

© 2026 Passage Bio. All rights reserved. Corporate Presentation Redefining the Course of Neurodegenerative Conditions January 2026

Forward - Looking Statement This presentation includes “forward - looking statements” within the meaning of, and made pursuant to the safe harbor provisions of, the Private Securities Litigation Reform Act of 1995, including, but not limited to: our expectations about timing and execution of anticipated milestones, including the progress of clinical studies and the availability of clinical data from such trials; timing of feedback from re gul atory authorities; the potential of our product candidates versus other treatment options and clinical candidates; our expectations about cash runwa y; the ability of PBFT02 to treat FTD - GRN or FTD - C9orf72 , and the potential development of other product candidates. These forward - looking statements may be accompanied by such words as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “ma y,” “might,” “plan,” “potential,” “possible,” “will,” “would,” and other words and terms of similar meaning. These statements involve risks and un cer tainties that could cause actual results to differ materially from those reflected in such statements, including: our ability to develop and obta in regulatory approval for our product candidates; the timing and results of preclinical studies and clinical trials; risks associated with clinical tr ials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during cl inical trials, the timing of and our ability to obtain and maintain regulatory approvals ; our expectations about the willingness of healthcare professionals to use our product candidates, the timing, or amount, the occurrence of adverse safety events; the risk that positive results in a preclinical study or clinical trial may not be replicated in subsequent trials or success in early stage clinical trials may not be predictive of res ults in later stage clinical trials; failure to protect and enforce our intellectual property, and other proprietary rights; our dependence on collaborato rs and other third parties for the development and manufacture of product candidates and other aspects of our business, which are outside of our fu ll control; the timing, or amount, of receipt of any potential future milestone and royalty payments; risks associated with current and poten tia l delays, work stoppages, or supply chain disruptions; and the other risks and uncertainties that are described in the Risk Factors section in documents the company files from time to time with the Securities and Exchange Commission (SEC), and other reports as filed with the SEC. P ass age Bio undertakes no obligation to publicly update any forward - looking statement, whether written or oral, that may be made from time t o time, whether as a result of new information, future developments or otherwise. January 2026 2
