
.2

Jasper Therapeutics BEACON & OLE Data Update January 8, 2026 .2

2 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Safe Harbor Statements Forward - Looking Statements This investor presentation and any accompanying oral presentation (together, this “Presentation”) contain forward - looking statements. All statements other than statements of historical fact contained in this Presentation, including statements regarding the future opportunities and prospects of Jasper Therapeutics, Inc. (together with its subsidiary, "Jasper" or the "Company"), including milestones, potential regulatory filings and the anticipated timing thereof, patient enrollment, future timelines, business strategy, and plans and objectives for future operations, are forward - looking statements. Jasper has based these forward - looking statements on its estimates and assumptions and its current expectations and projections about future events. These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those contained in the "Risk Factors" section of the Company's Annual Report on Form 10 - K for the year ended December 31, 2024, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K that the Company has subsequently filed or may subsequently file with the SEC. In light of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this Presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of future events. Jasper undertakes no obligation to update publicly or revise any forward - looking statements for any reason after the date of this Presentation or to conform these statements to actual results or to changes in Jasper's expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither the Company nor its affiliates, advisers or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the date of this Presentation. Such data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company.
3 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION New BEACON and OLE data support advancing into a Phase 2b CSU study in 2H 2026 Briquilimab’s unique drug properties allow for rapid clinical efficacy while minimizing KIT related AEs • Briquilimab’s MOA (direct ligand/receptor blockade) and high Cmax leads to rapid mast cell depletion with deep UAS7 reduction and disease control in first 2 - 4 weeks • Drug clearance near end of 8 week dosing cycle allows for reduction of KIT related AEs and improved profile for patients Briquilimab continues to demonstrate rapid and durable clinical responses • 10 additional patients enrolled in the BEACON study – Eight (6 briquilimab + 2 placebo) in Cohort 9.1(240mg 180mg Q8W) and 2 in Cohort 8.1(240mg Q8W) • New Cohort 9.1: 83% (5 of 6) of briquilimab patients achieved a CR by week 3, with 67% (4 of 6) CRs at 12 weeks Briquilimab was well tolerated and demonstrates a favorable chronic safety profile • OLE (n=63, 180mg Q8W median duration > 200 days) shows deepening efficacy with low incidence of KIT related AEs Data support commencing a Phase 2b study as part of a CSU registrational program in 2H 2026 • Phase 2b study expected to be a 75 - 100 patient multi - site study evaluating two effective dose regimens vs. placebo Briquilimab continues to demonstrate differentiated efficacy & safety profile

BEACON & OLE Data Update Dr. Daniel Adelman, Acting CMO Jasper Therapeutics Open - Label Extension Study +
5 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION January 2026 BEACON Study & OLE Study Data Update Briquilimab continues to demonstrate rapid and durable clinical responses • BEACON Cohort 9.1 patients reaffirms strong efficacy and safety profile • Mean reduction in UAS7 scores of 31 points at week 12 • Rapid onset of effect with CR or well controlled disease reported in the majority of patients by week 2 • 83% (5 of 6) of