

Nasdaq: TIVC | www.tivichealth.com ® ir@tivichealth.com – January 2026 – Jennifer Ernst, CEO, Tivic Health Harnessing the immune system to save lives and improve clinical outcomes • Late - stage, highly de - risked assets • Competitively differentiated treatments for radiation damage, oncology, and immune system modification • Experienced management team • Targeting multiple high value inflection points expected in the next 18 - 24 months Nasdaq: TIVC

Nasdaq: TIVC | www.tivichealth.com Slide # 2 Safe Harbor This presentation contains forward - looking statements . All statements other than statements of historical facts contained in this presentation may be forward - looking statements . Statements regarding our future results of operations and financial position, economic performance, business strategy and plans and objectives of management for future operations, including, among others, statements regarding our expected growth, acquisition strategies, investments, and future capital expenditures are all forward looking statements . Without limiting the generality of the foregoing, words such as “may,” “will,” “should,” “expect,” “believe,” “anticipate,” “intend,” “could,” “estimate,” “target,” “project,” “might,” “plan,” “predict” or “continue” or the negative or other variations thereof or comparable terminology are intended to identify forward - looking statements . We caution you that any such forward - looking statements are not guarantees of future performance, and are subject to risks, assumptions and uncertainties that are difficult to predict and beyond our ability and control . Although we believe that the expectations reflected in these forward - looking statements are reasonable as of the date made, actual results may prove to be materially different from the results expressed or implied by the forward - looking statements . Any differences could be caused by a number of factors, including but not limited to : the future development of our products and product candidates ; our plans to seek, and ability to obtain, regulatory approval for our product candidates ; timing and progress of clinical development of our product candidates ; our ability to commercialize products arising out of our programs ; our future development of Entolimod and Entolasta ; changes to our business strategy ; our ability to successfully generate revenue opportunities via our CDMO subsidiary ; the potential opportunities that may be available to us and our product candidates in the future ; our anticipated needs for working capital ; our ability to secure additional financing ; regulatory or legal developments in the United States and other countries ; and market and other conditions ; macroeconomic factors, including tariffs and economic uncertainty arising from geopolitical tensions . Many of the important factors that will determine these results are beyond our ability to control or predict . Accordingly, you should not