January 2026 3 Redefining the Course of Neurodegenerative Conditions * Based on cash, cash equivalents, and marketable securities as of December 31, 2025. Advancing clinical stage, potential best - in - class, one - time progranulin raising gene therapy for FTD Pursuing preclinical development of differentiated gene therapy approach in Huntington’s disease Cash runway expected into 1Q 2027*
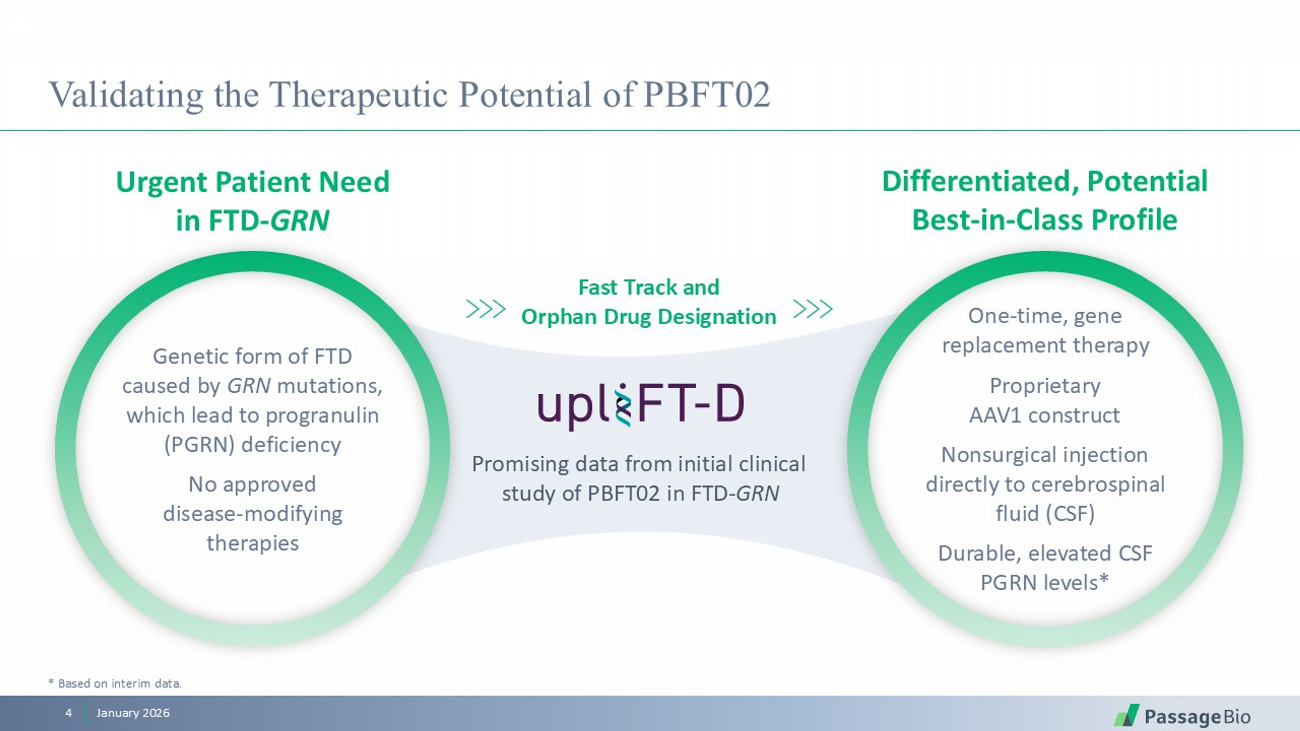
Validating the Therapeutic Potential of PBFT02 January 2026 4 * Based on interim data. Urgent Patient Need in FTD - GRN Differentiated, Potential Best - in - Class Profile Fast Track and Orphan Drug Designation Promising data from initial clinical study of PBFT02 in FTD - GRN Genetic form of FTD caused by GRN mutations, which lead to progranulin (PGRN) deficiency No approved disease - modifying therapies One - time, gene replacement therapy Proprietary AAV1 construct Nonsurgical injection directly to cerebrospinal fluid (CSF) Durable, elevated CSF PGRN levels*
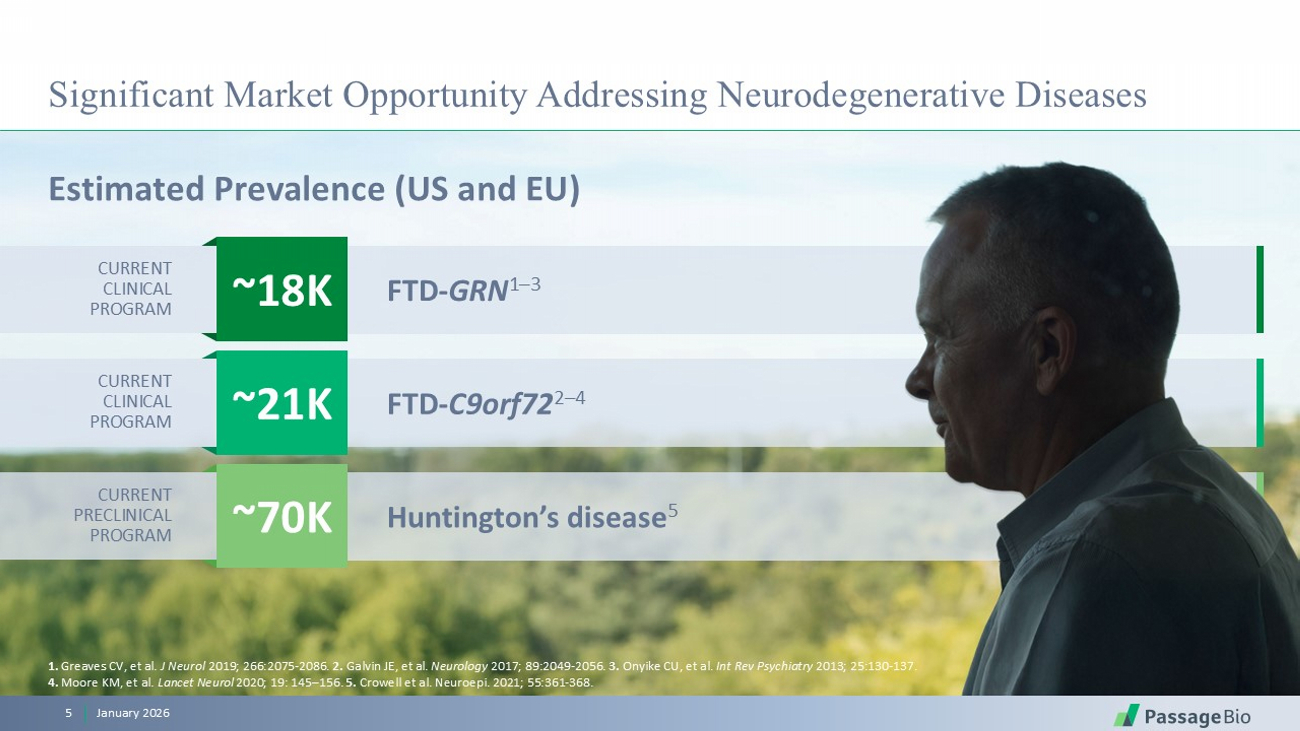
Significant Market Opportunity Addressing Neurodegenerative Diseases Estimated Prevalence (US and EU) January 2026 5 Huntington’s disease 5 FTD - C9orf72 2 – 4 FTD - GRN 1 – 3 ~18K ~21K ~70K 1. Greaves CV, et al. J Neurol 2019; 266:2075 - 2086. 2. Galvin JE, et al. Neurology 2017; 89:2049 - 2056. 3. Onyike CU, et al. Int Rev Psychiatry 2013; 25:130 - 137. 4. Moore KM, et al. Lancet Neurol 2020; 19: 145 – 156. 5. Crowell et al. Neuroepi. 2021; 55:361 - 368. CURRENT PRECLINICAL PROGRAM CURRENT CLINICAL PROGRAM CURRENT CLINICAL PROGRAM

PBFT02 Frontotemporal Dementia
OVERVIEW • Fatal adult - onset neurodegenerative disease affecting the frontal and temporal lobes of the brain, characterized by a decline in behavior, language, and executive function • One of the most common causes of early - onset dementia worldwide, disproportionately affecting individuals aged 40 – 65 years CLINICAL SYMPTOMS Disease progression is rapid and degenerative, including loss of speech, loss of expression, behavioral changes, and immobility Frontotemporal Dementia (FTD): A Devastating Adult Disease On average, people with FTD live 8 years after the onset of symptoms January 2026 7 Loss of inhibition Apathy Social withdrawal Hyperorality (mouthing of objects) Ritualistic compulsive behaviors
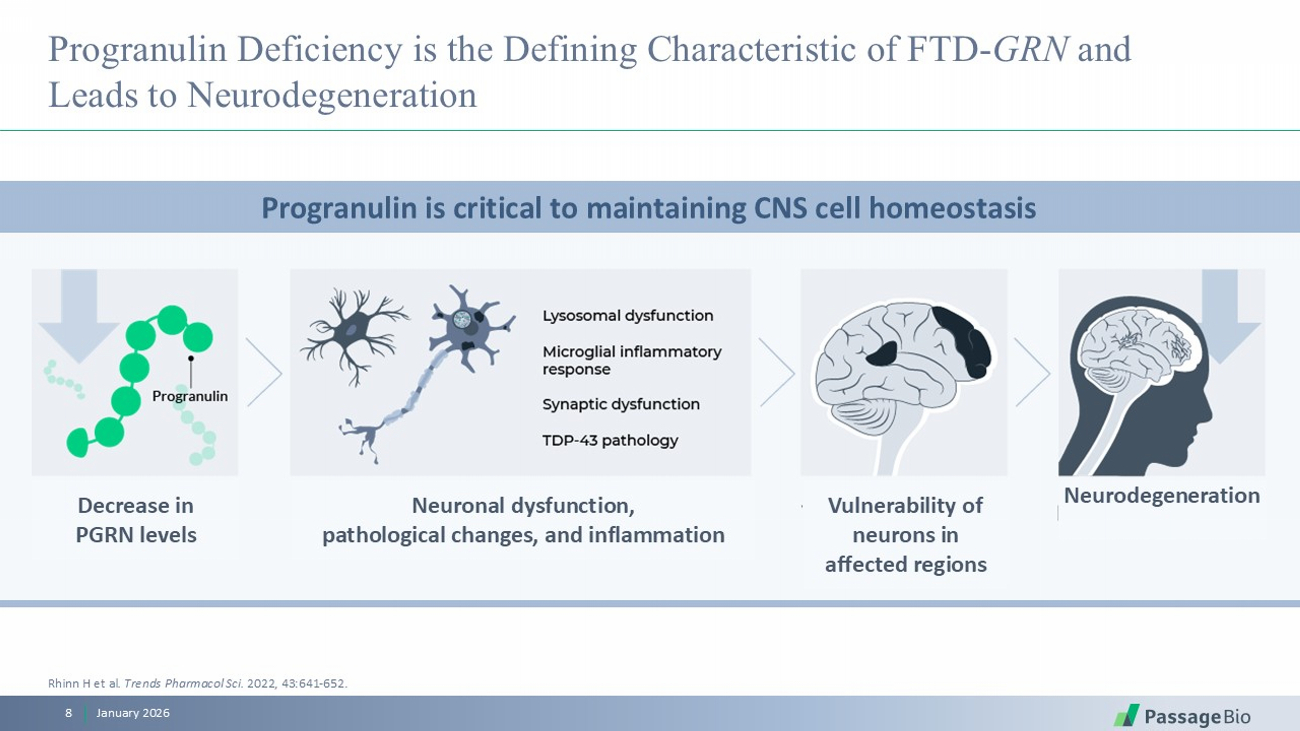
Progranulin Deficiency is the Defining Characteristic of FTD - GRN and Leads to Neurodegeneration January 2026 8 Progranulin is critical to maintaining CNS cell homeostasis Rhinn H et al . Trends Pharmacol Sci . 2022, 43:641 - 652. Decrease in PGRN levels Neuronal dysfunction, pathological changes, and inflammation Vulnerability of neurons in affected regions Neurodegeneration
