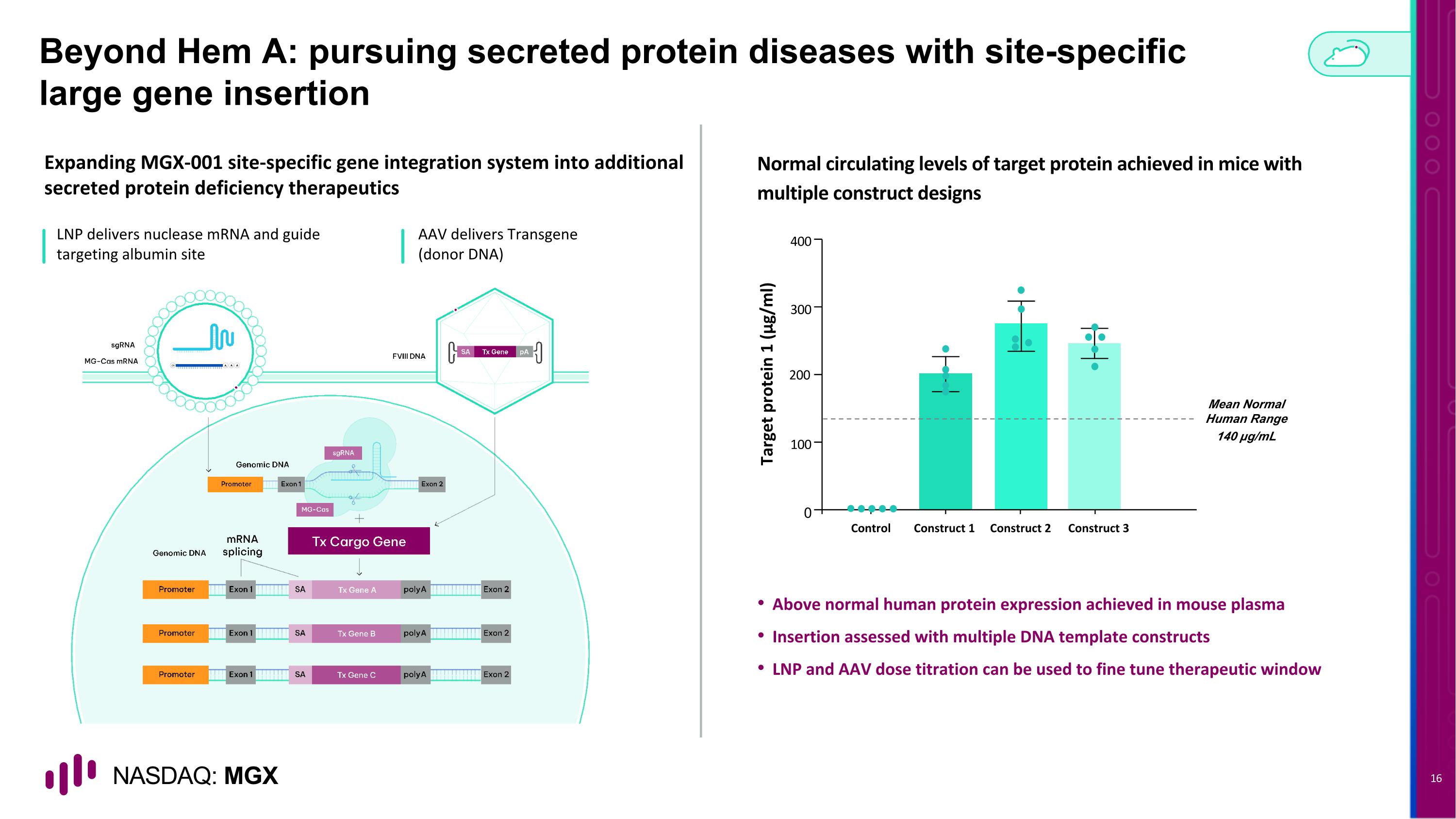
Beyond Hem A: pursuing secreted protein diseases with site-specific large gene insertion Expanding MGX-001 site-specific gene integration system into additional secreted protein deficiency therapeutics Normal circulating levels of target protein achieved in mice with multiple construct designs LNP delivers nuclease mRNA and guide targeting albumin site Above normal human protein expression achieved in mouse plasma Insertion assessed with multiple DNA template constructs LNP and AAV dose titration can be used to fine tune therapeutic window Mean Normal Human Range 140 µg/mL Target protein 1 (μg/ml) 0 100 200 300 400 Construct 1 Construct 2 Control Construct 3 AAV delivers Transgene (donor DNA)
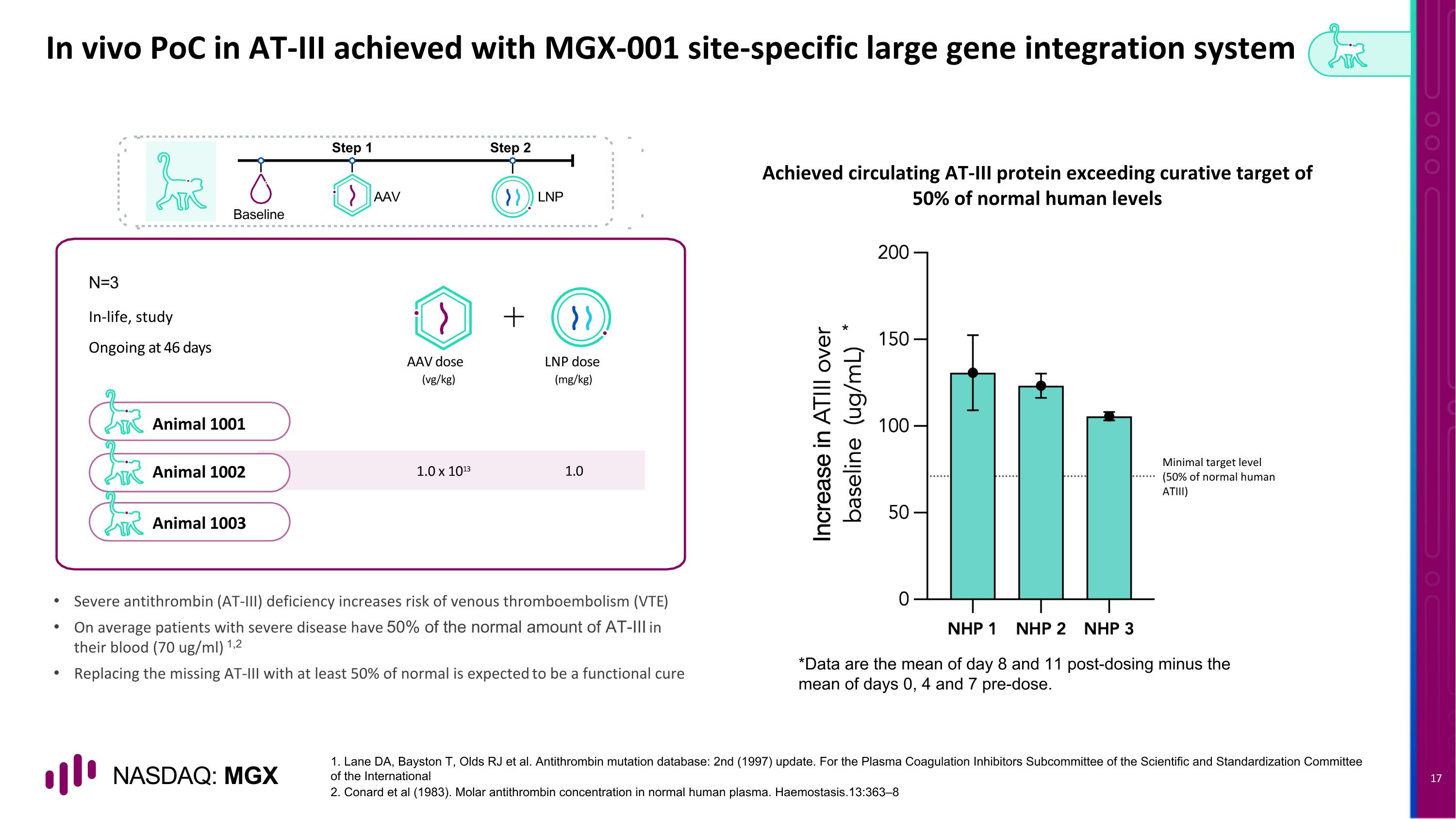
Animal 1001 Animal 1002 Animal 1003 In vivo PoC in AT-III achieved with MGX-001 site-specific large gene integration system N=3 AAV LNP Baseline Step 1 Step 2 In-life, study Ongoing at 46 days AAV dose LNP dose (vg/kg) (mg/kg) 1.0 x 1013 1.0 1. Lane DA, Bayston T, Olds RJ et al. Antithrombin mutation database: 2nd (1997) update. For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International 2. Conard et al (1983). Molar antithrombin concentration in normal human plasma. Haemostasis.13:363–8 Achieved circulating AT-III protein exceeding curative target of 50% of normal human levels Severe antithrombin (AT-III) deficiency increases risk of venous thromboembolism (VTE) On average patients with severe disease have 50% of the normal amount of AT-III in their blood (70 ug/ml) 1,2 Replacing the missing AT-III with at least 50% of normal is expected to be a functional cure Minimal target level (50% of normal human ATIII) * *Data are the mean of day 8 and 11 post-dosing minus the mean of days 0, 4 and 7 pre-dose.
Pursuing cardiometabolic indications in partnership TTR AGT APOC3 Undisclosed Current indications: MGX's in vivo genome editing complements Ionis leadership in cardiometabolic space. 4 targets: two co-development and co-commercialization options. Multibillion dollar TAM.

Team Jian Irish, PhD, MBA President and CEO Pamela Wapnick, MBA CFO Katalin Kauser, PhD, MD, Sc.D SVP, Translational Biology Board of Directors: Executive Leadership: Willard H. Dere, MD Professor Emeritus, Internal Medicine, University of Utah Eric Bjerkholt, MBA CFO, Mirum Pharmaceuticals, Inc. Jürgen Eckhardt, MD, MBA EVP Global Head, Business Development and Licensing, Head of Leaps by Bayer Laurence Reid, PhD Former CEO, Decibel Therapeutics Brian C. Thomas, PhD Founder, former CEO of Metagenomi, Inc. Jian Irish, PhD, MBA President and CEO, Metagenomi Therapeutics, Inc. Matthew Wein, JD General Counsel and Corporate Secretary Alan Brooks, PhD SVP, Research Mark Leonard, PhD SVP, Technical Operations

Thank you