
.2

Jasper Therapeutics ETESIAN Data + BEACON Investigation Update December 2025

2 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Safe Harbor Statements Forward - Looking Statements This investor presentation and any accompanying oral presentation (together, this “Presentation”) contain forward - looking statem ents. All statements other than statements of historical fact contained in this Presentation, including statements regarding the future opportunit ies and prospects of Jasper Therapeutics, Inc. (together with its subsidiary, "Jasper" or the "Company"), including milestones, potential regulatory fili ngs and the anticipated timing thereof, patient enrollment, future timelines, business strategy, and plans and objectives for future operations, are forward - lo oking statements. Jasper has based these forward - looking statements on its estimates and assumptions and its current expectations and projections about futur e events. These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those contained in the "Risk Fa cto rs" section of the Company's Annual Report on Form 10 - K for the year ended December 31, 2024, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K that the Company has subsequently filed or may subsequently file with the SEC. In light of these risks, uncertainties and assumptions, the forward - lo oking events and circumstances discussed in this Presentation are inherently uncertain and may not occur, and actual results could differ materially and adv ers ely from those anticipated or implied in the forward - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of future events. Jasper undertakes no obligation to update publicly or revise any forward - looking statements for any reason after the date of thi s Presentation or to conform these statements to actual results or to changes in Jasper's expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither the Company nor its affiliates, adv ise rs or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or repr ese ntatives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the da te of this Presentation. Such data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company.

3 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION BEACON Trial Investigation Results • Ronald Martell, CEO Jasper Therapeutics • Dr. Daniel Adelman, Acting CMO Jasper Therapeutics BEACON Redosing Data & KOL Feedback • Dr. Martin Metz, Professor of Dermatology and Allergy, Charité – Universitätsmedizin Berlin ETESIAN Trial Interim Results • Dr. Daniel Adelman, Acting CMO Jasper Therapeutics Q&A Session Agenda