patients achieved a CR at week 3 after initial 240mg loading dose • 67% (4 of 6) of patients reported a CR at week 12 on the 180mg maintenance dose • CIndU OLE – Durable clinical response demonstrated in CIndU patients with repeat - dose briquilimab • 65% (11 of 17) of CIndU patients achieved CR or PR at week 16 (8 weeks from last dose) • CSU OLE – Progressive UAS7 reductions over time supports potential for a maintenance dose in CSU • 75% (27 of 36) of patients achieved CR or WC disease at 12 weeks Briquilimab continues to demonstrate the potential for a differentiated safety profile Data gathered to - date sufficient to enable final dose selection for CSU Phase 2b study
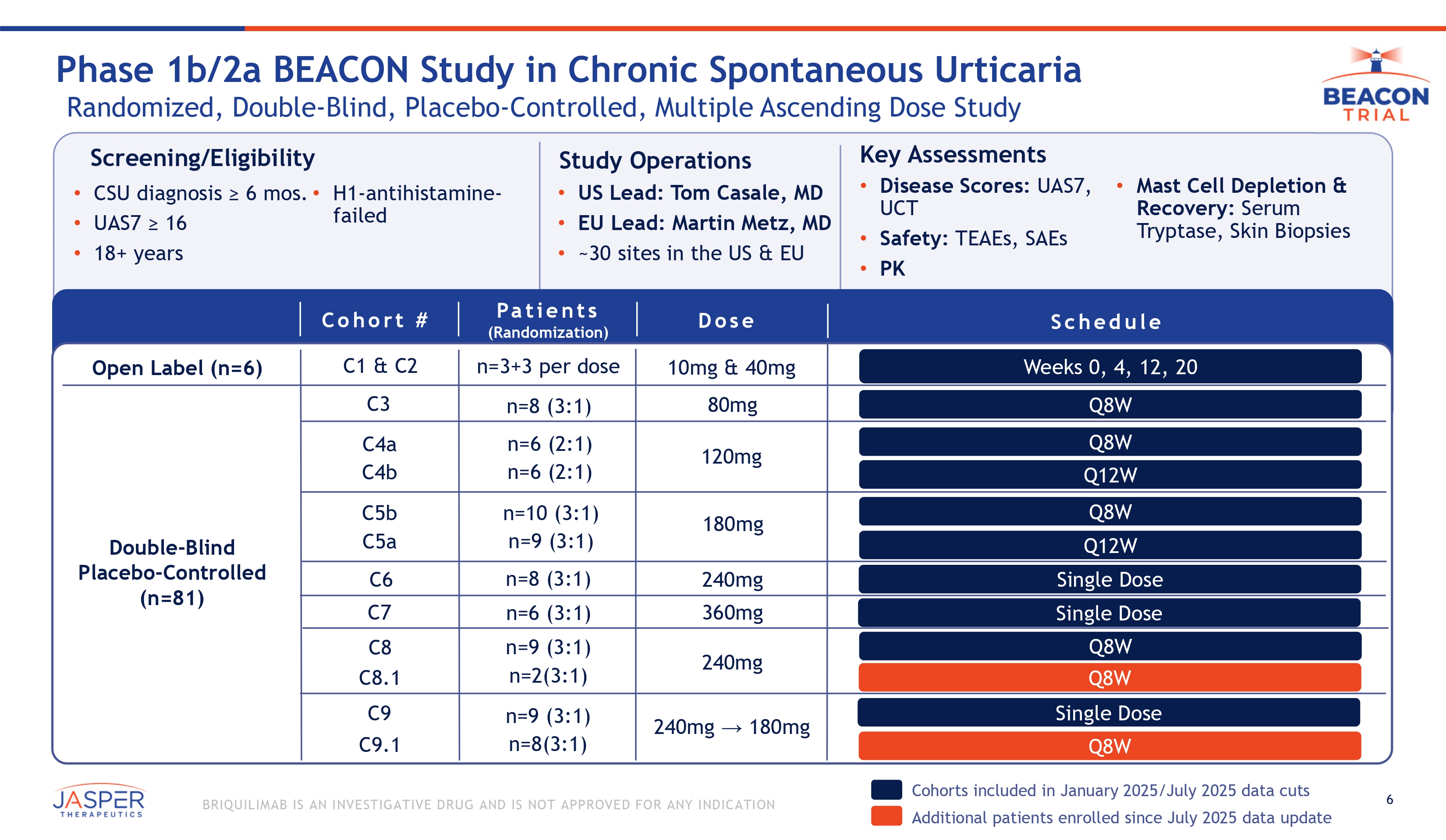
6 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION • UAS7 ≥ 16 • 18+ years Screening/Eligibility • CSU diagnosis ≥ 6 mos. • H1 - antihistamine - failed Study Operations • US Lead: Tom Casale, MD • EU Lead: Martin Metz, MD • ~30 sites in the US & EU Key Assessments • Disease Scores: UAS7, UCT • Safety: TEAEs, SAEs • PK • Mast Cell Depletion & Recovery: Serum Tryptase, Skin Biopsies Phase 1b/2a BEACON Study in Chronic Spontaneous Urticaria Randomized, Double - Blind, Placebo - Controlled, Multiple Ascending Dose Study Pa t i e n t s (Randomization) Dose Sch e du l e n=3+3 per dose n=8 (3:1) 10mg & 40mg 80mg Open Label (n=6) Double - Blind Placebo - Controlled (n=81) Cohorts included in January 2025/July 2025 data cuts Additional patients enrolled since July 2025 data update Weeks 0, 4, 12, 20 Q8W Q8W Q12W Q8W Q12W Single Dose 120mg 180mg 240mg 240mg → 180mg 360mg Single Dose Q8W Cohor t # C1 & C2 C4a C4b C5b C5a C3 C7 n=10 (3:1) n=9 (3:1) n=6 (2:1) n=6 (2:1) Single Dose n=9 (3:1) n=8(3:1) 240mg C6 n=8 (3:1) n=6 (3:1) n=9 (3:1) n=2(3:1) C9 C9.1 C8 C8.1 Q8W Q8W
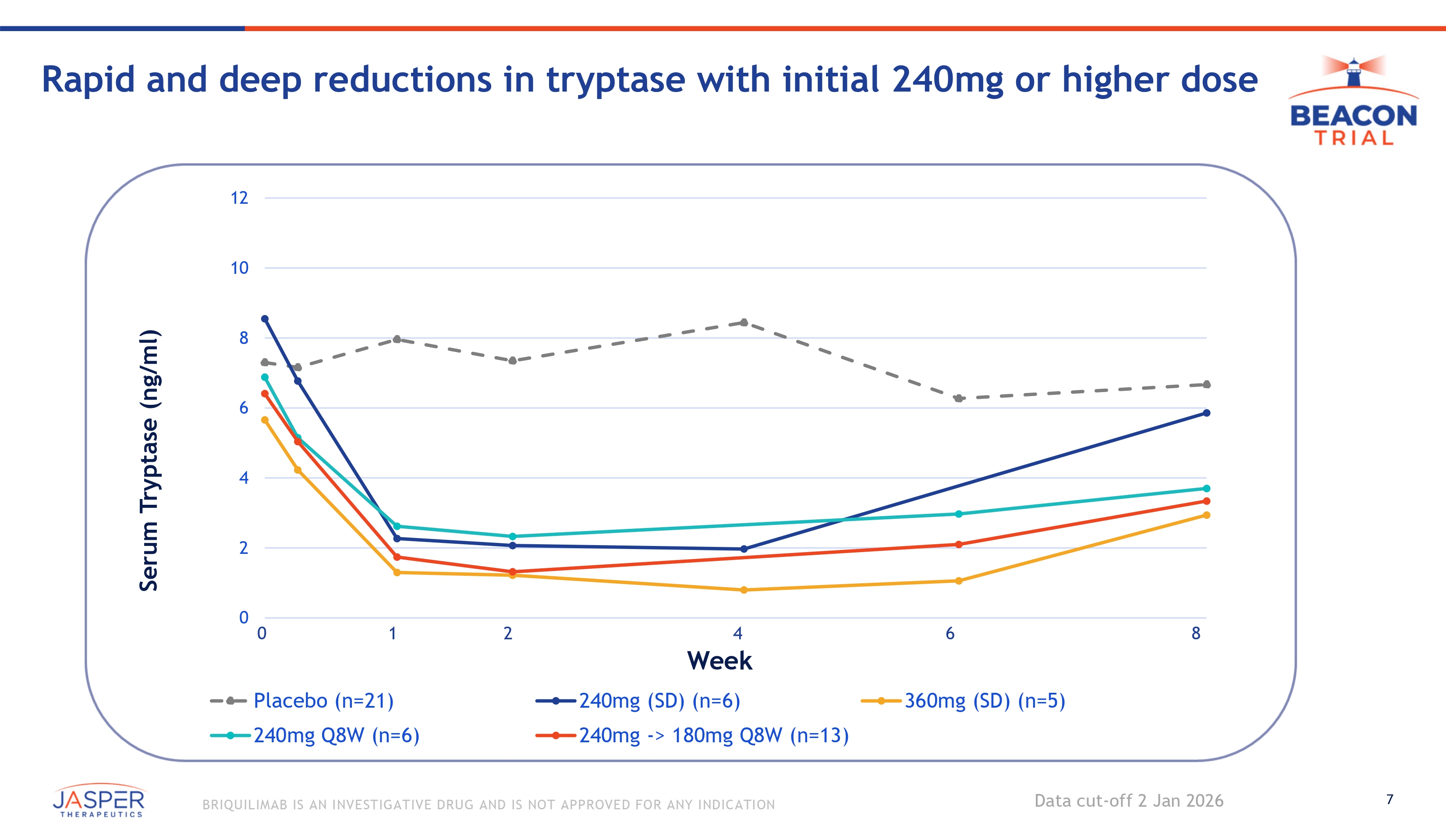