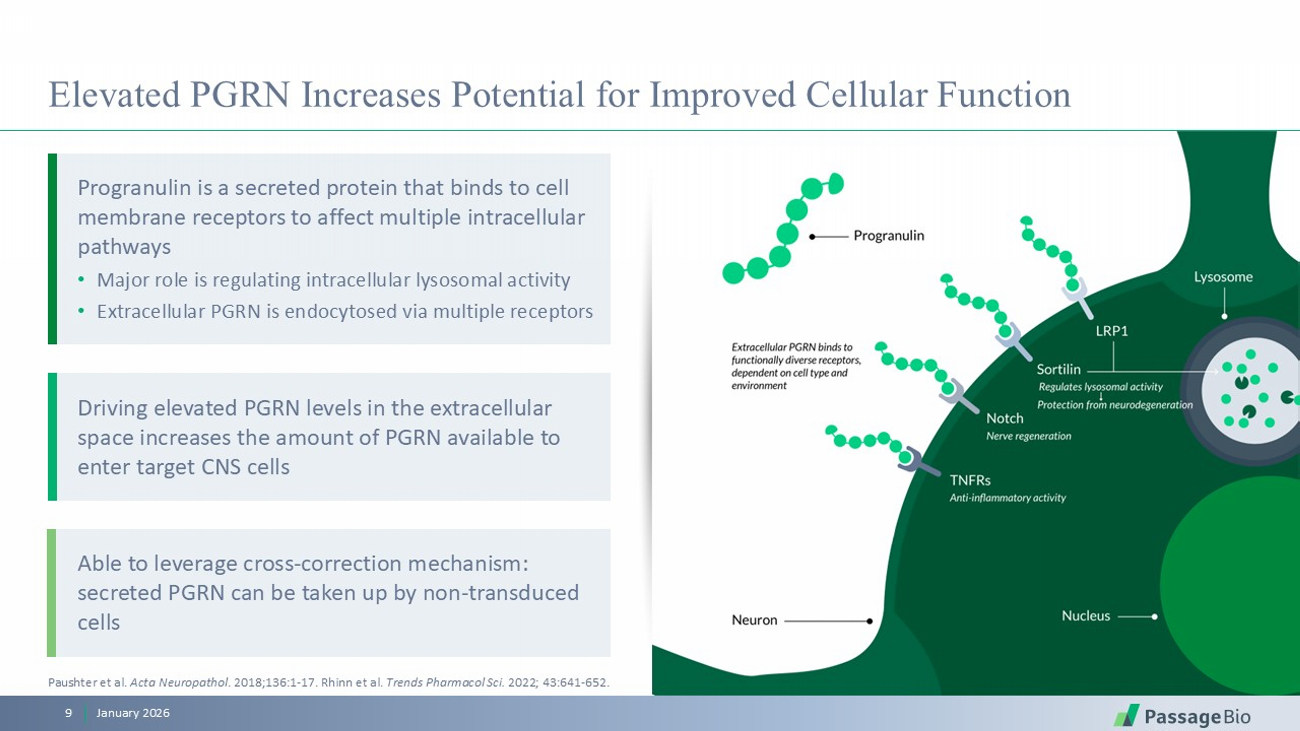
Elevated PGRN Increases Potential for Improved Cellular Function January 2026 9 Paushter et al. Acta Neuropathol . 2018;136:1 - 17. Rhinn et al. Trends Pharmacol Sci. 2022; 43:641 - 652. Driving elevated PGRN levels in the extracellular space increases the amount of PGRN available to enter target CNS cells Able to leverage cross - correction mechanism: secreted PGRN can be taken up by non - transduced cells Progranulin is a secreted protein that binds to cell membrane receptors to affect multiple intracellular pathways • Major role is regulating intracellular lysosomal activity • Extracellular PGRN is endocytosed via multiple receptors
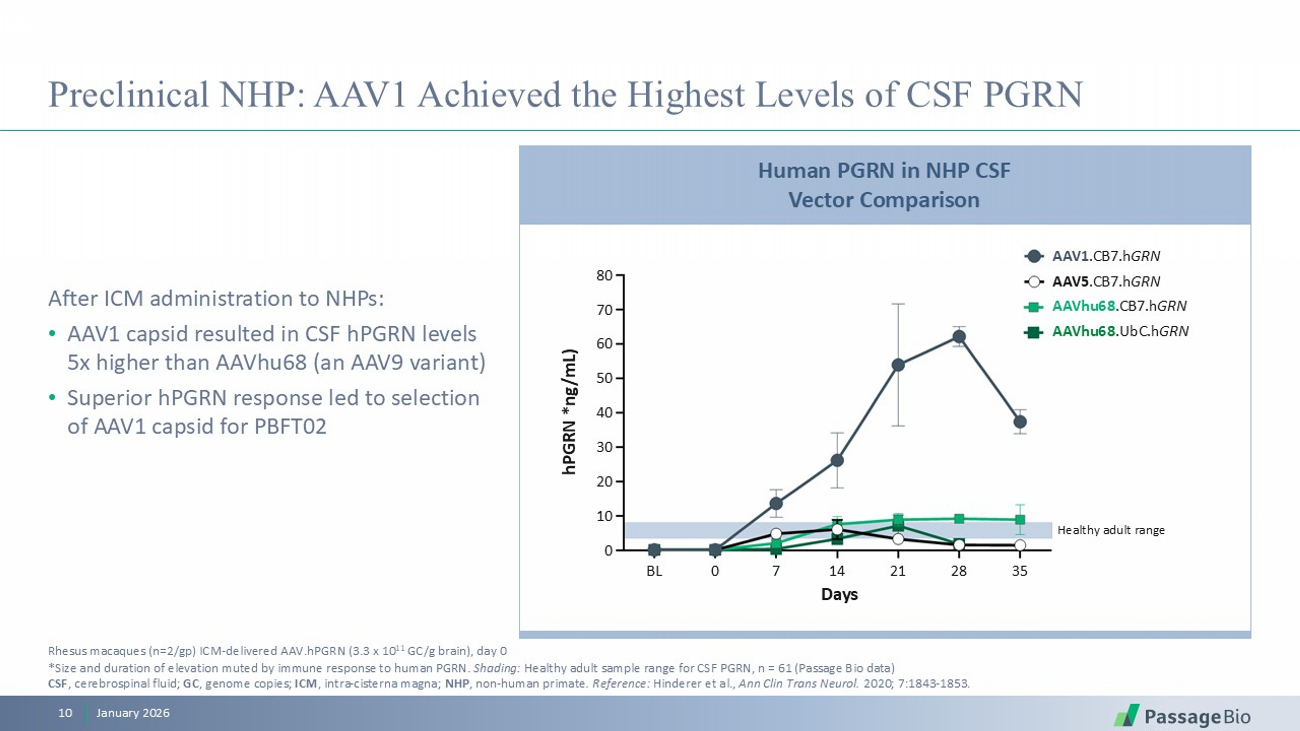
Preclinical NHP: AAV1 Achieved the Highest Levels of CSF PGRN January 2026 10 After ICM administration to NHPs: • AAV1 capsid resulted in CSF hPGRN levels 5x higher than AAVhu68 (an AAV9 variant) • Superior hPGRN response led to selection of AAV1 capsid for PBFT02 Rhesus macaques (n=2/ gp ) ICM - delivered AAV.hPGRN (3.3 x 10 11 GC/g brain), day 0 * Size and duration of elevation muted by immune response to human PGRN. Shading: Healthy adult sample range for CSF PGRN, n = 61 (Passage Bio data) CSF , cerebrospinal fluid; GC , genome copies; ICM , intra - cisterna magna; NHP , non - human primate . Reference: Hinderer et al., Ann Clin Trans Neurol. 2020; 7:1843 - 1853. Human PGRN in NHP CSF Vector Comparison Healthy adult range Days BL 0 7 14 21 28 35 hPGRN *ng/mL) 80 70 60 50 40 30 20 10 0 AAV1 .CB7.h GRN AAV5 .CB7.h GRN AAVhu68 .CB7.h GRN AAVhu68 .UbC.h GRN
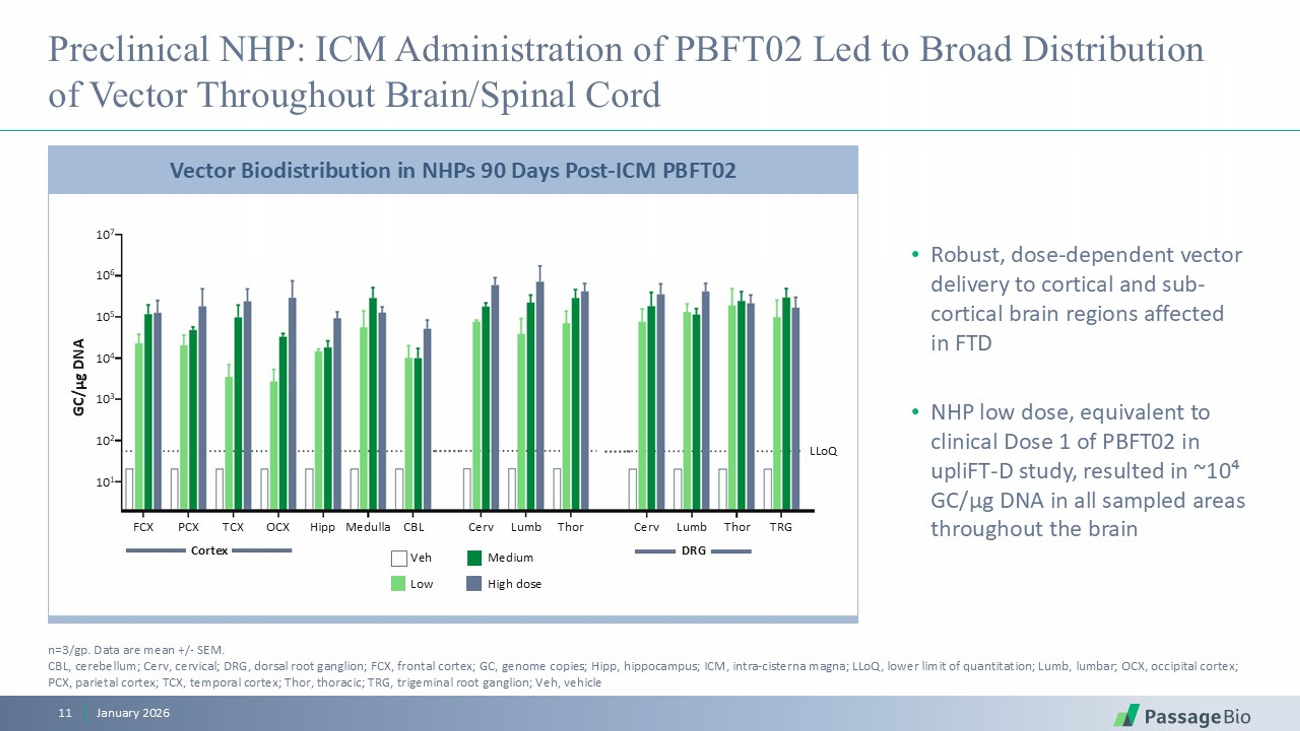
Preclinical NHP: ICM Administration of PBFT02 Led to Broad Distribution of Vector Throughout Brain/Spinal Cord • Robust, dose - dependent vector delivery to cortical and sub - cortical brain regions affected in FTD • NHP low dose, equivalent to clinical Dose 1 of PBFT02 in upliFT - D study, resulted in ~10⁴ GC/ μ g DNA in all sampled areas throughout the brain January 2026 11 n=3/ gp . Data are mean +/ - SEM. CBL, cerebellum; Cerv , cervical; DRG, dorsal root ganglion; FCX, frontal cortex; GC, genome copies; Hipp, hippocampus; ICM, intra - cisterna magna; LLoQ , lower limit of quantitation; Lumb, lumbar; OCX, occipital cortex; PCX, parietal cortex; TCX, temporal cortex; Thor, thoracic; TRG, trigeminal root ganglion; Veh, vehicle Vector Biodistribution in NHPs 90 Days Post - ICM PBFT02 GC/µg DNA 10 7 10 6 10 5 10 4 10 3 10 2 10 1 FCX PCX TCX OCX Hipp Medulla CBL Cerv Lumb Thor Cerv Lumb Thor TRG LLoQ Veh Low Medium High dose Cortex DRG