4 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION BEACON Study Cohort 8 and Cohort 9 Initial Results Anomalous efficacy observations led to internal investigation being undertaken July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg 180mg Q8W) showed an u nexpected lack of clinical response • No US patients (n=10) achieved Complete Response(CR) or Well Controlled (WC) UAS7 by week 12 • All were dosed using lot A34954, which was the first time it was used in the BEACON study • 2 of 3 patients at EU sites achieved CRs, however EU sites used a different drug lot (A34955) Jasper immediately replaced lot A34954 with lot A34955 (used in OLE and EU BEACON sites) Jasper also launched an internal investigation into the results • Comprehensive review of manufacturing records, drug handling, site training/logs and data handling • Recovery and testing by JSPR & independent labs of drug product samples from across the supply chain • Review of all US sites and all 10 US patients, including protocol adherence, patient medical histories, patient screening and all PK/PD/efficacy data A KOL panel reviewed the internal investigation findings, including full patient dossiers, and provided their input

BEACON Trial Internal Investigation Results Dr. Daniel Adelman, Acting CMO Jasper Therapeutics
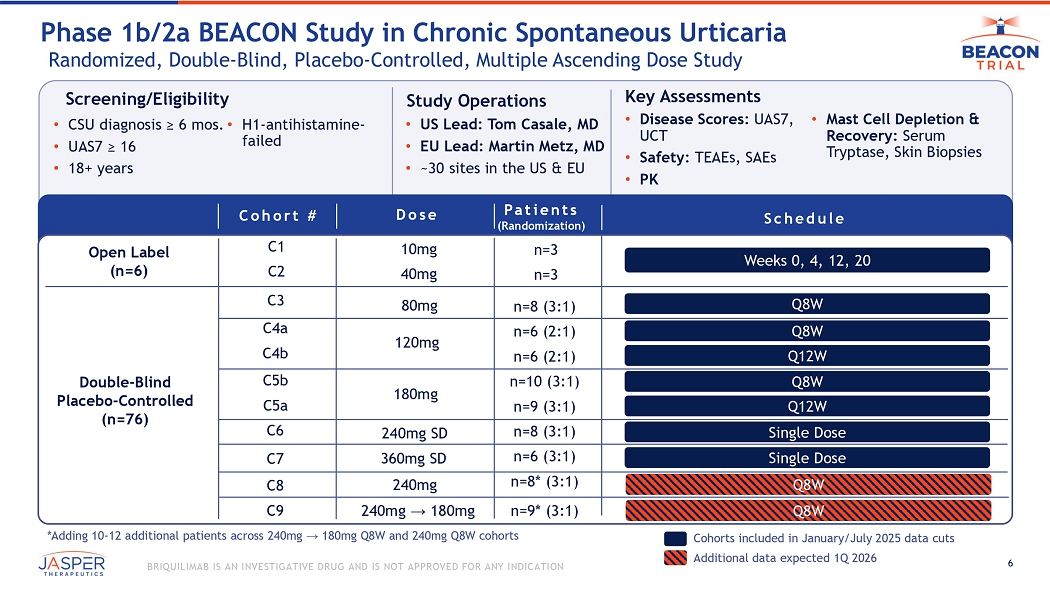
6 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Screening/Eligibility • CSU diagnosis ≥ 6 mos. • UAS7 ≥ 16 • 18+ years • H1 - antihistamine - failed Study Operations • US Lead: Tom Casale, MD • EU Lead: Martin Metz, MD • ~30 sites in the US & EU Key Assessments • Disease Scores: UAS7, UCT • Safety: TEAEs, SAEs • PK • Mast Cell Depletion & Recovery: Serum Tryptase, Skin Biopsies Phase 1b/2a BEACON Study in Chronic Spontaneous Urticaria Randomized, Double - Blind, Placebo - Controlled, Multiple Ascending Dose Study Patients (Randomization) Dose Schedule n=3 n=3 n=8 (3:1) n=6 (2:1) n=6 (2:1) n=10 (3:1) n=9 (3:1) n=8 (3:1) n= 6 (3:1) n=8* (3:1) n=9* (3:1) 10mg 40mg 80mg Open Label ( n =6) Double - Blind Placebo - Controlled ( n = 76 ) Cohorts included in January/July 2025 data cuts Weeks 0, 4, 12, 20 Q8W Q 8W Q12W Q8W Q12W Single Dose Single Dose 120mg 180mg 240mg SD 240mg → 180mg 240mg *Adding 10 - 12 additional patients across 240mg → 180mg Q8W and 240mg Q8W cohorts Additional data expected 1Q 2026 Q8W Q8W Cohort # C1 C2 C4a C4b C5b C5a C8 C3 C6 C7 C9 36 0mg SD
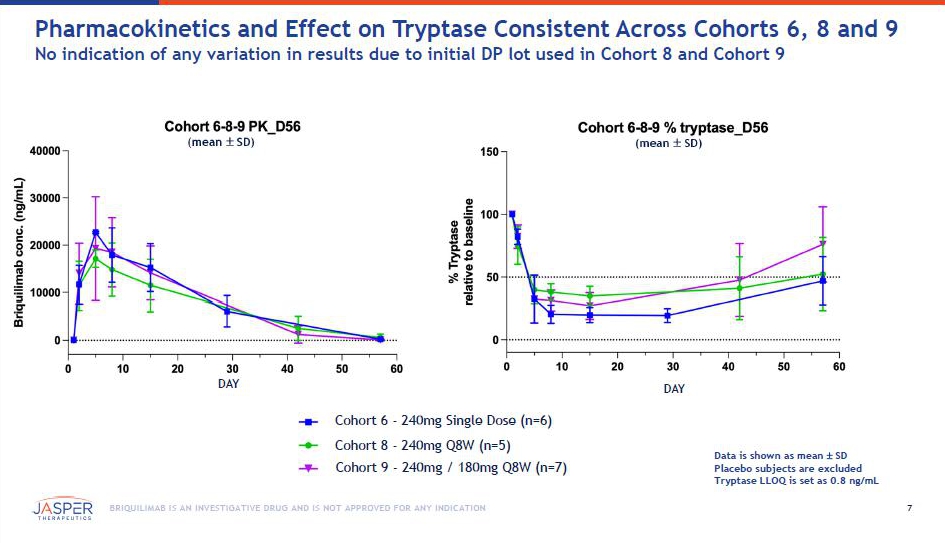