7 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Rapid and deep reductions in tryptase with initial 240mg or higher dose Data cut - off 2 Jan 2026 Serum Tryptase (ng/ml) 12 10 8 6 4 2 360mg (SD) (n=5) 0 0 1 Placebo (n=21) 240mg Q8W (n=6) 2 4 Week 240mg (SD) (n=6) 240mg - > 180mg Q8W (n=13) 6 8
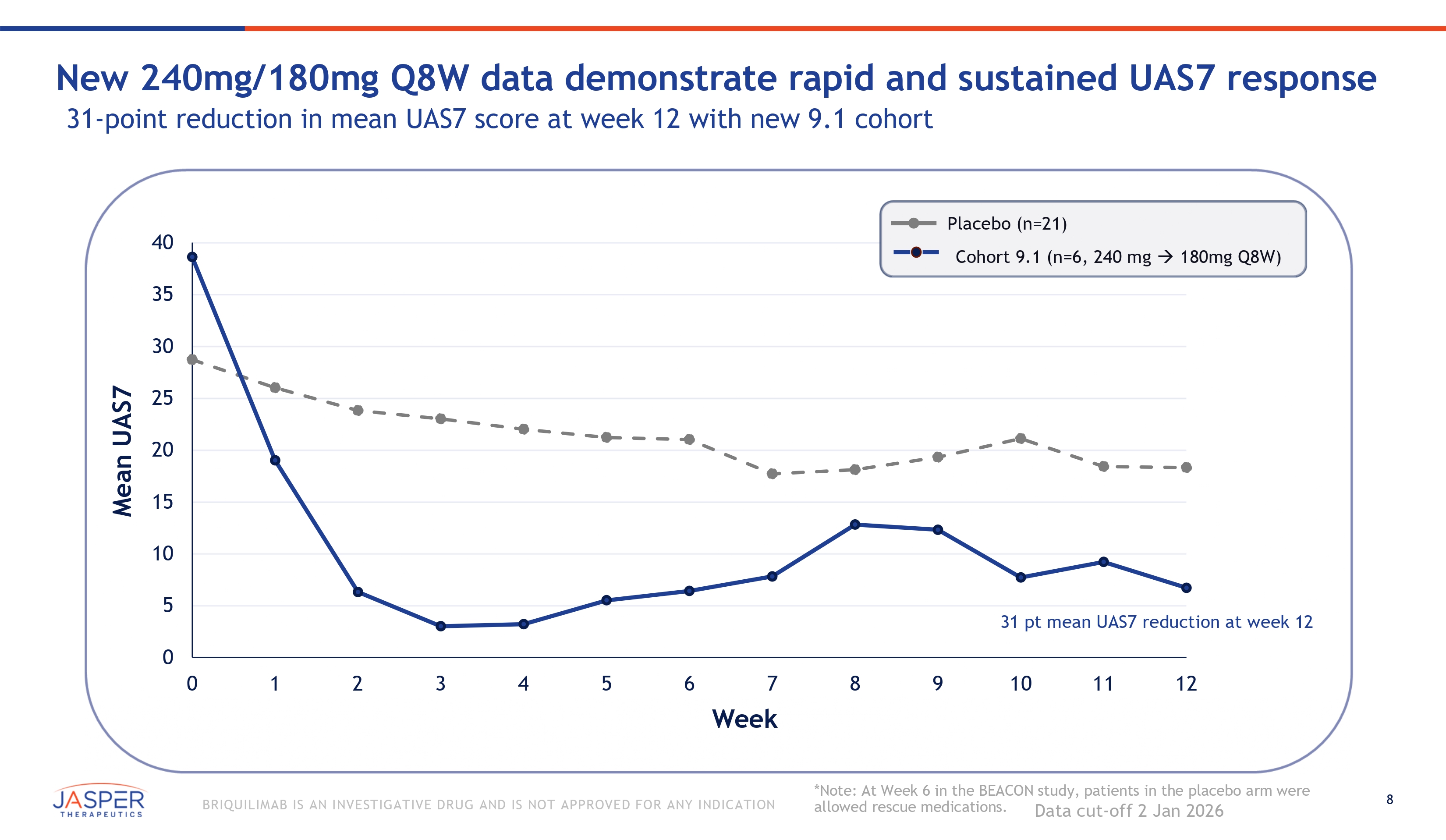
8 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION New 240mg/180mg Q8W data demonstrate rapid and sustained UAS7 response 31 - point reduction in mean UAS7 score at week 12 with new 9.1 cohort Mean UAS7 0 35 30 25 20 15 10 5 40 0 1 2 3 4 5 6 7 Week 8 9 Placebo (n=21) Cohort 9.1 (n=6, 240 mg 180mg Q8W) 31 pt mean UAS7 reduction at week 12 10 11 12 *Note: At Week 6 in the BEACON study, patients in the placebo arm were allowed rescue medications. Data cut - off 2 Jan 2026
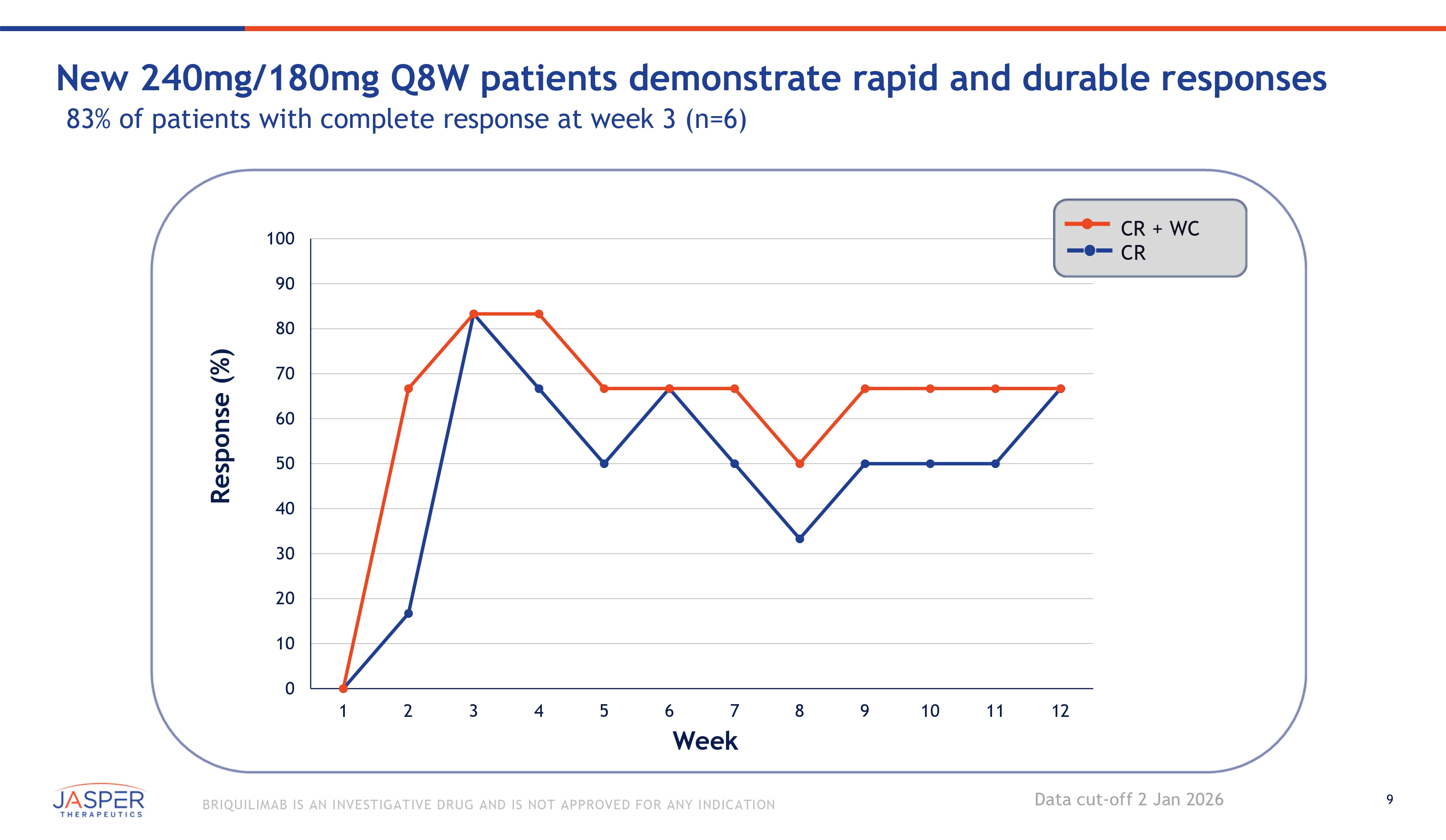
9 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Response (%) 90 80 70 60 50 40 30 20 10 0 100 1 2 3 4 5 6 7 Week 8 9 10 11 12 CR + WC CR New 240mg/180mg Q8W patients demonstrate rapid and durable responses 83% of patients with complete response at week 3 (n=6) Data cut - off 2 Jan 2026
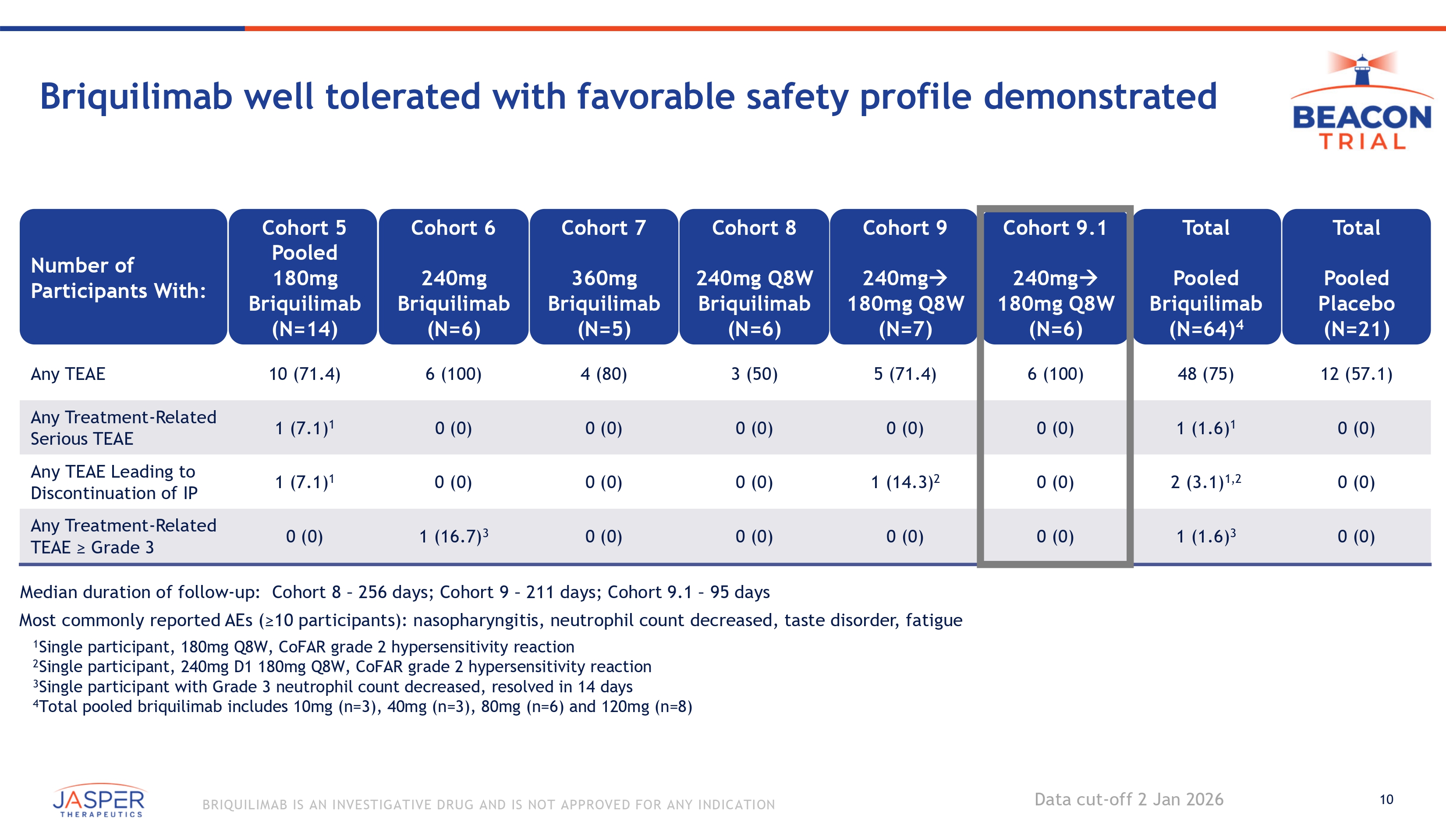
10 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab well tolerated with favorable safety profile demonstrated Total Pooled Placebo (N= 21 ) 12 ( 57 . 1 ) Total Pooled Briquilimab (N=64) 4 48 (75) Cohort 9.1 240mg 180mg Q8W (N=6) 6 (100) Cohort 9 240mg 180mg Q8W (N=7) 5 (71.4) Cohort 8 240mg Q8W Briquilimab (N=6) 3 (50) Cohort 7 360mg Briquilimab (N=5) 4 (80) Cohort 6 240mg Briquilimab (N=6) 6 (100) Cohort 5 Pooled 180mg Briquilimab (N=14) 10 (71.4) Number of Participants With: Any TEAE 0 (0) 1 (1.6) 1 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 1 (7.1) 1 Any Treatment - Related Serious TEAE 0 (0) 2 (3.1) 1,2 0 (0) 1 (14.3) 2 0 (0) 0 (0) 0 (0) 1 (7.1) 1 Any TEAE Leading to Discontinuation of IP 0 (0) 1 (1.6) 3 0 (0) 0 (0) 0 (0) 0 (0) 1 (16.7) 3 0 (0) Any Treatment - Related TEAE ≥ Grade 3 Median duration of follow - up: Cohort 8 – 256 days; Cohort 9 – 211 days; Cohort 9.1 – 95 days Most commonly reported AEs (≥10 participants): nasopharyngitis, neutrophil count decreased, taste disorder, fatigue 1 Single participant, 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 2 Single participant, 240mg D1 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 3 Single participant with Grade 3 neutrophil count decreased, resolved in 14 days 4 Total pooled briquilimab includes 10mg (n=3), 40mg (n=3), 80mg (n=6) and 120mg (n=8) Data cut - off 2 Jan 2026
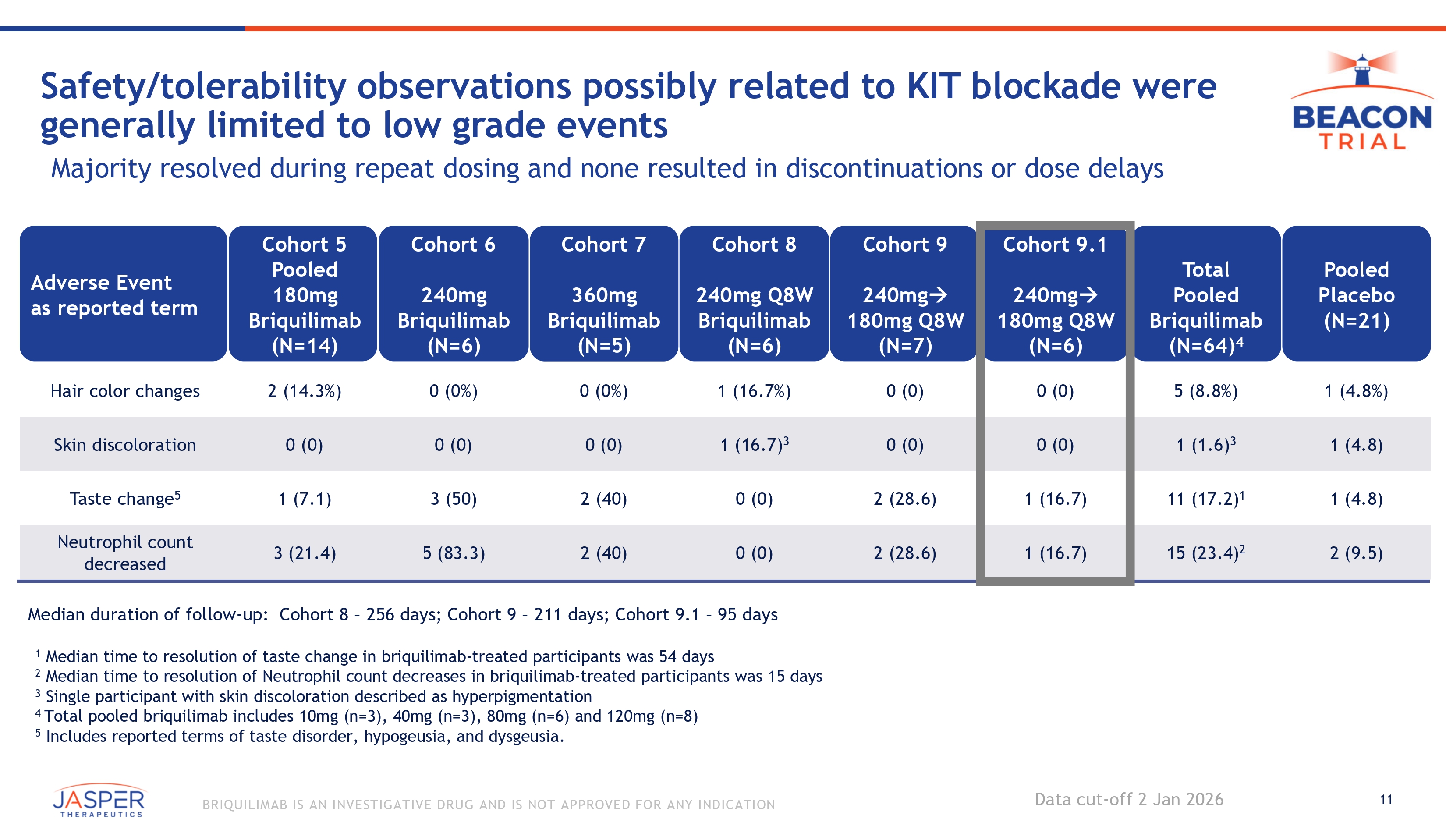
11 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Safety/tolerability observations possibly related to KIT blockade were generally limited to low grade events Majority resolved during repeat dosing and none resulted in discontinuations or dose delays Pooled Placebo (N= 21 ) 1 ( 4 . 8 % ) Total Pooled Briquilimab (N=64) 4 5 (8.8%) Cohort 9.1 240mg 180mg Q8W (N=6) 0 (0) Cohort 9 240mg 180mg Q8W (N=7) 0 (0) Cohort 8 240mg Q8W Briquilimab (N=6) 1 (16.7%) Cohort 7 360mg Briquilimab (N=5) 0 (0%) Cohort 6 240mg Briquilimab (N=6) 0 (0%) Cohort 5 Pooled 180mg Briquilimab (N=14) 2 (14.3%) Adverse Event as reported term Hair color changes 1 (4.8) 1 (1.6) 3 0 (0) 0 (0) 1 (16.7) 3 0 (0) 0 (0) 0 (0) Skin discoloration 1 (4.8) 11 (17.2) 1 1 (16.7) 2 (28.6) 0 (0) 2 (40) 3 (50) 1 (7.1) Taste change 5 2 (9.5) 15 (23.4) 2 1 (16.7) 2 (28.6) 0 (0) 2 (40) 5 (83.3) 3 (21.4) Neutrophil count decreased Median duration of follow - up: Cohort 8 – 256 days; Cohort 9 – 211 days; Cohort 9.1 – 95 days 1 Median time to resolution of taste change in briquilimab - treated participants was 54 days 2 Median time to resolution of Neutrophil count decreases in briquilimab - treated participants was 15 days 3 Single participant with skin discoloration described as hyperpigmentation 4 Total pooled briquilimab includes 10mg (n=3), 40mg (n=3), 80mg (n=6) and 120mg (n=8) 5 Includes reported terms of taste disorder, hypogeusia, and dysgeusia. Data cut - off 2 Jan 2026