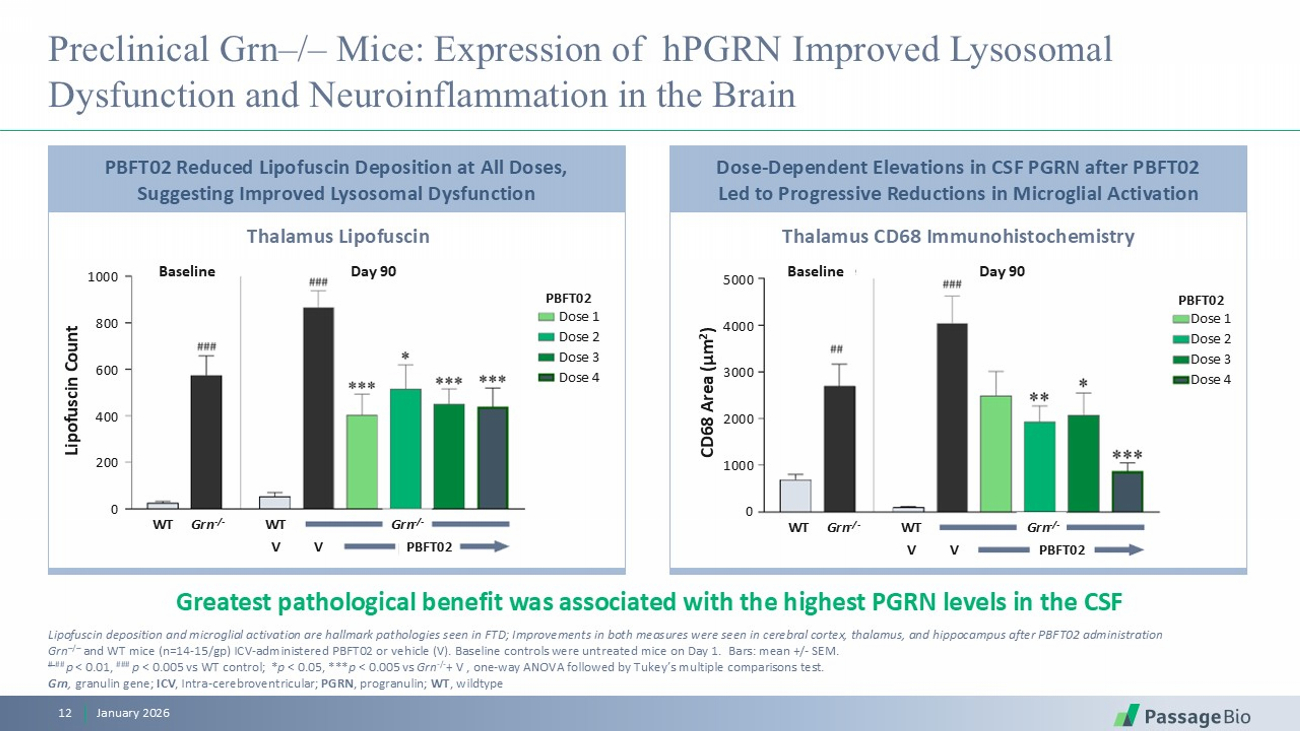
Preclinical Grn – / – Mice: Expression of hPGRN Improved Lysosomal Dysfunction and Neuroinflammation in the Brain January 2026 12 Greatest pathological benefit was associated with the highest PGRN levels in the CSF Lipofuscin deposition and microglial activation are hallmark pathologies seen in FTD; Improvements in both measures were seen in cerebral cortex, thalamus, and hippocampus after PBFT02 administration Grn – / – and WT mice (n=14 - 15/ gp ) ICV - administered PBFT02 or vehicle (V). Baseline controls were untreated mice on Day 1. Bars: mean +/ - SEM. # ## p < 0.01, ### p < 0.005 vs WT control; * p < 0.05, *** p < 0.005 vs Grn - / - + V , one - way ANOVA followed by Tukey’s multiple comparisons test . Grn , granulin gene; ICV , Intra - cerebroventricular; PGRN , progranulin; WT , wildtype Thalamus Lipofuscin Thalamus CD68 Immunohistochemistry PBFT02 Reduced Lipofuscin Deposition at All Doses, Suggesting Improved Lysosomal Dysfunction Dose - Dependent Elevations in CSF PGRN after PBFT02 Led to Progressive Reductions in Microglial Activation Lipofuscin Count 1000 800 600 400 200 0 Baseline Day 90 Baseline Day 90 CD68 Area (µm 2 ) 5000 4000 3000 2000 1000 0 PBFT02 Dose 1 Dose 2 Dose 3 Dose 4 PBFT02 Dose 1 Dose 2 Dose 3 Dose 4 WT Grn - / - WT Grn - / - WT Grn - / - WT Grn - / - V V PBFT02 V V PBFT02
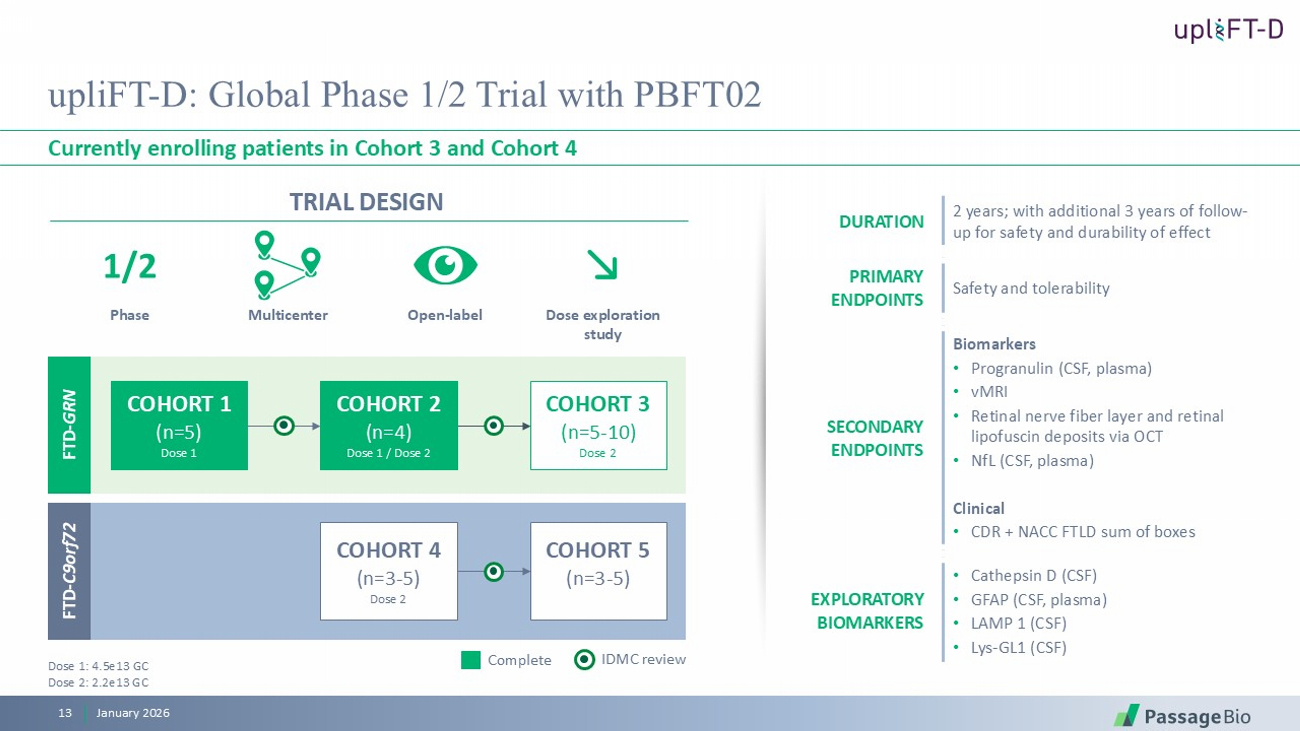
TRIAL DESIGN upliFT - D: Global Phase 1/2 Trial with PBFT02 Currently enrolling patients in Cohort 3 and Cohort 4 January 2026 13 2 years; with additional 3 years of follow - up for safety and durability of effect DURATION Safety and tolerability PRIMARY ENDPOINTS Biomarkers • Progranulin (CSF, plasma) • vMRI • Retinal nerve fiber layer and retinal lipofuscin deposits via OCT • NfL (CSF, plasma) Clinical • CDR + NACC FTLD sum of boxes SECONDARY ENDPOINTS • Cathepsin D (CSF) • GFAP (CSF, plasma) • LAMP 1 (CSF) • Lys - GL1 (CSF) EXPLORATORY BIOMARKERS Multicenter O pen - label D ose exploration study Phase 1/2 IDMC review Complete Dose 1: 4.5e13 GC Dose 2: 2.2 e13 GC COHORT 1 (n=5) Dose 1 COHORT 2 (n=4) Dose 1 / Dose 2 COHORT 3 (n=5 - 10) Dose 2 FTD - GRN COHORT 4 (n=3 - 5) Dose 2 COHORT 5 (n=3 - 5) FTD - C9orf72
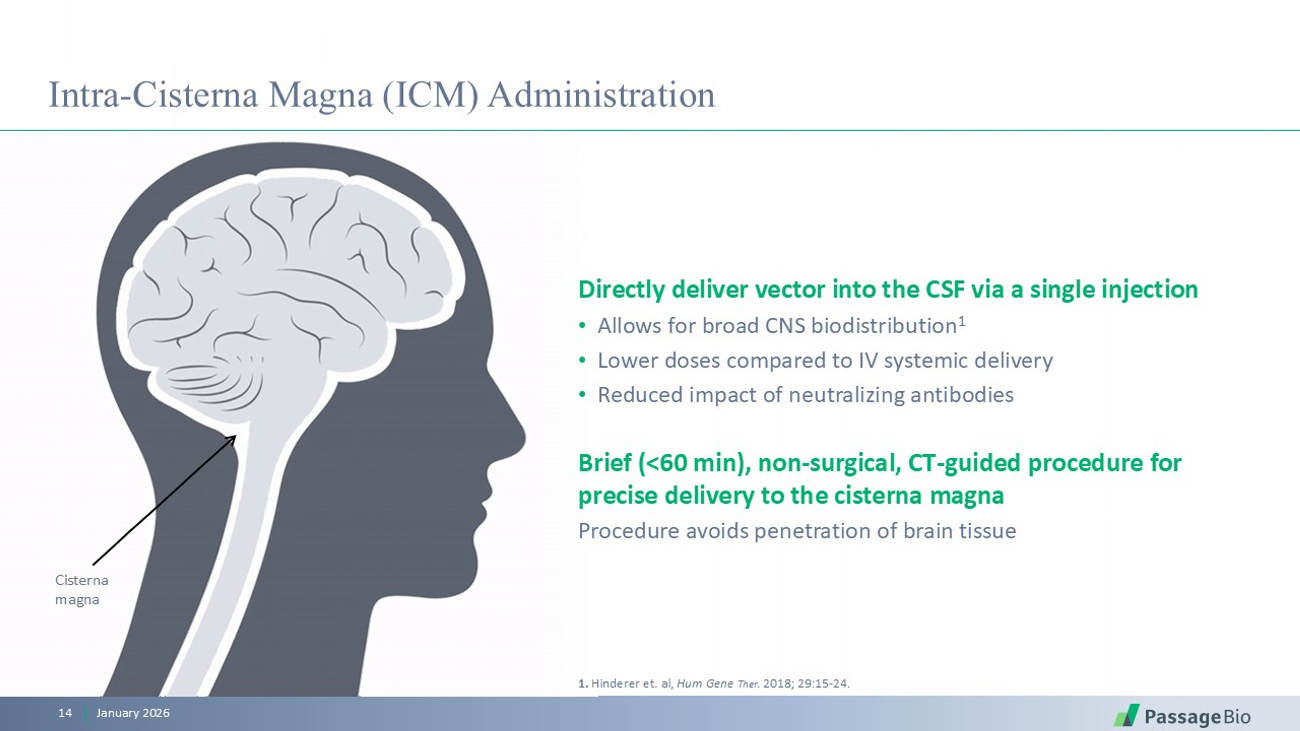
Cisterna magna Intra - Cisterna Magna (ICM) Administration January 2026 14 Directly deliver vector into the CSF via a single injection • Allows for broad CNS biodistribution 1 • Lower doses compared to IV systemic delivery • Reduced impact of neutralizing antibodies Brief (<60 min), non - surgical, CT - guided procedure for precise delivery to the cisterna magna Procedure avoids penetration of brain tissue 1. Hinderer et. al, Hum Gene Ther . 2018; 29:15 - 24 .