place undue reliance on any such forward - looking statements . Any forward - looking statement speaks only as of the date on which it is made, and, except as otherwise required by law, we do not undertake any obligation to publicly update or review any forward - looking statement, whether as a result of new information, future developments or otherwise . We cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements . In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject . These statements are based upon information available to us as of the date made, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information . These statements are inherently uncertain, and you are cautioned not to place undue reliance upon these statements . Unless otherwise indicated, information contained in this presentation concerning our industry, competitive position and the markets in which we operate is based on information from independent industry and research organizations, other third - party sources, as well as data from our internal research, and are based on assumptions made by us upon reviewing such data, and our experience in, and knowledge of, such industry and markets, which we believe to be reasonable . In addition, projections, assumptions and estimates of the future performance of the industry in which we operate, and our future performance are necessarily subject to uncertainty and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent parties and by us . This presentation shall not constitute an offer to sell or the solicitation of an offer to buy our securities .

Nasdaq: TIVC | www.tivichealth.com Experienced Management Team Company Facts Late - stage biologics Founded: 2016 IPO: 2021 Pivot: 2025 ~50 Employees Integrated manufacturing Nasdaq: TIVC
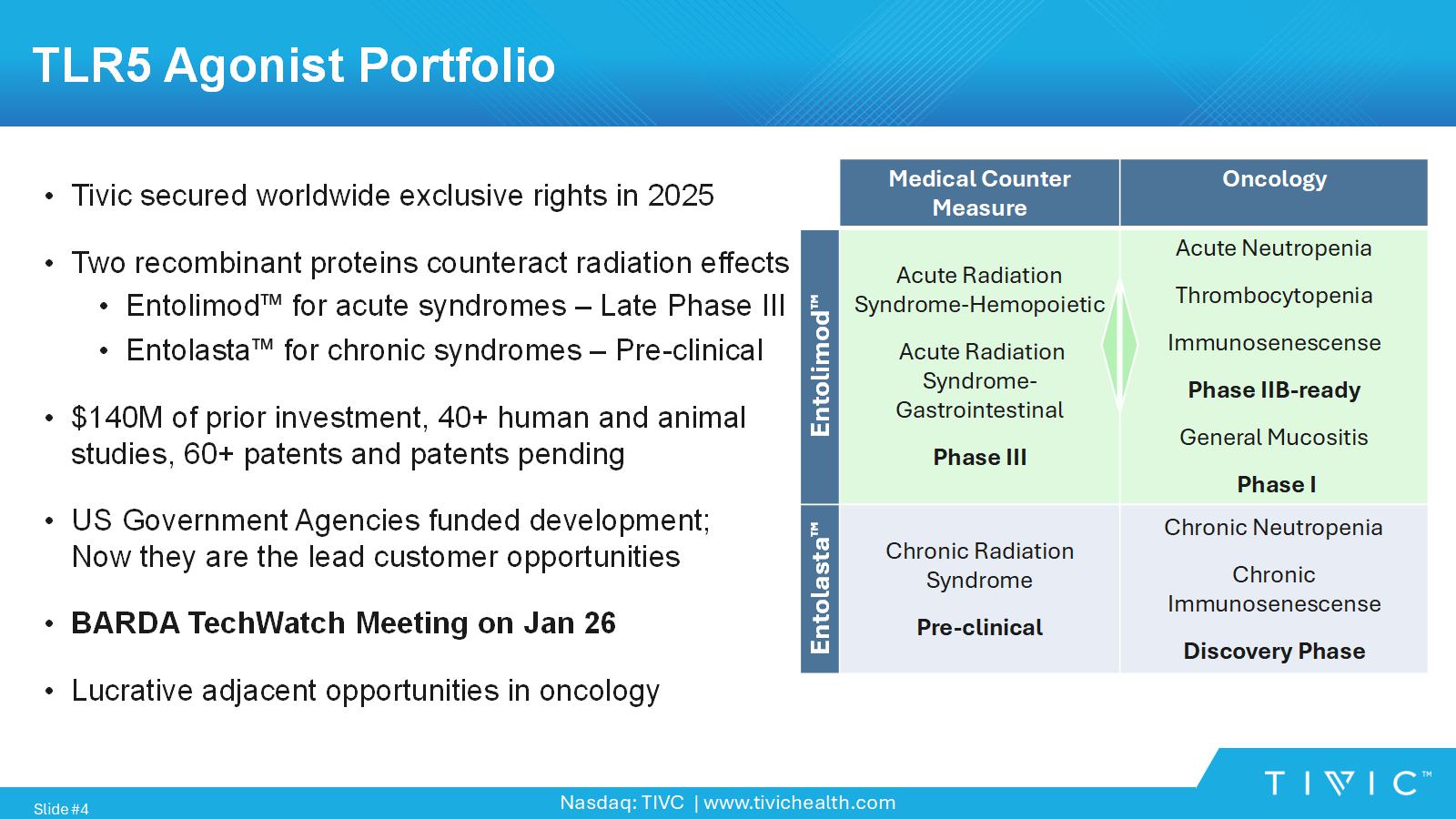
Nasdaq: TIVC | www.tivichealth.com TLR5 Agonist Portfolio • Tivic secured worldwide exclusive rights in 2025 • Two recombinant proteins counteract radiation effects • Entolimod for acute syndromes – Late Phase III • Entolasta for chronic syndromes – Pre - clinical • $140M of prior investment, 40+ human and animal studies, 60+ patents and patents pending • US Government Agencies funded development; Now they are the lead customer opportunities • BARDA TechWatch Meeting on Jan 26 • Lucrative adjacent opportunities in oncology Slide # 4 Oncology Medical Counter Measure Acute Neutropenia Thrombocytopenia Immunosenescense Phase IIB - ready General Mucositis Phase I Acute Radiation Syndrome - Hemopoietic Acute Radiation Syndrome - Gastrointestinal Phase III Entolimod Chronic Neutropenia Chronic Immunosenescense Discovery Phase Chronic Radiation Syndrome Pre - clinical Entolasta
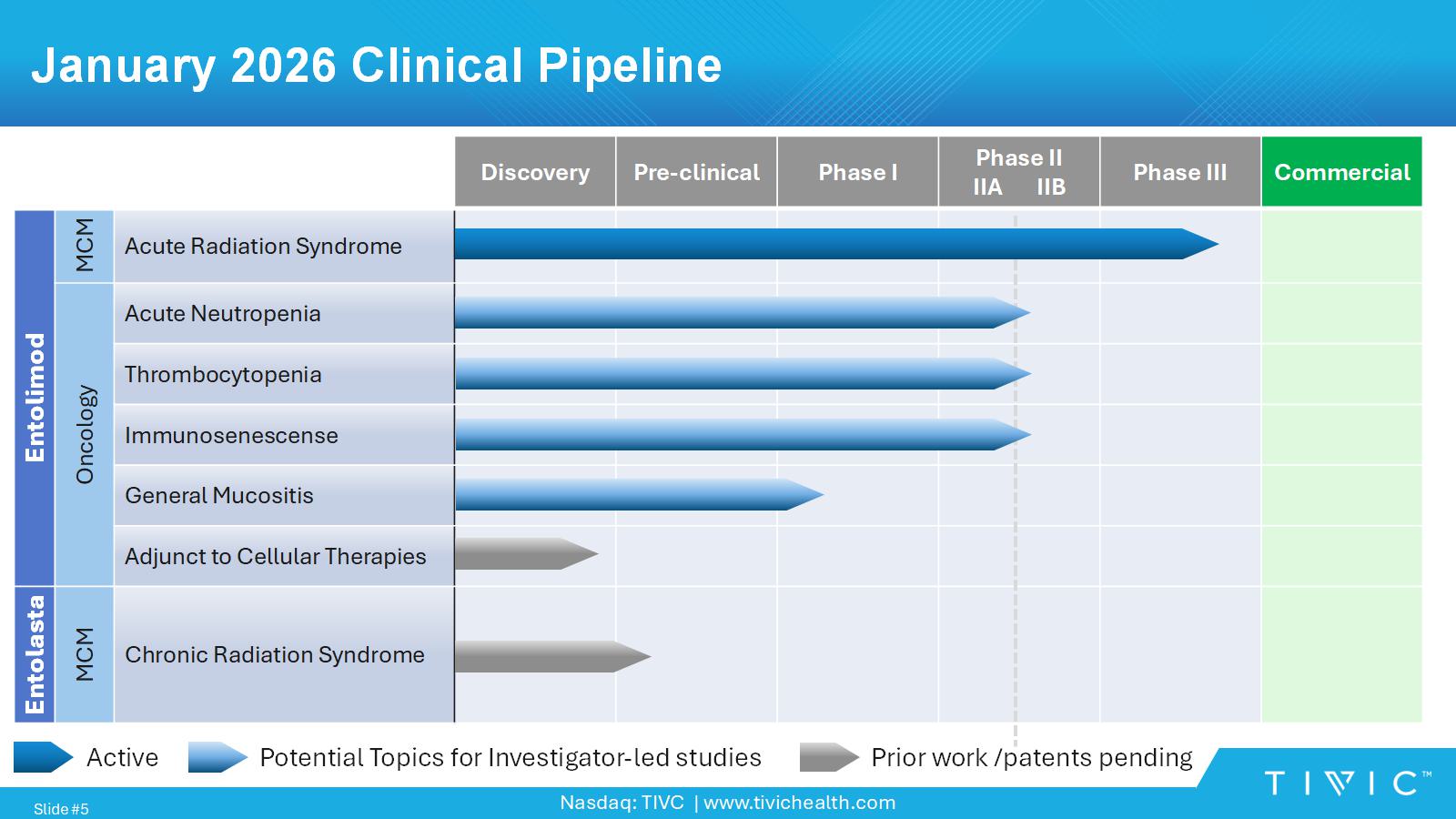
Nasdaq: TIVC | www.tivichealth.com January 2026 Clinical Pipeline Slide # 5 Commercial Phase III Phase II IIA IIB Phase I Pre - clinical Discovery Acute Radiation Syndrome MCM Entolimod Acute Neutropenia Oncology Thrombocytopenia Immunosenescense General Mucositis Adjunct to Cellular Therapies Chronic Radiation Syndrome MCM Entolasta Active Potential Topics for Investigator - led studies Prior work /patents pending