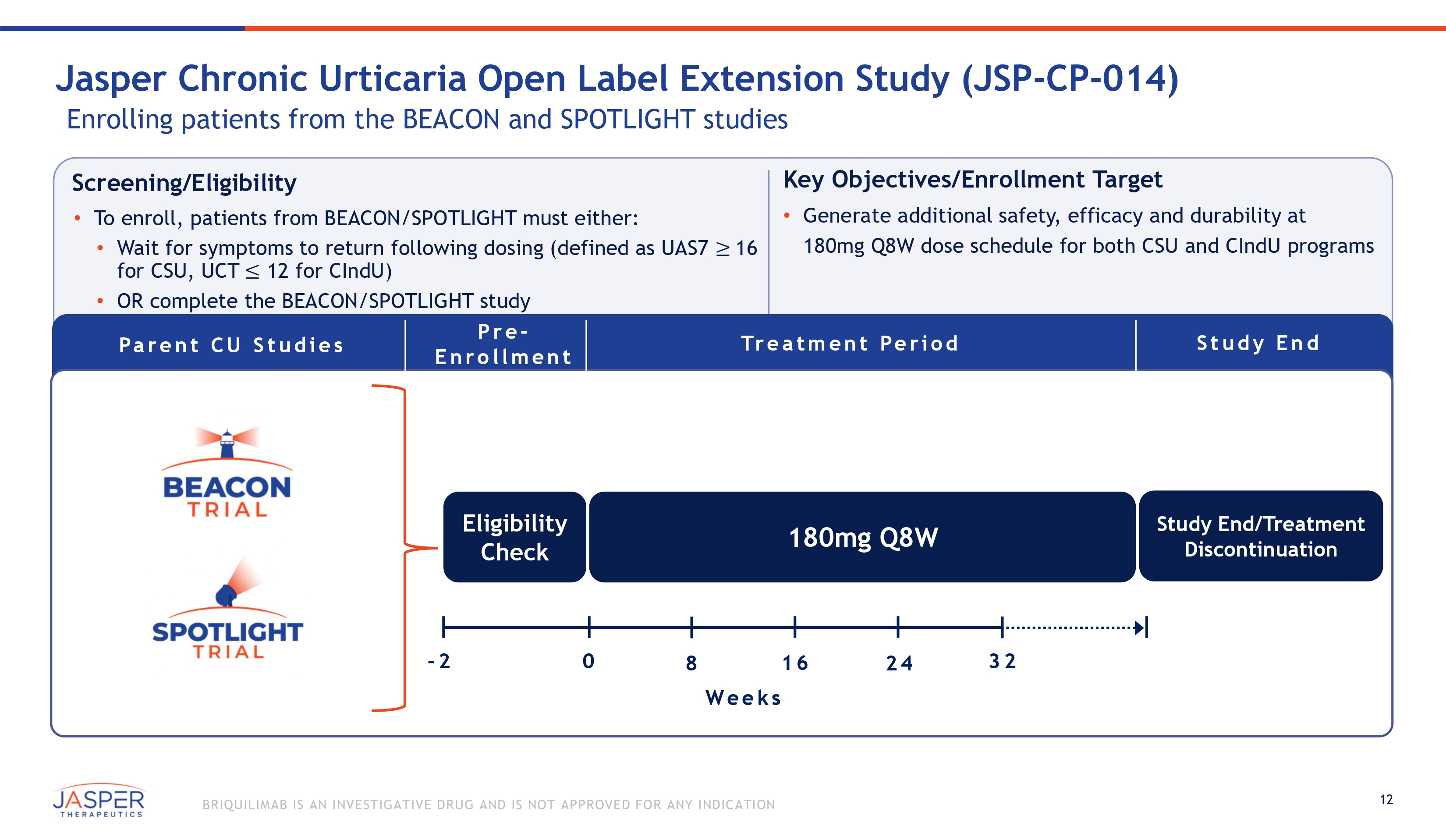
12 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Screening/Eligibility • To enroll, patients from BEACON/SPOTLIGHT must either: • Wait for symptoms to return following dosing (defined as UAS7 ≥ 16 for CSU, UCT ≤ 12 for CIndU) • OR complete the BEACON/SPOTLIGHT study Key Objectives/Enrollment Target • Generate additional safety, efficacy and durability at 180mg Q8W dose schedule for both CSU and CIndU programs Jasper Chronic Urticaria Open Label Extension Study (JSP - CP - 014) Enrolling patients from the BEACON and SPOTLIGHT studies Pa r e n t C U S t u d i e s Tr e a t m e n t Pe r i o d Pre - E n rollment 180mg Q8W Eligibility Check We e k s - 2 0 8 16 24 32 Study End/Treatment Discontinuation Stu d y End
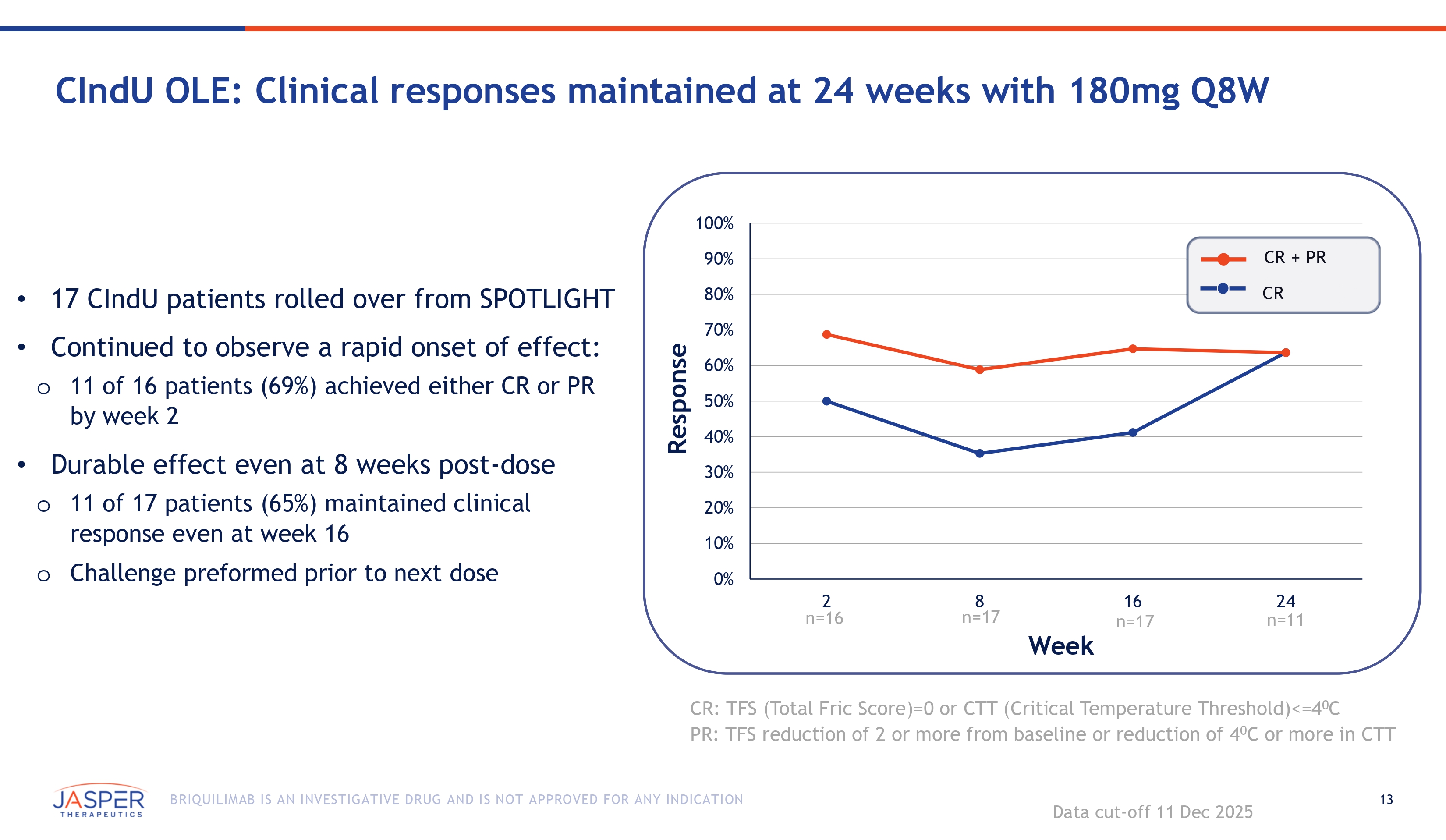
13 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION CIndU OLE: Clinical responses maintained at 24 weeks with 180mg Q8W • 17 CIndU patients rolled over from SPOTLIGHT • Continued to observe a rapid onset of effect: o 11 of 16 patients (69%) achieved either CR or PR by week 2 • Durable effect even at 8 weeks post - dose o 11 of 17 patients (65%) maintained clinical response even at week 16 o Challenge preformed prior to next dose Response Week CR: TFS (Total Fric Score)=0 or CTT (Critical Temperature Threshold)<=4 0 C PR: TFS reduction of 2 or more from baseline or reduction of 4 0 C or more in CTT Data cut - off 11 Dec 2025 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 2 n=16 8 n=17 16 n=17 24 n=11 CR + PR CR
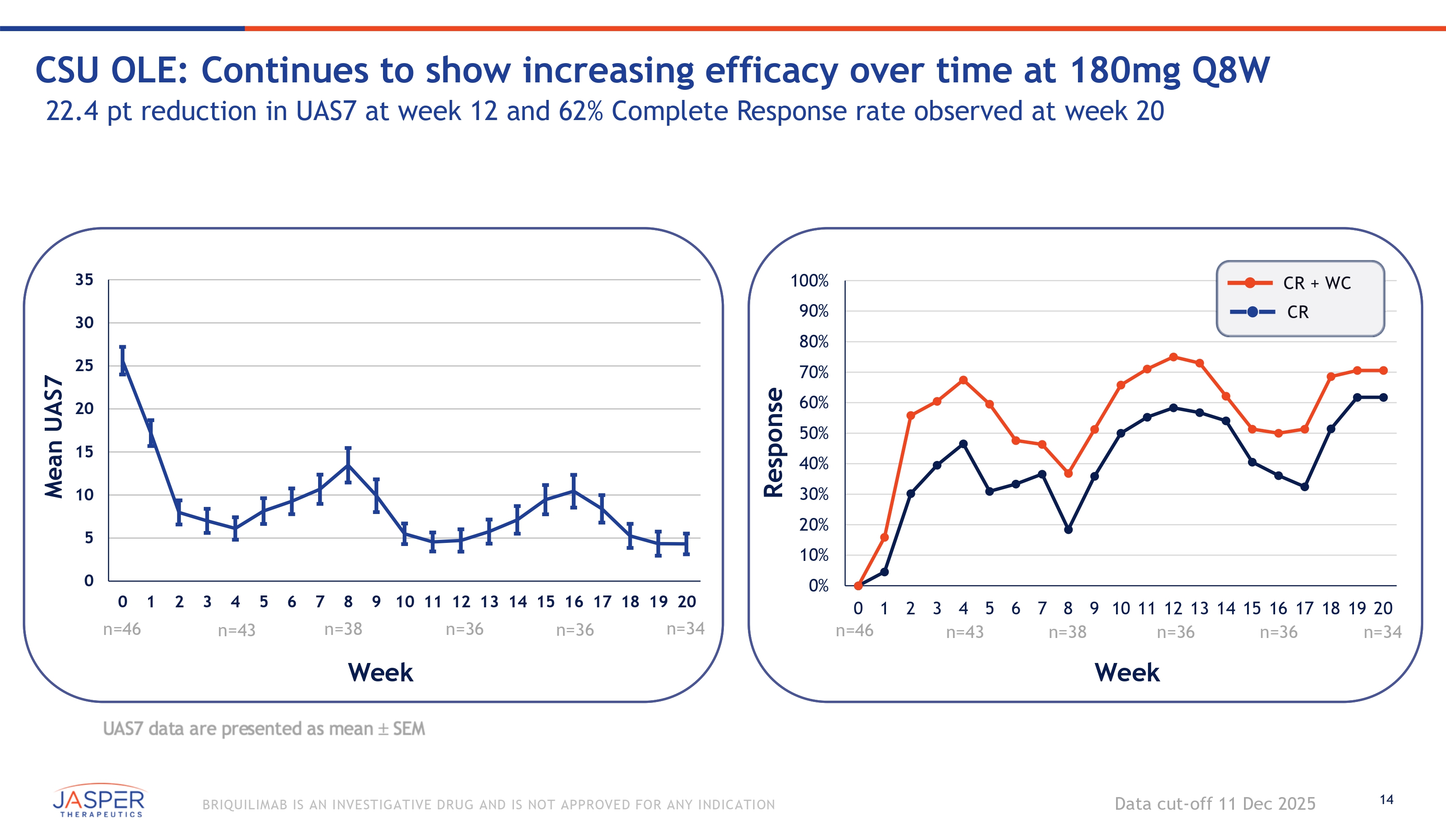