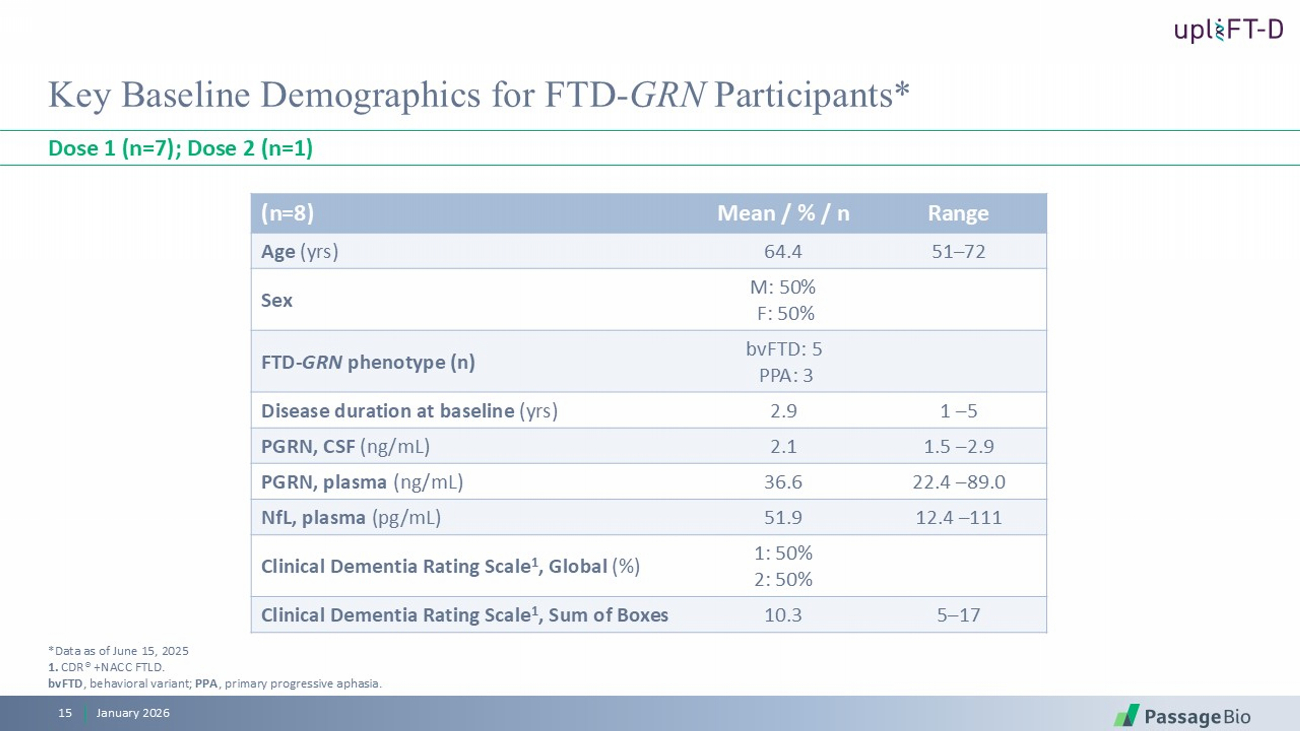
Key Baseline Demographics for FTD - GRN Participants* Dose 1 (n=7); Dose 2 (n=1) Range Mean / % / n (n=8) 51 – 72 64.4 Age (yrs) M: 50% F: 50% Sex bvFTD : 5 PPA: 3 FTD - GRN phenotype (n) 1 – 5 2.9 Disease duration at baseline (yrs) 1.5 – 2.9 2.1 PGRN, CSF (ng/mL) 22.4 – 89.0 36.6 PGRN, plasma (ng/mL) 12.4 – 111 51.9 NfL , plasma ( pg /mL) 1: 50% 2: 50% Clinical Dementia Rating Scale 1 , Global (%) 5 – 17 10.3 Clinical Dementia Rating Scale 1 , Sum of Boxes *Data as of June 15, 2025 1. CDR® +NACC FTLD. bvFTD , behavioral variant; PPA , primary p rogressive aphasia . January 2026 15
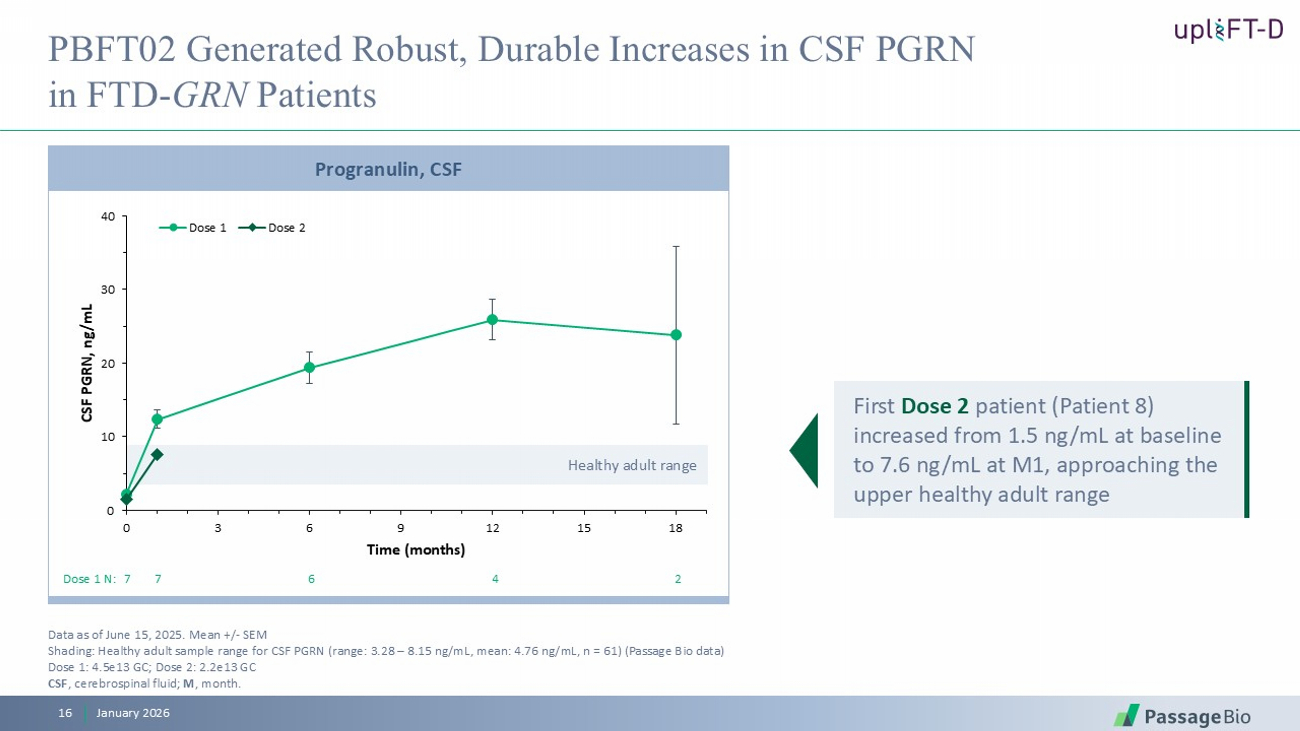
Healthy adult range 0 10 20 30 40 0 3 6 9 12 15 18 CSF PGRN, ng/mL Time (months) Dose 1 Dose 2 Progranulin, CSF PBFT02 Generated Robust, Durable Increases in CSF PGRN in FTD - GRN Patients Data as of June 15, 2025. Mean +/ - SEM Shading: Healthy adult sample range for CSF PGRN (range: 3.28 – 8.15 ng/mL, mean: 4.76 ng/mL, n = 61) (Passage Bio data) Dose 1: 4.5e13 GC; Dose 2: 2.2e13 GC CSF , c erebrospinal f luid ; M , month . Dose 1 N: 7 7 6 4 2 January 2026 16 First Dose 2 patient (Patient 8) increased from 1.5 ng/mL at baseline to 7.6 ng/mL at M1, approaching the upper healthy adult range
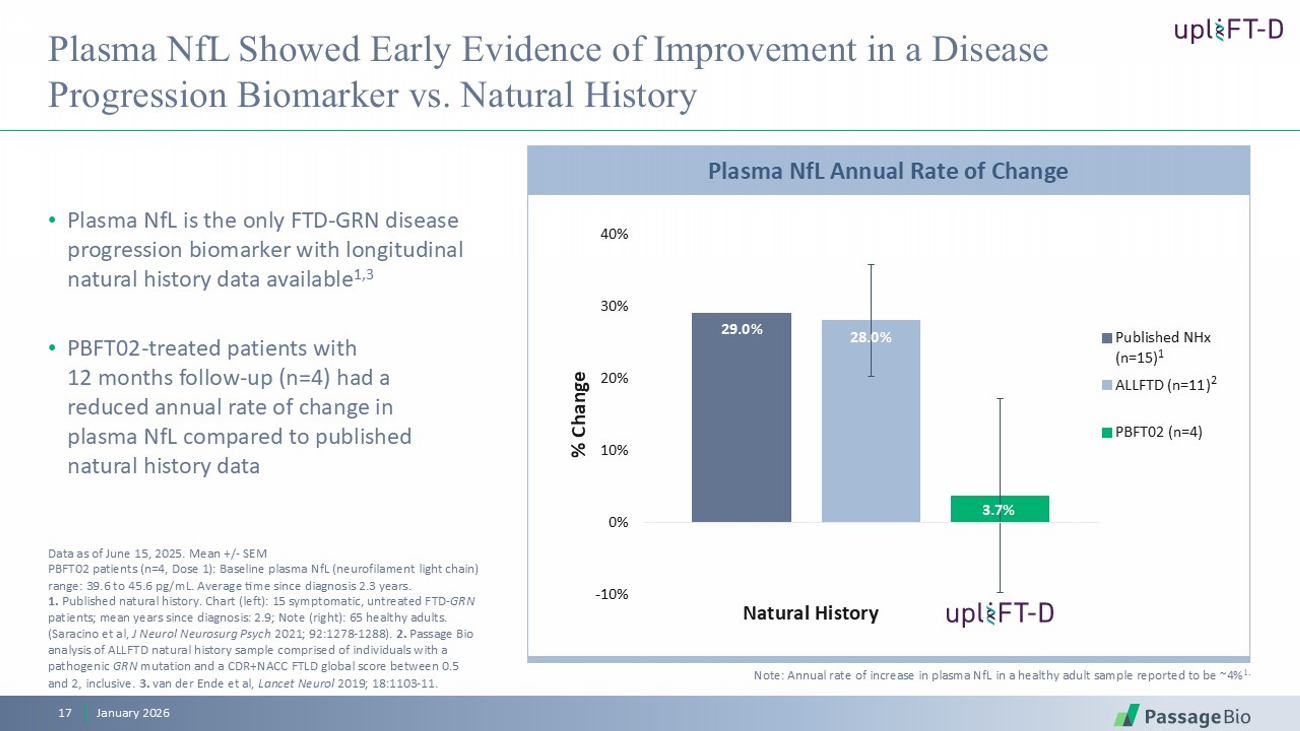
Plasma NfL Showed Early Evidence of Improvement in a Disease Progression Biomarker vs. Natural History • Plasma NfL is the only FTD - GRN disease progression biomarker with longitudinal natural history data available 1,3 • PBFT02 - treated patients with 12 months follow - up (n=4) had a reduced annual rate of change in plasma NfL compared to published natural history data January 2026 17 29.0% 28.0% 3.7% -10% 0% 10% 20% 30% 40% % Change Published NHx (n=15) ALLFTD (n=11) PBFT02 (n=4) Natural History 1 2 Note: Annual rate of increase in plasma NfL in a healthy adult sample reported to be ~4% 1. Plasma NfL Annual Rate of Change Data as of June 15, 2025. Mean +/ - SEM PBFT02 patients (n=4, Dose 1): Baseline plasma NfL (neurofilament light chain) range: 39.6 to 45.6 pg / mL. Average time since diagnosis 2.3 years. 1. Published natural history. Chart (left): 15 symptomatic, untreated FTD - GRN patients; mean years since diagnosis: 2.9; Note (right): 65 healthy adults. (Saracino et al, J Neurol Neurosurg Psych 2021; 92:1278 - 1288). 2. Passage Bio analysis of ALLFTD natural history sample comprised of individuals with a pathogenic GRN mutation and a CDR+NACC FTLD global score between 0.5 and 2, inclusive. 3. van der Ende et al, Lancet Neurol 2019; 18:1103 - 11.