The Radiation Issue Ionizing radiation destroys fast - growing cells
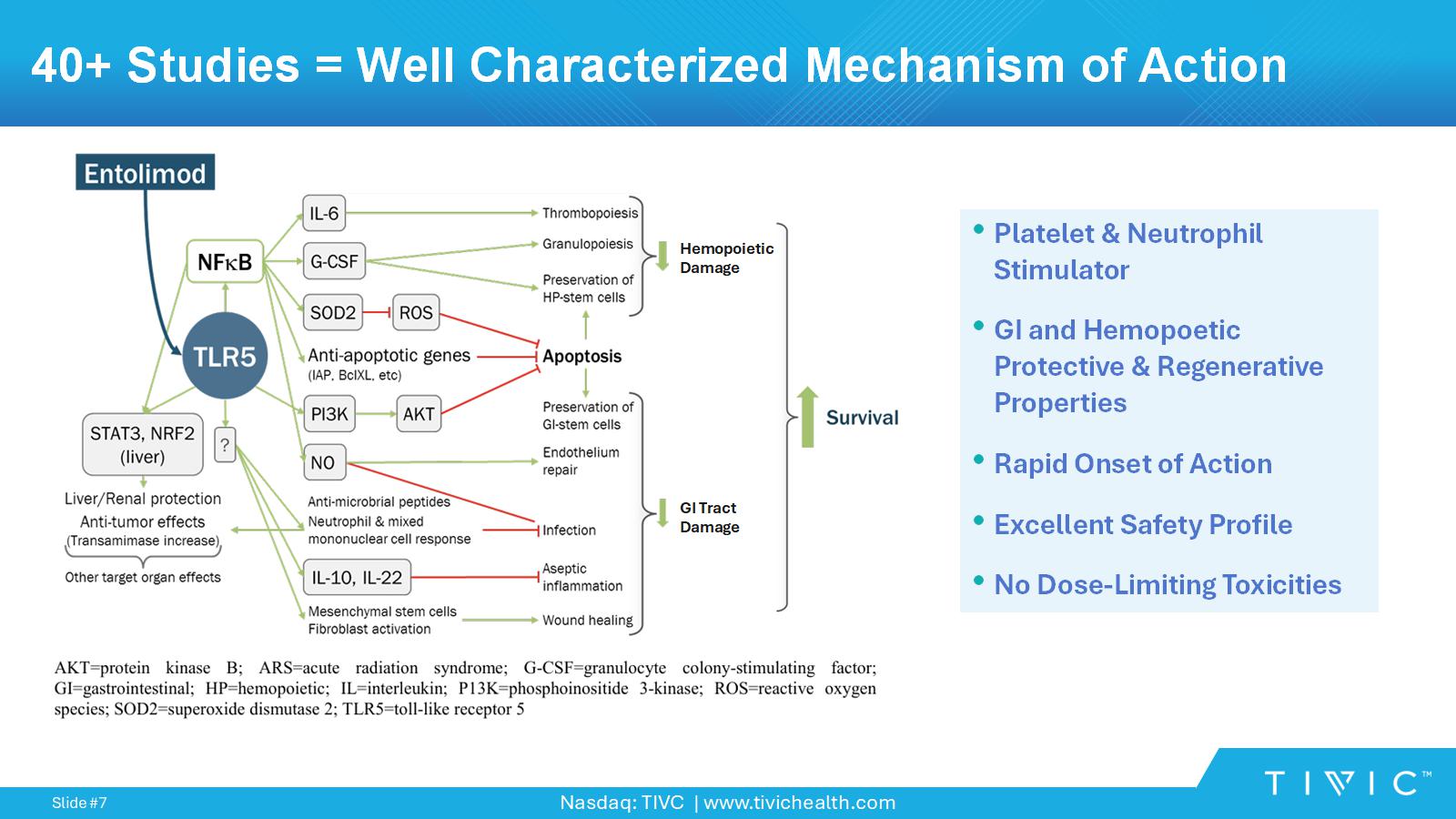
Nasdaq: TIVC | www.tivichealth.com Slide # 7 40+ Studies = Well Characterized Mechanism of Action Hemopoietic Damage GI Tract Damage • Platelet & Neutrophil Stimulator • GI and Hemopoetic Protective & Regenerative Properties • Rapid Onset of Action • Excellent Safety Profile • No Dose - Limiting Toxicities
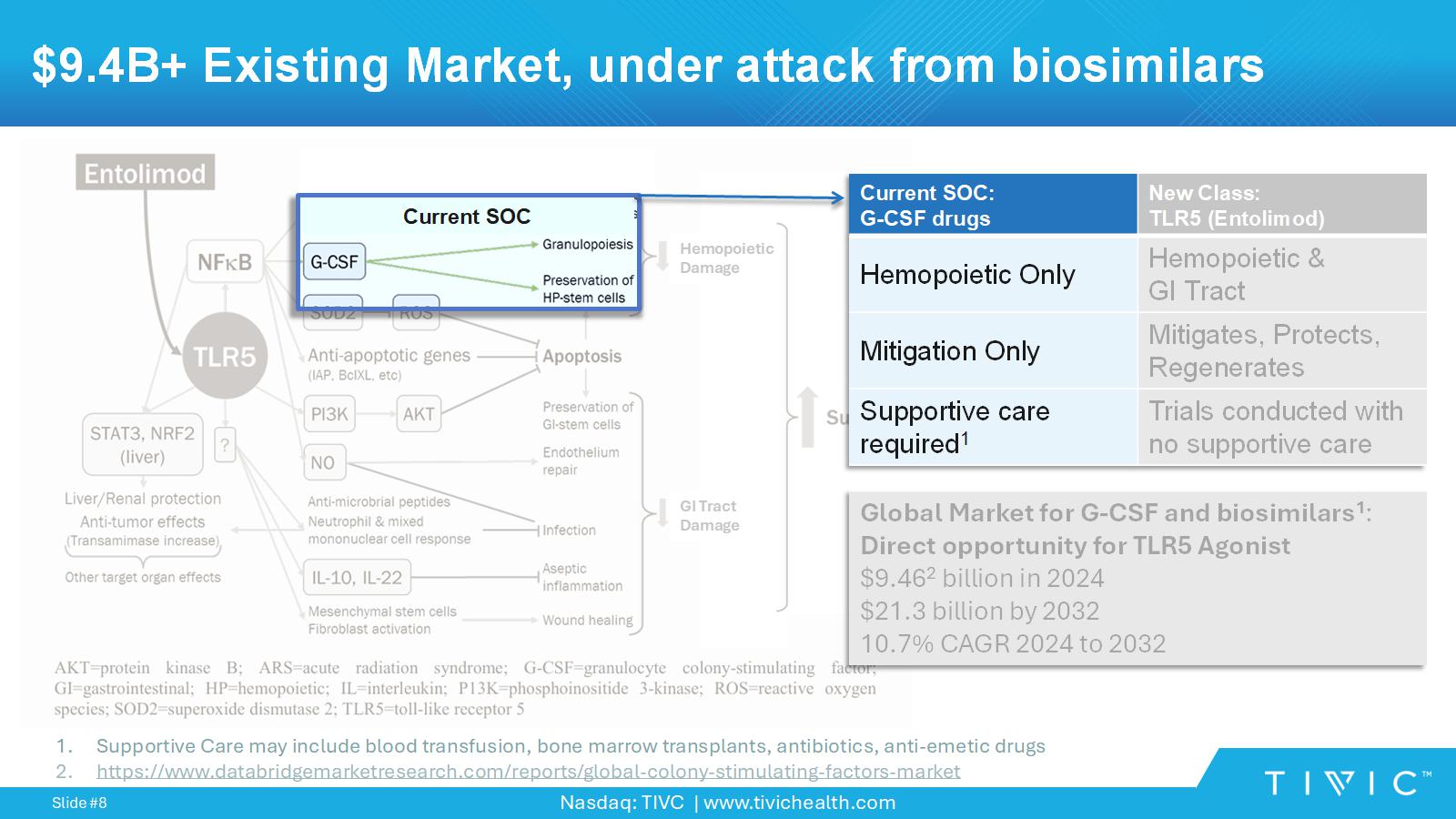
Nasdaq: TIVC | www.tivichealth.com Slide # 8 $9.4B+ Existing Market, under attack from biosimilars GI Tract Damage GI Tract Damage Hemopoietic Damage Current SOC New Class: TLR5 (Entolimod) Current SOC: G - CSF drugs Hemopoietic & GI Tract Hemopoietic Only Mitigates, Protects, Regenerates Mitigation Only Trials conducted with no supportive care Supportive care required 1 Global Market for G - CSF and biosimilars 1 : Direct opportunity for TLR5 Agonist $9.46 2 billion in 2024 $21.3 billion by 2032 10.7% CAGR 2024 to 2032 1. Supportive Care may include blood transfusion, bone marrow transplants, antibiotics, anti - emetic drugs 2. https://www.databridgemarketresearch.com/reports/global - colony - stimulating - factors - market
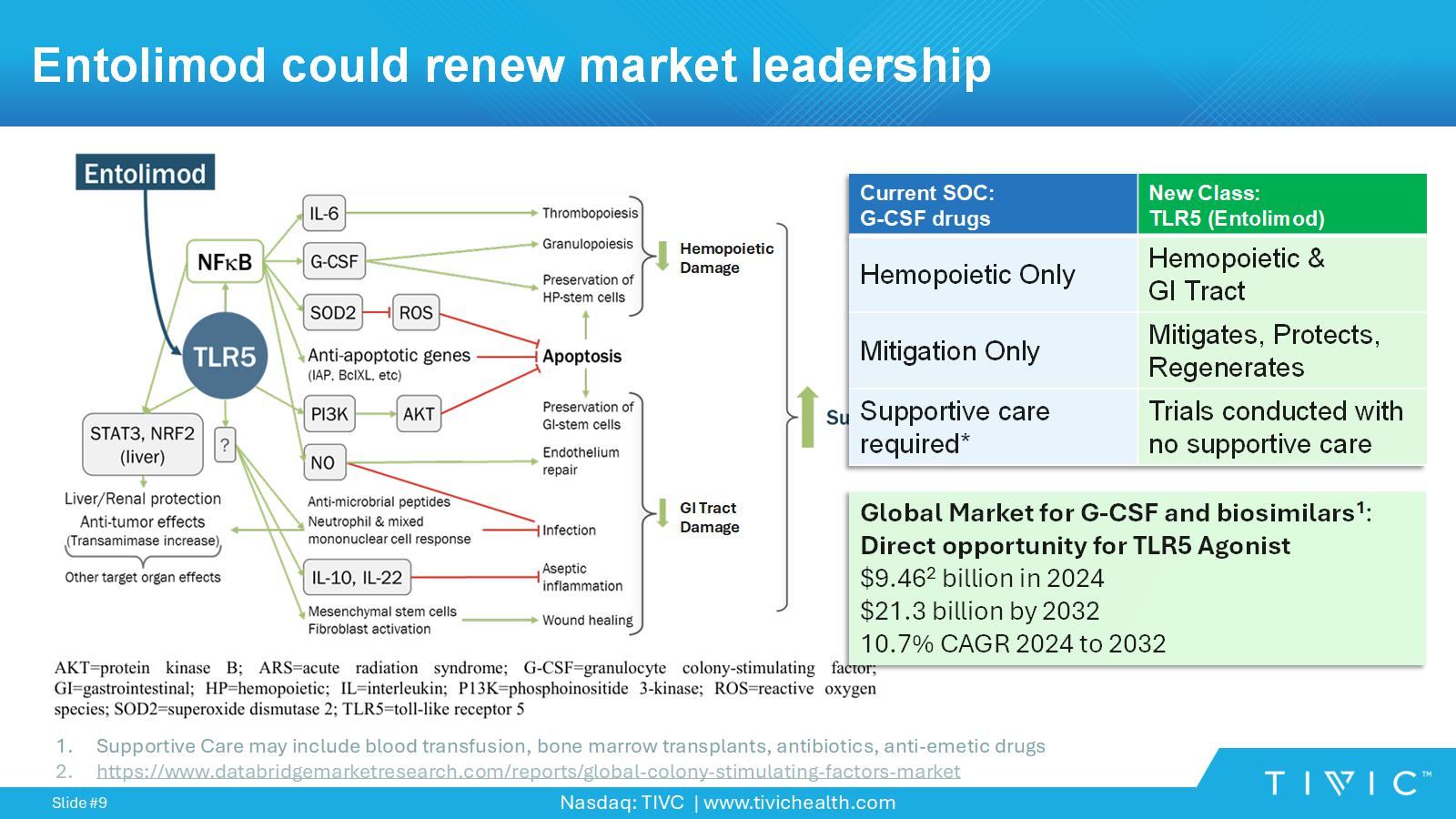