14 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION 0 5 10 15 20 25 30 35 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 n=46 CSU OLE: Continues to show increasing efficacy over time at 180mg Q8W 22.4 pt reduction in UAS7 at week 12 and 62% Complete Response rate observed at week 20 Data cut - off 11 Dec 2025 Mean UAS7 n=43 n=38 n=36 n=36 Week 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Response CR + WC CR n=38 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 n=46 n=43 n=36 n=36 n=34 Week n=34
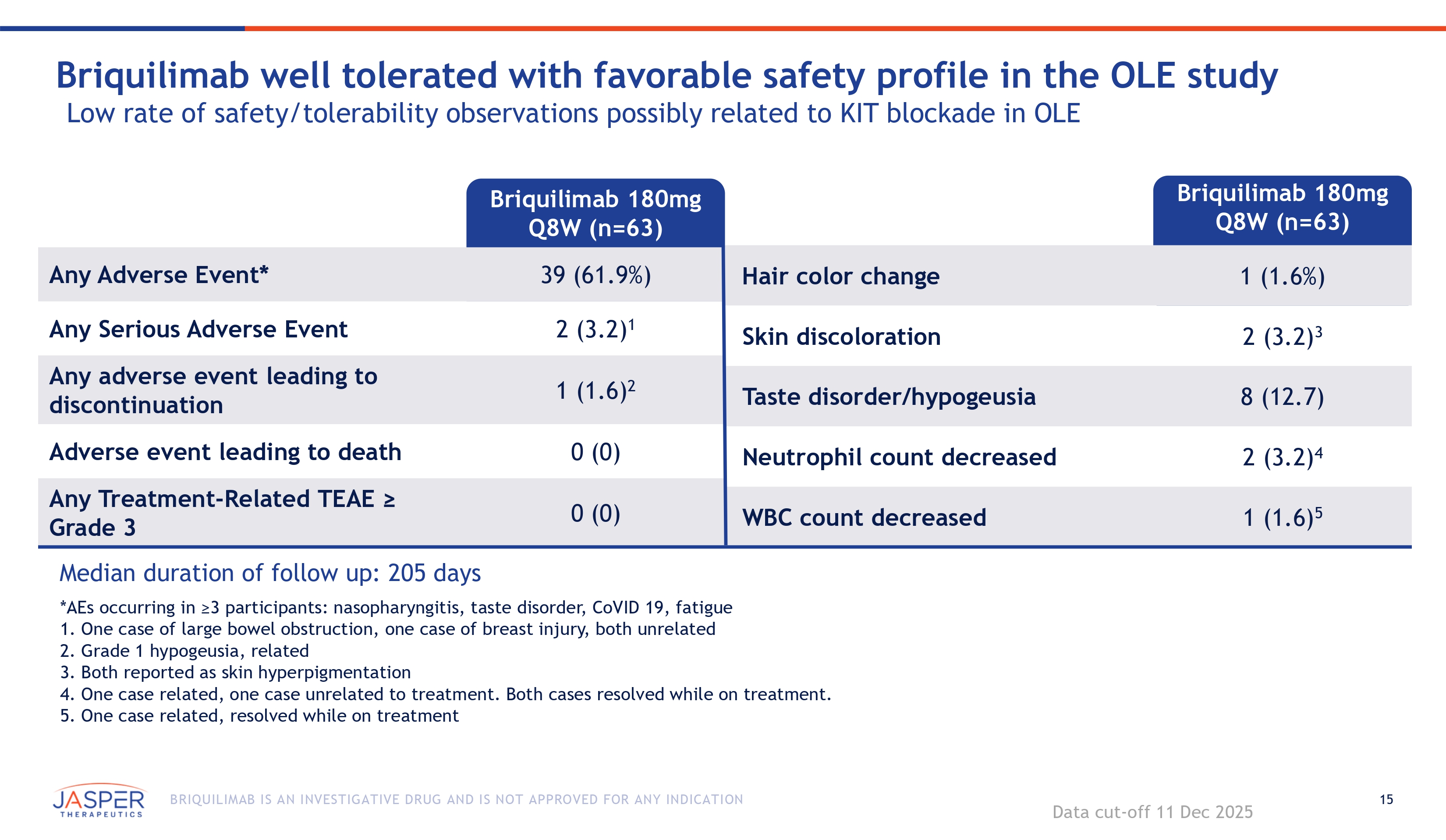
15 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab 180mg Q8W (n=63) Briquilimab well tolerated with favorable safety profile in the OLE study Low rate of safety/tolerability observations possibly related to KIT blockade in OLE Briquilimab 180mg Q8W (n=63) 1 (1.6%) Hair color change 39 (61.9%) Any Adverse Event* 2 (3.2) 3 Skin discoloration 2 (3.2) 1 Any Serious Adverse Event 1 (1.6) 2 Any adverse event leading to discontinuation 8 (12.7) Taste disorder/hypogeusia 2 (3.2) 4 Neutrophil count decreased 0 (0) Adverse event leading to death 0 (0) Any Treatment - Related TEAE ≥ Grade 3 1 (1.6) 5 WBC count decreased Median duration of follow up: 205 days *AEs occurring in ≥3 participants: nasopharyngitis, taste disorder, CoVID 19, fatigue 1. One case of large bowel obstruction, one case of breast injury, both unrelated 2. Grade 1 hypogeusia, related 3. Both reported as skin hyperpigmentation 4. One case related, one case unrelated to treatment. Both cases resolved while on treatment. 5. One case related, resolved while on treatment Data cut - off 11 Dec 2025

Conclusion and Next Steps Jeet Mahal, CEO
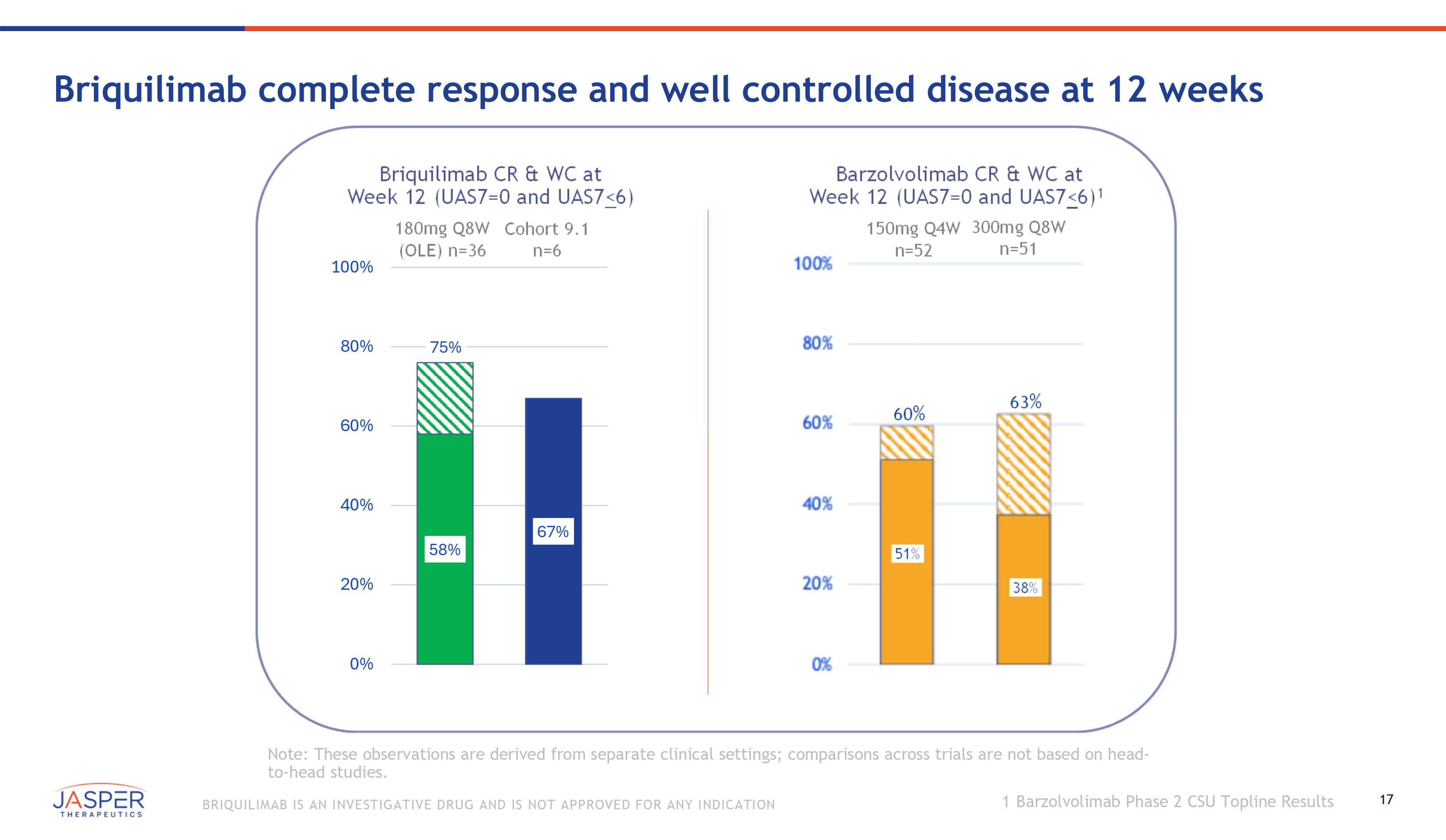