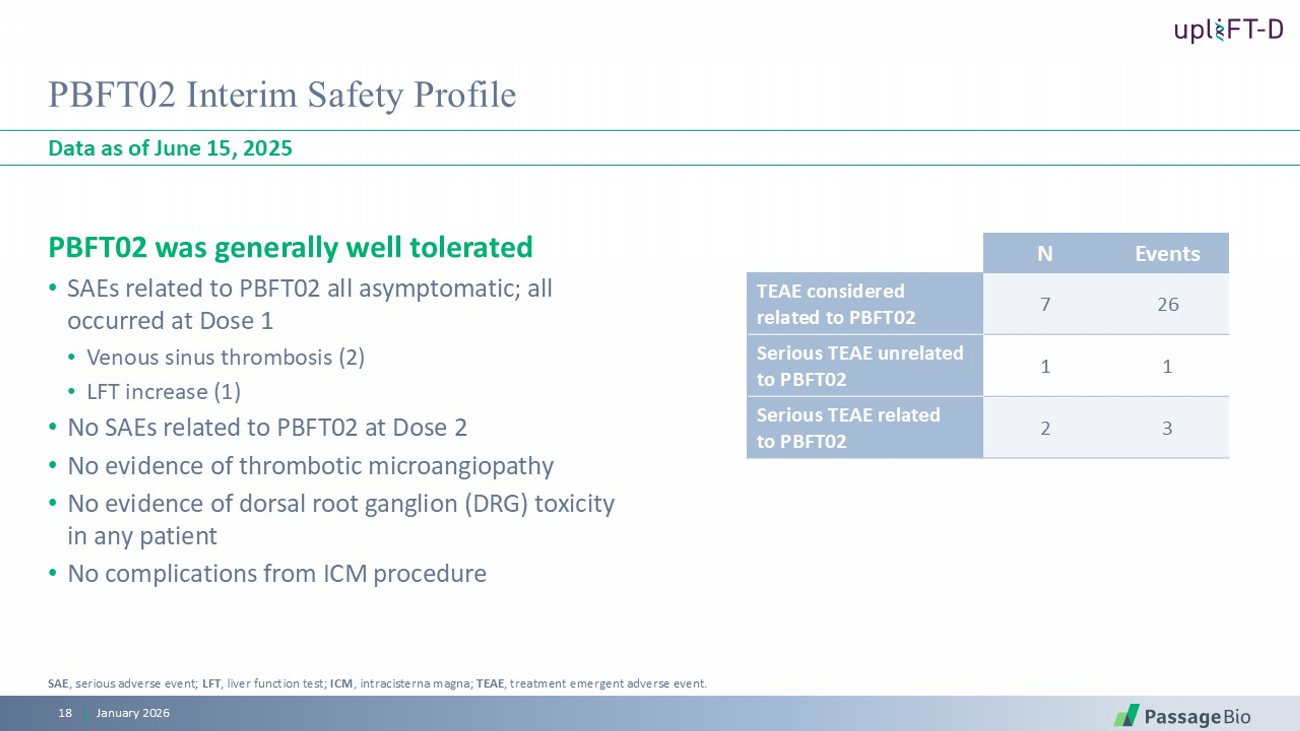
PBFT02 Interim Safety Profile PBFT02 was generally well tolerated • SAEs related to PBFT02 all asymptomatic; all occurred at Dose 1 • Venous sinus thrombosis (2) • LFT increase (1) • No SAEs related to PBFT02 at Dose 2 • No evidence of thrombotic microangiopathy • No evidence of dorsal root ganglion (DRG) toxicity in any patient • No complications from ICM procedure Data as of June 15, 2025 January 2026 18 Events N 26 7 TEAE considered related to PBFT02 1 1 Serious TEAE unrelated to PBFT02 3 2 Serious TEAE related to PBFT02 SAE , serious adverse event; LFT , liver function test; ICM , intracisterna magna; TEAE , treatment emergent adverse event.
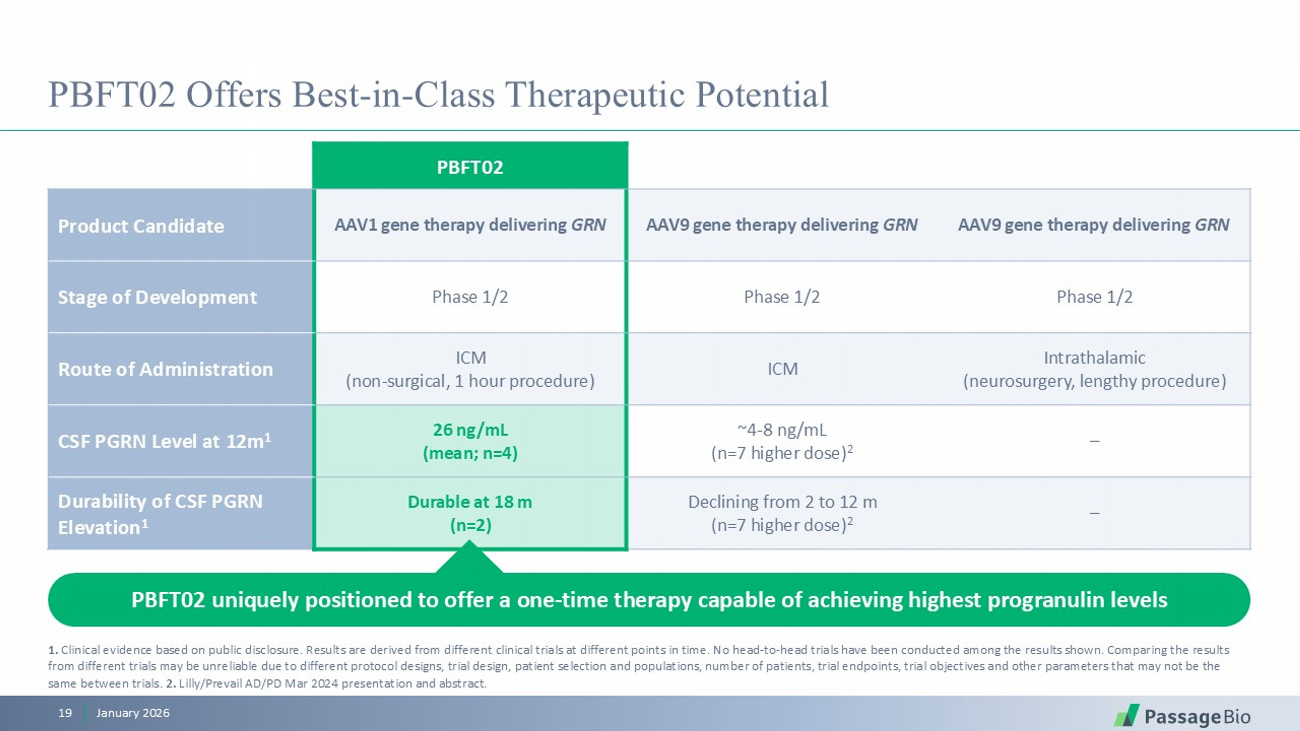
PBFT02 Offers Best - in - Class Therapeutic Potential PBFT02 AAV9 gene therapy delivering GRN AAV9 gene therapy delivering GRN AAV1 gene therapy delivering GRN Product Candidate Phase 1/2 Phase 1/2 Phase 1/2 Stage of Development Intrathalamic (neurosurgery, lengthy procedure) ICM ICM (non - surgical, 1 hour procedure) Route of Administration – ~4 - 8 ng/mL (n=7 higher dose) 2 26 ng/mL (mean; n=4) CSF PGRN Level at 12m 1 – Declining from 2 to 12 m (n=7 higher dose) 2 Durable at 18 m (n=2) Durability of CSF PGRN Elevation 1 PBFT02 uniquely positioned to offer a one - time therapy capable of achieving highest progranulin levels January 2026 19 1. Clinical evidence based on public disclosure. Results are derived from different clinical trials at different points in time. N o head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unreliable due to different protocol designs, trial design, patient selection and populations, n umb er of patients, trial endpoints, trial objectives and other parameters that may not be the same between trials. 2. Lilly/Prevail AD/PD Mar 2024 presentation and abstract.
PBFT02 has Potential to Correct Underlying Pathology in FTD - GRN, FTD - C9orf72 and ALS January 2026 20 TDP - 43 pathology is a hallmark of multiple neurodegenerative diseases 1 • TDP - 43 mislocalizes from nucleus to cytoplasm • Forms inclusion bodies associated with neurodegeneration
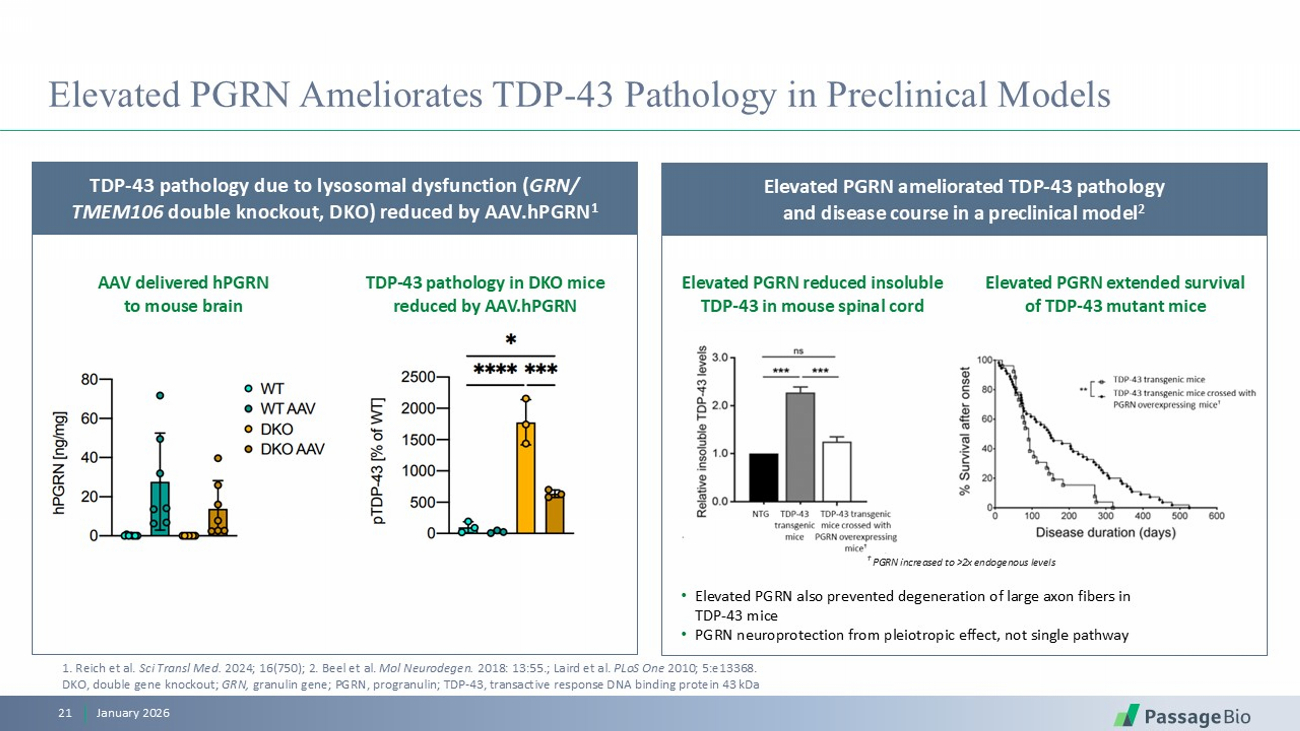
Elevated PGRN Ameliorates TDP - 43 Pathology in Preclinical Models January 2026 21 TDP - 43 pathology due to lysosomal dysfunction ( GRN / TMEM106 double knockout, DKO) reduced by AAV.hPGRN 1 AAV delivered hPGRN to mouse brain TDP - 43 pathology in DKO mice reduced by AAV.hPGRN Elevated PGRN reduced insoluble TDP - 43 in mouse spinal cord Elevated PGRN extended survival of TDP - 43 mutant mice Elevated PGRN ameliorated TDP - 43 pathology and disease course in a preclinical model 2 • Elevated PGRN also prevented degeneration of large axon fibers in TDP - 43 mice • PGRN neuroprotection from pleiotropic effect, not single pathway 1 . Reich et al. Sci Transl Med. 2024; 16(750) ; 2. Beel et al. Mol Neurodegen . 2018: 13:55. ; Laird et al. PLoS One 2010; 5:e13368. DKO, double gene knockout; GRN, granulin gene; PGRN, progranulin ; TDP - 43, t ransactive response DNA binding protein 43 kDa † PGRN increased to >2x endogenous levels

• AAV delivery of functional GRN gene to express new PGRN, increasing levels both intra - and extra - cellularly • Preserves all natural pathways to properly traffic PGRN intracellularly where it is needed • ICM route of administration enables low doses of AAV and broad CNS biodistribution • Non - surgical, brief procedure (< 60 minutes) • Promise of a one - time therapy for patients • Durable elevation of CSF PGRN 1 PBFT02: Summary of Approach January 2026 22 1. Interim data from upliFT - D as of June 15, 2025. A novel and potentially transformative therapy for FTD - GRN patients

Huntington’s Disease Preclinical Program
OVERVIEW • Fatal, monogenic, autosomal dominant neurodegenerative disease • Caused by trinucleotide (CAG) expansion in the huntingtin ( HTT ) gene resulting in mutant huntingtin ( mHTT ) protein expression • More than 200,000 people estimated to be at risk in the US 1 CLINICAL SYMPTOMS • Symptom onset typically occurs between 30 – 50 years old • Characterized by progressive motor, cognitive, and behavioral deterioration, due to neuronal dysfunction then degeneration Huntington’s Disease: A Fatal Neurodegenerative Disease with No Disease - Modifying Therapy Average life expectancy after symptom onset is 15 – 20 years January 2026 24 Motor deterioration Cognitive deterioration Behavioral deterioration 1. HDSA; Fisher and Hayden Mov Disord . 29:105 - 14, 2014.