Nasdaq: TIVC | www.tivichealth.com Slide # 9 Entolimod could renew market leadership Hemopoietic Damage GI Tract Damage New Class: TLR5 (Entolimod) Current SOC: G - CSF drugs Hemopoietic & GI Tract Hemopoietic Only Mitigates, Protects, Regenerates Mitigation Only Trials conducted with no supportive care Supportive care required* Global Market for G - CSF and biosimilars 1 : Direct opportunity for TLR5 Agonist $9.46 2 billion in 2024 $21.3 billion by 2032 10.7% CAGR 2024 to 2032 1. Supportive Care may include blood transfusion, bone marrow transplants, antibiotics, anti - emetic drugs 2. https://www.databridgemarketresearch.com/reports/global - colony - stimulating - factors - market
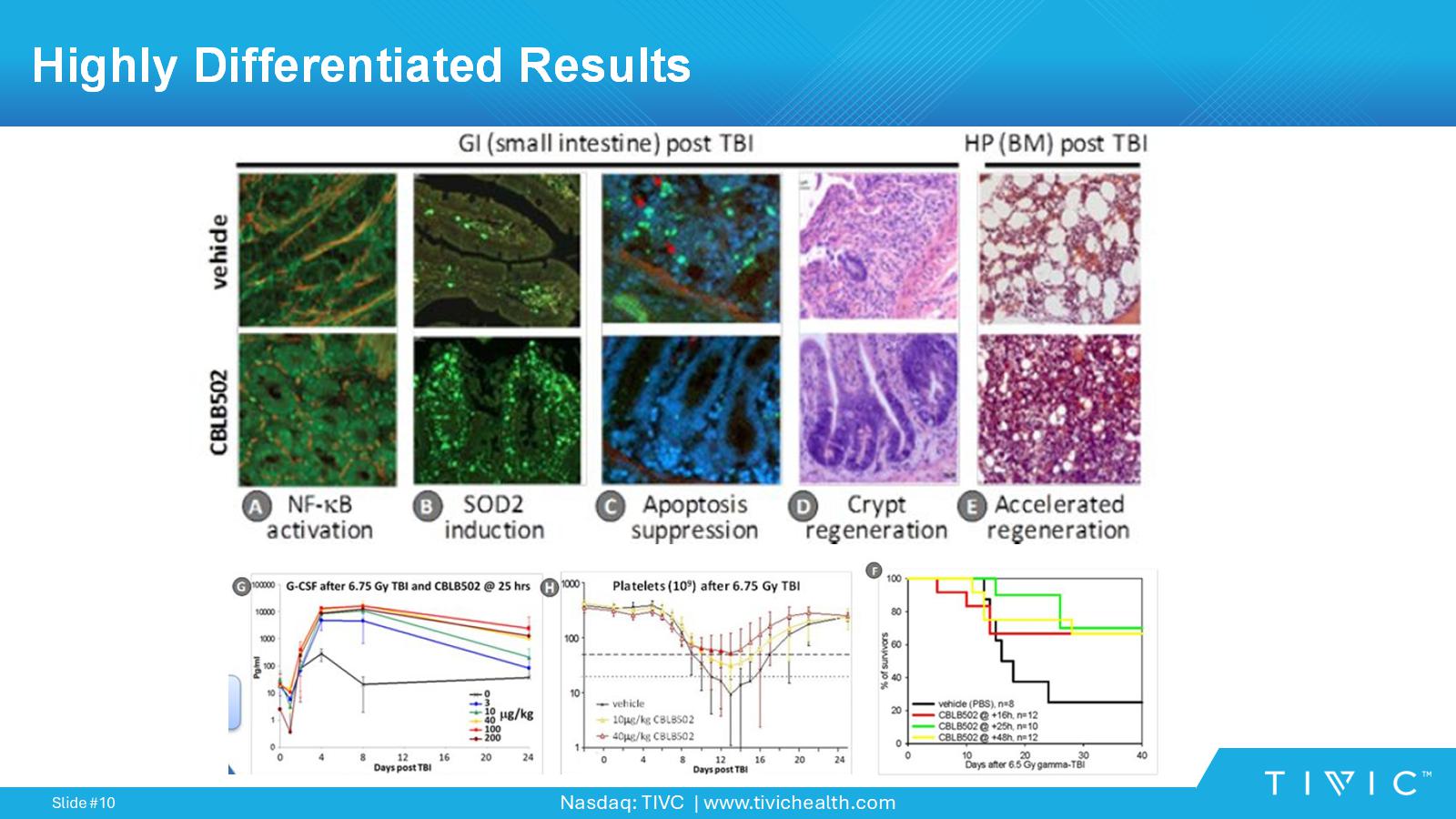
Nasdaq: TIVC | www.tivichealth.com Slide # 10 10 Highly Differentiated Results
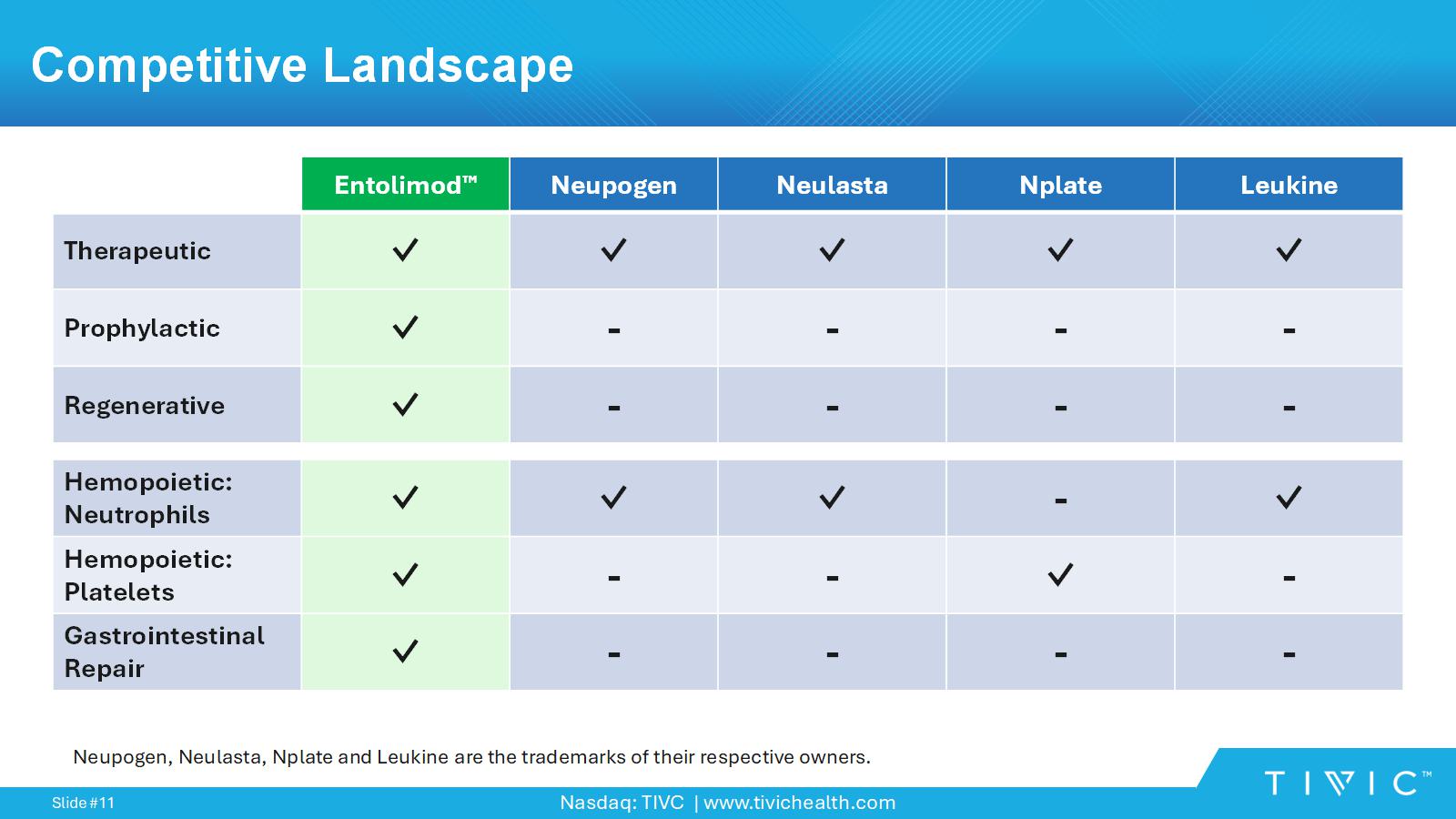
Nasdaq: TIVC | www.tivichealth.com Slide # 11 Competitive Landscape Leukine Nplate Neulasta Neupogen Entolimod ✓ ✓ ✓ ✓ ✓ Therapeutic - - - - ✓ Prophylactic - - - - ✓ Regenerative ✓ - ✓ ✓ ✓ Hemopoietic: Neutrophils - ✓ - - ✓ Hemopoietic: Platelets - - - - ✓ Gastrointestinal Repair Neupogen, Neulasta, Nplate and Leukine are the trademarks of their respective owners.
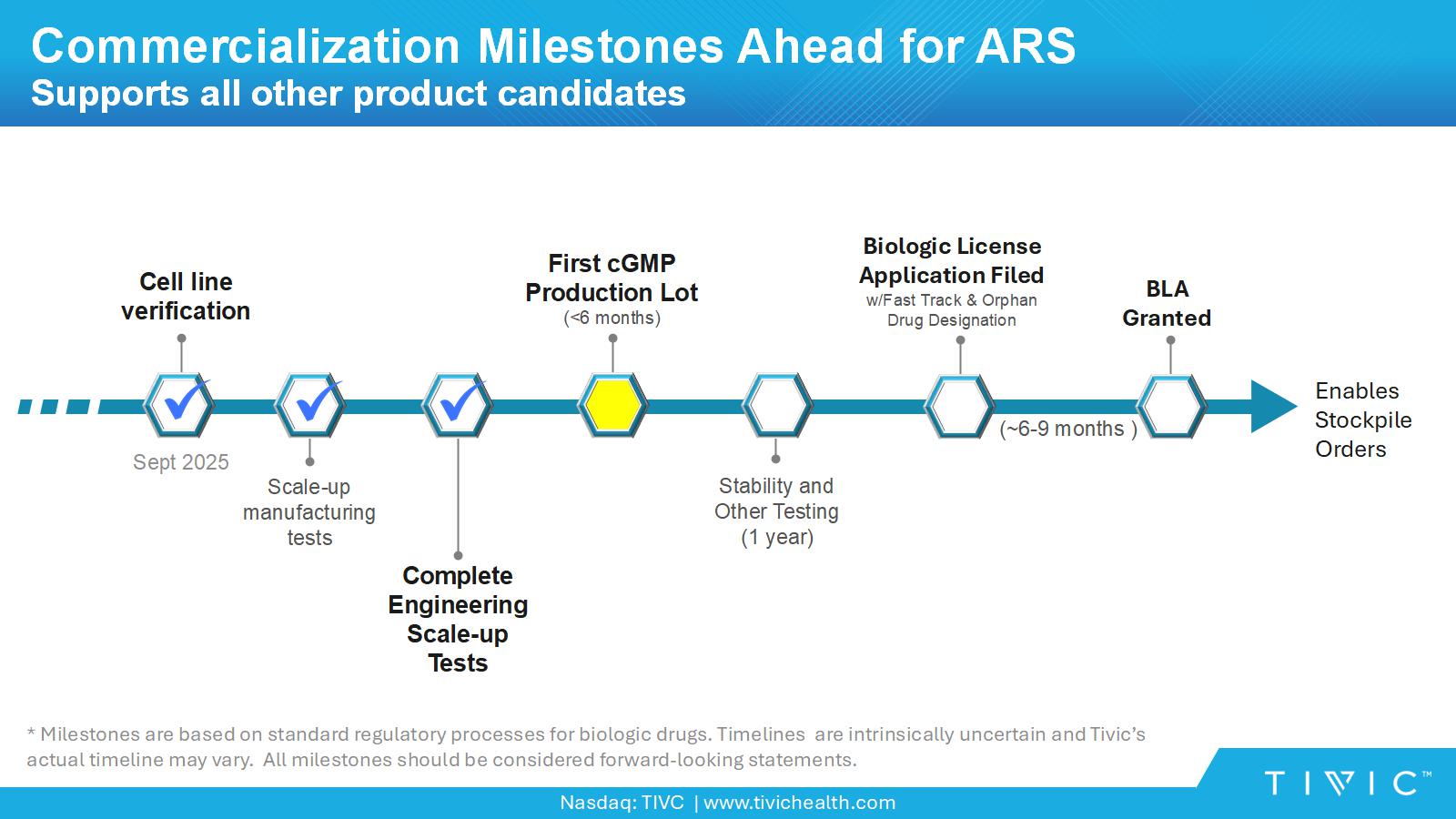
Nasdaq: TIVC | www.tivichealth.com Complete Engineering Scale - up Tests Commercialization Milestones Ahead for ARS Supports all other product candidates Scale - up manufacturing tests First cGMP Production Lot (<6 months) * Milestones are based on standard regulatory