7 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Pharmacokinetics and Effect on Tryptase Consistent Across Cohorts 6, 8 and 9 No indication of any variation in results due to initial DP lot used in Cohort 8 and Cohort 9 (mean SD) DAY Cohort 6 - 240mg Single Dose (n=6) Cohort 8 - 240mg Q8W (n=5) Cohort 9 - 240mg / 180mg Q8W (n=7) (mean SD) DAY Data is shown as mean SD Placebo subjects are excluded Tryptase LLOQ is set as 0.8 ng/mL0
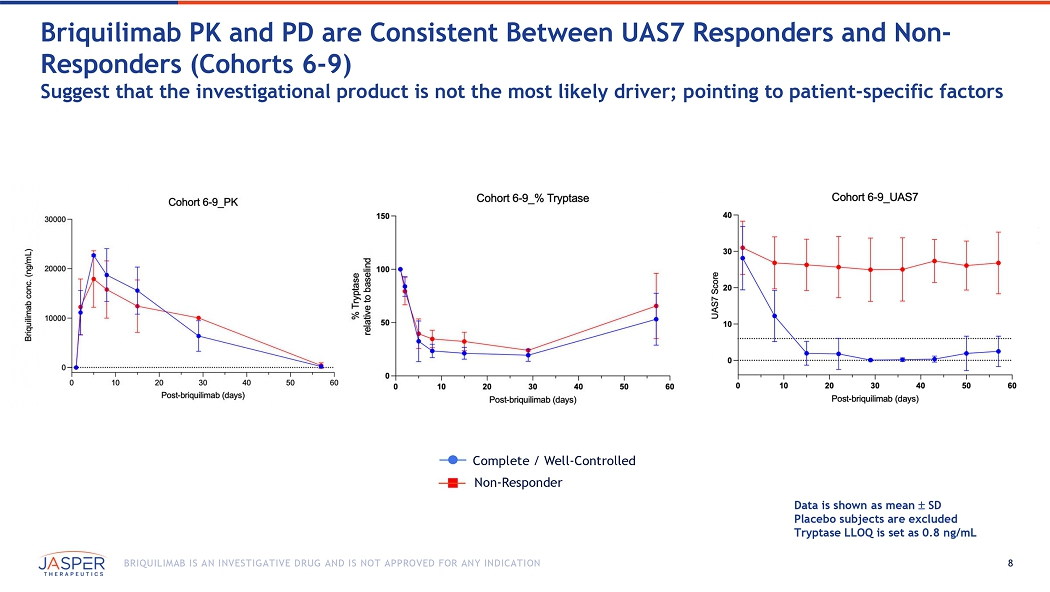
8 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Data is shown as mean SD Placebo subjects are excluded Tryptase LLOQ is set as 0.8 ng/mL Briquilimab PK and PD are Consistent B etween UAS7 Responders and Non - Responders (Cohorts 6 - 9) Suggest that the investigational product is not the most likely driver; pointing to patient - specific factors Complete / Well - Controlled Non - Responder
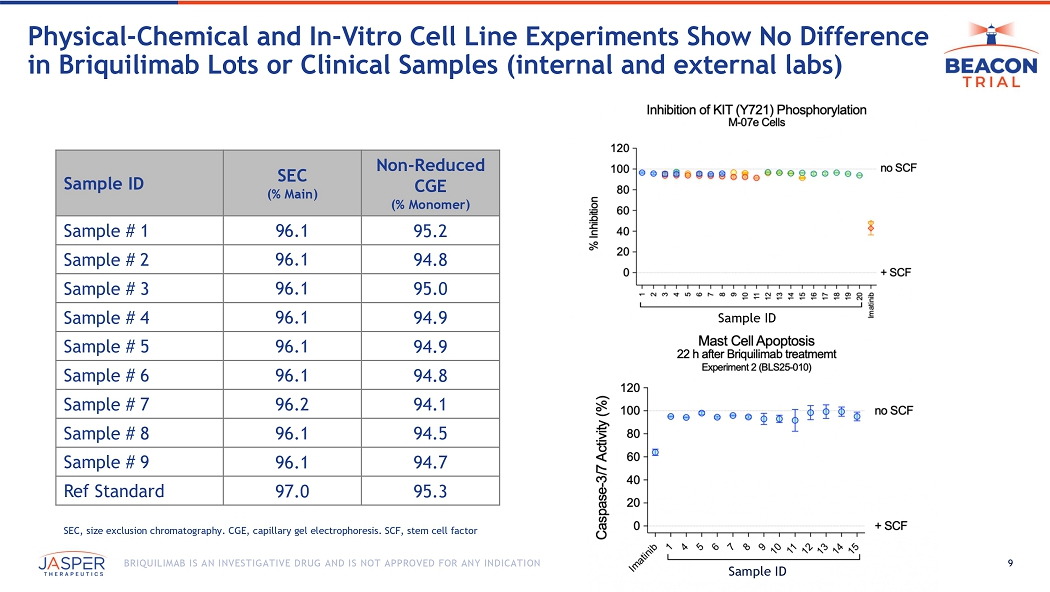
9 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Physical - Chemical and In - Vitro Cell Line Experiments Show No Difference in Briquilimab Lots or Clinical Samples (internal and external labs) Non - Reduced CGE (% Monomer) SEC (% Main) Sample ID 95.2 96.1 Sample # 1 94.8 96.1 Sample # 2 95.0 96.1 Sample # 3 94.9 96.1 Sample # 4 94.9 96.1 Sample # 5 94.8 96.1 Sample # 6 94.1 96.2 Sample # 7 94.5 96.1 Sample # 8 94.7 96.1 Sample # 9 95.3 97.0 Ref Standard Sample ID Sample ID SEC, size exclusion chromatography. CGE, capillary gel electrophoresis. SCF, stem cell factor

10 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Clinical Operations, Data Management and Site Investigations No deviations in drug management, clinical conduct or data entry/handling noted • On - site investigations • Drug substance and drug product manufacturing • Drug kitting, shipment and storage • Injection preparation, timing and storage, types of syringes, injection volume and site • Patient and site data entry, data management and analysis Site investigations • Site specific screening and dosing data reviewed for each patient • Comprehensive patient folios prepared for each patient and reviewed by CSU KOL panel • One new site enrolled 5 patients into active arms of Cohort 8 & 9 with no CR or WC responses • Community - based, clinical research center • All patients had minimal documented medical history of CSU or past treatments for CSU Internal investigation indicates anomalous results are due to patient specific factors • No deviations or issues with Drug Product or Drug Substance were noted

BEACON Trial Investigation KOL Review & Redosing Data Dr. Martin Metz, Professor of Dermatology and Allergy Charité – Universitätsmedizin Berlin