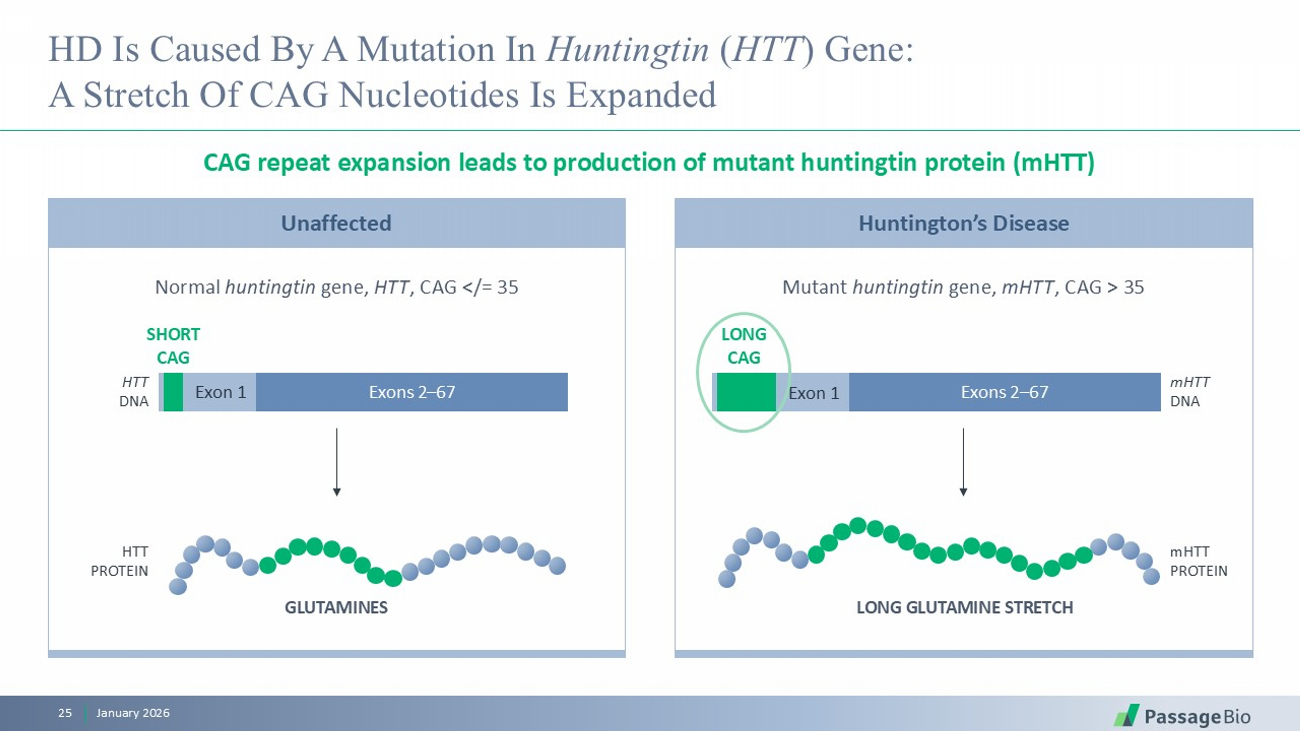
Unaffected HD Is Caused By A Mutation In Huntingtin ( HTT ) Gene: A Stretch Of CAG Nucleotides Is Expanded CAG repeat expansion leads to production of mutant huntingtin protein ( mHTT ) January 2026 25 Normal huntingtin gene, HTT , CAG </= 35 Mutant huntingtin gene, mHTT , CAG > 35 GLUTAMINES Exon 1 Exons 2 – 67 SHORT CAG HTT DNA LONG GLUTAMINE STRETCH HTT PROTEIN Exon 1 Exons 2 – 67 LONG CAG mHTT DNA mHTT PROTEIN Huntington’s Disease
DNA Repair (DR) Proteins Play a Key Role in CAG Repeat Expansion • CAG expansion above a certain threshold leads to neurodegeneration • Longer CAG repeats associated with worse disease pathology • CAG expansion occurs at different rates in different neurons, and is fastest in the caudate and putamen brain regions which degenerate first January 2026 26 1. Genetic Modifiers of Huntington’s Disease ( GeM - HD) Consortium, Nat Genet . 2025; 57:1426 - 36. 2. Dragileva E et. al, Neurobio Dis . 2009; 33:37 - 47. 3 . Mouro Pinto et. al, Nat Genet . 2025; 57:314 - 322. In Huntington’s disease (HD), the CAG repeat in the HTT gene can elongate over time, termed somatic instability • In the presence of certain CAG motifs, MSH3 can erroneously incorporate CAGs into DNA, leading to CAG expansion • In HD: certain genetic variants altering MSH3 function are associated with delayed onset and slowed progression 1 • In HD mice: MSH3 is essential for CAG expansion, and MSH3 knock - down reduced somatic instability and HTT pathology 2,3 MSH3, a DR protein, is a key driver of somatic instability
Program Status Our Approach: Decrease MSH3 to Reduce Somatic Instability in the HTT Gene Expect to declare a clinical candidate in 2H 2026 January 2026 27 Developing a differentiated approach to decrease MSH3 expression via AAV delivery of a miRNA Proof - of - concept studies completed, with additional preclinical studies ongoing Plan to utilize an optimized intraparenchymal delivery approach • One - time delivery • Direct delivery to critical brain regions • Reduced total procedure time • Limited peripheral exposure to reduce safety risks

Looking Ahead
Completed development of high - productivity, suspension - based manufacturing process Single production lot estimated to yield >1,000 doses 1 with >70% full capsids Robust Manufacturing Process Aligned with the FDA on an analytical approach to establish comparability of suspension - based process Process Comparability Plan Developed assay and reached alignment with FDA on suitability of assay for PBFT02 release Functional Potency Assay Critical Manufacturing Milestones Achieved to Enable Late - Stage Development of PBFT02 January 2026 29 1. Estimated yield based on Dose 2.