processes for biologic drugs. Timelines are intrinsically uncertain and Tivic’s actual timeline may vary. All milestones should be considered forward - looking statements. Stability and Other Testing (1 year) Cell line verification Sept 2025 Biologic License Application Filed w/Fast Track & Orphan Drug Designation BLA Granted Enables Stockpile Orders (~6 - 9 months )

Nasdaq: TIVC | www.tivichealth.com From ARS to Oncology • Engagements in progress with US Agencies • Demonstrated unique therapeutic value • 3x increase in survival rate for ARS • Secured production capacity • International stockpile opportunities Slide # 13 Acute Radiation Syndrome
Nasdaq: TIVC | www.tivichealth.com From ARS to Oncology: the Billion Dollar Move Entolimod is a first - in - class host - protection platform • Clinical work in ARS offers strong foundation in oncology Based on mechanism of action, Entolimod could: • Reduce severe treatment - limiting toxicities • Preserve delivery of high - value oncology therapies • Create multiple, compounding revenue streams Slide # 14 Acute Radiation Syndrome Oncology
Nasdaq: TIVC | www.tivichealth.com The problem • Radiation toxicity remains the primary limiter in cancer treatment • Severe radiation side effects drive • Hospitalizations • Treatment interruptions • Compliance • Underperformance of premium therapies (e.g. cell therapies) • Radiation toxicity is under - monetized ; it’s a value leak that hurts patients, payers and pharma • Mitigating radiation damage should reduce treatment - related side effects, reduce treatment interruptions, improve compliance, and allow increased radiation dosing Slide # 15
Nasdaq: TIVC | www.tivichealth.com Entolimod as host protection • Entolimod activates protective pathways of the innate immune system • Supports: • Neutrophil, platelet and GI endothelial recovery • Bone marrow regeneration • GI crypt preservation and regeneration • Our goal is to enable patients to stay on therapy, on schedule . Entolimod is intended to protect the patient so standard and advanced cancer therapies can be delivered as intended. Slide # 16

Nasdaq: TIVC | www.tivichealth.com Recent News: Velocity Bioworks – a Tivic Subsidiary • Newly created CDMO subsidiary Slide # 17 An integrated CDMO focused on speed to clinic Guarantees Tivic access to CMC and cGMP manufacturing Accelerates time - to - market Accelerates new formulations and doses Creates potential new revenue opportunities Strategically located: San Antonio Stock Images

Nasdaq: TIVC | www.tivichealth.com ® ir@tivichealth.com – January 2026 – Jennifer Ernst, CEO, Tivic Health Harnessing the immune system to save lives and improve clinical outcomes • Late - stage, highly de - risked assets • Competitively differentiated treatments for radiation damage, oncology, and immune system modification • Experienced management team • Targeting multiple high value inflection points expected in the next 18 - 24 months Nasdaq: TIVC