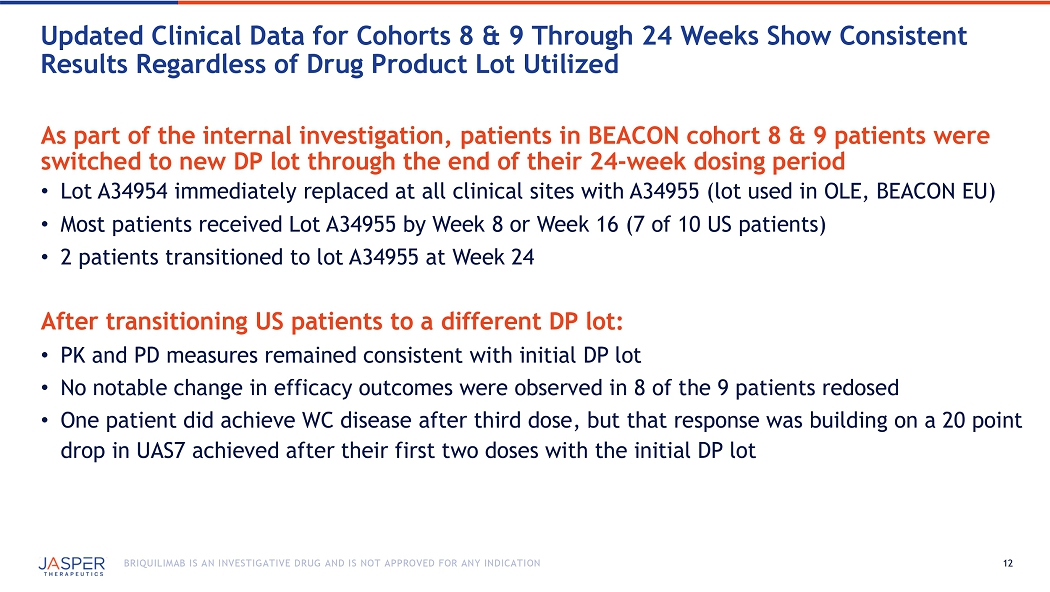
12 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION As part of the internal investigation, patients in BEACON cohort 8 & 9 patients were switched to new DP lot through the end of their 24 - week dosing period • Lot A34954 immediately replaced at all clinical sites with A34955 ( lot used in OLE , BEACON EU ) • Most patients received Lot A34955 by Week 8 or Week 16 (7 of 10 US patients) • 2 patients transitioned to lot A34955 at Week 24 After transitioning US patients to a different DP lot: • PK and PD measures remained consistent with initial DP lot • No notable change in efficacy outcomes were observed in 8 of the 9 patients redosed • One patient did achieve WC disease after third dose, but that response was building on a 20 point drop in UAS7 achieved after their first two doses with the initial DP lot Updated Clinical Data for Cohorts 8 & 9 Through 24 Weeks Show Consistent Results Regardless of Drug Product Lot Utilized
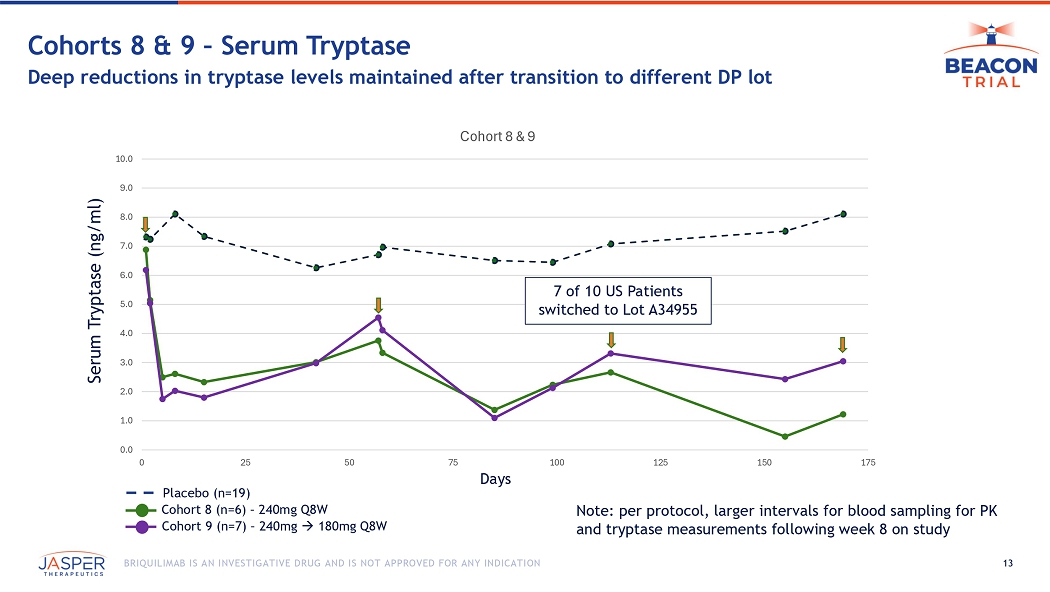
13 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 0 25 50 75 100 125 150 175 Cohort 8 & 9 Cohorts 8 & 9 – Serum Tryptase Deep reductions in tryptase levels maintained after transition to different DP lot 7 of 10 US Patients switched to Lot A34955 Days Serum Tryptase (ng/ml) Cohort 8 (n=6) – 240mg Q8W Cohort 9 (n=7) – 240mg 180mg Q8W Note: per protocol, larger intervals for blood sampling for PK and tryptase measurements following week 8 on study Placebo (n=19)
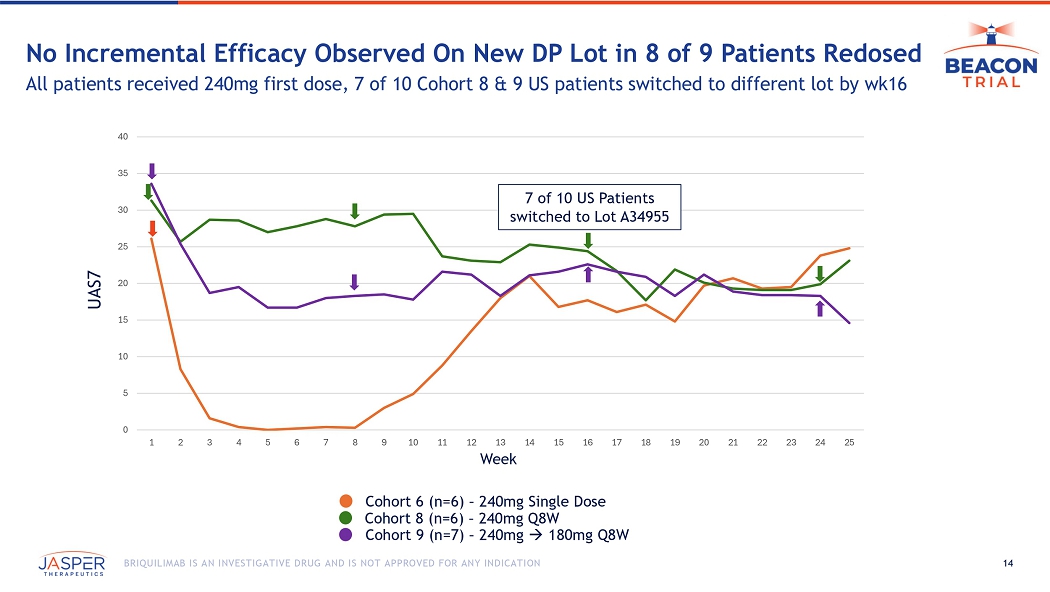
14 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION No Incremental Efficacy Observed On New DP Lot in 8 of 9 Patients Redosed All patients received 240mg first dose, 7 of 10 Cohort 8 & 9 US patients switched to different lot by wk16 0 5 10 15 20 25 30 35 40 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Week UAS7 7 of 10 US Patients switched to Lot A34955 Cohort 8 (n=6) – 240mg Q8W Cohort 9 (n=7) – 240mg 180mg Q8W Cohort 6 (n=6) – 240mg Single Dose
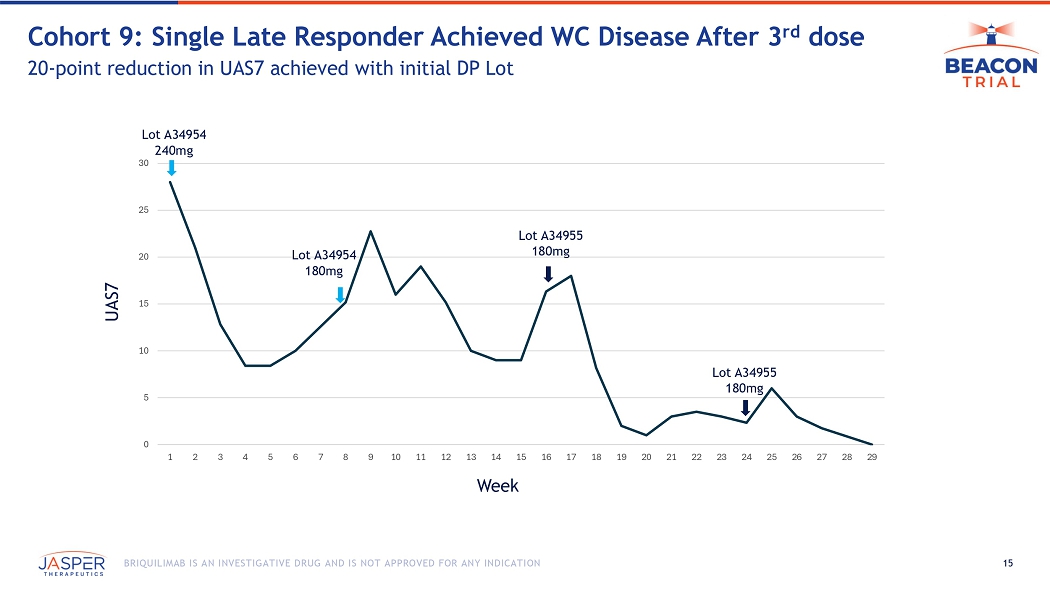
15 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Cohort 9: Single Late Responder Achieved WC Disease After 3 rd dose 20 - point reduction in UAS7 achieved with initial DP Lot 0 5 10 15 20 25 30 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
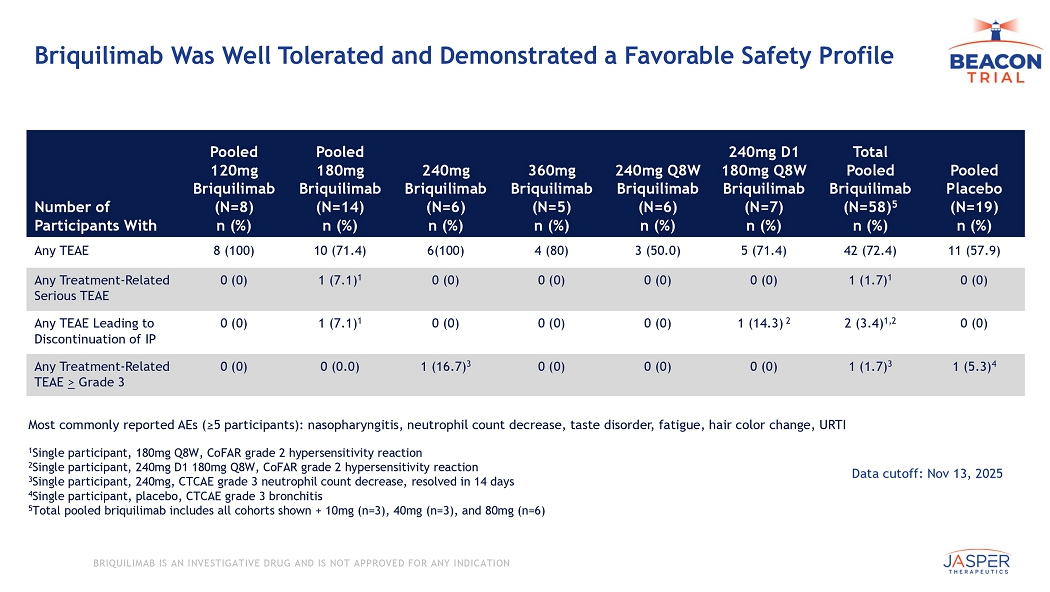