17 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab complete response and well controlled disease at 12 weeks Note: These observations are derived from separate clinical settings; comparisons across trials are not based on head - to - head studies. 51% 38% Briquilimab CR & WC at Barzolvolimab CR & WC at Week 12 (UAS7=0 and UAS7<6) Week 12 (UAS7=0 and UAS7<6) 1 180mg Q8W Cohort 9.1 150mg Q4W 300mg Q8W (OLE) n=36 n=6 n=52 n=51 60 % 63 % 58% 67% 75% 0% 20% 40% 60% 80% 100% 1 Barzolvolimab Phase 2 CSU Topline Results
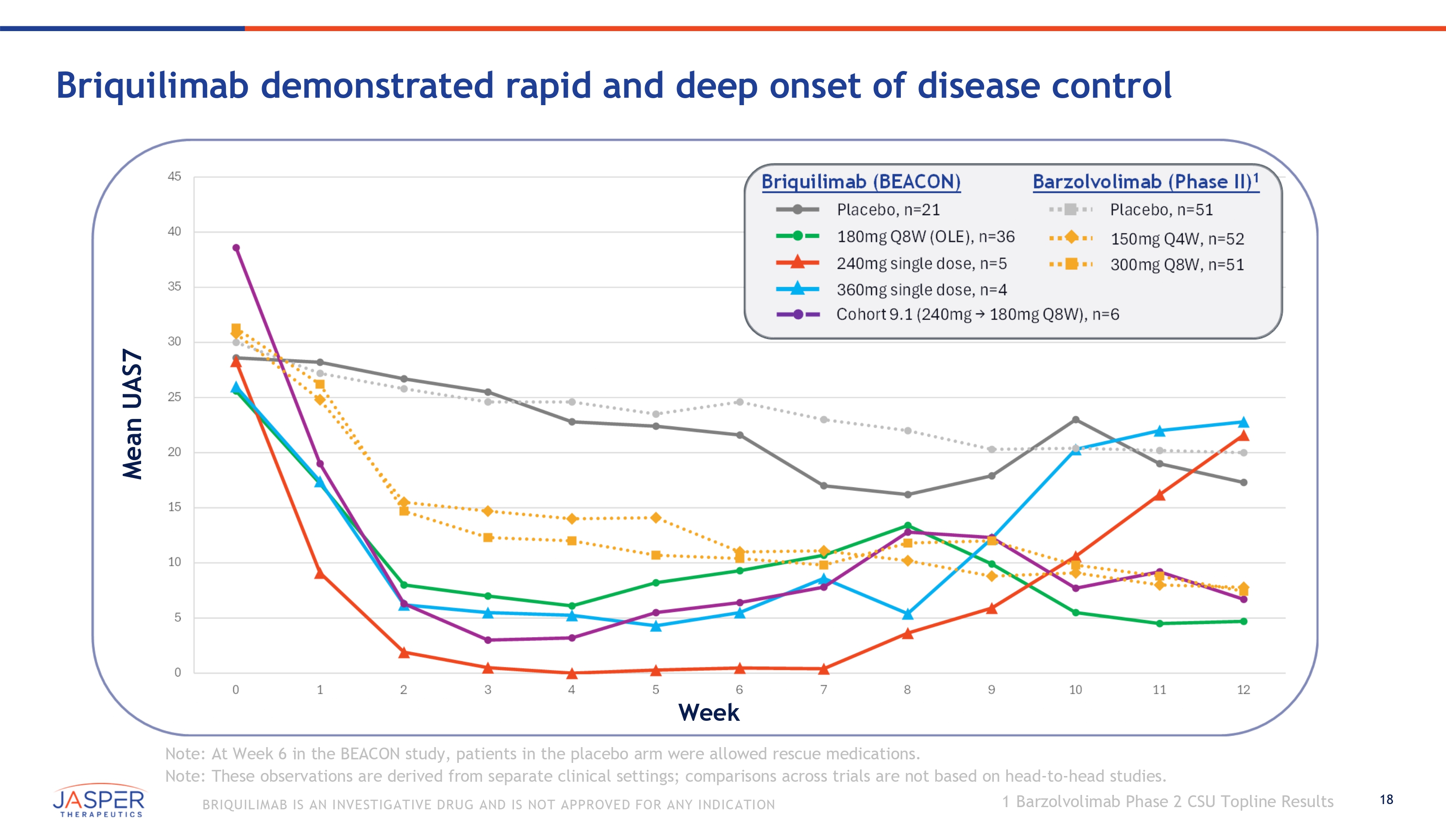
18 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION Briquilimab demonstrated rapid and deep onset of disease control 45 Briquilimab (BEACON) Barzolvolimab (Phase II) 1 Placebo, n=21 Placebo, n=51 40 180mg Q8W (OLE), n=36 150mg Q4W, n=52 240mg single dose, n=5 300mg Q8W, n=51 35 360mg single dose, n=4 Cohort 9.1 (240mg . 180mg Q8W), n=6 30 25 20 15 10 5 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Week Note: At Week 6 in the BEACON study, patients in the placebo arm were allowed rescue medications. Note: These observations are derived from separate clinical settings; comparisons across trials are not based on head - to - head studies. Mean UAS7 1 Barzolvolimab Phase 2 CSU Topline Results
19 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION January 2026 Chronic Urticaria Data Update Briquilimab demonstrates rapid efficacy and a favorable chronic safety profile • Rapid and durable clinical responses • Repeat dose safety data now available for more than 50 patients across BEACON and OLE studies • KIT related AEs continue to be predominantly low frequency, transient, low - grade events that resolved while on study Data gathered to - date sufficient to enable dose selection for CSU Phase 2b study • Targeting commencement of Phase 2b CSU study 2H 2026 Totality of data from BEACON, SPOTLIGHT and OLE support a compelling profile for briquilimab in chronic urticarias • Briquilimab’s unique drug properties allow for rapid disease control while minimizing KIT - related AEs

Jasper Therapeutics NASDAQ: JSPR January 2026