1 FTD - GRN is a rapidly progressing disease with no approved disease - modifying therapies and a substantial unmet clinical need 2 Multiple recent gene therapy precedents demonstrate FDA receptivity to a single - arm registrational approach 3 Existing, well - structured FTD natural history studies with >300 FTD - GRN patients Plan to Initiate Discussions with the FDA on a Registrational Study Design in 1H 2026 Rationale for a single - arm registrational study January 2026 30
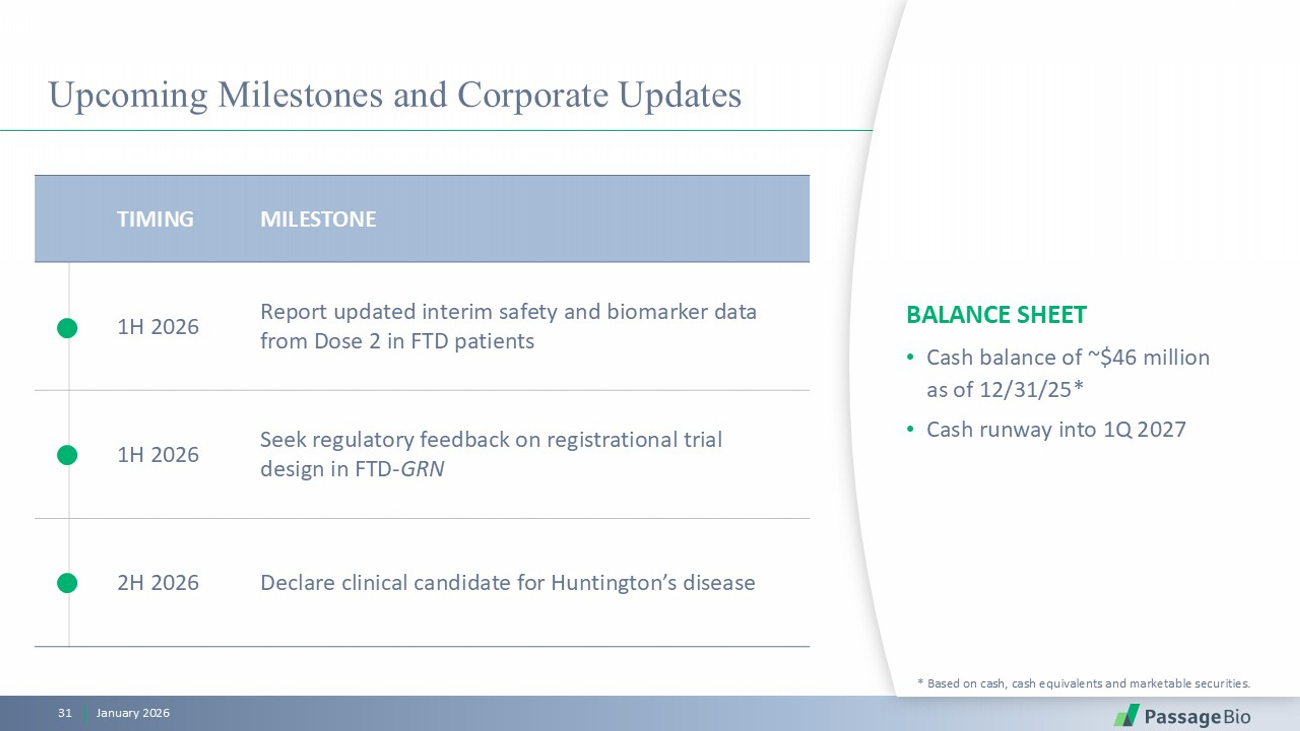
Upcoming Milestones and Corporate Updates BALANCE SHEET • Cash balance of ~$46 million as of 12/31/25* • Cash runway into 1Q 2027 * Based on cash, cash equivalents and marketable securities. MILESTONE TIMING Report updated interim safety and biomarker data from Dose 2 in FTD patients 1H 2026 Seek regulatory feedback on registrational trial design in FTD - GRN 1H 2026 Declare clinical candidate for Huntington’s disease 2H 2026 January 2026 31
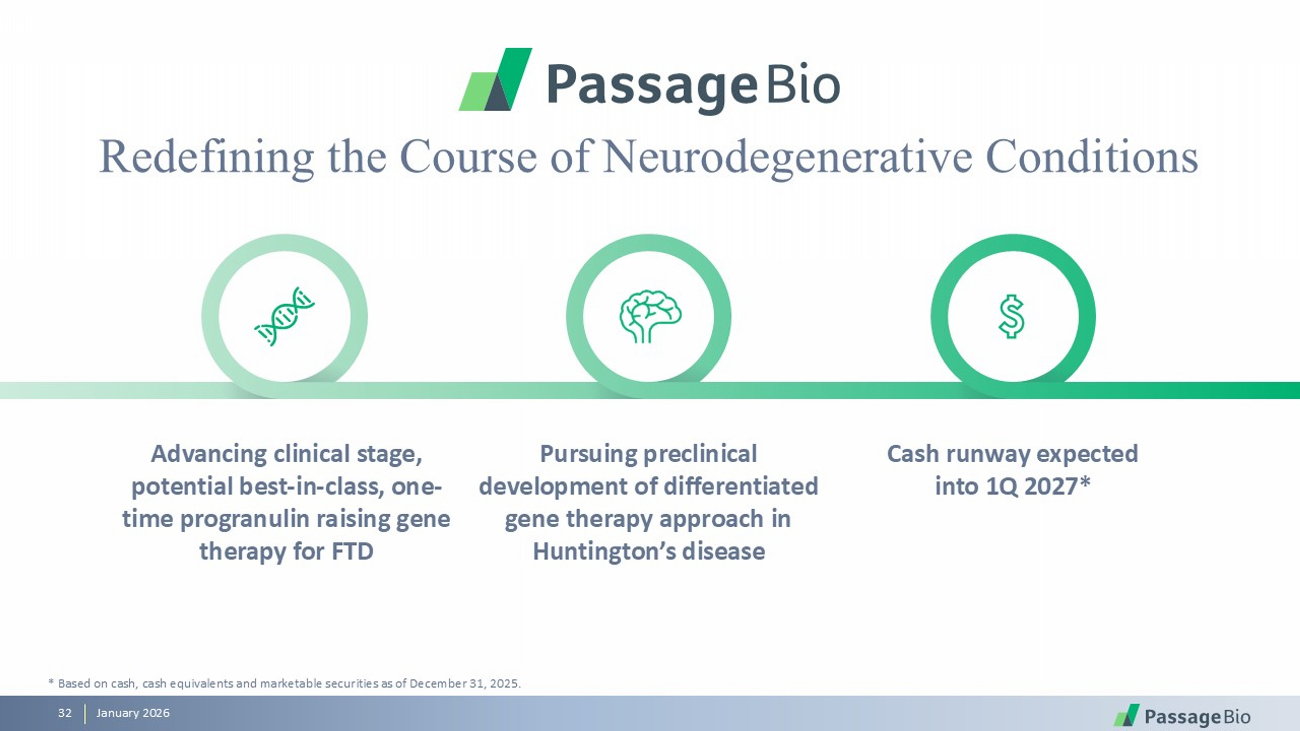
January 2026 32 * Based on cash, cash equivalents and marketable securities as of December 31, 2025. Redefining the Course of Neurodegenerative Conditions Advancing clinical stage, potential best - in - class, one - time progranulin raising gene therapy for FTD Pursuing preclinical development of differentiated gene therapy approach in Huntington’s disease Cash runway expected into 1Q 2027*

© 2026 Passage Bio. All rights reserved. passagebio.com Thank you!
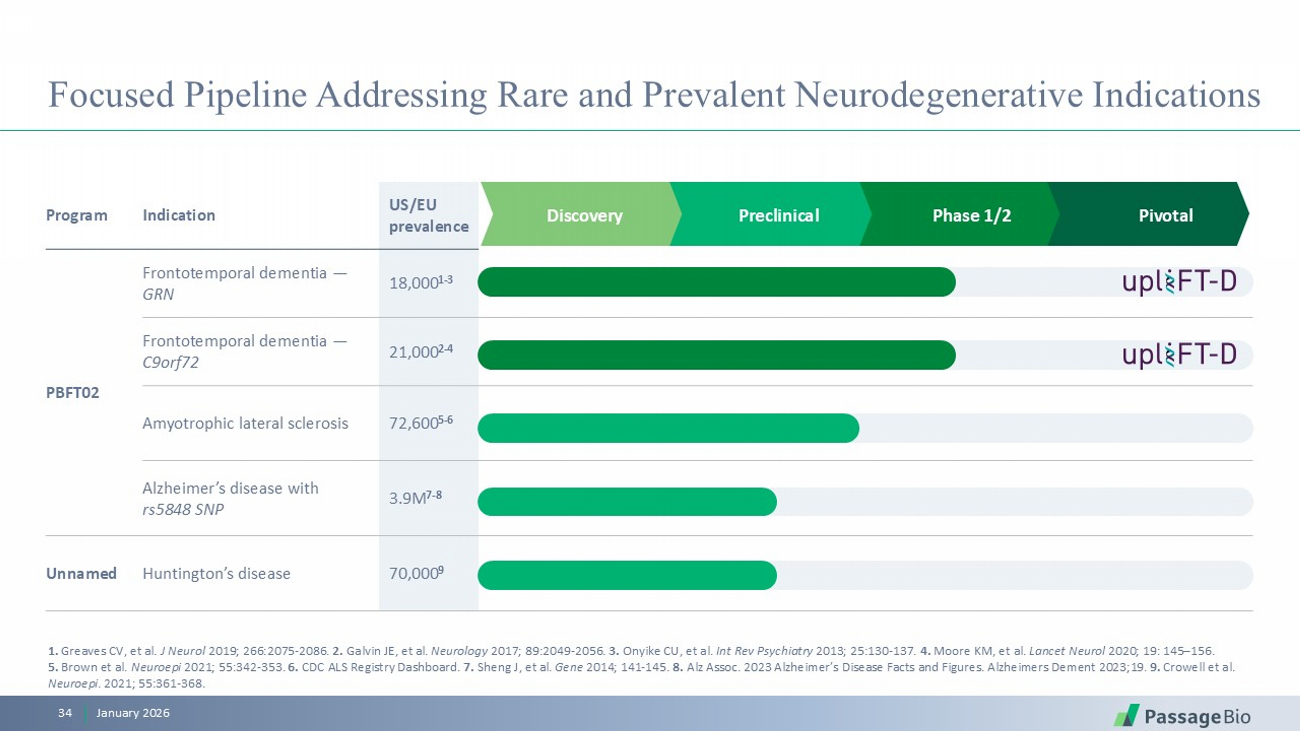
Pivotal Phase 1/2 Preclinical Discovery US/EU prevalence Indication Program 18,000 1 - 3 Frontotemporal dementia — GRN PBFT02 21,000 2 - 4 Frontotemporal dementia — C9orf72 72,600 5 - 6 Amyotrophic lateral sclerosis 3.9M 7 - 8 Alzheimer’s disease with rs5848 SNP 70,000 9 Huntington’s disease Unnamed Focused Pipeline Addressing Rare and Prevalent Neurodegenerative Indications 1. Greaves CV, et al. J Neurol 2019; 266:2075 - 2086. 2. Galvin JE, et al. Neurology 2017; 89:2049 - 2056 . 3. Onyike CU, et al. Int Rev Psychiatry 2013; 25:130 - 137 . 4. Moore KM, et al. Lancet Neurol 2020; 19: 145 – 156. 5. Brown et al. Neuroepi 2021; 55:342 - 353. 6. CDC ALS Registry Dashboard . 7. Sheng J, et al. Gene 2014; 141 - 145. 8. Alz Assoc. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2023;19. 9. Crowell et al. Neuroepi . 2021; 55:361 - 368. January 2026 34

LEADERSHIP TEAM Demonstrated Leadership BOARD OF DIRECTORS Maxine Gowen, Ph.D. Chairwoman Athena Countouriotis , M.D. Avenzo Therapeutics Derrell Porter, M.D. cTRL Therapeutics Dolan Sondhi, Ph.D. Weill Cornell Medicine Sandip Kapadia Harmony Biosciences Thomas Kassberg Former CBO Ultragenyx William Chou, M.D. President and Chief Executive Officer Deep experience in rare disease, CNS disorders and genetic medicines Eden Fucci SVP Technical Operations Stuart Henderson Chief Business Officer William Chou, M.D. President and Chief Executive Officer Kathleen Borthwick Chief Financial Officer Karl Whitney, Ph.D. SVP Global Regulatory Affairs Sue Browne, Ph.D. Chief Scientific Officer January 2026 35