CONFIDENTIAL BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab Was Well Tolerated and Demonstrated a Favorable Safety Profile Pooled Placebo (N=19) n (%) Total Pooled Briquilimab (N=58) 5 n (%) 240mg D1 180mg Q8W Briquilimab (N=7) n (%) 240mg Q8W Briquilimab (N=6) n (%) 360mg Briquilimab (N=5) n (%) 240mg Briquilimab (N=6) n (%) Pooled 180mg Briquilimab (N=14) n (%) Pooled 120mg Briquilimab (N=8) n (%) Number of Participants With 11 (57.9) 42 (72.4) 5 (71.4) 3 (50.0) 4 (80) 6(100) 10 (71.4) 8 (100) Any TEAE 0 (0) 1 (1.7) 1 0 (0) 0 (0) 0 (0) 0 (0) 1 (7.1) 1 0 (0) Any Treatment - Related Serious TEAE 0 (0) 2 (3.4) 1,2 1 (14.3) 2 0 (0) 0 (0) 0 (0) 1 (7.1) 1 0 (0) Any TEAE Leading to Discontinuation of IP 1 (5.3) 4 1 (1.7) 3 0 (0) 0 (0) 0 (0) 1 (16.7) 3 0 (0.0) 0 (0) Any Treatment - Related TEAE > Grade 3 1 Single participant, 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 2 Single participant, 240mg D1 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 3 Single participant, 240 mg, CTCAE grade 3 neutrophil count decrease, resolved in 14 days 4 Single participant, placebo, CTCAE grade 3 bronchitis 5 Total pooled briquilimab includes all cohorts shown + 10mg (n=3), 40mg (n=3), and 80mg (n=6) Most commonly reported AEs (≥5 participants): nasopharyngitis, neutrophil count decrease, taste disorder, fatigue, hair color ch ange, URTI Data cutoff: Nov 13, 2025
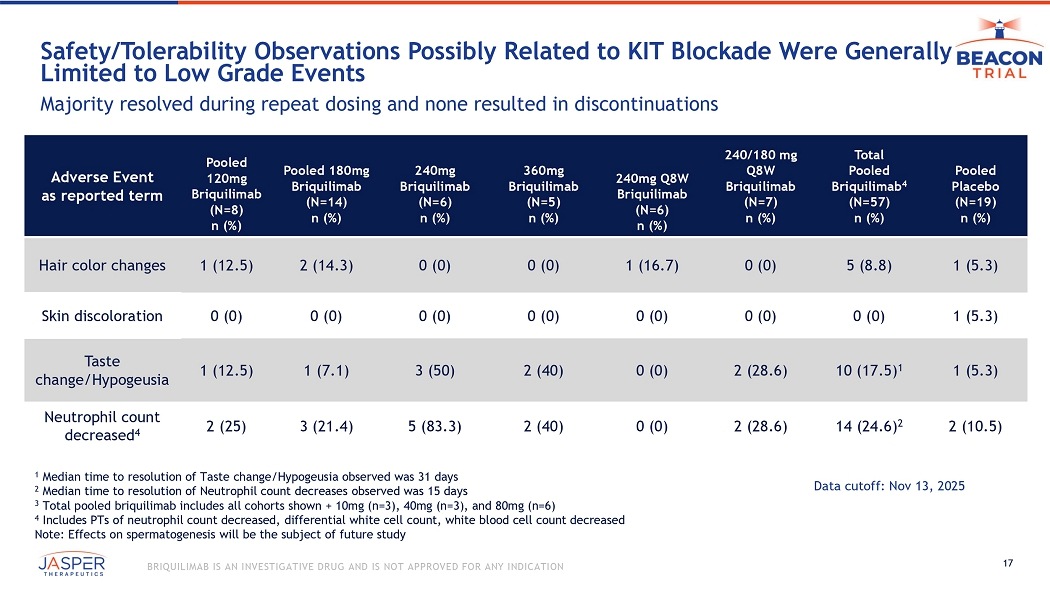
17 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Safety/Tolerability O bservations P ossibly R elated to KIT Blockade W ere G enerally L imited to Low G rade E vents Majority resolved during repeat dosing and none resulted in discontinuations Pooled Placebo (N=19) n (%) Total Pooled Briquilimab 4 (N=57) n (%) 240/180 mg Q8W Briquilimab (N=7) n (%) 240mg Q8W Briquilimab (N=6) n (%) 360mg Briquilimab (N=5) n (%) 240mg Briquilimab (N=6) n (%) Pooled 180mg Briquilimab (N=14) n (%) Pooled 120mg Briquilimab (N=8) n (%) Adverse Event as reported term 1 (5.3) 5 (8.8) 0 (0) 1 (16.7) 0 (0) 0 (0) 2 (14.3) 1 (12.5) Hair color changes 1 (5.3) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Skin discoloration 1 (5.3) 10 (17.5) 1 2 (28.6) 0 (0) 2 (40) 3 (50) 1 (7.1) 1 (12.5) Taste change/Hypogeusia 2 (10.5) 14 (24.6) 2 2 (28.6) 0 (0) 2 (40) 5 (83.3) 3 (21.4) 2 (25) Neutrophil count decreased 4 1 Median time to resolution of Taste change/Hypogeusia observed was 31 days 2 Median time to resolution of Neutrophil count decreases observed was 15 days 3 Total pooled briquilimab includes all cohorts shown + 10mg (n=3), 40mg (n=3), and 80mg (n=6) 4 Includes PTs of neutrophil count decreased, differential white cell count, white blood cell count decreased Note: Effects on spermatogenesis will be the subject of future study Data cutoff: Nov 13, 2025
18 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Investigation Conclusions Anomalous efficacy does not appear to be the result of any issue with the DP or study conduct • No issues or deviations noted in the testing done on DS and DP throughout the supply chain • Redosing Cohort 8 & 9 patients with different DP lot did not drive a different outcome • No protocol deviations, no issues with site training, no issues with study conduct Unexpected efficacy results appear to largely be the result of patient selection • Based on KOL panel review of the totality of the data, including the data on patients redosed with replacement DP lot, their feedback is as follows: • 9 of 10 patients that did not respond do not appear to have CSU • Not uncommon as CSU is a diagnosis by exclusion, other CSU studies have shown 25 - 30% of patients are incorrectly diagnosed KOL panel recommendations to ensure quality patient selection • Ensure sites utilized have a certified Immunologist/Dermatologist with a history of diagnosing and treating CSU patients • Expanded review of patient history during screening including visual records of lesions • Larger sample size in future studies should mitigate impact of non - MC driven CSU patients

Briquilimab in Allergic Asthma Dr. Daniel Adelman, Acting CMO Jasper Therapeutics
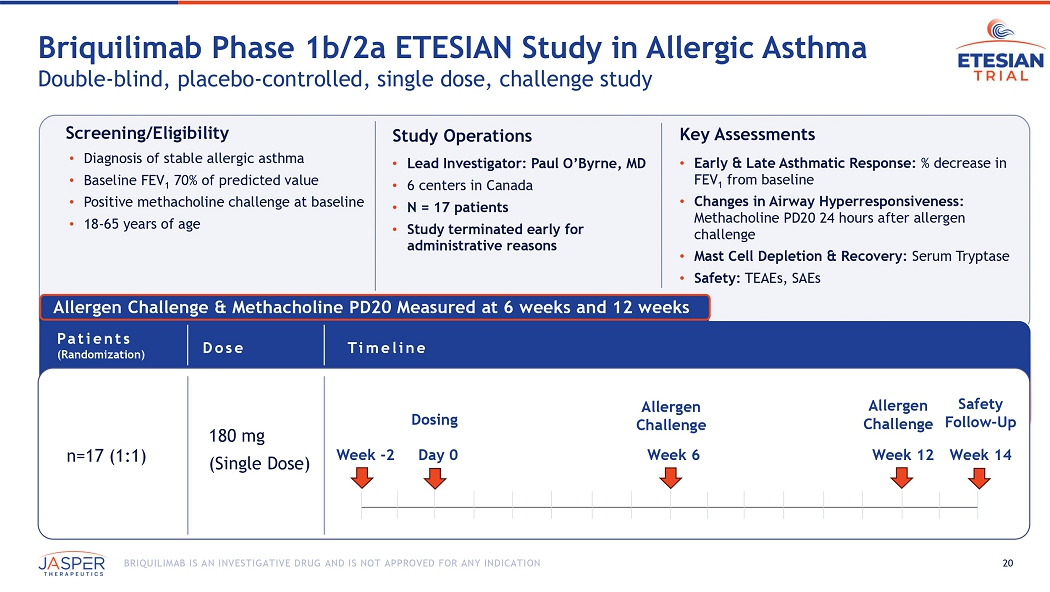
20 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Allergen Challenge & Methacholine PD20 Measured at 6 weeks and 12 weeks Briquilimab Phase 1b/2a ETESIAN Study in Allergic A sthma Double - blind, placebo - controlled, single dose, challenge study n=17 (1:1) 180 mg (Single Dose) Dose Timeline Patients (Randomization) Day 0 Week 12 Week 6 Allergen Challenge Allergen Challenge Week 14 Safety Follow - Up Week - 2 Dosing Screening/Eligibility • Diagnosis of stable allergic asthma • Baseline FEV 1 70% of predicted value • Positive methacholine challenge at baseline • 18 - 65 years of age Study Operations • Lead Investigator: Paul O’Byrne, MD • 6 centers in Canada • N = 17 patients • Study terminated early for administrative reasons Key Assessments • Early & Late Asthmatic Response: % decrease in FEV 1 from baseline • Changes in Airway Hyperresponsiveness: Methacholine PD20 24 hours after allergen challenge • Mast Cell Depletion & Recovery: Serum Tryptase • Safety: TEAEs, SAEs
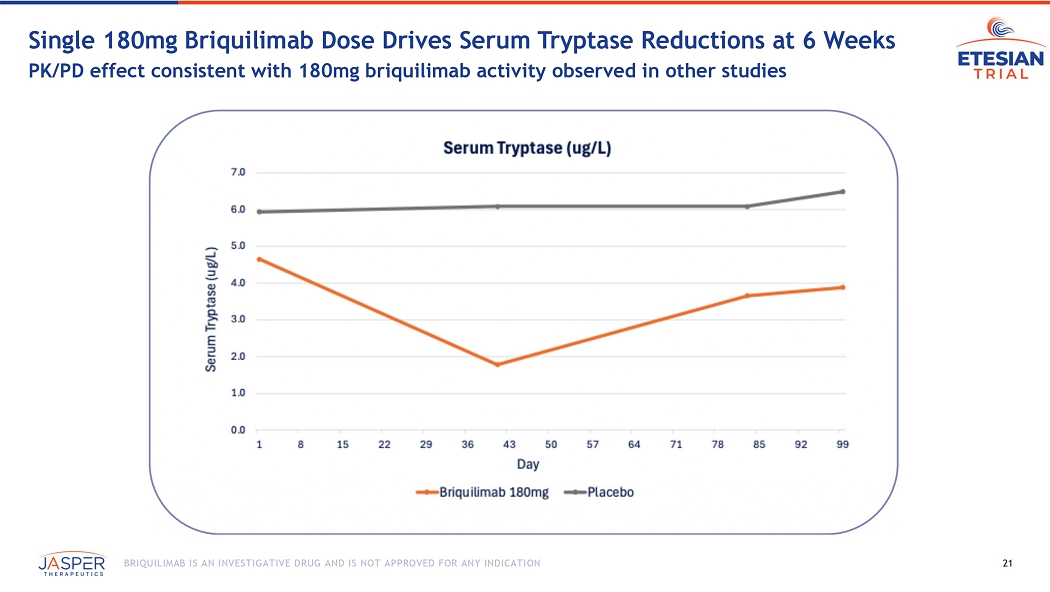
21 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Single 180mg Briquilimab Dose Drives Serum Tryptase Reductions at 6 Weeks PK/PD effect consistent with 180mg briquilimab activity observed in other studies
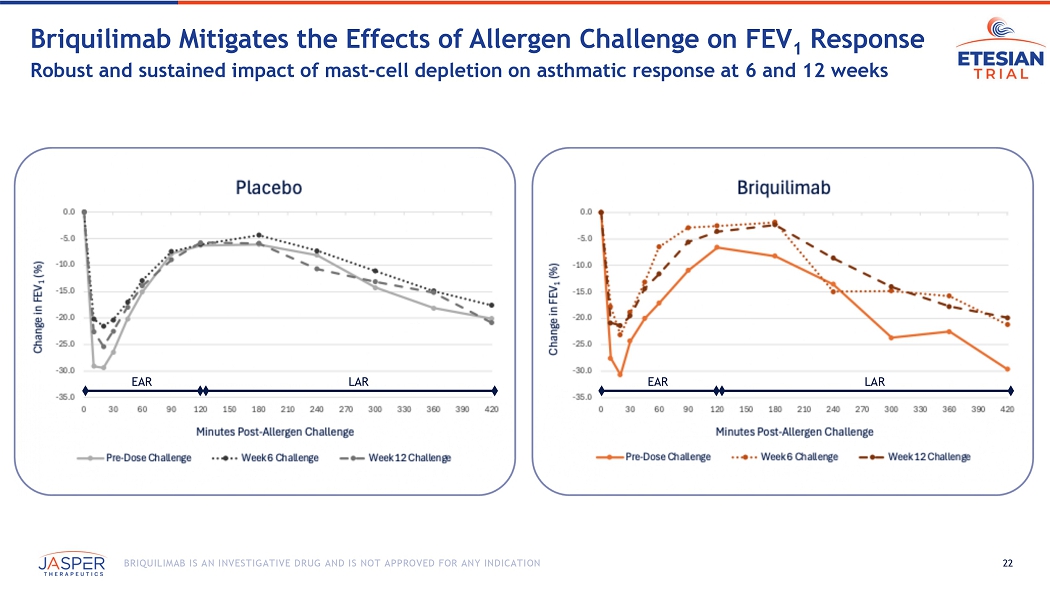
22 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab M itigates the Effects of Allergen Challenge on FEV 1 Response Robust and sustained impact of mast - cell depletion on asthmatic response at 6 and 12 weeks EAR LAR EAR LAR
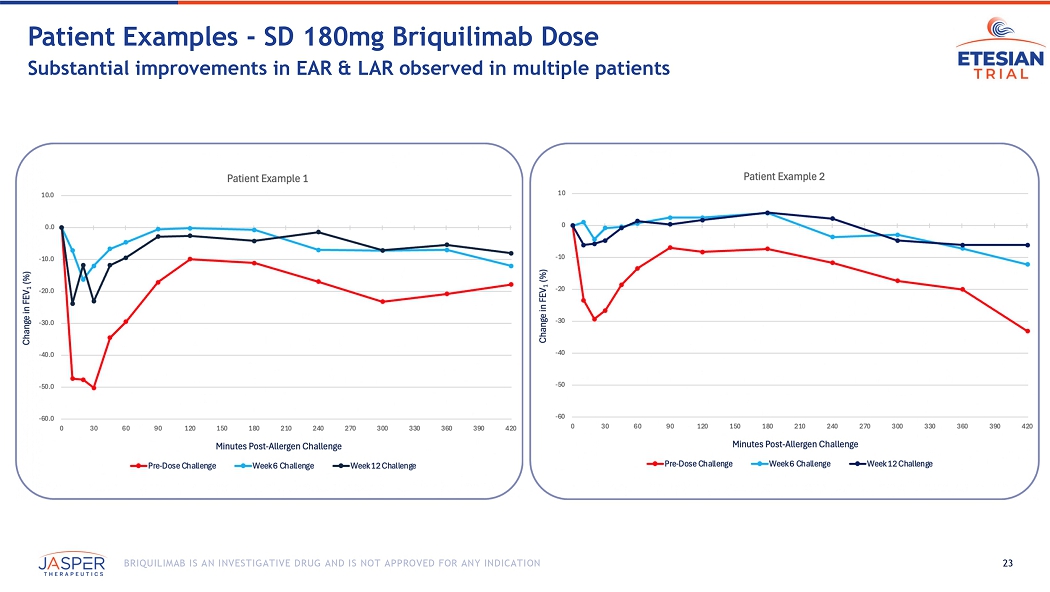
23 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Patient Examples - SD 180mg Briquilimab Dose Substantial improvements in EAR & LAR observed in multiple patients
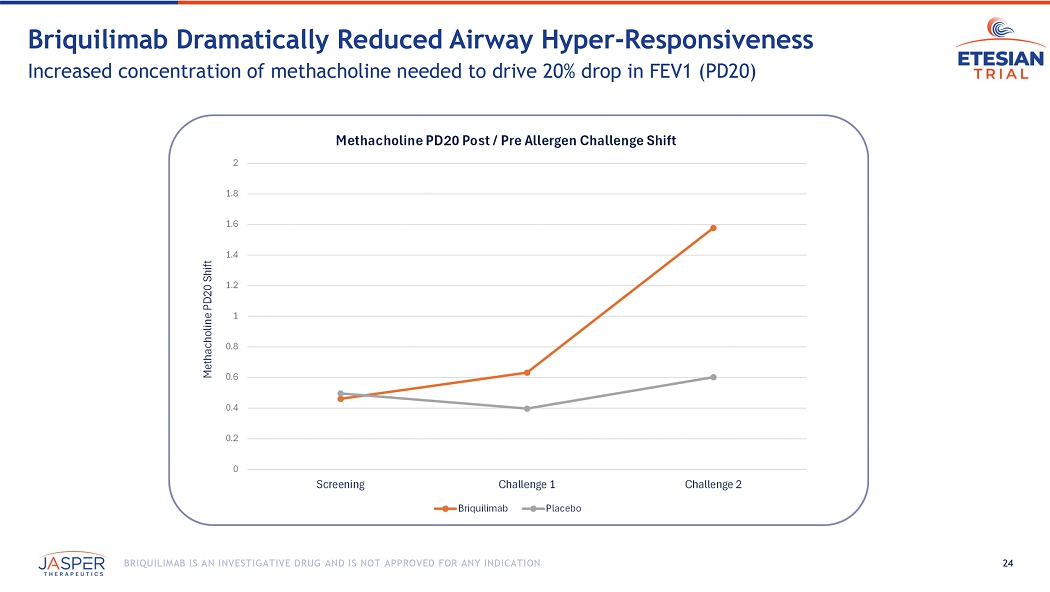
24 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab Dramatically Reduced Airway Hyper - Responsiveness Increased concentration of methacholine needed to drive 20% drop in FEV1 (PD20) 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 2 Screening Challenge 1 Challenge 2 Methacholine PD20 Shift Methacholine PD20 Post / Pre Allergen Challenge Shift Briquilimab Placebo
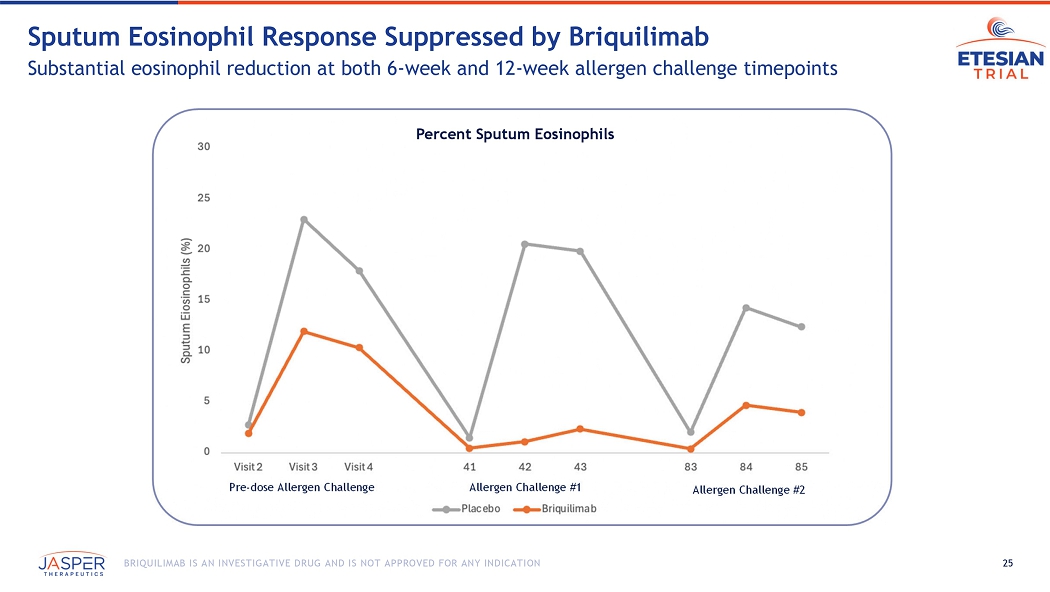
25 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Sputum Eosinophil Response Suppressed by Briquilimab Substantial eosinophil reduction at both 6 - week and 12 - week allergen challenge timepoints Pre - dose Allergen Challenge Allergen Challenge #1 Allergen Challenge #2 Percent Sputum Eosinophils
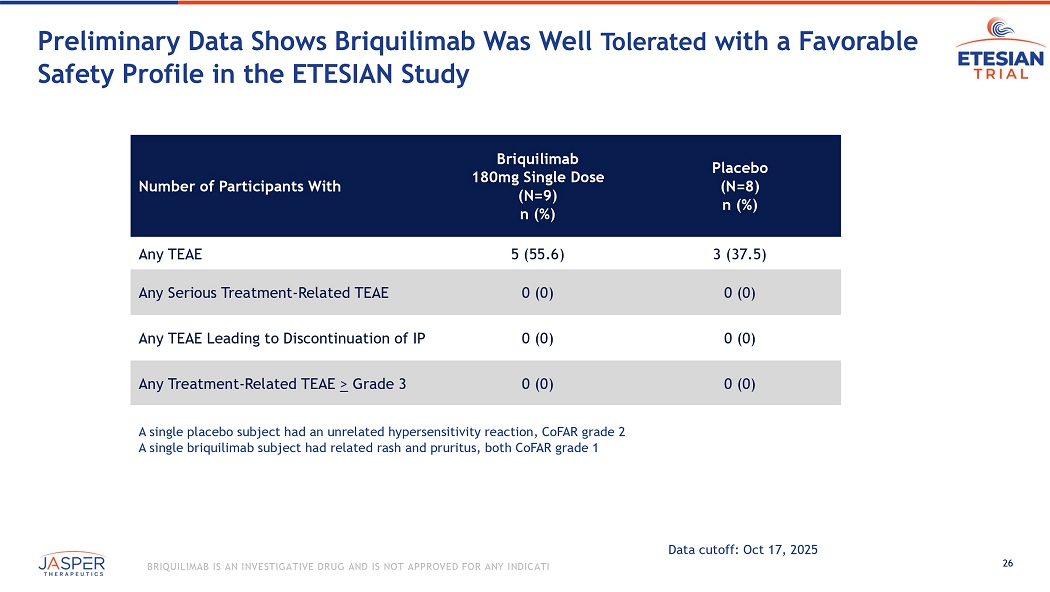
26 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Preliminary Data Shows Briquilimab Was Well Tolerated with a Favorable Safety Profile in the ETESIAN Study Placebo (N=8) n (%) Briquilimab 180mg Single Dose (N=9) n (%) Number of Participants With 3 (37.5) 5 (55.6) Any TEAE 0 (0) 0 (0) Any Serious Treatment - Related TEAE 0 (0) 0 (0) Any TEAE Leading to Discontinuation of IP 0 (0) 0 (0) Any Treatment - Related TEAE > Grade 3 A single placebo subject had an unrelated hypersensitivity reaction, CoFAR grade 2 A single briquilimab subject had related rash and pruritus, both CoFAR grade 1 Data cutoff: Oct 17, 2025
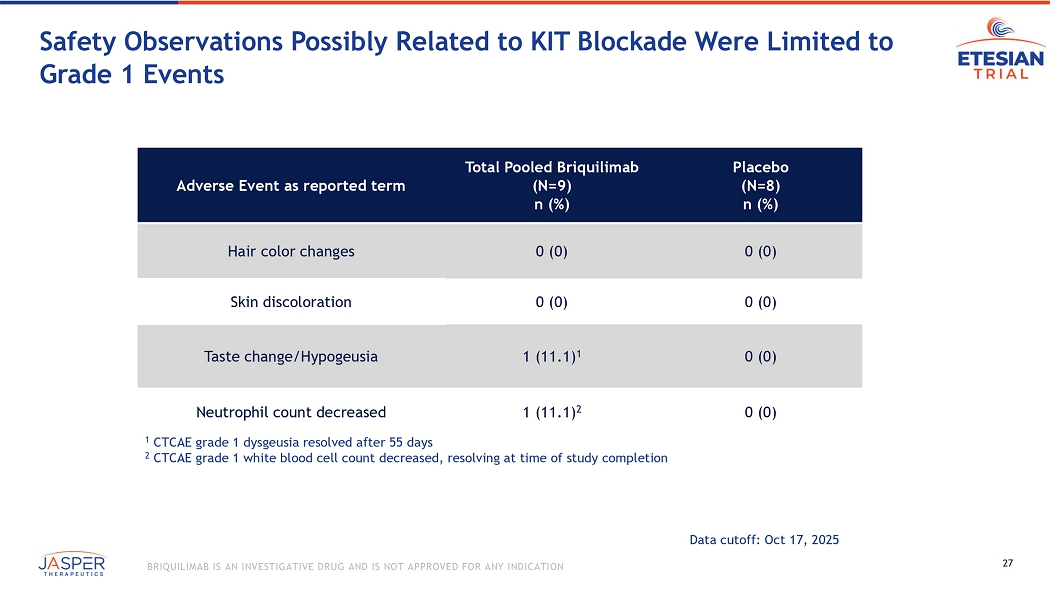
27 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Placebo (N=8) n (%) Total Pooled Briquilimab (N=9) n (%) Adverse Event as reported term 0 (0) 0 (0) Hair color changes 0 (0) 0 (0) Skin discoloration 0 (0) 1 (11.1) 1 Taste change/Hypogeusia 0 (0) 1 (11.1) 2 Neutrophil count decreased 1 CTCAE grade 1 dysgeusia resolved after 55 days 2 CTCAE grade 1 white blood cell count decreased, resolving at time of study completion Data cutoff: Oct 17, 2025 Safety Observations Possibly Related to KIT Blockade Were Limited to Grade 1 Events
28 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION ETESIAN Study Summary First time a potent KIT - specific therapeutic targeting mast cells has demonstrated potential for the treatment of asthma PK/PD demonstrated deep & sustained biologic activity in key biomarkers, including: • Serum tryptase • Sputum eosinophils Robust improvements observed in both aspects of the challenge study: • Improvement in mean FEV1 response seen at week 6 and week 12 in allergen challenge • Reductions in airway hyper - responsiveness observed in the methacoline challenge Data suggest that mast cells play a central role in airway inflammation • Given that the mast cell may be a central actor in both T2 high and T2 low disease, further developme nt in the broader asthma population is warranted • Next steps being evaluated, including potential dose - ranging/repeat dose studies in asthma

Next Steps Ron Martell, CEO
30 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Program Status and Next Steps Briquilimab development in mast - cell driven diseases continues to advance in multiple indications CSU - Briquilimab continues to demonstrate rapid onset, deep clinical response and favorable safety profile • More than 24pt drop in UAS7 with 82% CR and 91% WC disease by week 4 with single dose (240mg SD & 360mg SD, n=11) • Highly effective in OLE study at 180mg Q8W with 73% CR and 82% WC disease at 12 weeks (n=11) • Continued favorable safety profile, r epeat dose of 240mg Q8W and 240mg/180mg Q8W were generally well tolerated CSU - Additional BEACON and OLE data expected in 1H Q1 2026 will enable Phase 2b dose selection • Efficacy and safety data on additional patients enrolled in C8 (240mg Q8W) & C9 (240mg 180mg Q8W) • 24+ weeks of safety data on original patients enrolled in C8 and C9 • 20+ weeks of efficacy and safety data on ~40 CSU patients in OLE study (180mg Q8W) • Consistent PK/PD profile will enable rapid and robust population exposure analysis and dose selection CindU – Multi - dose data in CIndU expected in Q1 2026 data update • 15+ weeks of efficacy and safety data on ~15 CIndU patients in OLE study (180mg Q8W) Asthma – ETESIAN data provide strong proof of concept for briquilimab MOA in asthma • The initial results demonstrate the potential to reduce both airway hypersensitivity and the release of eosinophils • Both of which are key factors in managing chronic asthma and reducing exacerbations. • Jasper evaluating next steps to advance briquilimab in chronic asthma

Jasper Therapeutics NASDAQ: JSPR December 2025